DNA polymerase proofreading: active site switching catalyzed by the bacteriophage T4 DNA polymerase
|
|
|
- Lauren Baker
- 6 years ago
- Views:
Transcription
1 Nucleic Acids Research, 2007, Vol. 35, No. 16 Published online 15 August 2007 doi: /nar/gkm591 DNA polymerase proofreading: active site switching catalyzed by the bacteriophage T4 DNA polymerase Elizabeth Fidalgo da Silva and Linda J. Reha-Krantz* Department of Biological Sciences, University of Alberta, Edmonton, Alberta T6G 2E9, Canada Received April 17, 2007; Revised June 9, 2007; Accepted July 18, 2007 ABSTRACT DNA polymerases achieve high-fidelity DNA replication in part by checking the accuracy of each nucleotide that is incorporated and, if a mistake is made, the incorrect nucleotide is removed before further primer extension takes place. In order to proofread, the primer-end must be separated from the template strand and transferred from the polymerase to the exonuclease active center where the excision reaction takes place; then the trimmed primer-end is returned to the polymerase active center. Thus, proofreading requires polymeraseto-exonuclease and exonuclease-to-polymerase active site switching. We have used a fluorescence assay that uses differences in the fluorescence intensity of 2-aminopurine (2AP) to measure the rates of active site switching for the bacteriophage T4 DNA polymerase. There are three findings: (i) the rate of return of the trimmed primer-end from the exonuclease to the polymerase active center is rapid, `500 s 21 ; (ii) T4 DNA polymerase can remove two incorrect nucleotides under single turnover conditions, which includes presumed exonucleaseto-polymerase and polymerase-to-exonuclease active site switching steps and (iii) proofreading reactions that initiate in the polymerase active center are not intrinsically processive. INTRODUCTION DNA polymerase proofreading removes misincorporated nucleotides at the primer-end (1,2), which significantly improves the fidelity of DNA replication (3). Since increased epithelial tumors are observed in mice that express an exonuclease-deficient DNA polymerase d, DNA polymerase proofreading is important in preventing mutations that lead to cancer (4). DNA polymerase proofreading was first demonstrated to be a major determinant of replication fidelity for the bacteriophage T4 DNA polymerase (2,5,6) and this DNA polymerase continues to be a valuable model for studies of proofreading, especially for Family B DNA polymerases, which include the eukaryotic DNA polymerases d and e and several viral DNA polymerases (7,8). The T4 DNA polymerase proofreading pathway has at least four steps (9,10). During chromosome replication, the proofreading pathway is initiated in the polymerase active center when an incorrect nucleotide is inserted (step 1), which hinders further primer elongation (2,3,11,12). The end of the primer strand is then separated from the template and transferred to the exonuclease active center (step 2), which requires a b hairpin structure in the exonuclease domain to form stable exonuclease complexes (13 15). The terminal nucleotide is cleaved from the primer-end in the exonuclease active center (step 3) and then the trimmed primer-end is returned to the polymerase active center where nucleotide incorporation can resume (step 4). Genetic studies indicate that four of the five protein domains of the T4 DNA polymerase are involved in the proofreading pathway (16). The genetic studies are corroborated by structural studies, which find significant conformational differences for the exonuclease, palm and thumb domains in polymerase complexes compared to exonuclease complexes (15,17 20). There are still unanswered questions about how the primer-end is shuttled back-and-forth between the polymerase and exonuclease active centers, which we address here. Many DNA polymerases are normally tethered to the DNA by a protein clamp, which is necessary for processive DNA replication. The phage T4 clamp, the product of gene 45, is also reported to stimulate proofreading (21,22), but is the clamp essential for processive transfer of the primer-end from the polymerase to the exonuclease active center and for transfer of the trimmed primer-end from the exonuclease back to the polymerase active center? We proposed that the clamp is essential for processive proofreading that initiates in the polymerase active center because greater intrinsic processivity in nucleotide incorporation is observed for mutant DNA polymerases that have reduced ability to initiate the proofreading pathway, while reduced processivity in primer extension is detected for mutant DNA polymerases that proofread more (23). *To whom correspondence should be addressed. Tel: ; Fax: ; Linda.Reha-Krantz@ualberta.ca ß 2007 The Author(s) This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License ( by-nc/2.0/uk/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
2 Nucleic Acids Research, 2007, Vol. 35, No Intrinsic proofreading, however, is observed for the T4 DNA polymerase and the closely related RB69 DNA polymerase without its clamp (12,15). Coupled removal of an incorrect nucleotide and primer extension were observed under single turnover conditions in the presence of a heparin trap; however, it is not clear in these experiments if the T4 DNA polymerase first bound the DNA substrate in the polymerase or the exonuclease active center. If the T4 DNA polymerase bound the mismatched DNA initially in the polymerase active center, then the entire proofreading pathway beginning from strand separation and transfer of the primer-end from the polymerase to the exonuclease active center can be carried out without enzyme dissociation. However, if the T4 DNA polymerase can form exonuclease complexes directly without first forming polymerase complexes, then just the steps of hydrolysis and transfer of the trimmed primerend from the exonuclease to the polymerase active center have been demonstrated to be processive in the absence of the clamp. Another outstanding question is the rate of active site switching. Proofreading during ongoing DNA replication is restricted primarily to incorrect nucleotides at the primer-end because the rate of primer extension for a matched primer terminus is much greater than the rate for initiation of the proofreading pathway, but replicative DNA polymerases have poor ability to extend a mismatched primer terminus, which then tips the balance in favor of proofreading (3,9,11). Thus, there is a kinetic barrier to initiation of the proofreading pathway, which suggests that the rate of polymerase-to-exonuclease active site switching will be relatively slow. In contrast, transfer of the trimmed primer-end from the exonuclease to polymerase active center could be rapid if the corrected primer-end returns to the polymerase active center unassisted (15). We have developed a fluorescence assay using the fluorescent adenine base analog 2-aminopurine (2AP) to examine shuttling of the primer-end between the polymerase and exonuclease active centers during the proofreading reaction catalyzed by the T4 DNA polymerase. This assay depends on two observations: (i) T4 DNA polymerase recognizes a terminal 2AP-T base pair as a mismatch and preferentially proofreads the mismatch before primer extension (24), which we confirm in experiments reported here and (ii) T4 DNA polymerase forms distinct fluorescent complexes with different levels of fluorescence intensity depending if 2AP is in the n or +1 position in the template strand (25 29). Moderately, fluorescent exonuclease complexes are formed preferentially with 2AP in the n position of the template strand (Figure 1A) and highly fluorescent complexes are formed with DNA labeled at the +1 position (Figure 1B) in which the primer-end is bound in the polymerase active center. Thus, exonucleolytic proofreading of DNA in which 2AP is initially in the n position will produce an increase in fluorescence intensity as the moderately fluorescent exonuclease complexes are converted to the highly fluorescent complexes with 2AP in the +1 position. Since the primer-end is initially in the exonuclease active center and is then transferred to the polymerase active Figure 1. DNA substrates with the base analog 2-aminopurine (P). center after the terminal nucleotide is removed to form the highly fluorescent +1 complexes, the rate of increase in 2AP fluorescence intensity provides information on the rate of exonuclease-to-polymerase active site switching. We performed this experiment with wild-type and exonuclease-deficient T4 DNA polymerases under presteady-state, single-turnover conditions in which heparin was used to trap any free T4 DNA polymerase (12). An increase in fluorescence intensity was observed for the wild-type T4 DNA polymerase at the rate of 145 3s 1, but not for an exonuclease-deficient T4 DNA polymerase. These results are discussed with respect to the overall proofreading reaction, active site switching, structural implications and replication fidelity of the wild-type and proofreading defective T4 DNA polymerases. MATERIALS AND METHODS DNA polymerases Expression, purification and characterization of the wildtype and mutant D112A/E114A- and W213S-DNA polymerases were done as described previously (30,31). DNA substrates The 2AP-containing DNA substrates are described in Figure 1. The substrates were prepared as described previously (13,14,25). The 3 0 terminus of the template strand of the DNA duplexes was protected from enzyme binding by attachment of a biotin (b) group (BiotinTEG- CPG, Glen Research). The 2AP phosphoramidite was purchased from Glen Research. All oligonucleotides were purified by gel electrophoresis. The primer and template strands were annealed in buffer containing HEPES (ph 7.5) and 50 mm NaCl with a 20% excess of the oligonucleotide without 2AP to ensure complete hybridization of the 2AP containing strand. The non-2ap containing DNA substrates used for Figure 2 were synthesized using standard procedures and purified by gel electrophoresis. The annealing conditions were the same as used for the 2AP-containing oligonucleotides. The template strand was in 20% excess to ensure
3 5454 Nucleic Acids Research, 2007, Vol. 35, No. 16 Figure 2. Coupled processive proofreading and nucleotide incorporation. (A) DNA substrates. The reaction conditions are described in Materials and Methods section. (B) Test of the heparin trap under the conditions described by Reddy et al. (12). When heparin at 1 mg/ml was added to reactions before the addition of the wild-type T4 DNA polymerase no exonuclease activity (lane 1) or primer extension activity (lane 3) were detected. Full exonuclease (lane 2) and primer extension (lane 4) activities were detected in the absence of heparin. (C) Primerextension reactions in the presence of 1 mg/ml heparin with the matched DNA substrate and dctp (lanes 1, 3 and 5) and the mismatched DNA substrate with datp and dctp (2,4,6). The wildtype T4 DNA polymerase (lanes 1 and 2) carried out processive primer extension with the matched DNA (lane 1) and processive proofreading and primer extension reactions with the mismatched DNA (lane 2). The W213S-DNA polymerase had less ability to fully extend the matched DNA (lane 3) and almost no ability to carry out processive proofreading and primer extension reactions with the mismatched DNA (lane 4). The D112A/E114A-DNA polymerase extended the matched DNA substrate as efficiently as the wild-type enzyme (lane 5), but this mutant DNA polymerase had no ability to extend the mismatched DNA substrate. A control reaction with no DNA polymerase is in lane 7. complete hybridization of the 32 P-labeled primer strand, which was labeled using a standard T4 polynucleotide kinase labeling procedure (12). Processive (single-turnover) proofreading and incorporation reactions with 32 P-labeled DNA substrates Reaction mixtures (20 ml) contained 50 nm DNA, 150 nm DNA polymerase, 25 mm HEPES (ph 7.5), 50 mm NaCl, 1 mm DTT, 0.5 mm EDTA and 100 mm dntps as indicated. The reactions were first pre-incubated at 378C and then started by the addition of a solution of Mg 2+ /heparin to give a final concentration of 8 mm Mg 2+ and 1 or 0.1 mg/ml heparin as indicated (Sigma, 3000 average molecular weight from porcine intestinal mucosa). Reactions were stopped after 15 s by addition of 20 ml gel loading solution (95% formamide, 20 mm EDTA, and xylene cyanol and bromphenol blue dyes). Reddy et al. (12) used heparin at 1 mg/ml, but we find that 0.1 mg/ml is sufficient (10). The reaction products were separated on DNA sequencing type gels containing 15% acrylamide and 8 M urea. The 32 P-labeled products were visualized by using a PhosphorImager (Molecular Dynamics, Inc.). Exonuclease and primer extension reactions with 32 P-labeled DNA substrates under non-processive (multiple-turnover) conditions The same reaction conditions were used as described above for single-turnover reactions except that the heparin trap was omitted. Processive (single-turnover) hydrolysis and active site switching reactions determined by using changes in 2AP fluorescence intensity Stopped-flow experiments were performed with the Applied Photophysics SX.18 MV instrument, which allowed us to determine the pre-steady-state kinetics of selected aspects of the proofreading pathway. Excitation was at 310 nm; a 320 nm cutoff filter was used. The temperature in the sample-handling unit was maintained at C. Reactions were initiated by mixing equal volumes of a solution of T4 DNA polymerase DNA complexes, which contained 400 nm DNA labeled at the n position with 2AP (Figure 1A), 1000 nm T4 DNA polymerase, 25 mm HEPES (ph 7.5), 50 mm NaCl, 1 mm DTT and 0.5 mm EDTA with a second solution containing 16 mm MgCl 2, 0.2 mg/ml heparin, 25 mm HEPES (ph 7.5), 50 mm NaCl and 1 mm DTT. After mixing, the final concentrations of reaction components were 200 nm DNA polymerase DNA complexes, 25 mm HEPES (ph 7.5), 50 mm NaCl, 1 mm DTT, 0.25 mm EDTA, 8 mm MgCl 2 and 0.1 mg/ml heparin. The optimal DNA and enzyme concentrations to ensure full complex formation were determined by titration experiments (25). In general, a 52-fold excess of DNA polymerase over DNA produces maximal complex formation for DNA concentrations from 200 to 600 nm. Curves were fit either to single (monophasic) or double (biphasic) exponential equations. Six or more runs were performed with each set of reaction conditions; mean values were calculated.
