HTA RR/07/06 September 2007 Mobile Computed Tomography Scanner for Head and Neck Imaging
|
|
|
- Clara Sara Conley
- 6 years ago
- Views:
Transcription
1 HA HTA Brief offers prompt assessment to new or undecided technologies that may be of value or concern to the HA. It serves as a quick reference to inform care providers and practitioners of the safety, efficacy, opinion and/or state of technology diffusion in other parts of the world. We caution readers to interpret our report in light of the literature search and evidence presented, and on the role of HTA to provide information to aid decision-making on a system level rather than for individual patients. Besides technical efficiency, the suitability of a technology in public health services also depends on its cost-effectiveness, health and economic impacts on the society, resources available to the service provider and competing needs of other patients. By making technical efficiency explicit, one is in a better position to evaluate the other considerations. HTA RR/07/06 September 2007 Mobile Computed Tomography Scanner for Head and Neck Imaging Prepared by TSANG, Kwai-fan Ice; CHEUNG, Tsz-fung Ian; LIU, Hing-wing Background / Introduction Computed tomography (CT) scanning is a valuable diagnostic tool. Besides generating high-quality images of internal body structures, it enables threedimensional reconstruction of images such that their display need not be restricted to the conventional axial view. CT technology contributes remarkably to diagnostic accuracy and management strategy. A timely CT scan can be life-saving. However, conventional high-resolution flat-bed CT scanners are huge, weighing up to 4,000 kg and require a high-voltage power supply to run the massive cooling system, and hence, are installed in climate-controlled and lead-shielded radiology suites. To bring CT technology into emergency settings and operating theatres, mobile CT scanners were introduced in the 80s, for example, the TomoScan M (designed by Analogic and sold by Philips Medical Systems.) The first-generation mobile CT scanners were, however, limited by image quality, speed, size (still too large to be moved easily to patient bedside) and had too many wires and cables. Lately, NeuroLogica launched CereTom, a compact and lightweight scanner equipped with wheels, runs on wall outlets in combination with batteries, and a translating gantry for selecting the scan plane, which makes it suitable for use at the bedside. The model received pre-market approval from FDA in July Page 1 of 5
2 The system generates up to 8 slices per revolution. Its 25cm field of view limits usage primarily to head and neck imaging. ii) What changes (and resources) are required, other than a mobile CT scanner, to run an efficient mobile CT service? Mobile CT scanners have been used in a number of settings, in particular, intra-operatively to facilitate imageguided surgery, and in the ICU to avoid transporting critically ill patients to and from the fixed CT suite. 2 iii) Will the benefit generated, in terms of patient outcome, justify the opportunity cost required to run a mobile CT service? Review Questions While there is little concern over the safety and technical feasibility of approved mobile CT scanners, questions facing healthcare providers are: i) What is the added value, in terms of patient outcome, of having a mobile CT scanner in an acute hospital already equipped with a fixed CT scanner? 1 PMA (K051765) was granted on the basis that Use of the NL3000 CereTom does not result in any new potential safety risks. The equipment performs as well in its intended use as devices currently on the market. 2 Gunnarsson T, Theodorsson A, Karlsson P, Fridriksson S, Boström S, Persliden J, et al. Mobile computerized tomography scanning in the neurosurgery intensive care unit: increase in patient safety and reduction of staff workload. J Neurosurg Sep;93(3): Page 2 of 5
3 Literature Search HTA Agencies A. Agence d évaluation des technologies et des modes d intervention en santé (AETMIS) The Québec government agency responsible for health services and technology assessment, and reports to Québec's Minister of Health and Social services. Web-site: B. Agency for Healthcare Research and Quality (AHRQ) A Public Health Service agency in the Department of Health and Human Services (HHS) and reports to the US HHS Secretary. Web-site: C. Australian Safety and Efficacy Register of New Interventional Procedures Surgical (ASERNIP-S). Website: D. Blue Cross and Blue Shield Association s Technology Evaluation Center. Website: E. Canadian Agency for Drugs and Technologies in Health (CADTH) An independent, not-for-profit agency funded by Canadian federal, provincial, and territorial governments. Website: F. Catalan Agency for Health Technology Assessment (CAHTA) / Agència d'avaluació de Tecnologia i Recerca Mèdiques de Catalunya (AATRM) A public non-profit company reporting to the Catalan Health Service. Web-site: G. Committee for Evaluation and Diffusion of Innovative Technologies (CEDIT) A hospital-based agency for the assessment of medical technology and responsible for formulating advice for the Director General of the Assistance Publique-Hôpitaux de Paris (AP-HP). Website: H. Hayes, Inc. An independent health technology assessment organization. Website: I. Health Canada A federal department in Canada. Website: J. Health Services/Technology Assessment Text National Library of Medicine A free, web-based resource of full-text documents. Website: K. International Network of Agencies for Health Technology Assessment (INAHTA) The Network stretches from North and Latin America to Europe, Australia, and Page 3 of 5
4 New Zealand. Website: L. Medical Services Advisory Committee (MSAC) To advise the Minister for Health and Ageing in Australia. Website: M. National Centre for Biotechnology Information (NCBI). Website: N. National Coordinating Centre for Health Technology Assessment (NCCHTA) Developed the NHS HTA programme under contract from the Department of Health s Research and Development Division. Website: O. National Horizon Scanning Centre Provides advance notice of significant new and emerging health technologies to the Department of Health, England. Website: P. National Institute for Health and Clinical Excellence (NICE) An independent organization. Website: Q. Ontario Health Technology Advisory Committee (OHTAC) Provides advice to the health care system, including the Ministry of Health and Long-Term Care. Website: R. Swedish Council on Technology Assessment in Health Care An independent public authority. Website: S. Trip Database. Website: Other Databases T. Cochrane Collaboration An international not-for-profit organization. Website: U. German Cochrane Centre A centre of the Cochrane Collaboration. Website: V. MEDLINE (1996 to August Week ) W. EMBASE (1996 to 2007 Week 34) Page 4 of 5
5 Evidence-base Recommendation FDA granted 510k premarket approval to CereTom mobile CT scanner on the bases that it is substantially equivalent to previously cleared predicate devices. 3 To answer the review questions raised, we scanned HTA agency web-sites, the Cochrane databases, MEDLINE and EMBASE for evidence on the safety and efficacy of mobile CT scanning for head and neck and its impact on patient outcome. While we found a number of papers on successful application of mobile CT in operating theatre and ICU, none of them reported impact of the technology on patient outcome. In summary, no published study satisfying the search criteria could be identified. Given the information available, the best conclusion one can draw is (i) CereTom mobile CT scanner is FDA approved, indicating its safety and performance are substantially equivalent to predicate devices previously cleared by FDA; (ii) Evidence on the relative safety and benefit of mobile CT scanning, as compare to fixed CT scanning, in terms of patient outcome, is currently lacking. There is insufficient evidence to support widespread adoption of mobile CT technology in the existing radiology services. The decision to install a mobile CT scanner in addition to a fixed one is primarily a cost-benefit consideration in service planning, subject to other factors pertinent to a particular healthcare setting. The following considerations may be relevant in assisting such a decision making: (i) Application of CereTom mobile CT scanner is primarily confined to head and neck imaging. (ii) While it is more convenient and safe to scan critically ill patients at the bedside, the margin of benefit that can be gained is uncertain due to lack of evidence. (iii) Given budgetary constraint, it is necessary to consider the opportunity cost in running a mobile CT service, which includes direct fixed cost (e.g. machine cost, maintenance cost), direct variable cost (e.g. staff salaries, consumables), and indirect cost (facility modifications such as lifts, bed design and spacing.) 3 FDA PMA (K051765): Page 5 of 5
