EUROPEAN PATENT OFFICE U.S. PATENT AND TRADEMARK OFFICE CPC NOTICE OF CHANGES 397 DATE: 1 AUGUST 2017 PROJECT RP0002
|
|
|
- Wesley Fowler
- 6 years ago
- Views:
Transcription
1 EUROPEAN PATENT OFFICE U.S. PATENT AND TRADEMARK OFFICE CPC NOTICE OF CHANGES 397 The following classification changes will be effected by this Notice of Changes: Action Subclass Group(s) Symbols deleted: A61N 2001/086 A61N 2001/34 A61N 1/36032 A61N 1/36035 A61N 2001/36039 Symbols newly created: A61N 1/086 A61N 1/36002 A61N 1/3603 A61N 1/36031 A61N 1/36034 A61N 1/36036 A61N 1/36038 A61N 1/36039 A61N 1/3604 A61N 1/36062 A61N 1/3614 A61N 1/3629 A61N 1/36842 A61N 1/36843 A61N 1/37254 A61N 1/37512 A61N 1/37514 A61N 1/37516 A61N 1/37518 A61N 1/3904 A61N 1/39044 A61N 1/39046 A61N 1/39622 A61N 1/39624 Title wording change: A61N 1/08 A61N 2001/083 A61N 1/36 A61N 1/36067 A61N 1/36075 A61N 1/36078 A61N 1/36082 A61N 1/36085 A61N 1/36089
2 Action Subclass Group(s) A61N 1/36096 A61N 1/36103 A61N 1/36107 A61N 1/3611 A61N 1/36121 A61N 1/36135 A61N 1/36153 A61N 1/36157 A61N 1/36164 A61N 1/36175 A61N 1/36189 A61N 1/3627 A61N 1/3628 A61N 1/36521 A61N 1/3684 A61N 1/3702 A61N 1/3706 A61N 1/3712 A61N 1/37247 A61N 1/37252 A61N 1/38 A61N 1/3962 New Definitions: A61N 1/086 A61N 1/3603 A61N 1/3604 A61N 1/3614 A61N 1/3629 A61N 1/3684 A61N 1/3904 A61N 1/3962 A61N 1/36002 A61N 1/36031 A61N 1/36034 A61N 1/36036 A61N 1/36038 A61N 1/36039 A61N 1/36062 A61N 1/36842 A61N 1/36843 A61N 1/37254 A61N 1/37512 A61N 1/37514 A61N 1/37516 A61N 1/37518 A61N 1/39044 A61N 1/39046 A61N 1/39622 A61N 1/39624
3 Action Subclass Group(s) Modified Definitions: A61N 1/08 A61N 1/36 A61N 1/372 Scheme Warning Notices to be added: A61N 1/08 A61N 2001/083 A61N 1/36 A61N 1/36002 A61N 1/36014 A61N 1/3603 A61N 1/36031 A61N 1/36034 A61N 1/36036 A61N 1/36038 A61N 1/3606 A61N 1/36062 A61N 1/36135 A61N 1/3614 A61N 1/36153 A61N 1/36157 A61N 1/3616 A61N 1/362 A61N 1/3629 A61N 1/3684 A61N 1/36842 A61N 1/36843 A61N 1/37252 A61N 1/37254 A61N 1/375 A61N 1/37512 A61N 1/37514 A61N 1/37516 A61N 1/37518 A61N 1/39 A61N 1/3904 A61N 1/39044 A61N 1/39046 A61N 1/3962 A61N 1/39622 A61N 1/39624 The following subclasses/groups are also impacted by this Notice of Changes (indicate subclasses/groups outside of the project scope, such as those listed in the CRL): A61B, A61F, A61M, H04R
4 This Notice of Changes includes the following [Check the ones included]: 1. CLASSIFICATION SCHEME CHANGES A. New, Modified or Deleted Group(s) B. New, Modified or Deleted Warning Notice(s) C. New, Modified or Deleted Note(s) D. New, Modified or Deleted Guidance Heading(s) 2. DEFINITIONS (New or Modified) A. DEFINITIONS (Full definition template) B. DEFINITIONS (Definitions Quick Fix) 3. REVISION CONCORDANCE LIST (RCL) 4. CHANGES TO THE CPC-TO-IPC CONCORDANCE LIST (CICL) 5. CROSS-REFERENCE LIST (CRL)
5 1. CLASSIFICATION SCHEME CHANGES A. New, Modified or Deleted Group(s) SUBCLASS A61N - ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY Type* Symbol Indent Level Number of dots (e.g. 0, 1, 2) Title (new or modified) CPC only text should normally be enclosed in {curly brackets}** C A61N1/08 2 Arrangements or circuits for monitoring, protecting, controlling or indicating {(for external stimulators A61N1/3603; for implantable neurostimulators A61N1/36128; for heart stimulators A61N1/37; for defibrillators A61N1/3925)} Transferred to # A61N1/08, A61N1/3603 C A61N2001/083 3 {Monitoring integrity of contacts, e.g. by impedance measurement} A61N2001/083, A61N1/3603 N A61N1/086 3 {Magnetic resonance imaging [MRI] compatible leads} D A61N2001/086 3 <administrative transfer to A61N1/086> D A61N2001/34 3 <administrative transfer to A61N1/36021> C A61N1/36 3 for stimulation A61N1/36, A61N1/36002 N A61N1/ {Cancer treatment, e.g. tumour} U A61N1/ {of motor muscles, e.g. for walking assistance} C A61N1/ {External stimulators, e.g. with patch electrodes (external pacemakers A61N1/3625)} U A61N1/ {with leads or electrodes penetrating the skin} E A61N1/ {for treatment of pain} E A61N1/ {for treating a mental or cerebral condition} U A61N1/ {for aversion therapy} A61N1/36014, A61N1/3603, A61N1/36031, A61N1/36034
6 Type* Symbol Indent Level Number of dots (e.g. 0, 1, 2) Title (new or modified) CPC only text should normally be enclosed in {curly brackets}** Transferred to # N A61N1/ {Control systems} D A61N1/ <administrative transfer to A61N1/36036> N A61N1/ {using physiological parameters for adjustment} N A61N1/ {specified by the stimulation parameters} D A61N1/ <administrative transfer to A61N1/3604> Q A61N1/ {of the outer, middle or inner ear} A61N1/36036, A61N1/36038, A61N1/36039 N A61N1/ {Cochlear stimulation} N A61N1/ {fitting procedures} D A61N2001/ <administrative transfer to A61N1/36025 > N A61N1/ {for correcting spinal deformities, e.g. scoliosis} U A61N1/ {of grafted tissue, e.g. skeletal muscle} C A61N1/ {adapted for a particular treatment} A61N1/3606, A61N1/36062 N A61N1/ {Spinal stimulation} U A61N1/ {Epilepsy} M A61N1/ {Movement disorders, e.g. tremor or Parkinson disease (stimulating motor muscle A61N1/36003)} M A61N1/ {Headache or migraine} M A61N1/ {Inducing or controlling sleep or relaxation (non-implantable stimulator A61M 21/00)} M A61N1/ {Cognitive or psychiatric applications, e.g. dementia or Alzheimer's disease} M A61N1/ {Eating disorders or obesity} M A61N1/ {Addiction or withdrawal from substance abuse such as alcohol or drugs} M A61N1/ {Mood disorders, e.g. depression, anxiety or panic disorder}
7 Type* Symbol Indent Level Number of dots (e.g. 0, 1, 2) Title (new or modified) CPC only text should normally be enclosed in {curly brackets}** Transferred to # M A61N1/ {Neuro-rehabilitation; Repair or reorganisation of neural tissue, e.g. after stroke} M A61N1/ {Sexual dysfunction (stimulating genital organs A61N1/36007)} M A61N1/ {Respiration control (stimulating respiratory organs A61N1/3601)} M A61N1/ {Production of neurotransmitters; Modulation of genes expression} C A61N1/ {using physiological parameters} A61N1/36135, A61N1/3614 U A61N1/ {with automatic adjustment} N A61N1/ {based on impedance measurement} U A61N1/ {for improving safety} C A61N1/ {Voltage (A61N1/3616 takes precedence)} C A61N1/ {Current (A61N1/3616 takes precedence)} E A61N1/ {Voltage density or current density} M A61N1/ {Sub-threshold or non-excitatory signals (non-excitatory signals to the heart A61N1/3628)} M A61N1/ {Pulse width or duty cycle} M A61N1/ {using modulation techniques} C A61N1/362 4 Heart stimulators (heart defibrillators A61N1/39) M A61N1/ {for treating a mechanical deficiency of the heart, e.g. congestive heart failure or cardiomyopathy} M A61N1/ {using sub-threshold or nonexcitatory signals} N A61N1/ {in combination with non-electric therapy} U A61N1/365 5 controlled by a physiological parameter, e.g. heart potential {(evoked response A61N1/371)} M A61N1/ {the parameter being derived from measurement of an electrical impedance} A61N1/36153, A61N1/3616 A61N1/36157, A61N1/3616 A61N1/362, A61N1/3629
8 Type* Symbol Indent Level Number of dots (e.g. 0, 1, 2) Title (new or modified) CPC only text should normally be enclosed in {curly brackets}** U A61N1/ {the parameter being measured by means of ultrasound} U A61N1/ {controlled by body position or posture} U A61N1/ {controlled by body motion, e.g. acceleration} U A61N1/ {controlled by body or blood temperature} U A61N1/ {controlled by chemical substances in blood} U A61N1/ {controlled by blood pressure} U A61N1/ {controlled by blood flow rate, e.g. blood velocity or cardiac output} U A61N1/ {controlled by mechanical motion of the heart wall, e.g. measured by an accelerometer or microphone} U A61N1/ {controlled by two or more physical parameters} U A61N1/ {controlled by the heart rate variability} U A61N1/368 6 comprising more than one electrode co-operating with different heart regions {(A61N1/3622, A61N1/3627 take precedence)} U A61N1/ {with a variable atrioventricular delay} C A61N1/ {for stimulating the heart at multiple sites of the ventricle or the atrium} N A61N1/ {Multi-site stimulation in the same chamber} N A61N1/ {Bi-ventricular stimulation} U A61N1/ {configured for selecting the electrode configuration on a lead (A61N1/3688 takes precedence)} U A61N1/ {configured for switching the pacing mode, e.g. from AAI to DDD} U A61N1/37 5 Monitoring; Protecting Transferred to # A61N1/3684, A61N1/36842, A61N1/36843
9 Type* Symbol Indent Level Number of dots (e.g. 0, 1, 2) Title (new or modified) CPC only text should normally be enclosed in {curly brackets}** M A61N1/ {Physiological parameters (A61N1/365 takes precedence; evoked response A61N1/371)} U A61N1/ {Circuits specially adapted therefor, e.g. for sensitivity control} M A61N1/ {Pacemaker parameters (stimulation threshold A61N1/371)} U A61N1/ {for power depletion} U A61N1/371 6 {Capture, i.e. successful stimulation} M A61N1/ {Auto-capture, i.e. automatic adjustment of the stimulation threshold} U A61N1/ {Atrial capture} U A61N1/ {with reduction of residual polarisation effects} U A61N1/ {Monitoring of or protection against external electromagnetic fields or currents} U A61N1/372 4 Arrangements in connection with the implantation of stimulators U A61N1/ {Microstimulators, e.g. implantable through a cannula} U A61N1/ {Means for communicating with stimulators} U A61N1/ {characterised by the communication link, e.g. acoustic or tactile} U A61N1/ {Circuits for electromagnetic coupling} U A61N1/ {Shape or location of the implanted or external antenna} U A61N1/ {Aspects of the external programmer} U A61N1/ {providing test stimulations} M A61N1/ {User interfaces, e.g. input or presentation means} C A61N1/ {Details of algorithms or data aspects of communication system, e.g. handshaking, transmitting specific data or segmenting data} Transferred to # A61N1/37252, A61N1/37254
