NIH Forms E Changes for Human Subject and/or Clinical Trial Applications Attached are the Forms E application pages with the most significant changes
|
|
|
- Colleen Leonard
- 5 years ago
- Views:
Transcription
1 NIH Forms E Changes for Human Subject and/or Clinical Trial Applications Attached are the Forms E application pages with the most significant changes (pages 11-16). Many of the new fields require a short answer, rather than an attached document. This information will be needed from the PI in order to complete the application. Helpful information about the field is included in the blue boxes. Forms E is used for applications submitted with due dates on or after January 25, 218. Page 11: Additional questions if No is answered to involvement of human subjects. Additional attachment required if using human specimens and/or data. If Yes to human subjects, additional attachment(s) may be required for delayed onset studies including human subject study records. Here is the first place that Clinical Trial is checked and correct FOA must be used. Moving forward FOAs will distinguish between CT accepted, excluded, or optional. Page 12: This page is included in applications that accept clinical trials. Notice in section 1.4 that you answer all four of the questions to determine if this is a clinical trial. A NIH decision tree can be found at and this should be determined early in your decision process to submit an application. Section 2 covers Study Population Characteristics. New questions and attachments for inclusion and recruitment are covered here. Note the F and K application notes about clinical trials at the bottom of the page. Page 13: This is the new Inclusion Enrollment Report for Planned Enrollment and only rows and columns applicable can be filled (does not include unknown). Foreign locations are listed by country. Page 14: This is the new Inclusion Enrollment Report for Cumulative (Actual) Enrollment and only rows and columns applicable can be used (includes unknown). Pages 15-16: Section 3 Protection and Monitoring Plans. New questions about multi-site, single IRB protocols and appointments of DSMB boards. Section 4 Protocol Synopsis for Clinical Trials only. These fields are mainly short description and drop-down lists that will be needed to complete the CT application. Please familiarize yourself with these requirements if you are planning to submit a CT application.
2 PHS Human Subjects and Clinical Trials Information OMB Number: Expiration Date: 3/31/22 Please complete the human subjects section of the Research & Related Other Project Information form prior to completing this form. The following items are taken from the Research & Related Other Project Information form and displayed here for your reference. Any changes to these fields must be made on the Research & Related Other Project Information form and may impact the data items you are required to complete on this form. Are Human Subjects Involved? Yes No Is the Project Exempt from Federal regulations? Yes No Exemption number: If No to Human Subjects Does the proposed research involve human specimens and/or data? Yes No If Yes, provide an explanation of why the application does not involve human subjects research. Add Attachment Delete Attachment View Attachment Skip the rest of the PHS Human Subjects and Clinical Trials Information Form. If Yes to Human Subjects Add a record for each proposed Human Subject Study by selecting Add New Study or Add New Delayed Onset Study as appropriate. Delayed onset studies are those for which there is no well-defined plan for human subject involvement at the time of submission, per agency policies on Delayed Onset Studies. For delayed onset studies, you will provide the study name and a justification for omission of human subjects study information. Other Requested Information Add Attachment Delete Attachment View Attachment Study Record(s) Click here to extract the Human Subject Study Record Attachment Attach human subject study records using unique filenames. 1) Please attach Human Subject Study 1 Add Attachment Delete Attachment View Attachment Delayed Onset Study(ies) Study Title Anticipated Clinical Trial? Justification Add Attachment Delete Attachment View Attachment Updated: July 17, 217 FORMS-E Series Page 11 of 36
3 Check Form for Errors Save Study Record: PHS Human Subjects and Clinical Trials Information * Always required field OMB Number: Expiration Date: 3/31/22 Section 1 - Basic Information 1.1. * Study Title (each study title must be unique) 1.2. * Is this Study Exempt from Federal Regulations? Yes No 1.3. Exemption Number * Clinical Trial Questionnaire If the answers to all four questions below are yes, this study meets the definition of a Clinical Trial. 1.4.a. Does the study involve human participants? Yes No 1.4.b. Are the participants prospectively assigned to an intervention? Yes No 1.4.c. Is the study designed to evaluate the effect of the intervention on the participants? Yes No 1.4.d. Is the effect that will be evaluated a health-related, biomedical, or behavioral outcome? Yes No 1.5. Provide the ClinicalTrials.gov Identifier (e.g., NCT ) for this trial, if applicable Section 2 - Study Population Characteristics 2.1. Conditions or Focus of Study 2.2. Eligibility Criteria 2.3. Age Limits Minimum Age Maximum Age 2.4. Inclusion of Women, Minorities, and Children Add Attachment Delete Attachment View Attachment 2.5. Recruitment and Retention Plan Add Attachment Delete Attachment View Attachment 2.6. Recruitment Status 2.7. Study Timeline Add Attachment Delete Attachment View Attachment 2.8. Enrollment of First Subject Inclusion Enrollment Report(s) Add Inclusion Enrollment Report Updated: July 17, 217 FORMS-E Series Page 12 of 36
4 Inclusion Enrollment Report 1. * Using an Existing Dataset or Resource Yes No 2. * Enrollment Location Type Domestic Foreign 3. Enrollment Country(ies) 4. Enrollment Location(s) 5. Comments Planned Ethnic Categories Racial Categories Not Hispanic or Latino Hispanic or Latino Total American Indian/ Alaska Native Asian Native Hawaiian or Other Pacific Islander Black or African American White More than One Race Total Updated: July 17, 217 FORMS-E Series Page 13 of 36
5 Cumulative (Actual) Ethnic Categories Racial Categories Not Hispanic or Latino Unknown/ Not Hispanic or Latino Unknown/ Not Unknown/Not Ethnicity Unknown/ Not Total American Indian/ Alaska Native Asian Native Hawaiian or Other Pacific Islander Black or African American White More than One Race Unknown or Not Total Report 1 of 1 Updated: July 17, 217 FORMS-E Series Page 14 of 36
6 Section 3 - Protection and Monitoring Plans 3.1. Protection of Human Subjects Add Attachment Delete Attachment View Attachment 3.2. Is this a multi-site study that will use the same protocol to conduct non-exempt human subjects research at more than one domestic site? Yes No N/A If yes, describe the single IRB plan Add Attachment Delete Attachment View Attachment 3.3. Data and Safety Monitoring Plan Add Attachment Delete Attachment View Attachment 3.4. Will a Data and Safety Monitoring Board be appointed for this study? Yes No 3.5. Overall Structure of the Study Team Add Attachment Delete Attachment View Attachment Section 4 - Protocol Synopsis 4.1. Brief Summary 4.2. Study Design 4.2.a. Narrative Study Description 4.2.b. Primary Purpose 4.2.c. Interventions Intervention Type Name Description 4.2.d. Study Phase Is this an NIH-defined Phase III clinical trial? Yes No 4.2.e. Intervention Model 4.2.f. Masking Yes No Participant Care Provider Investigator Outcomes Assessor Updated: July 17, 217 FORMS-E Series Page 15 of 36
