SAMPLE SENDING MANAGEMENT
|
|
|
- Bernice Rice
- 6 years ago
- Views:
Transcription
1 SAMPLE SENDING MANAGEMENT CIBOMA/ Phase IV.III, multicenter, open, randomized treatment study to evaluate the efficacy of maintenance therapy with capecitabine (X) after standard adjuvant chemotherapy in patients with operable, hormone receptor and HER2neu negative breast cancer 1.- INFORMED CONSENT. TRIPLE NEGATIVE TEST (ATTACHMENT I) The potentially eligible patients, should sign the informed consent form for the sending of the sample to the central laboratory at the initiation of the treatment with standard adjuvant chemotherapy. Even if the hormone receptor and HER2 expression tests are carried out routinely in each hospital s laboratory, if the result is negative, the tests will be repeated in a central laboratory in Madrid (Spain), to confirm the negative result. Only samples of those patients that present negative estrogen and progesterone receptors in the immunohistochemical test carried out routinely in the investigation center before the administration of standard chemotherapy will be sent to the central laboratory. 2.- COURIER REQUEST. Please, contact the following people to request a courier to fetch the sample: - Esther Mahillo (emahillo@geicam.org) - Mª Ángeles Jimeno (majimeno@geicam.org) - Mª Carmen Cámara (mccamara@geicam.org) - Elena Gutiérrez (egutierrez@geicam.org) - Andrés Hernando (ahernando@ciboma.org) All the above indicated people should be stated in the with copy" section of the fetch requesting FILL THE ATTACHMENT II AND SEND IT BY FAX TO THE CIBOMA MAIN OFFICES IN SPAIN ( ). 4.- FILL A COMMERCIAL INVOICE (ATTACHMENT III). Page 1 of 11
2 5.- PREPARATION AND SENDING OF THE SAMPLES TO THE CENTRAL LABORATORY (ATTACHMENT IV: INTERNATIONAL RULES OF BIOLOGICAL SAMPLE SENDINGS). Along with the necessary wrappers or containers for the correct sending of the biological samples, the courier will deliver a note with 3 copies, one will be the sending ticket, other will be the copy for the courier and the third one should be sticked on the sending material in the attached adhesive window sticker. 6.- ATTACHED DOCUMENTS THAT SHOULD BE INCLUDED IN THE PACKING BOX ALONG WITH THE SAMPLE: ATTACHMENT II: DETERMINATION OF THE TRIPLE NEGATIVE. ATTACHMENT III: COMMERCIAL INVOICE. Page 2 of 11
3 CARRIER FOR THE SAMPLE FETCHING: TNT For any additional clarification please contact the CIBOMA main offices in Spain. Telephone: or by to: Page 3 of 11
4 ATTACHMENT I: PRE-SCREENING INFORMED CONSENT. TRIPLE NEGATIVE TEST FOR CIBOMA STUDY INTRODUCTION AND OBJECTIVES Your doctor is considering you for participation in a clinical study (also known as clinical trial). You will be able to take part in this trial once all the other treatments which you have been administered for your breast cancer have been completed. Before we continue talking about your possible participation in the study, other tests will first have to be carried out on your tumor sample, known as hormone receptor and HER2 expression tests. These tests can be carried out with a sample we already have, so it will not be necessary to carry out more biopsies, operations or blood draws. The hormone receptor and HER2 expression tests will be carried out routinely in your hospital laboratory. If the result is negative, the tests will be repeated in a central laboratory in Madrid, Spain, to confirm the negative result. The aim of this document is to provide you with sufficient information for you to understand the possible risks and benefits which may be involved in these additional tests and confirmations. If you decide later to participate in the clinical trial, your doctor will ask you to read and sign a document which contains more details on the trial. HORMONE RECEPTOR TEST Breast tissue contains some hormone receptors, called estrogens and progesterones, which regulate many of the processes related with the feminine sex. Approximately 70% of breast cancers contain these receptors. These hormone receptors are absent from about 20% of tumors, and these tumors are called receptor negative. In the case of your tumor, your doctor has determined using a technique called immunohistochemical staining, that it has negative hormone receptors, which is one of the two conditions necessary for your participation in the clinical trial. HER2 TEST HER2 is a protein which is produced in high levels in about 20% of breast cancer tumors. In approximately the other 80%, high levels of this protein are not detected. If you belong to this group (HER2 negative), you can participate in the clinical trial. The negative result for hormone receptors and HER2 must be confirmed in the central laboratory. The test material will be kept in the central laboratory for future reference. The laboratories will process the samples, using one of the following methods: - Immunohistochemical staining (IHC). - Fluorescence in situ hybridation (FISH). PATIENT AND INVESTIGATOR: SIGN AND DATE TWO COPIES OF INFORMED CONSENT. KEEP ONE COPY IN CLINICAL RECORDS. GIVE THE OTHER COPY TO THE PATIENT. Page 4 of 11
5 ADDITIONAL TESTS If you do participate in study CIBOMA in the future, another two tests will be carried out on the material sent to the central laboratory, to determine if your tumor produces high levels of a protein called cytokeratin 5 and of another protein called HER1, which is also known as EGF receptor. The process which will be used for these tests is immunohistochemical staining. POTENTIAL BENEFITS It is possible that you do not obtain any personal benefit from the additional tests carried out on your tumor sample, although the information on the factors analyzed may be useful for directing your future treatment. Your doctor will provide you with the results of these tests. ADDITIONAL COSTS OR PAYMENTS You will not have to pay for the hormone receptor and HER2 tests, or for any additional test. Nor will you be paid for agreeing to the analyses. DATA CONFIDENTIALITY You have a right to privacy and all the information which is collected for this study will be kept confidential within legal limits. In Spain, the handling of data of a personal nature is regulated by the Constitutional Law 15/1999, dated 13 December Your consent for handling your personal data and for allowing the use of the same can be revoked. You can exercise your right to access, rectification and cancellation, by asking your doctor, who will put you in contact with the sponsor. If the hormone receptor and HER2 levels are positive, and you cannot participate in the clinical trial, your doctor will file your signed and dated pre-screening informed consent in your clinical records, along with the results of the tests and the medical observations from the prescreening period. If the hormone receptor and HER2 levels are negative, you will be able to participate in the CIBOMA clinical trial. In this case, your results will be collected by your doctor and sent to CIBOMA, Madrid, Spain, where the research database for the study is located. Your original clinical records may be analyzed during or after the study by CIBOMA or its designated representatives, the health authorities and the local Ethics Committee representatives. You do not have to option to request that your medical records are not analyzed. PATIENT AND INVESTIGATOR: SIGN AND DATE TWO COPIES OF INFORMED CONSENT. KEEP ONE COPY IN CLINICAL RECORDS. GIVE THE OTHER COPY TO THE PATIENT. Page 5 of 11
