OPHTHALMOLOGY FIELD ADVISORY COMMITTEE MEETING Virtual Teleconference October 20, :00 PM 3:00 PM EST
|
|
|
- Darlene Sparks
- 6 years ago
- Views:
Transcription
1 OPHTHALMOLOGY FIELD ADVISORY COMMITTEE MEETING Virtual Teleconference October 20, :00 PM 3:00 PM EST Reference/Issue 1:00 Pharmacy EST Dr. Linda Margulies Discussion Guests: Deb Khachikian, PharmD, Kelly Echevarria, PharmD, Pharmacy Benefits Management (PBM) representatives Restasis 1) Patents transferred to the Saint Regis Mohawk tribe to circumvent a patent challenge from generic drug makers. 2) 78,446 prescriptions written for VA patients over last 12 months 3) Court recently invalidated Restasis patents Mohawk-Tribe-Deal-As-Court-Invalidates-Restasis-Patents Intracameral antibiotics at the time of surgery 1) Conference call 8/18/17 with Kelly Echevarria PharmD PBM Program Manager 2) Discussion at Ophthalmology Field Advisory Committiee meeting 10/20/17 a. Discussed the use of intracameral antibiotics and the indications a. Vigamox brand (moxifloxacin) undiluted is directly injected inside the eye at the conclusion of eye surgery b. Vigamox intracameral is commonly used at the VA and in the private sector (Kaiser Permanente has an SOP (J Cataract Refract Surg. 2013; 39: ). Northern California NCHCS and Houston VA and developed SOPs c. Generic moxifloxacin now available for use instead of brand Vigamox d. Local VA pharmacy sites are requesting or mandating ophthalmologists to use the generic drug e. Concerns raised by ophthalmology if the generic moxifloxacin is safe to use intracamerally and requested information and guidance from PBM. f. Ophthalmology discussed generic drugs do not always have the same efficacy and safety profile as brand drugs and mentioned examples. g. One site (Boston) is purchasing Cefuroxxime from local compounding pharmacy for intracameral injections at a low cost. h. Pharmacy discussed safety concerns with compounded medications. Ie. outbreak of meningitis with compounded products was harmful. Hundreds affected with several deaths. i. VA issued guidance in 2014: Veterans Health Administration (VHA) Pharmacy Benefits Management Services (PBM) Guidance for Compounded Sterile Products (CSPs) j. Some data from Europe suggests resistance to vigamox is on the rise 1
2 a. PBM representatives Deb Khachikian, PharmD and Kelly Echevarria, PharmD will investigate further generic versus brand moxifloxacin for safety and efficacy. b. FAC members requested specific written guidelines to the field from PBM c. Interim conference call occurred on October 27, with ophthalmology FAC members and PBM representatives to further discuss safety of using generic moxifloxacin. d. The Interim conference call discussed the following: PBM representatives have contacted Sandoz and determined that moxifloxacin is an Authorized Generic meaning it is produced by Alcon and is the exact same product as the Alcon brand vigamox but marketed under a different label. PBM representatives have requested this information in writing from Sandoz. Ophthalmology requested a delay on mandating the use of generic moxifloxacin at local sites until the ophthalmology concerns are resolved and requested PBM communicate this to the field. PBM will have their VISNs Pharmacy Call on Nov 13 and will discuss the above. A recent TASS outbreak occurred at a VA and Amy Chomsky and the Surgical Advisory Board will investigate further to see if any relationship to intracameral generic vigamox. Links to information on Authorized Generics included newdrugapplicationandagenerics/ucm htm Anti VEGF use in the VA: 1) Some providers wish to use different lot numbers of Anti-VEGF medications when bilateral injections are done 2) Ophthalmologists are unable to obtain different lot numbers from local VA Pharmacy and from vendors. 3) VA Evidence-based Synthesis Program: Comparative Clinical and Economic Effectiveness of Anti-VEGF Agents completed: Results were a. No clear, consistent, clinically meaningful differences between anti-vegf drugs were found for most effectiveness and harms outcomes. b. Aflibercept may provide a greater visual acuity benefit than the other agents among patients with low baseline visual acuity over the short-term, but the longer-term findings are unclear and more trials assessing the effects of aflibercept are needed. c. Compounded bevacizumab treatment is likely to be the most cost-effective of the 3 drugs. d. In choosing amongst these drugs, clinicians may also need to consider factors such as patient preference, 2
3 individual treatment response, convenience, and distance to facility. 4) No new update regarding guidance from FDA for aliquoted Avastin (FDA USP 797, Compounding Pharmacies). FDA document is still in draft form, but latest 2017 draft changed duration of time for aliquoted Avastin to 8 hours post puncture. 5) Currently, due to FDA draft guidelines, the VA policy remains at one vial of avastin is used per patient. a. PBM representatives Debbie Khachikian, PharmD will research the issue of obtaining different lot numbers of Anti-VEGF medications for bilateral injections b. PBM will continue to monitor FDA draft guidelines for avastin use. Omidria 1) Alternative is intracameral PF epinephrine a. Recent Shortage of epinephrine -- b. Potential for mix up with epinephrine (re: Par Pharmaceutical adding tartaric acid to formulation) c. VA now has epinephrine (NDC # ) product that can be used in intraocular surgeries. 2) Alternative is pupil expansion ring 3) Increasing use of toric IOLs --- need for continued dilation late into surgical procedure 4) Alpha agonist use in Veteran population may be higher than community 5) Recent articles (2016 & 2017) since PBM review show need for malyugan rings decreased 8% to 3% with Omidria. Though new data showed low level evidence retrospective chart reviews and omidria administered by unapproved method of intracameral injection instead of irrigation a. J Cataract Refract Surg 2017; 43: b. Patient Preference and Adherence 2016: c. Clinical Ophthalmology 2017: a. PBM awaiting several other retrospective studies with manuscripts in preparation b. PBM planning to re-review Omidria in 2018 for possible formulary addition once manuscripts available Lifitegrast 5.0% Ophthalmic Solution (XIIDRA) 1) New medication for dry eye disease 2) PBM completed monograph review. 3) High cost $320/month 3
4 a. Lifitegrast was not added to VA formulary and is available as NFDR Avenova 1) Is a non detergent base spray product for eyelid and lash hygiene that removes microorganisms and debris for dry eyes and blepharitis 2) Patients are requesting this item at the VA via their private or VCP ophthalmologist a. PBM representative Debbie Khachikian, PharmD will research this item further to see what information is available and if a drug monograph should be done Ozurdex Implant 1) Has been added to national VA formulary in 2017 with prior authorization criteria that was agreed upon by the ophthalmology Field Advisory Group. a. Ophthalmologists may need to check with their local pharmacy sites, if Ozurdex does not appear on their local formulary menu. Quick orders may be established at local sites to make ordering easier and to meet the criteria for use. Olopatadine 0.7% (Pazeo) 1) Added to the national VA formulary in day expiration of multi-use dilating eyedrops 1) Request from Wiley Chambers to PBM: This is a request to amend VHA Directive by deleting section 15.b.9. The request is being made to remove a potential safety hazard to patients. Section 15.b.9. currently reads Use of multi-dose products stored in ward stock centralized areas and outpatient surgical and procedure clinics must be labeled with an expiration date upon opening or entering the multi-dose product (e.g., to include but not limited to parenterals, insulin, ophthalmic drops) that does not exceed 28 days; or a shorter expiration date when recommended by the manufacturer. 2) Response from Puri Subramaniam: The that the current VHA Directive is based on concurrence by Ophthalmology on the 28 day limitation any request for a change would need to come from [FAC] since [FAC] agreed to the current policy. 3) Wiley maintains that USP <797> does not apply to the use of approved ophthalmic drug products used as labeled 4