7 4.2.g. Allocation 4.3. Outcome Measures Name Type Time Frame Brief Description 4.4. Statistical Design and Power Add Attachment Delete Attachment View Attachment 4.5. Subject Participation Duration 4.6. Will the study use an FDA-regulated intervention? Yes No 4.6.a. If yes, describe the availability of Investigational Product (IP) and Investigational New Drug (IND)/Investigational Device Exemption (IDE) status Add Attachment Delete Attachment View Attachment 4.7. Dissemination Plan Add Attachment Delete Attachment View Attachment Section 5 - Other Clinical Trial-related Attachments 5.1. Other Clinical Trial-related Attachments Add Attachments Delete Attachments View Attachments Updated: July 17, 217 FORMS-E Series Page 16 of 36
PHS Human Subjects and Clinical Trials Information
 PHS Human Subjects and Clinical Trials Information OMB Number: 925-1 Expiration Date: 3/31/22 Please complete the human subjects section of the Research & Related Other Project Information form prior to
PHS Human Subjects and Clinical Trials Information OMB Number: 925-1 Expiration Date: 3/31/22 Please complete the human subjects section of the Research & Related Other Project Information form prior to
Human Subjects Requirements for NIH and AHRQ Applications: Overview of Changes
 Human Subjects Requirements for NIH and AHRQ Applications: Overview of Changes Martha E. Payne, PhD, MPH Office of Research Development, Duke University School of Medicine Objectives for Today What has
Human Subjects Requirements for NIH and AHRQ Applications: Overview of Changes Martha E. Payne, PhD, MPH Office of Research Development, Duke University School of Medicine Objectives for Today What has
Major Changes to NIH & AHRQ Submissions with Human Subjects
 Major Changes to NIH & AHRQ Submissions with Human Subjects Good Clinical Practice New Application Forms Single IRB Clinical Trial Review Criteria Get Ready, Get Set, Apply Early. Registration & Reporting
Major Changes to NIH & AHRQ Submissions with Human Subjects Good Clinical Practice New Application Forms Single IRB Clinical Trial Review Criteria Get Ready, Get Set, Apply Early. Registration & Reporting
HOW TO MAINTAIN &POPULATE THE PHS HUMAN SUBJECTS AND CLINICAL TRIALS INFORMATION FORM (HSCT 1.0) IN KC PROPOSALS
 KC Proposal Instructions for populating the PHS Human Subjects and Clinical Trial Information Form 1.0 Quick Reference Card HOW TO MAINTAIN &POPULATE THE PHS HUMAN SUBJECTS AND CLINICAL TRIALS INFORMATION
KC Proposal Instructions for populating the PHS Human Subjects and Clinical Trial Information Form 1.0 Quick Reference Card HOW TO MAINTAIN &POPULATE THE PHS HUMAN SUBJECTS AND CLINICAL TRIALS INFORMATION
Welcome Baby Impact Evaluation Request for Proposals FREQUENTLY ASKED QUESTIONS. Information Webinar
 Last Updated: December 18, 2015 Welcome Baby Impact Evaluation Request for Proposals FREQUENTLY ASKED QUESTIONS Information Webinar Please note that some responses may have been refined to more clearly
Last Updated: December 18, 2015 Welcome Baby Impact Evaluation Request for Proposals FREQUENTLY ASKED QUESTIONS Information Webinar Please note that some responses may have been refined to more clearly
NIH Clinical Trials Updates: Putting it All Together. Why the changes to NIH-funded studies involving human subjects?
 NIH Clinical Trials Updates: Putting it All Together Sherry Mills, MD MPH Director Office of Extramural Programs Office of Extramural Research NIH 1 Why the changes to NIH-funded studies involving human
NIH Clinical Trials Updates: Putting it All Together Sherry Mills, MD MPH Director Office of Extramural Programs Office of Extramural Research NIH 1 Why the changes to NIH-funded studies involving human
New NIH Clinical Trial Requirements for Grants & Contracts 2017/2018
 New NIH Clinical Trial Requirements for Grants & Contracts 2017/2018 Changes in Human Subjects Research Grant Application Process Clinical Trial Applications IRB Single IRB Certificates of Confidentiality
New NIH Clinical Trial Requirements for Grants & Contracts 2017/2018 Changes in Human Subjects Research Grant Application Process Clinical Trial Applications IRB Single IRB Certificates of Confidentiality
2018 ABA Model Diversity Survey
 2018 ABA Model Diversity Survey PLEASE NOTE: You will not be able to save your entries. Please see the pdf version of the survey on the homepage, gather all of your firm data, and plan accordingly. You
2018 ABA Model Diversity Survey PLEASE NOTE: You will not be able to save your entries. Please see the pdf version of the survey on the homepage, gather all of your firm data, and plan accordingly. You
2018 ABA Model Diversity Survey
 2018 ABA Model Diversity Survey PLEASE NOTE: You will not be able to save your entries. Please see the pdf version of the survey on the homepage and plan accordingly. PURPOSE: The American Bar Association
2018 ABA Model Diversity Survey PLEASE NOTE: You will not be able to save your entries. Please see the pdf version of the survey on the homepage and plan accordingly. PURPOSE: The American Bar Association
ClinicalTrials.gov Updates. Gabrielle Gaspard Assistant Director Human Research Compliance
 ClinicalTrials.gov Updates Gabrielle Gaspard Assistant Director Human Research Compliance 02.12.18 1 Posting Requirements for ClinicalTrials.gov Reporting Requirement ICMJE Policy (effective in 2005) FDAAA
ClinicalTrials.gov Updates Gabrielle Gaspard Assistant Director Human Research Compliance 02.12.18 1 Posting Requirements for ClinicalTrials.gov Reporting Requirement ICMJE Policy (effective in 2005) FDAAA
Doing Human Subjects Research? Changing NIH Policies May Impact You
 Doing Human Subjects Research? Changing NIH Policies May Impact You Reforms & Initiatives To enhance the stewardship of research involving human subjects, NIH is implementing the following: All Research
Doing Human Subjects Research? Changing NIH Policies May Impact You Reforms & Initiatives To enhance the stewardship of research involving human subjects, NIH is implementing the following: All Research
NIH GRANTEE SUPPORT FOR MANAGEMENT OF HUMAN SUBJECTS AND CLINICAL TRIALS STUDY INFORMATION
 NIH GRANTEE SUPPORT FOR MANAGEMENT OF HUMAN SUBJECTS AND CLINICAL TRIALS STUDY INFORMATION DAWN CORBETT, MPH NIH INCLUSION POLICY OFFICER OFFICE OF EXTRAMURAL PROGRAMS OFFICE OF EXTRAMURAL RESEARCH INCLUSION@MAIL.NIH.GOV
NIH GRANTEE SUPPORT FOR MANAGEMENT OF HUMAN SUBJECTS AND CLINICAL TRIALS STUDY INFORMATION DAWN CORBETT, MPH NIH INCLUSION POLICY OFFICER OFFICE OF EXTRAMURAL PROGRAMS OFFICE OF EXTRAMURAL RESEARCH INCLUSION@MAIL.NIH.GOV
EQUAL EMPLOYMENT OPPORTUNITY (E.E.O.) WORKFORCE STATISTICS FORM
 CITY OF URBANA HUMAN RELATIONS DIVISION 400 SOUTH VINE ST. URBANA, ILLINOIS 61801 (217) 384-2455 (phone); 328-8288 (fax) hro@urbanaillinois.us Requested by: Approved by: Office Use Only (09/15) Certification
CITY OF URBANA HUMAN RELATIONS DIVISION 400 SOUTH VINE ST. URBANA, ILLINOIS 61801 (217) 384-2455 (phone); 328-8288 (fax) hro@urbanaillinois.us Requested by: Approved by: Office Use Only (09/15) Certification
CONTACT INFORMATION. PHRC Office. Josephine O Driscoll-Davis. Michael Ducey
 PHRC Office CONTACT INFORMATION 116 Huntington Ave., 10 th Floor Boston, MA 02116 http://healthcare.partners.org/phsirb Josephine O Driscoll-Davis Assistant Director Administrative Chair (617) 424-4107
PHRC Office CONTACT INFORMATION 116 Huntington Ave., 10 th Floor Boston, MA 02116 http://healthcare.partners.org/phsirb Josephine O Driscoll-Davis Assistant Director Administrative Chair (617) 424-4107
Terry Sousa UMass Medical School PRS Administrator for Clinicaltrials.gov
 Terry Sousa UMass Medical School PRS Administrator for Clinicaltrials.gov What is ClinicalTrials.gov? National registry of federally and privately supported research studies Why register and report results?
