Morris Animal. FOUNDATION Golden Retriever Lifetime Study. Golden Retreiver Lifetime Study Biopsy Kit: Collection & Shipping Instructions
|
|
|
- Theodora Bennett
- 6 years ago
- Views:
Transcription
1 Morris Animal FOUNDATION Golden Retriever Lifetime Study Golden Retreiver Lifetime Study Biopsy Kit: Collection & Shipping Instructions
2
3 Tips for Participating Veterinarians Thank you for your participation in this groundbreaking study. We understand study visits involve extra time and effort. Please review the following list of tips that may help streamline the process for you and your staff. If you have any other ideas, please feel free to share, them with the study team or on the Golden Retriever Lifetime Study Veterinarians Facebook page. If you have questions or need assistance, the customer care team is available 8 a.m. 6 p.m. EST at 855-4GR-DOGS, or grdogs@caninelifetimehealth.org. We recommend having a team of three clinic staff members perform the blood draw one for gentle control, one for managing the venipuncture, and one to handle the numerous blood tubes required. As soon as possible after the annual study examination and sample collection, log on to caninelifetimehealth.org to complete the veterinary questionnaire for your patient. (You must be on the Canine Lifetime Health Project website and not the general Morris Animal Foundation website.) Write your username and password in each study patient s chart where it is easy to find. Your username is your address and is case sensitive. Your password never needs to be changed. For the annual questionnaire you will report on health events, medications and vaccines given throughout the previous 12 months. If your software system does not show problem lists, medications and vaccines separately, consider maintaining an annual list for each category within the patient chart for easy review. You will need the vaccine manufacturer and lot number. A hard copy of the veterinary questionnaire is included in each kit. You can complete it as you perform the exam or dictate to a staff member. A knowledgeable staff member can enter the annual exam information online from the completed hard copy questionnaire or from your medical records. Please schedule extra time the day of a study examination for you or a trusted staff member to complete the online questionnaire. You can skip a question by using the table of contents on the right side of the screen (instead of clicking next, which requires the current page to be complete). MAF BVSKIB-v2 If you have any questions, please contact us at 855.4GR.DOGS ( ) 3
4 1 Biopsy Sample Collection Overview Collection Process: 1 Place a representative sample of the tumor into formalin at a standard ratio of 1 part sample to 10 parts buffered formalin. Store at room temperature. 2 Place a tumor sample less than 5 mm 3 (size of a pencil eraser) in one vial of RNAlater. Circle DISEASED on the label. Store at room temperature. 3 Place a sample of healthy tissue adjacent to the tumor less than 5 mm 3 (size of a pencil eraser) in a separate vial of RNAlater. Circle HEALTHY on the label. Store at room temperature. Please refer to the following sections for instructions on: 1 Biopsy Sample Fixation 2 Clinical Pathology Sample Collection 3 Sample Packaging and Shipping 4 Sample Reporting Refer to Appendices for guidance on: Appendix 1 Fixation and Shipping of Small or Large Specimens Appendix 2 Identification of Surgical Margins Appendix 3 Tissue Coding The biopsy kit contents should be kept together in the labeled biopsy kit box or envelope and stored at room temperature. (Please check expiration dates on formalin jars prior to biopsy collection.) Please contact the study team at 855.4GR.DOGS ( ) for advice or assistance with any sample submission or to request replacement or additional supplies. Read all instructions before sample collection. 4 If you have any questions, please contact us at 855.4GR.DOGS ( ) MAF BVSKIB-v2
5 Biopsy Sample Collection Requirements 1 1 Excise tissue 2 Separate tissue into appropriate sections 3 Mark tissue as necessary 4 Place tissue into respective jars 5 Label jars appropriately 6 Document necessary information 7 Package shipment(s) 8 Wait for histopathology results to post to dog s online account* 9 Complete online study Additional Veterinary Visit form 10 Discuss the finding with your client * You will not receive any results from the RNAlater samples. ANTECH Diagnostics Majority of the tumor in the formalin for immediate biopsy analysis. Fisher BioServices Portion of the tissue (labeled diseased) and adjacent tissue (labeled healthy) in RNAlater for longterm storage. MAF BVSKIB-v2 If you have any questions, please contact us at 855.4GR.DOGS ( ) 5
6 1 Biopsy Sample Fixation Three Biopsy Specimens Total are Requested 1 Place a representative sample of the tumor in formalin at a standard ratio of 1 part sample to 10 parts buffered formalin. Store at room temperature. 2 Place a tumor sample less than 5 mm 3 (size of a pencil eraser) in one vial of RNAlater. Circle DISEASED on the label. Store at room temperature. 3 Place a sample of adjacent healthy tissue less than 5 mm 3 (size of a pencil eraser) in a separate vial of RNAlater. Circle HEATLHY on the label. Store at room temperature. Formalin Tumor specimens should be placed into formalin immediately after surgical excision unless tissue marking with dye or suture is necessary (see Appendices 1 and 2). Samples should be placed in enough formalin to achieve a final ratio of 1 part sample to 10 parts formalin. Please contact our study team at if you need formalin jars. Formalin jars should be stored at room temperature and shipped the same day of collection (unless collected Friday through Sunday). RNAlater The tumor sample to be placed into the provided tube of RNAlater should be a piece of tissue no larger than 5 mm on each of the three sides (5 mm 3 or size of a pencil eraser). After surgical excision, immediately immerse the sample into the tube of RNAlater and replace the cap securely. Circle 'DISEASED' on the label. The sample of adjacent healthy tissue to be placed into a separate tube of RNAlater also should be a piece of tissue no larger than 5 mm on each of the three sides (5 mm 3 or size of a pencil eraser). After surgical excision of the healthy tissue, immediately immerse the sample into a separate tube of RNAlater and replace the cap securely. Circle 'HEALTHY' on the label. When surgical margins are important, select a non-marginal area of the tumor for the sample intended for RNAlater. RNAlater vials should be stored at room temperature and shipped the same day of collection (unless collected Friday through Sunday). If the entire tumor sample is <1 cm on each side, place half into formalin and half into RNAlater. If surgical margins are important, then margins and proper sampling for histopathological diagnosis take priority. Please be sure to collect the sample of adjacent healthy tissue and place in a separate vial of RNAlater and circle 'HEALTHY' on the label. 6 If you have any questions, please contact us at 855.4GR.DOGS ( ) MAF BVSKIB-v2
7 Clinical Pathology Sample Collection 2 Blood Collection To collect blood, please use the Vacutainer blood collection system included in this kit and: The butterfly catheter attached to the Vacutainer needle holder when collecting from the cephalic or lateral saphenous vein or The straight needle attached to the Vacutainer needle holder when collecting from the jugular vein If there are complications and you are not able to obtain all the blood required in one visit, ALL blood samples will have to be redrawn during a follow-up visit using replacement supplies. To request replacement supplies, please call the study team at 855.4GR.DOGS ( ). Using the Vacutainer holder and either the butterfly or straight needle provided, draw blood in the following order: 1 Fill the 3 ml EDTA* ANTECH tube (purple top; no barcode) verify the information on the label and print the date on the label. 2 Fill the 10 ml EDTA* biorepository tube (purple top; barcode: 094XXXXXXB10, EDTA.) 3 Fill the four 10 ml serum tubes (red tops; no barcodes.) * To prevent clotting, immediately invert the EDTA tubes several times to mix the blood with the anticoagulant. You will need to collect at least 53 ml of blood. If necessary, use a needle and syringe (not supplied) to collect additional blood to top off any underfilled tubes. Read all instructions before collecting sample. MAF BVSKIB-v2 If you have any questions, please contact us at 855.4GR.DOGS ( ) 7
8 2 Clinical Pathology Sample Collection (Cont.) Serum Isolation Allow the four serum tubes (red tops) to clot for at least 45 minutes and then centrifuge per your clinic s normal protocol (we recommend 1,100 1,300 RCF for 10 minutes). After centrifugation, remove the Vacutainer cap from each of the four serum tubes (red tops) and using the 3 ml transfer pipette provided (DO NOT POUR), transfer the serum as follows: Place 3 ml of serum into the 3 ml ANTECH serum tube. The black line on the left side of the tube label indicates the appropriate fill volume. (white top; no barcode.) Verify the information on the label and print the date on the label. Place 10 ml of serum into the 10 ml biorepository serum transport vial (red screw top; bar code: 94XXXXXXB20, SERUM.) If additional serum is needed to achieve the required volumes, try re-centrifuging the blood remaining in the tubes. Urine Collection Collect a minimum of 10 ml of urine via free catch or cystocentesis. A transfer pipette is provided for your convenience to: Place a minimum of 5 ml of urine into the ANTECH urine tube (green top; no barcode.) Verify the information on the label and print the date on the label. Place a minimum of 5 ml of urine into the biorepository urine tube (green top; bar code: 094XXXXXXB30, URINE.) 8 If you have any questions, please contact us at 855.4GR.DOGS ( ) MAF BVSKIB-v2
9 Clinical Pathology Sample Collection (Cont.) 2 Fecal Collection If the owner did not bring the dog s fecal sample to the visit, please obtain a sample from the patient during the visit. Transfer a marble-sized sample (approximately 1 g) of feces into each of the following: 30 ml ANTECH fecal collection tube, (white top; no barcode) Verify the information on the label and print the date on the label. Biorepository fecal collection tube, (brown top; bar code: 094XXXXXXB60, FECAL.) Toenail Trimmings Collection Collect 5 10 toenail trimmings from the dog. Place the toenail trimmings into the biorepository 2 ml plastic cryogenic tube, (blue screw top; bar code: 094XXXXXXB50, NAIL.) Hair Collection Cut a lock of hair 1/4 in diameter (about the diameter of a wooden pencil) and a minimum of 2 long as close to the root as possible.* Place the lock of hair into the 3.5 ml biorepository tube, (orange screw top; bar code: 094XXXXXXB40, HAIR.) Proceed to shipping instructions on the next page. * Please ask the owner for the preferred collection site. MAF BVSKIB-v2 If you have any questions, please contact us at 855.4GR.DOGS ( ) 9
