Report to the Board June 2015
|
|
|
- Amanda Wiggins
- 5 years ago
- Views:
Transcription
1 10-11 June 2015 SUBJECT: EBOLA VACCINE AND MITIGATION PLAN Report of: Authored by: Agenda item: 14 Seth Berkley, MD, Chief Executive Officer G. Vanden Bossche, Stefano Malvolti, Aurelia Nguyen, Alan Brooks, Patience Masanhu, Magdi Ibrahim, Maryse Dugue Category: For Information Strategic goal: Alliance operations Section A: Overview 1. Purpose of the report 1.1 The purpose of this report is to update the Board on the latest developments relevant to Gavi s involvement in the development of an Ebola vaccine, on the activities in the three Ebola affected countries as well as on the discussions held in the past month at the Programme and Policy Committee and at the Research & Development Ebola summit organised by the World Health Organisation. 2. Recommendations 2.1 This report is for information only. 3. Executive summary This paper provides an update on Gavi s continued efforts to implement previous Board decisions and on the adaptation of those efforts to the evolution of the epidemic. New external developments are described below whereas the approach taken to adapt to these developments is outlined in points below. 3.1 Evolution of the epidemic: To date (May 17 th ), there have been a total of 26,933 reported confirmed, probable, and suspected cases of Ebola Virus Disease (EVD) in Guinea, Liberia and Sierra Leone with 11,120 reported deaths (including over 500 deaths in health workers). Overall, the geographic area of transmission is now primarily restricted to the coastal areas of these countries. The outbreak in Liberia was declared over on May 9 th. A recent substantial resurgence in cases has been observed in Guinea with total of 27 confirmed cases in the last week compared to 7 in the previous one. Following a steep decline from December to late January, the weekly case incidence now seems to have flattened out in 1
2 Sierra Leone, hovering between 2 and 12 cases per week. Capacity for improved community engagement, case investigation, and targeted, active surveillance continues to be strengthened in areas of continuing transmission to ensure that remaining chains of transmission are detected, contained, and brought to an end. 3.2 Clinical trials: Only one out of the 5 vaccine candidates for which an Expression of Interest (EOI) towards Gavi procurement was submitted to UNICEF is currently being tested in phase III clinical trials in Guinea and Sierra Leone (Merck vaccine). Because of the substantial decline in the number of Ebola cases, however, the likelihood that the ongoing clinical trials will generate conclusive efficacy data to support a normal regulatory approval process is very limited. Other candidate vaccines have still not completed phase II (Johnson and Johnson) or phase I (Tianjin CanSino Biotechnology and Novovax) or have not been able to move on into phase III for lack of Ebola cases (e.g. GSK vaccine tested in the phase II/ III trial in Liberia that was terminated ahead of schedule). 3.3 Supply and procurement of vaccines: several challenges exist because of the current state of development of the vaccine candidates, including: the ambition to support multiple manufacturers in a market characterised by a lack of predictability on the desired volume of regimens; and a lack of knowledge of the preferred vaccine for procurement. The Secretariat, UNICEF SD and WHO are working closely together to develop a funding mechanism and procurement options that comply with the vaccination scenarios, which are currently most likely to occur while ensuring continued engagement of vaccine manufacturers in ensuring preparedness for a potential future outbreak. The Secretariat is leading with UNICEF SD the engagement with manufacturers of the Ebola vaccines that have progressed further in the development process. This effort aims at agreeing on the most appropriate mechanisms and contract structure for the immediate procurement of vaccines to be used in the at risk population or for stockpiling. Additionally the negotiation aims at enabling adequate responsiveness to an evolving epidemic and sufficient flexibility to respond to evolving progress on vaccine development efforts to maximise the effective use of funds and public health benefits. 3.4 The Secretariat is working with the partners to ensure full readiness to deploy a vaccine as soon as available and recommended for use, and to ensure timely support country to recovery efforts. 3.5 Deployment of the Ebola vaccine: the Ebola Vaccine Deployment Work Group, which consists of global health agencies and Ebola affected countries, has developed operational plans, guidance documents and tools for different Ebola vaccination roll-out scenarios. These plans target the at-risk population of health care and community workers as well as contacts of cases. These documents are designed to help immunisation programme managers and their partners to plan, organise, implement and evaluate Ebola Vaccine deployment activities. The group is also focusing on other activities critical to vaccine roll-out, including social mobilisation 2
3 and community engagement, and monitoring and evaluation. The draft supply chain management strategy is available and will be further refined. To ensure preparedness the group is also developing tools to strengthen management of financial resources, for example macro planning and light application processes. Learning from clinical trials and from the emergency response activities (e.g. resistance to health care interventions in the communities) potential obstacles to implementation are carefully analysed and incorporated into the deployment plans. Vaccine roll-out in the identified target population is not expected to start before the first quarter of 2016 given the evolving timelines of the ongoing phase III trials. 3.6 Recovery of immunisation and health systems: Gavi is working to support national restoration efforts which include catch-up campaigns - to reach those children who missed their routine vaccines - and broader interventions supporting national efforts to strengthen immunisation programmes as a key element of the primary health care system. Restoration of routine immunisation and health system recovery are critical to enable the three countries to face potential epidemic outbreaks over the next 12 to 18 months, and to regain confidence of communities. Countries have now started to shift attention from the Ebola response to longer-term recovery. All three countries have submitted their routine immunisation and health system recovery plans with support from partners. Joint missions undertaken in the first quarter of 2015 by Gavi and partners have enabled the National Interagency Coordinating Mechanisms to finalise and validate immunisation recovery plans. These plans were harmonised with the long-term national health system recovery strategies in preparation, ensuring complementarity with other funding partners support. The immunisation recovery plans take into account the absorptive capacity of each country level and envisage the appropriate arrangements for financial management through technical partners. All three have been recommended for approval by the High Level Review Panel in May Prompt resumption of immunisation activities, supported by these resources from Gavi, are critical to avoid outbreaks of vaccine-preventable diseases. Small size measles outbreaks have occurred in the three countries and immediate response measures been implemented, however the re-establishment of appropriate coverage levels will be key to avoid new outbreaks of larger size. As cases of meningitis are reported in Guinea, the country has planned to conduct a Meningitis A immunisation campaign next October. Civil Society Organisations from the three countries are also working to develop proposals related to the decision by the Board to allocate US$ 500k to their role in rebuilding confidence in the health system. Gavi, Global Fund, WHO and others will be meeting with countries the week of 8 June 2015 to coordinate support for the long-term recovery efforts, prior to health system strengthening applications expected later in Fund raising: The evolving dynamic of the epidemic and status of clinical trial results triggers a fund raising trade-off between ensuring sufficient funds to guarantee both preventive vaccine deployment in at risk 3
4 populations and preparedness for reactive vaccine roll-out against the total funding needs and uncertainty for the use of donor funds. Manufacturers are seeking input from Gavi, and Gavi is working to ensure that capacity is not funded that will not be required. In this context successful fundraising requires sufficient precision in understanding the most likely demand. The Secretariat has been working to maintain alignment between the resource requirements and the evolving requirements of this epidemic so to be able to finalise an investment case that accurately reflects an appropriate level of financing needed for the initiative. 3.8 During the latest Programme and Policy Committee (PPC) held in Geneva on May 4-6, Gavi support to countries in their efforts to quickly reactivate their immunisation systems was discussed. Special attention was dedicated to the need to minimise risk of emerging epidemics of other vaccine preventable diseases, in particular measles. The PPC also discussed Gavi engagement in the re-establishment of health systems with particular focus on the tailoring of this support to countries absorption capacity and on the need for tight alignment of Gavi s effort with the one of the other development partners active in the field. Finally the PPC discussed whether and how Gavi should engage in future efforts aimed at improving preparedness and enabling a more effective response to Ebolalike emergencies. PPC members agreed that many of the issues raised during this discussion would merit further discussion and agreed that while it would not be possible at the October 2015 meeting due to other priorities it should be incorporated into the PPC workplan. 3.9 In the context of EVD and other global public health threats, the World Health Organization has recently convened a Summit on Ebola Research and Development (R&D) on May in Geneva. The main objective of the Summit was to look at some of the lessons learned during the Ebola outbreak and to use this experience to brainstorm on how to better manage R&D efforts to improve preparedness and timely response to future infectious diseases emergencies. There was a general consensus amongst all participants that current R&D efforts should be continued and integrated into a broader set of Public Health response measures. With regard to the role of vaccines, Gavi s CEO highlighted a number of scenarios where vaccines fall short of impact because not optimised (e.g. current Ebola vaccine candidates), not available in sufficient quantities (e.g. in case of pandemic influenza) or not sufficiently advanced into clinical development (e.g. pilot vaccines for MERS, SARS, etc.). Based on the discussion and recommendations from key stakeholders, the WHO will initiate the development of a draft Blueprint for research and development preparedness in the context of global public health threats. Ultimately, the goal of the Blueprint will be to foster effective mechanisms for efficient collaboration and information sharing across all the organisations involved and to expedite the discovery, development, assessment and access to effective and safe health technologies to prevent and control infectious diseases of epidemic and pandemic potential. 4
