Pharmaceuticals in the Environment: Potential and requirements of soft technology targeting user behaviour
|
|
|
- Doris Hall
- 5 years ago
- Views:
Transcription
1 Pharmaceuticals in the Environment: Potential and requirements of soft technology targeting user behaviour Results from the EU FP7 project PHARMAS Rodrigo Vidaurre, Ecologic Institute Konrad Götz, ISOE
2 The PHARMAS project Natural science will close knowledge gaps related to 12 target molecules (antibiotics and anti-cancer drugs), by e.g.: determining human and animal exposure to target molecules producing probabilistic estimates of risk caused by exposure of wildlife and humans to the selected pharmaceuticals go beyond typical substance-by-substance risk assessments by investigating toxicity of realistic mixtures identifying stable transformation products and investigate their concentrations and (eco)toxicology 5/16/2013 2
3 The PHARMAS project Social / behavioural science will develop a prototype for an information / classification system for PIE for Europe, by: exploring stakeholder needs and content requirements, evaluating scientific and socio-economic impact, develop a prototype for a web-based classification system (based on Swedish experience): easily accessible DSS for practitioners (e.g. physicians, pharmacists) providing straightforward action alternatives 5/16/2013 3
4 5/16/2013 4
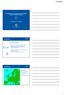
5 Tests and Assays WP2 - Effects OECD tests exposing species to drugs Cytotoxic assays e.g. Comet, micronucleus
6 % contribution to total sum of toxic units TU-Distribution (Algae, S1-F) Fish, L1-F WP3 - Mixtures
7 WP4 - Risk assesment
8 Stakeholder requirements of an environmental information/ classification system for PIE WP6 - Prototype information system WP 6.1 Rodrigo Vidaurre, Isabelle Turcotte, Eleftheria Kampa Ecologic Institute
9 Methodology Qualitative, in-depth interviews (~ 60 minutes) Topics: attitude towards system, evaluation of use and impacts, information requirements, own use (e.g. decision-making), characteristics and design, risk perceptions. Characteristics and functions of system left open 16 May
10 Methodology 12 stakeholder groups: 1. Env. authorities (including RBOs) / Chemical authorities 6 interviewees 2. Pharmaceutical Industry 3 " 3. Water industry 2 " 4. Drinking Water authorities 2 " 5. Research organizations (different disciplines) 5 " 6. Medicines Authorities 3 " 7. Medical associations 2 " 8. Pharmacies / Pharmacy Associations 1 " 9. Consumer NGOs 1 " 10. Environmental NGOs 2 " 11. Public Health authorities 1 " 12. Pharmaceutical Waste/Recycling Companies + 1 " Total : 29 interviewees 16 May
11 Main findings a) Attitudes and exp. impacts Approval all through: Widespread potential uptake: Environ. impacts: Economic impacts: Behavioural impacts (doctors/public): 100% approve system 62% would use most opinions + + predominant (few opinions) diverging opinions 16 May
12 Main findings b) Information requirements Strong and widespread requirements through most groups for: physico-chemical data toxicity and ecotoxicity behaviour in environment behaviour in water TPs sales and volumes data environmental levels 16 May
13 Main findings Strongest requirements: b) Information requirements water actors, research actors, env NGOs. 16 May
14 Water actors Research inst. Env. NGOs 16 May
15 Main findings Strongest requirements: Less strong requirements: b) Information requirements water actors, research actors, env NGOs. environmental authorities 16 May
16 Env. authorities Gaps related to human effects / human toxicology 16 May
17 Main findings Strongest requirements: Less strong requirements: b) Information requirements water actors, research actors, env NGOs. environmental authorities Some requirements: pharmaceutical industry 16 May
18 Pharma industry 16 May
19 Main findings Strongest requirements: Less strong requirements: b) Information requirements water actors, research actors, env NGOs. environmental authorities Some requirements: Minimal requirements: pharmaceutical industry doctors / pharmacists 16 May
20 Doctors / pharmacists 16 May
21 Main findings c) Two approaches for IS on PIE Two (non-exclusive!!!) concepts: 1) Knowledge-base approach Collects wide array of information, e.g. intrinsic properties, environmental behaviour data on environmental occurrence, further information (e.g. behaviour in WWTPs) Strong stakeholder support (many affected by data gaps) Basis for development of specific DSS (e.g. WWTP processes). Used for science and transparency in emerging env. issues. 16 May
22 Main findings c) Two approaches for IS on PIE Some Knowledge Base examples: 16 May
23 Main findings c) Two approaches for IS on PIE 2) DSS for doctors / pharmacists / patients Information on environmental performance of substances, i.e. limited to: a) intrinsic properties, b) substance s environmental risk / hazard. Aims to influence behaviour routines and increase awareness: Possible criteria when choosing otherwise equivalent pharmaceuticals Improved disposal of medicines. Could incentive companies to develop products with lower impact. 16 May
24 Main findings c) Two approaches for IS on PIE Classification system for doctors / patients has widespread support, but. Strong pull for more data, stakeholders affected by gaps To increase impact, system should go beyond needs of doctors / pharmacists, and include elements of Knowledge-base approach Multiplier effects (e.g. wastewater treatment) More chances of uptake and use / impact Fass.se already provides (some) additional data 16 May
25 Thank you for your attention. Rodrigo Vidaurre Ecologic Institute, Pfalzburger Str , D Berlin Tel. +49 (30) , Fax +49 (30) May
CLICO WP 4 Policy Mapping and Development
 CLICO WP 4 Policy Mapping and Development Policies that address the interface between climate change, water conflicts and human security Presented by Rodrigo Vidaurre Ecologic Institute (Berlin) In-depth
CLICO WP 4 Policy Mapping and Development Policies that address the interface between climate change, water conflicts and human security Presented by Rodrigo Vidaurre Ecologic Institute (Berlin) In-depth
EU RESEARCH STRATEGY Nanotechnologies and Advanced Materials Safe Nanotechnology. Dr. Georgios Katalagarianakis European Commission
 EU RESEARCH STRATEGY Nanotechnologies and Advanced Materials 2018 2020 Safe Nanotechnology Dr. Georgios Katalagarianakis European Commission Shaping Europe's Future June 2015 February 2017 June 2017 Towards
EU RESEARCH STRATEGY Nanotechnologies and Advanced Materials 2018 2020 Safe Nanotechnology Dr. Georgios Katalagarianakis European Commission Shaping Europe's Future June 2015 February 2017 June 2017 Towards
Green Week 2012 EUREAU position on emerging pollutants in the water cycle
 Green Week 2012 EUREAU position on emerging pollutants in the water cycle Dr. Frank Sacher, EUREAU Commission I A WORD ABOUT EUREAU European umbrella federation of national water and wastewater associations
Green Week 2012 EUREAU position on emerging pollutants in the water cycle Dr. Frank Sacher, EUREAU Commission I A WORD ABOUT EUREAU European umbrella federation of national water and wastewater associations
ECO-PHARMACO- STEWARDSHIP (EPS) PILLAR 1 - RESEARCH & DEVELOPMENT: INTELLIGENCE-LED ASSESSMENT OF PHARMACEUTICALS IN THE ENVIRONMENT (ipie)
 ECO-PHARMACO- STEWARDSHIP (EPS) PILLAR 1 - RESEARCH & DEVELOPMENT: INTELLIGENCE-LED ASSESSMENT OF PHARMACEUTICALS IN THE ENVIRONMENT (ipie) PILLAR 1 - RESEARCH & DEVELOPMENT: INTELLIGENCE-LED ASSESSMENT
ECO-PHARMACO- STEWARDSHIP (EPS) PILLAR 1 - RESEARCH & DEVELOPMENT: INTELLIGENCE-LED ASSESSMENT OF PHARMACEUTICALS IN THE ENVIRONMENT (ipie) PILLAR 1 - RESEARCH & DEVELOPMENT: INTELLIGENCE-LED ASSESSMENT
Pharmaceuticals. and Senate Bill 1757
