RESEARCH ARTICLE Polyethylenimine/DNA complexes shielded by transferrin target gene expression to tumors after systemic application
|
|
|
- Silvia Stewart
- 6 years ago
- Views:
Transcription
1 (2001) 8, Nature Publishing Group All rights reserved /01 $ RESEARCH ARTICLE Polyethylenimine/DNA complexes shielded by transferrin target gene expression to tumors after systemic application R Kircheis, L Wightman, A Schreiber, B Robitza, V Rössler, M Kursa and E Wagner Boehringer Ingelheim Austria, Dr Boehringer Gasse 5 11, A-1121 Vienna, Austria Systemic application of positively charged polycation/dna complexes has been shown to result in predominant gene expression in the lungs. Targeting gene expression to other sites, eg distant tumors, is hampered by nonspecific interactions largely due to the positive surface charge of transfection complexes. In the present study we show that the positive surface charge of PEI (25 kda branched or 22 kda linear)/dna complexes can be efficiently shielded by covalently incorporating transferrin at sufficiently high densities in the complex, resulting in a dramatic decrease in nonspecific interactions, eg with erythrocytes, and decreased gene expression in the lung. Systemic application of transferrinshielded PEI/DNA complexes into A/J mice bearing subcut- aneously growing Neuro2a tumors via the tail vein resulted in preferential (100- to 500-fold higher) luciferase reporter gene expression in distant tumors as compared with the major organs including the lungs. Tumor targeting is also demonstrated by DNA uptake and -galactosidase gene expression in tumor cells. Assessing DNA distribution following systemic application significant amounts of DNA were found in the liver and tumor. However, in the liver, DNA was mainly taken up by Kupffer cells and degraded without significant transgene expression. In the tumor, DNA was associated mainly with tumor cells and frequently found near structures which resemble primitive blood vessels. Gene Therapy (2001) 8, Keywords: gene therapy; tumor targeting; polyethylenimine; PEI; in vivo gene transfer; polyplex Introduction Although there are a variety of vectors available which show effective gene transfer in cell culture, 1 5 efficient and target specific gene delivery in vivo is still the major challenge in gene therapy. In vivo gene delivery faces a variety of additional obstacles such as anatomical size constraints, interactions with biological fluids, extracellular matrix, and binding to a broad variety of nontarget cell types. Transfection complexes have to be soluble, small enough to pass physiological barriers, 6 specific for binding to the target cells, 7 but inert against both body fluids 8,9 and unspecific interactions with tissues and cells, 9,10 such as the reticuloendothelial system (RES) Among the various nonviral vectors available, the polycation polyethylenimine (PEI) is receiving broader attention due to its specific features to combine DNA condensing with an intrinsic endosomolytic activity. 4 PEI has shown high transfection efficacy in vitro as well as in a variety of applications in vivo. 4,14 18 Recent studies indicate that lower molecular weight PEI molecules (22 kda, 25 kda) may be more advantageous compared with the high molecular weight PEI (800 kda), particularly for in vivo application High reporter gene expression was found with complexes using the linear 22 kda PEI in topical 6 and after systemic application with particularly high Correspondence: R Kircheis Received 7 June 2000; accepted 30 September 2000 expression in the lungs However, specific targeting of gene expression to organs other than the lung, eg to distant tumors, seems to be more difficult. 20 In order to achieve target specificity we and others have coupled cell-binding ligands such as transferrin, antibodies and carbohydrates to PEI, combining the intrinsic activities of PEI with receptor-mediated uptake mechanisms. 7,21 23 Transferrin PEI/DNA complexes have shown high activity in a broad variety of applications in vitro 7,24 and promising results in topical application in vivo. 24 Recently, we compared different types of delivery vectors for systemic application in tumor-bearing mice. 25 These studies have demonstrated that the physical and colloidal parameters of transfection complexes such as particle size, stability and surface charge critically influence DNA biodistribution, toxicity and gene transfer efficacy in vivo. 9,25,26 Positively charged PEI800/DNA complexes resulted in preferential expression in the lung, but were also associated with systemic toxicity. 25 Nonspecific interactions with plasma components, erythrocytes and nontarget cells may contribute to lung expression but also to the toxic side-effects observed after systemic application. 10,26 Shielding of the positive surface charge by coating the complexes with polyethylene glycol (PEG) inhibited these nonspecific interactions, resulting in preferential expression in tumor. Furthermore, lung expression and toxicity were dramatically decreased. 25,26 However, the efficacy of reporter gene delivery of PEGylated Tf-PEI(800)/DNA complexes was at least one order of magnitude lower compared with
2 optimized adenovirus enhanced transfection (AVET) complexes. In the present study, we present new formulations of transferrin polyethylenimine (Tf-PEI) complexes with improved efficacy in tumor delivery and gene expression. Low molecular weight PEI molecules (25 kda or 22 kda) and increased densities of transferrin in the complex were used to increase efficacy and decrease nonspecific interaction. We show that low molecular weight PEIs are more favorable for systemic in vivo application than 800 kda PEI. Furthermore, we demonstrate that transferrin can efficiently shield the positive surface charge of the PEI/DNA complexes and protect from nonspecific interactions, resulting in tumor targeted gene delivery after systemic application in vivo. 29 Results Aggregation of erythrocytes by PEI/DNA complexes: influence of PEI molecular weight Previously, we had shown that positively charged PEI800/DNA complexes, where DNA is complexed with PEI of 800 kda molecular weight (PEI800), are prone to a broad variety of nonspecific interactions with plasma components and blood cells. 26 Particularly, aggregation of erythrocytes by positively charged complexes can result in occlusion of capillaries in the lungs which could account for the high toxicity observed after systemic application of PEI800/DNA complexes. 10,25 PEI/DNA complexes using different molecular weight PEI molecules, eg linear 22 kda (PEI22), or branched 25 kda (PEI25), were compared with PEI800 for their ability to aggregate freshly collected, washed murine erythrocytes in vitro. PEI/DNA complexes were prepared at similar polycation (nitrogen) to DNA (phosphate) ratio (N/P = 6), and had similar high positive surface charge of +30 to approximately 35 mv as estimated by zetapotential measurement. After 1 h incubation the aggregation of erythrocytes was evaluated. Strong aggregation of erythrocytes was found with PEI800/DNA complexes, while DNA complexes with low molecular weight PEIs, in particular linear PEI22, showed lower aggregation (Figure 1). A lower erythrocyte aggregation by the low molecular weight PEIs indicates a more favorable feature for systemic application in vivo. Control experiments were performed which showed that small amounts of free PEI as present at moderate N/P ratios 18 after removal of complexes by centrifugation have no major contribution to the erythrocyte aggregation. Free PEI (without DNA) at amounts as used for complexation of DNA resulted in significant erythrocyte aggregation, but were even more pronounced in fusion of the erythrocytes with the plastic surface (data not shown). Shielding of the positive surface charge of PEI/DNA complexes by transferrin In spite of the differences in the degree of erythrocyte aggregation between different PEIs, in all cases the positive charge of the PEI/DNA complexes, a requirement for efficient complexation of the DNA, is also responsible for the nonspecific interactions and lung expression. 10,25 Shielding of the positive surface charge should further inhibit these nonspecific effects. PEI/DNA complexes were generated by mixing DNA Figure 1 Aggregation of erythrocytes by PEI/DNA complexes. Fresh blood was collected from A/J mice, and immediately mixed with 20 l heparin. Washed fresh murine erythrocytes were incubated with different PEI/DNA complexes (N/P = 6) for 1 h at 37 C. Representative pictures of erythrocytes incubated with DNA complexes formed with the PEI800, PEI25, or PEI22 versus control erythrocytes are shown. (pcmvl) with PEI25 at N/P ratio of 4.8 and the zetapotential was measured (Figure 2a). To study the effect of transferrin incorporation into the complex on the surface charge of the PEI25/DNA complexes, the PEI25 was partially or completely replaced by Tf-PEI (transferrin-conjugated PEI25 at a molar ratio Tf:PEI of 1:1) as described in Materials and methods. PEI25/DNA complexes (N/P = 4.8) have a high positive surface charge with a zeta-potential of approximately +35 mv. Incorporation of transferrin even at low concentrations (1 part Tf-PEI plus 9 parts PEI25, ie (1:10)) into complexes significantly decreased the zeta-potential. Complexes where PEI was completely replaced by Tf-PEI had a zeta-potential of zero or slightly negative, however, transfection efficacy was low (data not shown). When transfection efficiency and zeta-potential were correlated, a ratio of 1 part Tf- PEI plus 3 parts PEI25 was deemed to be optimal for in vivo application (high transfection efficency combined with a low zeta-potential); in all further studies this ratio Tf-PEI25/PEI25 (1 + 3) was used. Figure 2b shows the zeta-potential of Tf- PEI/PEI/DNA complexes and PEI/DNA complexes over a range of N/P ratios. PEI/DNA complexes with either branched PEI25 or linear PEI22 are shown. Both PEI25/DNA and PEI22/DNA complexes have a high positive zeta-potential. In contrast, replacement of one quarter of the PEI by Tf-PEI significantly decreased the zeta-potential at all N/P ratios tested. At N/P = 4.8, both Tf-PEI/PEI25/DNA and Tf-PEI/PEI22/DNA complexes had nearly neutral zeta-potentials. The optimal Tf- PEI/PEI25/DNA complexes were tested for erythrocyte aggregation in comparison with the positive surface charged PEI25/DNA complexes. PEI25/DNA complexes (N/P = 4.8, zeta-potential +35 mv) induced a pronounced aggregation of erythrocytes (Figure 2c, center). In contrast, incorporation of Tf-PEI into complexes (similar N/P = 4.8, but low zeta-potential +7 mv) significantly decreased erythrocyte aggregation (Figure 2c, left).
3 30 c Figure 2 Shielding of positive surface charge of PEI/DNA complexes by transferrin. (a) PEI25/DNA complexes were generated at N/P = 4.8 and the zeta-potential (mv) was measured. To study the effect of transferrin on the surface charge of the DNA complex PEI25 was partially or completely replaced by Tf-PEI. (b) The zeta-potential of Tf-PEI/PEI/DNA complexes (1 part Tf-PEI and 3 parts PEI) and PEI/DNA complexes was measured over a range of N/P ratios. PEI/DNA complexes with either branched PEI25 or linear PEI22 were tested. (c) Incorporation of transferrin decreases aggregation of erythrocytes. Washed fresh murine erythrocytes were incubated with PEI25/DNA or Tf-PEI/PEI25/DNA complexes (N/P = 4.8) for 1 h at 37 C. Untreated erythrocytes are shown for control. Gene delivery after systemic application PEI/DNA (pcmvl) complexes, coding for the luciferase reporter gene, with or without Tf-PEI were tested for systemic application in tumor-bearing mice. One million Neuro 2a cells were set subcutaneously in syngeneic A/J mice; after approximately 2 weeks well vascularised tumors had developed. Transfection complexes were injected systemically into mice bearing subcutaneously growing Neuro2a tumors via the tail vein. For assessment of gene delivery luciferase activity was measured in the various organs and tumor 24 h after injection of transfection complexes (Figure 3). Compared with the 800 kda PEI, which shows significant toxicity at all N/P ratios tested (see also Ref. 25), the low molecular weight (25 kda and 22 kda) PEI-based transfection complexes were generally well tolerated after systemic application at N/P ratios of 4 9. At higher N/P ratios, toxicity could be found, particularly with PEI25/DNA complexes (data not shown). Application of PEI/DNA complexes without transferrin resulted in a nonspecific reporter gene expression pattern with significant gene expression observed in the lung and tumor, and varying levels in the other various organs (Figure 3a c). In contrast, Tf-PEI/PEI/DNA complexes targeted gene expression to the tumor with expression levels two log units higher than in major organs including the lungs. Besides targeting to the tumor, the absolute expression levels in tumors were also approximately one log unit higher with Tf-containing complexes as compared with Tf-free complexes (Figure 3e and f). Transferrin polylysine/dna complexes applied for comparison showed only marginal activity with some low expression in the liver and marginal expression in the tumor (Figure 3g). Other control animals were injected with naked DNA (pcmvl) or with a recombinant baculovirus coding for the luciferase gene under control of a CMV promotor. With naked DNA, no significant gene expression was found at any site apart from the injection site (Figure 3d, see also Ref. 25). It should be noted that injections were performed slowly and without higher pressure, in contrast to a recently published experimental protocol which used hydrodynamic pressure and showed significant gene delivery to the liver and other organs. 27,28 Recombinant luciferase-baculovirus led to significant expression in a variety of major organs with highest expression in the liver, but only very low expression in the tumor (Figure 3h). Comparison of the gene expression data with the physical parameters of the transfection complexes suggests a correlation between the positive zeta-potential and predominant lung expression, while expression in tumors correlates with a low or nearly neutral surface charge. The negligible tumor expression with TfpLys/ DNA complexes compared with Tf-PEI/PEI/DNA complexes obviously reflects the much lower transfection efficacy of polylysine compared with PEI mainly due to the poor endosomal release with polylysine. Complexes shown in Figure 3 were prepared at 75 mm NaCl (0.5 HBS). At physiological salt concentration, particularly PEI22 is known to form bigger particles or aggregates. 6 This aggregation was also observed for the 0.5 HBS ionic strength used in the present study. Particle size decreases when complexes are prepared at low ionic strength. 6,26 However, previously we had found that PEI800/DNA complexes prepared at low ionic strength
4 31 Figure 3 Gene expression after systemic gene delivery into tumor-bearing mice. Transfection complexes with the pcmvl plasmid were prepared in 75 mm NaCl, 20 mm Hepes at a DNA concentration of 200 g/ml, and iso-osmolarity was adjusted by addition of 2.5% glucose (final). Transfection complexes (50 g per mouse) were injected into the tail vein of Neuro2a tumor-bearing A/J mice. Transgene expression at 24 h after application was measured by luciferase assay. Luciferase activity represents mean ± s.e.m., n = 9 (b, c, e, f), 6 (d), 4 (a), 3 (g, h). One million relative light units (RLU) correspond to 2 ng luciferase. Luciferase background levels of untreated animals are below 200 RLU per organ (not shown). Note, there was significant toxicity in (a) with two of four animals dead. There was no toxicity in the other groups. (a) PEI800/DNA (N/P = 6), (b) PEI25/DNA (N/P = 4.8), (c) PEI22/DNA (N/P = 4.8), (d) naked DNA, (e) Tf-PEI/PEI25/DNA (N/P = 4.8), (f) Tf-PEI/PEI22/DNA (N/P = 4.8), (g) Tf-pLys/DNA or (h) recombinant baculovirus expressing luciferase (10 10 particles/0.2 ml).
