Guidance on Request for Information on Rapid Response Platform Technologies for Epidemic Preparedness
|
|
|
- Evan Gibson
- 6 years ago
- Views:
Transcription
1 Guidance on Request for Information on Rapid Response Platform Technologies for Epidemic Preparedness Purpose of this request for information The Bill & Melinda Gates Foundation (BMGF; also referred to as the Foundation ) and the Coalition for Epidemic Preparedness Innovations (CEPI) are collaborating to develop a landscape assessment of the rapid response vaccine and monoclonal antibody technologies that could be used to address future emerging diseases. The Request for Information (RFI) will be used to populate that landscape, as well as to influence the development of a Call for Proposals (CfP) by CEPI on rapid response platform technologies specifically for vaccine development. The CEPI CfP is anticipated to be issued by early June Background The increased number of emerging infectious disease (EID) outbreaks over the past decade [1], exemplified by the 2014 Ebola virus disease (EVD) outbreak in West Africa and the Zika virus disease epidemic in the Americas [2], have highlighted the gaps in our ability to respond with interventions rapidly enough to reduce disease spread and associated morbidity and mortality. There is currently no way of predicting if emerging infectious disease pathogens will cause local outbreaks or widespread epidemics. Therefore, it is important to advance new technologies that reduce the total response time from recognition of a pathogen with epidemic potential to the achievement of protective immunity in the target population. New vaccine technology platforms have been developed that can be used to rapidly make vaccines against newly identified pathogens. They have the potential to accelerate development timelines by relying on their track record in safety and immunogenicity from other relevant antigens and pathogens. This Request for Information (RFI) will support future investments into systems and technologies enabling rapid response vaccine development, production, and regulatory approval. The aim is to foster the development of vaccine platform technologies that can be adapted to novel antigens, produced at large scale, and that are likely to be safe and provide protection in humans. CEPI plans to provide funding support for vaccine development through an open Call for Proposals process. A strategic objective of CEPI is to advance development of vaccine technology platforms that enable a rapid response to immunize at-risk populations against emerging infectious diseases. This objective comes in addition to improving preparedness by facilitating vaccine development for specific prioritized infectious diseases, as identified by the WHO R&D Blueprint. CEPI aims to fund candidates with data from late preclinical evaluation in relevant animal models and/or clinical phase I/II trials until they are ready for testing in clinical phase III trials. Objective of this RFI To encourage expressions of interest, and to generate information vital for the scoping and structure of the upcoming Call for Proposals to be issued by CEPI, focused on vaccine platforms for rapid response. Disease scope Respondents should indicate the pathogens for which their platform would be suitable. Safety and immunogenicity data with respect to these pathogens should be highlighted. The vaccine technology platforms should be also capable of being adapted to two or more of the WHO priority pathogens.
2 Intended recipients for this RFI This RFI will be open for all interested parties within the field of vaccine development. Information submitted through this RFI will be owned by CEPI and the Foundation, stored in a secure database and accessed only by the two organizations. None of the information obtained through this RFI will be shared publicly. Minimum requirement for those submitting information to this RFI Organizations (not-for-profit, public, academic, NGO, governmental and other organizations) that can provide information needed to map the landscape of the rapid response technologies that could be used to address future emerging diseases. Preclinical data, ideally showing proof of concept of the platform, in relevant animal models We are seeking developers who believe that their platform technology and manufacturing operations can eventually meet most (or all) the following attributes: Target a 16-week timeframe, ideally shorter, for release of product for clinical trials after identification of antigen Completion of phase II trials demonstrating clinical proof of concept within 2-3 years Produce sufficient doses (e.g. >1,000,000) rapidly enough to impact an emerging outbreak Technology scope and use of information The RFI will identify platform technologies and manufacturing capabilities that can potentially improve vaccine preparedness and response needs against priority emerging disease and as-of-yet unknown threats. CEPI and the Foundation will work closely to ensure alignment on design of a future CfP, funding and support activities, and all information from the RFI will be shared with both organisations. Relevance of this RFI to future funding by CEPI The information gathered in this RFI will be considered in the shaping of the scope, budget and evaluation criteria of the CEPI CfP on Vaccine Platform Technologies. The request will be launched in late May-early June and will be open to all entities that meet the criteria specified in the CfP. Applicants who submitted proposals to the CEPI Lassa-MERS-Nipah CfP issued on 19 January 2017 are encouraged to also adapt their applications to this RFI format for informing the upcoming CfP for Vaccine Platform Technologies. The upcoming CfP for Vaccine Platform Technologies will focus on demonstrating safety and immunogenicity of vaccine platforms suitable for multiple pathogens. Key attributes to be addressed in the response Sound scientific rationale for the technical platform (e.g., why the products produced with this platform would be protective), based on: o Principle of antigen presentation, formulation, delivery and administration o Method of delivery and administration Feasibility of advancing platform from current readiness state of technology (stage of development) through to phase II including: o Details of development path through to regulatory agreement to deliver in an emergency response o Clear understanding and articulation of platform risks and reasonableness of proposed solutions to these challenges (most significant technical, regulatory & IP risks)
3 Time to develop material for clinical evaluation, including Clinical Trial Material (CTM) availability - as defined as the time from sequence/organism definition to phase 1 CTM released. Manufacturing scalability and speed; including rationale for manufacturing processes/technologies supporting the platform and their suitability for large scale production and delivery in an emergency, timelines to manufacture different bulk/fill-finished dose equivalent volumes Application potential to new pathogens, including: o Types of epidemic/endemic disease the platform may be used for (e.g., viral, bacterial, mosquito-borne, parasitic, etc.) o Target antigens and diseases for which proof-of-concept data in animal models has been developed How to respond to this RFI An RFI template is attached with all fields being mandatory for completion. The RFI should communicate the proposer s concept/technology and prospects for clinical assessment and long term readiness capabilities of the technology. The total length of the response should not exceed 5 (five) pages using the template and format (including font size) provided. Submissions after the deadline or those that exceed five pages in length will not be accepted. Deadline Submissions should be received by 3 pm U.S. Pacific Standard Time on 24 th of April Submit the completed RFI template directly to EpidemicRFP@gatesfoundation.org. Your submission should include only a completed RFI template. Other attachments, including cover letters and supplemental information will not be accepted or reviewed. Definitions The term rapid response platform technologies refers to technological approaches to vaccine research, development, testing and manufacturing that may lead to development of a vaccine/therapeutic for an as of yet unknown pathogen with accelerated timelines. Platforms may include expression systems, vectors, delivery technologies, adjuvants and other approaches linked to vaccine development against target pathogens. The underpinning technologies should be applicable to vaccine candidates for two or more pathogens. Rapid response refers to the time from identification of the pathogen/genetic sequence to develop, produce, and release supplies for clinical evaluation as well as the time required to produce sufficient quantities of vaccine or therapeutic to have a meaningful impact in outbreak settings. In addition, regulatory acceptance will be affected by safety and immunogenicity track record. Therefore, the timelines related to development, production scale-up, clinical testing, and regulatory review are important elements when evaluating the capability of a specific technology to improve response time.
