Measuring Potential Return on Investment in Pharmacovigilance: A Framework
|
|
|
- Marilynn Morton
- 5 years ago
- Views:
Transcription
1 Measuring Potential Return on Investment in Pharmacovigilance: A Framework Andy Stergachis, PhD Professor, Epidemiology & Global Health Director, Global Medicines Program Joseph Babigumira, MD, PhD Assistant Professor, Global Health Presentation at IOM, Washington, DC September 13, 2013
2 Benefits: Medicines are Among the Most Important Health Interventions Rx-Related Best Buys in Health: Vaccinate children Prevent and treat childhood pneumonia, diarrhea, and malaria Attack the spread of HIV, including providing antiretroviral medications Treat TB patients Disease Control Priorities Project,
3 Examples of Serious and Unexpected Adverse Drug Reactions Medicine Chloramphenicol Clioquinol Erythromycin estolate Fluothane Methyldopa Oral contraceptives Practolol Reserpine Statins Thalidomide Adverse reaction Aplastic anaemia Myelooptic neuropathy (SMON) Cholestatic hepatitis Hepatocellular hepatitis Hemolytic anemia Thromboembolism Sclerosing peritonitis Depression Rhabdomyolisis Congenital malformations Source: WHO Policy Perspectives on Medicines: Pharmacovigilance-ensuring the safe use of medicines. Geneva: WHO, October 2004.
4 What is Pharmacovigilance? The science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other possible medicine-related problems (WHO)
5 Context & Need for Pharmacovigilance Rapid scale-up of medicine-centric public health delivery programs New medicines in pipeline drugs & vaccines Short- and long-term toxicities Product quality issues Emergence of drug resistance Vulnerable, understudied populations
6
7 Sources of Safety Information Premarketing studies in humans Preclinical studies Spontaneous reporting of adverse events Stimulated reporting of adverse events Active surveillance prospective cohort, records linkage, registries Medical literature, including pharmacy journals Alerts from other regulatory agencies and WHO Media
8 Risk Management Options No change Monitor experience while watching and waiting More intensive data gathering Restrict product availability Suspend procurement of products Withdraw product from local approved or essential medicines list Communicate new or reinforced information to health professionals and the public Modify treatment guidelines
9 Pharmacovigilance Potential Tangible Benefits PV can protect the public s health by identifying risks and risk factors of adverse drug events (ADEs) in a timely manner and acting upon such information to prevent or mitigate risks PV can identify previously undetected ADEs and detects and can prevent irrational use of medicines, medication errors, and medical product defects Information collected through PV allows for the assessment of the risks and benefits throughout a medicine s life-cycle However many low- and middle-income countries lack fullyfunctional PV programs
10 Pharmacovigilance in LMICs Gaps in infrastructure, resources, training, and methodologies Low number of AE reports Limited active surveillance Few countries allocate budgets to PV, but some public health programs and donor organizations are supporting PV activities PV usually conducted separately in many countries through parallel and often poorly coordinated systems PV has not kept pace
11 Malaria-endemic countries submitted only 1.2% of all of the ADR reports Only 60 out of 21,312 ADR reports were related to ACTs, 51 of which were coming from four sub- Saharan African countries.
12 Resource Constraints and Investment Decisions LMICs (and increasingly development partners) face severe resource constraints and must prioritize among competing priorities Given that the set up of PV capacity and maintenance of PV activity is potentially costly : Should policy makers invest scarce resources in PV? What is the potential return on investment (ROI) for money spent on PV? i.e. How many $ would be gained for every $ spent on PV? How would the potential ROI in PV systems compare with other public health investments? Rigorous assessments of the ROI in national PV systems have not been reported in the literature
13 Objectives To provide a framework for policy makers and development partners at the country level to assess the potential return on investment (ROI) on resources spent on pharmacovigilance (To develop a generic analytic tool that is customizable at the country level using context-relevant data)
14 Model and Methods We developed a framework for a decision analytic return on investment (ROI) model The model compares the four PV classification groups: (1) no PV, (2) basic PV, (3) semi-functional PV, and (4) functional PV. The investment represents an itemized costing of resources needed to set up and maintain different levels of PV activity The returns represent the monetized reduction in ADR-related out-patient visits and hospitalizations, reduction in mortality, and reduction in ADR-related regimen switches ROI = Net Benefit/Incremental Cost
15 Framework
16 Areas of Performance of PV Systems Policy, law, and regulation System, structure, and stakeholder coordination Signal generation and data management Risk assessment and evaluation Risk management and communication
17 Classification Group 1 Countries have no capacity or only minimal capacity for PV Group 2 Countries have basic structure in place Group 3 Countries have the capacity to collect and evaluate safety data on the basis of legal and organizational structure Group 4 Countries have performing PV systems to detect, evaluate, and prevent medicine safety issues
18 Model introduction page (Figures are placeholders for demo only)
19 Comparators
20 Decision Analytic Model Diagram
21 Probabilities (Figures are placeholders for demo only)
22 Investment (Figures are placeholders for demo only)
23 Examples of Resources Required for Implementing PV at the National Level Capital resources Equipment Office furniture IT equipment Vehicles Books Recurrent (monthly) resources Office space (sq. meter) Personnel Physicians Pharmacists Nurses Clinical pharmacologist Epidemiologist Driver Support staff Materials and supplies Utilities Travel Mass media Meetings Serial publications Web hosting Database subscriptions
24 Return (Figures are placeholders for demo only)
25 Model Engine (Figures are placeholders for demo only)
26 Hypothetical Results (Figures are placeholders for demo only)
27 Summary We developed a framework for a decision analytic return on investment (ROI) model that compares four PV classification groups The investment represents an itemized costing of resources needed to set up & maintain different levels of PV activity The returns represent reductions in ADR-related out-patient visits and hospitalizations, reduction in mortality, and reduction in ADR-related regimen switches More work is needed
28 Clin Pharmacol Ther May;93(5):
29
30
31 Available Metrics for PV Minimum Requirements for a Functional Pharmacovigilance System. The Global Fund and the WHO Indicator-Based PV Assessment Tool (IPAT) Proposed Set of Indicators for Monitoring and Evaluation of Pharmacovigilance Activities (ICIUM 2011 Presentation) Miscellaneous Others pdf.usaid.gov/pdf_docs/pnads167.pdf
32 Monitoring and Evaluation Framework Comprehensive HIV and AIDS Care, Management and Treatment Plan for South Africa: Public Health Program
33 Key Inputs Needed to be Estimated 1. Monetary value of the investment, 2. Probability of different events including reductions in adverse events due to increased PV activity, and 3. Costs of different averted outcomes such as mortality, hospitalizations, and OP visits.
34 Next Steps Needed Data from systematic reviews of the literature, database analyses, and Delphi surveys with panels of experts Framework should be tested using real-world data for validity and assumptions??software-based user-friendly and interactive tool that is customizable for use at the national level
35 Project team Global Medicines Program, Department of Global Health, University of Washington, Seattle, WA Joseph Babigumira Andy Stergachis Lou Garrison Strengthening Pharmaceutical Systems (SPS), Washington DC Hye Lynn Choi Jude Nwokike World Health Organization Collaborating Centre for Advocacy and Training in Pharmacovigilance, Accra, Ghana Alexander Dodoo Acknowledge the support of USAID) under the terms of Cooperative Agreement number GHN-A The contents are the responsibility of the authors and do not necessarily reflect the views of USAID or the United States Government.
