PEACH CREEK A NATURALLY ACIDIC DRAINAGE NEAR GIBBONS CREEK LIGNITE MINE, TEXAS 1. Jan K. Horbaczewski 2. Abstract
|
|
|
- Ada Haynes
- 6 years ago
- Views:
Transcription
1 PEACH CREEK A NATURALLY ACIDIC DRAINAGE NEAR GIBBONS CREEK LIGNITE MINE, TEXAS 1 by Jan K. Horbaczewski 2 Abstract Peach Creek near Gibbons Creek lignite mine in east-central Texas offers an example of a drainage that is naturally acidic as opposed to drainages that may be acidic due to mining activities. The acidity in Peach Creek is caused by the oxidation of iron disulfide (pyrite) that is present in the undisturbed geological formations. The pyrite appears to be exposed to oxidizing conditions where groundwater flow intersects surface topography. This is usually along tributaries of Peach Creek which are more incised rather than in the main channel of Peach Creek which is less incised. The oxidation of the pyrite results in low ph values and relatively high salinity with elevated sulfate concentrations. In some tributaries the ph is low enough to maintain ferric iron in solution. But the ph is increased when these waters mix with the less acid water in the main channel. Where the ph change is large enough, a characteristic reddish-brown precipitate of iron oxyhydroxides appears in the stream-bed below the confluence. Although aluminum was not analyzed for, there are indications that aluminum oxyhydroxides are also precipitated due to ph changes. The acidity is most evident at times of low flow when it is not diluted by rainfall runoff. Introduction The purpose of this paper is to review the naturally occurring acidity of Peach Creek, an undisturbed drainage in the vicinity of Texas Municipal Power Agency s (TMPA) Gibbons Creek lignite mine in eastcentral Texas. In particular, this paper examines how the acidity varies with time and with location in the Peach Creek drainage system and why these effects may not be evident in the course of routine baseline monitoring. As had been previously discussed (Horbaczewski, 2007), acid seeps and acid drainages occur naturally in the pre-mining condition in Texas lignite areas. These features were recognized by early settlers and have given rise to the numerous Sulphur Creeks and Sulphur Springs to be found on maps of these areas. The sulphur terminology probably arose from the hydrogen sulfide (H 2 S) smell of rotten eggs associated with these formations. But the other related sulfide in the lignite-bearing strata is iron disulfide or pyrite (FeS 2 ), also known as fools gold. The significance of the iron disulfide is that it is decomposed by atmospheric oxygen to form sulfuric acid that when concentrated may adversely impact plant and animal life. 1 Paper presented at the 30 th Annual Surface Mine Reclamation Workshop, College Station, Texas, October 1-2, Workshop sponsored by the Texas Cooperative Extension Service and the Texas Agricultural Experiment Station. 2 Jan K. Horbaczewski is Mine, Land, and Environmental Manager, Texas Municipal Power Agency, Bryan, TX janh@texasmpa.org 1
2 It should be noted that both the hydrogen sulfide and the iron disulfide are natural constituents of the local lignite-bearing geological formations. They are products of the same highly anaerobic reducing conditions in which vegetation was prevented from decaying and gradually became converted to lignite approximately 35 million years ago in the late Eocene of the Tertiary period. The Peach Creek Drainage Basin Peach Creek is located in Grimes County, Texas, east of the reclaimed Gibbons Creek mine and flows into Gibbons Creek just east of Farm-to-Market Road (FM) 244 and south of State Highway (SH) 30 (Figure 1). The entire Peach Creek watershed is approximately 6.4 miles long and covers an area of 5,397 acres (just over 8 square miles). The streambed falls 201 feet from elevation 413 feet above mean sea level (msl) to 212 feet above msl on average 32 feet per mile (Texas Municipal Power Agency, 1993a, Section.129 Surface Water Information). Figure 1 Map of Peach Creek drainage basin Peach Creek was studied intensively because mining activities were to move east across FM 244 into the Peach Creek drainage basin. But in 1996 TMPA switched from the relatively high-sulfur lignite of the Gibbons Creek mine to low-sulfur compliance coal from the Powder River Basin in Wyoming. The Gibbons Creek lignite mine was closed in February 1996 and the Peach Creek drainage was never disturbed by mining. Data from the baseline studies, however, remain and form the basis of this paper. 2
3 The surface water baseline studies were of two types: Surface water monitoring at downstream site SWPC2 Special-purpose survey along the main channel and tributaries of Peach Creek. Surface water monitoring at downstream site SWPC2 (MK004) Surface water monitoring station SWPC2 (Figure 2), also known as MK004, is located on the downstream end of Peach Creek, approximately 1,200 yards from its discharge into Gibbons Creek. Approximately 2,954 acres (4.6 square miles) of the Peach Creek watershed are upstream of monitoring station SWPC2 (MK004). Figure 2 Surface water monitoring station SWPC2 on Peach Creek Studies completed on the basis of data collected at this site include the following: Baseline characterization based on data collected between February 1989 and January 1990 Statistical analysis of data collected between March 1985 and November 1992 Time sequence of data collected between January 1990 and April 1994 Baseline water data collected between July 1991 and September 1992 Baseline characterization based on data collected between February 1989 and January 1990 A baseline characterization based on monthly monitoring at site SWPC2 over the period February 20, 1989 to January 20, 1990 was prepared for TMPA by Blackwell & Woods, Consulting Engineers (Texas Municipal Power Agency, 1991). The report found ph readings in the range ph 4.7 ph 6.2, Total Dissolved Solids (TDS) in the range 451 mgl -1 1,021 mgl -1. Analyses of major cations and anions were 3
4 not included. The monitoring period was recorded as one of severe drought and therefore no flow data were collected even though the monitoring station was inspected on a weekly basis. Statistical analysis of data collected between March 1985 and November 1992 A statistical analysis that included the chemistry of Peach Creek, based on sampling data from site SWPC2 over the period March 1985 to November 1992, was compiled by TMPA Staff in a report entitled Surface Water Quality Atlas for the Gibbons Creek/Navasota River Drainage Area (Texas Municipal Power Agency, 1993b). This analysis of variance covered 15 surface water monitoring stations in the area of the Gibbons Creek lignite mine and the Gibbons Creek Steam Electric Station. The database included the following parameters: Total Dissolved Solids (TDS), Total Suspended Solids (TSS), Silver (Ag), Arsenic (As), Barium (Ba), Cadmium (Cd), Chromium (Cr), Copper (Cu), Iron (Fe), Mercury (Hg), Manganese (Mn), Nickel (Ni), Lead (Pb), Selenium (Se), Zinc (Zn), and Fluoride (F). In comparison to the mean values of the data from all 15 stations, Peach Creek exhibited: Significantly higher TDS content, with average concentrations in excess of 500 mgl -1. Significantly higher Cr, Mn, Ni, and Zn contents. Significantly lower TSS and As contents. Time sequence of data collected between January 1990 and April 1994 A time sequence of ph data is available for the same surface water monitoring station SWPC2. These data cover the period January April The results are presented in Table 1 in relation to rainfall over the preceding week (as a surrogate for flow in the creek). Table 1 ph of water at monitoring station SWPC2 in relation to rainfall Date ph (s.u.) Rainfall* (in) 02/14/ /10/ /9/ /14/ /5/ /10/ /31/ /13/ /9/ /28/ /1/ /8/ /4/ /12/ /8/ /4/ /15/ /6/ * Total rainfall over preceding week Baseline water data collected between July 1991 and September 1992 The fourth study performed with data collected at monitoring station SWPC2 (MK004) included water chemistry and flow data that were submitted to the Railroad Commission of Texas in a permit application (Texas Municipal Power Agency, 1993a, Section.129 Surface Water Information). These data were 4
5 collected by Morrison Knudsen Corporation on a monthly basis over the period July 1991 September On a few occasions, the sampling was performed during storm events to test the variability of the chemistry with flow. The report made the following findings: Unlike the other drainages with intermittent flow, Peach Creek was determined to have continual flow at site MK004 (SWPC2). This was attributed to sources other than direct overland runoff from storm events, such as seeps or springs. The report concluded that they were fed by shallow groundwater due to rainfall infiltration. This conclusion is supported by the relationships between stream flow (in cubic feet per second cfs) and the chemistry of Peach Creek water. Although not perfect, the relationships show that as stream flow increases, the ph generally increases (Figure 3) and total dissolved salts (TDS) and sulfate concentrations generally decrease (Figure 4). This supports the theory that at times of low stream flow, saline (mostly sulfate) groundwater is the main component of the water, whereas at times of high flow, the main component is near-neutral, non-saline rainfall runoff. The study concluded that the elevated sulfate concentrations and the lower ph were probably due to oxidation of naturally occurring pyrite in the geologic strata within the Peach Creek drainage basin. Figure 3 Relationship between stream flow and ph at SWPC2 5