10 3 Sample Packaging & Shipping Placing A Service Call For FedEx Shipment(s) Place a service call to FedEx directly at Let them know you have packages for pickup and tell them that the shipping cost is being billed to ANTECH Diagnostics and Fisher BioServices. If your clinic is inadvertently charged any fees, please contact the study team at 855.4GR.DOGS ( ). You will use the provided FedEx labels for shipments. Samples can ONLY be shipped Monday through Thursday. Please contact the study team at 855.4GR.DOGS ( ) for advice or assistance with any sample submission or to request replacement or additional supplies. 10 If you have any questions, please contact us at 855.4GR.DOGS ( ) MAF BVSKIB-v2
11 Sample Packaging & Shipping (Cont.) 3 ANTECH 1 Clinical Pathology Verify all four tubes (no bar codes) are properly filled and labeled, including the date. Place the fecal (white top), 3 ml serum (white top), 3 ml EDTA (purple top) and 5 ml urine (green top) tubes in the ANTECH zip-closure bag. In the top header of the ANTECH Diagnostics requisition form, provide the following information: your clinic name, address, , phone and fax number. Complete the information (doctor, date, client name, pet name, species, breed and age) in the upper right corner of the form. All laboratory tests are ordered with code 87007; this is a no-charge study code, so you must use this requisition form and verify that the study code is preprinted on the form. Failure to do so may result in charges to your clinic. No additional tests may be run with this order. If you fail to use the provided requisition form, your clinic will not receive the lab results. Retain the bottom copy of the form for your records. Place the completed top copy of the ANTECH Diagnostics requisition form into the ANTECH zip-closure bag (which should already contain the necessary samples). Place the zip-closure bag inside the ANTECH Diagnostics shipping box and seal the box with one of the provided tape strips (from the front of the plastic sleeve). 2 Histopathology Ensure all formalin container lids are tightly sealed. Tape lids to bottles. Handwrite the date and tissue code (see Appendix 3) on the label of all formalin jars. Place the labeled formalin jar(s)*, together with 1 absorbing sheet per formalin jar, into the 5 x8 zip-closure bag and seal the bag. Place the sealed bag containing the labeled formalin jar(s) plus the completed ANTECH Diagnostics histopathology requisition form along with any other documents or photos into an ANTECH Diagnostics zip-closure bag and seal the bag. *If using supplies you have on hand at the clinic, please label the jars with the required information. Acct: Dog ID: 094-XXXXXX Owner: Last Name, Dog Name Date: Tissue Type: Tissue Code: MAF BVSKIB-v2 If you have any questions, please contact us at 855.4GR.DOGS ( ) 11
12 3 Sample Packaging & Shipping (Cont.) 3 Packaging see figure 1 Place the sealed double bag of formalin jars and documents, and the box containing clinical pathology samples, into the FedEx Biological Substance Pak. Close the envelope by removing the clear strip to expose the adhesive seal. Complete your return address on the right side of the provided FedEx Expanded Billable Stamp labeled for ANTECH Diagnostics, affix it to the indicated location on the FedEx Biological Substance Pak. Retain the left half of the label for your records and tracking. Call FedEx at to arrange for a pickup. Figure 1: ANTECH Shipment ANTECH Samples: Histopath and clinical pathology report will be shared at no charge. 12 If you have any questions, please contact us at 855.4GR.DOGS ( ) MAF BVSKIB-v2
13 Sample Packaging & Shipping (Cont.) 3 Biorepository 1 Clinical Pathology Fill out the Shipment Inventory Form (Fisher BioServices) Golden Retriever Lifetime Study and include the patient information, collection date, collection times, sample shipment inventory, and any optional comments. Place the following three samples into the Styrofoam shipping container:* - 5 ml of urine 10 ml green-top tube - 10 ml of EDTA blood purple-top tube - 10 ml of serum red-top tube Close the Styrofoam shipping container and place it inside a zip-closure Lab-Loc Biohazard bag. Also place the following five items inside the zipclosure Lab-Loc Biohazard bag: - Fecal sample tube brown-top tube - Nails cryogenic tube blue-top tube - Hair cryogenic tube orange-top tube - Absorbent paper towel - Shipping inventory form 2 RNAlater There should usually be two vials of RNAlater shipped together - one labeled DISEASED and one labeled HEALTHY. Fill out the Tissue Submission Form for Tissues in RNAlater (Fisher BioServices) Golden Retriever Lifetime Study. Ensure you have handwritten the date and tissue code (see Appendix 3) on the label of all RNAlater tubes and circled DISEASED or HEALTHY on the appropriate vials. Place both labeled RNAlater tubes, together with one absorbing sheet, into the padded envelope and seal the envelope. Place the sealed padded envelope and Submission Form for Tissues in RNAlater into a Fisher Lab-loc biohazard zip-closure bag and seal the bag. * NOTE: All tubes for the biorepository shipment are barcoded. MAF BVSKIB-v2 If you have any questions, please contact us at 855.4GR.DOGS ( ) 13
14 3 Sample Packaging & Shipping (Cont.) 3 Packaging see figure 2 Place the Fisher Bioservices Lab-loc biohazard zip-closure bag containing the biopsy samples and the zip-lock bag containing the clinical pathology samples into the FedEx Biological Substance Pak and seal the envelope by removing the clear seal to expose the adhesive strip. On the provided FedEx air shipment bill labeled FedEx Express US Airbill, complete the required From section. Retain the top copy for your records. Place the shipment bill inside of the provided clear FedEx mailer pouch and seal the pouch. Peel the backing from the pouch and affix the pouch to the indicated location on the FedEx Biological Substance Pak. Call FedEx at to arrange for a pickup. Figure 2: Fisher Bioservices Shipment Fisher BioServices Samples: Healthy and diseased tissue and whole blood, serum, urine, feces, hair and toenails for long-term storage. 14 If you have any questions, please contact us at 855.4GR.DOGS ( ) MAF BVSKIB-v2
15 Sample Reporting 4 You will receive the test results from the samples you ship to ANTECH Diagnostics within three to five business days on your patient s record through your online study account at caninelifetimehealth. org. The results are posted under the tab labeled Lab Results. The results will come as two separate reports: one report for the biopsy and a second report for the clinical pathology. If malignant histopathology results are received, log on at caninelifetimehealth.org/secure to complete an Additional Veterinary Visit (AVV) form for your patient. You will have the option to print a hard copy of this form. You can access the AVV form by selecting the appropriate patient from your My Patients page. You will see a red-labeled button on the dog s Golden Retriever Lifetime Study tab. If you have any questions, please do not hesitate to the study team at grdogs@ caninelifetimehealth.org or call toll-free at 855.4GR.DOGS ( ). We are here to help! Figure 3: Additional Veterinary Visit Form My Patient - Fido Dog Information Name: Fido Dog ID: Breed: Golden Retirever Sex: Intact Male Birth Date: 01/01/2013 Owner Information Name: Jane Doe jdoe@mail.com Visit Date Information Visit Date: 01/01/2016 (Scheduled) Results will upload to your patient s online study account. MAF BVSKIB-v2 If you have any questions, please contact us at 855.4GR.DOGS ( ) 15
16 5 Appendix: 1 Fixation and Shipping of Small or Large Specimens Specimen shipping can be complicated when dealing with very small or very large tissues that cannot be routinely processed. Samples too large to fix as a whole (e.g., spleen) should be sectioned into portions and submitted in separate, appropriately labeled formalin jars. An annotated digital photograph or sketch of the original specimen to depict sectioning and orientation along with area(s) of gross lesions should accompany the samples. Dye margins before sectioning if appropriate. For an amputated limb, remove as much of the noncancerous tissue as possible, and then immerse the tumor into an appropriate-size container of formalin. Very small samples, such as endoscopic or punch biopsies, should be placed into the provided tissue cassette, the Dog Study ID# labeled with a #2 pencil, and then put into a formalin container for shipping. Do not use gauze sponges or cardboard because tissue may become compromised upon retrieval. Very small samples may, at times, yield insufficient artifact-free section for diagnosis; therefore, submission of multiple specimens from a single lesion is preferable. For luminal organs (e.g., intestine, uterus, large vessels), gently flush the intact lumen with formalin. For long sections of luminal tissues, make a partial longitudinal incision, leaving areas of interest (e.g., resection sites, mass) intact, or submit three labeled sections (e.g., proximal, middle and distal). Thin, flat samples (e.g., urinary bladder, stomach, diaphragm) should be placed directly into a formalin container. Larger flat samples can be sutured onto a flat piece of cardboard presoaked in formalin or water before immersion into a formalin container. Regional or draining lymph nodes associated with the primary tumor, including those that result in limb amputation, warrant microscopic examination. To ensure proper evaluation, consider dissection or biopsy of the regional lymph node during surgery. Submit the lymph node in a separate, appropriately labeled container. 16 If you have any questions, please contact us at 855.4GR.DOGS ( ) MAF BVSKIB-v2
