Value of DSC in characterization and optimization of protein stability as compared to other thermal stability assays
|
|
|
- Claud Brooks
- 6 years ago
- Views:
Transcription
1 Value of DSC in characterization and optimization of protein stability as compared to other thermal stability assays 韩佩韦 Malvern Instruments
2 Complex task of characterization and optimization of protein stability Chemical stress (ph, ionic strength, etc.) Physical stress (filtration, temperature, air-liquid interface) Freeze-thaw-induced stress
3 Challenge: numerous causes of protein instability matched by numerous remedies to be tested empirically Buffering agents Amino acids Osmolytes Sugars and carbohydrates Proteins and polymers Salts Surfactants Ligands... Stabilization might be achieved through : changes in bulk solutions properties specific interaction with protein inhibition of a degradation pathway
4 Buffer attribute 2 Profiling and optimization of protein stability remains a combination of trial-and-error and rational approaches Understanding of factors (intrinsic 60 and extrinsic) critical to protein stability on the molecular 50 level is needed for implementation of the rational approach 40 to optimization of protein stability Buffer attribute 1 10
5 Multiple biophysical methods used for characterization of protein stability Mass spectrometry (MS) Circular dichroism (CD) Fourier transform infrared spectroscopy (FTIR) Raman spectroscopy X-ray crystallography Nuclear magnetic resonance (NMR) Near-UV CD SEC HPLC Fluorescence Static and dynamic light scattering (SLS and DLS) Differential scanning calorimetry (DSC) Analytical ultracentrifugation (AUC)
6 Issues: Stability Indicator: Existing solutions: Thermal stability as generic indicator of protein stability Long-term costly stability studies of multiple samples. Involve lengthy incubation times & many analysis rounds for multiple samples. Protein long-term stability often correlates with thermal stability Assessment and optimization of protein stability through studies of thermal stability
7 Existing ways to assess thermal stability: Subject protein solution to a temperature up-scan and monitor changes in a property DSC IF DSF UV DLS&SLS CD BIOCHEM. ASSAY Rheology / Potency
8 What are we actually measuring? Instrumental readout Macroscopic average property 1st principle physical relations assumptions simplification Property, process Molecular level
9 DSC instrument readout is directly related to the thermal unfolding event Differential Scanning Calorimetry DSC Directly monitors thermal unfolding event by measuring changes in apparent excess heat capacity of protein sample during temperature up-scan Circular Dichroism Spectropolarimetry In a limited set of buffers follows unfolding through changes of CD spectrum sensitive to secondary and tertiary structure of the protein. Light scattering techniques: DLS and DSLS Indirectly monitor thermal unfolding of proteins through changes in protein size and extent of protein aggregation Differential Scanning Fluorescence, DSF Indirectly monitors thermal unfolding of proteins through changes in hydrophobic area or content of beta-sheet structure accessible to environment-sensitive fluorescent dye Fluorimetery, intrinsic fluorescence Indirectly and locally follows thermal unfolding of proteins by monitoring changes in intrinsic fluorescence of tryptophan and tyrosine residues
10 Differential Scanning Calorimetry, DSC Thermal stability of proteins at different conditions (buffers, excipients, adjuvants, ligands) Temperature is ramped Thermal denaturation of protein is monitored
11 MicroCal VP-Capillary DSC System m 130 µl cell volume
12 DSC offers universal thermal stability assay Heat as universal readout directly related to protein unfolding event No optical artifacts, works in turbid solutions Works under controlled conditions in most of buffers Allows to study protein samples as they are Minimum assay development Delivers multiple descriptors of protein stability
13 Protein unfolding monitored in DSC
14 Protein unfolding monitored in DSC
15 Protein unfolding monitored in DSC
16 Protein unfolding monitored in DSC
17 Protein unfolding monitored in DSC
18 Protein unfolding monitored in DSC
19 Protein unfolding monitored in DSC
20 Protein unfolding monitored in DSC
21 DSC thermogram provides multiple descriptors of protein thermal stability T m - thermal transition midpoint, multiple T m for multiple domains T onset - onset of unfolding DT 1/2 - width of a peak at half maximum hight DH cal, DH vh calorimetric and van t Hoff enthalpy Assessment of oligomerization and aggregation propensity Percent reversibility of the transition Kinetics of irreversible process DC p for reversible unfolding
22 Insights on protein stability accessible with DSC Shifts in Tm values ( C): Higher temperature = more stable Lower temperature = less stable Shifts in Tonset values ( C): Higher temperature = more stable Lower temperature = less stable Lowering of DH, area under the curve: decrease in the content of folded protein DH cal /DH vh ratio Measure of the size of the cooperative unit Changes in T 1/2 values (transition width, C): Smaller width = more cooperative unfolding usually associated with a compact structure Larger width less cooperative usually associated with a relaxed, partially unfolded structure Courtesy of Dr. Katherine E. Bowers, FUJIFILM Diosynth Biotechnologies U.S.A., Inc.
23 C p (kj K -1 mol -1 ) Use Tm to compare native, altered and mutant forms Native Mutant Phosphorylated Complexed Temperature ( C)
24 Tm as stability indicating parameter. Case study 1. DSC sensitive to changes in stability arising from a common chemical degradation pathway For each of the studied proteins (2 mabs and 2 fusion proteins), the melting temperature (Tm) decreased linearly as a function of oxidation. For one protein, DSC was shown to be a leading indicator of decreased antigen binding suggesting a subtle conformation change may be underway that can be detected using DSC prior to any observable impact on product potency. Arthur, K. K., Dinh, N. and Gabrielson, J. P. (2015), Technical Decision Making with Higher Order Structure Data: Utilization of Differential Scanning Calorimetry to Elucidate Critical Protein Structural Changes Resulting from Oxidation. J. Pharm. Sci., 104: doi: /jps.24313
25 Different T m at different protein concentrations indicative of protein oligomerization/ aggregation propensity Monomeric or not? T m shifts detect oligomers T m may shift with sample concentration Monomer Tm is concentration independent Monomeric protein Oligomeric protein
26 Cp (kcal/mole/ o C) Multiple Tms. DSC allows to address protein stability on domain level. Case study 2. Protein engineering guided by DSC. 150 Minor differences in primary sequence can have a big impact on antibody stability Stability of each domain can be assessed The least stable Fab variant expressed poorly and quickly formed high MW aggregates. Most stable antibody construct identified based on Tm of the Fab domain T m 2 = 73.3 C T m 1 = 60.4 C T m 3 = 82.0 C Temperature ( o C) Demarest et al, Malvern Application note
27 Cp (kcal/mole/ o C) Tonset. Shift in onset of thermal unfolding correlates with protein stability and aggregation propensity. Case study 3. Cp (kcal/mole/ o C) Data: BpaseTBSLa_cp Model: MN2State Chi^2 = T m ± DH1 2.19E3 ±181 DH v E5 ±1.06E4 T m ± DH2 1.41E4 ±2.8E3 DH v E4 ±4.94E3 T m ± DH3 4.01E4 ±2.68E3 DH v E5 ±2.92E3 onset at 12ºC BPase 8.3uM=0.23mg/ml BB293 in 20mM TBS + 200mM L-alanine ph 8.0 Protein X Construct Temperature ( o C) Tm=34.5ºC DSC: 10ºC upward shift of the lowtemperature transition stabilizes Protein X kinetically onset at 22ºC Protein X Construct 2 Tm=40ºC ca 0.22 mg/ml BPase BB384 in 20 mm TBS, ph % DMSO Data: BB384TBS05_cp Model: MN2State Chi^2 = T m ± DH1 877 ±93.6 DH v 1 4E5 ±5.32E4 T m ± DH2 1.14E4 ±932 DH v E4 ±4.06E3 T m ± DH3 2.64E4 ±939 DH v 3 1E5 ±2.15E Temperature ( o C) Construct 1 Construct 2 SEC SEC: Protein X homogeneity and stability to aggregation has dramatically increased
28 T 1/2 and Tm. Multiple metrics of protein thermal stability make buffer optimization /preformulation funnel more efficient. Case study selected Rank by T m 19 formulations tested Focus with T 1/2 5 focused on Katherine E. Bowers, Malvern Application Note
29 DH cal. Area under DSC curve is an indicator of the content of folded protein material. DSC can be used to assess quality of recombinant proteins. Case study 5. Malvern Application Note
30 DH cal /DH vh ratio as indicator of size of cooperative unit for thermal unfolding DH vh = DH cal cooperative unit and molecular weight are the same: largely reversible unfolding of one single domain DH vh < DH cal - cooperative unit is smaller than molecular weight: intermediates DH vh > DH cal cooperative unit is bigger than the molecular weight: oligomers or overestimated concentration of folded protein
31 Cp (cal/ o C) Ratio of calorimetric to van t Hoff enthalpy DH/DH vh, can be indicative of protein oligomerization state. Case study Data: Data2_cp Model: MN2State Chi^2/DoF = T m ±0.021 DH 3376 ±17.2 DH v 1.014E54 ±640 mgst Temperature (Deg. C) Ratio of van t Hoff to calorimetric enthalpy 3 indicates that protein unfolds as an oligomer (possibly trimer).