 Pharmaceuticals and Senate Bill 1757 Jessica Huybregts TCEQ Water Supply Division Public Drinking Water Section 512/239-4709 What are Pharmaceuticals? Prescription medications
Pharmaceuticals and Senate Bill 1757 Jessica Huybregts TCEQ Water Supply Division Public Drinking Water Section 512/239-4709 What are Pharmaceuticals? Prescription medications
The use of ecosystem-based approaches to climate change adaptation and mitigation: Barriers and success factors
 The use of ecosystem-based approaches to climate change adaptation and mitigation: Barriers and success factors Sandra Naumann Ecologic Institute Overview Introduction Study carried out by: Ecologic Institute
The use of ecosystem-based approaches to climate change adaptation and mitigation: Barriers and success factors Sandra Naumann Ecologic Institute Overview Introduction Study carried out by: Ecologic Institute
Public consultation on pharmaceuticals in the environment
 Contribution ID: 1bdef173-cd9f-44e0-a6bb-b9fef643f15d Date: 16/02/2018 12:07:54 Public consultation on pharmaceuticals in the environment Fields marked with * are mandatory. About this consultation This
Contribution ID: 1bdef173-cd9f-44e0-a6bb-b9fef643f15d Date: 16/02/2018 12:07:54 Public consultation on pharmaceuticals in the environment Fields marked with * are mandatory. About this consultation This
Recommendations for Improving Environmental Risk Assessment of Pharmaceuticals
 MISTRAPHARMA POLICYBRIEF Recommendations for Improving Environmental Risk Assessment of Pharmaceuticals MISTRAPHARMA Identification and Reduction of Environmental Risks Caused by Human Pharmaceuticals
MISTRAPHARMA POLICYBRIEF Recommendations for Improving Environmental Risk Assessment of Pharmaceuticals MISTRAPHARMA Identification and Reduction of Environmental Risks Caused by Human Pharmaceuticals
Industrial Safety Challenges and Advanced Materials
 Industrial Safety Challenges and Advanced Materials Prof.dr.ir.. Walter BOGAERTS EuMaT Executive Office European Technology Platform on Advanced Materials and Technologies www.eumat.org FP7 NMP What are
Industrial Safety Challenges and Advanced Materials Prof.dr.ir.. Walter BOGAERTS EuMaT Executive Office European Technology Platform on Advanced Materials and Technologies www.eumat.org FP7 NMP What are
Global Forum on Competition
 Unclassified DAF/COMP/GF/WD(2014)51 DAF/COMP/GF/WD(2014)51 Unclassified Organisation de Coopération et de Développement Économiques Organisation for Economic Co-operation and Development 14-Feb-2014 English
Unclassified DAF/COMP/GF/WD(2014)51 DAF/COMP/GF/WD(2014)51 Unclassified Organisation de Coopération et de Développement Économiques Organisation for Economic Co-operation and Development 14-Feb-2014 English
Setting the scene. CECs are ubiquitous and uncertain
 OECD workshop on Managing Contaminants of Emerging Concern in Surface Waters: Scientific developments and cost-effective policy responses, 5 February 2018 Summary Note Key messages Contaminants of emerging
OECD workshop on Managing Contaminants of Emerging Concern in Surface Waters: Scientific developments and cost-effective policy responses, 5 February 2018 Summary Note Key messages Contaminants of emerging
Externality Research in the Area of Agriculture and Its Impact on German Policy-Making
 Externality Research in the Area of Agriculture and Its Impact on German Policy-Making Holger Gerdes Ecologic Institute Relation to PhD topic Multi-criteria evaluation of ecosystem services as decision
Externality Research in the Area of Agriculture and Its Impact on German Policy-Making Holger Gerdes Ecologic Institute Relation to PhD topic Multi-criteria evaluation of ecosystem services as decision
Policy principles for a competitive healthcare environment
 Policy principles for a competitive healthcare environment Pharmaceutical Research and Development Industry Malta Association (PRIMA) PRIMA is an affiliate of the European Federation of Pharmaceutical
Policy principles for a competitive healthcare environment Pharmaceutical Research and Development Industry Malta Association (PRIMA) PRIMA is an affiliate of the European Federation of Pharmaceutical
Constraints to wastewater treatment and reuse in Mediterranean Partner Countries - Project recommendations
 Constraints to wastewater treatment and reuse in Mediterranean Partner Countries - Project recommendations Eleftheria Kampa Ecologic Institute, Berlin Partners: Redouane Choukr-Allah (IAVCHA); Mohamed
Constraints to wastewater treatment and reuse in Mediterranean Partner Countries - Project recommendations Eleftheria Kampa Ecologic Institute, Berlin Partners: Redouane Choukr-Allah (IAVCHA); Mohamed
HOW BUSINESS AND ORGANIZATIONS AROUND THE WORLD ARE ADDRESSING THE WATER STEWARDSHIP AGENDA
 HOW BUSINESS AND ORGANIZATIONS AROUND THE WORLD ARE ADDRESSING THE WATER STEWARDSHIP AGENDA 3 rd Karachi International Water Conference Adrian Sym, CEO, Alliance for Water Stewardship November 21 st, 2017
HOW BUSINESS AND ORGANIZATIONS AROUND THE WORLD ARE ADDRESSING THE WATER STEWARDSHIP AGENDA 3 rd Karachi International Water Conference Adrian Sym, CEO, Alliance for Water Stewardship November 21 st, 2017
International cooperation for transboundary water pollution control:
 International cooperation for transboundary water pollution control: The Rhine River example Dr. Darla Nickel Ecologic Institute Table of content Transboundary river basins in Europe and Germany The legislative
International cooperation for transboundary water pollution control: The Rhine River example Dr. Darla Nickel Ecologic Institute Table of content Transboundary river basins in Europe and Germany The legislative
Factors Supporting a Sustainable European Biosimilar Medicines Market PROJECT SUMMARY FOR EXTERNAL COMMUNICATION
 Market Access Factors Supporting a Sustainable European Biosimilar Medicines Market PROJECT SUMMARY FOR EXTERNAL COMMUNICATION A study undertaken by GfK Market Access on behalf of the European Biosimilars
Market Access Factors Supporting a Sustainable European Biosimilar Medicines Market PROJECT SUMMARY FOR EXTERNAL COMMUNICATION A study undertaken by GfK Market Access on behalf of the European Biosimilars
Transnational Action Program on Emerging. Substances. The TAPES project. Closing Conference Brussels, 24 September
 Transnational Action Program on Emerging Substances The TAPES project Closing Conference Brussels, 24 September 2015 www.tapes-interreg.eu The project Project aims and activities The closing conference
Transnational Action Program on Emerging Substances The TAPES project Closing Conference Brussels, 24 September 2015 www.tapes-interreg.eu The project Project aims and activities The closing conference
FACTORS SUPPORTING A SUSTAINABLE EUROPEAN BIOSIMILAR MEDICINES MARKET
 FACTORS SUPPORTING A SUSTAINABLE EUROPEAN BIOSIMILAR MEDICINES MARKET A study undertaken by GfK Market Access on behalf of the European Biosimilars Group, a sector group of EGA, about the future sustainability
FACTORS SUPPORTING A SUSTAINABLE EUROPEAN BIOSIMILAR MEDICINES MARKET A study undertaken by GfK Market Access on behalf of the European Biosimilars Group, a sector group of EGA, about the future sustainability
Pharmaceutical Marketing. lecture Two Microenvironment Environment Lecturer: Enas Abu-Qudais
 Pharmaceutical Marketing lecture Two Microenvironment Environment Lecturer: Enas Abu-Qudais Marketing Environment There are various factors/ forces, that directly or indirectly influence the organizations
Pharmaceutical Marketing lecture Two Microenvironment Environment Lecturer: Enas Abu-Qudais Marketing Environment There are various factors/ forces, that directly or indirectly influence the organizations
Swedish ac+vi+es. Pharmaceu)cals in the environment. Maria Wallin Swedish Ministry of the Environment and Energy. Make ideas work!
