Properties of Matter. Chemical Properties and Effects on Pollutant Fate. Characteristics of Chemical Changes. Physical Characteristics
|
|
|
- Anis Armstrong
- 6 years ago
- Views:
Transcription
1 Properties of Matter Chemical Properties and Effects on Pollutant Fate Physical Characteristics Characteristics of Chemical Changes Melting point Boiling point Vapor pressure Color State (solid, liquid, gas, plasma) Density Electrical conductivity Solubility Adsorption to a surface Hardness Reaction with acids Reaction with bases (alkalis) Reaction with oxygen (combustion) Ability to act as oxidizing agent Ability to act as reducing agent Reaction with other elements Decomposition into simpler substances Corrosion 1
2 Chemical Fate Processes Example: PESTICIDE FATES TRANSPORT TRANSFORMATION/ DEGRADATION SORPTION VOLATILIZATION BIOLOGICAL PROCESSES Photo-Decomposition Pest Uptake Application Volatilization ADSORPTION Atmospheric losses Crop Removal RUNOFF (water and sediment) Chemical Decomposition Leaching (water) Biological Decomposition Chemical Transport Processes RUNOFF EROSION WIND LEACHING MOVEMENT IN STREAMS OR GROUNDWATER Transformation and Degradation Processes Biological Transformations due to microorganisms aerobic anaerobic facultative Most Important reaction for many chemicals 2
3 Transformation and Degradation Processes Chemical Transformations Hydrolysis Oxidation-Reduction Photochemical Processes Only in the presence of light Only on or near the surface Transformation and Degradation Processes Usually assumed to follow a first-order linear function dc = C dt This leads to an exponential decay function for the concentration C = C e t 0 Transformation and Degradation Processes is the Degradation Coefficient is related to the Half-Life of the chemical in the environment (T H ) The Half-Life is the time required for the concentration of the chemical to become half of the initial value. Usually expressed in days. = T H EXAMPLE Metolachlor: T H =90 days Initial Agricultural Application Concentration = 1.5 kg/ha Estimate the concentration at 120 days = = / day (120) C = 1.5e = kg / ha 3
4 SOIL-WATER ADSORPTION PARTITIONING COEFFICIENT Properties Influencing Adsorption C s Concentration in Soil C s = d C w d 1 Linear, Instantaneous, Reversible Adsorption Model Organic Matter Content Clay Content Soil Water Content Soil Bulk Density Soil Temperature Concentration in Water C w Chemical Properties Influencing Adsorption Electronic Structure (ionic or non-ionic) Water Solubility Solution Composition Solution Concentration ph Organic Carbon Partitioning Coefficient d = oc % OC = % OM d = oc (%OC /100) /1.724 (%OM /172.4) 4
5 Chemical Transport Pathways d > 250: The chemical is so strongly adsorbed that very little will be transported except with eroded soil particles. 250 > d > 50 Transport depends on the sediment concentration of the runoff. 50 > d > 1: Enough will stay in solution that most will be transported with surface runoff water. Chemical Transport Pathways 1 > d > 0.1: The loss pathway will depend on the amount of infiltration occurring prior to initiation of surface runoff. d < 0.1: The chemical is so weakly adsorbed that the first rainfall will wash it into the soil profile before initiation of runoff. 3/28/2006 Loss pathways 17 VOLATILIZATION Of concern for surface-located chemicals Affected by temperature and soil water content adsorptivity and concentration vapor pressure and solubility (Henrys Constant) Biological Processes affecting Chemicals Interception Washoff Uptake/Penetration Translocation Metabolism/Decay 5
6 Q Reaction Quotient and Chemical Equilibrium aa + 2 k1 k bb cc + dd = b c d { C} { D} products a { A} { B} reactants reaction quotient = = c d { C} { D} k1 a b { A} { B} k 2 eq = = Acid-Base Equilibria: Brönsted-Lowry Model An acid donates a proton (H + ) A base accepts a proton HA is used to denote a generic acid HA + H 2 O A - + H 3 O + Where H 3 O + = hydronium ion Or HA H + + A - Where A - = conjugate base At equilibrium, Q = eq Brönsted-Lowry Model For an acid: For a base: B - + H 2 O HB + OH - where eq eq = a = + { H }{ A } { HA} B - = base HB = conjugate acid = b = { HB}{ OH } { B } For every acid, there has to be a conjugate base. For every base, there has to be a conjugate acid. Strong Acids Strong acids have relatively high a s. Example: HClO 4 a = 10 7 HCl a = 10 3 H 2 SO 4 a = 10 3 HNO 3 a =
7 Graphical Solutions (pc ph Diagrams) Graphical Solutions (pc ph Diagrams) Introduction and Review of Solubility Equilibria When the ions of a sparingly soluble salt are brought together in solution, it is observed that if the concentration of these ions is sufficiently large, a solid is formed that will settle from the solution. For example, consider the reaction: Ca 2+ (aq) + CO 3 2- (aq) CaCO 3(s) If the concentration of the calcium and carbonate is not excessively high, the formation of calcium carbonate may be observed to be time dependent. (It will take a period of time for visible CaCO 3(s) to form). Precipitation has therefore been concluded to be a two-step process: 1. Nucleation condensation of ions to very small particles. 2. Particle growth agglomeration/growth of these ions as a result of diffusion of ions from solution. In order for nucleation to occur, solute ions attraction must be strong enough to displace solvent molecules separating the ions. Also the collision frequency between the ions must be sufficiently high to promote nucleation. Supersaturated solutions do exist. Precipitation does not occur instantaneously. 7
8 Solubility Equilibrium: Slightly Soluble Salts In general, for a slightly soluble compound C a A b, the dissolution equation is: C a A b(s) ac (aq) + ba (aq) And the solubility equilibrium expression for an ideal solution: = sp [ C] a [ A] b Where sp = solubility product (equilibrium constant) [C] = cation concentration [A] = anion concentration Remember that the concentration of the solid is not included and is assumed to be 1. sp ALWAYS refers to the dissolution reaction and determines the components of a saturated solution. If [C] a [A] b is less than sp, no precipitate forms. If [C] a [A] b is greater than sp, precipitate forms in proportion to the reverse of the dissolution reaction until [C] a [A] b equals sp. Concept and Importance of Bioaccumulation It is a process by which persistent environmental pollution leads to the uptake and accumulation of one or more contaminants, by organisms in an ecosystem. The amount of a pollutant available for exposure depends on its persistence and the potential for its bioaccumulation. 8