32 Existing ways to assess thermal stability: Can DSC make a difference? DSC IF DSF UV DLS&SLS CD BIOCHEM. ASSAY Rheology / Potency
33 How DSC performs against other thermal stability assays? Case study 8. Buffer optimization conducted with DSC, DSF and IF. Thermal stability screening of >500 samples 3 different proteins (2 mabs and one receptor protein 30 different buffer conditions. Thermal unfolding of the protein was followed with DSC, DSF and IF.
34 Proteostat Tm C Sypro Orange Tm C When it works DSF correlates rather well with DSC (Tm1) Screens at: 0.3 mg/ml R²= DSF R²= DSF DSC Tm C
35 Proteostat Tm C Sypro Orange Tm C When it works DSF correlates rather well with DSC (Tm1) Screens at: 0.3 mg/ml R²= BUT does DSF always work? DSF R²= DSF DSC Tm C
36 DSF is not truly universal assay DSF is not applicable to all proteins and all buffer compositions. One out of 3 proteins in this study was not amenable to DSF with either of the extrinsic dyes SyproOrange or ENZO. Self-associating excipients corrupted the readout of DSF. Domain resolution was not achieved with DSF
37 DSF ProteoStat Enzo DSF Sypro Orange MicroCal Cap DSC automated Performance varies a lot between DSC and DSF techniques Technique Protein mab1 mab2 % samples amenable to analysis % samples with domain resolution % samples amenable to auto data analysis Receptor X 100 one peak 100 mab mab Receptor X failed mab mab Receptor X failed
38 Domain resolution generally only achieved in DSC Domain resolution for all buffers was only achieved with DSC. DSF is less effective when looking at domains and their stabilization. Peak amplitude in DSF is not domain-specific. Direct nature of DSC readout ensures robustness of baseline definition and domain resolution.
39 Why/when data on thermal stability of individual domains can be of importance Guiding protein engineering work Comparing proteins produced with different expressions systems (glycosylation effect on domain stability) Relating changes in functional activity to stability changes of individual domains Selecting optimal condition during pre/formulation studies Characterization of Higher Order Structure (HOS) Comparability analysis
40 DSC vs IF in the buffer screen of mab1 sample. IF data can be difficult to interpret IF readout susceptible to optical artefacts like quenching, innerfiltering, aggregation, and light scattering
41 DSC has non-optical readout. DSC measurements are not affected by sample turbidity or presence of self-associating reagents in the buffers. DSF DSC DSC can be applied to the analysis of samples from long-term and stress-stability studies DSC gives means to assess conformational equivalence/biocomparability of protein batches originating from different processes and sources
42 DSC readout is directly related to protein concentration and conformational state. DSC thermogram is a fingerprint for assessment of conformational equivalence and Higher Order Structure. Three lots manufactured at different sites DSC verifies no difference in stability and solubility between DP lots Lot 3 Lot 2 Lot 1 Reference Malvern Application Note
43 Summary DSC is truly universal and generic assay. DSC is the only thermal stability (TS) method with direct readout DSC is information rich (multiple descriptors of thermal unfolding) allowing for rational data interpretation for protein characterization and stability screening DSC thermograms are sample specific, quantitative and reproducible. Allows for similarity analysis. DSC is a well-established tool used to validate other TS assays.
44 With Differential Scanning Calorimetry you can: Characterize protein stability Characterize domain structures Assess oligomerization state Screen engineered and recombinant proteins for stability and manufacturability Determine optimum solution conditions for protein purification and formulation development Assess biocomparability and biosimilarity Study/screen protein-ligand interaction
45 Thank you for your attention! Questions?
Use of ITC/DSC for the studies of biomolecularstability and interaction of excipients with proteins. Natalia Markova
 Use of ITC/DSC for the studies of biomolecularstability and interaction of excipients with proteins Natalia Markova MIBio2012 Outline Brief intro to the specifics of protein stability profiling and optimization
Use of ITC/DSC for the studies of biomolecularstability and interaction of excipients with proteins Natalia Markova MIBio2012 Outline Brief intro to the specifics of protein stability profiling and optimization
Regulatory Consideration for the Characterization of HOS in Biotechnology Products
 Regulatory Consideration for the Characterization of HOS in Biotechnology Products Maria Teresa Gutierrez Lugo, Ph.D. OBP/CDER/FDA 5 th International Symposium on Higher Order Structure of Protein Therapeutics
Regulatory Consideration for the Characterization of HOS in Biotechnology Products Maria Teresa Gutierrez Lugo, Ph.D. OBP/CDER/FDA 5 th International Symposium on Higher Order Structure of Protein Therapeutics
nanodsf 2bind: Your service provider for biophysical characterization of proteins Precisely revealing protein folding and stability
 nanodsf Precisely revealing protein folding and stability 2bind: Your service provider for biophysical characterization of proteins This booklet was written and designed by 2bind 08 2015 Any reproduction
nanodsf Precisely revealing protein folding and stability 2bind: Your service provider for biophysical characterization of proteins This booklet was written and designed by 2bind 08 2015 Any reproduction
Zetasizer ZS Helix (DLS/Raman) For Characterization of Pharmaceuticals and Biopharmaceuticals
 Zetasizer ZS Helix (DLS/Raman) For Characterization of Pharmaceuticals and Biopharmaceuticals Benefits of Zetasizer ZS Helix What it provides Develop mechanistic insight into oligomerization and aggregation
Zetasizer ZS Helix (DLS/Raman) For Characterization of Pharmaceuticals and Biopharmaceuticals Benefits of Zetasizer ZS Helix What it provides Develop mechanistic insight into oligomerization and aggregation
High-throughput Biophysical Analysis of Protein Stability: Comparability Assessments and Formulation Development
 High-throughput Biophysical Analysis of Protein Stability: Comparability Assessments and Formulation Development David B. Volkin Department of Pharmaceutical Chemistry, Macromolecule and Vaccine Stabilization
High-throughput Biophysical Analysis of Protein Stability: Comparability Assessments and Formulation Development David B. Volkin Department of Pharmaceutical Chemistry, Macromolecule and Vaccine Stabilization
ARBRE-P4EU Consensus Protein Quality Guidelines for Biophysical and Biochemical Studies Minimal information to provide
 ARBRE-P4EU Consensus Protein Quality Guidelines for Biophysical and Biochemical Studies Minimal information to provide Protein name and full primary structure, by providing a NCBI (or UniProt) accession
ARBRE-P4EU Consensus Protein Quality Guidelines for Biophysical and Biochemical Studies Minimal information to provide Protein name and full primary structure, by providing a NCBI (or UniProt) accession
Degradation of Biological Products and Impact on Higher Order Structure
 Degradation of Biological Products and Impact on Higher Order Jason K. Cheung, BioProcess Development Biologics and Vaccines Date: May 16, 2016 AAPS-NBC Sunrise Session Analytical Toolkit 2 Analytical
Degradation of Biological Products and Impact on Higher Order Jason K. Cheung, BioProcess Development Biologics and Vaccines Date: May 16, 2016 AAPS-NBC Sunrise Session Analytical Toolkit 2 Analytical
Stability of sub-unit vaccines measured with the UNit label-free stability platform
 Application Note Stability of sub-unit vaccines measured with the UNit label-free stability platform Introduction Sub-unit vaccines and the need for adjuvants Of the many classes of vaccines that have
Application Note Stability of sub-unit vaccines measured with the UNit label-free stability platform Introduction Sub-unit vaccines and the need for adjuvants Of the many classes of vaccines that have
Challenges in Optimizing Formulations for Multi- Antigen Vaccines
 Engineering Conferences International ECI Digital Archives Vaccine Technology IV Proceedings Spring 5-22-212 Challenges in Optimizing Formulations for Multi- Antigen Vaccines Lakshmi Khandke Pfizer Global
Engineering Conferences International ECI Digital Archives Vaccine Technology IV Proceedings Spring 5-22-212 Challenges in Optimizing Formulations for Multi- Antigen Vaccines Lakshmi Khandke Pfizer Global
Prometheus Series Product Information
 Prometheus Series Product Information Prometheus Instruments for nanodsf www.nanotemper-technologies.com Prometheus Series NanoTemper Technologies offers nanodsf technology with the Prometheus Series.
