TransCelerate s Clinical Quality Management System: From a Vision to a Conceptual Framework
|
|
|
- Virgil Wheeler
- 6 years ago
- Views:
Transcription
1 Sponsored Special Section by TransCelerate BioPharma: Analytical Report TransCelerate s Clinical Quality Management System: From a Vision to a Conceptual Framework Therapeutic Innovation & Regulatory Science 2016, Vol. 50(4) ª The Author(s) 2016 Reprints and permission: sagepub.com/journalspermissions.nav DOI: / tirs.sagepub.com Ann Meeker-O Connell, MS 1, Leslie M. Sam, BA 2, Nanci Bergamo, BS 3, and Janis A. Little, MS 4 Abstract The Quality Management System (QMS) initiative of TransCelerate BioPharma Inc has identified potential benefits that could be captured from the development of a flexible, proactive clinical QMS conceptual framework for clinical research. Such a framework would aid organizations in seamlessly managing the complex clinical trial environment and, ultimately, in expediting delivery of needed treatments to patients. This article chronicles the evolution of a TransCelerate concept paper describing a proposed clinical QMS framework and reviews feedback from varied global clinical trial stakeholders during socialization of the concept paper. Many stakeholders recognized the potential for the concept paper to inform development of a harmonized International Council for Harmonisation (ICH) guideline, providing needed clarity from regulators on their expectations for QMS in the clinical realm. Accordingly, the article also describes TransCelerate s efforts to work with regulators to facilitate harmonization on this important topic and reviews ongoing work to develop additional tools and resources that may support organizations in evaluating whether and how they might translate the conceptual framework principles into practice. Keywords TransCelerate, QMS, clinical development, risk management, issue management, knowledge management, clinical quality Introduction Organizations engaged in drug development face an increasingly challenging environment: an expanding global footprint for clinical trials, evolving regulatory requirements, dynamic partnership models, and rapidly evolving technology. At the same time, many organizations are also innovating how trials are designed and conducted, adding to the complexity. Managing this complexity could be greatly enhanced by an effective and efficient Quality Management System (QMS). However, what constitutes an effective and efficient QMS is not well defined for clinical development. This article provides an update on the activities of a TransCelerate initiative developing a flexible, holistic, clinical QMS conceptual framework to address this gap. 1 Initial Development of a Vision and Outline for a Clinical QMS Conceptual Framework This TransCelerate effort started with identifying the gap in regulatory guidance for clinical trials. Prior to this, many organizations had been attempting to implement the International Council for Harmonisation Guidance Q10 (ICH Q10) for Good Manufacturing Practices (GMP), 2 or to follow other international standards dedicated to quality, such as ISO methodology. 3 None, however, suited the unique circumstances of clinical trials. This issue, and other related quality issues, led TransCelerate to work to develop a vision and outline for a clinical QMS conceptual framework. 1 This outline incorporated feedback from TransCelerate member companies on their existing clinical QMS, perspectives on QMS from other nonpharmaceutical industries, and key concepts (ie, risk-based thinking) from the revised International Organization for Standardization 9001 standard (ISO 9001:2015). 4 In particular, interviews with TransCelerate member companies revealed that some are using the GMP QMS framework (ie, ICH Q10) for their clinical development activities, leading to the challenge of translating a rule-based GMP QMS framework 1 BioResearch Quality and Compliance, Johnson & Johnson, 1 Johnson and Johnson Plaza, New Brunswick, NJ, USA 2 Global Quality Systems, Eli Lilly and Company, Indianapolis, IN, USA 3 Clinical and Medical Quality Operations, Sanofi, Bridgewater, NJ, USA 4 Global R&D Quality, Allergan Actavis, Bridgewater, NJ, USA Submitted 20-Mar-2016; accepted 2-May-2016 Corresponding Author: Ann Meeker-O Connell, MS, BioResearch Quality and Compliance, Johnson & Johnson, 1 Johnson and Johnson Plaza, New Brunswick, NJ 08933, USA. ameekero@its.jnj.com
2 398 Therapeutic Innovation & Regulatory Science 50(4) into a judgment-based clinical environment. These interviews also indicated that existing clinical QMS are neither optimally efficient nor effective and are frequently overengineered and reactive in nature. Rather than helping to manage the complexity of clinical development, existing clinical QMS add to it. Also, too often, QMS are considered the responsibility of a Quality function, furthering the misconception that Quality is the sole owner of quality. During socialization of the vision and outline, stakeholders across the clinical development ecosystem concurred that a holistic, flexible approach to the design and implementation of a clinical QMS would be a key foundation for addressing these challenges. Stakeholders agreed that recurring quality issues could be better addressed (and in many cases, prevented) through a risk-based, clinicalfocused QMS. In doing so, perceptions about clinical research could be transformed, especially for the most important stakeholders the patients. Discussions with health authorities, in particular, provided valuable insights into the current state of clinical QMS design and implementation. First, health authorities were unaware that, because of the absence of a harmonized guidance for a clinical QMS, organizations were using the GMP QMS described in ICH Q10 for their clinical development activities. These health authorities acknowledged the challenges with conducting clinical research using ICH Q10. Further, they noted that organizations are missing opportunities to apply knowledge management and drive continuous improvement through a clinical-focused QMS. Development of the Concept Paper The concept paper described in this article (see the Appendix) builds upon the initial outline and feedback received from stakeholders and presents a fully articulated conceptual framework. The following principles guided the development of this concept paper: 1. The framework described in the concept paper must support a shift from reactive to proactive, to preventing risks from becoming issues that undermine clinical development activities. 2. The framework must be flexible and adaptable to account for the diversity of organizations that may be interested in voluntarily implementing it. In keeping with the ultimate goal of transitioning the proposed clinical QMS framework to ICH to support harmonization, the level of detail in the concept paper is intended to be consistent with formal guidance. In order to allow the QMS to be adapted broadly, fine details for implementation have been deliberately excluded from the concept paper. For example, there are many different methods for designing processes and delivering associated training, and organizations should be free to utilize the methods most suited to their needs. Organizations seeking more tactical guidance will be able to leverage separate element-specific publications and other resources being developed by the TransCelerate QMS initiative. By applying the approach described in the concept paper, organizations should benefit from decreased time, energy, effort, and resources devoted to re-work and labor intensive issue resolution. Health authorities will also benefit from this right the first time approach that permits their reviewers to focus on regulatory science. In addition, the framework, through its focus on prospective dialogue and mutual understanding of expectations for partnered activities, should enhance clinical development collaborations (eg, vendors, sites, and co-development partners). Over time, use of the framework could transform the concept of quality to what we do as an organization in day-to-day clinical development activities and decision making. With strong leadership commitment to quality and a focus on continuous improvement, organizations should experience fewer quality-related delays in bringing needed safe and effective treatments to patients. Stakeholder Feedback on the Concept Paper External stakeholders including health authorities, industry and trade associations, and others have generally expressed support for the conceptual framework, with several common themes emerging. One theme was that an organized framework for a clinical QMS would benefit patients by minimizing and/or eliminating delays in bringing products to market, whether due to recurring errors or other inefficiencies within the clinical trial process. Multiple health authorities noted that the consistency afforded by sponsors using the conceptual framework might streamline their application reviews, expediting decision making and potentially speeding patient access to medicines (Figure 1). Another theme was that a unified approach within an organization that encourages cross-functional collaboration could remove silos within the organization that impede efficient operations. Lack of clarity in expectations and communication within an organization and with an organization s partners would be reduced and potentially eliminated. Stakeholders identified that efficiencies from the framework may also extend to those with whom clinical trial sponsors engage in designing, conducting, and overseeing trials. Clinical site-related organizations indicated they could also benefit from such a framework in 2 ways: (1) the conceptual framework might support their own quality management activities, and (2) the approach in the conceptual framework might make sponsors better partners to them. Another party echoed this latter statement, suggesting that by considering the external context in which trials are conducted, sponsors would be better able to tailor their quality oversight appropriately. However, while stakeholders strongly agreed that a harmonized regulatory guidance was welcome, a few expressed concerns about how such guidance might be applied by industry and regulators. With the concept paper potentially informing regulatory guidance development, the TransCelerate QMS
3 Meeker-O Connell et al 399 Figure 1. Summary of health authority feedback on the TransCelerate concept paper. ANVISA, Agência Nacional de Vigilância Sanitária (Brazilian Health Surveillance Agency); BfArM, Bundesinstitut für Arzneimittel und Medizinprodukte (Federal Institute for Drugs and Medical Devices, Germany); CFDA, China Food and Drug Administration; COFEPRIS, Comisión Federal para la Protección contra Riesgos Sanitarios (Federal Commission for the Protection against Sanitary Risk, Mexico); CQMS, Clinical Quality Management System; EMA, European Medicines Agency; FDA, Food and Drug Administration (United States); ICH, International Council for Harmonisation; MFDS, Ministry of Food and Drug Safety (South Korea); PMDA, Pharmaceuticals and Medical Devices Agency (Japan). initiative is actively working to alleviate these concerns. For example, some stakeholders worried that the principles-based conceptual framework might evolve to a more rules-based guidance that does not allow for clinical and scientific judgment. To address this, TransCelerate has engaged in dialogue with a broad range of health authorities throughout the concept paper s development, emphasizing the need for flexibility and tailoring. Initiative members will continue to engage with ICH through appropriate channels to reiterate the core principle of flexibility if a harmonized guidance is undertaken. Such guidance should establish that alternative approaches and tailoring are encouraged, as long as the underlying requirements of applicable statutes and regulations related to clinical development are satisfied. Other stakeholders identified a need for more practical direction on how to implement the conceptual framework within their organizations. As noted, the TransCelerate QMS initiative is currently developing more tactical guidance for key elements (such as Issue Management and Knowledge Management) that will support organizations that choose to voluntarily implement the conceptual framework. However, where relevant, the concept paper includes examples of practical applications of framework elements to support the concept paper s use by a diverse range of organizations (including those new to clinical research). Additional resources for change management are also being developed to support both those organizations that may be developing a clinical-focused QMS and those with an existing clinical QMS who may be interested in comparing it to the conceptual framework to identify potential enhancements. Finally, some stakeholders questioned how a clinical QMS relates to other quality management initiatives, such as clinical quality-by-design, 5 risk-based monitoring, 6,7 and the proposed integrated addendum to ICH E6 that explicitly mentions a system to manage quality throughout the design, conduct, recording, evaluation, reporting and archiving of clinical trials. 8 The concept paper addresses these connections, clarifying that a clinical QMS integrates trial-level quality management activities such as quality-by-design and risk-based monitoring into a holistic picture of quality for an organization.