6 Figure 4 Relationships between stream flow and TDS and sulfate concentrations at SWPC2 Special-purpose survey along the main channel and tributaries of Peach Creek In order to better understand the baseline surface water chemistry observed at monitoring station SWPC2, it was decided in 1994 to perform a survey of the variability of ph and salinity along the main channel and tributaries of Peach Creek (Figure 1). This more detailed survey was performed by staff from TMPA, Morrison Knudsen Corporation and Navasota Mining Company on June 10, Analyses of ph and electrical conductivity (E.C.) were performed in the field and samples were delivered for more detailed analysis to Inter-Mountain Laboratories, Inc. (IML) of College Station, Texas. Partial surveys for verification purposes were performed on July 7, 1994 and July 29, Water Chemistry Changes at Three Confluences along Peach Creek In particular, samples of surface water were collected at three confluences of tributaries within the main Peach Creek channel (Figure 5). In this paper, they are described as the Upper Peach Creek Confluence (sampling Sites 8, 9, and 7), the Middle Peach Creek Confluence (Sites 12, 14, and 13), and the Lower Peach Creek Confluence (Sites 16, 15, and 17). The results of the analyses of samples collected on June 10, 1994 are summarized in Table 2. 6
7 Figure 5 Location of the three Peach Creek confluences sampled on June 10, 1994 Table 2 Chemistry of water at the three Peach Creek confluences on June 10, 1994 ph TDS Ca Mg K Na SO 4 Cl SO 4 Ca / Na / (field) / Cl Mg Cl (s.u.) (mgl -1 ) (meql -1 ) ratio Ca / SO 4 Site 8 (upstream) Site 9 (tributary) Site 7 (downstream) Calculated contribution from tributary Site 12 (upstream) Site 14 (tributary) Site 13 (downstream) Calculated contribution from tributary Site 16 (upstream) Site 15 (tributary) Site 17 (downstream) Calculated contribution from tributary Upper Peach Creek Confluence 4.1 2, , , % 19% 19% 0% 17% 16% 0% Middle Peach Creek Confluence 4.0 1, % 88%? 50% 89% 89% 89% Lower Peach Creek Confluence , % 11% 8% 0% 9% 7% 11%
8 The Upper Peach Creek Confluence is marked by the mixing of strongly acidic water (ph 3.5) from the tributary to the north (Site 9) with only slightly less acidic water (ph 4.1) in the main Peach Creek channel (Site 8) to give water of an intermediate acidity (ph 3.9) at Site 7 downstream of the confluence. This confluence is also characterized by the mixing of highly saline water from the tributary (TDS of 3,872 mgl -1 at Site 9) with moderately saline water in the main Peach Creek channel (TDS of 2,160 mgl -1 at Site 8). Based on the dilution of the TDS and of the major cations and anions, it is estimated that the proportion of flow contributed by the tributary was about 16% - 19% at the time of sampling. Almost half of the salinity of the water in the tributary is due to the sulfate ion alone. The sulfate to chloride ratio (at 3.7) is much higher than the general background level (around 1.5 or less), which suggests that the chemistry of the water in the tributary is strongly influenced by the products of oxidation of metal sulfides such as pyrite. The water is acidic because there is a deficit of calcium and other bases (cations) to balance the sulfate anion (the calcium to sulfate ratio is 0.5 or less). In contrast, the sodium to chloride ratio is close to 1.0 at most sites suggesting simple solution of pre-existing sodium chloride salt from the geological formation. Although analyses for iron were not run, it is evident that there must have been a considerable amount of iron in solution coming from the tributary. Mixing of the tributary water with the less acidic water from the Peach Creek channel upstream of the confluence (Figure 6) evidently caused precipitation of the iron resulting in the characteristic reddish-brown staining downstream of the confluence (Figure 7). Figure 6 Peach Creek sampling site 8 (upstream of confluence) 8
9 Figure 7 Peach Creek sampling site 7 (downstream of confluence) 9
10 In contrast, the Middle Peach Creek Confluence is marked by the mixing of almost neutral water (ph 6.7) from the tributary to the north (Site 14) with strongly acidic water (ph 4.0) in the main Peach Creek channel (Site 12) to give water of an intermediate acidity (ph 6.4) downstream of the confluence (Site 13). Based on the dilution of other constituents in the water, the proportion of flow contributed by the tributary was about 89% at the time of sampling. From all the chemical indicators, the effect of the tributary is to dilute and neutralize the acidity of the water in the main Peach Creek channel. The milky appearance of the water at Site 13 downstream of the mixing zone (Figure 8) may indicate the precipitation of aluminum hydroxide which, at the nearby Gibbons Creek mine, has been observed to occur in the range of ph 4.6 to ph 4.9 (Horbaczewski, 2001; Horbaczewski, 2006). Figure 8 Middle Peach Creek confluence (photograph taken from Site 13; Site 14 on tributary to the left; Site 12 in main channel to the right) The Lower Peach Creek Confluence marks another ph reversal. Strongly acidic water (ph 3.8) from the tributary to the south (Site 15) mixes with very slightly acidic water (ph 5.9) in the main Peach Creek channel (Site 16) to give water of an intermediate acidity (ph 5.1) downstream of the confluence at Site 17 (Figure 9). Based on the dilution of other constituents in the water, the proportion of flow contributed by the tributary is estimated at 7% - 12% at the time of sampling. Just as at the Middle Peach Creek Confluence, the milky appearance of the water at Site 17 may be due to the precipitation of aluminum hydroxide. In summary, the water in the main channel of Peach Creek at the time of sampling displayed major changes in chemistry as it flowed past each of the three tributaries described. There may have been more such changes that were missed in the survey. For example, the upper reaches of the main channel showed nearneutral ph values (ph 6.2 at Site 5 and ph 5.9 a few hundred yards farther downstream at Site 6). However, by the time the water flow reached Site 8 just above the Upper Peach Creek Confluence it was already down to ph 4.1. Similar ph changes may also occur along the tributaries themselves. 10
11 Figure 9 Lower Peach Creek confluence (photograph taken from Site 17; Site 16 on main channel to the left; Site 15 on tributary to the right) Variations in ph and E.C. with time The initial sampling was performed on June 10, 1994, and was partially repeated a few weeks later on July 7 and July 29, The changes in ph and E.C. over that period are summarized in Table 3. Table 3 ph and E.C. data from Peach Creek surveys Sampling site ph (standard units) Electrical Conductivity (mmho/cm) 06/10/94 07/7/94 07/29/94 06/10/94 07/7/94 07/29/ (f) 4.7 (f) 4.6 (f) (l) 4.4 (l) (f) 3.0 (f) 4.2 (f) (l) 4.0 (l) (f) 4.0 (f) 4.0 (f) (l) 3.7 (l) (f) 3.4 (f) 3.5 (f) (l) 3.3 (l) (f) (l) (f) (l) (f) (l) (f) 3.4 (f) 2.9 (f) (l) 3.6 (l) 3.5 (l) (f) 6.1 (f) 4.8 (f) (l) 6.1 (l) 6.4 (l) (f) 5.1 (l) 4.9 (f) 4.4 (l) 4.4 (f) 4.5 (l) Notes: (f) field determination of ph. (l) laboratory determination of ph (performed by IML on the same day as sampled). 11
12 In general, the data are consistent with some slight decreases in ph accompanied by slight increases in E.C., suggesting less dilution by rainfall runoff as the summer progressed. Interpretation The following discussion is an interpretation of the data presented earlier. Source of stream flow Stream flow may be composed of two major sources surface water runoff from rainfall and base flow from groundwater discharges. At the time of the survey on June 10, 1994, there had not been any significant rain for almost a month (Table 4) and flow rates were low, as evident at Site 8 (Figure 10). The flow at SWPC2 is estimated to have been less than 0.5 cfs on that day (approximately 225 gallons per minute gpm), compared to a flow of 8.31 cfs (3,750 gpm) measured during a storm event on February 27, 1992 (Texas Municipal Power Agency, 1993a, Section.129 Surface Water Information). Table 4 Rainfall distribution for January-June 1994 Date Rainfall (inches) 01/11/ /13/ /17/ /24/ /27/ /28/ /30/ /10/ /11/ /21/ /23/ /2/ /7/ /9/ /16/ /28/ /6/ /12/ /16/ /20/ /25/ /3/ /11/ /14/ /16/ /29/ /30/ /3/ /13/ /15/ /20/ /21/ /23/ Total January-June Note: Rainfall events greater than 1 inch shown in bolded type. 12
13 Figure 10 Evidence of low flow over rock ledge at Site 8 on June 10, 1994 As already discussed, the combination of low ph and relatively high sulfate concentrations suggests a groundwater origin for some of the stream flow. A groundwater baseline investigation performed by Morrison Knudsen Corporation over the period August 1991 to June 1992 suggested three potential sources of groundwater in the Peach Creek area the 4525 aquifer (Yuma sand), the 5525 sand and the 5527 sand (Texas Municipal Power Agency, 1993a, Section.146 Probable Hydrologic Consequences). The 4525 sand was considered to be the most probable source because it is sufficiently well developed for the mapping of a potentiometric surface and the derivation of a groundwater flow direction; the 5525 and 5527 sands were discontinuous and did not constitute mappable aquifer units. Studies of the 4525 aquifer showed that the groundwater flow was generally to the east. However, this eastward flow was strongly controlled by significant structural faults. For example, the strike-oriented fault running along the down-dip edge of the P5 Mine Block has a vertical displacement of 150 feet (with a downthrow to the north-west). The channeling of the groundwater flow results in an elevated potentiometric surface, which the study found to intersect, and to discharge into, a surface water drainage east of the mine block. The geography of the area suggests that this is probably the same acidic tributary, sampled at Site 9, that drains to the Upper Peach Creek Confluence. Generation of acidity Thus, the generation of acidity in the Peach Creek system is probably different to that previously described (Horbaczewski, 2007): Normally, the natural oxidation of pyrite in geological formations occurs from the surface downwards. Its progress is determined by the rate at which atmospheric oxygen and rain water can advance through the resisting geological formations. A fractionation of sorts develops resulting in a geochemical profile, one of 13