5 Puri Subramaniam Pharmd D is the USP expert and any changes would need to be initiated through the clinical group. a. Additional discussion occurred at the follow up interim meeting on October 27, 2017 with PBM representatives and Ophthalmology. Joint Commission Standards, reference material, and VA policy were reviewed. b. Members agreed that the 28 day expiration of multii-use dilating drops made sense for patient safety and would not request a change to the policy at this time. JAMA Ophthalmol. 2014;132(12): doi: /jamaophthalmol Handling post-op meds 1) No consistent practice. Some solutions in the field: a. Patient picks up post-op meds on POD 1 at the Pharmacy b. Order sets created for cataract surgery. Antibiotics and steroids sent to OR from Pharmacy for each patient. Dispensed immediately at surgery and placed in Eye Kit for patients to take home. c. Pre/post op drops mailed to patients prior to surgery. Patient brings drops on the day of surgery. The RN uses the antibiotic, but uses dilation drops from their pixis med unit. The RN then returns the antibiotics back to the Vet to use post op. a. PBM is not involved in how facilities handle posts op medications and this would need to be addressed at the local sites. Compounding Pharmacies 1) Leiter s will have both a 503A and a 503B pharmacy as part of their organization. 2) VA given the green light to use Burderer for EDTA per a representative from the National compounding group as long as we verify that they are not doing batch compounding and that we have their recipe for making this product. Lidocaine Gel 1) Dermatology products such as Akorn s lidocaine 2% jelly, while required to have a low bioburden, are not required to be sterile. This product is not approved for ophthalmic use 2) Akten (lidocaine hydrochloride ophthalmic gel) 3.5% is sterile and approved for ocular surface anesthesia during ophthalmic procedures. 5
6 Miscellaneous Items 1) Discussion occurred as to the PBM process and mechanisms for Ophthalmology FAC members to be involved with reviewing monographs and PBM decisions. 2) Sandoz Prednisolone --- reports from field that less effective than brand prednisolone inadvertent inflammation 3) Zirgan use restriction dropped. Had been restricted as second line therapy to Ophthalmology. 4) Difluprednate (Durezol) criteria for use changed. No longer restricted to Ophthalmology for recalcitrant inflammation. PBM Web Site Links 1) To view the ophthalmic formulary according to drug class, use this link and search for Ophthalmology with OP. Spreadsheets)/VA%20National%20Formulary-by%20class-July%202016(2).xls 2) For monographs or criteria for nonformulary drugs through the formulary drug listing., go to clinical guidance tab on the SharePoint site then select the Drug Monographs or Criteria for Use tabs. Drugs are listed alphabetically. 6
AMENDED Draft Report of the Compounding Workgroup
 AMENDED Draft Report of the Compounding Workgroup This report summarizes the actions taken by a workgroup convened by the Board of Pharmacy pursuant to the enactment clause of HB 1035 passed during the
AMENDED Draft Report of the Compounding Workgroup This report summarizes the actions taken by a workgroup convened by the Board of Pharmacy pursuant to the enactment clause of HB 1035 passed during the
CORPORATE PRESENTATION January 2019
 CORPORATE PRESENTATION January 2019 1 DISCLAIMER This presentation contains forward-looking statements about Outlook Therapeutics, Inc. ( Outlook Therapeutics or the Company ) based on management s current
CORPORATE PRESENTATION January 2019 1 DISCLAIMER This presentation contains forward-looking statements about Outlook Therapeutics, Inc. ( Outlook Therapeutics or the Company ) based on management s current
Quality is Our Promise.
 Quality is Our Promise. Our goal at KRS Global Biotechnology is to provide the highest quality pharmaceutical preparations. We accomplish this with an unrivaled quality assurance and quality control program
Quality is Our Promise. Our goal at KRS Global Biotechnology is to provide the highest quality pharmaceutical preparations. We accomplish this with an unrivaled quality assurance and quality control program
Part 7: Clinical investigations
 Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 11979-7 Third edition 2014-09-01 Ophthalmic implants Intraocular lenses Part 7: Clinical investigations Implants ophtalmiques Lentilles intraoculaires
Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 11979-7 Third edition 2014-09-01 Ophthalmic implants Intraocular lenses Part 7: Clinical investigations Implants ophtalmiques Lentilles intraoculaires
psivida Transforms into Commercial Stage Specialty BioPharmaceutical Company ASCRS April 12, 2018 NASDAQ: EYPT
 psivida Transforms into Commercial Stage Specialty BioPharmaceutical Company OIS @ ASCRS April 12, 2018 NASDAQ: EYPT Forward Looking SAFE HARBOR STATEMENTS UNDER THE PRIVATE SECURITIES LITIGATION REFORM
psivida Transforms into Commercial Stage Specialty BioPharmaceutical Company OIS @ ASCRS April 12, 2018 NASDAQ: EYPT Forward Looking SAFE HARBOR STATEMENTS UNDER THE PRIVATE SECURITIES LITIGATION REFORM
Microbiology Testing: USP requirements for Sterile and Nonsterile Preparations Webinar Q&A
 USP Antimicrobial Effectiveness Testing Microbiology Testing: Webinar Q&A 1. If a base ingredient is designed for the purpose of compounding a specific type of preparation does this forgo the necessity
USP Antimicrobial Effectiveness Testing Microbiology Testing: Webinar Q&A 1. If a base ingredient is designed for the purpose of compounding a specific type of preparation does this forgo the necessity
Pharmaceutical Compounding Sterile and Nonsterile Preparations. Jeanne Sun, PharmD
 Pharmaceutical Compounding Sterile and Nonsterile Preparations Jeanne Sun, PharmD Agenda Legal Framework Overview Pharmaceutical Compounding Nonsterile Preparations Pharmaceutical Compounding
Pharmaceutical Compounding Sterile and Nonsterile Preparations Jeanne Sun, PharmD Agenda Legal Framework Overview Pharmaceutical Compounding Nonsterile Preparations Pharmaceutical Compounding
COMPOUND MEDICATION NON-FORMULARY (QUALIFIED HEALTH PLANS)
 COMPOUND MEDICATION NON-FORMULARY (QUALIFIED HEALTH PLANS) Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit
COMPOUND MEDICATION NON-FORMULARY (QUALIFIED HEALTH PLANS) Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit
Overview. A brief from
 A brief from Dec 2017 Getty Images What Are Compounded Drugs, and How Can They Be Kept Safe? Drug Quality and Security Act plays key role in important but potentially high-risk aspect of health care Overview
A brief from Dec 2017 Getty Images What Are Compounded Drugs, and How Can They Be Kept Safe? Drug Quality and Security Act plays key role in important but potentially high-risk aspect of health care Overview
CGMP Requirements for Investigational Products