Terry Sousa UMass Medical School PRS Administrator for Clinicaltrials.gov What is ClinicalTrials.gov? National registry of federally and privately supported research studies Why register and report results?
Compliance Department RESEARCH COMPLIANCE MEMORANDUM
 Compliance Department RESEARCH COMPLIANCE MEMORANDUM To: UCD Health Chairs & Chief Administrator Officers From: Nirali Patel, Compliance Manager Kathy Olson, Research Compliance Analyst Re: Department
Compliance Department RESEARCH COMPLIANCE MEMORANDUM To: UCD Health Chairs & Chief Administrator Officers From: Nirali Patel, Compliance Manager Kathy Olson, Research Compliance Analyst Re: Department
Address: Education. Grade Completed
 APPLICATION FOR EMPLOYMENT AN EQUAL OPPORTUNITY EMPLOYER Virginia Bank & Trust is an equal opportunity and affirmative action employer. We comply with federal, state and/or local laws that prohibit discrimination
APPLICATION FOR EMPLOYMENT AN EQUAL OPPORTUNITY EMPLOYER Virginia Bank & Trust is an equal opportunity and affirmative action employer. We comply with federal, state and/or local laws that prohibit discrimination
POLICY AND PROCEDURE MANUAL
 POLICY AND PROCEDURE MANUAL PBRC: POLICY NO. 378.00 ORIGIN DATE: 5/25/2016 IMPACTS: CLINICAL RESEARCH LAST REVISED: 1/1/2017 SUBJECT: SOURCE: PURPOSE PENNINGTON BIOMEDICAL CLINICALTRIALS.GOV POLICY INSTITUTIONAL
POLICY AND PROCEDURE MANUAL PBRC: POLICY NO. 378.00 ORIGIN DATE: 5/25/2016 IMPACTS: CLINICAL RESEARCH LAST REVISED: 1/1/2017 SUBJECT: SOURCE: PURPOSE PENNINGTON BIOMEDICAL CLINICALTRIALS.GOV POLICY INSTITUTIONAL
ELEMENTS OF A DATA MONITORING PLAN
 ELEMENTS OF A DATA MONITORING PLAN Definitions Data Monitoring: The regular evaluation of data and documentation collected during a study to ensure both adherence to the approved investigative plan and
ELEMENTS OF A DATA MONITORING PLAN Definitions Data Monitoring: The regular evaluation of data and documentation collected during a study to ensure both adherence to the approved investigative plan and
December 7, CFPB Annual Employee Survey
 December 7, 2012 2012 CFPB Annual Employee Survey Introduction Interpretation of results: Approximately 74 percent of CFPB employees responded to the first annual employee survey conducted by the Consumer
December 7, 2012 2012 CFPB Annual Employee Survey Introduction Interpretation of results: Approximately 74 percent of CFPB employees responded to the first annual employee survey conducted by the Consumer
Supportive Intervention Services, LLC
 Application for Employment Please Print Legibly. Fill out Application Completely and Answer Each Question. Supportive Intervention Services, LLC is Equal Opportunity, Affirmative Action employers. No question
Application for Employment Please Print Legibly. Fill out Application Completely and Answer Each Question. Supportive Intervention Services, LLC is Equal Opportunity, Affirmative Action employers. No question
University of West Florida Recruitment Checklist
 University of West Florida Recruitment Checklist This checklist has been prepared to assist you in recruiting for positions at UWF. For additional assistance, please contact April Sargent, asargent@uwf.edu,
University of West Florida Recruitment Checklist This checklist has been prepared to assist you in recruiting for positions at UWF. For additional assistance, please contact April Sargent, asargent@uwf.edu,
INVESTIGATIONAL DEVICE EXEMPTION APPLICATION. IDE Title (if title being used)
 INVESTIGATIONAL DEVICE EXEMPTION APPLICATION IDE Title (if title being used) Name of Sponsor Investigator, MD X Professor, Department Icahn School of Medicine at Mount Sinai Date of Submission Form version
INVESTIGATIONAL DEVICE EXEMPTION APPLICATION IDE Title (if title being used) Name of Sponsor Investigator, MD X Professor, Department Icahn School of Medicine at Mount Sinai Date of Submission Form version
PENNSYLVANIA TURNPIKE COMMISSION
 PENNSYLVANIA TURNPIKE COMMISSION RETENTION OF AN ENGINEERING FIRM General Consulting Engineer Services Reference No. 3-214 The Pennsylvania Turnpike Commission (PTC) intends to issue a Request for Proposals
PENNSYLVANIA TURNPIKE COMMISSION RETENTION OF AN ENGINEERING FIRM General Consulting Engineer Services Reference No. 3-214 The Pennsylvania Turnpike Commission (PTC) intends to issue a Request for Proposals
ClinicalTrials.gov Registration Guide
 ClinicalTrials.gov Registration Guide The Food and Drug Administration Amendments Act (FDAAA), National Institutes of Health (NIH) and International Committee of Medical Journal Editors (ICMJE) require
ClinicalTrials.gov Registration Guide The Food and Drug Administration Amendments Act (FDAAA), National Institutes of Health (NIH) and International Committee of Medical Journal Editors (ICMJE) require
EMPLOYMENT APPLICATION
 EMPLOYMENT APPLICATION The Maryland Judiciary is an Equal Opportunity Employer Please print or type all information. Please complete all relevant sections. Application may be rejected if information is
EMPLOYMENT APPLICATION The Maryland Judiciary is an Equal Opportunity Employer Please print or type all information. Please complete all relevant sections. Application may be rejected if information is
EMPLOYMENT APPLICATION The Maryland Judiciary is an Equal Opportunity Employer
 EMPLOYMENT APPLICATION The Maryland Judiciary is an Equal Opportunity Employer INSTRUCTIONS: Please print or type all information. Please complete all relevant sections. Your application may be rejected
EMPLOYMENT APPLICATION The Maryland Judiciary is an Equal Opportunity Employer INSTRUCTIONS: Please print or type all information. Please complete all relevant sections. Your application may be rejected
Affirmative Action Plan Executive Summary
 Affirmative Action Plan 2016 Executive Summary 1 Executive Summary Introduction Signed into law in 1965 by President Johnson, Executive Order 11246 required federal contractors to adopt an affirmative
Affirmative Action Plan 2016 Executive Summary 1 Executive Summary Introduction Signed into law in 1965 by President Johnson, Executive Order 11246 required federal contractors to adopt an affirmative
US Special Operations Command Human Research Protection Office
 S- US Special Operations Command Human Research Protection Office Human Research Protocol Submission Form for Headquarters Level Administrative Review of Extramural* Research PURPOSE: All United States
S- US Special Operations Command Human Research Protection Office Human Research Protocol Submission Form for Headquarters Level Administrative Review of Extramural* Research PURPOSE: All United States
DIVERSITY IN WORKFORCE RECRUITMENT
 DIVERSITY IN WORKFORCE RECRUITMENT CPW DEFINES DIVERSITY AS Veteran, disability, gender, ethnicity, and varying age classes. 56.8% 32.3 4.4 5.6 71.1% 20.6 4.1 3.2 21.3 85.2% 10.8 3.8 Connections with
DIVERSITY IN WORKFORCE RECRUITMENT CPW DEFINES DIVERSITY AS Veteran, disability, gender, ethnicity, and varying age classes. 56.8% 32.3 4.4 5.6 71.1% 20.6 4.1 3.2 21.3 85.2% 10.8 3.8 Connections with
Retail / Office Building
 FOR LEASE > RETAIL / BUILDING Property Highlights > ±2,261 - ±3,680 SF Available > Fronting busy Contra Costa Blvd (27,235 ADT) > Located at the entrance to DVC Plaza Center re-development > Ground floor
FOR LEASE > RETAIL / BUILDING Property Highlights > ±2,261 - ±3,680 SF Available > Fronting busy Contra Costa Blvd (27,235 ADT) > Located at the entrance to DVC Plaza Center re-development > Ground floor
This report was obtained (via FOIA) and posted by AltGov2.
