THE CHEMISTRY AND TECHNOLOGY OF MAGNESIA
|
|
|
- Tobias Phillips
- 6 years ago
- Views:
Transcription
1 THE CHEMISTRY AND TECHNOLOGY OF MAGNESIA MARK A. SHAND Premier Chemicals, LLC Findlay, Ohio A JOHN WILEY & SONS, INC. PUBLICATION
2
3 THE CHEMISTRY AND TECHNOLOGY OF MAGNESIA
4
5 THE CHEMISTRY AND TECHNOLOGY OF MAGNESIA MARK A. SHAND Premier Chemicals, LLC Findlay, Ohio A JOHN WILEY & SONS, INC. PUBLICATION
6 Copyright # 2006 by John Wiley & Sons, Inc. All rights reserved. Published by John Wiley & Sons, Inc., Hoboken, New Jersey. Published simultaneously in Canada. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording, scanning, or otherwise, except as permitted under Section 107 or 108 of the 1976 United States Copyright Act, without either the prior written permission of the Publisher, or authorization through payment of the appropriate per-copy fee to the Copyright Clearance Center, Inc., 222 Rosewood Drive, Danvers, MA 01923, (978) , fax (978) , or on the web at Requests to the Publisher for permission should be addressed to the Permissions Department, John Wiley & Sons, Inc., 111 River Street, Hoboken, NJ 07030, (201) , fax (201) , or online at permission. Limit of Liability/Disclaimer of Warranty: While the publisher and author have used their best efforts in preparing this book, they make no representations or warranties with respect to the accuracy or completeness of the contents of this book and specifically disclaim any implied warranties of merchantability or fitness for a particular purpose. No warranty may be created or extended by sales representatives or written sales materials. The advice and strategies contained herein may not be suitable for your situation. You should consult with a professional where appropriate. Neither the publisher nor author shall be liable for any loss of profit or any other commercial damages, including but not limited to special, incidental, consequential, or other damages. For general information on our other products and services or for technical support, please contact our Customer Care Department within the United States at (800) , outside the United States at (317) or fax (317) Wiley also publishes its books in a variety of electronic formats. Some content that appears in print may not be available in electronic formats. For more information about Wiley products, visit our web site at Library of Congress Cataloging-in-Publication Data: Shand, Mark A. The chemistry and technology of magnesia/mark A. Shand. p. cm. Includes index. ISBN-13: (cloth) ISBN-10: (cloth) 1. Magnesium oxide. 2. Magnesium oxide Industrial applications. 3. Magnesium oxide Safety measures. I. Title. TP889.S dc Printed in the United States of America
7 CONTENTS Preface Acknowledgments xv xvii 1 History of Magnesia History of Magnesia, 1 Bibliography, 4 2 Formation and Occurrence of Magnesite and Brucite Introduction, Sedimentary Magnesite Basis for Carbonate Deposition, Secondary Nodular Magnesite, Biogenic Carbonate, Serpentine Alteration by Hydrothermal Processes, Cryptocrystalline Magnesite Formation by Infiltration, Crystalline Magnesite Replacement of Limestone and Dolomite, Brucite, Worldwide Occurrence of Magnesite and Brucite, United States and Canada, Brazil, 17 v
8 vi CONTENTS Australia, China, North Korea, Nigeria, South Africa, India, Saudi Arabia, Iran, Greece, Turkey, Serbia and Bosnia, Austria, Russia, Slovakia, Spain, Physical and Chemical Properties of Magnesite, Chemical and Physical Properties of Brucite, 33 Bibliography, 35 References, 35 3 Synthetic Magnesia Introduction, Composition of Seawater and Brines, Seawater Chemistry, Brine Extraction, Sump Leaching Phase, Preparation Phase, Production Phase, Evaporite Production, Subsurface Brines, Process Description, Precipitation Reaction, Influence of Reaction Conditions on Mg(OH) 2 Particle Morphology, Dolime/Lime Requirements, Seawater Pretreatment, 48
9 CONTENTS vii Precipitation Process, Settling and Compaction, Washing, Filtration, Brine Precipitation, Calcination, Grinding, Packaging, Sampling and Testing and In-Process Quality Control, Dolime, Seawater, Reactor, Settling/Thickener, Washing, Filtration, Calcining, Grinding, Finished Product, Aman Process, Pyrohydrolysis of Magnesium Chloride Hexahydrate, General Properties of Synthetic Magnesia, 60 Bibliography, 60 References, 60 4 Mining and Processing Magnesite Mining Operations, Overburden Removal, Drilling, Bench Height, Hole Diameter, Burden and Spacing, Subdrilling, Hole Stemming, Blast Hole Pattern, Blast Timing, 67
10 viii CONTENTS Blasting Agents, Secondary Blasting, Chemical Contour and Muck Maps, Processing Magnesite, Ore Removal and Primary Crushing, Gyratory and Cone Crushers, Jaw Crushers, Roll Crushers, Size Separation, Screening, Pneumatic (Air) Classification, Hydroclones, Gravity Concentration, Float Sink Separation, Froth Floatation, Floatation Reagents, Floatation Machines, Tertiary Crushing, Postcalcination Screening and Grinding, 81 References, 81 5 Calcination of Magnesium Hydroxide and Carbonate Calcination of Magnesite, Energy Requirement for Calcination Process, Effect of Time and Temperature, Kinetics of Calcination, Stone Size, Calcination of Magnesium Hydroxide, Energy Requirement for Calcination Process, Decomposition Mechanism, Kinetics of Decomposition, Effect of Time and Temperature, 94 References, 96 6 Furnaces and Kilns Introduction, 97
11 CONTENTS ix 6.2 Multiple-Hearth Furnaces, Single Progressive Rabble (Four Arms per Hearth), Full Progressive Rabble (Four Arms per Hearth), Back Rabble (Four Arms per Hearth), Full Progressive Rabble (Two Arms per Hearth), Refractory Linings, Horizontal Rotary Kilns, External Water Coolers, Shaft Kilns, Ore Charging, Discharge, Modern Shaft Kiln, Double-Inclined Kiln, Multichamber Kiln, Annular Shaft Kiln, Parallel-Flow Regenerative Kiln, Postcalcination Processing Introduction, Grinding, Ring-Roller Mills, Ball Mills, 117 Bibliography, 119 Reference, Physical and Chemical Properties of Magnesium Oxide Introduction, Physical Properties of Magnesium Oxide, Chemical Properties of Magnesium Oxide, Dissolution of Magnesium Oxide, Surface Structures of MgO, Molecular Adsorption on MgO, Chemisorption of Various Molecules on MgO, 129 Bibliography, 130 References, 131
12 x CONTENTS 9 Other Magnesia Products Production of Hard-Burned Magnesia, Production of Dead-Burned Magnesia, Sintering, Sinter Aids, Production Methods, Fused Magnesia, Refractory-Grade Fused Magnesia, Magnesium Hydroxide Slurry, Production of Synthetic Magnesium Hydroxide Slurry, Hydration of Magnesium Oxide, Hydration Kinetics and Mechanisms, Testing and Quality Control of Magnesium Hydroxide Slurry, Purification by Carbonation of Magnesium Hydroxide Slurry, Precipitation of Magnesium Carbonate from Bicarbonate Solution, 153 References, Water and Wastewater Applications for Magnesia Products Introduction to Applications, Industrial Wastewater Treatment, Advantages of Magnesium Hydroxide in Wastewater Treatment, Safety, ph Control, Metals Removal, Sludge Volume and Dewatering, Treatment Methods, Handling Requirements, Environmental Impact, Adsorption of Dyes on Magnesium Hydroxide, Biological Wastewater Treatment, Aerobic Processes, 164
13 CONTENTS xi Nitrification, Anaerobic Digestion, Bioflocculation and Solids Settling, Phosphorus Removal from Wastewater and Struvite Formation, Odor and Corrosion Control in Sanitary Collection Systems, Addition to Raw Sewage, Crown Spraying, Acid Mine Drainage, Silica Removal from Industrial Plant Water, Mechanism of Silica Removal, Factors Controlling the Removal of Silica, 174 References, Magnesia in Polymer Applications Magnesium Hydroxide as a Flame Retardant for Polymer Applications, Flame-Retardant Mechanisms, Properties Required of Magnesium Hydroxide for Flame-Retardant Applications, Surface Treatment, Stearic Acid, Silanes, Novel Applications for Magnesium Hydroxide as a Flame Retardant, Polymer Curing and Thickening, Sheet Molding Compound (SMC), Synthetic Rubber, 185 Bibliography, 186 References, Environmental Applications Flue Gas Desulfurization, Regenerative Process, Process Description, Once-Through Process, 192
14 xii CONTENTS Kawasaki Process, Dravo Thiosorbic Process with Magnesium Hydroxide Recovery, Sorbtech Process, Remediation Applications, Nuclear Waste Disposal, Hazardous Spill Cleanup, Antibacterial Activity of Magnesium Oxide Powder, Carbon Dioxide Sequestration Using Brucite, 197 Bibliography, 198 References, Role of Magnesium in Animal, Plant, and Human Nutrition Role of Magnesium in Plant Nutrition, Uptake of Magnesium from the Soil, Functions of Magnesium in Plant Growth, Magnesium Fertilizers, Magnesium in Animal Nutrition, Ruminant Animals, Magnesium Oxide Requirements for Animal Nutrition, Factors Affecting Magnesium Utilization, Magnesium Bioavailability, Preventing Grass Tetany by Magnesium Fertilization, Preventing Grass Tetany by Oral Supplementation, Magnesium Requirements of Swine, Magnesium Requirements of Poultry, Magnesium in Dairy Ration Buffers, Magnesium in Human Health and Nutrition, Health Benefits of Magnesium, 209 Bibliography, 210 References, Magnesium Salts and Magnesium Metal Magnesium Acetate, Magnesium Alkyls, 215
15 CONTENTS xiii 14.3 Magnesium Chloride, Magnesium Nitrate, Magnesium Sulfate, Magnesium Soaps, Magnesium Overbase Sulfonates, Magnesium Peroxide, Magnesium Metal Production, Thermal Processes, Electrolytic Production, 221 Bibliography, Pulp Applications Sulfite Pulping, Magnefite Pulping Process, Pulping Liquor Preparation, Pulping Process, Chemical Recovery, Pulp Bleaching, Oxygen Bleaching and Delignification, Hydrogen Peroxide Bleaching, Deinking, 229 Bibliography, 229 References, Magnesia Cements Introduction, Magnesium Oxychloride Cement, Phase Formation, Water Resistance of MOC Cement, Magnesium Oxysulfate (MOS) Cement, Thermal Insulative and Fire Resistance Properties of Sorel Cement, Thermal Insulation, Fire Resistance, Magnesium Phosphate Cement, Reaction Mechanism, 235
16 xiv CONTENTS Magnesium Phosphate Cement Derived from Ammonium Dihydrogen Phosphate, Magnesium Phosphate Cement Derived from Diammonium Phosphate, Magnesium Phosphate Cement Derived from Ammonium Polyphosphate, Magnesium Phosphate Cement Derived from Potassium Dihydrogen Phosphate, 238 Bibliography, 238 References, Miscellaneous Magnesia Applications Sugar Manufacture, Clarification and Filtration, Reduction in Scaling, Additional Benefits, Chrome Tanning of Leather, Magnesia as a Catalyst Support, Example of Magnesium Oxide as Catalyst, Fuel Additives, High-Temperature Corrosion, Low-Temperature Corrosion, Types of Additives for High-Temperature Corrosion, Types of Additives for Low-Temperature Corrosion, Well-Drilling Fluids, Nanoparticulate Magnesia, Synthesis of Nanoparticulate Magnesia, Chemical and Catalytic Properties of Nanocrystals, Transformer Steel Coating, 254 Bibliography, 254 References, 254 Appendix 257 Index 263
17 PREFACE This book attempts to encompass all things magnesia. Although the magnesia industry is similar in many respects to the much larger lime industry, there is to my knowledge no text concentrating solely on magnesia. There are, however, several excellent texts covering the lime industry. Unfortunately, in these books magnesia is practically a footnote. Although lime and limestone production far exceeds that of the magnesia industry (total lime production in the United States in 2004 was 20.4 million metric tonnes, compared with a total magnesia production of 280,000 metric tonnes), magnesia is still an important chemical and maintains many niche applications. By far, the largest consumer of magnesia worldwide is the refractory industry, which consumed about 56% of the magnesia in the United States in 2004, the remaining 44% being used in agricultural, chemical, construction, environmental, and other industrial applications. This text starts with the geological occurrences of magnesite and brucite, followed by the processing of magnesite to the end product magnesium oxide. The production of magnesium hydroxide and magnesium oxide by precipitation from seawater and brine sources is also introduced, along with details on the wide range of applications in which magnesia is utilized. These applications span animal feed to wastewater treatment, catalyst support and fertilizers to the production of pulp and paper. Like many industries, a certain amount of jargon arises as the industry matures, and the magnesia industry is no exception to this. However, there may be some confusion as to which compound magnesia really applies. xv
18 xvi PREFACE My definition is that the term magnesia is a generalization for magnesium oxide, whether it is derived from natural magnesite or extracted from seawater or brine. The term magnesite, in the strictest sense, refers to the mineral consisting of magnesium carbonate, but the same term is often used for the oxide, that is, dead-burned magnesite, the term even being used when the oxide has been produced from seawater or brine sources. The major products produced by the magnesia industry are magnesium carbonate (magnesite); magnesium hydroxide, both natural (brucite) and that derived from seawater and brine; magnesium oxide, which in itself has a number of categories, namely, light-burned or caustic-calcined MgO, hard-burn MgO, and dead-burn MgO, or as it is otherwise known, periclase; and the last category, fused magnesia. Light-burn or caustic-calcined MgO refers to a product that has been calcined at the lower end of the temperature spectrum, typically F. This product typically has the highest reactivity and greatest specific surface area of the entire magnesium oxide category. Hard-burn MgO is calcined at a higher temperature, F, and has a correspondingly lower reactivity and surface area. Dead-burn MgO, or periclase, is produced at temperatures above 28008F, which having a very small surface area, makes it unreactive. Finally, fused magnesia, produced at temperatures above the fusion point of magnesium oxide (28008C), is the least reactive.
