Extrapolation of in vitro effects to in vivo embryotoxicity Basic requirements for prediction model development. Han van de Sandt, PhD
|
|
|
- Anthony Blankenship
- 6 years ago
- Views:
Transcription
1 Extrapolation of in vitro effects to in vivo embryotoxicity Basic requirements for prediction model development Han van de Sandt, PhD
2 TNO: Netherlands Organisation for Applied Scientific Research Established in 1932 (by Act of Parliament) Independent RTO Revenue Generating - Not for Profit 5500 employees Serving 5 Core Areas 2
3 Method validation Assessment of reliability and relevance of a test procedure for a specific purpose Regulatory decision or In-house screening The intended use of the data generated by the method determines the required characteristics 3
4 REACH information requirements Annexes VII to X Higher tonnage more data Annex XI Rules for adaptation of the standard testing regimes Testing does not appear scientifically necessary Use of existing data Weight of evidence Qualitative or Quantitative Structure Activity Relationships In vitro methods ( suitable ; sufficiently well developed ) Grouping of substances and read-across approach Testing is technically not possible Substance-tailored exposure-driven testing 4
5 Validated methods or suitable methods? International validation of in vitro assays aims at: Making predictions for all chemicals General regulatory acceptance of method Hartung et al., ATLA 32, (2004) 5
6 Validated methods or suitable methods? Suitable methods mentioned in Annex XI of REACH E.g. ECVAM criteria for entry of a test into the pre-validation process Mechanistic basis of the test method and endpoint(s) evaluated Purpose of the test method and proposed practical application Relevance of the test method (preliminary evidence on predictive capacity) Preliminary self-assessment of the protocol optimization level Information on the prediction model applied Discussions ongoing on requirements 6
7 Prediction Models Definition (Worth and Balls, 2004): Unambiguous algorithm for converting (in vitro) data into predictions of a pharmaco-toxicological endpoint in animals or humans Test system (scientific basis) Prediction model (predictive capacity) (partial) replacement assay 7
8 Prediction Models Sensitivity to variation Dependence on training set Availability and quality of in vivo data for establishing predictive value predicting what? (Dis)similarity of in vivo and in vitro read-outs use of surrogate markers defining mechanisms / processes Categories or exact quantification (e.g. NOAEL / LOAEL) Selection of the reference test / data 8
9 Improving prediction models Biology (mechanistic understanding) Data (statistical analysis) PM 9
10 In vivo prenatal/developmental study (OECD TG 414) Read-outs Interauterine death Altered growth Structural abnormalities Growth and development Sexual maturation Gamete production and release Post-natal development Fertilisation Classification & Labelling R61 - may cause harm to the unborn R63 - possible risk of harm to the unborn Parturition Foetal development Implantation Zygote transport Embryogenesis 10
11 Validation of in vitro assays for embryotoxicity Embryonic Stem cell Test (EST) Model: mouse ES differentiating to beating cardiomyocytes Exposure time: 10 days Endpoints: cytotoxicity and cell differentiation Whole Embryo Culture (WEC) Model: cultured rat embryos with 1-5 somites Exposure time: 2 days Endpoints: malformation and morphological score Micromass (MM) Model: limb bud cell cultures from rat embryo Exposure time: 7 days Endpoints: cytotoxicity and cell differentiation 11
12 Classification on the basis of in vitro assays ECVAM validation study Class 1: non-embryotoxic chemicals not developmentally toxic at maternally toxic exposure Class 2: weakly embryotoxic chemicals of intermediate activity (excluding receptor mechanisms) Class 3: strongly embryotoxic chemicals which are developmentally toxic in all species tested, including multiple developmental effects and with a high A/D ratio Variable data on in vivo effects; Classification of test chemicals on basis of expert judgment 12
13 Validation of in vitro assays for embryotoxicity General conclusions Good concordance in vitro in vivo classification (71-80%) 100% predictivity for strong embryotoxicants Lack of discrimination between none and weak embryotoxicity Limitations Number of chemicals in training and test set Availability of in vivo data Link to regulatory practice Kinetic aspects and metabolic (de)activation 13
14 Use of in vitro data in risk assessment What is the purpose? In house prioritizing / screening Classification & Labeling Regulatory risk assessment Full replacement: stand-alone test / test batteries Tiered approaches Integrated Testing Strategies Substance grouping and read-across 14
15 Full replacement: in vitro test batteries Reference test ( all chemicals) Sexual maturation Growth and development Gamete production and release Post-natal development Fertilisation Parturition Zygote transport Foetal development Implantation Embryogenesis 15
16 Full replacement: in vitro test batteries In vitro approach chem group A chem group B Sexual maturation Growth and development Gamete production and release Post-natal development Fertilisation Parturition Zygote transport Foetal development Implantation Embryogenesis 16
17 Tiered approaches (I) - Further testing of all toxic' substances - High sensitivity required for in vitro test In vitro test Non-toxic Toxic In vivo test Non-toxic Toxic 17
18 Tiered approaches (II) - Further testing of all non-toxic' substances - High specificity required for in vitro test In vitro test Toxic Non-toxic In vivo test Toxic Non-toxic 18
19 Integrated Testing Strategies (Q)SARs Exposure Scenarios Read Across Endpoint information? TESTING In vitro Existing information 19
20 Grouping of substances and read-across Similarities may be based on: a common functional group common precursor and/or breakdown products constant pattern in changing of potency of properties 20
21 In vitro read across: filling in vivo data gaps Classification No classification In vivo data point In vitro data point Functional or chemical descriptor 21
22 In vitro in vivo extrapolation: kinetic aspects Predicting systemic toxicity on the basis of in vitro tests Information on kinetics & metabolism of the compound is essential Local exposure CELL in vitro toxicity test ORGANISM in vitro toxicity test CELL ORGAN Systemic exposure 22
23 Dose (LOAEL) Estimation of corresponding dose PK-Model Systemic exposure levels at target tissue Estimation of relevant systemic exposure Pathological effect in target tissue In vitro toxicological effect (EC 50 ) 23
24 Classification of embryotoxicity on the basis of EST 2-ME 2-MAA 2-EE 2-EAA RA 5-FU MTX Toxic effect levels In vitro IC50 3T3 (mg/l) > In vitro IC50 ES- D3 (mg/l) In vitro ID50 (mg/l) Classification (strong, weak or nonembryotoxic) In vitro Non Weak Non Weak Strong Strong Strong In vivo Weak Weak Weak Weak Strong Strong Strong 5-fluorouracil (5-FU), 2-methoxyethanol (2-ME), 2-methoxyacetic acid (2-MAA), 2-ethoxyethanol (2-EE), 2-ethoxyacetic acid (2-EAA), Retinoic acid (RA), Methotrexate (MTX) 24