 This report was obtained (via FOIA) and posted by AltGov2 www.altgov2.org/fevs 2014 CFPB annual employee survey December 2014 Introduction Interpretation of results More than 83 percent of the CFPB employee
This report was obtained (via FOIA) and posted by AltGov2 www.altgov2.org/fevs 2014 CFPB annual employee survey December 2014 Introduction Interpretation of results More than 83 percent of the CFPB employee
Amarex Clinical Research Washington DC metro area A Product Development Services Company. From Lab to Market Approval
 Amarex Clinical Research Washington DC metro area A Product Development Services Company Regulatory Strategy From Lab to Market Approval Strategy Implementation Pre-Clinical Assays Global Clinical Trials
Amarex Clinical Research Washington DC metro area A Product Development Services Company Regulatory Strategy From Lab to Market Approval Strategy Implementation Pre-Clinical Assays Global Clinical Trials
Toward Greater Transparency and Rigor
 Toward Greater Transparency and Rigor TRANSPARENCY: ClinicalTrials.gov registration and results reporting The Center for Clinical & Translational Science 205.934.7442 ccts@uab.edu www.uab.edu/ccts @cctsnetwork
Toward Greater Transparency and Rigor TRANSPARENCY: ClinicalTrials.gov registration and results reporting The Center for Clinical & Translational Science 205.934.7442 ccts@uab.edu www.uab.edu/ccts @cctsnetwork
Mayor Carter s Vision for Saint Paul
 Mayor Carter s Vision for Saint Paul Topics & Objectives: 3 Saint Paul s Landscape Population: 285,068 60.1% White 39.9% POC Saint Paul Workforce: 2,942 FT 73% White 27% POC 30% Women May 13, 2013. (MPR
Mayor Carter s Vision for Saint Paul Topics & Objectives: 3 Saint Paul s Landscape Population: 285,068 60.1% White 39.9% POC Saint Paul Workforce: 2,942 FT 73% White 27% POC 30% Women May 13, 2013. (MPR
EEOC File Creation and Submission
 EEOC File Creation and Submission Step 1: Run Employee EEO-1 Detail Report Navigate to Report Navigator >> HR Compliance >> PER00200 report. This will report will help determine which employees have not
EEOC File Creation and Submission Step 1: Run Employee EEO-1 Detail Report Navigate to Report Navigator >> HR Compliance >> PER00200 report. This will report will help determine which employees have not
Changed EEO Minority Codes in Payroll and the. Payroll, Human Resource Suite
 in Payroll and the Human Resource Suite Modules Affected: Versions Affected: Payroll, Human Resource Suite Version 6 and 7 (Software levels 7.6c3.16, 9.6c3.16, 8.7c2.16, and 9.7c2.16) The discussion of
in Payroll and the Human Resource Suite Modules Affected: Versions Affected: Payroll, Human Resource Suite Version 6 and 7 (Software levels 7.6c3.16, 9.6c3.16, 8.7c2.16, and 9.7c2.16) The discussion of
UC DAVIS OFFICE OF RESEARCH AAHRPP Preparation UC Davis Human Research Part III Investigator Manual. Cindy Gates IRB Administration
 UC DAVIS OFFICE OF RESEARCH AAHRPP Preparation UC Davis Human Research Part III Investigator Manual Cindy Gates IRB Administration What is the purpose of the INVESTIGATOR MANUAL? The manual is designed
UC DAVIS OFFICE OF RESEARCH AAHRPP Preparation UC Davis Human Research Part III Investigator Manual Cindy Gates IRB Administration What is the purpose of the INVESTIGATOR MANUAL? The manual is designed
Request for Proposals. For An. Assessment Study of Disproportionate Minority Contact (DMC) with the North Carolina Juvenile Justice System
 Request for Proposals For An Assessment Study of Disproportionate Minority Contact (DMC) with the North Carolina Juvenile Justice System Issued By: The Governor s Crime Commission, Juvenile Justice Planning
Request for Proposals For An Assessment Study of Disproportionate Minority Contact (DMC) with the North Carolina Juvenile Justice System Issued By: The Governor s Crime Commission, Juvenile Justice Planning
Faculty Profile
 2013-14 Faculty Profile Prepared by the Office of Institutional Research Wayne State University 2013-14 Faculty Profile Preface This profile contains information about full-time faculty at Wayne State
2013-14 Faculty Profile Prepared by the Office of Institutional Research Wayne State University 2013-14 Faculty Profile Preface This profile contains information about full-time faculty at Wayne State
PharmaSUG 2012 Paper #IB01
 PharmaSUG 2012 Paper #IB01 Adapt and Survive: Implementing the new ICH Development Safety Update Report (DSUR) Patricia L. Gerend, Genentech Inc., South San Francisco, CA Rajkumar Sharma, Genentech Inc.,
PharmaSUG 2012 Paper #IB01 Adapt and Survive: Implementing the new ICH Development Safety Update Report (DSUR) Patricia L. Gerend, Genentech Inc., South San Francisco, CA Rajkumar Sharma, Genentech Inc.,
Policy Appendix IV: DRCR.net Industry Collaboration Policies Version 6.0, August 2, 2017
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 DRCR.net is committed to: performing rigorous multi-center clinical
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 DRCR.net is committed to: performing rigorous multi-center clinical
Clinical Trial Diversification Better Practices
 Clinical Trial Diversification Better Practices TOPIC 1(A): DIVERSITY AWARENESS MATERIALS FOR SPONSORS These materials were first completed on 10-April, 2015 These materials were last updated on 8-May,
Clinical Trial Diversification Better Practices TOPIC 1(A): DIVERSITY AWARENESS MATERIALS FOR SPONSORS These materials were first completed on 10-April, 2015 These materials were last updated on 8-May,
WALTON COUNTY IS A DRUG FREE WORKPLACE! PERSONAL DATA Please print in black or blue ink or type DO NOT use pencil.
