REF/2013/02/ CTRI Website URL -
|
|
|
- Hugo Warner
- 6 years ago
- Views:
Transcription
1 Clinical Trial Details (PDF Generation Date :- Mon, 23 Apr :26:25 GMT) CTRI Number Last Modified On 16/03/2013 Post Graduate Thesis Type of Trial Type of Study Study Design Public Title of Study Scientific Title of Study CTRI/2013/03/ [Registered on: 04/03/2013] - Trial Registered Retrospectively No al Other (Specify) [Hair Care] Other rritation potential of the given hair colour samples- Patch test Single application closed patch test to evaluate the safety of hair colour formulations on healthy human subjects. Secondary IDs if Any Secondary ID Identifier Details of Principal Investigator or overall Trial Coordinator (multi-center study) Details Contact Person (Scientific Query) SRC/CD/398, Version 01, Dated :January 21,2013 Protocol Number Details of Principal Investigator V.G.Vaze College Mumbai (Suburban) Details Contact Person (Scientific Query) V.G.Vaze College Details Contact Person (Public Query) Details Contact Person (Public Query) V.G.Vaze College page 1 / 5
2 Source of Monetary or Material Support Primary Sponsor Details of Secondary Sponsor Countries of Recruitment Sites of Study Details of Ethics Committee Regulatory Clearance Status from DCGI Health Condition / Problems Studied / Comparator Agent Source of Monetary or Material Support > MARICO LTD, Marks, Plot no: 23 C,Mahal Industrial Estate, Near Ahura Centre,Mahakali Caves Road,Andheri( East) Type of Sponsor NIL List of Countries of Principal Investigator Ms Meghana Surve Primary Sponsor Details MARICO LTD Marks, Plot no: 23 C, Mahal Industrial Estate, Near Ahura Centre,Mahakali Caves Road,Andheri( East), Mumbai , Other [HAIR CARE] NIL of Site Site Phone/Fax/ The KET s, Scientific Research Centre, Cosmetology Division, The Kelkar Education Trust s, Scientific Research Centre,Cosmetology Division, 4th floor, V. G. Vaze college campus, Mithagar Road, Mulund (E), Mumbai , Mumbai (Suburban) Mumbai (Suburban) Mumbai (Suburban) meghana@kelkarcosm etology.com of Committee Approval Status Date of Approval Is Independent Ethics Committee? Independent Scientific & Ethics Committee Status Health Type Healthy Human Volunteers Approved 24/01/2013 No Date No Date Specified Condition skin patch test Type Details 1.Haircode Herbal Hair Dye- Natural Black i.e. 24 hrs. of patch application),day 3 (24 hrs. of patch removal),day 4 (48 hrs. of page 2 / 5
3 Inclusion Criteria 2.Haircode Active Herbal Powder Hair color- Natural Black 3.Haircode Active Herbal Powder Hair color- Natural Brown 4.Haircode Active Herbal Powder Hair color- Mehendi Red patch removal) and Day 9 (1 week removal), Day 4 (48 hrs. of patch removal)and Day 9 (1 week 5.EGY EGY EGY EGY-004 Comparator Agent 7.(Experimental Comparator):Sodium Lauryl Sulphate 3% Solution page 3 / 5
4 Exclusion Criteria Age From Age To Gender Year(s) Year(s) Both Inclusion Criteria Details Is a female/ male in generally good health,is between the ages of 18 and 50 years,has not participated in a similar clinical investigation in the past four weeks,is willing to give a written informed consent and come for regular observation. Details Exclusion Criteria A known history or present condition of allergic response to any cosmetic products,skin disease (e.g. psoriasis, atopic dermatitis or other cutaneous manifestations on the scalp), which would interfere with the test readings,medications (e.g. steroids or antihistamines), which would compromise the study,pregnant or lactating Method of Generating Random Sequence Method of Concealment Blinding/Masking Case Record Numbers Open Label Primary Outcome Outcome Timepoints Irritation Potential 0hrs, 24 hrs, 48 hrs and 1 week after patch removal. Secondary Outcome Outcome Timepoints Investigational products to be tested for irritancy test On each subject s back one patch of each investigational product will be applied on Day 1. This will be 9 days study. For all the products and positive control the subjects will be assessed by the study dermatologist on Day 1 (Day of patch application), Day 2 (0 hrs. of patch removal Day 3 (24 hrs. of patch removal),day 4 (48 hrs. of patch after patch removal). Target Sample Size Phase of Trial Phase 2 Date of First Enrollment () Date of First Enrollment (Global) Estimated Duration of Trial Recruitment Status of Trial (Global) Recruitment Status of Trial () Publication Details Brief Summary Total Sample Size=24 Sample Size from =24 28/01/2013 No Date Specified Years=0 Months=0 Days=9 Completed Skin Irritation Test: Irritants are substances that damage the skin. The damage will depend upon the nature, concentration and duration of exposure. Irritation is page 4 / 5
5 Powered by TCPDF ( REF/2013/02/ manifested as inflammatory responses such as erythema (redness), Oedema (swelling), and vesiculation and finally to an intense suppurate reaction without the involvement of the immune system. The irritation potential of a substance can be assessed by human patch test. page 5 / 5
REF/2014/07/ CTRI Website URL -
 Clinical Trial Details (PDF Generation Date :- Mon, 01 Jan 2018 18:09:26 GMT) CTRI Number Last Modified On 18/06/2015 Post Graduate Thesis Type of Trial Type of Study Study Design Public Title of Study
Clinical Trial Details (PDF Generation Date :- Mon, 01 Jan 2018 18:09:26 GMT) CTRI Number Last Modified On 18/06/2015 Post Graduate Thesis Type of Trial Type of Study Study Design Public Title of Study
REFCTRI/2010/ CTRI Website URL -
 Clinical Trial Details (PDF Generation Date :- Mon, 29 Jan 2018 06:45:50 GMT) CTRI Number CTRI/2010/091/000391 [Registered on: 21/04/2010] - Last Modified On 26/07/2016 Post Graduate Thesis Type of Trial
Clinical Trial Details (PDF Generation Date :- Mon, 29 Jan 2018 06:45:50 GMT) CTRI Number CTRI/2010/091/000391 [Registered on: 21/04/2010] - Last Modified On 26/07/2016 Post Graduate Thesis Type of Trial
Review PROCESS, decision making PROCESS and communication BY
 Review PROCESS, decision making PROCESS and communication BY ethics committee Dr. Reneega Gangadhar, Professor & Head Department of Pharmacology Govt. Medical college Trivandrum Outline Review process
Review PROCESS, decision making PROCESS and communication BY ethics committee Dr. Reneega Gangadhar, Professor & Head Department of Pharmacology Govt. Medical college Trivandrum Outline Review process
Volunteering for Clinical Trials
 Volunteering for Clinical Trials Volunteering for Clinical Trials When considering volunteering for a clinical trial, it is important to make an informed decision. Below are answers to frequently asked
Volunteering for Clinical Trials Volunteering for Clinical Trials When considering volunteering for a clinical trial, it is important to make an informed decision. Below are answers to frequently asked