Prometheus Series Product Information Prometheus Instruments for nanodsf www.nanotemper-technologies.com Prometheus Series NanoTemper Technologies offers nanodsf technology with the Prometheus Series.
Formulation Optimization Using the HUNK
 Formulation Optimization Using the HUNK Application Note Measuring ΔG: The Development Pathway to the Optimal Drug Product The development of successful biologics requires formulations that extend shelf
Formulation Optimization Using the HUNK Application Note Measuring ΔG: The Development Pathway to the Optimal Drug Product The development of successful biologics requires formulations that extend shelf
Best Practices in Formulation and Lyophilization Development Proteins, mabs and ADCs
 Best Practices in Formulation and Lyophilization Development Proteins, mabs and ADCs Best Practices in Formulation and Lyophilization Development The ultimate goal of formulation development is a stable
Best Practices in Formulation and Lyophilization Development Proteins, mabs and ADCs Best Practices in Formulation and Lyophilization Development The ultimate goal of formulation development is a stable
Ten Lessons for the Formulation Development of Monoclonal Antibodies from Multimodal Thermal Unfolding Case Studies
 Ten Lessons for the Formulation Development of Monoclonal Antibodies from Multimodal Thermal Unfolding Case Studies Mark Brader Protein Pharmaceutical Development Biogen Idec PEGS Boston essential protein
Ten Lessons for the Formulation Development of Monoclonal Antibodies from Multimodal Thermal Unfolding Case Studies Mark Brader Protein Pharmaceutical Development Biogen Idec PEGS Boston essential protein
AdvanceBio HIC: a Hydrophobic HPLC Column for Monoclonal Antibody (mab) Variant Analysis
 Application Note Biologics Development AdvanceBio HIC: a Hydrophobic HPLC Column for Monoclonal Antibody (mab) Variant Analysis Using the Agilent 16 Infinity II Bio-Inert LC Authors Andrew Coffey and Sandeep
Application Note Biologics Development AdvanceBio HIC: a Hydrophobic HPLC Column for Monoclonal Antibody (mab) Variant Analysis Using the Agilent 16 Infinity II Bio-Inert LC Authors Andrew Coffey and Sandeep
for MA and Beyond John Gabrielson CASSS 2 nd International Symposium on Higher Order Structure of Protein Therapeutics
 Higher Order Structure Characterization for MA and Beyond John Gabrielson CASSS 2 nd International Symposium on Higher Order Structure of Protein Therapeutics February 12, 2013 Proteins form multiple levels
Higher Order Structure Characterization for MA and Beyond John Gabrielson CASSS 2 nd International Symposium on Higher Order Structure of Protein Therapeutics February 12, 2013 Proteins form multiple levels
When proteins are being developed as therapeutics, an important consideration is both the inherent conformational
 Using Differential Scanning Calorimetry in Understanding the Correlation Between Thermal Stability and Protein Stability: A Case Study Written by Jie Wen, Yijia Jiang, Kathryn Hymes*, Ke Gong*, and Linda
Using Differential Scanning Calorimetry in Understanding the Correlation Between Thermal Stability and Protein Stability: A Case Study Written by Jie Wen, Yijia Jiang, Kathryn Hymes*, Ke Gong*, and Linda
Development of High Concentration cetuximab Formulations using Ultrafiltration and Precipitation Techniques
 Dissertation zur Erlangung des Doktorgrades der Fakultat fur Chemie und Pharmazie der Ludwig-Maximilians-Universitat Munchen Development of High Concentration cetuximab Formulations using Ultrafiltration
Dissertation zur Erlangung des Doktorgrades der Fakultat fur Chemie und Pharmazie der Ludwig-Maximilians-Universitat Munchen Development of High Concentration cetuximab Formulations using Ultrafiltration
Automated Chemical Denaturation as a Tool to Evaluate Protein Stability and Optimize the Formulation of Biologics
 Automated Chemical Denaturation as a Tool to Evaluate Protein Stability and Optimize the Formulation of Biologics Ernesto Freire Johns Hopkins University Baltimore, MD 21218 ef@jhu.edu AVIA 2304 Automated
Automated Chemical Denaturation as a Tool to Evaluate Protein Stability and Optimize the Formulation of Biologics Ernesto Freire Johns Hopkins University Baltimore, MD 21218 ef@jhu.edu AVIA 2304 Automated
Assistant Professor Mohammad A Alsenaidy, MSc, PhD, Saudi Arabia
 GaBI Scientific Meetings First GCC Stakeholder Meeting on Approval Process, Interchangeability/Substitution and Safety of Biosimilars 20 November 2017, Holiday Inn Izdihar Riyadh, Saudi Arabia Assistant
GaBI Scientific Meetings First GCC Stakeholder Meeting on Approval Process, Interchangeability/Substitution and Safety of Biosimilars 20 November 2017, Holiday Inn Izdihar Riyadh, Saudi Arabia Assistant
FORMULATION & LYOPHILIZATION CYCLE DEVELOPMENT OF AN ADC
 FORMULATION & LYOPHILIZATION CYCLE DEVELOPMENT OF AN ADC A Case Study Wendy Saffell-Clemmer Director, R&D Baxter BioPharma Solutions Antibody Drug Conjugates (ADCs) are complex molecules consisting of
FORMULATION & LYOPHILIZATION CYCLE DEVELOPMENT OF AN ADC A Case Study Wendy Saffell-Clemmer Director, R&D Baxter BioPharma Solutions Antibody Drug Conjugates (ADCs) are complex molecules consisting of
Protein Stability Analysis Using the Optim Patrick Celie NKI Protein Facility, Amsterdam
 Protein Stability Analysis Using the Optim 1000 Patrick Celie NKI Protein Facility, Amsterdam NKI Protein Facility Fundamental and translation cancer research ~ 650 scientists + supporting personnel Connected
Protein Stability Analysis Using the Optim 1000 Patrick Celie NKI Protein Facility, Amsterdam NKI Protein Facility Fundamental and translation cancer research ~ 650 scientists + supporting personnel Connected
Forced Degradation of Protein Therapeutics
 Forced Degradation of Protein Therapeutics Wim Jiskoot Division of Drug Delivery Technology Leiden Academic Centre for Drug Research (LACDR) AAPS Annual Meeting Orlando, FL 28 October 2015 Forced degradation
Forced Degradation of Protein Therapeutics Wim Jiskoot Division of Drug Delivery Technology Leiden Academic Centre for Drug Research (LACDR) AAPS Annual Meeting Orlando, FL 28 October 2015 Forced degradation
Regulatory Perspectives on Higher Order Structure Evaluations for Protein Products. Emily Shacter, Ph.D.
