2.1 Please indicate whether, according to Article 3 of the present Decision, the current report is:
|
|
|
- Bertina Bond
- 5 years ago
- Views:
Transcription
1 FORMAT FOR THE PRESENTATION OF THE RESULT OF DELIBERATE RELEASE INTO THE ENVIRONMENT OF GENETICALLY MODIFIED HIGHER PLANTS IN ACCORDANCE WITH ARTICLE 10 OF DIRECTIVE 2001/18/EC 1 General information 1.1 European notification number: B/ES/12/ Member State of notification: Spain 1.3 Date of consent and consent number: 20/04/12 Aragón. 2 Report status 2.1 Please indicate whether, according to Article 3 of the present Decision, the current report is: - the final report - a post-release monitoring report - final - intermediary 3 Characteristics of the release 3.1 Scientific name of the recipient organism: Zea mays 3.2 Transformation event(s) (acronym(s) or vectors 1 used (if transformation event identity not available): pag6573, 9 eventos de transformación : 10581, 10586, 10600, 10602, 10607, 10608, 10672, y Unique identifier, if available: NONE 3.4 Please provide the following information as well as the field(s) layout: 1 In the case of small-scale field trials where several lines may be tested, the vectors used should be mentioned, which gives insight into the introduced traits and/or genetic elements. In the case of large-scale trials, the number of events notified is limited to only one or a few events. 1
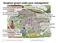
2 Geographical location(s) (administrative region and, where appropriate, grid reference) Size of the release site(s) ( 2 ) (m2) Identity ( 3 ) and approximate number of GM higher plants per event actually released (number of seeds/plants per m2) Duration of the release(s) (from (day/month/year until (d/m/y) ÉJEA DE LOS CABALLEROS, ZARAGOZA 3850 m2 used, m2 of GMO plants GMO plants 6/06/2012 sowing 3/12/2012 harvest MONZÓN (2), HUESCA 3850 m2 used, m2 of GMO plants GMO plants 6/06/2012 sowing 1/11/2012 harvest In all cases 8 rows of non GMO maize of similar cycle was sown in the laterals of the trial. At the beginning and end of the trial a plot 3 meters long of non GMO maize of similar cycle is sown. ( 2 ) Specify the size of the GM area and, where appropriate, the size of the non-gm area (e.g. non-gm border) ( 3 ) Vectors used 2
3 The trial design was the same in both locations, basic plot of 3 m long, m between plots, 0,72 cm between rows, two rows per plot, four replications. The reps were sown as follows: EJEA DE LOS CABALLEROS y MONZÓN (2): BORDER NON TRANSGENIC REP-1 REP-2 REP-3 REP-4 BORDER NON TRANSGENIC BORDER NON TRANSGENIC BORDER NON TRANSGENIC 3
4 Each replication and herbicide treatment as the following layout: Dose 0 Dose 1X Dose 1,5X Dose 3X Dose 4,5X Dose 6X LG3490 LG3490 LG3490 LG3490 LG3490 LG IG581 IG581 IG581 IG581 IG581 IG IG586 IG586 IG586 IG586 IG586 IG IG600 IG600 IG600 IG600 IG600 IG IG602 IG602 IG602 IG602 IG602 IG IG607 IG607 IG607 IG607 IG607 IG LG3490 HA6981 HA6981 HA6981 HA6981 HA IG608 IG608 IG608 IG608 IG608 IG IG672 IG672 IG672 IG672 IG672 IG IG675 IG675 IG675 IG675 IG675 IG IG773 IG773 IG773 IG773 IG773 IG HA6981 HA6981 HA6981 HA6981 HA6981 HA IH581 IH581 IH581 IH581 IH581 IH IH586 IH586 IH586 IH586 IH586 IH IH600 IH600 IH600 IH600 IH600 IH IH602 IH602 IH602 IH602 IH602 IH IH607 IH607 IH607 IH607 IH607 IH IH608 IH608 IH608 IH608 IH608 IH IH672 IH672 IH672 IH672 IH672 IH IH675 IH675 IH675 IH675 IH675 IH IH773 IH773 IH773 IH773 IH773 IH LG3490 LG3490 LG3490 LG3490 LG3490 LG LG3490 LG3490 LG3490 LG3490 LG3490 LG
5 4 Any kind of product that the notifier intends to notify at later stage 4.1 Does the notifier intend to notify the released transformation event(s) as product(s) for placing on the market under Community legislation(s) at a later stage? 4.2 Yes Unknown to date If yes, indicate the country (ies) of notification:.. If yes, specify for which use(s): - Import. - Cultivation (e ;g ; seed/planting material production). - Food. - Feed. - Pharmaceutical use (or processing for pharmaceutical use). - Processing for pour. Food use Feed use Industrial use. - Others (specify): 5 Type(s) of deliberate release(s) Please select the main type(s) (in boxes) as well as subtype(s) of the release(s). In the case of multi-sites, multi-events and/or multi-annual release(s), please provide a general overview of the type(s) of deliberate release(s) which has/have been carried out for the full duration of the consent. Please tick the appropriate type(s): 5.1 Deliberate release(s) for research purposes 5.2 Deliberate release(s) for development purposes - Event screening. X - Proof of concept 2 X. 2 For example, testing the new trait under environmental conditions. 5
6 - Agronomic performances (e.g. efficiency/selectivity of plant protection product, yield capacity, germination capacity, crop establishment, plant vigour, plant height, susceptibility to climatic factors/diseases, etc.) (specify). Resistance to Lepidoptera insects (Sesamia and Ostrinia) of different transformation events, resistance to Gliphosate, height of plants. - Altered agronomic properties (e.g. disease/pest/drought/frost-resistance, etc.) (specify). Resistance to Lepidoptera insects, (Sesamia and Ostrinia) of different transformation events, resistance to herbicide Gliphosate. - Altered qualitative properties (prolonged shelf-life, enhanced nutritional value, modified composition, etc.) (specify). - Stability of the expression. - Multiplication of lines. - Hybrid vigour study. - Molecular farming 3. - Phyto-remediation. - Others : (specify) Official testing - Variety registration on a national variety catalogue DUS (=Distinctness, Uniformity and Stability) VCU (=Value of Cultivation and Use) - Others: (specify):. 5.4 Herbicide authorization 5.5 Deliberate release(s) for demonstration purposes 5.6 Seeds multiplication 3 «Molecular farming» means the production of substances (for instance, proteins, pharmaceuticals) by plants, which have been genetically modified for a particular trait. Molecular farming could be defined as well as the production of plant-synthesised pharmaceuticals, plant-made pharceuticals, plant-based proteins production, etc. 6
7 5.7 Deliberate release(s) for biosafety/risk assessment research - Vertical gene transfer studies. Out-crossing with conventional crops Out-crossing with wild relatives - Horizontal gene transfer studies (gene transfer to micro-organisms). - Management of volunteers. - Potential changes in persistence or dispersal. - Potential invasiveness. - Potential effects on target organisms. The transformation events provide resistance to larvae of Lepidoptera that attack maize plants. Adult insects are not affected. The plants are resistant to Gliphosate herbicide. - Potential effects on non-target organisms. No effect has been observe don non-target organism. The populations of Lepidoptera insects were evaluated by placing insect s traps in the borders and in between the rows of GMO plants, no difference was observed. - Observation of resistant relatives. NO - Observation of resistant insects. NO - Others: (describe) Other(s) type(s) of deliberate release(s): (describe) :... 6 Method(s), result(s) of the release, management and monitoring Measure(s) in respect of any risk to human health or the environment. 6.1 Risk management measure(s) Please report the risk-management measures, which have been used to avoid or minimise the spread of the GMO(s) outside the site(s) of release, and in particular those measures: - Which were not originally notified in the application, - Which were applied in addition to the conditions in the consent, - Which the consent required only under certain conditions (e.g. dry periods, flooding), 7
8 - For which the consent allowed the notifier a choice among different measures. Tick the examples where appropriate: Before the sowing/planting: - Clear labelling of the GM seeds (distinct from other seeds/tubers/etc.) (describe). The GMO seeds were placed in individual envelops of 100 seeds, each envelop labeled with the corresponding transformation event 10581, 10586, 10600, 10602, 10607, 10608, 10672, and 10773, and the name of the experimental variety (IG, IH). - Segregation during the processing and transport of the seed/planting material (describe the method involved; provide example(s) of containment to prevent spillage during the processing and transport). The envelops were placed, in the sowing order in cardboard boxes then in a polypropylene bag, sealed before transport. The seeds preparation was done in the Laboratory of Limagrain in France. Transport was made in a Limagrain car by persons aware of the nature of the seeds. - Destruction of superfluous seeds/planting material (describe the method involved). After sowing, the remaining seeds were brought back to the laboratory for destruction. The planter was carefully cleaned on the experimental field (in the borders). - Temporal isolation (specify). No temporal isolation. - Rotation (specify the previous crop). Ejea de los Caballeros: cereal Monzón (2): ray-grass - Other(s): (specify) During the sowing/planting activities: - Method of sowing/planting. Planting was using a sowing machine for experimental trials. A system of auto cleaning sends the remaining seeds in a container where the seeds are collected. - Emptying and cleaning of the sowing machinery on the field of release. The sowing machine was cleaned on the release site; remaining seeds were collected after opening the sowing elements. - Segregation during the sowing (provide example of containment to prevent spillage during the sowing/planting). 8
9 Each bag of seeds was open only on the release site; after control of the label and of the position of the plot (using a map/design of the assay), the seeds were poured into the sowing elements. - Other(s): (specify) During the period of release: - Isolation distance (x meters) From sexually compatible commercial plant species. Ejea de los Caballeros: 245 m from maize Monzón (2): 240 m from maize From sexually compatible wild relatives. Maize has no wild relatives in Spain - Border rows (with the same crop or a different one, with a non-transgenic crop, x meters, etc). At least 8 border rows of non GM maize. - Cage/net/fence/signpost (specify). No - Pollen trap (specify). No - Removal of GM inflorescences before flowering (indicate the frequency of removal). No - Removal of bolters/relatives/hybrid partners (indicate the frecuency of the removal, x metres around the GM field, etc). No - Other(s): (specify) At the end of the release: - Harvest/destruction methods (of crop or part of it) / other means (e.g.: sampling) (describe). - Harvest / destruction before the ripeness of the seeds. - Effective removal of plant parts. Harvest by combine all the plants, burying in the site the grain, chopping of the plant parts and bury in the liberation site. - Segregated storage and transport of crop/waste (provide examples of containment to prevent spillage of collected seeds/crops/wastes). All cobs and plants were destroyed. - Clean up of machinery on the release site. 9
