Introduction Methods of Analysis Near Infrared Spectrophotometer (NIRS) High Performance Thin Layer Chromatography (HPTLC)...
|
|
|
- Shawn Dennis
- 6 years ago
- Views:
Transcription
1 REPORT ON KAV A ANALY S IS (JULY)
2 Table of Contents Introduction... 3 Methods of Analysis... 4 Near Infrared Spectrophotometer (NIRS)... 4 High Performance Thin Layer Chromatography (HPTLC)... 4 Thin Layer Chromatography (TLC)... 4 Colorimetry... 4 Standard Operating Procedures... 6 Kava Analysis... 6 Trends in kava quality... 7 Revenue earned from Tests... 8 Conclusion... 9 Recommendations... 9 References
3 Introduction Kava, Piper methysticum, is a traditional drink of and a number of Pacific countries such as Fiji, Tonga, Samoa and Palau. The active components in kava are kavalactones. The six major kavalactones are: [1] methysticin, [2] dehydromethysticin, [3] kavain, [4] yangonin, [5] desmethoxy yangonin,[6] dihydrokavain. has 12 varieties of noble kava, 79 varieties of medicinal kava and 44 varieties of two-day kava. From these three groups of kavas, the noble and medicinal can be differientiated from the two day kavas by the concentration of dihydrokavain, kavain, yangonin, methysticin and desmethoxyyangonin, in this order, or with Kavain being in highest concentration followed by dihydrokavain and then the rest of the other kavalactones. During the mid-1990 s to the year 2000, experienced an export boom which saw large amounts of kava being exported into Europe to Nutriceutical companies looking for new herbal supplements to combat stress, depression and other health issues. Farmers and Exporters spurred by the kava boom did not place quality as a priority. This oversight in quality control saw the export of peelings along with roots and chips. A number of deaths from liver poisoning due to the consumption of pills and other kava based products resulted in the ban of kava to Europe. This ban lasted from 2001 through to the early part of This saw a loss of VT5 billion annually for Pacific Island Countries (PINA, 2012). In 2002, the Kava Act was drafted so as to create quality standards for the export of Kava. The Act basically outlines the standards for the farming of kava and for the exportation of Kava. (Kava Act, 2002) While the standards outlined in the Act are mandatory, the lack of a Regulation to implement this has made it difficult to regulate the Act. In the 11 th session of the Coordinating Committee for North America and South West Pacific (CCNSWP) held in Tonga in 2010, Tonga presented the discussion paper of a proposal for new work on the development of an international standard for dry kava products. In the 12 th session of its meeting in Madang, Papua new Guinea in 2012, it was agreed that would take the lead on collecting more evidence on the safety of kava. More work is still required to be carried out on this standard. The High Level Kava Conference held in in early 2012 highlighted the importance of quality control of kava and the economic effects that the EU ban had on the annual revenue of all Pacific island countries who are major exporters. Renewed efforts were made then to continue the fight towards lifting the EU ban and to collect more scientific evidence for the development of an international standard for Kava. To date, BfARM the German Pharmaceutical company which caused the EU ban, has lost its court case and the EU has lifted the ban on kava exports. The lesson to be learnt from this experience is to ensure the quality of our kava exports is maintained through regular testing and traceability. 3
4 Methods of Analysis The common method of analysis is high pressure liquid chromatography (HPLC). This method was employed in the late 1990 s by one of the export companies, however, it is very expensive to maintain as it requires special analytical columns and chemicals specifically for HPLC. Near Infrared Spectrophotometer (NIRS) This was the initial method of analysis used at this laboratory. While it was fast and did not require the use of expensive chemicals it required annual calibration of the machine which would have cost VT1,000,000 which would be more than the revenue generated from the analysis. Due to this high cost, PICTURE 1: HPTLC PLATE SHOWING THE INDIVIDUAL KAVALACTONES AND FLAVOKAVAINS it was necessary to find another method of analysis. High Performance Thin Layer Chromatography (HPTLC) This method of analysis was investigated and found to be a lot more accurate than NIRS in that it was able to detect small differences between the noble types and non-noble types of kava. (Lebot V., Do, TK and Legendre, L. 2014). This method can only take a minimum of samples at a time on a 10 x 20 cm HPTLC plate [Picture 1]. One analysis session takes 2 hours and in one day 144 to 160 samples could be analysed. In terms of chemicals, it uses 35mLs of a mixture of dioxane, hexane and ethyl acetate per analysis. Due to the low throughput of samples at the current time, it would not be possible to use this method of analysis. Thin Layer Chromatography (TLC) This method is similar to that of HPTLC but is simpler and does not require high set up costs. It is however, a qualitative method. This means that it can determine the difference between noble and non-noble kava but it is not able to quantify the individual kavalactones. Colorimetry 4
5 This method uses the different colours of the acetone extract to identify the kava variety. The kava powder when mixed with acetone gives different coloured solutions. The colour of the solution determines the kava variety with noble varieties giving a yellow solution, two day varieties giving an orange to dark orange solution and wild varieties giving brown to dark brown solution [Picture 2]. PICTURE 2: EXTRACTS OF NOBLE AND NON-NOBLE KAVA. FROM THE FAR LEFT THE FIRST FOUR TEST TUBES SHOW THE YELLOE COLOUR OF THE NOBLE VARIETIES, THE NEXT FIVE TEST TUBES SHOW THE ORANGE COLOUR OF THE TWO DAY VARIETIES, THE LAST THREE TEST TUBES ON THE RIGHT SHOW THE COLOUR OF THE WILD KAVA VARIETIES. This is the simplest and easiest method to use to determine the differences between noble and nonnoble kava through the colour of the extract. In terms of set up costs, it requires the use of a UV-Vis spectrometer and can be used in conjunction with the TLC method. In terms of costs to the Exporter it would not be expensive to run and in the long run may be developed as a test kit for exporters. 5
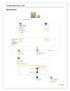
6 Standard Operating Procedures Over the past three years a process has been set up for analysis. This is illustrated in the flow diagram illustrated in Figure 1. Kava sample brought in Sample recorded Sample tested Results recorded Results and Certificate sent Analysis paid Invoice sent FIGURE 1: FLOW CHART OF ANALYTICAL PROCEDURE Details of this process can be viewed in the Policy and Procedures Manual for the laboratory [Annex 1]. Kava Analysis Exporter (July) South Seas Commodities Kava Store The Kava House Kava Emporium VRRP Valele Trust De Quiros Exporters American Kava Association (AKA) VCMB 9 1 Kava House Nakamal trading 1 Hugaituva Kava Store 1 Private Individuals TOTAL
7 TABLE 1: EXPORTERS VERSUS NUMBER OF SAMPLES ANALYSED In 2012, analysis of kava commenced with the first 14 samples coming from South Seas commodities, a kava exporting company which exports primarily to New Caledonia. Kava samples were analyzed using the Near Infrared Spectrophotometer (NIRS) initially as a pilot study. It was slow to take off [Table 1] as Exporters were not aware of the service or were unsure about the accreditation status of the laboratory. In 2013, a problem with the accuracy of the NIRS caused analysis to be stopped in 2013 hence the low number of analysis. Figure 2 gives a picture of the Percentage of Kava samples analysed per Exporter by Kava type (noble or two day) who have utilized the testing services of the Food Technology Development Centre and Analytical Unit to verify the quality of their samples. Certain Exporters have increased the number of samples they have brought in for analysis while others have reduced their samples. This fluctuation in analyses is directly related to the lack of implementation of Kava Standards given that it is a mandatory standard. FIGURE 2: COMPARISON OF NOBLE AND TWO DAY KAVA BROUGHT IN BY EXPORTERS FOR ANALYSIS Trends in kava quality 7
