Techno-Economic Analysis of a Cost-Effective Treatment of Flowback and Produced. A thesis presented to. the faculty of. In partial fulfillment
|
|
|
- Christopher Parsons
- 6 years ago
- Views:
Transcription
1 Techno-Economic Analysis of a Cost-Effective Treatment of Flowback and Produced Waters via an Integrated Precipitative Supercritical Process A thesis presented to the faculty of the Russ College of Engineering and Technology of Ohio University In partial fulfillment of the requirements for the degree Master of Science Xiao Dong May Xiao Dong. All Rights Reserved.
2 2 This thesis titled Techno-Economic Analysis of a Cost-Effective Treatment of Flowback and Produced Waters via an Integrated Precipitative Supercritical Process by XIAO DONG has been approved for the Department of Mechanical Engineering and the Russ College of Engineering and Technology by David Bayless Loehr Professor of Mechanical Engineering Dennis Irwin Dean, Russ College of Engineering and Technology
3 3 ABSTRACT DONG, XIAO., M.S., May 2015, Mechanical Engineering Techno-Economic Analysis of a Cost-Effective Treatment of Flowback and Produced Waters via an Integrated Precipitative Supercritical Process Director of Thesis: David Bayless The use of hydraulic fracturing for shale oil and gas development generates large quantities of flowback and produced (F/P) water as by-products. The current high treatment cost of F/P water presents a large obstacle to the full development and profitability of shale oil and gas. The challenge is to find an economical and technological feasible treatment method for the enormous amounts of F/P water produced from hydraulic fracturing with significantly less cost than current practices. A new proposed treatment method is the Integrated Precipitative Supercritical (IPSC) process. The objective of this thesis was to conduct a techno-economic analysis of the IPSC process, and was to evaluate its economic competitiveness for treatment of F/P water. The techno economic analysis of the IPSC process was built using Aspen process software and Microsoft Excel. The Aspen model simulated the IPSC process and the cost analysis was built on the results of the Aspen model. The cost analysis indicated an average cost of $6.33/bbl of F/P water treatment, and the sensitivity analysis indicated a possible range from $2.93/bbl to $16.03/bbl for the IPSC process. This techno-economic analysis confirmed the IPSC process economic competitiveness compared to existing practices.
4 4 DEDICATION To my parents
5 5 ACKNOWLEDGMENTS I would like thank my advisors, Dr. Bayless and Dr. Trembly, for helping me every step of the way. Their excellent guidance made everything possible. I would also like to thank my committee members Dr. Natalie Kruse Daniels and Dr. Ben Stuart for their valuable advice and support.
6 6 TABLE OF CONTENTS Page Abstract... 3 Dedication... 4 Acknowledgments... 5 List of Tables... 9 List of Figures Chapter 1 - Introduction Background Shale Gas Waste Water from Fracking Fracking Water Treatment Treatment Methods/Technologies/Cost IPSC Process Brief Description of Objectives Objective Objective Chapter 2 Literature Review Introduction Aspen Modeling Waste Water Composition Particle Sizes Settling Velocity Heat Exchanger Heat Transfer Physical Property Modeling in the Supercritical Region Heat Transfer for a Cylinder Shell with Insulation Cost-Analysis Modeling Capital Cost Estimation Cost of Operation Estimation Chapter 3 Model Simulation Introduction... 35
7 7 3.2 Aspen Model Aspen Modeling Physical Property Setup Precipitation Chemistry in Aspen Sand Filter UV Unit Precipitation Process NORM Unit Supercritical Area Cost Model Total Capital Cost Total Operating Cost Cost/Barrel Model Validation Chapter 4 Senstivity Analysis Introduction Sensitivity Analysis Flow Rate Water Chemistry Inlet Water Temperature Precipitation Separation Efficiency Cost of Precipitation Agents NORM Concentration Material of Construction Solids Waste Disposal Cost Equipment Lifetime Cost of Utilities Sulfation Only Option Cost Range Chapter 5 - Discussion Final Cost per Barrel of the IPSC Process Sensitivity Analysis... 91
8 Chapter 6 Conclusions Chapter 7 - Recommendations Validation of Precipitation Process Modeling Multivariate Sensitivity Analysis Incorporation of Engineering Design Constraints References Appendix A - VBA Settling Equation Code Appendix B - Hydrocyclone Quote Appendix C - Fortran Code for Transfer Function between the Ambient and Supercritical Area Appendix D - Fortran Code for Change in Ion Enthalpy due to Temperature and Reaction/Solids Produced Appendix E - UV Unit Quotation Appendix F - Plastic Tank Appendix G - Pump Quotation Appendix H - Generation/Truck Quotes
9 9 LIST OF TABLES Page Table 1.1: Estimated Water Consumption Numbers for Horizontal Drilling and Hydraulic Fracturing Process in Various Shale Plays...17 Table 1.2: Commercial F/P Water Management Technology Characteristics...19 Table 1.3: Input Variables for Sensitivity Analysis...24 Table 2.1: Constituents and Concentration in Fracking Water...26 Table 2.2: Convective Heat Transfer Coefficient for Sensible Heat Transfer...28 Table 2.3: Convective Heat Transfer Coefficient for Condensing Heat Transfer...29 Table 2.4: Convective Heat Transfer Coefficient for Vaporizing Heat Transfer...29 Table 2.5: Cost Factors...34 Table 3.1: Chemical Components in the Aspen Model...38 Table 3.2: Reaction Equations for the Aspen Model...38 Table 3.3: Cost Analysis for the Precipitation Agent Costs...48 Table 3.4: Sandfilter Pump Cost...49 Table 3.5: Settling Tank Cost...50 Table 3.6: Precipitation Pump Cost...51 Table 3.7: NORM Column Cost...53 Table 3.8: Heat Exchanger Cost for Various Configurations...55 Table 3.9: Reactor Shell Cost...56 Table 3.10: Capital Costs and Annualized Cost of Capital for All Equipment...59 Table 3.11: Raw Material Unit Cost...60
10 10 Table 3.12: Operating Costs...62 Table 3.13: Final Cost/Barrel...63 Table 3.14: Validation of the Sulfation Step...63 Table 3.15: Cost Comparison: Experimental vs. Aspen Model...64 Table 4.1: Input Variables for Sensitivity Analysis...66 Table 4.2: Cost/bbl vs. Flowrate...67 Table 4.3: Range of Ion Concentrations...68 Table 4.4: Linear Regression for Final Cost vs Ion...70 Table 4.5: Linear Regression for each Ion Removal...73 Table 4.6: Cost/bbl vs. Agent Cost Relationship...74 Table 4.7: Final Cost vs NORM Concentration Table...77 Table 4.8: Final Cost vs Salts Disposal Cost Table...82 Table 4.9: Final Cost vs. Equipment Lifetime...84 Table 4.10: Range of IPSC Final Costs...86 Table 4.11: Probably Range of IPSC Final Costs...87 Table 5.1: Cost Comparison with Existing Practices...91 Table 5.2: Values for the Price Ranges...93 Table 5.3: Cost Comparison with Existing Practices...94
11 11 LIST OF FIGURES Page Figure 1.1: U.S. Shale Deposits...16 Figure 1.2: Shale Gas Leads Growth in Total Gas Production Through Figure 1.3: IPSC Process...21 Figure 3.1: Aspen Model PDF...35 Figure 3.2: Sandfilter Unit...39 Figure 3.3: UV Unit...40 Figure 3.4: Sulfation...42 Figure 3.5: Softening...42 Figure 3.6: Hydrolysis...42 Figure 3.7: Calculation Block for Ion Compensation...45 Figure 3.8: Supercritical Area...47 Figure 4.1: Cost/bbl vs. Flowrate...67 Figure 4.2 Cost/bbl vs. Water Chemistry...68 Figure 4.3: Cost/bbl vs. Water Chemistry for Individual Ions...69 Figure 4.4: Cost/bbl vs. Inlet Temperature...71 Figure 4.5: Cost/bbl vs. Ion Removal...72 Figure 4.6: Cost/bbl vs. Ion Removal for Each Ion...73 Figure 4.7: Cost/bbl vs. Agent Cost...74 Figure 4.8:. Cost/bbl vs. Agent Cost...75 Figure 4.9: Cost/bbl vs. Agent Cost...75
12 12 Figure 4.10: Final Cost vs NORM Concentration...77 Figure 4.11: Final Cost vs Pump Material of Construction...79 Figure 4.12: Final Cost vs Heat Exchanger Material of Construction...80 Figure 4.13: Final Cost vs Supercritical Reactor Material of Construction...80 Figure 4.14: Final Cost vs Sulfate Disposal Cost...81 Figure 4.15: Final Cost vs Salts Disposal Cost...82 Figure 4.16: Final Cost vs Equipment Lifetime...83 Figure 4.17: Final Cost vs Equipment Lifetime for each Equipment...84 Figure 4.18: Final Cost vs Natural Gas Price...85 Figure 5.1: Cost Breakdown...89 Figure 5.2: Cost Breakdown for Cost of Raw Materials...89 Figure 5.3: Cost Breakdown- Sulfation...90 Figure 5.4: Price Ranges of Sensitivity Analysis...93
13 13 CHAPTER 1 - INTRODUCTION 1.1 Background Oil and gas in shale deposits has historically been a source of energy considered economically and/or technologically challenging to extract. With the recent application of hydraulic fracturing (fracking) technology to release trapped shale gas/oil, shale has become a significant source of energy. With more than 70 major shale oil/gas basins that have been identified in countries outside the United States, shale resources are now estimated to account for more than 40% of the world s recoverable gas reserves [1]. A large amount of water is needed for fracturing as fracturing fluid. On average U.S. shale wells require around 45 million gallons of water per hydraulic fracturing completed [2]. In addition, several compounds, such as acids, inhibitors, and biocide, are used to enhance the fracturing process. These compounds compose up to 0.5% by weight of the hydraulic fracturing fluid [2]. After fracking fluid is pumped into the ground, a portion of the fluid flows back up the well. Not only does the fracking fluid itself require treatment after the fracking process, fracking also unlocks additional underground brine water that flows back along with the shale gas/oil which is termed produced water. Typically, 10 70% of frack fluid returns to the surface during the initial period of production (<4 weeks from onset of gas production), and is referred to as flowback [3]. Both flowback and produced (F/P) water are byproduct waste water generated from fracking. With the recent enormous interest and activity in hydraulic fracturing, a significant obstacle that arises is the treatment of fracking generated waste water. National produced water volume estimates range from
14 14 15 to 20 billion barrels each year [4], which is equivalent to billion liters per day. Currently, three main methods of disposal exist for water unsuited for direct discharge, deep well injection, treatment for surface discharge, and treatment for reuse. Public Owned Treatment Works (POTWs) are being used as an option for surface discharge, but most POTWs are not designed to remove large volumes of solids (TDS or total dissolved solids) or naturally occurring radiative materials (NORM). Along with the limited particle treatment range, the existing POTWs are having trouble handling the increasing amount of waste water being generated without affecting plant effluent quality [5]. Industry also uses class II deep saline disposal sites for Salt Water Disposal (SWD). The cost of SWD can range from $ /bbl for transportation, and $ /bbl for injection, which results with average costs ranging from $ /bbl nationwide [6]. Along with the high cost, many proven and unproven environment issues are associated with SWD, such as ground water contamination and earthquakes [5]. Currently, treatments for reuse include thermal evaporation/condensation, reverse osmosis, natural evaporation, freeze thaw, crystallization, filtration, ozone, and integrated hybrid processing, which range in costs from $ /bbl [6]. Many of these methods require storing the waste water, and transportation of the waste water off-site to treatment facilities. Costs for the filtering process treatment range from $ /bbl alone. Also, transportation costs, from $ /bbl, must be added. With the addition of storage, transport and other costs, the price of treatment can reach $3.00/bbl to $30.30/bbl.
15 15 The current high treatment cost presents a large obstacle to the full development and profitability of shale gas, especially with current low prices for shale gas and oil. The challenge is to find an economical and technological feasible treatment method for the enormous amounts of produced water from hydraulic fracturing for on-site reuse with significantly less cost than current practices. The objectives of this thesis are to conduct a techno-economic analysis of an integrated precipitative super-critical water treatment (IPSC) process and evaluate its economic competitiveness for treatment of F/P water. 1.2 Shale Gas Shales are fine-grained sedimentary rocks that can be rich sources of petroleum and natural gas. Oil shale is defined as any shale rock that contains more than 5% by volume of kerogen [7], and shale gas refers to natural gas that is trapped within shale formations. Shale deposits are found across the globe, and the minimum estimated global shale oil reserves are now estimated to be over three trillion bbl. [8]. The U.S. contains the largest reserves of shale deposits, and the distribution of U.S. shale deposits is shown in Figure 1.1. Over the past decade, the hydraulic fracturing and horizontal drilling has allowed development of large volumes of shale gas that were previously uneconomical to produce. The production of natural gas from shale formations has rejuvenated the natural gas industry in the United States [10]. In reports published by the U.S. EIA [1], in 2000, shale gas accounted for only 2% of United States gas production; by 2010 shale gas increased to 23% of production. The outlook for shale gas is expected to increase further, and this trend is seen in Figure 1.2. With more than 70 major shale gas basins identified
16 16 in countries outside the United States, shale resources are now estimated to account for more than 40% of the world s recoverable gas reserves [1]. Figure 1.1: U.S. Shale Deposits [9] Figure 1.2: Shale Gas Leads Growth in Total Gas Production Through 2040 [11]
17 Waste Water from Fracking The technology that has allowed the unprecedented shale gas exploration and development is hydraulic fracturing (fracking). Fracking is the process that expands fractures in the shale via injection of high pressure fracking fluid [12]. Fracturing fluid is a combination of water, proppants such as sand, and other chemicals. The exact makeup of the fracking fluid varies, but, in general, each hydraulic fracturing of a well requires 3 7 million gallons of fluids [5]. Table 1.1 shows a comparison of water required for hydraulic fracking in various shale basins across the U.S. Table 1.1: Estimated Water Consumption Numbers for Horizontal Drilling and Hydraulic Fracturing Process in Various Shale Plays [9], [13], [14] Shale Type Drilling Process Water Consumption Fracturing Process Water Consumption (gal/well) Eagle Ford 125,000 6,000,000 Marcellus 85,000 5,500,000 Haynesville 600,000 5,000,000 Fayetteville 65,000 4,900,000 Barnett 250,000 3,800,000 Niobrara 300,000 3,000,000 The fracking fluid consists of 90% 95% water, 5% 10% sand proppant, and 0.1% 1% of chemical additives, such as friction reducers, biocides, acids, and gelling agents [5]. A large volume of waste water is generated from each well after hydraulic fracturing is completed. Flowback water, as mentioned previously, is the portion of the injected hydraulic fracturing and native formation fluids that return in the days
18 18 after a well has been fractured and typically represents 10% 20% of the injected hydraulic fracturing volume. Brine formation water or produced water, as mentioned previously, is generally considered to be the fluids generated from and around the gasbearing formation after the period of flowback fluid production and when the well is in the production phase. Produced water, may reach levels of several barrels per day. Industry s preferred practice is to reuse F/P water as fracturing fluid in subsequent well development due to the cost benefits of reuse [15]. However, the physical and chemical properties of F/P water vary considerably depending on the geographic location of the field, the geologic formation from where the water was produced, and the type of hydrocarbon product being produced [4]. Constituents contained within F/P water include suspended solids, bacteria, organics, total dissolved solids (TDS), and naturally occurring radioactive material (NORM) [16]. Due to the high level of chemical species, most waste water cannot be directly reused. The Marcellus Shale is currently one of the largest shale formations being developed in the U.S., and only a small fraction of F/P water generated by Marcellus Shale wells is directly reused. Its high TDS concentrations makes filtration/direct reuse an infeasible option for much of the waste water generated [15]. 1.4 Fracking Water Treatment Treatment of the large volume of waste water generated by hydraulic fracturing is an enormous challenge. Current treatment methods also have technological limitations. In some shales, such as the Marcellus, both high levels of TDS and total suspended solids (TSS) in the water are challenges to existing technologies. Table 1.2 lists advantages and disadvantages of existing technologies [5].
19 19 Table 1.2: Commercial F/P Water Management Technology Characteristics [5] Technology/ Funding Organization Membrane Separation Reverse Osmosis Electrodialysis Nanofiltration Micro/Ultrafiltration Thermal Technologies Chemical Precipitation Advantages Mature technology used for seawater/brackish desalination Small process footprint and mobility Used in industrial waste water treatment Capable of treating TDS, Si, and dissolved organics Mature technologies Capable of treating water containing 100, ,000 ppm TDS Processes capable to treating TSS, TDS, heavy metals, and volatile/semivolatile organics High quality water product Good for zero discharge requirements Mature technology used in commercial water treatment/disposal facilities Capable of treating water with high TDS content (>100,000 ppm) Disadvantages Applicable for water containing <25,000-45,000 ppm TDS Extensive fouling caused by TDS short membrane life Unable to remove NORM Concentrate requires treatment or disposal High treatment costs when accounting for necessary balance of plant and concentrate treatment/disposal Large physical plant size Not suitable for mobile applications Low heat integration/high energy costs Low water recovery Requires more energy Unable to remove NORM Concentrate requires further treatment/disposal High treatment costs >$2.60/bbl Can produce hazardous salts requiring expensive disposal Minimal NORM treatment capability Large chemicals consumption Increases TDS content
20 Treatment Methods/Technologies/Cost Off-site disposal is one method currently used for F/P treatment. Argonne National Laboratory [6] listed treatment costs of many off-site disposal methods across the United States. Disposal costs can range from $0.30/bbl to $105.00/bbl [6]. Evaporation of produced water is most commonly used in the western states of Wyoming, Colorado, Utah, and New Mexico. The disposal costs ranges $0.40/bbl and $3.95/bbl, but can reach up to $84.00/bbl. Although widely used in the western states, evaporation is not practical in Eastern states and shales due to higher relative humidity [6]. Burial in landfills is available for produced water across the U.S., however the general requirement of solidification can increase the costs [6]. Volume-based costs range between $3.00/bbl to $22.00/bbl. Weight-based costs vary significantly by state, but generally goes from $15.00/ton to $80.00/ton, with Mississippi and Louisiana reporting costs of $128.00/ton and $250.00/ton, respectively [6]. Cavern disposal is a competitive option for produced water in Texas for a cost between $0.30/bbl and $10.00/bbl [6]. Discharge of produced water under an NPDES permit occurs in Pennsylvania and in Wyoming [6], with the costs ranging between $2.25/bbl and $2.75/bbl in Pennsylvania, and between $2.50/bbl and $3.50/bbl in Wyoming [6]. Recycling, or reuse of water after treatment, of produced water is not widely reported with one company in California charging $5.00/bbl, and another company in
21 21 Oklahoma charging a cost of $25.00/load [6]. Thermal treatment of produced water costs range from $10.50/bbl to $105.00/bbl [6]. Since all of the methods listed above are off-site disposal methods, transportation costs needs to be added to the total cost. The cost for transportation can add $ /bbl to the final price of treatment. Overall, current costs for disposal methods range from $ /bbl [6]. 1.6 IPSC Process Ohio University is developing an Integrated Precipitative Supercritical Process (IPSC) to convert F/P water into a clean water product suitable for reuse as a hydraulic fracturing fluid or direct discharge. This process integrates solids filtering, ultra-violet light (UV) treatment, and chemical precipitation technologies, and an advanced supercritical water reactor [15]. It uses commercially available solids filtering, UV treatment, and precipitation technologies with an advanced supercritical reactor (SCR) unit design to cost-effectively convert F/P water into a clean water product which may be reused as a fracking fluid or safely discharged to the local environment [15]. The IPSC process contains five main subprocesses, and is shown in Figure 1.3. Figure 1.3: IPSC process
22 22 The sand filter and other filters in the first region are mainly used to remove TSS elements. F/P water discharged from an unconventional shale gas well or intermittent storage enters a series of filtration devices, including a hydrocyclone and sand filter to remove suspended solids [15]. The solids-free F/P water is then treated by UV light in an enclosed reactor in the second region. The UV light inactivates bacteria DNA, preventing their ability to replicate and foul downstream equipment or sour future reservoirs in the case of water reuse. The third region is the precipitation sub-process which includes three precipitation steps. Sulfation, softening, and hydrolysis precipitation steps are used to remove dissolved hazardous and scaling constituents [15]. The NORM removal region is used to remove mainly Ra-226 and Ra-228 and other harmful NORM constituents. The final supercritical region is the most heat/work intensive unit. The water after NORM removal is pressurized past Bar via a high pressure pump, and is heated to 350 C by the heat exchanger. The water then enters the supercritical reactor and is heated to 380 C to achieve maximum salt precipitation and removal. Also, catalysts in the reactor decompose hydrocarbons in the water. The treated water exits the reactor and travel through the heat exchanger to pre-heat water prior to the reactor. Preliminary bench test and techno-economic evaluations have indicated IPSC s ability to treat produced water at a significantly lower cost ($1.41/bbl) compared to existing treatment or disposal methodologies ($ /bbl) [15]. The preliminary techno-economic analysis needs to be revised and updated with more detailed information and analysis to provide a more accurate estimation of the cost per barrel for the IPSC process. The economic information for the IPSC process will be critical as a
23 23 comparison against other treatment methods as cost is the main obstacle for shale energy development. The key objectives of this study will be to develop an Aspen -based model, techno - economic analysis of the new proposed IPSC process, and to perform sensitivity analyses on the major process variables inputs utilizing the model template. 1.7 Brief Description of Objectives Objective 1 The first objective of this thesis is to develop an Aspen -based model and techno - economic analysis of the IPSC process. Aspects of the Aspen model will be validated. The process model will be built through Aspen Plus to calculate outputs of the IPSC process with inputs and operating conditions. The techno-economic analysis will determine the cost per barrel of the process for a fully developed commercial sized IPSC process. The results of the Aspen model will be verified and validated by comparing the model results and literature data. The Aspen model and techno-economic analysis of the process will determine a cost per barrel for the proposed IPSC process. The final cost per barrel will help compare the economics of the new IPSC process against the economics of current waste disposal methods Objective 2 The second objective is to perform sensitivity analyses on the major process variables inputs utilizing the model template. The input variables listed in Table 1.3 are assumed to be potentially significant to the final cost per barrel. Sensitivity analysis will be performed for each variable in Table 1.3.
