A61N. Definition statement. Relationships with other classification places CPC - A61N
|
|
|
- Sheryl Hudson
- 5 years ago
- Views:
Transcription
1 A61N ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY (measurement of bioelectric currents A61B; surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body A61B 18/00; anaesthetic apparatus in general A61M; incandescent lamps H01K; infra-red radiators for heating H05B) Specially adapted apparatus, instruments, devices, or processes for the following types of therapy: electrotherapy, e.g. iontophoresis; magnetotherapy; radiation therapy; ultrasound therapy. Components having specialized structural features that limit their use to the apparatus, instruments, or devices for the types of therapy appropriate for this subclass. Relationships with other classification places Several subclasses provide for subject matter that is used for therapy. The relationship between these subclasses in regard to the type of therapy covered by each is as follows: Subclass A61N provides for medical treatment-type therapy by apparatus or methods utilizing forms of energy other than direct mechanical energy when they are not used for surgical purposes and they are intended to either destroy, control, or advance the recovery of sick or abnormal cells within body tissue while minimizing undesirable destruction of adjoining healthy cells, or treat other types of abnormal conditions of the body (e.g., disease, impaired organ, injured limb). In particular, subclass A61N covers implanted devices, when the implanted device is not a replacement or supplementation of an actual portion of the organ or part, utilizing forms of energy other than direct mechanical energy to stimulate organs or body parts in a manner that facilitates, regulates, or improves their normal functioning. Subclass A61H provides for massage and physical-type therapy apparatus or methods for the treatment of disease or disability (i.e., an abnormal condition of the body) by utilization of direct mechanical energy. The apparatus or methods appropriate for this subclass do not surgically alter any portion of the bodies during treatment. Usually, the massage and therapy apparatus and methods of this subclass are intended to facilitate the healing of diseases, reduce the impact of injuries, or beneficially influence the condition of disabled body parts by physically moving a part of a body (e.g., devices for exercising a passive body member) solely by direct physical contact with, or stimulation of, external surfaces of the body or naturally occurring cavities in the body. However, stimulation of internal organs or body parts, such as artificial respiration and stimulation of the heart, when it is done by direct mechanical energy is also considered proper for this subclass. Subclass A61K and Subclass A61P provide for drug-type therapy using chemical compounds or medicinal preparations specially adapted for use in healing, benefiting, or destroying abnormal conditions of the body (e.g., diseases, birth defects) and the specific therapeutic activities that the chemical compounds and medicinal preparations are used for. Subclass A61B provides for surgery and surgical-type therapy that alters or repairs organs and body parts. It also provides for any surgical or diagnostic apparatus or methods that would otherwise be proper for subclass A61H or A61N when the apparatus or methods are used for both purposes or combined together. In particular, subclass A61B provides for surgical instruments, devices, or 1
2 A61N (continued) CPC - A61N methods for transferring non-mechanical forms of energy to or from the body (e.g., electromagnetic radiation surgery) to alter or repair it. Limiting references This place does not cover: Apparatus for diagnosis or surgery combined with, or applicable also as, therapy apparatus A61B Apparatus for radiation diagnosis A61B 6/00 Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body (e.g., radiation surgery, electrosurgery) Instruments, implements, tools, or methods specially adapted so as to limit their use to only animals Apparatus or methods utilizing direct mechanical energy in therapy intended to treat disease or disability Percussion or vibration massage apparatus (e.g. using supersonic vibration) Apparatus or methods for stimulating specific reflex points by heating or cooling within the cell-life limits A61B 18/00 A61D A61H A61H 23/00 A61H 39/06 Devices for applying radioactive material to the body A61M 37/0069, A61N 5/10 Measuring bioelectrical signals of the body or body parts A61B 5/04 Surgical accessories A61B 90/00 Artificial substitutes or replacements for parts of the body A61F 2/00 Heating or cooling appliances for medical or therapeutic treatment A61F 7/00 Saunas A61H 33/06 Radiopharmaceuticals A61K 51/12 Anaesthetic apparatus in general Artificial hearts and circulatory assistance means intended for implantation A61M A61M 1/12 Pressure infusion pumps A61M 5/142 Devices or methods to cause a change in the state of consciousness by mechanical, optical or acoustical means, e.g. audiovisual stimuli Specific therapeutic activity of chemical compounds or medicinal preparations Measurement of nuclear X-radiation Techniques for handling of particles or electromagnetic radiation Incandescent lamps having general utility Infrared radiators for heating A61M 21/00 A61P G01T G21K H01K H05B 2
3 A61N 1/00 Electrotherapy; Circuits therefor (A61N 2/00 takes precedence; irradiation apparatus A61N 5/00) Details about electric fields which directly provide a therapeutic effect or which render the delivery of therapeutic substances possible; details about (components of) apparatuses which generate these electric fields. Limiting references This place does not cover: High frequency currents for surgery instead of therapy (i.e. higher temperatures so as to destroy tissue instead of healing tissue) A61B 18/12 Irradiation apparatus A61N 5/00 Measuring blood pressure / blood flow A61B 5/02 Measuring blood output from heart A61B 5/029 Detecting specific parameters of the ECG cycle A61B 5/0452 Detecting muscle signals A61B 5/0488 Detecting urogenital signals A61B 5/04882 Detecting gastrointestinal signals A61B 5/04884 Measuring electrical impedance of a portion of the human body A61B 5/053 Measuring movement of the entire body or parts thereof A61B 5/11 Diagnosis using ultrasound in general A61B 8/00 Measuring blood pressure by ultrasound A61B 8/04 Measuring blood flow by ultrasound A61B 8/06 Gastric anti-eating devices using electrical stimulation A61F 5/0026 Devices implantable in the eye e.g. ocular inserts A61F 9/0017 Devices for eye surgery A61F 9/007 Mechanical stimulation of ear nerves A61F 11/04 Heart stimulation by mechanical means A61H 31/004 Stimulating specific reflex points using electric currents A61H 39/002 Percussion or vibration massage with electrotherapy A61H 2201/10 Acoustically, mechanically or optically inducing sleep or relaxation A61M 21/02 Electrically conditioning the air e.g. by ionising B60H 3/0071 Applying static electricity for purifying the air F24F 3/166 Recognising signal patterns per se G06K 9/
4 A61N 1/00 (continued) CPC - A61N ICT specially adapted for therapies or health-improving plans (e.g. for handling prescriptions, for steering therapy or for monitoring patient compliance) relating to mechanical, radiation or invasive therapies (e.g. surgery, laser therapy, dialysis or acupuncture) G16H 20/40 Deaf aid sets H04R 25/00 Special rules of classification Additional information could be either specific details about components, signals or algorithms which are not related to the invention as such; or less preferred embodiments which are shortly mentioned (e.g. inventive specification of cochlear implants which can be used for retinal implants as well). Documents with such information should be given an Indexing Code. Synonyms and Keywords In patent documents, the following abbreviations are often used: AAI AED AEI AV AVD AVI BPM CHD CHF CO CRT DBS DDD DDDR DDI ECG EKG HR ICD IMD IPG NS PAVB PESP PMT single chamber atrial pacemaker with inhibited mode automatic external defibrillator atrial escape interval atrioventricular atrioventricular delay atrioventricular interval beats per minute coronary heart disease chronic heart failure cardiac output cardiac resynchronisation therapy deep brain stimulation biventricular pacemaker e stimulation rate with dual mode DDD pacemaker with pacing rate dependent on sensor output biventricular pacemaker with inhibited mode electrocardiogram electrocardiogram heart rate implantable cardioverter defibrillator implantable medical device implantable pulse generator neural stimulation post-atrial ventricular blanking postextrasystolic potentiation pacemaker mediated tachycardia 4
5 A61N 1/00 (continued) CPC - A61N SV SVT VDD VF VT VVI stroke volume supraventricular tachycardia pacemaker that senses the atrium and ventricle and paces the ventricle with dual mode ventricular fibrillation ventricular tachycardia single chamber ventricular pacemaker with inhibited mode A61N 1/04 Electrodes {(electrosurgical electrodes A61B 18/14)} Details about the electrodes and their associated leads specially adapted for delivering electrical therapeutic stimulation. Patient's garment with incorporated electrodes for medical monitoring A41D 13/1281 Electrodes specially adapted for ECG A61B 5/0408 Electrodes specially adapted for EEG A61B 5/0478 Electrodes specially adapted for EMG A61B 5/0492 Electrodes for electrosurgery A61B 18/14 Stents and instruments for their placement A61F 2/82 Stimulating specific reflex points using electric currents A61H 39/002 Anchoring by balloon expansion A61M 25/10 Form details about flexible conductors per se H01B 7/04 Form details about conductors for implantation in a human body H01B 7/048 Special rules of classification Details about the stimulating device as such, about the therapeutic signal and/or about the stimulating algorithm should be classified in A61N 1/36-A61N 1/3712. A61N 1/08 Arrangements or circuits for monitoring, protecting, controlling or indicating {(for external stimulators A61N 1/3603; for implantable neurostimulators A61N 1/36128; for heart stimulators A61N 1/37; for defibrillators A61N 1/3925)} Protecting the functioning of electrical therapeutic stimulation devices, especially against MRI. 5