 Regulatory Perspectives on Higher Order Structure Evaluations for Protein Products Emily Shacter, Ph.D. Chief, Laboratory of Biochemistry U.S. Food and Drug Administration Center for Drug Evaluation and
Regulatory Perspectives on Higher Order Structure Evaluations for Protein Products Emily Shacter, Ph.D. Chief, Laboratory of Biochemistry U.S. Food and Drug Administration Center for Drug Evaluation and
Understanding the Effects of Common Mobile Phase Additives on the Performance of Size Exclusion Chromatography
 Understanding the Effects of Common Mobile Phase Additives on the Performance of Size Exclusion Chromatography 1 Presentation Importance of aggregate analysis in BioPharma manufacturing Analytical techniques
Understanding the Effects of Common Mobile Phase Additives on the Performance of Size Exclusion Chromatography 1 Presentation Importance of aggregate analysis in BioPharma manufacturing Analytical techniques
Modular DSP approaches for complex non-mab molecules
 Modular DSP approaches for complex non-mab molecules Dr. Stefan R. Schmidt MBA SVP Process Science & Production Agenda 1 Introduction 2 3 4 5 Process related impurities Product related impurities Virus
Modular DSP approaches for complex non-mab molecules Dr. Stefan R. Schmidt MBA SVP Process Science & Production Agenda 1 Introduction 2 3 4 5 Process related impurities Product related impurities Virus
Cryoconcentration effects during freeze/thaw processing of bulk protein, and possible consequences of frozen storage on protein aggregation
 Cryoconcentration effects during freeze/thaw processing of bulk protein, and possible consequences of frozen storage on protein aggregation Satish K Singh 20 July 2010 Abstract Frozen storage of proteins
Cryoconcentration effects during freeze/thaw processing of bulk protein, and possible consequences of frozen storage on protein aggregation Satish K Singh 20 July 2010 Abstract Frozen storage of proteins
Off to the races: rapid buffer exchange, protein quantification and stability screening with Junior, Lunatic and Uncle
 Application Note Off to the races: rapid buffer exchange, protein quantification and stability screening with Junior, Lunatic and Uncle Introduction The conformational, chemical and colloidal stability
Application Note Off to the races: rapid buffer exchange, protein quantification and stability screening with Junior, Lunatic and Uncle Introduction The conformational, chemical and colloidal stability
MICROCAL PEAQ-DSC BIOMOLECULAR STABILITY ANALYSIS FOR THE REGULATED ENVIRONMENT LABEL-FREE ANALYSIS MICROCALORIMETRY
 LABEL-FREE ANALYSIS MICROCALORIMETRY MICROCAL PEAQ-DSC BIOMOLECULAR STABILITY ANALYSIS FOR THE REGULATED ENVIRONMENT Biomolecular stability analysis for the regulated environment INTRODUCTION TO DIFFERENTIAL
LABEL-FREE ANALYSIS MICROCALORIMETRY MICROCAL PEAQ-DSC BIOMOLECULAR STABILITY ANALYSIS FOR THE REGULATED ENVIRONMENT Biomolecular stability analysis for the regulated environment INTRODUCTION TO DIFFERENTIAL
Characterization of mab aggregation using a Cary 60 UV-Vis Spectrophotometer and the Agilent 1260 Infinity LC system
 Characterization of mab aggregation using a Cary 60 UV-Vis Spectrophotometer and the Agilent 1260 Infinity LC system Application Note Biopharmaceuticals Authors Arunkumar Padmanaban and Sreelakshmy Menon
Characterization of mab aggregation using a Cary 60 UV-Vis Spectrophotometer and the Agilent 1260 Infinity LC system Application Note Biopharmaceuticals Authors Arunkumar Padmanaban and Sreelakshmy Menon
Suppl. Table I. Design of mutations within the BIIB4 and BIIB5 scfvs for screening in a thermal challenge assay.
 Suppl. Table I. Design of mutations within the and scfvs for screening in a thermal challenge assay. scfv position (Kabat numbering) Residue Frequency a Covariation DEEK or Rosetta c scfv position (Kabat
Suppl. Table I. Design of mutations within the and scfvs for screening in a thermal challenge assay. scfv position (Kabat numbering) Residue Frequency a Covariation DEEK or Rosetta c scfv position (Kabat
Accelerating the development of optimized therapeutic protein formulations using Differential Scanning Calorimetry
 Accelerating the development of optimized therapeutic protein formulations using Differential Scanning Calorimetry LABEL-FREE ANALYSIS MICROCALORIMETRY Biomolecules for therapeutic use must be stabilized
Accelerating the development of optimized therapeutic protein formulations using Differential Scanning Calorimetry LABEL-FREE ANALYSIS MICROCALORIMETRY Biomolecules for therapeutic use must be stabilized
Mass Spectrometry for Characterization of Monoclonal Antibodies: Regulatory Considerations
 Mass Spectrometry for Characterization of Monoclonal Antibodies: Regulatory Considerations Jun Park, Ph.D. Division of Monoclonal Antibodies Office of Biotechnology Products OPS/CDER Food and Drug Administration
Mass Spectrometry for Characterization of Monoclonal Antibodies: Regulatory Considerations Jun Park, Ph.D. Division of Monoclonal Antibodies Office of Biotechnology Products OPS/CDER Food and Drug Administration
Formulation Development
 Formulation Development Application Note NT-PR-013 Use of iformulate and nanodsf for Fast and Precise Protein Formulation Development Dennis Breitsprecher*, Rajiv Nayar #, *NanoTemper Technologies GmbH,
Formulation Development Application Note NT-PR-013 Use of iformulate and nanodsf for Fast and Precise Protein Formulation Development Dennis Breitsprecher*, Rajiv Nayar #, *NanoTemper Technologies GmbH,
Formulation Aspects in Biosimilar Development. Kedar S. Gokhale, PhD. Lupin Ltd. (Biotech)
 Formulation Aspects in Biosimilar Development Kedar S. Gokhale, PhD. Lupin Ltd. (Biotech) Typical Protein Formulation Components of a protein formulation Active Ingredient Buffer Tonicity modifier Stabilizer
Formulation Aspects in Biosimilar Development Kedar S. Gokhale, PhD. Lupin Ltd. (Biotech) Typical Protein Formulation Components of a protein formulation Active Ingredient Buffer Tonicity modifier Stabilizer
High Throughput Biophysical Approaches to Preformulation Characterization and Comparability Analysis of Protein Drugs and Macromolecular Vaccines
 High Throughput Biophysical Approaches to Preformulation Characterization and Comparability Analysis of Protein Drugs and Macromolecular Vaccines David B. Volkin, Ph.D. Higuchi Distinguished Professor
High Throughput Biophysical Approaches to Preformulation Characterization and Comparability Analysis of Protein Drugs and Macromolecular Vaccines David B. Volkin, Ph.D. Higuchi Distinguished Professor
Size-exclusion chromatography TT30 sample was analyzed using a Tosoh Haas TSK Gel G3000SW XL 7.8 mm 30cm column at 1 ml/min and 280 nm detection.