25 Toxic effect levels: comparison in vitro and in vivo data In vitro ID50 (mg/l) In vivo LOAEL 2-ME ppm 2-MAA EE ppm 2-EAA RA mg/kg 5-FU MTX mg/kg 0.1 mg/kg Direct translation in vitro to in vivo effect levels not possible Extrapolation steps necessary 25
26 In vitro/in silico prediction of effect level of 2-ME In vitro effect levels determined with EST (ID50) ID50 2-MAA = 1539 µm 1 Extrapolation rules ID50>ECplasma Extrapolation rule 1: ID50 = ECplasma Internal exposure (EC plasma) EC plasma 2-MAA = 1539 µm 2-ME 2-MAA Inhaled Exhaled air air Lung 2 In silico modeling PBPK Venous Blood Fat Richly perfused Poorly perf Liver Kegc Arterial Blood 2-MAA Kmaac Venous Blood Fat Richly perfused Poorly perfused Liver Arterial Blood Kex Predicted effect level, scaled from in vitro In vivo effect level 2-ME = 326 ppm 26
27 Comparison predicted and measured effect levels Route of exposure In vitro/in silico (predicted in vivo) In vivo (observed in vivo) Ratio pred/obs 2-ME Inhalation 326 ppm 50 ppm EE Inhalation 354 ppm 100 ppm 3.5 RA Oral 5.45 mg/kg 6 mg/kg FU Subcutaneous 0.05 mg/kg 10 mg/kg MTX Intravenous 0.13 mg/kg 0.1 mg/kg 1.3 Verwei et al., Toxicology Letters (2006) 165 (1),
28 In vitro in vivo extrapolation: further development and evaluation Extension of database Development of generic kinetic model for extrapolation from internal to external exposure levels Implementation of metabolic fraction in in vitro embryotoxicity tests 28
29 Conclusions Formal validation of new in vitro methods is preferred for maximal impact in regulatory risk assessment, but is costly and time consuming Proof of method suitability (limited application) will enhance introduction of innovations and reduce costs and animal use Use of in vitro data for prediction of embryotoxicity is feasible, provided that kinetics are taken into account. Proof-of-principle of in vitro / in silico approach has been shown, but needs to be further developed and evaluated 29
30 Acknowledgements TNO (NL) BfR/Zebet (D) RIVM (NL) Miriam Verwei, Cyrille Krul Dinant Kroese, Andreas Freidig Ine Waalkens-Berendsen, André Wolterbeek Mariska Tegelenbosch-Schouten Horst Spielmann, Andrea Seiler Aldert Piersma Dutch Ministry of Social Affairs and Employment EU 6 th Framework Programme ReProTect 30
Safety Evaluation Study Using Embryonic Stem (ES) Cells
 Safety Evaluation Study Using Embryonic Stem (ES) Cells Sumitomo Chemical Co., Ltd. Environmental Health Science Laboratory Nobuyuki HORIE Hashihiro HIGUCHI Satoshi KAWAMURA Koichi SAITO Noriyuki SUZUKI
Safety Evaluation Study Using Embryonic Stem (ES) Cells Sumitomo Chemical Co., Ltd. Environmental Health Science Laboratory Nobuyuki HORIE Hashihiro HIGUCHI Satoshi KAWAMURA Koichi SAITO Noriyuki SUZUKI
Safety Evaluation Study Using Embryonic Stem (ES) Cells
 Safety Evaluation Study Using Embryonic Stem (ES) Cells Nobuyuki HORIE Hashihiro HIGUCHI Satoshi KAWAMURA Koichi SAITO Noriyuki SUZUKI Embryonic stem (ES) cells are pluripotent stem cells that have the
Safety Evaluation Study Using Embryonic Stem (ES) Cells Nobuyuki HORIE Hashihiro HIGUCHI Satoshi KAWAMURA Koichi SAITO Noriyuki SUZUKI Embryonic stem (ES) cells are pluripotent stem cells that have the
International Pre-validation and Validation Studies
 Symposium 20th Anniversary of ZEBET at BfR and 50 Years of the 3Rs Principle October 26-27, 2009 Federal Institute for Risk Assessment (BfR), Berlin, International Pre-validation and Validation Studies
Symposium 20th Anniversary of ZEBET at BfR and 50 Years of the 3Rs Principle October 26-27, 2009 Federal Institute for Risk Assessment (BfR), Berlin, International Pre-validation and Validation Studies
Recommendations for registrants to improve data quality in registration dossiers for chemicals 1000 tpa Based on the project REACH Compliance
 TEXTE 65/2018 Recommendations for registrants to improve data quality in registration dossiers for chemicals 1000 tpa Based on the project REACH Compliance TEXTE 65/2018 Environmental Research of the
TEXTE 65/2018 Recommendations for registrants to improve data quality in registration dossiers for chemicals 1000 tpa Based on the project REACH Compliance TEXTE 65/2018 Environmental Research of the
Workshop: Applying Exposure Science to Increase the Utility of Non-Animal Data in Efficacy and Safety Testing, 16 Feb. 2017
 Workshop: Applying Exposure Science to Increase the Utility of Non-Animal Data in Efficacy and Safety Testing, 16 Feb. 2017 Presentation Outline Should we account for kinetics in vitro and in vivo for
Workshop: Applying Exposure Science to Increase the Utility of Non-Animal Data in Efficacy and Safety Testing, 16 Feb. 2017 Presentation Outline Should we account for kinetics in vitro and in vivo for
COMMISSION REGULATION (EU) / of
 EUROPEAN COMMISSION Brussels, 3.12.2018 C(2018) 7942 final COMMISSION REGULATION (EU) / of 3.12.2018 amending Regulation (EC) No 1907/2006 of the European Parliament and of the Council on the Registration,
EUROPEAN COMMISSION Brussels, 3.12.2018 C(2018) 7942 final COMMISSION REGULATION (EU) / of 3.12.2018 amending Regulation (EC) No 1907/2006 of the European Parliament and of the Council on the Registration,
JSAAE & JaCVAM Joint Workshop International Trends on 3Rs in Animal Experiments 14 February 2011, Tokyo
 JSAAE & JaCVAM Joint Workshop International Trends on 3Rs in Animal Experiments 14 February 2011, Tokyo The FP7 Project AXLR8 Accelerating the transition to a toxicity pathway-based paradigm for chemical
JSAAE & JaCVAM Joint Workshop International Trends on 3Rs in Animal Experiments 14 February 2011, Tokyo The FP7 Project AXLR8 Accelerating the transition to a toxicity pathway-based paradigm for chemical
STUDIES TO EVALUATE THE SAFETY OF RESIDUES OF VETERINARY DRUGS IN HUMAN FOOD: GENERAL APPROACH TO TESTING
 VICH GL33 (SAFETY: GENERAL APPROACH) October 2002 For implementation at Step 7 - Final STUDIES TO EVALUATE THE SAFETY OF RESIDUES OF VETERINARY DRUGS IN HUMAN FOOD: GENERAL APPROACH TO TESTING Recommended
VICH GL33 (SAFETY: GENERAL APPROACH) October 2002 For implementation at Step 7 - Final STUDIES TO EVALUATE THE SAFETY OF RESIDUES OF VETERINARY DRUGS IN HUMAN FOOD: GENERAL APPROACH TO TESTING Recommended
Chemical Safety Assessment Under REACH
 Chemical Safety Assessment Under REACH Dinant Kroese TNO knowledge for business CONTENTS 1. Registration requirements 2. Core tools under REACH 3. Chemical Safety Assessment (CSA) Human Health 4. Our experience
Chemical Safety Assessment Under REACH Dinant Kroese TNO knowledge for business CONTENTS 1. Registration requirements 2. Core tools under REACH 3. Chemical Safety Assessment (CSA) Human Health 4. Our experience
The Dose: Toxicokinetics for Human Health Risk Assessment
 The Dose: Toxicokinetics for Human Health Risk Assessment John C. Lipscomb, PhD, DABT, ATS U.S. Environmental Protection Agency Office of Research and Development National Center for Environmental Assessment