4 Guidance on Request for Information template General Information, Prospective Grantee/Vendor Information, Submission Information Please provide the requested organizational information The amount of funding requested, as well as the duration of the project, must be included 1. Concept Statement: Please provide the following information: Key features of the platform technology, including an overview of the proposed mechanism of target antigen presentation, supported by an analysis of rationale, key assumptions and any source data from previous or current application of the platform on other diseases. Include a summary of available proof-of concept preclinical and clinical data for the platform. Include an assessment of strengths and limitations of the proposed platform technology and its characteristics. List of infectious pathogens that could be used for demonstration of the platform properties and allow for its evaluation in terms of feasibility and anticipated use potential in an outbreak setting. Include rationale, key assumptions and any existing evidence on platform performance to support your suggested list of pathogens against which the platform has application potential. Please describe any prior interaction with and current guidance from competent regulatory authorities about the development of the candidate platform as well as the proposed regulatory pathway and rationale for accelerating regulatory approval of candidate vaccines during an epidemic response. Specifically address the question of why regulatory authorities should be comfortable granting conditional approval and/or licensure for a vaccine developed against a novel disease using the candidate platform without traditional large safety and efficacy evaluations. Manufacturing processes/technologies for the platform and their suitability for scale-up production and delivery in response to an outbreak. Include a description of timelines for bulk and fill-finish manufacturing, including ramp-up times to meet pilot scale demand of 200,000 doses and surge demand of 1 million+ dose scenarios. Note: Surge capacity of 2-5 million and > 5 million doses should be highlighted, if achievable. Provide an outline of current in-house manufacturing capacities or existing contract manufacturing partnerships to meet pilot scale and surge demand. Clarify feasibility of process scale-up with existing in-house capacity or contract manufacturing partnerships and any need to establish new capacity required to meet pilot scale and surge demand scenarios respectively. 2. Organizational capabilities, experience and track record: The purpose of this section is to allow the applicant to provide a brief, high-level summary of their organization s technical capabilities and track record for research and development.
5 3. Risks/Challenges: The purpose of this section is to encourage the applicant to provide a balanced and logical risk assessment for the proposed concept/technology. A preliminary, high-level mitigation plan for key risks is also requested. 4. Sub-awards: Partners and/or vendors that will be engaged and paid by the applicant to complete the Project Scope should be listed under sub-awards, with one line per sub-awardee (please add rows as necessary, but do not exceed the guidelines for overall document length). For example, a CMO may be engaged to manufacture test batches or engineering runs. 5. Level and sources of support for this project: In this section please describe the total cost estimate, how much is already covered and what funding level you are likely to require through a potential investment opportunity issued by CEPI or BMGF. In this description please outline contingency plans and alternate funding options. The extent of the anticipated cost requirements already covered by other funding sources to advance the platform from current to future state must be disclosed, and distinguished from the potential need for funding from CEPI to possibly advance the platform concept to a defined readiness state. In addition, requests for funding from other sources which overlap with the funding requested in this proposal should be noted. Applications to BMGF, Welcome Trust, BARDA and CEPI as additional funding sources must be disclosed. Important information for applicants: 1. The applicant must have freedom to operate as related to IP, including consent from others where applicable. 2. Your concept note submissions should not contain sensitive or confidential information - responses will be reviewed by BMGF and CEPI, and could be shared with other partners to these organisations. 3. The RFI process does not constitute a guarantee of funding in any CFP process that arises from the data gathered here. References 1. Jones, K.E., et al., Global trends in emerging infectious diseases. Nature, (7181): p Sands, P., C. Mundaca-Shah, and V.J. Dzau, The Neglected Dimension of Global Security--A Framework for Countering Infectious-Disease Crises. N Engl J Med, (13): p
Accelerating Vaccine Development for Epidemic Preparedness: New Vaccines for a Safer World. Richard Hatchett, MD CEO, CEPI
 Accelerating Vaccine Development for Epidemic Preparedness: New Vaccines for a Safer World Richard Hatchett, MD CEO, CEPI Photo: Daniel Berehulak, The New York Times CEPI s Gestation (1) February 2016
Accelerating Vaccine Development for Epidemic Preparedness: New Vaccines for a Safer World Richard Hatchett, MD CEO, CEPI Photo: Daniel Berehulak, The New York Times CEPI s Gestation (1) February 2016
CEPI Coalition for Epidemic Preparedness Innovations. CEPI a model for funding other initiatives and areas
 CEPI Coalition for Epidemic Preparedness Innovations CEPI a model for funding other initiatives and areas Foto: Daniel Berehulak, The New York Times Testing of an Ebola vaccine a successful but suboptimal
CEPI Coalition for Epidemic Preparedness Innovations CEPI a model for funding other initiatives and areas Foto: Daniel Berehulak, The New York Times Testing of an Ebola vaccine a successful but suboptimal
A new Model of Vaccine R&D Global Health Public Private Partnership : The Global Health Vaccine Center of Innovation (GHVCI)
 A new Model of Vaccine R&D Global Health Public Private Partnership : The Global Health Vaccine Center of Innovation (GHVCI) Jean Lang avp R&D sanofi pasteur 9eme Rencontre Nationale des Directeurs de
A new Model of Vaccine R&D Global Health Public Private Partnership : The Global Health Vaccine Center of Innovation (GHVCI) Jean Lang avp R&D sanofi pasteur 9eme Rencontre Nationale des Directeurs de
Licenced Ebola Vaccine
 The Market Shaping Goal Shape vaccine markets to ensure adequate supply of appropriate, quality vaccines at low and sustainable prices for developing countries. Supply and Procurement Roadmap Licenced
The Market Shaping Goal Shape vaccine markets to ensure adequate supply of appropriate, quality vaccines at low and sustainable prices for developing countries. Supply and Procurement Roadmap Licenced
ACCELERATING VACCINE DELIVERY AND DEVELOPMENT
 ACCELERATING VACCINE DELIVERY AND DEVELOPMENT Evolving Vaccine Ecosystem Technologies and Partnerships David Robinson Bill & Melinda Gates Foundation Bill & Melinda Gates Foundation WE SUPPORT VACCINE
ACCELERATING VACCINE DELIVERY AND DEVELOPMENT Evolving Vaccine Ecosystem Technologies and Partnerships David Robinson Bill & Melinda Gates Foundation Bill & Melinda Gates Foundation WE SUPPORT VACCINE
MAIN OUTCOMES OF DISCUSSION FROM WHO CONSULTATION ON NUCLEIC ACID VACCINES. I. Knezevic, R. Sheets Feb, 2018 Geneva, Switzerland
 MAIN OUTCOMES OF DISCUSSION FROM WHO CONSULTATION ON NUCLEIC ACID VACCINES I. Knezevic, R. Sheets 21-23 Feb, 2018 Geneva, Switzerland CONTEXT OF DISCUSSION WHO Consultation held to determine whether the
MAIN OUTCOMES OF DISCUSSION FROM WHO CONSULTATION ON NUCLEIC ACID VACCINES I. Knezevic, R. Sheets 21-23 Feb, 2018 Geneva, Switzerland CONTEXT OF DISCUSSION WHO Consultation held to determine whether the
ZIKAVAX PARTNERSHIP. Dr Odile LEROY DCVMN Seoul 26 th September 2017
 ZIKAVAX PARTNERSHIP Dr Odile LEROY DCVMN Seoul 26 th September 2017 1 Product Development Partnership THE EUROPEAN VACCINE INITIATIVE IS A PRODUC T DEVELOPMENT PARTNERSHIP WHICH AIMS TO ACCELERATE THE
