Item 36 has not been slated for public release in 2011.
|
|
|
- Herbert Pope
- 5 years ago
- Views:
Transcription
1 S Use the power-plant diagrams and information below to answer questions Power Plants 35. Which describes a chemical change and a physical change that take place during the production of electricity in the coal-fired power plant (Figure 1)? S Lake Intake Figure 1: Coal-Fired Power Plant Coal Supply Stack Conveyor Belt Turbine Generator Steam Line Long- Distance Power Lines Boiler Cooling Water Figure 2: Hydroelectric Power Plant Generator Turbine Condenser Long-Distance Power Lines Diagrams Courtesy of Tennessee Valley Authority 116; 8S0053PSAXA0008B An electric power plant in Toledo, Ohio, is powered by coal (see Figure 1). The coal is Form E SP06 (1) burned to boil water and produce steam. The steam turns a turbine, which causes the generator to produce electricity. River The power plant in Belleville, West Virginia, is hydroelectric (see Figure 2). Water from the lake behind the dam falls on the turbine blades. The turbine turns, which causes the generator to produce electricity. 289; 8S0053PSXXX0000X A. The coal moving along the conveyor belt is a chemical change. The coal burning to give off heat and carbon dioxide gas is a physical change. B. The coal burning to give off heat and carbon dioxide gas is a chemical change. The boiling water turning to steam is a physical change. C. The boiling water turning to steam is a chemical change. The steam turning the turbine is a physical change. D. The steam turning the turbine is a chemical change. The turbine running the generator to produce electricity is a physical change. Item 36 has not been slated for public release in The hydroelectric plant (Figure 2) uses a renewable energy resource. Which statement describes why this resource is considered renewable? A. Heated water turns the turbine. The used water flows upstream back directly into the lake and may be used again right away. B. Falling water turns the turbine. The used water flows upstream back directly into the lake and may be used again right away. C. Heated water turns the turbine. The water is returned to the river downstream, so it may continue in the water cycle. D. Falling water turns the turbine. The water is returned to the river downstream, so it may continue in the water cycle. 6162; 8S0053PSCXC0009D
2 41. In any physical or chemical process, what two quantities are always conserved? A. matter and total energy B. light and acoustic energy C. density and thermal energy D. gravity and potential energy 5444; 8S0000PSDXR0727A Form F SP08 (8)
3 Properties of Substances A scientist measures out 15 ml each of baking soda, salt, sugar, iron filings, and corn starch. She runs a series of procedures and records her results in the table below. The first column in the table indicates the procedure. The remaining columns indicate her results for each substance. Procedure Baking Soda Salt Sugar Iron Filings Corn Starch Measure Mass Measure Volume 19 g 21 g 18 g 118 g 8 g 15 ml 15 ml 15 ml 15 ml 15 ml 42. According to the information in the table, what comparison can the scientist make about the densities of sugar and corn starch? A. Sugar is more dense than corn starch. B. Corn starch is more dense than sugar. C. Sugar and corn starch have the same density. D. Both sugar and cornstarch are more dense than salt. 8926; 8S0193PSAXC0002A FT Form F SP06 (33) S 44. What two changes does sugar undergo when heated in a spoon over a flame? A. two physical changes B. two chemical changes C. a chemical change and then a physical change D. a physical change and then a chemical change 8927; 8S0193PSAXC0003D FT Form F SP06 (34) Heat in Spoon Over Flame Add 3 Drops of Vinegar Mix With Iodine Solution 621 8S0193PSXXX0000X No Change No Change Fizzes and Melts; Then Burns and Turns Black No Change No Reaction at First; Next Day, Rust Had Formed No Reaction at First; Next Day, Rust Had Formed Burns Turns Black or Dark Purple 43. The scientist observes the rust on the sample of iron filings and dries the sample thoroughly. The scientist weighs the entire sample and finds that it has more mass than the original 118 grams. What explains this increase in mass? A. The sample gained mass during drying. B. The sample gained mass during a physical change. C. The sample gained mass during a change of state. D. The sample gained mass during a chemical change. 9646; 8S0193PSAXA0008D
4 S7. A student studying rock densities needs to measure the volume of a small rock sample to the nearest milliliter (ml). The student knows that the rock sample has a volum of at least 5 ml. Which tool should the student use to get the most accurate measure of the volume of water displaced by the rock? A. 100 ml 80 ml 60 ml 40 ml 50 ml B. 25 ml C. 25 ml 20 ml 15 ml 10 ml 5 ml 10 ml D. 5 ml 52; 8S0000SIAXD0129C Form B SP07 (18) 0 ml
5 S26. The following steps describe the process of generating electricity by burning coal in a power plant: 1. The coal fire converts liquid water to steam. 2. The steam rotates fanlike blades of a mechanical turbine. 3. The shaft of the turbine spins an electric generator. 4. The electric power is delivered to the consumer over long-distance power lines. Which energy transformation occurs during this process? A. Nuclear energy of the steam is converted to thermal energy of the turbine. B. Mechanical energy from the turbine is converted to electric energy in the generator. C. Electric energy from the generator is converted to chemical energy in the power lines. D. Chemical energy from the generator is converted to electric energy in the power lines. 135; 8S0000PSDXR0227B
6 Baking Soda and Vinegar Reaction Students perform a chemistry experiment by mixing baking soda with vinegar. They mix 4 grams of baking soda with 50 grams of vinegar in a 6-gram plastic cup as shown below. The cup is left uncovered. The students record the mass and temperature of the mixture every 5 seconds. They continue these observations until 10 seconds after they see the mixture stop bubbling. Their data are shown in the table below. 30. Which graphs show the trends in the mass (M) and temperature (T) change during the baking soda and vinegar reaction shown in the diagram? M T A. (seconds) Mass (grams) Temperature (degrees Celsius) B. M T M S31. The data in the table show the temperature of the cup-baking soda-vinegar system. The temperature of the surrounding air was not measured. Assuming that the total amount of energy remains constant, what conclusion about energy transfer does the temperature data support? A. Thermal energy was lost from the surrounding air and the system. B. Thermal energy was gained by the system and the surrounding air. C. Thermal energy was transferred from the surrounding air to the system. 32. The data show that the mass of the cup and its contents decreases while the mixture is bubbling. In your Answer Document, explain why the mass of the cup and its contents at 25 seconds is less than the initial total mass of the cup, baking soda and vinegar. Include in your answer a valid scientific reason on which you based your explanation. (2 points) C. D. M T T D. Thermal energy was transferred from the system to the surrounding air. 0320; 8S0247PSDXC0004D
