IN SEARCH OF LOW COST TITANIUM: THE FRAY FARTHING CHEN (FFC) CAMBRIDGE PROCESS. S J Oosthuizen Materials Science & Manufacturing, CSIR, Pretoria
|
|
|
- Donald Horton
- 6 years ago
- Views:
Transcription
1 IN SEARCH OF LOW COST TITANIUM: THE FRAY FARTHING CHEN (FFC) CAMBRIDGE PROCESS Materials Science & Manufacturing, CSIR, Pretoria Abstract This article explores the Fray Farthing Chen (FFC) Cambridge Process, a novel method for the electrochemical de-oxidation of metal oxides in molten salt, discovered at the University of Cambridge in The process was hailed as a highly promising, potentially low cost, novel method for the production of titanium metal direct from its oxides. The article should inform researchers in the field of some of the challenges in the commercialisation of a novel, high profile process involving multiple stakeholders. The author, former senior process engineer at British Titanium Plc, the company originally tasked with commercialising titanium production via the FFC Cambridge process, reviews the latest literature and discusses past and present progress in the pursuit of low cost titanium metal via this process. Topics explored include the history of the process, attempts at commercialisation, NASA s alternative application, and present status of the process. 1 Introduction Titanium offers advantages over many alternative metals in terms of weight, density corrosion, maintenance and lifetime costs. The main barrier to realizing the benefits associated with titanium in numerous applications have been the high cost of raw material and secondary processing; as compared to the alternatives, e.g. stainless steel and aluminium. Present industrial production methods for titanium occur by the reduction of titanium tetrachloride (TiCl 4 ) with magnesium (Kroll process) or sodium (Hunter process). After more than 60 years of research and development, titanium yet holds the promise of a process capable of significantly reducing titanium product cost, ideally by more than 30%. A process merely delivering a sponge product, aimed at replacing Kroll sponge alone, does not have potential for large reduction in overall titanium cost. Significant cost savings can only be achieved by also reducing the large number of process steps required to process the sponge to mill product, including sponge purification, comminution, electrode forming, Vacuum Arc Re-melting, Hot and cold rolling. Presently, economy of scale in the production of titanium dictates that it is most cost effective to cast the largest possible size ingot. This however leads to significant material waste in downstream processing, contributing to the existence of a large titanium scrap industry. Page 1
2 Titanium powder / granules material can be used in several powder metallurgy processes, e.g. laser forming and Powder Injection Moulding, to directly produce final product in fewer steps. Also, rather than machining intricate components from wrought billet, by creating near net shape parts, titanium powder metallurgy can decrease processing cost and material wastage. Commercially available titanium powder is however costly, as it requires titanium sponge or ingot as starting material. To obtain titanium as a powder requires further processing of the sponge or ingot, e.g. via the plasma rotating electrode process, where a rotating bar of titanium is subjected to gas plasma, the molten droplets are atomised and collected as titanium powder. The FFC Cambridge process is a metallurgical processing technology developed at the University of Cambridge in 1997, based on the counter-intuitive discovery that ceramics like titanium dioxide can be used as electrodes in a molten salt reactor 1. It has now been demonstrated that oxygen can be separated from most metal oxides by the FFC process, including production of titanium from titanium dioxide, chromium from chromites and silicon metal from silica. The process was hailed as a highly promising, potentially low cost, novel method for the production of titanium metal and several other metals and alloys. It has been more than a decade since the discovery of the process, and millions of dollars has been invested in the development of the FFC Cambridge process to date. There is, despite numerous apparent benefits, presently no commercial scale facility employing the process for metal or alloy production. This article examines the FFC Process, its relative benefits, the challenges faced in its commercialisation, and its present status. The basis of research is the personal experience of the author whilst active as senior process engineer at British Titanium Plc, the now defunct company originally tasked with commercialising titanium production via the FFC Cambridge process. The article also reviews the latest literature, and discusses past and present progress in the pursuit of low cost titanium metal via this process. Topics explored include the history of the process, attempts at commercialisation, NASA s alternative application, and present status of the process. 2. Discussion: The FFC Cambridge Process The invention of the FFC Cambridge process involved three people: Dr George Chen, Prof. Derek Fray and Dr. Tom W. Farthing. It resulted, "completely out of expectation" from a University of Cambridge research program aimed at removing oxygen from Group IV metals, particularly the alpha case on titanium and alloys by molten salt electrolysis. It was found that upon applying a voltage to an insulating metal oxide in a molten alkaline earth chloride a small amount of reduction takes place in the porous pellet, causing it to become an electronically conducting electrode. The ionization of oxygen then takes place throughout the pellet, with the oxygen anions dissolving in CaCl 2, where they diffuse quickly out of the pellet into the melt and eventually discharge at the anode, leaving low oxygen metal at the cathode. Page 2
3 The first patent on the process was filed in 1998 and the invention was first announced in the September 2000 edition of Nature Magazine 1, following which the FFC Process attracted significant interest and international support for its development and rapid commercialization, including initial financial support from the US Office of Naval Research (ONR) and the Defense Advanced Research Projects Agency (DARPA). 2.1 Process Description The process takes place in a molten salt medium, and is limited to operating temperatures above the melting point of the utilized salt, typically being in the range of C. The salt is typically a molten alkali halide, with a preference for CaCl 2 due to the high solubility of oxygen (primarily as CaO) in the CaCl 2 melt. Metal oxide reduction via the FFC Cambridge (FFC) process is very simple in that the oxide to be reduced is rendered cathodic in molten alkaline earth chloride, such as molten CaCl 2 at 950C. By applying a voltage below the decomposition potential of the salt (for CaCl 2 it is 3V), it has been found that ionization of oxygen is the dominant cathode reaction, rather than alkaline earth metal deposition. For titanium the suggested overall cathode reaction was given as 2 : TiO 2 + 4e - Ti + 2O 2- (in CaCl 2 ) The de-oxidation process is driven by the externally applied voltage over an anode (usually carbon) and cathode, comprising of the metal oxide to be reduced held in a conductive metal basket. The negative voltage at the cathode drives the release of oxygen ions into the CaCl 2 melt, and the oxygen ions react at the carbon anode to evolve CO or CO 2. The overall CaO content is unchanged over the course of the reduction, and the process occurs under the decomposition potential of the salt, hence the process does not consume the electrolyte. The technique has been applied to reduce a large number of metal oxides to the metals, including titanium, zirconium, chromium, niobium, silicon, tantalum, uranium and nickel. The metal oxide is then directly de-oxidised to metal or an alloy, where the starting material was a mixture of metal oxides. 2.2 Process Benefits When comparing the FFC process with the industrial processes for titanium production (Table 1), it can be seen that there are significant differences, which could lead to significant process advantages. Page 3
4 Table 1. Comparison of industrial titanium production processes with FFC Process Process Feedstock Reductant Byproducts Duration Product Kroll Hunter TiCl 4 TiCl 4 Magnesium Sodium MgCl 2 NaCl Batches, up to 7 days Sponge Sponge FFC TiO 2 Applied current (electrons) Pellets / Powder CO, CO 2 (from carbon anode) Semicontinuous, 8-24 hours The FFC process in the production of titanium has been widely claimed to offer the benefits of: a) An elegant, single stage process. The Kroll and Hunter processes employ slow, batch wise reduction of aggressive/reactive chemicals to deliver titanium sponge, which requires capital and labor intensive product recovery, handling and processing. The batches of titanium sponge are produced over several days in steel vessels, delivering around 10 tons of titanium sponge per vessel. The reactions are highly exothermic, and utilise reactive and hazardous raw materials. When looking at materials handling, the Kroll process requires 380kg of TiCl 4 and more than 100kg magnesium to produce 100 kg titanium and 380 kg MgCl 2 byproduct. In the FFC process it is possible to simply load a cathode of metal oxide, complete the electrodeoxidation step in a relatively short period, and retrieve a spent cathode of deoxidized metal post reduction. Multiple cathode and anode arrangements can also be simultaneously processed in a single molten salt bath, with the scale of production easily increased. The FFC process requires only 160kg of TiO 2 to produce 100kg of titanium. b) Low Environmental Impact. The only significant consumable cost for the FFC process is carbon (consumed at the anode). Some research has been conducted to test anode materials other than carbon, as an inert anode could be used to produce oxygen rather than CO 2, eliminating generation of the greenhouse gas. The FFC process can also process naturally occurring minerals, e.g. rutile (a natural form of TiO 2 ), whereas the TiCl 4 feedstock for the Kroll and Hunter processes requires the carbochlorination of rutile. Presently CaCl 2 is the preferred molten salt medium. It is a waste product from the chemical industry and whilst containing very few impurities, is inexpensively available. The material is also given the same toxicity as sodium chloride so there are no problems in handling or disposal. Salt byproduct from the Hunter process is often discarded, and magnesium chloride formed as byproduct in the Kroll process is again electrolyzed to produce hazardous chlorine and magnesium for recycle. In comparison the feedstock Page 4
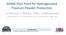