4 Nucleic Acids Research, 2007, Vol. 35, No Multiple turnover proofreading reactions determined by using changes in 2AP fluorescence intensity The same reaction conditions were used as described above for single-turnover reactions except that the heparin trap was omitted. RESULTS Processive (single-turnover) proofreading and incorporation reactions with 32 P-labeled DNA substrates Reddy et al. (12) used heparin to obtain single-turnover (single-encounter) conditions for exonuclease and nucleotide incorporation reactions with the T4 DNA polymerase; any DNA polymerase molecules that dissociate from the DNA template were prevented from rebinding to the DNA substrate by forming stable complexes with heparin. Heparin is indeed a useful trap for the T4 DNA polymerase. If 1 mg/ml heparin, the concentration used by Reddy et al. (12), is added to exonuclease or primerextension reactions with the matched DNA substrate (Figure 2A) before the addition of the T4 DNA polymerase, no activity is detected (Figure 2B, lanes 1 and 3). In the absence of heparin, exonucleolytic degradation (Figure 2B, lane 2) and full primer extension (Figure 2B, lane 4) are observed. Single-turnover primer extension reactions with matched and mismatched DNA substrates (Figure 2A) were carried out with the wild-type T4 DNA polymerase. Reaction components were first pre-incubated in the absence of Mg 2+ and then the reactions were initiated by the addition of a solution of Mg 2+ /heparin. In the primer extension reaction with the matched DNA substrate in which the only nucleotide provided was dctp, the wild-type T4 DNA polymerase fully extended most of the primer by two nucleotides; only a small amount of partially extended +1 product was detected (Figure 2C, lane 1). Although the wild-type T4 DNA polymerase has a potent exonuclease activity, only traces of products less than the length of the primer strand were observed (Figure 2C, lane 1). Degradation products were detected because the only nucleotide provided in these reactions was dctp, which means that if there was any primer degradation first removal of the terminal dtmp, then another dtmp, etc. (Figure 1A) the primer could not be resynthesized. Thus, the wild-type T4 DNA polymerase formed primarily polymerase complexes with the matched DNA substrate that were poised for nucleotide incorporation rather than exonuclease complexes poised for primer degradation. Primer extension was also detected with the mismatched T-T DNA substrate (Figure 2A) in which dctp and datp were provided (Figure 2C, lane 2). Because the wild-type T4 DNA polymerase cannot efficiently extend a mismatched primer-end (2,11,12), the primer extension observed with the mismatched DNA substrate must have been preceded by removal of the incorrect terminal dtmp, which was followed by transfer of the trimmed primer-end from the exonuclease to the polymerase active center, incorporation of damp and then incorporation of two dcmps. All steps were performed without dissociation of the DNA polymerase since the heparin trap was present. To further demonstrate that exonucleolytic proofreading of the mismatched primer terminus is required before the primer can be extended, experiments were repeated with the exonuclease deficient D112A/E114A-DNA polymerase under the same conditions used for the wild-type T4 DNA polymerase. The D112A/E114A-DNA polymerase has an alanine substitution for an essential aspartate (D112) residue in the exonuclease active center and, as a consequence, has almost no detectable 3 0! 5 0 exonuclease activity (31). While the D112A/E114A-DNA polymerase extended the matched DNA substrate under singleturnover conditions (Figure 2C, lane 5) as efficiently as the wild-type T4 DNA polymerase (Figure 2C, lane 1), no extension was observed for the mismatched DNA substrate (Figure 2C, lane 6). We also tested the ability of the W213S-DNA polymerase to carry out primer extension reactions of the matched and mismatched DNA substrates under single-turnover conditions. The W213S-DNA polymerase replicates DNA with reduced fidelity in vivo (16), which is due to reduced exonuclease activity. Significantly less degradation of single-stranded DNA was observed for the W213S-DNA polymerase compared to the wild-type T4 DNA polymerase in multiple-turnover reactions (Figure 3; compare wild-type activity in lanes 1 and 2 to that of the W213S-DNA polymerase in lanes 3 and 4). Even less exonuclease activity was detected for the W213S-DNA polymerase on double-stranded DNA (compare wild-type activity in lane 5 to that of the W213S-DNA polymerase in lane 8). In single-turnover reactions with the W213S- DNA polymerase and the matched DNA substrate, dcmp incorporation was observed (Figure 2C, lane 3), but primer extension was not as efficient as observed for the wild-type and D112A/E114A-DNA polymerases (Figure 2C, lanes 1 and 5, respectively). In single-turnover reactions with the W213S-DNA polymerase and the mismatched DNA substrate (Figure 2C, lane 4), a small amount of +2 extension product was detected, which indicates that the W213S-DNA polymerase can catalyze only a very limited processive proofreading-nucleotide incorporation reaction. T4 DNA polymerase recognizes the terminal 2AP-T base pair as a mismatch The DNA substrate labeled with 2AP in the n (terminal) position of the primer strand (Figure 1C) was labeled with 32 P at the 5 0 -end of the primer strand. Single-turnover experiments were performed as were done for the reactions shown in Figure 2C except that the concentration of heparin was reduced from 1 to 0.1 mg/ml, which we found to be as effective (10). Reactions contained dttp, dctp and datp; thus, successful primer extension will extend the primer by 4 nt. The wild-type T4 DNA polymerase produced the +4 extension product under single turnover conditions with the heparin trap (Figure 4, lane 2), but much less primer extension was observed for the W213S-DNA polymerase (Figure 4, lane 1).
5 5456 Nucleic Acids Research, 2007, Vol. 35, No. 16 Figure 3. The W213S-DNA polymerase has reduced exonuclease activity. All reactions were incubated for 15 s at 378C. Reactions with 25 nm single-stranded DNA and 25 nm or 50 nm enzyme are shown in lanes 1 and 3 and lanes 2 and 4, respectively. Exonuclease reactions with the wild-type T4 DNA polymerase are in lanes 1 and 2; reactions with the W213S-DNA polymerase are in lanes 3 and 4. Exonuclease reactions with 25 nm double-stranded DNA and 50 nm enzyme are in lanes 5 (wild-type) and 8 (W213S-DNA polymerase). Primer extension reactions with the wild-type and W213S-DNA polymerases are in lanes 6 and 7, respectively. No primer extension product was detected for the D112A/ E114A-DNA polymerase (data not shown), which indicates that extension of the 2AP-T terminal base pair cannot be done under single turnover conditions by this mutant enzyme. Thus, the processive primer extension reaction first required removal of the terminal 2AP nucleotide from the primer-end, then transfer of the trimmed primer-end to the polymerase active center and finally nucleotide incorporation. The reactions shown in Figure 4 demonstrate that the terminal 2AP-T base pair with 2AP in the terminal position of the primer strand is recognized as a mismatch Figure 4. Proofreading of the 2AP-T terminal base pair occurs before primer extension; single turnover conditions. Primer extension reactions were performed with the DNA substrate described in Figure 1C, which has 2AP at the primer terminus. Reactions contained DNA labeled at the 5 0 -end of the primer strand with 32 P, DNA polymerase, buffer, datp, dttp and dctp; reactions were initiated with a solution of Mg 2+ /heparin as described in Materials and Methods section. Full primer extension was observed for the wild-type T4 DNA polymerase (lane 2), but not for the exonuclease-deficient W213S-DNA polymerase (lane 1). A control reaction with no enzyme is shown in lane 3. by the T4 DNA polymerase. The same is true if 2AP is in the n position in the template strand and T is in the terminal position of the primer strand (25,28). The DNA substrate labeled with 2AP in the n position of the template strand (Figure 1A) is used in the next experiments. Determining the rate of the proofreading reaction catalyzed by the wild-type T4 DNA polymerase using changes in 2AP fluorescence intensity; single turnover conditions The previous experiments demonstrate that the T4 DNA polymerase can proofread a T-T mismatch (Figure 2C) and a 2AP-T terminal base pair (Figure 4) and then incorporate nucleotides without dissociating from the DNA substrate; however, it is not possible to determine from these experiments if the proofreading pathway initiated in the polymerase or the exonuclease active center or in both. These experiments also do not provide information about the rate of active site switching. Both questions were addressed by measuring the increase in 2AP fluorescence intensity in stopped-flow experiments for the conversion of the DNA substrate with 2AP in the n position in the template strand to the DNA with 2AP in the +1 position (the DNAs are described in Figure 1A and B, respectively). Two rates were detected for removal of the 2AP nucleotide from the primer-end under multiple turnover conditions for DNA substrates like the DNA described in
6 Nucleic Acids Research, 2007, Vol. 35, No Figure 1C (13,14,32); thus, two rates are also expected for the removal of dtmp opposite template 2AP for the DNA substrate described in Figure 1A. The two rates observed in previous experiments were explained by the proposal that the DNA primer/template exists in two states in solution: (i) an annealed state, which is the substrate used by the T4 DNA polymerase for forming complexes with the primer/template bound in the polymerase active center and (ii) a melted state, which is the preferred substrate for forming exonuclease complexes in which the end of the primer strand is bound in the exonuclease active center (32). The faster of the two rates was attributed to proofreading reactions that initiated in the exonuclease active center (activated complexes) and the slower rate was attributed to reactions in which the template/primer was first bound in the polymerase active center and, thus, were initially inactive for the hydrolysis reaction. If two rates are detected in the presence of the heparin trap for removal of dtmp opposite template 2AP, then complexes formed initially with the primer/template in the polymerase active center and complexes formed with the primerend bound in the exonuclease active center can both support processive proofreading reactions. If a single rate is observed, then only one type of complex can carry out the proofreading reaction processively. For reactions initiating in the exonuclease active center, a rate as fast as 100 to 4200 s 1 may be observed, which are the reported rates for hydrolysis of the terminal phosphodiester bond in the exonuclease active center (9,13). A 510-fold slower rate is expected for proofreading reactions that initiate in the polymerase active center (9,13,14,32). A solution of moderately fluorescent complexes was formed with the wild-type T4 DNA polymerase and DNA labeled in the n position in the template strand (Figure 1A). This solution was mixed with an equal volume of a second solution containing Mg 2+ /heparin in the stopped-flow, which produced an increase in fluorescence intensity at the observed rate, k obs = 145 3s 1 (Figure 5A). The curve was best fit by a single exponential equation. Because a single rate was observed in the range of the reported hydrolysis rate, processive proofreading appears to be detected only for complexes in which the primer-end was bound initially in the exonuclease active center. Thus, the addition of Mg 2+ to the preformed complexes triggered the following chain of steps: excision of the terminal phosphodiester bond in the exonuclease active center to remove dtmp and transfer of the trimmed primer-end to the polymerase active center to form the highly fluorescent complexes in which 2AP is now in the +1 position. In experiments with the 32 P-labeled DNAs (Figures 2C and 4), nucleotide incorporation follows return of the trimmed primer-end to the polymerase active center. The highly fluorescent complexes with 2AP in the +1 position are also poised for nucleotide incorporation since these complexes bind the correct nucleotide rapidly within the dead time of the stopped-flow instrument (26,29). No increase in fluorescence intensity was detected in reactions with the proofreading deficient W213S-DNA polymerase, as expected since this mutant DNA polymerase has only very limited ability to carry out processive Figure 5. Time courses for conversion of exonuclease complexes to polymerase complexes. (A) Single turnover conditions. A solution of complexes formed with the wild-type T4 DNA polymerase and DNA labeled at the n position in the template strand with 2AP (Figure 1A) was mixed with a second solution containing Mg 2+ /heparin as described in Materials and Methods section. Fluorescence intensity increased at the rate of 145 s 1.(B) Multiple turnover conditions. The experimental conditions described above were repeated except that heparin was omitted. The increase in fluorescence intensity was biphasic; the best curve fit was achieved using a double exponential equation. The faster rate was 106 s 1 and the slower rate was 11 s 1 (Table 1). proofreading as demonstrated in the primer extension assays (Figures 2C and 4). Determining the rate of the proofreading reactions catalyzed by the wild-type and W213S-DNA polymerases using changes in 2AP fluorescence intensity; multiple turnover conditions The above experiments were repeated without the heparin trap. The increase in fluorescence intensity was biphasic in reactions with the wild-type T4 DNA polymerase. The best curve fit was achieved by using a double exponential equation; the faster rate was s 1 and the slower rate was 11 1s 1 (Figure 5B, Table 1). The two rates indicate that two distinct populations of complexes were formed initially: one population (40% of the complexes) can form the highly fluorescent +1 complexes at about