24 24 Table 1.3: Input Variables for Sensitivity Analysis Inputs Variables Flow rate Water chemistry Inlet temperature Ion (,, ) removal efficiency due to Precipitation Cost of precipitate agents NORM concentration Material of construction Solids disposal cost Equipment lifetime Price of utilities
25 25 CHAPTER 2 LITERATURE REVIEW 2.1 Introduction This chapter provides a review of work conducted by previous investigators that is applicable to the objectives and recommendations of this thesis. This chapter also lists technical background information and methods critical for building the model and sensitive analysis. 2.2 Aspen Modeling According to Aspen s own description, Aspen Plus is the Chemical industry s leading process simulation software. It is a comprehensive chemical process modeling system, used by the world s leading chemical and specialty chemical organizations, and related to industries to design and improve their process plants [17]. It is common practice in industry to use Aspen Plus software to model processes for techno-economic evaluations of potential processes scale up Waste Water Composition As mentioned before, constituents contained within waste water include suspended solids, bacteria, organics, total dissolved solids (TDS), and NORM. Table 2.1 presents constituents and a range of their concentrations in waste water from the Marcellus Shale Particle Sizes The particle size of the solid particles is a very important parameter for determining the solids-liquid separation in the sand filter, precipitation, and supercritical process.
26 26 Table 2.1: Constituents and Concentration in Fracking Water [16, 18-20] Constituent Total Suspended Solids Bacteria Aerobic Sulphate Reducing Total Dissolved Solids Ba Ca Fe Mg Mn Sr Na NORM Hydrocarbons Concentration 9, ,000 mg/l cfu/ml cfu/ml 30, ,000 mg/l 2,300-6,500 mg/l 5,100-18,000 mg/l mg/l 1,300-4,400 mg/l 2-5 mg/l 1,000-6,900 mg/l 23,000-57,300 mg/l 0-10,000 pci/l mg/l The majority of total suspended solids are assumed to be fracking sand and sand particles. Based on industry technical sheets [21-22], the average sand particle is around 50 microns. The particle size for precipitated solids is around 20 microns [23]. Particle size for precipitated solids after the supercritical process is around 50 microns [24] Settling Velocity To calculate the settling velocity, and the area of a settling tank required for the settling velocity, the following equations from (2.1) to (2.6) are used. is the buoyance force, is the force due to drag, is the force due to gravity, is the density of the water, is the density of the particle, is the diameter of the particle, is the cross sectional area of the particle, is the coefficient of drag, is the Reynolds number, is the flowrate, and is the settling velocity in question. Eq (2.5) is used to determine the,, for Reynolds number from 1 to 100. [25]
27 27 (2.1) (2.2) (2.3) (2.4) (2.5) (2.6) Heat Exchanger Heat Transfer The heat exchanger of choice in this model is the shell and tube heat exchanger due their wide availability in the chemical industry and their ability to handle high pressures and high flowrates. The required heat transfer area is given by Equation (2.7) [26]. To calculate area,, the heat duty per unit time, the heat transfer coefficient, and log mean temperature difference are needed. The heat duty per unit time is calculated in equation (2.8) [26]. The heat transfer coefficient is highly sensitive to wall thickness, fouling factor, thermal conductivity, and flow conditions. Fouling is likely a very significantly concern for the IPSC process as the large ion concentrations are very likely to deposit on the heat exchanger walls. Fouling reduces the heat transfer efficiency as deposition reduces the pipe diameter and flowrate, and the deposition also reduces the conduction transfer. With fouling, a larger area is needed to achieve the same heat transfer. The area needed for heat transfer due to fouling,, is calculated in Equation (2.9), where is the overall heat transfer coefficient for a clean heat exchanger, is the fouling factor, and is the area needed for heat transfer for clean system [26].
28 28 (2.7) (2.8) (2.9) Based on Kakac and Liu s work [26], the heat transfer coefficient,, for shell and tube heat exchangers can be determined in equation (2.10), where is convective heat transfer coefficient of the shell side and where is convective heat transfer coefficient of the tube side, and is the thermal conductivity of the metal. (2.10) the thermal conductivity of the metal are found in tabled values [26], and depend only on the metal itself. However,, the convective heat transfer coefficient, is dependent on flow conditions (pressure, temperature, flow rate) of the heat transfer medium as well. There are two methods of finding the value of. The first method is to use tabulated values such as those listed in Table 2.2, 2.3, 2.4, where they list values ranges for applicable medium used in this study. Table 2.2: Convective Heat Transfer Coefficient for Sensible Heat Transfer [26] Medium Convective Heat Transfer Coefficient ) Sensible Heat Transfer Water 5,000-7,500 Light Organics 1,500-2,000 Med Organics 750-1,500 Heavy Organics (heating) Heavy Organics (cooling) Very Heavy Organics (heating) Very Heavy Organics (cooling)
29 29 Table 2.3: Convective Heat Transfer Coefficient for Condensing Heat Transfer [26] Medium Convective Heat Transfer Coefficient ) Condensing Heat Transfer Water 8,000-12,000 Light Organics 2,000-5,000 Med Organics 1,500-4,000 Heavy Organics 6,00-2,000 Table 2.4: Convective Heat Transfer Coefficient for Vaporizing Heat Transfer [26] Medium Convective Heat Transfer Coefficient ) Vaporizing Heat Transfer Water 4,000-15,000 Light Organics 750-3,000 Med Organics 600-2,500 Heavy Organics 400-1,500 The second method is to calculate the value based on the flow conditions, and this is described in equations (2.11) to (2.14) [26]. is the Nusselt number, and Equations (2.11) and (2.12) describes finding of the shell side, where is the Reynolds number, and is the Prandtl Number, and is the mass flowrate of the fluid medium, is the cross-section area of the shell, is the tube outside diameter, is the dynamic viscosity of the fluid. and are physical properties of the fluid, and can be determined from tabulated values [26]. Equations (2.13) and (2.14) describes finding of the tube side, where is the Reynolds number, and is the Prandtl Number, and is the mass flowrate of the fluid medium, is the cross-section area of the tube, is the tube inside diameter, is the dynamic viscosity of the fluid. After the Nusselt number is
30 30 determined,, the convective heat transfer coefficient can be found using equation (2.15) where is the length of the tube/shell, and is the thermal conductivity of the fluid. (2.11) * (2.12) (2.13) * (2.14) (2.15) Physical Property Modeling in the Supercritical Region The solubility of,, in the supercritical region is modeled with the equations (2.16), (2.17), (2.18). is modeled by equation (2.16) where is the solubility, is the density of the supercritical water, and is the temperature of the water [27]. (2.16) is modeled by equation (2.17) where is the equilibrium constant, is the density of the supercritical water, and is the temperature of the water. for is 0, b for is and is 3.44 [27]. ( ) (2.17) is modeled by equation (2.18) where is the equilibrium constant, is the density of the supercritical water, and is the temperature of the water. for is -441, b for is and is 2.52 [27].
31 31 ( ) (2.18) Heat Transfer for a Cylinder Shell with Insulation The heat transfer around the reactor can be modeled similar to an insulated cylinder. The main mode of heat transfer and heat loss is conduction. The amount of heat loss per unit time is described in equation (2.19), where is the heat loss per unit time,, the change in temperature,, the area of heat transfer, and, the overall heat transfer resistance. The value can be calculated in equation (2.20), where t1, k1 is the thickness and thermal conductivity of material 1 [28]. (2.19) ( ) (2.20) 2.3 Cost-Analysis Modeling The main cost-analysis methodology is based off the work established by Turton et al [29] for the estimation of new chemical process economics Capital Cost Estimation Vendor quotes are generally considered the most accurate method of determining the individual cost of process equipment. If available, vendor quotes are used to determine the cost of the process equation in this model. If vendor quotes are unavailable, the module costing technique from Turton et al [29] is used to estimate the cost of the equation. For this effort, three types of equipment are modeled using Turton s technique: pumps that are described with Equation 2.21, process vessel described by Equation 2.22,
32 32 and the packing towers described by Equation , where the cost module for each component is then calculated with Equation (2.21) (2.22) (2.23) (2.24) is the cost for a similar equipment in base condition (ambient operating pressure, temperature, carbon steel). The factor accounts for the change in price for different operating pressures for similar equipment, and the value of depends on the value of the operating pressure. The factor accounts for the change in price for different material of construction for similar equipment, and the value of depends on the material of construction. The final equation for, the module cost of the equipment, includes all of direct cost and indirect cost of purchase and installation of that equipment. This model accounts for inflation using the Chemical Engineering Plant Cost Index I, (CEPCI index) where and is for year k and year n respectively. is adjusted using Equation (2.25) (2.25) The total modular cost,, is shown in Equitation (2.26). C TM is the sum of individual equipment costs adjusted for a cost factor (in this case, 1.18) to describe the extra cost associated with construction/building/permit and all other auxiliary items associated with a new process.
33 33 (2.26) In the case where vendor quotes are available for a specific price for the specific capacity of that equipment. To estimate the cost of a similar equipment with different capacity, the industry standard 6-10 th rule will be used [29]. Equation (2.27) is the 6-10 th rule, and is used for estimating equipment cost from vendor quotations. is the cost of the equipment with capacity, and is the cost of the similar equipment with capacity (2.27) Cost of Operation Estimation The operation cost is dependent on five main factors described in this section: Capital Cost (FCI), Cost of operating labor (, Cost of utilities (, Cost of solids waste disposal ( ), and Cost of raw materials (. There are also operation and/or manufacturing cost associated with operations of the process, and they are listed in Table 2.5. These costs are estimated by multiplying a factor to one of the five main operation costs (FCI,,,, ), and the typical ranges of the multiplying factors are listed in Table 2.5. The total cost of all operations,, is the summation of all the costs listed in Table 2.5.
34 34 Table 2.5: Cost Factors [29] Cost Items Typical Range of Multiplying Factors 1. Direct Manufacturing Cost a. Raw Materials b. Solids Waste Disposal c. Utilities d. Operating Labor e. Direct supervisory and clerical labor f. Maintenance and repairs g. Operating supplies h. Laboratory charges i. Patents and royalties (0-0.06) 2. Fixed Manufacturing Cost a. Depreciation 0.1 b. Local taxes and insurance ( ) c. Plant overhead costs ( )( + ) 3. General Manufacturing Expenses a. Administration costs (0.15)( + ) b. Distribution and selling costs ( ) c. Research and development 0.05
35 35 CHAPTER 3 MODEL SIMULATION 3.1 Introduction This chapter discusses meeting the first objective, which was building the Aspen model, building cost analysis model, and validating the models. 3.2 Aspen Model Figure 3.1 illustrates the process flow of the Aspen Model for the IPSC process, and includes the Sand Filter Area, Precipitation Area, and Supercritical Area. The model was constructed in Aspen Plus software utilizing process design constraints, physical (thermodynamic, fluid, chemical) properties, and reaction kinetics to serve as relational equations between inputs and outputs. The process design constraints, initial operating parameters, and other inputs such as flowrate, water chemistry, and precipitant agents used in the model were based on the initial IPSC design proposal and literature review. Figure 3.1: Aspen Model PDF
36 Aspen Modeling Physical Property Setup Aspen models a process, and determines desired process information based on user defined inputs. Aspen contains physical properties databases with thermodynamic properties for common chemicals. Two main groups of property methods for describing properties of multiphase and multicomponent process streams are the equation of state methods and activity coefficient methods. Different property determination methods are more accurate for different applications and conditions. Because the majority of the constituents in the model s IPSC process water were dissolved ions, the modeling property method of ELECNRTL was employed. The ELECNRTL method is an activity coefficient method that uses the NRTL (Non Random Two Liquid) model as the base method with adjustments for electrolytes properties [17]. Aspen Technologies recommended the ELECNRTL method for electrolyte systems below medium pressure (< 100 bar). The IPSC process prior to the high pressure pump, including the Sand Filter, UV Unit, Precipitation Unit, and the NORM Unit, was at ambient temperature and standard pressure conditions, and the ELECNRTL method served as the property method basis for this region. However, the ELECNRTL method is less accurate above medium pressure conditions (>100 bar). The supercritical region of the IPSC process was at a pressure of around 221 Bar and temperatures above 375 C. Equations of state methods are more accurate for the high pressures and temperatures of the supercritical region s physical property system. Equations of states methods are often used to model many power
37 37 generation plants, which often uses supercritical water. The PG (Peng-Robinson), R-K (Redlich-Kwong), and experimentally compiled Steam Tables were the three main methods suggested by Aspen Technologies for use in Power System Applications [17]. Although all the equations of state methods were viable in the supercritical region, the PG and R-K modeling equations were the least accurate near the critical point. Since the reactor operated right around the super-critical point, empirical Steam Tables were used Precipitation Chemistry in Aspen As stated in section 2.1.3, F/P water contains constituents at varying concentrations, and Table 2.1 describes the range of ion concentrations for F/P water. The Aspen model simulated the ions phase, concentration, and reactions, and all chemical components used in the Aspen model are listed below in Table 3.1. The concentrations of each component was based on the values from Table 2.1, and assumed the mid value (50 percentile) of each ion range. In the Aspen model, the concentration of was based on the concentration of positive ions, and was used to balance the ion charge in the Aspen model. The precipitation process involves electrolyte chemistry reactions, shown in Table 3.2 contains the reaction equations that were modeled in the Aspen Model. After Table 3.2 equations were inputted into Aspen, Aspen calculated the reactions kinetics and product concentrations using its physical properties database. Aspen then outputted the results of the reaction equations.
38 38 Table 3.1: Chemical Components in the Aspen Model Chemicals Components mg/l 100 GPM 4,400 3,950 11,550 2, ,182 40, ,500 Table 3.2: Reaction Equations for the Aspen Model Reaction Equations
39 Sand Filter The main goal of the sand filter component is to filter total suspended solids (TSS), the majority of which are sand particles. The proposed liquid-solids separation process unit is the hydrocyclone. Figure 3.2 shows the Sand Filter Area, where the inlet F/P water was modeled by the stream F/P, and enters into the hydrocyclone unit SANDFILT. The hydrocyclone unit separated the sand into the SAND stream and the rest of the process water into stream AFTSAND. All TSS were assumed to be inert particles with diameters based on industry fracking sand size, 50 microns [21-22]. By supplying the desired removal efficiency and particle diameter, the Aspen hydrocyclone model calculated and optimized the pressure drop, hydrocyclone diameter, and the number of hydrocyclones needed. Figure 3.2: Sandfilter Unit
40 UV Unit The main goal of the UV unit is to inactivate bacteria via ultra violet light. From a process prospective, the heater model in Aspen best emulated the process characteristics of the UV unit. Figure 3.3 is the UV unit. The AFTSAND process stream entered into the UV unit, and exited out of UV heating unit in the process stream AFTUV. Figure 3.3: UV Unit Precipitation Process The precipitation process is the step that removes a significant proportion of the TDS. There are three different precipitation processes: sulfation, softening, and hydrolysis. All three sub-processes were identical in the model with the exception of the precipitate agent used. For each sub-process in practice, precipitate agent was added to
41 41 the process stream in the mixing tank, and the precipitation reaction occurred in the tank. The precipitated solids were then removed via either gravimetric settling or hydrocyclone separation. Two modeling components were required for each sub-area include the mixer and a general separation unit. The mixing tank was modeled by the mixer unit in Aspen. The precipitation reactions occurred in the mixing tank. Solids removal was modeled by a general separation unit, where Aspen calculated the solids based on a user inputted solids-liquid separation efficiency. Based on both gravimetric settling calculations (see Appendix A), and technical data from hydrocyclone operation (see Appendix B), 99% efficiency was used for the precipitated solids removal from the process stream. Figure 3.4 shows a schematic of the sulfation part of the precipitation process, where the main process stream AFTUV entered into the mixing unit SULFTANK along with the stream H2SO4 which contained the precipitation agent sulfuric acid. After mixing in SULFTANK, the two process streams were combined into the stream INSULF. The process stream INSULF then entered into the general separation unit SULFSEP, which modeled the solids liquid separation, and separated the solids into the stream SO4SOLID, and the main process stream exited as AFTSULF. Figures 3.5 and 3.6 show schematics of the softening and hydrolysis portions of the precipitation process. They followed the same process flow as the sulfation process except the precipitation agent is replaced with sodium carbonate and sodium hydroxide for the softening and the hydrolysis process respectively.
42 42 Figure 3.4: Sulfation SULFSEP SOFTTANK SOFTSEP AFTSULF INSOFT AFTSOFT NA2CO3 CO3SOLID Figure 3.5: Softening SOFTSEP HYDRTANK HYDROSEP AFTSOFT INHYDRO AFTHYDRO NAOH OHSOLID Figure 3.6: Hydrolysis
43 Ion Removal Efficiency A Design Spec Block implemented with Fortran code was used to calculate the amount of precipitate agent needed based on the removal percentage specified by user input. User entered the desired efficiency into Excel and the efficiency was transferred via the Fortran code into Aspen (see Appendix C) ph The use of precipitation agents can drastically change the ph level of the stream. The ph is crucial for determining material of construction of tanks as high/low levels of ph induces aggressive corrosion. Aspen was able to directly calculate ph based on the ion concentration in the process stream NORM Unit The main goal of the NORM unit is to remove radiative materials, mainly in the form of Ra-226 and Ra-228 from the stream. The packing column for NORM removal only changed the process conditions by requiring a pressure drop. Since this did not significantly change the process conditions, the NORM unit was not modeled in Aspen, and only modeled in the Cost-Analysis (Section ) Supercritical Area Properties As described before in Section 3.2.1, the supercritical region is under extremely high pressure and high temperature. Therefore the ELECNRTL property method was no longer valid. The Equations of State (EOS) methods broke down around the supercritical region [17]. The most accurate method was to use empirical steam tables for modeling
44 44 the supercritical region. Because the Steam Table method was used for the supercritical region, only water can be modeled in the supercritical region. A Transfer Function Block was used to assign the properties of the outlet of the ambient side to the inlet of the supercritical region, accounting for chemicals in the water Pump The main goal of the pump is to increase the pressure of the system. It was modeled with the pump model in Aspen, which calculated the power input required based on the inlet pressure and desired outlet pressure of 221 bar Heat Exchanger The main goal of the heat exchanger unit is to recycle part of the heat coming off the supercritical reactor. The inlet and outlet temperatures of the cold side, and inlet temperature of the hot side were set by design criteria, and the Aspen model calculated the other conditions based on an energy balance. The inlet cold temperature was the temperature of the inlet process stream. The outlet cold temperature was also the temperature of the inlet stream going into the supercritical reactor, and was limited at 350 C to prevent early deposition. The inlet hot temperature was set by the temperature coming off the reactor, which is 380 C Supercritical Reactor The main goal of the supercritical reactor is to heat the fluid past supercritical temperature to 380 C to change the polarity of the water so that most of the dissolved solids precipitates out of solution. It is assumed that the solids separate from the fluid via gravimetric settling similar to the precipitation unit. This reactor was also modeled by
45 45 two units in Aspen with a heater to emulate the heating requirements and a general separation unit for the solids separation. The breaking down of hydrocarbons via catalyst was not simulated in the Aspen model. The heater model calculated the power input requirement, and general separation unit efficiency was calculated outside of the Aspen model Ions Thermochemistry Change Due to Change in Temperature As the discussed in the previous section, ions were not modeled in the supercritical region. To compensate for the ion thermochemistry change due to change in temperature, a calculation block was introduced as seen in Figure 3.7. Figure 3.7: Calculation Block for Ion Compensation
46 46 HSC software is an industry database that contains physical properties for chemicals and chemical reactions at different temperature and pressures. HSC was used to retrieve the enthalpy change from 25 C to 350 C and 350 C to 380 C for,,,,,,. FORTRAN code in the calculation block seen in Figure 3.7 was used to add the ion thermochemistry change as described in Equation (3.1). (See Appendix D) (3.1) Ion Removal in the Supercritical Area Since the supercritical area in the Aspen model only contained water, it could not use reaction equations in the supercritical region. The amount of precipitation was calculated by using the solubility of,, as described in Section The solubility equations from Section were built into the FORTRAN code in the calculation block seen in Figure 3.7, and was used to calculate the amount of solids produced in the supercritical reactor (See Appendix D). The change in enthalpy due to the precipitation reaction at 380 C was determined in HSC software for,,. The change in enthalpy per mole multiplied by the moles of precipitated solids is the total enthalpy change due to precipitation. The amount of precipitated solids was based on the calculation described in Section Figure 3.8 shows a schematic of the supercritical area in Aspen. The temperature, pressure, and mass flowrate of the water in stream AFTHYDRO were transferred to stream Inlet via the Transfer Function Block. The process stream inlet was first pressurized by the pump. As described in Section , since the process stream
47 47 only models water, the user-defined unit IONHEAT compensated for the extra heat for the ions change in enthalpy from ambient to 350 C, while IONHEAT2 compensated for the extra heat for the ions change in enthalpy from 350 C to 380 C. As described in Section , since the process stream only modeled water, the user-defined unit IONREA compensated for the ions change in enthalpy due to the precipitation reactions. The heat exchanger was modeled by the unit HX, and the supercritical reactor was modeled by the heater unit SCR. Figure 3.8: Supercritical Area 3.3 Cost Model The cost model was built in Microsoft Excel using outputs from the Aspen model as inputs for the cost model. The cost-analysis methodology was based on the cost analysis methods from Turton [29] as described in section 2.3. Aspen results were directly linked so that any change in the Aspen model was automatically changed in the Excel model. As an example, Table 3.3 shows the cost analysis for the precipitation agent costs from the Excel model. The first column titled amount is the Aspen output that is directly entered into the Excel Model. In the example in Table 3.3, the amount of precipitation agent needed was calculated by the Aspen model. The second column
48 48 ($/ton) is the chemical prices for the precipitation agents, and Excel calculated the cost of the raw materials per year based on the Aspen results in the orange block and chemical prices in the purple block. A similar technique was used for the capital equipment cost estimation and other operating cost. Table 3.3: Cost Analysis for the Precipitation Agent Costs Amount (kmol/hr) $/ton Cost ($/yr) Sulfuric Acid $25.00 $41,143 Sodium Carbonate $ $1,029,438 Sodium Hydroxide $ $1,289,386 Total Cost $2,359, Total Capital Cost Sand Filter The method for cost estimation of the sand filter was via vendor quotation for a hydrocyclone sized to meet the known conditions. For the assumed particle of 50 microns as discussed in Section 3.2., and a separation efficiency of 90%, one vendor (Well Minerals) quoted a cost for hydrocyclone of $2,000 (see Appendix B). The required pressure drop is 50 psi, requiring a pump to compensate for the pressure drop. The pump cost was estimated using the equipment module technique, and required the pump power for cost estimation. The power required for the pump was estimated in Equation (3.2) based on the pressure drop and flowrate of the process stream. The bare module cost of the pump,, was the both direct and indirect cost of the pump, and it was estimated with the equations and factor listed in Table 3.4.