6 A61N 1/08 (continued) CPC - A61N This group only relates to protection (especially against MRI) for electrical therapeutic stimulation devices and not for other types of medical devices such as drug pumps. The purpose of this protection is to protect the functioning of the medical device. Limiting references This place does not cover: For external stimulators A61N 1/3603 For implantable neurostimulators A61N 1/36128 Monitoring or protecting for heart stimulators A61N 1/37 Monitoring or protecting for defibrillators A61N 1/3925 Neutralising atmospheric radiation A61N 1/16 Mattresses for protecting against e.g. static electricity or radiation A47C 31/004 Dynamisation and other esoteric therapies using medicinal preparations obtained by wave energy A61K 41/0004 NMR gyrometers G01C 19/60 Measuring electric variables Instruments for measuring magnetic variables involving magnetic resonance G01R G01R 33/20 Provisions within MR facilities for enhancing safety during MR G01R 33/288 Control of generator output per se H02P, H03L Screening of apparatus or components against electric or magnetic fields H05K 9/00 Special rules of classification Protecting the functioning of the medical device constitutes the difference with A61N 1/16, which relates to protecting the human body. E.g. pacemaker circuitry protecting the pacemaker from the field of a TV antenna should go to A61N 1/08 whereas as special building arrangement for protecting a person from the same field to A61N 1/16. Synonyms and Keywords In patent documents, the following abbreviations are often used: MR MRI NMR magnetic resonance magnetic resonance imaging nuclear magnetic resonance 6
7 A61N 1/086 {Magnetic resonance imaging [MRI] compatible leads} Leads (both implantable and external) having features that mitigate the effects of unwanted electric currents induced by a Magnetic Resonance Imaging [MRI] device or field. A61N 1/16 Screening or neutralising undesirable influences from {or using,} atmospheric or terrestrial radiation or fields {(using atmospheric electricity or earth currents H05F 3/00)} Screening or neutralising atmospheric or terrestrial radiation or fields with the purpose of protecting the human body. The purpose of this protection is to protect the human body. Limiting references This place does not cover: Electrostimulators protected for electromagnetic interference A61N 1/08 Measuring magnetic field characteristics of the earth G01V 3/00 Carrying-off electrical charges using atmospheric electricity or earth currents H05F 3/00 Special rules of classification Protecting the human body constitutes the difference with A61N 1/08, which relates to protecting the functioning of the medical device. E.g. pacemaker circuitry protecting the pacemaker from the field of a TV antenna should go to A61N 1/08 whereas as special building arrangement for protecting a person from the same field to A61N 1/16. A61N 1/08 takes precedence over A61N 1/16. A61N 1/36 for stimulation Details about the therapeutic signal and/or about the stimulation algorithm, constructional and/or circuitry details, telemetry details. 7
8 A61N 1/36 (continued) CPC - A61N Details about the electrodes or the leads A61N 1/04, A61N 1/06 Applying constant continuous current A61N 1/20 - A61N 1/306 Electromedical belts A61N 1/321 Electromedical brushes, combs, massage devices A61N 1/322 Inteference currents A61N 1/323 Iontophoresis A61N 1/325 Promoting growth of cells A61N 1/326 Electroporation A61N 1/327 Improving skin appearance A61N 1/328 A61N 1/36002 {Cancer treatment, e.g. tumour} Covers devices (both implanted and external to the body) that treat cancer by electrical stimulation. A61N 1/3603 {Control systems} Covers features of control systems used by electrical stimulation devices that are external to the body (e.g. switches/buttons used for controlling TENS devices, control displays for pain relief devices, controlling modes of operation for muscle stimulators, algorithms/flow charts for controlling external stimulation devices, etc.). A61N 1/36031 {using physiological parameters for adjustment} Device control systems under A61N 1/3603, which adjust therapeutic electrical stimulation based upon at least one sensed physiological parameter. 8
9 A61N 1/36034 {specified by the stimulation parameters} Device control systems under A61N 1/3603, which are characterised by their stimulation parameters (e.g. frequency, amplitude, pulse width, multiphasic, combination of waveforms, etc.). A61N 1/36036 {of the outer, middle or inner ear} Covers therapeutic devices that electrically stimulate portions of a patient's outer, middle, or inner ear. A61N 1/36038 {Cochlear stimulation} Devices under A61N 1/36036 that electrically stimulate a patient's cochlea. A61N 1/36039 {fitting procedures} Devices and methods used for fitting a patient with a cochlear stimulation device or setting a cochlea stimulation parameter (as described in A61N 1/36038) (This subgroup is intended for devices having more than a general mention of fitting or stimulation parameters). A61N 1/3604 {for correcting spinal deformities, e.g. scoliosis} Electrical stimulation devices used for correcting spinal deformities. A61N 1/36062 {Spinal stimulation} Includes implantable neurostimulation devices and associated methods used to perform spinal stimulation (as described in A61N 1/3605). 9
10 A61N 1/3614 {based on impedance measurement} Control systems for implantable neural stimulation devices (under A61N 1/3605) which measure impedance (This subgroup is intended for devices having more than a general mention of impedance). A61N 1/3629 {in combination with non-electric therapy} Heart stimulation devices (e.g. pacemakers) under (A61N 1/362) which also include an additional nonelectric therapy to the heart or other body area (e.g. drug therapy, nanotherapy, gene therapy, etc.). A61N 1/3684 {for stimulating the heart at multiple sites of the ventricle or the atrium} Heart stimulation devices under A61N 1/368, which include dual chamber pacemakers (e.g. pacers using DDD modes of operation) and pacing devices that electrically stimulate multiple sites within the 4 chambers of the heart. A61N 1/36842 {Multi-site stimulation in the same chamber} Covers heart stimulation devices under A61N 1/3684 having more than one stimulation site within the same heart chamber (e.g. two stimulation electrodes in the right ventricle that deliver separate and/or different stimulation to each electrode, etc.). A61N 1/36843 {Bi-ventricular stimulation} Covers heart stimulation devices which stimulate both the right ventricle [RV] and the left ventricle [LV]. 10
11 A61N 1/372 Arrangements in connection with the implantation of stimulators Specifications going beyond the implantable medical device in isolation, such as communicating with, delivering power to, or testing of the implantable medical device. Structural details of (parts of) the implantable medical device, e.g. casing or feedthrough. Details about leads and electrodes A61N 1/04 - A61N 1/06 Transmitting measured data to processing apparatus A61B 5/0002 Transferring electromagnetic energy to implanted prostheses A61F 2250/0001 Transferring data to implanted prostheses A61F 2250/0001 Materials for catheters A61L 29/00 Materials for coating of implanted articles A61L 31/08 Testing electrical condition of batteries per se G01R 31/36 Transmitting signals with a radio link per se G08C 17/02 ICT specially adapted for therapies or health-improving plans (e.g. for handling prescriptions, for steering therapy or for monitoring patient compliance) relating to mechanical, radiation or invasive therapies (e.g. surgery, laser therapy, dialysis or acupuncture) G16H 20/40 ICT specially adapted for the operation of medical equipment or devices G16H 40/60 Sealing of leads to lead-through insulators per se H01B 17/303 Feedthrough capacitors per se H01G 4/35 Batteries per se H01M Manufacture of small size batteries per se H01M 10/0436 Electrical connections per se H01R Electrical connections for medical use H01R 13/5224 Adapters for connecting two parts per se H01R 31/06 Electrical connectors for medicine and surgery H01R 2201/12 Charging batteries per se H02J 7/00 Charging batteries by inductive or capacitive coupling H02J 7/025 Near-field (inductive) transmission systems per se H04B 5/00 Radio transmission systems per se H04B 7/00 Transmission of digital information per se Transmitting circuits using pulse with modulation or pulse position modulation H04L H04L 25/
12 A61N 1/37254 {Pacemaker or defibrillator security, e.g. to prevent or inhibit programming alterations by hackers or unauthorised individuals} Devices and methods used to provide security (both programming and physical) for electrical stimulation devices (in particular pacemakers and defibrillators) to combat hackers and other unauthorised persons from accessing/re-programming electrical stimulation devices. A61N 1/37512 {Pacemakers} Covers device features related to the construction of pacemakers (i.e. housings/can details, material, shape, etc.). A61N 1/37514 {Brain implants} Covers device features related to the construction of brain implants (i.e. housing or can details, material, shape, etc.). Burr Hole Covers A61B 90/00 A61N 1/37516 {Intravascular implants} Covers device features related to the construction of intravascular implants (i.e. housing details, material, shape, etc.). 12
13 A61N 1/37518 {Anchoring of the implants, e.g. fixation} Covers anchoring/fixation details of electrical stimulation devices. A61N 1/38 for producing shock effects Electrolytic capacitors per se H01G 9/10 For producing shock effects per se H05C 1/00 Synonyms and Keywords In patent documents, the following abbreviations are often used: AF VF atrial fibrillation ventricular fibrillation A61N 1/3904 {External heart defibrillators [EHD]} Covers external heart defibrillators (e.g. Automatic External Defibrillators [AEDs], semi-automatic external defibrillators, manual) and methods for constructing and using them. A61N 1/39044 {in combination with cardiopulmonary resuscitation [CPR] therapy} External defibrillators and methods for using them for performing or assisting in cardiopulmonary resuscitation [CPR] therapy. Cardiopulmonary Resuscitation [CPR] therapy A61H 31/00 13