 Analytical ultracentrifugation (AUC) and size exclusion chromatography (SEC-HPLC) were used to assess TT30 molecular weight distribution. The sedimentation distribution of TT30 is shown in Fig. S1A. The
Analytical ultracentrifugation (AUC) and size exclusion chromatography (SEC-HPLC) were used to assess TT30 molecular weight distribution. The sedimentation distribution of TT30 is shown in Fig. S1A. The
Quantify protein stability with ΔG
 Technical Note Quantify protein stability with ΔG Introduction Biologics stability determinations often rely on forced degradation methods to rank constructs or optimize formulations. The most well-established
Technical Note Quantify protein stability with ΔG Introduction Biologics stability determinations often rely on forced degradation methods to rank constructs or optimize formulations. The most well-established
Using sequence analysis and design to mitigate formulation concerns during pre-clinical development
 Using sequence analysis and design to mitigate formulation concerns during pre-clinical development Margaret Ricci, Riki Stevenson, Holly Huang, Francis Kinderman, Heather Franey, Stephen Brych, Xichdao
Using sequence analysis and design to mitigate formulation concerns during pre-clinical development Margaret Ricci, Riki Stevenson, Holly Huang, Francis Kinderman, Heather Franey, Stephen Brych, Xichdao
Localized Higher Order Structures of mabs and ADCs Investigated by MS-based Protein Footprinting
 Localized Higher Order Structures of s and ADCs Investigated by MS-based Protein Footprinting Lucy Pan, John Valliere-Douglass and Oscar Salas-Solano 6 th International Symposium on the Higher Order Structure
Localized Higher Order Structures of s and ADCs Investigated by MS-based Protein Footprinting Lucy Pan, John Valliere-Douglass and Oscar Salas-Solano 6 th International Symposium on the Higher Order Structure
GE Healthcare Life Sciences. Development and optimization of protein formulations
 GE Healthcare Life Sciences Development and optimization of protein formulations Introduction Formulation optimization is best performed early in the development of a biopharmaceutical. Since biopharmaceuticals
GE Healthcare Life Sciences Development and optimization of protein formulations Introduction Formulation optimization is best performed early in the development of a biopharmaceutical. Since biopharmaceuticals
Structural Aspects of Immunogenicity: Aggregates
 Structural Aspects of Immunogenicity: Aggregates Ronald Smulders 19 November 2009 Pharmaceutical Sciences & Drug Metabolism EIP-Protein Characterization Subcommittee Mission of the EIP-PCS: To discuss
Structural Aspects of Immunogenicity: Aggregates Ronald Smulders 19 November 2009 Pharmaceutical Sciences & Drug Metabolism EIP-Protein Characterization Subcommittee Mission of the EIP-PCS: To discuss
CONSEQUENCES OF SAMPLE AGE ON BIOTHERAPEUTIC HIGHER ORDER STRUCTURE: INSIGHTS FROM NATIVE ION MOBILITY-MASS SPECTROMETRY METHODS
 COSEQUECES OF SAMPLE AGE O BIOTHERAPEUTIC HIGHER ORDER STRUCTURE: ISIGHTS FROM ATIVE IO MOBILITY-MASS SPECTROMETRY METHODS Richard Kerr ORISE Post-Doctoral Fellow US FDA, Division of Pharmaceutical Analysis
COSEQUECES OF SAMPLE AGE O BIOTHERAPEUTIC HIGHER ORDER STRUCTURE: ISIGHTS FROM ATIVE IO MOBILITY-MASS SPECTROMETRY METHODS Richard Kerr ORISE Post-Doctoral Fellow US FDA, Division of Pharmaceutical Analysis
Technical Challenges in the Development of Biosimilars. E. Morrey Atkinson, PhD Interphex May 1, 2012
 Technical Challenges in the Development of Biosimilars E. Morrey Atkinson, PhD Interphex May 1, 2012 FDA Guidance on Biosimilarity Guidance for Industry: Scientific Consideration in Demonstrating Biosimilarity
Technical Challenges in the Development of Biosimilars E. Morrey Atkinson, PhD Interphex May 1, 2012 FDA Guidance on Biosimilarity Guidance for Industry: Scientific Consideration in Demonstrating Biosimilarity
Biosimilar Profiling Application Note NT-PR-010
 Biosimilar Profiling Application Note NT-PR-010 Rapid and Precise Biosimilar Candidate Profiling by nanodsf Dennis Breitsprecher 1, Florian Beck 2 and Lukasz Kacprzyk 2 1 NanoTemper Technologies GmbH,
Biosimilar Profiling Application Note NT-PR-010 Rapid and Precise Biosimilar Candidate Profiling by nanodsf Dennis Breitsprecher 1, Florian Beck 2 and Lukasz Kacprzyk 2 1 NanoTemper Technologies GmbH,
Extracting Pure Proteins from Cells
 Extracting Pure Proteins from Cells 0 Purification techniques focus mainly on size & charge 0 The first step is homogenization (grinding, Potter Elvejhem homogenizer, sonication, freezing and thawing,
Extracting Pure Proteins from Cells 0 Purification techniques focus mainly on size & charge 0 The first step is homogenization (grinding, Potter Elvejhem homogenizer, sonication, freezing and thawing,
Characterisation of proteins
 Characterisation of proteins Stephan Uebel, Microchemistry Core Facility, MPI of Biochemistry Proteincharacterization Why? Proteincharacterization is done for peace of mind The key to a good Itc experiment
Characterisation of proteins Stephan Uebel, Microchemistry Core Facility, MPI of Biochemistry Proteincharacterization Why? Proteincharacterization is done for peace of mind The key to a good Itc experiment
The Present State and Future Outlook for Characterizing the Higher Order Structure (HOS) of Protein Drugs in the Biopharmaceutical Industry
 The Present State and Future Outlook for Characterizing the Higher Order Structure (HOS) of Protein Drugs in the Biopharmaceutical Industry Steven Berkowitz, Senior Principal Scientist Biogen Idec 2 nd
The Present State and Future Outlook for Characterizing the Higher Order Structure (HOS) of Protein Drugs in the Biopharmaceutical Industry Steven Berkowitz, Senior Principal Scientist Biogen Idec 2 nd
Stability of Biopharmaceuticals: Past, Present and Future. Paul Varley, Vice President, Biopharmaceutical Development
 Stability of Biopharmaceuticals: Past, Present and Future Paul Varley, Vice President, Biopharmaceutical Development Agenda 1 The last 20 years or so 2 Where we are now 3 The future 2 Early Process - THEN
Stability of Biopharmaceuticals: Past, Present and Future Paul Varley, Vice President, Biopharmaceutical Development Agenda 1 The last 20 years or so 2 Where we are now 3 The future 2 Early Process - THEN
Challenges in peptide mapping mass spectrometry of biopharmaceuticals
 Challenges in peptide mapping mass spectrometry of biopharmaceuticals Will Burkitt, Ben Holmes, Johanna Paris, Nisha Patel, John O Hara 07.MAR.2018 Characterisation within UCB 2 Context to the type of
Challenges in peptide mapping mass spectrometry of biopharmaceuticals Will Burkitt, Ben Holmes, Johanna Paris, Nisha Patel, John O Hara 07.MAR.2018 Characterisation within UCB 2 Context to the type of
3D Structure of Biologics in a Convenient Immunoassay Format
 3D Structure of Biologics in a Convenient Immunoassay Format Xing Wang, Ph.D. Array Bridge Inc. 5/25/2017 1 Topics Covered Today Why New Technologies? Technology Development. Case Studies for Novel and
3D Structure of Biologics in a Convenient Immunoassay Format Xing Wang, Ph.D. Array Bridge Inc. 5/25/2017 1 Topics Covered Today Why New Technologies? Technology Development. Case Studies for Novel and
CBI Toolbox Tour 2015
 CBI Toolbox Tour 2015 Thermophoresis (NanoTemper) NT.115 & NT.LabelFree Images: NanoTemper Circular Dichroism Jasco J-1500 Spectrometer Six Position Turreted Peltier Temperature Control System Automated