10 Type* Symbol Indent Level Number of dots (e.g. 0, 1, 2) Title (new or modified) CPC only text should normally be enclosed in {curly brackets}** N A61N1/ {Pacemaker or defibrillator security, e.g. to prevent or inhibit programming alterations by hackers or unauthorised individuals} U A61N1/ {Alerting the patient} U A61N1/ {Changing the program; Upgrading firmware} U A61N1/ {characterised by the modulation technique} U A61N1/ {characterised by means for reducing power consumption during telemetry} U A61N1/ {characterised by communication with experts in remote locations using a network} U A61N1/ {Communication to several implantable medical devices within one patient} C A61N1/375 5 Constructional arrangements, e.g. casings N A61N1/ {Pacemakers} N A61N1/ {Brain implants} N A61N1/ {Intravascular implants} N A61N1/ {Anchoring of the implants, e.g. fixation} U A61N1/ {Details of casing-lead connections} U A61N1/ {Feedthroughs} U A61N1/ {Casings with electrodes thereon, e.g. leadless stimulators} U A61N1/ {Packaging of the components within the casing} U A61N1/378 5 Electrical supply U A61N1/ {producing a voltage above the power source level} U A61N1/ {generated by biological activity or substance, e.g. body movement} Transferred to # A61N1/375, A61N1/37512, A61N1/37514, A61N1/37516, A61N1/37518
11 Type* Symbol Indent Level Number of dots (e.g. 0, 1, 2) Title (new or modified) CPC only text should normally be enclosed in {curly brackets}** Transferred to # U A61N1/ {from an external energy source} M A61N1/38 3 for producing shock effects U A61N1/385 4 {Devices for inducing an abnormal cardiac function, e.g. fibrillation} C A61N1/39 4 Heart defibrillators A61N1/39, A61N1/3904, A61N1/39044, A61N1/39046 N A61N1/ {External heart defibrillators [EHD]} N A61N1/ {in combination with cardiopulmonary resuscitation [CPR] therapy} N A61N1/ {User protection from shock} U A61N1/ {characterised by the form of the shockwave} U A61N1/ {Output circuitry therefor, e.g. switches} U A61N1/ {characterised by shock pathway, e.g. by electrode configuration} U A61N1/ {Monitoring; Protecting} U A61N1/ {Protecting, e.g. back-up systems} U A61N1/ {Monitoring output parameters} U A61N1/ {for threshold determination} U A61N1/395 5 {for treating atrial fibrillation} U A61N1/ {Implantable devices for applying electric shocks to the heart, e.g. for cardioversion} C A61N1/ {in combination with another heart therapy} N A61N1/ {Pacing therapy} N A61N1/ {Pain reduction therapy} U A61N1/ {Constructional arrangements, e.g. casings (A61N1/375 takes precedence)} U A61N1/ {Power supply (A61N1/378 takes precedence)} U A61N1/ {High voltage charging circuitry} A61N1/3962, A61N1/39622, A61N1/39624
12 Type* Symbol Indent Level Number of dots (e.g. 0, 1, 2) Title (new or modified) CPC only text should normally be enclosed in {curly brackets}** U A61N1/ {characterised by the timing or triggering of the shock} U A61N1/ {User interfaces for automatic external defibrillators} Transferred to # *N = new entries where reclassification into entries is involved; C = entries with modified file scope where reclassification of documents from the entries is involved; Q = new entries which are firstly populated with documents via administrative transfe rs from deleted (D) entries. Afterwards, the transferred documents into the Q entry will either stay or be mov ed to more appropriate entries, as determined by intellectual reclassification; E= existing entries with enlarged file scope, which receive documen ts from C or D entries, e.g. when a limiting reference is removed from the entry title; M = entries with no change to the file scope (no reclassification); D = deleted entries; F = frozen entries will be deleted once reclassification of documents from the entries is completed; U = entries that are unchanged. NOTES: **No {curly brackets} are used for titles in CPC only subclasses, e.g. C12Y, A23Y; 2000 series symbol titles of groups found at the end of schemes (orthogonal codes); or the Y section titles. The {curly brackets} are used for 2000 series symbol titles found interspersed throughout the main trunk schemes (breakdown codes). For U groups, the minimum requirement is to include the U group located immediately prior to the N group or N group array, in order to show the N group hierarchy and improve the readability and understanding of the scheme. Always include the symbol, indent level and title of the U group in the table above. All entry types should be included in the scheme changes table above for better understanding of the overall scheme change picture. Symbol, indent level, and title are required for all types except D which requires only a symbol. # Transferred to column must be completed for all C, D, F, and Q type entries. F groups will be deleted once reclassification is completed. When multiple symbols are included in the Transferred to column, avoid using ranges of symbols in order to be as precise as possible. For administrative transfer of documents, the following text should be used: < administrative transfer to XX> or <administrative transfer to XX and YY simultaneously> when administrative transfer of the same documents is to more than one place. Administrative transfer to main trunk groups is assumed to be invention information, unless otherwise indicated, and to 2000 series groups is assumed to be additional information.
13 B. New, Modified or Deleted Warning notice(s) SUBCLASS A61N - ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY Type* Location Old Warning notice New/Modified Warning notice N A61N1/08 Group A61N1/08 is impacted by reclassification into group A61N1/3603. Groups A61N1/08 and A61N1/3603 should be considered in order to perform a complete search. N A61N2001/083 Group A61N2001/083 is impacted by reclassification into group A61N1/3603. Groups A61N2001/083 and A61N1/3603 should be considered in order to perform a complete search. N A61N1/36 Group A61N1/36 is impacted by reclassification into group A61N1/ Groups A61N1/36 and A61N1/36002 should be considered in order to perform a complete search. N A61N1/36002 Group A61N1/36002 is incomplete pending reclassification of documents from group A61N1/36. Groups A61N1/36002 and A61N1/36 should be considered in order to perform a complete search N A61N1/36014 Group A61N1/36014 is impacted by reclassification into groups A61N1/3603, A61N1/36031 and A61N1/ All groups listed in this Warning should be considered in order to perform a complete search. N A61N1/3603 Group A61N1/3603 is incomplete pending reclassification of documents from groups A61N1/08, A61N2001/083 and A61N1/ All groups listed in this Warning should be considered in order to perform a complete search. N A61N1/36031 Group A61N1/36031 is incomplete pending reclassification of documents from group A61N1/ Groups A61N1/36031 and A61N1/36014 should be considered in order to perform a complete search. N A61N1/36034 Group A61N1/36034 is incomplete pending reclassification of documents from group A61N1/ Groups A61N1/36034 and A61N1/36014 should be considered in order to perform a complete search.
14 Type* Location Old Warning notice New/Modified Warning notice N A61N1/36036 Group A61N1/36036 is impacted by reclassification into groups A61N1/36038 and A61N1/ Groups A61N1/36036, A61N1/36038 and A61N1/36039 should be considered in order to perform a complete search. N A61N1/36038 Groups A61N1/36038 and A61N1/36039 are incomplete pending reclassification of documents from group A61N1/ Groups A61N1/36036, A61N1/36038 and A61N1/36039 should be considered in order to perform a complete search N A61N1/3606 Group A61N1/3606 is impacted by reclassification into group A61N1/ Groups A61N1/3606 and A61N1/36062 should be considered in order to perform a complete search. N A61N1/36062 Group A61N1/36062 is incomplete pending reclassification of documents from group A61N1/3606. Groups A61N1/36062 and A61N1/3606 should be considered in order to perform a complete search N A61N1/36135 Group A61N1/36135 is impacted by reclassification into group A61N1/3614. Groups A61N1/36135 and A61N1/3614 should be considered in order to perform a complete search. N A61N1/3614 Group A61N1/3614 is incomplete pending reclassification of documents from group A61N1/ Groups A61N1/3614 and A61N1/36135 should be considered in order to perform a complete search N A61N 1/36153 Group A61N 1/36153 is impacted by reclassification into group A61N 1/3616. Groups A61N 1/36153 and A61N 1/3616 should be considered in order to perform a complete search. N A61N 1/36157 Group A61N 1/36157 is impacted by reclassification into group A61N 1/3616. Groups A61N 1/36157 and A61N 1/3616 should be considered in order to perform a complete search. N A61N 1/3616 Group A61N 1/3616 is incomplete pending reclassification of documents from groups A61N 1/36153 and A61N 1/ Groups A61N 1/3616, A61N 1/36153, and A61N 1/36157 should be considered in order to perform a complete search.