7 5458 Nucleic Acids Research, 2007, Vol. 35, No. 16 Table 1. Proofreading and active site switching rates determined under single and multiple turnover conditions Reaction conditions WT T4 DNA pol (Figure 5A) DNA: Figure 1A, single Removal of one nucleotide WT T4 DNA pol (Figure 5B) DNA: Figure 1A, multiple Removal of one nucleotide W213S-DNA pol DNA: Figure 1A, multiple Removal of one nucleotide WT T4 DNA pol (Figure 6) DNA: Figure 1D, single Removal of two nucleotides Removal of the first nucleotide Removal of the second nucleotide Proofreading rates (s 1 ) Active site switching rates (s 1 ) exo-to-pol: (0.4) (0.6) pol-to-exo: exo-to-pol/ pol-toexo: Details of the reactions and the calculation of reaction rates are described in the text. Figure 6. Time course for removal of two incorrect nucleotides; single turnover conditions. A solution of complexes formed with the wild-type T4 DNA polymerase and the mismatched DNA substrate with two terminal incorrect nucleotides (Figure 1D) was mixed with a second solution containing Mg 2+ /heparin as described in Materials and Methods section. Fluorescence intensity decreased at the rate of 55 s 1 (Table 1). the same rate as detected in the presence of the heparin trap and a second population (60% of the complexes) that forms the +1 complexes at a 10-fold slower rate (Table 1). Since a single 145 s 1 rate was observed in the presence of the heparin trap, only the population of complexes that proofreads at the apparent rate of 106 s 1 rate appears to carry out the proofreading reaction processively. Since heparin prevents detection of the slower 11 s 1 rate, complexes responsible for this rate must initially be inactive and can convert to active complexes only by an intervening dissociation, heparin-sensitive step. A single rate of s 1 was observed for the W213S-DNA polymerase (Table 1). This slow rate is consistent with the severely reduced ability of this mutant DNA polymerase to degrade single- and double-stranded DNA (Figure 3). Determining the rate for removal of two incorrect nucleotides The T4 DNA polymerase and the closely related RB69 DNA polymerase can remove two incorrect nucleotides and then extend the primer terminus under single-turnover conditions in the presence of the heparin trap (12,15). We repeated the above experiments with the DNA substrate illustrated in Figure 1D, which has two incorrect G nucleotides at the end of the primer strand and 2AP is in the n position in the template strand. Moderately, fluorescent exonuclease complexes are formed with this DNA substrate (25,28). After removal of the two incorrect G nucleotides, 2AP will be in the +2 position (Figure 1D). T4 DNA polymerase complexes formed with 2AP in the +2 position of the template strand are only weakly fluorescent (25). Thus, removal of the two terminal incorrect G nucleotides is expected to produce an overall decrease in fluorescence intensity, but will there be an intervening increase in fluorescence intensity after removal of the terminal incorrect nucleotide since 2AP will be transiently in the +1 position? Highly fluorescent +1 complexes are not expected to be formed after removal of the first incorrect nucleotide since these complexes are not detected if the terminal base pair is mismatched (28). No increase in fluorescence intensity was observed; fluorescence intensity decreased at the rate of 55 2s 1 (Figure 6, Table 1). The same rate of decrease in fluorescence intensity was also observed without the heparin trap, which indicates that none of the complexes formed during the process of removing two incorrect nucleotides are sensitive to the heparin trap. The T4 DNA polymerase exonuclease activity degrades single-stranded DNA one nucleotide at a time from the 3 0 -end; hence, the rate of 55 s 1 for removing two nucleotides is the combined rate for two consecutive excision steps. If removal of the penultimate incorrect nucleotide occurs at the rate of 145 s 1, as determined for removal of a single incorrect nucleotide (Figure 5A), then it is possible to calculate the overall rate for removal of the terminal incorrect nucleotide by using the following equation: 1/k removal of 2 nucleotides =1/k removal of 1st nucleotide +1/k removal of 2nd nucleotide. By rearranging the equation, 1/k removal of 1st nucleotide =1/k removal of 2 nucleotides 1/k removal of 2nd nucleotide = 1/55 1/145. Thus, k removal of 1st nucleotide = 88.5 s 1. The slower apparent rate for removal of the terminal nucleotide compared to the rate for removal of the second incorrect nucleotide suggests that there are extra steps for removal of the terminal nucleotide. We propose that after removal of the terminal nucleotide, the trimmed primerend is returned to the polymerase active center. This proposal is reasonable since the correctness of the primerend can only be examined in the polymerase active center where hydrogen bonding between the terminal base on the primer strand and the complementary template base can
8 Nucleic Acids Research, 2007, Vol. 35, No be evaluated as well as the geometry of the terminal base pair (29,33). Such a mechanism must exist in order to explain how exonucleolytic proofreading is limited primarily to the removal of incorrect nucleotides. If the primer-end is found to be incorrect, then the primer-end is returned to the exonuclease active center for a second cycle of excision, and then the further trimmed primer-end is returned to the polymerase active center. DISCUSSION We developed a fluorescence assay that uses changes in 2AP fluorescence intensity in 2AP-labeled DNA to monitor the proofreading reaction catalyzed by the T4 DNA polymerase. T4 DNA polymerase forms moderately fluorescent exonuclease complexes with duplex DNA substrates labeled with 2AP in the n position of the template strand (Figure 1A) and highly fluorescent complexes with the primer-end bound in the polymerase active center for DNAs labeled at the +1 position in the template strand (Figure 1B). Thus, exonucleolytic proofreading of the DNA substrate labeled initially with 2AP in the n position in the template strand will produce an increase in fluorescence intensity due to formation of the highly fluorescent complexes with 2AP in the +1 position. The rate of increase in fluorescence intensity is a measure of the overall reaction; k obs under single turnover conditions was 145 3s 1 (Figure 5A). We used this assay to confirm the results of Reddy et al. (12) that the wild-type T4 DNA polymerase can catalyze a processive proofreading reaction without accessory proteins, but only for reactions that initiate in the exonuclease active center. Only a single proofreading rate of 145 s 1 was detected in the presence of the heparin trap (Figure 5A), but two rates of 106 and 11 s 1 were detected in the absence of heparin (Figure 5B). We (32) and others (9) proposed that the T4 DNA polymerase can form two types of complexes [E-D] exo complexes that are active for hydrolysis of the terminal nucleotide and [E-D] pol complexes that are inactive for hydrolysis. [E-D] exo complexes react quickly with Mg 2+ to give a burst of product. Since the 145 and 106 s 1 rates are in the range of the reported hydrolysis rate for the T4 DNA polymerase (9,13), these rates are likely produced from active [E-D] exo complexes. Under multiple turnover conditions, inactive [E-D] pol complexes can convert slowly to active [E-D] exo complexes, at the rate of 11 s 1 in experiments reported here (Figure 5B, Table 1). This slow rate is not detected in the presence of the heparin trap, which indicates that conversion from an inactive to an active state involves enzyme dissociation. This point is discussed again later with respect to the clamped or tethered DNA polymerase. The 2AP fluorescence assay can also be used to determine the rates for active site switching. The rate of increase in fluorescence intensity for conversion of the moderately fluorescent exonuclease complexes with 2AP in the n position to the highly fluorescent polymerase complexes with 2AP in the +1 position is a measure of the overall rate for the proofreading pathway that initiates in the exonuclease active center. The terminal phosphodiester bond of the primer strand is hydrolyzed in the exonuclease active center and then the trimmed primerend is transferred from the exonuclease to the polymerase active center where the highly fluorescent +1 complexes are formed. These steps can be described by the following equation: 1/k obs = 1/145 = 1/k hydrolysis +1/k exo-to-pol transfer +1/k +1complexes. The combined rates for exonucleaseto-polymerase transfer of the trimmed primer-end and for formation of the highly fluorescent +1 complexes can be calculated if the hydrolysis rate is known. The hydrolysis rate catalyzed by the T4 DNA polymerase is reported to be 100 s 1 for degradation of single-stranded DNA (9) and from 176 to 228 s 1 (13) for removal of the 2AP nucleotide from the 3 0 -end of single-stranded DNA. Because the hydrolysis rate must be faster than the observed overall rate of 145 3s 1 detected in our experiments, the true hydrolysis rate is likely closer to 200 s 1, the average rate reported for removal of a terminal 2AP nucleotide (13). The combined rates for exonuclease-to-polymerase switching and formation of the highly fluorescent complex with 2AP in the +1 position can be calculated from the following equation: [1/k exo-to-pol transfer +1/k +1complexes ]=1/k obs 1/k hydrolysis = 1/145 1/200; therefore, [k exo-to-pol transfer + k +1complexes ] = 526 s 1 (Table 1). Thus, once the hydrolysis reaction takes place, the trimmed primer-end is returned rapidly to the polymerase active center in position to resume nucleotide incorporation. The efficient proofreading reaction that initiates in the exonuclease active center has several implications for understanding proofreading by the T4 DNA polymerase and Family B DNA polymerases in general. First, the template strand is likely bound in the polymerase active center when the primer-end is bound in the exonuclease active center. Intuitively, it makes sense for the template strand to be held in the polymerase active center during proofreading to ensure that the trimmed primer-end will be returned to the polymerase active center in correct alignment, otherwise frameshift mutations will be produced. It is also important that proofreading be limited to only removing incorrect nucleotides in order to prevent gratuitous degradation of the newly synthesized DNA, which would slow DNA replication and waste dntps. Severely reduced DNA replication is observed in T4 infections with mutant DNA polymerases that catalyze excessive proofreading (23,34). These potential problems with proofreading can be reduced if the trimmed primerend is returned to the polymerase active center in position to resume replication after an incorrect nucleotide is removed. If the primer-end is matched, primer extension will be the favored reaction; however, if the primer-end is not correct or if the primer-end is misaligned, then another cycle of proofreading will be favored over primer extension. Several observations are consistent with the proposal that the template strand is held in the polymerase active center when the primer-end is bound in the exonuclease active center. Moderate fluorescence enhancement is observed for 2AP in the n and +1 positions in the template strand in exonuclease complexes (25,29,35), which is the starting point of the fluorescence assay shown in Figure 5A. The rapid transfer (4500 s 1 )ofthe