 PREP #6 CGMP Requirements for Investigational Products Ji-Eun Kim, RPh, PhD Research Pharmacist Regulatory Affairs Office of Research Compliance December 6, 2016 1 CME Disclosure Statement Northwell Health
PREP #6 CGMP Requirements for Investigational Products Ji-Eun Kim, RPh, PhD Research Pharmacist Regulatory Affairs Office of Research Compliance December 6, 2016 1 CME Disclosure Statement Northwell Health
I. Purpose. II. Definitions. Last Approval Date
 Investigational Drugs and Biologics Page 1 of 13 I. Purpose The purpose of this policy is to establish procedures for the proper control, storage, use and handling of investigational drugs and biologics
Investigational Drugs and Biologics Page 1 of 13 I. Purpose The purpose of this policy is to establish procedures for the proper control, storage, use and handling of investigational drugs and biologics
Testimony of Molly Ventrelli Vice President, Regulatory Affairs Fresenius Kabi USA, LLC
 Testimony of Molly Ventrelli Vice President, Regulatory Affairs Fresenius Kabi USA, LLC U.S. House of Representatives Energy and Commerce Committee Subcommittee on Health Subcommittee January 30, 2018
Testimony of Molly Ventrelli Vice President, Regulatory Affairs Fresenius Kabi USA, LLC U.S. House of Representatives Energy and Commerce Committee Subcommittee on Health Subcommittee January 30, 2018
Learning Objectives. What is a Compounding Pharmacist? Disclosure
 PHARMACY COMPOUNDING: Update on Trends & Regulations Michael Roberge, RPh Certified Compounding Pharmacist Owner, Compounded Solutions in Pharmacy LLC Monroe, CT Learning Objectives Define pharmacy compounding
PHARMACY COMPOUNDING: Update on Trends & Regulations Michael Roberge, RPh Certified Compounding Pharmacist Owner, Compounded Solutions in Pharmacy LLC Monroe, CT Learning Objectives Define pharmacy compounding
Cancer Vanguard. Biosimilars Trust Policy Template
 Cancer Vanguard Biosimilars Trust Policy Template Aim of this document: The document provides generic guidance and outline for the development of local trust policies in relation to the adoption of biosimilars
Cancer Vanguard Biosimilars Trust Policy Template Aim of this document: The document provides generic guidance and outline for the development of local trust policies in relation to the adoption of biosimilars
Where Quality Meets Flexibility
 Where Quality Meets Flexibility is an industry leading 503B Outsourcing Facility providing sterile and non-sterile compounding services to hospitals, surgery centers, clinics, researchers & patients nationwide.
Where Quality Meets Flexibility is an industry leading 503B Outsourcing Facility providing sterile and non-sterile compounding services to hospitals, surgery centers, clinics, researchers & patients nationwide.
Regulation of Biosimilars in Canada
 Regulation of Biosimilars in Canada Session 2: Global Regulatory Trends of Biosimilars GBC 2018 June 28, 2018 Stephanie Hardy Office of Policy and International Collaboration Biologics and Genetic Therapies
Regulation of Biosimilars in Canada Session 2: Global Regulatory Trends of Biosimilars GBC 2018 June 28, 2018 Stephanie Hardy Office of Policy and International Collaboration Biologics and Genetic Therapies
Pain Full Drug Shortages
 Pain Full Drug Shortages Barbara Higgins, Pharm.D Assistant Director Pharmacy, Medication Use Systems University of Michigan Hospitals and Health System Ann Arbor, MI Objectives Describe actions taken
Pain Full Drug Shortages Barbara Higgins, Pharm.D Assistant Director Pharmacy, Medication Use Systems University of Michigan Hospitals and Health System Ann Arbor, MI Objectives Describe actions taken
Emmett T. Cunningham, Jr., M.D., PhD., M.P.H
 ICO World Ophthalmology Roundtable on Leadership Development (WORLD) Five Trends in Ophthalmic Innovation Poised to Impact Global Eye Health Emmett T. Cunningham, Jr., M.D., PhD., M.P.H Department of Ophthalmology,
ICO World Ophthalmology Roundtable on Leadership Development (WORLD) Five Trends in Ophthalmic Innovation Poised to Impact Global Eye Health Emmett T. Cunningham, Jr., M.D., PhD., M.P.H Department of Ophthalmology,
Why Do I Test, What Do I Test & When Do I Test It? Ross Caputo, PhD Chief Technical Officer Eagle Analytical Services
 Why Do I Test, What Do I Test & When Do I Test It? Ross Caputo, PhD Chief Technical Officer Eagle Analytical Services Disclosures I, Ross Caputo, declare no conflicts of interest, real or apparent, and
Why Do I Test, What Do I Test & When Do I Test It? Ross Caputo, PhD Chief Technical Officer Eagle Analytical Services Disclosures I, Ross Caputo, declare no conflicts of interest, real or apparent, and
Review of Medicare Part B Avastin and Lucentis Treatments for Age-Related Macular Degeneration (A )
 DEPARTMENT OF HEALTH & HUMAN SERVICES Office of Inspector General Washington, D.C. 20201 September 6, 2011 TO: Donald M. Berwick, M.D. Administrator Centers for Medicare & Medicaid Services FROM: /Daniel
DEPARTMENT OF HEALTH & HUMAN SERVICES Office of Inspector General Washington, D.C. 20201 September 6, 2011 TO: Donald M. Berwick, M.D. Administrator Centers for Medicare & Medicaid Services FROM: /Daniel
Sterile Compounding elearning Course Curriculum 28 lessons with 33 hours of CE. Fundamentals of Sterile Compounding (8 lessons/8 hours CE)
 Sterile Compounding elearning Course Curriculum 28 lessons with 33 hours of CE Fundamentals of Sterile Compounding (8 lessons/8 hours CE) The History of Compounding and USP Sterile Compounding Chapters
Sterile Compounding elearning Course Curriculum 28 lessons with 33 hours of CE Fundamentals of Sterile Compounding (8 lessons/8 hours CE) The History of Compounding and USP Sterile Compounding Chapters
2016 Glaukos Corporation. August 2016
 August 2016 DISCLAIMER All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will
August 2016 DISCLAIMER All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will
Harmonizing FDA Regulation and the Practice of Pharmacy: Challenges and Opportunities. Plus an update on PET Drug User Fees
 Harmonizing FDA Regulation and the Practice of Pharmacy: Challenges and Opportunities Plus an update on PET Drug User Fees Michael Nazerias - Vice President, RA/QA PETNET Solutions, Inc. (a Siemens Company)
Harmonizing FDA Regulation and the Practice of Pharmacy: Challenges and Opportunities Plus an update on PET Drug User Fees Michael Nazerias - Vice President, RA/QA PETNET Solutions, Inc. (a Siemens Company)
FDA Update on Compounding
 FDA Update on Compounding Julie Dohm, JD, PhD Senior Science Advisor for Compounding, Center for Drug Evaluation and Research; Agency Lead on Compounding, FDA Compounding A Snapshot Compounded drugs: Are
FDA Update on Compounding Julie Dohm, JD, PhD Senior Science Advisor for Compounding, Center for Drug Evaluation and Research; Agency Lead on Compounding, FDA Compounding A Snapshot Compounded drugs: Are
Santen Acquisition of InnFocus, Developer of MicroShunt Glaucoma Implant Device
 Santen Acquisition of InnFocus, Developer of MicroShunt Glaucoma Implant Device Akira Kurokawa President & CEO August 2, 2016 Copyright 2016 Santen Pharmaceutical Co., Ltd. All rights reserved. 1 External
Santen Acquisition of InnFocus, Developer of MicroShunt Glaucoma Implant Device Akira Kurokawa President & CEO August 2, 2016 Copyright 2016 Santen Pharmaceutical Co., Ltd. All rights reserved. 1 External
Investigator s Handbook
 Page 96 CHAPTER 11 Investigational Drugs, Agents, Biologics, and Devices Investigational Drugs/Investigational Biologics (Test Articles) A new drug/agent or biologic that is used in a clinical investigation.