8 Percentage Trends in Kava Type Analysed from 2012-July July Year Noble Two Day FIGURE 3: COMPARISON OF KAVA TYPES ANALYSED SINCE 2012 TO MID-JULY Since kava analysis commenced there has been a marked increase in the noble varieties analyzed. It must be noted here that this data reflects samples that have been brought in for analysis only. Figure 3 shows the trend in the quality of kava types brought in for analysis for the past 3 years. While some Exporters are ensuring that all kava exported are analyzed, there are still a certain number who do not check the quality of the kava exported. This is evident in Kava Exporters residing in Santo. Current arrangements with the Commodities Marketing Board (VCMB) have seen attempts by the Board to collect samples for analysis with the Exporter paying for these tests. Experience has seen that Kava consignments that have been exported into the United States, through the American Kava Association [Figure 2], have had samples sent back to for testing as per the Food Drug Administration (FDA) rules. While there has been an improvement in the quality of kava exported, there is room for more improvement especially in the regulating of Exports. It must be noted that the data presented come from only a small number of Exporters based in and the occasional sample from Santo. Revenue earned from Tests Year Total Samples analyzed Total revenue earned No fees charged ,600 8
9 , (July) 38 38,000 Total , 600 estimates TABLE 2: REVENUE EARNED PER YEAR [NOTE: VALUES ARE CALCULATED FROM THE NUMBER OF SAMPLES ANALYSED IN OUR DATABASE ONLY AND IS NOT FROM THE DEPT OF FINANCE. THERE MAY BE DIFFERENCES IN VALUES] During the first year of analysis, no fees were charged as the Analytical Unit was trialing out the tests as this was the first time that the Unit was analyzing kava. The low number of samples and revenue in 2013 was related directly to problems with the NIRS machine not giving accurate readings. Analysis increased in 2014 due to the use of the colorimetric method of analysis. The fees that are currently charged for analysis are 1000VT for samples that have not been ground up as extra time is taken to powder the samples, while VT500 is charged for powdered samples. A fee of VT500 is charged for the Certificate of Analysis (COA). At the current time there is no legal framework allowing for fees to be prescribed so the fees will remain at this level until such time. Conclusion Kava analysis will come ever more important as more and more importing countries become more knowledgeable about kava. This means that they will demand good quality kava. For this reason, it is necessary that the Kava Act is implemented to ensure that the loss of revenue experienced during the EU ban is not repeated. It also means that alternative markets need to be sought closer to home. The lesson learnt from the EU ban was that quality and proof of quality should never be overlooked. Recommendations The following are recommendations made to improve the current testing capacity Encourage Bio-security and Department of Agriculture and Rural Development (DARD) to enforce Kava Regulations to implement the Kava Act to continue to improve the quality of kava exported. Increase the awareness of farmers on the negative impacts of poor quality kava on the market. Enhance testing and calibration records and standard operating procedures Equip the analytical unit with appropriate equipment to increase its current testing capacity 9
10 Develop the colorimetric method of analysis to be used in conjunction with the TLC method as a quick and less costly method for routine analysis. Work towards GLP (Good laboratory practices) Certification for Kava Analysis. 10
11 References Pacific Kava Producers losing over VT5 Billion in EU Ban. PINA, Date Kava Act, Government, Food Chem May 15;151: doi: /j.foodchem Epub 2013 Nov 27. Date of Access 3/8/2015 ftp://ftp.fao.org/codex/meetings/ccnaswp/ccnaswp12/na12_08e.pdf. Date of Access 3/8/
12 Annex 1
13 Table of Contents INTRODUCTION... Error! Bookmark not defined. SUMMARY OF ANALYSIS PROCESS... Error! Bookmark not defined. 1.0 ANALYSIS REQUEST... Error! Bookmark not defined. A.R.1.1. Analysis request form... Error! Bookmark not defined. 2.0 RECEIVING SAMPLES... Error! Bookmark not defined. D.B.C. 2.1 Commodities Sample Receipt Database... Error! Bookmark not defined. D.B.I. 2.2 Import Sample Receipt Database... Error! Bookmark not defined. 3.0 ANALYSING SAMPLES... Error! Bookmark not defined. 4.0 ANALYSIS Report... Error! Bookmark not defined. AR 4.1 Certificate of Analysis and Analysis Report... Error! Bookmark not defined. 5.0 PAYMENT... Error! Bookmark not defined. PYT 5.1 PYT 5.2 Invoice... Error! Bookmark not defined. Receipt... Error! Bookmark not defined.
14 Ministry of Tourism, Trade, Commerce and Tel INTRODUCTION The key to quick and efficient laboratory testing lies in the processes and procedures in a laboratory and how well laboratory technicians know and implement them. Knowing what to do in a laboratory will ensure that quality is maintained at all times. This Policy and Procedures Manual serves as a guide to all current and new staff of the Food Technology Development Centre and Analytical Unit as to the policies that guide the standard operating procedures of this laboratory and the rationale behind them. This guide is to ensure a standard procedure is in place on how to go about the day today running of the Laboratory and maintain transparency and Traceability. As time goes by additional policies and procedures will be added to ensure that these policies are as up-to-date as possible. QUALITY POLICY STATEMENT To ensure accurate and timely analytical testing services and to continuously meet or exceed the stated or implied expectation our customers through day-to-day interactions. Effective date: April 1 st, 2012 a) Management commitment to good professional practice and quality of services provided to the customer: Tests are always carried out in accordance with stated standardized methods and customers requirements. Requests to perform tests that may jeopardize an objective result or have a low validity are rejected. b) Standards of service include: a. Customer satisfaction b. Accurate c. Timely Excellence in the workplace is promoted by providing all employees with the knowledge, training, and tools necessary to allow for the completion of accurate and timely work.
15 Ministry of Tourism, Trade, Commerce and Tel SUMMARY OF ANALYSIS PROCESS Process Procedure Form 1. Analysis Request Form AR Sample Receipt Database D.B.K Sample Analysis Laboratory Manual Worksheets 4. Analysis Report AR 4.1 Certificate of Analysis and Analytical 5. Payment Invoice Receipt
16 Ministry of Tourism, Trade, Commerce and Tel ANALYSIS REQUEST Policy: All information pertaining to analysis shall be recorded and given correctly and in an efficient manner and shall be with regards to the test required for the sample in question only. Rationale: Customers who request analysis must always be given the information regarding analysis as correctly and efficiently as possible in order to make it easy for them to make a decision about whether to analyze or not. Procedure: AR 1.1 Record information in Analysis Request form Find out what kind of test the customer wants. a. If Customer tells you the type of test they want then follow procedure 1.2. b. If the Customer does not know what type of test is require for their sample follow procedure Give details about the test, a. Payment method b. When Customer should expect their test results c. Other information about the particular test they are requesting Inform the customer about the different tests and what they determine in terms of their product and similar information to procedure Place request form on the IN tray on the Laboratory Technicians desk. Forms: CHF1.1 Analysis Request Form Other Documents: Confidentiality Policy,
17 Ministry of Tourism, Trade, Commerce and A.R.1.1. Analysis request form Date of Receipt: Reference Number Office use only Client Information Client Name Company Name Company Address Telephone Number Address Sample Information Sample Name Sample Type Sample Description
18 Ministry of Tourism, Trade, Commerce and Testing Information Type Of Test.. ph.. Iron test Iron Content Cut Test % Fat Kava Quality test Moisture SAP test HPTLC Esterification Value
19 2.0 RECEIVING SAMPLES Ministry of Tourism, Trade, Commerce and Policy: All samples received for analysis must be recorded on the Sample Receipt form, labeled and recorded in appropriate database immediately on day of receipt. Rationale: Procedure: It is good laboratory practice to record all samples received for analysis on a database or record book. This makes it easy to collect statistics on tested samples and also for traceability purposes. All samples recorded are given a laboratory sample number which is used on all forms related to the sample Upon receiving a sample, record all information in the sample analysis request form in the Database in the Main station PC. The analysis request form will determine what type of sample is to be analyzed and what tests need to be carried out Find the appropriate worksheet needed for this testing method and attach it to the sample Place the sample along with its worksheet in the For Analysis tray. Forms: Analysis Database (main computer) For Kava samples, Open Data base D.B.K. 1.1 For Cocoa sample, open Database D.B.CC 1.2 For coconut oil sample, open Data base D.B.E.C. 1.3 For Imported foods, Open Data base D.B.I. 1.4 Other Documents: Worksheets [Laboratory Manual]