CBI Toolbox Tour 2015 Thermophoresis (NanoTemper) NT.115 & NT.LabelFree Images: NanoTemper Circular Dichroism Jasco J-1500 Spectrometer Six Position Turreted Peltier Temperature Control System Automated
Physical Methods in Models of Cataract Disease. O. P. Srivastava Department of Vision Science University of Alabama at Birmingham
 Physical Methods in Models of Cataract Disease O. P. Srivastava Department of Vision Science University of Alabama at Birmingham Function of the Lens: Refraction Lens Specific Structural Proteins (α-,
Physical Methods in Models of Cataract Disease O. P. Srivastava Department of Vision Science University of Alabama at Birmingham Function of the Lens: Refraction Lens Specific Structural Proteins (α-,
Fluorescent dyes as tools to detect changes in higher order. protein structures
 Fluorescent dyes as tools to detect changes in higher order Wim Jiskoot protein structures native stressed protein + bound dye protein native intermediate unfolded unbound dye partially unfolded aggregate
Fluorescent dyes as tools to detect changes in higher order Wim Jiskoot protein structures native stressed protein + bound dye protein native intermediate unfolded unbound dye partially unfolded aggregate
Analytical similarity: Lessons from the first US biosimilar
 Analytical similarity: Lessons from the first US biosimilar 2nd FDA/PQRI Conference on Advancing Product Quality Oct. 5-7, 2015 Corinna Sonderegger, Head Pharmaceutical Development Sandoz Biopharmaceuticals,
Analytical similarity: Lessons from the first US biosimilar 2nd FDA/PQRI Conference on Advancing Product Quality Oct. 5-7, 2015 Corinna Sonderegger, Head Pharmaceutical Development Sandoz Biopharmaceuticals,
9/16/2011. Fluorescent Dye-based Methods to Detect Changes in Higher Order Structures. Protein aggregation
 9/16/211 Fluorescent Dye-based Methods to Detect Changes in Higher Order Structures Wim Jiskoot native protein native stressed protein + bound dye intermediate unfolded unbound dye partially unfolded aggregate
9/16/211 Fluorescent Dye-based Methods to Detect Changes in Higher Order Structures Wim Jiskoot native protein native stressed protein + bound dye intermediate unfolded unbound dye partially unfolded aggregate
A Comprehensive Workflow to Optimize and Execute Protein Aggregate Studies
 A Comprehensive Workflow to Optimize and Execute Protein Aggregate Studies Combining Size Exclusion Chromatography with Method Development and Light Scattering Application Note Biotherapeutics and Biosimilars
A Comprehensive Workflow to Optimize and Execute Protein Aggregate Studies Combining Size Exclusion Chromatography with Method Development and Light Scattering Application Note Biotherapeutics and Biosimilars
Develop A Highly Similar" Biosimilar Compound: Lessons Learnt
 Develop A Highly Similar" Biosimilar Compound: Lessons Learnt Hui-Chun Li, Ph.D. Sr. Manager of Process Science 2015 Feb 05 3rd Biologics World Taiwan 2015 SPIN OFF ACQUISITION Development Center for Biotechnology
Develop A Highly Similar" Biosimilar Compound: Lessons Learnt Hui-Chun Li, Ph.D. Sr. Manager of Process Science 2015 Feb 05 3rd Biologics World Taiwan 2015 SPIN OFF ACQUISITION Development Center for Biotechnology
Analysis of Protein Biopharmaceuticals
 Analysis of Protein Biopharmaceuticals Comprehensive cgmp Services at Every Stage of Drug Development Amazing where you can go At Solvias, we work closely with you Solvias provides comprehensive services
Analysis of Protein Biopharmaceuticals Comprehensive cgmp Services at Every Stage of Drug Development Amazing where you can go At Solvias, we work closely with you Solvias provides comprehensive services
Pharmaceutical Development for ADCs: Not as Simple as ABC
 Pharmaceutical Development for ADCs: Not as Simple as ABC Pharmaceutical Development for ADCs Formulation, process, and analytical development for antibody-drug conjugates, or ADCs, is complex. While the
Pharmaceutical Development for ADCs: Not as Simple as ABC Pharmaceutical Development for ADCs Formulation, process, and analytical development for antibody-drug conjugates, or ADCs, is complex. While the
Characterization of biopharmaceutical stability with Differential Scanning Calorimetry: Candidate selection for developability
 Characterization of biopharmaceutical stability with Differential Scanning Calorimetry: Candidate selection for developability LABEL-FREE ANALYSIS MICROCALORIMETRY PROTEIN HIGHER ORDER STRUCTURE PROTEIN
Characterization of biopharmaceutical stability with Differential Scanning Calorimetry: Candidate selection for developability LABEL-FREE ANALYSIS MICROCALORIMETRY PROTEIN HIGHER ORDER STRUCTURE PROTEIN
Expert Consensus on Quality Control and Preclinical Evaluation of Antibody-Drug Conjugates
 Expert Consensus on Quality Control and Preclinical Evaluation of Antibody-Drug Conjugates National Institutes for Food and Drug Control July 20 th, 2018 Contents 1. Overview... 3 2. Manufacturing... 4
Expert Consensus on Quality Control and Preclinical Evaluation of Antibody-Drug Conjugates National Institutes for Food and Drug Control July 20 th, 2018 Contents 1. Overview... 3 2. Manufacturing... 4
SUPPLEMENTARY INFORMATION
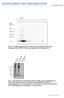 doi:10.1038/nature10324 Fig. S1: Two dimensional IEF/SDS-PAGE/Western blot analysis of RBC lysate crosslinked with 4 mm BS 3. The blot was probed with αsyn antibody C20. Fig. S2: SDS-PAGE/silver stain
doi:10.1038/nature10324 Fig. S1: Two dimensional IEF/SDS-PAGE/Western blot analysis of RBC lysate crosslinked with 4 mm BS 3. The blot was probed with αsyn antibody C20. Fig. S2: SDS-PAGE/silver stain
ALP (alkaline phosphatase) calibrators were analyzed manually in microtiter plates to find the linearity range by following this protocol:
 Exam Mol 3008 May 2009 Subject 1 (15p) ALP (alkaline phosphatase) calibrators were analyzed manually in microtiter plates to find the linearity range by following this protocol: Reaction solutions: 50
Exam Mol 3008 May 2009 Subject 1 (15p) ALP (alkaline phosphatase) calibrators were analyzed manually in microtiter plates to find the linearity range by following this protocol: Reaction solutions: 50
Demonstrating Biosimilarity of a Therapeutic Protein Product to a Reference Product ICH Guidance
 Demonstrating Biosimilarity of a Therapeutic Protein Product to a Reference Product ICH Guidance Derek Bradley M.Sc. BioClin Research Laboratories 13 th May 2016 Agenda Brief overview of biosimilars ICH
Demonstrating Biosimilarity of a Therapeutic Protein Product to a Reference Product ICH Guidance Derek Bradley M.Sc. BioClin Research Laboratories 13 th May 2016 Agenda Brief overview of biosimilars ICH
Assessment of Active Biopharmaceutical Ingredients Prior To and Following Removal of Interfering Excipients
 Assessment of Active Biopharmaceutical Ingredients Prior To and Following Removal of Interfering Excipients Andrew J Reason and Howard R Morris BioPharmaSpec Ltd, and BioPharmaSpec Inc, The EMA guideline
Assessment of Active Biopharmaceutical Ingredients Prior To and Following Removal of Interfering Excipients Andrew J Reason and Howard R Morris BioPharmaSpec Ltd, and BioPharmaSpec Inc, The EMA guideline
Case Study: A Phase-Driven Approach to the Development and Lifecycle Management of Potency Assays. Spring in New England!!!