19 ACKNOWLEDGMENTS First and foremost, I am indebted to my wife Karen, whose encouragement kept me working during periods when my enthusiasm waned, and also to my kids, Alex, Victoria, and Madalyn, whose persistent question haven t you finished that book yet? also provided the needed impetus to get the text finished. I also owe a debt of gratitude to John Gehret and the board of directors of Premier Chemicals, LLC for allowing me the opportunity to write the book. I would also like to especially thank my long-time friend and mentor, Dr. Ronald Wardle, along with John Noble of Vesuvius USA and Mark Wajer of Martin Marietta Magnesia Specialties for diligently correcting the text and providing helpful suggestions. I would also like to thank Lynden Johnson for increasing my knowledge of the process of mining magnesite. Finally, I would like to thank Gerry Spoors, Dr. Theofilos Zampetakis, and John Turner for their affirmation that this book should be written, and to John Wiley & Sons for agreeing to publish said text. xvii
20
21 1 HISTORY OF MAGNESIA 1.1 HISTORY OF MAGNESIA Magnesia alba, otherwise known to alchemists as white magnesia or mild magnesian earth, is known today as magnesite or magnesium carbonate, MgCO 3. Magnesia nigra, however, refers to black manganese oxide, MnO 2. Both of these names are derived from Magnesia, Mágvh sıá, which is a prefecture in Thessaly, Greece. Manganese and magnesium, as well as iron, are abundant in the form of oxides and carbonates in this region, and these minerals were referred to as stones from Magnesia. The iron oxides present in magnesia were in the form of magnetic magnetite or lodestone, and both magnesia alba and magnesia nigra contain large amounts of magnetite, thus making them magnetic. This explains why magnesium and magnet are both derived from the place name Magnesia. In alchemical terms, magnesia meant a stone shining like silver and was purported to be an ingredient of the philosopher s stone. In the more modern sense of the word, it is thought to have originated from magnes carneus, which means flesh magnet from the way it stuck strongly to the lips. Bergman s essay (Bergman et al ) De Magnesia claimed that the Roman Count di Palma prepared a white powder that he claimed was a panacea for all diseases. The white powder was called magnesia alba, or Count Palma s powder, and its origin was a closely guarded secret. The Chemistry and Technology of Magnesia, by Mark A. Shand Copyright # 2006 John Wiley & Sons, Inc. 1
22 2 HISTORY OF MAGNESIA In 1701 M.B. Valentini (Valentini, 1707) prepared magnesia alba by the calcination of the residue remaining after evaporating the mother liquor to dryness from the preparation of niter or potassium nitrate. To add even more to the confusion, it is apparent that at least three minerals of different chemical composition were called magnesia: (1) magnesius lapis, which referred to magnetic magnetite, (2) magnesia nigra, which refers to pyrolusite (MnO 2 ), and (3) a silver-white mineral that was probably steatite or talc. At the beginning of the eighteenth century the term manganes was employed for the manganese mineral and magnesia for the white mineral. However, the difference between magnesia nigra and magnesius lapis was not demonstrated until the middle of the eighteenth century. Hoffman (Hoffman, 1729) was the first to recognize the differences between magnesia and lime. He stated that an alkaline earth prepared by the reaction of a bitter salt (Epsom salts) with a fixed alkali differed from lime. Whereas lime gave a sparingly soluble salt with sulfuric acid that was nearly without taste, magnesia alba gave a bitter soluble salt. However, it was not until 1754 that magnesia was finally distinguished from lime by Joseph Black. Black was the first person to recognize that magnesium was an element. Black, a prominent professor of anatomy and chemistry at Edinburgh, showed that magnesia alba (magnesium carbonate), when heated, evolved fixed air (carbon dioxide). The residue from this heating, calcined magnesia (magnesium oxide), was lighter and more alkaline than the basic carbonate. Limestone (calcium carbonate) was found to behave in the same manner. Black also demonstrated that magnesia alba produced a soluble sulfate in contrast to lime. He gave the alkaline earth the name magnesia. Black s thesis (Black, 1777) presented in June 1754, On the Acid Humour Arising from Food and Magnesia Alba dealt primarily with the value of magnesia as an antacid. In 1808 Humphrey Davy (Davy, 1808) proved definitively that magnesia is the oxide of a metal, which he named magnium. At this juncture, the term magnesium was being used by some to define metallic manganese. Davy s technique involved mixing moistened alkaline-earth oxide with mercuric sulfide (cinnabar) and placing the paste onto a platinum plate. A drop of mercury was dropped into a depression made in the paste and the whole covered with naphtha. The platinum plate and drop of mercury were then connected to the poles of a voltaic pile. The resultant amalgam that formed on the mercury pole was then transferred to a glass tube and the mercury distilled off. Davy described the characteristics of magnesium as follows (Davy, 1808) The metal from magnesia appears to react with the glass, especially before all the mercury has distilled off. In one experiment, in which I interrupted the distillation before the mercury had been completely removed, the metal appeared
23 1.1 HISTORY OF MAGNESIA 3 as a solid body, which exhibited the same white color and the same luster as the other metals of the alkalide-earths. It immediately sank to the bottom of the water although surrounded by gas bubbles, formed magnesia. It changed quickly in the atmosphere, a white crust forming, and finally it disintegrated into a white powder, which proved to be magnesia. Eventually, much to the consternation of Davy, the name magnesium was adopted for the metal in magnesia alba and manganese for the metal in pyrolusite. Michael Faraday produced magnesium metal in 1833 by the electrolysis of fused anhydrous magnesium chloride. The mineralogical term magnesite was first applied to a series of magnesium salts (carbonate, sulfate, nitrate, and chloride) by J.C. Delaméthrie (Delaméthrie, 1795) in The same term was also being applied to magnesium carbonates and silicates by A. Brongniart (Brongniart, 1807). Deposits of natural magnesium carbonate were discovered at Hrubschütz in Moravia, which is now Hrubšice in the Czech Republic, and named Kohlensaurer Talkerde by W.A. Lampadius in 1800 (Lampadius, 1800). C.F. Ludwig described these minerals as talcum carbonatum in The use of the term magnesite was first restricted to the carbonate minerals by D.L.G. Karsten in 1808 (Karsten, 1808). The name magnesite gradually grew in acceptance. Deposits of magnesite were found in Austria and Greece during the later half of the nineteenth century, and around the same time magnesite mines were opened in Canada. In 1886 magnesite was discovered in California and commercial mining commenced around 1900, and in 1913 production of magnesia commenced in Pennsylvania. The development of the magnesite industry was accelerated in 1914 by the outbreak of World War I as supplies from Austria and Greece were cut-off by the blockade of central European powers. Magnesite was found in Stevens County, Washington, in 1916 and mining started in During World War I, California magnesite came from small deposits in the Porterville district, which were operated in a crude fashion by small owners or contractors. The deposits situated at Magnesite, California, which is a short distance from Portersville, were developed in a more systematic manner, and after the war formed the nucleus of a sound mining and production operation. However, much of the early magnesite technology was developed in the Portersville district, such as the mechanical beneficiation of magnesite and the calcining of magnesite to develop a product with specific and controllable characteristics. It was also at Portersville that the first high-purity crystalline magnesium oxide was produced in a rotary kiln. Commercial production of refractorygrade magnesia was also in operation in the Livermore district of California by Western Mine and the Bald Eagle mine operation by Westvaco Chlorine products in Stanislaus County, California.