 Positions Applied For: WALTON COUNTY EMPLOYMENT APPLICATION 303 South Hammond Drive, Suite 331 Monroe, Georgia 30655 Office: 770-267-1329, Fax: 770-267-1415, Job Line: 770-267-1986 Email: hr.resume@co.walton.ga.us
Positions Applied For: WALTON COUNTY EMPLOYMENT APPLICATION 303 South Hammond Drive, Suite 331 Monroe, Georgia 30655 Office: 770-267-1329, Fax: 770-267-1415, Job Line: 770-267-1986 Email: hr.resume@co.walton.ga.us
NIH funded Human Subjects Research/Clinical Trials and the Transition to Forms E. How Do Changing NIH Policies Impact Me?
 NIH funded Human Subjects Research/Clinical Trials and the Transition to Forms E How Do Changing NIH Policies Impact Me? LCOM Informational Sessions Nov. 14, 16 2017 Jeralyn Haraldsen, PhD Grant Proposal
NIH funded Human Subjects Research/Clinical Trials and the Transition to Forms E How Do Changing NIH Policies Impact Me? LCOM Informational Sessions Nov. 14, 16 2017 Jeralyn Haraldsen, PhD Grant Proposal
PART III EPA POSITIONS APPLICANT TRACKING
 PART III EPA POSITIONS APPLICANT TRACKING 1 Niner Talent User s Guide Table of Contents 4 Introduction 4 Contents 4 Process Overview 4 2.1 Initiator: How to Post a Position 5 Introduction 5 Policies 5
PART III EPA POSITIONS APPLICANT TRACKING 1 Niner Talent User s Guide Table of Contents 4 Introduction 4 Contents 4 Process Overview 4 2.1 Initiator: How to Post a Position 5 Introduction 5 Policies 5
Diversity & Inclusion Strategic Plan NextConnect Marketing Summit Atlanta, GA April 24-25, 2017
 & Inclusion Strategic Plan 2017 NextConnect Marketing Summit Atlanta, GA April 24-25, 2017 OFFICE OF DIVERSITY nfff 2 OFFICE OF DIVERSITY "As the nation becomes more diverse, so too must Scouting if we
& Inclusion Strategic Plan 2017 NextConnect Marketing Summit Atlanta, GA April 24-25, 2017 OFFICE OF DIVERSITY nfff 2 OFFICE OF DIVERSITY "As the nation becomes more diverse, so too must Scouting if we
Outline of Discussion
 This final rule is intended to better protect human subjects involved in research, while facilitating valuable research and reducing burden, delay, and ambiguity for investigators. These revisions are
This final rule is intended to better protect human subjects involved in research, while facilitating valuable research and reducing burden, delay, and ambiguity for investigators. These revisions are
CTSI NIH KL2 SCHOLAR MULTIDISCIPLINARY PROGRAM APPLICATION INSTRUCTIONS
 INSTRUCTIONS Note: Please DO NOT submit any supplementary materials (e.g., appendices) as these will not be reviewed. Submit only the requested documents. The application packet must be: Typed using Arial
INSTRUCTIONS Note: Please DO NOT submit any supplementary materials (e.g., appendices) as these will not be reviewed. Submit only the requested documents. The application packet must be: Typed using Arial
PITTSBURGH REGIONAL DIVERSITY SURVEY RESULTS JOB SECTORS
 PITTSBURGH REGIONAL DIVERSITY SURVEY RESULTS JOB SECTORS Sample sizes: Job Sector Frequency Percent 286 11.2 902 35.4 Health Care & 340 13.4 Information 111 4.4 328 12.9 Professional, 298 11.7 159 6.2
PITTSBURGH REGIONAL DIVERSITY SURVEY RESULTS JOB SECTORS Sample sizes: Job Sector Frequency Percent 286 11.2 902 35.4 Health Care & 340 13.4 Information 111 4.4 328 12.9 Professional, 298 11.7 159 6.2
ClinicalTrials.gov REGISTRATION REQUIREMENTS
 ClinicalTrials.gov REGISTRATION REQUIREMENTS Background: What is it? ClinicalTrials.gov is a public registry that provides easy access to information on clinical studies, both clinical trials and observational
ClinicalTrials.gov REGISTRATION REQUIREMENTS Background: What is it? ClinicalTrials.gov is a public registry that provides easy access to information on clinical studies, both clinical trials and observational
Somali Youth Development Fund: Greater Minnesota Capacity Building 2017
 Greater Minnesota Capacity Building 2017 Capacity Building 2017 The Somali Youth Development Fund seeks to support strength-based, holistic youth development approaches that value the whole child in the
Greater Minnesota Capacity Building 2017 Capacity Building 2017 The Somali Youth Development Fund seeks to support strength-based, holistic youth development approaches that value the whole child in the
PENNSYLVANIA TURNPIKE COMMISSION
 PENNSYLVANIA TURNPIKE COMMISSION RETENTION OF AN ENGINEERING or PROJECT MANAGEMENT FIRM Program Management Services for All Electronic Toll (AET) Conversion Project Reference No. 3-233 The Pennsylvania
PENNSYLVANIA TURNPIKE COMMISSION RETENTION OF AN ENGINEERING or PROJECT MANAGEMENT FIRM Program Management Services for All Electronic Toll (AET) Conversion Project Reference No. 3-233 The Pennsylvania
The Johns Hopkins Institute for Clinical and Translational Research
 ICTR The Johns Hopkins Institute for Clinical and Translational Research INTRODUCTION TO CLINICALTRIALS.GOV LINDA POST, RN, BSN, CCRP RESEARCH NAVIGATOR JUNE 28, 2013 Development of this resource was supported
ICTR The Johns Hopkins Institute for Clinical and Translational Research INTRODUCTION TO CLINICALTRIALS.GOV LINDA POST, RN, BSN, CCRP RESEARCH NAVIGATOR JUNE 28, 2013 Development of this resource was supported
Clinical Trials: Registration, Results Reporting, and Data Sharing
 Clinical Trials: Registration, Results Reporting, and Data Sharing Jerry Sheehan Assistant Director for Policy Development National Library of Medicine National Institutes of Health OECD Expert Workshop
Clinical Trials: Registration, Results Reporting, and Data Sharing Jerry Sheehan Assistant Director for Policy Development National Library of Medicine National Institutes of Health OECD Expert Workshop
APPLICATION FOR EMPLOYMENT
 APPLICATION FOR EMPLOYMENT Ryan Logistics, Inc. is an equal opportunity employer. We are committed to our policy of providing equal employment opportunity to employees and job applicants in a manner consistent
APPLICATION FOR EMPLOYMENT Ryan Logistics, Inc. is an equal opportunity employer. We are committed to our policy of providing equal employment opportunity to employees and job applicants in a manner consistent
* indicates required field
 Application All applications are due midnight January 22, 2017. You may start your application and resume at a later time. In order to save your application, you must choose Save & Resume Later, otherwise
Application All applications are due midnight January 22, 2017. You may start your application and resume at a later time. In order to save your application, you must choose Save & Resume Later, otherwise
Volunteer Application Kentucky Cooperative Extension Service
 Volunteer Application Kentucky Cooperative Extension Service Kentucky Cooperative Extension Service takes seriously its obligation to provide a safe environment for all persons involved in volunteer activities.