Introduction to Clinical Research
 Introduction to Clinical Research What is Clinical Research? Clinical research is medical research that involves people like you. People volunteer to participate in carefully conducted investigations that
Introduction to Clinical Research What is Clinical Research? Clinical research is medical research that involves people like you. People volunteer to participate in carefully conducted investigations that
Pre-Screening revised checklist for BA/BE NOC for Export Purpose
 Pre-Screening revised checklist for BA/BE NOC for Export Purpose O/o Drugs Controller General (India) Directorate General of Health Services FDA Bhawan, New Delhi (With effective from 25 th April 2014)
Pre-Screening revised checklist for BA/BE NOC for Export Purpose O/o Drugs Controller General (India) Directorate General of Health Services FDA Bhawan, New Delhi (With effective from 25 th April 2014)
INNOVATION ALMOST 180 MILLION EUROS AREAS OF INNOVATION
 From health to beauty INNOVATION IMAGINING FUTURE PRODUCTS 40 Investments in R&D reflect the importance that we attach to the discovery and development of innovative therapeutic and well-being solutions,
From health to beauty INNOVATION IMAGINING FUTURE PRODUCTS 40 Investments in R&D reflect the importance that we attach to the discovery and development of innovative therapeutic and well-being solutions,
REF/2015/09/ CTRI Website URL -
 Clinical Trial Details (PDF Generation Date :- Wed, 07 Feb 2018 16:42:23 GMT) CTRI Number Last Modified On 26/08/2016 Post Graduate Thesis Type of Trial Type of Study Study Design Public Title of Study
Clinical Trial Details (PDF Generation Date :- Wed, 07 Feb 2018 16:42:23 GMT) CTRI Number Last Modified On 26/08/2016 Post Graduate Thesis Type of Trial Type of Study Study Design Public Title of Study
REQUEST FOR AUTHORISATION TO THE COMPETENT AUTHORITY: REQUEST FOR OPINION OF THE ETHICS COMMITTEE:
 Annex 1: Clinical trial Application Form REQUEST FOR AUTHORISATION OF A CLINICAL TRIAL ON A MEDICINAL PRODUCT FOR HUMAN USE TO THE COMPETENT AUTHORITIES AND FOR OPINION OF THE ETHICS COMMITTEES IN THE
Annex 1: Clinical trial Application Form REQUEST FOR AUTHORISATION OF A CLINICAL TRIAL ON A MEDICINAL PRODUCT FOR HUMAN USE TO THE COMPETENT AUTHORITIES AND FOR OPINION OF THE ETHICS COMMITTEES IN THE
Office for Human Subject Protection. University of Rochester
 POLICY 1. Purpose Outline the responsibilities and regulatory requirements when conducting human subject research that involves the use of drugs, agents, biological products, or nutritional products (e.g.,
POLICY 1. Purpose Outline the responsibilities and regulatory requirements when conducting human subject research that involves the use of drugs, agents, biological products, or nutritional products (e.g.,
Journal of Clinical Urology (JCU): Declaration Guidelines for Authors
 Table of Contents 1. Case Report or Case Series... 2 Required Declarations... 2 Example of a completed declarations section:... 2 Example of text that should be used if any declaration is not relevant
Table of Contents 1. Case Report or Case Series... 2 Required Declarations... 2 Example of a completed declarations section:... 2 Example of text that should be used if any declaration is not relevant
ISO In vivo UVAPF Testing
 SUPPORTING SKINCARE Testing STEPS 3. Product Application This ISO 24442 method is is mandated in Japan and Korea and an accepted option for E.U. and some other Countries. Supportable Claims UVA Protection
SUPPORTING SKINCARE Testing STEPS 3. Product Application This ISO 24442 method is is mandated in Japan and Korea and an accepted option for E.U. and some other Countries. Supportable Claims UVA Protection
Employment Application Date:
 Employment Application Date: APPLICANT INFORMATION Name: Home Phone: Cell: Address: City: ST, ZIP: Driver s Lic: Email: Dear Stepping Stone School Applicant Thank you for choosing Stepping Stone School
Employment Application Date: APPLICANT INFORMATION Name: Home Phone: Cell: Address: City: ST, ZIP: Driver s Lic: Email: Dear Stepping Stone School Applicant Thank you for choosing Stepping Stone School
Editor-in-Chief, 23rd March 2017
 Author s response to reviews Title: Single Use and Conventional Bronchoscopes for Broncho alveolar Lavage (BAL) in research: A comparative study (NCT 02515591) Authors: Seher Raza Zaidi (seher.zaidi@lstmed.ac.uk)
Author s response to reviews Title: Single Use and Conventional Bronchoscopes for Broncho alveolar Lavage (BAL) in research: A comparative study (NCT 02515591) Authors: Seher Raza Zaidi (seher.zaidi@lstmed.ac.uk)
NOTICE OF SUBSTANTIAL AMENDMENT
 NOTICE OF SUBSTANTIAL AMENDMENT For use in the case of all research other than clinical trials of investigational medicinal products (CTIMPs). For substantial amendments to CTIMPs, please use the EU-approved
NOTICE OF SUBSTANTIAL AMENDMENT For use in the case of all research other than clinical trials of investigational medicinal products (CTIMPs). For substantial amendments to CTIMPs, please use the EU-approved
}"'ileno. CT/25/2011-DCG (I) Directorate General of Health Services Office of Drugs Controller General (India)
 }"'ileno. CT/25/2011-DCG (I) To, Mis VCB India Pvt. Ltd. 504, Peninsula Towers, Peninsula Corporate Park, Ganpatrao Kadam Marg, Lower Parol (W) Mumbai FDA Bhawan, New Delhi Dated Kotla Road, Subject: Export
}"'ileno. CT/25/2011-DCG (I) To, Mis VCB India Pvt. Ltd. 504, Peninsula Towers, Peninsula Corporate Park, Ganpatrao Kadam Marg, Lower Parol (W) Mumbai FDA Bhawan, New Delhi Dated Kotla Road, Subject: Export
Good Clinical Practice (GCP) & Clinical Trial Registries
 Good Clinical Practice (GCP) & Clinical Trial Registries The Fifth Annual Pharmaceutical Regulatory and Compliance Congress and Best Practice Forum November 14-17, 2004 Kate Maloney, RN, MS, CPHQ Manager,
Good Clinical Practice (GCP) & Clinical Trial Registries The Fifth Annual Pharmaceutical Regulatory and Compliance Congress and Best Practice Forum November 14-17, 2004 Kate Maloney, RN, MS, CPHQ Manager,
Systems and Business Intelligence Department. Head of Systems and Business Intelligence
 JOB DESCRIPTION Job Title: Grade: Hours: Location: Department: Accountable to: Systems Support Officer Support Grade E 37 hours per week (pro rata) Framwellgate Moor Campus Systems and Business Intelligence
JOB DESCRIPTION Job Title: Grade: Hours: Location: Department: Accountable to: Systems Support Officer Support Grade E 37 hours per week (pro rata) Framwellgate Moor Campus Systems and Business Intelligence
Guidelines and criteria for seeking approval to conduct clinical trials in Ayurveda, Siddha and Unani system.