36 Back-up Slide
PHARMACOVIGILANCE METRICS
 PHARMACOVIGILANCE METRICS Andy Stergachis, PhD, RPh Professor of Epidemiology and Global Health Director, Global Medicines Program University of Washington, Seattle, WA stergach@uw.edu MEASURING PERFORMANCE
PHARMACOVIGILANCE METRICS Andy Stergachis, PhD, RPh Professor of Epidemiology and Global Health Director, Global Medicines Program University of Washington, Seattle, WA stergach@uw.edu MEASURING PERFORMANCE
11.1 TRACKING COMMODITY FINANCIAL FLOWS
 FIGURE 11-1. THE LOGISTICS CYCLE THE LOGISTICS CYCLE CHAPTER 11 FINANCING Serving Customers Cost of the health care products that are required by the health care system Source of funding for these products
FIGURE 11-1. THE LOGISTICS CYCLE THE LOGISTICS CYCLE CHAPTER 11 FINANCING Serving Customers Cost of the health care products that are required by the health care system Source of funding for these products
Pharmacovigilance Approach to Challenges with Use of Medicines. Oksana Lebega USAID SIAPS project Antalya, Turkey December 10-13, 2013
 Pharmacovigilance Approach to Challenges with Use of Medicines Oksana Lebega USAID SIAPS project Antalya, Turkey December 10-13, 2013 Background Development of Pharmacovigilance (1) Growing market of pharmaceutical
Pharmacovigilance Approach to Challenges with Use of Medicines Oksana Lebega USAID SIAPS project Antalya, Turkey December 10-13, 2013 Background Development of Pharmacovigilance (1) Growing market of pharmaceutical
Medicines Safety in WHO: promoting best practices in Pharmacovigilance. Dr Shanthi Pal Medicines Safety Programme Manager WHO (HQ)
 Medicines Safety in WHO: promoting best practices in Pharmacovigilance Dr Shanthi Pal Medicines Safety Programme Manager WHO (HQ) 1 Birth of modern pharmacovigilance Thalidomide Phocomelia 1961 2 16th
Medicines Safety in WHO: promoting best practices in Pharmacovigilance Dr Shanthi Pal Medicines Safety Programme Manager WHO (HQ) 1 Birth of modern pharmacovigilance Thalidomide Phocomelia 1961 2 16th
Emerging study designs - The need of real world evidence to validate safety and efficacy results from clinical trials
 14MAR2018 1 Emerging study designs - The need of real world evidence to validate safety and efficacy results from clinical trials Symposium Drug Research Academy March 14 2018 Atheline Major-Pedersen MD,
14MAR2018 1 Emerging study designs - The need of real world evidence to validate safety and efficacy results from clinical trials Symposium Drug Research Academy March 14 2018 Atheline Major-Pedersen MD,
Advance Topics in Pharmacoepidemiology. Risk Management. Conflict of Interest Declaration. Benefit Harm Profile?
 Advance Topics in Pharmacoepidemiology Risk Management 2012 Mid-Year ISPE Meeting Miami, April 21-23, 2012 Ariel E., Arias MD, PhD - Fac. Pharmacy; Université de Montréal - Biologics & Genetic Therapies
Advance Topics in Pharmacoepidemiology Risk Management 2012 Mid-Year ISPE Meeting Miami, April 21-23, 2012 Ariel E., Arias MD, PhD - Fac. Pharmacy; Université de Montréal - Biologics & Genetic Therapies
SIAPS assistance is grouped into four technical areas
 Our specific approaches to each of the five result areas follows Governance Under SIAPS, our approach to improving governance and accountability focuses on establishing transparent management systems grounded
Our specific approaches to each of the five result areas follows Governance Under SIAPS, our approach to improving governance and accountability focuses on establishing transparent management systems grounded
A Risk-Based Resource Allocation Framework for Pharmaceutical Quality Assurance for Medicines Regulatory Authorities in Low- and Middle-Income
 A Risk-Based Resource Allocation Framework for Pharmaceutical Quality Assurance for Medicines Regulatory Authorities in Low- and Middle-Income Countries June 2018 Contact Information Joseph B. Babigumira,
A Risk-Based Resource Allocation Framework for Pharmaceutical Quality Assurance for Medicines Regulatory Authorities in Low- and Middle-Income Countries June 2018 Contact Information Joseph B. Babigumira,
PHARMACOVIGILANCE IN AND EMERGING MARKETS: AN INDUSTRY PERSPECTIVE. Shashidhar Swamy (Head, Pharmacovigilance) Wockhardt limited, Mumbai
 PHARMACOVIGILANCE IN AND EMERGING MARKETS: AN INDUSTRY PERSPECTIVE Shashidhar Swamy (Head, Pharmacovigilance) Wockhardt limited, Mumbai 1 Disclaimer The content expressed in this presentation is solely
PHARMACOVIGILANCE IN AND EMERGING MARKETS: AN INDUSTRY PERSPECTIVE Shashidhar Swamy (Head, Pharmacovigilance) Wockhardt limited, Mumbai 1 Disclaimer The content expressed in this presentation is solely
Pharmacovigilance Methods. The Spectrum of PV
 Pharmacovigilance Methods The Spectrum of PV PV Methods: which one? Cohort Event Monitoring Spontaneous Reporting EHR Mining Intensified ADR Reporting Targeted Reporting Record Linkage Geraldine Hill,
Pharmacovigilance Methods The Spectrum of PV PV Methods: which one? Cohort Event Monitoring Spontaneous Reporting EHR Mining Intensified ADR Reporting Targeted Reporting Record Linkage Geraldine Hill,
REPORT ON MONITORING OF ADVERSE DRUG REACTIONS DUE TO ANTIMALARIAL USE IN TANZANIA
 Doc. No. TFDA/DMC/CTPV/003 TANZANIA FOOD AND RUGS AUTHORITY REPORT ON MONITORING OF ADVERSE DRUG REACTIONS DUE TO ANTIMALARIAL USE IN TANZANIA 2006-2008 P. O. Box 77150, EPI Mabibo, Off Mandela Road, Dar
Doc. No. TFDA/DMC/CTPV/003 TANZANIA FOOD AND RUGS AUTHORITY REPORT ON MONITORING OF ADVERSE DRUG REACTIONS DUE TO ANTIMALARIAL USE IN TANZANIA 2006-2008 P. O. Box 77150, EPI Mabibo, Off Mandela Road, Dar
Regulatory system strengthening
 SIXTY-SEVENTH WORLD HEALTH ASSEMBLY A67/32 Provisional agenda item 15.6 14 March 2014 Regulatory system strengthening Report by the Secretariat 1. The Executive Board at its 134th session noted an earlier
SIXTY-SEVENTH WORLD HEALTH ASSEMBLY A67/32 Provisional agenda item 15.6 14 March 2014 Regulatory system strengthening Report by the Secretariat 1. The Executive Board at its 134th session noted an earlier
WHO Pharmacovigilance strategies of interest to IPC stakeholders
 WHO Pharmacovigilance strategies of interest to IPC stakeholders Shanthi Pal Medicines Safety Programme Manager Essential Medicines and Health Products WHO WHO focus on building PV systems in LMIC Europe
WHO Pharmacovigilance strategies of interest to IPC stakeholders Shanthi Pal Medicines Safety Programme Manager Essential Medicines and Health Products WHO WHO focus on building PV systems in LMIC Europe
The Role of the Pharmacist in Pharmacovigilance A Regulatory Perspective
 The Role of the Pharmacist in Pharmacovigilance A Regulatory Perspective Almath Spooner, Irish Medicines Board Pharmaceutical Society of Ireland National Pharmacy Summit, November 2008. Presentation Topics
The Role of the Pharmacist in Pharmacovigilance A Regulatory Perspective Almath Spooner, Irish Medicines Board Pharmaceutical Society of Ireland National Pharmacy Summit, November 2008. Presentation Topics
Pharmacovigilance Practice in Pharmaceutical Industry. From Adverse Event Collection Risk Management