6 If you can, and you decide to, participate in the CIBOMA study, your data may be analyzed in any of the member countries of the Coalition, to determine how many patients have tumors with the same characteristics as yours (called triple-negative). CIBOMA may send the results to the health authorities of the CIBOMA countries which are participating in this study, present them in medical meetings, or publish them in specialized journals, so that other doctors may know about them. If you withdraw from the study, the information gathered about you up to the moment of withdrawal will continue to be used. The representatives of CIBOMA, of the health authorities and of the local ethics committee may be permitted to inspect your clinical record to ensure that the information gathered is correct. In these cases, you may be identified personally. It is possible that CIBOMA may need to analyze the prescreening data again and do new statistical tests on it. The results of this study may be used for future medical research. Your participation in the study CIBOMA is described in another consent sheet. OBTAINING ADDITIONAL INFORMATION We encourage you to ask all the questions you want at any time during the study. If problems emerge or if you have more questions on prescreening, or on the study or your rights as a patient, call Dr.. on..(tel.). BASIS OF PARTICIPATION The participation in prescreening procedures for the CIBOMA study is voluntary. If you do not want to participate, you will not lose any of the benefits to which you otherwise have a right nor will you suffer any penalty. PATIENT AND INVESTIGATOR: SIGN AND DATE TWO COPIES OF INFORMED CONSENT. KEEP ONE COPY IN CLINICAL RECORDS. GIVE THE OTHER COPY TO THE PATIENT. Page 6 of 11
7 PATIENT DECLARATION AND SIGNATURE PHASE IV.III, MULTICENTER, OPEN, RANDOMIZED TREATMENT STUDY TO EVALUATE THE EFFICACY OF MAINTENANCE THERAPY WITH CAPECITABINE (X) AFTER STANDARD ADJUVANT CHEMOTHERAPY IN PATIENTS WITH OPERABLE, HORMONE RECEPTOR AND HER2neu NEGATIVE BREAST CANCER Study number: CIBOMA/ I,... have read the information sheet which has been provided to me and I understand the aim of screening for the analysis of hormone receptor and HER2 expression. I have been able to ask questions about the study. I have received satisfactory answers to my questions. I have received sufficient information about the study. I have spoken with... I understand that my participation is voluntary. I understand that I can withdraw from the study: 1. whenever I want 2. without having to give explanations 3. without affecting my medical care I freely give my agreement to participate in this screening for the analysis of hormone receptors and HER2 in a sample of my primary breast cancer. Date Signature of participant: I have fully explained the relevant details of this study to the above-named patient. Date: Signature of investigator: PATIENT AND INVESTIGATOR: SIGN AND DATE TWO COPIES OF INFORMED CONSENT. KEEP ONE COPY IN CLINICAL RECORDS. GIVE THE OTHER COPY TO THE PATIENT. Page 7 of 11
8 ATTACHMENT II: DETERMINATION OF THE TRIPLE NEGATIVE BY THE CENTRAL LABORATORY CIBOMA/ : Phase IV.III, multicenter, open, randomized treatment study to evaluate the efficacy of maintenance therapy with capecitabine (X) after standard adjuvant chemotherapy in patients with operable, hormone receptor and HER2neu negative breast cancer. Investigator name: Investigator fax: Hospital name: No. of registry of the patient: Birth date: / / DETERMINATION OF THE TRIPLE NEGATIVE BY THE SITE: Test Estrogen receptors Progesterone receptors Biochemical method Immunohistochemistry HER2neu FISH Immunohistochemistry Signature: DETERMINATION BY THE CENTRAL LABORATORY: Immunohistochemistry HER2neu Cytokeratins 5/6 EGF receptor (EGFR) Estrogen receptors FISH Inmunohistochemistry Inmunohistochemistry Progesterone receptors Immunohistochemistry Determination date: Signature: Note: Once the result have been confirmed it will be necessary to send this document by fax to the CIBOMA main offices in Spain ( ). Page 8 of 11
9 ATTACHMENT III: COMMERCIAL INVOICE SENT BY: ºData and address of the person that send the sampleº Name: Address: COMMERCIAL INVOICE City/P. Code: No.: ºNot applicableº Country: Tel/Fax. No: SENT TO: ºData and address of the person that receive the sampleº AIRBILL No.: ºDelivery note No.º Name: Attn: Date: Sending date No. of Address: pieces: ºUnit numberº City/P. Code: Total Gross Weight: ºTotal gross weightº Country: Total Net Weight: ºTotal net weightº Tel/Fax. No.: CARRIER: Full description of goods Custom Commodity. Code C. Origin Unit Value & Currency ºDescription of the sending materialº Subt Value & Currency Total Value & Currency VALUE FOR CUSTOMS PURPOSE ONLY NO COMMERCIAL VALUE REASON FOR EXPORT TERMS OF DELIVERY IF IT REGARDS: The exporter of the product covered by this document (custom autorization No.: declares that, except where otherwise clearly, these products are of preferential origin. Name: Signature: Place and date: Page 9 of 11
10 ATTACHMENT IV: INTERNATIONAL RULES FOR BIOLOGICAL SAMPLE SENDING Picture 1 Material for the sending of biological samples Pictures 2 and 3 The piece is wrapped in bubble paper (WRAPPER 1) Pictures 4, 5 and 6 The piece is introduced into a plastic bag that will be hermetically closed (WRAPPER 2) Pictures 7, 8 and 9 The bag is introduced into a sending box and is properly identified (WRAPPER 3) Page 10 of 11
11 FLOW CHART SAMPLE SENDING MANAGEMENT Standard Chemotherapy Estrogen and progesterone RH negative The patient signs the Informed Consent Form for sending the samples to the central laboratory Patient registry in the CIBOMA intranet Preparation and sending of the sample to the central laboratory Determination of the triple negative in the central laboratory NO Not eligible patient YES Eligible patient The patient signs the Informed Consent Form for the inclusion in the CIBOMA/ study Patient randomization by means of the CIBOMA intranet Page 11 of 11
Genentech, Inc., its research partners, collaborators, assignees, licensees or designees. Invitation to Participate
 CONSENT AND RESEARCH AUTHORIZATION TO DONATE BLOOD AND TISSUE SAMPLES FOR FUTURE RESEARCH PURPOSES YALE UNIVERSITY SCHOOL OF MEDICINE YALE-NEW HAVEN HOSPITAL 200 FR. 4 Study Title: An Open-Label, Phase
CONSENT AND RESEARCH AUTHORIZATION TO DONATE BLOOD AND TISSUE SAMPLES FOR FUTURE RESEARCH PURPOSES YALE UNIVERSITY SCHOOL OF MEDICINE YALE-NEW HAVEN HOSPITAL 200 FR. 4 Study Title: An Open-Label, Phase
Nanthealth Patient Informed Consent
 Patient Informed Consent Why am I being asked to give consent? You are being asked to sign this consent form because you want to have a molecular/genomic analysis performed to help your physician understand
Patient Informed Consent Why am I being asked to give consent? You are being asked to sign this consent form because you want to have a molecular/genomic analysis performed to help your physician understand
Related Donor Informed Consent to Participate in Research
 Related Donor Informed Consent to Participate in Research This is an informed consent document for a research study that your family member is participating in. This document will inform you about the
Related Donor Informed Consent to Participate in Research This is an informed consent document for a research study that your family member is participating in. This document will inform you about the
The patient must address the treating physician directly if he/she has any questions or requires information regarding the test results.