 Guidelines and criteria for seeking approval to conduct clinical trials in Ayurveda, Siddha and Unani system. Application for seeking approval of the Central Government for conduct of clinical trial in
Guidelines and criteria for seeking approval to conduct clinical trials in Ayurveda, Siddha and Unani system. Application for seeking approval of the Central Government for conduct of clinical trial in
Tel: Bradbury Centre Fax: Aldermoor Road. APPLICATION FOR EMPLOYMENT
 Tel: 023 8072 1234 Bradbury Centre Fax: 023 8051 3473 300 Aldermoor Road www.roseroad.org.uk Southampton jobs@roseroad.org.uk SO16 5NA APPLICATION FOR EMPLOYMENT Your application will be judged solely
Tel: 023 8072 1234 Bradbury Centre Fax: 023 8051 3473 300 Aldermoor Road www.roseroad.org.uk Southampton jobs@roseroad.org.uk SO16 5NA APPLICATION FOR EMPLOYMENT Your application will be judged solely
The Los Angeles Child Guidance Clinic
 The Los Angeles Child Guidance Clinic Today s Date: APPLICATION FOR EMPLOYMENT It is the policy of THE LOS ANGELES CHILD GUIDANCE CLINIC to provide equal employment opportunity to all qualified applicants
The Los Angeles Child Guidance Clinic Today s Date: APPLICATION FOR EMPLOYMENT It is the policy of THE LOS ANGELES CHILD GUIDANCE CLINIC to provide equal employment opportunity to all qualified applicants
INVESTIGATIONAL DEVICE EXEMPTION APPLICATION. IDE Title (if title being used)
 INVESTIGATIONAL DEVICE EXEMPTION APPLICATION IDE Title (if title being used) Name of Sponsor Investigator, MD X Professor, Department Icahn School of Medicine at Mount Sinai Date of Submission Form version
INVESTIGATIONAL DEVICE EXEMPTION APPLICATION IDE Title (if title being used) Name of Sponsor Investigator, MD X Professor, Department Icahn School of Medicine at Mount Sinai Date of Submission Form version
REFCTRI/2010/ CTRI Website URL -
 Clinical Trial Details (PDF Generation Date :- Sun, 18 Feb 2018 17:17:30 GMT) CTRI Number CTRI/2010/091/000254 [Registered on: 09/09/2010] - Last Modified On 05/03/2013 Post Graduate Thesis Type of Trial
Clinical Trial Details (PDF Generation Date :- Sun, 18 Feb 2018 17:17:30 GMT) CTRI Number CTRI/2010/091/000254 [Registered on: 09/09/2010] - Last Modified On 05/03/2013 Post Graduate Thesis Type of Trial
Health, Safety and Environment (HSE) Policy
 Health, Safety and Environment (HSE) Policy Policy Code: HR-015 Version: 6.0 Effective Date: 3 April 17 Purpose: As the principal health, safety and environment policy, this policy seeks to set the approach
Health, Safety and Environment (HSE) Policy Policy Code: HR-015 Version: 6.0 Effective Date: 3 April 17 Purpose: As the principal health, safety and environment policy, this policy seeks to set the approach
Topical sirna for management of androgenic alopecia and oily skin Quark Pharmaceuticals, Inc.
 Topical sirna for management of androgenic alopecia and oily skin 1 2017 Quark Pharmaceuticals, Inc. About Quark Founded in 1993; privately held Late stage pharmaceutical company with 2 Phase 3 programs,
Topical sirna for management of androgenic alopecia and oily skin 1 2017 Quark Pharmaceuticals, Inc. About Quark Founded in 1993; privately held Late stage pharmaceutical company with 2 Phase 3 programs,
What We Learned Running Investigator Initiated Trials
 What We Learned Running Investigator Initiated Trials James D. Lewis, MD, MSCE Division of Gastroenterology Center for Clinical Epidemiology & Biostatistics University of Pennsylvania Hans Herfarth, MD,
What We Learned Running Investigator Initiated Trials James D. Lewis, MD, MSCE Division of Gastroenterology Center for Clinical Epidemiology & Biostatistics University of Pennsylvania Hans Herfarth, MD,
SAE Reporting Timelines, Causality Assessment And Compensation. Pawandeep Kaur Associate Medical Director CDSA
 SAE Reporting Timelines, Causality Assessment And Compensation Pawandeep Kaur Associate Medical Director CDSA Objective Background Important definitions SAE Reporting Timelines Causality Assessment Compensation
SAE Reporting Timelines, Causality Assessment And Compensation Pawandeep Kaur Associate Medical Director CDSA Objective Background Important definitions SAE Reporting Timelines Causality Assessment Compensation
Application form UNIVERSITY OF NOTTINGHAM MEDICAL SCHOOL ETHICS COMMITTEE
 Application form UNIVERSITY OF NOTTINGHAM MEDICAL SCHOOL ETHICS COMMITTEE In completing this form please refer to the attached Notes of Guidance Application for approval of all studies involving Healthy
Application form UNIVERSITY OF NOTTINGHAM MEDICAL SCHOOL ETHICS COMMITTEE In completing this form please refer to the attached Notes of Guidance Application for approval of all studies involving Healthy
2017 Clinical Trials Data Library
 2017 Clinical Trials Data Library Copyright 2017 CenterWatch. Investigator Databank Total Active Principal Investigators Worldwide Active Investigators Worldwide Global Distribution of Investigative Sites
2017 Clinical Trials Data Library Copyright 2017 CenterWatch. Investigator Databank Total Active Principal Investigators Worldwide Active Investigators Worldwide Global Distribution of Investigative Sites
11. Type of Research * Type of research maybe: 1. Biomedical research: research conducted primarily in lab setting
 Definition of Data elements required for submission to NMRR * Indicates data is required for submission # Fields * Definition Title, Identification and Study organization 1. Research Title * Research official
Definition of Data elements required for submission to NMRR * Indicates data is required for submission # Fields * Definition Title, Identification and Study organization 1. Research Title * Research official
Streamlining IRB Procedures for Expanded Access
 Streamlining IRB Procedures for Expanded Access Marjorie A. Speers, Ph.D. Executive Director, WCG Foundation Richard Klein Director, FDA Patient Liaison Program Office of Health and Constituent Affairs
Streamlining IRB Procedures for Expanded Access Marjorie A. Speers, Ph.D. Executive Director, WCG Foundation Richard Klein Director, FDA Patient Liaison Program Office of Health and Constituent Affairs
Yorkshire Wildlife Park Job Application Form
 Yorkshire Wildlife Park Job Application Form Title of post applied for: Job Ref: Please refer to the accompanying guidance notes before completing this form. Please write clearly in black ink or type.