5 and products of the FFC process are non-toxic, and the process does not produce a solid waste. c) Direct production of alloys. Through de-oxidation of mixtures of oxides it is possible to directly produce alloys via the FFC process, with no need for melting. It is then able to produce impossible alloys which could not previously be made via conventional routes that involve melting the metals, due to the fact that the individual melting and boiling points of the constituent metals differ, or where the metals are immiscible. It could therefore be possible to e.g. produce an alloy of aluminium (boiling point 2467 C) and tungsten (melting point of 3422 C) directly from a mixture of the metal oxides. Some of the earliest attempts at alloy production via the FFC process were aimed at the Ti-6Al-4V alloy, which is extensively used in aerospace, medical, marine, and chemical processing applications. d) Near Net Shape Components and Low Cost Powder Materials losses and high scrap rates in aerospace applications are high, the amount of titanium purchased vs. that which ends up in a final part is claimed to be 10:1. It is claimed 5 that the FFC process may improve that ratio to 5:1. The FFC process can potentially offer near net shape parts, by pre-forming / moulding oxide powder to the intended shape product. A further advantage is that the solid product formed is a porous agglomeration of fine particles, capable of being crushed to produce titanium powder. In Figure 1 is shown pre-forms (B) produced from a metal oxide (A). Figure 1. FFC Process: From mineral to metal Page 5
6 e) Oxygen production / ILMENOX: In 2004, NASA called for the development of technologies that would allow In-Situ Resource Utilization (ISRU) of lunar materials. The aim of ISRU technologies are to allow the use of space resources on site to produce useful items, significantly reducing the launch mass, cost, and risk of near and long-term space exploration. Oxygen production for rocket propulsion promises by far the greatest cost and mass saving of any off-world in-situ resource utilization (ISRU). Since the 1960s most work on lunar resource utilisation focused on the mineral ilmenite (FeTiO 3 ) as feedstock for in-situ oxygen production, as samples returned by six Apollo and three Luna missions have shown that ilmenite occurs in high abundance (up 15 to 20%) in Lunar Basalt. In conventional ISRU processes oxygen is extracted from ilmenite through a reaction with hydrogen: FeTiO 3 + H 2 Fe + TiO 2 + H 2 O This process is unsatisfactory as it extracts only 10% oxygen per weight of ilmenite, requires a feed of hydrogen, and also further electrolysis stages for the separation of products. The titanium dioxide (TiO 2 ) is left unreduced, robbing the process of 40% oxygen by weight and potentially valuable titanium metal. The author submitted a proposal suggesting a variation of the FFC process as to be developed to extract oxygen from lunar ilmenite. The ILMENOX process could potentially extract >90% of the oxygen present in the FeTiO 3 compound. This would allow roughly 32kg of oxygen to be produced for every 100 kg lunar ilmenite processed, which could then be used as propellant and for human habitation. Liquid oxygen is a primary component of rocket fuel, contributing as much as 85 percent by weight, and its production outside the earth s gravity well would be very beneficial for extra planetary missions. NASA allocated funding to the value of $14.3 Million for a 4 year project, aimed at developing an oxygen production technology based on the FFC Cambridge process. The project, dubbed ILMENOX, was continued with some success at both the Cambridge laboratories and at partnering institution, the Florida Institute of Technology. 2.3 Process challenges A number of problems were observed, requiring solution prior to the FFC process being ready for commercial production of titanium metal. These issues included: a) Current Efficiency: It was found that current efficiencies were not as high as expected, getting progressively worse as the de-oxidation reaction neared completion. As the levels of oxygen decreases in the cathode it is possible that calcium metal, which is also soluble in Page 6
7 CaCl 2, be produced. Such dissolved calcium leads to a more electronically conductive melt, and measurable reduction in current efficiency. There is also a concern that on larger scale operations, carbon particles from the anode may build up in the cell and cause short circuiting. A further concern 2 is that CO 2 may dissolve in the melt as carbonate ions (CO 3 2- ); carbonate ions will react at the anode to deposit carbon both contaminating the product and acting as a parasitic reaction. As electricity consumption is a major contributor to the cost of FFC produced metals, current inefficiencies from the above mentioned issues may lead to high operating costs. b) Incomplete / partial reduction If the Kroll and Hunter processes are not operated with a sufficient stoichiometric excess of reductant (15-20%), partial reduction of TiCl 4 to sub-chlorides (TiCl 2 and TiCl 3 ) can occur. In comparison the FFC process proceeds from TiO 2 to titanium metal via numerous sub-oxides 2 (Ti 4 O 7, Ti 3 O 3, Ti 3 O 5, TiO), and incomplete reduction would leave these sub-oxides at the core of the TiO 2 pre-form/pellet. Analyses also confirmed the presence of calcium titanate, CaTiO 3 and calcium titanite CaTi 2 O 4 in partially reduced pellets 2. c) Product Purity To compete with the existing industrial processes, titanium product from the FFC process must meet the minimum specifications of CP (Commercially Pure) Grade Titanium in terms of chemical composition. These specifications determine upper limits for e.g. oxygen, nitrogen, chlorine, carbon and iron present in the titanium product. There are significant challenges in meeting the strict standards required for titanium when utilising the FFC process. Interstitial oxygen and nitrogen strongly affects the properties of titanium metal and alloys. Standards are then most often concerned with the levels of oxygen and nitrogen present in the product. When compared to the Kroll and Hunter processes, which start with TiCl 4 as raw material and virtually no oxygen present in the system, the FFC process starts with TiO 2 and has to get the oxygen remaining in the material down to less than 250ppm. This is no small task as oxygen is highly reactive with, and also soluble in, titanium metal. In the Kroll and Hunter processes significant amounts of iron can be found in the titanium sponge due to contamination from the steel reactor walls. This is lessened in the FFC process which only utilizes a conductive metal cage to hold the material to be Page 7
8 deoxidised. Iron, nickel and similar contamination from materials of construction are then expected to be lower in FFC product. The deoxidised pellets may sinter, trapping chlorides in the microstructure which cannot be leached. Titanium powders with chloride content higher than 50ppm are known to present problems in downstream sintering, reaching full density when compacted, and during the welding of final products. 2.4 Commercial challenges A number of issues influenced the commercialisation of the FFC Process. Intellectual property rights to the FFC Process are protected by 25 patent families, covering e.g. production of novel alloys, superconducting and shape-memory alloys via the process. The licensing structure was complex and caused some controversy, as per the following summary: In 1999 the initial patent was granted 4, with Cambridge University Technical Services (CUTS) owning the head license to the technology and being responsible for stimulating commercialisation. In 2000 CUTS issued a sub-licence to Qinetiq (formerly UK Defence Evaluation Research Agency) for production of titanium via the FFC process. In December 2000, Mr James Hamilton, chairman of South African exploration company, Bushveld Alloys, funded a pilot plant in exchange for the exclusive rights to the FFC process for the production of bulk titanium and titanium alloys??. The sub-licence was issued to British Titanium Plc (BTi) by Qinetiq, founded and headed by Mr Hamilton. BTi managed to win contracts with the US Office of Naval Research in 2000, and again in 2002 towards research and development of the FFC process for titanium and its alloys. In September 2002 the US Defence Advanced Research Agency (DARPA) funded a $12m project allowing US titanium manufacturer TIMET to purchase a non-exclusive license from BTi and attempt scaling up the process in the US. In 2002 Cambridge University spins-out Metalysis (formerly FFC Metals). Metalysis is granted an exclusive world wide licence to the FFC process by CUTS for metals and alloys, excluding titanium above 40% by weight. The company was initially funded by the University of Cambridge Venture Capital Fund to the value of 250,000. In 2004 BTi won a $14m contract with NASA to pursue the in-house developed ILMENOX technology towards production of oxygen from lunar ilmenite simulant. In 2005 Metalysis attracted 5m in venture capital to commercialise the FFC process. In April 2005 CUTS transfers all rights/the head license to the FFC Cambridge process to Metalysis. In December 2005, BTi receives notice from Qinetiq of termination of its sublicence to exploit the FFC process as Metalysis had apparently terminated the licensed rights to the FFC process for titanium granted to Qinetiq. Page 8
9 In February 2006 BTi claimed damages of more than $400m from QinetiQ and Metalysis. In April 2006 BTi lost a Security for Costs hearing and entered Administration. In April 2008 BTi was liquidated and the case for damages was struck out 6. Metalysis has raised >$23m in venture capital and grant funding to date 6, and is proceeding with development of the FFC process. In April 2010 Metalysis claimed 5 to be at the point of commissioning a pilot production cell. The cell is said to represent a capital outlay of single digit millions of dollars and to have the capability to produce titanium in a 4-12 hour cycle at commercial scale (thousands of pounds). Formatted: Bulleted + Level: 1 + Aligned at: 0.63 cm + Tab after: 1.27 cm + Indent at: 1.27 cm The brief summary above indicates only the activities of the main license holders, excluding developments at other organisations e.g. Norsk Titanium AS which was formed with the intent to utilise the FFC process for titanium production in Norway, having the support of BTi and researchers at Cambridge University 2. Norsk Titanium s connections with Norsk Hydro allowed access to vast experience in molten salts processing (Hydro having magnesium and aluminium production facilities) and more sophisticated laboratory facilities. Experiments were jointly conducted at laboratories in Porsgrunn, and by having improved monitoring and control of the process the team was able to achieve reduction of TiO 2 to titanium in less than 24hrs. BHP Billiton (BHP) also expressed interest in the FFC process for titanium production, but eventually developed a parallel technology, the so-called Polar process. In 2006 Metalysis acquired the Polar process from BHP in exchange for equity and a seat on the board of Metalysis Titanium Ltd., a company formed as high volume titanium subsidiary of Metalysis 6. 3 Conclusions From an optimistic, highly publicised introduction the FFC process has now been the focus of much previous development, numerous scientific studies and attempts at commercialisation by various organisations. The FFC process is versatile and can still offer a wide range of advantages over existing processes, advantages which could positively impact the cost of titanium and titanium products. The study of the issues around the commercialisation of the high profile FFC process, hailed as a breakthrough in the production of low cost titanium, can guide decision makers and role players active in the pursuit of novel titanium production processes. The combination of technical challenges, issues around licensing, and conflict and litigation between stakeholders in the FFC process has negatively impacted commercialisation efforts. It can also be observed that, despite the relatively large amounts invested in the process, the standard innovation curve involved in the development and commercialisation of a new process was followed, including the shake-out and consolidation of competing firms. Page 9