7 Density Experiment The two graduated cylinders pictured can hold the same amount of water and use the same scale. A student measures the masses of two metal balls. One ball is made of aluminum and the other ball is made of lead. The student adds 50 ml of water to each graduated cylinder and then drops one metal ball into each graduated cylinder. 100 ml 100 ml 50 ml 50 ml Aluminum Lead 296 8S0060PSXXX0000X 3. Which tool did the student use to measure the mass of each metal ball? A. ruler B. timer C. balance D. graduated cylinder S0060SIAXR0006C 4. The student includes the sentence below in the write-up of this investigation. The lead ball has a measured mass of 113 grams. Which kind of scientific statement is this sentence? A. inference B. prediction C. explanation D. observation 5. The teacher asks the student to use the information she collected to compare the density of the two metal balls. The mass of the lead ball is 113 grams and the mass of the aluminum ball is 27 grams. What can the student infer about the density of these metal balls based on this investigation? A. The density of both metal balls is the same. B. The density of both metal balls is less than the density of the water. C. The density of the lead ball is less than the density of the aluminum ball. D. The density of the lead ball is greater than the density of the aluminum ball. S 6. In repeating the investigation, the student accidentally drops and steps on the lead ball. This action changes its shape from a sphere to an egg-shaped solid. The lead ball is placed back into the graduated cylinder. In your Answer Document, predict what effect, if any, this change has on the amount of water displaced by the lead ball. Explain your prediction. (2 points) S0060PSAXD0003S
8 15. The table below lists the densities of several materials. Material Density (g/ml) Limestone 2.70 Magnesium 1.74 Sulfur 2.07 Water 1.00 Wax 0.95 Which material will float in water? A. limestone B. magnesium C. sulfur D. wax 6459
9 16. Students do an investigation on the reaction of baking soda with vinegar. They create a data table to record the mass (grams) and temperature (degrees Celsius) of the mixture every 5 seconds. Several pieces of data are missing from the table (seconds) Mass (grams) Temperature (degrees Celsius) In your Answer Document, give one example of how the incomplete data make it difficult for the students to draw conclusions about the changes that occur when baking soda reacts with vinegar. Explain how the incomplete data will affect the ability of other students to reproduce the experiment. (2 points)
Ohio Achievement Assessments
 hio Department of Education tudent Name: Ohio Achievement Assessments Grade 8 tudent Test Booklet pring 2011 This test was originally administered to students in pring 2011. Not all items from the pring
hio Department of Education tudent Name: Ohio Achievement Assessments Grade 8 tudent Test Booklet pring 2011 This test was originally administered to students in pring 2011. Not all items from the pring
Two variables in this investigation are location of trap and size of jar.
 An eighth-grade class wants to identify a representative sample of the crawling and flying insects living in the schoolyard. The students build and set five traps like the one shown below. They place the
An eighth-grade class wants to identify a representative sample of the crawling and flying insects living in the schoolyard. The students build and set five traps like the one shown below. They place the
Ohio Achievement Test Grade 8 Science. May Answer Key and Scoring Guidelines
 Ohio Achievement Test Grade 8 Science May 2007 Answer Key and Ohio Department of Education, Rev. September 2011 Page 1 of 13 Grade 8 Science Answer Key May 2007 Content Item No. Type Content Standard Standard
Ohio Achievement Test Grade 8 Science May 2007 Answer Key and Ohio Department of Education, Rev. September 2011 Page 1 of 13 Grade 8 Science Answer Key May 2007 Content Item No. Type Content Standard Standard
Lesson 5 Energy. OAA Science Lesson 5 52
 Lesson 5 Energy OAA Science Lesson 5 52 Name Date Period Student Lesson 5: Energy Reference Sheet: Energy - is the ability to do work or cause change - can be changed from one form to another - cannot
Lesson 5 Energy OAA Science Lesson 5 52 Name Date Period Student Lesson 5: Energy Reference Sheet: Energy - is the ability to do work or cause change - can be changed from one form to another - cannot
Copyright 2014 Science Doodles
 Copyright 2014 Science Doodles If a scientist comes across an unknown element, could she use density to identify the element? A. Yes, because each element has a specific density. B. No, because density
Copyright 2014 Science Doodles If a scientist comes across an unknown element, could she use density to identify the element? A. Yes, because each element has a specific density. B. No, because density
DENSITY of Solids and Liquids
 Regents Earth Science Name: Date: Lab # DENSITY of Solids and Liquids Introduction: Density is the term used to describe the relationship between the mass of an object and its volume. Under given conditions
Regents Earth Science Name: Date: Lab # DENSITY of Solids and Liquids Introduction: Density is the term used to describe the relationship between the mass of an object and its volume. Under given conditions
Chemistry Attitudes, Skills, & Knowledge Survey (CASKS) Form 3
 Chemistry Attitudes, Skills, & Knowledge Survey (CASKS) Form 3 Directions to Students: Do not open this booklet until you are told to do so. Please respond to the following items by marking the best answer
Chemistry Attitudes, Skills, & Knowledge Survey (CASKS) Form 3 Directions to Students: Do not open this booklet until you are told to do so. Please respond to the following items by marking the best answer
caustic soda (strong alkali) [1]
![caustic soda (strong alkali) [1] caustic soda (strong alkali) [1]](/thumbs/80/82022264.jpg) 3 1 Litmus is made from a plant pigment. It is red when placed in an acidic solution. It is blue when placed in an alkaline solution. It is purple when neutral. Examiner's (a) What do we call substances
3 1 Litmus is made from a plant pigment. It is red when placed in an acidic solution. It is blue when placed in an alkaline solution. It is purple when neutral. Examiner's (a) What do we call substances
Acids and alkalis/simple chemical reactions
 Medway LEA Advisory Service Acids and alkalis/simple chemical reactions 7E & 7F 30 min 30 marks Q1-L3, Q2-L4, Q3-L4, Q4-L5, Q5-L6, Q6-L6 1. (a) Reshma had a mixture of iron filings and sand. What could
Medway LEA Advisory Service Acids and alkalis/simple chemical reactions 7E & 7F 30 min 30 marks Q1-L3, Q2-L4, Q3-L4, Q4-L5, Q5-L6, Q6-L6 1. (a) Reshma had a mixture of iron filings and sand. What could
1) An object weighs 75.7 kg. What is this weight in lbs? [1 lb = grams]
![1) An object weighs 75.7 kg. What is this weight in lbs? [1 lb = grams] 1) An object weighs 75.7 kg. What is this weight in lbs? [1 lb = grams]](/thumbs/89/98796796.jpg) CHEMISTRY 51 SPRING 2016 NAME: Exam 1 Multiple Choice (2 pts each) 1) An object weighs 75.7 kg. What is this weight in lbs? [1 lb = 453.6 grams] a) 34.3 pounds b) 167 pounds c) 343 pounds d) 16.7 pounds
CHEMISTRY 51 SPRING 2016 NAME: Exam 1 Multiple Choice (2 pts each) 1) An object weighs 75.7 kg. What is this weight in lbs? [1 lb = 453.6 grams] a) 34.3 pounds b) 167 pounds c) 343 pounds d) 16.7 pounds
CRHS Academic Chemistry Unit 1 Matter and Change HOMEWORK. Due Date Assignment On-Time (100) Late (70)