The Dose: Toxicokinetics for Human Health Risk Assessment John C. Lipscomb, PhD, DABT, ATS U.S. Environmental Protection Agency Office of Research and Development National Center for Environmental Assessment
Integration of non-testing tools: a weight of evidence approach. Dinant Kroese TNO Quality of Life Zeist, The Netherlands
 Integration of non-testing tools: a weight of evidence approach Dinant Kroese TNO Quality of Life Zeist, The Netherlands Generation of information under REACH Gathering all existing data Data sharing Animal
Integration of non-testing tools: a weight of evidence approach Dinant Kroese TNO Quality of Life Zeist, The Netherlands Generation of information under REACH Gathering all existing data Data sharing Animal
The Long Range Science Strategy (LRSS) of Cosmetics Europe
 The Long Range Science Strategy (LRSS) of Cosmetics Europe Repeated dose systemic toxicity 2016 Bas Blaauboer Aim of LRSS: Development of a strategy to determine safety of cosmetic ingredients and products
The Long Range Science Strategy (LRSS) of Cosmetics Europe Repeated dose systemic toxicity 2016 Bas Blaauboer Aim of LRSS: Development of a strategy to determine safety of cosmetic ingredients and products
Cosmetics Europe LRSS Programme
 Cosmetics Europe LRSS Programme 2016-20 Rob Taalman Objectives of CE Research To deliver tools & strategies for animal-free safety assessment Accurate Robust Efficient ultimately accepted by regulators
Cosmetics Europe LRSS Programme 2016-20 Rob Taalman Objectives of CE Research To deliver tools & strategies for animal-free safety assessment Accurate Robust Efficient ultimately accepted by regulators
Exposure informed testing under REACH
 Exposure informed testing under REACH SETAC Europe Special Science Symposium REACH, October 23-24 2008 Theo Vermeire (RIVM), Marja van de Bovenkamp (RIVM), Hans Marquart (TNO) 1 2 Outline Background Exposure
Exposure informed testing under REACH SETAC Europe Special Science Symposium REACH, October 23-24 2008 Theo Vermeire (RIVM), Marja van de Bovenkamp (RIVM), Hans Marquart (TNO) 1 2 Outline Background Exposure
Tox21: Opportunities& Challenges. Richard A. Becker Ph.D., DABT American Chemistry Council
 Tox21: Opportunities& Challenges Richard A. Becker Ph.D., DABT American Chemistry Council October 21, 2010 Opportunities and Challenges The manifest challenge is to develop in vitro and in silico approaches
Tox21: Opportunities& Challenges Richard A. Becker Ph.D., DABT American Chemistry Council October 21, 2010 Opportunities and Challenges The manifest challenge is to develop in vitro and in silico approaches
How to bring your registration dossier in compliance with REACH Tips and Hints - Part 5
 How to bring your registration dossier in compliance with REACH Tips and Hints - Part 5 Read-across Niklas Andersson 12 February Read-across - Definition Read-across is a technique for predicting endpoint
How to bring your registration dossier in compliance with REACH Tips and Hints - Part 5 Read-across Niklas Andersson 12 February Read-across - Definition Read-across is a technique for predicting endpoint
Progress and Future Directions in Integrated Systems Toxicology. Mary McBride Agilent Technologies
 Progress and Future Directions in Integrated Systems Toxicology Mary McBride Agilent Technologies 1 Toxicity testing tools of the late 20 th century Patchwork approach to testing dates back to the 1930
Progress and Future Directions in Integrated Systems Toxicology Mary McBride Agilent Technologies 1 Toxicity testing tools of the late 20 th century Patchwork approach to testing dates back to the 1930
TOXICITY STUDIES. Mr.D.Raju M.Pharm., Lecturer
 TOXICITY STUDIES Mr.D.Raju M.Pharm., Lecturer INTRODUCTION A drug is a single substance or mixture of substances used for diagnosis, treatment, mitigation or prevention of disease ; restoring, correcting
TOXICITY STUDIES Mr.D.Raju M.Pharm., Lecturer INTRODUCTION A drug is a single substance or mixture of substances used for diagnosis, treatment, mitigation or prevention of disease ; restoring, correcting
C.3.9. Repeated Dose Dermal Toxicity: 21/28-Day Study (OECD TG 410)
 C.3.9. REPEATED DOSE DERMAL TOXICITY: 21-28-DAY STUDY (OECD TG 410) 557 C.3.9. Repeated Dose Dermal Toxicity: 21/28-Day Study (OECD TG 410) Status: Assay validated by the OECD. 841. Modalities detected:
C.3.9. REPEATED DOSE DERMAL TOXICITY: 21-28-DAY STUDY (OECD TG 410) 557 C.3.9. Repeated Dose Dermal Toxicity: 21/28-Day Study (OECD TG 410) Status: Assay validated by the OECD. 841. Modalities detected:
A Battery of In Vitro Assays for Prenatal Developmental Toxicity Testing of Petroleum Substances
 A Battery of In Vitro Assays for Prenatal Developmental Toxicity Testing of Petroleum Substances LENNY KAMELIA (lenny.kamelia@wur.nl) 12 th Concawe Symposium March 21 st, 2017 Antwerp, Belgium Petroleum
A Battery of In Vitro Assays for Prenatal Developmental Toxicity Testing of Petroleum Substances LENNY KAMELIA (lenny.kamelia@wur.nl) 12 th Concawe Symposium March 21 st, 2017 Antwerp, Belgium Petroleum
S2(R1) Revision of the Guidance on Genotoxicity Testing and Data Interpretation for Pharmaceuticals Intended for Human Use
 S2(R1) Revision of the Guidance on Genotoxicity Testing and Data Interpretation for Pharmaceuticals Intended for Human Use Peter Kasper BfArM and S2(R1)EWP International Conference on Harmonisation of
S2(R1) Revision of the Guidance on Genotoxicity Testing and Data Interpretation for Pharmaceuticals Intended for Human Use Peter Kasper BfArM and S2(R1)EWP International Conference on Harmonisation of
2017 PROGRAM Hands-On Training
 - FORMAT - - REGISTRATION FEE - 2 days training From Thursday morning to Friday evening 590 euros* without VAT Lectures Thursday morning Laboratory practices Rest of the time Training certificate can be
- FORMAT - - REGISTRATION FEE - 2 days training From Thursday morning to Friday evening 590 euros* without VAT Lectures Thursday morning Laboratory practices Rest of the time Training certificate can be
BUNDESINSTITUT FÜR RISIKOBEWERTUNG
 BUNDESINSTITUT FÜR RISIKOBEWERTUNG Mehrfachrückstände von Pflanzenschutzmitteln in Lebensmitteln Teil III Internationale Bewertungskonzepte für Mehrfachrückstände 10.11.2005 Cumulative Risk Assessment:
BUNDESINSTITUT FÜR RISIKOBEWERTUNG Mehrfachrückstände von Pflanzenschutzmitteln in Lebensmitteln Teil III Internationale Bewertungskonzepte für Mehrfachrückstände 10.11.2005 Cumulative Risk Assessment:
Acceptance of New Technology. Richard Phillips, ExxonMobil Petroleum & Chemical CEFIC LRI 11th Annual Workshop, 19 November 2009
 Acceptance of New Technology Richard Phillips, ExxonMobil Petroleum & Chemical CEFIC LRI 11th Annual Workshop, 19 November 2009 Overview Acceptance of New Technology Within LRI Nanotechnology New Approaches
Acceptance of New Technology Richard Phillips, ExxonMobil Petroleum & Chemical CEFIC LRI 11th Annual Workshop, 19 November 2009 Overview Acceptance of New Technology Within LRI Nanotechnology New Approaches
What can be done from regulatory side?