10 The article broadly discussed both technical and commercial issues related to process commercialisation to date, and as such does not delve deep into specifics. Much literature is still being generated and it is expected that the process will yet form the basis of many future scientific investigations. There now remains one well funded company tasked with developing the FFC process. Claims of the imminent commissioning of a facility capable of large scale production of titanium powder via the FFC process have been made. With scale-up often comes yet unknown technical issues, and these will form the basis of future research. 4 References 1. G. Z. Chen, D. J. Fray, T. W. Farthing "Direct Electrochemical Reduction of Titanium Dioxide to Titanium in Molten Calcium Chloride". Nature, 407: Schwandt, C., Doughty, G.R. & Fray, D.J The FFC-Cambridge Process for Titanium Metal Winning. Key Engineering Materials, 436: Bertolini, M., Shaw, L., England, L., Rao1, K., Deane, J. & Collins The FFC Cambridge Process for Production of Low Cost Titanium and Titanium Powders. J. Key Engineering Materials, 436: D.J. Fray, T.W. Farthing, Z. Chen: Patent WO (1999) 5. Bertolini, M Metalysis Launching Pilot Cell. International Titanium Association Newsletter, April(1): 3 6. Metalysis Ltd [Online] Available from: [Accessed: ] The Author Shaan Oosthuizen, Senior Process Engineer, CSIR 2006 PRESENT SENIOR PROCESS ENGINEER, CSIR, PRETORIA, SOUTH AFRICA The Council for Scientific & Industrial Research (CSIR) is Africa s largest Scientific and Research organization: Active at the division of Materials Science and Manufacturing, focused on primary metals production processes. Page 10
11 PROJECT MANAGER / LEAD ENGINEER, BTI (SECONDED TO NASA), CAMBRIDGE, UK Negotiated and project managed a $14.8M Cost Reimbursement contract with NASA to develop the ILMENOX process for lunar ilmenite utilisation to ANSI 748 standards and a strict timeline SENIOR DEVELOPMENT ENGINEER, BRITISH TITANIUM PLC (BTI), CAMBRIDGE, UK Research, development and commercialisation of the FFC Cambridge process, a revolutionary metal production technology discovered at the University of Cambridge in TECHNOLOGY RESEARCH MANAGER, LIBRARY HOUSE, CAMBRIDGE, UK Library House assists venture capital & private equity investors internationally by reducing the risk & cost of finding and analyzing investment opportunities in innovation based companies: Active in identifying high potential companies/technologies; completing technology audits, techno-economic and feasibility studies, company profiles; assisting in technology transfer, innovation management and investment rounds from seed to IPO COMMERCIAL DIRECTOR, HEAD PORTER, CAMBRIDGE, UK Head Porter is a wireless technology start-up company founded by four students at the University of Cambridge to develop applications for the youth market. Founded May 02, Head Porter was acquired in Feb 04 by Carat Media Group at a valuation of 1M. Page 11
The FFC Process for the production of metals and metal alloys 2 nd -5 th October 2011 K Rao, I Mellor, M Conti, J Deane, L Grainger and G Dhariwal
 The FFC Process for the production of metals and metal alloys 2 nd -5 th October 2011 K Rao, I Mellor, M Conti, J Deane, L Grainger and G Dhariwal Metalysis Ltd. UK 1 Overview The FFC and Kroll processes
The FFC Process for the production of metals and metal alloys 2 nd -5 th October 2011 K Rao, I Mellor, M Conti, J Deane, L Grainger and G Dhariwal Metalysis Ltd. UK 1 Overview The FFC and Kroll processes
Direct reduction processes for titanium oxide in mol. The final publication is available at Instructions for use
 Title Direct reduction processes for titanium oxide in mol Author(s)Suzuki, Ryosuke O. CitationJOM : Journal of the Minerals, Metals and Materials Issue Date 2007-01 Doc URL http://hdl.handle.net/2115/50035
Title Direct reduction processes for titanium oxide in mol Author(s)Suzuki, Ryosuke O. CitationJOM : Journal of the Minerals, Metals and Materials Issue Date 2007-01 Doc URL http://hdl.handle.net/2115/50035
Fundamental Aspects of Calciothermic Process to Produce Titanium
 Materials Transactions, Vol. 45, No. 5 (2004) pp. 1660 to 1664 Special Issue on Recent Research and Developments in Titanium and Its Alloys #2004 The Japan Institute of Metals Fundamental Aspects of Calciothermic
Materials Transactions, Vol. 45, No. 5 (2004) pp. 1660 to 1664 Special Issue on Recent Research and Developments in Titanium and Its Alloys #2004 The Japan Institute of Metals Fundamental Aspects of Calciothermic
Topic 2.7 EXTRACTION OF METALS. Extraction of Iron Extraction of Aluminium Extraction of Titanium Recycling
 Topic 2.7 EXTRACTION OF METALS Extraction of Iron Extraction of Aluminium Extraction of Titanium Recycling EXTRACTING METALS FROM THEIR ORES Most metals do not occur native. They exist in compounds, usually
Topic 2.7 EXTRACTION OF METALS Extraction of Iron Extraction of Aluminium Extraction of Titanium Recycling EXTRACTING METALS FROM THEIR ORES Most metals do not occur native. They exist in compounds, usually
Iron Removal from Titanium Ore using Selective Chlorination and Effective Utilization of Chloride Wastes
 Iron Removal from Titanium Ore using Selective Chlorination and Effective Utilization of Chloride Wastes Ryosuke Matsuoka 1 and Toru H. Okabe 2 1 Graduate School of Engineering, The University of Tokyo
Iron Removal from Titanium Ore using Selective Chlorination and Effective Utilization of Chloride Wastes Ryosuke Matsuoka 1 and Toru H. Okabe 2 1 Graduate School of Engineering, The University of Tokyo
Institute of Industrial Science (IIS) and Okabe Lab.
 Institute of Industrial Science (IIS) and Okabe Lab. Ryosuke Matsuoka Graduate School of Engineering, The University of Tokyo 1 Institute of Industrial Science (IIS) http://www.iis.u-tokyo.ac.jp/ 2 About
Institute of Industrial Science (IIS) and Okabe Lab. Ryosuke Matsuoka Graduate School of Engineering, The University of Tokyo 1 Institute of Industrial Science (IIS) http://www.iis.u-tokyo.ac.jp/ 2 About
2.7 EXTRA QUESTIONS MS. heat or C (1) 2 (b) electrolysis (1) molten or with cryolite (1) 2 [4]
![2.7 EXTRA QUESTIONS MS. heat or C (1) 2 (b) electrolysis (1) molten or with cryolite (1) 2 [4] 2.7 EXTRA QUESTIONS MS. heat or C (1) 2 (b) electrolysis (1) molten or with cryolite (1) 2 [4]](/thumbs/79/78837475.jpg) 2.7 EXTRA QUESTIONS MS 1. (a) C + Cl 2 (or in eq n ) (1) heat or 500 1000 C (1) 2 (b) electrolysis (1) molten or with cryolite (1) 2 [4] 2. Equation(s) for iron Fe 2 O 3 + 3C 2Fe + 3CO (1) (3CO) (3CO 2
2.7 EXTRA QUESTIONS MS 1. (a) C + Cl 2 (or in eq n ) (1) heat or 500 1000 C (1) 2 (b) electrolysis (1) molten or with cryolite (1) 2 [4] 2. Equation(s) for iron Fe 2 O 3 + 3C 2Fe + 3CO (1) (3CO) (3CO 2
Production of Titanium Powder Directly from Titanium Ore by Preform Reduction Process (PRP)
 Production of Titanium Powder Directly from Titanium Ore by Preform Reduction Process (PRP) Haiyan Zheng 1 & Toru H. Okabe 2 1: Department of Materials Engineering, Graduate School of Engineering, The
Production of Titanium Powder Directly from Titanium Ore by Preform Reduction Process (PRP) Haiyan Zheng 1 & Toru H. Okabe 2 1: Department of Materials Engineering, Graduate School of Engineering, The
The TEMPER and Free Form Titanium Fabrication Initiatives at The Center for Advanced Mineral and Metallurgical Processing, USA
 The TEMPER and Free Form Titanium Fabrication Initiatives at The Center for Advanced Mineral and Metallurgical Processing, USA By C. G. ANDERSON, P. J. MIRANDA, and L. G. TWIDWELL The Center for Advanced
The TEMPER and Free Form Titanium Fabrication Initiatives at The Center for Advanced Mineral and Metallurgical Processing, USA By C. G. ANDERSON, P. J. MIRANDA, and L. G. TWIDWELL The Center for Advanced
OXIDES BY ELECTRO-DEOXIDATION imilllll
 REDUCTION OF TITANIUM DIOXIDE AND OTHER METAL OXIDES B Y.. REDUCTION OF TITANIUM DIOXIDE AND OTHER METAL OXIDES BY ELECTRO-DEOXIDATION imilllll Dere.J.Fray Department of Materials Science and Metallurgy
REDUCTION OF TITANIUM DIOXIDE AND OTHER METAL OXIDES B Y.. REDUCTION OF TITANIUM DIOXIDE AND OTHER METAL OXIDES BY ELECTRO-DEOXIDATION imilllll Dere.J.Fray Department of Materials Science and Metallurgy
PRODUCTION AND REFINING OF METALS (electrolytic C25); PRETREATMENT OF RAW MATERIALS
 CPC - C22B - 2017.08 C22B PRODUCTION AND REFINING OF METALS (electrolytic C25); PRETREATMENT OF RAW MATERIALS Metallurgical or chemical processes for producing or recovering metals from metal compounds,
CPC - C22B - 2017.08 C22B PRODUCTION AND REFINING OF METALS (electrolytic C25); PRETREATMENT OF RAW MATERIALS Metallurgical or chemical processes for producing or recovering metals from metal compounds,
Fundamental Study on Titanium Production Process by Disproportionation of TiCl 2 in MgCl 2 Molten Salt
 Kyoto International Forum for Environment and Energy Fundamental Study on Titanium Production Process by Disproportionation of TiCl 2 in MgCl 2 Molten Salt Taiji Oi and Toru H. Okabe Institute of Industrial
Kyoto International Forum for Environment and Energy Fundamental Study on Titanium Production Process by Disproportionation of TiCl 2 in MgCl 2 Molten Salt Taiji Oi and Toru H. Okabe Institute of Industrial
An investigation of electro-deoxidation process for producing titanium from dense titanium dioxide cathode
 An investigation of electro-deoxidation process for producing titanium from dense titanium dioxide cathode Zhi-Yuan CHEN 1),2)*, Kuo-Chih CHOU 1),2) and Fu-Shen LI 2) 1) State Key Laboratory of Advanced
An investigation of electro-deoxidation process for producing titanium from dense titanium dioxide cathode Zhi-Yuan CHEN 1),2)*, Kuo-Chih CHOU 1),2) and Fu-Shen LI 2) 1) State Key Laboratory of Advanced
Metals and alloys for biomedical applications
 http://pioneer.netserv.chula.ac.th/~pchedtha/ Metals and alloys for biomedical applications Fundamental of Materials Engineering 2189202 Chedtha Puncreobutr Department of Metallurgical Engineering Chulalongkorn
http://pioneer.netserv.chula.ac.th/~pchedtha/ Metals and alloys for biomedical applications Fundamental of Materials Engineering 2189202 Chedtha Puncreobutr Department of Metallurgical Engineering Chulalongkorn
Q1.A student investigated simple cells using the apparatus shown in the figure below.