4 400 Therapeutic Innovation & Regulatory Science 50(4) Ultimately the benefits, including the potential to reduce and eliminate rework, address challenges in collaboration and prevent recurring issues surpass the potential risks of a harmonized guidance (eg, the potential for increased inspectional scrutiny of QMS). Without a clearly defined clinical QMS conceptual framework, existing challenges will continue unabated. Quality will continue to be viewed as belonging to a singular function ( Quality ) rather than being owned by everyone involved in clinical development. Organizations will continue to expend resources unnecessarily and experience success in fixing problems rather than preventing them from occurring. Organizations will continue to face delays in getting approval to market their drugs as a result of preventable quality issues. Additionally, performance, both internally and in partnerships, will continue to be assessed primarily on speed of delivery rather than on proactive and collaborative management of risks and, ultimately, quality and timely delivery. Without the benefit of a clearly articulated clinical QMS framework, organizations may define their QMS reactively, in response to inspection findings. This reactive approach misses opportunities to strategically design a framework focused on the broad range of stakeholders, not only the expectations of inspectors, and to create an environment of continual improvement that supports innovation across clinical development. Finally, in the absence of the benefits such a QMS can provide, confidence in pharmaceutical research may decline and delays in bringing important therapeutic treatments to patients will continue. Conclusion and Next Steps This publication summarizes the journey that TransCelerate has taken to develop a clinical QMS conceptual framework. This framework will permit various stakeholders to apply a tailored approach to implementing a clinical-focused QMS that fits their specific organizational context and needs. Such a tailored clinical QMS will result in a clear and concise approach for all stages of clinical development and will benefit all clinical trial stakeholders. While ICH considers whether clinical QMS will be a topic for harmonization, TransCelerate QMS initiative members will continue to support the ICH process through public comment and ongoing dialogue with stakeholders. Initiative members will also continue to seek feedback and build upon the concepts articulated in the paper. Work to further elaborate 2 QMS elements, Issue Management and Knowledge Management, is ongoing. TransCelerate will develop additional detailed tactical guidance and resources for other QMS elements to support organizations interested in proactively and progressively adopting the proposed framework. These resources, like the conceptual framework itself, are intended to be tailored to organizational needs. Change management resources will also be developed and issued throughout Finally, to support companies who have elected to embrace this approach, Trans- Celerate will develop and communicate measures of clinical QMS effectiveness and share additional best practices and examples of practical application of the conceptual framework. Appendix: Enhancing Quality and Efficiency in Clinical Development Through a Clinical QMS Conceptual Framework 1. Introduction A Quality Management System (QMS) is an integrated approach through which an organization systematically defines quality objectives taking into account both its strategic objectives and applicable regulatory requirements and develops and implements the infrastructure, including the foundations, organizational structure, processes, and resources, required to achieve these objectives. QMS are successfully used to promote and manage quality and to improve performance in complex environments in industries ranging from aviation to information technology. While QMS have become ubiquitous across many industries, their adoption in the clinical development area has been challenging. This paper therefore offers a conceptual framework that will facilitate clinical development organizations in developing a QMS that is proactive, flexible, and tailored to the clinical environment. A QMS tailored to clinical development and the unique requirements of clinical development stakeholders can 1. support organizations in holistically and systematically managing quality across all clinical development activities from first-in-human trials through postmarketing studies, including noninterventional trials; 2. give organizations the means to efficiently achieve their clinical quality and organizational objectives; 3. Provide an organization s leadership with an integrated view of whether these objectives are being met and risks to patients and to data quality are appropriately managed systematically; 4. support organizations appropriately managing clinical development-related risks; 5. reduce recurring quality-related issues that undermine patient safety and data integrity and consume resources; 6. promote a culture of quality and knowledge management, permitting an organization to capitalize on opportunities to enhance its clinical development activities (ie, continuous improvement); and 7. ultimately increase confidence in clinical research. A clinical QMS enables consistent and efficient delivery of reliable data that may be used by an organization, its partners, health authorities, health care providers, and patients to make informed decisions concerning medicinal products. Moreover, in a research setting, a clinical-focused QMS will enable the consistent conduct and appropriate oversight of research that is
5 Meeker-O Connell et al 401 Figure A1. Relationships across clinical Quality Management Systems, quality-by-design, and risk-based monitoring. sound and ethical. Research participants will have assurance that the data generated from their participation will be used to advance scientific knowledge, and ultimately, patients can be confident that information they receive about medicines is based on sound research. 2. Scope This conceptual framework outlines a clinical QMS focused on the development of drugs, biologics, and vaccines from first-inhuman trials through postmarketing clinical activities, including noninterventional studies. Medical device development may be subject to existing GMP-focused QMS requirements, but this framework may support clinical aspects of their development. This framework provides a flexible, risk-based approach that can facilitate developing a clinical QMS that can continue the quality oversight provided by a preclinical QMS; integrate with a manufacturing QMS for clinical trial product supply; and transition to commercial manufacturing quality management. 2 Safety data collection, review, and reporting in the context of clinical development activities fall within the scope of a clinical QMS and should closely align with other pharmacovigilance activities to demonstrate end-to-end oversight of product safety. 3. Guiding Principles The conceptual framework described in this concept paper will enable development of a flexible and proactive clinical QMS through the following: a. encouraging an environment in which leadership drives a culture of quality, focused on prevention, in which all individuals are responsible for quality; b. facilitating a common understanding of quality management concepts as applied to clinical development; c. encouraging organizations to develop and improve their clinical QMS, regardless of the organization s size and operating context, and to align with other organizational QMS; d. overcoming a siloed approach to quality within organizations and ensuring a sustained focus on the fundamental goal of clinical development: serving the needs of patients and health care professionals and improving global health; e. highlighting the broad elements of a QMS tailored to a dynamic clinical environment; f. taking a holistic, clinical development wide view of quality, integrating quality measures from trial-level
6 402 Therapeutic Innovation & Regulatory Science 50(4) Figure A2. Foundations and elements of a clinical Quality Management System (QMS) conceptual framework. and program-level quality activities across all of clinical development; g. facilitating the efficient integration of new models, such as clinical quality by design (QbD) approaches; and h. encouraging data-driven decision making by capitalizing on available data, information, and knowledge and, as appropriate, technology advances. 4. Relationship of a Clinical QMS With Trial-Level Quality Activities A clinical QMS provides for clinical development wide management of quality, integrating trial-, program-, and functional-level quality activities. Clinical QbD is an overarching trial-level quality management approach that builds quality into trial design and operations through prospective identification of critical to quality aspects of a specific trial and subsequently focusing efforts on preventing (eg, through trial design) and/or mitigating errors that matter (ie, those errors that would materially impact the safety of patients or the credibility of conclusions based on trial data). 5 QbD sits within the broader clinical QMS and is itself an anchor for quality control activities such as risk-based monitoring (RBM). RBM is a quality control activity that can be used to identify and respond swiftly to errors that matter in a trial (TransCelerate s RBM initiative materials can be found at mainc.com/initiatives/risk-based-monitoring). Figure A1 depicts how QMS, QbD, and trial-level quality control activities such as RBM are related. 5. Foundational Aspects of a Clinical QMS Conceptual Framework Figure A2 shows the foundational aspects and conceptual elements recommended for a clinical QMS. The foundational aspects are described in this section and the elements in Sections 6 and Understanding the Context To develop an effective and efficient clinical QMS, the organization should evaluate and understand the external and internal environment (Figure A3) in which it operates. This evaluation will permit tailored development, refinement, and implementation of an organization s clinical QMS based on the unique aspects of the organization. Ready access to information relevant to the internal and external context is strongly facilitated by an effective knowledge management framework (see Section 6.6) External considerations An understanding of the organization s customers and other stakeholders and their requirements related to quality will shape the design of a clinical QMS. The range of external stakeholders for a clinical QMS can be broad, given the complex nature of clinical development. Each organization should define key stakeholders for their clinical development activities and perform an assessment of their views. This information will inform not only the clinical QMS design but the manner and cadence of engagement of these stakeholders, as applicable. The following are examples of external stakeholders whose needs and expectations should be considered: a. Clinical trial participants and the broader patient population, who may provide valuable input at all stages of the development process. b. Health authorities, including each applicable health authority s interpretation and application of its regulations and guidance, changes in this interpretation/application over time, and health authority decision making regarding product approvals. c. Institutional review boards, ethics committees, and data privacy boards with an interest in oversight of participant safety, privacy, and data protection.
7 Meeker-O Connell et al 403 Figure A3. Examples of external and internal considerations. d. Clinical investigators and site personnel who rely on objective evidence from prior development work to determine whether to be involved in current and future trials and studies. e. Partners, including sponsors/co-development partners, contract research organizations (CROs), and other stakeholders engaged in or supporting clinical development that require clarity of objectives and ongoing transparency and collaboration. f. Health care providers who rely on the quality of data from clinical development for making decisions in the clinic. g. Payers who utilize data from clinical trials, postmarketing studies, and real-world evidence to make coverage decisions. The organization should also monitor for new or evolving legislation, regulations, guidance and/or other significant changes in the external environment relevant to its activities and its partners. The evolution of regulatory policy and advances in regulatory science may all impact the design and operations of a clinical QMS. These regulatory intelligence activities are directly relevant to processes, risk management, issue management, and knowledge management Internal considerations An organization s strategic objectives (including its quality objectives), the plans for achieving them, its governance structure, and its culture strongly influence how a clinical QMS will be designed and implemented. Careful consideration should be given to how a clinical QMS will align with strategic objectives, as a QMS framework should support their achievement while maintaining compliance with applicable regulatory requirements. Similarly, a clinical QMS should be tailored and integrated within the governance structure, with flexibility to account for changes in governance. In turn, the governance structure should support the QMS. For example, considering the size and complexity of the organization, the appropriate governance structure can facilitate swift transparent decision making, avoid the development of silos within the organization, and facilitate enterprise wide input into the clinical QMS. The organization s culture can strongly influence successful QMS design and implementation. The organization should critically assess the current culture and value drivers within the organization to understand the extent to which these may support or hinder an effective QMS. Attention should be given to proactively addressing any cultural challenges that may impede effective and efficient design and sustained implementation of a clinical QMS. The organization should consult with the range of internal stakeholders who may impact, be impacted by, or perceive that they are impacted by a clinical QMS and/or the quality of clinical development. Internal stakeholders extend beyond the clinical development organization and may include, but are not limited to, legal, regulatory affairs, drug safety/pharmacovigilance, human resources, product manufacturing, procurement, finance, and staff members from other functions. These consultations may also identify process touchpoints and overlaps between functions and support evaluation of whether processes may be aligned, streamlined, and coordinated where possible, while avoiding a one-size-fits-all environment. For example, the organization may identify that there are many different training-related processes and systems across the enterprise, which may be aligned and streamlined while still accounting for necessary variations across disciplines Leadership Commitment to Quality An organization s senior leadership is accountable and responsible for defining and documenting expectations for quality and communicating the importance of quality to the organization. This message should be consistent, credible, and tangible, such that each individual understands how quality applies to him or her and other stakeholders.