 Name Period CRHS Academic Chemistry Unit 1 Matter and Change HOMEWORK Due Date Assignment On-Time (100) Late (70) 1.1 1.2 1.3 Warm Ups Extra Credit Notes, Homework, Exam Reviews and Their KEYS located
Name Period CRHS Academic Chemistry Unit 1 Matter and Change HOMEWORK Due Date Assignment On-Time (100) Late (70) 1.1 1.2 1.3 Warm Ups Extra Credit Notes, Homework, Exam Reviews and Their KEYS located
NCERT solution for Physical and
 NCERT solution for Physical and chemical changes 1 Question 1 Classify the changes involved in the following processes as physical or chemical changes: (a) Photosynthesis (b) Dissolving sugar in water
NCERT solution for Physical and chemical changes 1 Question 1 Classify the changes involved in the following processes as physical or chemical changes: (a) Photosynthesis (b) Dissolving sugar in water
Topic 1 Review Questions Set 1
 Topic 1 Review Questions Set 1 Base your answers to questions 1-5 on your knowledge of Earth Science, the Earth Science Reference Tables, and the diagrams below. The diagrams represent three samples of
Topic 1 Review Questions Set 1 Base your answers to questions 1-5 on your knowledge of Earth Science, the Earth Science Reference Tables, and the diagrams below. The diagrams represent three samples of
SC.8.P.8.4 Classify and compare substances on the basis of characteristic physical properties that can be demonstrated or measured; for example,
 SC.8.P.8.4 Classify and compare substances on the basis of characteristic physical properties that can be demonstrated or measured; for example, density, thermal or electrical conductivity, solubility,
SC.8.P.8.4 Classify and compare substances on the basis of characteristic physical properties that can be demonstrated or measured; for example, density, thermal or electrical conductivity, solubility,
Appendix D OB-SCERTAINER ACTIVITY. This activity will required the student to discover the maze pattern inside the Ob-Scertainer with
 Appendix D OB-SCERTAINER ACTIVITY This activity will required the student to discover the maze pattern inside the Ob-Scertainer with opening it. They gently tilted and rotated the Ob-Scertainer to hear
Appendix D OB-SCERTAINER ACTIVITY This activity will required the student to discover the maze pattern inside the Ob-Scertainer with opening it. They gently tilted and rotated the Ob-Scertainer to hear
SCI-6 SOL Practice Questions_6th Grade Exam not valid for Paper Pencil Test Sessions
 SCI-6 SOL Practice Questions_6th Grade Exam not valid for Paper Pencil Test Sessions [Exam ID:392MWT 1 The quality of pond water can be determined by identifying the number and types of organisms found
SCI-6 SOL Practice Questions_6th Grade Exam not valid for Paper Pencil Test Sessions [Exam ID:392MWT 1 The quality of pond water can be determined by identifying the number and types of organisms found
Iron filings (Fe) 56g IRON + SULPHUR IRON SULPHIDE
 W.S.51. Chemical reactions. All of the different materials around us have been formed by chemical reactions from about one hundred simple elements. The diagram below shows a chemical reaction between the
W.S.51. Chemical reactions. All of the different materials around us have been formed by chemical reactions from about one hundred simple elements. The diagram below shows a chemical reaction between the
CRHS Academic Chemistry Unit 1 Matter and Change HOMEWORK. Due Date Assignment On-Time (100) Late (70)
 Name KEY Period CRHS Academic Chemistry Unit 1 Matter and Change HOMEWORK Due Date Assignment On-Time (100) Late (70) 1.1 1.2 1.3 Warm Ups Notes, Homework, Exam Reviews and Their KEYS located on CRHS Academic
Name KEY Period CRHS Academic Chemistry Unit 1 Matter and Change HOMEWORK Due Date Assignment On-Time (100) Late (70) 1.1 1.2 1.3 Warm Ups Notes, Homework, Exam Reviews and Their KEYS located on CRHS Academic
13. Friction changes mechanical energy into heat energy.
 1. What basic form of energy is present in radioactive substances. A) nuclear B) chemical C) mechanical D) electrical 2. What basic form of energy is present in a blowing wind? A) nuclear B) chemical C)
1. What basic form of energy is present in radioactive substances. A) nuclear B) chemical C) mechanical D) electrical 2. What basic form of energy is present in a blowing wind? A) nuclear B) chemical C)
Cambridge International Examinations Cambridge International General Certificate of Secondary Education
 www.xtremepapers.com Cambridge International Examinations Cambridge International General Certificate of Secondary Education *2872917612* PHYSICAL SCIENCE 0652/61 Paper 6 Alternative to Practical October/November
www.xtremepapers.com Cambridge International Examinations Cambridge International General Certificate of Secondary Education *2872917612* PHYSICAL SCIENCE 0652/61 Paper 6 Alternative to Practical October/November
Station Two Guide. Endothermic and exothermic reactions. A Vocabulary
 Part One: Baking Soda and Vinegar Station Two Guide How is the temperature of vinegar affected when combined with baking soda? Baking soda Measuring cups Vinegar expand kinetic energy potential energy
Part One: Baking Soda and Vinegar Station Two Guide How is the temperature of vinegar affected when combined with baking soda? Baking soda Measuring cups Vinegar expand kinetic energy potential energy
Creation Settings. can be separated into two or more pure substances by distillation. can be separated into two or more substances by chromatography.
 Page 1 of 10 TEST BANK (ACCT3321_201_1220) > CONTROL PANEL > POOL MANAGER > POOL CANVAS Pool Canvas Add, modify, and remove questions. Select a question type from the Add drop-down list and click Go to
Page 1 of 10 TEST BANK (ACCT3321_201_1220) > CONTROL PANEL > POOL MANAGER > POOL CANVAS Pool Canvas Add, modify, and remove questions. Select a question type from the Add drop-down list and click Go to
Name Honors Chemistry / /
 Name Honors Chemistry / / SOL Questions Chapter 1 Each of the following questions below appeared on an SOL Chemistry Exam. For each of the following bubble in the correct answer on your scantron. 1. The
Name Honors Chemistry / / SOL Questions Chapter 1 Each of the following questions below appeared on an SOL Chemistry Exam. For each of the following bubble in the correct answer on your scantron. 1. The
Paper 2. Year 9 science test. Remember: First name. Last name. Class. Date
 Sc KEY STAGE 3 Year 9 science test TIER 5 7 Paper 2 First name Last name Class Date Please read this page, but do not open your booklet until your teacher tells you to start. Write your name, your class
Sc KEY STAGE 3 Year 9 science test TIER 5 7 Paper 2 First name Last name Class Date Please read this page, but do not open your booklet until your teacher tells you to start. Write your name, your class
Look at the measuring cylinders. What happened to the volume of the water and the wax after freezing? the volume of water... the volume of wax...
 1. Meera poured 7 cm 3 of water into a measuring cylinder. She poured 7 cm 3 of melted wax into another measuring cylinder. She put both measuring cylinders into a freezer for 24 hours. water before freezing
1. Meera poured 7 cm 3 of water into a measuring cylinder. She poured 7 cm 3 of melted wax into another measuring cylinder. She put both measuring cylinders into a freezer for 24 hours. water before freezing
Name Class Date. What is an energy resource? How do we use nonrenewable energy resources? What are renewable energy resources?