ZIKAVAX PARTNERSHIP Dr Odile LEROY DCVMN Seoul 26 th September 2017 1 Product Development Partnership THE EUROPEAN VACCINE INITIATIVE IS A PRODUC T DEVELOPMENT PARTNERSHIP WHICH AIMS TO ACCELERATE THE
NIAID Resources to Facilitate Medical Countermeasure Development
 NIAID Resources to Facilitate Medical Countermeasure Development Paula Bryant, Ph.D. Senior Scientific Officer Concept Acceleration Program Biodefense, Research Resources, and Translational Research (OBRRTR)
NIAID Resources to Facilitate Medical Countermeasure Development Paula Bryant, Ph.D. Senior Scientific Officer Concept Acceleration Program Biodefense, Research Resources, and Translational Research (OBRRTR)
WHO Blood Regulators Network (BRN)
 Distribution: General English only WHO Blood Regulators Network (BRN) Position Paper on Use of Convalescent Plasma, Serum or Immune Globulin Concentrates as an Element in Response to an Emerging Virus*
Distribution: General English only WHO Blood Regulators Network (BRN) Position Paper on Use of Convalescent Plasma, Serum or Immune Globulin Concentrates as an Element in Response to an Emerging Virus*
HIV VACCINE PROGRAM and P5 PARTNERSHIP
 HIV VACCINE PROGRAM and P5 PARTNERSHIP Considerations for a Pan-African HIV Vaccine Development Agenda Mar. 16-17, Kigali, Rwanda Silvija Staprans, PhD Senior Program Officer, HIV OUTLINE BMGF STRATEGY
HIV VACCINE PROGRAM and P5 PARTNERSHIP Considerations for a Pan-African HIV Vaccine Development Agenda Mar. 16-17, Kigali, Rwanda Silvija Staprans, PhD Senior Program Officer, HIV OUTLINE BMGF STRATEGY
An R&D Blueprint for action to prevent epidemics. Report to the Scientific Advisory Group 8-9 February 2017
 An R&D Blueprint for action to prevent epidemics Report to the Scientific Advisory Group 8-9 February 2017 Why an R&D Blueprint? The Ebola epidemic has demonstrated that it is possible to accelerate R&D
An R&D Blueprint for action to prevent epidemics Report to the Scientific Advisory Group 8-9 February 2017 Why an R&D Blueprint? The Ebola epidemic has demonstrated that it is possible to accelerate R&D
Data & Materials Sharing Agreement. Collaboration for AIDS Vaccine Discovery. Clinical Trials Data Sharing Addendum & Related Information
 Data & Materials Sharing Agreement Collaboration for AIDS Vaccine Discovery Clinical Trials Data Sharing Addendum & Related Information I. Overview of this Document The primary purpose of this document
Data & Materials Sharing Agreement Collaboration for AIDS Vaccine Discovery Clinical Trials Data Sharing Addendum & Related Information I. Overview of this Document The primary purpose of this document
A Potential Innovative CMC Solution: Responding To Public Health Needs With An Accelerated Clinical Pathway A Vaccine Example
 A Potential Innovative CMC Solution: Responding To Public Health Needs With An Accelerated Clinical Pathway A Vaccine Example January 2018 Natalie A. Christian Integrated Development and Supply Team Lead
A Potential Innovative CMC Solution: Responding To Public Health Needs With An Accelerated Clinical Pathway A Vaccine Example January 2018 Natalie A. Christian Integrated Development and Supply Team Lead
From research to large-scale use
 Accelerating access to Ebola vaccines From research to large-scale use Dr Marie-Paule Kieny Assistant Director General HIS 1 Number of cases Ebola response roadmap: Situation report 3 December 2014 Sierra
Accelerating access to Ebola vaccines From research to large-scale use Dr Marie-Paule Kieny Assistant Director General HIS 1 Number of cases Ebola response roadmap: Situation report 3 December 2014 Sierra
Anticipating Epidemics Science and Research December Dr Marie-Paule Kieny Assistant Director-General Health Systems and Innovation
 Anticipating Epidemics Science and Research December 2015 Dr Marie-Paule Kieny Assistant Director-General Health Systems and Innovation Preparing for the inevitable With more frequent travel, globalized
Anticipating Epidemics Science and Research December 2015 Dr Marie-Paule Kieny Assistant Director-General Health Systems and Innovation Preparing for the inevitable With more frequent travel, globalized
CBER Regulatory Updates: Initiatives for Product Review and Licensure
 CBER Regulatory Updates: Initiatives for Product Review and Licensure CASSS CMC Strategy Forum Japan 2018 December 3, 2018 Robin Levis, Ph.D. Division of Viral Products Office of Vaccines Research and
CBER Regulatory Updates: Initiatives for Product Review and Licensure CASSS CMC Strategy Forum Japan 2018 December 3, 2018 Robin Levis, Ph.D. Division of Viral Products Office of Vaccines Research and
The Critical Path to TB Drug Regimens Initiative
 The Critical Path to TB Drug Regimens Initiative Jan Gheuens, MD, PhD Global Health, Bill & Melinda Gates Foundation Addis Ababa, August 18th, 2010 The new tools that are needed to fight TB are on their
The Critical Path to TB Drug Regimens Initiative Jan Gheuens, MD, PhD Global Health, Bill & Melinda Gates Foundation Addis Ababa, August 18th, 2010 The new tools that are needed to fight TB are on their
RSV Vaccine Development Status Update
 Photo: PATH/Doune Porter RSV Vaccine Development Status Update WHO RSV Surveillance Pilot 18-20 Dec 2017 Washington DC Deborah Higgins PATH Photo credit CENTER FOR VACCINE INNOVATION AND ACCESS PATH RSV
Photo: PATH/Doune Porter RSV Vaccine Development Status Update WHO RSV Surveillance Pilot 18-20 Dec 2017 Washington DC Deborah Higgins PATH Photo credit CENTER FOR VACCINE INNOVATION AND ACCESS PATH RSV
STATUS OF REVIEWS, AUTHORIZATIONS AND OVERSIGHT FOR CLINICAL TRIALS IN THE WHO AFRICAN REGION. Technical Document
 30 August 2017 REGIONAL COMMITTEE FOR AFRICA ORIGINAL: ENGLISH Sixty-seventh session Victoria Falls, Republic of Zimbabwe, 28 August 1 September 2017 Agenda item 17 STATUS OF REVIEWS, AUTHORIZATIONS AND
30 August 2017 REGIONAL COMMITTEE FOR AFRICA ORIGINAL: ENGLISH Sixty-seventh session Victoria Falls, Republic of Zimbabwe, 28 August 1 September 2017 Agenda item 17 STATUS OF REVIEWS, AUTHORIZATIONS AND
Identification Selection Definition Execution. Schedule.
 SCHEDULE DEVELOPMENT Schedule development is the identification, definition and sequencing of activities that are required to be performed for successful completion of the project scope. Identification
SCHEDULE DEVELOPMENT Schedule development is the identification, definition and sequencing of activities that are required to be performed for successful completion of the project scope. Identification
STATUS OF REVIEWS, AUTHORIZATIONS AND OVERSIGHT FOR CLINICAL TRIALS IN THE WHO AFRICAN REGION. Technical Document
 15 June 2017 REGIONAL COMMITTEE FOR AFRICA ORIGINAL: ENGLISH Sixty-seventh session Victoria Falls, Republic of Zimbabwe, 28 August 1 September 2017 Provisional agenda item 17 STATUS OF REVIEWS, AUTHORIZATIONS
15 June 2017 REGIONAL COMMITTEE FOR AFRICA ORIGINAL: ENGLISH Sixty-seventh session Victoria Falls, Republic of Zimbabwe, 28 August 1 September 2017 Provisional agenda item 17 STATUS OF REVIEWS, AUTHORIZATIONS
R&D for potential vaccines for the Ebola virus
 R&D for potential vaccines for the Ebola virus Update 4 March 2015 Roberto Bertollini MD MPH Chief Scientist and WHO Representative to the EU Gathering knowledge Since August 2014, series of consultations
R&D for potential vaccines for the Ebola virus Update 4 March 2015 Roberto Bertollini MD MPH Chief Scientist and WHO Representative to the EU Gathering knowledge Since August 2014, series of consultations
Preparedness for the inevitable: overcoming obstacles to a timely and effective R&D response. The WHO Blueprint Team
 Preparedness for the inevitable: overcoming obstacles to a timely and effective R&D response The WHO Blueprint Team Context of the Blueprint WHO Executive Board 1 (January 2015) WHO Summit on Ebola R&D
Preparedness for the inevitable: overcoming obstacles to a timely and effective R&D response The WHO Blueprint Team Context of the Blueprint WHO Executive Board 1 (January 2015) WHO Summit on Ebola R&D
EBOLA AND OTHER AFRICAN VIRAL HAEMORRHAGIC FEVERS
 EBOLA AND OTHER AFRICAN VIRAL HAEMORRHAGIC FEVERS In response to the 2014 West African Ebola epidemic, last year s G-FINDER survey tracked funding for Ebola R&D for the first time, capturing FY2014 investments.
