Stem Cell Regenerative Therapy
|
|
|
- Lucas Pitts
- 6 years ago
- Views:
Transcription
1 Stem Cell Regenerative Therapy Standards & Guidelines January 2018 v1 Serving the public by guiding the medical profession
2 Approval Date: November 30, 2017 Originating Committee: Advisory Committee on Non-Hospital Surgical Facilities
3 Table of Contents This document addresses privileges and standards specific to Stem Cell Regenerative Therapy and is intended to be followed in addition to the College s standards for Non-Hospital Surgical Facilities. Preamble Physicians Providing Stem Cell Services: Qualifications Intraoperative Image Guidance Assisting Personnel: Qualifications Procedures Preoperative Evaluation Intra-Operative Management: Anesthesia Medical Records Harvesting Systems Stem Cell Collection & Separation Equipment Stem Cell Collection & Separation Discharging the Patient... 7 Resources... 8 Stem Cell Regenerative Therapy i CPSA: January 2018 v1 Copyright 2017 College of Physicians & Surgeons of Alberta
4 Preamble This document addresses privileges and standards for Stem Cell Regenerative Therapy and is intended to supplement the College s standards for Non-Hospital Surgical Facilities (NHSF). Physicians may only provide services that involve Adipose-Derived Stem/Stromal Cells (ADSC) and/or Bone Marrow Aspirate Concentrate (BMAC) in a non-hospital facility in Alberta that is accredited by College of Physicians & Surgeons of Alberta (CPSA). These CPSA Stem Cell Regenerative Therapy Standards are requirements which are in addition to those stipulated in Government of Canada regulations for cell therapy products in humans. Alberta physicians and NHSFs shall comply with these CPSA Standards. Further, if analysis of the stem cell aspirate or fluoroscopic guidance is necessary, then separate CPSA accreditation will be also be required by the respective College programs of Diagnostic Laboratory Medicine and/or Diagnostic Imaging. Physicians who perform Regenerative Therapy in an NHSF are encouraged to participate in active collaborations to identify outcome measures and aggregate outcomes data in addition to making their methods and results available for peer review. The College s accreditation prevue does not imply the endorsement of stem cell regenerative therapy by the CPSA. Rather, the oversights as a regulating body regarding the health services occurring within the non-hospital surgical facility are consistent with competent medical care. Stem Cell Regenerative Therapy 1 CPSA: January 2018 v1 Copyright 2017 College of Physicians & Surgeons of Alberta
5 1.0 Physicians Providing Stem Cell Services: Qualifications 1.1 Physicians who perform Regenerative Therapy in an NHSF shall: a. Be a physician licensed to practice medicine in Alberta; -and - b. Be certified as a specialist in the appropriate field with evidence of recognized training in Regenerative Therapy; -or- c. Have evidence of appropriate and sufficient training with experience that is suitable to the Registrar. 1.2 A satisfactory postgraduate training program acceptable to the Registrar for sufficient training and experience shall include clear demonstration and evidence of: a. Peer training preceptors with credentials acceptable to the Registrar; b. Both didactic and hands-on training; c. A clear description of the amount and the nature of the hands-on training, including: i. The selection of procedures; ii. The preparation of patients; iii. Maintenance of asepsis in non-hospital settings; iv. Intra-operative patient monitoring; v. Post-operative care and follow-up; vi. Quality improvement in surgical services; vii. Hands-on training in the surgical technique of bone marrow aspiration and/or liposuction; viii. Processing and delivery of the aspirate. 1.3 The Registrar may require a preliminary assessment prior to approving procedure privileges when there is insufficient information about the physician s training, experience or performance to satisfy the College. 1.4 The physician shall maintain competence through continuing education in the procedures performed. 1.5 Adipose derived stem cell aspiration is considered by the College as a form of the liposuction surgical procedure: a. Eligibility for privileges in adipose tissue aspiration shall include a minimum of one year of a residency training program resulting in demonstrated competency with the procedure; -or- b. Completion of postgraduate training in adipose tissue aspiration commensurate with the background of surgical training and experience of the applicant which is acceptable to the Registrar. Stem Cell Regenerative Therapy 2 CPSA: January 2018 v1
6 1.6 Conditions may be attached to privileges in adipose stem cell harvesting, restricting physicians to one or more of the following: a. Specified anatomical sites; b. Volume of the aspirate to a maximum of 240 ml; c. Type of anesthesia; d. Procedural Technique (collection, isolation and administration). 1.7 Bone marrow derived stem cell aspiration is considered by the College as a form of the bone biopsy surgical procedure: a. Eligibility for privileges in bone marrow aspiration shall include a minimum of one year of a residency training program resulting in demonstrated competency with the procedure; -or- b. Completion of postgraduate training in bone marrow biopsy commensurate with the background of surgical training and experience of the applicant which is acceptable to the Registrar. 1.8 Conditions may be attached to privileges in bone marrow stem cell harvesting, restricting physicians to one or more of the following: a. Specified anatomical sites; b. Maximum volume of aspirate; c. Bone Marrow derived stem cell aspirate to a maximum of 120 ml; d. Type of anesthesia; e. Procedural Technique (Collection, Isolation and administration). 1.9 All physicians who administer patient-managed gas inhalation anesthesia shall maintain current certification in health care provider cardiopulmonary resuscitation/aed and ACLS and meet all requirements listed in these accreditation standards under section 7.0 Intra-Operative Management: Anesthesia. Stem Cell Regenerative Therapy 3 CPSA: January 2018 v1
7 2.0 Intraoperative Image Guidance 2.1 Ultrasound guidance a. Point of care ultrasound does not require specific physician approval from the CPSA as stated in the Bylaws of the College subsection 36 (6) (a). 2.2 Fluoroscopic guidance a. Fluoroscopy aided services shall require separate accreditation by the CPSA Diagnostic Imaging Accreditation Program. i. All physicians who perform guided fluoroscopy shall be recognized as a specialist in radiology with CPSA modality approval; or- ii. Obtain modality approval as a non-radiologist with restricted fluoroscopy applicable to that modality as specified within the CPSA Diagnostic Imaging Accreditation Physician Approvals training requirement standards. 2.3 All facilities which provide fluoroscopy shall comply with CPSA Diagnostic Imaging Accreditation Standards specific to that modality. 3.0 Assisting Personnel: Qualifications 3.1 At least one assistant who is continuously in attendance of the patient shall have documented training and experience with: a. Procedural Technique (Collection, Isolation and administration); b. Monitoring and documentation of vital signs; c. Maintaining aseptic fields and instruments; d. Assisting in emergency procedures including the use of a bag-valve-mask device and current certification in cardiopulmonary resuscitation/aed use; f. g. Infection Prevention and Control, including medical device reprocessing; and Assembly, calibration, programming and/or operation of equipment. 4.0 Procedures 4.1 There shall be written procedures for all surgical procedures in the facility involving adipose tissue aspiration and/or bone marrow aspiration. 4.2 There shall be written procedures for all non-surgical procedures in the facility involved in the process of the stem cell regenerative therapy. Stem Cell Regenerative Therapy 4 CPSA: January 2018 v1
8 5.0 Preoperative Evaluation 5.1 The pre-operative evaluation by the surgeon shall also include the following determinations: a. Absence of contraindications; b. A pre-anesthetic assessment for patient-administered nitrous oxide not exceeding 50% of concentration inhalation which includes: i. A review of the patient's clinical record; ii. A medical interview with the patient; iii. A physical examination relative to anesthetic aspects of care; iv. A review and ordering of tests as indicated; v. A review or request for medical consultations as necessary for patient assessment and planning of peri-operative care; vi. Orders for pre-operative preparation such as fasting, medication, or other instructions as indicated; and, vii. A signed consent for the procedure/anesthetic that is part of the patient's clinical record. 6.0 Intra-Operative Management: Anesthesia 6.1 The College recognizes patient-administered gas inhalation as a form of anesthesia due to its sedating properties which may obtund awareness and protective reflexes. 6.2 The gas inhalation provision shall be from the appropriate nitrous oxide compressed medical gas provider as Entonox, the homogeneous gas containing a mix of 50% oxygen and 50% nitrous oxide compressed into a cylinder. This includes the Demand Valve/Regulator/Mask/Hose for use with Entonox cylinders. Substitution shall not occur. 6.3 Patient-administered gas inhalation shall only occur with: a. Documentation of prescreening for: i. Inability to follow verbal instructions; ii. Intoxication with alcohol or drugs; iii. Altered mental status; iv. Medication allergy or sensitivity. b. Documentation of successful patient teaching/ instruction with return demonstration of competency to self-administer. c. Monitoring and documentation of patient tolerance. 6.4 Patients undergoing patient-administered gas inhalation shall receive adequate instruction by qualified facility staff prior to use with documentation in the patient record. 6.5 Continual patient monitoring with vital signs documented at a minimum of every 5 minutes during the administration of gas inhalation shall occur via a second individual. This second individual shall be trained on gas inhalation equipment, set up, administration, patient monitoring and vital sign documentation and shall not be assisting in the surgical procedure. 6.6 No additional sedating medications shall be administered prior to or during patient-administered gas inhalation. Stem Cell Regenerative Therapy 5 CPSA: January 2018 v1