17 Appendix: 2 5 Identification of Surgical Margins The primary means of indicating surgical margins is by marking with tissue dye and/or placement of sutures. It is imperative that a corresponding written description of these tissue demarcations be provided. The best practice is to have the surgeon dye the margins before submission to the laboratory as the surgeon can best identify these areas. Guidelines for the Appropriate Marking of Tissues: Marking of tissue margins should occur as soon as possible post-excision. The tissue should be blotted dry prior to marking. Marking should be done using the provided dye. Place dye only on regions that are of specific interest or are true surgical margins. Complete submersion of the sample in dye is not recommended. To prevent excess dye from washing off or coating insignificant areas, allow the dye to dry completely (approximately 5 to 10 minutes) before placing the specimen in formalin. Some discoloration of the formalin with dye is expected. For larger specimens that require bread loafing (i.e., incomplete parallel cuts approximately 1 cm apart), dye the tissue and allow dye to dry prior to cutting. MAF BVSKIB-v2 If you have any questions, please contact us at 855.4GR.DOGS ( ) 17
18 5 Appendix: 3 Tissue Coding Code Description Additional Indications 70 Other tissue, source Tissue: Diseased Healthy 71 Adrenal Gland Left Right Both 72 Bone None 73 Bone Marrow None 74 Brain None 75 Colon None 76 Duodenum None 77 Esophagus None 78 Eye Left Right Both 79 Gonads Left Right Both 80 Heart None 81 Ileocecocolic Junction None 82 Ileum None 83 Jejunum None 84 Kidney Left Right Both 85 Liver None 86 Lung Specify Lobe: 87 Lymph Node Left Right : : Axillary Mesenteric Prescapular Mandibular Popliteal Other: 88 Oral Cavity None 89 Pancreas None 90 Parathyroid Gland None 91 Prostate None 92 Rectum None 93 Skeletal Muscle None 94 Skin None 95 Spinal Cord None 96 Spleen None 97 Stomach None 98 Thyroid None 99 Urinary Bladder None Please contact the study team at for advice or assistance with any sample submission, or to request replacement or additional supplies. 18 If you have any questions, please contact us at 855.4GR.DOGS ( ) MAF BVSKIB-v2
19 MAF BVSKIB-v2 If you have any questions, please contact us at 855.4GR.DOGS ( ) 19
20 6 About Morris Animal Foundation Morris Animal Foundation is a nonprofit organization that invests in science to advance animal health. The foundation is a global leader in funding scientific studies for companion animals, horses and wildlife. Since its founding in 1948, Morris Animal Foundation has invested in studies that have led to significant breakthroughs in diagnostics, treatments, preventions and cures to benefit animals worldwide. Learn more at morrisanimalfoundation.org. Thank You to Our Partners FOUNDING PARTNER The Mark & Bette Morris Family Foundation PLATINUM PARTNERS GOLD SPONSORS Golden Retriever Foundation Hadley and Marion Stuart Foundation Zoetis GOLDEN CHAMPIONS Mars Veterinary Please contact the study team at for advice or assistance with any sample submission or to request replacement or additional supplies. 20 If you have any questions, please contact us at 855.4GR.DOGS ( ) MAF BVSKIB-v2
Morris Animal. FOUNDATION Golden Retriever Lifetime Study
 Morris Animal FOUNDATION Golden Retriever Lifetime Study Golden Retreiver Lifetime Study Annual Veterinary Sample Kit: Collection & Shipping Instructions Tips for Participating Veterinarians Thank you
Morris Animal FOUNDATION Golden Retriever Lifetime Study Golden Retreiver Lifetime Study Annual Veterinary Sample Kit: Collection & Shipping Instructions Tips for Participating Veterinarians Thank you
PLATELET-ORIENTED INHIBITION IN NEW TIA
 PLATELET-ORIENTED INHIBITION IN NEW TIA AND MINOR ISCHEMIC STROKE ANCILLARY BIOMARKERS STUDY BLOOD SPECIMEN PROCEDURE MANUAL US Sites DNA Only DNA and Plasma CONTENTS I. DNA ONLY SPECIMEN PROCEDURES...
PLATELET-ORIENTED INHIBITION IN NEW TIA AND MINOR ISCHEMIC STROKE ANCILLARY BIOMARKERS STUDY BLOOD SPECIMEN PROCEDURE MANUAL US Sites DNA Only DNA and Plasma CONTENTS I. DNA ONLY SPECIMEN PROCEDURES...
BioSpecimen Exchange for Neurological Disorders (BioSEND) LBD Training Webinar
 BioSpecimen Exchange for Neurological Disorders (BioSEND) LBD Training Webinar BioSEND Training Webinar Overview 1. Study Reminders 2. Site Equipment 3. LBD Biospecimen Collection Protocol 4. Study Visit
BioSpecimen Exchange for Neurological Disorders (BioSEND) LBD Training Webinar BioSEND Training Webinar Overview 1. Study Reminders 2. Site Equipment 3. LBD Biospecimen Collection Protocol 4. Study Visit
BioSpecimen Exchange for Neurological Disorders (BioSEND) LBD Training Webinar
 BioSpecimen Exchange for Neurological Disorders (BioSEND) LBD Training Webinar BioSEND Training Staff Tatiana Foroud Colleen Mitchell Scott Kaiser BioSEND Training Webinar Overview 1. Study Reminders 2.
BioSpecimen Exchange for Neurological Disorders (BioSEND) LBD Training Webinar BioSEND Training Staff Tatiana Foroud Colleen Mitchell Scott Kaiser BioSEND Training Webinar Overview 1. Study Reminders 2.
MAY Research Blood and Tissue Kit Instructions
 Kit Inventory Instruction sheet Requisition Form Tissue Collection Supplies (2) Ambient Transport Bag (2) 4mL orange cap cryovials (2) 6mL Formalin (2) specimen labels ( 4 per sheet) (1) graduated pipet
Kit Inventory Instruction sheet Requisition Form Tissue Collection Supplies (2) Ambient Transport Bag (2) 4mL orange cap cryovials (2) 6mL Formalin (2) specimen labels ( 4 per sheet) (1) graduated pipet
BioSpecimen Exchange for Neurological Disorders, BioSEND
 NINDS Biorepository BioSpecimen Exchange for Neurological Disorders, BioSEND Training Webinar Presented by: Scott Kaiser Webinar Overview 1. Site Equipment 2. BioSEND Kit Contents and Ordering Sample Labelling
NINDS Biorepository BioSpecimen Exchange for Neurological Disorders, BioSEND Training Webinar Presented by: Scott Kaiser Webinar Overview 1. Site Equipment 2. BioSEND Kit Contents and Ordering Sample Labelling
BioSpecimen Exchange for Neurological Disorders (BioSEND) Parkinsonism Biomarker Subtypes Training Webinar
 BioSpecimen Exchange for Neurological Disorders (BioSEND) Parkinsonism Biomarker Subtypes Training Webinar BioSEND Training Webinar Overview 1. Study Reminders 2. Site Equipment 3. PBS Biospecimen Collection
BioSpecimen Exchange for Neurological Disorders (BioSEND) Parkinsonism Biomarker Subtypes Training Webinar BioSEND Training Webinar Overview 1. Study Reminders 2. Site Equipment 3. PBS Biospecimen Collection
RISK Stratification and Identification of Immunogenetic and Microbial Markers of Rapid Disease Progression in Children with Crohn s Disease
 RISK Stratification and Identification of Immunogenetic and Microbial Markers of Rapid Disease Progression in Children with Crohn s Disease RISK LAB MANUAL 4/22/2011 Risk Stratification Study LAB MANUAL
RISK Stratification and Identification of Immunogenetic and Microbial Markers of Rapid Disease Progression in Children with Crohn s Disease RISK LAB MANUAL 4/22/2011 Risk Stratification Study LAB MANUAL
Pathology and Correlative Science Instructions for NSABP Foundation Protocol FB-11
 Pathology and Correlative Science Instructions for NSABP Foundation Protocol FB-11 A Phase II Randomized Study Evaluating the Biological and Clinical Effects of the Combination of Palbociclib with Letrozole
Pathology and Correlative Science Instructions for NSABP Foundation Protocol FB-11 A Phase II Randomized Study Evaluating the Biological and Clinical Effects of the Combination of Palbociclib with Letrozole
Special Specimen Collection Procedures for Coagulation Testing
 Special Specimen Collection Procedures for Coagulation Testing The accuracy of hemostasis testing depends upon the quality of the specimen submitted. Hemostasis specimens must be properly collected, labeled,
Special Specimen Collection Procedures for Coagulation Testing The accuracy of hemostasis testing depends upon the quality of the specimen submitted. Hemostasis specimens must be properly collected, labeled,
Copyright 2017 Aegis Sciences Corporation All Rights Reserved
 Oral Fluid Collection Healthcare Collection Site Preparation 1. The collection site must have all the supplies needed to complete a specimen collection (e.g. collection kits, ink pens, Custody and Control
Oral Fluid Collection Healthcare Collection Site Preparation 1. The collection site must have all the supplies needed to complete a specimen collection (e.g. collection kits, ink pens, Custody and Control
Processing, Aliquoting, Recording and Storing of C1 Biospecimens
 Processing, Aliquoting, Recording and Storing of C1 Biospecimens NHLBI Pulmonary Vascular Disease Phenomics Program Funded by the National Heart, Lung, and Blood Institute of the National Institutes of
Processing, Aliquoting, Recording and Storing of C1 Biospecimens NHLBI Pulmonary Vascular Disease Phenomics Program Funded by the National Heart, Lung, and Blood Institute of the National Institutes of
General Information and Guidelines
 General Information and Guidelines Please refer to the test information on the website for specific guidelines on type of specimen submitted. The following is intended to serve as a guideline. We do request
General Information and Guidelines Please refer to the test information on the website for specific guidelines on type of specimen submitted. The following is intended to serve as a guideline. We do request
Blood-Based Biospecimen Training Slides
 Neurodegeneration in Aging Down Syndrome: A Longitudinal Study of Cognition and Biomarkers of Alzheimer's Disease in collaboration with The National Cell Repository for Alzheimer s Disease (NCRAD) Blood-Based
Neurodegeneration in Aging Down Syndrome: A Longitudinal Study of Cognition and Biomarkers of Alzheimer's Disease in collaboration with The National Cell Repository for Alzheimer s Disease (NCRAD) Blood-Based
HISTOPATHOLOGY INTRODUCTION
 HISTOPATHOLOGY INTRODUCTION Surgical, anatomical and consultative pathology services are available through pathologists in the Department of Pathology. The services available include: Routine surgical
HISTOPATHOLOGY INTRODUCTION Surgical, anatomical and consultative pathology services are available through pathologists in the Department of Pathology. The services available include: Routine surgical
Surgical/Cytology Specimen Collection and Handling
 Surgical/Cytology Specimen Collection and Handling To ensure that hospital departments and outside sources submitting specimens to the surgical pathology department follow the established methods to guard
Surgical/Cytology Specimen Collection and Handling To ensure that hospital departments and outside sources submitting specimens to the surgical pathology department follow the established methods to guard
Specimen labeling and shipping protocol.