15 Type* Location Old Warning notice New/Modified Warning notice N A61N1/362 Group A61N1/362 is impacted by reclassification into group A61N1/3629. Groups A61N1/362 and A61N1/3629 should be considered in order to perform a complete search. N A61N1/3629 Group A61N1/3629 is incomplete pending reclassification of documents from group A61N1/362. Groups A61N1/3629 and A61N1/362 should be considered in order to perform a complete search N A61N1/3684 Group A61N1/3684 is impacted by reclassification into groups A61N1/36842 and A61N1/ Groups A61N1/3684, A61N1/36842 and A61N1/36843 should be considered in order to perform a complete search. N A61N1/36842 Group A61N1/36842 is incomplete pending reclassification of documents from group A61N1/3684. Groups A61N1/36842 and A61N1/3684 should be considered in order to perform a complete search. N A61N1/36843 Group A61N1/36843 is incomplete pending reclassification of documents from group A61N1/3684. Groups A61N1/36843 and A61N1/3684 should be considered in order to perform a complete search. N A61N1/37252 Group A61N1/37252 is impacted by reclassification into group A61N1/ Groups A61N1/37252 and A61N1/37254 should be considered in order to perform a complete search. N A61N1/37254 Group A61N1/37254 is incomplete pending reclassification of documents from group A61N1/ Groups A61N1/37254 and A61N1/37252 should be considered in order to perform a complete search. N A61N1/375 Group A61N1/375 is impacted by reclassification into groups A61N1/37512, A61N1/37514, A61N1/37516 and A61N1/ All groups listed in this Warning should be considered in order to perform a complete search. N A61N1/37512 Group A61N1/37512 is incomplete pending reclassification of documents from group A61N1/375. Groups A61N1/37512 and A61N1/375 should be considered in order to perform a complete search. N A61N1/37514 Group A61N1/37514 is incomplete pending reclassification of documents from group A61N1/375. Groups A61N1/37514
16 Type* Location Old Warning notice New/Modified Warning notice and A61N1/375 should be considered in order to perform a complete search. N A61N1/37516 Group A61N1/37516 is incomplete pending reclassification of documents from group A61N1/375. Groups A61N1/37516 and A61N1/375 should be considered in order to perform a complete search. N A61N1/37518 Group A61N1/37518 is incomplete pending reclassification of documents from group A61N1/375. Groups A61N1/37518 and A61N1/375 should be considered in order to perform a complete search. N A61N1/39 Group A61N1/39 is impacted by reclassification into groups A61N1/3904, A61N1/39044 and A61N1/ All groups listed in this Warning should be considered in order to perform a complete search. N A61N1/3904 Group A61N1/3904 is incomplete pending reclassification of documents from group A61N1/39. Groups A61N1/3904 and A61N1/39 should be considered in order to perform a complete search. N A61N1/39044 Group A61N1/39044 is incomplete pending reclassification of documents from group A61N1/39. Groups A61N1/39044 and A61N1/39 should be considered in order to perform a complete search. N A61N1/39046 Group A61N1/39046 is incomplete pending reclassification of documents from group A61N1/39. Groups A61N1/39046 and A61N1/39 should be considered in order to perform a complete search. N A61N1/3962 Group A61N1/3962 is impacted by reclassification into groups A61N1/39622 and A61N1/ Groups A61N1/3962, A61N1/39622 and A61N1/39624 should be considered in order to perform a complete search. N A61N1/39622 Group A61N1/39622 is incomplete pending reclassification of documents from group A61N1/3962. Groups A61N1/39622 and A61N1/3962 should be considered in order to perform a complete search. N A61N1/39624 Group A61N1/39624 is incomplete pending reclassification of documents from group A61N1/3962. Groups A61N1/39624 and A61N1/3962 should be considered in order to perform a complete search. *N = new warning, M = modified warning, D = deleted warning
17 NOTE: The Location column only requires the symbol PRIOR to the location of the warning. No further directions such as before or after are required.
18 2. A. DEFINITIONS (new) A61N 1/086 {Magnetic Resonance Imaging [MRI] compatible leads} Leads (both implantable and external) having features that mitigate the effects of unwanted electric currents induced by a Magnetic Resonance Imaging [MRI] device or field.
19 A61N 1/36002 {Cancer treatment, e.g. tumour} Covers devices (both implanted and external to the body) that treat cancer by electrical stimulation.
20 A61N 1/3603 {Control Systems} Covers features of control systems used by electrical stimulation devices that are external to the body (e.g. switches/buttons used for controlling TENS devices, control displays for pain relief devices, controlling modes of operation for muscle stimulators, algorithms/flow charts for controlling external stimulation devices, etc.).
21 A61N 1/36031 {using physiological parameters for adjustment} Device control systems under A61N1/3603, which adjust therapeutic electrical stimulation based upon at least one sensed physiological parameter.
22 A61N 1/36034 {specified by the stimulation parameters} Device control systems under A61N1/3603, which are characterised by their stimulation parameters (e.g. frequency, amplitude, pulse width, multiphasic, combination of waveforms, etc.).
23 A61N 1/36036 {of the outer, middle or inner ear} Covers therapeutic devices that electrically stimulate portions of a patient s outer, middle, or inner ear.
24 A61N 1/36038 {Cochlear stimulation} Devices under A61N1/36036 that electrically stimulate a patient s cochlea.
25 A61N 1/36039 {fitting procedures} Devices and methods used for fitting a patient with a cochlear stimulation device or setting a cochlea stimulation parameter (as described in A61N1/36038) (This subgroup is intended for devices having more than a general mention of fitting or stimulation parameters).
26 A61N1/3604 {for correcting spinal deformities, e.g. scoliosis} Electrical stimulation devices used for correcting spinal deformities.
27 A61N1/36062 {Spinal stimulation} Includes implantable neurostimulation devices and associated methods used to perform spinal stimulation (as described in A61N1/3605).
28 A61N1/3614 {based on impedance measurement} Control systems for implantable neural stimulation devices (under A61N1/3605) which measure impedance (This subgroup is intended for devices having more than a general mention of impedance).
29 A61N1/3629 {in combination with non-electric therapy} Heart stimulation devices (e.g. pacemakers) under (A61N1/362) which also include an additional non-electric therapy to the heart or other body area (e.g. drug therapy, nanotherapy, gene therapy, etc.).
30 A61N1/3684 {for stimulating the heart at multiple sites of the ventricle or the atrium} Heart stimulation devices under A61N1/368, which include dual chamber pacemakers (e.g. pacers using DDD modes of operation) and pacing devices that electrically stimulate multiple sites within the 4 chambers of the heart.
31 A61N1/36842 {Multi-site stimulation in the same chamber} Covers heart stimulation devices under A61N1/3684 having more than one stimulation site within the same heart chamber (e.g. two stimulation electrodes in the right ventricle that deliver separate and/or different stimulation to each electrode, etc.).
32 A61N1/36843 {Bi-ventricular stimulation} Covers heart stimulation devices which stimulate both the right ventricle [RV] and the left ventricle [LV].
33 A61N1/37254 {Pacemaker or defibrillator security, e.g. to prevent or inhibit programming alterations by hackers or unauthorised individuals} Devices and methods used to provide security (both programming and physical) for electrical stimulation devices (in particular pacemakers and defibrillators) to combat hackers and other unauthorised persons from accessing/re-programming electrical stimulation devices.
34 A61N1/37512 {Pacemakers} Covers device features related to the construction of pacemakers (i.e. housings/can details, material, shape, etc.).
35 A61N1/37514 {Brain implants} Covers device features related to the construction of brain implants (i.e. housing or can details, material, shape, etc.). Informative references Attention is drawn to the following places, which may be of interest for search: Burr Hole Covers A61B90/00
36 A61N1/37516 {Intravascular implants} Covers device features related to the construction of intravascular implants (i.e. housing details, material, shape, etc.).
37 A61N1/37518 {Anchoring of the implants, e.g. fixation} Covers anchoring/fixation details of electrical stimulation devices.
38 A61N1/3904 {External Heart Defibrillators [EHD]} Covers external heart defibrillators (e.g. Automatic External Defibrillators [AEDs], semiautomatic external defibrillators, manual) and methods for constructing and using them.
39 A61N1/39044 {in combination with cardiopulmonary resuscitation [CPR] therapy} External defibrillators and methods for using them for performing or assisting in cardiopulmonary resuscitation [CPR] therapy. Informative references Attention is drawn to the following places, which may be of interest for search: Cardiopulmonary Resuscitation [CPR] therapy A61H31/00
40 A61N1/39046 {User protection from shock} External defibrillators which include features that protect a user or patient from an accidental or unwanted shock.
41 A61N1/3962 {in combination with another heart therapy} Implantable defibrillator which in addition to electrical shock therapy, also provides another form of heart therapy (e.g. drug therapy, gene therapy, nanotherapy, etc.).
42 A61N1/39622 {Pacing therapy} Implantable defibrillators under A61N1/3962 which apply pacing therapy in addition to defibrillation shocks.
43 A61N1/39624 {Pain reduction therapy} Implantable defibrillators under A61N1/3962 which apply electrical stimulation therapy for pain reduction.
44 2. A. DEFINITIONS (modified) A61N1/36 Insert: The entire Limiting reference table from the Limiting reference section into a new Informative reference section. Limiting references Delete: The entire Limiting reference section including table.
45 2. B. DEFINITIONS QUICK FIX Symbol Location of change (e.g., section title) Existing reference symbol or text Action; New symbol; New text A61N1/08 Limiting References Insert the following new row: For external stimulators A61N1/3603 A61N1/08 Limiting References Insert the following new row: A61N1/372 Limiting References Details about leads and electrodes A61N1/04 - A61N1/06 A61N1/372 Informative references For implantable neurostimulators A61N1/36128 DELETE the entire section Insert the following new row: Details about leads and electrodes A61N1/04 - A61N1/06 NOTES: The table above is used for corrections or modifications to existing definitions, e.g. delete an entire definition or part thereof; propose new wording or modify wording of a section, change the symbol the definition is associated with, change or delete a reference symbol, etc. Do not delete (F) symbol definitions.
46 3. REVISION CONCORDANCE LIST (RCL) Type* From CPC Symbol (existing) To CPC Symbol(s) C A61N1/08 A61N1/08, A61N1/3603 C A61N2001/083 A61N2001/083, A61N1/3603 D A61N2001/086 <administrative transfer to A61N1/086> D A61N2001/34 <administrative transfer to A61N1/36021> C A61N1/36 A61N1/36, A61N1/36002 C A61N1/36014 A61N1/36014, A61N1/3603, A61N1/36031, A61N1/36034 D A61N1/36032 <administrative transfer to A61N1/36036> D A61N1/36035 <administrative transfer to A61N1/3604> Q A61N1/36036 A61N1/36036, A61N1/36038, A61N1/36039 D A61N2001/36039 <administrative transfer to A61N1/36025 > C A61N1/3606 A61N1/3606, A61N1/36062 C A61N1/36135 A61N1/36135, A61N1/3614 C A61N1/36153 A61N1/36153, A61N1/3616 C A61N1/36157 A61N1/36157, A61N1/3616 C A61N1/362 A61N1/362, A61N1/3629 C A61N1/3684 A61N1/3684, A61N1/36842, A61N1/36843 C A61N1/37252 A61N1/37252, A61N1/37254 C A61N1/375 A61N1/375, A61N1/37512, A61N1/37514, A61N1/37516, A61N1/37518 C A61N1/39 A61N1/39, A61N1/3904, A61N1/39044, A61N1/39046 C A61N1/3962 A61N1/3962, A61N1/39622, A61N1/39624 * C = entries with modified file scope where reclassification of documents from the entries is involved; Q = new entries whic h are firstly populated with documents via administrative transfers from deleted (D) entries. Afterwards, the transferred documents into the Q entry will either stay or be moved to more appropriate entries, as determined by intellectual reclassification; D = deleted entries. NOTES: Only C, D, and Q type entries are included in the table above. When multiple symbols are included in the To column, avoid using ranges of symbols in order to be as precise as possible. For administrative transfer of documents, the following text should be used: < administrative transfer to XX> or <administrative transfer to XX and YY simultaneously> when administrative transfer of the same documents is to more than one place. Administrative transfer to main trunk groups is assumed to be invention information, unless otherwise indicated, and to 2000 series groups is assumed to be additional information.