9 6.7 Patients undergoing patient-administered gas inhalation shall be continuously evaluated with at least the following: a. Visualization of patient s use (technique, frequency, pallor and respiratory effort) under appropriate lighting; b. Pulse oximeter with audible signal recognition; c. Apparatus to measure blood pressure with an appropriately sized cuff; and d. Vital signs documented at a minimum of every 5 minutes. 7.0 Medical Records 7.1 The peri-operative record shall contain a designated section and be completed for: a. Unique identifier and expiry date of stem cell collection/separation/administration device(s); b. The anatomical site(s) prepped and number of times accessed for collection; c. The volumes and time of aspirate; d. The volumes and time of stem cells isolated; e. The anatomical sites prepped, treated, time and the volume of stem cells injected per site; f. The use of guided techniques, if any; g. Surgical technique complications, if any; h. Amounts of aspirate and stem cell isolate discarded in appropriate medical waste container(s) for human tissue; i. Documentation of vital signs at a minimum of every 5 minutes during the procedure. 8.0 Harvesting Systems 8.1 Due to their inability to filter or sterilize the end product, all harvesting systems shall demonstrate the ability to avoid contaminants of debris and ambient air particulates at all stages of the stem cell process. 8.2 Commercial harvest kits, centrifuges and other specialized medical equipment shall be approved for use by Health Canada. 8.3 Design specifications shall comply with the manufacturer s instructions. 8.4 If facilities augment the specialized medical equipment and centrifuges with the preparation of their own inhouse harvesting kit, the following criteria shall be met: a. The kits is comprised of only commercially prepared sterile medical supplies (i.e. syringes, 3 way stopcock, etcetera); b. There is unique identifier and expiry date labeling on the in-house harvest equipment for tracking; Stem Cell Regenerative Therapy 6 CPSA: January 2018 v1
10 c. Processing of the specimen outside of the sterile field shall meet all requirements listed within the Section 11.0 Stem Cell Collection & Separation Equipment. 9.0 Stem Cell Collection & Separation Equipment 9.1 The intraoperative procedure shall be restricted to simple processing steps of anticoagulants containing antimicrobial properties, enzymatic digestion and centrifugation. 9.2 The stem cells concentrate shall not be compounded or administered with other substances Stem Cell Collection & Separation 10.1 Stem cell collection and separation done outside of the sterile field shall adhere to current good tissue practices as per Health Canada s Guidance Document for Cell, Tissue and Organ Establishments Safety of Human Cells, Tissues and Organs for Transplantation The collection and separation of the specimen/stem cell product shall be performed by a physician approved by the Registrar to perform stem cell procedures or by an appropriately trained a regulated health care professional designated to those responsibilities by the Medical Director Stem cells collected and separated in the facility shall be restricted to minimally manipulated autologous cells of the patient Stringent aseptic procedures and technique shall be followed during the collection and separation of the specimen/stem cell product Collection, separation and administration containers shall be clearly labeled with the patient s name, second identifier in addition to date and time of collection The stem cell separation process from collection to administration shall only occur in the operating room with the patient present and attended by the CPSA approved physician an appropriately trained a regulated health care professional designated to those responsibilities by the Medical Director Separated blood, bone marrow and adipose tissue products shall be used within the time frame specified by the equipment manufacturer and shall not exceed three (3) hours post-collection Discharging the Patient 11.1 The physician approved for patient-administered gas inhalation service shall remain on the premises of the NHSF until the patient meets documented pre-determined recovery criteria using a validated grading system (e.g. Analgesia and Anesthesia,Current Researches, Volume 49(6): , Nov-Dec A Postanesthetic Recovery Score by J.A. Aldrete and D. Kroulik.) Instructions not to drive or operate hazardous equipment for 24 hours after receiving patient-administered gas inhalation shall be given to the patient and accompanying adult. Stem Cell Regenerative Therapy 7 CPSA: January 2018 v1
11 Resources Aesthetic Surgery Journal. Current Evidence for Clinical Efficacy of Platelet Rich Plasma in Aesthetic Surgery: A Systematic Review. Frautschi B. et al; CA, USA; AHCTA. Harmonising Standards in HSCT: AHCTA Initiatives. K. Loper: Alliance of harmonization of cellular therapy accreditation; Annual Review of Biomedical Engineering. Intraoperative Stem Cell Therapy, Beato Coelho M. et al; Palo Alto CA; Australian Government Department of Health. Regulation of autologous stem cell therapies, Discussion paper for consultation; Australian Government Department of Health Therapeutic Goods Administration; Biological Science Section; Australia; Canadian Blood Services. Background Paper for the Tissue Expert Committee: How can the Canadian tissue donation and transplantation system best ensure consistent safety and quality; Canadian Blood Services Organ and Tissues Donation and Transplantation Steering Committee; Ottawa Clinical, Cosmetic and Investigational Dermatology. Autologous fat grafting: use of closed syringe microcannula system for enhanced autologous structural grafting. Alexander R. & Harrell D.: Clinical, Cosmetic and Investigational Dermatology; Cell Stem Cell. Oversight for Clinical Uses of Autologous Adult Stem Cells: Lessons from International Regulations; Lysaght T.et al: Cambridge, MA; Cell Stem Cell. New Japanese Initiative on Stem Cell Therapies; Konomi K.et al: Cambridge, MA; Centers for Disease Control and Prevention. Summary of Infection Prevention Practices in Dental Settings. National Center for Chronic Disease Prevention and Health Promotion. GA, USA; CLSI. Essential Tools for Implementation and Management of a Point-of-Care Testing Program. CLSI document POCT04, 3rd Ed. Wayne, PA: Clinical and Laboratory Standards Institute; CLSI. Quality Management: Approaches to Reducing Errors at the Point of Care; Approved Guideline. CLSI document POCT07- A. Wayne, PA: Clinical and Laboratory Standards Institute; CLSI. Selection Criteria for Point-of-Care Testing Devices; Approved Guideline. CLSI document POCT09-A. Wayne, PA: Clinical and Laboratory Standards Institute; CSA. Point-of-care testing (POCT) - Requirements for quality and competence. CAN/CSA Z :2006(E); Mississauga, ON; CSA. Blood and blood components. CAN/CSA-Z902-10(R0215); Mississauga, ON; CSA. Cells, Tissues, & Organs for Transplantation & Assisted Reproduction: General Requirements CAN/CSA-Z ; Mississauga, ON; CSTM. Standards for Hospital Transfusion Services, Version 4. Canadian Society of Transfusion Medicine; Markham ON; Europeon Spine Journal. A new simplified technique for producing platelet-rich plasma: a short technical note. Marlovit S. et al: Switzerland; GOC. Biologics, Radiopharmaceutical and Genetic Therapies, GOC, Confirmed access GOC. Guidance Document for Cell, Tissue and Organ Establishments - Safety of Human Cells, Tissues and Organs for Transplantation, GOC, Stem Cell Regenerative Therapy 8 CPSA: January 2018 v1
12 genetic-therapies/regulatory-initiatives/cells-tissues-organs/guidance-document-safety-human-cells-tissues-organstransplantation.html#a1; Confirmed access GOC. Guidance on Drug Establishment Liscenses and Drug Estabilishment Liscensing Fees, GOC, licences/directives-guidance-documents-policies/guidance-drug-establishment-licences-drug-establishment-licensing-fees html, Confirmed access GOC. Guidance Document: Preparation of Clinical Trial Applications for use of Cell Therapy Products in Humans, GOC, Confirmed access GOD. Platelet Rich Plasma (PRP) Guidelines, Government of Dubai, Dubai Health Authority; Dubai, FACT-JACIE. Hematopoietic Cellular Therapy Accreditation Manual 6 th Ed; E. McGrath: Netherlands; FACT-JACIE. FACT-Jacie International Standards for Hematopoietic Cellular Therapy, Product Collection, Processing, and Administration 6 th Ed-V 6.1; McGrath E.: Netherlands; Journal of Cutaneous and Aesthetic Surgery. Principles and Methods of Preparation of Platelet-Rich Plasma: A Review and Author s Perspective. Dhurat D. & Sukesh M.S.;.; India NICE. Platelet-rich plasma injections for osteoarthritis of the knee; Interventional procedure guideline. London UK; NICE. Autologous blood injection for tendinopathy; Interventional procedure guideline. London UK; PIDAC. Infection and Control for Clinical Office Practice. Public Health Ontario; ON; The International Cellular Medical Society Guidelines for the Use of Platelet Rich Plasma; International Cellular Medicine Society; NV, USA; Revista Brasileira De Ortopedia. Platelet-rich plasma (PRP) applied during total knee arthoroplasty; Guerreiro J. et al: Brazil; WHO. WHO best practices for injections and related procedures toolkit. World Health Organization; Switzerland; WHO. WHO guidelines on drawing blood: best practices in phlebotomy. World Health Organization; Switzerland; Stem Cell Regenerative Therapy 9 CPSA: January 2018 v1
The Ultimate Guide To STEM CELL THERAPY. for Joint Disorders
 The Ultimate Guide To STEM CELL THERAPY for Joint Disorders Who Are We? The Regenerative Orthopedic Clinic is a state-of-the-art medical facility that offers a number of services for non-surgical joint
The Ultimate Guide To STEM CELL THERAPY for Joint Disorders Who Are We? The Regenerative Orthopedic Clinic is a state-of-the-art medical facility that offers a number of services for non-surgical joint
Both health-care facility classification and area classification, as per CSA, determine the design requirements.