24 4 HISTORY OF MAGNESIA An immense deposit of medium- to low-quality magnesite exists in Steven County, Washington, which was exploited by the Northwest Magnesite Company during World War I. Operations were centered on the towns of Chewelah and Valley. It was here that the first use of froth flotation to beneficiate magnesite was employed to reduce silica and lime content. A very large deposit of dolomite, magnesite, and brucite in the Paradise Range, Nye County, Nevada, has been known since about 1927 when brucite was discovered by Harry Springer. Drilling by U.S. Brucite in 1930 and 1931 revealed the presence of considerable quantities of magnesite adjacent to the brucite. From 1931 to 1933, the U.S. Geological Survey mapped the deposit and estimated that there were 71 million tons of magnesite and brucite bearing rock. Both magnesite and brucite were mined by Basic Ores, Inc., and the Sierra Magnesite Company. Currently, the only magnesite deposit being exploited in the United States is the one at Gabbs, Nevada. BIBLIOGRAPHY Black, J. (1777). Experiments upon Magnesia ALBA, Quick-Lime, and other Alkaline Substances. W. Creech: Edinburgh. Bergman, T. O., Cullen, E. and Beddoes, T. ( ). Physical and Chemical Essays. J. Murray: London. Brongniart, A. (1807). Traité Élémentaire de Mineralogic, , Paris. Davy, H. (1808). Electro-chemical researches on the decomposition of the Earths; with observations on the metals obtained from the alkaline Earths, and on the amalgam procured form ammonia. Philosophical Transactions of the Royal Society of London, 98, pp Delamétheric, J. C. (1975). Théorie de la Terre, Paris. Greenberg, A. (2000). A Chemical History Tour: Picturing Chemistry from Alchemy to Modern Molecular Science. Wiley-Interscience: New York. Hoffman, F. (1729). Dissertationum physico-chymicarne Haloe Magdeburigicae. Karsten, D. L. G. (1808). Mineralogische Tabellen, , Berlin. Knibbs, N. (1924). Lime and Magnesia. D. Van Nostrand: New York. Lampadius, M. A. (1800). Sammlung Praktisch-Chemischer Abhandlungen, Dresden. Mellor, J. W. (1960). A Comprehensive Treatise on Inorganic and Theoretical Chemistry, Vol. IV. Wiley: New York. Seaton, M. Y. (1942). Production and Properties of the Commercial Magnesia. Mining Tech., Valentini, M. B. (1707). Relatio de Magnesia alba, novo, genuino, polychresto et innixio pharmaco purgante Roma nuper advecto. Giessa Hassoram: Italy. Weeks, M. E. (1968). Discovery of the Elements. J. Chem. Ed., 7th ed
25 2 FORMATION AND OCCURRENCE OF MAGNESITE AND BRUCITE 2.1 INTRODUCTION Magnesium is the eighth most abundant element in the solar system and constitutes about 2% of Earth s crust. It is also the third most abundant element in solution in seawater, with a concentration of about 1300 ppm. The element magnesium is composed of three stable isotopes: 24 Mg (78.6%), 25 Mg (10.1%), and 26 Mg (11.3%), with an average atomic weight of More than 60 magnesium-containing minerals are known. The most important rock-forming minerals containing magnesium are the chlorites, the pyroxene and amphibole group minerals, dolomite, and magnesium calcite (calcite with some of the Ca replaced by Mg). Magnesium is also present in magnesite (MgCO 3 ), and the hydrated carbonates such as nesquehonite (MgCO 3.3H 2 O) and lansfordite (MgCO 3.5H 2 O) as well in brucite [Mg(OH) 2 ]. In addition there is a series of basic magnesium carbonates having the empirical formula xmgco 3.yMg(OH) 2.zH 2 O. These include hydromagnesite [4MgCO 3.Mg(OH) 2.4H 2 O] and artinite [MgCO 3. Mg(OH) 2.3H 2 O]. Magnesium also occurs in salt deposits such as carnallite (KMgCl 3.6H 2 O), epsomite (MgSO 4.7H 2 O), and kieserite (MgSO 4.H 2 O). It has been estimated that the total mass of the sedimentary layer in Earth s crust is tonnes (Wedephol, 1968). Magnesium is present in the sediment mainly in dolomite and phyllosilicates, such as chlorite and The Chemistry and Technology of Magnesia, by Mark A. Shand Copyright # 2006 John Wiley & Sons, Inc. 5
26 6 FORMATION AND OCCURRENCE OF MAGNESITE AND BRUCITE glauconite. Carbonate rocks constitute about 8 wt % of the total sediments (Goldschmidt, 1954). Dolomite makes up about 30 wt % of the carbonates in the sedimentary layer and contains tonnes Mg. Magnesite is the magnesium end member of an isomorphous series of carbonates. An increase in calcium content results first in dolomite and then calcite as the Ca end member. Since the difference between the ionic diameters of Mg 2þ and Fe 2þ (Mg 2þ ¼ 0.65 Å, Fe 2þ ¼ 0.79 Å), is not as great as that between Ca 2þ (0.99 Å) and Mg 2þ, Fe and Mg substitute for each other and form the isomorphic series from magnesite through breunnerite (5 30% FeCO 3 ) to siderite (FeCO 3 ), and from dolomite to CaCO 3.FeCO 3 ; see Figure 2.1. This isomorphic miscibility results in the presence of both calcite and dolomite in magnesite deposits as mechanical admixtures. However, iron is bound within the magnesite crystal lattice. Pure magnesite is rarely found, and the natural mineral tends to occur part way along an isomorphic series. Four main types of magnesite deposits have been described to date: 1. Magnesite as a sedimentary rock 2. Magnesite as an alteration of serpentine 3. Magnesite as a vein filling 4. Magnesite as a replacement of limestone and dolomite Figure 2.1 Isomorphic series substitution of Fe for Mg to form brunnerite.
27 2.2 SEDIMENTARY MAGNESITE BASIS FOR CARBONATE DEPOSITION 7 There are two physical forms of magnesite: cryptocrystalline or amorphous magnesite and crystalline, macrocrystalline, or bone magnesite. 2.2 SEDIMENTARY MAGNESITE BASIS FOR CARBONATE DEPOSITION Carbonate compounds are relatively insoluble, and Table 2.1 lists the solubility constants for a number of geologically important carbonates. Since these minerals are relatively insoluble, carbonates are precipitated at relatively low carbonate and counterion concentrations. As an example, a solution containing MCa 2þ will be precipitated by a concentration of CO 3 2- in excess of M. This occurs because the product of the two ionic concentrations exceeds the solubility constant for calcium carbonate; see Equation (2.1): ½Ca 2þ Š½CO 2 3 Š¼K sp ¼ 10 8:32 (2:1) Since the solubility of calcium carbonate is considerably less than that of magnesium carbonate, evaporation and concentration of salt lakes and lagoons must have produced calcium carbonate deposits initially. The brine would gradually be depleted of calcium ion and enriched with magnesium. Eventually, a condition would be reached where the brine concentration of the magnesium ion and carbonate exceeds the solubility constant of magnesite, and precipitation would proceed; see Equation (2.2): ½Mg 2þ Š½CO 2 3 Š¼K sp ¼ 10 5 (2:2) Sedimentary deposits of cryptocrystalline magnesite occur either in lagoons, salt lakes, or freshwater lakes (lacustrine). The genesis of magnesite in saltwater requires specific conditions for it to occur: a reducing alkaline environment, a high concentration of magnesium sulfate, and a concentration TABLE 2.1 Solubility Constants of Geologically Important Carbonates Compound (K sp ) Ref. CaCO Latimer and Hildebrand (1942) MgCO 3.3H 2 O Latimer and Hildebrand (1942) MgCO Weast and Astle (1982) CaMg(CO 3 ) Stumm and Morgan (1981) Source: Adapted from H. L. Lutz, Geomicrobiology, Marcel Dekker, New York, 2002, p. 191.
28 8 FORMATION AND OCCURRENCE OF MAGNESITE AND BRUCITE of CO 2 above 380 mg/l, a low Ca content (below 50 mg/l), and the presence of H 2 S, ammonia, or organic salts, along with an elevated temperature. It is thought that magnesium precipitates as the hydroxide first [Mg(OH) 2 ], which is subsequently altered by reaction with carbonate ion to form MgCO 3.xH 2 O. Dehydration then follows to form MgCO 3. Massive magnesite formation in freshwater lacustrine environments proceeds from magnesium derived from either hot solutions emanating from magma or from weathering of ultrabasic rock or serpentinites Secondary Nodular Magnesite A variation of the sedimentary process occurs when the host ultrabasic rock is weathered by water, the eroded material being transported and deposited in a distant lacustrine environment. Here, magnesite was precipitated as concretions along with impurities in a mud matrix. The recrystallization of the magnesite would then have occurred through additional uptake of carbon dioxide from the atmosphere. Wave movement on the lake during crystallization carried away noncrystallized impurities such as SiO 2,Fe 2 O 3,Al 2 O 3, and CaO from the newly formed magnesite nodules. These impurities are then deposited on the exterior and in the pores of the magnesite nodule. The resultant sand clay matrix with embedded magnesite nodules is covered with soil and compacted, and over time a silica-rich encrustation forms over the nodule. The percolation of bicarbonate-rich subsurface waters through the deposit results in raising the magnesite density (Schmid, 1987) Biogenic Carbonate A significant portion of the insoluble carbonate deposits at Earth s surface is of biogenic origin, while the remainder is the result of magmatic and metamorphic activity. The biological fixation of carbon as carbonate involves bacteria, fungi, and algae, and the carbonate can be deposited both extraand intracellular. A part of magnesium present in the oceans is withdrawn by marine CaCO 3 -secreting organisms, and this Mg replaces a part of the Ca in the hard parts of the organism. The quantity of Ca replaced depends upon the phylogenic position of the organism. Algae have the highest (5 wt %) and barnacles the lowest Mg concentration (0.9 wt %) (Chave, 1954). Sedimentary deposits of magnesite have been described as having either a biogenic origin or a chemical route via direct precipitation. These deposits are cryptocrystalline in form. Actinomycetes bacteria, which belong to the genus Streptomyces, are thought to have played a role in the formation of