14 the most striking features of which is an oxidation-reduction ( redox ) boundary at a depth of about 27 feet. Geological materials above the redox boundary have a characteristic tan, brown, or red coloration indicative of oxidized (ferric) iron (Fe 3+ ) oxy-hydroxides and decomposed organic matter. Geological materials below the redox boundary have dark greenish-gray colors due to the presence of reduced (ferrous) iron (Fe 2+ ) and undecomposed organic matter, including lignite seams. But in the Peach Creek situation, there is an additional complication in which percolating rainwater mixes with discharging groundwater in the vicinity of the redox boundary. The generation of acidity suggests that there is fresh pyritic material available for oxidation and this must mean that the reactions are occurring above a redox boundary. On the other hand, oxidation cannot occur if conditions are reducing as in anaerobic conditions where there is saturation with water. This suggests that a relationship as shown in Figure 11 may be present with a water table or potentiometric surface underlying a redox boundary. However, this is no more than an interpretation and has not been tested in the field. Figure 11 Interpretation of relationship between water table/potentiometric surface and redox boundary 14
15 Neutralization of acidity The acidity generated at various sites in the Peach Creek system was also neutralized or partly neutralized as it flowed past various confluences where it mixed with water of different ph. In some cases, the ph changes crossed the critical ph ranges or fences for the precipitation of iron hydroxides and aluminum hydroxides. These ranges (Figure 12) were derived earlier from observations of the precipitation of these minerals at the nearby Gibbons Creek lignite mine (Horbaczewski, 2001). Interpretation of this graph suggests the following: At the Upper Peach Creek Confluence very acidic water (ph 3.5) from the tributary (Site 9) mixed with less acidic water (ph 4.1) from the main channel (Site 8). Downstream of this confluence (Site 7 with ph 3.9), there is visible evidence of precipitation of iron oxyhydroxides from the reddish-brown staining of the stream-bed. The ph fence for iron hydroxide precipitation is in the general range of ph ph 3.4. This suggests that, at times, the ph of the water from the tributary had a ph lower than this range, which is consistent with the laboratory testing of a sample collected on July 29, 1994 that showed ph 3.3 (Table 3). The water downstream of the Upper Peach Creek Confluence was still sufficiently acidic to stay below the aluminum hydroxide fence so aluminum was able to stay in solution. Figure 12 ph profile of Peach Creek vs. precipitation ranges of iron and aluminum oxyhydroxides Note: The ph ranges of the Al(OH) 3 and Fe(OH) 3 fences are based on Horbaczewski (2001) However, at the Middle Peach Creek Confluence, the injection of nearly neutral (ph 6.7) water from a tributary (Site 14) raises the downstream acidity to ph 6.4 (Site 13), crossing the aluminum hydroxide fence and apparently precipitating aluminum hydroxide from solution as evidenced in the milky appearance of the water (Figure 8). 15
16 At the Lower Peach Creek Confluence, there is another addition of very acidic (ph 3.8) water from a tributary (Site 15). This lowers the ph of Peach Creek from ph 5.9 (Site 16) to ph 5.1 (Site 17). The water from the tributary crosses the aluminum hydroxide fence and that may account for the somewhat milky appearance of the water at Site 17 (Figure 9). It is possible that water in the tributary had even lower ph values to start with but had become partly neutralized even before reaching the confluence. Finally, the water in Peach Creek appears to have received some more acidic drainage because by the time it reaches monitoring station SWPC2 it is at ph 4.6. At this ph aluminum becomes soluble again. Thus, in just the three confluences observed, the water chemistry crossed the iron hydroxide fence at least once and possibly twice, and the aluminum hydroxide fence at least three times and possibly four times. There may have been additional crossings of this nature within other segments of the Peach Creek drainage. The chemistry of the water at monitoring station SWPC2 is therefore a composite of many reactions, which have been masked by numerous neutralizations and dilutions. Conclusions The following conclusions may be drawn from this investigation: Peach Creek is fed by groundwater as well as rainfall runoff. The groundwater contribution from some of the tributaries is very acidic (less than ph 3.5). Water was acidic enough to hold iron and aluminum in solution. Mixing with water of higher phs at various confluences caused the precipitation of visible iron hydroxides and presumed aluminum hydroxides. The water that finally reached the monitoring station SWPC2 was a composite of at least several reactions resulting from partial neutralizations and dilutions. The data from station SWPC2 were therefore difficult to interpret. The acidity is due to natural oxidation of pyrite in the geological formations. Oxidation of pyrite is known to generate water of even greater acidity than that observed in this study. It is possible that more detailed investigations would identify the sources of the acid drainage and their original ph values. Acknowledgments Special thanks are due to Dr. Lloyd Hossner who reviewed an earlier paper on acid seeps (Horbaczewski, 2007) and encouraged further investigation. The special survey conducted on June 10, 1994 was performed by Eric Lancaster, Wayne Godsey, Tom Carter, and Jan Horbaczewski. Figures 1, 3, 4, 10, and 11 were drafted at TMPA by Rachel Brandt, with assistance from Joe Garcia (Figure 10) and Morriss Barney (Figures 3 and 4). The photographs were taken by the author. References Horbaczewski, J. K. (2001) Neutralization of acid mine pit water at Gibbons Creek lignite mine. Society for Mining, Metallurgy, and Exploration, Inc., Transactions 2001, Vol. 310, pp
17 Horbaczewski, J. K. (2006) Aluminum precipitation in acidic pit lakes at the Gibbons Creek lignite mine, Texas, USA: Field observations vs. laboratory simulations, pp In: Proceedings of the 7 th International Conference on Acid Rock Drainage (ICARD), March 26-30, 2006, St. Louis, MO. R. I. Barnhisel (ed.) Published by the American Society of Mining and Reclamation (ASMR), 3134 Montavesta Road, Lexington, KY Horbaczewski, J. K. (2007) Mine-related and natural acid seeps at Gibbons Creek Lignite Mine, Texas. Paper presented at the 28 th Annual Surface Mine Reclamation Workshop, College Station, Texas. October 4-5, Texas Cooperative Extension and Texas Agricultural Experiment Station. Texas Municipal Power Agency (1991) GCLM IV Mining Revision Application, Section.129 (Surface Hydrology). Prepared for TMPA by Blackwell & Woods, March Texas Municipal Power Agency (1993a) GCLM V Surface Mining and Reclamation Permit Application. Prepared for TMPA by Morrison Knudsen Corporation, March Texas Municipal Power Agency (1993b) Surface Water Quality Atlas for the Gibbons Creek/Navasota River Drainage Area, between March 1985 and November
Pebble Project Surface Water Quality Program Streams, Seeps, and Ponds
 Day1_1445_Surface Water Quality_PLP_McCay.mp3 Pebble Project Surface Water Quality Program Streams, Seeps, and Ponds Mark Stelljes SLR International February 1, 2012 Outline Stream Program Overview Results
Day1_1445_Surface Water Quality_PLP_McCay.mp3 Pebble Project Surface Water Quality Program Streams, Seeps, and Ponds Mark Stelljes SLR International February 1, 2012 Outline Stream Program Overview Results
EPA Primary. (mg/l as CaCO3) (mg/l as CaCO3)
 NORTH TEXAS MUNICIPAL WATER DISTRICT - Wylie Water Analysis Jan-2018 Mineral Analysis Raw Treated Standards Residue on Evaporation 412 456 500 1000 Silica (SiO2) 3.63 3.41 Iron (Fe) 0.378 0.259 0.3 0.3
NORTH TEXAS MUNICIPAL WATER DISTRICT - Wylie Water Analysis Jan-2018 Mineral Analysis Raw Treated Standards Residue on Evaporation 412 456 500 1000 Silica (SiO2) 3.63 3.41 Iron (Fe) 0.378 0.259 0.3 0.3
Lecture 1: Introduction
 Islamic University of Gaza Environmental Engineering Department Water Treatment EENV 4331 Lecture 1: Introduction Dr. Fahid Rabah 1 1.1 Water Cycle and Water Resources 2 1.2 Water Distribution on Earth
Islamic University of Gaza Environmental Engineering Department Water Treatment EENV 4331 Lecture 1: Introduction Dr. Fahid Rabah 1 1.1 Water Cycle and Water Resources 2 1.2 Water Distribution on Earth
Warm Mineral Springs Sampling by Sarasota County
 Warm Mineral Springs Sampling by Sarasota County John Ryan, Kathryn Meaux, Rene Janneman and Jon S. Perry Sarasota County Environmental Services Sarasota, Florida September 11 Warm Mineral Springs is a