 ACCREDITATION STANDA RDS HEATING, VENTILATION AND AIR-CONDITIONING SYSTEM (HVAC) Class 3 Facility: Local Anesthesia Heating, ventilation and air-conditioning system standards are a primary infection prevention
ACCREDITATION STANDA RDS HEATING, VENTILATION AND AIR-CONDITIONING SYSTEM (HVAC) Class 3 Facility: Local Anesthesia Heating, ventilation and air-conditioning system standards are a primary infection prevention
INTRODUCTION OF BIOCELLULAR REGENERATIVE MEDICINE
 Evolution of Cell-Based Therapies Over the past decade, great strides have been made in the understanding and potentials of guided cell-based therapies. As an evolving expansion of palpation guided injection
Evolution of Cell-Based Therapies Over the past decade, great strides have been made in the understanding and potentials of guided cell-based therapies. As an evolving expansion of palpation guided injection
6TH EDITION OF FACT-JACIE STANDARDS A QUICK SUMMARY. BHS JACIE Day, 6 March 2015 Eoin McGrath JACIE Operations Manager
 6TH EDITION OF FACT-JACIE STANDARDS A QUICK SUMMARY BHS JACIE Day, 6 March 2015 Eoin McGrath JACIE Operations Manager CONTENTS Review process Sources of information Selection of main changes and developments
6TH EDITION OF FACT-JACIE STANDARDS A QUICK SUMMARY BHS JACIE Day, 6 March 2015 Eoin McGrath JACIE Operations Manager CONTENTS Review process Sources of information Selection of main changes and developments
Radiography Curriculum Analysis
 Program Number Program Name Date / /20 Radiography Curriculum Analysis DIRECTIONS: Determine the course(s) in which each of the following content area is covered and enter the course number(s) and/or title(s).
Program Number Program Name Date / /20 Radiography Curriculum Analysis DIRECTIONS: Determine the course(s) in which each of the following content area is covered and enter the course number(s) and/or title(s).
Sharon Tindle, MS, CQA (ASQ) QA Manager, BMT Tissue Services Mount Sinai Hospital, New York, NY. June 7, 2016
 Sharon Tindle, MS, CQA (ASQ) QA Manager, BMT Tissue Services Mount Sinai Hospital, New York, NY June 7, 2016 1 Brief description of the Mount Sinai Cellular Therapy Laboratory Overview of BM transplant
Sharon Tindle, MS, CQA (ASQ) QA Manager, BMT Tissue Services Mount Sinai Hospital, New York, NY June 7, 2016 1 Brief description of the Mount Sinai Cellular Therapy Laboratory Overview of BM transplant
BUILT TO CONCENTRATE. Magellan is an autologous concentration system that delivers concentrated platelets and cells at the point of care.
 BUILT TO CONCENTRATE Magellan is an autologous concentration system that delivers concentrated platelets and cells at the point of care. DELIVER PERSONALIZED MEDICINE Every patient has a unique biology
BUILT TO CONCENTRATE Magellan is an autologous concentration system that delivers concentrated platelets and cells at the point of care. DELIVER PERSONALIZED MEDICINE Every patient has a unique biology
FDA Regulatory, Compliance and Policy Developments: 361 HCT/Ps
 FDA Regulatory, Compliance and Policy Developments: 361 HCT/Ps September 27, 2018 Presentation by: Elaine H. Tseng Partner FDA and Life Sciences Group King & Spalding Discussion with: Thomas Poché Vice
FDA Regulatory, Compliance and Policy Developments: 361 HCT/Ps September 27, 2018 Presentation by: Elaine H. Tseng Partner FDA and Life Sciences Group King & Spalding Discussion with: Thomas Poché Vice
Regulatory framework for Stem Cell and Products review in India. Presentation By: Dr. V G Somani Joint Drugs Controller (India) CDSCO (HQ)
 Regulatory framework for Stem Cell and Products review in India Presentation By: Dr. V G Somani Joint Drugs Controller (India) CDSCO (HQ) 1 Outline of Presentation Legal Provisions MCI Code of Ethics regulation
Regulatory framework for Stem Cell and Products review in India Presentation By: Dr. V G Somani Joint Drugs Controller (India) CDSCO (HQ) 1 Outline of Presentation Legal Provisions MCI Code of Ethics regulation
Canadian Cancer Clinical Trials Network Portfolio Eligibility Criteria and Guidelines
 Canadian Cancer Clinical Trials Network Portfolio Eligibility Criteria and Guidelines 1. Introduction The Canadian Cancer Clinical Trials Network (3CTN) will support a portfolio of academic clinical trials
Canadian Cancer Clinical Trials Network Portfolio Eligibility Criteria and Guidelines 1. Introduction The Canadian Cancer Clinical Trials Network (3CTN) will support a portfolio of academic clinical trials
Regulation of Cell and Gene Therapy Products in Canada
 Regulation of Cell and Gene Therapy Products in Canada Canadian Blood and Bone Marrow Transplant Group June 8, 2018 Nadine Kolas, PhD Senior Policy Analyst Blood, Cells, Tissues and Organs Biologics and
Regulation of Cell and Gene Therapy Products in Canada Canadian Blood and Bone Marrow Transplant Group June 8, 2018 Nadine Kolas, PhD Senior Policy Analyst Blood, Cells, Tissues and Organs Biologics and
T H E R A P Y REGENERATIVE MEDICINE NATURAL EFFECTIVE LIFE-CHANGING
 S T E M C E L L T H E R A P Y REGENERATIVE MEDICINE NATURAL EFFECTIVE LIFE-CHANGING STEM CELL THERAPY Early stem cell research has traditionally been associated with the controversial use of embryonic
S T E M C E L L T H E R A P Y REGENERATIVE MEDICINE NATURAL EFFECTIVE LIFE-CHANGING STEM CELL THERAPY Early stem cell research has traditionally been associated with the controversial use of embryonic
Quality Program: Supplies and Reagents
 Quality Program: Supplies and Reagents J. Wade Atkins, MS, MT(ASCP) SBB, CQA (ASQ) Supervisor, Quality Assurance and Regulatory Affairs Department of Transfusion Medicine, Clinical Center, NIH Disclosures:
Quality Program: Supplies and Reagents J. Wade Atkins, MS, MT(ASCP) SBB, CQA (ASQ) Supervisor, Quality Assurance and Regulatory Affairs Department of Transfusion Medicine, Clinical Center, NIH Disclosures:
Polidocanol Endovenous Microfoam (PEM) Comprehensive Treatment for Great Saphenous Vein System (GSV) Incompetence
 Polidocanol Endovenous Microfoam (PEM) Comprehensive Treatment for Great Saphenous Vein System (GSV) Incompetence By Ariel D. Soffer, MD, FACC Dr. Ariel David Soffer-Bio NCVH Vein Forum Fellow of the American
Polidocanol Endovenous Microfoam (PEM) Comprehensive Treatment for Great Saphenous Vein System (GSV) Incompetence By Ariel D. Soffer, MD, FACC Dr. Ariel David Soffer-Bio NCVH Vein Forum Fellow of the American
FACT Common Standards for Cellular Therapies, Second Edition. Summary of Changes
 FACT Common Standards for Cellular Therapies, Second Edition Summary of Changes This document summarizes major changes made to the Second Edition FACT Common Standards for Cellular Therapies. This summary
FACT Common Standards for Cellular Therapies, Second Edition Summary of Changes This document summarizes major changes made to the Second Edition FACT Common Standards for Cellular Therapies. This summary
How You Can Use Stem Cells
 How You Can Use Stem Cells Dr. Ralph T Bright Macquarie Cosmetic Medicine 21B Bathurst Street Liverpool NSW 2170 www.cosmeticmedicine.com.au (02) 9824 3044 Stem Cell Transfer Many of the people in this
How You Can Use Stem Cells Dr. Ralph T Bright Macquarie Cosmetic Medicine 21B Bathurst Street Liverpool NSW 2170 www.cosmeticmedicine.com.au (02) 9824 3044 Stem Cell Transfer Many of the people in this
6/2/17. Updates on Regulatory Issues for Clinical Use of Biologics. Purpose. Biologics. Andrew G. Geeslin, MD
 Updates on Regulatory Issues for Clinical Use of Biologics Andrew G. Geeslin, MD Pre-Course: The Use of Biologics to Treat Sports Medicine Pathology June 3, 2017 11th Biennial ISAKOS Congress 2017, Shanghai,
