$29.00 $30.00 Blood Glucose Testing (190.20) (Also see Medicare Preventative Services Quick Reference) Carcinoembryonic Antigen (CEA) (190.
|
|
|
- Rodney Clifton Lambert
- 5 years ago
- Views:
Transcription
1 TEST DESCRIPTION INCLUDES TESTS NATIONAL COVERAGE DETERMINATIONS (NCD S) Est. cost Alpha Fetoprotein (190.25) alpha-fetoprotein, serum $35.96 Blood Counts (CBC) (190.15) Blood count; automated differential white blood cell (WBC) count blood smear, microscopic examination with manual differential WBC count blood smear, microscopic examination without manual differential WBC count Blood counts, spun microhematocrit Blood counts, hematocrit (Hct) Blood counts, hemoglobin blood counts, complete (CBC), automated (Hgb, Hct, RBC, WBC and platelet count) and automated differential WBC count Blood counts, complete (CBC), automated (Hcb, Hct, RBC, WBC and platelet count) Blood count; manual cell count (erythrocyte, leukocyte, or platelet) each Blood counts, leukocyte (WBC) automated Blood count; platelet, automated $24.00 $37.62 $11.88 $30.00 Blood Glucose Testing (190.20) Carcinoembryonic Antigen (CEA) (190.26) Glucose; quantitative, blood (except reagent strip) Glucose; blood, reagent strip Glucose; blood by glucose monitoring device cleared by the FDA specifically for home use $ Carcinoembryonic Antigen (CEA) $59.40 Collagen Crosslinks (190.19) Collagen Cross Links, any method $ Culture-Bacterial (Urine) (190.12) Culture, bacterial; quantitative colony count, urine Culture, bacterial; with isolation and presumptive identification of isolates, urine $49.50 Digoxin Therapeutic Drug Assay (190.24) Fecal Occult Blood (190.34) Gamma Glutamyl Transferase (GGT) (190.32) Glycated Hemoglobin/Glycated Protein (190.21) Hepatitis Panel (Acute) (190.33) HIV Testing (Prognosis including monitoring) (190.13) Digoxin (therapeutic drug assay) $ Blood, occult, by peroxidase activity (eg guaiac); feces, 1-3 simultaneous determinations Blood, occult, by peroxidase activity (eg guaiac), qual. Feces; single spec (dig exam) Glutamyltransferase, gamma (GGT) Glycated protein Hemoglobin; glycated (A1C) Hemoglobin; glycated (A1C) by device cleared by FDA for home use Acute Hepatitis Panel Includes: Hepatitis B surface antigen (HBsAg) Hepatitis C antibody Hepatitis B core antibody (HBcAb), IgM Antibody Hepatitis A antibody (HAAb), IgM Antibody Infectious agent detection by nucleic acid (DNA or RNA); HIV-1 quantification Infectious agent detection by nucleic acid (DNA or RNA); HIV-2 quantification $89.00 $39.60 $ $342.00
2 HIV Testing (Diagnosis) (190.14) HLA (Histocompatibility Testing (190.1) Human Chorionic Gonadotropin (HCG) (190.27) Lipids Testing (190.23) Pap Smear screening Partial Thromboplastin Time (PTT) (190.16) Prostate Specific Antigen (PSA) (190.31) Qual. or semi-quant. Immunoassays performed by multiple step $ methods; HTLV or HIV antibody, confirmatory test (ie, Western Blot) Qual. or semi-quant. Immunoassays performed by multiple step $55.00 methods; HIV-I Qual. or semi-quant. Immunoassays performed by multiple step $55.00 methods; HIV-II Qual. or semi-quant. Immunoassays performed by multiple step $ methods; HIV-I and HIV-II, single assay Infectious agent antigen detection by enzyme immunoassay $ technique, qual. or semiquant., multiple step, HIV-I Infectious agent antigen detection by enzyme immunoassay technique, qual. or semiquant., multiple step, HIV-II Infectious agent detection by nucleic acid (DNA or RNA); HIV-I, direct probe technique Infectious agent detection by nucleic acid (DNA or RNA); HIV-I, direct $ probe technique HIV-I, amplified probe technique Infectious agent detection by nucleic acid (DNA or RNA); HIV-II, direct probe technique Infectious agent detection by nucleic acid (DNA or RNA); HIV-II, direct probe technique HIV-I, amplified probe technique HLA B-27 $ Gonadotropin, chorionic (HCG) quantitative Lipid Panel Apolipoprotein, each Cholesterol, serum, total Lipoprotein, blood; electrophoretic separation and quantitation Lipoprotein, blood; high resolution fractionation and quantitation of lipoprotein including subclasses when performed Lipoprotein, direct measurement; high density cholesterol (HDL) Lipoprotein, direct measurement; VLDL cholesterol Direct measurement; LDL cholesterol Triglycerides G0145 Screening Cytopathology, automated thin layer preparation P3000 Screening Papanicolaou smear (conventional Pap) $54.36 $ $12.68 $58.00 $ $23.76 $45.54 $17.92 $74.26 $ Thromboplastin time, partial (PTT); plasma or whole blood Prostate Specific Antigen (PSA); complexed (direct measurement) Prostate Specific Antigen (PSA); total Prostate Specific Antigen (PSA); free G0103 Prostate Cancer Screening; Prostate Specific Antigen Test (PSA) $56.94 $95.00 $56.94 Prothrombin Time (PT) (190.17) Prothrombin time $15.84 Serum Iron Studies (190.18) Ferritin Iron Iron Binding Capacity Transferrin $49.50 $27.72 $27.72 $109.00
3 Thyroid Testing (190.22) (CA 15-3/27.29) (190.29) (CA-125) (190.28) (CA19-9) (190.30) Thyroxine; total Thyroxine; free Thyroid Stimulating Hormone (TSH) Thyroid Hormone (T3 or T4) uptake or thyroid hormone binding ratio (THBR) $39.60 $ Immunoassay for tumor antigen, quantitative; CA 15-3 / $ Immunoassay for tumor antigen, quantitative, CA 125 $ Immunoassay for tumor antigen, quantitative; CA 19-9 $ INDIANA PART B LOCAL COVERAGE DETERMINATIONS (LCD S) CONTRACTOR MAC PART B, WISCONSIN PHYSICIANS SERVICE CORPORATION (08102) Gamma globulin (Immunoglobulin) IGE Allergen specific IgE; Quantitative or semi-quantitative, each allergen Allergen specific IgE; Qualitative multiallergen screen (dipstick, paddle or disc) Allergy Testing and Allergy Immunotherapy (L36402) (Frequency Guideline exists) Biomarkers in Cardiovascular Risk Assessment (L36523) Cytogenetic Studies (L34655) Apolipoprotein, Each Cystatin C Homocysteine Lipoprotein (A) Lipoprotein, blood; Electrophoretic separation and quantitation Lipoprotein, blood; high resolution fractionations and quantitation of lipoproteins including lipoprotein subclasses when performed (eg, electrophoresis, ultracentrifugation) Lipoprotein, direct measurement; LDL cholesterol Natriuretic peptide C-reactive protein; high sensitivity (HSCRP) Tissue culture for non-neoplastic disorders; lymphocyte Tissue culture for non-neoplastic disorders; skin or other solid tissue biopsy Tissue culture for non-neoplastic disorders; amniotic fluid or chorionic villus cells Tissue culture for non-neoplastic disorders; bone marrow, blood cells Chromosome analysis; count 5 cells, I karyotype, with banding Chromosome analysis; count cells, 2 karyotypes, with banding Chromosome analysis; analyze cells Chromosome analysis, in situ for amniotic fluid cells, count cells from 6-12 colonies, 1 karyotype, with banding Molecular cytogenetics; DNA probe, each (e.g. FISH) Molecular cytogenetics; interphase in situ hybridization, analyze cells Chromosome analysis; additional karyotypes, each study Cytogenetics and molecular cytogenetics, interpretation and report $21.64 $19.07 Multiple- please $ $ $59.40 $90.00 $58.00 $ $45.54 $99.00 $79.20