9 5460 Nucleic Acids Research, 2007, Vol. 35, No. 16 trimmed primer-end from the exonuclease to the polymerase active center to form the highly fluorescent +1 complexes (the end point of the fluorescence assay shown in Figure 5A) is also consistent with the template strand being held in the polymerase active center since the +1 complexes are poised for rapid nucleotide incorporation (26,29). Furthermore, the ability of the T4 DNA polymerase to remove two incorrect nucleotides under single turnover conditions (Figure 6) suggests that the trimmed primer-end can be efficiently shuttled back-andforth between the exonuclease and polymerase active centers, which can only reasonably occur if the template strand remains bound in the polymerase active center. How does the T4 DNA polymerase transfer the primerend between the polymerase and exonuclease active centers? The apparent rapid rate for return of the trimmed primer-end to the polymerase active center 4500 s 1 indicates that exonuclease-to-polymerase switching is rapid once the terminal phosphodiester bond is cleaved. Thus, the primer-end may spring back to the polymerase active center unassisted once the terminal phosphodiester bond is cleaved. This proposal is supported by the observation that while a b hairpin structure in the exonuclease domain is important for forming stable exonuclease complexes (10,13 15), this structure is not needed for return of the trimmed primer-end to the polymerase active center (15). However, deletion of the loop in the b hairpin structure reduces the ability of the RB69 DNA polymerase to remove two incorrect nucleotides (15). Thus, the b hairpin may have a role in assisting further strand separation and reformation of exonuclease complexes for the second proofreading cycle. The overall rate for removing two incorrect nucleotides is 55 s 1 (Table 1, Figure 6). The calculated overall rate for removing the first incorrect nucleotide is 88.5 s 1 if removal of the second nucleotide occurs at the same rate as removal of a singly incorrect nucleotide 145 s 1.We propose that the trimmed primer-end is returned to the polymerase active center after removal of an incorrect nucleotide where the accuracy of the primer-end is evaluated based on the ability of the primer-end to form hydrogen bonds with the complementary template bases. Thus, the 88.5 s 1 rate includes transfer of the primer-end back-and-forth between the exonuclease and polymerase active centers plus an intervening evaluation of the primerend in the polymerase active center. The rate for these combined exo-to-pol/evaluation/pol-to-exo steps can be calculated from the following equation: 1/k exo-to-pol/evaluation/pol-to-exo =1/k removal of 1st nucleotide 1/k hydrolysis =1/ /200 = ; thus, k exo-to-pol/evaluation/pol-toexo = 159 s 1 (Table 1). Processive proofreading for reactions that initiate in the exonuclease active center appear to be limited to removal of two incorrect nucleotides because removal of three incorrect nucleotides is not reported to occur (12) or to take place less efficiently than removal of one or two incorrect nucleotides (15). We conclude from this observation that the removal of a third incorrect nucleotide involves a heparin-sensitive step that is not present for removal of the first two incorrect nucleotides. This step is presumably slower than enzyme dissociation. What is the role of the clamp in proofreading? We could not detect any intrinsic processive proofreading for reactions that initiated in the polymerase active center (Figure 5A), but proofreading is stimulated by the clamp protein, the product of T4 gene 45 (21,22). Proofreading could be stimulated if the clamp allows intramolecular polymerase-to-exonuclease switching without dissociation of the DNA polymerase from the DNA. If this is the case, then tethering the DNA polymerase to the DNA allows strand separation and transfer of the primer-end from the polymerase to the exonuclease active center, even if the rate is slower than the rate for enzyme dissociation. Another possibility is that polymerase-to-exonuclease active site switching is intermolecular. Given the efficient ability of the T4 DNA polymerase to proofread mismatched DNAs by forming exonuclease complexes directly without first forming polymerase complexes [Figures 5A and 6; (12,15,32)], the T4 DNA polymerase may normally dissociate from the DNA substrate when a wrong nucleotide is incorporated, even when clamped to the DNA, and then rebind to form exonuclease complexes with the mismatched DNA. The mismatched DNA may be rebound by the same or another DNA polymerase. The proposal of enzyme dissociation has merit because the concept of processivity in DNA replication has recently been redefined for the T4 DNA polymerase. Yang et al. (36) demonstrated that T4 DNA polymerases exchange during DNA replication and that this exchange requires the clamp. Since the clamp can potentially bind the replicating DNA polymerase and a spare DNA polymerase, incorporation of a wrong nucleotide may lead to dissociation of the replicating DNA polymerase and then the spare DNA polymerase forms an exonuclease complex with the mismatched DNA (20,37). The role of the clamp then is to provide a locally high concentration of spare DNA polymerases at replication forks for exonucleolytic proofreading. This proposal could explain why reduced concentrations of DNA polymerase d in yeast produces a mutator phenotype (38). If reduced concentrations of DNA polymerase d means that there is not always a tethered spare for exonucleolytic proofreading, then proofreading would be reduced. This situation could also provide increased opportunity for replication by a translesion DNA polymerase such as DNA polymerase z, which lacks proofreading activity. This scenario also provides a possible mechanism to explain how DNA polymerase d can proofread for DNA polymerase a (39) or for a translesion DNA polymerase to take over replication when DNA damage blocks replicative DNA polymerases (36). One last point to consider is what happens with mutant DNA polymerases that have reduced ability to catalyze the exonuclease reaction. The exonuclease deficient D112A/E114A-DNA polymerase has almost no detectable ability to carry out removal of an incorrect terminal nucleotide or to extend the mismatched primer terminus under single-turnover conditions (Figure 2C, lane 6); the W213S-DNA polymerase has only limited ability to do so (Figure 2C, lane 4). The W213S-DNA polymerase slowly removed (1.5 s 1 ) the terminal dtmp nucleotide from the DNA substrate with 2AP in the n position and this activity
10 Nucleic Acids Research, 2007, Vol. 35, No Figure 7. Proofreading pathways catalyzed by the bacteriophage T4 DNA polymerase. Proofreading pathways that initiate in the exonuclease active center are illustrated for a single terminal incorrect nucleotide (I) and for two terminal incorrect nucleotides (II). Proofreading that initiates in the polymerase active center is illustrated by pathway III. Details are presented in the Discussion section. was observed only in the absence of the heparin trap (Table 1). Increased base substitution mutations are observed for both mutant DNA polymerases in vivo as expected if proofreading activity is reduced (16). Mutant T4 DNA polymerases with reduced ability to catalyze the hydrolysis reaction also produce increased frameshift mutations, which is not observed to the same extent for other mutant DNA polymerases that are defective in proofreading but still retain significant hydrolysis activity (40, unpublished data). One intriguing question is what happens if the terminal nucleotide is not removed, as is expected to be the case for the hydrolysis-defective DNA polymerases? Will the uncorrected primer-end be returned to the polymerase active center? If so, will the primer-end be correctly aligned? Since increased frameshift mutagenesis is observed for mutant DNA polymerases that are deficient in cleaving the terminal phosphodiester bond, strand misalignments may be a consequence of aberrant proofreading reactions. SUMMARY The proofreading pathway catalyzed by the bacteriophage T4 DNA polymerase is presented in Figure 7. The experiments presented in this report begin with preformed exonuclease complexes, but association rates were determined in previous experiments and range from 70 to 120 s 1 depending on the DNA sequence, which correspond to bimolecular association rates of M 1 s 1 (14,32). In pathway I for removal of a single incorrect nucleotide, exonuclease complexes are formed directly in which the primer-end is bound in the exonuclease active center and the template strand is bound in the polymerase active center. Hydrolysis (200 s 1 ) and rapid transfer (4500 s 1 ) of the trimmed primer-end to the polymerase active center produces a DNA polymerase complex (identified by an asterisk ) that is poised to resume rapid nucleotide incorporation. For DNA substrates with two incorrect nucleotides at the primer-end (pathway II), exonuclease complexes are again formed. The terminal wrong (W) nucleotide is excised, then we propose that the trimmed primer-end is transferred to the polymerase active center (step a) as happens for removal of a single wrong nucleotide. However, since the primer-end still has a wrong nucleotide, the incorrect primer-end is returned to the exonuclease active center (step b) for a second cycle of excision. The overall rate for steps a + b is calculated to be 159 s 1 (Table 1). The second wrong nucleotide is then removed as described for pathway I. Intrinsic processive proofreading was not detected for reactions that initiate in the polymerase active center, pathway III. We observed a rate of 11 s 1 in multiple turnover reactions for the DNA substrate used in experiments reported here (Table 1), which involves dissociation of the DNA polymerase from a complex that is inactive for the excision reaction and then formation of an active exonuclease complex. Although the apparent rate for initiating the proofreading reaction in the polymerase active center is slow, 11 s 1, this rate is still much faster than the rate for extending a mismatched primer terminus (11), but is slower than the rate for extension of a matched primer terminus (26,29). Thus, the 11 s 1 rate is a barrier to gratuitous proofreading, but is fast enough to prevent extension of a
Welcome to Class 18! Lecture 18: Outline and Objectives. Replication is semiconservative! Replication: DNA DNA! Introductory Biochemistry!
 Lecture 18: Outline and Objectives Welcome to Class 18! Introductory Biochemistry! l DNA Replication! l DNA polymerase! l the enzymatic reaction! l proofreading and accuracy! l DNA synthesis! l origins
Lecture 18: Outline and Objectives Welcome to Class 18! Introductory Biochemistry! l DNA Replication! l DNA polymerase! l the enzymatic reaction! l proofreading and accuracy! l DNA synthesis! l origins
Evaluating The Effects of Enhanced Processivity and Metal Ions on Translesion DNA Replication Catalyzed by The Bacteriophage T4 DNA Polymerase
 Cleveland State University EngagedScholarship@CSU Chemistry Faculty Publications Chemistry Department 5-16-2003 Evaluating The Effects of Enhanced Processivity and Metal Ions on Translesion DNA Replication
Cleveland State University EngagedScholarship@CSU Chemistry Faculty Publications Chemistry Department 5-16-2003 Evaluating The Effects of Enhanced Processivity and Metal Ions on Translesion DNA Replication
Questions from chapters in the textbook that are relevant for the final exam
 Questions from chapters in the textbook that are relevant for the final exam Chapter 9 Replication of DNA Question 1. Name the two substrates for DNA synthesis. Explain why each is necessary for DNA synthesis.
Questions from chapters in the textbook that are relevant for the final exam Chapter 9 Replication of DNA Question 1. Name the two substrates for DNA synthesis. Explain why each is necessary for DNA synthesis.