9 Basic Factors Affecting Bioaccumulation Water, soil, air, plants, and any of their combinations can be an ecosystem for chemical bioaccumulation. Bioaccumulants tend to be persistent, stable, and lipophilic environmental pollutants. Chemicals tending to move freely within an organism s body are less likely to be accumulated by organisms. Uptake of Bioaccumulants The uptake of many bioaccumulants by organisms is typically initiated by passive transport, as chemical molecules tend to move from high to low concentration. This first step is affected by the bioaccumulant s lipophilicity and water solubility. Some chemicals also have a high affinity for binding with proteins or the ability to dissolve in fats, thus prolonging the storage of these substances inside an organism. Breakdown of Pollutants The biological breakdown of chemicals is called metabolism; this ability varies among individual species. Some chemicals are highly fat-soluble but are easily metabolized; these chemicals do not accumulate in organisms. Thus, biological breakdown is one of the factors leading to one of the two specific consequences of chemical bioaccumulation: bioconcentration or biomagnification. BAF, BCF, BMF Bioaccumulation Factor (BAF) is the ratio of a test chemical s concentration in a test organism s tissues to that in the surrounding medium, when all potential uptake mechanisms are included. Bioconcentration Factor (BCF) is a specific case of BAF, when the uptake is only from the surrounding medium. Biomagnification Factor (BMF) is the ratio of a test chemical s concentration in the tissues of an organism, to that in the organism s prey. 9
10 Numerical Criteria for Bioaccumulation Potential In the USA, chemicals are considered bioaccumulative if they have a degradation half-life > 30 days; or If they have a bioconcentration factor greater than 1,000; or If their log ow is greater than 4.2. These values are lower (i.e., more health conservative) than those set forth by Canada and many other Western countries. Bioconcentration Factor (I) Many bioconcentration factor (BCF) assessments are based on aquatic measurements because fish provides a rich lipophilic microenvironment for bioaccumulation. BCF is typically measured as the ratio of the concentration of a chemical in a test organism to the chemical s concentration in the surrounding medium. For many lipophilic chemicals, BCFs can be calculated using the regression equation: log BCF = x (log ow). Bioconcentration Factor (III) For a bioconcentration factor (BCF) to be estimated from a site-specific study, three (3) conditions should be met. For a BCF to be estimated from a laboratory study, five (5) conditions should be met. These conditions include: sufficient duration for observation; a subthreshold test levels; and the use of test guidelines acceptable to the regulatory authorities. 10
GetIPM.com Children fall ill after pesticides sprayed -- 2,4-D applied to lawns near family's home Over-the-Counter Herbicides Pose a Significant Thre
 Environmental Fate of Herbicides: The Disappearing Act Adam Hixson BASF, The Chemical Company GetIPM.com Children fall ill after pesticides sprayed -- 2,4-D applied to lawns near family's home Over-the-Counter
Environmental Fate of Herbicides: The Disappearing Act Adam Hixson BASF, The Chemical Company GetIPM.com Children fall ill after pesticides sprayed -- 2,4-D applied to lawns near family's home Over-the-Counter
CHAPTER # 4. Fate of Pollutants in the Environment
 CHAPTER # 4 Fate of Pollutants in the Environment Once a pesticide is introduced into the environment, whether through an application, a disposal or a spill, it is influenced by many processes. These processes
CHAPTER # 4 Fate of Pollutants in the Environment Once a pesticide is introduced into the environment, whether through an application, a disposal or a spill, it is influenced by many processes. These processes
Environmental Fate of Pesticides. Dr. James N. McCrimmon Abraham Baldwin Agricultural College
 Environmental Fate of Pesticides Dr. James N. McCrimmon Abraham Baldwin Agricultural College Public Concerns Health Quality of Life Environment Toxic Waste Chemicals vs. Natural Right-to-Know Public Concerns
Environmental Fate of Pesticides Dr. James N. McCrimmon Abraham Baldwin Agricultural College Public Concerns Health Quality of Life Environment Toxic Waste Chemicals vs. Natural Right-to-Know Public Concerns
Herbicide Behavior in Soil Section 4
 Herbicide Behavior in Soil Section 4 Why is it important to understand herbicide behavior in soil? That behavior can affect: success or failure of weed control presence or absence of crop injury persistence
Herbicide Behavior in Soil Section 4 Why is it important to understand herbicide behavior in soil? That behavior can affect: success or failure of weed control presence or absence of crop injury persistence
Aerobic Biodegradation of Organic Compounds
 Lecture 6.b., Aerobic and Anaerobic Biodegradation of Organic Compounds, Modeling Biodegradation and Bioconcentration and Accumulation in Aquatic Organisms Conrad (Dan) Volz, DrPH, MPH Bridgeside Point
Lecture 6.b., Aerobic and Anaerobic Biodegradation of Organic Compounds, Modeling Biodegradation and Bioconcentration and Accumulation in Aquatic Organisms Conrad (Dan) Volz, DrPH, MPH Bridgeside Point
Proposed Rule on Conventional Pesticides (40 CFR Part 158) May 3-4, 2005 Holiday Inn Rosslyn 1900 N. Fort Myer Drive Arlington, VA 22209
 Proposed Rule on Conventional Pesticides (40 CFR Part 158) May 3-4, 2005 Holiday Inn Rosslyn 1900 N. Fort Myer Drive Arlington, VA 22209 Environmental Fate and Effects Division Environmental Fate Data
Proposed Rule on Conventional Pesticides (40 CFR Part 158) May 3-4, 2005 Holiday Inn Rosslyn 1900 N. Fort Myer Drive Arlington, VA 22209 Environmental Fate and Effects Division Environmental Fate Data
33. Fate of pesticides in soil and plant.
 33. Fate of pesticides in soil and plant. What Happens to Pesticides When a pesticide is released into the environment many things happen to it. Sometimes what happens is beneficial. For example, the leaching
33. Fate of pesticides in soil and plant. What Happens to Pesticides When a pesticide is released into the environment many things happen to it. Sometimes what happens is beneficial. For example, the leaching
4.0 ENVIRONMENTAL TRANSPORT AND FATE PRINCIPAL FINDINGS
 4.0 ENVIRONMENTAL TRANSPORT AND FATE PRINCIPAL FINDINGS Both EDB and EDC: o Have low to moderate sorptive affinity for aquifer solids o Are relatively mobile in groundwater o Can volatilize from solution
4.0 ENVIRONMENTAL TRANSPORT AND FATE PRINCIPAL FINDINGS Both EDB and EDC: o Have low to moderate sorptive affinity for aquifer solids o Are relatively mobile in groundwater o Can volatilize from solution
Bio 430: Chemicals in the environment. Jeffrey Jenkins Department of Environmental and Molecular Toxicology Oregon State University
 Bio 430: Chemicals in the environment Jeffrey Jenkins Department of Environmental and Molecular Toxicology Chemical fate: transformation and transport within and between Soil-Air-Water-Biota Source: U.S.
Bio 430: Chemicals in the environment Jeffrey Jenkins Department of Environmental and Molecular Toxicology Chemical fate: transformation and transport within and between Soil-Air-Water-Biota Source: U.S.