 Pharmaceu)cals in the environment Make ideas work! Swedish ac+vi+es Maria Wallin Swedish Ministry of the Environment and Energy Tuesday 6 th September 2016 HCWH Europe 2016 Text (leg align, 28).. hand
Pharmaceu)cals in the environment Make ideas work! Swedish ac+vi+es Maria Wallin Swedish Ministry of the Environment and Energy Tuesday 6 th September 2016 HCWH Europe 2016 Text (leg align, 28).. hand
Figure 1. Environmental cycle of pharmaceuticals and resistant bacteria
 1. Huize Aarde Stichting Huize Aarde ( Home Earth ) in Enschede, The Netherlands is founded in 1992 in order to strengthen social responsibility in research, policy, management, production and consumption.
1. Huize Aarde Stichting Huize Aarde ( Home Earth ) in Enschede, The Netherlands is founded in 1992 in order to strengthen social responsibility in research, policy, management, production and consumption.
Orphan Medicinal Products
 Development process of Orphan Medicinal Products 1 Did you know that... A rare disease is officially defined as a lifethreatening or chronically debilitating condition that affects no more than 5 in 10,000
Development process of Orphan Medicinal Products 1 Did you know that... A rare disease is officially defined as a lifethreatening or chronically debilitating condition that affects no more than 5 in 10,000
Topic: Intelligent prediction and identification of environmental risks posed by human medicinal products
 Topic: Intelligent prediction and identification of environmental risks posed by human medicinal products All information regarding future IMI Call topics is indicative and subject to change. Final information
Topic: Intelligent prediction and identification of environmental risks posed by human medicinal products All information regarding future IMI Call topics is indicative and subject to change. Final information
EPAA 3Rs Science Award 2012
 EPAA 3Rs Science Award 2012 Systematic approach to investigate outliers of the fish embryo test to increase its predictive capacity and applicability domain for acute fish toxicity and beyond Nils Klüver,
EPAA 3Rs Science Award 2012 Systematic approach to investigate outliers of the fish embryo test to increase its predictive capacity and applicability domain for acute fish toxicity and beyond Nils Klüver,
ACCOUNTABILITY AND TRANSPARENCY IN PHARMA SUPPLY CHAIN 6/26/2018. Presentation to UN 2018 Public Service Forum. Richard Bergström External Lead Pharma
 ACCOUNTABILITY AND TRANSPARENCY IN PHARMA SUPPLY CHAIN Presentation to UN 2018 Public Service Forum Richard Bergström External Lead Pharma Enabling trust 1 SICPA: GLOBAL ROLE Protection of 85+% world currency
ACCOUNTABILITY AND TRANSPARENCY IN PHARMA SUPPLY CHAIN Presentation to UN 2018 Public Service Forum Richard Bergström External Lead Pharma Enabling trust 1 SICPA: GLOBAL ROLE Protection of 85+% world currency
Green Pharmacy Campaign Helping Communities Safely Dispose of Unused Medicines
 Green Pharmacy Campaign Helping Communities Safely Dispose of Unused Medicines www.teleosis.org Teleosis Institute 1521B 5th Street Berkeley, CA 94710 (510) 558-7285 Why Green Pharmacy? Of the 4 billion
Green Pharmacy Campaign Helping Communities Safely Dispose of Unused Medicines www.teleosis.org Teleosis Institute 1521B 5th Street Berkeley, CA 94710 (510) 558-7285 Why Green Pharmacy? Of the 4 billion
ECO-PHARMACO- STEWARDSHIP (EPS) A HOLISTIC ENVIRONMENTAL RISK MANAGEMENT PROGRAM
 ECO-PHARMACO- STEWARDSHIP (EPS) A HOLISTIC ENVIRONMENTAL RISK MANAGEMENT PROGRAM A HOLISTIC ENVIRONMENTALRISK MANAGEMENT PROGRAM) Executive Summary...3 The European Pharmaceutical Industry s Role and Mission
ECO-PHARMACO- STEWARDSHIP (EPS) A HOLISTIC ENVIRONMENTAL RISK MANAGEMENT PROGRAM A HOLISTIC ENVIRONMENTALRISK MANAGEMENT PROGRAM) Executive Summary...3 The European Pharmaceutical Industry s Role and Mission
FRANCISCO P. TRANQUILINO, M.D., FPCP
 FRANCISCO P. TRANQUILINO, M.D., FPCP PHAP Consultant on Ethics Matters Chair, Ethics Committee, Philippine College of Physicians Special Assistant to the Dean and College Secretary University of the Philippines
FRANCISCO P. TRANQUILINO, M.D., FPCP PHAP Consultant on Ethics Matters Chair, Ethics Committee, Philippine College of Physicians Special Assistant to the Dean and College Secretary University of the Philippines
EU health policy. Strategy for the pharmaceutical industry and biosimilars. Salvatore D'Acunto. DG Research. DG Internal Market. DG Health & Consumers
 Strategy for the pharmaceutical industry and biosimilars Salvatore D'Acunto European Commission Enterprise and Industry Directorate-General London, 3 April 2014 EU health policy DG Research R&D, innovation
Strategy for the pharmaceutical industry and biosimilars Salvatore D'Acunto European Commission Enterprise and Industry Directorate-General London, 3 April 2014 EU health policy DG Research R&D, innovation
Heavily Modified Water Bodies (HMWB) in the WFD
 Heavily Modified Water Bodies (HMWB) in the WFD Eleftheria Kampa, Ecologic 12/10/2007 - Belluno ESWG Conference Content of this presentation Hydromorphology & HMWB in the WFD Provisional identification
Heavily Modified Water Bodies (HMWB) in the WFD Eleftheria Kampa, Ecologic 12/10/2007 - Belluno ESWG Conference Content of this presentation Hydromorphology & HMWB in the WFD Provisional identification
FIP STATEMENT OF POLICY Pharmacist's authority in pharmaceutical product selection: therapeutic interchange and substitution
 Pharmacist's authority in pharmaceutical product selection: therapeutic interchange and substitution Purpose: The purpose of this document is to provide a set of recommendations on therapeutic interchange
Pharmacist's authority in pharmaceutical product selection: therapeutic interchange and substitution Purpose: The purpose of this document is to provide a set of recommendations on therapeutic interchange
The ecosystem-based approach to adaptation: Concepts and implementation
 The ecosystem-based approach to adaptation: Concepts and implementation Timo Kaphengst Sandra Naumann Ecologic Institute Overview Introduction/Definition Methodological steps Two project examples Actors
The ecosystem-based approach to adaptation: Concepts and implementation Timo Kaphengst Sandra Naumann Ecologic Institute Overview Introduction/Definition Methodological steps Two project examples Actors
Alternatives to Animal Testing
 Alternatives to Animal Testing ILSI Europe's 2017 Annual Symposium 30-31 March 2017 Dr. Katrin Schütte DG Environment, European Commission Contents 1) 1) Directive 2010/63/EU From legislation to application
Alternatives to Animal Testing ILSI Europe's 2017 Annual Symposium 30-31 March 2017 Dr. Katrin Schütte DG Environment, European Commission Contents 1) 1) Directive 2010/63/EU From legislation to application
What can be done from regulatory side?