 The replication of DNA Kornberg 1957 Meselson and Stahl 1958 Cairns 1963 Okazaki 1968 DNA Replication The driving force for DNA synthesis. The addition of a nucleotide to a growing polynucleotide
The replication of DNA Kornberg 1957 Meselson and Stahl 1958 Cairns 1963 Okazaki 1968 DNA Replication The driving force for DNA synthesis. The addition of a nucleotide to a growing polynucleotide
Chapter 11 DNA Replication and Recombination
 Chapter 11 DNA Replication and Recombination Copyright Copyright 2009 Pearson 2009 Pearson Education, Education, Inc. Inc. 11.1 DNA is reproduced by Semiconservative Replication The complementarity of
Chapter 11 DNA Replication and Recombination Copyright Copyright 2009 Pearson 2009 Pearson Education, Education, Inc. Inc. 11.1 DNA is reproduced by Semiconservative Replication The complementarity of
Molecular Biology: General Theory
 Molecular Biology: General Theory Author: Dr Darshana Morar Licensed under a Creative Commons Attribution license. DNA REPLICATION DNA replication is the process of duplicating the DNA sequence in the
Molecular Biology: General Theory Author: Dr Darshana Morar Licensed under a Creative Commons Attribution license. DNA REPLICATION DNA replication is the process of duplicating the DNA sequence in the
Molecular Biology: General Theory
 Molecular Biology: General Theory Author: Dr Darshana Morar Licensed under a Creative Commons Attribution license. DNA REPLICATION DNA replication is the process of duplicating the DNA sequence in the
Molecular Biology: General Theory Author: Dr Darshana Morar Licensed under a Creative Commons Attribution license. DNA REPLICATION DNA replication is the process of duplicating the DNA sequence in the
* + * RecA * + + for RecA-dependent strand + + MIT Department of Biology 7.28, Spring Molecular Biology
 MIT Department of Biology 7.28, Spring 2005 - Molecular Biology 1) Question 1. 1A. You are studying the function of in vitro along with a lab partner. You run several reactions to examine the requirements
MIT Department of Biology 7.28, Spring 2005 - Molecular Biology 1) Question 1. 1A. You are studying the function of in vitro along with a lab partner. You run several reactions to examine the requirements
Genetics and Genomics in Medicine Chapter 3. Questions & Answers
 Genetics and Genomics in Medicine Chapter 3 Multiple Choice Questions Questions & Answers Question 3.1 Which of the following statements, if any, is false? a) Amplifying DNA means making many identical
Genetics and Genomics in Medicine Chapter 3 Multiple Choice Questions Questions & Answers Question 3.1 Which of the following statements, if any, is false? a) Amplifying DNA means making many identical
Feedback D. Incorrect! No, although this is a correct characteristic of RNA, this is not the best response to the questions.
 Biochemistry - Problem Drill 23: RNA No. 1 of 10 1. Which of the following statements best describes the structural highlights of RNA? (A) RNA can be single or double stranded. (B) G-C pairs have 3 hydrogen
Biochemistry - Problem Drill 23: RNA No. 1 of 10 1. Which of the following statements best describes the structural highlights of RNA? (A) RNA can be single or double stranded. (B) G-C pairs have 3 hydrogen
Supplementary Information for
 Supplementary Information for Conformational landscapes of DNA polymerase I and mutator derivatives establish fidelity checkpoints for nucleotide insertion Hohlbein et al. 1 Supplementary Figure S1. Wt
Supplementary Information for Conformational landscapes of DNA polymerase I and mutator derivatives establish fidelity checkpoints for nucleotide insertion Hohlbein et al. 1 Supplementary Figure S1. Wt
DNA REPLICATION. Anna Onofri Liceo «I.Versari»
 DNA REPLICATION Anna Onofri Liceo «I.Versari» Learning objectives 1. Understand the basic rules governing DNA replication 2. Understand the function of key proteins involved in a generalised replication
DNA REPLICATION Anna Onofri Liceo «I.Versari» Learning objectives 1. Understand the basic rules governing DNA replication 2. Understand the function of key proteins involved in a generalised replication
Chapter 9 Preview - DNA
 Chapter 9 Preview - DNA Multiple Choice Identify the choice that best completes the statement or answers the question. 1. In order to show that DNA in cell extracts is responsible for genetic transformation
Chapter 9 Preview - DNA Multiple Choice Identify the choice that best completes the statement or answers the question. 1. In order to show that DNA in cell extracts is responsible for genetic transformation
CGTAAGAGTACGTCCAGCATCGGF ATCATTATCTACATCXXXXXTACCATTCATTCAGATCTCACTATCGCATTCTCATGCAGGTCGTAGCCXS
 5 CGTAAGAGTACGTCCAGCATCGGF ATCATTATCTACATCXXXXXTACCATTCATTCAGATCTCACTATCGCATTCTCATGCAGGTCGTAGCCXS 29 Supplementary Figure 1 Sequence of the DNA primer/template pair used in Figure 1bc. A 23nt primer is
5 CGTAAGAGTACGTCCAGCATCGGF ATCATTATCTACATCXXXXXTACCATTCATTCAGATCTCACTATCGCATTCTCATGCAGGTCGTAGCCXS 29 Supplementary Figure 1 Sequence of the DNA primer/template pair used in Figure 1bc. A 23nt primer is
Syllabus for GUTS Lecture on DNA and Nucleotides
 Syllabus for GUTS Lecture on DNA and Nucleotides I. Introduction. DNA is the instruction manual for how to build a living organism here on earth. The instructions in DNA are propagated to future generations
Syllabus for GUTS Lecture on DNA and Nucleotides I. Introduction. DNA is the instruction manual for how to build a living organism here on earth. The instructions in DNA are propagated to future generations
Replication. Obaidur Rahman
 Replication Obaidur Rahman DIRCTION OF DNA SYNTHESIS How many reactions can a DNA polymerase catalyze? So how many reactions can it catalyze? So 4 is one answer, right, 1 for each nucleotide. But what
Replication Obaidur Rahman DIRCTION OF DNA SYNTHESIS How many reactions can a DNA polymerase catalyze? So how many reactions can it catalyze? So 4 is one answer, right, 1 for each nucleotide. But what
Enzymes used in DNA Replication
 Enzymes used in DNA Replication This document holds the enzymes used in DNA replication, their pictorial representation and functioning. DNA polymerase: DNA polymerase is the chief enzyme of DNA replication.
Enzymes used in DNA Replication This document holds the enzymes used in DNA replication, their pictorial representation and functioning. DNA polymerase: DNA polymerase is the chief enzyme of DNA replication.
All This For Four Letters!?! DNA and Its Role in Heredity
 All This For Four Letters!?! DNA and Its Role in Heredity What Is the Evidence that the Gene Is DNA? By the 1920s, it was known that chromosomes consisted of DNA and proteins. A new dye stained DNA and
All This For Four Letters!?! DNA and Its Role in Heredity What Is the Evidence that the Gene Is DNA? By the 1920s, it was known that chromosomes consisted of DNA and proteins. A new dye stained DNA and
III. Detailed Examination of the Mechanism of Replication A. Initiation B. Priming C. Elongation D. Proofreading and Termination
 Outline for Replication I. General Features of Replication A. Semi-Conservative B. Starts at Origin C. Bidirectional D. Semi-Discontinuous II. Proteins and Enzymes of Replication III. Detailed Examination
Outline for Replication I. General Features of Replication A. Semi-Conservative B. Starts at Origin C. Bidirectional D. Semi-Discontinuous II. Proteins and Enzymes of Replication III. Detailed Examination
Chapter 13 Section 2: DNA Replication
 Chapter 13 Section 2: DNA Replication Opening Activity DNA is considered to be a relatively stable molecule. What gives it this stability, even though the hydrogen bonds between the nitrogen bases are
Chapter 13 Section 2: DNA Replication Opening Activity DNA is considered to be a relatively stable molecule. What gives it this stability, even though the hydrogen bonds between the nitrogen bases are
DNA Metabolism. I. DNA Replication. A. Template concept: 1. How can you make a copy of a molecule? 2. Complementary Hydrogen bonding
 DNA Metabolism I. DNA Replication A. Template concept: 1. How can you make a copy of a molecule? 2. Complementary Hydrogen bonding B. DNA replication follows a set of fundamental rules 1. Semiconservative
DNA Metabolism I. DNA Replication A. Template concept: 1. How can you make a copy of a molecule? 2. Complementary Hydrogen bonding B. DNA replication follows a set of fundamental rules 1. Semiconservative
Exam 2 Key - Spring 2008 A#: Please see us if you have any questions!
 Page 1 of 5 Exam 2 Key - Spring 2008 A#: Please see us if you have any questions! 1. A mutation in which parts of two nonhomologous chromosomes change places is called a(n) A. translocation. B. transition.
Page 1 of 5 Exam 2 Key - Spring 2008 A#: Please see us if you have any questions! 1. A mutation in which parts of two nonhomologous chromosomes change places is called a(n) A. translocation. B. transition.
Fidelity of Escherichia coli DNA Polymerase III Holoenzyme
 THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 272, No. 44, Issue of October 31, pp. 27919 27930, 1997 1997 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. Fidelity of
THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 272, No. 44, Issue of October 31, pp. 27919 27930, 1997 1997 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. Fidelity of
RNA Expression of the information in a gene generally involves production of an RNA molecule transcribed from a DNA template. RNA differs from DNA
 RNA Expression of the information in a gene generally involves production of an RNA molecule transcribed from a DNA template. RNA differs from DNA that it has a hydroxyl group at the 2 position of the
RNA Expression of the information in a gene generally involves production of an RNA molecule transcribed from a DNA template. RNA differs from DNA that it has a hydroxyl group at the 2 position of the
Recitation CHAPTER 9 DNA Technologies
 Recitation CHAPTER 9 DNA Technologies DNA Cloning: General Scheme A cloning vector and eukaryotic chromosomes are separately cleaved with the same restriction endonuclease. (A single chromosome is shown
Recitation CHAPTER 9 DNA Technologies DNA Cloning: General Scheme A cloning vector and eukaryotic chromosomes are separately cleaved with the same restriction endonuclease. (A single chromosome is shown
DNA: Structure & Replication
 DNA Form & Function DNA: Structure & Replication Understanding DNA replication and the resulting transmission of genetic information from cell to cell, and generation to generation lays the groundwork
DNA Form & Function DNA: Structure & Replication Understanding DNA replication and the resulting transmission of genetic information from cell to cell, and generation to generation lays the groundwork
Fig. 16-7a. 5 end Hydrogen bond 3 end. 1 nm. 3.4 nm nm
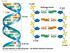 Fig. 16-7a end Hydrogen bond end 1 nm 3.4 nm 0.34 nm (a) Key features of DNA structure end (b) Partial chemical structure end Fig. 16-8 Adenine (A) Thymine (T) Guanine (G) Cytosine (C) Concept 16.2: Many
Fig. 16-7a end Hydrogen bond end 1 nm 3.4 nm 0.34 nm (a) Key features of DNA structure end (b) Partial chemical structure end Fig. 16-8 Adenine (A) Thymine (T) Guanine (G) Cytosine (C) Concept 16.2: Many
Amplified segment of DNA can be purified from bacteria in sufficient quantity and quality for :
 Transformation Insertion of DNA of interest Amplification Amplified segment of DNA can be purified from bacteria in sufficient quantity and quality for : DNA Sequence. Understand relatedness of genes and
Transformation Insertion of DNA of interest Amplification Amplified segment of DNA can be purified from bacteria in sufficient quantity and quality for : DNA Sequence. Understand relatedness of genes and
September 19, synthesized DNA. Label all of the DNA strands with 5 and 3 labels, and clearly show which strand(s) contain methyl groups.
