European Commission, Brussels. Requirements for facilities and acceptance criteria for the disposal of metallic mercury 07.
|
|
|
- Corey Shelton
- 6 years ago
- Views:
Transcription
1 , Brussels Requirements for facilities and acceptance criteria for the disposal of metallic mercury /2009/ April 2010 DISCLAIMER: This document is distributed as prepared by GmbH, and is the result of the scientific work carried out by its authors. Any subjective information contained therein should be perceived as expressing the views of its authors rather than those of the European Commission. Beratungsgesellschaft für integrierte Problemlösungen
2 Reference number /2009/530302/ETU/G.2.2 ii Content 1 Background and objectives General background Legal background Objectives of the project References Methodology Overall methodological approach Detailed methodology for the identification of options and the review of the state of the art; approach for information gathering Overview of information gathering Literature search Questionnaire Expert interviews and site visits Data base search Detailed description of the screening analysis and the selection of options including the elaboration of basic acceptance criteria Detailed description of the assessment methodology including the elaboration of fine tuned acceptance criteria and the recommendation list References Identification of options Review of the hazardous characteristics of metallic mercury Specific properties of liquid mercury related to storage Occurrence of mercury Basic physico-chemical properties Toxic effects Classification Occupational exposure limit values Hazardous properties related to the environment Transformation and transport of mercury Overview of the behaviour in the environment Environmental limit values related to mercury Conclusions...57
3 Reference number /2009/530302/ETU/G2 iii 4.4 References Review of legislation, policy and best practice International agreements European legislation Legal requirements for all storage facilities Legal requirements for above-ground storage facilities Legal requirements for underground disposal Additional considerations for salt mines Additional considerations for hard rock Legislation at Member State level National legislation on mercury and mercury-containing waste National legislation on leachate limit values of mercury Germany Sweden UK Legislation of non-eu countries Norway USA Policy Initiatives UNEP Mercury Programme WHO International Conference on Mercury HELCOM PARCOM / OSPAR References Review of the state of the art of storage and disposal options General considerations Review of underground disposal operations Potential host rocks Salt rock Hard rock formations Radioactive waste Review of above-ground storage Europe USA...143
4 Reference number /2009/530302/ETU/G2 iv 6.4 Review of containment Container systems currently in use Environmental and safety aspects Container material Conclusions References Review of immobilization, solidification and other appropriate technologies for metallic mercury waste Sulphur stabilization Technical background: Economic information Environmental information Use of the technology Overview of patents Further details concerning the realization of the process Sulphur Polymer Stabilisation/Solidification SPSS Technical background Economic information Environmental information Use of the technology Overview of patents Further details concerning realization of the process Amalgamation Technical background Economic background Environmental background Use of the technology Overview of patents Phosphate ceramic/glass stabilization: Chemical bonded phosphate ceramic (CBPC) Technical background Economic background Environmental background Use of technology Overview of patents Solidification/encapsulation...195
5 Reference number /2009/530302/ETU/G2 v 7.6 Encapsulation of stabilized mercury with cement Technical background Economic background Environmental background Overview of patents Further details concerning realization of the process Conclusion References Screening analysis of options Identification of minimum requirements for storage options Feasibility of options Acceptance criteria for metallic mercury and appropriate containment, procedure for the acceptance at the storage facility Acceptance criteria for metallic mercury Appropriate containment Acceptance procedure Option 1l: permanent storage of liquid mercury in salt mines Technical minimum requirements Environmental minimum requirements Economic minimum requirements Feasibility of implementation Summary: option 1l Option 2l: temporary storage of liquid mercury in salt mines Technical minimum requirements Environmental minimum requirements Economic minimum requirements Feasibility of implementation Summary: Option 2l Option 3l: permanent storage of liquid mercury in deep underground hard rock formations Technical minimum requirements Summary: option 3l Option 4l: temporary storage of liquid mercury in deep underground hard rock formations Technical minimum requirements Environmental minimum requirements...236
6 Reference number /2009/530302/ETU/G2 vi Economic minimum requirements Feasibility of implementation Summary: option 4l Option 5l: temporary storage of liquid mercury in above-ground facilities Technical minimum requirements Environmental minimum requirements Economic minimum requirements Feasibility of implementation Summary: option 5l Option 6: Pre-treatment Technical, environmental and economic minimum requirements Technical minimum requirements Environmental minimum requirements Economic minimum requirements Assessment of pre-treatment technologies Technology overview of Option Feasibility of immobilization techniques Minimum acceptance criteria for stabilised mercury Feasibility of permanent storage of pre-treated elemental mercury Summary Option 6l and its permanent storage Summary of the screening analysis References Summary of acceptance criteria and additional facility related requirements Proposed acceptance criteria for metallic mercury and additional facility related requirements Proposed acceptance criteria for stabilized mercury and additional facility related requirements Assessment of options Economic assessment of the options Environmental assessment of the options Overview on the result of the assessment Conclusions and Recommendations Annexes...282
7 Reference number /2009/530302/ETU/G2 vii 12.1 Annex 1: Questionnaire Annex 2: Literature overview Annex 3: Data base research - results Annex 4: Physico-chemical properties of metallic mercury and products resulting from different immobilisation technologies Annex 5: Summary of technologies available in large-scale application...315
8 Reference number /2009/530302/ETU/G2 viii Index of tables Table 1-1: Sources of mercury supply in 2005 [UNEP 2006]...13 Table 1-2: EU mercury consumption estimates in 2007 (tonnes) [MEMO _EN]...13 Table 1-3: Overview of chlor-alkali plants still using mercury, September 2009 (source: Euro Chlor)...14 Table 1-4: Estimated amount of excess mercury that has to be safely stored...18 Table 2-1: Overview of important studies relevant for the project...26 Table 4-1: Solubility of Hg and Hg compounds in water...43 Table 4-2: Change of solubility (in ppm) against the temperature [Mersade 2007A]...47 Table 4-3: Risk phrases and classification of mercury...49 Table 4-4: Hazard class, category codes and hazard statement codes of mercury...49 Table 4-5: Environmental Quality Standards (EQS) set for mercury in Directive 2008/105/EC...56 Table 5-1: Requirements for all types of mercury storage facilities according to Directive N EC 1102/ Table 5-2: General requirements for all classes of landfills according to Directive 1999/31/EC, Annex I...71 Table 5-3: Control and monitoring procedures for all classes of landfills according to Directive 1999/31/EC, Annex III...73 Table 5-4: Control and monitoring procedures for all classes of landfills according to Directive 1999/31/EC, Annex III...74 Table 5-5: Procedures for the acceptance of waste according to Decision 2003/33/EC, Annex...75 Table 5-6: Mercury leaching limit values for different landfill types and standards according to Decision 2003/33/EC...77 Table 5-7: Requirements for above ground mercury storage according to Regulation (EC) N 1102/ Table 5-8: Requirements for mercury storage in above-ground disposal facilities according to Directive 1996/82/EC...82 Table 5-9: Site specific risk assessment for underground disposal according to Decision 2003/33/EC, Appendix A...85 Table 5-10: Requirements for mercury storage in salt mines according to Directive N EC 1102/ Table 5-11: Requirements for salt mines according to Decision 2003/33/EC...88 Table 5-12: Requirements for mercury storage in hard rock formations according to Directive N EC 1102/
9 Reference number /2009/530302/ETU/G2 ix Table 5-13: Requirements for deep storage in hard rocks according to Decision 2003/33/EC...90 Table 5-14: Overview of Member State legislation concerning mercury and mercurycontaining waste...91 Table 5-15: Member States mercury leaching limit values for landfills (more stringent or additional to Decision 2003/33/EC)...93 Table 5-16: Requirements for deep storage in salt mines according to German legislation...95 Table 6-1: Overview of literature related to the storage of liquid mercury Table 6-2: Overview of properties of salt rock Table 6-3: Overview of properties of crystalline rock Table 6-4: Overview of properties of Argillaceous rock, Clay / claystone Table 6-5: Summary of Estimates of Total Storage Costs (US Dollars) for 40 Years [USEPA 2007a] Table 6-6: Tested equipment [Muñoz, 2009], presentation: Mr. Ramos Table 7-1: Sulphur stabilization: overview of the relevant literature Table 7-2: Sulphur Polymer Stabilisation/Solidification: overview of the relevant literature Table 7-3: Amalgamation: overview of the relevant literature Table 7-4: Phosphate ceramic/glass stabilization: overview of the relevant literature Table 7-5: Cement solidification: overview of the relevant literature Table 7-6: Overview on existing pre-treatment technologies for liquid mercury Table 8-1: Summary of the assessment of used pre-treatment technologies against minimum requirements Table 8-2: Summary of the assessment of pre-treatment technologies Table 8-3: Assessment of feasibility requirements Table 8-4: Results of the evaluation of the options for storage of liquid mercury...259
10 Reference number /2009/530302/ETU/G2 x Index of figures Figure 1-1: Legal background...17 Figure 2-1: Overview of the methodological approach...22 Figure 2-2: Overview of possible options to be assessed...23 Figure 2-3: Systematic data collection...25 Figure 3-1: Overview of options...38 Figure 4-1: Relative solubility of elemental mercury in different salt water concentrations (NaCl and KCl) [GRS 2008A]...44 Figure 4-2: Diagram of the biogeochemical mercury cycle...55 Figure 6-1: Illustration of possible releases of mercury related to the temporary or permanent storage of mercury Figure 6-2: Protection layers for the storage of mercury Figure 6-3: Metallic mercury storage at the Defense National Stockpile Center (source: DNSC) Figure 6-4: Examples of standard mercury steel containers used by Mayasa (source: Mayasa) Figure 6-5: typical mercury storage flask [DNSC 2007] Figure 6-6: Overpacking concept of mercury containing flask [DNSC 2007] Figure 6-7: Packaging instruction for liquid mercury according to ADR Figure 7-1: Overview of immobilisation technologies for metallic mercury Figure 7-2: Leaching behaviour of stabilized waste with different Hg loads and at different ph values Figure 8-1: Decision scheme for the selection of suitable pre-treatment processes...245
11 Reference number /2009/530302/ETU/G2 xi List of Abbreviations: ADR AISI ASTM BFS CBPC DNSC EPA Agreement on Dangerous Goods by Road American Iron and Steel Institute American Society for Testing and Materials Blast furnace slag Chemically bonded phosphate ceramic Defense National Stockpile Center Environmental protection agency Hg-Regulation Regulation (EC) N 1102/2008 IATA IMO MERSADE MM EIS OPC RID RCRA SPC SPSS TCLP UNEP WAC International Air Transport Association International Maritime Organisation Mercury Safety Deposit Mercury Management Environmental Impact Statement Ordinary Portland cement Regulations concerning the International Transport of Dangerous Goods by Rail Resource Conservation and Recovery Act, US Sulphur polymer cement Sulphur polymer stabilization/solidification Toxicity characteristic leaching procedure United Nations Environment Programme Waste acceptance criteria
12 12 1 Background and objectives 1.1 General background Metallic mercury as well as most of its compounds are highly toxic to humans and the environment. High doses can be fatal to humans, but even relatively low doses can have serious adverse health effects, e.g. on the reproductive system. Mercury is considered a global persistent pollutant; once entering in the environment it cannot be broken down to any harmless form. Mercury can be found in almost all environmental compartments, such as the atmosphere, soil or water systems all over the world. Current environmental concentrations are a result of anthropogenic and natural sources. Mercury is the only metallic chemical element being liquid at standard conditions of temperature and pressure. In particular in its gaseous form mercury is transported globally via the atmosphere. Due to its bioaccumulation through the food chain, the consumption of fish is by far the most significant source of mercury exposure in humans. Due to its high toxicity to humans, ecosystems and wildlife, especially if chemically converted to methyl mercury, there is now a world-wide common effort to reduce both demand and supply of mercury. In 2009, the UN Environment Programme Governing Council agreed to take steps towards a comprehensive legally binding international agreement on mercury. The Council of the European Union had already supported this approach towards an international agreement by adopting Conclusions on the specific issue in December 2008 [EU Council 2008]. Mercury emissions In 2005 the global atmospheric emissions of mercury from natural sources were estimated to be 400 1,300 tonnes per year from oceans and 500 1,000 tonnes per year from land. The global atmospheric emissions of mercury from human activities were estimated in the same range between 1,220 2,900 tonnes [UNEP 2009]. The major sources of anthropogenic mercury emissions worldwide are from fossil fuel combustion for power and heating (878 tonnes), artisanal and small-scale gold production (350 tonnes), metal production (ferrous and non-ferrous, excluding gold) (200 tonnes), cement production (189 tonnes), waste incineration, waste and others (125 tonnes) [UNEP 2009]. Sources of elemental mercury At present Kyrgyzstan is the only country mining mercury for export, China s mercury mining is for domestic consumption only [UNEP 2009]. Before 2003 Europe was a major exporter of mercury (around 25% of the total supply) [MEMO _EN]. The main production site in Europe was the mine in Almadén in Spain where primary production of metallic mercury came from cinnabar extraction. In 2003 the production of virgin
13 Reference number /2009/530302/ETU/G2 13 mercury in Almadén stopped and the export of mercury from Europe declined significantly. Mercury mining in Slovenia (Idrija mine) and Italy (Monte Amiata) ceased several years ago (1995 in Slovenia and 1976 in Italy). Apart from primary production from cinnabar ore, mercury can also be obtained as a secondary product along with the production of other materials e.g. zinc or tin. Nowadays, the recovery of mercury from waste materials containing mercury e.g. thermometers, measuring devices, etc is also a source of elemental mercury. The estimated average global supply (and demand) of metallic mercury is around 3,000 (2008) tonnes per year. Based on [UNEP 2006], the main sources of mercury on the global market are summarized in the following table: Table 1-1: Sources of mercury supply in 2005 [UNEP 2006] Sector Mercury supply (metric tonnes) range Primary mercury mining 1,350-1,600 By-product Recycled mercury from chlor-alkali wastes Recycled mercury others Mercury from chlor-alkali cells (decommissioning) (Stocked) Total 3,000-3,800 Use of mercury In 2007 the demand for mercury was estimated at more than 320 t in the 27 EU Member States. The following table gives an overview of the most important uses of metallic mercury in Europe: Table 1-2: EU mercury consumption estimates in 2007 (tonnes) [MEMO _EN] sector Mercury demand (metric tonnes) range Chlor-alkali plants Batteries 7-25 Dental amalgam Measuring and control equipment 7-17 Switches and electrical control 0-1 Lighting (energy-efficient lamps) Chemicals Other uses Total
14 Reference number /2009/530302/ETU/G2 14 In general the use of mercury is declining at both global and EU levels [EU COM 2005]. One reason is the increased availability of mercury-free alternatives e.g. mercury-free production of chlorine or mercury-free thermometers. On the other hand the use of mercury is increasingly being banned or restricted by legal provisions such as restrictions for batteries (Directive 91/157/EEC 1 ). In Europe the most important industry related to the use of mercury is the chemical industry with its sub-sector chlorine production. In the so called mercury cell process mercury is essential for the production process of chlorine 2. Currently the European chlorine industry represented by Euro Chlor, the European association of the chlor-alkali industry has an agreement with the state-owned Miñas de Almadén and y Arrayanes (MAYASA) in Spain. According to the agreement MAYASA receives all excess mercury from western European chlorine producers and places it on the market instead of virgin mercury. As a consequence MAYASA ceased mercury mining in Phase out of mercury The European chlorine industry committed itself to voluntarily phasing out the mercury-based chlorine plants or conversion to non-mercury technologies (e.g. membrane technology) by As a consequence, an amount of around 8,000-9,000 t of metallic mercury is expected to arise from the decommissioned plants of the chlor-alkali industry within the next decade [Euro Chlor 2009]. Table 1-3: Overview of chlor-alkali plants still using mercury, September 2009 (source: Euro Chlor) Country N of plants Country N of plants Belgium 3 Poland 1 Czech Rep. 2 Romania 1 Finland 1 Slovak Rep. 1 France 6 Spain 7 Germany 4* Sweden 1 Greece 1 Switzerland 1 Hungary 1 UK 1 Italy 2 TOTAL 33 * In Germany, a total of 6 plants use mercury cell technology; however 2 plants are excepted from the voluntary phase out as they are not chlor-alkali plants and have a different product range. The ongoing substitution process of mercury in products and in particular the decommissioning of 1 Council Directive 91/157/EEC of 18 March 1991 on batteries and accumulators containing certain dangerous substances, OJ L 078,
15 Reference number /2009/530302/ETU/G2 15 mercury cell plants has led to a situation in which increasing amounts of mercury are available on the market. Therefore efforts have to be made to phase out surplus mercury; withdraw it from circulation and find solutions for a permanent and safe final storage. 1.2 Legal background Community Mercury Strategy On 28 January 2005 the Community Strategy Concerning Mercury [EU COM 2005] was published formulating the key objective to reduce mercury levels in the environment and human exposure as mercury poses a threat within the Community and globally. The mercury strategy lists 20 actions which should support the overall objectives. Among others, the following two actions have been included in this strategy: Action 5: As a pro-active contribution to a proposed globally organised effort to phase out primary production of mercury and to stop surpluses re-entering the market as described in section 10, the Commission intends to propose an amendment to Regulation (EC) No. 304/2003 to phase out the export of mercury from the Community by Action 9: The Commission will take action to pursue the storage of mercury from the chlor-alkali industry, according to a timetable consistent with the intended phase out of mercury exports by In the first instance the Commission will explore the scope for an agreement with the industry. Mercury Regulation To implement the above stated actions, the Council and European Parliament adopted on the Regulation on the banning of exports and the safe storage of metallic mercury (Regulation (EC) No 1102/2008, OJ L304 of 14/11/08, p.75). The export ban starts on 15 March 2011 and affects metallic mercury, cinnabar ore, mercury (I) chloride, mercury (II) oxide and mixtures of metallic mercury with other substances including alloys of mercury, with a concentration of at least 95 wt % Hg. Furthermore, the Regulation lays down that from 15 March 2011 metallic mercury from the following sources should be considered as waste (Article 2, Regulation (EC) No 1102/2008): Metallic mercury that is no longer used in the chlor-alkali industry Metallic mercury gained from the cleaning of natural gas Metallic mercury gained from non-ferrous mining and smelting operations Metallic mercury extracted from cinnabar ore in the Community as from 15 March 2011 In order to provide for possibilities of a safe storage of the above mentioned metallic mercury waste within the Community, Article 3 of Regulation (EC) No 1102/2008 constitutes suitable options both for permanent and temporary storage in appropriate containments (by derogation from Article 5
16 Reference number /2009/530302/ETU/G2 16 (3)(a) of Directive 1999/31 3 ): temporary storage for more than one year or permanent storage in o o salt mines adapted for the disposal of metallic mercury, or in deep underground, hard rock formations providing a level of safety and confinement equivalent to that of those salt mines; or temporary storage for more than one year in above-ground facilities dedicated to and equipped for the temporary storage of metallic mercury (In this case, the criteria set out in section 2.4 of the Annex to Decision 2003/33/EC 4 shall not apply). Article 3 (1) also sets out that all other provisions (except Article 5 (3)(a)) of Directive 1999/31/EC and Decision 2003/33/EC shall apply to the above described storage options for liquid mercury. In addition, in case of a temporary above ground storage Directive 96/82/EC 5 (Seveso Directive, see also chapter 5) applies to the storage facility and the corresponding requirements (e.g. establishment of a safety management system) have to be fulfilled. Article 4 of Regulation (EC) No 1102/2008 stipulates that the safety assessment which is required for a safe underground storage under Decision 2003/33/EC should be complemented by specific requirements to address the particular risks specific to the storage of metallic mercury. Furthermore, acceptance criteria should be developed for metallic mercury either temporarily or permanently stored in appropriate underground or above-ground facilities. 3 Council directive 1999/31/EC of 26 April 1999 on the landfill of waste (OJ L14, , p.10) 4 Council Decision of 19 December 2002 establishing criteria and procedures for the acceptance of waste at landfills pursuant to Article 16 of and Annex II to Directive 1999/31/EC 5 Council Directive 96/82/EC of 9 December 1996 on the control of major-accident hazards involving dangerous substances (OJ L 10, , p ) as amended by Directive 2003/105/EC; also referred to as the Seveso II Directive.
17 Reference number /2009/530302/ETU/G2 17 Regulation 1102/ Export ban of metallic mercury - Metallic mercury from specific sources has to be considered as waste Derogation from Article 5(3)(a) of Directive 1999/31/EC Underground storage permanent or temporary (>1 year) Above-ground storage temporary (>1 year) Definition of requirements for storage facilities and acceptance criteria for metallic mercury Amending of Annex I, II, III of Directive 1999/31/EC Annex I: General requirements for all classes of landfills Annex II: Waste acceptance criteria and procedures Annex III: Control and Monitoring procedures in operation and after-care phase + Decision 2003/33/EC - Criteria for above ground storage - Criteria for underground storage - Site specific safety assessment (Annex A) Figure 1-1: Legal background Against this background the Commission is requested, in order to ensure the proper application and enforcement of the Regulation (EC) No 1102/2008, to propose requirements for the three specific types of storage facilities (salt mine, hard rock, above ground) as well as acceptance criteria for metallic mercury going to such facilities by amending annexes I, II and III of Directive 1999/31/EC. Consequences of the Regulation As a consequence of the Regulation large amounts of metallic mercury which are currently considered as raw material will become waste, and adequate safe storage or disposal options have to be identified. The following amounts of metallic mercury are expected to be stored/disposed of in the next few years:
18 Reference number /2009/530302/ETU/G2 18 Table 1-4: Estimated amount of excess mercury that has to be safely stored Activity Metallic mercury that is no longer used in the chlor-alkali industry Metallic mercury gained from the cleaning of natural gas Metallic mercury gained from non-ferrous smelting operations Metallic mercury extracted from cinnabar ore in the Community as from 15 March 2011 Estimated amount of excess mercury which has to be safely stored ~8,000 t 9,000 t (~ 700 m³) by 2020 [Euro Chlor 2009] ~26t/a [Concorde 2006] ~53t/a [Concorde 2006] No mining activities currently or anticipated [COWI 2008] estimates that quantities of mercury in non-ferrous ores and in natural gas gives a total of tonnes of mercury per year potentially recoverable as a by-product from these sources, of which tonnes are already being recovered. A possible extension of storage obligation to metallic mercury from other sources will be based on the outcome of an information exchange organized by the Commission (Article 8 of the Mercury Regulation). In December 2008 Euro Chlor announced a voluntary agreement to ensure the safe storage of surplus mercury from the European chlor-alkali industry, once a ban on mercury exports from the European Union takes effect in This voluntary commitment was formally acknowledged by an EC Recommendation on 22 December 2008 (C (2008) 8422). According to the Regulation, no final disposal operations for metallic mercury should be permitted until the special requirements and acceptance criteria for the storage or disposal of metallic mercury are adopted. 1.3 Objectives of the project The overall objective of the study is to provide the Commission with a solid knowledge base for fulfilling the tasks resulting from Article 4 (3) of the Regulation (EC) N 1102/2008. The Regulation requires that for the storage options as defined in Article 3(1)(a) and (b) requirements for the different types of storage facilities as well as acceptance criteria for metallic mercury going to such facilities are established by amending the annexes I, II and III of Directive 1999/31/EC. The study will provide: an overview of treatment techniques for metallic mercury before storage (solidification and others), assessing the stage of development already reached (concept, laboratory phase, pilot phase or proven large-scale application; costs),
19 Reference number /2009/530302/ETU/G2 19 an overview of feasible storage options (permanent or temporary) for metallic mercury as well as treated (solidified/stabilized) mercury Based on the outcome of the overview a set of draft requirements and draft acceptance criteria for metallic mercury a set of draft requirements and draft acceptance criteria for stabilized mercury will be elaborated for the different types of permanent and temporary storage. For the elaboration of these draft requirements and acceptance criteria the following principles as stated in the Mercury Regulation will be taken into consideration: The storage conditions in a salt mine or in deep underground, hard rock formations, adapted for the disposal of metallic mercury, should notably meet the principles of o o o o protection of groundwater against mercury prevention of vapour emissions of mercury impermeability to gas and liquids of the surroundings and in case of permanent storage of firmly encapsulating the wastes at the end of the mines' deformation process. The safety assessment required for underground storage under Decision 2003/33/EC will be complemented by specific requirements and will be made applicable to non-underground storage to ensure storage that is safe for human health and the environment. The above-ground storage conditions should notably meet o o o o the principles of reversibility of storage, protection of mercury against meteoric water, impermeability towards soils and prevention of vapour emissions of mercury. The above-ground storage of metallic mercury should be considered as a temporary solution. To achieve the overall objective, all feasible options will be assessed and compared in order to provide the Commission with a recommendation of how to best fulfill the tasks resulting from Article 4(3) of the Regulation (EC) N 1102/2008.
20 Reference number /2009/530302/ETU/G References [Concorde 2004] Concorde EastWest Spr., Mercury flows in Europe and the world: the impact of decommissioned chlor-alkali plants, February [Concorde 2006] Concorde EastWest Spr., Mercury flows and safe storage of surplus mercury, [Concorde 2009] Concorde sprl, Assessment of excess mercury in Asia, , May ay2009.pdf [COWI 2007] COWI, Follow-up study on the implementation of Directive 1999/31/EC on, June 2007 the landfill of waste in EU-25, Final Report - Findings of the Study 7-implementation_eu_25_2007_cowi_report.pdf [COWI 2008] COWI A/S and Concorde East/West Sprl, Options for reducing mercury use in products and applications, and the fate of mercury already circulating in society, December [EIA EU 2005] Communication from the Commission to the Council and the European Parliament on Community Strategy Concerning Mercury, EXTENDED IMPACT ASSESSMENT, [EU COM 2001], Integrated Pollution Prevention and Control (IPPC) - Reference Document on Best Available Techniques in the Chlor-Alkali Manufacturing industry -, [EU COM 2002] Report from the Commission to the Council concerning mercury from the Chlor-alkali industry, COM (2002) 489 final, =COMfinal&an_doc=2002&nu_doc=489 [EU COM 2005] Communication from the Commission to the Council and the European Parliament,
21 Reference number /2009/530302/ETU/G2 21 Community Strategy Concerning Mercury, COM (2005) 20 final, 28 January 2005, Brussels [EU COM 2005A] Commission staff working paper, Annex to the Communication from the Commission to the Council and the European Parliament, Community Strategy Concerning Mercury, Extended Impact Assessment, SEC(2005) 101, 28 January 2005, Brussels, f [EU COM 2006], Report on the International Mercury Conference - How to reduce mercury supply and demand, Brussels October 2006, [EU COM 2006A], Integrated Pollution Prevention and Control Reference Document on Best Available Techniques for the Waste Treatments Industries, August 2006 ftp://ftp.jrc.es/pub/eippcb/doc/wt_bref_0806.pdf [MEMO _EN] Questions & Answers on the EU Mercury Strategy, MEMO/08/808 Brussels, 22 December aged=0&language=en&guilanguage=en [UNEP 2007] Draft technical guidelines on the environmentally sound management of mercury wastes, 2007, [UNEP 2009] UNEP, Draft technical guidelines on the environmentally sound management of mercury wastes, 4th Draft, April 2009,
22 22 Reference number /2009/530302/ETU/G2 2 Methodology 2.1 Overall methodological approach Against the described background and objectives, the methodological approach of the project is visualized in the following figure: Figure 2-1: Overview of the methodological approach For the first step of this methodology (Identification of options against the legal background) a review of the existing pre-treatment technologies, disposal facilities for temporary and permanent storage and of different types of containment has been carried out. With regard to disposal facilities, apart from existing hazardous waste landfills (above ground and underground), experience from radioactive waste disposal is also included in the review. An overview of the current status of knowledge explains the hazardous properties and characteristics of metallic mercury with a special focus on its behaviour in the environment. An overview of existing legal requirements, policies and best practice related to the disposal of mercury waste in Europe as well as on an international level is provided. With this investigation a pool of options is generated. The various options of the pool can also be combined (e.g. temporary storage + pre-treatment + permanent storage) and in this way form a broad basis for all potential solutions related to the problem of the disposal of liquid mercury. The objective of this initial work is to acquire a current and updated status on the scientific and technical knowledge related to the storage or disposal of mercury. The results provide various options that all comply with the legal requirements. This outcome can be roughly characterized as
23 Reference number /2009/530302/ETU/G2 23 follows and forms the basis for the further assessments, identification of necessary acceptance criteria and combinations of options (see steps 2 and 3 of the overall methodology, Figure 2-1). Metallic mercury waste Pre-treatment options No pre-treatment Solidified mercury (non metallic mercury) Various storage possibilities according to Directive 1999/31/EC Options: underground storage in salt mines Options: storage in deep underground, hard rock formations Options: above ground storage Permanent Temporary Permanent Temporary Temporary Underground Above ground Figure 2-2: Overview of possible options to be assessed Data and information sources for step 1 have been: extensive literature research patent analyses expert interviews site visits questionnaire survey All these information sources have been used by the project team to generate the pool of options (for more details see section 2.2.1).
24 Reference number /2009/530302/ETU/G2 24 The second step of the methodology foresees a screening analysis (see Figure 2-1). One target of this screening analysis is to exclude options from further investigation if there is no reasonable possibility to realize them in compliance with minimum technical, environmental and economic criteria. A second target of this step is to identify basic acceptance criteria that need to be linked to the options (or to combinations of options) to fulfill the minimum criteria. Within the screening analysis, the feasibility of options related to their implementation under time constraints and required resources for realization are also investigated. The screening analysis results in a short list of feasible options which will be further assessed. Option Currently feasibly I Permanent storage of liquid mercury in salt rock No / YES /? II III Permanent storage of liquid mercury in deep underground hard rock formations No / YES /? For further details see section 2.3. The third step of the methodology covers the assessment of options or combinations of options (and corresponding acceptance criteria) that remain after the screening analysis on a short list. Environmental and economic targets will be used to basically evaluate the options within the assessment. After the basic evaluation, potential combinations of options and fine tuning of corresponding acceptance criteria take place and the evaluation is repeated for a final overview on the appropriateness of options which then found their way to the list of recommendations. Options that are best in all or in selected criteria then find their way into the list of recommendations. The list of recommendations will also cover the required amendments of annexes I, II and III of Directive 1999/31/EC. The findings of the study were presented in a workshop in November 2009 and discussed with interested stakeholders. All received comments were taken into consideration for this report.
25 Reference number /2009/530302/ETU/G Detailed methodology for the identification of options and the review of the state of the art; approach for information gathering Overview of information gathering The review of the state of the art and state of the development is based on information provided by the relevant experts on relevant studies and other systematically identified documents or publications. Systematic data collection Literature search Review of important studies Web search Libraries Questionnaire; expert interviews, site visits MS and international authorities Industrial and scientific experts NGOs Data base search Patent data bases Scientific data bases - Environmental data bases - Technical data bases - Health data bases Figure 2-3: Systematic data collection All options that could be identified with these information sources have been collected and characterized in a pool of options. The results are documented in chapters 3, 4, 5 and 7 of this report Literature search At the beginning of the study an intensive literature search was carried out to identify relevant information. Studies already carried out on the topic of the disposal and storage of metallic mercury have been investigated in detail and the bibliographic references thereof were evaluated in order to
26 Reference number /2009/530302/ETU/G2 26 identify further relevant literature sources and contact details of relevant experts. The following studies provided an important input to the project: Table 2-1: Overview of important studies relevant for the project Reference [Env Canada 2001] National Office of Pollution prevention, Environment Canada, The Development of retirement and long term storage options of mercury, Draft final report, Ontario, June 2001 [Env Canada 2004] Environment Canada, Mercury and the environment, Internet document: last update , accessed on 29 June 2009 [DNSC 2004B] Defense National Stockpile Center, Final Mercury Management Environmental Impact Statement, Volume I, 2004 [SOU 2008A] Statens offentliga Utredningar (SOU) 2008: 19: Att slutförvara långlivat farligt avfall i undermarksdeponi i berg Permanent storage of long-lived hazardous waste in underground deep bedrock depositories, SOU 2008: 10 April 2008 [UNEP 2009] UNEP, Draft technical guidelines on the environmentally sound management of Short description of the content Environmental Canada has published a draft report (prepared by the Consultants SENSE) on the development of retirement and long-term storage options for mercury. The study evaluated 67 technologies including disposal options in mines using the Kepner-Tregoe ranking technique and reviewed a further 9 technologies but did not rank them because there was insufficient information. This homepage provides an overview of the current state of knowledge related to the properties of liquid mercury. The Defense Logistics Agency (DLA), USA, prepared a Mercury Management Environmental Impact Statement (MMEIS). In 2003/2004 a Mercury Management Environmental Impact Assessment (MM EIS) was carried out to find the most appropriate way of how to deal with the stored mercury in future. This study commissioned by the Swedish government analysed the permanent storage of mercury in deep bedrock and salt mines. The report provides an account of permanent storage options for mercury-containing waste, and the requirements and risks attendant to the permanent storage of liquid mercury. A summary on the key findings of the study is available in English [SOU 2008] These draft technical guidelines provide a broad overview of the current state of knowledge related to the properties of mercury and its compounds, its behaviour in the environment but also information
27 Reference number /2009/530302/ETU/G2 27 mercury wastes, 4th Draft, April 2009 [USEPA 2002c] Preliminary analysis of alternatives for the long term management of excess mercury, EPA/600/R-03/048 AUGUST 2002 [DOE 2009] U.S. Department of Energy, Interim Guidance on Packaging, Transportation, Receipt, Management, and Long-Term Storage of Elemental Mercury, U.S. Department of Energy Office of Environmental Management Washington, D.C., November 13, 2009 on the storage of liquid mercury. This study describes a systematic method for comparing options for the long-term management of surplus elemental mercury in the US, using the analytic hierarchy process. A limited scope multicriteria decision analysis was performed. Alternatives were evaluated against criteria that included costs, environmental performance, compliance with current regulations, implementation considerations, technology maturity, potential risks to the public and workers, and public perception. This document is intended to provide general guidance on standards and illustrative procedures that are current, consistent, and best suited for supporting the DOE program for the receipt, management, and long-term storage of mercury generated in the United States. As such, this interim guidance provides a framework for the standards and procedures associated with a DOEdesignated elemental mercury storage facility with a focus on the RCRA permitting of such a facility and planning for that storage facility s needs. Apart from the above-mentioned studies, the world wide web has also been used for individual searches for specific information by using specific key word combinations according to individual search purposes. The identified documents have been evaluated related to their relevance and an evaluation of the bibliographic references was carried out to identify further documents Questionnaire Relevant experts of Member States authorities as well as non-eu country authorities, industrial and scientific experts and international NGOs dealing with this topic were contacted within the scope of a questionnaire survey in June 2009 (for a list of contacted experts, see separate excel file 6 ). The experts were asked to provide information on relevant research / scientific activities related to pretreatment techniques and disposal options of metallic mercury waste as well as on relevant contact persons. 6 A separate excel file including the contacted institutions and experts has been provided to the European Commission. Due to reasons of confidentiality, this list is not included as an annex to this report.
28 Reference number /2009/530302/ETU/G2 28 In total, the questionnaire (see Annex 1) was sent to 43 institutions. In total 26 questionnaires have been sent back. The feedback on the questionnaire was quite diverse and it varied from very extensive answers including links, documents and experts for further investigations, to very short answers due to lack of information or no relevance of the topic Expert interviews and site visits Received information was evaluated with respect to its content. Key persons were contacted with the intention of obtaining specific additional information. In this way the most relevant information sources could be systematically identified and an information exchange with selected experts was initiated. Expert interviews were carried out with various concerned stakeholders such as Treatment technology providers / developers Operators of underground disposal facilities Operators of above ground storage facilities (not landfills) for liquid mercury Member States experts on landfill and mercury European Associations (e.g. Euro Chlor) In addition, site visits at an underground disposal site and treatment technology provider took place to receive more detailed information on the disposal process and treatment technology. This approach turned out to be the most efficient way of targeted data collection. It was additionally supplemented by a systematic literature search in selected data bases. Another valuable input for the project was a workshop initiated by IKIMP 7. The workshop Safe storage and disposal of redundant mercury (13 & 14 October, 2009) offered a good platform for information exchange and discussion of technologies and storage options with experts. 7 IKIMP: Integrating Knowledge to Inform Mercury Policy,
29 Reference number /2009/530302/ETU/G Data base search An intensive data base search was carried out to identify relevant scientific literature. The search covered patent data bases as well as environmental, technical and health related data bases. The data bases were scanned using appropriate key words such as mercury and immobilization and mercury and stabilization. The identified results were evaluated based on the available abstracts for their relevance to the project. Relevant persons and companies cited in the patents were contacted with the intention of obtaining specific additional information. A detailed overview of the data base search results is attached as Annex 3 to this report. Patent data bases With regard to relevant patents, the following data bases were used for the search: German patent information system (DEPATIS 8 ) provided by the German Patent and Trade Mark Office. It enables online searches in patent publications from around the world stored in the database of DEPATIS, the in-house patent information system of the German Patent and Trade Mark Office. Europe's network of patent data bases (esp@cenet 9 ). Its interface is available in most European languages. It contains over 60 million patent documents from all over the world. These patent data bases have been searched using the following search terms and their combinations: mercury, stabilization, solidification, immobilization. The patents resulting from the search, preceding patents and patents that are cited in the search results have been evaluated for their usefulness to the review of the state of the art. Relevant persons and companies cited in the patents where contacted with the intention of obtaining specific additional information. Scientific data bases With regard to scientific publications, the following data bases were used for the literature search: Dialog Dissertation Abstracts Online (35), Dialog Enviroline (40) Dialog WasteInfo (110) Federal Research in Progress (FEDRIP) (File 266) UFORDAT 10 : (Data base of the German EPA on research projects) ULIDAT 11 (Data base of the German EPA on environmental literature) 8 x&session=c23b66f330d8b2a5ae5f763b d8ee58f67400&stamp=
30 Reference number /2009/530302/ETU/G2 30 These data bases have been systematically searched for relevant literature by using appropriate key words (e.g. mercury, stabilization) and key word combinations (see Annex 3). Search results were screened for their relevance for the project objectives. Relevant studies were evaluated with respect to their content and their bibliographic references. Selected authors of key studies have been contacted with the intention of obtaining specific additional information. The identified documents have been evaluated based on the available abstracts. At the end of each section the section specific references are listed. Annex 2 includes a compilation of all identified relevant literature for this study.
31 Reference number /2009/530302/ETU/G Detailed description of the screening analysis and the selection of options including the elaboration of basic acceptance criteria The following flow chart describes the methodology for the first phase of the screening analysis: Pool of options resulting from step 1 Acceptance criteria to be combined with options Options + acceptance criteria for further investigation Technical minimum requirements Options that have to be excluded as they do not fulfill the minimum requirements/ acceptance criteria Acceptance criteria to be combined with options Options + acceptance criteria for further investigation Environmental minimum requirements Options that have to be excluded as they do not fulfill the minimum requirements/ acceptance criteria Acceptance criteria to be combined with options Options + acceptance criteria for further investigation Economic minimum requirements Options that have to be excluded as they do not fulfill the minimum requirements/ acceptance criteria Candidates (options + corresponding acceptance criteria) for further investigation
32 Reference number /2009/530302/ETU/G2 32 In the first step, technical minimum requirements are established that are basically justified by the requirements for safe and sustainable solutions. Examples of such minimum requirements are: - Protection of groundwater against mercury in cases of permanent underground storage - Impermeability to gas and liquids of the surroundings (permanent underground storage) - Large scale application or pilot plant available (applicable for pre-treatment technologies) In relation to an option, these criteria find themselves translated into facility related requirements and acceptance criteria for the waste, which need to be fulfilled to make the option a real solution. Facility related requirements directly address the disposal facility or the pre-treatment technology, such as Effectiveness of the geological barrier in terms of migration time for mercury to the biosphere (permanent storage) >1 million years Installation of a permanent mercury vapour monitoring system Acceptance criteria directly address the waste and its properties, the waste container or the handling of the waste, such as Acceptance only of certified containers Purity of the mercury to be accepted: >99.9% per weight If the defined additional acceptance criteria cannot be fulfilled by an option, the corresponding option has to be excluded from further investigations. The same procedure as described for the minimum technical criteria is carried out for minimum environmental/health and minimum economic criteria. Environmental and health related minimum criteria are for example the compliance with existing occupational exposure limits. A corresponding facility related requirement might be the installation of a permanent monitoring system with a certain level of sensitivity. The following flow chart describes the methodology for the second phase of the screening analysis. For this second phase, only options and combinations of options are considered that have not been excluded in the first phase:
33 Reference number /2009/530302/ETU/G2 33 Pool of options resulting from step 1 Candidates as outcome of first phase of screening analysis Feasibility related to time frame requirements for large scale implementation failed Option not regarded as feasible OK Feasibility related to costs required for large scale implementation failed Option not regarded as feasible OK Feasible option + acceptance criteria for comparative assessment The first criterion that is checked concerns the feasibility under the given framework of time. Feasible solutions need to be available together with fulfilled acceptance criteria at the latest by 15 March 2011 for large scale applications. If options for permanent solutions cannot fulfill this feasibility criterion they need to be combined with temporary storage options to bridge the period up to the implementation of the option. The second criterion covers the costs that are required to enable a large scale application. Currently, there are some uncertainties on the quantities of liquid mercury that need to be disposed of in the years after Feasibility under economic conditions is granted, if with reasonable costs a flexibility related to the required annual capacity is provided.
34 Reference number /2009/530302/ETU/G Detailed description of the assessment methodology including the elaboration of fine tuned acceptance criteria and the recommendation list The screening analysis was followed by an assessment of remaining options or combinations of options on their strengths and weaknesses. Within this assessment environmental and economic targets have been used to basically evaluate the options. After the basic evaluation, potential combinations of options and fine tuning of corresponding acceptance criteria took place and the evaluation was repeated for a final overview on the appropriateness of options which then found their way to the list of recommendations. If an option or a combination of options fulfills all acceptance criteria, in principle it can be chosen by industry. So the question might come up why an assessment and a following recommendation list are necessary at all. The answer on such questions and correspondingly the justification of the final assessment is to offer industry an information and decision basis where they can see the advantages of options under different criteria. This might lead to a preference of solutions that provide environmental advantages against other options with equal costs. Also a preference might be generated for less expensive solutions with the same level of environmental safeness. For the assessment direct environmental and economic target criteria are set up such as: Hg-Emissions and corresponding risks for human health or the environment Risk for accidents or handling problems Overall costs Required investment costs Costs of temporary storage of liquid mercury prior to treatment The final result is then summarized in the recommendation list including a written justification.
35 Reference number /2009/530302/ETU/G References [DNSC 2004B] Defense National Stockpile Center, Final Mercury Management Environmental Impact Statement, Volume I, 2004 [DOE 2009] U.S. Department of Energy, Interim Guidance on Packaging, Transportation, Receipt, Management, and Long-Term Storage of Elemental Mercury, U.S. Department of Energy Office of Environmental Management Washington, D.C., November 13, 2009, pdf [Env Canada 2001] National Office of Pollution prevention, Environment Canada, The Development of retirement and long term storage options of mercury, Draft final report, Ontario, June 2001 [Env Canada 2004] Environment Canada, Mercury and the environment, Internet document: last update , accessed on 29 June 2009 [SOU 2008A] Miljödepartementet, Att slutförvara långlivat farligt avfall i undermarksdeponi i berg, ISBN , 2008 [UNEP 2009] UNEP, Draft technical guidelines on the environmentally sound management of mercury wastes, 4th Draft, April 2009, [USEPA 2002c] Hugh W. McKinnon, Preliminary analysis of alternatives for the long term management of excess mercury, EPA/600/R-03/048, 2002,
36 Reference number /2009/530302/ETU/G Identification of options Article 3 (1) Regulation (EC) N 1102/2008 prescribes possible storage options for liquid mercury By way of derogation from Article 5(3)(a) of Directive 1999/31/EC, metallic mercury that is considered as waste may, in appropriate containment, be a. temporarily stored for more than one year or permanently stored (disposal operations D 15 or D 12 respectively, as defined in Annex II A of Directive 2006/12/EC) in salt mines adapted for the disposal of metallic mercury, or in deep underground, hard rock formations providing a level of safety and confinement equivalent to that of those salt mines; or b. temporarily stored (disposal operation D 15, as defined in Annex II A of Directive 2006/12/EC) for more than one year in above-ground facilities dedicated to and equipped for the temporary storage of metallic mercury. In this case, the criteria set out in section 2.4 of the Annex to Decision 2003/33/EC shall not apply. The other provisions of Directive 1999/31/EC and Decision 2003/33/EC shall apply to points (a) and (b). The term temporarily is not clearly defined in the Regulation. It is simply stated that temporary means more than one year but no upper limit is defined. The term stored is defined via the reference to Annex II A (Disposal operations) of the waste directive 2006/12/EC: D12: Permanent storage (e.g. emplacement of containers in a mine, etc.) D15: Storage pending any of the operations numbered D1 to D14 (excluding temporary storage, pending collection, on the site where it is produced) The expression adapted for the disposal of metallic mercury implies that, in order to fulfil the requirements especially established in Regulation 1102/2008, in particular the recitals (11) and (12) on a storage that is safe for human health and the environment, not only the mercury waste has to fulfil requirements and acceptance criteria, but also the potential storage facilities. The term appropriate containment refers to the aspect that various types of containment might be required, depending on the storage time and facility. Against the above-described background, the following 5 options for the storage of liquid mercury (options are marked with an l ) have been derived:
37 Reference number /2009/530302/ETU/G2 37 Option 1l: Option 2l: Option 3l: Option 4l: Option 5l: Permanent storage of liquid mercury in salt mines Temporary storage of liquid mercury in salt mines Permanent storage of liquid mercury in deep underground hard rock formations Temporary storage of liquid mercury in deep underground hard rock formations Temporary storage of liquid mercury in above ground facilities Apart from the storage/disposal of liquid mercury, the possibility of pre-treating liquid mercury has also to be taken into consideration. Article 8 (2) Regulation 1102/2008 requires that the Commission shall keep under review ongoing research activities on safe disposal options, including solidification of metallic mercury. Therefore, in addition to the above-stated options, the following option shall also be taken into consideration: Option 6l: Pre-treatment of liquid mercury After the pre-treatment process a stabilised and solid (waste) product is the result, with quite different properties compared to liquid mercury. The resulting product is no longer metallic mercury and thus the storage provisions laid down in Regulation (EC) N 1108/2008 will no longer apply. Therefore, depending on its properties, various storage options are possible following existing legal requirements. The storage options referring to pre-treated or stabilised mercury are marked with an s : Option 1s: Option 3s: Option 7s: Permanent storage of pre-treated mercury in salt mines Permanent storage of pre-treated mercury in deep underground hard rock formations Permanent storage of pre-treated mercury in above ground facilities Another temporary storage (options 2s, 4s, 5s) after the pre-treatment does not provide the requested type of a solution. Therefore for the pre-treated (stabilised) product only permanent storage options are considered further. The following diagram presents an overview of all relevant options related to the storage of metallic mercury which will be investigated within this study:
38 Reference number /2009/530302/ETU/G2 38 Metallic mercury waste No pre-treatment Pre-treatment options Underground storage in salt mines Storage in deep underground, hard rock formations Temporary above ground storage Option 6l Solidified mercury (non metallic mercury) Option 5l Permanent Temporary Permanent Temporary Option 1l Option 2l Option 3l Option 4l Permanent underground storage in salt mines Permanent storage in deep underground, hard rock formations Permanent above ground storage Option 1s Option 3s Option 7s Figure 3-1: Overview of options Option 1l: Permanent storage of metallic mercury in salt mines This option covers the permanent storage of metallic mercury in salt mines. It must be determined which minimum requirements salt mines have to fulfil for safe permanent storage of metallic mercury. Option 2l: Temporary storage of metallic mercury in salt mines The temporary storage (>1 year) of metallic mercury in salt mines requires partly different requirements compared to permanent storage. In cases of temporary storage, the retrievability of the metallic mercury has to be taken into consideration. In addition, other criteria related to the containment apply, in comparison with permanent storage. Option 3l: Permanent storage of metallic mercury in deep underground hard rock formations Storage in deep underground hard rock formations is only possible according to Regulation
39 Reference number /2009/530302/ETU/G /2008 in case the level of safety and confinement is equal to those of salt mines. Option 4l: Temporary storage of metallic mercury in deep underground hard rock formations This option considers the temporary underground storage in deep hard rock formation. The same requirements apply as for salt mines. According to Decision 2003/33/EC, deep storage in hard rock is defined here as an underground storage at a depth of several hundred metres, where hard rock includes various igneous rocks, e.g. granite or gneiss, it may also include sedimentary rocks, e.g. limestone and sandstone. Option 5l: Temporary storage of liquid mercury in above-ground facilities The storage of liquid mercury above ground is only foreseen as a temporary form of storage. In cases of above ground storage, the facility has to fulfil the criteria of reversibility of the storage. Aboveground facilities have to comply with the requirements of Directive 1996/82/EC (Seveso Directive, see chapter 5). Option 6l: Pre-treatment of mercury Option 6 differs from the other options as it does not investigate the storage or disposal of metallic mercury but investigates the available solidification possibilities for liquid mercury. This option includes several sub-options using various pre-treatment technologies. In chapter 7 techniques currently under development or already available have been introduced. By means of a screening analysis, the most promising pre-treatment technologies will be selected for further investigation. Relevant sub-options will then be defined. After pre-treatment of the mercury a suitable permanent storage option has to be identified for the stabilised mercury. Further temporary storage following pre-treatment does not provide the required adequate solution therefore, after pre-treatment only permanent storage options are considered further. Option 1s: Permanent storage of pre-treated mercury in salt mines This option covers the permanent storage of pre-treated mercury in salt mines. It must be determined which minimum requirements salt mines have to fulfil for a safe permanent storage of pre-treated mercury. In addition, the criteria (e.g. leaching rate) which pre-treated mercury has to fulfil to be accepted for permanent storage in underground salt mines, will be examined. Option 3s: Permanent storage of pre-treated mercury in deep underground hard rock formations Option 3s investigates the permanent storage of pre-treated metallic mercury in deep underground hard rock formations. The minimum requirements which hard rock formations have to fulfil for safe permanent storage of pre-treated mercury, will be examined. In addition, the criteria (e.g. leaching rate) which pre-treated mercury has to fulfil to be accepted for permanent storage in underground hard rock formations, will be examined.
