Clinical Pharmacology
|
|
|
- Caroline McBride
- 5 years ago
- Views:
Transcription
1 Clinical Pharmacology Gene Williams, Ph.D. Team Leader, Division of Clinical Pharmacology V Office of Clinical Pharmacology (OCP) Office of Translational Sciences (OTS) Center for Drug Evaluation and Research (CDER) 1
2 Goals Approval High quality instructions for use: individualization of dose Avoid post-marketing requirements / commitments 2
3 Developmental Milestones First-in-Human Trial Phase 2 Trial End-of-Phase 2 Meeting Phase 3 Trial Pre-NDA/BLA meeting 3
4 First-in-Human Trial, 1 of 2 Safety = hold issues, recommendation to allow trial to proceed, microdose can make non-relevant add sub-bullets Entry criteria e.g., renal impairment Monitoring of cardiac safety e.g., QT C Drug-drug interactions (DDI) drug as substrate/victim most often the concern Food effect (not relevant for IV) 4
5 First-in-Human Trial, 2 of 2 Efficacy = non-hold issues, recommendations for trial design Goal is near optimal dose regimen and imaging conditions (timing, machine) prior to confirmatory trials Process is completed in Phase 2, but begins in first trial Superior images to alternatives which have been studied Assured only when > 3 doses and > 3 imaging windows Accuracy and precision impacts Phase 2 design / success Sufficient PK sampling DDI / food 5
6 Pharmacokinetics Useful for drug development goals, not only package insert Phase 1/Phase 2: improve selection of imaging timing, timing of repeat dosing, and amount of repeat dose Eventual goal is to correlate concentrations with clinical outcomes: collect information in Phase 3 Bioanalytical Method Validation Topicals PK needed to correlate with safety outcomes Demonstration of non-absorption may include acquiring PK in Phase 3 6
7 Phase 2 Trial Safety = hold issues, recommendation to allow trial to proceed Same as Phase 1 trial, but informed by Phase 1 information including use of PK for dose adjustments Efficacy = non-hold issues, recommendations for trial design Completion of discovery of near optimal dose and imaging conditions for use in Phase 3 Sufficient PK sampling linearity: issue for later specific population studies 7
8 End-of-Phase 2 Meeting, 1 of 2 Near Optimal Dose: efficacy as well as safety Food Effect (not relevant for IV) Acquire Agency input on data to address Specific Populations in NDA/BLA Q.: What to measure in future studies? Info: Identity of major active (imaging, toxicity for non-microdose) metabolites Q. What data in subjects with organs impairment are needed? Info: Route of elimination and excretion of parent and major active metabolites 8
9 End-of-Phase 2 Meeting, 2 of 2 Acquire Agency input on data to address Specific Populations in NDA/BLA Q. What in vivo drug interaction studies with new drug as victim are needed? Info: parent and major metabolites as substrates (e.g., CYP enzymes and transporters) Q. What future in vivo drug interaction studies with new drug as perpetrator are needed? Info: Parent and major metabolites as inhibitors and inducers (CYP enzymes and transporters, not applicable to microdose) 9
10 Phase 3 Trial, 1 of 2 Safety = hold issues, recommendation to allow trial to proceed Same as Phase 2 trial, but informed by Phase 2 information including use of PK for dose adjustments 10
11 Phase 3 Trial, 2 of 2 Efficacy = non-hold issues, recommendations for trial design Evaluate if dose is near optimal (e.g., review EOP 2 meeting) Sufficient PK sampling to inform dose adjustment during or at end of trial; sampling all patients maximizes information (PK-imaging and PK-safety) Adjust dose for typical patient Adjust dose for specific population, or determine doseadjustment not needed 11
12 Pre-NDA/BLA Meeting Review of data acquired to fulfill recommendations made at the Endof-Phase 2 meeting Review of organization of future application: study reports, datasets 12
13 Clinical Pharmacology Guidance Page mation/guidances/ucm htm This list is not comprehensive (e.g., pregnancy, pharmacogenomics, pediatrics) Exposure-Response Relationships Study Design, Data Analysis, and Regulatory Applications Pharmacokinetics in Patients with Impaired Renal Function Study Design, Data Analysis, and Impact on Dosing and Labeling Pharmacokinetics in Patients with Impaired Hepatic Function: Study Design, Data Analysis, and Impact on Dosing and Labeling Drug Interaction Studies--Study Design, Data Analysis, Implications for Dosing, and Labeling Recommendations 13
14 Biopharamceutics Guidance Page mation/guidances/ucm htm Below not comprehensive; biopharmaceutics often not relevant to IV Bioanalytical Method Validation relevant to all PK Bioavailability and Bioequivalence Studies Submitted in NDAs or INDs General Considerations case-by-case relevance for IVs Food-Effect Bioavailability and Fed Bioequivalence Studies Clinical Lactation Studies--Study Design, Data Analysis, and Recommendations for Labeling not relevant for IVs 14
15 Acknowledgments Brian Booth, Deputy Director, DCPV DMIP: Louis Marzella, Betsy Ballard, Adebayo(Bayo) Laniyonu, Kyong(Kaye) Kang, Nushin Todd 15
16 End END 16
17 Dose Adjustment Example No dose change needed for renal impairment 17
Celerion s Symposia Series: Bridging the Gap from Phase I to Proof-of-Concept. San Francisco, CA Tue 8 th, Apr 2014
 Celerion s Symposia Series: Bridging the Gap from Phase I to Proof-of-Concept San Francisco, CA Tue 8 th, Apr 2014 Mind the Gap: Elements of a Bridging Strategy J. Fred Pritchard, Ph.D. Vice President,
Celerion s Symposia Series: Bridging the Gap from Phase I to Proof-of-Concept San Francisco, CA Tue 8 th, Apr 2014 Mind the Gap: Elements of a Bridging Strategy J. Fred Pritchard, Ph.D. Vice President,
Date/Lecturer Class Topic Readings 01/28/10 Brookman
 Course Director: Sheldon, Ph.D. 01/28/10 1 I. Course Description and Requirements II. Historical Perspectives and Overview of Drug Development a. Evolution of the pharmaceutical industry b. Changing development
Course Director: Sheldon, Ph.D. 01/28/10 1 I. Course Description and Requirements II. Historical Perspectives and Overview of Drug Development a. Evolution of the pharmaceutical industry b. Changing development
CATEGORY Advertising. CATEGORY Biopharmaceutics. CATEGORY Biosimilarity
 Guidance Agenda: New & Revised Draft Guidances CDER is Planning to Publish During Calendar Year 2015 (See the Good Guidance Practices (GGPs) regulation on this Web page or 21 CFR 10.115 for details about
Guidance Agenda: New & Revised Draft Guidances CDER is Planning to Publish During Calendar Year 2015 (See the Good Guidance Practices (GGPs) regulation on this Web page or 21 CFR 10.115 for details about
Guidance for Industry
 Guidance for Industry Submission of Abbreviated Reports and Synopses in Support of Marketing Applications U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation
Guidance for Industry Submission of Abbreviated Reports and Synopses in Support of Marketing Applications U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation
FDA Guidance, Clinical Pharmacology, and Regulatory Science
 Principles of Clinical Pharmacology NIH, April 25, 2013 FDA Guidance, Clinical Pharmacology, and Regulatory Science Carl Peck, MD UCSF Center for Drug Development Science Washington DC and San Francisco
Principles of Clinical Pharmacology NIH, April 25, 2013 FDA Guidance, Clinical Pharmacology, and Regulatory Science Carl Peck, MD UCSF Center for Drug Development Science Washington DC and San Francisco
The Role of Clinical Pharmacology in HCV Drug Development: Regulatory Experience
 The Role of Clinical Pharmacology in HCV Drug Development: Regulatory Experience Islam R. Younis, Ph.D. Team Leader Office of Clinical Pharmacology Center for Drug Evaluation and Research U.S. Food and
The Role of Clinical Pharmacology in HCV Drug Development: Regulatory Experience Islam R. Younis, Ph.D. Team Leader Office of Clinical Pharmacology Center for Drug Evaluation and Research U.S. Food and
Structure and Mandate of FDA
 Structure and Mandate of FDA Leonard Sacks, M.D. Office of Medical Policy Center for Drug Evaluation and Research FDA FDA Clinical Investigator Training Course November 13, 2018 Mission of regulatory agencies
Structure and Mandate of FDA Leonard Sacks, M.D. Office of Medical Policy Center for Drug Evaluation and Research FDA FDA Clinical Investigator Training Course November 13, 2018 Mission of regulatory agencies
The Role of a Clinical Statistician in Drug Development By: Jackie Reisner
 The Role of a Clinical Statistician in Drug Development By: Jackie Reisner Types of studies within clinical development Phase I Phase II Phase III Phase IV Phase I First Human Dose (FHD) Young healthy
The Role of a Clinical Statistician in Drug Development By: Jackie Reisner Types of studies within clinical development Phase I Phase II Phase III Phase IV Phase I First Human Dose (FHD) Young healthy
Maximizing opportunities towards achieving clinical success D R U G D I S C O V E R Y. Report Price Publication date
 F o r a c l e a r e r m a r k e t p e r s p e c t i v e Early Stage Drug Safety Strategies & Risk Management Maximizing opportunities towards achieving clinical success D R U G D I S C O V E R Y Report
F o r a c l e a r e r m a r k e t p e r s p e c t i v e Early Stage Drug Safety Strategies & Risk Management Maximizing opportunities towards achieving clinical success D R U G D I S C O V E R Y Report
Outline CLINICALLY RELEVANT SPECIFICATIONS. ISPE Process Validation Conference September 2017 Bethesda, MD
 CLINICALLY RELEVANT SPECIFICATIONS Patrick J Marroum Ph.D. Senior Director and ACOS Senior Research Fellow Department of Clinical Pharmacology and Pharmacometrics Abbvie Pharmaceuticals Outline CMC variables
CLINICALLY RELEVANT SPECIFICATIONS Patrick J Marroum Ph.D. Senior Director and ACOS Senior Research Fellow Department of Clinical Pharmacology and Pharmacometrics Abbvie Pharmaceuticals Outline CMC variables
Conducting Successful pre-ind Meetings to Reach FDA Concurrence for Sound 505(b)(2) Development
 Conducting Successful pre-ind Meetings to Reach FDA Concurrence for Sound 505(b)(2) Development 2015 AAPS Annual Meeting and Exposition October 28, 2015 Kimberly Raines, Ph.D. Lead Pharmacologist Quality
Conducting Successful pre-ind Meetings to Reach FDA Concurrence for Sound 505(b)(2) Development 2015 AAPS Annual Meeting and Exposition October 28, 2015 Kimberly Raines, Ph.D. Lead Pharmacologist Quality
Clinical Trials A Closer Look
 The Food and Drug Administration (FDA) is the main consumer watchdog for numerous products: Drugs and biologics (prescription and over-the counter) Food Medical devices Animal feed and drugs Cosmetics
The Food and Drug Administration (FDA) is the main consumer watchdog for numerous products: Drugs and biologics (prescription and over-the counter) Food Medical devices Animal feed and drugs Cosmetics
Guidance for Industry
 Guidance for Industry M4S: The CTD Safety U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research
Guidance for Industry M4S: The CTD Safety U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research
Application of PGx in PK at FDA: Experience and Expectations
 Application of PGx in PK at FDA: Experience and Expectations EMEA-EFPIA Workshop Integrating PGx into Early Drug Development London UK 19 December 2008 Lawrence J. Lesko, Ph.D., F.C.P. Office of Clinical
Application of PGx in PK at FDA: Experience and Expectations EMEA-EFPIA Workshop Integrating PGx into Early Drug Development London UK 19 December 2008 Lawrence J. Lesko, Ph.D., F.C.P. Office of Clinical
Basic Principles for Conducting Phase I Trials in the Japanese Population Prior to Global Clinical Trials
 Administrative Notice October 27, 2014 To: Prefectural Health Department (Bureau) Evaluation and Licensing Division, Pharmaceutical and Food Safety Bureau, Ministry of Health, Labour and Welfare Basic
Administrative Notice October 27, 2014 To: Prefectural Health Department (Bureau) Evaluation and Licensing Division, Pharmaceutical and Food Safety Bureau, Ministry of Health, Labour and Welfare Basic
Introduction to clinical trials
 Introduction to clinical trials Definition of a clinical trial A research activity that involves administration of a test treatment to some experimental unit in order to evaluate the treatment. Key words
Introduction to clinical trials Definition of a clinical trial A research activity that involves administration of a test treatment to some experimental unit in order to evaluate the treatment. Key words
8. Clinical Trial Assessment Phase II
 8. Clinical Trial Assessment Phase II Junko Sato, PhD Office of New Drug I, PMDA Disclaimer: The information within this presentation is based on the presenter s expertise and experience, and represents
8. Clinical Trial Assessment Phase II Junko Sato, PhD Office of New Drug I, PMDA Disclaimer: The information within this presentation is based on the presenter s expertise and experience, and represents
Introduction to clinical trials Magnus Kjaer
 Introduction to clinical trials 2016-01-20 Magnus Kjaer One Definition of Clinical Trial NIH 2014 A research study in which one or more human subjects are prospectively assigned to one or more interventions
Introduction to clinical trials 2016-01-20 Magnus Kjaer One Definition of Clinical Trial NIH 2014 A research study in which one or more human subjects are prospectively assigned to one or more interventions
Guideline on the reporting of physiologically based pharmacokinetic (PBPK) modelling and simulation