5 Section B: Progress Update as of May 20th 4. Update on the outbreak as per the May 20th situation report. 4.1 In the week to 17 May, a total of 27 new confirmed cases were reported in Guinea and 8 in Sierra Leone. This is the highest weekly total of confirmed cases of Ebola virus disease (EVD) for over a month and represents a substantial increase compared with the 9 cases (in Guinea and Sierra Leone combined) reported the previous week. Also the geographical area of transmission has expanded compared with recent weeks. 4.2 Whereas in Sierra Leone almost all of the cases could be traced to previous registered contacts of a previous Ebola case, a total of 9 of the 27 cases reported from Guinea originated from an unknown source. Most of the other confirmed cases were linked to the attendance of a funeral. 4.3 Community engagement and effective contact tracing in battling the disease have proven particularly challenging in Sierra Leone and even more so in Guinea, thus making disease transmission particularly hard to combat effectively and rendering contact tracing in the area very difficult; this may explain why chains of transmission continue to evade detection in several areas and underscores the need to improve community engagement strategies to make contact tracing more effective. 5. Update on clinical Ebola vaccine development and potential regulatory pathways as per May 20th 5.1 Because of the lack of Ebola cases, randomised controlled trials in Liberia with the two lead candidate vaccines rvsv-zebov (Merck/New Link/Public Health Canada) and ChAd3-ZEBOV (GSK/ NIAID) will no longer be conducted as phase III studies, as initially planned, but as phase II studies, enabling collection of more extensive safety data. 5.2 In Guinea, a Phase III trial (rvsv-zebov) started in March 2015; the study comprises a ring vaccination study and a cohort study in front-line workers (FLWs). So far, 53 rings have been enrolled (the final target to enrol 200 rings has now shifted to 100 rings comprising 50 persons each) and 486 FLWs have been vaccinated (the final target is to immunise about 1,500 FLWs). Given the current evolution of the epidemic, preliminary data from this study are not expected to be available before the end of August. 5.3 Another phase III trial using rvsv-zebov (stepped wedge), called the Sierra Leone Trial to Introduce a Vaccine against Ebola (STRIVE), started on April 8 and plans to assess the safety and efficacy of the vaccine among 8000 health workers (about 1670 individuals immunised as on May 16). The study completion is anticipated before the end of Other manufacturers are about to finalise their phase I study reports (Novavax; Ebola GP vaccine comprising a recombinant nanoparticle with Matrix-M adjuvant) or are preparing for the start of their phase II trials (Tianjin Cansino Biotechnology Inc.; recombinant adenovirus Ebola 5
6 vaccine comprising a lyophilised type-5 adenovector). Johnson & Johnson is in the process of finalizing their phase II trials (Janssen/ Bavarian Nordic; Ad26/MVA vaccine). 5.5 While the overall fall off in Ebola cases in the 3 affected countries is obviously welcome from an individual and public health viewpoint, it poses a threat to the successful conclusion of ongoing pivotal vaccine trials. Despite adaptation of clinical trial protocols to the current epidemic situation, clinical efficacy data may not be conclusive or even meaningful. The likely inability of the trials to reach conclusions on clinical efficacy has raised the question as to whether vaccine candidates could be regulated through alternative regulatory pathways (e.g., accelerated/ conditional approval or approval based on animal rule). This is currently subject of considerable debate among regulatory authorities as immune correlates of protection have not been unambiguously identified, animal models have not been validated and the perceived risk-benefit balance of the vaccine keeps shifting with the further waning of the epidemic. 5.6 Given the dramatic consequences of the Ebola epidemic, especially in populations who are at high risk of exposure (e.g., front line workers and contacts), there is still a possibility, that a current lead candidate vaccine would gain a recommendation from WHO for emergency use. Such recommendation could be backed by an alternative regulatory approval process based upon a combination of animal and human data. This scenario will require collection of additional safety data through extensive post-authorisation surveillance. The approval granted under these exceptional circumstances may only be temporary unless additional clinical efficacy and safety data would ultimately enable full licensure according to a routine regulatory approval process. 5.7 Depending on the evolution of the epidemic, it is likely that WHO will investigate whether the epidemic still qualifies as a Public Health Emergency of International Concern before the clinical trial data become available. This may also prompt the question as to whether the option of a regulatory pathway of approval for use outside the normal process of a full registration from a National Regulatory Authority should be maintained. Gavi is monitoring closely the evolving regulatory scenario; regulatory agencies continue to be actively engaged and attempting to be flexible and helpful. 5.8 Based on the current pace of enrolment in clinical trials and the subsequent regulatory assessments, it is highly unlikely that a WHO recommendation will be granted before the end of As a result it is also increasingly unlikely that any investigational Ebola vaccine will be available for procurement and deployment before the first quarter of Update on Procurement of Ebola Vaccine 6.1 In December 2014, the Gavi Board authorized up to US$ 300 million during 2015/16 to support production and procurement of up to 12 million courses of first generation Ebola vaccine and a global stockpile of 6
7 vaccines from The Gavi Executive Committee (EC) has the authority to approve both the funding structure and the final number of courses to be procured contingent on WHO recommendations. 6.2 The declining Ebola disease incidence is having some implications for the anticipated immediate needs for vaccine production and procurement, which was first addressed at the EC in March In that meeting a recommendation was made to shift the planning to a medium demand scenario while retaining some flexibility to achieve greater volumes if required. This reflected a shift away from the assumption of large scale vaccination of the adult population of the three affected countries used in December Since March a further decrease in the number of Ebola cases has occurred and as a direct consequence the likelihood of a WHO recommendation for use and the anticipated need for large volume of vaccine courses in 2015 have both become less likely. Gavi is now addressing the challenge of keep on adapting to the evolving situation while retaining the principles that drove the initial decision to fund vaccine production and procurement. Managing this trade-off means establishing a financing structure that allows to ensure, on one hand that sufficient production capacity can be made available for immediate deployment and stockpiling, on the other hand that manufacturers receive sufficient incentives to continue their development efforts. These goals are pursued while balancing stewardship of the funds committed to Ebola. 6.4 To accommodate the evolved and still uncertain situation without compromising our principles or flexibility to respond to a resurgence of the current epidemic or a potential future outbreak, the Secretariat is currently working on a funding strategy proposal, which would allow to take into consideration different scenarios for addressing current and potential future needs for Ebola vaccines. 7. Update on the preparatory activities for deployment and surveillance/ monitoring of Ebola vaccine immunisation 7.1 Based on the most recent information on the clinical trials and on the potential alternative regulatory pathway is highly unlikely that an Ebola vaccine will be deployed before early The Ebola vaccine deployment partner team was set up with WHO, UNICEF, USAID, CDC, BMGF and Gavi Secretariat with two face-to-face meetings in January and February. Four subgroups have been created to focus on supply and procurement, vaccine implementation and surveillance/ monitoring/ evaluation, and social mobilisation and community engagement. Deployment scenarios are being developed for vaccination of health care workers and at-risk populations; other scenarios are to be developed depending on the evolution of the epidemic. 7.3 Preparatory activities are being conducted to account for appropriate cold chain equipment and handling of deep-frozen vaccines (as currently 7
8 implemented for the clinical trials). The use of candidate vaccines in clinical studies is supported by stability data at current storage and distribution temperature (-60 C). Gavi and other funders are working to ensure manufacturers proceed with requested stability plans assessing storage temperatures that would allow the authorised vaccines to be aligned with other EPI vaccines. 7.4 A roadmap defining the required step for the recommendation for deployment of an Ebola vaccine (once data and results from regulatory review are available) has been produced recently and will be discussed at the next SAGE meeting. The document states that recommendations for vaccine use and country implementation cannot be made at this stage. The need for complete and detailed data to be made available from the pivotal vaccine trials to inform any recommendation is highlighted as well as the importance of public health decision-making being based on clinical safety and efficacy endpoints. 7.5 In the meantime, guidance documents and standard operating procedures have been drafted to deal with critically important aspects of vaccine deployment: accommodation of potential vaccine scenarios, training of health care workers, work-plans and supply (cold chain and logistics) for vaccine roll-out, risk and crisis communication, community awareness/ education and social mobilisation, and a template for requesting funding. An approval process for release of funds is being discussed and will be developed in consultation with partners. 7.6 Likewise, a partner team has been set up with WHO, UNICEF and CDC to organise surveillance and monitoring of adverse events following immunisation. The budget for consultants and economic evaluation has been prepared and submitted to the steering group. 8. Update on the recovery of routine immunisation activities and Health Systems in the countries 8.1 Liberia, Guinea and Sierra Leone's routine immunisation (RI) programmes and coverage levels have been severely affected by the recent Ebola outbreak. In all three countries, vaccine coverage has dropped by at least 20-40%, and outbreaks of measles have been reported in the last three to six months, with the majority of measles cases and related deaths occurring in Sierra Leone, and most recently in Guinea (three since mid- February). Since there is an immediate need to deal with the drop in coverage and the large number of under-immunised/unimmunised children, Gavi currently regards the recovery of routine immunisation activities as the highest Ebola priority. Hence, the Alliance has worked at different levels (Headquarters, Regional and in-country) to readily implement the Gavi Board approved elements contributing to EPI recovery efforts. A transparent, tailored approval process for release of approximately US$ 12.5 million of Alliance s funds for EPI recovery efforts against the country plans has been established. 8