4 Drug Testing (L34645) 80305: Drug test(s), presumptive, any number of drug classes. Any number of devices or procedures (eg, immunoassay); capable of being read by direct optical observation only (eg, dipsticks, cups, cards, cartridges) includes sample validation when performed, per date of service : Drug test(s), presumptive, any number of drug classes. Any number of devices or procedures (eg, immunoassay); read by instrument assisted direct optical observation )eg, dipsticks, cups, cards, cartridges), includes sample validation when performed, per date of service : Drug test(s), presumptive, any number of drug classes, any number of devices or procedures, by instrument chemistry analyzers (eg, utilizing immunoassay {eg, EIA, ELISA, EMIT, FPIA, IA, IMS, RIA}, chromatography (eg, GC, HPLC), and mass spectrometry either with or without chromatography, (eg, DART, DESI, GC-MS, GC-MS/MS, LC-MS, LC-MS/MS, LDTD, MALDI, TOF) includes sample validation when performed, per date of service Definitive drug classes - Alcohols drug(s) or substance(s), definitive, qualitative or quantitative, not otherwise specified; 7 or more. G Drug test(s), definitive, utilizing drug identification methods able to identify stereoisomers), including but not limited to GC/MS (any type single or tandem) and LC/MS (any type, single or tandem and excluding immunoassays (eg, IA, EIA, ELISA, EMIT, FPIA) and enzymatic methods (eg, alcohol dehydrogenase)); qualitative or quantitative, all source(s), includes specimen validity testing, per day. 1-7 drug class(es), including metabolite(s), if performed. G Drug test(s), definitive, utilizing drug identification methods able to identify stereoisomers), including but not limited to GC/MS (any type single or tandem) and LC/MS (any type, single or tandem and excluding immunoassays (eg, IA, EIA, ELISA, EMIT, FPIA) and enzymatic methods (eg, alcohol dehydrogenase)); qualitative or quantitative, all source(s), includes specimen validity testing, per day drug classes, including metabolite(s), if performed. G0482: Drug test(s), definitive, utilizing (1) drug identification methods able to identify stereoisomers), including, but not limited to GC/MS (any type, single or tandem) and LC/MS (any type, single or tandem and excluding immunoassays (e.g., IA, EIA, ELISA, EMIT, FPIA) and enzymatic methods (e.g., alcohol dehydrogenase)), (2) stable isotope or other universally recognized internal standards in all samples (e.g., to control for matrix effects, interferences and variations in signal strength), and (3) method or drug-specific calibration and matrix-matched quality control material (e.g., to control for instrument variations and mass spectral drift); qualitative or quantitative, all sources, includes specimen validity testing, per day; drug class(es), including metabolite(s) if performed G0483: Drug test(s), definitive, utilizing (1) drug identification methods able to identify stereoisomers), including, but not limited to GC/MS (any type, single or tandem) and LC/MS (any type, single or tandem and excluding immunoassays (e.g., IA, EIA, ELISA, EMIT, FPIA) and enzymatic methods (e.g., alcohol dehydrogenase)), (2) stable isotope or other universally recognized internal standards in all samples (e.g., to control for matrix effects, interferences and variations in signal strength), and (3) method or drug-specific calibration and matrix-matched quality control material (e.g., to control for instrument variations and mass spectral drift); qualitative or quantitative, all sources, includes specimen validity testing, per day; 22 or more drug class(es), including metabolite(s) if performed
5 Flow Cytometry (L34651) Genetic Testing for: (L36400) Factor II Prothrombin Factor V Leiden MTHFR Vitamin D Assay Testing (L34658) Flow Cytometry, cell cycle or DNA analysis Flow Cytometry, cell surface, cytoplasmic or nuclear marker, technical component only, first marker Flow Cytometry, cell surface, cytoplasmic, or nuclear marker, technical component only; each additional marker (list separately in addition to code for first marker Flow Cytometry, interpretation; 2-8 markers Flow Cytometry, interpretation; 9-15 markers Flow Cytometry, interpretation; 16 or more markers 81240: F2 (prothrombin, coagulation factor II) (eg. Hereditary hypercoagulability) gene analysis, 20210G>A variant : F5 (coagulation factor V)(eg. Hereditary hypercoagulability) gene analysis, Leiden variant : MTHFR (5,10-methylguanine-DNA methyltransferase)(eg. Hereditary hypercoagulability) gene analysis, common variants (eg. 677T, 1298C) Calcifediol (25-OH Vitamin D-3) Vitamin D; 1, 25 dihydroxy, includes fraction(s), if performed $ schedule $ $ $ $ $217.00
$29.00 $30.00 Blood Glucose Testing (190.20) (Also see Medicare Preventative Services Quick Reference) Carcinoembryonic Antigen (CEA) (190.
 TEST DESCRIPTION INCLUDES TESTS NATIONAL COVERAGE DETERMINATIONS (NCD S) Est. cost Alpha Fetoprotein (190.25) 82105 alpha-fetoprotein, serum $35.96 Blood Counts (CBC) (190.15) 85004 -- Blood count; automated
TEST DESCRIPTION INCLUDES TESTS NATIONAL COVERAGE DETERMINATIONS (NCD S) Est. cost Alpha Fetoprotein (190.25) 82105 alpha-fetoprotein, serum $35.96 Blood Counts (CBC) (190.15) 85004 -- Blood count; automated
Payment Policy: Physician s Office Lab Testing Reference Number: CC.PP.055 Product Types: ALL
 Payment Policy: Physician s Office Lab Testing Reference Number: CC.PP.055 Product Types: ALL Effective Date: 11/01/2017 Last Review Date: Coding Implications Revision Log See Important Reminder at the
Payment Policy: Physician s Office Lab Testing Reference Number: CC.PP.055 Product Types: ALL Effective Date: 11/01/2017 Last Review Date: Coding Implications Revision Log See Important Reminder at the
No. Type of Sample Test Method. Chemistry
 Chemistry 1. Serum, NaF Plasma, Heparinized Plasma Glucose Enzymatic Reference Method with Hexokinase: Cobas c501, Cobas 2. Serum, Heparinized Plasma Blood Urea Nitrogen (BUN) Kinetic Test with Urease
Chemistry 1. Serum, NaF Plasma, Heparinized Plasma Glucose Enzymatic Reference Method with Hexokinase: Cobas c501, Cobas 2. Serum, Heparinized Plasma Blood Urea Nitrogen (BUN) Kinetic Test with Urease
Approved by: Thomas M. Driskill, Jr. President & CEO
 HAWAII HEALTH SYSTEMS C O R P O R A T I O N Touching Lives Everyday" Policies and Procedures Subject: Unlisted Laboratory Procedure Codes Quality Through Compliance Issued by: Corporate Compliance Committee
HAWAII HEALTH SYSTEMS C O R P O R A T I O N Touching Lives Everyday" Policies and Procedures Subject: Unlisted Laboratory Procedure Codes Quality Through Compliance Issued by: Corporate Compliance Committee
Laboratory Tests Chronic Renal Deficiency (CRD) Patients (NCD )
 Policy Number 190.10 Approved By UnitedHealthcare Medicare Reimbursement Policy Committee Current Approval Date 03/26/2014 IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY This policy is applicable to UnitedHealthcare
Policy Number 190.10 Approved By UnitedHealthcare Medicare Reimbursement Policy Committee Current Approval Date 03/26/2014 IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY This policy is applicable to UnitedHealthcare
BlueCare Tennessee Lab Exclusion List
 The Primary Criteria for the Exclusion List is as follows: Test Results needed in an Outpatient Setting to Facilitate Immediate care of the Patient. NOTE: Codes added during the Q2 2016 review are indicated