Yorkshire Wildlife Park Job Application Form Title of post applied for: Job Ref: Please refer to the accompanying guidance notes before completing this form. Please write clearly in black ink or type.
Employment Application
 For Office Use Only Date: Time: ID: Shoe Size: Availability: Employment Application Please return to: Stateside Foods Ltd. 31-32 Great Bank Road Wingates Ind. Park Westhoughton Bolton BL5 3XU Tel: 01942
For Office Use Only Date: Time: ID: Shoe Size: Availability: Employment Application Please return to: Stateside Foods Ltd. 31-32 Great Bank Road Wingates Ind. Park Westhoughton Bolton BL5 3XU Tel: 01942
Classroom Discussion Guide on Ethics and Public Health Emergencies
 Classroom Discussion Guide on Ethics and Public Health Emergencies This guide provides discussion questions and topics based on the Presidential Commission for the Study of Bioethical Issues report, Ethics
Classroom Discussion Guide on Ethics and Public Health Emergencies This guide provides discussion questions and topics based on the Presidential Commission for the Study of Bioethical Issues report, Ethics
Official Letter from the DOH
 Issued Date 2009/04/02 Issued by DOH Ref. No 0980316268 RE The DOH issued an official letter to announce the implementation of the Guideline for BA/BE Studies on April 2, 2009 (Ref. No. 0980316265). Please
Issued Date 2009/04/02 Issued by DOH Ref. No 0980316268 RE The DOH issued an official letter to announce the implementation of the Guideline for BA/BE Studies on April 2, 2009 (Ref. No. 0980316265). Please
DIRECTOR OF CAMPUS SAFETY
 JOB DESCRIPTION DIRECTOR OF CAMPUS SAFETY AT HOLTON-ARMS SCHOOL LOCATION BENEFITS REPORTS TO Bethesda, MD (Washington, DC area) Competitive salary and generous benefits package Chief Financial Officer
JOB DESCRIPTION DIRECTOR OF CAMPUS SAFETY AT HOLTON-ARMS SCHOOL LOCATION BENEFITS REPORTS TO Bethesda, MD (Washington, DC area) Competitive salary and generous benefits package Chief Financial Officer
REF/2011/04/ CTRI Website URL -
 Clinical Trial Details (PDF Generation Date :- Wed, 03 Jan 2018 22:58:35 GMT) CTRI Number Last Modified On 06/03/2013 Post Graduate Thesis Type of Trial Type of Study Study Design Public Title of Study
Clinical Trial Details (PDF Generation Date :- Wed, 03 Jan 2018 22:58:35 GMT) CTRI Number Last Modified On 06/03/2013 Post Graduate Thesis Type of Trial Type of Study Study Design Public Title of Study
A Phase I/IIa Study of Human Anti-CD38 Antibody MOR03087 in Relapsed/Refractory Multiple Myeloma
 1 von 5 10.12.2013 10:33 A service of the U.S. National Institutes of Health Trial record 1 of 1 for: MOR202C101 Previous Study Return to List Next Study A Phase I/IIa Study of Human Anti-CD38 Antibody
1 von 5 10.12.2013 10:33 A service of the U.S. National Institutes of Health Trial record 1 of 1 for: MOR202C101 Previous Study Return to List Next Study A Phase I/IIa Study of Human Anti-CD38 Antibody
Summary of Provisions in 21 st Century Cures Act (H.R. 6) as passed by full House of Representatives, July 10, 2015
 Pediatric-Specific Provisions Summary of Provisions in 21 st Century Cures Act (H.R. 6) as passed by full House of Representatives, July 10, 2015 Requires the NIH to complete a strategic plan, and in the
Pediatric-Specific Provisions Summary of Provisions in 21 st Century Cures Act (H.R. 6) as passed by full House of Representatives, July 10, 2015 Requires the NIH to complete a strategic plan, and in the
WCG ACADEMY COURSE OVERVIEW
 WCG ACADEMY COURSE OVERVIEW Investigator Course Title Documentation of Informed Consent Duration Ethical Principles Underlying Consent Documentation Long Form Short Form Consent Records Special Considerations
WCG ACADEMY COURSE OVERVIEW Investigator Course Title Documentation of Informed Consent Duration Ethical Principles Underlying Consent Documentation Long Form Short Form Consent Records Special Considerations
About Clinical Trials
 About Clinical Trials A guide for people with CF and their families INSIDE Who Can Participate in a Clinical Trial? Clinical Research Basics Who Is Involved in Clinical Research? Informed Consent Your
About Clinical Trials A guide for people with CF and their families INSIDE Who Can Participate in a Clinical Trial? Clinical Research Basics Who Is Involved in Clinical Research? Informed Consent Your
APPLICATION INSTRUCTIONS INSTITUTIONAL REVIEW BOARD. Revised January 2010 Page 1
 APPLICATION INSTRUCTIONS INSTITUTIONAL REVIEW BOARD Revised January 2010 Page 1 The IRB Proposal Includes: 1. All documents, other than the informed consent form, should be understandable to an informed
APPLICATION INSTRUCTIONS INSTITUTIONAL REVIEW BOARD Revised January 2010 Page 1 The IRB Proposal Includes: 1. All documents, other than the informed consent form, should be understandable to an informed
Standard Operating Procedures Guidelines for Good Clinical Practice
 SOP # CRSC-105 Effective Date 10-22-2013 Version # 1 Version Date 7-30-2013 Standard Operating Procedures Guidelines for Good Clinical Practice Purpose: This SOP outlines the steps required to follow FDA
SOP # CRSC-105 Effective Date 10-22-2013 Version # 1 Version Date 7-30-2013 Standard Operating Procedures Guidelines for Good Clinical Practice Purpose: This SOP outlines the steps required to follow FDA
Sponsorship of Clinical Research Studies
 Sponsorship of Clinical Research Studies Category: Summary: Equality Impact Assessment undertaken: Policy The UK Policy Framework for Health and Social Care 2017 (UKPF) and The Medicines for Human Use
Sponsorship of Clinical Research Studies Category: Summary: Equality Impact Assessment undertaken: Policy The UK Policy Framework for Health and Social Care 2017 (UKPF) and The Medicines for Human Use
Clinical Research: A Multifaceted Discipline