47 4. CHANGES TO THE CPC-TO-IPC CONCORDANCE LIST (CICL) CPC IPC Action* A61N1/086 A61N1/08 NEW A61N2001/086 DELETE A61N2001/34 DELETE A61N1/36002 A61N1/36 NEW A61N1/3603 A61N1/36 NEW A61N1/36031 A61N1/36 NEW A61N1/36032 DELETE A61N1/36034 A61N1/36 NEW A61N1/36035 DELETE A61N1/36036 A61N1/36 NEW A61N1/36038 A61N1/36 NEW A61N1/36039 A61N1/36 NEW A61N2001/36039 DELETE A61N1/3604 A61N1/36 NEW A61N1/36062 A61N1/36 NEW A61N1/3614 A61N1/36 NEW A61N1/3629 A61N1/362 NEW A61N1/36842 A61N1/368 NEW A61N1/36843 A61N1/368 NEW A61N1/37254 A61N1/372 NEW A61N1/37512 A61N1/375 NEW A61N1/37514 A61N1/375 NEW A61N1/37516 A61N1/375 NEW A61N1/37518 A61N1/375 NEW A61N1/3904 A61N1/39 NEW A61N1/39044 A61N1/39 NEW A61N1/39046 A61N1/39 NEW A61N1/39622 A61N1/39 NEW A61N1/39624 A61N1/39 NEW *Action column: For an (N) or (Q) entry, provide an IPC symbol and complete the Action column with NEW. For an existing CPC main trunk entry or indexing entry where the existing IPC symbol needs to be changed, provide an updated IPC symbol and complete the Action column with UPDATED. For a (D) CPC entry or indexing entry complete the Action column with DELETE. IPC symbol does not need to be included in the IPC column. For an (N) 2000 series CPC entry which is positioned within the main trunk scheme (breakdown code) provide an IPC symbol and complete the action column with NEW. For an (N) 2000 series CPC entry positioned at the end of the CPC scheme (orthogonal code), with no IPC equivalent, complete the IPC column with CPCONLY and complete the action column with NEW. NOTES: F symbols are not included in the CICL table above. E and M symbols are not included in the CICL table above unless a change to the existing IPC is desired.
48 5. CROSS-REFERENCE LIST (CRL) Scheme references impacted by this revision project Location of reference Referenced subclass or Action; New reference symbol; New text to be changed group to be changed A61B5/076 A61N1/362 A61N1/3605, A61N1/362 A61F11/04 A61N1/36032 A61N1/36036 H04R25/00 A61N1/36032 A61N1/36036 Definitions references impacted by this revision project Location of reference to be changed Referenced subclass or group to be changed Section of definition Action; New reference symbol; New text A61F11/04 A61N1/36032 Limiting references A61N1/36036 A61M (subclass) A61N2001/34 Limiting references A61N1/36021 A61M21/02 A61N2001/34 Informative A61N1/36021 References A61M21/02 A61N1/3601 A61N1/36A5 Informative References Delete both symbols and description H04R25/00 A61N1/36032 Limiting references A61N1/36036 H04R 25/606 A61N 1/36032 Informative A61N 1/36036 References H04R27/02 A61N1/36032 Limiting references A61N1/36036 NOTES: The CRL tables above are used for changes to locations outside of the project scope. Changes to references in scheme titles or definitions inside the project scope will be reflected in the scheme change template or one of the definition templates. In addition to other changes proposed in the tables above, in the column titled Referenced subclass or group to be changed, referenced D symbols should indicate an action of delete or should indicate a replacement symbol and referenced F symbols should indicate a replacement symbol. When a reference is deleted, text related to that reference will also be deleted unless other references or a range of references associated with the same text remain.
EUROPEAN PATENT OFFICE U.S. PATENT AND TRADEMARK OFFICE CPC NOTICE OF CHANGES 479 DATE: MAY 1, 2018 PROJECT RP0492
 EUROPEAN PATENT OFFICE U.S. PATENT AND TRADEMARK OFFICE CPC NOTICE OF CHANGES 479 The following classification changes will be effected by this Notice of Changes: Action Subclass Group(s) SCHEME: Symbols
EUROPEAN PATENT OFFICE U.S. PATENT AND TRADEMARK OFFICE CPC NOTICE OF CHANGES 479 The following classification changes will be effected by this Notice of Changes: Action Subclass Group(s) SCHEME: Symbols
EUROPEAN PATENT OFFICE U.S. PATENT AND TRADEMARK OFFICE CPC NOTICE OF CHANGES 473 DATE: JANUARY 1, 2018 PROJECT RP0486
 EUROPEA PATET OFFICE U.S. PATET AD TRADEMARK OFFICE CPC OTICE OF CHAGES 473 The following classification changes will be effected by this otice of Changes: Action Subclass Group(s) SCHEME: Symbols Deleted:
EUROPEA PATET OFFICE U.S. PATET AD TRADEMARK OFFICE CPC OTICE OF CHAGES 473 The following classification changes will be effected by this otice of Changes: Action Subclass Group(s) SCHEME: Symbols Deleted:
EUROPEAN PATENT OFFICE U.S. PATENT AND TRADEMARK OFFICE CPC NOTICE OF CHANGES 325 DATE: AUGUST 1, 2017 PROJECT MP0128
 EUROPEAN PATENT OFFICE U.S. PATENT AND TRADEMARK OFFICE CPC NOTICE OF CHANGES 325 The following classification s will be effected by this Notice of Changes: Action Subclass Group(s) Title wording s: 1/00
EUROPEAN PATENT OFFICE U.S. PATENT AND TRADEMARK OFFICE CPC NOTICE OF CHANGES 325 The following classification s will be effected by this Notice of Changes: Action Subclass Group(s) Title wording s: 1/00
EUROPEAN PATENT OFFICE U.S. PATENT AND TRADEMARK OFFICE CPC NOTICE OF CHANGES 265 DATE: AUGUST 1, 2016 PROJECT RP0307. Action Subclass Group(s)
 EUROPEAN PATENT OFFICE U.S. PATENT AND TRADEMARK OFFICE CPC NOTICE OF CHANGES 265 The following classification changes will be effected by this Notice of Changes: Action Subclass Group(s) Symbols deleted:
EUROPEAN PATENT OFFICE U.S. PATENT AND TRADEMARK OFFICE CPC NOTICE OF CHANGES 265 The following classification changes will be effected by this Notice of Changes: Action Subclass Group(s) Symbols deleted:
A61N. Definition statement. Relationships with other classification places CPC - A61N
 A61N ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY (measurement of bioelectric currents A61B; surgical instruments, devices or methods for transferring non-mechanical forms of energy
A61N ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY (measurement of bioelectric currents A61B; surgical instruments, devices or methods for transferring non-mechanical forms of energy
A61N. Definition statement. Relationships with other classification places CPC - A61N
 A61N ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY (measurement of bioelectric currents A61B; surgical instruments, devices or methods for transferring non-mechanical forms of energy
A61N ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY (measurement of bioelectric currents A61B; surgical instruments, devices or methods for transferring non-mechanical forms of energy
EUROPEAN PATENT OFFICE U.S. PATENT AND TRADEMARK OFFICE CPC NOTICE OF CHANGES 558 DATE: AUGUST 1, 2018 PROJECT RP0075 A61K 38/1764
 EUROPEAN PATENT OFFICE U.S. PATENT AND TRADEMARK OFFICE CPC NOTICE OF CHANGES 558 The following classification changes will be effected by this Notice of Changes: Action* Subclass Group(s) SCHEME: Symbols
EUROPEAN PATENT OFFICE U.S. PATENT AND TRADEMARK OFFICE CPC NOTICE OF CHANGES 558 The following classification changes will be effected by this Notice of Changes: Action* Subclass Group(s) SCHEME: Symbols
1. RF ablation guarding circuits for EIT
 1. RF ablation guarding circuits for EIT Synopsis: Intracardiac and endovascular ablation therapies are widespread in their use in the treatment of a variety of cardiac arrhythmias as well as renal artery
1. RF ablation guarding circuits for EIT Synopsis: Intracardiac and endovascular ablation therapies are widespread in their use in the treatment of a variety of cardiac arrhythmias as well as renal artery
ISO INTERNATIONAL STANDARD. Implants for surgery Active implantable medical devices Part 2: Cardiac pacemakers
 INTERNATIONAL STANDARD Provläsningsexemplar / Preview ISO 14708-2 First edition 2005-10-01 Implants for surgery Active implantable medical devices Part 2: Cardiac pacemakers Implants chirurgicaux Dispositifs
INTERNATIONAL STANDARD Provläsningsexemplar / Preview ISO 14708-2 First edition 2005-10-01 Implants for surgery Active implantable medical devices Part 2: Cardiac pacemakers Implants chirurgicaux Dispositifs
Implants for surgery Active implantable medical devices. Part 3: Implantable neurostimulators
 Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 14708-3 Second edition 2017-04 Implants for surgery Active implantable medical devices Part 3: Implantable neurostimulators Implants chirurgicaux
Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 14708-3 Second edition 2017-04 Implants for surgery Active implantable medical devices Part 3: Implantable neurostimulators Implants chirurgicaux
The technologies behind next-gen neuromodulation devices
 WHITEPAPER The technologies behind next-gen neuromodulation devices With the use closed-loop devices becoming more widespread in the development neuromodulation therapies, the ability to support smaller
WHITEPAPER The technologies behind next-gen neuromodulation devices With the use closed-loop devices becoming more widespread in the development neuromodulation therapies, the ability to support smaller
EUROPEAN PATENT OFFICE U.S. PATENT AND TRADEMARK OFFICE CPC NOTICE OF CHANGES 523 DATE: MAY 1, 2018 PROJECT MP0332. Action Subclass Group(s)
 EUROPEAN PATENT OFFICE U.S. PATENT AND TRADEMARK OFFICE CPC NOTICE OF CHANGES 523 The following classification changes will be effected by this Notice of Changes: Action Subclass Group(s) Modified Definitions
EUROPEAN PATENT OFFICE U.S. PATENT AND TRADEMARK OFFICE CPC NOTICE OF CHANGES 523 The following classification changes will be effected by this Notice of Changes: Action Subclass Group(s) Modified Definitions
Draft Guidance for Industry and Food and Drug Administration Staff Class II Special Controls Guidance Document: External Pacemaker Pulse Generator
 Reprinted from FDA s website by Draft Guidance for Industry and Food and Drug Administration Staff Class II Special Controls Guidance Document: External Pacemaker Pulse Generator DRAFT GUIDANCE This guidance
Reprinted from FDA s website by Draft Guidance for Industry and Food and Drug Administration Staff Class II Special Controls Guidance Document: External Pacemaker Pulse Generator DRAFT GUIDANCE This guidance
EUROPEAN PATENT OFFICE U.S. PATENT AND TRADEMARK OFFICE CPC NOTICE OF CHANGES 647 DATE: FEBRUARY 1, 2019 PROJECT RP0573 G06F 19/00
 EUROPEAN PATENT OFFICE U.S. PATENT AND TRADEMARK OFFICE The following classification changes will be effected by this Notice of Changes: Action Subclass Group(s) SCHEME: Symbols Deleted: C40B 30/02 C40B