EBOLA AND OTHER AFRICAN VIRAL HAEMORRHAGIC FEVERS In response to the 2014 West African Ebola epidemic, last year s G-FINDER survey tracked funding for Ebola R&D for the first time, capturing FY2014 investments.
DMTC Technology Readiness Levels Guideline
 Technology Readiness Levels Technology Readiness Levels (TRLs) are used as standardised numerical indicators of the level of maturity of a technology. The standard TRL definitions are given in Table 1.
Technology Readiness Levels Technology Readiness Levels (TRLs) are used as standardised numerical indicators of the level of maturity of a technology. The standard TRL definitions are given in Table 1.
Regulatory Challenges for the Licensure of Future Vaccines
 Regulatory Challenges for the Licensure of Future Vaccines Tong Wu, Ph.D. Bacterial & Combination Vaccine Division, BGTD, Health Canada June 26-29, 2018, Seoul, Korea, the Global Bio Conference 1 Disclaimer
Regulatory Challenges for the Licensure of Future Vaccines Tong Wu, Ph.D. Bacterial & Combination Vaccine Division, BGTD, Health Canada June 26-29, 2018, Seoul, Korea, the Global Bio Conference 1 Disclaimer
ibio, Inc. Holds Annual Meeting in College Station, Texas
 April 7, 2016 ibio, Inc. Holds Annual Meeting in College Station, Texas NEW YORK, NY -- (Marketwired) -- 04/07/16 -- ibio, Inc. (NYSE MKT: IBIO) - Speaking to shareholders at the ibio, Inc. (NYSE MKT:
April 7, 2016 ibio, Inc. Holds Annual Meeting in College Station, Texas NEW YORK, NY -- (Marketwired) -- 04/07/16 -- ibio, Inc. (NYSE MKT: IBIO) - Speaking to shareholders at the ibio, Inc. (NYSE MKT:
Development of plasma therapies for emerging infectious diseases. Dr Glenn Smith Biological Science Section Scientific Evaluation Branch
 Development of plasma therapies for emerging infectious diseases Dr Glenn Smith Biological Science Section Scientific Evaluation Branch June 2017 Therapeutic Goods Administration (TGA) A part of the Australian
Development of plasma therapies for emerging infectious diseases Dr Glenn Smith Biological Science Section Scientific Evaluation Branch June 2017 Therapeutic Goods Administration (TGA) A part of the Australian
MRC s Pathways to Translation.. Louise Jones, Feb 13 th 2015, LSTM
 MRC s Pathways to Translation.. Louise Jones, Feb 13 th 2015, LSTM MRC Remit and Partners MRC: basic research to early clinical trials Underpinning and aetiological Prevention Detection and diagnosis Treatment
MRC s Pathways to Translation.. Louise Jones, Feb 13 th 2015, LSTM MRC Remit and Partners MRC: basic research to early clinical trials Underpinning and aetiological Prevention Detection and diagnosis Treatment
Feasibility Analysis Workbook
 Feasibility Analysis Workbook A feasibility analysis, or feasibility study, is used to assess the strengths and weaknesses of a proposed project, policy, product or service for its capability to achieve
Feasibility Analysis Workbook A feasibility analysis, or feasibility study, is used to assess the strengths and weaknesses of a proposed project, policy, product or service for its capability to achieve
Page 1 of 11. WHO Target Product Profile for multivalent filovirus vaccines: providing long-term protection to high-risk populations.
 Page 1 of 11 WHO Target Product Profile for multivalent filovirus vaccines: providing long-term protection to high-risk populations November 2016 Page 2 of 11 Target Audience The target audience for this
Page 1 of 11 WHO Target Product Profile for multivalent filovirus vaccines: providing long-term protection to high-risk populations November 2016 Page 2 of 11 Target Audience The target audience for this
2015/2016 Year for Vaccine Development. Vasee Moorthy MD PhD Team Leader Vaccine Development
 2015/2016 Year for Vaccine Development Vasee Moorthy MD PhD Team Leader Vaccine Development Product Development for Vaccines Advisory Committee Established in 2014 to fill strategic gap in our advisory
2015/2016 Year for Vaccine Development Vasee Moorthy MD PhD Team Leader Vaccine Development Product Development for Vaccines Advisory Committee Established in 2014 to fill strategic gap in our advisory
EDCTP perspective on biopreparedness and related capacity development in Africa
 EDCTP perspective on biopreparedness and related capacity development in Africa Consultation workshop on Preparedness for emerging diseases Dr. Michael Makanga EDCTP Executive Director. 29 September 2016
EDCTP perspective on biopreparedness and related capacity development in Africa Consultation workshop on Preparedness for emerging diseases Dr. Michael Makanga EDCTP Executive Director. 29 September 2016
Vector control. REGIONAL COMMITTEE Provisional Agenda item 8.4. SEA/RC70/10 Maldives 6 10 September July Seventieth Session
 REGIONAL COMMITTEE Provisional Agenda item 8.4 Seventieth Session SEA/RC70/10 Maldives 6 10 September 2017 18 July 2017 Vector control Major vector-borne diseases account for an estimated 17% of the global
REGIONAL COMMITTEE Provisional Agenda item 8.4 Seventieth Session SEA/RC70/10 Maldives 6 10 September 2017 18 July 2017 Vector control Major vector-borne diseases account for an estimated 17% of the global
EMERGING INFECTIOUS DISEASE R&D SCOPE DOCUMENT
 EMERGING INFECTIOUS DISEASE R&D SCOPE DOCUMENT This document sets out the research and development (R&D) activities that are included within the scope of the Policy Cures Research/G-FINDER survey of global
EMERGING INFECTIOUS DISEASE R&D SCOPE DOCUMENT This document sets out the research and development (R&D) activities that are included within the scope of the Policy Cures Research/G-FINDER survey of global
Guide for Terms of Reference
 Centre for Research on Impact Evaluation (CRIE) Guide for Terms of Reference Template 1. Introduction and Description of the Intervention (1.1) Purpose, objectives and intended outcomes [Set out the purpose,
Centre for Research on Impact Evaluation (CRIE) Guide for Terms of Reference Template 1. Introduction and Description of the Intervention (1.1) Purpose, objectives and intended outcomes [Set out the purpose,
Introduction of Development Center for Biotechnology TAIWAN
 Introduction of Development Center for Biotechnology TAIWAN DCB Nonprofit Organization Founded in 1984 Funded Mainly by Ministry of Economic Affairs (MOEA), National Science Council and the Industry 394
Introduction of Development Center for Biotechnology TAIWAN DCB Nonprofit Organization Founded in 1984 Funded Mainly by Ministry of Economic Affairs (MOEA), National Science Council and the Industry 394
Synthetic vaccine research and development. Comprehensive and innovative synthetic biology solutions and technologies
 Synthetic vaccine research and development Comprehensive and innovative synthetic biology solutions and technologies From plan to product, Thermo Fisher Scientific supports your synthetic vaccine goals
Synthetic vaccine research and development Comprehensive and innovative synthetic biology solutions and technologies From plan to product, Thermo Fisher Scientific supports your synthetic vaccine goals
REQUEST FOR PROPOSALS
 REQUEST FOR PROPOSALS DEVELOPMENT OF REPORTS ON GAPS IN PRIMARY HEALTH CARE RESEARCH AND IMPLEMENTATION IN LOW AND MIDDLE-INCOME COUNTRIES TO ACCELERATE IMPROVEMENT Proposals for: Development of a report
REQUEST FOR PROPOSALS DEVELOPMENT OF REPORTS ON GAPS IN PRIMARY HEALTH CARE RESEARCH AND IMPLEMENTATION IN LOW AND MIDDLE-INCOME COUNTRIES TO ACCELERATE IMPROVEMENT Proposals for: Development of a report
Developing Vaccines for Neglected Diseases
 Developing Vaccines for Neglected Diseases Vaccine Technologies II Albufeira, Portugal June 5 th, 2008 Douglas Holtzman, Ph.D., M.P.H. Senior Program Officer, Global Health Program Bill & Melinda Gates