Updates on Regulatory Issues for Clinical Use of Biologics Andrew G. Geeslin, MD Pre-Course: The Use of Biologics to Treat Sports Medicine Pathology June 3, 2017 11th Biennial ISAKOS Congress 2017, Shanghai,
SUR-421, Surgical Technology Pharmacology, 1 cr
 SUR-421, Surgical Technology Pharmacology, 1 cr COURSE DESCRIPTION: Provides information needed to calculate and handle drugs in the operating room. Provides an overview of the administration and general
SUR-421, Surgical Technology Pharmacology, 1 cr COURSE DESCRIPTION: Provides information needed to calculate and handle drugs in the operating room. Provides an overview of the administration and general
LIP IMPLANT UPPER LIP: SIZE MM LOWER LIP: SIZE MM
 INFORMED CONSENT FOR LIP IMPLANT UPPER LIP: SIZE MM LOWER LIP: SIZE MM PLEASE REVIEW AND BRING WITH YOU ON THE DAY OF YOUR PROCEDURE PATIENT NAME KAROL A. GUTOWSKI, MD, FACS AESTHETIC SURGERY CERTIFIED
INFORMED CONSENT FOR LIP IMPLANT UPPER LIP: SIZE MM LOWER LIP: SIZE MM PLEASE REVIEW AND BRING WITH YOU ON THE DAY OF YOUR PROCEDURE PATIENT NAME KAROL A. GUTOWSKI, MD, FACS AESTHETIC SURGERY CERTIFIED
Section 8. To introduce the basic theory and principles of collecting blood for Intraoperative Cell Salvage (ICS)
 Section 8 Practicalities Blood Collection Aim To introduce the basic theory and principles of collecting blood for Intraoperative Cell Salvage (ICS) Learning Outcomes To identify the equipment used for
Section 8 Practicalities Blood Collection Aim To introduce the basic theory and principles of collecting blood for Intraoperative Cell Salvage (ICS) Learning Outcomes To identify the equipment used for
FDA s Same Surgical Procedure Draft Guidance Could Stifle Use of Autologous Stem Cell Therapies
 FDA s Same Surgical Procedure Draft Guidance Could Stifle Use of Autologous Stem Cell Therapies Executive Summary, June 2015 Life Sciences Practice Group AUTHOR Areta L. Kupchyk Nixon Peabody LLP Washington,
FDA s Same Surgical Procedure Draft Guidance Could Stifle Use of Autologous Stem Cell Therapies Executive Summary, June 2015 Life Sciences Practice Group AUTHOR Areta L. Kupchyk Nixon Peabody LLP Washington,
Starting from Scratch: Experiences with a Focused Apheresis Service
 Starting from Scratch: Experiences with a Focused Apheresis Service University of Virginia Tamila Kindwall-Keller, DO, MS September 21, 2018 Disclosures Medical Monitor for BMT CTN 1301 Back Up Medical
Starting from Scratch: Experiences with a Focused Apheresis Service University of Virginia Tamila Kindwall-Keller, DO, MS September 21, 2018 Disclosures Medical Monitor for BMT CTN 1301 Back Up Medical
Application of ISO/IEC 17011:2004 in the Field of Medical Device Quality Management Systems (ISO 13485)
 IAF Mandatory Document Application of ISO/IEC 17011:2004 in the Field of Medical Device Quality Management Systems (ISO 13485) Issue 3 (IAF MD 8:2017) Issued: 09 June 2017 Application Date: 09 June 2018
IAF Mandatory Document Application of ISO/IEC 17011:2004 in the Field of Medical Device Quality Management Systems (ISO 13485) Issue 3 (IAF MD 8:2017) Issued: 09 June 2017 Application Date: 09 June 2018
Japan Update - Implementation of PMD Act - March, 2015
 Japan Update - Implementation of PMD Act - March, 2015 Topics Implementation of PMD Act; Revision of Pharmaceutical Affairs Law (PAL) PMDA Medical Device Training Seminar 2 Implementation of PMD Act: The
Japan Update - Implementation of PMD Act - March, 2015 Topics Implementation of PMD Act; Revision of Pharmaceutical Affairs Law (PAL) PMDA Medical Device Training Seminar 2 Implementation of PMD Act: The
FDA Regulation of Stem Cell Therapies. Regulation of adipose derived stromal preps
 2018 FDA Regulation of Stem Cell Therapies Regulation of adipose derived stromal preps EMERGING NEW FIELD BUT STILL A TODDLER Regenerative medicine technologies for orthopedic medicine application has
2018 FDA Regulation of Stem Cell Therapies Regulation of adipose derived stromal preps EMERGING NEW FIELD BUT STILL A TODDLER Regenerative medicine technologies for orthopedic medicine application has
Stem Cells Canadian Perspective
 Stem Cells Canadian Perspective John Laffey Critical Illness and Injury Research Centre, Keenan Research Centre, St Michael s Hospital. Departments of Anesthesia, Physiology and Interdepartmental Division
Stem Cells Canadian Perspective John Laffey Critical Illness and Injury Research Centre, Keenan Research Centre, St Michael s Hospital. Departments of Anesthesia, Physiology and Interdepartmental Division
Discover TruPRP. PRP the way you want it.
 Discover TruPRP PRP the way you want it. Discover TruPRP Discover the quality of Magellan TruPRP. The Magellan technology provides an automated dual spin processing system that can deliver (PRP) Platelet
Discover TruPRP PRP the way you want it. Discover TruPRP Discover the quality of Magellan TruPRP. The Magellan technology provides an automated dual spin processing system that can deliver (PRP) Platelet
MEDICINES CONTROL COUNCIL
 MEDICINES CONTROL COUNCIL GUIDELINE FOR A LICENCE TO MANUFACTURE, IMPORT, EXPORT OR DISTRIBUTE MEDICAL DEVICES & IVDs This guideline is intended to provide recommendations to applicants wishing to submit
MEDICINES CONTROL COUNCIL GUIDELINE FOR A LICENCE TO MANUFACTURE, IMPORT, EXPORT OR DISTRIBUTE MEDICAL DEVICES & IVDs This guideline is intended to provide recommendations to applicants wishing to submit
Osteobiologic B O N E G R A F T I N G S O L U T I O N S
 Osteobiologic BONE GRAFTING SOLUTIONS The AlloSource Advantage Delivering Quality and Safety * Allografts labeled Sterile R are aseptically processed and packaged and then subjected to gamma irradiation.
Osteobiologic BONE GRAFTING SOLUTIONS The AlloSource Advantage Delivering Quality and Safety * Allografts labeled Sterile R are aseptically processed and packaged and then subjected to gamma irradiation.
GUIDELINES FOR ISSUING AND RETURNING BLOOD COMPONENTS AND BLOOD PRODUCTS WITHIN A FACILITY
 GUIDELINES FOR ISSUING AND RETURNING BLOOD COMPONENTS AND BLOOD PRODUCTS WITHIN A FACILITY 1. Policy Statement 1.1 A policy shall be in place to ensure traceability of all blood components and blood products
GUIDELINES FOR ISSUING AND RETURNING BLOOD COMPONENTS AND BLOOD PRODUCTS WITHIN A FACILITY 1. Policy Statement 1.1 A policy shall be in place to ensure traceability of all blood components and blood products
Glossary to The Practice Standards for Medical Imaging and Radiation Therapy
 Glossary to The Practice Standards for Medical Imaging and Radiation Therapy Accuracy Ability of the bone mineral densitometry system to measure the true value of an object. Act anything done, being done,
Glossary to The Practice Standards for Medical Imaging and Radiation Therapy Accuracy Ability of the bone mineral densitometry system to measure the true value of an object. Act anything done, being done,
Platelet Concentrate in Total Knees BIOLOGICS. This brochure is for International use only. It is not for distribution in the United States.
 Platelet Concentrate in Total Knees BIOLOGICS This brochure is for International use only. It is not for distribution in the United States. Platelet Concentrate in Total Knees Total Knee Arthroplasty Total
Platelet Concentrate in Total Knees BIOLOGICS This brochure is for International use only. It is not for distribution in the United States. Platelet Concentrate in Total Knees Total Knee Arthroplasty Total
The patient must address the treating physician directly if he/she has any questions or requires information regarding the test results.