14 A61N 1/39046 {User protection from shock} External defibrillators which include features that protect a user or patient from an accidental or unwanted shock. A61N 1/3962 {in combination with another heart therapy} Implantable defibrillator which in addition to electrical shock therapy, also provides another form of heart therapy (e.g. drug therapy, gene therapy, nanotherapy, etc.). A61N 1/39622 {Pacing therapy} Implantable defibrillators under A61N 1/3962 which apply pacing therapy in addition to defibrillation shocks. A61N 1/39624 {Pain reduction therapy} Implantable defibrillators under A61N 1/3962 which apply electrical stimulation therapy for pain reduction. A61N 1/44 Applying ionised fluids {(ion generators H01J 37/00)} Baths for special therapeutic purposes A61H 33/00 Baths for specific parts of the body A61H 35/00 Ionised inhalator A61M 15/02 Ion generators H01J 37/00 Generating ions into the atmosphere H01T 23/00 14
15 A61N 2/00 Magnetotherapy Use of static or dynamic magnetic fields for treating humans or animals, where the magnetic field itself provides the therapeutic effect. Limiting references This place does not cover: Using a magnetic field for diagnosis A61B 5/05, G01R 33/00 Medicinal preparations obtained with a magnetic field A61K 41/00 Releasing a medicament through the use of a magnetic field B03C 1/00 A61N 5/00 Radiation therapy (ultrasound therapy A61N 7/00; devices or apparatus applicable to both therapy and diagnosis A61B 6/00) Therapeutic treatment of the human or animal body with electromagnetic or particle radiation. Therapy implies the curing of a disease or malfunction of the body and includes prophylactic treatment like killing of germs, bacteria in or on the body by means of radiation as well as treatment of symptoms like pain (anaesthesia) by means of radiation. Limiting references This place does not cover: Magnetotherapy A61N 2/00 Ultrasound therapy A61N 7/00 Radiation imaging or diagnosis A61B 6/00 Surgery with radiation (cf. special rules below) A61B 18/18, A61F 9/008, A61C 1/0046 Treatment by heat, sauna A61F 7/00, A61H 33/00 Sterilization or disinfection of articles or products by radiation A61L 2/08 Lighting Irradiation devices for articles or products G21K 5/00 Lithography with a particle beam H01J 37/30 Ion implantation in semiconductors H01L 21/26 F21 15
16 A61N 5/00 (continued) CPC - A61N Application-oriented references Examples of places where the subject matter of this place is covered when specially adapted, used for a particular purpose, or incorporated in a larger system: Devices or apparatus applicable to both therapy and diagnosis A61B 6/00 Radiation measurement devices, dosimeters, beam monitors G01T, e.g. G01T 1/02, G01T 1/161, G01T 1/2914 Accelerator Technology H05H, e.g. H05H 13/00, H05H 7/10 X-ray tubes H01J 35/00, H01J 35/32 Beam transport, forming, focussing, filtering G21K, e.g. G21K 1/00, G21K 1/10, G21K 5/04, G21K 5/10 Radiation Shielding G21F 1/00, G21F 3/00; G21F 5/00 Protection of tissue around treatment site A61B 90/04 Site contact feedback, abutting means A61B 2090/065, A61B 90/03 Patient positioning A61B 6/04, A61B 90/10 Patient movement or breathing monitoring A61B 5/11, A61B 5/113 Markers A61B 90/39 Navigation, image assistance A61B 90/36, A61B 6/12, A61B 8/12 Sensing at the treatment site A61B 2017/00022, A61B 2018/00636, A61B 5/0048, A61B 5/053, A61B 5/0059 Photosensitizers, radiosensitisers A61K 41/00 Radionuclides, radiopharmaceuticals A61K 51/00 Radiation sources G21G 1/00, G21G 4/00 Vapour or discharge lamps, tubes H01J 61/00 Light guides G02B 6/00, A61B 18/22 Filters G02B 5/20 LEDs H01L 33/00 Lasers H01S 3/00, A61B 18/20, A61C 1/0046, A61F 9/008 Psychological treatments A61M 21/00, A61M 2021/0044, A61M 2021/0055 Acupuncture A61H 39/00 Teeth whitening by light A61C 19/066 16
17 A61N 5/00 (continued) CPC - A61N Curing resins within the teeth A61C 19/003 ICT specially adapted for therapies or health-improving plans (e.g. for handling prescriptions, for steering therapy or for monitoring patient compliance) relating to mechanical, radiation or invasive therapies (e.g. surgery, laser therapy, dialysis or acupuncture) G16H 20/40 Devices for implanting pellets or seeds A61M 25/10 (Balloon) catheters A61M 25/10 Balloons for catheter applicators A61B 2017/22051 Irradiation of blood outside the patient (ex-vivo) A61M 1/3681 Medical informatics, patient data management A61B 34/00 Special rules of classification The IPC group A61M36/00 is not used: these devices are instead classified in A61N 5/10, since their purpose is therapeutic. Surgery vs. Therapy In contrast to the therapeutic applications covered by this group, surgery is characterized by the immediate and direct destruction or transformation of the tissue (ablation, vaporization, coagulation...). For therapy, the effect in the tissue is induced with rather lower intensity and power levels and/or longer pulse durations (photothermal, photochemical reactions, biostimulation...). Thus, for instance, cell destruction by photoactivation of an agent is to be classified in A61N 5/062. Some devices may be used for both applications depending on the operation mode. Note that A61N 5/1084 covers focussed radiotherapy applications like "gamma knife" and stereotactic radiotherapy which are sometimes also called "radiosurgery", which is not to be confused with surgery in the sense of A61B 18/00. Light therapy (A61N 5/06) The scheme in A61N 5/06 contains several independent classification trees covering different aspects of a light therapy apparatus. The therapeutic application is meant to be classified in A61N 5/0613 and lower hierarchical groups thereof for external treatments or A61N 5/0601 and lower hierarchical groups thereof for internal treatments. Further aspects are then classified in codes according to the area of treatment (subgroups of A61N 2005/0635), the type of light source used (subgroups of A61N 2005/065), and the wavelength of therapeutic light (subgroups of A61N 2005/0658). Aspects like coherence of the therapeutic light (A61N 2005/067) and polarisation (A61N 2005/073) are also to be coded. For some aspects, no suitable code is available and in such cases, sometimes the scheme of a neighbouring field is used: subgroups of A61B 2018/00773 or A61B 2017/00022 (feedback at the treatment site), A61B 2018/2255 (features at the distal end of a light guide, e.g. diffusers). Note: devices of A61N 5/0614 (tanning) do not need to be classified in A61N 2005/0661 since the ultraviolet light is there by necessity. Particle and X-ray therapy (A61N 5/10) In the A61N 5/10 part, there is a similar aspect coded in A61N 2005/1085 (type of particles, e.g. ions, electrons, neutrons, low energy photons etc.). This code is obligatory for the particles specified in the 17
18 A61N 5/00 (continued) CPC - A61N subgroups of A61N 2005/1085. Only normal energy (MeV) photons are not coded in this tree, since it concerns the default situation. The group A61N 5/01 should not be used for radiotherapy gantries, since a rotating gantry is common and not worth to classify. Synonyms and Keywords In patent documents, the following abbreviations are often used: IMRT IMAT IGRT MLC SAS (O)LED DRR SOBP DVH EPI OAR Intensity Modulated Radiation Therapy Intensity Modulated Arc Ttherapy Image Guided Radiation Therapy Multileaf Collimator Step And Shot (Organic) Light Emitting Diode Digitally Reconstructed Radiograph Spread-Out Bragg Peak Dose Volume Histogram Electronic Portal Imaging Organ At Risk A61N 5/025 {Warming the body, e.g. hyperthermia treatment} Heating by RF A61N 1/403 Heating by infrared radiation A61N 5/0625 Heating by other appliances A61F 7/00 Hot air bath A61H 33/06 A61N 5/0601 {Apparatus for use inside the body} Illuminating body cavities A61B 1/06 By intraoral means A61B 6/145 By using a source unit in the interior of the body A61B 6/4057 Devices for heating or cooling body cavities A61F 7/12 18
19 A61N 5/0601 (continued) CPC - A61N X-ray tubes having a small cross-section to facilitate introduction into small cavities H01J 35/32 A61N 5/0618 {Psychological treatment} Other devices or methods to cause a change in the state of consciousness; Devices for producing or ending sleep by mechanical, optical, or acoustical means, e.g. for hypnosis A61M 21/00 A61N 5/0619 {Acupuncture} Acupuncture in general A61H 39/00 A61N 5/0624 {for eliminating microbes, germs, bacteria on or in the body} Sterilization of articles or products by radiation A61L 2/08 A61N 5/0625 {Warming the body, e.g. hyperthermia treatment} Heating by RF A61N 1/403 Heating using microwaves A61N 5/025 Heating by other appliances A61F 7/00 Hot air bath A61H 33/06 19
20 A61N 5/10 X-ray therapy; Gamma-ray therapy; Particle-irradiation therapy (A61N 5/01 takes precedence) Limiting references This place does not cover: Devices for producing movement of radiation source during therapy A61N 5/01 Radiation diagnosis, e.g. combined with radiation therapy A61B 6/00 Irradiation devices in general G21K 1/00 X-ray tubes, Lenard tubes H01J 35/00 X-ray techniques, in particular circuits for feeding or controlling X-ray tubes H05G A61N 5/1002 {Intraluminal radiation therapy} Intraluminal catheters in general A61M 25/00 A61N 5/1007 {Arrangements or means for the introduction of sources into the body} Needle guides in general A61B 17/3403 Apparatus for implanting surgical devices A61B 17/3468 Devices for implanting seeds or pellets in general A61M 37/
21 A61N 5/1029 {Radioactive dressings} FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, E.G. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS A61F Dressings in general A61L 15/00 A61N 7/00 Ultrasound therapy (lithotripsy A61B 17/22, A61B 17/225; massage using supersonic vibration A61H 23/00 {; using ultrasound for introducing media into the body A61M 37/0092}) Ultrasound therapy of the human or animal body. Also included is the thermal destruction of tissue in the focal point of a high intensity ultrasound beam. Limiting references This place does not cover: Removal of calculi, thromboses etc. by ultrasound A61B 17/22004 Extracorporeal shock waves (with mainly mechanical effect, e.g. lithotripters) A61B 17/225 Cutting using ultrasonic vibrations, e.g. with vibrating knife A61B 17/ Devices creating a mechanical shock wave by laser light A61B 18/26 Apparatus for introducing media into the body using ultrasonic waves, phonophoresis A61M 37/0092 Ultrasound diagnosis A61B 8/00 Accessories for surgery or diagnosis A61B 90/00 Ultrasound imaging during surgical procedures A61B 2090/378 Percussion or vibration massage A61H 23/00 Generating mechanical vibrations B06B 1/00 Transmitting, conducting and directing sound in general G10K 11/00 21