The Primary Criteria for the Exclusion List is as follows: Test Results needed in an Outpatient Setting to Facilitate Immediate care of the Patient. NOTE: Codes added during the Q2 2016 review are indicated
CAP Accreditation Checklists 2017 Edition
 CAP Accreditation Checklists 2017 Edition The College of American Pathologists (CAP) accreditation checklists contain the CAP accreditation program requirements, developed on more than 50 years of insight
CAP Accreditation Checklists 2017 Edition The College of American Pathologists (CAP) accreditation checklists contain the CAP accreditation program requirements, developed on more than 50 years of insight
CAP Accreditation Checklists 2016 Edition
 CAP Accreditation Checklists 2016 Edition The College of American Pathologists (CAP) accreditation checklists contain the CAP accreditation program requirements, developed on more than 50 years of insight
CAP Accreditation Checklists 2016 Edition The College of American Pathologists (CAP) accreditation checklists contain the CAP accreditation program requirements, developed on more than 50 years of insight
Our Lady's Hospital Navan Ireland East Hospital Group
 Ireland East Hospital Group Department of Pathology, Navan, Co. Meath Testing Laboratory Registration number: 215MT is accredited by the Irish National Board (INAB) to undertake testing as detailed in
Ireland East Hospital Group Department of Pathology, Navan, Co. Meath Testing Laboratory Registration number: 215MT is accredited by the Irish National Board (INAB) to undertake testing as detailed in
SCOPE OF ACCREDITATION TO ISO/IEC 17043:2010. ACCUTEST INC Fraserwood Court Burnaby, CANADA V5J 5H7 Louise Wilkinson
 SCOPE OF ACCREDITATION TO ISO/IEC 17043:2010 ACCUTEST INC 9-8980 Fraserwood Court Burnaby, CANADA V5J 5H7 Louise Wilkinson 604 500 8788 PROFICIENCY TESTING PROVIDER Valid To: June 30, 2022 Certificate
SCOPE OF ACCREDITATION TO ISO/IEC 17043:2010 ACCUTEST INC 9-8980 Fraserwood Court Burnaby, CANADA V5J 5H7 Louise Wilkinson 604 500 8788 PROFICIENCY TESTING PROVIDER Valid To: June 30, 2022 Certificate
The Future of Reimbursement for Lab Developed Mass Spec Assays
 The Future of Reimbursement for Lab Developed Mass Spec Assays Charles Root, PhD CodeMap, LLC Schaumberg, IL AACC/MSSS Mass Spectrometry and Separation Sciences for Lab Medicine Oct 1 2, 2015 1 The Future
The Future of Reimbursement for Lab Developed Mass Spec Assays Charles Root, PhD CodeMap, LLC Schaumberg, IL AACC/MSSS Mass Spectrometry and Separation Sciences for Lab Medicine Oct 1 2, 2015 1 The Future
Reflex Testing. General Laboratory Reflex Testing
 Reflex Testing Page 1 of 10 Introduction General Laboratory Reflex Testing Blood Bank and Coagulation Reflex Testing Microbiology Reflex Testing Introduction Diagnostic Laboratory Services offers medically
Reflex Testing Page 1 of 10 Introduction General Laboratory Reflex Testing Blood Bank and Coagulation Reflex Testing Microbiology Reflex Testing Introduction Diagnostic Laboratory Services offers medically
Reflex Testing. General Laboratory Reflex Testing
 Reflex Testing Page 1 of 10 Introduction General Laboratory Reflex Testing Blood Bank and Coagulation Reflex Testing Microbiology Reflex Testing Introduction Diagnostic Laboratory Services offers medically
Reflex Testing Page 1 of 10 Introduction General Laboratory Reflex Testing Blood Bank and Coagulation Reflex Testing Microbiology Reflex Testing Introduction Diagnostic Laboratory Services offers medically
BlueCare Tennessee Lab Exclusion List
 BlueCare Tennessee is an Independent Licensee of the BlueCross BlueShield Association The Primary Criteria for the Exclusion List is as follows: Test Results needed in an Outpatient Setting to Facilitate
BlueCare Tennessee is an Independent Licensee of the BlueCross BlueShield Association The Primary Criteria for the Exclusion List is as follows: Test Results needed in an Outpatient Setting to Facilitate
King George's Medical University UP., Lucknow center for advance research
 SUMMER/WINTER: SHORT TERM TRAINING COURSE-DRAFT DURATION: 30 DAYS Molecular Biology Unit-Genomics 10:00 to 12:00 AM 2:00 to 4:00 PM Lecture 1: Basics of Molecular Biology- DNA and RNA Blood Collection
SUMMER/WINTER: SHORT TERM TRAINING COURSE-DRAFT DURATION: 30 DAYS Molecular Biology Unit-Genomics 10:00 to 12:00 AM 2:00 to 4:00 PM Lecture 1: Basics of Molecular Biology- DNA and RNA Blood Collection
Mental Health and Addictions Program Resource Manual
 Title: Physician Assistant Ordering Lab Tests for CKHA Mental Health and Addictions Program Patients (Medical Directive #2) Policy Number: MHAP#1-275 Chatham-Kent Health Alliance embraces a philosophy
Title: Physician Assistant Ordering Lab Tests for CKHA Mental Health and Addictions Program Patients (Medical Directive #2) Policy Number: MHAP#1-275 Chatham-Kent Health Alliance embraces a philosophy
Bureau of Laboratory Quality Standards Page 1 of 7
 Blood Bank 1. Clotted Blood, EDTA Blood 2. Clotted Blood, EDTA Blood Immunology ABO Grouping Rh Typing Cell And Serum Grouping: Agglutination Cell Grouping: Agglutination 3. EDTA Blood CD4 Count Flowcytometry:
Blood Bank 1. Clotted Blood, EDTA Blood 2. Clotted Blood, EDTA Blood Immunology ABO Grouping Rh Typing Cell And Serum Grouping: Agglutination Cell Grouping: Agglutination 3. EDTA Blood CD4 Count Flowcytometry:
EPIC Procedure Code (EAP)
 45511111 36415 $3.00 Routine Venipuncture 60036415 36415 $3.00 Routine Venipuncture 4700000 47000 $90.00 Incision Procedure of Liver 60047000 47000 $1,304.00 Incision Procedure of Liver 7020026 26 Professional
45511111 36415 $3.00 Routine Venipuncture 60036415 36415 $3.00 Routine Venipuncture 4700000 47000 $90.00 Incision Procedure of Liver 60047000 47000 $1,304.00 Incision Procedure of Liver 7020026 26 Professional
BIOMAN 2015 IMMUNOASSAYS. Barbara Bielska Northampton Community College Tannersville, PA
 BIOMAN 2015 IMMUNOASSAYS Barbara Bielska Northampton Community College Tannersville, PA 1 Immunoassays An immunoassay is a biochemical test that measures the presence or concentration of a molecules (typically
BIOMAN 2015 IMMUNOASSAYS Barbara Bielska Northampton Community College Tannersville, PA 1 Immunoassays An immunoassay is a biochemical test that measures the presence or concentration of a molecules (typically
IMMUNOCHEMICAL TECHNIQUES
 24 IMMUNOCHEMICAL TECHNIQUES 24.1 INTRODUCTION All vertebrates have advanced immune system. The more complex the organism the more advanced the immune system. The immune system of mammals has evolved over
24 IMMUNOCHEMICAL TECHNIQUES 24.1 INTRODUCTION All vertebrates have advanced immune system. The more complex the organism the more advanced the immune system. The immune system of mammals has evolved over
Industry Recommended Payment Decision Either 1) Gapfill, or 2) Various combined crosswalk calculations to multiple existing laboratory codes
 Calendar Year 2014 Centers for Medicare and Medicaid Services (CMS) New and Reconsidered Clinical Laboratory Fee Schedule (CLFS) Test Codes And Preliminary Payment Determinations Reconsideration Codes
Calendar Year 2014 Centers for Medicare and Medicaid Services (CMS) New and Reconsidered Clinical Laboratory Fee Schedule (CLFS) Test Codes And Preliminary Payment Determinations Reconsideration Codes
EXAM COVER SHEET. Course Code: CLS 432. Course Description: Clinical Biochemistry. Example Past Exam Modal Answer.