 Q1.A student investigated simple cells using the apparatus shown in the figure below. If metal 2 is more reactive than metal 1 then the voltage measured is positive. If metal 1 is more reactive than metal
Q1.A student investigated simple cells using the apparatus shown in the figure below. If metal 2 is more reactive than metal 1 then the voltage measured is positive. If metal 1 is more reactive than metal
FUNDAMENTAL STUDY ON MAGNESIOTHERMIC REDUCTION OF TITANIUM SUBCHLORIDES. O. Takeda a and T. H. Okabe *
 FUNDAMENTAL STUDY ON MAGNESIOTHERMIC REDUCTION OF TITANIUM SUBCHLORIDES O. Takeda a and T. H. Okabe * Institute of Industrial Science, The University of Tokyo 4-6-1 Komaba, Meguro-ku, Tokyo 153-8505, Japan
FUNDAMENTAL STUDY ON MAGNESIOTHERMIC REDUCTION OF TITANIUM SUBCHLORIDES O. Takeda a and T. H. Okabe * Institute of Industrial Science, The University of Tokyo 4-6-1 Komaba, Meguro-ku, Tokyo 153-8505, Japan
Production of Iron and Steels
 MME 131: Lecture 24 Production of Iron and Steels Prof. A.K.M.B. Rashid Department of MME BUET, Dhaka Topics to discuss 1. Importance of iron and steels 2. The smelting of iron in Blast Furnace 3. The
MME 131: Lecture 24 Production of Iron and Steels Prof. A.K.M.B. Rashid Department of MME BUET, Dhaka Topics to discuss 1. Importance of iron and steels 2. The smelting of iron in Blast Furnace 3. The
Materials Selection Case Study 3 Eco-Properties and Composites. Professors: Anne Mertens and Davide Ruffoni Assistant: Tommaso Maurizi Enrici
 Materials Selection Case Study 3 Eco-Properties and Composites Professors: Anne Mertens and Davide Ruffoni Assistant: Tommaso Maurizi Enrici Monday, November 19, 2018 Mechanical Properties Case Studies
Materials Selection Case Study 3 Eco-Properties and Composites Professors: Anne Mertens and Davide Ruffoni Assistant: Tommaso Maurizi Enrici Monday, November 19, 2018 Mechanical Properties Case Studies
Niobium Powder Production in Molten Salt by Electrochemical Pulverization
 Niobium Powder Production in Molten Salt by Electrochemical Pulverization Boyan Yuan * and Toru H. Okabe ** *: Graduate Student, Department of Materials Engineering, University of Tokyo **: Associate Professor,
Niobium Powder Production in Molten Salt by Electrochemical Pulverization Boyan Yuan * and Toru H. Okabe ** *: Graduate Student, Department of Materials Engineering, University of Tokyo **: Associate Professor,
Shaun Moss Mars Society Australia June 2006
 Shaun Moss Mars Society Australia shaunmoss@yahoo.com.au June 2006 Steel is Good! Arguably the most useful material on Earth:! Cheap! Strong! Lightweight! Recyclable! Versatile! The basic material for
Shaun Moss Mars Society Australia shaunmoss@yahoo.com.au June 2006 Steel is Good! Arguably the most useful material on Earth:! Cheap! Strong! Lightweight! Recyclable! Versatile! The basic material for
A NOVEL RECYCLING PROCESS OF TITANIUM METAL SCRAPS BY USING CHLORIDE WASTES
 Title of Publication Edited by TMS (The Minerals, Metals & Materials Society), Year A NOVEL RECYCLING PROCESS OF TITANIUM METAL SCRAPS BY USING CHLORIDE WASTES Haiyan Zheng 1 and Toru H. Okabe 2 1 Graduate
Title of Publication Edited by TMS (The Minerals, Metals & Materials Society), Year A NOVEL RECYCLING PROCESS OF TITANIUM METAL SCRAPS BY USING CHLORIDE WASTES Haiyan Zheng 1 and Toru H. Okabe 2 1 Graduate
Powder Metallurgy Preparation of metal powders by Atomization Electrolytic process Reduction. 15CY104: Material Technology SRM University 2
 Powder Metallurgy Preparation of metal powders by Atomization Electrolytic process Reduction 15CY104: Material Technology SRM University 2 Powder metallurgy is a term covering a wide range of ways in which
Powder Metallurgy Preparation of metal powders by Atomization Electrolytic process Reduction 15CY104: Material Technology SRM University 2 Powder metallurgy is a term covering a wide range of ways in which
CO forms CO 2. forms. (a) The coke reacts with the oxygen in the air to form carbon dioxide. C + O 2
 1 Iron is extracted from the ore hematite in the Blast Furnace. waste gases firebrick lining raw materials: coke, C iron ore, Fe 2 O 3 limestone, CaCO 3 CO forms air slag molten iron CO 2 forms (a) The
1 Iron is extracted from the ore hematite in the Blast Furnace. waste gases firebrick lining raw materials: coke, C iron ore, Fe 2 O 3 limestone, CaCO 3 CO forms air slag molten iron CO 2 forms (a) The
Electricity and Chemistry
 Electricity and Chemistry Electrochemistry: It is a branch of chemistry that deals with the reactions involving the conversion of chemical energy into electrical energy and vice-versa. Electrochemical
Electricity and Chemistry Electrochemistry: It is a branch of chemistry that deals with the reactions involving the conversion of chemical energy into electrical energy and vice-versa. Electrochemical
Extracting and using metals. ores. native. Only the most unreactive metals such as gold and platinum are found as native metals.