 13 December 2018 EMA/CHMP/458101/2016 Committee for Medicinal Products for Human Use (CHMP) Guideline on the reporting of physiologically based pharmacokinetic (PBPK) modelling and simulation Draft agreed
13 December 2018 EMA/CHMP/458101/2016 Committee for Medicinal Products for Human Use (CHMP) Guideline on the reporting of physiologically based pharmacokinetic (PBPK) modelling and simulation Draft agreed
21st Annual International Conference on Drug-Drug Interactions. Zach Mitts Northwest Account Manager
 21st Annual International Conference on Drug-Drug Interactions Zach Mitts Northwest Account Manager How memorable can a 5-minute presentation be? COMPANY HISTORY From the work of our Founder, Dr. Andrew
21st Annual International Conference on Drug-Drug Interactions Zach Mitts Northwest Account Manager How memorable can a 5-minute presentation be? COMPANY HISTORY From the work of our Founder, Dr. Andrew
Drug Development and Incrementally Modified Drugs : Regulatory Perspective. American Association of Pharmaceutical Scientists October 27, 2015
 Drug Development and Incrementally Modified Drugs : Regulatory Perspective American Association of Pharmaceutical Scientists October 27, 2015 Larissa Lapteva, M.D., M.H.S., Division of Therapeutic Performance
Drug Development and Incrementally Modified Drugs : Regulatory Perspective American Association of Pharmaceutical Scientists October 27, 2015 Larissa Lapteva, M.D., M.H.S., Division of Therapeutic Performance
Introduction to Drug Design and Discovery
 Introduction to Drug Design and Discovery Course: Drug Design Course code: 0510412 Dr. Balakumar Chandrasekaran Dr. Bilal Al-Jaidi Assistant Professors, Pharmaceutical Medicinal Chemistry, Faculty of Pharmacy,
Introduction to Drug Design and Discovery Course: Drug Design Course code: 0510412 Dr. Balakumar Chandrasekaran Dr. Bilal Al-Jaidi Assistant Professors, Pharmaceutical Medicinal Chemistry, Faculty of Pharmacy,
From Bench to Bedside. Russ H. Read June 23, 2014
 From Bench to Bedside Russ H. Read June 23, 2014 Talent Required Scientists, clinicians, technicians, chemists, biologists, pharmacologists, toxicologists, research associates, regulatory experts, manufacturing
From Bench to Bedside Russ H. Read June 23, 2014 Talent Required Scientists, clinicians, technicians, chemists, biologists, pharmacologists, toxicologists, research associates, regulatory experts, manufacturing
Acceptance of Foreign Clinical Data A U.S. Perspective
 Acceptance of Foreign Clinical Data A U.S. Perspective Robert L. Justice, M.D., M.S. 9 th Kitasato University Harvard School of Public Health Symposium Disclaimer The views expressed in this presentation
Acceptance of Foreign Clinical Data A U.S. Perspective Robert L. Justice, M.D., M.S. 9 th Kitasato University Harvard School of Public Health Symposium Disclaimer The views expressed in this presentation
Clara Li, MS VP, Regulatory and Strategic Development Clinipace, Inc.
 Clara Li, MS VP, Regulatory and Strategic Development Clinipace, Inc. Part of Public Health Service, within Department of Health & Human Services PHS consists of FDA, CDC, NIH, and other public health
Clara Li, MS VP, Regulatory and Strategic Development Clinipace, Inc. Part of Public Health Service, within Department of Health & Human Services PHS consists of FDA, CDC, NIH, and other public health
Overview of Recently Approved 505(b)(2) New Drug Applications ( ): Role of Clinical Pharmacology
 Regulatory Overview of Recently Approved 505(b)(2) New Drug Applications (2010 2012): Role of Clinical Pharmacology The Journal of Clinical Pharmacology XX(XX) 1 7 2014, The American College of Clinical
Regulatory Overview of Recently Approved 505(b)(2) New Drug Applications (2010 2012): Role of Clinical Pharmacology The Journal of Clinical Pharmacology XX(XX) 1 7 2014, The American College of Clinical
Road Map to the ITC Transporter White Paper For Drug Development
 Road Map to the ITC Transporter White Paper For Drug Development Highlights of: Membrane Transporters in Drug Development, Kathleen Giacomini (UCSF), Shiew-Mei Huang (FDA), Donald J. Tweedie (Boehringer
Road Map to the ITC Transporter White Paper For Drug Development Highlights of: Membrane Transporters in Drug Development, Kathleen Giacomini (UCSF), Shiew-Mei Huang (FDA), Donald J. Tweedie (Boehringer
Investigational New Drug Application
 Investigational New Drug Application Regulatory Sponsor: Funding Sponsor: Study Product: Protocol Number: Name of the Sponsor-Investigator Department Name Address Phone Number Name of Primary Funding Institution
Investigational New Drug Application Regulatory Sponsor: Funding Sponsor: Study Product: Protocol Number: Name of the Sponsor-Investigator Department Name Address Phone Number Name of Primary Funding Institution
Guideline on the qualification and reporting of physiologically based pharmacokinetic (PBPK) modelling and simulation
 1 2 3 21 July 2016 EMA/CHMP/458101/2016 Committee for Medicinal Products for Human Use (CHMP) 4 5 6 7 Guideline on the qualification and reporting of physiologically based pharmacokinetic (PBPK) modelling
1 2 3 21 July 2016 EMA/CHMP/458101/2016 Committee for Medicinal Products for Human Use (CHMP) 4 5 6 7 Guideline on the qualification and reporting of physiologically based pharmacokinetic (PBPK) modelling
FDA Guidance, Clinical Pharmacology, And Regulatory Science
 Principles of Clinical Pharmacology NIH, April 25, 2013 FDA Guidance, Clinical Pharmacology, And Regulatory Science Carl Peck, MD UCSF Center for Drug Development Science Washington DC and San Francisco
Principles of Clinical Pharmacology NIH, April 25, 2013 FDA Guidance, Clinical Pharmacology, And Regulatory Science Carl Peck, MD UCSF Center for Drug Development Science Washington DC and San Francisco
Current Guidelines for Drug-drug Interaction Evaluation
 Current Guidelines for Drug-drug Interaction Evaluation Odette A Fahmi, PhD DDI-Edge Consulting, LLC June 2018 21 ST International Conference on Drug-Drug Interactions OUTLINE Recommended DDI approaches
Current Guidelines for Drug-drug Interaction Evaluation Odette A Fahmi, PhD DDI-Edge Consulting, LLC June 2018 21 ST International Conference on Drug-Drug Interactions OUTLINE Recommended DDI approaches
Pharmacokinetics. Processes, Mathematics, and Applications. Second Edition. Peter G. Welling. Institut de Recherche Jouveinal
 Pharmacokinetics Processes, Mathematics, and Applications Second Edition Peter G. Welling Institut de Recherche Jouveinal ACS Professional Reference Book American Chemical Society Washington, DC Contents
Pharmacokinetics Processes, Mathematics, and Applications Second Edition Peter G. Welling Institut de Recherche Jouveinal ACS Professional Reference Book American Chemical Society Washington, DC Contents
Development of a new medicinal product. as. MUDr. Martin Votava, PhD.