29 2.2 SEDIMENTARY MAGNESITE BASIS FOR CARBONATE DEPOSITION 9 hydromagnesite (Cañaveras et al., 1999). These bacteria were found associated with moonmilk deposits containing hydromagnesite in Altamira in northern Spain. Moonmilk is a soft, white, claylike substance present on the walls of many caves. It consists mainly of calcite but can also contain appreciable quantities of magnesium. The magnesium-containing minerals present in moonmilk consist of any of the following: hydromagnesite, magnesite, huntite, nesquehonite, and dolomite. When the minerals constituents of moonmilk are dissolved in a weak acid, there remains a large quantity of organic matter, which consists mainly of such bacteria as Macromonas bipunctata, along with Actinomycetes, and algae. The larger mineral bodies in calcite moonmilk consist of rods with a diagonal grain impressed on the surface of the rods, the combined effect resembling an ear of corn. In many moonmilk samples these rods are enmeshed in a net of calcite filaments about 0.1 mm in width. It is believed that the filaments are associated with microbes that serve as nuclei for growth of the calcite bodies. The hypothesis of microbial mediation in the formation of magnesium carbonate minerals have been reported by Pontoizeau et al. (1987) who have suggested the involvement of sulfate-reducing bacteria in the genesis of huntite and magnesite in Sabkhat el Melah (Tunisia). The relationship between magnesium carbonates and sulfate-reducing bacteria have often been noticed (Hardie, 1987; Lasemi et al., 1989; Middleburg et al., 1990). Sulfate-reducing bacteria (SRB), such as Desulfovibrio species, which are obligate anaerobes, are able to oxidize organic matter by using the oxygen of sulfates as the terminal electron acceptor, see reaction (2.3). SRB produce energy by consuming sulfate and oxidizing the carbon of organic matter. During this metabolic process, bicarbonate (HCO 3 2 ) and carbonate ions are produced that may combine with magnesium in high-mg 2þ /Ca 2þ brines to form magnesite (Pontoizeau et al., 1987). C 106 H 291 O 124 N 16 P 1 þ 53SO 2 4 þ 14H 2 O! 53H 2 S þ 106HCO 3 þ HPO 2 4 þ 16NH 4 þ 14OH (2:3) This reaction produces the accumulation of the bicarbonate ion along with an increase in the alkalinity due to the formation of the hydroxyl ion, which also favors carbonate precipitation. Therefore, the precipitation of high-mg carbonates in sediments will be favored by: (1) anoxic conditions allowing the growth of SRB, (2) the presence of organic matter, (3) the presence of sulfate, and (4) a high Mg 2þ /Ca 2þ ratio. These conditions are encountered in continental salt lakes and flats. During the course of sedimentation, calcium ion is depleted from solution by precipitation of the carbonates
CHLOR-ALKALI INDUSTRY
 CHLOR-ALKALI INDUSTRY The chlor-alkali industry represents of three major industrial chemicals: Soda ash (sodium carbonate-na 2 CO 3 ) Caustic soda (sodium hydroxide-naoh) Chlorine (Cl 2 ) These chemicals
CHLOR-ALKALI INDUSTRY The chlor-alkali industry represents of three major industrial chemicals: Soda ash (sodium carbonate-na 2 CO 3 ) Caustic soda (sodium hydroxide-naoh) Chlorine (Cl 2 ) These chemicals
CEMENT MANUFACTURING PROCESS
 CEMENT MANUFACTURING PROCESS Definition: Defined as a product material obtained by calcination of calcareous (a material containing lime) and argillaceous (a material which contain silica) materials. According
CEMENT MANUFACTURING PROCESS Definition: Defined as a product material obtained by calcination of calcareous (a material containing lime) and argillaceous (a material which contain silica) materials. According
by Dimitris E. Tsakonitis (Refractory Business Unit Manager, GRECIAN MAGNESITE S.A Polygyros, GREECE)
 Presentation 20/06/2000 MAGNESIA FOR REFRACTORY APPLICATIONS by Dimitris E. Tsakonitis (Refractory Business Unit Manager, GRECIAN MAGNESITE S.A. 631 00 Polygyros, GREECE) 1. MAGNESIA Magnesia or Magnesium
Presentation 20/06/2000 MAGNESIA FOR REFRACTORY APPLICATIONS by Dimitris E. Tsakonitis (Refractory Business Unit Manager, GRECIAN MAGNESITE S.A. 631 00 Polygyros, GREECE) 1. MAGNESIA Magnesia or Magnesium
MINERALOGICAL STUDY AND PROPERTIES OF MAGNESIA REFRACTORIES DERIVED FROM EVIAN MAGNESITE
 ελτίο της Ελληνικής Γεωλογικής Εταιρίας τοµ. XXXVI, 2004 Πρακτικά 10 ου ιεθνούς Συνεδρίου, Θεσ/νίκη Απρίλιος 2004 Bulletin of the Geological Society of Greece vol. XXXVI, 2004 Proceedings of the 10 th
ελτίο της Ελληνικής Γεωλογικής Εταιρίας τοµ. XXXVI, 2004 Πρακτικά 10 ου ιεθνούς Συνεδρίου, Θεσ/νίκη Απρίλιος 2004 Bulletin of the Geological Society of Greece vol. XXXVI, 2004 Proceedings of the 10 th
GENARAL INTRODUCTION TO METALLURGY :Std: XI-CHEMISTRY
 GENARAL INTRODUCTION TO METALLURGY :Std: XI-CHEMISTRY 1. What is matrix? The ore is generally associated with rock impurities like clay, sand etc. called gangue or matrix 2. What is mineral? The natural
GENARAL INTRODUCTION TO METALLURGY :Std: XI-CHEMISTRY 1. What is matrix? The ore is generally associated with rock impurities like clay, sand etc. called gangue or matrix 2. What is mineral? The natural
1. Scaling. H.O.: H-5/21, KRISHNA NAGAR, DELHI Tel.: , Fax:
 Boiler Water Problems and Its Causes Water is the essential medium for steam generation. Conditioning it properly can increase the efficiency of boiler and as well as extend the boiler s life. Treating
Boiler Water Problems and Its Causes Water is the essential medium for steam generation. Conditioning it properly can increase the efficiency of boiler and as well as extend the boiler s life. Treating
Soda Ash ( Sodium carbonate) Manufacture
 Soda Ash ( Sodium carbonate) Manufacture Pertinent properties Mol. Wt. 106 M.P. 851deg.C. B.P. Decomposes Soluble in water 8.9 gm/100gm at 20 deg.cel. Grade s: 99% sodium carbonate washing soda ( Na 2
Soda Ash ( Sodium carbonate) Manufacture Pertinent properties Mol. Wt. 106 M.P. 851deg.C. B.P. Decomposes Soluble in water 8.9 gm/100gm at 20 deg.cel. Grade s: 99% sodium carbonate washing soda ( Na 2
Flue Gas Desulphurization (FGD) plant 2 x 600 MW Coal based Thermal power plant Cuddalore, Tamil Nadu. By MK Parameswaran 23 rd Dec, 2016
 Flue Gas Desulphurization (FGD) plant 2 x 600 MW Coal based Thermal power plant Cuddalore, Tamil Nadu. By MK Parameswaran 23 rd Dec, 2016 Introduction Flue Gas Desulfurization is a process of removing
Flue Gas Desulphurization (FGD) plant 2 x 600 MW Coal based Thermal power plant Cuddalore, Tamil Nadu. By MK Parameswaran 23 rd Dec, 2016 Introduction Flue Gas Desulfurization is a process of removing
» Talc is a native, hydrous magnesium silicate, sometimes containing a small proportion of aluminum silicate.
 Change to read: Talc» Talc is a native, hydrous magnesium silicate, sometimes containing a small proportion of aluminum silicate. Packaging and storage Preserve in well closed containers. Identification
Change to read: Talc» Talc is a native, hydrous magnesium silicate, sometimes containing a small proportion of aluminum silicate. Packaging and storage Preserve in well closed containers. Identification
5072 CHEMISTRY (NEW PAPERS WITH SPA) TOPIC 9: METALS 5067 CHEMISTRY (NEW PAPERS WITH PRACTICAL EXAM) TOPIC 9: METALS
 5072 CHEMISTRY (NEW PAPERS WITH SPA) TOPIC 9: METALS 5067 CHEMISTRY (NEW PAPERS WITH PRACTICAL EXAM) TOPIC 9: METALS SUB-TOPIC 9.3 TO 5 EXTRACTION OF METALS; RECYLING OF METALS; IRON LEARNING OUTCOMES
5072 CHEMISTRY (NEW PAPERS WITH SPA) TOPIC 9: METALS 5067 CHEMISTRY (NEW PAPERS WITH PRACTICAL EXAM) TOPIC 9: METALS SUB-TOPIC 9.3 TO 5 EXTRACTION OF METALS; RECYLING OF METALS; IRON LEARNING OUTCOMES
Chapter 3 Ecosystem Ecology. Tuesday, September 19, 17
 Chapter 3 Ecosystem Ecology Reversing Deforestation in Haiti Answers the following: Why is deforestation in Haiti so common? What the negative impacts of deforestation? Name three actions intended counteract
Chapter 3 Ecosystem Ecology Reversing Deforestation in Haiti Answers the following: Why is deforestation in Haiti so common? What the negative impacts of deforestation? Name three actions intended counteract
2.2 Nutrient Cycles in Ecosystems. Review How energy flows What is the difference between a food chain, food web, and food pyramid?
 2.2 Nutrient Cycles in Ecosystems Review How energy flows What is the difference between a food chain, food web, and food pyramid? https://www.youtube.com/watch?v=xhr1iebeops https://www.youtube.com/watch?v=alusi_6ol8m
2.2 Nutrient Cycles in Ecosystems Review How energy flows What is the difference between a food chain, food web, and food pyramid? https://www.youtube.com/watch?v=xhr1iebeops https://www.youtube.com/watch?v=alusi_6ol8m
Agricultural Lime Recommendations Based on Lime Quality
 ID-163 Agricultural Lime Recommendations Based on Lime Quality University of Kentucky College of Agriculture, Food and Environment Cooperative Extension Service E.L. Ritchey, L.W. Murdock, D. Ditsch, and
ID-163 Agricultural Lime Recommendations Based on Lime Quality University of Kentucky College of Agriculture, Food and Environment Cooperative Extension Service E.L. Ritchey, L.W. Murdock, D. Ditsch, and
GRECIAN MAGNESITE S.A ELENI ISKOU SALES SPECIALIST
 GRECIAN MAGNESITE S.A ELENI ISKOU SALES SPECIALIST In 1959 Grecian Magnesite was established by Mr G. Portolos. Privately owned company. Head quarters in Athens Mines and production facilities in Yerakini,
GRECIAN MAGNESITE S.A ELENI ISKOU SALES SPECIALIST In 1959 Grecian Magnesite was established by Mr G. Portolos. Privately owned company. Head quarters in Athens Mines and production facilities in Yerakini,
Chemical Activation of Low Calcium Fly Ash Part 1: Identification of Suitable Activators and their Dosage
 Chemical Activation of Low Calcium Fly Ash Part 1: Identification of Suitable Activators and their Dosage P. Arjunan 1, M. R. Silsbee 2, and D. M. Roy 2, 1 Custom Building Products, 6515, Salt Lake Ave,
Chemical Activation of Low Calcium Fly Ash Part 1: Identification of Suitable Activators and their Dosage P. Arjunan 1, M. R. Silsbee 2, and D. M. Roy 2, 1 Custom Building Products, 6515, Salt Lake Ave,
CERAMIC MATERIALS I. Asst. Prof. Dr. Ayşe KALEMTAŞ. Office Hours: Thursday, 09:30-10:30 am.