 Order Form 1. Ordering of the FoundationOne Service The FoundationOne Service comprises the genetic analysis of tumour tissue and the compilation of a comprehensive report on any mutations found in the
Order Form 1. Ordering of the FoundationOne Service The FoundationOne Service comprises the genetic analysis of tumour tissue and the compilation of a comprehensive report on any mutations found in the
Annex VIIIB Paragraphs to be included in the Informed Consent Form for the collection and use of biological samples in clinical trials
 DEPARTAMENTO DE MEDICAMENTOS DE USO HUMANO Annex VIIIB Paragraphs to be included in the Informed Consent Form for the collection and use of biological samples in clinical trials Version 20 th December
DEPARTAMENTO DE MEDICAMENTOS DE USO HUMANO Annex VIIIB Paragraphs to be included in the Informed Consent Form for the collection and use of biological samples in clinical trials Version 20 th December
FREQUENTLY ASKED QUESTIONS
 CLINICAL TRIALS FREQUENTLY ASKED QUESTIONS PARTICIPATING IN A CLINICAL TRIAL What is a clinical trial? Clinical trials are research studies that involve people. Through clinical trials, researchers find
CLINICAL TRIALS FREQUENTLY ASKED QUESTIONS PARTICIPATING IN A CLINICAL TRIAL What is a clinical trial? Clinical trials are research studies that involve people. Through clinical trials, researchers find
TERMS OF SALE FOR COMPANIES
 I GENERAL INFORMATION 1. Seller s details:, al. 29 Listopada 94, 31-406 Kraków, National Court Register Number: 0000113750, Tax Identification Number: PL2220684266, National Business Registry Number: 276435920,
I GENERAL INFORMATION 1. Seller s details:, al. 29 Listopada 94, 31-406 Kraków, National Court Register Number: 0000113750, Tax Identification Number: PL2220684266, National Business Registry Number: 276435920,
A GUIDE TO THIS REFLECTIONS B RESEARCH STUDY IF YOU RE FIGHTING BREAST CANCER, YOU RE NOT ALONE
 A GUIDE TO THIS REFLECTIONS B327-02 RESEARCH STUDY IF YOU RE FIGHTING BREAST CANCER, YOU RE NOT ALONE Do you have breast cancer that has spread to outside the breast? Has your tumor tested positive for
A GUIDE TO THIS REFLECTIONS B327-02 RESEARCH STUDY IF YOU RE FIGHTING BREAST CANCER, YOU RE NOT ALONE Do you have breast cancer that has spread to outside the breast? Has your tumor tested positive for
Rules of Human Experimentation
 Rules of Human Experimentation Elaine Larson CUMC IRB Chair Associate Dean for Research, School of Nursing Professor of Epidemiology, Mailman School of Public Health Oversight for Human Research Office
Rules of Human Experimentation Elaine Larson CUMC IRB Chair Associate Dean for Research, School of Nursing Professor of Epidemiology, Mailman School of Public Health Oversight for Human Research Office
GUIDE THE GUIDE TO GETTING AN ONCOTYPE DX TEST
 STEP BY STEP GUIDE STEP BY STEP THE GUIDE TO GETTING AN ONCOTYPE DX TEST ONCOTYPE DX IS A TEST THAT MAY HELP BREAST CANCER PATIENTS DETERMINE IF THEY WILL BENEFIT FROM CHEMOTHERAPY Choosing the optimal
STEP BY STEP GUIDE STEP BY STEP THE GUIDE TO GETTING AN ONCOTYPE DX TEST ONCOTYPE DX IS A TEST THAT MAY HELP BREAST CANCER PATIENTS DETERMINE IF THEY WILL BENEFIT FROM CHEMOTHERAPY Choosing the optimal
National Study of Nephrotic Syndrome (NephroS) Information Sheet for Adult Patients
 National Study of Nephrotic Syndrome (NephroS) Information Sheet for Adult Patients You are being invited to participate in a research study. Before you decide, it is important for you to understand why
National Study of Nephrotic Syndrome (NephroS) Information Sheet for Adult Patients You are being invited to participate in a research study. Before you decide, it is important for you to understand why
Generic lab muanual Practical examples on the shipping instructions.
 Generic lab muanual Practical examples on the shipping instructions. Site 123 Dr. Investigator Address 5 March 2014 Dear Doctor, World Courier has been asked by ABC to co-ordinate the delivery of samples
Generic lab muanual Practical examples on the shipping instructions. Site 123 Dr. Investigator Address 5 March 2014 Dear Doctor, World Courier has been asked by ABC to co-ordinate the delivery of samples
Brain Tumour Australia Information FACT SHEET 22 Clinical Trials: Questions and Answers
 FACT SHEET 22 Clinical Trials: Questions and Answers What is a clinical trial? Clinical trials are research studies that answer scientific questions and try to find better ways to prevent, screen for,
FACT SHEET 22 Clinical Trials: Questions and Answers What is a clinical trial? Clinical trials are research studies that answer scientific questions and try to find better ways to prevent, screen for,
EIGHT BASIC ELEMENTS OF INFORMED CONSENT
 1/6 This guidance addresses: o Eight Basic elements of informed consent o Additional elements of informed consent o Other information required by the IRB EIGHT BASIC ELEMENTS OF INFORMED CONSENT Required
1/6 This guidance addresses: o Eight Basic elements of informed consent o Additional elements of informed consent o Other information required by the IRB EIGHT BASIC ELEMENTS OF INFORMED CONSENT Required
Central Laboratory Services. Anatomic Pathology Histology Services
 Central Laboratory Services Anatomic Pathology Histology Services Oncology and Beyond... There is no question that innovations in technology have the potential to significantly impact a number of clinical
Central Laboratory Services Anatomic Pathology Histology Services Oncology and Beyond... There is no question that innovations in technology have the potential to significantly impact a number of clinical
2013 Continuing Compliance Master Series Checklist Updates for Anatomic Pathology. Gerald A. Hoeltge, MD, FCAP July 17,
 2013 Continuing Compliance Master Series Checklist Updates for Anatomic Pathology Gerald A. Hoeltge, MD, FCAP July 17, 2013 www.cap.org Today s Presenter Gerald A. Hoeltge, MD FCAP Chair, Checklists Committee
2013 Continuing Compliance Master Series Checklist Updates for Anatomic Pathology Gerald A. Hoeltge, MD, FCAP July 17, 2013 www.cap.org Today s Presenter Gerald A. Hoeltge, MD FCAP Chair, Checklists Committee
TheraLin. Universal Tissue Fixative Enabling Molecular Pathology
 TheraLin Universal Tissue Fixative Enabling Molecular Pathology TheraLin Universal Tissue Fixative Enabling Molecular Pathology Contents Page # TheraLin Universal Tissue Fixative 3 Introduction 5 Easy
TheraLin Universal Tissue Fixative Enabling Molecular Pathology TheraLin Universal Tissue Fixative Enabling Molecular Pathology Contents Page # TheraLin Universal Tissue Fixative 3 Introduction 5 Easy
Participant Information
 If you need an interpreter, please let us know You are invited to donate your tissue to the Auckland Regional Tissue Bank. This patient information sheet and consent form explains what is involved in donating
If you need an interpreter, please let us know You are invited to donate your tissue to the Auckland Regional Tissue Bank. This patient information sheet and consent form explains what is involved in donating
07/30/2013. Record of Revisions IRB Page 1 of 17
 Current Protocol Version 7.1 (Continuing Review) Approved July 30, 2013 Version 7.1 (Amendment) September 6, 2012 Version 7.0 (Continuing Review) July 30, 2012 Version 6.0 (Continuing Review) July 30,
Current Protocol Version 7.1 (Continuing Review) Approved July 30, 2013 Version 7.1 (Amendment) September 6, 2012 Version 7.0 (Continuing Review) July 30, 2012 Version 6.0 (Continuing Review) July 30,
Caris Molecular Profiling Shipper Kit Instructions
 Caris Molecular Profiling Shipper Kit Instructions Preparing the Shipper Kit Step by Step procedure You need a Caris Paraffin Block and Slide Shipper Kit. If you don t have shipper kits stored at your
Caris Molecular Profiling Shipper Kit Instructions Preparing the Shipper Kit Step by Step procedure You need a Caris Paraffin Block and Slide Shipper Kit. If you don t have shipper kits stored at your
REQUEST FOR RETINOBLASTOMA TESTING
 PATIENT INFORMATION REQUEST FOR RETINOBLASTOMA TESTING Please provide the following information. We cannot perform your test without ALL of this information. PLEASE PRINT ALL ANSWERS FIRST NAME MI LAST
PATIENT INFORMATION REQUEST FOR RETINOBLASTOMA TESTING Please provide the following information. We cannot perform your test without ALL of this information. PLEASE PRINT ALL ANSWERS FIRST NAME MI LAST
PHARMACY, MEDICINES & POISONS BOARD
 PHARMACY, MEDICINES & POISONS BOARD PHARMACY GUIDELINES FOR INVESTIGATIONAL DRUGS A. Purpose These guidelines are principally derived and adapted from guidelines from various sources within the SADC region.