 CHAPTER 5 4 Energy Resources SECTION Energy and Energy Resources BEFORE YOU READ After you read this section, you should be able to answer these questions: What is an energy resource? How do we use nonrenewable
CHAPTER 5 4 Energy Resources SECTION Energy and Energy Resources BEFORE YOU READ After you read this section, you should be able to answer these questions: What is an energy resource? How do we use nonrenewable
Name: Date: Block: IP 670 Conservation of Energy Notes
 Name: Date: Block: IP 670 Conservation of Energy Notes The Law of Conservation of Energy states! energy cannot be or! Energy can only be changed in form (transformed from one type to another) For a bouncing
Name: Date: Block: IP 670 Conservation of Energy Notes The Law of Conservation of Energy states! energy cannot be or! Energy can only be changed in form (transformed from one type to another) For a bouncing
STAAR Vocabulary noticing something about the world around you. using clues to find the answer. everything everywhere. to sort into groups
 Observation noticing something about the world around you Inference using clues to find the answer Matter everything everywhere Classify to sort into groups Physical Property something you observe with
Observation noticing something about the world around you Inference using clues to find the answer Matter everything everywhere Classify to sort into groups Physical Property something you observe with
Year 7 Chemistry HW Questions
 Year 7 Chemistry HW Questions 37 minutes 56 marks Page 1 of 15 Q1. Molly used a ph sensor to test different liquids. She dipped the probe of the sensor into each liquid and recorded the ph value in a table.
Year 7 Chemistry HW Questions 37 minutes 56 marks Page 1 of 15 Q1. Molly used a ph sensor to test different liquids. She dipped the probe of the sensor into each liquid and recorded the ph value in a table.
Kansas Corn: Ethanol - Corn Mash and Distillation High School Student Lab Packet
 Kansas Corn: Ethanol - Corn Mash and Distillation High School Student Lab Packet Overview In this lab, students will learn about ethanol and its important role in our world s ever-increasing demand for
Kansas Corn: Ethanol - Corn Mash and Distillation High School Student Lab Packet Overview In this lab, students will learn about ethanol and its important role in our world s ever-increasing demand for
Kansas Corn: Ethanol - Corn Mash and Distillation High School Student Lab Packet
 Kansas Corn: Ethanol - Corn Mash and Distillation High School Student Lab Packet Overview In this lab, students will learn about ethanol and its important role in our world s everincreasing demand for
Kansas Corn: Ethanol - Corn Mash and Distillation High School Student Lab Packet Overview In this lab, students will learn about ethanol and its important role in our world s everincreasing demand for
WATER. Name Date. Survey/Posttest
 WATER Date 1. What happens to the level of the water in the straw when the water in the bottle is heated? A. The water level goes down. B. The water level stays the same. C. The water level goes up. Why
WATER Date 1. What happens to the level of the water in the straw when the water in the bottle is heated? A. The water level goes down. B. The water level stays the same. C. The water level goes up. Why
Chapters 1&2 Practice Problems
 Name Chapters 1&2 Practice Problems Multiple Choice No Calculators Allowed! 1. Which physical state of matter exhibits the greatest change in volume with changes in temperature or pressure? a) solid b)
Name Chapters 1&2 Practice Problems Multiple Choice No Calculators Allowed! 1. Which physical state of matter exhibits the greatest change in volume with changes in temperature or pressure? a) solid b)
Generating Electricity
 Worksheet 3 Generating Electricity In most power stations, electricity is generated by burning fuels. Coal, oil and natural gas are the common fuels for generating electricity. Major parts of a power station
Worksheet 3 Generating Electricity In most power stations, electricity is generated by burning fuels. Coal, oil and natural gas are the common fuels for generating electricity. Major parts of a power station
Science 8. Unit 1. Booklet
 Science 8 Unit 1 Mixture and Flow of Matter Booklet Name: Class: 1 TOPIC 1 REINFORCEMENT The Particle Model Goal Demonstrate your understanding of the particle model and changes of state. BLM 1-1 Answer
Science 8 Unit 1 Mixture and Flow of Matter Booklet Name: Class: 1 TOPIC 1 REINFORCEMENT The Particle Model Goal Demonstrate your understanding of the particle model and changes of state. BLM 1-1 Answer
Cambridge International Examinations Cambridge Checkpoint
 Cambridge International Examinations Cambridge Checkpoint SCIENCE 1113/02 Paper 2 For Examination from 2014 SPECIMEN PAPER Candidates answer on the Question Paper. Additional Materials: Pen Calculator
Cambridge International Examinations Cambridge Checkpoint SCIENCE 1113/02 Paper 2 For Examination from 2014 SPECIMEN PAPER Candidates answer on the Question Paper. Additional Materials: Pen Calculator
STATE DA VINCI DECATHLON 2017
 STATE DA VINCI DECATHLON 2017 CELEBRATING THE ACADEMIC GIFTS OF STUDENTS IN YEARS 7 & 8 SCIENCE TEAM NUMBER 1 The Coal Paradox: We can't live without it. But can we survive with it? On a scorching August
STATE DA VINCI DECATHLON 2017 CELEBRATING THE ACADEMIC GIFTS OF STUDENTS IN YEARS 7 & 8 SCIENCE TEAM NUMBER 1 The Coal Paradox: We can't live without it. But can we survive with it? On a scorching August
Chemistry Quiz #1 Review
 Name: Chemistry Quiz 1 Review Page 1 of 6 Date: Please check your answers at http://leetz.weebly.com Chemistry Quiz #1 Review Part A: Density Calculations 1. The density of sodium is 0.97 g/cm 3. A sample
Name: Chemistry Quiz 1 Review Page 1 of 6 Date: Please check your answers at http://leetz.weebly.com Chemistry Quiz #1 Review Part A: Density Calculations 1. The density of sodium is 0.97 g/cm 3. A sample
Directions: Complete the following prior to class. Be prepared to show your work on the board.