22 A61N 7/00 (continued) CPC - A61N Special rules of classification A61N 7/00 Ultrasound therapy with negligible thermal effect. Treatment area not reduced to a focal point. E.g. devices for bone fracture healing. A61N 2007/0004 The subgroups define various application areas on the body to be treated or therapeutic applications. Headgroup A61N 2007/0004 not to be used for classification. A61N 7/02 Localized ultrasound hyperthermia, high intensity focused ultrasound (HIFU). The effect is primary a thermal one of substantially heating tissue above the normal temperature or even above the point of tissue destruction. Mechanical percussion to destroy calculi is to be classified in A61B 17/22004 or A61B 17/225 in case of external shockwaves. A61N 7/022 Intracavitary localized hyperthermia. The probe or transducer is located within a body cavity. Glossary of terms In this place, the following terms or expressions are used with the meaning indicated: HIFU Phased array transducer high intensity focused ultrasound transducer consisting of an array of small transducers individually adressable with a time delay to steer the focal point of the beam 22
A61N. Definition statement. Relationships with other classification places CPC - A61N
 A61N ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY (measurement of bioelectric currents A61B; surgical instruments, devices or methods for transferring non-mechanical forms of energy
A61N ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY (measurement of bioelectric currents A61B; surgical instruments, devices or methods for transferring non-mechanical forms of energy
EUROPEAN PATENT OFFICE U.S. PATENT AND TRADEMARK OFFICE CPC NOTICE OF CHANGES 397 DATE: 1 AUGUST 2017 PROJECT RP0002
 EUROPEAN PATENT OFFICE U.S. PATENT AND TRADEMARK OFFICE CPC NOTICE OF CHANGES 397 The following classification changes will be effected by this Notice of Changes: Action Subclass Group(s) Symbols deleted:
EUROPEAN PATENT OFFICE U.S. PATENT AND TRADEMARK OFFICE CPC NOTICE OF CHANGES 397 The following classification changes will be effected by this Notice of Changes: Action Subclass Group(s) Symbols deleted:
Medical Electronic Devices
 Medical Electronic Devices Basic Electronics (Electronic devices, Amplifiers, A/D, D/A) Electrodes EEG Deep brain stimulator ECG Cardiac Pacemakers External Defibrillators EMG Neuromuscular Electrical
Medical Electronic Devices Basic Electronics (Electronic devices, Amplifiers, A/D, D/A) Electrodes EEG Deep brain stimulator ECG Cardiac Pacemakers External Defibrillators EMG Neuromuscular Electrical
Revised Examination Guidelines for Industrially Applicable Inventions (Provisional Translation)
 Revised Examination Guidelines for Industrially Applicable Inventions (Provisional Translation) Examination Guidelines for Patent and Utility Model Part II: REQUIREMENTS FOR PATENTABILITY Chapter 1 Industrially
Revised Examination Guidelines for Industrially Applicable Inventions (Provisional Translation) Examination Guidelines for Patent and Utility Model Part II: REQUIREMENTS FOR PATENTABILITY Chapter 1 Industrially
Medical instrumentationi 11/19/2010
 Medical instrumentationi BIOEN 302 11/19/2010 Medical instrumentation Definition: instrument for sensing, diagnostics, therapeutics or surgery of human being. 2 Medical instrumentation Definition: instrument
Medical instrumentationi BIOEN 302 11/19/2010 Medical instrumentation Definition: instrument for sensing, diagnostics, therapeutics or surgery of human being. 2 Medical instrumentation Definition: instrument
ISO INTERNATIONAL STANDARD. Implants for surgery Active implantable medical devices Part 2: Cardiac pacemakers
 INTERNATIONAL STANDARD Provläsningsexemplar / Preview ISO 14708-2 First edition 2005-10-01 Implants for surgery Active implantable medical devices Part 2: Cardiac pacemakers Implants chirurgicaux Dispositifs
INTERNATIONAL STANDARD Provläsningsexemplar / Preview ISO 14708-2 First edition 2005-10-01 Implants for surgery Active implantable medical devices Part 2: Cardiac pacemakers Implants chirurgicaux Dispositifs
Appendix 1 Canadian Standards Association Standards for Equipment
 Can J Anesth/J Can Anesth (2018) Appendix 1 Canadian Standards Association Standards for Equipment Legend Example: C22.2 NO. 0.1-FM85 (C1999) F Indicates the standard is written in French. M Indicates
Can J Anesth/J Can Anesth (2018) Appendix 1 Canadian Standards Association Standards for Equipment Legend Example: C22.2 NO. 0.1-FM85 (C1999) F Indicates the standard is written in French. M Indicates
ADDIS ABABA UNIVERSITY CENTER OF BIOMEDICAL ENGINEERING
 ADDIS ABABA UNIVERSITY CENTER OF BIOMEDICAL ENGINEERING November 2013 History of Biomedical Engineering Definition of Biomedical Engineering Achievements of Biomedical Engineering Streams in Biomedical
ADDIS ABABA UNIVERSITY CENTER OF BIOMEDICAL ENGINEERING November 2013 History of Biomedical Engineering Definition of Biomedical Engineering Achievements of Biomedical Engineering Streams in Biomedical
Classification of MDs under the European MDR. Joachim Makowski. Joachim Makowski, MED-RAS GmbH, a partner to confinis ag
 Classification of MDs under the European MDR Joachim Makowski 1 Table of content MDR references Context of classification Key changes Actions required 2 3 Context of classification Context of classification
Classification of MDs under the European MDR Joachim Makowski 1 Table of content MDR references Context of classification Key changes Actions required 2 3 Context of classification Context of classification
MDR ID: Definition: Applicable:
 MDR Classification: (Reference Medical Device Regulation EU 2017/745, Annex VIII) Product: Product Name 1. DURATION OF USE MDR ID: Definition: Applicable: - Invasive Device: Continue Go to 2. Invasive
MDR Classification: (Reference Medical Device Regulation EU 2017/745, Annex VIII) Product: Product Name 1. DURATION OF USE MDR ID: Definition: Applicable: - Invasive Device: Continue Go to 2. Invasive
MEDICAL ELECTRONICS. Subject Title : Medical Electronics Subject Code : EC Hours Per Week : 04 Hours Per Semester : 64 TOPIC ANALYSIS
 MEDICAL ELECTRONICS Subject Title : Medical Electronics Subject Code : EC Hours Per Week : 04 Hours Per Semester : 64 SL. No TOPIC ANALYSIS Major Topics Hours Allotted UNIT I 1 Bio medical system and bio
MEDICAL ELECTRONICS Subject Title : Medical Electronics Subject Code : EC Hours Per Week : 04 Hours Per Semester : 64 SL. No TOPIC ANALYSIS Major Topics Hours Allotted UNIT I 1 Bio medical system and bio
Implants for surgery Active implantable medical devices. Part 3: Implantable neurostimulators
 Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 14708-3 Second edition 2017-04 Implants for surgery Active implantable medical devices Part 3: Implantable neurostimulators Implants chirurgicaux
Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 14708-3 Second edition 2017-04 Implants for surgery Active implantable medical devices Part 3: Implantable neurostimulators Implants chirurgicaux
1. RF ablation guarding circuits for EIT
 1. RF ablation guarding circuits for EIT Synopsis: Intracardiac and endovascular ablation therapies are widespread in their use in the treatment of a variety of cardiac arrhythmias as well as renal artery
1. RF ablation guarding circuits for EIT Synopsis: Intracardiac and endovascular ablation therapies are widespread in their use in the treatment of a variety of cardiac arrhythmias as well as renal artery
Medical Device Regulatory Requirements for South Africa
 Medical Device Regulatory Requirements for South Africa Disclaimer: The information contained on this website is derived from public sources and is current to the best of our knowledge. For detailed and
Medical Device Regulatory Requirements for South Africa Disclaimer: The information contained on this website is derived from public sources and is current to the best of our knowledge. For detailed and
Attention is drawn to the following places, which may be of interest for search:
 CPC - B33Y - 2017.08 B33Y ADDITIVE MANUFACTURING, i.e. MANUFACTURING OF THREE-DIMENSIONAL [3-D] OBJECTS BY ADDITIVE DEPOSITION, ADDITIVE AGGLOMERATION OR ADDITIVE LAYERING, e.g. BY 3-D PRINTING, STEREOLITHOGRAPHY
CPC - B33Y - 2017.08 B33Y ADDITIVE MANUFACTURING, i.e. MANUFACTURING OF THREE-DIMENSIONAL [3-D] OBJECTS BY ADDITIVE DEPOSITION, ADDITIVE AGGLOMERATION OR ADDITIVE LAYERING, e.g. BY 3-D PRINTING, STEREOLITHOGRAPHY
Draft Guidance for Industry and Food and Drug Administration Staff Class II Special Controls Guidance Document: External Pacemaker Pulse Generator
 Reprinted from FDA s website by Draft Guidance for Industry and Food and Drug Administration Staff Class II Special Controls Guidance Document: External Pacemaker Pulse Generator DRAFT GUIDANCE This guidance
Reprinted from FDA s website by Draft Guidance for Industry and Food and Drug Administration Staff Class II Special Controls Guidance Document: External Pacemaker Pulse Generator DRAFT GUIDANCE This guidance
Glossary to The Practice Standards for Medical Imaging and Radiation Therapy
 Glossary to The Practice Standards for Medical Imaging and Radiation Therapy Accuracy Ability of the bone mineral densitometry system to measure the true value of an object. Act anything done, being done,
Glossary to The Practice Standards for Medical Imaging and Radiation Therapy Accuracy Ability of the bone mineral densitometry system to measure the true value of an object. Act anything done, being done,
EUROPEAN PATENT OFFICE U.S. PATENT AND TRADEMARK OFFICE CPC NOTICE OF CHANGES 473 DATE: JANUARY 1, 2018 PROJECT RP0486
 EUROPEA PATET OFFICE U.S. PATET AD TRADEMARK OFFICE CPC OTICE OF CHAGES 473 The following classification changes will be effected by this otice of Changes: Action Subclass Group(s) SCHEME: Symbols Deleted:
EUROPEA PATET OFFICE U.S. PATET AD TRADEMARK OFFICE CPC OTICE OF CHAGES 473 The following classification changes will be effected by this otice of Changes: Action Subclass Group(s) SCHEME: Symbols Deleted:
EUROPEAN PATENT OFFICE U.S. PATENT AND TRADEMARK OFFICE CPC NOTICE OF CHANGES 523 DATE: MAY 1, 2018 PROJECT MP0332. Action Subclass Group(s)
 EUROPEAN PATENT OFFICE U.S. PATENT AND TRADEMARK OFFICE CPC NOTICE OF CHANGES 523 The following classification changes will be effected by this Notice of Changes: Action Subclass Group(s) Modified Definitions
EUROPEAN PATENT OFFICE U.S. PATENT AND TRADEMARK OFFICE CPC NOTICE OF CHANGES 523 The following classification changes will be effected by this Notice of Changes: Action Subclass Group(s) Modified Definitions
Act anything done, being done, or to be done; the process of doing. Synonymous with procedure and clinical services.