PHARMACY, MEDICINES & POISONS BOARD PHARMACY GUIDELINES FOR INVESTIGATIONAL DRUGS A. Purpose These guidelines are principally derived and adapted from guidelines from various sources within the SADC region.
SUNY Upstate Medical University. Stephen J. Glatt, Ph.D. Stephen V. Faraone, Ph.D
 SUNY Upstate Medical University Stephen J. Glatt, Ph.D. Stephen V. Faraone, Ph.D. 315-464-7742 315-464-3113 Study Title: Longitudinal Family/Molecular Genetic Study to Evaluate Research Domain Criteria
SUNY Upstate Medical University Stephen J. Glatt, Ph.D. Stephen V. Faraone, Ph.D. 315-464-7742 315-464-3113 Study Title: Longitudinal Family/Molecular Genetic Study to Evaluate Research Domain Criteria
annex 3. Template consent form for biobanking
 annex 3. Template consent form for biobanking This template is based on Public Population Project in Genomics and Society (P3G) database resources. General considerations The information brochure and consent
annex 3. Template consent form for biobanking This template is based on Public Population Project in Genomics and Society (P3G) database resources. General considerations The information brochure and consent
CLINICAL TRIALS: WHAT YOU NEED TO KNOW
 CLINICAL TRIALS: WHAT YOU NEED TO KNOW www.kidney.org National Kidney Foundation's Kidney Disease Outcomes Quality Initiative Did you know that the National Kidney Foundation's Kidney Disease Outcomes
CLINICAL TRIALS: WHAT YOU NEED TO KNOW www.kidney.org National Kidney Foundation's Kidney Disease Outcomes Quality Initiative Did you know that the National Kidney Foundation's Kidney Disease Outcomes
Terms and Conditions of the online sales of the TATRYSKI pass. General Commercial Terms and Conditions TERMS AND CONDITIONS OF THE ONLINE SALE OF
 Terms and Conditions of the online sales of the TATRYSKI pass General Commercial Terms and Conditions TERMS AND CONDITIONS OF THE ONLINE SALE OF THE TATRYSKI ENTITLEMENTS ARTICLE 1. DEFINITIONS 1. Seller
Terms and Conditions of the online sales of the TATRYSKI pass General Commercial Terms and Conditions TERMS AND CONDITIONS OF THE ONLINE SALE OF THE TATRYSKI ENTITLEMENTS ARTICLE 1. DEFINITIONS 1. Seller
"From Bedside to Bench and Back: regulatory requirements for collaborations between pharma industry and academia
 "From Bedside to Bench and Back: regulatory requirements for collaborations between pharma industry and academia Damir Hamamdžić D.V.M., Ph.D. Office of Research Regulatory Affairs Rutgers University RWJMS
"From Bedside to Bench and Back: regulatory requirements for collaborations between pharma industry and academia Damir Hamamdžić D.V.M., Ph.D. Office of Research Regulatory Affairs Rutgers University RWJMS
Participant Copy. No. Participation is voluntary. Your decision will not affect your health care at Mayo Clinic in any way.
 Name and Clinic Number IRB # 08-007049 00 Consent form approved July 16, 2015; This consent valid through July 15, 2016; 1. General Information About This Research Study Study Title: Mayo Clinic Biobank
Name and Clinic Number IRB # 08-007049 00 Consent form approved July 16, 2015; This consent valid through July 15, 2016; 1. General Information About This Research Study Study Title: Mayo Clinic Biobank
Illumina Clinical Services Laboratory
 Illumina Clinical Services Laboratory CLIA Certificate No.: 05D1092911 Illumina Clinical Services Laboratory TruGenome Technical Sequence Data Test Requisition Form The Illumina Clinical Services Laboratory
Illumina Clinical Services Laboratory CLIA Certificate No.: 05D1092911 Illumina Clinical Services Laboratory TruGenome Technical Sequence Data Test Requisition Form The Illumina Clinical Services Laboratory
INSTRUCTIONS: HOW TO COMPLETE THE AVASTIN PATIENT
 INSTRUCTIONS: HOW TO COMPLETE THE AVASTIN PATIENT ASSISTANCE PROGRAM ENROLLMENT FORM Phone: (888) 249-4918 Fax: (888) 249-4919 www.avastinaccesssolutions.com Enrollment Requirements Complete the Enrollment
INSTRUCTIONS: HOW TO COMPLETE THE AVASTIN PATIENT ASSISTANCE PROGRAM ENROLLMENT FORM Phone: (888) 249-4918 Fax: (888) 249-4919 www.avastinaccesssolutions.com Enrollment Requirements Complete the Enrollment
The Importance of Feasibility Studies for Oncology Clinical Trials
 The Importance of Feasibility Studies for Oncology Clinical Trials www.ppdi.com Executive Summary The segmenting of the oncology patient population has increased the challenges in designing clinical trials.
The Importance of Feasibility Studies for Oncology Clinical Trials www.ppdi.com Executive Summary The segmenting of the oncology patient population has increased the challenges in designing clinical trials.
Billing Contact: Phone: Research Funding: NIH Funded Industry Funded Department Funded Short Study Protocol Title:
 Please submit this application to CPRS@mednet.ucla.edu at least 2-3 weeks prior to the beginning of the research for approval. All UCLA studies using patient medical record numbers/identifiers must be
Please submit this application to CPRS@mednet.ucla.edu at least 2-3 weeks prior to the beginning of the research for approval. All UCLA studies using patient medical record numbers/identifiers must be
Date: Date of start of procedure: Authorisation/ positive opinion: Date: Date:
 NOTIFICATION OF A SUBSTANTIAL AMENDMENT TO A CLINICAL TRIAL ON A MEDICINAL PRODUCT FOR HUMAN USE TO THE COMPETENT AUTHORITIES AND FOR OPINION OF THE ETHICS COMMITTEES IN THE COMMUNITY For official use:
NOTIFICATION OF A SUBSTANTIAL AMENDMENT TO A CLINICAL TRIAL ON A MEDICINAL PRODUCT FOR HUMAN USE TO THE COMPETENT AUTHORITIES AND FOR OPINION OF THE ETHICS COMMITTEES IN THE COMMUNITY For official use:
Objectives Discuss the importance of proper data collection. Identify the types of data collected for clinical trials. List potential source documents
 Data Management in Clinical Trials Introduction to the Principles and Practice of Clinical Research January 24, 2011 Diane St. Germain, RN, MS, CRNP Nurse Consultant Division of Cancer Prevention National
Data Management in Clinical Trials Introduction to the Principles and Practice of Clinical Research January 24, 2011 Diane St. Germain, RN, MS, CRNP Nurse Consultant Division of Cancer Prevention National
Surgical/Cytology Specimen Collection and Handling
 Surgical/Cytology Specimen Collection and Handling To ensure that hospital departments and outside sources submitting specimens to the surgical pathology department follow the established methods to guard
Surgical/Cytology Specimen Collection and Handling To ensure that hospital departments and outside sources submitting specimens to the surgical pathology department follow the established methods to guard
IRB USE ONLY Approval Date: September 10, 2013 Expiration Date: September 10, 2014
 Approval : September 10, 2013 Expiration : September 10, 2014 DESCRIPTION: You are invited to donate your excess sperm and/or testicular biopsy material, as well as your somatic cells (ordinary body cells
Approval : September 10, 2013 Expiration : September 10, 2014 DESCRIPTION: You are invited to donate your excess sperm and/or testicular biopsy material, as well as your somatic cells (ordinary body cells
What is the purpose of the Tissue Bank and the Research it will be used for?