8 404 Therapeutic Innovation & Regulatory Science 50(4) Leaders at all levels should visibly support this message and emphasize the importance of a collaborative approach to quality. Leaders should communicate that a quality function is an enabling function, to support and advise the business, not the function wholly responsible for ensuring quality. In addition to formal communication, leadership commitment can be demonstrated in a variety of ways including serving as a visible quality advocate; modeling desired behaviors to shape and reinforce a culture of quality; assuring sufficient resources and adequately trained staff is available across the organization to support all functions with a role in ensuring clinical quality; and rewarding proactive mitigation of risks to quality. An organization s senior leadership should have ongoing insight into the effectiveness and efficiency of its clinical QMS as well as a holistic view of the status of significant quality risks and issues (see Section 7.2). These senior leaders are accountable and responsible for defining the overall risk attitude of the organization and verifying that decisions to accept, avoid, mitigate, or transfer significant risks are aligned (see Section 6.4). They are also accountable and responsible for making decisions about actions required to address significant quality issues. Senior leaders may delegate their responsibility for ongoing management oversight. However, these leaders retain accountability and should ensure that staff members to whom these responsibilities are delegated have the appropriate authority, competence, and resources to carry them out effectively. Finally, given the dynamic nature of clinical development and the ongoing evolution of health authority requirements for quality, senior leadership should periodically review quality expectations and update them, as appropriate, to ensure stakeholder needs and requirements continue to be met Organizational Commitment to Quality Senior leaders and managers throughout the organization should cultivate an environment in which each individual takes ownership of quality in his or her role and holds himself or herself and others accountable for quality. The organization should empower individuals to address quality concerns by providing formal channels for communication and escalation without fear of reprisal, and the organization should provide opportunities for open and ongoing dialogue about quality. Individuals should be encouraged to identify and bring forward more effective and efficient ways to undertake activities Continuous Improvement of the Clinical QMS The organization should monitor the internal and external context for changes that might necessitate modification of its clinical QMS. The organization should seek opportunities to enhance effectiveness and/or efficiency of its clinical QMS. This is generally accomplished by leveraging outputs from the organization s knowledge management activities (Section 6.6), ongoing monitoring of process effectiveness and efficiency, conducting periodic self-assessments or internal audits, and engaging in benchmarking. Modification of an organization s clinical QMS or its individual elements should be supported by appropriate change management activities Elements of a Clinical QMS Conceptual Framework to Integrate Quality Across Clinical Development The following elements should generally be prospectively developed to integrate quality into clinical development activities: Processes; Resources, Roles, and Responsibilities; Partnering; Risk Management; Issue Management; Knowledge Management; and Documentation Supporting Achievement of Quality. These elements frequently intersect; for example, both knowledge management and issue management provide background information supporting risk management activities. Risk management in turn helps to define which potential issues might meet the threshold for action if they were to materialize Processes Processes should be well defined and characterized prior to determining which require supporting procedural documentation and which do not. Process mapping and other business process management tools may support clear articulation and understanding of processes and evaluation of the need for formal written procedures. Clear and concise procedural documents (eg, policies, standard operating procedures, and work instructions) should be developed as necessary to ensure consistency in process execution. Where processes cut across multiple functions, development of procedural documents may be best accomplished through cross-disciplinary teams. Leadership commitment to quality (Section 5.2) should also be reflected through review and approval of procedural documents by appropriate levels of leadership. The organization should identify the appropriate level and detail of procedural documentation needed to support clinical development. Procedural documents should be fit for purpose and should facilitate individuals in readily identifying their roles and associated responsibilities and the context of their roles within the broader process. Consideration should be given to including links to other relevant procedural documents. Procedural documentation should be created or modified primarily to address changes in the underlying process (eg, improvements in process efficiency identified through continuous improvement activities), address evolving customer requirements (eg, health authority requirements), or mitigate risks to quality objectives. Before developing a procedural document to mitigate a risk, the organization should verify that this action will meaningfully reduce the risk or eliminate its root cause. The
9 Meeker-O Connell et al 405 organization should also periodically assess information from issue management activities to identify whether issues could be alleviated through process redesign and/or the development, revision, and/or retirement of procedural documents. Knowledge management activities may also identify changes that warrant re-evaluation of processes and associated documents. These activities may also identify redundant documents that could be retired, simplifying the procedural document infrastructure. For any new or revised procedural document, the organization should consider the burden of implementation (including change management activities and training needs) and sustaining the process relative to the risk or requirement being addressed by the procedure. Organizations should craft procedural documents to be less sensitive to organizational changes to avoid frequent administrative revisions (eg, referring to generic roles instead of functional titles or specific individuals by name). The organization should determine how staff will be made aware of new and/or amended procedural documents and should determine appropriate architecture and methods to permit ready access by staff members. The organization should clearly identify those procedural documents that are considered mandatory (ie, represent regulatory or organizational requirements) and those that represent best practices and can aid staff in carrying out their activities. Training on procedural documents should be similarly focused and streamlined. Consideration should be given to what training is considered part of the core curriculum for those engaged in a given activity and which training could be delivered just in time (ie, training timed to the need to carry out a specific activity) Resources, Roles, and Responsibilities The organization should prospectively evaluate the required resources and skillsets for achieving its clinical strategy. The organization s leadership should ensure that appropriate resources are available, including both material resources (systems, standards, training environments) and staff. For staff members, leaders should ensure that there is clarity in roles, responsibilities, and accountability for operational effectiveness and quality at all levels throughout the organization, including with its partners. Leaders at all levels should manage resources in a proactive manner, by anticipating resource and expertise needs according to the strategic direction of the organization, its evolving risk profile, as well as the evolving regulatory environment. All staff, including temporary personnel and contractors, should be qualified by appropriate education, training, and/or experience to perform the activities for which they are responsible ( right people in the right place ). Prospective consideration should be given to the knowledge and competencies required for each role and how these competencies and knowledge will be achieved Partnering In a clinical development setting, partnerships include both collaborations in which organizations jointly develop a product (eg, co-development agreements) as well as outsourcing activities (eg, data management or clinical monitoring). Partnering involves forging successful strategic relationships. The organization should prospectively consider the needs, expectations, and limitations of all parties involved in a partnership as well as the risks posed by the activities to be carried out in such partnerships. The parties should be transparent about known risks relevant to the partnered activities and should agree upon an appropriate strategy for proactively identifying, assessing, and addressing risks throughout the partnership. While the Sponsor or Marketing Authorization Holder has ultimate accountability for quality, each party should take ownership of quality for its contribution to the partnership. A common understanding should be established among all parties regarding their specific roles in the overall quality of the partnered activities and how oversight of quality will be maintained. This should include their respective roles and responsibilities and how issues will be managed and escalated. Where applicable, the parties should prospectively determine how to bridge their respective quality systems in the management of the partnered activity. The parties should prospectively document their agreement regarding expectations for how activities will be conducted and overseen, communications (including escalations) will be managed, and performance will be measured. The level of documentation and oversight of partnered activities should be commensurate with the risks of the activities. This documentation may be as simple as a contractual clause or, where necessary, could be contained in a stand-alone quality agreement. The parties should periodically monitor and discuss the performance of all aspects of the partnership and identify and implement any needed improvements. Table A1 provides examples of points to consider during when establishing a partnership between a sponsor and CRO. It includes potential tools or resources that may support or document decisions made in these discussions Risk Management Overview Risk management enables an organization to achieve both organizational and quality objectives by taking explicit account of uncertainty about achieving these objectives and making informed decisions about how best to manage that uncertainty (ISO ). Risk management should ideally be integrated into day-to-day decision making throughout the organization. An organization s senior leadership should communicate the value of risk management and demonstrate their consideration of risk in decision making (eg, when setting organizational strategic plans).
10 406 Therapeutic Innovation & Regulatory Science 50(4) Table A1. Points to Consider When Establishing a CRO/Sponsor Partnership. Points to Consider 1. What are the specific tasks or activities that each party will carry out? Is there a mutual understanding of the scope of each activity (where it begins and where it ends)? 2. What are the roles and responsibilities of the various staff and functions at the CRO and sponsor? 3. Is there need for a formal governance structure for the relationship? Who has decision rights and in what context? 4. Which SOPs will be followed (including consideration of any training requirements)? 5. Is non-gcp-related training required for CRO staff (eg, policies on business conduct or privacy)? 6. What are the key risks related to the activities being undertaken in partnership? To whom and when should information about new or changing risks and/or issues be communicated (including escalations)? 7. What specific expectations does each party have related to quality and compliance? Are there specific considerations based on the activities and/or geography in which the activities may take place? 8. How will specific key performance indicators (KPIs) and key quality indicators (KQIs) be monitored and reviewed? 9. Where, how, and by whom will required records be maintained during the partnership and after its completion? Potential Tools 1. Detailed scope of work/task ownership matrix 2. Responsible, Accountable, Consulted, Informed (RACI) chart by role 3. Governance charter 4. Table of SOPs identifying which SOPs will be used for which activity 5. Training curricula 6. (a) Risk register listing key risks for the partnership/partnered activities and plans for mitigation; (b) communication plan; (c) escalation pathway 7. Quality charter 8. Metrics scorecard/dashboard 9. Record retention plan, including media (eg, paper, electronic) Abbreviations: CRO, contract research organization; GCP, Good Clinical Practice; SOP, standard operating procedure. Risk management is a process through which risks are identified and characterized to permit timely and data-driven decisions about whether and how they need to be addressed. Care should be taken to distinguish between issues (events that have occurred) and risks (potential events with a range of potential consequences). Focusing on risks and their management allows organizations to shift to a proactive and predictive state, prioritizing resources to address the most significant strategic, operational, quality, and compliance-related risks. The following are examples of these types of risks: a. Strategic risk: Will engaging in licensing opportunities enhance an organization s product portfolio and ability to serve patients? b. Operational risks: Willtheorganizationbeableto deliver investigational product in a timely manner for all clinical activities throughout the lifecycle of a compound s development such that patient safety is not impacted by disruptions in treatment? c. Quality risks: Will the primary endpoint criteria be consistently interpreted by clinical investigators across a development program, such that health authorities and others relying on the aggregated data find them credible? d. Compliance risks: Will the organization be able to meet varied regional regulatory requirements for trial registration and disclosure? The organization should prospectively define a framework for how it will manage risks effectively and efficiently, including how risk management will be integrated with other activities (eg, in setting the thresholds for issue triage; see Section 6.5). A generic risk management framework and process that can be tailored to any organization is described in ISO The framework and associated activities should permit the organization to differentiate significant risks from those with minimal impact on patient safety, data integrity/scientific rigor, regulatory compliance, and trust in the organization. Finally, the framework should enable the integration of risks across functions and clinical development programs to provide leadership with a holistic view of significant risks and their mitigations Components of a Risk Management Process A risk management process generally consists of the following: a. Understanding the context: Risk management activities should leverage information about the internal and external environment (Section 5.1 and Figure A3) to identify changes that might create risks. For example, a significant decrease in staffing in a key function may create operational and compliance challenges if overall workload is unchanged. b. Risk assessment: Risk assessment consists of three steps: risk identification, risk analysis, and risk evaluation. A variety of risk assessment techniques and