 Act anything done, being done, or to be done; the process of doing. Synonymous with procedure and clinical services. Action plan A program or method that explains the actions or steps to be taken. Advanced-practice
Act anything done, being done, or to be done; the process of doing. Synonymous with procedure and clinical services. Action plan A program or method that explains the actions or steps to be taken. Advanced-practice
BME101 Introduction to Biomedical Engineering Medical Imaging Özlem BİRGÜL Ankara University Department of Biomedical Engineering
 BME101 Introduction to Biomedical Engineering Medical Imaging Özlem BİRGÜL Ankara University Department of Biomedical Engineering Outline What is Medical Imaging? History of Medical Imaging X-Ray Imaging
BME101 Introduction to Biomedical Engineering Medical Imaging Özlem BİRGÜL Ankara University Department of Biomedical Engineering Outline What is Medical Imaging? History of Medical Imaging X-Ray Imaging
EUROPEAN PATENT OFFICE U.S. PATENT AND TRADEMARK OFFICE CPC NOTICE OF CHANGES 521 DATE: MAY 1, 2018 PROJECT DP0192
 EUROPEAN PATENT OFFICE U.S. PATENT AND TRADEMARK OFFICE CPC NOTICE OF CHANGES 521 The following classification changes will be effected by this Notice of Changes: Action Subclass Group(s) DEFINITIONS:
EUROPEAN PATENT OFFICE U.S. PATENT AND TRADEMARK OFFICE CPC NOTICE OF CHANGES 521 The following classification changes will be effected by this Notice of Changes: Action Subclass Group(s) DEFINITIONS:
Freshman/Sophomore Junior Senior
 Freshman/Sophomore Junior Senior Course Recommendations Mathematics (6 courses) MA 1021 MA 1024 (Calculus I) (Calculus IV) MA 1022 MA 2051 (Calculus II) (Differential Equations) MA 1023 MA 2611 (Calculus
Freshman/Sophomore Junior Senior Course Recommendations Mathematics (6 courses) MA 1021 MA 1024 (Calculus I) (Calculus IV) MA 1022 MA 2051 (Calculus II) (Differential Equations) MA 1023 MA 2611 (Calculus
MEDICAL DEVICES : Guidance document
 EUROPEAN COMMISSION DG ENTERPRISE Directorate G Unit 4 - Pressure Equipment, Medical Devices, Metrology MEDICAL DEVICES : Guidance document MEDDEV 2. 1/2 rev 2 26 April 1994 GUIDELINES RELATING TO THE
EUROPEAN COMMISSION DG ENTERPRISE Directorate G Unit 4 - Pressure Equipment, Medical Devices, Metrology MEDICAL DEVICES : Guidance document MEDDEV 2. 1/2 rev 2 26 April 1994 GUIDELINES RELATING TO THE
Glossary to The Practice Standards for Medical Imaging and Radiation Therapy
 0 0 Glossary to The Practice Standards for Medical Imaging and Radiation Therapy Accuracy Ability of the bone mineral densitometry system to measure the true value of an object. Act anything done, being
0 0 Glossary to The Practice Standards for Medical Imaging and Radiation Therapy Accuracy Ability of the bone mineral densitometry system to measure the true value of an object. Act anything done, being
UNIT I PHYSIOLOGYAND TRANSDUCERS
 SRI VENKATESWARA COLLEGE OF ENGINEERING AND TECHNOLOGY TIRUPACHUR DEPARTMENT OF ELECTRICAL AND ELECTRONICS ENGINEERING 1. What is meant by cell? EI 2311 BIOMEDICAL INSTRUMENTATION 2 Mark Questions With
SRI VENKATESWARA COLLEGE OF ENGINEERING AND TECHNOLOGY TIRUPACHUR DEPARTMENT OF ELECTRICAL AND ELECTRONICS ENGINEERING 1. What is meant by cell? EI 2311 BIOMEDICAL INSTRUMENTATION 2 Mark Questions With
Urodynamic Measurement System
 ID name description 1 CO2 Monitor Bedside carbon dioxide respiratory monitors. 2 Breathing Monitor Bedside monitors for continuous measurement of carbon dioxide and/or oxygen. 3 Electrohydraulic Lithotripter
ID name description 1 CO2 Monitor Bedside carbon dioxide respiratory monitors. 2 Breathing Monitor Bedside monitors for continuous measurement of carbon dioxide and/or oxygen. 3 Electrohydraulic Lithotripter
Assembly Solutions. OmniCure. Ablation Catheters. Challenge. Solution. Benefit. Repeatable assembly of electrodes within ablation catheters.
 OmniCure Ablation Catheters Assembly Solutions Challenge Repeatable assembly of electrodes within ablation catheters. Solution The OmniCure S2000 UV Spot Curing System with Closed-Loop Feedback technology
OmniCure Ablation Catheters Assembly Solutions Challenge Repeatable assembly of electrodes within ablation catheters. Solution The OmniCure S2000 UV Spot Curing System with Closed-Loop Feedback technology
TechSearch International, Inc.
 Future Vision of Medical Electronics E. Jan Vardaman President TechSearch International, Inc. www.techsearchinc.com The Future: The Bionic Age Vision: So that the blind may see Hearing: So that the deaf
Future Vision of Medical Electronics E. Jan Vardaman President TechSearch International, Inc. www.techsearchinc.com The Future: The Bionic Age Vision: So that the blind may see Hearing: So that the deaf
ARTIFICAL VISION. Regina Leung, Michelle Ngai
 ARTIFICAL VISION http://www.sfn.org/skins/main/images/brainbriefings/big.nov.jpg http://www.ubergizmo.com/photos/2006/4/wireless-ocular-implant.jpg http://www.crystalinks.com/eyeblind.gif Regina Leung,
ARTIFICAL VISION http://www.sfn.org/skins/main/images/brainbriefings/big.nov.jpg http://www.ubergizmo.com/photos/2006/4/wireless-ocular-implant.jpg http://www.crystalinks.com/eyeblind.gif Regina Leung,
Attention is drawn to the following places, which may be of interest for search:
 CPC - F04D - 2017.05 F04D NON-POSITIVE DISPLACEMENT PUMPS Non positive displacement pumps for liquids, for elastic fluids, or for liquids and elastic fluids, whether rotary or not having pure rotation.
CPC - F04D - 2017.05 F04D NON-POSITIVE DISPLACEMENT PUMPS Non positive displacement pumps for liquids, for elastic fluids, or for liquids and elastic fluids, whether rotary or not having pure rotation.
MEMBERSHIP APPLICATION FORM
 PERSATUAN PERALATAN PERUBATAN MALAYSIA (MALAYSIA MEDICAL DEVICE ASSOCIATION) (0596-05-7) MMDA Secretariat Suite A-807, Level 8 Block A, Kelana Square No: 17, Jalan SS7/26, Kelana Jaya 47301 Petaling Jaya,
PERSATUAN PERALATAN PERUBATAN MALAYSIA (MALAYSIA MEDICAL DEVICE ASSOCIATION) (0596-05-7) MMDA Secretariat Suite A-807, Level 8 Block A, Kelana Square No: 17, Jalan SS7/26, Kelana Jaya 47301 Petaling Jaya,
NONTRADITIONAL MANUFACTURING PROCESSES
 NONTRADITIONAL MANUFACTURING PROCESSES Lasers & Laser Beam Machining Basic NTM Process Groups: * Thermal NTM Processes - Laser Beam Machining (LBM) - Electron Beam Machining (EBM) - Plasma Arc Machining
NONTRADITIONAL MANUFACTURING PROCESSES Lasers & Laser Beam Machining Basic NTM Process Groups: * Thermal NTM Processes - Laser Beam Machining (LBM) - Electron Beam Machining (EBM) - Plasma Arc Machining
Date: May 26, 2015 Page 1
 Part I. Answer these questions by marking the best answer among the choices given: [2 points each] 1. Ethics and morals differ in that a. Only one of them should be followed b. Ethics are for professionals
Part I. Answer these questions by marking the best answer among the choices given: [2 points each] 1. Ethics and morals differ in that a. Only one of them should be followed b. Ethics are for professionals
China-EU Competition Week: Abbott's Acquisition of St. Jude Medical
 China-EU Competition Week: Abbott's Acquisition of St. Jude Medical Anti-monopoly Bureau, MOFCOM Tang Yu March 2017 Part I: basic information of the case Part II: definition of relevant markets and competitive
China-EU Competition Week: Abbott's Acquisition of St. Jude Medical Anti-monopoly Bureau, MOFCOM Tang Yu March 2017 Part I: basic information of the case Part II: definition of relevant markets and competitive
6/6/2017 ELECTROSURGERY OBJECTIVES. Electro Surgery Unit Safety Copyright Chesapeake Bay Perioperative Consortium,
 Electro Surgery Unit Safety Copyright Chesapeake Bay Perioperative Consortium,. updated 2017 ELECTROSURGERY OBJECTIVES The learner will be able to identify and define general electrosurgical terms The
Electro Surgery Unit Safety Copyright Chesapeake Bay Perioperative Consortium,. updated 2017 ELECTROSURGERY OBJECTIVES The learner will be able to identify and define general electrosurgical terms The
Standard and Directives on Medical Devices
 Standard and Directives on Medical Devices Definition A Medical Device is identified by means of its INTENDED PURPOSE Intended to treat, prevent or control physiological characteristics of a living being
Standard and Directives on Medical Devices Definition A Medical Device is identified by means of its INTENDED PURPOSE Intended to treat, prevent or control physiological characteristics of a living being
Electrocautery 101 New York Society of Gastrointestinal Endoscopy 31 st Annual Summer Fellows Course
 Electrocautery 101 New York Society of Gastrointestinal Endoscopy 31 st Annual Summer Fellows Course Renee Williams, MD Assistant Professor of Medicine NYU School of Medicine Division of Gastroenterology