 Development of a new medicinal product as. MUDr. Martin Votava, PhD. Development and registration of a medicinal product Costs of development: 800 mil USD Time to develop: 10 years Successfulness: 0,005%
Development of a new medicinal product as. MUDr. Martin Votava, PhD. Development and registration of a medicinal product Costs of development: 800 mil USD Time to develop: 10 years Successfulness: 0,005%
SPECIFIC COMMENTS ON TEXT
 1500 K Street, NW Suite 1100 Washington, DC 20005 Telephone: +1 202.230.5621 Email: info@iqconsortium.org Website: https://iqconsortium.org IQ CONSORTIUM COMMENTS ON Draft Guidance for Industry Clinical
1500 K Street, NW Suite 1100 Washington, DC 20005 Telephone: +1 202.230.5621 Email: info@iqconsortium.org Website: https://iqconsortium.org IQ CONSORTIUM COMMENTS ON Draft Guidance for Industry Clinical
BIOSTATISTICAL METHODS FOR TRANSLATIONAL & CLINICAL RESEARCH
 BIOSTATISTICAL METHODS FOR TRANSLATIONAL & CLINICAL RESEARCH Phase 0 Trials: EARLY-PHASE CLINICAL TRIALS CLINICAL PHASE Clinical Studies: Class of all scientific approaches to evaluate Disease Prevention,
BIOSTATISTICAL METHODS FOR TRANSLATIONAL & CLINICAL RESEARCH Phase 0 Trials: EARLY-PHASE CLINICAL TRIALS CLINICAL PHASE Clinical Studies: Class of all scientific approaches to evaluate Disease Prevention,
Guideline for Bioequivalence Studies of Generic Products. December 22, 1997
 Guideline for Bioequivalence Studies of Generic Products December 22, 1997 Index Section 1: Introduction Section 2: Terminology Section 3: Tests A. Oral conventional dosage forms and enteric coated products
Guideline for Bioequivalence Studies of Generic Products December 22, 1997 Index Section 1: Introduction Section 2: Terminology Section 3: Tests A. Oral conventional dosage forms and enteric coated products
Question and Answer Guide Regarding Notification on Practical Operations of Electronic Study Data Submissions
 Administrative Notice April 27, 2015 To: Prefectrual Health Department (Bureau) Evaluation and Licensing Division, Pharmaceutical and Food Safety Bureau, Ministry of Health, Labour and Welfare Question
Administrative Notice April 27, 2015 To: Prefectrual Health Department (Bureau) Evaluation and Licensing Division, Pharmaceutical and Food Safety Bureau, Ministry of Health, Labour and Welfare Question
Natural Products and Drug Discovery
 Natural Products and Drug Discovery Secondary metabolism Building blocks & Phytochemicals Drug discovery 26-Oct-18 2 Secondary metabolism: Biochemical reactions derived from primary metabolic pathways
Natural Products and Drug Discovery Secondary metabolism Building blocks & Phytochemicals Drug discovery 26-Oct-18 2 Secondary metabolism: Biochemical reactions derived from primary metabolic pathways
CRYSTAL CITY V REDUX: GUIDANCE FOR INDUSTRY BIOANALYTICAL METHOD VALIDATION DRAFT GUIDANCE (2013) VI. ADDITIONAL ISSUES
 CRYSTAL CITY V REDUX: GUIDANCE FOR INDUSTRY BIOANALYTICAL METHOD VALIDATION DRAFT GUIDANCE (2013) VI. ADDITIONAL ISSUES DELAWARE VALLEY DRUG METABOLISM DISCUSSION GROUP STEVE PICCOLI, BMS RAND JENKINS,
CRYSTAL CITY V REDUX: GUIDANCE FOR INDUSTRY BIOANALYTICAL METHOD VALIDATION DRAFT GUIDANCE (2013) VI. ADDITIONAL ISSUES DELAWARE VALLEY DRUG METABOLISM DISCUSSION GROUP STEVE PICCOLI, BMS RAND JENKINS,
CATEGORY Advertising. CATEGORY Biopharmaceutics. CATEGORY Biosimilarity
 CATEGORY Advertising Guidance Agenda: New & Guidances CDER is Planning to Publish During Calendar Year 2016 (See the Good Guidance Practices (GGPs) regulation on this Web page or 21 CFR 10.115 for details
CATEGORY Advertising Guidance Agenda: New & Guidances CDER is Planning to Publish During Calendar Year 2016 (See the Good Guidance Practices (GGPs) regulation on this Web page or 21 CFR 10.115 for details
Workflow for PBPK Modeling to Support Pediatric Research and Development
 Workflow for PBPK Modeling to Support Pediatric Research and Development Jeff Barrett, PhD Vice-President, Interdisciplinary Pharmacometrics Program Sanofi 1 Industrial Strength PBPK 2 Outline Industrial
Workflow for PBPK Modeling to Support Pediatric Research and Development Jeff Barrett, PhD Vice-President, Interdisciplinary Pharmacometrics Program Sanofi 1 Industrial Strength PBPK 2 Outline Industrial
Guideline on the use of pharmacogenetic methodologies in the pharmacokinetic evaluation of medicinal products
 12 December 2011 EMA/CHMP/37646/2009 Committee for Medicinal Products for Human Use (CHMP) Guideline on the use of pharmacogenetic methodologies in the pharmacokinetic evaluation of medicinal products
12 December 2011 EMA/CHMP/37646/2009 Committee for Medicinal Products for Human Use (CHMP) Guideline on the use of pharmacogenetic methodologies in the pharmacokinetic evaluation of medicinal products
How PK and PD analyses drive dataset designs
 PharmaSUG 2018 - Paper AA-11 ABSTRACT How PK and PD analyses drive dataset designs David B. Radtke and Brian A. Moser, Eli Lilly and Company Historically, the format of pharmacokinetic analysis datasets
PharmaSUG 2018 - Paper AA-11 ABSTRACT How PK and PD analyses drive dataset designs David B. Radtke and Brian A. Moser, Eli Lilly and Company Historically, the format of pharmacokinetic analysis datasets
Toxicology - Problem Drill 24: Toxicology Studies in Pharmaceutical Development
 Toxicology - Problem Drill 24: Toxicology Studies in Pharmaceutical Development No. 1 of 10 1. regulates all the drugs products manufactured and sold in the USA. (A) EMEA (B) IND (C) FDA (D) NDA (E) OSHA
Toxicology - Problem Drill 24: Toxicology Studies in Pharmaceutical Development No. 1 of 10 1. regulates all the drugs products manufactured and sold in the USA. (A) EMEA (B) IND (C) FDA (D) NDA (E) OSHA
Impurities in Drugs: Monitoring, Safety and Regulation The Israel Chapter of PDA
 Overview of exploratory INDs Impurities in Drugs: Monitoring, Safety and Regulation The Israel Chapter of PDA July, 15 16, 2008 David Jacobson-Kram, Ph.D. DABT Office of New Drugs Center for Drug Evaluation
Overview of exploratory INDs Impurities in Drugs: Monitoring, Safety and Regulation The Israel Chapter of PDA July, 15 16, 2008 David Jacobson-Kram, Ph.D. DABT Office of New Drugs Center for Drug Evaluation
* For Smarties. Design of Clinical Drug Development Programs* Objectives 4/19/2012. Disclaimer
 Design of Clinical Drug Development Programs* * For Smarties Dr Christopher D Breder 4/12 1 Disclaimer The views expressed in this talk represent my opinions and do not necessarily represent the views
Design of Clinical Drug Development Programs* * For Smarties Dr Christopher D Breder 4/12 1 Disclaimer The views expressed in this talk represent my opinions and do not necessarily represent the views
Design of Clinical Drug Development Programs*
 Design of Clinical Drug Development Programs* * For Smarties Dr Christopher D Breder 4/03/14 1 1 Disclaimer The views expressed in this talk represent my opinions and do not necessarily represent the views
Design of Clinical Drug Development Programs* * For Smarties Dr Christopher D Breder 4/03/14 1 1 Disclaimer The views expressed in this talk represent my opinions and do not necessarily represent the views
Defining Clinical Benefit in Clinical Trials: FDA Perspective
 Defining Clinical Benefit in Clinical Trials: FDA Perspective Jessica J. Lee, MD, MMSc Medical Team Leader Division of Gastroenterology and Inborn Errors Products Center for Drug Evaluation and Research
Defining Clinical Benefit in Clinical Trials: FDA Perspective Jessica J. Lee, MD, MMSc Medical Team Leader Division of Gastroenterology and Inborn Errors Products Center for Drug Evaluation and Research
DMTC Technology Readiness Levels Guideline
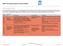 Technology Readiness Levels Technology Readiness Levels (TRLs) are used as standardised numerical indicators of the level of maturity of a technology. The standard TRL definitions are given in Table 1.