 Pharmacovigilance Practice in Pharmaceutical Industry From Adverse Event Collection Risk Management Hani Mickail, MD Head Global Clinical Safety Operations - Novartis Pharmacovigilance Workshop 23-24 October
Pharmacovigilance Practice in Pharmaceutical Industry From Adverse Event Collection Risk Management Hani Mickail, MD Head Global Clinical Safety Operations - Novartis Pharmacovigilance Workshop 23-24 October
Improving Access to Malaria Medicines in Zambia
 Logistics Brief Improving Access to Malaria Medicines in Zambia A healthcare worker manages artemisininbased combination therapies at a clinic in Zambia. USAID DELIVER PROJECT 2009 The Essential Medicines
Logistics Brief Improving Access to Malaria Medicines in Zambia A healthcare worker manages artemisininbased combination therapies at a clinic in Zambia. USAID DELIVER PROJECT 2009 The Essential Medicines
Practising Safety THE ROLE OF PHARMACOVIGILANCE IN PHARMACOECONOMICS
 Practising Safety THE ROLE OF PHARMACOVIGILANCE IN PHARMACOECONOMICS Coimbatore, India London, United Kingdom Disclaimer The views and opinions expressed in this presentation do not necessarily reflect
Practising Safety THE ROLE OF PHARMACOVIGILANCE IN PHARMACOECONOMICS Coimbatore, India London, United Kingdom Disclaimer The views and opinions expressed in this presentation do not necessarily reflect
Working with an Integrated Supply Chain
 Working with an Integrated Supply Chain Key Concept Challenges and Opportunities of Integrated Supply Chains for Contraceptive Security Countries are moving from vertical supply chains for different programs
Working with an Integrated Supply Chain Key Concept Challenges and Opportunities of Integrated Supply Chains for Contraceptive Security Countries are moving from vertical supply chains for different programs
Malaria Research Capability Strengthening in Africa
 July 2005 UNICEF/UNDP/World Bank/WHO Special Programme for Research & Training in Tropical Diseases (TDR) Multilateral Initiative on Malaria (MIM) Malaria Research Capability Strengthening in Africa INTRODUCTION
July 2005 UNICEF/UNDP/World Bank/WHO Special Programme for Research & Training in Tropical Diseases (TDR) Multilateral Initiative on Malaria (MIM) Malaria Research Capability Strengthening in Africa INTRODUCTION
Importance of Pharmacovigilance for Pharmaceutical Industry
 Importance of Pharmacovigilance for Pharmaceutical Industry JARIR AT THOBARI, MD, DPHARM, PHD FACULTY OF MEDICINE GADJAH MADA UNIVERSITY YOGYAKARTA, INDONESIA Role of Pharma Company Globally Investment
Importance of Pharmacovigilance for Pharmaceutical Industry JARIR AT THOBARI, MD, DPHARM, PHD FACULTY OF MEDICINE GADJAH MADA UNIVERSITY YOGYAKARTA, INDONESIA Role of Pharma Company Globally Investment
Health Canada s NHP Vigilance Activities
 Health Canada s NHP Vigilance Activities Canadian Health Food Association (CHFA) East October 3, 2013 Scott Sawler Director General Marketed Health Products Directorate Overview NHP Program & MHPD Activities
Health Canada s NHP Vigilance Activities Canadian Health Food Association (CHFA) East October 3, 2013 Scott Sawler Director General Marketed Health Products Directorate Overview NHP Program & MHPD Activities
Risk Based Approach To Complaint Handling
 Risk Based Approach To Complaint Handling Khaudeja Bano, M.D. Senior Medical Director, Medical Device Safety Head, AbbVie Inc. Life Sciences Product Complaints Congress Europe Dublin, Ireland December
Risk Based Approach To Complaint Handling Khaudeja Bano, M.D. Senior Medical Director, Medical Device Safety Head, AbbVie Inc. Life Sciences Product Complaints Congress Europe Dublin, Ireland December
Surveillance Part 2. Marcia A. Testa, MPH, PhD Harvard School of Public Health
 Surveillance Part 2 Marcia A. Testa, MPH, PhD Harvard School of Public Health Periodic Population-based Surveys Used for surveillance if surveys are repeated on a regular basis Careful attention to the
Surveillance Part 2 Marcia A. Testa, MPH, PhD Harvard School of Public Health Periodic Population-based Surveys Used for surveillance if surveys are repeated on a regular basis Careful attention to the
MPharm programme in Pharmacovigilance and Pharmacoepidemiology
 MPharm programme in Pharmacovigilance and Pharmacoepidemiology Prof Martie S Lubbe Director: Medicine Usage in South Africa Faculty of Health Sciences Medicine Usage in South Africa Research focus has
MPharm programme in Pharmacovigilance and Pharmacoepidemiology Prof Martie S Lubbe Director: Medicine Usage in South Africa Faculty of Health Sciences Medicine Usage in South Africa Research focus has
High burden to high impact: a targeted malaria response. Malaria Policy Advisory Committee (MPAC) October 2018, Geneva
 High burden to high impact: a targeted malaria response Malaria Policy Advisory Committee (MPAC) October 2018, Geneva Malaria in numbers 445 000 216m 12b 60 90 2 47 6.5b 10+1 The problem Million cases
High burden to high impact: a targeted malaria response Malaria Policy Advisory Committee (MPAC) October 2018, Geneva Malaria in numbers 445 000 216m 12b 60 90 2 47 6.5b 10+1 The problem Million cases
JOB DESCRIPTION. Job title: Supply Chain Specialist Location: Abuja, Nigeria. Department: Management Length of contract: 5 years
 JOB DESCRIPTION Job title: Supply Chain Specialist Location: Abuja, Nigeria Donor title: Supply Chain Expert Department: Management Length of contract: 5 years Role type: National Grade: 9 Travel involved:
JOB DESCRIPTION Job title: Supply Chain Specialist Location: Abuja, Nigeria Donor title: Supply Chain Expert Department: Management Length of contract: 5 years Role type: National Grade: 9 Travel involved:
Explaining EDCTP2 and its perspective on Capacity Building for PV of products used in managing PRDs
 Explaining EDCTP2 and its perspective on Capacity Building for PV of products used in managing PRDs 16 th Annual meeting of the International Society of Pharmacovigilance Jean Marie Vianney Habarugira,
Explaining EDCTP2 and its perspective on Capacity Building for PV of products used in managing PRDs 16 th Annual meeting of the International Society of Pharmacovigilance Jean Marie Vianney Habarugira,
Regulatory Aspects of Pharmacovigilance
 Regulatory Aspects of Pharmacovigilance Deirdre Mc Carthy Pia Caduff-Janosa Training Course Uppsala 2012 Agenda Risk based approach to spontaneous reporting (incl clinical trials) -> Pia Caduff-Janosa
Regulatory Aspects of Pharmacovigilance Deirdre Mc Carthy Pia Caduff-Janosa Training Course Uppsala 2012 Agenda Risk based approach to spontaneous reporting (incl clinical trials) -> Pia Caduff-Janosa
Post-Marketing Surveillance Studies
 Surveillance Part 1 Post-Marketing Surveillance Studies Marcia A. Testa, MPH, PhD Harvard School of Public Health The time period after regulatory approval and after the product can be purchased and used
Surveillance Part 1 Post-Marketing Surveillance Studies Marcia A. Testa, MPH, PhD Harvard School of Public Health The time period after regulatory approval and after the product can be purchased and used
Guidelines for Detecting & Reporting Adverse Drug Reactions