Electrocautery 101 New York Society of Gastrointestinal Endoscopy 31 st Annual Summer Fellows Course Renee Williams, MD Assistant Professor of Medicine NYU School of Medicine Division of Gastroenterology
Introduction to Biomedical Instruments Biology
 Introduction to Biomedical Instruments Biology It deals with wide spectrum of Life sciences i.e. plants, animals, Insects or in nutshell all living organisms. Study of only human being out of these is
Introduction to Biomedical Instruments Biology It deals with wide spectrum of Life sciences i.e. plants, animals, Insects or in nutshell all living organisms. Study of only human being out of these is
Today we are going to talk about principles of electrosurgery. Electrosurgery came in to wide use because of the need to control bleeding during
 Today we are going to talk about principles of electrosurgery. Electrosurgery came in to wide use because of the need to control bleeding during operative procedures. 1 Surgeons have used electrical energy
Today we are going to talk about principles of electrosurgery. Electrosurgery came in to wide use because of the need to control bleeding during operative procedures. 1 Surgeons have used electrical energy
Glossary of Terms Specific to the general field of Light Therapy
 Glossary of Terms Specific to the general field of Light Therapy Absorption When light quanta travel through tissue, the energy is eventually absorbed by some component within the tissue. When energy is
Glossary of Terms Specific to the general field of Light Therapy Absorption When light quanta travel through tissue, the energy is eventually absorbed by some component within the tissue. When energy is
Cardiac Rhythm Management // Patient Brochure // BioMonitor 2. BioMonitor 2. Patient Information
 Cardiac Rhythm Management // Patient Brochure // BioMonitor 2 BioMonitor 2 Patient Information BioMonitor 2 We Listen to Your Heart Dear patient, Your doctor has decided to implant a cardiac monitor BioMonitor
Cardiac Rhythm Management // Patient Brochure // BioMonitor 2 BioMonitor 2 Patient Information BioMonitor 2 We Listen to Your Heart Dear patient, Your doctor has decided to implant a cardiac monitor BioMonitor
Notification of a Body in the framework of a technical harmonization directive
 Notification of a Body in the framework of a technical harmonization directive From : Department of Health Medicines and Healthcare Products Regulatory Agency (MHRA) 151 Buckingham Palace Road, Victoria,
Notification of a Body in the framework of a technical harmonization directive From : Department of Health Medicines and Healthcare Products Regulatory Agency (MHRA) 151 Buckingham Palace Road, Victoria,
Senior Science. Total marks 100. Section I Pages marks This section has two parts, Part A and Part B
 2012 HIGHER SCHOOL CERTIFICATE EXAMINATION Senior Science Total marks 100 General Instructions Reading time 5 minutes Working time hours Write using black or blue pen Black pen is preferred Draw diagrams
2012 HIGHER SCHOOL CERTIFICATE EXAMINATION Senior Science Total marks 100 General Instructions Reading time 5 minutes Working time hours Write using black or blue pen Black pen is preferred Draw diagrams
Biomedical Engineering Seminar
 Biomedical M.A.Sc. Biomedical M.A.Sc. Biomedical with Specialization in Data Science M.A.Sc. Biomedical with Specialization in Bioinformatics M.Eng. Biomedical M.Eng. Biomedical with Concentration in Clinical
Biomedical M.A.Sc. Biomedical M.A.Sc. Biomedical with Specialization in Data Science M.A.Sc. Biomedical with Specialization in Bioinformatics M.Eng. Biomedical M.Eng. Biomedical with Concentration in Clinical
IMDRF Working Group Improving the Quality of International Standards for Regulatory Use
 IMDRF Working Group Improving the Quality of International Standards for Regulatory Use Progress Report Dr. Matthias Neumann, Lead Federal Ministry of Health, Germany IMDRF 11 14 March 2017 Vancouver,
IMDRF Working Group Improving the Quality of International Standards for Regulatory Use Progress Report Dr. Matthias Neumann, Lead Federal Ministry of Health, Germany IMDRF 11 14 March 2017 Vancouver,
BIOMEDICAL ENGINEERING
 index INTRODUCTION BIOMEDICAL INSTRUMENTATION SCOPE OF THE LABORATORY: OBJECTIVES PROFILE OF THE COURSE MANUALS AND SOFTWARE POWER SUPPLIES BASE FRAME WITH POWER SUPPLY, INTERFACE TO PC AND VIRTUAL INSTRUMENTATION
index INTRODUCTION BIOMEDICAL INSTRUMENTATION SCOPE OF THE LABORATORY: OBJECTIVES PROFILE OF THE COURSE MANUALS AND SOFTWARE POWER SUPPLIES BASE FRAME WITH POWER SUPPLY, INTERFACE TO PC AND VIRTUAL INSTRUMENTATION
Manufacture of composite layers, workpieces, or articles, comprising metallic powder, by sintering the powder, with or without compacting;
 CPC - B22F - 2018.05 B22F WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER (processes or devices for granulating materials in general B01J 2/00; making ceramics
CPC - B22F - 2018.05 B22F WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER (processes or devices for granulating materials in general B01J 2/00; making ceramics
Hearing aids; part_2: hearing aids with automatic gain control circuits; amendment_1 N
 Status: 2014-September Document Reference Publication IEC 60118-0 1983 N IEC 60118-0 AMD 1 1994-01 N IEC 60118-1 1995-04 N IEC 60118-1 AMD 1 1998-07 N IEC 60118-1 Edition 3.1 1999-01 N IEC 60118-12 1996-09
Status: 2014-September Document Reference Publication IEC 60118-0 1983 N IEC 60118-0 AMD 1 1994-01 N IEC 60118-1 1995-04 N IEC 60118-1 AMD 1 1998-07 N IEC 60118-1 Edition 3.1 1999-01 N IEC 60118-12 1996-09
Endovascular Device Testing A Survey of Requirements for Product Validation
 MET A4 1p Endovascular Device Testing Info Sheet-02.qxp_MET A4 1p Endovascular Device Testing Info Sheet.qxp 05/04/2016 09:58 Page 1 Endovascular Device Testing A Survey of Requirements for Product Validation
MET A4 1p Endovascular Device Testing Info Sheet-02.qxp_MET A4 1p Endovascular Device Testing Info Sheet.qxp 05/04/2016 09:58 Page 1 Endovascular Device Testing A Survey of Requirements for Product Validation
Iran's Capabilities in the field of Medical Equipment. Dr.Mahmoud Biglar. General Director Medical Devices Department Food and Drug Organization
 Iran's Capabilities in the field of Medical Equipment Dr.Mahmoud Biglar General Director Medical Devices Department Food and Drug Organization Overview: Medical Equipment Department, Aims & Mission Imports,
Iran's Capabilities in the field of Medical Equipment Dr.Mahmoud Biglar General Director Medical Devices Department Food and Drug Organization Overview: Medical Equipment Department, Aims & Mission Imports,
U.S. Census Bureau Electromedical Equipment and Analytical Instruments MA334A(10) Issued July 2011
 U.S. Census Bureau Electromedical Equipment and Analytical Instruments - 2010 MA334A(10) Issued July 2011 Address inquiries concerning these data to Investment Goods Industries Branch, U.S. Department
U.S. Census Bureau Electromedical Equipment and Analytical Instruments - 2010 MA334A(10) Issued July 2011 Address inquiries concerning these data to Investment Goods Industries Branch, U.S. Department
The Healthcare System
 Biomedical Engineering: The Healthcare System What is the healthcare system? The healthcare system is the organization of people, institutions, and resources that deliver healthcare services to meet society
Biomedical Engineering: The Healthcare System What is the healthcare system? The healthcare system is the organization of people, institutions, and resources that deliver healthcare services to meet society
MEGADYNE. MEGA 2000 Patient Return Electrode System. Frequently Asked Questions
 MEGADYNE MEGA 2000 Patient Return Electrode System Frequently Asked Questions Do any of MEGADYNE's products contain latex? Is the system FDA approved? How is the MEGA 2000 Patient Return Electrode system
MEGADYNE MEGA 2000 Patient Return Electrode System Frequently Asked Questions Do any of MEGADYNE's products contain latex? Is the system FDA approved? How is the MEGA 2000 Patient Return Electrode system
Version 1.0: abc. General Certificate of Education. Health and Social Care 8621/8623 HC14. Mark Scheme examination January series
 Version 1.0: 0108 abc General Certificate of Education Health and Social Care 8621/8623 HC14 Mark Scheme 2008 examination January series Mark schemes are prepared by the Principal Examiner and considered,
Version 1.0: 0108 abc General Certificate of Education Health and Social Care 8621/8623 HC14 Mark Scheme 2008 examination January series Mark schemes are prepared by the Principal Examiner and considered,
Electromagnetic Radiation Fundamentals
 IBE 204.1 Electromagnetic Radiation Fundamentals v1.0 Bringing together technology and design methods to provide the information needed to create healthy homes and workplaces. IBE 204.1 Electromagnetic
IBE 204.1 Electromagnetic Radiation Fundamentals v1.0 Bringing together technology and design methods to provide the information needed to create healthy homes and workplaces. IBE 204.1 Electromagnetic
