NIH Public Access Author Manuscript Curr Protoc Immunol. Author manuscript; available in PMC 2008 November 25.
|
|
|
- Dortha Howard
- 5 years ago
- Views:
Transcription
1 NIH Public Access Author Manuscript Published in final edited form as: Curr Protoc Immunol August ; CHAPTER: Unit doi: / im0112s82. TECHNIQUES IN ASEPTIC RODENT SURGERY Shelley L. Hoogstraten-Miller 1 and Patricia A. Brown 2 1 National Human Genome Research Institute, Bethesda, Maryland 2 Office of Laboratory Animal Welfare, Bethesda, Maryland Abstract Performing aseptic survival surgery in rodents can be challenging. This unit describes some basic principles to assist clinicians, researchers, and technicians in becoming proficient in performing aseptic rodent surgery. Key Terms aseptic surgery; technique; rodent; surgical preparation; instrument preparation; suture material; anesthesia; analgesia; surgical gloves Unit Introduction Performing aseptic survival surgery in rodents can be challenging. Unlike in larger species where there is a dedicated surgical suite and several personnel to assist the surgeon, rodent surgery is most commonly performed alone. This means the surgeon must induce, maintain, and recover the animal from anesthesia, as well as, surgically prepare the animal, and perform the surgery aseptically. The following principles described in the Guide (NAS, 1996) apply to rodent surgery: 1. Appropriate pre-operative and post-operative care of animals in accordance with established veterinary medical and nursing practices is required. 2. All survival surgery will be performed by using aseptic procedures, including sterile gloves, masks, sterile instruments, and aseptic techniques. 3. A dedicated surgical facility is not required for rodents but surgery must be performed using aseptic techniques. 4. Research personnel will be appropriately qualified and trained in all procedures to ensure that good surgical technique is practiced. Shelley Hoogstraten-Miller, DVM, MS, DACLAM, National Human Genome Research Institute/National Institutes of Health, Building 49/3A14 MSC4472, 49 Convent Drive, Bethesda, MD 20892, T: (301) , F: (301) , E: shoogstr@mail.nih.gov, Patricia Brown, VMD, MS, Office of Laboratory Animal Welfare/National Institutes of Health, RKL1, Suite 360, MSC 7982, 6705 Rockledge Drive, Bethesda, MD , T: , F: , E: brownp@mail.nih.gov. Diskette Information: MAC, Microsoft Word , File name(s): Techniques in Aseptic Rodent Surgery final.doc, Techniques in Aseptic Rodent Surgery legends and figures.doc Publisher's Disclaimer: This unit was prepared by Patricia Brown in her private capacity. No official support or endorsement by the National Institutes of Health or the United States Department of Health and Human Services is intended or should be inferred.
2 Hoogstraten-Miller and Brown Page 2 Strategic Planning Good surgical technique includes asepsis, gentle tissue handling, minimal dissection of tissue, appropriate use of instruments, effective hemostasis, and correct use of suture materials and patterns. Aseptic surgery is performed using procedures that limit microbial contamination so that significant infection or suppuration does not occur. This includes preparation of the animal, preparation of the surgeon, sterilization of instruments, supplies, and implanted materials, and the use of operative techniques to reduce the likelihood of infection (NIH ARAC, 2005). Aseptic technique is achieved, in part, by the pre-surgical planning that begins during protocol development in consultation with your veterinarian. This includes identification of personnel, their roles and training needs, equipment and supplies required for the procedures planned, the location and nature of the facilities in which the procedures will be conducted, and pre- and post-operative care. 1. Choosing the Surgical Area Characteristics of a good surgical area include an uncluttered area that is easily organized and disinfected, and free of debris and equipment not related to surgery (figure ). The area should be dedicated for the duration of the procedure, but can be used for other purposes when not being used for surgery. Avoid locations that are beneath supply ducts to minimize contamination from dust. Avoid high traffic areas such as those near doorways to prevent unnecessary interruptions and creation of air turbulence and contamination of the surgical field. 2. Considerations for Anesthesia and Analgesia, an Introduction (See Unit 1.4) Select the anesthetic based on the type of surgical procedure, the length of the surgical procedure, the equipment available and the expertise of those who will be responsible for administering the anesthetic (Flecknell, 1996; Kohn, 1997; Swindle, 2002). Consideration must also be given to the application of pre-, intra-, and post-operative analgesia. Analgesics can be injected, applied topically in a drop-wise fashion to the surgical area and/or supplied in the food or water. Inhalant gas anesthetics are administered using precision calibrated vaporizers (Figure ). When using gas anesthetics you must account for scavenging of waste gases. One acceptable method of scavenging is the use of a downdraft table (Figure ). It is important not to completely cover the surface of the downdraft table. This will cause a loss in its ability to effectively scavenge gases. Downdraft tables are usually only effective up to a height of 6 8 inches from the surface. Do not use induction chambers taller than this for induction of anesthesia. Placing the chamber in a chemical fume hood (Figure ) or a type IIB biosafety cabinet (Figure ) that is vented to the outside are other methods than can be used to scavenge waste anesthetic gases. A charcoal canister (Figure ) attached to the part of the breathing circuit for expired gases can also be used for scavenging. Charcoal canisters must be weighed before, and after, each use and must be replaced after an increase in the recommended weight. Depending on the size of the canister and the manufacturer s recommendations, the canister should also be weighed during especially long procedures to assure its continued effectiveness. Injectable anesthetics are widely used in rodent surgeries (e.g., ketamine/xylazine mixtures, pentobarbital, tribromoethanol). Controlled substances (pentobarbital, ketamine) require additional record keeping. If using injectable anesthetics, it is important to weigh each animal and dose each according to its body weight.
3 Hoogstraten-Miller and Brown Page 3 Some anesthetics, such as ketamine, abolish the blink reflex. Anesthetized animals should have their corneas protected with an ophthalmic lubricant. To avoid contamination of the lubricant, do not touch the tip of the tube to the skin or eye surface. Anesthetized animals must be monitored during the procedure to assure that they stay in the proper anesthetic plane. Do not allow them to get too light or too deep. Once the anesthetic has been given time to take effect, the anesthetic plane can be assessed by pinching the toes, tail or ear of the animal. Any reaction from the animal indicates that the animal is too light and that additional anesthetic should be given. Monitoring should include inspection of the mucous membranes and exposed tissues. This will give an indication of tissue perfusion and oxygenation. The color should be a bright pink to red and not dusky gray or blue. Respiratory pattern and frequency is also easily monitored and will give an indication of anesthetic depth and other potential complications. Various types of instrumentation can assist in monitoring the anesthetized patient. Core body temperature can be monitored in rodents, including mice. Pulse oximetry, capnography, and electrocardiograms can be used in larger rodents to monitor pulse, oxygenation, and heart rate. Monitoring instruments must be properly calibrated, as inaccurate information may be misleading and could result in a compromised condition or fatalities. The most frequent complication of small animal anesthesia is hypothermia resulting in prolonged recovery or death of the animal. Animals should be provided with a heat source during the pre-operative, intra-operative, and postoperative periods. Because of the high airflow, the risk of hypothermia is heightened when using downdraft tables, chemical fume hoods, or biosafety cabinets. The safest devices for providing heat to anesthetized animals are circulating hot water blankets or instant heat devices. These devices must be covered with a paper towel or other insulation so that the animal does not come in direct contact with the hot surface. Slide warmers can also be used as a heat source during recovery. By placing the recovery cage on the slide warmer it will be pre-warmed and ready to accept the animal once the surgery is complete. Use a thermometer to measure the temperature at the level of the animal. The temperature not exceed 85 to 95 F (29.4 to 32.2 C). Heat lamps and electric heating pads can be very dangerous and should be used with great caution. 3. Instrument Preparation Planning the surgical procedure requires consideration of the instruments required for the procedure and what method of instrument sterilization will be used. There are three commonly used methods for instrument sterilization: a. Steam autoclave or ethylene oxide. When using one of these methods a simple paper peel pack (Figure ) or a complex pack (Figure ) is used. A simple peel pack contains small numbers of small to medium sized instruments. A complex pack consists of overlapping cloth or paper drapes folded together and sealed with indicator tape. It can contain a large collection of instruments of various sizes (Knecht, 1987). Tip protectors should be added to delicate instruments or those with sharp points. Delicate instruments, materials for implantation such as catheters or items that otherwise may melt or become damaged when heated can be sterilized using ethylene oxide. The packs must be sufficiently aerated to prevent toxic effects from residual gas. This may require hours.