EUROPEAN PATENT OFFICE U.S. PATENT AND TRADEMARK OFFICE The following classification changes will be effected by this Notice of Changes: Action Subclass Group(s) SCHEME: Symbols Deleted: C40B 30/02 C40B
BMEn 5910: Neuromodulation
 BMEn 5910: Spring 2011 (3 credits) Course Information Course Instructor: Course Meetings: Course Website: Professor Matt Johnson E-mail: john5101@umn.edu (please include BMEn 5910 in the subject) Office:
BMEn 5910: Spring 2011 (3 credits) Course Information Course Instructor: Course Meetings: Course Website: Professor Matt Johnson E-mail: john5101@umn.edu (please include BMEn 5910 in the subject) Office:
MEDICAL ELECTRONICS. Subject Title : Medical Electronics Subject Code : EC Hours Per Week : 04 Hours Per Semester : 64 TOPIC ANALYSIS
 MEDICAL ELECTRONICS Subject Title : Medical Electronics Subject Code : EC Hours Per Week : 04 Hours Per Semester : 64 SL. No TOPIC ANALYSIS Major Topics Hours Allotted UNIT I 1 Bio medical system and bio
MEDICAL ELECTRONICS Subject Title : Medical Electronics Subject Code : EC Hours Per Week : 04 Hours Per Semester : 64 SL. No TOPIC ANALYSIS Major Topics Hours Allotted UNIT I 1 Bio medical system and bio
EUROPEAN PATENT OFFICE U.S. PATENT AND TRADEMARK OFFICE CPC NOTICE OF CHANGES 521 DATE: MAY 1, 2018 PROJECT DP0192
 EUROPEAN PATENT OFFICE U.S. PATENT AND TRADEMARK OFFICE CPC NOTICE OF CHANGES 521 The following classification changes will be effected by this Notice of Changes: Action Subclass Group(s) DEFINITIONS:
EUROPEAN PATENT OFFICE U.S. PATENT AND TRADEMARK OFFICE CPC NOTICE OF CHANGES 521 The following classification changes will be effected by this Notice of Changes: Action Subclass Group(s) DEFINITIONS:
Every Day is a CardioDay. Speed. Accuracy. Ease of Use. CardioDay
 Every Day is a CardioDay Speed. Accuracy. Ease of Use. CardioDay Designed for the complexity and pace of today s Holter ECG workloads Speed Analytical Speed CardioDay harnesses the speed and standardization
Every Day is a CardioDay Speed. Accuracy. Ease of Use. CardioDay Designed for the complexity and pace of today s Holter ECG workloads Speed Analytical Speed CardioDay harnesses the speed and standardization
Every Day is a CardioDay. Speed. Accuracy. Ease of Use. CardioDay
 Every Day is a CardioDay Speed. Accuracy. Ease of Use. CardioDay Managing Holter volume can be challenging for many ECG departments. Is there a way to maintain your high quality standards while still achieving
Every Day is a CardioDay Speed. Accuracy. Ease of Use. CardioDay Managing Holter volume can be challenging for many ECG departments. Is there a way to maintain your high quality standards while still achieving
Medical instrumentationi 11/19/2010
 Medical instrumentationi BIOEN 302 11/19/2010 Medical instrumentation Definition: instrument for sensing, diagnostics, therapeutics or surgery of human being. 2 Medical instrumentation Definition: instrument
Medical instrumentationi BIOEN 302 11/19/2010 Medical instrumentation Definition: instrument for sensing, diagnostics, therapeutics or surgery of human being. 2 Medical instrumentation Definition: instrument
Class 7: Methods in Research By: Ray
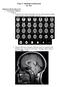 Class 7: Methods in Research By: Ray Method in Brain Research 1. Non-Invasive (Human) o Imaging Computerized (Axial) Tomography (X-rays). Static pictures and high spatial resolution. Horizontal plane only.
Class 7: Methods in Research By: Ray Method in Brain Research 1. Non-Invasive (Human) o Imaging Computerized (Axial) Tomography (X-rays). Static pictures and high spatial resolution. Horizontal plane only.
Urgent Field Safety Notice
 Urgent Field Safety Notice December 2017 Subject: Pacemakers Technical programming information on Minute Ventilation Sensor. Ref.: 92186345-FA Product name Models VALITUDE CRT-P U125, U128 ACCOLADE Pacemakers
Urgent Field Safety Notice December 2017 Subject: Pacemakers Technical programming information on Minute Ventilation Sensor. Ref.: 92186345-FA Product name Models VALITUDE CRT-P U125, U128 ACCOLADE Pacemakers
Lesson 7A Specialized Cells, Stem Cells & Cellular Differentiation
 Lesson 7A Specialized Cells, Stem Cells & Cellular Differentiation Learning Goals I can explain the concept of cell differentiation and cell specialization. I can explain how the cell structure relates
Lesson 7A Specialized Cells, Stem Cells & Cellular Differentiation Learning Goals I can explain the concept of cell differentiation and cell specialization. I can explain how the cell structure relates
MEDICAL DEVICES : Guidance document
 EUROPEAN COMMISSION DG ENTERPRISE Directorate G Unit 4 - Pressure Equipment, Medical Devices, Metrology MEDICAL DEVICES : Guidance document MEDDEV 2. 1/2 rev 2 26 April 1994 GUIDELINES RELATING TO THE
EUROPEAN COMMISSION DG ENTERPRISE Directorate G Unit 4 - Pressure Equipment, Medical Devices, Metrology MEDICAL DEVICES : Guidance document MEDDEV 2. 1/2 rev 2 26 April 1994 GUIDELINES RELATING TO THE
2018 REVIEW CATEGORIES
 2018 REVIEW CATEGORIES 100 Neuro 101 Neuro: Acquisition 102 Neuro: Processing 103 Neuro: Neonatal & Pediatric - Normal Development 104 Neuro: Neonatal & Pediatric - Clinical Studies 105 Neuro: Normal Aging
2018 REVIEW CATEGORIES 100 Neuro 101 Neuro: Acquisition 102 Neuro: Processing 103 Neuro: Neonatal & Pediatric - Normal Development 104 Neuro: Neonatal & Pediatric - Clinical Studies 105 Neuro: Normal Aging
AEON PHOCUS. Electromagnetic Steering of Interventional Instruments. phone +41 (0) fax +41 (0)
 AEON PHOCUS Electromagnetic Steering of Interventional Instruments aeon scientific AG Rütistrasse 12 8952 Schlieren-Zürich Switzerland phone +41 (0)44 738 89 00 fax +41 (0)44 738 89 01 www.aeon-scientific.com
AEON PHOCUS Electromagnetic Steering of Interventional Instruments aeon scientific AG Rütistrasse 12 8952 Schlieren-Zürich Switzerland phone +41 (0)44 738 89 00 fax +41 (0)44 738 89 01 www.aeon-scientific.com
Disclaimer. Cardiostim June
 Disclaimer This presentation contains management preliminary estimates and forward-looking statements, including information related to Sorin projected financial performance and the expected development
Disclaimer This presentation contains management preliminary estimates and forward-looking statements, including information related to Sorin projected financial performance and the expected development
A niche Contract Research Organisation. Dr Tina Soulis, CEO
 A niche Contract Research Organisation A niche Contract Research Organisation Dr Tina Soulis, CEO 1 Introducing The Florey (Institute of Neuroscience and Mental Health) The largest Brain research capability
A niche Contract Research Organisation A niche Contract Research Organisation Dr Tina Soulis, CEO 1 Introducing The Florey (Institute of Neuroscience and Mental Health) The largest Brain research capability
Package Design Innovation
 Package Design Innovation Reveal ICM Sterile Packaging Solution March 2-4, 2010 www.healthpack.net Acknowledgments Agenda ICM/Reveal Product Overview Previous Sterile Barrier System Design for Reveal Previous
Package Design Innovation Reveal ICM Sterile Packaging Solution March 2-4, 2010 www.healthpack.net Acknowledgments Agenda ICM/Reveal Product Overview Previous Sterile Barrier System Design for Reveal Previous
Medical Electronic Devices
 Medical Electronic Devices Basic Electronics (Electronic devices, Amplifiers, A/D, D/A) Electrodes EEG Deep brain stimulator ECG Cardiac Pacemakers External Defibrillators EMG Neuromuscular Electrical
Medical Electronic Devices Basic Electronics (Electronic devices, Amplifiers, A/D, D/A) Electrodes EEG Deep brain stimulator ECG Cardiac Pacemakers External Defibrillators EMG Neuromuscular Electrical
ARTIFICAL VISION. Regina Leung, Michelle Ngai
 ARTIFICAL VISION http://www.sfn.org/skins/main/images/brainbriefings/big.nov.jpg http://www.ubergizmo.com/photos/2006/4/wireless-ocular-implant.jpg http://www.crystalinks.com/eyeblind.gif Regina Leung,
ARTIFICAL VISION http://www.sfn.org/skins/main/images/brainbriefings/big.nov.jpg http://www.ubergizmo.com/photos/2006/4/wireless-ocular-implant.jpg http://www.crystalinks.com/eyeblind.gif Regina Leung,
UNIT I PHYSIOLOGYAND TRANSDUCERS
 SRI VENKATESWARA COLLEGE OF ENGINEERING AND TECHNOLOGY TIRUPACHUR DEPARTMENT OF ELECTRICAL AND ELECTRONICS ENGINEERING 1. What is meant by cell? EI 2311 BIOMEDICAL INSTRUMENTATION 2 Mark Questions With
SRI VENKATESWARA COLLEGE OF ENGINEERING AND TECHNOLOGY TIRUPACHUR DEPARTMENT OF ELECTRICAL AND ELECTRONICS ENGINEERING 1. What is meant by cell? EI 2311 BIOMEDICAL INSTRUMENTATION 2 Mark Questions With
Revised Examination Guidelines for Industrially Applicable Inventions (Provisional Translation)
 Revised Examination Guidelines for Industrially Applicable Inventions (Provisional Translation) Examination Guidelines for Patent and Utility Model Part II: REQUIREMENTS FOR PATENTABILITY Chapter 1 Industrially
Revised Examination Guidelines for Industrially Applicable Inventions (Provisional Translation) Examination Guidelines for Patent and Utility Model Part II: REQUIREMENTS FOR PATENTABILITY Chapter 1 Industrially
- MRI Safety Update - RF Induced Heating. Society for Medical Innovation and Technology
 - MRI Safety Update - RF Induced Heating presented to Society for Medical Innovation and Technology 11-14 May 2006 Pebble Beach, Monterey, CA, USA Jeffrey L. Helfer Objective of this Presentation Share
- MRI Safety Update - RF Induced Heating presented to Society for Medical Innovation and Technology 11-14 May 2006 Pebble Beach, Monterey, CA, USA Jeffrey L. Helfer Objective of this Presentation Share
What is your patient wearing? By Jeff Gastorf, DO FACOFP
 What is your patient wearing? By Jeff Gastorf, DO FACOFP Disclosures None Importance of medical devices Patient Engagement Importance to society What is the importance of medical devices? Doctor Engagement
What is your patient wearing? By Jeff Gastorf, DO FACOFP Disclosures None Importance of medical devices Patient Engagement Importance to society What is the importance of medical devices? Doctor Engagement
Simple, intuitive and accessible MRI solution for preclinical research. M-Series Compact MRI Systems
 Simple, intuitive and accessible MRI solution for preclinical research M-Series Compact MRI Systems Application Oriented Imaging Anatomy and Morphology In vivo soft tissue imaging for morphological characterization.