5 32 (Hepes buffered 5% glucose) have a significantly lower transfection efficacy in vitro and in vivo compared with complexes formed at physiological salt concentrations (HBS, Hepes-buffered saline). 9,26 On the other hand, Goula and colleagues 6,17 had shown high transfection efficacy in the lung with PEI22/DNA complexes formed at low ionic strength. Therefore, we compared here the efficacy of transfection complexes prepared at 75 mm NaCl with complexes prepared at low ionic strength (Hepes or water with 5% glucose) for systemic in vivo application. The gene expression pattern indicated tumor targeting for all Tf-containing complexes, however, differences were found between PEI25-based complexes and PEI22-based complexes. While the transfection efficacy of Tf-PEI/PEI25/DNA complexes was decreased more than 10-fold when the complexes were formed at low ionic strength (data not shown), Tf-PEI/PEI22/DNA complexes almost retained their activity (Figure 4b, compare with Figure 3f). The efficacy of transferrin-free PEI22/DNA complexes was strongly increased when formed at low ionic strength, however, with high lung expression which is in accordance with published data. 6,17,18 The high expression in the lung with PEI22/DNA complexes formed at low ionic strength obviously correlates with a more in vivo compatible par- ticle size of a few hundred nanometers compared with the bigger aggregates formed at higher ionic strength. 6,19 Next, complexes formed at N/P = 4.8 were compared with complexes formed at higher N/P ratios. Increasing N/P ratio dramatically increased the overall transfection activity of transferrin-free PEI22/DNA complexes in all major organs, however, this increase was not observed in the tumor (Figure 4a and c). Luciferase expression in the lung was approximately two log units higher as compared with other organs. In contrast, incorporation of transferrin retained a low zeta-potential even at high N/P ratios, resulting in predominant tumor targeted gene expression (Figure 4b and d). These data give further support to the notion that gene expression in the major organs including lung correlates with excess of positive charge while targeting to the distant tumor correlates with a low or neutral charge. The dependency of gene expression in tumors on the applied amount of DNA complex was investigated (Figure 5a). A clear correlation between the amount of DNA and the level of expression up to a dose of 50 g DNA was observed. Increasing the DNA dose above 75 g per application, however, did not further increase the levels of luciferase gene expression in the tumors. The kinetics of luciferase reporter gene expression was Figure 4 Transfection complexes formed at low ionic strength. Effect of the N/P ratio. Transfection complexes with the pcmvl plasmid were prepared in 20 mm Hepes at a DNA concentration of 200 g/ml and iso-osmolarity was adjusted by addition of 5% glucose (final). Transfection complexes (50 g per mouse) were injected into the tail vein of Neuro2a tumor-bearing A/J mice. Transgene expression at 24 h after application was measured by luciferase assay. Luciferase activity represents mean ± s.e.m., n = 6 (a,b), 3 (c,d). Luciferase background levels of untreated animals are below 200 RLU per organ. (a) PEI22/DNA (N/P = 4.8), (b) Tf-PEI/PEI22/DNA (N/P = 4.8), (c) PEI22/DNA (N/P = 7.2), (d) Tf-PEI/PEI22/DNA (N/P = 7.2).
6 33 Figure 6 -galactosidase expression in the tumor. Tf-PEI/PEI25/DNA (pcmv ) transfection complexes (N/P = 4.8, prepared at 75 mm NaCl, 20 mm Hepes, 2.5% glucose, 50 g per mouse) were injected into the tail vein of Neuro2a tumor-bearing A/J mice. Transgene expression at 24 h after application was visualized by X-gal staining and evaluated under microscope, 100 magnification. tumor, Tf-PEI/PEI25/DNA (pcmv ) complexes coding for the -galactosidase reporter gene were injected into tumor-bearing mice. Gene expression was found to be distributed over large regions of the tumor, but in a heterogeneous pattern with focal areas showing strong transgene expression (visualized after short X-gal exposure) and broad areas without any visible gene expression. Transfected cells were often found near to lacuna-like structures which resemble immature vessels (Figure 6). No blue staining was detected in any of the control animals injected with similar transfection complexes using an expression plasmid coding for the luciferase gene. Figure 5 (a) Dependency of gene expression in the tumor on the DNA dose. Tf-PEI/PEI25/DNA (pcmvl) transfection complexes (N/P = 4.8, prepared at 75 mm NaCl, 20 mm Hepes, 2.5% glucose) were injected into the tail vein of Neuro2a tumor-bearing A/J mice. Transgene expression in the tumor at 24 h after application was measured by luciferase assay. Luciferase activity represents mean ± s.e.m., n 3. (b, c) Kinetics of gene expression in the tumor after systemic application. Tf-PEI/PEI25/DNA (pcmvl) transfection complexes (N/P = 4.8, prepared at 75 mm NaCl, 20 mm Hepes, 2.5% glucose, 50 g DNA per mouse) were injected into the tail vein of Neuro2a tumor-bearing A/J mice once (b) or twice at the following days (day 0 and day 1) (c). Transgene expression at indicated time-points was measured by luciferase assay. Luciferase activity represents mean ± s.e.m., n = 3. evaluated after single or repeated application (Figure 5b and c). Expression after single application is transient and declined after a few days (Figure 5b). However, repeated application at day 0 and day 1 resulted in higher luciferase expression levels, and significant luciferase activity was detectable 1 week after application (Figure 5c). For histological assessment of gene expression in the DNA distribution after systemic application DNA biodistribution of Tf-PEI/PEI/DNA (pcmvl) transfection complexes was studied by Southern blot analysis hybridizing with a pcmvl-specific probe as described in Materials and methods. 25 Using this technique intact DNA can be easily distinguished from partially degraded DNA. Figure 7a and b shows the DNA distribution 4 h after application of transferrin-shielded complexes (b) or unshielded complexes (a) analyzed in the major organs and tumor. Highest amounts of (total, intact and partially degraded) DNA were detected in the liver in both cases. However, with transferrin-shielded complexes significant amounts of DNA were detected also in the tumor. A major part of the total DNA was found to be already degraded 4 h after application, eg with more than 95% of the total DNA in the liver quantified being partially degraded. Estimation of intact DNA (Figure 7c and d) revealed major differences in the DNA biodistribution between unshielded and transferrin-shielded complexes. With unshielded complexes the highest amounts were found in the liver. Significant amounts were also found in the lungs, spleen, and some small amounts in the tumor (Figure 7c). In contrast, with transferrin-shielded complexes highest amounts of intact DNA were detected in the tumor followed by the liver (Figure 7d). In other organs such as lungs, spleen and kidney only small amounts of DNA were detected. For comparison of DNA delivery and transgene expression animals were analyzed for luciferase reporter gene expression 24 h after application (Figure 7e and f). A good correlation between DNA biodistribution pattern and gene expression pattern was found particularly for
7 34 Figure 7 Biodistribution of plasmid DNA and reporter gene expression after systemic gene delivery. PEI25/DNA (pcmvl) complexes (a, c, e) or Tf- PEI/PEI25/DNA complexes (b, d, f) (both N/P = 4.8, prepared at 75 mm NaCl, 20 mm Hepes, 2.5% glucose, 50 gdna per mouse) were injected into the tail vein of Neuro2a tumor-bearing A/J mice. (a-d) DNA biodistribution at 4 h after injection was studied by Southern blot hybridization with a probe specific for pcmvl as described in Materials and methods. The amounts of total DNA (a, b) and intact DNA (c, d) were determined. Black bars, intact DNA; grey bars, partially degraded DNA. (e, f) Transgene expression at 24 h after application was measured by luciferase assay. Data represent mean ± s.e.m., n = 3. tumor and lung. A six-fold higher accumulation of intact DNA (or more than 10-fold higher accumulation of total DNA) in the tumor after application of transferrinshielded complexes correlated with a more than 20-fold higher gene expression compared with unshielded complexes. In contrast, a five-fold decrease in intact DNA accumulation in the lung with transferrin-shielded complexes correlated with a more than 30-fold lower gene expression in the lung compared with unshielded complexes. Different from organs such as tumor or lungs, in the liver a significant DNA accumulation with both unshielded as well as shielded complexes did not result in high gene expression. The liver is well known for its central role in removal of foreign particles from the circulation 13 mainly by the Kupffer cells. We followed the fate of the transfection complexes in the liver after systemic application using DNA labeled with rhodamine-pna clamp, 29 which is commercially available as pgenegrip. Figure 8a shows histological fluorescence images of the liver 4 h after systemic application of transfection complexes. Significant uptake of rhodamine-labeled DNA was found in large areas of the liver lobes (Figure 8a). Uptake of DNA was associated with single cells scattered throughout the
8 a c a b 35 d c f b e g f d e Figure 8 Uptake of plasmid DNA in the liver after systemic application visualized by immunofluorescence staining. Tf-PEI/PEI25/DNA transfection complexes containing rhodamine-labeled plasmid DNA (pgenegrip) (N/P = 4.8, prepared at 75 mm NaCl, 20 mm Hepes, 2.5% glucose, 50 gdna per mouse) were injected into the tail vein of Neuro2a tumorbearing A/J mice. DNA distribution in the tissue can be followed by the red fluorescence of pgenegrip. Cell nuclei are visualized by DAPI staining (b, f). Endothelial cells were stained using anti-cd31 antibody (FITClabeled, a, b). Kupffer cells were stained by F4/80 antibody (FITC-labeled, d f). Slides were evaluated under a Zeiss Axioskop II fluorescence microscope equipped with a Hamamatsu CCD camera. (a, b) 100 original magnification, (c f) 630 original magnification. tissue (compare DAPI staining of cell nuclei, Figure 8b). There was no obvious association between DNA uptake and endothelial cells as assessed by anti-cd31 staining. Staining with an F4/80 antibody (FITC-labeled), which is specific for macrophage-like cells, indicated an almost exclusive uptake of DNA by Kupffer cells while no uptake was found in the surrounding hepatocytes (cells visualized by DAPI staining of the nuclei (Figure 8c f). The histological data together with the DNA distribution and luciferase gene expression data (Figure 7) indicate that transfection complexes are taken up mainly by Kupffer cells in the liver and rapidly degraded without significant transgene expression. Histological distribution of gene expression in the tumor The distribution of transfection complexes in the tumor 4 h after administration was visualized using rhodaminelabeled DNA (pgenegrip). In contrast to the DNA distribution in the liver, uptake of DNA in the tumor was much more heterogeneous with focal areas of DNA uptake and large areas of the tumor without significant uptake (Figure 9a). Within these foci of uptake, the DNA was associated with the majority of the cells. Most of the cells were attributed as tumor cells as determined by cell morphology and negative staining with the macrophagespecific F4/80 antibody (Figure 9b). However, not all DNA was associated with tumor cells, some was also found associated with extracellular matrix (Figure 9f). Figure 9 Uptake of plasmid DNA in the tumor after systemic application visualized by immunofluorescence staining. Tf-PEI/PEI25/DNA transfection complexes containing rhodamine-labeled plasmid DNA (pgenegrip) (N/P = 4.8, prepared at 75 mm NaCl, 20 mm Hepes, 2.5% glucose, 50 gdna per mouse) were injected into the tail vein of Neuro2a tumorbearing A/J mice. DNA distribution in the tissue can be followed by the red fluorescence of pgenegrip. Cell nuclei are visualized by DAPI staining (all, except c). Endothelial cells were stained using anti-cd31 antibody (FITC-labeled, e). Slides were evaluated under a Zeiss Axioskop II fluorescence microscope equipped with a Hamamatsu CCD camera. (a) 100 original magnification, (c, d, e) 200 original magnification, (b, f) 400 original magnification, (g) 630 original magnification. DNA uptake in tumor cells was often found near to structures which may represent primitive blood vessels, and occasionally, labeled DNA was found associated with endothelial cells as characterized by immunofluorescence staining with an antibody against the endothelial cell marker CD31 (Figure 9c e). Discussion The physical and colloidal parameters of transfection complexes, such as particle size, charge and stability are critical factors which determine DNA biodistribution and expression profile in vivo. One has to consider them as the basis for the rational design of gene delivery vectors for in vivo application. 13,25,26,30 35 Unprotected naked DNA is obviously rapidly degraded in the blood circulation. Recently, a specific experimental application protocol has been reported which uses rapid injection of very large volumes of plasmid DNA solutions into the tail vein of mice associated with a transient cardiac congestion and hydrodynamic pressure of the DNA solution into the organs connected to the vena cava inferior and resulting in high gene expression in organs such as the liver. 27,28 This specific experimental application protocol may be applicable for