 CERAMIC MATERIALS I Office Hours: Thursday, 09:30-10:30 am. akalemtas@mu.edu.tr, akalemtas@gmail.com, Phone: 211 19 17 Metallurgical and Materials Engineering Department CLASSIFICATION OF CERAMICS Ceramic
CERAMIC MATERIALS I Office Hours: Thursday, 09:30-10:30 am. akalemtas@mu.edu.tr, akalemtas@gmail.com, Phone: 211 19 17 Metallurgical and Materials Engineering Department CLASSIFICATION OF CERAMICS Ceramic
Module: 5 Lecture: 24
 Module: 5 Lecture: 24 CEMENT MANUFACTURE MANUFACTURE It involves the following steps 1. Mixing of raw material 2. Burning 3. Grinding 4. Storage and packaging 1. Mixing of raw material Mixing can be done
Module: 5 Lecture: 24 CEMENT MANUFACTURE MANUFACTURE It involves the following steps 1. Mixing of raw material 2. Burning 3. Grinding 4. Storage and packaging 1. Mixing of raw material Mixing can be done
Corrosion and Odor Control in Wastewater Systems
 Corrosion and Odor Control in Wastewater Systems Topics to be covered Hydrogen Sulfide Formation and Consequences What has changed since the Clean Water Act Corrosion in Wastewater systems Liquid Phase
Corrosion and Odor Control in Wastewater Systems Topics to be covered Hydrogen Sulfide Formation and Consequences What has changed since the Clean Water Act Corrosion in Wastewater systems Liquid Phase
Unit Treatment Processes in Water and Wastewater Engineering
 Unit Treatment Processes in Water and Wastewater Engineering T J Casey AQUAVARRA RESEARCH LIMITED 22A Brookfield Avenue Blackrock Co. Dublin. October 2006 Author s Note Water and wastewater treatment technology
Unit Treatment Processes in Water and Wastewater Engineering T J Casey AQUAVARRA RESEARCH LIMITED 22A Brookfield Avenue Blackrock Co. Dublin. October 2006 Author s Note Water and wastewater treatment technology
Center for Advanced Sustainable Iron & Steel Making
 Proposed Project 10: Effects of Carbonate Minerals on Filtration Rates Objective(s): Determine how carbonate minerals that either occur naturally in the ore, or are added as flux, affect the filtration
Proposed Project 10: Effects of Carbonate Minerals on Filtration Rates Objective(s): Determine how carbonate minerals that either occur naturally in the ore, or are added as flux, affect the filtration
Ecosystems: Nutrient Cycles
 Ecosystems: Nutrient Cycles Greeks, Native Peoples, Buddhism, Hinduism use(d) Earth, Air, Fire, and Water as the main elements of their faith/culture Cycling in Ecosystems the Hydrologic Cycle What are
Ecosystems: Nutrient Cycles Greeks, Native Peoples, Buddhism, Hinduism use(d) Earth, Air, Fire, and Water as the main elements of their faith/culture Cycling in Ecosystems the Hydrologic Cycle What are
Removing Heavy Metals from Wastewater
 Removing Heavy Metals from Wastewater Engineering Research Center Report David M. Ayres Allen P. Davis Paul M. Gietka August 1994 1 Removing Heavy Metals From Wastewater Introduction This manual provides
Removing Heavy Metals from Wastewater Engineering Research Center Report David M. Ayres Allen P. Davis Paul M. Gietka August 1994 1 Removing Heavy Metals From Wastewater Introduction This manual provides
Properties A Metal B Non- metal Electronic configuration?? Nature of oxides?? Oxidizing or reducing action?? Conduction of heat and electricity??
 CLASS: X NCERT (CBSE) SCIENCE: Chemistry Page: 1 Question 1: Compare the properties of a typical metal and a non-metal on the basis of the following. Fill in Column A, B. Properties A Metal B Non- metal
CLASS: X NCERT (CBSE) SCIENCE: Chemistry Page: 1 Question 1: Compare the properties of a typical metal and a non-metal on the basis of the following. Fill in Column A, B. Properties A Metal B Non- metal
WHY DO WE NEED NITROGEN?? Nitrogen is needed to make up DNA and protein!
 Nitrogen Cycle 2.2 WHY DO WE NEED NITROGEN?? Nitrogen is needed to make up DNA and protein! In animals, proteins are vital for muscle function. In plants, nitrogen is important for growth. NITROGEN Nitrogen
Nitrogen Cycle 2.2 WHY DO WE NEED NITROGEN?? Nitrogen is needed to make up DNA and protein! In animals, proteins are vital for muscle function. In plants, nitrogen is important for growth. NITROGEN Nitrogen
Application of the AGF (Anoxic Gas Flotation) Process
 Application of the AGF (Anoxic Gas Flotation) Process Dennis A. Burke Environmental Energy Company, 6007 Hill Road NE, Olympia, WA 98516 USA (E-mail: dennis@makingenergy.com http//www.makingenergy.com)
Application of the AGF (Anoxic Gas Flotation) Process Dennis A. Burke Environmental Energy Company, 6007 Hill Road NE, Olympia, WA 98516 USA (E-mail: dennis@makingenergy.com http//www.makingenergy.com)
*20GSD5201* Double Award Science: Chemistry. Unit C2 Higher Tier TUESDAY 9 JUNE 2015, AFTERNOON [GSD52] *GSD52* *G5802* TIME 1 hour 15 minutes.
![*20GSD5201* Double Award Science: Chemistry. Unit C2 Higher Tier TUESDAY 9 JUNE 2015, AFTERNOON [GSD52] *GSD52* *G5802* TIME 1 hour 15 minutes. *20GSD5201* Double Award Science: Chemistry. Unit C2 Higher Tier TUESDAY 9 JUNE 2015, AFTERNOON [GSD52] *GSD52* *G5802* TIME 1 hour 15 minutes.](/thumbs/76/73786999.jpg) Centre Number Candidate Number General Certificate of Secondary Education 2015 Double Award Science: Chemistry Unit C2 Higher Tier [GSD52] *GSD52* *G5802* *GSD52* TUESDAY 9 JUNE 2015, AFTERNOON TIME 1
Centre Number Candidate Number General Certificate of Secondary Education 2015 Double Award Science: Chemistry Unit C2 Higher Tier [GSD52] *GSD52* *G5802* *GSD52* TUESDAY 9 JUNE 2015, AFTERNOON TIME 1
Iron filings (Fe) 56g IRON + SULPHUR IRON SULPHIDE
 W.S.51. Chemical reactions. All of the different materials around us have been formed by chemical reactions from about one hundred simple elements. The diagram below shows a chemical reaction between the
W.S.51. Chemical reactions. All of the different materials around us have been formed by chemical reactions from about one hundred simple elements. The diagram below shows a chemical reaction between the
Worldwide Pollution Control Association
 Worldwide Pollution Control Association WPCA-Duke Energy FGD Wastewater Treatment Seminar March 7, 2013 All presentations posted on this website are copyrighted by the Worldwide Pollution Control Association
Worldwide Pollution Control Association WPCA-Duke Energy FGD Wastewater Treatment Seminar March 7, 2013 All presentations posted on this website are copyrighted by the Worldwide Pollution Control Association
W. R. Grace & Co.-Conn. Cobalt Oxide Product Stewardship Summary
 W. R. Grace & Co.-Conn. Cobalt Oxide Product Stewardship Summary I. Overview Cobalt oxide is a component in Grace hydroprocessing catalysts used globally by the petroleum industry for the refining of crude
W. R. Grace & Co.-Conn. Cobalt Oxide Product Stewardship Summary I. Overview Cobalt oxide is a component in Grace hydroprocessing catalysts used globally by the petroleum industry for the refining of crude
Basic Survival Chemistry
 Basic Survival Chemistry Francis Glode hclo3.tk Notation: The numbers in parentheses indicate the reaction temperature in Celsius. RT indicates that the reaction proceeds at room temperature.
Basic Survival Chemistry Francis Glode hclo3.tk Notation: The numbers in parentheses indicate the reaction temperature in Celsius. RT indicates that the reaction proceeds at room temperature.
REMOVAL OF MANGANESE FROM ACID MINE DRAINAGE
 REMOVAL OF MANGANESE FROM ACID MINE DRAINAGE I. INTRODUCTION Raymond J. Lovett Department of Chemistry West Virginia University Morgantown, WV 26506 Manganese is a common component of both neutral and
REMOVAL OF MANGANESE FROM ACID MINE DRAINAGE I. INTRODUCTION Raymond J. Lovett Department of Chemistry West Virginia University Morgantown, WV 26506 Manganese is a common component of both neutral and
O 3, SIO 2. O, CAO, Al 2 AND MG IN ANT-HILL SOIL SAMPLES WITHIN ABRAKA TOWN IN NIGERIA
 Int. J. Agric.Sc & Vet.Med. 2014 Ekakitie A O and Osakwe A A, 2014 Research Paper ISSN 2320-3730 www.ijasvm.com Vol. 2, No. 3, August 2014 2014 www.ijasvm.com. All Rights Reserved DETERMINATION OF FE 2,
Int. J. Agric.Sc & Vet.Med. 2014 Ekakitie A O and Osakwe A A, 2014 Research Paper ISSN 2320-3730 www.ijasvm.com Vol. 2, No. 3, August 2014 2014 www.ijasvm.com. All Rights Reserved DETERMINATION OF FE 2,
JOHN BASCHAB JON PIOT
 T H E PROFESSIONAL SERVICES FIRM BIBLE JOHN BASCHAB JON PIOT John Wiley & Sons, Inc. T H E PROFESSIONAL SERVICES FIRM BIBLE T H E PROFESSIONAL SERVICES FIRM BIBLE JOHN BASCHAB JON PIOT John Wiley & Sons,
T H E PROFESSIONAL SERVICES FIRM BIBLE JOHN BASCHAB JON PIOT John Wiley & Sons, Inc. T H E PROFESSIONAL SERVICES FIRM BIBLE T H E PROFESSIONAL SERVICES FIRM BIBLE JOHN BASCHAB JON PIOT John Wiley & Sons,
Wastewater Treatment Processes
 Wastewater Treatment Processes (Sep 27 th and 28 th, 2016) by Dr. Arun Kumar (arunku@civil.iitd.ac.in) Objective: To learn about processes used in tertiary treatment Courtesy: Dr. Irene Xagoraraki, MSU,
Wastewater Treatment Processes (Sep 27 th and 28 th, 2016) by Dr. Arun Kumar (arunku@civil.iitd.ac.in) Objective: To learn about processes used in tertiary treatment Courtesy: Dr. Irene Xagoraraki, MSU,
2.2 Nutrient Cycles in Ecosystems Name: Date: (Reference: BC Science 10 pp. 68 to 91) Block: NUTRIENT CYCLING IN THE BIOSPHERE. nutrients: aka.
 2.2 Nutrient Cycles in Ecosystems Name: Date: (Reference: BC Science 10 pp. 68 to 91) Block: NUTRIENT CYCLING IN THE BIOSPHERE nutrients: stores: aka Nutrients are accumulated for short or long periods
2.2 Nutrient Cycles in Ecosystems Name: Date: (Reference: BC Science 10 pp. 68 to 91) Block: NUTRIENT CYCLING IN THE BIOSPHERE nutrients: stores: aka Nutrients are accumulated for short or long periods
OCN 201 Chemical Oceanography Class Notes, Fall 2014 The origin of sea salt Chris Measures, Department of Oceanography
 OCN 201 Chemical Oceanography Class Notes, Fall 2014 The origin of sea salt Chris Measures, Department of Oceanography 1 Introduction Everyone knows that the sea is salty but what exactly is the salt in
OCN 201 Chemical Oceanography Class Notes, Fall 2014 The origin of sea salt Chris Measures, Department of Oceanography 1 Introduction Everyone knows that the sea is salty but what exactly is the salt in
WATER QUALITY AND STANDARDS Vol. II -Salinization of Soils - Hideyasu Fujiyama, Yasumoto Magara
 SALINIZATION OF SOILS Hideyasu Fujiyama Professor of Agriculture, Tottori University, Tottori, Japan Yasumoto Magara Professor of Engineering, Hokkaido University, Sapporo, Japan Keywords: Capillary effect;
SALINIZATION OF SOILS Hideyasu Fujiyama Professor of Agriculture, Tottori University, Tottori, Japan Yasumoto Magara Professor of Engineering, Hokkaido University, Sapporo, Japan Keywords: Capillary effect;
ph Management and Lime Material Selection and Application
 ph Management and Lime Material Selection and Application Quirine M. Ketterings Cornell University Nutrient Management Spear Program http://nmsp.cals.cornell.edu Acidity and ph Acidity = H + and Al 3+
ph Management and Lime Material Selection and Application Quirine M. Ketterings Cornell University Nutrient Management Spear Program http://nmsp.cals.cornell.edu Acidity and ph Acidity = H + and Al 3+
REMOVAL OF HARDNESS BY PRECIPITATION
 REMOVAL OF HARDNESS BY PRECIPITATION Hardness divalent cations If hardness is too high Ca 2+ + Mg 2+ + Fe 2+ + Mn 2+ + Sr 2+... precipitation of soap, scaling on pipes, boilers, cooling towers, heat exchangers.