11 Meeker-O Connell et al 407 Figure A4. Applying risk management to regulatory requirements regarding clinical trial disclosure. considerations for their use are described in ISO A risk assessment yields an understanding of how significant a risk is to objectives and leads to a decision on whether or not a risk requires action. Risks are identified and then each is analyzed by considering the impact (significance) of its consequences, how likely it is that these consequences will occur, and the effectiveness of existing risk mitigations. The combination of the impact and likelihood, accounting for the effect of existing mitigations, is the current level of risk. Risks are evaluated by comparing the current level of risk to the level of risk the organization will accept (Section 5.1.2) to determine whether actions need to be taken to reduce the risk. c. Risk mitigation: Action(s) are identified that will address the risk. Organizations should consider a range of options including avoiding the risk, changing its likelihood and/or consequences, transferring the risk (eg, through insurance), or accepting the risk by informed decision. A key question to consider is whether proposed actions will reduce the risk to an acceptable level. If not, then these activities will add burden with little benefit. d. Risk monitoring and review: The organization should evaluate whether actions taken to address risks are achieving the desired outcome. Issue management will provide valuable insight into risk mitigation strategies that may not be optimal. When an issue occurs, risk assessment should be revisited to determine whether the risk of the issue occurring was not identified during assessment; whether risk mitigations were appropriately designed but not implemented; and/or whether new risk mitigation activities are needed to appropriately control the risk. In addition, the organization should periodically assess whether changes in the internal or external context (eg, new regulations) or accumulating information from knowledge and issue management activities (eg, trend analyses) might yield new risks or modify existing risk management strategies. e. Communication and consultation: Appropriate communication and consultation with stakeholders, including health authorities as applicable, should occur at all stages of the risk management process. Example: Application of risk management The following is a specific example of how risk management can be applied in clinical research. An organization may face an array of regulatory requirements related to disclosing clinical trials on public registries (eg, One risk would be if new clinical trials meeting registration requirements were not appropriately disclosed on one or more of these registries. The organization could brainstorm on the potential causes and consequences of this risk (Figure A4). For example, lacking no mechanism to quickly notify those responsible for clinical trial registration of a new trial might lead to no or late registration, with the potential for loss of trust in the organization and regulatory action such as fines or penalties. The organization could determine that the impact of this potential failure to register a trial would be too significant and the likelihood too high without specific mitigations for this scenario. This should prompt dialogue and decisions on what actions will be taken to prevent or mitigate the risk (eg, creating a process step at protocol approval to mandate sharing of information with relevant staff or making site activation dependent on the trial information having been submitted for registration). These mitigations can then be subject to periodic monitoring and review, with defined thresholds for when an issue related to trial registration would become a significant issue (ie, an Issue that Matters, as defined below) Issue Management An effective issue management framework will improve identification, investigation/assessment, escalation, and communication of significant issues (ie, Issues that Matter ). Issues that Matter are issues that materially impact any of the following:
12 408 Therapeutic Innovation & Regulatory Science 50(4) Figure A5. Conceptual approach for defining and triaging issues. Patient safety, rights, and well-being; Data integrity and/or scientific rigor; Compliance with regulatory requirements; Trust in the clinical research enterprise. Such a framework will provide end-to-end management of issues and support an effective Corrective and Preventive Actions (CAPA) process commensurate with the impact of the issue. An effective framework will also enhance risk management strategies and drive continuous improvement through predictive analytics. With an effective issue management framework, significant issues should not recur, drugs may get to patients faster, and resource requirements of drug development may be reduced, all contributing to the improvement of the quality of clinical studies Issue detection Within any organization, issues can be reported or detected by examining information from multiple sources. Use of methodologies that integrate information from different sources may result in identification of issues that would not be found from any single source alone. For example, linking investigator site audit/inspection data to clinical operations investigator databases can be leveraged for detecting issues Issue documentation and investigation An issue management framework will enable consistent recording and investigation of Issues that Matter (Figure A5). As described below (Section 6.5.3), Issues that Matter should be investigated (gathering of pertinent information to facilitate analysis), analyzed (determining the underlying root cause[s] of the issue), and corrected/prevented via a robust CAPA plan. There should be ongoing assessment of any immediate corrections needed as well as the need for escalation to management and reporting to regulatory agencies. Issues not meeting the threshold for significance should be documented as appropriate to enable trending. This documentation should be
13 Meeker-O Connell et al 409 Figure A6. Example of issue triage thresholds for a clinical trial. commensurate with the complexity of the issue and potential impact. Examples of Issues that Matter: Over a 1-year period, the Sponsor repeatedly failed to send Suspected Unexpected Serious Adverse Reactions (SUSAR) reports for an investigational drug to multiple health authorities. An electronic Patient-Reported Outcomes (epro) system that was used across multiple development programs did not have an audit trail, bringing into question whether patients entered the data themselves and whether these data had been inappropriately changed. Figure A6 shows how an organization that has prospectively identified risk (or potential issues ) related to a trial and set thresholds for action will be well positioned to quickly act when an Issue that Matters materializes The CAPA process Issues that Matter should be managed by a CAPA process. The following are the basic elements of an effective and sustainable CAPA process: a. Taking immediate action to prevent further negative impact from the issue (correction). b. Identifying the accountable person(s) for: 1. Investigating the issue (gathering the necessary information); 2. Determining the underlying root cause of the issue (eg, through performing root cause analysis); and 3. Performing a holistic assessment of the scope and impact of the issue to understand whether it is isolated or systemic. c. Identifying the accountable person(s) to develop a robust CAPA plan with at least the following elements: 1. Actions that address the identified root causes; 2. Implementation details including assigned responsibilities and timelines; and 3. Effectiveness checks to ensure the actions achieve the desired outcome and future occurrences are prevented. d. Implementing the CAPA plan, including effectiveness checks. e. Verifying the long-term sustainability of the corrective and preventive actions. Delays or significant challenges with the execution of the CAPA process should be escalated to an appropriate level of management Trending and analytics An effective issue management framework should foster continuous improvement and enhance risk management through detection of trends that may represent systemic issues or risks. Trend analysis is also a vital input to risk assessment (eg, providing information on the likelihood of an event occurring). Effective trend analysis can be achieved with the following: a. Organizational commitment and resources b. Timely, comprehensive, complete, and meaningful data/information c. Identification of critical data/information from organizational processes and activities
14 410 Therapeutic Innovation & Regulatory Science 50(4) d. Method for accessing/analyzing the data/information in knowledge management repositories (eg, data/information structured for ease of retrieval) Trending activities may lead to the identification of new Issues that Matter for which appropriate actions should be taken. For example: it was discovered that a patient had not given informed consent for a blood draw for a substudy. Over a 4-month period, the same issue was detected at multiple sites across multiple trials. Collectively, these issues were elevated to an Issue that Matters Knowledge Management A knowledge management (KM) framework enables the effective and successful implementation of a clinical QMS. The goal of managing knowledge is to improve organizational performance by getting the right information to the right people at the right time (ie, critical knowledge). 12 Loss of knowledge or the failure to apply knowledge may have negative consequences for organizations and most importantly, for patients. Consider the following scenario where a company identified a series of drug candidates with unique mechanisms of action that could potentially transform the care of patients and address a high unmet medical need. Delays plagued the development program of the innovator company due to key staff departures and lack of project team access to either relevant prior knowledge or across-team learnings. As a result, the company ended up being delayed to market and access by patients to transformational medicines was delayed by almost 2 years. Examples of root causes that could have been addressed with a knowledge management strategy and approach include the following: Knowledge and insights from previous and concurrent clinical programs were not documented and shared across clinical development teams. Company culture resulted in silos which did not actively promote sharing across clinical development teams; therefore, subject matter expertise and experience were not fully leveraged. There was no procedure in place to capture knowledge when key staff members left development programs; thus, historical context and lessons learned were lost. Data and information related to each asset were maintained in different repositories and were not easily accessible or searchable. Implementing a structured approach to KM within clinical development can enable organizations to effectively utilize knowledge, thereby accelerating delivery of new medicines to patients by a. increasing productivity by enabling employees to search, find, and apply knowledge faster; b. achieving and improving quality and compliance through leveraging best practices and lessons learned; and c. enhancing the ability to maintain and sustain institutional knowledge so that it can be shared and applied at an organizational level. A KM framework includes strategies and processes designed to identify, capture, structure, value, leverage, and share the organization s intellectual assets to enhance the clinical development organization s performance and the performance of QMS elements including issue management and risk management. Fundamentals of this framework include a. the capture, storage, and accessibility of institutional explicit (formal and systematic) and tacit (personal expertise) knowledge, and b. its assimilation, interpretation, dissemination, application, and maintenance within an organization Types of knowledge There are two types of knowledge the organization should consider: explicit and tacit. Explicit knowledge is visible and easily recognized, shared, documented, and accessed. Examples include tangible items such as procedural documents and clinical development plans. Tacit knowledge is harder to identify, document, and transfer as it is mainly acquired from the experience of individuals or groups (eg, subject matter experts and trial teams). The loss of tacit knowledge may be most evident when a key employee leaves an organization. In cases where tacit knowledge cannot readily be made explicit, mechanisms for collaboration and communication will help the exchange and maintenance of this knowledge Knowledge management conceptual framework A knowledge-sharing culture and continuous improvement of the KM framework are important foundational aspects of the KM framework employed by the organization. Organizations should take steps to address behaviors that may impede knowledge sharing (eg, the perception that knowledge is power). A KM framework may include the following: a. People responsible for sponsorship, contribution, and application of knowledge, and the maintenance and credibility of the captured and stored knowledge. b. Processes that drive and support the implementation of knowledge management within the organization. c. Content that includes and enables access to and retrieval of critical knowledge and/or subject matter experts. d. Technology as an enabler to facilitate capture, use, and maintenance of critical knowledge over time.
15 Meeker-O Connell et al Considerations for a knowledge management framework The following considerations can further support the implementation of an effective KM framework: a. Understand the Knowledge Needs: Identify the critical knowledge necessary to drive optimal outcomes tailored to organizational needs. b. Assess Opportunity: Understand where improvement of knowledge flow may provide opportunity for enhanced execution to improve critical business outcomes. c. Design and Implement Solutions: Define and deploy mechanisms to enable and improve knowledge access and sharing. d. Improve and Sustain: Assess performance against anticipated outcomes and adjust as necessary. Assess opportunities for continuous improvement to sustain the framework over time Documentation Supporting Achievement of Quality The level of documentation for any clinical development related process should be commensurate with the risks of the activity and the significance of the activity to achieving quality objectives and meeting stakeholder requirements. How, where, and for what period of time documentation will be retained should be established prospectively. 7. Elements of a Clinical QMS Conceptual Framework That Support Continuous Improvement The following elements should be developed to evaluate the overall status and health of the clinical QMS and as part of continuous improvement: Assessing the Clinical Quality Management System and Management Review Assessing the Clinical Quality Management System To ensure the initial implementation and ongoing effectiveness and efficiency of its clinical QMS, the organization should prospectively define how the elements and foundational aspects will be evaluated. These activities provide the organization with insight into whether the QMS design is appropriate and continues to be fit for purpose. The performance of the QMS elements should be assessed both individually and in aggregate. These assessments are an important input to management review (Section 7.2). An effective plan for assessment typically includes the following steps: a. Assess the current state against the clinical QMS conceptual framework. b. Identify gaps and/or inefficiencies as well as interdependencies between elements. c. Evaluate the impact of the gaps and/or inefficiencies and identify what improvements/enhancements are needed. d. Prioritize development and implementation of identified improvements/enhancements. e. Recommend actions to senior leadership (ie, during management review). A baseline assessment should be performed, followed by periodic evaluations at appropriate intervals as part of continuous improvement or in response to changes in the organization s environment. For example, a change in the organization s portfolio (part of the organization s internal and external context) should trigger an evaluation of the continued suitability of the existing clinical QMS elements. Assessing the current state against the clinical QMS framework generally occurs first on an individual element basis, comparing what an organization has in place vs. the concepts described for that element. For example, in Figure A7, an organization could determine that it has implemented most, but not all, items needed for maturity level 2 for the Issue Management element. This information can then be utilized to evaluate impact and identify what improvements or enhancements may be required, based on the organization s needs. This assessment against the clinical QMS conceptual framework can also be done in aggregate. Overall QMS maturity may be determined, and the organization can identify gaps and/or inefficiencies as well as interdependencies between the elements Management Review Senior leadership with the appropriate accountability and decision-making authority for clinical development should periodically review the performance of the QMS to ensure that it a. continues to fulfill the quality objectives, b. remains aligned with the strategic direction of the organization, c. is supported by appropriate resources, and d. provides benefits that exceed the burden of maintenance. The frequency, structure, and content of the management reviews should be based on the maturity of the QMS and the complexity of the organization. During this review, senior leaders should determine the need for action and communicate decisions to all appropriate levels within the organization. Figure A8 provides examples of potential data and information that may be considered during management reviews as well as examples of potential outcomes from these reviews.
16 412 Therapeutic Innovation & Regulatory Science 50(4) Figure A7. Individual element assessment for issue management. Potential Inputs to Management Review Progress toward Quality Objectives Outputs from QMS maturity assessment Audits and inspections Status of Significant Risks Metrics on"issues that Matter" and associated CAPA completion and effectiveness Commitments to regulatory authorities Changes in the internal and external environment Key Process changes Process performance Partnership performance Resource challenges Follow-up actions from previous MRs Potential Outputs from Management Review Affirmation that quality objectives remain appropriate and relevant and aligned with the strategic direction of the organization Affirmation that the QMS remains fit-for-purpose Improvements to the QMS Updated risk profile Prioritization of resources and activities (eg, mitigation of key risks, improvements to the clinical QMS) Figure A8. Potential inputs to and outputs from management review. CAPA, Corrective and Preventative Actions; MR, management review; QMS, Quality Management System.