10 Cleaned in site - Destination of the waste, treatment of waste/ surplus yield/plant residues (describe). Buried on the experimental release site. - Post-harvest treatment and cultivation measures on the release site (describe the method for preparing and managing the release site at the end of the release, including cultivation practices). Soil preparation for next crop that is not maize. Other(s): (describe): Post-harvest measures: Please indicate which measures were taken on the release site after harvest: Frequency of visits (average) Monthly. - Subsequent crop (specify). Ejea de los Caballeros: cereal, barley Monzón (2): cereal - Crop rotation (specify). - Fallow/no crop (specify). - Superficial soil work / no deep ploughing. - False-sowing beds. - Control of volunteers (specify intervals and duration). - Appropriate chemical treatment(s) (specify). - Appropriate soil treatment(s) (specify). - Other(s) (specify) Other(s) measure(s): (describe) The monthly visits make sure there is no re-grown of maize. Not found till now Emergency plan(s). Indicate: a) If the release proceeded as planned: Yes X 10
11 No (describe for which reason, e.g. vandalism, climatic conditions, etc.) No incidence to report b) if measures according to the emergency plan(s) (Article 6(2)(a)(vi) and Annex III.B of Directive 2001/18/EC) had to be taken: No X Yes (describe) 6.2 Post-release monitoring measures Due to the fact that the current report format can be used for the final and post-release monitoring report(s), the notifier is asked to clearly make the difference between both types of report through this section 2 of Chapter 6. Please indicate whether - The post-release monitoring plan will start (in the case of a final report, after the last harvest of the GM higher plants). - The post-release monitoring plan is ongoing (in the case of an intermediary postrelease monitoring report). The monitoring plan post-release goes on. The monthly visits have nothing to report till now. - The post-release monitoring plan has been completed (in the case of the final postrelease monitoring report). - No post-release monitoring plan has to be fulfilled. The results of this monitoring are meant to confirm or invalidate earlier assumptions in the risk assessment. According to the aforementioned cases, please indicate which monitoring measure(s) will be/are/were taken and where (on the release site/near the site (e.g. on fields edges)). Please be aware that all post-release monitoring measures taken during the whole post-release period shall be indicated here. Specify: - Monitoring measures within site Duration: one year Frequency of visits (average): monthly Observation of resistant relatives. Observation of resistant insects. 11
12 Control of volunteers (specify intervals and duration). Control of volunteers for one year monthly, nothing to report till now. Monitoring of gene flow (specify). Appropriate chemical treatment(s) and/or soil treatment(s). Others (specify). - Monitoring measures of adjacent areas: Duration: One year, at the same time of the visits to the release site, to date no incidence or re-growth to report in the adjacent plots. Frequency of visits (average): monthly Area monitored: Observation of resistant relatives. Observation of resistant insects. Resistance to insects was not observed, there was no development of Lepidoptera in the transgenic plants. The small size of the experiment and the reduced number of transgenic plants are not giving favorable conditions for development of resistance. Control of volunteers X Control of volunteers is to be done for a year by monthly visits, nothing found to date. and/or monitoring of feral populations (specify intervals and duration). Monitoring of gene flow (specify). Appropriate chemical treatment(s) and/or soil treatment(s). Others (specify). 6.3 Plan for observation(s)/methods(s) involved In this section the observation plan and the methods used to collect the effects which have to be reported under the next section (section 6.4) need to be specified. Any amendments or modifications to the plan as proposed in the application and the SNIF 4 part B need to be specified in detail. During the time between the notification and the final report submission, new scientific insights or methods may be developed which cause a change in the methods used. In particular these modifications need to be specified under this section. During the field trial observations made do not lead to change the conclusions of the risk assessment made in the application. No changes were found, as compared to conventional maize in terms of persistence or invasiveness, advantages, potential of transfer of genetic material, or biological interactions, etc. The difference found was the tolerance to the herbicide glyphosate which is the trait introduced into these transgenic maize plants. The other difference found is the resistance to insects. In this first step of the project the damage to maize is measured as the length of tunnels originated by Sesamia under natural attack. No Ostrinia attack or of other insect could be 4 Summary notification information format (=SNIF) 12
13 documented, in the specific traps set for Sesamia and Ostrinia we have only found Sesamia. 6.4 Observed effect(s) Explanatory note. All results of the deliberate release(s) in respect of any risk for human health or the environment shall be stated, without prejudice to whether the results indicate that any risk is increased, reduced or remains unchanged. The main objectives of the information given in this section are: - to confirm or invalidate any assumption regarding the occurrence and impact of potential effect(s) of the GMO(s) which was/were identified in the environmental risk assessment, - to identify effect(s) of the GMO(s) which was/were not anticipated in the environmental risk assessment. The observed effect(s)/interaction(s) of the GMO(s) - with respect to any risk to human health, - with respect to any risk to the environment shall be reported under this section. Particular attention shall be drawn to unexpected and unintended effect(s). Nothing has been detected in effects over the human health or environment, different to the effects related to a normal maize cultivation in the relative to agriculture land preparation, etc. No unexpected or unintended effect to report, we have to report that the GMO plants were resistant to Sesamia, resistance measurable by the absence of tunnels inside the GMO plants, the non transgenic controls had an average of 130 cm of tunnels inside the stem of plants. Indications as regards the effects, that the notifier may have to report, are provided hereunder. The effects have obviously to be considered in the light of the crop, the new trait, the receiving environment as well as the conclusions of the environmental risk assessment, which is carried out on a case-by-case basis. In order to structure the information and to facilitate and efficient search within the given information, the notifier shall use, as far as possible, specific keywords to fill in the text fields under Chapter 6, especially sections 6.4.2, and A most updated list of those specific keywords is available on the Internet at: Expected effect(s) 13
14 This section concerns «expected effects», that is to say, potential effects which were already identified in the environmental risk assessment of the notification and could therefore be anticipated. Notifiers should supply data from the deliberate release(s) which validate the assumptions made in the environmental risk assessment. No effect could be detected into biodiversity in general different to what can happen to a cultivated non GMO maize, except that the GMO plants were resistant to Sesamia, resistance measurable by the absence of tunnels inside the GMO plants, the non transgenic controls had an average of 130 cm of tunnels inside the stem of plants Unexpected effect(s) 5 Unexpected effects refer to effects on human health or the environment which were not foreseen or identified in the environmental risk assessment of the notification. This part of the report should contain any information with regard to unexpected effects or observations relevant for the initial environmental risk assessment. In case of any observed unexpected effects or observations, this section should be as detailed as possible to allow a proper interpretation of the data Other information Notifiers are encouraged to supply information, which is outside the scope of the notification but which might be relevant to the field trials in question. This may also include observations of beneficial effects. No effect could be detected into biodiversity in general different to what can happen to a cultivated non GMO maize, except that the GMO plants were resistant to Sesamia, resistance measurable by the absence of tunnels inside the GMO plants, the non transgenic controls had an average of 130 cm of tunnels inside the stem of plants. 7 Conclusion In this chapter, the notifier should specify the conclusions drawn and the measures taken or to be taken on the basis of the results of the release with regard to further release(s) and where appropriate, make reference to any kind of product the notifier intends to notify at a later stage. In this first step of the project the objective was to confirm that the plants were resistant to the attack of Lepidoptera larvae, this was already know for Ostrinia, and now we have confirmed again that are also resistant to Sesamia. We have the intention to continue the evaluation of the same plant, in different locations to control the different insects that attack maize. We also intend to observe the specific effect on target organism. 5 Without prejudice to Article 8 OF Directive 2001/18/EC as regards handling of modifications or new information. 14