49 49 (3.2) Table 3.4: Sandfilter Pump Cost [29] Equipment Pump (centrifugal) Reference Equation (2.21) = 2.5 for Stainless Steel = 1 (Pressure < 10 bar) Reference Equation (2.24) Estimation of the cost of the hydrocyclone for different flowrates was based on the $2,000 vendor quotation and the 6-10 th rule. The pump cost was determined by Turton s method [29], and the bare module of the pump was adjusted for inflation via Equation (3.3). The CEPCI for 2001,, was 397, and the CEPCI for 2013,, was [30]. The hydrocyclone quote was attained in The total cost for the sand filtering process was the sum of the pump price and $2000 hydrocyclone cost. (3.3) UV Unit The cost of the UV unit was directly acquired from a vendor quote (see Appendix E). The UV quote was for a UV unit treating water at 100 GPM and attained in Precipitation Process Precipitation Process Settling Tank Method Initially, a settling tank was assumed to be feasible for the solids separation after the precipitation process. As described in Section 2.1.3, the settling velocity equations were modeled in Excel to calculate the required base area for the settling tank. (see Appendix A for Excel VBA code for the calculating the settling velocity) Based on the
50 50 work of [24], the particle diameter was assumed to be around 20 microns. From the required base area, and a height assumed to be three feet, the volume of settling tank was calculated. With the volume as an input, the estimated cost was modeled using Table 3.5. Equipment Table 3.5: Settling Tank Cost [29] Process Vessel Reference Equation (2.22) For Carbon Steel = 1 (Pressure < 10 bar) Reference Equation (2.24) However, calculations indicated that a settling tank using gravimetric settling would require a base area with radius of 100 inches. A settling tank with such a large area would not be feasible for a mobile unit. A second method, described next, estimated the cost if a hydrocyclone were to be used for solids separation for the precipitation solids separation Precipitation Process Hydrocyclone For the calculation for the cost of a tank for mixing, the volume of the tank was needed, and this depended on the residence time of the reaction and the flowrate. The residence time was assumed to be five minutes for complete reaction, the required mixing tank volume was determined with Equation (3.4). The assumed particle diameter was around 20 microns. With the same required efficiency, based on vendor consultations, the same hydrocyclone design from the Sandfilter area worked for this application (see Appendix B).
51 51 The estimated pressure drop is 50 psi, requiring a pump. The pump cost was estimated using the equipment module technique, and required the pump power for cost estimation. The power required for the pump was estimated in Equation (3.5) [31] based on the pressure drop and flowrate of the stream. The bare module cost of the pump,, was the direct and indirect cost of the pump, and it was estimated with the equations and factor listed in Table 3.6. (3.4) (3.5) Table 3.6: Precipitation Pump Cost [29] Equipment Pump (centrifugal) Reference Equation (2.21) = 2.5 for Stainless Steel = 1 (Pressure < 10 bar) Reference Equation (2.24) Estimations of the cost of the hydrocyclone and mixing tank for different flowrates are based off the previously described vendor quotations and the 6-10 th rule. The mixing tank quotation was based on the pricing by plasticmart.com for plastic water tanks (See Appendix F). The pump bare module was adjusted for inflation via Equation (3.6). The CEPCI for 2001,, is 397, and the CEPCI for 2013,, is The hydrocyclone quote and mixing tank cost did not need to be adjusted as the quotations were attained in (3.6)
52 52 The total capital cost for just the sulfation process was the sum of the pump price, mixing tank price, and $2000 hydrocyclone cost, and was the same cost as the hydrolysis and softening process. The precipitation agent holding tank for cost, metering equipment cost, and mixing cost were include in the total capital cost through contingency factor in equation The same hydrocyclone and tank cost estimation method was used for the sulfation, hydrolysis, and softening process. The total cost of the precipitation process was the sum of the sulfation, hydrolysis, and softening processes NORM A packed column with sorbent material is used for the NORM removal. The sorbent was assumed to be zeolite material with costs estimated as part of the cost of raw material as described in later sections. The packing column vessel was modeled as a process vessel as described in Table 3.7. The volume required for the packing column was the sum of the packing material needed for removal of NORM for the anticipated volume of water to be processed in a day and with a conservative residence time of five minutes. As there is a pressure drop across the packing material, a conservative estimate of 50 psi was assumed, and a suitable pump was assumed to be needed to compensate for the pressure drop. The cost of pump was modeled in Table 3.7 as well along with Equation (3.7) to adjust for inflation. (3.7) The total cost of the NORM area was the sum of the pump and process vessel.
53 Supercritical Area The total cost of the equipment in the supercritical area was the sum of the high pressure pump cost, heat exchanger cost, and supercritical reactor cost. Table 3.7: NORM Column Cost [29] Equipment Packing Tower Pump (centrifugal) Reference Equation (2.23) Reference Equation (2.21) For Stainless Steel = 2.5 for Stainless Steel = 1 (Pressure < 10 bar) = 1 (Pressure < 10 bar) Reference Equation (2.24) Reference Equation (2.24) Pump Vendor quotes were used to predict the cost of the high pressure pump. A single 100 GPM 221 Bar pump was not technically feasible for either positive displacement pumps or centrifugal pumps due the combination of both high pressure and high flowrate. After reviewing options, ten 10-GPM pumps connected in parallel was found to have achieved the same results. Based on vendor quotes, one 10 GPM pump that can raise the fluid pressure to over 221 bar would cost $22,000 (See Appendix G). Thus ten pumps would cost $220,000. For the range of 10 GPM to 200 GPM, the linear equation in (3.8) was used to predict the cost of the pump based on flowrate. (3.8) Heat Exchanger The high pressure of the heat exchanger is out of range for the bare module cost method as the Turton did not develop cost approximations for heat exchangers above 150
54 54 Bar. However, Woods et al [32] described a method for estimating the cost of high pressure heat exchangers based on the required heat exchange area. The Aspen model calculated the required heat exchange area, and Equation (2.9) was used to calculate the final heat exchange area adjusted for fouling caused by the accumulation of precipitated scale. A fouling factor of = was used for very brackish water [29]. Equations (3.9), (3.10), and (3.11) from Woods et al [32] were used. For this model, = 3 for stainless steel tubes and shell, = 2.56 for 3,200 psi, and = 2.3 for labor and installation costs. Since the paper was published in 1976, a CEPCI of for 1976 was used to adjust for inflation. ) (3.10) (3.11) One of the key unknowns for determining the heat exchanger area was the overall heat transfer coefficient, U. Many methods exist for determining the values of the heat exchangers discussed in Section The value was determined from a combination of the resistance due to conduction and convection as seen in the Equation (3.12). Table 3.8 lists the different methods to calculate and values, and their final cost per barrel. This table shows that even the different values do not significantly impact the final cost per barrel. Since the final cost per barrel was not influenced by changes in values, the conservative tabulated values from Table 2.4, 2.5, 2.6 were used for the model. Table 3.8 also compares using one heat exchanger vs two heat exchangers in terms of cost saving. For using two exchangers, one would be a condenser unit and one
55 55 would be the heat exchanger. Table 3.8 shows that there is no difference between one and two heat exchangers. Since there is no difference in price, only one heat exchanger is used. Table 3.8: Heat Exchanger Cost for Various Configurations Method for Calculating H Final Cost/bbl Single HX (Calculated Method) $6.30 Single HX (High Tabulated Values) $6.31 Single HX (Conservative Tabulated Values ) $6.33 Two HX (Calculated Method) $6.30 Two HX (High Tabulated Values) $6.31 Two HX (Conservative Tabulated Values) $ Supercritical Reactor The supercritical reactor costing was divided into three main cost components: the reactor shell, insulation, and the control valves As described in Section 2.1.3, the settling velocity equations were modeled in Excel to calculate the required base area for the process vessel. The precipitated solids particle diameter was assumed to be 50 microns [24]. From the required base area, and a height assumed to be three feet, the volume of settling tank was calculated. The volume of reactor was the input for the cost estimation in Table 3.9. The reactor shell was modeled as a process vessel., pressure factor, was dependent on the internal pressure,, and outer diameter,, of the vessel. The wall thickness was needed to calculate the outer diameter. The wall thickness was based on the equations (3.13) and (3.14) from the ASME code [33], where was the pressure, was the radius, and was the maximum
56 allowable stress of the material of construction, which was found from the ASME code [33]. 56 Table 3.9: Reactor Shell Cost [29] Equipment Process Vessel Reference Equation (2.22) For Carbon Steel Reference Equation (2.24) (3.13) (3.14) Refractory insulates the reactor, and limits the heat loss through the reactor walls. The thickness of the refractory material was assumed to be 0.02 meters based on the thickness of one layer of insulation, and it was assumed that the whole inside of the reactor was lined with refractory material. The cost of the refractory material was 0.99/square feet based on a vendor quote. The cost of the control valves was based on a vendor consultation, and ranged from $2000 to $15,000. The price of $15,000 was used as a conservative estimate. The total cost of the supercritical reactor was the combined cost of all three components: reactor vessel shell, refractory, and control valves Generation Unit An onsite power generation unit is needed to provide electric power for heating the reactor, powering the pumps, and operating other powered equipment. Based on vendor quotes (see Appendix H), the price of the generation unit for different flowrate
57 57 conditions was determined with the 6-10 th rule as shown in equation (3.15) where Power1 and Power0 were the power required for flowrate 1 and flowrate 0 respectively, and where and were costs for flowrate 1 and flowrate 0 respectively [29]. (3.15) Truck Cost The envisioned IPSC unit will be deployed on-site, and will be carried by a flatbed trailer. Vendor quotations suggested a range of $50,000 to $150,000 for the truck and $5,000 to $50,000 for the trailer (see Appendix H). A mid-range value of $120,000 was assumed for the truck and $30,000 for the trailer. Based on vendor quotes, the price of the truck for different flowrate conditions was determined with the 6-10 th rule [29]. The total cost of the mobile base was the sum of the truck and trailer Final Total Capital Cost As a major capital investment (>$ 1 Million), it is likely that the capital cost would be financed. Equation (3.16) [29] calculated the annual equipment cost per year including the interest cost, where was the nominal interest rate, and n was length of the loan in years. The final capital cost of each component and the annual cost of capital was calculated and shown in Table (3.16) Total Operating Cost The operating cost is the cost associated with the normal operation of the process, and depends on the cost of solids waste disposal, cost of raw materials, cost of utilities, cost of operating labor, and the capital cost from Section
58 Cost of Solids Waste Disposal The main by-products of the process are precipitation solids, and the NORM that needs to be treated. The cost for solids waste disposal depends on the hazardous nature of the material. and were considered as hazardous due to the hazardous classification of any barium and strontium solids waste. The salts such as the chlorides and oxides were considered non-hazardous. There is also the possibility of the chlorides used as de-icing road salt, which could potentially bring a resale value. The cost for nonhazardous solids waste disposal was $33/ton [29]. The cost of the hazardous waste disposal ranged from $200/ton to $2000/ton [29, 35]. A quotation from California indicates $200/ton was the more realistic estimate for hazardous solids disposal cost [34]. For this model, the sulfates and NORM removal material was modeled as hazardous at a treatment cost of $250/ton, the oxides and carbonates as non-hazardous at $33/ton, and chlorides at $0/ton for use as de-icing agent Cost of Utilities All the process equipment including heating of the supercritical water is powered by electricity. The total required power input of the entire process was the sum of the power requirements of all the process equipment, and was determined through the Aspen model. For cost calculations, it was assumed that the mobile generation unit converts on-site available natural gas to electric power. However there was an efficiency loss due to the conversion of natural gas to electric power. Equation (3.17) was used to calculate the total power input needed in the form of natural gas. The heating value of natural gas was determined from [36], and the price of natural gas was determined from
59 59 the U.S. Dept of Energy [37]. The conversion efficiency for most turbines were around 30% [38], and 30% was assumed for the efficiency of natural gas to electric power conversion. (3.17) Table 3.10: Capital Costs and Annualized Cost of Capital for All Equipment Annual Equipment Cost ($) Equipment Lifetime Cost/yr ($/yr) Payment ($/yr) Sand Filter $21, $2, $2,911 UV Unit $15, $1, $2,022 Sulftank $21, $2, $2,898 Softtank $21, $2, $2,898 Hydrotank $21, $2, $2,898 NORM $61, $6, $8,296 Pump $222, $23, $29,941 HX $123, $13, $16,685 SCReactor $123, $12, $16,627 Generation $749, $78, $101,061 Truck $120, $12, $16,176 Total $1,501,594 $158,063 $202,412 Total Equipment Cost $1,771,881 $186,514 $238, Cost of Raw Materials There are two main raw materials needs for the process, the NORM removal sorbent material and the agents used for chemical precipitation. The amount needed for each agent was determined by the amount of ions in the stream, and the separation efficiency required. Once the amount of ions in the stream, and separation efficiency
60 60 were inputted into the Aspen Model, as described in Section 3.2.1, the Aspen model calculated the amount of agent needed. The amount of zeolite (NORM absorption) material required was based on experimental results. The preliminary experiments from Ohio University indicated 0.5 g of zeolite was needed for removal of 1 pci of NORM. The amount of each raw material needed for one year was then multiplied by its unit price to calculate the cost of raw materials per year. The price of each chemical was determined using the Chemical Market Index [39] and [40] for zeolites, and is listed in Table Table 3.11: Raw Material Unit Cost Chemical ($/ton) Sulfuric Acid $25.00 Sodium Carbonate $ Sodium Hydroxide $ Clinoptilolite $ Cost of Labor The labor wage was determined using the Bureau of Labor Statistics data [41]. The wage for wastewater treatment operators in the Parkersburg- WV area was $17.89/hour [41]. It was that assumed that the process will require two operators. The final labor cost was the hourly wages multiplied by the number of workers multiplied by the hours worked Final Total Operation Cost The total operating cost per year was calculated and shown in Table It was based on the cost analysis developed by Turton [29]. Because the process was assumed to
61 61 be mobile with minimal overhead, the direct supervisory and clerical labor factor and plant overhead costs were assumed to be low, and the low value of the range were used in the cost-analysis. Due to the process being high pressure and high salinity, it was assumed that there are frequent maintenance and repairs. The high end of the cost factors were used for the maintenance and repairs and operating supplies. Because there was no laboratory, new research and development, or patent costs associated with IPSC process itself, laboratory, new research and development, and patents costs were assumed to be zero Cost/Barrel The Cost per Barrel (average cost) was found as a combination of the total capital cost/yr and total operating cost/yr, the capacity factor, C.F., and the barrels treated/year as shown in Equation (3.18). A conservative estimate of 0.75 was used for the capacity factor. The barrels treated/yr was determined by Equation (3.19), and the final cost per barrel is listed in Table (3.18) ( ) (3.19) 3.4 Model Validation Because one of the largest costs of the IPSC unit is the operation of the precipitation units, validation efforts were focused on this aspect of the model. Lab experiments measuring the precipitation parameter were performed, and this experimental data was used to validate the Aspen Model for the precipitation area.
62 62 Table 3.12: Operating Costs Typical Range Value Used Cost ($/yr) Direct Manufacturing Cost Raw Materials 1 1 $2,379,359 Solids Waste Disposal 1 1 $997,619 Utilities 1 1 $2,443,538 Operating Labor 1 1 $313,647 Direct supervisory and clerical labor ( ) COL 0.1 $31,365 Maintenance and repairs ( )FCI 0.1 $177,188 Operating Supplies ( ) Maintenance and repairs 0.2 $35,438 Laboratory charges ( )COL 0 $0 Patents and royalties (0-0.06)COM 0 $0 Fixed Manufacturing Costs Depreciation 0.1FCI 0 $0 Local taxes and insurance ( )FCI $56,700 Plant Overhead Costs ( )*(COL+DSCL+MR) 0.5 $261,100 General Manufacturing Costs Administration costs 0.15(COL + DSCL+MR) 0.15 $78,330 Distribution and selling costs ( )COM 0.11 $837,271 Research and development 0.05COM 0 $0 Total Annual Operating Cost $7,611,556
63 63 Table 3.13: Final Cost/Barrel Total Capital Cost (/yr) $238,846 Total Operating Cost (/yr) $7,611,556 Flow Rate (gal/min) 100 Capacity Factor 0.75 Barrels Treated (/yr) 939,195 Treatment Cost ($/bbl) $6.33 Table 3.14 shows the comparison of the experimental results and the modeling results for the sulfation step. The conditions of the Aspen Model simulated those of the experimental conditions to ensure consistency. Ion Concentration Table 3.14: Validation of the Sulfation Step Ba++ Removal Efficiency Sulfuric Required- Experimental (kmol) Sulfuric Required- Aspen (kmol) Error Percentage low 90% % low 50% % mid 90% % mid 50% % high 90% % high 50% % Aspen used an equilibrium approach to model the removal, the theoretical stoichiometric removal ratio, where one mole of sulfuric acid was needed to react with one mole of to form barium sulfate as seen in Equation (3.20). (3.20)
64 64 The difference percentage between the Aspen model outcomes and experimental ratio was less than 20%. This validated that the predicted outcome of the Aspen model was fairly close that to of experimental data. Since Aspen used an equilibrium approach to model the removal, the Aspen neglected effects such as co-precipitation and reaction kinetics and this explains the difference between the Aspen equilibrium model and experimental results. Table 3.15 shows the cost difference in the final cost per barrel, and it was less than $0.09 per barrel, or less than 1.5% difference between the experimental and Aspen results for the overall process cost per barrel. With a small difference in the estimated final cost per barrel by the using the experimental results compared to the Aspen results, the Aspen model gave a very close approximation and estimation of the real IPSC process. Table 3.15: Cost Comparison: Experimental vs. Aspen Model Final Cost ($/bbl) - Experimental Data $5.73 Final Cost ($/bbl) - Aspen Model $5.64
65 65 CHAPTER 4 SENSTIVITY ANALYSIS 4.1 Introduction This chapter discusses meeting the second objective, which is performing sensitivity analysis on the cost model. Table 4.1 contains a list of variables for which sensitivity analysis was performed, and the range of the variable that was tested. The sensitivity analysis was conducted utilizing the Aspen model and the cost analysis developed in Chapter 3. For each sensitivity analysis, the variable being tested was changed while holding the other variables constant. The range of each variable being tested, and the sensitivity analysis itself will be described in detail in the following sections. While one variable is undergoing sensitivity analysis, the default value in Table 4.1 was used for the other variables to ensure they were being held constant. 4.2 Sensitivity Analysis Flow Rate For the purpose of this analysis, the flow rate was user determined. The default flow rate was 100 GPM with the lower limit of 10 GPM. The upper limit was set at 200 GPM as the 200 GPM process will require over 15 MW, which could not implemented in a mobile application. Figure 4.1 shows a graph of the final cost per barrel versus the flowrate of the process, and Table 4.2 shows the values associated with the analysis. The trend of the graph shows an inverse relationship exists between the cost per barrel and flowrate, and based on a regression test, the final cost per barrel was approximated by equation (4.1). (4.1)
66 66 Table 4.1: Input Variables for Sensitivity Analysis Inputs Variables Range Default Value Flow rate 10 to 200 GPM 100 GPM Water chemistry Reference Table 2.1 Middle of Range (50%tile) Inlet temperature -5 C to 35 C 25 C Ion (,, ) 50% - 99% 90% removal removal efficiency due to Precipitation Cost of precipitate agents Sulfuric Acid $25.00/ton -$130.00/ton Sodium Carbonate $165.00/ton -$350.00/ton Sodium Hydroxide $150.00/ton -$900.00/ton Sulfuric Acid $25.00/ton Sodium Carbonate $165.00/ton Sodium Hydroxide $400.00/ton NORM concentration 0 to 10,000 pci 5,000 pci Material of construction SS/Ni Alloy/Hastelloy SS/SS/Ni Alloy Alloy/Ti Solids disposal cost $0/ton to $2000/ton Sulfates - $275/ton Chlorides - $0/ton Equipment lifetime 0.25 years to 15 years 9.5 years Price of utilities $2/MMBtu to $6/MMBtu $3.5/MMBtu Equations (4.1) takes the form of a reciprocal function, where the average cost of the process per barrel goes down as the flowrate goes up. This indicates that there is an economy of scale for the IPSC process. Within the constraints of a mobile process, the results from Figure 4.1 indicates that the maximum flowrate of 200 GPM should be used. The reciprocal function also indicated diminishing returns for cost savings as the flowrate increases. The average cost savings between 25 and 50 GPM was found to be $1.66 per barrel, while the cost savings between 125 and 150 GPM was found to be only $0.04 per barrel.