Singapore Clinical Research Professional (CRP) /Clinical Research Coordinator Society (CRCS) Forum
 Singapore Clinical Research Professional (CRP) /Clinical Research Coordinator Society (CRCS) Forum 25 August 2017 TOPIC: Issue Management / Quality Risk Management Implications with ICH GCP E6 (R2) and
Singapore Clinical Research Professional (CRP) /Clinical Research Coordinator Society (CRCS) Forum 25 August 2017 TOPIC: Issue Management / Quality Risk Management Implications with ICH GCP E6 (R2) and
An integrated model approach to improve the management of marketed products
 Insight brief Regulatory and safety integration An integrated model approach to improve the management of marketed products Leo Dodds, Principal, Quintiles Advisory Services John Rogers, Engagement Leader,
Insight brief Regulatory and safety integration An integrated model approach to improve the management of marketed products Leo Dodds, Principal, Quintiles Advisory Services John Rogers, Engagement Leader,
Q10 PHARMACEUTICAL QUALITY SYSTEM
 Q10 PHARMACEUTICAL QUALITY SYSTEM This draft guidance, when finalized, will represent the Food and Drug Administration's (FDA's) current thinking on this topic. It does not create or confer any rights
Q10 PHARMACEUTICAL QUALITY SYSTEM This draft guidance, when finalized, will represent the Food and Drug Administration's (FDA's) current thinking on this topic. It does not create or confer any rights
inventivhealthclinical.com The Goal: Reducing Uncertainty A Word on RBM
 Seeing Around Corners: Risk Assessment Is the Foundation of Risk-Based Monitoring By: Jeff Fetterman, President ParagonRX, an inventiv Health Company, Michael Macri, Director, Strategic Services, inventiv
Seeing Around Corners: Risk Assessment Is the Foundation of Risk-Based Monitoring By: Jeff Fetterman, President ParagonRX, an inventiv Health Company, Michael Macri, Director, Strategic Services, inventiv
TransCelerate Overview. Tozheg Roshankar
 TransCelerate Overview Tozheg Roshankar 11, Aug, 2016 TransCelerate is a not for profit entity created to drive collaboration Our vision To improve the health of people around the world by accelerating
TransCelerate Overview Tozheg Roshankar 11, Aug, 2016 TransCelerate is a not for profit entity created to drive collaboration Our vision To improve the health of people around the world by accelerating
The Role of the CRO in Effective Risk-Based Monitoring
 New Whitepaper The Role of the CRO in Effective Risk-Based Monitoring The clinical trial industry is evolving. In an effort to improve participant safety and data integrity, regulators are encouraging
New Whitepaper The Role of the CRO in Effective Risk-Based Monitoring The clinical trial industry is evolving. In an effort to improve participant safety and data integrity, regulators are encouraging
CDER Perspective: Challenges in Clinical Trials and the Path Forward
 CDER Perspective: Challenges in Clinical Trials and the Path Forward Pharmaceutical Compliance Congress and Best Practices Forum November 3, 2011 Ann Meeker O Connell, MS, CCEP Associate Director (Acting),
CDER Perspective: Challenges in Clinical Trials and the Path Forward Pharmaceutical Compliance Congress and Best Practices Forum November 3, 2011 Ann Meeker O Connell, MS, CCEP Associate Director (Acting),
BEST PRACTICES GUIDE COMPLYING WITH THE ICH E6(R2) ADDENDUM
 BEST PRACTICES GUIDE COMPLYING WITH THE ICH E6(R2) ADDENDUM Six steps to ensuring risk-based quality management in clinical trials EVOLVING REGULATIONS ON RISK-BASED MONITORING In 2016, the International
BEST PRACTICES GUIDE COMPLYING WITH THE ICH E6(R2) ADDENDUM Six steps to ensuring risk-based quality management in clinical trials EVOLVING REGULATIONS ON RISK-BASED MONITORING In 2016, the International
POSITION PROFILE FOR THE CHIEF OF THE WINNIPEG POLICE SERVICE. Last updated October, 2015
 POSITION PROFILE FOR THE CHIEF OF THE WINNIPEG POLICE SERVICE Last updated October, 2015 1 PREFACE The Winnipeg Police Board is required by Section 21 of Manitoba s Police Services Act to appoint a person
POSITION PROFILE FOR THE CHIEF OF THE WINNIPEG POLICE SERVICE Last updated October, 2015 1 PREFACE The Winnipeg Police Board is required by Section 21 of Manitoba s Police Services Act to appoint a person
For all the talk about how risk-based
 October 2017 A CenterWatch Feature Article Reprint Volume 24, Issue 10 Tracking adoption of risk assessment and RBM Growing utilization but wide variation in approaches and impact Tracking the adoption
October 2017 A CenterWatch Feature Article Reprint Volume 24, Issue 10 Tracking adoption of risk assessment and RBM Growing utilization but wide variation in approaches and impact Tracking the adoption
The Ohio State University Human Resources Strategic Plan
 Human Resources 2018-2023 Strategic Plan Finalized: May 16, 2018 Delivering HR Excellence. Inspiring People. Leading Change. HR.OSU.EDU 1590 N. High Street, Suite 300 Columbus, OH 43201 614-292-1050 Table
Human Resources 2018-2023 Strategic Plan Finalized: May 16, 2018 Delivering HR Excellence. Inspiring People. Leading Change. HR.OSU.EDU 1590 N. High Street, Suite 300 Columbus, OH 43201 614-292-1050 Table
BEST PRACTICES IN Talent Management Article Title Format
 SCHOONOVER ASSOCIATES WHITE PAPER BEST PRACTICES IN Talent Management Article Title Format SCHOONOVER ASSOCIATES, LLC. 2015 Dr. Stephen C. Schoonover President, Schoonover Associates, LLC Contents Executive
SCHOONOVER ASSOCIATES WHITE PAPER BEST PRACTICES IN Talent Management Article Title Format SCHOONOVER ASSOCIATES, LLC. 2015 Dr. Stephen C. Schoonover President, Schoonover Associates, LLC Contents Executive
PORTLAND PUBLIC SCHOOLS HUMAN RESOURCE SERVICES AND DELIVERY
 PORTLAND PUBLIC SCHOOLS HUMAN RESOURCE SERVICES AND DELIVERY January 2013 Overview Portland Public School District (the District or PPS) contracted with AKT to create a new vision and values for their
PORTLAND PUBLIC SCHOOLS HUMAN RESOURCE SERVICES AND DELIVERY January 2013 Overview Portland Public School District (the District or PPS) contracted with AKT to create a new vision and values for their
Recommendations for Strengthening the Investigator Site Community
 Recommendations for Strengthening the Investigator Site Community October 2017 CTTI MISSION: To develop and drive adoption of practices that will increase the quality and efficiency of clinical trials
Recommendations for Strengthening the Investigator Site Community October 2017 CTTI MISSION: To develop and drive adoption of practices that will increase the quality and efficiency of clinical trials
Risk Management in Clinical Trials in Today s ICH-GCP(R2) Framework
 Clinical Research in Resource Limited Settings: Mission Impossible or Role Model for Future Drug Development? Risk Management in Clinical Trials in Today s ICH-GCP(R2) Framework Ingrid Klingmann, MD, PhD,
Clinical Research in Resource Limited Settings: Mission Impossible or Role Model for Future Drug Development? Risk Management in Clinical Trials in Today s ICH-GCP(R2) Framework Ingrid Klingmann, MD, PhD,
Textvergleich. Verglichene Dokumente MEDIA3917.pdf. ICH_Q10_Step4.pdf
 Textvergleich Verglichene Dokumente MEDIA3917.pdf ICH_Q10_Step4.pdf Übersicht 2270 Wort/Wörter hinzugefügt 1338 Wort/Wörter gelöscht 3558 Wort/Wörter übereinstimmend 157 Block/Blöcke übereinstimmend Blättern
Textvergleich Verglichene Dokumente MEDIA3917.pdf ICH_Q10_Step4.pdf Übersicht 2270 Wort/Wörter hinzugefügt 1338 Wort/Wörter gelöscht 3558 Wort/Wörter übereinstimmend 157 Block/Blöcke übereinstimmend Blättern
IT GOVERNANCE AND MANAGED SERVICES Creating a win-win relationship
 IT GOVERNANCE AND MANAGED SERVICES Creating a win-win relationship TABLE OF CONTENTS IT Governance and Managed Services 3 ROLE OF IT GOVERNANCE AND OUTSOURCING 3 IT GOVERNANCE AND THE OUTSOURCING CONTRACT
IT GOVERNANCE AND MANAGED SERVICES Creating a win-win relationship TABLE OF CONTENTS IT Governance and Managed Services 3 ROLE OF IT GOVERNANCE AND OUTSOURCING 3 IT GOVERNANCE AND THE OUTSOURCING CONTRACT
Example Approach To Non-Profit Organizations. ExeComp Solutions Compensation Advisory Services May 2014
 Example Approach To Non-Profit Organizations ExeComp Solutions Compensation Advisory Services May 2014 ExeComp Solutions LLC (ECS) appreciates the opportunity to present our services to The Museum We are
Example Approach To Non-Profit Organizations ExeComp Solutions Compensation Advisory Services May 2014 ExeComp Solutions LLC (ECS) appreciates the opportunity to present our services to The Museum We are
Quality Management System Guidance. ISO 9001:2015 Clause-by-clause Interpretation
 Quality Management System Guidance ISO 9001:2015 Clause-by-clause Interpretation Table of Contents 1 INTRODUCTION... 4 1.1 IMPLEMENTATION & DEVELOPMENT... 5 1.2 MANAGING THE CHANGE... 5 1.3 TOP MANAGEMENT
Quality Management System Guidance ISO 9001:2015 Clause-by-clause Interpretation Table of Contents 1 INTRODUCTION... 4 1.1 IMPLEMENTATION & DEVELOPMENT... 5 1.2 MANAGING THE CHANGE... 5 1.3 TOP MANAGEMENT
Sponsor/CRO Partnership Optimization
 Insight Brief Sponsor/CRO Partnership Optimization Developing a CRO Governance Model Geoff Garabedian, Vice President and Managing Director, Consulting at Quintiles Josh Samon, Principal Life Sciences
Insight Brief Sponsor/CRO Partnership Optimization Developing a CRO Governance Model Geoff Garabedian, Vice President and Managing Director, Consulting at Quintiles Josh Samon, Principal Life Sciences
Enterprise Risk Management Framework
 Enterprise Risk Management Framework 2018 Johnson & Johnson 1 2 Introduction In order to deliver value to our consumers, patients, caregivers, employees, communities and shareholders, we at Johnson & Johnson
Enterprise Risk Management Framework 2018 Johnson & Johnson 1 2 Introduction In order to deliver value to our consumers, patients, caregivers, employees, communities and shareholders, we at Johnson & Johnson
CDER Perspective: Good Clinical Practice
 CDER Perspective: Good Clinical Practice Sensible Guidelines Symposium, 25 May 2012 Ann Meeker-O Connell FDA, CDER, Office of Compliance Office of Scientific Investigations The views presented in this
CDER Perspective: Good Clinical Practice Sensible Guidelines Symposium, 25 May 2012 Ann Meeker-O Connell FDA, CDER, Office of Compliance Office of Scientific Investigations The views presented in this
Core Values and Concepts
 Core Values and Concepts These beliefs and behaviors are embedded in highperforming organizations. They are the foundation for integrating key performance and operational requirements within a results-oriented
Core Values and Concepts These beliefs and behaviors are embedded in highperforming organizations. They are the foundation for integrating key performance and operational requirements within a results-oriented
Stepping Into the Future of Pharmacovigilance
 PAREXEL White Paper Stepping Into the Future of Pharmacovigilance The What, Why and How of Knowledge Process Outsourcing Mature biopharmaceutical products with established safety profiles frequently represent
PAREXEL White Paper Stepping Into the Future of Pharmacovigilance The What, Why and How of Knowledge Process Outsourcing Mature biopharmaceutical products with established safety profiles frequently represent
City of Saskatoon Business Continuity Internal Audit Report
 www.pwc.com/ca City of Saskatoon Business Continuity Internal Audit Report June 2018 Executive Summary The City of Saskatoon s (the City ) Strategic Risk Register identifies Business Continuity as a high
www.pwc.com/ca City of Saskatoon Business Continuity Internal Audit Report June 2018 Executive Summary The City of Saskatoon s (the City ) Strategic Risk Register identifies Business Continuity as a high