 Guidelines for Detecting & Reporting Adverse Drug Reactions For Healthcare Professionals Rational Drug Use and Pharmacovigilance Department- JFDA (2014) Guidelines for Detecting & Reporting Adverse Drug
Guidelines for Detecting & Reporting Adverse Drug Reactions For Healthcare Professionals Rational Drug Use and Pharmacovigilance Department- JFDA (2014) Guidelines for Detecting & Reporting Adverse Drug
Pharmacovigilance and Patient Safety. Dr M. Roy Jobson Chairperson: Pharmacovigilance Committee of the MCC
 Pharmacovigilance and Patient Safety Dr M. Roy Jobson Chairperson: Pharmacovigilance Committee of the MCC Outline Pharmacovigilance The South African Medicines Control Council (MCC) The National Adverse
Pharmacovigilance and Patient Safety Dr M. Roy Jobson Chairperson: Pharmacovigilance Committee of the MCC Outline Pharmacovigilance The South African Medicines Control Council (MCC) The National Adverse
Disproportionality statistics for signal detection
 Disproportionality statistics for signal detection Andrew Bate Senior Director, Analytics Team Lead, Epidemiology ISOP-UMC Training Course Mysore, 13 January 2015 Disclosures I am a full time employee
Disproportionality statistics for signal detection Andrew Bate Senior Director, Analytics Team Lead, Epidemiology ISOP-UMC Training Course Mysore, 13 January 2015 Disclosures I am a full time employee
Pharmaceutical Sector Governance in the Middle East and North Africa Region A Regional Review by the World Bank
 Pharmaceutical Sector Governance in the Middle East and North Africa Region A Regional Review by the World Bank Presented at the Workshop on Governance of Pharmaceuticals in MENA Amman, Jordan June 6,
Pharmaceutical Sector Governance in the Middle East and North Africa Region A Regional Review by the World Bank Presented at the Workshop on Governance of Pharmaceuticals in MENA Amman, Jordan June 6,
The value partnership between NHS, industry and other providers
 The value partnership between NHS, industry and other providers Dr Richard Greville, Director Wales and Director Distribution & Supply, ABPI 12 April 2016 The Value Partnership between NHS, Industry and
The value partnership between NHS, industry and other providers Dr Richard Greville, Director Wales and Director Distribution & Supply, ABPI 12 April 2016 The Value Partnership between NHS, Industry and
CASE STUDY BUILDING A JUST CULTURE CLIENT: WELLSPAN HEALTH
 CASE STUDY BUILDING A JUST CULTURE CLIENT: WELLSPAN HEALTH RISK MANAGEMENT SOFTWARE U S T CU ORGANIZATIONAL SNAPSHOT WellSpan Health is an integrated health system that serves more than 650,000 people
CASE STUDY BUILDING A JUST CULTURE CLIENT: WELLSPAN HEALTH RISK MANAGEMENT SOFTWARE U S T CU ORGANIZATIONAL SNAPSHOT WellSpan Health is an integrated health system that serves more than 650,000 people
Ensuring Quality of Donated Medicines and Supplies during a Global Health Emergency
 Ensuring Quality of Donated Medicines and Supplies during a Global Health Emergency May 2018 Christine Chacko Public Sector During times of global health emergency, the potential to introduce substandard
Ensuring Quality of Donated Medicines and Supplies during a Global Health Emergency May 2018 Christine Chacko Public Sector During times of global health emergency, the potential to introduce substandard
The EU Risk Management Plan - a tool to address the uncertainties at the time of approval, and manage the risks of medicines
 The EU Risk Management Plan - a tool to address the uncertainties at the time of approval, and manage the risks of medicines Health care uncertainty assessment workshop Session 3: The challenges of health
The EU Risk Management Plan - a tool to address the uncertainties at the time of approval, and manage the risks of medicines Health care uncertainty assessment workshop Session 3: The challenges of health
Phuoc V. Le, MD, MPH Assistant Professor, Internal Medicine and Pediatrics University of California, San Francisco
 To address emerging infections, we must invest in enduring systems: The kinetics and dynamics of health systems strengthening Authors:* Sonak Pastakia, PharmD, MPH, BCPS Associate Professor of Pharmacy
To address emerging infections, we must invest in enduring systems: The kinetics and dynamics of health systems strengthening Authors:* Sonak Pastakia, PharmD, MPH, BCPS Associate Professor of Pharmacy
Mali: Strengthening Multi-Sectoral Coordination using a Total Market Approach
 Mali: Strengthening Multi-Sectoral Coordination using a Total Market Approach A pathway for delivering health impact Strengthening Multi-Sectoral Coordination using a Total Market Approach Palladium is
Mali: Strengthening Multi-Sectoral Coordination using a Total Market Approach A pathway for delivering health impact Strengthening Multi-Sectoral Coordination using a Total Market Approach Palladium is
Darshil Shahh et al, JGTPS, 2015, Vol. 6(3):
 ISSN- 2230-7346 Journal of Global Trends in Pharmaceutical Sciences Journal home page: www.jgtps.com PHARMACEUTICAL RISK MANAGEMENT PLAN: A TOOL FOR PHARMACEUTICAL INDUSTRY Ravindra Trivedi, Darshil Shah
ISSN- 2230-7346 Journal of Global Trends in Pharmaceutical Sciences Journal home page: www.jgtps.com PHARMACEUTICAL RISK MANAGEMENT PLAN: A TOOL FOR PHARMACEUTICAL INDUSTRY Ravindra Trivedi, Darshil Shah
EVOLUTION OF THE VISIBILITY AND ANALYTICS NETWORK IN SOUTH AFRICA
 EVOLUTION OF THE VISIBILITY AND ANALYTICS NETWORK IN SOUTH AFRICA ROB BOTHA - PwC Public Sector Chief of Party Global Health Supply Chain Technical Assistance AGENDA The South African context Visibility
EVOLUTION OF THE VISIBILITY AND ANALYTICS NETWORK IN SOUTH AFRICA ROB BOTHA - PwC Public Sector Chief of Party Global Health Supply Chain Technical Assistance AGENDA The South African context Visibility
JOB DESCRIPTION. Field Logistics Manager
 Job Title: Department: Reports To: Direct Reports: Field Logistics Manager Operations Area Coordinator JOB DESCRIPTION (3) Logistics Officers (Fleet, Warehouse, and Health Commodities) Indirect Reports:
Job Title: Department: Reports To: Direct Reports: Field Logistics Manager Operations Area Coordinator JOB DESCRIPTION (3) Logistics Officers (Fleet, Warehouse, and Health Commodities) Indirect Reports:
The Right Data for the Right Questions:
 The Right Data for the Right Questions: Evidentiary Needs as a Guide to Data Source Selection David Thompson, PhD Senior Vice President Louise Parmenter, PhD Global Head of Operations Real-World & Late
The Right Data for the Right Questions: Evidentiary Needs as a Guide to Data Source Selection David Thompson, PhD Senior Vice President Louise Parmenter, PhD Global Head of Operations Real-World & Late
2017 Call for Proposals EDCTP-TDR Clinical Research and Development Fellowships
 2017 Call for Proposals EDCTP-TDR Clinical Research and Development Fellowships Call Identifier: TMA2017IF The purpose of this Call for Proposals is to provide funding towards actions that aim to support
2017 Call for Proposals EDCTP-TDR Clinical Research and Development Fellowships Call Identifier: TMA2017IF The purpose of this Call for Proposals is to provide funding towards actions that aim to support
Szabolcs Barotfi Ph.D.