 Specimen labeling and shipping protocol. SENDING YOUR URINE OR ORAL FLUID SPECIMENS TO THE LABORATORY Preparing specimens for the laboratory 2 Specimen labeling and shipping We ask that you refer to the
Specimen labeling and shipping protocol. SENDING YOUR URINE OR ORAL FLUID SPECIMENS TO THE LABORATORY Preparing specimens for the laboratory 2 Specimen labeling and shipping We ask that you refer to the
35. BIOREPOSITORY CORE URINE SPECIMENS COLLECTION, PROCESSING AND SHIPPING
 35. BIOREPOSITORY CORE URINE SPECIMENS COLLECTION, PROCESSING AND SHIPPING 35.1 Purpose Participants who enroll in the PVDOMICS study with be asked to provide biological specimens (blood and urine). The
35. BIOREPOSITORY CORE URINE SPECIMENS COLLECTION, PROCESSING AND SHIPPING 35.1 Purpose Participants who enroll in the PVDOMICS study with be asked to provide biological specimens (blood and urine). The
PATHOLOGY LABORATORY SERVICES
 VANDERBILT PATHOLOGY LABORATORY SERVICES VANDERBILT UNIVERSITY MEDICAL CENTER Request for Neuropathology Consultation Division of Neuropathology C-2318 Medical Center North Nashville, TN 37232-2561 Phone
VANDERBILT PATHOLOGY LABORATORY SERVICES VANDERBILT UNIVERSITY MEDICAL CENTER Request for Neuropathology Consultation Division of Neuropathology C-2318 Medical Center North Nashville, TN 37232-2561 Phone
HOW TO ORDER Revised
 HOW TO ORDER Revised 2015 9 29 How to Order Animal Health Testing Our Mission We have been working hard to get the word out about the risks of zoonotic infection to the veterinary and medical communities
HOW TO ORDER Revised 2015 9 29 How to Order Animal Health Testing Our Mission We have been working hard to get the word out about the risks of zoonotic infection to the veterinary and medical communities
34. PVDOMICS BIOSPECIMEN BLOOD PROCESSING AND SHIPPING. Chapter 35 describes collection, processing and shipment of urine specimens.
 34. PVDOMICS BIOSPECIMEN BLOOD PROCESSING AND SHIPPING 34.1 Purpose and Background Participants who enroll in the PVDOMICS study will be asked to provide biological samples (blood and urine) for OMICS
34. PVDOMICS BIOSPECIMEN BLOOD PROCESSING AND SHIPPING 34.1 Purpose and Background Participants who enroll in the PVDOMICS study will be asked to provide biological samples (blood and urine) for OMICS
APPENDIX D1. BLOOD SPECIMEN PROCEDURES
 APPENDIX D1. BLOOD SPECIMEN PROCEDURES Blood Specimen Collection & Storage Supplies: Red-top vacutainer tubes Lavender vacutainer tube 10mL white-top cryogenic vial 10mL blue-top cryogenic vial WIND Study
APPENDIX D1. BLOOD SPECIMEN PROCEDURES Blood Specimen Collection & Storage Supplies: Red-top vacutainer tubes Lavender vacutainer tube 10mL white-top cryogenic vial 10mL blue-top cryogenic vial WIND Study
Pointers on Shipping: Clinical Samples, Biological Substance Category B and Environmental Test Samples
 Pointers on Shipping: Clinical Samples, Biological Substance Category B and Environmental Test Samples At FedEx, we understand the importance of ensuring the safe shipping of clinical samples such as human
Pointers on Shipping: Clinical Samples, Biological Substance Category B and Environmental Test Samples At FedEx, we understand the importance of ensuring the safe shipping of clinical samples such as human
Alzheimer s Disease Center (ADC) New Site Training for the National Cell Repository for Alzheimer s Disease (NCRAD) Summer 2015
 Alzheimer s Disease Center (ADC) New Site Training for the National Cell Repository for Alzheimer s Disease (NCRAD) Summer 2015 NCRAD Leaders Tatiana Foroud, Principal Investigator Kelley Faber, Project
Alzheimer s Disease Center (ADC) New Site Training for the National Cell Repository for Alzheimer s Disease (NCRAD) Summer 2015 NCRAD Leaders Tatiana Foroud, Principal Investigator Kelley Faber, Project
Biofluids Collection Training Slides
 Longitudinal Early-onset Alzheimer s Disease Study in collaboration with The National Centralized Repository for Alzheimer s Disease and Related Dementias (NCRAD) Biofluids Collection Training Slides Contact
Longitudinal Early-onset Alzheimer s Disease Study in collaboration with The National Centralized Repository for Alzheimer s Disease and Related Dementias (NCRAD) Biofluids Collection Training Slides Contact
Zika Testing: Specimen Collection, Packaging and Shipping
 August, 2017 New Jersey Public Health and Environmental Laboratories Technical Bulletin Supplement Update Zika Testing: Specimen Collection, Packaging and Shipping SAMPLE SPECIFICATIONS FOR ZIKA Trioplex
August, 2017 New Jersey Public Health and Environmental Laboratories Technical Bulletin Supplement Update Zika Testing: Specimen Collection, Packaging and Shipping SAMPLE SPECIFICATIONS FOR ZIKA Trioplex
INTERNATIONAL SHIPPING GUIDE. Send testing to Mayo Clinic from around the world
 INTERNATIONAL SHIPPING GUIDE Send testing to Mayo Clinic from around the world Contents Ship to us from around the world 3 - Prior to shipping your first order, follow these steps 3 Step 1. Pre-shipping
INTERNATIONAL SHIPPING GUIDE Send testing to Mayo Clinic from around the world Contents Ship to us from around the world 3 - Prior to shipping your first order, follow these steps 3 Step 1. Pre-shipping
Specimen Collection and Preparation
 Specimen Collection and Preparation Laboratory test results are dependent on the quality of the specimen submitted. It is important that all specimens and request forms be properly labeled with the name
Specimen Collection and Preparation Laboratory test results are dependent on the quality of the specimen submitted. It is important that all specimens and request forms be properly labeled with the name
WELCOME FROM YOUR PROJECT TEAM!