 Clinical Research: A Multifaceted Discipline Anuradha Kulkarni a, Arun Bhatt b* a Senior Associate - Medical & Regulatory Affairs, Clininvent Research Pvt Ltd, A-302, Everest Chambers, Marol Naka, Andheri
Clinical Research: A Multifaceted Discipline Anuradha Kulkarni a, Arun Bhatt b* a Senior Associate - Medical & Regulatory Affairs, Clininvent Research Pvt Ltd, A-302, Everest Chambers, Marol Naka, Andheri
Guidelines: c. Providing the investigational drug for the requested use will not interfere with the initiation, conduct, or completion of clinical
 Guidelines for the Submission of an Expanded Access IND to Permit Diagnosis, Monitoring or Treatment of Intermediate-size Patient Populations with an Investigational Drug or a REMS-restricted, Approved
Guidelines for the Submission of an Expanded Access IND to Permit Diagnosis, Monitoring or Treatment of Intermediate-size Patient Populations with an Investigational Drug or a REMS-restricted, Approved
Interpharma Praha a.s.
 C C 2 C 2 C C C C C C C PhC Eucapil : a topical 2% fluridil solution in rubbing alcohol for androgenetic alopecia Ph Ac Bz Ac 2 F 3 C 2 Androgens and hair loss Androgens play a pivotal role in first promoting
C C 2 C 2 C C C C C C C PhC Eucapil : a topical 2% fluridil solution in rubbing alcohol for androgenetic alopecia Ph Ac Bz Ac 2 F 3 C 2 Androgens and hair loss Androgens play a pivotal role in first promoting
Recruitment and Selection Policy and Procedure
 Recruitment and Selection Policy and Procedure 1 Purpose and Scope Having the right people in the right place at the right time is crucial to Ambitious about Autism (AaA) and Ambitious about Autism Schools
Recruitment and Selection Policy and Procedure 1 Purpose and Scope Having the right people in the right place at the right time is crucial to Ambitious about Autism (AaA) and Ambitious about Autism Schools
Project Management Standards Applied to Complex Clinical Trials. Disclaimer
 Project Management Standards Applied to Complex Clinical Trials Alexander Gissler, PMP ProjectPharm Ltd., Owner & Director 1 Disclaimer The views and opinions expressed in the following PowerPoint slides
Project Management Standards Applied to Complex Clinical Trials Alexander Gissler, PMP ProjectPharm Ltd., Owner & Director 1 Disclaimer The views and opinions expressed in the following PowerPoint slides
Int. J. Pharm. Sci. Rev. Res., 31(2), March April 2015; Article No. 04, Pages: A Review on Drug Approval in Regulated and Non-Regulated Markets
 Review Article A Review on Drug Approval in Regulated and Non-Regulated Markets Vemuri Pavan Kumar, N Vishal Gupta* Pharmaceutical Quality Assurance Group, Department of Pharmaceutics, JSS College of Pharmacy,
Review Article A Review on Drug Approval in Regulated and Non-Regulated Markets Vemuri Pavan Kumar, N Vishal Gupta* Pharmaceutical Quality Assurance Group, Department of Pharmaceutics, JSS College of Pharmacy,
Jackson Municipal Airport Authority Director of Business Development, Marketing & Communications
 Jackson Municipal Airport Authority Director of Business Development, Marketing & Communications The Director of Business Development, Marketing & Communications is responsible for overseeing the development,
Jackson Municipal Airport Authority Director of Business Development, Marketing & Communications The Director of Business Development, Marketing & Communications is responsible for overseeing the development,
Designing Safe and Efficient Phase I Studies to Expedite Clinical Development
 Designing Safe and Efficient Phase I Studies to Expedite Clinical Development Mario Tanguay, B.Pharm, Ph.D. Vice President, Scientific & Regulatory Affairs, Anapharm Guest Professor, Faculty of Pharmacy,
Designing Safe and Efficient Phase I Studies to Expedite Clinical Development Mario Tanguay, B.Pharm, Ph.D. Vice President, Scientific & Regulatory Affairs, Anapharm Guest Professor, Faculty of Pharmacy,
Florida State University Policy 7-IRB-
 Florida State University Policy 7-IRB- Title of Policy: Institutional Review Board Jurisdiction/Applicability Responsible Executive: Gary K. Ostrander Approving Official: Gary K. Ostrander Effective Date:
Florida State University Policy 7-IRB- Title of Policy: Institutional Review Board Jurisdiction/Applicability Responsible Executive: Gary K. Ostrander Approving Official: Gary K. Ostrander Effective Date:
Primary Care mcta 2013: Guidance for use
 GUIDANCE ON USE OF THE MODEL CLINICAL TRIAL AGREEMENT FOR PHARMACEUTICAL AND BIOPHARMACEUTICAL INDUSTRY SPONSORED RESEARCH IN PRIMARY CARE (PRIMARY CARE mcta, 2013 VERSION) Background to the development
GUIDANCE ON USE OF THE MODEL CLINICAL TRIAL AGREEMENT FOR PHARMACEUTICAL AND BIOPHARMACEUTICAL INDUSTRY SPONSORED RESEARCH IN PRIMARY CARE (PRIMARY CARE mcta, 2013 VERSION) Background to the development
Section 1 APPLICANT INFORMATION: Please submit a resume with this Application for Employment. First Name Middle Name Last Name
 Employment Application Utopian Academy for the Arts is an equal opportunity employer, dedicated to a policy of nondiscrimination in employment on any basis including age, sex, color, race, creed, national
Employment Application Utopian Academy for the Arts is an equal opportunity employer, dedicated to a policy of nondiscrimination in employment on any basis including age, sex, color, race, creed, national
Joint statement on public disclosure of results from clinical trials
 Joint statement on public disclosure of results from clinical trials Signatories on 18 May 2017 European Commission for Horizon 2020 Societal Challenge Health Demographic Change and Wellbeing (joined on
Joint statement on public disclosure of results from clinical trials Signatories on 18 May 2017 European Commission for Horizon 2020 Societal Challenge Health Demographic Change and Wellbeing (joined on
COLORADO MILITARY ACADEMY, INC.