4 Hoogstraten-Miller and Brown Page 4 b. Cold sterilization. Glutaraldehyde solutions are effective if instruments are exposed for the proper length of time and expiration dates of prepared solutions are observed (usually 28 to 30 days). c. Dry heat sterilization. Hot bead sterilizers (Figure ) sterilize only the tips of the instruments. The beads must be pre-heated to the recommended temperature and the instruments exposed for the recommended time. Flash dry heat sterilizers (Figure ) sterilize the entire instrument and also requires adherence to the recommended temperature and exposure time. For both methods, gross debris must be removed from the instrument before sterilizing and the instruments must be allowed to cool before touching tissues. Alcohol provides disinfection not sterilization and should not be used to sterilize instruments. 4. Selection of Wound Closure Materials The selection of the type and size of suture material should be done in advance of the surgical procedure. A 3-0 suture thickness or smaller is best. Cutting and reverse cutting needles have sharp edges and are best used for skin suturing. Non-cutting, taper or round needles are used for suturing easily torn tissues such as peritoneum, muscle or intestine. If ligation of vessels or suturing of tissues other than skin is necessary during surgery, an absorbable material such as polyglactin 910, polyglycolic acid, polydioxanone, polyglyconate, or chromic gut should be used. For skin closure, non-absorbable suture such as polypropylene, nylon, stainless steel wound clips or staples may be used. Most rodents will gnaw at any externalized sutures, so a buried suture line or wound clips are recommended. Cyanoacrylate surgical adhesives may be used to close incisions or to close the area between sutures. Silk is a non-absorbable suture material that can cause tissue reactions and may wick microorganisms into the wound. It is best used for cardiovascular procedures only and not for closure (Knecht, 1987). Basic Protocol 1: Surgical Preparation of the Animal Materials Once everything for the surgery is pre-selected and organized, the animal can be anesthetized and surgically prepped. Surgical preparation includes removal of the hair and disinfection of the surgical site. Surgical preparation of the animal should occur in a location different than that used for performing the surgeries. This will help to prevent hair and dander from getting on the sterile packs. If space constraints or requirements for use of the down draft table, chemical fume hood, or biosafety cabinet necessitates a single location for prepping and surgery, then the bench towel used to prep the animal should be replaced before performing the surgery. The surgical pack, if already open, must be covered with a sterile drape to prevent contamination with hair. Mini clipper with no blade Gauze pads (2 2 ) Adhesive tape (1 2 wide) Cotton-tipped applicators 70% alcohol Iodophor or chlorhexidine scrub
5 Hoogstraten-Miller and Brown Page 5 Performing Surgery 1. Using the clipper remove the hair from the surgical site. In mice, an easy alternative to clipping the fur is to remove it by plucking. Hair follicles in mice are usually in telogen or resting phase, and hair can be removed without injury. It is a fast and easy method that does not leave stubble. 2. Dab the clipped or plucked area with a piece of adhesive tape or moistened gauze to pick up loose hair that could otherwise migrate into the incision. 3. Use a gauze pad or cotton-tipped applicator to prep the surgical site with alternating scrubs of an iodophor and 70% alcohol. Use a circular motion beginning at the center of the shaved area and working toward the periphery.. Never go back to the center with the same sponge. For small incision sites cotton-tipped applicators work best. Alternative scrubs such as chlorhexidine may also be used 4. Repeat the alternating scrubs at least 3 times. Be careful not to excessively wet the animal as this can exacerbate hypothermia. The pre-surgical preparation should have included consideration of the surgical technique that will be used: sterile surgical gloves (Basic Protocol 2) or clean exam gloves (Alternate Basic Protocol 2) with a tips-only technique. Proper surgical attire for both techniques consists of cap, mask, and clean lab coat. Basic Protocol 2: Sterile Surgical Gloves Materials Using sterile surgical gloves allows you to touch all areas of the sterile surgical field and surgical instruments with your gloved hand. Surgical attire; cap, mask, lab coat Sterile surgical gloves Surgical instruments and equipment Surgical drape Anesthetized and surgically prepped animal 1. Don cap, mask, and clean lab coat. 2. Ready the sterile surgical instruments on a sterile surgical field. If using surgical packs, verify that the sterilization indicator has turned the appropriate color before using. Simple-peel packs are opened in a manner that preserves the sterility of the inside surface. Do not touch the inside surface as it can be used as a sterile field on which to keep the instruments. Complex surgical packs are also opened in such a way as to keep the inside surface of the wrapping sterile so that it can be used as a sterile field. Instruments in cold sterilant solutions must be removed from the solution and rinsed with sterile water, saline or alcohol. This is very important, as the sterilization solution is very irritating to tissues. Rinsed instruments must be placed on a sterile field. Dry heat sterilized instrument must also be placed on a sterile surgical field. Remember that bead sterilizers only sterilize the tips of the instruments.
6 Hoogstraten-Miller and Brown Page 6 3. Open all other sterile equipment, such as scalpels and suture material. Open these items in such a way as to prevent contamination of the item and the surgical pack. 4. Place the anesthetized and surgically prepped animal on the warming device that has been covered with a clean paper bench towel. 5. Don the surgical gloves as described below (Video ) to prevent contamination of the outer surface of the glove (Knecht,1987). a. Open the glove packet in such a way that prevents contamination of the inner surface. b. With one hand, lift a glove from the opened packet by its turned-down cuff. c. Pull the glove onto the opposite hand with a rotating motion. Do not touch the outside surface of the glove. d. Place the gloved fingers beneath the cuff of the other glove. e. With the gloved fingers under the cuff, pull the glove onto the ungloved hand. The folded cuff protects the gloved hand from contamination. f. Pull the cuff of the glove up and over the cuff of the lab coat. g. Slip the fingers under the cuff of the first glove to pull it over the lab coat cuff 6. Organize the instruments. It is helpful to point all the tips in one direction and place them in the order used (Figure ). Between surgeries or during breaks in surgeries cover the tips of the instruments with sterile gauze or drapes (Figure ). 7. Drape the surgical site. The most common drape is a paper drape (Figure ). It may be precut or one in which you must cut your own hole. The disadvantage of paper drapes is that they usually cover the entire animal, making patient monitoring difficult. Plastic drapes (Figure ), usually with an adhesive, offer the advantage of more visibility and better patient monitoring. Sterile gauze sponges (Figure ) can also be used for drapes. If using the tips-only technique you must handle the drape only by its edges so that it does not become contaminated. 8. Perform the surgical procedure. 9. Close the surgical wound in layers (e.g., body wall, subcuticular space, skin). 10. Move animal to a warm cage for recovery. 11. Sterilize or sanitize instruments between surgeries. If multiple surgeries are performed each day and multiple surgical packs are not available, the instruments should be rinsed with 70% alcohol or flash sterilized between surgeries. Remember, alcohol will disinfect, not sterilize. Alternatively, a glass bead sterilizer can be used to sterilize the tips of the instruments. Remember to allow instruments to cool before touching tissues! 12. Rinse gloves with 70% alcohol between surgeries. If you have had to handle another animal to anesthetize and prep it, you should change gloves before performing the next surgery.
7 Hoogstraten-Miller and Brown Page 7 Alternate Basic Protocol 2: Clean Exam Gloves Using clean exam gloves and a tips-only technique restricts you to using only the sterile working ends of the surgical instruments to manipulate the surgical field. The gloved, but not sterile, hand must never touch the working end of the instruments, the suture, suture needle, or any part of the surgical field. This technique is useful when working alone and manipulation of non-sterile objects (e.g., anesthesia machines, microscopes, lighting) is required. Additional Materials (also see Basic Protocol 2) Commentary Clean exam gloves (not sterile surgical gloves) 1. Don cap, mask, and clean lab coat. 2. Place the anesthetized and surgically prepped animal on the warming device that has been covered with a clean paper bench towel. 3. Don clean examination gloves. 4. Place the sterile tips of the instruments on a sterile gauze sponge or drape to prevent contamination (Figure ). 5. Open all other sterile equipment, such as scalpels and suture material. Open these items in such a way as to prevent contamination of the item and the sterile tips of the instruments. 6. Drape the surgical site. Handle the drape only by its edges so that it does not become contaminated. 7. Continue as described in steps 8 through 12 above When performing multiple rodent surgeries it is a good idea to have staging areas for the different steps of the procedure. Whenever possible, animals waiting for surgery should be kept at a visual and olfactory distance from those animals undergoing surgery. As you are performing your surgery, you should be aware of the space that is not sterile between your pack and the draped animal (Figure ). Do not lay instruments in this space. They will become contaminated. While performing surgery, be careful not to get paper or cloth instrument drapes wet (Figure ). Wet material acts as a wick to pull bacteria through from the non-sterile surface below. When this occurs the instruments should be considered contaminated and re-sterilized before further use. You must keep the recovering patient warm. Do not lay recovering animals directly on the bedding. They may aspirate and asphyxiate. Recovery from anesthesia can also be aided by the administration of warmed fluids given subcutaneously or intraperitoneally. In neonates, or animals recovering from prolonged surgical procedures, hypoglycemia can be a problem leading to post-surgical complications. These animals may benefit from the administration of oral glucose. Glucose solutions should never be given subcutaneously (SQ) or intraperitoneally (IP). Animals may be returned to their holding area once they are awake and appear to be making a normal recovery. Be sure to mark the cage card with the surgical procedure performed and the date.