Technology Readiness Levels Technology Readiness Levels (TRLs) are used as standardised numerical indicators of the level of maturity of a technology. The standard TRL definitions are given in Table 1.
Extrapolation in Pediatric Product Development: Practical Application of the Principle of Scientific Necessity
 Extrapolation in Pediatric Product Development: Practical Application of the Principle of Scientific Necessity Robert Skip Nelson, MD PhD Deputy Director and Senior Pediatric Ethicist Office of Pediatric
Extrapolation in Pediatric Product Development: Practical Application of the Principle of Scientific Necessity Robert Skip Nelson, MD PhD Deputy Director and Senior Pediatric Ethicist Office of Pediatric
Accelerating Therapeutic Development through a look at current Regulatory Applications A Non-Clinical Perspective
 Accelerating Therapeutic Development through a look at current Regulatory Applications A Non-Clinical Perspective Imran M. Khan, Ph.D. Division of Psychiatry Center for Drug Evaluation and Research FDA
Accelerating Therapeutic Development through a look at current Regulatory Applications A Non-Clinical Perspective Imran M. Khan, Ph.D. Division of Psychiatry Center for Drug Evaluation and Research FDA
Preclinical studies needed in the development of human pharmaceutical drugs role of toxicology and risk assessment
 Preclinical studies needed in the development of human pharmaceutical drugs role of toxicology and risk assessment Hubert Dirven Lead Validation Unit - NPI Amersham Health (GE Healthcare), Oslo Preclinical
Preclinical studies needed in the development of human pharmaceutical drugs role of toxicology and risk assessment Hubert Dirven Lead Validation Unit - NPI Amersham Health (GE Healthcare), Oslo Preclinical
The views and opinions expressed in the following PowerPoint slides are
 Indications, Dosage and Administration, Clinical Studies, Clinical Pharmacology sections of a CCDS Presented by: Mary Jane Boyle Head, Worldwide Product Labeling Merck & Co., 13-October-2011 The views
Indications, Dosage and Administration, Clinical Studies, Clinical Pharmacology sections of a CCDS Presented by: Mary Jane Boyle Head, Worldwide Product Labeling Merck & Co., 13-October-2011 The views
Bioanalytical Support to In Vitro Studies
 Bioanalytical Support to In Vitro Studies Presenter: Tim Sangster (on behalf of EBF (mini-workshop)) Open Symposium 2017 November 17 th 2017 Barcelona http://www.europeanbioanalysisforum.eu Agenda Ø Scope
Bioanalytical Support to In Vitro Studies Presenter: Tim Sangster (on behalf of EBF (mini-workshop)) Open Symposium 2017 November 17 th 2017 Barcelona http://www.europeanbioanalysisforum.eu Agenda Ø Scope
Drug Development: Why Does it Cost so Much? Lewis J. Smith, MD Professor of Medicine Director, Center for Clinical Research Associate VP for Research
 Drug Development: Why Does it Cost so Much? Lewis J. Smith, MD Professor of Medicine Director, Center for Clinical Research Associate VP for Research Drug Development Process by which new chemical entities
Drug Development: Why Does it Cost so Much? Lewis J. Smith, MD Professor of Medicine Director, Center for Clinical Research Associate VP for Research Drug Development Process by which new chemical entities
An Introduction to Clinical Research and Development
 Bay Clinical R&D Services An Introduction to Clinical Research and Development The Complex Process by which New Drugs are Tested in Humans Anastassios D. Retzios, Ph.D. Outline of Presentation What is
Bay Clinical R&D Services An Introduction to Clinical Research and Development The Complex Process by which New Drugs are Tested in Humans Anastassios D. Retzios, Ph.D. Outline of Presentation What is
Phase 1 Clinical Studies First-In-Human (FIH) <Chapter 31> Pharmacologically-Guided Dose Escalation
 Phase 1 Clinical Studies First-In-Human (FIH) Pharmacologically-Guided Dose Escalation Jerry M. Collins, Ph.D. Developmental Therapeutics Program Division of Cancer Treatment and Diagnosis,
Phase 1 Clinical Studies First-In-Human (FIH) Pharmacologically-Guided Dose Escalation Jerry M. Collins, Ph.D. Developmental Therapeutics Program Division of Cancer Treatment and Diagnosis,
Role of Academic Investigators in Drug Development
 DTRCS Regulatory Education Seminar, June 12, 2007 Role of Academic Investigators in Drug Development Howard Lee, MD, PhD Associate Adjunct Professor Director, Center for Drug Terminology Sponsor Investigator
DTRCS Regulatory Education Seminar, June 12, 2007 Role of Academic Investigators in Drug Development Howard Lee, MD, PhD Associate Adjunct Professor Director, Center for Drug Terminology Sponsor Investigator
Approval Review of Generic Drugs. Office of Generic/OTC Drugs, PMDA Kazuyuki SAITO, Ph.D.