Nitrogen cycle Important steps
 Nitrogen cycle Nitrogen cycle Important steps Stage1 Entry and Accumulation Ammonia is introduced into the water via tropical fish waste, uneaten food, and decomposition. These will break down into ammonia
Nitrogen cycle Nitrogen cycle Important steps Stage1 Entry and Accumulation Ammonia is introduced into the water via tropical fish waste, uneaten food, and decomposition. These will break down into ammonia
2.4 Period 3. Na Mg Al Si P S Cl Ar
 2.4 Period 3 Period 3 Na Mg Al Si P S Cl Ar Periodicity: Periodicity: The repeating trends in physical and chemical properties of elements as you go across the Periodic Table Periods often show gradual
2.4 Period 3 Period 3 Na Mg Al Si P S Cl Ar Periodicity: Periodicity: The repeating trends in physical and chemical properties of elements as you go across the Periodic Table Periods often show gradual
RAAF Appendix ENV-E: Scenario 5 1 (44)
 RAAF Appendix ENV-E: Scenario 5 1 (44) RAAF Appendix ENV-E Scenario 5 5.1 Description This scenario covers the category approach for which the read-across hypothesis is based on transformation to common
RAAF Appendix ENV-E: Scenario 5 1 (44) RAAF Appendix ENV-E Scenario 5 5.1 Description This scenario covers the category approach for which the read-across hypothesis is based on transformation to common
ENVIRONMENTAL GEOLOGY - GEOL 406/506
 ENVIRONMENTAL GEOLOGY - GEOL 406/506 Glossary of useful Terms: 1. Abiotic: not living. 2. A b s o r p t i o n: the penetration of atoms, ions, or molecules into the bulk mass of substrate. 3. Acclimation:
ENVIRONMENTAL GEOLOGY - GEOL 406/506 Glossary of useful Terms: 1. Abiotic: not living. 2. A b s o r p t i o n: the penetration of atoms, ions, or molecules into the bulk mass of substrate. 3. Acclimation:
AP*Chemistry Solubility Equilibria
 AP*Chemistry Solubility Equilibria SOLUBILITY EQUILIBRIA (K sp, THE SOLUBILITY PRODUCT) Saturated solutions of salts are another type of chemical equilibria. Remember those solubility rules? The fine print
AP*Chemistry Solubility Equilibria SOLUBILITY EQUILIBRIA (K sp, THE SOLUBILITY PRODUCT) Saturated solutions of salts are another type of chemical equilibria. Remember those solubility rules? The fine print
MSE 352 Engineering Ceramics II
 Kwame Nkrumah University of Science & Technology, Kumasi, Ghana MSE 352 Engineering Ceramics II Ing. Anthony Andrews (PhD) Department of Materials Engineering Faculty of Mechanical and Chemical Engineering
Kwame Nkrumah University of Science & Technology, Kumasi, Ghana MSE 352 Engineering Ceramics II Ing. Anthony Andrews (PhD) Department of Materials Engineering Faculty of Mechanical and Chemical Engineering
How Ecosystems Work Section 2
 Objectives List the three stages of the carbon cycle. Describe where fossil fuels are located. Identify one way that humans are affecting the carbon cycle. List the tree stages of the nitrogen cycle. Describe
Objectives List the three stages of the carbon cycle. Describe where fossil fuels are located. Identify one way that humans are affecting the carbon cycle. List the tree stages of the nitrogen cycle. Describe
Section 2: The Cycling of Materials
 Section 2: The Cycling of Materials Preview Bellringer Objectives The Carbon Cycle How Humans Affect the Carbon Cycle The Nitrogen Cycle Decomposers and the Nitrogen Cycle The Phosphorus Cycle Section
Section 2: The Cycling of Materials Preview Bellringer Objectives The Carbon Cycle How Humans Affect the Carbon Cycle The Nitrogen Cycle Decomposers and the Nitrogen Cycle The Phosphorus Cycle Section
Chapter Overview. Water molecule. Atomic Structure. Hydrogen Bonding. Hydrogen Bonding. CHAPTER 5 Water and Seawater
 Chapter Overview CHAPTER 5 Water and Seawater Water has many unique thermal and dissolving properties. Seawater is mostly water molecules but has dissolved substances. Ocean is layered by salinity and
Chapter Overview CHAPTER 5 Water and Seawater Water has many unique thermal and dissolving properties. Seawater is mostly water molecules but has dissolved substances. Ocean is layered by salinity and
Nutrient Cycling in an Aquatic Ecosystem
 Nutrient Cycling in an Aquatic Ecosystem 2.1 Productivity 2.2 Oxygen 2.3 Salinity 2.4 Carbon 2.5 Nitrogen 2.6 Phosphorous 2.7 Iron 2.8 Sulphur 2.9 Silica 2.3 Salinity of Inland Waters The salinity of freshwaters
Nutrient Cycling in an Aquatic Ecosystem 2.1 Productivity 2.2 Oxygen 2.3 Salinity 2.4 Carbon 2.5 Nitrogen 2.6 Phosphorous 2.7 Iron 2.8 Sulphur 2.9 Silica 2.3 Salinity of Inland Waters The salinity of freshwaters
Overzicht voortgang discussiepunten. Eric Verbruggen
 Overzicht voortgang discussiepunten Eric Verbruggen Discussion points 19-03-2015 Contents 1. Introduction 2. Compartment 3. Persistence 4. Bioaccumulation Discussion Points 19-03-2015 Timeline of EU PBT
Overzicht voortgang discussiepunten Eric Verbruggen Discussion points 19-03-2015 Contents 1. Introduction 2. Compartment 3. Persistence 4. Bioaccumulation Discussion Points 19-03-2015 Timeline of EU PBT
Cycles in Nature Standard 1 Objective 2:
 Cycles in Nature Standard 1 Objective 2: Explain relationships between matter cycles and Energy a) use diagrams to trace the movement of matter through a cycle b) Explain how water is a limiting factor
Cycles in Nature Standard 1 Objective 2: Explain relationships between matter cycles and Energy a) use diagrams to trace the movement of matter through a cycle b) Explain how water is a limiting factor
How Ecosystems Work Section 2. Chapter 5 How Ecosystems Work Section 2: Cycling of Materials DAY 1
 Chapter 5 How Ecosystems Work Section 2: Cycling of Materials DAY 1 The Carbon Cycle The carbon cycle is the movement of carbon from the nonliving environment into living things and back Carbon is the
Chapter 5 How Ecosystems Work Section 2: Cycling of Materials DAY 1 The Carbon Cycle The carbon cycle is the movement of carbon from the nonliving environment into living things and back Carbon is the
Economics of equal masses of NaOH and Cl 2 being produced
 Chlorine and its Compounds Issue #1: the production of 2 : the chloralkali process 2Na + 2H 2 O > 2NaOH + 2 + H 2 Economics of equal masses of NaOH and 2 being produced Electrolysis: Anode reaction! (aq)
Chlorine and its Compounds Issue #1: the production of 2 : the chloralkali process 2Na + 2H 2 O > 2NaOH + 2 + H 2 Economics of equal masses of NaOH and 2 being produced Electrolysis: Anode reaction! (aq)
Introduction. Wetland System. A Wetland Scene at Lorne C. Henderson Conservation Area near Petrolia
 Wetland Treatment of Wastewater This monograph, one in a series of single issue documents that deal with our local environment, has been prepared by the Sarnia-Lambton Environmental Association in co-operation
Wetland Treatment of Wastewater This monograph, one in a series of single issue documents that deal with our local environment, has been prepared by the Sarnia-Lambton Environmental Association in co-operation
NOTEBOOK. Table of Contents: 9. Properties of Water 9/20/ Water & Carbon Cycles 9/20/16
 NOTEBOOK Table of Contents: 9. Properties of Water 9/20/16 10. Water & Carbon Cycles 9/20/16 NOTEBOOK Assignment Page(s): Agenda: Tuesday, September 20, 2016 Properties of Water Water & Carbon Cycles 1.
NOTEBOOK Table of Contents: 9. Properties of Water 9/20/16 10. Water & Carbon Cycles 9/20/16 NOTEBOOK Assignment Page(s): Agenda: Tuesday, September 20, 2016 Properties of Water Water & Carbon Cycles 1.