20 Ministry of Tourism, Trade, Commerce and D.B.C. 2.1 Commodities Sample Receipt Database ID Date Receipt Client Name Owners sample code Lab. Sample code 1 3/27/2012 VKS 10 10B2412 Sample Description Method of Analysis Date of Analysis Analysis Result Price per analysis Invoice number Dried kava roots & chips NIRS 27/03/12 Noble VT /2012 Reference Number 01VKE VAT Amount Owed Receipt Number Date of Payment ,125VT /27/12 D.B.I. 2.2 Import Sample Receipt Database ID Date Receipt Client Name Owners sample code Lab. Sample code Sample Description Method of Analysis Date of Analysis Analysis Result Price per analysis Invoice number Reference Number ,125VT /27/12 VAT Amount Owed Receipt Numbe r Date of Payment
21 Ministry of Tourism, Trade, Commerce and 3.0 ANALYSING SAMPLES Policy: All samples received must be analyzed according to procedures in the official Laboratory manual. Rationale: All test methods used in this laboratory can be found in the Laboratory Manual. Test methods that are beyond our capabilities are not recorded. Procedure: Refer to Laboratory Manual for appropriate Test method for the sample. Forms: Refer to Laboratory Manual for Worksheets and Analysis Result Sheets. Other Documents: Laboratory Manual.-Volume 1 : CHEMISTRY
22 Ministry of Tourism, Trade, Commerce and 4.0 ANALYSIS Report Policy: Analyses shall be reported on the official analysis results (AR) form and certificate of analysis (COA) only with endorsement by the designated head of the laboratory. Rationale: All results reported must be on the official laboratory letterhead only and signed off by the head of the laboratory. Results that have been reported otherwise will not be regarded as official and as having come from the laboratory. Procedure: 4.1 Prepare a Certificate of Analysis (COA) and an Analysis Report (AR) from the data on the appropriate worksheet. 4.2 Submit the CoA and AR to the Manager for approval and signature 4.3 Place CoA and AR in the CoA and AR box or if payment was received for analysis on deposit of sample then a scanned copy of CoA and AR should be ed immediately to the client. Forms: Analysis Report Form Certificate of Analysis Other Documents: Worksheets
23 Ministry of Tourism, Trade, Commerce and Tel AR 4.1 Certificate of Analysis and Analysis Report Kava Sample Receipt and Analysis Report Form Reference: 29VRRP Date of Receipt: 30/04/2015 Sample Type: Powder Clients Name and Address: Telephone: address: Test Results: VRRP Limited Method of Analysis: TLC and Qualitative Sample code % Kavalactone TLC results Chemotype Qualitative Type Test 1. DMY 2. DHK 3. Y 4. K 5. DHM 6. M 445B300415/ TKK Noble yellow 456B300415/ PP Noble yellow Comments: Both kava samples are of noble kava variety and can therefore be used for consumption or commercial purposes. Key: Noble=yellow; Two-day= orange; Wild=brown Analyst: Beverly Nishai Report Authorised by: _Ruth Amos Date: 13/05/2015
24 Ministry of Tourism, Trade, Commerce and Tel CERTIFIC ATE O F AN ALY S I S Reference 29VRRP Date 13/05/2015 Clients Name & Address VRRP Limited - vrrp@vanuatu.com.vu Method of Analysis TLC and Qualitative Type Test Number of Samples Tested: 2 Names of Samples tested: TLC Results Qualitative Type Test 455B Noble Yellow 456B Noble Yellow Authorised by: Analysts name Analysts signature Date Ruth Amos 13/05/2015 Manager Signature Date &Stamp
25 Ministry of Tourism, Trade, Commerce and Tel PAYMENT Policy: Analysis results shall only be released after payments have been made. Rationale: All analyses must be paid before the results can be released to the Customer as there has been experience in the past of results being released with no payments ever been made even after invoices have been sent numerous times to the customer concerned. Procedure: 5.1. Prepare an invoice for the analysis carried out invoice to client and Manager and inform client that hard copies of the CoA and AR are ready to be collected at the FTDC-AU office 5.3. Write a receipt in the Government Receipt Book and give the first copy (original) to the client with the COA and AR. Forms: Invoice Other Documents: Receipt book Government receipt
26 Ministry of Tourism, Trade, Commerce and Tel PYT 5.1 Invoice
27 Ministry of Tourism, Trade, Commerce and Tel PYT 5.2 Receipt
Pacific Kava Export Industry Overview
 Pacific Kava Export Industry Overview Kava a rich cultural heritage Kava exports Kava is an important agricultural commodity for a number of Pacific Island Countries (PICs), forming an integral part of
Pacific Kava Export Industry Overview Kava a rich cultural heritage Kava exports Kava is an important agricultural commodity for a number of Pacific Island Countries (PICs), forming an integral part of
THE VANUATU COCONUT INDUSTRY
 SUB-REGIONAL WORKSHOP ON THE TRADE AND ENVIRONMENT DIMENSIONS IN THE FOOD AND FOOD PROCESSING INDUSTRIES IN THE PACIFIC THE MINISTRY OF TRADES, COMMERCE & INDUSTRY Introduction: 7 TH - 8 TH JUNE 2006 SUVA,
SUB-REGIONAL WORKSHOP ON THE TRADE AND ENVIRONMENT DIMENSIONS IN THE FOOD AND FOOD PROCESSING INDUSTRIES IN THE PACIFIC THE MINISTRY OF TRADES, COMMERCE & INDUSTRY Introduction: 7 TH - 8 TH JUNE 2006 SUVA,
Customs Approval to Stuff Container. Freight Forwarder Books Space on Vessel. Permission to Stuff Container. Book Space. Container Made Available
 High Level Process Pre-clearance Process 1 2 3 4 Ministries Departments and Agencies (MDA) Process Scans Commercial Invoice Freight Forwarder Books Space on Vessel Book Space Customs Approval to Stuff
High Level Process Pre-clearance Process 1 2 3 4 Ministries Departments and Agencies (MDA) Process Scans Commercial Invoice Freight Forwarder Books Space on Vessel Book Space Customs Approval to Stuff
LIMS interfaces. R.D. McDowall. The role of a LIMS MA R C H/ A P RI L
 3 8 LIMS interfaces To obtain sufficient tangible benefits from a Laboratory Information Management System (LIMS), the system must be interfaced to analytical instruments inside the laboratory and to other
3 8 LIMS interfaces To obtain sufficient tangible benefits from a Laboratory Information Management System (LIMS), the system must be interfaced to analytical instruments inside the laboratory and to other
Beautiful Business Intelligence Just for Engineers
 2017 FEATURES Beautiful Business Intelligence Just for Engineers EngineerOffice Power Your Office. Empower Yourself. www.bqe.com info@bqe.com 1 Get ready for the all-new and more-powerful EngineerOffice
2017 FEATURES Beautiful Business Intelligence Just for Engineers EngineerOffice Power Your Office. Empower Yourself. www.bqe.com info@bqe.com 1 Get ready for the all-new and more-powerful EngineerOffice
 Overview Stability testing is an essential part of drug development which ensures the quality, safety and efficacy of the drug for the lifetime of the drug product. Appropriate storage conditions can only
Overview Stability testing is an essential part of drug development which ensures the quality, safety and efficacy of the drug for the lifetime of the drug product. Appropriate storage conditions can only
Outsource or in-house? The evolving role of analytical services in pharmaceutical manufacturing
 Outsource or in-house? The evolving role of analytical services in pharmaceutical manufacturing By Impact Analytical Introduction About Impact Analytical Impact Analytical is a contract analytical testing
Outsource or in-house? The evolving role of analytical services in pharmaceutical manufacturing By Impact Analytical Introduction About Impact Analytical Impact Analytical is a contract analytical testing
QUALITY AGREEMENT. The following Agreement has been concluded between
 QUALITY AGREEMENT The following Agreement has been concluded between LLC LABORATORIES The contract laboratory and service provider 1625 Trinity Dr., Unit 11 Mississauga, Ontario Canada L5T 1W9 Hereinafter
QUALITY AGREEMENT The following Agreement has been concluded between LLC LABORATORIES The contract laboratory and service provider 1625 Trinity Dr., Unit 11 Mississauga, Ontario Canada L5T 1W9 Hereinafter
Skills Assessment Support (SAS) General Occupations - Required Document Checklist
 All uploaded documents need to be high quality colour scans of the original documents. Identity Documents: Passport Bio Page/ Birth Certificate: It is preferable that you upload a coloured copy of your
All uploaded documents need to be high quality colour scans of the original documents. Identity Documents: Passport Bio Page/ Birth Certificate: It is preferable that you upload a coloured copy of your
The American Proficiency Institute is the most innovative. and widely accepted proficiency testing program available.