Page 96 CHAPTER 11 Investigational Drugs, Agents, Biologics, and Devices Investigational Drugs/Investigational Biologics (Test Articles) A new drug/agent or biologic that is used in a clinical investigation.
FDA s Bar Code Label Requirements for Human Drug Products. General Questions Related to Drugs and Biologics
 FDA s Bar Code Label Requirements for Human Drug Products General Questions Related to Drugs and Biologics 1. Which medical products should carry a bar code? For example, should all prescription and over-the-counter
FDA s Bar Code Label Requirements for Human Drug Products General Questions Related to Drugs and Biologics 1. Which medical products should carry a bar code? For example, should all prescription and over-the-counter
Ensuring Quality Pharmacy Compounding: Implications for Pharmaceutics Education
 Ensuring Quality Pharmacy Compounding: Implications for Pharmaceutics Education Loyd V. Allen, Jr., Ph.D. Professor & Chair Emeritus University of Oklahoma HSC College of Pharmacy Editor-in in-chief International
Ensuring Quality Pharmacy Compounding: Implications for Pharmaceutics Education Loyd V. Allen, Jr., Ph.D. Professor & Chair Emeritus University of Oklahoma HSC College of Pharmacy Editor-in in-chief International
October 11, I. Interpretation of Commercially Available
 [Submitted electronically to www.regulations.gov] Division of Dockets Management (HFA-305) U.S. Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Re: FDA Docket FDA-2016-D-1309
[Submitted electronically to www.regulations.gov] Division of Dockets Management (HFA-305) U.S. Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Re: FDA Docket FDA-2016-D-1309
Sterile Compounding elearning Course Curriculum 28 lessons with 33 hours of CE. Fundamentals of Sterile Compounding (8 lessons/8 hours CE)
 Sterile Compounding elearning Course Curriculum 28 lessons with 33 hours of CE CriticalPoint s Sterile Compounding elearning curriculum is written by industry experts and covers both Chapter and
Sterile Compounding elearning Course Curriculum 28 lessons with 33 hours of CE CriticalPoint s Sterile Compounding elearning curriculum is written by industry experts and covers both Chapter and
Infection Prevention for Pharmacy Compounding
 Infection Prevention for Pharmacy Compounding Samuel M. Eberwein, PharmD, MS, BCPS November 5, 2018 Infection Prevention Starts with Regulation Compounding Regulation has a Rich History 2019 USP
Infection Prevention for Pharmacy Compounding Samuel M. Eberwein, PharmD, MS, BCPS November 5, 2018 Infection Prevention Starts with Regulation Compounding Regulation has a Rich History 2019 USP
Infection and Intravenous Drug Delivery: A Long and Tumultuous Relationship
 Infection and Intravenous Drug Delivery: A Long and Tumultuous Relationship Eric Wenzler, PharmD, BCPS, AAHVIP Assistant Professor University of Illinois at Chicago Disclosures I have no actual or potential
Infection and Intravenous Drug Delivery: A Long and Tumultuous Relationship Eric Wenzler, PharmD, BCPS, AAHVIP Assistant Professor University of Illinois at Chicago Disclosures I have no actual or potential
Gaining Momentum in Gene Therapy
 Gaining Momentum in Gene Therapy Corporate Deck March 6, 2019 Forward-looking Statements Statements contained in this document regarding matters, events, statistics, or clinical or financial results that
Gaining Momentum in Gene Therapy Corporate Deck March 6, 2019 Forward-looking Statements Statements contained in this document regarding matters, events, statistics, or clinical or financial results that
Compounding Unit-of-Use Kits
 Compounding Unit-of-Use Kits White Paper by Loyd V. Allen PhD, RPh Professor Emeritus, College of Pharmacy, University of Oklahoma Former Chairperson, USP Pharmacy Compounding Expert Committee Editor in
Compounding Unit-of-Use Kits White Paper by Loyd V. Allen PhD, RPh Professor Emeritus, College of Pharmacy, University of Oklahoma Former Chairperson, USP Pharmacy Compounding Expert Committee Editor in
Drug Products, Labeling, and Packaging
 442 Pharmaceutical Industry: Drug Products, Labeling, and Packaging Positions Drug Products, Labeling, and Packaging Ready-to-Administer Packaging for Hazardous Drug Products Intended for Home Use (1711)
442 Pharmaceutical Industry: Drug Products, Labeling, and Packaging Positions Drug Products, Labeling, and Packaging Ready-to-Administer Packaging for Hazardous Drug Products Intended for Home Use (1711)
XYLOCAINE National Drug Code Directory
 63323-489-27 XYLOCAINE National Drug Code Directory The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration (FDA) with a current list of all drugs
63323-489-27 XYLOCAINE National Drug Code Directory The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration (FDA) with a current list of all drugs
OBG: The Ocular Liquid Bandage A crosslinked hyaluronic acid (CMHA-S) eye drop for corneal wounds and epitheliopathies, including dry eye NASDAQ: EYEG
 OBG: The Ocular Liquid Bandage A crosslinked hyaluronic acid (CMHA-S) eye drop for corneal wounds and epitheliopathies, including dry eye NASDAQ: EYEG Follow us on Facebook, LinkedIn and Twitter EyeGate
OBG: The Ocular Liquid Bandage A crosslinked hyaluronic acid (CMHA-S) eye drop for corneal wounds and epitheliopathies, including dry eye NASDAQ: EYEG Follow us on Facebook, LinkedIn and Twitter EyeGate
BUPIVACAINE HYDROCHLORIDE AND EPINEPHRINE National Drug Code Directory
 0409-9046-01 BUPIVACAINE HYDROCHLORIDE AND EPINEPHRINE National Drug Code Directory The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration (FDA)
0409-9046-01 BUPIVACAINE HYDROCHLORIDE AND EPINEPHRINE National Drug Code Directory The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration (FDA)
Ophthalmic implants Intraocular lenses. Part 7: Clinical investigations of intraocular lenses for the correction of aphakia
 Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 11979-7 Fourth edition 2018-03 Ophthalmic implants Intraocular lenses Part 7: Clinical investigations of intraocular lenses for the correction
Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 11979-7 Fourth edition 2018-03 Ophthalmic implants Intraocular lenses Part 7: Clinical investigations of intraocular lenses for the correction
INVESTIGATIONAL DRUG MANAGEMENT OVERVIEW
 NORTHWELL HEALTH OFFICE OF RESEARCH COMPLIANCE STUDY FEASIBILITY AND APPROVAL Where can I learn about the investigational drug? Protocol Investigator s brochure Product insert or prescribing information