 EXAM COVER SHEET Course Code: CLS 432 Course Description: Clinical Biochemistry Example Past Exam Modal Answer Duration: 2 hour 1st semester 1432/1433 Part 1 Multiple choice questions Answer all the questions
EXAM COVER SHEET Course Code: CLS 432 Course Description: Clinical Biochemistry Example Past Exam Modal Answer Duration: 2 hour 1st semester 1432/1433 Part 1 Multiple choice questions Answer all the questions
Coding Essentials for Laboratories 2018
 Coding Essentials for Laboratories 2018 An Easy-to-Use Tool for Coding and Reimbursement Compliance Prepared and Published By: MedLearn Publishing A Division of MedLearn Media, Inc. 445 Minnesota Street,
Coding Essentials for Laboratories 2018 An Easy-to-Use Tool for Coding and Reimbursement Compliance Prepared and Published By: MedLearn Publishing A Division of MedLearn Media, Inc. 445 Minnesota Street,
CHAP10-CPTcodes _final doc Revision Date: 1/1/2018
 CHAP10-CPTcodes80000-89999_final10312017.doc Revision Date: 1/1/2018 CHAPTER X PATHOLOGY / LABORATORY SERVICES CPT CODES 80000-89999 FOR NATIONAL CORRECT CODING INITIATIVE POLICY MANUAL FOR MEDICARE SERVICES
CHAP10-CPTcodes80000-89999_final10312017.doc Revision Date: 1/1/2018 CHAPTER X PATHOLOGY / LABORATORY SERVICES CPT CODES 80000-89999 FOR NATIONAL CORRECT CODING INITIATIVE POLICY MANUAL FOR MEDICARE SERVICES
Coding Essentials for Laboratories 2017
 Coding Essentials for Laboratories 2017 An Easy-to-Use Tool for Coding and Reimbursement Compliance Prepared and Published By: MedLearn Publishing A Division of Panacea Healthcare Solutions, Inc. 287 East
Coding Essentials for Laboratories 2017 An Easy-to-Use Tool for Coding and Reimbursement Compliance Prepared and Published By: MedLearn Publishing A Division of Panacea Healthcare Solutions, Inc. 287 East
Fetal Screen Fetal screen Positive Kleihauer-Betke
 Reflex Test Protocols Reflex tests fall into 2 categories: standard industry practice (example: sensitivities, interpretations, and confirmations) and institutional practice based on staff clinical practice
Reflex Test Protocols Reflex tests fall into 2 categories: standard industry practice (example: sensitivities, interpretations, and confirmations) and institutional practice based on staff clinical practice
MGH Pathology Service Laboratory Reflex Test Protocols 2011 Approved by MGH Medical Policy Committee 07/27/2011
 Approved by MGH Medical Policy Committee 07/27/2011 Reflex Test Protocols Reflex tests fall into 2 categories: standard industry practice (example: sensitivities and confirmations) and institutional practice
Approved by MGH Medical Policy Committee 07/27/2011 Reflex Test Protocols Reflex tests fall into 2 categories: standard industry practice (example: sensitivities and confirmations) and institutional practice
Chapter 10 Analytical Biotechnology and the Human Genome
 Chapter 10 Analytical Biotechnology and the Human Genome Chapter Outline Enzyme tests and biosensors DNA-based tests DNA analysis technologies Human genome and genome-based analytical methods 1 Enzyme-based
Chapter 10 Analytical Biotechnology and the Human Genome Chapter Outline Enzyme tests and biosensors DNA-based tests DNA analysis technologies Human genome and genome-based analytical methods 1 Enzyme-based
Immunological Applications. Chapter 8: Background
 Immunological Applications Chapter 8: Background The Immune System Types of Immunity Innate The natural immunity present at birth Acquired A specific response to foreign substances. Some cells remember
Immunological Applications Chapter 8: Background The Immune System Types of Immunity Innate The natural immunity present at birth Acquired A specific response to foreign substances. Some cells remember
Antigen-antibody reactions with labeled reagents
 Antigen-antibody reactions with labeled reagents Department of Immunology Faculty of Medicine University of Belgrade ANTIGEN ANTIBODY REACTIONS WITH LABELED REAGENTS Enzyme immunoassay Radioimmunoassay
Antigen-antibody reactions with labeled reagents Department of Immunology Faculty of Medicine University of Belgrade ANTIGEN ANTIBODY REACTIONS WITH LABELED REAGENTS Enzyme immunoassay Radioimmunoassay
CERTIFICATE OF ACCREDITATION
 CERTIFICATE OF ACCREDITATION In terms of section 22(2) (b) of the Accreditation for Conformity Assessment, Calibration and Good Laboratory Practice Act, 2006 (Act 19 of 2006), read with sections 23(1),
CERTIFICATE OF ACCREDITATION In terms of section 22(2) (b) of the Accreditation for Conformity Assessment, Calibration and Good Laboratory Practice Act, 2006 (Act 19 of 2006), read with sections 23(1),
M 313 OCT 0 1 Z015 ORDER OF THE MINISTER OF HEALTH PROVINCE OF BRITISH COLUMBIA. Class of referring practitioner. Schedule
 Ministerial Order No. M 313 PROVINCE OF BRITISH COLUMBIA ORDER OF THE MINISTER OF HEALTH I, Terry Lake, Minister of Health order that: Schedules in respect of the benefits that may be requested by the
Ministerial Order No. M 313 PROVINCE OF BRITISH COLUMBIA ORDER OF THE MINISTER OF HEALTH I, Terry Lake, Minister of Health order that: Schedules in respect of the benefits that may be requested by the
Laboratory Accreditation Program Laboratory Activity Menu SU: ALL
 Page 1 of 19 03/16/ 05:40 AM All Common Common (CAP Office use) Albumin, body fluid Albumin, serum/plasma Alkaline phosphatase, serum/plasma ALT, serum/plasma Amylase, serum/plasma AST, serum/plasma Bilirubin,
Page 1 of 19 03/16/ 05:40 AM All Common Common (CAP Office use) Albumin, body fluid Albumin, serum/plasma Alkaline phosphatase, serum/plasma ALT, serum/plasma Amylase, serum/plasma AST, serum/plasma Bilirubin,
Proteomics And Cancer Biomarker Discovery. Dr. Zahid Khan Institute of chemical Sciences (ICS) University of Peshawar. Overview. Cancer.