15 DATE: Elorz 18 December Enrique Sánchez-Monge 15
1.3 Date of consent and consent number: 15/04/11 Aragón, 24/06/11 Navarra.
 FORMAT FOR THE PRESENTATION OF THE RESULT OF DELIBERATE RELEASE INTO THE ENVIRONMENT OF GENETICALLY MODIFIED HIGHER PLANTS IN ACCORDANCE WITH ARTICLE 10 OF DIRECTIVE 2001/18/EC 1 General information 1.1
FORMAT FOR THE PRESENTATION OF THE RESULT OF DELIBERATE RELEASE INTO THE ENVIRONMENT OF GENETICALLY MODIFIED HIGHER PLANTS IN ACCORDANCE WITH ARTICLE 10 OF DIRECTIVE 2001/18/EC 1 General information 1.1
THE RESULT OF DELIBERATE RELEASE INTO THE ENVIRONMENT OF GENETICALLY MODIFIED HIGHER PLANTS IN ACCORDANCE WITH ARTICLE 10 OF DIRECTIVE 2001/18/EC
 THE RESULT OF DELIBERATE RELEASE INTO THE ENVIRONMENT OF GENETICALLY MODIFIED HIGHER PLANTS IN ACCORDANCE WITH ARTICLE 10 OF DIRECTIVE 2001/18/EC 1 GENERAL INFORMATION 1.1 European notification number:
THE RESULT OF DELIBERATE RELEASE INTO THE ENVIRONMENT OF GENETICALLY MODIFIED HIGHER PLANTS IN ACCORDANCE WITH ARTICLE 10 OF DIRECTIVE 2001/18/EC 1 GENERAL INFORMATION 1.1 European notification number:
THE RESULT OF DELIBERATE RELEASE INTO THE ENVIRONMENT OF GENETICALLY MODIFIED HIGHER PLANTS IN ACCORDANCE WITH ARTICLE 10 OF DIRECTIVE 2001/18/EC
 THE RESULT OF DELIBERATE RELEASE INTO THE ENVIRONMENT OF GENETICALLY MODIFIED HIGHER PLANTS IN ACCORDANCE WITH ARTICLE 10 OF DIRECTIVE 2001/18/EC 1 GENERAL INFORMATION 1.1 European notification number:
THE RESULT OF DELIBERATE RELEASE INTO THE ENVIRONMENT OF GENETICALLY MODIFIED HIGHER PLANTS IN ACCORDANCE WITH ARTICLE 10 OF DIRECTIVE 2001/18/EC 1 GENERAL INFORMATION 1.1 European notification number:
FINAL REPORT OF THE EXPERIMENTAL RELEASE INTO THE ENVIRONMENT OF GENETICALLY MODIFIED SUGARBEET EVENT H7-1
 REPORT TO PRESENT THE RESULTS OF THE DELIBERATE RELEASE OF GENETICALLY MODIFIED HIGHER PLANTS INTO THE ENVIRONMENT ACCORDING TO ARTICLE 10 OF DIRECTIVE 2001/18/EC FINAL REPORT OF THE EXPERIMENTAL RELEASE
REPORT TO PRESENT THE RESULTS OF THE DELIBERATE RELEASE OF GENETICALLY MODIFIED HIGHER PLANTS INTO THE ENVIRONMENT ACCORDING TO ARTICLE 10 OF DIRECTIVE 2001/18/EC FINAL REPORT OF THE EXPERIMENTAL RELEASE
RESULT OF DELIBERATE RELEASE INTO THE ENVIRONMENT OF GENETICALLY MODIFIED HIGHER PLANTS IN ACCORDANCE WITH ARTICLE 10 OF DIRECTIVE 2001/18/EC
 RESULT OF DELIBERATE RELEASE INTO THE ENVIRONMENT OF GENETICALLY MODIFIED HIGHER PLANTS IN ACCORDANCE WITH ARTICLE 10 OF DIRECTIVE 2001/18/EC 1 GENERAL INFORMATION 1.1 European notification number: B/RO/07/12
RESULT OF DELIBERATE RELEASE INTO THE ENVIRONMENT OF GENETICALLY MODIFIED HIGHER PLANTS IN ACCORDANCE WITH ARTICLE 10 OF DIRECTIVE 2001/18/EC 1 GENERAL INFORMATION 1.1 European notification number: B/RO/07/12
POSTRELEASE MONITORING REPORT
 Syngenta Agro SRL Victoria Park 73-81 Bucuresti-Ploiesti Str. Floor 4, 013685 Bucharest, Romania Correspondence address: Tel. +40 21 5281200 Fax. +40 21 5281299 e-mail: andrei.marutescu@syngenta.com POSTRELEASE
Syngenta Agro SRL Victoria Park 73-81 Bucuresti-Ploiesti Str. Floor 4, 013685 Bucharest, Romania Correspondence address: Tel. +40 21 5281200 Fax. +40 21 5281299 e-mail: andrei.marutescu@syngenta.com POSTRELEASE
1.3 Date of consent and consent number: 09/04/2013 B/ES/13/17
 FORMAT FOR THE PRESENTATION OF THE RESULT OF DELIBERATE RELEASE INTO THE ENVIRONMENT OF GENETICALLY MODIFIED HIGHER PLANTS IN ACCORDANCE WITH ANNEX XI OF ROYAL DECREE 178/2004 1 General information 1.1
FORMAT FOR THE PRESENTATION OF THE RESULT OF DELIBERATE RELEASE INTO THE ENVIRONMENT OF GENETICALLY MODIFIED HIGHER PLANTS IN ACCORDANCE WITH ANNEX XI OF ROYAL DECREE 178/2004 1 General information 1.1
POSTRELEASE MONITORING REPORT
 Syngenta Agro SRL Victoria Park 73-81 Bucuresti-Ploiesti Str. Floor 4, 013685 Bucharest, Romania Correspondence address: Tel. +40 21 5281200 Fax. +40 21 5281299 e-mail: andrei.marutescu@syngenta.com POSTRELEASE
Syngenta Agro SRL Victoria Park 73-81 Bucuresti-Ploiesti Str. Floor 4, 013685 Bucharest, Romania Correspondence address: Tel. +40 21 5281200 Fax. +40 21 5281299 e-mail: andrei.marutescu@syngenta.com POSTRELEASE
POSTRELEASE MONITORING REPORT
 Syngenta Agro SRL Victoria Park 73-81 Bucuresti-Ploiesti Str. Floor 4, 013685 Bucharest, Romania Correspondence address: Tel. +40 21 5281200 Fax. +40 21 5281299 e-mail: andrei.marutescu@syngenta.com POSTRELEASE
Syngenta Agro SRL Victoria Park 73-81 Bucuresti-Ploiesti Str. Floor 4, 013685 Bucharest, Romania Correspondence address: Tel. +40 21 5281200 Fax. +40 21 5281299 e-mail: andrei.marutescu@syngenta.com POSTRELEASE
POSTRELEASE MONITORING REPORT
 Syngenta Agro SRL Victoria Park 73-81 Bucuresti-Ploiesti Str. Floor 4, 013685 Bucharest, Romania Correspondence address: Tel. +40 21 5281200 Fax. +40 21 5281299 e-mail: andrei.marutescu@syngenta.com POSTRELEASE
Syngenta Agro SRL Victoria Park 73-81 Bucuresti-Ploiesti Str. Floor 4, 013685 Bucharest, Romania Correspondence address: Tel. +40 21 5281200 Fax. +40 21 5281299 e-mail: andrei.marutescu@syngenta.com POSTRELEASE
3.4 Please provide the following information as well as the field(s) layout:
 FORMAT FOR THE PRESENTATION OF THE RESULT OF DELIBERATE RELEASE INTO THE ENVIRONMENT OF GENETICALLY MODIFIED HIGHER PLANTS IN ACCORDANCE WITH ARTICLE 10 OF DIRECTIVE 2001/18/EC 1 GENERAL INFORMATION 1.1
FORMAT FOR THE PRESENTATION OF THE RESULT OF DELIBERATE RELEASE INTO THE ENVIRONMENT OF GENETICALLY MODIFIED HIGHER PLANTS IN ACCORDANCE WITH ARTICLE 10 OF DIRECTIVE 2001/18/EC 1 GENERAL INFORMATION 1.1
1 GENERAL INFORMATION
 FORMAT FOR THE PRESENTATION OF THE RESULT OF DELIBERATE RELEASE INTO THE ENVIRONMENT OF GENETICALLY MODIFIED HIGHER PLANTS IN ACCORDANCE WITH ARTICLE 10 OF DIRECTIVE 2001/18/EC 1 GENERAL INFORMATION 1.1
FORMAT FOR THE PRESENTATION OF THE RESULT OF DELIBERATE RELEASE INTO THE ENVIRONMENT OF GENETICALLY MODIFIED HIGHER PLANTS IN ACCORDANCE WITH ARTICLE 10 OF DIRECTIVE 2001/18/EC 1 GENERAL INFORMATION 1.1
(COMMISSION DECISION 2003/701/EC)
 FORMAT FOR THE PRESENTATION OF THE RESULT OF DELIBERATE RELEASE INTO THE ENVIRONMENT OF GENETICALLY MODIFIED HIGHER PLANTS IN ACCORDANCE WITH ARTICLE 10 OF DIRECTIVE 2001/18/EC (COMMISSION DECISION 2003/701/EC)
FORMAT FOR THE PRESENTATION OF THE RESULT OF DELIBERATE RELEASE INTO THE ENVIRONMENT OF GENETICALLY MODIFIED HIGHER PLANTS IN ACCORDANCE WITH ARTICLE 10 OF DIRECTIVE 2001/18/EC (COMMISSION DECISION 2003/701/EC)
REPORT ON RESULTS. Notification B/ES/04/19. Trials in the Autonomous Region of Aragon. (In accordance with Annex XI of RD 178/2004, of 30 th January)
 REPORT ON RESULTS Notification B/ES/04/19 Trials in the Autonomous Region of Aragon (In accordance with Annex XI of RD 178/2004, of 30 th January) 1. GENERAL INFORMATION 1.1 European notification number
REPORT ON RESULTS Notification B/ES/04/19 Trials in the Autonomous Region of Aragon (In accordance with Annex XI of RD 178/2004, of 30 th January) 1. GENERAL INFORMATION 1.1 European notification number
REPORT ON RESULTS. Notification B/ES/04/18. (In accordance with Annex XI of RD 178/2004, of 30 th January)
 REPORT ON RESULTS Notification B/ES/04/18 (In accordance with Annex XI of RD 178/2004, of 30 th January) 1. GENERAL INFORMATION 1.1 European notification number B/ES/04/18 1.2 Member State of Notification
REPORT ON RESULTS Notification B/ES/04/18 (In accordance with Annex XI of RD 178/2004, of 30 th January) 1. GENERAL INFORMATION 1.1 European notification number B/ES/04/18 1.2 Member State of Notification
Consent for Notification No. B/IE/06/01
 Headquarters, PO Box 3000, Johnstown Castle Estate, County Wexford, Ireland Consent for Notification No. B/IE/06/01 Consent to a deliberate release of GM potato lines (with improved resistance to late