Warm Mineral Springs Sampling by Sarasota County John Ryan, Kathryn Meaux, Rene Janneman and Jon S. Perry Sarasota County Environmental Services Sarasota, Florida September 11 Warm Mineral Springs is a
Clyde Mine Discharge/Tenmile Creek Water Quality Final Report
 Clyde Mine Discharge/Tenmile Creek Water Quality Final Report November 01, 2016 Background In follow-up to the Pennsylvania Department of Environmental Protection s (DEP) December 15, 2015, Tenmile Creek
Clyde Mine Discharge/Tenmile Creek Water Quality Final Report November 01, 2016 Background In follow-up to the Pennsylvania Department of Environmental Protection s (DEP) December 15, 2015, Tenmile Creek
Globeville Landing Outfall Surface Water. December 12, 2017 Andrew Ross, Jon Novick Denver Department of Public Health & Environment
 Globeville Landing Outfall Surface Water December 12, 2017 Andrew Ross, Jon Novick Denver Department of Public Health & Environment Introductions Andrew Ross Environmental Program Manager, DDPHE Jon Novick
Globeville Landing Outfall Surface Water December 12, 2017 Andrew Ross, Jon Novick Denver Department of Public Health & Environment Introductions Andrew Ross Environmental Program Manager, DDPHE Jon Novick
2.0 Scope of Work. 3.0 Stream Discharge Measurements. Technical Memorandum City of Farmers Branch Page 2
 Technical Memorandum City of Farmers Branch Page 2 over the No. 1 dam. Discharge of commingled water from the reservoirs must be of sufficient quality to meet the Surface Water Quality Standards of Segment
Technical Memorandum City of Farmers Branch Page 2 over the No. 1 dam. Discharge of commingled water from the reservoirs must be of sufficient quality to meet the Surface Water Quality Standards of Segment
Peach Creek Watershed
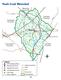 SH 304 Peach Creek Watershed Bastrop Peach Cree FM 713 Caldwell Peach Creek, Sandy Fork Copperas Creek 1803C Peach Creek Fayette FM 1054 US 90 Waelder US IH US 90 FM 1680 IH 10 Dry Run Gonzales SH 97 Peach
SH 304 Peach Creek Watershed Bastrop Peach Cree FM 713 Caldwell Peach Creek, Sandy Fork Copperas Creek 1803C Peach Creek Fayette FM 1054 US 90 Waelder US IH US 90 FM 1680 IH 10 Dry Run Gonzales SH 97 Peach
Golden Sunlight Mine Bio-Treatment of Acid Producing Waste. By Rory Tibbals Operations Superintendent
 Golden Sunlight Mine Bio-Treatment of Acid Producing Waste By Rory Tibbals Operations Superintendent Golden Sunlight Mine Gold Producing Mine 2.5 Million Ounces Produced 20 Year Operation Ore and All
Golden Sunlight Mine Bio-Treatment of Acid Producing Waste By Rory Tibbals Operations Superintendent Golden Sunlight Mine Gold Producing Mine 2.5 Million Ounces Produced 20 Year Operation Ore and All
Impact of Coal Mine Reclamation Using Coal Combustion By-products (CCBs) on Groundwater Quality: Two Case Studies
 Impact of Coal Mine Reclamation Using Coal Combustion By-products (CCBs) on Groundwater Quality: Two Case Studies C.-M. Cheng 1,, T. Butalia 1, W. Wolfe 1, R. Baker 1 N. Yencho 1, N. Mauger 1, J. Massey-Norton
Impact of Coal Mine Reclamation Using Coal Combustion By-products (CCBs) on Groundwater Quality: Two Case Studies C.-M. Cheng 1,, T. Butalia 1, W. Wolfe 1, R. Baker 1 N. Yencho 1, N. Mauger 1, J. Massey-Norton
Mount Polley Mining Corporation an Imperial Metals company Box 12 Likely, BC V0L 1N0 T F
 Mount Polley Mining Corporation an Imperial Metals company Box 12 Likely, BC V0L 1N0 T 250.790.2215 F 250.790.2613 November 24, 2016 Ministry of Environment Mining Operations Environmental Protection 2080
Mount Polley Mining Corporation an Imperial Metals company Box 12 Likely, BC V0L 1N0 T 250.790.2215 F 250.790.2613 November 24, 2016 Ministry of Environment Mining Operations Environmental Protection 2080
Summary of Preliminary Results of EPA Remedial Investigation Molycorp molybdenum mine Questa, New Mexico
 Summary of Preliminary Results of EPA Remedial Investigation Molycorp molybdenum mine Questa, New Mexico Prepared by Steve Blodgett, Technical Advisor Rio Colorado Reclamation Committee Introduction On
Summary of Preliminary Results of EPA Remedial Investigation Molycorp molybdenum mine Questa, New Mexico Prepared by Steve Blodgett, Technical Advisor Rio Colorado Reclamation Committee Introduction On
PIT LAKES LIABILITY OR LEGACY? David Allen (MBS Environmental) Karen Ganza (MBS Environmental) Rob Garnham (Groundwater Resource Management)
 PIT LAKES LIABILITY OR LEGACY? David Allen (MBS Environmental) Karen Ganza (MBS Environmental) Rob Garnham (Groundwater Resource Management) PRESENTATION OUTLINE Introduction Examples of Pit Lakes Important
PIT LAKES LIABILITY OR LEGACY? David Allen (MBS Environmental) Karen Ganza (MBS Environmental) Rob Garnham (Groundwater Resource Management) PRESENTATION OUTLINE Introduction Examples of Pit Lakes Important
Flambeau Mine On-Site Surface Water Monitoring Program (See Appendix I for maps with site locations)
 Flambeau Mine On-Site Surface Water Monitoring Program (See Appendix I for maps with site locations) Controlling Document and Specifications COC Stipulation Monitoring Plan (Dec 2007) Frequency: Spring/Fall,
Flambeau Mine On-Site Surface Water Monitoring Program (See Appendix I for maps with site locations) Controlling Document and Specifications COC Stipulation Monitoring Plan (Dec 2007) Frequency: Spring/Fall,
An Evaluation of Recent Allegations of Trace Metal Contamination At the McDermott and Ernest Mine Sites
 An Evaluation of Recent Allegations of Trace Metal Contamination At the McDermott and Ernest Mine Sites Tim Kania, P.G. 4/14/2009 Introduction At a November 13, 2008 meeting at the Rachel Carson State
An Evaluation of Recent Allegations of Trace Metal Contamination At the McDermott and Ernest Mine Sites Tim Kania, P.G. 4/14/2009 Introduction At a November 13, 2008 meeting at the Rachel Carson State
A Semi-Passive Bioreactor for Treatment of a Sulfate and Metals Contaminated Well Field Nacimiento Mine, New Mexico
 A Semi-Passive Bioreactor for Treatment of a Sulfate and Metals Contaminated Well Field Nacimiento Mine, New Mexico Timothy K. Tsukamoto, Ph.D. tkttim@gmail.com Why Semi-passive? PASSIVE ACTIVE Limestone
A Semi-Passive Bioreactor for Treatment of a Sulfate and Metals Contaminated Well Field Nacimiento Mine, New Mexico Timothy K. Tsukamoto, Ph.D. tkttim@gmail.com Why Semi-passive? PASSIVE ACTIVE Limestone
APPENDIX 15-L SEDIMENT QUALITY MODELLING
 APPENDIX 15-L SEDIMENT QUALITY MODELLING TM Memorandum DATE: January 31, 2013 TO: FROM: SUBJECT: Michael Henry and Chris Burns Kelsey Norlund, Lesley Shelley, and Brenda Bailey KSM Project Loadings and
APPENDIX 15-L SEDIMENT QUALITY MODELLING TM Memorandum DATE: January 31, 2013 TO: FROM: SUBJECT: Michael Henry and Chris Burns Kelsey Norlund, Lesley Shelley, and Brenda Bailey KSM Project Loadings and
Dynamic Waste Water Modeling for Coal Burning Power Plants
 2017 World of Coal Ash (WOCA) Conference in Lexington, KY - May 9-11, 2017 http://www.flyash.info/ Dynamic Waste Water Modeling for Coal Burning Power Plants Greg P. Behrens 1, Mike T. Damian 2, and Steven
2017 World of Coal Ash (WOCA) Conference in Lexington, KY - May 9-11, 2017 http://www.flyash.info/ Dynamic Waste Water Modeling for Coal Burning Power Plants Greg P. Behrens 1, Mike T. Damian 2, and Steven
9. Cadmium, Cd (atomic no. 48)
 9. Cadmium, Cd (atomic no. 48) - Cd is comparatively rare in the environment - Median concentrations of Cd in soils and sediments range from about 0.04 to 1.8 mg/kg. - Cadmium concentrations are elevated
9. Cadmium, Cd (atomic no. 48) - Cd is comparatively rare in the environment - Median concentrations of Cd in soils and sediments range from about 0.04 to 1.8 mg/kg. - Cadmium concentrations are elevated
RIMINI ROAD IMPROVEMENT PROJECT RESTORATION OF ABANDONED MINE SITES PROJECT DRAFT REPORT
 RIMINI ROAD IMPROVEMENT PROJECT RESTORATION OF ABANDONED MINE SITES PROJECT DRAFT REPORT Prepared by U.S. Army Corps of Engineers Omaha District Omaha, Nebraska August 2004 Table of Contents 1 Introduction...
RIMINI ROAD IMPROVEMENT PROJECT RESTORATION OF ABANDONED MINE SITES PROJECT DRAFT REPORT Prepared by U.S. Army Corps of Engineers Omaha District Omaha, Nebraska August 2004 Table of Contents 1 Introduction...
A Hydrologic Study of the North Hills, Helena, Montana
 A Hydrologic Study of the North Hills, Helena, Montana Andrew Bobst, Kirk Waren, James Swierc, and Jane Madison MBMG Groundwater Investigations Program 10/6/11 MT AWRA Background Groundwater Steering Committee
A Hydrologic Study of the North Hills, Helena, Montana Andrew Bobst, Kirk Waren, James Swierc, and Jane Madison MBMG Groundwater Investigations Program 10/6/11 MT AWRA Background Groundwater Steering Committee
Special Publication SJ2004-SP22 St. Johns River Water Supply Project Surface Water Treatment and Demineralization Study
 Special Publication SJ2004-SP22 St. Johns River Water Supply Project Surface Water Treatment and Demineralization Study Preliminary Raw Water Characterization TECHNICAL MEMORANDUM PRELIMINARY RAW WATER
Special Publication SJ2004-SP22 St. Johns River Water Supply Project Surface Water Treatment and Demineralization Study Preliminary Raw Water Characterization TECHNICAL MEMORANDUM PRELIMINARY RAW WATER
Draft Fact Sheet Butte County Stable Isotope Recharge Study
 Agenda Item #4 Draft Fact Sheet Butte County Stable Isotope Recharge Study Purpose of the Study: To develop a better understanding of how various water sources contribute to recharge of Butte County groundwater.
Agenda Item #4 Draft Fact Sheet Butte County Stable Isotope Recharge Study Purpose of the Study: To develop a better understanding of how various water sources contribute to recharge of Butte County groundwater.