 The American Proficiency Institute is the most innovative and widely accepted proficiency testing program available. 1159 Business Park Drive Traverse City, Michigan 49686 email: foodtest@foodpt.com www.foodpt.com
The American Proficiency Institute is the most innovative and widely accepted proficiency testing program available. 1159 Business Park Drive Traverse City, Michigan 49686 email: foodtest@foodpt.com www.foodpt.com
APPLICATION PACK FOR THE ECOLABEL
 APPLICATION PACK FOR THE ECOLABEL PART 1: Guidance notes ISSUED BY: Insert name of Competent Body and contact details (address, telephone and fax numbers, Email address) Date of issue: Page 1 of 12 Introduction
APPLICATION PACK FOR THE ECOLABEL PART 1: Guidance notes ISSUED BY: Insert name of Competent Body and contact details (address, telephone and fax numbers, Email address) Date of issue: Page 1 of 12 Introduction
ISO Client Program and Audit Manual
 1 Great Western Registrar LLC ISO Client Program and Audit Manual Based on the requirements of ISO17021 and Associated ANAB Accreditation Rules 04/02/2018 Created and Approved by: Karey S. Yates, President/Owner
1 Great Western Registrar LLC ISO Client Program and Audit Manual Based on the requirements of ISO17021 and Associated ANAB Accreditation Rules 04/02/2018 Created and Approved by: Karey S. Yates, President/Owner
Section 13 Operating Procedures for Importers
 OF&G ORGANIC STANDARDS AND CERTIFICATION MANUAL Section 13 13.0 Contents Page This Section explains the operating procedures, which must be followed by a registered importer of organic products. These
OF&G ORGANIC STANDARDS AND CERTIFICATION MANUAL Section 13 13.0 Contents Page This Section explains the operating procedures, which must be followed by a registered importer of organic products. These
MANUAL FOR THE ORGANIC CERTIFICATION OF WILD COLLECTED PLANTS
 MANUAL FOR THE ORGANIC CERTIFICATION OF WILD COLLECTED PLANTS Centre for Research, Information and Action in Africa Southern Africa Development and Consulting (CRIAA SA-DC), 2008 This manual is available
MANUAL FOR THE ORGANIC CERTIFICATION OF WILD COLLECTED PLANTS Centre for Research, Information and Action in Africa Southern Africa Development and Consulting (CRIAA SA-DC), 2008 This manual is available
Warehousing Process - Land
 High Level Process Pre-clearance Process 1 2 Ministries Departments and Agencies (MDA) Process Scans Commercial Invoice Unique Consignment Reference (UCR) Bond Process Removal Bond Premises Bond MDA Permit
High Level Process Pre-clearance Process 1 2 Ministries Departments and Agencies (MDA) Process Scans Commercial Invoice Unique Consignment Reference (UCR) Bond Process Removal Bond Premises Bond MDA Permit
ISO/IEC 17025: 2017 General Requirements for the Competence of Testing & Calibration of Laboratories
 An Intensive 5 Day Training Course ISO/IEC 17025: 2017 General Requirements for the Competence of Testing & Calibration of Laboratories 02-06 Dec 2018, Dubai 01-05 Dec 2019, Dubai 09-MAY-18 This course
An Intensive 5 Day Training Course ISO/IEC 17025: 2017 General Requirements for the Competence of Testing & Calibration of Laboratories 02-06 Dec 2018, Dubai 01-05 Dec 2019, Dubai 09-MAY-18 This course
STANDARD OPERATING PROCEDURES. Handling and working with Analytical Standards
 Page: 1 of 6 1.Purpose The purpose of this Standard Operating Procedure is to establish a standardized procedure of using United States Pharmacopoeia (USP) standards, detailing the procedure of qualification,
Page: 1 of 6 1.Purpose The purpose of this Standard Operating Procedure is to establish a standardized procedure of using United States Pharmacopoeia (USP) standards, detailing the procedure of qualification,
COMMISSION OF THE EUROPEAN COMMUNITIES. Proposal for a COUNCIL REGULATION
 COMMISSION OF THE EUROPEAN COMMUNITIES Brussels, 22.01.2001 COM(2000) 536 final 2000/0232 (ACC) Proposal for a COUNCIL REGULATION on procedures to facilitate the issue of movement certificates EUR.1, the
COMMISSION OF THE EUROPEAN COMMUNITIES Brussels, 22.01.2001 COM(2000) 536 final 2000/0232 (ACC) Proposal for a COUNCIL REGULATION on procedures to facilitate the issue of movement certificates EUR.1, the
5. When two companies are open what function cannot be performed in the secondary window?
 4. What does the toggle function do? a) enables a second company file to be opened b) enables the user to view the version of QuickBooks that the client has c) allows the user to have more than one window
4. What does the toggle function do? a) enables a second company file to be opened b) enables the user to view the version of QuickBooks that the client has c) allows the user to have more than one window
Sending Shipments to Germany
 Ultimate Introductory Guide: Sending Shipments to Germany.com Why Germany? Germany is the undisputed economic engine of Europe. Germany has the largest Economy in Europe and is fourthlargest economy in
Ultimate Introductory Guide: Sending Shipments to Germany.com Why Germany? Germany is the undisputed economic engine of Europe. Germany has the largest Economy in Europe and is fourthlargest economy in
NGSP Level II Laboratory Information Packet
 NGSP Level II Laboratory Information Packet LEVEL II LABORATORY CERTIFICATION General Instructions: If a laboratory is interested in certifying their method as traceable to the Diabetes Control and Complications
NGSP Level II Laboratory Information Packet LEVEL II LABORATORY CERTIFICATION General Instructions: If a laboratory is interested in certifying their method as traceable to the Diabetes Control and Complications
European Pharmacopoeia Reference Standards A Lodi, Head of the Laboratory Department, EDQM, Council of Europe
 European Pharmacopoeia Reference Standards A Lodi, Head of the Laboratory Department, EDQM, Council of Europe Content Ph. Eur. Reference Standards General notices, terms and definitions Key attributes
European Pharmacopoeia Reference Standards A Lodi, Head of the Laboratory Department, EDQM, Council of Europe Content Ph. Eur. Reference Standards General notices, terms and definitions Key attributes
FIDIC for PRACTITIONERS
 Nestor Bildungsinstitut GmbH Federation of International Consulting Engineers FIDIC for PRACTITIONERS TRAINING COURSES presents Two-Day Course on: Management of Claims and Disputes resolution under the
Nestor Bildungsinstitut GmbH Federation of International Consulting Engineers FIDIC for PRACTITIONERS TRAINING COURSES presents Two-Day Course on: Management of Claims and Disputes resolution under the
AS&D Client Program and Audit Manual
 1 Great Western Registrar LLC AS&D Client Program and Audit Manual Based on ISO 17021, AS9104, Associated ANAB Accreditation Rules & ICOP Resolutions 04/02/2018 Created and Approved by: Karey S. Yates,
1 Great Western Registrar LLC AS&D Client Program and Audit Manual Based on ISO 17021, AS9104, Associated ANAB Accreditation Rules & ICOP Resolutions 04/02/2018 Created and Approved by: Karey S. Yates,
Incremental GMPs. Presented by: Karen S. Ginsbury For: IFF October 31, Nov 02, PCI Pharmaceutical Consulting Israel Ltd.
 Incremental GMPs Presented by: Karen S. Ginsbury For: IFF October 31, Nov 02, 2017 1/55 Sound Science Good Development Practice Traceability R&D Tox I II III IV What You Need Here Can t be generated here
Incremental GMPs Presented by: Karen S. Ginsbury For: IFF October 31, Nov 02, 2017 1/55 Sound Science Good Development Practice Traceability R&D Tox I II III IV What You Need Here Can t be generated here
Membership Registration Form. Company Details
 Membership Registration Form This form allows you to become a voting or non-voting member of GS1 Healthcare, the global healthcare user group. GS1 Healthcare welcomes the following stakeholders as voting
Membership Registration Form This form allows you to become a voting or non-voting member of GS1 Healthcare, the global healthcare user group. GS1 Healthcare welcomes the following stakeholders as voting
Overview of Good Food Laboratory Practices
 Overview of Good Food Laboratory Practices Dr. Anne Bridges Technical Director, AACC International International Workshop & Training Program On Good Food Laboratory Practices Jointly Organized By Food
Overview of Good Food Laboratory Practices Dr. Anne Bridges Technical Director, AACC International International Workshop & Training Program On Good Food Laboratory Practices Jointly Organized By Food
EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL
 Ref. Ares(2013)148102-05/02/2013 EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL Health systems and products Medicinal products quality, safety and efficacy Brussels, SANCO/D/6/SF/mg/ddg1.d.6(2013)179367
Ref. Ares(2013)148102-05/02/2013 EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL Health systems and products Medicinal products quality, safety and efficacy Brussels, SANCO/D/6/SF/mg/ddg1.d.6(2013)179367
For certification, manufacturers must perform a sample comparison with a Secondary Reference Laboratory (SRL) following the NGSP protocol.
 NGSP Manufacturer Information Packet MANUFACTURER CERTIFICATION General Instructions: If a manufacturer is interested in certifying a method as traceable to the Diabetes Control and Complications Trial
NGSP Manufacturer Information Packet MANUFACTURER CERTIFICATION General Instructions: If a manufacturer is interested in certifying a method as traceable to the Diabetes Control and Complications Trial
CUSTOMS AND BORDER MANAGEMENT REFERENCE GUIDE CUSTOMS GENERAL REFUND ERRORS
 12 November20 CUSTOMS AND BORDER MANAGEMENT REFERENCE GUIDE CUSTOMS GENERAL REFUND ERRORS Revision: 88 Page 1 of 14 12 November20 TABLE OF CONTENTS 1 PURPOSE 3 2 SCOPE 3 3 REFERENCES 3 3.1 Legislation
12 November20 CUSTOMS AND BORDER MANAGEMENT REFERENCE GUIDE CUSTOMS GENERAL REFUND ERRORS Revision: 88 Page 1 of 14 12 November20 TABLE OF CONTENTS 1 PURPOSE 3 2 SCOPE 3 3 REFERENCES 3 3.1 Legislation
 Email: info@saferworkplace.com.au / Web: ENROLMENT FORM Training Program: Authorised Drug Collection Officer (ADCO) Date you would like to attend: / / STUDENT DETAILS: Please complete all required fields
Email: info@saferworkplace.com.au / Web: ENROLMENT FORM Training Program: Authorised Drug Collection Officer (ADCO) Date you would like to attend: / / STUDENT DETAILS: Please complete all required fields
IMPORTANT: PLEASE READ BEFORE COMPLETING YOUR APPLICATION
 IMPORTANT: PLEASE READ BEFORE COMPLETING YOUR APPLICATION 1. Appoint your EFQM Representative The first step each organisation has to take when joining our community is to choose one individual from the
IMPORTANT: PLEASE READ BEFORE COMPLETING YOUR APPLICATION 1. Appoint your EFQM Representative The first step each organisation has to take when joining our community is to choose one individual from the
FIDIC for PRACTITIONERS
 beruflich aufwärts Nestor Bildungsinstitut GmbH Federation of International Consulting Engineers FIDIC for PRACTITIONERS TRAINING COURSES PACKAGE DEAL Module 1: CONTRACT MANAGEMENT UNDER THE FIDIC CONDITIONS
beruflich aufwärts Nestor Bildungsinstitut GmbH Federation of International Consulting Engineers FIDIC for PRACTITIONERS TRAINING COURSES PACKAGE DEAL Module 1: CONTRACT MANAGEMENT UNDER THE FIDIC CONDITIONS
SUPPLIER POLICIES AND PROCEDURES
 SUPPLIER POLICIES AND PROCEDURES Contents i. New Item Introduction... 3 ii. Return Privileges... 3 iii. Stock Issues... 3 iv. Pricing... 3 v. Invoice Payment & Reconciliation... 3 vi. Date Codes... 4 vii.