9 36 certain regional application, but obviously is not practical for systemic application in big animals or humans. Under normal application, without hydrodynamic pressure, as used in the present study, the DNA has to be protected, eg by condensation with cationic lipids or polymers to survive in the blood circulation. Since condensation of the DNA is usually achieved at an excess of the polycation, transfection complexes will have a positive surface charge. The positive surface charge is also essential for uptake of the complexes into the cells unless cell-binding ligands are present. The requirement for an excess of positive charge poses a major problem for systemic application, because positively charged complexes are prone to interact nonspecifically with a variety of components in the blood, other body fluids, extracellular matrix and nontarget cells. These nonspecific interactions result in destabilization of the complexes, their removal and degradation, or nonspecific uptake with undesired effects. The observed aggregation of erythrocytes clearly illustrates the potential of these nonspecific interactions in causing toxic side-effects. Aggregation of erythrocytes can result in occlusion of capillaries and may be one of the leading pathogenetic mechanisms for lung embolism previously observed after application of PEI800/DNA complexes. 10,25 The causal correlation between erythrocyte aggregation and systemic toxicity is supported by the fact that increasing N/P ratios of the polycation/dna complex is also associated with increased erythrocyte aggregation and increasing toxicity. This correlation was found with PEIs of different polymer sizes. Beside the dependency on the N/P ratio there was, however, also a dependency on the type of PEI molecule used. PEI800 showed the most pronounced erythrocyte aggregation and is by far the most toxic for systemic application. DNA complexes with the lower molecular weight PEIs were generally well tolerated at N/P ratios which guarantee colloidal stability (N/P = 4 9), and toxicity was found only at higher N/P ratios. These data, together with the erythrocyte aggregation, also support the notion that the lower molecular weight PEIs are generally more favorable for in vivo use as compared with the high molecular weight PEI800. 6,15,17,18 It is interesting to note the different behavior of DNA complexes with linear PEI22 and branched PEI25 at different salt concentrations. PEI25-based complexes formed at low ionic strength had significantly lower activity than complexes formed at higher ionic strength, a similar observation as reported previously for PEI800/DNA complexes. 9,26 In contrast, PEI22/DNA complexes had high activity when also prepared at low ionic strength. Moreover, formation at low ionic strength even seems to be a prerequisite for the high expression in the lungs of unmodified PEI22 complexes Following our previous studies with PEGylated Tf- PEI800/DNA complexes, we show that shielding of the positive surface charge of PEI/DNA complexes is crucial for bypassing so-called, first pass organs such as the lungs, and to target gene delivery specifically to distant tumors irrespective of which PEI species is used. This can be achieved by the incorporation of transferrin a highly hydrophilic negatively charged protein which is abundant in serum at sufficient densities in the complex. As has been repeatedly shown for lipoplexes and polyplexes, the positive surface charge leads to an expression pattern with highest expression in the lungs and lower expression in other major organs including heart, liver and kidney. 30,31,35 37 In particular, unmodified PEI/DNA complexes with the linear 22 kda PEI have shown impressive lung expression, which was also observed in this study. Incorporation of transferrin at sufficient densities significantly decreased the surface charge to a nearly neutral zeta-potential and resulted in targeted gene delivery to subcutaneously growing tumors. Similarly, Xu and colleagues 38 have reported targeting of DNA containing liposomes to distant tumors by noncovalent coating with transferrin. However, sufficient stability of the binding of transferrin to the complex seems to be important in the case of PEI/DNA complexes since coating of preformed positively charged PEI/DNA complexes by just addition of free transferrin reduced the zeta-potential as well as lung expression, however, it did not result in tumor-specific delivery in our tumor model (RK, unpublished data). The particle size of complexes is another parameter which can be crucial for targeting to the tumor. To get transfection complexes through the circulation and to pass the spleen and liver organs which are specialized in filtration and removal of foreign particles a small and stable particle size is obviously a prerequisite. However, to make the most advantage of the differences in fenestration and leakiness of tumor vasculature in comparison with normal vasculature, obviously, particle sizes which are bigger than the fenestration of normal vasculature (eg in the liver fenestration of approximately 100 nm can be assumed) can favor selective accumulation of transfection complexes in the tumor tissue compared with normal tissues. After the tumor has been reached a higher uptake by proliferating cells, 42 as well as active targeting of the transferrin receptor resulting in receptormediated endocytosis, 3,7 may contribute to a predominant gene expression in the tumor cells. When following the biodistribution of the DNA complexes, a major part of the DNA was found in the liver, however, most of the DNA was already degraded 4 h after application. Histological data using rhodaminelabeled DNA showed a significant uptake of DNA by Kupffer cells while virtually no uptake was observed in hepatocytes. The histological data correlate with the very low luciferase reporter gene expression found in the liver. DNA uptake by Kupffer cells was homogeneously distributed over whole liver lobes, illustrating again the function of the liver for filtration and degradation of foreign particles, while uptake of DNA in the tumor was very heterogeneous. The focal areas of labeled DNA in the tumor can be correlated with the focal -galactosidase reporter gene expression. Histology of these areas often showed lacuna-like structures which, in some cases, resembled primitive blood vessels indicating areas where transfection complexes can more easily leave the blood circulation and penetrate into the tumor. The intensive staining for -galactosidase expression (found with short X-gal exposure times) demonstrates considerable transgene expression in these areas. In comparison with the transfected regions, large areas of the tumor without DNA uptake and without gene expression indicate that the distribution through the tissue and efficacy of gene transfer need further optimization. In the present study, DNA uptake and gene expression in the tumor were found mainly in tumor cells, with occasional DNA uptake also by endothelial cells, differ-
10 ent to studies with lipoplexes, which showed DNA uptake primarily by endothelial cells and virtually no uptake by tumor cells. 43 In conclusion, we show that incorporation of transferrin can efficiently shield the positive surface charge of polyplexes. Shielding of the surface charge is essential for targeting gene delivery and expression to distant targets, ie tumors, which unlike first pass organs such as the lungs cannot be reached directly following systemic intravenous application. Using transferrin-modified PEIs of low molecular weight more than 10-fold higher efficacy of gene expression in the tumor was found compared with PEGylated Tf-PEI800 complexes described previously. 25,26 Beside the higher transfection efficacy, low molecular weight PEIs were also less toxic than high molecular weight PEI800 which seems to correlate with the lower erythrocyte aggregation observed. Assessing DNA distribution in the tumor, DNA was found mainly near to structures which resemble primitive blood vessels. As also demonstrated here, distribution of DNA and gene expression are very heterogenous and large amounts of DNA were degraded by the RES. Further improvement in the formulation aspects in combination with additional levels of specificity such as controllable transcription/translation 44,45 and improvement of the intracellular steps of the gene transfer such as nuclear uptake 46,47 should finally result in therapeutically useful vector systems. Materials and methods Chemicals and expression vectors Polyethylenimine (PEI), branched, molecular weight 25 kda and 800 kda, were obtained from Aldrich (Milwaukee, WI, USA) and Fluka (Buchs, Switzerland) (50% w/v), respectively. Linear PEI (22 kda) was purchased from Euromedex (Soufferlweyersheim, France) or more recently, from MBI Fermentas (St Leon-Rot, Germany). Human transferrin (iron-free) was obtained from Biotest (Dreieich, Germany). Transferrin-PEI (Tf-PEI, 25 kda) conjugate synthesis: This was performed similarly as described previously for Tf-PEI (800 kda) conjugates 7 with some modifications. A solution of PEI (25 kda) as HCl salt in water was subjected to gel filtration on a Sephadex G-25 superfine (Pharmacia, Uppsala, Sweden) column. Liquid chromatography was performed with a Merck-Hitachi L-6220 pump, a L-4500A UV-VIS detector. The content of PEI in the fractions was determined by ninhydrin assay and measured spectrophotometrically at 570 nm. The amount of transferrin was determined by absorption measurement at 280 nm. A solution of transferrin in 30 mm sodium acetate buffer (ph 5) was subjected to gel filtration on a Sephadex G-25 superfine (Pharmacia) column. The resulting solution was cooled to 0 C, and three equivalents of sodium periodate in 30 mm sodium acetate buffer (ph 5) were added. The mixture was kept on ice, in the dark, for 90 min. For removal of the low molecular weight products an additional gel filtration (Sephadex G- 25 superfine, 30 mm sodium acetate buffer ph 5) was performed; this yielded a solution containing oxidized transferrin (monitoring: UV absorbtion at 280 nm). The modified transferrin solution (30 mm sodium acetate ph 5) was promptly added (within 15 min) to the PEI (25) solution (0.25 m sodium chloride) at a molar ratio of 1/1.2 and vigorously mixed at room temperature. The ph of the mixed solutions was adjusted to 7.3 by the addition of 2 m Hepes ph 7.9. After 30 min, four portions of sodium cyanoborohydride (1 mg per 10 mg transferrin) were added at 1 h intervals. After 19 h, the salt concentration was adjusted to 0.5 m by addition of 3 m sodium chloride. The reaction mixture was loaded on to a cation-exchange column (Bio-Rad (Hercules, CA, USA) Macro-Prep high S HR 10/10) and fractionated with a salt gradient from 0.5 m to 3.0 m sodium chloride (with a constant content of 20 mm Hepes ph 7.3). The major amount of conjugate eluted between 2.0 m and 3.0 m salt. After dialysis against 2 l of HBS ph 7.3 (150 mm sodium chloride, 20 mm Hepes ph 7.3), the conjugate (designated as Tf-PEI) was obtained with a molar ratio of transferrin/pei = 1/ Samples were diluted to 1 mg/ml PEI concentration by addition of HBS ph 7.3. Iron was incorporated by addition of 1.25 l 10 mm iron (III) citrate buffer (containing 200 mm citrate and adjusted to ph 7.8 by sodium bicarbonate addition) per milligram transferrin. The conjugates were aliquoted, shock-frozen in liquid nitrogen and kept at 80 C. Plasmids: The plasmids pcmvl coding for the Photinus pyralis luciferase gene and pcmv coding for -galactosidase, were purified using Endofree Plasmid Giga kit (Qiagen, Hilden, Germany). Endotoxin (LPS) levels were 0.1 endotoxin units/50 g DNA as determined by Limulus Amebocyte Lysate assay (BioWhittaker, Walkersville, MD, USA). Rhodamine-labeled DNA coding for GFP (pgenegrip) was purchased from Systems (San Diego, CA, USA). A recombinant baculovirus expressing the luciferase reporter gene under the control of a CMV promotor similar to that described by Hofmann et al 48 was a gift of Dr Tamas Schweighoffer, Boehringer Ingelheim Austria. The detailed construction of this recombinant baculovirus will be described elsewhere (Schweighoffer et al, manuscript in preparation). Cells and animals Murine Neuro2A neuroblastoma cells (ATCC (Rockville, MD, USA) CCL 131) were cultured in RPMI 1640 medium/10% FCS. Syngeneic A/J mice (7 8 weeks, female) were purchased from Harlan (Bicester, UK). Preparation of transfection complexes PEI/DNA complexes: These were prepared by flash-mixing of indicated amounts of plasmid DNA with PEI at indicated N/P ratios (N/P = molar ratio of PEI nitrogen to DNA phosphate, eg N/P = 4.8: 32 g PEI per 50 g DNA). Transferrin-PEI/DNA complexes: These were prepared similarly with the exception that PEI was partially replaced by the Tf-PEI conjugate, eg [1 + 3], N/P = 4.8 were formed by flash-mixing of a mixture of 8 g Tf-PEI and 24 g PEI (25 kda) with 50 g DNA. Unless indicated, complexes were prepared at DNA concentration of 200 g/ml in 0.5 HBS (ie 75 mm NaCl, 20 mm Hepes ph 7.4). To ensure iso-osmolarity, glucose (from a 50% stock solution) was added to a final concen- 37