 Extracting and using metals Only the most unreactive metals such as gold and platinum are found as native metals. ores All the other metals we use are extracted from their ores by chemical processes. native
Extracting and using metals Only the most unreactive metals such as gold and platinum are found as native metals. ores All the other metals we use are extracted from their ores by chemical processes. native
Supplementary Material: Development of the Fray-Farthing-Chen Cambridge Process: Towards the Sustainable Production of Titanium and Its Alloys
 Supplementary Material: Development of the Fray-Farthing-Chen Cambridge Process: Towards the Sustainable Production of Titanium and Its Alloys DI HU 1, 2, 4, ALEKSEI DOLGANOV 1, MINGCHAN MA 1, BIYASH BHATTACHARYA
Supplementary Material: Development of the Fray-Farthing-Chen Cambridge Process: Towards the Sustainable Production of Titanium and Its Alloys DI HU 1, 2, 4, ALEKSEI DOLGANOV 1, MINGCHAN MA 1, BIYASH BHATTACHARYA
Ti Titanium. Leading the Way in the Production of Plasma Atomized Spherical Metal Powders. Specialists in Powders for Additive Manufacturing
 Leading the Way in the Production of Plasma Atomized Spherical Metal Powders Specialists in Powders for Additive Manufacturing 22 Ti 47.867 Titanium 10+ years servicing major customers AP&C: Large Scale
Leading the Way in the Production of Plasma Atomized Spherical Metal Powders Specialists in Powders for Additive Manufacturing 22 Ti 47.867 Titanium 10+ years servicing major customers AP&C: Large Scale
Strong under tension and compression. Malleable. Low density. Have a dull appearance. Good conductors of electricity and heat
 Revision from Year 10: Properties of Metals and Non-Metals Read CC pp182-183 Use arrows to link the properties with the materials: Strong under tension and compression Malleable Low density Have a dull
Revision from Year 10: Properties of Metals and Non-Metals Read CC pp182-183 Use arrows to link the properties with the materials: Strong under tension and compression Malleable Low density Have a dull
GMAW (MIG) / FCAW / MCAW
 Welding Processes GMAW () / FCAW / MCAW Gas Metal Arc Welding (GMAW), Flux Cored Arc Welding (FCAW) and Metal Cored Arc Welding (MCAW) Gas Metal Arc Welding (GMAW) GMA commonly referred to as Metal Inert
Welding Processes GMAW () / FCAW / MCAW Gas Metal Arc Welding (GMAW), Flux Cored Arc Welding (FCAW) and Metal Cored Arc Welding (MCAW) Gas Metal Arc Welding (GMAW) GMA commonly referred to as Metal Inert
EXTRACTIVE METALLURGY
 EXTRACTIVE METALLURGY Extractive metallurgy is the practice of removing valuable metals from an ore and refining the extracted raw metals into a purer form. In order to convert a metal oxide or sulfide
EXTRACTIVE METALLURGY Extractive metallurgy is the practice of removing valuable metals from an ore and refining the extracted raw metals into a purer form. In order to convert a metal oxide or sulfide
Chemical Phenomena in Titanium Production. DS van Vuuren SACI January 2011
 Chemical Phenomena in Titanium Production DS van Vuuren SACI 2011 19 January 2011 Outline Background Routes to produce titanium Some basic physical properties Main process routes and key physical properties
Chemical Phenomena in Titanium Production DS van Vuuren SACI 2011 19 January 2011 Outline Background Routes to produce titanium Some basic physical properties Main process routes and key physical properties
ELECTRODEOXIDATION OF SOLID Fe 2 O 3 IN MOLTEN CaCl 2 TO PRODUCE IRON
 ELECTRODEOXIDATION OF SOLID Fe 2 O 3 IN MOLTEN CaCl 2 TO PRODUCE IRON Geir Haarberg & Odne Burheim Norwegian University of Science and Technology, Norway Derek Fray University of Cambridge, United Kingdom
ELECTRODEOXIDATION OF SOLID Fe 2 O 3 IN MOLTEN CaCl 2 TO PRODUCE IRON Geir Haarberg & Odne Burheim Norwegian University of Science and Technology, Norway Derek Fray University of Cambridge, United Kingdom
RECYCLING PROCESS FOR TANTALUM AND SOME OTHER METAL SCRAPS
 EPD Congress 2004 Edited by TMS (The Minerals, Metals & Materials Society), Year RECYCLING PROCESS FOR TANTALUM AND SOME OTHER METAL SCRAPS Ryosuke Matsuoka 1, Kunio Mineta 1, Toru H. Okabe 2 1 Graduate
EPD Congress 2004 Edited by TMS (The Minerals, Metals & Materials Society), Year RECYCLING PROCESS FOR TANTALUM AND SOME OTHER METAL SCRAPS Ryosuke Matsuoka 1, Kunio Mineta 1, Toru H. Okabe 2 1 Graduate
Metal Powder - the Raw Material of Future Production
 Metal Powder - the Raw Material of Future Production BY GÜNTER BUSCH* SYNOPSIS Alongside Mobile Internet, Cloud Computing, Robotics, Energy Storage and Autonomous Vehicles, Additive Manufacturing is one
Metal Powder - the Raw Material of Future Production BY GÜNTER BUSCH* SYNOPSIS Alongside Mobile Internet, Cloud Computing, Robotics, Energy Storage and Autonomous Vehicles, Additive Manufacturing is one
I. PHYSICAL PROPERTIES. PROPERTY METALS NON-METALS 1.Lustre Metals have shining surface. They do not have shining surface.
 Elements can be classified as metals and non-metals on the basis of their properties. Example of some metals are : Iron (Fe), Aluminium (Al), Silver (Ag), Copper (Cu) Examples of some non-metals are :
Elements can be classified as metals and non-metals on the basis of their properties. Example of some metals are : Iron (Fe), Aluminium (Al), Silver (Ag), Copper (Cu) Examples of some non-metals are :
Introduction to PM. Marco Actis Grande
 Introduction to PM Marco Actis Grande What is PM? Materials forming technique Create powders (metallic & non-metallic) Assemble them into artefacts of desired shape Cause the powder particles to adhere
Introduction to PM Marco Actis Grande What is PM? Materials forming technique Create powders (metallic & non-metallic) Assemble them into artefacts of desired shape Cause the powder particles to adhere
Suggest one reason why spoons are electroplated. ... Why is hydrogen produced at the negative electrode and not sodium?
 Q1.This question is about electrolysis. (a) Metal spoons can be coated with silver. This is called electroplating. Suggest one reason why spoons are electroplated. (b) When sodium chloride solution is
Q1.This question is about electrolysis. (a) Metal spoons can be coated with silver. This is called electroplating. Suggest one reason why spoons are electroplated. (b) When sodium chloride solution is
ADMA Pilot Plant for Hydrogenated Titanium Powder Production
 ADMA Pilot Plant for Hydrogenated Titanium Powder Production A. Klevtsov, V. Moxson, V.Duz, V.Sukhoplyuyev ADMA Products Inc., 2035 Midway Drive, Twinsburg, Ohio 44087, USA Presentation overview ADMA innovative
ADMA Pilot Plant for Hydrogenated Titanium Powder Production A. Klevtsov, V. Moxson, V.Duz, V.Sukhoplyuyev ADMA Products Inc., 2035 Midway Drive, Twinsburg, Ohio 44087, USA Presentation overview ADMA innovative
GENERAL PRINCIPLES AND PROCE ISOLATION ISOL ELEMENTS
 Unit 6 GENERAL PRINCIPLES AND PROCE PR OCESSE SSES S OF ISOLATION ISOL OF ELEMENTS I. Multiple Choice Questions (Type-I) 1. In the extraction of chlorine by electrolysis of brine. oxidation of Cl ion to
Unit 6 GENERAL PRINCIPLES AND PROCE PR OCESSE SSES S OF ISOLATION ISOL OF ELEMENTS I. Multiple Choice Questions (Type-I) 1. In the extraction of chlorine by electrolysis of brine. oxidation of Cl ion to
REFINING OF FERRO-SILICON
 REFINING OF FERRO-SILICON Arun P Bhattacharya National Metallurgical Laboratory, Jamshedpur 831 007 1. Introduction The ferro-silicon required for making electrical steels must have low content of aluminium,
REFINING OF FERRO-SILICON Arun P Bhattacharya National Metallurgical Laboratory, Jamshedpur 831 007 1. Introduction The ferro-silicon required for making electrical steels must have low content of aluminium,
METALS AND THEIR COMPOUNDS
 METALS AND THEIR COMPOUNDS Metals are elements whose atoms ionize by electron loss, while non-metals are elements whose atoms ionize by electron gain. Metals are in groups 1, 2 and 3 of the periodic table.
METALS AND THEIR COMPOUNDS Metals are elements whose atoms ionize by electron loss, while non-metals are elements whose atoms ionize by electron gain. Metals are in groups 1, 2 and 3 of the periodic table.
Topic 9 National 4 Chemistry Summary Notes. Metals and Alloys. Materials
 Topic 9 National 4 Chemistry Summary Notes Metals and Alloys LI 1 Materials Materials are all substances and include: metals ceramics plastics natural substances novel substances. Materials can be used
Topic 9 National 4 Chemistry Summary Notes Metals and Alloys LI 1 Materials Materials are all substances and include: metals ceramics plastics natural substances novel substances. Materials can be used
Secondary Steelmaking 1 Synthetic slag practice, injection ladle metallurgy, deoxidation
 17 Secondary Steelmaking 1 Synthetic slag practice, injection ladle metallurgy, deoxidation Topics to discuss... Secondary steelmaking Synthetic slag practice Injection ladle metallurgy Deoxidation Secondary
17 Secondary Steelmaking 1 Synthetic slag practice, injection ladle metallurgy, deoxidation Topics to discuss... Secondary steelmaking Synthetic slag practice Injection ladle metallurgy Deoxidation Secondary
Direct Powder Rolling (DPR) of Titanium. Light Metals Flagship D.Cantin, P.Kean, N.A. Stone, R.Wilson, M.A. Gibson, D.Ritchie, R.
 Direct Powder Rolling (DPR) of Titanium. Light Metals Flagship D.Cantin, P.Kean, N.A. Stone, R.Wilson, M.A. Gibson, D.Ritchie, R. Rajakumar Titanium 2010 Orlando, Florida 3-6 October 2010 Manufacture of
Direct Powder Rolling (DPR) of Titanium. Light Metals Flagship D.Cantin, P.Kean, N.A. Stone, R.Wilson, M.A. Gibson, D.Ritchie, R. Rajakumar Titanium 2010 Orlando, Florida 3-6 October 2010 Manufacture of
 Question 6.1: Copper can be extracted by hydrometallurgy but not zinc. Explain. The reduction potentials of zinc and iron are lower than that of copper. In hydrometallurgy, zinc and iron can be used to
Question 6.1: Copper can be extracted by hydrometallurgy but not zinc. Explain. The reduction potentials of zinc and iron are lower than that of copper. In hydrometallurgy, zinc and iron can be used to
5072 CHEMISTRY (NEW PAPERS WITH SPA) TOPIC 9: METALS 5067 CHEMISTRY (NEW PAPERS WITH PRACTICAL EXAM) TOPIC 9: METALS
 5072 CHEMISTRY (NEW PAPERS WITH SPA) TOPIC 9: METALS 5067 CHEMISTRY (NEW PAPERS WITH PRACTICAL EXAM) TOPIC 9: METALS SUB-TOPIC 9.3 TO 5 EXTRACTION OF METALS; RECYLING OF METALS; IRON LEARNING OUTCOMES
5072 CHEMISTRY (NEW PAPERS WITH SPA) TOPIC 9: METALS 5067 CHEMISTRY (NEW PAPERS WITH PRACTICAL EXAM) TOPIC 9: METALS SUB-TOPIC 9.3 TO 5 EXTRACTION OF METALS; RECYLING OF METALS; IRON LEARNING OUTCOMES
THE ANALOGY AND DIFFERENCES BETWEEN ALUMINIUM AND TITANIUM ELECTROWINNING
 THE ANALOGY AND DIFFERENCES BETWEEN ALUMINIUM AND TITANIUM ELECTROWINNING DS van Vuuren 1, AD Engelbrecht, TD Hadley 1 Materials Science and Manufacturing, CSIR Correspondence to: DS van Vuuren, Materials
THE ANALOGY AND DIFFERENCES BETWEEN ALUMINIUM AND TITANIUM ELECTROWINNING DS van Vuuren 1, AD Engelbrecht, TD Hadley 1 Materials Science and Manufacturing, CSIR Correspondence to: DS van Vuuren, Materials
not to be republished NCERT GENERAL PRINCIPLES AND PROCE ISOLATION ISOL ELEMENTS Unit I. Multiple Choice Questions (Type-I)
 I. Multiple Choice Questions (Type-I) 1. In the extraction of chlorine by electrolysis of brine. (i) (ii) (iii) (iv) oxidation of Cl ion to chlorine gas occurs. reduction of Cl ion to chlorine gas occurs.