Volunteer Application Kentucky Cooperative Extension Service Kentucky Cooperative Extension Service takes seriously its obligation to provide a safe environment for all persons involved in volunteer activities.
All applicants are considered without regard to race, color, religion, sex, national origin, age, veteran status, or disability.
 EMPLOYMENT APPLICATION Kershaw County Government 515 Walnut Street. Camden, SC 29020 Telephone: (803) 425-1500 Website Address: http://www.kershaw.sc.gov All applicants are considered without regard to
EMPLOYMENT APPLICATION Kershaw County Government 515 Walnut Street. Camden, SC 29020 Telephone: (803) 425-1500 Website Address: http://www.kershaw.sc.gov All applicants are considered without regard to
Joint statement on public disclosure of results from clinical trials
 Joint statement on public disclosure of results from clinical trials Signatories on 18 May 2017 European Commission for Horizon 2020 Societal Challenge Health Demographic Change and Wellbeing (joined on
Joint statement on public disclosure of results from clinical trials Signatories on 18 May 2017 European Commission for Horizon 2020 Societal Challenge Health Demographic Change and Wellbeing (joined on
GENERAL INFORMATION. Permanent Address: No. Street City State Zip
 Transit Management of Denton County Human Resources Department 1101 Teasley Lane, Denton, TX 76205 Fax: 940-218-1625 APPLICATION FOR EMPLOYMENT (Staff) Thank you for considering applying for a position
Transit Management of Denton County Human Resources Department 1101 Teasley Lane, Denton, TX 76205 Fax: 940-218-1625 APPLICATION FOR EMPLOYMENT (Staff) Thank you for considering applying for a position
4.1. Target Accrual Rate: The Target Accrual Goal divided by the Expected Duration (in days) of the trial.
 POLICY #: COM-102 Page: 1 of 5 1. POLICY STATEMENT: The Scientific Review Committee (SRC) is responsible for monitoring the accrual and evaluating the scientific merit of each interventional clinical research
POLICY #: COM-102 Page: 1 of 5 1. POLICY STATEMENT: The Scientific Review Committee (SRC) is responsible for monitoring the accrual and evaluating the scientific merit of each interventional clinical research
BUREAU OF CONSUMER FINANCIAL PROTECTION DECEMBER BCFP Annual Employee Survey Results
 BUREAU OF CONSUMER FINANCIAL PROTECTION DECEMBER 2018 2018 BCFP Annual Employee Survey Results Introduction Interpretation of results More than 72 percent of the Bureau of Consumer Financial Protection
BUREAU OF CONSUMER FINANCIAL PROTECTION DECEMBER 2018 2018 BCFP Annual Employee Survey Results Introduction Interpretation of results More than 72 percent of the Bureau of Consumer Financial Protection
EMPLOYMENT APPLICATION
 EMPLOYMENT APPLICATION CDAC Behavioral Healthcare, Inc. 3804 North Ninth Avenue Pensacola, Florida 32503 (850) 434-2724 CDAC Behavioral Healthcare, Inc. is an equal opportunity employer and in compliance
EMPLOYMENT APPLICATION CDAC Behavioral Healthcare, Inc. 3804 North Ninth Avenue Pensacola, Florida 32503 (850) 434-2724 CDAC Behavioral Healthcare, Inc. is an equal opportunity employer and in compliance
Best Time to Contact You At Home is: : AM/PM to : AM/PM
 M. Luis Construction Co., Inc. Application for Employment : 410-545-0641 Fax: 410-545-0643 WE ARE AN EQUAL OPPORTUNITY EMPLOYER. We consider applicants for all positions without regard to race, color religion,
M. Luis Construction Co., Inc. Application for Employment : 410-545-0641 Fax: 410-545-0643 WE ARE AN EQUAL OPPORTUNITY EMPLOYER. We consider applicants for all positions without regard to race, color religion,
PART III EPA POSITIONS APPLICANT TRACKING
 PART III EPA POSITIONS APPLICANT TRACKING 1 Niner Talent User s Guide Table of Contents 4 Introduction 4 Contents 4 Process Overview 4 2.1 Initiator: How to Post a Position 5 Introduction 5 Policies 5
PART III EPA POSITIONS APPLICANT TRACKING 1 Niner Talent User s Guide Table of Contents 4 Introduction 4 Contents 4 Process Overview 4 2.1 Initiator: How to Post a Position 5 Introduction 5 Policies 5
CHECKLIST: Pre-review
 11-5-2014 1 of 5 The purpose of this checklist is to provide support for IRB staff conducting pre-review. This checklist is to be completed by the IRB staff, signed, dated, and retained with the IRB protocol
11-5-2014 1 of 5 The purpose of this checklist is to provide support for IRB staff conducting pre-review. This checklist is to be completed by the IRB staff, signed, dated, and retained with the IRB protocol
PART II FACULTY POSITIONS
 PART II FACULTY POSITIONS Page 1 of 48 NinerTalent User s Guide for Faculty Positions Table of Contents NinerTalent User s Guide for FACULTY Positions... 4 Overview... 4 Introduction... 4 Format... 4 Section
PART II FACULTY POSITIONS Page 1 of 48 NinerTalent User s Guide for Faculty Positions Table of Contents NinerTalent User s Guide for FACULTY Positions... 4 Overview... 4 Introduction... 4 Format... 4 Section
Policy Appendix IV: DRCR.net Industry Collaboration Policies Version 5.0, February 2, 2009
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 DRCR.net is committed to: performing rigorous multi-center clinical
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 DRCR.net is committed to: performing rigorous multi-center clinical
SUPPORT FOR COMPREHENSIVE CLIMATE CHANGE & CLEAN ENERGY POLICY AMONG RACIAL & ETHNIC GROUPS
 SUPPORT FOR COMPREHENSIVE CLIMATE CHANGE & CLEAN ENERGY POLICY AMONG RACIAL & ETHNIC GROUPS African American, Latino and other racial and ethnic communities often bear the brunt of global warming 1 pollution.
SUPPORT FOR COMPREHENSIVE CLIMATE CHANGE & CLEAN ENERGY POLICY AMONG RACIAL & ETHNIC GROUPS African American, Latino and other racial and ethnic communities often bear the brunt of global warming 1 pollution.