International Standards for the Professional Practice of Internal Auditing (Standards)
 INTERNATIONAL STANDARDS FOR THE PROFESSIONAL PRACTICE OF INTERNAL AUDITING (STANDARDS) Attribute Standards 1000 Purpose, Authority, and Responsibility The purpose, authority, and responsibility of the
INTERNATIONAL STANDARDS FOR THE PROFESSIONAL PRACTICE OF INTERNAL AUDITING (STANDARDS) Attribute Standards 1000 Purpose, Authority, and Responsibility The purpose, authority, and responsibility of the
GxP Auditing, Remediation, and Staff Augmentation
 GxP Auditing, Remediation, and Staff Augmentation TABLE OF CONTENTS 3 Introduction 4 GxP Auditing 4 GMP Auditing 5 GCP Auditing 5 GLP Auditing 6 Pharmacovigilance Auditing 6 Vendor/Supplier Auditing 7
GxP Auditing, Remediation, and Staff Augmentation TABLE OF CONTENTS 3 Introduction 4 GxP Auditing 4 GMP Auditing 5 GCP Auditing 5 GLP Auditing 6 Pharmacovigilance Auditing 6 Vendor/Supplier Auditing 7
ICH E6 Guideline R 2 優良臨床試驗指引修改及增編附錄
 ICH E6 Guideline R 2 優良臨床試驗指引修改及增編附錄 Mandy Liu 劉文婷 COQM/ Boehringer Ingelheim Taiwan Ltd Former Team leader GCP inspection team/ CDE Member of Expert Working Group of ICH E6 2016.11.23 Statement of the
ICH E6 Guideline R 2 優良臨床試驗指引修改及增編附錄 Mandy Liu 劉文婷 COQM/ Boehringer Ingelheim Taiwan Ltd Former Team leader GCP inspection team/ CDE Member of Expert Working Group of ICH E6 2016.11.23 Statement of the
Strategic Plan. Uniting to care & cure
 2017-2020 Strategic Plan Uniting to care & cure Table of Contents Message from the President & CEO Page 2 Overview Page 3 Mission Page 4 Core Values Page 5 2017-2020 Objectives & Strategies Page 6 Mission
2017-2020 Strategic Plan Uniting to care & cure Table of Contents Message from the President & CEO Page 2 Overview Page 3 Mission Page 4 Core Values Page 5 2017-2020 Objectives & Strategies Page 6 Mission
Enterprise Risk Management: Developing a Model for Organizational Success. White Paper
 Enterprise Risk Management: Developing a Model for Organizational Success White Paper January 2009 Overview Less than a decade ago, Enterprise Risk Management (ERM) was an unfamiliar concept. Today, the
Enterprise Risk Management: Developing a Model for Organizational Success White Paper January 2009 Overview Less than a decade ago, Enterprise Risk Management (ERM) was an unfamiliar concept. Today, the
ICMI PROFESSIONAL CERTIFICATION
 ICMI PROFESSIONAL CERTIFICATION Contact Center Management Competencies The ICMI Professional Certification Contact Center Management Competencies specify job role-specific knowledge, skills and abilities
ICMI PROFESSIONAL CERTIFICATION Contact Center Management Competencies The ICMI Professional Certification Contact Center Management Competencies specify job role-specific knowledge, skills and abilities
GxP Auditing, Remediation, and Staff Augmentation
 GxP Auditing, Remediation, and Staff Augmentation TABLE OF CONTENTS 3 Introduction 4 GxP Auditing 4 GMP Auditing 5 GCP Auditing 6 GLP Auditing 7 Pharmacovigilance Auditing 7 Vendor/Supplier Auditing 8
GxP Auditing, Remediation, and Staff Augmentation TABLE OF CONTENTS 3 Introduction 4 GxP Auditing 4 GMP Auditing 5 GCP Auditing 6 GLP Auditing 7 Pharmacovigilance Auditing 7 Vendor/Supplier Auditing 8
the council initiative on public engagement
 public engagement at the city of edmonton share your voice shape our city the council initiative on public engagement new public engagement practice and implementation roadmap final report CITY OF EDMONTON
public engagement at the city of edmonton share your voice shape our city the council initiative on public engagement new public engagement practice and implementation roadmap final report CITY OF EDMONTON
Inside of a ring or out, ain t nothing wrong with going down. It s staying down that s wrong. Muhammad Ali
 MANAGING OPERATIONAL RISK IN THE 21 ST CENTURY White Paper Series Inside of a ring or out, ain t nothing wrong with going down. It s staying down that s wrong. Muhammad Ali 2 In today s competitive and
MANAGING OPERATIONAL RISK IN THE 21 ST CENTURY White Paper Series Inside of a ring or out, ain t nothing wrong with going down. It s staying down that s wrong. Muhammad Ali 2 In today s competitive and
Implementation Guides
 Implementation Guides Implementation Guides assist internal auditors in applying the Definition of Internal Auditing, the Code of Ethics, and the Standards and promoting good practices. Implementation
Implementation Guides Implementation Guides assist internal auditors in applying the Definition of Internal Auditing, the Code of Ethics, and the Standards and promoting good practices. Implementation
FRAMEWORK OF CHARACTERISTICS OF A QUALIFIED SITE TEAM: How Does Yours Measure Up?
 FRAMEWORK OF CHARACTERISTICS OF A QUALIFIED SITE TEAM: How Does Yours Measure Up? The following framework of characteristics focuses on attributes that are within the control of investigators and their
FRAMEWORK OF CHARACTERISTICS OF A QUALIFIED SITE TEAM: How Does Yours Measure Up? The following framework of characteristics focuses on attributes that are within the control of investigators and their
GxP Auditing, Remediation, and Quality System Resourcing
 GxP Auditing, Remediation, and Quality System Resourcing TABLE OF CONTENTS 3 Introduction 4 GxP Auditing 4 GMP Auditing 5 GCP Auditing 6 GLP Auditing 7 Pharmacovigilance Auditing 7 Vendor/Supplier Auditing
GxP Auditing, Remediation, and Quality System Resourcing TABLE OF CONTENTS 3 Introduction 4 GxP Auditing 4 GMP Auditing 5 GCP Auditing 6 GLP Auditing 7 Pharmacovigilance Auditing 7 Vendor/Supplier Auditing
STRATEGIC PLAN. FISCAL YEARS 2018 to 2022 SAFETY WORKS
 STRATEGIC PLAN FISCAL YEARS 2018 to 2022 SAFETY WORKS TSSA has developed an ambitious plan to ensure we effectively reduce safety risks and provide value to our customers and stakeholders. STRATEGIC PLAN
STRATEGIC PLAN FISCAL YEARS 2018 to 2022 SAFETY WORKS TSSA has developed an ambitious plan to ensure we effectively reduce safety risks and provide value to our customers and stakeholders. STRATEGIC PLAN
INTEGRITY MANAGEMENT CONTINUOUS IMPROVEMENT. Foundation for an Effective Safety Culture
 INTEGRITY MANAGEMENT CONTINUOUS IMPROVEMENT Foundation for an Effective Safety Culture June 2011 Foundation for an Effective Safety Culture describes the key elements of organizational culture and business
INTEGRITY MANAGEMENT CONTINUOUS IMPROVEMENT Foundation for an Effective Safety Culture June 2011 Foundation for an Effective Safety Culture describes the key elements of organizational culture and business
Leveraging IT risk management to boost competitive advantage
 Pharmaceuticals and Life Sciences Leveraging IT risk management to boost competitive advantage Achieving integrated information technology, governance, risk, and compliance Table of contents The heart
Pharmaceuticals and Life Sciences Leveraging IT risk management to boost competitive advantage Achieving integrated information technology, governance, risk, and compliance Table of contents The heart
Meeting sponsor s criteria : strategy to collaborate, engage and deliver
 Meeting sponsor s criteria : strategy to collaborate, engage and deliver Frédérique Couttet Clinical Trial Manager 4th Annual Outsourcing in Clinical Trials Europe Brussels, Switzerland, May 21 22, 2014
Meeting sponsor s criteria : strategy to collaborate, engage and deliver Frédérique Couttet Clinical Trial Manager 4th Annual Outsourcing in Clinical Trials Europe Brussels, Switzerland, May 21 22, 2014
HCCA Compliance Institute : Intersection of Internal Audit & Compliance. April 17, Agenda. Where are we today?
 HCCA Institute 2018 708: Intersection of & April 17, 2018 Agenda Objectives Where are we today? Corporate Integrity: The intersection of, and Privacy Questions 2 Where are we today? 3 1 Regulatory change
HCCA Institute 2018 708: Intersection of & April 17, 2018 Agenda Objectives Where are we today? Corporate Integrity: The intersection of, and Privacy Questions 2 Where are we today? 3 1 Regulatory change
How it works: Questions from the OCAT 2.0
 Social Sector Practice How it works: Questions from the OCAT 2.0 OCAT 2.0 is an updated and improved version of our original OCAT survey. It asks nonprofit staff to rate their organization s operational
Social Sector Practice How it works: Questions from the OCAT 2.0 OCAT 2.0 is an updated and improved version of our original OCAT survey. It asks nonprofit staff to rate their organization s operational
Active Essex Risk Management Strategy
 Active Essex Risk Management Strategy 2017-2021 November 2017 Contents 1. Policy Statement 2. Statement of Commitment 3. Risk Management Framework 4. Risk Appetite 5. Risk Maturity 6. Risk Management Levels
Active Essex Risk Management Strategy 2017-2021 November 2017 Contents 1. Policy Statement 2. Statement of Commitment 3. Risk Management Framework 4. Risk Appetite 5. Risk Maturity 6. Risk Management Levels
CAPABILITY MATRIX FOR PROFESSIONAL STAFF HANDBOOK
 CAPABILITY MATRIX FOR PROFESSIONAL STAFF HANDBOOK INTRODUCTION TO THE CAPABILITY MATRIX This handbook is intended to help you understand the Capability Matrix and how it will be used at UoN. The Capability
CAPABILITY MATRIX FOR PROFESSIONAL STAFF HANDBOOK INTRODUCTION TO THE CAPABILITY MATRIX This handbook is intended to help you understand the Capability Matrix and how it will be used at UoN. The Capability
CORROSION MANAGEMENT MATURITY MODEL
 CORROSION MANAGEMENT MATURITY MODEL CMMM Model Definition AUTHOR Jeff Varney Executive Director APQC Page 1 of 35 TABLE OF CONTENTS OVERVIEW... 5 I. INTRODUCTION... 6 1.1 The Need... 6 1.2 The Corrosion
CORROSION MANAGEMENT MATURITY MODEL CMMM Model Definition AUTHOR Jeff Varney Executive Director APQC Page 1 of 35 TABLE OF CONTENTS OVERVIEW... 5 I. INTRODUCTION... 6 1.1 The Need... 6 1.2 The Corrosion
PROCESS LED TRANSFORMATION & SUSTAINABILITY
 PROCESS LED TRANSFORMATION & SUSTAINABILITY BUSINESS PROCESS MANAGEMENT USE CASE IMPRIVA Inc. 101A Clay St. #196 San Francisco, CA 94111 877.838.9714 www.impriva.com US Content Pain Point 3 Key Terms 3
PROCESS LED TRANSFORMATION & SUSTAINABILITY BUSINESS PROCESS MANAGEMENT USE CASE IMPRIVA Inc. 101A Clay St. #196 San Francisco, CA 94111 877.838.9714 www.impriva.com US Content Pain Point 3 Key Terms 3
OPERATIONAL EXCELLENCE ACROSS THE ERO ENTERPRISE: Adding Value to the Compliance Monitoring and Enforcement Program
 OPERATIONAL EXCELLENCE ACROSS THE ERO ENTERPRISE: Adding Value to the Compliance Monitoring and Enforcement Program A Discussion Paper By the Midwest Reliability Organization I. INTRODUCTION This discussion
OPERATIONAL EXCELLENCE ACROSS THE ERO ENTERPRISE: Adding Value to the Compliance Monitoring and Enforcement Program A Discussion Paper By the Midwest Reliability Organization I. INTRODUCTION This discussion
VETERINARY DRUGS DIRECTORATE HEALTH PRODUCTS AND FOOD BRANCH HEALTH CANADA STRATEGIC PLAN
 VETERINARY DRUGS DIRECTORATE HEALTH PRODUCTS AND FOOD BRANCH HEALTH CANADA STRATEGIC PLAN APRIL 2004 - MARCH 2007 TABLE OF CONTENTS INTRODUCTION... Page 2 of 12 ORGANIZATIONAL PROFILE AND HISTORY... Page
VETERINARY DRUGS DIRECTORATE HEALTH PRODUCTS AND FOOD BRANCH HEALTH CANADA STRATEGIC PLAN APRIL 2004 - MARCH 2007 TABLE OF CONTENTS INTRODUCTION... Page 2 of 12 ORGANIZATIONAL PROFILE AND HISTORY... Page
Guideline on Good Pharmacovigilance Practices Module V- Pharmacovigilance System Master File
 Guideline on Good Pharmacovigilance Practices Module V- Pharmacovigilance System Master File Turkish Medicines and Medical Devices Agency 16.02.2015 CHAPTER I... 2 1.1. Introduction... 2 CHAPTER II...