 Worksheet L4X Student s Name Directions: Complete the following prior to class. Be prepared to show your work on the board. 1) Convert 30 ml to oz, qt, L, gal. 2) Convert 16 oz to kg, lb, tons, g. Page
Worksheet L4X Student s Name Directions: Complete the following prior to class. Be prepared to show your work on the board. 1) Convert 30 ml to oz, qt, L, gal. 2) Convert 16 oz to kg, lb, tons, g. Page
Energy & Power Unit 5, Lesson 1 Explanation
 Energy & Power 5.1.1 Unit 5, Lesson 1 Explanation The Unit Big Idea The designed world is the product of a design process, which provides ways to turn resources - materials, tools and machines, people,
Energy & Power 5.1.1 Unit 5, Lesson 1 Explanation The Unit Big Idea The designed world is the product of a design process, which provides ways to turn resources - materials, tools and machines, people,
LESSON hat is density? 24
 LESSON hat is density? 24 Rocks are heavy. A small box of rocks has a large. Yet a box of the same size containing corks has a small. How can objects that are the same size have different es? Scientists
LESSON hat is density? 24 Rocks are heavy. A small box of rocks has a large. Yet a box of the same size containing corks has a small. How can objects that are the same size have different es? Scientists
OC22 Show that approximately one fifth of the air is oxygen; show that there is CO 2 and water vapour in air
 Chemistry: 10. Air and Oxygen Please remember to photocopy 4 pages onto one sheet by going A3 A4 and using back to back on the photocopier Syllabus OC21 Understand that air is a mixture of gases, and state
Chemistry: 10. Air and Oxygen Please remember to photocopy 4 pages onto one sheet by going A3 A4 and using back to back on the photocopier Syllabus OC21 Understand that air is a mixture of gases, and state
The Production of Electricity Power from Natural Gas. Image Source: Mscalora
 The Production of Electricity Power from Natural Gas Image Source: Mscalora How does it work? Natural Gas can be converted to Electricity using a Gas Turbine Power Plant While gas turbines can power up
The Production of Electricity Power from Natural Gas Image Source: Mscalora How does it work? Natural Gas can be converted to Electricity using a Gas Turbine Power Plant While gas turbines can power up
PHYSICAL SCIENCE 0652/06
 www.xtremepapers.com Centre Number Candidate Number Name CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education PHYSICAL SCIENCE 0652/06 Paper 6 Alternative to Practical
www.xtremepapers.com Centre Number Candidate Number Name CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education PHYSICAL SCIENCE 0652/06 Paper 6 Alternative to Practical
SCIENCE Course of Study
 6 th GRADE SPECIAL EDUCATION SCIENCE Course of Study Findlay City Schools 2005 6 TH GRADE BENCHMARK: (A) Use skills of scientific inquiry processes (e.g., hypothesis, record keeping, description and explanation).
6 th GRADE SPECIAL EDUCATION SCIENCE Course of Study Findlay City Schools 2005 6 TH GRADE BENCHMARK: (A) Use skills of scientific inquiry processes (e.g., hypothesis, record keeping, description and explanation).
DETERMINING THE DENSITY OF LIQUIDS & SOLIDS - worksheet
 DETERMINING THE DENSITY OF LIQUIDS & SOLIDS - worksheet 17 Density, like color, odor, melting point, and boiling point, is a physical property of matter. Therefore, density may be used in identifying matter.
DETERMINING THE DENSITY OF LIQUIDS & SOLIDS - worksheet 17 Density, like color, odor, melting point, and boiling point, is a physical property of matter. Therefore, density may be used in identifying matter.
Copper Odyssey. Chemical Reactions of Copper
 Name Lab Partner(s) Copper Odyssey Chemical Reactions of Copper Date Period Elemental copper metal will be converted into copper (II) ion and then brought through a series of compound conversions until
Name Lab Partner(s) Copper Odyssey Chemical Reactions of Copper Date Period Elemental copper metal will be converted into copper (II) ion and then brought through a series of compound conversions until
Calculating Ballast By Troy Averill
 Calculating Ballast By Troy Averill Lesson Overview: Students use the dimensions of the cargo hold of a ship s hull to calculate the volume of material that can be contained. Students will then use the
Calculating Ballast By Troy Averill Lesson Overview: Students use the dimensions of the cargo hold of a ship s hull to calculate the volume of material that can be contained. Students will then use the
CHY1F (JUN08CHY1F01) General Certifi cate of Secondary Education June Unit Chemistry C1. CHEMISTRY Unit Chemistry C1.
 Surname Other Names For Examiner s Use Centre Number Candidate Number Candidate Signature General Certifi cate of Secondary Education June 2008 SCIENCE B Unit Chemistry C1 CHEMISTRY Unit Chemistry C1 CHY1F
Surname Other Names For Examiner s Use Centre Number Candidate Number Candidate Signature General Certifi cate of Secondary Education June 2008 SCIENCE B Unit Chemistry C1 CHEMISTRY Unit Chemistry C1 CHY1F
CHAPTER 2 HW PRACTICE SOLUTIONS
 CHAPTER 2 HW PRACTICE SOLUTIONS SIGNIFICANT FIGURES 1.) How many significant figures are shown in each of the following numbers? a. 0.0320 3 SF c. 503.10 5 SF e. 0.00702 3 SF b. 8000 1 SF d. 91,000,000
CHAPTER 2 HW PRACTICE SOLUTIONS SIGNIFICANT FIGURES 1.) How many significant figures are shown in each of the following numbers? a. 0.0320 3 SF c. 503.10 5 SF e. 0.00702 3 SF b. 8000 1 SF d. 91,000,000
1. The diagram below represents a solid object with a density of 3 grams per cubic centimeter.
 1. The diagram below represents a solid object with a density of 3 grams per cubic centimeter. What is the mass of this object? A) 0.5 g B) 2 g C) 18 g D) 36 g Base your answers to questions 2 through
1. The diagram below represents a solid object with a density of 3 grams per cubic centimeter. What is the mass of this object? A) 0.5 g B) 2 g C) 18 g D) 36 g Base your answers to questions 2 through
London Examinations IGCSE
 Centre No. Paper Reference Surname Initial(s) Candidate No. Signature Paper Reference(s) 4335/03 4437/08 London Examinations IGCSE Chemistry 4335 Paper 3 Science (Double Award) 4437 Paper 8 Foundation
Centre No. Paper Reference Surname Initial(s) Candidate No. Signature Paper Reference(s) 4335/03 4437/08 London Examinations IGCSE Chemistry 4335 Paper 3 Science (Double Award) 4437 Paper 8 Foundation
GREENHOUSE LAB EXPERIMENTAL FOUNDATIONS OF GLOBAL CLIMATE CHANGE BROUGHT ON BY CARBON DIOXIDE POLLUTION
 GREENHOUSE LAB EXPERIMENTAL FOUNDATIONS OF GLOBAL CLIMATE CHANGE BROUGHT ON BY CARBON DIOXIDE POLLUTION STUDENT VERSION: PART 1...1-10 PART 2...11-14 PART 3...15-17 TEACHER INFORMATION PAGES: PART 1...18-26
GREENHOUSE LAB EXPERIMENTAL FOUNDATIONS OF GLOBAL CLIMATE CHANGE BROUGHT ON BY CARBON DIOXIDE POLLUTION STUDENT VERSION: PART 1...1-10 PART 2...11-14 PART 3...15-17 TEACHER INFORMATION PAGES: PART 1...18-26
Period 24 Solutions: Energy and Water
 Period 24 Solutions: Energy and Water 24.1 The Earth s Water Cycle 1) Components of the Earth s water cycle a) What can happen to some of the water in lakes, rivers, oceans, and in the soil as the Sun
Period 24 Solutions: Energy and Water 24.1 The Earth s Water Cycle 1) Components of the Earth s water cycle a) What can happen to some of the water in lakes, rivers, oceans, and in the soil as the Sun
Chem 5, Spring 2016 Exam 1 (Chapter 1 and 2)
 Chem 5, Spring 2016 Exam 1 (Chapter 1 and 2) NAME 95 pt Mark the answers for Questions 1-41 on your Scantron. Each Question is worth 2 pt. In some cases you will be asked to mark more than one answer.