 Federal Agency for Medicines and Health Products (FAMHP) What can be done from regulatory side? 9th Annual ecopa Workshop November 29-30, 2008, Brussels Dr. Sonja BEKEN Non-Clinical Assessor, Registration
Federal Agency for Medicines and Health Products (FAMHP) What can be done from regulatory side? 9th Annual ecopa Workshop November 29-30, 2008, Brussels Dr. Sonja BEKEN Non-Clinical Assessor, Registration
Nanotechnology in Fertilizers and Supplements
 Nanotechnology in Fertilizers and Supplements February 21, 2017 Nathalie Decan, Ph.D. Safety Evaluator, Fertilizer Safety Section Canadian Food Inspection Agency 2007 Her Majesty the Queen in Right of
Nanotechnology in Fertilizers and Supplements February 21, 2017 Nathalie Decan, Ph.D. Safety Evaluator, Fertilizer Safety Section Canadian Food Inspection Agency 2007 Her Majesty the Queen in Right of
Making the entire pharmaceutical chain sustainable. Bengt Mattson. Policy Manager, LIF (Manager,CSR & Environmental Affairs, Pfizer AB)
 Making the entire pharmaceutical chain sustainable Bengt Mattson Policy Manager, LIF (Manager,CSR & Environmental Affairs, Pfizer AB) www.ansvarsblogg.se Twitter: CSRBengt LIF, Feb 15, 2017 Sustainability
Making the entire pharmaceutical chain sustainable Bengt Mattson Policy Manager, LIF (Manager,CSR & Environmental Affairs, Pfizer AB) www.ansvarsblogg.se Twitter: CSRBengt LIF, Feb 15, 2017 Sustainability
Workshop on Access to and Uptake of Biosimilar Medicinal Products
 EUROPEAN COMMISSION Directorate-General for Internal Market, Industry, Entrepreneurship and SMEs Consumer, Environmental and Health Technologies Biotechnology and Food Supply Chain Workshop on Access to
EUROPEAN COMMISSION Directorate-General for Internal Market, Industry, Entrepreneurship and SMEs Consumer, Environmental and Health Technologies Biotechnology and Food Supply Chain Workshop on Access to
SCIENTIFIC COMMITTEE ON TOXICITY, ECOTOXICITY AND THE ENVIRONMENT (CSTEE)
 EUROPEAN COMMISSION HEALTH & CONSUMER PROTECTION DIRECTORATE-GENERAL Directorate C - Public Health and Risk Assessment C7 - Risk assessment Brussels, C7/GF/csteeop/edta-env/100903 D(03) SCIENTIFIC COMMITTEE
EUROPEAN COMMISSION HEALTH & CONSUMER PROTECTION DIRECTORATE-GENERAL Directorate C - Public Health and Risk Assessment C7 - Risk assessment Brussels, C7/GF/csteeop/edta-env/100903 D(03) SCIENTIFIC COMMITTEE
Quality Quality problems r API used used in essent essen ial t ial medicines : Role e of of WHO and and its its pre pr lifi qua ca i ttion on progr
 Quality problems for API used in essential medicines : Role of WHO and its pre qualification programme Corinne POUGET Consultant Pavia 11 May 2012 1 A few key figures 2010 WW Pharmaceutical market: a USD
Quality problems for API used in essential medicines : Role of WHO and its pre qualification programme Corinne POUGET Consultant Pavia 11 May 2012 1 A few key figures 2010 WW Pharmaceutical market: a USD
Wendy Poirier, Director of Towers Watsons Canadian Health & Group Benefits Practice talks about the Canadian Rx Coalition
 EXCLUSIVE INTERVIEW: Wendy Poirier, Director of Towers Watsons Canadian Health & Group Benefits Practice talks about the Canadian Rx Coalition Today I am pleased to interview Wendy Poirier Director of
EXCLUSIVE INTERVIEW: Wendy Poirier, Director of Towers Watsons Canadian Health & Group Benefits Practice talks about the Canadian Rx Coalition Today I am pleased to interview Wendy Poirier Director of
Chemically synthesized proteins referencing biological medicinal products
 Chemically synthesized proteins referencing biological medicinal products A EuropaBio white paper Calling for: - Equal assessment transparency - Equal measures for traceability and adverse event reporting
Chemically synthesized proteins referencing biological medicinal products A EuropaBio white paper Calling for: - Equal assessment transparency - Equal measures for traceability and adverse event reporting
Engagement with stakeholders
 Engagement with stakeholders 2 nd International Awareness Session - The EU medicines regulatory system and the European Medicines Agency Presented by Juan Garcia Burgos and Marie-Helene Pinheiro on 8 March
Engagement with stakeholders 2 nd International Awareness Session - The EU medicines regulatory system and the European Medicines Agency Presented by Juan Garcia Burgos and Marie-Helene Pinheiro on 8 March
Implications of the FDA Draft Guidance on Biosimilars for Clinicians: What We Know and Don t Know
 368 Journal of the National Comprehensive Cancer Network Implications of the FDA Draft Guidance on Biosimilars for Clinicians: What We Know and Don t Know Edward Li, PharmD, BCOP, and James M. Hoffman,
368 Journal of the National Comprehensive Cancer Network Implications of the FDA Draft Guidance on Biosimilars for Clinicians: What We Know and Don t Know Edward Li, PharmD, BCOP, and James M. Hoffman,
The importance of regulatory science in a societal and industrial perspective Annual Conference CORS 24 November 2016
 The importance of regulatory science in a societal and industrial perspective Annual Conference CORS 24 November 2016 Marianne Kock, Head of Global Regulatory Affairs & Managing Director, Ferring Pharmaceuticals
The importance of regulatory science in a societal and industrial perspective Annual Conference CORS 24 November 2016 Marianne Kock, Head of Global Regulatory Affairs & Managing Director, Ferring Pharmaceuticals
12/10/2011. Development of sterile medicinal products in Dutch hospital pharmacy SEHP Santiago de Compostella. Introduction.