40 Reference number /2009/530302/ETU/G2 40 Option 7s: Permanent storage of pre-treated mercury in above-ground facilities Regulation (EC) N 1102/2008 prescribes the temporary or permanent storage options for metallic mercury only. Following pre-treatment (stabilisation / solidification), mercury is no longer metallic (liquid). Therefore, permanent above-ground storage of the resulting product (stabilised/solidified mercury) might also be an option. Consequently, option 7s is introduced as permanent storage above ground.
41 Reference number /2009/530302/ETU/G Review of the hazardous characteristics of metallic mercury Mercury is particularly related to specific risks due to its persistent, bio-accumulative and toxic characteristics. This section is dedicated to providing a brief review of metallic mercury's hazardous properties in view of defining appropriate acceptance criteria for its temporary and long term disposal. The brief overview of the hazardous characteristics is to a large extent based on information that has been generated by Environment Canada ([Env Canada 2004] 12 ], the information is available at the web site Mercury and the Environment ), [WHO 2003] and [UNEP 2009]. Additional information used within this section is specifically cited. 4.1 Specific properties of liquid mercury related to storage Occurrence of mercury In nature, mercury has three possible oxidation states. Elemental or metallic mercury (Hg 0 ) has no electric charge. Mercury is also found in two positively charged, or cationic, states, Hg 2+ (mercuric) and Hg 1+ (mercurous). The mercuric cation is more stable and is generally associated with inorganic molecules, such as sulphur (in the mineral cinnabar), chlorine (mercuric chloride), oxygen and hydroxyl ions. Hg 2+ and Hg 1+ are also found in organic (carbon based) substances such as dimethylmercury (Me 2 Hg) 13 or methylmercury (MeHg) 14 which are far more toxic than inorganic forms of mercury and bioaccumulate in the tissues of living organisms. Since mercury can be adsorbed easily into small particles of matter, some scientists use the notation Hg(p) to represent elemental mercury attached to or absorbed onto a particle. Mercury is a persistent chemical and once released to the environment, it will stay there in one of its forms. It is converted into its various forms through a range of abiotic and biogeochemical transformations and during atmospheric transportation. The most common natural occurrence of mercury is cinnabar (HgS), with large deposits in Spain (Almadén) and Slovenia (Idrija) Basic physico-chemical properties The list of physico-chemical parameters in Annex 4 is mainly restricted to those parameters that enable an assessment of the mobilisation and transport mechanisms of the relevant substances Structural formula: (CH 3 ) 2 Hg 14 Structural formula: CH 3 Hg
42 Reference number /2009/530302/ETU/G2 42 With regard to mobilisation and transport from stored substances, these parameters were selected in order to assess possible risks of releases. Relevant substances are elemental mercury and all mercury compounds that will potentially be stored (temporarily or permanently). Organic mercury compounds (e.g. methyl mercury) will not be stored or disposed of. However, it should be noted once again that mercury compounds that are released can be easily transformed under environmental conditions into organic compounds and may cause severe environmental and health risks. Mercury is a naturally occurring element. Its atomic number is 80, its atomic mass is grams per mole and its specific density is g/cm 3 at 25 C. Mercury has a melting point of C and a boiling point of 357 C. It is the only metal to remain in liquid form at room temperature. Droplets of liquid mercury are shiny and silver-white with a high surface tension, appearing rounded when on flat surfaces. The liquid is highly mobile and droplets combine easily due to low viscosity. The metal is a fair conductor of electricity, but a poor conductor of heat. Under high pressure (1.2 GPa, 12,000 bar) and at room temperature, mercury becomes solid [Funtikov 2009], [Mercury 65GPa 1993]. According to a report from the Fraunhofer Institut Bauphysik (building construction physics) [Fraunhofer 273], at a pressure of 200 bar, mercury is capable of penetrating into micropores with a radius of 3.7 nm. Solubility ([g/l], [g/kg], [mol/l] or [mol/kg]) Solubility is the quantity of a particular substance that can dissolve in a particular solvent (yielding a saturated solution). It is a measure indicating how easily a substance may dissolve and mobilise from a stored material and is transported via a water pathway. The solubility of elemental mercury in naturally occurring water depends on various parameters such as the composition of the solution or ph. The solubility of metallic mercury in distilled water is indicated in literature with ~0.3µmol/L (~60µg/l) at 25 C [Hagelberg 2006]. In [USEPA 2007], the solubility of mercury in water is indicated as 0.28µmol/l (56µg/l) at 25 C. In 2006 a study was published which investigated the solubility of elemental mercury in three different liquid matrices (L1: 1 mmoll -1 NaCl and 1 mmol NaHCO 3, L2: as L1 with 1.8 mmol conc. H 2 SO 4 and L3: leachate of crunched concrete) and at three different mercury/solution ratios [Hagelberg 2006]. Due to the very small number of repetitions with equal parameters, lack of equilibrium conditions, improper equipment materials (PTFE tubes instead of glass), among some other inconsistencies, the test results are only of limited value. In comparison to elemental mercury the solubility of mercury compounds is very different:
43 Reference number /2009/530302/ETU/G2 43 Table 4-1: Solubility of Hg and Hg compounds in water Mercury compound Solubility of Hg in water Hg Hg = 1.6*10-4 mmol/l (2.9 *10-5 g/l) [GRS 2008] HgS HgS = 7*10-21 mmol/l (1.40*10-27 g/l) [SPC 2009] HgO HgO = 8.9mmol/l (1.5 g/l) [GRS 2008A] HgCl 2 HgCl 2 = 6,000mmol/l (1,200 g/l) [GRS 2008A] While pure mercury sulphide has a significantly lower solubility compared to elemental mercury, the presence of HgO or HgCl 2 which might be due to impurities (< 1%) in mercury sulphide increases the solubility of HgS. In 2003 a laboratory study was conducted [Sakar 2003] to investigate the solubility of mercury in the presence of amphoteric oxides of iron, an electron acceptor. Investigations were performed with and without chloride in solution, a rather ubiquitous component of mercury wastes. Mercury solubility decreased in the presence of iron oxides, suggesting adsorption of mercury ions at the oxide-water interface. There was indirect evidence of formation of ionic mercury due to oxidation of elemental mercury in the presence of free iron in solution. Mercury solubility generally increased in the presence of chloride in solution because of the formation of weakly adsorbing mercury-chloro complexes. Information related to the influence of salt and salt solutions to solubility of mercury is very limited. In the presentation [GRS 2008A], first results have been presented on solubility of mercury at different concentrations of NaCl and KCl. It can be seen that the solubility of mercury in pure water is 0.3 µmol/l, whereas in a saturated NaCl (~6mol/l) solution the solubility is reduced to 0.16 µmol/l. This relationship is nearly linear but flattens with increasing NaCl concentrations. In the case of KCl, fewer solubility values are available (5 different concentrations) at lower concentration levels (< 1 mol/l), but it can be predicted that the Hg solubility in KCl solutions is similar to that in NaCl solutions. Some salts have a reverse effect on the solubility of mercury. NaSCN and (CH 3 ) 4 NBr for example are salts which increase the solubility of mercury with increasing salt concentration. The solubility of pure mercury in salt solutions is illustrated in the diagram below.
44 Reference number /2009/530302/ETU/G2 44 Figure 4-1: Relative solubility of elemental mercury in different salt water concentrations (NaCl and KCl) [GRS 2008A] These data coincide with the results of other studies such as Effects of salts on the solubility of elemental mercury in water [Sanemasa 1981] and The solubility of elemental mercury vapor in water [Sanemasa 1975]. The main result of the studies is that there is a linear decrease at least until a 1 molar solution is reached of the solubility of mercury in saturated potassium or sodium solutions. The solubility is significantly lower (half) compared to distilled water. Tests have also been made to demonstrate the temperature dependency of mercury vapour in pure water and sea water. It can be seen that the solubility increases exponentially with the temperature starting with 0.1 µmol/l (19.2 µg/l) at 5 C and up to 9 µmol/l (1,800 µg/l) at 100 C. The solubility in sea water is about 20% less than that in pure water [Sanemasa 1975]. Solubility product ((mol/l) n ) With the mean value of the solubility product (K sp ), it is possible to determine the dissolved concentration of a solid s constituents in solution assuming it has reached equilibrium. The solubility product constant is the simplified equilibrium constant (K sp ) defined for equilibrium between a solid and its respective ions in a solution. Its value indicates the degree to which a compound dissociates in water. The higher the solubility product constant, the more soluble is the compound. As elemental mercury only consists of one element, the solubility product is not relevant. But in the case of pre-treatment to immobilize or solidify mercury, the solubility product of the resulting solid form is important. The solubility products of relevant pre-treated mercury compounds (e.g. HgS) are included in Annex 4.
45 Reference number /2009/530302/ETU/G2 45 Leachability (mg/l or mg/kg) Leachability is a specific measure to assess how a pollutant that is contained in a stored material contributes to possible groundwater contamination if water seeps into and through the stored material. It indicates to what extent the mercury compounds are mobilised in the waste matrix and transported out of the stored material. The leachability of waste is often used for the classification of a waste. Depending on the leaching behaviour, the waste is allocated to different landfill categories (WAC Decision 2003/33/EC). The leachability is of particular interest for pre-treated (solidified) mercury. It is linked to the conditions of the pre-treatment process and the stability and homogeneity of the solidified mercury thus attained. Therefore general statements are not possible. Product-specific leaching values are included in Annex 4. Volatility (mg/kg) or vapour pressure (Pa) Volatility is a measure indicating how easily a substance may be evaporated and mobilised from a stored material and transported via an atmospheric pathway. Mercury has a relatively high vapour pressure of 0.3 Pa at 25 C [WHO 2003] and the highest volatility of any metal. The vapour pressure increases with temperature. The vapour is colour- and odourless and due to its high molar mass heavier than air and therefore mercury concentration is higher at ground level. Reactivity of mercury with other substances Reactivity indicates under which conditions the stored substance may react and be transformed to other substances that may be more easily mobilised and/or transported. Mercury can be elemental, monovalent or bivalent. Monovalent bonds are always bimolecular: Hg 2 X 2 and bivalent bonds are always monomolecular: HgX 2. Mercury has a positive redox potential which makes it noble and therefore does not tend to oxidise. [Ho Wi 1995] A reaction with oxygen takes place above ~300 C in air and decomposes again at 400 C. Pure mercury does not interact with ambient air but in the case of contaminated mercury an oxide layer is formed on the surface of the mercury [Ho Wi 1995]. Formation of HgO should be avoided due to its higher solubility compared to elemental mercury. [GRS 2008A] Mercury can react with halogens and more easily with sulphur but not with phosphorus, nitrate, hydrogen or carbon. [Ho Wi 1995] Reaction with chlorine can result in Hg 2 Cl 2 (low water solubility) or in HgCl 2 (very high water solubility). The formation of HgCl 2 should be avoided due to its very high water solubility. In general it can be stated that Hg(I) molecules are more stable and therefore less soluble than Hg(II) molecules. Both kinds of chlorides can be generated by sublimation. [Ho Wi 1995]
46 Reference number /2009/530302/ETU/G2 46 Mercury has a very high affinity to sulphur resulting in very stable mercury(ii)sulphide (HgS). This is the reason why natural sources of mercury are mostly HgS (cinnabar) reservoirs. Elemental mercury cannot be attacked by dilute HCl or H 2 SO 4 solutions, and then only slowly in the case of dilute HNO 3. [Ho Wi 1995] Mercury combines with other metals such as copper, gold, zinc, aluminium, nickel, tin, silver or selenium and forms mercury alloys known as amalgams. Amalgams are semi-solid solutions obtained by dissolution of mercury in the solid metal [Mersade 2007A]. Mercury destroys the passivation layer of aluminium which normally protects aluminium from oxidation. Blank aluminium can be oxidised again and the corrosion process is ongoing. Therefore, aluminium is not a suitable material for the storage of mercury. [Aluminium 2004] Iron on the other hand does not dissolve in elemental mercury and can therefore be used as container material. [Ho Wi 1995] Elemental mercury does not react with glass or ceramic products. An interaction of elemental mercury with certain plastic material is possible. [Hagelberg 2006] reported an adsorption of elemental mercury at plastic tubes. Within the scope of the Life project MERSADE (see chapter ) a literature review has been carried out concerning corrosion problems in mercury [Mersade 2007A]. It was concluded that literature referring to corrosion problems on metal containment used for the storage of liquid mercury is practically nil. In the following, the main findings of the review are described (the information is based on [MERSADE 2007A]): According to the document, at low temperature and static conditions, liquid metal corrosion is not an important factor. Therefore, steel and ceramic materials are appropriate for the storage of liquid mercury. Plain carbon steel is virtually unattached by mercury under non flowing conditions or isothermal conditions. Working temperatures up to 540 C are possible. The addition of Titanium (10 ppm) might increase the operating temperatures up to 650 C, but in this case elements with a higher affinity for oxygen than titanium, such as Na or Mg, are required to prevent oxidation of the titanium and loss of its inhibitive action. On the contrary, the presence of either a temperature gradient or liquid flow might lead to a drastic attack of the containment. It is stated that the solubility of metals (e.g. iron, nickel) in mercury increases with temperature.
47 Reference number /2009/530302/ETU/G2 47 Table 4-2: Change of solubility (in ppm) against the temperature [Mersade 2007A] 260 C 538 C Iron Nickel Chromium 0, As a consequence, low solubility of metals in mercury results in a low corrosion rate. Although the addition of elements such as chromium, titanium, silicon and molybdenum, alone or in combination, show high resistance up to 600 C, other alloying elements might have a contrary effect. For example, Nickel tends to have adverse effects on iron-based and cobalt-based alloys as it tends to form intermetallic compounds with mercury, lead and bismuth. In addition, experiences related to the use of liquid mercury as a target for a proton beam in a Spallation Neutron Source (SNS) facility have been included in the report. Tests have been carried out with various alloys, flow rates and temperatures. Due to contradicting results in the corrosion investigations, an extrapolation to static or different dynamic conditions, other temperatures or a long-term mercury storage condition is not recommended. Within the scope of the Mersade project, practical investigation also took place. Storage containers and pipe systems which have been in use for several years for the storage of liquid mercury at the storage facility at Almadén were analysed for potential attack by the stored mercury [Muñoz, 2009]. Another important information source related to potential corrosive effects of metallic mercury with containers are the investigations carried out by the Oak Ridge National Laboratory (ORNL), USA. The ORNL analysed storage containers which have been used for almost 40 years for the storage of metallic mercury. Detailed information on the outcome and conclusions of both projects are included in sections and Adsorption of mercury Ionic forms of mercury are strongly adsorbed by soils and sediments and are desorbed slowly. Clay minerals optimally adsorb mercury ions at ph 6. Iron oxides also adsorb mercury ions in neutral soils. Most mercury ions are adsorbed by organic matter (mainly fulvic and humic acids) in acidic soils. When organic matter is not present, mercury becomes relatively more mobile in acid soils and can evaporate to the atmosphere or leach to groundwater (Ref. 1.5). [US EPA 2007] Octanol/water partition coefficient (Kow) The octanol/water partition coefficient is a measure to indicate the hydrophobicity of a substance. It can give an indication of how easily a compound might be taken up in groundwater to pollute waterways. In the field of hydrogeology it is used to predict and model the migration of dissolved
48 Reference number /2009/530302/ETU/G2 48 hydrophobic organic compounds in soil and groundwater. Several studies have been carried out concerning the 1-Octanol/Water partition coefficient of mercury. The 1-octanol/water partition coefficient of metallic mercury was measured as a useful parameter for predicting the environmental behaviour and fate of mercury. The partition coefficient obtained was 4.15 ± 0.20 at 298 K [Okouchi 1985] and is indirectly proportional to the temperature, having a value of 3.80 at 35 C. Mercury is therefore a lipophile element, which has a higher solubility in octanol instead of water. The coefficient depends on the temperature and it decreases with an increase in temperature. The partition coefficient of metallic mercury is very low in comparison to non-polar organic compounds such as benzene, tetrachloromethane or PCBs. Therefore, it was concluded that metallic mercury has a tendency for further concentration in the atmosphere. [OKOUCHI 1985] Toxic effects The severity of mercury's toxic effects depends on the form and concentration of mercury and the route of exposure. Exposure to elemental mercury can result in effects on the nervous system, including tremors, memory loss and headaches. Other symptoms include bronchitis, weight loss, fatigue, gastrointestinal problems, gingivitis, excitability, thyroid enlargement, unstable pulse, and toxicity to the kidneys. Adverse effects to human health from exposure to elemental mercury are summarised in the draft technical guidelines on the environmentally sound management of mercury wastes (see [UNEP 2009], section 1.3.2). Exposure to inorganic mercury can affect the kidneys, causing immune-mediated kidney toxicity. Effects may also include tremors, loss of co-ordination, slower physical and mental responses, gastric pain, vomiting, bloody diarrhoea and gingivitis. Adverse effects to human health from exposure to inorganic mercury compounds are summarised in the draft technical guidelines on the environmentally sound management of mercury wastes (see [UNEP 2009], section 1.3.3). Symptoms of methylmercury toxicity, also known as Minamata disease, range from tingling of the skin, numbness, lack of muscle coordination, tremors, tunnel vision, loss of hearing, slurred speech, skin rashes, abnormal behaviour (such as fits of laughter), intellectual impairment, to cerebral palsy, coma and death, depending on the level of exposure. In addition, methylmercury has been classified as a possible human carcinogen by the U.S. Environmental Protection Agency. More recently, additional findings have described adverse cardiovascular and immune system effects at very low exposure levels. Prenatal exposure to organic mercury, even at levels that do not appear to affect the mother, may
49 Reference number /2009/530302/ETU/G2 49 depress the development of the central nervous system and may cause psychomotor retardation for affected children. Mild neurological and developmental delays may occur in infants ingesting methylmercury in breast milk. Affected children may exhibit reduced coordination and growth, lower intelligence, poor hearing and verbal development, cerebral palsy and behavioural problems. Adverse effects to human health from exposure to inorganic mercury compounds are summarised in the draft technical guidelines on the environmentally sound management of mercury wastes (see [UNEP 2009], section 1.3.1) Classification The classification of mercury (EC ; CAS ) according to Directive 67/548/EEC 15 is as follows: Table 4-3: Risk phrases and classification of mercury Classification Repr. Cat. 2; R61 T+; R26 Risk phrases R61: May cause harm to the unborn child. R26: Very toxic by inhalation. T; R48/23 R48/23: Toxic: danger of serious damage to health by prolonged exposure through inhalation. N; R R50/53: Very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. The preliminary classification of mercury according to Regulation (EC) N 1272/ is as follows: Table 4-4: Hazard class, category codes and hazard statement codes of mercury Hazard Class and Category Code(s) Acute Tox. 3 * STOT RE 2 * Aquatic Acute 1 Aquatic Chronic 1 Hazard statement Code(s) H331: Toxic if inhaled H373: May cause damage to organs through prolonged or repeated exposure H400: Very toxic to aquatic life H410: Very toxic to aquatic life with long lasting effects 15 Council Directive 67/548/EEC of 27 June 1967 on the approximation of laws, regulations and administrative provisions relating to the classification, packaging and labelling of dangerous substances, OJ 196, Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006 (Text with EEA relevance), OJ L 353, , p
50 Reference number /2009/530302/ETU/G Occupational exposure limit values Acute exposure (>0.1 mg mercury/m 3 ) to mercury vapour causes adverse effects to human health [UNEP 2009]. Therefore, many EU Member States implemented occupational exposure limit (OEL, eight hour average) values for mercury and its inorganic divalent compounds (as Hg) ranging from 0.03 mg/m³ in Lithuania, Sweden, Slovakia to 0.1 mg/m³ in Germany [EU OSHA 2007, GESTIS 2009, TRGS 900]. Currently, Poland is the only country with an OEL (eight hour average) for metallic vapour of mercury with a value of mg/m³ [GESTIS 2009]. On the European level no corresponding indicative value is available but [SCOEL 2007] recommended an 8-hour TWA of 0.02 mg mercury/m³ for elemental mercury and inorganic divalent mercury compounds. A biological limit value (BLV) of 10 µg Hg/l blood and 30 µg Hg/g creatinine in urine is also recommended by [SCOEL 2007]. The UNEP recommended health-based exposure limit value for metallic mercury is mg Hg/m³ for long-term exposure as the time weighted average (TWA). This means the time weighted average concentration for a normal 8-hour day and 40-hour workweek, to which nearly all workers can be repeatedly exposed without adverse effect [UNEP 2009]. However, recent studies suggest that mercury may have no threshold below which adverse effects do not occur [UNEP 2009]. In the USA 0.1 mg/m 3 is also declared as the ceiling limit value for mercury vapour (concentration cannot exceed this value at any time) [OSHA 2009]. Threshold limit values (TLV) are available for elemental mercury being mg/m³ and 0.01 mg/m 3 for organic mercury [US EP 2007]. Referring to [NIOSH 2005] the Time Weighted Average (TWA) for an 8-hour day should not exceed 0.05 mg/m Hazardous properties related to the environment Transformation and transport of mercury Natural transformations and environmental pathways of mercury are very complex and are greatly affected by local conditions. The environmental fate and the impacts of anthropogenic mercury emissions depend on a range of biogeochemical interactions affecting mercury in its various physical states and chemical forms. There are two main types of reactions in the mercury cycle that convert mercury through its various forms: oxidation-reduction and methylation-demethylation. In oxidation-reduction reactions, mercury is either oxidized to a higher valence state (e.g. from relatively inert Hg 0 to the more reactive Hg 2+ ) or reduced (e.g. from Hg 2+ to Hg 0 ). Most relevant environmental pathways are short or long range atmospheric transport mechanisms
51 Reference number /2009/530302/ETU/G2 51 and transport in water/sediment systems (e.g. river or marine systems). In general, the form of mercury in the environment varies with the season, with changes in organic matter, nutrient and oxygen levels and hydrological interactions within an ecosystem. In addition, the quantity and forms of mercury are, to a large extent, a function of emission sources and transportation processes. All of these variables in turn affect the global mercury budget. Mercury oxidation The oxidation of elemental mercury (Hg 0 ) in the atmosphere is an important mechanism involved in the deposition of mercury on land and water. Hg 0 can volatilize relatively easily and be emitted into the atmosphere, where it may be transported on wind currents for a year or more and be redeposited in the environment for further cycling. In contrast, Hg 2+ has an atmospheric residence time of less than two weeks due to its solubility in water, low volatility and reactive properties. Hence, when (Hg 0 ) is converted to Hg 2+, it can be rapidly taken up in rain water, snow, or adsorbed onto small particles, and be subsequently deposited in the environment through "wet" or "dry" deposition [Selin 2009]. In the Arctic, the conversion of Hg 0 to Hg 2+ in the atmosphere occurs very rapidly in a phenomenon known as "mercury depletion" at the end of dark polar winters. When the sun rises in the spring, atmospheric Hg 0 is converted photochemically to Hg 2+ in the presence of reactive chemicals released from sea salt (for example, bromine and chlorine ions) and mercury levels in the atmosphere are "depleted" as the Hg 2+ is then deposited on snow and ice surfaces. As a consequence, a pulse of reactive mercury enters the Arctic environment when the short lived growing season is beginning. It remains a research question as to what fraction of this reactive mercury is converted to toxic methylmercury and taken up by animals and plants. Mercury Methylation In an aquatic environment under suitable conditions, mercury is bioconverted to methylmercury, by a chemical process called Methylation [Wood 1974]. In the Methylation process, mercury is transformed into methylmercury when the oxidized, or mercuric species (Hg 2+ ), gains a methyl group (CH 3 ). The methylation of Hg 2+ is primarily a biological process resulting in the production of highly toxic and bioaccumulative methylmercury compounds (MeHg + ) that build up in living tissue and increase in concentration up the food chain, from microorganisms like plankton, to small fish, then to fish eating-species such as otters and loons, and humans. The formation of methylmercury is critically important due to its highly toxic, bioaccumulative and persistent nature. A variety of micro-organisms, particularly methanogenic (methane producing) and sulphate-dependant bacteria are thought to be involved in the conversion of Hg 2+ to MeHg under anaerobic (oxygen poor) conditions found, for example, in wetlands and river sediments, as well as in certain soils. Methylation occurs primarily in aquatic, low ph (acidic) environments with high concentrations of organic matter.
52 Reference number /2009/530302/ETU/G2 52 Rates of bio-methylation are a function of environmental variables affecting mercuric ion availability as well as the population sizes of methylating microbes. Alkalinity, or ph, plays a strong role in regulating the process because it is affected by, and in turn affects, the adsorption of various forms of mercury on soil, clay and organic matter particles, thus influencing mercuric ion availability. Acid rain may increase biomethylation as more MeHg is formed under acidic conditions. [Env Canada 2004]. In several reports it is stated that bioavailability of mercury for methylation is increased in the presence of neutral dissolved Hg complexes HgS 0 (aq) [Benoit 1999], [Drott 2007]. It is also described that high dissolved sulphide concentration favour the creation of disulfide complexes, primarily HgHS2-, which reduces the bioavailability of mercury for methylation. [Hammerschmidt 2008] [Benoit 1999]. Tests showed that not dissolved Hg or mercury sulphide gave no significant relationship with the specific methylation rate constant. [Drott 2007] [Benoit 2001]. Some substances have been identified to have an inhibiting effect on the methylation process as iron sulphides. This is probably due to the decrease of neutral Hg(II)-sulphide complexes via formation of charged Hg(II)polysulfides [[Liu 2009]. However, sulphate may stimulate growth of certain methylating microbes. Organic matter can stimulate microbial populations, reduce oxygen levels, and therefore increase bio-methylation. Biomethylation increases in warmer temperatures when biological productivity is high, and decreases during the winter. Atmospheric long range transport Mercury in the atmosphere is broadly divided into gas form and particulate form. Most of the mercury in the general atmosphere is in gas form (95% or more). Gaseous mercury includes mercury vapour, inorganic compounds (chlorides and oxides), and alkyl mercury (primarily methylmercury [JPHA 2001]. The volatility of elemental mercury (Hg 0 ) enables mercury to travel in a multi-step sequence of emission to the atmosphere, transportation, deposition and re-emission. As a result, mercury from point source emissions may remain localized in the environment, or may be transported regionally and even globally. Atmospheric transport is likely the primary mechanism by which Hg 0 is distributed throughout the environment, unlike many pollutants that follow erosion or leaching pathways. Mercury can enter the atmosphere as a gas or bound to other airborne particles and circulates until removal. Removal occurs primarily through the "wet" deposition of Hg 2+ in rainfall, however it can also occur in the presence of snow, fog, or through direct, or "dry", deposition. Approximately 98% of the estimated 5000 tonnes of mercury in the atmosphere is Hg 0 vapour, emitted from human activities, contaminated soils and water, as well as natural sources [Env Canada 2004]. This gas is readily transported and has a mean atmospheric residence time of about one year to one and a half years [Selin 2009]. The transformation of insoluble Hg 0 to its more reactive and
53 Reference number /2009/530302/ETU/G2 53 water-soluble form, Hg 2+, is thought to provide the mechanism for the deposition of Hg 0 emissions to land and water. Hg 0 oxidation may also be affected by concentrations of other atmospheric pollutants such as ozone, sulphur dioxide and soot. Additional research is needed in order to predict corresponding mercury deposition rates. Mercury Deposition Following release to the atmosphere and depending on its physical/chemical form, mercury can be either deposited in the vicinity of the emission source, or subjected to long-range atmospheric transport via air masses. Because the uptake of Hg 0 in cloud water is relatively slow, this process may be responsible for the deposition of mercury far from its source and may be important when considering global mercury pollution. Gaseous Hg +2 and particulate mercury (Hg(p), mercury adsorbed onto other particulate matter) emissions generally undergo direct wet or dry deposition to the earth's surface locally. These species have relatively short residence times in the atmosphere ranging from hours to months. Gaseous Hg +2 has a residence time of 5 to 14 days in the atmosphere, and may travel tens to hundreds of kilometres. Particulate forms of mercury (Hg(p)) tend to fall out closer to the source of emissions, with larger particles falling out faster than smaller ones. The sitespecific deposition of mercury is variable, and is affected by conditions such as meteorology, temperature and humidity, solar radiation and emission characteristics (speciation, source, stack height, etc.). Atmospheric Circulation Atmospheric circulation processes may play an important role in determining where airborne mercury is eventually deposited. Mercury, like other semi-volatile compounds such as PCBs, is thought to participate in a global distillation phenomenon that transfers chemical emissions from equatorial, subtropical and temperate regions to the polar regions via the "grasshopper effect". When this phenomenon takes place, an emitted compound re-enters the atmosphere by volatilizing after initial deposition, and continues over time to "hop" through the environment in the direction of the prevailing winds, favouring accumulation in the colder regions of the planet. During the summer months, major air currents in the northern hemisphere lead to the Arctic, and once there, a contaminant can no longer gain enough heat energy for another "hop" out of the Arctic. The net result is a concentration of contaminants in the Arctic at odds with the relative sparsity of emissions sources in the region. Other pathways (other than atmospheric long range transport) In addition to atmospheric pathways, mercury can be transported through river systems in their sediment loads, or in aqueous solution. The transport distance may be long or short. Where mercury is carried on particles, the distance is limited by sedimentation. Transport of contaminants via particles tends to halt at riverine lakes or reservoirs since heavy sedimentation can occur there. Transport also occurs along ocean currents. Transport of mercury underground depends particularly on the ph conditions in the hydrogeological and geochemical conditions. Deep groundwater is generally neutral to alkaline, under which
54 Reference number /2009/530302/ETU/G2 54 conditions Hg tends to be immobilised due to adsorption to mineral surfaces. Deep environments tend to be reducing, under which conditions mercury tends to be immobilised due to precipitation as sulphur compounds. The adsorption of mercury compounds is positively correlated with the cation exchange capacity of the geo-environment. The mercury is adsorbed by certain clays (e.g. naturally occurring clays or montmorillonite/bentonite). [Heath 2006] The oceans are considered the ultimate sink for mercury because Hg 2+ deposited from the atmosphere can settle to oceanic depths where it is reduced and precipitates as insoluble mercuric sulphide. It is thought that approximately one third of the total current mercury emissions cycle between the oceans and the atmosphere, and that 20 to 30% of oceanic emissions, are re-emitted from prior anthropogenic sources Overview of the behaviour in the environment Mercury is a persistent, mobile and bioaccumulative element in the environment and is retained in organisms. Mercury in the aquatic environment is changed to various forms, mainly methylmercury, methylated from mercury. As a consequence mercury permanently exists in the environment and its chemical form availability to living organism change over time depending on the environmental conditions. Figure 4-2 shows the main chemical reactions and pathways of mercury in the atmosphere, water, soil, and in sediments and the effect of bioaccumulation.
55 Reference number /2009/530302/ETU/G2 55 Figure 4-2: Diagram of the biogeochemical mercury cycle Figure 4-2 illustrates the main chemical reactions and pathways of mercury in the atmosphere, water, soil, and in sediments. Pathways include leaching and runoff, emission from natural and anthropogenic sources, volatilization, deposition from the atmosphere, and sedimentationresuspension. Methylation-demethylation, oxidation-reduction, and complexation are the chemical reactions shown. The diagram also illustrates bioaccumulation of mercury in a fish food chain. (Source: Env Canada 2004) Environmental limit values related to mercury Water Within the European Community, Directive 2008/105/EC 17 on environmental quality standards in the field of water policy establishes Environmental Quality Standards (EQS) for mercury and its compounds. 17 Directive 2008/105/EC on environmental quality standards in the field of water policy, amending and subsequently, repealing Council Directives 82/176/EEC, 83/513/EEC, 84/156/EEC, 84/491/EEC, 86/280/EEC and amending Directive 2000/60/EC of the European Parliament and of the Council, 16 December 2008 (OJ L 348, , p )
56 Reference number /2009/530302/ETU/G2 56 Table 4-5: Environmental Quality Standards (EQS) set for mercury in Directive 2008/105/EC AA-EQS Inland surface waters (3) AA-EQS Other surface waters MAC-EQS Inland surface waters (3) MAC-EQS Other surface waters Mercury and its 0.05 (9) 0.05 (9) compounds AA: annual average; MAC: maximum allowable concentration, Unit: [μg/l] In addition, in article 3(2)(a) an EQS for biota is set for mercury and its compounds of 20 µg/kg based on prey tissue (wet weight), choosing the most appropriate indicator from fish, molluscs, crustaceans and other biota. In cases where Member States do not apply the EQS for biota they shall introduce stricter EQS for water in order to achieve the same level of protection. On the international level [WHO 2004] is proposing a limit value of 1 μg/litre for total mercury in water. Air Within the European Community no common limit value for mercury concentration is set. Directive 2004/107/EC 18 establishing target values for the concentration of arsenic, cadmium, mercury, nickel and polycyclic aromatic hydrocarbons in ambient air does not introduce specific target values for mercury, while for the other substances such targets are introduced (Annex I of the Directive). On the international level, the WHO prescribes an air quality guideline value of 1 μg Hg 0 /m 3 as an annual average concentration and 0.2 μg/m 3 for long-term inhalation exposure to elemental mercury vapour [WHO 2007]. [UNEP 2009] also describes exposure levels (RELs) for Hg 0 established for the general non-occupational population, based on US American and Canadian limits: 0.3 μg/m 3 from the US EPA (chronic reference air concentration) 0.2 μg/m 3 from the US Agency for Toxic Substances and Disease Registry (minimal risk level for chronic inhalation exposure) 0.09 μg/m 3 from the California Environmental Protection Agency (inhalation reference exposure) 0.06 μg/m 3 from Health Canada (chronic tolerable air concentration) 18 Directive 2004/107/EC of the European Parliament and of the Council of 15 December 2004 relating to arsenic, cadmium, mercury, nickel and polycyclic aromatic hydrocarbons in ambient air, 16. December 2008 (OJ L 23, , p. 3 16)
57 Reference number /2009/530302/ETU/G Conclusions Mercury is a persistent, mobile and bioaccumulative element in the environment and is retained in organisms [Env Canada 2004]. In particular the methylation - transformation of mercury compounds to the highly toxic form methylmecury has to be taken into consideration for the storage of liquid mercury but also for mercury compounds. Methylation occurs primarily in aquatic, low ph environments with high concentration of organic matter but also other parameters might influence the formation of methylmecury [Env Canada 2004], [Wood 1974]. Therefore in case of the storage of mercury a special focus should be laid on the potential formation of methylmercury. Metallic mercury has specific properties which have to be taken into consideration for the assessment of possible storage options. In particular its high vapour pressure and its liquid state at room temperature might entail problems in handling and long term storage. Information on leaching values for mercury and mercury compounds are available in literature and are summarized in Annex 4. Although information is available related to the solubility of mercury and mercury compounds in water [Hagelberg 2006], [USEPA 2007], information to the influence of salt and salt solutions to solubility of mercury is still very limited. Results from available investigations ([GRS 2008A], [Sanemasa 1981]) give first indications of a decreased solubility of mercury in salt solutions. But further research is required to verify these results [GRS 2008A]. Information on the reactivity of mercury with other substances is described in chemical literature [Ho Wi 1995]. With reference to the corrosiveness of mercury with possible container material only very limited literature has been found [Mersade 2007A]. Most important information is available from two projects carried out by ONRL (Oak Ridge National Laboratory), USA and within the Life Project MERSADE 19 (detailed results see 6.4.3). Acute exposure to mercury vapour causes adverse effects to human health. Therefore occupational exposure limit values are established in many countries [EU OSAH 2007], [GESTIS 2009]. On European level a limit value of 0.02 mg Hg/m³ (TWA, 8 h) for elemental mercury and inorganic divalent mercury compounds is recommended by [SCOEL 2007]. Environmental limit values are available on EU level for water 20. On international level also air quality guideline values are established [WHO 2007] LIFE is the EU s financial instrument supporting environmental and nature conservation projects throughout the EU, as well as in some candidate, acceding and neighbouring countries, for further information see Directive 2008/105/EC
58 Reference number /2009/530302/ETU/G References [Aluminium 2004] Corrosion of Aluminium, Christian Vargel, ISBN: , 2004 [ATSDR 1999] U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES, Public Health Service, Agency for Toxic Substances and Disease Registry, TOXICOLOGICAL PROFILE FOR MERCURY, 1999; [Benoit 1999] Benoit, J.M., Mason, R.P., Gilmour, C.C., Estimation of mercury-sulfide speciation in sediment pore waters using octanol-water partitioning and implications for availability to methylating bacteria, Environmental Toxicology and Chemistry, Vol. 18, No. 10, pp , [Benoit 2001] Benoit, J.M., Gilmour, C.C., Mason, R.P., The influence of sulphide on solid-phase mercury bioavailability for methylation by pure cultures of Desulfobulbus propionicus, (2001), Environmental Science and Technology, 35 (1), pp , 2001 [CCOHS 1998] Canadian Centre for Occupational Health & Safety, Chemical profile mercury, preparation date 1998, copyright [Drott 2007] Drott, a., Lambertsson, L., Björn, E., Skyllberg, U., Importance of dissolved neutral mercury sulfides for methyl mercury production in contaminated sediments, Environmental Science and Technology, 41 (7), pp , 2007 [Env Canada 2004] Environment Canada, Mercury and the environment, Internet document: last update , accessed on 29 June 2009 [EU OSHA 2007] Exploratory Survey of Occupational Exposure Limits for Carcinogens, Mutagens and Reprotoxic substances at EU Member States Level, European Agency for Safety and Health at Work, European Risk Observatory Report [Euro Chlor 2009] Euro Chlor, Metallic mercury (Hg0) The biological effects of long-time, low to moderate exposures, Science dossier 13, February 2009 [Fraunhofer 273] M. Krus, H.M. Künzel, Das Wasseraufnahmeverhalten von Betonbaustoffen, IBP-Mitteilung 273, Fraunhofer Institut für Bauphysik
59 Reference number /2009/530302/ETU/G2 59 [Funtikov 2009] High Temperature 2009, Vol. 47, No. 2, pp , A. I. FUTNIKOV, Shock Adiabat, Phase Diagram, and Viscosity of Mercury at a Pressure up to 50 GPa. [Gestis 2009] [GRS 2008A] Gesellschaft für Anlagen- und Reaktorsicherheit (GRS) mbh, Solubility of metallic mercury and mercury compounds in saline solutions, presentation by Horst-Jürgen Herbert and Sven Hagemann GRS, Braunschweig, 2008, Germany [Hagelberg 2006] Hagelberg, Erik, Örebro University, Institutionen för naturvetenskap, Department of Natural Sciences, The matrix dependent solubility and speciation of mercury, 2006, [Hammerschmidt 2008] Hammerschmidt Chad R., Fitzgerald William F., Balcom Prentiss H., Visscher Pieter T., Organic matter and sulfide inhibit methylmercury production in sediments of New York/New Jersey Habor, Marine Chemistry 109 (2008) [Heath 2006] Mike Heath, Health environmental and safety questions related to the underground storage/disposal of mercury over time, Presentation at the EEB Conference on EU Mercury surplus management and mercury-use restrictions in measuring and control equipment, Brussels, 19 June 2006; [Ho Wi 1995] Lehrbuch der Anorganischen Chemie 101. Auflage Hollemann Wiberg, 1995 [JPHA 2001] Japan Public Health Association: Preventive Measures against Environmental Mercury Pollution and Its Health Effects, Japan, [Liu 2009] Liu, J., Valsaraj, K.T., Delaune, Inhibition of mercury methylation by iron sulfides in an anoxic sediment, Environmental Engineering Science, 26 (4), pp , 2009 [Mercury 65GPa] Rapid communication, Physical Review B, Volume 48, Number 18, , 1 November 1993-II, Olaf Schulte and Wilfried B. Holzapfel, Phase Diagram for mercury up to 65 GPa and 500 K [OKOUCHI 1985] Okouchi S., Sasaki, S., The 1-octanol/water partition coefficient of mercury, Bulletin of the Chemical Society of Japan, Vol.58, No.11(1985)pp , e=3401&lang=en&from=jnlabstract
60 Reference number /2009/530302/ETU/G2 60 [Sakar 2003] Sarkar, Dibyendu, Preliminary studies on mercury solubility in the presence of iron oxide phases using static headspace analysis, Environmental Geosciences; December 2003; v. 10; no. 4; p ; DOI: /eg [Sanemasa 1975] Isao Sanemasa, The solubility of elemental mercury vapor in water, Bulletin of the chemical society of Japan, Vol 48(6), =1795&lang=en&from=jnlabstract [Sanemasa 1981] Isao Sanemasa, Effects of salts on the solubility of elemental mercury vapor in water, Bulletin of the chemical society of Japan, Vol 54(4), =1040&lang=en&from=jnlabstract [SCOEL 2007] SCOEL, Recommendation from the Scientific Committee on Occupational Exposure Limits for elemental mercury and inorganic divalent mercury compounds, SCOEL/SUM/84, May 2007, [Selin 2009] Selin, Noelle E., Global Biogeochemical Cycling of Mercury: A Review, Department of Earth, Atmospheric, and Planetary Sciences, Massachusetts Institute of Technology, Cambridge, Massachusetts ; Annu. Rev. Environ. Resour :43 63, DOI /annurev.environ [SPC 2009] [TRGS 900] Technische Regeln für Gefahrstoffe. Arbeitsplatzgrenzwerte. Ausgabe: Januar 2006, zuletzt geändert und ergänzt: GMBl Nr. 28 S. 605 (v ), Z/Gefahrstoffe/TRGS/pdf/TRGS-900.pdf [UNEP 2002] UNEP, Global mercury assessment, 2002, [UNEP 2009] UNEP, Draft technical guidelines on the environmentally sound management of mercury wastes, 4th Draft, April 2009, [USEPA 2007] U.S. Environmental Protection Agency, Treatment Technologies For Mercury in Soil, Waste, and Water, EPA-542-R , 2007,
61 Reference number /2009/530302/ETU/G2 61 [WHO 2004] Guidelines for Drinking-water quality 3rd edition. Geneva, World Health Organization, [WHO 2005] World Health Organisation, Mercury in Drinking-water, Background document for development of WHO Guidelines for Drinking-water Quality, 2005, [WHO 2006] World Health Organisation, Guidelines for drinking-water quality incorporating first addendum. Vol. 1, Recommendations. 3rd ed.electronic version for the Web, 2006, [WHO 2007] World Health Organisation, Preventing disease through healthy environments exposure to mercury, A major public health concerns, Geneve 2007, [Wood 1974] Wood, J.M.: Biological Cycles for Toxic Elements in the Environment, Science, 15, , 1974 [ZERO Hg 2006] Zero Mercury working group, EU Mercury Surplus Management and Mercury-Use Restrictions in Measuring and Control Equipment, Report from the EEB Conference, October 2006,
62 Reference number /2009/530302/ETU/G Review of legislation, policy and best practice 5.1 International agreements UNEP The United Nations Environment Programme established the UNEP Mercury Programme with the aim of delivering activities on mercury and to support negotiations of an international instrument for the control of mercury (for more information about the UNEP Mercury Programme, see chapter 5.5 [UNEP 2009B]. In order to strengthen the UNEP Mercury Programme, a series of Governing Council (GC) Decisions have been established since 2001: GC Decision 21/5 Mercury assessment (2001), GC Decision 22/4 V Chemicals; Mercury Programme (2003), GC Decision 23/9 IV Chemicals management; Mercury Programme (2005), GC Decision 24/3 IV Chemicals management; Mercury (2007), GC Decision 25/5 III Chemicals management, including mercury; Mercury (2009). Decision GC 21/5 from 2001 focused on the assessment of available information and the provision of a summary description of existing scientific and technical information, needs and data gaps referring to information like the global nature and anthropogenic source of mercury, the long-range transport, pathways and deposition, source of releases and production patterns of mercury as well as prevention and control technologies and practice. As regards mercury waste, one aim of the Decision GC 21/5 was to describe ongoing actions and to compile information about future plans at national, sub-regional and regional levels for controlling releases and limiting use and exposures, including waste management practice. Decision GC 22/4 from 2003 announced the Mercury Programme and promoted first actions to reduce the risks of mercury. It proposed again the improvement of the scientific basis on mercury and mercury components. The decision requested to enhance risk communication on mercury, to reduce anthropogenic releases and the demand for and the uses of mercury, to identify subsidisation of mercury mining and to cooperate with other international organisations. With regard to mercury waste, the decision proposed a reduction of releases from waste streams and to improve collection and exchange of information on disposal. GC Decision 23/9 from 2005 included the management on other chemicals, in particular lead and
63 Reference number /2009/530302/ETU/G2 63 cadmium. The decision concentrated on the cooperation between UNEP and other multilateral environmental organisations and agreements. With regard to mercury, it repeated the requests of the previous decision promoting national, regional and global actions, both immediate and longterm, to reduce and eliminate the use of mercury. It firstly included specific provisions on processes (the chlor-alkali process) and products containing mercury (batteries containing mercury) to be phased out. With regard to mercury waste, the decision promoted more precisely the development of environmentally sound disposal and remediation practices. GC Decision 24/3 firstly established a strategic approach to international chemicals management in The reduction in mercury supply was identified as a global priority. The aim was to establish a legally binding instrument on mercury at international level. With regard to mercury waste, the decision urged governments to gather information on the options and solutions for the management of waste containing mercury and mercury components as well as for the long-term storage of mercury instead of allowing this mercury to be sold on the global marketplace. In consequence, the Draft technical guidelines on the environmentally sound management of mercury wastes were prepared in 2007 [UNEP 2007] containing information on: the application for mercury waste prevention and minimisation; guidance on environmentally sound management (EMS) criteria and practice of mercury waste; the chemical analysis of mercury in waste; treatment of mercury waste and recovery of mercury; long-term storage and disposal of mercury waste. In February 2009, the UNEP Governing Council Decision 25/5 considered further work required on mercury, and agreed to a number of activities, in particular: the elaboration of a legally binding instrument on mercury, including provisions to be considered by the intergovernmental negotiating committee; a study on various types of mercury emitting sources; interim activities to reduce risks to human health and the environment; an update of the 2008 report on Global Atmospheric Mercury Assessment; Sources, Emissions and Transport. The reduction of the supply of mercury and the enhancement of capacity for its environmentally sound storage is set out as a further priority. Furthermore, the Draft technical guidelines on the environmentally sound management of mercury wastes was further elaborated [UNEP 2009].
64 Reference number /2009/530302/ETU/G2 64 Basel Convention The Basel Convention on the Control of Transboundary Movements of Hazardous Wastes and their Disposal is the most comprehensive global environmental agreement on hazardous and other wastes. The Secretariat of the Basel Convention is administered by UNEP. The Convention came into force in 1992 and has 172 Parties. It aims at the protection of human health and the environment against the adverse effects resulting from the generation, management, transboundary movements and disposal of hazardous and other wastes by stipulating that any transboundary movement of wastes (export, import, transit) is permitted only when the movement itself and the disposal of the wastes are environmentally sound [Basel 2010]. The parties of the Basel Convention have the right to prohibit the import of hazardous wastes or other wastes for disposal. In this case they have to inform the other Parties of their decision (Article 4 of the Basel Convention). Article 4 (2) contains the major general provisions for the Parties of the Basel Convention including the obligation to take appropriate measures to: Reduce the generation of hazardous and other wastes Ensure the availability of adequate disposal facilities Prevent pollution while handling and managing the waste Reduce transboundary movements to a minimum Not allow export of wastes to countries where the import of the waste is prohibited Ensure that information about the transboundary movement is provided to other states Prevent the import of waste if environmental sound management cannot be assured Co-operate Mercury and mercury compounds are included as entry Y29 in Annex I, which lists hazardous wastes that shall be controlled. In case of transboundary movements of such wastes a notification document is required which has to be forwarded to the competent authorities of the involved countries (Art. 6 of the Basel Convention). Additionally mercury containing wastes are listed in Annex VIII of the Basel convention as A1010 (metal wastes and wastes consisting of different alloys, amongst mercury) and A1030 (waste having as constitutes or contaminants mercury and mercury compounds). Also in other entries wastes are listed which may contain mercury, in particular A 1180 (mercuryswitches), A1170 (batteries), A 2030 (waste catalysts), A2060 (fly-ashes), A3170 (wastes from production of aliphatic halogenated hydrocarbons), A4010 (wastes from production of pharmaceutical products), A4020 (clinical wastes), A4080 (explosive wastes) and A 4160 (spent activated carbon) [UNEP 2009].