67 67 $16.00 $14.00 $12.00 Cost/bbl ($/bbl) $10.00 $8.00 $6.00 $4.00 $2.00 $ Flowrate (GPM) Figure 4.1: Cost/bbl vs. Flowrate Table 4.2: Cost/bbl vs. Flowrate Flowrate (GPM) Cost/bbl $13.48 $8.63 $6.97 $6.49 $6.33 $6.24 $6.20 $ Water Chemistry The ion levels at the inlet IPSC process significantly impacts the final cost per barrel of the process, because it impacts the amount of precipitative agents used. Table 4.3 shows the ion concentrations ranges for each ion used in the sensitivity analysis, and was based on the literature reference from Table % was the highest end of the expected ion concentration range, and the 0% was the lowest end of the ion concentration range. 10% was 10 percent of the difference between the high and low end of the range in addition to the lowest end of the ion range.
68 68 Table 4.3: Range of Ion Concentrations Ions 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% Ba 2+ 2,300 2,720 3,140 3,560 3,980 4,400 4,820 5,240 5,660 6,080 6,500 Sr 2+ 1,000 1,590 2,180 2,770 3,360 3,950 4,540 5,130 5,720 6,310 6,900 Ca 2+ 5,100 6,390 7,680 8,970 10,260 11,550 12,840 14,130 15,420 16,710 18,000 Mg 2+ 1,300 1,610 1,920 2,230 2,540 2,850 3,160 3,470 3,780 4,090 4,400 Fe Mn Na+ 23,000 26,430 29,860 33,290 36,720 40,150 43,580 47,010 50,440 53,870 57,300 Figure 4.2 shows the results of the sensitivity analysis for changes in water chemistry of the inlet process water. A linear regression of the data in Figure 4.2 yielded Equation (4.2). The slope of indicated that for every 1% or 0.01 of the difference between the assumed low and high range increase in all ion TDS levels means a increase in final cost per barrel. ( ) (4.2) $9.00 $8.00 $7.00 y = x R² = Cost/bbl ($/bbl) $6.00 $5.00 $4.00 $3.00 $2.00 $1.00 $0.00 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% % in Addition to the Lowest Level of Ions Figure 4.2: Cost/bbl vs. Water Chemistry
69 69 The sensitivity analysis also indicated the price of the IPSC process ranged from $4.42 to $8.51 due to differences in the F/P water ion concentration. The results indicated that the cost per barrel to process fracking water using the IPSC process has a notable dependence on the ion concentrations in the inlet F/P water. Figure 4.3 shows the same analysis for each individual ion while holding the other ions at the middle value (50%) of each ion range. Table 4.4 shows the linear regression for each ion. Fe and Mn were given a value of 0 due to the low levels of each ion. Table 4.4 ranks the ions from the most to the least sensitive, with being the most sensitivity, and and the least. One percent increase in concentration corresponds to an increase of $ per barrel in the final price. $7.50 $7.00 Cost/bbl ($/bbl) $6.50 $6.00 $5.50 Ba++ Sr++ Ca++ Mg++ Fe++ Mn++ Na+ $5.00 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% % in Addition to the Lowest Level of Ions Figure 4.3: Cost/bbl vs. Water Chemistry for Individual Ions
70 70 Table 4.4: Linear Regression for Final Cost vs Ion Ion Equation Cost/bbl ($/bbl) = * (Ion %) Cost/bbl ($/bbl) = * (Ion %) Cost/bbl ($/bbl) = * (Ion %) Cost/bbl ($/bbl) = * (Ion %) Cost/bbl ($/bbl) = * (Ion %) Inlet Water Temperature The temperature of the process inlet water depends on the ambient temperature around the water storage tank before it enters the process. While the air temperature might vary from as much as -40 C to 40 C, the large thermal mass of the storage tank and the degree of salinity limits the water temperature range from -5 C to 35 C. As seen in Figure 4.4, the inlet water temperature has a minimal effect on the final cost per barrel of treatment. The maximum difference between the lowest and highest cost per barrel at a range of -5 C to 35 C was only $0.04 per barrel. The minimum cost per barrel is around 25 C while the maximum is around 35 C. Temperature change impacted two components of the final cost per barrel. An increase in temperature reduces the heat loss from the supercritical reactor, which reduces the heat input. However, an increase in the temperature also increases the ion solubility, and higher ion solubility means more precipitation agent was needed to produce the same amount of precipitation solids. From -5 C to 25 C, the effect of the heat loss from the reactor is greater than that of the increase solubility, and the final cost per barrel is reduced slightly as temperature
71 71 increases. However, from 25 C to 35 C, the effect of the heat loss from the reactor is less than that of the increase in solubility, and the final cost per barrel increases as the temperature increases. Cost/bbl ($/bbl) $7.00 $6.80 $6.60 $6.40 $6.20 $6.00 $5.80 $5.60 $5.40 $5.20 $ Temperature Figure 4.4: Cost/bbl vs. Inlet Temperature Precipitation Separation Efficiency The amount of precipitation agent needed for the IPSC process is dependent upon several factors, one of which is the desired separation ion removal percentage of,, in the precipitation process, which is a user defined input. As described in Section 3.2.4, the user setted the ion removal percentage of,, in the precipitation process using Aspen. The Aspen model calculated the amount of precipitation agent needed to achieve the desire ion removal percentage. 50% to 99%
72 72 removal was used in this sensitivity analysis. Figure 4.5 shows the final cost per barrel vs. removal percentage of,, ranging from 50% to 99%. From Figure 4.5, the relationship between percentage removal and cost per barrel generally followed a positive linear relationship. Using a linear regression approximation, shown in Equation (4.4), results indicated that a cost of $0.025 per barrel was required to remove an additional 1% of,, ions. ( ) (4.3) Figure 4.6 shows the final cost per barrel vs. removal percentage for each type of salt ion (,, ) while holding the removal percentage for all other ions constant at 90%. Table 4.5 shows the results of linear regression analysis for the costs of removal per barrel for each ion. $6.80 $6.60 $6.40 y = 2.493x R² = Cost/bbl ($/bbl) $6.20 $6.00 $5.80 $5.60 $5.40 $5.20 $ % 54% 57% 61% 64% 68% 71% 75% 78% 82% 85% 89% 92% 96% 99% Percentage Removal Figure 4.5: Cost/bbl vs. Ion Removal
73 73 $6.50 $6.40 Cost/bbl ($/bbl) $6.30 $6.20 $6.10 $6.00 Sr++ Ca++ Mg++ $5.90 $ % 54% 57% 61% 64% 68% 71% 75% 78% 82% 85% 89% 92% 96% 99% Percentage Removal Figure 4.6: Cost/bbl vs. Ion Removal for Each Ion Table 4.5: Linear Regression for each Ion Removal Ion Equation Cost/bbl ($/bbl) = * (% Removal) Cost/bbl ($/bbl) = * (% Removal) Cost/bbl ($/bbl) = * (% Removal) Figure 4.6 shows that all 3 ion removal efficiency are generally linearly correlated with the final cost/bbl Cost of Precipitation Agents The cost of the precipitation agents,,, and are dependent on the market for chemicals. Historic market prices, current price ranges, and future chemical price forecasts were used to determine a range for each sensitivity analysis [39].
74 74 Table 4.6 shows the range of chemical price used in this sensitivity analysis. Figures 4.7, 4.8, 4.9 show the sensitivity analysis for price change in,, and respectively. As stated in Table 4.1, 90% ion removal is assumed for all ions. The graphs show linear relationship for each agent cost. Table 4.6: Cost/bbl vs. Agent Cost Relationship Chemical Low Price ($/ton) High Price ($/ton) Relationship Sulfuric Acid $25.00 $ Cost/bbl ($/bbl) = * Cost/ton Sodium Carbonate $ $ Cost/bbl ($/bbl) = * Cost/ton Sodium Hydroxide $ $ Cost/bbl ($/bbl) = * Cost/ton $7.00 $6.80 $6.60 $6.40 Cost/bbl ($/bbl $6.20 $6.00 $5.80 $5.60 $5.40 $5.20 $5.00 $- $20.00 $40.00 $60.00 $80.00 $ $ $ Cost ($/ton) Figure 4.7: Cost/bbl vs. Agent Cost ( )
75 75 $7.40 $7.20 Cost/bbl ($/bbl $7.00 $6.80 $6.60 $6.40 $6.20 $6.00 $- $50.00 $ $ $ $ $ $ $ Cost ($/ton) Figure 4.8: Cost/bbl vs. Agent Cost ( ) $8.00 $7.50 Cost/bbl ($/bbl $7.00 $6.50 $6.00 $5.50 $5.00 $- $ $ $ $ $ $ $ $ $ $1, Cost ($/ton) Figure 4.9: Cost/bbl vs. Agent Cost ( )
76 76 From the equations of the Table 4.6, the final cost/barrel is most sensitive to price changes in sodium carbonate, followed by the sodium hydroxide, and finally sulfuric acid. The sodium carbonate is 2X more significant than sulfuric acid, while sodium hydroxide is 2.5X more significant than sulfuric acid. Due to the large range of price possibilities for sodium hydroxide, there is also the largest price range difference. The potential maximum final cost per barrel due to sodium hydroxide prices is higher than that of sodium carbonate due to the price volatility of sodium hydroxide, even though its sensitivity is lower than that of sodium carbonate NORM Concentration As described in Table 2.1, there is a range of possible NORM concentrations for F/P water. The range of possible NORM concentrations of 0 pci to 10,000 pci was based on the literature data from Table 2.1. Figure 4.10 shows how the NORM concentration affects the final cost per barrel, and Table 4.7 shows the values associated with the graph. Based on the graph, the difference between the maximum and minimum prices due to NORM concentrations changes is only $0.06. The final cost per barrel is not very sensitivity to the NORM concentration in the F/P inlet water.
77 77 $6.36 $6.35 Cost/bbl ($/bbl) $6.34 $6.33 $6.32 y = 5E-06x R² = $6.31 $ NORM Concentration (pci/l) Figure 4.10: Final Cost vs NORM Concentration Table 4.7: Final Cost vs NORM Concentration Table NORM Concentration (pci) Cost/Barrel ($/bbl) $6.31 $6.31 $6.32 $6.32 $6.33 $6.34 $6.35 $ Material of Construction The cost associated with each potential type of fabrication material (e.g. low carbon stainless to nickel alloyed metals) can be tested by changing the material factor in the cost equation via Turton s method [29]. In Turton s method, by changing the material factor also accounts for both the change in material price and the change in labor cost associated with the material construction. Three major capital equipment costs are highly sensitive to different materials of construction: The high pressure pump, heat exchanger and supercritical reactor. Figures 4.11, 4.12, 4.13 show the sensitivity analysis of pump, heat exchanger and supercritical reactor respectively due to the difference of
78 78 material of construction. Although there exist many types of stainless steel such as SS 316 and SS 304, Turton s method only considers the genetic stainless steel for cost estimation. Thus, for this sensitivity analysis, it is assumed that the cost differences between different types of stainless steel are negligible. The use of a Hastelloy pump compared to a stainless steel pump only increases the final cost per barrel by only $0.04. This suggests that Hastelloy pump might be a viable option for the process, especially if it can increase the lifetime of pump, which would reduce the cost per barrel of the final process. The small price increase for a materials upgrade for the pump suggests that Hastelloy might be a viable choice. In Figure 4.12, a SS/CS is stainless steel shell and carbon steel tubes, with the shell material on the left side, and tube material on the right. Based on Figure 4.12, Monel appears to be a viable material for both tubes and shell of the heat exchanger. Monel, or a nickel alloy, could significantly enhance corrosion resistance, while only increasing the price $0.01 compared to the stainless steel option for both the shell and tubes. The titanium option also could increase corrosion resistance, however at a greater cost of $0.25 per barrel. The Monel/Monel option is a viable material update due to its low cost increase, and potential significant process improvement. The use of nickel cladding and titanium cladding are choices for improving the corrosion resistance for the supercritical reactor. The use of nickel cladding or titanium cladding for the material of construction only adds less than $0.01 to the final cost per barrel compared to the use of stainless steel. The use of pure nickel or titanium adds $0.03 to $0.04 per barrel respectively to the final cost per barrel compared to the use of
79 79 stainless steel. Nickel or titanium cladding offer the same basic corrosion resistance as pure nickel or pure titanium, while only adding less $0.01 per barrel. $6.44 $6.42 $6.40 $6.38 $6.36 $6.34 $6.32 $6.30 $6.28 SS Hastelloy Figure 4.11: Final Cost vs Pump Material of Construction Solids Waste Disposal Cost The key question for the solids disposal sensitivity analysis is the classification of barium sulfate and chloride salts. As discussed previously, waste disposal cost ranged from $0/ton for re-use in other applications, nonhazardous disposal at $33/ton, and hazardous disposal from $200/ton to $2000/ton [29]. This sensitivity analysis tested for the range of solids disposal cost from $0/ton to $2000/ton for both sulfates and chlorides.
80 80 $6.55 $6.50 $6.45 $6.40 $6.35 $6.30 $6.25 $6.20 Figure 4.12: Final Cost vs Heat Exchanger Material of Construction $6.40 $6.38 $6.36 $6.34 $6.32 $6.30 $6.28 $6.26 CS SS Clad SS Ni Alloy Clad Ni Alloy Ti Clad Ti Figure 4.13: Final Cost vs Supercritical Reactor Material of Construction
81 81 Figure 4.14 shows the effect of the disposal method for barium sulfate on the final cost per barrel. Figure 4.15 shows the effect of disposal method (and thus cost) for chloride salts on the final cost per barrel. Table 4.8 shows the values from Figure 4.14 and Figure 4.15, and their respective regressions. For the sulfates, classification between hazardous and non-hazardous represents a difference of $0.67/bbl. The default hazardous treatment cost was $250/ton [34]. However, if the disposal cost are to reach $2,000/ton, the final cost could reach $11.03 per barrel. However, the sodium chloride salts represents the most sensitive solids product due to change in waste disposal cost, which can range from $6.33, if sodium chloride is re-used and disposal cost is $0/ton, to $61.69, if sodium chloride disposal reaches $2,000/ton. The sensitivities of calcium chlorides and magnesium chlorides are very small compared to that of sodium chloride. $12.00 $10.00 Cost/bbl ($/bbl) $8.00 $6.00 $4.00 $2.00 $0.00 $- $ $ $1, $1, $2, Disposal Cost ($/ton) Figure 4.14: Final Cost vs Sulfate Disposal Cost
82 82 Cost/bbl ($/bbl) $70.00 $60.00 $50.00 $40.00 $30.00 $20.00 $10.00 $0.00 $- $ $ $1, $1, $2, NaCl MgCl2 CaCl2 All Salts Disposal Cost ($/ton) Figure 4.15: Final Cost vs Salts Disposal Cost Table 4.8: Final Cost vs Salts Disposal Cost Table Solids Disposal Cost ($/ton) $ - $33.00 $ $2, Relationship Sulfates $5.58 $5.66 $6.13 $11.02 Cost/bbl ($/bbl) = * Cost/ton Sodium Chloride $6.33 $7.16 $11.87 $61.69 Cost/bbl ($/bbl) = * Cost/ton Magnesium Chloride $6.33 $6.34 $6.37 $6.75 Cost/bbl ($/bbl) = * Cost/ton Calcium Chloride $6.33 $6.35 $6.45 $7.52 Cost/bbl ($/bbl) = * Cost/ton All Chloride Salts $6.33 $7.19 $12.03 $63.28 Cost/bbl ($/bbl) = * Cost/ton Equipment Lifetime The lifetime of each equipment significantly impacts the final cost per barrel. The default lifetime for each equipment was 9.5 years. Three months was set as the lowest possible equipment lifetime, while 15 years was assumed as the longest possible lifetime. The range of equipment lifetime from three months to 15 years was tested for each individual process equipment. Figure 4.16 shows the how the equipment lifetime affects the final cost per barrel. Figure 4.17 shows the how the equipment lifetime affects the
83 83 final cost per barrel for each individual equipment type while holding the others at a default lifetime of 9.5 years. Both figures show inverse relationships between the final cost per barrel vs. equipment lifetime. A shorter equipment lifetime exponentially increases the final cost per barrel. If all equipment failed at three months, the final cost per barrel could potentially increase to $14.99 per barrel, while a lifetime of 15 years for all equipment could reduce treatment costs to $6.25 per barrel. In terms of individual equipment, sensitivity should be positively correlated with the price of the capital equipment. This is seen in Table 4.9 as the Truck is the most sensitive, followed by the generation unit, and Supercritical Reactor. This data should be used with the data from section Material of Construction to minimize the final cost/bbl. More expensive materials of construction for the heat exchanger, pump and reactor could extend the equipment lifetime, and should be weighed against the increase in price for the material update. Cost/bbl ($/bbl) $16.00 $15.00 $14.00 $13.00 $12.00 $11.00 $10.00 $9.00 $8.00 $7.00 $ Equipment Lifetime (yr) Figure 4.16: Final Cost vs Equipment Lifetime
84 84 Cost/bbl ($/bbl) $12.00 $11.00 $10.00 $9.00 $8.00 $7.00 $ Equipment Lifetime (yr) Sand Filter UV Unit Sulftank Softtank Hydrotank NORM Pump HX SCReactor Generation Figure 4.17: Final Cost vs Equipment Lifetime for each Equipment Table 4.9: Final Cost vs. Equipment Lifetime Equipment Lifetime (yrs) Sand Filter $6.40 $6.47 $6.36 $6.35 $6.35 $6.34 $6.33 $6.33 UV Unit $6.38 $6.43 $6.35 $6.35 $6.34 $6.33 $6.33 $6.33 Sulftank $6.40 $6.47 $6.36 $6.35 $6.35 $6.34 $6.33 $6.33 Softtank $6.40 $6.47 $6.36 $6.35 $6.35 $6.34 $6.33 $6.33 Hydrotank $6.40 $6.47 $6.36 $6.35 $6.35 $6.34 $6.33 $6.33 NORM $6.52 $6.72 $6.42 $6.39 $6.37 $6.34 $6.33 $6.33 Pump $7.01 $7.72 $6.65 $6.53 $6.47 $6.37 $6.33 $6.32 HX $6.71 $7.11 $6.51 $6.44 $6.41 $6.35 $6.33 $6.32 SCReactor $6.71 $7.11 $6.51 $6.44 $6.41 $6.35 $6.33 $6.32 Generation $8.62 $11.03 $7.41 $7.01 $6.81 $6.45 $6.33 $6.29 Truck $6.70 $7.08 $6.51 $6.44 $6.41 $6.35 $6.33 $6.33 Total $10.54 $14.99 $8.32 $7.58 $7.21 $6.54 $6.33 $6.25
85 Cost of Utilities The change in natural gas price and its effect on the final cost is shown in Figure The cost of natural gas is dependent the market for energy. Based on the U.S. energy market price trends for the past 10 years [37], the price range of natural gas from $2.00/MMBtu to $6.00/MMBtu was used for the sensitivity analysis. Equation (4.4) shows the regression of the natural gas sensitive analysis. ( ) (4.4) From Equation (4.4), it is evident that the final cost per barrel is sensitive to a price change in natural gas, as a $1/MMBtu increase in natural gas represents $0.629 increase in the final cost per barrel. The high and low potential final cost per barrel due to high and low natural gas price is from $5.40 to $7.90. $8.50 $8.00 $7.50 Cost/bbl ($/bbl) $7.00 $6.50 $6.00 $5.50 $5.00 $0.00 $1.00 $2.00 $3.00 $4.00 $5.00 $6.00 $7.00 Gas Cost ($/MMBTU) Figure 4.18: Final Cost vs Natural Gas Price
86 Sulfation Only Option Using only sulfation in the precipitation process is possibly cheaper than using all three precipitation steps due to the high cost of and. The cost of sulfation only and the full process was compared assuming all input variables at the default values from Table 4.1. The final cost per barrel for only sulfation is $4.13 per barrel compared to the final cost of $6.33 per barrel for the full precipitation process Cost Range Using the results of the sensitivity analysis, a range can be developed for the final cost per barrel of the IPSC processes. For the IPSC low price, the low end of all the sensitivity analysis inputs were assumed, and for the IPSC high price, the high end of all the sensitivity analysis inputs were assumed. Thus the lowest price per barrel of the IPSC is $2.32 and the highest price of the IPSC process is $140.42, while the mid value price of $6.33 is the most probably value for the IPSC process. Table 4.10: Range of IPSC Final Costs IPSC Low Price IPSC Mid Price IPSC High Price $2.32 $6.33 $ However, the range in Table 4.10 also assumes the high and low ends of the flowrate and ion separation efficiency, which can be both set by the operator. Table 4.10 also assumes that the hazardous treatment cost can reach $2000/ton, which is unlikely due to more recent cost information [34]. The low and high price for the IPSC process in Table 4.10 is the absolute possible minimum and maximum of the process. Table 4.11
87 87 shows the more likely high and low cost per barrel of the IPSC process, where the process was set at 100 GPM, 90% ion separation efficiency of,, in the precipitation process, and the hazardous solids waste disposal cost was set at $250/ton. Table 4.11: Probably Range of IPSC Final Costs IPSC Low Price IPSC Mid Price IPSC High Price $2.93 $6.33 $16.03
88 88 CHAPTER 5 - DISCUSSION 5.1 Final Cost per Barrel of the IPSC Process From Section 3.4, the cost-analysis shows that the likely cost of the IPSC process is $6.33 per barrel. A breakdown of the cost analysis reveals how each component contributes to the final cost per barrel. Using the cost analysis from Section 3.4, the components of the IPSC process are displayed in Figure 5.1. This pie chart shows a breakdown of the final cost per barrel. The capital cost contributes only to 11% of the total cost. This indicates that the IPSC is not a capital intensive process, and the 6% labor cost indicates that it is not a labor intensive process either. The majority of the costs is due to the high cost of utility to power the process and the cost of the raw materials needed mostly for the separation process. The combination of both make up 69% of the final cost per barrel. The key to reducing the final cost is to find a solution to reduce the utility cost and raw material cost. To isolate the major reason for the high cost of raw material, a further breakdown is seen in Figure 5.2 for all three precipitation agents. It is evident that hydrolysis and softening contributes to 98% of the agent cost. If the IPSC process can eliminate the use of sodium carbonate and sodium hydroxide, then the final cost per barrel will be reduced. This is the rationale behind potentially using the IPSC process with only sulfation in the precipitation step.