 Development of sterile medicinal products in Dutch hospital pharmacy SEHP 2011 Santiago de Compostella Introduction Willem Meulenhoff (1971) Production Pharmacist at NV Organon (MSD) Head of the Production
Development of sterile medicinal products in Dutch hospital pharmacy SEHP 2011 Santiago de Compostella Introduction Willem Meulenhoff (1971) Production Pharmacist at NV Organon (MSD) Head of the Production
Public Interest Incorporated Foundation BioSafety Research Center
 Public Interest Incorporated Foundation BioSafety Research Center http://www.anpyo.or.jp Ver. 201801 Building #1(Test and research annex) Building #2 (Administrat ion office) Building #3 (Toxicity test
Public Interest Incorporated Foundation BioSafety Research Center http://www.anpyo.or.jp Ver. 201801 Building #1(Test and research annex) Building #2 (Administrat ion office) Building #3 (Toxicity test
Bioscientists Do you have the Bayer Spirit?
 CropScience HealthCare MaterialScience Business Services Industry Services Technology Services www.mybayerjob.com Bioscientists Do you have the Bayer Spirit? Research and Development, Screening, Toxicology,
CropScience HealthCare MaterialScience Business Services Industry Services Technology Services www.mybayerjob.com Bioscientists Do you have the Bayer Spirit? Research and Development, Screening, Toxicology,
Bi Regional Consultation on Good Governance
 Pharmaceutical Services Ministry of Health Malaysia GGM PROGRAMME IN MALAYSIA WHO Collaborating Centre For Regulatory Control of Pharmaceuticals Bi Regional Consultation on Good Governance 9 th November
Pharmaceutical Services Ministry of Health Malaysia GGM PROGRAMME IN MALAYSIA WHO Collaborating Centre For Regulatory Control of Pharmaceuticals Bi Regional Consultation on Good Governance 9 th November
Health Job Family: Pharmacy Tech Progression
 Cornell University Staff Compensation Program Generic Job Profile Summaries Compensation Services 353 Pine Tree Road, East Hill Plaza, Ithaca, NY 14850 (607) 254-8355 compensation@cornell.edu www.hr.cornell.edu
Cornell University Staff Compensation Program Generic Job Profile Summaries Compensation Services 353 Pine Tree Road, East Hill Plaza, Ithaca, NY 14850 (607) 254-8355 compensation@cornell.edu www.hr.cornell.edu
Assessment of the potential of ecosystem-based approaches to climate change adaptation and mitigation in Europe
 Assessment of the potential of ecosystem-based approaches to climate change adaptation and mitigation in Europe Timo Kaphengst Ecologic Institute Authors: Sandra Naumann, Gerardo Anzaldua, Holger Gerdes,
Assessment of the potential of ecosystem-based approaches to climate change adaptation and mitigation in Europe Timo Kaphengst Ecologic Institute Authors: Sandra Naumann, Gerardo Anzaldua, Holger Gerdes,
May Patient Reported Outcomes: Strategies for gaining or expanding market access ES SAMPLE PAGES SA. A FirstWord ExpertViews Dossier Report
 A FirstWord ExpertViews Dossier Report Published Copyright 2016 Doctor s Guide Publishing Limited All rights reserved. No part of this publication may be reproduced or used in any form or by any means
A FirstWord ExpertViews Dossier Report Published Copyright 2016 Doctor s Guide Publishing Limited All rights reserved. No part of this publication may be reproduced or used in any form or by any means
- A6/1 - Regulatory authority Month and year Microbial Pest Control Agent (Name) page of - A6/1 - APPENDIX 6
 - A6/1 - - A6/1 - APPENDIX 6 FORMAT FOR THE LISTING OF END POINTS TO BE INCLUDED IN THE REASONED STATEMENT OF THE OVERALL CONCLUSIONS DRAWN BY THE REGULATORY AUTHORITY (LEVEL 2)8 General remark: Testing
- A6/1 - - A6/1 - APPENDIX 6 FORMAT FOR THE LISTING OF END POINTS TO BE INCLUDED IN THE REASONED STATEMENT OF THE OVERALL CONCLUSIONS DRAWN BY THE REGULATORY AUTHORITY (LEVEL 2)8 General remark: Testing
The Future of Market Access A FirstWord ExpertViews Dossier Report
 AM PL E PA G ES S A G ES S A FirstWord ExpertViews Dossier Report Published Copyright 2016 Doctor s Guide Publishing Limited All rights reserved. No part of this publication may be reproduced or used in
AM PL E PA G ES S A G ES S A FirstWord ExpertViews Dossier Report Published Copyright 2016 Doctor s Guide Publishing Limited All rights reserved. No part of this publication may be reproduced or used in
The Competition Council launched for public consultation the report on sector inquiry on pharma market
 The Competition Council launched for public consultation the report on sector inquiry on pharma market One of the conclusions of the sector inquiry on pharma sector carried out by the Competition Council
The Competition Council launched for public consultation the report on sector inquiry on pharma market One of the conclusions of the sector inquiry on pharma sector carried out by the Competition Council
Europe Fights Back against Counterfeit Medication with the European Falsified Medicines Directive (2011/62/CE)
 Europe Fights Back against Counterfeit Medication with the European Falsified Medicines Directive (2011/62/CE) The instances of falsified medicines in Europe are increasing at a dramatic rate. Medication
Europe Fights Back against Counterfeit Medication with the European Falsified Medicines Directive (2011/62/CE) The instances of falsified medicines in Europe are increasing at a dramatic rate. Medication
Electricity Policies
 Mainstreaming Climate Change into EU Electricity Policies 1, Eleftheria Vasileiadou 2, Ajay Gandhi 2 1 2 Institute for Environmental Studies, Vrije Universiteit Amsterdam Research Domain Sustainable Solutions
Mainstreaming Climate Change into EU Electricity Policies 1, Eleftheria Vasileiadou 2, Ajay Gandhi 2 1 2 Institute for Environmental Studies, Vrije Universiteit Amsterdam Research Domain Sustainable Solutions
A Comparison of the Acute Aquatic Ecotoxicity of Surfactants used in Cleaning Products with Natural Ingredients
 File: 162704239 Michael Rochon Cogent Environmental Solutions Ltd 18 Massari Street Caledon ON L0N 1C0 Via e-mail: cogentenvironmental@rogers.com Dear Mike: Reference: A Comparison of the Acute Aquatic
File: 162704239 Michael Rochon Cogent Environmental Solutions Ltd 18 Massari Street Caledon ON L0N 1C0 Via e-mail: cogentenvironmental@rogers.com Dear Mike: Reference: A Comparison of the Acute Aquatic
Al- Hayat Company for Studies and Research, Al- Isra' Building, Sufian st., Nablus P.O.Box 167, Palestine
 Journal of Pharmacy and Pharmacology 4 (2016) 17-22 doi: 10.17265/2328-2150/2016.01.004 D DAVID PUBLISHING Qusai N. Al-Shahed, Anhar Assali and Ruba Najjar Al- Hayat Company for Studies and Research, Al-