Developing Vaccines for Neglected Diseases Vaccine Technologies II Albufeira, Portugal June 5 th, 2008 Douglas Holtzman, Ph.D., M.P.H. Senior Program Officer, Global Health Program Bill & Melinda Gates
European Technology Platform for Global Animal Health. Action Plan
 European Technology Platform for Global Animal Health Action Plan European Technology T Platform for Global Animal Health Action Plan 2 Table of contents Executive Summary 7 Chapter 1: The Action Plan
European Technology Platform for Global Animal Health Action Plan European Technology T Platform for Global Animal Health Action Plan 2 Table of contents Executive Summary 7 Chapter 1: The Action Plan
Boosting Decent Employment for Africa s Youth. Request for Concept Notes
 Boosting Decent Employment for Africa s Youth Request for Concept Notes Submission deadline: October 8, 2018 i Table of Contents 1. About the partner organizations... 1 2. Background and rationale... 2
Boosting Decent Employment for Africa s Youth Request for Concept Notes Submission deadline: October 8, 2018 i Table of Contents 1. About the partner organizations... 1 2. Background and rationale... 2
1 R01 AI VMD HALFORD, W
 1 R01 AI104935-01 2 VMD 1R01AI104935-01 ILLIAM CRITIQUE 1: Significance: 5 Investigator(s): 2 Innovation: 5 Approach: 8 Environment: 2 Overall Impact: This application proposes to develop a recombinant
1 R01 AI104935-01 2 VMD 1R01AI104935-01 ILLIAM CRITIQUE 1: Significance: 5 Investigator(s): 2 Innovation: 5 Approach: 8 Environment: 2 Overall Impact: This application proposes to develop a recombinant
Marburg and Ebola viruses. Stephan Becker Philipps-Universität Marburg
 Marburg and Ebola viruses Stephan Becker Philipps-Universität Marburg Filoviridae (order Mononegavirales) Matrix (VP40) Surface glycoprotein (GP) Lipid envelope Ribonucleoprotein complex (vrna, NP, VP35,
Marburg and Ebola viruses Stephan Becker Philipps-Universität Marburg Filoviridae (order Mononegavirales) Matrix (VP40) Surface glycoprotein (GP) Lipid envelope Ribonucleoprotein complex (vrna, NP, VP35,
Guide for Terms of Reference
 Centre for Research on Impact Evaluation (CRIE) Guide for Terms of Reference In this document we provide a guideline to write Terms of Reference (here-in-after ToR) in view of a counterfactual impact evaluation
Centre for Research on Impact Evaluation (CRIE) Guide for Terms of Reference In this document we provide a guideline to write Terms of Reference (here-in-after ToR) in view of a counterfactual impact evaluation
Request for Proposals (RFP)
 Request for Proposals (RFP) Issued on: October 30, 2018 For: Management and operational review and planning for Alive & Thrive s Africa programs Estimated Period of Performance: December 2018 March 2019
Request for Proposals (RFP) Issued on: October 30, 2018 For: Management and operational review and planning for Alive & Thrive s Africa programs Estimated Period of Performance: December 2018 March 2019
Planning of Application
 2016 Good Registration Management Regulatory Science Center of Excellence Pilot Workshop Planning of Application Moderator: Speaker: Jin Shun, AbbVie Thean Soo Lo, J&J Sannie Chong, Roche Agenda 10:00~10:05
2016 Good Registration Management Regulatory Science Center of Excellence Pilot Workshop Planning of Application Moderator: Speaker: Jin Shun, AbbVie Thean Soo Lo, J&J Sannie Chong, Roche Agenda 10:00~10:05
May 9, Meeting Summary. Facilitating Antibacterial Drug Development
 May 9, 2012 Meeting Summary Facilitating Antibacterial Drug Development Origins of the Current Public Health Crisis of Antibacterial Resistance Antibacterial drugs play a critical role in the ability to
May 9, 2012 Meeting Summary Facilitating Antibacterial Drug Development Origins of the Current Public Health Crisis of Antibacterial Resistance Antibacterial drugs play a critical role in the ability to
1 R21 AI A1 2 VMD HALFORD, W
 1 R21 AI081072-01A1 2 VMD 1R21AI081072-01A1 ILLIAM RESUME AND SUMMARY OF DISCUSSION: The proposed study is to develop safe and effective live attenuated vaccines against herpes simplex virus 2 by using
1 R21 AI081072-01A1 2 VMD 1R21AI081072-01A1 ILLIAM RESUME AND SUMMARY OF DISCUSSION: The proposed study is to develop safe and effective live attenuated vaccines against herpes simplex virus 2 by using
Better vaccines and healthier life: PATH s innovative approach to developing rotavirus vaccines in China
 Better vaccines and healthier life: PATH s innovative approach to developing rotavirus vaccines in China 14 th Annual Global DCVMN meeting Hanoi, October 7-9, 2013 Jean-Marie Préaud Vaccine Development
Better vaccines and healthier life: PATH s innovative approach to developing rotavirus vaccines in China 14 th Annual Global DCVMN meeting Hanoi, October 7-9, 2013 Jean-Marie Préaud Vaccine Development
Office for Human Subject Protection. University of Rochester
 POLICY 1. Purpose Outline the responsibilities and regulatory requirements when conducting human subject research that involves the use of drugs, agents, biological products, or nutritional products (e.g.,
POLICY 1. Purpose Outline the responsibilities and regulatory requirements when conducting human subject research that involves the use of drugs, agents, biological products, or nutritional products (e.g.,
The Power of Partnership
 The Foundation for Science and Technology 25 March, 2015 London Debate on from the Response to the Ebola Outbreak Chapter One The Power of Partnership 2 1 Some Key Points So Far Dec 2013 First Ebola case
The Foundation for Science and Technology 25 March, 2015 London Debate on from the Response to the Ebola Outbreak Chapter One The Power of Partnership 2 1 Some Key Points So Far Dec 2013 First Ebola case
Competencies Checklist for CE. Tier 1 Core Public Health Competencies Checklist
 Competencies Checklist for CE Student Name: Area of Concentration: Project Title: Tier 1 Core Public Health Competencies Checklist Domain #1: Analytic/Assessment Skills Describes factors affecting the
Competencies Checklist for CE Student Name: Area of Concentration: Project Title: Tier 1 Core Public Health Competencies Checklist Domain #1: Analytic/Assessment Skills Describes factors affecting the
Joint scientific and ethics reviews of clinical trial applications. Dr Diadié Maïga, WHO/AFRO
 Joint scientific and ethics reviews of clinical trial applications Dr Diadié Maïga, WHO/AFRO 2 Outline Introduction African Vaccine Regulatory Forum (AVAREF) Joint Review Conclusion Introduction (1) Health
Joint scientific and ethics reviews of clinical trial applications Dr Diadié Maïga, WHO/AFRO 2 Outline Introduction African Vaccine Regulatory Forum (AVAREF) Joint Review Conclusion Introduction (1) Health
Special Considerations and Utility of Modeling and Simulation for Pediatric Medical Countermeasures: Introduction
 Special Considerations and Utility of Modeling and Simulation for Pediatric Medical Countermeasures: Introduction Dionna Green, M.D. Medical Officer Pediatric Clinical Pharmacology Staff Office of Clinical
Special Considerations and Utility of Modeling and Simulation for Pediatric Medical Countermeasures: Introduction Dionna Green, M.D. Medical Officer Pediatric Clinical Pharmacology Staff Office of Clinical
The Integrated Support and Assurance Process (ISAP): detailed guidance on assuring novel and complex contracts
 The Integrated Support and Assurance Process (ISAP): detailed guidance on assuring novel and complex contracts Part C: Guidance for NHS trusts and NHS foundation trusts Published by NHS England and NHS