THE ABATEMENT PLAN. beneficial abatement plan. For the purpose of defining the pollution potential of surface waters
 THE ABATEMENT PLAN GENERAL PLAN Preventing surface waters from entering deep mine workings apparently offers the most beneficial abatement plan. For the purpose of defining the pollution potential of surface
THE ABATEMENT PLAN GENERAL PLAN Preventing surface waters from entering deep mine workings apparently offers the most beneficial abatement plan. For the purpose of defining the pollution potential of surface
Evaluating acid rock drainage from road cuts in Tennessee
 Evaluating acid rock drainage from road cuts in Tennessee Michael Bradley, U.S. Geological Survey In cooperation with Tennessee Department of Transportation 14th Annual Technical Forum Geohazards Impacting
Evaluating acid rock drainage from road cuts in Tennessee Michael Bradley, U.S. Geological Survey In cooperation with Tennessee Department of Transportation 14th Annual Technical Forum Geohazards Impacting
QUESTIONNAIRE FOR GENERAL WTP REQUESTED DATA FOR THE WATER TREATMENT PLANT (WTP) - Application : groundwater (well pit); urban wastewater;
 REQUESTED DATA FOR THE WATER TREATMENT PLANT (WTP) - Application : ENVIRONMENTAL DATA - Temperature min. : C - Temperature max. : C - Altitude (m.a.s.l) : m - Plant location (geographic area, seismic zone,
REQUESTED DATA FOR THE WATER TREATMENT PLANT (WTP) - Application : ENVIRONMENTAL DATA - Temperature min. : C - Temperature max. : C - Altitude (m.a.s.l) : m - Plant location (geographic area, seismic zone,
Butte Highlands Joint Venture Mine Design, Operations, and Closure Conference Mine Water Treatment Short Course
 Butte Highlands Joint Venture Mine Design, Operations, and Closure Conference Mine Water Treatment Short Course May 3, 2015 Butte Highlands Joint Venture Highland Mining (ISR Capital) ISR Capital a private
Butte Highlands Joint Venture Mine Design, Operations, and Closure Conference Mine Water Treatment Short Course May 3, 2015 Butte Highlands Joint Venture Highland Mining (ISR Capital) ISR Capital a private
CANADA-BRITISH COLUMBIA WATER QUALITY MONITORING AGREEMENT
 CANADA-BRITISH COLUMBIA WATER QUALITY MONITORING AGREEMENT WATER QUALITY ASSESSMENT OF THE ISKUT RIVER BELOW JOHNSON RIVER, BRITISH COLUMBIA (199 7) PREPARED BY: T. C. E. DESSOUKI B.C. MINISTRY OF ENVIRONMENT
CANADA-BRITISH COLUMBIA WATER QUALITY MONITORING AGREEMENT WATER QUALITY ASSESSMENT OF THE ISKUT RIVER BELOW JOHNSON RIVER, BRITISH COLUMBIA (199 7) PREPARED BY: T. C. E. DESSOUKI B.C. MINISTRY OF ENVIRONMENT
FGD as a Soil Amendment for Mine Reclamation Warren A. Dick
 FGD as a Soil Amendment for Mine Reclamation Warren A. Dick School of Environment and Natural Resources Ohio State University, Wooster, OH 220-263-3877; dick.5@osu.edu FGD Properties of Value for Mineland
FGD as a Soil Amendment for Mine Reclamation Warren A. Dick School of Environment and Natural Resources Ohio State University, Wooster, OH 220-263-3877; dick.5@osu.edu FGD Properties of Value for Mineland
Backfilled Pits Laboratory-scale Tests for Assessing Impacts on Groundwater Quality
 Page 1 This paper was first presented at AusIMM Life-of-Mine Conference in Brisbane, Australia on 28-30 September, 2016. Introduction Closure options under consideration at some sites include backfilling
Page 1 This paper was first presented at AusIMM Life-of-Mine Conference in Brisbane, Australia on 28-30 September, 2016. Introduction Closure options under consideration at some sites include backfilling
PROTECTING GROUNDWATER IN MINING AREAS. MME Workshop Duluth 2015 Bruce Olsen
 PROTECTING GROUNDWATER IN MINING AREAS MME Workshop Duluth 2015 Bruce Olsen WHY PROTECT GROUNDWATER? Principal Source of Drinking Water in MN Recharges Many Lakes and Streams Extensively Used to Irrigate
PROTECTING GROUNDWATER IN MINING AREAS MME Workshop Duluth 2015 Bruce Olsen WHY PROTECT GROUNDWATER? Principal Source of Drinking Water in MN Recharges Many Lakes and Streams Extensively Used to Irrigate
Water Management in Queensland Coal Seam Gas. John Walsh, PhD CETCO Energy Services
 Water Management in Queensland Coal Seam Gas John Walsh, PhD CETCO Energy Services Background: Water Management in the Coal Seam Gas industry in Queensland is a complex and expensive undertaking Huge pipeline
Water Management in Queensland Coal Seam Gas John Walsh, PhD CETCO Energy Services Background: Water Management in the Coal Seam Gas industry in Queensland is a complex and expensive undertaking Huge pipeline
Contaminated Extraction Pit treated to allow dewatering for continuation of mining works
 Contaminated Extraction Pit treated to allow dewatering for continuation of mining works Name Customer name withheld Site Location South East Queensland Site Problem ph, Acidity and Dissolved Metals Water
Contaminated Extraction Pit treated to allow dewatering for continuation of mining works Name Customer name withheld Site Location South East Queensland Site Problem ph, Acidity and Dissolved Metals Water
Base Metal and Iron Ore Mining
 Multilateral Investment Guarantee Agency Environmental Guidelines for Base Metal and Iron Ore Mining Industry Description and Practices This document addresses the mining of base metal ores (copper, lead
Multilateral Investment Guarantee Agency Environmental Guidelines for Base Metal and Iron Ore Mining Industry Description and Practices This document addresses the mining of base metal ores (copper, lead
WEF Collection Systems Conference Evaluation of High ph Infiltration Water and Recycled Bedding Material
 Evaluation of High ph Infiltration Water and Recycled Bedding Material Keith Hobson, PE, BCEE, FOX Engineering Associates, Inc. ABSTRACT The purpose of this evaluation was to determine the cause and source
Evaluation of High ph Infiltration Water and Recycled Bedding Material Keith Hobson, PE, BCEE, FOX Engineering Associates, Inc. ABSTRACT The purpose of this evaluation was to determine the cause and source
is the consumptive rate which is the amount of water consumed through evaporation and transpiration (evapotranspiration).
 HYDROLOGY Hydrologic data were used to determine the amount of surface water entering the underground mine pools of the region, which are the sources of the thirty-one (31) discharges in the watershed.
HYDROLOGY Hydrologic data were used to determine the amount of surface water entering the underground mine pools of the region, which are the sources of the thirty-one (31) discharges in the watershed.
1556 by Georgius Agricola. In the United States 10,000 miles of streams and surface
 USE OF SULFATE REDUCING BACTERIA IN ACID MINE DRAINAGE TREATMENT Thomas J. Powers U. S. Environmental Protection Agency Risk Reduction Engineering Laboratory 26 West Martin Luther King Drive Cincinnati,
USE OF SULFATE REDUCING BACTERIA IN ACID MINE DRAINAGE TREATMENT Thomas J. Powers U. S. Environmental Protection Agency Risk Reduction Engineering Laboratory 26 West Martin Luther King Drive Cincinnati,
REPORT ON RABBIT CREEK SPECIAL STUDY SUBWATERSHED 5.19
 REPORT ON RABBIT CREEK SPECIAL STUDY SUBWATERSHED 5.19 Sabine River Authority of Texas August 31, 23 Prepared in Cooperation with the Texas Commission on Environmental Quality Under the Authorization of
REPORT ON RABBIT CREEK SPECIAL STUDY SUBWATERSHED 5.19 Sabine River Authority of Texas August 31, 23 Prepared in Cooperation with the Texas Commission on Environmental Quality Under the Authorization of
Effects of Precipitation on the Acid Mine Drainage Impacted Hewett Fork Watershed Understanding Storm Response
 Effects of Precipitation on the Acid Mine Drainage Impacted Hewett Fork Watershed Understanding Storm Response Zeb Martin Ohio University Contents Project Overview Objectives of the Research Project Area
Effects of Precipitation on the Acid Mine Drainage Impacted Hewett Fork Watershed Understanding Storm Response Zeb Martin Ohio University Contents Project Overview Objectives of the Research Project Area
Decision of the Alabama Surface Mining Commission on the Petition of the Black Warrior River Keeper to Designate Certain Lands as Unsuitable for Coal
 Decision of the Alabama Surface Mining Commission on the Petition of the Black Warrior River Keeper to Designate Certain Lands as Unsuitable for Coal Mining Alabama Surface Mining Commission October 28,
Decision of the Alabama Surface Mining Commission on the Petition of the Black Warrior River Keeper to Designate Certain Lands as Unsuitable for Coal Mining Alabama Surface Mining Commission October 28,
Pebble Lake - Water Quality Report
 Emerald Lakes Village, Oakland County January 8 th, 218 Pebble Lake - Water Quality Report The goal of this testing protocol was to monitor various water quality parameters of the lake, compare results
Emerald Lakes Village, Oakland County January 8 th, 218 Pebble Lake - Water Quality Report The goal of this testing protocol was to monitor various water quality parameters of the lake, compare results
CDF062 Colby Lake Probabilistic Water Quality, Version 2 January 31, 2013
 This form has been developed to document changes to the NorthMet Project and/or Project SDEIS Water Modeling resulting from the water modeling process. The forms will be used during the water modeling
This form has been developed to document changes to the NorthMet Project and/or Project SDEIS Water Modeling resulting from the water modeling process. The forms will be used during the water modeling
The Impact of Surface Coal Mining on Water Quality in the Northern Great Plains
 The Impact of Surface Coal Mining on Water Quality in the Northern Great Plains by Joseph D. Friedlander The North American Coal Corporation Presented at the 2015 National Meeting of the American Society
The Impact of Surface Coal Mining on Water Quality in the Northern Great Plains by Joseph D. Friedlander The North American Coal Corporation Presented at the 2015 National Meeting of the American Society
Earth Science Department, University of Arkansas at Little Rock, 2801 S University Ave Little Rock, AR 72204
 Determining the Extent of Contamination and Potential Health Effects from Water Quality of the Oklahoma Section of the Historic Tri-State Mining District William Ketcheside 1 (wdketcheside@ualr.edu), Laura
Determining the Extent of Contamination and Potential Health Effects from Water Quality of the Oklahoma Section of the Historic Tri-State Mining District William Ketcheside 1 (wdketcheside@ualr.edu), Laura
WOLVERINE MINE MONITORING AND SURVEILLANCE PLAN 2011 ANNUAL REPORT
 WOLVERINE MINE MONITORING AND SURVEILLANCE PLAN 211 ANNUAL REPORT Prepared for: Yukon Energy, Mines and Resources Prepared by: Yukon Zinc Corporation Vancouver, British Columbia March 31, 212 Wolverine
WOLVERINE MINE MONITORING AND SURVEILLANCE PLAN 211 ANNUAL REPORT Prepared for: Yukon Energy, Mines and Resources Prepared by: Yukon Zinc Corporation Vancouver, British Columbia March 31, 212 Wolverine
The surface water hydrology of the site has been logically divided into six phases of monitoring, analyses, and investigation as outlined below:
 SURFACE WATER HYDROLOGY The surface water hydrology of the site has been logically divided into six phases of monitoring, analyses, and investigation as outlined below: Sample Station Locations and Descriptions