Journal of Pharmacy and Pharmacology 4 (2016) 17-22 doi: 10.17265/2328-2150/2016.01.004 D DAVID PUBLISHING Qusai N. Al-Shahed, Anhar Assali and Ruba Najjar Al- Hayat Company for Studies and Research, Al-
The Danish Generic Medicines Industry Association (IGL)
 The Danish Generic Medicines Industry Association (IGL) comments on the concept paper on the delegated act for a unique identifier for medicinal products for human use, and its verification. The Danish
The Danish Generic Medicines Industry Association (IGL) comments on the concept paper on the delegated act for a unique identifier for medicinal products for human use, and its verification. The Danish
Re: Therapeutic Goods Administration, Consultation: Nomenclature of Biological Medicines
 September 7, 2017 Biological Science Section Therapeutic Goods Administration (TGA) PO Box 100 Woden ACT 2606 Re: Therapeutic Goods Administration, Consultation: Nomenclature of Biological Medicines Dear
September 7, 2017 Biological Science Section Therapeutic Goods Administration (TGA) PO Box 100 Woden ACT 2606 Re: Therapeutic Goods Administration, Consultation: Nomenclature of Biological Medicines Dear
Healthcare provider perspective. Vision from a hospital pharmacist on bar coding of pharmaceuticals and implementation of GS1 standards
 Healthcare provider perspective Vision from a hospital pharmacist on bar coding of pharmaceuticals and implementation of GS1 standards Prof. Pascal BONNABRY GS1 Global Forum Brussels, February 23, 2010
Healthcare provider perspective Vision from a hospital pharmacist on bar coding of pharmaceuticals and implementation of GS1 standards Prof. Pascal BONNABRY GS1 Global Forum Brussels, February 23, 2010
The water sectors contribution to reducing pollution by pharmaceuticals
 The water sectors contribution to reducing pollution by pharmaceuticals Water and Pharmaceuticals - insights and perspectives for health and environment IWA Sweden Uppsala 12-13 April 2016 Anders Finnson
The water sectors contribution to reducing pollution by pharmaceuticals Water and Pharmaceuticals - insights and perspectives for health and environment IWA Sweden Uppsala 12-13 April 2016 Anders Finnson
POLICY POSITION ON NAMING OF BIOTECHNOLOGY-DERIVED THERAPEUTIC PROTEINS. October 31, 2006
 POLICY POSITION ON NAMING OF BIOTECHNOLOGY-DERIVED THERAPEUTIC PROTEINS October 31, 2006 POLICY POSITION ON NAMING OF BIOTECHNOLOGY-DERIVED THERAPEUTIC PROTEINS 1 This is a joint position statement of
POLICY POSITION ON NAMING OF BIOTECHNOLOGY-DERIVED THERAPEUTIC PROTEINS October 31, 2006 POLICY POSITION ON NAMING OF BIOTECHNOLOGY-DERIVED THERAPEUTIC PROTEINS 1 This is a joint position statement of
Managing active pharmaceutical ingredients (API) in manufacturing effluent Kelly Kappler Johnson & Johnson April 26, 2017
 Managing active pharmaceutical ingredients (API) in manufacturing effluent Kelly Kappler Johnson & Johnson April 26, 2017 Agenda 1 2 3 4 Importance of managing active pharmaceutical ingredients (API) in
Managing active pharmaceutical ingredients (API) in manufacturing effluent Kelly Kappler Johnson & Johnson April 26, 2017 Agenda 1 2 3 4 Importance of managing active pharmaceutical ingredients (API) in
Maximizing Market Access: THE 5 MOST CRITICAL QUESTIONS TO ASK WHEN LAUNCHING A SPECIALTY DRUGS
 Maximizing Market Access: THE 5 MOST CRITICAL QUESTIONS TO ASK WHEN LAUNCHING A SPECIALTY DRUGS by Jan Nielsen, division president, Access & Patient Support Are You Asking the Right Questions? We ve all
Maximizing Market Access: THE 5 MOST CRITICAL QUESTIONS TO ASK WHEN LAUNCHING A SPECIALTY DRUGS by Jan Nielsen, division president, Access & Patient Support Are You Asking the Right Questions? We ve all
Off-label use of medicinal products in the EU STAMP Expert Group 14 March 2017
 Off-label use of medicinal products in the EU STAMP Expert Group 14 March 2017 Study on off-label use of medicinal products in the European Union The information and views set out in the study report are
Off-label use of medicinal products in the EU STAMP Expert Group 14 March 2017 Study on off-label use of medicinal products in the European Union The information and views set out in the study report are
EU MILESTONES ON ENDOCRINE DISRUPTING CHEMICALS (EDCS): OFFICIAL COMMITMENTS AND LEGISLATIVE ACTION ON EDCS IN THE EU
 EU MILESTONES ON ENDOCRINE DISRUPTING CHEMICALS (EDCS): OFFICIAL COMMITMENTS AND LEGISLATIVE ACTION ON EDCS IN THE EU January 2014 October 1998 - the European Parliament adopted a Resolution calling upon
EU MILESTONES ON ENDOCRINE DISRUPTING CHEMICALS (EDCS): OFFICIAL COMMITMENTS AND LEGISLATIVE ACTION ON EDCS IN THE EU January 2014 October 1998 - the European Parliament adopted a Resolution calling upon
MULTICHANNEL MARKETING FOR PHARMA
 02 AN INTRODUCTION TO MULTICHANNEL MARKETING FOR PHARMA Inspiring audiences. Motivating change. Thinking beyond. www.wearecouch.com COUCH medical communications 1 Introduction Consumers these days have
02 AN INTRODUCTION TO MULTICHANNEL MARKETING FOR PHARMA Inspiring audiences. Motivating change. Thinking beyond. www.wearecouch.com COUCH medical communications 1 Introduction Consumers these days have
Psychological Mapping of Medical Practitioner for Brand Association
 Europ. J. Econ. Bus. DOI: http://dx.doi.org/10.20936/ejeb/160102 ORIGINAL ARTICLE Psychological Mapping of Medical Practitioner for Brand Association Reshma Nasreen Associate Professor, Faculty of Management,
Europ. J. Econ. Bus. DOI: http://dx.doi.org/10.20936/ejeb/160102 ORIGINAL ARTICLE Psychological Mapping of Medical Practitioner for Brand Association Reshma Nasreen Associate Professor, Faculty of Management,
Better Access, Better Health A guide to the generic and biosimilars medicines market and pricing
 Better Access, Better Health A guide to the generic and biosimilars medicines market and pricing Section 1: About the BGMA & Generics The British Generic Manufacturers Association (BGMA) is made up of
Better Access, Better Health A guide to the generic and biosimilars medicines market and pricing Section 1: About the BGMA & Generics The British Generic Manufacturers Association (BGMA) is made up of
Developed Markets Italy,Portugal,Spain
 Developed Markets Italy,Portugal,Spain Istanbul, 15 th June 2007 Hugo Carradinha Health Economics Affairs Manager Italy Current Market Share Italian Pharmaceutical Market Source: IMF data 03/07 Italy -
Developed Markets Italy,Portugal,Spain Istanbul, 15 th June 2007 Hugo Carradinha Health Economics Affairs Manager Italy Current Market Share Italian Pharmaceutical Market Source: IMF data 03/07 Italy -