Simple, intuitive and accessible MRI solution for preclinical research M-Series Compact MRI Systems Application Oriented Imaging Anatomy and Morphology In vivo soft tissue imaging for morphological characterization.
Simple, intuitive and accessible MRI solution for preclinical research. M-Series Compact MRI Systems
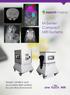 Simple, intuitive and accessible MRI solution for preclinical research M-Series Compact MRI Systems Application Oriented Imaging Molecular Imaging Using Contrast Agents Detection and quantification of
Simple, intuitive and accessible MRI solution for preclinical research M-Series Compact MRI Systems Application Oriented Imaging Molecular Imaging Using Contrast Agents Detection and quantification of
Tissue Engineering and the Brain. Susan Perry Bioengineering Program Lehigh University
 Tissue Engineering and the Brain Susan Perry Bioengineering Program Lehigh University ...all the most acute, most powerful, and most deadly diseases, and those which are most difficult to be understood
Tissue Engineering and the Brain Susan Perry Bioengineering Program Lehigh University ...all the most acute, most powerful, and most deadly diseases, and those which are most difficult to be understood
Tissue Engineering and the Brain. Susan Perry Bioengineering Program Lehigh University
 Tissue Engineering and the Brain Susan Perry Bioengineering Program Lehigh University ...all the most acute, most powerful, and most deadly diseases, and those which are most difficult to be understood
Tissue Engineering and the Brain Susan Perry Bioengineering Program Lehigh University ...all the most acute, most powerful, and most deadly diseases, and those which are most difficult to be understood
Focus on Cardiac Devices. with
 Focus on Cardiac Devices with We are focused FDA Class II and III therapeutic and diagnostic device product development and manufacturing A mature, well-developed ISO-13485 quality system Efficient and
Focus on Cardiac Devices with We are focused FDA Class II and III therapeutic and diagnostic device product development and manufacturing A mature, well-developed ISO-13485 quality system Efficient and
1: Executive Summary The Brain Industry Revealed Neuropharmaceuticals Cell Therapies Neurodevices Neurodiagnostics Investment Risks and Opportunities
 1: Executive Summary The Brain Industry Revealed Neuropharmaceuticals Cell Therapies Neurodevices Neurodiagnostics Investment Risks and Opportunities Translating Neuroscience into Neurotech The Neurotech
1: Executive Summary The Brain Industry Revealed Neuropharmaceuticals Cell Therapies Neurodevices Neurodiagnostics Investment Risks and Opportunities Translating Neuroscience into Neurotech The Neurotech
FUNDING OPPORTUNITIES FAIRE: NATIONAL INSTITUTES OF HEALTH
 FUNDING OPPORTUNITIES FAIRE: NATIONAL INSTITUTES OF HEALTH ERC Biennial Meeting Theme: Preparing Tomorrow s Leaders October 28, 2014 Grace C.Y. Peng, Ph.D. The NIH Mission NIH is the steward of medical
FUNDING OPPORTUNITIES FAIRE: NATIONAL INSTITUTES OF HEALTH ERC Biennial Meeting Theme: Preparing Tomorrow s Leaders October 28, 2014 Grace C.Y. Peng, Ph.D. The NIH Mission NIH is the steward of medical
Cardiac Rhythm Management // Patient Brochure // BioMonitor 2. BioMonitor 2. Patient Information
 Cardiac Rhythm Management // Patient Brochure // BioMonitor 2 BioMonitor 2 Patient Information BioMonitor 2 We Listen to Your Heart Dear patient, Your doctor has decided to implant a cardiac monitor BioMonitor
Cardiac Rhythm Management // Patient Brochure // BioMonitor 2 BioMonitor 2 Patient Information BioMonitor 2 We Listen to Your Heart Dear patient, Your doctor has decided to implant a cardiac monitor BioMonitor
Electrical Safety. Hsiao-Lung Chan, Ph.D. Dept Electrical Engineering Chang Gung University, Taiwan
 Electrical Safety Hsiao-Lung Chan, Ph.D. Dept Electrical Engineering Chang Gung University, Taiwan chanhl@mail.cgu.edu.tw Electro-medical equipment safety standard International electrotechnical commission
Electrical Safety Hsiao-Lung Chan, Ph.D. Dept Electrical Engineering Chang Gung University, Taiwan chanhl@mail.cgu.edu.tw Electro-medical equipment safety standard International electrotechnical commission
Delft
 1 Established in 2014; Youngest section of EWI-ME Mainly involved in theme Health and Well-Being Wouter Serdijn (full prof.); Vasiliki Giagka (assist. prof.), since Sept. 2015; Virgilio Valente (assist.
1 Established in 2014; Youngest section of EWI-ME Mainly involved in theme Health and Well-Being Wouter Serdijn (full prof.); Vasiliki Giagka (assist. prof.), since Sept. 2015; Virgilio Valente (assist.
Integrating sensors into health diagnostic systems
 Integrating sensors into health diagnostic systems May 16 2007 NanoEXPO Conference Prepared by Diana Hodgins, Managing Director ETB Email: diana.hodgins@etb.co.uk SIXTH FRAMEWORK PROGRAMME Information
Integrating sensors into health diagnostic systems May 16 2007 NanoEXPO Conference Prepared by Diana Hodgins, Managing Director ETB Email: diana.hodgins@etb.co.uk SIXTH FRAMEWORK PROGRAMME Information
IoT and Behavior Change: Can We Build a GPS for Our Brains?
 IoT and Behavior Change: Can We Build a GPS for Our Brains? Rich Fletcher Research Scientist, MIT Asst Prof, UMass Medical School Sept 2016 Introduction 2000-2010 Rich Fletcher Health and Disease in Middle
IoT and Behavior Change: Can We Build a GPS for Our Brains? Rich Fletcher Research Scientist, MIT Asst Prof, UMass Medical School Sept 2016 Introduction 2000-2010 Rich Fletcher Health and Disease in Middle
NeuroPort Array PN 4382, 4383, 6248, and 6249 Instructions for Use
 630 Komas Drive Suite 200 Salt Lake City UT 84108 USA P +1 801.582.5533 F +1 801.582.1509 www.blackrockmicro.com NeuroPort Array PN 4382, 4383, 6248, and 6249 Instructions for Use Revision 2.00 / LB-0612
630 Komas Drive Suite 200 Salt Lake City UT 84108 USA P +1 801.582.5533 F +1 801.582.1509 www.blackrockmicro.com NeuroPort Array PN 4382, 4383, 6248, and 6249 Instructions for Use Revision 2.00 / LB-0612
Final project topics
 Final project topics BIOEN 327 Autumn 2016 1 Mechanical heart valve Clinical relevance: replace failing valves Physiological principles: heart as a pump, blood viscosity, vascular dynamics Engineering
Final project topics BIOEN 327 Autumn 2016 1 Mechanical heart valve Clinical relevance: replace failing valves Physiological principles: heart as a pump, blood viscosity, vascular dynamics Engineering
ISO/TS TECHNICAL SPECIFICATION. Assessment of the safety of magnetic resonance imaging for patients with an active implantable medical device
 TECHNICAL SPECIFICATION ISO/TS 10974 First edition 2012-05-01 Assessment of the safety of magnetic resonance imaging for patients with an active implantable medical device Évaluation de la sécurité de
TECHNICAL SPECIFICATION ISO/TS 10974 First edition 2012-05-01 Assessment of the safety of magnetic resonance imaging for patients with an active implantable medical device Évaluation de la sécurité de
SI8000 Live Cell Imaging System
 SI8000 Live Cell Imaging System Sony Biotechnology Inc. SI8000 Cell Motion Imaging System The Sony SI8000 Live Cell Imaging System detects and quantifies cellular motion using proprietary video processing
SI8000 Live Cell Imaging System Sony Biotechnology Inc. SI8000 Cell Motion Imaging System The Sony SI8000 Live Cell Imaging System detects and quantifies cellular motion using proprietary video processing
Clinical Evaluation of a New Computer Based Expert Management System of Pacemaker Diagnostic Functions
 December 2000 471 Clinical Evaluation of a New Computer Based Expert Management System of Pacemaker Diagnostic Functions D. IGIDBASHIAN, G. LONARDI, M. GEMELLI, M. BARBIERO Cardiology Department, Electrophysiology
December 2000 471 Clinical Evaluation of a New Computer Based Expert Management System of Pacemaker Diagnostic Functions D. IGIDBASHIAN, G. LONARDI, M. GEMELLI, M. BARBIERO Cardiology Department, Electrophysiology
Individualized Therapies
 Individualized Therapies Alexander Medvedev Information Technology, Uppsala University, SWEDEN alexander.medvedev@it.uu.se Individualized Therapies Background Focus on medical technology Uppsala University
Individualized Therapies Alexander Medvedev Information Technology, Uppsala University, SWEDEN alexander.medvedev@it.uu.se Individualized Therapies Background Focus on medical technology Uppsala University
R Series. Confidence Comes with Knowing. You Are Code-Ready
 R Series Confidence Comes with Knowing You Are Code-Ready The First and Only Code-Ready Defibrillator The worst time to find out a defibrillator isn t ready is at the code. Quick action is essential. Stress
R Series Confidence Comes with Knowing You Are Code-Ready The First and Only Code-Ready Defibrillator The worst time to find out a defibrillator isn t ready is at the code. Quick action is essential. Stress
R Series. Confidence Comes with Knowing. You Are Code-Ready
 R Series Confidence Comes with Knowing You Are Code-Ready The First and Only Code-Ready Defibrillator The worst time to find out a defibrillator isn t ready is at the code. Quick action is essential. Stress
R Series Confidence Comes with Knowing You Are Code-Ready The First and Only Code-Ready Defibrillator The worst time to find out a defibrillator isn t ready is at the code. Quick action is essential. Stress
Cooperative Patent Classification at the USPTO
 Cooperative Patent Classification at the USPTO Jerry Lorengo - Director Technology Center 1600 Organic Chemistry & Biotechnology PIUG Biotech Conference San Francisco, CA Feb 2015 Overview CPC Milestones
Cooperative Patent Classification at the USPTO Jerry Lorengo - Director Technology Center 1600 Organic Chemistry & Biotechnology PIUG Biotech Conference San Francisco, CA Feb 2015 Overview CPC Milestones
Lehman Conference March 2006
 Lehman Conference March 2006 Forward Looking Statements Some of the statements made in the presentation whether written or oral may be forward-looking statements within the meaning of Section 27A of the
Lehman Conference March 2006 Forward Looking Statements Some of the statements made in the presentation whether written or oral may be forward-looking statements within the meaning of Section 27A of the
TechSearch International, Inc.