SURFACE WATER HYDROLOGY The surface water hydrology of the site has been logically divided into six phases of monitoring, analyses, and investigation as outlined below: Sample Station Locations and Descriptions
Semi-Passive Bioreactors and RCTS Lime Treatment of Acid Mine Drainage
 Semi-Passive Bioreactors and RCTS Lime Treatment of Acid Mine Drainage Timothy K. Tsukamoto, Ph.D. TKT Consulting, LLC TKTtim@gmail.com Bridging the Gap Between Passive and Active PASSIVE ACTIVE Limestone
Semi-Passive Bioreactors and RCTS Lime Treatment of Acid Mine Drainage Timothy K. Tsukamoto, Ph.D. TKT Consulting, LLC TKTtim@gmail.com Bridging the Gap Between Passive and Active PASSIVE ACTIVE Limestone
Drinking Water Supply and
 Drinking Water Supply and Health Engineered Water Systems Water and Health 80% of sickness in the world is caused by inadequate water supply or sanitation 40% of the world population does not have access
Drinking Water Supply and Health Engineered Water Systems Water and Health 80% of sickness in the world is caused by inadequate water supply or sanitation 40% of the world population does not have access
Water Quality Protection Issues for New Water Supply Wells
 Water Quality Protection Issues for New Water Supply Wells Water Quality Protection Issues for New Water Supply Wells Project siting Facility water balance Supply well water quality analysis Supply well
Water Quality Protection Issues for New Water Supply Wells Water Quality Protection Issues for New Water Supply Wells Project siting Facility water balance Supply well water quality analysis Supply well
HYDROGEOCHEMISTRY AND TREATMENT OF ACID MINE DRAINAGE IN SOUTHERN CHINA' by Guo Fang2 and Yu Hong 2
 HYDROGEOCHEMISTRY AND TREATMENT OF ACID MINE DRAINAGE IN SOUTHERN CHINA' by Guo Fang2 and Yu Hong 2 Abstract. Coal mines and various sulfide ore deposits are widely distributed in Southern China. Acid
HYDROGEOCHEMISTRY AND TREATMENT OF ACID MINE DRAINAGE IN SOUTHERN CHINA' by Guo Fang2 and Yu Hong 2 Abstract. Coal mines and various sulfide ore deposits are widely distributed in Southern China. Acid
ROLE OF ACCELERATED OXIDATION FOR REMOVAL OF METALS FROM MINE DRAINAGE 1
 ROLE OF ACCELERATED OXIDATION FOR REMOVAL OF METALS FROM MINE DRAINAGE 1 Don Budeit 2 Abstract. The kinetics of iron oxidation can dictate the effectiveness of both passive and active mine drainage treatment
ROLE OF ACCELERATED OXIDATION FOR REMOVAL OF METALS FROM MINE DRAINAGE 1 Don Budeit 2 Abstract. The kinetics of iron oxidation can dictate the effectiveness of both passive and active mine drainage treatment
University of Nairobi Department of Geology Presentation on, POLLUTION OF GROUNDWATER IN URBAN AREAS OF KENYA; FOCUSING NAIROBI CITY
 University of Nairobi Department of Geology Presentation on, POLLUTION OF GROUNDWATER IN URBAN AREAS OF KENYA; FOCUSING NAIROBI CITY presented by, NAME: NKONGE MURUNGI ELIUD REG NO: I13/3167/2008 COURSE:
University of Nairobi Department of Geology Presentation on, POLLUTION OF GROUNDWATER IN URBAN AREAS OF KENYA; FOCUSING NAIROBI CITY presented by, NAME: NKONGE MURUNGI ELIUD REG NO: I13/3167/2008 COURSE:
Acid Mine Water Reclamation using the ABC Process. Abstract. Introduction
 1 Acid Mine Water Reclamation using the ABC Process M de Beer 1, J. P Maree 2, J. Wilsenach 1, S Motaung 1, L Bologo 1, V Radebe 1 1 Natural Resources and the Environment, CSIR, P O Box 395, Pretoria,
1 Acid Mine Water Reclamation using the ABC Process M de Beer 1, J. P Maree 2, J. Wilsenach 1, S Motaung 1, L Bologo 1, V Radebe 1 1 Natural Resources and the Environment, CSIR, P O Box 395, Pretoria,
Understanding your EAL agricultural soil results
 Understanding your EAL agricultural soil results An EAL agricultural soil test report holds a wealth of information. To assist in its interpretation, please refer to the colour coded text below and within
Understanding your EAL agricultural soil results An EAL agricultural soil test report holds a wealth of information. To assist in its interpretation, please refer to the colour coded text below and within
CTB3365x Introduction to Water Treatment
 CTB3365x Introduction to Water Treatment D4a Groundwater treatment Doris van Halem Ever wondered where your drinking water comes from? Well, chances are that you are sitting on it as we speak. Welcome
CTB3365x Introduction to Water Treatment D4a Groundwater treatment Doris van Halem Ever wondered where your drinking water comes from? Well, chances are that you are sitting on it as we speak. Welcome
Sandies Creek Watershed
 SH 97 Sandies Creek Watershed Gonzales De Witt FM 1116 Sandies Creek SH 72 FM 240 FM 466 Sandies Creek Karnes Smiley Elm Creek FM 108 US 87 SH 80 FM 1117 Guadalupe FM 1681 Wilson Nixon 13657 1803B 1803B
SH 97 Sandies Creek Watershed Gonzales De Witt FM 1116 Sandies Creek SH 72 FM 240 FM 466 Sandies Creek Karnes Smiley Elm Creek FM 108 US 87 SH 80 FM 1117 Guadalupe FM 1681 Wilson Nixon 13657 1803B 1803B
LAKE FORK OF THE GUNNISON RIVER WATERSHED: WATER QUALITY DATA ANALYSIS AND SUMMARY
 LAKE FORK OF THE GUNNISON RIVER WATERSHED: WATER QUALITY DATA ANALYSIS AND SUMMARY By: Ashley Bembenek July 21, 2011 Lake Fork Watershed 3 sub-watersheds Upper Lake Fork (ULF) Henson Creek (HC) Lower Lake
LAKE FORK OF THE GUNNISON RIVER WATERSHED: WATER QUALITY DATA ANALYSIS AND SUMMARY By: Ashley Bembenek July 21, 2011 Lake Fork Watershed 3 sub-watersheds Upper Lake Fork (ULF) Henson Creek (HC) Lower Lake
Interpreting Irrigation Water Quality Reports
 Interpreting Irrigation Water Quality Reports By Dara M Park, L. B. McCarty, and Sarah A. White. Clemson University Water Chemistry What? Water is not just H 2O? What else could possibly be in there? As
Interpreting Irrigation Water Quality Reports By Dara M Park, L. B. McCarty, and Sarah A. White. Clemson University Water Chemistry What? Water is not just H 2O? What else could possibly be in there? As
SOME CONSIDERATIONS WHEN APPLYING LIMESTONE/ROCK PHOSPHATE MATERIALS ON TO ACID PYRITIC SPOILS
 SOME CONSIDERATIONS WHEN APPLYING LIMESTONE/ROCK PHOSPHATE MATERIALS ON TO ACID PYRITIC SPOILS Bill Evangelou U. M. Sainju E. Portig Agronomy Department University of Kentucky ABSTRACT Published in the
SOME CONSIDERATIONS WHEN APPLYING LIMESTONE/ROCK PHOSPHATE MATERIALS ON TO ACID PYRITIC SPOILS Bill Evangelou U. M. Sainju E. Portig Agronomy Department University of Kentucky ABSTRACT Published in the
ACID MINE DRAINAGE TREATMENT VIA ALKALINE INJECTION TECHNOLOGY 1
 ACID MINE DRAINAGE TREATMENT VIA ALKALINE INJECTION TECHNOLOGY 1 G.A. Canty and J.W. Everett 2 Abstract. The Oklahoma Conservation Commission conducted a demonstration project to investigate the feasibility
ACID MINE DRAINAGE TREATMENT VIA ALKALINE INJECTION TECHNOLOGY 1 G.A. Canty and J.W. Everett 2 Abstract. The Oklahoma Conservation Commission conducted a demonstration project to investigate the feasibility
Appendix C1: Batch Kinetics Tests
 Appendix C1: Batch Kinetics Tests This appendix contains the entire data set for the batch kinetics tests for the potential biofilter components. These tests were performed to provide estimates of optimal
Appendix C1: Batch Kinetics Tests This appendix contains the entire data set for the batch kinetics tests for the potential biofilter components. These tests were performed to provide estimates of optimal
Concentration of Metals (Cd, Hg, Ag; mean +SD) in sediment samples collected from Pit Specific Sediment Chemistry Monitoring for CMP 1 in May 2014.
 Figure 1: Concentration of Metals (Cr, Cu, Ni, Pb, Zn, As; mean +SD) in sediment samples collected from Pit Specific Sediment Chemistry Monitoring for CMP 1 in May 2014. Figure 2: Concentration of Metals
Figure 1: Concentration of Metals (Cr, Cu, Ni, Pb, Zn, As; mean +SD) in sediment samples collected from Pit Specific Sediment Chemistry Monitoring for CMP 1 in May 2014. Figure 2: Concentration of Metals
Lecture 5. Exchange Reactions. Cation exchange Salt/Sodium Affected Soils Acid Soils
 Lecture 5 Exchange Reactions Cation exchange Salt/Sodium Affected Soils Acid Soils General Classes (layer build-up) of Phyllosilicate Minerals: Layer Type Charge Trioctahedral Dioctahedral 1 octahedra
Lecture 5 Exchange Reactions Cation exchange Salt/Sodium Affected Soils Acid Soils General Classes (layer build-up) of Phyllosilicate Minerals: Layer Type Charge Trioctahedral Dioctahedral 1 octahedra
Please write the balanced net ionic reaction for each one. Then answer the accompanying question.
 AP Chemistry Net Ionic Rx Practice Test A CLASS SET PLEASE RETURN!! Please write the balanced net ionic reaction for each one. Then answer the accompanying question. 1) A piece of potassium is dropped
AP Chemistry Net Ionic Rx Practice Test A CLASS SET PLEASE RETURN!! Please write the balanced net ionic reaction for each one. Then answer the accompanying question. 1) A piece of potassium is dropped
CE 370. Wastewater Characteristics. Quality. Wastewater Quality. The degree of treatment depends on: Impurities come from:
 CE 37 Wastewater Characteristics Quality Wastewater Quality The degree of treatment depends on: Influent characteristics Effluent characteristics Impurities come from: Domestic activities Industrial activities
CE 37 Wastewater Characteristics Quality Wastewater Quality The degree of treatment depends on: Influent characteristics Effluent characteristics Impurities come from: Domestic activities Industrial activities
MILAF: INTEGRAL MANAGEMENT OF ARSENICAL SLUDGE, TREATMENT AND RECOVERY OF BY-PRODUCTS OF ACID WATERS FROM SMELTER PLANTS
 MILAF: INTEGRAL MANAGEMENT OF ARSENICAL SLUDGE, TREATMENT AND RECOVERY OF BY-PRODUCTS OF ACID WATERS FROM SMELTER PLANTS ABSTRACT ULRIKE BROSCHEK, CECILIA VIDAL, LUIS BRAVO and GILDA ZUÑIGA Environmental
MILAF: INTEGRAL MANAGEMENT OF ARSENICAL SLUDGE, TREATMENT AND RECOVERY OF BY-PRODUCTS OF ACID WATERS FROM SMELTER PLANTS ABSTRACT ULRIKE BROSCHEK, CECILIA VIDAL, LUIS BRAVO and GILDA ZUÑIGA Environmental
Use of Flue Gas Desulfurization By-Product for Mine Sealing and Abatement of Acid Mine Drainage
 Use of Flue Gas Desulfurization By-Product for Mine Sealing and Abatement of Acid Mine Drainage Ben J. Stuart 1, Gary Novak 2, Harry Payne 3, and Carl S. Togni 4 1 Ohio University, Department of Civil
Use of Flue Gas Desulfurization By-Product for Mine Sealing and Abatement of Acid Mine Drainage Ben J. Stuart 1, Gary Novak 2, Harry Payne 3, and Carl S. Togni 4 1 Ohio University, Department of Civil
ABSTRACT: Clean sampling and analysis procedures were used to quantify more than 70 inorganic chemical constituents (including 36 priority
 ABSTRACT: Clean sampling and analysis procedures were used to quantify more than 70 inorganic chemical constituents (including 36 priority pollutants), organic carbon and phenols, and other characteristics
ABSTRACT: Clean sampling and analysis procedures were used to quantify more than 70 inorganic chemical constituents (including 36 priority pollutants), organic carbon and phenols, and other characteristics
THE RELATION OF WATER TO STRIP-MINE OPERATION
 THE RELATION OF WATER TO STRIP-MINE OPERATION GEORGE P. HANNA, JR. Director, Water Resources Center, Engineering Experiment Station Water is a major factor in all problems relating to strip-mine reclamation.