11 38 tration of 2.5% (w/v). To generate small particles complexes were formed in water (or 20 mm Hepes, ph 7.3), and 5% (w/w) glucose was added. Systemic application of transfection complexes in tumorbearing mice A/J mice were injected subcutaneously with Neuro2a cells. After 2 weeks, when tumors had reached approximately mm in size, transfection complexes were applied. Transfection complexes (250 l per mouse) were injected slowly without pressure into the tail vein. At indicated time-points after injection animals were killed and the indicated tissues were resected, frozen in liquid nitrogen, and stored at 80 C. Determination of DNA biodistribution after systemic application Southern blot technique: This was performed as described previously 25 with minor modifications. Four hours after injection animals were killed and organs were resected and homogenized in 250 mm Tris-buffer, ph 7.5, using an IKA-homogenizer, frozen in liquid nitrogen, and stored at 80 C. Isolation of DNA was performed according to the QIAamp Tissue Kit protocol (Qiagen, Cat. No ). Southern blots were hybridized with a probe generated from plasmid pcmvl and washed as described in the DIG Labeling and Detection Kit (Boehringer Mannheim, Mannheim, Germany, Cat. No ). Immunological detection was done with Vistra ECF Substrate (Amersham RPN5785, Bucks, UK) that can be detected in a Phosphor Imager (Molecular Dynamics, Wayzata, MN, USA). Histological examination of DNA distribution: For immunohistological examination of DNA distribution animals were injected with transfection complexes containing 50 g pgenegrip vectors (rhodamine-labeled DNA coding for GFP). Four hours after injection animals were killed and the tumors were resected and snap-frozen in liquid nitrogen. Cryosections (thickness 6 7 m) were prepared by means of a microtom (at 20 C). DNA distribution in the tissues was visualized under a Zeiss Axioskop II fluorescence microscope (Zeiss, Jena, Germany) equipped with a Hamamatsu CCD camera (Hamamatsu, Phototonics, Hamamatsu City, Japan). Determination of reporter gene expression after systemic application Luciferase reporter gene expression assay: For examination of luciferase reporter gene expression animals were injected with transfection complexes with the pcmvl plasmid coding for luciferase. Animals were killed at indicated time-points by cervical dislocation. Organs were resected and homogenized in 250 mm Tris-buffer, ph 7.5, using an IKA-homogenizer, frozen in liquid nitrogen, and stored at 80 C. Luciferase activity in the tissue lysate was measured using a Lumat LB9507 instrument (Berthold, Bad Wildbad, Germany) as described previously. 25 Luciferase background ( RLU) was subtracted from each value and transfection efficacy is expressed as RLU per organ. One million RLU correspond approximately to 2 ng luciferase. Protein assay: To provide the possibility of normalizing luciferase expression to the average weight of the organs, the protein content of the organs was estimated using standard protein assay. The protein contents of the various organs are: liver, 120 mg; tumor, 51 mg; kidneys, 31 mg; lungs, 26 mg; heart, 17 mg; and spleen, 13 mg. Histological examination of -galactosidase gene expression: For immunohistological examination of gene expression animals were injected with transfection complexes containing 50 g pcmv plasmid coding for galactosidase, or for control, with similar complexes containing 50 g pcmvl plasmid coding for luciferase. Twenty-four hours after injection tumors were resected and snap-frozen in liquid nitrogen. Cryosections (thickness 6 7 m) were prepared by means of a microtom (at 20 C). Expression for -galactosidase reporter gene was estimated by X-gal staining as described. 25 Briefly, microslides were incubated for 4 h with X-gal substrate (0.2% of 5-bromo-4-chloro-3-indolyl- -d-galactopyranoside; Sigma, St Louis, MO, USA) at 37 C, washed, counterstained with eosin, and embedded in DAKO (Carpinteria, CA, USA) Faramount mounting medium. Immunofluorescence staining For immunofluorescence staining microslides were blocked with PBS/1% BSA for 10 min, and 10% goat serum for 15 min. For specific staining of macrophages slides were incubated with the primary antibody: antimouse macrophage F4/80 (rat), Serotec (Oxford, UK); clone: Cl:A3 1 (F4/80), diluted 1:50, and incubated for 1 h. After washing three times, slides were incubated with the secondary antibodies: F(ab ) 2 goat anti-rat IgG, FITC-labeled, Serotec diluted 1:50 for 30 min. For specific staining of endothelial cells slides were incubated with an anti-murine CD31 (rat) mab-fitc-labeled, Pharmingen, (San Diego, CA, USA) diluted 1:50, and incubated for 1 h. Slides were washed three times, mounted with DAKO fluorescent mounting medium, and evaluated under a Zeiss Axioskop II fluorescence microscope equipped with a Hamamatsu CCD camera. For visualization of cell nuclei slides were stained with DAPI (Boehringer Mannheim, 1 g/ml in methanol). Measurement of particle size and zeta-potential Particle size of transfection complexes was measured by laser-light scattering using a Malvern Zetasizer 3000 (Malvern Instruments, Malvern, Worcs, UK) as described previously. 9,26 The presented data are means of several measurements (n 3), with each measurement averaging the data of 10 subruns. For estimation of the surface charge transfection complexes were diluted in 10 mm NaCl and the zeta-potential was measured using a Malvern Zetasizer The data represent the means of at least three measurements. Erythrocyte aggregation assay Fresh blood from A/J mice was collected and immediately mixed with 20 l heparin. Erythrocytes were washed three times, and suspended in Ringer s solution. Washed erythrocyte suspension (1 ml) was mixed with the indicated amount of PEI/DNA or Tf-PEI/PEI/DNA complexes (17 g DNA) and incubated for 1 h at 37 C in six-well plates (Nunc, Rochester, NY, USA); erythrocyte
12 aggregation was evaluated under a microscope. To distinguish whether aggregation of erythrocytes with PEI/DNA complexes is due to the positively charged complexes or to free PEI, 18 in control experiments the complexes were removed by centrifugation (Eppendorf centrifuge, U/min, 20 min) and the supernatant containing free PEI was added to the erythrocytes. Removal of the PEI/DNA complexes was confirmed by measuring DNA concentration before and after centrifugation. Acknowledgements We thank Dr Tamas Schweighoffer, Boehringer Ingelheim Austria, GmbH, for the gift of the recombinant luciferase-expressing baculovirus. This work was supported by a European Community grant. References 1 Behr JP. Gene transfer with synthetic cationic amphiphiles: prospects for gene therapy (review). Bioconj Chem 1994; 5: Felgner JH et al. Enhanced gene delivery and mechanism studies with a novel series of cationic lipid formulations. J Biol Chem 1994; 269: Wagner E et al. Coupling of adenovirus to transferrinpolylysine/dna complexes greatly enhances receptormediated gene delivery and expression of transfected genes. Proc Natl Acad Sci USA 1992; 89: Boussif O et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo polyethylenimine. Proc Natl Acad Sci USA 1995; 92: Tang MX, Redemann CT, Szoka FC Jr. In vitro gene delivery by degraded polyamidoamine dendrimers. Bioconj Chem 1996; 7: Goula D et al. Size, diffusibility and transfection performance of linear PEI/DNA complexes in the mouse central nervous system. 1998; 5: Kircheis R et al. Coupling of cell-binding ligands to polyethylenimine for targeted gene delivery. 1997; 4: Plank C, Mechtler K, Szoka F, Wagner E. Activation of the complement system by synthetic DNA complexes: a potential barrier for intravenous gene delivery. Hum Gene Ther 1996; 7: Ogris M et al. PEGylated DNA/transferrin PEI complexes: reduced interaction with blood components, extended circulation in blood and potential for systemic gene delivery. Gene Therapy 1999; 6: Kircheis R, Wagner E. Polycation/DNA complexes for in vivo gene delivery. Gene Ther Regul 2000; 1: Gregoriadis G (ed.). Liposomes as Drug Carriers: Recent Trends and Progress. John Wiley and Sons: New York, Papahadjopoulos D et al. Sterically stabilized liposomes: improvements in pharmacokinetics and antitumor therapeutic efficacy. Proc Natl Acad Sci USA 1991; 88: Mahato RI et al. Physicochemical and pharmacokinetic characteristics of plasmid DNA/cationic liposome complexes. J Pharmacol Sci 1995; 84: Boussif O, Zanta MA, Behr J-P. Optimized galenics improve in vitro gene transfer with cationic molecules up to a thousandfold. 1996; 3: Abdallah B et al. A powerful nonviral vector for in vivo gene transfer into the adult mammalian brain: polyethylenimine. Hum Gene Ther 1996; 7: Ferrari S et al. ExGen 500 is an efficient vector for gene delivery to lung epithelial cells in vitro and in vivo. 1997; 4: Goula D et al. Polyethylenimine-based intravenous delivery of transgenes to mouse lung. 1998; 5: Zou S-M, Erbacher P, Remy J-S, Behr J-P. Systemic linear polyethylenimine (L-PEI)-mediated gene delivery in the mouse. J Gene Med 2000; 2: Goula D et al. Rapid crossing of the pulmonary endothelial barrier by polyethylenimine/ DNA complexes. 2000; 7: Coll J-L et al. In vivo delivery to tumors of DNA complexed with linear polyethylenimine. Hum Gene Ther 1999; 10: Zanta MA, Boussif O, Adib A, Behr JP. In vitro gene delivery to hepatocytes with galactosylated polyethylenimine. Bioconjug Chem 1997; 8: Erbacher P, Remy JS, Behr JP. Gene transfer with synthetic virus-like particles via the integrin-mediated endocytosis pathway. 1999; 6: Diebold SS et al. Efficient gene delivery into human dendritic cells by adenovirus polyethylenimine and Mannose polyethylenimine transfection. Hum Gene Ther 1999; 10: Wightman L, Patzelt E, Wagner E, Kircheis R. Development of transferrin-polycation/dna based vectors for gene delivery to melanoma cells. J Drug Target 1999; 7: Kircheis R et al. Polycation-based DNA complexes for tumortargeted gene delivery in vivo. J Gene Med 1999; 1: Ogris M et al. The size of DNA/transferrin PEI complexes is an important factor for gene expression in cultured cells. Gene Therapy 1998; 5: Liu F, Song YK, Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Therapy 1999; 6: Zhang G, Budker V, Wolff J. High levels of foreign gene expression in hepatocytes after tail vein injections of naked DNA. Hum Gene Ther 1999; 10: Zelphati O, Liang X, Hobart P, Felgner PL. Gene chemistry: functionally and conformationally intact fluorescent plasmid DNA. Hum Gene Ther 1999; 10: Liu F, Qi H, Huang L, Liu D. Factors controlling the efficiency of cationic lipid-mediated transfection in vivo via intravenous administration. 1997; 4: Liu Y et al. Factors influencing the efficiency of cationic liposome-mediated intravenous gene delivery. Nat Biotechnol 1997; 15: Dash PR et al. Factors affecting blood clearance and in vivo distribution of polyelectrolyte complexes for gene delivery. Gene Therapy 1999; 6: Wolfert MA et al. Polyelectrolyte vectors for gene delivery: influence of cationic polymer on biophysical properties of complexes formed with DNA. Bioconjug Chem 1999; 10: Dash PR et al. Decreased binding to proteins and cells of polymeric gene delivery vectors surface modified with multivalent hydrophilic polymer and retargeting through attachment of transferrin. J Biol Chem 2000; 275: Li S, Huang L. In vivo gene transfer via intravenous administration of cationic lipid-protamine DNA (LPD) complexes. Gene Therapy 1997; 4: Templeton NS et al. Improved DNA: liposome complexes for increased systemic delivery and gene expression. Nat Biotechnol 1997; 15: Song YK, Liu F, Chu S, Liu D. Characterization of cationic liposome-mediated gene transfer in vivo by intravenous administration. Hum Gene Ther 1997; 8: Xu L et al. Transferrin-liposome-mediated systemic p53 gene therapy in combination with radiation results in regression of human head and neck cancer xenografts. Hum Gene Ther 1999; 10: Gerlowski LE, Jain RK. Microvascular permeability of normal and neoplastic tissues. Microvasc Res 1986; 31: Yuan F et al. Vascular permeability in a human tumor xenograft: molecular size dependence and cutoff size. Cancer Res 1995; 55: Hobbs SK et al. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci USA 1998; 95:
Gene therapy has gained significant attention over the past two decades as a potential method for treating genetic disorders such as severe combined
 Abstract Gene therapy has gained significant attention over the past two decades as a potential method for treating genetic disorders such as severe combined immunodeficiency, cystic fibrosis, and Parkinson
Abstract Gene therapy has gained significant attention over the past two decades as a potential method for treating genetic disorders such as severe combined immunodeficiency, cystic fibrosis, and Parkinson
jetpei In vitro Transfection Protocol
 jetpei Cationic polymer transfection reagent In vitro Transfection Protocol 101-05 0.5 ml (250 transfections in 24-well plates) 101-05N 0.5 ml 50ml of 150 mm NaCl (250 transfections in 24-well plates)
jetpei Cationic polymer transfection reagent In vitro Transfection Protocol 101-05 0.5 ml (250 transfections in 24-well plates) 101-05N 0.5 ml 50ml of 150 mm NaCl (250 transfections in 24-well plates)
Pierce Protein Transfection Reagent Kit
 INSTRUCTIONS Pierce Protein Transfection Reagent Kit 89850 Number Description MAN0016365 Rev. A.0 Pub. Part No. 2161369.2 89850 Pierce Protein Transfection Reagent Kit, sufficient reagent for 24 reactions
INSTRUCTIONS Pierce Protein Transfection Reagent Kit 89850 Number Description MAN0016365 Rev. A.0 Pub. Part No. 2161369.2 89850 Pierce Protein Transfection Reagent Kit, sufficient reagent for 24 reactions
DNA delivery and DNA Vaccines
 DNA delivery and DNA Vaccines Last Time: Today: Reading: intracellular drug delivery: enhancing cross priming for vaccines DNA vaccination D.W. Pack, A.S. Hoffman, S. Pun, and P.S. Stayton, Design and
DNA delivery and DNA Vaccines Last Time: Today: Reading: intracellular drug delivery: enhancing cross priming for vaccines DNA vaccination D.W. Pack, A.S. Hoffman, S. Pun, and P.S. Stayton, Design and
THE DELIVERY EXPERTS. in vivo-jetpei DNA & sirna Delivery Protocol PROTOCOL
 THE DELIVERY EXPERTS in vivo-jetpei DNA & sirna Delivery Protocol PROTOCOL DESCRIPTION in vivo-jetpei is a linear polyethylenimine, which mediates efficient nucleic acid (DNA, shrna, sirna, oligonucelotides,
THE DELIVERY EXPERTS in vivo-jetpei DNA & sirna Delivery Protocol PROTOCOL DESCRIPTION in vivo-jetpei is a linear polyethylenimine, which mediates efficient nucleic acid (DNA, shrna, sirna, oligonucelotides,
in vivo-jetpei -Man Nucleic Acid Delivery Protocol
 in vivo-jetpei -Man Nucleic Acid Delivery Protocol Company Information... 2 Product Information... 3 In vivo Delivery Protocol... 5 1. Reagents required... 5 2. Recommended amount of nucleic acid and injection
in vivo-jetpei -Man Nucleic Acid Delivery Protocol Company Information... 2 Product Information... 3 In vivo Delivery Protocol... 5 1. Reagents required... 5 2. Recommended amount of nucleic acid and injection
in vivo-jetpei DNA & sirna delivery reagent
 in vivo-jetpei DNA & sirna Delivery Protocol Company Information... 2 Product Information... 3 In vivo Delivery Protocol... 5 1. Reagents required... 5 2. Recommended amount of nucleic acid and injection
in vivo-jetpei DNA & sirna Delivery Protocol Company Information... 2 Product Information... 3 In vivo Delivery Protocol... 5 1. Reagents required... 5 2. Recommended amount of nucleic acid and injection
Convoy TM Transfection Reagent
 Convoy TM Transfection Reagent Catalog No.11103 0.25ml (40-80 transfections in 35mm dishes) Catalog No.11105 0.5 ml (80-165 transfections in 35mm dishes) Catalog No.11110 1.0 ml (165-330 transfections
Convoy TM Transfection Reagent Catalog No.11103 0.25ml (40-80 transfections in 35mm dishes) Catalog No.11105 0.5 ml (80-165 transfections in 35mm dishes) Catalog No.11110 1.0 ml (165-330 transfections
Page 1 of 5. Product Name Label Quantity Product No. Cy 3 10 µg (~0.75 nmol) MIR Cy µg (~7.5 nmol) MIR 7901
 Page 1 of 5 Label IT RNAi Delivery Control Product Name Label Quantity Product No. Cy 3 10 µg (~0.75 nmol) MIR 7900 Label IT RNAi Delivery Control Cy 3 100 µg (~7.5 nmol) MIR 7901 Fluorescein 10 µg (~0.75
Page 1 of 5 Label IT RNAi Delivery Control Product Name Label Quantity Product No. Cy 3 10 µg (~0.75 nmol) MIR 7900 Label IT RNAi Delivery Control Cy 3 100 µg (~7.5 nmol) MIR 7901 Fluorescein 10 µg (~0.75
Methods Western blot analysis of plg Quantification of plasminogen accumulation by ELISA Immunohistochemical analysis
 Methods Western blot analysis of plg Wild-type mice first received a standardized burn wound and then were intravenously administered 2 mg of human plg (Omnio AB, Umeå, Sweden). 24 hours after wounding
Methods Western blot analysis of plg Wild-type mice first received a standardized burn wound and then were intravenously administered 2 mg of human plg (Omnio AB, Umeå, Sweden). 24 hours after wounding
SUPPLEMENTAL MATERIAL. Supplemental Methods:
 SUPPLEMENTAL MATERIAL Supplemental Methods: Immunoprecipitation- As we described but with some modifications [22]. As part of another ongoing project, lysate from human umbilical vein endothelial cells
SUPPLEMENTAL MATERIAL Supplemental Methods: Immunoprecipitation- As we described but with some modifications [22]. As part of another ongoing project, lysate from human umbilical vein endothelial cells
Tumor tissues or cells were homogenized and proteins were extracted using
 SUPPLEMENTAL MATERIALS AND METHODS Western Blotting Tumor tissues or cells were homogenized and proteins were extracted using T-PER tissue protein extraction buffer. Protein concentrations were determined
SUPPLEMENTAL MATERIALS AND METHODS Western Blotting Tumor tissues or cells were homogenized and proteins were extracted using T-PER tissue protein extraction buffer. Protein concentrations were determined
jetpei -FluoF in vitro DNA Transfection Protocol
 jetpei -FluoF in vitro DNA Transfection Protocol Company Information... 2 Product Information... 3 Contact our Technical Assistance and Scientific Advice Service... 4 1.Transfection protocol... 5 1. 1.
jetpei -FluoF in vitro DNA Transfection Protocol Company Information... 2 Product Information... 3 Contact our Technical Assistance and Scientific Advice Service... 4 1.Transfection protocol... 5 1. 1.
64 CuCl 2 in 50 µl 0.1N NaOAc buffer, and 20 µg of each DOTA-antibody conjugate in 40 µl
 Number of DOTA per antibody The average number of DOTA chelators per antibody was measured using a reported procedure with modifications (1,2). Briefly, nonradioactive CuCl 2 (80-fold excess of DOTA antibodies)
Number of DOTA per antibody The average number of DOTA chelators per antibody was measured using a reported procedure with modifications (1,2). Briefly, nonradioactive CuCl 2 (80-fold excess of DOTA antibodies)
in vivo-jetpei Nucleic Acid Delivery Protocol
 in vivo-jetpei Nucleic Acid Delivery Protocol DESCRIPTION in vivo-jetpei is a linear polyethylenimine, which mediates efficient nucleic acid (DNA, shrna, sirna, oligonucleotides, ) delivery to a wide range
in vivo-jetpei Nucleic Acid Delivery Protocol DESCRIPTION in vivo-jetpei is a linear polyethylenimine, which mediates efficient nucleic acid (DNA, shrna, sirna, oligonucleotides, ) delivery to a wide range
Rat Neurotrophin-3 ELISA Kit (rnt-3-elisa)
 Rat Neurotrophin-3 ELISA Kit (rnt-3-elisa) Cat. No. EK0474 96 Tests in 8 x 12 divisible strips Background Neurotrophin-3 (NT-3) is a new member of the nerve growth factor gene family which plays an important
Rat Neurotrophin-3 ELISA Kit (rnt-3-elisa) Cat. No. EK0474 96 Tests in 8 x 12 divisible strips Background Neurotrophin-3 (NT-3) is a new member of the nerve growth factor gene family which plays an important
Mag4C-Ad Kit - Results
 Mag4C-Ad Kit - Results Mag4C-Ad Kit Magnetic capture for viral concentration & storage buffer for superior viral preservation OZ Biosciences is delighted to announce the launching of a new product Mag4C-Ad
Mag4C-Ad Kit - Results Mag4C-Ad Kit Magnetic capture for viral concentration & storage buffer for superior viral preservation OZ Biosciences is delighted to announce the launching of a new product Mag4C-Ad
ExGen 500 in vitro Transfection Reagent
 ExGen 500 in vitro Transfection Reagent Catalog No: 53087 Lot No: XXXXX Supplied as: liquid Stability: store at +4 C Background ExGen 500, polyethylenimine, belongs to an efficient new class of non-viral,
ExGen 500 in vitro Transfection Reagent Catalog No: 53087 Lot No: XXXXX Supplied as: liquid Stability: store at +4 C Background ExGen 500, polyethylenimine, belongs to an efficient new class of non-viral,
Protein Delivery Reagent
 Protein Delivery Reagent Cat. # Contents Quantity BP502401 BioPORTER Reagent, dried 1 tube (24 rxns.) β-galactosidase control protein 10 µg (100 µg/ml) FITC-antibody control protein 10 µg (100 µg/ml) (fluorescein-labeled
Protein Delivery Reagent Cat. # Contents Quantity BP502401 BioPORTER Reagent, dried 1 tube (24 rxns.) β-galactosidase control protein 10 µg (100 µg/ml) FITC-antibody control protein 10 µg (100 µg/ml) (fluorescein-labeled
Supplementary Information Temperature-responsive Gene Silencing by a Smart Polymer
 Supplementary Information Temperature-responsive Gene Silencing by a Smart Polymer Mingming Wang, Yiyun Cheng * Shanghai Key Laboratory of Regulatory Biology, School of Life Sciences, East China Normal
Supplementary Information Temperature-responsive Gene Silencing by a Smart Polymer Mingming Wang, Yiyun Cheng * Shanghai Key Laboratory of Regulatory Biology, School of Life Sciences, East China Normal
Supplementary Figure 1. CryoTEM images of the barcoded nanoparticles (a) and
 a b Supplementary Figure 1. CryoTEM images of the barcoded nanoparticles (a) and size measurements of the particles (b). Liposomes were loaded with DNA barcodes and were imaged using cryo-tem and measured
a b Supplementary Figure 1. CryoTEM images of the barcoded nanoparticles (a) and size measurements of the particles (b). Liposomes were loaded with DNA barcodes and were imaged using cryo-tem and measured
jetpei -Macrophage in vitro DNA transfection reagent PROTOCOL
 jetpei - in vitro DNA transfection reagent PROTOCOL DESCRIPTION jetpei - allows DNA transfection of macrophages and macrophage-like cells. It contains a mannose-conjugated linear polyethylenimine that
jetpei - in vitro DNA transfection reagent PROTOCOL DESCRIPTION jetpei - allows DNA transfection of macrophages and macrophage-like cells. It contains a mannose-conjugated linear polyethylenimine that
ExGen 500 in vitro Transfection Reagent (#R0511, 1ml (for in vitro transfections))
 1 ExGen 500 in vitro Transfection Reagent (#R0511, 1ml (for 100-200 in vitro transfections)) Cationic polymer transfection reagent Concentration 5.47mM in nitrogen residues or 10µM in PEI. Reagent no Supplied
1 ExGen 500 in vitro Transfection Reagent (#R0511, 1ml (for 100-200 in vitro transfections)) Cationic polymer transfection reagent Concentration 5.47mM in nitrogen residues or 10µM in PEI. Reagent no Supplied
Instructions. Fuse-It-siRNA. Shipping and Storage. Overview. Kit Contents. Specifications. Note: Important Guidelines
 Membrane fusion is a highly efficient method for transfecting various molecules and particles into mammalian cells, even into sensitive and primary cells. The Fuse-It reagents are cargo-specific liposomal
Membrane fusion is a highly efficient method for transfecting various molecules and particles into mammalian cells, even into sensitive and primary cells. The Fuse-It reagents are cargo-specific liposomal
Data Sheet. TCR activator / PD-L1 - CHO Recombinant Cell line Cat. #: 60536
 Data Sheet TCR activator / PD-L1 - CHO Recombinant Cell line Cat. #: 60536 Product Description Recombinant CHO-K1 cells constitutively expressing human PD-L1 (Programmed Cell Death 1 Ligand 1, CD274, B7