 WELCOME FROM YOUR PROJECT TEAM! LabCorp Clinical Trials has been selected by University of California San Francisco to perform the DNA Extraction for Protocol POINT NCT00991029. As such, we have the pleasure
WELCOME FROM YOUR PROJECT TEAM! LabCorp Clinical Trials has been selected by University of California San Francisco to perform the DNA Extraction for Protocol POINT NCT00991029. As such, we have the pleasure
COMBINE FGF23 and R01 Kit. Procedure Manual (Draft)
 UW Medicine SCHOOL OF MEDICINE COMBINE FGF23 and R01 Kit Procedure Manual (Draft) Clinical Research Laboratory Study Coordinator Kim van Leuven, MT(ASCP) kvleuven@u.washington.edu (206) 543-9032 Laboratory
UW Medicine SCHOOL OF MEDICINE COMBINE FGF23 and R01 Kit Procedure Manual (Draft) Clinical Research Laboratory Study Coordinator Kim van Leuven, MT(ASCP) kvleuven@u.washington.edu (206) 543-9032 Laboratory
Unless otherwise directed, collect the following specimens from each person who may have been exposed:
 Shipping Instructions for Specimens Collected from People Who May Have Been Exposed to Chemical-Terrorism Agents Section One: Collecting and Labeling Specimens Required Specimens Unless otherwise directed,
Shipping Instructions for Specimens Collected from People Who May Have Been Exposed to Chemical-Terrorism Agents Section One: Collecting and Labeling Specimens Required Specimens Unless otherwise directed,
Laboratory Sample Manual
 Laboratory Sample Manual RCI BMT 13-TLEC/PBMTC LTE1401 Natural History and Biology of Late Effects Following Hematopoietic Cell Transplant for Childhood Hematologic Malignancies Page 1 I. Study Contact
Laboratory Sample Manual RCI BMT 13-TLEC/PBMTC LTE1401 Natural History and Biology of Late Effects Following Hematopoietic Cell Transplant for Childhood Hematologic Malignancies Page 1 I. Study Contact
Systemic Synuclein Sampling Study. Biospecimen Collection, Processing, and Shipment Manual
 S4 Biospecimen Collection, Processing, and Shipment Manual 25 February 2016 1 Table of Contents 1.0 Biorepository Information 5 1.1 Biorepository Contacts 1.2 Hours of Operation 1.3 Holiday Schedules and
S4 Biospecimen Collection, Processing, and Shipment Manual 25 February 2016 1 Table of Contents 1.0 Biorepository Information 5 1.1 Biorepository Contacts 1.2 Hours of Operation 1.3 Holiday Schedules and
Systemic Synuclein Sampling Study. Biospecimen Collection, Processing, and Shipment Manual
 Systemic Synuclein Sampling Study S4 Biospecimen Collection, Processing, and Shipment Manual Version 2.0 of the S4 Biologics Manual is to be used in conjunction with Version 2.0 of the S4 Protocol 21 March
Systemic Synuclein Sampling Study S4 Biospecimen Collection, Processing, and Shipment Manual Version 2.0 of the S4 Biologics Manual is to be used in conjunction with Version 2.0 of the S4 Protocol 21 March
Pathology and Correlative Science Instructions for NRG Oncology Trials: San Francisco Bank Kits Version
 Pathology and Correlative Science Instructions for NRG Oncology Trials: San Francisco Bank Kits Version Note: In an effort to go Green and reduce paper the NRGBB-SF will no longer include a full instruction
Pathology and Correlative Science Instructions for NRG Oncology Trials: San Francisco Bank Kits Version Note: In an effort to go Green and reduce paper the NRGBB-SF will no longer include a full instruction
Title: NonGyn Cytology Specimen Submission and Handling
 Approver(s): William Pitts, M.D. Date Created: 09/04/2013 Date Approved: 10/21/2013 Next Review Date: 10/21/2015 Principle: The laboratory establishes procedures for patient preparation, collection methods,
Approver(s): William Pitts, M.D. Date Created: 09/04/2013 Date Approved: 10/21/2013 Next Review Date: 10/21/2015 Principle: The laboratory establishes procedures for patient preparation, collection methods,
ROUTINE SPECIMEN COLLECTION BY SPECIMEN TYPE
 1. AUTOPSY EYE ROUTINE SPECIMEN COLLECTION BY SPECIMEN TYPE a. Arrangements: No special arrangements necessary. i. Immerse the specimen in a sufficient quantity of fixative so that the eye is covered completely
1. AUTOPSY EYE ROUTINE SPECIMEN COLLECTION BY SPECIMEN TYPE a. Arrangements: No special arrangements necessary. i. Immerse the specimen in a sufficient quantity of fixative so that the eye is covered completely
Both you and the responsible veterinary staff must sign the form affirming a match of the dog with the PERMANENT ID# AND THE BLOOD SAMPLE.
 Veterinary Appointment Blood can be collected by your veterinarian or a licensed veterinary technician and must be processed according to the instructions below. OptiGen does not supply a sampling kit,
Veterinary Appointment Blood can be collected by your veterinarian or a licensed veterinary technician and must be processed according to the instructions below. OptiGen does not supply a sampling kit,
KCC Path-Core Page 1 of 5
 Instructions for Sample preparation for Paraffin embedding PLEASE NOTE: There is no one-size-fits-all method of tissue preparation for all experimental designs. Before harvesting tissue, you need to assess
Instructions for Sample preparation for Paraffin embedding PLEASE NOTE: There is no one-size-fits-all method of tissue preparation for all experimental designs. Before harvesting tissue, you need to assess
SITE PROCEDURES NASAL SWAB
 SITE PROCEDURES NASAL SWAB Nasal Swab Collection Nasal swab specimen labels EXAKT-PAK One Pak shipping system Cryogenic specimen vial with transport medium Outer transport tube with absorbent material
SITE PROCEDURES NASAL SWAB Nasal Swab Collection Nasal swab specimen labels EXAKT-PAK One Pak shipping system Cryogenic specimen vial with transport medium Outer transport tube with absorbent material
Phlebotomist Instructions
 Phlebotomist Instructions July 2016 This guide is offered to our vendors and contracted companies to help them safely and legally package and ship medical specimens to Mayo Medical Laboratories for testing.
Phlebotomist Instructions July 2016 This guide is offered to our vendors and contracted companies to help them safely and legally package and ship medical specimens to Mayo Medical Laboratories for testing.
Departments of Electron Microscopy and Neuropathology
 Departments of Electron Microscopy and Neuropathology Method for preserving muscle and nerve biopsies (Procedures 1 and 2) From each biopsy, pieces of tissue should be taken for the following: 1. Frozen
Departments of Electron Microscopy and Neuropathology Method for preserving muscle and nerve biopsies (Procedures 1 and 2) From each biopsy, pieces of tissue should be taken for the following: 1. Frozen
ARMED FORCES REPOSITORY OF SPECIMEN SAMPLES FOR THE IDENTIFICATION OF REMAINS COLLECTION INSTRUCTIONS
 ARMED FORCES REPOSITORY OF SPECIMEN SAMPLES FOR THE IDENTIFICATION OF REMAINS COLLECTION INSTRUCTIONS 1. Purpose The following DNA collection instructions are designed to give specific direction to installations/sites
ARMED FORCES REPOSITORY OF SPECIMEN SAMPLES FOR THE IDENTIFICATION OF REMAINS COLLECTION INSTRUCTIONS 1. Purpose The following DNA collection instructions are designed to give specific direction to installations/sites
WELCOME FROM YOUR PROJECT TEAM!
 WELCOME FROM YOUR PROJECT TEAM! LabCorp Clinical Trials has been selected by University of California San Francisco to perform the DNA Extraction for Protocol POINT NCT00991029. As such, we have the pleasure
WELCOME FROM YOUR PROJECT TEAM! LabCorp Clinical Trials has been selected by University of California San Francisco to perform the DNA Extraction for Protocol POINT NCT00991029. As such, we have the pleasure
SAMPLE PROCESSING SOP. Purpose: To standardize a processing procedure for sample aliquoting in the ACRL.