 Approval Review of Generic Drugs Office of Generic/OTC Drugs, PMDA Kazuyuki SAITO, Ph.D. Outline of Presentation Introduction Approval Review of Generic Drugs Equivalency review Conformity audit Conclusion
Approval Review of Generic Drugs Office of Generic/OTC Drugs, PMDA Kazuyuki SAITO, Ph.D. Outline of Presentation Introduction Approval Review of Generic Drugs Equivalency review Conformity audit Conclusion
New TB Drugs Approval in Thailand
 New TB Drugs Approval in Thailand Tharnkamol Chanprapaph,, Ph.D. Drug Control Division, Thai Food and Drug Administration Third Annual Open Forum on Key Issues in Tuberculosis Drug Development, New Delhi,
New TB Drugs Approval in Thailand Tharnkamol Chanprapaph,, Ph.D. Drug Control Division, Thai Food and Drug Administration Third Annual Open Forum on Key Issues in Tuberculosis Drug Development, New Delhi,
* For Smarties 4/21/2016. Design of Clinical Drug Development Programs* Disclaimer. Objectives
 Design of Clinical Drug Development Programs* * For Smarties Dr Christopher D Breder 4/21/16 1 Disclaimer The views expressed in this talk represent my opinions and do not necessarily represent the views
Design of Clinical Drug Development Programs* * For Smarties Dr Christopher D Breder 4/21/16 1 Disclaimer The views expressed in this talk represent my opinions and do not necessarily represent the views
From Discovery to Development of new Drugs. and pitfalls along the way. by Kim Dekermendjian, PhD in Medicine BD & Key Account manager
 From Discovery to Development of new Drugs. and pitfalls along the way by Kim Dekermendjian, PhD in Medicine BD & Key Account manager The roots of Drug Discovery Before 20 th century the term didn't exists,
From Discovery to Development of new Drugs. and pitfalls along the way by Kim Dekermendjian, PhD in Medicine BD & Key Account manager The roots of Drug Discovery Before 20 th century the term didn't exists,
BIOSTATISTICAL METHODS
 BIOSTATISTICAL METHODS FOR TRANSLATIONAL & CLINICAL RESEARCH Phase 0 Trials: EARLY-PHASE CLINICAL TRIALS Steps to New Drug Discovery Get idea for drug target Develop a bioassay Screen chemical compounds
BIOSTATISTICAL METHODS FOR TRANSLATIONAL & CLINICAL RESEARCH Phase 0 Trials: EARLY-PHASE CLINICAL TRIALS Steps to New Drug Discovery Get idea for drug target Develop a bioassay Screen chemical compounds
Official Letter from the DOH
 Issued Date 2009/04/02 Issued by DOH Ref. No 0980316268 RE The DOH issued an official letter to announce the implementation of the Guideline for BA/BE Studies on April 2, 2009 (Ref. No. 0980316265). Please
Issued Date 2009/04/02 Issued by DOH Ref. No 0980316268 RE The DOH issued an official letter to announce the implementation of the Guideline for BA/BE Studies on April 2, 2009 (Ref. No. 0980316265). Please
S9 Nonclinical Evaluation for Anticancer Pharmaceuticals
 S9 Nonclinical Evaluation for Anticancer Pharmaceuticals This draft guidance, when finalized, will represent the Food and Drug Administration's (FDA's) current thinking on this topic. It does not create
S9 Nonclinical Evaluation for Anticancer Pharmaceuticals This draft guidance, when finalized, will represent the Food and Drug Administration's (FDA's) current thinking on this topic. It does not create
Injectable modified release products
 Guideline on the pharmacokinetic and clinical evaluation of modified release dosage forms (EMA/CPMP/EWP/280/96 Corr1) Injectable modified release products Dr Sotiris Michaleas, National Expert for the
Guideline on the pharmacokinetic and clinical evaluation of modified release dosage forms (EMA/CPMP/EWP/280/96 Corr1) Injectable modified release products Dr Sotiris Michaleas, National Expert for the
Microdose Radiopharmaceutical Diagnostic Drugs: Nonclinical Study Recommendations Guidance for Industry
 Microdose Radiopharmaceutical Diagnostic Drugs: Nonclinical Study Recommendations Guidance for Industry U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation
Microdose Radiopharmaceutical Diagnostic Drugs: Nonclinical Study Recommendations Guidance for Industry U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation
Regulatory Approaches for New Drugs: BA/BE of Topical Drug Products
 Regulatory Approaches for New Drugs: BA/BE of Topical Drug Products CAPT E. Dennis Bashaw, Pharm.D Director, Division of Clinical Pharmacology-3 Office of Clinical Pharmacology Office of Translational
Regulatory Approaches for New Drugs: BA/BE of Topical Drug Products CAPT E. Dennis Bashaw, Pharm.D Director, Division of Clinical Pharmacology-3 Office of Clinical Pharmacology Office of Translational
NOTICE OF COMPLIANCE WITH CONDITIONS - QUALIFYING NOTICE
 Therapeutic Products Directorate Holland Cross, Tower "B" 6th Floor, 1600 Scott Street Address Locator #3106B OTTAWA, Ontario K1A 0K9 January 10, 2014 Dossier Identifier: E141793 Control No.: 152211 [employee
Therapeutic Products Directorate Holland Cross, Tower "B" 6th Floor, 1600 Scott Street Address Locator #3106B OTTAWA, Ontario K1A 0K9 January 10, 2014 Dossier Identifier: E141793 Control No.: 152211 [employee
Regulatory Updates in Clinical Pharmacology, Nonclinical Development, and Translational Medicine
 Regulatory Updates in Clinical Pharmacology, Nonclinical Development, and Translational Medicine Luana Pesco Koplowitz, MD, PhD, FCP, FFPM President and Chief Medical & Scientific Officer DUCK FLATS Pharma,
Regulatory Updates in Clinical Pharmacology, Nonclinical Development, and Translational Medicine Luana Pesco Koplowitz, MD, PhD, FCP, FFPM President and Chief Medical & Scientific Officer DUCK FLATS Pharma,
Question Yes No. 1. Is the study interventional (a clinical trial)? Study Type data element is Interventional
 Checklist for Evaluating Whether a Clinical Trial or Study is an Applicable Clinical Trial (ACT) Under 42 CFR 11.22(b) for Clinical Trials Initiated on or After January 18, 2017 1 (NOT FOR SUBMISSION 2
Checklist for Evaluating Whether a Clinical Trial or Study is an Applicable Clinical Trial (ACT) Under 42 CFR 11.22(b) for Clinical Trials Initiated on or After January 18, 2017 1 (NOT FOR SUBMISSION 2
CPTR Mission, Structure & Goals for Innovation
 CPTR Mission, Structure & Goals for Innovation Dr. Debra Hanna, PhD Executive Director Critical Path to TB Drug Regimens Event title - DD Month YYYY - Location The Challenge Collaborating for Cures 7 March
CPTR Mission, Structure & Goals for Innovation Dr. Debra Hanna, PhD Executive Director Critical Path to TB Drug Regimens Event title - DD Month YYYY - Location The Challenge Collaborating for Cures 7 March
Saudi Pharmacist Licensure Examination (SPLE)
 Saudi Pharmacist Licensure Examination (SPLE) 1 SPLE Overview This document provides important information about the topics covered on the examination and the competency areas in which candidates will