Headquarters, PO Box 3000, Johnstown Castle Estate, County Wexford, Ireland Consent for Notification No. B/IE/06/01 Consent to a deliberate release of GM potato lines (with improved resistance to late
Draft COMMISSION DECISION
 COMISIONOFTHEUROPEANCOMUNITIES Brussels, D003698/01 Draft COMMISSION DECISION of [ ] concerning the placing on the market, in accordance with Directive 2001/18/EC of the European Parliament and of the
COMISIONOFTHEUROPEANCOMUNITIES Brussels, D003698/01 Draft COMMISSION DECISION of [ ] concerning the placing on the market, in accordance with Directive 2001/18/EC of the European Parliament and of the
Environmental risk assessment for experimental releases. Jeremy Sweet. JT Environmental Consultants Ltd, Cambridge CB24 5JA, UK
 Environmental risk assessment for experimental releases Jeremy Sweet JT Environmental Consultants Ltd, Cambridge CB24 5JA, UK GM crop Field Trial Applications to CAs Competent Authorities will require
Environmental risk assessment for experimental releases Jeremy Sweet JT Environmental Consultants Ltd, Cambridge CB24 5JA, UK GM crop Field Trial Applications to CAs Competent Authorities will require
Deliberate releases B/BE/10/V1 Final report
 Deliberate releases B/BE/10/V1 Final report 1. General information 1.1. European notification number B/BE/10/V1 Two separate notification numbers were given to differentiate between the legal responsibilities
Deliberate releases B/BE/10/V1 Final report 1. General information 1.1. European notification number B/BE/10/V1 Two separate notification numbers were given to differentiate between the legal responsibilities
COMMISSION OF THE EUROPEAN COMMUNITIES. Draft COMMISSION DECISION
 COMMISSION OF THE EUROPEAN COMMUNITIES Brussels, D003697/01 Draft COMMISSION DECISION of [ ] concerning the placing on the market, in accordance with Directive 2001/18/EC of the European Parliament and
COMMISSION OF THE EUROPEAN COMMUNITIES Brussels, D003697/01 Draft COMMISSION DECISION of [ ] concerning the placing on the market, in accordance with Directive 2001/18/EC of the European Parliament and
EUROPEAN COMMISSION AGRICULTURE DIRECTORATE-GENERAL DIRECTORATE-GENERAL JOINT RESEARCH CENTRE ARGUMENTAIRE ON CO-EXISTENCE OF GM CROPS
 EUROPEAN COMMISSION AGRICULTURE DIRECTORATE-GENERAL DIRECTORATE-GENERAL JOINT RESEARCH CENTRE Brussels, May 17 2002 ARGUMENTAIRE ON CO-EXISTENCE OF GM CROPS WITH CONVENTIONAL AND ORGANIC CROPS 1- WHAT
EUROPEAN COMMISSION AGRICULTURE DIRECTORATE-GENERAL DIRECTORATE-GENERAL JOINT RESEARCH CENTRE Brussels, May 17 2002 ARGUMENTAIRE ON CO-EXISTENCE OF GM CROPS WITH CONVENTIONAL AND ORGANIC CROPS 1- WHAT
COMMISSION OF THE EUROPEAN COMMUNITIES COMMISSION RECOMMENDATION. of 23 July 2003
 COMMISSION OF THE EUROPEAN COMMUNITIES Brussels, 23 July 2003 C(2003) COMMISSION RECOMMENDATION of 23 July 2003 on guidelines for the development of national strategies and best practices to ensure the
COMMISSION OF THE EUROPEAN COMMUNITIES Brussels, 23 July 2003 C(2003) COMMISSION RECOMMENDATION of 23 July 2003 on guidelines for the development of national strategies and best practices to ensure the
(Acts whose publication is not obligatory) COMMISSION
 L 189/36 29.7.2003 II (Acts whose publication is not obligatory) COMMISSION COMMISSION RECOMMDATION of 23 July 2003 on guidelines for the development of national strategies and best practices to ensure
L 189/36 29.7.2003 II (Acts whose publication is not obligatory) COMMISSION COMMISSION RECOMMDATION of 23 July 2003 on guidelines for the development of national strategies and best practices to ensure
The genetically modified maize is proposed to be used as any other maize.
 Opinion of the Scientific Committee on Plants Regarding "Submission for Placing on the Market of Glufosinate Tolerant Corns ( Zea Mays) Transformation Event T25" by the Agrevo Company (NOTIFICATION C/F/95/12/07)
Opinion of the Scientific Committee on Plants Regarding "Submission for Placing on the Market of Glufosinate Tolerant Corns ( Zea Mays) Transformation Event T25" by the Agrevo Company (NOTIFICATION C/F/95/12/07)
Stewardship and Integrated Pest Management for generic / off patent GM crops
 Stewardship and Integrated Pest Management for generic / off patent GM crops Georges FREYSSINET 1 Stewardship and Integrated Pest Management for generic GM crops Generic Off Patent GM crops Stewardship
Stewardship and Integrated Pest Management for generic / off patent GM crops Georges FREYSSINET 1 Stewardship and Integrated Pest Management for generic GM crops Generic Off Patent GM crops Stewardship
Secretary-General of the European Commission, signed by Mr Jordi AYET PUIGARNAU, Director
 COUNCIL OF THE EUROPEAN UNION Brussels, 12 November 2013 (OR. es) 16120/13 Interinstitutional File: 2013/0368 (NLE) AGRILEG 151 ENV 1062 PROPOSAL From: date of receipt: 12 November 2013 To: No. Cion doc.:
COUNCIL OF THE EUROPEAN UNION Brussels, 12 November 2013 (OR. es) 16120/13 Interinstitutional File: 2013/0368 (NLE) AGRILEG 151 ENV 1062 PROPOSAL From: date of receipt: 12 November 2013 To: No. Cion doc.:
COMPREHENSIVE GLOSSARY OF TERMS
 COMPREHENSIVE GLOSSARY OF TERMS Accidental release: Any unintended release of regulated plant material into the environment, food and/or feed chains. For the purposes of these Guidelines, any breach of
COMPREHENSIVE GLOSSARY OF TERMS Accidental release: Any unintended release of regulated plant material into the environment, food and/or feed chains. For the purposes of these Guidelines, any breach of
COMMISSION OF THE EUROPEAN COMMUNITIES. Proposal for a COUNCIL DECISION
 COMMISSION OF THE EUROPEAN COMMUNITIES Brussels, 04.07.2002 COM(2002) 362 final Proposal for a COUNCIL DECISION establishing pursuant to Directive 2001/18/EC of the European Parliament and of the Council
COMMISSION OF THE EUROPEAN COMMUNITIES Brussels, 04.07.2002 COM(2002) 362 final Proposal for a COUNCIL DECISION establishing pursuant to Directive 2001/18/EC of the European Parliament and of the Council
Bayer CropScience LP Summary_EFSA-GMO-RX-XXX.pdf CC1: 09 Jan 2018 Oilseed rape T45 Page 1 of 7
 Oilseed rape T45 Page 1 of 7 Request for Renewal of the Authorization for placing on the market of products containing or produced from genetically modified oilseed rape T45 (ACS-BNØØ8-2) resulting from
Oilseed rape T45 Page 1 of 7 Request for Renewal of the Authorization for placing on the market of products containing or produced from genetically modified oilseed rape T45 (ACS-BNØØ8-2) resulting from
Summary Notification Information Format
 BIJLAGE 12 Summary Notification Information Format A. General information A1. Details of notification Notification Number Member State Belgium Date of Acknowledgement Title of the Project Scientific field
BIJLAGE 12 Summary Notification Information Format A. General information A1. Details of notification Notification Number Member State Belgium Date of Acknowledgement Title of the Project Scientific field
Official Journal of the European Union. (Acts whose publication is obligatory)
 5.11.2003 L 287/1 I (Acts whose publication is obligatory) REGULATION (EC) No 1946/2003 OF THE EUROPEAN PARLIAMT AND OF THE COUNCIL of 15 July 2003 on transboundary movements of genetically modified organisms
5.11.2003 L 287/1 I (Acts whose publication is obligatory) REGULATION (EC) No 1946/2003 OF THE EUROPEAN PARLIAMT AND OF THE COUNCIL of 15 July 2003 on transboundary movements of genetically modified organisms
Appendix 4. Insect Protected Maize Farmer Questionnaire - User s Manual
 Appendix 4. Insect Protected Maize Farmer Questionnaire - User s Manual Insect Protected Maize Farmer Questionnaire USER S MANUAL 1 General introduction to the insect protected maize farmer questionnaire