NORTHWELL HEALTH OFFICE OF RESEARCH COMPLIANCE STUDY FEASIBILITY AND APPROVAL Where can I learn about the investigational drug? Protocol Investigator s brochure Product insert or prescribing information
Profiles in CSP Insourcing: Scripps Health
 Profiles in CSP Insourcing: Scripps Health Robert Eastin, Pharm.D. Director, Central Pharmacy and Shared Services Scripps Health Hospital Profile Scripps Health is a private, nonprofit integrated health
Profiles in CSP Insourcing: Scripps Health Robert Eastin, Pharm.D. Director, Central Pharmacy and Shared Services Scripps Health Hospital Profile Scripps Health is a private, nonprofit integrated health
Fresenius Kabi Compounding Canton, MA
 Fresenius Kabi Compounding Canton, MA Fresenius Kabi - Boston Compounding Andy Basso Operations Director Agenda Existential question: Why am I here? Who is Fresenius-Kabi? What does our Canton Facility
Fresenius Kabi Compounding Canton, MA Fresenius Kabi - Boston Compounding Andy Basso Operations Director Agenda Existential question: Why am I here? Who is Fresenius-Kabi? What does our Canton Facility
2017 Glaukos Corporation. August 2017
 August 2017 DISCLAIMER All statements other than statements of historical facts included in this presentation that address activities, events or developments that we expect, believe or anticipate will
August 2017 DISCLAIMER All statements other than statements of historical facts included in this presentation that address activities, events or developments that we expect, believe or anticipate will
CADTH COMMON DRUG REVIEW. Procedure for the CADTH Common Drug Review
 CADTH COMMON DRUG REVIEW Procedure for the CADTH Common Drug Review AUGUST 2014 RECORD OF UPDATES TO THE PROCEDURE FOR THE CADTH COMMON DRUG REVIEW Update Original June 2003 1 January 2005 2 July 2005
CADTH COMMON DRUG REVIEW Procedure for the CADTH Common Drug Review AUGUST 2014 RECORD OF UPDATES TO THE PROCEDURE FOR THE CADTH COMMON DRUG REVIEW Update Original June 2003 1 January 2005 2 July 2005
Received: ; Accepted: HOME PARENTERAL NUTRITION IN STERILE PRODUCTS. SenthilKumar Krishnan *, D. R.Nagesh
 International Journal of Universal Pharmacy and Bio Sciences 1(2): November-December2012 INTERNATIONAL JOURNAL OF UNIVERSAL PHARMACY AND BIO SCIENCES Pharmaceutical Sciences Review Article!!! Received:
International Journal of Universal Pharmacy and Bio Sciences 1(2): November-December2012 INTERNATIONAL JOURNAL OF UNIVERSAL PHARMACY AND BIO SCIENCES Pharmaceutical Sciences Review Article!!! Received:
Gaining Momentum in Gene Therapy
 Gaining Momentum in Gene Therapy Corporate Presentation November 2018 Forward-looking Statements Statements contained in this document regarding matters, events, statistics, or clinical or financial results
Gaining Momentum in Gene Therapy Corporate Presentation November 2018 Forward-looking Statements Statements contained in this document regarding matters, events, statistics, or clinical or financial results
September 2, Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm Rockville, MD 20852
 September 2, 2014 Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Re: FDA Draft Guidance: Current Good Manufacturing Practice Interim
September 2, 2014 Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Re: FDA Draft Guidance: Current Good Manufacturing Practice Interim
The Right Prescription for Working with Investigational Drug Service at BMC
 The Right Prescription for Working with Investigational Drug Service at BMC Andrew Schoch Pharmacy Intern Northeastern University Class of 2010 Hyeseon Hong, Pharm.D. IDS Pharmacy Manager What is Investigational
The Right Prescription for Working with Investigational Drug Service at BMC Andrew Schoch Pharmacy Intern Northeastern University Class of 2010 Hyeseon Hong, Pharm.D. IDS Pharmacy Manager What is Investigational
Our vision APL
 Our vision We want be the most attractive partner and workplace for development and manufacturing of extemporaneous drug formulations and pharmaceuticals. APL 2018 2 Our task Our task is to develop and
Our vision We want be the most attractive partner and workplace for development and manufacturing of extemporaneous drug formulations and pharmaceuticals. APL 2018 2 Our task Our task is to develop and
Pharmacy Compounding: Infection Prevention
 Pharmacy Compounding: Infection Prevention Sam Eberwein, PharmD, MS, BCPS Clinical Manager, Sterile Products Area, Perioperative Services, & Special Formulations UNC Medical Center Department of Pharmacy
Pharmacy Compounding: Infection Prevention Sam Eberwein, PharmD, MS, BCPS Clinical Manager, Sterile Products Area, Perioperative Services, & Special Formulations UNC Medical Center Department of Pharmacy
Extending Beyond Use Dating for Compounded Preparations Webinar Q&A
 Stability Studies 1. If potency over time studies are not acceptable to extend BUD, then why is it offered? Potency point-in-time studies only indicate the potency of a compounded preparation at that specific
Stability Studies 1. If potency over time studies are not acceptable to extend BUD, then why is it offered? Potency point-in-time studies only indicate the potency of a compounded preparation at that specific
Interim Policy on Compounding Using Bulk Drug Substances Under Section 503B of the Federal Food, Drug, and Cosmetic Act
 Interim Policy on Compounding Using Bulk Drug Substances Under Section 503B of the Federal Food, Drug, and Cosmetic Act Guidance for Industry U.S. Department of Health and Human Services Food and Drug
Interim Policy on Compounding Using Bulk Drug Substances Under Section 503B of the Federal Food, Drug, and Cosmetic Act Guidance for Industry U.S. Department of Health and Human Services Food and Drug
Extending Beyond Use Dating for Compounded Preparations Webinar Q&A
 Stability Studies 1. If potency over time studies are not acceptable to extend BUD, then why is it offered? Potency point-in-time studies only indicate the potency of a compounded preparation at that specific
Stability Studies 1. If potency over time studies are not acceptable to extend BUD, then why is it offered? Potency point-in-time studies only indicate the potency of a compounded preparation at that specific
Next Generation Eye Care
 Next Generation Eye Care Company Overview Ocular Science is an innovative biotech and pharmaceutical company that offers paradigm-shifting eye care products to physicians and their patients in a cost-effective
Next Generation Eye Care Company Overview Ocular Science is an innovative biotech and pharmaceutical company that offers paradigm-shifting eye care products to physicians and their patients in a cost-effective