MEDICAL EQUIPMENT (1) TOPIC 1: RECORDING AND PROCESSING OF BIOSIGNALS
 MEDICAL EQUIPMENT (1) TOPIC 1: RECORDING AND PROCESSING OF BIOSIGNALS Term 1 2013/14 Prof. Yasser Mostafa Kadah www.k-space.org Measurement Basics Measuring is the experimental determination of a measured
MEDICAL EQUIPMENT (1) TOPIC 1: RECORDING AND PROCESSING OF BIOSIGNALS Term 1 2013/14 Prof. Yasser Mostafa Kadah www.k-space.org Measurement Basics Measuring is the experimental determination of a measured
PERIYAR UNIVERSITY SALEM CERTIFICATE IN RADIO IMAGE TECHNOLOGY (1Years) SYLLABUS / REGULATIONS. Annexure - 14
 PERIYAR UNIVERSITY SALEM 636 011. Annexure - 14 PERIYAR INSTITUTE OF DISTANCE EDUCATION [PRIDE] CERTIFICATE IN RADIO IMAGE TECHNOLOGY (1Years) SYLLABUS / REGULATIONS [Candidates admitted from 2007-2008
PERIYAR UNIVERSITY SALEM 636 011. Annexure - 14 PERIYAR INSTITUTE OF DISTANCE EDUCATION [PRIDE] CERTIFICATE IN RADIO IMAGE TECHNOLOGY (1Years) SYLLABUS / REGULATIONS [Candidates admitted from 2007-2008
IBE ELECTROMAGNETICS
 International Institute for Bau- biologie & Ecology IBE 204.3 IBE 204.3 ELECTROMAGNETICS BRINGING TOGETHER TECHNOLOGY AND DESIGN METHODS TO PROVIDE THE INFORMATION NEEDED TO CREATE HEALTHY HOMES AND WORKPLACES
International Institute for Bau- biologie & Ecology IBE 204.3 IBE 204.3 ELECTROMAGNETICS BRINGING TOGETHER TECHNOLOGY AND DESIGN METHODS TO PROVIDE THE INFORMATION NEEDED TO CREATE HEALTHY HOMES AND WORKPLACES
Best Total Solutions for Breast Cancer Diagnosis & Treatment
 Best Total Solutions for Breast Cancer Diagnosis & Treatment 2015 Best Medical International, Inc. Best Medical International, Inc. 7643 Fullerton Road, Springfield, VA 22153 USA v24_08182015_web Best
Best Total Solutions for Breast Cancer Diagnosis & Treatment 2015 Best Medical International, Inc. Best Medical International, Inc. 7643 Fullerton Road, Springfield, VA 22153 USA v24_08182015_web Best
RADIATION ONCOLOGY RESIDENCY PROGRAM Competency Evaluation of Resident
 Resident s Name: RADIATION ONCOLOGY RESIDENCY PROGRAM Competency Evaluation of Resident Rotation: PHYS 705: Clinical Rotation 3 Inclusive dates of rotation: Aug. 25, 2015 Feb. 25, 2016 Director or Associate
Resident s Name: RADIATION ONCOLOGY RESIDENCY PROGRAM Competency Evaluation of Resident Rotation: PHYS 705: Clinical Rotation 3 Inclusive dates of rotation: Aug. 25, 2015 Feb. 25, 2016 Director or Associate
Blue Marble University. Fast Track Combination Bachelor of Science (B.S.) and Doctor of Science (D.Sc.) in Biomedical engineering
 Blue Marble University Fast Track Combination Bachelor of Science (B.S.) and Doctor of Science (D.Sc.) in Biomedical engineering 5 Year program which you can enter right after High School And Complete
Blue Marble University Fast Track Combination Bachelor of Science (B.S.) and Doctor of Science (D.Sc.) in Biomedical engineering 5 Year program which you can enter right after High School And Complete
B63J AUXILIARIES ON VESSELS. Definition statement. Relationships with other classification places. References. Limiting references
 CPC - B63J - 2018.05 B63J AUXILIARIES ON VESSELS Auxiliaries on vessels Arrangements of installations for producing fresh water Arrangements of installations for ventilating, heating, cooling, or air conditioning
CPC - B63J - 2018.05 B63J AUXILIARIES ON VESSELS Auxiliaries on vessels Arrangements of installations for producing fresh water Arrangements of installations for ventilating, heating, cooling, or air conditioning
Basics of Technology. Lecturer Mgr. Vladan Bernard, Ph.D. Tuition 2nd semester. Range of education. Type of education
 Subject Basics of Technology Lecturer Mgr. Vladan Bernard, Ph.D. Tuition 2nd semester Type of subject Compulsory Range of education 2 hours Self-study 28 hours Type of education Lecture Completion Oral
Subject Basics of Technology Lecturer Mgr. Vladan Bernard, Ph.D. Tuition 2nd semester Type of subject Compulsory Range of education 2 hours Self-study 28 hours Type of education Lecture Completion Oral
Simple, intuitive and accessible MRI solution for preclinical research. M-Series Compact MRI Systems
 Simple, intuitive and accessible MRI solution for preclinical research M-Series Compact MRI Systems Application Oriented Imaging Anatomy and Morphology In vivo soft tissue imaging for morphological characterization.
Simple, intuitive and accessible MRI solution for preclinical research M-Series Compact MRI Systems Application Oriented Imaging Anatomy and Morphology In vivo soft tissue imaging for morphological characterization.
Measuring Tissue Perfusion and PO2 in Conscious Animals to Investigate Organ Failure
 Webinar Q&A Report: Measuring Tissue Perfusion and PO2 in Conscious Animals to Investigate Organ Failure 1. In what animal models has this system been used? The OxyLite and OxyFlo systems have been used
Webinar Q&A Report: Measuring Tissue Perfusion and PO2 in Conscious Animals to Investigate Organ Failure 1. In what animal models has this system been used? The OxyLite and OxyFlo systems have been used
2 / 1. SUMMARY public, should be well documented, and should be reviewed on a regular basis to determine whether it continues to meet the operational
 1. Summary This Report is concerned with the protection of individuals who may be exposed to radiation emitted by x-ray equipment and both sealed and unsealed radioactive sources in the practice of veterinary
1. Summary This Report is concerned with the protection of individuals who may be exposed to radiation emitted by x-ray equipment and both sealed and unsealed radioactive sources in the practice of veterinary
ArcCHECK & 3DVH. The Ultimate 4D Patient QA Solution. Your Most Valuable QA and Dosimetry Tools
 ArcCHECK & 3DVH The Ultimate 4D Patient QA Solution Your Most Valuable QA and Dosimetry Tools THE 4D QA GOLD STANDARD The ArcCHECK is the world s most selected independent 4D measurement array. Simply
ArcCHECK & 3DVH The Ultimate 4D Patient QA Solution Your Most Valuable QA and Dosimetry Tools THE 4D QA GOLD STANDARD The ArcCHECK is the world s most selected independent 4D measurement array. Simply
MEDICAL PHYSICS (MED PHYS)
 Medical Physics (MED PHYS) 1 MEDICAL PHYSICS (MED PHYS) MED PHYS/PHYSICS 265 INTRODUCTION TO MEDICAL PHYSICS Primarily for premeds and other students in the medical and biological sciences. Applications
Medical Physics (MED PHYS) 1 MEDICAL PHYSICS (MED PHYS) MED PHYS/PHYSICS 265 INTRODUCTION TO MEDICAL PHYSICS Primarily for premeds and other students in the medical and biological sciences. Applications
Simple, intuitive and accessible MRI solution for preclinical research. M-Series Compact MRI Systems
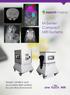 Simple, intuitive and accessible MRI solution for preclinical research M-Series Compact MRI Systems Application Oriented Imaging Molecular Imaging Using Contrast Agents Detection and quantification of
Simple, intuitive and accessible MRI solution for preclinical research M-Series Compact MRI Systems Application Oriented Imaging Molecular Imaging Using Contrast Agents Detection and quantification of
What is your patient wearing? By Jeff Gastorf, DO FACOFP
 What is your patient wearing? By Jeff Gastorf, DO FACOFP Disclosures None Importance of medical devices Patient Engagement Importance to society What is the importance of medical devices? Doctor Engagement
What is your patient wearing? By Jeff Gastorf, DO FACOFP Disclosures None Importance of medical devices Patient Engagement Importance to society What is the importance of medical devices? Doctor Engagement
BTEC Unit 1 Physics Revision. Learning Aim E: Energy Stores, Energy Transfers and Energy Transformations
 BTEC Unit 1 Physics Revision Learning Aim E: Energy Stores, Energy Transfers and Energy Transformations Forms of Energy and Their Uses Kinetic (Mechanical) Description and Use The energy in moving objects.
BTEC Unit 1 Physics Revision Learning Aim E: Energy Stores, Energy Transfers and Energy Transformations Forms of Energy and Their Uses Kinetic (Mechanical) Description and Use The energy in moving objects.
CLEANING OR DEGREASING OF METALLIC MATERIAL BY CHEMICAL METHODS OTHER THAN ELECTROLYSIS (polishing compositions C09G; detergents in general C11D)
 CPC - C23G - 2017.08 C23G CLEANING OR DEGREASING OF METALLIC MATERIAL BY CHEMICAL METHODS OTHER THAN ELECTROLYSIS (polishing compositions C09G; detergents in general C11D) Definition statement This place
CPC - C23G - 2017.08 C23G CLEANING OR DEGREASING OF METALLIC MATERIAL BY CHEMICAL METHODS OTHER THAN ELECTROLYSIS (polishing compositions C09G; detergents in general C11D) Definition statement This place
Wave Characteristics. National 4 Physics - Waves and Radiation Answer File. Longitudinal and transverse waves 1. A transverse, B longitudinal.