Appendix 4. Insect Protected Maize Farmer Questionnaire - User s Manual Insect Protected Maize Farmer Questionnaire USER S MANUAL 1 General introduction to the insect protected maize farmer questionnaire
Public information sheet
 Public information sheet AVENTIS CROPSCIENCE N.V. Field evaluation with genetically modified pod shatter resistant oilseed rape European Notification number B/BE/01/V5 Upon advice of the Biosafety Council
Public information sheet AVENTIS CROPSCIENCE N.V. Field evaluation with genetically modified pod shatter resistant oilseed rape European Notification number B/BE/01/V5 Upon advice of the Biosafety Council
GMO & Food Safety. Presented By: Dr. Yasser Mostafa Quality & Food Safety Manager MARS KSA
 GMO & Food Safety Presented By: Dr. Yasser Mostafa Quality & Food Safety Manager MARS KSA Contents: What are genetically modified (GM) organisms and GM foods? Why are GM foods produced? Are GM foods assessed
GMO & Food Safety Presented By: Dr. Yasser Mostafa Quality & Food Safety Manager MARS KSA Contents: What are genetically modified (GM) organisms and GM foods? Why are GM foods produced? Are GM foods assessed
Public information sheet
 Public information sheet AVENTIS CROPSCIENCE N.V. Field evaluation of hybrid and parental Brassica juncea lines European Notification number B/BE/01/V3 Upon advice of the Biosafety Council and the Service
Public information sheet AVENTIS CROPSCIENCE N.V. Field evaluation of hybrid and parental Brassica juncea lines European Notification number B/BE/01/V3 Upon advice of the Biosafety Council and the Service
Official Journal of the European Communities. (Acts whose publication is not obligatory) COMMISSION
 L 200/22 EN 30.7.2002 II (Acts whose publication is not obligatory) COMMISSION COMMISSION DECISION of 24 July 2002 establishing guidance notes supplementing AnnexII to Directive 2001/18/EC of the European
L 200/22 EN 30.7.2002 II (Acts whose publication is not obligatory) COMMISSION COMMISSION DECISION of 24 July 2002 establishing guidance notes supplementing AnnexII to Directive 2001/18/EC of the European
Guidance on requirements for efficacy data for zonal evaluation of a plant protection product in the Northern Zone
 Guidance on requirements for efficacy data for zonal evaluation of a plant protection product in the Northern Zone June, 2011 The present document is based on Directive 93/71/EEC amending the Commission
Guidance on requirements for efficacy data for zonal evaluation of a plant protection product in the Northern Zone June, 2011 The present document is based on Directive 93/71/EEC amending the Commission
June, 2011 Guidance on requirements for efficacy data for zonal evaluation of a plant protection product in the Northern Zone
 htttp://agrsci.au.dk/en/institutter/institut-for-plantebeskyttelse-og-skadedyr/pesticidforskning-ogmiljoekemi/guidance-on-requirements-for-efficacy-data/ June, 2011 Guidance on requirements for efficacy
htttp://agrsci.au.dk/en/institutter/institut-for-plantebeskyttelse-og-skadedyr/pesticidforskning-ogmiljoekemi/guidance-on-requirements-for-efficacy-data/ June, 2011 Guidance on requirements for efficacy
The following are answers to frequently asked questions
 Genetically Medified Organisms Production, Regulation, and Maricoting The following are answers to frequently asked questions about what constitutes genetically modified organisms and foods, and how these
Genetically Medified Organisms Production, Regulation, and Maricoting The following are answers to frequently asked questions about what constitutes genetically modified organisms and foods, and how these
Problem Formulation in Environmental Risk Assessment for Genetically Modified Crops
 Problem Formulation in Environmental Risk Assessment for Genetically Modified Crops Alan Gray Centre for Ecology and Hydrology UK The genetic basis of unintended effects in modified plants Ottawa, 14-15
Problem Formulation in Environmental Risk Assessment for Genetically Modified Crops Alan Gray Centre for Ecology and Hydrology UK The genetic basis of unintended effects in modified plants Ottawa, 14-15
Environmental Risk Assessment
 EFSA NGO Consultation: 22 February 2006 Guidance document for the risk assessment of genetically modified plants and derived food and feed Environmental Risk Assessment Chapter III D, Sections 8-10, Jeremy
EFSA NGO Consultation: 22 February 2006 Guidance document for the risk assessment of genetically modified plants and derived food and feed Environmental Risk Assessment Chapter III D, Sections 8-10, Jeremy
Decree of the Ministry of Social Affairs and Health on the deliberate release of genetically modified organisms
 Ministry of Social Affairs and Health, Finland N.B. Unofficial translation. Legally valid only in Finnish and Swedish No 110/2005 Decree of the Ministry of Social Affairs and Health on the deliberate release
Ministry of Social Affairs and Health, Finland N.B. Unofficial translation. Legally valid only in Finnish and Swedish No 110/2005 Decree of the Ministry of Social Affairs and Health on the deliberate release
Application for authorization of stacked MIR604 x GA21 maize in the European Union under Regulation (EC) No 1829/2003 PART II: SUMMARY
 Syngenta MIR604 x GA21 maize Page 1 of 34 Application for authorization of stacked MIR604 x GA21 maize in the European Union under Regulation (EC) No 1829/2003 The information contained in this document
Syngenta MIR604 x GA21 maize Page 1 of 34 Application for authorization of stacked MIR604 x GA21 maize in the European Union under Regulation (EC) No 1829/2003 The information contained in this document
Tips for Biotech Trial Conduct
 Tips for Biotech Trial Conduct James A. Mickelson, Ph.D. Research Scientist Pioneer Hi-Bred International, Inc. Johnston, Iowa World-Wide GMO Acreage Introduction This increase in GMO acreage is the result
Tips for Biotech Trial Conduct James A. Mickelson, Ph.D. Research Scientist Pioneer Hi-Bred International, Inc. Johnston, Iowa World-Wide GMO Acreage Introduction This increase in GMO acreage is the result
Official Journal of the European Union DIRECTIVES
 L 67/30 9.3.2018 DIRECTIVES COMMISSION DIRECTIVE (EU) 2018/350 of 8 March 2018 amending Directive 2001/18/EC of the European Parliament and of the Council as regards the environmental risk assessment of
L 67/30 9.3.2018 DIRECTIVES COMMISSION DIRECTIVE (EU) 2018/350 of 8 March 2018 amending Directive 2001/18/EC of the European Parliament and of the Council as regards the environmental risk assessment of
Environmental release of plants with novel traits in Canada: A product-based approach to regulatory oversight
 Environmental release of plants with novel traits in Canada: A product-based approach to regulatory oversight Cindy Pearson CFIA Plant Biosafety Office OUTLINE Canadian regulatory framework for biotechnology
Environmental release of plants with novel traits in Canada: A product-based approach to regulatory oversight Cindy Pearson CFIA Plant Biosafety Office OUTLINE Canadian regulatory framework for biotechnology
GM maize. Stakeholder Consultation on Animal Feeding Studies and in-vitro Studies in GMO Risk Assessment. (3-4 December 2012, Vienna)
 Human Health Environment Socio-Economy GM maize Stakeholder Consultation on Animal Feeding Studies and in-vitro Studies in GMO Risk Assessment (3-4 December 2012, Vienna) www.grace-fp7.eu Top countries
Human Health Environment Socio-Economy GM maize Stakeholder Consultation on Animal Feeding Studies and in-vitro Studies in GMO Risk Assessment (3-4 December 2012, Vienna) www.grace-fp7.eu Top countries
Key Words: glyphosate-resistant corn; genetically engineered corn; corn pollen; pollen transport; corn seed purity, transgene.