 Order Form 1. Ordering of the FoundationOne Service The FoundationOne Service comprises the genetic analysis of tumour tissue and the compilation of a comprehensive report on any mutations found in the
Order Form 1. Ordering of the FoundationOne Service The FoundationOne Service comprises the genetic analysis of tumour tissue and the compilation of a comprehensive report on any mutations found in the
FDA Approach to the Regulation of Hematopoietic Stem/Progenitor Cells (HPC)
 FDA Approach to the Regulation of Hematopoietic Stem/Progenitor Cells (HPC) Ellen Lazarus, M.D. Medical Officer Division of Human Tissues Office of Cellular, Tissue, and Gene Therapies FDA proposed approach
FDA Approach to the Regulation of Hematopoietic Stem/Progenitor Cells (HPC) Ellen Lazarus, M.D. Medical Officer Division of Human Tissues Office of Cellular, Tissue, and Gene Therapies FDA proposed approach
Review of the ATMP Regulation
 Review of the ATMP Regulation Joint DIA/MHRA Workshop on ATMPs London, 18 November 2011 Dr. Christian K Schneider Committee for Advanced Therapies (CAT) European Medicines Agency (EMA), London, UK I attend
Review of the ATMP Regulation Joint DIA/MHRA Workshop on ATMPs London, 18 November 2011 Dr. Christian K Schneider Committee for Advanced Therapies (CAT) European Medicines Agency (EMA), London, UK I attend
 2015 State of the Science in Transfusion Medicine Overarching themes and specific questions for RBCs What s in the [RBC] bag? Identify and quantify the components of RBC products to improve the quality,
2015 State of the Science in Transfusion Medicine Overarching themes and specific questions for RBCs What s in the [RBC] bag? Identify and quantify the components of RBC products to improve the quality,
Policies Concerning Survival Surgery of Mice
 Policies Concerning Survival Surgery of Mice Date approved: October 25, 2016 Purpose This document details the minimum standards for survival (recovery) surgery in all laboratory rodent species. Responsibility
Policies Concerning Survival Surgery of Mice Date approved: October 25, 2016 Purpose This document details the minimum standards for survival (recovery) surgery in all laboratory rodent species. Responsibility
Diagnostic Laboratory New Facility Registration Pre-Assessment Data Verification Form
 Diagnostic Laboratory New Facility Registration Pre-Assessment Data Verification Form Notes and Instructions: Section 4: For submission of required documentation, embed document into table beside the specific
Diagnostic Laboratory New Facility Registration Pre-Assessment Data Verification Form Notes and Instructions: Section 4: For submission of required documentation, embed document into table beside the specific
Regenerative Medicine. Speaker Disclosures. What is regenerative medicine? 6/22/2016
 Regenerative Medicine Halland Chen, MD Speaker Disclosures I disclose that I am not a consultant or have any financial interests. 2 What is regenerative medicine? Regeneration of human cells, tissues,
Regenerative Medicine Halland Chen, MD Speaker Disclosures I disclose that I am not a consultant or have any financial interests. 2 What is regenerative medicine? Regeneration of human cells, tissues,
Good Manufacturing Practice for Advanced Therapy Medicinal Products
 Good Manufacturing Practice for Advanced Therapy Medicinal Products Response on behalf of members of the ATMP-working group in the Netherlands and Belgium Erasmus University Medical Center, Department
Good Manufacturing Practice for Advanced Therapy Medicinal Products Response on behalf of members of the ATMP-working group in the Netherlands and Belgium Erasmus University Medical Center, Department
Evaluation & Decision Guides
 SURGERY STRATEGIC CLINICAL NETWORK EVIDENCE DECISION SUPPORT PROGRAM Evaluation & Decision Guides 2014 Revision (v3) New ideas & Improvements Department of Surgery Evidence Decision Support Program Resource
SURGERY STRATEGIC CLINICAL NETWORK EVIDENCE DECISION SUPPORT PROGRAM Evaluation & Decision Guides 2014 Revision (v3) New ideas & Improvements Department of Surgery Evidence Decision Support Program Resource
Special Issue. Mesoblast Limited
 Overview is an Australian biotechnology company committed to the commercialization of novel treatments for orthopedic conditions by using its unique adult stem cell technology for the regeneration and
Overview is an Australian biotechnology company committed to the commercialization of novel treatments for orthopedic conditions by using its unique adult stem cell technology for the regeneration and
Challenges in Capturing Long Term Follow up of Recipients of Genetically Modified Cells. Cell Therapy Liaison Meeting January, 2018
 Challenges in Capturing Long Term Follow up of Recipients of Genetically Modified Cells Cell Therapy Liaison Meeting January, 2018 Outline Development of the Cellular Therapy Registry Standardized Data
Challenges in Capturing Long Term Follow up of Recipients of Genetically Modified Cells Cell Therapy Liaison Meeting January, 2018 Outline Development of the Cellular Therapy Registry Standardized Data
Regulatory Issues in Human Subjects Research
 Regulatory Issues in Human Subjects Research Ian McNiece, PhD University of Miami Human Subjects Research Require IRB approval Studies of new drugs or applications of drugs require an FDA approved IND
Regulatory Issues in Human Subjects Research Ian McNiece, PhD University of Miami Human Subjects Research Require IRB approval Studies of new drugs or applications of drugs require an FDA approved IND
Point-of-Care Cell Processing Devices A Manufacturer s Perspective Clinical trials, regulatory compliance, and commercialization.
 Philadelphia, 2013 Point-of-Care Cell Processing Devices A Manufacturer s Perspective Clinical trials, regulatory compliance, and commercialization. Brian Barnes, Ph.D. VP, Clinical and Regulatory Affairs
Philadelphia, 2013 Point-of-Care Cell Processing Devices A Manufacturer s Perspective Clinical trials, regulatory compliance, and commercialization. Brian Barnes, Ph.D. VP, Clinical and Regulatory Affairs
DRAFT MEDICAL DEVICE GUIDANCE DOCUMENT
 November 2015 DRAFT DRAFT MEDICAL DEVICE GUIDANCE DOCUMENT REQUIREMENTS FOR LABELLING OF MEDICAL DEVICES Medical Device Authority MINISTRY OF HEALTH MALAYSIA Contents Page Preface... iii 1 Introduction.
November 2015 DRAFT DRAFT MEDICAL DEVICE GUIDANCE DOCUMENT REQUIREMENTS FOR LABELLING OF MEDICAL DEVICES Medical Device Authority MINISTRY OF HEALTH MALAYSIA Contents Page Preface... iii 1 Introduction.
Agenzia Italiana del Farmaco
 Agenzia Italiana del Farmaco European Regulation on Advanced Therapies Cristina Pintus Head of European Relations Unit and Coordinator of the Advanced Therapy Project Italian Medicines Agency Proposal
Agenzia Italiana del Farmaco European Regulation on Advanced Therapies Cristina Pintus Head of European Relations Unit and Coordinator of the Advanced Therapy Project Italian Medicines Agency Proposal
3/20/2017. R 3 Repair Paradigm REGENERATIVE MEDICINE GLOSSARY OF STEM CELL PLATFORMS. Limitations of Embryonic Stem Cells
 R 3 Repair Paradigm CENTER FOR REGENERATIVE MEDICINE Regenerative Medicine Regulatory And Practice Management Focus Replacement ACL/UCL Reconstruction Regeneration Next generation therapy Repair Rejuvenation
R 3 Repair Paradigm CENTER FOR REGENERATIVE MEDICINE Regenerative Medicine Regulatory And Practice Management Focus Replacement ACL/UCL Reconstruction Regeneration Next generation therapy Repair Rejuvenation
HCT/P Deviations & Reporting. 13 th ANNUAL FDA & The Changing Paradigm for HCT/P Regulation February 13-15, 2017
 HCT/P Deviations & Reporting CASE STUDIES FROM TISSUE AND EYE BANKS AND CELLULAR THERAPY. 13 th ANNUAL FDA & The Changing Paradigm for HCT/P Regulation February 13-15, 2017 Tissue Bank Perspective Ashley
HCT/P Deviations & Reporting CASE STUDIES FROM TISSUE AND EYE BANKS AND CELLULAR THERAPY. 13 th ANNUAL FDA & The Changing Paradigm for HCT/P Regulation February 13-15, 2017 Tissue Bank Perspective Ashley
Andrew Finnerty General Manager - CCMI
 Andrew Finnerty General Manager - CCMI Manufacturing Human Mesenchymal Stem Cells for Clinical Trials Quality Considerations Biopharma and Pharma RQA Regional Forum Bioclin Laboratories, Athlone 13 May
Andrew Finnerty General Manager - CCMI Manufacturing Human Mesenchymal Stem Cells for Clinical Trials Quality Considerations Biopharma and Pharma RQA Regional Forum Bioclin Laboratories, Athlone 13 May
College of Physicians and Surgeons of Saskatchewan Laboratory Quality Assurance Program. Policy Manual Edition
 College of Physicians and Surgeons of Saskatchewan Laboratory Quality Assurance Program Policy Manual 2016 Edition LABORATORY QUALITY ASSURANCE POLICY MANUAL SUMMARY OF POLICY MANUAL CHANGES The following
College of Physicians and Surgeons of Saskatchewan Laboratory Quality Assurance Program Policy Manual 2016 Edition LABORATORY QUALITY ASSURANCE POLICY MANUAL SUMMARY OF POLICY MANUAL CHANGES The following
Inspections, Compliance, Enforcement, and Criminal Investigations
 Page 1 of 5 Home Inspections, Compliance, Enforcement, and Criminal Investigations Enforcement Actions Warning Letters Inspections, Compliance, Enforcement, and Criminal Investigations Thomas E. Young,
Page 1 of 5 Home Inspections, Compliance, Enforcement, and Criminal Investigations Enforcement Actions Warning Letters Inspections, Compliance, Enforcement, and Criminal Investigations Thomas E. Young,
07/30/2013. Record of Revisions IRB Page 1 of 17
 Current Protocol Version 7.1 (Continuing Review) Approved July 30, 2013 Version 7.1 (Amendment) September 6, 2012 Version 7.0 (Continuing Review) July 30, 2012 Version 6.0 (Continuing Review) July 30,
Current Protocol Version 7.1 (Continuing Review) Approved July 30, 2013 Version 7.1 (Amendment) September 6, 2012 Version 7.0 (Continuing Review) July 30, 2012 Version 6.0 (Continuing Review) July 30,
GENReports: Market & Tech Analysis
 GENReports: Market & Tech Analysis > Enal Razvi, Ph.D. Managing Director Select Biosciences, Inc. enal@selectbio.us! There! is! much! interest! of! late! in! the! deployment! of! adipose! Assue! and!adiposebderived!stem!cells!for!regeneraave!medicine!