Involvement of consumers in the HTA activities of INAHTA members
 Involvement of consumers in the HTA activities of INAHTA members Report on a survey Document prepared by:: David Hailey (IHE), Sophie Werkö (SBU), Rugayah Bakri (MaHTAS), Alun Cameron (ASERNIP-S), Britta
Involvement of consumers in the HTA activities of INAHTA members Report on a survey Document prepared by:: David Hailey (IHE), Sophie Werkö (SBU), Rugayah Bakri (MaHTAS), Alun Cameron (ASERNIP-S), Britta
OPTIMAL USE PROGRAM DRUG Therapeutic Review Framework and Process
 OPTIMAL USE PROGRAM DRUG Therapeutic Review Framework and Process Version: 3.0 Publication Date: June 27, 2018 Report Length: 16 Pages Revision History From time to time, CADTH may amend the therapeutic
OPTIMAL USE PROGRAM DRUG Therapeutic Review Framework and Process Version: 3.0 Publication Date: June 27, 2018 Report Length: 16 Pages Revision History From time to time, CADTH may amend the therapeutic
JUN (k) Summary Philips Medical Systems (Cleveland) Inc. "Brilliance Volume" 1. Submitter. 3. Predicate Device Information
 510(k) Summary Philips Medical Systems (Cleveland) Inc. "Brilliance Volume" JUN - 6 2006 This summary of this 510(k) provides safety and effectiveness information submitted in accordance with the requirements
510(k) Summary Philips Medical Systems (Cleveland) Inc. "Brilliance Volume" JUN - 6 2006 This summary of this 510(k) provides safety and effectiveness information submitted in accordance with the requirements
Environmental Scan Process
 CADTH Environmental Scan Process May 2015 Version 1.0 REVISION HISTORY Periodically, this document will be revised as part of ongoing process improvement activities. The following version control table,
CADTH Environmental Scan Process May 2015 Version 1.0 REVISION HISTORY Periodically, this document will be revised as part of ongoing process improvement activities. The following version control table,
Finding the Evidence: Tools and Techniques for Literature Searching
 Finding the Evidence: Tools and Techniques for Literature Searching April 24, 2015 Webinar for SHLA CADTH CADTH is an independent, not-for-profit producer and broker of health technology assessments. Federal,
Finding the Evidence: Tools and Techniques for Literature Searching April 24, 2015 Webinar for SHLA CADTH CADTH is an independent, not-for-profit producer and broker of health technology assessments. Federal,
Medical Technology: Cost Impact. Medical Technology EBPA 2008 EBPA 2008 EBPA 2008
 Medical Technology: Cost Impact Employee Benefit Planning Association November 6, 2008 Mark Lyons, RPh, MBA Director, Care Facilitation Consulting Healthcare Costs continue to rise Current medical costs
Medical Technology: Cost Impact Employee Benefit Planning Association November 6, 2008 Mark Lyons, RPh, MBA Director, Care Facilitation Consulting Healthcare Costs continue to rise Current medical costs
High performance comes easily
 High performance comes easily Philips MX 16-slice CT Easy from any The CT solution Your days may not be getting any easier, but now your CT solution is. The remarkably easy-to-use Philips MX 16-slice CT
High performance comes easily Philips MX 16-slice CT Easy from any The CT solution Your days may not be getting any easier, but now your CT solution is. The remarkably easy-to-use Philips MX 16-slice CT
Medical Technology: Cost Impact
 Medical Technology: Cost Impact Employee Benefit Planning Association November 6, 2008 Mark Lyons, RPh, MBA Director, Care Facilitation Consulting Healthcare Costs continue to rise Current medical costs
Medical Technology: Cost Impact Employee Benefit Planning Association November 6, 2008 Mark Lyons, RPh, MBA Director, Care Facilitation Consulting Healthcare Costs continue to rise Current medical costs
CANADIAN COORDINATING OFFICE FOR HEALTH TECHNOLOGY ASSESSMENT (CCOHTA)
 CANADIAN COORDINATING OFFICE FOR HEALTH TECHNOLOGY ASSESSMENT (CCOHTA) CCOHTA s Transition to the Canadian Health Technology Agency Five-Year Business Plan 2006 2011 For Public Distribution Approved by
CANADIAN COORDINATING OFFICE FOR HEALTH TECHNOLOGY ASSESSMENT (CCOHTA) CCOHTA s Transition to the Canadian Health Technology Agency Five-Year Business Plan 2006 2011 For Public Distribution Approved by
2014 CLINICAL POLICY UNIT. Dr Vesna Kupresan
 2014 CLINICAL POLICY UNIT Dr Vesna Kupresan Healthcare Industry Challenges Medical inflation Cost drivers Aging population Increased utilization of healthcare Growing chronic disease burden Rapid evolution
2014 CLINICAL POLICY UNIT Dr Vesna Kupresan Healthcare Industry Challenges Medical inflation Cost drivers Aging population Increased utilization of healthcare Growing chronic disease burden Rapid evolution
1. Executive Summary
 1. Executive Summary 1.1 General The fluoroscope is defined as an instrument used chiefly in industry and in the practice of medicine for observing the internal structure of objects (such as the living
1. Executive Summary 1.1 General The fluoroscope is defined as an instrument used chiefly in industry and in the practice of medicine for observing the internal structure of objects (such as the living
Reducing Unnecessary Medical Imaging Exposure
 Reducing Unnecessary Medical Imaging Exposure CDR Sean M. Boyd, MPH, USPHS U.S. Food and Drug Administration Center for Devices and Radiological Health April 30, 2010 Objectives FDA s role in ensuring
Reducing Unnecessary Medical Imaging Exposure CDR Sean M. Boyd, MPH, USPHS U.S. Food and Drug Administration Center for Devices and Radiological Health April 30, 2010 Objectives FDA s role in ensuring
The time has come. Philips GEMINI TF PET/CT with TruFlight technology
 The time has come Philips GEMINI TF PET/CT with TruFlight technology TruFlight has arrived Time-of-flight technology has always held the promise of better PET imaging. But it took Philips to harness its
The time has come Philips GEMINI TF PET/CT with TruFlight technology TruFlight has arrived Time-of-flight technology has always held the promise of better PET imaging. But it took Philips to harness its
THE NEW 640 SLICE CT SCANNER
 THE NEW 640 SLICE CT SCANNER www.ahdubai.com The American Hospital Dubai has recently acquired a state-ofthe-art computerized tomography (CT) scanner that houses a variety of intelligent and industry-leading
THE NEW 640 SLICE CT SCANNER www.ahdubai.com The American Hospital Dubai has recently acquired a state-ofthe-art computerized tomography (CT) scanner that houses a variety of intelligent and industry-leading
COVERAGE ELIGIBILITY OF SERVICES ASSOCIATED WITH A CLINICAL TRIAL
 Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Medical Coverage Guideline must be read in its
Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Medical Coverage Guideline must be read in its
Hybrid Operating Room Market Research Report - Global Forecast Till 2023
 Report Information More information from: https://www.marketresearchfuture.com/reports/862 Hybrid Operating Room Market Research Report - Global Forecast Till 2023 Report / Search Code: MRFR/HC/0360-HCRR
Report Information More information from: https://www.marketresearchfuture.com/reports/862 Hybrid Operating Room Market Research Report - Global Forecast Till 2023 Report / Search Code: MRFR/HC/0360-HCRR
The Drug Approval Process in Canada
 The Drug Approval Process in Canada Ryan Clarke Saturday, April 26, 2014 Overview Process Drug approvals Pricing Common Drug Review Listings (QC) Process Public drug formularies are impacted by federal,
The Drug Approval Process in Canada Ryan Clarke Saturday, April 26, 2014 Overview Process Drug approvals Pricing Common Drug Review Listings (QC) Process Public drug formularies are impacted by federal,
Copyright. Jeremiah J. Kelly (2015). All rights reserved. Further dissemination without express written consent strictly prohibited.