REMOVAL OF HARDNESS BY PRECIPITATION Hardness divalent cations If hardness is too high Ca 2+ + Mg 2+ + Fe 2+ + Mn 2+ + Sr 2+... precipitation of soap, scaling on pipes, boilers, cooling towers, heat exchangers.
REFRACTORIES. Definition Classification Properties Manufacture of refractory bricks Properties and applications of Refractory bricks
 Topics REFRACTORIES Definition Classification Properties Manufacture of refractory bricks Properties and applications of Refractory bricks Definition Substances which can with stand high temperature without
Topics REFRACTORIES Definition Classification Properties Manufacture of refractory bricks Properties and applications of Refractory bricks Definition Substances which can with stand high temperature without
Cycles of Ma,er. Lesson Overview. Lesson Overview. 3.4 Cycles of Matter
 Lesson Overview Cycles of Ma,er Lesson Overview 3.4 Cycles of Matter THINK ABOUT IT A handful of elements combine to form the building blocks of all known organisms. Organisms cannot manufacture these
Lesson Overview Cycles of Ma,er Lesson Overview 3.4 Cycles of Matter THINK ABOUT IT A handful of elements combine to form the building blocks of all known organisms. Organisms cannot manufacture these
2015 UTAH ASPHALT CONFERENCE AGGREGATE PRODUCTION 101. Kilgore Companies and Kimball Equipment
 2015 UTAH ASPHALT CONFERENCE AGGREGATE PRODUCTION 101 Kilgore Companies and Kimball Equipment Table of Contents Types of Aggregates used in Asphalt Production Extraction Processes Primary Crushing Secondary
2015 UTAH ASPHALT CONFERENCE AGGREGATE PRODUCTION 101 Kilgore Companies and Kimball Equipment Table of Contents Types of Aggregates used in Asphalt Production Extraction Processes Primary Crushing Secondary
USE OF CHEMICALS TO CONTROL ODORS AND CORROSION IN WASTEWATER SYSTEMS
 USE OF CHEMICALS TO CONTROL ODORS AND CORROSION IN WASTEWATER SYSTEMS By Robert P.G. Bowker, P.E. Bowker & Associates, Inc. Portland, Maine Florida Water Environment Association Air Quality Workshop February
USE OF CHEMICALS TO CONTROL ODORS AND CORROSION IN WASTEWATER SYSTEMS By Robert P.G. Bowker, P.E. Bowker & Associates, Inc. Portland, Maine Florida Water Environment Association Air Quality Workshop February
Success story on mineral carbonation of CO 2
 www.energytech.aalto.fi/en/ Success story on mineral carbonation of CO 2 Success story on mineral carbonation of CO 2 Pushing academic research towards industrial scale through advanced modelling and piloting
www.energytech.aalto.fi/en/ Success story on mineral carbonation of CO 2 Success story on mineral carbonation of CO 2 Pushing academic research towards industrial scale through advanced modelling and piloting
MICROBES IN ECOLOGY INTRODUCTION
 MICROBES IN ECOLOGY INTRODUCTION - Microbes usually live in communities and rarely as individuals They are Present in every known ecosystem Over 99% of microbes contribute to the quality of human life
MICROBES IN ECOLOGY INTRODUCTION - Microbes usually live in communities and rarely as individuals They are Present in every known ecosystem Over 99% of microbes contribute to the quality of human life
IMPROVEMENT OF CONCRETE DURABILITY BY COMPLEX MINERAL SUPER-FINE POWDER
 277 IMPROVEMENT OF CONCRETE DURABILITY BY COMPLEX MINERAL SUPER-FINE POWDER Chen Han-bin, Chen Jian-xiong, Xiao Fei, and Cui Hong-ta College of Material Science, Chongqing University, Chongqing, PRC Abstract
277 IMPROVEMENT OF CONCRETE DURABILITY BY COMPLEX MINERAL SUPER-FINE POWDER Chen Han-bin, Chen Jian-xiong, Xiao Fei, and Cui Hong-ta College of Material Science, Chongqing University, Chongqing, PRC Abstract
Ron Zevenhoven. Åbo Akademi University, Turku, Finland
 CO 2 fixation by serpentinite Ron Zevenhoven Åbo Akademi University, Turku, Finland Åbo Akademi University Thermal and Flow Engineering 20500 Turku Finland 22/05/2013 1 Overview Background and scope CO
CO 2 fixation by serpentinite Ron Zevenhoven Åbo Akademi University, Turku, Finland Åbo Akademi University Thermal and Flow Engineering 20500 Turku Finland 22/05/2013 1 Overview Background and scope CO
Chemistry 145 Exam number 4 name 11/19/98 # Faraday s constant is 96,500 c/mole of electrons.
 Chemistry 145 Exam number 4 name 11/19/98 # Faraday s constant is 96,500 c/mole of electrons. A.(16) An electrochemical cell is prepared with a strip of manganese metal dipping in to a 1.0 M MnSO 4 solution
Chemistry 145 Exam number 4 name 11/19/98 # Faraday s constant is 96,500 c/mole of electrons. A.(16) An electrochemical cell is prepared with a strip of manganese metal dipping in to a 1.0 M MnSO 4 solution
OC30 Conduct a qualitative experiment to detect the presence of dissolved solids in water samples, and test water for hardness (soap test)
 Chemistry: 6. Water Please remember to photocopy 4 pages onto one sheet by going A3 A4 and using back to back on the photocopier Syllabus OC14 Use cobalt chloride paper or anhydrous copper sulfate to test
Chemistry: 6. Water Please remember to photocopy 4 pages onto one sheet by going A3 A4 and using back to back on the photocopier Syllabus OC14 Use cobalt chloride paper or anhydrous copper sulfate to test
XRF S ROLE IN THE PRODUCTION OF MAGNESIUM METAL BY THE MAGNETHERMIC METHOD
 Copyright(c)JCPDS-International Centre for Diffraction Data 2001,Advances in X-ray Analysis,Vol.44 398 XRF S ROLE IN THE PRODUCTION OF MAGNESIUM METAL BY THE MAGNETHERMIC METHOD H. L. Baker Northwest Alloys,
Copyright(c)JCPDS-International Centre for Diffraction Data 2001,Advances in X-ray Analysis,Vol.44 398 XRF S ROLE IN THE PRODUCTION OF MAGNESIUM METAL BY THE MAGNETHERMIC METHOD H. L. Baker Northwest Alloys,
Water Pollution & Quality. Dr. Deniz AKGÜL Marmara University Department of Environmental Engineering
 Water Pollution & Quality Dr. Deniz AKGÜL Marmara University Department of Environmental Engineering IMPORTANCE OF WATER Life on planet Earth would be impossible without water. All life forms, from simple
Water Pollution & Quality Dr. Deniz AKGÜL Marmara University Department of Environmental Engineering IMPORTANCE OF WATER Life on planet Earth would be impossible without water. All life forms, from simple
Waste Acid Recycling Technology by Slag
 Technical Report NIPPON STEEL & SUMITOMO METAL TECHNICAL REPORT No. 109 JULY 2015 Waste Acid Recycling Technology by Slag UDC 669. 184. 28 : 621. 794. 48 Shigeharu MATSUBAYASHI* Abstract In this study,
Technical Report NIPPON STEEL & SUMITOMO METAL TECHNICAL REPORT No. 109 JULY 2015 Waste Acid Recycling Technology by Slag UDC 669. 184. 28 : 621. 794. 48 Shigeharu MATSUBAYASHI* Abstract In this study,
Changes to the Atmosphere
 Changes to the Atmosphere 49 minutes 49 marks Page of 24 Q. The amount of carbon dioxide in the Earth s atmosphere has changed since the Earth was formed. The amount of carbon dioxide continues to change
Changes to the Atmosphere 49 minutes 49 marks Page of 24 Q. The amount of carbon dioxide in the Earth s atmosphere has changed since the Earth was formed. The amount of carbon dioxide continues to change
East Maui Watershed Partnership Adapted from Utah State University and University of Wisconsin Ground Water Project Ages 7 th -Adult
 INTRODUCTION What is groundwater? Water contained in saturated soil and rock materials below the surface of the earth. It is not NEW water, but is recycled water through the hydraulic cycle. The source
INTRODUCTION What is groundwater? Water contained in saturated soil and rock materials below the surface of the earth. It is not NEW water, but is recycled water through the hydraulic cycle. The source
EFFECTS ON SETTING, STRENGTH AND WATER RESISTANCE OF SOREL CEMENT ON MIXING FLY ASH AS AN ADDITIVE
 Research Paper ISSN 2278 0149 www.ijmerr.com Vol. 3, No. 2, April, 2014 2014 IJMERR. All Rights Reserved EFFECTS ON SETTING, STRENGTH AND WATER RESISTANCE OF SOREL CEMENT ON MIXING FLY ASH AS AN ADDITIVE
Research Paper ISSN 2278 0149 www.ijmerr.com Vol. 3, No. 2, April, 2014 2014 IJMERR. All Rights Reserved EFFECTS ON SETTING, STRENGTH AND WATER RESISTANCE OF SOREL CEMENT ON MIXING FLY ASH AS AN ADDITIVE
METALS AND THEIR COMPOUNDS
 METALS AND THEIR COMPOUNDS Metals are elements whose atoms ionize by electron loss, while non-metals are elements whose atoms ionize by electron gain. Metals are in groups 1, 2 and 3 of the periodic table.
METALS AND THEIR COMPOUNDS Metals are elements whose atoms ionize by electron loss, while non-metals are elements whose atoms ionize by electron gain. Metals are in groups 1, 2 and 3 of the periodic table.