Chem 5, Spring 2016 Exam 1 (Chapter 1 and 2) NAME 95 pt Mark the answers for Questions 1-41 on your Scantron. Each Question is worth 2 pt. In some cases you will be asked to mark more than one answer.
Science Class 8 Topic: Elements And Compounds Reinforcement Worksheet
 Science Class 8 Topic: Elements And Compounds Reinforcement Worksheet Name: Sec: Date: Q1.Choose the best answer. 1. Which of the following is an element? a) steam b) sugar c)dry ice d) sulphur 2. Which
Science Class 8 Topic: Elements And Compounds Reinforcement Worksheet Name: Sec: Date: Q1.Choose the best answer. 1. Which of the following is an element? a) steam b) sugar c)dry ice d) sulphur 2. Which
ES 106 Laboratory # 1 PROPERTIES OF WATER
 ES 106 Laboratory # 1 PROPERTIES OF WATER 1-1 Introduction What are the physical and chemical properties of water that make it so unique and necessary for living things? When you look at water, taste and
ES 106 Laboratory # 1 PROPERTIES OF WATER 1-1 Introduction What are the physical and chemical properties of water that make it so unique and necessary for living things? When you look at water, taste and
Florida LAFS. English Language Arts INSTRUCTION
 Florida LAFS 5 English Language Arts INSTRUCTION Table of Contents UNIT 1 Key Ideas and Details in Informational Text............ 8 Standards Lesson 1 Finding Main Ideas and Details............ 10 5.RI.1.2,
Florida LAFS 5 English Language Arts INSTRUCTION Table of Contents UNIT 1 Key Ideas and Details in Informational Text............ 8 Standards Lesson 1 Finding Main Ideas and Details............ 10 5.RI.1.2,
The table below shows some of the properties of the elements cobalt and nickel.
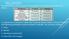 BELL RINGER The table below shows some of the properties of the elements cobalt and nickel. A scientist has a sample of metal that could be either cobalt or nickel. Which of the following properties could
BELL RINGER The table below shows some of the properties of the elements cobalt and nickel. A scientist has a sample of metal that could be either cobalt or nickel. Which of the following properties could
Triple Beam Balance: add the three together: 700g + 20g + 2.9g = 722.9g Metric base unit for mass is gram.
 6 th Grade 2 nd Nine Week CSA Study Guide 2015-16 SOL 6.1b: Make precise and consistent measurements and estimations. Graduated Cylinder: read from the lowest part of the water line curve. Reading: 56ml
6 th Grade 2 nd Nine Week CSA Study Guide 2015-16 SOL 6.1b: Make precise and consistent measurements and estimations. Graduated Cylinder: read from the lowest part of the water line curve. Reading: 56ml
1 Elements. TAKE A LOOK 2. Identify Look at the illustration and identify one source of iron that comes to Earth from somewhere else.
 CHAPTER 4 1 Elements SECTION Elements, Compounds, and Mixtures BEFORE YOU READ After you read this section, you should be able to answer these questions: What is an element? How do elements differ from
CHAPTER 4 1 Elements SECTION Elements, Compounds, and Mixtures BEFORE YOU READ After you read this section, you should be able to answer these questions: What is an element? How do elements differ from
May 2018 for entry September 2018
 May 2018 for entry September 2018 Information for Candidates: 1. Write your name and school on this page. 2. Write all of your answers in the spaces provided. If you need additional paper then please ask
May 2018 for entry September 2018 Information for Candidates: 1. Write your name and school on this page. 2. Write all of your answers in the spaces provided. If you need additional paper then please ask
Total Grade /150 Checked by
 FIRST LETTER OF YOUR LAST NAME CHEMISTRY 1127 EXAM I NAME (PRINT) SECTION SIGNATURE TA PLEASE READ THE FOLLOWING INSTRUCTIONS Do NOT begin the exam until asked to do so. There are 8 numbered pages, a useful
FIRST LETTER OF YOUR LAST NAME CHEMISTRY 1127 EXAM I NAME (PRINT) SECTION SIGNATURE TA PLEASE READ THE FOLLOWING INSTRUCTIONS Do NOT begin the exam until asked to do so. There are 8 numbered pages, a useful
CHM101 Lab - Energy Grading Rubric
 Name Team Name CHM101 Lab - Energy Grading Rubric To participate in this lab you must have splash- proof goggles, proper shoes and attire. Criteria Points possible Points earned Lab Performance Printed
Name Team Name CHM101 Lab - Energy Grading Rubric To participate in this lab you must have splash- proof goggles, proper shoes and attire. Criteria Points possible Points earned Lab Performance Printed
Reactivity Series. Question Paper. Save My Exams! The Home of Revision. Module Double Award (Paper 1C) Chemistry of the Elements.
 Save My Exams! The Home of Revision For more awesome GCSE and A level resources, visit us at www.savemyexams.co.uk Reactivity Series Question Paper Level GCSE Subject Chemistry Exam Board Edexcel IGCSE
Save My Exams! The Home of Revision For more awesome GCSE and A level resources, visit us at www.savemyexams.co.uk Reactivity Series Question Paper Level GCSE Subject Chemistry Exam Board Edexcel IGCSE
Experiment #3. Density and Specific Gravity.
 Experiment #3. Density and Specific Gravity. Goals 1. To measure and calculate the density and specific gravity of various substances. 2. To use significant figures correctly in calculations. Background
Experiment #3. Density and Specific Gravity. Goals 1. To measure and calculate the density and specific gravity of various substances. 2. To use significant figures correctly in calculations. Background
Iÿtÿÿeÿ/ÿrh Jÿtcket. Tÿlchÿwÿeÿ
 o 0 Iÿtÿÿeÿ/ÿrh Jÿtcket Tÿlchÿwÿeÿ Elements, Compounds, and Mixtures Classify each of the pictures below by placing the correct label in the blanks below: A= Element D-- Mixture of compounds B= Compound
o 0 Iÿtÿÿeÿ/ÿrh Jÿtcket Tÿlchÿwÿeÿ Elements, Compounds, and Mixtures Classify each of the pictures below by placing the correct label in the blanks below: A= Element D-- Mixture of compounds B= Compound
Energy and Energy Resources
 Energy and Energy Resources Energy Defined as the ability to do work or the ability to cause change. Two types of energy: Kinetic energy- energy of motion; anything that moves has kinetic energy, cars,
Energy and Energy Resources Energy Defined as the ability to do work or the ability to cause change. Two types of energy: Kinetic energy- energy of motion; anything that moves has kinetic energy, cars,
Topic P3 Sustainable Energy Homework booklet
 Name Key terms and spellings on back page Topic P3 Sustainable Energy Homework booklet Due Date Teacher Comment Homework 1 Homework 2 Homework 3 Homework 4 Homework One: Energy and Power Stations Add these
Name Key terms and spellings on back page Topic P3 Sustainable Energy Homework booklet Due Date Teacher Comment Homework 1 Homework 2 Homework 3 Homework 4 Homework One: Energy and Power Stations Add these
SIMPLE CHEMICAL REACTIONS
 SIMPLE CHEMICAL REACTIONS 1. Magnesium burns in air giving a very bright light. (a) Complete the word equation below to show this reaction. magnesium +...... The diagram shows four gas-jars. Each contains
SIMPLE CHEMICAL REACTIONS 1. Magnesium burns in air giving a very bright light. (a) Complete the word equation below to show this reaction. magnesium +...... The diagram shows four gas-jars. Each contains
Chemistry 8402/2 8402/2. (Jun ) AQA Level 1/2 Certificate June Paper 2 TOTAL. Time allowed 1 hour 30 minutes
 Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials Question Mark AQA Level 1/2 Certificate June 2014 Chemistry 8402/2 Paper 2 Tuesday 10 June
Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials Question Mark AQA Level 1/2 Certificate June 2014 Chemistry 8402/2 Paper 2 Tuesday 10 June
Minnesota Comprehensive Assessments-Series III
 Name Minnesota Comprehensive Assessments-Series III Science Item Sampler Grade 8 ITEM SAMPLERS ARE NOT SECURE TEST MATERIALS. THIS ITEM SAMPLER TEST BOOK MAY BE COPIED OR 18 Point State of Minnesota Copyright
Name Minnesota Comprehensive Assessments-Series III Science Item Sampler Grade 8 ITEM SAMPLERS ARE NOT SECURE TEST MATERIALS. THIS ITEM SAMPLER TEST BOOK MAY BE COPIED OR 18 Point State of Minnesota Copyright
Calorie Unit to measure amount of energy in foods and fuels.