 PARTICIPANT INFORMATION SHEET King s College London Haemato-Oncology Tissue Bank: The Collection and Storage of Blood and Tissue for Use in Future Studies into the Causes, Diagnosis and Treatment of Haematological
PARTICIPANT INFORMATION SHEET King s College London Haemato-Oncology Tissue Bank: The Collection and Storage of Blood and Tissue for Use in Future Studies into the Causes, Diagnosis and Treatment of Haematological
BRAIN UK UK Brain Archive Information Network
 BRAIN UK UK Brain Archive Information Network TISSUE REQUEST FORM Version 1.4 Date: 11 February 2011-1 - Ref: 11/SC/0056 GUIDANCE NOTES Please complete all sections of this form and send an electronic
BRAIN UK UK Brain Archive Information Network TISSUE REQUEST FORM Version 1.4 Date: 11 February 2011-1 - Ref: 11/SC/0056 GUIDANCE NOTES Please complete all sections of this form and send an electronic
FACTS YOU NEED TO KNOW UNITED STATES OF AMERICA
 CLINICAL TRIALS BROCHURE FACTS YOU NEED TO KNOW Thousands of men across the United States suffer from the difficult effects of prostate cancer and often undergo treatment that does not produce optimal
CLINICAL TRIALS BROCHURE FACTS YOU NEED TO KNOW Thousands of men across the United States suffer from the difficult effects of prostate cancer and often undergo treatment that does not produce optimal
Mazzitti & Sullivan EAP Services Notice of Privacy Practices
 This notice describes how medical information about you may be used and disclosed and how you can get access to this information. Please review it carefully. Mazzitti & Sullivan EAP Services Notice of
This notice describes how medical information about you may be used and disclosed and how you can get access to this information. Please review it carefully. Mazzitti & Sullivan EAP Services Notice of
Guidance for Sponsors, Institutional Review Boards, Clinical Investigators and FDA Staff
 Guidance for Sponsors, Institutional Review Boards, Clinical Investigators and FDA Staff Guidance on Informed Consent for In Vitro Diagnostic Device Studies Using Leftover Human Specimens that are Not
Guidance for Sponsors, Institutional Review Boards, Clinical Investigators and FDA Staff Guidance on Informed Consent for In Vitro Diagnostic Device Studies Using Leftover Human Specimens that are Not
OCREVUS Start Form. Instructions for Patients. Instructions for Health Care Providers
 Instructions for Patients By completing this form: You will enroll in support services from Genentech You can apply to the Genentech Access to Care Foundation (GATCF) to determine if you are eligible to
Instructions for Patients By completing this form: You will enroll in support services from Genentech You can apply to the Genentech Access to Care Foundation (GATCF) to determine if you are eligible to
Clinical trial information leaflet and consent
 Informed consent 1(7) Clinical trial information leaflet and consent General You must provide sufficient information on the rights of clinical trial subjects, the purpose and nature of the trial, the methodologies
Informed consent 1(7) Clinical trial information leaflet and consent General You must provide sufficient information on the rights of clinical trial subjects, the purpose and nature of the trial, the methodologies
Stanford University IRB Guidance On Data and Tissue Repositories
 Stanford University IRB Guidance On Data and Tissue Repositories Databases, registries (data banks), and repositories (tissue banks) all involve the collection and storage of information and/or biological
Stanford University IRB Guidance On Data and Tissue Repositories Databases, registries (data banks), and repositories (tissue banks) all involve the collection and storage of information and/or biological
APPLICATION FORM. Application for a Recognised Research Ethics Committee (REC) Opinion on a Clinical Trial on a Medicinal Product for Human Use.
 APPLICATION FORM Application for a Recognised Research Ethics Committee (REC) Opinion on a Clinical Trial on a Medicinal Product for Human Use. Form 1 This application form should be completed and submitted
APPLICATION FORM Application for a Recognised Research Ethics Committee (REC) Opinion on a Clinical Trial on a Medicinal Product for Human Use. Form 1 This application form should be completed and submitted
International Transfers of Personal Data at sanofi-aventis R & D
 International Transfers of Personal Data at sanofi-aventis R & D Pierre-Yves Lastic, PhD Senior Director, Standards Management & Data Privacy Sanofi-aventis R&D CONFERENCE ON INTERNATIONAL TRANSFERS OF
International Transfers of Personal Data at sanofi-aventis R & D Pierre-Yves Lastic, PhD Senior Director, Standards Management & Data Privacy Sanofi-aventis R&D CONFERENCE ON INTERNATIONAL TRANSFERS OF
VERSION: 21 st June Date of Publication: 15 th March C/ CAMPEZO, 1 EDIFICIO MADRID Tel.: Fax:
 Memorandum on Collaboration and Exchange of Information between the Spanish Agency of Medicinal Products and Medical Devices and Ethics Committees for investigation with medicinal products VERSION: 21
Memorandum on Collaboration and Exchange of Information between the Spanish Agency of Medicinal Products and Medical Devices and Ethics Committees for investigation with medicinal products VERSION: 21
Understanding clinical research trials
 Understanding clinical research trials Dr. Isabelle Fleury Medical Hemato-oncologist Hôpital Maisonneuve-Rosemont CIUSSS de l Est-de-l Île-de-Montréal Goals of this presentation Better understand clinical
Understanding clinical research trials Dr. Isabelle Fleury Medical Hemato-oncologist Hôpital Maisonneuve-Rosemont CIUSSS de l Est-de-l Île-de-Montréal Goals of this presentation Better understand clinical
About Clinical Trials
 About Clinical Trials A guide for people with CF and their families INSIDE Who Can Participate in a Clinical Trial? Clinical Research Basics Who Is Involved in Clinical Research? Informed Consent Your
About Clinical Trials A guide for people with CF and their families INSIDE Who Can Participate in a Clinical Trial? Clinical Research Basics Who Is Involved in Clinical Research? Informed Consent Your
Learning about Clinical Trials
 Learning about Clinical Trials A Guide for Individuals and Their Loved Ones INTRODUCTION Clinical trials help researchers answer important medical questions, providing information that may help with the
Learning about Clinical Trials A Guide for Individuals and Their Loved Ones INTRODUCTION Clinical trials help researchers answer important medical questions, providing information that may help with the
PARTNERS HUMAN RESEARCH COMMITTEE. Human Tissues: Brief Primer on Research Use and Requirement for Partners IRB Review
 PARTNERS HUMAN RESEARCH COMMITTEE Human Tissues: Brief Primer on Research Use and Requirement for Partners IRB Review For the purposes of this Primer, tissue is defined as any biological specimen obtained
PARTNERS HUMAN RESEARCH COMMITTEE Human Tissues: Brief Primer on Research Use and Requirement for Partners IRB Review For the purposes of this Primer, tissue is defined as any biological specimen obtained
EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL INVESTIGATIONAL MEDICINAL PRODUCTS' (NIMPS) (REV. 1, MARCH 2011)
 Ref. Ares(2011)300458-18/03/2011 EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL Public health and Risk assessment Pharmaceuticals Brussels, 18/03/2011 SANCO/C/8/SF/cg/a.5.001(2011)332855
Ref. Ares(2011)300458-18/03/2011 EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL Public health and Risk assessment Pharmaceuticals Brussels, 18/03/2011 SANCO/C/8/SF/cg/a.5.001(2011)332855
Completion instructions Comprehensive guarantee authorisation (CGU) and Deferment of payment authorisation (DPO)
 1 (5) 1. APPLICATION Enter the name and code of authorisation(s) applied for: comprehensive guarantee CGU, Deferment of payment DPO. 2. APPLICANT S DETAILS 1.1 Application type Enter the type of application:
1 (5) 1. APPLICATION Enter the name and code of authorisation(s) applied for: comprehensive guarantee CGU, Deferment of payment DPO. 2. APPLICANT S DETAILS 1.1 Application type Enter the type of application:
Pathology and Correlative Science Instructions for NSABP Foundation Protocol FB-11
 Pathology and Correlative Science Instructions for NSABP Foundation Protocol FB-11 A Phase II Randomized Study Evaluating the Biological and Clinical Effects of the Combination of Palbociclib with Letrozole
Pathology and Correlative Science Instructions for NSABP Foundation Protocol FB-11 A Phase II Randomized Study Evaluating the Biological and Clinical Effects of the Combination of Palbociclib with Letrozole
Short Title: CCI Biobank
 Patient Label Stamp or letterhead of the department Patient Information Donation, Storage and Use of Biological Materials and Collection, Processing and Use of Data of the CCI Biobank, Medical Center -
Patient Label Stamp or letterhead of the department Patient Information Donation, Storage and Use of Biological Materials and Collection, Processing and Use of Data of the CCI Biobank, Medical Center -
Demystifying Audits. Audits and Audit Preparation 5/23/2016. What is an Audit?