Slide 1. Slide 2. Slide 3. Hardness. Concentration is. What s the concentration of red triangles? What s in your pipes? 500 ml
 Slide 1 Hardness What s in your pipes? Slide 2 What s the concentration of red triangles? 500 ml 1 g 1 g 1 g A. 10 B. 10 C. D. 1 g 1 g It s all of the above! Slide 3 Concentration is any statement of the
Slide 1 Hardness What s in your pipes? Slide 2 What s the concentration of red triangles? 500 ml 1 g 1 g 1 g A. 10 B. 10 C. D. 1 g 1 g It s all of the above! Slide 3 Concentration is any statement of the
Hard water. Hard and Soft Water. Hard water. Hard water 4/2/2012
 Hard and Soft Water is the type of water that has high mineral content (in contrast with soft water). minerals primarily consist of calcium (Ca 2+ ), and magnesium (Mg 2+ ) metal cations, and sometimes
Hard and Soft Water is the type of water that has high mineral content (in contrast with soft water). minerals primarily consist of calcium (Ca 2+ ), and magnesium (Mg 2+ ) metal cations, and sometimes
By-Products from EAF Dust Recycling and Their Valorisation. Vlad POPOVICI
 By-Products from EAF Dust Recycling and Their Valorisation Bredero Shaw, Canada 5 th Global Slag Conference, Brussels, 23-24 November 2009 Agenda Electric Arc Furnace Dust Global Production EAF Dust Recycling
By-Products from EAF Dust Recycling and Their Valorisation Bredero Shaw, Canada 5 th Global Slag Conference, Brussels, 23-24 November 2009 Agenda Electric Arc Furnace Dust Global Production EAF Dust Recycling
85 Q.51 Which of the following carbonates would give the metal when heated with carbon? (1) MgCO 3 (2) PbCO 3 (3) K 2 CO 3 (4) CuCO 3
 Metal and metal reactivity / Section 2 / Sect2pp.doc / S. W. Tse / P.1 85 Q.51 Which of the following carbonates would give the metal when heated with carbon? (1) MgCO 3 (2) PbCO 3 (3) K 2 CO 3 (4) CuCO
Metal and metal reactivity / Section 2 / Sect2pp.doc / S. W. Tse / P.1 85 Q.51 Which of the following carbonates would give the metal when heated with carbon? (1) MgCO 3 (2) PbCO 3 (3) K 2 CO 3 (4) CuCO
concentration of acid in mol / dm 3 temperature / C ti / min
 1 (a A small piece of marble, calcium carbonate, was added to 5 cm 3 of hydrochloric acid at 25 C. The time taken for the reaction to stop was measured. CaCO 3 (s) + 2HCl(aq) CaCl 2 (aq) + CO 2 (g) + H
1 (a A small piece of marble, calcium carbonate, was added to 5 cm 3 of hydrochloric acid at 25 C. The time taken for the reaction to stop was measured. CaCO 3 (s) + 2HCl(aq) CaCl 2 (aq) + CO 2 (g) + H
THE PERIODIC TABLE. Chapter 15
 THE PERIODIC TABLE Chapter 15 MENDELEEV 1869 The first version of the periodic table was published by Dmitri Mendeleev, a Russian chemist He arranged the elements in order of increasing atomic mass This
THE PERIODIC TABLE Chapter 15 MENDELEEV 1869 The first version of the periodic table was published by Dmitri Mendeleev, a Russian chemist He arranged the elements in order of increasing atomic mass This
NCEA Level 1 Chemistry (90933) 2012 page 1 of 5. Q Evidence Achievement Achievement with Merit Achievement with Excellence NØ N1 N2 A3 A4 M5 M6 E7 E8
 Assessment Schedule 2012 NCEA Level 1 Chemistry (90933) 2012 page 1 of 5 Chemistry: Demonstrate understanding of aspects of selected elements (90933) Evidence Statement Q Evidence with Merit with Excellence
Assessment Schedule 2012 NCEA Level 1 Chemistry (90933) 2012 page 1 of 5 Chemistry: Demonstrate understanding of aspects of selected elements (90933) Evidence Statement Q Evidence with Merit with Excellence
CHAPTER 6. Natural Mineral Mineral Content Elements In The Minerals Bauxite Aluminium oxide Aluminium, oxygen. Cassiterite Tin oxide Tin, oxygen
 CHAPTER 6 6.1 Minerals Found In The Earth s Crust mineral : is a naturally occurring solid element or compound with a definite crystalline structure and chemical composition. natural elements : gold, silver
CHAPTER 6 6.1 Minerals Found In The Earth s Crust mineral : is a naturally occurring solid element or compound with a definite crystalline structure and chemical composition. natural elements : gold, silver
Mon. Tues. Wed. Thurs. Fri. AM or PM B
 Name: (cf. Honesty Declaration Statement on page 20) Laboratory Day (circle) Lab Room Locker Lab. Session (circle) Lab. Section Mon. Tues. Wed. Thurs. Fri. AM or PM B Date experiment is performed MARK:
Name: (cf. Honesty Declaration Statement on page 20) Laboratory Day (circle) Lab Room Locker Lab. Session (circle) Lab. Section Mon. Tues. Wed. Thurs. Fri. AM or PM B Date experiment is performed MARK:
TITANIUM DIOXIDE. SYNONYMS Titania; CI Pigment white 6; CI (1975) No ; INS No. 171 DEFINITION DESCRIPTION FUNCTIONAL USES CHARACTERISTICS
 TITANIUM DIOXIDE Prepared at the 71 st JECFA (2009) and published in FAO JECFA Monographs 7 (2009), superseding specifications prepared at the 67 th JECFA (2006) and published in FAO JECFA Monographs 3
TITANIUM DIOXIDE Prepared at the 71 st JECFA (2009) and published in FAO JECFA Monographs 7 (2009), superseding specifications prepared at the 67 th JECFA (2006) and published in FAO JECFA Monographs 3
MATERIAL SAFETY DATA SHEET FUTUREGARDEN, INC Updated 11/01/04
 MATERIAL SAFETY DATA SHEET FUTUREGARDEN, INC Updated 11/01/04 SECTION I COMPANY AND PRODUCT INFORMATION MANUFACTURER S NAME Futuregarden, Inc ADDRESS (number, street, city, state, zip code) 931 C. Conklin
MATERIAL SAFETY DATA SHEET FUTUREGARDEN, INC Updated 11/01/04 SECTION I COMPANY AND PRODUCT INFORMATION MANUFACTURER S NAME Futuregarden, Inc ADDRESS (number, street, city, state, zip code) 931 C. Conklin
WASTEWATER TREATMENT
 WASTEWATER TREATMENT Every community produces both liquid and solid wastes. The liquid portion-wastewater-is essentially the water supply of the community after it has been fouled by a variety of uses.
WASTEWATER TREATMENT Every community produces both liquid and solid wastes. The liquid portion-wastewater-is essentially the water supply of the community after it has been fouled by a variety of uses.
Fused Magnesia Trends. Global Outlook. Asım Bilge MagForum 2016, Vienna Kümaş Manyezit San. A.Ş.
 Fused Magnesia Trends & Global Outlook Asım Bilge MagForum 2016, Vienna Kümaş Manyezit San. A.Ş. Contents Fused Magnesia Global FM Market, Demand and Prices Effects of Global Economy Issues Kümaş FM Production
Fused Magnesia Trends & Global Outlook Asım Bilge MagForum 2016, Vienna Kümaş Manyezit San. A.Ş. Contents Fused Magnesia Global FM Market, Demand and Prices Effects of Global Economy Issues Kümaş FM Production
Evaluation copy. Total Dissolved Solids. Computer INTRODUCTION
 Total Dissolved Solids Computer 12 INTRODUCTION Solids are found in streams in two forms, suspended and dissolved. Suspended solids include silt, stirred-up bottom sediment, decaying plant matter, or sewage-treatment
Total Dissolved Solids Computer 12 INTRODUCTION Solids are found in streams in two forms, suspended and dissolved. Suspended solids include silt, stirred-up bottom sediment, decaying plant matter, or sewage-treatment
Electricity and Chemistry
 Electricity and Chemistry Electrochemistry: It is a branch of chemistry that deals with the reactions involving the conversion of chemical energy into electrical energy and vice-versa. Electrochemical
Electricity and Chemistry Electrochemistry: It is a branch of chemistry that deals with the reactions involving the conversion of chemical energy into electrical energy and vice-versa. Electrochemical
Cycling and Biogeochemical Transformations of N, P and S
 Cycling and Biogeochemical Transformations of N, P and S OCN 401 - Biogeochemical Systems Reading: Schlesinger, Chapter 6 1. Nitrogen cycle Soil nitrogen cycle Nitrification Emissions of N gases from soils
Cycling and Biogeochemical Transformations of N, P and S OCN 401 - Biogeochemical Systems Reading: Schlesinger, Chapter 6 1. Nitrogen cycle Soil nitrogen cycle Nitrification Emissions of N gases from soils
Leigh Creek Magnesite Revisited 2010
 ARCHER EXPLORATION LIMITED Leigh Creek Magnesite Revisited 2010 Sept 2010 www.archerexploration.com.au ASX : AXE Disclaimer Competent persons statement The exploration results reported herein, insofar
ARCHER EXPLORATION LIMITED Leigh Creek Magnesite Revisited 2010 Sept 2010 www.archerexploration.com.au ASX : AXE Disclaimer Competent persons statement The exploration results reported herein, insofar
3 The Formation, Mining, and Use of Minerals
 CHAPTER 3 3 The Formation, Mining, and Use of Minerals SECTION Minerals of the Earth s Crust BEFORE YOU READ After you read this section, you should be able to answer these questions: How do minerals form?
CHAPTER 3 3 The Formation, Mining, and Use of Minerals SECTION Minerals of the Earth s Crust BEFORE YOU READ After you read this section, you should be able to answer these questions: How do minerals form?
Topic 2.7 EXTRACTION OF METALS. Extraction of Iron Extraction of Aluminium Extraction of Titanium Recycling
 Topic 2.7 EXTRACTION OF METALS Extraction of Iron Extraction of Aluminium Extraction of Titanium Recycling EXTRACTING METALS FROM THEIR ORES Most metals do not occur native. They exist in compounds, usually
Topic 2.7 EXTRACTION OF METALS Extraction of Iron Extraction of Aluminium Extraction of Titanium Recycling EXTRACTING METALS FROM THEIR ORES Most metals do not occur native. They exist in compounds, usually
Effect of Water Composition on Flotation of Lead and Zinc Sulphide Ore
 ABSTRACT 533 Effect of Water Composition on Flotation of Lead and Zinc Sulphide Ore Ünzile Yenial*, Gülay Bulut Mineral Processing Engineering Department, Mining Faculty, Istanbul Technical University,
ABSTRACT 533 Effect of Water Composition on Flotation of Lead and Zinc Sulphide Ore Ünzile Yenial*, Gülay Bulut Mineral Processing Engineering Department, Mining Faculty, Istanbul Technical University,
Table of Contents. Preface...
 Preface... xi Chapter 1. Metallurgical Thermochemistry... 1 1.1. Introduction... 1 1.2. Quantities characterizing the state of a system and its evolution... 3 1.2.1. The types of operations... 3 1.2.2.
Preface... xi Chapter 1. Metallurgical Thermochemistry... 1 1.1. Introduction... 1 1.2. Quantities characterizing the state of a system and its evolution... 3 1.2.1. The types of operations... 3 1.2.2.
Chapter 16 Minerals: A Nonrenewable Resource
 Lecture Outline: Chapter 16 Minerals: A Nonrenewable Resource I. Introduction to Minerals A. Minerals are elements or compounds of elements that occur naturally in Earth s crust and have precise chemical
Lecture Outline: Chapter 16 Minerals: A Nonrenewable Resource I. Introduction to Minerals A. Minerals are elements or compounds of elements that occur naturally in Earth s crust and have precise chemical
SRI RAMAKRISHNA INSTITUTE OF TECHNOLOGY COIMBATORE First Year BE/B.TECH ( ) Engineering Chemistry- I
 SRI RAMAKRISHNA INSTITUTE OF TECHNOLOGY COIMBATORE-641010 1. Define hard water and soft water? First Year BE/B.TECH (2012-2013) Engineering Chemistry- I UNIT-I- Water Technology Water which does not produce
SRI RAMAKRISHNA INSTITUTE OF TECHNOLOGY COIMBATORE-641010 1. Define hard water and soft water? First Year BE/B.TECH (2012-2013) Engineering Chemistry- I UNIT-I- Water Technology Water which does not produce
Industrial Solutions. Pyroprocessing. for the minerals industry. High-quality and innovative solutions for individual application.