 Statutory Framework for Devices Medical Devices Investigational Use Application IDE (21 CFR 812) Abbreviated IDE Exempt Pre-Market Approval Applications 510(k) Pre-marketing Notification (21 CFR 807(e))
Statutory Framework for Devices Medical Devices Investigational Use Application IDE (21 CFR 812) Abbreviated IDE Exempt Pre-Market Approval Applications 510(k) Pre-marketing Notification (21 CFR 807(e))
Press Presse Press Presse
 Press Presse Press Presse Healthcare Sector Imaging & IT Division Erlangen, November 30, 2009 Siemens Introduces its New Power Couple at RSNA 2009 Tim and Dot* redefine productivity in MRI up to 30 percent
Press Presse Press Presse Healthcare Sector Imaging & IT Division Erlangen, November 30, 2009 Siemens Introduces its New Power Couple at RSNA 2009 Tim and Dot* redefine productivity in MRI up to 30 percent
This template is to be used by companies willing to submit an overview of relevant
 Briefing book template for pharmaceuticals to support a multi-hta Early Dialogue (ED) December 13 th, 2013 This template is to be used by companies willing to submit an overview of relevant information
Briefing book template for pharmaceuticals to support a multi-hta Early Dialogue (ED) December 13 th, 2013 This template is to be used by companies willing to submit an overview of relevant information
Antihemophilic Factor (Recombinant BDD) Fc Fusion Protein (Eloctate): Treatment Cost Comparison and Budget Impact Analysis
 CADTH TECHNOLOGY REVIEW Antihemophilic Factor (Recombinant BDD) Fc Fusion Protein (): Treatment Cost Comparison and Budget Impact Analysis Product Line: Technology Review Version: 1.0 Issue Number: 2 Publication
CADTH TECHNOLOGY REVIEW Antihemophilic Factor (Recombinant BDD) Fc Fusion Protein (): Treatment Cost Comparison and Budget Impact Analysis Product Line: Technology Review Version: 1.0 Issue Number: 2 Publication
Interventional CT: More Procedures, Reduced Dose, Improved Speed at Minimal Cost
 www.usa.siemens.com/healthcare Interventional CT: More Procedures, Reduced Dose, Improved Speed at Minimal Cost St. Nicholas Hospital Answers for life. Key Benefits Clinical: Improved image quality including
www.usa.siemens.com/healthcare Interventional CT: More Procedures, Reduced Dose, Improved Speed at Minimal Cost St. Nicholas Hospital Answers for life. Key Benefits Clinical: Improved image quality including
GE Healthcare. The new MAC * 800
 GE Healthcare The new MAC * 800 Fits your needs wherever you are. Advanced ECG technology can make a real difference in patient care. As long as it s working for you and not the other way around. The new
GE Healthcare The new MAC * 800 Fits your needs wherever you are. Advanced ECG technology can make a real difference in patient care. As long as it s working for you and not the other way around. The new
Real World Evidence for the Canadian Market
 WHITE PAPER REAL WORLD EVIDENCE August 2013 Real World Evidence for the Canadian Market WHITE PAPER 1 8.2 The Business of Pharmaceutical Marketing 8.2.1 Real World Evidence for the Canadian Pharmaceutical
WHITE PAPER REAL WORLD EVIDENCE August 2013 Real World Evidence for the Canadian Market WHITE PAPER 1 8.2 The Business of Pharmaceutical Marketing 8.2.1 Real World Evidence for the Canadian Pharmaceutical
Delivering the NIHR Central Commissioning Facility
 Delivering the NIHR Central Commissioning Facility About the NIHR The National Institute for Health Research is funded through the Department of Health to improve the health and wealth of the nation through
Delivering the NIHR Central Commissioning Facility About the NIHR The National Institute for Health Research is funded through the Department of Health to improve the health and wealth of the nation through
Computer-Aided Surgical Navigation Coding Guide Neurosurgery. May 1, 2009
 Computer-Aided Surgical Navigation Coding Guide Neurosurgery May 1, 2009 Please direct any questions to: Kim Brew Manager, Reimbursement and Therapy Access Medtronic Surgical Technologies (904) 279-7569
Computer-Aided Surgical Navigation Coding Guide Neurosurgery May 1, 2009 Please direct any questions to: Kim Brew Manager, Reimbursement and Therapy Access Medtronic Surgical Technologies (904) 279-7569
Progress in X-Ray & MR
 Progress in X-Ray & MR Michiel Manuel Analyst Meeting June 15 th, 2005 X-Ray & MR: Agenda Introduction General X-Ray Cardio/Vascular X-Ray Magnetic Resonance China growth opportunity Conclusion 2 X-Ray
Progress in X-Ray & MR Michiel Manuel Analyst Meeting June 15 th, 2005 X-Ray & MR: Agenda Introduction General X-Ray Cardio/Vascular X-Ray Magnetic Resonance China growth opportunity Conclusion 2 X-Ray
Disclosures. Laboratory Stakeholders. IVD vs. LDT. FDA Regulation of Laboratory Developed Tests 10/2/2015. FDA Regulation of LDTs
 Disclosures FDA Regulation of Laboratory Developed Tests Beaumont Health System, 24 th Annual Symposium on Molecular Pathology September 16, 2015 Roger D. Klein, MD JD Director, Molecular Pathology Clinical
Disclosures FDA Regulation of Laboratory Developed Tests Beaumont Health System, 24 th Annual Symposium on Molecular Pathology September 16, 2015 Roger D. Klein, MD JD Director, Molecular Pathology Clinical
Disinvestment. Overview of disinvestment experiences and challenges in selected countries. Project report
 Disinvestment Overview of disinvestment experiences and challenges in selected countries Project report HTA-Projektbericht Nr: 057 ISSN 1992-0488 ISSN online 1992-0496 Disinvestment Overview of disinvestment
Disinvestment Overview of disinvestment experiences and challenges in selected countries Project report HTA-Projektbericht Nr: 057 ISSN 1992-0488 ISSN online 1992-0496 Disinvestment Overview of disinvestment
E v e r y t h i n g y o u n e e d...