65 Reference number /2009/530302/ETU/G2 65 The Basel Convention not only aims at a controlled transboundary movement but also at the environmentally sound disposal of the wastes. Disposal means any operation specified in Annex IV to the Basel Convention (Article 2 (4) of the Basel Convention). Annex IV includes the whole list of operations which do not lead to the possibility of resource recovery, recycling, reclamation, direct reuse or alternative uses (D operations, as similar listed in the Waste Framework Directive) and operations which may lead to such that possibility (R operations, as similar listed in the Waste Framework Directive). However environmental sound management (ESM) is only generally described within Article 2 (8) of the Basel Convention as taking all practicable steps to ensure that hazardous wastes or other wastes are managed in a manner which will protect human health and the environment against the adverse effects which may result from such wastes. CLRTAP Since 1979 the Convention on Long-range Transboundary Air Pollution (CLRTAP) has addressed major environmental problems of the UNECE 21 region through scientific collaboration and policy negotiation. The aim of the Convention is to limit and, as far as possible, gradually reduce and prevent air pollution including long-range transboundary air pollution. Parties develop policies and strategies to combat the discharge of air pollutants through exchanges of information, consultation, research and monitoring. The Convention has been extended by eight protocols that identify specific measures to be taken by Parties to cut their emissions of air pollutants, amongst them the Protocol on Heavy Metals 22 which was adopted by the Executive Body (EB) on 24 June 1998 entering into force in It targets the three particularly harmful metals cadmium, lead and mercury. One of the main obligations is the reduction of emissions from these metals by aiming at the cut of emissions from industrial sources (iron and steel industry, non-ferrous metal industry), combustion processes (power generation, road transport) and waste incineration. Therefore, it lays down stringent limit values for emissions from stationary sources and suggests best available techniques (BAT) for these sources, such as special filters or scrubbers for combustion sources or mercury-free processes. The Protocol also requires Parties to phase out leaded petrol and reduce emissions from other products (e.g. mercury in batteries, electrical components (thermostats, switches), measuring devices (thermometers, manometers, barometers), fluorescent lamps, dental amalgam, pesticides and paint. Each party shall reduce its total annual emissions into the atmosphere for mercury to the level of the emissions in the reference year 1990, or an alternative year from 1985 to United Nations Economic Commission for Europe with currently 56 Member States from the European continent 22 The 1998 Protocol on Heavy Metals to the 1979 Convention on long-range transboundary air pollution, 24 June 1998 in Aarhus (Denmark)
66 Reference number /2009/530302/ETU/G European legislation Elemental mercury At present, mercury is not seen as waste on the European Community level, but considered as raw material. Excess mercury from the decommissioning of chlor-alkali plants as well as liquid mercury gained from e.g. recycling processes of products is sold to mercury dealing companies (e.g. Mayasa) and re-sold as raw material for various applications. Large amounts are also exported to non-eu countries. Therefore specific requirements related to the disposal of liquid mercury have not been needed so far. The situation changed with the publication of Regulation (EC) No 1102/ on the banning of exports and the safe storage of metallic mercury. Safe storage options for metallic mercury are needed for the near future within the Community as the ban starts from 15 March 2011 and affects metallic mercury, cinnabar ore, mercury (I) chloride, mercury (II) oxide and mixtures of metallic mercury with other substances including alloys of mercury, with a concentration of at least 95 wt % Hg (recital 5 of Regulation (EC) No 1102/2008). In order to provide possibilities for a safe storage of the above-mentioned metallic mercury waste within the Community, Article 3 of Regulation (EC) No 1102/2008 constitutes suitable options, both for permanent and temporary storage in appropriate containments: temporary storage for more than one year or permanent storage in salt mines adapted for the disposal of metallic mercury, temporary storage for more than one year or permanent storage in deep underground, hard rock formations providing a level of safety and confinement equivalent to that of those salt mines, temporary storage for more than one year in above-ground facilities dedicated to and equipped for the temporary storage of metallic mercury. For this purpose Article 5 (3)(a) of Directive 1999/31 24 shall be derogated. In addition, Article 4 of Regulation (EC) No 1102/2008 stipulates that the safety assessment which is required for a safe underground storage under Decision 2003/33/EC 25 should be complemented by specific requirements resulting from the specific risk of the storage of metallic mercury. Furthermore, acceptance criteria should be developed for metallic mercury either temporarily or permanently 23 Regulation (EC) No 1102/2008 of the European Parliament and of the Council of 22 October 2008 on the banning of exports of metallic mercury and certain mercury compounds and mixtures and the safe storage of metallic mercury (OJ L304 of 14/11/08, p.75-79), also referred to as the Mercury Regulation. 24 Council Directive 1999/31/EC of 26 April 1999 on the landfill of waste (OJ L 182, , p. 1 19), also referred to as the Landfill Directive. 25 Council Decision of 19 December 2002 establishing criteria and procedures for the acceptance of waste at landfills pursuant to Article 16 of and Annex II to Directive 1999/31/EC (OJ L 11, , p ), also referred to as the WAC Decision.
67 Reference number /2009/530302/ETU/G2 67 stored in appropriate underground or above-ground facilities. So far no waste code exists for elemental mercury on the European level. With regard to transport of liquid mercury, the containers and transport operations have to fulfil the specific requirements set for the different types of transports inter alia stated in the European Agreement Concerning the International Carriage of Dangerous Goods by Road (ADR), Regulations concerning the Transport of Dangerous Goods by Rail (RID), International Maritime Organisation (IMO) or International Air Transport Association (IATA). Waste containing mercury For waste containing mercury (not liquid), requirements concerning disposal exist at the European as well as at Member State levels. Directive 1999/31/EC together with Decision 2003/33/EC in particular lay down which requirements storage facilities (landfills) in general have to fulfil and which acceptance criteria in particular have to be fulfilled for a certain type of landfill. This includes technical standards, acceptance procedures, limit values, monitoring and control activities. More stringent protective measures at Member States level are possible. Several waste codes for waste containing mercury exist, depending on its source of origin listed in the European Waste Catalogue with the following EWC numbers: * wastes containing mercury from natural gas purification, * wastes containing mercury from inorganic chemical processes, * barium sulphate sludge containing mercury, * waste from gas cleaning containing mercury, * components containing mercury, * mercury-containing batteries, * construction and demolition wastes containing mercury, * fluorescent tubes and other mercury-containing waste. Apart from the specifically addressed mercury-containing waste other types of waste may also contain mercury or mercury compounds such as waste types specified as containing heavy metals or containing hazardous substances (Directive 2000/532/EC 26 ). 26 Commission Decision of 3 May 2000 replaces Decision 94/3/EC establishing a list of wastes pursuant to Article 1(a) of Council Directive 75/442/EEC on waste and Council Decision 94/904/EC establishing a list of hazardous waste pursuant to Article 1(4) of Council Directive 91/689/EEC on hazardous waste (notified under document number C(2000) 1147) (OJ L 226, , p. 3 24).
68 Reference number /2009/530302/ETU/G2 68 Apart from Regulation (EC) N 1102/2008, the following legal documents at the European level have to be considered for evaluating the storage requirements of metallic mercury and mercury containing waste as they are referred to in Regulation (EC) N 1102/2008: Directive 2006/12/EC 27 and Directive 2008/98/EC 28 ( Waste Framework Directives ) Directive 1999/31/EC ( Landfill Directive ), Decision 2003/33/EC ( WAC Decision ), Directive 1996/82/EC 29 ( Seveso II Directive ), Regulation (EC) N 1013/ ( Waste Shipment Regulation ), Directive 2004/35/CE 31 ( Environmental Liability Directive ). Directive 85/337/EEC 32 ( Environmental Impact Assessment Directive ) was also taken into account, though not mentioned in the Mercury Regulation, as certain storage facilities need to comply with this Directive. These documents are evaluated in the following chapter with regard to possible storage facilities for metallic mercury and mercury containing waste including above ground and underground facilities such as salt mines and deep underground hard rock formation. The evaluation focuses on the extraction of requirements for the various storage types laid down in the above-mentioned legislation. However, issues such as transport and handling of mercury and mercury waste will also be tackled within the following chapters. Further national legislation have been screened for additional requirements for mercury disposal and disposal of hazardous waste e.g. in underground facilities. 27 Directive 2006/12/EC of the European Parliament and of the Council of 5 April 2006 on waste (OJ L 114, , p. 9 21), also referred as Waste Framework Directive. 28 Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on waste and repealing certain Directives(OJ L 312, , p. 3 29), also referred as new Waste Framework Directive. 29 Council Directive 96/82/EC of 9 December 1996 on the control of major-accident hazards involving dangerous substances (OJ L 10, , p ) as amended by Directive 2003/105/EC; also referred to as the Seveso II Directive. 30 Regulation N o 1013/2006 of the European Parliament and of the Council of 14 June 2006 on shipments of waste (OJ L 190, , p.1-98), also referred to as the Waste Shipment Regulation. 31 Directive 2004/35/CE of the European Parliament and of the Council of 21 April 2004 on environmental liability with regard to the prevention and remedying of environmental damage (OJ L 143, , p ), also referred to as Environmental Liability Directive. 32 Council Directive 85/337/EEC of 27 June 1985 on the assessment of the effects of certain public and private projects on the environment (OJ L 175, , p ) with last amendment from 25 June 2003, also referred as Environmental Impact Assessment Directive.
69 Reference number /2009/530302/ETU/G Legal requirements for all storage facilities Regulation (EC) N 1102/2008 ( Mercury Regulation ) The Mercury Regulation sets specific requirements for the disposal of metallic mercury. In general, the provisions from the Landfill Directive and the WAC Decision are applicable with few exemptions for specific types of landfills. The requirements listed in Table 5-1are applicable for all types of mercury disposal facilities, while some specifications are made for above-ground facilities and for storage in salt-mines and deep underground hard rock formations (see chapters to 5.2.5). Table 5-1: Requirements for all types of mercury storage facilities according to Directive N EC 1102/2008 Requirements for all types of mercury storage facilities according to Regulation EC N 1102/2008 Requirement / source Objective [Regulation 1102/2008, Recital 6] Provisions [Regulation 1102/2008, Recital 8 and Article 3(1)] Containment [Regulation 1102/2008, Article 3 (1)] Safety assessment [Regulation 1102/2008, Article 4(1)] Visual inspection [Regulation 1102/2008, Article 4(2)] Specification Safe storage should be ensured All provisions of Directive 1999/31/EC shall apply; except Article 5(3)(a) (= not accepting liquid waste at landfills) Assuring financial security (provision in Article 8(a)(iv) of Landfill Directive) including period of closure and after care Directive 2004/35/CE on environmental liability applies to mercury storage facilities Storage of metallic mercury in appropriate containment Safety assessment for all mercury storage facilities Covering particular risks of metallic mercury and its containment arising from natural and long-term properties Permit for storage according to Landfill Directive shall include requirements for regular visual inspections of the containers, Installation of appropriate vapour detection equipment to detect leak Directive 2006/12/EC and Directive 2008/98/EC ( Waste Framework Directives ) The new Waste Framework Directive (Directive 2008/98/EC), which has to transposed into national law by the Member States by 12 December 2010 is repealing the old Waste Framework Directive (Directive 2006/12/EC) and incorporating and repealing the Hazardous Waste Directive 33 and the Waste Oil Directive Council Directive 91/689/EEC of 12 December 1991 on hazardous waste (OJ L 337, , 20 27) with last amendment from 19 November 2008, also referred as Hazardous Waste Directive. 34 Council Directive 75/439/689/EEC of 16 June 1975 on the disposal of waste oils (OJ L 194, , p ) as Waste Oil Directive.
70 Reference number /2009/530302/ETU/G2 70 The new Framework Directive requires more stringent waste reduction and waste prevention efforts. Member States must ensure that waste is recovered or disposed of without endangering human health and the environment and that the waste amount disposed of is reduced to a minimum by kind of measures and effective tools to minimise waste generation. Amongst other issues, the new WFD provides a further clarification and differentiation of the waste hierarchy, modifies definitions as regards e.g. the end-of-waste status, by-products and classification of treatment operations and changes requirements for the preparation of waste management plans. The Directive emphasizes new waste management targets, encourages waste reduction and gives a new dimension to prevention as Member States are obliged to draw up and implement waste prevention programmes not later than Also the producer responsibility is extended in order to strengthen the re-use, prevention as well as recycling and other recovery of waste. The New Waste Framework Directive also sets new recycling targets which have to be achieved by In addition, the Directive sets out more stringent provisions for authorisation and registration. With the new Waste Framework Directive, the Hazardous Waste Directive is repealed with effect from 12 December No reference in Mercury Regulation is therefore made to the Hazardous Waste Directive. However some parts of the Hazardous Waste Directive were incorporated into Directive 2008/98/EC, especially Annex III, describing properties of waste (which are classified as hazardous). Annexes I (describing categories or generic types of waste which are classified as hazardous) and Annex II (listing constitutes of waste which are classified as hazardous) are not incorporated in the new Waste Framework Directive. Directive 1999/31/EC ( Landfill Directive ) The Landfill Directive defines the relevant terms (e.g. waste, treatment options, and technical terms), sets the scope and the landfill classes, specifies the waste and treatment options acceptable and not acceptable at different landfill classes and explains the requirements for a permit, waste acceptance and the control and monitoring as well as the closure and after-care procedures. The focus of this report is only on the provisions being of relevance for the issue of mercury storage and the deviation of specific criteria for mercury storage. According to Directive 1999/31/EC, landfill means a waste disposal site for the deposit of waste onto or into land (i.e. underground), including [ ] a permanent site (i.e. more than one year) which is used for temporary storage of waste (Article 2 (g)) but excluding Facilities where waste is unloaded in order to permit its preparation for further transport for recovery, treatment or disposal elsewhere, and Storage of waste prior to recovery or treatment for a period of less than three years as a general rule, or
71 Reference number /2009/530302/ETU/G2 71 Storage of waste prior to disposal for a period of less than one year. According to Article 7 of Directive 1999/31/EC a landfill needs a permit, which contains information about the identity of the applicant and the operator, the description of the types and total quantity of waste, the proposed capacity, a description of the site, the proposed methods for pollution prevention and control and the proposed plan for the closure and after-care procedures. Furthermore an impact assessment following Council Directive 85/337/EEC might be required and has to be added to the permit. The provisions that all types of landfill have to fulfil are set out in Annex I of Directive 1999/31/EC and summarised in Table 5-2. Table 5-2: General requirements for all classes of landfills according to Directive 1999/31/EC, Annex I Requirements for all classes of landfills according to Directive 1999/31/EC, Annex I Requirement / source Location [Directive 1999/31/EC, Annex I, section 1] Specification Location of landfill must take into consideration requirements related to: Distance to residential and recreational areas, waterways, water bodies, agricultural and urban sites Existence of groundwater, coastal water or nature protection zones Geological and hydrological conditions Risk of flooding, subsidence, landslides or avalanches Protection of nature or cultural patrimony Landfill can only be authorised if requirements or measures indicate, that they do not pose a serious risk. Water and leachate management [Directive 1999/31/EC, Annex I, section 2] Measures (possible exceptions for inert landfills): Control water from precipitations entering into the landfill Prevent surface water and/or groundwater from entering Collect contaminated water and leachate (exception possible) Treat contaminated water and leachate Protection of soil and water [Directive 1999/31/EC, Annex I, section 3] Measures (possible exceptions for inert landfills): Landfill must be situated to prevent pollution to soil, groundwater, surface water and to ensure efficient collection of leachate Combination of geological barrier and bottom liner during passive phase/postclosure Combination of geological barrier and top liner during operational/active phase Geological barrier determined by geological and hydro-geological conditions below and in the vicinity of a landfill providing sufficient attenuation capacity to prevent a potential risk
72 Reference number /2009/530302/ETU/G2 72 Requirements for all classes of landfills according to Directive 1999/31/EC, Annex I Requirement / source Specification Landfill base and sites consist of mineral layer (specified for each class; artificial layer possible) Leachate collection and sealing system including artificial sealing liner and drainage layer (exception possible for inert waste) Surface sealing layer dependent on landfill class Gas control [Directive 1999/31/EC, Annex I, section 4] Nuisances and hazards [Directive 1999/31/EC, Annex I, section 6] Stability [Directive 1999/31/EC, Annex I, section 6] Barriers [Directive 1999/31/EC, Annex I, section 7] Control the accumulation and migration of landfill gas Collection and treatment of landfill gas (for landfills receiving biodegradable waste) Measures to minimise nuisance and hazards through emissions of odours and dust; wind-blown materials; noise and traffic; birds, vermin and insects; formation of aerosols; fires Stability of the mass of waste and associated structures; avoid slippages Where artificial barrier, geological substratum stable to prevent settlement that causes damage to barrier Secured to prevent free access Gates shall be closed outside operating hours System to detect and discourage illegal dumping Annex II of Directive 1999/31/EC prescribes general waste acceptance criteria proposing the following three-level hierarchy [Directive 1999/31/EC, Annex II, section 3]: Basic characterisation (each type of waste, exemption for waste types where impractical) o determination according to standardised analysis and behaviour-testing methods, o short and long-term leaching behaviour, o characteristic properties of the waste. Compliance testing (at regular intervals, at least once a year) o periodical testing by simpler standardised analysis and behaviour-testing methods, o determine whether a waste complies with permit conditions and/or specific reference criteria, o focus on key variables and behaviour identified by basic characterisation. On-site verification (each load of waste)
73 Reference number /2009/530302/ETU/G2 73 o rapid test methods to confirm that each shipment/load of waste is the same as in the basic characterisation and described in accompanying documents, o visual inspection of each load of waste before and after unloading at the landfill site. The acceptance procedures shall as far as possible be based on standardised waste analysing methods. Furthermore, they shall respect corresponding limit values for the properties of waste to be accepted. Therefore, Member States shall establish a national list of waste to be accepted or refused at each class of landfills. These lists shall be used to establish site specific lists [Directive 1999/31/EC, Annex II, section 2]. The general waste acceptance procedure is concretised by Decision 2003/33/EC, Annex including information on the function of each level of testing, the fundamental requirements, the testing methods and the limit values to be fulfilled. A first guideline to define what kind of waste should be accepted at each landfill class is given in the Landfill Directive [Directive 1999/31/EC, Annex II, section 4], summarised as follows: Table 5-3: Control and monitoring procedures for all classes of landfills according to Directive 1999/31/EC, Annex III Preliminary criteria for waste acceptance according to Directive 1999/31/EC, Annex II Landfill class Inert waste landfills Waste to be generally accepted Only inert waste as defined in Article 2(e) accepted (=not undergoing significant physical, chemical or biological transformations, not dissolving, burning, physically or chemically reacting, biodegrading, adversely affecting, no rise in environmental pollution or harm to human health, leachability and ecotoxicity of leachate insignificant) Non-hazardous waste landfills Hazardous waste landfills Only waste type not covered by Directive 91/689/EEC 35 Covered by Directive 91/689/EEC (preliminary list) Prior treatment required if contents or leachability is high enough to constitute short term occupational and environmental risk Additionally, the acceptance of waste at each landfill type depends on the leaching properties of the waste. Leaching limit values are defined for each class of landfills in the Annex of the WAC Decision (see description below). Wastes which contain mercury and mercury compounds are defined as hazardous waste as they are listed in entry C16 in Annex II of the Hazardous Waste Directive (when they fulfil the properties described in Annex III of the same Directive). The Hazardous Waste Directive will be incorporated 35 Council Directive 91/689/EEC of 12 December 1991 on hazardous waste (OJ L 337, , p. 20) with last amendment from 19 November 2008, also referred as Hazardous Waste Directive
74 Reference number /2009/530302/ETU/G2 74 and repealed by the new Waste Framework Directive (Directive 2008/98/EC) by 12 December The new Waste Framework Directive does not adopt the list of constitutes of the wastes. However, it adopts also in its Annex III the list of properties of waste which render it hazardous. In consequence following the new Waste Framework Directive wastes containing mercury and mercury compounds are defined as hazardous if fulfilling the properties listed in Annex III of Directive 2008/98/EC. In case of stable and non-reactive waste provision 2.3 of the Annex of Decision 2003/33/EC allows the disposal of hazardous wastes in landfills for non-hazardous waste if such wastes have been rendered stable and non-reactive. Stable, non-reactive means that the leaching behaviour of the waste will not change adversely in the long-term, under landfill design conditions or foreseeable accidents (Provision 2.3 of Annex of Decision 2003/33/EC): In the waste alone (for example, by biodegradation), Under the impact of long-term ambient conditions (for example, water, air, temperature, mechanical constraints), By the impact of other wastes (including waste products such as leachate and gas). Annex III of the Landfill Directive lays down control and monitoring procedures during operation as well as for the after-care phase of a landfill. The purpose of these procedures is to check that: the waste has been accepted to disposal in accordance with the criteria set for the category of landfill in question, the processes within the landfill proceed as desired, the environmental protection systems are functioning fully as intended, the permit conditions for the landfill are fulfilled [Annex III, point 1 of Directive 1999/31/EC]. The control and monitoring programmes have to cover the following areas: Table 5-4: Control and monitoring procedures for all classes of landfills according to Directive 1999/31/EC, Annex III Control and monitoring procedures for all classes of landfills according to Directive 1999/31/EC, Annex III Requirement / source Meteorological data [Directive 1999/31/EC, Annex III, section 2] Emission data [Directive 1999/31/EC, Annex III, section 3] Specification MS decide how to collect data (in situ, national, etc.) If required for evaluating leachate behaviour data of precipitation, temperature, wind, evaporation and atmospheric humidity data have to be collected according to given schedule Data on water, leachate and gas control including: collection of leachate and surface water if present (volume and composition) at representative points according to guidelines
75 Reference number /2009/530302/ETU/G2 75 Control and monitoring procedures for all classes of landfills according to Directive 1999/31/EC, Annex III Requirement / source Specification collection of surface water down- and up-stream gas monitoring representative for each section of landfill according to given frequency and analysis and according to permit Protection of groundwater [Directive 1999/31/EC, Annex III, section 4] Sampling at measuring points for groundwater at inflow and outflow region according to Sampling Guideline Monitoring based on parameters according to local conditions and level of groundwater according to given schedule Determination of trigger level for change of groundwater composition in permit Topography [Directive 1999/31/EC, Annex III, section 5] Data according to given schedule on: structure and composition of landfill setting behaviour of the landfill body Decision 2003/33/EC ( WAC Decision ) Decision 2003/33/EC lays down specific requirements which have to be fulfilled by storage facilities (landfills). Furthermore, the decision determines waste acceptance criteria for each type of waste to be accepted at a certain type of landfill. More stringent protective measures at Member States level are possible. This could be of particular relevance with reference to the limit values for cadmium and mercury (see introduction to Annex of Decision 2003/33/EC). The WAC Decision specifies in its Annex the procedure for waste acceptance at landfills as laid down in Annex II, section 3 of the Landfill Directive. The procedures are generally applicable for all types of landfills. The following procedures are specified: Table 5-5: Procedures for the acceptance of waste according to Decision 2003/33/EC, Annex Procedures for the acceptance of waste according to Decision 2003/33/EC, Annex Requirement / Source Function of the basic characterisation [Decision 2003/33/EC, Annex, section 1.1.1] (a) (b) (c) (d) Specification Basic information on the waste ( type and origin, composition, consistency, leachability and where necessary and available other characteristic properties) Basic information for understanding the behaviour of waste in landfills and options for treatment as laid out in Article 6(a) of the Landfill Directive Assessing waste against limit values Detection of key variables (critical parameters) for compliance testing and options for simplification of compliance testing (leading to a significant decrease of constituents to be measured, but only after demonstration of relevant
76 Reference number /2009/530302/ETU/G2 76 Procedures for the acceptance of waste according to Decision 2003/33/EC, Annex Requirement / Source Fundamental requirements for basic characterisation [Decision 2003/33/EC, Annex, section 1.1.2] Compliance testing [Decision 2003/33/EC, Annex, section 1.2] On-site verification [Decision 2003/33/EC, Annex, section 1.3] Specification information). Characterisation may deliver ratios between basic characterisation and results of simplified test procedures as well as frequency for compliance testing. Necessary data: (a) Source and origin of waste (b) Information on process producing waste (description, characteristics of raw materials and products) (c) Description of pre-treatment applied, or statement why no treatment (d) Data on composition of waste and leaching behaviour, where relevant (e) Appearance of waste (smell, colour, physical form) (f) Code according to the European waste list 36 (Commission Decision 2001/118/EC) (1) (g) Relevant hazard properties ( Annex III to Hazardous Waste Directive) for mirror entries (h) Information to prove that the waste does not fall under the exclusions of Article 5(3) of the Landfill Directive (liquid, explosive, corrosive etc.) (i) Landfill class at which the waste maybe accepted (j) If necessary, additional precautions to be taken at landfill (k) Check if waste can be recycled or recovered Parameters, scope and frequency of testing determined in basic characterisation (key variables) Same testing method as in basic characterisation Keeping of records Visual inspection of waste before and after unloading at the landfill Same waste as described in accompanying documents / same as basic characterisation The extent of laboratory testing between basic characterisation and compliance testing is dependent on the type of waste and if the waste is regularly generated within the same process or not. For waste regularly generated, information on compositional range, variability of properties and key variables are also necessary. Waste does not need testing if it is produced within the same process in the same installation or within a defined and already tested process. There is also no testing required, if the necessary information is well known and duly justified [Annex, Section 1.3 (a) and (b), Decision 2003/33/EC]. 36 Decision 2001/118/EC amending Decision 2000/532/EC as regards the list of wastes, 16 January 2001 (OJL 47, , p. 1-31)
77 Reference number /2009/530302/ETU/G2 77 In addition, short lists with EWC codes according to the European waste list are established by the WAC Decision for wastes acceptable without testing. The acceptances of all other wastes primarily depend on their leaching properties, which are laid down in the Annex to the WAC Decision [Section 2.1 to 2.4.]. The storage options of mercury containing waste depend mainly on the leaching limit values for Hg as summarized for each landfill type in the following table: Table 5-6: Mercury leaching limit values for different landfill types and standards according to Decision 2003/33/EC Mercury leaching limit values for different landfill types according to Decision 2003/33/EC, Annex Landfill type L/S =2 l/kg mg/kg dry substance L/S =10 l/kg mg/kg dry substance C 0 (percolating test) mg/l Criteria for landfills for inert waste Criteria for granular non-hazardous waste accepted in the same cell as stable non-reactive hazardous waste Criteria for hazardous waste acceptable at landfills for non-hazardous waste Criteria for waste acceptable for landfills for hazardous waste Member States have the possibility to determine more stringent requirements (such as more stringent leaching limit values, see chapter 5.3). In general, the limit values given are valid for all kinds of storage facilities. However, in the case of hazardous waste disposed of in underground disposal facilities, the leaching limit values are not valid. In such cases the waste has to be compliant with the site specific safety assessment [Annex, point 2.5 of Decision 2003/33/EC]. The sampling and test methods which have to be used to determine the leachability are set in Section 3 of the Annex to the Decision 2003/33/EC. Leaching tests have to be made in accordance with EN 12457/1-4 Leaching-Compliance test for leaching of granular waste materials and sludges. (Part 1: L/S=2 l/kg particle size <4mm; Part 2: L/S=10 l/kg particle size < 4mm; Part 3: L/S=2 and 8 l/kg particle size <4mm and Part 4: L/S=2 l/kg particle size <10mm). The WAC Decision foresees that Member States have to set criteria for monolithic waste to provide the same level of environmental protection as for granular waste. In many Member States the crunched monolithic waste has to fulfil the same leaching limit values as the granular waste. The percolating test has to be carried out in accordance with pren (CEN/TS 14405:2004) Leaching behaviour test Up flow percolation test for inorganic constituents. Each of the Member States has to decide which of the leaching tests shall be used. Mercury containing waste which exceeds the indicated limit value set for a specific type of landfill has to be treated again to reduce the content of mercury or to be stabilised to reduce the
78 Reference number /2009/530302/ETU/G2 78 leachability. Apart from the general requirements, the WAC Decision contains specific requirements for underground disposal facilities (see chapter 5.2.3) and for salt mines (see chapter 5.2.4) and hard rock formation (see chapter 5.2.5). Regulation (EC) N 1013/2006 ( Waste Shipment Regulation ) If only a few storage sites for metallic mercury within the European Union would be in operation, mercury has to be transported throughout Europe. For such waste transports the Waste Shipment Regulation has to be complied with. It states that shipments of waste destined for disposal operation shall be subject to prior written notification and consent procedure [Article 3(1)(a) of Regulation (EC) N 1013/2006]. Recital 10 of the Mercury Regulation makes it clear that the Mercury Regulation should be without prejudice to the Waste Shipment Regulation. This means that transboundary transports of mercury destined for disposal within the European Union have to follow the notification procedures. The notifier has to submit the notification document to the competent authority in the country of dispatch the country from which the shipment is planned to be initiated [Article 4 of Regulation (EC) N 1013/2006]. Then the competent authorities at the point of dispatch and destination and in the case of transit, the competent authority/authorities of the transit countries, have to agree to the shipment (consent) or not agree to the shipment (objection). In general, one reason for objection for a shipment of waste destined for disposal which can be raised by the authorities of dispatch and destination is laid down in Article 11(1)(a) of the Waste Shipment Regulation. The Article refers to: the principles of proximity, priority for recovery and the principle of self-sufficiency as defined in Directive 2006/12/EC 37. If one of the authorities believes that one or more of the principles is not being complied with it can make a reasoned objection. The principles are alike defined in Directive 2008/98/EC (new Waste Framework Directive) which repeals Directive 2006/12/EC and which has to be transposed into national legislation by the Member States by 12 December In the case of a disposal of mercury, Recital 10 of the Mercury Regulation now encourages the authorities of dispatch and destination to avoid raising such reasoned objections based on the listed 37 Directive 2006/12/EC of the European Parliament and of the Council of 5 April 2006 on waste (OJ L 114, , p. 9 21), also referred as Waste Framework Directive.
79 Reference number /2009/530302/ETU/G2 79 principles outlined in Article 11(1)(a) of the Waste Shipment Regulation. The article is not applicable for shipments of metallic mercury and certain mercury compounds destined for disposal in another EU Member State. As a justification, the Mercury Regulation refers to Article 11(3) of the Waste Shipment Regulation stating that where the production of wastes in very small quantities and the setting up of a new specialised installation is uneconomic, Article 11(1)(a) will not apply. However, reasoned objections by the competent authority of dispatch might be based on Article 11(1)(b) interference with national legislation related to environmental protection, public order, public safety or health protection of the country or Article 11(1) (e) right of Member States pursuant to Article 4(1) of the Basel Convention to prohibit import of hazardous waste or of waste listed in Annex II (household residues, residues from incineration of household wastes). In consequence, all procedures and requirements of the Waste Shipment Regulation are applicable for the shipment of mercury and mercury-containing waste but reasoned objections based on Article 11(1)(a) cannot be made by the authorities. Directive 2004/35/EC 38 ( Environmental Liability Directive ) The objective of the Directive on environmental liability with regard to the prevention and remedying of environmental damage is to establish a common framework for the prevention and remedying of environmental damage at a reasonable cost to society. It applies to occupational activities which present a risk for environmental damage (land, water, protected species and natural habitats), or human health [Recital 8 and 9 as well as Article 2 (1) of Directive 2004/35/EC]. As laid down in the Mercury Regulation, it applies also to all storage facilities for metallic mercury [Recital 8 of Regulation EC N o 1102/2008]. The criteria for measuring damage are laid down in Annex I of the Directive. By implementing the polluter pays principle, an operator causing environmental damage or creating an imminent threat of such damage shall, in principle, bear the cost of the necessary preventive or remedial measures. In cases where a competent authority acts by itself or through a third party (in the place of an operator) the authority shall ensure that the cost incurred is recovered from the operator [Recital 18 and Article 8(1) of Directive 2004/35/EC]. For such purposes the competent authority may require the operator to provide information on any imminent threat or suspicion of threat of environmental damage and to take necessary preventive measures. Additionally, the authority may give to the operator instructions to be followed on a necessary preventive measure or to take the measure itself [Article 5 (3) of Directive 2004/35/EC]. Annex II of the Directive lays down the framework to choose the appropriate remediation measure. Member States shall establish financial security instruments and financial guarantees enabling the 38 Directive 2004/35/EC of the European Parliament and of the Council of 21 April 2004 on environmental liability with regard to the prevention and remedying of environmental damage (OJ L 143, , p ), also referred as Environmental Liability Directive.
80 Reference number /2009/530302/ETU/G2 80 operators to cover their responsibilities under the Directive [Article 14 of Directive 2004/35/EC]. Directive 85/337/EEC ( Environmental Impact Assessment Directive ) The Directive on Environmental Impact Assessment (EIA) was introduced in 1985 and was amended in Member States had to transpose the amended EIA Directive by 14 March 1999 at the latest. The EIA procedure ensures that environmental consequences of projects are identified and assessed before authorisation is given. The public can give its opinion and all results are taken into account in the authorisation procedure of the project. The EIA Directive outlines which project categories shall be made subject to an EIA, which procedure shall be followed and the content of the assessment. In terms of waste disposal, Annex I, 9 and 10 lays down, that the following facilities require an EIA: Waste disposal installations for the incineration, chemical treatment as defined in Annex IIA to Directive 75/442/EEC 39 under heading D9, or landfill of hazardous waste (i.e. waste to which Directive 91/689/EEC 39 applies). Waste disposal installations for the incineration or chemical treatment as defined in Annex IIA to Directive 75/442/EEC 39 under heading D9 of nonhazardous waste with a capacity exceeding 100 tonnes per day. That means, that landfill for hazardous waste do require an EIA. Also installations for incineration or chemical treatment (operation D9) for hazardous waste and for non-hazardous waste if exceeding the capacity of 100 t/day do require an EIA. For operations listed in Annex II Member States shall determine though a case-by-case examination or/and the setting of thresholds or criteria which projects listed shall be subject to an EIA. Criteria which could be used are listed as a proposal in Annex III. Article 5 in connection with Annex VI includes the information to be provided by the developer of the project Legal requirements for above-ground storage facilities Regulation (EC) N 1102/2008 ( Mercury Regulation ) Metallic mercury may be temporarily stored for more than one year in above-ground facilities [Article 3(1)(b) of Regulation (EC) N 1102/2008]. The above-ground storage of metallic mercury shall be considered as a temporary solution [Recital 12 of Regulation (EC) N 1102/2008] Requirements to be applied for all mercury storage facilities are listed in Table References to repealed Directives shall be construed as references to new Directives in accordance with the correlations table, thus meaning Directive 2006/12/EC Annex IIA and Directive 2008/98/EC Annex I.
81 Reference number /2009/530302/ETU/G2 81 Table 5-7: Requirements for above ground mercury storage according to Regulation (EC) N 1102/2008 Requirements for above ground mercury storage according to Regulation (EC) N 1102/2008 Requirement / source Provisions [Regulation 1102/2008, Recital 7] Seveso II Directive [Regulation 1102/2008, Recital 9 and Article 3 (1)] Equipment Specification All provisions of Directive 1999/31/EC shall apply; except Article 5(3)(a) = not accepting liquid waste at landfills All provisions of Decision 2003/33/EC shall apply, except criteria set out in section 2.4 of the Annex (criteria for waste acceptable at landfills for hazardous waste) Above-ground storage for more than one year in facilities dedicated to and equipped for this purpose Council Directive 96/82/EC of 9 December 1996 on the control of major-accident hazards involving dangerous substances should apply Dedicated and equipped for the temporary storage of mercury [Regulation 1102/2008, Recital 9] Safety assessment [Regulation 1102/2008, Recital 11] Principles [Regulation 1102/2008, Recital 12] Containment [Regulation 1102/2008, Article 3 (1)] Reversibility [Regulation 1102/2008, Article 3 (1)(b)] Safety assessment required under WAC Decision for underground storage applicable also for above ground storage Complemented by specific requirements No final disposal operation permitted until special requirements and acceptance criteria are adopted Principle of reversibility of storage Protection of mercury against meteoric water Impermeability towards soils Prevention of vapour emissions of mercury Temporary storage in above-ground facilities in appropriate containment Facility has to be dedicated to and equipped for temporary storage of metallic mercury Directive 1999/31/EC ( Landfill Directive ) / Decision 2003/33/EC ( WAC Decision ) Neither the Landfill Directive nor the WAC Decision provide specifications on disposal exclusively for above ground storage. The defined requirements are valid for all types of storage, including above and underground storage. For the deposit of liquid mercury, an exemption is made for Article 5 (3) (a) of the Landfill Directive in which liquid waste is not to be accepted at landfills. Additionally, Section 2.4 of the Annex to the WAC Decision, setting out the criteria for waste acceptable at landfills for hazardous waste, will not apply for temporary storage of mercury in above ground facilities [Recital 7 and Article 3(1)(b) of Regulation (EC) N 1102/2008]. Furthermore, Recital 11 of the Mercury Regulation states that the safety assessment laid down in the
82 Reference number /2009/530302/ETU/G2 82 WAC Decision required for underground storage (see Table 5-9) shall also be applicable to nonunderground mercury disposal. Directive 1996/82/EC ( Seveso II Directive ) Recital 9 and Article 3(1) of Regulation (EC) N 1102/2008 states that for temporary storage of metallic mercury for more than one year in above-ground facilities, Council Directive 96/82/EC on the control of major-accident hazards involving dangerous substances shall apply. The Seveso II Directive aims at the prevention of major accidents that involve dangerous substances and the limitation of their negative consequences for humans and the environment [Article 1 of Directive 96/82/EC] by requiring the operator of facilities and establishments where dangerous substances are involved, to notify the establishment to a competent authority. The notification includes information on the location of the facility, the responsible person, dangerous substances or category of substances involved, etc. [Article 6 of Directive 96/82/EC]. Additionally, the operator has to prepare the following documents: A major-accident prevention policy laid down in a safety report (including information on the safety management system, the major hazards arising from the operation, operational control and maintenance measures, planning for emergencies, monitoring performance, etc.) [Article 7 and 9 and Annex III of Directive 96/82/EC]; An emergency plan (including internal emergency plans made known to the staff of the facility and external plans to be made known to the general public) [Article 11 and Annex IV of Directive 96/82/EC]. The safety report has to be reviewed at least every five years or earlier where justified by new facts; development of new technical knowledge etc. [Article 9(5) of Directive 96/82/EC]. Guidance on the preparation of such safety reports is available, e.g. at [Seveso Guidance 2005]. For the disposal of mercury in above ground storage, the requirements listed in Table 5-8 are of relevance for the planning of the facility. Table 5-8: Requirements for mercury storage in above-ground disposal facilities according to Directive 1996/82/EC Requirements for mercury storage in above-ground disposal facilities according to Directive 1996/82/EC Requirement / source Adaptation of site [Directive 1996/82/EC, Recital 17 and Article 9] Specification By means of a safety report it shall be demonstrated that: a major-accident prevention policy and a safety management system for implementing have been put into effect in accordance with the information set out in Annex III; major-accident hazards have been identified and the necessary measures have been taken to prevent such accidents and to limit their consequences for man and the environment;
83 Reference number /2009/530302/ETU/G2 83 Requirements for mercury storage in above-ground disposal facilities according to Directive 1996/82/EC Requirement / source Domino effect [Directive 1996/82/EC, Recital 18] Location [Directive 1996/82/EC, Recital 22] Specification adequate safety and reliability have been incorporated into the design, construction, operation and maintenance of any installation, storage facility, equipment and infrastructure connected with its operation which are linked to major-accident hazards inside the establishment; internal emergency plans have been drawn up and information has been supplied to enable the external plan to be drawn up in order to take the necessary measures in the event of a major accident; In addition the safety report shall provide sufficient information to the competent authorities to enable decisions to be made in terms of the siting of new activities or developments around existing establishments. In order to reduce the risk of domino effects, where establishments are sited so close together so as to increase the probability and possibility of major accidents, or aggravate their consequences, there should be provision for the exchange of appropriate information and cooperation on public information Suitable distance between areas of: substantial public use areas of particular natural interest or sensitivity Taking account of additional technical measures so that the risk to individuals is not increased Legal requirements for underground disposal Regulation (EC) N 1102/2008 ( Mercury Regulation ) Another possibility for the storage of metallic mercury is the storage in a hazardous underground facility permitted for the disposal of mercury containing waste. For such facilities, the requirements for all mercury storage facilities (see Table 5-1) have to be applied. The safety assessment as described in Decision 2003/33/EC for underground storage is applicable for the underground storage of mercury. There are no further specifications for disposal in underground facilities. A few specifications however are made for underground disposal in salt mines (see chapter 5.2.4) and in hard rock formations (see chapter 5.2.5). Directive 1999/31/EC ( Landfill Directive ) The Landfill Directive defines underground storage as permanent waste storage facilities in a deep geological cavity such as a salt or potassium mine [Article 2 (g)]. It is also defined that landfill as disposal onto or into land includes underground storage [Article 2 (f) of Directive 1999/31/EC]. Therefore, in general, the provisions of the Landfill Directive apply also to underground disposal. However, Member States can declare for underground storage exemptions regarding the following
84 Reference number /2009/530302/ETU/G2 84 provisions [Article 3 (5) of Directive 1999/31/EC]: Monitoring and analysing of landfill gas (provision in Article 13(d), Annex I, point 4 and Annex III, point 3), Water control and leachate management (provision in Article 13(d), Annex I, point 2 and Annex III, point 3), Measures to protect soil, groundwater or surface water and collection of leachate (Annex I, point 3 and Annex III, point 4), Measures to minimise nuisance and hazards (Annex I, point 5), Reporting of meteorological data (Annex III, point 2), Data on the structure and composition of the landfill body and settling behaviour (provision of Annex III, point 5). The control of water from precipitations entering into the landfill body has to be performed for underground disposal as well, because the first indent of Article 13 (d) is excluded from the exemption. Article 16 and Annex II, point 1 of the Landfill Directive proposes that the development of specific criteria, test methods and associated limit values shall be set for each landfill class, including specific landfill types such as underground storage. These specifications are set out in the WAC Decision. Decision 2003/33/EC ( WAC Decision ) For underground storage facilities no leaching limits are established in Decision 2003/33/EC. The acceptance of waste though in underground storage facilities depends on a site-specific safety assessment which is obligatory for each underground storage facility. Waste may be only accepted if it is compatible with the assessment. For the underground storage of inert waste and non-hazardous waste, the same leaching limit values as for other landfills apply. In case of storage of hazardous waste in underground storage, such leaching limit values do not apply. Only the compliance with the site-specific safety assessment is of relevance. The acceptance procedures however are the same as for other landfill types [Annex, point 2.5]. The procedure for carrying out a site-specific safety assessment is defined in Appendix A of Decision 2003/33/EC. It includes: 2. Safety philosophy for underground storage: all types, 3. Acceptance criteria for underground storage: all sites, 4. Additional considerations: salt mines,
85 Reference number /2009/530302/ETU/G Additional considerations: hard rock. The safety philosophy chapter covers the importance of the geological barrier and demands the permanent isolation of wastes from the biosphere by such a barrier. To demonstrate the long-term safety of the installation and prevent the discharge of pollutants into the groundwater, a site-specific risk assessment has to be carried out. The specifications to the risk assessment analysis set out in Appendix A, section 1 of Directive 2003/33/EC are highlighted and described in Table 5-9. Table 5-9: Site specific risk assessment for underground disposal according to Decision 2003/33/EC, Appendix A Site-specific risk assessment for underground disposal according to Decision 2003/33/EC, Appendix A Requirement / source Specification Identification The deposited waste ( the hazard ) [Decision 2003/33/EC, Appendix A, The biosphere and possibly groundwater ( the receptor ) point 1.2, para 1] The pathway by which the substances may reach the biosphere, and The assessment of impact of substances that may reach the biosphere Analysis of host rock Analysis of host rock [Decision 2003/33/EC, Appendix A, Taking into account conditions stated in Annex I, point 1, 6, 7 of point 1.2, para 2] Directive 1999/31/EC referring to location, stability and barriers Demonstration of suitability of strata for establishing storage Acceptance criteria Referring to local conditions [Decision 2003/33/EC, Appendix A, Taking into account overall system of waste, engineered structures point 1.2, para 3] and cavities and host rock body Time and measures Assessment for operational and post-operational phase [Decision 2003/33/EC, Appendix A, Development of control and safety measures and waste acceptance point 1.2, para 4] criteria Geological assessment Investigation of: [Decision 2003/33/EC, Appendix A, rocks, soils, topography point 1.2.1] location, frequency and structure of faults and fractures in surrounding geological strata Seismic activity Alternative location Geomechanical assessment Demonstrate that: [Decision 2003/33/EC, Appendix A, no risk of major deformation that could impair the operability point 1.2.2] no risk of major deformation that could provide a pathway to the biosphere no risk of collapse during operation deposited material has necessary stability compatible with the properties of the host rock Hydrogeological assessment Investigation of: [Decision 2003/33/EC, Appendix A, Hydraulic properties and groundwater flow patterns point 1.2.3] hydraulic conductivity of the rock mass fractures
86 Reference number /2009/530302/ETU/G2 86 Site-specific risk assessment for underground disposal according to Decision 2003/33/EC, Appendix A Requirement / source Specification hydraulic gradients Geochemical assessment Investigation of: [Decision 2003/33/EC, Appendix A, present groundwater composition and potential evolution over point 1.2.4] time nature and abundance of fracture-filling mineral rock composition Biosphere impact assessment Baseline studies to define local natural background levels of [Decision 2003/33/EC, Appendix A, relevant substances point 1.2.5] Assessment operational of phase Demonstrate that there is/are: [Decision 2003/33/EC, Appendix A, stable cavities point 1.2.6] no unacceptable risk of pathways between waste and biosphere no unacceptable risk affecting the operation of the facility no reaction with rock in any physical or chemical way, which could impair strength and tightness of rock Identification of: waste inventory, facility management, scheme of operation accidents leading to pathway to biosphere operational risks Development of: contingency measures Long-term assessment Investigation of: [Decision 2003/33/EC, Appendix A, barriers, e.g. waste quality, engineered structures, back filling, point 1.2.7] sealing performance of host rock surrounding strata and overburden groundwater flow, barrier efficiency, natural attenuation, leaching of waste changes over geological time scenarios of consequences on release of waste reflecting long-term evolution of biosphere, geosphere and underground storage (not taking into account containers/lining due to limited lifetime) Assessment of the surface Reception facilities must be designed and operated to: reception facilities prevent harm to human health [Decision 2003/33/EC, 1.2.8] prevent harm to the local environment fulfil same requirements as other waste reception facilities Assessment of other risks separation of waste disposal from other mining activities [Decision 2003/33/EC, Appendix A, no acceptance of hazardous substances which harm human health point 1.2.9] (e.g. pathogenic germs) Section 2 of Appendix A of the WAC Decision includes an overview of waste types which are excluded from underground storage and considerations related to the acceptance of waste for underground
87 Reference number /2009/530302/ETU/G2 87 storage. Wastes that may undergo undesired physical, chemical or biological transformation after it has been deposited are generally excluded from underground storage. Liquid waste is in general also excluded from storage in underground disposal (Appendix A, 2.1 of Decision 2003/33/EC with reference to Article 5(3) of Directive 1999/31/EC). Wastes not excluded by this list are suitable for underground storage including inert wastes, nonhazardous and hazardous wastes. In general, the acceptance of waste is based on the site-specific risk assessment demonstrating the level of isolation from the biosphere (see table: Table 5-9). Member States may also establish a list of wastes which are acceptable at underground storage facilities [Appendix A, 2.2 of Decision 2003/33/EC]. Sections 3 and 4 include some further considerations related to the safety of underground storage in salt mines and hard rock, described in more detail in chapter and Additional considerations for salt mines Regulation (EC) N 1102/2008 ( Mercury Regulation ) Another possibility for the storage of metallic mercury is the storage in salt mines, as salt is considered to provide total containment. For a salt mine, permitted for the storage of mercury, the requirements for all mercury storage facilities (see Table 5-1) have to be applied. Additionally, some further requirements are made, listed in Table Table 5-10: Requirements for mercury storage in salt mines according to Directive N EC 1102/2008 Requirements for mercury storage in salt mines according to Regulation EC N 1102/2008 Requirement / source Safety assessment [Regulation 1102/2008, Recital 11] Reversibility Specification Safety assessment required under WAC Decision for underground storage also for salt mines Adapted for the disposal of metallic mercury Meet principle of protection of groundwater against mercury Prevention of vapour emissions of mercury Impermeability to gas and liquids of the surroundings Firmly encapsulating the waste at the end of the mine s deformation process (for permanent storage) Facility has to be adapted for storage of metallic mercury [Regulation 1102/2008, Article 3 (1)(a)]
88 Reference number /2009/530302/ETU/G2 88 Directive 1999/31/EC ( Landfill Directive ) Apart from the definition in Article 2 of Directive 1999/31/EC stating that salt mines may be used as underground storage facilities, the Landfill Directive does not provide specifications on disposal in salt mines. Decision 2003/33/EC ( WAC Decision ) In addition to the requirements set in Appendix A of Decision 2003/33/EC, section 1 and 2 referring to the safety assessment and the acceptance criteria (see chapter 5.2.3), section 3 refers especially to disposal in salt mines including the requirements specified in Table Table 5-11: Requirements for salt mines according to Decision 2003/33/EC Requirements for salt mines according to Decision 2003/33/EC, Appendix A Requirement / source Specification Encapsulation Rock surrounding the waste acts as host rock in which waste is [Decision 2003/33/EC, Appendix A, encapsulated 3.1, para 1] Geological barrier The storage site must be located between overlying and [Decision 2003/33/EC, Appendix A, underlying impermeable rock strata to prevent groundwater 3.1, bullett 2] from entering and liquids and gases from escaping Shafts and boreholes must be sealed during operation Shafts and boreholes must be hermetically closed after operation Disposal area must be sealed with a hydraulically impermeable dam (according to calculated hydraulically operative pressure corresponding to depth) when mineral extraction still ongoing Failure scenarios Salt is considered to provide total containment [Decision 2003/33/EC, Appendix A, Cases of accidents where waste can come into contact with the 3.1, bullet 3] biosphere have to be assessed Events in geological time (earth movements, erosion e.g. associated with sea-level rise) have to be assessed Long-term assessment Designation of the salt rock as barrier rock for the long term [Decision 2003/33/EC, Appendix A, assessment 3.2, para 1] Stability and Integrity Stability of the host rock has to be assured during the operation [Decision 2003/33/EC, Appendix A, phase 3.2, para 2] The integrity of the geological barrier has to be assured over unlimited time Subsidence of the overburden or other defects are acceptable only if rupture-free transformation will occur
89 Reference number /2009/530302/ETU/G Additional considerations for hard rock Regulation (EC) N 1102/2008 ( Mercury Regulation ) For the disposal of mercury in hard rock formations, the requirements for all mercury storage facilities (see Table 5-1) have to be applied. Some additional requirements are outlined in the Mercury Regulation listed in Table Table 5-12: Requirements for mercury storage in hard rock formations according to Directive N EC 1102/2008 Requirements for mercury storage in hard rock formations according to Regulation EC N 1102/2008 Requirement / source Safety assessment [Regulation 1102/2008, Recital 11] Containment [Regulation 1102/2008, Article 3 (1)] Reversibility [Regulation 1102/2008, Article 3 (1)(a)] Specification Safety assessment required under the WAC Decision for underground storage also for deep underground hard rock formations Adapted for the disposal of metallic mercury Meet principle of protection of groundwater against mercury Prevention of vapour emissions of mercury Impermeability to gas and liquids of the surroundings Firmly encapsulating the waste at the end of the mine s deformation process (for permanent storage) Storage in deep underground hard rock formations in appropriate containment Providing a level of safety and confinement equivalent to salt mines Directive 1999/31/EC ( Landfill Directive ) The Landfill Directive does not provide specifications on disposal in hard rock formations. Decision 2003/33/EC ( WAC Decision ) According to Decision 2003/33/EC deep storage in hard rocks is defined as an underground storage at several hundred metres depth, where hard rock includes various igneous rocks, e.g. granite or gneiss or also sedimentary rocks, e.g. limestone and sandstone. In addition to the requirements set out in Appendix A of Decision 2003/33/EC, section 1 and 2 referring to the safety assessment and the acceptance criteria (see chapter 5.2.3), section 4 refers especially to the disposal in hard rock formations including the requirements specified in Table 5-13.