Contents 1. OVERVIEW APPLICABILITY TRIAL INFORMATION PHARMACY REGISTRATION & SET-UP PHARMACY ACTIVATION PATI
 CONTACT DETAILS For further information on trial drugs, trial protocol, dosing, drug supply and distribution, please contact: Trial Coordinator Name: Trial Coordinator Phone: 020 7679 9860 Email: ctc.animate@ucl.ac.uk
CONTACT DETAILS For further information on trial drugs, trial protocol, dosing, drug supply and distribution, please contact: Trial Coordinator Name: Trial Coordinator Phone: 020 7679 9860 Email: ctc.animate@ucl.ac.uk
Pharmacy. Medication. Checks
 Pharmacy Medication Checks Module 2 Table of Contents Section A... 1 A.1 Clinical Medication Order Check... 1 A.2 Final Product and Computer Order Entry Check... 1 Section B... 2 B.1 Documentation of Pharmacy
Pharmacy Medication Checks Module 2 Table of Contents Section A... 1 A.1 Clinical Medication Order Check... 1 A.2 Final Product and Computer Order Entry Check... 1 Section B... 2 B.1 Documentation of Pharmacy
PREVAIL Partnership for Research on Ebola Virus in Liberia
 PREVAIL Partnership for Research on Ebola Virus in Liberia DEPLOYMENT EXPERIENCE LIBERIA, AFRICA 2018 Holly Van Lew, Pharm.D., BCPS Advance Practice Pharmacist Indian Health Service Phoenix Indian Medical
PREVAIL Partnership for Research on Ebola Virus in Liberia DEPLOYMENT EXPERIENCE LIBERIA, AFRICA 2018 Holly Van Lew, Pharm.D., BCPS Advance Practice Pharmacist Indian Health Service Phoenix Indian Medical
CEOCFO Magazine. Keith Ignotz President & CEO
 CEOCFO Magazine ceocfointerviews.com All rights reserved Issue: January 28, 2019 OcuMedic has Conquered the Ability to Deliver Drugs from a Contact Lens that has Eluded the Industry for 50 Years Replacing
CEOCFO Magazine ceocfointerviews.com All rights reserved Issue: January 28, 2019 OcuMedic has Conquered the Ability to Deliver Drugs from a Contact Lens that has Eluded the Industry for 50 Years Replacing
Corporate Presentation February 2016
 Corporate Presentation February 2016 Issuer Free Writing Prospectus dated February 25, 2016 Filed Pursuant to Rule 433 Registration Statement No. 333 209454 Forward Looking Statements This presentation
Corporate Presentation February 2016 Issuer Free Writing Prospectus dated February 25, 2016 Filed Pursuant to Rule 433 Registration Statement No. 333 209454 Forward Looking Statements This presentation
Compounding Problems: 17-Hydroxprogesterone Caproate (17Pc)
 Compounding Problems: 17-Hydroxprogesterone Caproate (17Pc) C Andrew Combs MD PhD Obstetrix Medical Group, San Jose, California Disclosure of Grant Support: I am the PI of an Obstetrix trial of 17Pc for
Compounding Problems: 17-Hydroxprogesterone Caproate (17Pc) C Andrew Combs MD PhD Obstetrix Medical Group, San Jose, California Disclosure of Grant Support: I am the PI of an Obstetrix trial of 17Pc for
Guidance for Industry Compounding Animal Drugs from Bulk Drug Substances
 #230 Guidance for Industry Compounding Animal Drugs from Bulk Drug Substances DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this
#230 Guidance for Industry Compounding Animal Drugs from Bulk Drug Substances DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this
Inspections, Compliance, Enforcement, and Criminal Investigations
 Home Inspections, Compliance, Enforcement, and Criminal Investigations Enforcement Actions Warning Letters Inspections, Compliance, Enforcement, and Criminal Investigations Bernard H. Doft, M.D. 6/12/13
Home Inspections, Compliance, Enforcement, and Criminal Investigations Enforcement Actions Warning Letters Inspections, Compliance, Enforcement, and Criminal Investigations Bernard H. Doft, M.D. 6/12/13
Comparative Effectiveness Research. Informing Public and Private Payer Decision-Making Brian Sweet, Chief Pharmacy Officer June 24, 2010
 Comparative Effectiveness Research Informing Public and Private Payer Decision-Making Brian Sweet, Chief Pharmacy Officer June 24, 2010 How Evidence Begins No Evidence Beginning of Human Testing FDA Approval
Comparative Effectiveness Research Informing Public and Private Payer Decision-Making Brian Sweet, Chief Pharmacy Officer June 24, 2010 How Evidence Begins No Evidence Beginning of Human Testing FDA Approval
DEVICES ARE KEY TO DRUG EFFICACY
 pump up your performance Co-Development DEVICES ARE KEY TO DRUG EFFICACY The intranasal route is widely used for both prescription and over-the-counter drugs. It is an attractive option for topical drugs
pump up your performance Co-Development DEVICES ARE KEY TO DRUG EFFICACY The intranasal route is widely used for both prescription and over-the-counter drugs. It is an attractive option for topical drugs
INNOVATIVE COMPOUNDING SERVICES
 INNOVATIVE COMPOUNDING SERVICES We are excited to introduce our innovative compounded oral rinses that may offer a safe and effective alternative for the management of side effects associated with chemotherapy
INNOVATIVE COMPOUNDING SERVICES We are excited to introduce our innovative compounded oral rinses that may offer a safe and effective alternative for the management of side effects associated with chemotherapy
NOVARTIS AG (Exact name of the registrant as specified in its charter)
 UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM SD SPECIALIZED DISCLOSURE REPORT NOVARTIS AG (Exact name of the registrant as specified in its charter) Switzerland 1-15024
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM SD SPECIALIZED DISCLOSURE REPORT NOVARTIS AG (Exact name of the registrant as specified in its charter) Switzerland 1-15024
American Heart Association/American Stroke Association Statement on Drug Formularies
 American Heart Association/American Stroke Association Statement on Drug Formularies Underlined terms are defined on page 4 of the document. 1. The AHA supports a formulary system that: Assures access
American Heart Association/American Stroke Association Statement on Drug Formularies Underlined terms are defined on page 4 of the document. 1. The AHA supports a formulary system that: Assures access
STIMULI TO THE REVISION PROCESS
 Page 1 of 6 STIMULI TO THE REVISION PROCESS Stimuli articles do not necessarily reflect the policies of the USPC or the USP Council of Experts USP's Nomenclature Initiatives Angela G. Long, M.S.; Andrzej
Page 1 of 6 STIMULI TO THE REVISION PROCESS Stimuli articles do not necessarily reflect the policies of the USPC or the USP Council of Experts USP's Nomenclature Initiatives Angela G. Long, M.S.; Andrzej
USP800: A quick summary and disposal. Brenda Denson, Pharm.D.