9 8.2 One of the most critical considerations in implementing Gavi support in Ebola-affected countries is the pre-existing weakness of country health systems (in particular, limited health workforces), community concerns about re-engaging with health systems, and countries limited capacity to absorb additional resources. The closure of many health facilities and the loss of large numbers of health care workers (371 cases and 179 deaths in Liberia, for example) have adversely affected primary health care services. The closure has also had an impact on financing of immunisation services: in Guinea, the Ebola epidemic control has a budget of about US$ 150 million and the coordination reports directly to the president, adding complexity to the role of the Ministry of Health. 8.3 To help restore of EPI programmes in the immediate term, Gavi is therefore supporting interventions proposed by countries aimed at: restoring trust in the health system through social mobilisation and CSOs; planning and conducting catch-up campaigns with multiple antigens; supporting programme management; strengthening logistics and ensuring adequate supply of vaccines and infection protection control equipment; and accelerating recruitment and training of healthcare workers. These restoration plans in the three countries have been aligned with the National Recovery plans 8.4 Liberia, with support from WHO and UNICEF, prepared a draft EPI recovery plan in late The plan was discussed during a Gavi field visit, and immediate priorities for Gavi funding were identified with the Minister of Health and the Alliance partners, and submitted for ICC review and approval in March The estimated cost is US$ 2.9 million over 12 months. The immediate response has already resulted in two rounds of periodic intensification of routine immunisation (PIRI) campaigns targeting MCV (December 2014 and February 2015) and currently two additional SIAs (polio and measles) being conducted. Further intensification of EPI strengthening efforts is planned for the second half of Guinea has prepared its EPI recovery plan, with the support of an external consultant. The recovery plan was discussed with national stakeholders and technical partners and approved by the ICC in April The plan is estimated to cost US$ 6 million over 12 to 15 months (including a measles SIA). A measles outbreak response immunisation (ORI) was performed in two districts in the first quarter of 2015, with the support of UNICEF. A second ORI, covering 10 districts, was conducted from April 2015, targeting children 6 months to 10 years of age, and the World Bank funded the Measles Outbreak Response Plan. A meningitis outbreak may be under way but has not been officially declared yet. Up to the 20 th of May of this year, 191 suspected cases were notified by the districts, with 16 deaths; out of 178 cases serology tested, 102 were found positive (95 Neisseria Meningitis A, 6 Streptococcus pneumoniae and 1 Haemophilus Influenza Type b). While the country is preparing to launch a national immunisation campaign, Gavi has released US$ 334,518 to UNICEF for social mobilization/ communication equipment/ maintenance of the cold chain and US$ 1,987,018 to WHO for other operational costs. Preparing 9
10 the micro-planning for the campaign even with the support of the technical partners will be a challenging undertaking. 8.6 Sierra Leone's recovery plan has been drafted and was reviewed by Gavi during a mission in March, with priority interventions identified for financial support approved by the HSCC/ICC. It is estimated at US$ 4.1 million. A polio SIA is planned for April/May Additionally, a polio and measles ORI (for children aged 9-59 months) is planned in the framework of a multi-intervention campaign, Mother Child Health Week, with a financial contribution released in May from Gavi to support this effort. 8.7 Disbursement of financial support for the EPI recovery plan has been worked out with countries and partners to ensure best use of resource, timely availability of funding and to prevent any risk of misuse of funding. Depending on financial management agreements in place, funds will be disbursed either through partners or through government systems, with adequate reporting in place. 8.8 Plans for the use of the funds over the coming months, as well as previously approved HSS grants, were reviewed by the High Level Review Panel in May and recommended for approval. The country plans are followed country-level coordination to ensure Gavi s support was harmonised with that of other funding partners. The country plans are including, where appropriate, reallocation of existing HSS commitments in countries (approximately US$ 900,000 for Guinea, US$ 3.6 million for Sierra Leone and US$ 400,000 for Liberia). Proposals to help rebuild confidence in health services and immunisation are anticipated to be submitted by CSOs by the end of May. Finally, Guinea has requested, as part of its recovery plans, that Gavi waives its 2014 and 2015 co-financing requirements, while Liberia and Sierra Leone have requested a 2015 cofinancing waiver, having already fulfilled their 2014 co-financing obligations. 8.9 Alliance partners including WHO, UNICEF, the Gates Foundation, the US Centers for Disease Control, the World Bank, CSOs and the Global Fund are coordinating support for longer-term health system recovery efforts. They are also collaborating to ensure Gavi's support complements World Bank and other resources that will be committed to national Health System Resilience Plans. The approximate doubling of HSS support approved by the Gavi Board will be most critical in 2016 and beyond when emergency funds begin to decrease. A tailored process for approving the HSS grants through the IRC, most likely in September, will be established Three main principles have been employed in preparing and finalising the recovery plans: providing a response that is tailored to the specific needs of the countries, financial mitigation (channelling funding through technical partners) and harmonisation and accountability in implementing the plan (putting in place a steering committee in charge of the follow-up and agree with other financial partners and stakeholders on an M&E framework to document the implementation process). 10
11 9. Fundraising: key steps and timelines through mid Following the Board approval of the Ebola envelope, the Secretariat has been working to fully understand the resource requirements in the face of an ever-changing epidemic and to finalise an investment case that accurately reflects an appropriate level of financing needed for the initiative. The Resource Mobilisation team has engaged with the African Development Bank (AfDB) to seek their support in the fundraising effort. The AfDB has pledged US$ 50 million towards Gavi s Ebola initiative. 9.2 The forthcoming investment case could allow for flexible fundraising to accommodate different levels of support depending on the various stages of vaccine development and outbreak trajectory. The case will also highlight the key uncertainties (e.g. around Ebola outbreak trajectory) that still remain and which could influence the amount of funds that could potentially be needed over the long-term. Section C: Implications 10. Impact on potential funding envelopes 10.1 It is worth noting that there are a number of funding expectations for Gavi irrespective of how the outbreak develops. In all cases, Gavi will be funding the committed Recovery envelope ($45M). In addition, Gavi s commitment to address the Ebola vaccine market failure may include incurring some production scale up and indirect manufacturing costs in addition to direct production costs. Therefore, if the number of doses procured is low, the funding envelope will not necessarily decrease linearly with the number of doses procured. 11. Use of the Country Tailored Approach 11.1 As consequence of the continuing emergency situation in the affected countries, the Secretariat is handling all activities related to the preparation and deployment of an Ebola vaccine as well as the recovery activities for health systems and existing immunisation programmes utilising the flexibility granted by the Country Tailored Approach for Emergency Situations. This approach is required to ensure that all necessary interventions can be implemented and that those activities unfold in a timely manner. Governance and operations have been variably affected in the health systems of these countries and normal authorisation and implementation practices may not always be viable. This situation creates a trade-off between speed necessary for a prompt response to outbreaks of Ebola or VPDs and control necessary to ensure appropriate stewardship of the funds. The utilisation of the Country Tailored Approach allows Gavi through its Senior Country Managers and the in-county partners representative to design specific route of support that minimise the risk on the grant management side. In this sense funds for the recovery plans, including the technical assistance in Guinea and Sierra Leone, are being transferred to the partners. Specific monitoring 11
12 systems will be established to make sure that control of funding flows is ensured even in this especially difficult setting. 12. Looking forward 12.1 While we have made good progress since our last update in March, to get a more precise picture it will be important to gain further clarity and/or traction in a few priority areas. Consequently, in the coming weeks/months, the Secretariat and the Alliance partners will focus on: (a) Continuing to track outbreak evolution and clinical trial developments in order to assess their implications for Gavi's Ebola activities (b) Understanding manufacturers development plans and explain the vaccination scenarios we anticipate to facilitate our negotiations on funding mechanisms and commitments (c) Refining manufacturer cost and subsidy estimates, providing estimates for funding and refining the procurement and stockpiling scenarios as needed (d) Aligning on a preferred funding/contracting mechanism with manufacturers (e) Finalising high level operational plans for vaccination of the target population for each affected country (f) Agree with country partners on a clear performance framework to ensure that the activities aimed at restoring immunisation coverage in the affected countries will be implemented timely during the next transitional period of 18 months. (g) Working with Ministries of Health and partners on finalizing national health recovery plans (h) Finalising the investment case and continuing dialogue with donors 12
Report to the Board 2-3 December 2015
 2-3 December 2015 SUBJECT: EBOLA UPDATE Report of: Authored by: Agenda item: 16 Seth Berkley, MD, Chief Executive Officer G. Vanden Bossche, Stefano Malvolti, Aurelia Nguyen, Alan Brooks, Patience Masanhu,
2-3 December 2015 SUBJECT: EBOLA UPDATE Report of: Authored by: Agenda item: 16 Seth Berkley, MD, Chief Executive Officer G. Vanden Bossche, Stefano Malvolti, Aurelia Nguyen, Alan Brooks, Patience Masanhu,
R&D for potential vaccines for the Ebola virus
 R&D for potential vaccines for the Ebola virus Update 4 March 2015 Roberto Bertollini MD MPH Chief Scientist and WHO Representative to the EU Gathering knowledge Since August 2014, series of consultations
R&D for potential vaccines for the Ebola virus Update 4 March 2015 Roberto Bertollini MD MPH Chief Scientist and WHO Representative to the EU Gathering knowledge Since August 2014, series of consultations
Licenced Ebola Vaccine
 The Market Shaping Goal Shape vaccine markets to ensure adequate supply of appropriate, quality vaccines at low and sustainable prices for developing countries. Supply and Procurement Roadmap Licenced
The Market Shaping Goal Shape vaccine markets to ensure adequate supply of appropriate, quality vaccines at low and sustainable prices for developing countries. Supply and Procurement Roadmap Licenced
Preparations for Ebola Vaccine Deployment
 Preparations for Ebola Vaccine Deployment Ebola vaccine and vaccination - Session 8 Meeting of the Strategic Advisory Group of Experts on Immunization (SAGE) Geneva, 14 16 April 2015 Dr Marie-Pierre Preziosi,