Better Health, LLC. Cultural Competency and Minority Recruitment Plan. June 1
 Better Health, LLC. Cultural Competency and Minority Recruitment Plan June 1 2013 CULTURAL COMPETENCY and MINORITY RECRUITMENT PLAN REFORM AND NON REFORM POPULATION June 1, 2013 A. Introduction Some studies
Better Health, LLC. Cultural Competency and Minority Recruitment Plan June 1 2013 CULTURAL COMPETENCY and MINORITY RECRUITMENT PLAN REFORM AND NON REFORM POPULATION June 1, 2013 A. Introduction Some studies
New Hire Information Packet
 2019 The information contained in this package should be read thoroughly, completed, and brought with you on your first day of employment. UNION HOSPITAL OF CECIL COUNTY HUMAN RESOURCES WELCOME Dear New
2019 The information contained in this package should be read thoroughly, completed, and brought with you on your first day of employment. UNION HOSPITAL OF CECIL COUNTY HUMAN RESOURCES WELCOME Dear New
City of Sanford Employment Application
 City of Sanford Employment Application 225 E. Weatherspoon St P.O. Box 3729 Sanford, NC 27331 www.sanfordnc.net An Equal Opportunity/Affirmative Action Employer Phone: (919) 777-1131 Fax: (919) 774-8712
City of Sanford Employment Application 225 E. Weatherspoon St P.O. Box 3729 Sanford, NC 27331 www.sanfordnc.net An Equal Opportunity/Affirmative Action Employer Phone: (919) 777-1131 Fax: (919) 774-8712
New Hire Information Packet
 2018 The information contained in this package should be read thoroughly, completed, and brought with you on your first day of employment. UNION HOSPITAL OF CECIL COUNTY HUMAN RESOURCES WELCOME Dear New
2018 The information contained in this package should be read thoroughly, completed, and brought with you on your first day of employment. UNION HOSPITAL OF CECIL COUNTY HUMAN RESOURCES WELCOME Dear New
Human Research Participant Protection Program Institutional Review Board (IRB)
 Human Research Participant Protection Program Institutional Review Board (IRB) Procedure 1: Determining whether a research activity needs IRB review and approval 1. Subject All research activities that
Human Research Participant Protection Program Institutional Review Board (IRB) Procedure 1: Determining whether a research activity needs IRB review and approval 1. Subject All research activities that
https://dev.e-irb.jhmi.edu/eirb2/sd/resourceadministration/project/printsmartforms?proje...
 Print: - *EXTERNAL IRB APPLICATION - TEST* Page 1 of 10 Date: Tuesday, June 13, 2017 4:29:22 PM Print Close 1 - General Information ID: 1. * Principal Investigator: Click Select to choose PI: 2. * Will
Print: - *EXTERNAL IRB APPLICATION - TEST* Page 1 of 10 Date: Tuesday, June 13, 2017 4:29:22 PM Print Close 1 - General Information ID: 1. * Principal Investigator: Click Select to choose PI: 2. * Will
Investigational New Drug Development Steps for CRCs
 Investigational New Drug Development Steps for CRCs Natalie Nardone, PhD Program Manager, Department of Medicine CRC Instructor, Office of Clinical Research Contact: natalie.nardone@ucsf.edu Learning Objectives
Investigational New Drug Development Steps for CRCs Natalie Nardone, PhD Program Manager, Department of Medicine CRC Instructor, Office of Clinical Research Contact: natalie.nardone@ucsf.edu Learning Objectives
**ATTENTION APPLICANTS PLEASE READ CAREFULLY** VILLAGE OF ADDISON, ILLINOIS - HUMAN RESOURCES DEPARTMENT APPLICANT INFORMATION SHEET
 **ATTENTION APPLICANTS PLEASE READ CAREFULLY** VILLAGE OF ADDISON, ILLINOIS - HUMAN RESOURCES DEPARTMENT APPLICANT INFORMATION SHEET A. INDIVIDUALS APPLYING FOR EMPLOYMENT WHEN THERE IS NO CURRENT OPENING
**ATTENTION APPLICANTS PLEASE READ CAREFULLY** VILLAGE OF ADDISON, ILLINOIS - HUMAN RESOURCES DEPARTMENT APPLICANT INFORMATION SHEET A. INDIVIDUALS APPLYING FOR EMPLOYMENT WHEN THERE IS NO CURRENT OPENING
Counts and Density of All Jobs in Work Selection Area in All Workers
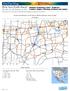 OnTheMap Work Area Profile Report All Jobs for All Workers in. Created by the U.S. Census Bureau s OnTheMap http://onthemap.ces.census.gov on 02/18/2014 s and Density of All Jobs in Work Selection Area
OnTheMap Work Area Profile Report All Jobs for All Workers in. Created by the U.S. Census Bureau s OnTheMap http://onthemap.ces.census.gov on 02/18/2014 s and Density of All Jobs in Work Selection Area
Protocol Submission Form
 Protocol Submission Form 1. Administrative Information Name of Submitting Party: Street: Province/State: Postal/Zip Code: Phone: ( ) E-mail Address: Fax: ( ) Other: ( ) Date: 2. General Study Information
Protocol Submission Form 1. Administrative Information Name of Submitting Party: Street: Province/State: Postal/Zip Code: Phone: ( ) E-mail Address: Fax: ( ) Other: ( ) Date: 2. General Study Information
Are you legally eligible for employment in the United States? Yes No When will you be available to begin work?
 R and G Construction Co. Employment Application 2694 County Road 6 Highway 59 South Marshall, MN 56258 Phone: (507) 537-1473 Fax: (507) 537-0513 Desired Driver Equipment Operator Laborer Mechanic Date
R and G Construction Co. Employment Application 2694 County Road 6 Highway 59 South Marshall, MN 56258 Phone: (507) 537-1473 Fax: (507) 537-0513 Desired Driver Equipment Operator Laborer Mechanic Date
RMHC Range Mental Health Center PO Box 1188 Virginia, MN 55792
 RMHC Range Mental Health Center PO Box 1188 Virginia, MN 55792 EMPLOYMENT APPLICATION Range Mental Health Center is an Equal Opportunity/Affirmative Action Employer Complete this form in full. Do not write
RMHC Range Mental Health Center PO Box 1188 Virginia, MN 55792 EMPLOYMENT APPLICATION Range Mental Health Center is an Equal Opportunity/Affirmative Action Employer Complete this form in full. Do not write
Counts and Density of All Jobs in Work Selection Area in All Workers
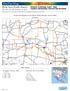 OnTheMap Work Area Profile Report All Jobs for All Workers in. Created by the U.S. Census Bureau s OnTheMap http://onthemap.ces.census.gov on 02/18/2014 s and Density of All Jobs in Work Selection Area
OnTheMap Work Area Profile Report All Jobs for All Workers in. Created by the U.S. Census Bureau s OnTheMap http://onthemap.ces.census.gov on 02/18/2014 s and Density of All Jobs in Work Selection Area
PLANNING FOR SUCCESS. Sunday, November 17, 2013 Community Volunteer Leadership Conference Nashville, TN 8:45 a.m. 10:30 a.m.