Section 2: The Cycling of Matter
 Section 2: The Cycling of Matter Preview Classroom Catalyst Objectives The Carbon Cycle How Humans Affect the Carbon Cycle The Nitrogen Cycle Decomposers and the Nitrogen Cycle The Phosphorus Cycle Section
Section 2: The Cycling of Matter Preview Classroom Catalyst Objectives The Carbon Cycle How Humans Affect the Carbon Cycle The Nitrogen Cycle Decomposers and the Nitrogen Cycle The Phosphorus Cycle Section
Lesson Overview. Cycles of Matter. Lesson Overview. 3.4 Cycles of Matter
 Lesson Overview 3.4 THINK ABOUT IT A handful of elements combine to form the building blocks of all known organisms. Organisms cannot manufacture these elements and do not use them up, so where do essential
Lesson Overview 3.4 THINK ABOUT IT A handful of elements combine to form the building blocks of all known organisms. Organisms cannot manufacture these elements and do not use them up, so where do essential
Nutrient Cycling & Soils
 Nutrient Cycling & Soils tutorial by Paul Rich Outline 1. Nutrient Cycles What are nutrient cycles? major cycles 2. Water Cycle 3. Carbon Cycle 4. Nitrogen Cycle 5. Phosphorus Cycle 6. Sulfur Cycle 7.
Nutrient Cycling & Soils tutorial by Paul Rich Outline 1. Nutrient Cycles What are nutrient cycles? major cycles 2. Water Cycle 3. Carbon Cycle 4. Nitrogen Cycle 5. Phosphorus Cycle 6. Sulfur Cycle 7.
Pest Management Regulatory Agency: Aquatic exposure modelling for exposure assessment in support of the regulation of pest control products in Canada
 Pest Management Regulatory Agency: Aquatic exposure modelling for exposure assessment in support of the regulation of pest control products in Canada 242nd ACS National Meeting August 28, 2011 Greg Malis,
Pest Management Regulatory Agency: Aquatic exposure modelling for exposure assessment in support of the regulation of pest control products in Canada 242nd ACS National Meeting August 28, 2011 Greg Malis,
CEE 370 Environmental Engineering Principles
 Updated: 4 November 2015 Print version CEE 370 Environmental Engineering Principles Lecture #25 Water Quality Management III: Laes & toxic models Reading: Mihelcic & Zimmerman, Chapter 7 Reading: Davis
Updated: 4 November 2015 Print version CEE 370 Environmental Engineering Principles Lecture #25 Water Quality Management III: Laes & toxic models Reading: Mihelcic & Zimmerman, Chapter 7 Reading: Davis
Guidance for the derivation of environmental risk limits Part 11. Variables and default values used in the ERL guidance documents. version 1.
 Guidance for the derivation of environmental risk limits Part 11. Variables and default values used in the ERL guidance documents version 1.0 Colophon RIVM 2015 Parts of this publication may be reproduced,
Guidance for the derivation of environmental risk limits Part 11. Variables and default values used in the ERL guidance documents version 1.0 Colophon RIVM 2015 Parts of this publication may be reproduced,
C = W/a a = Q + v s A + kv C=C o e -λt. 7. Lakes (reactors) in series (SS)
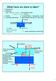 What have we done to date? 1. Regulations 2. Hydrology (ΔS = Rivers + Precip. + Groundwater disch. Outflow Evap. GW out ) 3. Kinetics 4. Eutrophication description, model 5. Limnology (field trips) 6.
What have we done to date? 1. Regulations 2. Hydrology (ΔS = Rivers + Precip. + Groundwater disch. Outflow Evap. GW out ) 3. Kinetics 4. Eutrophication description, model 5. Limnology (field trips) 6.
Cycles of Ma,er. Lesson Overview. Lesson Overview. 3.4 Cycles of Matter
 Lesson Overview Cycles of Ma,er Lesson Overview 3.4 Cycles of Matter THINK ABOUT IT A handful of elements combine to form the building blocks of all known organisms. Organisms cannot manufacture these
Lesson Overview Cycles of Ma,er Lesson Overview 3.4 Cycles of Matter THINK ABOUT IT A handful of elements combine to form the building blocks of all known organisms. Organisms cannot manufacture these
CHEMICAL COMPOSITION OF NATURAL WATERS
 CHEMICAL COMPOSITION OF NATURAL WATERS DISSOVLED GASES Oxygen (and E h ) Why important? product of photosynthesis needed for aerobic respiration - Much of an aquatic organisms energy budget is devoted
CHEMICAL COMPOSITION OF NATURAL WATERS DISSOVLED GASES Oxygen (and E h ) Why important? product of photosynthesis needed for aerobic respiration - Much of an aquatic organisms energy budget is devoted
New insight into pesticide partition coefficient K d for modelling pesticide fluvial transport with the SWAT model
 New insight into pesticide partition coefficient K d for modelling pesticide fluvial transport with the SWAT model Laurie BOITHIAS, Sabine SAUVAGE, Raghavan SRINIVASAN, Jeff ARNOLD, José-Miguel SANCHEZ-PEREZ
New insight into pesticide partition coefficient K d for modelling pesticide fluvial transport with the SWAT model Laurie BOITHIAS, Sabine SAUVAGE, Raghavan SRINIVASAN, Jeff ARNOLD, José-Miguel SANCHEZ-PEREZ
Solubility Equilibrium
 Solubility Equilibrium This is an X-ray of the large intestine. The patient was given a drink of barium sulfate to drink which is insoluble (does NOT dissolve) and gives the doctor a better image. Barium
Solubility Equilibrium This is an X-ray of the large intestine. The patient was given a drink of barium sulfate to drink which is insoluble (does NOT dissolve) and gives the doctor a better image. Barium
Chapter 4: Advanced Wastewater Treatment for Phosphorous Removal
 ENGI 9605 Advanced Wastewater Treatment Chapter 4: Advanced Wastewater Treatment for Phosphorous Removal Winter 2011 Faculty of Engineering & Applied Science 4.1 Phosphorous in wastewaters 1. Common forms
ENGI 9605 Advanced Wastewater Treatment Chapter 4: Advanced Wastewater Treatment for Phosphorous Removal Winter 2011 Faculty of Engineering & Applied Science 4.1 Phosphorous in wastewaters 1. Common forms
The Biosphere Chapter 3. What Is Ecology? Section 3-1
 The Biosphere Chapter 3 What Is Ecology? Section 3-1 Interactions and Interdependence Ecology is the scientific study of interactions among organisms and between organisms and their environment, or surroundings.
The Biosphere Chapter 3 What Is Ecology? Section 3-1 Interactions and Interdependence Ecology is the scientific study of interactions among organisms and between organisms and their environment, or surroundings.