89 89 Figure 5.1: Cost Breakdown Figure 5.2: Cost Breakdown for Cost of Raw Materials
90 90 Figure 5.3 shows the cost breakdown for the sulfation only process. As shown by the results from Section 4.13, the final cost per barrel is $4.13 per barrel if only sulfation is used. The elimination of expensive sodium carbonate and sodium hydroxide is major reason that final cost of using only sulfation is $2.20 lower than the full precipitation. The cost of the raw materials has been drastically reduced to less than 4% of the total cost. This shows that although the full IPSC process is already cheaper than existing technologies, by only using sulfation as the precipitation step, greater cost savings could be realized. Figure 5.3: Cost Breakdown for the case of using only sulfation for precipitation Table 5.1 shows a comparison of the final cost per barrel of both the full IPSC process and IPSC process with only sulfation compared to the cost per barrel of existing
91 91 treatment technologies. It is evident both the full IPSC process at $6.33 per barrel and the sulfation-only IPSC process at $4.13 per barrel are both economically competitive against existing treatment technologies. As discussed before, there exists a large range of costs per barrel for existing technologies. Current existing costs largely depend on the location of the shale. On the higher end of existing practices, the price ranges from $25 per barrel to $105 per barrel [42], and both the IPSC with the full precipitation process and IPSC with only the sulfation are significantly cheap than the more expensive existing practices. On the lower end, existing practices range from $3 - $8 per barrel [15], however both the IPSC with the full precipitation process or IPSC with sulfation only compares very favorably with the costs of existing technologies. Table 5.1: Cost Comparison with Existing Practices IPSC Full Precipitation IPSC Sulfation Only Existing Practices (Lower End) Existing Practices (Higher End) $6.33 $4.13 $3.00-$8.00 $25.00-$ Sensitivity Analysis Using the cost model and assuming 100 GPM of flow and 90% removal of,, in the precipitation steps, the final predicted cost of the process is $6.33 per barrel. However, as seen in the sensitivity analysis, there is a significant variation in the cost per barrel as a function of other process parameters. Figure 5.4 shows the comparison of the final cost per barrel of different sensitivity factors that was determined
92 92 in Chapter 4. For example, the sensitivity analysis in Chapter 4 determined that the final cost per barrel due to the change in chloride salt solids waste disposal cost ranges from $6.33 to $63.00 per barrel, while final cost per barrel due to the change in temperature ranges from $6.33 to $6.35 per barrel. Both of these ranges are seen Figure 5.4, and the numerical values are seen in Table 5.2. The comparisons ranks the input variable from the largest range to the smallest range. The sensitivity factors reveals how inputs affect the final cost per barrel, and based on this, the top factors should be the first ones to be addressed with potential engineering design or process solutions. It is evident that the classification of the produced salts significantly impacts the final cost per barrel of the process. A $50 per barrel price difference exists between classifying the produced salts for reuse vs. classifying the produced salts as the most expensive hazardous material. If a small addition to the process can ensure that the produced salts can be classified as a non-hazardous waste, or the salt can be guaranteed for reuse as a deicing agent, the final cost per barrel of the IPSC process is closer to the expected value of $6.33 per barrel. As shown in Figure 4.11, the cost per barrel for IPSC treatment ranges from $2.93 to $ While there is some sensitivity to material prices, ion concentrations, and energy prices, which are all affected by the geographic location of possible deployment sites, the range of possible costs compare favorably to the costs of current technology, as seen in Table 5.3.
93 93 All Chloride Salts Solids Disposal Cost Equipment Lifetime Flowrate All Sulfate Salts Solids Disposal Cost Water Chemistry Utilities Sodium Hydroxide Cost Ion Removal Percentage (50%-99%) Sodium Carbonate Cost HX Material of Construction Sulfuric Acid Cost Pump Material of Construction SCR Material of Construction NORM Concentration Temperature IPSC Final Cost/bbl $5.00 $15.00 $25.00 $35.00 $45.00 $55.00 $65.00 Figure 5.4: Price Ranges of Sensitivity Analysis Table 5.2: Values for the Price Ranges Sensitivity Analysis Low High All Chloride Salts Solids Disposal Cost $6.33 $63.29 Equipment Lifetime $6.26 $15.00 Flowrate $6.13 $13.48 All Sulfate Salts Solids Disposal Cost $5.59 $11.03 Water Chemistry $4.42 $8.51 Utilities $5.40 $7.91 Sodium Hydroxide Cost $5.62 $7.79 Ion Removal Percentage (50%-99%) $5.33 $6.55 Sodium Carbonate Cost $6.33 $7.38 HX Material of Construction $6.29 $6.48 Sulfuric Acid Cost $6.33 $6.50 Pump Material of Construction $6.33 $6.43 SCR Material of Construction $6.32 $6.40 NORM Concentration $6.31 $6.37 Temperature $6.33 $6.39
94 94 IPSC Low Price Table 5.3: Cost Comparison with Existing Practices IPSC Mid Price IPSC High Price Existing Practices (Lower End) Existing Practices (Higher End) $2.93 $6.33 $16.03 $3.00-$8.00 $25.00-$ Different geographic locations have different temperature conditions and shales of different locations contain different ion concentrations. The small sensitivity in final cost per barrel for the IPSC process due to changes in ion concentration and changes in temperature makes the IPSC process deployable in different geographic locations. The small sensitivity of the final cost per barrel for the IPSC process due to changes in precipitation agent cost and changes in energy prices makes the IPSC process cost less susceptible to volatility related to market forces. Chemical prices and energy prices are dictated by market forces, and can fluctuate in short periods of time. However, the small change in IPSC final cost per barrel even with large changes in chemical and energy prices makes the IPSC final cost per barrel very stable. This price stability is an economic advantage and important characteristic. Based on this techno-economic analysis, the most likely cost per barrel of the ISPC process is $6.33 with a possible range from $2.93 to $ This competitive price and small range of price difference indicates that the IPSC process is economically feasible as a treatment method for F/P water, and may mitigate problems related to F/P water management for shale oil and gas producers.
95 95 CHAPTER 6 CONCLUSIONS The first objective of this thesis was to develop an Aspen -based model and techno - economic analysis of the IPSC process. Aspects of the Aspen model were to be validated with experimental data. Chapter 3 described how the process model was built through Aspen to calculate and simulate outputs of the IPSC process. Chapter 3 also described the techno-economic analysis that determined the final cost per barrel of the process for a fully developed commercial sized IPSC process. Finally, in Chapter 3, the results of the Aspen model were verified and validated by comparing the model results of the precipitation step with experimental results. The second objective was to perform sensitivity analyses on the selected ten major process variables inputs utilizing the model template. Chapter 4 described the sensitivity analysis on these ten variable inputs. The objectives of this thesis are met based on the results of Chapter 3 and Chapter 4. Section 3.2 described how the IPSC model was modeled in Aspen with the Aspen model seen in Figure 3.1. The cost analysis was built on the results of the Aspen model, and was described in Section 3.3. The final cost per barrel of the IPSC predicted by the techno-economic analysis was listed in Table Section 3.4 described how the Aspen model was validated using experimental results. The second objective was to perform sensitivity analysis on the major process variables inputs utilizing the model template. Chapter 4 described the ranges of each potential input and the results of the sensitivity analyses on the inputs utilizing the model template. Sections , , and also described a potential range of the final cost per barrel of the IPSC using the results of the sensitivity analysis, and this range of
96 cost per barrel was compared to other existing F/P water treatment practices to determine the economic competitiveness of the IPSC process. 96
97 97 CHAPTER 7 - RECOMMENDATIONS This chapter focuses on recommendations stemming from the results of this thesis. Thesis recommendations are presented with the goal of improving the cost estimation of the IPSC process provided by the techno-economic analysis and to point to potential areas of future investigation to improve the cost viability of the IPSC process. 7.1 Validation of Precipitation Process Modeling The first recommendation is to validate the softening/hydrolysis results of the Aspen model with experimental results. Because the Aspen model used an equilibrium method to model the precipitation steps, the Aspen model neglected the effects of kinetic effects and co-precipitation effects. Experiments are planned to simulate the softening and hydrolysis processes, and the experiments will determine a more accurate prediction regarding the amount of precipitation agents needed for the precipitation step. Once the experimental results are available, validation similar to the one performed in Section 3.4 can be conducted for the softening and hydrolysis processes. By comparing the final cost per barrel based on the experimental results and final cost per barrel based on the Aspen model, one can see if the negation of the effects of kinetics and co-precipitation are significant to the final cost per barrel. If negation of the effects are not significant, then the Aspen model is strengthen. Using the experimental data to validate the Aspen models of softening and hydrolysis will further enhance the validity of the Aspen model, and will further validate the accuracy of the Aspen process model.
98 Multivariate Sensitivity Analysis The second recommendation is to conduct multivariate sensitivity analyses compared to the single variable changes done in the sensitivity analyses of this thesis. Many input factors are related and correlated, which may not be evident in single variable sensitivities. For example, equipment lifetime can be enhanced with a more expensive material of construction. While more expensive materials increase costs, longer equipment lifetime may reduce the final cost per barrel. Another more evident example is the relationship between the input factors of ion removal percentage vs. the cost of the precipitation agent. Higher ion removal requires more precipitation agents, which increase the final cost per barrel, but an increase in the cost of the precipitation agent will also increase the final cost per barrel. Multivariable analysis can establish relationships between input factors, and provides more accurate sensitivity analysis of the IPSC process. 7.3 Incorporation of Engineering Design Constraints The third recommendation is to refine the cost-analysis model with experimental data and engineering design constraints when further experimental results have been found and more engineering designs parameters have been determined. More accurate empirical data pertaining to the IPSC process will give a more accurate estimation of the final cost per barrel than the general literature reference data. Similarly, more finalized engineering designs will also enhance the accuracy of the final cost estimation. For example, Aspen used equilibrium methods to model the precipitation process, and certain parameters of the cost analysis such as the particle size diameters of
99 99 the precipitation solids and supercritical solids were based on literature data. Experiments are planned to analyze the particle sizes and precipitation solids concentrations under IPSC process. Once these experiments are conducted, the results can be directly integrated into the techno-economic model for the effects on hydrocyclonic separation, tank size, and heat exchanger fouling (among other factors) can be updated. Updating the Aspen model will be facilitated by an instructional video that can be found. Another example is that of the overall heat transfer coefficient of the heat exchanger that was based on typical values in similar conditions. However, with further engineering design, such as the heat exchanger piping layout and pipe diameter, a more accurate heat transfer coefficient can be determined. A more accurate heat transfer coefficient will lead to a better cost analysis with higher accuracy of the heat exchanger cost and energy requirements.
100 100 REFERENCES [1] U.S. Energy Information Administration (US EIA) (2011b), World shale gas resources: An initial assessment of 14 regions outside the United States, US EIA., Washington, D. C., Rep., [2] P. Williams, Oil shales and their analysis, Fuel, vol. 62, no. 7, pp , [3] American Petroleum Institute (API), Water management associated with hydraulic fracturing, Am. Pet. Inst., Washington, D. C., Rep. HF2, June [4] C. Clark, J. Veil, Produced water volumes and management practices in the United States, Argonne National Laboratory., Lemont, IL., Report No.: DE-AC02-06CH11357, [5] K. Maloney, D. Yoxtheimer, Production and disposal of waste materials from gas and oil extraction from the Marcellus Shale Play in Pennsylvania, Environmental Practice., vol. 14, no. 4, Dec [6] M. Pruder, J. Veil, Offsite commercial disposal of oil and gas exploration and production waste: Availability, options, and costs, Argonne National Laboratory., Lemont, IL., Report No.: ANL/EVS/R-06/5, [7] H. I. Petersen and A. Mathiesen, Variations in Composition, Petroleum Potential & Kinetics of Ordovician - Miocene Type I & Type I-II Source Rocks (Oil Shales): Implications for Hydrocarbon Generation Characterestics, J. Pet. Geol., vol. 33, no. 1, pp , [8] R. Kleinberg, Oil Shales & Hydrates Subgroup of the Technology Task Group of the NPC Committee on Global Oil & Gas, Houston, [9] INTEK, Review of Emerging Resources: U.S. Shale Gas and Shale Oil Plays, Washington, D.C, [10] What is shale gas and why is it important?. Internet: Dec. 5, 2012 [Dec. 16, 2013] [11] A. Sieminski, In: Outlook for shale gas and tight oil development in the U.S, in Deloitte Energy Conference., May 21, 2013; Washington, DC. ; [12] U.S. Department of Energy (US DOE) (2009), Modern shale gas development in the United States: A primer, DOE., Washington, D. C. Rep., DoE-FG26-04NT15455, 116 pp., 2009.
101 101 [13] M. Mantell, EPA Hydraulic Fracturing Study Technical Workshop # 4 Water Resources Management Produced Water Reuse and Recycling Challenges and Opportunities Across Major Shale Plays, Oklahoma City, [14] Chesapeake Energy, Hydraulic Fracturing, [Online]. Available: es_fracking.pdf. [13] API, Hydraulic Fracturing: Unlocking America s Natural Gas Resources, American Petroleum Institute, Washington, D.C, Jul [15] J.Trembly, Cost-Effective Treatment of Flowback and Produced Waters via an Integrated Precipitative Supercritical Process, Ohio University, Athens, OH., REQUEST FOR PROPOSAL: RFP2011UN001. [16] A. Gaudlip, L. Paugh, T. Hayes, In: Marcellus shale water management challenges in Pennsylvania, in Society of Petroleum Engineers Shale Gas Production Conference., November 16-18, 2008; Fort Worth, TX. ; [17] Aspen Physical Property Methods and Models, 11.1th ed., Aspen Technology, Inc., Cambridge, MA, [18] R. Welch, D. Rychel, Produced water from oil and gas operations in the onshore lower 48 states. Phase I Report., US Department of Energy National Energy Technology Laboratory, Report No.: DE-AD26-01NT00249, [19] A. Gaudlip, L. Paugh, T. Hayes, An integrated framework for treatment and management of produced water, in Society of Petroleum Engineers Shale Gas Production Conference., November 16-18, 2008; Fort Worth, TX. ; [20] H. Acharya et al., Cost effective recovery of low-tds frac flowback water for re-use, GE Global Research., Niskayuna, NY, Rep. DE-FE , March 31, [21] M. O Driscoll, Frac Sand Frenzy Focus on supply & demand for hydraulic fracturing sand, in Silica Arabia, 2012: Jeddah, March 2012 [22] Frac Sand & Proppant Characteristics. Nov, 2009 [Nov. 29, 2014]. [23] F. Armellini, J. Tester, and G. Hong, Precipitation of sodium chloride and sodium sulfate in water from sub- to supercritical conditions: 150 to 550 C, 100 to 300 bar, J. Supercrit. Fluids, vol. 7, no. 3, pp , 1994.
102 102 [24] F. Armellini, J. Tester, and G. Hong, Precipitation of sodium chloride and sodium sulfate in water from sub- to supercritical conditions: 150 to 550 C, 100 to 300 bar, J. Supercrit. Fluids, vol. 7, no. 3, pp , [25] N. Cheng, Comparison of formulas for drag coefficient and settling velocity of spherical particles, Powder Technology, vol. 189, pp , [26] S. Kakac, H. Liu, Heat Exchangers Selection, Rating, and Thermal Design, 2 nd ed. Boca Raton, FL: CRC Press, [27] M. Yizhak, Scw as a Green Solvent, in Supercritical Water A Green Solvent: Properties & Uses, 10th ed., Hoboken: John Wiley & Sons, 2012, pp [28] A. Chapman., Heat Transfer, 4 th ed. New York: Macmillan Publishing Company, [29] R. Turton et al., Analysis, Synthesis, and Design of Chemical Processes, 3 rd ed. Upper Saddle River, NJ: Prentice Hall, [30] Chemical Engineering Plant Cost Index (CEPCI), Chemical Engineering Vol. 121, Issue 11, pp , [31] [online] 2014, (Accessed: 1 December 2014). [32] R. Felder, R. Rousseau, Elementary Principles of Chemical Processes, 3 rd ed. Wiley, [33] D. Woods, S. Anderson, S. Norman, Evaluation of Capital Cost Data: Heat Exchangers, The Canadian Journal of Chemical Engineering, vol. 54, pp , [34] ASME, Section VIII, Division 1: Design and Fabrication of Pressure Vessels, Section VIII, [35] Mobile Generation Unit, Internet: Dec. 16, 2013 [Dec. 22, 2013] [36] Mobile Generation Unit, Internet: Dec. 16, 2013 [Dec. 22, 2013]
103 103 [37] Department of Toxic Substances Control Fee Summary, Dept. of Toxic Substances Control., Sacramento, CA, [38] Technologies and management strategies for hazardous waste control. [electronic resource]. n.p.: Washington, D.C. : Congress, of the U.S., Office of Technology Assessment, [1983], OHIO UNIV - MAIN's Catalog, EBSCOhost (accessed January 5, 2015). [39] Lower and Higher Heating Values of Gas, Liquid and Solid Fuels [online]. Available: quid_and_solid_fuels.pdf [40] SHORT-TERM ENERGY OUTLOOK, Internet: Dec. 10, 2013 [Dec. 16, 2013] [41] M. Moran, H. Shapiro, Fundamentals of Engineering Thermodynamics, 6 th ed. Wiley, [42] Indicative Chemical Prices A-Z, Internet: Aug. 28, 2006 [Dec. 22, 2013] [43] H. Zhao, G. Vance, G. Ganjegunte, M. Urynowicz, Use of zeolites for treating natural gas co-produced water in Wyoming, USA, Desalination, vol. 228, pp , 2008 [44] Overview of BLS Wage Data by Area and Occupation. Internet: Dec. 16, 2013 [Dec. 22, 2013] [45] P. Boschee, Handling Produced Water from Hydraulic Fracturing, Oil & Gas Facilities, no. February, pp , Feb-2012.