 Federal Agency for Medicines and Health Products (FAMHP) What can be done from regulatory side? 9th Annual ecopa Workshop November 29-30, 2008, Brussels Dr. Sonja BEKEN Non-Clinical Assessor, Registration
Federal Agency for Medicines and Health Products (FAMHP) What can be done from regulatory side? 9th Annual ecopa Workshop November 29-30, 2008, Brussels Dr. Sonja BEKEN Non-Clinical Assessor, Registration
Detection of toxicity to reproduction for human pharmaceuticals. Explanatory slides agreed by EWG members
 Detection of toxicity to reproduction for human pharmaceuticals Explanatory slides agreed by EWG members 2 October 2017 International Council for Harmonisation of Technical Requirements for Pharmaceuticals
Detection of toxicity to reproduction for human pharmaceuticals Explanatory slides agreed by EWG members 2 October 2017 International Council for Harmonisation of Technical Requirements for Pharmaceuticals
Current challenges from Evaluation point of view - Introduction case studies
 Current challenges from Evaluation point of view - Introduction case studies Expert Workshop Dealing with Uncertainty of Non-Test Methods under REACH 23-24 September 2010 Wim De Coen ECHA Evaluation I
Current challenges from Evaluation point of view - Introduction case studies Expert Workshop Dealing with Uncertainty of Non-Test Methods under REACH 23-24 September 2010 Wim De Coen ECHA Evaluation I
NON-ANIMAL APPROACHES TO SAFETY ASSESSMENT OF COSMETIC PRODUCTS. Cutting-Edge Science and Constant Innovation: The Keys to Success
 NON-ANIMAL APPROACHES TO SAFETY ASSESSMENT OF COSMETIC PRODUCTS Cutting-Edge Science and Constant Innovation: The Keys to Success 2 NON-ANIMAL APPROACHES TO SAFETY ASSESSMENT OF COSMETIC PRODUCTS Cutting-Edge
NON-ANIMAL APPROACHES TO SAFETY ASSESSMENT OF COSMETIC PRODUCTS Cutting-Edge Science and Constant Innovation: The Keys to Success 2 NON-ANIMAL APPROACHES TO SAFETY ASSESSMENT OF COSMETIC PRODUCTS Cutting-Edge
Current State of the Science in Chemical Risk Assessment
 Current State of the Science in Chemical Risk Assessment M.E. (Bette) Meek McLaughlin Centre University of Ottawa, Ottawa, Canada Email:bmeek@uottawa.ca Outline The Evolution of Regulatory Mandates & Implications
Current State of the Science in Chemical Risk Assessment M.E. (Bette) Meek McLaughlin Centre University of Ottawa, Ottawa, Canada Email:bmeek@uottawa.ca Outline The Evolution of Regulatory Mandates & Implications
THE FUTURE OF IN VITRO SCREENING IN THE DEVELOPMENT OF NEW DRUGS. Hajime Kojima, JaCVAM, NIHS, Japan
 THE FUTURE OF IN VITRO SCREENING IN THE DEVELOPMENT OF NEW DRUGS Hajime Kojima, JaCVAM, NIHS, Japan 1 3Rs of animal use (Russel and Burch 1959) Reduction (of animal use) Refinement (to lessen pain or distress
THE FUTURE OF IN VITRO SCREENING IN THE DEVELOPMENT OF NEW DRUGS Hajime Kojima, JaCVAM, NIHS, Japan 1 3Rs of animal use (Russel and Burch 1959) Reduction (of animal use) Refinement (to lessen pain or distress
Challenges Faced in Interpreting Biomonitoring Data in a Risk Context
 Challenges Faced in Interpreting Biomonitoring Data in a Risk Context Sean M. Hays Summit Toxicology Understanding Human Biomonitoring A Workshop Presented by ISRTP Sacramento, CA June 16, 2005 The Critical
Challenges Faced in Interpreting Biomonitoring Data in a Risk Context Sean M. Hays Summit Toxicology Understanding Human Biomonitoring A Workshop Presented by ISRTP Sacramento, CA June 16, 2005 The Critical
Needs and Future Perspectives of Modelling Skin Permeability and Metabolism
 Needs and Future Perspectives of Modelling Skin Permeability and Metabolism Mark Cronin School of Pharmacy and Chemistry Liverpool John Moores University England E-mail: m.t.cronin@ljmu.ac.uk Reasons for
Needs and Future Perspectives of Modelling Skin Permeability and Metabolism Mark Cronin School of Pharmacy and Chemistry Liverpool John Moores University England E-mail: m.t.cronin@ljmu.ac.uk Reasons for
THE SYSTEMATIC REVIEW OF MECHANISTIC DATA IN IRIS ASSESSMENTS
 THE SYSTEMATIC REVIEW OF MECHANISTIC DATA IN IRIS ASSESSMENTS Catherine Gibbons, Ph.D. U.S. EPA The views expressed in this presentation are those of the author and do not necessarily reflect the views
THE SYSTEMATIC REVIEW OF MECHANISTIC DATA IN IRIS ASSESSMENTS Catherine Gibbons, Ph.D. U.S. EPA The views expressed in this presentation are those of the author and do not necessarily reflect the views
ADVERSE OUTCOME PATHWAYS: WHERE DO YOU START?
 ADVERSE OUTCOME PATHWAYS: WHERE DO YOU START? Terry Schultz Presented at: Adverse Outcome Pathways 101: The How and Why of Development and Use. Phoenix, AZ 25 March 2014 THE AIM OF MY PRESENTATION is to
ADVERSE OUTCOME PATHWAYS: WHERE DO YOU START? Terry Schultz Presented at: Adverse Outcome Pathways 101: The How and Why of Development and Use. Phoenix, AZ 25 March 2014 THE AIM OF MY PRESENTATION is to
Update of the WHO/IPCS Mode of Action Framework
 Update of the WHO/IPCS Mode of Action Framework ECETOC/WHO/IPCS Mode of Action Workshop Vienna, February 21 st 22 nd, 2013 Presented by: M.E. (Bette) Meek University of Ottawa bmeek@uottawa.ca Outline
Update of the WHO/IPCS Mode of Action Framework ECETOC/WHO/IPCS Mode of Action Workshop Vienna, February 21 st 22 nd, 2013 Presented by: M.E. (Bette) Meek University of Ottawa bmeek@uottawa.ca Outline
ILSI AGRICULTURAL CHEMICAL SAFETY ASSESSMENT (ACSA) PROJECT: A TIERED TESTING APPROACH. November 18, 2005
 ILSI AGRICULTURAL CHEMICAL SAFETY ASSESSMENT (ACSA) PROJECT: A TIERED TESTING APPROACH Ann Blacker, PhD, DABT Head of Regulatory Toxicology Bayer CropScience November 18, 2005 HESI ACSA Technical Committee
ILSI AGRICULTURAL CHEMICAL SAFETY ASSESSMENT (ACSA) PROJECT: A TIERED TESTING APPROACH Ann Blacker, PhD, DABT Head of Regulatory Toxicology Bayer CropScience November 18, 2005 HESI ACSA Technical Committee
Chagas Disease Drug Discovery Entering a New Era. Eric Chatelain, PhD Head of Drug Discovery
 Chagas Disease Drug Discovery Entering a New Era Eric Chatelain, PhD Head of Drug Discovery ICOPA Meeting, Mexico, 12 th August 2014 Chagas Disease Effective immune responses provide control of the infection
Chagas Disease Drug Discovery Entering a New Era Eric Chatelain, PhD Head of Drug Discovery ICOPA Meeting, Mexico, 12 th August 2014 Chagas Disease Effective immune responses provide control of the infection
Workshop 5.15 In vitro metabolism: Applications in pharmacology and toxicology. Metabolic Activation for In Vitro Systems
 Workshop 5.15 In vitro metabolism: Applications in pharmacology and toxicology Metabolic Activation for In Vitro Systems Angelika Langsch and Heinz Nau Department of Food Toxicology, University of Veterinary
Workshop 5.15 In vitro metabolism: Applications in pharmacology and toxicology Metabolic Activation for In Vitro Systems Angelika Langsch and Heinz Nau Department of Food Toxicology, University of Veterinary
Risk Management under the Chemicals Management Plan
 Risk Management under the Chemicals Management Plan Hazard Characterization and Tools for Health Risk Assessment under CMP Stakeholder Advisory Council meeting Health Canada PAHO Workshop Lima, Peru November
Risk Management under the Chemicals Management Plan Hazard Characterization and Tools for Health Risk Assessment under CMP Stakeholder Advisory Council meeting Health Canada PAHO Workshop Lima, Peru November
Alternative Testverfahren und intelligente Teststrategien Position der EU-Kommission. Thomas Hartung & ECVAM Team.