Lecture 11. Solubility of Ionic Compounds in Water
 Lecture 11 Solubility of Ionic Compounds in Water Soluble Compounds Exceptions Acetates, Nitrates, Chlorates Na +, K +, NH 4 + compounds None None Halides ( Cl -, Br -, I - ) Ag +, Hg 2 2+, Cu +, Pb 2+
Lecture 11 Solubility of Ionic Compounds in Water Soluble Compounds Exceptions Acetates, Nitrates, Chlorates Na +, K +, NH 4 + compounds None None Halides ( Cl -, Br -, I - ) Ag +, Hg 2 2+, Cu +, Pb 2+
BASICS OF WASTEWATER TREATMENT
 BASICS OF WASTEWATER TREATMENT Knowing the decisioning criteria relevant to site and drain field suitability, i.e., soil properties, can be enhanced by an understanding of some of the basics of wastewater
BASICS OF WASTEWATER TREATMENT Knowing the decisioning criteria relevant to site and drain field suitability, i.e., soil properties, can be enhanced by an understanding of some of the basics of wastewater
Applications of Acids, Bases, and Neutralization Reactions
 Applications of Acids, Bases, and Neutralization Reactions Agricultural Impacts and Uses The successful growth of plants in agriculture depends on many conditions that must be met for optimal plant growth.
Applications of Acids, Bases, and Neutralization Reactions Agricultural Impacts and Uses The successful growth of plants in agriculture depends on many conditions that must be met for optimal plant growth.
10/18/2010 THINK ABOUT IT CHAPTER 3 THE BIOSHPERE RECYCLING IN THE BIOSPHERE RECYCLING IN THE BIOSPHERE
 THINK ABOUT IT CHAPTER 3 THE BIOSHPERE 3.4 Mrs. Michaelsen A handful of elements combine to form the building blocks of all known organisms. Organisms cannot manufacture these elements and do not use them
THINK ABOUT IT CHAPTER 3 THE BIOSHPERE 3.4 Mrs. Michaelsen A handful of elements combine to form the building blocks of all known organisms. Organisms cannot manufacture these elements and do not use them
Study Questions Exam 5
 Study Questions Exam 5 1. List three best management practices intended to reduce the loss of nutrients from agroecosystems. No problem. 2. Explain how buffer strips work. Runoff enters at higher velocity,
Study Questions Exam 5 1. List three best management practices intended to reduce the loss of nutrients from agroecosystems. No problem. 2. Explain how buffer strips work. Runoff enters at higher velocity,
NUTRIENT CYCLES REVIEW
 52 Name A.P. Environmental Science Date Mr. Romano NUTRIENT CYCLES REVIEW 1. Which of the following chain of events would occur as a result of land clearing/deforestation? (vocabulary check: efflux means
52 Name A.P. Environmental Science Date Mr. Romano NUTRIENT CYCLES REVIEW 1. Which of the following chain of events would occur as a result of land clearing/deforestation? (vocabulary check: efflux means
Uptake and translocation of non-ionised pollutants by plants
 Uptake and translocation of non-ionised pollutants by plants Richard H. Bromilow Transfer of organic pollutants from soil to plants Reading University, September 25/26, 2007 Pesticides applied directly
Uptake and translocation of non-ionised pollutants by plants Richard H. Bromilow Transfer of organic pollutants from soil to plants Reading University, September 25/26, 2007 Pesticides applied directly
5/6/2015. Matter is recycled within and between ecosystems.
 Biogeochemical Cycles/ Nutrient Cycles Biogeochemical Cycle Evaporation Water Cycle Transpiration Condensation Precipitation Runoff Vocabulary Seepage Root Uptake Carbon Cycle Phosphorus Cycle Nitrogen
Biogeochemical Cycles/ Nutrient Cycles Biogeochemical Cycle Evaporation Water Cycle Transpiration Condensation Precipitation Runoff Vocabulary Seepage Root Uptake Carbon Cycle Phosphorus Cycle Nitrogen
Lecture 18. Soil Acidity, Alkalinity, and Socidity
 Lecture 18 Soil Acidity, Alkalinity, and Socidity 1 Questions ow can acidification occur in soils? ow does p affects availability of N, P, K? ow can acidic soils be managed? Define a saline and sodic soil.
Lecture 18 Soil Acidity, Alkalinity, and Socidity 1 Questions ow can acidification occur in soils? ow does p affects availability of N, P, K? ow can acidic soils be managed? Define a saline and sodic soil.
Cycling and Biogeochemical Transformations of N, P and S
 Cycling and Biogeochemical Transformations of N, P and S OCN 401 - Biogeochemical Systems Reading: Schlesinger, Chapter 6 1. Nitrogen cycle Soil nitrogen cycle Nitrification Emissions of N gases from soils
Cycling and Biogeochemical Transformations of N, P and S OCN 401 - Biogeochemical Systems Reading: Schlesinger, Chapter 6 1. Nitrogen cycle Soil nitrogen cycle Nitrification Emissions of N gases from soils
3 3 Cycles of Matter Slide 1 of 33
 1 of 33 Recycling in the Biosphere Recycling in the Biosphere Energy and matter move through the biosphere very differently. Unlike the one-way flow of energy, matter is recycled within and between ecosystems.
1 of 33 Recycling in the Biosphere Recycling in the Biosphere Energy and matter move through the biosphere very differently. Unlike the one-way flow of energy, matter is recycled within and between ecosystems.
Nutrient Cycling in Soils
 Nutrient Cycling in Soils Kristin A. Fisher, Ph.D. Nutrient Management Specialist Agricultural Nutrient Management Program University of Maryland, College Park Nutrient Cycling in Soils N, P & K cycles
Nutrient Cycling in Soils Kristin A. Fisher, Ph.D. Nutrient Management Specialist Agricultural Nutrient Management Program University of Maryland, College Park Nutrient Cycling in Soils N, P & K cycles
CHAPTER 5 Water and Seawater
 1 2 3 4 5 6 7 8 9 10 11 12 13 CHAPTER 5 Water and Seawater Chapter Overview Water has many unique thermal and dissolving properties. Seawater is mostly water molecules but has dissolved substances. Ocean
1 2 3 4 5 6 7 8 9 10 11 12 13 CHAPTER 5 Water and Seawater Chapter Overview Water has many unique thermal and dissolving properties. Seawater is mostly water molecules but has dissolved substances. Ocean
Cycles of Matter. Slide 1 of 33. End Show. Copyright Pearson Prentice Hall
 Cycles of Matter 1 of 33 The purpose of this lesson is to learn the water, carbon, nitrogen, and phosphorus cycles. This PowerPoint will provide most of the required information you need to accomplish
Cycles of Matter 1 of 33 The purpose of this lesson is to learn the water, carbon, nitrogen, and phosphorus cycles. This PowerPoint will provide most of the required information you need to accomplish
Water Runoff and the Environment
 This guided activity explores the process of erosion and pollution caused by water runoff, using 3D Molecular Design s and paper clips. It also demonstrates how protective covers such as perennial grasses
This guided activity explores the process of erosion and pollution caused by water runoff, using 3D Molecular Design s and paper clips. It also demonstrates how protective covers such as perennial grasses
Oxidation Reactions. This oxide will from only if thermodynamics favour a reaction of the form: M + O 2 = MO 2. Which must form rapidly (favourable(
 Oxidation of s Oxidation is a general term used to define the reaction between a metal or alloy and its environment. s or alloys are oxidised when heated to elevated temperatures es in air or highly oxidised
Oxidation of s Oxidation is a general term used to define the reaction between a metal or alloy and its environment. s or alloys are oxidised when heated to elevated temperatures es in air or highly oxidised
10/17/ Cycles of Matter. Recycling in the Biosphere. How does matter move among the living and nonliving parts of an ecosystem?