THE RELATION OF WATER TO STRIP-MINE OPERATION GEORGE P. HANNA, JR. Director, Water Resources Center, Engineering Experiment Station Water is a major factor in all problems relating to strip-mine reclamation.
4.0 SAN GABRIEL RIVER WATERSHED MANAGEMENT AREA
 4.0 SAN GABRIEL RIVER WATERSHED MANAGEMENT AREA 4.1 Watershed Description 4.1.1 Watershed Land Use, Percent Impervious, and Population Land use in the San Gabriel River Watershed Management Area is approximately
4.0 SAN GABRIEL RIVER WATERSHED MANAGEMENT AREA 4.1 Watershed Description 4.1.1 Watershed Land Use, Percent Impervious, and Population Land use in the San Gabriel River Watershed Management Area is approximately
Dredged Material and Acid Sulfate Soils
 Dredged Material and Acid Sulfate Soils Biogeochemistry of Upland Placement of Dredged Sediments on Delta Peatland Soils Sediment ph and Attenuation of Arsenic, Copper, TDS/salinity, Nitrate Nitrogen,
Dredged Material and Acid Sulfate Soils Biogeochemistry of Upland Placement of Dredged Sediments on Delta Peatland Soils Sediment ph and Attenuation of Arsenic, Copper, TDS/salinity, Nitrate Nitrogen,
AQUIFER STUDIES RELATED TO GEOLOGIC STORAGE OF CO 2
 AQUIFER STUDIES RELATED TO GEOLOGIC STORAGE OF CO 2 Presenter: Rebecca C. Becky Smyth Gulf Coast Carbon Center,, Jackson School of Geosciences, The University of Texas at Austin ACKNOWLEDGEMENTS Fellow
AQUIFER STUDIES RELATED TO GEOLOGIC STORAGE OF CO 2 Presenter: Rebecca C. Becky Smyth Gulf Coast Carbon Center,, Jackson School of Geosciences, The University of Texas at Austin ACKNOWLEDGEMENTS Fellow
THE EFFECTS OF EXTREMELY HIGH DENSITY SEPTIC SYSTEMS ON SURFACE WATER QUALITY IN GWINNETT COUNTY, GEORGIA
 THE EFFECTS OF EXTREMELY HIGH DENSITY SEPTIC SYSTEMS ON SURFACE WATER QUALITY IN GWINNETT COUNTY, GEORGIA John Anderson Georgia Perimeter College Lawrence Kiage Georgia State University Geographic Setting
THE EFFECTS OF EXTREMELY HIGH DENSITY SEPTIC SYSTEMS ON SURFACE WATER QUALITY IN GWINNETT COUNTY, GEORGIA John Anderson Georgia Perimeter College Lawrence Kiage Georgia State University Geographic Setting
PREDICTED VERSUS ACTUAL WATER QUALITY AT HARDROCK MINE SITES: EFFECT OF INHERENT GEOCHEMICAL AND HYDROLOGIC CHARACTERISTICS 1
 PREDICTED VERSUS ACTUAL WATER QUALITY AT HARDROCK MINE SITES: EFFECT OF INHERENT GEOCHEMICAL AND HYDROLOGIC CHARACTERISTICS 1 Ann Maest 2, James Kuipers, Kim MacHardy, and Gregory Lawson Abstract. The
PREDICTED VERSUS ACTUAL WATER QUALITY AT HARDROCK MINE SITES: EFFECT OF INHERENT GEOCHEMICAL AND HYDROLOGIC CHARACTERISTICS 1 Ann Maest 2, James Kuipers, Kim MacHardy, and Gregory Lawson Abstract. The
FORTUNE MINERALS LIMITED 140 Fullarton Street, Suite 1902, London, Ontario, Canada N6A 5P2 Tel ~ Fax
 FORTUNE MINERALS LIMITED 140 Fullarton Street, Suite 1902, London, Ontario, Canada N6A 5P2 Tel. 519-858-8188 ~ Fax. 519-858-8155 September 30, 2011 Chuck Hubert Environmental Assessment Officer Mackenzie
FORTUNE MINERALS LIMITED 140 Fullarton Street, Suite 1902, London, Ontario, Canada N6A 5P2 Tel. 519-858-8188 ~ Fax. 519-858-8155 September 30, 2011 Chuck Hubert Environmental Assessment Officer Mackenzie
WEST VIRGINIA DEPARTMENT OF ENVIRONMENTAL PROTECTION OFFICE OF OIL AND GAS. GENERAL WATER POLLUTION CONTROL PERMIT Permit Number: GP-WV-1-07
 WEST VIRGINIA DEPARTMENT OF ENVIRONMENTAL PROTECTION OFFICE OF OIL AND GAS GENERAL WATER POLLUTION CONTROL PERMIT Permit Number: GP-WV-1-07 FACT SHEET, RATIONALE AND INFORMATION FOR GENERAL PERMIT FOR
WEST VIRGINIA DEPARTMENT OF ENVIRONMENTAL PROTECTION OFFICE OF OIL AND GAS GENERAL WATER POLLUTION CONTROL PERMIT Permit Number: GP-WV-1-07 FACT SHEET, RATIONALE AND INFORMATION FOR GENERAL PERMIT FOR
Pennsylvania Senior Environment Corps. Table of Contents Part 2 Getting Started:. 21 Chemical Analysis... 22
 Table of Contents Part 2 Getting Started:. 21 Chemical Analysis.... 22 3 Chapter 2: Getting Started 21 Chemical Analysis of the Water Dependent on your area, you may measure for several parameters. In
Table of Contents Part 2 Getting Started:. 21 Chemical Analysis.... 22 3 Chapter 2: Getting Started 21 Chemical Analysis of the Water Dependent on your area, you may measure for several parameters. In
Statewide Characterization of Oklahoma s Major Aquifers
 Statewide Characterization of Oklahoma s Major Aquifers Mark Belden, Brittany McCall, Sarah Yepez Water Quality Programs Division, OWRB Oklahoma Clean Lakes & Watersheds Conference April 5-6, 2017 Groundwater
Statewide Characterization of Oklahoma s Major Aquifers Mark Belden, Brittany McCall, Sarah Yepez Water Quality Programs Division, OWRB Oklahoma Clean Lakes & Watersheds Conference April 5-6, 2017 Groundwater
Kashi Banerjee Ph.D.; P.E.; BCEE. Moon Township, PA Andrea Laybauer
 Metals Precipitation Kashi Banerjee Ph.D.; P.E.; BCEE Veolia Water Solutions & Technologies Moon Township, PA 15108 Andrea Laybauer Introduction Metals in Mining Wastes Type of Mines and Ore Characteristics
Metals Precipitation Kashi Banerjee Ph.D.; P.E.; BCEE Veolia Water Solutions & Technologies Moon Township, PA 15108 Andrea Laybauer Introduction Metals in Mining Wastes Type of Mines and Ore Characteristics
SALINITY MANAGEMENT IN PROCESSING TOMATOES. Brenna Aegerter and Michelle Leinfelder-Miles UC Cooperative Extension San Joaquin County
 SALINITY MANAGEMENT IN PROCESSING TOMATOES Brenna Aegerter and Michelle Leinfelder-Miles UC Cooperative Extension San Joaquin County WHERE DO SALTS COME FROM? Irrigation water is the primary source of
SALINITY MANAGEMENT IN PROCESSING TOMATOES Brenna Aegerter and Michelle Leinfelder-Miles UC Cooperative Extension San Joaquin County WHERE DO SALTS COME FROM? Irrigation water is the primary source of
Low Maintenance Passive Treatment Systems for Mining Site Contaminants. Robert C. Thomas, Ph.D
 Low Maintenance Passive Treatment Systems for Mining Site Contaminants Robert C. Thomas, Ph.D Acid rock drainage (ARD) forms when sulfide minerals are exposed to oxygen and water during large-scale land
Low Maintenance Passive Treatment Systems for Mining Site Contaminants Robert C. Thomas, Ph.D Acid rock drainage (ARD) forms when sulfide minerals are exposed to oxygen and water during large-scale land
APPENDIX A. This is a comprehensive map of the areas that will orient the. This map shows the cross-sectional Area A to B at which the
 APPENDIX A EXPLANATION OF MAPS AND PLANS 1. LOCATION MAP - PLATE 1 This is a comprehensive map of the areas that will orient the numbers indicated on the Flow Data Charts interested reader of general location
APPENDIX A EXPLANATION OF MAPS AND PLANS 1. LOCATION MAP - PLATE 1 This is a comprehensive map of the areas that will orient the numbers indicated on the Flow Data Charts interested reader of general location
2. GUIDELINES FOR THE EVALUATION OF DRINKING-WATER QUALITY FOR HUMAN CONSUMPTION WITH REGARD TO CHEMICAL, PHYSICAL AND BACTERIOLOGICAL QUALITY
 THE WATER ACT, 1956 (ACT 54 OF 1956 ) AND ITS REQUIREMENTS IN TERMS OF WATER SUPPLIES FOR DRINKING WATER AND FOR WASTE WATER TREATMENT AND DISCHARGE INTO THE ENVIRONMENT 1. INTRODUCTION The provisions
THE WATER ACT, 1956 (ACT 54 OF 1956 ) AND ITS REQUIREMENTS IN TERMS OF WATER SUPPLIES FOR DRINKING WATER AND FOR WASTE WATER TREATMENT AND DISCHARGE INTO THE ENVIRONMENT 1. INTRODUCTION The provisions
ATTENUATION OF METAL CONCENTRATIONS IN TERRACE RESERVOIR, CONEJOS COUNTY, COLORADO, MAY 1994 THROUGH MAY 1995
 ATTENUATION OF METAL CONCENTRATIONS IN TERRACE RESERVOIR, CONEJOS COUNTY, COLORADO, MAY 1994 THROUGH MAY 1995 By Robert W. Stogner, Sr. U.S. Geological Survey, WRD 201 W. 8th Street, Suite 200 Pueblo,
ATTENUATION OF METAL CONCENTRATIONS IN TERRACE RESERVOIR, CONEJOS COUNTY, COLORADO, MAY 1994 THROUGH MAY 1995 By Robert W. Stogner, Sr. U.S. Geological Survey, WRD 201 W. 8th Street, Suite 200 Pueblo,