 Application for Employment Location of the School (being remodeled so please email application) 360 Command View Colorado Springs, CO (719)576-9838 OFFICE USE ONLY Position: Application Received: (Page
Application for Employment Location of the School (being remodeled so please email application) 360 Command View Colorado Springs, CO (719)576-9838 OFFICE USE ONLY Position: Application Received: (Page
Human Research Protection Program Guidance for Human Research Determination
 Human Research Protection Program Guidance for Human Research Determination I.1.A The sole purpose of the Institutional Review Board (IRB), as defined in federal statutes, is the protection of human subjects
Human Research Protection Program Guidance for Human Research Determination I.1.A The sole purpose of the Institutional Review Board (IRB), as defined in federal statutes, is the protection of human subjects
APPLICATION FORM. - Guidance -
 APPLICATION FORM - Guidance - This application form will consist of three sections. Section 1 will ask you to provide us with yours personal details; Section 2 is relevant to your education, qualifications
APPLICATION FORM - Guidance - This application form will consist of three sections. Section 1 will ask you to provide us with yours personal details; Section 2 is relevant to your education, qualifications
EMPLOYMENT APPLICATION The Maryland Judiciary is an Equal Opportunity Employer
 EMPLOYMENT APPLICATION The Maryland Judiciary is an Equal Opportunity Employer INSTRUCTIONS: Please print or type all information. Please complete all relevant sections. Your application may be rejected
EMPLOYMENT APPLICATION The Maryland Judiciary is an Equal Opportunity Employer INSTRUCTIONS: Please print or type all information. Please complete all relevant sections. Your application may be rejected
Policy 1.15 Sexual Harassment Frequently Asked Questions (Romantic and/or Sexual Relations)
 1. What relationships or behaviors are really prohibited? Teachers (faculty, instructors, staff, GAs, undergraduate TAs) are prohibited from engaging in romantic and/or sexual relationships with students
1. What relationships or behaviors are really prohibited? Teachers (faculty, instructors, staff, GAs, undergraduate TAs) are prohibited from engaging in romantic and/or sexual relationships with students
Understanding clinical research trials
 Understanding clinical research trials Dr. Isabelle Fleury Medical Hemato-oncologist Hôpital Maisonneuve-Rosemont CIUSSS de l Est-de-l Île-de-Montréal Goals of this presentation Better understand clinical
Understanding clinical research trials Dr. Isabelle Fleury Medical Hemato-oncologist Hôpital Maisonneuve-Rosemont CIUSSS de l Est-de-l Île-de-Montréal Goals of this presentation Better understand clinical
Data & Materials Sharing Agreement. Collaboration for AIDS Vaccine Discovery. Clinical Trials Data Sharing Addendum & Related Information
 Data & Materials Sharing Agreement Collaboration for AIDS Vaccine Discovery Clinical Trials Data Sharing Addendum & Related Information I. Overview of this Document The primary purpose of this document
Data & Materials Sharing Agreement Collaboration for AIDS Vaccine Discovery Clinical Trials Data Sharing Addendum & Related Information I. Overview of this Document The primary purpose of this document
Patient Handbook on Stem Cell Therapies
 Patient Handbook on Stem Cell Therapies WWW.ISSCR.ORG WWW.CLOSERLOOKATSTEMCELLS.ORG Patient Handbook on Stem Cell Therapies Introduction We have all heard about the extraordinary promise that stem cell
Patient Handbook on Stem Cell Therapies WWW.ISSCR.ORG WWW.CLOSERLOOKATSTEMCELLS.ORG Patient Handbook on Stem Cell Therapies Introduction We have all heard about the extraordinary promise that stem cell
WMA Declaration of Helsinki - Ethical
 WMA Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects Download the PDF file Adopted by the 18 th WMA General Assembly, Helsinki, Finland, June 1964 and amended
WMA Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects Download the PDF file Adopted by the 18 th WMA General Assembly, Helsinki, Finland, June 1964 and amended
Preservation and Skin Microflora: A Conflict? Understanding the Role of Skin Microflora in Cosmetic Formulation
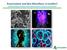 Preservation and Skin Microflora: A Conflict? Understanding the Role of Skin Microflora in Cosmetic Formulation Importance of the Skin Microbiome Introduction Skin microbiome discovery is a revolutionary
Preservation and Skin Microflora: A Conflict? Understanding the Role of Skin Microflora in Cosmetic Formulation Importance of the Skin Microbiome Introduction Skin microbiome discovery is a revolutionary
C O N F I D E N T I A L
 C O N F I D E N T I A L EMPLOYMENT APPLICATION FORM IMPORTANT NOTES FOR APPLICANTS 1. Please fully complete this form personally. Read it through first then answer all questions and make sure you sign
C O N F I D E N T I A L EMPLOYMENT APPLICATION FORM IMPORTANT NOTES FOR APPLICANTS 1. Please fully complete this form personally. Read it through first then answer all questions and make sure you sign
Author Signature: Date: 10 October 2017 The author is signing to confirm the technical content of this document
 SOP Title: Investigator s Brochure Content, Design, Amendments, Filing & Distribution Author: Clinical Research and Development Office (CRDO) Author Signature: Date: 10 October 2017 The author is signing
SOP Title: Investigator s Brochure Content, Design, Amendments, Filing & Distribution Author: Clinical Research and Development Office (CRDO) Author Signature: Date: 10 October 2017 The author is signing
The EFGCP Report on The Procedure for the Ethical Review of Protocols for Clinical Research Projects in Europe (Update: April 2011) Ireland
 The Procedure for the Ethical Review of Protocols for Clinical Research Projects in Europe (Update: April 2011) Ireland Question 1: What laws or regulations apply to an application for conducting a clinical
The Procedure for the Ethical Review of Protocols for Clinical Research Projects in Europe (Update: April 2011) Ireland Question 1: What laws or regulations apply to an application for conducting a clinical
Hospital Advertisements in Newspapers, Is It Still Worth It?