The Integrated Support and Assurance Process (ISAP): detailed guidance on assuring novel and complex contracts Part C: Guidance for NHS trusts and NHS foundation trusts Published by NHS England and NHS
Development and challenges to monoclonal antibodies for passive immunization
 Development and challenges to monoclonal antibodies for passive immunization Erin Sparrow 21 June 2017 1 A brief history of serum derived passive immunization Concept developed by von Behring & Kitasato
Development and challenges to monoclonal antibodies for passive immunization Erin Sparrow 21 June 2017 1 A brief history of serum derived passive immunization Concept developed by von Behring & Kitasato
IMI2 Ebola and other filoviral haemorrhagic fevers programme. Webinar for SRG and SC 3 November 2014
 IMI2 Ebola and other filoviral haemorrhagic fevers programme Webinar for SRG and SC 3 November 2014 Background Ebola virus diseases (EVD) is a rare and deadly disease Filoviruses such as Ebola spread directly
IMI2 Ebola and other filoviral haemorrhagic fevers programme Webinar for SRG and SC 3 November 2014 Background Ebola virus diseases (EVD) is a rare and deadly disease Filoviruses such as Ebola spread directly
World Bank Africa Gender Innovation Lab (GIL) Call for Expressions of Interest: About the World Bank Africa Region Gender Innovation Lab (GIL)
 World Bank Africa Gender Innovation Lab (GIL) Call for Expressions of Interest: Seeking NGOs, governments, donors, and private sector firms with interventions designed to improve women s land tenure security
World Bank Africa Gender Innovation Lab (GIL) Call for Expressions of Interest: Seeking NGOs, governments, donors, and private sector firms with interventions designed to improve women s land tenure security
Efficient Project Management of Outsourced Biopharmaceutical Development and Manufacturing Programs
 Efficient Project Management of Outsourced Biopharmaceutical Development and Manufacturing Programs Thomas C. Ransohoff and Howard L. Levine, Ph.D. BioProcess Technology Consultants Inc. Presented at IBC
Efficient Project Management of Outsourced Biopharmaceutical Development and Manufacturing Programs Thomas C. Ransohoff and Howard L. Levine, Ph.D. BioProcess Technology Consultants Inc. Presented at IBC
NIH-RAID: A ROADMAP Program
 NIH-RAID: A ROADMAP Program (Rapid Access to Interventional Development} A Program designed to facilitate the development of new therapeutics The NIH-RAID Pilot Program is intended to reduce some of the
NIH-RAID: A ROADMAP Program (Rapid Access to Interventional Development} A Program designed to facilitate the development of new therapeutics The NIH-RAID Pilot Program is intended to reduce some of the
Request for Proposals Facilitating Utility Smart Energy Education (FUSED)
 Request for Proposals Facilitating Utility Smart Energy Education (FUSED) Specialized DER Professional Training for Utility Employees Technical point of contact: Business point of contact: Jenny Senff
Request for Proposals Facilitating Utility Smart Energy Education (FUSED) Specialized DER Professional Training for Utility Employees Technical point of contact: Business point of contact: Jenny Senff
Regulation of Microbiota- Based Products
 Regulation of Microbiota- Based Products LCDR Matthew Steele, PhD Team Leader, Regulatory Review Branch 1 Division of Vaccines and Related Products Applications CBER/OVRR My presentation is an informal
Regulation of Microbiota- Based Products LCDR Matthew Steele, PhD Team Leader, Regulatory Review Branch 1 Division of Vaccines and Related Products Applications CBER/OVRR My presentation is an informal
HIV ENV MANUFACTURING WORKSHOP A workshop sponsored by the Division of AIDS/NIAID/NIH and the Global HIV Vaccine Enterprise
 HIV ENV MANUFACTURING WORKSHOP A workshop sponsored by the Division of AIDS/NIAID/NIH and the Global HIV Vaccine Enterprise Strategies for Facilitating Regulatory Pathways Tanya Scharton-Kersten, PhD Senior
HIV ENV MANUFACTURING WORKSHOP A workshop sponsored by the Division of AIDS/NIAID/NIH and the Global HIV Vaccine Enterprise Strategies for Facilitating Regulatory Pathways Tanya Scharton-Kersten, PhD Senior
Rational approach to selection and clinical development of TB vaccine candidates
 Tuberculosis 92S1 (2012) S25 S29 Rational approach to selection and clinical development of TB vaccine candidates Lew Barker a, *, Luc Hessel b, Barry Walker c a Aeras, 1405 Research Boulevard, Rockville,
Tuberculosis 92S1 (2012) S25 S29 Rational approach to selection and clinical development of TB vaccine candidates Lew Barker a, *, Luc Hessel b, Barry Walker c a Aeras, 1405 Research Boulevard, Rockville,
Request for Proposals State and Territorial Health Leadership Initiative (SHLI)
 Request for Proposals State and Territorial Health Leadership Initiative (SHLI) Overview and Summary Information The Association of State and Territorial Health Officials (ASTHO) is the voice of state
Request for Proposals State and Territorial Health Leadership Initiative (SHLI) Overview and Summary Information The Association of State and Territorial Health Officials (ASTHO) is the voice of state
Accelerating Innovation in Global Health Crises The Fighting Ebola and Combating Zika and Future Threats Grand Challenge
 Accelerating Innovation in Global Health Crises The Fighting Ebola and Combating Zika and Future Threats Grand Challenge What will we do with what we ve learned? This was a test of the world s ability
Accelerating Innovation in Global Health Crises The Fighting Ebola and Combating Zika and Future Threats Grand Challenge What will we do with what we ve learned? This was a test of the world s ability
Regulated Studies at UTMB
 Regulated Studies at UTMB 1 st Annual Summit of Academic Excellence in Quality Assurance & Regulatory Science 18 19 May 2017 Melissa Eitzen, MT (ASCP), MS, RQAP GLP Director, Regulatory Operations 1 Schedule
Regulated Studies at UTMB 1 st Annual Summit of Academic Excellence in Quality Assurance & Regulatory Science 18 19 May 2017 Melissa Eitzen, MT (ASCP), MS, RQAP GLP Director, Regulatory Operations 1 Schedule
Summary of Provisions in 21 st Century Cures Act (H.R. 6) as passed by full House of Representatives, July 10, 2015
 Pediatric-Specific Provisions Summary of Provisions in 21 st Century Cures Act (H.R. 6) as passed by full House of Representatives, July 10, 2015 Requires the NIH to complete a strategic plan, and in the
Pediatric-Specific Provisions Summary of Provisions in 21 st Century Cures Act (H.R. 6) as passed by full House of Representatives, July 10, 2015 Requires the NIH to complete a strategic plan, and in the
PERtussIS COrrelates of Protection Europe. Newsletter Issue 1. periscope-project.eu
 PERtussIS COrrelates of Protection Europe Newsletter Issue 1 periscope-project.eu Editorial Welcome to the first issue of our Newsletter! We are very pleased to present the first e-newsletter of the PERISCOPE
PERtussIS COrrelates of Protection Europe Newsletter Issue 1 periscope-project.eu Editorial Welcome to the first issue of our Newsletter! We are very pleased to present the first e-newsletter of the PERISCOPE
IDSA is pleased to offer the following comments on specific priority areas identified by FDA:
 October 31, 2018 Shashi Malhotra Office of Acquisitions & Grants Services (OAGS) Food and Drug Administration Telephone: 240-402-7592 Email: Shashi.Malhotra@fda.hhs.gov SUBJECT: NOT-FD-18-16: Development
October 31, 2018 Shashi Malhotra Office of Acquisitions & Grants Services (OAGS) Food and Drug Administration Telephone: 240-402-7592 Email: Shashi.Malhotra@fda.hhs.gov SUBJECT: NOT-FD-18-16: Development
Course Agenda. Day One