 AgBioForum Volume 4, Number 2 2001 Pages 87-92 CROSS POLLINATION FROM GENETICALLY ENGINEERED CORN: WIND TRANSPORT AND SEED SOURCE John M. Jemison, Jr. & Michael E. Vayda 1 Pollen transport from genetically
AgBioForum Volume 4, Number 2 2001 Pages 87-92 CROSS POLLINATION FROM GENETICALLY ENGINEERED CORN: WIND TRANSPORT AND SEED SOURCE John M. Jemison, Jr. & Michael E. Vayda 1 Pollen transport from genetically
Innovative IPM solutions for winter wheat-based rotations: cropping systems assessed in Denmark
 Applied Crop Protection 2014 X Innovative IPM solutions for winter wheat-based rotations: cropping systems assessed in Denmark Per Kudsk, Lise Nistrup Jørgensen, Bo Melander & Marianne LeFebvre Introduction
Applied Crop Protection 2014 X Innovative IPM solutions for winter wheat-based rotations: cropping systems assessed in Denmark Per Kudsk, Lise Nistrup Jørgensen, Bo Melander & Marianne LeFebvre Introduction
English for Agriculture
 English for Agriculture Boosting Adult System Education In Agriculture - AGRI BASE Erasmus+ K2 Action Strategic Partnership UNIT 5 CULTIVATION, PLANTING AND HARVESTING EQUIPMENT Reading comprehension Before
English for Agriculture Boosting Adult System Education In Agriculture - AGRI BASE Erasmus+ K2 Action Strategic Partnership UNIT 5 CULTIVATION, PLANTING AND HARVESTING EQUIPMENT Reading comprehension Before
Biosafety Issues and Risk Assessment in Transgenic Crops
 Biosafety Issues and Risk Assessment in Transgenic Crops K.C. Bansal National Research Centre on Plant Biotechnology Indian Agricultural Research Institute New Delhi 110 012 kailashbansal@hotmail.com Biotechnology
Biosafety Issues and Risk Assessment in Transgenic Crops K.C. Bansal National Research Centre on Plant Biotechnology Indian Agricultural Research Institute New Delhi 110 012 kailashbansal@hotmail.com Biotechnology
Class IX Chapter 15 Improvement in Food Resources Science
 Class IX Chapter 15 Improvement in Food Resources Science Question 1: What do we get from cereals, pulses, fruits and vegetables? (i) Cereals provide us with carbohydrates. Also, they are a rich source
Class IX Chapter 15 Improvement in Food Resources Science Question 1: What do we get from cereals, pulses, fruits and vegetables? (i) Cereals provide us with carbohydrates. Also, they are a rich source
QUESTIONNAIRE about the socio-economic implications of the placing on the market of GMOs for cultivation. Contact Details
 QUESTIONNAIRE about the socio-economic implications of the placing on the market of GMOs for cultivation Contact Details Member State: Cyprus Name of ministry/ies contact Person/s: Environment Department,
QUESTIONNAIRE about the socio-economic implications of the placing on the market of GMOs for cultivation Contact Details Member State: Cyprus Name of ministry/ies contact Person/s: Environment Department,
EUROPEAN COMMISSION HEALTH AND FOOD SAFETY DIRECTORATE-GENERAL
 EUROPEAN COMMISSION HEALTH AND FOOD SAFETY DIRECTORATE-GENERAL sante.ddg2.g.5(2016)4037470 SUMMARY REPORT OF THE JOINT MEETING OF THE STANDING COMMITTEE ON PLANTS, ANIMALS, FOOD AND FEED (Genetically Modified
EUROPEAN COMMISSION HEALTH AND FOOD SAFETY DIRECTORATE-GENERAL sante.ddg2.g.5(2016)4037470 SUMMARY REPORT OF THE JOINT MEETING OF THE STANDING COMMITTEE ON PLANTS, ANIMALS, FOOD AND FEED (Genetically Modified
DECISIONS. (Only the Dutch and French texts are authentic) (Text with EEA relevance)
 21.12.2018 L 327/65 DECISIONS COMMISSION IMPLEMTING DECISION (EU) 2018/2045 of 19 December 2018 renewing the authorisation for the placing on the market of products containing, consisting of or produced
21.12.2018 L 327/65 DECISIONS COMMISSION IMPLEMTING DECISION (EU) 2018/2045 of 19 December 2018 renewing the authorisation for the placing on the market of products containing, consisting of or produced
EFSA-GMO-NL PART VII SUMMARY
 Pioneer Overseas Corporation, Inc. 4114 Maize Page 1 APPLICATION FOR AUTHORISATION OF GENETICALLY MODIFIED PLANTS AND DERIVED FOOD AND FEED IN ACCORDANCE WITH REGULATION (EC) No 1829/2003 4114 MAIZE (DP-ØØ4114-3
Pioneer Overseas Corporation, Inc. 4114 Maize Page 1 APPLICATION FOR AUTHORISATION OF GENETICALLY MODIFIED PLANTS AND DERIVED FOOD AND FEED IN ACCORDANCE WITH REGULATION (EC) No 1829/2003 4114 MAIZE (DP-ØØ4114-3
Sorghum grown under poor management
 Sorghum grown under poor management Compaction of soil with tracking animals and tractors Use of poor seeds No hedges to protect the soil from erosion by wind Burning of crop residues or overgrazing of
Sorghum grown under poor management Compaction of soil with tracking animals and tractors Use of poor seeds No hedges to protect the soil from erosion by wind Burning of crop residues or overgrazing of
Incident Report GENETICALLY MODIFIED WHEAT 2018
 Incident Report GENETICALLY MODIFIED WHEAT 2018 Her Majesty the Queen in Right of Canada (Canadian Food Inspection Agency), 2018. CFIA P0951-18 ISBN: 978-0-660-26780-7 Catalogue No.: A104-141/2018E-PDF
Incident Report GENETICALLY MODIFIED WHEAT 2018 Her Majesty the Queen in Right of Canada (Canadian Food Inspection Agency), 2018. CFIA P0951-18 ISBN: 978-0-660-26780-7 Catalogue No.: A104-141/2018E-PDF
Chapter 15: Improvement in Food Resources Science
 Chapter 15: Improvement in Food Resources Science Page No: 204 1. What do we get from cereals, pulses, fruits and vegetables? Cereals provide us with carbohydrates. Also, they are a rich source of energy.
Chapter 15: Improvement in Food Resources Science Page No: 204 1. What do we get from cereals, pulses, fruits and vegetables? Cereals provide us with carbohydrates. Also, they are a rich source of energy.
APPLICATION FOR CONFINED FIELD TRIAL
 APPLICATION FOR CONFINED FIELD TRIAL INSTRUCTIONS: This application form must be completed for each plant species. The application may include more than one event of the same plant species as long as the
APPLICATION FOR CONFINED FIELD TRIAL INSTRUCTIONS: This application form must be completed for each plant species. The application may include more than one event of the same plant species as long as the
The EU Legislation on GMOs
 The EU Legislation on GMOs An overview Update December 2011 Damien Plan, Guy Van den Eede EUR 25228 EN - 2012 The mission of the JRC-IHCP is to protect the interests and health of the consumer in the framework
The EU Legislation on GMOs An overview Update December 2011 Damien Plan, Guy Van den Eede EUR 25228 EN - 2012 The mission of the JRC-IHCP is to protect the interests and health of the consumer in the framework
Class IX Chapter 15 Improvement in Food Resources Science
 What do we get from cereals, pulses, fruits and vegetables? (i) Cereals provide us with carbohydrates. Also, they are a rich source of energy. (ii) Pulses give us proteins. (iii) Fruits and vegetables
What do we get from cereals, pulses, fruits and vegetables? (i) Cereals provide us with carbohydrates. Also, they are a rich source of energy. (ii) Pulses give us proteins. (iii) Fruits and vegetables
 Question 1: What do we get from cereals, pulses, fruits and vegetables? (i) Cereals provide us with carbohydrates. Also, they are a rich source of energy. (ii) Pulses give us proteins. (iii) Fruits and
Question 1: What do we get from cereals, pulses, fruits and vegetables? (i) Cereals provide us with carbohydrates. Also, they are a rich source of energy. (ii) Pulses give us proteins. (iii) Fruits and
Spread of genetically modified (GM) oilseed rape Relevance for Schleswig-Holstein Land, Germany
 Spread of genetically modified (GM) oilseed rape Relevance for Schleswig-Holstein Land, Germany Ulrike Middelhoff, Wilhelm Windhorst, Ecology Centre, University of Kiel, Germany e-mail: uli@ecology.uni-kiel.de
Spread of genetically modified (GM) oilseed rape Relevance for Schleswig-Holstein Land, Germany Ulrike Middelhoff, Wilhelm Windhorst, Ecology Centre, University of Kiel, Germany e-mail: uli@ecology.uni-kiel.de
ROUNDUP READY CANOLA CROP MANAGEMENT PLAN (CMP)
 ROUNDUP READY CANOLA CROP MANAGEMENT PLAN (CMP) Objective. The Roundup Ready canola Crop Management Plan details strategies that can be implemented on-farm to manage risks to the integrity of grain supply-chains
ROUNDUP READY CANOLA CROP MANAGEMENT PLAN (CMP) Objective. The Roundup Ready canola Crop Management Plan details strategies that can be implemented on-farm to manage risks to the integrity of grain supply-chains
Workshop Herbicide-Tolerant varieties EWRS Working Group, Frankfurt, 21 May 2014
 Workshop Herbicide-Tolerant varieties EWRS Working Group, Frankfurt, 21 May 2014 Assessing the effect of changes of agricultural practices accompanying herbicide-tolerant crops on agricultural biodiversity.
Workshop Herbicide-Tolerant varieties EWRS Working Group, Frankfurt, 21 May 2014 Assessing the effect of changes of agricultural practices accompanying herbicide-tolerant crops on agricultural biodiversity.