90 Reference number /2009/530302/ETU/G2 90 Table 5-13: Requirements for deep storage in hard rocks according to Decision 2003/33/EC Requirement for deep storage in hard rock according to Decision 2003/33/EC, Appendix A Requirement / source Definition [Decision 2003/33/EC, Appendix A, 4] Construction [Decision 2003/33/EC, Appendix A, 4.1 para 1] Safety philosophy [Decision 2003/33/EC, Appendix A, 4.1 para 2] Safety philosophy [Decision 2003/33/EC, Appendix A, 4.1 para 3] Groundwater [Decision 2003/33/EC, Appendix A, 4.1 para 4] Gas formation [Decision 2003/33/EC, Appendix A, 4.1 para 5] Specification Site must be located in hard rock several hundred metres in depth Composed of hard rock including igneous rocks and sedimentary rocks To be passive with no need for maintenance Allows recovery of waste and future corrective measures No negative environmental effects or liabilities should fall upon future generations Isolation of the waste from biosphere, natural attenuation of any pollutants leaking from the waste Extended periods of time (several thousands of years) Former mines where mining activity has come to an end New storage facilities The storage site has to be constructed so that natural attenuation of the surrounding strata mediates the effect of pollutants to the extent that they have no irreversible negative effects on the environment The storage site must be located below the groundwater table No direct discharge of pollutants into the groundwater Prevent deterioration of the status of all bodies of groundwater Assessment of paths to and in the biosphere and impact of variability on the geohydraulic system Gas formation must be considered
91 Reference number /2009/530302/ETU/G Legislation at Member State level National legislation on mercury and mercury-containing waste A good overview of existing national legislation related to mercury and mercury containing waste exceeding EU legislation is provided by [COWI 2008] (section 5). The legislation about mercury waste requirements in the Member States concentrates on the following issues: Requirements on treatment methods of mercury-containing waste, e.g. lamps, equipment or amalgam residues (AT); Restriction of the concentration of mercury in certain products and materials, e.g. engine oils and compost (AT); Classification of mercury-containing products as hazardous waste, e.g. thermometers, electrical equipment, batteries etc. (AT); Restriction of incineration and co-incineration of waste containing mercury (AT, BE, FR); Prohibition of mixing mercury-containing wastes with other wastes for preparation of a mix principally used as a fuel or other means to generate energy (NL); Emission limit values for mercury from crematoria (BE); Requirements for the handling and treatment of dental amalgam (AT, BE, UK); Restriction of export of waste containing mercury (NL, SE, UK, FI, SE). Additionally, some Member States have set specific requirements for the treatment, landfilling and storage of mercury and mercury-containing waste, which are listed in more detail in Table Table 5-14: Overview of Member State legislation concerning mercury and mercury-containing waste Description of Member State legislation for mercury and mercury-containing waste Scope MS Description Legislation Ban on landfilling of BE Landfilling of mercury is prohibited (in Flanders) [VLAREM 1995] mercury Ban on landfilling of mercury- containing NL waste Restriction of landfilling Ban on mercury-containing waste, by-products, measuring and control equipment, e.g. thermometers [COWI 2008] and batteries containing mercury Mercury limit value for landfilling is 1-20 mg/kg TS depending on landfill class of mercury- containing AT Exception: mercury as sulphide: 3,000 mg/kg TS waste Waste exceeding limit values has to be decontaminated or stored in underground landfills [DeponieVO 2008] BE Mercury limit value for landfilling is 0.5% of organic or inorganic compounds (in Flanders) Exception: mercury as sulphide [VLAREM 1995]
92 Reference number /2009/530302/ETU/G2 92 Description of Member State legislation for mercury and mercury-containing waste Scope MS Description Legislation In practice, threshold value of 100 mg/kg (also limit for toxic waste in BE) FI Mercury limit value for industrial waste deposit area is < 40 ppm Waste above limit value must be deposited in landfills or hazardous landfills (special permission) Supplementary requirements for solubility of mercury from wastes in landfills [COWI 2008] Mercury containing waste that exceeds 0,1 % by SE weight shall be finally disposed in underground [personal storage. information Mr. The rules mentioned above do not apply for mercury Carl Mikael waste that is covered by the Regulation (EC) Strauss, 1102/2008. Swedish EPA] From the 1 of January 2010 it is possible to grant an exemption from the rules of underground storage. FI Mercury-containing waste is neutralised or treated in well-controlled sulphidation reactor before landfilling [COWI 2008] Stabilisation using hydraulic binders is required on the [COWI 2008], Treatment of mercurycontaining waste going Stabilisation/solidification landfilling of hazardous leachable fraction for storage in landfills. FR [FNADE/ADEME 2006] to landfills waste Lowest standard for permitting waste treatment NL installations is separating mercury and recovering other fractions e.g. metals, glass [COWI 2008] Treatment of mercurycontaining waste going FR Solidification is required for storage in salt mines [COWI 2008] to salt mines Treatment of mercurycontaining waste going to bedrock SE Specific requirements for pre-treatment are under development, including solidification and stabilisation as mercury sulphide [COWI 2008] National legislation on leachate limit values of mercury Directive 1999/31/EC together with the WAC Decision lay down which requirements storage facilities (landfills) in general have to fulfil and which acceptance criteria waste has to fulfil to be accepted at a certain type of landfill. More stringent protective measures at Member States level are possible and are mentioned in particular in the Annex of Decision 2003/33/EC (introduction): In accordance with Article 176 of the Treaty, Member States are not prevented from maintaining or introducing more stringent protective measures than those established in this Annex, provided that such measures are compatible with the Treaty. Such measures
93 Reference number /2009/530302/ETU/G2 93 shall be notified to the Commission. This could be of particular relevance with reference to the limit values for cadmium and mercury in section 2. Most of the EU Member States adopted the limit values set in the WAC Decision, while only a few countries implemented more stringent and additional leaching limit values for mercury. Those stricter values are summarised in Table Table 5-15: Member States mercury leaching limit values for landfills (more stringent or additional to Decision 2003/33/EC) Member States mercury leaching limit values for landfills (stricter or additional to Decision 2003/33/EC) Landfill type L/S =2 l/kg mg/kg dry substance L/S =10 l/kg mg/kg dry substance C 0 (percolating test) mg/l dry EU criteria for landfills for inert waste Luxembourg [Legislation ] EU criteria for landfills for non-hazardous waste Austria (residual waste, e.g. incineration residues) [DeponieVO 2008] 0.1 Denmark (non-hazardous landfills in a noncoastal location)[miljøministeriet 2009] Germany (non-hazardous waste with low organic compounds)[depvereinfachv 2009] 0.05* Italy [Decreto 2003] 0.05* Luxembourg [Legislation ] 0.02 EU criteria for hazardous waste acceptable at landfills for non-hazardous waste UK / Northern Ireland [Schedule ] 0.02 EU criteria for waste acceptable for landfills for hazardous waste Austria [DeponieVO 2008] 0.5 Denmark (hazardous landfills in a non-coastal location)[miljøministeriet 2009] Italy [Decreto 2003] 0.5* Luxembourg [Legislation ] 0.1 UK / Northern Ireland [Schedule ] 0.4 *Unit [mg/l] is used instead of [mg/kg]; values have been converted In the following, the legal situation of some selected countries with underground disposal facilities is described in more detail.
94 Reference number /2009/530302/ETU/G Germany Due to the lack of appropriate storage facilities, many EU Member States export Hg-containing waste to other EU countries. Germany is the main importing country of mercury containing waste. On the basis of information by [COWI 2008], Germany is also the only country importing mercury containing waste for permanent storage (D12). Due to its hazardous and leaching properties Hg-containing waste is typically disposed of in hazardous underground facilities (salt mines). Leaching requirements for above-ground facilities are typically not met. Waste exceeding the limit values for above ground landfills can be stored in underground facilities (no limit values related to mercury concentration, for example) if it fulfils the site-specific waste acceptance criteria for the underground disposal site. In Germany hazardous waste is only allowed to be deposited in salt rock due to the lack of hard rock formations fulfilling the requirements of safe long-term storage. Therefore, in the new legislation (see below) only requirements for underground disposals in salt rock are defined. On 16 July 2009 the ordinance on the simplification of waste disposal regulations 40 came into force. This regulation has already taken into consideration Regulation (EC) No 1102/2008 as regards the long-term storage of mercury. Following the regulation, the long-term storage of metallic mercury is possible in landfill class III (above ground) and landfill class IV (underground storage). The class IV landfills have been recently introduced for the purpose of mercury storage in Germany. Liquid wastes are forbidden in long-term storage. An exception is made for the long-term storage of liquid mercury ( 23, para 2 of the Simplification Ordinance). The exception adopts the provisions set down in Article 3 of Regulation (EC) No 1102/2008 [Kabinett 2008, questionnaire survey]. With regard to above-ground storage (landfill class III), the landfill has to be dedicated for the storage of mercury and needs to be operationally and technically equipped for this purpose. In the case of underground storage (landfill class IV) the landfill has to be adapted for the purpose of disposing of metallic mercury and this has to be taken into particular consideration in the site-specific safety assessment. Wastes accepted into long-term storage facilities need to have a written certification granting the planned recovery or disposal operation ( 23, para 3 of the Simplification Ordinance). The requirements referring to the site specific risk assessment set out in the WAC Decision, Appendix A have been specified so far in German legislation in the Technical Instruction on Waste 41. With the Simplification Ordinance, the Technical Instruction on Waste is no longer in place since The requirements are now set down in the Ordinance on Landfills 42 which is included in the Simplification Ordinance. 40 Verordnung zur Vereinfachung des Deponierechts vom 27. April 2009 (Bundesgesetzblatt Jahrgang 2009 Teil I Nr. 22, ausgegeben zu Bonn am 29. April 2009), also referred to as the Simplification Ordinance 41 Zweite allgemeine Verwaltungsvorschrift zum Abfallgesetz (TA Abfall) vom 12. März 1991 (GMBl. Nr. 8 S. 139) last amendment on 21. März Verordnung über Deponien und Langzeitlager (Deponieverordnung - DepV) vom 27. April 2009 (BGBl. I S. 900), also referred to as the Landfill Ordinance.
95 Reference number /2009/530302/ETU/G2 95 In order to obtain permission for the disposal of hazardous waste in salt rock in class IV landfills, a long-term safety record is necessary in Germany. This concerns in particular site-specific circumstances, including scheduled and non-scheduled (hypothetical) incidents. The long-term safety record is essentially based on the results of the other two individual records, the: record of geotechnical stability, and safety record for the operational phase. The record of geotechnical stability in particular plays a key role in evaluating the long-term effectiveness and integrity of the salt barrier. If the complete enclosure has been verified by the record of geotechnical stability, there is no need for any model calculations of unplanned incidents and no need for a long-term safety record for model calculations on pollutant dissemination [Annex 2, 2.1.1]. The geotechnical stability has to be proven by a report from an expert in rock mechanics requiring very detailed information about rock behaviour and rock mechanics based on geotechnical laboratory experiments, on-site measurements and computational rock-mechanical modelling [Annex 2, 2.1.4]. The long-time safety record summarises the information on the entire system waste/underground structure/rock body. The record requires comprehensive information for example about the natural barriers of the host rock, the technical barriers and events that could endanger the whole encapsulation (earthquakes, volcanism, leaks in boreholes etc.) Annex 2 of the Simplification Ordinance includes instructions on the maintenance of long-term safety records within the context of site-related safety assessments for mines in salt rock and applied in practice. Most of the requirements have been laid down since 2002 and are already included in the old Ordinance of Landfills 43. Recently implemented are the requirements laid down in Annex 2, point 3 and 4 of the Waste Ordinance, addressing the closure of the deep underground disposal facility in salt mines and the documentation of access to the mines after closure (including for example the filling of pillars, introduction of a safety zone and documentation of waste filled). Table 5-16includes the provisions for salt mines that complement the requirements of European legislation. Table 5-16: Requirements for deep storage in salt mines according to German legislation Requirement for deep storage in salt mines according to German Landfill Ordinance Requirement / source Specification Location / geological barriers The salt barrier rock must have / must be: [Landfill Ordinance, Annex 2, point 1] impermeable against gas and liquids adequate spatial spread adequate unworked salt thickness, being sufficiently large that the barrier function is not impaired in the long term gradually enclosing the waste by its convergence behaviour, 43 Verordnung über Deponien und Langzeitlager (Deponieverordnung - DepV) vom 24. Juli 2002 (BGBl. I S. 2807), mit Änderungen vom 13.Dezember 2006.
96 Reference number /2009/530302/ETU/G2 96 Requirement for deep storage in salt mines according to German Landfill Ordinance Requirement / source Geotechnical stability [Landfill Ordinance, Annex point 2, 2 and 2.1.4] Geological properties [Landfill Ordinance, Annex 2, point ] Specification and at the end of the deformation process, of encapsulating it solidly stable cavities at least during the operational and closing phase of the landfill storage is prohibited in regional areas where the earth movement intensity of the value 8 according to the MSKscale 44 is above 99% In addition to the provisions set out in the WAC Decision regarding the site-specific safety assessment (Appendix A), the record of geotechnical stability is required (see above) In addition to the provisions set out in the WAC Decision regarding the geological assessment, the following concretisations are made: Geological barrier; vertical distance from salt roof zone to nearest upper underground, excavations; distances of horizontal cavities from salt rock edges and vertical distance from the footwall; thickness of the entire salt deposit or salt rock body Degree of exploration of deposit Exploratory bore holes from above and below ground Stratigraphy in mining territory (including thicknesses, rock face transitions) Material composition of salt deposit with ratio of salt rock to potash rock, clays, anhydrites, carbonate rock Salt deposit structure/interior construction, structural development including movements of the salt deposit and its environment, convergence, bearings and underlays of deposit, edge formation, transformations at surface of salt deposit, position and formation of potential alkali Degree of tectonic stress on the salt structure, predominant fault directions Geological cross-sections through the drifts Geothermal depth level Regional seismic activity in past and present Subrosion, formation of earth subsidence on surface Halokinesis 44 The Medvedev-Sponheuer-Karnik scale, also known as the MSK or MSK-64, is a macro seismic intensity scale used to evaluate the severity of ground shaking on the basis of observed effects in an area of the earthquake occurrence. The scale ranges from 1 to denotes damaging (Many people find it difficult to stand, even outdoors. Furniture may be overturned. Waves may be seen on very soft ground. Older structures partially collapse or sustain considerable damage. Large cracks and fissures opening up, rock falls). See e.g.
97 Reference number /2009/530302/ETU/G2 97 Requirement for deep storage in salt mines according to German Landfill Ordinance Requirement / source Drifts [Landfill Ordinance, Annex 2, point ] Hydrological Assessment [Landfill Ordinance, Annex 2, point ] Waste Information [Landfill Ordinance, Annex 2, point ] Waste Information [Landfill Ordinance, Annex 2, ] Specification In addition to the provisions set out in the WAC Decision regarding the site-specific safety assessment (Appendix A), information about drifts is required, including: Layout (depth of drifts, cavity volume, drift cross-sections, shafts, staple-pits, spiral, chutes and ramps, horizontal spread of drifts, location and deepness of all shafts in drifts, area and location of levels and sub-levels, distance between levels and sub-levels, levels connected to a filling station on air shaft, location and size of planned storage cavities) Safety (stability of shafts, drifts, staple-pits and working areas; roof subsidence, flaking due to impact and footwall risers in vicinity of mining territory; solution inflows, cause and origin, gas release/risk; petroleum/natural gas occurrences; safety pillars to overburden/edges/base /solution cavities/bore holes/shafts/neighbouring mines; existing exploratory bore holes from above and below ground; insulated parts of drift and those that need to be insulated) In addition to the provisions set out in the WAC Decision regarding the hydrological assessment, the following concretisations are made: Stratigraphy, petrography, tectonics, thickness and storage conditions of layers in the overburden and adjacent rock Details of the structure of aquifers and details of groundwater movement Permeability and flow speeds Mineralisation of groundwater, groundwater chemism, location of saltwater / freshwater boundary Use of groundwater, designated and planned drinking water and healing water conservation areas and priority areas Location, formation and properties of overground watercourses and stagnant waterbodies and those in water-filled underground caverns In addition to the provisions set out in the WAC Decision (Appendix A), information about waste is required (e.g. waste types, quantities and properties, geomechanical behaviour of waste, reaction behaviour). In addition to the provisions set out in the WAC Decision (Appendix A), information about waste is required (e.g. waste types, quantities and properties, geomechanical behaviour of waste, reaction behaviour). The old Ordinance on Landfills from 2002 included a leaching limit value for mercury in landfill class
98 Reference number /2009/530302/ETU/G2 98 IV disposal in rock other than salt rock - being 0,001 mg/l Hg. However, this limit value was not transposed into the revised Landfill Ordinance (2009). Under the amended Landfill Ordinance only salt mines are covered by the landfill class IV. Since in salt mines total containment and permanent isolation from the biosphere is assumed, leaching limit values are not required. Furthermore, the Federal Mining Act 45 includes requirements for underground storage. Nevertheless, it is not relevant to the storage of hazardous waste, as underground storage is defined as storage of gas, liquid and solid materials without containment Sweden Sweden is recognised as having the most far-reaching approach to mercury waste. Sweden has a national environmental goal and legislation stating that the use of mercury shall be phased out. In addition, mercury waste shall be deposited in final storage underground to eliminate emissions and to isolate mercury from the biosphere. Since 1 August 2005 Sweden implemented an ordinance regarding mercury in waste (Waste Ordinance 2001:1063) which states: Waste that contains at least 0.1 percent by weight mercury and is not in a permanent landfill shall be placed in deep underground disposal by 1 January 2015 at the latest. It is not allowed to dispose of mercury waste before 1 January 2015 in a way that prevents terminal storage in bedrock. [COWI 2008, questionnaire survey] The Swedish ordinance regarding mercury in waste (Waste ordinance 2001:1063) states: Waste that contains at least 0,1 % by weight mercury and is not in a permanent landfill shall be placed in deep underground disposal. The rules mentioned above do not apply for mercury waste that is covered by the Regulation (EC) 1102/2008. [personal information Mr. Carl Mikael Strauss, Swedish EPA] The characteristics of deep underground disposal must however be viewed in the light of other barriers such as containment, and if the waste is stabilised or not. Both salt mines and underground hard rock formations can fulfil the requirements [Questionnaire survey, SE] UK The Environmental Permitting System of England and Wales of Regulation gives effect to the permitting requirements of Articles 9 & 10 of the EU Waste Framework Directive to ensure that waste is recovered or disposed of without endangering human health or the environment. The EU 45 Bundesberggesetz vom 13. August 1980 (BGBl. I S. 1310), mit Änderungen vom 31. Juli 2009 (BGBl. I S. 2585) 46 Environmental Permitting (England and Wales) Regulations 2007
99 Reference number /2009/530302/ETU/G2 99 Landfill Directive and the WAC Decision are both implemented by Regulation 2007, Schedule for England and Wales. The provisions of both documents have been taken over with minor differences regarding the acceptance and limit values laid down in 6 to 8 in Schedule 10. The provisions set for disposal facilities are unaffected. The same is valid for Northern Ireland 48 and Scotland Legislation of non-eu countries Norway Since January 2008, the Norwegian Pollution Control Authority banned the use of mercury in all products within the country (Norwegian Product Regulation, Produktforskriften). In addition, the importing, exporting and selling of products containing mercury or mercury compounds is forbidden. There are limited exemptions for some areas of use until December At the moment, it is very uncertain if elemental mercury will be allowed for export from Norway, it depends on whether the authorities will regard elemental mercury as a product or not [Kystverket 2008]. In Norway, there is a need to store mercury containing waste from zinc-production (one site). Mercury containing waste from zinc-production is treated for final disposal. The mercury-residue from zinc-production is cemented in sarcophagi and placed in a bedrock hall at the production site [NO 2005, COWI 2008]. There are no emissions of mercury reported from this activity [COWI 2008]. In the future, there might be a need to store unexpected mercury-waste, as in 2003 a submarinewreck from the World War II was discovered, containing large amounts of mercury. Historic documents state that the submarine contains 65 tons of metallic mercury which is stored in steel ampoules. The area surrounding the submarine is monitored, but so far no decision has been made about bringing the mercury cargo up from the ocean floor. Following the statement of [NO 2005], Norway prefers a terminal disposal in a safe manner that meets standards for long-term environmentally sound management USA The US approach relating to governmental as well as non-governmental surplus metallic mercury is long-term storage (>40 years) in appropriate above ground facilities. In 2008 the US Congress adopted the Mercury Export Ban Act of 2008 [US ban 2008]. This Act 47 Schedule 10: Provision in relation to landfill to the Environmental Permitting (England and Wales) Regulations 2007, 48 Schedule 2 (General requirements for landfills) of the Landfill Regulations (Northern Ireland) 2003 with amendments from 2004 and The Environment Act 1995, The Criteria And Procedures For The Acceptance Of Waste At Landfills (Scotland) Direction 2005 [Scotland Directive 2005]
100 Reference number /2009/530302/ETU/G2 100 prohibits on the one hand the sale, distribution, or transfer of elemental mercury by federal agencies and on the other hand, it bans the export of elemental mercury from the United States effective from 1 January In addition, the Act directs the DOE (Department of Energy) to provide storage facilities by 2013 to accept and store excess mercury sent to it from commercial mercury recyclers, gold mines generating mercury as a by-product, and chlor-alkali plants. By 1 January 2010 the DOE has to designate one or more facilities for the purpose of long-term management and storage of elemental mercury generated in the USA. Currently, seven sites are in discussion as possible future storage facilities for metallic mercury. The DOE is carrying out an Environmental Impact Statement (EIS) to identify the most appropriate storage facilities. The relevant facility(ies) constructed, existing or modified facilities must comply with the corresponding requirements of section 5 (d) of the Act Management Standards for Facilities, including the requirements of the Solid Waste Disposal Act, as amended by the Resource Conservation and Recovery Act (RCRA). The DOE already stores about 1,200 tons of state-owned mercury at the Y-12 National Security complex in Oak Ridge, Tennessee. The Environmental Protection Agency (EPA) estimates that 7,500 to 10,000 metric tons of elemental mercury from private sources would be eligible for storage over the next 40 years. In November 2009 the DOE published Interim Guidance on Packaging, Transport, Receipt, Management, and Long-Term Storage of Elemental Mercury [DOE 2009]. These interim guidelines a framework for the standards and procedures associated with a DOE-designated elemental mercury storage facility with a focus on the RCRA permitting of such a facility and planning for that storage facility s needs. Apart from the above mentioned 1,200 metric tons currently stored by the DOE, the Department of Defense (DOD) has stored approximately 4,436 metric tons of government-owned elemental mercury in three above ground locations for more than 40 years. Until 1994 this governmental owned mercury was sold as a commodity. After the environmental and health risks related to mercury became more and more obvious, the DOD halted the selling of elemental mercury. In 2003/2004 a Mercury Management Environmental Impact Assessment (MM EIS) was carried out to find the most appropriate way of dealing with the stored mercury in future. The MM EIS considered the following options: Maintaining all the sites, Consolidating the mercury for storage at one site. Selling the elemental mercury on the market. The addressed storage period was 40 years. The MM EIS evaluated consolidated storages in warehouses as well as in igloos designed for the storage of army materials. As a result of the MM EIS the preferred alternative was the consolidation of mercury storage at one
101 Reference number /2009/530302/ETU/G2 101 site. The selected warehouse (Hawthorne Army Depot) was not one of the existing mercury storage sites. This decision was based on a combination of environmental, economic and technical factors, policy considerations and public and stakeholder comments [DNSC 2004]: Consolidating the DNSC mercury inventory at one site results in negligible-to-minor environmental impacts at that site and improves environmental conditions at sites from which the mercury would be removed; Human health risks to the public are negligible for normal operations and negligible-to-low for facility and transportation accidents; Ecological risks are negligible for normal operations and negligible-to-low for facility and transportation accidents with dry deposition. Ecological risks are negligible-to-moderate for facility and transportation accidents if it is raining during an accident which results in a release of mercury and a fire; Consolidating the mercury inventory simplifies storage operations and results in economies of scale (i.e., fewer resources required to manage the mercury inventory); Consolidating the excess mercury inventory facilitates DNSC s long-term closure strategy at the sites from which the mercury is removed; Removing DNSC s excess mercury inventory is consistent with the national security mission of Y-12; and The stored DNSC commodity-grade elemental mercury will be available for future use. The environmental impact of the temporary storage itself could be expected to be negligible. Both of the storage alternatives were assessed as having negligible human health and ecological risks, considering both routine operations and the risk of facility accidents. The consolidated storage option was seen as presenting slightly higher (but still low and short term) risk, connected with transporting the mercury. A concern with temporary storage is that there is a possibility that the storage facilities might be neglected or damaged in the future. However, the DLA assessed the risks over a 40 year period, from a variety of accident scenarios such as fires, earthquakes, vehicle and aircraft crashes, etc., to be negligible. The MM EIS also took into consideration underground storage as well as pre-treatment options (stabilisation of the waste). These possibilities have not been further evaluated due to the following reasons: Below-ground facilities such as bunkers and mines were considered but not evaluated as bunkers would be similar to the evaluated igloos at Hawthorn. Due to the limited availability of existing mines, inspection considerations, additional material handling, and regulatory issues, the storage in mines was not considered to be a reasonable alternative.
102 Reference number /2009/530302/ETU/G2 102 A pre-treatment of the metallic mercury to a stabilised, less toxic form before storage was also eliminated from a detailed analysis. According to the DNSC, treatment and storage would result in additional environmental impacts and costs, without significant benefits as in their opinion metallic mercury can be safely stored and it is the preferred form in most industrial processes requiring mercury. Based on the immaturity of the bulk mercury treatment technologies and the lack of a way forward approved by the EPA for treatment and disposal of elemental mercury, the disposal in a qualified landfill after pre-treatment was not evaluated in detail in the MM EIS. 5.5 Policy Initiatives UNEP Mercury Programme Under the UNEP Mercury Programme (see chapter 5.1) the UNEP Global Mercury Partnership is the main mechanism for the delivery of immediate actions related to mercury. Its overall objective is to protect human health and the global environment from the release of mercury and its compounds by minimizing and, where feasible, ultimately eliminating global, anthropogenic mercury releases to air, water and land [UNEP 2009B]. The partnership working areas currently identified include: Mercury Management in Artisanal and Small-Scale Gold Mining, Mercury Control from Coal Combustion, Mercury Reduction in the Chlor-alkali Sector, Mercury Reduction in Products, Mercury Air Transport and Fate Research, Mercury Waste Management, Mercury Supply and Storage. The objective of the Mercury Waste Management partnership is to minimise and, where feasible, eliminate unintentional mercury releases to air, water and land from mercury wastes. Activities within the waste partnership area are: to support the Basel Convention on the Control of Transboundary Movements of Hazardous Wastes and their Disposal, to draft Technical Guidelines on EMS of Mercury Waste,
103 Reference number /2009/530302/ETU/G2 103 to execute the Mercury waste management project, to add requirements on the technical and economic assessment of mercury-containing tailings. As a consequence of the Mercury Supply and Storage partnership the project Reduce Mercury Supply and Investigate Mercury Storage Solutions has been initiated in two regions within the: Asian mercury storage project, Latin America and the Caribbean mercury storage project. After the establishment of regional advisory groups formed by interested organisations and governments assessment reports on excess mercury supply in Asia [Concorde 2009] and Latin America [UNEP 2009 A] for the period have been prepared as a basis for further activities. During inception workshops in Bangkok (Thailand, 4-5 March ) and Montevideo (Uruguay, April ) these assessment reports have been discussed and possible long-term storage options have been presented. The regional advisory group will now explore and analyse the presented options. In exploring options, the range of factors needed to establish a safe long-term storage or repository facilities will be explored, including criteria (e.g. costs and benefits, social and political acceptability, technical and environmental factors, infrastructure and regulatory requirements) for site selection. Following the development of the options, a feasibility study which further explores the suitability of a proposed site will be undertaken. Country selection for a mercury storage site will be dependent on the results of the analysis and site suitability. Furthermore, it will be based on an agreement within the region. These mercury storage projects complement a mercury waste project (funded by Norway) that aims to improve the technical guidelines on the environmentally sound management of mercury waste done in coordination with the Secretariat of Basel Convention (SBC). Long-term storage is critical in the ESM of mercury waste. The mercury waste project is underway to being implemented in two countries in Asia, being managed by UNEP Chemicals, and in two countries in Latin America, being managed by the SBC WHO The World Health Organisation has been dealing with the issue of mercury, mercury exposure and the risks to human health and the environment and for many years focusing on topics as follows: Exposure to mercury and effects on human health and health risks from food intake [WHO 2008]; 50 Workshop documents are available at: 51 Workshop documents are available at:
104 Reference number /2009/530302/ETU/G2 104 Risks from long-range transboundary air pollution of heavy metals and mercury content in environmental compartments (soil, air, water) [WHO 2007a]; Setting of exposure limit values for mercury, e.g. for air and water exposure [WHO 2007]; Estimating the environmental burden on diseases based on mercury exposure [WHO 2008]; Elaborating policy papers for mercury issues, e.g. [WHO 2005a]. The WHO is organizing workshops with policy makers on the issue of mercury and mercury exposure. The organisation is also proposing strategic actions to eliminate mercury-related diseases including the [WHO 2007]: The use of mercury-free alternatives, e.g. for manometers and thermometers, The development of mercury clean-up and waste-handling, storage and safe-handling procedures, The promotion of environmentally sound management of health-related waste containing mercury (as set out in the UN Basel Convention on the Control of Trans-boundary Movements of Hazardous Wastes and their Disposal) International Conference on Mercury The International Conferences on Mercury as a Global Pollutant (ICMGP) have been held periodically since the 1990s and are the major international forum for formal presentation and discussion of scientific advances concerning environmental mercury. The most recent conference was held in China in May 2009 (see: HELCOM The Helsinki Commission for the Convention Protection of the Marine Environment of the Baltic Sea Area (HELCOM) focuses on environmental issues relevant for the protection of the Baltic Sea area. The contamination with heavy metals and mercury in particular has been one of the concerns for some years. HELCOM is regularly estimating and reporting data, for example about the atmospheric emissions of mercury and mercury emissions from anthropogenic sources for neighbouring European countries PARCOM / OSPAR 15 Governments of the western coasts and catchments of Europe are members of the Commission for the Protection of the marine Environment of the North-East Atlantic. OSPAR investigated mercury as hazardous substance of concern as early as the 1980s, e.g. [PARCOM 1981, 1982] and elaborated
105 Reference number /2009/530302/ETU/G2 105 for example recommendations on land-based sources of mercury pollution and also addressed specific industrial sectors such as the chlor-alkali industry [PARCOM, 1981a and 1982a, 1985]. More recent agreements from OSPAR focus on the management of contaminated, dredged material [OSPAR 2009] and the realisation of coordinated monitoring programmes [OSPAR 2008].
106 Reference number /2009/530302/ETU/G References [Basel 2010] Concorde 2009] Concorde sprl, Assessment of excess mercury in Asia, , May 2009, ay2009.pdf [COWI 2008] COWI A/S and Concorde East/West Sprl, Options for reducing mercury use in products and applications, and the fate of mercury already circulating in society, December [Decreto 2003] Criteri di ammissibilità dei rifiuti in discarica. Ministero dell'ambiente e della tutela del territorio, 13 marzo 2003, Italy [Deponieverordnung 2008] 39. Verordnung des Bundesministers für Land- und Forstwirtschaft, Umwelt und Wasserwirtschaft über Deponien, Januar 2008, Germany [DepVereinfachV 2009] Verordnung zur Vereinfachung des Deponierechts, Germany 27. April 2009, [DOE 2009] U.S. Department of Energy, Interim Guidance on Packaging, Transportation, Receipt, Management, and Long-Term Storage of Elemental Mercury, U.S. Department of Energy Office of Environmental Management Washington, D.C., November 13, 2009, (dated% ).pdf [DNSC 2004] Defense National Stockpile Center, Record of Decision for the Mercury Management EIS, April 2004 [FNADE/ADEME 2006] Feedback on the French system, stabilisation/solidification-landfilling of hazardous waste, FNADE/ADEME, 2006 [IKIMP 2009] Briefing note for participants for "Workshop on Safe Storage and Disposal of Redundant Mercury", St Anne s College, Oxford (UK), 13th & 14th October, 2009,
107 Reference number /2009/530302/ETU/G [Kabinett 2008] Begründung Verordnung zur Vereinfachung des Deponierechts (Stand Kabinettbeschluss am ) [Kystverket 2008] Det Norske Veritas AS, Kystverket Norwegian Coastal Administration - Salvage of U Supplementary studies - disposal, report NO , Revision N 01, [Legisation ] Recueil de Legislation No 36 Mise en décharge des déchets (Legislation 36), 2 mars 2006, Luxembourg [Miljøministeriet 2009] 252 af 31/ Bek. om deponeringsanlæg, 31. marts 2009, Denmark [NO 2005] Stakeholder meeting in Brussels 8 September Additional questions, Answers from the Norwegian authorities [OSPAR 2008] OSPAR Coordinated Environmental Monitoring Programme (CEMP) (This agreement replaces agreement ), Brest 2008 [PARCOM 1981] PARCOM Recommendation 81/1 on Other Land-Based Sources of Mercury Pollution (Thermometers, Batteries, Dental Filters), Brussels 1981 [PARCOM 1981b] PARCOM Decision 81/2 on Limit Values for Existing Brine Recirculation Chlor-Alkali Plants (exit of the factory site), Brussels 1981 [PARCOM 1982] PARCOM Recommendation 82/1 on Other Land-Based Sources of Mercury Pollution, Copenhagen 1982 [PARCOM 1982a] PARCOM Decision 82/1 on New Chlor-Alkali Plants Using Mercury Cells, Copenhagen 1982
108 Reference number /2009/530302/ETU/G2 108 [PARCOM 1985] PARCOM Recommendation 85/1 on Limit Values for Mercury Emissions in Water from Existing Brine Recirculation Chlor-Alkali Plants (exit of factory site), Brussels 1985 [Schedule ] The Landfill (Amendment) Regulations (Northern Ireland) 2004 Statutory, Rule 2004 No. 297, The Landfill (Amendment) Regulations (Northern Ireland) 2004 [Scotland Directive 2005] The Environment Act 1995, The Criteria And Procedures For The Acceptance Of Waste At Landfills (Scotland) Direction 2005 [Seveso Guidance 2005] Guidance on the preparation of a safety report to meet the requirements of Directive 96/82/EC as amended by Directive 2003/105/EC (Seveso II), Report EUR EN, Institute for the protection and security of the citizen, Major accident hazardous bureau, European Commission, DG Joint Research Centre, [UNEP 2007] Draft technical guidelines on the environmentally sound management of mercury wastes, 2007, [UNEP 2009] UNEP, Draft technical guidelines on the environmentally sound management of mercury wastes, 4th Draft, April 2009, [UNEP 2009 A] UNEP Chemicals, EXCESS MERCURY SUPPLY IN LATIN AMERICA AND THE CARIBBEAN, , ASSESSMENT REPORT, July inal_1july09.pdf [UNEP 2009 B] [US ban 2008] Mercury export ban Act 2008, Public Law Oct, 14., 2008, 122 Stat. 4341, [VLAREM 1995] VLAREM II: Order of the Flemish Government of 1 June 1995 concerning General and Sectoral provisions relating to Environmental Safety, 1th June 1995, Belgium
109 Reference number /2009/530302/ETU/G2 109 [WHO 2005a] World Health Organisation, Policy Paper: Mercury in health care, August 2005; [WHO 2007] World Health Organisation, Preventing disease through healthy environments exposure to mercury, A major public health concerns, Geneva 2007 [WHO 2007a] World Health Organisation, risks of heavy metals from long-range transboundary air pollution, Joint WHO/Convention Task Force on the Health Aspects of Air Pollution, Germany 2007 [WHO 2008] World Health Organisation, Assessing the environmental burden of disease at national and local levels. Environmental Burden of Disease Series, No. 16, Geneva 2008
110 Reference number /2009/530302/ETU/G Review of the state of the art of storage and disposal options 6.1 General considerations Almost all mercury compounds are toxic and can be dangerous at very low levels in both aquatic and terrestrial ecosystems. Mercury is a persistent substance. It can build up or bioaccumulate in living organisms and inflict increasing levels of harm on higher order species such as predatory fish and fish eating birds and mammals through a process known as "biomagnification". In the environment, mercury and its compounds are readily transformed from one form to another and transported over long and short distances (see chapter 4) Consequently, each contamination of the environment due to anthropogenic releases of mercury or its compounds should be prevented or minimised during its extraction, use, transport and temporary storage or permanent disposal. Against the background of the present project, the focus here is laid upon the risk that mercury or its compounds are released under permanent or temporary storage conditions and related pretreatment and disposal operations. Corresponding risks are anthropogenic releases of mercury in the short and long term due to leaching or evaporation or solid releases (e.g. dust) from pre-treatment (immobilisation) and storage/disposal operations and storage/disposal vapor pretreatment disposal operations storage/ disposal solids (e.g. dust) leachate Figure 6-1: Illustration of possible releases of mercury related to the temporary or permanent storage of mercury
111 Reference number /2009/530302/ETU/G2 111
112 Reference number /2009/530302/ETU/G2 112 Pre-treatment (immobilisation) before storage or disposal of metallic mercury may require additional transport and/or handling of the mercury and may be related to additional releases of mercury. An overview of possible pre-treatment technologies and relevant emissions is provided in chapter 7. The disposal of hazardous waste generally aims at the isolation of hazardous substances from the biosphere and groundwater. Related risks are possible due to the release and transport of hazardous substances from the storage or disposal site. The permeability / impermeability of the geological barrier depends on the site-specific hydraulic conductivity and the possible appearance of fractures of the host rock. The corresponding risk depends on the substance properties and on artificial and natural barriers between the waste and the biosphere. The risk depends therefore on the following parameters: physico-chemical parameters of the hazardous waste (e.g. stability, solubility, reactivity) technical parameters of the containments (e.g. material and thickness of the containment, properties of sealants) and the disposal facility (e.g. engineered barriers) natural parameters of the facility (e.g. geology, hydrology) Once mercury waste is temporarily or permanently stored, the risk of releases depends on the waste itself (substance/mixture and state of the substance) and the short and long term transmissibility 52 of the artificial and geological barriers that separate the waste from the environment. The number and in particular the effectiveness of these barriers define the protection of the environment against adverse effects from the stored waste. This so called multi-barrier concept is in particular applicable to underground storage but also above-ground storage typically consists of more than one barrier to prevent hazardous chemicals released from the waste from entering the biosphere. Geological barrier(s) (far field) Artificial barrier(s) (near-field) Waste (source term) Figure 6-2: Protection layers for the storage of mercury The environmental risk of stored mercury waste is therefore related to the short and long-term transmissibility for liquid mercury, leachate and vapour as possible source of releases, its possible 52 Transmissibility means the permeability of the barriers for components of the waste.
113 Reference number /2009/530302/ETU/G2 113 environmental transport and related environmental and/or human exposure. It must be assessed in the light of geological and artificial barriers (such as containment, back fillings) and the state of the waste (e.g. whether it is stabilised or not). For the acceptance of hazardous waste in underground storage sites, a site-specific safety assessment must be carried out (according to section 2.5 of Council Decision 2003/33/EC and as defined in Annex A of that Decision). The long term assessment should be assessed without taking account of the containment of the waste and cavity lining (see section Council Decision 2003/33/EC) as it is assumed that the containment will not persist in the long term. Waste may be accepted only if it is compatible with the site-specific safety assessment. For above-ground facilities, waste acceptance criteria are established in Decision 2003/33/EC. The transmissibility of the barriers is related to the physico-chemical properties of the components of the stored waste. For the assessment of potential releases and associated risks, a set of physicochemical parameters of mercury and relevant mercury compounds is relevant, such as volatility, solubility, leachability, reactivity, and the octanol/water partition coefficient. An overview of the most important parameters is given in chapter 4. Relevant substances are elemental mercury and all mercury compounds that will potentially be stored (temporarily or permanently). Organic mercury compounds (e.g. methyl mercury) will not be stored or disposed of. However, it should be noted once again that metallic mercury released in the environment may be transformed into organic compounds and cause severe environmental and health risks. Particularly when considering the underground storage/disposal, specific factors affecting the behaviour of mercury in the host rock and the geological environment need to be considered apart from the waste properties and the storage system (layout, containments, storage place and conditions, monitoring, access conditions, closure strategy, sealing and backfilling, depth of the storage place). These include particularly (see [Heath 2006]): Geology (lithology, structure, stability) Hydrogeological factors (hydraulic gradient, hydraulic conductivity / permeability) Geochemical factors (ph, redox, other cations and complexation agents in solution) Retardation/attenuation processes o o o o o Hydrodynamic dispersion Diffusion Sorption and ion exchange (ph dependent) Precipitation and co-precipitation (redox dependent) Microbiological factors
114 Reference number /2009/530302/ETU/G2 114 Establishment of distribution coefficient, Kd (equilibrium ratio of the sorbed to dissolved concentrations) Experiences related to the underground storage of liquid waste mercury are not available as mercury is still a valuable product used for various applications worldwide (see chapter 1.1). Up-to-date relevant experience or investigations are available from permanent storage of mercury containing waste in salt mines (in particular German experience from storage of hazardous waste) (see section 6.2.2) permanent storage in deep hard rock underground formations of hazardous waste (Swedish experience on deep bedrock and stabilisation as HgS) (see section 6.2.3) In addition, specific experience and investigation is available from the worldwide work on underground disposal of radioactive waste which relies particularly on the principle of isolating the radioactive waste from the biosphere for a very long time (see section 6.2.4). The review related to the temporary storage of metallic mercury above ground is based on experience from the temporary storage of liquid mercury in the USA and of Mayasa in Almadén (former mercury mine), which stores and handles significant quantities of mercury as a product (see section 6.3). The containment of the waste is in particular important for the temporary storage of liquid mercury as it has to ensure a safe containment of the waste for a certain period of time. For long-term storage the major function of the container is to ensure a safe handling of the waste before storage (and for a certain time period until the waste cell is closed). An overview of the containers currently used in Europe for the transport and storage of liquid mercury (as a product) and as well as the packaging system used in the USA for a foreseen storage period of 40 years, are described in section 6.4). 6.2 Review of underground disposal operations Underground disposal is based on the principle of isolating waste from the biosphere in geological formations where it is expected to remain stable over a very long time. Information on experience from current underground storage of liquid mercury is not available. In the European Union the acceptance of liquid waste is forbidden in landfills (Article 5(3)(a), Directive 1999/31/EC). A review of the state-of-the-art of disposal operations for hazardous waste, and in particular metallic mercury, in salt mines or deep underground hard rock formations can take account of experience with the underground disposal of hazardous waste and radioactive waste. Experience with the disposal of mercury-containing waste and other hazardous waste has been available for several decades (e.g. underground waste disposal since 1972 in a German salt mine). Valuable information can also be drawn from experience in the underground disposal of radioactive
115 Reference number /2009/530302/ETU/G2 115 waste. In order to ensure an appropriate level of safety via the geological barrier, underground disposal of radioactive waste is usually carried out in depths ranging from several hundred to about one thousand metres (see e.g. [IAEA 2009]). Although the properties of radioactive waste are somewhat different to liquid mercury, 53 experiences from research in particular related to geological requirements of host rocks like the stability are also valid for the permanent storage of liquid mercury. The most relevant sources of information related to the underground disposal which were consulted are listed below: Table 6-1: Overview of literature related to the storage of liquid mercury Review of important literature related to underground storage options Reference [BGR 2007] BGR, Bundesanstalt für Geowissenschaften und Rohstoffe, Nuclear waste disposal in Germany - Investigation and evaluation of regions with potentially suitable host rock formations for a geologic nuclear repository, Hannover/Berlin, April 2007 [GRS 2008] GRS, Gesellschaft für Anlagen- und Reaktorsicherheit (GRS) mbh, Öko-Institute e.v., Institut für angewandte Ökologie, Endlagerung wärmeentwickelnder radioaktiver Abfälle in Deutschland, Anhang Wirtsgesteine Potentielle Wirtsgesteine und Eigenschaften, Anhang zu GRS-247, ISBN , Braunschweig/Darmstadt, September 2008 [IAEA 2009] Geological Disposal of Radioactive Waste: Technological Implications for Retrievability 78_web.pdf Content This study summarizes the findings related to a geological disposal of nuclear waste in Germany including minimum requirements for the host rock. This annex comprises main properties of possible host rock for the disposal of radioactive waste in Germany. It provides a broad overview of the different properties of potential host rocks including their advantages and disadvantages in view of a safe long term storage. This report provides an overview of the current status of geological disposal of radioactive waste. The report assesses the technological implications of retrievability in geological disposal concepts. Scenarios for retrieving emplaced waste packages are considered, and the publication aims to identify and describe any related technological provisions that should be incorporated into the design, construction, operational and closure phases of a 53 Radioactive waste is typically solid or immobilised, partly heat generating and the hazardousness decreases over a long period. In contrast to this, metallic mercury waste is primarily liquid, it does not generate heat and its hazardousness remains stable over unlimited time.
116 Reference number /2009/530302/ETU/G2 116 repository. [KEMAKTA 2007] Lars Olof Höglund and Sara Södergren, Aspects on final disposal of mercury The need for waste stabilization, 22 March 2007 The purpose of this document was to establish background documentation for the proposal of the Hg-Regulation. [Popov 2006] V. Popov, R. Pusch, Disposal of Hazardous waste in underground mines, Wit Press, Southhampton, Boston, 2006 [SOU 2008A] Statens offentliga Utredningar (SOU) 2008: 19: Att slutförvara långlivat farligt avfall i undermarksdeponi i berg - Permanent storage of long-lived hazardous waste in underground deep bedrock depositories,, SOU 2008: 10 April 2008 This book contains a collection of articles presenting the current experiences in the utilization of underground mines for the safe storage of hazardous waste. The book provides a broad overview of mines in Europe (active and inactive). In addition, articles by various authors to the following topics are included: Criteria for selection of repository mines, engineered barriers, stability analysis of mines, risk assessment of underground repositories. This study commissioned by the Swedish government analyses the permanent storage of mercury in deep bedrock and salt mines. The report provides an account of permanent storage options for mercury-containing waste, and the requirements and risks attendant to the permanent storage of liquid mercury. A summary on the key findings of the study is available in English [SOU 2008] [SOU 2001] NATURVÅRDSVERKET, A Safe Mercury Repository, A translation of the Official Report SOU 2001:58, Report 8105, January 2003 This report was prepared before the Hg-Regulation entered into force. It recommends that waste containing at least one percent mercury by weight be taken to a permanent deep bedrock repository (at least 400m). The repository should isolate mercury from the biosphere for a very long period, preferably more than 1,000 years. The repository should require no maintenance to avoid burdening future generations. A list of all references used for this chapter is provided in chapter 6.5. Additional information received personally during the research is also included in this section Potential host rocks In Europe, many deep mines of different types of rock exist which might be generally suitable for the storage of hazardous waste [Popov 2006]. Depending on their geological formation, mines which are currently still in use might be used as hazardous waste disposal sites in future. Information on existing underground disposal sites is mainly available for EU 15. But also in the Eastern European
117 Reference number /2009/530302/ETU/G2 117 countries underground disposal sites are in place (e.g. Slovenia) or planned (e.g. Poland). Appropriate host rock for disposal of metallic mercury according to Council Decision 2003/33/EC are salt rocks and hard rocks (igneous rocks, e.g. granite or gneiss including also sedimentary rocks e.g. limestone or sandstone). Furthermore, deep storage in hard rock with an appropriate depth is defined as an underground storage at several hundred metres depth (WAC Decision, Appendix A (4)). In a geological sense, the term hard rock includes igneous rocks (e.g. granite or basalt), metamorphic rocks (e.g. slate, marble, gneiss, schist) and sedimentary rocks (e.g. sandstone, shale, limestone). Salt rock is a specific sedimentary rock. Further, it can be differentiated between consolidated and nonconsolidated rocks. Consolidated rocks consist of a mixture of minerals with primary solid matrix material. Examples are breccia/conglomerate, sandstone or claystone. Non-consolidated rocks are non-bound fragmental rocks without solid matrix material. Examples are gravel, sand or clay. Each type of consolidated hard rock is theoretically possible for underground disposal sites for mercury. This generally corresponds to experiences from disposal options for radioactive waste according to which preferred host rocks could be hard rock (i.e. crystalline igneous rock), clay rock (i.e. igneous sedimentary rock) and salt rock. Underground laboratories for testing and building confidence in disposal technologies for the disposal of radioactive waste have been built in all types of potential host rocks [IAEA 2009]. The host rock properties are decisive for the design and the operational and environmental safety (in the short and long term) of an underground disposal site and other relevant aspects (e.g. retrievability, costs, etc.). Relevant properties of potential host rocks are in particular available from experience in underground disposal of radioactive waste (see [IAEA 2009]), [BGR 2007] and [GRS 2008]. In the following, an overview is provided on the properties, available experiences, economic and environmental information of potential host rocks for the storage of liquid mercury.