 S U R G I C A L T A B L E E v e r y t h i n g y o u n e e d... E a s y m a x S u r g i c a l Ta b l e S T E R I S, A M A J O R H E A L T H C A R E P L A Y E R STERIS Surgical Technologies SARAN USA STERIS
S U R G I C A L T A B L E E v e r y t h i n g y o u n e e d... E a s y m a x S u r g i c a l Ta b l e S T E R I S, A M A J O R H E A L T H C A R E P L A Y E R STERIS Surgical Technologies SARAN USA STERIS
Pilot Socioeconomic Impact Analysis of CFI and CIHR Funding: Medical Imaging R&D
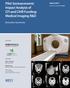 Pilot Socioeconomic Impact Analysis of CFI and CIHR Funding: Medical Imaging R&D March 2013 RTI Project Number 0213097 Executive Summary Prepared for Prepared by Alan C. O Connor RTI International Albert
Pilot Socioeconomic Impact Analysis of CFI and CIHR Funding: Medical Imaging R&D March 2013 RTI Project Number 0213097 Executive Summary Prepared for Prepared by Alan C. O Connor RTI International Albert
Innovations in Drug Pricing and Reimbursement:
 ADVISORY REPORT AM PL E PA G ES S A S G ES A FirstWord Dossier Advisory report and Reimbursement Published Copyright 2016 Doctor s Guide Publishing Limited Part of the FirstWord Dossier family of reports
ADVISORY REPORT AM PL E PA G ES S A S G ES A FirstWord Dossier Advisory report and Reimbursement Published Copyright 2016 Doctor s Guide Publishing Limited Part of the FirstWord Dossier family of reports
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: clinical_trial_services 3/2002 2/2018 2/2019 8/2018 Description of Procedure or Service Clinical trials are
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: clinical_trial_services 3/2002 2/2018 2/2019 8/2018 Description of Procedure or Service Clinical trials are
How Logistics Enables the Healthcare Revolution. Richard Holmes Managing Director of Polar Speed Distribution Ltd, a UPS company
 How Logistics Enables the Healthcare Revolution Richard Holmes Managing Director of Polar Speed Distribution Ltd, a UPS company Introduction There is a revolution taking place in healthcare delivery. Patient-centred
How Logistics Enables the Healthcare Revolution Richard Holmes Managing Director of Polar Speed Distribution Ltd, a UPS company Introduction There is a revolution taking place in healthcare delivery. Patient-centred
Template for comments
 WHO concept note and collaboration proposal for International Nomenclature, coding and classification of medical devices (ICMD) Name of person(s) submitting comments Eileen G. Erinoff Institution/organization:
WHO concept note and collaboration proposal for International Nomenclature, coding and classification of medical devices (ICMD) Name of person(s) submitting comments Eileen G. Erinoff Institution/organization:
COCIR CT MANUFACTURERS VOLUNTARY COMMITMENT REGARDING CT DOSE OPTIMIZATION Annual Report
 COCIR CT MANUFACTURERS VOLUNTARY COMMITMENT REGARDING CT DOSE OPTIMIZATION 2015 Annual Report Preamble In 2010 discussions took place between COCIR and the Heads of European Radiation Competent Authorities
COCIR CT MANUFACTURERS VOLUNTARY COMMITMENT REGARDING CT DOSE OPTIMIZATION 2015 Annual Report Preamble In 2010 discussions took place between COCIR and the Heads of European Radiation Competent Authorities
HTA methodology at HIQA. Conor Teljeur
 HTA methodology at HIQA Conor Teljeur What is HTA? Health technology assessment (HTA) is a multidisciplinary process that summarises information about the medical, social, economic and ethical issues related
HTA methodology at HIQA Conor Teljeur What is HTA? Health technology assessment (HTA) is a multidisciplinary process that summarises information about the medical, social, economic and ethical issues related
Page 1 of 8. Better informed health care through better clinical guidelines
 Introduction The Guidelines International Network (G-I-N) Australian and New Zealand Regional Community was established to provide a forum to support the collaboration of organisations and individuals
Introduction The Guidelines International Network (G-I-N) Australian and New Zealand Regional Community was established to provide a forum to support the collaboration of organisations and individuals
Cell Therapy Development Past, present and future
 Cell Therapy Development Past, present and future 20 th Annual ISCT Meeting Matthew Durdy, CBO April 2014 ct.catapult.org.uk Catapult is a Technology Strategy Board programme Cell Therapy Past: Pioneering
Cell Therapy Development Past, present and future 20 th Annual ISCT Meeting Matthew Durdy, CBO April 2014 ct.catapult.org.uk Catapult is a Technology Strategy Board programme Cell Therapy Past: Pioneering
Single Drug Technology Assessment Processes Across Health Technology Assessment Organizations
 CADTH ENVIRONMENTAL SCAN Single Drug Technology Assessment Processes Across Health Technology Assessment Organizations Product Line: Environmental Scan Version: 1.0 Issue Number: 55 Publication Date: November
CADTH ENVIRONMENTAL SCAN Single Drug Technology Assessment Processes Across Health Technology Assessment Organizations Product Line: Environmental Scan Version: 1.0 Issue Number: 55 Publication Date: November
Convergence and difference in HTA approaches in UK, Germany and France: reflections on recent and proposed changes
 Convergence and difference in HTA approaches in UK, Germany and France: reflections on recent and proposed changes Professor Ron Akehurst School of Health and Related Research Content of Talk During this
Convergence and difference in HTA approaches in UK, Germany and France: reflections on recent and proposed changes Professor Ron Akehurst School of Health and Related Research Content of Talk During this
EUnetHTA JA2 Guideline Choice of comparator(s) WP 7 GUIDELINE
 GUIDELINE COMPARATORS & COMPARISONS Criteria for the choice of the most appropriate comparator(s) Summary of current policies and best practice recommendations Adapted version (2015) based on Comparators
GUIDELINE COMPARATORS & COMPARISONS Criteria for the choice of the most appropriate comparator(s) Summary of current policies and best practice recommendations Adapted version (2015) based on Comparators
Summary Pediatric Device Stakeholders Meetings June 28, October 4 & 25, and November 1, 2004
 Summary Pediatric Device Stakeholders Meetings June 28, October 4 & 25, and November 1, 2004 In an ongoing effort to improve therapeutics and diagnostics for pediatric populations, the American Academy
Summary Pediatric Device Stakeholders Meetings June 28, October 4 & 25, and November 1, 2004 In an ongoing effort to improve therapeutics and diagnostics for pediatric populations, the American Academy
Future bound. Philips Ingenuity Core
 Future bound Philips Ingenuity Core High reliability Low-dose, high-quality imaging and coverage, and the ability to personalize image quality* patient by patient. Expect excellence in routine imaging,
Future bound Philips Ingenuity Core High reliability Low-dose, high-quality imaging and coverage, and the ability to personalize image quality* patient by patient. Expect excellence in routine imaging,
Submission of comments on COMMISSION NOTICE ON THE APPLICATION OF ARTICLES 3, 5 AND 7 OF REGULATION (EC) NO 141/2000 ON ORPHAN MEDICINAL PRODUCTS
 Ref. Ares(2016)807620-16/02/2016 15 February 2016 Submission of comments on COMMISSION NOTICE ON THE APPLICATION OF ARTICLES 3, 5 AND 7 OF REGULATION (EC) NO 141/2000 ON ORPHAN MEDICINAL PRODUCTS Response
Ref. Ares(2016)807620-16/02/2016 15 February 2016 Submission of comments on COMMISSION NOTICE ON THE APPLICATION OF ARTICLES 3, 5 AND 7 OF REGULATION (EC) NO 141/2000 ON ORPHAN MEDICINAL PRODUCTS Response
Medical Device Single Audit Program (MDSAP) Copyright 2014 BSI. All rights reserved.