SUPPLIER POLICIES AND PROCEDURES Contents i. New Item Introduction... 3 ii. Return Privileges... 3 iii. Stock Issues... 3 iv. Pricing... 3 v. Invoice Payment & Reconciliation... 3 vi. Date Codes... 4 vii.
MASTERING PROJECT MANAGEMENT - PROJECT MANAGEMENT PROFESSIONAL ( PMP ) CERTIFICATION SEMINAR
 MASTERING PROJECT MANAGEMENT - PROJECT MANAGEMENT PROFESSIONAL ( PMP ) CERTIFICATION SEMINAR Monday - Friday June 25-29, 2018 Book and pay before 4 June 2018 and get a discount per delegate. i This course
MASTERING PROJECT MANAGEMENT - PROJECT MANAGEMENT PROFESSIONAL ( PMP ) CERTIFICATION SEMINAR Monday - Friday June 25-29, 2018 Book and pay before 4 June 2018 and get a discount per delegate. i This course
Agilent TRS100 Raman. Quantitative Pharmaceutical Analysis System
 Agilent TRS100 Raman Quantitative Pharmaceutical Analysis System Agilent TRS100 Raman Streamlined Quality Control Fast Test hundreds of intact tablets or capsules in minutes Simple Quantify active pharmaceutical
Agilent TRS100 Raman Quantitative Pharmaceutical Analysis System Agilent TRS100 Raman Streamlined Quality Control Fast Test hundreds of intact tablets or capsules in minutes Simple Quantify active pharmaceutical
Sub: Tender for HPLC System
 INDIAN ASSOCITION FOR THE CULTIVATION OF SCIENCE 2A & 2B, Raja S.C.Mullick Road, Jadavpur, Kolkata-700032 TENDER NOTICE NO: OC/JG/SB/S1/OC-52/2013/14/28 dated 01.07.2014 Sub: Tender for HPLC System Sealed
INDIAN ASSOCITION FOR THE CULTIVATION OF SCIENCE 2A & 2B, Raja S.C.Mullick Road, Jadavpur, Kolkata-700032 TENDER NOTICE NO: OC/JG/SB/S1/OC-52/2013/14/28 dated 01.07.2014 Sub: Tender for HPLC System Sealed
NOTIFICATION. Islamabad, the 10 th February, 2000.
 NOTIFICATION Islamabad, the 10 th February, 2000. S.R.O. 258-(1)/2000. In exercise of the powers conferred by section 33 of the Pakistan Environmental Protection Act, 1997 (XXXIV of 1997), read with clause
NOTIFICATION Islamabad, the 10 th February, 2000. S.R.O. 258-(1)/2000. In exercise of the powers conferred by section 33 of the Pakistan Environmental Protection Act, 1997 (XXXIV of 1997), read with clause
SPC/CRGA 46 (16) Paper 3 ORIGINAL: ENGLISH
 SPC/CRGA 46 (16) Paper 3 ORIGINAL: ENGLISH FORTY-SIXTH MEETING OF THE COMMITTEE OF REPRESENTATIVES OF GOVERNMENTS AND ADMINISTRATIONS (Noumea, New Caledonia, 28 30 June 2016i) AGENDA ITEM 3: STRATEGIC
SPC/CRGA 46 (16) Paper 3 ORIGINAL: ENGLISH FORTY-SIXTH MEETING OF THE COMMITTEE OF REPRESENTATIVES OF GOVERNMENTS AND ADMINISTRATIONS (Noumea, New Caledonia, 28 30 June 2016i) AGENDA ITEM 3: STRATEGIC
Introduction to Bridging Your Business concept
 1 Introduction to Bridging Your Business concept 1 Chapter 1 Introduction to Bridging Your Business concept When you are comparing year-over-year sales results, very easily the net difference between 2
1 Introduction to Bridging Your Business concept 1 Chapter 1 Introduction to Bridging Your Business concept When you are comparing year-over-year sales results, very easily the net difference between 2
Documenta tion and Records
 Documenta tion and Records Page 1 of 30 Training Outcome of the Module: After completing this module, you will be able to: Recognize the importance of procedures Recognize the importance of record keeping
Documenta tion and Records Page 1 of 30 Training Outcome of the Module: After completing this module, you will be able to: Recognize the importance of procedures Recognize the importance of record keeping
DRUGS CONTROL ORGANISATION
 DRUGS CONTROL ORGANISATION Medical & health Department, Swasthya Bhawan, Tilak Marg Jaipur Email address- drugscontrolraj@gmail.com Phone - 01412221760 Check List for documents to be submitted for Grant
DRUGS CONTROL ORGANISATION Medical & health Department, Swasthya Bhawan, Tilak Marg Jaipur Email address- drugscontrolraj@gmail.com Phone - 01412221760 Check List for documents to be submitted for Grant
Surveillance and CoP clearance
 INFORMATION for MANUFACTURERS regarding: Surveillance and CoP clearance EC Directives and ECE Regulations Vehicle Category L, M, N, O, T, separate technical units, systems and components. INFORMATION for
INFORMATION for MANUFACTURERS regarding: Surveillance and CoP clearance EC Directives and ECE Regulations Vehicle Category L, M, N, O, T, separate technical units, systems and components. INFORMATION for
All data should be sent by from the laboratory and from the SRL by directly to the NGSP Network Coordinator (above address).