Data Sheet TCR activator / PD-L1 - CHO Recombinant Cell line Cat. #: 60536 Product Description Recombinant CHO-K1 cells constitutively expressing human PD-L1 (Programmed Cell Death 1 Ligand 1, CD274, B7
Supporting Information
 Supporting Information Chan et al. 10.1073/pnas.0903849106 SI Text Protein Purification. PCSK9 proteins were expressed either transiently in 2936E cells (1), or stably in HepG2 cells. Conditioned culture
Supporting Information Chan et al. 10.1073/pnas.0903849106 SI Text Protein Purification. PCSK9 proteins were expressed either transiently in 2936E cells (1), or stably in HepG2 cells. Conditioned culture
Hypoxyprobe -1 Green Kit Kit contents:
 Updated 2015 1 PRODUCT INSERT Hypoxyprobe, Inc 121 Middlesex Turnpike Burlington, MA 01803 USA www.hypoxyprobe.com Hypoxyprobe -1 Green Kit Kit contents: Solid pimonidazole HCl (Hypoxyprobe -1) FITC conjugated
Updated 2015 1 PRODUCT INSERT Hypoxyprobe, Inc 121 Middlesex Turnpike Burlington, MA 01803 USA www.hypoxyprobe.com Hypoxyprobe -1 Green Kit Kit contents: Solid pimonidazole HCl (Hypoxyprobe -1) FITC conjugated
ProductInformation. Genomic DNA Isolation Kit. Product No. GDI-3 Technical Bulletin No. MB-275 May 2000 TECHNICAL BULLETIN
 Genomic DNA Isolation Kit Product No. GDI-3 Technical Bulletin No. MB-275 May 2000 TECHNICAL BULLETIN ProductInformation Product Description Sigma s Genomic DNA Isolation Kit isolates genomic DNA from
Genomic DNA Isolation Kit Product No. GDI-3 Technical Bulletin No. MB-275 May 2000 TECHNICAL BULLETIN ProductInformation Product Description Sigma s Genomic DNA Isolation Kit isolates genomic DNA from
Kazuki N. Sugahara, Tambet Teesalu, Priya Prakash Karmali, Venkata Ramana Kotamraju, Lilach
 Cancer Cell, Volume 16 Supplemental Data Tissue-Penetrating Delivery of Compounds and Nanoparticles into Tumors Kazuki N. Sugahara, Tambet Teesalu, Priya Prakash Karmali, Venkata Ramana Kotamraju, Lilach
Cancer Cell, Volume 16 Supplemental Data Tissue-Penetrating Delivery of Compounds and Nanoparticles into Tumors Kazuki N. Sugahara, Tambet Teesalu, Priya Prakash Karmali, Venkata Ramana Kotamraju, Lilach
Immunofluorescence Confocal Microscopy of 3D Cultures Grown on Alvetex
 Immunofluorescence Confocal Microscopy of 3D Cultures Grown on Alvetex 1.0. Introduction Immunofluorescence uses the recognition of cellular targets by fluorescent dyes or antigen-specific antibodies coupled
Immunofluorescence Confocal Microscopy of 3D Cultures Grown on Alvetex 1.0. Introduction Immunofluorescence uses the recognition of cellular targets by fluorescent dyes or antigen-specific antibodies coupled
Zenon Goat IgG Labeling Kits
 Product Information Revised: 19 June 2007 Quick Facts Storage upon receipt: 2 6 C Protect from light Abs/Em: See Table 1 Unlabeled IgG antibody Zenon labeling reagent (labeled Fab fragment) Introduction
Product Information Revised: 19 June 2007 Quick Facts Storage upon receipt: 2 6 C Protect from light Abs/Em: See Table 1 Unlabeled IgG antibody Zenon labeling reagent (labeled Fab fragment) Introduction
Approaches to Nanoparticle Targeting. Mahmoud R. Jaafari, PhD Prof. of Pharmaceutics and Pharmaceutical Nanotechnology
 Approaches to Nanoparticle Targeting Mahmoud R. Jaafari, PhD Prof. of Pharmaceutics and Pharmaceutical Nanotechnology Principles of drug targeting Drug targeting is the systemic or local administration
Approaches to Nanoparticle Targeting Mahmoud R. Jaafari, PhD Prof. of Pharmaceutics and Pharmaceutical Nanotechnology Principles of drug targeting Drug targeting is the systemic or local administration
NUVEC. Non-viral adjuvant delivery system for vaccines and cancer treatments. Allan Hey, Head of CMC, N4 Pharma Ltd
 NUVEC Non-viral adjuvant delivery system for vaccines and cancer treatments Allan Hey, Head of CMC, N4 Pharma Ltd 1 N4 Pharma plc o Established in 2014 o Listed on Alternative Investment Market (AIM) in
NUVEC Non-viral adjuvant delivery system for vaccines and cancer treatments Allan Hey, Head of CMC, N4 Pharma Ltd 1 N4 Pharma plc o Established in 2014 o Listed on Alternative Investment Market (AIM) in
Qi Peng, Fujie Chen, Zhenlin Zhong,* Renxi Zhuo
 Electronic Supplementary Information Enhanced Gene Transfection Capability of Polyethylenimine by Incorporating Boronic Acid Groups Qi Peng, Fujie Chen, Zhenlin Zhong,* Renxi Zhuo Key Laboratory of Biomedical
Electronic Supplementary Information Enhanced Gene Transfection Capability of Polyethylenimine by Incorporating Boronic Acid Groups Qi Peng, Fujie Chen, Zhenlin Zhong,* Renxi Zhuo Key Laboratory of Biomedical
EZ-10 SPIN COLUMN GENOMIC DNA MINIPREPS KIT HANDBOOK
 EZ-0 SPIN COLUMN GENOMIC DNA MINIPREPS KIT HANDBOOK (Bacteria, Plant, Animal, Blood) Version 8 Rev 05/0/03 EZ-0 Genomic DNA Kit Handbook Table of Contents Introduction Limitations of Use Features Applications
EZ-0 SPIN COLUMN GENOMIC DNA MINIPREPS KIT HANDBOOK (Bacteria, Plant, Animal, Blood) Version 8 Rev 05/0/03 EZ-0 Genomic DNA Kit Handbook Table of Contents Introduction Limitations of Use Features Applications
F4/80, CD11b, Gr-1, NK1.1, CD3, CD4, CD8 and CD19. A-antigen was detected with FITCconjugated
 SDC MATERIALS AND METHODS Flow Cytometric Detection of A-Antigen Expression Single cell suspensions were prepared from bone marrow, lymph node and spleen. Peripheral blood was obtained and erythrocytes
SDC MATERIALS AND METHODS Flow Cytometric Detection of A-Antigen Expression Single cell suspensions were prepared from bone marrow, lymph node and spleen. Peripheral blood was obtained and erythrocytes
User s Guide MegaTran 1.0 Transfection Reagent
 User s Guide MegaTran 1.0 Transfection Reagent Package Contents and Storage Conditions... 2 Related products... 2 Introduction... 2 Production and Quality Assurance:... 3 Experimental Procedures... 3 Transfection
User s Guide MegaTran 1.0 Transfection Reagent Package Contents and Storage Conditions... 2 Related products... 2 Introduction... 2 Production and Quality Assurance:... 3 Experimental Procedures... 3 Transfection
Electronic Supplementary Information. MMP2-targeting and redox-responsive PEGylated chlorin e6
 Electronic Supplementary Information MMP2-targeting and redox-responsive PEGylated chlorin e6 nanoparticles for cancer near-infrared imaging and photodynamic therapy Wenxiu Hou 1,2, Fangfang Xia 1, Carla
Electronic Supplementary Information MMP2-targeting and redox-responsive PEGylated chlorin e6 nanoparticles for cancer near-infrared imaging and photodynamic therapy Wenxiu Hou 1,2, Fangfang Xia 1, Carla
1 Electronic Supplementary information. 2 Co-assembling FRET nanomedicine with self-indicating drug
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2018 1 Electronic Supplementary information 2 Co-assembling FRET nanomedicine with self-indicating drug
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2018 1 Electronic Supplementary information 2 Co-assembling FRET nanomedicine with self-indicating drug
Hypoxyprobe Plus Kit
 Updated 2017 1 PRODUCT INSERT Hypoxyprobe, Inc 121 Middlesex Turnpike Burlington, MA 01803 USA www.hypoxyprobe.com Hypoxyprobe Plus Kit (HPI Part # HP2-XXX) Kit contents: Solid pimonidazole HCl (Hypoxyprobe
Updated 2017 1 PRODUCT INSERT Hypoxyprobe, Inc 121 Middlesex Turnpike Burlington, MA 01803 USA www.hypoxyprobe.com Hypoxyprobe Plus Kit (HPI Part # HP2-XXX) Kit contents: Solid pimonidazole HCl (Hypoxyprobe
Nonionic Polymer Enhancement of Aggregate Removal in Ion Exchange
 Nonionic Polymer Enhancement of Aggregate Removal in Ion Exchange and Hydroxyapatite Chromatography Pete Gagnon, Richard Richieri, Simin Zaidi 12th Annual Waterside Conference, San Juan, Puerto Rico, April
Nonionic Polymer Enhancement of Aggregate Removal in Ion Exchange and Hydroxyapatite Chromatography Pete Gagnon, Richard Richieri, Simin Zaidi 12th Annual Waterside Conference, San Juan, Puerto Rico, April
Nature Medicine doi: /nm.2548
 Supplementary Table 1: Genotypes of offspring and embryos from matings of Pmm2 WT/F118L mice with Pmm2 WT/R137H mice total events Pmm2 WT/WT Pmm2 WT/R137H Pmm2 WT/F118L Pmm2 R137H/F118L offspring 117 (100%)
Supplementary Table 1: Genotypes of offspring and embryos from matings of Pmm2 WT/F118L mice with Pmm2 WT/R137H mice total events Pmm2 WT/WT Pmm2 WT/R137H Pmm2 WT/F118L Pmm2 R137H/F118L offspring 117 (100%)
PRODUCT DATA SHEET. Carboxylated Fluorescent Gold Nanoparticles. Description. Characteristics
 PRODUCT DATA SHEET Carboxylated Fluorescent Gold Nanoparticles Description Cytodiagnostics carboxylated fluorescent gold nanoparticles is a unique product that combines our Cyto fluorescent dyes and gold
PRODUCT DATA SHEET Carboxylated Fluorescent Gold Nanoparticles Description Cytodiagnostics carboxylated fluorescent gold nanoparticles is a unique product that combines our Cyto fluorescent dyes and gold
Arrest-In Transfection Reagent Catalog numbers: ATR1740, ATR1741, ATR1742, ATR1743
 Arrest-In Transfection Reagent Catalog numbers: ATR1740, ATR1741, ATR1742, ATR1743 Technical support: 1-888-412-2225 Page 1 Arrest-In Transfection Reagent Catalog numbers: ATR1740, ATR1741, ATR1742, ATR1743
Arrest-In Transfection Reagent Catalog numbers: ATR1740, ATR1741, ATR1742, ATR1743 Technical support: 1-888-412-2225 Page 1 Arrest-In Transfection Reagent Catalog numbers: ATR1740, ATR1741, ATR1742, ATR1743
Human VEGFR2/KDR ELISA Kit (hvegfr-elisa)
 Human VEGFR2/KDR ELISA Kit (hvegfr-elisa) Cat. No. EK0544 96 Tests in 8 x 12 divisible strips Background Vascular endothelial growth factor receptor 2 (VEGFR-2), also known as kinase insert domain receptor
Human VEGFR2/KDR ELISA Kit (hvegfr-elisa) Cat. No. EK0544 96 Tests in 8 x 12 divisible strips Background Vascular endothelial growth factor receptor 2 (VEGFR-2), also known as kinase insert domain receptor
3. Close the bottom end of the column and apply the packing device on top. Pump water through the upper adaptor to remove air.
 INSTRUCTIONS FOR USE WorkBeads Protein A Product name Pack size Article number WorkBeads Protein A Bulk Media 1.5 ml 40605001 Bulk Media 5 ml 40605002 Bulk Media 10 ml 40605003 Bulk Media 100 ml 40605004
INSTRUCTIONS FOR USE WorkBeads Protein A Product name Pack size Article number WorkBeads Protein A Bulk Media 1.5 ml 40605001 Bulk Media 5 ml 40605002 Bulk Media 10 ml 40605003 Bulk Media 100 ml 40605004
Application Note. Authors. Abstract. Biopharmaceuticals
 Characterization of monoclonal antibodies on the Agilent 126 Infinity Bio-inert Quaternary LC by Size Exclusion Chromatography using the Agilent BioSEC columns Application Note Biopharmaceuticals Authors
Characterization of monoclonal antibodies on the Agilent 126 Infinity Bio-inert Quaternary LC by Size Exclusion Chromatography using the Agilent BioSEC columns Application Note Biopharmaceuticals Authors
Hypoxyprobe RED PE Kit (HPI Catalog # HP11-XXX)
 Updated 2019 1 PRODUCT INSERT Hypoxyprobe, Inc 121 Middlesex Turnpike Burlington, MA 01803 USA www.hypoxyprobe.com Hypoxyprobe RED PE Kit (HPI Catalog # HP11-XXX) Kit contents: Solid pimonidazole HCl (Hypoxyprobe
Updated 2019 1 PRODUCT INSERT Hypoxyprobe, Inc 121 Middlesex Turnpike Burlington, MA 01803 USA www.hypoxyprobe.com Hypoxyprobe RED PE Kit (HPI Catalog # HP11-XXX) Kit contents: Solid pimonidazole HCl (Hypoxyprobe
Hypoxyprobe -1 Plus Kit
 November 29, 2007 1 PRODUCT INSERT Natural Pharmacia International, Inc 121 Middlesex Turnpike Burlington, MA 01803 USA Hypoxyprobe -1 Plus Kit Kit contents: Solid pimonidazole HCl (Hypoxyprobe -1) FITC
November 29, 2007 1 PRODUCT INSERT Natural Pharmacia International, Inc 121 Middlesex Turnpike Burlington, MA 01803 USA Hypoxyprobe -1 Plus Kit Kit contents: Solid pimonidazole HCl (Hypoxyprobe -1) FITC
AdvanceBio HIC: a Hydrophobic HPLC Column for Monoclonal Antibody (mab) Variant Analysis
 Application Note Biologics Development AdvanceBio HIC: a Hydrophobic HPLC Column for Monoclonal Antibody (mab) Variant Analysis Using the Agilent 16 Infinity II Bio-Inert LC Authors Andrew Coffey and Sandeep
Application Note Biologics Development AdvanceBio HIC: a Hydrophobic HPLC Column for Monoclonal Antibody (mab) Variant Analysis Using the Agilent 16 Infinity II Bio-Inert LC Authors Andrew Coffey and Sandeep
Chitosans: Natural Origin Cationic Polymers as Non-viral Vectors
 produced a PEG-PEI with the aim of reducing toxicity whilst retaining transfection efficiency of higher molecular weight PEI (Ahn et al., 2002). Toxicity was reduced (80 % viability of control) compared
produced a PEG-PEI with the aim of reducing toxicity whilst retaining transfection efficiency of higher molecular weight PEI (Ahn et al., 2002). Toxicity was reduced (80 % viability of control) compared
For quantitative detection of mouse IGF-1 in serum, body fluids, tissue lysates or cell culture supernatants.