Preparations for Ebola Vaccine Deployment Ebola vaccine and vaccination - Session 8 Meeting of the Strategic Advisory Group of Experts on Immunization (SAGE) Geneva, 14 16 April 2015 Dr Marie-Pierre Preziosi,
Report to the Board December 2014
 10-11 December 2014 SUBJECT: Report of: Authored by: Agenda item: 05 ACCELERATING ACCESS TO EBOLA VACCINES AND COUNTRY PERSPECTIVE Robert Newman, Managing Director, Policy and Performance Alan Brooks,
10-11 December 2014 SUBJECT: Report of: Authored by: Agenda item: 05 ACCELERATING ACCESS TO EBOLA VACCINES AND COUNTRY PERSPECTIVE Robert Newman, Managing Director, Policy and Performance Alan Brooks,
From research to large-scale use
 Accelerating access to Ebola vaccines From research to large-scale use Dr Marie-Paule Kieny Assistant Director General HIS 1 Number of cases Ebola response roadmap: Situation report 3 December 2014 Sierra
Accelerating access to Ebola vaccines From research to large-scale use Dr Marie-Paule Kieny Assistant Director General HIS 1 Number of cases Ebola response roadmap: Situation report 3 December 2014 Sierra
STATUS OF REVIEWS, AUTHORIZATIONS AND OVERSIGHT FOR CLINICAL TRIALS IN THE WHO AFRICAN REGION. Technical Document
 30 August 2017 REGIONAL COMMITTEE FOR AFRICA ORIGINAL: ENGLISH Sixty-seventh session Victoria Falls, Republic of Zimbabwe, 28 August 1 September 2017 Agenda item 17 STATUS OF REVIEWS, AUTHORIZATIONS AND
30 August 2017 REGIONAL COMMITTEE FOR AFRICA ORIGINAL: ENGLISH Sixty-seventh session Victoria Falls, Republic of Zimbabwe, 28 August 1 September 2017 Agenda item 17 STATUS OF REVIEWS, AUTHORIZATIONS AND
STATUS OF REVIEWS, AUTHORIZATIONS AND OVERSIGHT FOR CLINICAL TRIALS IN THE WHO AFRICAN REGION. Technical Document
 15 June 2017 REGIONAL COMMITTEE FOR AFRICA ORIGINAL: ENGLISH Sixty-seventh session Victoria Falls, Republic of Zimbabwe, 28 August 1 September 2017 Provisional agenda item 17 STATUS OF REVIEWS, AUTHORIZATIONS
15 June 2017 REGIONAL COMMITTEE FOR AFRICA ORIGINAL: ENGLISH Sixty-seventh session Victoria Falls, Republic of Zimbabwe, 28 August 1 September 2017 Provisional agenda item 17 STATUS OF REVIEWS, AUTHORIZATIONS
Dialogue 1: Partnerships in support of strengthening health systems: Building resilience to pandemics 1
 ECONOMIC AND SOCIAL COUNCIL PARTNERSHIPS FORUM Dialogue 1: Partnerships in support of strengthening health systems: Building resilience to pandemics 1 Introduction 28 May 2015, 11:00 a.m. 1:00 p.m. UN
ECONOMIC AND SOCIAL COUNCIL PARTNERSHIPS FORUM Dialogue 1: Partnerships in support of strengthening health systems: Building resilience to pandemics 1 Introduction 28 May 2015, 11:00 a.m. 1:00 p.m. UN
Update from the Business Continuity Working Group
 Agenda item: 13 Report title: Report by: Action: Update from the Business Continuity Working Group Steve Jones, Head of Facilities, Resources and Quality Assurance, stjones@gmc-uk.org, 0161 923 6287 To
Agenda item: 13 Report title: Report by: Action: Update from the Business Continuity Working Group Steve Jones, Head of Facilities, Resources and Quality Assurance, stjones@gmc-uk.org, 0161 923 6287 To
CEPI Coalition for Epidemic Preparedness Innovations. CEPI a model for funding other initiatives and areas
 CEPI Coalition for Epidemic Preparedness Innovations CEPI a model for funding other initiatives and areas Foto: Daniel Berehulak, The New York Times Testing of an Ebola vaccine a successful but suboptimal
CEPI Coalition for Epidemic Preparedness Innovations CEPI a model for funding other initiatives and areas Foto: Daniel Berehulak, The New York Times Testing of an Ebola vaccine a successful but suboptimal
One Year Later, Where Does the U.S. Response to Ebola Stand?
 One Year Later, Where Does the U.S. Response to Ebola Stand? Kaiser Family Foundation, Washington, DC Jen Kates, PhD Vice President & Director, Global Health & HIV Policy Kaiser Family Foundation jkates@kff.org
One Year Later, Where Does the U.S. Response to Ebola Stand? Kaiser Family Foundation, Washington, DC Jen Kates, PhD Vice President & Director, Global Health & HIV Policy Kaiser Family Foundation jkates@kff.org
Current context and challenges; stopping the epidemic; and preparedness in non-affected countries and regions
 EXECUTIVE BOARD Special session on Ebola Provisional agenda item 3 EBSS/3/2 EXECUTIVE BOARD 136th session 9 January 2015 Provisional agenda item 9.4 Current context and challenges; stopping the epidemic;
EXECUTIVE BOARD Special session on Ebola Provisional agenda item 3 EBSS/3/2 EXECUTIVE BOARD 136th session 9 January 2015 Provisional agenda item 9.4 Current context and challenges; stopping the epidemic;
Ebola Virus disease in West Africa : challenges and lessons learned
 Ebola Virus disease in West Africa : challenges and lessons learned Pr N. Shindo Dr S.C. Briand Pandemic and Epidemic Diseases Department WHO Geneva EVD outbreak in West Africa in Numbers From24 March
Ebola Virus disease in West Africa : challenges and lessons learned Pr N. Shindo Dr S.C. Briand Pandemic and Epidemic Diseases Department WHO Geneva EVD outbreak in West Africa in Numbers From24 March
Ebola outbreak in West Africa : Shift in paradigm
 Ebola outbreak in West Africa : Shift in paradigm Dr S.C Briand, Director Pandemic and Epidemic Diseases department, WHO Geneva EVD West Africa: many "first time" Enormous epidemic in the affected countries
Ebola outbreak in West Africa : Shift in paradigm Dr S.C Briand, Director Pandemic and Epidemic Diseases department, WHO Geneva EVD West Africa: many "first time" Enormous epidemic in the affected countries
Guidance on Request for Information on Rapid Response Platform Technologies for Epidemic Preparedness
 Guidance on Request for Information on Rapid Response Platform Technologies for Epidemic Preparedness Purpose of this request for information The Bill & Melinda Gates Foundation (BMGF; also referred to
Guidance on Request for Information on Rapid Response Platform Technologies for Epidemic Preparedness Purpose of this request for information The Bill & Melinda Gates Foundation (BMGF; also referred to
Ebola Virus Disease in West Africa: From Crisis to More Resilient Health Systems
 Ebola Virus Disease in West Africa: From Crisis to More Resilient Health Systems Patricio V. Marquez Lead Health Specialist World Bank Health, Nutrition and Population Global Practice April 15, 2015 Outline
Ebola Virus Disease in West Africa: From Crisis to More Resilient Health Systems Patricio V. Marquez Lead Health Specialist World Bank Health, Nutrition and Population Global Practice April 15, 2015 Outline
Opportunities to find leverage for polio transition integration with other global health initiatives
 Opportunities to find leverage for polio transition integration with other global health initiatives Diana Chang Blanc, WHO Geneva IVB Transition Independent Monitoring Board, London 2 November 2017 1
Opportunities to find leverage for polio transition integration with other global health initiatives Diana Chang Blanc, WHO Geneva IVB Transition Independent Monitoring Board, London 2 November 2017 1
Accelerating Vaccine Development for Epidemic Preparedness: New Vaccines for a Safer World. Richard Hatchett, MD CEO, CEPI
 Accelerating Vaccine Development for Epidemic Preparedness: New Vaccines for a Safer World Richard Hatchett, MD CEO, CEPI Photo: Daniel Berehulak, The New York Times CEPI s Gestation (1) February 2016
Accelerating Vaccine Development for Epidemic Preparedness: New Vaccines for a Safer World Richard Hatchett, MD CEO, CEPI Photo: Daniel Berehulak, The New York Times CEPI s Gestation (1) February 2016
EBOLA RESPONSE PHASE 3
 EBOLA RESPONSE PHASE 3 Framework for achieving and sustaining a resilient zero SEPTEMBER 2015 Photo: WHO/M. Winkler WHO/EVD/Guidance/Strategy/15.3 World Health Organization 2015 All rights reserved. The
EBOLA RESPONSE PHASE 3 Framework for achieving and sustaining a resilient zero SEPTEMBER 2015 Photo: WHO/M. Winkler WHO/EVD/Guidance/Strategy/15.3 World Health Organization 2015 All rights reserved. The
IMI2 Ebola and other filoviral haemorrhagic fevers programme. Webinar for SRG and SC 3 November 2014
 IMI2 Ebola and other filoviral haemorrhagic fevers programme Webinar for SRG and SC 3 November 2014 Background Ebola virus diseases (EVD) is a rare and deadly disease Filoviruses such as Ebola spread directly
IMI2 Ebola and other filoviral haemorrhagic fevers programme Webinar for SRG and SC 3 November 2014 Background Ebola virus diseases (EVD) is a rare and deadly disease Filoviruses such as Ebola spread directly
The Power of Partnership
 The Foundation for Science and Technology 25 March, 2015 London Debate on from the Response to the Ebola Outbreak Chapter One The Power of Partnership 2 1 Some Key Points So Far Dec 2013 First Ebola case
The Foundation for Science and Technology 25 March, 2015 London Debate on from the Response to the Ebola Outbreak Chapter One The Power of Partnership 2 1 Some Key Points So Far Dec 2013 First Ebola case
Effective Vaccine Management
 WHO & UNICEF Joint Statement DRAFT VERSION Effective Vaccine Management 1 P a g e The Context In the early days of EPI since its launch 40 years ago at the World Health Assembly (in May 1974), vaccines
WHO & UNICEF Joint Statement DRAFT VERSION Effective Vaccine Management 1 P a g e The Context In the early days of EPI since its launch 40 years ago at the World Health Assembly (in May 1974), vaccines
Accelerated Ebola Vaccine Development: Phase 1-2
 15 April 2015 Vasee Moorthy MRCP PhD Accelerated Ebola Vaccine Development: Phase 1-2 WHO calls for accelerated ebola vaccine development We call on the international vaccine community to accelerate development
15 April 2015 Vasee Moorthy MRCP PhD Accelerated Ebola Vaccine Development: Phase 1-2 WHO calls for accelerated ebola vaccine development We call on the international vaccine community to accelerate development
Standard Competencies Framework for the Immunization Workforce
 Standard Framework for the Immunization Workforce Immunization Program Initiative Supporting the objective of the Expanded Program on Immunization DRAFT December 20, 2018 Version 2.1 Dedicated to the health
Standard Framework for the Immunization Workforce Immunization Program Initiative Supporting the objective of the Expanded Program on Immunization DRAFT December 20, 2018 Version 2.1 Dedicated to the health
Johnson & Johnson Announces Start of Clinical Trial of Ebola Vaccine Regimen in Sierra Leone
 Press Contacts: Ernie Knewitz +1 (732) 524-6623 +1 (917) 697-2318 Seema Kumar +1 (908) 405-1144 Investor Contacts: Louise Mehrotra +1 (732) 524-6491 Lesley Fishman +1 (732) 524-3922 Ronan Collins (EU)
Press Contacts: Ernie Knewitz +1 (732) 524-6623 +1 (917) 697-2318 Seema Kumar +1 (908) 405-1144 Investor Contacts: Louise Mehrotra +1 (732) 524-6491 Lesley Fishman +1 (732) 524-3922 Ronan Collins (EU)
Accelerating access to Ebola vaccines. Dr Manica Balasegaram, MSF Access Campaign Annecy, January 2015
 Accelerating access to Ebola vaccines Dr Manica Balasegaram, Annecy, January 2015 Introduction A strategic approach is key to accelerate access to Ebola vaccines; often parallel rather than sequential
Accelerating access to Ebola vaccines Dr Manica Balasegaram, Annecy, January 2015 Introduction A strategic approach is key to accelerate access to Ebola vaccines; often parallel rather than sequential
7TH E-NEWSLETTER. 7 th EBOVAC2. e-newsletter
 7 th EBOVAC2 e-newsletter November 2018 1 Welcome to the EBOVAC2 e-newsletter! EBOVAC2: Getting up to date The EBOVAC2 project is one of 8 projects funded under the IMI Ebola+ program that was launched
7 th EBOVAC2 e-newsletter November 2018 1 Welcome to the EBOVAC2 e-newsletter! EBOVAC2: Getting up to date The EBOVAC2 project is one of 8 projects funded under the IMI Ebola+ program that was launched
Page 1 of 11. WHO Target Product Profile for multivalent filovirus vaccines: providing long-term protection to high-risk populations.