104 104 APPENDIX A - VBA SETTLING EQUATION CODE Function vel(x As Double, dp As Double, rhop As Double, rhof As Double, gra As Double, mu As Double) As Double Dim diff Dim i As Integer Dim f, fprime As Double diff = 1 Do While Abs(diff) > f = (0.15 * ((rhof * dp / mu) ^ 0.687) * (x ^ 1.687)) + x - (1 / 18) * (dp ^ 2) * (rhop - rhof) * gra * (1 / mu) fprime = * (((rhof * dp) / mu) ^ 0.687) * (x ^ 0.687) + 1 vel = x - (f / fprime) diff = vel - x x = vel Loop End Function
105 105 APPENDIX B - HYDROCYCLONE QUOTE Ohio University Coal Research Center Solid Polyurethane Cyclone WSNA Proposal #:WCW081214BRM LINS August 12, 2014
106 106 Kissick Engineered Products & Services, Inc W. Fair Ave. Lancaster, OH fax August 12, 2014 Xiao Dong Ohio University Coal Research Center Athens, OH Dear Xiao, Solid Polyurethane Cyclone Quotation WCW081214BRM LINS Please find attached our proposal for the Cavex Polyurethane Cyclone. Bid Equipment Cyclone One (1) CAVEX Model (150-06P CVX) Cyclone with a 40mm Solid Polyurethane Vortex Finder and 20mm Spigot Liner. Includes Overflow Pipe and Spiral Contoured Inlet Head Liner for low turbulence and longer wear life. Price for one (1) Cyclone.....$1, Price Schedule All pricing has been given in US dollars and is Ex-Works North American Point of assembly. This pricing does not include any duty, freight, export packaging, or taxes of any kind. Pricing is based on 2014 award with delivery in Freight Actual freight costs will be prepaid and added to the order. F.O.B. Point is Gallatin, TN. Delivery Schedule Lead-time is 2 weeks after receipt of order but is subject to review of shop loading at the time of order. Proposal Validity This proposal is valid for your acceptance for 30 days. Commercial Conditions
107 107 Kissick Engineered Products & Services, Inc W. Fair Ave. Lancaster, OH fax Warranty New Products manufactured by WMNA shall be free of defects in material and workmanship for a period of one (1) year from the date of shipment of the Product by WMNA to Buyer; WMNA does not warrant components of WMNA Products, which are not manufactured by WMNA. Refer to WMNA's General Conditions of Sale for complete warranty details. Comments, Clarifications or Exceptions Equipment is based upon customer duty conditions only. The Weir Cavex model 150CVX6 Polyurethane Cyclone is a good candidate to handle the 100 GPM and resist heavy corrosion. Quote DOES NOT include: Off Loading Equipment Power and / or Control Cables Equipment Support Framework or Structure Automation and Instrumentation Field Erection and Installation Pressure or Performance Monitors (Except as Noted herin) Any Item not Specifically Noted Herein Attached documentation is for reference purposes only. If you would like to proceed with this order please address your Purchase Order as follows: Kissick EPS, Inc West Fair Ave Lancaster, OH FAX Thank you for this valued opportunity to present our proposal, we trust that we have assessed your requirements correctly and that our proposal meets with your approval. Please do not hesitate to contact us should you require any additional information or clarification. Kind Regards, Brad for Jon House
108 108 Warman International CAVEX Flow Curve Model : 150CVX6 Ref : 150V6-41 Feed Chamber Liner Vortex Finder Part No. Diameter Part No. Diameter 06035CVX41 41 mm 06080CVX40 40 mm 06080CVX50 50 mm 06080CVX60 60 mm
109 APPENDIX C - FORTRAN CODE FOR TRANSFER FUNCTION BETWEEN THE BP = AP BT = AT BW = AW AMBIENT AND SUPERCRITICAL AREA 109
110 110 APPENDIX D - FORTRAN CODE FOR CHANGE IN ION ENTHALPY DUE TO TEMPERATURE AND REACTION/SOLIDS PRODUCED IONTOT = NA + CA + MG + BA + SR + FE + MN Ion1 = -(NA* CA* MG* CL*-98.4) Ion1a = -(FE* MN* SR* Ba*-57.87) IONHEAT = (Ion1+Ion1a)* /3600 Ion2 = -(NA* CA* MG* CL*-57.11) Ion2a = -(FE* MN* SR* Ba*-16.93) IONHEAT2 = (Ion2+Ion2a)* /3600 rhoscw = Tscw = NaSat = 10**( *LOG10(rhoscw) - (1233.4/Tscw)) NaSatmol = NaSat*(1.0/ )*(1.0/55.51) NaSatTot = NaSatmol*flow NaP = Na - NaSattot if (NaSatTot.gt.0) then NaREA = (-NaP * * )/ else NaREA = 0 end if MGCAF = 10**( *LOG10(rhoscw)) Kmg = MGCAF/(rhoscw**3.44) Mgp = Mg*(1-Kmg) MGREA = (-Mgp* * )/ CACAF = 10**((-441.0/Tscw) *LOG10(rhoscw)) Kca = CACAF/(rhoscw**2.52) Cap = CA*(1-Kca) CAREA = (-CAP* * )/3600.0
111 IONREA = NaREA + MGRea + CaRea 111
112 112 APPENDIX E - UV UNIT QUOTATION Website: gayq#.vpiagpnf-t8
113 113 APPENDIX F - PLASTIC TANK Website:
114 114 APPENDIX G - PUMP QUOTATION 1311 Freese Works Place, Galion, OH Phone: Fax: East 76th Street, Cincinnati, OH Phone: Fax: Power Unit Quotation April 28, 2014 To: Xiao Company: Ohio University xd386012@ohio.edu Ph: Power Unit Quote # System Specifications Application Flow: 8.5 GPM Very Salty Ambient Fluid Temp Pressure: 3750 PSI HP: E.B.H.P. Unit Specifications Pump: Triplex Plunger Pump #3501 Standard Features Include: Die Cast Aluminum Crankcase, Stainless Steel Valves and Seats, Chrome-Moly Crankshaft, Oversized Bearings, 316SS Inlet Manifold, Duplex Stainless Steel Discharge Head, Buna Seal Material 1-1/2 NPTF x 3/4 NPTF Ports Motor: 25 HP, 3 Phase, /460 Volt, 60 Htz, TEFC, 1750 RPM, # T Frame, Inverter Duty, Weg Motor Brand Base: Steel Epoxy Coated Base Plate and Belt Guard # Remote oil drain. Bolt Down Shock Mounts Base Plate Dimensions 50 L x 30 W Drive Package: # R Includes: Sheaves, Hubs, Belts, and Keys. Pump will be running at RPM ** Power unit will be performance tested and accessories set to system specifications **
115 115 Regulator The main pressure regulator, use to control the system discharge pressure SS Construction, 2000 to 4000 PSI, 2.5 to 25 GPM User will supply the bypass hose (*) Pressure Gauge The pressure gauge is glycerin filled for consistent, accurate readings. A built Model 6087 in snubber reduces pressure fluctuations. Stainless Steel Material, 0 to 6000 PSI (*) Back-Up/Relief ValvThe relief valve provides back up protection to assure complete pressure Model relief for maximum system protection. The relief valve is set approximately 300 psi over operating system pressure. 304SS Material, PSI Installation A flooded inlet to 70 PSI is recommended for optimum performance. The supply tank should be 6 to 10 times the system capacity with a least two baffles. At least 3 feet of flexible hose is recommended on the inlet and discharge of the pump. Maximum fluid temperature, for the pump, is 160 degrees F. A qualified electrician should perform all electrical connections. If using reclaim water it is recommended to filter down to 40 microns, or 5 micron if reclaim contains abrasives. Service and Training Service Video Video training for rebuilding fluid end. Standard with unit. Service/Spec. Manuals Data sheet with all individual components and a Standard with unit. Service manual to rebuild fluid end. Technical Support Inside technical sales staff to assist with technical questions **Sample Photo. Photo may not reflect your exact quotation** User needs to supply the motor starter Customer needs to confirm the fluid compatibility to stainless steel material, and seal material Experimental Unit
116 116 Options: Pulsation Dampener add $1, Net EA Stainless Steel, NBR(Buna) Bladder Material, Pre-charged at Cat Pumps Would be mounted near pump discharge port. Would assist in reducing the discharge pressure spikes Net Pricing Your Net Pricing on the Power Unit Listed Above $22, Net Ea The above price is F.O.B. Minneapolis, MN Approximate Weight 850 lbs This Power Unit Quote is valid for 90 Days. Delivery, 4 to 5 Week, Est Please advise freight carrier Thank you, for allowing me the opportunity to quote this unit to you. I look forward to working with you on this Power Unit project. Please contact me if you have any questions or comments regarding this quote. Best Regards, Zach Baker Application Engineer zbaker@buckeyepumps.com Buckeye Pumps, Inc.
117 117 APPENDIX H - GENERATION/TRUCK QUOTES GTG_1614EM_SGT400Mobiles_60Hz.doc CFAS Enterprises Inc. After Market Utility Power Equipment Brokerage Mailto:Staff@CFASPower.com Turbine URL: Reciprocating URL: 10 x New Siemens SGT MW 60Hz (DF) Gas Turbine Mobile Generator Set
118 118 Asking Price: 7.375MM (British Pounds) Each Download Pdf (Large Document-May Take Some Time)
119 119 CFAS Enterprises Inc. After Market Utility Power Equipment Brokerage Turbine URL: Reciprocating URL: GTG_1291SB_Orenda.doc I Used 5.5MW Trailer Mounted Orenda OFT-370 Gas Turbine Generator Unit Asking Price: USD $300,000 Plus Delivery Will require some repairs to make operational. Gas Turbine Model: Orenda OFT-370 Gas Turbine Engine Serial Number: 5513 Generator Serial Number: Truck Cost: Website: Trucks-Teacher-Notes.pdf
120 !!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!! Thesis and Dissertation Services!
Evaluation of Abandoned Mine Drainage as a water supply for hydraulic fracturing
 Evaluation of Abandoned Mine Drainage as a water supply for hydraulic fracturing E. Barbot, M. Li, K. Gregory, R. Vidic University of Pittsburgh Carnegie Mellon University Project funded by the US Department
Evaluation of Abandoned Mine Drainage as a water supply for hydraulic fracturing E. Barbot, M. Li, K. Gregory, R. Vidic University of Pittsburgh Carnegie Mellon University Project funded by the US Department
The Use of Electro-Coagulation Technology to Treat Hydrofracturing Flowback Water and Other Oil and Gas Field Wastewaters
 The Use of Electro-Coagulation Technology to Treat Hydrofracturing Flowback Water and Other Oil and Gas Field Wastewaters Randy D. Horsak, PE Principal Engineer Marcellus Shale Water Group, LLC Houston,
The Use of Electro-Coagulation Technology to Treat Hydrofracturing Flowback Water and Other Oil and Gas Field Wastewaters Randy D. Horsak, PE Principal Engineer Marcellus Shale Water Group, LLC Houston,
George E. King SPE GCS 27 October 2011
 Treating Produced Water For Shale Fracs A U S T R A L I A A R G E N T I N A C A N A D A E G Y P T N O R T H S E A U. S. C E N T R A L U. S. G U L F George E. King SPE GCS 27 October 2011 Horn River 5000
Treating Produced Water For Shale Fracs A U S T R A L I A A R G E N T I N A C A N A D A E G Y P T N O R T H S E A U. S. C E N T R A L U. S. G U L F George E. King SPE GCS 27 October 2011 Horn River 5000
Water / Fluid Treatment Technologies and the Art of Applying Technology to Address Reuse and Recycling Challenges in the Oil and Gas Field
 Water / Fluid Treatment Technologies and the Art of Applying Technology to Address Reuse and Recycling Challenges in the Oil and Gas Field Thomas Steinke Johnson Screens Global Applications Manager Fluid
Water / Fluid Treatment Technologies and the Art of Applying Technology to Address Reuse and Recycling Challenges in the Oil and Gas Field Thomas Steinke Johnson Screens Global Applications Manager Fluid
Collection and Treatment of Flowback and Produced Waters from Hydraulic Fracturing. Edwin Pinero Veolia Water North America
 Collection and Treatment of Flowback and Produced Waters from Hydraulic Fracturing Edwin Pinero Veolia Water North America The Issue Oil and gas production using enhanced techniques is NOT new Hydraulic
Collection and Treatment of Flowback and Produced Waters from Hydraulic Fracturing Edwin Pinero Veolia Water North America The Issue Oil and gas production using enhanced techniques is NOT new Hydraulic
EXPERIENCES IN SHALE WASTE WATER MANAGEMENT An Update on the Issues Associated with Water in the United States Shale Gas Sector
 Shale Gas: A Game changer in Energy May 17, 2013 EXPERIENCES IN SHALE WASTE WATER MANAGEMENT An Update on the Issues Associated with Water in the United States Shale Gas Sector Devesh Sharma Managing Director
Shale Gas: A Game changer in Energy May 17, 2013 EXPERIENCES IN SHALE WASTE WATER MANAGEMENT An Update on the Issues Associated with Water in the United States Shale Gas Sector Devesh Sharma Managing Director
RECENT DEVELOPMENTS AND OPPORTUNITIES FOR RE-USE OF PRODUCED WATER. Presented by: Richard Bost, P.E., P.G. (Texas) I2M Associates
 RECENT DEVELOPMENTS AND OPPORTUNITIES FOR RE-USE OF PRODUCED WATER Presented by: Richard Bost, P.E., P.G. (Texas) I2M Associates 1 Co-Authors and Acknowledgements Many thanks to those who did the most
RECENT DEVELOPMENTS AND OPPORTUNITIES FOR RE-USE OF PRODUCED WATER Presented by: Richard Bost, P.E., P.G. (Texas) I2M Associates 1 Co-Authors and Acknowledgements Many thanks to those who did the most
The Application of Produced Water Treatment and Water Blending in Shale Resource Development
 The Application of Produced Water Treatment and Water Blending in Shale Resource Development Authors: J. D. Arthur, P.E., SPEC; and David Alleman New York Water Environment Association 2013 Spring Technical
The Application of Produced Water Treatment and Water Blending in Shale Resource Development Authors: J. D. Arthur, P.E., SPEC; and David Alleman New York Water Environment Association 2013 Spring Technical
OsmoBC Integrated Membrane Systems
 OsmoBC Integrated Membrane Systems For Industrial Wastewater Treatment Fluid Technology Solutions, Inc. OsmoF2O FO Membranes HBCR High Brine Concentrator OsmoZLD Treatment Process INTEGRA Disk Filtration
OsmoBC Integrated Membrane Systems For Industrial Wastewater Treatment Fluid Technology Solutions, Inc. OsmoF2O FO Membranes HBCR High Brine Concentrator OsmoZLD Treatment Process INTEGRA Disk Filtration
Water/Energy Sustainability Symposium 2010 Annual Forum Pittsburgh, Pennsylvania September 26-29, 2010
 CONSIDERATIONS FOR TREATING WATER ASSOCIATED WITH SHALE GAS DEVELOPMENT GROUNDWATER PROTECTION COUNCIL Water/Energy Sustainability Symposium 2010 Annual Forum Pittsburgh, Pennsylvania September 26-29,
CONSIDERATIONS FOR TREATING WATER ASSOCIATED WITH SHALE GAS DEVELOPMENT GROUNDWATER PROTECTION COUNCIL Water/Energy Sustainability Symposium 2010 Annual Forum Pittsburgh, Pennsylvania September 26-29,
Techno-economic Assessment of Water Management Solutions
 Techno-economic Assessment of Water Management Solutions Shale Gas Water Management Marcellus Initiative 2011 Dr. Radisav Vidic, University of Pittsburgh Dr. Tom Hayes, Gas Technology Institute Steve Hughes
Techno-economic Assessment of Water Management Solutions Shale Gas Water Management Marcellus Initiative 2011 Dr. Radisav Vidic, University of Pittsburgh Dr. Tom Hayes, Gas Technology Institute Steve Hughes
We weren t planning to talk about it, but since you asked... CEE With special thanks to Dr. Brian Rahm of the NY State Water Resources Institute
 We weren t planning to talk about it, but since you asked... CEE 3510 With special thanks to Dr. Brian Rahm of the NY State Water Resources Institute Hydraulic fracturing ( fracking ) is used to enhance
We weren t planning to talk about it, but since you asked... CEE 3510 With special thanks to Dr. Brian Rahm of the NY State Water Resources Institute Hydraulic fracturing ( fracking ) is used to enhance
Detail on Concentrate Handling and Disposal Options
 Detail on Concentrate Handling and Disposal Options A number of options are available for disposing of concentrate including direct disposal as well as additional handling and/or treatment designed to
Detail on Concentrate Handling and Disposal Options A number of options are available for disposing of concentrate including direct disposal as well as additional handling and/or treatment designed to
CHALLENGES AND ADVANCEMENTS IN PRODUCED WATER REUSE AND RECYCLING
 CHALLENGES AND ADVANCEMENTS IN PRODUCED WATER REUSE AND RECYCLING MATTHEW E. MANTELL, P.E. ENGINEERING ADVISOR - COMPLETIONS TECHNOLOGY 2013 OKLAHOMA GOVERNORS WATER CONFERENCE PRESENTATION OVERVIEW Chesapeake
CHALLENGES AND ADVANCEMENTS IN PRODUCED WATER REUSE AND RECYCLING MATTHEW E. MANTELL, P.E. ENGINEERING ADVISOR - COMPLETIONS TECHNOLOGY 2013 OKLAHOMA GOVERNORS WATER CONFERENCE PRESENTATION OVERVIEW Chesapeake
EMERGING CHALLENGES IN PRODUCED WATER MANAGEMENT
 EMERGING CHALLENGES IN PRODUCED WATER MANAGEMENT MARCH 22, 2017 David Lampert, Assistant Professor Civil & Environmental Engineering Oklahoma State University PRESENTATION OVERVIEW Oil and gas production
EMERGING CHALLENGES IN PRODUCED WATER MANAGEMENT MARCH 22, 2017 David Lampert, Assistant Professor Civil & Environmental Engineering Oklahoma State University PRESENTATION OVERVIEW Oil and gas production
Aquatech Energy Services
 Aquatech Energy Services Upstream Oil & Gas Water Management Services Total Water Management Total Water Management For Oil & Gas Producers More Than 30 Years Experience Serving The Oil & Gas Industry
Aquatech Energy Services Upstream Oil & Gas Water Management Services Total Water Management Total Water Management For Oil & Gas Producers More Than 30 Years Experience Serving The Oil & Gas Industry
Current NETL Water Management R&D Efforts Drilling Engineering Association Quarterly Forum
 Current NETL Water Management R&D Efforts Drilling Engineering Association Quarterly Forum Bill Pike, LTI/NETL MASTER Module 4b Outline Introduction to NETL Shale/Water Issues Overview Updates on Selected
Current NETL Water Management R&D Efforts Drilling Engineering Association Quarterly Forum Bill Pike, LTI/NETL MASTER Module 4b Outline Introduction to NETL Shale/Water Issues Overview Updates on Selected
PREPARED BY KEN PANDYA AWTS, INC TRIPLE CROWN LN PLANO, TX OFFICE: APRIL 11, 2012
 HIGH EFFICIENCY SOFTENING PROCESS (HESP ) FOR FRACK FLOWBACK / PRODUCED WATER TREATMENT SPONSORED BY OGRES, IN ASSOCIATION WITH CITY OF GRAND PRAIRIE, TEXAS PREPARED BY KEN PANDYA AWTS, INC. 1817 TRIPLE
HIGH EFFICIENCY SOFTENING PROCESS (HESP ) FOR FRACK FLOWBACK / PRODUCED WATER TREATMENT SPONSORED BY OGRES, IN ASSOCIATION WITH CITY OF GRAND PRAIRIE, TEXAS PREPARED BY KEN PANDYA AWTS, INC. 1817 TRIPLE
Green FSRU for the future
 Green FSRU for the future Presentation at GREEN4SEA Athens April 6 th 2016 Dr. John Kokarakis Vice President Technology & Business Development, Africa, S. Europe Hellenic, Black Sea & Middle East Zone
Green FSRU for the future Presentation at GREEN4SEA Athens April 6 th 2016 Dr. John Kokarakis Vice President Technology & Business Development, Africa, S. Europe Hellenic, Black Sea & Middle East Zone
The Next Oilfield Step:
 The Next Oilfield Step: A Circular Economy Approach to Reuse, Recycle, and Reduce Production Water IADC ART SPARK Tank Houston, Texas 04-April-2018 A Fresh (Water) Opportunity Supply Side THE Limiting
The Next Oilfield Step: A Circular Economy Approach to Reuse, Recycle, and Reduce Production Water IADC ART SPARK Tank Houston, Texas 04-April-2018 A Fresh (Water) Opportunity Supply Side THE Limiting
OriginClear Water Treatment with Innovative Desalination Techniques High TDS Water Treatment for Oil & Gas Markets in Oman
 OriginClear Water Treatment with Innovative Desalination Techniques High TDS Water Treatment for Oil & Gas Markets in Oman A Technology & A Process Electro Water Separation (EWS) A first stage technology