 Szabolcs Barotfi Ph.D. Manufacturer: Bayer Withdrawn in 2001 (4 years after registration) Financial loss: 1.2 bn $ Manufacturer: MSD Withdrawn in 2004 (5 years after registration) Financial loss: 2 bn
Szabolcs Barotfi Ph.D. Manufacturer: Bayer Withdrawn in 2001 (4 years after registration) Financial loss: 1.2 bn $ Manufacturer: MSD Withdrawn in 2004 (5 years after registration) Financial loss: 2 bn
Viewpoint. National Pharmacovigilance Program
 Viewpoint National Pharmacovigilance Program Sandeep B. Bavdekar* Sunil Karande* Introduction Pharmacovigilance is the science and activities relating to the detection, assessment, understanding and prevention
Viewpoint National Pharmacovigilance Program Sandeep B. Bavdekar* Sunil Karande* Introduction Pharmacovigilance is the science and activities relating to the detection, assessment, understanding and prevention
Systems for Improved Access to Pharmaceuticals and Services Program, Mozambique: Program Description
 Systems for Improved Access to s and Services Program, Mozambique: Program Description October 2012 Systems for Improved Access to s and Services Program, Mozambique: Program Description Abdel Raouf Qawwas
Systems for Improved Access to s and Services Program, Mozambique: Program Description October 2012 Systems for Improved Access to s and Services Program, Mozambique: Program Description Abdel Raouf Qawwas
REQUIRED DOCUMENT FROM HIRING UNIT
 Terms of reference GENERAL INFORMATION Title: Performance Framework/M&E Coordinator for Developing the Global-Fund Funding Request on HIV and AIDS Intervention Program (National Consultant) Project Name:
Terms of reference GENERAL INFORMATION Title: Performance Framework/M&E Coordinator for Developing the Global-Fund Funding Request on HIV and AIDS Intervention Program (National Consultant) Project Name:
JOB DESCRIPTION. Department: Management Length of contract: 5 years. Role type: National Grade: 10
 JOB DESCRIPTION Job title: State Project Manager Location: Jigawa, Kaduna, Kano, Katsina, Lagos, Yobe (6 positions) Department: Management Length of contract: 5 years Role type: National Grade: 10 Travel
JOB DESCRIPTION Job title: State Project Manager Location: Jigawa, Kaduna, Kano, Katsina, Lagos, Yobe (6 positions) Department: Management Length of contract: 5 years Role type: National Grade: 10 Travel
Access, Affordability and Innovation Focus on High Cost Medicines: Facts and Potential Options
 Access, Affordability and Innovation Focus on High Cost Medicines: Facts and Potential Options Ashoke Bhattacharjya, PhD Robert E Campbell Pharmaceutical Seminar Rutgers Business School Feb 15, 2017 1
Access, Affordability and Innovation Focus on High Cost Medicines: Facts and Potential Options Ashoke Bhattacharjya, PhD Robert E Campbell Pharmaceutical Seminar Rutgers Business School Feb 15, 2017 1
The Future of Pharmaceutical Quality
 The Future of Pharmaceutical Quality First SQA/BIRS Center Meeting Purdue University Polytechnic Institute Louis W. Yu, Ph.D. Chief Quality Officer Unique Challenges of the Pharmaceutical Industry a. The
The Future of Pharmaceutical Quality First SQA/BIRS Center Meeting Purdue University Polytechnic Institute Louis W. Yu, Ph.D. Chief Quality Officer Unique Challenges of the Pharmaceutical Industry a. The
1201 Maryland Avenue SW, Suite 900, Washington, DC ,
 1201 Maryland Avenue SW, Suite 900, Washington, DC 20024 202-962-9200, www.bio.org June 19, 2008 Dockets Management Branch (HFA-305) Food and Drug Administration 5600 Fishers Lane, Rm. 1061 Rockville,
1201 Maryland Avenue SW, Suite 900, Washington, DC 20024 202-962-9200, www.bio.org June 19, 2008 Dockets Management Branch (HFA-305) Food and Drug Administration 5600 Fishers Lane, Rm. 1061 Rockville,
A practical guide to achieving and maintaining global oversight and ensuring end-to-end pharmacovigilance
 FOR PHARMA & LIFE SCIENCES WHITE PAPER A practical guide to achieving and maintaining global oversight and ensuring end-to-end pharmacovigilance The increasing complexity of today s pharmaceutical industry
FOR PHARMA & LIFE SCIENCES WHITE PAPER A practical guide to achieving and maintaining global oversight and ensuring end-to-end pharmacovigilance The increasing complexity of today s pharmaceutical industry
Palladium s Total Market Approach (TMA)
 Palladium s Total Market Approach (TMA) A pathway for delivering health impact Building Momentum for Total Market Growth Palladium is committed to building new pathways to creating greater health equity,
Palladium s Total Market Approach (TMA) A pathway for delivering health impact Building Momentum for Total Market Growth Palladium is committed to building new pathways to creating greater health equity,
PHARMA CONGRESS. Pharmacovigilance and Drug Safety: Assessing Future Regulatory and Compliance Developments. October 28, 2008
 PHARMA CONGRESS October 28, 2008 Pharmacovigilance and Drug Safety: Assessing Future Regulatory and Compliance Developments Beverly H. Lorell, MD Senior Medical & Policy Advisor King & Spalding LLP Assessing
PHARMA CONGRESS October 28, 2008 Pharmacovigilance and Drug Safety: Assessing Future Regulatory and Compliance Developments Beverly H. Lorell, MD Senior Medical & Policy Advisor King & Spalding LLP Assessing
Adverse Event Reporting
 Adverse Event Reporting AE Case Receipt When we receive a case, we induct it through a well-oiled process that reduces the number of subsequent queries, classifies events appropriately, and increases the
Adverse Event Reporting AE Case Receipt When we receive a case, we induct it through a well-oiled process that reduces the number of subsequent queries, classifies events appropriately, and increases the
PHARMACOVIGILANCE-AN EMERGENCE
 PHARMACOVIGILANCE-AN EMERGENCE A Case Study By Dr Deven V Parmar 1 and Dr. Dharani Munirathinam 2, USA ( 1 Faculty at Fellow American College Of Clinical Pharmacology 2 Pharmacovigilance consultant, ACCP
PHARMACOVIGILANCE-AN EMERGENCE A Case Study By Dr Deven V Parmar 1 and Dr. Dharani Munirathinam 2, USA ( 1 Faculty at Fellow American College Of Clinical Pharmacology 2 Pharmacovigilance consultant, ACCP
Second Africa TB Regional Conference on Management of TB Medicines. Africa TB Conference 2012, Zanzibar December 5-7, 2012
 Second Africa TB Regional Conference on Management of TB Medicines Africa TB Conference 2012, Zanzibar December 5-7, 2012 Country experience in planning, quantification and supply of MDR- TB medicines
Second Africa TB Regional Conference on Management of TB Medicines Africa TB Conference 2012, Zanzibar December 5-7, 2012 Country experience in planning, quantification and supply of MDR- TB medicines
HARMONIZATION PROCESS FOR MEDICINES REGULATION IN THE SADC REGION MANDISA HELA HELA ON ON BEHALF OF SADC SADC SECRETARIAT GABORONE, BOTSWANA