 USP800: A quick summary and disposal Brenda Denson, Pharm.D. Objectives Review current USP800 guidelines on destruction of hazardous medications. Illustrate how a local pharmacy demonstrates compliance
USP800: A quick summary and disposal Brenda Denson, Pharm.D. Objectives Review current USP800 guidelines on destruction of hazardous medications. Illustrate how a local pharmacy demonstrates compliance
Policy Position. Pharmacy-mediated interchangeability for Similar Biotherapeutic Products (SBPs)
 Pharmacy-mediated interchangeability for Similar Biotherapeutic Products (SBPs) Geneva, April 2016 Appropriate use of biotherapeutics including SBPs - SBPs, also known as biosimilars, are developed to
Pharmacy-mediated interchangeability for Similar Biotherapeutic Products (SBPs) Geneva, April 2016 Appropriate use of biotherapeutics including SBPs - SBPs, also known as biosimilars, are developed to
Drug Shortages are Plentiful
 Have you ever reported to work and encountered this scenario? Drug Shortages are Plentiful Travis Hunerdosse, Pharm.D. Clinical Specialist, Drug Use Policy/Formulary Management Rush University Medical
Have you ever reported to work and encountered this scenario? Drug Shortages are Plentiful Travis Hunerdosse, Pharm.D. Clinical Specialist, Drug Use Policy/Formulary Management Rush University Medical
Specialty Drug Management Is it possible? John R. Adler President/Consultant ELMC RxSolutions, LLC
 Specialty Drug Management Is it possible? John R. Adler President/Consultant ELMC RxSolutions, LLC Defining Specialty Drugs Categories/Types of Specialty Drugs Brands Garden Variety Orphan Super Orphan
Specialty Drug Management Is it possible? John R. Adler President/Consultant ELMC RxSolutions, LLC Defining Specialty Drugs Categories/Types of Specialty Drugs Brands Garden Variety Orphan Super Orphan
Sarpy County Purchasing Department
 Sarpy County Purchasing Department SARPY COUNTY COURTHOUSE 1210 GOLDEN GATE DRIVE, SUITE 1220 PAPILLION, NE 68046 Brian Hanson, Purchasing Agent (402) 593 2349 Debby Peoples, Asst. Purchasing Agent (402)
Sarpy County Purchasing Department SARPY COUNTY COURTHOUSE 1210 GOLDEN GATE DRIVE, SUITE 1220 PAPILLION, NE 68046 Brian Hanson, Purchasing Agent (402) 593 2349 Debby Peoples, Asst. Purchasing Agent (402)
CMS , Medicare Benefit Policy Manual, Chapter 15- Section 50 - Drugs and Biologicals:
 Billing and Coding Guidelines for Drugs and Biologics (Non-chemotherapy) L 34741 Medicare Excerpts: CMS 100-02, Medicare Benefit Policy Manual, Chapter 15- Section 50 - Drugs and Biologicals: 50.2 - Determining
Billing and Coding Guidelines for Drugs and Biologics (Non-chemotherapy) L 34741 Medicare Excerpts: CMS 100-02, Medicare Benefit Policy Manual, Chapter 15- Section 50 - Drugs and Biologicals: 50.2 - Determining
IBM Micromedex care delivery Evidence-based clinical decision support for healthcare decision-makers
 IBM Micromedex care delivery Evidence-based clinical decision support for healthcare decision-makers WELLNESS AND PREVENTION REDUCING COSTS BETTER USE OF PATIENT DATA AND ANALYTICS IMPROVING QUALITY OF
IBM Micromedex care delivery Evidence-based clinical decision support for healthcare decision-makers WELLNESS AND PREVENTION REDUCING COSTS BETTER USE OF PATIENT DATA AND ANALYTICS IMPROVING QUALITY OF
Secundum Artem. USP Chapter <795> Pharmaceutical Compounding - Nonsterile Preparations INTRODUCTION BACKGROUND VOLUME 13 NUMBER 4
 VOLUME 13 NUMBER 4 Secundum Artem Current & Practical Compounding Information for the Pharmacist. USP Chapter Pharmaceutical Compounding - Nonsterile Preparations GOALS AND OBJECTIVES Goal: The goal
VOLUME 13 NUMBER 4 Secundum Artem Current & Practical Compounding Information for the Pharmacist. USP Chapter Pharmaceutical Compounding - Nonsterile Preparations GOALS AND OBJECTIVES Goal: The goal
MICHIGAN PHARMACY LAW UPDATE Objectives. Areas of Focus Pharmacy Compounding 3/9/2016
 MICHIGAN PHARMACY LAW UPDATE 2016 Eric Roath, Pharm.D. Director of Professional Practice Michigan Pharmacists Association Objectives 1. Identify the changes that have occurred in healthcare and pharmacy
MICHIGAN PHARMACY LAW UPDATE 2016 Eric Roath, Pharm.D. Director of Professional Practice Michigan Pharmacists Association Objectives 1. Identify the changes that have occurred in healthcare and pharmacy
About Clinical Trials: What They Can Mean For You?
 About Clinical Trials: What They Can Mean For You? Karl Cremer, PharmD and AnnKatrin Petersen, MD, MS January 25, 2012 ClinicalStudy.org Owned and operated by PRI, Inc. 5927 Balfour Court, Suite 201 Carlsbad,
About Clinical Trials: What They Can Mean For You? Karl Cremer, PharmD and AnnKatrin Petersen, MD, MS January 25, 2012 ClinicalStudy.org Owned and operated by PRI, Inc. 5927 Balfour Court, Suite 201 Carlsbad,
Prescription Drug Pricing. Page 1
 Prescription Drug Pricing Page 1 Prescription Drugs As a Percent of Total National Health Expenditures, 1991-2014 12% 10% 8% 6% 4% 2% 0% 1991 1992 1993 1994 1995 1996 1997 1998 1999 2000 2001 2002 2003
Prescription Drug Pricing Page 1 Prescription Drugs As a Percent of Total National Health Expenditures, 1991-2014 12% 10% 8% 6% 4% 2% 0% 1991 1992 1993 1994 1995 1996 1997 1998 1999 2000 2001 2002 2003
THE BIOLOGIC DRUG MARKET. ebook: The current state of biologic drugs and the challenges ahead
 THE BIOLOGIC DRUG MARKET 2 0 1 7 HOW DO DRUGS AND BIOLOGICS DIFFER? This ebook answers that question, and so much more. OptumRx has summarized the complex and expensive world of biologic drugs for you.
THE BIOLOGIC DRUG MARKET 2 0 1 7 HOW DO DRUGS AND BIOLOGICS DIFFER? This ebook answers that question, and so much more. OptumRx has summarized the complex and expensive world of biologic drugs for you.
Kingston Health Sciences Centre Pharmacy Services Investigational Drug Service (IDS) COST ESTIMATE REQUEST FORM Version July 2017
 Please complete the KHSC Pharmacy Services Study Request Form and ATTACH the Form to your TRAQ DSS FORM prior to submission. If you forgot to attach the Form to your TRAQ DSS FORM prior to submission,
Please complete the KHSC Pharmacy Services Study Request Form and ATTACH the Form to your TRAQ DSS FORM prior to submission. If you forgot to attach the Form to your TRAQ DSS FORM prior to submission,
Kingston Health Sciences Centre Pharmacy Services Investigational Drug Service (IDS) COST ESTIMATE REQUEST FORM Version June 2018
 Please complete the KHSC Pharmacy Services Study Request Form and ATTACH the Form to your TRAQ DSS FORM prior to submission. If you forgot to attach the Form to your TRAQ DSS FORM prior to submission,
Please complete the KHSC Pharmacy Services Study Request Form and ATTACH the Form to your TRAQ DSS FORM prior to submission. If you forgot to attach the Form to your TRAQ DSS FORM prior to submission,
Policies Approved by the 2018 ASHP House of Delegates
 House of Delegates Policies Approved by the 2018 ASHP House of Delegates 1801 Unit Dose Packaging Availability To advocate that pharmaceutical manufacturers provide all medications used in health systems
House of Delegates Policies Approved by the 2018 ASHP House of Delegates 1801 Unit Dose Packaging Availability To advocate that pharmaceutical manufacturers provide all medications used in health systems
Santen Pharmaceutical Co., Ltd. Financial Performance and Outlook Year Ended March 31, 2005
 Santen Pharmaceutical Co., Ltd. Financial Performance and Outlook Year Ended March 31, 2005 Summary of Year Ended March 2005; Progress of 2003-2005 Medium-term Management Plan; Returning Profit to Shareholders;
Santen Pharmaceutical Co., Ltd. Financial Performance and Outlook Year Ended March 31, 2005 Summary of Year Ended March 2005; Progress of 2003-2005 Medium-term Management Plan; Returning Profit to Shareholders;
Pediatric Exclusivity and Drug Development Requirements in the Overall Pediatric Population. Prepared by Beckloff Associates, Inc.