I. Multiple Choice Questions (Type-I) 1. In the extraction of chlorine by electrolysis of brine. (i) (ii) (iii) (iv) oxidation of Cl ion to chlorine gas occurs. reduction of Cl ion to chlorine gas occurs.
I. PHYSICAL PROPERTIES PROPERTY METALS NON-METALS
 Elements can be classified as metals and non-metals on the basis of their properties. Example of some metals are : Iron (Fe), Aluminium (Al), Silver (Ag), Copper (Cu) Examples of some non-metals are :
Elements can be classified as metals and non-metals on the basis of their properties. Example of some metals are : Iron (Fe), Aluminium (Al), Silver (Ag), Copper (Cu) Examples of some non-metals are :
Rusting is an example of corrosion, which is a spontaneous redox reaction of materials with substances in their environment.
 CORROSION WHAT IS CORROSION? Corrosion is the deterioration of a metal as a result of chemical reactions between it and the surrounding environment. Rusting is an example of corrosion, which is a spontaneous
CORROSION WHAT IS CORROSION? Corrosion is the deterioration of a metal as a result of chemical reactions between it and the surrounding environment. Rusting is an example of corrosion, which is a spontaneous
ATOM STRUCTURE AND BONDING OF METALS
 ATOM STRUCTURE AND BONDING OF METALS The atom is composed of a small, central nucleus, which contains protons and neutrons. Shells, or energy levels of electrons surround this nucleus. These electrons
ATOM STRUCTURE AND BONDING OF METALS The atom is composed of a small, central nucleus, which contains protons and neutrons. Shells, or energy levels of electrons surround this nucleus. These electrons
CLEANER PRODUCTION GUIDELINES IN SMELTING INDUSTRIESS
 2015 CLEANER PRODUCTION GUIDELINES IN COPPER SMELTING INDUSTRIESS Gujarat Cleaner Production Centre (Established by Industries & Mines Department, GoG) ENVIS Centre on: Cleaner Production/Technology Supported
2015 CLEANER PRODUCTION GUIDELINES IN COPPER SMELTING INDUSTRIESS Gujarat Cleaner Production Centre (Established by Industries & Mines Department, GoG) ENVIS Centre on: Cleaner Production/Technology Supported
Innovative Process for Manufacturing Hydrogenated Titanium Powder for Solid State Production of P/M Titanium Alloy Components
 Innovative Process for Manufacturing Hydrogenated Titanium Powder for Solid State Production of P/M Titanium Alloy Components O.M. Ivasishin 1, M.V.Matviychuk 1, C.A.Lavender 2, G.I.Abakumov 3, V.A.Duz
Innovative Process for Manufacturing Hydrogenated Titanium Powder for Solid State Production of P/M Titanium Alloy Components O.M. Ivasishin 1, M.V.Matviychuk 1, C.A.Lavender 2, G.I.Abakumov 3, V.A.Duz
SAMPLE PAGES PAGES. Extraction of metals from metal oxides. mixture of iron sand and coal are heated as they move down kiln, by force of gravity
 Unit 11.5 Metals and Non-metals Topic 3: Extraction of metals and corrosion In the previous two Topics we looked at the physical and chemical properties of metals. In Topic 3 we now examine how metals
Unit 11.5 Metals and Non-metals Topic 3: Extraction of metals and corrosion In the previous two Topics we looked at the physical and chemical properties of metals. In Topic 3 we now examine how metals
METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON- FERROUS METALS
 XXXX C22 METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON- FERROUS METALS Note(s) [2012.01] (1) Processes or devices specific to the transformation of iron ore or iron carbonyl into
XXXX C22 METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON- FERROUS METALS Note(s) [2012.01] (1) Processes or devices specific to the transformation of iron ore or iron carbonyl into
C1.3 METALS AND THEIR USES
 C1.3 METALS AND THEIR USES Q1.Where copper ore has been mined there are areas of land that contain very low percentages of copper compounds. One way to extract the copper is to grow plants on the land.
C1.3 METALS AND THEIR USES Q1.Where copper ore has been mined there are areas of land that contain very low percentages of copper compounds. One way to extract the copper is to grow plants on the land.
An Introduction to Powder Metallurgy
 An Introduction to Powder Metallurgy F. THÜMMLER Dr.-Ing.habil, FIM Professor Emeritus for Materials University and Nuclear Research Centre Karlsruhe and R. OBERACKER Dr.-Ing., Central Laboratory Institute
An Introduction to Powder Metallurgy F. THÜMMLER Dr.-Ing.habil, FIM Professor Emeritus for Materials University and Nuclear Research Centre Karlsruhe and R. OBERACKER Dr.-Ing., Central Laboratory Institute
Welding Engineering Prof. Dr. D. K. Dwivedi Department of Mechanical and Industrial Engineering Indian Institute of Technology, Roorkee
 Welding Engineering Prof. Dr. D. K. Dwivedi Department of Mechanical and Industrial Engineering Indian Institute of Technology, Roorkee Module - 4 Arc Welding Processes Lecture - 1 SMAW- 1 So, dear students,
Welding Engineering Prof. Dr. D. K. Dwivedi Department of Mechanical and Industrial Engineering Indian Institute of Technology, Roorkee Module - 4 Arc Welding Processes Lecture - 1 SMAW- 1 So, dear students,
HYDROGEN UPTAKE RATES FOR GRADE 12 TITANIUM IN HOT ALKALINE BRINE MEDIA
 HYDROGEN UPTAKE RATES FOR GRADE 12 TITANIUM IN HOT ALKALINE BRINE MEDIA J. S. Grauman & D. E. Cooper TIMET Division, Titanium Metals Corporation of America P.O. Box 2128 Henderson, NV 89015 INTRODUCTION
HYDROGEN UPTAKE RATES FOR GRADE 12 TITANIUM IN HOT ALKALINE BRINE MEDIA J. S. Grauman & D. E. Cooper TIMET Division, Titanium Metals Corporation of America P.O. Box 2128 Henderson, NV 89015 INTRODUCTION
COOPERATIVE PATENT CLASSIFICATION
 CPC C COOPERATIVE PATENT CLASSIFICATION CHEMISTRY; METALLURGY (S omitted) METALLURGY C22 METALLURGY (of iron C21); FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS (production of
CPC C COOPERATIVE PATENT CLASSIFICATION CHEMISTRY; METALLURGY (S omitted) METALLURGY C22 METALLURGY (of iron C21); FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS (production of
Anhui University of Technology, School of Metallurgy and Resource, Maanshan, China. (Received 14 October 2012; accepted 28 January 2013)
 J. Min. Metall. Sect. B-Metall. 49 (1) B (2013) 71-76 Journal of Mining and Metallurgy, Section B: Metallurgy DEPOLARIZED -BASED GAS ANODES FOR ELECTROWINNING OF SILVER IN MOLTEN CHLORIDES S. Xiao a,*,
J. Min. Metall. Sect. B-Metall. 49 (1) B (2013) 71-76 Journal of Mining and Metallurgy, Section B: Metallurgy DEPOLARIZED -BASED GAS ANODES FOR ELECTROWINNING OF SILVER IN MOLTEN CHLORIDES S. Xiao a,*,
The following steps are used in the powder metallurgy techniques:
 Advantages of Powder Metallurgy Virtually unlimited choice of alloys and non metallicswith associated properties. * A variety of metal or non metal powders can be used. * Refractory materials are popularly
Advantages of Powder Metallurgy Virtually unlimited choice of alloys and non metallicswith associated properties. * A variety of metal or non metal powders can be used. * Refractory materials are popularly
A.D. Ryabtsev*, A.A. Troyansky*, O.V. Tarlov, V.V. Pashinsky*, M.G. Benz**, V.N. Radchenko*** *Donetsk State Technical University, Ukraine
 THE DEVELOPMENT OF TECHNOLOGY OF HIGH- QUALITY INGOT MANUFACTURING FROM METALS WITH HIGH REACTION ABILITY (Cr,Ti,V AND OTHERS) BY THE ESR METHOD UNDER ACTIVE CALCIUM-CONTAINING SLAG SYSTEMS. A.D. Ryabtsev*,
THE DEVELOPMENT OF TECHNOLOGY OF HIGH- QUALITY INGOT MANUFACTURING FROM METALS WITH HIGH REACTION ABILITY (Cr,Ti,V AND OTHERS) BY THE ESR METHOD UNDER ACTIVE CALCIUM-CONTAINING SLAG SYSTEMS. A.D. Ryabtsev*,
The ERMS Synthetic Rutile Process
 The ERMS Synthetic Rutile Process Ultra-high Grade SR by leaching ilmenite with HCl Intertech, Miami February 5, 2003 Overview! History of ERMS SR! Austpac s technologies! ERMS, EARS, LTR, ilmenite leaching!
The ERMS Synthetic Rutile Process Ultra-high Grade SR by leaching ilmenite with HCl Intertech, Miami February 5, 2003 Overview! History of ERMS SR! Austpac s technologies! ERMS, EARS, LTR, ilmenite leaching!