118 Reference number /2009/530302/ETU/G Salt rock Properties Salt host rocks exist in different geological formations as layered salt and salt domes, usually of a sodium or potassium type. Both geological formations may in principle be used for disposal purposes. The following table summarizes the main properties of salt rock related to its suitability as a host rock (source: [GRS 2008], [GRS 2009], [Popov 2006]): Table 6-2: Overview of properties of salt rock Criteria Permeability Mechanical Strength: Deformation behaviour: Stability of cavities: In situ stresses: Dissolution behaviour: Sorption behaviour: Properties Very low (practically impermeable) Medium Visco-plastic (creep) Self-supporting Isotropic High Very low Salt rock is very dry, it contains no free water and offers very good isolation of the waste. Under natural disposal conditions rock salt is practically impermeable to gases and liquids. Together with an overlying and underlying impermeable rock strata (e.g. claystone), it acts as a geological barrier intended to prevent groundwater entering the landfill and, where necessary, effectively to stop liquids or gases escaping from the disposal area (see Council Decision 2003/33/EC). On the other hand, salt rocks are highly soluble, thus any access of water would cause severe consequences on the host rock. Salt rock is perfectly impermeable with respect to water and gas [Popov 2006] as a consequence no gas producing materials should be stored to avoid an increase of pressure in the rock. Recent research give indications that in case of gas generation there will be no explosion and the gas will not escape via a macroscopic fracture as assumed so far. According to [Popp 2007] recent research micro fractures occur in the near field along existing grain boundaries with the effect that an increased volume is available for the generated gas. It can be concluded that the permeability of the surrounding salt rock decreases in the near field and is only relevant for a defined area around the stored material. It stops as soon as the pressure does not increase anymore. [Brückner 2003, Popp 2007] Salt rocks generally have a low sorption capability (see [GRS 2009]. Information related to its sorption capacity for mercury has not been identified. The hydraulic conductivity of rock salt is very low. In [GRS 2008] the following values have been indicated for rock salt:
119 Reference number /2009/530302/ETU/G2 119 Average depth of N of Hydraulic conductivity (Kf in m/s) samples samples Range Median value Rock salt m x x x A liner is usually not required in salt formations. Here, rock creep is a continuous process leading to rock deformation in response to lithostatic pressure. Salt creep will close the void space around waste packages in the emplacement cells, leading to complete encapsulation. The creep rate depends on in situ stress (increasing with depth) and temperature. The investigation of the structure of layered salt mines is easier compared to salt domes, and well established investigation methods are available [GSR 2008]. In particular, the presence of brine in local lenses or irregular structures or fissures may cause difficulties for a safe storage. Therefore, the presence of such structures has to be excluded via a site-specific safety assessment. [Popov 2006]. Due to its plastic deformation behaviour the salt rock encapsulates wastes in the long term. The encapsulation process is enhanced by backfill material. Given that salt encapsulation is one of the main safety elements of a disposal concept in salt, it is advantageous to backfill the emplacement cells rapidly after waste emplacement, and keeping the disposal cells open has never been considered in the German salt based repository concept (for disposal of radioactive waste) [IAEA 2009]. The most appropriate backfilling material for salt rock is crushed salt rock with a major barrier function [Popov 2006]. At the German underground Herfa-Neurode waste disposal site, salt dams are filled up or stone walls are built in order to separate the storage cells and to facilitate the ventilation of the disposal site. The operators of the Herfa-Neurode disposal site assume that the galleries of the underground salt mine will be completely closed within some thousand years 54. Long-lasting seals in the form of plugs in the shafts leading down to the repository level are required since water inflow from shallow soil and rock can cause very difficult problems [Popov 2006]. The plug system has to be adapted to site specific requirements Experience of underground disposal of hazardous waste in salt mines Hazardous wastes including mercury contaminated solid wastes have been deposited in underground salt mines for several decades in Europe. Therefore, an extensive knowledge base on all repository relevant properties of rock salt and salt formations is available (in particular in Germany). In Europe, salt mines are currently authorised for the underground disposal of hazardous waste only in Germany and the UK. Poland is currently considering using specific salt mines for the disposal of hazardous waste. 54 Personal Communication, Dr. Lukas, K&S Entsorgung GmbH,
120 Reference number /2009/530302/ETU/G2 120 In France, the first underground landfill was opened in February 1999 in a potash-salt mine in Wittelsheim, France-Alsace, with a licensed capacity of 320,000 tons (for 30 years). 13 different waste types including galvanisation sludge, spent catalysts and residues from waste incineration were licensed. The deposit of explosive and flammable wastes was forbidden. In September 2002, a fire broke out in the underground landfill which may have been caused by improper disposal. As a consequence of the fire, the landfill and the adjacent mine were closed. [UBA DE 2004] Salt mine in the UK A relatively new salt mine deposit for the storage of hazardous waste has been operating in Winsford, Cheshire, United Kingdom since 2005 (Minosus rock salt mine). The site is permitted according to the IPPC directive and has a licence for selected waste codes. At Minosus rock salt mine, waste disposal takes place at a specific 30-hectare worked-out area of the mine and its activities will have no impact upon either continuing rock salt extraction or upon the area dedicated to and used for archiving and document storage. The site consists of a 200 million year-old bed of rock salt formation and the hazardous waste is disposed of at a depth of 170m. The storage capacity of the mine is 2 million tonnes of hazardous waste over the next 20 years including incinerator and heavy industry waste and asbestos. Up to 100,000 tonnes of suitably packaged wastes can be handled each year. [Minosus 2009] The facility is licensed to handle different categories of waste including hazardous waste such as air pollution control residues some of which will be disposed of and some stored. The range of waste accepted includes ashes with dangerous substances, which might also be mercury. Currently, the facility is not permitted to receive mercury wastes (source: questionnaire survey reply UK). Under its Environment Agency permit, Minosus can accept 42 different categories of waste included in the European Waste Catalogue. A further 24 potential waste categories are permissible but are subject to Environment Agency improvement orders. [Minosus 2009] While the Minosus facility is exempt from the need to meet the leaching limit values imposed by the Waste Acceptance Criteria Decision, the company does have its own parameters for waste acceptance 55. The waste acceptance procedure follows the provisions of Directive 2003/33/EC (see chapter 5). The containers will be opened upon arrival for further sampling to verify their contents and then sealed again before being taken underground. Before the permit was given to the Minosus mine extensive research and assessments have been carried out related to the long-term safety of the storage site. No other waste management facility, save for those in the nuclear industry, has been as deeply researched and assessed as the Minosus facility [extract of the report commissioned by the Environment Agency and prepared by Cranfield University in 2004, [Minosus 2009]. The elaborated scenarios look forward as far as 50,000 years into the future. 55
121 Reference number /2009/530302/ETU/G2 121 Salt mines in Germany Germany has many years of experience in the storage of hazardous waste in underground landfills. In total, 3 companies are authorised to permanently store mercury containing waste in 5 salt mines whereof one of the sites is not currently in operation. Official long term safety and risk analysis studies exist for these disposal sites. Storage is carried out in depths of several hundred metres. The following is a synopsis of information (on capacities, acceptance criteria, site specific assessment, other information) from the following underground disposal salt mines (UDSM): Germany, Herfa-Neurode (Hesse) underground waste disposal Germany, Zielitz (Saxony-Anhalt) underground waste disposal Germany, Borth (North Rhine-Westphalia) underground waste disposal (valid permit but not in operation) Germany, Heilbronn (Kochendorf, Baden-Württemberg) underground waste disposal Germany, Sondershausen underground waste disposal All in all, approximately three million tonnes of hazardous waste have been disposed of in the two disposal sites Herfa-Neurode (since 1972) and Zielitz (since 1995). Herfa-Neurode was the first worldwide, and is still the biggest hazardous waste underground disposal salt mine. Sondershausen has been in operation since 2006 and Heilbronn since At Heilbronn, Herfa-Neurode, Zielitz and Sondershausen a large variety of waste codes are authorised for underground waste disposal. Apart from the specifically addressed mercurycontaining waste (e.g * wastes containing mercury from inorganic chemical processes) also other types of waste may contain mercury or mercury compounds such as waste types specified as containing heavy metals or containing hazardous substances (Directive 2000/532/EC ). According to information from the operator of the two disposal sites Herfa-Neurode and Zielitz, several ten-thousands of tonnes could be disposed of in a short time frame. Annual technical capacities in Zielitz are 70,000 tonnes, in Herfa-Neurode 200,000 tonnes. Currently authorised capacities at the smaller Zielitz facility amount to approximately two million tonnes (an extension of the capacities is possible). Sondershausen has a remaining storage volume of more than one million m³. Operation of the underground disposals Prior to the transport of the waste to the facility the generator/owner of the waste has to obtain the facilities approval to transport the waste to the facility. For the approval the waste owner has to send a description and analysis of the composition of the waste to the facility owner. After a first check at the disposal site, the documents have to be sent to the relevant authorities and the
122 Reference number /2009/530302/ETU/G2 122 acceptance of the waste has to be approved by the authority. Typical operations in an underground disposal in salt mines include the following [information based on a site visit to Herfa-Neurode]: The waste is transported to the underground disposal site by truck or train. Depending on the waste identity (toxicity, ph, residual moisture, share of particulate matter), appropriate containers (steel panel barrels, steel panel containers or large bags) are used for the transport of the waste. At reception the waste documents, the delivered amounts and the packaging are checked and random samples of the waste are analysed (degassing, visual inspection, chemical composition). Waste is only unloaded if the waste is identified as indicated in the waste documents and fulfils specific waste acceptance criteria. Otherwise the disposal of the waste is rejected. Accepted waste is unloaded and transported to the shaft where it is then transported underground. Underground special purpose vehicles bring the waste to the place of final disposal in the mine. The waste is unloaded from the vehicles and stacked in staples of steel panel barrels, steel panel containers or large bags. Walls are built up and separate the single material groups from each other. As soon as a field is filled, it is closed off with up to 15-metre-wide dams. The underground disposal sites can be organised like warehouses. A sample of each waste is stored in a sample room underground. Storage place and storage time can be documented and waste can be removed from the mine if required. Safety aspects Long term All salt mines can provide proof that the waste is securely isolated, long-term and completely from the biosphere on the basis of an officially accepted certificate issued by an independent institution. The German salt mines are classified as landfill for hazardous waste, underground disposal, D HAZ. Accordingly, they are subject to special requirements listed in Appendix A of Council Decision 2003/33/EC. Operational phase The German salt mines are all acknowledged as certified waste management facilities. In order to minimise risks such as fire, explosion, toxic gas, unintended reactions, unacceptable smell, infections or radioactive contamination, waste is only accepted if it corresponds to specific waste acceptance criteria (see 56, 57, 58, 59 ) (in German)
123 Reference number /2009/530302/ETU/G2 123 In accordance with Decision 2003/33/EC, waste is only accepted if random tests have proven that the waste has the identity as indicated in the corresponding waste documents and fulfils the waste acceptance criteria. Otherwise the disposal of the waste is rejected. Depending on the waste identity the waste is disposed of in appropriate containers (steel panel barrels, steel panel containers or large bags) and eventually an appropriate inner packaging in order to facilitate the handling of the waste (e.g. during sampling) and/or to protect the containers from corrosive waste. Salt mines are typically equipped with a ventilation system. According to the information received from the operator of Herfa-Neurode, the salt mine is equipped with a permanent monitoring system which apart from other parameters already monitors the mercury concentration in the air Economic information hazardous waste in salt mines According to information from the operators of the disposal sites Herfa-Neurode, Zielitz, Niederrhein, Heilbronn and Sondershausen (all in Germany), the costs for disposal of 1 tonne of hazardous waste is approximately euros, irrespective of the hazardousness of the disposed waste (e.g. metallic mercury or pre-treated mercury). The only condition is that the site-specific waste acceptance criteria are fulfilled. The upper end of the price already includes additional costs which might result from specific storage requirements for hazardous waste (e.g. separate chamber, isolated area). The prices are based on recent conditions. Depending on additional requirements that facilities have to fulfil for the storage of liquid mercury (e.g. regular monitoring and inspection), the price might be higher. The costs for temporary storage in salt mines depend on the necessary additional monitoring, inspection requirements and the costs for the retrieval of the stored material Environmental and safety aspects related to the storage of hazardous waste in salt mines Due to its plastic deformation behaviour, salt rock may completely enclose metallic mercury in a gastight and impermeable geological barrier. Under natural disposal conditions, rock salt is practically impermeable to gases and liquids. [BGR 2007] A study [Siemann 2007] investigated the origin and migration behaviour of mineral bonded gases in evaporite (salt rock). The study concludes that gases which have been generated during the sedimentation and diagense (forming of the rock) have not moved significantly before they have been finally fixed in the investigated salt rock. This means that the gases have been fixed in the salt rock for 250 million years. Undisturbed salt rock can therefore be seen as gas tight even in cases of the easily migrating hydrogen molecule (in German) 59 (in German)
124 Reference number /2009/530302/ETU/G2 124 In long term storage, the only effective barrier to prevent hazardous waste entering the environment is the salt rock and its specific isolation criteria. Therefore, a minimum thickness of the salt layer is needed around the waste to ensure the safe encapsulation of it. For short-term storage, additional engineered barriers, such as containment or constructed barriers, can be applied. For the storage of radioactive waste, minimum requirements for the thickness of the host rock have been established to ensure a safe storage [BRG 2007]. These criteria are included in section On the basis of literature available on the subject (e.g. [Popov 2006], [IAEA 2009]), salt mines in general are seen as appropriate for the storage of hazardous waste. But only mines located several hundred metres below the ground surface should be considered as appropriate for storage of hazardous waste [Popov 2006]. [Env Canada 2001] also included the disposal of mercury waste in conventional mines and solution mines in its analysis. While solution mines 60 have been assessed as less appropriate with regard to health, safety, environment and plant operations, the disposal of mercury waste in conventional mines (e.g. slat, potash, gypsum, limestone or underground granite) has been assessed as highly suitable for the disposal of excess mercury. But only on condition that pre-treated waste containing mercury is placed in a stable semi-soluble form in containers. According to [Env Canada 2001] conventional mines could also be used as a long-term underground warehouse, if retrievability for recycling were desired. [USEPA 2002c] included in the analysis of alternatives for the long-term management of excess mercury the temporary storage of liquid (bulk) mercury as well as the disposal of pre-treated (stabilised) mercury waste. In particular, the temporary storage of liquid mercury in an already existing mine cavity has been evaluated as an appropriate storage option for liquid mercury. In Germany, the disposal of liquid mercury in salt mines is seen as a long term safe solution as long as all legal requirements are fulfilled and the long-term assessment of the underground facility allows the storage of liquid mercury (source: questionnaire survey German EPA). However, until now only very limited information is available related to the behaviour of liquid mercury in salt rock. First research results relating to the solubility of metallic mercury and mercury compounds in saline solutions are available but have to be further investigated [GRS 2008A, personnel information: Mr. Hagemann, GRS]. Indications suggest that the solubility of mercury in salt solutions is lower compared to pure water [GRS 2008A] but is nevertheless significantly higher compared to mercury sulphide for example, see also section According to information from German authorities, a project is planned to test the behaviour of metallic mercury in salt and salt solutions. The intended start of this project is in 2010 (source: 60 Mines which have been created by solution mining which means the extraction of the materials from the earth by leaching and fluid recovery.
125 Reference number /2009/530302/ETU/G2 125 questionnaire survey German EPA, personal communication Ms. Hempen, BMU 61 ). According to the information received from the German Environment Ministry, permanent storage of metallic mercury in a German salt mine would not be authorised before the results of the study are available. Most probably the planned project also includes investigations about the behaviour of stabilised mercury or mercury compounds in salt rock. There are also concerns related to salt host rock as a permanent storage site for liquid mercury. A Swedish report [SOU 2008] states that salt mines have properties which enable the waste to be completely enclosed. But for this to occur it is important that the deformations occur without cracks, and the shafts, inspection drill-holes and the like that link the terminal storage facility to flowing groundwater are properly sealed. If waste contamination leaks from the salt formation, it is crucial that the surrounding rock has a natural ability to immobilise it, to ameliorate the effects of a leak. In the report, possible scenarios for the permanent underground storage of liquid mercury in salt mines and related potential environmental risks (see [SOU 2008] Safety analysis and scenarios for salt mine storage ) are described. The main concerns are: Possible sinking of the heavy mercury (which is seen as a long process that can take place over hundreds or thousands of years) and thus increased risk of liquid mercury coming in contact with open fissures Salt rock formations are affected by convergence, thus the waste is subject to pressure over time which might result in it being squeezed out, into the access shaft for example. Fissures in the salt rock might result in a release of the liquid mercury or mercury vapour into the biosphere. Chemical reaction in the storage site (e.g. reaction between mercury and containment) might result in gas formation and a corresponding pressurisation with the risk of mercury being pressed out through sealing plugs, fissures or pores of the rock. Corrective measures and retrieval of waste is more difficult in cases where liquid mercury is stored without containers; in addition, mercury might very efficiently leach through existing pores and fissures and the ability of mercury to penetrate might also cause new pores and fissures. Possible plug leaks due to very high petrostatic pressure at greater depths. As a consequence an effective enclosure of the mercury at a depth of 500 m would require plugs with a very dense structure (max. pore radius: nm). 61 BMU: Bundesministerium für Umwelt, Naturschutz und Reaktorsicherheit (Federal Ministry for the Environment, Nature Conservation and Nuclear Safety, Berlin)
126 Reference number /2009/530302/ETU/G2 126 During a workshop at Oxford in October in particular the lack of information related to the behaviour of mercury and mercury compounds in salt rock has been raised as major concern. No post-closure models related to the long term behaviour of liquid mercury or other mercury compounds are available up to now. According to expert opinions expressed during the workshop, post-closure models developed for the disposal of radioactive waste could be adapted to the specific characteristics of mercury Conclusions: salt rock Valuable information on the properties of salt rock is available in particular from the research for a safe nuclear waste disposal ([GRS 2008], [BGR 2007]). In particular, the salt rock properties such as gas and liquid impermeability, total encapsulation of the waste, very low hydraulic conductivity and high stability of cavities qualify salt rock as a host rock for metallic mercury as well as for other mercury compounds [GRS 2008], [Popov 2006]. Apart from the rock properties the stability of the formation, the overlying impermeable strata and the exclusion of water entering the storage site are crucial for underground storage sites in salt mines [Popov 2006], [WAC Decision]. The geological properties of existing underground disposals sites in salt rock in Europe which might be relevant for the permanent or temporary storage of liquid mercury are well investigated to reduce the probability of unexpected incidents. A site-specific risk assessment as outlined in the WAC decision, Appendix A and prepared by independent experts or institutions is crucial to determine the effectiveness of the host rock as a geological barrier and its capability to isolate the waste from the biosphere over a very long time. Based on the site specific risk assessment a list of waste is derived which is allowed to be stored in the salt mine. In salt mines only waste can be stored which is specifically permitted for the site. In Europe currently 5 underground salt mines are authorised as underground disposal sites for hazardous waste. Experience with regard to the storage of liquid mercury as well as large amounts of stabilised mercury (e.g. mercury sulphide) in salt rock is not yet available. The only experience available is from storage of mercury containing waste in a salt mine in Germany over several decades. Several studies ([USEPA 2002c], [Env Canada 2001]) assessed salt mines as an appropriate option for stabilised mercury. [USEPA 2002c] evaluated the temporary storage of metallic mercury in existing cavities in salt mines as a possible option. In general salt mines are seen as safe disposal options for hazardous waste ([Popov 2006], [IAEA 2009], but concerns related to a permanent storage of metallic mercury still remain [SOU 2008] due to its specific properties. Up to now, specific studies or risk assessments related to the behaviour of metallic mercury in salt rock are still missing presentations/
127 Reference number /2009/530302/ETU/G Hard rock formations Properties of crystalline hard rocks In the following, the most important properties of crystalline rock (e.g. granite and metamorphic rocks) are summarised (source: [GRS 2008], [GRS 2009], [Popov 2006]: Table 6-3: Overview of properties of crystalline rock Criteria Permeability Hydraulic conductivity Mechanical Strength: Deformation behaviour: Stability of cavities: In situ stresses: Dissolution behaviour: Sorption behaviour: Properties Very low (unfractured) to high (fractured) Very low to high High Brittle High (unfractured) to low (strongly fractured) Anisotropic Very low Medium to high The hydraulic conductivity of crystalline rock depends to a great extent on its physical state (whether fractured or not). Unfractured crystalline rock has a low hydraulic conductivity. In [GRS 2008] the following values have been indicated for crystalline rock: Average depth of N of Hydraulic conductivity (Kf in m/s) samples samples Range Median value Granite m x x x Gneiss x x x The permeability of the rock is highly dependent on whether it is fractured or not. In situ stress (anisotropic) in hard rock formations and the typical deformation behaviour (brittle) may lead to fractures in the host rock (see [GRS 2009]). Hard rocks are effectively self-supporting and minimal engineered support and maintenance is required to prevent failure of the rock walls in the emplacement cells and access drifts. Maintenance of rock support, if necessary at all, is not expected to be required over extended periods (see [IAEA 2009]). Crystalline rock has excellent stability of the drifts and rooms even at large depths but it has a relatively high permeability [Popov 2006]. The creep potential of crystalline rock is very low and thus self-healing is unimportant.
128 Reference number /2009/530302/ETU/G2 128 In the case of hard-rocks (crystalline and sedimentary), total containment is not possible (due to its brittle deformation behaviour, cracks and faults in the host rock may occur and liquids and gases could escape from a hard rock depository). In such cases, an underground storage needs to be constructed in a way that natural attenuation of the surrounding strata mediates the effect of pollutants to the extent that they have no irreversible negative effects on the environment. This means that the capacity of the near environment (engineered barriers) to attenuate and degrade pollutants as well as the state of the waste (e.g. solid waste with a low solubility and volatility) will determine the acceptability of a release from such a facility (see Council Decision 2003/33/EC). The investigation of the rock structure of crystalline rock (granite) is very limited in particular with respect to hydraulic conductivity [GSR 2008]. The homogeneity of the rock is strongly site-related and examination of a homogenous rock structure is very complex [GRS 2008]. Low permeability is only guaranteed in unfractured rocks. In the case of fractured rocks, engineered barriers (such as appropriate containers, backfillings) are required to avoid contamination of the environment. For the backfilling of rooms and drifts, dense clay material rich in smectites seem to be the most appropriate material for crystalline rock. Following an article by Pusch published in [Popov 2006] German Friedland Ton appears to represent an optimum with respect to costs and good isolating properties. The article refers to a study published in 2007 by Roland Pusch [Pusch 2007] which investigated whether toxic, non-radioactive chemical waste can be safely stored underground. A major issue of the study was to develop techniques for the isolation of hazardous waste primarily mercury in solid and solidified form (batteries). Various techniques for preparation and application of the clay-based materials have been tested and found to be very effective as near-field isolation of solid waste represented by mercury batteries. The best isolating medium turned out to be dense clay material applied in the form of pre-compacted blocks of clay powder or as on-site compacted clay layers. According to the study, deep abandoned mines appear to be suitable for the disposal of solid hazardous waste because of low costs and suitable chemical conditions. The study concluded that solid or solidified mercury waste and other solidified hazardous waste can be isolated from the biosphere for hundreds of thousands of years and that subsequent groundwater contamination will be lower than stipulated by the EU. The study also covers estimations of the rock mechanical stability around drifts and rooms suitable for disposal of such waste. Dense clay (bentonite) is also recommended by [BGR 2007] as appropriate backfilling material for crystalline rock. Experiences related to the storage of waste in crystalline rock are available but only for stabilised waste.
129 Reference number /2009/530302/ETU/G Properties of other sedimentary hard rocks (e.g. claystone) Argillaceous rock covers a wide range of rock types from plastic clays, with transitional types, to strongly consolidated and partially fractured claystones. Argillaceous rock formations in France (Callovo-Oxfordian), Canada (Ordovician argilites) and Switzerland (Opalinus Clay) are highly consolidated sediments. In the following, the most important properties of argillaceous rock (e.g. granite and metamorphic rocks) are summarised (source: [GRS 2008], [GRS 2009], [Popov 2006]): Table 6-4: Properties Permeability Overview of properties of Argillaceous rock, Clay / claystone Argillaceous rock, Clay / claystone Very low to low Hydraulic conductivity Mechanical strength Very low Low to medium Deformation behaviour Plastic to brittle Stability of cavities In situ stresses Dissolution behaviour Sorption behaviour Artificial reinforcement required Anisotropic Very low Very high Argillaceous rock has a very low hydraulic conductivity but poor stability and the vicinity of the drifts may be very conductive. In [GRS 2008] the following values related to hydraulic conductivity have been indicated for argillaceous rock: Average depth of N of Hydraulic conductivity (Kf in m/s) samples samples Range Median value Argillaceous rock m x x x Argillaceous rock formations possess relatively high mechanical strength, depending on the particular structure (fracturing) and mineralogy of the rock. However, these may exhibit some plastic behaviour, which progressively reduces fracturing but they may also lead to excavation damage zones around excavations in the repository, depending on the support and rock characteristics. Appropriate support would be required for operational safety, although it is considered that excavations could be kept open with suitable maintenance over extended periods. In argillaceous
130 Reference number /2009/530302/ETU/G2 130 rock, short term support (from a few months to some years) is often provided by means of rock bolts with metallic arches, metallic meshes and/or shotcrete. Concrete linings can subsequently be deployed to provide mechanical stability for a longer period. In the case of Boom Clay in Belgium, mechanical support by liner systems is required. Regular maintenance of the excavation lining may be necessary should the access to excavation remain open to enable easy access to the waste emplacement cell. The frequency and scale of any maintenance work will depend on the deformation rate of the rock at the proposed depth and on the design and properties of the lining. In-situ-stress in clay rock formations (anisotropic) and the typical deformation behaviour (plastic to brittle) may lead to fractions in the host rock. Cavities are often not self-stable but must be supported by mechanical structures (see [GRS 2009]). The investigation of the rock structure of consolidated argillaceous rock is possible by means of boreholes and other geophysical methods as they have a limited thickness and composition [GRS 2008]. According to [GRS 2008] argillaceous rock is generally assumed to have adequate strength for the construction and maintenance of underground drifts, but the stability of drifts can only be guaranteed by additional reinforcement and supporting measures. These measures are particularly complex and expensive in unconsolidated clays, therefore storage in consolidated clays is more appropriate. Analogous to crystalline rock, clay material rich in smectites are particularly relevant as backfilling material due to their high isolating potential. [Popov 2006]. See also backfilling crystalline rock. Argillaceous rocks have proven their long-term effectiveness as geological barriers where they form tight seals, for example above hydrocarbon reservoirs. Mineralogical, geochemical and geotechnical investigations of argillaceous rocks are currently being conducted in international rock laboratories. Little information is available due to a lack of mining experience with these rocks [GRS 2008] Experience of underground disposal of mercury in hard rock formations Although several hard rock mines (active and inactive) exist in Europe, experience with the disposal of mercury in hard rock formations is very limited. In deep underground hard rock formations typically solid industrial waste such as fly-ash from incineration plants is stored [Popov 2006]. These waste types might contain small amounts of Hg but only in a solid matrix. Sweden There is no underground disposal for mercury waste at the moment in Sweden. However it has been assessed that Swedish bedrock should be able to meet specific requirements [SOU 2008].
131 Reference number /2009/530302/ETU/G2 131 The Swedish government has commissioned an inquiry into permanent deep bedrock storage of mercury-containing waste. The inquiry commenced in mid-2005 and a final report was presented on 31 January The report analyses the permanent storage of mercury in deep bedrock and salt mines. A summary of the report (in English) provides an account of permanent storage options for mercury-containing waste, and the requirements and risks attendant to the permanent storage of liquid mercury [SOU 2008]. According to the report, the technical conditions to build secure underground depositories in stable geological formations are very good. Deep bedrock deposition in mines or at an existing bedrock facility enables the permanent storage of long-lived hazardous waste, providing both technical advantages and extensive safety margins. The latter point is naturally dependent on the enclosure of the waste in a massive geological barrier. Deposition of long-lived, potentially hazardous waste in underground depositories provides safety advantages that markedly exceed the current European practice of surface storage for this type of waste. [SOU 2008] This report states further that all waste, including metallic mercury, must be appropriately stabilised prior to deposition. Direct deposition of metallic mercury for example in steel containers as an alternative to the storage of stabilised mercury has disadvantages in terms of safe deposition, and raises new issues which currently lack an adequate knowledge base. Clarification of these key issues is required to consider the deposition of liquid mercury as a serious alternative. Therefore the report states that for practical adaptation, it is reasonable that the necessary safety analyses in case of the deposition of liquid mercury demonstrate that safety margins correspond to what can be achieved with stabilised mercury deposited in deep geological formations, such as Swedish bedrock [SOU 2008]. Norway According to a Norwegian report [Kystverket 2008], it could be a problem to find a suitable location for deep geological disposal in Norway. Though Norwegian storage locations may fulfil the criteria of stable physical and chemical conditions there is the problem that most storage locations in rock are relatively shallow (<100 m) and not at several hundred metres depth as required in the WAC Decision. In Norway there is a need to store mercury containing waste from zinc-production (one site). Mercury from zinc-production is a by-product and is treated as waste for final disposal. The mercuryresidue from zinc-production is cemented into sarcophagi and placed in a bedrock hall at the production site. [NO 2005] Another Norwegian study has investigated the environmental, safety and health consequences from salvaging mercury and mercury-contaminated sediments from a sunken submarine [Kystverket 2008]. At least two facilities have permits for disposal of mercury containing waste (mercury content max. 10%). Possible storage locations and costs for the disposal of hazardous waste are included in the study. The following possible underground storage locations are cited in the study:
132 Reference number /2009/530302/ETU/G2 132 NOAH AS, Langøya, Norway NOAH is Norway s largest disposal facility for hazardous waste. It has a permit to receive a total of 622,000 metric tons of different types of waste per year, including 322,000 metric tons of inorganic hazardous waste per year. Since the year 2000, NOAH has received approximately 200,000 tons of mercury waste (10% Hg). It has developed a stabilisation method in cooperation with the University of Oslo, where mercury is absorbed into gypsum and iron hydroxide. The maximum allowed discharge of mercury to water is kg/day. NOAH is situated on the island of Langøya and waste can be transported directly to the island by ship. Miljøteknikk Terrateam AS, Mo i Rana, Norway Miljøteknikk Terrateam has a large disposal facility in the rock caverns of the former steel works in Mo i Rana. Miljøteknikk Terrateam has a permit to receive 70,000 metric tons of inorganic hazardous waste per year. The waste has to be stabilised/solidified before placement into the rock cavern. Maximum allowed leaching of waste containing mercury which has been stabilised/solidified is 0.01 mg Hg/l. The leached amount is determined by using the United States TCLP 63 (Toxicity Characteristic Leaching Procedure) test. There are also other possible disposal facilities in Norway: Boliden Odda AS, Odda Boliden Odda has large rock caverns for disposal of mainly jarosite-bearing sludge from smelters, but also other waste streams containing mercury sulphide compounds. They have 14 large rock caverns and each is 75, ,000 m 3. The waste is placed in plastic drums and is then cast in concrete in the rock caverns. BIR (Bergen Interkommunale Renholdsverk), Hordaland BIR has a disposal facility for hazardous waste in a rock cavern in Stendafjellet. Its permit would probably have to be revised to be able to receive mercury. Disposal of mercury waste in Norway (allowed for waste with max 10% Hg) will need stabilisation prior to disposal. According to [Kystverket 2008] binders for stabilisation could be gypsum, cement, sulphur and sulphides. The report recommends a temporary storage while immobilisation technologies are developed. Temporary storage could typically be in salt mines (which are already available), rock caverns, preferably in deep bedrock permanent depositories in order to achieve non-oxidative conditions [Kystverket 2008]. 63 The leaching tests of various literatures refer to different standards, which make a direct comparison of the results impossible. In the United States the toxicity characteristic leaching procedure (TCLP) is typically used, whereas in Europe the leaching standard (EN 12457/1-4) is used. (The rarely-used percolating test (pren 14405) is also possible.) With the American TCLP test, a liquid/solid ratio of 20 is used whereas the European leaching test uses a liquid/solid ratio of 10 or 2. Hence, the same material results in higher concentration values in the European measurements.
133 Reference number /2009/530302/ETU/G Economic information hazardous waste in hard rock In 2001 the report [SOU 2001] published by the Swedish EPA estimated the cost of a deep bedrock repository having a capacity of about 1,000-20,000 tonnes of high-level mercury waste to be about SEK million. This represents a cost of approximately SEK 250, ,000 per tonne of pure mercury. The higher figure represents storage of mixed waste such as process waste containing 1-10% mercury. The report [SOU 2008A] contains updated and more detailed information relating to expected storage costs of stabilised mercury. The figures below were received from Johan Gråberg, Swedish Ministry of Environment, and give an overview of estimated costs for the construction of a permanent deep bedrock storage of mercurycontaining waste in connection with an existing bedrock facility (all cost figures have been converted from SEK to Euro by using an exchange rate of ~0.1 euro = 1 SEK). - The estimated investment cost for a deep bedrock storage established adjacent to an existing or former mine or bedrock facility is around 900-1,500 euros per m³ at 10,000m³ stored volume or euros per m³ at 100,000 m³ deposited volume. - The construction of an entrance ramp is estimated to cost around 5,000 euros/meter. - The cost for an underground deep bedrock depository is around 50 euros per excavated m³ volume. - Further costs for equipment such as pumps, cables, ventilation, lights etc should be added to these costs. The investment cost for equipment is estimated at percent of the construction cost. In addition to this, operational costs should be added for pumping and ventilation (100, ,000 euros/year), staff (100, ,000 euros/year) as well as costs for loading, unloading and transportation of the waste (20,000 euros/year). In total, operative expenses amount to 250, ,000 euros/year. The report [Kystverket 2008] did not make any assumptions about costs relating to the storage of elemental mercury. The report only refers to the assumptions made in the Swedish Report [SOU 2001] Environmental and safety aspects related to the storage of hazardous waste in hard rock Total enclosure of the waste by the host rock is not possible in hard rock depositories [SOU 2008]. Due to its brittle deformation behaviour, hard rock cannot encapsulate metallic mercury or mercury compounds. Therefore, additional artificial or engineered barriers are needed to ensure a safe encapsulation of 64 Exchange rate (October 2009): 10 SEK = around 1 euro
134 Reference number /2009/530302/ETU/G2 134 the hazardous waste over a very long time. Although hard rock has a very low hydraulic conductivity and gas permeability under the condition it is unfractured the investigation on the homogeneity of the rock is very complex [GRS 2008]. It is difficult to exclude the occurrence of fractures or faults for a relevant dimension of the host rock [GRS 2008]. Containers, which for instance might provide an important additional safety factor for the storage of metallic mercury, cannot be considered for long-term storage (see Decision 2003/33/EC, Appendix A, point 1.2.7). Therefore considerations for long-term safety might be based solely on engineered barriers. A presentation prepared by the Swedish environmental agency [Eriksson 2006] made the following recommendation relating to underground storage in bedrock (the Swedish solution for mercury waste): - the responsibility for safe storage rests on the waste owners - mercury in waste streams should be extracted and converted into an insoluble form - the storage facility should be located at least 400m below ground in granite bedrock The Swedish EPA concluded as Swedish mercury strategy [Eriksson 2006] that it should; Reduce emissions as far as possible Phase out use in products and processes Collect mercury already in use Effect terminal disposal In addition, the fundamental properties of the surrounding bedrock have been defined as follows (for stabilised waste only) [Eriksson 2006], [Höglund 2009]: Low water permeability Absence of major fracture zones Chemically stable environment Mechanically stable environment Reduced risk of unintentional disturbance Long transport paths to the surface Dilution potential in the recipient bedrock
135 Reference number /2009/530302/ETU/G2 135 The presence of ground water flow in hard rock formations cannot be excluded, but the exchange rate of deep groundwater in hard rock is expected to be very low [Höglund 2009]. Investigations and estimates on possible maximum mercury releases from stored mercury sulphide have been made for a specific underground mine in Sweden. The study concluded that a maximum release of g mercury/year might be possible under the given conditions (underground water flow). With such a release rate, existing environmental limit values would be upheld [Höglund 2009]. For comparison, the hypothetical release rates calculated for non-stabilized mercury waste without any engineered barriers and with adequate engineered barriers have been calculated. In addition it was estimated that the effect of chemical stabilisation of metallic mercury would further reduce the release rates by a factor of 100 in all alternatives [Höglund 2009]. In the studies [Env Canada 2001] and [USEPA 2003] the permanent storage of pre-treated (stabilised) mercury is assessed as an appropriate solution for the storage of excess mercury. In [USEPA 2002c] the temporary storage of liquid (bulk) mercury in existing mine cavities has been identified as a possible option. In the report [SOU 2008], the storage of liquid mercury in deep underground hard rock formations is not recommended Conclusions: hard rock formations In hard rock formations a total enclosure of the waste by the host rock is not possible ( [Council Decision 2003/33/EC] Appendix A, Nr. 4.1). Thus, the attenuation and degradation capacity of artificial barriers determine the long term safety of deep underground hard rock formations ([Höglund 2009], [Popov 2006]). Valuable information on the properties of hard rock (crystalline rock and argillaceous rock) are available from the intensive research for nuclear waste deposits ([GRS 2008],[GRS 2009], [IAEA 2009]). Possible fractures in the hard rock (in particular crystalline rock) and the resulting higher permeability and higher hydraulic conductivity of hard rock formations might cause releases of liquid mercury or mercury vapour into the biosphere [GRS 2008]. Due to the possible presence of groundwater flows in hard rock formations, storage of liquid mercury is seen as more critical due to the higher solubility in comparison to storage of solidified mercury with its lower solubility ([GRS 2008], [Höglund 2009]). Where storage of stabilised mercury is concerned (e.g. in form of mercury sulphide), the hydraulic situation has to be very carefully taken into consideration to avoid non-acceptable emissions from the storage site into the biosphere via groundwater flows ([Höglund 2009], WAC Decision, Appendix A, Nr. 4.1). Experience with regard to the storage of metallic mercury as well as stabilised mercury (e.g. mercury sulphide) in hard rock formations is not yet available. In deep underground hard rock formations typically solid industrial waste such as fly-ash from incineration plants are stored with small amounts
136 Reference number /2009/530302/ETU/G2 136 of mercury but only in a solid matrix [Popov 2006]. In Norway two underground facilities have a permit for the storage of stabilised mercury containing waste with a maximal content of mercury of 10% [Kystverket 2008]. In addition, a Swedish study assessed Swedish bedrock to be able to meet specific requirements for the storage of stabilised mercury [SOU 2008]. Hard rock formations are seen in particular suitable for the storage of stabilised mercury [SOU 2008], [Höglund 2009]. Literature promoting the storage of metallic mercury in hard rock formations has not been found Radioactive waste The goal of radioactive waste disposal is passive isolation of waste so that it does not result in undue exposure to radiation for humans or the environment, now or in the future. This objective can be achieved by isolating radioactive materials in a disposal system that is located, designed, constructed, operated and enclosed such that any potential hazard to human health is kept acceptably low over required periods of time [IAEA 1994]. This goal is generally comparable with the disposal of mercury. As a consequence, experience made with the geological disposal of radioactive waste may contribute to establishing strategies for the disposal of mercury. Apart from the composition and substance properties, the main differences between mercury waste and radioactive waste under the point of view of storage are that the Hazardousness of radioactive waste decreases over a long period of time radioactive waste is partly heat-generating The aims of geological disposal of radioactive waste are, among others, to contain the waste until most of the radioactivity has decayed and to delay any significant migration of radionuclides to the biosphere until much of the radioactivity has decayed [IAEA 2006]. Though the hazardousness of radioactive waste reduces in the long term - according to the half-lives of the corresponding radionuclides - it still might take several thousand or even millions of years to decay to zero. It has to be noted that the hazardousness of metallic mercury does not diminish, even in the long term Experience from underground disposal of radioactive waste By the late 1970s, it had become clear that underground disposal was the internationally accepted approach for most types of solid radioactive waste. Towards the end of the 1980s, the issue of radioactive waste and its management was becoming increasingly important in the political sphere. It
137 Reference number /2009/530302/ETU/G2 137 was seen as one of the technically unresolved issues of nuclear power. [IAEA 2002]. The IAEA radioactive waste classification system provides a framework for defining a generic approach to radioactive waste management. The system can be linked to potential disposal options for various waste categories based on their specific characteristics, with specific activity and longevity of radioactive constituents being the key distinguishing parameters. High-level and longlived radioactive wastes require a higher degree of isolation and should be predominantly disposed of in geological formations (i.e., emplacement in engineered structures at depths of hundreds of metres). In principle, the higher the activity and the longer the half-life of major radiocontaminants, the deeper the facility should be. In addition, some national approaches to disposal prefer the emplacement of all types of radioactive waste (short and long-lived, low and high level) in geological formations. [IAEA 2007] In developing any disposal system concept, reliance is placed on a multi-barrier system approach in which both the site characteristics and the engineered (technical) barriers, namely the waste form and the packaging, together contribute to the isolation of the radioactive waste from the environment for time periods that are sufficiently long for radionuclides to decay to acceptably low levels. [IAEA 2007] In the meantime, considerable experience has been made concerning the search for appropriate disposal sites, the construction of waste disposal facilities and their operation. However, even today much needs to be done concerning siting, construction and operation of spent fuel and radioactive waste disposal facilities, even if some countries make considerable progress in the establishment of geological disposal facilities [IAEA 2009b]. It is noteworthy that in the disposal strategy for nuclear waste, the retrievability of waste becomes increasingly important [IAEA 2009]. The reversibility of waste management options may also be an important issue in developing disposal strategies for mercury due to various reasons. Arguments for and against retrievability of radioactive waste are listed in [IAEA 2009] (see section 3.1), several of these arguments can also be considered valid for mercury disposal. A very important activity related to the disposal of high level waste and other long-lived waste is the selection of an appropriate underground disposal site. Such a site should have favourable natural confinement characteristics for the waste types under consideration, and should be suitable for implementing all necessary engineered barriers to prevent or retard the potential movement of radionuclides from the disposal system to the accessible environment. Since the natural characteristics of the site play an important role in the disposal concept, site selection activities should be given major consideration in the overall development of an appropriate disposal system [IAEA 1994]. The process of selecting appropriate sites for underground disposal is underway in several countries. Geological disposal of radioactive waste is based on the isolation of waste within the geosphere in locations where it is expected to be stable over a very long time. Repository concepts and potential host rocks differ between countries. The main host rocks considered are igneous crystalline and
138 Reference number /2009/530302/ETU/G2 138 volcanic rocks, argillaceous clay rocks and salts. The choice of host rock is mainly governed by the availability of suitable geological formations of convenient thickness and geological setting. Underground laboratories for testing and building confidence in disposal technologies have been built in all types of potential host rocks [IAEA 2009] Environmental and safety aspects relating to the underground disposal of radioactive waste The long term safety of a geological repository for radioactive waste is based on the concepts of defence in depth and isolation that is provided by the combined effects of multiple, man-made and natural barriers. The definition of an engineered barrier system refers to the container, backfill and buffer sealing materials, and any man-made component that is designed to isolate radioactive waste and limit its release and transport over long periods of time [IAEA 2009]. Scientifically based and well developed exposure models relating to the post-closure safety of geological disposal of long-lived radioactive waste are available and might be adjusted in 3-5 years to predict the long term behavior of mercury or mercury compounds. Information relating to potential host rocks is available and suitable, and can be used as input for such models. The quality and reliability of the post-closure model is based on the reliability of the input data [source: expert discussion at the Oxford workshop 65 ]. These data include information on the specific behaviour of liquid mercury under underground storage conditions (e.g. possible interaction with the salt rock, behaviour under pressure). Operational and long-term safety have to be proven by corresponding safety assessments of the operational phase and long term safety assessments. For example, the German Government recently published safety requirements concerning the final storage of radioactive waste (see [BMU 2009]. Safety standards for the geological disposal of radioactive waste were published by the International Atomic Energy Agency in The report sets out the objective and criteria for the protection of human health and the environment during the operation of geological disposal facilities, as well as for the time after such facilities are closed. It also establishes the requirements for ensuring their safety. According to the report, geological disposal facilities for radioactive waste are designed to ensure both operational safety and post-closure safety. Operational safety is provided by means of engineered features and operational controls. Post closure safety is provided by engineered and geological barriers. [IAEA 2006] Minimum requirements and criteria for geological repositories for radioactive waste have been published in [BGR 2007]. The main objective of this report was to identify suitable host rock formations for a nuclear repository in Germany. Following the report, geoscientific criteria must now 65 presentations/
139 Reference number /2009/530302/ETU/G2 139 be given priority in the site selection process as they define the geological barrier function of the geological repositories: The identification of regions was therefore conducted at the first step by applying the following internationally recognised geoscientific and host rock independent exclusion criteria and minimum requirements compiled in 2002 by the Committee on a Site Selection Procedure for Repository Sites (AkEnd 66 ): - Seismic activity: In the repository area, the seismic activities to be expected must not exceed Earthquake Zone 1 according to DIN Volcanic activity: In the repository area, there must neither be any quaternary nor any expected future volcanism. - The thickness of the isolating rock zone must be at least 100m and must consist of rock types to which a field hydraulic conductivity of less than m per second can be assigned. - The depth of the top of the required isolating rock zone must be at least 300m. - The repository mine must lie no deeper than 1,500m. - The isolating rock zone must have an areal extension that permits the realisation of a repository (minimum 10km² in clay stone). - There must be no findings or data which give rise to doubts as to whether the geoscientific minimum requirements regarding field hydraulic conductivity, thickness and extent of the isolating rock zone can be fulfilled over a period of time in the order of magnitude of one million years.... In the second evaluation step, the following criteria are also considered in the selection process because they are considered to be of crucial geoscientific importance for rock salt and argillaceous rocks. Their application led to the exclusion of additional regions: - The 1995 BGR study defined a minimum thickness of 500m for rock salt deposits in salt domes (300m roof sequence, plus 100m for the underground workings in the mine, plus 100m underneath the mine). BGR is of the opinion that these criteria are still valid today. - The 1995 study stipulated a salt roof of at least 300m above the repository zone in salt domes. The cover rock overlying the top of the salt dome should be at least 200m thick and consist of horizons impermeable to water. - The 1995 BGR study assumed that the minimum area required for a nuclear repository in a salt dome should be 9km 2 for the repository itself. This takes into consideration an outer protective shell with thicknesses of at least 200m, plus a safety margin of at least 20% so that 66 Arbeitskreis Auswahlverfahren Endlagerstandort (AkEnd), this working group was established by the German Environmental Ministry to elaborate criteria and methods for the selection of repositories for the disposal of nuclear waste
140 Reference number /2009/530302/ETU/G2 140 adequate reserve areas are available, and to ensure that the safety margins are not jeopardised by unexpected intercalations of anhydrite, potash seams, etc. The 3km 2 area postulated by AkEnd 2002 is therefore considered to be inadequate. - Another exclusion criterion included for rock salt was the stipulation that the salt body is not affected by any other mining or drilling. - Argillaceous rock formations buried to depths below 1000m are expected to be affected by very difficult rock mechanical conditions, giving rise to very high costs for the excavation and operation of a repository. Another difficulty in the use of argillaceous rocks at depths >1000m is associated with the relatively low heat conductivity of these rocks and the higher temperatures prevailing at such depths. This will lead to considerable technical problems if waste generating large amounts of heat is emplaced. One of the criteria for argillaceous rock formations included in the evaluation was therefore the restriction to depths between 300 and 1000 m below ground level Conclusion Although the disposal of radioactive waste is carried out under different conditions compared to liquid mercury, one principal common aspect is the safe long-term isolation of the hazardous waste material from the biosphere [IAEA 2009]. Therefore, in particular the research and investigation into appropriate host rocks and their function as a geological barrier for a safe storage are relevant for the underground storage of metallic mercury. In particular the exclusion criteria and minimum requirements for depository sites should be taken into consideration when defining acceptance criteria and minimum requirements for the underground storage of metallic or pre-treated mercury. [BGR 2007] published minimum requirements and criteria for geological repositories for radioactive waste. Exposure models related to the post-closure safety of geological disposal of long-lived radioactive waste are available and might be adjusted in 3-5 years to predict the long term behaviour of mercury or mercury compounds 67. Important for these model calculations are reliable input data presentations/
141 Reference number /2009/530302/ETU/G Review of above-ground storage According to Article 3(1)(b) of Regulation (EC) No 1102/2008, metallic mercury that is considered as waste may, in appropriate containment, be temporarily stored for more than one year in aboveground facilities dedicated to and equipped for the temporary storage of metallic mercury. This provision is by way of derogation from Article 5(3)(a) of Directive 1999/31/EC and allows thus to dispose of metallic mercury as liquid waste. Following Regulation (EC) No 1102/2008 above-ground storage of metallic mercury should only be considered as a temporary solution. Therefore, on the one hand the principle of reversibility of storage has to be followed and on the other hand the requirements of protection against meteoric water, impermeability towards soils and prevention of vapour emissions of mercury have to be met in the best way (recital 12). Contrary to underground disposal sites with natural (geological) barriers, above ground facilities mainly have artificial barriers as protection against releases of metallic mercury. These artificial barriers include constructive measures like buildings, special flooring and in particular the packaging system of the waste. Apart from these artificial barriers, adequate surveillance and security measures (e.g. fencing, restricted access, emergency plans) have to be implemented as additional protective measures. The storage of metallic mercury is quite common due to the fact that at present liquid mercury is not considered as a waste but as a raw material. Consequently, experiences are already available relating to the handling, packaging, transport and temporary storage of metallic mercury as a product. The main purpose of these activities is not the storage of metallic mercury over a long period but the stockpiling for a limited short-term period. However, existing experiences can be used to define future criteria for a possible temporary above-ground storage of metallic mercury. The suitability of existing packaging and storage conditions for long-term storage have to be investigated and where necessary adapted. In the following, the two most important existing above-ground warehouses/storage facilities for liquid mercury in Europe (Almadén) and in the USA (DNSC) are described in detail. Mercury as a product is also stored by other companies but only in smaller amounts, for example in chlor-alkali plants, recycling plants.