 HARMONIZATION PROCESS FOR MEDICINES REGULATION IN THE SADC REGION MANDISA HELA HELA ON ON BEHALF OF OF SADC SADC SECRETARIAT GABORONE, Map of SADC Member-States Southern African African Development Community
HARMONIZATION PROCESS FOR MEDICINES REGULATION IN THE SADC REGION MANDISA HELA HELA ON ON BEHALF OF OF SADC SADC SECRETARIAT GABORONE, Map of SADC Member-States Southern African African Development Community
Pharmacovigilance & Signal Detection
 Pharmacovigilance & Signal Detection 30 th International Conference on Pharmacoepidemiology & Therapeutic Risk Management Pre-conference educational session Thursday, October 23, 2014; 2:00-6:00pm Semi-automated,
Pharmacovigilance & Signal Detection 30 th International Conference on Pharmacoepidemiology & Therapeutic Risk Management Pre-conference educational session Thursday, October 23, 2014; 2:00-6:00pm Semi-automated,
External Defibrillator Improvement Initiative
 External Defibrillator Improvement Initiative November 2010 Center for Devices and Radiological Health U.S. Food and Drug Administration External Defibrillator Improvement Initiative Table of Contents
External Defibrillator Improvement Initiative November 2010 Center for Devices and Radiological Health U.S. Food and Drug Administration External Defibrillator Improvement Initiative Table of Contents
Hosted health partnerships
 EXECUTIVE BOARD EB134/42 134th session 16 December 2013 Provisional agenda item 11.4 Hosted health partnerships Report by the Secretariat 1. In January 2013 the Executive Board adopted decision EB132(10),
EXECUTIVE BOARD EB134/42 134th session 16 December 2013 Provisional agenda item 11.4 Hosted health partnerships Report by the Secretariat 1. In January 2013 the Executive Board adopted decision EB132(10),
A Review of Constraints to Using Data for Decision Making. Recommendations to Inform the Design of Interventions
 A Review of Constraints to Using Data for Decision Making Recommendations to Inform the Design of Interventions A Review of Constraints to Using Data for Decision Making Recommendations to Inform the Design
A Review of Constraints to Using Data for Decision Making Recommendations to Inform the Design of Interventions A Review of Constraints to Using Data for Decision Making Recommendations to Inform the Design
Safety of Medicinal Products in Ukraine: Assessment of the Pharmacovigilance System and its Performance
 Safety of Medicinal Products in Ukraine: Assessment of the Pharmacovigilance System and its Performance Oksana Lebega Jude Nwokike Helena Walkowiak July 2012 Strengthening Pharmaceutical Systems Center
Safety of Medicinal Products in Ukraine: Assessment of the Pharmacovigilance System and its Performance Oksana Lebega Jude Nwokike Helena Walkowiak July 2012 Strengthening Pharmaceutical Systems Center
EPSA EC CONSULTATION:
 EPSA EC CONSULTATION: STRATEGY TO BETTER PROTECT PUBLIC HEALTH BY STRENGTHENING AND RATIONALISING EU PHARMACOVIGILANCE: PUBLIC CONSULTATION ON LEGISLATIVE PROPOSALS 1 Executive Summary: The European Pharmaceutical
EPSA EC CONSULTATION: STRATEGY TO BETTER PROTECT PUBLIC HEALTH BY STRENGTHENING AND RATIONALISING EU PHARMACOVIGILANCE: PUBLIC CONSULTATION ON LEGISLATIVE PROPOSALS 1 Executive Summary: The European Pharmaceutical
THE BENEFIT/RISK BALANCE DURING THE LIFE CYCLE OF DRUGS IN JAPAN
 THE BENEFIT/RISK BALANCE DURING THE LIFE CYCLE OF DRUGS IN JAPAN 2 CONTENTS 1. Balancing Benefit and Risk 2. Risk Manager 3. Risk Management Plan (RMP) 4. PMDA s view on Pharmaceutical Affairs 1. BALANCING
THE BENEFIT/RISK BALANCE DURING THE LIFE CYCLE OF DRUGS IN JAPAN 2 CONTENTS 1. Balancing Benefit and Risk 2. Risk Manager 3. Risk Management Plan (RMP) 4. PMDA s view on Pharmaceutical Affairs 1. BALANCING
A joint document by the GF and WHO 2
 Towards a global strategy 1 on Pharmacovigilance (PV) A joint document by the GF and WHO 2 PLAN of this document: Executive Summary 1- Background 2- Overall goal of this initiative 3- Driving principles
Towards a global strategy 1 on Pharmacovigilance (PV) A joint document by the GF and WHO 2 PLAN of this document: Executive Summary 1- Background 2- Overall goal of this initiative 3- Driving principles
Action points and notes. UNFPA, UN House, Copenhagen Denmark
 Action points and notes IPC Meeting 30 th November 02 nd December 2011 UNFPA, UN House, Copenhagen Denmark Action points - Share draft documents on diagnostics to be shared with IPC members for review
Action points and notes IPC Meeting 30 th November 02 nd December 2011 UNFPA, UN House, Copenhagen Denmark Action points - Share draft documents on diagnostics to be shared with IPC members for review
Ethical Issues, Community Perspective and Standards of Care for Clinical Research on Women & Children in Africa
 Ethical Issues, Community Perspective and Standards of Care for Clinical Research on Women & Children in Africa Holly Rawizza, M.D. Harvard PEPFAR Nigeria/APIN Plus ICASA Dakar, Senegal 3 December 2008
Ethical Issues, Community Perspective and Standards of Care for Clinical Research on Women & Children in Africa Holly Rawizza, M.D. Harvard PEPFAR Nigeria/APIN Plus ICASA Dakar, Senegal 3 December 2008
WHO s IMS Organizational Structure Overview
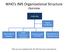 WHO s IMS Organizational Structure Overview Leadership Partner Coordination Information & Planning Health Expertise & Operations Operations Support and Logistics Management & Administration *this structure
WHO s IMS Organizational Structure Overview Leadership Partner Coordination Information & Planning Health Expertise & Operations Operations Support and Logistics Management & Administration *this structure
Effective application of Risk Management techniques to Drug Safety: a pragmatic approach
 Effective application of Risk Management techniques to Drug Safety: a pragmatic approach Dr Mark Perrott WCI Consulting ltd 19th October 2009 1 Disclaimer The views and opinions expressed in the following
Effective application of Risk Management techniques to Drug Safety: a pragmatic approach Dr Mark Perrott WCI Consulting ltd 19th October 2009 1 Disclaimer The views and opinions expressed in the following
JOB DESCRIPTION. Job title: Epidemiologist Location: Maputo, Mozambique frequent visits to Provinces
 JOB DESCRIPTION Job title: Epidemiologist Location: Maputo, Mozambique frequent visits to Provinces Department: Technical Length of contract: 4 years Role type: National Grade: 9 Travel involved: Up to
JOB DESCRIPTION Job title: Epidemiologist Location: Maputo, Mozambique frequent visits to Provinces Department: Technical Length of contract: 4 years Role type: National Grade: 9 Travel involved: Up to