 NGSP Level I Laboratory Information Packet LEVEL I LABORATORY CERTIFICATION General Instructions: If a laboratory is interested in directly certifying their method as traceable to the Diabetes Control
NGSP Level I Laboratory Information Packet LEVEL I LABORATORY CERTIFICATION General Instructions: If a laboratory is interested in directly certifying their method as traceable to the Diabetes Control
IAB LEVEL 1 AWARD IN MANUAL AND COMPUTERISED BOOKKEEPING (QCF)
 CONTENTS IAB LEVEL 1 AWARD IN MANUAL AND COMPUTERISED BOOKKEEPING (QCF) Qualification Accreditation Number 601/3790/3 (Accreditation review date 31 st December 2016) QUALIFICATION SPECIFICATION 1. Introduction
CONTENTS IAB LEVEL 1 AWARD IN MANUAL AND COMPUTERISED BOOKKEEPING (QCF) Qualification Accreditation Number 601/3790/3 (Accreditation review date 31 st December 2016) QUALIFICATION SPECIFICATION 1. Introduction
GUIDELINES ON THE IMPORT AND EXPORT OF REGISTERED MEDICINES
 GUIDELINES ON THE IMPORT AND EXPORT OF REGISTERED MEDICINES 1.0 INTRODUCTION 1.1. PREAMBLE The Medicines Control Authority of Zimbabwe (MCAZ) is a regulatory body established by the Medicines and Allied
GUIDELINES ON THE IMPORT AND EXPORT OF REGISTERED MEDICINES 1.0 INTRODUCTION 1.1. PREAMBLE The Medicines Control Authority of Zimbabwe (MCAZ) is a regulatory body established by the Medicines and Allied
Business Manager. Enhancements Version January
 Business Manager Enhancements Version 3.39 January 2019 www.farmplan.co.uk 01594 545022 support@farmplan.co.uk Contents Contents... 1 Installation Instructions... 3 Business Manager Enhancements... 4 Making
Business Manager Enhancements Version 3.39 January 2019 www.farmplan.co.uk 01594 545022 support@farmplan.co.uk Contents Contents... 1 Installation Instructions... 3 Business Manager Enhancements... 4 Making
Regulatory Expectations, Standards & Guidelines
 Regulatory Expectations, Standards & Guidelines Regulatory Requirements Pharmacopeias Good Automated Manufacturing Practice (GAMP) 21 CFR Part 11 and Annex 11 Consequences of Non-Compliance 22 Regulatory
Regulatory Expectations, Standards & Guidelines Regulatory Requirements Pharmacopeias Good Automated Manufacturing Practice (GAMP) 21 CFR Part 11 and Annex 11 Consequences of Non-Compliance 22 Regulatory
IAB Level 1 Award in Bookkeeping (RQF) Qualification Specification
 IAB Level 1 Award in Bookkeeping (RQF) Qualification Specification Contents 1 Introduction to the qualification... 2 2 Statement of level... 2 3 Aims... 2 4 Target groups... 3 5 Entry requirements... 3
IAB Level 1 Award in Bookkeeping (RQF) Qualification Specification Contents 1 Introduction to the qualification... 2 2 Statement of level... 2 3 Aims... 2 4 Target groups... 3 5 Entry requirements... 3
PACIFIC AGRICULTURE POLICY BANKS
 PART 1: EXECUTIVE SUMMARY PACIFIC AGRICULTURE POLICY BANKS Agriculture and fisheries is the lifeblood of Pacific countries. Just two years ago, little was known publicly about what a country Government
PART 1: EXECUTIVE SUMMARY PACIFIC AGRICULTURE POLICY BANKS Agriculture and fisheries is the lifeblood of Pacific countries. Just two years ago, little was known publicly about what a country Government
COMPUTERIZED SYSTEM VALIDATION
 www.kevintech.com Current Practices of COMPUTERIZED SYSTEM VALIDATION Kevin ISSUE.1/2017 CONNECT INSIDE - Basics of Computerized System Validation - Life Cycle of Computerized System The less manual the
www.kevintech.com Current Practices of COMPUTERIZED SYSTEM VALIDATION Kevin ISSUE.1/2017 CONNECT INSIDE - Basics of Computerized System Validation - Life Cycle of Computerized System The less manual the
WHO PUBLIC INSPECTION REPORT (WHOPIR) Quality Control Laboratory. Uganda (NDQCL-NDA) Mulago Hill, P.O. Box 23096, Kampala, Uganda. NDA,
 SOP 408.4 Annex D 20, avenue Appia CH-1211 Geneva 27 Switzerland Tel central +41 22 791 2111 Fax central +41 22 791 3111 www.who.int WHO PUBLIC INSPECTION REPORT (WHOPIR) Quality Control Laboratory Part
SOP 408.4 Annex D 20, avenue Appia CH-1211 Geneva 27 Switzerland Tel central +41 22 791 2111 Fax central +41 22 791 3111 www.who.int WHO PUBLIC INSPECTION REPORT (WHOPIR) Quality Control Laboratory Part
ISPE-FDA 3 rd Annual CGMP Conference 2 4 June 2014 Baltimore, MD. Detecting GMP Data Integrity Issues
 Detecting GMP Data Integrity Issues Elaine Eborall Senior Director, GMP Compliance, Americas and Asia Pacific Compliance and External Collaboration ISPE-FDA cgmp Conference Baltimore, Maryland 2-4 June
Detecting GMP Data Integrity Issues Elaine Eborall Senior Director, GMP Compliance, Americas and Asia Pacific Compliance and External Collaboration ISPE-FDA cgmp Conference Baltimore, Maryland 2-4 June
Town of Lake Placid, Florida
 ACCOUNTS PAYABLE / PAYROLL CLERK Town of Lake Placid, Florida ACCOUNTS PAYBLE / PAYROLL CLERK This is a statistical, accounting and financial position for the Town of Lake Placid. Work performed requires
ACCOUNTS PAYABLE / PAYROLL CLERK Town of Lake Placid, Florida ACCOUNTS PAYBLE / PAYROLL CLERK This is a statistical, accounting and financial position for the Town of Lake Placid. Work performed requires
Part Time General Office Secretary 2017 Application for Employment
 An Equal Opportunity Employer 325 N. O Plaine Road Gurnee, IL 60031 Phone: 847-599-7500 www.gurnee.il.us The Village of Gurnee Department accepts for employment and promotes its employees without regard
An Equal Opportunity Employer 325 N. O Plaine Road Gurnee, IL 60031 Phone: 847-599-7500 www.gurnee.il.us The Village of Gurnee Department accepts for employment and promotes its employees without regard
Innovate 2 Succeed Invitation to Tender External Evaluator - June 2017
 1. Introduction: Innovate 2 Succeed Invitation to Tender External Evaluator - June 2017 1.1 Invitation to Tender This is an invitation to submit a tender to provide services ( Services ) to meet the requirements,
1. Introduction: Innovate 2 Succeed Invitation to Tender External Evaluator - June 2017 1.1 Invitation to Tender This is an invitation to submit a tender to provide services ( Services ) to meet the requirements,
Identification of the need and uses of a reference standard
 Identification of the need and uses of a reference standard Stefan Almeling Deputy Head of Laboratory Department, EDQM, Council of Europe TOPICS General / Definition Role in the monograph How established
Identification of the need and uses of a reference standard Stefan Almeling Deputy Head of Laboratory Department, EDQM, Council of Europe TOPICS General / Definition Role in the monograph How established
General European OMCL Network (GEON) QUALITY MANAGEMENT DOCUMENT
 General European OMCL Network (GEON) QUALITY MANAGEMENT DOCUMENT PA/PH/OMCL (08) 73 R3 QUALIFICATION OF EQUIPMENT CORE DOCUMENT Full document title and reference Document type Qualification of Equipment
General European OMCL Network (GEON) QUALITY MANAGEMENT DOCUMENT PA/PH/OMCL (08) 73 R3 QUALIFICATION OF EQUIPMENT CORE DOCUMENT Full document title and reference Document type Qualification of Equipment
LAW ON STANDARDS AND TECHNICAL REGULATIONS
 For informative purposes NATIONAL ASSEMBLY ------------------------ No: /2006/PL-UBTVQH11 SOCIALIST REPUBLIC OF VIETNAM INDEPENDENCE FREEDOM - HAPPINESS LAW ON STANDARDS AND TECHNICAL REGULATIONS Pursuant
For informative purposes NATIONAL ASSEMBLY ------------------------ No: /2006/PL-UBTVQH11 SOCIALIST REPUBLIC OF VIETNAM INDEPENDENCE FREEDOM - HAPPINESS LAW ON STANDARDS AND TECHNICAL REGULATIONS Pursuant
MONTHLY BOOKKEEPING SERVICES AGREEMENT
 MONTHLY BOOKKEEPING SERVICES AGREEMENT Client: July 14, 2018 Company Address Phone City ST Zip email Owner / contact The purpose of this agreement is to ensure a complete understanding between us. It will
MONTHLY BOOKKEEPING SERVICES AGREEMENT Client: July 14, 2018 Company Address Phone City ST Zip email Owner / contact The purpose of this agreement is to ensure a complete understanding between us. It will
IAB LEVEL 3 CERTIFICATE IN COMPUTERISED ACCOUNTING FOR BUSINESS (QCF)
 IAB LEVEL 3 CERTIFICATE IN COMPUTERISED ACCOUNTING FOR BUSINESS (QCF) (Qualification Accreditation Number 500/9622/9) Accreditation end date 31 st December 2012 CONTENTS QUALIFICATION SPECIFICATION 1.
IAB LEVEL 3 CERTIFICATE IN COMPUTERISED ACCOUNTING FOR BUSINESS (QCF) (Qualification Accreditation Number 500/9622/9) Accreditation end date 31 st December 2012 CONTENTS QUALIFICATION SPECIFICATION 1.
Guide for the preparation of quality programs for licensing of companies performing BPE regulated work.