142 Reference number /2009/530302/ETU/G Europe The information related to the storage of liquid mercury at Mayasa is based on the information received by the questionnaire survey, information available on the internet 68 and additional personal information from Mr. Ramos, Mayasa. In Europe, the Spanish state-owned company Miñas de Almadén (MAYASA), the operator of the former mercury mine, is the major company dealing with liquid mercury, apart from other sources, mainly received from decommissioned chlor-alkali plants. The company uses a reconverted auxiliary above-ground building as a warehouse for the storage of the mercury. The installation is located above a former mercury mine. The metallic mercury is either stored in flasks (34.5kg net), containers (1 tonne) or bulk tanks. The flasks and containers are also used for the transport of liquid mercury and thus fulfil the requirements of transport regulations (for further information on containment, see section 6.4). The filling and re-filling of tanks with mercury takes place via pipes and valves. Displaced air during filling activities is extracted and cleaned via special filters with activated carbon. The purity of the stored mercury is 99.9%. In case the delivered mercury does not fulfil this criterion, a cleaning of the mercury takes place before storage. The bulk tanks are stored in a collecting basin made of concrete which is capable to collect all mercury included in the bulk tanks. All the areas in which mercury is handled, stored or packaged are equipped with specially treated (waterproof) flooring (epoxy based paint) which avoid the infiltration of the metallic mercury in the event of accidental spillage. In addition, the floors have a slight slope directed to a central collecting basin. Although gas displacement systems and activated carbon filters are installed, mercury emissions from operational processes (e.g. filling of tanks) cannot be completely avoided. The storage building is equipped with appropriate Hg-emission monitoring systems. The measurement results are regularly evaluated. Accompanying studies related to possible impacts of mercury emissions have been carried out under the Mersade project (see information below). According to these studies, direct impacts of the emissions to the environmental surroundings are expected at a maximum distance (along the direction of the prevailing wind) of 300m from the central point of the installation. The Hg-emissions from the storage site are estimated (by modeling) to be around 15kg per year [personal communication Mr. Ramos, Mayasa]. Life Project MERSADE Currently, an EU financed Life project 69 with the title Mercury Safety Deposit (Acronym: MERSADE, project reference: MERSADE LIFE06 ENV/ES/PREP/03) is being carried out by Miñas de Almadén y Arrayanes, S.A. (Mayasa) together with its partners CENIM (Centro Nacional de Investigaciones Metalúrgicas) and the University of Castilla la Mancha LIFE is the EU s financial instrument supporting environmental and nature conservation projects throughout the EU, as well as in some candidate, acceding and neighbouring countries, for further information see
143 Reference number /2009/530302/ETU/G2 143 Based on the expertise on handling and storage of mercury of Miñas de Almadén S.A., and by the description of the current and operational installations, this project aims to develop technical support for a plan (for the next 50 years) for defining the packaging to be used during the transport from plants to the site where it is deposited, the procedure for handling the metal and the construction of a prototype installation for depositing surplus mercury coming from the EU. [Mersade 2007] The project is expected to develop a model for the safe deposit of bulk mercury that meets strict safety requirements and prevents mercury emissions after closure. Within the project, measures will be elaborated to minimise emissions during the operational workings through a wide range of passive systems of safety in design and construction, and with a programme of permanent vigilance and an intervention plan if required. The project also includes practical investigations of existing storage containers to identify the most appropriate material for long-term storage. More detailed information on preliminary results and recommendations are available in section The second substantial part of the MERSADE project covers the development of a stabilization/solidification process for mercury and mercury containing wastes. Preliminary information on the developed technology and the resulting product is available in section The project started in October 2006 and was expected to be finalized in September Following information from Mr. Ramos, the project manager, a six-month extension of the project duration was agreed with the EC. Thus the final results of the project will only be available by end of March USA The information related to the storage of liquid mercury at DNSC is based on information available from the MM EIS [DNSC 2004], [DNSC 2004A] [DNSC 2004B], [DNSC 2007], [Hogue 2007] and personal information from Mr. Dennis Lynch (DNSC). In the USA, experiences relating to long-term storage periods already exist. Government owned liquid mercury which is no longer used for military purposes has already been stockpiled for more than 40 years in four above-ground warehouses. The Defense National Stockpile Center (DNSC) stores its liquid mercury in 30-gal drums, each containing six steel flasks that individually hold 34.5kg of the liquid metal in above-ground warehouses. In total, 4,436 metric tons are stockpiled by the Defense Department and 1,200 metric tons stockpiled by the Energy Department (see also chapter 5). As a result of a Mercury Management Environmental Impact Statement (MM EIS) the DNSC is currently in the process of consolidating its mercury holdings from facilities in New Jersey, Indiana, and Ohio at one site, the Hawthorne Army Depot in Nevada. This depot was selected as a future
144 Reference number /2009/530302/ETU/G2 144 storage site for liquid mercury currently stockpiled. The depot fulfilled the requirements set out for the storage site. The defined storage period is 40 years. The selected mercury storage warehouse at Hawthorne Army depot has to be upgraded to fulfill the required safety standards for long-term storage of metallic mercury. In general, the following safety requirements and level of protection have to be fulfilled by the warehouse [DNSC 2004]: Sealed warehouse floors (without drains) with epoxy mercury-resistant sealer (Intrusion protection) Intrusion detection Adequate lighting for inspection Static ventilation All doors fitted with 3 inch containment dikes that are incorporated into floor sealant systems Heat, smoke and fire detection system monitored continuously Fire protection system (active fire suppression system, fire extinguisher and alarm system) Closely controlled access (Security systems) Regular monitoring (routine monitoring and inspections of mercury) Protective equipment and supplies Emergency procedures (spill prevention control and response procedures) Positive contact intrusion detection on all doors, windows and vents monitored continuously Ramped containment dikes Figure 6-3: Metallic mercury storage at the Defense National Stockpile Center (source: DNSC)
145 Reference number /2009/530302/ETU/G2 145 The warehouses at the Hawthorne Army Depot are constructed with concrete support columns, steel roof trusses, and transite roofing. The warehouses have concrete floors and walls (resistant to fire). Another option at the Hawthorne Army Depot is the use of earth-mounded storage buildings (igloos).the site has 393 empty, usable igloos. The igloos are made of steel-reinforced concrete and covered with about 2ft (1m) of soil. The mercury could be stored in about 125 igloos. Analogues to the existing warehouses the new site will have approved Spill Prevention Control and Countermeasures and Installation Spill Contingency Plans to ensure that the appropriate response to a spill is made. State and local emergency response teams are aware of the mercury storage. In case of a mercury spill, an appropriate response would occur and the spill would be cleaned up to applicable standards. Public access to the storage site is restricted by a security system, including guards, locked warehouses, and other measures. Warehouses are kept locked except for inspections and other periodic maintenance work. In addition to security, perimeter fencing, and closely controlled access comparable to the levels of protection at the current mercury storage sites, DNSC would work with local authorities to ensure that even the most unlikely scenarios would be handled properly. Maintenance and Inspection Apart from the technical safety measures, periodic maintenance activities and inspections of the stored mercury by appropriately trained DNSC or contract personnel are essential to ensure that it is safe and secure. Inspections have to be conducted by trained personnel and include the following methods: visual examinations mercury vapor monitoring using state-of-the-art equipment. In 2002, the DNSC issued the Environmental Inspection Plan for Mercury in Storage (Appendix 4 A in the Defense National Stockpile Operations and Logistics Storage Manual). The main purpose of this manual is to improve the inspection and reporting process for mercury storage. The plan also documents the correct storage and control measures that are required for the protection, safety, and health of workers and the public, and protection of the environment. The manual provides procedures for: Frequency of inspections Temperature, barometric pressure, and humidity measurement Vapour monitoring Visual inspection Documentation and records
146 Reference number /2009/530302/ETU/G2 146 Corrective action In case the DNSC action level of mg Hg/m³ is exceeded or if metallic mercury is found during a visual inspection, an investigation has to be initiated to determine the cause. Any defects in the packaging have to be quickly corrected. Costs The facility at Hawthorne will be operated by a contractor. DNSC estimates that storage of mercury at Hawthorne will cost $.0515 per lb per year, for a total of a little more than $500,000 per year for the military's entire stockpile of mercury [Hogue 2007]. Cost estimates are also available associated with the permanent, private sector storage of elemental mercury as a method of safe management of excess non-federal mercury supply. The USEPA study [USEPA 2007a] examined the costs of private sector storage under two storage scenarios: a storage facility that uses rented warehouses and a storage facility that includes construction of warehouses specifically for mercury storage. Estimates of total storage costs assume that over a 40-year period, either 7,500 or 10,000 metric tons of excess mercury supply will require storage. Table 6-5: Summary of Estimates of Total Storage Costs (US Dollars) for 40 Years [USEPA 2007a] Storage Capacity Total Cost Estimates Rent Scenario Build Scenario 7,500 ton Total Project Costs (undiscounted) million million Net Present Value of Total Project Costs million million Annualized Costs million million Annualized Costs per pound ,000 ton Total Project Costs (undiscounted) million million Net Present Value of Total Project Costs million million Annualized Costs million million Annualized Costs per pound Note: present value calculation assumes a seven percent discount rate. 6.4 Review of containment Container systems currently in use The packaging system is an integrated element of a safe storage of metallic mercury in particular in the case of temporary storage. It is an engineered barrier which is designed to ensure operational
147 Reference number /2009/530302/ETU/G2 147 safety during interim storage, transport and waste package handling operations, and may provide a long term containment function [IAEA 2009]. In the following, the standard steel containers used in Europe for the transport and stockpile of liquid mercury as raw material are described. In addition, the foreseen packaging system for the storage of metallic mercury at the DNSC is described. The system is designed to be safe for a period of 40 years Europe The information related to the packaging of liquid mercury is based on the information available, personal information from Mr. M. Ramos, Mayasa. Currently, for the transport and stockpile of liquid mercury standard gas and liquid-tight steel flasks (34.5 kg net 70 ) and containers (1 metric ton net) are in use in Europe. Both are UN-approved (see also section below) and meet the requirements for transport on the road (ADR 71 ), by rail (RID 72 ) and ship (IMO 73 ). In addition, the smaller flasks meet the requirements for the shipment by air (IATA 74 ). Both containers are made of steel with a lacquered interior. For further information on the container material see section Figure 6-4: Examples of standard mercury steel containers used by Mayasa (source: Mayasa) The international unit of measurement of mercury, a 34.5 kg flask, is originally from Almadén and equal to three old Castilian arrobas of 11.5 kg Agreement on Dangerous Goods by Road Regulations concerning the Intl Transport of Dangerous Goods by Rail International Maritime Organisation International Air Transport Association
148 Reference number /2009/530302/ETU/G2 148
149 Reference number /2009/530302/ETU/G2 149 The flasks are suitable for strapped to standard wooden pallets (115 cm X 115 cm x 13.5 cm). The 1 metric tonne containers have a height of 66.2cm and a diameter of 70cm. Costs: The costs for the current carbon steel flask mainly used (34.5 kg) are around 10/flask, for a 1 tonne container the costs are around 700 [personal information: M. Ramos, Mayasa]. Other figures vary from 600 to 1,100 (stainless steel) for the 1 tonne container [personal information by Euro Chlor] USA (DNSC) The information relating to the packaging of liquid mercury is based on the information available from the MM EIS [DNSC 2004B], [DNSC 2007], [Hogue 2007] and personal information from Mr. Dennis Lynch (DNSC). The DNSC has 4,436 metric tons of mercury in inventory. The purity of the mercury is between 99.5 and 99.9%. In total, the metallic mercury is stored in 128,662 steel flasks. The mercury inventory is contained in flasks made of 0.2-in (0.5-cm) thick, low-carbon steel. Each flask contains 34.5kg (76lb) of liquid mercury and is sealed with a threaded pipe plug. Figure 6-5 shows the dimensions of a typical flask. Currently, two types of flasks are in use. Newer flasks are seamless and thus they are not as susceptible to leakage as the older, welded flasks. The older flasks have already been in use for 50 years. Figure 6-5: typical mercury storage flask [DNSC 2007] In order to prepare the older flasks for the long-term storage of 40 years, an overpacking of the flasks took place. This overpacking consists of a 30 gal (114 l) air & liquid tight steel drum. Each drum is made of 16-gauge, carbon steel and has a removable lid. The drums are lined with an epoxy-phenolic coating and to improve handling in case of possible leaks, the flasks are enclosed a 6-mil, plastic bag
150 Reference number /2009/530302/ETU/G2 150 and sealed with wire ties. Six flasks are placed in each drum with an absorbent mat on the bottom. The absorbent mat also serves as a cushioning material. Cardboard dividers that are 1/4-in (0.64-cm) thick keep the flasks separated (see picture below). The drums are closed with a half inch rubber gasket and a bolt, providing a water- and air-tight seal. Figure 6-6: Overpacking concept of mercury containing flask [DNSC 2007] Before being placed in the drum, each flask was removed from its pallet, cleaned by a mercury vacuum cleaner, and checked for leakage. The plug on each flask was also checked to make sure that it was secure. The drums are stored on metal catch trays made of 12-gauge, painted carbon steel. Each catch tray is 1-in (2.5-cm) deep and can collect the contents of several flasks. The catch trays rest on wooden pallets. In order for pallets to be easily inspected, they are not stacked at the consolidation site. The overpack drums meet the U.S. Department of Transportation s (DOT s) packaging requirements for shipping hazardous materials by highway and rail (Title 49 Code of Federal Regulations [CFR] (d)(2)). Overpacked mercury would be stored at a consolidated storage site for 40 years. The DNSC assumes that the overpack drums would not fail during that time. The overpack drums would be opened during the last year of storage, and the flasks would be checked for leaks. The DNSC assumes that some flasks would leak and would need to be replaced. Waste flasks would be moved to a treatment facility for retort and reclamation of scrap metal. The treatment and disposal or recycling of wastes are not evaluated in this MM EIS because these activities would occur in commercial facilities with permits for routinely performing these types of activities. Of the 108,386 flasks inspected at the New Haven, Somerville, and Warren depots, only eight flasks, all from the New Haven Depot, were found to be leaking. The eight flasks were replaced and the old flasks were placed in a 55-gal (208-1) drum and sent to a licensed commercial facility for treatment and disposal.
151 Reference number /2009/530302/ETU/G2 151 The DNSC mercury at Y 12 is contained in newer, seamless flasks that hold 76 lb (34kg). The flasks are not overpacked. They are stored in groups of 45 on wooden pallets that measure 38 in by 38 in by 20 in (96 cm by 96 cm by 51 cm). The overpacking procedure was accompanied by an independent study of mercury vapor reading carried out by the State University of New York (SUNY) and New Jersey institute of Technology (NJIT) [DNSC 2007]. The results showed mercury levels to be below established background reading [DNSC 2007]. Costs: This overpacking costs about $20 per flask [Hogue 2007]. The center looked into consolidating the metal into containers holding 1 metric ton of mercury. But DNSC did not pursue this option because it would have cost about $100 per flask and posed a greater risk of releasing mercury than did leaving it in the smaller containers. DNSC assumes that 0.74 percent of mercury containers will require replacement every 40 years, at a cost of $99.79 per container. Dividing the replacement percentage by four and multiplying it by the replacement cost per container yields the unit cost per pound of inspecting mercury containers once every ten years. [USEPA 2007a] Environmental and safety aspects Transport and handling Currently only the transport requirements for dangerous goods apply to the transport of liquid mercury. The transport of liquid mercury is regulated under the various transport regulations ADR, RID, IMO, IATA. Specific packaging provisions for liquid mercury are established and only packaging complying with the provisions of these regulations is allowed for transport. Mercury is classified as follows: UN N 2809 Class 8: corrosive substances classification code: C9 (other corrosive substances, liquid) Special provisions: 5 kg (maximum net quantity per inner packaging in case of combination packaging) packaging group: III (substance presenting low danger) Packaging instructions: P800 Mixed packaging provisions: M15 The following packaging instructions apply for the transport of liquid mercury (UN N 2809):
152 Reference number /2009/530302/ETU/G2 152 Figure 6-7: Packaging instruction for liquid mercury according to ADR
153 Reference number /2009/530302/ETU/G2 153 In the case of elemental mercury no longer being transported as a product but as waste, additional provisions have to be taken into consideration, in particular: Regulation 1013/2006/EC 75 on shipment of waste requiring a notification procedure for all wastes destined for disposal involved in transboundary transport (see chapter 5 legal assessment ) Directive 91/689/EEC 76 on hazardous waste and Directive 2006/12/EC 77 on waste requiring a record of waste including information on quantity, nature, origin, destination, frequency of collection, mode of transport and treatment method. Documentary evidence that the management operations have been carried out to be kept for at least three years The specific requirements laid down for waste transports according to ADR National requirements for signing waste transport, e.g. according to the German legislation 78, signing the transport with an A for waste Improper handling of metallic mercury might result in mercury emissions with adverse effects to workers and the environment. No mercury specific provisions are implemented on EU level but the general established occupational and health regulations have to be taken into consideration during the handling and transport of metallic mercury (e.g. compliance with existing occupational limit values for mercury). To avoid improper handling which might result in mercury releases Euro Chlor has implemented the following specific requirements for the safe handling and transport of liquid mercury to Almadén (Annex 2 Technical requirements to the Euro Chlor Voluntary Agreement on Safe Storage of Decommissioned Mercury): General - Mercury shall be delivered to the storage site as a liquid in hermetically sealed containers ready for storage. - The containers will be placed in a dedicated area in the storage site. 75 Regulation N 1013/2006 of the European Parliament and of the Council of 14 June 2006 on shipments of waste (OJ L 190, , p.1-98), also referred as the Waste Shipment Regulation 76 Council Directive 91/689/EEC of 12 December 1991 on hazardous waste (OJ L 337, , p. 20) with last amendment from 19 November 2008, also referred as Hazardous Waste Directive 77 Directive 2006/12/EC of the European Parliament and of the Council of 5 April 2006 on waste (OJ L 114, , p. 9 21) 78 Act for Promoting Closed Substance Cycle Waste Management and Ensuring Environmentally Compatible Waste Disposal (Kreislaufwirtschafts- und Abfallgesetz - KrW-/AbfG), 27 September 1994 (BGBl I 1994, 2705)
154 Reference number /2009/530302/ETU/G2 154 Containers: - The containers will be made of steel, with top connection only (no bottom valves) and should have ADR/RID approval for transportation. The containers will normally have a capacity in the region of 1 tonne of mercury. Containers of other capacities may be used if appropriate. - The containers will be used for transportation and storage to avoid further manipulation of mercury on the storage site. - The containers will have a visible indication of their empty and full weights. Preparation and filling operation: - Before filling the containers, residual sodium concentration in the mercury will be checked to ensure that there is no risk of hydrogen production. - The container shall not be completely filled to avoid overpressure by thermal expansion. - After filling, the container will be hermetically closed. The filled containers will be weighted for the quantity of mercury; sealed and properly identified: product with UN code, danger signs, amount, sender, date and reference number to trace the origin. Loading and unloading of containers - During loading and unloading trucks or rail wagons, all precautions will be taken to avoid any spill and emergency aspiration equipment will be ready to collect accidental spillage. All members of Euro Chlor still operating chlor-alkali plants using the mercury technology, signed the agreement and thus have to take into consideration the above stated requirements. Furthermore Euro Chlor published Guidelines for the preparation for permanent storage of metallic mercury above ground or in underground mines [Euro Chlor 2007] including detailed information on required quality, containment and packaging of mercury resulting from decommissioned chlor-alkali plants. It is stated in the document that Mercury [ ] may be contaminated, so it is necessary to purify it before transfer to storage containers. The most likely contaminants are water-soluble (specifically sodium, which has the potential to generate hydrogen in storage). In addition the presence of radioactive traces, which are used to measure the plant mercury inventory, should be avoided. [Euro Chlor 2007] Mercury from decommissioned chlor-alkali plants have some small metallic contaminants, like iron, nickel, copper usually not detectable (< 20 mg/kg each) [personal information by Euro Chlor].
155 Reference number /2009/530302/ETU/G Container material Containers must withstand all anticipated levels of handling, storage, stacking, loading and unloading conditions and should not become adversely affected by changes in atmospheric conditions, pressure, temperature and humidity [UNEP 2009]. With respect to the containment of metallic mercury the following primary aspects have to be fulfilled: Reaction stability against its content (also in case of impurities) Reaction stability against the surrounding environment Mechanical stability Suitability for transport (avoidance of additional re-filling) Air and liquid tightness Monitoring possibilities Container suitability is largely related to the form and foreseen storage period of the metallic mercury. In the case of permanent underground storage, the containment is not seen as a protection measure anymore as the stability of any packaging system cannot be expected for a period of >1,000 years. Apart from the above described steel containers currently in use, glass containers are also discussed as possible containers for liquid mercury since glass will not react with mercury. However, due to its low pressure resistance, fragility and strength, appropriate surrounding packaging (casing) has to be designed to avoid breakage during transport and handling. Teflon might also be suitable. No specific information has been found to packaging systems made of glass and teflon. Pure iron flasks might also be an appropriate material for the storage of liquid mercury as iron does not react with mercury and it is more stable than glass. The problem with iron is that depending on the storage environment (e.g. saline solutions) iron might corrode. An appropriate coating or second layer might by necessary. No specific information on pure iron flasks could be identified. In the case of storage of liquid mercury over a long time, possible reactions of mercury with the containment have to be taken into consideration. Although for example pure iron does not react with pure mercury, impurities in the mercury may result in a possible reaction and thus an attack of the containment. For pre-treated metallic mercury, the requirements to be fulfilled would be different than those applying to metallic mercury. Solidified mercury might either be stored in drums or large packs depending on the structure of the final product.
156 Reference number /2009/530302/ETU/G2 156 In the following, the preliminary findings of the MERSADE project relating to corrosion by liquid mercury of the container material are described. One objective of the MERSADE project is to identify appropriate container material for the safe long term storage of metallic mercury. Apart from a literature review (see section 4.1.2), practical investigations also took place with tanks and flasks in use for several years. The Oak Ridge National Laboratory (ORNL) also carried out an extensive assessment of mercury containers to identify the most appropriate container material as well as container size Corrosion by liquid mercury of the container material (steel) Preliminary results of the MERSADE project The following information is based on [Muñoz, 2009] and [Mersade 2009A] and additional personal information from Mr. Ramos, Mayasa. One major objective of Mersade was to identify appropriate container material for the storage of liquid mercury. Therefore, in a first step the storage containers that have been in use for several years for the storage of liquid mercury at Almadén have been analysed for potential effects resulting from the mercury. The stored mercury at Almadén has a purity of 99.9%. The following equipment has been investigated. Table 6-6: Tested equipment [Muñoz, 2009], presentation: Mr. Ramos Capacity Thickness container material Container material Flask kg Not indicated Plain carbon steel (low C, Mn, P steel, DD13) Flask kg Not indicated Plain carbon steel (low C, Mn, P steel, DD13) In use since >7 years 6 years Flask kg 4mm Plain carbon steel (low 30 years C, Mn, P steel, not DD13) Container 1 1 tonne Not indicated AISI 316L 79 >10 years Container 2 1 tonne Not indicated AISI 304L 79 6 years Deposit of scale Not indicated 7mm AISI steel (304L Not indicated C Content limit) Bulk tank-25 Not indicated 8mm AISI 304 steel 25 years Pipes / 3.5mm AISI steel 25 years 79 Classification according to AISI = American Iron and Steel Institute
157 Reference number /2009/530302/ETU/G2 157 In addition, two samples of the stored mercury have been analyzed to identify possible impurities. In particular one sample shows bismuth, sodium, manganese and potassium concentrations of around 200 ppm and calcium was identified in a concentration of up to 250 ppm. Other substances such as Ag, Pb, Zn, Al have been found in concentrations below 10 ppm. The following conclusions have been drawn: Conclusions: packaging (flasks, containers) samples had a good optical appearance with no significant damage FLASK 1: some iron oxides were observed on the damaged areas as well as below the protective coating on specimens taken from non damaged areas Profile depletion by the GDOES technique for Cr, Ni and Mn shows that for CONT.-1 (AISI 316L) the depleted areas are deeper (2,5 μm) than for CONT.-2 (AISI 304) (1 μm). Additionally, for CONT.-1 the depth of the affected zone increases up to 4 μm when the specimens evaluated were taken from the bottom of the tank. FLASK-30 shows a deeper damage since the average thickness of the iron oxide layer may reach 30-40μm which is about 1% of the thickness of the steel, reaching up to 200μm, 5% of the thickness. Conclusions: Installations (pipes, tanks): Intergranular attack on the surface, but the depth of damage on the steel is rather small Bulk tank 25, which has been used for Hg storage for 25 years, showed small amounts of damage of 40µm depth (0,5% of the total thickness) --->max 5,000 years (8mm thickness) The deposit of scale showed the same results: 20µm (<0,3% of the total thickness) - max. 8,750 years (7mm thickness) Pipe: Under flowing conditions, the attack was more severe, resulting in a regression of the surface and increased roughness of the surface. Due to the elevated presence of impurities in the mercury, it is not possible to conclude that the identified attacks can be attributed exclusively to the mercury. Preliminary overall conclusion resulting from the MERSADE project: Stainless steel AISI 304 shows a good performance in metallic mercury under static and isothermal conditions, since after 25 years the steel only shows a slight attack on the surface. These results suggest that this steel grade seems suitable for constructing the long term storage depository.
158 Reference number /2009/530302/ETU/G2 158 The planned prototype for a bulk storage container will be constructed with stainless steel AISI Assessment of mercury storage containers by the Oak Ridge National Laboratory (ORNL) The ORNL has been working for several years on the assessment of mercury storage containers to identify the most appropriate container material as well as container size for the storage of liquid mercury. In the following, a summary of the research activities is provided, based on presentations [ORNL 2009] and [ORNL 2009A]. The main findings of the research are: - Mild steel and stainless steel containers are immune to pure mercury (purity >99,5%) for anticipated exposure conditions and are appropriate for long-term storage - Avoid acceptance of unknown compositions of Hg, at least until more information is available - Mercury is compatible with iron and mild steel up to ~400 C (solubility of iron in Hg << 0.1 ppm at RT, mercury does not chemically wet steel at RT in the presence of air) - The evaluation of flasks with a life time of up to ~50 years service confirmed the absence of steel interaction with mercury - Welds are likely to be the weakest point in containers The outcome of the research activities has been used as input for research on the design of appropriate storage containers. As acceptable container materials, carbon steel (ASTM A36 minimum) or stainless steel (~316L) have been identified. Carbon steel is recommended as it has further advantages compared to stainless steel. Stainless steel is more than twice the cost of carbon steel has lower material strength but provides better exterior corrosion protection than carbon steel The purity of the stored mercury should be at least 99.5% and the remaining impurities within it should not be capable of corroding carbon or stainless steel (i.e., nitric acid solutions, chloride salt solutions, or water). For a protective coating for the exterior surface of the containers, epoxy paint or electro plating are recommended. For the inner surface, no protective coating is required for as long as mercury meets purity requirements and no water is present inside the container.
159 Reference number /2009/530302/ETU/G2 159 For a plug, a National Pipe Thread (NPT) plug with Teflon tape is recommended, as it provides an excellent seal at low cost. The presentation [ORNL 2009A] also includes a comparison of storage containers with different sizes (3 l flask, 1, 2, 3 and 10 metric tonne containers) and the pros and cons relating to their storage function. Based on the results of the above described investigations, DOE published in November 2009 Interim Guidance on Packaging, Transportation, Receipt, Management, and Long-Term Storage of Elemental Mercury [DOE 2009]. The document provides a framework for the standards and procedures associated with a DOEdesignated elemental mercury storage facility (see chapter 5.4.2) with a focus on the RCRA (Resource Conservation and Recovery Act) permitting of such a facility and planning for that storage facility s needs. This document provides general guidance on standards and illustrative procedures that are current, consistent, and best suited for supporting the DOE program for the receipt, management, and long-term storage of mercury generated in the United States. The document lays down that a detailed analysis of the purity of the elemental mercury has to be prepared. This purity analysis shall confirm a minimum purity of 99.5% (per volume) and list all impurities and their weight percent of content. The total liquid shipment per container is on a volume basis, and the percent impurities are on a weight basis. The impurities shall not be capable of corroding carbon or stainless steel. To prevent degradation of the container, nitric acid solutions, chloride salts solutions, water, and other possible corrosion agents are prohibited. The mercury shall be free of any added radiological components Conclusions Above ground storage of the product liquid mercury has already been practiced for several years and experiences with the storage of large quantities of liquid mercury are available in particular in the USA and Spain. Also experiences are available related to the handling, packaging, transport of metallic mercury. In Europe, the Spanish state-owned company Miñas de Almadén (MAYASA), the operator of the former mercury mine, is the major company dealing with liquid mercury. According to an agreement with Euro Chlor, MAYASA receives all excess mercury from western European chlorine producers. The required minimum purity for the acceptance of mercury is > 99.9%. Currently, for the transport of liquid mercury, standard gas and liquid-tight steel flasks (34.5 kg net) and containers (1 metric ton net) are in use in Europe. The storage at Almadén also takes place in bulk tanks which are stored in collecting basins capable to collect all mercury included in the bulk tanks. In the USA, government owned liquid mercury (more than 5,500 metric tons) which is no longer used for military purposes has already been stockpiled for more than 40 years in four above-ground
160 Reference number /2009/530302/ETU/G2 160 warehouses. It is planned to store this metallic mercury for another 40 years in a selected warehouse [DNSC 2004]. The selection of the warehouse was accompanied by intensive research related to minimum requirements for the storage site and the containment ([ONRL 2009], [ONRL 2009A]). The purity of the stored mercury is above 99.5%. Intensive research related to appropriate containers for the storage of metallic mercury has been carried out in specific projects in the US ([ONRL 2009], [ONRL 2009A]) and in Spain ([Muñoz, 2009], [Mersade 2009A]). Within both projects, containers actually used since several years/decades for the storage of metallic mercury have been analysed on possible effects by the stored mercury. Based on the results from the analytical investigations of the storage containers, requirements related to container material suitable for long term storage have been derived. Both concluded that suitable container material is available for a temporary storage of metallic mercury. Recently, the Department of Energy (DOE) published Interim Guidance on Packaging, Transportation, Receipt, Management, and Long-Term Storage of Elemental Mercury [DOE 2009] which is based on the outcome of the above described investigations.
161 Reference number /2009/530302/ETU/G References [BGR 2007] BGR, Bundesanstalt für Geowissenschaften und Rohstoffe, Nuclear waste disposal in Germany - Investiagtion and evaluation of regions with potentially suitable host rock formations for a geologic nuclear repository, Hannover/Berlin, April 2007, HostRoc kformations en,templateid=raw,property=publicationfile.pdf/wastedisposal_hostrockformations _en.pdf [BMU 2009] Bundesministerium für Umwelt, Natur und Reaktorsicherheit, Sicherheitsanforderungen an die Endlagerung wärmeentwickelnder radioaktiver Abfälle, Berlin, 2009, pdf [Brückner 2003] Brückner, D.; Lindert, A., Wiedemann, M., The Bernburg Test Cavern - A Model Study of Cavern Abandoment, SMRI Fall Meeting, 5-8. Oct. 2003, Chester, UK, 69 89, 2003 [Council Decision 2003/33/EC] Council Decision, of 19 December 2002 establishing criteria and procedures for the acceptance of waste at landfills pursuant to Article 16 of and Annex II to Directive 1999/31/EC (2003/33/EC) [DNSC 2003] Defense National Stockpile Center, Draft Mercury Management Environmental Impact Statement, 2003 [DNSC 2004] Defense National Stockpile Center, Record of Decision for the Mercury Management EIS, April 2004 [DNSC 2004A] Defense National Stockpile Center, Final Mercury Management Environmental Impact Statement, Executive Summary, 2004 [DNSC 2004B] Defense National Stockpile Center, Final Mercury Management Environmental Impact Statement,Volume I, 2004 [DNSC 2004C] Defense National Stockpile Center, Human Health and Ecological Risk Assessment Report for the Mercury Management EIS, Volume II, 2004 [DNSC 2007] Defense National Stockpile Center, Fact Sheet: Mercury Over-Packing, Storage & Transportation, May 2007 [DNSC 2007A] Defense National Stockpile Center, Fact Sheet: Somerville Depot, February 2007
162 Reference number /2009/530302/ETU/G2 162 [DOE 2009] U.S. Department of Energy, Interim Guidance on Packaging, Transportation, Receipt, Management, and Long-Term Storage of Elemental Mercury, U.S. Department of Energy Office of Environmental Management Washington, D.C., November 13, 2009, ).pdf [Env Canada 2001] National Office of Pollution prevention, Environment Canada, The Development of retirement and long term storage options of mercury, Draft final report, Ontario, June 2001 [Eriksson 2006] L. Eriksson, Swedish policy for a mercury free environment, presentation, Swedish Environmental Protection Agency [Euro Chlor 2007] Euro Chlor, Guidelines for the preparation for permanent storage of metallic mercury above ground or in underground mines, Env Prot 19, 1st Edition, October 2007 [EU COM 2001], Integrated Pollution Prevention and Control (IPPC) - Reference Document on Best Available Techniques in the Chlor-Alkali Manufacturing industry -, [FZK 2007] Forschungszentrum Karlsruhe in der Helmhotz - Gemeinschaft: Schwerpunkte zukünftiger FuE- Arbeiten bei der Endlagerung radioaktiver Abfälle ( ), Förderkonzept des BMWT, Dezember [Gibb 2000] Fergus Gibb, A new scheme for the deep geological disposal of high-level radioactive waste, Journal of the Geological Society, Jan [GRS 2008] GRS, Gesellschaft für Anlagen- und Reaktorsicherheit (GRS) mbh, Öko-Institute e.v., Institut für angewandte Ökologie, Endlagerung wärme entwickelnder radioaktiver Abfälle in Deutschland, Anhang Wirtsgesteine Potentielle Wirtsgesteine und Eigenschaften, Anhang zu GRS-247, ISBN , Braunschweig/Darmstadt, September [GRS 2009] GSR Gesellschaft für Anlagen- und Reaktorsicherheit, Legislation and Technical Aspects of Regulations on Waste Containing Mercury in Europe and Germany, presentation by Thomas Brasser at the Latin American Mercury Storage Project Inception workshop, Montevideo, Uruguay, April 22-23, 2009 [Heath 2006] Mike Heath, Health environmental and safety questions related to the underground storage/disposal of mercury over time, Presentation at the EEB Conference on EU Mercury surplus management and
163 Reference number /2009/530302/ETU/G2 163 mercury-use restrictions in measuring and control equipment, Brussels, 19 June 2006; [Hogue 2007] Cheryl Hogue, Mercury Excess, congress and EPA probe possibility of long-term storage of liquid metal, Chemical & Engineering News, July 2, 2007, Volume 85, Number 27, pp [Höglund 2009] Höglund, Lars Olof, Underground storage and disposal in hard rock based on a chemically-stable mercury solid, Presented at Workshop of Safe Storage and Disposal of Redundant Mercury, St Anne s College, Oxford, 13th and 14th October, 2009; [IAEA 1994] SITING OF GEOLOGICAL DISPOSAL FACILITIES - A Safety Guide, 1994, [IAEA 2002] Issues relating to safety standards on the geological disposal of radioactive waste, Proceedings of a specialists meeting held in Vienna, June 2001, June 2002, [IAEA 2003] Technical Reports Series No. 413, Scientific and Technical Basis for the Geological Disposal of Radioactive Wastes, Vienna [IAEA 2007] Disposal Aspects of Low and Intermediate Level Decommissioning Waste, Results of a coordinated research project , IAEA-TECDOC-1572, December [IAEA 2009] Geological Disposal of Radioactive Waste: Technological Implications for Retrievability [IAEA 2009b] Joint Convention on the Safety of Spent Fuel Management and on the Safety of Radioactive Waste Management, Third Review Meeting of the Contracting Parties 11 to 20 May 2009, Vienna, Austria, SUMMARY REPORT, 20 May [IfG 2007] Institut für Gebirgsmechanik GmbH, Gebirgsmechanische Zustandsanalyse des Tragsystems der Schaftanlage Asse II, Kurzbericht, November 2007 [K+S 2009] K+S, Underground Waste Disposal, presentation by Alexander Baart at the Latin American Mercury Storage Project Inception workshop, Montevideo, Uruguay, April 22-23, 2009;
164 Reference number /2009/530302/ETU/G2 164 [KEMAKTA 2007] Lars Olof Höglund and Sara Södergren, Aspects on final disposal of mercury - The need for waste stabilisation, 22 March 2007 [Kystverket 2008] Det Norske Veritas AS, Kystverket Norwegian Coastal Administration - Salvage of U Supplementary studies - disposal, report NO , Revision N 01, [Mersade 2007] M. Ramos, Estimation of figures for total quantity for possible storage from EU countries and in adhesion process taking in account the caustic-soda industry and others., Status Report Literature review, T 1.2, Life Project Number Life06 ENV/ES/PRE/03, July 2007; ore%20inside%20eu%20after%20export%20ban%20mayasa.pdf [Mersade 2007 A] M. Ramos, Literature review concerning corrosion problems in mercury and stabilisation of liquid Hg, Status Report Literature review, T 1.3 and T 1.4, Life Project Number Life06 ENV/ES/PRE/03, February 2007; ercury%20corrosion%20and%20stabilisation%20of%20liquid%20hg.pdf [Mersade 2007 B] P. Higueras, J. M. Esbrí, Literature review concerning environmental mercury monitoring, Status Report, Life Project Number Life06 ENV/ES/PRE/03, March 2007; mental%20mercury%20mon.pdf [Mersade 2009] Process for the Stabilization of Liquid mercury, via mercury sulfide, by the use of polymeric sulfur, F.A. López, A. López-Delgado and F.J. Alguacil, Consejo superior de investicadiones cientificas (CSIC), Centor nacional de investigations metalúrgicas (CENIM) [Minosus 2009] [Muñoz, 2009] C. Muñoz, M.T. Dorado, A. G mez-coedo, J.J. de Damborenea, A. Conde, Corrosión en depsitos de almacenmiento de mercurio, 2009, [Nirex 2004] United Kingdom Nirex Limited: A Review of the Deep Borehole Disposal Concept for Radioactive Waste, Nirex Report no. N/108, June 2004 [NO 2005] Stakeholder meeting in Brussels 8 September 2005, Additional questions, Answers from the Norwegian authorities
165 Reference number /2009/530302/ETU/G2 165 [öko institut 2007] Methodenentwicklung für die ökologische Bewertung der Entsorgung gefährlicher Abfälle unter und über Tage und Anwendung auf ausgewählte Abfälle, [ORNL 2009] Pawel S. J., Oak Ridge National Laboratory, Assessment of Mercury Storage Containers, Presentation October 2009, [ORNL 2009A] Carroll, Adam J., Oak Ridge National Laboratory, Design of Mercury Storage Containers, Presentation October 2009, [Popov 2006] V. Popov, R. Pusch, Disposal of Hazardous waste in underground mines, Wit Press, Southhampton, Boston, 2006 [Popp 2007] Popp. T.; Wiedemann, M.; Böhnel, H., Minkley, W.; Manthei, G., Untersuchungen zur Barriereintegrität im Hinblick auf das Ein-Endlager-Konzept,Institut für Gebirgsmechanik GmbH, Leipzig, UFOPLAN-Vorhaben: SR 2470, Ergebnisbericht, 2007 [Pusch 2007] Pusch, Roland, Project on underground disposal of toxic chemical waste like mercury batteries, Roland Pusch, Geodevelopment International AB, Lund, 2007 [Siemann 2007] M. Siemann, Herkunft und Migration mineralgebundener Gase der Zechstein 2 Schichten in Zielitz, Technische Universität Clausthal Institut für Endlagerforschung Fachgebiet Mineralogie, Geochemie, Salzlagerstätten, published in Kali und Steinsalz, ISSN , Heft 3/2007, page 26, [SKB 1999] Deep repository for long-lived low- and intermediate-level waste, Preliminary safety assessment. Swedish Nuclear Fuel and Waste Management Co (SKB), Stockholm, 1999 [SKB 2000] What requirements does the KBS-3 repository make on the host rock? Geoscientific suitability indicators and criteria for siting and site evaluation. Swedish Nuclear Fuel and Waste Management Co (SKB), Stockholm, 2000 [SOU 2001] NATURVÅRDSVERKET, A Safe Mercury Repository, A translation of the Official Report SOU 2001:58, Report 8105 January 2003, [SOU 2008] Statens offentliga Utredningar (SOU) 2008: 19 Permanent storage of long-lived hazardous waste in underground deep bedrock depositories, Summary of key findings, SOU 2008: 10 April 2008
166 Reference number /2009/530302/ETU/G2 166 [SOU 2008A] Miljödepartementet, Att slutförvara långlivat farligt avfall i undermarksdeponi i berg, ISBN , 2008 [Spiegel 2007] Gau in der Grube, Michael Fröhlingsdorf, Sebastian Knauer, 17/2007 [UBA DE 2004] Umweltbundesamt, Background paper on permanent storage in salt mines prepared by the Federal Environment Agency, Berlin, Germany, 29 July 2004; [UNEP 2009] UNEP, Draft technical guidelines on the environmentally sound management of mercury wastes, 4th Draft, April 2009, [USEPA 2002c] Hugh W. McKinnon, Preliminary analysis of alternatives for the long term management of excess mercury, EPA/600/R-03/048, 2002, [USEPA 2004] Application of the analytic hierarchy process to compare alternatives for the long-term management of surplus mercury, Paul Randall, Linda Brown, Larry Deschaine, John Dimarzio, Geoffrey Kaiser, John Vierow, 6 January 2004 USEPA 2007a] US EPA, Mercury Storage Cost Estimates, final report, November
167 Reference number /2009/530302/ETU/G Review of immobilization, solidification and other appropriate technologies for metallic mercury waste Metallic mercury is liquid and has a high vapour pressure at 20 C. These properties make the storage as well as the disposal of mercury difficult due to possible risks for the environment. In addition, the high vapour pressure of liquid mercury may result in mercury emissions during handling, storage and/or disposal. To avoid these negative effects, intensive investigations took place during the last year to develop technologies to stabilize or immobilize liquid mercury before storage. In the following, these technologies are designated pre-treatment technologies. The main purpose of the pre-treatment technologies is to improve the handling reduce possible risks by reducing the volatility and/or toxicity reduce possible risks by improving the leaching properties The use of pre-treatment technologies may also influence the required safety measures for aboveground as well as underground storage facilities. For example, in the case of liquid mercury storage in an above-ground facility, damage to the packaging may lead to a release of mercury emissions to the environment, depending on the implemented safety measures (e.g. collecting basin, air monitoring system). The risk of accidental release of mercury is significantly reduced or even non-existent if liquid mercury is solidified and stored as solid waste. With regard to underground disposal in particular, the enhanced leaching properties might be arguments for a pre-treatment of metallic mercury before temporary or permanent storage in order to minimise the risk of mercury being released into the environment from the storage site. On the other hand, pre-treatment may also result in additional emissions of mercury during the treatment process and additional handling operations. For subsequent temporary or permanent storage the changed properties and environmental behaviour of the pre-treated material have to be taken into consideration, for instance long-term stability and safety of the compound (e.g. under mechanical pressure in underground disposal) completeness of the reaction leachability retrievability of elemental mercury
168 Reference number /2009/530302/ETU/G2 168 long-term experiences/tests In addition, environmental, health and safety-related impacts during the treatment process have to be considered. The pre-treatment of metallic mercury aims at the immobilisation of the mercury. There can be differentiation between stabilization and solidification depending on the technology used. Pretreatment can also be a combination of both. According to Decision 2000/532/EC, stabilisation is defined in the following manner: Stabilisation processes change the dangerousness of the constituents in the waste and thus transform hazardous waste into non-hazardous waste. It should be taken into account, that solidification processes only change the physical state of the waste by using additives, (e.g. liquid into solid) without changing the chemical properties of the waste. Similar definitions of stabilization and solidification are provided by UNEP [UNEP 2009]: Stabilization refers to techniques that chemically reduce the hazard potential of a waste by converting the contaminants into less soluble, mobile, or toxic forms. The physical nature and handling characteristics of the waste are not necessarily changed by stabilization; and Solidification refers to techniques that encapsulate the waste, forming a solid material, and does not necessarily involve a chemical interaction between the contaminants and the solidifying additives. The product of solidification, often known as the waste form, may be a monolithic block, a clay-like material, a granular particulate, or some other physical form commonly considered solid. In stabilisation processes, metallic mercury is blended with a substance (e.g. sulphur) or substances which react chemically to a new, less volatile, soluble or toxic compound. In the case of solidification, the mercury is simply embedded in a solid matrix without forming a new mercury compound by a chemical reaction. The matrix is solid either because the melting point of the matrix is well above room temperature or due to a curing process. Often the term encapsulation is used for the solidification process. Encapsulations are commonly used in the treatment of hazardous waste. Encapsulation (solidification) is applied to prevent hazardous waste from coming into contact with potential leaching agents. Encapsulation can further be split into Microencapsulation and Macroencapsulation. Microencapsulation means a process of surrounding or enveloping one substance with another substance on a very small scale, yielding capsules that might range from less than one micron to several hundred microns. In the case of metallic mercury, the core material would be mercury with a wall / coating material around it. Macroencapsulation involves pouring the encasing material over and around a large mass of the core material which should be encapsulated; thereby enclosing it in a solidified block. The two processes can also be combined. Cement, Portland cement and lime are the most commonly used materials for solidification/
169 Reference number /2009/530302/ETU/G2 169 encapsulation of hazardous metal waste; however, research continues into the use of other binders and additives to enhance performance of the final waste form and to reduce project costs. [USEPA2002] The following figure provides an overview of applied and discussed immobilization technologies for elemental mercury. Figure 7-1: Overview of immobilisation technologies for metallic mercury In the following, the currently discussed pre-treatment options for metallic mercury are presented. The overview includes technologies which are only available as patents as well as technologies already in a trial stage. In many cases patents or literature refer to the same process (e.g. sulphur stabilisation) but under different process conditions. Therefore, as a first step, the process in general is described and afterwards variations of the process are listed and described. An overview of the examined literature is provided at the beginning of each process description. The literature search resulted in a long list of patents and other scientific literature dealing with the stabilization of contaminated wastes, including mercury contaminated waste. These patents include the separation of Hg from the waste. As the focus of the project is the stabilization of elemental Hg, these patents are not useful in the light of the project background and have not been taken into consideration. The main purpose of this section is to provide an overview of the state of development of currently discussed immobilisation technologies relevant for liquid mercury. Based on this overview the preselection of the most promising technologies will take place (see section 8.9). Therefore, information already available related to these aspects is included as preliminary information in this section (e.g. solubility). In addition, the state of realisation of the technology is indicated.