8 Hoogstraten-Miller and Brown Page 8 The Guide (NRC, 1996) states that the application of prophylactic antibiotics is not a substitute for the practice of proper aseptic surgery. If prophylactic antibiotics must be used, for example in gastrointestinal surgery or an accidental break in aseptic technique, choose an appropriate antibiotic and give it at the dose and for the length of time recommended by the veterinarian. In guinea pigs and hamsters, the use of inappropriate antibiotics can cause fatalities. Post-operative care does not end with the return of the animal to its home environment. Animals must be monitored for several days after the surgical procedure for the development of postsurgical complications and for the continued need for analgesics. Food intake may be difficult to monitor in rodents, especially if they are group housed. However, if post-operative animals are singly housed and food rations are supplied in measured amounts this can be a useful monitoring tool. A more practical and sensitive method of monitoring the animal is daily weighing of the animal. While subtle changes in the animal s activity or appetite may not be clinically observed, changes in weight will be quickly detected allowing appropriate clinical intervention to be instituted. It is important to remember that some analgesics may depress the appetite causing secondary weight loss. This weight loss must be differentiated from that which occurs in an animal that is not feeling well. Supplying a softer, more palatable, easily accessible diet may encourage the animal to eat. The animal s hydration can be monitored by tenting the skin along the back of the animal. In a well-hydrated animal, the skin should quickly fall back into place when released. If an animal is dehydrated, the skin will be slow to return to its original place. When this occurs, your veterinarian should be consulted for the appropriate use of subcutaneous or intraperitoneal fluids. Wound closures should be removed at 10 to 14 days post-operatively. Suture scissors or staple removers should be used. Supplementary Material References Cited Refer to Web version on PubMed Central for supplementary material. Flecknell, PA. Laboratory Animal Anaesthesia. 2. Academic Press; London: Knecht, CD.; Algernon, RA.; Williams, DJ., et al. Fundamental Techniques in Veterinary Surgery. WB Saunders Company; Philadelphia, PA: Kohn, DH.; Wixson, SK.; White, WJ.; Benson, GJ. Anesthesia and Analgesia in Laboratory Animals. Academic Press; San Diego, CA: National Institutes of Health Animal Research Advisory Committee. Guidelines for Survival Rodent Surgery National Research Council. Guide for the Care and Use of Laboratory. National Academy Press; Washington, DC: Veterinary Care; p Swindle, MM.; Vogler, GA.; Fulton, LK., et al. Preanesthesia, anesthesia, analgesia, and euthanasia. In: Fox, JG.; Anderson, LC.; Loew, FM.; Quimby, FW., editors. Laboratory Animal Medicine. Academic Press; San Diego, CA: p
9 Hoogstraten-Miller and Brown Page 9 Figure Good surgical site (left) and poor surgical site (right).
10 Hoogstraten-Miller and Brown Page 10 Figure Anesthesia machine with precision calibrated vaporizer.
11 Hoogstraten-Miller and Brown Page 11 Figure Downdraft table.
12 Hoogstraten-Miller and Brown Page 12 Figure Chemical fume hood.
13 Hoogstraten-Miller and Brown Page 13 Figure Type IIB biosafety cabinet.
14 Hoogstraten-Miller and Brown Page 14 Figure Charcoal canister.
15 Hoogstraten-Miller and Brown Page 15 Figure Simple peel pack.
16 Hoogstraten-Miller and Brown Page 16 Figure Complex surgical pack.
17 Hoogstraten-Miller and Brown Page 17 Figure Hot bead sterilizer.
18 Hoogstraten-Miller and Brown Page 18 Figure Flash dry heat sterilizer.
19 Hoogstraten-Miller and Brown Page 19 Figure Organize the surgical instruments.
20 Hoogstraten-Miller and Brown Page 20 Figure Between surgeries, the tips of the instruments should be covered.
21 Hoogstraten-Miller and Brown Page 21 Figure Paper surgical drape
22 Hoogstraten-Miller and Brown Page 22 Figure Plastic adhesive surgical drape.
23 Hoogstraten-Miller and Brown Page 23 Figure Sterile gauze pads used for surgical drapes.
24 Hoogstraten-Miller and Brown Page 24 Figure Sterile tips of the instruments are placed on a sterile field.
25 Hoogstraten-Miller and Brown Page 25 Figure Arrows indicate space between drape and instruments that is not sterile.
26 Hoogstraten-Miller and Brown Page 26 Figure A wet area on a paper or cloth drape acts as a wick to pull bacteria through from the non-sterile surface below.
27 Hoogstraten-Miller and Brown Page 27 Video Donning sterile surgical gloves.
Policies Concerning Survival Surgery of Mice
 Policies Concerning Survival Surgery of Mice Date approved: October 25, 2016 Purpose This document details the minimum standards for survival (recovery) surgery in all laboratory rodent species. Responsibility
Policies Concerning Survival Surgery of Mice Date approved: October 25, 2016 Purpose This document details the minimum standards for survival (recovery) surgery in all laboratory rodent species. Responsibility
liquids. 3) Mist/wipe the work surface wiped with disinfectant before conducting surgery.
 Institutional Animal Care Program Title: RODENT SURVIVAL SURGERY Policy#: IACP 004 Date in Effect: 12/14/07 Rev #: 01 Rev Date: 12/14/12 Rev #: 02 Rev Date: 03/08/13 Rev #: 03 Rev Date: 12/13/13 Rev #:
Institutional Animal Care Program Title: RODENT SURVIVAL SURGERY Policy#: IACP 004 Date in Effect: 12/14/07 Rev #: 01 Rev Date: 12/14/12 Rev #: 02 Rev Date: 03/08/13 Rev #: 03 Rev Date: 12/13/13 Rev #:
STANDARD OPERATING PROCEDURES DIVISION OF COMPARATIVE MEDICINE UNIVERSITY OF SOUTH FLORIDA
 STANDARD OPERATING PROCEDURES DIVISION OF COMPARATIVE MEDICINE UNIVERSITY OF SOUTH FLORIDA SOP# 412.6 Date Issued: 9/02 Date Revised: 6/17 Page 1 of 5 TITLE: SCOPE: RESPONSIBILITY: PURPOSE: All Animal
STANDARD OPERATING PROCEDURES DIVISION OF COMPARATIVE MEDICINE UNIVERSITY OF SOUTH FLORIDA SOP# 412.6 Date Issued: 9/02 Date Revised: 6/17 Page 1 of 5 TITLE: SCOPE: RESPONSIBILITY: PURPOSE: All Animal
Section A Definitions
 Guidelines for Surgical Procedures in Rodents, Birds, and Cold-Blooded Vertebrates The University of Texas at Austin Institutional Animal Care and Use Committee These guidelines have been written to assist
Guidelines for Surgical Procedures in Rodents, Birds, and Cold-Blooded Vertebrates The University of Texas at Austin Institutional Animal Care and Use Committee These guidelines have been written to assist
Animal Care and Use Program Policy & Procedures Rodent Surgery. To outline the proper procedures for the performance of rodent surgery
 Animal Care and Use Program Policy & Procedures Rodent Surgery Objective: Author: To outline the proper procedures for the performance of rodent surgery Chandra D. Williams, DVM, DACLAM Reviewed: 09/24/2012;
Animal Care and Use Program Policy & Procedures Rodent Surgery Objective: Author: To outline the proper procedures for the performance of rodent surgery Chandra D. Williams, DVM, DACLAM Reviewed: 09/24/2012;
Office of Research Compliance (ORC) Institutional Animal Care and Use Committee (IACUC) Surgery & Post-Operative Care
 Office of Research Compliance (ORC) Institutional Animal Care and Use Committee (IACUC) Surgery & Post-Operative Care FULL POLICY CONTENTS Scope Background Policy Statements References Effective: 7.2016
Office of Research Compliance (ORC) Institutional Animal Care and Use Committee (IACUC) Surgery & Post-Operative Care FULL POLICY CONTENTS Scope Background Policy Statements References Effective: 7.2016
Rodent Anesthesia, Surgery and Analgesia
 Florida Atlantic University Institutional Animal Care and Use Committee SOP Performance Standard: To minimize the risks associated with anesthesia and surgical procedures and reduce post-operative pain.
Florida Atlantic University Institutional Animal Care and Use Committee SOP Performance Standard: To minimize the risks associated with anesthesia and surgical procedures and reduce post-operative pain.