 Proteomics And Cancer Biomarker Discovery Dr. Zahid Khan Institute of chemical Sciences (ICS) University of Peshawar Overview Proteomics Cancer Aims Tools Data Base search Challenges Summary 1 Overview
Proteomics And Cancer Biomarker Discovery Dr. Zahid Khan Institute of chemical Sciences (ICS) University of Peshawar Overview Proteomics Cancer Aims Tools Data Base search Challenges Summary 1 Overview
Meets Standards AFTER REVIEW ( 2.5 pts) Fail to meet the standard safety precautions, counseled one time
 Morgan State University 2014 MDTC 411 CLINICAL CHEMISTRY AND URINALYSIS PRACTICUM SUGGESTED LABORATORY ROTATION SCORING RUBRIC Please attach the student s test record to this evaluation Clinical Chemistry
Morgan State University 2014 MDTC 411 CLINICAL CHEMISTRY AND URINALYSIS PRACTICUM SUGGESTED LABORATORY ROTATION SCORING RUBRIC Please attach the student s test record to this evaluation Clinical Chemistry
NEW YORK STATE APPROVED TEST LIST
 BIO 4300 Acylcarnitine - Plasma Tandem Mass Spectroscopy Plasma BIO 4310 Carnitine Determination - Plasma Tandem Mass Spectroscopy Plasma BIO 4130, 4260 Creatine and Guanidinoacetate Determination Tandem
BIO 4300 Acylcarnitine - Plasma Tandem Mass Spectroscopy Plasma BIO 4310 Carnitine Determination - Plasma Tandem Mass Spectroscopy Plasma BIO 4130, 4260 Creatine and Guanidinoacetate Determination Tandem
2111: ALL Post-HCT. Add/ Remove/ Modify. Manual Section. Date. Description. Comprehensive Disease- Specific Manuals
 2111: ALL Post-HCT The Acute Lymphoblastic Leukemia Post-HCT Data Form is one of the Comprehensive Report Forms. This form captures ALL-specific post-hct data such as: planned treatments post-hct, the
2111: ALL Post-HCT The Acute Lymphoblastic Leukemia Post-HCT Data Form is one of the Comprehensive Report Forms. This form captures ALL-specific post-hct data such as: planned treatments post-hct, the
Outline. Impact on Human Diseases Basis for Molecular Assay Novel Biomarkers
 MOLECULAR DIAGNOSITICS 1 Outline Concept of Molecular Diagnostics History of Molecular Diagnostics Impact on Human Diseases Basis for Molecular Assay Novel Biomarkers 2 Molecular Diagnosis Molecular diagnosis
MOLECULAR DIAGNOSITICS 1 Outline Concept of Molecular Diagnostics History of Molecular Diagnostics Impact on Human Diseases Basis for Molecular Assay Novel Biomarkers 2 Molecular Diagnosis Molecular diagnosis
CERTIFICATE OF ACCREDITATION
 CERTIFICATE OF ACCREDITATION LANCET LABORATORIES TANZANIA CONSERVATION HOUSE Registration No: PHL-B2/DSM-KIN/AUT/09 Facility Accreditation Number: MED 016 is a SADCAS accredited Medical Laboratory provided
CERTIFICATE OF ACCREDITATION LANCET LABORATORIES TANZANIA CONSERVATION HOUSE Registration No: PHL-B2/DSM-KIN/AUT/09 Facility Accreditation Number: MED 016 is a SADCAS accredited Medical Laboratory provided
Schedule of Accreditation issued by United Kingdom Accreditation Service 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK
 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK Viapath Pathology Department of Clinical Biochemistry Bedford Hospital NHS Trust Kempston Road Bedford MK42 9DJ Contact: Dr W S Wassif Tel:
2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK Viapath Pathology Department of Clinical Biochemistry Bedford Hospital NHS Trust Kempston Road Bedford MK42 9DJ Contact: Dr W S Wassif Tel:
Cytogenetics Laboratory: Laboratory Information, Clinical Case Reports, and Proficiency Testing Participation
 Cytogenetics Laboratory: Laboratory Information, Clinical Case Reports, and Proficiency Testing Participation Complete one form for each cytogenetics laboratory associated with the training program. List
Cytogenetics Laboratory: Laboratory Information, Clinical Case Reports, and Proficiency Testing Participation Complete one form for each cytogenetics laboratory associated with the training program. List
Bureau of Laboratory Quality Standards Page 1 of 7
 1. Serum Anti Human Immunodeficiency Virus 2. Serum Hepatitis B Virus Surface Antigen - Gelatin Particle Agglutination (GPA) : Serodia HIV 1/2 - Immuno Chromatography : Wandfo HIV 1/2 - Immunocomb (indirect
1. Serum Anti Human Immunodeficiency Virus 2. Serum Hepatitis B Virus Surface Antigen - Gelatin Particle Agglutination (GPA) : Serodia HIV 1/2 - Immuno Chromatography : Wandfo HIV 1/2 - Immunocomb (indirect
PROF. DR. ASMAA HUSSEIN DIRECTOR OF THE MOLECULAR BIOLOGY RESEARCH UNIT
 BY PROF. DR. ASMAA HUSSEIN DIRECTOR OF THE MOLECULAR BIOLOGY RESEARCH UNIT Immunoassay are based on the strong and highly specific interaction occurring between antigens (Ag)) and antibodies (Ab). Ag Ab
BY PROF. DR. ASMAA HUSSEIN DIRECTOR OF THE MOLECULAR BIOLOGY RESEARCH UNIT Immunoassay are based on the strong and highly specific interaction occurring between antigens (Ag)) and antibodies (Ab). Ag Ab
February 15, RE: Nebraska Methodist Hospital Pathology Center Special Bulletin ANNUAL NOTICE TO PHYSICIANS 2017.
 February 15, 2017 RE: Nebraska Methodist Hospital Pathology Center Special Bulletin ANNUAL NOTICE TO PHYSICIANS 2017 Dear Partner, We are enclosing for your review the Annual Notice to Physicians for medical
February 15, 2017 RE: Nebraska Methodist Hospital Pathology Center Special Bulletin ANNUAL NOTICE TO PHYSICIANS 2017 Dear Partner, We are enclosing for your review the Annual Notice to Physicians for medical
Original Policy Date
 MP 2.04.75 Genetic Testing Medical Policy Section Medicine Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Created Local Policy/12:2013 Return to Medical Policy Index Disclaimer Our
MP 2.04.75 Genetic Testing Medical Policy Section Medicine Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Created Local Policy/12:2013 Return to Medical Policy Index Disclaimer Our
***Before submitting your application, please review the checklist on the last page.***
 Maryland Department of Health and Mental Hygiene Office of Health Care Quality Laboratory Licensing Programs Spring Grove Center Bland Bryant Building 55 Wade Avenue, Catonsville, MD 21228 Phone: 410.402.8025
Maryland Department of Health and Mental Hygiene Office of Health Care Quality Laboratory Licensing Programs Spring Grove Center Bland Bryant Building 55 Wade Avenue, Catonsville, MD 21228 Phone: 410.402.8025
Quest Tennessee Representatives
 VIA FACSIMILE February (Insert Date), 2018 Dear Valued Customer: We are writing to provide you with the necessary information for your office to transition its laboratory services for BCBS TN s BlueCare
VIA FACSIMILE February (Insert Date), 2018 Dear Valued Customer: We are writing to provide you with the necessary information for your office to transition its laboratory services for BCBS TN s BlueCare
May 21, Dear Valued OSF Lab Customer,
 Dear Valued OSF Lab Customer, OSF System Laboratory is committed to providing you with the high quality products and services you expect to meet your patient s needs by continually assessing processes
Dear Valued OSF Lab Customer, OSF System Laboratory is committed to providing you with the high quality products and services you expect to meet your patient s needs by continually assessing processes
FORENSIC SEROLOGY. Chapter PRENTICE HALL 2008 Pearson Education, Inc. Upper Saddle River, NJ 07458
 Chapter 8 FORENSIC SEROLOGY 8-1 Nature of Blood The word blood refers to a highly complex mixture of cells, enzymes, proteins, and inorganic substances. Plasma, which is the fluid portion of blood, is
Chapter 8 FORENSIC SEROLOGY 8-1 Nature of Blood The word blood refers to a highly complex mixture of cells, enzymes, proteins, and inorganic substances. Plasma, which is the fluid portion of blood, is
So we can separate antigens into their components and allow them to react with their antibodies
 Ag-ab reactions As for single immunodiffusion, double immunodiffusion can be also combined with electrophoresis to speed up the reaction, and in this case the test is called immunoelectrophoresis. So electrophoresis