Saudi Pharmacist Licensure Examination (SPLE) 1 SPLE Overview This document provides important information about the topics covered on the examination and the competency areas in which candidates will
From Drug Discovery to IND Saving Time and Money Through Smart Study Design
 From Drug Discovery to IND Saving Time and Money Through Smart Study Design Stephen Madden BSc PhD CBiol FSB Head, Metabolism and Pharmacokinetics Charles River Preclinical Services, Edinburgh Outline
From Drug Discovery to IND Saving Time and Money Through Smart Study Design Stephen Madden BSc PhD CBiol FSB Head, Metabolism and Pharmacokinetics Charles River Preclinical Services, Edinburgh Outline
International Consortium For Innovation & Quality in Pharmaceutical Development
 International Consortium For Innovation & Quality in Pharmaceutical Development s on Draft Guidance: FDA Draft Guidance: Investigational Enzyme Replacement Therapy Products: Nonclinical Assessment (draft
International Consortium For Innovation & Quality in Pharmaceutical Development s on Draft Guidance: FDA Draft Guidance: Investigational Enzyme Replacement Therapy Products: Nonclinical Assessment (draft
PRECLINICAL DRUG DISCOVERY AND DEVELOPMENT BIOBOOT CAMP 2016
 PRECLINICAL DRUG DISCOVERY AND DEVELOPMENT BIOBOOT CAMP 2016 1 FROM DISCOVERY TO COMMERCIALIZATION At least 10 years on average Average spend of $1.4B Less than 12% of drugs entering Phase I are approved
PRECLINICAL DRUG DISCOVERY AND DEVELOPMENT BIOBOOT CAMP 2016 1 FROM DISCOVERY TO COMMERCIALIZATION At least 10 years on average Average spend of $1.4B Less than 12% of drugs entering Phase I are approved
FDA s Critical Path Initiative and Drug Development
 FDA s Critical Path Initiative and Drug Development Duu-Gong Wu, PhD Executive Director, PharmaNet Consulting The views expressed herein are solely those of the author and do not necessarily reflect the
FDA s Critical Path Initiative and Drug Development Duu-Gong Wu, PhD Executive Director, PharmaNet Consulting The views expressed herein are solely those of the author and do not necessarily reflect the
Introduction to Drug Development in Commercializing Biomedical Technology
 Introduction to Drug Development in Commercializing Biomedical Technology Kevin W. Hunt, Ph.D. Director of Biopharmaceutical Product Development Office of Technology Development and Head of Translational
Introduction to Drug Development in Commercializing Biomedical Technology Kevin W. Hunt, Ph.D. Director of Biopharmaceutical Product Development Office of Technology Development and Head of Translational
FDA Perspective on the Preclinical Evaluation of Biological Therapies for Cancer
 FDA Perspective on the Preclinical Evaluation of Biological Therapies for Cancer Yongjie Zhou, M.D., Ph.D. FDA/CBER/OCTGT/DCEPT Yongjie.zhou@fda.hhs.gov isbtc Global Regulatory Summit October 29, 2008
FDA Perspective on the Preclinical Evaluation of Biological Therapies for Cancer Yongjie Zhou, M.D., Ph.D. FDA/CBER/OCTGT/DCEPT Yongjie.zhou@fda.hhs.gov isbtc Global Regulatory Summit October 29, 2008
Agreed with W. Cornell Graduate Program and Tri-I
 Drug Development Course From Molecule to Prescription Weill Cornell Graduate School - Tri-Institutional Therapeutics Discovery Institute ABOUT THIS COURSE This course has been designed in collaboration
Drug Development Course From Molecule to Prescription Weill Cornell Graduate School - Tri-Institutional Therapeutics Discovery Institute ABOUT THIS COURSE This course has been designed in collaboration
EUROPEAN COMMISSION ENTERPRISE DIRECTORATE-GENERAL
 EUROPEAN COMMISSION ENTERPRISE DIRECTORATE-GENERAL Single market : management & legislation for consumer goods Pharmaceuticals : regulatory framework and market authorisations Brussels, ENTR/CT 5.2 Final
EUROPEAN COMMISSION ENTERPRISE DIRECTORATE-GENERAL Single market : management & legislation for consumer goods Pharmaceuticals : regulatory framework and market authorisations Brussels, ENTR/CT 5.2 Final
The Impact of Pharmaceutical Sciences on Healthcare Vinod P. Shah, Ph.D., Pharmaceutical Consultant
 The Impact of Pharmaceutical Sciences on Healthcare Vinod P. Shah, Ph.D., Pharmaceutical Consultant Southern African Regional and International Symposium SARI 2014 MCC/AAPS Symposium Pretoria, S Africa,
The Impact of Pharmaceutical Sciences on Healthcare Vinod P. Shah, Ph.D., Pharmaceutical Consultant Southern African Regional and International Symposium SARI 2014 MCC/AAPS Symposium Pretoria, S Africa,
Pain Therapeutics, Inc. Pain Therapeutics Announces Update on Drug Portfolio
 For More Information Contact: Peter S. Roddy Vice President and Chief Financial Officer Pain Therapeutics, Inc. proddy@paintrials.com 512 501 2450 FOR IMMEDIATE RELEASE Pain Therapeutics Announces Update
For More Information Contact: Peter S. Roddy Vice President and Chief Financial Officer Pain Therapeutics, Inc. proddy@paintrials.com 512 501 2450 FOR IMMEDIATE RELEASE Pain Therapeutics Announces Update
Update on DDI Guidance of the FDA - In Vitro
 Update on DDI Guidance of the FDA - In Vitro Xinning Yang, Ph.D. Policy lead Guidance & Policy Team (GPT) Office of Clinical Pharmacology (OCP) Office of Translational Sciences (OTS) Center for Drug Evaluation
Update on DDI Guidance of the FDA - In Vitro Xinning Yang, Ph.D. Policy lead Guidance & Policy Team (GPT) Office of Clinical Pharmacology (OCP) Office of Translational Sciences (OTS) Center for Drug Evaluation
European Medicines Agency Evaluation of Medicines for Human Use COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) DRAFT
 European Medicines Agency Evaluation of Medicines for Human Use London, 27 July 2005 EMEA/CHMP/89249/2004 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) DRAFT Guideline on the Clinical Investigation
European Medicines Agency Evaluation of Medicines for Human Use London, 27 July 2005 EMEA/CHMP/89249/2004 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) DRAFT Guideline on the Clinical Investigation
The course is taught by a team of UNC faculty and industry scientists. The course has been designed to provide the students an understanding of
 Drug Metabolism MOPH 810 Fall 2009 Classes: 80 minute class twice a week Course Director: Dhiren R. Thakker Eshelman School of Pharmacy, UNC-Chapel Hill The course is taught by a team of UNC faculty and
Drug Metabolism MOPH 810 Fall 2009 Classes: 80 minute class twice a week Course Director: Dhiren R. Thakker Eshelman School of Pharmacy, UNC-Chapel Hill The course is taught by a team of UNC faculty and
Application of PBPK Models in Assessment of Bioequivalence
 Application of PBPK Models in Assessment of Bioequivalence Robert Lionberger, Ph.D. Acting Director Office of Research and Standards Office of Generic Drugs Center for Drug Evaluation and Research, FDA