 Course Agenda BioImmersion: Biotech for the Non-Scientist A three-day, in-depth course that provides the background required for understanding today s fast-paced biotech marketplace. Beginning with an
Course Agenda BioImmersion: Biotech for the Non-Scientist A three-day, in-depth course that provides the background required for understanding today s fast-paced biotech marketplace. Beginning with an
State and Territorial Health Agency Organizational Structures and Partnerships for Mosquito Control Management
 State and Territorial Health Agency Organizational Structures and Partnerships for Mosquito Control Management Findings from ASTHO s 2017 Mosquito Control Survey August 2018 National Headquarters 2231
State and Territorial Health Agency Organizational Structures and Partnerships for Mosquito Control Management Findings from ASTHO s 2017 Mosquito Control Survey August 2018 National Headquarters 2231
Request for Proposal RFP #
 Request for Proposal RFP # 2018-002 PATH Immunization Data: Evidence for Action, Strategic Communications Support I. Summary of Deadlines Release of Request for Proposal Confirmation of interest due Fact-finding
Request for Proposal RFP # 2018-002 PATH Immunization Data: Evidence for Action, Strategic Communications Support I. Summary of Deadlines Release of Request for Proposal Confirmation of interest due Fact-finding
COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS NOTE FOR GUIDANCE 1 : DNA VACCINES NON-AMPLIFIABLE IN EUKARYOTIC CELLS FOR VETERINARY USE
 The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Veterinary Use CVMP/IWP/07/98-FINAL COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS NOTE FOR GUIDANCE 1 : DNA VACCINES
The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Veterinary Use CVMP/IWP/07/98-FINAL COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS NOTE FOR GUIDANCE 1 : DNA VACCINES
Modeling Zoonoses to Identify Potential Vulnerabilities and Data Gaps: Rift Valley Fever in the United States
 Modeling Zoonoses to Identify Potential Vulnerabilities and Data Gaps: Rift Valley Fever in the United States David M. Hartley, PhD Nicole Leahy, PhD and Holly Gaff, PhD University of Maryland School of
Modeling Zoonoses to Identify Potential Vulnerabilities and Data Gaps: Rift Valley Fever in the United States David M. Hartley, PhD Nicole Leahy, PhD and Holly Gaff, PhD University of Maryland School of
MEDICINES CONTROL COUNCIL
 PRE-REGISTRATION CONSULTATION MEETING MATERIAL CHECKLIST MEDICINES CONTROL COUNCIL PRE-REGISTRATION CONSULTATION MEETING MATERIAL The CHECKLIST is to be used for the preparation of a formal request to
PRE-REGISTRATION CONSULTATION MEETING MATERIAL CHECKLIST MEDICINES CONTROL COUNCIL PRE-REGISTRATION CONSULTATION MEETING MATERIAL The CHECKLIST is to be used for the preparation of a formal request to
Phase Appropriate GMPs for IMPs. Presented by: Karen S. Ginsbury For: IFF, October 31, Nov 02, 2017
 Phase Appropriate GMPs for IMPs Presented by: Karen S. Ginsbury For: IFF, October 31, Nov 02, 2017 1 Lets start with References https://mhrainspectorate.blog.gov.uk/2016/0 5/20/manufacture-of-investigationalmedicinal-products-frequently-askedquestions/
Phase Appropriate GMPs for IMPs Presented by: Karen S. Ginsbury For: IFF, October 31, Nov 02, 2017 1 Lets start with References https://mhrainspectorate.blog.gov.uk/2016/0 5/20/manufacture-of-investigationalmedicinal-products-frequently-askedquestions/
Research Opportunities Program Guidelines
 July 2015 2015-16 Research Opportunities Program Guidelines Prevention Office Ministry of Labour Table of Contents 1. General Information...2 2. Priority Areas...2 3. Funding Parameters...6 4. Application
July 2015 2015-16 Research Opportunities Program Guidelines Prevention Office Ministry of Labour Table of Contents 1. General Information...2 2. Priority Areas...2 3. Funding Parameters...6 4. Application
Context and objectives of the workshop on platform technologies. 21 July 2016 David Wood and Virginia Benassi
 Context and objectives of the workshop on platform technologies 21 July 2016 David Wood and Virginia Benassi 2nd Technical workshop on platform technologies Geneva, Switzerland, 21 July 2016 Platform Technologies
Context and objectives of the workshop on platform technologies 21 July 2016 David Wood and Virginia Benassi 2nd Technical workshop on platform technologies Geneva, Switzerland, 21 July 2016 Platform Technologies
BIO-INNOVATION FOR HEALTH: SOUTH AFRICA
 BIO-INNOVATION FOR HEALTH: SOUTH AFRICA SA s Burden of Disease SA MRC is committed to addressing the burden of disease that impacts health Cause of death Deaths % HIV/AIDS 180,870 29.4 Hypertensive heart
BIO-INNOVATION FOR HEALTH: SOUTH AFRICA SA s Burden of Disease SA MRC is committed to addressing the burden of disease that impacts health Cause of death Deaths % HIV/AIDS 180,870 29.4 Hypertensive heart
Investing in Research, Development and Innovation for Global Health
 The Malaria Advocacy Working Group (MAWG) of the Roll Back Malaria Partnership s (RBM) contribution to the European Commission consultation on the Green Paper - From Challenges to Opportunities: Towards
The Malaria Advocacy Working Group (MAWG) of the Roll Back Malaria Partnership s (RBM) contribution to the European Commission consultation on the Green Paper - From Challenges to Opportunities: Towards
WHO s IMS Organizational Structure Overview
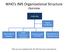 WHO s IMS Organizational Structure Overview Leadership Partner Coordination Information & Planning Health Expertise & Operations Operations Support and Logistics Management & Administration *this structure
WHO s IMS Organizational Structure Overview Leadership Partner Coordination Information & Planning Health Expertise & Operations Operations Support and Logistics Management & Administration *this structure
Topics Covered. FDA s Role in Expediting the Development of Novel Medical Products. How a Regulatory Agency Comes into Existence 3/5/2018
 FDA s Role in Expediting the Development of Novel Medical Products Peter Marks, M.D., Ph.D. Director Center for Biologics Evaluation and Research Topics Covered Brief history of FDA Expediting product
FDA s Role in Expediting the Development of Novel Medical Products Peter Marks, M.D., Ph.D. Director Center for Biologics Evaluation and Research Topics Covered Brief history of FDA Expediting product
Guideline on requirements for the production and control of immunological veterinary medicinal products
 14 June 2012 EMA/CVMP/IWP/206555/2010 Committee for Medicinal Products for Veterinary Use (CVMP) Guideline on requirements for the production and control of immunological veterinary Draft agreed by Immunologicals
14 June 2012 EMA/CVMP/IWP/206555/2010 Committee for Medicinal Products for Veterinary Use (CVMP) Guideline on requirements for the production and control of immunological veterinary Draft agreed by Immunologicals
Lessons learnt from the Ebola outbreak A regulatory position
 www.pei.de Lessons learnt from the Ebola outbreak A regulatory position Jan Mueller-Berghaus Paul-Ehrlich-Institut Federal Institute for Vaccines and Biomedicines, Germany The views expressed in this presentation
www.pei.de Lessons learnt from the Ebola outbreak A regulatory position Jan Mueller-Berghaus Paul-Ehrlich-Institut Federal Institute for Vaccines and Biomedicines, Germany The views expressed in this presentation
Innovative Medicines Initiative (IMI) Future funding opportunities. Catherine Brett, IMI Conferencia H2020 y Salud Madrid, Spain 8 November 2017
 Innovative Medicines Initiative (IMI) Future funding opportunities Catherine Brett, IMI Conferencia H2020 y Salud Madrid, Spain 8 November 2017 Outline What is IMI and why do we need it? How does IMI work?