 and Drug Development Requirements in the Overall Pediatric Population 1 INTRODUCTION AND OVERVIEW Prepared by Beckloff Associates, Inc. Although children suffer from many of the same diseases as adults
and Drug Development Requirements in the Overall Pediatric Population 1 INTRODUCTION AND OVERVIEW Prepared by Beckloff Associates, Inc. Although children suffer from many of the same diseases as adults
Toxicology - Problem Drill 24: Toxicology Studies in Pharmaceutical Development
 Toxicology - Problem Drill 24: Toxicology Studies in Pharmaceutical Development No. 1 of 10 1. regulates all the drugs products manufactured and sold in the USA. (A) EMEA (B) IND (C) FDA (D) NDA (E) OSHA
Toxicology - Problem Drill 24: Toxicology Studies in Pharmaceutical Development No. 1 of 10 1. regulates all the drugs products manufactured and sold in the USA. (A) EMEA (B) IND (C) FDA (D) NDA (E) OSHA
Ghalib Abbasi, RPh, MS, PharmD Pharmacy Technology Consultant Florida, USA
 Ghalib Abbasi, RPh, MS, PharmD Pharmacy Technology Consultant Florida, USA Disclosure Information IV Robotics and Workflow: Design and Integration with other Healthcare and Regulatory Components Ghalib
Ghalib Abbasi, RPh, MS, PharmD Pharmacy Technology Consultant Florida, USA Disclosure Information IV Robotics and Workflow: Design and Integration with other Healthcare and Regulatory Components Ghalib
USP CHAPTER <797> INTRODUCTION RISK LEVELS
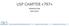 USP CHAPTER INTRODUCTION RISK LEVELS Introduction: The objective of this chapter is to describe conditions and practices to prevent harm, including death, to patients that could result from 1) microbial
USP CHAPTER INTRODUCTION RISK LEVELS Introduction: The objective of this chapter is to describe conditions and practices to prevent harm, including death, to patients that could result from 1) microbial
CONVENIENCE FOR PATIENTS
 the dermal experience Co-Development CONVENIENCE FOR PATIENTS The dermal delivery route is well established. Millions of patients globally rely on delivery systems for topical and systemic treatments administered
the dermal experience Co-Development CONVENIENCE FOR PATIENTS The dermal delivery route is well established. Millions of patients globally rely on delivery systems for topical and systemic treatments administered
Compounding Pharmacy Assessment Questionnaire (CPAQ )
 Compounding Pharmacy Assessment Questionnaire (CPAQ ) The International Academy of Compounding Pharmacists represents more than 2,700 pharmacists and other professionals who specialize in the provision
Compounding Pharmacy Assessment Questionnaire (CPAQ ) The International Academy of Compounding Pharmacists represents more than 2,700 pharmacists and other professionals who specialize in the provision
2018 Glaukos Corporation. January 2018
 2018 Glaukos Corporation 1 January 2018 Disclaimer All statements other than statements of historical facts included in this presentation that address activities, events or developments that we expect,
2018 Glaukos Corporation 1 January 2018 Disclaimer All statements other than statements of historical facts included in this presentation that address activities, events or developments that we expect,
ASBM Biosimilars. Canada Prescribers and Biosimilars October, Kevin Olson, CEO Industry Standard Research
 ASBM Biosimilars Canada Prescribers and Biosimilars October, 2017 Kevin Olson, CEO Industry Standard Research KevinO@ISRreports.com Table of contents Page 3 Methodology 5 Sample Characteristics 6 Executive
ASBM Biosimilars Canada Prescribers and Biosimilars October, 2017 Kevin Olson, CEO Industry Standard Research KevinO@ISRreports.com Table of contents Page 3 Methodology 5 Sample Characteristics 6 Executive
SUR-421, Surgical Technology Pharmacology, 1 cr
 SUR-421, Surgical Technology Pharmacology, 1 cr COURSE DESCRIPTION: Provides information needed to calculate and handle drugs in the operating room. Provides an overview of the administration and general
SUR-421, Surgical Technology Pharmacology, 1 cr COURSE DESCRIPTION: Provides information needed to calculate and handle drugs in the operating room. Provides an overview of the administration and general
RapidFACT: Accelerated Formulation Development for Poorly Soluble Drugs and Modified Release Products
 RapidFACT: Accelerated Formulation Development for Poorly Soluble Drugs and Modified Release Products Kevin Kane, Scientific Director, BCP 7 th Annual Global Drug Delivery & Formulation Summit 28 th August
RapidFACT: Accelerated Formulation Development for Poorly Soluble Drugs and Modified Release Products Kevin Kane, Scientific Director, BCP 7 th Annual Global Drug Delivery & Formulation Summit 28 th August
Managing Risk and Uncertainty Through the Drug Life cycle. Recent FDA Initiatives. Theresa Mullin, PhD
 Managing Risk and Uncertainty Through the Drug Life cycle Recent FDA Initiatives Theresa Mullin, PhD Director Office of Strategic Programs US FDA Center for Drug Evaluation and Research October 14, 2014
Managing Risk and Uncertainty Through the Drug Life cycle Recent FDA Initiatives Theresa Mullin, PhD Director Office of Strategic Programs US FDA Center for Drug Evaluation and Research October 14, 2014
EyeGate Pharmaceuticals, Inc.
 EyeGate Pharmaceuticals, Inc. Providing innovative products that enhance drug efficacy and patient compliance to improve vision Corporate Presentation Forward Looking Statements Some of the matters discussed
EyeGate Pharmaceuticals, Inc. Providing innovative products that enhance drug efficacy and patient compliance to improve vision Corporate Presentation Forward Looking Statements Some of the matters discussed
DELIVERING SOLUTIONS FOR PATIENTS
 a clear vision for eyecare Co-Development DELIVERING SOLUTIONS FOR PATIENTS Patients suffer because of preservatives in eye drops. Scientific research has shown that preservatives cause irritations, allergies
a clear vision for eyecare Co-Development DELIVERING SOLUTIONS FOR PATIENTS Patients suffer because of preservatives in eye drops. Scientific research has shown that preservatives cause irritations, allergies
Repeated-dose ocular instillation toxicity study: a survey of its study design on the basis of common technical documents in Japan
 Fundamental Toxicological Sciences (Fundam. Toxicol. Sci.) Vol.4, No.2, 95-99, 2017 95 Original Article Repeated-dose ocular instillation toxicity study: a survey of its study design on the basis of common
Fundamental Toxicological Sciences (Fundam. Toxicol. Sci.) Vol.4, No.2, 95-99, 2017 95 Original Article Repeated-dose ocular instillation toxicity study: a survey of its study design on the basis of common
March 4, Dockets Management Branch (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm Rockville, MD 20852
 March 4, 2014 Dockets Management Branch (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Re: Docket No. FDA 2013-N-1523: Request for Nominations: Drug Products that
March 4, 2014 Dockets Management Branch (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Re: Docket No. FDA 2013-N-1523: Request for Nominations: Drug Products that
LUNCH AND LEARN. Current Regulatory Landscape for Sterile Compounding: Part 1. June 8, 2018
 LUNCH AND LEARN Current Regulatory Landscape for Sterile Compounding: Part 1 June 8, 2018 Featured Speaker: Fred Massoomi, PharmD, FASHP Senior Director of Health system & Hospital Services Visante, Inc.
LUNCH AND LEARN Current Regulatory Landscape for Sterile Compounding: Part 1 June 8, 2018 Featured Speaker: Fred Massoomi, PharmD, FASHP Senior Director of Health system & Hospital Services Visante, Inc.