 Demystifying Audits Darlene Kitterman, MBA Director, Investigator Support & Integration Services OCTRI May 26, 2016 Audits and Audit Preparation What is an Audit? A systematic and independent examination
Demystifying Audits Darlene Kitterman, MBA Director, Investigator Support & Integration Services OCTRI May 26, 2016 Audits and Audit Preparation What is an Audit? A systematic and independent examination
Study Files and Filing
 Study Files and Filing The current version of all Hillingdon Hospital R&D Guidance Documents and Standard Operating Procedures are available from the R&D Intranet and Internet sites: www.ths.nhs.uk/departments/research/research.htm
Study Files and Filing The current version of all Hillingdon Hospital R&D Guidance Documents and Standard Operating Procedures are available from the R&D Intranet and Internet sites: www.ths.nhs.uk/departments/research/research.htm
Billing Contact: Phone: Type of Research: NIH Funded Industry Funded Department Funded Study Name: CTRC Protocol: Start Date: End Date:
 Please submit this application to CPRS@mednet.ucla.edu prior to beginning your research protocol. Please allow up to 2-3 weeks for the processing and approval of this application. All UCLA studies using
Please submit this application to CPRS@mednet.ucla.edu prior to beginning your research protocol. Please allow up to 2-3 weeks for the processing and approval of this application. All UCLA studies using
MTS Real-Time Data Usage Declaration (for Direct Users)
 January 2018 version MTS Real-Time Data Usage Declaration (for Direct Users) 1 of 12 This document is a declaration required by EuroMTS Limited (hereinafter Licensor and/or MTS ) for Direct Users to specify
January 2018 version MTS Real-Time Data Usage Declaration (for Direct Users) 1 of 12 This document is a declaration required by EuroMTS Limited (hereinafter Licensor and/or MTS ) for Direct Users to specify
CADTH COMMON DRUG REVIEW. Procedure for the CADTH Common Drug Review
 CADTH COMMON DRUG REVIEW Procedure for the CADTH Common Drug Review AUGUST 2014 RECORD OF UPDATES TO THE PROCEDURE FOR THE CADTH COMMON DRUG REVIEW Update Original June 2003 1 January 2005 2 July 2005
CADTH COMMON DRUG REVIEW Procedure for the CADTH Common Drug Review AUGUST 2014 RECORD OF UPDATES TO THE PROCEDURE FOR THE CADTH COMMON DRUG REVIEW Update Original June 2003 1 January 2005 2 July 2005
GUIDELINES FOR TISSUE COLLECTION FOR RESEARCH AUTOPSY
 GUIDELINES FOR TISSUE COLLECTION FOR RESEARCH AUTOPSY 1. Informed consent for the above tissue collection can be obtained by: a. Physicians who have completed the CITI/Miami Human Subjects Research Educational
GUIDELINES FOR TISSUE COLLECTION FOR RESEARCH AUTOPSY 1. Informed consent for the above tissue collection can be obtained by: a. Physicians who have completed the CITI/Miami Human Subjects Research Educational
REQUEST FOR RETINOBLASTOMA TESTING
 REQUEST FOR RETINOBLASTOMA TESTING Please provide the following information. We cannot perform your test without ALL of this information. PLEASE PRINT ALL ANSWERS PATIENT INFORMATION* FIRST NAME MI LAST
REQUEST FOR RETINOBLASTOMA TESTING Please provide the following information. We cannot perform your test without ALL of this information. PLEASE PRINT ALL ANSWERS PATIENT INFORMATION* FIRST NAME MI LAST
The Children s Hospital of Philadelphia Department of Pathology and Laboratory Medicine
 TheChildren shospitalofphiladelphia DepartmentofPathologyandLaboratoryMedicine Muscle Biopsy - General Instructions The Division of Neuropathology, Department of Pathology and Laboratory Medicine, Children
TheChildren shospitalofphiladelphia DepartmentofPathologyandLaboratoryMedicine Muscle Biopsy - General Instructions The Division of Neuropathology, Department of Pathology and Laboratory Medicine, Children
Details: Approval: Distribution & Storage:
 Details: Author: Neville Young Quality Assurance Manager SOP Pages: Version No. of replaced SOP: Effective date of replaced SOP: NA NA Approval: Version No: of the SOP being approved. Name of person approving
Details: Author: Neville Young Quality Assurance Manager SOP Pages: Version No. of replaced SOP: Effective date of replaced SOP: NA NA Approval: Version No: of the SOP being approved. Name of person approving
Instruction document of the Spanish Agency of Medicines and Medical Devices for conducting clinical trials in Spain
 DEPARTMENT OF MEDICINAL PRODUCTS FOR HUMAN USE Instruction document of the and Medical Devices for conducting clinical trials in Spain Version 9 dated 22 nd February 2018 Date of publication: 24 April
DEPARTMENT OF MEDICINAL PRODUCTS FOR HUMAN USE Instruction document of the and Medical Devices for conducting clinical trials in Spain Version 9 dated 22 nd February 2018 Date of publication: 24 April
IRB USE ONLY Approval Date: September 10, 2013 Expiration Date: September 10, 2014
 Approval : September 10, 2013 Expiration : September 10, 2014 DESCRIPTION: You are invited to donate your leftover frozen eggs and/or ovarian tissues generated during your fertility preservation treatment
Approval : September 10, 2013 Expiration : September 10, 2014 DESCRIPTION: You are invited to donate your leftover frozen eggs and/or ovarian tissues generated during your fertility preservation treatment
consent. With the exception, such as impossible to obtain informed consent objectively or there is no risk of the clinical trial for subjects, the res
 The Technical Guidelines for In Vitro Diagnostic Reagents Clinical Trial Article 1 In vitro diagnostic reagents clinical trial (include comparison experiment with marketed products) refers to the systematically
The Technical Guidelines for In Vitro Diagnostic Reagents Clinical Trial Article 1 In vitro diagnostic reagents clinical trial (include comparison experiment with marketed products) refers to the systematically
Morgan Memorial Goodwill Industries Running for Great Kids 2015 Boston Marathon Team Application
 1 Morgan Memorial Goodwill Industries Running for Great Kids 2015 Boston Marathon Team Application Applications will be accepted on a rolling basis. Send completed applications to: Erin Flaherty Barfield
1 Morgan Memorial Goodwill Industries Running for Great Kids 2015 Boston Marathon Team Application Applications will be accepted on a rolling basis. Send completed applications to: Erin Flaherty Barfield
NPHL Packaging & Shipping Revisions
 NPHL Packaging & Shipping Revisions Karen Stiles, SM(ASCP) CM State Training Coordinator Assistant Chemical Terrorism Laboratory Coordinator Nebraska Public Health Laboratory 402-559-3590 kstiles@unmc.edu
NPHL Packaging & Shipping Revisions Karen Stiles, SM(ASCP) CM State Training Coordinator Assistant Chemical Terrorism Laboratory Coordinator Nebraska Public Health Laboratory 402-559-3590 kstiles@unmc.edu
Are subsidiaries/affiliates to be included? Yes No If Yes, list names and addresses:
 Instructions for completing this agreement The employer or the employer representative must complete the entire Application form with signature. The agent must sign and date this agreement. A signed copy
Instructions for completing this agreement The employer or the employer representative must complete the entire Application form with signature. The agent must sign and date this agreement. A signed copy