 SAMPLE PROCESSING SOP Purpose: To standardize a processing procedure for sample aliquoting in the ACRL. Procedure: 1. Processing: a. One unit (or vial) of the sample type is thawed overnight in the walk-in
SAMPLE PROCESSING SOP Purpose: To standardize a processing procedure for sample aliquoting in the ACRL. Procedure: 1. Processing: a. One unit (or vial) of the sample type is thawed overnight in the walk-in
GENERAL SPECIMEN COLLECTION AND HANDLING HANDBOOK
 GENERAL SPECIMEN COLLECTION AND HANDLING HANDBOOK Building 27, Ibn Sina Building, Block B, Ground floor, Dubai Healthcare City, U.A.E. ABOUT US After working 20 years in USA, Dow Healthcare Management
GENERAL SPECIMEN COLLECTION AND HANDLING HANDBOOK Building 27, Ibn Sina Building, Block B, Ground floor, Dubai Healthcare City, U.A.E. ABOUT US After working 20 years in USA, Dow Healthcare Management
The Children s Hospital of Philadelphia Department of Pathology and Laboratory Medicine
 TheChildren shospitalofphiladelphia DepartmentofPathologyandLaboratoryMedicine Muscle Biopsy - General Instructions The Division of Neuropathology, Department of Pathology and Laboratory Medicine, Children
TheChildren shospitalofphiladelphia DepartmentofPathologyandLaboratoryMedicine Muscle Biopsy - General Instructions The Division of Neuropathology, Department of Pathology and Laboratory Medicine, Children
ARMED FORCES REPOSITORY OF SPECIMEN SAMPLES FOR THE IDENTIFICATION OF REMAINS COLLECTION INSTRUCTIONS
 ARMED FORCES REPOSITORY OF SPECIMEN SAMPLES FOR THE IDENTIFICATION OF REMAINS COLLECTION INSTRUCTIONS 1. Purpose The following DNA collection instructions are designed to give specific direction to installations/sites
ARMED FORCES REPOSITORY OF SPECIMEN SAMPLES FOR THE IDENTIFICATION OF REMAINS COLLECTION INSTRUCTIONS 1. Purpose The following DNA collection instructions are designed to give specific direction to installations/sites
BIOSPECIMEN COLLECTION SOP Phenotype, Genotype, and Biomarkers (PGB) in ALS and Related Disorders
 The purpose of this Standard Operating Procedure (SOP) is to describe the instructions for collection, processing, and storage of collected specimens for the PGB Study. 1. Definitions 1. EDTA An EDTA tube
The purpose of this Standard Operating Procedure (SOP) is to describe the instructions for collection, processing, and storage of collected specimens for the PGB Study. 1. Definitions 1. EDTA An EDTA tube
Instructions for Use: Duodenoscope Sampling Kit
 Instructions for Use: Duodenoscope Sampling Kit Brand Name of Product Generic Name of Product Product Code Number(s) Intended Use Duodenoscope Sampling Kit Endoscope sampling and culturing kit CK-250,
Instructions for Use: Duodenoscope Sampling Kit Brand Name of Product Generic Name of Product Product Code Number(s) Intended Use Duodenoscope Sampling Kit Endoscope sampling and culturing kit CK-250,
Manual of Procedures Version 2.1 May, 2017
 Specimen Collection, Processing and Internet-based Conversational Engagement Clinical Trial (I-CONECT) in collaboration with The National Cell Repository for Alzheimer s Disease (NCRAD) Manual of Procedures
Specimen Collection, Processing and Internet-based Conversational Engagement Clinical Trial (I-CONECT) in collaboration with The National Cell Repository for Alzheimer s Disease (NCRAD) Manual of Procedures
3.1 GENERAL SPECIMEN REQUIREMENTS See test menu for test-specific requirements
 3.1 GENERAL SPECIMEN REQUIREMENTS See test menu for test-specific requirements For all cases, include the following (see test-specific specimen requirements below): A. Completed PhenoPath requisition form
3.1 GENERAL SPECIMEN REQUIREMENTS See test menu for test-specific requirements For all cases, include the following (see test-specific specimen requirements below): A. Completed PhenoPath requisition form
INSTRUCTION MANUAL FOR LABORATORY AND SPECIMEN PROCEDURES
 INSTRUCTION MANUAL FOR LABORATORY AND SPECIMEN PROCEDURES CARDIOVASCULAR INFLAMMATION REDUCTION TRIAL (CIRT) Sponsor: Brigham and Women s Hospital All individuals connected with the clinical phase of this
INSTRUCTION MANUAL FOR LABORATORY AND SPECIMEN PROCEDURES CARDIOVASCULAR INFLAMMATION REDUCTION TRIAL (CIRT) Sponsor: Brigham and Women s Hospital All individuals connected with the clinical phase of this
Biofluids Collection Training Slides
 A Phase 2 Randomized Double-Blind Placebo-Controlled Trial to Evaluate the Efficacy and Safety of BHV-4157 in Patients with Mild to Moderate Alzheimer s Disease (T2 PROTECT AD) & The National Centralized
A Phase 2 Randomized Double-Blind Placebo-Controlled Trial to Evaluate the Efficacy and Safety of BHV-4157 in Patients with Mild to Moderate Alzheimer s Disease (T2 PROTECT AD) & The National Centralized
Standard Operating Procedure. Packing and Shipping of Ambient, Refrigerated, Frozen and Combined Biological Samples
 Standard Operating Procedure Packing and Shipping of Ambient, Refrigerated, Frozen and Combined Biological Samples I. POLICY: The Puerto Rico Clinical and Translational Research Consortium (PRCTRC) ensure
Standard Operating Procedure Packing and Shipping of Ambient, Refrigerated, Frozen and Combined Biological Samples I. POLICY: The Puerto Rico Clinical and Translational Research Consortium (PRCTRC) ensure
INSTRUCTIONS FOR SUBMITTING EYE SPECIMENS THE OPHTHALMIC PATHOLOGY LABORATORY HOUSTON METHODIST HOSPITAL
 Page 1 of 5 INSTRUCTIONS FOR SUBMITTING EYE SPECIMENS THE OPHTHALMIC PATHOLOGY LABORATORY HOUSTON METHODIST HOSPITAL Table of Contents Sample Aqueous humor 1 Vitreous 1 Corneal scraping for infectious
Page 1 of 5 INSTRUCTIONS FOR SUBMITTING EYE SPECIMENS THE OPHTHALMIC PATHOLOGY LABORATORY HOUSTON METHODIST HOSPITAL Table of Contents Sample Aqueous humor 1 Vitreous 1 Corneal scraping for infectious
Proper Labeling of Sample Tubes: Tiger Top/SST 5.0 ml Screw Cap Tube
 Proper Labeling of Sample Tubes: Tiger Top/SST 5.0 ml Screw Cap Tube Tiger Top/SST 5.0 ml Screw Cap Tube Apply barcode label starting at the top of the sample tube (the end closest to the cap). The barcode
Proper Labeling of Sample Tubes: Tiger Top/SST 5.0 ml Screw Cap Tube Tiger Top/SST 5.0 ml Screw Cap Tube Apply barcode label starting at the top of the sample tube (the end closest to the cap). The barcode
1. Introduction. 2. Responsibilities. 3. Equipment. 4. Method. Blood tube ID and sample preparation No: 009D
 Blood tube ID and sample preparation No: 009D 1. Introduction Accurate identification and preparation of samples is vital to ensure success of the study. It is essential that each sample is individually
Blood tube ID and sample preparation No: 009D 1. Introduction Accurate identification and preparation of samples is vital to ensure success of the study. It is essential that each sample is individually
I-SPOT Laboratory Manual Log of Changes from Version 3 to Version 4
 Log of Changes from Version 3 to Version 4 Throughout document changed Footer from July 2013 Version 3 to November 2013 Version 4 Throughout document changed aliquot to cryovial Page 2 Updated Page numbers
Log of Changes from Version 3 to Version 4 Throughout document changed Footer from July 2013 Version 3 to November 2013 Version 4 Throughout document changed aliquot to cryovial Page 2 Updated Page numbers
Unique patient demographic identifiers: Name, Date of Birth (DOB), medical record number (MRN), Social security number (SS#)
 I. Purpose of Procedure (Principle) To standardize the process for obtaining the maximum volume (up to 20 milliliters) of blood from a patient for Blood culture testing while observing aseptic technique.
I. Purpose of Procedure (Principle) To standardize the process for obtaining the maximum volume (up to 20 milliliters) of blood from a patient for Blood culture testing while observing aseptic technique.
Laboratory (LAB) prepares sample kits prior to Epidemiologist Investigator (EPI) arrival.
 Standard Operating Procedure for Sample Collection of Heavy Metals (As, Hg, U, Cd, Mn, Se), Creatinine, Phthalate Metabolites, 2,4-DCP, 2,5-DCP and Pyrethroids for Biomonitoring A. Scope and Purpose The
Standard Operating Procedure for Sample Collection of Heavy Metals (As, Hg, U, Cd, Mn, Se), Creatinine, Phthalate Metabolites, 2,4-DCP, 2,5-DCP and Pyrethroids for Biomonitoring A. Scope and Purpose The
ADAPTIVE BIOTECHNOLOGIES DIAGNOSTIC PORTAL: ACCOUNT LOGIN
 ADAPTIVE BIOTECHNOLOGIES DIAGNOSTIC PORTAL: ACCOUNT LOGIN 1. Create an account. To set up your account for our online order entry and reporting system, please go to: https://diagnostics.adaptivebiotech.com/account/login.
ADAPTIVE BIOTECHNOLOGIES DIAGNOSTIC PORTAL: ACCOUNT LOGIN 1. Create an account. To set up your account for our online order entry and reporting system, please go to: https://diagnostics.adaptivebiotech.com/account/login.
Biofluids Collection Training Slides
 North American Prodromal Synucleinopathy (NAPS) & The National Centralized Repository for Alzheimer s Disease and Related Dementias (NCRAD) Biofluids Collection Training Slides Contact Information Questions?
North American Prodromal Synucleinopathy (NAPS) & The National Centralized Repository for Alzheimer s Disease and Related Dementias (NCRAD) Biofluids Collection Training Slides Contact Information Questions?
Biofluids Collection Training Slides
 North American Prodromal Synucleinopathy (NAPS) & The National Centralized Repository for Alzheimer s Disease and Related Dementias (NCRAD) Biofluids Collection Training Slides Contact Information Questions?
North American Prodromal Synucleinopathy (NAPS) & The National Centralized Repository for Alzheimer s Disease and Related Dementias (NCRAD) Biofluids Collection Training Slides Contact Information Questions?