 Alternative Testverfahren und intelligente Teststrategien Position der EU-Kommission Thomas Hartung & ECVAM Team Institute for Health and Consumer Protection (IHCP) Ispra (Va), Italy http://ecvam.jrc.it
Alternative Testverfahren und intelligente Teststrategien Position der EU-Kommission Thomas Hartung & ECVAM Team Institute for Health and Consumer Protection (IHCP) Ispra (Va), Italy http://ecvam.jrc.it
Human Vss Prediction. James Yates AstraZeneca. Disclaimer 10/25/2011
 0/25/20 Human Vss Prediction Part 2: Comparative Assessment of Prediction Methods of Human Volume of Distribution R.D.O JONES,H.M. JONES, M. ROWLAND, C.R. GIBSON, J.W.T. YATES, J.Y. CHIEN, B. J. RING,
0/25/20 Human Vss Prediction Part 2: Comparative Assessment of Prediction Methods of Human Volume of Distribution R.D.O JONES,H.M. JONES, M. ROWLAND, C.R. GIBSON, J.W.T. YATES, J.Y. CHIEN, B. J. RING,
Fig. 4. A two-compartment pharmacokinetic model.
 3.4 Pharmacokinetic (PK) modeling and simulations Noora Sjöstedt, Wilma Kiander, Heidi Kidron 3.4.1 Introduction (Hanna Kortejärvi) Pharmacokinetic modeling and simulations can be performed with different
3.4 Pharmacokinetic (PK) modeling and simulations Noora Sjöstedt, Wilma Kiander, Heidi Kidron 3.4.1 Introduction (Hanna Kortejärvi) Pharmacokinetic modeling and simulations can be performed with different
EFSA Guidance on the Submission of a. Evaluation: Toxicological data
 Committed since 2002 to ensuring that Europe s food is safe EFSA Guidance on the Submission of a Dossier on Food Enzymes for Safety Evaluation: Toxicological data Prof. Karl-Heinz Engel CEF Panel member
Committed since 2002 to ensuring that Europe s food is safe EFSA Guidance on the Submission of a Dossier on Food Enzymes for Safety Evaluation: Toxicological data Prof. Karl-Heinz Engel CEF Panel member
An NGO Perspective on Regulatory Acceptance of Non-animal Data and Related Issues. Martin Stephens, Ph.D. The Humane Society of the United States
 An NGO Perspective on Regulatory Acceptance of Non-animal Data and Related Issues Martin Stephens, Ph.D. The Humane Society of the United States Animal Use Numbers Rabbits 222,167 Guinea pigs 203,098 Hamsters
An NGO Perspective on Regulatory Acceptance of Non-animal Data and Related Issues Martin Stephens, Ph.D. The Humane Society of the United States Animal Use Numbers Rabbits 222,167 Guinea pigs 203,098 Hamsters
Three recently approved in vivo genotoxicity test guidelines
 Three recently approved in vivo genotoxicity test guidelines (Revised in February 2018) a. Title of the test guideline (year of original adoption, year of update) In Vitro Mammalian Cell Gene Mutation
Three recently approved in vivo genotoxicity test guidelines (Revised in February 2018) a. Title of the test guideline (year of original adoption, year of update) In Vitro Mammalian Cell Gene Mutation
Moving Towards Version 2.0 of Toxicity Testing in the 21 st Century and Application to Regulatory Decision Making
 Moving Towards Version 2.0 of Toxicity Testing in the 21 st Century and Application to Regulatory Decision Making ECHA Topical Scientific Workshop on New Approach Methodologies April 20, 2016 Rusty Thomas
Moving Towards Version 2.0 of Toxicity Testing in the 21 st Century and Application to Regulatory Decision Making ECHA Topical Scientific Workshop on New Approach Methodologies April 20, 2016 Rusty Thomas
IN VITRO TOXICITY TESTING: TECHNOLOGIES AND GLOBAL MARKETS
 IN VITRO TOXICITY TESTING: TECHNOLOGIES AND GLOBAL MARKETS PHM017E January 2014 Robert Hunter Project Analyst ISBN: 1-56965-661-4 BCC Research 49 Walnut Park, Building 2 Wellesley, MA 02481 USA 866-285-7215
IN VITRO TOXICITY TESTING: TECHNOLOGIES AND GLOBAL MARKETS PHM017E January 2014 Robert Hunter Project Analyst ISBN: 1-56965-661-4 BCC Research 49 Walnut Park, Building 2 Wellesley, MA 02481 USA 866-285-7215
Generation and Preliminary Assessment of a Zebrafish Teratogenicity Assay. An Overview of How BMS Generated the Assay
 Generation and Preliminary Assessment of a Zebrafish Teratogenicity Assay An Overview of How BMS Generated the Assay Karen Augustine, Ph.D. Discovery Toxicology Bristol-Myers Squibb Zebrafish as a Developmental
Generation and Preliminary Assessment of a Zebrafish Teratogenicity Assay An Overview of How BMS Generated the Assay Karen Augustine, Ph.D. Discovery Toxicology Bristol-Myers Squibb Zebrafish as a Developmental
Technical Notes for Guidance. on the assessment of technical equivalence of substances regulated under Directive 98/8/EC
 Technical Notes for Guidance on the assessment of technical equivalence of substances regulated under Directive 98/8/EC These Technical Notes for Guidance were adopted during the 29 th meeting of representatives
Technical Notes for Guidance on the assessment of technical equivalence of substances regulated under Directive 98/8/EC These Technical Notes for Guidance were adopted during the 29 th meeting of representatives
MATERIAL SAFETY DATA SHEET
 Page 1 of 7 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 7 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
David Owen Shell Chemicals Ltd ISRTP Workshop, Baltimore November 2005
 REACH: alternatives and tiered strategies David Owen Shell Chemicals Ltd ISRTP Workshop, Baltimore November 2005 Contents Background and scope Components of REACH Information requirements Implementation
REACH: alternatives and tiered strategies David Owen Shell Chemicals Ltd ISRTP Workshop, Baltimore November 2005 Contents Background and scope Components of REACH Information requirements Implementation
New Approaches to Chemical Risk Assessment
 New Approaches to Chemical Risk Assessment Wednesday, 11 May, 13.30-15.00 IAFP s European Symposium on Food Safety 2016, Athens, Greece New Approaches in Chemical Risk Assessment Dr Ans Punt RIKILT Wageningen
New Approaches to Chemical Risk Assessment Wednesday, 11 May, 13.30-15.00 IAFP s European Symposium on Food Safety 2016, Athens, Greece New Approaches in Chemical Risk Assessment Dr Ans Punt RIKILT Wageningen
Guidance for Setting Occupational Exposure Limits: Emphasis on Data-Poor Substances
 Guidance for Setting Occupational Exposure Limits: Emphasis on Data-Poor Substances ISSN-0773-8072-101 Brussels, September 2006 Copyright ECETOC AISBL European Centre for Ecotoxicology and Toxicology of
Guidance for Setting Occupational Exposure Limits: Emphasis on Data-Poor Substances ISSN-0773-8072-101 Brussels, September 2006 Copyright ECETOC AISBL European Centre for Ecotoxicology and Toxicology of
Maximizing opportunities towards achieving clinical success D R U G D I S C O V E R Y. Report Price Publication date
 F o r a c l e a r e r m a r k e t p e r s p e c t i v e Early Stage Drug Safety Strategies & Risk Management Maximizing opportunities towards achieving clinical success D R U G D I S C O V E R Y Report
F o r a c l e a r e r m a r k e t p e r s p e c t i v e Early Stage Drug Safety Strategies & Risk Management Maximizing opportunities towards achieving clinical success D R U G D I S C O V E R Y Report
Comparative Study of Regulatory Requirements for Biologics Filing in United States and European Union
 Comparative Study of Regulatory Requirements for Biologics Filing in United States and European Union Mr. Shashi Kumar Yadav Assistant Professor Sri Indu Institute of Pharmacy Hyderabad Outline Introduction
Comparative Study of Regulatory Requirements for Biologics Filing in United States and European Union Mr. Shashi Kumar Yadav Assistant Professor Sri Indu Institute of Pharmacy Hyderabad Outline Introduction