 PLANNING FOR SUCCESS Sunday, November 17, 2013 Community Volunteer Leadership Conference Nashville, TN 8:45 a.m. 10:30 a.m. Breakout Overview An opportunity for Staff and Volunteer Partner Teams to: Discuss
PLANNING FOR SUCCESS Sunday, November 17, 2013 Community Volunteer Leadership Conference Nashville, TN 8:45 a.m. 10:30 a.m. Breakout Overview An opportunity for Staff and Volunteer Partner Teams to: Discuss
Regulatory Binder: Set-up and Maintenance
 Regulatory Binder: Set-up and Maintenance Introduction Federal and state regulations, institutional policy, and good clinical and research practices require investigators to maintain documents related
Regulatory Binder: Set-up and Maintenance Introduction Federal and state regulations, institutional policy, and good clinical and research practices require investigators to maintain documents related
District Five-Year
 GATEWAY TECHNICAL COLLEGE District Five-Year 2015-2019 Equal Opportunity / Affirmative Action Plan Submitted by: Jacqueline Morris Director, Staffing TABLE OF CONTENTS SECTION I Equal Opportunity/Affirmative
GATEWAY TECHNICAL COLLEGE District Five-Year 2015-2019 Equal Opportunity / Affirmative Action Plan Submitted by: Jacqueline Morris Director, Staffing TABLE OF CONTENTS SECTION I Equal Opportunity/Affirmative
Best Practices in Outreach
 Best Practices in Outreach The webinar will begin shortly. For Audio: 877-713-0446, Conference Code: 101 725 8988 Please mute your computer speakers & phone line during this webinar. Center for Parent
Best Practices in Outreach The webinar will begin shortly. For Audio: 877-713-0446, Conference Code: 101 725 8988 Please mute your computer speakers & phone line during this webinar. Center for Parent
Assessing Your Agency s Ethical Culture
 PUBLIC SERVICE ETHICS International City/County Management Association Assessing Your Agency s Ethical Culture This survey has been designed to assist your agency in gauging its ethical climate. Instructions:
PUBLIC SERVICE ETHICS International City/County Management Association Assessing Your Agency s Ethical Culture This survey has been designed to assist your agency in gauging its ethical climate. Instructions:
TRIANGLE HEALTH ALLIANCE New Hire Information Packet
 2017 TRIANGLE HEALTH ALLIANCE The information contained in this package should be read thoroughly, completed, and brought with you on your first day of employment. WELCOME Dear New Employee, Welcome to
2017 TRIANGLE HEALTH ALLIANCE The information contained in this package should be read thoroughly, completed, and brought with you on your first day of employment. WELCOME Dear New Employee, Welcome to
2014 Employee Satisfaction Survey. New Mexico State University
 2014 Employee Satisfaction Survey New Mexico State University Total number of employees at NMSU, all locations = 3,938 Total number of clicks of interest = 2,314 Number of useable responses = 1,668 Number
2014 Employee Satisfaction Survey New Mexico State University Total number of employees at NMSU, all locations = 3,938 Total number of clicks of interest = 2,314 Number of useable responses = 1,668 Number
Kohler Distributing Company 150 Wagaraw Road Hawthorne, NJ 07506
 Kohler Distributing Company 150 Wagaraw Road Hawthorne, NJ 07506 APPLICATION FOR EMPLOYMENT Kohler Distributing Company is an Equal Opportunity Employer Please fill in this application completely and truthfully.
Kohler Distributing Company 150 Wagaraw Road Hawthorne, NJ 07506 APPLICATION FOR EMPLOYMENT Kohler Distributing Company is an Equal Opportunity Employer Please fill in this application completely and truthfully.
The 21st Century Challenge: Water Policy in the Context of Climate Change
 The 21st Century Challenge: Policy in the Context of Climate Change Arnold Vedlitz, Ph.D. Bob Bullock Chair in Government and Public Policy Director Institute for Science, Technology and Public Policy
The 21st Century Challenge: Policy in the Context of Climate Change Arnold Vedlitz, Ph.D. Bob Bullock Chair in Government and Public Policy Director Institute for Science, Technology and Public Policy
HUMAN RESOURCES DEPARTMENT 100 South Myrtle Avenue, P.O. Box 4748 Clearwater, FL
 HUMAN RESOURCES DEPARTMENT 100 South Myrtle Avenue, P.O. Box 4748 Clearwater, FL 33756 727-562-4870 APPLICATION FOR EMPLOYMENT Apply on-line: www.myclearwater.com Date Recv'd: A City application is required
HUMAN RESOURCES DEPARTMENT 100 South Myrtle Avenue, P.O. Box 4748 Clearwater, FL 33756 727-562-4870 APPLICATION FOR EMPLOYMENT Apply on-line: www.myclearwater.com Date Recv'd: A City application is required
Safety Sensitive Positions Employment Application
 Transit Management of Denton County Human Resources Department 1101 Teasley Lane, Denton, TX 76205 Fax: 940-218-1625 Email: jobs@dentontransit.com Safety Sensitive Positions Employment Application te to
Transit Management of Denton County Human Resources Department 1101 Teasley Lane, Denton, TX 76205 Fax: 940-218-1625 Email: jobs@dentontransit.com Safety Sensitive Positions Employment Application te to
GlaxoSmithKline (GSK) Response to World Health Organization International Clinical Trials Registry Platform (ICTRP) Consultation September 2005
 GlaxoSmithKline (GSK) Response to World Health Organization International Clinical Trials Registry Platform (ICTRP) Consultation September 2005 Introduction GlaxoSmithKline (GSK) is committed to enhancing
GlaxoSmithKline (GSK) Response to World Health Organization International Clinical Trials Registry Platform (ICTRP) Consultation September 2005 Introduction GlaxoSmithKline (GSK) is committed to enhancing
APPLICATION FOR EMPLOYMENT
 APPLICATION FOR EMPLOYMENT We consider applicants for all positions without regard to race, color, religion, sex, national origin, age, or disability, or any other legally protected status. (PLEASE PRINT)
APPLICATION FOR EMPLOYMENT We consider applicants for all positions without regard to race, color, religion, sex, national origin, age, or disability, or any other legally protected status. (PLEASE PRINT)
APPLICATION INSTRUCTIONS INSTITUTIONAL REVIEW BOARD. Revised January 2010 Page 1
 APPLICATION INSTRUCTIONS INSTITUTIONAL REVIEW BOARD Revised January 2010 Page 1 The IRB Proposal Includes: 1. All documents, other than the informed consent form, should be understandable to an informed
APPLICATION INSTRUCTIONS INSTITUTIONAL REVIEW BOARD Revised January 2010 Page 1 The IRB Proposal Includes: 1. All documents, other than the informed consent form, should be understandable to an informed
PRE-SEARCH ACTIVITIES
 SUMMARY OF AFFIRMATIVE ACTION RECRUITING ACTIVITY FOR FACULTY AND NON -TEACHING PROFESSIONAL POSITIONS STATE UNIVERSITY OF NEW YORK This form is required in order to summarize applicant flow and recruitment
SUMMARY OF AFFIRMATIVE ACTION RECRUITING ACTIVITY FOR FACULTY AND NON -TEACHING PROFESSIONAL POSITIONS STATE UNIVERSITY OF NEW YORK This form is required in order to summarize applicant flow and recruitment
NEIGHBORHOOD PROPERTIES, INC.
 NEIGHBORHOOD PROPERTIES, INC. APPLICATION FOR EMPLOYMENT Neighborhood Properties, Inc. is an equal opportunity employer and does not discriminate on the basis of race, religion, national origin, ancestry,
NEIGHBORHOOD PROPERTIES, INC. APPLICATION FOR EMPLOYMENT Neighborhood Properties, Inc. is an equal opportunity employer and does not discriminate on the basis of race, religion, national origin, ancestry,
Fulfilling the American Dream with the CPA profession. Richard J. Caturano, CPA, CGMA Chairman of the AICPA Board of Directors
 Fulfilling the American Dream with the CPA profession Richard J. Caturano, CPA, CGMA Chairman of the AICPA Board of Directors American Institute of CPAs American Institute of CPAs American Institute of
Fulfilling the American Dream with the CPA profession Richard J. Caturano, CPA, CGMA Chairman of the AICPA Board of Directors American Institute of CPAs American Institute of CPAs American Institute of