Innovative Medicines Initiative (IMI) Future funding opportunities Catherine Brett, IMI Conferencia H2020 y Salud Madrid, Spain 8 November 2017 Outline What is IMI and why do we need it? How does IMI work?
NATIONAL PRIORITIES:
 NATIONAL PRIORITIES: CONTINUOUS MANUFACTURING OF MEDICAL COUNTERMEASURES R. Thomas Warf, Director Manufacturing, Facilities, and Engineering HHS/ASPR/BARDA June 27, 2016 Resilient People. Healthy Communities.
NATIONAL PRIORITIES: CONTINUOUS MANUFACTURING OF MEDICAL COUNTERMEASURES R. Thomas Warf, Director Manufacturing, Facilities, and Engineering HHS/ASPR/BARDA June 27, 2016 Resilient People. Healthy Communities.
Expanded Access. to Investigational Drugs & Biologics. for Treatment Use
 SJMHS Research Compliance Office Guidance Document Expanded Access to Investigational Drugs & Biologics for Treatment Use May 2015 1 Expanded Access to Investigational Drugs & Biologics for Treatment Use
SJMHS Research Compliance Office Guidance Document Expanded Access to Investigational Drugs & Biologics for Treatment Use May 2015 1 Expanded Access to Investigational Drugs & Biologics for Treatment Use
Expanding Hydropower and Pumped Storage s Contribution to Grid Resiliency and Reliability RFI#: DE-FOA
 DATE: February 21, 2018 Expanding Hydropower and Pumped Storage s Contribution to Grid Resiliency and Reliability RFI#: DE-FOA-0001886 SUBJECT: Request for Information (RFI) RESPONSE DUE: April 6, 2018
DATE: February 21, 2018 Expanding Hydropower and Pumped Storage s Contribution to Grid Resiliency and Reliability RFI#: DE-FOA-0001886 SUBJECT: Request for Information (RFI) RESPONSE DUE: April 6, 2018
Building a PMO: A Blueprint for Success
 Building a PMO: A Blueprint for Success BRIEF As corporations seek every possible competitive and operational advantage, project management is gaining new momentum and importance. Some organisations are
Building a PMO: A Blueprint for Success BRIEF As corporations seek every possible competitive and operational advantage, project management is gaining new momentum and importance. Some organisations are
Forthcoming Calls for Proposals (10/11) Barcelona 28 June 2013
 Forthcoming Calls for Proposals (10/11) Barcelona 28 June 2013 Indicative list Proposed topic /11 /11 /11 Identification and validation of innovative clinical endpoints for Osteoarthritis European genotype-phenotype
Forthcoming Calls for Proposals (10/11) Barcelona 28 June 2013 Indicative list Proposed topic /11 /11 /11 Identification and validation of innovative clinical endpoints for Osteoarthritis European genotype-phenotype
Project Planning & Scheduling
 Project Planning & Scheduling 2 Objectives To introduce and discuss key concepts and techniques for planning and scheduling major projects To provide ideas for development of a practice session to apply
Project Planning & Scheduling 2 Objectives To introduce and discuss key concepts and techniques for planning and scheduling major projects To provide ideas for development of a practice session to apply
The U.S. Response to Ebola: Status of the FY2015 Emergency Ebola Appropriation
 The U.S. Response to Ebola: Status of the FY2015 Emergency Ebola Appropriation Jen Kates, Josh Michaud, Adam Wexler, and Allison Valentine The Ebola outbreak of 2014 was a global wake-up call regarding
The U.S. Response to Ebola: Status of the FY2015 Emergency Ebola Appropriation Jen Kates, Josh Michaud, Adam Wexler, and Allison Valentine The Ebola outbreak of 2014 was a global wake-up call regarding
The U.S. Response to Ebola: Status of the FY2015 Emergency Ebola Appropriation
 November 2015 Issue Brief The U.S. Response to Ebola: Status of the FY2015 Emergency Ebola Appropriation Jen Kates, Josh Michaud, Adam Wexler, and Allison Valentine Overview The Ebola outbreak of 2014
November 2015 Issue Brief The U.S. Response to Ebola: Status of the FY2015 Emergency Ebola Appropriation Jen Kates, Josh Michaud, Adam Wexler, and Allison Valentine Overview The Ebola outbreak of 2014
WAVA Update to LSDF Board
 WAVA Update to LSDF Board December 7, 2010 Larry Corey WAVA Science Director Incoming President of the Fred Hutchinson Cancer Center Harlan Patterson WAVA Executive Director 1 Washington Vaccine Alliance
WAVA Update to LSDF Board December 7, 2010 Larry Corey WAVA Science Director Incoming President of the Fred Hutchinson Cancer Center Harlan Patterson WAVA Executive Director 1 Washington Vaccine Alliance
Supporting Clinical Research Centres: A PDP Model
 Supporting Clinical Research Centres: A PDP Model Annaléne Nel, Chief Medical Officer Global Health Trials Meeting Cape Town, 1 February 2013 Overview o The PDP Model o Product Development Process o Research
Supporting Clinical Research Centres: A PDP Model Annaléne Nel, Chief Medical Officer Global Health Trials Meeting Cape Town, 1 February 2013 Overview o The PDP Model o Product Development Process o Research
Evaluation: annual report
 EXECUTIVE BOARD EB137/7 137th session 8 May 2015 Provisional agenda item 8.2 Evaluation: annual report 1. The Executive Board at its 131st session approved the WHO evaluation policy. 1 The policy requires
EXECUTIVE BOARD EB137/7 137th session 8 May 2015 Provisional agenda item 8.2 Evaluation: annual report 1. The Executive Board at its 131st session approved the WHO evaluation policy. 1 The policy requires
Expanded Access and the Individual Patient IND
 Expanded Access and the Individual Patient IND Research Wednesdays April 26, 2017 Erika Segear Johnson, PhD, RAC Associate Director of Regulatory Affairs Office of Regulatory Affairs and Quality Office
Expanded Access and the Individual Patient IND Research Wednesdays April 26, 2017 Erika Segear Johnson, PhD, RAC Associate Director of Regulatory Affairs Office of Regulatory Affairs and Quality Office
2018 Call for Letters of Intent LOI Guidelines and Submission Instructions
 2018 Call for Letters of Intent LOI Guidelines and Submission Instructions This call is being issued to support the development of SCN s funding application to the Networks of Centres of Excellence program.
2018 Call for Letters of Intent LOI Guidelines and Submission Instructions This call is being issued to support the development of SCN s funding application to the Networks of Centres of Excellence program.