INSTITUTIONAL ANIMAL USER TRAINING PROGRAM Online Courses and Hands-On Workshops Outline
 INSTITUTIONAL ANIMAL USER TRAINING PROGRAM Online Courses and Hands-On Workshops Outline - 2018 Table of Contents I. Institutional Animal User Training Program Steps... 3 II. Governing Institutional Policies
INSTITUTIONAL ANIMAL USER TRAINING PROGRAM Online Courses and Hands-On Workshops Outline - 2018 Table of Contents I. Institutional Animal User Training Program Steps... 3 II. Governing Institutional Policies
Chapter 2 Basic Equipment for Microsurgical Experiment
 Chapter 2 Basic Equipment for Microsurgical Experiment Peter Balaz Abstract Unlike of human organ transplantation, the most of the experimental procedures in small animal are performed under magnification
Chapter 2 Basic Equipment for Microsurgical Experiment Peter Balaz Abstract Unlike of human organ transplantation, the most of the experimental procedures in small animal are performed under magnification
SOP: Blood Collection in the Mouse, Retro-orbital Bleed
 SOP: Blood Collection in the Mouse, Retro-orbital Bleed These SOPs were developed by the Office of the University Veterinarian and reviewed by Virginia Tech IACUC to provide a reference and guidance to
SOP: Blood Collection in the Mouse, Retro-orbital Bleed These SOPs were developed by the Office of the University Veterinarian and reviewed by Virginia Tech IACUC to provide a reference and guidance to
SOP: Blood Collection in the Mouse, Tail Vein
 SOP: Blood Collection in the Mouse, Tail Vein These SOPs were developed by the Office of the University Veterinarian and reviewed by Virginia Tech IACUC to provide a reference and guidance to investigators
SOP: Blood Collection in the Mouse, Tail Vein These SOPs were developed by the Office of the University Veterinarian and reviewed by Virginia Tech IACUC to provide a reference and guidance to investigators
3.03 Understand support services
 3.03 Understand support services Clean and Decontaminate the Healthcare Environment Manage Hazardous Materials and Wastes Manage and Store Materials 3.03 Understand support services 2 Clean and Decontaminate
3.03 Understand support services Clean and Decontaminate the Healthcare Environment Manage Hazardous Materials and Wastes Manage and Store Materials 3.03 Understand support services 2 Clean and Decontaminate
COLLECTION OF SMALL AMOUNTS OF BLOOD FROM TAIL TIP MICROSAMPLING IN MICE
 UBC Animal Care Guidelines SOP: ACC 2014 Tech12 microsampling in mice. Submitted by: Shelly McErlane Last date revised: Date approved: June 4, 2014 COLLECTION OF SMALL AMOUNTS OF BLOOD FROM TAIL TIP MICROSAMPLING
UBC Animal Care Guidelines SOP: ACC 2014 Tech12 microsampling in mice. Submitted by: Shelly McErlane Last date revised: Date approved: June 4, 2014 COLLECTION OF SMALL AMOUNTS OF BLOOD FROM TAIL TIP MICROSAMPLING
Standard Operating Procedure for performing a muscle biopsy
 Standard Operating Procedure for performing a muscle biopsy Effective date: 31.10.2016 Review due date: 30.08.2018 Original Author Name: Richard Metcalfe and Abdullah Alghannam Date: 14.12.2012 Position:
Standard Operating Procedure for performing a muscle biopsy Effective date: 31.10.2016 Review due date: 30.08.2018 Original Author Name: Richard Metcalfe and Abdullah Alghannam Date: 14.12.2012 Position:
Guidelines for Survival Bleeding of Mice and Rats
 Guidelines for Survival Bleeding of Mice and Rats These guidelines have been developed to assist investigators and Institutional Animal Care and Use Committees (IACUC) in their choice and application of
Guidelines for Survival Bleeding of Mice and Rats These guidelines have been developed to assist investigators and Institutional Animal Care and Use Committees (IACUC) in their choice and application of
STONEHILL COLLEGE. Lab Inspection Checklist
 General Considerations: Does the location minimize traffic and contamination? Is the location secure? Are the animal areas separate from personnel areas? Is there a separation by species and/or disease
General Considerations: Does the location minimize traffic and contamination? Is the location secure? Are the animal areas separate from personnel areas? Is there a separation by species and/or disease
Type: Embedded within the selected substance, indicated when creating the substance.
 Overview: Substance Table: Each procedure will include a substance table, either on the main page of the procedure (immediately after selecting the procedure type), embedded elsewhere within a procedure,
Overview: Substance Table: Each procedure will include a substance table, either on the main page of the procedure (immediately after selecting the procedure type), embedded elsewhere within a procedure,
SOP: Blood Collection from the Tail Vein in Rats
 SOP: Blood Collection from the Tail Vein in Rats These SOPs were developed by the Office of the University Veterinarian and reviewed by Virginia Tech IACUC to provide a reference and guidance to investigators
SOP: Blood Collection from the Tail Vein in Rats These SOPs were developed by the Office of the University Veterinarian and reviewed by Virginia Tech IACUC to provide a reference and guidance to investigators
Policy 19 Fluid Administration and Collection in Rodents Version 1.0 Approval Date: 12/12/12
 Policy 19 Fluid Administration and Collection in Rodents Version 1.0 Approval Date: 12/12/12 Purpose This policy was designed to provide investigators with reference values related to the administration
Policy 19 Fluid Administration and Collection in Rodents Version 1.0 Approval Date: 12/12/12 Purpose This policy was designed to provide investigators with reference values related to the administration
Vertebrate Animal Use Protocol for Research or Testing
 AUP #: ORC USE ONLY Vertebrate Animal Use Protocol for Research or Testing Date Received: Instructions and Researcher Certifications (Failure to follow may result in a delay in processing) Complete this
AUP #: ORC USE ONLY Vertebrate Animal Use Protocol for Research or Testing Date Received: Instructions and Researcher Certifications (Failure to follow may result in a delay in processing) Complete this
3M Tegaderm PICC/CVC Securement Device + I.V. Advanced Securement Dressing Product Description
 3M Tegaderm PICC/CVC Securement Device + I.V. Advanced Securement Dressing Product Description 3M Tegaderm PICC/CVC Securement Device + I.V. Advanced Securement Dressing ( Securement System ) is designed
3M Tegaderm PICC/CVC Securement Device + I.V. Advanced Securement Dressing Product Description 3M Tegaderm PICC/CVC Securement Device + I.V. Advanced Securement Dressing ( Securement System ) is designed
3.03 Understand support services
 3.03 Understand support services Clean and Decontaminate the Healthcare Environment Manage Hazardous Materials and Wastes Manage and Store Materials 3.03 Understand support services 2 Clean and Decontaminate
3.03 Understand support services Clean and Decontaminate the Healthcare Environment Manage Hazardous Materials and Wastes Manage and Store Materials 3.03 Understand support services 2 Clean and Decontaminate
COLLECTION OF SMALL AMOUNTS OF BLOOD FROM TAIL TIP MICROSAMPLING IN RATS
 UBC Animal Care Guidelines SOP: ACC 2014 Tech13 Collection of small amounts of blood from tail tip microsampling in rats. Submitted by: Shelly McErlane Last date revised: Date approved: June 4, 2014 COLLECTION
UBC Animal Care Guidelines SOP: ACC 2014 Tech13 Collection of small amounts of blood from tail tip microsampling in rats. Submitted by: Shelly McErlane Last date revised: Date approved: June 4, 2014 COLLECTION
Animal cell and tissue culture. Lab 1
 Animal cell and tissue culture Lab 1 Tissue culture Laboratory Safety Outline Lab Safety Biohazards Biosafety Levels Biosafety Cabinets Decontamination Biological Waste Introduction A cell culture laboratory
Animal cell and tissue culture Lab 1 Tissue culture Laboratory Safety Outline Lab Safety Biohazards Biosafety Levels Biosafety Cabinets Decontamination Biological Waste Introduction A cell culture laboratory
Packaging & Sterilization
 Packaging & Sterilization Packaging & Sterilization 1 Dr. Norman Clinical Consultant General Sterilization Packaging System in Hospitals Sterilization Packaging System Disposable Reusable Crepe paper Woven
Packaging & Sterilization Packaging & Sterilization 1 Dr. Norman Clinical Consultant General Sterilization Packaging System in Hospitals Sterilization Packaging System Disposable Reusable Crepe paper Woven
Guidelines for the Use of Cytotoxic or Chemotherapeutic Drugs
 Guidelines for the Use of Cytotoxic or Chemotherapeutic Drugs Caltech Environment, Health and Safety 1200 E. California Blvd. M/C 25-6 Pasadena, CA 91125 626.395.6727 safety@caltech.edu www.safety.caltech.edu
Guidelines for the Use of Cytotoxic or Chemotherapeutic Drugs Caltech Environment, Health and Safety 1200 E. California Blvd. M/C 25-6 Pasadena, CA 91125 626.395.6727 safety@caltech.edu www.safety.caltech.edu
Micro-Electrode Arrays (MEA), Micro-Wire Arrays (MWA)
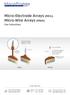 MicroProbes for Life Science Micro-Electrode Arrays (MEA), Micro-Wire Arrays (MWA) User Instructions The distance between contacts sites could be between 0.1-1.0 mm 2 mm (recommended) Different lengths
MicroProbes for Life Science Micro-Electrode Arrays (MEA), Micro-Wire Arrays (MWA) User Instructions The distance between contacts sites could be between 0.1-1.0 mm 2 mm (recommended) Different lengths
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) PACIFIC LUTHERAN UNIVERSITY TACOMA, WA PHONE: (253) FAX: (253)
 INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) PACIFIC LUTHERAN UNIVERSITY TACOMA, WA 98447-0001 PHONE: (253)535-7126 FAX: (253) 535-8760 PROTOCOL FOR ANIMAL CARE AND USE IACUC Approval Form (Created
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) PACIFIC LUTHERAN UNIVERSITY TACOMA, WA 98447-0001 PHONE: (253)535-7126 FAX: (253) 535-8760 PROTOCOL FOR ANIMAL CARE AND USE IACUC Approval Form (Created
Standard Operating Procedure Safe Autoclave Operations
 Standard Operating Procedure Safe Autoclave Operations The purpose of this instructional document is to introduce and familiarize the reader to the standard operating procedures for the safe use of autoclaves.
Standard Operating Procedure Safe Autoclave Operations The purpose of this instructional document is to introduce and familiarize the reader to the standard operating procedures for the safe use of autoclaves.