 Industrial Solutions Pyroprocessing for the minerals industry. High-quality and innovative solutions for individual application. 2 3 ThyssenKrupp Polysius Minerals Pyroprocessing As one of the world s
Industrial Solutions Pyroprocessing for the minerals industry. High-quality and innovative solutions for individual application. 2 3 ThyssenKrupp Polysius Minerals Pyroprocessing As one of the world s
AMMONIA REMOVAL FROM DIGESTED SLUDGE SUPERNATANT
 AMMONIA REMOVAL FROM DIGESTED SLUDGE SUPERNATANT J. Suschka and S. Popławski University of Bielsko-Biała, Institute of Environmental Protection and Engineering, ul. Willowa 2, 43-309 Bielsko-Biała, Poland
AMMONIA REMOVAL FROM DIGESTED SLUDGE SUPERNATANT J. Suschka and S. Popławski University of Bielsko-Biała, Institute of Environmental Protection and Engineering, ul. Willowa 2, 43-309 Bielsko-Biała, Poland
At Home in North America
 At Home in North America PRECIOUS METALS BASE METALS (POLYMETALLIC) Gold, Silver, Platinum, Palladium Copper, Zinc, Lead, Nickel, Gold, Silver Precious Metals Recovery Technology SPECIALTY METALS & MINERALS
At Home in North America PRECIOUS METALS BASE METALS (POLYMETALLIC) Gold, Silver, Platinum, Palladium Copper, Zinc, Lead, Nickel, Gold, Silver Precious Metals Recovery Technology SPECIALTY METALS & MINERALS
Hydrology and Water Quality. Water. Water 9/13/2016. Molecular Water a great solvent. Molecular Water
 Hydrology and Water Quality Water Molecular Water Exists as an equilibrium But equilibrium altered by what is dissolved in it Water Molecular Water a great solvent In reality, water in the environment
Hydrology and Water Quality Water Molecular Water Exists as an equilibrium But equilibrium altered by what is dissolved in it Water Molecular Water a great solvent In reality, water in the environment
Heavy metal stabilization in EAFD using magnesia and Sorel cements
 31 st International Conference of Society for Environmental Geochemistry & Health Heavy metal stabilization in EAFD using magnesia and Sorel cements 22-26 June 2015 E. Ntinoudi 1, H. Yiannoulakis 2, Th.
31 st International Conference of Society for Environmental Geochemistry & Health Heavy metal stabilization in EAFD using magnesia and Sorel cements 22-26 June 2015 E. Ntinoudi 1, H. Yiannoulakis 2, Th.
Third-Party Technology Verification of the Hydropath Technology at the Robert W. Hite Treatment Facility, Denver, Colorado
 TECHNICAL MEMORANDUM Third-Party Technology Verification of the Hydropath Technology at the Robert W. Hite Treatment Facility, Denver, Colorado PREPARED FOR: PREPARED BY: DATE: Tal Journo/HydroFLOW USA
TECHNICAL MEMORANDUM Third-Party Technology Verification of the Hydropath Technology at the Robert W. Hite Treatment Facility, Denver, Colorado PREPARED FOR: PREPARED BY: DATE: Tal Journo/HydroFLOW USA
Compounds & Reactions Week 1. Writing Formulas & Balancing Equations. Write the chemical formula for each molecular (covalent) compound.
 Compounds & Reactions Week 1 Name Writing Formulas & Balancing Equations Write the chemical formula for each ionic compound. 1. Lithium fluoride 2. Copper (II) chloride 3. Manganese (II) oxide 4. Potassium
Compounds & Reactions Week 1 Name Writing Formulas & Balancing Equations Write the chemical formula for each ionic compound. 1. Lithium fluoride 2. Copper (II) chloride 3. Manganese (II) oxide 4. Potassium
SOFTWARE EVOLUTION AND MAINTENANCE
 SOFTWARE EVOLUTION AND MAINTENANCE A PRACTITIONER S APPROACH PRIYADARSHI TRIPATHY KSHIRASAGAR NAIK SOFTWARE EVOLUTION AND MAINTENANCE SOFTWARE EVOLUTION AND MAINTENANCE A Practitioner s Approach PRIYADARSHI
SOFTWARE EVOLUTION AND MAINTENANCE A PRACTITIONER S APPROACH PRIYADARSHI TRIPATHY KSHIRASAGAR NAIK SOFTWARE EVOLUTION AND MAINTENANCE SOFTWARE EVOLUTION AND MAINTENANCE A Practitioner s Approach PRIYADARSHI
Lecture 24 Microbially Influenced Corrosion (MIC) Definitions, Environments and Microbiology
 Lecture 24 Microbially Influenced Corrosion (MIC) Definitions, Environments and Microbiology Keywords: Microbial Corrosion, Microorganisms, Biofouling. Introduction Microbially-influenced corrosion (MIC)
Lecture 24 Microbially Influenced Corrosion (MIC) Definitions, Environments and Microbiology Keywords: Microbial Corrosion, Microorganisms, Biofouling. Introduction Microbially-influenced corrosion (MIC)
Trace Elements in. Elements. Trace Elements and Minor. Elements 2/25/2009
 Trace Elements in Carbonates A Short Course VU March, 2009 Peter Swart University of Miami Trace Elements and Minor Elements Minor Elements (Usually concentrations over 1000 ppm) Strontium Aragonite-7000
Trace Elements in Carbonates A Short Course VU March, 2009 Peter Swart University of Miami Trace Elements and Minor Elements Minor Elements (Usually concentrations over 1000 ppm) Strontium Aragonite-7000
A Tailor Made Approach for the Beneficiation of Phosphate Rock
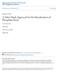 Engineering Conferences International ECI Digital Archives Beneficiation of Phosphates VII Proceedings Spring 3-29-2015 A Tailor Made Approach for the Beneficiation of Phosphate Rock Leonid Lisiansky Mike
Engineering Conferences International ECI Digital Archives Beneficiation of Phosphates VII Proceedings Spring 3-29-2015 A Tailor Made Approach for the Beneficiation of Phosphate Rock Leonid Lisiansky Mike
SINTER BASICITY INCREASE THROUGH INSERTION OF MAGNESIUM SILICATE MINILUMPS BY ALBA FERNÁNDEZ, PAMELA DIAZ, ESTEBAN RUISÁNCHEZ, JAVIER MARTÍNEZ *
 SINTER BASICITY INCREASE THROUGH INSERTION OF MAGNESIUM SILICATE MINILUMPS BY ALBA FERNÁNDEZ, PAMELA DIAZ, ESTEBAN RUISÁNCHEZ, JAVIER MARTÍNEZ * SYPNOPSIS PASEK Dunite is an ultramaphic rock with a basic
SINTER BASICITY INCREASE THROUGH INSERTION OF MAGNESIUM SILICATE MINILUMPS BY ALBA FERNÁNDEZ, PAMELA DIAZ, ESTEBAN RUISÁNCHEZ, JAVIER MARTÍNEZ * SYPNOPSIS PASEK Dunite is an ultramaphic rock with a basic
FORM 26R CHEMICAL ANALYSIS OF RESIDUAL WASTE ANNUAL REPORT BY THE GENERATOR INSTRUCTIONS GENERAL INFORMATION
 COMMONWEALTH OF PENNSYLVANIA DEPARTMENT OF ENVIRONMENTAL PROTECTION BUREAU OF WASTE MANAGEMENT FORM 26R CHEMICAL ANALYSIS OF RESIDUAL WASTE ANNUAL REPORT BY THE GENERATOR INSTRUCTIONS GENERAL INFORMATION
COMMONWEALTH OF PENNSYLVANIA DEPARTMENT OF ENVIRONMENTAL PROTECTION BUREAU OF WASTE MANAGEMENT FORM 26R CHEMICAL ANALYSIS OF RESIDUAL WASTE ANNUAL REPORT BY THE GENERATOR INSTRUCTIONS GENERAL INFORMATION
Pelletizing technologies for iron ore
 Pelletizing technologies for iron ore The Outokumpu partnership As one of the world s leading developers and suppliers of technology, Outokumpu Technology designs and delivers plants, processes and equipment
Pelletizing technologies for iron ore The Outokumpu partnership As one of the world s leading developers and suppliers of technology, Outokumpu Technology designs and delivers plants, processes and equipment
Water Pollution. Objective: Name, describe, and cite examples of the eight major types of water pollution.
 Water Pollution Objective: Name, describe, and cite examples of the eight major types of water pollution. Types of Water Pollution Water pollutants are divided into eight categories: 1. Sediment pollution
Water Pollution Objective: Name, describe, and cite examples of the eight major types of water pollution. Types of Water Pollution Water pollutants are divided into eight categories: 1. Sediment pollution
Chemistry CH1FP. (Jun15CH1FP01) General Certificate of Secondary Education Foundation Tier June Unit Chemistry C1. Unit Chemistry C1 TOTAL
 Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials Question Mark Science A Unit Chemistry C1 Chemistry Unit Chemistry C1 Tuesday 9 June 2015 General
Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials Question Mark Science A Unit Chemistry C1 Chemistry Unit Chemistry C1 Tuesday 9 June 2015 General
The Cycling of Matter
 Section 2 Objectives Describe the short-term and long-term process of the carbon cycle. Identify one way that humans are affecting the carbon cycle. List the three stages of the nitrogen cycle. Describe
Section 2 Objectives Describe the short-term and long-term process of the carbon cycle. Identify one way that humans are affecting the carbon cycle. List the three stages of the nitrogen cycle. Describe
Manufacture of Iron & Steel. Prepared By: John Cawley
 Manufacture of Iron & Steel Prepared By: John Cawley Presentation Objectives Identify the basic steps in the production of steel. Identify the properties and uses of iron ore and pig iron. Differentiate
Manufacture of Iron & Steel Prepared By: John Cawley Presentation Objectives Identify the basic steps in the production of steel. Identify the properties and uses of iron ore and pig iron. Differentiate
The Carbon cycle. Atmosphere, terrestrial biosphere and ocean are constantly exchanging carbon
 The Carbon cycle Atmosphere, terrestrial biosphere and ocean are constantly exchanging carbon The oceans store much more carbon than the atmosphere and the terrestrial biosphere The oceans essentially
The Carbon cycle Atmosphere, terrestrial biosphere and ocean are constantly exchanging carbon The oceans store much more carbon than the atmosphere and the terrestrial biosphere The oceans essentially
AP Chemistry A. Allan Chapter 18 - The Representative Elements: Groups 1A through 4A
 AP Chemistry A. Allan Chapter 18 - The Representative Elements: Groups 1A through 4A 18.1 A Survey of the Representative Elements A. Basic Trends 1. Metals tend to lose electrons and form cations 2. Nonmetals
AP Chemistry A. Allan Chapter 18 - The Representative Elements: Groups 1A through 4A 18.1 A Survey of the Representative Elements A. Basic Trends 1. Metals tend to lose electrons and form cations 2. Nonmetals
Module: 9 Lecture: 40
 Module: 9 Lecture: 40 AMMONIUM SULFATE INTRODUCTION Ammonium sulfate containing 21% nitrogen is another important nitrogenous fertilizer. It occurs naturally as the mineral mascagnite and offers many advantages
Module: 9 Lecture: 40 AMMONIUM SULFATE INTRODUCTION Ammonium sulfate containing 21% nitrogen is another important nitrogenous fertilizer. It occurs naturally as the mineral mascagnite and offers many advantages