 Page 1 of 11 WHO Target Product Profile for multivalent filovirus vaccines: providing long-term protection to high-risk populations November 2016 Page 2 of 11 Target Audience The target audience for this
Page 1 of 11 WHO Target Product Profile for multivalent filovirus vaccines: providing long-term protection to high-risk populations November 2016 Page 2 of 11 Target Audience The target audience for this
Queensland Strategy for Disaster Resilience. Making Queensland the most disaster resilient state in Australia
 Queensland Strategy for Disaster Resilience Making Queensland the most disaster resilient state in Australia 2 Foreword Message from the Deputy Premier As Queenslanders, we know full well what it is to
Queensland Strategy for Disaster Resilience Making Queensland the most disaster resilient state in Australia 2 Foreword Message from the Deputy Premier As Queenslanders, we know full well what it is to
WHO-MSD Collaboration to Bring an Ebola Vaccine to the Populations in Need
 WHO-MSD Collaboration to Bring an Ebola Vaccine to the Populations in Need Jules Millogo, MD, MSc Director, Public Health Partnerships Merck & Co, Inc. North Wales, PA, USA V920: rvsvδg-zebov-gp Vaccine
WHO-MSD Collaboration to Bring an Ebola Vaccine to the Populations in Need Jules Millogo, MD, MSc Director, Public Health Partnerships Merck & Co, Inc. North Wales, PA, USA V920: rvsvδg-zebov-gp Vaccine
The U.S. Response to Ebola: Status of the FY2015 Emergency Ebola Appropriation
 The U.S. Response to Ebola: Status of the FY2015 Emergency Ebola Appropriation Jen Kates, Josh Michaud, Adam Wexler, and Allison Valentine The Ebola outbreak of 2014 was a global wake-up call regarding
The U.S. Response to Ebola: Status of the FY2015 Emergency Ebola Appropriation Jen Kates, Josh Michaud, Adam Wexler, and Allison Valentine The Ebola outbreak of 2014 was a global wake-up call regarding
Combating Infectious Diseases Takes a Community: Lessons From the Ebola Epidemic
 Combating Infectious Diseases Takes a Community: Lessons From the 2014 2015 Ebola Epidemic May 1, 2015 Nahid Bhadelia, MD, MA Director of Infection Control and Medical Response, National Emerging Infectious
Combating Infectious Diseases Takes a Community: Lessons From the 2014 2015 Ebola Epidemic May 1, 2015 Nahid Bhadelia, MD, MA Director of Infection Control and Medical Response, National Emerging Infectious
To effectively manage risks to supply chain performance, a public health supply chain manager should understand the following: THE LOGISTICS CYCLE
 12 12 CHAPTER 12 SUPPLY CHAIN RISK MANAGEMENT FIGURE 11-1. THE LOGISTICS CYCLE Serving Customers WHAT A SUPPLY CHAIN MANAGER NEEDS TO KNOW: As detailed in this handbook, making health commodities available
12 12 CHAPTER 12 SUPPLY CHAIN RISK MANAGEMENT FIGURE 11-1. THE LOGISTICS CYCLE Serving Customers WHAT A SUPPLY CHAIN MANAGER NEEDS TO KNOW: As detailed in this handbook, making health commodities available
Regulatory system strengthening
 SIXTY-SEVENTH WORLD HEALTH ASSEMBLY A67/32 Provisional agenda item 15.6 14 March 2014 Regulatory system strengthening Report by the Secretariat 1. The Executive Board at its 134th session noted an earlier
SIXTY-SEVENTH WORLD HEALTH ASSEMBLY A67/32 Provisional agenda item 15.6 14 March 2014 Regulatory system strengthening Report by the Secretariat 1. The Executive Board at its 134th session noted an earlier
The U.S. Response to Ebola: Status of the FY2015 Emergency Ebola Appropriation
 November 2015 Issue Brief The U.S. Response to Ebola: Status of the FY2015 Emergency Ebola Appropriation Jen Kates, Josh Michaud, Adam Wexler, and Allison Valentine Overview The Ebola outbreak of 2014
November 2015 Issue Brief The U.S. Response to Ebola: Status of the FY2015 Emergency Ebola Appropriation Jen Kates, Josh Michaud, Adam Wexler, and Allison Valentine Overview The Ebola outbreak of 2014
Emergency Supply Chain Preparedness Overview Guide
 Emergency Supply Chain Preparedness Overview Guide June 2018 1 OVERVIEW CONTENTS Purpose of this overview: To provide sponsors, senior leadership, and interested partners with an introduction to chain
Emergency Supply Chain Preparedness Overview Guide June 2018 1 OVERVIEW CONTENTS Purpose of this overview: To provide sponsors, senior leadership, and interested partners with an introduction to chain
Ebola virus disease outbreak in western Africa
 Action Plan Strategic Objectives 6. Promote innovation and research on new drugs International surveillance networks and information sharing on promising research Active role in research for governments
Action Plan Strategic Objectives 6. Promote innovation and research on new drugs International surveillance networks and information sharing on promising research Active role in research for governments
Ministerial Review Terms of Reference. Better responses to natural disasters and other emergencies in New Zealand
 Ministerial Review Terms of Reference Better responses to natural disasters and other emergencies in New Zealand 1. Purpose This review will provide advice to the Minister of Civil Defence on the most
Ministerial Review Terms of Reference Better responses to natural disasters and other emergencies in New Zealand 1. Purpose This review will provide advice to the Minister of Civil Defence on the most
TED Challenge: Tracking & Tracing Vaccines in the GAVI Alliance Supply Chain
 TED Challenge: Tracking & Tracing Vaccines in the GAVI Alliance Supply Chain 15 February 2013 Vaccines are a powerful but sensitive technology. They have to be stored at a certain temperature, or they
TED Challenge: Tracking & Tracing Vaccines in the GAVI Alliance Supply Chain 15 February 2013 Vaccines are a powerful but sensitive technology. They have to be stored at a certain temperature, or they
Trade-Related Assistance: What Do Recent Evaluations Tell Us?