 Global Journal of Health Science; Vol. 9, No. 5; 2017 ISSN 1916-9736 E-ISSN 1916-9744 Published by Canadian Center of Science and Education Hospital Advertisements in Newspapers, Is It Still Worth It?
Global Journal of Health Science; Vol. 9, No. 5; 2017 ISSN 1916-9736 E-ISSN 1916-9744 Published by Canadian Center of Science and Education Hospital Advertisements in Newspapers, Is It Still Worth It?
IRB-GCP and Timelines. Andrew Majewski, MSc. 1 st DOLF Meeting Washington University School of Medicine St Louis, Missouri-USA October th, 2010
 IRB-GCP and Timelines Andrew Majewski, MSc. 1 st DOLF Meeting Washington University School of Medicine St Louis, Missouri-USA October 11-14 th, 2010 1 Factors that affect Timelines Finalized Protocol Finalized
IRB-GCP and Timelines Andrew Majewski, MSc. 1 st DOLF Meeting Washington University School of Medicine St Louis, Missouri-USA October 11-14 th, 2010 1 Factors that affect Timelines Finalized Protocol Finalized
Work Instruction - Research Billing Risk
 THE UNIVERSITY OF TEXAS HEALTH SCIENCE CENTER AT SAN ANTONIO Work Instruction - Research Billing Risk Velos eresearch Version 9.2 Version: 2.0, 04/03/2015 Revision History Version/Amendment #: Version
THE UNIVERSITY OF TEXAS HEALTH SCIENCE CENTER AT SAN ANTONIO Work Instruction - Research Billing Risk Velos eresearch Version 9.2 Version: 2.0, 04/03/2015 Revision History Version/Amendment #: Version
Additional Responsibilities
 Cecil Harford USBC Association Position Title: Association Manager Position Summary: Association Manager is responsible for overseeing the operations of the association, providing administrative support
Cecil Harford USBC Association Position Title: Association Manager Position Summary: Association Manager is responsible for overseeing the operations of the association, providing administrative support
Job Application Form
 Post Applied for: Post Number: Job Application Form Closing Date: Interview Date: Please complete this form fully using black ink or type. Please ensure that all sections are completed and that any gaps
Post Applied for: Post Number: Job Application Form Closing Date: Interview Date: Please complete this form fully using black ink or type. Please ensure that all sections are completed and that any gaps
ClinicalTrials.gov Registration Guide
 ClinicalTrials.gov Registration Guide The Food and Drug Administration Amendments Act (FDAAA), National Institutes of Health (NIH) and International Committee of Medical Journal Editors (ICMJE) require
ClinicalTrials.gov Registration Guide The Food and Drug Administration Amendments Act (FDAAA), National Institutes of Health (NIH) and International Committee of Medical Journal Editors (ICMJE) require
Human Research Protection Program Good Clinical Practice Guidance for Investigators Regulatory File Essential Documents
 Guidance for s Regulatory File Essential s All principal investigators must maintain a regulatory binder or file system, which contains all study documentation. These records may be reviewed at the time
Guidance for s Regulatory File Essential s All principal investigators must maintain a regulatory binder or file system, which contains all study documentation. These records may be reviewed at the time
SPF (PRELIMINARY TEST) DETERMINATION OF THE SOLAR PROTECTION FACTOR (SPF) ON A SMALL NUMBER OF VOLUNTEERS FOLLOWING COLIPA METHOD
 SPF (PRELIMINARY TEST) DETERMINATION OF THE SOLAR PROTECTION FACTOR (SPF) ON A SMALL NUMBER OF VOLUNTEERS FOLLOWING COLIPA METHOD Record no. 1303M26SP2 BIOSELECT BIOSELECT OLIVE SUN PROTECTIVE FACE & BODY
SPF (PRELIMINARY TEST) DETERMINATION OF THE SOLAR PROTECTION FACTOR (SPF) ON A SMALL NUMBER OF VOLUNTEERS FOLLOWING COLIPA METHOD Record no. 1303M26SP2 BIOSELECT BIOSELECT OLIVE SUN PROTECTIVE FACE & BODY
Compliance Department RESEARCH COMPLIANCE MEMORANDUM
 Compliance Department RESEARCH COMPLIANCE MEMORANDUM To: UCD Health Chairs & Chief Administrator Officers From: Nirali Patel, Compliance Manager Kathy Olson, Research Compliance Analyst Re: Department
Compliance Department RESEARCH COMPLIANCE MEMORANDUM To: UCD Health Chairs & Chief Administrator Officers From: Nirali Patel, Compliance Manager Kathy Olson, Research Compliance Analyst Re: Department
EIGHT BASIC ELEMENTS OF INFORMED CONSENT
 1/6 This guidance addresses: o Eight Basic elements of informed consent o Additional elements of informed consent o Other information required by the IRB EIGHT BASIC ELEMENTS OF INFORMED CONSENT Required
1/6 This guidance addresses: o Eight Basic elements of informed consent o Additional elements of informed consent o Other information required by the IRB EIGHT BASIC ELEMENTS OF INFORMED CONSENT Required
 Overview Stability testing is an essential part of drug development which ensures the quality, safety and efficacy of the drug for the lifetime of the drug product. Appropriate storage conditions can only
Overview Stability testing is an essential part of drug development which ensures the quality, safety and efficacy of the drug for the lifetime of the drug product. Appropriate storage conditions can only
HUMAN RESOURCES DEPARTMENT 100 South Myrtle Avenue, P.O. Box 4748 Clearwater, FL
 HUMAN RESOURCES DEPARTMENT 100 South Myrtle Avenue, P.O. Box 4748 Clearwater, FL 33756 727-562-4870 APPLICATION FOR EMPLOYMENT Apply on-line: www.myclearwater.com Date Recv'd: A City application is required
HUMAN RESOURCES DEPARTMENT 100 South Myrtle Avenue, P.O. Box 4748 Clearwater, FL 33756 727-562-4870 APPLICATION FOR EMPLOYMENT Apply on-line: www.myclearwater.com Date Recv'd: A City application is required
A GUIDE TO THIS REFLECTIONS B RESEARCH STUDY IF YOU RE FIGHTING BREAST CANCER, YOU RE NOT ALONE
 A GUIDE TO THIS REFLECTIONS B327-02 RESEARCH STUDY IF YOU RE FIGHTING BREAST CANCER, YOU RE NOT ALONE Do you have breast cancer that has spread to outside the breast? Has your tumor tested positive for
A GUIDE TO THIS REFLECTIONS B327-02 RESEARCH STUDY IF YOU RE FIGHTING BREAST CANCER, YOU RE NOT ALONE Do you have breast cancer that has spread to outside the breast? Has your tumor tested positive for
GENDER PAY GAP REPORT