 Medical Device Single Audit Program (MDSAP) International Medical Device Regulatory Forum (IMDRF) IMDRF Management Committee (MC) regulators: Australia, Brazil, Canada, China, the European Union, Japan
Medical Device Single Audit Program (MDSAP) International Medical Device Regulatory Forum (IMDRF) IMDRF Management Committee (MC) regulators: Australia, Brazil, Canada, China, the European Union, Japan
Whole-of-Government Approaches: Two Canadian Examples
 Whole-of-Government Approaches: Two Canadian Examples Denise Kouri François Gagnon IUHPE Vancouver, June 12, 2007 Presentation overview Who we are and why we think this subject is important What are whole-of-government
Whole-of-Government Approaches: Two Canadian Examples Denise Kouri François Gagnon IUHPE Vancouver, June 12, 2007 Presentation overview Who we are and why we think this subject is important What are whole-of-government
Venue. Ultrasound for the critical moment. Simple. Fast. Precise. gehealthcare.com
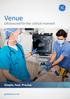 Venue Ultrasound for the critical moment Simple. Fast. Precise. gehealthcare.com In the critical moment, every second matters. Seamless flat display Make cleaning a breeze. Convenient button probe Control
Venue Ultrasound for the critical moment Simple. Fast. Precise. gehealthcare.com In the critical moment, every second matters. Seamless flat display Make cleaning a breeze. Convenient button probe Control
Philips: a focused leader in HealthTech
 Philips: a focused leader in HealthTech Frans van Houten, CEO Royal Philips 36th Annual J.P. Morgan Healthcare Conference, San Francisco, USA January 9, 2018 Important information Forward looking statements
Philips: a focused leader in HealthTech Frans van Houten, CEO Royal Philips 36th Annual J.P. Morgan Healthcare Conference, San Francisco, USA January 9, 2018 Important information Forward looking statements
Information Day Leeds, UK 27 October 2015 Treating and managing disease
 The societal challenge 'Health, demographic change and well-being' Work programme 2016-2017 Information Day Leeds, UK 27 October 2015 Treating and managing disease Image credit: Adam Fagen Rallying for
The societal challenge 'Health, demographic change and well-being' Work programme 2016-2017 Information Day Leeds, UK 27 October 2015 Treating and managing disease Image credit: Adam Fagen Rallying for
A l l t h a t y o u n e e d...
 S U R G I C A L T A B L E S A l l t h a t y o u n e e d... S u r g i c a l Ta b l e Advanced Technology E a s y m a x Clinical Versatility S T E R I S, A M A J O R H E A L T H C A R E P L A Y E R STERIS
S U R G I C A L T A B L E S A l l t h a t y o u n e e d... S u r g i c a l Ta b l e Advanced Technology E a s y m a x Clinical Versatility S T E R I S, A M A J O R H E A L T H C A R E P L A Y E R STERIS
Prequalification of in vitro diagnostics - Technical Update 24 November 2015
 Prequalification of in vitro diagnostics - Technical Update 24 November 2015 Copenhagen, Denmark 22-26 November 2015 1 Changes assessment Copenhagen, Denmark 22-26 November 2015 2 Changes to a Prequalified
Prequalification of in vitro diagnostics - Technical Update 24 November 2015 Copenhagen, Denmark 22-26 November 2015 1 Changes assessment Copenhagen, Denmark 22-26 November 2015 2 Changes to a Prequalified
Advanced solutions for the Healthcare sector
 TECHNICAL CERAMICS Morgan Advanced Materials For all inquiries, please contact our specialist sales and marketing offices: Morgan Advanced Materials is a global engineering company offering world-leading
TECHNICAL CERAMICS Morgan Advanced Materials For all inquiries, please contact our specialist sales and marketing offices: Morgan Advanced Materials is a global engineering company offering world-leading
Recommendation on elements required to support the medical plausibility and the assumption of significant benefit for an orphan designation
 2 March 2010 EMA/COMP/15893/2009 Final Committee for Orphan Medicinal Products (COMP) Recommendation on elements required to support the medical plausibility and the assumption of significant benefit for
2 March 2010 EMA/COMP/15893/2009 Final Committee for Orphan Medicinal Products (COMP) Recommendation on elements required to support the medical plausibility and the assumption of significant benefit for
Patient doses from CT examinations in region of Prishtina, Kosovo
 Patient doses from CT examinations in region of Prishtina, Kosovo Sehad KADIRI, Gëzim HODOLLI, Kostandin DOLLANI Institute of Occupational Medicine, Radiation Protection Service, Obiliq, Kosovo Institute
Patient doses from CT examinations in region of Prishtina, Kosovo Sehad KADIRI, Gëzim HODOLLI, Kostandin DOLLANI Institute of Occupational Medicine, Radiation Protection Service, Obiliq, Kosovo Institute
Statements on the Regulation of Laboratory Developed Tests
 Statements on the Regulation of Laboratory Developed Tests Current Regulatory Gaps and Perspectives on Oversight of LDTs American Cancer Society Cancer Action Network says Molecular tests, in particular,
Statements on the Regulation of Laboratory Developed Tests Current Regulatory Gaps and Perspectives on Oversight of LDTs American Cancer Society Cancer Action Network says Molecular tests, in particular,
Position Paper (I) Healthcare Provider Advisory Council Interoperability of Information Technology Systems
 I. i Introduction Position Paper (I) Towards the end of 2011, GS1 Healthcare established the Healthcare Provider Advisory Council (HPAC) to be the forum for sharing and discussing the practical realities
I. i Introduction Position Paper (I) Towards the end of 2011, GS1 Healthcare established the Healthcare Provider Advisory Council (HPAC) to be the forum for sharing and discussing the practical realities
CDEC FINAL RECOMMENDATION
 CDEC FINAL RECOMMENDATION APIXABAN (Eliquis Bristol-Myers Squibb Canada and Pfizer Canada) Indication: Venous Thromboembolic Events Recommendation: The Canadian Drug Expert Committee (CDEC) recommends
CDEC FINAL RECOMMENDATION APIXABAN (Eliquis Bristol-Myers Squibb Canada and Pfizer Canada) Indication: Venous Thromboembolic Events Recommendation: The Canadian Drug Expert Committee (CDEC) recommends
Occams Business Research & Consultancy
 Occams Business Research & Consultancy http://www.marketresearch.com/occams Business Research Consultancy v4040/ Publisher Sample Phone: 800.298.5699 (US) or +1.240.747.3093 or +1.240.747.3093 (Int'l)
Occams Business Research & Consultancy http://www.marketresearch.com/occams Business Research Consultancy v4040/ Publisher Sample Phone: 800.298.5699 (US) or +1.240.747.3093 or +1.240.747.3093 (Int'l)
HTA Principles Survey Questionnaire
 I. Introduction This survey aims to inform health care decision makers in their rational and efficacious applications of health technology assessment (HTA) in Asia, while adhering to key established benchmarks.