OriginClear Water Treatment with Innovative Desalination Techniques High TDS Water Treatment for Oil & Gas Markets in Oman A Technology & A Process Electro Water Separation (EWS) A first stage technology
New Technologies for Gas Exploration and Production
 New Technologies for Gas Exploration and Production D.B. Burnett Global Petroleum Research Institute Texas A&M University Rich Haut Houston Area Research Center Tom Williams Matagorda Redfish Society GPRI
New Technologies for Gas Exploration and Production D.B. Burnett Global Petroleum Research Institute Texas A&M University Rich Haut Houston Area Research Center Tom Williams Matagorda Redfish Society GPRI
Characterization of Marcellus Shale and Barnett Shale Flowback Waters and Technology Development for Water Reuse
 Characterization of Marcellus Shale and Barnett Shale Flowback Waters and Technology Development for Water Reuse Tom Hayes, Gas Technology Institute USEPA Shale Gas Hydraulic Fracturing Workshop #4 Arlington,
Characterization of Marcellus Shale and Barnett Shale Flowback Waters and Technology Development for Water Reuse Tom Hayes, Gas Technology Institute USEPA Shale Gas Hydraulic Fracturing Workshop #4 Arlington,
Shale gas operation & water resource preservation. PECC, November 2013
 Shale gas operation & water resource preservation PECC, November 2013 Part I Veolia and the oil and gas industry Water, a very sensitive raw material for oil and gas firms Oil and gas firms use more of
Shale gas operation & water resource preservation PECC, November 2013 Part I Veolia and the oil and gas industry Water, a very sensitive raw material for oil and gas firms Oil and gas firms use more of
Management of Water from CCS Project Number 49607
 Management of Water from CCS Project Number 49607 Christopher Harto Argonne National Laboratory U.S. Department of Energy National Energy Technology Laboratory Carbon Storage R&D Project Review Meeting
Management of Water from CCS Project Number 49607 Christopher Harto Argonne National Laboratory U.S. Department of Energy National Energy Technology Laboratory Carbon Storage R&D Project Review Meeting
Novel Treatment Technologies for Desalination and Selective Ion Removal
 Novel Treatment Technologies for Desalination and Selective Ion Removal Lucy Mar Camacho, Ph.D. Department of Environmental Engineering Texas A&M University - Kingsville Eagle Ford Center for Research,
Novel Treatment Technologies for Desalination and Selective Ion Removal Lucy Mar Camacho, Ph.D. Department of Environmental Engineering Texas A&M University - Kingsville Eagle Ford Center for Research,
A Water Solutions Company
 STW Water Process & Technologies A Subsidiary of STW Resources Holding A Water Solutions Company OTCQB: STWS STW Water Process & Technologies Capabilities for Municipal & Industrial Applications STW WATER
STW Water Process & Technologies A Subsidiary of STW Resources Holding A Water Solutions Company OTCQB: STWS STW Water Process & Technologies Capabilities for Municipal & Industrial Applications STW WATER
Shale gas & Hydraulic fracturing. Dr. Jürg M. Matter Ocean & Earth Science University of Southampton
 Shale gas & Hydraulic fracturing Dr. Jürg M. Matter Ocean & Earth Science University of Southampton J.Matter@southampton.ac.uk Definitions unconventional hydrocarbons unconventional gas shale gas tight
Shale gas & Hydraulic fracturing Dr. Jürg M. Matter Ocean & Earth Science University of Southampton J.Matter@southampton.ac.uk Definitions unconventional hydrocarbons unconventional gas shale gas tight
Chemical Precipitation and Ballasted Flocculation
 Chemical Precipitation and Ballasted Flocculation Rationale to Recycle Fracing Flowback and Produced Water As the unconventional oil/gas industry faces an evolving regulatory environment, increasing public
Chemical Precipitation and Ballasted Flocculation Rationale to Recycle Fracing Flowback and Produced Water As the unconventional oil/gas industry faces an evolving regulatory environment, increasing public
J. Daniel Arthur, P.E., ALL Consulting. Author. Ground Water Protection Council January 2009 San Antonio, Texas. Presented at
 Prudent and Sustainable Water Management and Disposal Alternatives Applicable to Shale Gas Development Author J. Daniel Arthur, P.E., ALL Consulting Presented at Ground Water Protection Council January
Prudent and Sustainable Water Management and Disposal Alternatives Applicable to Shale Gas Development Author J. Daniel Arthur, P.E., ALL Consulting Presented at Ground Water Protection Council January
Drilling for Natural Gas in the Marcellus and Utica Shales: Environmental Regulatory Basics
 January 2014 Introduction This fact sheet provides a basic overview of natural gas drilling in the Marcellus and Utica Shale regions of Ohio and the potential environmental issues associated with these
January 2014 Introduction This fact sheet provides a basic overview of natural gas drilling in the Marcellus and Utica Shale regions of Ohio and the potential environmental issues associated with these
RECYCLING PRODUCED & FLOWBACK WASTEWATER FOR FRACKING TECHNOLOGY. Clarifying misconceptions on the obstacles for frac water reuse
 RECYCLING PRODUCED & FLOWBACK WASTEWATER FOR FRACKING by Eli Gruber, President & CEO Ecologix Environmental Systems Clarifying misconceptions on the obstacles for frac water reuse TECHNOLOGY 01110000 01110101
RECYCLING PRODUCED & FLOWBACK WASTEWATER FOR FRACKING by Eli Gruber, President & CEO Ecologix Environmental Systems Clarifying misconceptions on the obstacles for frac water reuse TECHNOLOGY 01110000 01110101
Zero Liquid Discharge for Water Remediation. Bill Berzins, M.A.Sc. P.Eng.
 Zero Liquid Discharge for Water Remediation Bill Berzins, M.A.Sc. P.Eng. Zero Liquid Discharge Strategy Technology Selection Case Studies Recommendations Project Portfolio: Design-Build-Operate-Transfer
Zero Liquid Discharge for Water Remediation Bill Berzins, M.A.Sc. P.Eng. Zero Liquid Discharge Strategy Technology Selection Case Studies Recommendations Project Portfolio: Design-Build-Operate-Transfer
Water Issues Relating to Unconventional Oil and Gas Production
 Water Issues Relating to Unconventional Oil and Gas Production John Veil 410 212 0950 john@veilenvironmental.com www.veilenvironmental.com National Research Council Workshop on the Development of Unconventional
Water Issues Relating to Unconventional Oil and Gas Production John Veil 410 212 0950 john@veilenvironmental.com www.veilenvironmental.com National Research Council Workshop on the Development of Unconventional
Shale Gas (D: unkonventionelles Erdgas )
 1 Shale Gas (D: unkonventionelles Erdgas ) Aus: W. Zittel (ASPO) Kurzstudie Unkonventionelles Erdgas, 2010 Shale + Tight Gas (worldwide resources) In total: shale gas volume convential gas tight gas 50%
1 Shale Gas (D: unkonventionelles Erdgas ) Aus: W. Zittel (ASPO) Kurzstudie Unkonventionelles Erdgas, 2010 Shale + Tight Gas (worldwide resources) In total: shale gas volume convential gas tight gas 50%
Addressing increasing wastewater volumes in industrial and oil & gas operations using thermal systems
 Water Arabia 2013 Conference & Exhibition Innovative Water and Wastewater Technologies for a Sustainable Environment 4 to 6 February, 2013 Le Meridien Khobar, Saudi Arabia Addressing increasing wastewater
Water Arabia 2013 Conference & Exhibition Innovative Water and Wastewater Technologies for a Sustainable Environment 4 to 6 February, 2013 Le Meridien Khobar, Saudi Arabia Addressing increasing wastewater
THE MARCELLUS SHALE AN ENERGY GAME
 THE MARCELLUS SHALE AN ENERGY GAME CHANGER? James Patrick Dougherty McNees Wallace & Nurick LLC October 4, 2010 2010 McNees Wallace & Nurick LLC McNees Wallace & Nurick LLC is a fullservice law firm that
THE MARCELLUS SHALE AN ENERGY GAME CHANGER? James Patrick Dougherty McNees Wallace & Nurick LLC October 4, 2010 2010 McNees Wallace & Nurick LLC McNees Wallace & Nurick LLC is a fullservice law firm that
Product Models & Specifications
 water Treatment Technology for the Upstream Oil & Gas Industry Business Philosophy The AquaTex COG product line is proprietary reclamation, pretreatment, advanced treatment and recycling technology designed
water Treatment Technology for the Upstream Oil & Gas Industry Business Philosophy The AquaTex COG product line is proprietary reclamation, pretreatment, advanced treatment and recycling technology designed
Advanced Treatment of Flowback Water Using Magnetic Ballast Clarification and Vortex Generating Membrane Systems for Discharge
 Advanced Treatment of Flowback Water Using Magnetic Ballast Clarification and Vortex Generating Membrane Systems for Discharge Compensation Committee Report Ben Pakzadeh, Ph.D., P.E. Presented to: Marcellus,
Advanced Treatment of Flowback Water Using Magnetic Ballast Clarification and Vortex Generating Membrane Systems for Discharge Compensation Committee Report Ben Pakzadeh, Ph.D., P.E. Presented to: Marcellus,
Changes in the Quantity and Quality of Produced Water from Appalachian Shale Energy Development and their Implications for Water Reuse
 Changes in the Quantity and Quality of Produced Water from Appalachian Shale Energy Development and their Implications for Water Reuse Radisav D. Vidic Department of Civil and Environmental Engineering,
Changes in the Quantity and Quality of Produced Water from Appalachian Shale Energy Development and their Implications for Water Reuse Radisav D. Vidic Department of Civil and Environmental Engineering,
VOLUME 06 ISSUE 07-JULY 2013
 VOLUME 06 ISSUE 07-JULY 2013 Michael Dejak, Eco-Tec Inc., Canada, unveils a next generation water filtration technology for the oil and gas industry. T here is a need in many segments of the oil and gas
VOLUME 06 ISSUE 07-JULY 2013 Michael Dejak, Eco-Tec Inc., Canada, unveils a next generation water filtration technology for the oil and gas industry. T here is a need in many segments of the oil and gas
Arthur W. Rose Professor Emeritus of Geochemistry Penn State University
 Arthur W. Rose Professor Emeritus of Geochemistry Penn State University Flowback Chemistry of flowback Source of flowback brine Environmental Problems Evan Dresel MS 1985 Conventional brines, recognition
Arthur W. Rose Professor Emeritus of Geochemistry Penn State University Flowback Chemistry of flowback Source of flowback brine Environmental Problems Evan Dresel MS 1985 Conventional brines, recognition
Modutech S.r.l. WDS SEAWATER DROPLET SYSTEM FOR FRESH WATER SUPPLY. Ing. Alessandro Cariani
 Modutech S.r.l. WDS SEAWATER DROPLET SYSTEM FOR FRESH WATER SUPPLY Ing. Alessandro Cariani The world's water consumption rate is doubling every 20 years, outpacing by two times the rate of population growth.
Modutech S.r.l. WDS SEAWATER DROPLET SYSTEM FOR FRESH WATER SUPPLY Ing. Alessandro Cariani The world's water consumption rate is doubling every 20 years, outpacing by two times the rate of population growth.
THESIS PRECIPITATION AND REMOVAL OF IONIC COMPOUNDS FROM PRODUCED WATER: OBSERVED VERSUS MODELED RESULTS. Submitted by.
 THESIS PRECIPITATION AND REMOVAL OF IONIC COMPOUNDS FROM PRODUCED WATER: OBSERVED VERSUS MODELED RESULTS Submitted by Xiaochen Yang Department of Civil and Environmental Engineering In partial fulfillment
THESIS PRECIPITATION AND REMOVAL OF IONIC COMPOUNDS FROM PRODUCED WATER: OBSERVED VERSUS MODELED RESULTS Submitted by Xiaochen Yang Department of Civil and Environmental Engineering In partial fulfillment
Approaches with Recycle, Treatment, and Disposal of Flowback and Produced Water and the ABCs of Managing NORM in the Marcellus Shale Region
 Approaches with Recycle, Treatment, and Disposal of Flowback and Produced Water and the ABCs of Managing NORM in the Marcellus Shale Region Mark Gannon, PE, PMP Alex Lopez, CHP AMEC Environment & Infrastructure
Approaches with Recycle, Treatment, and Disposal of Flowback and Produced Water and the ABCs of Managing NORM in the Marcellus Shale Region Mark Gannon, PE, PMP Alex Lopez, CHP AMEC Environment & Infrastructure
SECTION 1. The Technology Behind Recycling Produced Water and Hydraulic Fracturing Flowback Fluid
 SECTION 1 The Technology Behind Recycling Produced Water and Hydraulic Fracturing Flowback Fluid Presented by Dr. David Stewart, PE Stewart Environmental Consultants, LLC Fort Collins, CO The Technology
SECTION 1 The Technology Behind Recycling Produced Water and Hydraulic Fracturing Flowback Fluid Presented by Dr. David Stewart, PE Stewart Environmental Consultants, LLC Fort Collins, CO The Technology
Project Name: Melut Basin Oil Development. Client Name: PDOC, Petrolium. Development Oman Company. EPC: Dodsal. Consultant: Mott MacDonald
 Produced Water Treatment Project Profile Project Name: Melut Basin Oil Development Client Name: PDOC, Petrolium EPC: Dodsal Consultant: Mott MacDonald Plant Location: Adar/ Agordeed Field Scope: Design,
Produced Water Treatment Project Profile Project Name: Melut Basin Oil Development Client Name: PDOC, Petrolium EPC: Dodsal Consultant: Mott MacDonald Plant Location: Adar/ Agordeed Field Scope: Design,
Summary: Saltworks Technologies
 Summary: There are four types of shale waters, each with unique properties, uses, and treatment approaches. Economic shale water management strategies require you to explore: treatment options, treatment
Summary: There are four types of shale waters, each with unique properties, uses, and treatment approaches. Economic shale water management strategies require you to explore: treatment options, treatment
Chemical precipitation out performs filtration in flowback and produced water recycling operations.
 Chemical precipitation out performs filtration in flowback and produced water recycling operations. Abstract The Shalewater Solutions water recycling approach harmonizes technology and simplicity. Treatment
Chemical precipitation out performs filtration in flowback and produced water recycling operations. Abstract The Shalewater Solutions water recycling approach harmonizes technology and simplicity. Treatment
Saving Energy and Water. Working with High Recovery Water Treatment Plants
 Saving Energy and Water Working with High Recovery Water Treatment Plants > Saving Energy and Water Working with High Recovery Treatment Plants Reverse Osmosis - Benefits o Reliable and Consistent Technology
Saving Energy and Water Working with High Recovery Water Treatment Plants > Saving Energy and Water Working with High Recovery Treatment Plants Reverse Osmosis - Benefits o Reliable and Consistent Technology
Placeholder for photo
 Placeholder for photo Consultancy and Construction Firm 7500 staff worldwide, 1800 staff UK Water and Energy Bringing Our Global Unconventional Experience to the UK Hydrocarbon Strategy and Water/Brine
Placeholder for photo Consultancy and Construction Firm 7500 staff worldwide, 1800 staff UK Water and Energy Bringing Our Global Unconventional Experience to the UK Hydrocarbon Strategy and Water/Brine
Overview. Heavy Metal Removal Ceramic Microfiltration Presentation. Brackish Water as a New Water Resource For Developing Domestic Energy
 Brackish Water as a New Water Resource For Developing Domestic Energy Energy issues with water and its associated costs for development Drought conditions are requiring the investigation of different water
Brackish Water as a New Water Resource For Developing Domestic Energy Energy issues with water and its associated costs for development Drought conditions are requiring the investigation of different water
IWC ZLD: New Silica Based Inhibitor Chemistry Permits Cost Effective Water Conservation for HVAC and Industrial Cooling Towers
 IWC 07-11 ZLD: New Silica Based Inhibitor Chemistry Permits Cost Effective Water Conservation for HVAC and Industrial Cooling Towers Report by Dan Duke Water Conservation Technology International water-cti.com
IWC 07-11 ZLD: New Silica Based Inhibitor Chemistry Permits Cost Effective Water Conservation for HVAC and Industrial Cooling Towers Report by Dan Duke Water Conservation Technology International water-cti.com
NEMC Challenges of Managing Water in the Marcellus Play. Nick Inkenhaus Water Resources Engineer
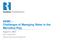 NEMC Challenges of Managing Water in the Marcellus Play August 5, 2013 December 22, 2011 Nick Inkenhaus Water Resources Engineer Water Requirements for Marcellus Shale Completion typically requires 3-5
NEMC Challenges of Managing Water in the Marcellus Play August 5, 2013 December 22, 2011 Nick Inkenhaus Water Resources Engineer Water Requirements for Marcellus Shale Completion typically requires 3-5
Carden. CARDEN WATER SYSTEMS, LLC 3440 E. Broadway Phoenix, Arizona Water Systems. Industry Applications
 Carden Water Systems Industry Applications CARDEN WATER SYSTEMS, LLC 3440 E. Broadway Phoenix, Arizona 85040 Carden-A New Competitive Advantage CARDEN Water Systems, LLC (CWS), an Arizona LLC, is based
Carden Water Systems Industry Applications CARDEN WATER SYSTEMS, LLC 3440 E. Broadway Phoenix, Arizona 85040 Carden-A New Competitive Advantage CARDEN Water Systems, LLC (CWS), an Arizona LLC, is based
Water Technologies & Solutions Water Technologies & Solutions. water treatment solutions for unconventional fuels
 Water Technologies & Solutions Water Technologies & Solutions water treatment solutions for unconventional fuels addressing today s challenges Today, corporations, individuals and governmental bodies are
Water Technologies & Solutions Water Technologies & Solutions water treatment solutions for unconventional fuels addressing today s challenges Today, corporations, individuals and governmental bodies are
Lifecycle Water Management Considerations & Challenges for Marcellus Shale Gas Development
 Lifecycle Water Management Considerations & Challenges for Marcellus Shale Gas Development Author: J. Daniel Arthur, P.E., ALL Consulting Presented at: The Independent Oil & Gas Association of New York
Lifecycle Water Management Considerations & Challenges for Marcellus Shale Gas Development Author: J. Daniel Arthur, P.E., ALL Consulting Presented at: The Independent Oil & Gas Association of New York
New York State Regulatory/Permitting Process and Practical Considerations for Publicly Owned Treatment Works (POTWs) to Treat Flowback Water
 New York State Regulatory/Permitting Process and Practical Considerations for Publicly Owned Treatment Works (POTWs) to Treat Flowback Water Presented by: Elizabeth M. Davis Rodney L. Aldrich, P.E. Sterling
New York State Regulatory/Permitting Process and Practical Considerations for Publicly Owned Treatment Works (POTWs) to Treat Flowback Water Presented by: Elizabeth M. Davis Rodney L. Aldrich, P.E. Sterling
Water supplied by Marafiq does not meet the process requirements.