 Case Study: A Phase-Driven Approach to the Development and Lifecycle Management of Potency Assays CASSS Bioassays 2016: Scientific Approaches & Regulatory Strategies Session Potency Assays: Cell-based
Case Study: A Phase-Driven Approach to the Development and Lifecycle Management of Potency Assays CASSS Bioassays 2016: Scientific Approaches & Regulatory Strategies Session Potency Assays: Cell-based
Conjugated protein biotherapeutics
 B i o P r o c e s s TECHNICAL Mass Spectrometric Conjugate Characterization Process Qualification of Recombinant Protein Hapten Conjugation Miao-Fang Lin, Margo Wilson, Michael V. Murray, and Greg W. Adams
B i o P r o c e s s TECHNICAL Mass Spectrometric Conjugate Characterization Process Qualification of Recombinant Protein Hapten Conjugation Miao-Fang Lin, Margo Wilson, Michael V. Murray, and Greg W. Adams
Thermo Scientific MAbPac HIC Columns. Novel Hydrophobic Interaction HPLC Columns. Designed for Monoclonal Antibody Analysis
 Thermo Scientific MAbPac HIC Columns Novel Hydrophobic Interaction HPLC Columns Designed for Monoclonal Antibody Analysis Introduction The Thermo Scientific MAbPac HIC column family is designed for separations
Thermo Scientific MAbPac HIC Columns Novel Hydrophobic Interaction HPLC Columns Designed for Monoclonal Antibody Analysis Introduction The Thermo Scientific MAbPac HIC column family is designed for separations
Quantitative Evaluation of the Ability of Ionic Liquids to Offset the Cold- Induced Unfolding of Proteins
 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2014 Supplimentary informations Quantitative Evaluation of the Ability of Ionic Liquids
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2014 Supplimentary informations Quantitative Evaluation of the Ability of Ionic Liquids
Practical Considerations in Developing High Concentration Antibody Formulations
 Practical Considerations in Developing High Concentration Antibody Formulations Qingyan Hu Formulation Development Group Regeneron Pharmaceuticals DDF Summit, 28 29 Aug 2017 Outline High concentration
Practical Considerations in Developing High Concentration Antibody Formulations Qingyan Hu Formulation Development Group Regeneron Pharmaceuticals DDF Summit, 28 29 Aug 2017 Outline High concentration
Peptide libraries: applications, design options and considerations. Laura Geuss, PhD May 5, 2015, 2:00-3:00 pm EST
 Peptide libraries: applications, design options and considerations Laura Geuss, PhD May 5, 2015, 2:00-3:00 pm EST Overview 1 2 3 4 5 Introduction Peptide library basics Peptide library design considerations
Peptide libraries: applications, design options and considerations Laura Geuss, PhD May 5, 2015, 2:00-3:00 pm EST Overview 1 2 3 4 5 Introduction Peptide library basics Peptide library design considerations
Analysis of NFU-1 metallocofactor binding-site substitutions impacts on iron sulfur cluster coordination and protein structure and function
 Analysis of NFU-1 metallocofactor binding-site substitutions impacts on iron sulfur cluster coordination and protein structure and function Nathaniel A. Wesley 1,, Christine Wachnowsky 1,2,, Insiya Fidai
Analysis of NFU-1 metallocofactor binding-site substitutions impacts on iron sulfur cluster coordination and protein structure and function Nathaniel A. Wesley 1,, Christine Wachnowsky 1,2,, Insiya Fidai
UV Fluorescence Polarization as a Means to Investigate Protein Conformational and Mass Change
 A p p l i c a t i o n N o t e UV Fluorescence Polarization as a Means to Investigate Protein Conformational and Mass Change Using Intrinsic Tryptophan Fluorescence in Conjunction with UV-capable Polarizers
A p p l i c a t i o n N o t e UV Fluorescence Polarization as a Means to Investigate Protein Conformational and Mass Change Using Intrinsic Tryptophan Fluorescence in Conjunction with UV-capable Polarizers
David Volkin. Department of Pharmaceutical Chemistry, Macromolecule and Vaccine Stabilization Center
 New Analytical Approaches and Data Visualization Tools to Assess Protein Higher-Order Structure and Stability as Applied to Comparability Assessments David Volkin Department of Pharmaceutical Chemistry,
New Analytical Approaches and Data Visualization Tools to Assess Protein Higher-Order Structure and Stability as Applied to Comparability Assessments David Volkin Department of Pharmaceutical Chemistry,
In Vitro Monitoring of the Formation of Pentamers from the Monomer of GST Fused HPV 16 L1
 This journal is The Royal Society of Chemistry 213 In Vitro Monitoring of the Formation of Pentamers from the Monomer of GST Fused HPV 16 L1 Dong-Dong Zheng, a Dong Pan, a Xiao Zha, ac Yuqing Wu,* a Chunlai
This journal is The Royal Society of Chemistry 213 In Vitro Monitoring of the Formation of Pentamers from the Monomer of GST Fused HPV 16 L1 Dong-Dong Zheng, a Dong Pan, a Xiao Zha, ac Yuqing Wu,* a Chunlai
Antibody-Drug Conjugate Characterization and Quality Assurance
 Antibody-Drug Conjugate Characterization and Quality Assurance Sarah Kennett Division of Monoclonal Antibodies Office of Biotechnology Products OPS/CDER/FDA October 12, 2011 1 Disclaimer The views and
Antibody-Drug Conjugate Characterization and Quality Assurance Sarah Kennett Division of Monoclonal Antibodies Office of Biotechnology Products OPS/CDER/FDA October 12, 2011 1 Disclaimer The views and
Structural basis of a novel PD-L1 nanobody for immune checkpoint blockade
 Structural basis of a novel PD-L1 nanobody for immune checkpoint blockade Supplementary Materials Supplementary methods Table S1-S Figure S1-S 1 1 1 1 1 1 1 1 1 0 1 0 Supplementary Methods Competitive
Structural basis of a novel PD-L1 nanobody for immune checkpoint blockade Supplementary Materials Supplementary methods Table S1-S Figure S1-S 1 1 1 1 1 1 1 1 1 0 1 0 Supplementary Methods Competitive
Thermal Analysis. Dr. Lidia Tajber School of Pharmacy and Pharmaceutical Sciences, Trinity College Dublin
 Thermal Analysis Dr. Lidia Tajber School of Pharmacy and Pharmaceutical Sciences, Trinity College Dublin Characterisation for Pharma Active pharmaceutical ingredients (API, drugs) Organic molecules, peptides,
Thermal Analysis Dr. Lidia Tajber School of Pharmacy and Pharmaceutical Sciences, Trinity College Dublin Characterisation for Pharma Active pharmaceutical ingredients (API, drugs) Organic molecules, peptides,
Antibody-Drug Conjugates The Road to the Current State. Nila Das, Ph.D. American Drug Delivery & Formulation Summit San Diego, CA June 13, 2016
 Antibody-Drug Conjugates The Road to the Current State Nila Das, Ph.D. American Drug Delivery & Formulation Summit San Diego, CA June 13, 2016 1 Current Status of ADCs C&E News. 2014, 92(3): 13-21 2 Current
Antibody-Drug Conjugates The Road to the Current State Nila Das, Ph.D. American Drug Delivery & Formulation Summit San Diego, CA June 13, 2016 1 Current Status of ADCs C&E News. 2014, 92(3): 13-21 2 Current
Introduction to Assay Development
 Introduction to Assay Development A poorly designed assay can derail a drug discovery program before it gets off the ground. While traditional bench-top assays are suitable for basic research and target
Introduction to Assay Development A poorly designed assay can derail a drug discovery program before it gets off the ground. While traditional bench-top assays are suitable for basic research and target
Physical Aging and Fragility of Amorphous Sucrose by DSC
 Physical Aging and Fragility of Amorphous Sucrose by DSC R. Bruce Cassel, Ph.D. TA Instruments, 109 Lukens Drive, New Castle DE 19720, USA ABSTRACT A method for quantifying physical aging and fragility
Physical Aging and Fragility of Amorphous Sucrose by DSC R. Bruce Cassel, Ph.D. TA Instruments, 109 Lukens Drive, New Castle DE 19720, USA ABSTRACT A method for quantifying physical aging and fragility
Chapter 6. Techniques of Protein and Nucleic Acid Purification
 Chapter 6 Techniques of Protein and Nucleic Acid Purification Considerations in protein expression and purification Protein source Natural sources Recombinant sources Methods of lysis and solubilization
Chapter 6 Techniques of Protein and Nucleic Acid Purification Considerations in protein expression and purification Protein source Natural sources Recombinant sources Methods of lysis and solubilization
Small-Scale Physicochemical and Biophysical Characterization to Enable Peptide Delivery in Discovery
 Small-Scale Physicochemical and Biophysical Characterization to Enable Peptide Delivery in Discovery Candice Alleyne, Andrew Leithead, and Ellen Minnihan Senior Scientists, Discovery Pharmaceutical Sciences