In-Country shipment : How to safely ship human blood samples from suspected Ebola cases within a country by road, rail and sea
 In-Country shipment : How to safely ship human blood samples from suspected Ebola cases within a country by road, rail and sea Step 1: Before handling the sample, prepare all shipping equipment (1) Manage
In-Country shipment : How to safely ship human blood samples from suspected Ebola cases within a country by road, rail and sea Step 1: Before handling the sample, prepare all shipping equipment (1) Manage
Minnesota Urolith Center. How to prepare and package samples
 Minnesota Urolith Center How to prepare and package samples Preparing your sample Please submit samples dry Bloody uroliths should be rinsed gently in water and allowed to air dry. Preparing your sample
Minnesota Urolith Center How to prepare and package samples Preparing your sample Please submit samples dry Bloody uroliths should be rinsed gently in water and allowed to air dry. Preparing your sample
Department of Laboratories St. Louis, MO ISSUE DATE: June 2007 REVISION DATE: May 2012 REVIEWED DATE: May 2018 PRINCIPLE:
 Page 1 of 7 PROCEDURE: LEAD ESA LEADCARE II / HEALTHY KIDS EXPRESS ISSUE DATE: June 2007 REVISION DATE: May 2012 REVIEWED DATE: May 2018 PRINCIPLE: The LEADCARE II System relies on electrochemistry and
Page 1 of 7 PROCEDURE: LEAD ESA LEADCARE II / HEALTHY KIDS EXPRESS ISSUE DATE: June 2007 REVISION DATE: May 2012 REVIEWED DATE: May 2018 PRINCIPLE: The LEADCARE II System relies on electrochemistry and
Hawaii Island Rat Lungworm Working Group Jarvi Lab Veterinary Sample Submission Protocol. Non-human animal blood and CSF Protocol
 Hawaii Island Rat Lungworm Working Group Jarvi Lab Veterinary Sample Submission Protocol Thank you for your interest and concerns regarding Rat Lungworm in Hawaii. Our facility has a species specific biological
Hawaii Island Rat Lungworm Working Group Jarvi Lab Veterinary Sample Submission Protocol Thank you for your interest and concerns regarding Rat Lungworm in Hawaii. Our facility has a species specific biological
ADAPTIVE BIOTECHNOLOGIES DIAGNOSTIC PORTAL: ACCOUNT LOGIN
 ACCOUNT LOGIN 1. Create an account. To set up your account for our online order entry and reporting system, please go to: https://diagnostics.adaptivebiotech.com/account/login. After signing up, please
ACCOUNT LOGIN 1. Create an account. To set up your account for our online order entry and reporting system, please go to: https://diagnostics.adaptivebiotech.com/account/login. After signing up, please
TITLE: LIVE/DEAD VIABILITY FOR CLINICAL SAMPLES
 Paul K. Wallace, Ph.D. Director Roswell Park Cancer Institute Elm & Carlton Streets Voice:(716) 845-8471 Buffalo, NY 14263 Fax:(716) 845-8806 FILE NAME FL-SRP-2090.00 Live/Dead Viability for Clinical Samples
Paul K. Wallace, Ph.D. Director Roswell Park Cancer Institute Elm & Carlton Streets Voice:(716) 845-8471 Buffalo, NY 14263 Fax:(716) 845-8806 FILE NAME FL-SRP-2090.00 Live/Dead Viability for Clinical Samples
To help you become familiar and comfortable with the different supplies and materials required for HIV rapid testing.
 Module 7 Preparation for Testing Supplies and Kits Purpose To help you become familiar and comfortable with the different supplies and materials required for HIV rapid testing. Pre-requisite Modules Learning
Module 7 Preparation for Testing Supplies and Kits Purpose To help you become familiar and comfortable with the different supplies and materials required for HIV rapid testing. Pre-requisite Modules Learning
Laboratory Manual. Insights on Selected Procoagulation markers and Outcomes in stroke Trial. I-SPOT Laboratory Manual
 Laboratory Manual Insights on Selected Procoagulation markers and Outcomes in stroke Trial (Response to Insulin Administration and Blood Glucose Control) November 2013December 2015 Version 54 1 Table of
Laboratory Manual Insights on Selected Procoagulation markers and Outcomes in stroke Trial (Response to Insulin Administration and Blood Glucose Control) November 2013December 2015 Version 54 1 Table of
Laboratory Manual. Insights on Selected Procoagulation markers and Outcomes in stroke Trial. I-SPOT Laboratory Manual. December 2015 Version 5 1
 Laboratory Manual Insights on Selected Procoagulation markers and Outcomes in stroke Trial (Response to Insulin Administration and Blood Glucose Control) December 2015 Version 5 1 Table of Contents Title
Laboratory Manual Insights on Selected Procoagulation markers and Outcomes in stroke Trial (Response to Insulin Administration and Blood Glucose Control) December 2015 Version 5 1 Table of Contents Title
NOTE: Do NOT overfill sharps containers; there should be NO more than 50ml of residual liquid in the mailback kit when returned
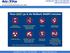 These DO go in the Mailback SHARPS Containers Sharps Waste Such As: Needles & syringes Razor blades Orthodon?c wires Scalpel blades & lancets Glass pipebes, slides & tubes Broken, contaminated glass Staples
These DO go in the Mailback SHARPS Containers Sharps Waste Such As: Needles & syringes Razor blades Orthodon?c wires Scalpel blades & lancets Glass pipebes, slides & tubes Broken, contaminated glass Staples
Coverage>100X. Sensitivity>99%. Specificity 100%. NGS
 Coverage>100X. Sensitivity>99%. Specificity 100%. NGS Coverage 15.000 X. Sensitivity 99%. Specificity 100% without false positives. o o Sample requirements and shipping information (solid biopsy) Only
Coverage>100X. Sensitivity>99%. Specificity 100%. NGS Coverage 15.000 X. Sensitivity 99%. Specificity 100% without false positives. o o Sample requirements and shipping information (solid biopsy) Only
Immunoassay Kit Catalog # KCA0021. Canine. C-Reactive Protein
 Immunoassay Kit Catalog # KCA0021 Canine C-Reactive Protein BioSource International, Inc. 542 Flynn Road Camarillo, California 93012 USA Tel: 805-987-0086 800-242-0607 FAX: 805-987-3385 email: tech.support@biosource.com
Immunoassay Kit Catalog # KCA0021 Canine C-Reactive Protein BioSource International, Inc. 542 Flynn Road Camarillo, California 93012 USA Tel: 805-987-0086 800-242-0607 FAX: 805-987-3385 email: tech.support@biosource.com
Human soluble TREM-2 ELISA Kit
 Human soluble TREM-2 ELISA Kit Cat.No: DEIA-SY005 Lot. No. (See product label) Size 96T Intended use For the quantitative measurement of human soluble TREM-2 concentrations in serum, plasma. General Description
Human soluble TREM-2 ELISA Kit Cat.No: DEIA-SY005 Lot. No. (See product label) Size 96T Intended use For the quantitative measurement of human soluble TREM-2 concentrations in serum, plasma. General Description
Creating Storage and Shipment Logs for Frozen Biorepository Specimens
 Creating Storage and Shipment Logs for Frozen Biorepository Specimens NHLBI Pulmonary Vascular Disease Phenomics Program Funded by the National Heart, Lung, and Blood Institute of the National Institutes
Creating Storage and Shipment Logs for Frozen Biorepository Specimens NHLBI Pulmonary Vascular Disease Phenomics Program Funded by the National Heart, Lung, and Blood Institute of the National Institutes
GUIDE THE GUIDE TO GETTING AN ONCOTYPE DX TEST
 STEP BY STEP GUIDE STEP BY STEP THE GUIDE TO GETTING AN ONCOTYPE DX TEST ONCOTYPE DX IS A TEST THAT MAY HELP BREAST CANCER PATIENTS DETERMINE IF THEY WILL BENEFIT FROM CHEMOTHERAPY Choosing the optimal
STEP BY STEP GUIDE STEP BY STEP THE GUIDE TO GETTING AN ONCOTYPE DX TEST ONCOTYPE DX IS A TEST THAT MAY HELP BREAST CANCER PATIENTS DETERMINE IF THEY WILL BENEFIT FROM CHEMOTHERAPY Choosing the optimal
Minnesota Urolith Center HOW TO SUBMIT UROLITH SAMPLES
 Minnesota Urolith Center HOW TO SUBMIT UROLITH SAMPLES SUBMIT SAMPLES DRY Rinse bloody uroliths gently in water and air dry SUBMIT SAMPLES DRY Rinse bloody uroliths gently in water and air dry SUBMIT SAMPLES
Minnesota Urolith Center HOW TO SUBMIT UROLITH SAMPLES SUBMIT SAMPLES DRY Rinse bloody uroliths gently in water and air dry SUBMIT SAMPLES DRY Rinse bloody uroliths gently in water and air dry SUBMIT SAMPLES
Specimen Collection and Preparation
 Specimen Collection and Preparation Laboratory test results are dependent on the quality of the specimen submitted. It is important that all specimens and requisitions be properly labeled with the full
Specimen Collection and Preparation Laboratory test results are dependent on the quality of the specimen submitted. It is important that all specimens and requisitions be properly labeled with the full
Veterinarian s Guide to Maximizing Biopsy Results
 Veterinarian s Guide to Maximizing Biopsy Results Veterinarian s Guide to Maximizing Biopsy Results F. Yvonne Schulman DVM, Diplomate ACVP This edition first published 2016 2016 John Wiley & Sons, Inc.
Veterinarian s Guide to Maximizing Biopsy Results Veterinarian s Guide to Maximizing Biopsy Results F. Yvonne Schulman DVM, Diplomate ACVP This edition first published 2016 2016 John Wiley & Sons, Inc.
Collection, Handling, and Transport of Food Samples to NM SLD
 Collection, Handling, and Transport of Food Samples to NM SLD The Environmental Microbiology Laboratory (EM) Section at NM SLD routinely conducts the microbiological testing of food and water samples.
Collection, Handling, and Transport of Food Samples to NM SLD The Environmental Microbiology Laboratory (EM) Section at NM SLD routinely conducts the microbiological testing of food and water samples.