170 Reference number /2009/530302/ETU/G Sulphur stabilization The most common natural occurrence of mercury is as cinnabar (HgS) from which metallic mercury is derived. Therefore, one of the most important and well investigated approaches is the reconversion of liquid mercury close to its natural state as HgS. The production of HgS can result in two different types, alpha-hgs (Cinnabar) and beta-hgs (metacinnabar). Pure alpha-hgs (intensive red colour) has a slightly lower water solubility compared to pure beta-hgs (black colour). Natural occurring alpha-hgs crystals are called cinnabar and cinnabar ore and are the most common mercury modification in nature. The process has been described and investigated in many different patents and other scientific literature. The following table offers an overview of the relevant documents found relating to this process. The information on the general process is summarised in section (Technical background). In the case of specific process conditions or specific variations, these are described together with the state of development in section (Use of the technology). Table 7-1: Sulphur stabilization: overview of the relevant literature Relevant literature overview for sulphur stabilisation Reference Salvage of U-864 supplementary studies disposal [Kystverket 2008] Treatment of elemental mercury [US ] Advances in Encapsulation Technologies for the Management of Mercury-contaminated Hazardous Wastes [USEPA 2002b] Preliminary analysis of alternatives for the long term management of excess mercury. [USEPA 2002c] Economic and Environmental Analysis of Technologies to Treat Mercury and Dispose in a Waste Containment Facility. [USEPA 2005] Mersade [Mersade 2007a] Stabilisation of metallic mercury [SAKAB DELA 2009] Disposal of wastes containing mercury [CA ] Production of non-fading cinnabar from the elements [DE453523] Mercury immobilization, A requirement for permanent disposal of mercury waste in Sweden [ÖREBRO 2006] Method for stabilization of metallic mercury using Content General description of the process General description of the process General description of the process General description of the process and assessment General description of the process and assessment General description of the process Description of a pilot scale plant Treatment of waste with sulphuric acid and neutralisation with lime slurry Mixing of mercury and sulphur together with a solution of 1:1 monosulphide Production of HgS with different sulphur and mercury ratio. Different solubility tests analyses for elemental mercury. Reaction of mercury metal with sulphur in the solid
171 Reference number /2009/530302/ETU/G2 171 Relevant literature overview for sulphur stabilisation Reference Content sulphur [US A1] state. Mercury / sulphur molar ratio of 1/1 to 1/3. Disposal of elemental mercury via sulphur reaction by milling [Lopez 2008] Preparation of mercury sulphide [US ] Verfahren und Vorrichtung zur Herstellung von Quecksilber zur anschließenden Entsorgung [EP A2] Verfahren und Vorrichtung zur Herstellung von Quecksilber zur anschließenden Entsorgung [EP A2] Mercury wastes evaluation of Treatment of mercury surrogated waste [USEPA 2002] Mercury wastes evaluation of bulk elemental mercury [USEPA 2002a] Formation of HgS in non stoichiometric conditions with mechanical energy (ball mill). HgS production with sulphur and sodiumsulphide Batch process to produce Mercury sulphide, established by DELA Continuous process to produce Mercury sulphide, established by DELA Comparison of four different stabilizing surrogated sludges Comparison of three vendors stabilizing bulk elemental mercury Technical background: Due to their strong affinity, sulphur and mercury form a very stable product either as beta HgS (meta-cinnabar, black, beta-phase) or alpha HgS (cinnabar, red, alpha phase). Naturally bonded mercury is mainly found in combination with sulphur, forming the alpha-phase of a crystal, so called Cinnabar. Solid beta HgS can be converted by thermal treatment into the alpha form. Alpha-HgS is more stable with lower leaching levels and therefore the most favourable reaction product. The production of alpha-hgs needs a more precise adjustment of production parameters compared to the production of beta-hgs. In general, HgS is produced by blending mercury and sulphur under ambient conditions for a certain time, until mercury(ii)sulphide is produced. To start the reaction process, a certain activation energy is required which may be provided by intensive mixing of the blend. Among other factors, higher shear rates and temperatures during the process support the production of the alpha phase, whereas a longer process time favours the creation of beta cinnabar. Excessively long milling in the presence of oxygen can lead to the production of mercury(ii)oxide. As HgO has a higher water solubility than HgS, its creation should be avoided by milling under inert atmospheric conditions or addition of an antioxidant (e.g. sodium sulphide). The process is robust and relatively simple to carry out. The HgS is insoluble in water and non-volatile, chemically stable and unreactive, being attacked only by concentrated acids. As a fine powdery material its handling is subject to specific requirements (e.g. risk of dust releases). This stabilisation process leads to an increase of the volume by a factor of ~300% and of the weight by ~16-60% compared to elemental mercury.
172 Reference number /2009/530302/ETU/G2 172 Leaching values from beta-hgs products are typically much higher than alpha HgS products (production of beta-hgs seems to be more prone to impurities compared to alpha-hgs), therefore beta-hgs is often not the endpoint of a possible pre-treatment process but the starting point for further treatment processes (e.g. SPSS, see section 7.2) Economic information The costs of this process depend on the costs for the raw material (e.g. sulphur, ~ 0.25/kg) and on the process conditions. The process conditions include energy costs for the milling process, heating if required, duration of the process, etc Environmental information The physico-chemical properties of the products have been collected and included in Annex 4. Available data about the solubility product in water have been found which is, for alpha-hgs = 2*10-54 and for beta HgS = 2*10-53 at 25 C [SPC 2009]. Both solubility products are very low, but the alpha phase is the more stable modification related to lower solubility and lower leaching values Use of the technology As already stated above, this process has been described and investigated by many different scientific institutions and companies. The most developed process from the implementation point of view is the DELA process, for which a pilot plant already exists. A second process for the production of mercury(ii) sulphide is from Bethlehem apparatus. Stabilisation of metallic mercury [SAKAB/DELA 2009] [EP A2] In this invention a pilot plant for the production of mercury sulphide is described. The process involves mixing of mercury and sulphur. The resulting HgS is a powder with about 16% increased weight compared to the original elemental mercury. The pilot plant has a batch size of 5 kg of elemental mercury and the process duration is between 90 and 240 minutes. According to SAKAB/DELA, a line with a capacity of about 3 to 6 tonnes per day can be constructed. The apparatus is kept below ambient pressure to avoid Hg vapour emissions. According to information from SAKAB/DELA, the costs for the pre-treatment and subsequent disposal are estimated at around 2,000/t. At present, further tests are being carried out. Method and apparatus for generating mercury(ii) sulphide from elemental mercury [Bethlehem apparatus] The information for this process has been directly provided by the relevant company (Bethlehem apparatus) by telephone conversation and exchange. No literature could be identified which describes and evaluates this process. The product of the process is a mercury sulphide crystal grown in a controlled temperature and
173 Reference number /2009/530302/ETU/G2 173 pressure atmosphere. They use slightly more sulphur than is required for stochiometric combination. By weight the crystal is 16 per cent sulphur and 84 per cent mercury. When mixed with polyethylene the mix is 75 % polyethylene, 21 % mercury and 4 % sulphur. Disposal of elemental mercury via sulphur reaction by milling [Lopez 2008] According to this reference, the reaction between elemental Hg and S in non-stoichiometric conditions was facilitated by means of mechanical energy provided by a planetary ball mill comprising of four stainless steel balls. The leaching behaviour of the product against milling time was checked. The process was carried out at lab scale using ca. 25 g of elemental Hg. Mersade [2007a] In the most extended processes mercury reacts with powdered sulphur and/or liquid sulphur (polysulfide) to form mercuric sulphide. Mercuric sulphide is the most stable compound formed between mercury and sulphur. It exists in two stable forms. Ones in the black cubic tetrahedral form (metacinnabar) and the other stable form is the red hexagonal form found in natures as cinnabar. Both forms are insoluble in water and in acidic solutions. In alkaline solutions with excess of sulphur anions HgS is solubilized. In the stabilization of soluble mercury in mercury-containing materials by the formation of insoluble mercury sulphides, it is desirable to minimize the formation of mercury polysulfide complexes which can be eluted or leached from deposits in effluents which would contain Hg concentrations higher than desired. This can be accomplished by the selection of the inorganic sulphur compound. Mercury polysulphide formation may also be minimized or eliminated by the addition of a polysulphide inhibitor (alkali metal sulphite, alkali metal bisulphite and alkali metal metabisulphite). Mercury wastes evaluation of treatment of mercury surrogated waste [USEPA 2002] In this study, different pre-treatment technologies had been compared: sulphur stabilization, SPSS, amalgamation and formation of mercuric sulphide followed by cement-containing proprietary stabilization. The product from the mercury sulphide process has a mercury content of 72 wt % (after encapsulation). Therefore the product has increased by 38.9 % by weight on a dry basis compared to the mercury surrogated waste. The mercury sulphide was soil-like. Mercury wastes evaluation of bulk elemental mercury [USEPA 2002a] In this study three pre-treatment technologies had been compared: sulphur stabilization, SPSS and amalgamation. The mercury sulphide process (sulphur stabilisation) is a multi-step process that can be stopped at a given stage dependent on what the performance specification is. The first step (primary stabilization) consists of conversion of elemental mercury to mercuric sulphide (beta-hgs). This step fits the EPA definition of elemental mercury amalgamation. The primary product is then subject to micro and macro encapsulation utilizing a range of polymeric and other agents to attain the desired product specification. The final product was a bead-like material that had a top diameter of 9.5 mm.
174 Reference number /2009/530302/ETU/G2 174 The product resulting from the primary stabilisation (sulphur stabilisation) has a mercury content of 55 wt%. Therefore the product has increased by 81.8 % by weight on a dry basis compared to the mercury surrogated waste. The mercury sulphide form was soil-like. After the encapsulation process the product from the mercury sulphide process has a mercury content of 44 wt%. Therefore the product has increased by 127 % by weight on a dry basis compared to the mercury surrogated waste. The final form was microencapsulated pellets. The leaching values for the final pellets are dependent on the ph value and were about 0.001mg/l at ph=2, ~0.01mg/l at ph = 8, and ~0.1 mg/l at ph = 12. Economic and Environmental analyses of Technologies to treat mercury and dispose in a waste containment facility [USEPA2005] Information available for the Option B (sulphur stabilisation process) process closely parallels that available for Option A (SPSS process). The treatment of elemental mercury was evaluated by EPA; TCLP testing of treated elemental mercury was conducted by DOE. In addition, existing general mercuric sulphide formation data can similarly be applied to the sulphur-based Option B technology. USEPA data show a trend in leaching results with respect to ph, with results lowest in acidic conditions and highest in basic conditions (see Figure B-1). The results were consistently below the UTS level at all but the highest range of ph. The Option B process has been used for the treatment of approximately 7600 kg (3.5 tons) of radioactive elemental mercury since Therefore the process is in active use and development. Preliminary analysis of alternatives for the long term management for excess mercury [USEPA 2002c] Raw materials for the ADA / Permafix treatment process include a sulphur-based reagent. The treated material can be a granular material or a monolithic material. Permafix proposed to treat 880 flasks of mercury per week (66,800 lb) and generate gallon drums. This represents a volume increase of 14 times. The vendor estimates it would take three years to process the 4,890 tons of mercury stockpile. The ADA amalgamation process, a batch process, consists of combining liquid mercury with a proprietary sulphur mixture in a pug mill; in one application a 60-liter capacity pug mill was used for treatment of an elemental mercury waste. Treatment of the liquid mercury was conducted by adding powdered sulphur to the pug mill, while a specific amount of mercury was poured into the mill. While the processing of mercury in the pug mill was performed without the addition of heat, the reaction of mercury with sulphur is exothermic at room temperature, and the mixture increases in temperature during processing Overview of patents In the following, a short overview is provided on available patents relating to the sulphur stabilisation process. All these tests have been carried out on a laboratory scale. Production of non-fading cinnabar from the elements [DE453523]
175 Reference number /2009/530302/ETU/G2 175 In the described process 20g of mercury and 3.2g of sulphur are added under stirring to a solution of potassium monosulphide. The process includes several steps and takes at least 2 hours. During the process the solution is slightly yellow. Alternatively, potassium sulphide can be used as a reactant with subsequent treatment with potassium monosulphide. To promote the mixing process, glass beads can be added. Treatment of elemental mercury [US ] A brief description of a sulphur stabilisation process, involving a chemical reaction between sulphur and mercury to form mercury sulphide, which may be effected by blending and grinding a mixture of mercury and elemental sulphur under ambient conditions. The process is robust and relatively simple to carry out, and the mercury(ii) sulphide which is produced is insoluble and non-volatile in water and chemically stable and unreactive, being attacked only by concentrated acids. However, some refinement of process control is required with this method and, although no suffering from the same problems as liquid mercury, in terms of volatility and proneness to leaching, mercury(ii)sulphide is still a toxic material requiring disposal and, as a fine powdery material which presents its own handling difficulties. Verfahren und Vorrichtung zur Herstellung von Quecksilber zur anschließenden Entsorgung [EP A2] The invention is a process for the production of mercury sulphide. The reactants are elemental mercury and elemental sulphide or a sulphur connection. The process takes place at 50 C and/or reduced pressure (0.95 bar instead of 1 bar). The temperature of 50 C increases the amount of mercury in the vapour phase and therefore the production of mercuric sulphide is promoted. The product shall be produced in a discontinuous process. Verfahren und Vorrichtung zur Herstellung von Quecksilber zur anschließenden Entsorgung [EP A2] The invention is a process for the production of mercury sulphide. The reactants are elemental mercury and elemental sulphide or a sulphur connection. The process shall takes at temperatures above the boiling point of mercury (>580 C). The temperature can be increase in case a lower pressure is used. The high temperature increases the amount of mercury in the vapour phase and therefore the production of mercuric sulphide is promoted. The product shall be produced in a continuous process. Method for stabilization of metallic mercury using sulphur [US A1] This patent describes tests which have been carried out on a laboratory scale, with an amount of about 55g of elemental mercury and on a semi-pilot scale with 1kg of elemental mercury. During 9 test series, the influence of the presence of a grinding agent, the ratio of Hg/S, stirring rate, temperature and the degree of filling the machine is studied.
176 Reference number /2009/530302/ETU/G2 176 Preparation of mercury sulphide [US ] Within this technique, elemental mercury, elemental sulphur and sodium polysulphide are mixed to produce a finely divided dispersion of mercury sulphate. Due to its large surface area and the resulting negative leaching behaviour mercury sulphate is not suitable for disposal. During several experiments on a laboratory scale ( g elemental Hg), different ratios of Hg, S and Na 2 S have been investigated. Disposal of wastes containing mercury [CA ] This patent describes a waste treatment technology in which the waste is treated with a minor amount of sulphuric acid in the presence of an oxidizing agent to convert the metallic mercury to mercury compounds. The waste is blended with a sufficient amount of spent pickle liquor to convert the mercury compounds to basic mercuric sulphate. The sulphate is then precipitated as insoluble basic mercuric carbonate by adding lime. The amount of lime has to be sufficient to precipitate substantially all the mercury sulphate as basic mercuric carbonate to yield a solid mass comprising an insoluble form of mercury and having a small proportion of liquid containing less than 10 ppb of soluble mercury. The process does not describe a production of mercuric sulphide, but a production for mercury carbonate. The process includes a sulphur connection (sulphuric acid) and is therefore included in this chapter Further details concerning the realization of the process Two companies have been identified which utilise sulphur stabilization in pilot plants or large scale laboratory application which could provide additional information on technical, environmental and economic aspects of the technology. The information included is based on personal communication with the companies Sulphur stabilisation according to SAKAB / DELA In the following, the technology of SAKAB / DELA is described. The scaling up of the process is currently taking place. Reactants Process description Process description and equipment The Reactants are technical sulphur and technical elemental mercury which was received from the chlor-alkali industry and was not further processed before treatment. The process which is used by DELA is a sulphuring method capable of treating elemental mercury. The reactor is filled with elemental sulphur (slight stoichiometric excess of sulphur) and mercury, and if needed, with additives. The additives can be added to receive a granular product instead of a powder. The inner atmosphere of the reactor is filled with
177 Reference number /2009/530302/ETU/G2 177 Process conditions Throughput Emissions Energy consumption Expected operational costs Patent Implementation time Implementation costs Process description and equipment nitrogen. The process is carried out with 0.1 bar absolute, which is 0.9 bar below atmospheric pressure. The total quantity of sulphur is added into the reaction vessel. Afterwards, the elemental mercury is continually added to the vessel within approximately 15 to 20 minutes. The temperature is monitored and the reactor can be cooled to prevent a temperature increase which might occur due to energy generated by the mixing process and the exothermic reaction of mercury and sulphide. After about two hours the product can be removed from the vessel. Most of the tests so far have been performed without heating. Additional heating of the HgS at the end of the process (~250 C) leads to a higher alpha-hgs content and thus an increased quality of the product... It is foreseen that the pilot-scale facility will have a heating option and that the whole process will take place at a temperature between 100 and 200 C. Currently, a laboratory scale reactor with volume of about 5 liters exists. The process is carried out in batches with a processing time of about 120 minutes ( min) per batch. Due to the use of a vacuum (100 mbar) in the reaction vessel, a dust filter system and an activated carbon filter, the Hg-emissions should be close to zero (no measurement results available). The energy requirements of the process have not been assessed. According to estimates, the costs will be about 2,000/tonne, packaging, transport and final underground disposal in salt mines of the produced HgS included. DE A1, EP , EP A2 In February 2010, DELA has installed a large scale application which shall be capable of stabilizing 1,000 tonnes of elemental mercury each year. Due to the lack of large scale testing experiments so far no parameter adjustments or test results could be provided. No information is provided for implementation costs. Final product Product stability Resulting Product The final product is cinnabar (red). No elemental mercury (silver) or metacinnabar (black) could be detected in an X-ray structure analyses. It is a fine powder with a density of g/cm 3. The single crystals have a density of about 8.2g/cm 3. The leaching limit values from test runs under stable conditions range between 0.01 mg/kg and 0.04 mg/kg with an average value of mg Hg/kg (tests according to EN12457/1-4).. Tests show that the product is stable up to 350 C. In its current state, the product of the laboratory-
178 Reference number /2009/530302/ETU/G2 178 Volume and weight Emissions from the product Resulting Product scale facility could be disposed of on hazardous and non-hazardous landfills according to the WAC Decision 2003/33/EC (hazardous landfills 0.2 mg/l (2 mg/kg) and non-hazardous 0.02 mg/l (0.2 mg/kg)). The volume of the mercury sulphide powder is about six times the volume of elemental mercury. The weight is increased by about 16%. Mercury vapour tests have been performed. However, no mercury vapour could be detected (LOD=0.003 mg/m 3 ). If additives are introduced, the product form can be changed into a granular form (1-4mm). Thus, dust emissions could be further reduced and handling facilitated Sulphur stabilization according to Bethlehem Apparatus Bethlehem Apparatus is situated in the United States. Their patent is still pending but they have a developed and running, stable process. Reactants Process description Process conditions Throughput Emissions Energy consumption Expected operational costs Patent Implementation time Implementation costs Process description and equipment The reactants are sulphur and elemental mercury. The use of polyethylene to produce pellets was abandoned. Elemental mercury is brought into contact with elemental sulphur. resulting in HgS. The crystal formation is considered to be very sensitive to temperature and pressure changes. No information available Early runs have been batch sizes of about 22.5kg of mercury. The last few runs have been in the range of 90kg. It was decided to work with 45kg batches due to easy processability and possible reruns in 24 hour periods. It is planned to attach 10 or 20 units to a single mercury feed. With such a set-up, the operating system will be capable of processing 500 to 1000kg of mercury per day. The process takes place in a sealed container and no emissions should occur. This container is capable of holding 1-10 bar pressure at 530 C. No information is provided for energy consumption. The stabilization costs are about 5-6 $ / pound which is about 8,000 to 9,000 /tonne of elemental mercury. The patent has been approved but the official number has not been received yet. The application is: U.S. Patent Application No. 12/255,403, Title: A METHOD AND APPARATUS FOR GENERATING MERCURY (II) SULFIDE ELEMENTAL MERCURY No information is provided for the implementation time. About 700,000 for a facility to stabilize 300t per year.
179 Reference number /2009/530302/ETU/G2 179 Resulting Product Final Product The product is a powder with the bulk of the material of approximately 50 mesh size. It easily breaks down into less than 250 mesh size. When removed from the reaction chamber there are also clumps with a diameter of approximately 1 cm. The product of the treatment is HgS and was compared with data on file for naturally occurring cinnabar using x- ray diffraction. The results show complete similarity. No elemental mercury could be detected when a pellet was analysed in computer aided tomography. Product stability The leaching values which were measured had an average of mg/kg (EPA TCLP) Volume and weight The powder density is about 5g/cm 3. Emissions from the No emissions from the product are known. product
180 Reference number /2009/530302/ETU/G Sulphur Polymer Stabilisation/Solidification SPSS The Sulphur Polymer Stabilisation Solidification (SPSS) process is based on the process of sulphur stabilisation with the advantage that, in the case of SPSS, the final product is monolithic with a low surface area. This favours the vapour and leaching performance of the product. Table 7-2: Sulphur Polymer Stabilisation/Solidification: overview of the relevant literature Relevant literature overview for Sulphur Polymer Stabilisation/Solidification Reference Mercury Bakeoff: Technology comparison for the treatment of mixed waste mercury contaminated soils at BNL [Mercury Bakeoff 1999] Mersade Mercury Safety Deposit [Mersade 2007a] Sulphur polymer stabilization/solidification (SPSS) treatability of simulated mixed-waste mercury contaminated sludge [Waste Management 2002] Advances in Encapsulation Technologies for the management of Mercury-contaminated Hazardous Wastes [EPA 2002b] Economic and Environmental analyses of technologies to treat mercury and dispose of it in a waste containment facility [USEPA 2005] Treatment Technologies for Mercury in Soil, Waste and Water [USEPA 2007] Determination of acute Hg emissions form solidified-stabilized cement waste forms [ORNL 2002] Using Sulphur Polymer Stabilisation/Solidification Process to Treat Residual Mercury Wastes form Gold Mining Operations [Brookhaven-Newmont 2003] Advances in Encapsulation Technologies for the Management of Mercury-Contaminated Hazardous Wastes [USEPA 2002b] Sulphur polymer solidification/stabilization of elemental mercury waste. [Waste Management 2001] Process for the stabilization of liquid mercury, via mercury sulphide, by the use of polymeric Content General description of the SPSS process for mercury contaminated soils. General description of different stabilization, solidification techniques, among others SPSS. General description of the SPSS process for a surrogated sludge contaminated with 5000 ppm Hg General description of different immobilization techniques and cost estimates General information about costs and techniques for sulphur stabilization, sulphur polymer stabilization/solidification and amalgamation. General description of techniques and cost estimates General information about SPSS Batch scale SPSS treatment. Comparison of different stabilization techniques (SPSS, CBPC, Macroencapsulation) from different literature sources. Tests for SPSS by adding triisobutyl phosphine and sodium sulphide are presented. Physical and chemical data of SPSS of mercury
181 Reference number /2009/530302/ETU/G2 181 Relevant literature overview for Sulphur Polymer Stabilisation/Solidification Reference sulphur [Mersade 2009] Emerging Technologies in Hazardous Waste Management [ACS Kazakhstan 2000] Mercury wastes evaluation of bulk elemental Mercury [USEPA 2002a] Mercury wastes evaluation of Treatment of mercury surrogated waste [USEPA 2002] Treatment of mercury containing waste [US B1] Method and apparatus for stabilizing liquid elemental mercury [US B1] Process for the encapsulation and stabilization of radioactive, hazardous material [US ] Content Production of SPC and encapsulation of contaminated phosphor gypsum Comparison of three vendors stabilizing bulk elemental mercury Comparison of four different stabilizing surrogated sludges Stabilizing mercury containing waste with SPC and encapsulation Detailed experiment description of stabilizing elemental mercury with sulphur and calcium polysulphide to receive a monolithic product. General description of encapsulation with sulphur cement Technical background Sulphur polymer stabilization is a modification of sulphur stabilization. Within this process elemental mercury reacts with sulphur to mercury(ii)sulphide. Simultaneously, the HgS is encapsulated and thus the final product is a monolith. The process relies on the use of ~95 wt% of elemental sulphur and 5% of organic polymer modifiers also called sulphur polymer cement (SPC). The SPC can be dicyclopentadiene or oligomers of cyclopentadiene. The process has to be carried out at a relatively high temperature of about 135 C, which may lead to some volatilization and thus emission, of the mercury during the process. In any event, the process requires the provision of an inert atmosphere in order to prevent the formation of water soluble mercury(ii)oxide. In the case of SPC, beta-hgs is obtained. The addition of sodium sulphide nonahydrat results in alpha-hgs as a product. A relatively high Hg load of the monolith (~70%) can be achieved with this process, as there is no chemical reaction of the matrix required to set and cure. The process is robust and relatively simple to implement and the product of it is very insoluble in water, has a high resistance to corrosive environment, is resistant to freeze-thaw cycles and has a high mechanical strength. During the process, volatile losses are liable to occur and therefore appropriate engineering controls are needed. Engineering controls to avoid possible ignition and explosions are also necessary. Additionally, the volume of the resulting waste material is considerably increased.
182 Reference number /2009/530302/ETU/G2 182 Polysulphide is added to elemental mercury and sulphur in order to obtain a monolithic product, but the synthesis of a mercury polysulphide complex, with a higher leaching value compared to mercury sulphide, shall be avoided. This can be done by adding the sulphur first and in a second step the sulphur polymer. Formation of mercury polysulphide can also be avoided by adding a polysulphide inhibitor. The generation of toxic H 2 S can be inhibited by limiting the exposure of the stabilizing inorganic sulphur compounds to air and sunlight or by adding antioxidants Economic information According to various studies, the costs vary from 2.88 $/kg (~2 /kg) elemental mercury [USEPA2002b] and between 2.6 $ and 26 $/kg (~ 2 and 20/kg) [USEPA 2005] of treated elemental mercury. The wide range of costs from the report [USEPA 2005] is due to variation of different parameters, which are: technology (ADA or DOE process, whereas the ADA process is considered to be twice expensive compared to the DOE process), mobile and stationary construction, with or without macroencapsulation and considered amount of mercury to be treated (5,000 or 25,000 tonnes) Environmental information The physical chemical properties of the products have been collected and included in Annex 4. Available data about the leachability of the SPSS product are in the range of ~0.02mg/l [Brookhaven- Newmont 2003] and the volatility is about mg/kg (18 C) [Waste Management 2001] Use of the technology Using Sulphur Polymer Stabilisation/Solidification Process to Treat Residual Mercury Wastes from Gold Mining Operations [Brookhaven-Newmont 2003] Two experiments are described, which have been performed with 500 and 250ml mercury and a waste load of 33 to 37%. 2% of hydrated sodium sulphide and 65 to 61% SPC have been used. The products were approximately cylindrical pellets with a largest dimension of 9.5mm. The TCLP results for Hg have been between and 0.039mg/l. Advances in Encapsulation Technologies for the Management of Mercury-Contaminated Hazardous Wastes USEPA 2002b], Sulphur polymer solidification/stabilization of elemental mercury waste. [Waste Management 2001] In this reports the same process is described, which is the SPSS treatment of radioactive Hg with different additives. The report includes examples for the optimisation of the SPSS process with additives in a 5 gallon, heavy gauge steel drum. Triisobutyl phosphine sulphide and sodium sulphide have been tested as additives. The additives (except pure Triisobutyl phosphine) improved the leaching behaviour as well
183 Reference number /2009/530302/ETU/G2 183 as the reaction time. Instead of 16 hours, the process could be finalized within 8 hours. This process is a two step single-vessel process. Mercury SPC and quartz cobbles were placed in the drum, which was covered and then purged with argon through one of the vents. In the first step equal weights of mercury and SPC were mixed in the reaction vessel assuring a six fold, molar excess of sulphur to mercury which facilitates a faster reaction. Prior to mixing the reaction vessel was purged with argon. The vessel was heated to ~ 40 C with agitation to accelerate the mercuric sulphide reaction. Once the mercury had completely reacted with the sulphur, extra SPC was added and the temperature was increased to ± 5 C with agitation, until the mixture melted. The molten product was then poured into metal cans where it cooled into a monolithic waste form. The final product had a mercury load of 33.3 %. The untreated material had a TCLP of 2.64mg/l whereas the SPSS treated material had a TCLP of between 0.02 and 0.4mg/l. Adding 3% triisobutyl phosphine to the SPC changed the TCLP to >0.4mg/l and using 3% Na 2 S.9H 2 O to the SPC resulted in a TCPL of between to 0.05mg/l. Therefore, adding 3% of Na 2 S.9H 2 O generated the best results. Additional information is available on the ph dependency. Process for the stabilization of liquid mercury, via mercury sulphide, by the use of polymeric sulphur [Mersade 2009] In this study, the physical characterization and durability of a SPSS concrete with approximately 50% of filler, sand and gravel as well as 30% of mercury are measured. It has been tested that the comprehensive strength is about 57 N/mm 2, the flexural strength is about 8.5 N/mm², the density is about 3.1g/cm³ and the porosity is less than 2%. Emerging technologies in hazardous waste management [ACS Kazakhstan 2000] This report includes the production of SPC and its use for the stabilization of phosphorgypsum sand waste. In the described process, molten sulphur (140 C) was reacted with a mixture of 2.5% of polyester grade dicyclopentadiene and 2.5% of a proprietary reactive polymer. After 4 hours, the molten SPC was cooled and solidified. The use of 3% sodium sulphide resulted in a TCLP mercury concentration of 26µg/l. Based on the observation from different tests at different times, it is assumed that leachable mercury may decrease over time. Mercury wastes evaluation of bulk elemental Mercury [USEPA 2002a] The technologies compared in the report are: SPSS sulphur stabilization with micro and macro encapsulation and amalgamation. The SPSS process is conducted in two stages. The first step is a reaction between elemental mercury and powdered sulphur polymer cement to generate mercuric sulphide (HgS). During reaction the vessel is placed under inert nitrogen gas to prevent mercuric oxide (HgO) formation and heated to 40 C to enhance the sulphide formation. The purpose of this first step is to chemically stabilize the mercury. The purpose of the second step is to solidify the product. The mixture is heated to 130 C to melt the thermoplastic sulphur binder. It is then poured into a mould. On cooling the reacted
184 Reference number /2009/530302/ETU/G2 184 sulphide particles become microencapsulated within the monolithic sulphur matrix The mercury content of the product from the SPSS process was 33 wt% and had an therefore an increase of 203% by weight. The volume increase is 1,500%. The final form was monolithic and it was estimated that mercury losses to air were about 0.3%. The leaching values for the final pellets are dependent on the ph value and were about 0.01 mg/l at ph=2, ~30 mg/l at ph = 8, 0.01mg/l at ph = 11 and ~140mg/l at ph = Overview of patents Treatment of mercury containing waste [US B1] In this patent, several examples for a SPSS have been performed. The tests were made with 5kg of elemental mercury and 5kg SPC (containing 5% elemental sulphur). Additionally, 3% of the additives sodium sulphide, triisobutyl phosphine sulphide and a 1:1 mixture of both have been added. Untreated mercury had a TCLP concentration of 2.64mg/l and SPC without any additive had a TCLP concentration of 0.02mg/l (if the processing time was enough for a complete reaction). Adding 3% triisobutyl phosphine sulphide resulted in a TCLP concentration of 0.42mg/l whereas 3% sodium sulphide resulted in a TCLP concentration of By adding 1.5% triisobutyl phosphine sulphide and 1.5% sodium sulphide to the mixture, the final product had a TCLP concentration of 0.064mg/l. Vapour tests show that the vapour pressure of Hg sharply decrease over the span of one week from approximately 37µg/l to approximately 3µg/l. The decrease in Hg vapour is explained by the theory of an ongoing curing process of elemental mercury with free sulphur in the matrix. Process for the encapsulation and stabilisation of radioactive, hazardous material [US ] The patent provides a detailed description of the process of encapsulation of hazardous waste. The waste loading is about 40 w/w% waste, 52.5% modified sulphur cement, 7% anhydrous sodium sulphide and 0.5 % glass fibres to increase the physical strength of the product. Compressive strength and leaching tests were performed but the focus is not set on mercury. Method and apparatus for stabilizing liquid elemental mercury [US B1] In this work various tests with elemental mercury containing waste and mercury chlorine were performed. A promising example was the experiment to combine 13.5kg of mercury with 6.75kg of elemental sulphur, 2.7 litres of calcium polysulphide and 6.75kg of sand. The leaching test resulted in 0.1mg/l; whereas a test without the sand resulted in 2mg/l.
185 Reference number /2009/530302/ETU/G Further details concerning realization of the process Two companies have been identified which apply the SPSS process at least on a laboratory scale and which could provide additional information on technical, environmental and economic aspects of the technology. The information included is based on personal communication with the companies SPSS According to ADA Technology ADA Technology is the owner of the technology only. The licensee is M&EC, a company in Oak Ridge, TN. Process description and equipment Reactants Process description Process conditions Throughput The reactants of the process are elemental mercury, sulphur, polysulfide (calcium polysulphide, or sodium polysulphide) and sand It is a batch process consisting of combining elemental mercury with a proprietary sulphur mixture in a pug mill. Treatment of the liquid mercury was conducted by adding powdered sulphur to the pug mill, while a preweighed amount of mercury was poured into the mill. As the mill continues to mix and the reaction takes place, additional substances as sand or water can be added to provide temperature control and sufficient volume for efficient mixing to take place. While the processing of mercury in the pug mill is performed without heating, the reaction of mercury with sulphur is exothermic at room temperature. and the temperature of the mixture increases but shall not exceed 100 C. No further information than that provided in the process description could be provided for the process conditions. A batch size of 50 kg has already been used which would result in a daily throughput of 250 kg/day. A scale up to 375kg/batch is considered possible by the vendor. In this case the yearly throughput is expected to be 1,000t/year if five mixers are used in parallel. All together, 10 metric tonnes of radioactive mercury has already been stabilized by the Company. Emissions Off-gas is passed through a High Efficiency Particulate Airfilter (HEPA), and then passed through a sulphur-impregnated carbon filter. Mercury vapour concentration above the plug mill is below the threshold limit
186 Reference number /2009/530302/ETU/G2 186 Process description and equipment value (TLV) of 50 mg/m 3. Energy consumption No information could be provided for energy consumption. Expected costs operational No information could be provided for operational costs. Implementation costs Patent Implementation time No information could be provided for implementation costs. US 6,403,044 B1 10t of waste have already been stabilised by 50 kg/batch. But no information could be provided for a larger facility e.g. 375 kg/batch Resulting product Final product Product stability Volume and weight Emissions from the product The final product is a granular waste, which consist of HgS and sulphur polymer cement, and can be poured into drums. For this product, only leaching values (TCLP) at different ph values are available. The lowest leaching behaviour can be achieved at a ph value of 2 with mg/l. In a more or less linear trend the leaching value reaches a maximum of ~0.1 mg/l at ph value of 12. The weight of the material increases by about 100 % and the volume increases by about 2200 %. No emissions from the product except leaching are known.
187 Reference number /2009/530302/ETU/G SPSS according to DOE The patent assignee is Brookhaven Natural Laboratory, which is one of ten laboratories overseen and primarily funded by the office of science of the U.S. Department of energy (DOE). The process results in a product containing traces of mercury, further investigations are on hold due to economic reasons. Process description and equipment Reactants Process description Process conditions Throughput Emissions The reactants are elemental mercury, sulphur polymer cement (SPC) and sodium sulphide. This process is a two stage single vessel (vertical mixer/dryer) batch process that results in mercuric sulphide stabilised in a sulphur polymer matrix. In the first step, mercury is reacted with powdered sulphur polymer cement and additives to form a stable mercury sulphide compound. Next, the chemically stabilized mixture is melted in a sulphur polymer matrix, mixed and cooled to form a monolithic solid waste form in which the stabilized mercury particles are microencapsulated within a sulphur polymer matrix [USEPA 2002c]. In the first reaction step the reactor is heated to C and in a second step to 135 C. The whole process takes place under an inert gas atmosphere (nitrogen or argon). A 1 ft 3 (0.03 m 3 ) mixer has already been realized, capable of stabilizing about 20 kg mercury per shift. Assumptions have been provided for the following mixer sizes. 10 m 3 mixers could stabilize about 7,600 kg/day, 1.8 m 3 mixers have a daily throughput of 1,400 kg and 0.28 m 3 mixers have a daily throughput of 270 kg/day. All these assumptions are based on an average batch time of twelve hours and two shifts per day. The process produces some mercury vapour, so a ventilation system is required to filter out the vapour. Since the process is carried out at a high temperature (135 C), heat exchangers are included in the ventilation system. A liquid nitrogen cryogenic trap condenses the mercury vapour and it is recycled back into the process. Trials have shown that 99.7 % of the mercury is retained in the product. Energy consumption No information could be provided for the energy consumption of the process. Expected operational costs No information could be provided for the expected operational costs of the process. From a different study [USEPA 2003] an estimated full scale cost is
188 Reference number /2009/530302/ETU/G2 188 Process description and equipment provided with about 2.88 $/kg (~2,000 /t). Implementation costs No information could be provided for the implementation costs of the process. Patent US 6,399,849 Implementation time No information could be provided for the implementation time of the process. Resulting product Final product Product stability Volume and weight Emissions from the product The product is a monolithic structure with a mercury content of 33%, 65% sulphur polymer cement and 2% sodium sulphide. In order to determine leaching behavior, the TCLP process was used for different ph values. The results have been in a range of and 45 mg/l. The reason for this wide range of leaching behaviour was not the ph dependency but a small amount of elemental mercury which was still existing in the final product. It is considered by the inventors that by adjusting the processing methodology (e.g. mixing method, introduction of waste material) the product quality can be increased and that the process can be controlled better. No further work has been done so far in this field. The Volume of the product is about times the original elemental mercury whereas the weight increased by a factor of 3. The mercury content of the final product is 33 % No emissions from the product except leaching are known.
189 Reference number /2009/530302/ETU/G Amalgamation Many metals interact with liquid mercury and an alloy is formed. These alloys are called amalgams. If the amount of the non-mercury metal is small, the amalgam is still liquid and the viscosity of the amalgam increases with higher concentration of the non-mercury metal in the amalgam. Table 7-3: Amalgamation: overview of the relevant literature Relevant literature overview for amalgamation Literature Treatment Technologies for Mercury in Soil, Waste and Water [USEPA 2007] Advances in Encapsulation Technologies for the management of Mercurycontaminated Hazardous Wastes [USEPA 2002b] Determination of acute Hg emissions from solidified-stabilised cement waste forms [ORNL 2002] Economic and Environmental analyses of technologies to treat mercury and disposed of in a waste containment facility [USEPA 2005] Mersade Mercury Safety Deposit [Mersade 2007a] Mercury wastes evaluation of Treatment of mercury surrogated waste [USEPA 2002] Mercury wastes evaluation of Bulk elemental Mercury [USEPA 2002a] Process for treating mercury in preparation for Disposal [US ] Treatment of elemental mercury [WO A2] and [US A1] Content General description of techniques and cost estimates General description of different immobilization techniques and cost estimates General description of the volatile behaving of mercury after amalgamation. General information about costs and techniques for sulphur stabilisation, sulphur polymer stabilisation/solidification and amalgamation. General description about different stabilization, solidification techniques, among others amalgamation. Comparison of four different stabilizing surrogated sludges Comparison of three vendors stabilizing bulk elemental mercury Mercury is mixed with an inorganic powder (copper, zinc, nickel and sulphur) resulting in a permanent bonding of the mercury to the powder in a solid form. Amalgamation as a first step, followed by a cementation process with Ordinary Portland Cement (OPC) Technical background Amalgamation means the dissolution and solidification of mercury in other metals such as copper, selenium, nickel, zinc and tin, resulting in a solid, non-volatile product. The amalgamating metal is preferably provided in the form of a fine powder, thereby providing the maximum surface area and promoting increased efficiency of reaction. In general, the preferred amalgamation metal is copper.
190 Reference number /2009/530302/ETU/G2 190 Amalgamation is a subset of solidification technologies and does not involve a chemical reaction. Different amalgamation processes exist: aqueous and non-aqueous. The non-aqueous process is suitable for elemental mercury. This process involves the mixing of finely divided powder into liquid mercury, forming a solidified amalgam. This technology is a speedy process for the treatment of elemental mercury. However, mercury in the resulting amalgam is susceptible to volatilization or hydrolysis. Therefore, amalgamation is typically used in combination with an encapsulation technology. Disadvantages come from the difficulties to scale up and the need for dilute nitric acid to achieve high efficiency. The use of nickel has to be considered critically due to its hazardous properties. In addition, prices of potentially suitable metals are relatively high Economic background The prices of the metals used for the amalgamation (Cu ~ 3/kg, Zn ~ 1 /kg, Sn 9/kg) [LME] as well as the adverse raw material/elemental mercury ratio of suggested 3:1 result in relatively high costs of this technology. (HgCu = 9/kg treated mercury, HgZn = 3/kg and HgSn 27/kg) Environmental background The physical-chemical properties of the products have been collected and included in Annex 4. Available data about amalgams is mainly indicated at 0.2 mg/l (TCLP) and a further encapsulation step is therefore recommended Use of the technology Mercury wastes: evaluation of treatment of mercury surrogated waste [USEPA 2002] The technologies compared in this report are: sulphur stabilisation, SPSS, amalgamation and formation of mercuric sulphide followed by cement-containing stabilization. The waste load for the amalgamation process followed by a precipitation of stable salt was ~45 wt% and had an increase of 120% by weight. The final form was soil-like and it was estimated that the mercury loss to air was about 0.05%. Mercury wastes: evaluation of Bulk elemental Mercury [USEPA 2002a] The technologies compared in this report are: sulphur stabilisation, SPSS and amalgamation. The waste load for the amalgamation process followed by a precipitation of stable salt was ~20 wt% and had an increase of 400% by weight. The final form was monolithic. The leaching values for the final pellets are dependent on the ph value and have been about 30 mg/l at ph=2, ~0.2mg/l at ph = 8, 0.1mg/l at ph = 11 and ~0.02mg/l at ph = 12.
191 Reference number /2009/530302/ETU/G2 191 In a separate test, mercury and selenium were heated and allowed to react in the vapour phase. The leaching value of the mercuric selenide was tested at different chlorine concentrations in the water. At ph 7 the addition of 500 ppm of chloride increased solubility from 0.007mg/l to 0.021mg/l Overview of patents Process for treating mercury in preparation for disposal [US ] In different experiments, amalgamation of 1 pint of mercury was tested. In the case of amalgamation with copper it was determined, that a compound agitation with a copper/mercury ratio of 3:1 for 40 minutes provided an optimum result. The product is a powder with a copper appearance, satisfactory for disposal in landfills (for US conditions). In the case of a copper/mercury ratio of 1:1 elemental mercury remained even after 45 min of compound agitation. Another test showed that 2 hours of reciprocal agitation with a copper/mercury ratio of 3:1 yielded an unacceptable high amount of liquid mercury. In a final experiment, sulphur was used instead of copper. After 20 minutes of compound agitation of a mixture with a sulphur/mercury ratio of 3:1 the mercury was solidified. However a mercuric sulphide gas was noticed. Treatment of elemental mercury [WO A2] and [US A1] Amalgamation tests have been performed and the greatest success was observed when copper was added to mercury in a ratio of 2:3. In addition, a 1:1 w/w of a 0.1 M diluted aqueous nitric acid should be employed for optimum results. This mixture was subjected to vigorous agitation until the amalgam reaction was completed. The amalgam sludge is suitable for treatment with an appropriate cementitious particulate filler material such as OPC (or a mixture of a blast furnace slag (BFS) with Ordinary Portland Cement in a ratio of 3:1). The whole process is conducted at room temperature. The amalgamation stage is complete after 5-10 minutes whereas the curing of the BFS and OPC takes 24 to 48 hours. The mercury concentration of the final product is about 14%. The final product shall be suitable for immediate disposal (according to the US legal requirements).
192 Reference number /2009/530302/ETU/G Phosphate ceramic/glass stabilization: Chemical bonded phosphate ceramic (CBPC) The first efforts to stabilize mercury or mercury compounds with phosphate glass started in In the relevant patent, HgO was stabilized with different phosphates and metal oxides. The product of this technology is a Chemically Bonded Phosphate Ceramic (CBPC). Table 7-4: Phosphate ceramic/glass stabilization: overview of the relevant literature Relevant literature overview for phosphate ceramic/glass stabilization Literature Content Method for producing chemically bonded phosphate ceramics and for stabilizing contaminants encapsulated therein utilizing reducing agents[uswagh Singh] Advances in Encapsulation Technologies for the Management of Mercury contaminated Hazardous Wastes [USEPA 2002b] Polymer coating for immobilizing soluble ions in a phosphate ceramic product [US A] Evaluation of chemically bonded phosphate ceramics for mercury stabilization of mixed synthetic waste [USEPA 2003] Chemically bonded phosphate ceramics for stabilization and solidification of mixed waste [USWagh] Mercury stabilization in chemically bonded phosphate ceramics [USWagh 2000] Mercury containing phosphate glass [US ] General information about CBPC and magnesium potassium phosphate (MKP) and examples with different wastes. General description of different immobilization techniques and cost estimates General description for CBPC and applying a polymer coating to the exterior surface of the CBPC product Leaching tests of CBPC containing Hg- and HgCl 2 contaminated wastes. Cost estimation Experience on bench scale stabilization of various waste streams containing Hg in the CBPC process. Detailed explanation for producing a CBPC with mercury contaminated waste and improvement by adding Na 2 S or K 2 S. Process description of the production of mercury phosphate glass with the reactants HgO, P 2 O 5 and a metal of Group I-II, lead or aluminum.
193 Reference number /2009/530302/ETU/G Technical background Chemically bonded phosphate ceramics (CBPCs) are fabricated by an acid-base reaction between calcinated magnesium oxide (MgO) and mono-potassium-phosphate (KH 2 PO 4 ) in solution to form a hard dense ceramic of magnesium potassium phosphate hydrate. For this purpose calcinated magnesium oxide powder and monopotassium phosphate is stirred under an aqueous condition to produce Magnesium potassium phosphate (MKP). In a second step, the MKP is combined with the mercury. The process temperature is low (<80 C) and therefore little hazardous off-gasses arise and no secondary waste is generated. CBPC treatment of elemental Mercury will form low solubility chemical bonded phosphate solids (Hg 3 (PO 4 ) 2 ), but a further improved stabilization by forming HgS in a first step, can be realised with a small amount of sodium sulphide (Na 2 S) or potassium sulphide (K 2 S). The sulphides significantly improve the performance of the final CBPC waste and are therefore recommended. An excess of sulphide will increase the leachability and therefore careful processing is needed. The product of the CBPC process can have a mercury load as high as 78% with a density of 1.8g/cm³. The immobilisation is a result of chemical stabilisation and a physical encapsulation (solidification). Studies have been carried out to show stabilization of waste streams only, which were contaminated with small amount of mercury. In the case of elemental mercury, some significant work will have to be carried out to develop a process to treat mercury in large quantities, though theoretically this can be achieved. An advantage for phosphate glass is the high physical stability Economic background The total costs, including raw materials, labour and disposal for the CBPC process is about $/kg (~ 10/kg) elemental mercury [USEPA 2002b] Environmental background The physical-chemical properties of the products have been collected and included in Annex 4. The solubility of Hg 3 (PO 4 )2 is 1.4*10-8 mol/l and for HgHPO 4 = 2.8*10-7. This is equal to a mercury concentration of 2.8 and 56µg/l respectively. Even though these values are very low, the leaching value of HgS is much lower (4.5 *10-25 mol/l or µg/l) [USWagh 2000].
194 Reference number /2009/530302/ETU/G Use of technology Evaluation of chemically bonded phosphate ceramics for mercury stabilization of mixed synthetic waste [USEPA 2003] In this evaluation, CBPCs of elemental mercury with a concentration of 50% and 70% Hg in the stabilised waste form have been produced. For the 50% load, 300g Hg were mixed with 2g Na 2 S and 160g water. After 10 minutes of mixing, it was combined with 300g MKP binder. For the 70% load, 400g Hg were mixed with 2.67 g Na 2 S and 120g water. After 10 minutes of mixing, it was combined with 172g MKP binder. These mixtures were transferred into plastic vertical cylindrical moulds and allowed to set until solidified. The moulds were cured by air-drying for about three weeks. In the case of untreated Hg the leaching behaviour is ~250mg/l at ph of 2 and ~35µg/l at ph 12. Stabilised waste with 50% Hg had a leaching concentration of ~3mg/l at ph 2 and ~8µg/l at ph 12. Stabilised waste with 70% Hg had a leaching concentration of ~6mg/l at ph 2 and ~1.4 mg/l at ph 12. The results are shown in Figure 7-2. Figure 7-2: Leaching behaviour of stabilized waste with different Hg loads and at different ph values.
195 Reference number /2009/530302/ETU/G2 195 Chemically bonded phosphate ceramics for stabilization and solidification of mixed waste [USWagh] In this report the CBPC technology to encapsulate different wastes is described. Among other wastes, the encapsulation of Hg-contaminated wastes from light bulbs is also presented. The examples were performed in 5-gals drums with a waste load of about 40%. For the Hg-contaminated wastes, potassium sulphide was added. The final product had a TCLP leaching value of 0.05 ppb of Hg in the leaching water. Mercury stabilization in chemically bonded phosphate ceramics [USWagh 2000] This report describes different types of wastes that have been treated to form CBPC and to bind mercury as Hg 3 (PO 4 ) 2 within the ceramic. It was shown that the limit value of TCLP 0.2 mg/l could not be reached. Therefore, it is recommended to add a sulphide as Na 2 S or K 2 S to receive HgS which is encapsulated within the ceramic. An excess of sulphide favours the formation of HgSO 4 which has a disadvantageously high solubility product. The waste was added to the binder mixture (K 2 S, MgO, and KH 2 PO 4 ) and to a stoichiometric amount of water. The mixture was mixed for 30 minutes and poured into a mould to set within 2 hours. The hard and dense ceramic was stored for 3 weeks for good curing. Leaching tests and long term leaching tests delivered sufficient stability for EPA limits Overview of patents Mercury containing phosphate glass [US ] In this patent different combinations of HgO, Li 2 O and P 2 O 6 have been used to produce phosphate glass. The focus was to produce a glass with good optical characteristics. The mercury content in the different glasses is between ~30 to 70% with a density between 3 to 6.5g/cm Solidification/encapsulation The following techniques are used for hazardous waste treatment. No reports have been published which cover the encapsulation of elemental mercury but the processes shall be briefly described here for a complete overview of stabilisation encapsulation techniques. All these processes only encapsulate mercury but do not interact chemically with the mercury: Polyethylene Encapsulation [US-EPA2002] The polyethylene encapsulation is dependent on the extruder used, a macro-encapsulation process or a combined micro- and macro-encapsulation process. Low density polyethylene (LDPE) is less prone to cratering and cracking than high density polyethylene (HDPE). The resulting material has a high mechanical strength, flexibility and chemical resistance. The waste load can be up to 70% and the equipment is commercially available. The disadvantage is that this process requires higher temperature and therefore Hg emissions from the process can occur. This technique can be combined with a stabilisation process.