Utilization of Geochemical Parameters in Developing a Conceptual Site Model
 Utilization of Geochemical Parameters in Developing a Conceptual Site Model Annual Risk Assessment & Remediation Seminar MSECA, Indianapolis, IN October 29 2012 µg/l Steven R. Irvin & Jim V. Rouse Acuity
Utilization of Geochemical Parameters in Developing a Conceptual Site Model Annual Risk Assessment & Remediation Seminar MSECA, Indianapolis, IN October 29 2012 µg/l Steven R. Irvin & Jim V. Rouse Acuity
East TX Test Site (1/2 Treated)
 1 East TX Test Site (1/2 Treated) 2 CATION EXCHANGE CAPACITY ( CEC ) It is a measure of the quantity of cations reversibly adsorbed per unit weight of soil. CEC is expressed in meq/100 g of mass (meq is
1 East TX Test Site (1/2 Treated) 2 CATION EXCHANGE CAPACITY ( CEC ) It is a measure of the quantity of cations reversibly adsorbed per unit weight of soil. CEC is expressed in meq/100 g of mass (meq is
RECOMMENDED SCOPE OF ACCREDITATION (For Testing Laboratories) * Test Method / Standard against which tests are performed
 Sl Product / Material of test 1 Wastewater Colour, true colour units, 2 Turbidity, NTU 3 Total dissolved solids mg/1, IS-3025 (Part 4) -1983 2005: 2120 /C IS-3025 (Part 10)-1984 2005: 2130/B IS-3025 (Part
Sl Product / Material of test 1 Wastewater Colour, true colour units, 2 Turbidity, NTU 3 Total dissolved solids mg/1, IS-3025 (Part 4) -1983 2005: 2120 /C IS-3025 (Part 10)-1984 2005: 2130/B IS-3025 (Part
S TAR-ORION S OUTH D IAMOND P ROJECT E NVIRONMENTAL I MPACT S TATEMENT. Appendix A. Mine Site Water Balance and Water Quality
 S TAR-ORION S OUTH D IAMOND P ROJECT E NVIRONMENTAL I MPACT S TATEMENT Appendix 6.2.7-A Mine Site Water Balance and Water Quality TABLE OF CONTENTS S TAR-ORION S OUTH D IAMOND P ROJECT E NVIRONMENTAL I
S TAR-ORION S OUTH D IAMOND P ROJECT E NVIRONMENTAL I MPACT S TATEMENT Appendix 6.2.7-A Mine Site Water Balance and Water Quality TABLE OF CONTENTS S TAR-ORION S OUTH D IAMOND P ROJECT E NVIRONMENTAL I
Groundwater and Surface Water Overview of the Lochend Area, Alberta
 Groundwater and Surface Water Overview of the Lochend Area, Alberta The Lochend Industry Producers Group (LIPG) conducted a hydrogeological / hydrological study in the Lochend operating field. The objectives
Groundwater and Surface Water Overview of the Lochend Area, Alberta The Lochend Industry Producers Group (LIPG) conducted a hydrogeological / hydrological study in the Lochend operating field. The objectives
Background Information on the. Peace River Basin
 Background Information on the Peace River Basin Resource Conservation & Development Department August 24 Background Physiography The Peace River drainage basin occupies large parts of Polk, Hardee, DeSoto,
Background Information on the Peace River Basin Resource Conservation & Development Department August 24 Background Physiography The Peace River drainage basin occupies large parts of Polk, Hardee, DeSoto,
Tenmile Creek Sampling Summary
 Tenmile Creek Sampling Summary December 15, 2015 Background In 2014, DEP s California District Mining Office collected surface water samples from three locations on Tenmile Creek (TMC) in the vicinity
Tenmile Creek Sampling Summary December 15, 2015 Background In 2014, DEP s California District Mining Office collected surface water samples from three locations on Tenmile Creek (TMC) in the vicinity
Evaluation of FGD-Gypsum to Improve Forage Production and Reduce Phosphorus Losses from Piedmont Soils
 211 World of Coal Ash (WOCA) Conference May 9-12, 211 in Denver, CO, USA http://www.flyash.info/ Evaluation of FGD-Gypsum to Improve Forage Production and Reduce Phosphorus Losses from Piedmont Soils Harry
211 World of Coal Ash (WOCA) Conference May 9-12, 211 in Denver, CO, USA http://www.flyash.info/ Evaluation of FGD-Gypsum to Improve Forage Production and Reduce Phosphorus Losses from Piedmont Soils Harry
Approaches to Treatment of Very High Acidity Wastewater
 Approaches to Treatment of Very High Acidity Wastewater AIChE International Society for Water Solutions Industrial Water Use and Reuse Workshop Strategies for Sustainable Water Management for Mining Kevin
Approaches to Treatment of Very High Acidity Wastewater AIChE International Society for Water Solutions Industrial Water Use and Reuse Workshop Strategies for Sustainable Water Management for Mining Kevin
State of Nevada Department of Conservation and Natural Resources Division of Environmental Protection Laboratory Scope of Accreditation
 Matrix: CWA (Non Potable Water) Discipline: Chemistry ASTM D516-07 ASTM D6888-09 ASTM D7511-09e2 EPA 160.4 EPA 1631E EPA 1664A EPA 1664A (SGT-HEM) EPA 180.1 Sulfate 8/1/2018 Cyanide, Amenable 8/1/2018
Matrix: CWA (Non Potable Water) Discipline: Chemistry ASTM D516-07 ASTM D6888-09 ASTM D7511-09e2 EPA 160.4 EPA 1631E EPA 1664A EPA 1664A (SGT-HEM) EPA 180.1 Sulfate 8/1/2018 Cyanide, Amenable 8/1/2018
Water Resources on PEI: an overview and brief discussion of challenges
 Water Resources on PEI: an overview and brief discussion of challenges Components: Components and links Atmospheric water Surface water (including glacial water) Groundwater Links: Precipitation (atm(
Water Resources on PEI: an overview and brief discussion of challenges Components: Components and links Atmospheric water Surface water (including glacial water) Groundwater Links: Precipitation (atm(
Chemical Testing of Drinking Water
 Chemical Testing of Drinking Water Adapted from: An original Creek Connections activity. Water Chemistry Grade Level: all Duration: 50 minutes Setting: lab or classroom Summary: Students will conduct chemistry
Chemical Testing of Drinking Water Adapted from: An original Creek Connections activity. Water Chemistry Grade Level: all Duration: 50 minutes Setting: lab or classroom Summary: Students will conduct chemistry
ACID MINE DRAINAGE TREATMENT IN GREENS RUN BY AN ANOXIC LIMESTONE DRAIN
 ACID MINE DRAINAGE TREATMENT IN GREENS RUN BY AN ANOXIC LIMESTONE DRAIN Introduction Troy Titchenell and Jeff Skousen Anker Energy and West Virginia University From its headwaters in Pocahontas, Randolph
ACID MINE DRAINAGE TREATMENT IN GREENS RUN BY AN ANOXIC LIMESTONE DRAIN Introduction Troy Titchenell and Jeff Skousen Anker Energy and West Virginia University From its headwaters in Pocahontas, Randolph
GROUNDWATER ASSESSMENT MONITORING REPORT APRIL 2014
 Tennessee Valley Authority John Sevier Fossil Plant GROUNDWATER ASSESSMENT MONITORING REPORT APRIL 2014 Prepared by Amos L. Smith, PG Chattanooga, Tennessee June 4, 2014 DOCUMENT CERTIFICATION I certify
Tennessee Valley Authority John Sevier Fossil Plant GROUNDWATER ASSESSMENT MONITORING REPORT APRIL 2014 Prepared by Amos L. Smith, PG Chattanooga, Tennessee June 4, 2014 DOCUMENT CERTIFICATION I certify
Acidity and Alkalinity:
 Evaluation of Pollution Sources to Lake Glenville Quarterly Report December 2018 Kimberlee K Hall, PhD Environmental Health Program, Western Carolina University Summary Chemical and microbial analysis
Evaluation of Pollution Sources to Lake Glenville Quarterly Report December 2018 Kimberlee K Hall, PhD Environmental Health Program, Western Carolina University Summary Chemical and microbial analysis
SPECIFICATION NO.1197S Addendum No.4 Attachment D. Appendix L- Ground Water Quality Data and Revised Construction Groundwater Discharge Plan
 SPECIFICATION NO.1197S Addendum No.4 Attachment D Appendix L- Ground Water Quality Data and Revised Construction Groundwater Discharge Plan Page 1 of 2 Specification No. 1197S Addendum No. 4 ATTACHMENT
SPECIFICATION NO.1197S Addendum No.4 Attachment D Appendix L- Ground Water Quality Data and Revised Construction Groundwater Discharge Plan Page 1 of 2 Specification No. 1197S Addendum No. 4 ATTACHMENT