Post-completion OPTIONAL PRACTICAL TRAINING (OPT) Employment authorization following graduation
 Post-completion OPTIONAL PRACTICAL TRAINING (OPT) Employment authorization following graduation Definition: Optional practical training (OPT) is defined in the F-1 regulations as "temporary employment
Post-completion OPTIONAL PRACTICAL TRAINING (OPT) Employment authorization following graduation Definition: Optional practical training (OPT) is defined in the F-1 regulations as "temporary employment
Data Quality and Integrity: From Clinical Monitoring to Marketing Approval
 Data Quality and Integrity: From Clinical Monitoring to Marketing Approval Nancy Detich, Ph.D., C.C.R.P. Senior Scientist, Clinical Strategy 18 November 2010 1 Objectives Identify the importance of accuracy,
Data Quality and Integrity: From Clinical Monitoring to Marketing Approval Nancy Detich, Ph.D., C.C.R.P. Senior Scientist, Clinical Strategy 18 November 2010 1 Objectives Identify the importance of accuracy,
PROTOCOL DRAFTING GUIDE
 MUHC Research Ethics Board (Neurosciences & Psychiatry) Comité d éthique de la recherche du MUHC (Neurosciences & psychiatrie) PROTOCOL DRAFTING GUIDE LIST OF ITEMS TO BE INCLUDED IN A PROTOCL FOR RESEARCH
MUHC Research Ethics Board (Neurosciences & Psychiatry) Comité d éthique de la recherche du MUHC (Neurosciences & psychiatrie) PROTOCOL DRAFTING GUIDE LIST OF ITEMS TO BE INCLUDED IN A PROTOCL FOR RESEARCH
ORGANIZATION AND ROLE OF A PHASE I ONCOLOGY UNIT. Dr Philippe CASSIER Centre Léon Bérard, Lyon
 ORGANIZATION AND ROLE OF A PHASE I ONCOLOGY UNIT Dr Philippe CASSIER Centre Léon Bérard, Lyon Outline Cancer & Oncology Drug development in oncology Specificity of phase I trials in oncology Study design
ORGANIZATION AND ROLE OF A PHASE I ONCOLOGY UNIT Dr Philippe CASSIER Centre Léon Bérard, Lyon Outline Cancer & Oncology Drug development in oncology Specificity of phase I trials in oncology Study design
A.R.K. DRIVE SHIPPING INSTRUCTIONS
 A.R.K. DRIVE SHIPPING INSTRUCTIONS PAY ATTENTION, TAKE YOUR TIME, AND FOCUS! READ ALL INSTRUCTIONS COMPLETELY AND FOLLOW THEM EXACTLY, OR YOU WILL NOT RECEIVE THE A.R.K. DATA! I have extremely valid reasons
A.R.K. DRIVE SHIPPING INSTRUCTIONS PAY ATTENTION, TAKE YOUR TIME, AND FOCUS! READ ALL INSTRUCTIONS COMPLETELY AND FOLLOW THEM EXACTLY, OR YOU WILL NOT RECEIVE THE A.R.K. DATA! I have extremely valid reasons
An information leaflet for individuals with a family history of cancer. DNA Banking Saving your DNA for the future
 An information leaflet for individuals with a family history of cancer DNA Banking Saving your DNA for the future Why have I been given this leaflet? You may have been given this leaflet because you have
An information leaflet for individuals with a family history of cancer DNA Banking Saving your DNA for the future Why have I been given this leaflet? You may have been given this leaflet because you have
Single Unit Mandatory Training Workbook
 Blackpool Teaching Hospitals NHS Foundation Trust NHS Single Unit Mandatory Training Workbook Blood Collection Page 1 IMPORTANT: PLEASE READ Completion of the workbook does not constitute a full competence
Blackpool Teaching Hospitals NHS Foundation Trust NHS Single Unit Mandatory Training Workbook Blood Collection Page 1 IMPORTANT: PLEASE READ Completion of the workbook does not constitute a full competence
Handling and Shipping of Infectious Substances for Research/Clinical Trials
 OFFICE FOR RESEARCH MELBOURNE HEALTH STANDARD OPERATING PROCEDURE: SOP012 Handling and Shipping of Infectious Substances for Research/Clinical Trials Prepared by Sarah Rickard Position Manager of Research
OFFICE FOR RESEARCH MELBOURNE HEALTH STANDARD OPERATING PROCEDURE: SOP012 Handling and Shipping of Infectious Substances for Research/Clinical Trials Prepared by Sarah Rickard Position Manager of Research
Good Clinical Practice. Martin Rose, MD, JD February 8, 2018 ASQ
 Good Clinical Practice Martin Rose, MD, JD February 8, 2018 ASQ Disclaimer The views expressed in this presentation are those of the presenter and do not necessarily represent the official position of
Good Clinical Practice Martin Rose, MD, JD February 8, 2018 ASQ Disclaimer The views expressed in this presentation are those of the presenter and do not necessarily represent the official position of
Protection of Research Participants: The IRB Process and the Winds of Change
 Protection of Research Participants: The IRB Process and the Winds of Change Ethics in Patient-Oriented Research October 12, 2011 Sharon Friend Director, OHRPP Overview Charge and Function of the IRB Quick
Protection of Research Participants: The IRB Process and the Winds of Change Ethics in Patient-Oriented Research October 12, 2011 Sharon Friend Director, OHRPP Overview Charge and Function of the IRB Quick
SWOG ONCOLOGY RESEARCH PROFESSIONAL (ORP) MANUAL STUDY PROTOCOL CHAPTER 14 REVISED: OCTOBER 2015
 THE STUDY PROTOCOL The study protocol is a written document detailing how a clinical trial is conducted. The elements of a protocol include: 1. Trial design and organization; 2. Study objectives; 3. Background
THE STUDY PROTOCOL The study protocol is a written document detailing how a clinical trial is conducted. The elements of a protocol include: 1. Trial design and organization; 2. Study objectives; 3. Background
Clinical Trial Performance Metrics
 Clinical Trial Performance Metrics Clinical trial performance metrics? Data points that provide insight into operational and quality of performance. Objectives of Clinical Trial Performance Metrics The
Clinical Trial Performance Metrics Clinical trial performance metrics? Data points that provide insight into operational and quality of performance. Objectives of Clinical Trial Performance Metrics The
Beryllium Testing Information and Consent Form
 Beryllium Testing Information and Consent Form What is the purpose of the beryllium testing in the NSSP? The main purpose of this beryllium testing is to determine whether or not former Department of Energy
Beryllium Testing Information and Consent Form What is the purpose of the beryllium testing in the NSSP? The main purpose of this beryllium testing is to determine whether or not former Department of Energy
Compassionate Use Navigator Information for Physicians
 Compassionate Use Navigator Information for Physicians Contact: Elena Gerasimov, Program Director, Elena@kidsvcancer.org. As a physician, you must have wished there would be more treatment options for
Compassionate Use Navigator Information for Physicians Contact: Elena Gerasimov, Program Director, Elena@kidsvcancer.org. As a physician, you must have wished there would be more treatment options for
Standard Operating Procedures Guidelines for Good Clinical Practice
 SOP # CRSC-105 Effective Date 10-22-2013 Version # 1 Version Date 7-30-2013 Standard Operating Procedures Guidelines for Good Clinical Practice Purpose: This SOP outlines the steps required to follow FDA
SOP # CRSC-105 Effective Date 10-22-2013 Version # 1 Version Date 7-30-2013 Standard Operating Procedures Guidelines for Good Clinical Practice Purpose: This SOP outlines the steps required to follow FDA
ATTACHMENTS. Attachment 1 "Your Rights under the FMLA of 1993" Notice to be posted of employee rights under FMLA.