Guideline on Good Pharmacovigilance Practices Module V- Pharmacovigilance System Master File Turkish Medicines and Medical Devices Agency 16.02.2015 CHAPTER I... 2 1.1. Introduction... 2 CHAPTER II...
Corporate Governance Guidelines of Surgery Partners, Inc.
 Corporate Governance Guidelines of Surgery Partners, Inc. SELECTION AND COMPOSITION OF BOARD OF DIRECTORS Role of the Board The basic responsibility of the board of directors (the Board ) of Surgery Partners,
Corporate Governance Guidelines of Surgery Partners, Inc. SELECTION AND COMPOSITION OF BOARD OF DIRECTORS Role of the Board The basic responsibility of the board of directors (the Board ) of Surgery Partners,
International Standards for the Professional Practice of Internal Auditing (Standards)
 Attribute Standards 1000 Purpose, Authority, and Responsibility The purpose, authority, and responsibility of the internal audit activity must be formally defined in an internal audit charter, consistent
Attribute Standards 1000 Purpose, Authority, and Responsibility The purpose, authority, and responsibility of the internal audit activity must be formally defined in an internal audit charter, consistent
Risk-Based Monitoring - Prospective from CRO
 Risk-Based Monitoring - Prospective from CRO New Trends in Clinical Trials Taipei 2016 Tong Guo, PhD Head of Biostatistics, Africa & Asia QuintilesIMS Copyright 2015 Quintiles Disclaimer The views and
Risk-Based Monitoring - Prospective from CRO New Trends in Clinical Trials Taipei 2016 Tong Guo, PhD Head of Biostatistics, Africa & Asia QuintilesIMS Copyright 2015 Quintiles Disclaimer The views and
Reducing Risks and Reaping Rewards
 a consumer goods technology whitepaper Reducing Risks and How an Enterprise Quality Management Solution Benefits PRODUCED BY As food markets become more global, the risk of compromise to food safety increases,
a consumer goods technology whitepaper Reducing Risks and How an Enterprise Quality Management Solution Benefits PRODUCED BY As food markets become more global, the risk of compromise to food safety increases,
T E A L C O N S U L T I N G L T D I S O A G U I D E
 T E A L C O N S U L T I N G L T D I S O 4 4 0 0 1 A G U I D E W H A T I S I S O 4 4 0 0 1? There is much talk about collaboration but for many the concept seems ad hoc and without a clear perspective as
T E A L C O N S U L T I N G L T D I S O 4 4 0 0 1 A G U I D E W H A T I S I S O 4 4 0 0 1? There is much talk about collaboration but for many the concept seems ad hoc and without a clear perspective as
Implementing Category Management for Common Goods and Services
 Implementing Category Management for Common Goods and Services Darbi Dillon Office of Federal Procurement Policy 1800 G Street NW, Washington DC 20006 Audit Tax Advisory Grant Thornton LLP 333 John Carlyle
Implementing Category Management for Common Goods and Services Darbi Dillon Office of Federal Procurement Policy 1800 G Street NW, Washington DC 20006 Audit Tax Advisory Grant Thornton LLP 333 John Carlyle
Appropriate Control Strategies Eliminate the Need for Redundant Testing of Pharmaceutical Products
 Date: April 23, 2012 Appropriate Control Strategies Eliminate the Need for Redundant Testing of Pharmaceutical Products Key Messages Importation quality control testing for pharmaceutical, biological/biotechnology
Date: April 23, 2012 Appropriate Control Strategies Eliminate the Need for Redundant Testing of Pharmaceutical Products Key Messages Importation quality control testing for pharmaceutical, biological/biotechnology
Policy Governance Manual
 Policy Governance Manual Introduction The Policy Governance Manual (the Manual') assists policy owners, policy authors, teams and business units in the review, revision, development and implementation
Policy Governance Manual Introduction The Policy Governance Manual (the Manual') assists policy owners, policy authors, teams and business units in the review, revision, development and implementation
SAFETY AND HEALTH AUDIT STRATEGY Safety & Health Services Safety and Health Audit Strategy Version 1.0
 SAFETY AND HEALTH AUDIT STRATEGY 2016-2019 Safety & Health Services Contents 1. INTRODUCTION... 1 2. AIMS AND OBJECTIVES... 1 3. DEFINITIONS... 1 AUDIT... 1 ASSURANCE... 1 AUDIT SPONSOR... 1 AUDIT OPINION...
SAFETY AND HEALTH AUDIT STRATEGY 2016-2019 Safety & Health Services Contents 1. INTRODUCTION... 1 2. AIMS AND OBJECTIVES... 1 3. DEFINITIONS... 1 AUDIT... 1 ASSURANCE... 1 AUDIT SPONSOR... 1 AUDIT OPINION...
A Model for CAS Self Assessment
 Introduction An effective Contractor Assurance System integrates contractor management, supports corporate parent governance and facilitates government oversight systems. The purpose of a CAS is threefold:
Introduction An effective Contractor Assurance System integrates contractor management, supports corporate parent governance and facilitates government oversight systems. The purpose of a CAS is threefold:
RESEARCH SUPPORT SERVICES FRAMEWORK. Streamlining the management and governance of R&D studies in the NHS
 RESEARCH SUPPORT SERVICES FRAMEWORK Streamlining the management and governance of R&D studies in the NHS Page 1 of 22 Contents 1. INTRODUCTION... 3 How to use this document... 3 Background... 4 Purpose
RESEARCH SUPPORT SERVICES FRAMEWORK Streamlining the management and governance of R&D studies in the NHS Page 1 of 22 Contents 1. INTRODUCTION... 3 How to use this document... 3 Background... 4 Purpose
Follow-up Audit of the CNSC Performance Measurement and Reporting Frameworks, November 2011
 Audit of Mobile Telecommunication Devices June 21, 2012 Follow-up Audit of the CNSC Performance Measurement and Reporting Frameworks, November 2011 Presented to the Audit Committee on November 21, 2016
Audit of Mobile Telecommunication Devices June 21, 2012 Follow-up Audit of the CNSC Performance Measurement and Reporting Frameworks, November 2011 Presented to the Audit Committee on November 21, 2016
Application of King III Corporate Governance Principles
 Application of Corporate Governance Principles / 1 This table is a useful reference to each of the principles and how, in broad terms, they have been applied by the Group. The information should be read
Application of Corporate Governance Principles / 1 This table is a useful reference to each of the principles and how, in broad terms, they have been applied by the Group. The information should be read
RISK MANAGEMENT FRAMEWORK OF THE CGIAR SYSTEM
 RISK MANAGEMENT FRAMEWORK OF THE CGIAR SYSTEM Approved by the System Council at its 5 th meeting (SC/M5/DP12) 10 November 2017 CGIAR System Organization Page 1 of 9 Introduction 1. The scope of CGIAR s
RISK MANAGEMENT FRAMEWORK OF THE CGIAR SYSTEM Approved by the System Council at its 5 th meeting (SC/M5/DP12) 10 November 2017 CGIAR System Organization Page 1 of 9 Introduction 1. The scope of CGIAR s
IS&T Leadership Job Description
 IS&T Leadership Job Description December 1, 2015 IT Leadership Job Description December 1, 2015 Page i Table of Contents General Characteristics... 1 Job Path... 2 Explanation of Proficiency Level Definitions...
IS&T Leadership Job Description December 1, 2015 IT Leadership Job Description December 1, 2015 Page i Table of Contents General Characteristics... 1 Job Path... 2 Explanation of Proficiency Level Definitions...
IT Governance Overview
 IT Governance Overview Contents Executive Summary... 3 What is IT Governance?... 4 Strategic Vision and IT Guiding Principles... 4 Campus-Wide IT Strategic Vision... 4 IT Guiding Principles... 4 The Scope
IT Governance Overview Contents Executive Summary... 3 What is IT Governance?... 4 Strategic Vision and IT Guiding Principles... 4 Campus-Wide IT Strategic Vision... 4 IT Guiding Principles... 4 The Scope
Introduction. The Assessment consists of:
 ESG / Sustainability Governance Assessment: A Roadmap to Build a Sustainable Board By Coro Strandberg President, Strandberg Consulting www.corostrandberg.com November 2018 Introduction This is a tool for
ESG / Sustainability Governance Assessment: A Roadmap to Build a Sustainable Board By Coro Strandberg President, Strandberg Consulting www.corostrandberg.com November 2018 Introduction This is a tool for
THE CULTURE CANVAS A Working Guide and Checklist to Support the Development of a High-Performing Culture
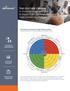 denison TM THE CULTURE CANVAS A Working Guide and Checklist to Support the Development of a High-Performing Culture The Denison Model of High Performance A Systems Approach to Understanding and Managing
denison TM THE CULTURE CANVAS A Working Guide and Checklist to Support the Development of a High-Performing Culture The Denison Model of High Performance A Systems Approach to Understanding and Managing
The table below compares to the 2009 Essential Elements and the 2018 Enhanced Data Stewardship Elements
 October 8, 2018 The Essential Elements of Accountability were developed by a multi-stakeholder group that met in Dublin Ireland as the Global Accountability Dialogue. The Essential Elements provided granularity
October 8, 2018 The Essential Elements of Accountability were developed by a multi-stakeholder group that met in Dublin Ireland as the Global Accountability Dialogue. The Essential Elements provided granularity
The power of the Converge platform lies in the ability to share data across all aspects of risk management over a secure workspace.