 KEY DNA Replication and Mutation September 19, 2011 1. Below is a short DNA sequence located on the E. coli chromosome. In class we talked about how during the process of DNA replication, an enzyme adds
KEY DNA Replication and Mutation September 19, 2011 1. Below is a short DNA sequence located on the E. coli chromosome. In class we talked about how during the process of DNA replication, an enzyme adds
DNA Replication semiconservative replication conservative replication dispersive replication DNA polymerase
 DNA Replication DNA Strands are templates for DNA synthesis: Watson and Crick suggested that the existing strands of DNA served as a template for the producing of new strands, with bases being added to
DNA Replication DNA Strands are templates for DNA synthesis: Watson and Crick suggested that the existing strands of DNA served as a template for the producing of new strands, with bases being added to
BIO 311C Spring Lecture 34 Friday 23 Apr.
 BIO 311C Spring 2010 1 Lecture 34 Friday 23 Apr. Summary of DNA Replication in Prokaryotes origin of replication initial double helix origin of replication new growing polynucleotide chains Circular molecule
BIO 311C Spring 2010 1 Lecture 34 Friday 23 Apr. Summary of DNA Replication in Prokaryotes origin of replication initial double helix origin of replication new growing polynucleotide chains Circular molecule
The GeneEditor TM in vitro Mutagenesis System: Site- Directed Mutagenesis Using Altered Beta-Lactamase Specificity
 Promega Notes Magazine Number 62, 1997, p. 02 The GeneEditor TM in vitro Mutagenesis System: Site- Directed Mutagenesis Using Altered Beta-Lactamase Specificity By Christine Andrews and Scott Lesley Promega
Promega Notes Magazine Number 62, 1997, p. 02 The GeneEditor TM in vitro Mutagenesis System: Site- Directed Mutagenesis Using Altered Beta-Lactamase Specificity By Christine Andrews and Scott Lesley Promega
DNA damage alters DNA polymerase d to a form that exhibits increased discrimination against modified template bases and mismatched primers
 Published online 11 December 2008 Nucleic Acids Research, 2009, Vol. 37, No. 2 647 657 doi:10.1093/nar/gkn1000 DNA damage alters DNA polymerase d to a form that exhibits increased discrimination against
Published online 11 December 2008 Nucleic Acids Research, 2009, Vol. 37, No. 2 647 657 doi:10.1093/nar/gkn1000 DNA damage alters DNA polymerase d to a form that exhibits increased discrimination against
Wang Tian, Ying T. Hwang, and Charles B. C. Hwang*
 JOURNAL OF VIROLOGY, Sept. 2008, p. 8937 8941 Vol. 82, No. 17 0022-538X/08/$08.00 0 doi:10.1128/jvi.00911-08 Copyright 2008, American Society for Microbiology. All Rights Reserved. The Enhanced DNA Replication
JOURNAL OF VIROLOGY, Sept. 2008, p. 8937 8941 Vol. 82, No. 17 0022-538X/08/$08.00 0 doi:10.1128/jvi.00911-08 Copyright 2008, American Society for Microbiology. All Rights Reserved. The Enhanced DNA Replication
Proofreading, post-replication modification of DNA. Mitesh Shrestha
 Proofreading, post-replication modification of DNA Mitesh Shrestha Proofreading During DNA replication (copying), most DNA polymerases can check their work with each base that they add. This process is
Proofreading, post-replication modification of DNA Mitesh Shrestha Proofreading During DNA replication (copying), most DNA polymerases can check their work with each base that they add. This process is
PCR OPTIMIZATION AND TROUBLESHOOTING
 PCR OPTIMIZATION AND TROUBLESHOOTING Amplification of each DNA fragment can occur only under the defined conditions which are provided by a reaction mixture. If no positive PCR result can be obtained,
PCR OPTIMIZATION AND TROUBLESHOOTING Amplification of each DNA fragment can occur only under the defined conditions which are provided by a reaction mixture. If no positive PCR result can be obtained,
The flow of Genetic information
 The flow of Genetic information http://highered.mcgrawhill.com/sites/0072507470/student_view0/chapter3/animation dna_replication quiz_1_.html 1 DNA Replication DNA is a double-helical molecule Watson and
The flow of Genetic information http://highered.mcgrawhill.com/sites/0072507470/student_view0/chapter3/animation dna_replication quiz_1_.html 1 DNA Replication DNA is a double-helical molecule Watson and
DNA Replication Fidelity
 DNA Replication Fidelity Errol C Friedberg, UT Southwestern Medical Center, Dallas, Texas, USA Paula L Fischhaber, UT Southwestern Medical Center, Dallas, Texas, USA DNA polymerases are proteins that catalyse
DNA Replication Fidelity Errol C Friedberg, UT Southwestern Medical Center, Dallas, Texas, USA Paula L Fischhaber, UT Southwestern Medical Center, Dallas, Texas, USA DNA polymerases are proteins that catalyse
BCMB Chapters 34 & 35 DNA Replication and Repair
 BCMB 3100 - Chapters 34 & 35 DNA Replication and Repair Semi-conservative DNA replication DNA polymerase DNA replication Replication fork; Okazaki fragments Sanger method for DNA sequencing DNA repair
BCMB 3100 - Chapters 34 & 35 DNA Replication and Repair Semi-conservative DNA replication DNA polymerase DNA replication Replication fork; Okazaki fragments Sanger method for DNA sequencing DNA repair
Genetic Information: DNA replication
 Genetic Information: DNA replication Umut Fahrioglu, PhD MSc DNA Replication Replication of DNA is vital to the transmission of genomes and the genes they contain from one cell generation to the other.
Genetic Information: DNA replication Umut Fahrioglu, PhD MSc DNA Replication Replication of DNA is vital to the transmission of genomes and the genes they contain from one cell generation to the other.
BCMB Chapters 34 & 35 DNA Replication and Repair
 BCMB 3100 - Chapters 34 & 35 DNA Replication and Repair Semi-conservative DNA replication DNA polymerase DNA replication Replication fork; Okazaki fragments Sanger method for DNA sequencing DNA repair
BCMB 3100 - Chapters 34 & 35 DNA Replication and Repair Semi-conservative DNA replication DNA polymerase DNA replication Replication fork; Okazaki fragments Sanger method for DNA sequencing DNA repair
Student name ID # Second Mid Term Exam, Biology 2020, Spring 2002 Scores Total
 Second Mid Term Exam, Biology 2020, Spring 2002 Scores 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. Total 1 1. Matching (7 pts). Each answer is used exactly once Helicase
Second Mid Term Exam, Biology 2020, Spring 2002 Scores 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. Total 1 1. Matching (7 pts). Each answer is used exactly once Helicase
DNA replication. - proteins for initiation of replication; - proteins for polymerization of nucleotides.
 DNA replication Replication represents the duplication of the genetic information encoded in DNA that is the crucial step in the reproduction of living organisms and the growth of multicellular organisms.
DNA replication Replication represents the duplication of the genetic information encoded in DNA that is the crucial step in the reproduction of living organisms and the growth of multicellular organisms.
Multiple choice questions (numbers in brackets indicate the number of correct answers)
 1 Multiple choice questions (numbers in brackets indicate the number of correct answers) February 1, 2013 1. Ribose is found in Nucleic acids Proteins Lipids RNA DNA (2) 2. Most RNA in cells is transfer
1 Multiple choice questions (numbers in brackets indicate the number of correct answers) February 1, 2013 1. Ribose is found in Nucleic acids Proteins Lipids RNA DNA (2) 2. Most RNA in cells is transfer
DNA Replication I Biochemistry 302. Bob Kelm January 24, 2005
 DNA Replication I Biochemistry 302 Bob Kelm January 24, 2005 Watson Crick prediction: Each stand of parent DNA serves as a template for synthesis of a new complementary daughter strand Fig. 4.12 Proof
DNA Replication I Biochemistry 302 Bob Kelm January 24, 2005 Watson Crick prediction: Each stand of parent DNA serves as a template for synthesis of a new complementary daughter strand Fig. 4.12 Proof
The Size and Packaging of Genomes
 DNA Replication The Size and Packaging of Genomes Vary greatly in size Ø Smallest viruses- 4 or 5 genes Ø Escherichia coli- 4,288 genes Ø Human cell- 20,000 to 25,000 genes E. coli 4 million base pairs
DNA Replication The Size and Packaging of Genomes Vary greatly in size Ø Smallest viruses- 4 or 5 genes Ø Escherichia coli- 4,288 genes Ø Human cell- 20,000 to 25,000 genes E. coli 4 million base pairs
Recombinant DNA Technology
 History of recombinant DNA technology Recombinant DNA Technology (DNA cloning) Majid Mojarrad Recombinant DNA technology is one of the recent advances in biotechnology, which was developed by two scientists
History of recombinant DNA technology Recombinant DNA Technology (DNA cloning) Majid Mojarrad Recombinant DNA technology is one of the recent advances in biotechnology, which was developed by two scientists
7.1 Techniques for Producing and Analyzing DNA. SBI4U Ms. Ho-Lau
 7.1 Techniques for Producing and Analyzing DNA SBI4U Ms. Ho-Lau What is Biotechnology? From Merriam-Webster: the manipulation of living organisms or their components to produce useful usually commercial
7.1 Techniques for Producing and Analyzing DNA SBI4U Ms. Ho-Lau What is Biotechnology? From Merriam-Webster: the manipulation of living organisms or their components to produce useful usually commercial
Technical tips Session 4
 Technical tips Session 4 Biotinylation assay: Streptavidin is a small bacterial protein that binds with high affinity to the vitamin biotin. This streptavidin-biotin combination can be used to link molecules
Technical tips Session 4 Biotinylation assay: Streptavidin is a small bacterial protein that binds with high affinity to the vitamin biotin. This streptavidin-biotin combination can be used to link molecules
3.A.1 DNA and RNA: Structure and Replication
 3.A.1 DNA and RNA: Structure and Replication Each DNA polymer is made of Nucleotides (monomer) which are made of: a) Phosphate group: Negatively charged and polar b) Sugar: deoxyribose- a 5 carbon sugar
3.A.1 DNA and RNA: Structure and Replication Each DNA polymer is made of Nucleotides (monomer) which are made of: a) Phosphate group: Negatively charged and polar b) Sugar: deoxyribose- a 5 carbon sugar
NEW PARADIGM of BIOTECHNOLOGY - GENET BIO. GeNet Bio Global Gene Network
 NEW PARADIGM of BIOTECHNOLOGY - GENET BIO GeNet Bio Global Gene Network GENET BIO DNA AMPLIFICATION PRODUCTS GUIDE Keynote of Products Prime TaqTM DNA Polymerase Prime TaqTM Premix ExPrime TaqTM DNA Polymerase
NEW PARADIGM of BIOTECHNOLOGY - GENET BIO GeNet Bio Global Gene Network GENET BIO DNA AMPLIFICATION PRODUCTS GUIDE Keynote of Products Prime TaqTM DNA Polymerase Prime TaqTM Premix ExPrime TaqTM DNA Polymerase
produces an RNA copy of the coding region of a gene
 1. Transcription Gene Expression The expression of a gene into a protein occurs by: 1) Transcription of a gene into RNA produces an RNA copy of the coding region of a gene the RNA transcript may be the
1. Transcription Gene Expression The expression of a gene into a protein occurs by: 1) Transcription of a gene into RNA produces an RNA copy of the coding region of a gene the RNA transcript may be the
DNA Replication * OpenStax
 OpenStax-CNX module: m45475 1 DNA Replication * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0 By the end of this section, you will be able
OpenStax-CNX module: m45475 1 DNA Replication * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0 By the end of this section, you will be able
Gene Expression- Protein Synthesis
 Gene Expression- Protein Synthesis Protein synthesis is the key to expression of biological information - Structural Protein: bones, cartilage - Contractile Proteins: myosin, actin - Enzymes - Transport
Gene Expression- Protein Synthesis Protein synthesis is the key to expression of biological information - Structural Protein: bones, cartilage - Contractile Proteins: myosin, actin - Enzymes - Transport
DNA: The Genetic Material. Chapter 14. Genetic Material
 DNA: The Genetic Material Chapter 14 Genetic Material Frederick Griffith, 1928 Streptococcus pneumoniae, a pathogenic bacterium causing pneumonia 2 strains of Streptococcus: - S strain virulent - R strain
DNA: The Genetic Material Chapter 14 Genetic Material Frederick Griffith, 1928 Streptococcus pneumoniae, a pathogenic bacterium causing pneumonia 2 strains of Streptococcus: - S strain virulent - R strain
Fidelity of DNA polymerase
 Fidelity of DNA polymerase Shape selectivity: DNA polymerase's conformational change for determination of fidelity for each nucleotide Induced fit: Structure determines function Matched nucleotide Fidelity
Fidelity of DNA polymerase Shape selectivity: DNA polymerase's conformational change for determination of fidelity for each nucleotide Induced fit: Structure determines function Matched nucleotide Fidelity
DNA Replication * Robert Bear David Rintoul. Based on DNA Replication by OpenStax
 OpenStax-CNX module: m47204 1 DNA Replication * Robert Bear David Rintoul Based on DNA Replication by OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution
OpenStax-CNX module: m47204 1 DNA Replication * Robert Bear David Rintoul Based on DNA Replication by OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution
Lesson Overview DNA Replication
 12.3 THINK ABOUT IT Before a cell divides, its DNA must first be copied. How might the double-helix structure of DNA make that possible? Copying the Code What role does DNA polymerase play in copying DNA?