 Labour and Advanced Education Guide for the preparation of quality programs for licensing of companies performing BPE regulated work. Company Name: QC Program issue#: Revision# Overview: 1. This guideline
Labour and Advanced Education Guide for the preparation of quality programs for licensing of companies performing BPE regulated work. Company Name: QC Program issue#: Revision# Overview: 1. This guideline
EA-KENYA UNILEVER KENYA LIMITED
 EA-KENYA UNILEVER KENYA LIMITED GUIDANCE FOR SUPPLIERS GOLDEN RULES: SUPPLIER S ROLE IN THE UNILEVER PAYMENT PROCESS Please find below the key steps to be taken to ensure your invoice is paid on time Step
EA-KENYA UNILEVER KENYA LIMITED GUIDANCE FOR SUPPLIERS GOLDEN RULES: SUPPLIER S ROLE IN THE UNILEVER PAYMENT PROCESS Please find below the key steps to be taken to ensure your invoice is paid on time Step
2. Procedure for scrutiny of applications by the officer or person concerned:
 Operational Guidelines to the State Biodiversity Boards for Processing of Applications for Access to Biological Resources received under section 7 of the Biological Diversity Act, 2002 1. Preamble and
Operational Guidelines to the State Biodiversity Boards for Processing of Applications for Access to Biological Resources received under section 7 of the Biological Diversity Act, 2002 1. Preamble and
Lessons from Pharmaceutical Laboratory related FDA Warning Letters
 Lessons from Pharmaceutical Laboratory related FDA Warning Letters The Agilent Critical Compliance Seminar 2016 Ludwig Huber Ludwig_huber@labcompliance.com Overview FDA Inspections and reports GMP compliance
Lessons from Pharmaceutical Laboratory related FDA Warning Letters The Agilent Critical Compliance Seminar 2016 Ludwig Huber Ludwig_huber@labcompliance.com Overview FDA Inspections and reports GMP compliance
Facility and Property Management Professional
 3 Day Program on Facility and Property Management Professional Venue: Radisson Blu Residence Dubai Marina, Dubai - UAE Dates: 12-14 December 2017 Delivery Type: Group Live Pre-requisites: None Duration:
3 Day Program on Facility and Property Management Professional Venue: Radisson Blu Residence Dubai Marina, Dubai - UAE Dates: 12-14 December 2017 Delivery Type: Group Live Pre-requisites: None Duration:
Delivering Successful Projects within the Oil & Gas Industry
 An Intensive 5 Day Training Course Delivering Successful Projects within the Oil & Gas Industry Understand How to Gain Maximum Economic Recovery on the Project Investment 22-26 Jul 2018, New York 11-15
An Intensive 5 Day Training Course Delivering Successful Projects within the Oil & Gas Industry Understand How to Gain Maximum Economic Recovery on the Project Investment 22-26 Jul 2018, New York 11-15
Laboratory Challenges with TNI Implementation and Laboratory Audits
 Laboratory Challenges with TNI Implementation and Laboratory Audits December 14, 2017 Dr. Donna Ferguson, Director, Monterey County Public Health Laboratory Dr. Martina McGarvey, Director, Pennsylvania
Laboratory Challenges with TNI Implementation and Laboratory Audits December 14, 2017 Dr. Donna Ferguson, Director, Monterey County Public Health Laboratory Dr. Martina McGarvey, Director, Pennsylvania
Country presentation MAURITIUS
 Country presentation MAURITIUS GALVmed/OIE Stakeholder Workshop On The Harmonisation Of The Registration Of Veterinary Medicinal Products JOHANNESBURG 09-11 May 2017 STATUS OF REQUIREMENTS FOR REGISTRATION
Country presentation MAURITIUS GALVmed/OIE Stakeholder Workshop On The Harmonisation Of The Registration Of Veterinary Medicinal Products JOHANNESBURG 09-11 May 2017 STATUS OF REQUIREMENTS FOR REGISTRATION
ISPM NO. 15 REGISTRATIONS
 ISPM NO. 15 REGISTRATIONS Application for registration of manufacturers and treatment providers for solid wood packaging material intended for export. The completed application form must be couriered (not
ISPM NO. 15 REGISTRATIONS Application for registration of manufacturers and treatment providers for solid wood packaging material intended for export. The completed application form must be couriered (not
CHAPTER IV SUPPLY CHAIN MANAGEMENT - GLOAL DISTRIBUTION NETWORK PROCESSES IN PHARMA INDUSTRY
 CHAPTER IV SUPPLY CHAIN MANAGEMENT - GLOAL DISTRIBUTION NETWORK PROCESSES IN PHARMA INDUSTRY An Overview of Distribution Operation The Distribution Operations in DR Reddy s Laboratories can be divided
CHAPTER IV SUPPLY CHAIN MANAGEMENT - GLOAL DISTRIBUTION NETWORK PROCESSES IN PHARMA INDUSTRY An Overview of Distribution Operation The Distribution Operations in DR Reddy s Laboratories can be divided
Operations Advantage Program
 The Operations Advantage Program HANDOUT The Operations Advantage Program Delegate and improve the bookkeeping, payroll, and reporting operations of your company with full-service bookkeeping, payroll,
The Operations Advantage Program HANDOUT The Operations Advantage Program Delegate and improve the bookkeeping, payroll, and reporting operations of your company with full-service bookkeeping, payroll,
Banking: Conduct of Business sourcebook. Chapter 3. Distance communications
 Banking: Conduct of Business sourcebook Chapter Distance BCOBS : Distance Section.1 : Distance marketing.1 Distance marketing.1.1 Application This section applies to a firm that carries on any distance
Banking: Conduct of Business sourcebook Chapter Distance BCOBS : Distance Section.1 : Distance marketing.1 Distance marketing.1.1 Application This section applies to a firm that carries on any distance
TRAINING DELEGATE FORM
 TRAINING DELEGATE FORM Kindly fill in and send this form with all your delegate information and provide proof of payment. Please email the proof of payment and training delegate form to support@propworx.co.za.
TRAINING DELEGATE FORM Kindly fill in and send this form with all your delegate information and provide proof of payment. Please email the proof of payment and training delegate form to support@propworx.co.za.
Good Laboratory Practice (GLP) Philip Cunningham St Vincent s s Hospital Sydney
 Good Laboratory Practice (GLP) Philip Cunningham St Vincent s s Hospital Sydney Pharmaceutical clinical trials may require research or non-routine routine laboratory test results for study endpoints Why?
Good Laboratory Practice (GLP) Philip Cunningham St Vincent s s Hospital Sydney Pharmaceutical clinical trials may require research or non-routine routine laboratory test results for study endpoints Why?
Exporting Guide. Helpful tips and advice if you are thinking about exporting.
 Exporting Guide Helpful tips and advice if you are thinking about exporting. Choosing how to export. If you are planning to export goods overseas you will need to determine a few factors about the process
Exporting Guide Helpful tips and advice if you are thinking about exporting. Choosing how to export. If you are planning to export goods overseas you will need to determine a few factors about the process
GUIDE FOR CECT DEPOSITORS
 SPANISH TYPE CULTURE COLLECTION http://www.cect.org GUIDE FOR CECT DEPOSITORS 1. Request via e-mail whether the CECT can accept the strain to be deposited, providing taxonomic identification and growth
SPANISH TYPE CULTURE COLLECTION http://www.cect.org GUIDE FOR CECT DEPOSITORS 1. Request via e-mail whether the CECT can accept the strain to be deposited, providing taxonomic identification and growth
Application for registration of manufacturers and treatment providers for solid wood packaging material intended for export.
 Application for registration of manufacturers and treatment providers for solid wood packaging material intended for export. The completed application form and supporting documents must be couriered (not
Application for registration of manufacturers and treatment providers for solid wood packaging material intended for export. The completed application form and supporting documents must be couriered (not
Power the Bench. Power the Bench An Enhanced Strategy for Data Integrity
 Power the Bench Power the Bench An Enhanced Strategy for Data Integrity Acquire workflow control, traceability and data automation with laboratory bench top instruments and balances fully integrated in
Power the Bench Power the Bench An Enhanced Strategy for Data Integrity Acquire workflow control, traceability and data automation with laboratory bench top instruments and balances fully integrated in
Qualification of Highway Product Manufacturers Through the Use of NTPEP Audits
 Standard Practice for Qualification of Highway Product Manufacturers Through the Use of NTPEP Audits NTPEP Designation: [SP01] (2018) National Transportation Product Evaluation Program 444 North Capitol
Standard Practice for Qualification of Highway Product Manufacturers Through the Use of NTPEP Audits NTPEP Designation: [SP01] (2018) National Transportation Product Evaluation Program 444 North Capitol
Whether you take in a lot of money. or you collect pennies
 Whether you take in a lot of money or you collect pennies ..it is important to maintain good cash handling procedures: Segregation of Duties Security Reconciliation Management Review Documentation It s
Whether you take in a lot of money or you collect pennies ..it is important to maintain good cash handling procedures: Segregation of Duties Security Reconciliation Management Review Documentation It s