A Strategy for Reducing Animal Use in the U.S. EPA's Endocrine Disruption Screening Program
 A Strategy for Reducing Animal Use in the U.S. EPA's Endocrine Disruption Screening Program Catherine Willett, PhD Science Policy Advisor Patricia Bishop, M.S. Research Associate Kristie Sullivan, MPH
A Strategy for Reducing Animal Use in the U.S. EPA's Endocrine Disruption Screening Program Catherine Willett, PhD Science Policy Advisor Patricia Bishop, M.S. Research Associate Kristie Sullivan, MPH
SEURAT-1: Why predictive safety science is important to regulatory acceptance of alternative methods
 1 SEURAT-1: Why predictive safety science is important to regulatory acceptance of alternative methods Ian A Cotgreave Swedish Toxicology Sciences Research Center On behalf of all SEURAT-1 partners 10
1 SEURAT-1: Why predictive safety science is important to regulatory acceptance of alternative methods Ian A Cotgreave Swedish Toxicology Sciences Research Center On behalf of all SEURAT-1 partners 10
Human embryonic stem cells for in-vitro developmental toxicity testing
 Human embryonic stem cells for in-vitro developmental toxicity testing HESI workshop on alternatives assays for developmental toxicity Raimund Strehl Cellartis The company Founded in early 2001, University
Human embryonic stem cells for in-vitro developmental toxicity testing HESI workshop on alternatives assays for developmental toxicity Raimund Strehl Cellartis The company Founded in early 2001, University
Chapter 21. Toxicity Testing
 Chapter 21 Toxicity Testing Toxicity Testing There are two purposes of toxicity testing. There is a quantitative effort to elucidate a dose effect relationship There is a qualitative determination of the
Chapter 21 Toxicity Testing Toxicity Testing There are two purposes of toxicity testing. There is a quantitative effort to elucidate a dose effect relationship There is a qualitative determination of the
Cost Reduction in REACH Alternatives to Testing ChemicalWatch EXPO Berlin, April 2017 Peter Jenkinson CEHTRA
 Consultancy for Environmental and Human Toxicology and Risk Assessment Science Beyond Regulatory Compliance Cost Reduction in REACH Alternatives to Testing ChemicalWatch EXPO Berlin, April 2017 Peter Jenkinson
Consultancy for Environmental and Human Toxicology and Risk Assessment Science Beyond Regulatory Compliance Cost Reduction in REACH Alternatives to Testing ChemicalWatch EXPO Berlin, April 2017 Peter Jenkinson
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP)
 European Medicines Agency Evaluation of Medicines for Human Use London, 22 February 2006 EMEA/CHMP/BMWP/32775/2005 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) ANNEX TO GUIDELINE ON SIMILAR BIOLOGICAL
European Medicines Agency Evaluation of Medicines for Human Use London, 22 February 2006 EMEA/CHMP/BMWP/32775/2005 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) ANNEX TO GUIDELINE ON SIMILAR BIOLOGICAL
COMMISSION STAFF WORKING DOCUMENT IMPACT ASSESSMENT
 EUROPEAN COMMISSION Brussels, 15.6.2016 SWD(2016) 211 final PART 4/16 COMMISSION STAFF WORKING DOCUMENT IMPACT ASSESSMENT Defining criteria for identifying endocrine disruptors in the context of the implementation
EUROPEAN COMMISSION Brussels, 15.6.2016 SWD(2016) 211 final PART 4/16 COMMISSION STAFF WORKING DOCUMENT IMPACT ASSESSMENT Defining criteria for identifying endocrine disruptors in the context of the implementation
OPINION "REPORT FOR ESTABLISHING THE TIMETABLE FOR PHASING OUT ANIMAL TESTING FOR THE PURPOSE OF THE COSMETICS DIRECTIVE"
 THE SCIENTIFIC COMMITTEE ON COSMETIC PRODUCTS AND NON-FOOD PRODUCTS INTENDED FOR CONSUMERS OPINION CONCERNING "REPORT FOR ESTABLISHING THE TIMETABLE FOR PHASING OUT ANIMAL TESTING FOR THE PURPOSE OF THE
THE SCIENTIFIC COMMITTEE ON COSMETIC PRODUCTS AND NON-FOOD PRODUCTS INTENDED FOR CONSUMERS OPINION CONCERNING "REPORT FOR ESTABLISHING THE TIMETABLE FOR PHASING OUT ANIMAL TESTING FOR THE PURPOSE OF THE
MATERIAL SAFETY DATA SHEET
 Page 1 of 7 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 7 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Guidance on information requirements and chemical safety assessment. Chapter R.5: Adaptation of information requirements
 Guidance on information requirements and chemical safety assessment Chapter R.5: Adaptation of information requirements Version: 2.1 December 2011 LEGAL NOTICE This document contains guidance on REACH
Guidance on information requirements and chemical safety assessment Chapter R.5: Adaptation of information requirements Version: 2.1 December 2011 LEGAL NOTICE This document contains guidance on REACH
Version January 2007
 Version January 2007 COMMISSION GUIDELINE ON THE FORMAT AND CONTENT OF APPLICATIONS FOR AGREEMENT OR MODIFICATION OF A PAEDIATRIC INVESTIGATION PLAN AND REQUESTS FOR WAIVERS OR DEFERRALS AND CONCERNING
Version January 2007 COMMISSION GUIDELINE ON THE FORMAT AND CONTENT OF APPLICATIONS FOR AGREEMENT OR MODIFICATION OF A PAEDIATRIC INVESTIGATION PLAN AND REQUESTS FOR WAIVERS OR DEFERRALS AND CONCERNING
Detection of Toxicity to Reproduction for Human Pharmaceuticals
 Detection of Toxicity to Reproduction for Human Pharmaceuticals Paving the Way for Alternative Assays in the 3 rd Revision of ICH S5 1 st EMA Workshop on Non-Animal Approaches, Oct 5 th 2017 Günter Waxenecker
Detection of Toxicity to Reproduction for Human Pharmaceuticals Paving the Way for Alternative Assays in the 3 rd Revision of ICH S5 1 st EMA Workshop on Non-Animal Approaches, Oct 5 th 2017 Günter Waxenecker
Medical Device Testing. Andrew Makin, MSc, ERT, MRSB Scientific Director CiToxLAB Scantox, Denmark
 Medical Device Testing Andrew Makin, MSc, ERT, MRSB Scientific Director CiToxLAB Scantox, Denmark Medical device Risk evaluation Efficacy Biocompatibility Clinical testing Pharmaceutical product Preclinical
Medical Device Testing Andrew Makin, MSc, ERT, MRSB Scientific Director CiToxLAB Scantox, Denmark Medical device Risk evaluation Efficacy Biocompatibility Clinical testing Pharmaceutical product Preclinical
Transmission to CHMP July Adoption by CHMP for release for consultation 20 July Start of consultation 31 August 2017
 1 2 3 29 August 2017 EMA/CHMP/ICH/544278/1998 Committee for Human Medicinal Products 4 ICH S5 (R3) guideline on reproductive toxicology: 5 detection of toxicity to reproduction for human 6 pharmaceuticals
1 2 3 29 August 2017 EMA/CHMP/ICH/544278/1998 Committee for Human Medicinal Products 4 ICH S5 (R3) guideline on reproductive toxicology: 5 detection of toxicity to reproduction for human 6 pharmaceuticals
Applicability of the AOP for Assessing Causality of Observations in Epidemiological Studies
 Applicability of the AOP for Assessing Causality of Observations in Epidemiological Studies Presented by: M.E. Meek, University of Ottawa bmeek@uottawa.ca 2 Outline Background on Adverse Outcome Pathways
Applicability of the AOP for Assessing Causality of Observations in Epidemiological Studies Presented by: M.E. Meek, University of Ottawa bmeek@uottawa.ca 2 Outline Background on Adverse Outcome Pathways
MATERIAL SAFETY DATA SHEET
 Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
EUSAAT Linz (Austria) October The JaCVAM validation study of the ROS in vitro photoxicity assay
 EUSAAT2013 - Linz (Austria) 15-18 October 2012 The JaCVAM validation study of the ROS in vitro photoxicity assay Dr. med. Horst Spielmann Professor for Regulatory Toxicology, FU Berlin & Sate Animal Welfare