Production of -TiAl-Ingots by Aluminothermic Reduction of TiO 2 and Refining by ESR
 EMC 2005 September 18 th -21 th, Dresden Production of -TiAl-Ingots by Aluminothermic Reduction of TiO 2 and Refining by ESR Stoephasius, Jan-Christoph; Friedrich, Bernd; IME Process Metallurgy and Metal
EMC 2005 September 18 th -21 th, Dresden Production of -TiAl-Ingots by Aluminothermic Reduction of TiO 2 and Refining by ESR Stoephasius, Jan-Christoph; Friedrich, Bernd; IME Process Metallurgy and Metal
Edexcel GCSE Chemistry. Topic 4: Extracting metals and equilibria. Obtaining and using metals. Notes.
 Edexcel GCSE Chemistry Topic 4: Extracting metals and equilibria Obtaining and using metals Notes 4.1 Deduce the relative reactivity of some metals, by their reactions with water, acids and salt solutions
Edexcel GCSE Chemistry Topic 4: Extracting metals and equilibria Obtaining and using metals Notes 4.1 Deduce the relative reactivity of some metals, by their reactions with water, acids and salt solutions
CaCl2 - CaF2 at ca. 800
 United States Patent (19) Sharma 54 MOLTEN SALT PROCESS FOR PRODUCING TITANIUM OR ZIRCONIUM POWDER 76 Inventor: Ram A. Sharma, 2951 Homewood Dr., Troy, Mich. 48098 21 Appl. No.: 09/065,817 22 Filed: Apr.
United States Patent (19) Sharma 54 MOLTEN SALT PROCESS FOR PRODUCING TITANIUM OR ZIRCONIUM POWDER 76 Inventor: Ram A. Sharma, 2951 Homewood Dr., Troy, Mich. 48098 21 Appl. No.: 09/065,817 22 Filed: Apr.
Metallurgy, Alloys, and Applications p. 1 Introduction and Overview p. 3 Major Groups of Copper and Copper Alloys p. 3 Properties of Importance p.
 Preface p. vii Metallurgy, Alloys, and Applications p. 1 Introduction and Overview p. 3 Major Groups of Copper and Copper Alloys p. 3 Properties of Importance p. 3 Fabrication Characteristics p. 5 Alloy
Preface p. vii Metallurgy, Alloys, and Applications p. 1 Introduction and Overview p. 3 Major Groups of Copper and Copper Alloys p. 3 Properties of Importance p. 3 Fabrication Characteristics p. 5 Alloy
CHLOR-ALKALI INDUSTRY
 CHLOR-ALKALI INDUSTRY The chlor-alkali industry represents of three major industrial chemicals: Soda ash (sodium carbonate-na 2 CO 3 ) Caustic soda (sodium hydroxide-naoh) Chlorine (Cl 2 ) These chemicals
CHLOR-ALKALI INDUSTRY The chlor-alkali industry represents of three major industrial chemicals: Soda ash (sodium carbonate-na 2 CO 3 ) Caustic soda (sodium hydroxide-naoh) Chlorine (Cl 2 ) These chemicals
General Principle of Isolation of Elements (NCERT)
 Question 6.1: Copper can be extracted by hydrometallurgy but not zinc. Explain. The reduction potentials of zinc and iron are lower than that of copper. In hydrometallurgy, zinc and iron can be used to
Question 6.1: Copper can be extracted by hydrometallurgy but not zinc. Explain. The reduction potentials of zinc and iron are lower than that of copper. In hydrometallurgy, zinc and iron can be used to
FLUID BED CHLORINATION PILOT PLANT AT MINTEK. A Kale and K Bisaka Mintek
 FLUID BED CHLORINATION PILOT PLANT AT MINTEK Mintek Abstract A chlorination pilot plant having capacity to produce 5-10 kg/hr of titanium tetrachloride is in operation at the chlorination facility in Mintek.
FLUID BED CHLORINATION PILOT PLANT AT MINTEK Mintek Abstract A chlorination pilot plant having capacity to produce 5-10 kg/hr of titanium tetrachloride is in operation at the chlorination facility in Mintek.
Reactivity Series. Question Paper. Cambridge International Examinations. Score: /39. Percentage: /100
 Reactivity Series Question Paper Level Subject Exam oard Topic Sub-Topic ooklet O Level hemistry ambridge International Examinations Metals Reactivity Series Question Paper Time llowed: 47 minutes Score:
Reactivity Series Question Paper Level Subject Exam oard Topic Sub-Topic ooklet O Level hemistry ambridge International Examinations Metals Reactivity Series Question Paper Time llowed: 47 minutes Score:
40 Non-ferrous metallurgy, metal alloys, electrometallurgy, refinement of metals and metal alloys
 The German Patent Classification, Class 40 Page 1 40 Non-ferrous metallurgy, metal alloys, electrometallurgy, refinement of metals and metal alloys 40a 40a 40b 40b 40c 40c 40d 40d Metallurgy (IPC: C22B)
The German Patent Classification, Class 40 Page 1 40 Non-ferrous metallurgy, metal alloys, electrometallurgy, refinement of metals and metal alloys 40a 40a 40b 40b 40c 40c 40d 40d Metallurgy (IPC: C22B)
Direct electrochemical reduction of titanium-bearing compounds to titanium-silicon alloys in molten calcium chloride
 Direct electrochemical reduction of titanium-bearing compounds to titanium-silicon alloys in molten calcium chloride Xingli ZOU, and Xionggang LU Shanghai Key Laboratory of Modern Metallurgy and Materials
Direct electrochemical reduction of titanium-bearing compounds to titanium-silicon alloys in molten calcium chloride Xingli ZOU, and Xionggang LU Shanghai Key Laboratory of Modern Metallurgy and Materials
Converting Steel Industry Waste into Profits. Presentation by Mike Turbott Managing Director
 Converting Steel Industry Waste into Profits Presentation by Mike Turbott Managing Director 1 Disclaimer This presentation is provided to you for information purposes only and should not be construed as
Converting Steel Industry Waste into Profits Presentation by Mike Turbott Managing Director 1 Disclaimer This presentation is provided to you for information purposes only and should not be construed as
Advances and Innovations in the Extraction of Aluminum, Magnesium, Lithium, and Titanium
 Advances and Innovations in the Extraction of Aluminum, Magnesium, Lithium, and Titanium Donald R. Sadoway Department of Materials Science & Engineering Massachusetts Institute of Technology Cambridge,
Advances and Innovations in the Extraction of Aluminum, Magnesium, Lithium, and Titanium Donald R. Sadoway Department of Materials Science & Engineering Massachusetts Institute of Technology Cambridge,
EXPERIENCE AND RESULTS FROM RUNNING OF A 1 KG TI SCALE KROLL REACTOR. S A C Hockaday and K Bisaka Pyrometallurgy Division, MINTEK
 EXPERIENCE AND RESULTS FROM RUNNING OF A 1 KG TI SCALE KROLL REACTOR Pyrometallurgy Division, MINTEK Abstract Ti metal is conventionally produced by magnesiothermic reduction of titanium tetrachloride
EXPERIENCE AND RESULTS FROM RUNNING OF A 1 KG TI SCALE KROLL REACTOR Pyrometallurgy Division, MINTEK Abstract Ti metal is conventionally produced by magnesiothermic reduction of titanium tetrachloride
Materials are all substances and include metals, ceramics and plastics as well as natural and new substances.
 National 4 Materials It is hard to imagine life without mobile gadgets such as iphones, ipads and MP3 players. Yet twenty years ago these handy gadgets such as the mobile phone where bigger and cost five
National 4 Materials It is hard to imagine life without mobile gadgets such as iphones, ipads and MP3 players. Yet twenty years ago these handy gadgets such as the mobile phone where bigger and cost five
Use of Direct Reduced Iron in the Electric Furnace P CaO + MgO... o. 20. Mn Cr... <o.o05
 Use of Direct Reduced Iron in the Electric Furnace DIRECTLY reduced iron is coming more and more into the headlines of the steelmaking industry and interest is widespread and increasing. Much work has
Use of Direct Reduced Iron in the Electric Furnace DIRECTLY reduced iron is coming more and more into the headlines of the steelmaking industry and interest is widespread and increasing. Much work has
Disruptive Process Technology Delivering a Sustainable Energy Future
 Disruptive Process Technology Delivering a Sustainable Energy Future ii Vanadium coproduct from the Chinese steel boom helped create the business case for the vanadium battery gigafactory, and home of
Disruptive Process Technology Delivering a Sustainable Energy Future ii Vanadium coproduct from the Chinese steel boom helped create the business case for the vanadium battery gigafactory, and home of
GRADE 10: Chemistry 2. UNIT 10AC.2 11 hours. The chemical industry. Resources. About this unit. Previous learning. Expectations
 GRADE 10: Chemistry 2 The chemical industry UNIT 10AC.2 11 hours About this unit This unit is the second of six units on chemistry for Grade 10 advanced. The unit is designed to guide your planning and
GRADE 10: Chemistry 2 The chemical industry UNIT 10AC.2 11 hours About this unit This unit is the second of six units on chemistry for Grade 10 advanced. The unit is designed to guide your planning and
Reactivity Series. Question Paper. Save My Exams! The Home of Revision. Module Double Award (Paper 1C) Chemistry of the Elements.