GENReports: Market & Tech Analysis > Enal Razvi, Ph.D. Managing Director Select Biosciences, Inc. enal@selectbio.us! There! is! much! interest! of! late! in! the! deployment! of! adipose! Assue! and!adiposebderived!stem!cells!for!regeneraave!medicine!
Tissue cleaning and sterilization provide an additional level of safety
 Managing biologics Understanding tissue processing Third in a series on managing bone allografts. In the October issue, articles included were Allografts: Overview of the process; and Donor screening:
Managing biologics Understanding tissue processing Third in a series on managing bone allografts. In the October issue, articles included were Allografts: Overview of the process; and Donor screening:
Therapeutic Apheresis Services. Annual Review 2015/16
 Therapeutic Apheresis Services Annual Review 2015/16 1 OVERVIEW NHS Blood and Transplant (NHSBT) is a major provider of Therapeutic Apheresis Services (TAS) in the NHS. At the end of each financial year,
Therapeutic Apheresis Services Annual Review 2015/16 1 OVERVIEW NHS Blood and Transplant (NHSBT) is a major provider of Therapeutic Apheresis Services (TAS) in the NHS. At the end of each financial year,
Development of non-substantially manipulated cell-based ATMPs 1 : flexibility introduced via the application of the risk-based approach
 3 July 2017 EMA/CAT/216556/2017 Inspections, Human Medicines, Pharmacovigilance and Committees Division Development of non-substantially manipulated cell-based ATMPs 1 : flexibility introduced via the
3 July 2017 EMA/CAT/216556/2017 Inspections, Human Medicines, Pharmacovigilance and Committees Division Development of non-substantially manipulated cell-based ATMPs 1 : flexibility introduced via the
A.P.A.G. Activated Plasma Albumin Gel
 A.P.A.G. Activated Plasma Albumin Gel How Does This Work In a new wound, platelets are the first cells which arrive. They aggregate to stop the bleeding and then release substances (growth factors, cytokines,
A.P.A.G. Activated Plasma Albumin Gel How Does This Work In a new wound, platelets are the first cells which arrive. They aggregate to stop the bleeding and then release substances (growth factors, cytokines,
I. Purpose. II. Definitions. Last Approval Date
 Investigational Drugs and Biologics Page 1 of 13 I. Purpose The purpose of this policy is to establish procedures for the proper control, storage, use and handling of investigational drugs and biologics
Investigational Drugs and Biologics Page 1 of 13 I. Purpose The purpose of this policy is to establish procedures for the proper control, storage, use and handling of investigational drugs and biologics
Insight to Gene Techno Science Co.,Ltd
 Unlimited drug discovery from the beginning Ticker symbol: 4584 Insight to Gene Techno Science Co.,Ltd November 2017 2 Corporate Overview Chief Executive Founded March 2001 Masaharu Tani, President Listed
Unlimited drug discovery from the beginning Ticker symbol: 4584 Insight to Gene Techno Science Co.,Ltd November 2017 2 Corporate Overview Chief Executive Founded March 2001 Masaharu Tani, President Listed
MEDICAL DEVICE GUIDANCE DOCUMENT REQUIREMENTS FOR LABELLING OF MEDICAL DEVICES
 January 2018 Second Edition MEDICAL DEVICE GUIDANCE DOCUMENT REQUIREMENTS FOR LABELLING OF MEDICAL DEVICES Medical Device Authority MINISTRY OF HEALTH MALAYSIA Contents Page Preface.... iii 1 Introduction....
January 2018 Second Edition MEDICAL DEVICE GUIDANCE DOCUMENT REQUIREMENTS FOR LABELLING OF MEDICAL DEVICES Medical Device Authority MINISTRY OF HEALTH MALAYSIA Contents Page Preface.... iii 1 Introduction....
Bone Marrow Concentration. For customized cellular concentrations of platelet-rich plasma from bone marrow aspirate
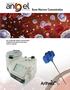 ARTHREX TM SYSTEM Bone Marrow Concentration For customized cellular concentrations of platelet-rich plasma from bone marrow aspirate Arthrex Angel System Technology is what sets Angel apart from the competition.
ARTHREX TM SYSTEM Bone Marrow Concentration For customized cellular concentrations of platelet-rich plasma from bone marrow aspirate Arthrex Angel System Technology is what sets Angel apart from the competition.
Computer-Aided Surgical Navigation Coding Guide Neurosurgery. May 1, 2009
 Computer-Aided Surgical Navigation Coding Guide Neurosurgery May 1, 2009 Please direct any questions to: Kim Brew Manager, Reimbursement and Therapy Access Medtronic Surgical Technologies (904) 279-7569
Computer-Aided Surgical Navigation Coding Guide Neurosurgery May 1, 2009 Please direct any questions to: Kim Brew Manager, Reimbursement and Therapy Access Medtronic Surgical Technologies (904) 279-7569
Apheresis M. Flores, BSN, RN, HP (ASCP)
 Apheresis M. Flores, BSN, RN, HP (ASCP) Objectives: 1. Discuss Apheresis and key concepts 2. Discuss : Cell Collection Extracorporeal Photopheresis (ECP) 1 What is Apheresis? Apheresis: Greek word meaning
Apheresis M. Flores, BSN, RN, HP (ASCP) Objectives: 1. Discuss Apheresis and key concepts 2. Discuss : Cell Collection Extracorporeal Photopheresis (ECP) 1 What is Apheresis? Apheresis: Greek word meaning
STANDARDS FOR HEMATOPOIETIC PROGENITOR CELL COLLECTION, PROCESSING & TRANSPLANTATION
 STANDARDS FOR HEMATOPOIETIC PROGENITOR CELL COLLECTION, PROCESSING & TRANSPLANTATION FROM THE JOINT ACCREDITATION COMMITTEE OF ISCT-EUROPE AND EBMT Second Edition - Europe June 2003 COPYRIGHT 2003 JOINT
STANDARDS FOR HEMATOPOIETIC PROGENITOR CELL COLLECTION, PROCESSING & TRANSPLANTATION FROM THE JOINT ACCREDITATION COMMITTEE OF ISCT-EUROPE AND EBMT Second Edition - Europe June 2003 COPYRIGHT 2003 JOINT
Human Tissue Intended for Transplantation Skin, corneas, sclera, bone, heart valves, blood vessels, pericardium, tendons, cartilage, fascia
 TITLE / DESCRIPTION: DEPARTMENT: Human Tissue Intended for Transplantation Skin, corneas, sclera, bone, heart valves, blood vessels, pericardium, tendons, cartilage, fascia Surgical Services PERSONNEL:
TITLE / DESCRIPTION: DEPARTMENT: Human Tissue Intended for Transplantation Skin, corneas, sclera, bone, heart valves, blood vessels, pericardium, tendons, cartilage, fascia Surgical Services PERSONNEL:
Act anything done, being done, or to be done; the process of doing. Synonymous with procedure and clinical services.