I. Introduction This survey aims to inform health care decision makers in their rational and efficacious applications of health technology assessment (HTA) in Asia, while adhering to key established benchmarks.
USE OF PHANTOMS AND IMAGE DATASETS FOR
 USE OF PHANTOMS AND IMAGE DATASETS FOR REGULATORY DECISION MAKING Nicholas Petrick. Ph.D. Division of Imaging, Diagnostics and Software Reliability (DIDSR) Office of Science and Engineering Labs Center
USE OF PHANTOMS AND IMAGE DATASETS FOR REGULATORY DECISION MAKING Nicholas Petrick. Ph.D. Division of Imaging, Diagnostics and Software Reliability (DIDSR) Office of Science and Engineering Labs Center
CQIE MRI PROCEDURES. American College of Radiology Clinical Research Center. Centers for Quantitative Imaging Excellence LEARNING MODULE
 Centers for Quantitative Imaging Excellence LEARNING MODULE CQIE MRI PROCEDURES American College of Radiology Clinical Research Center Imaging Core Laboratory v.2 Centers for Quantitative Imaging Excellence
Centers for Quantitative Imaging Excellence LEARNING MODULE CQIE MRI PROCEDURES American College of Radiology Clinical Research Center Imaging Core Laboratory v.2 Centers for Quantitative Imaging Excellence
CYTOTOXIC TRACEABILITY AND PATIENT WRISTBANDS
 CYTOTOXIC TRACEABILITY AND PATIENT WRISTBANDS Pr Pascal BONNABRY Head of pharmacy Global GS1 Healthcare Conference Dubai, April 18, 2016 Introduction A full traceability of the medication process is the
CYTOTOXIC TRACEABILITY AND PATIENT WRISTBANDS Pr Pascal BONNABRY Head of pharmacy Global GS1 Healthcare Conference Dubai, April 18, 2016 Introduction A full traceability of the medication process is the
pan-canadian Oncology Drug Review Final Economic Guidance Report Pomalidomide (Pomalyst) for Multiple Myeloma July 31, 2014
 pan-canadian Oncology Drug Review Final Economic Guidance Report Pomalidomide (Pomalyst) for Multiple Myeloma July 31, 2014 DISCLAIMER Not a Substitute for Professional Advice This report is primarily
pan-canadian Oncology Drug Review Final Economic Guidance Report Pomalidomide (Pomalyst) for Multiple Myeloma July 31, 2014 DISCLAIMER Not a Substitute for Professional Advice This report is primarily
Networked intermediate care
 Networked intermediate care IntelliVue MP40 and MP50 patient monitors Connected to more The IntelliVue family of networked patient monitors gives care teams throughout the hospital more of the information
Networked intermediate care IntelliVue MP40 and MP50 patient monitors Connected to more The IntelliVue family of networked patient monitors gives care teams throughout the hospital more of the information
Lessons learned from around the world
 Lessons learned from around the world Some opportunities for Australian Healthcare Terry Hoy AEW Matthews Memorial Travelling Scholarship 2017 March 20 to April 10 2017 1 About us 12 Health Services in
Lessons learned from around the world Some opportunities for Australian Healthcare Terry Hoy AEW Matthews Memorial Travelling Scholarship 2017 March 20 to April 10 2017 1 About us 12 Health Services in
SPECIFICATION CT SIMULATOR FOR BLACK LION HOSPITAL, ADDIS ABEBA, ETHIOPIA
 SPECIFICATION CT SIMULATOR FOR BLACK LION HOSPITAL, ADDIS ABEBA, ETHIOPIA 1. Scope This specification describes the requirements for the supply, delivery, installation, and acceptance testing of a CT Simulator
SPECIFICATION CT SIMULATOR FOR BLACK LION HOSPITAL, ADDIS ABEBA, ETHIOPIA 1. Scope This specification describes the requirements for the supply, delivery, installation, and acceptance testing of a CT Simulator
Canadian Cancer Clinical Trials Network Portfolio Eligibility Criteria and Guidelines
 Canadian Cancer Clinical Trials Network Portfolio Eligibility Criteria and Guidelines 1. Introduction The Canadian Cancer Clinical Trials Network (3CTN) will support a portfolio of academic clinical trials
Canadian Cancer Clinical Trials Network Portfolio Eligibility Criteria and Guidelines 1. Introduction The Canadian Cancer Clinical Trials Network (3CTN) will support a portfolio of academic clinical trials
Technologies scoping report
 In response to an enquiry from the Scottish Health Council, Healthcare Improvement Scotland Technologies scoping report Number 16 July 2013 What approaches have been taken and efforts made to ensure public
In response to an enquiry from the Scottish Health Council, Healthcare Improvement Scotland Technologies scoping report Number 16 July 2013 What approaches have been taken and efforts made to ensure public
Human-centred and human-powered: an infusion device to offer a real alternative
 Human-centred and human-powered: an infusion device to offer a real alternative Abstract Despite the many brands and models of drug infusion delivery devices available there are only a few different basic
Human-centred and human-powered: an infusion device to offer a real alternative Abstract Despite the many brands and models of drug infusion delivery devices available there are only a few different basic
GE Healthcare. OEC 9800 Plus. A Classic Choice
 GE Healthcare OEC 9800 Plus A Classic Choice g Proprietary OEC technology for superb, high quality images Powerful 15 kw generator Smart Metal Smart Window Auto Trak Auto Histo Image Quality The OEC brand
GE Healthcare OEC 9800 Plus A Classic Choice g Proprietary OEC technology for superb, high quality images Powerful 15 kw generator Smart Metal Smart Window Auto Trak Auto Histo Image Quality The OEC brand
GE Healthcare. OEC 9800 Plus. A Classic Choice
 GE Healthcare OEC 9800 Plus A Classic Choice g Classic is something of lasting worth with a timeless quality. High quality images from a dependable, flexible C-arm. That s what more than 11,000 OEC 9800
GE Healthcare OEC 9800 Plus A Classic Choice g Classic is something of lasting worth with a timeless quality. High quality images from a dependable, flexible C-arm. That s what more than 11,000 OEC 9800
CONNECT. Materials Management. The Foundational Role of Group Purchasing in Canadian Healthcare and how Suppliers Can Participate
 Materials Management MM CONNECT Fall/Winter 2017 Welcome to Materials Management CONNECT, HealthPRO s bi-annual newsletter for Materials Management The Foundational Role of Group Purchasing in Canadian
Materials Management MM CONNECT Fall/Winter 2017 Welcome to Materials Management CONNECT, HealthPRO s bi-annual newsletter for Materials Management The Foundational Role of Group Purchasing in Canadian
A Guide for Assessing Health Research Knowledge Translation (KT) Plans