Winter Oilseed Rape Varieties
 Winter Oilseed Rape Varieties Irish Recommended List for 2013 Sowing The Department of Agriculture, Food and the Marine An Roinn Talmhaíochta, Bia agus Mara CONTENTS CROPS EVALUATION and CERTIFICATION
Winter Oilseed Rape Varieties Irish Recommended List for 2013 Sowing The Department of Agriculture, Food and the Marine An Roinn Talmhaíochta, Bia agus Mara CONTENTS CROPS EVALUATION and CERTIFICATION
Cartagena Protocol on Biosafety. Dr. Manoranjan Hota Additional Director Ministry of Environment and Forests New Delhi
 Cartagena Protocol on Biosafety Dr. Manoranjan Hota Additional Director Ministry of Environment and Forests New Delhi hota@nic.in 29 December, 2006 GENETIC BASICS CELL: SMALLEST UNIT OF LIFE NUCLEUS: BRAIN
Cartagena Protocol on Biosafety Dr. Manoranjan Hota Additional Director Ministry of Environment and Forests New Delhi hota@nic.in 29 December, 2006 GENETIC BASICS CELL: SMALLEST UNIT OF LIFE NUCLEUS: BRAIN
Regulations Regarding Co-existence of Genetically Modified Crops
 Republic of Latvia Cabinet Regulation No. 78 Adopted 10 February 2015 Regulations Regarding Co-existence of Genetically Modified Crops Issued pursuant to Section 5, Paragraph one, Clause 4 of the Law On
Republic of Latvia Cabinet Regulation No. 78 Adopted 10 February 2015 Regulations Regarding Co-existence of Genetically Modified Crops Issued pursuant to Section 5, Paragraph one, Clause 4 of the Law On
Chapter 5. The Carriage of Genetically Modified (GM) Crops
 Chapter 5 The Carriage of Genetically Modified (GM) Crops The term GMO (genetically modified organism) refers to any organism whose genetic makeup has been altered using genetic engineering. In the instance
Chapter 5 The Carriage of Genetically Modified (GM) Crops The term GMO (genetically modified organism) refers to any organism whose genetic makeup has been altered using genetic engineering. In the instance
Developing New GM Products and Detection Methods
 Developing New GM Products and Detection Methods Dave Grothaus Monsanto Company Slides Thanks to: International Life Sciences Institute Crop Life International Indus try Colleagues Hope Hart - Syngenta
Developing New GM Products and Detection Methods Dave Grothaus Monsanto Company Slides Thanks to: International Life Sciences Institute Crop Life International Indus try Colleagues Hope Hart - Syngenta
14 FARMING PRACTICES Land preparation. - To control the growth of weeds; - To shape the seedbed (into ridges, beds, or mounds).
 14 FARMING PRACTICES An enumerator working in farm surveys needs a basic understanding of the agricultural operations done by the farmers during the crop season. It is on these subjects that he will be
14 FARMING PRACTICES An enumerator working in farm surveys needs a basic understanding of the agricultural operations done by the farmers during the crop season. It is on these subjects that he will be
European Coexistence Bureau. Summary of conclusions of the 2 nd meeting of the Technical Working Group for Potato. 2-3 of May 2016, Seville, Spain
 European Coexistence Bureau Summary of conclusions of the 2 nd meeting of the Technical Working Group for Potato 2-3 of May 2016, Seville, Spain The 2 nd Meeting of the Technical Working Group for Potato
European Coexistence Bureau Summary of conclusions of the 2 nd meeting of the Technical Working Group for Potato 2-3 of May 2016, Seville, Spain The 2 nd Meeting of the Technical Working Group for Potato
APPLICATION FOR INTENTIONAL INTRODUCTION (CONDUCT A TRIAL RELEASE) OF GENETICALLY MODIFIED ORGANISMS (GMOs) INTO THE ENVIRONMENT OF SOUTH AFRICA
 DIRECTORATE GENETIC RESOURCES Private Bag X973, Pretoria, 0001 Harvest House Room 167, 30 Hamilton Street, Arcadia, Pretoria, 0002 Tel: 12 319 6382, Fax: 12 319 6298, E-mail: NompumeleloM@daff.gov.za APPLICATION
DIRECTORATE GENETIC RESOURCES Private Bag X973, Pretoria, 0001 Harvest House Room 167, 30 Hamilton Street, Arcadia, Pretoria, 0002 Tel: 12 319 6382, Fax: 12 319 6298, E-mail: NompumeleloM@daff.gov.za APPLICATION
Release into Environment of Genetically Modified Organisms Act 1
 Issuer: Riigikogu Type: act In force from: 01.07.2014 In force until: 31.07.2014 Translation published: 27.06.2014 Release into Environment of Genetically Modified Organisms Act 1 Amended by the following
Issuer: Riigikogu Type: act In force from: 01.07.2014 In force until: 31.07.2014 Translation published: 27.06.2014 Release into Environment of Genetically Modified Organisms Act 1 Amended by the following
GENETICALLY MODIFIED ORGANISM PRESENTATION. 13 September 2013 by Department of Trade and Industry
 GENETICALLY MODIFIED ORGANISM PRESENTATION 13 September 2013 by Department of Trade and Industry Introduction - A genetically modified organism (GMO) means any organism; the genes (or genetic material)
GENETICALLY MODIFIED ORGANISM PRESENTATION 13 September 2013 by Department of Trade and Industry Introduction - A genetically modified organism (GMO) means any organism; the genes (or genetic material)
Environmental monitoring plan for soybean MON 89788
 Environmental monitoring plan for soybean MON 89788 1. Introduction The GMO panel has carried out the scientific assessment of the genetically modified soybean MON89788 and considered that the soybean
Environmental monitoring plan for soybean MON 89788 1. Introduction The GMO panel has carried out the scientific assessment of the genetically modified soybean MON89788 and considered that the soybean
ALINORM 03/12A APPENDIX X
 108 DRAFT CODE OF PRACTICE FOR THE PREVENTION AND REDUCTION OF MYCOTOXIN CONTAMINATION IN CEREALS, INCLUDING ANNEXES ON OCHRATOXIN A, ZEARALENONE, FUMONISINS AND TRICOTHECENES (AT STEP 8 OF THE PROCEDURE)
108 DRAFT CODE OF PRACTICE FOR THE PREVENTION AND REDUCTION OF MYCOTOXIN CONTAMINATION IN CEREALS, INCLUDING ANNEXES ON OCHRATOXIN A, ZEARALENONE, FUMONISINS AND TRICOTHECENES (AT STEP 8 OF THE PROCEDURE)
GMO.1 Genetically Modified Organisms
 GMO.1 Genetically Modified Organisms Index GMO.1 Genetically Modified Organisms GMO.1.1 GMO.1.2 GMO.1.3 Description & Expectations Eligibility Rules Notifications GMO.2 GMO.2.1 GMO.2.2 GMO.2.3 GMO.2.4
GMO.1 Genetically Modified Organisms Index GMO.1 Genetically Modified Organisms GMO.1.1 GMO.1.2 GMO.1.3 Description & Expectations Eligibility Rules Notifications GMO.2 GMO.2.1 GMO.2.2 GMO.2.3 GMO.2.4
Calibre BMR Forage Sorghum
 Calibre BMR Forage Sorghum Sorghum bicolor x sudanese Early to mid maturing, Brown Mid Rib sorghum x Sudan grass hybrid 12 gene BMR now delivering new high quality in the forage market Low Lignin = highly
Calibre BMR Forage Sorghum Sorghum bicolor x sudanese Early to mid maturing, Brown Mid Rib sorghum x Sudan grass hybrid 12 gene BMR now delivering new high quality in the forage market Low Lignin = highly
PP 1/296 (1) Principles of efficacy evaluation for low-risk plant protection products
 EPPO - Licenced for Guest #0000u0000 Bulletin OEPP/EPPO Bulletin (2017) 47 (3), 297 304 ISSN 0250-8052. DOI: 10.1111/epp.12396 European and Mediterranean Plant Protection Organization Organisation Européenne
EPPO - Licenced for Guest #0000u0000 Bulletin OEPP/EPPO Bulletin (2017) 47 (3), 297 304 ISSN 0250-8052. DOI: 10.1111/epp.12396 European and Mediterranean Plant Protection Organization Organisation Européenne
Using Problem Formulation to Develop a Regulatory ERA. Hanoi, 27 th June Mónica García Alonso Estel Consult Ltd.