Innovative Products. Dr Arnout R. H. Fischer and Prof Dr J (Hans) C M van Trijp Marketing and Consumer Behaviour Group
 Benefit Communications - How and when to communicate the science Innovative Products Dr Arnout R. H. Fischer and Prof Dr J (Hans) C M van Trijp Marketing and Consumer Behaviour Group Marketing functional
Benefit Communications - How and when to communicate the science Innovative Products Dr Arnout R. H. Fischer and Prof Dr J (Hans) C M van Trijp Marketing and Consumer Behaviour Group Marketing functional
Need for improved risk assessment in Europe
 May 2005 NoMiracle Newsletter No. 1 Need for improved risk assessment in Europe To support current and future European strategies, in particular for environment and health, there is an urgent need for
May 2005 NoMiracle Newsletter No. 1 Need for improved risk assessment in Europe To support current and future European strategies, in particular for environment and health, there is an urgent need for
An industry database of curated API ecotox data and PNECs
 An industry database of curated API ecotox data and PNECs Presented at the IWA Sweden Conference 2016 on Water and Pharmaceuticals insights and perspectives for health and environment D.J. Caldwell, 12
An industry database of curated API ecotox data and PNECs Presented at the IWA Sweden Conference 2016 on Water and Pharmaceuticals insights and perspectives for health and environment D.J. Caldwell, 12
Pharmaceutical Sector Governance in the Middle East and North Africa Region A Regional Review by the World Bank
 Pharmaceutical Sector Governance in the Middle East and North Africa Region A Regional Review by the World Bank Presented at the Workshop on Governance of Pharmaceuticals in MENA Amman, Jordan June 6,
Pharmaceutical Sector Governance in the Middle East and North Africa Region A Regional Review by the World Bank Presented at the Workshop on Governance of Pharmaceuticals in MENA Amman, Jordan June 6,
Re : EC Consultation Paper Revision of the variations regulations
 Merck Sharp & Dohme (Europe) Inc. Lisette Vromans Clos du Lynx 5 Lynx Binnenhof Regulatory Policy Brussel 1200 Bruxelles EU & Most of World 32 2 776 6211 31 412 66 2755 Fax 31 412 66 2571 e-mail : lisette.vromans@merck.com
Merck Sharp & Dohme (Europe) Inc. Lisette Vromans Clos du Lynx 5 Lynx Binnenhof Regulatory Policy Brussel 1200 Bruxelles EU & Most of World 32 2 776 6211 31 412 66 2755 Fax 31 412 66 2571 e-mail : lisette.vromans@merck.com
Pharmacology & Regulatory Affairs
 2 nd International Conference & Expo on Pharmacology & Regulatory Affairs 06-08 August, 2018 Berlin, Germany Theme Cutting Edge Research Works in Pharmacy & Drug Development About Conference: Cenetri Publishing
2 nd International Conference & Expo on Pharmacology & Regulatory Affairs 06-08 August, 2018 Berlin, Germany Theme Cutting Edge Research Works in Pharmacy & Drug Development About Conference: Cenetri Publishing
NORMAN Network of reference laboratories for monitoring of emerging substances
 WFD Lille 2007 NORMAN Network of reference laboratories for monitoring of emerging substances Jaroslav Slobodník, Valeria Dulio Environmental Institute slobodnik@ei.sk Emerging environmental substances
WFD Lille 2007 NORMAN Network of reference laboratories for monitoring of emerging substances Jaroslav Slobodník, Valeria Dulio Environmental Institute slobodnik@ei.sk Emerging environmental substances
Putting the Criteria into Practice
 Putting the Criteria into Practice A Description of the Criteria & Evaluation Matrix Christopher Stopes, UK Outline Purpose How Criteria Matrix is used Structure - Application, Evaluation, Comparison:
Putting the Criteria into Practice A Description of the Criteria & Evaluation Matrix Christopher Stopes, UK Outline Purpose How Criteria Matrix is used Structure - Application, Evaluation, Comparison:
1. Identification of the substance/mixture and of the company/undertaking. Identified uses : Mixture for production of finished medicinal products.
 1. Identification of the substance/mixture and of the company/undertaking Designation : Actilyse, solid (Bulk),
1. Identification of the substance/mixture and of the company/undertaking Designation : Actilyse, solid (Bulk),
Review of the List of restricted substances under RoHS II
 Review of the List of restricted substances under RoHS II 2 nd Stakeholder Meeting 12 May 13 1 Review of the List of restricted substances under RoHS II- 2nd Stakeholder Meeting, Brussels, 13.5.2013 Proposal
Review of the List of restricted substances under RoHS II 2 nd Stakeholder Meeting 12 May 13 1 Review of the List of restricted substances under RoHS II- 2nd Stakeholder Meeting, Brussels, 13.5.2013 Proposal
Water reuse and water quality aspects in Europe
 Water reuse and water quality aspects in Europe Dr. Ulf Stein Ecologic Institute, Berlin CAPACITIE Training Course, Berlin U.Stein Table of contents Definition Why is water reuse important? Uses for recycled
Water reuse and water quality aspects in Europe Dr. Ulf Stein Ecologic Institute, Berlin CAPACITIE Training Course, Berlin U.Stein Table of contents Definition Why is water reuse important? Uses for recycled
Experience with Serialization Implementation as one powerful measure to protect our patients. Françoise Hirth
 Experience with Serialization Implementation as one powerful measure to protect our patients Françoise Hirth Objectives 1 2 3 Supply Chain integrity & product security A worldwide concern Pharma Industry
Experience with Serialization Implementation as one powerful measure to protect our patients Françoise Hirth Objectives 1 2 3 Supply Chain integrity & product security A worldwide concern Pharma Industry
2 Pharmacovigilance. 2.1 Fundamentals of Pharmacovigilance
 Pharmacovigilance 2 Pharmacovigilance 2 In this chapter, the fundamentals of pharmacovigilance are outlined with a particular emphasis on the role of healthcare professionals in reporting adverse drug
Pharmacovigilance 2 Pharmacovigilance 2 In this chapter, the fundamentals of pharmacovigilance are outlined with a particular emphasis on the role of healthcare professionals in reporting adverse drug
GLOBAL CHEMICAL REGISTRATIONS: TESTING STRATEGIES AND CMO COMMUNICATION
 GLOBAL CHEMICAL REGISTRATIONS: TESTING STRATEGIES AND CMO COMMUNICATION HPAPI Summit June 21, 2017 Jessica Graham, Ph.D., DABT Senior Research Investigator, Product Quality and Occupational Toxicology
GLOBAL CHEMICAL REGISTRATIONS: TESTING STRATEGIES AND CMO COMMUNICATION HPAPI Summit June 21, 2017 Jessica Graham, Ph.D., DABT Senior Research Investigator, Product Quality and Occupational Toxicology