 Measuring Energy: Calorie Unit to measure amount of energy in foods and fuels. One calorie = amount of heat energy needed to raise the temperature of 1 gram of water by 1 degree Celsius. Watt Unit used
Measuring Energy: Calorie Unit to measure amount of energy in foods and fuels. One calorie = amount of heat energy needed to raise the temperature of 1 gram of water by 1 degree Celsius. Watt Unit used
Density. How tightly the atoms are packed together in an object
 Density How tightly the atoms are packed together in an object Mass vs Weight Mass is the amount of matter in an object. (it doesn t change) Weight is determined based on the amount of gravity pulling
Density How tightly the atoms are packed together in an object Mass vs Weight Mass is the amount of matter in an object. (it doesn t change) Weight is determined based on the amount of gravity pulling
v = 5.00cm x 3.00cm x 2.00cm 2. What is the volume of a marble that has a mass of 3.0 g and a density of 2.7 g/ml? Formula (density) Substitution
 Name: STUDY GUIDE: MEASUREMENT, DENSITY, MASS, VOLUME, AND DIMENSIONAL ANALYSIS (2016) 1. A solid block is 5.00 cm high, 3.00 cm wide, and 2.00 cm long. It has a mass of 129 g. What is the density? Formula
Name: STUDY GUIDE: MEASUREMENT, DENSITY, MASS, VOLUME, AND DIMENSIONAL ANALYSIS (2016) 1. A solid block is 5.00 cm high, 3.00 cm wide, and 2.00 cm long. It has a mass of 129 g. What is the density? Formula
Metals and reactivity series Question paper 1
 Metals and reactivity series Question paper 1 Level GCSE Subject Chemistry Exam Board CCEA Topic Metals and reactivity series Sub-Topic Metals and reactivity series Booklet Question paper 1 Time Allowed:
Metals and reactivity series Question paper 1 Level GCSE Subject Chemistry Exam Board CCEA Topic Metals and reactivity series Sub-Topic Metals and reactivity series Booklet Question paper 1 Time Allowed:
Kansas Corn: Ethanol - Corn Mash and Distillation
 Kansas Corn: Ethanol - Corn Mash and Distillation This lab is made possible with the support and content contributions of the Kansas Corn Commission. Grade Level: High School Overview In this lab, students
Kansas Corn: Ethanol - Corn Mash and Distillation This lab is made possible with the support and content contributions of the Kansas Corn Commission. Grade Level: High School Overview In this lab, students
Angel International School - Manipay 3 rd Term Examination July, 2015
 Grade 08 Angel International School - Manipay 3 rd Term Examination July, 2015 Chemistry Duration: 2 Hours Index No:- Part I Choose the correct answer and underline it. 1. Which of the following correctly
Grade 08 Angel International School - Manipay 3 rd Term Examination July, 2015 Chemistry Duration: 2 Hours Index No:- Part I Choose the correct answer and underline it. 1. Which of the following correctly
Cambridge International Examinations Cambridge Primary Checkpoint
 Cambridge International Examinations Cambridge Primary Checkpoint SCIENCE 0846/01 Paper 1 April 2016 Candidates answer on the Question Paper. Additional Materials: Pen Calculator Pencil Ruler 45 minutes
Cambridge International Examinations Cambridge Primary Checkpoint SCIENCE 0846/01 Paper 1 April 2016 Candidates answer on the Question Paper. Additional Materials: Pen Calculator Pencil Ruler 45 minutes
Cambridge International Examinations Cambridge International General Certificate of Secondary Education
 Cambridge International Examinations Cambridge International General Certificate of Secondary Education *5349025480* CO-ORDINATED SCIENCES 0654/63 Paper 6 Alternative to Practical October/November 2015
Cambridge International Examinations Cambridge International General Certificate of Secondary Education *5349025480* CO-ORDINATED SCIENCES 0654/63 Paper 6 Alternative to Practical October/November 2015
Paper 2. Year 9 science test. Remember: First name. Last name. Class. Date
 Sc KEY STAGE 3 Year 9 science test TIER 3 6 Paper 2 First name Last name Class Date Please read this page, but do not open your booklet until your teacher tells you to start. Write your name, your class
Sc KEY STAGE 3 Year 9 science test TIER 3 6 Paper 2 First name Last name Class Date Please read this page, but do not open your booklet until your teacher tells you to start. Write your name, your class
DETERMINING THE DENSITY OF LIQUIDS & SOLIDS
 DETERMINING THE DENSITY OF LIQUIDS & SOLIDS Density, like color, odor, melting point, and boiling point, is a physical property of matter. Therefore, density may be used in identifying matter. Density
DETERMINING THE DENSITY OF LIQUIDS & SOLIDS Density, like color, odor, melting point, and boiling point, is a physical property of matter. Therefore, density may be used in identifying matter. Density
Eton College King s Scholarship Examination SCIENCE 1 (Theory) (60 minutes) Candidate Number:
 Eton College King s Scholarship Examination 2017 SCIENCE 1 (Theory) (60 minutes) Candidate Number: Remember to write your candidate number on every sheet in the space provided. You should attempt ALL the
Eton College King s Scholarship Examination 2017 SCIENCE 1 (Theory) (60 minutes) Candidate Number: Remember to write your candidate number on every sheet in the space provided. You should attempt ALL the
ENERGY FUN. Extending Fun With Energy In Your Classroom CURRICULUM GUIDE. GRADES 3 through 5 with. Meets. Next Generation Science Standards
 JEFF BOYER PRODUCTIONS presents CURRICULUM GUIDE FUN ENERGY GRADES 3 through 5 with Extending Fun With Energy In Your Classroom This study guide is meant to build on the enthusiasm and curiosity of your
JEFF BOYER PRODUCTIONS presents CURRICULUM GUIDE FUN ENERGY GRADES 3 through 5 with Extending Fun With Energy In Your Classroom This study guide is meant to build on the enthusiasm and curiosity of your
Human Dependence on Natural Resources
 You use Earth s resources every day. When you eat cereal with milk for breakfast, you use resources from plants and animals. When you ride the bus to school, you use energy (fuel) resources. When you take
You use Earth s resources every day. When you eat cereal with milk for breakfast, you use resources from plants and animals. When you ride the bus to school, you use energy (fuel) resources. When you take
How Do You Choose Cookware?