 3-4 NOVEMBER 2008 CONFERENCE CENTRE, PARIS Background Document for Session III Trade-Related Assistance: What Do Recent Evaluations Tell Us? MAIN FINDINGS AND KEY RECOMMENDATIONS 1 Identify the most adequate
3-4 NOVEMBER 2008 CONFERENCE CENTRE, PARIS Background Document for Session III Trade-Related Assistance: What Do Recent Evaluations Tell Us? MAIN FINDINGS AND KEY RECOMMENDATIONS 1 Identify the most adequate
PARTNERSHIP FOR MARKET READINESS (PMR) IMPLICATIONS OF THE PARIS AGREEMENT ON PMR ACTIVITIES Workshop Summary Monday 25 April, 2016 Lima, Peru
 PARTNERSHIP FOR MARKET READINESS (PMR) IMPLICATIONS OF THE PARIS AGREEMENT ON PMR ACTIVITIES Workshop Summary Monday 25 April, 2016 Lima, Peru 1.0 Introduction 1. This note summarizes discussions held
PARTNERSHIP FOR MARKET READINESS (PMR) IMPLICATIONS OF THE PARIS AGREEMENT ON PMR ACTIVITIES Workshop Summary Monday 25 April, 2016 Lima, Peru 1.0 Introduction 1. This note summarizes discussions held
European Centre for Disease Prevention and Control. Call for expression of interest for Seconded National Experts (ECDC/2017/SNE)
 European Centre for Disease Prevention and Control Call for expression of interest for Seconded National Experts (ECDC/2017/SNE) Applications are invited for the secondment at the European Centre for Disease
European Centre for Disease Prevention and Control Call for expression of interest for Seconded National Experts (ECDC/2017/SNE) Applications are invited for the secondment at the European Centre for Disease
MANAGING BETTER THE WORLD FOOD SYSTEM AND ADDRESSING THE CHALLENGES OF FOOD SECURITY IN THE 21 ST CENTURY: CONTRIBUTION OF THE UN SYSTEM HIGH LEVEL
 MANAGING BETTER THE WORLD FOOD SYSTEM AND ADDRESSING THE CHALLENGES OF FOOD SECURITY IN THE 21 ST CENTURY: CONTRIBUTION OF THE UN SYSTEM HIGH LEVEL TASK FORCE ON THE GLOBAL FOOD SECURITY CRISIS ODI: 11
MANAGING BETTER THE WORLD FOOD SYSTEM AND ADDRESSING THE CHALLENGES OF FOOD SECURITY IN THE 21 ST CENTURY: CONTRIBUTION OF THE UN SYSTEM HIGH LEVEL TASK FORCE ON THE GLOBAL FOOD SECURITY CRISIS ODI: 11
BUDGET REVISION OF SO FOR APPROVAL BY THE EXECUTIVE DIRECTOR
 BUDGET REVISION OF SO FOR APPROVAL BY THE EXECUTIVE DIRECTOR 6) To: Division Room Approval and Date Ms. Ertharin Cousin Executive Director OiC OED 6G30 5) Released for Approval: Division Room Signature
BUDGET REVISION OF SO FOR APPROVAL BY THE EXECUTIVE DIRECTOR 6) To: Division Room Approval and Date Ms. Ertharin Cousin Executive Director OiC OED 6G30 5) Released for Approval: Division Room Signature
Lessons learnt from the Ebola outbreak A regulatory position
 www.pei.de Lessons learnt from the Ebola outbreak A regulatory position Jan Mueller-Berghaus Paul-Ehrlich-Institut Federal Institute for Vaccines and Biomedicines, Germany The views expressed in this presentation
www.pei.de Lessons learnt from the Ebola outbreak A regulatory position Jan Mueller-Berghaus Paul-Ehrlich-Institut Federal Institute for Vaccines and Biomedicines, Germany The views expressed in this presentation
ACTIVITY WORK PLAN CONTENTS
 ACTIVITY WORK PLAN Activity Program Organisation Activity Plan Timeframe Strengthening the capacity, efficiency and effectiveness of Clinical Trials Networks through the Australian Clinical Trials Alliance
ACTIVITY WORK PLAN Activity Program Organisation Activity Plan Timeframe Strengthening the capacity, efficiency and effectiveness of Clinical Trials Networks through the Australian Clinical Trials Alliance
Management Board 16 June 2011 Budapest, Hungary
 mb 16 06 11 item 9 doc 7 Update on EFSA s science strategy Management Board 16 June 2011 Budapest, Hungary Subject : Update on EFSA s science strategy Document number: mb 16 06 11 item 9 doc 7 Submitted
mb 16 06 11 item 9 doc 7 Update on EFSA s science strategy Management Board 16 June 2011 Budapest, Hungary Subject : Update on EFSA s science strategy Document number: mb 16 06 11 item 9 doc 7 Submitted
Strengthening National Ebola Survivor Networks: Successes and Challenges
 REFLECTION Strengthening National Ebola Survivor Networks: Successes and Challenges INTRODUCTION The 2014 2016 Ebola outbreak in West Africa resulted in more than 11,300 deaths and over 10,000 survivors
REFLECTION Strengthening National Ebola Survivor Networks: Successes and Challenges INTRODUCTION The 2014 2016 Ebola outbreak in West Africa resulted in more than 11,300 deaths and over 10,000 survivors
CONSULATE GENERAL OF JAPAN 601 UNION STREET, SUITE 500 SEATTLE, WASHINGTON PHONE (206) FAX (206) NEWS RELEASE
 CONSULATE GENERAL OF JAPAN 601 UNION STREET, SUITE 500 SEATTLE, WASHINGTON 98101 PHONE (206)682-9107 FAX (206)624-9097 NEWS RELEASE April 23, 2015 For Immediate Release Contact: info@se.mofa.go.jp Update
CONSULATE GENERAL OF JAPAN 601 UNION STREET, SUITE 500 SEATTLE, WASHINGTON 98101 PHONE (206)682-9107 FAX (206)624-9097 NEWS RELEASE April 23, 2015 For Immediate Release Contact: info@se.mofa.go.jp Update
actions to be undertaken
 Ref. Ares(2017)6260317-20/12/2017 FOLLOW-UP ON THE LESSONS LEARNED AND RECOMMENDATIONS OF THE EVALUATION OF IPA CROSS BORDER Recommendations, Final report Recommendations on the future of IPA CBC Restructuring
Ref. Ares(2017)6260317-20/12/2017 FOLLOW-UP ON THE LESSONS LEARNED AND RECOMMENDATIONS OF THE EVALUATION OF IPA CROSS BORDER Recommendations, Final report Recommendations on the future of IPA CBC Restructuring
Anticipating Epidemics Science and Research December Dr Marie-Paule Kieny Assistant Director-General Health Systems and Innovation
 Anticipating Epidemics Science and Research December 2015 Dr Marie-Paule Kieny Assistant Director-General Health Systems and Innovation Preparing for the inevitable With more frequent travel, globalized
Anticipating Epidemics Science and Research December 2015 Dr Marie-Paule Kieny Assistant Director-General Health Systems and Innovation Preparing for the inevitable With more frequent travel, globalized
Major changes brought about by
 Major changes brought about by the AGIR process in the Sahel and West Africa november 2017 UEMOA SAHEL AND Club WEST AFRICA Secretariat 1. Introduction Stakeholders, committed to tackling the long-term
Major changes brought about by the AGIR process in the Sahel and West Africa november 2017 UEMOA SAHEL AND Club WEST AFRICA Secretariat 1. Introduction Stakeholders, committed to tackling the long-term
Vaccine Clinical Trials
 Vaccine Clinical Trials Simon Agwale, PhD CEO Innovative Biotech Ltd, Keffi, Nigeria Chair, Developing countries Coordinating Committee (DCCC)-European and Developing Countries Clinical Trials Partnership
Vaccine Clinical Trials Simon Agwale, PhD CEO Innovative Biotech Ltd, Keffi, Nigeria Chair, Developing countries Coordinating Committee (DCCC)-European and Developing Countries Clinical Trials Partnership
JOINT STATEMENT BY THE MINISTRY OF ENERGY OF THE RUSSIAN FEDERATION AND THE INTERNATIONAL ENERGY AGENCY. Paris October 2011
 JOINT STATEMENT BY THE MINISTRY OF ENERGY OF THE RUSSIAN FEDERATION AND THE INTERNATIONAL ENERGY AGENCY Paris 18-19 October 2011 1. Our discussions at the IEA Energy Ministerial have underlined the need
JOINT STATEMENT BY THE MINISTRY OF ENERGY OF THE RUSSIAN FEDERATION AND THE INTERNATIONAL ENERGY AGENCY Paris 18-19 October 2011 1. Our discussions at the IEA Energy Ministerial have underlined the need
Evaluation: annual report
 EXECUTIVE BOARD EB137/7 137th session 8 May 2015 Provisional agenda item 8.2 Evaluation: annual report 1. The Executive Board at its 131st session approved the WHO evaluation policy. 1 The policy requires
EXECUTIVE BOARD EB137/7 137th session 8 May 2015 Provisional agenda item 8.2 Evaluation: annual report 1. The Executive Board at its 131st session approved the WHO evaluation policy. 1 The policy requires
REPORT 2014/119 INTERNAL AUDIT DIVISION
 INTERNAL AUDIT DIVISION REPORT 2014/119 Audit of the support provided by the United Nations Mission in Liberia to build the capacity of the Liberian National Police Overall results relating to the effectiveness
INTERNAL AUDIT DIVISION REPORT 2014/119 Audit of the support provided by the United Nations Mission in Liberia to build the capacity of the Liberian National Police Overall results relating to the effectiveness
Management Response to the Recommendations of the Summary Evaluation Report of WFP s Ebola Crisis Response: Guinea, Liberia and Sierra Leone
 Executive Board First Regular Session Rome, 20 23 February 2017 Distribution: General Date: 20 January 2017 Original: English Agenda Item 6 WFP/EB.1/2017/6-B/Add.1 Evaluation Reports For consideration
Executive Board First Regular Session Rome, 20 23 February 2017 Distribution: General Date: 20 January 2017 Original: English Agenda Item 6 WFP/EB.1/2017/6-B/Add.1 Evaluation Reports For consideration
Realisation of the SDGs in Countries Affected by Conflict and Fragility: The Role of the New Deal Conceptual Note
 Realisation of the SDGs in Countries Affected by Conflict and Fragility: The Role of the New Deal Conceptual Note This publication was made possible, in part, thanks to the generous support of the European
Realisation of the SDGs in Countries Affected by Conflict and Fragility: The Role of the New Deal Conceptual Note This publication was made possible, in part, thanks to the generous support of the European
Work plan for enhancing the management and administration of UNCTAD
 Distr.: Restricted 7 September 2012 English only Trade and Development Board Fifty-ninth session Geneva, 17 28 September 2012 Item 12 of the provisional agenda Matters requiring action by the Board in
Distr.: Restricted 7 September 2012 English only Trade and Development Board Fifty-ninth session Geneva, 17 28 September 2012 Item 12 of the provisional agenda Matters requiring action by the Board in
EBOLA SITUATION REPORT
 25 NOVEMBER 215 1 GUINEA Total confirmed cases (by week, 215) LIBERIA 1 5 3 5 SUMMARY A cluster of three confirmed cases of Ebola virus disease (EVD) were reported from Liberia in the week to 22 November.