 File: GCBE-R ATTACHMENTS Attachment 1 "Your Rights under the FMLA of 1993" Notice to be posted of employee rights under FMLA. Attachment 2 "Certification of Health Care Provider" If requested, employee
File: GCBE-R ATTACHMENTS Attachment 1 "Your Rights under the FMLA of 1993" Notice to be posted of employee rights under FMLA. Attachment 2 "Certification of Health Care Provider" If requested, employee
DIAGNOSE AND TREAT WITH ANTIBODIES THAT RECOGNIZE NATIVE HUMAN PROTEIN EPITOPES IN BLOOD AND TISSUE
 DIAGNOSE AND TREAT WITH ANTIBODIES THAT RECOGNIZE NATIVE HUMAN PROTEIN EPITOPES IN BLOOD AND TISSUE EXECUTIVE SUMMARY Oncologists reviewing blood and tissue test results face the same problems: Is the
DIAGNOSE AND TREAT WITH ANTIBODIES THAT RECOGNIZE NATIVE HUMAN PROTEIN EPITOPES IN BLOOD AND TISSUE EXECUTIVE SUMMARY Oncologists reviewing blood and tissue test results face the same problems: Is the
Order Management. Order is Displayed on Seller Center. Customer Orders an Item. Packing. Shipping. Item Delivered to Customer
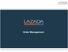 1 Order Management Customer Orders an Item Order is Displayed on Seller Center Packing Shipping Item Delivered to Customer Click Ready to Ship for Delivery Order Management 1. Go to orders 2. Click the
1 Order Management Customer Orders an Item Order is Displayed on Seller Center Packing Shipping Item Delivered to Customer Click Ready to Ship for Delivery Order Management 1. Go to orders 2. Click the
The Importance of Participating in Clinical Trials in Alzheimer s Disease. Amanda G. Smith, M.D. Medical Director USF Health Byrd Alzheimer Institute
 The Importance of Participating in Clinical Trials in Alzheimer s Disease Amanda G. Smith, M.D. Medical Director USF Health Byrd Alzheimer Institute First and foremost Clinical trials and studies are a
The Importance of Participating in Clinical Trials in Alzheimer s Disease Amanda G. Smith, M.D. Medical Director USF Health Byrd Alzheimer Institute First and foremost Clinical trials and studies are a
HOW TO ORDER Revised
 HOW TO ORDER Revised 2015 9 29 How to Order Animal Health Testing Our Mission We have been working hard to get the word out about the risks of zoonotic infection to the veterinary and medical communities
HOW TO ORDER Revised 2015 9 29 How to Order Animal Health Testing Our Mission We have been working hard to get the word out about the risks of zoonotic infection to the veterinary and medical communities
Sea Dwelling Creatures, Inc W. 104 th St. Los Angeles, Ca fax New Customer Check List
 Sea Dwelling Creatures, Inc. 5515 W. 104 th St. Los Angeles, Ca 90045 310 676-9697 fax 310 676-9699 New Customer Check List Dear New Customer, Attached please find all the paperwork required to establish
Sea Dwelling Creatures, Inc. 5515 W. 104 th St. Los Angeles, Ca 90045 310 676-9697 fax 310 676-9699 New Customer Check List Dear New Customer, Attached please find all the paperwork required to establish
Celldex Compassionate Use Policy
 Celldex Compassionate Use Policy Introduction Currently, none of Celldex s investigational products are available for compassionate use given their early stage of clinical development. The policy outlined
Celldex Compassionate Use Policy Introduction Currently, none of Celldex s investigational products are available for compassionate use given their early stage of clinical development. The policy outlined
NON-INTERVENTIONAL STUDY ABSTRACT FOR EXTERNAL DISCLOSURE
 NON-INTERVENTIONAL STUDY ABSTRACT FOR EXTERNAL DISCLOSURE Title: KIMS (Pfizer International Metabolic Database) Date of Abstract: 25 February 2015 Keywords: Growth hormone deficiency, Genotropin, hypopituitarism.
NON-INTERVENTIONAL STUDY ABSTRACT FOR EXTERNAL DISCLOSURE Title: KIMS (Pfizer International Metabolic Database) Date of Abstract: 25 February 2015 Keywords: Growth hormone deficiency, Genotropin, hypopituitarism.
UFCW HRQ F A Q HRQ website:
 UFCW HRQ F A Q HRQ website: www.ufcwhrq.com Contents Health Risk Questionnaire... 3 What is the Health Risk Questionnaire (HRQ... 3 Where do I complete the HRQ?... 3 How long will the HRQ take?... 3 How
UFCW HRQ F A Q HRQ website: www.ufcwhrq.com Contents Health Risk Questionnaire... 3 What is the Health Risk Questionnaire (HRQ... 3 Where do I complete the HRQ?... 3 How long will the HRQ take?... 3 How
HANDLING AND SHIPPING OF INFECTIOUS SUBSTANCES FOR CLINICAL TRIALS
 STANDARD OPERATING PROCEDURE HANDLING AND SHIPPING OF INFECTIOUS SUBSTANCES FOR CLINICAL TRIALS Standard Operating Procedure Western Health SOP reference 012 Version: 2.0 dated December 2015 Effective
STANDARD OPERATING PROCEDURE HANDLING AND SHIPPING OF INFECTIOUS SUBSTANCES FOR CLINICAL TRIALS Standard Operating Procedure Western Health SOP reference 012 Version: 2.0 dated December 2015 Effective
Coverage>100X. Sensitivity>99%. Specificity 100%. NGS
 Coverage>100X. Sensitivity>99%. Specificity 100%. NGS Coverage 15.000 X. Sensitivity 99%. Specificity 100% without false positives. o o Sample requirements and shipping information (solid biopsy) Only
Coverage>100X. Sensitivity>99%. Specificity 100%. NGS Coverage 15.000 X. Sensitivity 99%. Specificity 100% without false positives. o o Sample requirements and shipping information (solid biopsy) Only
2018/2019 final draft National Genomic Test Directory FAQ
 2018/2019 final draft National Genomic Test Directory FAQ August 2018 1 Contents 1 Introduction... 3 2 About the Test Directory... 3 3 Frequently asked questions... 4 3.1 Where has the Test Directory been
2018/2019 final draft National Genomic Test Directory FAQ August 2018 1 Contents 1 Introduction... 3 2 About the Test Directory... 3 3 Frequently asked questions... 4 3.1 Where has the Test Directory been
STUDY INFORMATION Principal Investigator: Phone: Primary Coordinator: Phone:
 Please submit this application to CPRS@mednet.ucla.edu at your earliest convenience, at least 2-3 weeks prior to the beginning of the research for approval. All UCLA studies using patient medical record
Please submit this application to CPRS@mednet.ucla.edu at your earliest convenience, at least 2-3 weeks prior to the beginning of the research for approval. All UCLA studies using patient medical record
Laws and Regulations. Valuation. Assists
 Laws and Regulations When importing merchandise from foreign countries there are certain trade laws and regulations. U.S. Customs oversees the compliance of those laws. Our International Department is
Laws and Regulations When importing merchandise from foreign countries there are certain trade laws and regulations. U.S. Customs oversees the compliance of those laws. Our International Department is
1. INTRODUCTION AND INSTRUCTIONS
 1. INTRODUCTION AND INSTRUCTIONS The School of Medicine HR Compliance Self Assessment As we mentioned in our HRG compliance briefings in vember 2013, here is the School of Medicine HR Compliance Self Assessment
1. INTRODUCTION AND INSTRUCTIONS The School of Medicine HR Compliance Self Assessment As we mentioned in our HRG compliance briefings in vember 2013, here is the School of Medicine HR Compliance Self Assessment