 Act anything done, being done, or to be done; the process of doing. Synonymous with procedure and clinical services. Action plan A program or method that explains the actions or steps to be taken. Advanced-practice
Act anything done, being done, or to be done; the process of doing. Synonymous with procedure and clinical services. Action plan A program or method that explains the actions or steps to be taken. Advanced-practice
Office for Human Subject Protection. University of Rochester
 POLICY 1. Purpose Outline the responsibilities and regulatory requirements when conducting human subject research that involves the use of drugs, agents, biological products, or nutritional products (e.g.,
POLICY 1. Purpose Outline the responsibilities and regulatory requirements when conducting human subject research that involves the use of drugs, agents, biological products, or nutritional products (e.g.,
PIC/S GMP GUIDE FOR BLOOD ESTABLISHMENTS
 PHARMACEUTICAL INSPECTION CONVENTION PHARMACEUTICAL INSPECTION CO-OPERATION SCHEME PE 005-2 1 July 2004 PIC/S GMP GUIDE FOR BLOOD ESTABLISHMENTS PIC/S July 2004 Reproduction prohibited for commercial purposes.
PHARMACEUTICAL INSPECTION CONVENTION PHARMACEUTICAL INSPECTION CO-OPERATION SCHEME PE 005-2 1 July 2004 PIC/S GMP GUIDE FOR BLOOD ESTABLISHMENTS PIC/S July 2004 Reproduction prohibited for commercial purposes.
Adult Stem Cells for Chronic Pain. Dr. John Hughes, DO January 24 th, 2018
 Adult Stem Cells for Chronic Pain Dr. John Hughes, DO January 24 th, 2018 Dr. John Hughes, DO Doctor of Osteopathy From Georgia Arizona College of Osteopathic Medicine - 2007 Aspen Integrative Medicine
Adult Stem Cells for Chronic Pain Dr. John Hughes, DO January 24 th, 2018 Dr. John Hughes, DO Doctor of Osteopathy From Georgia Arizona College of Osteopathic Medicine - 2007 Aspen Integrative Medicine
Contact details: Anna Silvani
 Consultation Document Good Manufacturing Practice for Advanced Therapy Medicinal Products MolMed comments to the DG SANTE consultation on GMPs for ATMPs pursuant to Article 5 of Regulation 1394/2007. MolMed
Consultation Document Good Manufacturing Practice for Advanced Therapy Medicinal Products MolMed comments to the DG SANTE consultation on GMPs for ATMPs pursuant to Article 5 of Regulation 1394/2007. MolMed
Arthrex Angel TM System. Indication-specific PRP and PRF gel preparations
 Arthrex Angel TM System Indication-specific PRP and PRF gel preparations Arthrex Angel TM System The Arthrex Angel TM System is a revolutionary production system for platelet-rich plasma (PRP) formulations
Arthrex Angel TM System Indication-specific PRP and PRF gel preparations Arthrex Angel TM System The Arthrex Angel TM System is a revolutionary production system for platelet-rich plasma (PRP) formulations
Inspections, Compliance, Enforcement, and Criminal Investigations
 Home Inspections, Compliance, Enforcement, and Criminal Investigations Enforcement Actions Warning Letters Inspections, Compliance, Enforcement, and Criminal Investigations Celltex Therapeutics Corporation
Home Inspections, Compliance, Enforcement, and Criminal Investigations Enforcement Actions Warning Letters Inspections, Compliance, Enforcement, and Criminal Investigations Celltex Therapeutics Corporation
Special Report. medicine? Peter Marks, M.D., Ph.D., and Scott Gottlieb, M.D.
 The new england journal of medicine Special Report Balancing Safety and Innovation for Cell-Based Regenerative Medicine Peter Marks, M.D., Ph.D., and Scott Gottlieb, M.D. Regenerative medicine is a field
The new england journal of medicine Special Report Balancing Safety and Innovation for Cell-Based Regenerative Medicine Peter Marks, M.D., Ph.D., and Scott Gottlieb, M.D. Regenerative medicine is a field
Standard Operating Procedure
 TITLE AFFECTED EFFECTIVE DATE IDENTIFICATION DONOR PROGRAM 1 of 8 I. PRINCIPLE As a member of the Radiation Injury Treatment Network (RITN), the Bone Marrow Donor Program whose Donor Center and Apheresis
TITLE AFFECTED EFFECTIVE DATE IDENTIFICATION DONOR PROGRAM 1 of 8 I. PRINCIPLE As a member of the Radiation Injury Treatment Network (RITN), the Bone Marrow Donor Program whose Donor Center and Apheresis
Regulatory frameworks of regenerative medicines and products review in Japan
 Regulatory frameworks of regenerative medicines and products review in Japan August 27th, 2018 Kenji KUROIWA Deputy Director, Medical Devices Evaluation Division Ministry of Health, Labour and Welfare,
Regulatory frameworks of regenerative medicines and products review in Japan August 27th, 2018 Kenji KUROIWA Deputy Director, Medical Devices Evaluation Division Ministry of Health, Labour and Welfare,
Stem Cells, Regenerative Medicine and cgmp (GTP)
 Stem Cells, Regenerative Medicine and cgmp (GTP) Encompass Stem cell based therapies activities Collection source Purification Isolation from other cell types if needed Manipulation Minimal vs Moderate
Stem Cells, Regenerative Medicine and cgmp (GTP) Encompass Stem cell based therapies activities Collection source Purification Isolation from other cell types if needed Manipulation Minimal vs Moderate
Outpatient Spine Surgery The Next Five Years Richard Wohns,, M.D., JD, MBA Neospine,, Puget Sound Region, Washington. Disclosures.
 Outpatient Spine Surgery The Next Five Years Richard Wohns,, M.D., JD, MBA Neospine,, Puget Sound Region, Washington Becker s s 12th Annual Spine, Orthopedic & Pain Management-Driven ASC Conference + The
Outpatient Spine Surgery The Next Five Years Richard Wohns,, M.D., JD, MBA Neospine,, Puget Sound Region, Washington Becker s s 12th Annual Spine, Orthopedic & Pain Management-Driven ASC Conference + The
FINAL DOCUMENT. International Medical Device Regulators Forum. Medical Device Regulatory Audit Reports
 FINAL DOCUMENT International Medical Device Regulators Forum Title: Authoring Group: Medical Device Regulatory Audit Reports IMDRF MDSAP Working Group Date: 2 October 2015 Toshiyoshi Tominaga, IMDRF Chair
FINAL DOCUMENT International Medical Device Regulators Forum Title: Authoring Group: Medical Device Regulatory Audit Reports IMDRF MDSAP Working Group Date: 2 October 2015 Toshiyoshi Tominaga, IMDRF Chair
-Regulation (EC) No.1394/2007 -Regulation (EC) No. 668/2009
 Introduction to Advanced Therapy Medicinal Products Regulation -Regulation (EC) No.1394/2007 -Regulation (EC) No. 668/2009 -Directive 2009/120/EC Dr. Maura O Donovan F.R.C.O.G. MA MD M.R.C.P.I. CAT member
Introduction to Advanced Therapy Medicinal Products Regulation -Regulation (EC) No.1394/2007 -Regulation (EC) No. 668/2009 -Directive 2009/120/EC Dr. Maura O Donovan F.R.C.O.G. MA MD M.R.C.P.I. CAT member
An introductory guide to the medical device regulation (MDR) and the in vitro diagnostic medical device regulation (IVDR)
 An introductory guide to the medical device regulation (MDR) and the in vitro diagnostic medical device regulation (IVDR) devices.implementation@mhra.gov.uk 1 Introduction - How to use this guide Navigate
An introductory guide to the medical device regulation (MDR) and the in vitro diagnostic medical device regulation (IVDR) devices.implementation@mhra.gov.uk 1 Introduction - How to use this guide Navigate
MEDICAL DEVICE GUIDANCE
 June 2018 MEDICAL DEVICE GUIDANCE GN-23: Guidance on Labelling for Medical Devices Revision 1 CONTENTS PREFACE... 3 1. INTRODUCTION... 4 2. LABELLING REQUIREMENTS... 7 2.1. General Guidelines... 7 2.2.
June 2018 MEDICAL DEVICE GUIDANCE GN-23: Guidance on Labelling for Medical Devices Revision 1 CONTENTS PREFACE... 3 1. INTRODUCTION... 4 2. LABELLING REQUIREMENTS... 7 2.1. General Guidelines... 7 2.2.