 Mouse IGF-1 ELISA Kit (migf-1-elisa) Cat. No. EK0378 96 Tests in 8 x 12 divisible strips Background Insulin-like growth factor 1 (IGF-1), also known as somatomedin C, is a polypeptide protein hormone similar
Mouse IGF-1 ELISA Kit (migf-1-elisa) Cat. No. EK0378 96 Tests in 8 x 12 divisible strips Background Insulin-like growth factor 1 (IGF-1), also known as somatomedin C, is a polypeptide protein hormone similar
Beta3 integrin promotes long-lasting activation and polarization of Vascular Endothelial Growth Factor Receptor 2 by immobilized ligand
 SUPPLEMENTAL FIGURES Beta3 integrin promotes long-lasting activation and polarization of Vascular Endothelial Growth Factor Receptor 2 by immobilized ligand C. Ravelli et al. FIGURE S. I Figure S. I: Gremlin
SUPPLEMENTAL FIGURES Beta3 integrin promotes long-lasting activation and polarization of Vascular Endothelial Growth Factor Receptor 2 by immobilized ligand C. Ravelli et al. FIGURE S. I Figure S. I: Gremlin
Production of FITC conjugate
 Production of FITC conjugate # If you can purify IgG utilising Protein G column or by caprylic acid + ammonium sulfate method instead of the following precipitation method (Step A), the
Production of FITC conjugate # If you can purify IgG utilising Protein G column or by caprylic acid + ammonium sulfate method instead of the following precipitation method (Step A), the
Supplementary Figure 1 A green: cytokeratin 8
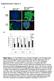 Supplementary Figure 1 A green: cytokeratin 8 green: α-sma red: α-sma blue: DAPI blue: DAPI Panc-1 Panc-1 Panc-1+hPSC Panc-1+hPSC monoculture coculture B Suppl. Figure 1: A, Immunofluorescence staining
Supplementary Figure 1 A green: cytokeratin 8 green: α-sma red: α-sma blue: DAPI blue: DAPI Panc-1 Panc-1 Panc-1+hPSC Panc-1+hPSC monoculture coculture B Suppl. Figure 1: A, Immunofluorescence staining
BEH.462/3.962J Molecular Principles of Biomaterials Spring 2003
 Lecture 17: Drug targeting Last time: Today: Intracellular drug delivery Drug targeting Reading: T.J. Wickham, Ligand-directed targeting of genes to the site of disease, Nat. Med. 9(1) 135-139 (2003) Drug
Lecture 17: Drug targeting Last time: Today: Intracellular drug delivery Drug targeting Reading: T.J. Wickham, Ligand-directed targeting of genes to the site of disease, Nat. Med. 9(1) 135-139 (2003) Drug
jetpei Cationic polymer transfection reagent
 jetpei Cationic polymer transfection reagent Cat# GDSP10105 (0.5 ml) Cat# GDSP10110 (1.0 ml) Cat# GDSP10140 (4 x 1.0 ml) developed by PolyPlus-transfection Product Description jetpei is a powerful transfection
jetpei Cationic polymer transfection reagent Cat# GDSP10105 (0.5 ml) Cat# GDSP10110 (1.0 ml) Cat# GDSP10140 (4 x 1.0 ml) developed by PolyPlus-transfection Product Description jetpei is a powerful transfection
Electronic Supplementary Information
 Electronic Supplementary Information FRET-based probing to gain direct information on sirna sustainability in live cells: Asymmetric degradation of sirna strands Seonmi Shin, a Hyun-Mi Kwon, b Kyung-Sik
Electronic Supplementary Information FRET-based probing to gain direct information on sirna sustainability in live cells: Asymmetric degradation of sirna strands Seonmi Shin, a Hyun-Mi Kwon, b Kyung-Sik
Detection of antibody-stained cell surface and intracellular protein targets with the Agilent 2100 bioanalyzer. Application
 Detection of antibody-stained cell surface and intracellular protein targets with the Agilent 2100 bioanalyzer Application Gerd Luedke and Tobias Preckel Abstract This Application Note describes how the
Detection of antibody-stained cell surface and intracellular protein targets with the Agilent 2100 bioanalyzer Application Gerd Luedke and Tobias Preckel Abstract This Application Note describes how the
Multi-Purpose Transfection Reagents
 Product Information & Instruction Manual Multi-Purpose Transfection Reagents Cat. No: ScreenFect A S-3001 S-3001-2 S-3001-3 ScreenFect A-plus S-6001 S-6001-2 S-6001-3 www.incella.com Contents 1. Characteristics
Product Information & Instruction Manual Multi-Purpose Transfection Reagents Cat. No: ScreenFect A S-3001 S-3001-2 S-3001-3 ScreenFect A-plus S-6001 S-6001-2 S-6001-3 www.incella.com Contents 1. Characteristics
Rat IGF-1 ELISA Kit (rigf-1-elisa)
 Rat IGF-1 ELISA Kit (rigf-1-elisa) Cat. No. EK0377 96 Tests in 8 x 12 divisible strips Background Insulin-like growth factor 1 (IGF-1), also known as somatomedin C, is a polypeptide protein hormone similar
Rat IGF-1 ELISA Kit (rigf-1-elisa) Cat. No. EK0377 96 Tests in 8 x 12 divisible strips Background Insulin-like growth factor 1 (IGF-1), also known as somatomedin C, is a polypeptide protein hormone similar
In Vitro Study of Receptor-Mediated Silica Nanoparticles Delivery across Blood-Brain Barrier
 Supporting Information In Vitro Study of Receptor-Mediated Silica Nanoparticles Delivery across Blood-Brain Barrier Yang Song, Dan Du, Lei Li, Jun Xu, Prashanta Dutta *,, and Yuehe Lin *, School of Mechanical
Supporting Information In Vitro Study of Receptor-Mediated Silica Nanoparticles Delivery across Blood-Brain Barrier Yang Song, Dan Du, Lei Li, Jun Xu, Prashanta Dutta *,, and Yuehe Lin *, School of Mechanical
ReliaBLOT TM. IP/Western Blot Reagents Cat. No. WB120, rabbit
 ReliaBLOT TM Introduction: IP/Western Blot Reagents Cat. No. WB120, rabbit ReliaBLOT TM IP/Western Blot Reagents and Procedures (patent pending) provide an improved method for the detection of immunoprecipitated
ReliaBLOT TM Introduction: IP/Western Blot Reagents Cat. No. WB120, rabbit ReliaBLOT TM IP/Western Blot Reagents and Procedures (patent pending) provide an improved method for the detection of immunoprecipitated
Quantitative Imaging of Tumor Associated Macrophages and Their Response to Therapy Using 64Cu-Labeled Macrin
 Supporting Information for Quantitative Imaging of Tumor Associated Macrophages and Their Response to Therapy Using 64Cu-Labeled Macrin Hye-Yeong Kim 1,2+, Ran Li 1+, Thomas S.C. Ng 1, Gabriel Courties
Supporting Information for Quantitative Imaging of Tumor Associated Macrophages and Their Response to Therapy Using 64Cu-Labeled Macrin Hye-Yeong Kim 1,2+, Ran Li 1+, Thomas S.C. Ng 1, Gabriel Courties
A histone H1-binding-aptide based apoptosis-imaging probe for monitoring
 Electronic Supplementary Material (ESI) for MedChemComm. This journal is The Royal Society of Chemistry 2017 Supporting Information A histone H1-binding-aptide based apoptosis-imaging probe for monitoring
Electronic Supplementary Material (ESI) for MedChemComm. This journal is The Royal Society of Chemistry 2017 Supporting Information A histone H1-binding-aptide based apoptosis-imaging probe for monitoring
OPPF-UK Standard Protocols: Mammalian Expression
 OPPF-UK Standard Protocols: Mammalian Expression Joanne Nettleship joanne@strubi.ox.ac.uk Table of Contents 1. Materials... 3 2. Cell Maintenance... 4 3. 24-Well Transient Expression Screen... 5 4. DNA
OPPF-UK Standard Protocols: Mammalian Expression Joanne Nettleship joanne@strubi.ox.ac.uk Table of Contents 1. Materials... 3 2. Cell Maintenance... 4 3. 24-Well Transient Expression Screen... 5 4. DNA
Data Sheet PD-1 / NFAT - Reporter - Jurkat Recombinant Cell Line Catalog #: 60535
 Data Sheet PD-1 / NFAT - Reporter - Jurkat Recombinant Cell Line Catalog #: 60535 Product Description Recombinant Jurkat T cell expressing firefly luciferase gene under the control of NFAT response elements
Data Sheet PD-1 / NFAT - Reporter - Jurkat Recombinant Cell Line Catalog #: 60535 Product Description Recombinant Jurkat T cell expressing firefly luciferase gene under the control of NFAT response elements
Human OSM/Oncostatin M ELISA Kit
 Human OSM/Oncostatin M ELISA Kit CATALOG NO: IRKTAH5205 LOT NO: SAMPLE INTENDED USE For quantitative detection of human OSM in cell culture supernates, serum and plasma (heparin, EDTA). BACKGROUND OSM(Oncostatin
Human OSM/Oncostatin M ELISA Kit CATALOG NO: IRKTAH5205 LOT NO: SAMPLE INTENDED USE For quantitative detection of human OSM in cell culture supernates, serum and plasma (heparin, EDTA). BACKGROUND OSM(Oncostatin
Cell were phenotyped using FITC-conjugated anti-human CD3 (Pharmingen, UK)
 SUPPLEMENTAL MATERIAL Supplemental Methods Flow cytometry Cell were phenotyped using FITC-conjugated anti-human CD3 (Pharmingen, UK) and anti-human CD68 (Dako, Denmark), anti-human smooth muscle cell α-actin
SUPPLEMENTAL MATERIAL Supplemental Methods Flow cytometry Cell were phenotyped using FITC-conjugated anti-human CD3 (Pharmingen, UK) and anti-human CD68 (Dako, Denmark), anti-human smooth muscle cell α-actin
Data Sheet CD137/NF-κB Reporter - HEK293 Recombinant Cell Line Catalog # 79289
 Data Sheet CD137/NF-κB Reporter - HEK293 Recombinant Cell Line Catalog # 79289 Background Human CD137 (4-1BB; TNFRS9) is an inducible co-stimulatory molecule that activates T cells. CD137:CD137L-mediated
Data Sheet CD137/NF-κB Reporter - HEK293 Recombinant Cell Line Catalog # 79289 Background Human CD137 (4-1BB; TNFRS9) is an inducible co-stimulatory molecule that activates T cells. CD137:CD137L-mediated
Mag4C-Lv Kit - Results
 Mag4C-Lv Kit - Results Mag4C-Lv Kit Magnetic capture for viral concentration & storage buffer for superior viral preservation OZ Biosciences is delighted to announce the launching of a new product Mag4C-Lv
Mag4C-Lv Kit - Results Mag4C-Lv Kit Magnetic capture for viral concentration & storage buffer for superior viral preservation OZ Biosciences is delighted to announce the launching of a new product Mag4C-Lv
Star poly(β-amino esters) obtained from the combination of linear poly(β-amino esters) and polyethyleneimine
 Supporting information Star poly(β-amino esters) obtained from the combination of linear poly(β-amino esters) and polyethyleneimine Xiaobei Huang,, Dezhong Zhou,,* Ming Zeng, Fatma Alshehri, Xiaolin Li,
Supporting information Star poly(β-amino esters) obtained from the combination of linear poly(β-amino esters) and polyethyleneimine Xiaobei Huang,, Dezhong Zhou,,* Ming Zeng, Fatma Alshehri, Xiaolin Li,
Epo (Mouse) ELISA Kit
 Epo (Mouse) ELISA Kit Catalog Number KA1998 96 assays Version: 04 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of the Assay...
Epo (Mouse) ELISA Kit Catalog Number KA1998 96 assays Version: 04 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of the Assay...