 A Guide for Assessing Health Research Knowledge Translation (KT) Plans Developed as part of a research study being conducted by investigators from the Centre for Addiction and Mental Health and McMaster
A Guide for Assessing Health Research Knowledge Translation (KT) Plans Developed as part of a research study being conducted by investigators from the Centre for Addiction and Mental Health and McMaster
COCIR CT Manufacturers Voluntary Commitment Regarding CT Dose 2014 Annual Report
 Preamble COCIR CT Manufacturers Voluntary Commitment Regarding CT Dose 2014 Annual Report This 2 nd Annual Report defines the COCIR CT manufacturers voluntary commitment to HERCA as a result of the meeting
Preamble COCIR CT Manufacturers Voluntary Commitment Regarding CT Dose 2014 Annual Report This 2 nd Annual Report defines the COCIR CT manufacturers voluntary commitment to HERCA as a result of the meeting
Update on Real World Evidence Data Collection
 Update on Real World Evidence Data Collection STAMP, 10 March 2016 Presented by Dr Peter Arlett EMA An agency of the European Union What is Real-world evidence? Big data = umbrella term describing large
Update on Real World Evidence Data Collection STAMP, 10 March 2016 Presented by Dr Peter Arlett EMA An agency of the European Union What is Real-world evidence? Big data = umbrella term describing large
SKF linear motion solutions for medical equipment
 SKF linear motion solutions for medical equipment The Power of Knowledge Engineering The way to care In today s medical world, comfort and safety are key requirements. Hospital environments become more
SKF linear motion solutions for medical equipment The Power of Knowledge Engineering The way to care In today s medical world, comfort and safety are key requirements. Hospital environments become more
Discovery NM630 Innovation to expand your care.
 GE Healthcare Discovery NM630 Innovation to expand your care. Focus on what matters most. Your reason for being is the health of countless patients who come to you for care. This is why GE Healthcare strives
GE Healthcare Discovery NM630 Innovation to expand your care. Focus on what matters most. Your reason for being is the health of countless patients who come to you for care. This is why GE Healthcare strives
Public release of clinical information in drug submissions and medical device applications
 Public release of clinical information in drug submissions and medical device applications Health Products and Food Branch March 10, 2017 Health Canada is the federal department responsible for helping
Public release of clinical information in drug submissions and medical device applications Health Products and Food Branch March 10, 2017 Health Canada is the federal department responsible for helping
Current Market Dynamics and Future Vision of the Care Cycle
 Current Market Dynamics and Future Vision of the Care Cycle Tim Irish Analysts Meeting June 15 th, 2005 Overview Market and Customer trends The Care Cycle and Molecular Medicine 2 Healthcare is the world
Current Market Dynamics and Future Vision of the Care Cycle Tim Irish Analysts Meeting June 15 th, 2005 Overview Market and Customer trends The Care Cycle and Molecular Medicine 2 Healthcare is the world
HEALTH TECHNOLOGY ASSESSMENTS IN KOREA
 HEALTH TECHNOLOGY ASSESSMENTS IN KOREA July 24, 2012 HTA and Coverage Decisions Conference Taipei, Taiwan Jeonghoon Ahn, PhD Senior Director National Evidence-based healthcare Collaborating Agency (NECA)
HEALTH TECHNOLOGY ASSESSMENTS IN KOREA July 24, 2012 HTA and Coverage Decisions Conference Taipei, Taiwan Jeonghoon Ahn, PhD Senior Director National Evidence-based healthcare Collaborating Agency (NECA)
NICE methods of technology appraisal
 NICE methods of technology appraisal Zoe Garrett Senior Technical Adviser, Centre for Health Technology Evaluation National Institute for Health and Care Excellence (NICE) Contents Function of NICE Work
NICE methods of technology appraisal Zoe Garrett Senior Technical Adviser, Centre for Health Technology Evaluation National Institute for Health and Care Excellence (NICE) Contents Function of NICE Work
ABPI response to European Commission consultation on advanced therapy medicinal products
 ABPI response to European Commission consultation on advanced therapy medicinal products 28 March 2013 ABPI response to European Commission consultation on the regulation of advanced therapy medicinal
ABPI response to European Commission consultation on advanced therapy medicinal products 28 March 2013 ABPI response to European Commission consultation on the regulation of advanced therapy medicinal
Stakeholder Consultation Strategy
 Stakeholder Consultation Strategy Strengthening of the EU cooperation on Health Technology Assessment (HTA) 1. Context The present stakeholder consultation aims to provide broad and high quality information
Stakeholder Consultation Strategy Strengthening of the EU cooperation on Health Technology Assessment (HTA) 1. Context The present stakeholder consultation aims to provide broad and high quality information
Ensuring the Safety and Quality of Products and Services
 At the Terumo Group, we strive to enhance product quality and achieve continuous improvement in quality systems and processes to promise safety and reliability to medical settings. High product quality
At the Terumo Group, we strive to enhance product quality and achieve continuous improvement in quality systems and processes to promise safety and reliability to medical settings. High product quality
Filtration Media. Steve Nelson
 OC-1 Filtration Media Steve Nelson OC-1 The holy grail in the pool industry is the pre loaded filter. Everyone wants a filter which is as convenient as a sand filter - (back washable, effective, low maintenance)
OC-1 Filtration Media Steve Nelson OC-1 The holy grail in the pool industry is the pre loaded filter. Everyone wants a filter which is as convenient as a sand filter - (back washable, effective, low maintenance)
innovations worth funding
 europe MedInvest Scanner Starowislna St. 32/6 31-032 Krakow, Poland we identify innovations worth funding +48 123789579 +1 8455821595 + 44 2033320494 office@medinvestscanner.com www.medinvestscanner.com
europe MedInvest Scanner Starowislna St. 32/6 31-032 Krakow, Poland we identify innovations worth funding +48 123789579 +1 8455821595 + 44 2033320494 office@medinvestscanner.com www.medinvestscanner.com
Philips PET/CT Gemini GXL. Total performance. Total confidence.