25 NOVEMBER 215 1 GUINEA Total confirmed cases (by week, 215) LIBERIA 1 5 3 5 SUMMARY A cluster of three confirmed cases of Ebola virus disease (EVD) were reported from Liberia in the week to 22 November.
Raising awareness and commitment to VC s and needs through advocacy and social mobilisation;
 To improve the quality of life of vulnerable children (VCs) and to ensure that they enjoy their basic human rights, through 5 strategic interventions, namely: Raising awareness and commitment to VC s and
To improve the quality of life of vulnerable children (VCs) and to ensure that they enjoy their basic human rights, through 5 strategic interventions, namely: Raising awareness and commitment to VC s and
EBOLA IN DEMOCRATIC REPUBLIC OF CONGO (DRC) CRISIS INFO #2 06/06/2018
 EBOLA IN DEMOCRATIC REPUBLIC OF CONGO (DRC) CRISIS INFO #2 06/06/2018 1. Global overview 1.1. Context So far, the outbreak (officially declared on 8 th of May) has affected the Bikoro (Bikoro and Ikoko
EBOLA IN DEMOCRATIC REPUBLIC OF CONGO (DRC) CRISIS INFO #2 06/06/2018 1. Global overview 1.1. Context So far, the outbreak (officially declared on 8 th of May) has affected the Bikoro (Bikoro and Ikoko
Joint Assessment of National Health Strategies and Plans
 Joint Assessment of National Health Strategies and Plans Version 2: September 2011 Joint Assessment of National Health Strategies and Plans Joint Assessment Tool: the attributes of a sound national strategy
Joint Assessment of National Health Strategies and Plans Version 2: September 2011 Joint Assessment of National Health Strategies and Plans Joint Assessment Tool: the attributes of a sound national strategy
GLOBAL LOGISTICS CLUSTER PREPAREDNESS WORKSHOP
 GLOBAL LOGISTICS CLUSTER PREPAREDNESS WORKSHOP 4-6 DECEMBER 2018 CAIRO, EGYPT http://www.logcluster.org/preparedness 1 Introduction The inaugural Global Logistics Cluster (GLC) Preparedness Workshop took
GLOBAL LOGISTICS CLUSTER PREPAREDNESS WORKSHOP 4-6 DECEMBER 2018 CAIRO, EGYPT http://www.logcluster.org/preparedness 1 Introduction The inaugural Global Logistics Cluster (GLC) Preparedness Workshop took
Standard Project Report 2015
 Standard Project Report 2015 Reporting Period: 1 January - 31 December 2015 WEST AFRICA (DAKAR) Logistics Common Services for the Humanitarian Community's Response to the Ebola Virus Disease Outbreak in
Standard Project Report 2015 Reporting Period: 1 January - 31 December 2015 WEST AFRICA (DAKAR) Logistics Common Services for the Humanitarian Community's Response to the Ebola Virus Disease Outbreak in
THE VIETNAM PARTNERSHIP GROUP ON AID EFFECTIVENESS
 THE VIETNAM PARTNERSHIP GROUP ON AID EFFECTIVENESS ACTION PLAN 2008 Introduction 1. This Action Plan is based on the vision that Vietnam will achieve the objectives and targets in the Hanoi Core Statement
THE VIETNAM PARTNERSHIP GROUP ON AID EFFECTIVENESS ACTION PLAN 2008 Introduction 1. This Action Plan is based on the vision that Vietnam will achieve the objectives and targets in the Hanoi Core Statement
Management Response. OPEV is in fact an integral part of the Bank s Roadmap. As part of Action Area 4 of the Roadmap, it has reviewed
 A F R I C A N D E V E L O P M E N T B A N K G R O U P Management Response T GROUPE DE LA BANQUE AFRICAINE DE DEVELOPPEMENT he African Development Bank welcomes this independent evaluation of its implementation
A F R I C A N D E V E L O P M E N T B A N K G R O U P Management Response T GROUPE DE LA BANQUE AFRICAINE DE DEVELOPPEMENT he African Development Bank welcomes this independent evaluation of its implementation
Report on the status of staffing of the Secretariat
 Meeting of the Board 13 15 December 2016 Apia, Samoa Provisional agenda item 15 GCF/B.15/14 7 December, 2016 Report on the status of staffing of the Secretariat Summary At its twelfth meeting, the Board
Meeting of the Board 13 15 December 2016 Apia, Samoa Provisional agenda item 15 GCF/B.15/14 7 December, 2016 Report on the status of staffing of the Secretariat Summary At its twelfth meeting, the Board
Support for national planning & capacity building UN-Water Global Annual Assessment of Sanitation and Drinking-Water (GLAAS)
 Global Framework for Action on Sanitation and Water Supply (GF4A) FREQUENTLY ASKED QUESTIONS Working Document July 21, 2009 General 1. Why do we need the GF4A? 2. What is the GF4A and how does the GF4A
Global Framework for Action on Sanitation and Water Supply (GF4A) FREQUENTLY ASKED QUESTIONS Working Document July 21, 2009 General 1. Why do we need the GF4A? 2. What is the GF4A and how does the GF4A
Realisation of the SDGs in Countries Affected by Conflict and Fragility: The Role of the New Deal. Conceptual Note
 Realisation of the SDGs in Countries Affected by Conflict and Fragility: The Role of the New Deal Conceptual Note Realisation of the SDGs in Countries Affected by Conflict and Fragility: the Role of the
Realisation of the SDGs in Countries Affected by Conflict and Fragility: The Role of the New Deal Conceptual Note Realisation of the SDGs in Countries Affected by Conflict and Fragility: the Role of the
2017 NON FINANCIAL RESOURCE REQUIREMENTS GPEI DONOR CONTRIBUTIONS
 2017 NON FINANCIAL RESOURCE REQUIREMENTS GPEI DONOR CONTRIBUTIONS Background The Global Polio Eradication Initiative (GPEI) is financed through a range of public and private donations. The Financial Resource
2017 NON FINANCIAL RESOURCE REQUIREMENTS GPEI DONOR CONTRIBUTIONS Background The Global Polio Eradication Initiative (GPEI) is financed through a range of public and private donations. The Financial Resource
Climate Change and Health in Small Island Developing States:
 Climate Change and Health in Small Island Developing States: WHO Special Initiative in partnership with UNFCCC Secretariat and Fijian Presidency of COP-23 Contents The Challenge... 2 1. Health impacts
Climate Change and Health in Small Island Developing States: WHO Special Initiative in partnership with UNFCCC Secretariat and Fijian Presidency of COP-23 Contents The Challenge... 2 1. Health impacts
WFP West Africa Regional Bureau REGIONAL SPECIAL OPERATION SO
 WFP West Africa Regional Bureau REGIONAL SPECIAL OPERATION SO 200773 Country: West Africa Regional Bureau (OMD) Ghana, Guinea, Sierra Leone, and Liberia Type of project: Special Operation Title: Logistics
WFP West Africa Regional Bureau REGIONAL SPECIAL OPERATION SO 200773 Country: West Africa Regional Bureau (OMD) Ghana, Guinea, Sierra Leone, and Liberia Type of project: Special Operation Title: Logistics
Implementing the. Reaching. Approach. A Guide for District Health Management Teams. Revised August Africa REGIONAL OFFICE FOR
 Implementing the Reaching Every District Approach A Guide for District Health Management Teams Revised August 2008 REGIONAL OFFICE FOR Africa 2 Implementing the Reaching Every District Approach Reaching
Implementing the Reaching Every District Approach A Guide for District Health Management Teams Revised August 2008 REGIONAL OFFICE FOR Africa 2 Implementing the Reaching Every District Approach Reaching
Finding Efficiencies in Zambia s Immunisation Supply Chain
 Finding Efficiencies in Zambia s Immunisation Supply Chain A Priority on Prevention Vaccines are at the core of Zambia s efforts to prevent childhood death and illness and move towards prevention rather
Finding Efficiencies in Zambia s Immunisation Supply Chain A Priority on Prevention Vaccines are at the core of Zambia s efforts to prevent childhood death and illness and move towards prevention rather
Global Ebola Vaccine Implementation Team (GEVIT) Regional Workshop
 MEETING REPORT Global Ebola Vaccine Implementation Team (GEVIT) Regional Workshop 27 to 29 October 2015, Geneva, Switzerland Rev. 1 World Health Organization 2016. All rights reserved. The designations
MEETING REPORT Global Ebola Vaccine Implementation Team (GEVIT) Regional Workshop 27 to 29 October 2015, Geneva, Switzerland Rev. 1 World Health Organization 2016. All rights reserved. The designations
RESEARCH SUPPORT SERVICES FRAMEWORK. Streamlining the management and governance of R&D studies in the NHS
 RESEARCH SUPPORT SERVICES FRAMEWORK Streamlining the management and governance of R&D studies in the NHS Page 1 of 22 Contents 1. INTRODUCTION... 3 How to use this document... 3 Background... 4 Purpose
RESEARCH SUPPORT SERVICES FRAMEWORK Streamlining the management and governance of R&D studies in the NHS Page 1 of 22 Contents 1. INTRODUCTION... 3 How to use this document... 3 Background... 4 Purpose
Canada s Archives: A vision and areas of focus for
 Canada s Archives: A vision and areas of focus for 2015-2025 Community consultation In January 2014, the archival community convened the Canadian Archives Summit: Towards a New Blueprint for Canada s Recorded
Canada s Archives: A vision and areas of focus for 2015-2025 Community consultation In January 2014, the archival community convened the Canadian Archives Summit: Towards a New Blueprint for Canada s Recorded
Round Table on Evaluation Reports Strategic evaluation of the pilot country strategic plans. 9 November 2018
 Round Table on Evaluation Reports Strategic evaluation of the pilot country strategic plans 9 November 2018 0 Strategic evaluation of the CSP pilots (2017 mid-2018) Recommendations from the evaluation
Round Table on Evaluation Reports Strategic evaluation of the pilot country strategic plans 9 November 2018 0 Strategic evaluation of the CSP pilots (2017 mid-2018) Recommendations from the evaluation
The National Improvement Strategy for Policing. A Consultation Draft Version 1.1