Pharmacovigilance. Dr. Ganesh Uchit, MD Pfizer, India
 Pharmacovigilance 1 Dr. Ganesh Uchit, MD Pfizer, India DISCLAIMER The presentation is intended for educational purposes only and does not replace independent professional judgement. Statements of fact
Pharmacovigilance 1 Dr. Ganesh Uchit, MD Pfizer, India DISCLAIMER The presentation is intended for educational purposes only and does not replace independent professional judgement. Statements of fact
Saudi FDA Drug Approval Process. Dr. Mohammed A. Alquwaizani Consultant- Chief of the scientific office Saudi Food and Drug Authority
 Saudi FDA Drug Approval Process Dr. Mohammed A. Alquwaizani Consultant- Chief of the scientific office Saudi Food and Drug Authority Objective Describe the drug regulation practice in Saudi Arabia and
Saudi FDA Drug Approval Process Dr. Mohammed A. Alquwaizani Consultant- Chief of the scientific office Saudi Food and Drug Authority Objective Describe the drug regulation practice in Saudi Arabia and
Malaria Research Capability Strengthening in Africa
 October 2004 UNICEF/UNDP/World Bank/WHO Special Programme for Research & Training in Tropical Diseases (TDR) Multilateral Initiative on Malaria (MIM) Malaria Research Capability Strengthening in Africa
October 2004 UNICEF/UNDP/World Bank/WHO Special Programme for Research & Training in Tropical Diseases (TDR) Multilateral Initiative on Malaria (MIM) Malaria Research Capability Strengthening in Africa
GLOBAL PARTNERSHIP TO STOP TB STOP TB PARNTERSHIP SECRETARIAT 2003 WORKPLAN AND BUDGET EXECUTIVE SUMMARY
 GLOBAL PARTNERSHIP TO STOP TB STOP TB PARNTERSHIP SECRETARIAT 2003 WORKPLAN AND BUDGET EXECUTIVE SUMMARY DOCUMENT 6a Stop TB Coordinating Board, 28-29 October 2002, Cape Town, South Africa The Stop TB
GLOBAL PARTNERSHIP TO STOP TB STOP TB PARNTERSHIP SECRETARIAT 2003 WORKPLAN AND BUDGET EXECUTIVE SUMMARY DOCUMENT 6a Stop TB Coordinating Board, 28-29 October 2002, Cape Town, South Africa The Stop TB
Belgian Centre for Pharmacotherapeutic Information
 Pharmacovigilance and Risk Management Ann Van Ermen Belgian Centre for Pharmacotherapeutic Information (www.bcfi.be be ; www.cbip.be) be) and Belgian Centre for Pharmacovigilance Gent, August 26, 2007
Pharmacovigilance and Risk Management Ann Van Ermen Belgian Centre for Pharmacotherapeutic Information (www.bcfi.be be ; www.cbip.be) be) and Belgian Centre for Pharmacovigilance Gent, August 26, 2007
Identification and traceability of biological products. Dr Peter De Veene EU QPPV, Roche On behalf of EBE
 Identification and traceability of biological products Dr Peter De Veene EU QPPV, Roche On behalf of EBE Outline Biopharmaceuticals & Pharmacovigilance Traceability & Pharmacovigilance Legal Requirement
Identification and traceability of biological products Dr Peter De Veene EU QPPV, Roche On behalf of EBE Outline Biopharmaceuticals & Pharmacovigilance Traceability & Pharmacovigilance Legal Requirement
The Middle East. 15 Countries*, 5 Time Zones. Population > 333 millions** GDP: $3.13 Trillions*** 58% of world oil reserves
 Pharmaco-Economical Overview of Healthcare Omar Rifi, R.Ph., M.B.A. January, 2012-1- The Middle East 15 Countries*, 5 Time Zones Population > 333 millions** GDP: $3.13 Trillions*** 58% of world oil reserves
Pharmaco-Economical Overview of Healthcare Omar Rifi, R.Ph., M.B.A. January, 2012-1- The Middle East 15 Countries*, 5 Time Zones Population > 333 millions** GDP: $3.13 Trillions*** 58% of world oil reserves
Thijs J Giezen, PharmD, MSc, PhD The Netherlands
 Thijs J Giezen, PharmD, MSc, PhD The Netherlands Hospital Pharmacist, Foundation Pharmacy for Hospitals in Haarlem, The Netherlands Member of the Biosimilar Medicinal Product Working Party of European
Thijs J Giezen, PharmD, MSc, PhD The Netherlands Hospital Pharmacist, Foundation Pharmacy for Hospitals in Haarlem, The Netherlands Member of the Biosimilar Medicinal Product Working Party of European
Policy Position. Pharmacy-mediated interchangeability for Similar Biotherapeutic Products (SBPs)
 Pharmacy-mediated interchangeability for Similar Biotherapeutic Products (SBPs) Geneva, April 2016 Appropriate use of biotherapeutics including SBPs - SBPs, also known as biosimilars, are developed to
Pharmacy-mediated interchangeability for Similar Biotherapeutic Products (SBPs) Geneva, April 2016 Appropriate use of biotherapeutics including SBPs - SBPs, also known as biosimilars, are developed to
Quality check of spontaneous adverse drug reaction reporting forms of different countries y
 pharmacoepidemiology and drug safety (2010) Published online in Wiley Online Library (wileyonlinelibrary.com).2004 ORIGINAL REPORT Quality check of spontaneous adverse drug reaction reporting forms of
pharmacoepidemiology and drug safety (2010) Published online in Wiley Online Library (wileyonlinelibrary.com).2004 ORIGINAL REPORT Quality check of spontaneous adverse drug reaction reporting forms of
Overview of FDA s Sentinel Initiative
 Overview of FDA s Sentinel Initiative Judy Racoosin, Sentinel Initiative Scientific Lead, Office of Medical Policy, Center for Drug Evaluation and Research, U.S. Food and Drug Administration September
Overview of FDA s Sentinel Initiative Judy Racoosin, Sentinel Initiative Scientific Lead, Office of Medical Policy, Center for Drug Evaluation and Research, U.S. Food and Drug Administration September
Pharmacovigilance and Drug Safety Compliance Update GAP Analysis: How We Get to Where We Want to Be
 Pharmacovigilance and Drug Safety Compliance Update GAP Analysis: How We Get to Where We Want to Be John P. Ford Sidley Austin LLP Core PV Concepts Risk identification, collection of information Risk assessment,
Pharmacovigilance and Drug Safety Compliance Update GAP Analysis: How We Get to Where We Want to Be John P. Ford Sidley Austin LLP Core PV Concepts Risk identification, collection of information Risk assessment,
Antimicrobial Stewardship
 Antimicrobial Stewardship Safe Table Webcast / EQUIP Workbook for Alaska Meg Kilcup, PharmD, Director Safe Medication Practices WSHA March 14, 2017 Objectives Overview of the EQUIP Jump Start Stewardship
Antimicrobial Stewardship Safe Table Webcast / EQUIP Workbook for Alaska Meg Kilcup, PharmD, Director Safe Medication Practices WSHA March 14, 2017 Objectives Overview of the EQUIP Jump Start Stewardship
Pharmacovigilance System in Russia and the EAEU
 Pharmacovigilance System in Russia and the EAEU Authors: Sergey Simeniv CEO X7 Research, CRO; Olga Latysheva Head of Pharmacovigilance Department X7 Research, CRO; Dmitry Kryuchkov Executive Director X7
Pharmacovigilance System in Russia and the EAEU Authors: Sergey Simeniv CEO X7 Research, CRO; Olga Latysheva Head of Pharmacovigilance Department X7 Research, CRO; Dmitry Kryuchkov Executive Director X7