 2 of 33 3-3 Cycles of Matter How does matter move among the living and nonliving parts of an ecosystem? 3 of 33 Recycling in the Biosphere Recycling in the Biosphere Energy and matter move through the
2 of 33 3-3 Cycles of Matter How does matter move among the living and nonliving parts of an ecosystem? 3 of 33 Recycling in the Biosphere Recycling in the Biosphere Energy and matter move through the
Chapter 5 Water & Seawater. Chapter 5 Water & Seawater
 Chapter 5 Water & Seawater Chapter 5 Water & Seawater Chapter Overview Water has many unique thermal and dissolving properties. Seawater is mostly water molecules but has dissolved substances. Ocean water
Chapter 5 Water & Seawater Chapter 5 Water & Seawater Chapter Overview Water has many unique thermal and dissolving properties. Seawater is mostly water molecules but has dissolved substances. Ocean water
Ethyl Xanthate. Identifier of Ethyl Xanthate EINECS or ELINCS number CAS name and CAS number
 Ethyl Xanthate Ethyl Xanthate is one of these chemicals where no or little data is available regarding their environmental impact. This chemical substance is not classified in the Annex I of Directive
Ethyl Xanthate Ethyl Xanthate is one of these chemicals where no or little data is available regarding their environmental impact. This chemical substance is not classified in the Annex I of Directive
Role of organic matter, soil microorganisms, and downward mobility in determining fate of pesticides in managed turfgrass systems
 Role of organic matter, soil microorganisms, and downward mobility in determining fate of pesticides in managed turfgrass systems Fred H. Yelverton North Carolina State University Introduction 74 agricultural
Role of organic matter, soil microorganisms, and downward mobility in determining fate of pesticides in managed turfgrass systems Fred H. Yelverton North Carolina State University Introduction 74 agricultural
BAE 820 Physical Principles of Environmental Systems
 BAE 820 Physical Principles of Environmental Systems Fate and transport of pollutants in the water environment Dr. Zifei Liu The global hydrologic cycle The mass of water on Earth remains fairly constant
BAE 820 Physical Principles of Environmental Systems Fate and transport of pollutants in the water environment Dr. Zifei Liu The global hydrologic cycle The mass of water on Earth remains fairly constant
The rest of this article describes four biogeochemical cycles: the water cycle, carbon cycle, nitrogen cycle, and phosphorous cycle.
 BIOGEOCHEMICAL CYCLES The chemical elements and water that are needed by living things keep recycling over and over on Earth. These cycles are called biogeochemical cycles. They pass back and forth through
BIOGEOCHEMICAL CYCLES The chemical elements and water that are needed by living things keep recycling over and over on Earth. These cycles are called biogeochemical cycles. They pass back and forth through
Just what is Acid Rain?
 Acid Rain Just what is Acid Rain? Acid Rain is the term used to describe the ways in which acid precipitates out of the atmosphere. Acid Rain is more accurately termed acid deposition. There are two types
Acid Rain Just what is Acid Rain? Acid Rain is the term used to describe the ways in which acid precipitates out of the atmosphere. Acid Rain is more accurately termed acid deposition. There are two types
BIOGEOCHEMICAL CYCLES
 BIOGEOCHEMICAL CYCLES BIOGEOCHEMICAL CYCLES A biogeochemical cycle or cycling of substances is a pathway by which a chemical element or molecule moves through both biotic and abiotic compartments of Earth.
BIOGEOCHEMICAL CYCLES BIOGEOCHEMICAL CYCLES A biogeochemical cycle or cycling of substances is a pathway by which a chemical element or molecule moves through both biotic and abiotic compartments of Earth.
Cycling and Biogeochemical Transformations of N, P, S, and K
 Cycling and Biogeochemical Transformations of N, P, S, and K OCN 401 - Biogeochemical Systems 24 September 2013 Reading: Schlesinger & Bernhardt, Chapter 6 1. Nitrogen cycle Soil nitrogen cycle Nitrification
Cycling and Biogeochemical Transformations of N, P, S, and K OCN 401 - Biogeochemical Systems 24 September 2013 Reading: Schlesinger & Bernhardt, Chapter 6 1. Nitrogen cycle Soil nitrogen cycle Nitrification
Freshwater Wetlands: Functions & Conservation. ENVIRTHON Workshop 2016 University of Massachusetts Amherst Deborah J.
 Freshwater Wetlands: Functions & Conservation ENVIRTHON Workshop 2016 University of Massachusetts Amherst Deborah J. Henson, PhD, CPSS What is a Wetland? Legal Definition:...those areas that are inundated
Freshwater Wetlands: Functions & Conservation ENVIRTHON Workshop 2016 University of Massachusetts Amherst Deborah J. Henson, PhD, CPSS What is a Wetland? Legal Definition:...those areas that are inundated
Schematic diagram of a cooling water system
 Cooling Tower Water Treatment Cooled water is needed for, example, air conditioners, manufacturing processes or power generation. A cooling tower is an equipment used to reduce the temperature of a water
Cooling Tower Water Treatment Cooled water is needed for, example, air conditioners, manufacturing processes or power generation. A cooling tower is an equipment used to reduce the temperature of a water
Oxygen. Oxygen is one of the fundamental resources required by life forms on Earth. Aquatic ecosystems have a wide assortment of life forms.