 Wave Characteristics Longitudinal and transverse waves 1. A transverse, B longitudinal. 2. (a) Stretch the spring and move sideways, back and fore at right angles to length of spring. (b) Stretch the spring
Wave Characteristics Longitudinal and transverse waves 1. A transverse, B longitudinal. 2. (a) Stretch the spring and move sideways, back and fore at right angles to length of spring. (b) Stretch the spring
Sectoral patterns of technical change: Towards a taxonomy and a theory
 Sectoral patterns of technical change: Towards a taxonomy and a theory 기술경영협동과정 박사 4학기 유광용/ 김동희 Contents Keith PAVITT, Research Policy 13 (1984) 343-373 Chapter 1 Summary Chapter 2 Critiques Chapter 3
Sectoral patterns of technical change: Towards a taxonomy and a theory 기술경영협동과정 박사 4학기 유광용/ 김동희 Contents Keith PAVITT, Research Policy 13 (1984) 343-373 Chapter 1 Summary Chapter 2 Critiques Chapter 3
Research Involving FDA Regulated Devices Policy 606 Version: 1.1 POLICY
 POLICY 1. Purpose Outline the responsibilities and regulatory requirements when conducting human subject research that involves the use of devices under the oversight of the Food and Drug Administration
POLICY 1. Purpose Outline the responsibilities and regulatory requirements when conducting human subject research that involves the use of devices under the oversight of the Food and Drug Administration
Urgent Field Safety Notice
 Urgent Field Safety Notice December 2017 Subject: Pacemakers Technical programming information on Minute Ventilation Sensor. Ref.: 92186345-FA Product name Models VALITUDE CRT-P U125, U128 ACCOLADE Pacemakers
Urgent Field Safety Notice December 2017 Subject: Pacemakers Technical programming information on Minute Ventilation Sensor. Ref.: 92186345-FA Product name Models VALITUDE CRT-P U125, U128 ACCOLADE Pacemakers
(12) Patent Application Publication (10) Pub. No.: US 2006/ A1
 (19) United States US 20060135953A1 (12) Patent Application Publication (10) Pub. No.: US 2006/0135953 A1 Kania et al. (43) Pub. Date: (54) TISSUE ABLATION SYSTEM INCLUDING GUIDEWIRE WITH SENSINGELEMENT
(19) United States US 20060135953A1 (12) Patent Application Publication (10) Pub. No.: US 2006/0135953 A1 Kania et al. (43) Pub. Date: (54) TISSUE ABLATION SYSTEM INCLUDING GUIDEWIRE WITH SENSINGELEMENT
OUR WISH LIST RESEARCH EQUIPMENT
 OUR WISH LIST RESEARCH EQUIPMENT YOU CAN MAKE A TANGIBLE DIFFERENCE! The South Australian Health and Medical Research Institute (SAHMRI) is one of the most exciting developments in the field of health
OUR WISH LIST RESEARCH EQUIPMENT YOU CAN MAKE A TANGIBLE DIFFERENCE! The South Australian Health and Medical Research Institute (SAHMRI) is one of the most exciting developments in the field of health
OUR WISH LIST RESEARCH EQUIPMENT
 OUR WISH LIST RESEARCH EQUIPMENT WITH YOUR PHILANTHROPIC SUPPORT, WE CAN WORK TOGETHER TO COMPLETE OUR FULLY-FUNCTIONAL FACILITY BY PURCHASING THE CUTTING-EDGE EQUIPMENT AND RESOURCES TO SUPPORT SAHMRI
OUR WISH LIST RESEARCH EQUIPMENT WITH YOUR PHILANTHROPIC SUPPORT, WE CAN WORK TOGETHER TO COMPLETE OUR FULLY-FUNCTIONAL FACILITY BY PURCHASING THE CUTTING-EDGE EQUIPMENT AND RESOURCES TO SUPPORT SAHMRI
Advanced solutions for the Healthcare sector
 TECHNICAL CERAMICS Morgan Advanced Materials For all inquiries, please contact our specialist sales and marketing offices: Morgan Advanced Materials is a global engineering company offering world-leading
TECHNICAL CERAMICS Morgan Advanced Materials For all inquiries, please contact our specialist sales and marketing offices: Morgan Advanced Materials is a global engineering company offering world-leading
Medicines (Database of Medical Devices) Regulations 2003
 Reprint as at Medicines (Database of Medical Devices) (SR 2003/325) Silvia Cartwright, Governor-General Order in Council At Wellington this 17th day of November 2003 Present: Her Excellency the Governor-General
Reprint as at Medicines (Database of Medical Devices) (SR 2003/325) Silvia Cartwright, Governor-General Order in Council At Wellington this 17th day of November 2003 Present: Her Excellency the Governor-General
The role of thermal spray in medical applications
 The role of thermal spray in medical applications Rajan Bamola Traditionally, thermal spray in the medical industry has been linked to the spraying of either titanium or hydroxyapatite on dental, spinal
The role of thermal spray in medical applications Rajan Bamola Traditionally, thermal spray in the medical industry has been linked to the spraying of either titanium or hydroxyapatite on dental, spinal
This place covers: all treatments of molten iron or steel, including the apparatuses specifically related thereto, e.g. converter, electric furnace.
 CPC - C21C - 2017.05 C21C PROCESSING OF PIG-IRON, e.g. REFINING, MANUFACTURE OF WROUGHT- IRON OR STEEL; TREATMENT IN MOLTEN STATE OF FERROUS ALLOYS (refining metals in general C22B 9/00) all treatments
CPC - C21C - 2017.05 C21C PROCESSING OF PIG-IRON, e.g. REFINING, MANUFACTURE OF WROUGHT- IRON OR STEEL; TREATMENT IN MOLTEN STATE OF FERROUS ALLOYS (refining metals in general C22B 9/00) all treatments
Measurement of ion channel functions under in vitro conditions. Dr. Norbert Nagy Research Associate Department of Pharmacology and Pharmacotherapy
 Measurement of ion channel functions under in vitro conditions Dr. Norbert Nagy Research Associate Department of Pharmacology and Pharmacotherapy Topics: -Electrophysiological techniques for basic research
Measurement of ion channel functions under in vitro conditions Dr. Norbert Nagy Research Associate Department of Pharmacology and Pharmacotherapy Topics: -Electrophysiological techniques for basic research
BIOMEDICAL SIGNAL AND IMAGE PROCESSING
 BIOMEDICAL SIGNAL AND IMAGE PROCESSING EE 5390-001 SYLLABUS Instructor: Wei Qian, Ph.D. Professor of Electrical and Computer Engineering Medical Signal and Image Computerized Processing Scheme for Medical
BIOMEDICAL SIGNAL AND IMAGE PROCESSING EE 5390-001 SYLLABUS Instructor: Wei Qian, Ph.D. Professor of Electrical and Computer Engineering Medical Signal and Image Computerized Processing Scheme for Medical
The Role of Tomosynthesis Imaging at Our Institute and X-ray Dose Optimization
 R/F The Role of Tomosynthesis Imaging at Our Institute and X-ray Dose Optimization Tetsuta Izumi R.T. Osaka International Cancer Institute, Diagnostic and Interventional Radiology Section 1 Osaka International
R/F The Role of Tomosynthesis Imaging at Our Institute and X-ray Dose Optimization Tetsuta Izumi R.T. Osaka International Cancer Institute, Diagnostic and Interventional Radiology Section 1 Osaka International
Focus on Cardiac Devices. with
 Focus on Cardiac Devices with We are focused FDA Class II and III therapeutic and diagnostic device product development and manufacturing A mature, well-developed ISO-13485 quality system Efficient and
Focus on Cardiac Devices with We are focused FDA Class II and III therapeutic and diagnostic device product development and manufacturing A mature, well-developed ISO-13485 quality system Efficient and
Types of product/intervention
 January 2017 This document contains information and guidance to assist users in selecting the appropriate type when reporting a medical product or intervention. This guidance is provided and governed by
January 2017 This document contains information and guidance to assist users in selecting the appropriate type when reporting a medical product or intervention. This guidance is provided and governed by
UCD Biomedical Engineering Dr. Simon Kelly Dr. Eoin O Cearbhaill
 UCD Biomedical Engineering Dr. Simon Kelly Dr. Eoin O Cearbhaill School of Electrical and Electronic Engineering School of Mechanical and Materials Engineering BE and ME Biomedical Engineering Overview
UCD Biomedical Engineering Dr. Simon Kelly Dr. Eoin O Cearbhaill School of Electrical and Electronic Engineering School of Mechanical and Materials Engineering BE and ME Biomedical Engineering Overview
HOW TO APPLY FOR MEDICAL DEVICE REGISTRATION UNDER MEDICAL DEVICE ACT 2012 (ACT 737)
 MDA/GL No 1: July 2013 Guidelines for implementation of medical device regulatory system HOW TO APPLY FOR MEDICAL DEVICE REGISTRATION UNDER MEDICAL DEVICE ACT 2012 (ACT 737) [Appendix 4 Schedule 3 Medical
MDA/GL No 1: July 2013 Guidelines for implementation of medical device regulatory system HOW TO APPLY FOR MEDICAL DEVICE REGISTRATION UNDER MEDICAL DEVICE ACT 2012 (ACT 737) [Appendix 4 Schedule 3 Medical
Name... Class... Date...
 Close up on cell membranes Specification references 4.1.1 Cell structure Aims The incredibly narrow cell membrane is all that separates the cell interior from its surroundings. You will see that the membrane
Close up on cell membranes Specification references 4.1.1 Cell structure Aims The incredibly narrow cell membrane is all that separates the cell interior from its surroundings. You will see that the membrane
Integrated CNS and CV monitoring via telemetry in behavior studies
 Measurement Behavior 2012 - Tutorial - Integrated CNS and CV monitoring via telemetry in behavior studies Thomas Penning European Manager at Data Sciences International The Gold Standard in Physiologic
Measurement Behavior 2012 - Tutorial - Integrated CNS and CV monitoring via telemetry in behavior studies Thomas Penning European Manager at Data Sciences International The Gold Standard in Physiologic
Lesson 7A Specialized Cells, Stem Cells & Cellular Differentiation
 Lesson 7A Specialized Cells, Stem Cells & Cellular Differentiation Learning Goals I can explain the concept of cell differentiation and cell specialization. I can explain how the cell structure relates
Lesson 7A Specialized Cells, Stem Cells & Cellular Differentiation Learning Goals I can explain the concept of cell differentiation and cell specialization. I can explain how the cell structure relates
1. Quantity & Quality of Infrared: Not all infrared is created equally
 BUYER S GUIDE: WHAT TO LOOK FOR IN INFRARED SAUNAS 4 Most Important Factors to Consider With so much information on the internet, researching infrared saunas can be confusing. There are hundreds of saunas
BUYER S GUIDE: WHAT TO LOOK FOR IN INFRARED SAUNAS 4 Most Important Factors to Consider With so much information on the internet, researching infrared saunas can be confusing. There are hundreds of saunas
Out Reach Report BME 402 Alan Meyer, John McGuire, Wan-Ting Kou, Albert Wang
 Out Reach Report BME 402 Alan Meyer, John McGuire, Wan-Ting Kou, Albert Wang 1.0 Out Reach Event Summary As engineers, we are driven to gain and implement knowledge. However it is not simply enough for
Out Reach Report BME 402 Alan Meyer, John McGuire, Wan-Ting Kou, Albert Wang 1.0 Out Reach Event Summary As engineers, we are driven to gain and implement knowledge. However it is not simply enough for
Experimental Medical Device Studies in Canada
 Experimental Medical Device Studies in Canada Stephen A Hoption Cann, PhD Clinical Professor Chair, UBC Clinical Research Ethics Board School of Population & Public Health University of British Columbia
Experimental Medical Device Studies in Canada Stephen A Hoption Cann, PhD Clinical Professor Chair, UBC Clinical Research Ethics Board School of Population & Public Health University of British Columbia
Systems & Biomedical Engineering Department. Systems and Biomedical Engineering Department Faculty of Engineering Cairo University
 Systems & Biomedical Engineering Department Agenda Students' Mindsets and Careers Biomedical Engineering Biomedical Engineering Careers in Egypt Non-Biomedical Careers in Egypt Courses Agenda Students'
Systems & Biomedical Engineering Department Agenda Students' Mindsets and Careers Biomedical Engineering Biomedical Engineering Careers in Egypt Non-Biomedical Careers in Egypt Courses Agenda Students'