 The National Improvement Strategy for Policing A Consultation Draft Version 1.1 2 The National Improvement Strategy for Policing A Consultation Draft Version 1.1 Introduction 1. With the agreement of the
The National Improvement Strategy for Policing A Consultation Draft Version 1.1 2 The National Improvement Strategy for Policing A Consultation Draft Version 1.1 Introduction 1. With the agreement of the
Polio tender overview. Industry Consultation UNICEF Supply Division January 2012
 Polio tender overview Industry Consultation UNICEF Supply Division 25-26 January 2012 Presentation Overview OPV Update: 2011 OPV Supply & Demand: 2012 Tender: strategic issues including Polio (OPV & IPV)
Polio tender overview Industry Consultation UNICEF Supply Division 25-26 January 2012 Presentation Overview OPV Update: 2011 OPV Supply & Demand: 2012 Tender: strategic issues including Polio (OPV & IPV)
Civil Society Engagement Draft Concept Note
 International Health Partnership and Related Initiatives (IHP+) Civil Society Engagement Draft Concept Note This draft note provides the background, objectives and options for active engagement of civil
International Health Partnership and Related Initiatives (IHP+) Civil Society Engagement Draft Concept Note This draft note provides the background, objectives and options for active engagement of civil
WHO s IMS Organizational Structure Overview
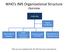 WHO s IMS Organizational Structure Overview Leadership Partner Coordination Information & Planning Health Expertise & Operations Operations Support and Logistics Management & Administration *this structure
WHO s IMS Organizational Structure Overview Leadership Partner Coordination Information & Planning Health Expertise & Operations Operations Support and Logistics Management & Administration *this structure
Oral Polio Vaccine Supply Outlook. UNICEF Supply Division
 Oral Polio Vaccine Outlook UNICEF Division September 2014 Oral Polio Vaccine (OPV) Outlook September 2014 This update reports on anticipated OPV availability during 2H 2014 and 1Q 2015 for intensified
Oral Polio Vaccine Outlook UNICEF Division September 2014 Oral Polio Vaccine (OPV) Outlook September 2014 This update reports on anticipated OPV availability during 2H 2014 and 1Q 2015 for intensified
2014 Climate Change Summit. Chair s Summary
 2014 Climate Change Summit Chair s Summary The purpose of the 2014 Climate Summit was to raise political momentum for a meaningful universal climate agreement in Paris in 2015 and to galvanize transformative
2014 Climate Change Summit Chair s Summary The purpose of the 2014 Climate Summit was to raise political momentum for a meaningful universal climate agreement in Paris in 2015 and to galvanize transformative
Financial Management Harmonization & Alignment Frequently Asked Questions (FAQ) & Guidance
 Financial Management Harmonization & Alignment Frequently Asked Questions (FAQ) & Guidance Introduction The harmonization of Financial Management (FM) arrangements by Development Partners (DPs) can assist
Financial Management Harmonization & Alignment Frequently Asked Questions (FAQ) & Guidance Introduction The harmonization of Financial Management (FM) arrangements by Development Partners (DPs) can assist
Final Report. Some key points which were highlighted during the meeting included:
 Final Report Executive Summary The World Organisation for Animal Health (OIE) hosted the first Global Conference on Biological Threat Reduction in Paris, 30 June 2 July 2015. For the purposes of the Conference,
Final Report Executive Summary The World Organisation for Animal Health (OIE) hosted the first Global Conference on Biological Threat Reduction in Paris, 30 June 2 July 2015. For the purposes of the Conference,
A Framework for Resilience: PR19 and Beyond
 A Framework for Resilience: PR19 and Beyond January 2018 1. Introduction Anglian Water recently published its Strategic Direction Statement 2020-2045. Water is a longterm business. Our ability to provide
A Framework for Resilience: PR19 and Beyond January 2018 1. Introduction Anglian Water recently published its Strategic Direction Statement 2020-2045. Water is a longterm business. Our ability to provide
COUNTY DURHAM & DARLINGTON NHS FOUNDATION TRUST
 COUNTY DURHAM & DARLINGTON NHS FOUNDATION TRUST Introduction County Durham and Darlington NHS Foundation Trust is one of the largest integrated care providers in England, serving a population of around
COUNTY DURHAM & DARLINGTON NHS FOUNDATION TRUST Introduction County Durham and Darlington NHS Foundation Trust is one of the largest integrated care providers in England, serving a population of around
WHAT IS THE SESAR DEPLOYMENT PROGRAMME
 WHAT IS THE SESAR DEPLOYMENT PROGRAMME 2. What is the SESAR Deployment Programme? 2.1 The regulatory framework In line with the overall arrangement of the SESAR Programme (depicted within the previous
WHAT IS THE SESAR DEPLOYMENT PROGRAMME 2. What is the SESAR Deployment Programme? 2.1 The regulatory framework In line with the overall arrangement of the SESAR Programme (depicted within the previous
Compliance Challenges in Clinical Research Naïve Locations: Case Study STRIVE Clinical Trial for Ebola Vaccine in Sierra Leone
 Compliance Challenges in Clinical Research Naïve Locations: Case Study STRIVE Clinical Trial for Ebola Vaccine in Sierra Leone Presented By Seema Garg, MS, MBA, CQA, CSGB Manager, Quality Assurance Technical
Compliance Challenges in Clinical Research Naïve Locations: Case Study STRIVE Clinical Trial for Ebola Vaccine in Sierra Leone Presented By Seema Garg, MS, MBA, CQA, CSGB Manager, Quality Assurance Technical
TAMING COMPLEXITY ON MAJOR RAIL PROJECTS WITH A COLLABORATIVE SYSTEMS ENGINEERING APPROACH
 TAMING COMPLEXITY ON MAJOR RAIL PROJECTS WITH A COLLABORATIVE SYSTEMS ENGINEERING APPROACH Chris Rolison CEO, Comply Serve Limited The Collaborative Systems Engineering Approach Collaboration A system
TAMING COMPLEXITY ON MAJOR RAIL PROJECTS WITH A COLLABORATIVE SYSTEMS ENGINEERING APPROACH Chris Rolison CEO, Comply Serve Limited The Collaborative Systems Engineering Approach Collaboration A system
An R&D Blueprint for action to prevent epidemics. Report to the Scientific Advisory Group 8-9 February 2017
 An R&D Blueprint for action to prevent epidemics Report to the Scientific Advisory Group 8-9 February 2017 Why an R&D Blueprint? The Ebola epidemic has demonstrated that it is possible to accelerate R&D
An R&D Blueprint for action to prevent epidemics Report to the Scientific Advisory Group 8-9 February 2017 Why an R&D Blueprint? The Ebola epidemic has demonstrated that it is possible to accelerate R&D
International Consultant to Support the Coordination of the 2017 MR Follow up Vaccination Campaign, UNICEF Rwanda, 35 Working Days
 International Consultant to Support the Coordination of the 2017 MR Follow up Vaccination Campaign, UNICEF Rwanda, 35 Working Days Job Number: 506796 Locations: Africa: Rwanda Work Type: Consultancy If
International Consultant to Support the Coordination of the 2017 MR Follow up Vaccination Campaign, UNICEF Rwanda, 35 Working Days Job Number: 506796 Locations: Africa: Rwanda Work Type: Consultancy If
Health Impact of Ebola Crisis. Presentation for LSHTM / Options - Chris Lewis
 Health Impact of Ebola Crisis Presentation for LSHTM / Options - Chris Lewis Outline Introduction Ebola outbreak Impact of the crisis Timeline Early December 2013 - little boy in southern Guinea caught
Health Impact of Ebola Crisis Presentation for LSHTM / Options - Chris Lewis Outline Introduction Ebola outbreak Impact of the crisis Timeline Early December 2013 - little boy in southern Guinea caught
GGGI EVALUATION RULES
 GGGI EVALUATION RULES VERSION 1.0 Current version: Version 1.0 Authorized by: Date: 24 August 2017 Frank Rijsberman, Director General, GGGI 2 CONTENTS 1. INTRODUCTION... 4 1.1 About this policy... 4 1.2
GGGI EVALUATION RULES VERSION 1.0 Current version: Version 1.0 Authorized by: Date: 24 August 2017 Frank Rijsberman, Director General, GGGI 2 CONTENTS 1. INTRODUCTION... 4 1.1 About this policy... 4 1.2
Organisational review of UNICEF
 Organisational review of UNICEF Request for proposals 1. Introduction While many gains have been made for the world s children, their situation remains grave in many countries. Making progress towards
Organisational review of UNICEF Request for proposals 1. Introduction While many gains have been made for the world s children, their situation remains grave in many countries. Making progress towards
Organisational strategy
 2016 2020 Organisational strategy 2016 2020 1 Contents Vision 1 Responding to a Changing World 2 Sustainable Development Goal Focus 3 Working within the Plan International Federation 4 Purpose and Values
2016 2020 Organisational strategy 2016 2020 1 Contents Vision 1 Responding to a Changing World 2 Sustainable Development Goal Focus 3 Working within the Plan International Federation 4 Purpose and Values
The role of the health sector in the Strategic Approach to International Chemicals Management towards the 2020 goal and beyond
 SEVENTIETH WORLD HEALTH ASSEMBLY A70/36 Provisional agenda item 16.2 4 May 2017 The role of the health sector in the Strategic Approach to International Chemicals Management towards the 2020 goal and beyond
SEVENTIETH WORLD HEALTH ASSEMBLY A70/36 Provisional agenda item 16.2 4 May 2017 The role of the health sector in the Strategic Approach to International Chemicals Management towards the 2020 goal and beyond