HEALTHCARE IN GOMBALA
 HEALTHCARE IN GOMBALA Team 4 Saketh Chinni Deepti Nair Brandon Sturm Jing Wang Our Plan Today 2 Problem Statement Recommendation Gombala Analysis Implementation Operational Opeaoa Challenges Caeges & Caveats
HEALTHCARE IN GOMBALA Team 4 Saketh Chinni Deepti Nair Brandon Sturm Jing Wang Our Plan Today 2 Problem Statement Recommendation Gombala Analysis Implementation Operational Opeaoa Challenges Caeges & Caveats
Pharmacovigilance in Asia: The China Perspectives. Disclaimer
 Pharmacovigilance in Asia: The China Perspectives Rebecca Wang, MD, FRCP, FACC Head, Product Development Drug Safety Operation, Asia Pacific Roche Shanghai Disclaimer The views and opinions expressed in
Pharmacovigilance in Asia: The China Perspectives Rebecca Wang, MD, FRCP, FACC Head, Product Development Drug Safety Operation, Asia Pacific Roche Shanghai Disclaimer The views and opinions expressed in
Professor Kimme Hyrich, MD, PhD, FRCPC, UK
 GaBI Scientific Meetings 26 January 2017, Pullman London St Pancras, London, UK ROUNDTABLE ON REGISTRIES Practical Considerations for Registries making them work Professor Kimme Hyrich, MD, PhD, FRCPC,
GaBI Scientific Meetings 26 January 2017, Pullman London St Pancras, London, UK ROUNDTABLE ON REGISTRIES Practical Considerations for Registries making them work Professor Kimme Hyrich, MD, PhD, FRCPC,
Zimbabwe National Pharmacovigilance Policy Handbook
 Zimbabwe National Pharmacovigilance Policy Handbook 2 nd Edition, December 2016 106 Baines Avenue Tel: +263-4-708255/792165/736981 In collaboration with the Ministry Harare Mobile: +263 772145191/2/3 of
Zimbabwe National Pharmacovigilance Policy Handbook 2 nd Edition, December 2016 106 Baines Avenue Tel: +263-4-708255/792165/736981 In collaboration with the Ministry Harare Mobile: +263 772145191/2/3 of
Regional Medicines Optimisation Committee Briefing Best Value Biologicals: Adalimumab Update 2
 Regional Medicines Optimisation Committee Briefing Best Value Biologicals: Adalimumab Update 2 Adalimumab RMOC Update 1: https://www.sps.nhs.uk/articles/rmoc-briefing-paper-on-adalimumab/ The purpose of
Regional Medicines Optimisation Committee Briefing Best Value Biologicals: Adalimumab Update 2 Adalimumab RMOC Update 1: https://www.sps.nhs.uk/articles/rmoc-briefing-paper-on-adalimumab/ The purpose of
WORKING WITH COUNTRIES TO BRING NEW PRODUCTS TO MARKETS THE ACHIEVEMENTS OF AVAREF & REGIONAL HARMONISATION
 WORKING WITH COUNTRIES TO BRING NEW PRODUCTS TO MARKETS THE ACHIEVEMENTS OF AVAREF & REGIONAL HARMONISATION Outline Introduction Global Product Development Challenges Key Regulatory Challenges AVAREF History
WORKING WITH COUNTRIES TO BRING NEW PRODUCTS TO MARKETS THE ACHIEVEMENTS OF AVAREF & REGIONAL HARMONISATION Outline Introduction Global Product Development Challenges Key Regulatory Challenges AVAREF History
Pharmacovigilance: A Review
 ISSN 0976 3333 Available Online at www.ijpba.info International Journal of Pharmaceutical & Biological Archives 2011; 2(6):1569-1574 REVIEW ARTICLE Pharmacovigilance: A Review Avani Patel *1, D.Giles 1,
ISSN 0976 3333 Available Online at www.ijpba.info International Journal of Pharmaceutical & Biological Archives 2011; 2(6):1569-1574 REVIEW ARTICLE Pharmacovigilance: A Review Avani Patel *1, D.Giles 1,
The people of Haiti have long been overwhelmed by health problems related to
 Performance-Based Contracting With NGOs in Haiti by John Pollock The people of Haiti have long been overwhelmed by health problems related to rapid population growth, poverty, poor diet, and emerging diseases.
Performance-Based Contracting With NGOs in Haiti by John Pollock The people of Haiti have long been overwhelmed by health problems related to rapid population growth, poverty, poor diet, and emerging diseases.
2 Pharmacovigilance. 2.1 Fundamentals of Pharmacovigilance
 Pharmacovigilance 2 Pharmacovigilance 2 In this chapter, the fundamentals of pharmacovigilance are outlined with a particular emphasis on the role of healthcare professionals in reporting adverse drug
Pharmacovigilance 2 Pharmacovigilance 2 In this chapter, the fundamentals of pharmacovigilance are outlined with a particular emphasis on the role of healthcare professionals in reporting adverse drug
The Future of Drug Safety
 The Future of Drug Safety IOM Committee on the Assessment of the US Drug Safety System R. Alta Charo Warren P. Knowles Professor of Law & Bioethics University of Wisconsin Charge to the Committee Examine
The Future of Drug Safety IOM Committee on the Assessment of the US Drug Safety System R. Alta Charo Warren P. Knowles Professor of Law & Bioethics University of Wisconsin Charge to the Committee Examine
WHA65.29 Addressing the Global Shortages of Medicines and Vaccines. ISG June WHA Geneva
 WHA65.29 Addressing the Global Shortages of Medicines and Vaccines 1 CASE STUDY, PROGRESS AND ACTIVITIES 2 Informal survey on shortages of lopinavir/ ritonavir. Rapid root cause analysis noted planning
WHA65.29 Addressing the Global Shortages of Medicines and Vaccines 1 CASE STUDY, PROGRESS AND ACTIVITIES 2 Informal survey on shortages of lopinavir/ ritonavir. Rapid root cause analysis noted planning
Dialogue 1: Partnerships in support of strengthening health systems: Building resilience to pandemics 1
 ECONOMIC AND SOCIAL COUNCIL PARTNERSHIPS FORUM Dialogue 1: Partnerships in support of strengthening health systems: Building resilience to pandemics 1 Introduction 28 May 2015, 11:00 a.m. 1:00 p.m. UN
ECONOMIC AND SOCIAL COUNCIL PARTNERSHIPS FORUM Dialogue 1: Partnerships in support of strengthening health systems: Building resilience to pandemics 1 Introduction 28 May 2015, 11:00 a.m. 1:00 p.m. UN
CBA Workshop Series -3 Drug Safety (Pharmacovigilance) and Big Data. Larry Liu TCONNEX Inc.
 CBA Workshop Series -3 Drug Safety (Pharmacovigilance) and Big Data Larry Liu TCONNEX Inc. Larry.liu@tconnex.com Drug Safety Drug Safety is about Adverse Drug Reaction or ADR ADR is any unintended and
CBA Workshop Series -3 Drug Safety (Pharmacovigilance) and Big Data Larry Liu TCONNEX Inc. Larry.liu@tconnex.com Drug Safety Drug Safety is about Adverse Drug Reaction or ADR ADR is any unintended and
Addressing the global shortages of medicines, and the safety and accessibility of children s medication
 EXECUTIVE BOARD EB138/41 138th session 18 December 2015 Provisional agenda item 10.5 Addressing the global shortages of medicines, and the safety and accessibility of children s medication Report by the
EXECUTIVE BOARD EB138/41 138th session 18 December 2015 Provisional agenda item 10.5 Addressing the global shortages of medicines, and the safety and accessibility of children s medication Report by the