Ag-ab reactions As for single immunodiffusion, double immunodiffusion can be also combined with electrophoresis to speed up the reaction, and in this case the test is called immunoelectrophoresis. So electrophoresis
Immunological Techniques in Research and Clinical Medicine. Philip L. Cohen, M.D. Chief of Rheumatology, LKSOM 10 March 2016
 Immunological Techniques in Research and Clinical Medicine Philip L. Cohen, M.D. Chief of Rheumatology, LKSOM 10 March 2016 Antibodies Remarkable Tools for Research and Diagnosis You can make an antibody
Immunological Techniques in Research and Clinical Medicine Philip L. Cohen, M.D. Chief of Rheumatology, LKSOM 10 March 2016 Antibodies Remarkable Tools for Research and Diagnosis You can make an antibody
Schedule of Accreditation
 Schedule of Accreditation Organisation Name INAB Reg No Contact Name Address West North West Hospitals Group Galway University Hospital Dept Haematology 239MT Mary Kilcooley Contact Phone No 091 544419
Schedule of Accreditation Organisation Name INAB Reg No Contact Name Address West North West Hospitals Group Galway University Hospital Dept Haematology 239MT Mary Kilcooley Contact Phone No 091 544419
HOST DEFENSE SMALL GROUP PROBLEM SOLVING SESSION CLINICAL IMMUNOLOGIC ASSAYS-II
 HOST DEFENSE SMALL GROUP PROBLEM SOLVING SESSION CLINICAL IMMUNOLOGIC ASSAYS-II Monday, March 24, 2008 2:00 PM 4:00 PM Small Group Classrooms LEARNING GOAL Understanding in vitro assessment of immunologic
HOST DEFENSE SMALL GROUP PROBLEM SOLVING SESSION CLINICAL IMMUNOLOGIC ASSAYS-II Monday, March 24, 2008 2:00 PM 4:00 PM Small Group Classrooms LEARNING GOAL Understanding in vitro assessment of immunologic
Molecular characterization, detection & quantitation of biological products Purin Charoensuksai, PhD
 Molecular characterization, detection & quantitation of biological products Purin Charoensuksai, PhD Department of Biopharmacy, Faculty of Pharmacy, Silpakorn University Example of critical checkpoints
Molecular characterization, detection & quantitation of biological products Purin Charoensuksai, PhD Department of Biopharmacy, Faculty of Pharmacy, Silpakorn University Example of critical checkpoints
Immunodiagnosis SCBM 343 CLINICAL PATHOLOGY. Lect. Dr. Witchuda Payuhakrit
 Immunodiagnosis SCBM 343 CLINICAL PATHOLOGY Lect. Dr. Witchuda Payuhakrit witchuda.pay@mahidol.ac.th Objectives 2 Understand the antigen and antibody interaction Understand the principle of immunoassay
Immunodiagnosis SCBM 343 CLINICAL PATHOLOGY Lect. Dr. Witchuda Payuhakrit witchuda.pay@mahidol.ac.th Objectives 2 Understand the antigen and antibody interaction Understand the principle of immunoassay
Lecture 24. Autoimmunity. Origins of autoimmunity. Diseases. Immune Diseases - Chapter 16 - Allergies - Autoimmunity - Immunodeficiency
 Lecture 24 Immune Diseases - Chapter 16 - Allergies - Autoimmunity - Immunodeficiency Disease Diagnostics - Chapter 17 - Sample Collections - Phenotypic Method - Genotypic Method - Immunological Method
Lecture 24 Immune Diseases - Chapter 16 - Allergies - Autoimmunity - Immunodeficiency Disease Diagnostics - Chapter 17 - Sample Collections - Phenotypic Method - Genotypic Method - Immunological Method
Multianalyte Assays with Algorithmic Analyses (MAAA)
 Mr. Glenn McGuirk Centers for Medicare and Medicaid Services 7500 Security Blvd. Baltimore, Maryland 21244 Docket # CMS-1441-N Dear Mr. McGuirk: The American Association for Clinical Chemistry (AACC) welcomes
Mr. Glenn McGuirk Centers for Medicare and Medicaid Services 7500 Security Blvd. Baltimore, Maryland 21244 Docket # CMS-1441-N Dear Mr. McGuirk: The American Association for Clinical Chemistry (AACC) welcomes
1) Western Blot: It`s based on Electrophoresis.
 Today we will begin our lecture with Immunoassays. They are methods to detect proteins in samples or in cells rather than to purify them. We have two methods: 1) Western Blot 2) ELIZA 1) Western Blot:
Today we will begin our lecture with Immunoassays. They are methods to detect proteins in samples or in cells rather than to purify them. We have two methods: 1) Western Blot 2) ELIZA 1) Western Blot:
CERTIFICATE OF ACCREDITATION
 CERTIFICATE OF ACCREDITATION In terms of section 22(2) (b) of the Accreditation for Conformity Assessment, Calibration and Good Laboratory Practice Act, 2006 (Act 19 of 2006), read with sections 23(1),
CERTIFICATE OF ACCREDITATION In terms of section 22(2) (b) of the Accreditation for Conformity Assessment, Calibration and Good Laboratory Practice Act, 2006 (Act 19 of 2006), read with sections 23(1),
Classification under the IVD Regulation
 Classification under the IVD Regulation Nick Baker Head of IVD Notified Body LRQA Ltd Improving performance, reducing risk IVD regulation shift in regulatory scrutiny Under the current IVD directive 10-15%
Classification under the IVD Regulation Nick Baker Head of IVD Notified Body LRQA Ltd Improving performance, reducing risk IVD regulation shift in regulatory scrutiny Under the current IVD directive 10-15%
Immunoglobulins. (1 of 2)
 Immunoglobulins (1 of 2) Immunoglobulins (Igs) = antibodies Each B cell synthesizes Igs of single specificity for a specific epitope B cell receptors (BCRs) are the Igs on B cell surface Humoral immunity
Immunoglobulins (1 of 2) Immunoglobulins (Igs) = antibodies Each B cell synthesizes Igs of single specificity for a specific epitope B cell receptors (BCRs) are the Igs on B cell surface Humoral immunity
Schedule of Accreditation issued by United Kingdom Accreditation Service 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK
 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK Haematology Department Contact: Ian Diton Conquest Hospital Tel: +44 (0) 1323 417400 ex 4127 The Ridge E-Mail: ian.diton@nhs.net St Leonards
2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK Haematology Department Contact: Ian Diton Conquest Hospital Tel: +44 (0) 1323 417400 ex 4127 The Ridge E-Mail: ian.diton@nhs.net St Leonards
Part 1 examination. Clinical Biochemistry: First paper. Tuesday 26 September Candidates must answer FOUR questions only
 Tuesday 26 September 2017 Candidates must answer FOUR questions only Time allowed: three hours 1. Outline how pre-analytical factors can affect the results of clinical biochemistry tests and describe what
Tuesday 26 September 2017 Candidates must answer FOUR questions only Time allowed: three hours 1. Outline how pre-analytical factors can affect the results of clinical biochemistry tests and describe what
Fluorescent in-situ Hybridization
 Fluorescent in-situ Hybridization Presented for: Presented by: Date: 2 Definition In situ hybridization is the method of localizing/ detecting specific nucleotide sequences in morphologically preserved
Fluorescent in-situ Hybridization Presented for: Presented by: Date: 2 Definition In situ hybridization is the method of localizing/ detecting specific nucleotide sequences in morphologically preserved
Clinical Biochemistry 3H03 Session 1
 Clinical Biochemistry 3H03 Session 1 Dr J. Macri Associate Professor Department of Pathology and Molecular Medicine Clinical Biochemist Hamilton Regional Laboratory Medicine Program Your Most Important
Clinical Biochemistry 3H03 Session 1 Dr J. Macri Associate Professor Department of Pathology and Molecular Medicine Clinical Biochemist Hamilton Regional Laboratory Medicine Program Your Most Important
CMS CDLT Panel Meeting, September 25, 2017 Page 1 of 5
 September 25, 2017* The maximum number of voting panel members was nine. For those votes for which the sum does not equal nine, we ve indicated that one or more panel members did not vote on that. 1. 80410
September 25, 2017* The maximum number of voting panel members was nine. For those votes for which the sum does not equal nine, we ve indicated that one or more panel members did not vote on that. 1. 80410
Radioimmunoassay. Presented by Dr. A. Suneetha Dept. of Pharm. Analysis Hindu College of Pharmacy