 Oxygen Oxygen is one of the fundamental resources required by life forms on Earth. Aquatic ecosystems have a wide assortment of life forms. Oxygen is also required for some natural chemical decays. What
Oxygen Oxygen is one of the fundamental resources required by life forms on Earth. Aquatic ecosystems have a wide assortment of life forms. Oxygen is also required for some natural chemical decays. What
9.2.1 Similarities and trends in the properties of the Group II metals magnesium to barium and their compounds
 9.2 Group II Content 9.2.1 Similarities and trends in the properties of the Group II metals magnesium to barium and their compounds Learning Outcomes Candidates should be able to: (a) (b) (c) (d) describe
9.2 Group II Content 9.2.1 Similarities and trends in the properties of the Group II metals magnesium to barium and their compounds Learning Outcomes Candidates should be able to: (a) (b) (c) (d) describe
Cycling and Biogeochemical Transformations of N, P and S
 Cycling and Biogeochemical Transformations of N, P and S OCN 401 - Biogeochemical Systems Reading: Schlesinger,, Chapter 6 1. Nitrogen cycle Soil nitrogen cycle Nitrification Emissions of N gases from
Cycling and Biogeochemical Transformations of N, P and S OCN 401 - Biogeochemical Systems Reading: Schlesinger,, Chapter 6 1. Nitrogen cycle Soil nitrogen cycle Nitrification Emissions of N gases from
Earth Systems and Interactions
 CHAPTER The Earth System Earth Systems and Interactions What do you think? Read the three statements below and decide whether you agree or disagree with them. Place an A in the Before column if you agree
CHAPTER The Earth System Earth Systems and Interactions What do you think? Read the three statements below and decide whether you agree or disagree with them. Place an A in the Before column if you agree
Bell Ringer AP Practice
 Bell Ringer AP Practice 1) Reasons that the population size of an exotic species often grows rapidly when the species is introduced in a new environment include which of the following? i. The exotic species
Bell Ringer AP Practice 1) Reasons that the population size of an exotic species often grows rapidly when the species is introduced in a new environment include which of the following? i. The exotic species
3 3 Cycles of Matter
 3 3 Cycles of Matter Recycling in the Biosphere Energy - one way flow matter - recycled within and between ecosystems. biogeochemical cycles matter Elements, chemical compounds, and other forms passed
3 3 Cycles of Matter Recycling in the Biosphere Energy - one way flow matter - recycled within and between ecosystems. biogeochemical cycles matter Elements, chemical compounds, and other forms passed
Water Pollution & Quality. Dr. Deniz AKGÜL Marmara University Department of Environmental Engineering
 Water Pollution & Quality Dr. Deniz AKGÜL Marmara University Department of Environmental Engineering IMPORTANCE OF WATER Life on planet Earth would be impossible without water. All life forms, from simple
Water Pollution & Quality Dr. Deniz AKGÜL Marmara University Department of Environmental Engineering IMPORTANCE OF WATER Life on planet Earth would be impossible without water. All life forms, from simple
Biology 5868 ID Exam 1 February 23, 2007
 Ecotoxicology Name KEY Biology 5868 ID Exam 1 February 23, 2007 Be as specific as possible for all answers. Most of the questions have multiple parts; make sure to answer each part! Use diagrams, flowcharts,
Ecotoxicology Name KEY Biology 5868 ID Exam 1 February 23, 2007 Be as specific as possible for all answers. Most of the questions have multiple parts; make sure to answer each part! Use diagrams, flowcharts,
Corrosion. Lab. of Energy Conversion & Storage Materials. Produced by K. B. Kim
 Corrosion 대기환경에의한금속소재 (organic film coated steel) 의퇴화현상평가연구 Lab. of Energy Conversion & Storage Materials Produced by K. B. Kim Introduction AC Impedance Spectroscopy Application of AC Impedance to Corrosion
Corrosion 대기환경에의한금속소재 (organic film coated steel) 의퇴화현상평가연구 Lab. of Energy Conversion & Storage Materials Produced by K. B. Kim Introduction AC Impedance Spectroscopy Application of AC Impedance to Corrosion
CRISTALIZATION UNIT YUSRON SUGIARTO
 CRISTALIZATION UNIT YUSRON SUGIARTO COURSE OUTCOMES CO DESCRIBE the basic principles and applications of crystallization process. CALCULATE the yields, material and energy balance in crystallization. OUTLINES
CRISTALIZATION UNIT YUSRON SUGIARTO COURSE OUTCOMES CO DESCRIBE the basic principles and applications of crystallization process. CALCULATE the yields, material and energy balance in crystallization. OUTLINES
CTB3365x Introduction to Water Treatment
 CTB3365x Introduction to Water Treatment D4a Groundwater treatment Doris van Halem Ever wondered where your drinking water comes from? Well, chances are that you are sitting on it as we speak. Welcome
CTB3365x Introduction to Water Treatment D4a Groundwater treatment Doris van Halem Ever wondered where your drinking water comes from? Well, chances are that you are sitting on it as we speak. Welcome
Phosphorus Chemistry and Sequestration in Soil
 Phosphorus Chemistry and Sequestration in Soil Elizabeth (Libby) Dayton Research Scientist / Soil Environmental Chemistry School of Environment and Natural Resources Ohio State University Today s Presentation
Phosphorus Chemistry and Sequestration in Soil Elizabeth (Libby) Dayton Research Scientist / Soil Environmental Chemistry School of Environment and Natural Resources Ohio State University Today s Presentation
Environmental Models Fugacity approach
 Environmental Models Fugacity approach Equilibrium artition coefficients Definition, experimental assessment The fugacity approach to partition coefficients Fugacity definitions for a gas, a liquid, a
Environmental Models Fugacity approach Equilibrium artition coefficients Definition, experimental assessment The fugacity approach to partition coefficients Fugacity definitions for a gas, a liquid, a
Pennsylvania Senior Environment Corps. Table of Contents Part 2 Getting Started:. 21 Chemical Analysis... 22
 Table of Contents Part 2 Getting Started:. 21 Chemical Analysis.... 22 3 Chapter 2: Getting Started 21 Chemical Analysis of the Water Dependent on your area, you may measure for several parameters. In
Table of Contents Part 2 Getting Started:. 21 Chemical Analysis.... 22 3 Chapter 2: Getting Started 21 Chemical Analysis of the Water Dependent on your area, you may measure for several parameters. In
ENVIRONMENTAL CHEMICAL ANALYSIS III
 ENVIRONMENTAL CHEMICAL ANALYSIS III Chromatographic Methods Gas Chromatography (GC) Gas chromatography is a separation process in which a gaseous solute is carried through the column by a gaseous mobile
ENVIRONMENTAL CHEMICAL ANALYSIS III Chromatographic Methods Gas Chromatography (GC) Gas chromatography is a separation process in which a gaseous solute is carried through the column by a gaseous mobile
Biology Factors Modifying Contaminant Effects
 Biology 5868 Factors Modifying Contaminant Effects Modifying Factors Any characteristic of the organism or its surrounding environment that affects toxicity of a pollutant is considered to act as a Modifying
Biology 5868 Factors Modifying Contaminant Effects Modifying Factors Any characteristic of the organism or its surrounding environment that affects toxicity of a pollutant is considered to act as a Modifying
EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL. Safety of the Food chain Chemicals, contaminants, pesticides
 EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL Safety of the Food chain Chemicals, contaminants, pesticides Brussels, 25.09.2012 rev. 3 DG SANCO Working Document on "Evidence Needed to Identify
EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL Safety of the Food chain Chemicals, contaminants, pesticides Brussels, 25.09.2012 rev. 3 DG SANCO Working Document on "Evidence Needed to Identify
David Rowlings Institute for Sustainable Resources Queensland University of Technology
 How does carbon influence nitrogen availability and losses? David Rowlings Institute for Sustainable Resources Queensland University of Technology Outline Carbon cycle Global carbon cycle Soil carbon 3