EUSAAT2013 - Linz (Austria) 15-18 October 2012 The JaCVAM validation study of the ROS in vitro photoxicity assay Dr. med. Horst Spielmann Professor for Regulatory Toxicology, FU Berlin & Sate Animal Welfare
Stem Cel s Key Words:
 Stem Cells Key Words: Embryonic stem cells, Adult stem cells, ips cells, self-renewal, differentiation, pluripotent, multipotent, Inner cell mass, Nuclear transfer (Therapeutic cloning), Feeder cells,
Stem Cells Key Words: Embryonic stem cells, Adult stem cells, ips cells, self-renewal, differentiation, pluripotent, multipotent, Inner cell mass, Nuclear transfer (Therapeutic cloning), Feeder cells,
CONTRACT ASSAY SERVICES CONTRACT ASSAY SERVICES FOR STEM AND PROGENITOR CELLS
 CONTRACT ASSAY SERVICES CONTRACT ASSAY SERVICES FOR STEM AND PROGENITOR CELLS TABLE OF CONTENTS 3 4 5 6 7 Introduction Hematopoietic Stem and Progenitor Cell Assays Mesenchymal Stem and Progenitor Cell
CONTRACT ASSAY SERVICES CONTRACT ASSAY SERVICES FOR STEM AND PROGENITOR CELLS TABLE OF CONTENTS 3 4 5 6 7 Introduction Hematopoietic Stem and Progenitor Cell Assays Mesenchymal Stem and Progenitor Cell
LAWSONE OPINION OF THE SCIENTIFIC COMMITTEE ON COSMETIC PRODUCTS AND NON-FOOD PRODUCTS INTENDED FOR CONSUMERS. SCCNFP/0561/02, final.
 SCCNFP/0561/02, final OPINION OF THE SCIENTIFIC COMMITTEE ON COSMETIC PRODUCTS AND NON-FOOD PRODUCTS INTENDED FOR CONSUMERS CONCERNING LAWSONE Colipa n C146 adopted by the SCCNFP during the 19 th plenary
SCCNFP/0561/02, final OPINION OF THE SCIENTIFIC COMMITTEE ON COSMETIC PRODUCTS AND NON-FOOD PRODUCTS INTENDED FOR CONSUMERS CONCERNING LAWSONE Colipa n C146 adopted by the SCCNFP during the 19 th plenary
How to maintain a robust and objective scientific dialogue between government and stakeholder experts?
 How to maintain a robust and objective scientific dialogue between government and stakeholder experts? An NGO Perspective Angeliki Lyssimachou, PhD Environmental Toxicologist Workshop: What does the future
How to maintain a robust and objective scientific dialogue between government and stakeholder experts? An NGO Perspective Angeliki Lyssimachou, PhD Environmental Toxicologist Workshop: What does the future
Revidierte ICH M3 Auswirkungen auf die präklinische Arzneimittelentwicklung. Pharmakologie, ADME, Missbrauchs- Potential und Kombinations-Toxizität
 Revidierte ICH M3 Auswirkungen auf die präklinische Arzneimittelentwicklung Pharmakologie, ADME, Missbrauchs- Potential und Kombinations-Toxizität PD Dr. Elke Röhrdanz Präklinik Fachgebiet Klinische Prüfung
Revidierte ICH M3 Auswirkungen auf die präklinische Arzneimittelentwicklung Pharmakologie, ADME, Missbrauchs- Potential und Kombinations-Toxizität PD Dr. Elke Röhrdanz Präklinik Fachgebiet Klinische Prüfung
Guideline on the non-clinical requirements for radiopharmaceuticals
 1 2 3 15 November 2018 EMA/CHMP/SWP/686140/2018 Committee for Medicinal Products for Human Use (CHMP) 4 5 6 Draft Draft agreed by Safety Working Party October 2018 Adopted by CHMP for release for consultation
1 2 3 15 November 2018 EMA/CHMP/SWP/686140/2018 Committee for Medicinal Products for Human Use (CHMP) 4 5 6 Draft Draft agreed by Safety Working Party October 2018 Adopted by CHMP for release for consultation
ANNEX. 1. Annex I to Regulation (EC) No 1907/2006 is amended as follows:
 Ref. Ares(2017)4925011-09/10/2017 EN ANNEX 1. Annex I to Regulation (EC) No 1907/2006 is amended as follows: (a) Subsection 0.1. is replaced by the following: "0.1. The purpose of this Annex is to set
Ref. Ares(2017)4925011-09/10/2017 EN ANNEX 1. Annex I to Regulation (EC) No 1907/2006 is amended as follows: (a) Subsection 0.1. is replaced by the following: "0.1. The purpose of this Annex is to set
Guidance on information requirements and chemical safety assessment
 Guidance on information requirements and chemical safety assessment Chapter R.: Adaptation of information requirements October 0 Draft Update V.0 Guidance for the implementation of REACH LEGAL NOTICE This
Guidance on information requirements and chemical safety assessment Chapter R.: Adaptation of information requirements October 0 Draft Update V.0 Guidance for the implementation of REACH LEGAL NOTICE This
MATERIAL SAFETY DATA SHEET
 Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
VELTIS : INNOVATIVE ALBUMIN BASED TECHNOLOGY FOR HALF- LIFE EXTENSION AND OPTIMIZATION OF BIOTHERAPEUTICS
 VELTIS : INNOVATIVE ALBUMIN BASED TECHNOLOGY FOR HALF- LIFE EXTENSION AND OPTIMIZATION OF BIOTHERAPEUTICS Dr Mikael Bjerg Caspersen Industrial Biotechnology Conference August 10 th 2015 INNOVATIVE TECHNOLOGY
VELTIS : INNOVATIVE ALBUMIN BASED TECHNOLOGY FOR HALF- LIFE EXTENSION AND OPTIMIZATION OF BIOTHERAPEUTICS Dr Mikael Bjerg Caspersen Industrial Biotechnology Conference August 10 th 2015 INNOVATIVE TECHNOLOGY
drug discovery: Where are we now? How did we RSC February 2013
 The role of pharmacokinetics in drug discovery: Where are we now? How did we get th here??where are we going? Peter Webborn RSC February 2013 Why conduct PK studies in animals? The primary purpose of pre-clinical
The role of pharmacokinetics in drug discovery: Where are we now? How did we get th here??where are we going? Peter Webborn RSC February 2013 Why conduct PK studies in animals? The primary purpose of pre-clinical
The Use of Exposure Reconstruction to Link Exposure, Internal Dose, and Health Outcomes
 The Use of Exposure Reconstruction to Link Exposure, Internal Dose, and Health Outcomes Harvey Clewell Director, Center for Human Health Assessment CIIT Centers for Health Research Research Triangle Park,
The Use of Exposure Reconstruction to Link Exposure, Internal Dose, and Health Outcomes Harvey Clewell Director, Center for Human Health Assessment CIIT Centers for Health Research Research Triangle Park,
Main achievements & Progress in Gianni Dal Negro, GlaxoSmithKline Industry Co-Chair EPAA Steering Committee
 Main achievements & Progress in 2011 Gianni Dal Negro, GlaxoSmithKline Industry Co-Chair EPAA Steering Committee Overview of EPAA activities in 2011 Focus on Integrated Testing Strategies (annual theme)
Main achievements & Progress in 2011 Gianni Dal Negro, GlaxoSmithKline Industry Co-Chair EPAA Steering Committee Overview of EPAA activities in 2011 Focus on Integrated Testing Strategies (annual theme)
06/03/2009. Overview. Preclinical Support for Exploratory Phase I Clinical Trials. Micro-dosing IND. Pharmacological Active Single Dose IND
 Preclinical Support for Exploratory Phase I Clinical Trials Clive Joseph, DSRD Sandwich Overview Identify the most appropriate development paradigm - traditional vs alternative IND approach Confidence
Preclinical Support for Exploratory Phase I Clinical Trials Clive Joseph, DSRD Sandwich Overview Identify the most appropriate development paradigm - traditional vs alternative IND approach Confidence
11 th International Conference on Environmental Mutagens Foz du Iguaccu, Brazil; 7 November 2013
 Pig-a In Vivo Mutation Assay Workgroup Report 6 th International Workshop on Genotoxicity Testing Presented By: Bhaskar Gollapudi, Ph.D. Exponent, Inc. 11 th International Conference on Environmental Mutagens
Pig-a In Vivo Mutation Assay Workgroup Report 6 th International Workshop on Genotoxicity Testing Presented By: Bhaskar Gollapudi, Ph.D. Exponent, Inc. 11 th International Conference on Environmental Mutagens
Pharmacology. Chatchai Chinpaisal, Ph.D. Department of Pharmacology and Toxicology, Faculty of Pharmacy, Silpakorn University.