Biosafety Training. WVU Shared Research Facilities 2012
 Biosafety Training WVU Shared Research Facilities 2012 Training Overview DEFINE Biosafety, Biohazard, Biosafety Levels Protection (PPE, biosafety cabinets) CELL CULTURE Hazards (Blood Borne Pathogens)
Biosafety Training WVU Shared Research Facilities 2012 Training Overview DEFINE Biosafety, Biohazard, Biosafety Levels Protection (PPE, biosafety cabinets) CELL CULTURE Hazards (Blood Borne Pathogens)
Medical Device Reprocessing Assessment - General (Cleaning, Disinfection, and/or Sterilization)
 Medical Device Reprocessing Assessment - General (Cleaning, Disinfection, and/or Sterilization) Date: Office Name: Office Number: Responsible Physician: Assessor: M.1. General Yes No N/A If No Please Comment
Medical Device Reprocessing Assessment - General (Cleaning, Disinfection, and/or Sterilization) Date: Office Name: Office Number: Responsible Physician: Assessor: M.1. General Yes No N/A If No Please Comment
Texas A&M University-Commerce Institutional Animal Care and Use Committee Animal Care and Use Application Teaching
 Texas A&M University-Commerce Institutional Animal Care and Use Committee Animal Care and Use Application Teaching Principal Investigator Name: Department: Campus Address: Campus Phone Number: Email Rank:
Texas A&M University-Commerce Institutional Animal Care and Use Committee Animal Care and Use Application Teaching Principal Investigator Name: Department: Campus Address: Campus Phone Number: Email Rank:
IACUC POLICIES, PROCEDURES and GUIDELINES. Rodent Surgery
 Page 1 of 15 IACUC POLICIES, PROCEDURES and GUIDELINES Rodent Surgery 102.1 Purpose This document provides information and guidance for the performance of surgical procedures in rodents (guinea pigs, gerbils,
Page 1 of 15 IACUC POLICIES, PROCEDURES and GUIDELINES Rodent Surgery 102.1 Purpose This document provides information and guidance for the performance of surgical procedures in rodents (guinea pigs, gerbils,
Standard Operating Procedures. Strong Corrosives Strong Acids (SA) Acutely Toxic Chemicals Hydrofluoric Acid
 Standard Operating Procedures Strong Corrosives Strong Acids (SA) Acutely Toxic Chemicals Hydrofluoric Acid Department: Chemistry Date SOP was written: April 16, 2014 Principal Investigator: Dr. Greg Boyce
Standard Operating Procedures Strong Corrosives Strong Acids (SA) Acutely Toxic Chemicals Hydrofluoric Acid Department: Chemistry Date SOP was written: April 16, 2014 Principal Investigator: Dr. Greg Boyce
Purdue Animal Care and Use Committee (PACUC) APPLICATION TO USE VERTEBRATE ANIMALS IN RESEARCH, TEACHING, OR TESTING
 Principal Investigator/Project Director: Protocol Title: Coeus Protocol Number: Purdue Animal Care and Use Committee (PACUC) APPLICATION TO USE VERTEBRATE ANIMALS IN RESEARCH, TEACHING, OR TESTING IACUC
Principal Investigator/Project Director: Protocol Title: Coeus Protocol Number: Purdue Animal Care and Use Committee (PACUC) APPLICATION TO USE VERTEBRATE ANIMALS IN RESEARCH, TEACHING, OR TESTING IACUC
Amherst College. Health and Safety Manual for the Use of Recombinant DNA and Biological Agents Current as of May 14, 2010
 Amherst College Health and Safety Manual for the Use of Recombinant DNA and Biological Agents Current as of May 14, 2010 Introduction This manual contains the basic procedures to be followed for all work
Amherst College Health and Safety Manual for the Use of Recombinant DNA and Biological Agents Current as of May 14, 2010 Introduction This manual contains the basic procedures to be followed for all work
Guidelines for Selection and Use of Disinfectants
 Guidelines for Selection and Use of Disinfectants Ref: (a) APIC Guidelines for Infection Control Practice, American Journal of Infection Control; April 1990, Vol 18, 99-113. To assist health care professionals
Guidelines for Selection and Use of Disinfectants Ref: (a) APIC Guidelines for Infection Control Practice, American Journal of Infection Control; April 1990, Vol 18, 99-113. To assist health care professionals
ENVIRONMENTAL HEALTH & SAFETY
 ENVIRONMENTAL HEALTH & SAFETY Standard Operating Procedures for Safe Autoclave Operations The purpose of this document is to provide standard operating procedures for the safe use of autoclaves. Autoclaving
ENVIRONMENTAL HEALTH & SAFETY Standard Operating Procedures for Safe Autoclave Operations The purpose of this document is to provide standard operating procedures for the safe use of autoclaves. Autoclaving
Facility: Sterile Processing Assessment Date: CRITERIA ANSI/AAMI COMPLIANT Number Y N N/A Point of Use Gross contaminant is removed with
 Point of Use 6.2-6.3 Gross contaminant is removed with water Instruments are sorted Sharps removed Instruments placed back in original container Un-used instruments placed in bottom of basket Towel placed
Point of Use 6.2-6.3 Gross contaminant is removed with water Instruments are sorted Sharps removed Instruments placed back in original container Un-used instruments placed in bottom of basket Towel placed
Packaging Systems for Central Service Operations
 Lesson No. CRCST 132 (Technical Continuing Education - TCE) Sponsored by: by Carol Petro, RN, CRCST, CIS, CNOR OR Educator and Sterile Processing Educator Indiana University Health North Hospital Carmel,
Lesson No. CRCST 132 (Technical Continuing Education - TCE) Sponsored by: by Carol Petro, RN, CRCST, CIS, CNOR OR Educator and Sterile Processing Educator Indiana University Health North Hospital Carmel,
STANDARD OPERATING PROCEDURE #712 USE OF AZOXYMETHANE IN RODENTS
 STANDARD OPERATING PROCEDURE #712 USE OF AZOXYMETHANE IN RODENTS 1. PURPOSE This Standard Operating Procedure (SOP) describes the guidelines for the use of azoxymethane in rodents. 2. CONSIDERATIONS Azoxymethane
STANDARD OPERATING PROCEDURE #712 USE OF AZOXYMETHANE IN RODENTS 1. PURPOSE This Standard Operating Procedure (SOP) describes the guidelines for the use of azoxymethane in rodents. 2. CONSIDERATIONS Azoxymethane
EH&S. Sheet. Fact. Safe and Effective Use of Autoclaves. What are autoclaves? Factors for effective sterilization. Dry heat cycle - when to use
 Please post or circulate Fact heet nvironment, ealth and afety Information for the Berkeley Campus No. 33 Revised 04/04/11 afe and ffective Use of Autoclaves Autoclaves are easy to use but can pose a safety
Please post or circulate Fact heet nvironment, ealth and afety Information for the Berkeley Campus No. 33 Revised 04/04/11 afe and ffective Use of Autoclaves Autoclaves are easy to use but can pose a safety
Intravitreal and sub-retinal injections of plasmid DNA and electroporation in P0 pups
 Intravitreal and sub-retinal injections of plasmid DNA and electroporation in P0 pups Protocol modified from: Retinal Gene Delivery by raav and DNA Electroporation, Aditya Venkatesh et all, Current Protocols
Intravitreal and sub-retinal injections of plasmid DNA and electroporation in P0 pups Protocol modified from: Retinal Gene Delivery by raav and DNA Electroporation, Aditya Venkatesh et all, Current Protocols
Instructions for Use: Duodenoscope Sampling Kit
 Instructions for Use: Duodenoscope Sampling Kit Brand Name of Product Generic Name of Product Product Code Number(s) Intended Use Duodenoscope Sampling Kit Endoscope sampling and culturing kit CK-250,
Instructions for Use: Duodenoscope Sampling Kit Brand Name of Product Generic Name of Product Product Code Number(s) Intended Use Duodenoscope Sampling Kit Endoscope sampling and culturing kit CK-250,
Contact Information: Laboratory Supervisor: Denise Kind Laboratory Manager: Mat Ashby
 Biology and Wildlife STANDARD OPERATING PROCEDURE Autoclaving Location(s): Murie 215 Chemical(s): None Specific Hazards: o steam improper use of autoclave can expose user to dangerous steam burns o extremely
Biology and Wildlife STANDARD OPERATING PROCEDURE Autoclaving Location(s): Murie 215 Chemical(s): None Specific Hazards: o steam improper use of autoclave can expose user to dangerous steam burns o extremely
Ohio University IACUC Approved Guidelines Revised & Approved: April 3, 2017
 Ohio University IACUC Approved Guidelines A. Use of Controlled Substances in Research B. Endpoints Guidelines for Experimental Live Vertebrate Animals C. Live Vertebrate Definition D. Use of Non-Pharmaceutical
Ohio University IACUC Approved Guidelines A. Use of Controlled Substances in Research B. Endpoints Guidelines for Experimental Live Vertebrate Animals C. Live Vertebrate Definition D. Use of Non-Pharmaceutical
Needle Loading. Seed Sterilization. Shielding. Seed Handling. SeedVac Seed Slider. Seed Sterilization and Sorting Tray Seed Sterilization Pill Box
 Prepare Prostate Brachytherapy treatments fast and with minimal exposure. Standard Imaging can provide you with complete kits that easily provide everything needed to start a prostate implant program.
Prepare Prostate Brachytherapy treatments fast and with minimal exposure. Standard Imaging can provide you with complete kits that easily provide everything needed to start a prostate implant program.