 Save My Exams! The Home of Revision For more awesome GCSE and A level resources, visit us at www.savemyexams.co.uk Reactivity Series Question Paper Level GCSE Subject Chemistry Exam Board Edexcel IGCSE
Save My Exams! The Home of Revision For more awesome GCSE and A level resources, visit us at www.savemyexams.co.uk Reactivity Series Question Paper Level GCSE Subject Chemistry Exam Board Edexcel IGCSE
Titanium-Strength, Corrosion Properties And Uses
 Titanium-Strength, Corrosion Properties And Uses How do you consider Titanium, as an unusual material described for powerful jet engines? A costly metal that is discovered on the outer walls of a popular
Titanium-Strength, Corrosion Properties And Uses How do you consider Titanium, as an unusual material described for powerful jet engines? A costly metal that is discovered on the outer walls of a popular
GENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
 INTEXT QUESTIONS GENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS Question 6.1: Which of the ores mentioned in Table 6.1 can be concentrated by magnetic separation method? If the ore or the gangue
INTEXT QUESTIONS GENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS Question 6.1: Which of the ores mentioned in Table 6.1 can be concentrated by magnetic separation method? If the ore or the gangue
Title. Author(s)Osaki, Shogo; Sakai, Hiroshi; Suzuki, Ryosuke O. CitationJournal of the Electrochemical Society, 157(8): E117. Issue Date
 Title Direct Production of Ti-29Nb-13Ta-4.6Zr Biomedical A Author(s)Osaki, Shogo; Sakai, Hiroshi; Suzuki, Ryosuke O. CitationJournal of the Electrochemical Society, 157(8): E117 Issue Date 2010-06-03 Doc
Title Direct Production of Ti-29Nb-13Ta-4.6Zr Biomedical A Author(s)Osaki, Shogo; Sakai, Hiroshi; Suzuki, Ryosuke O. CitationJournal of the Electrochemical Society, 157(8): E117 Issue Date 2010-06-03 Doc
Introduction Fuel Cells
 Introduction Fuel Cells Fuel cell applications PEMFC PowerCell AB, S2 PEMFC, 5-25 kw Toyota Mirai a Fuel Cell Car A look inside The hydrogen tank 1. Inside Layer of polymer closest to the H2 gas 2. Intermediate
Introduction Fuel Cells Fuel cell applications PEMFC PowerCell AB, S2 PEMFC, 5-25 kw Toyota Mirai a Fuel Cell Car A look inside The hydrogen tank 1. Inside Layer of polymer closest to the H2 gas 2. Intermediate
Title. Author(s)Oka, Yuichi; Suzuki, Ryosuke O. CitationECS Transactions, 16(49): Issue Date Doc URL. Rights. Type.
 Title Direct Reduction of Liquid V2O5 in Molten CaCl2 Author(s)Oka, Yuichi; Suzuki, Ryosuke O. CitationECS Transactions, 16(49): 255-264 Issue Date 2009 Doc URL http://hdl.handle.net/2115/50032 Rights
Title Direct Reduction of Liquid V2O5 in Molten CaCl2 Author(s)Oka, Yuichi; Suzuki, Ryosuke O. CitationECS Transactions, 16(49): 255-264 Issue Date 2009 Doc URL http://hdl.handle.net/2115/50032 Rights
GRADE 8: Materials 3. UNIT 8M.3 10 hours. Uses of metals. Resources. About this unit. Previous learning. Expectations
 GRADE 8: Materials 3 Uses of metals UNIT 8M.3 10 hours About this unit This unit is the third of four units on materials for Grade 8. It is desirable that students should have studied Unit8P.3 on heat
GRADE 8: Materials 3 Uses of metals UNIT 8M.3 10 hours About this unit This unit is the third of four units on materials for Grade 8. It is desirable that students should have studied Unit8P.3 on heat
A study of preparation of titanium metal by the electrochemical reduction of titanium dioxide in molten salt
 Available online at www.sciencedirect.com Procedia Earth and Planetary Science 2 ( 211 ) 1 6 The Second International Conference on Mining Engineering and Metallurgical Technology A study of preparation
Available online at www.sciencedirect.com Procedia Earth and Planetary Science 2 ( 211 ) 1 6 The Second International Conference on Mining Engineering and Metallurgical Technology A study of preparation
Molten salt electrolysis of Titanium using a TiO 2 -C composite anode in halide electrolytes
 Molten salt electrolysis of Titanium using a TiO 2 -C composite anode in halide electrolytes Claudia A. Möller, Bernd Friedrich RWTH Aachen University, IME Process Metallurgy and Metals, Intzestrasse 3,
Molten salt electrolysis of Titanium using a TiO 2 -C composite anode in halide electrolytes Claudia A. Möller, Bernd Friedrich RWTH Aachen University, IME Process Metallurgy and Metals, Intzestrasse 3,
a service offered by the Hempel Special Metals Group
 Hot Isostatic Pressing of Near Net Shaped Parts a service offered by the Hempel Special Metals Group content introduction description of the method supply chain aspects & quality management applications
Hot Isostatic Pressing of Near Net Shaped Parts a service offered by the Hempel Special Metals Group content introduction description of the method supply chain aspects & quality management applications
Summary of findings from HYBRIT Pre-Feasibility Study
 Fe₂O₃ A joint venture between SSAB, LKAB and Vattenfall H₂O Summary of findings from HYBRIT Pre-Feasibility Study 2016 2017 This work was funded by the Swedish Energy Agency, SSAB, LKAB, and Vattenfall
Fe₂O₃ A joint venture between SSAB, LKAB and Vattenfall H₂O Summary of findings from HYBRIT Pre-Feasibility Study 2016 2017 This work was funded by the Swedish Energy Agency, SSAB, LKAB, and Vattenfall
BENCHMARKING SUMMARY BETWEEN SLM AND EBM RELATED TO POWDER RECYCLING.
 BENCHMARKING SUMMARY BETWEEN SLM AND EBM RELATED TO POWDER RECYCLING. In the ManSYS project a method has been specified which examines components for meeting required specifications (qualifying criteria)
BENCHMARKING SUMMARY BETWEEN SLM AND EBM RELATED TO POWDER RECYCLING. In the ManSYS project a method has been specified which examines components for meeting required specifications (qualifying criteria)
Al-Foam Production Scrap Source for Recycling?
 1 Al-Foam Production Scrap Source for Recycling? Dipl.-Ing. K. Jessen*, Prof. Dr.-Ing. B. Friedrich*, Dr.-Ing. G. Rombach** * IME, Process Metallurgy and Metal Recycling, RWTH Aachen ** Hydro Aluminium
1 Al-Foam Production Scrap Source for Recycling? Dipl.-Ing. K. Jessen*, Prof. Dr.-Ing. B. Friedrich*, Dr.-Ing. G. Rombach** * IME, Process Metallurgy and Metal Recycling, RWTH Aachen ** Hydro Aluminium
Lecture 17 Alternative Charge Materials in EAF
 Lecture 17 Alternative Charge Materials in EAF Contents: Introduction Types of metallic charge materials Carbon content in DRI Charging methods Key words: Sponge iron, DRI, electric arc furnace, UHP furnaces
Lecture 17 Alternative Charge Materials in EAF Contents: Introduction Types of metallic charge materials Carbon content in DRI Charging methods Key words: Sponge iron, DRI, electric arc furnace, UHP furnaces
Chemical reactions and electrolysis
 Chemical reactions and electrolysis Higher Revision Questions Name: Class: Date: Time: 95 minutes Marks: 95 marks Comments: Page of 29 (a) Magnesium metal is shaped to make magnesium ribbon. Explain why
Chemical reactions and electrolysis Higher Revision Questions Name: Class: Date: Time: 95 minutes Marks: 95 marks Comments: Page of 29 (a) Magnesium metal is shaped to make magnesium ribbon. Explain why
Development of new methodologies for InDustrial CO2-freE steel production by electrowinning SIDERWIN general presentation, 2018
 9, 1% Development of new methodologies for InDustrial CO2-freE steel production by electrowinning Partners 2 General presentation of SIDERWIN project General presentation of SIDERWIN project 1200000 1000000
9, 1% Development of new methodologies for InDustrial CO2-freE steel production by electrowinning Partners 2 General presentation of SIDERWIN project General presentation of SIDERWIN project 1200000 1000000
Current Activities of OS Process Using Molten CaO+CaCl 2
 Current Activities of OS Process Using Molten CaO+CaCl 2 Ryosuke O. Suzuki Faculty of Engineering, Hokkaido University 1. Direct Production of Ti-29Nb-13Ta-4.6Zr Biomedical Alloy Appling the principle
Current Activities of OS Process Using Molten CaO+CaCl 2 Ryosuke O. Suzuki Faculty of Engineering, Hokkaido University 1. Direct Production of Ti-29Nb-13Ta-4.6Zr Biomedical Alloy Appling the principle
Treatment of Spent Nuclear Fuel with Molten Salts
 Treatment of Spent Nuclear Fuel with Molten Salts Michael Goff Deputy Associate Laboratory Director Operations Nuclear Science and Technology Idaho National Laboratory 2008 Joint Symposium on Molten Salts
Treatment of Spent Nuclear Fuel with Molten Salts Michael Goff Deputy Associate Laboratory Director Operations Nuclear Science and Technology Idaho National Laboratory 2008 Joint Symposium on Molten Salts
Ti 6Al-4V is recommended for use at service temperatures up to approximately 350 C (660 F).
 Titanium Alloy Ti 6Al-4V Type Analysis Carbon (Maximum) 0.10 % Titanium Balance Aluminum 5.50 to 6.75 % Vanadium 3.50 to 4.50 % Nitrogen 0.05 % Iron (Maximum) 0.40 % Oxygen (Maximum) 0.020 % Hydrogen (Maximum)
Titanium Alloy Ti 6Al-4V Type Analysis Carbon (Maximum) 0.10 % Titanium Balance Aluminum 5.50 to 6.75 % Vanadium 3.50 to 4.50 % Nitrogen 0.05 % Iron (Maximum) 0.40 % Oxygen (Maximum) 0.020 % Hydrogen (Maximum)