Application of PBPK Models in Assessment of Bioequivalence Robert Lionberger, Ph.D. Acting Director Office of Research and Standards Office of Generic Drugs Center for Drug Evaluation and Research, FDA
Eurofins ADME BIOANALYSES Your partner in drug development
 Eurofins ADME BIOANALYSES Your partner in drug development Since 1987, you have placed your confidence in us. Our responsibility, to which my staff and I commit, is to consistently provide you with relevant
Eurofins ADME BIOANALYSES Your partner in drug development Since 1987, you have placed your confidence in us. Our responsibility, to which my staff and I commit, is to consistently provide you with relevant
FRAMEWORK OF SETTING CLINICALLY RELEVANT SPECIFICATIONS: APPROACH, INFORMATION NEEDED, AND CRITERIA
 FRAMEWORK OF SETTING CLINICALLY RELEVANT SPECIFICATIONS: APPROACH, INFORMATION NEEDED, AND CRITERIA DISSOLUTION AND TRANSLATIONAL MODELING STRATEGIES ENABLING PATIENT-CENTRIC PRODUCT DEVELOPMENT MAY 15-17,
FRAMEWORK OF SETTING CLINICALLY RELEVANT SPECIFICATIONS: APPROACH, INFORMATION NEEDED, AND CRITERIA DISSOLUTION AND TRANSLATIONAL MODELING STRATEGIES ENABLING PATIENT-CENTRIC PRODUCT DEVELOPMENT MAY 15-17,
Common Deficiences in Bioequivalence Studies and Biowaiver Requests submitted to the JFDA
 Common Deficiences in Bioequivalence Studies and Biowaiver Requests submitted to the JFDA DIMA S. SHAHIN, M.SC. MEMBER OF THE BIOEQUIVALENCE STUDIES TECHNICAL COMMITTEE REGISTRATION DEPARTMENT, DRUG DIRECTORATE-
Common Deficiences in Bioequivalence Studies and Biowaiver Requests submitted to the JFDA DIMA S. SHAHIN, M.SC. MEMBER OF THE BIOEQUIVALENCE STUDIES TECHNICAL COMMITTEE REGISTRATION DEPARTMENT, DRUG DIRECTORATE-
Role of Pharmacometrics in Drug Development and Regulatory Review:PMDA perspectives
 Role of Pharmacometrics in Drug Development and Regulatory Review:PMDA perspectives Naomi Nagai Ph.D. Office of New Drug IV/Advanced Review with Electronic Data Promotion Group Pharmaceuticals and Medical
Role of Pharmacometrics in Drug Development and Regulatory Review:PMDA perspectives Naomi Nagai Ph.D. Office of New Drug IV/Advanced Review with Electronic Data Promotion Group Pharmaceuticals and Medical
Use of Mechanistic Population Based PBPK Models in the Establishment and Application of IVIVCs. Nikunjkumar Patel, Simcyp Limited
 Use of Mechanistic Population Based PBPK Models in the Establishment and Application of IVIVCs Nikunjkumar Patel, Simcyp Limited IVIVC and Its Components CONVOLUTION VALIDATION DECONVOLUTION MODELS IVIVC
Use of Mechanistic Population Based PBPK Models in the Establishment and Application of IVIVCs Nikunjkumar Patel, Simcyp Limited IVIVC and Its Components CONVOLUTION VALIDATION DECONVOLUTION MODELS IVIVC
e-pkgene: A knowledge-based research tool for analysing the impact of genetics on drug exposure
 GENOME DATABASES e-pkgene: A knowledge-based research tool for analysing the impact of genetics on drug exposure Houda Hachad, 1 Casey Lynnette Overby, 2 Sophie Argon, 1 Catherine K. Yeung, 1 Isabelle
GENOME DATABASES e-pkgene: A knowledge-based research tool for analysing the impact of genetics on drug exposure Houda Hachad, 1 Casey Lynnette Overby, 2 Sophie Argon, 1 Catherine K. Yeung, 1 Isabelle
! Background. ! What is really new?! The new Section 7: Explorative Clinical Trials (ECTs) ! Consequences in General
 ! Background! What is really new?! The new Section 7: Explorative Clinical Trials (ECTs)! 5 Approaches (Table 3) for:! Microdose trials (7.1)! Single-Dose (SD) Trials at Sub-therapeutic Doses or Into the
! Background! What is really new?! The new Section 7: Explorative Clinical Trials (ECTs)! 5 Approaches (Table 3) for:! Microdose trials (7.1)! Single-Dose (SD) Trials at Sub-therapeutic Doses or Into the
A Life-cycle Approach to Dose Finding Studies
 A Life-cycle Approach to Dose Finding Studies Rajeshwari Sridhara, Ph.D. Director, Division of Biometrics V Center for Drug Evaluation and Research, USFDA This presentation reflects the views of the author
A Life-cycle Approach to Dose Finding Studies Rajeshwari Sridhara, Ph.D. Director, Division of Biometrics V Center for Drug Evaluation and Research, USFDA This presentation reflects the views of the author
S9 Implementation Working Group ICH S9 Guideline: Nonclinical Evaluation for Anticancer Pharmaceuticals Questions and Answers
 S9 Implementation Working Group ICH S9 Guideline: Nonclinical Evaluation for Anticancer Pharmaceuticals Questions and Answers Current version dated 8 June 2016 International Council for Harmonisation of
S9 Implementation Working Group ICH S9 Guideline: Nonclinical Evaluation for Anticancer Pharmaceuticals Questions and Answers Current version dated 8 June 2016 International Council for Harmonisation of
Beta-lactamase inhibition: A potted history of beta lactamase and lessons from recent development of betalactamase inhibiter combinations
 Beta-lactamase inhibition: A potted history of beta lactamase and lessons from recent development of betalactamase inhibiter combinations Dr Shampa Das, Senior Lecturer, Molecular and Clinical Pharmacology,
Beta-lactamase inhibition: A potted history of beta lactamase and lessons from recent development of betalactamase inhibiter combinations Dr Shampa Das, Senior Lecturer, Molecular and Clinical Pharmacology,
Drug Development & the FDA Pharmacy 309 November 2005
 Goals & Objectives Drug Development & the FDA Pharmacy 309 November 2005 Tom Hazlet, Pharm.D., Dr.P.H. H375S 206.616.2732 thazlet@u... Be able to describe major regulatory events in the drug & biologic,
Goals & Objectives Drug Development & the FDA Pharmacy 309 November 2005 Tom Hazlet, Pharm.D., Dr.P.H. H375S 206.616.2732 thazlet@u... Be able to describe major regulatory events in the drug & biologic,
The Drug Development Process and Design of Clinical Trials
 The Drug Development Process and Design of Clinical Trials Darlene Kitterman, MBA Director, Investigator Support & Integration Services September 24, 2014 Clinical Trial Design Guidance Clinical Trial:
The Drug Development Process and Design of Clinical Trials Darlene Kitterman, MBA Director, Investigator Support & Integration Services September 24, 2014 Clinical Trial Design Guidance Clinical Trial:
5/23/2016. The Drug Development Process and Design of Clinical Trials. Clinical Trial Design Guidance. Regulatory Definitions
 The Drug Development Process and Design of Clinical Trials Darlene Kitterman, MBA Director, Investigator Support & Integration Services May 25, 2016 Clinical Trial Design Guidance Clinical Trial: a prospective
The Drug Development Process and Design of Clinical Trials Darlene Kitterman, MBA Director, Investigator Support & Integration Services May 25, 2016 Clinical Trial Design Guidance Clinical Trial: a prospective