RNAPro SAL ORAL SPECIMEN COLLECTION SYSTEM. Catalog Number RPSAL-701. Transparent Plastic Compression Tube. Compression Seal
 RNAPro SAL ORAL SPECIMEN COLLECTION SYSTEM Catalog Number RPSAL-701 Transparent Plastic Compression Tube Splitting Unit Equipped with Variable Filtration Media Compression Seal Ergonomically Correct Handle
RNAPro SAL ORAL SPECIMEN COLLECTION SYSTEM Catalog Number RPSAL-701 Transparent Plastic Compression Tube Splitting Unit Equipped with Variable Filtration Media Compression Seal Ergonomically Correct Handle
AssayMax Human Transferrin ELISA Kit
 AssayMax Human Transferrin ELISA Kit Assaypro LLC 3400 Harry S Truman Blvd St. Charles, MO 63301 T (636) 447-9175 F (636) 395-7419 www.assaypro.com For any questions regarding troubleshooting or performing
AssayMax Human Transferrin ELISA Kit Assaypro LLC 3400 Harry S Truman Blvd St. Charles, MO 63301 T (636) 447-9175 F (636) 395-7419 www.assaypro.com For any questions regarding troubleshooting or performing
The kit is a sandwich enzyme immunoassay for in vitro quantitative measurement of CCSA-4 in. Reagents Quantity Reagents Quantity
 Catalog No: YLA0017HU 96 Test Human colon cancer-specific antigen-4(ccsa-4)elisa Kit FOR RESEARCH USE ONLY; NOT FOR THERAPEUTIC OR DIAGNOSTIC APPLICATIONS! PLEASE READ THROUGH ENTIRE procedure BEFORE BEGINNING!
Catalog No: YLA0017HU 96 Test Human colon cancer-specific antigen-4(ccsa-4)elisa Kit FOR RESEARCH USE ONLY; NOT FOR THERAPEUTIC OR DIAGNOSTIC APPLICATIONS! PLEASE READ THROUGH ENTIRE procedure BEFORE BEGINNING!
Section: Administration Date of Issue: 05/09/2011 Issued By: Administration Part: Pneumatic Tube System Revision #: Revision Date:
 STANDARD OPERATING PROCEDURE VETERINARY HEALTH COMPLEX Section: Administration Date of Issue: 05/09/2011 Issued By: Administration Part: Pneumatic Tube System Revision #: Revision Date: Pages: Board Approval
STANDARD OPERATING PROCEDURE VETERINARY HEALTH COMPLEX Section: Administration Date of Issue: 05/09/2011 Issued By: Administration Part: Pneumatic Tube System Revision #: Revision Date: Pages: Board Approval
How to safely ship human blood samples from suspected Ebola cases within a country by road, rail and sea
 INTERIM GUIDELINE How to safely ship human blood samples from suspected Ebola cases within a country by road, rail and sea 2014 Step 1: Before handling the sample, prepare all shipping equipment Step 1a:
INTERIM GUIDELINE How to safely ship human blood samples from suspected Ebola cases within a country by road, rail and sea 2014 Step 1: Before handling the sample, prepare all shipping equipment Step 1a:
Version 2.2 CONTENTS. Introduction 3. Frequently Asked Questions 4. Kit Contents 5. Materials and Equipment Needed 5. Standard Protocol 6
 Version 2.2 CONTENTS Introduction 3 Frequently Asked Questions 4 Kit Contents 5 Materials and Equipment Needed 5 Standard Protocol 6 Short Protocol 9 DNA Concentration Measurement 12 PCR Amplification
Version 2.2 CONTENTS Introduction 3 Frequently Asked Questions 4 Kit Contents 5 Materials and Equipment Needed 5 Standard Protocol 6 Short Protocol 9 DNA Concentration Measurement 12 PCR Amplification
Sputum Collect >1 ml expectorated lower respiratory specimen into a sterile, leakproof container. Transport Directly to lab at Room Temperature
 Clinical Specimen Selection for Agents of Bioterrorism Bacillus anthracis Anthrax Cutaneous lesions - Vesicular Stage Collect fluid from intact vesicles on sterile swab(s) from previously unopened vesicles.
Clinical Specimen Selection for Agents of Bioterrorism Bacillus anthracis Anthrax Cutaneous lesions - Vesicular Stage Collect fluid from intact vesicles on sterile swab(s) from previously unopened vesicles.
Human Myostatin, ELISA Kit (MSTN)
 Human Myostatin, ELISA Kit (MSTN) 96 Tests Catalog Number: MBS733837 Store all reagents at 2-8 C Valid Period: six months For samples: Cell culture fluid & body fluid & tissue homogenate Serum or blood
Human Myostatin, ELISA Kit (MSTN) 96 Tests Catalog Number: MBS733837 Store all reagents at 2-8 C Valid Period: six months For samples: Cell culture fluid & body fluid & tissue homogenate Serum or blood
STANDARD OPERATING PROCEDURE
 Effective Date: 12/1/2011 Page 1 of 12 Approval Name/Title Signature Date Authors: Jessica Landis Trial Implementation Manager Immune Tolerance Network Mary C. Philogene Associate Director, QA And Cell
Effective Date: 12/1/2011 Page 1 of 12 Approval Name/Title Signature Date Authors: Jessica Landis Trial Implementation Manager Immune Tolerance Network Mary C. Philogene Associate Director, QA And Cell
Alt-MSA Handbook Part 8: STC Administrative Tasks (Portfolio Materials Ordering, Pre-ID Label Generation and Placement, and Packing and Shipment)
 Handbook Part 8: STC Administrative Tasks (Portfolio Materials Ordering, Pre-ID Label Generation and Placement, and Packing and Shipment) This section of the Handbook contains information to be used by
Handbook Part 8: STC Administrative Tasks (Portfolio Materials Ordering, Pre-ID Label Generation and Placement, and Packing and Shipment) This section of the Handbook contains information to be used by
COLORIMETRIC SANDWICH ELISA KIT INSTRUCTION MANUAL
 Page 1 of 7 COLORIMETRIC SANDWICH ELISA KIT INSTRUCTION MANUAL This product is for research use ONLY and not for human or animal therapeutic or diagnostic use. Page 2 of 7 Contents Page 3 I. Supplied Materials:
Page 1 of 7 COLORIMETRIC SANDWICH ELISA KIT INSTRUCTION MANUAL This product is for research use ONLY and not for human or animal therapeutic or diagnostic use. Page 2 of 7 Contents Page 3 I. Supplied Materials:
Chapter 12: Computers and Specimen Handling and Processing
 Objectives Chapter 12: Computers and Specimen Handling and Processing 1. Define the key terms and abbreviations listed at the beginning of this chapter. 2. Describe components and elements of a computer,
Objectives Chapter 12: Computers and Specimen Handling and Processing 1. Define the key terms and abbreviations listed at the beginning of this chapter. 2. Describe components and elements of a computer,
Tamper Evident Anti-Doping Equipment from Versapak
 Phone: +44 (0)20 8333 5300 Email: info@versapak-anti-doping.com www.versapak-anti-doping.com Tamper Evident Anti-Doping Equipment from Versapak PRODUCT GUIDE Versapak Doping Control Limited has been manufacturing
Phone: +44 (0)20 8333 5300 Email: info@versapak-anti-doping.com www.versapak-anti-doping.com Tamper Evident Anti-Doping Equipment from Versapak PRODUCT GUIDE Versapak Doping Control Limited has been manufacturing
UNIVERSITY OF TOLEDO HEALTH SCIENCE CAMPUS
 UNIVERSITY OF TOLEDO HEALTH SCIENCE CAMPUS SUBJECT: SPECIMEN TRANSPORT IN Procedure No: S-08-011 COMPUTERIZED TUBE SYSTEM PROCEDURE STATEMENT All laboratory specimens will be transported in a manner consistent
UNIVERSITY OF TOLEDO HEALTH SCIENCE CAMPUS SUBJECT: SPECIMEN TRANSPORT IN Procedure No: S-08-011 COMPUTERIZED TUBE SYSTEM PROCEDURE STATEMENT All laboratory specimens will be transported in a manner consistent
GI DNA Extraction Kit
 GI DNA Extraction Kit REF: 655-01; 48 extractions (32 wells plate) 656-01; 48 extractions (96 wells plate) Store at 15 C-35 C For use with the Nextractor Instrument For Professional Use Only Savyon Diagnostics
GI DNA Extraction Kit REF: 655-01; 48 extractions (32 wells plate) 656-01; 48 extractions (96 wells plate) Store at 15 C-35 C For use with the Nextractor Instrument For Professional Use Only Savyon Diagnostics
GUIDELINES FOR SHIPPING NONPATHOGENIC BIOLOGICAL CULTURES AND NON-INFECTIOUS BIOLOGICAL MATERIALS
 GUIDELINES FOR SHIPPING NONPATHOGENIC BIOLOGICAL CULTURES AND NON-INFECTIOUS BIOLOGICAL MATERIALS Table of Contents General Information Regulations Definitions Recommended packing procedure Universal precautions
GUIDELINES FOR SHIPPING NONPATHOGENIC BIOLOGICAL CULTURES AND NON-INFECTIOUS BIOLOGICAL MATERIALS Table of Contents General Information Regulations Definitions Recommended packing procedure Universal precautions
Human Angiotensin 2 (Ang2) ELISA
 Human Angiotensin 2 (Ang2) ELISA For the quantitative determination of human Ang2 in serum, plasma, cell culture fluid and other biological fluids Cat. No. KT-52748 For Research Use Only. Not for use in
Human Angiotensin 2 (Ang2) ELISA For the quantitative determination of human Ang2 in serum, plasma, cell culture fluid and other biological fluids Cat. No. KT-52748 For Research Use Only. Not for use in