 Philips PET/CT Gemini GXL Total performance. Total confidence. Gemini GXL The one for all. Healthcare isn t about to slow down. Your best bet? 2 Accelerate. All patients. All applications. All the time.
Philips PET/CT Gemini GXL Total performance. Total confidence. Gemini GXL The one for all. Healthcare isn t about to slow down. Your best bet? 2 Accelerate. All patients. All applications. All the time.
Ontario Drinking Water Quality Standards
 Ontario Drinking Water Quality Standards Ingersoll, Ontario November 26, 2012 Tim Fletcher Standards Development Branch Environmental Sciences and Standards Division Ministry of the Environment Introduction
Ontario Drinking Water Quality Standards Ingersoll, Ontario November 26, 2012 Tim Fletcher Standards Development Branch Environmental Sciences and Standards Division Ministry of the Environment Introduction
MEDICINES CONTROL COUNCIL
 MEDICINES CONTROL COUNCIL GUIDELINE FOR A LICENCE TO MANUFACTURE, IMPORT, EXPORT OR DISTRIBUTE MEDICAL DEVICES & IVDs This guideline is intended to provide recommendations to applicants wishing to submit
MEDICINES CONTROL COUNCIL GUIDELINE FOR A LICENCE TO MANUFACTURE, IMPORT, EXPORT OR DISTRIBUTE MEDICAL DEVICES & IVDs This guideline is intended to provide recommendations to applicants wishing to submit
Medical Devices; Immunology and Microbiology Devices; Classification of the Brain Trauma
 This document is scheduled to be published in the Federal Register on 06/14/2018 and available online at https://federalregister.gov/d/2018-12760, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 06/14/2018 and available online at https://federalregister.gov/d/2018-12760, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE. Health and Social Care Directorate. Indicator Process Guide. Published December 2017
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Health and Social Care Directorate Indicator Process Guide Published December 2017 Please note that this is an interim factual update to the NICE Indicator
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Health and Social Care Directorate Indicator Process Guide Published December 2017 Please note that this is an interim factual update to the NICE Indicator
DENOMINATOR: All final reports for patients, regardless of age, undergoing a CT procedure
 Quality ID #363: Optimizing Patient Exposure to Ionizing Radiation: Search for Prior Computed Tomography (CT) Studies Through a Secure, Authorized, Media-Free, Shared Archive National Quality Strategy
Quality ID #363: Optimizing Patient Exposure to Ionizing Radiation: Search for Prior Computed Tomography (CT) Studies Through a Secure, Authorized, Media-Free, Shared Archive National Quality Strategy
New Product Innovation Leadership
 Philips Healthcare A Frost & Sullivan Position Paper Nadim Michel Daher TABLE OF CONTENTS SIGNIFICANCE OF THE NEW PRODUCT INNOVATION LEADERSHIP AWARD... 3 KEY INDUSTRY CHALLENGES ADDRESSED BY PHILIPS HEALTHCARE...
Philips Healthcare A Frost & Sullivan Position Paper Nadim Michel Daher TABLE OF CONTENTS SIGNIFICANCE OF THE NEW PRODUCT INNOVATION LEADERSHIP AWARD... 3 KEY INDUSTRY CHALLENGES ADDRESSED BY PHILIPS HEALTHCARE...
Improving the process for HTA of Medical Devices
 Improving the process for HTA of Medical Devices Rod Taylor, PhD Oriana Ciani, PhD Final Conference 13 th November 2015 Universita Bocconi Project Funded under FP7 Funded - HEALTH under FP7 - HEALTH Grant
Improving the process for HTA of Medical Devices Rod Taylor, PhD Oriana Ciani, PhD Final Conference 13 th November 2015 Universita Bocconi Project Funded under FP7 Funded - HEALTH under FP7 - HEALTH Grant
Ethics of Radiation Protection in Medicine
 Ethics of Radiation Protection in Medicine Richard J. Vetter, Ph.D. CHP Professor Emeritus Mayo Clinic First IRPA North American Workshop on the Ethics of Radiological Protection Baltimore, Maryland, USA
Ethics of Radiation Protection in Medicine Richard J. Vetter, Ph.D. CHP Professor Emeritus Mayo Clinic First IRPA North American Workshop on the Ethics of Radiological Protection Baltimore, Maryland, USA
Leeds July 2017 Matthew Durdy Chief Business Officer
 Leeds July 2017 Matthew Durdy Chief Business Officer The Cell and Gene Therapy Catapult 70m Development Facility 1,200m 2 Custom designed cell and gene therapy development facility Prime location in the
Leeds July 2017 Matthew Durdy Chief Business Officer The Cell and Gene Therapy Catapult 70m Development Facility 1,200m 2 Custom designed cell and gene therapy development facility Prime location in the
Biosimilars Market Update
 Biosimilars Market Update Panel: Matthew Brougham Consultant Economist, Brougham Consulting Inc Mark Jackson Consultant Pharmacist, TELUS Health Dr. Ed Keystone Professor of Medicine, University of Toronto
Biosimilars Market Update Panel: Matthew Brougham Consultant Economist, Brougham Consulting Inc Mark Jackson Consultant Pharmacist, TELUS Health Dr. Ed Keystone Professor of Medicine, University of Toronto
R/F. Experiences Using SONIALVISION safire and the Utility of Tomosynthesis. 1. Introduction. 2. Basics of Tomosynthesis.
 R/F Experiences Using SONIALVISION safire and the Utility of Tomosynthesis Radiology Division, Dokkyo Medical University Koshigaya Hospital Masahiro Nakajima Mr. Masahiro Nakajima 1. Introduction The hospital
R/F Experiences Using SONIALVISION safire and the Utility of Tomosynthesis Radiology Division, Dokkyo Medical University Koshigaya Hospital Masahiro Nakajima Mr. Masahiro Nakajima 1. Introduction The hospital
H-20 Page 1 of 5. Institutional Review Board Policy Manual HUMANITARIAN USE DEVICE (HUD) REQUIREMENTS
 H-20 Page 1 of 5 Institutional Review Board Policy Manual HUMANITARIAN USE DEVICE (HUD) REQUIREMENTS PURPOSE: To describe the requirements for McLeod Health IRB review of Humanitarian Use Devices (HUD).
H-20 Page 1 of 5 Institutional Review Board Policy Manual HUMANITARIAN USE DEVICE (HUD) REQUIREMENTS PURPOSE: To describe the requirements for McLeod Health IRB review of Humanitarian Use Devices (HUD).