 GENDER PAY GAP REPORT For the first time, all large UK companies employing 250+ people are required to report on their gender pay. The gender pay gap is not the same as equal pay. Equal pay ensures that
GENDER PAY GAP REPORT For the first time, all large UK companies employing 250+ people are required to report on their gender pay. The gender pay gap is not the same as equal pay. Equal pay ensures that
ABICH S.r.l. Analisi Biologiche e Chimiche Tossicologia, Ricerche e Servizi
 Page: 1 of 17 FINAL REPORT ASSESSMENT OF DERMAL COMPATIBILITY (irritant potential) Occlusive patch test in a single application (duration 48 h) Study N CF024/14-01 Study Protocol code REL/0185/2014/CLI/SAB
Page: 1 of 17 FINAL REPORT ASSESSMENT OF DERMAL COMPATIBILITY (irritant potential) Occlusive patch test in a single application (duration 48 h) Study N CF024/14-01 Study Protocol code REL/0185/2014/CLI/SAB
IMP Management and Accountability
 This is a controlled document. The master document is posted on the JRCO website and any print-off of this document will be classed as uncontrolled. Researchers and their teams may print off this document
This is a controlled document. The master document is posted on the JRCO website and any print-off of this document will be classed as uncontrolled. Researchers and their teams may print off this document
Regulatory and ethical requirements in medical devices studies. France
 Regulatory and ethical in medical devices studies France SECTIONS A.Type of research SECTIONS A.Type of research We have differentiated 8 types of research: Medical device alone with CE mark use within
Regulatory and ethical in medical devices studies France SECTIONS A.Type of research SECTIONS A.Type of research We have differentiated 8 types of research: Medical device alone with CE mark use within
Session 7 Clinical Trial Assessment Bioequivalence Studies
 L1 Session 7 Clinical Trial Assessment Bioequivalence Studies Presentation to APEC Preliminary Workshop on Review of Drug Development in Clinical Trials Celia Lourenco, PhD, Manager, Clinical Group I Office
L1 Session 7 Clinical Trial Assessment Bioequivalence Studies Presentation to APEC Preliminary Workshop on Review of Drug Development in Clinical Trials Celia Lourenco, PhD, Manager, Clinical Group I Office
US Department of Labor Equal Employment Opportunity Commission. Job Applicant Self-Declaration Dear Job Applicant,
 US Department of Labor Equal Employment Opportunity Commission Job Applicant Self-Declaration Dear Job Applicant, The Executive Order 11246 (E.O 11246) prohibits federal contractors and subcontractors
US Department of Labor Equal Employment Opportunity Commission Job Applicant Self-Declaration Dear Job Applicant, The Executive Order 11246 (E.O 11246) prohibits federal contractors and subcontractors
Ethics Committees/IRBs Today: Challenges for Efficiency and Quality
 Marjorie A. Speers, Ph.D. President and CEO Ethics Committees/IRBs Today: Challenges for Efficiency and Quality Copyright 2013 AAHRPP All rights reserved Goal Clinical Research Globally Access to patients
Marjorie A. Speers, Ph.D. President and CEO Ethics Committees/IRBs Today: Challenges for Efficiency and Quality Copyright 2013 AAHRPP All rights reserved Goal Clinical Research Globally Access to patients
QI, Research, Ethics Review Committees (aka, IRBs), and the SQUIRE 2.0 Guidelines. Greg Ogrinc, MD, MS December 6, 2015 IHI National Forum
 QI, Research, Ethics Review Committees (aka, IRBs), and the SQUIRE 2.0 Guidelines Greg Ogrinc, MD, MS December 6, 2015 IHI National Forum How has SQUIRE been used? Write articles Abstracts and posters
QI, Research, Ethics Review Committees (aka, IRBs), and the SQUIRE 2.0 Guidelines Greg Ogrinc, MD, MS December 6, 2015 IHI National Forum How has SQUIRE been used? Write articles Abstracts and posters
Expanded Access. to Investigational Drugs & Biologics. for Treatment Use
 SJMHS Research Compliance Office Guidance Document Expanded Access to Investigational Drugs & Biologics for Treatment Use May 2015 1 Expanded Access to Investigational Drugs & Biologics for Treatment Use
SJMHS Research Compliance Office Guidance Document Expanded Access to Investigational Drugs & Biologics for Treatment Use May 2015 1 Expanded Access to Investigational Drugs & Biologics for Treatment Use
Please note that if you have completed and sent this form electronically, you will be asked to sign it if you are invited to an interview.
 ROYAL BOROUGH OF KINGSTON APPLICATION FORM An equal opportunities employer The Royal Borough of Kingston is committed to safeguarding and promoting the welfare of children and young people and expects
ROYAL BOROUGH OF KINGSTON APPLICATION FORM An equal opportunities employer The Royal Borough of Kingston is committed to safeguarding and promoting the welfare of children and young people and expects
Application for Kielder Osprey Assistant
 Application for Kielder Osprey Assistant Please complete the form in full and return to Paula Turner (HR and Payroll Officer) by email to paula.turner@northwt.org.uk or to Northumberland Wildlife Trust,
Application for Kielder Osprey Assistant Please complete the form in full and return to Paula Turner (HR and Payroll Officer) by email to paula.turner@northwt.org.uk or to Northumberland Wildlife Trust,
Clinical Trial Project Manual Of Operations Template
 Clinical Trial Project Manual Of Operations Template A Manual of Procedures (MOP) is a handbook that guides a study's conduct and or the NIDCR Office of Clinical Trials Operations and Management (OCTOM).
Clinical Trial Project Manual Of Operations Template A Manual of Procedures (MOP) is a handbook that guides a study's conduct and or the NIDCR Office of Clinical Trials Operations and Management (OCTOM).
EEO Utilization Report
 EEO Utilization Report Organization Information Name: Hendry County Board Of County Commissioners City: LaBelle State: FL Zip: 33935 Type: County/Municipal Government (not law enforcement) Tue May 23 15:05:55
EEO Utilization Report Organization Information Name: Hendry County Board Of County Commissioners City: LaBelle State: FL Zip: 33935 Type: County/Municipal Government (not law enforcement) Tue May 23 15:05:55