 Future Vision of Medical Electronics E. Jan Vardaman President TechSearch International, Inc. www.techsearchinc.com The Future: The Bionic Age Vision: So that the blind may see Hearing: So that the deaf
Future Vision of Medical Electronics E. Jan Vardaman President TechSearch International, Inc. www.techsearchinc.com The Future: The Bionic Age Vision: So that the blind may see Hearing: So that the deaf
A CRITICAL REVIEW ON APPLICATION OF BIOMEDICAL SIGNAL PROCESSING IN MEDICINE
 A CRITICAL REVIEW ON APPLICATION OF BIOMEDICAL SIGNAL PROCESSING IN MEDICINE Dr.L.Latchu 1, Dr.N.Aruna Kumari 2, Kanakam Yamini Sushma Murali Priya 3, Mathamsetty Jaya Lakshmi 4, Garapati Anjali Devi 5
A CRITICAL REVIEW ON APPLICATION OF BIOMEDICAL SIGNAL PROCESSING IN MEDICINE Dr.L.Latchu 1, Dr.N.Aruna Kumari 2, Kanakam Yamini Sushma Murali Priya 3, Mathamsetty Jaya Lakshmi 4, Garapati Anjali Devi 5
PICC TIP TRACKING & CONFIRMATION WITHOUT CHEST X-RAY
 GETTING TO THE HEART OF THE MATTER Using x-ray to confirm picc tip placement wastes clinical care time, exposes patients to harmful radiation, and delays medical therapy. The Sherlock 3cg* Tip Confirmation
GETTING TO THE HEART OF THE MATTER Using x-ray to confirm picc tip placement wastes clinical care time, exposes patients to harmful radiation, and delays medical therapy. The Sherlock 3cg* Tip Confirmation
UCD Biomedical Engineering Dr. Simon Kelly Dr. Eoin O Cearbhaill
 UCD Biomedical Engineering Dr. Simon Kelly Dr. Eoin O Cearbhaill School of Electrical and Electronic Engineering School of Mechanical and Materials Engineering BE and ME Biomedical Engineering Overview
UCD Biomedical Engineering Dr. Simon Kelly Dr. Eoin O Cearbhaill School of Electrical and Electronic Engineering School of Mechanical and Materials Engineering BE and ME Biomedical Engineering Overview
Nexeon MedSystems Inc. Corporate Overview
 Nexeon MedSystems Inc. Corporate Overview www.nexeonmed.com Forward-looking Statements This information contains forward-looking statements which may be identified by their use of words like "plans," "expects,"
Nexeon MedSystems Inc. Corporate Overview www.nexeonmed.com Forward-looking Statements This information contains forward-looking statements which may be identified by their use of words like "plans," "expects,"
Implants for surgery Cardiac pacemakers. Part 2: Reporting of clinical performance of populations of pulse generators or leads
 Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 5841-2 Third edition 2014-08-01 Implants for surgery Cardiac pacemakers Part 2: Reporting of clinical performance of populations of pulse generators
Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 5841-2 Third edition 2014-08-01 Implants for surgery Cardiac pacemakers Part 2: Reporting of clinical performance of populations of pulse generators
Article: "Noninvasive and Targeted Gene Delivery into the Brain Using Microbubble-Facilit 1 / 16. Ultrasound" Malova Anna
 Article: "Noninvasive and Targeted Gene Delivery into the Brain Using Microbubble-Facilitated Focused Ultrasound" 06.04.2013 Article: "Noninvasive and Targeted Gene Delivery into the Brain Using 06.04.2013
Article: "Noninvasive and Targeted Gene Delivery into the Brain Using Microbubble-Facilitated Focused Ultrasound" 06.04.2013 Article: "Noninvasive and Targeted Gene Delivery into the Brain Using 06.04.2013
Stem cells in Development
 ANAT 2341 Embryology Lab 10 8 Oct 2009 Therapeutic Use of Stem Cells Practical Hurdles & Ethical Issues Stem cells in Development Blastocyst Cord blood Antonio Lee PhD Neuromuscular & Regenerative Medicine
ANAT 2341 Embryology Lab 10 8 Oct 2009 Therapeutic Use of Stem Cells Practical Hurdles & Ethical Issues Stem cells in Development Blastocyst Cord blood Antonio Lee PhD Neuromuscular & Regenerative Medicine
Stem cells in Development
 ANAT 2341 Embryology Lab 10 8 Oct 2009 Therapeutic Use of Stem Cells Practical Hurdles & Ethical Issues Stem cells in Development Blastocyst Cord blood Antonio Lee PhD Neuromuscular & Regenerative Medicine
ANAT 2341 Embryology Lab 10 8 Oct 2009 Therapeutic Use of Stem Cells Practical Hurdles & Ethical Issues Stem cells in Development Blastocyst Cord blood Antonio Lee PhD Neuromuscular & Regenerative Medicine
ACS COLLEGE OF ENGINEERING
 ACS COLLEGE OF ENGINEERING DEPARTMENT OF BIOMEDICAL ENGINEERING Clinical Instrumentation -II Lab Prelab & Postlab questions (2015-2016) YEAR III YEAR SEMESTER VI SEM SUBJECT NAME CLINICAL INSTRUMENTATION
ACS COLLEGE OF ENGINEERING DEPARTMENT OF BIOMEDICAL ENGINEERING Clinical Instrumentation -II Lab Prelab & Postlab questions (2015-2016) YEAR III YEAR SEMESTER VI SEM SUBJECT NAME CLINICAL INSTRUMENTATION
Appendix 1 Canadian Standards Association Standards for Equipment
 Can J Anesth/J Can Anesth (2018) Appendix 1 Canadian Standards Association Standards for Equipment Legend Example: C22.2 NO. 0.1-FM85 (C1999) F Indicates the standard is written in French. M Indicates
Can J Anesth/J Can Anesth (2018) Appendix 1 Canadian Standards Association Standards for Equipment Legend Example: C22.2 NO. 0.1-FM85 (C1999) F Indicates the standard is written in French. M Indicates
Research collaboration with Q Therapeutics and its founder Dr. Mahendra Rao. REPROCELL Inc., 2017
 Research collaboration with Q Therapeutics and its founder Dr. Mahendra Rao 1 29 th November, 2017 Collaboration with Q therapeutics to develop new ipsc therapies for CNS diseases REPROCELL announced a
Research collaboration with Q Therapeutics and its founder Dr. Mahendra Rao 1 29 th November, 2017 Collaboration with Q therapeutics to develop new ipsc therapies for CNS diseases REPROCELL announced a
Laurea Magistrale in «Ingegneria Elettronica» Presentazione corso di. «Chimica e Processi per la Microelettronica «Anno Accademico 2016/2017
 Laurea Magistrale in «Ingegneria Elettronica» Presentazione corso di «Chimica e Processi per la Microelettronica «Anno Accademico 2016/2017 Programma del modulo di Chimica e Processi per la Microelettronica
Laurea Magistrale in «Ingegneria Elettronica» Presentazione corso di «Chimica e Processi per la Microelettronica «Anno Accademico 2016/2017 Programma del modulo di Chimica e Processi per la Microelettronica
Systems & Biomedical Engineering Department. Systems and Biomedical Engineering Department Faculty of Engineering Cairo University
 Systems & Biomedical Engineering Department Agenda Students' Mindsets and Careers Biomedical Engineering Biomedical Engineering Careers in Egypt Non-Biomedical Careers in Egypt Courses Agenda Students'
Systems & Biomedical Engineering Department Agenda Students' Mindsets and Careers Biomedical Engineering Biomedical Engineering Careers in Egypt Non-Biomedical Careers in Egypt Courses Agenda Students'
Application Performance Monitoring Dashboard 7.2
 User Guide Focused Insights for SAP Solution Manager Document Version: 1.1 2017-07-31 ST-OST 200 SP 1 Typographic Conventions Type Style Example Example EXAMPLE Example Example EXAMPLE Description
User Guide Focused Insights for SAP Solution Manager Document Version: 1.1 2017-07-31 ST-OST 200 SP 1 Typographic Conventions Type Style Example Example EXAMPLE Example Example EXAMPLE Description
WhaleTeq Co., Ltd Company Profile and Introduction
 The Accurate and Com pliant Test Solution Provider WhaleTeq Co., Ltd Company Profile and Introduction Content About WhaleTeq Co., Ltd Global Customers Core Competences Vision Test Solutions Test System
The Accurate and Com pliant Test Solution Provider WhaleTeq Co., Ltd Company Profile and Introduction Content About WhaleTeq Co., Ltd Global Customers Core Competences Vision Test Solutions Test System
Plenary Session: An update of brain circuits in Parkinson s and Deep Brain Stimulation
 Plenary Session: An update of brain circuits in Parkinson s and Deep Brain Stimulation Deep Brain Stimulation Andres M Lozano, University of Toronto Deep brain stimulation is the delivery of an electrical
Plenary Session: An update of brain circuits in Parkinson s and Deep Brain Stimulation Deep Brain Stimulation Andres M Lozano, University of Toronto Deep brain stimulation is the delivery of an electrical
Innovative IONM solutions.
 Innovative IONM solutions H e l p i n g y o u h e l p o t h e r1 s www.cadwell.com/ionm Surgeries are inherently risky, especially those that involve the brain, spinal cord or nerves. Intraoperative neurophysiological
Innovative IONM solutions H e l p i n g y o u h e l p o t h e r1 s www.cadwell.com/ionm Surgeries are inherently risky, especially those that involve the brain, spinal cord or nerves. Intraoperative neurophysiological
INVESTOR PRESENTATION
 INVESTOR PRESENTATION May 2018 2016 BioTelemetry, Inc. All rights reserved 1 FORWARD LOOKING STATEMENTS This presentation includes certain forward-looking statements within the meaning of the "Safe Harbor"
INVESTOR PRESENTATION May 2018 2016 BioTelemetry, Inc. All rights reserved 1 FORWARD LOOKING STATEMENTS This presentation includes certain forward-looking statements within the meaning of the "Safe Harbor"
(12) Patent Application Publication (10) Pub. No.: US 2014/ A1
 (19) United States US 20140O84064A1 (12) Patent Application Publication (10) Pub. No.: US 2014/0084.064 A1 MAN (43) Pub. Date: Mar. 27, 2014 (54) BARCODE AND RFID READINGAPPARATUS (52) U.S. Cl. CPC...
(19) United States US 20140O84064A1 (12) Patent Application Publication (10) Pub. No.: US 2014/0084.064 A1 MAN (43) Pub. Date: Mar. 27, 2014 (54) BARCODE AND RFID READINGAPPARATUS (52) U.S. Cl. CPC...