1 st National Stakeholder Workshop Vanuatu National Green Export Review Warwick Le Lagoon Resort Port Vila, Vanuatu 27 August 2014
 1 st National Stakeholder Workshop Vanuatu National Green Export Review Warwick Le Lagoon Resort Port Vila, Vanuatu 27 August 2014 Third Session III: Copra/Coconut VCMB to Agri-Services Promotion and Regulation
1 st National Stakeholder Workshop Vanuatu National Green Export Review Warwick Le Lagoon Resort Port Vila, Vanuatu 27 August 2014 Third Session III: Copra/Coconut VCMB to Agri-Services Promotion and Regulation
Strategy Statement
 Strategy Statement 2017 2019 Contents Message from the State Chemist Mission, Vision, Values, Objective Context and Opportunities STRATEGIC GOALS 1 Support National Food and Feed Safety Programmes 2 Support
Strategy Statement 2017 2019 Contents Message from the State Chemist Mission, Vision, Values, Objective Context and Opportunities STRATEGIC GOALS 1 Support National Food and Feed Safety Programmes 2 Support
Warehousing Process - Sea
 High Level Process Pre-clearance Process 1 2 3 4 Ministries Departments and Agencies (MDA) Process Bond Process Shipping Agent Process Pre-clearance Movement Process Scans Bill of Lading (BL) and Commercial
High Level Process Pre-clearance Process 1 2 3 4 Ministries Departments and Agencies (MDA) Process Bond Process Shipping Agent Process Pre-clearance Movement Process Scans Bill of Lading (BL) and Commercial
Specific Accreditation Criteria
 Specific Accreditation Criteria ISO/IEC 17025 Application Document Materials - Annex Characterisation of industrial materials - General July 2018 Copyright National Association of Testing Authorities,
Specific Accreditation Criteria ISO/IEC 17025 Application Document Materials - Annex Characterisation of industrial materials - General July 2018 Copyright National Association of Testing Authorities,
Guidance Document. A Guide for the Validation and Approval of New Marine Biotoxin Test Methods. 10 April 2017
 Guidance Document A Guide for the Validation and Approval of New Marine Biotoxin Test Methods A guidance document issued by the Ministry for Primary Industries Title Guidance Document: Validation and Approval
Guidance Document A Guide for the Validation and Approval of New Marine Biotoxin Test Methods A guidance document issued by the Ministry for Primary Industries Title Guidance Document: Validation and Approval
OPPORTUNITIES FOR SRI LANKAN VALUE ADDED RICE BASED PRODUCTS IN KUWAIT
 OPPORTUNITIES FOR SRI LANKAN VALUE ADDED RICE BASED PRODUCTS IN KUWAIT Prepared by: Embassy of Sri Lanka, Kuwait February 2017 Opportunities for Sri Lankan Rice based Value added Products in Kuwait Page
OPPORTUNITIES FOR SRI LANKAN VALUE ADDED RICE BASED PRODUCTS IN KUWAIT Prepared by: Embassy of Sri Lanka, Kuwait February 2017 Opportunities for Sri Lankan Rice based Value added Products in Kuwait Page
Project Management for the Oil and Gas Industry
 An Intensive 5 Day Training Course Project Management for the Oil and Gas Industry Essential skills for Selecting & Delivering Challenging Projects in a Complex Environment 17-28 Jul 2017, Vienna 06-17
An Intensive 5 Day Training Course Project Management for the Oil and Gas Industry Essential skills for Selecting & Delivering Challenging Projects in a Complex Environment 17-28 Jul 2017, Vienna 06-17
Belgium-Brussels: HOME/2015/ISFP/PR/DRUG/0062 Waste water analysis report on the stimulant illicit drug markets in the EU 2016/S
 1 / 6 This notice in TED website: http://ted.europa.eu/udl?uri=ted:notice:254272-2016:text:en:html Belgium-Brussels: HOME/2015/ISFP/PR/DRUG/0062 Waste water analysis report on the stimulant illicit drug
1 / 6 This notice in TED website: http://ted.europa.eu/udl?uri=ted:notice:254272-2016:text:en:html Belgium-Brussels: HOME/2015/ISFP/PR/DRUG/0062 Waste water analysis report on the stimulant illicit drug
Quality Management Exporter Guideline. IRAQ ICIGI Program Rev 1.1, 18 April 2016
 Page 1 of 6 1. Purpose Country: Certificate: Parties: Republic of Iraq Pre-Import Inspection, Testing & Certification Program of Goods into the Republic of Iraq (ICIGI) Manufacturer / Trader 1.1 ICIGI
Page 1 of 6 1. Purpose Country: Certificate: Parties: Republic of Iraq Pre-Import Inspection, Testing & Certification Program of Goods into the Republic of Iraq (ICIGI) Manufacturer / Trader 1.1 ICIGI
The Move to 100% Testing of Incoming Raw Material. Ravi Kalyanaraman, Ph.D. Global Analytical Technology Bristol-Myers Squibb
 The Move to 100% Testing of Incoming Raw Material Ravi Kalyanaraman, Ph.D. Global Analytical Technology Bristol-Myers Squibb Current Situation Current procedure in pharmaceutical industry is to obtain
The Move to 100% Testing of Incoming Raw Material Ravi Kalyanaraman, Ph.D. Global Analytical Technology Bristol-Myers Squibb Current Situation Current procedure in pharmaceutical industry is to obtain
NATIONAL PLAN REPUBLIC OF PALAU
 WATER SAFETY PLANS PROGRAMME NATIONAL PLAN REPUBLIC OF PALAU OCTOBER 2006 Prepared during the National Water Safety Plan Training and Planning Workshop at Koror, Republic of Palau 9 to 13 October 2006.
WATER SAFETY PLANS PROGRAMME NATIONAL PLAN REPUBLIC OF PALAU OCTOBER 2006 Prepared during the National Water Safety Plan Training and Planning Workshop at Koror, Republic of Palau 9 to 13 October 2006.
OMCL Network of the Council of Europe QUALITY ASSURANCE DOCUMENT
 OMCL Network of the Council of Europe QUALITY ASSURANCE DOCUMENT PA/PH/OMCL (13) 113 2R Evaluation and Reporting of Results Core document Full document title and reference Evaluation and Reporting of Results
OMCL Network of the Council of Europe QUALITY ASSURANCE DOCUMENT PA/PH/OMCL (13) 113 2R Evaluation and Reporting of Results Core document Full document title and reference Evaluation and Reporting of Results
Notice of Secondment
 The Hague, 03/08/2015 File nº: Europol/2015/SNE/096 Notice of Secondment Europol is currently looking for a Seconded National Expert in the European Cybercrime Centre (Terminal) Expert in Online Fraud
The Hague, 03/08/2015 File nº: Europol/2015/SNE/096 Notice of Secondment Europol is currently looking for a Seconded National Expert in the European Cybercrime Centre (Terminal) Expert in Online Fraud
4.12. Ontario Clean Water Agency. Chapter 4 Section. Background. Follow-up on VFM Section 3.12, 2008 Annual Report. Ministry of the Environment
 Chapter 4 Section 4.12 Ministry of the Environment Ontario Clean Water Agency Follow-up on VFM Section 3.12, 2008 Annual Report Chapter 4 Follow-up Section 4.12 Background The Ontario Clean Water Agency
Chapter 4 Section 4.12 Ministry of the Environment Ontario Clean Water Agency Follow-up on VFM Section 3.12, 2008 Annual Report Chapter 4 Follow-up Section 4.12 Background The Ontario Clean Water Agency
G04 Receiving and Accepting Applications for Building Consents and Code Compliance Certificates
 G04 Receiving and Accepting Applications for Building Consents and Code Compliance Certificates Contents 1. INTRODUCTION... 1 2. DEFINITIONS FOR RECEIVING AND ACCEPTING APPLICATIONS FOR BUILDING CONSENTS,
G04 Receiving and Accepting Applications for Building Consents and Code Compliance Certificates Contents 1. INTRODUCTION... 1 2. DEFINITIONS FOR RECEIVING AND ACCEPTING APPLICATIONS FOR BUILDING CONSENTS,
QUESTIONS and ANSWERS NW IRONWORKERS DRUG FREE WORKPLACE PROGRAM DRUG AND ALCOHOL TESTING
 QUESTIONS and ANSWERS NW IRONWORKERS DRUG FREE WORKPLACE PROGRAM DRUG AND ALCOHOL TESTING 1. WHAT IS THE IRONWORKERS DRUG FREE WORKPLACE PROGRAM? The Northwest Ironworkers Employers Association, Ironworkers
QUESTIONS and ANSWERS NW IRONWORKERS DRUG FREE WORKPLACE PROGRAM DRUG AND ALCOHOL TESTING 1. WHAT IS THE IRONWORKERS DRUG FREE WORKPLACE PROGRAM? The Northwest Ironworkers Employers Association, Ironworkers
Codex Standards. Related to control, inspection and approval procedures. Patrick Sekitoleko Food Standards Officer Codex Secretariat
 Codex Standards Related to control, inspection and approval procedures Patrick Sekitoleko Food Standards Officer Codex Secretariat Codex is 2 a result of negotiations: the Codex Alimentarius a collection
Codex Standards Related to control, inspection and approval procedures Patrick Sekitoleko Food Standards Officer Codex Secretariat Codex is 2 a result of negotiations: the Codex Alimentarius a collection
WELCOME TO DHL CONNECT V3.3 USER GUIDE
 WELCOME TO DHL CONNECT V3.3 USER GUIDE SHIPPING CONVENIENCE ON YOUR PC. A powerful, easy-to-use shipping tool you can install on your PC within minutes. It gives you all the benefits of online shipping
WELCOME TO DHL CONNECT V3.3 USER GUIDE SHIPPING CONVENIENCE ON YOUR PC. A powerful, easy-to-use shipping tool you can install on your PC within minutes. It gives you all the benefits of online shipping
CONVENT OF THE SACRED HEART SCHOOL FOUNDATION FINANCIAL REGULATIONS
 CONVENT OF THE SACRED HEART SCHOOL FOUNDATION FINANCIAL REGULATIONS Approved by Convent of the Sacred Heart School Foundation, Board of Governors on 9 th October 2008 Policy Statement So that all officers
CONVENT OF THE SACRED HEART SCHOOL FOUNDATION FINANCIAL REGULATIONS Approved by Convent of the Sacred Heart School Foundation, Board of Governors on 9 th October 2008 Policy Statement So that all officers