How does carbon influence nitrogen availability and losses? David Rowlings Institute for Sustainable Resources Queensland University of Technology Outline Carbon cycle Global carbon cycle Soil carbon 3
Water Chemistry. Water 101
 Water Chemistry Water 101 I. Introduction A. Water is not pure Many different kinds of chemicals dissolved in it Ions, organic chemicals, organic matter, particulate matter, and gases can all be in water
Water Chemistry Water 101 I. Introduction A. Water is not pure Many different kinds of chemicals dissolved in it Ions, organic chemicals, organic matter, particulate matter, and gases can all be in water
Water Chemistry and Plant Performance
 Plant Operations and Laboratory Analysis Specialty Workshop Water Chemistry and Plant Performance September 1, 2010 Dan Miklos Water Environment Association Preserving & Enhancing Ohio s Water Environment
Plant Operations and Laboratory Analysis Specialty Workshop Water Chemistry and Plant Performance September 1, 2010 Dan Miklos Water Environment Association Preserving & Enhancing Ohio s Water Environment
Environmental Fate of Aquatic Herbicides
 Environmental Fate of Aquatic Herbicides UF-IFAS Aquatic Weed Control Short Course Michael Netherland, Ph.D US Army Engineer Research & Development Center US Army Corps of Engineers What Happens After
Environmental Fate of Aquatic Herbicides UF-IFAS Aquatic Weed Control Short Course Michael Netherland, Ph.D US Army Engineer Research & Development Center US Army Corps of Engineers What Happens After
ENVIRONMENTAL QUALITY Fall Semester 2011
 ENVIRONMENTAL QUALITY Fall Semester 2011 Instructor: Office: Office Hours: Course Text: Grading: Dr. George F. Vance 1007 Agricultural Hall, 766-2297, gfv@uwyo.edu Tuesday, Wednesday, Thursday 12:15-1:30,
ENVIRONMENTAL QUALITY Fall Semester 2011 Instructor: Office: Office Hours: Course Text: Grading: Dr. George F. Vance 1007 Agricultural Hall, 766-2297, gfv@uwyo.edu Tuesday, Wednesday, Thursday 12:15-1:30,
Environmental Fate of Herbicides. J. Ferrell Extension Weed Specialist
 Environmental Fate of Herbicides J. Ferrell Extension Weed Specialist What is Environmental Fate? Simple definition: what happens to the herbicide after it leaves the sprayer. Why Does it Matter Pesticides
Environmental Fate of Herbicides J. Ferrell Extension Weed Specialist What is Environmental Fate? Simple definition: what happens to the herbicide after it leaves the sprayer. Why Does it Matter Pesticides
IV Evaluation of research results
 IV Data contain errors which are derived from accidental, random, non-systematic and systematic characteristics of analytical methods. Environmental monitoring of chemical substances tends to investigate
IV Data contain errors which are derived from accidental, random, non-systematic and systematic characteristics of analytical methods. Environmental monitoring of chemical substances tends to investigate
2/11/16. Materials in ecosystems are constantly reused Three cycles: The Carbon Cycle The Nitrogen Cycle The Phosphorus Cycle
 Materials in ecosystems are constantly reused Three cycles: The Carbon Cycle The Nitrogen Cycle The Cycle Carbon is essential in proteins, fats, and carbohydrates, which make up all organisms Carbon cycle
Materials in ecosystems are constantly reused Three cycles: The Carbon Cycle The Nitrogen Cycle The Cycle Carbon is essential in proteins, fats, and carbohydrates, which make up all organisms Carbon cycle
3 3 Cycles of Matter. EOC Review
 EOC Review A freshwater plant is placed in a salt marsh. Predict the direction in which water will move across the plant s cell wall, and the effect of that movement on the plant. a. Water would move out
EOC Review A freshwater plant is placed in a salt marsh. Predict the direction in which water will move across the plant s cell wall, and the effect of that movement on the plant. a. Water would move out
Nutrient Cycling in Soils: The Big 3 and Carbon
 Fundamentals of Nutrient Management Nutrient Cycling in Soils: The Big 3 and Carbon Gurpal Toor Department of Environmental Science & Technology University of Maryland College Park @ToorUMD N deficiency
Fundamentals of Nutrient Management Nutrient Cycling in Soils: The Big 3 and Carbon Gurpal Toor Department of Environmental Science & Technology University of Maryland College Park @ToorUMD N deficiency
Water Quality. CE 370 Lecture 1. Global Distribution of Earth s s Water
 Water Quality CE 370 Lecture 1 Global Distribution of Earth s s Water Water Demand and Supply in Saudi Arabia Total Water Consumption = 22 billion m 3 /Year Water Demand Water Supply Industrial Domestic
Water Quality CE 370 Lecture 1 Global Distribution of Earth s s Water Water Demand and Supply in Saudi Arabia Total Water Consumption = 22 billion m 3 /Year Water Demand Water Supply Industrial Domestic
Geology 627, Hydrogeology Review questions for final exam h t 1/ 2
 Geology 67, Hydrogeology Review questions for final exam 004 Multiple choice and fill in the blank. There may be more than one correct choice for each question. 1. Which hydrogeologic quantities are represented
Geology 67, Hydrogeology Review questions for final exam 004 Multiple choice and fill in the blank. There may be more than one correct choice for each question. 1. Which hydrogeologic quantities are represented
3. [7 points] How many significant figures should there be in the answer to the following problem?
![3. [7 points] How many significant figures should there be in the answer to the following problem? 3. [7 points] How many significant figures should there be in the answer to the following problem?](/thumbs/83/87492512.jpg) 1 of 6 10/20/2009 3:52 AM 1. [7 points] What is the correct name for ZnF 2? (a) zinc fluorine (b) zinc (II) fluoride (c) zinc fluoride (d) fluoride zinc 2. [7 points] What is the correct name for P 2 O
1 of 6 10/20/2009 3:52 AM 1. [7 points] What is the correct name for ZnF 2? (a) zinc fluorine (b) zinc (II) fluoride (c) zinc fluoride (d) fluoride zinc 2. [7 points] What is the correct name for P 2 O
GRAVIMETRIC DETERMINATION OF SULFATE IN AN UNKNOWN SOLUTION
 GRAVIMETRIC DETERMINATION OF SULFATE IN AN UNKNOWN SOLUTION AIM The main objective of this experiment is to determine the concentration of sulfate ion in an unknown solution by using gravimetry. INTRODUCTION
GRAVIMETRIC DETERMINATION OF SULFATE IN AN UNKNOWN SOLUTION AIM The main objective of this experiment is to determine the concentration of sulfate ion in an unknown solution by using gravimetry. INTRODUCTION
Modeling Surface Water Contamination
 Modeling Surface Water Contamination One of the resources required for an ecosystem to function is an available source of fresh water This is quite true for human settlements as well: If you examine the
Modeling Surface Water Contamination One of the resources required for an ecosystem to function is an available source of fresh water This is quite true for human settlements as well: If you examine the
Chapter 17 Solubility and Complex Ion Equilibria
 Chem 1046 General Chemistry by Ebbing and Gammon, 8th Edition Prof George W.J. Kenney, Jr Last Update: 27Nov2008 Chapter 17 Solubility and Complex Ion Equilibria These Notes are to SUPPLIMENT the Text,
Chem 1046 General Chemistry by Ebbing and Gammon, 8th Edition Prof George W.J. Kenney, Jr Last Update: 27Nov2008 Chapter 17 Solubility and Complex Ion Equilibria These Notes are to SUPPLIMENT the Text,
Chapter 9. Dissolution and Precipitation Equilibria
 Chapter 9 Dissolution and Precipitation Equilibria 9-1 The Nature of Solubility Equilibria 9-2 The Solubility of Ionic Solids 9-3 Precipitation and the Solubility Product 9-4 The Effects of ph on Solubility
Chapter 9 Dissolution and Precipitation Equilibria 9-1 The Nature of Solubility Equilibria 9-2 The Solubility of Ionic Solids 9-3 Precipitation and the Solubility Product 9-4 The Effects of ph on Solubility
Streamwater Chemistry
 Streamwater Chemistry 1) Dissolved major ions 2) Suspended and dissolved organic matter 3) Dissolved nutrients and biological transformations 4) Dissolved gases 5) ph 1) Dissolved major ions TDS (Total
Streamwater Chemistry 1) Dissolved major ions 2) Suspended and dissolved organic matter 3) Dissolved nutrients and biological transformations 4) Dissolved gases 5) ph 1) Dissolved major ions TDS (Total