Adipose-Derived Stem Cell Therapy
 22 Adipose-Derived Stem Cell Therapy Damir Hudetz, MD, PhD, orthopaedic surgeon and Head of the Department of Orthopaedics at St. Catherine, Specialty Hospital in Zabok, Croatia, briefly described Stem
22 Adipose-Derived Stem Cell Therapy Damir Hudetz, MD, PhD, orthopaedic surgeon and Head of the Department of Orthopaedics at St. Catherine, Specialty Hospital in Zabok, Croatia, briefly described Stem
Double Syringe System Autologous Conditioned Plasma
 TM Double Syringe System Autologous Conditioned Plasma Autologous Conditioned Plasma Introduction Autologous blood products have created a growing interest for use in a number of orthopaedic therapies.
TM Double Syringe System Autologous Conditioned Plasma Autologous Conditioned Plasma Introduction Autologous blood products have created a growing interest for use in a number of orthopaedic therapies.
1. Executive Summary
 1. Executive Summary 1.1 General The fluoroscope is defined as an instrument used chiefly in industry and in the practice of medicine for observing the internal structure of objects (such as the living
1. Executive Summary 1.1 General The fluoroscope is defined as an instrument used chiefly in industry and in the practice of medicine for observing the internal structure of objects (such as the living
Operating Room Manual
 CHAPTER 6 SUPPLY CHAIN Lead Author: Tangwan Azefor, MD; Clinical Associate, Department of Anesthesiology & Critical Care Medicine; Johns Hopkins Bayview Medical Center Baltimore, MD Checklist 1. How many
CHAPTER 6 SUPPLY CHAIN Lead Author: Tangwan Azefor, MD; Clinical Associate, Department of Anesthesiology & Critical Care Medicine; Johns Hopkins Bayview Medical Center Baltimore, MD Checklist 1. How many
Appendix A CALIFORNIA POLYTECHNIC STATE UNIVERSITY, SAN LUIS OBISPO INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE
 Appendix A CALIFORNIA POLYTECHNIC STATE UNIVERSITY, SAN LUIS OBISPO INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE INSTRUCTIONS FOR: "PROTOCOL FOR ANIMAL USE" FORM NOTE: THIS IS NOT AN ORDER FORM. ORDERS
Appendix A CALIFORNIA POLYTECHNIC STATE UNIVERSITY, SAN LUIS OBISPO INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE INSTRUCTIONS FOR: "PROTOCOL FOR ANIMAL USE" FORM NOTE: THIS IS NOT AN ORDER FORM. ORDERS
Asterand Bioscience Strategic Alliances
 Asterand Bioscience Strategic Alliances Excellence through partnership www.asterandbio.com WHY ASTERAND BIOSCIENCE Asterand Bioscience has a well-established human tissue heritage, having been formed in
Asterand Bioscience Strategic Alliances Excellence through partnership www.asterandbio.com WHY ASTERAND BIOSCIENCE Asterand Bioscience has a well-established human tissue heritage, having been formed in
Paul Ashford Executive Director ICCBBA
 Introduction to ISBT 128 Paul Ashford Executive Director ICCBBA ICCBBA ICCBBA enhances safety for patients by managing the ISBT 128 international information standard for use in transfusion and transplantation.
Introduction to ISBT 128 Paul Ashford Executive Director ICCBBA ICCBBA ICCBBA enhances safety for patients by managing the ISBT 128 international information standard for use in transfusion and transplantation.
Autohemotherapy. Dermoelectroporation. a new science in skin care. The Effectiveness of Biological Therapy with maximum Comfort in total Safety
 a new science in skin care Dermoelectroporation Autohemotherapy The Effectiveness of Biological Therapy with maximum Comfort in total Safety A partnership: & Autohemotherapy Autohemotherapy can be considered
a new science in skin care Dermoelectroporation Autohemotherapy The Effectiveness of Biological Therapy with maximum Comfort in total Safety A partnership: & Autohemotherapy Autohemotherapy can be considered
Therapeutic Products Directorate. Medical Devices Bureau Performance Quarterly Report. Q2-2016/17 July through September
 Therapeutic Products Directorate Medical Devices Bureau Performance Quarterly Report Q2-2016/17 July through September Table of Contents OVERVIEW... 4 General Information... 4 The Application Review Process...
Therapeutic Products Directorate Medical Devices Bureau Performance Quarterly Report Q2-2016/17 July through September Table of Contents OVERVIEW... 4 General Information... 4 The Application Review Process...
Vertebrate Animal Use Protocol for Research or Testing
 AUP #: ORC USE ONLY Vertebrate Animal Use Protocol for Research or Testing Date Received: Instructions and Researcher Certifications (Failure to follow may result in a delay in processing) Complete this
AUP #: ORC USE ONLY Vertebrate Animal Use Protocol for Research or Testing Date Received: Instructions and Researcher Certifications (Failure to follow may result in a delay in processing) Complete this
CAP Accreditation Checklists 2016 Edition
 CAP Accreditation Checklists 2016 Edition The College of American Pathologists (CAP) accreditation checklists contain the CAP accreditation program requirements, developed on more than 50 years of insight
CAP Accreditation Checklists 2016 Edition The College of American Pathologists (CAP) accreditation checklists contain the CAP accreditation program requirements, developed on more than 50 years of insight
Quality Manual. Quality Manual. Vera Bioscience / Anu Life Sciences. April 2018
 Quality Manual Vera Bioscience / Anu Life Sciences April 2018 Page 1 of 15 TABLE OF CONTENTS Quality Manual Page 1. Company Overview 3 2. References 3 3. Exemptions, Alternatives and Variances 3 4. General
Quality Manual Vera Bioscience / Anu Life Sciences April 2018 Page 1 of 15 TABLE OF CONTENTS Quality Manual Page 1. Company Overview 3 2. References 3 3. Exemptions, Alternatives and Variances 3 4. General
Artist Proof: Clotalyst. Autologous Serum Collection System
 Artist Proof: 01-15-10 Clotalyst Autologous Serum Collection System Clotalyst Autologous Serum The kit is designed for the preparation of autologous serum that is to be mixed with PRP and autograft or
Artist Proof: 01-15-10 Clotalyst Autologous Serum Collection System Clotalyst Autologous Serum The kit is designed for the preparation of autologous serum that is to be mixed with PRP and autograft or
PHARMA/MEDICAL DEVICE COMPANIES
 PHARMA/MEDICAL DEVICE COMPANIES Company Information Company Division of Address City State Zip Phone Fax Web Email Company Services Provided please keep description brief (50 words or less) and factual:
PHARMA/MEDICAL DEVICE COMPANIES Company Information Company Division of Address City State Zip Phone Fax Web Email Company Services Provided please keep description brief (50 words or less) and factual:
3. Human Biomedical Research. Defining Human Biomedical Research
 PART B: SECTION III: HUMAN BIOMEDICAL RESEARCH HUMAN BIOMEDICAL RESEARCH 3. Human Biomedical Research Defining Human Biomedical Research 3.1. In this section, we consider what kinds of human biomedical
PART B: SECTION III: HUMAN BIOMEDICAL RESEARCH HUMAN BIOMEDICAL RESEARCH 3. Human Biomedical Research Defining Human Biomedical Research 3.1. In this section, we consider what kinds of human biomedical
Reporting Deviations of Biological Products and HCT/Ps. Ellen Areman Senior Consultant Biologics Consulting Group, Inc.
 Reporting Deviations of Biological Products and HCT/Ps Ellen Areman Senior Consultant Biologics Consulting Group, Inc. Relevant Legislation Public Health Service (PHS) Act Regulates biological products
Reporting Deviations of Biological Products and HCT/Ps Ellen Areman Senior Consultant Biologics Consulting Group, Inc. Relevant Legislation Public Health Service (PHS) Act Regulates biological products
COLLEGE OF CHIROPRACTORS OF ONTARIO GUIDE TO AN APPLICATION FOR A CERTIFICATE OF AUTHORIZATION FOR HEALTH PROFESSION CORPORATIONS
 COLLEGE OF CHIROPRACTORS OF ONTARIO GUIDE TO AN APPLICATION FOR A CERTIFICATE OF AUTHORIZATION FOR HEALTH PROFESSION CORPORATIONS As a result of amendments to the Regulated Health Professions Act (RHPA)
COLLEGE OF CHIROPRACTORS OF ONTARIO GUIDE TO AN APPLICATION FOR A CERTIFICATE OF AUTHORIZATION FOR HEALTH PROFESSION CORPORATIONS As a result of amendments to the Regulated Health Professions Act (RHPA)
Contact details: Ilaria Lucibello
 Assobiotec contribution to the European Commission Consultation Document Good anufacturing Practice for Advanced Therapy edicinal Products Issued 28-Jun-2016 Assobiotec is the Italian Association for the
Assobiotec contribution to the European Commission Consultation Document Good anufacturing Practice for Advanced Therapy edicinal Products Issued 28-Jun-2016 Assobiotec is the Italian Association for the