 WATER TREATMENT In Industries Water is used for: a. Drinking b. Cleaning c. Cooling d. Producing Steam e. Process requirement Why we need to treat water? For human consumption a. Water to be purified (Make
WATER TREATMENT In Industries Water is used for: a. Drinking b. Cleaning c. Cooling d. Producing Steam e. Process requirement Why we need to treat water? For human consumption a. Water to be purified (Make
POREX Tubular Membrane Filter (TMF ) Applied in a ZLD System as Critical Solid/Liquid Separation Process
 POREX Tubular Membrane Filter (TMF ) Applied in a ZLD System as Critical Solid/Liquid Separation Process Abstract Introduction Beijing Shougang Biomass Energy Technology Co., Ltd, a branch company of SHOUGANG
POREX Tubular Membrane Filter (TMF ) Applied in a ZLD System as Critical Solid/Liquid Separation Process Abstract Introduction Beijing Shougang Biomass Energy Technology Co., Ltd, a branch company of SHOUGANG
Opportunities and Freight Transportation Needs from Marcellus and Utica in Ohio
 Opportunities and Freight Transportation Needs from Marcellus and Utica in Ohio Ohio Conference on Freight Toledo, Ohio September 20, 2011 Founded in 1990 to provide professional services for port, multi
Opportunities and Freight Transportation Needs from Marcellus and Utica in Ohio Ohio Conference on Freight Toledo, Ohio September 20, 2011 Founded in 1990 to provide professional services for port, multi
A s populations increase and sources of highquality
 E-249 04-10 Desalination Methods for Producing Drinking Water *Justin K. Mechell and Bruce Lesikar A s populations increase and sources of highquality fresh drinking water decrease, many communities have
E-249 04-10 Desalination Methods for Producing Drinking Water *Justin K. Mechell and Bruce Lesikar A s populations increase and sources of highquality fresh drinking water decrease, many communities have
Thermal Distillation Technology for Management of Produced Water and Frac Flowback Water
 Water Technology Brief #2008-1 Thermal Distillation Technology for Management of Produced Water and Frac Flowback Water John A. Veil 1 Argonne National Laboratory May 13, 2008 Thermal distillation uses
Water Technology Brief #2008-1 Thermal Distillation Technology for Management of Produced Water and Frac Flowback Water John A. Veil 1 Argonne National Laboratory May 13, 2008 Thermal distillation uses
HEAVY INDUSTRY PLANT WASTEWATER TREATMENT, RECOVERY AND RECYCLE USING THREE MEMBRANE CONFIGURATIONS IN COMBINATION WITH AEROBIC TREATMENT A CASE STUDY
 HEAVY INDUSTRY PLANT WASTEWATER TREATMENT, RECOVERY AND RECYCLE USING THREE MEMBRANE CONFIGURATIONS IN COMBINATION WITH AEROBIC TREATMENT A CASE STUDY ABSTRACT Francis J. Brady Koch Membrane Systems, Inc.
HEAVY INDUSTRY PLANT WASTEWATER TREATMENT, RECOVERY AND RECYCLE USING THREE MEMBRANE CONFIGURATIONS IN COMBINATION WITH AEROBIC TREATMENT A CASE STUDY ABSTRACT Francis J. Brady Koch Membrane Systems, Inc.
A Modeling Framework for Shale Gas Wastewater Management. Jhih-Shyang Shih, Elaine Swiedler, Alan Krupnick
 A Modeling Framework for Shale Gas Wastewater Management Jhih-Shyang Shih, Elaine Swiedler, Alan Krupnick Motivation Wastewater flows growing even as production falls Well Drilled and Wastewater Generations
A Modeling Framework for Shale Gas Wastewater Management Jhih-Shyang Shih, Elaine Swiedler, Alan Krupnick Motivation Wastewater flows growing even as production falls Well Drilled and Wastewater Generations
Advanced technologies portfolio for Produced Water treatment and reuse proposed by VEOLIA
 Advanced technologies portfolio for Produced Water treatment and reuse proposed by VEOLIA M. HENDOU TECHNICAL DIRECTOR VEOLIA OIL & GAS GEOENERGY DAYS July 4, 2018 Produced water main components Water
Advanced technologies portfolio for Produced Water treatment and reuse proposed by VEOLIA M. HENDOU TECHNICAL DIRECTOR VEOLIA OIL & GAS GEOENERGY DAYS July 4, 2018 Produced water main components Water
Eutectic freeze crystallization: Application to process streams and waste water purification
 Chemical Engineering and Processing 37 (1998) 207 213 Eutectic freeze crystallization: Application to process streams and waste water purification F. van der Ham *, G.J. Witkamp, J. de Graauw, G.M. van
Chemical Engineering and Processing 37 (1998) 207 213 Eutectic freeze crystallization: Application to process streams and waste water purification F. van der Ham *, G.J. Witkamp, J. de Graauw, G.M. van
WATER AND SHALE GAS DEVELOPMENT
 WATER AND SHALE GAS DEVELOPMENT J. Daniel Arthur, P.E., PE SPEC Jon W. Seekins, Env. Sc. ALL Consulting National Association of Royalty Owners Annual Conference Pittsburgh, PA October 7, 2010 1 OVERVIEW
WATER AND SHALE GAS DEVELOPMENT J. Daniel Arthur, P.E., PE SPEC Jon W. Seekins, Env. Sc. ALL Consulting National Association of Royalty Owners Annual Conference Pittsburgh, PA October 7, 2010 1 OVERVIEW
The Use of Walnut Shell Filtration with Enhanced Synthetic Media for the Reduction and/or Elimination of Upstream Produced Water Treatment Equipment
 Siemens Water Solutions The Use of Walnut Shell Filtration with Enhanced Synthetic Media for the Reduction and/or Elimination of Upstream Produced Water Treatment Equipment White Paper January 2016 Researchers
Siemens Water Solutions The Use of Walnut Shell Filtration with Enhanced Synthetic Media for the Reduction and/or Elimination of Upstream Produced Water Treatment Equipment White Paper January 2016 Researchers
ISSN: [Utama et al., 8(1): January, 2019] Impact Factor: 5.164
![ISSN: [Utama et al., 8(1): January, 2019] Impact Factor: 5.164 ISSN: [Utama et al., 8(1): January, 2019] Impact Factor: 5.164](/thumbs/93/112396340.jpg) IJESRT INTERNATIONAL JOURNAL OF ENGINEERING SCIENCES & RESEARCH TECHNOLOGY DESALINATION REJECT WATER UTILIZATION for REVERSE OSMOSIS PLANT RAW WATER Komang Gede Nara Utama*, Eko Supriyanto*, Matradji**,
IJESRT INTERNATIONAL JOURNAL OF ENGINEERING SCIENCES & RESEARCH TECHNOLOGY DESALINATION REJECT WATER UTILIZATION for REVERSE OSMOSIS PLANT RAW WATER Komang Gede Nara Utama*, Eko Supriyanto*, Matradji**,
Water Treatment Technology
 Lecture 4: Membrane Processes Technology in water treatment (Part I) Water Treatment Technology Water Resources Engineering Civil Engineering ENGC 6305 Dr. Fahid Rabah PhD. PE. 1 Membrane Processes Technology
Lecture 4: Membrane Processes Technology in water treatment (Part I) Water Treatment Technology Water Resources Engineering Civil Engineering ENGC 6305 Dr. Fahid Rabah PhD. PE. 1 Membrane Processes Technology
Supercritical Water Coal Conversion with Aquifer-Based Sequestration of CO 2
 Supercritical Water Coal Conversion with Aquifer-Based Sequestration of CO 2 Profs. Reginald Mitchell, 1 Christopher Edwards 1 and Scott Fendorf 2 1 Mechanical Engineering Department 2 Department of Geological
Supercritical Water Coal Conversion with Aquifer-Based Sequestration of CO 2 Profs. Reginald Mitchell, 1 Christopher Edwards 1 and Scott Fendorf 2 1 Mechanical Engineering Department 2 Department of Geological
Hybrid RO & Softening Birjand Water Treatment Plant
 Hybrid RO & Softening Birjand Water Treatment Plant Ali Farahmand 1 *, Nassir Gifani 1, and Mohsen Farivar 1 1 ToossAb Consulting Engineers Co., Tehran, Iran (*correspondence: farahmandali@yahoo.com) FORMAT:
Hybrid RO & Softening Birjand Water Treatment Plant Ali Farahmand 1 *, Nassir Gifani 1, and Mohsen Farivar 1 1 ToossAb Consulting Engineers Co., Tehran, Iran (*correspondence: farahmandali@yahoo.com) FORMAT:
Treatability Study and Reverse Osmosis Pilot Study of Industrial Wastewater at a Wood Products Mill
 Treatability Study and Reverse Osmosis Pilot Study of Industrial Wastewater at a Wood Products Mill NC AWWA-WEA 2017 Annual Conference Randall Foulke, PE, BCEE, LEED AP Tracey Daniels, EI November 14,
Treatability Study and Reverse Osmosis Pilot Study of Industrial Wastewater at a Wood Products Mill NC AWWA-WEA 2017 Annual Conference Randall Foulke, PE, BCEE, LEED AP Tracey Daniels, EI November 14,
Understanding Pretreatment. WesTech Engineering, Inc. Salt Lake City, Utah, USA
 Understanding Pretreatment WesTech Engineering, Inc. Salt Lake City, Utah, USA Industrial Water Usage Water is required in almost every industry For: Cooling Boiler feed Process Drinking Cleaning In 2005
Understanding Pretreatment WesTech Engineering, Inc. Salt Lake City, Utah, USA Industrial Water Usage Water is required in almost every industry For: Cooling Boiler feed Process Drinking Cleaning In 2005
Revolution. NON-Chemical Treatment. Technology. for Scale Control. 500 different Applications NON TOXIC FOR POLLUTION PREVENTION AND GREEN TECHNOLOGY
 Revolution of NON-Chemical Treatment Technology for Scale Control in 500 different Applications NON TOXIC FOR POLLUTION PREVENTION AND GREEN TECHNOLOGY FILTERSORB SP3 Table of contents 1. Glossary of terms
Revolution of NON-Chemical Treatment Technology for Scale Control in 500 different Applications NON TOXIC FOR POLLUTION PREVENTION AND GREEN TECHNOLOGY FILTERSORB SP3 Table of contents 1. Glossary of terms
Industrial & Municipal Catalogue
 Industrial & Municipal Catalogue Low Maintenance Amiad filters are designed to perform and built to last. Low maintenance means high confidence, which allows our customers to focus on their core business.
Industrial & Municipal Catalogue Low Maintenance Amiad filters are designed to perform and built to last. Low maintenance means high confidence, which allows our customers to focus on their core business.
Utilizing Unconventional Water Sources for Industrial Reuse
 Technologies exist to economically treat any strength of acid mine drainage for industrial reuse. Utilizing Unconventional Water Sources for Industrial Reuse Reclamation and reuse of unconventional wastewater
Technologies exist to economically treat any strength of acid mine drainage for industrial reuse. Utilizing Unconventional Water Sources for Industrial Reuse Reclamation and reuse of unconventional wastewater
The Art of the Possible : Technical & Economic Challenges and Opportunities for Produced Water Recovery. Presented by JP Welch November 16, 2018
 The Art of the Possible : Technical & Economic Challenges and Opportunities for Produced Water Recovery Presented by JP Welch November 16, 2018 Veolia Water Technologies Veolia designs and implement solutions
The Art of the Possible : Technical & Economic Challenges and Opportunities for Produced Water Recovery Presented by JP Welch November 16, 2018 Veolia Water Technologies Veolia designs and implement solutions
Saltworks Technologies
 As regulations on FGD wastewater tighten, additional treatment is required. Often, this is chemically intense, and high cost. The best means to lower treatment costs is to reduce the volume of wastewater
As regulations on FGD wastewater tighten, additional treatment is required. Often, this is chemically intense, and high cost. The best means to lower treatment costs is to reduce the volume of wastewater
Fluid Mechanics, Heat Transfer, and Thermodynamics Fall Design Project. Production of Dimethyl Ether
 Fluid Mechanics, Heat Transfer, and Thermodynamics Fall 2001 Design Project Production of Dimethyl Ether We are investigating the feasibility of constructing a new, grass-roots, 50,000 tonne/y, (1 tonne
Fluid Mechanics, Heat Transfer, and Thermodynamics Fall 2001 Design Project Production of Dimethyl Ether We are investigating the feasibility of constructing a new, grass-roots, 50,000 tonne/y, (1 tonne
Chemical Engineering Plant Design 1
 Chemical Engineering Plant Design 1 Costing of Ethylene Oxide Plant CHE 4181 By: Abdullah Kurdi Khalid Almansoori Nasser Almakhmari Report Submitted: 12/2/2015 Instructor: Dr. Jonathan Whitlow Table of
Chemical Engineering Plant Design 1 Costing of Ethylene Oxide Plant CHE 4181 By: Abdullah Kurdi Khalid Almansoori Nasser Almakhmari Report Submitted: 12/2/2015 Instructor: Dr. Jonathan Whitlow Table of
Comparing Evaporative Technologies for the Recycling of Produced Waters
 Comparing Evaporative Technologies for the Recycling of Produced Waters Relatively recent advance refinements of evaporative technologies have enabled a cost effective solution for a variety of wastewater
Comparing Evaporative Technologies for the Recycling of Produced Waters Relatively recent advance refinements of evaporative technologies have enabled a cost effective solution for a variety of wastewater
Natural Gas Well Development in the Marcellus Shale: The Use of Fresh Water and Beyond
 Natural Gas Well Development in the Marcellus Shale: The Use of Fresh Water and Beyond Jason de Wolfe, Chief Oil and Gas Q: How much water does the industry use to develop the Marcellus Shale? Each well
Natural Gas Well Development in the Marcellus Shale: The Use of Fresh Water and Beyond Jason de Wolfe, Chief Oil and Gas Q: How much water does the industry use to develop the Marcellus Shale? Each well
Extreme Recovery Membrane Process and Zero Liquid Discharge Low Temperature Crystallization for Treating Scaling Mine Waters
 Extreme Recovery Membrane Process and Zero Liquid Discharge Low Temperature Crystallization for Treating Scaling Mine Waters Malcolm Man, Xiangchun Yin, Zhongyuan Zhou, Ben Sparrow, Susie Lee, Mitch Frank
Extreme Recovery Membrane Process and Zero Liquid Discharge Low Temperature Crystallization for Treating Scaling Mine Waters Malcolm Man, Xiangchun Yin, Zhongyuan Zhou, Ben Sparrow, Susie Lee, Mitch Frank
Crystallization A Viable and Green Solution to Industry s Wastewater Needs
 Marcellus, Utica, and Point Pleasant Shale: Energy Development and Enhancement by Hydraulic Fracturing Conference Crystallization A Viable and Green Solution to Industry s Wastewater Needs Jay N. Smith,
Marcellus, Utica, and Point Pleasant Shale: Energy Development and Enhancement by Hydraulic Fracturing Conference Crystallization A Viable and Green Solution to Industry s Wastewater Needs Jay N. Smith,
The role of water management in unlocking unconventional resources
 The role of water management in unlocking unconventional resources GARY CRISP (GHD), JOHN WALSH (GHD), MARK SHAW (GHD), CHRIS HERTLE (GHD) Introduction Water Management for unconventional hydrocarbons
The role of water management in unlocking unconventional resources GARY CRISP (GHD), JOHN WALSH (GHD), MARK SHAW (GHD), CHRIS HERTLE (GHD) Introduction Water Management for unconventional hydrocarbons
Technologies for Removing Contaminants from Produced Water
 Technologies for Removing Contaminants from Produced Water RICK MCCURDY SR. ENGINEERING ADVISOR CHEMICALS & WATER Ground Water Protection Council Annual UIC Conference February 12-14, 2018 Tulsa, Oklahoma
Technologies for Removing Contaminants from Produced Water RICK MCCURDY SR. ENGINEERING ADVISOR CHEMICALS & WATER Ground Water Protection Council Annual UIC Conference February 12-14, 2018 Tulsa, Oklahoma
Presentation Outline
 Presentation Outline Background on ECCV Brackish Water RO Project Options Considered for Concentrate Disposal Evaluation of Brine Minimization Alternatives Pilot Testing Results Conclusions & Recommendations
Presentation Outline Background on ECCV Brackish Water RO Project Options Considered for Concentrate Disposal Evaluation of Brine Minimization Alternatives Pilot Testing Results Conclusions & Recommendations
Altela, Inc. Treating water naturally
 Altela, Inc. Treating water naturally A Novel Solution for the Energy/Water Nexus: Low-cost Water Desalination Using Waste Heat from CSP 23 October 09 Water and Land for Renewable Energy in the Southwest
Altela, Inc. Treating water naturally A Novel Solution for the Energy/Water Nexus: Low-cost Water Desalination Using Waste Heat from CSP 23 October 09 Water and Land for Renewable Energy in the Southwest
Modular Oil & Gas Equipment Onshore & Offshore
 Modular Oil & Gas Equipment Onshore & Offshore Separators & Desalters AI Energy Solutions onshore and offshore oil process solutions offer innovative technologies packaged with global project management
Modular Oil & Gas Equipment Onshore & Offshore Separators & Desalters AI Energy Solutions onshore and offshore oil process solutions offer innovative technologies packaged with global project management
Chapter 9. Water Sourcing and Wastewater Disposal for Marcellus Shale Development in Pennsylvania
 CITE AS 32 Energy & Min. L. Inst. 9 (2011) Chapter 9 Water Sourcing and Wastewater Disposal for Marcellus Shale Development in Pennsylvania Kevin J. Garber 1 Jean M. Mosites Babst Calland Clements& Zomnir,
CITE AS 32 Energy & Min. L. Inst. 9 (2011) Chapter 9 Water Sourcing and Wastewater Disposal for Marcellus Shale Development in Pennsylvania Kevin J. Garber 1 Jean M. Mosites Babst Calland Clements& Zomnir,
What New Brunswick Needs to Safely Manage Wastewaters from Oil & Natural Gas Development: Smarter Regulations and Better Communication
 What New Brunswick Needs to Safely Manage Wastewaters from Oil & Natural Gas Development: Smarter Regulations and Better Communication Prepared by the New Brunswick Petroleum Alliance in partnership with
What New Brunswick Needs to Safely Manage Wastewaters from Oil & Natural Gas Development: Smarter Regulations and Better Communication Prepared by the New Brunswick Petroleum Alliance in partnership with
Alternative Approaches to Design Evaporator and Crystallizer Systems using OLI Software
 Alternative Approaches to Design Evaporator and Crystallizer Systems using OLI Software Ken Martins/CH2M HILL October 17, 2012 Outline Key Drivers for Zero Liquid Discharge (ZLD) Processes Overview of
Alternative Approaches to Design Evaporator and Crystallizer Systems using OLI Software Ken Martins/CH2M HILL October 17, 2012 Outline Key Drivers for Zero Liquid Discharge (ZLD) Processes Overview of
News. Power Generation 11. ph Control Reduces Acid Consumption in Neutralization Process up to 90 % Perspectives in Pure Water Analytics THORNTON
 Power Generation 11 Perspectives in Pure Water Analytics News THORNTON Leading Pure Water Analytics ph Control Reduces Acid Consumption in Neutralization Process up to 90 % THORNTON new instrumentation
Power Generation 11 Perspectives in Pure Water Analytics News THORNTON Leading Pure Water Analytics ph Control Reduces Acid Consumption in Neutralization Process up to 90 % THORNTON new instrumentation
STW Water Process & Technologies! A Water Solutions Company!
 STW Water Process & Technologies! A Water Solutions Company! Processing and Zero Liquid Discharge of Seawater, Brackish Water, Flowback & Produced Water STW/Salttech- July 2014 Midland, Texas STW/Salttech
STW Water Process & Technologies! A Water Solutions Company! Processing and Zero Liquid Discharge of Seawater, Brackish Water, Flowback & Produced Water STW/Salttech- July 2014 Midland, Texas STW/Salttech
Zero Blowdown Technology - ZBT Arizona Transportation Center
 Zero Blowdown Technology - ZBT Arizona Transportation Center A major transportation center in Arizona was designed to be certified by the USGBC LEED green building program to its highest level, Platinum.
Zero Blowdown Technology - ZBT Arizona Transportation Center A major transportation center in Arizona was designed to be certified by the USGBC LEED green building program to its highest level, Platinum.
Efficient volume reduction and concentration of mining waste water with direct contact evaporation technology
 Efficient volume reduction and concentration of mining waste water with direct contact evaporation technology Steven Panz 1, Wesley Young 2 1. President, Inproheat Industries, Canada 2. Chief Technical
Efficient volume reduction and concentration of mining waste water with direct contact evaporation technology Steven Panz 1, Wesley Young 2 1. President, Inproheat Industries, Canada 2. Chief Technical
Water Fit for Use. Avoiding the Unintended Consequences of Good Intentions
 Water Fit for Use Avoiding the Unintended Consequences of Good Intentions Ronald Tebbetts Industrial Technical Consultant, Global rtebbetts@nalco.com (225) 276-7769 Water Flow Diagram Steam Loss Venting
Water Fit for Use Avoiding the Unintended Consequences of Good Intentions Ronald Tebbetts Industrial Technical Consultant, Global rtebbetts@nalco.com (225) 276-7769 Water Flow Diagram Steam Loss Venting
WATER USED FOR HYDRAULIC FRACTURING: AMOUNTS, SOURCES, REUSE,
 WATER USED FOR HYDRAULIC FRACTURING: AMOUNTS, SOURCES, REUSE, AND DISPOSAL GROUND WATER PROTECTION COUNCIL 2011 Annual Forum Atlanta, GA September 24-28, 2011 David Alleman, ALL Consulting PROJECT FUNDING
WATER USED FOR HYDRAULIC FRACTURING: AMOUNTS, SOURCES, REUSE, AND DISPOSAL GROUND WATER PROTECTION COUNCIL 2011 Annual Forum Atlanta, GA September 24-28, 2011 David Alleman, ALL Consulting PROJECT FUNDING