12.3 THINK ABOUT IT Before a cell divides, its DNA must first be copied. How might the double-helix structure of DNA make that possible? Copying the Code What role does DNA polymerase play in copying DNA?
Chapters 31-32: Ribonucleic Acid (RNA)
 Chapters 31-32: Ribonucleic Acid (RNA) Short segments from the transcription, processing and translation sections of each chapter Slide 1 RNA In comparison with DNA RNA utilizes uracil in place of thymine
Chapters 31-32: Ribonucleic Acid (RNA) Short segments from the transcription, processing and translation sections of each chapter Slide 1 RNA In comparison with DNA RNA utilizes uracil in place of thymine
Rapid Learning Center Presents. Teach Yourself High School Biology in 24 Hours. and Functions
 Rapid Learning Center Chemistry :: Biology :: Physics :: Math Rapid Learning Center Presents Teach Yourself High School Biology in 24 Hours Gene e Structures and Functions High School Biology Rapid Learning
Rapid Learning Center Chemistry :: Biology :: Physics :: Math Rapid Learning Center Presents Teach Yourself High School Biology in 24 Hours Gene e Structures and Functions High School Biology Rapid Learning
Ligation Independent Cloning (LIC) Procedure
 Ligation Independent Cloning (LIC) Procedure Ligation Independent Cloning (LIC) LIC cloning allows insertion of DNA fragments without using restriction enzymes into specific vectors containing engineered
Ligation Independent Cloning (LIC) Procedure Ligation Independent Cloning (LIC) LIC cloning allows insertion of DNA fragments without using restriction enzymes into specific vectors containing engineered
The Genetic Material. The Genetic Material. The Genetic Material. DNA: The Genetic Material. Chapter 14
 DNA: Chapter 14 Frederick Griffith, 1928 studied Streptococcus pneumoniae, a pathogenic bacterium causing pneumonia there are 2 strains of Streptococcus: - S strain is virulent - R strain is nonvirulent
DNA: Chapter 14 Frederick Griffith, 1928 studied Streptococcus pneumoniae, a pathogenic bacterium causing pneumonia there are 2 strains of Streptococcus: - S strain is virulent - R strain is nonvirulent
DNA Replication II Biochemistry 302. January 25, 2006
 DNA Replication II Biochemistry 302 January 25, 2006 Following in Dad s footsteps Original A. Kornberg E. coli DNA Pol I is a lousy replicative enzyme. 400 molecules/cell but ~2 replication forks/cell
DNA Replication II Biochemistry 302 January 25, 2006 Following in Dad s footsteps Original A. Kornberg E. coli DNA Pol I is a lousy replicative enzyme. 400 molecules/cell but ~2 replication forks/cell
Essential Question. What is the structure of DNA, and how does it function in genetic inheritance?
 DNA Dr. Bertolotti Essential Question What is the structure of DNA, and how does it function in genetic inheritance? What is the role of DNA in hereditary? Transformation Transformation is the process
DNA Dr. Bertolotti Essential Question What is the structure of DNA, and how does it function in genetic inheritance? What is the role of DNA in hereditary? Transformation Transformation is the process
5-Formyl- and 5-carboxyl-cytosine reduce the rate and substrate specificity. of RNA polymerase II transcription
 SUPPLEMENTARY INFORMATION FOR 5-Formyl- and 5-carboxyl-cytosine reduce the rate and substrate specificity of RNA polymerase II transcription Matthew W. Kellinger 1, Chunxiao Song 2, Jenny Chong 1, Xingyu
SUPPLEMENTARY INFORMATION FOR 5-Formyl- and 5-carboxyl-cytosine reduce the rate and substrate specificity of RNA polymerase II transcription Matthew W. Kellinger 1, Chunxiao Song 2, Jenny Chong 1, Xingyu
Polymerase Chain Reaction
 Polymerase Chain Reaction = multiple rounds of in vitro DNA replication = a region of DNA lying between two regions of known sequence is amplified hundreds of millions of time within a matter of several
Polymerase Chain Reaction = multiple rounds of in vitro DNA replication = a region of DNA lying between two regions of known sequence is amplified hundreds of millions of time within a matter of several
Mechanisms of DNA Synthesis
 Mechanisms of DNA Synthesis Biochemistry 201 Advanced Molecular Biology January 19, 2000 Doug Brutlag Introduction Today's topic concerns the fundamental mechanism of DNA replication as exemplified by
Mechanisms of DNA Synthesis Biochemistry 201 Advanced Molecular Biology January 19, 2000 Doug Brutlag Introduction Today's topic concerns the fundamental mechanism of DNA replication as exemplified by
MBioS 503: Section 1 Chromosome, Gene, Translation, & Transcription. Gene Organization. Genome. Objectives: Gene Organization
 Overview & Recap of Molecular Biology before the last two sections MBioS 503: Section 1 Chromosome, Gene, Translation, & Transcription Gene Organization Joy Winuthayanon, PhD School of Molecular Biosciences
Overview & Recap of Molecular Biology before the last two sections MBioS 503: Section 1 Chromosome, Gene, Translation, & Transcription Gene Organization Joy Winuthayanon, PhD School of Molecular Biosciences
1. True or False? At the DNA level, recombination is initiated by a single stranded break in a DNA molecule.
 1. True or False? At the DNA level, recombination is initiated by a single stranded break in a DNA molecule. 2. True or False? Dideoxy sequencing is a chain initiation method of DNA sequencing. 3. True
1. True or False? At the DNA level, recombination is initiated by a single stranded break in a DNA molecule. 2. True or False? Dideoxy sequencing is a chain initiation method of DNA sequencing. 3. True
MCB 150: The Molecular and Cellular Basis of Life
 MCB 150 The Molecular and Cellular Basis of Life Mutations, Part II Today s Learning Catalytics Session ID is: 66727193 1 Exam II information: 343 students (59%) stayed the same or increased from Exam
MCB 150 The Molecular and Cellular Basis of Life Mutations, Part II Today s Learning Catalytics Session ID is: 66727193 1 Exam II information: 343 students (59%) stayed the same or increased from Exam
Answer Key. Microbial Genetics, BIO 410/510 Winter Quarter 2015 Exam III. Name. Student ID# p
 Answer Key Name Student ID# p 1.) For a typical lytic phage, describe two types of gene products that you would expect to be expressed early after infection? (2pts) Gene products that are associated directing
Answer Key Name Student ID# p 1.) For a typical lytic phage, describe two types of gene products that you would expect to be expressed early after infection? (2pts) Gene products that are associated directing
Fundamental Process. Unit Map. Unit
 Unit 3 Unit Map 3.A DNA replication, repair and recombination 120 3.B RNA synthesis and processing 137 3.C Protein synthesis and processing 151 3.D Control of gene expression at transcription and translation
Unit 3 Unit Map 3.A DNA replication, repair and recombination 120 3.B RNA synthesis and processing 137 3.C Protein synthesis and processing 151 3.D Control of gene expression at transcription and translation
DNA Replication II Biochemistry 302. Bob Kelm January 28, 2004
 DNA Replication II Biochemistry 302 Bob Kelm January 28, 2004 Conceptual model for proofreading based on kinetic considerations Fig. 24.44 stalling transient melting exonuclease site occupancy Following
DNA Replication II Biochemistry 302 Bob Kelm January 28, 2004 Conceptual model for proofreading based on kinetic considerations Fig. 24.44 stalling transient melting exonuclease site occupancy Following
Molecular Biology (2)
 Molecular Biology (2) DNA replication Mamoun Ahram, PhD Second semester, 2018-2019 Resources This lecture Cooper, pp. 191-207 2 Some basic information The entire DNA content of the cell is known as genome.
Molecular Biology (2) DNA replication Mamoun Ahram, PhD Second semester, 2018-2019 Resources This lecture Cooper, pp. 191-207 2 Some basic information The entire DNA content of the cell is known as genome.