Standard Operating Procedures. Regulated Carcinogens (RC) Formaldehyde
 Standard Operating Procedures Regulated Carcinogens (RC) Formaldehyde Department: Chemistry Date SOP was written: April 16, 2014 Principal Investigator: Dr. Greg Boyce Location: Whitaker Hall Room 243
Standard Operating Procedures Regulated Carcinogens (RC) Formaldehyde Department: Chemistry Date SOP was written: April 16, 2014 Principal Investigator: Dr. Greg Boyce Location: Whitaker Hall Room 243
Guide for Cleaning, Sterilization and Storage of Introducers for Caldera Medical Implants
 Guide for Cleaning, Sterilization and Storage of Introducers for Caldera Medical Implants M Manufactured by: Caldera Medical, Inc. 5171 Clareton Drive Agoura Hills, CA 91301 U.S. Toll Free: 866-4-CALDERA
Guide for Cleaning, Sterilization and Storage of Introducers for Caldera Medical Implants M Manufactured by: Caldera Medical, Inc. 5171 Clareton Drive Agoura Hills, CA 91301 U.S. Toll Free: 866-4-CALDERA
Generation of Mouse Spinal Cord Injury Oneil G. Bhalala *, Liuliu Pan, Hilary North, Tammy McGuire and John A. Kessler
 Generation of Mouse Spinal Cord Injury Oneil G. Bhalala *, Liuliu Pan, Hilary North, Tammy McGuire and John A. Kessler Department of Neurology, Northwestern University, Chicago, USA *For correspondence:
Generation of Mouse Spinal Cord Injury Oneil G. Bhalala *, Liuliu Pan, Hilary North, Tammy McGuire and John A. Kessler Department of Neurology, Northwestern University, Chicago, USA *For correspondence:
BIOSAFETY LEVEL 2 PROTOCOLS BIOPROCESS OPTIMIZATION LABORATORY - POULIOT BUILDING, ROOM 1541 ALAIN GARNIER JUNE 2007, LAVAL UNIVERSITY
 BIOSAFETY LEVEL 2 PROTOCOLS BIOPROCESS OPTIMIZATION LABORATORY - POULIOT BUILDING, ROOM 1541 ALAIN GARNIER JUNE 2007, LAVAL UNIVERSITY 2 Preamble This document describes the procedures to follow for safely
BIOSAFETY LEVEL 2 PROTOCOLS BIOPROCESS OPTIMIZATION LABORATORY - POULIOT BUILDING, ROOM 1541 ALAIN GARNIER JUNE 2007, LAVAL UNIVERSITY 2 Preamble This document describes the procedures to follow for safely
WICHITA STATE UNIVERSITY INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) ANIMAL PROTOCOL FORM
 WICHITA STATE UNIVERSITY INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) ANIMAL PROTOCOL FORM Date: Principal Investigator: Title of Project: Protocol #: Animal Species: Anticipated Start Date: Anticipated
WICHITA STATE UNIVERSITY INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) ANIMAL PROTOCOL FORM Date: Principal Investigator: Title of Project: Protocol #: Animal Species: Anticipated Start Date: Anticipated
Microorganisms are the agents of contamination, infection. Hence it becomes necessary to remove them from materials and areas.
 Mawada M.yahia Objectives 1.Define the terms sterilization, disinfectant and antiseptic. 2. Classify the different methods of sterilization 3. Rrealizes that heat is the most important method of sterilization.
Mawada M.yahia Objectives 1.Define the terms sterilization, disinfectant and antiseptic. 2. Classify the different methods of sterilization 3. Rrealizes that heat is the most important method of sterilization.
Institutional Biosafety Committee Policy: BIOSAFETY BEST PRACTICES FOR RESEARCH USE OF BIOLOGICAL TOXINS & VENOMS
 Institutional Biosafety Committee Policy: BIOSAFETY BEST PRACTICES FOR RESEARCH USE OF BIOLOGICAL TOXINS & VENOMS Applies to: Vanderbilt University (VU) Vanderbilt University Medical Center (MC) This document
Institutional Biosafety Committee Policy: BIOSAFETY BEST PRACTICES FOR RESEARCH USE OF BIOLOGICAL TOXINS & VENOMS Applies to: Vanderbilt University (VU) Vanderbilt University Medical Center (MC) This document
Because life is precious...
 Because life is precious... VISION MISSION COMPANY PROFILE FLEXIBILITY 6 7 8 9 MENALENE MENALON MENABOND MENASILK 18 18 19 19 MENASORB MENASORB FAST MENACRYL MENACRYL FAST MENA PD MENACAPRONE MENAGUT
Because life is precious... VISION MISSION COMPANY PROFILE FLEXIBILITY 6 7 8 9 MENALENE MENALON MENABOND MENASILK 18 18 19 19 MENASORB MENASORB FAST MENACRYL MENACRYL FAST MENA PD MENACAPRONE MENAGUT
Quality. you expect. you ll love. you require. Performance
 Quality you require Performance you ll love Value you expect Quality We measure our suppliers consistently on a wide variety of metrics and hold them to our standards. Value We leverage years of experience
Quality you require Performance you ll love Value you expect Quality We measure our suppliers consistently on a wide variety of metrics and hold them to our standards. Value We leverage years of experience
Standard Operating Procedure for Fluorouracil in Animals
 Standard Operating Procedure for Fluorouracil in Animals 1. Health hazards Fluorouracil or 5-FU (trademarked as Adrucil (IV), Carac (topical), Efudex and Efudix (topical)) is a drug that is a pyrimidine
Standard Operating Procedure for Fluorouracil in Animals 1. Health hazards Fluorouracil or 5-FU (trademarked as Adrucil (IV), Carac (topical), Efudex and Efudix (topical)) is a drug that is a pyrimidine
CAUTION: U.S. Federal law restricts this device to sale by or on the order of a licensed physician.
 TM CAUTION: U.S. Federal law restricts this device to sale by or on the order of a licensed physician. TABLE OF CONTENTS Section Port Styles 4 Description 5 Indications 5 Contraindications 5-6 Information
TM CAUTION: U.S. Federal law restricts this device to sale by or on the order of a licensed physician. TABLE OF CONTENTS Section Port Styles 4 Description 5 Indications 5 Contraindications 5-6 Information
Guide for Cleaning, Sterilization and Storage of Reusable Introducers
 Guide for Cleaning, Sterilization and Storage of Reusable Introducers M Manufactured by: Caldera Medical, Inc. 5171 Clareton Drive Agoura Hills, CA 91301 U.S. Toll Free: 866-4-CALDERA Telephone: 818-879-6555
Guide for Cleaning, Sterilization and Storage of Reusable Introducers M Manufactured by: Caldera Medical, Inc. 5171 Clareton Drive Agoura Hills, CA 91301 U.S. Toll Free: 866-4-CALDERA Telephone: 818-879-6555
SOP: Mouse Intravenous Injections
 SOP: Mouse Intravenous Injections These SOPs were developed by the Office of the University Veterinarian and reviewed by Virginia Tech IACUC to provide a reference and guidance to investigators during
SOP: Mouse Intravenous Injections These SOPs were developed by the Office of the University Veterinarian and reviewed by Virginia Tech IACUC to provide a reference and guidance to investigators during
University of Denver Environmental Health & Safety Department Laboratory Inspection Comment Sheet
 University of Denver Environmental Health & Safety Department Laboratory Inspection Comment Sheet The following briefly describes what EHS looks for during a typical laboratory inspection. Items in boxes
University of Denver Environmental Health & Safety Department Laboratory Inspection Comment Sheet The following briefly describes what EHS looks for during a typical laboratory inspection. Items in boxes
Contact: To sterilize or decontaminate uniformly, superheated steam must contact all areas of the load.
 Published on UC Davis Safety Services (https://safetyservices.ucdavis.edu) Effective Use of Autoclaves SafetyNet #: 26 Autoclave Use Autoclaves (steam sterilizers) are metal pressure vessels that are used
Published on UC Davis Safety Services (https://safetyservices.ucdavis.edu) Effective Use of Autoclaves SafetyNet #: 26 Autoclave Use Autoclaves (steam sterilizers) are metal pressure vessels that are used
B. The Facility Manager is responsible for ensuring all staff are adequately trained and experienced in autoclave sterilization procedures.