Hypoxyprobe -1 Pacific Blue Kit (HPI Catalog # HP15-XXX)
 Updated 2019 1 PRODUCT INSERT Hypoxyprobe, Inc 121 Middlesex Turnpike Burlington, MA 01803 USA www.hypoxyprobe.com Product Data Sheet for HP Pacific Blue M Picture: Pacific Blue anti pimonidazole mab on
Updated 2019 1 PRODUCT INSERT Hypoxyprobe, Inc 121 Middlesex Turnpike Burlington, MA 01803 USA www.hypoxyprobe.com Product Data Sheet for HP Pacific Blue M Picture: Pacific Blue anti pimonidazole mab on
Hypoxyprobe -1 Pacific Blue Kit (HPI Catalog # HP15-XXX)
 Updated 2018 1 PRODUCT INSERT Hypoxyprobe, Inc 121 Middlesex Turnpike Burlington, MA 01803 USA www.hypoxyprobe.com Product Data Sheet for HP Pacific Blue M Picture: Pacific Blue anti pimonidazole mab on
Updated 2018 1 PRODUCT INSERT Hypoxyprobe, Inc 121 Middlesex Turnpike Burlington, MA 01803 USA www.hypoxyprobe.com Product Data Sheet for HP Pacific Blue M Picture: Pacific Blue anti pimonidazole mab on
For in vitro killing assays with lysed cells, neutrophils were sonicated using a 550 Sonic
 Supplemental Information Cell Host & Microbe, Volume 8 Statins Enhance Formation of Phagocyte Extracellular Traps Ohn A. Chow, Maren von Köckritz-Blickwede, A. Taylor Bright, Mary E. Hensler, Annelies
Supplemental Information Cell Host & Microbe, Volume 8 Statins Enhance Formation of Phagocyte Extracellular Traps Ohn A. Chow, Maren von Köckritz-Blickwede, A. Taylor Bright, Mary E. Hensler, Annelies
Xfect Protein Transfection Reagent
 Xfect Protein Transfection Reagent Mammalian Expression Systems Rapid, high-efficiency, low-toxicity protein transfection Transfect a large amount of active protein Virtually no cytotoxicity, unlike lipofection
Xfect Protein Transfection Reagent Mammalian Expression Systems Rapid, high-efficiency, low-toxicity protein transfection Transfect a large amount of active protein Virtually no cytotoxicity, unlike lipofection
Titration of Fluorochrome-Conjugated Antibodies for Labeling Cell Surface Markers on Live Cells
 Titration of Fluorochrome-Conjugated Antibodies for Labeling Cell Surface Markers on Live Cells Ruud Hulspas 1 UNIT 6.29 1 Cytonome/ST, Boston, Massachusetts ABSTRACT Nonspecific antibody binding is best
Titration of Fluorochrome-Conjugated Antibodies for Labeling Cell Surface Markers on Live Cells Ruud Hulspas 1 UNIT 6.29 1 Cytonome/ST, Boston, Massachusetts ABSTRACT Nonspecific antibody binding is best
Application Note. Author. Abstract. Biopharmaceuticals. Verified for Agilent 1260 Infinity II LC Bio-inert System. Sonja Schneider
 Combining small-scale purification and analysis of monoclonal antibodies on one instrument Protein purification with high-volume injection using the Agilent 126 Infinity Bio-inert Quaternary LC System
Combining small-scale purification and analysis of monoclonal antibodies on one instrument Protein purification with high-volume injection using the Agilent 126 Infinity Bio-inert Quaternary LC System
Mobile Phase Optimization in SEC Method Development
 Application Note Pharma & Biopharma Mobile Phase Optimization in SEC Method Development Author Richard Hurteau Agilent Technologies, Inc., Wilgton, DE, USA Abstract Aggregation of monoclonal antibody (mab)
Application Note Pharma & Biopharma Mobile Phase Optimization in SEC Method Development Author Richard Hurteau Agilent Technologies, Inc., Wilgton, DE, USA Abstract Aggregation of monoclonal antibody (mab)
M X 500 µl. M X 1000 µl
 GeneGlide TM sirna Transfection Reagent (Catalog # M1081-300, -500, -1000; Store at 4 C) I. Introduction: BioVision s GeneGlide TM sirna Transfection reagent is a cationic proprietary polymer/lipid formulation,
GeneGlide TM sirna Transfection Reagent (Catalog # M1081-300, -500, -1000; Store at 4 C) I. Introduction: BioVision s GeneGlide TM sirna Transfection reagent is a cationic proprietary polymer/lipid formulation,
Supplementary Materials and Methods
 Supplementary Materials and Methods Reagents Supplementary Material (ESI) for Lab on a Chip RPMI medium, FBS, HEPES buffer solution, sodium pyruvate, penicillin, and streptomycin were obtained from Biological
Supplementary Materials and Methods Reagents Supplementary Material (ESI) for Lab on a Chip RPMI medium, FBS, HEPES buffer solution, sodium pyruvate, penicillin, and streptomycin were obtained from Biological
Contaminant human transferrin assay
 ILA Application Note Contaminant human transferrin assay INTRODUCTION Human transferrin is an 8, Dalton glycoprotein found in human serum that facilitates transport of iron between cells. The iron-poor
ILA Application Note Contaminant human transferrin assay INTRODUCTION Human transferrin is an 8, Dalton glycoprotein found in human serum that facilitates transport of iron between cells. The iron-poor
Human Transferrin / TF ELISA Pair Set
 Human Transferrin / TF ELISA Pair Set Catalog Number : SEK11019 To achieve the best assay results, this manual must be read carefully before using this product and the assay is run as summarized in the
Human Transferrin / TF ELISA Pair Set Catalog Number : SEK11019 To achieve the best assay results, this manual must be read carefully before using this product and the assay is run as summarized in the
HistoMark Double Staining Procedures. Where Better Science Begins.
 HistoMark Double Staining Procedures Where Better Science Begins www.kpl.com HistoMark Double Staining Procedures Researchers often need the ability to visualize multiple proteins in one tissue sample.
HistoMark Double Staining Procedures Where Better Science Begins www.kpl.com HistoMark Double Staining Procedures Researchers often need the ability to visualize multiple proteins in one tissue sample.
Les acides nucléiques thérapeutiques. Jean-Paul Behr Laboratoire de Chimie Génétique Faculté de Pharmacie Université de Strasbourg
 Académie Nationale de Pharmacie Les acides nucléiques thérapeutiques (re)vus par un chimiste Jean-Paul Behr Laboratoire de Chimie Génétique Faculté de Pharmacie Université de Strasbourg behr@unistra.fr
Académie Nationale de Pharmacie Les acides nucléiques thérapeutiques (re)vus par un chimiste Jean-Paul Behr Laboratoire de Chimie Génétique Faculté de Pharmacie Université de Strasbourg behr@unistra.fr
Supplementary Figure 1. Determination of the purity of CP. a, SDS-PAGE of CP and CP- PTX conjugate, and b, HPLC trace of purified CP.
 Supplementary Figure 1. Determination of the purity of CP. a, SDS-PAGE of CP and CP- PTX conjugate, and b, HPLC trace of purified CP. Supplementary Figure 2. Synthesis of CP-PTX conjugate. Supplementary
Supplementary Figure 1. Determination of the purity of CP. a, SDS-PAGE of CP and CP- PTX conjugate, and b, HPLC trace of purified CP. Supplementary Figure 2. Synthesis of CP-PTX conjugate. Supplementary
A Supramolecular Approach to Improve the Gene Transfection Efficacy of Dendrimers
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 A Supramolecular Approach to Improve the Gene Transfection Efficacy of Dendrimers Naimin Shao,
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 A Supramolecular Approach to Improve the Gene Transfection Efficacy of Dendrimers Naimin Shao,
Nature Biotechnology: doi: /nbt.4086
 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3
Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3
Hypoxyprobe Plus Kit
 Updated 2017 1 PRODUCT INSERT Hypoxyprobe, Inc 121 Middlesex Turnpike Burlington, MA 01803 USA www.hypoxyprobe.com Hypoxyprobe Plus Kit (HPI Part # HP2-XXX) Kit contents: Solid pimonidazole HCl (Hypoxyprobe
Updated 2017 1 PRODUCT INSERT Hypoxyprobe, Inc 121 Middlesex Turnpike Burlington, MA 01803 USA www.hypoxyprobe.com Hypoxyprobe Plus Kit (HPI Part # HP2-XXX) Kit contents: Solid pimonidazole HCl (Hypoxyprobe
Silica/Porphyrin Hybrid Nanotubes for In Vivo Cell Tracking
 Electronic Supplementary Information Silica/Porphyrin Hybrid Nanotubes for In Vivo Cell Tracking by Near-Infrared Fluorescence Imaging Koichiro Hayashi,* Michihiro Nakamura and Kazunori Ishimura Department
Electronic Supplementary Information Silica/Porphyrin Hybrid Nanotubes for In Vivo Cell Tracking by Near-Infrared Fluorescence Imaging Koichiro Hayashi,* Michihiro Nakamura and Kazunori Ishimura Department
AnaTag HiLyte Fluor 647 Protein Labeling Kit
 AnaTag HiLyte Fluor 647 Protein Labeling Kit Catalog # 72049 Kit Size 3 Conjugation Reactions This kit is optimized to conjugate HiLyte Fluor 647 SE to proteins (e.g., IgG). It provides ample materials
AnaTag HiLyte Fluor 647 Protein Labeling Kit Catalog # 72049 Kit Size 3 Conjugation Reactions This kit is optimized to conjugate HiLyte Fluor 647 SE to proteins (e.g., IgG). It provides ample materials
Supporting Information
 Supporting Information Deng et al. 10.1073/pnas.1515692112 SI Materials and Methods FPLC. All fusion proteins were expressed and purified through a three-step FPLC purification protocol, as described (20),
Supporting Information Deng et al. 10.1073/pnas.1515692112 SI Materials and Methods FPLC. All fusion proteins were expressed and purified through a three-step FPLC purification protocol, as described (20),
Human Neurotrophin-3 ELISA Kit
 Human Neurotrophin-3 ELISA Kit CATALOG NO: IRKTAH5200 LOT NO: SAMPLE INTENDED USE For quantitative detection of human Neurotrophin-3 in cell culture supernates and serum. BACKGROUND Neurotrophin-3 (NT-3)
Human Neurotrophin-3 ELISA Kit CATALOG NO: IRKTAH5200 LOT NO: SAMPLE INTENDED USE For quantitative detection of human Neurotrophin-3 in cell culture supernates and serum. BACKGROUND Neurotrophin-3 (NT-3)
Mouse PECAM-1/CD31 ELISA Kit (mpecam-elisa)
 Mouse PECAM-1/CD31 ELISA Kit (mpecam-elisa) Cat. No. EK0874 96 Tests in 8 x 12 divisible strips Background Platelet/endothelial cell adhesion molecule-1 (PECAM-1) is an important target GP in DITP. 1 PECAM-1/CD31
Mouse PECAM-1/CD31 ELISA Kit (mpecam-elisa) Cat. No. EK0874 96 Tests in 8 x 12 divisible strips Background Platelet/endothelial cell adhesion molecule-1 (PECAM-1) is an important target GP in DITP. 1 PECAM-1/CD31
K2 Transfection System
 K2 Transfection System DNA & RNA Transfection Kit for Mammalian Cells For order information, SDS, publications and application examples see www.biontex.com Product Order No. Size K2 Transfection System
K2 Transfection System DNA & RNA Transfection Kit for Mammalian Cells For order information, SDS, publications and application examples see www.biontex.com Product Order No. Size K2 Transfection System
Supplementary Data. In-Vivo Tumor Targeting and Spectroscopic Detection with Surface- Enhanced Raman Nanoparticle Tags
 Supplementary Data In-Vivo Tumor Targeting and Spectroscopic Detection with Surface- Enhanced Raman Nanoparticle Tags X.-M. Qian, 1 Xiang-Hong Peng, 2 Dominic O. Ansari, 1 Qiqin Yin-Goen, 3 Georgia Z.
Supplementary Data In-Vivo Tumor Targeting and Spectroscopic Detection with Surface- Enhanced Raman Nanoparticle Tags X.-M. Qian, 1 Xiang-Hong Peng, 2 Dominic O. Ansari, 1 Qiqin Yin-Goen, 3 Georgia Z.
For Research Use Only. Not for use in diagnostic procedures.
 Printed December 13, 2011 Version 1.0 For Research Use Only. Not for use in diagnostic procedures. DDDDK-tagged Protein PURIFICATION GEL with Elution Peptide (MoAb. clone FLA-1) CODE No. 3326 / 3327 PURIFICATION
Printed December 13, 2011 Version 1.0 For Research Use Only. Not for use in diagnostic procedures. DDDDK-tagged Protein PURIFICATION GEL with Elution Peptide (MoAb. clone FLA-1) CODE No. 3326 / 3327 PURIFICATION
Molecular Weight-Dependent Gene Transfection Activity of Unmodified and Galactosylated Polyethyleneimine on Hepatoma Cells and Mouse Liver
 ARTICLE doi:10.1016/s1525-0016(02)00053-9 Molecular Weight-Dependent Gene Transfection Activity of Unmodified and Galactosylated Polyethyleneimine on Hepatoma Cells and Mouse Liver Kensuke Morimoto, Makiya
ARTICLE doi:10.1016/s1525-0016(02)00053-9 Molecular Weight-Dependent Gene Transfection Activity of Unmodified and Galactosylated Polyethyleneimine on Hepatoma Cells and Mouse Liver Kensuke Morimoto, Makiya
A Comprehensive Workflow to Optimize and Execute Protein Aggregate Studies
 A Comprehensive Workflow to Optimize and Execute Protein Aggregate Studies Combining Size Exclusion Chromatography with Method Development and Light Scattering Application Note Biotherapeutics and Biosimilars
A Comprehensive Workflow to Optimize and Execute Protein Aggregate Studies Combining Size Exclusion Chromatography with Method Development and Light Scattering Application Note Biotherapeutics and Biosimilars