 Radioimmunoassay Presented by Dr. A. Suneetha Dept. of Pharm. Analysis Hindu College of Pharmacy Radioimmunoassay (RIA) is a very sensitive technique used to measure concentrations of antigens (for example
Radioimmunoassay Presented by Dr. A. Suneetha Dept. of Pharm. Analysis Hindu College of Pharmacy Radioimmunoassay (RIA) is a very sensitive technique used to measure concentrations of antigens (for example
The Cardiovascular System: Blood
 The Cardiovascular System: Blood The Functions of Blood General Overview Provides a system for rapid transport within the body Nutrients Hormones Waste products Respiratory gases Cells & Blood Components
The Cardiovascular System: Blood The Functions of Blood General Overview Provides a system for rapid transport within the body Nutrients Hormones Waste products Respiratory gases Cells & Blood Components
Competency 1: The student will demonstrate knowledge of the science of biotechnology
 Common Course Number: BSC-4420 Course Title: Biotechnology Catalog Course Description: This course will prepare students in the knowledge and proper use of laboratory techniques including but not limited
Common Course Number: BSC-4420 Course Title: Biotechnology Catalog Course Description: This course will prepare students in the knowledge and proper use of laboratory techniques including but not limited
IQCP ELIGIBILITY CROSSWALK TABLE
 IQCP ELIGIBILITY CROSSWALK TABLE: The first column lists those CLIA regulations (general QC and specialty/subspecialty) that are eligible for IQCP based on federal requirements. The second column designates
IQCP ELIGIBILITY CROSSWALK TABLE: The first column lists those CLIA regulations (general QC and specialty/subspecialty) that are eligible for IQCP based on federal requirements. The second column designates
CERTIFICATE OF ACCREDITATION
 CERTIFICATE OF ACCREDITATION In terms of section 22(2) (b) of the Accreditation for Conformity Assessment, Calibration and Good Laboratory Practice Act, 2006 (Act 19 of 2006), read with sections 23(1),
CERTIFICATE OF ACCREDITATION In terms of section 22(2) (b) of the Accreditation for Conformity Assessment, Calibration and Good Laboratory Practice Act, 2006 (Act 19 of 2006), read with sections 23(1),
BIOTECHNOLOGY P.G DIPLOMA Duration - 1 year MODULE I o INTRODUCTION TO GENERAL MICROBIOLOGY
 BIOTECHNOLOGY P.G DIPLOMA Duration - 1 year MODULE I o INTRODUCTION TO GENERAL MICROBIOLOGY o BASIC CONCEPTS Sterilization techniques Microbiological culture media and its importance Isolation of microorganisms
BIOTECHNOLOGY P.G DIPLOMA Duration - 1 year MODULE I o INTRODUCTION TO GENERAL MICROBIOLOGY o BASIC CONCEPTS Sterilization techniques Microbiological culture media and its importance Isolation of microorganisms
Exam MOL3007 Functional Genomics
 Faculty of Medicine Department of Cancer Research and Molecular Medicine Exam MOL3007 Functional Genomics Tuesday May 29 th 9.00-13.00 ECTS credits: 7.5 Number of pages (included front-page): 5 Supporting
Faculty of Medicine Department of Cancer Research and Molecular Medicine Exam MOL3007 Functional Genomics Tuesday May 29 th 9.00-13.00 ECTS credits: 7.5 Number of pages (included front-page): 5 Supporting
RE: Nebraska Methodist Hospital Pathology Center Special Bulletin ANNUAL NOTICE TO PHYSICIANS 2018
 February 1, 2018 RE: Nebraska Methodist Hospital Pathology Center Special Bulletin ANNUAL NOTICE TO PHYSICIANS 2018 Dear Partner, We are enclosing for your review the Annual Notice to Physicians for medical
February 1, 2018 RE: Nebraska Methodist Hospital Pathology Center Special Bulletin ANNUAL NOTICE TO PHYSICIANS 2018 Dear Partner, We are enclosing for your review the Annual Notice to Physicians for medical
CHAPTER 24. Immunology
 CHAPTER 24 Diagnostic i Microbiology and Immunology Growth-Dependent Diagnostic Methods Isolation of Pathogens from Clinical Specimens Proper sampling and culture of a suspected pathogen is the most reliable
CHAPTER 24 Diagnostic i Microbiology and Immunology Growth-Dependent Diagnostic Methods Isolation of Pathogens from Clinical Specimens Proper sampling and culture of a suspected pathogen is the most reliable
Prepared by Date Adopted Supersedes Procedure # Adapted from HPTN Policy. Review Date Revision Date Signature
 APPENDIX IV: LABORATORY QUALITY CONTROL POLICY Prepared by Date Adopted Supersedes Procedure # Adapted from HPTN Policy N/A Review Date Revision Date Signature Distributed to # of Copies Distributed to
APPENDIX IV: LABORATORY QUALITY CONTROL POLICY Prepared by Date Adopted Supersedes Procedure # Adapted from HPTN Policy N/A Review Date Revision Date Signature Distributed to # of Copies Distributed to
Supplementary Figure 1. Utilization of publicly available antibodies in different applications.
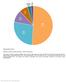 Supplementary Figure 1 Utilization of publicly available antibodies in different applications. The fraction of publicly available antibodies toward human protein targets is shown with data for the following
Supplementary Figure 1 Utilization of publicly available antibodies in different applications. The fraction of publicly available antibodies toward human protein targets is shown with data for the following
Strategies for Assessment of Immunotoxicology in Preclinical Drug Development
 Strategies for Assessment of Immunotoxicology in Preclinical Drug Development Rebecca Brunette, PhD Scientist, Analytical Biology SNBL USA Preclinical Immunotoxicology The study of evaluating adverse effects
Strategies for Assessment of Immunotoxicology in Preclinical Drug Development Rebecca Brunette, PhD Scientist, Analytical Biology SNBL USA Preclinical Immunotoxicology The study of evaluating adverse effects
HOST CELL PROTEIN & BIOPROCESSING REAGENT DEVELOPMENT
 HOST CELL PROTEIN & BIOPROCESSING REAGENT DEVELOPMENT INTRODUCTION Biopharmaceuticals require products to be free of residual host cell protein (HCP) contaminants from the bioprocessing workflow. To evaluate
HOST CELL PROTEIN & BIOPROCESSING REAGENT DEVELOPMENT INTRODUCTION Biopharmaceuticals require products to be free of residual host cell protein (HCP) contaminants from the bioprocessing workflow. To evaluate
Objectives. Understand and give examples of the problems which result from lack of comparability of patient results
 Objectives Understand and give examples of the problems which result from lack of comparability of patient results Understand the principles behind standardization and harmonization Understand the importance
Objectives Understand and give examples of the problems which result from lack of comparability of patient results Understand the principles behind standardization and harmonization Understand the importance
AUTOIMMUNE DISEASE ASSAYS
 product range 1 Corgenix PRODUCT RANGE 04-05 Antiphospholipid Syndrome Assays 06-10 AUTOIMMUNE DISEASE ASSAYS 11 Liver Disease Assays 11 Liver Fibrosis Markers 12 Cardiovascular Markers 12-13 Platelet
product range 1 Corgenix PRODUCT RANGE 04-05 Antiphospholipid Syndrome Assays 06-10 AUTOIMMUNE DISEASE ASSAYS 11 Liver Disease Assays 11 Liver Fibrosis Markers 12 Cardiovascular Markers 12-13 Platelet
How to Manage CMS Analyte Reporting Selections
 How to Manage CMS Analyte Reporting Selections What are CMS Analyte Reporting Selections? Proficiency testing (PT) is required for regulated analytes, as defined in Subpart I, Proficiency Testing Programs
How to Manage CMS Analyte Reporting Selections What are CMS Analyte Reporting Selections? Proficiency testing (PT) is required for regulated analytes, as defined in Subpart I, Proficiency Testing Programs
American Society of Cytopathology Core Curriculum in Molecular Biology
 American Society of Cytopathology Core Curriculum in Molecular Biology American Society of Cytopathology Core Curriculum in Molecular Biology Chapter 3 Molecular Techniques Fluorescence In Situ Hybridization