 Author: Amber Matthews Edited by Amy Chappelle 10/2/25 1 of 5 Responsible faculty: Phil Smith 10/2/25 (Signature/Date) PURPOSE A. Sterilization refers to the complete killing of all living organisms, including
Author: Amber Matthews Edited by Amy Chappelle 10/2/25 1 of 5 Responsible faculty: Phil Smith 10/2/25 (Signature/Date) PURPOSE A. Sterilization refers to the complete killing of all living organisms, including
INSTRUCTIONS FOR USE FOR:
 INSTRUCTIONS FOR USE FOR: en English INSTRUCTIONS FOR USE GORE ENFORM INTRAPERITONEAL BIOMATERIAL Carefully read all instructions prior to use. Observe all instructions, warnings, and precautions noted
INSTRUCTIONS FOR USE FOR: en English INSTRUCTIONS FOR USE GORE ENFORM INTRAPERITONEAL BIOMATERIAL Carefully read all instructions prior to use. Observe all instructions, warnings, and precautions noted
Effective Use of Autoclaves
 Published on UC Davis Safety Services (https://safetyservices.ucdavis.edu) Effective Use of Autoclaves SafetyNet #: 26 Autoclave Use Autoclaves (steam sterilizers) are metal pressure vessels that are used
Published on UC Davis Safety Services (https://safetyservices.ucdavis.edu) Effective Use of Autoclaves SafetyNet #: 26 Autoclave Use Autoclaves (steam sterilizers) are metal pressure vessels that are used
FACT SHEET : Using Autoclaves Safely
 CSULA Environmental Health and Safety Biosafety Office FACT SHEET : Using Autoclaves Safely Most science research laboratories on campus require the use of autoclaves. The primary purpose of the autoclave
CSULA Environmental Health and Safety Biosafety Office FACT SHEET : Using Autoclaves Safely Most science research laboratories on campus require the use of autoclaves. The primary purpose of the autoclave
SOP: Intravenous Injections in the Rat
 SOP: Intravenous Injections in the Rat These SOPs were developed by the Office of the University Veterinarian and reviewed by Virginia Tech IACUC to provide a reference and guidance to investigators during
SOP: Intravenous Injections in the Rat These SOPs were developed by the Office of the University Veterinarian and reviewed by Virginia Tech IACUC to provide a reference and guidance to investigators during
DUQUESNE UNIVERSITY. Biosafety Guidelines
 DUQUESNE UNIVERSITY Biosafety Guidelines Prepared by: Environmental Health and Safety Department TABLE OF CONTENTS Page Biosafety Guidelines 1 Biosafety Guidelines for PIs 2 Biosafety Levels 3 SOP Biohazardous
DUQUESNE UNIVERSITY Biosafety Guidelines Prepared by: Environmental Health and Safety Department TABLE OF CONTENTS Page Biosafety Guidelines 1 Biosafety Guidelines for PIs 2 Biosafety Levels 3 SOP Biohazardous
DUQUESNE UNIVERSITY. Biosafety Guidelines
 DUQUESNE UNIVERSITY Biosafety Guidelines Prepared by: Environmental Health and Safety Department TABLE OF CONTENTS Page Biosafety Guidelines 1 Biosafety Guidelines for PIs 2 Biosafety Levels 3 SOP Biohazardous
DUQUESNE UNIVERSITY Biosafety Guidelines Prepared by: Environmental Health and Safety Department TABLE OF CONTENTS Page Biosafety Guidelines 1 Biosafety Guidelines for PIs 2 Biosafety Levels 3 SOP Biohazardous
Presented by LINXObere Medizintechnik GmbHH
 Advanced Wound Closure Solutions By LINXOBERE Surgical Products Presented by LINXObere Medizintechnik GmbHH Needle Type Structure Symbol Main Applications Round Needles (Taper Point) Smallest possible
Advanced Wound Closure Solutions By LINXOBERE Surgical Products Presented by LINXObere Medizintechnik GmbHH Needle Type Structure Symbol Main Applications Round Needles (Taper Point) Smallest possible
Inspection Checklist for NIH BL3 Laboratories (7 CFR 331; 9 CFR 121; 42 CFR 73; NIH Guidelines)
 12(a) 12(a) 12(b) 12 (c)(3) An individual or entity required to register under this part must develop and implement a written biosafety plan that is commensurate with the risk of the agent or toxin, given
12(a) 12(a) 12(b) 12 (c)(3) An individual or entity required to register under this part must develop and implement a written biosafety plan that is commensurate with the risk of the agent or toxin, given
Appendix Detailed Description of the Posterolateral Intertransverse Process Fusion Procedure Performed in a Rat Model
 Page 1 of 7 Fig. E-1 Image of surgical dissection of L4-L5 posterolateral intertransverse process fusion in the rat. The transverse processes of L3, L4, and L5 are visible at the top of the image. Fig.
Page 1 of 7 Fig. E-1 Image of surgical dissection of L4-L5 posterolateral intertransverse process fusion in the rat. The transverse processes of L3, L4, and L5 are visible at the top of the image. Fig.
Standard Operating Procedure
 Section 1: Overview Page 1 of 5 Non-halogenated Organic Solvents Standard Operating Procedure Lab: 3724 Beckman Institute Department: Materials Science and Engineering PI: Paul V. Braun Written By: Eric
Section 1: Overview Page 1 of 5 Non-halogenated Organic Solvents Standard Operating Procedure Lab: 3724 Beckman Institute Department: Materials Science and Engineering PI: Paul V. Braun Written By: Eric
Guidelines for Rodent Survival Surgery
 Journal of Investigative Surgery, 22, 445 451, 2009 Copyright C Informa Healthcare USA, Inc. ISSN: 0894-1939 print / 1521-0553 online DOI: 10.3109/08941930903396412 SURGICAL RESEARCH GUIDELINES Guidelines
Journal of Investigative Surgery, 22, 445 451, 2009 Copyright C Informa Healthcare USA, Inc. ISSN: 0894-1939 print / 1521-0553 online DOI: 10.3109/08941930903396412 SURGICAL RESEARCH GUIDELINES Guidelines
(College / School / Department): College of Natural Sciences, School of Biological Sciences
 (College / School / Department): College of Natural Sciences, School of Biological Sciences RISK ASSESSMENT TITLE: Use of Bio Medical Science Laboratory Location / Building / Area: Brambell Building, C4
(College / School / Department): College of Natural Sciences, School of Biological Sciences RISK ASSESSMENT TITLE: Use of Bio Medical Science Laboratory Location / Building / Area: Brambell Building, C4
Use of Non-Pharmaceutical-Grade Chemicals and Other Substances in Research with Animals
 Use of Non-Pharmaceutical-Grade Chemicals and Other Substances in Research with Animals Patricia Brown, VMD, MS, DACLAM Director, OLAW, NIH Carol Clarke, DVM, DACLAM Staff Officer, AC, APHIS, USDA Christian
Use of Non-Pharmaceutical-Grade Chemicals and Other Substances in Research with Animals Patricia Brown, VMD, MS, DACLAM Director, OLAW, NIH Carol Clarke, DVM, DACLAM Staff Officer, AC, APHIS, USDA Christian
Principles of Rodent Aseptic Surgery & Perioperative Care
 Principles of Rodent Aseptic Surgery & Perioperative Care Resources Marcel Perret-Gentil University Veterinarian & Director Laboratory Animal Resources Center The University of Texas at San Antonio marcel.perret@utsa.edu
Principles of Rodent Aseptic Surgery & Perioperative Care Resources Marcel Perret-Gentil University Veterinarian & Director Laboratory Animal Resources Center The University of Texas at San Antonio marcel.perret@utsa.edu
Active Learning and Lab Activity Bioman Conference 2014
 Active Learning and Lab Activity Bioman Conference 2014 A. What is validation? Establishing documented evidence which provides a high degree of assurance that a specific process will consistently produce
Active Learning and Lab Activity Bioman Conference 2014 A. What is validation? Establishing documented evidence which provides a high degree of assurance that a specific process will consistently produce
Biology and Wildlife STANDARD OPERATING PROCEDURE Ethidium Bromide
 Biology and Wildlife STANDARD OPERATING PROCEDURE Ethidium Bromide 4 0 0 This SOP addresses only ethidium bromide. Users must also follow the electrophoresis SOP and any other SOPs relevant to their particular
Biology and Wildlife STANDARD OPERATING PROCEDURE Ethidium Bromide 4 0 0 This SOP addresses only ethidium bromide. Users must also follow the electrophoresis SOP and any other SOPs relevant to their particular
In-Country shipment : How to safely ship human blood samples from suspected Ebola cases within a country by road, rail and sea
 In-Country shipment : How to safely ship human blood samples from suspected Ebola cases within a country by road, rail and sea Step 1: Before handling the sample, prepare all shipping equipment (1) Manage
In-Country shipment : How to safely ship human blood samples from suspected Ebola cases within a country by road, rail and sea Step 1: Before handling the sample, prepare all shipping equipment (1) Manage
Radiation Safety Program Hunter College of the City University of New York
 Radiation Safety Program Hunter College of the City University of New York 1. Guidelines for All Users of Radioactive Materials This document presents the guidelines for all persons using radioactive materials.
Radiation Safety Program Hunter College of the City University of New York 1. Guidelines for All Users of Radioactive Materials This document presents the guidelines for all persons using radioactive materials.