Storage and Expression of Genetic Information
 Storage and Expression of Genetic Information 29. DNA structure, Replication and Repair ->Ch 25. DNA metabolism 30. RNA Structure, Synthesis and Processing ->Ch 26. RNA metabolism 31. Protein Synthesis
Storage and Expression of Genetic Information 29. DNA structure, Replication and Repair ->Ch 25. DNA metabolism 30. RNA Structure, Synthesis and Processing ->Ch 26. RNA metabolism 31. Protein Synthesis
MCB 110 SP 2005 Second Midterm
 MCB110 SP 2005 Midterm II Page 1 ANSWER KEY MCB 110 SP 2005 Second Midterm Question Maximum Points Your Points I 40 II 40 III 36 IV 34 150 NOTE: This is my answer key (KC). The graders were given disgression
MCB110 SP 2005 Midterm II Page 1 ANSWER KEY MCB 110 SP 2005 Second Midterm Question Maximum Points Your Points I 40 II 40 III 36 IV 34 150 NOTE: This is my answer key (KC). The graders were given disgression
Switching between polymerase and exonuclease sites in DNA polymerase
 Nucleic Acids Research, 214 1 doi: 1.193/nar/gku1353 Switching between polymerase and exonuclease sites in DNA polymerase Rais A. Ganai, Göran O. Bylund and Erik Johansson * Department of Medical Biochemistry
Nucleic Acids Research, 214 1 doi: 1.193/nar/gku1353 Switching between polymerase and exonuclease sites in DNA polymerase Rais A. Ganai, Göran O. Bylund and Erik Johansson * Department of Medical Biochemistry
Supplementary Figure 1. Analysis of replication rates by Pol. Nature Structural & Molecular Biology: doi: /nsmb.3207
 Supplementary Figure 1 Analysis of replication rates by Pol (a) Electrophoretic Mobility Shift Assay (EMSA) of Pol -DV binding to template-primer DNA (Fried, M. & Crothers, D.M. Equilibria and kinetics
Supplementary Figure 1 Analysis of replication rates by Pol (a) Electrophoretic Mobility Shift Assay (EMSA) of Pol -DV binding to template-primer DNA (Fried, M. & Crothers, D.M. Equilibria and kinetics
IN E. COLI WHAT IS THE FUNCTION OF DNA POLYMERASE III
 10 January, 2018 IN E. COLI WHAT IS THE FUNCTION OF DNA POLYMERASE III Document Filetype: PDF 312 KB 0 IN E. COLI WHAT IS THE FUNCTION OF DNA POLYMERASE III The actual replication enzyme in E. Both will
10 January, 2018 IN E. COLI WHAT IS THE FUNCTION OF DNA POLYMERASE III Document Filetype: PDF 312 KB 0 IN E. COLI WHAT IS THE FUNCTION OF DNA POLYMERASE III The actual replication enzyme in E. Both will
DNA Transcription. Visualizing Transcription. The Transcription Process
 DNA Transcription By: Suzanne Clancy, Ph.D. 2008 Nature Education Citation: Clancy, S. (2008) DNA transcription. Nature Education 1(1) If DNA is a book, then how is it read? Learn more about the DNA transcription
DNA Transcription By: Suzanne Clancy, Ph.D. 2008 Nature Education Citation: Clancy, S. (2008) DNA transcription. Nature Education 1(1) If DNA is a book, then how is it read? Learn more about the DNA transcription
Biochemistry 111. Carl Parker x A Braun
 Biochemistry 111 Carl Parker x6368 101A Braun csp@caltech.edu Central Dogma of Molecular Biology DNA-Dependent RNA Polymerase Requires a DNA Template Synthesizes RNA in a 5 to 3 direction Requires ribonucleoside
Biochemistry 111 Carl Parker x6368 101A Braun csp@caltech.edu Central Dogma of Molecular Biology DNA-Dependent RNA Polymerase Requires a DNA Template Synthesizes RNA in a 5 to 3 direction Requires ribonucleoside
3 Designing Primers for Site-Directed Mutagenesis
 3 Designing Primers for Site-Directed Mutagenesis 3.1 Learning Objectives During the next two labs you will learn the basics of site-directed mutagenesis: you will design primers for the mutants you designed
3 Designing Primers for Site-Directed Mutagenesis 3.1 Learning Objectives During the next two labs you will learn the basics of site-directed mutagenesis: you will design primers for the mutants you designed
Chromatographic Separation of the three forms of RNA Polymerase II.
 Chromatographic Separation of the three forms of RNA Polymerase II. α-amanitin α-amanitin bound to Pol II Function of the three enzymes. Yeast Pol II. RNA Polymerase Subunit Structures 10-7 Subunit structure.
Chromatographic Separation of the three forms of RNA Polymerase II. α-amanitin α-amanitin bound to Pol II Function of the three enzymes. Yeast Pol II. RNA Polymerase Subunit Structures 10-7 Subunit structure.
BS GENOMES. DNA replication and repair
 BS2009 - GENOMES DNA replication and repair REPLICATION GENERAL PRINCIPLES START Must be ready Must know where to start FINISH Must all finish Must ensure that each piece of DNA is replicated only once
BS2009 - GENOMES DNA replication and repair REPLICATION GENERAL PRINCIPLES START Must be ready Must know where to start FINISH Must all finish Must ensure that each piece of DNA is replicated only once
Guidelines for Preventing Contamination of PCR Reference Guidelines for Primer Design Estimation of Primer Melting Temperature
 Guidelines for Preventing Contamination of PCR During PCR more than 10 million copies of a template DNA are generated. Therefore, care must be taken to avoid contamination with other templates and amplicons
Guidelines for Preventing Contamination of PCR During PCR more than 10 million copies of a template DNA are generated. Therefore, care must be taken to avoid contamination with other templates and amplicons
Lecture Four. Molecular Approaches I: Nucleic Acids
 Lecture Four. Molecular Approaches I: Nucleic Acids I. Recombinant DNA and Gene Cloning Recombinant DNA is DNA that has been created artificially. DNA from two or more sources is incorporated into a single
Lecture Four. Molecular Approaches I: Nucleic Acids I. Recombinant DNA and Gene Cloning Recombinant DNA is DNA that has been created artificially. DNA from two or more sources is incorporated into a single
8.1. KEY CONCEPT DNA was identified as the genetic material through a series of experiments. 64 Reinforcement Unit 3 Resource Book
 8.1 IDENTIFYING DNA AS THE GENETIC MATERIAL KEY CONCEPT DNA was identified as the genetic material through a series of experiments. A series of experiments helped scientists recognize that DNA is the genetic
8.1 IDENTIFYING DNA AS THE GENETIC MATERIAL KEY CONCEPT DNA was identified as the genetic material through a series of experiments. A series of experiments helped scientists recognize that DNA is the genetic
Combinatorial Evolution of Enzymes and Synthetic pathways Using
 Supplementary data Combinatorial Evolution of Enzymes and Synthetic pathways Using One-Step PCR Peng Jin,, Zhen Kang,,, *, Junli Zhang,, Linpei Zhang,, Guocheng Du,, and Jian Chen, The Key Laboratory of
Supplementary data Combinatorial Evolution of Enzymes and Synthetic pathways Using One-Step PCR Peng Jin,, Zhen Kang,,, *, Junli Zhang,, Linpei Zhang,, Guocheng Du,, and Jian Chen, The Key Laboratory of
B. Incorrect! Ligation is also a necessary step for cloning.
 Genetics - Problem Drill 15: The Techniques in Molecular Genetics No. 1 of 10 1. Which of the following is not part of the normal process of cloning recombinant DNA in bacteria? (A) Restriction endonuclease
Genetics - Problem Drill 15: The Techniques in Molecular Genetics No. 1 of 10 1. Which of the following is not part of the normal process of cloning recombinant DNA in bacteria? (A) Restriction endonuclease
HIV-1 Reverse Transcriptase Polymerase and RNase H Active Sites Work Simultaneously and Independently
 JBC Papers in Press. Published on October 24, 2016 as Manuscript M116.753160 The latest version is at http://www.jbc.org/cgi/doi/10.1074/jbc.m116.753160 The two active sites of HIV-1 RT work simultaneously
JBC Papers in Press. Published on October 24, 2016 as Manuscript M116.753160 The latest version is at http://www.jbc.org/cgi/doi/10.1074/jbc.m116.753160 The two active sites of HIV-1 RT work simultaneously
CHAPTER 20 DNA TECHNOLOGY AND GENOMICS. Section A: DNA Cloning
 Section A: DNA Cloning 1. DNA technology makes it possible to clone genes for basic research and commercial applications: an overview 2. Restriction enzymes are used to make recombinant DNA 3. Genes can
Section A: DNA Cloning 1. DNA technology makes it possible to clone genes for basic research and commercial applications: an overview 2. Restriction enzymes are used to make recombinant DNA 3. Genes can
Masayoshi Honda, Jeehae Park, Robert A. Pugh, Taekjip Ha, and Maria Spies
 Molecular Cell, Volume 35 Supplemental Data Single-Molecule Analysis Reveals Differential Effect of ssdna-binding Proteins on DNA Translocation by XPD Helicase Masayoshi Honda, Jeehae Park, Robert A. Pugh,
Molecular Cell, Volume 35 Supplemental Data Single-Molecule Analysis Reveals Differential Effect of ssdna-binding Proteins on DNA Translocation by XPD Helicase Masayoshi Honda, Jeehae Park, Robert A. Pugh,
UNCC Biotechnology and Bioinformatics Camp. Dr. Jennifer Weller Summer 2010
 UNCC Biotechnology and Bioinformatics Camp Dr. Jennifer Weller Summer 2010 PCR Topic: the Polymerase Chain Reaction (PCR) 1988, Saiki, Mullis et al. proposed using a heat stable polymerase to carry out
UNCC Biotechnology and Bioinformatics Camp Dr. Jennifer Weller Summer 2010 PCR Topic: the Polymerase Chain Reaction (PCR) 1988, Saiki, Mullis et al. proposed using a heat stable polymerase to carry out
Chapter 20 Biotechnology
 Chapter 20 Biotechnology Manipulation of DNA In 2007, the first entire human genome had been sequenced. The ability to sequence an organisms genomes were made possible by advances in biotechnology, (the
Chapter 20 Biotechnology Manipulation of DNA In 2007, the first entire human genome had been sequenced. The ability to sequence an organisms genomes were made possible by advances in biotechnology, (the
Chapter 8 From DNA to Proteins. Chapter 8 From DNA to Proteins
 KEY CONCEPT Section 1 DNA was identified as the genetic material through a series of experiments. Griffith finds a transforming principle. Griffith experimented with the bacteria that cause pneumonia.
KEY CONCEPT Section 1 DNA was identified as the genetic material through a series of experiments. Griffith finds a transforming principle. Griffith experimented with the bacteria that cause pneumonia.
DNA Replication Query Query
 DNA Replication 1. Query: How is the origin opened during the initiation step of replication of E. coli DNA? What is the role of negative supercoiling in this process? How does the DnaA protein help the
DNA Replication 1. Query: How is the origin opened during the initiation step of replication of E. coli DNA? What is the role of negative supercoiling in this process? How does the DnaA protein help the
DNA metabolism. DNA Replication DNA Repair DNA Recombination
 DNA metabolism DNA Replication DNA Repair DNA Recombination Chutima Talabnin Ph.D. School of Biochemistry,Institute of Science, Suranaree University of Technology Central Dogma or Flow of genetic information
DNA metabolism DNA Replication DNA Repair DNA Recombination Chutima Talabnin Ph.D. School of Biochemistry,Institute of Science, Suranaree University of Technology Central Dogma or Flow of genetic information
DNA SEQUENCING BY SANGER METHOD
 DNA SEQUENCING BY SANGER METHOD First method described by Sanger and Coulson,1975 for DNA sequencing was called plus and minus. This method used E.coli DNA polymerase I and DNA ploymerase from bacteriophage
DNA SEQUENCING BY SANGER METHOD First method described by Sanger and Coulson,1975 for DNA sequencing was called plus and minus. This method used E.coli DNA polymerase I and DNA ploymerase from bacteriophage
High-fidelity DNA polymerases, such as Escherichia coli
 pubs.acs.org/biochemistry Terms of Use Prechemistry Nucleotide Selection Checkpoints in the Reaction Pathway of DNA Polymerase I and Roles of Glu710 and Tyr766 Oya Bermek, Nigel D. F. Grindley, and Catherine
pubs.acs.org/biochemistry Terms of Use Prechemistry Nucleotide Selection Checkpoints in the Reaction Pathway of DNA Polymerase I and Roles of Glu710 and Tyr766 Oya Bermek, Nigel D. F. Grindley, and Catherine
BIOCHEMISTRY REVIEW. Overview of Biomolecules. Chapter 11 DNA Replication
 BIOCHEMISTRY REVIEW Overview of Biomolecules Chapter 11 DNA Replication 2 3 4 5 6 7 8 9 Are You Getting It?? Which characteristics will be part of semi-conservative replication? (multiple answers) a) The
BIOCHEMISTRY REVIEW Overview of Biomolecules Chapter 11 DNA Replication 2 3 4 5 6 7 8 9 Are You Getting It?? Which characteristics will be part of semi-conservative replication? (multiple answers) a) The