 Pharmacology Chatchai Chinpaisal, Ph.D. Department of Pharmacology and Toxicology, Faculty of Pharmacy, Silpakorn University. 1 PHARMACODYNAMIC STUDIES A. Primary pharmacodynamics primary action in target
Pharmacology Chatchai Chinpaisal, Ph.D. Department of Pharmacology and Toxicology, Faculty of Pharmacy, Silpakorn University. 1 PHARMACODYNAMIC STUDIES A. Primary pharmacodynamics primary action in target
The WHO Framework for Combined Exposures and MOA/AOP Analysis; Implications for Euromix. Euromix Kick Off Meeting
 The WHO Framework for Combined Exposures and MOA/AOP Analysis; Implications for Euromix Euromix Kick Off Meeting M.E. (Bette) Meek University of Ottawa bmeek@uottawa.ca Contents of the WHO IPCS Framework
The WHO Framework for Combined Exposures and MOA/AOP Analysis; Implications for Euromix Euromix Kick Off Meeting M.E. (Bette) Meek University of Ottawa bmeek@uottawa.ca Contents of the WHO IPCS Framework
ICH Considerations. Oncolytic Viruses September 17, 2009
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH Considerations Oncolytic Viruses September 17, 2009 1. Introduction Oncolytic viruses
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH Considerations Oncolytic Viruses September 17, 2009 1. Introduction Oncolytic viruses
Validation of the 21 st Century Toxicology Toolbox: Challenges, Opportunities, and the Way Forward
 Validation of the 21 st Century Toxicology Toolbox: Challenges, Opportunities, and the Way Forward William S. Stokes 1, Judy Strickland 2, and Warren Casey 1 1 National Toxicology Program Interagency Center
Validation of the 21 st Century Toxicology Toolbox: Challenges, Opportunities, and the Way Forward William S. Stokes 1, Judy Strickland 2, and Warren Casey 1 1 National Toxicology Program Interagency Center
Approximately 5,000 high production volume (HPV) chemicals are now used by industries around the world
 PREFACE Approximately 5,000 high production volume (HPV) chemicals are now used by industries around the world although there are insufficient safety data for most of them. The Ministry of Economy, Trade
PREFACE Approximately 5,000 high production volume (HPV) chemicals are now used by industries around the world although there are insufficient safety data for most of them. The Ministry of Economy, Trade
The following sources of information were used to fill out the NanoRiskCat for nanosilver:
 Literature methodology/sources of information The following sources of information were used to fill out the NanoRiskCat for nanosilver: 1. Regulation (EC) No 1272/2008 OF THE EUROPEAN PARLIAMENT AND OF
Literature methodology/sources of information The following sources of information were used to fill out the NanoRiskCat for nanosilver: 1. Regulation (EC) No 1272/2008 OF THE EUROPEAN PARLIAMENT AND OF
Dosing for Controlled Exposure (DoCE): Dosing strategies for characterising in vitro dose-responses with increased relevance for in vivo extrapolation
 Dosing for Controlled Exposure (DoCE): Dosing strategies for characterising in vitro dose-responses with increased relevance for in vivo extrapolation Background Chemical safety assessments continue to
Dosing for Controlled Exposure (DoCE): Dosing strategies for characterising in vitro dose-responses with increased relevance for in vivo extrapolation Background Chemical safety assessments continue to
MATERIAL SAFETY DATA SHEET
 Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Printed paper and board: Priority setting strategy for toxicological assessment
 Printed paper and board: Priority setting strategy for toxicological assessment M. Van Bossuyt 1,2, E. Van Hoeck 1, T. Vanhaecke 2, V. Rogiers 2* and B. Mertens 1* 1 Food, Medicines and Consumer Safety,
Printed paper and board: Priority setting strategy for toxicological assessment M. Van Bossuyt 1,2, E. Van Hoeck 1, T. Vanhaecke 2, V. Rogiers 2* and B. Mertens 1* 1 Food, Medicines and Consumer Safety,
DMTC Technology Readiness Levels Guideline
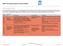 Technology Readiness Levels Technology Readiness Levels (TRLs) are used as standardised numerical indicators of the level of maturity of a technology. The standard TRL definitions are given in Table 1.
Technology Readiness Levels Technology Readiness Levels (TRLs) are used as standardised numerical indicators of the level of maturity of a technology. The standard TRL definitions are given in Table 1.
The Role of Chemistry in Adverse Outcome Pathways. Paul Russell & Steve Gutsell Safety and Environmental Assurance Centre, Unilever April 2013
 The Role of Chemistry in Adverse Outcome Pathways Paul Russell & Steve Gutsell Safety and Environmental Assurance Centre, Unilever April 2013 Contents Background Risk Assessment in FMCG industry Drivers
The Role of Chemistry in Adverse Outcome Pathways Paul Russell & Steve Gutsell Safety and Environmental Assurance Centre, Unilever April 2013 Contents Background Risk Assessment in FMCG industry Drivers
Pathway-based Approaches to Safety Assessment: development and use
 Pathway-based Approaches to Safety Assessment: development and use Molecular initiating event Intermediary steps Adverse Outcome Catherine Willett, PhD Director, Regulatory Testing Risk Assessment and
Pathway-based Approaches to Safety Assessment: development and use Molecular initiating event Intermediary steps Adverse Outcome Catherine Willett, PhD Director, Regulatory Testing Risk Assessment and
Guidance on information requirements and chemical safety assessment
 Guidance on information requirements and chemical safety assessment Chapter R.5: Adaptation of information requirements April 2010 Draft Update V.01 Guidance for the implementation of REACH LEGAL NOTICE
Guidance on information requirements and chemical safety assessment Chapter R.5: Adaptation of information requirements April 2010 Draft Update V.01 Guidance for the implementation of REACH LEGAL NOTICE