BIOMEDICAL ENGINEERING
 index INTRODUCTION BIOMEDICAL INSTRUMENTATION SCOPE OF THE LABORATORY: OBJECTIVES PROFILE OF THE COURSE MANUALS AND SOFTWARE POWER SUPPLIES BASE FRAME WITH POWER SUPPLY, INTERFACE TO PC AND VIRTUAL INSTRUMENTATION
index INTRODUCTION BIOMEDICAL INSTRUMENTATION SCOPE OF THE LABORATORY: OBJECTIVES PROFILE OF THE COURSE MANUALS AND SOFTWARE POWER SUPPLIES BASE FRAME WITH POWER SUPPLY, INTERFACE TO PC AND VIRTUAL INSTRUMENTATION
GE Healthcare. EchoPAC Dimension 06. Clinical workstation
 GE Healthcare EchoPAC Dimension 06 Clinical workstation Workflow that works for you. The freedom of choice extends to the echo lab with an information management solution that gives clinicians the ability
GE Healthcare EchoPAC Dimension 06 Clinical workstation Workflow that works for you. The freedom of choice extends to the echo lab with an information management solution that gives clinicians the ability
Sabrina Jedlicka 9/24/2012. NEUROENGINEERING: Interactions of Neurons and Materials
 Sabrina Jedlicka 9/24/2012 NEUROENGINEERING: Interactions of Neurons and Materials What is neuroengineering? Neuroengineering combines engineering and computational approaches to problems in basic and
Sabrina Jedlicka 9/24/2012 NEUROENGINEERING: Interactions of Neurons and Materials What is neuroengineering? Neuroengineering combines engineering and computational approaches to problems in basic and
PRODUCT BROCHURE GLOBAL COORDINATE MEASURING MACHINES
 PRODUCT BROCHURE GLOBAL COORDINATE MEASURING MACHINES 2 GLOBAL Coordinate Measuring Machines TAILORED FOR MAXIMUM PRODUCTIVITY Scalability and scope are the hallmarks of the GLOBAL coordinate measuring
PRODUCT BROCHURE GLOBAL COORDINATE MEASURING MACHINES 2 GLOBAL Coordinate Measuring Machines TAILORED FOR MAXIMUM PRODUCTIVITY Scalability and scope are the hallmarks of the GLOBAL coordinate measuring
Out Reach Report BME 402 Alan Meyer, John McGuire, Wan-Ting Kou, Albert Wang
 Out Reach Report BME 402 Alan Meyer, John McGuire, Wan-Ting Kou, Albert Wang 1.0 Out Reach Event Summary As engineers, we are driven to gain and implement knowledge. However it is not simply enough for
Out Reach Report BME 402 Alan Meyer, John McGuire, Wan-Ting Kou, Albert Wang 1.0 Out Reach Event Summary As engineers, we are driven to gain and implement knowledge. However it is not simply enough for
Will Stem Cells Finally Deliver Without Controversy?
 Will Stem Cells Finally Deliver Without Controversy? Keith Gary, Ph.D. Director of Program Development Kansas City Area Life Sciences Institute Olathe North Life Sciences 1 February 2012 What s the Buzz?
Will Stem Cells Finally Deliver Without Controversy? Keith Gary, Ph.D. Director of Program Development Kansas City Area Life Sciences Institute Olathe North Life Sciences 1 February 2012 What s the Buzz?
Classification of MDs under the European MDR. Joachim Makowski. Joachim Makowski, MED-RAS GmbH, a partner to confinis ag
 Classification of MDs under the European MDR Joachim Makowski 1 Table of content MDR references Context of classification Key changes Actions required 2 3 Context of classification Context of classification
Classification of MDs under the European MDR Joachim Makowski 1 Table of content MDR references Context of classification Key changes Actions required 2 3 Context of classification Context of classification
CE 561 Lecture Notes. Reference, AASHTO, Guide on Evaluation and Abatement of Traffic Noise, Set 9. General Characteristics
 CE 561 Lecture Notes Set 9 Reference, AASHTO, Guide on Evaluation and Abatement of Traffic Noise, 1993 General Characteristics Noise an unwarranted or excessive sound a form of environmental degradation
CE 561 Lecture Notes Set 9 Reference, AASHTO, Guide on Evaluation and Abatement of Traffic Noise, 1993 General Characteristics Noise an unwarranted or excessive sound a form of environmental degradation
Oracle Talent Management Cloud. What s New in Release 9
 Oracle Talent Management Cloud What s New in Release 9 30 April 2015 TABLE OF CONTENTS REVISION HISTORY... 4 OVERVIEW... 5 Give Us Feedback... 5 RELEASE FEATURE SUMMARY... 6 HCM COMMON FEATURES... 8 HCM
Oracle Talent Management Cloud What s New in Release 9 30 April 2015 TABLE OF CONTENTS REVISION HISTORY... 4 OVERVIEW... 5 Give Us Feedback... 5 RELEASE FEATURE SUMMARY... 6 HCM COMMON FEATURES... 8 HCM
ADDIS ABABA UNIVERSITY CENTER OF BIOMEDICAL ENGINEERING
 ADDIS ABABA UNIVERSITY CENTER OF BIOMEDICAL ENGINEERING November 2013 History of Biomedical Engineering Definition of Biomedical Engineering Achievements of Biomedical Engineering Streams in Biomedical
ADDIS ABABA UNIVERSITY CENTER OF BIOMEDICAL ENGINEERING November 2013 History of Biomedical Engineering Definition of Biomedical Engineering Achievements of Biomedical Engineering Streams in Biomedical
MLS the 3 rd Millennium Laser Therapy
 Jste:im MLS the 3 rd Millennium Laser Therapy UNITED STATES PATENT No.: US 8,251,982 B2 The patent obtained in the USA officially recognizes the uniqueness and originality of MLS MLS Multiwave Locked System
Jste:im MLS the 3 rd Millennium Laser Therapy UNITED STATES PATENT No.: US 8,251,982 B2 The patent obtained in the USA officially recognizes the uniqueness and originality of MLS MLS Multiwave Locked System
Attorney Docket No Date: 6 November 2007
 DEPARTMENT OF THE NAVY NAVAL UNDERSEA WARFARE CENTER DIVISION NEWPORT OFFICE OF COUNSEL PHONE: (401) 832-3653 FAX: (401) 832-4432 NEWPORT DSN: 432-3653 Attorney Docket No. 97966 Date: 6 November 2007 The
DEPARTMENT OF THE NAVY NAVAL UNDERSEA WARFARE CENTER DIVISION NEWPORT OFFICE OF COUNSEL PHONE: (401) 832-3653 FAX: (401) 832-4432 NEWPORT DSN: 432-3653 Attorney Docket No. 97966 Date: 6 November 2007 The
Rhythm Management Cardiac Rhythm Management and Electrophysiology
 Rhythm Management Cardiac Rhythm Management and Electrophysiology Joe Fitzgerald - Executive Vice President & President, Rhythm & Neuro Ken Stein, M.D. - Senior Vice President and CMO, Rhythm Management
Rhythm Management Cardiac Rhythm Management and Electrophysiology Joe Fitzgerald - Executive Vice President & President, Rhythm & Neuro Ken Stein, M.D. - Senior Vice President and CMO, Rhythm Management
RESPIRATORY SENSING LEAD
 RESPIRATORY SENSING LEAD 4340 Technical Manual ONLY Explanation of symbols on product or package labeling Caution, consult accompanying documents eifu indicator Consult electronic instructions for use
RESPIRATORY SENSING LEAD 4340 Technical Manual ONLY Explanation of symbols on product or package labeling Caution, consult accompanying documents eifu indicator Consult electronic instructions for use
Report on the Results of the Medical Devices Metrology and Standards Needs Workshop
 Report on the Results of the Medical Devices Metrology and Standards Needs Workshop Dr. Nicholas G. Dagalakis nicholas.dagalakis@nist.gov Outline Workshop Sessions Computer Assisted Surgical Navigation
Report on the Results of the Medical Devices Metrology and Standards Needs Workshop Dr. Nicholas G. Dagalakis nicholas.dagalakis@nist.gov Outline Workshop Sessions Computer Assisted Surgical Navigation
Corrosion of Steel in Concrete
 Corrosion of Steel in The rebar corrosion in concrete structures and the NDT techniques for corrosion rate measurement were previously discussed in another post. In the current post, the details of the
Corrosion of Steel in The rebar corrosion in concrete structures and the NDT techniques for corrosion rate measurement were previously discussed in another post. In the current post, the details of the
NEURON-SPECTRUM.NETω
 NEURON-SPECTRUM.NETω Software for Neuron-Spectrum Systems 8-256 channel acquisition User-friendly interface compatible with touchscreen displays Flexible stimulator settings analysis during acquisition
NEURON-SPECTRUM.NETω Software for Neuron-Spectrum Systems 8-256 channel acquisition User-friendly interface compatible with touchscreen displays Flexible stimulator settings analysis during acquisition
Best Total Solutions for Breast Cancer Diagnosis & Treatment
 Best Total Solutions for Breast Cancer Diagnosis & Treatment 2015 Best Medical International, Inc. Best Medical International, Inc. 7643 Fullerton Road, Springfield, VA 22153 USA v24_08182015_web Best
Best Total Solutions for Breast Cancer Diagnosis & Treatment 2015 Best Medical International, Inc. Best Medical International, Inc. 7643 Fullerton Road, Springfield, VA 22153 USA v24_08182015_web Best
What's New - Task Planning
 What's New - Task Planning by Dale Howard and Gary Chefetz With this chapter, teach yourself how to use Microsoft Project 2010 s new manual scheduling feature. This self-paced study guide includes hands-on
What's New - Task Planning by Dale Howard and Gary Chefetz With this chapter, teach yourself how to use Microsoft Project 2010 s new manual scheduling feature. This self-paced study guide includes hands-on
External Defibrillator Improvement Initiative
 External Defibrillator Improvement Initiative November 2010 Center for Devices and Radiological Health U.S. Food and Drug Administration External Defibrillator Improvement Initiative Table of Contents
External Defibrillator Improvement Initiative November 2010 Center for Devices and Radiological Health U.S. Food and Drug Administration External Defibrillator Improvement Initiative Table of Contents
Last Name First Name Middle Initial Previous Last Name(s)
 2018 PCC MRI Technologist Training Program Application Please check here if you applied to PCC s MRI Technologist Training Program in 2017. (PCC keeps all application materials on file for one year. Re-applicants
2018 PCC MRI Technologist Training Program Application Please check here if you applied to PCC s MRI Technologist Training Program in 2017. (PCC keeps all application materials on file for one year. Re-applicants
Date: May 26, 2015 Page 1
 Part I. Answer these questions by marking the best answer among the choices given: [2 points each] 1. Ethics and morals differ in that a. Only one of them should be followed b. Ethics are for professionals
Part I. Answer these questions by marking the best answer among the choices given: [2 points each] 1. Ethics and morals differ in that a. Only one of them should be followed b. Ethics are for professionals
See What's Coming in Oracle Talent Management Cloud
 See What's Coming in Oracle Talent Management Cloud Release 9 Release Content Document 1 TABLE OF CONTENTS REVISION HISTORY... 3 HCM COMMON FEATURES... 4 HCM Extracts... 4 Deliver Extracts Using HCM Connect...
See What's Coming in Oracle Talent Management Cloud Release 9 Release Content Document 1 TABLE OF CONTENTS REVISION HISTORY... 3 HCM COMMON FEATURES... 4 HCM Extracts... 4 Deliver Extracts Using HCM Connect...