 Converge Platform The transition to value-based care is breaking down the barriers between the CNO, CMO, and Chief Legal Counsel in managing enterprise risk. It s time to take a proactive systems approach
Converge Platform The transition to value-based care is breaking down the barriers between the CNO, CMO, and Chief Legal Counsel in managing enterprise risk. It s time to take a proactive systems approach
4/26. Analytics Strategy
 1/26 Qlik Advisory As a part of Qlik Consulting, Qlik Advisory works with Customers to assist in shaping strategic elements related to analytics to ensure adoption and success throughout their analytics
1/26 Qlik Advisory As a part of Qlik Consulting, Qlik Advisory works with Customers to assist in shaping strategic elements related to analytics to ensure adoption and success throughout their analytics
ENHANCING CLINICAL DATA QUALITY WITH SPECIALIZED FUNCTIONAL SERVICE PROVIDER MODEL
 Shaping the Future of Drug Development ENHANCING CLINICAL DATA QUALITY WITH SPECIALIZED FUNCTIONAL SERVICE PROVIDER MODEL Jim Baker Senior Vice President Clinical Research Services Cytel JIM BAKER Senior
Shaping the Future of Drug Development ENHANCING CLINICAL DATA QUALITY WITH SPECIALIZED FUNCTIONAL SERVICE PROVIDER MODEL Jim Baker Senior Vice President Clinical Research Services Cytel JIM BAKER Senior
Wildland Fire Management Working Group: Strategic Directions
 Wildland Fire Management Working Group: Strategic Directions 2014-2019 WFMWG Strategic Directions 2014-2019 14 August 2014 Table of Contents Introduction...2 Refocusing Our Efforts...3 Strategic Focus
Wildland Fire Management Working Group: Strategic Directions 2014-2019 WFMWG Strategic Directions 2014-2019 14 August 2014 Table of Contents Introduction...2 Refocusing Our Efforts...3 Strategic Focus
INTERNAL AUDIT OF PROCUREMENT AND CONTRACTING
 OFFICE OF THE COMMISSIONNER OF LOBBYING OF CANADA INTERNAL AUDIT OF PROCUREMENT AND CONTRACTING AUDIT REPORT Presented by: Samson & Associates February 20, 2015 TABLE OF CONTENT EXECUTIVE SUMMARY... I
OFFICE OF THE COMMISSIONNER OF LOBBYING OF CANADA INTERNAL AUDIT OF PROCUREMENT AND CONTRACTING AUDIT REPORT Presented by: Samson & Associates February 20, 2015 TABLE OF CONTENT EXECUTIVE SUMMARY... I
Institute for Collaborative Working
 Principles for Effective Adoption and Implementation of ISO 44001 Management Systems - Requirements Framework Introduction This document provides the high level principles for the adoption, implementation
Principles for Effective Adoption and Implementation of ISO 44001 Management Systems - Requirements Framework Introduction This document provides the high level principles for the adoption, implementation
QUALITY ASSURANCE. Pharma Quality Agreements: What Are They, and Why They Matter For Your Study ABSTRACT
 WHITE PAPER PRESENTED BY PREMIER RESEARCH Pharma Quality Agreements: What Are They, and Why They Matter For Your Study ABSTRACT Quality Agreements are an effective bridge to a successful future for companies
WHITE PAPER PRESENTED BY PREMIER RESEARCH Pharma Quality Agreements: What Are They, and Why They Matter For Your Study ABSTRACT Quality Agreements are an effective bridge to a successful future for companies
Risk Based Monitoring Initiative RISK-BASED MONITORING UPDATE - VOLUME 1
 Risk Based Monitoring Initiative RISK-BASED MONITORING UPDATE - VOLUME 1 RISK-BASED MONITORING UPDATE - VOLUME 1 1. Introduction TransCelerate s Risk-Based Monitoring (RBM) project had a very productive
Risk Based Monitoring Initiative RISK-BASED MONITORING UPDATE - VOLUME 1 RISK-BASED MONITORING UPDATE - VOLUME 1 1. Introduction TransCelerate s Risk-Based Monitoring (RBM) project had a very productive
Preparing your organization for a Human Resource Outsourcing implementation
 IBM Global Technology Services Thought Leadership White Paper April 2013 Preparing your organization for a Human Resource Outsourcing implementation How to collaborate for a more successful transition
IBM Global Technology Services Thought Leadership White Paper April 2013 Preparing your organization for a Human Resource Outsourcing implementation How to collaborate for a more successful transition
Requirements Analysis and Design Definition. Chapter Study Group Learning Materials
 Requirements Analysis and Design Definition Chapter Study Group Learning Materials 2015, International Institute of Business Analysis (IIBA ). Permission is granted to IIBA Chapters to use and modify this
Requirements Analysis and Design Definition Chapter Study Group Learning Materials 2015, International Institute of Business Analysis (IIBA ). Permission is granted to IIBA Chapters to use and modify this
Region of Peel Digital Strategy
 Region of Peel Digital Strategy 1 2 The Digital Strategy The Digital Strategy defines a shared digital mandate and strategic roadmap for the Region of Peel to meet the growing needs of its residents, employees
Region of Peel Digital Strategy 1 2 The Digital Strategy The Digital Strategy defines a shared digital mandate and strategic roadmap for the Region of Peel to meet the growing needs of its residents, employees
Federal Segment Architecture Methodology Overview
 Federal Segment Architecture Methodology Background In January 2008, the Federal Segment Architecture Working Group (FSAWG) was formed as a sub-team of the Federal CIO Council s Architecture and Infrastructure
Federal Segment Architecture Methodology Background In January 2008, the Federal Segment Architecture Working Group (FSAWG) was formed as a sub-team of the Federal CIO Council s Architecture and Infrastructure
Guidance Note: Corporate Governance - Board of Directors. January Ce document est aussi disponible en français.
 Guidance Note: Corporate Governance - Board of Directors January 2018 Ce document est aussi disponible en français. Applicability The Guidance Note: Corporate Governance - Board of Directors (the Guidance
Guidance Note: Corporate Governance - Board of Directors January 2018 Ce document est aussi disponible en français. Applicability The Guidance Note: Corporate Governance - Board of Directors (the Guidance
Increasing the Intensity and Effectiveness of Supervision
 Increasing the Intensity and Effectiveness of Supervision Consultative Document Guidance on Supervisory Interaction with Financial Institutions on Risk Culture 18 November 2013 Table of Contents Page
Increasing the Intensity and Effectiveness of Supervision Consultative Document Guidance on Supervisory Interaction with Financial Institutions on Risk Culture 18 November 2013 Table of Contents Page
Toronto Transit Commission Board. City Manager and Chief Executive Officer, TTC
 STAFF REPORT ACTION REQUIRED EX21.14 TTC Capital Program Delivery Review Date: September 21, 2016 To: From: Wards: Toronto Transit Commission Board City Manager and Chief Executive Officer, TTC All Reference
STAFF REPORT ACTION REQUIRED EX21.14 TTC Capital Program Delivery Review Date: September 21, 2016 To: From: Wards: Toronto Transit Commission Board City Manager and Chief Executive Officer, TTC All Reference
The Integrated Support and Assurance Process (ISAP): detailed guidance on assuring novel and complex contracts
 The Integrated Support and Assurance Process (ISAP): detailed guidance on assuring novel and complex contracts Part C: Guidance for NHS trusts and NHS foundation trusts Published by NHS England and NHS
The Integrated Support and Assurance Process (ISAP): detailed guidance on assuring novel and complex contracts Part C: Guidance for NHS trusts and NHS foundation trusts Published by NHS England and NHS
A High-Touch Approach to Improving Patient Access. Using field support to navigate reimbursement challenges
 A High-Touch Approach to Improving Patient Access Using field support to navigate reimbursement challenges For the brand and reimbursement teams who must develop commercial strategies for the biopharmaceutical
A High-Touch Approach to Improving Patient Access Using field support to navigate reimbursement challenges For the brand and reimbursement teams who must develop commercial strategies for the biopharmaceutical
Role of Board of Directors in Risk Management. CPA Erick Audi Thursday, 15 th November 2018
 Role of Board of Directors in Risk Management Presentation by: CPA Erick Audi Thursday, 15 th November 2018 Uphold public interest Presentation Agenda Introduction & Definitions Legal Provisions/Guidelines
Role of Board of Directors in Risk Management Presentation by: CPA Erick Audi Thursday, 15 th November 2018 Uphold public interest Presentation Agenda Introduction & Definitions Legal Provisions/Guidelines
A Framework for Audit Quality
 Ernst & Young Global Limited Becket House 1 Lambeth Palace Road London SE1 7EU Tel: +44 [0]20 7980 0000 Fax: +44 [0]20 7980 0275 www.ey.com Mr. James Gunn Technical Director International Auditing and
Ernst & Young Global Limited Becket House 1 Lambeth Palace Road London SE1 7EU Tel: +44 [0]20 7980 0000 Fax: +44 [0]20 7980 0275 www.ey.com Mr. James Gunn Technical Director International Auditing and
NOT PROTECTIVELY MARKED. HM Inspectorate of Constabulary in Scotland. Inspection Framework. Version 1.0 (September 2014)
 HM Inspectorate of Constabulary in Scotland Inspection Framework Version 1.0 (September 2014) Improving Policing across Scotland Introduction I am pleased to introduce the new HMICS Inspection Framework.
HM Inspectorate of Constabulary in Scotland Inspection Framework Version 1.0 (September 2014) Improving Policing across Scotland Introduction I am pleased to introduce the new HMICS Inspection Framework.
Practice Guide. Developing the Internal Audit Strategic Plan
 Practice Guide Developing the Internal Audit Strategic Plan JUly 2012 Table of Contents Executive Summary... 1 Introduction... 2 Strategic Plan Definition and Development... 2 Review of Strategic Plan...
Practice Guide Developing the Internal Audit Strategic Plan JUly 2012 Table of Contents Executive Summary... 1 Introduction... 2 Strategic Plan Definition and Development... 2 Review of Strategic Plan...
APPLICATION OF THE KING IV TM PRINCIPLES
 APPLICATION OF THE KING IV TM PRINCIPLES Ethical culture Good performance Effective control Legitimacy LEADERSHIP, ETHICS AND CORPORATE CITIZENSHIP Leadership 1 The Board should lead ethically and effectively
APPLICATION OF THE KING IV TM PRINCIPLES Ethical culture Good performance Effective control Legitimacy LEADERSHIP, ETHICS AND CORPORATE CITIZENSHIP Leadership 1 The Board should lead ethically and effectively
Organizational & Management Review Draft Executive Summary
 DRAFT DOCUMENT FOR DISCUSSION AA The Global Fund to Fight AIDS, Tuberculosis and Malaria Organizational & Management Review Draft Executive Summary November 5, 2007 As it enters its second half-decade,
DRAFT DOCUMENT FOR DISCUSSION AA The Global Fund to Fight AIDS, Tuberculosis and Malaria Organizational & Management Review Draft Executive Summary November 5, 2007 As it enters its second half-decade,
medicines, improving the health of people around the world.
 TransCelerate BioPharma Inc. is a non-profit organization with a mission to collaborate across the biopharmaceutical research and development community to identify, design and facilitate the implementation
TransCelerate BioPharma Inc. is a non-profit organization with a mission to collaborate across the biopharmaceutical research and development community to identify, design and facilitate the implementation
PART THREE: Work Plan and IV&V Methodology (RFP 5.3.3)
 PART THREE: Work Plan and IV&V Methodology (RFP 5.3.3) 3.1 IV&V Methodology and Work Plan 3.1.1 NTT DATA IV&V Framework We believe that successful IV&V is more than just verification that the processes
PART THREE: Work Plan and IV&V Methodology (RFP 5.3.3) 3.1 IV&V Methodology and Work Plan 3.1.1 NTT DATA IV&V Framework We believe that successful IV&V is more than just verification that the processes
Section I: Pharmaceuticals and Medical Devices
 SUPPLEMENT on HEALTHCARE INNOVATION Visionary Goals and Recommendations 51th Japan-U.S. Business Conference Japan-U.S. Business Council / U.S.-Japan Business Council November 14, 2014 The R&D-based pharmaceutical
SUPPLEMENT on HEALTHCARE INNOVATION Visionary Goals and Recommendations 51th Japan-U.S. Business Conference Japan-U.S. Business Council / U.S.-Japan Business Council November 14, 2014 The R&D-based pharmaceutical
Periodic Comprehensive Review of the External Auditor
 Periodic Comprehensive Review of the External Auditor TOOL FOR AUDIT COMMITTEES January 2014 ENHANCING AUDIT QUALITY AUDIT COMMITTEES iii Table of Contents Introduction 1 1. Determine the scope, timing
Periodic Comprehensive Review of the External Auditor TOOL FOR AUDIT COMMITTEES January 2014 ENHANCING AUDIT QUALITY AUDIT COMMITTEES iii Table of Contents Introduction 1 1. Determine the scope, timing
Compliance Program Effectiveness Guide
 Compliance Program Effectiveness Guide June 2017 This Guide is a comparison of: Compliance Program Elements New York State, Social Services Law 363-D Office of Inspector General (OIG) Compliance Program
Compliance Program Effectiveness Guide June 2017 This Guide is a comparison of: Compliance Program Elements New York State, Social Services Law 363-D Office of Inspector General (OIG) Compliance Program