 Cookin' Chem Activity 6 How Do You Choose Cookware? GOALS In this activity you will: Explore the concept of specific heat capacity. Experimentally determine the specific heat capacity of various substances.
Cookin' Chem Activity 6 How Do You Choose Cookware? GOALS In this activity you will: Explore the concept of specific heat capacity. Experimentally determine the specific heat capacity of various substances.
TEST I REVIEW. 1. All of the following are properties of antimony. Which one is not a
 TEST I REVIEW I Identify the letter of the choice that best completes the statement or answers the question. 1. All of the following are properties of antimony. Which one is not a physical property? a.
TEST I REVIEW I Identify the letter of the choice that best completes the statement or answers the question. 1. All of the following are properties of antimony. Which one is not a physical property? a.
CHEMISTRY WESTMINSTER SCHOOL THE CHALLENGE Thursday 28 April Time allowed: 30 minutes. Please write in black or blue ink.
 WESTMINSTER SCHOOL THE CHLLENGE 2016 CHEMISTRY Thursday 28 pril 2016 Time allowed: 30 minutes Please write in black or blue ink. Write your answers in the spaces provided. For examiner use only Total Mark
WESTMINSTER SCHOOL THE CHLLENGE 2016 CHEMISTRY Thursday 28 pril 2016 Time allowed: 30 minutes Please write in black or blue ink. Write your answers in the spaces provided. For examiner use only Total Mark
CHY1H (JUN09CHY1H01) General Certifi cate of Secondary Education June Unit Chemistry C1. CHEMISTRY Unit Chemistry C1.
 Surname Other Names For Examiner s Use Centre Number Candidate Number Candidate Signature General Certifi cate of Secondary Education June 2009 SCIENCE B Unit Chemistry C1 CHEMISTRY Unit Chemistry C1 CHY1H
Surname Other Names For Examiner s Use Centre Number Candidate Number Candidate Signature General Certifi cate of Secondary Education June 2009 SCIENCE B Unit Chemistry C1 CHEMISTRY Unit Chemistry C1 CHY1H
Density What is density? How do you measure density?
 Density What is density? How do you measure density? What is density? Density describes how much mass is in a given volume of a material. Mass: the amount of matter in an object. Volume: the amount of
Density What is density? How do you measure density? What is density? Density describes how much mass is in a given volume of a material. Mass: the amount of matter in an object. Volume: the amount of
Determining the Density of Unknown Substances
 Determining the Density of Unknown Substances Introduction: Most of the elements on the periodic table are metals and solids. These elements have observable properties that make it possible to identify
Determining the Density of Unknown Substances Introduction: Most of the elements on the periodic table are metals and solids. These elements have observable properties that make it possible to identify
Card #1/24. Describe how thermal energy is passed on in terms of ions Using these ideas explain how a convection current occurs
 Card #1/24 Card #2/24 Topic: Conduction Topic: Convection In what state of matter does conduction occur? In what states of matter does convection occur? Explain why it needs to be in this state? Define
Card #1/24 Card #2/24 Topic: Conduction Topic: Convection In what state of matter does conduction occur? In what states of matter does convection occur? Explain why it needs to be in this state? Define
B I N G-B I N G-T O E. FREE Space
 B I N G-B I N G-T O E FREE Space B I N G-B I N G-T O E GAME RULES Right side of room X Left side of room O 2 players from each team go head to head (standing by opposite team) Team may not help First team
B I N G-B I N G-T O E FREE Space B I N G-B I N G-T O E GAME RULES Right side of room X Left side of room O 2 players from each team go head to head (standing by opposite team) Team may not help First team
GCSE BITESIZE Examinations
 GCSE BITESIZE Examinations General Certificate of Secondary Education AQA SCIENCE A Unit Physics P1a AQA Chemistry Unit Physics P1a PHY1A (Energy and Electricity) (Energy and Electricity) FOUNDATION TIER
GCSE BITESIZE Examinations General Certificate of Secondary Education AQA SCIENCE A Unit Physics P1a AQA Chemistry Unit Physics P1a PHY1A (Energy and Electricity) (Energy and Electricity) FOUNDATION TIER
PHYSICAL AND CHEMICAL CHANGES CLASS 7. Types of changes: The changes are of two kinds, physical and chemical..
 PHYSICAL AND CHEMICAL CHANGES CLASS 7 Types of changes: The changes are of two kinds, physical and chemical.. Physical Properties of Substances Properties such as shape, size, colour and state of a substance
PHYSICAL AND CHEMICAL CHANGES CLASS 7 Types of changes: The changes are of two kinds, physical and chemical.. Physical Properties of Substances Properties such as shape, size, colour and state of a substance
Name: 2015 Non Common Entrance Examination Third and Fourth Form Entry. Science. Time Allowed : 1 hour
 Name: 2015 Non Common Entrance Examination Third and Fourth Form Entry Science Time Allowed : 1 hour Please write your name in the box above Answer as many questions as you can in the time available Calculators
Name: 2015 Non Common Entrance Examination Third and Fourth Form Entry Science Time Allowed : 1 hour Please write your name in the box above Answer as many questions as you can in the time available Calculators
Experiment: Measurements
 Experiment: Measurements I. INTRODUCTION Measurements are essential to experimental sciences such as chemistry, physics, biology, and geology. The measurements are usually made using the metric system
Experiment: Measurements I. INTRODUCTION Measurements are essential to experimental sciences such as chemistry, physics, biology, and geology. The measurements are usually made using the metric system
Practice with Properties of Matter. Practice with Physical and Chemical Changes. Practice with Mixtures and Pure Substances
 Chapter 1 Practice Problems Practice with Properties of Matter Indicate whether each of the following statements describes a physical or a chemical property. a) the normal color of elemental bromine is
Chapter 1 Practice Problems Practice with Properties of Matter Indicate whether each of the following statements describes a physical or a chemical property. a) the normal color of elemental bromine is
Administered December 2003
 STUDENT NAME DATE ID GRADE 5 SCIENCE Administered December 2003 Page 1 5 th Grade Science Interim 2; SAISD Standard 23/32; TAKS Commended; 30/32 DIRECTIONS Read each question and choose the best answer.
STUDENT NAME DATE ID GRADE 5 SCIENCE Administered December 2003 Page 1 5 th Grade Science Interim 2; SAISD Standard 23/32; TAKS Commended; 30/32 DIRECTIONS Read each question and choose the best answer.