 Hanoi, 27 th June 2011 Estel Consult Ltd. Using problem formulation in environmental risk assessments for regulatory dossiers Basic concepts Examples Considerations for the preparation of high quality
Hanoi, 27 th June 2011 Estel Consult Ltd. Using problem formulation in environmental risk assessments for regulatory dossiers Basic concepts Examples Considerations for the preparation of high quality
Application for import and use of genetically modified herbicide tolerant maize Event GA21 under Regulation (EC) No 1829/2003 PART II: SUMMARY
 Syngenta Event GA21 Page 1 of 29 Application for import and use of genetically modified herbicide tolerant maize Event GA21 under Regulation (EC) No 1829/2003 Syngenta Event GA21 Page 2 of 29 A. GENERAL
Syngenta Event GA21 Page 1 of 29 Application for import and use of genetically modified herbicide tolerant maize Event GA21 under Regulation (EC) No 1829/2003 Syngenta Event GA21 Page 2 of 29 A. GENERAL
Maize and Biodiversity: The Effects of Transgenic Maize in Mexico. Issues Summary. Prepared by Chantal Line Carpentier and Hans Herrmann
 Maize and Biodiversity: The Effects of Transgenic Maize in Mexico Issues Summary Prepared by Chantal Line Carpentier and Hans Herrmann Secretariat of the Commission for Environmental Cooperation of North
Maize and Biodiversity: The Effects of Transgenic Maize in Mexico Issues Summary Prepared by Chantal Line Carpentier and Hans Herrmann Secretariat of the Commission for Environmental Cooperation of North
Commission Directive 2008/62/EC
 Commission Directive 2008/62/EC of 20 June 2008 providing for certain derogations for acceptance of agricultural landraces and varieties which are naturally adapted to the local and regional conditions
Commission Directive 2008/62/EC of 20 June 2008 providing for certain derogations for acceptance of agricultural landraces and varieties which are naturally adapted to the local and regional conditions
Friends of the Earth inspires solutions to environmental problems, which make life better for people
 March 2008 Representation from Friends of the Earth on application for a part B consent from Leeds University to release genetically modified potatoes with improved resistance to potato cystnematodes (Application
March 2008 Representation from Friends of the Earth on application for a part B consent from Leeds University to release genetically modified potatoes with improved resistance to potato cystnematodes (Application
Genetically modified sugarcane and Eldana. Sandy Snyman Agronomist s Association Annual Symposium 27 October 2015
 Genetically modified sugarcane and Eldana Sandy Snyman Agronomist s Association Annual Symposium 27 October 2015 GM=Genetically modified What is GM? DNA/gene from one source is transferred to another organism
Genetically modified sugarcane and Eldana Sandy Snyman Agronomist s Association Annual Symposium 27 October 2015 GM=Genetically modified What is GM? DNA/gene from one source is transferred to another organism
PROCESS MANAGEMENT MANUAL FOR RELEASE INTO THE ENVIRONMENT OF GENETICALLY MODIFIED AGRICULTURAL ORGANISMS
 PROCESS MANAGEMENT MANUAL FOR RELEASE INTO THE ENVIRONMENT OF GENETICALLY MODIFIED AGRICULTURAL ORGANISMS By Adelaida Harries Seed Science Center/Biosafety Institute for Genetically Modified Agricultural
PROCESS MANAGEMENT MANUAL FOR RELEASE INTO THE ENVIRONMENT OF GENETICALLY MODIFIED AGRICULTURAL ORGANISMS By Adelaida Harries Seed Science Center/Biosafety Institute for Genetically Modified Agricultural
CODE OF CONDUCT Principles of Quality Assurance in Beet Seed Production
 CODE OF CONDUCT Principles of Quality Assurance in Beet Seed Production (Version of 22 October 2007, amended in October 2014) Summary The principles of quality assurance and measures highlighted in this
CODE OF CONDUCT Principles of Quality Assurance in Beet Seed Production (Version of 22 October 2007, amended in October 2014) Summary The principles of quality assurance and measures highlighted in this
NATIONAL ORGANIC PROGRAM APPLICATION & ORGANIC SYSTEM PLAN - FARMS
 NATIONAL ORGANIC PROGRAM APPLICATION & ORGANIC SYSTEM PLAN - FARMS 1 of 26 1. GENERAL INFORMATION: Name of Operation: Address: City: State: Zip Code: Mailing Address (if different): City: State: Zip Code:
NATIONAL ORGANIC PROGRAM APPLICATION & ORGANIC SYSTEM PLAN - FARMS 1 of 26 1. GENERAL INFORMATION: Name of Operation: Address: City: State: Zip Code: Mailing Address (if different): City: State: Zip Code:
NATIONAL BIOSAFETY AUTHORITY RISK ASSESSMENT REPORT
 1 NATIONAL BIOSAFETY AUTHORITY RISK ASSESSMENT REPORT APPLICATION FOR ENVIRONMENTAL RELEASE, CULTIVATION AND PLACING ON THE MARKET OF MON 810 EVENT IN MAIZE VARIETIES APPLICANT: KENYA AGRICULTURAL AND
1 NATIONAL BIOSAFETY AUTHORITY RISK ASSESSMENT REPORT APPLICATION FOR ENVIRONMENTAL RELEASE, CULTIVATION AND PLACING ON THE MARKET OF MON 810 EVENT IN MAIZE VARIETIES APPLICANT: KENYA AGRICULTURAL AND
INTERNATIONAL UNION FOR THE PROTECTION OF NEW VARIETIES OF PLANTS
 * ORIGINAL: English DATE: April 19, 2002 INTERNATIONAL UNION FOR THE PROTECTION OF NEW VARIETIES OF PLANTS GENEVA E GENERAL INTRODUCTION TO THE EXAMINATION OF DISTINCTNESS, UNIFORMITY AND STABILITY AND
* ORIGINAL: English DATE: April 19, 2002 INTERNATIONAL UNION FOR THE PROTECTION OF NEW VARIETIES OF PLANTS GENEVA E GENERAL INTRODUCTION TO THE EXAMINATION OF DISTINCTNESS, UNIFORMITY AND STABILITY AND
BIOSAFETY INSPECTOR'S MANUAL FOR CONFINED FIELD TRIAL REQUIREMENTS IN TANZANIA
 UNITED REPUBLIC OF TANZANIA BIOSAFETY INSPECTOR'S MANUAL FOR CONFINED FIELD TRIAL REQUIREMENTS IN TANZANIA A Procedural Guide for Biosafety Inspectors Conducting Inspections of Confined Field Trials of
UNITED REPUBLIC OF TANZANIA BIOSAFETY INSPECTOR'S MANUAL FOR CONFINED FIELD TRIAL REQUIREMENTS IN TANZANIA A Procedural Guide for Biosafety Inspectors Conducting Inspections of Confined Field Trials of
Genetically Modified Crops
 page 1/7 Scientific Facts on Genetically Modified Crops Source document: FAO (2004) Summary & Details: GreenFacts Context - We are regularly confronted with genetically modified foods, be it in the news
page 1/7 Scientific Facts on Genetically Modified Crops Source document: FAO (2004) Summary & Details: GreenFacts Context - We are regularly confronted with genetically modified foods, be it in the news
Unit 3: Sustainability and Interdependence
 Unit 3: Sustainability and Interdependence Sub-topic 3.2 Plant and Animal Breeding Page 1 of 17 On completion of this sub-topic I will be able to: understand that plant and animal breeding involves the
Unit 3: Sustainability and Interdependence Sub-topic 3.2 Plant and Animal Breeding Page 1 of 17 On completion of this sub-topic I will be able to: understand that plant and animal breeding involves the
GMO in seeds actual state of affairs
 GMO in seeds actual state of affairs The EU concept of co-existence costs of co-existence liability GMO free regions and zones Seed contamination, labelling and approval Save our Seeds Benedikt Haerlin
GMO in seeds actual state of affairs The EU concept of co-existence costs of co-existence liability GMO free regions and zones Seed contamination, labelling and approval Save our Seeds Benedikt Haerlin
SCIENTIFIC REPORT OF EFSA
 The EFSA Scientific Report (2009) 293, 1-18 SCIENTIFIC REPORT OF EFSA Public Consultation on the Updated Guidance Document of the Scientific Panel on Genetically Modified Organisms (GMO) for the risk assessment
The EFSA Scientific Report (2009) 293, 1-18 SCIENTIFIC REPORT OF EFSA Public Consultation on the Updated Guidance Document of the Scientific Panel on Genetically Modified Organisms (GMO) for the risk assessment
Maize MON MON NK603
 Maize MON 87427 MON 89034 NK603 Organisation: The European GMO-free Citizens (De Gentechvrije Burgers) Country: The Netherlands Type: Others a. Assessment: b. Food Safety Assessment: Toxicology The conclusions
Maize MON 87427 MON 89034 NK603 Organisation: The European GMO-free Citizens (De Gentechvrije Burgers) Country: The Netherlands Type: Others a. Assessment: b. Food Safety Assessment: Toxicology The conclusions
EUROPEAN PARLIAMENT. Committee on the Environment, Public Health and Consumer Policy
 EUROPEAN PARLIAMT 1999 2004 Committee on the Environment, Public Health and Consumer Policy 28 August 2002 PE 319.341/45-62 AMDMTS 45-62 Draft report (PE 319.341) Jonas Sjöstedt on the proposal for a European
EUROPEAN PARLIAMT 1999 2004 Committee on the Environment, Public Health and Consumer Policy 28 August 2002 PE 319.341/45-62 AMDMTS 45-62 Draft report (PE 319.341) Jonas Sjöstedt on the proposal for a European
LATITUDE. A seed treatment for the reduction of take-all in winter and spring wheat and winter barley
 F - Fungicide LATITUDE A seed treatment for the reduction of take-all in winter and spring wheat and winter barley This product contains a flowable suspension concentrate containing 125g/litre (11.83%
F - Fungicide LATITUDE A seed treatment for the reduction of take-all in winter and spring wheat and winter barley This product contains a flowable suspension concentrate containing 125g/litre (11.83%