American Society of Cytopathology Core Curriculum in Molecular Biology American Society of Cytopathology Core Curriculum in Molecular Biology Chapter 3 Molecular Techniques Fluorescence In Situ Hybridization
Metabolomics: Techniques and Applications ABRF
 Metabolomics: Techniques and Applications ABRF Sacramento, CA March 23, 2010 Overview Metabolomics Definitions Representative Project Sarcosine, a prostatic cancer biomarker Technical overview/ How we
Metabolomics: Techniques and Applications ABRF Sacramento, CA March 23, 2010 Overview Metabolomics Definitions Representative Project Sarcosine, a prostatic cancer biomarker Technical overview/ How we
Step-by-Step Description of ELISA
 Step-by-Step Description of ELISA The protocols in this kit rely on indirect antibody capture ELISA. The steps in this assay are: Step 1: Antigen is added to the wells of the microplate strip and incubated
Step-by-Step Description of ELISA The protocols in this kit rely on indirect antibody capture ELISA. The steps in this assay are: Step 1: Antigen is added to the wells of the microplate strip and incubated
Fetal Screen Fetal screen Positive Kleihauer-Betke
 Reflex Test Protocols Reflex tests fall into 2 categories: standard industry practice (example: sensitivities, interpretations, and confirmations) and institutional practice based on staff clinical practice
Reflex Test Protocols Reflex tests fall into 2 categories: standard industry practice (example: sensitivities, interpretations, and confirmations) and institutional practice based on staff clinical practice
Schedule of Accreditation issued by United Kingdom Accreditation Service 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK
 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK Rapid Response Laboratory Contact: Jacqueline Sutherland North Middlesex University Hospital Tel: +44 (0) 207 307 7342 Sterling Way E-Mail:
2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK Rapid Response Laboratory Contact: Jacqueline Sutherland North Middlesex University Hospital Tel: +44 (0) 207 307 7342 Sterling Way E-Mail:
Proteomics Background and clinical utility
 Proteomics Background and clinical utility H.H. Helgason MD Antoni van Leeuwenhoek Hospital The Netherlands Cancer Institute Amsterdam Introduction Background Definitions Protein biomarkers Technical aspects
Proteomics Background and clinical utility H.H. Helgason MD Antoni van Leeuwenhoek Hospital The Netherlands Cancer Institute Amsterdam Introduction Background Definitions Protein biomarkers Technical aspects
CHEMILUMINESCENCE ENZYME IMMUNOASSAY (CLIA) THYROID STIMULATING HORMONE (FERRITIN) Ferritin
 DIAGNOSTIC AUTOMATION, INC. 21250 Califa Street, Suite 102 and 116, Woodland hills CA 91367 USA Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com
DIAGNOSTIC AUTOMATION, INC. 21250 Califa Street, Suite 102 and 116, Woodland hills CA 91367 USA Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com
Office of. Integrated Surveillance and Informatics Services (ISIS) Standard Operating Procedures
 Office of Integrated Surveillance and Informatics Services (ISIS) Standard Operating Procedures GUIDE TO EVENT CLASSIFICATION AND LABORATORY INTERPRETATION Q:\RESOURCES\ISIS SURVEILLANCE\Case Classification
Office of Integrated Surveillance and Informatics Services (ISIS) Standard Operating Procedures GUIDE TO EVENT CLASSIFICATION AND LABORATORY INTERPRETATION Q:\RESOURCES\ISIS SURVEILLANCE\Case Classification
PROTEINS. *Adapted from Biotechnology: Science for the New Millennium by Ellyn Daugherty.
 PROTEINS Most biotech products have something to do with proteins either containing amino acids or entire proteins. To manufacture protein products, researchers must understand protein structure and function.
PROTEINS Most biotech products have something to do with proteins either containing amino acids or entire proteins. To manufacture protein products, researchers must understand protein structure and function.
Electrophoresis. Assays... INTERLAB ASSAYS. Instrument. Software Easy data management thanks to innovative Elfolab software. General Characteristic
 Software Easy data management thanks to innovative Elfolab software. Instrument Complete walk-away automation. Initial 52 results available within 50 minutes. Impressive 208 Serum Proteins samples per
Software Easy data management thanks to innovative Elfolab software. Instrument Complete walk-away automation. Initial 52 results available within 50 minutes. Impressive 208 Serum Proteins samples per
Gold Np s in Diagnosis Invitro Diagnosis. Name : Veera CSR, Chittepu
 Gold Np s in Diagnosis Invitro Diagnosis Name : Veera CSR, Chittepu Out line Nanodiagnosis is defined as application of nanotechnology to research in Diagnosis. Gold particles usage in Diagnosis, which
Gold Np s in Diagnosis Invitro Diagnosis Name : Veera CSR, Chittepu Out line Nanodiagnosis is defined as application of nanotechnology to research in Diagnosis. Gold particles usage in Diagnosis, which
Microchip Electrophoresis for Glycoprotein Separation. Analytical Strategy, September 30th, 2014 Karina Hasler & Adrian Müller
 Microchip Electrophoresis for Glycoprotein Separation Analytical Strategy, September 30th, 2014 Karina Hasler & Adrian Müller Karina Hasler & Adrian Müller 30.09.2014 1 Content Glycoproteins Available
Microchip Electrophoresis for Glycoprotein Separation Analytical Strategy, September 30th, 2014 Karina Hasler & Adrian Müller Karina Hasler & Adrian Müller 30.09.2014 1 Content Glycoproteins Available
ARCHITECT i1000sr ARCHITECT i2000sr ChemWell x 59 x x 61 x " x 18.75" x 21.5" n high volume q low volume
 Immunoassay Analyzers Abbott Diagnostics Abbott Diagnostics Awareness Technology Inc Abbott Park, Ill (847) 937-6100 www.abbottdiagnostics.com Abbott Park, Ill (847) 937-6100 www.abbottdiagnostics.com
Immunoassay Analyzers Abbott Diagnostics Abbott Diagnostics Awareness Technology Inc Abbott Park, Ill (847) 937-6100 www.abbottdiagnostics.com Abbott Park, Ill (847) 937-6100 www.abbottdiagnostics.com
Specific Accreditation Guidance. Scope of accreditation - service descriptors for animal health
 Specific Accreditation Guidance Scope of accreditation - service descriptors for animal health January 2019 Copyright National Association of Testing Authorities, Australia 2018 This publication is protected
Specific Accreditation Guidance Scope of accreditation - service descriptors for animal health January 2019 Copyright National Association of Testing Authorities, Australia 2018 This publication is protected
Physiology Unit 3 HEMATOLOGY
 Physiology Unit 3 HEMATOLOGY In Physiology Today Hematocrit Percentage of blood volume that is erythrocytes 45% in men 42% in women Average blood volume in is 5 L Plasma Large amounts of organic/inorganic
Physiology Unit 3 HEMATOLOGY In Physiology Today Hematocrit Percentage of blood volume that is erythrocytes 45% in men 42% in women Average blood volume in is 5 L Plasma Large amounts of organic/inorganic
NEW YORK STATE LABORATORY PERMIT CATEGORY DESCRIPTIONS
 NEW YORK STATE DEPARTMENT OF HEALTH WADSWORTH CENTER CLINICAL LABORATORY EVALUATION PROGRAM EMPIRE STATE PLAZA, P.O. BOX 509 ALBANY, NEW YORK 12201-0509 Telephone: (518) 485-5378 FAX: (518) 485-5414 E-MAIL:
NEW YORK STATE DEPARTMENT OF HEALTH WADSWORTH CENTER CLINICAL LABORATORY EVALUATION PROGRAM EMPIRE STATE PLAZA, P.O. BOX 509 ALBANY, NEW YORK 12201-0509 Telephone: (518) 485-5378 FAX: (518) 485-5414 E-MAIL:
Quantitative Proteomics: From Technology to Cancer Biology
 Quantitative Proteomics: From Technology to Cancer Biology Beyond the Genetic Prescription Pad: Personalizing Cancer Medicine in 2014 February 10-11, 2014 Thomas Kislinger Molecular Biomarkers in Body
Quantitative Proteomics: From Technology to Cancer Biology Beyond the Genetic Prescription Pad: Personalizing Cancer Medicine in 2014 February 10-11, 2014 Thomas Kislinger Molecular Biomarkers in Body