How to safely ship human blood samples from suspected Ebola cases within a country by road, rail and sea
 INTERIM GUIDELINE How to safely ship human blood samples from suspected Ebola cases within a country by road, rail and sea 2014 Step 1: Before handling the sample, prepare all shipping equipment Step 1a:
INTERIM GUIDELINE How to safely ship human blood samples from suspected Ebola cases within a country by road, rail and sea 2014 Step 1: Before handling the sample, prepare all shipping equipment Step 1a:
Rodent restrainers. Cylindrical restrainers: Broome Restrainers. Pastry bag restrainer: DecapiCone
 Clinical Techniques Rodent restrainers Cylindrical restrainers: Broome Restrainers Pastry bag restrainer: DecapiCone Rabbit restrainers Needles Gauge refers to diameter Smaller diameter is the larger number
Clinical Techniques Rodent restrainers Cylindrical restrainers: Broome Restrainers Pastry bag restrainer: DecapiCone Rabbit restrainers Needles Gauge refers to diameter Smaller diameter is the larger number
Autoclave Operation and Performance Verification
 SOP AMBL-010-A Page 1 of 10 Standard Operating Procedure AMBL-010-A Prepared: 7/17/2017 Revised: 6/9/2018 Prepared by: Terry E. Baxter Reviewed by: James E. Biddle Adam Bringhurst Autoclave Operation and
SOP AMBL-010-A Page 1 of 10 Standard Operating Procedure AMBL-010-A Prepared: 7/17/2017 Revised: 6/9/2018 Prepared by: Terry E. Baxter Reviewed by: James E. Biddle Adam Bringhurst Autoclave Operation and
Vertebrate Animal Biosafety Level 2 Criteria
 Vertebrate Animal Biosafety Level 2 Criteria Biosafety in Microbiological and Biomedical Laboratories (BMBL) 5 th Edition Section V Animal Biosafety Level 2 (ABSL-2): Animal Biosafety Level 2 builds upon
Vertebrate Animal Biosafety Level 2 Criteria Biosafety in Microbiological and Biomedical Laboratories (BMBL) 5 th Edition Section V Animal Biosafety Level 2 (ABSL-2): Animal Biosafety Level 2 builds upon
ARABIC CANADIAN MEDICAL CENTER CSSD POLICIES AND PROCEDURES
 CONTENTS Section Title 2 Disinfection, Packaging and Sterilization 3 Validation of Sterilization (Physical, Chemical and Biological) 4 Sterilizer Requalification 5 Sterile Package Shelf Life 6 Acquisition
CONTENTS Section Title 2 Disinfection, Packaging and Sterilization 3 Validation of Sterilization (Physical, Chemical and Biological) 4 Sterilizer Requalification 5 Sterile Package Shelf Life 6 Acquisition
SOP BIO-006 USE OF AUTOCLAVE FOR STERILIZATION OF MATERIALS AND BIOLOGICAL WASTE
 ENVIRONMENTAL AND EMERGENCY MANAGEMENT Environmental Health and Safety University Crossing Suite 140 Lowell MA 01854 http://www.uml.edu/eem/ SOP BIO-006 USE OF AUTOCLAVE FOR STERILIZATION OF MATERIALS
ENVIRONMENTAL AND EMERGENCY MANAGEMENT Environmental Health and Safety University Crossing Suite 140 Lowell MA 01854 http://www.uml.edu/eem/ SOP BIO-006 USE OF AUTOCLAVE FOR STERILIZATION OF MATERIALS
Instructions for Use READY TO USE
 READY TO USE Instructions for Use LifeCell Corporation One Millennium Way Branchburg, NJ 08876-3876 1.908.947.1215 1.800.367.5737 Fax: 1.908.947.1089 E-mail: customerservicegroup@lifecell.com www.lifecell.com
READY TO USE Instructions for Use LifeCell Corporation One Millennium Way Branchburg, NJ 08876-3876 1.908.947.1215 1.800.367.5737 Fax: 1.908.947.1089 E-mail: customerservicegroup@lifecell.com www.lifecell.com
INSTRUMENT PROCESSING FROM PREP/PACKAGING TO STORAGE. January 2016
 INSTRUMENT PROCESSING FROM PREP/PACKAGING TO STORAGE January 2016 Disclosures The opinions expressed in this presentation are those of the author and do not necessarily reflect the official position of
INSTRUMENT PROCESSING FROM PREP/PACKAGING TO STORAGE January 2016 Disclosures The opinions expressed in this presentation are those of the author and do not necessarily reflect the official position of
Objectives. The Perioperative Nurse s Role 6/24/2016. Instrument Processing & Sterilization Chesapeake Perioperative Consortium 2016
 Instrument Processing & Sterilization Chesapeake Perioperative Consortium 2016 Ensuring our patients get clean, sterile instruments every time. Objectives Define the perioperative nurse and surgical tech
Instrument Processing & Sterilization Chesapeake Perioperative Consortium 2016 Ensuring our patients get clean, sterile instruments every time. Objectives Define the perioperative nurse and surgical tech
Repliform. Tissue Regeneration Matrix. Instructions for Use. Distributed by:
 Repliform Tissue Regeneration Matrix Instructions for Use Distributed by: Boston Scientific Corporation 300 Boston Scientific Way Marlborough, MA 01752 Processed from Donated Human Tissue by: LifeCell
Repliform Tissue Regeneration Matrix Instructions for Use Distributed by: Boston Scientific Corporation 300 Boston Scientific Way Marlborough, MA 01752 Processed from Donated Human Tissue by: LifeCell
Section: Administration Date of Issue: 05/09/2011 Issued By: Administration Part: Pneumatic Tube System Revision #: Revision Date:
 STANDARD OPERATING PROCEDURE VETERINARY HEALTH COMPLEX Section: Administration Date of Issue: 05/09/2011 Issued By: Administration Part: Pneumatic Tube System Revision #: Revision Date: Pages: Board Approval
STANDARD OPERATING PROCEDURE VETERINARY HEALTH COMPLEX Section: Administration Date of Issue: 05/09/2011 Issued By: Administration Part: Pneumatic Tube System Revision #: Revision Date: Pages: Board Approval
GUIDELINES FOR LABORATORY USE OF CHEMICAL CARCINOGENS
 GUIDELINES FOR LABORATORY USE OF CHEMICAL CARCINOGENS UNIVERSITY RISK MANAGEMENT Occupational Safety and Health Programs 19 Hagood Avenue, Suite 908 Charleston SC 29425 843-792-3604 Revised: January 2015
GUIDELINES FOR LABORATORY USE OF CHEMICAL CARCINOGENS UNIVERSITY RISK MANAGEMENT Occupational Safety and Health Programs 19 Hagood Avenue, Suite 908 Charleston SC 29425 843-792-3604 Revised: January 2015
Sterilization Policy. Georgia Regents Medical Center Policy Library. Policy Owner: Epidemiology POLICY STATEMENT
 POLICY STATEMENT The ability to sterilize instruments and equipment for use during operative or other invasive procedures is critical to promoting successful patient outcomes and preventing infections.
POLICY STATEMENT The ability to sterilize instruments and equipment for use during operative or other invasive procedures is critical to promoting successful patient outcomes and preventing infections.
Material Safety Data Sheet
 Section 1. Product Identification PRODUCT NAME: 3.6V Lithium Battery PRODUCT CODE: LIR2450 Section 2 Composition/Information on Ingredient Section 3 - Hazards Identification Health Hazards (Acute and Chronic)
Section 1. Product Identification PRODUCT NAME: 3.6V Lithium Battery PRODUCT CODE: LIR2450 Section 2 Composition/Information on Ingredient Section 3 - Hazards Identification Health Hazards (Acute and Chronic)
Safe Operating Procedure
 Safe Operating Procedure (Revised 8/12) AUTOCLAVE OPERATION AND PERFORMANCE TESTING (For assistance, please contact EHS at (402) 472-4925, or visit our web site at http://ehs.unl.edu/) Autoclaves are used
Safe Operating Procedure (Revised 8/12) AUTOCLAVE OPERATION AND PERFORMANCE TESTING (For assistance, please contact EHS at (402) 472-4925, or visit our web site at http://ehs.unl.edu/) Autoclaves are used
IACUC Standard Operating Procedure SOP : Isolation of Hazardous Chemicals/Agents in Use in Rodents
 IACUC Standard Operating Procedure SOP-05-2017: Isolation of Hazardous Chemicals/Agents in Use in Rodents This document describes the procedures used when handling rodents exposed to hazardous chemicals/agents,
IACUC Standard Operating Procedure SOP-05-2017: Isolation of Hazardous Chemicals/Agents in Use in Rodents This document describes the procedures used when handling rodents exposed to hazardous chemicals/agents,
LABORATORY WASTE MANAGEMENT PROGRAMMES
 LABORATORY WASTE MANAGEMENT PROGRAMMES Sylvia Wanjiru Kamau INTERNATIONAL LIVESTOCK RESEARCH INSTITUTE LABORATORY MANAGEMENT AND EQUIPMENT OPERATIONS WORKSHOP. 17 TH -21 ST JUNE 2012 TRAINING OBJECTIVES
LABORATORY WASTE MANAGEMENT PROGRAMMES Sylvia Wanjiru Kamau INTERNATIONAL LIVESTOCK RESEARCH INSTITUTE LABORATORY MANAGEMENT AND EQUIPMENT OPERATIONS WORKSHOP. 17 TH -21 ST JUNE 2012 TRAINING OBJECTIVES
Laboratory Biosafety Plan
 DUKE NEUROBIOLOGY SCHOOL OF MEDICINE Laboratory Biosafety Plan Laboratory of Boris Kantor Laboratory Biohazards: Biosafety Level 1 Recombinant DNA cloning in E. coli K-12 and derivatives Adeno-Associated
DUKE NEUROBIOLOGY SCHOOL OF MEDICINE Laboratory Biosafety Plan Laboratory of Boris Kantor Laboratory Biohazards: Biosafety Level 1 Recombinant DNA cloning in E. coli K-12 and derivatives Adeno-Associated