Small-Scale Physicochemical and Biophysical Characterization to Enable Peptide Delivery in Discovery Candice Alleyne, Andrew Leithead, and Ellen Minnihan Senior Scientists, Discovery Pharmaceutical Sciences
The Role of Mass Spectrometry for Developing Biotherapeutics: Regulatory Perspectives
 The Role of Mass Spectrometry for Developing Biotherapeutics: Regulatory Perspectives Jun Park, Ph.D. Division of Monoclonal Antibodies Office of Biotechnology Products CDER/FDA CASSS, Applications of
The Role of Mass Spectrometry for Developing Biotherapeutics: Regulatory Perspectives Jun Park, Ph.D. Division of Monoclonal Antibodies Office of Biotechnology Products CDER/FDA CASSS, Applications of
Prometheus NT.48 Product Information
 Prometheus NT.48 Product Information Prometheus Instruments for nanodsf www.nanotemper-technologies.com Prometheus NT.48 NanoTemper Technologies offers nanodsf technology with the Prometheus Series. nanodsf
Prometheus NT.48 Product Information Prometheus Instruments for nanodsf www.nanotemper-technologies.com Prometheus NT.48 NanoTemper Technologies offers nanodsf technology with the Prometheus Series. nanodsf
ENZYME CATALYSTS FOR A BIOTECHNOLOGY-BASED CHEMICAL INDUSTRY
 ENZYME CATALYSTS FOR A BIOTECHNOLOGY-BASED CHEMICAL INDUSTRY Professor Frances H. Arnold California Institute of Technology DOE Contract DE-FG02-93CH10578 Quarterly Progress Report, September 29 - December
ENZYME CATALYSTS FOR A BIOTECHNOLOGY-BASED CHEMICAL INDUSTRY Professor Frances H. Arnold California Institute of Technology DOE Contract DE-FG02-93CH10578 Quarterly Progress Report, September 29 - December
R R Innovation Way P/N SECKIT-7830 Newark, DE 19711, USA Tel: Fax: Website: Published in November 2013
 5-100 Innovation Way Newark, DE 19711, USA Tel:302-3661101 Fax:302-3661151 Website: www.sepax-tech.com Published in November 2013 P/N SECKIT-7830 These Phases are developed based on innovative surface
5-100 Innovation Way Newark, DE 19711, USA Tel:302-3661101 Fax:302-3661151 Website: www.sepax-tech.com Published in November 2013 P/N SECKIT-7830 These Phases are developed based on innovative surface
Guide. recombinant DNA proteins. for the elaboration of monographs on synthetic peptides and. European Pharmacopoeia
 Guide for the elaboration of monographs on synthetic peptides and recombinant DNA proteins European Pharmacopoeia European Directorate for the Quality of Medicines & HealthCare Edition Council of Europe,
Guide for the elaboration of monographs on synthetic peptides and recombinant DNA proteins European Pharmacopoeia European Directorate for the Quality of Medicines & HealthCare Edition Council of Europe,
Comparison of ExoSAP-IT and ExoSAP-IT Express reagents to alternative PCR cleanup methods
 WHITE PAPER ExoSAP-IT PCR cleanup reagents Comparison of ExoSAP-IT and ExoSAP-IT Express reagents to alternative PCR cleanup methods Abstract Here we present superior workflow advantages of enzymatic PCR
WHITE PAPER ExoSAP-IT PCR cleanup reagents Comparison of ExoSAP-IT and ExoSAP-IT Express reagents to alternative PCR cleanup methods Abstract Here we present superior workflow advantages of enzymatic PCR
Changes to a Potency Bioassay for Biotechnology Products: a Regulatory Perspective Kathleen A. Clouse, Ph.D., Director Division of Monoclonal Antibodi
 Changes to a Potency Bioassay for Biotechnology Products: a Regulatory Perspective Kathleen A. Clouse, Ph.D., Director Division of Monoclonal Antibodies Office of Biotechnology Products Center for Drug
Changes to a Potency Bioassay for Biotechnology Products: a Regulatory Perspective Kathleen A. Clouse, Ph.D., Director Division of Monoclonal Antibodies Office of Biotechnology Products Center for Drug
Supplementary Note 1. Enzymatic properties of the purified Syn BVR
 Supplementary Note 1. Enzymatic properties of the purified Syn BVR The expression vector pet15b-syn bvr allowed us to routinely prepare 15 mg of electrophoretically homogenous Syn BVR from 2.5 L of TB-medium
Supplementary Note 1. Enzymatic properties of the purified Syn BVR The expression vector pet15b-syn bvr allowed us to routinely prepare 15 mg of electrophoretically homogenous Syn BVR from 2.5 L of TB-medium
Supporting Information
 Supporting Information Copper and zinc ions specifically promote non-amyloid aggregation of the highly stable human γ-d crystallin Liliana Quintanar, 1,* José A. Domínguez-Calva, 1 Eugene Serebryany, 2
Supporting Information Copper and zinc ions specifically promote non-amyloid aggregation of the highly stable human γ-d crystallin Liliana Quintanar, 1,* José A. Domínguez-Calva, 1 Eugene Serebryany, 2
Higher Order Structure: Route to QC testing? Carl Jone, UCB, Belgium CASSS AT in Europe, Brussels, Belgium March 2016
 Higher Order Structure: Route to QC testing? Carl Jone, UCB, Belgium CASSS AT in Europe, Brussels, Belgium 14-17 March 2016 Introduction 2 Many quality attributes measured Why is HOS usually performed?
Higher Order Structure: Route to QC testing? Carl Jone, UCB, Belgium CASSS AT in Europe, Brussels, Belgium 14-17 March 2016 Introduction 2 Many quality attributes measured Why is HOS usually performed?
Chromatography column for therapeutic protein analysis
 PRODUCT SPECIFICATIONS ProPac Elite WCX Column Chromatography column for therapeutic protein analysis Benefits Superior resolution power for proteins, monoclonal antibodies, and associated charge variants
PRODUCT SPECIFICATIONS ProPac Elite WCX Column Chromatography column for therapeutic protein analysis Benefits Superior resolution power for proteins, monoclonal antibodies, and associated charge variants
Rapid Kinetics with IR Protein folding examples
 Rapid Kinetics with IR Protein folding examples Time dependent data with FTIR Stop-flow methods - msec limits so far Continuous, micro-flow methods - < 100 µsec Rapid scan FT-IR - msec Multichannel laser
Rapid Kinetics with IR Protein folding examples Time dependent data with FTIR Stop-flow methods - msec limits so far Continuous, micro-flow methods - < 100 µsec Rapid scan FT-IR - msec Multichannel laser
Stability of Biological Products
 Stability of Biological Products Dr Jurgen Lindner Principal, BioPharma Consulting & Executive Secretary, APIMAA Biological Products Functional Proteins or Polypeptides (mab s, enzymes & inhibitors, growth
Stability of Biological Products Dr Jurgen Lindner Principal, BioPharma Consulting & Executive Secretary, APIMAA Biological Products Functional Proteins or Polypeptides (mab s, enzymes & inhibitors, growth
AAPS Short Course: Characterization Methods for Aggregates and Particles in Peptide and Small Protein Formulations
 AAPS Short Course: Characterization Methods for Aggregates and Particles in Peptide and Small Protein Formulations Sunday, October 25, 2015 Orange County Convention Center Orlando, Florida Logistics AAPS
AAPS Short Course: Characterization Methods for Aggregates and Particles in Peptide and Small Protein Formulations Sunday, October 25, 2015 Orange County Convention Center Orlando, Florida Logistics AAPS
Effect of Liquid Handling QC on Biological Assay Performance. 06 Feb 2012 Artel Tutorial Nathaniel Hentz, PhD
 Effect of Liquid Handling QC on Biological Assay Performance 06 Feb 2012 Artel Tutorial Nathaniel Hentz, PhD Outline Review accuracy vs. precision Review common liquid handler QC methods Artel MVS technology
Effect of Liquid Handling QC on Biological Assay Performance 06 Feb 2012 Artel Tutorial Nathaniel Hentz, PhD Outline Review accuracy vs. precision Review common liquid handler QC methods Artel MVS technology
Renaturation Basic Kit for Proteins
 Renaturation Basic Kit for Proteins Product Number 96827 Store at 2-8 C Application The Renaturation Basic Kit for Proteins is a rapid empirical screening method used to determine the best conditions for
Renaturation Basic Kit for Proteins Product Number 96827 Store at 2-8 C Application The Renaturation Basic Kit for Proteins is a rapid empirical screening method used to determine the best conditions for
Comparability Analysis of Protein Therapeutics by Bottom-Up LC-MS with Stable Isotope-Tagged Reference Standards
 Comparability Analysis of Protein Therapeutics by Bottom-Up LC-MS with Stable Isotope-Tagged Reference Standards 16 September 2011 Abbott Bioresearch Center, Worcester, MA USA Manuilov, A. V., C. H. Radziejewski
Comparability Analysis of Protein Therapeutics by Bottom-Up LC-MS with Stable Isotope-Tagged Reference Standards 16 September 2011 Abbott Bioresearch Center, Worcester, MA USA Manuilov, A. V., C. H. Radziejewski