Mapping the Patient Journey: Harnessing the power of big data and analytics A FirstWord ExpertViews Dossier Report
 AM PL E PA G ES S A S G ES data and analytics A FirstWord ExpertViews Dossier Report Published Copyright 2016 Doctor s Guide Publishing Limited All rights reserved. No part of this publication may be reproduced
AM PL E PA G ES S A S G ES data and analytics A FirstWord ExpertViews Dossier Report Published Copyright 2016 Doctor s Guide Publishing Limited All rights reserved. No part of this publication may be reproduced
VALUE OF COMPANION DIAGNOSTICS IN PERSONALISED MEDICINE
 VALUE OF COMPANION DIAGNOSTICS IN PERSONALISED MEDICINE Stimulating innovation for improving health through companion diagnostics 5 March 2015 I. Introduction Personalised healthcare provides targeted
VALUE OF COMPANION DIAGNOSTICS IN PERSONALISED MEDICINE Stimulating innovation for improving health through companion diagnostics 5 March 2015 I. Introduction Personalised healthcare provides targeted
HORIZON 2030 Executive Summary LPIA Workshop
 HORIZON 2030 Executive Summary LPIA Workshop 30th September 2017 1 CONTENTS P 4 1.0 Introduction 1.1 The Event & Objectives 1.2 The Agenda & Presentations 1.3 The Attendance 2.0 The profession 2.1 Role
HORIZON 2030 Executive Summary LPIA Workshop 30th September 2017 1 CONTENTS P 4 1.0 Introduction 1.1 The Event & Objectives 1.2 The Agenda & Presentations 1.3 The Attendance 2.0 The profession 2.1 Role
ASSESSMENT OF ENVIRONMENTAL AND HEALTH RISKS
 ASSESSMENT OF ENVIRONMENTAL AND HEALTH RISKS OF GMOS AND ASSOCIATED PESTICIDES PROJECT FOR A ONE-YEAR POSTDOCTORAL FELLOWSHIP FOR PR. G.E. SÉRALINI S TEAM, CAEN UNIVERSITY, FRANCE November 2014 1 PROPOSED
ASSESSMENT OF ENVIRONMENTAL AND HEALTH RISKS OF GMOS AND ASSOCIATED PESTICIDES PROJECT FOR A ONE-YEAR POSTDOCTORAL FELLOWSHIP FOR PR. G.E. SÉRALINI S TEAM, CAEN UNIVERSITY, FRANCE November 2014 1 PROPOSED
The ERA master file concept
 German Environment Agency Workshop How to achieve an appropriate Environmental Risk Assessment of Veterinary Medicinal Products The ERA master file concept Ines Rönnefahrt Federal Environment Agency, Germany
German Environment Agency Workshop How to achieve an appropriate Environmental Risk Assessment of Veterinary Medicinal Products The ERA master file concept Ines Rönnefahrt Federal Environment Agency, Germany
Resource efficiency targets and indicators
 Resource efficiency targets and indicators Dr. Martin Hirschnitz-Garbers Coordinator Resource Efficiency Ecologic Institute Table of content Development of the resource efficiency agenda Resource efficiency
Resource efficiency targets and indicators Dr. Martin Hirschnitz-Garbers Coordinator Resource Efficiency Ecologic Institute Table of content Development of the resource efficiency agenda Resource efficiency
European and German Waste Policy
 European and German Waste Policy Alexander Neubauer Ecologic Institute Table of content Definition of Waste European Waste Legislation as Framework Waste Management (General) Waste Treatment Legislation
European and German Waste Policy Alexander Neubauer Ecologic Institute Table of content Definition of Waste European Waste Legislation as Framework Waste Management (General) Waste Treatment Legislation
Risk Assessment in Pharmacy Practice
 Risk Assessment in Pharmacy Practice Executive Summary December 2010 Denham L. Phipps 1, Peter R. Noyce 1, Kieran Walshe 2, Dianne Parker 1, Darren M. Ashcroft 1 1 Centre for Innovation in Practice, School
Risk Assessment in Pharmacy Practice Executive Summary December 2010 Denham L. Phipps 1, Peter R. Noyce 1, Kieran Walshe 2, Dianne Parker 1, Darren M. Ashcroft 1 1 Centre for Innovation in Practice, School
TOWARDS A MANAGED OFF-LABEL USE OF DRUGS
 TOWARDS A MANAGED OFF-LABEL USE OF DRUGS CELINE VANNIEUWENHUYSEN, PIERRE SLEGERS, MATTIAS NEYT, FRANK HULSTAERT, SABINE STORDEUR, IRINA CLEEMPUT, IRM VINCK Unauthorised use vs use of unauthorised pharmaceuticals
TOWARDS A MANAGED OFF-LABEL USE OF DRUGS CELINE VANNIEUWENHUYSEN, PIERRE SLEGERS, MATTIAS NEYT, FRANK HULSTAERT, SABINE STORDEUR, IRINA CLEEMPUT, IRM VINCK Unauthorised use vs use of unauthorised pharmaceuticals
Cutting Edge Information
 Cutting Edge Information http://www.marketresearch.com/cutting Edge Information v3179/ Publisher Sample Phone: 800.298.5699 (US) or +1.240.747.3093 or +1.240.747.3093 (Int'l) Hours: Monday - Thursday:
Cutting Edge Information http://www.marketresearch.com/cutting Edge Information v3179/ Publisher Sample Phone: 800.298.5699 (US) or +1.240.747.3093 or +1.240.747.3093 (Int'l) Hours: Monday - Thursday:
Page 1/6. Printing date Reviewed on Identification of substance: Product details:
 Page 1/6 1 Identification of substance: Product details: Trade name: BSE60K Application of the substance / the preparation: Lubricant Manufacturer/Supplier: FUCHS EUROPE SCHMIERSTOFFE GMBH EXPORT DIVISION
Page 1/6 1 Identification of substance: Product details: Trade name: BSE60K Application of the substance / the preparation: Lubricant Manufacturer/Supplier: FUCHS EUROPE SCHMIERSTOFFE GMBH EXPORT DIVISION
Strategic trends facing the pharmaceutical industry and their implications for marketing skills development Received: 4th September, 2002
 Strategic trends facing the pharmaceutical industry and their implications for marketing skills development Received: 4th September, 2002 Gill Butler has worked in the pharmaceutical industry for 21 years,
Strategic trends facing the pharmaceutical industry and their implications for marketing skills development Received: 4th September, 2002 Gill Butler has worked in the pharmaceutical industry for 21 years,
Stakeholder education on biosimilar concepts - why does it matter globally?
 Stakeholder education on biosimilar concepts - why does it matter globally? Dr Virginia Acha Director, Regulatory Policy Europe, Middle East and Africa Amgen 26th Annual EuroMeeting 25-27 March 2014 ACV,
Stakeholder education on biosimilar concepts - why does it matter globally? Dr Virginia Acha Director, Regulatory Policy Europe, Middle East and Africa Amgen 26th Annual EuroMeeting 25-27 March 2014 ACV,
Foreign Affairs, Defence and Trade Select Committee Parliament Wellington. 24 March 2016
 Foreign Affairs, Defence and Trade Select Committee Parliament Wellington 24 March 2016 Dear Committee Members, Our apologies for a delayed written submission (attached) on the International treaty examination
Foreign Affairs, Defence and Trade Select Committee Parliament Wellington 24 March 2016 Dear Committee Members, Our apologies for a delayed written submission (attached) on the International treaty examination