196 Reference number /2009/530302/ETU/G2 196 Encapsulation with Asphalt [US-EPA2002] Asphalt micro-encapsulation can be used for encapsulation of different wastes. For mercury containing waste, cold-mix asphalt seems to be more appropriate than hot-mix asphalt due to the possible volatilization of mercury. As there is no chemical reaction between the asphalt and the mercury a stabilizing pre-treatment step is necessary. Encapsulation with Polyester and Epoxy resin [US-EPA2002] With polyester and epoxy resin encapsulation, waste loads of 50% have been reported but no information for the usability for metallic mercury is available. As there is no chemical reaction between the polyester nor the epoxy resin with the mercury, a stabilizing pre-treatment step is necessary. Encapsulation with Synthetic Elastomers [US-EPA2002] Synthetic rubbers have been used for microencapsulation and stabilisation of metal contaminated waste. As there is no chemical reaction between the synthetic elastomer with the mercury a stabilizing pre-treatment step is necessary. Encapsulation with Polysiloxane [US-EPA2002] Polysiloxane or ceramic silicon foam (CSF) have been used for the encapsulation of waste and consists of 50 wt% vinylpolydimethyl-siloxane, 20 wt% quartz, 25 wt% proprietary ingredients and less than 5 wt% water. The material sets at room temperature and is resistant to extreme temperatures, pressures and chemical exposure. The waste loading can be up to 50 wt%. As there is no chemical reaction between polysiloxane and the mercury, a stabilizing pre-treatment step is necessary. Sol gels encapsulation [US-EPA2002] Sol gels are a combination of organic polymers and inorganic ceramics. The polymer and silicon dioxide are combined first and then mixed with the waste and then solidified to encapsulate the waste. The temperature for this process is about 70 C and a waste loading of 30 to 70% can be achieved. DolocreteTM encapsulation [US-EPA2002], DolocreteTM is a calcined dolomitic binder material that can be used for microencapsulation of inorganic, organic and low-level radioactive waste. Encapsulation with calcium carbonate and magnesium oxide (CaCO 3 -MgO) [US B1] The hazardous waste material is added to a settable composition forming a slurry and allowing the slurry to set to encapsulate the waste material. The settable composition is a powdered, flowable cement composition, containing calcium carbonate and a caustic magnesium oxide. Different
197 Reference number /2009/530302/ETU/G2 197 additives such as aluminium sulphate of citric acid can be added to increase the performance. Encapsulation with ladle furnace slag [WO A1] When ladle furnace slag is subjected to an alkali-activated (2M NaOH) process with thermal treatment, the non-reactive ladle furnace slag undergoes a chemical reaction and forms a durable cementitious matrix capable of advantageously stabilizing mercury ions. The mercury ions are precipitated into stable heavy metal compounds such as mercury sulphides and are encapsulated by the matrix slurry as the matrix slurry sets into a monolith structure. The amount of mercury in comparison to the ladle furnace slag is 1%. 7.6 Encapsulation of stabilized mercury with cement Cement solidification is an encapsulation technique as listed in section 7.5. This technique is described separately because tests with a starting material of metallic mercury, which was pretreated before encapsulation, have already been carried out and a patent is available. This technique has only been realised on a laboratory scale. Table 7-5: Cement solidification: overview of the relevant literature Relevant literature overview for ordinary Portland cement solidification Literature Method for producing inorganic hardened body [JP ] Content General description of inorganic hardened body by using fibre and cement. Treatment of elemental mercury [WO A2] Encapsulation process [Lopez 2009] Procedimiento de estabilizacion de mercurio liquid mediante cemento polimerico de azufre, via sulfuro de mercurio [P ] Describes the encapsulation technique with ordinary portland cement (OPC). A pre-treatment technique is recommended. Description of a sulphur stabilization technology, combined with a cement encapsulation. Patent application for a process of a sulphur stabilization technology, combined with a cement encapsulation Technical background Cement (e.g. ordinary Portland cement [OPC]), acting as the cementitious filler material is used for the encapsulation of elemental mercury. To improve the leaching properties, a previous stabilisation step (amalgamation with Cu) is carried out. Additional inorganic fillers can be added to this process as pulverised fuel ash, hydrate lime, finely divided silica, limestone flour and organic and inorganic fluidizing agent and especially blast furnace slag (BFS). The ratio of the inorganic filler to the cementitious filler material can be in the range of 3:1 w/w. The immobilised mercury shall be mixed
198 Reference number /2009/530302/ETU/G2 198 in a ratio of 1:1 w/w with the filler material Economic background The cost estimate is $16.37 per kg for conventional Portland cement stabilization (including disposal) [US-EPA 2003] Environmental background No relevant environmental data have yet been found for the OPC encapsulation of mercury Overview of patents Treatment of elemental mercury [WO A2] In a first experiment, 80g of amalgam sludge (20g Hg, 30g Cu and 30ml dilute nitric acid) are stirred with 40g OPC and 120g BFS. Water was added to this mixture as necessary. The mixture was covered and allowed to stand for 48 hours at ambient temperature. The product was suitable for immediate disposal according to the US legislation requirements. In a second experiment, 100g mercury, 150g copper and 150ml 0.1 M nitric acid were intensively stirred for 30 minutes to receive an amalgam sludge. This sludge was mixed with 300 g BFS and 100 g OPC resulting in a Hg load of 14%. The mixture was poured into a mould and left for 24 hours for curing Further details concerning realization of the process One institution has been identified which applies the sulphur stabilization in combination with the encapsulation technique, which could provide additional information on technical, environmental and economic aspects of the technology Cement encapsulation technique according to MERSADE The information included is based on personal communication with the companies. The process was developed in the context of the EU Life-project Mersade. The technology is until now only performed on a semi-laboratory scale. A larger scaling up has not yet started.
199 Reference number /2009/530302/ETU/G2 199 Process description and equipment Reactants Process description Process conditions Throughput Emissions Energy consumption Expected operational costs Implementation costs The reactants are elemental mercury, elemental sulphur, polymeric sulphur, coarse and fine gravel, sand and CaCO 3. The concrete block has a mercury content of 30%. The stabilization takes place in a two-step process. In the first step the elemental mercury is stabilized with sulphur to meta-cinnabar with a planetary ball mill. In a second step this meta-cinnabar is incorporated in a polymeric S-concrete matrix, composed of gravel, sand, filler, elemental sulphur and modified sulphur. The concrete matrix is prepared at 140 C and at room temperature The facility is still only on a small scale, producing 6 kg of a final product per batch and a throughput of 4 kg/ Due to the laboratory scale, emissions can occur during the milling of sulphur and liquid mercury No information could be provided for the energy consumption of the process. The cost for the stabilization of metallic mercury at a full scale application is estimated to be between 15,000 and 17,000 /tonne metallic mercury. No information could be provided for the implementation costs of the process. Patent Patent application N P , priority date: 9 September 2009 [P ] Implementation time No information could be provided for the implementation time of the process. Resulting Product Final Product Product stability The final product is prepared in the form of a monolithic material of 16x16x4 cm. The shape of the ashlars can also be changed. The concrete blocks have a water absorption by capillary of 0.07 g/cm 2. The water permeability under low pressure (RILEM) shows no water absorption under low pressure. To determine the leaching behaviour the
200 Reference number /2009/530302/ETU/G2 200 Resulting Product TCLP procedure was used and the average value was ~0,102mg/l. The concrete block shows very good mechanical properties with a comprehensive strength of 57.2 ± 44 N/mm2 and a flexural strength of 8.5 ± 1.17 N/mm 2. Volume and weight Emissions from the product The density of the concrete block is about g/cm 3 and has a total porosity of ~2% and a closed porosity of ~0.6%. The mercury loaded concrete blocks have a higher density and lower pore volume than a mercury free reference. The reason is that it is expected that metacinnabar particles fill interparticle interstices and the higher size pores which exist in the initial S-concrete. The volume of the product is approximately 13 times higher than elemental mercury and the weight is increased by a factor of 3. No emissions from the product except leaching are known.
201 Reference number /2009/530302/ETU/G Conclusion Based on an extensive literature search including patent data bases, scientific data bases and other relevant recent publications numerous pre-treatment technologies have been identified. Wherever possible, the authors or companies, developing the technologies, have been approached directly to receive the most recent information on the state of the art of the technology and their state of implementation. The identified technologies could be allocated to 6 categories depending on the used technology or stabilization process (see Table 7-6). Apart from the technologies already realised in large scale application only very limited information on costs or environmental aspects of the process are available. An evaluation of the technologies against technical, environmental and economic requirements is included in section 8. In particular the sulphur stabilization and the SPSS technologies are already well developed and available at a full-scale application. Detailed data on operation conditions and final products as well as some information concerning costs are available and included in the previous section. In addition, an overview on the most important information related to technologies already realised in largescale application is compiled in Annex 5. Sulphur stabilisation The stabilisation with sulphur has been widely described in literature such as [DE453523], [CA ], [ÖREBRO 2006], [US ], [Lopez 2008], [Kystverket 2008], [Mersade 2007a], [USEPA 2002], [GRS 2009A] and [USEPA 2005]. Literature refers to the stabilisation of metallic mercury but also to mercury-containing waste. In general sulphur is seen as an appropriate stabilisation agent and the stabilisation process with sulphur is considered to be an effective stabilisation process. If testing results have been presented in literature [US EPA 2005], the sulphur containing stabilisation techniques show good test results with respect to the stability of the product. Due to intensive research work a continuous improvement of the process could be observed. At present, only two companies realised this process on a large-scale application, SAKAB/DELA, Germany and Bethlehem Apparatus, USA. Literature available related to the latest process conditions are patents, presentations [DELA 2009] or direct information from the companies. DELA has published some patents on the production of mercury sulphide. Patents are available for a continuous process [EP A2] as well as for a discontinuous process [EP A2]. The currently realised process refers to patent No. EP A2 (batch process).
202 Reference number /2009/530302/ETU/G2 202 Details of the process have been gathered by telephone conversation, s and a visit. During 2009 the process was continuously developed and the parameters have been adjusted. Since the end of 2009, stable and low leaching values are realised and the mercury concentration in the gaseous phase was measured and was below the limit of detection of the used analysis instrument (0.003 mg/m³). In February 2010 a large-scale application (installed capacity: 1,000 t/year) has been installed by the company DELA 80. At the moment no test results of the product could be provided. Recently, a patent of Bethlehem Apparatus concerning the stabilisation of metallic mercury with sulphide has been approved and an official number is expected in the near future 81. All the data related to the process developed by Bethlehem Apparatus has been gained by telephone conversation and a filled in questionnaire from the company. The quality of the stabilised product is comparable to the product resulting from the SAKAB/DELA process. The leaching value is in the same range and no unreacted metallic mercury could be detected when analysed with x-ray diffraction or by computer aided tomography. With both methods no mercury could be detected 82.Sulphur polymer stabilisation/solidification (SPSS) The different literatures ([Brookhaven_Newmont 2003], [US B1], [ACS Kazakhstan 2000], [USEPA 2002a]) describing the SPSS process developed by the Department of Energy (DOE) evaluate this pre-treatment technology as a stable process which is fully developed. Only one report ([USEPA 2002a, vendor A]) indicated leaching values - measured at different ph values which seemed higher than expected. After direct contact with DOE 83 it turned out that this technique still needs some R&D to optimize the technology and improve quality control (i.e. to ensure complete reaction of all the Hg and therefore consistently low leachability). The high leaching values reported in the report result from an incomplete stabilisation of the metallic mercury. Only 99.7% of the mercury is retained in the product. Apart from DOE another company (ADA Technology) has been identified which has developed a pretreatment process based on SPSS. The process is described in detail in literature (e.g. [USEPA 2002a, vendor B], [USEPA 2005]). In addition several discussions and exchanges took place with ADA Technologies as well as with M&EC, the licence holder of this technology. The installation costs of the facility are stated in the report [USEPA2005] to be about 2,000,000 and the estimated cost per year (for 1,000 t/year) have been set at about 2,700,000. According to ADA Technology this economic calculation of the report [USEPA 2005] is considered to be rather conservative. ADA Technology which has 15 years of experience in developing mercury stabilisation solutions indicated that on the 80 Miriam Ortheil, DELA GmbH, 6 January Bruce J. Lawrence, president, Bethlehem Apparatus Co. Inc., 5 January 2010, U.S. Patent Application No. 12/255, Bruce J. Lawrence, president, Bethlehem Apparatus Co. Inc., 19 August statement from Mr. Kalb, Division Head, Brookhaven National Laboratory,
203 Reference number /2009/530302/ETU/G2 203 basis of their experience mercuric sulphide is the most stable and least soluble form of mercury. 84 Amalgamation Technologies based on amalgamation of mercury with other metals are widely described (especially [USEPA 2007], [USEPA 2002a], [WO A2] [US ]), but the stability and suitability of the resulting amalgam for a final storage are highly questionable. Report [USEPA 2002a] compares leaching limit values of different pre-treatment technologies. The leaching values indicated for the amalgamation process (vendor C) are higher compared to the other pre-treatment technologies using sulphur as a stabilisation agent. Especially at lower ph values (ph <4) the poor quality of this stabilisation technology can be recognised. No information on a potential commercial use has been found. The poor stabilisation performance of amalgams is a general accepted opinion [USEPA 2002a] and no expert could be found who would favour amalgamation as a stabilisation technique. In many cases as in the patent [US ] amalgamation is combined with an encapsulation step. CBPC The stabilisation of metallic mercury by chemical bonded phosphate ceramic processes are well described in the literature, e.g. [US Wagh], [US Wagh Singh], [USWagh]). Evaluating the literature, the reader has the impression that this technology is ready to be used to stabilise metallic mercury. To verify this information Mr. Wagh was contacted. The following statement has been received by e- mail 85 : The phosphate bonded ceramic technology has not been used or demonstrated for elemental mercury. We have developed detailed solubility models to produce suitable formulation for treating metals, but we have not carried out any experimental work. It was also stated that still a lot of work has to be done to develop a process to treat mercury in large quantity, though theoretically this would be possible. Encapsulation A lot of information related to encapsulation processes has been identified. In particular [USEPA 2002b] describes encapsulation processes in detail. But all technologies deal with the encapsulation of mercury containing waste. Investigations using an encapsulation technique with metallic mercury could not be found. Numerous patents are available describing encapsulation of mercury contaminated waste (e.g. [WO A1]). A rough screening of these patents was carried out but no suitable technologies for the treatment of pure metallic mercury have been identified. Due to its liquid state, metallic mercury is completely different to mercury-contaminated waste (solid) and therefore stabilisation technologies cannot be easily transferred. Only one encapsulation technology with a prior sulphur stabilization of the metallic mercury shows promising results [Mersade 2009A]. It can be considered that the main stabilisation is due to the sulphurisation and not the encapsulation [Mersade 2007A]. Currently information only is available from the institution developing this technology. 84 statement from Mr. Jim Butz, vice president of Operations from ADA Technology, Inc from Mr. Wagh, dated
204 Reference number /2009/530302/ETU/G2 204 A patent promoting the encapsulation of immobilised mercury (either by sulphur stabilisation, sulphur polymer stabilisation/solidification, chemically bonded phosphate ceramic or copper) with Ordinary Portland Cement (OPC) is described by [WO A2]. A practical use of this technology could not be found. In the following table a short overview on the realised pre-treatment per categories is given:
205 205 Table 7-6: Overview on existing pre-treatment technologies for liquid mercury Existing pre-treatment technologies Process Company Elemental mercury Daily Throughput Complete Hg content Comments per batch for one existing line stabilisation in product Sulphur stabilisation Large scale application available but not DELA 5 kg 60 kg/day 84 wt% tested yet. No scaling up is planned but the parallel Bethlehem 50 kg 275 kg/day 84 wt% use of many small lines is proposed to apparatus meet quantity needs, when needed SPSS M&CE 50 kg 250 kg/day 50 wt% 10 tonnes already stabilised DOE 20 kg 40 kg/day X 33 wt% Incomplete reaction, presence of elemental mercury in the product Amalgamation The technology is currently not X X X X X economically used for Hg stabilisation CBPS The technology is currently not X X X X X economically used for Hg stabilisation Encapsulation The technology is currently not X X X X X without stabilisation economically used for Hg stabilisation Sulphurisation / Needed time period for a large scale MERSADE 2 kg 100 kg/day 30 wt% Encapsulation application: 3-5 years
206 Reference number /2009/530302/ETU/G References [ACS Kazakhstan 2000] Emerging Technologies in Hazardous Waste Management 8 D. William Tedder and Frederick G. Pohland, 2000 [Brookhaven Newmont 2003] Using the Sulfur Polymer Stabilization/Solidification Process to Treat Residual Mercury Wastes from Gold Mining Operations B. Bowerman, J. Adams, P.Kalb, R-Y Wan and M. LeVier February 2003, [CA ] McCord, Andrew T. and Wagner, lois E., Disposal of wastes containing mercury, Chem-Trol pollution Services [CENIM 2009] The application of sulphur concrete to the stabilization of Hg-contaminated soil, 1st Spanish national conference on advances in materials recycling and eco-energy, F.A. López, C.P. Román, I. Padilla, A. López-Delgado and F.J. Alguacil, 2009 [DELA 2009] Workshop on the safe storage and disposal of redundant mercury, Stabilisation of mercury for final disposal by formation of mercury sulphide, Miriam Ortheil, DELA, St Anne s College, Oxford (UK), 13 th & 14 th October, 2009 [DE453523] Herstellung von lichtecher Zinnober aus den Elementen, Deutsches Reich, Alexander Eibner, 7. April 1925 [EP A2] Verfahren und Vorrichtung zur Herstellung von Quecksilbersulfid zur anschließenden Entsorgung, Bonman Christian, EP A2 [EP A2] Verfahren und Vorrichtung zur Herstellung von Quecksilbersulfid zur anschließenden Entsorgung, Bonman Christian, EP A2 [GRS 2009A] GSR Gesellschaft für Anlagen- und Reaktorsicherheit, Technologies for the stabilization of elemental mercury and mercury-containing wastes, Final Report, GRS 252, ISBN , October 2009 [JP ] Method for producing inorganic hardened body, Suzuki Shinchi, Watanabe Hiroshi, Shimada Kyoko, JP , 2002 [Kystverket 2008] Det Norske Veritas AS, Kystverket Norwegian Coastal Administration - Salvage of U-864 -
207 Reference number /2009/530302/ETU/G Supplementary studies - disposal, report NO , Revision N 01, [LME] London metal exchange, [Lopez 2008] F.A. López, F.H. Alguacil, C.P. Roman, H. Tayibi and A. López-Delgado, Disposal of elemental mercury via sulphur reaction by milling, 2008 ; [Lopez 2009] Stabiliszation of mercury by sulphur concrete: Study of the Durability of the Materials obtained, F.A. López, C. Pérez, A. Guerrero, S. Goñi, F.J.Alguacil and A. López-Delgado, 1 st Spanish National Conference on Advances in Materials Recycling and Eco-Energy, Madrid, November 2009 [Mercury Bakeoff 1999] Mercury Bakeoff: Technology Comparison for the Treatment of Mixed Waste Mercury Contaminated Soils at BNL] P.D. Kalb, J.W. Adams, L.W. Milian, G. Penny, J. Brower, A. Lockwood Brookhaven National Laboratory 2 March 1999 [Mersade 2007 A] M. Ramos, Literature review concerning corrosion problems in mercury and stabilisation of liquid Hg, Status Report Literature review, T 1.3 and T 1.4, Life Project Number Life06 ENV/ES/PRE/03, February 2007; ercury%20corrosion%20and%20stabilisation%20of%20liquid%20hg.pdf [Mersade 2007 B] P. Higueras, J. M. Esbrí, Literature review concerning environmental mercury monitoring, Status Report, Life Project Number Life06 ENV/ES/PRE/03, March 2007; mental%20mercury%20mon.pdf [Mersade 2009] Process for the Stabilization of Liquid mercury, via mercury sulfide, by the use of polymeric sulfur, F.A. López, A. López-Delgado and F.J. Alguacil, Consejo superior de investicadiones cientificas (CSIC), Centor nacional de investigations metalúrgicas (CENIM) [ÖREBRO 2006] Margareta Svensson, Mercury immobilisation, A requirement for permanent disposal of mercury waste in Sweden, Mercury_immobilization.pdf 3rd February 2006 [ORNL-2002] MEASUREMENTS OF MERCURY RELEASED FROM SOLIDIFIED/STABILIZED WASTE FORMS FY Mercury pdf
208 Reference number /2009/530302/ETU/G [ORNL 2002a] Determination of acute Hg emissions form solidified -stabilized cement waste forms, C.H. Mattus, 2002 [P ] López FA, López-Delgado A, Alguacil FJ and Alonso M., Procedimiento de estabilizacion de mercurio liquid mediante cemento polimerico de azufre, via sulfuro de mercurio, P (2009) [SAKAB/ DELA 2009] Stabilization of metallic mercury, Fact sheet, Susanne Kummel, 2009 [SPC 2009] [Spiegel 2007] Gau in der Grube, Michael Fröhlingsdorf, Sebastian Knauer, 17/2007 [UNEP 2009] UNEP, Draft technical guidelines on the environmentally sound management of mercury wastes, 4th Draft, April 2009 [UNEP 2009 B] [USEPA 2002] Mary Cunningham, John Austin, Mike Morris, Evaluation of Treatment of Mercury Surrogate waste, final report, 2002 [USEPA 2002a] Mary Cunningham, John Austin, Mike Morris, Greg Hulet, Mercury wastes evaluation of treatment of bulk elemental mercury, 2002 [USEPA 2002b] Paul M. Randall, Sandip Chattopadhyay, Wendy E. Condit, Advances in encapsulation technologies for the management of mercury-contaminated hazardous wastes, 2002 [USEPA 2002c] Hugh W. McKinnon, Preliminary analysis of alternatives for the long term management of excess mercury, EPA/600/R-03/048, 2002, [USEPA 2003] Evaluation of chemically Bonded Phosphate Ceramics for Mercury Stabilization of a Mixed Synthetic Waste, Land Remediation and Pollution Control Division National Risk Management Research Center Sandip Chattopadhyay, Paul M. Randall, March [US EPA 2005] Paul Randall, Economic and Environmental Analysis of Technologies to Treat Mercury and Dispose in a Waste Containment Facility, April
209 Reference number /2009/530302/ETU/G [USEPA 2007] U.S. Environmental Protection Agency, Treatment Technologies For Mercury in Soil, Waste, and Water, EPA-542-R , 2007, [US A1] Christelle Riviere-Huc, Vincent Huc, Emilie Bosse, Method for stabilisation of metallic mercury using sulphur, Oblon, Spivak, Mccleland Maier & Neustadt, 24. January 2008 [US A1] Treatment of elemental mercury, Moore & Van Allen PLLC, Henry Boso Chan, Raymond Hall, 25. Sep [US ] Preparation of mercuric sulfide, Anthony Giordano, 30. October 1962 [US ] Mercury-containing phosphate glass University Park Woldemar A. Weyl 10. March 1970 [US ] Removal of mercury from effluent streams, Penwalt Corporation, Paul Francis Waltrich,05. December 1972 [US ] Process for treating mercury in preparation for disposal, Ecoflo Inc., Jeffrey C. Woodward, 23 July 1991 [US ] Stabilizing inorganic substrates, Harold W. Adams, 13. September 1994 [US ] Stabilizing inorganic substrates Harold W. Adams, 8. October 1996 [US ] Method of immobilizing toxic waste material and resultant products, Southwest Research Institute, William A. Mallow, Robert D. Young, 29. October 1996 [US ] Process for the encapsulation and stabilization of radioactive, hazardous and mixed wastes, Peter Colombo, Paul D. Kalb, John H. Heisser, US 5,678,234, 1997 [US B1] Encapsulation of hazardous waste materials, Dolomatrix International Limited, Dino Rechichi, 04. July 2002 [US B1] Treatment of mercury containing waste, Brookhaven Science Associates LLC, Paul D. Kalb, Dan Melamed, Bhavesh R Patel, Mark Fuhrmann, 04 July 2002
210 Reference number /2009/530302/ETU/G [US A] Polymer coating for immobilizing soluble ions in a phosphate ceramic product, Dileep Singh, Arun S. Wagh, Kartikey D. Patel, US 6,153,809, 2000 [US B1] John E. Litz, Thomas Broderick, Robin M. Stewart, Method and apparatus for stabilizing liquid elemental mercury, ADA Technology Inc., 11. July 2002 [US Wagh] Chemically Bonded Phosphate Ceramics for Stabilization and Solidification of mixed waste, Energy Technology Division Arun S. Wagh, Dileep Singh, Seung-Young Jeong, [US Wagh 2000] Mercury Stabilization in Chemically Bonded Phosphate Ceramics; Energy Technology Division Argonne National Laboratory Dilep Singh, Arun Wagh, Seung Young Jeong [US Wagh Singh] Method for producing chemically bonded phosphate ceramics and for stabilizing contaminants encapsulated therein utilizing reducing agents; United States Government; Dileep Singh, Arun Wagh, Seung-Young Jeong / nscUTZ/webviewable/ [Wagh-1] Personal information Mr. Wagh [Waste Management 2001] Sulfur Polymer Solidification/Stabilization of elemental mercury waste M. Fuhrmann, D. Melamed, P.D. Kalb, J.W. Adams, L.W. Milian 14 August 2001 [Waste Management 2001] Sulfur polymer solidification/stabilization of elemental mercury waste, Waste Management 22 (2002) , M. Fuhrmann, D. Melamed, P.D. Kalb, J.W. Adams, L.W. Milian, 2001 [Waste Management 2002] Sulfur polymer stabilization/solidification (SPSS) treatability of simulated mixed-waste mercury contaminated sludge J.W: Adams, B.S. Bowerman, P.D. Kalb February [WO A1] A method and composition for stabilizing waste mercury compounds using ladle furnace slag, Nanyang Technological University Sun, Darren Delai, Tay, Joo Hwa, Cheong, Hee Kiat, 06. May 2005 [WO A2] Treatment of elemental mercury, Nuclear Fuels PLC, Chan, Henry, Boso 22. March 2005
211 Reference number /2009/530302/ETU/G Screening analysis of options The main goal of this study (and also of the Hg-Regulation) is to find an economically viable permanent solution for the long-term storage of liquid mercury with minimized environmental impacts which prevent the re-entry of the waste mercury onto the market. For this purpose, existing options have to be investigated in a screening analysis. It should be noted that possible storage facilities have to fulfil the requirements set out in Directive 1999/31/EC (landfill directive, with the exception of Article 5 (3)(a)) and Decision 2003/33/EC (WAC decision, with the exception of section 2.4, Annex I). But as the existing provisions are established for the storage of solid waste it is necessary to investigate if these provisions are sufficient to ensure safe storage of metallic mercury with its specific properties (e.g. liquid state, high vapour pressure). In contrast to the other options, option 6 (pre-treatment of metallic mercury) consists of numerous sub options representing different pre-treatment technologies (for detailed information on the pretreatment technologies, see chapter 7). A differentiation is needed as each pre-treatment option is different in relation to its environmental performance, costs and in particular, maturity of the technologies. The main objective of the screening analysis is to evaluate the options identified in section 3 against minimum technical, environmental or economic requirements. An investigation will be carried out to determine whether appropriate minimum requirements are already available by applying existing and implemented legal provisions (e.g. in the WAC Decision) or if additional requirements for the facilities or criteria for the acceptance of the waste are necessary. Options which do not fulfil the minimum requirements will not be further investigated. The feasibility of the options as regards their implementation time and costs will also be taken into consideration. In the following, specific criteria will be described in detail. The screening analysis results in a short list of options which have been assessed as feasible for the storage of the mercury waste (metallic or in stabilized form) under the pre-condition that the derived additional facility-related requirements and acceptance criteria are fulfilled. The analysis is based on the information compiled in the review chapters 4-7.
212 Reference number /2009/530302/ETU/G Identification of minimum requirements for storage options Technical minimum requirements The technical minimum requirements which the options have to fulfil have been mainly derived from the provisions in Regulation (EC) No 1102/2008 but also other relevant minimum requirements related to the technical performance of the pre-treatment technologies are included in the screening analysis. For the permanent underground storage of liquid mercury in deep hard rock or salt formations, the following technical minimum requirements have to be fulfilled by the options (recital 11, Hg- Regulation): protection of groundwater against mercury prevention of vapour emissions of mercury impermeability to gas and liquids of the surroundings and firmly encapsulating the wastes at the end of the mines' deformation process Furthermore, deep underground hard rock formations have to provide a level of safety and confinement equal to those of salt mines. The Regulation also foresees appropriate containment for the storage (Article 3 (1)). The containment and the lining of the mercury have no barrier function in the underground long term storage (WAC Decision, Appendix A, Nr ) Therefore, the main function of the containment and the lining is to ensure a safe handling of the liquid mercury and a safe encapsulation of the liquid mercury until the cell and the salt mine are closed. For the temporary underground storage of liquid mercury in deep hard rock and salt formations, the following technical minimum requirements have to be fulfilled by the options (recital 11, Hg- Regulation): protection of groundwater against mercury prevention of vapour emissions of mercury impermeability to gas and liquids of the surroundings and reversibility/retrievability Temporary storage is only seen as bridging of the gap until a permanent solution is found. Therefore, storage should take place in a way that subsequent processing of the metallic mercury waste is not hindered or made impossible.
213 Reference number /2009/530302/ETU/G In the case of a temporary storage, the containment on the one hand ensures a safe handling and storage of the waste, and on the other hand it might also be part of the multi-barrier system to protect the biosphere against mercury emissions. Therefore, an appropriate containment suitable for the temporary storage conditions (salt mines or hard rock formations), as stated in the Regulation (EC) N 1102/2008, is also a minimum requirement for temporary storage. For the temporary storage of liquid mercury in above ground facilities, the following technical minimum requirements have to be fulfilled (recital 12, Hg-Regulation): reversibility protection of mercury against meteoric water impermeability towards soils and prevention of vapour emissions of mercury The same minimum requirements related to the containment apply as described above for the temporary storage in underground disposal sites. Environmental and health related minimum requirements Environmental minimum requirements are defined by existing environmental limit values. Options that are further investigated are only those that guarantee proper compliance with existing environmental limit values for example for leaching rates, underground water and/or air. Relevant limit values are included in chapter In addition, the protection of workers has to be ensured by complying with relevant occupational limit values and periodic monitoring. Varying occupational limit values have been established for mercury and its inorganic divalent compounds in the EU Member States. An overview on the relevant values is given in chapter 4.1.5) Economic minimum requirements Options will not be further examined that compared to viable options generate additional costs without providing technical or environmental benefits or added value. Technologies that have been evaluated (e.g. due to technical or economic aspects) as not suitable at the current state of development may be taken into consideration again (within an iterative investigation procedure) in case no adequate technology could be identified in the feasibility analysis and they might be expected to be a solution in the future, combined with the option of temporary storage now.
214 Reference number /2009/530302/ETU/G Feasibility of options In addition to the above-described minimum requirements, the feasibility of implementation is discussed. The feasibility of implementation evaluates the identified options against their availability until 15 March 2011 (date on which significant quantities of liquid mercury will be characterised as waste due to the entry into effect of the export ban regulation)and their general adequacy against the given background (capacity to store all expected mercury waste). The result of the feasibility assessment is also the basis for the decision if a temporary storage of liquid mercury is required because no suitable permanent solution would be available up to 15 March Feasibility of implementation Capacity available It is estimated that around 8,000-9,000t of liquid mercury would have to be stored within the next ten years. This amount would result in a net storage volume of around 700m³. Feasible options should have the capability to store this amount or volume, taking also into account additional space required for the packaging of liquid mercury. Experience available Experience already available related to large-scale operations and with the storage of comparable waste/products is required for proper implementation. Implementation time The implementation time of a possible option is also an important criterion for its large-scale feasibility. Options with an implementation time of more than 2 years in cases of temporary storage are not considered feasible, since a solution for a temporary storage at least, has to be found by 15 March In the case of permanent storage, no time restriction related to the implementation is foreseen since on the one hand a temporary storage can be used as an interim solution. On the other hand, investigations related to long-term safety of the stored mercury might be very time-consuming and should not be restricted. Implementation costs Implementation costs cover not only the costs for the practical implementation of the option, but also take account of estimated costs related to research still needed for the proper implementation of the option.
215 Reference number /2009/530302/ETU/G Acceptance criteria for metallic mercury and appropriate containment, procedure for the acceptance at the storage facility For a safe storage of the waste metallic mercury, its specific properties and components have to be defined for the acceptance at the storage site. In order to facilitate handling, it is recommended to establish only one set of acceptance criteria and procedure for metallic mercury and for the container, which is valid for all types of storage facilities (underground/above-ground, temporary/permanent) Acceptance criteria for metallic mercury The acceptance criteria are defined in a way that no unacceptable risks arise for the storage facility. Any deviation from these defined criteria might result in possible risks for either the facility surroundings or the workers, or both of these. To be accepted for the temporary or permanent storage waste metallic mercury should meet the following minimum acceptance criteria: - Purity of the mercury: > 99.9 % per weight - Max. metallic contaminates (like iron, nickel, copper): < 20 mg/kg each - Presence of sodium < 1 mg/kg - No residual radioactivity (e.g. from tracers used in the chlor-alkali industry) - No impurities capable of corroding carbon or stainless steel (e.g. nitric acid solution, chloride salts solutions, or water) Justification: The purity of the liquid mercury is the most important factor. The higher the purity, the lower the risk that the stored mercury contains impurities, which might interact with the containment or the storage environment. Experiences and investigations related to appropriate container material are available (MERSADE, see chapter and ORNL, see chapter ). These investigations are based on the examination of existing containers that have been used for several years for the storage of liquid mercury. The minimum purity of the stored mercury was 99.5% (ORNL) and 99.9% (MERSADE). [ORNL 2009] recommends only storing mercury with a purity of at least 99.5% (per volume) to avoid any unforeseeable reactions due to a lower purity of mercury. Mercury recovered from the chlor-alkali plants has a purity above 99.9% [personal information by Euro Chlor]. This value is currently also applied as minimum criteria for the stored liquid mercury at Almadén. All accepted mercury from chlor-alkali plants has to fulfil this criterion. In case this
216 Reference number /2009/530302/ETU/G requirement is not fulfilled, a purification of the mercury by distillation has to be carried out prior to the storage (see chapter 6.3.1). Following the precautionary principle it is recommended to establish a purity of 99.9% per weight for metallic mercury as this quality was used for the investigation and derivation of container material. With this purity the quality requirement of existing investigations is covered. Apart from the purity, also the content of certain contaminants should be known and if necessary excluded or restricted. Euro Chlor has an internal quality standard for mercury. Following this standard metallic contaminants, such as iron, nickel, copper should be below 20 mg/kg each [personal information by Euro Chlor]. Following Euro Chlor this quality can be achieved by chlor-alkali plants which present the most important source of metallic mercury to be stored. Therefore it is recommended to establish - at least for mercury coming from chlor-alkali plants this limit value. In addition, the presence of radioactive components (e.g. from tracers used in the chlor-alkali industry) and sodium has to be checked and avoided following the standard of Euro Chlor [Euro Chlor 2007]. According to [Euro Chlor 2007] sodium should be avoided due to the fact that it might produce hydrogen during the storage. The U.S. Department of Energy (DOE) published in November 2009 minimum acceptance criteria for temporarily stored mercury [DOE 2009], see also chapter Apart from the minimum purity requirement of 99.5% (per volume), the absence of any radiological components, the document set out that any impurities capable of corroding carbon or stainless steel, such as nitric acid, chloride salts, or water, should not be present in liquid mercury for permanent or temporary storage. It is recommended to introduce this criterion for the temporary and permanent storage liquid mercury in Europe as well. Currently at Almadén no limit value for sodium, metallic contaminants and radioactive components are established. Following information received by Mayasa (personal information Mr. Ramos) accepted mercury (not from chlor-alkali-plants) show for example concentrations of sodium up to 200 ppm. Also for other elements like potassium, calcium, boron concentration above the quality standard of Euro Chlor are accepted at Mayasa. However, here again the precautionary principle should be applied. To avoid any adverse effects during the storage due to the production of hydrogen it is therefore recommended to introduce the above mentioned criteria. It is further recommended to establish a specific waste code for metallic mercury from chlor-alkali plants fulfilling the above described criteria. Following the precautionary principle it is recommended to apply these criteria also for mercury resulting from other sources. Deviations from these criteria have to be checked and would need a justified case-by-case justification. Another argument for a specific waste code for metallic mercury is that it would be easier to track the route of the mercury from different sources. The screening analysis is based on the assumption that the stored liquid mercury fulfils the above
217 Reference number /2009/530302/ETU/G stated minimum requirements Appropriate containment The containment has to ensure a safe handling and storage of the waste over a certain time. In the case of a temporary storage, the containment might also be part of the multi-barrier system to protect the biosphere against mercury emissions. Based on the information provided in chapter the storage containers for liquid mercury have to fulfil the following minimum requirements: - Container material: Stainless steel (AISI 304, 316L) or carbon steel (ASTM A36 minimum) - Container has to be gas and liquid tight - Outer side of the container must be resistant against the storage conditions - Containers should be certified for the storage of mercury - Welds should be avoided as far as possible Justification: The type of container material is based on experiences and investigations related to appropriate container material (MERSADE, see chapter and ORNL, see chapter ). Only containers should be allowed which are gas and liquid tight, so no mercury or mercury vapour is able to escape from the container. The container should be certified for the storage of mercury. This can either be proven by a paper certificate from the producer including the type number or indicated in a data plate which is fixed at the container. These recommendations are technically obvious (see chapter 6.4) and required to adequately protect workers health and the environment. For above-ground storage long-term experience are in particular available from an existing aboveground warehouses/storage facilities for liquid mercury in Europe (Almadén) and in the USA (DNSC), see also chapter 6.3. These containers are also seen as appropriate for storage in underground disposal sites in particular in the dry atmosphere of salt mines. Welds are the weakest point in the container and should therefore be avoided as far as possible (see chapter ). Each facility can define which size of containers is acceptable (depends on the facility conditions) Acceptance procedure The standard waste acceptance procedure as defined in Directive 1999/31/EC and Decision 2003/33/EC shall apply for metallic mercury. Metallic mercury is only allowed to be accepted if it is
218 Reference number /2009/530302/ETU/G addressed in the site-specific risk assessment and included in the list of waste authorized to be stored at a specific site. Prior to the shipment of the mercury an approval by the facility operator is necessary. For this purpose the waste owner has to send information on the amount and characteristics of the waste to the storage facility. In addition, the following requirements shall be fulfilled: - Only acceptance of metallic mercury which fulfils the minimum requirements as set out in section (verification required either by sampling or a certificate issued by a certified person) - Visual inspection of the container, no acceptance of damaged, leaking or corroded containers - Only acceptance of containers with adequate labelling (at least according to the transport requirements) - Only acceptance of containers with a certificate which confirms the appropriateness for the storage of liquid mercury Justification: In order to ensure that a container fulfils the minimum requirements as mentioned in chapter a certificate is needed. By the mean of this certificate the operator of the storage facility is able to verify if the container is appropriate for the storage of liquid mercury. The certificate might also be a plate permanently fixed on the container - should include as a minimum, identification number of the container, container material, producer of the container, date of production and a confirmation that only mercury has been stored/transported in the container (exclusion of storage of products which might react with mercury or the container material). The acceptance procedure at underground storage facilities typically includes visual inspection, sampling and analysis of the received waste (WAC Decision). To avoid the opening of the mercury containers and thus possible mercury emissions, it is recommended that the acceptance of sealed containers accompanied by a certificate issued by a certified person - which verifies the quality of the mercury is possible. In the case of the acceptance of sealed containers it is crucial that there is a reliable proof that the containers only contain mercury which fulfils the minimum acceptance criteria as mentioned in section To avoid mercury is accepted which does not fulfil the minimum criteria, the filling and the sealing of the containers should be supervised by a certified person. It is essential that the supervising person has basic knowledge on the process and required quality of the mercury. With the requirement that a certified person should supervise the filling and sealing on the one hand it is assured that the person has a basic knowledge on the process and required quality of the mercy. On the other hand incorrect information or misuse of the certificate could be avoided.
219 Reference number /2009/530302/ETU/G The certificate, which has to be issued by the certified person, should include at least: - Name and address of the company (waste owner) - Place and date of packaging - The purity of the mercury (min. >99.9%) and, if relevant, description of the impurities (analytical report has to be provided) - Quantity of the mercury - Any specific comments - Signature One major advantage of the acceptance of sealed containers is that mercury emissions occurring during the opening process can be reduced and thus a possible exposure of workers with mercury can be avoided. In addition the risk of damaging of the plugs or improper re-closing of the flask - which might result in a release of small amounts of mercury during the storage - can be significantly reduced. On the other hand there is the risk that mercury is stored which does not fulfil the minimum quality requirements due to wrong information included in the certificate. In case of any suspicion that the quality criteria might not be met random samples should be carried out. Sealed containers accompanied by an incomplete certificates or certificates issued by unknown certification institutions have either to be rejected or the waste acceptance procedure as required by the WAC has to be applied. Record keeping In general the recordkeeping for hazardous waste is designed to track hazardous waste from its generation to final disposition. With respect to the record keeping of the basic characterisation and compliance testing no specific time frame is given in the Annex of the WAC Decision. Each Member State has to determine the period of time these records have to be kept. The time for sample keeping from the on-site verification is set by the WAC Decision for a minimum of one month. In case of temporary storage it is recommended that the documents have to be stored for at least 3 years after the termination of the storage. In case of a permanent storage of liquid mercury the Member State specific requirements should apply but the records should at least be kept until the closure of the disposal site. A plan of the storage area should be kept also after the closure of the storage site. Records and plans must be available for inspection by regulators.
220 Reference number /2009/530302/ETU/G Option 1l: permanent storage of liquid mercury in salt mines In chapter 6.2.2, an overview is provided on basic characteristics of salt rock formations and their qualification as a permanent disposal facility for hazardous waste. In the following, an evaluation of the options conforming to the minimum requirements described in chapter 8.1 is carried out. As already stated above, metallic mercury is only allowed to be stored in facilities which fulfil the requirements of the landfill directive and the WAC decision Technical minimum requirements In the case of permanent underground storage in salt mines, the potential storage site needs a permit as an underground landfill, including a site specific risk assessment as outlined in Appendix A of the WAC decision. The site-specific risk assessment has to include the following: 1. geological assessment; 2. geomechanical assessment; 3. hydrogeological assessment; 4. geochemical assessment; 5. biosphere impact assessment; 6. assessment of the operational phase; 7. long-term assessment; 8. assessment of the impact of all the surface facilities at the site 9. assessment of other risks (e.g. protection of workers) More detailed information on the site-specific risk assessment is included in section Based on the site-specific risk assessment, the list of acceptable waste has to be derived for each storage site. As a consequence, the storage of liquid mercury in underground facilities is only possible when it is demonstrated that the level of isolation from the biosphere is acceptable (WAC Decision, Appendix A, Nr. 2.3). Salt rock fulfils the requirement to be impermeable to gas and liquids (WAC Decision, Appendix A, Nr. 3.2). Therefore, in cases of vapour emissions of mercury from the waste after the sealing of the mine or disposal cell, these should then still remain enclosed in the salt rock. Due to its plastic properties, salt rock has a creeping potential, and thus a firm encapsulation of the waste at the end of the mines deformation process is possible. Decision 2003/33/EC describes the role of salt mines as follows: With the overlying and underlying impermeable rock strata (e.g.
221 Reference number /2009/530302/ETU/G anhydrite), it acts as a geological barrier intended to prevent groundwater entering the landfill and, where necessary, effectively to stop liquids or gases escaping from the disposal area. Its function as a geological barrier to protect groundwater against mercury strongly depends on the geological conditions of the salt rock. In particular the thickness and composition of the salt rock as well as the overlying and underlying impermeable strata (e.g. anhydrite or claystone) define the protection level of the storage facility. Therefore, minimum requirements for theses parameters in addition to the requirements already included in Decision 2003/33/EC are recommended to be established to prevent mercury from entering the biosphere due to inappropriate geological conditions. Although the disposal of radioactive waste is carried out under different conditions compared to metallic mercury, one principal aspect is the same the safe long-term isolation of the hazardous waste material from the biosphere. Intensive research related to safe storage of radioactive waste in geological deposits has been carried out (see section 6.2.4). Valuable information is available in particular on minimum requirements related to the geological effectiveness of salt rocks. Based on the findings of this research, exclusion and minimum requirements have been defined which an underground disposal site should fulfil for safe storage (see section ). Some of these minimum criteria, which are not already covered by the site-specific assessment as outlined in Appendix A of the WAC decision, are also seen as being relevant for the storage of liquid mercury. In particular, the minimum thickness of the isolating salt rock being at least 100m, as well as the minimum depth of the storage site being 300m, these conditions should be fulfilled as additional safety factors. In case a storage site does not fulfil these criteria (300m minimum depth and 100m minimum thickness of the isolation rock) it has to be proven by a separate document that due to other geological criteria or measures this deficit can be compensated. The determination of the effectiveness of the geological salt rock barrier by a time factor is seen as an appropriate criterion for the safe storage of liquid mercury in salt mines. It is proposed to set a time limit for which the geological barrier has to protect the biosphere against the entry of mercury from the storage site. For radioactive waste, a similar approach is currently being discussed (see section 6.2.4). Following the recommendations of , the radioactive waste has to be safely enclosed for one million years. Due to the fact that the hazardousness of mercury, in contrast to the hazardousness of radioactive waste, will not decrease over time, it is recommended to apply at least the same period of time for mercury as for radioactive waste. Therefore, a site-specific safety assessment has to be carried out including a long term safety assessment which verifies the effectiveness of the geological barrier against liquid mercury. By means of the assessment, it has to be proven that mercury will not pass the overlying impermeable strata and thus enter the biosphere for a period of time in the order of magnitude of one million years. The storage of liquid mercury has to take place in a separate cell to avoid any reaction of the storage containers with other chemicals. The cells have to be separated by salt barriers or other adequate artificial barriers. As a minimum, a distance of 100m should be kept from access shafts and other
222 Reference number /2009/530302/ETU/G waste storage areas to ensure a safe encapsulation. The container also has to fulfil the minimum requirements as set out in chapter Its main function is to ensure a safe handling and storage at least until the closure of the cell. During the operational phase of the storage cell, in case of spills or leaks, it is important that adequate measures are established to prevent liquid mercury from entering other parts of the salt mine. It is recommended to store the containers in collecting basins which are able to capture the total amount of the stored liquid mercury. The collecting basins can be constructed as pits in the salt rock or other constructed basins. Adequate linings and slopes should be installed which allow an easy collection of the mercury. The following table presents a summary of the outcome of the evaluation including the minimum requirements and the identified additional facility-related requirements. The last column of the table indicates if an option fulfils the requirements either due to the application of already existing provisions and/or by applying the identified additional requirements. Technical minimum requirements Geological barrier enables the protection of groundwater against mercury Geological barrier enables the prevention of vapour emissions of mercury Geological barrier ensures impermeability to gas and liquids of the surroundings The salt rock ensures a firm encapsulation of the waste at the end of the mines' deformation process Additional facility related requirements - Effectiveness of the geological barrier in terms of migration time for mercury to the biosphere >1 million years (verification by a site-specific assessment including a long term safety verification) - Minimum thickness of the isolating salt rock: 100m (justified exemption possible) - Minimum depth of the storage area: 300m (justified exemption possible) - Minimum distance from access shafts and other waste storage areas: 100m - No storage together with other waste Minimum requirements fulfilled - Storage of the liquid mercury containers in collecting basins able to catch the whole amount of stored mercury The check mark ( ) does not mean that any existing landfill does already fulfil the minimum requirements for the storage of metallic mercury. It simply indicates that if the storage site fulfils the described additional facility-related requirements (together with the requirements set out in the
223 Reference number /2009/530302/ETU/G landfill directive and the WAC Decision) it would be suitable for the storage of metallic mercury. If existing storage facilities are available which already fulfil the requirements or if there is still need for further investigation/research or approval by the authorities, this will be assessed under the category feasibility of implementation Environmental minimum requirements The criteria that no existing environmental limit values are allowed to be exceeded is not relevant for the permanent storage of liquid mercury in salt mines as the technical minimum requirements already imply a total enclosure of the liquid mercury in salt rocks for a certain time period. The protection of workers has to be ensured during the whole operational phase of the storage cell. Therefore, during this phase, adequate monitoring, control measures and inspection schemes have to be defined to avoid mercury emissions from the stored mercury due to leaking storage containers or improper handling. Proper ventilation is required and in case of any incidents, adequate protection equipment and emergency plans have to be available. In addition, workers have to be adequately informed and trained in case of any incidents. Mercury vapour monitoring system Annex III of the landfill directive already foresees specific requirements relating to a monitoring and after-care control. Where there is permanent storage of liquid mercury in salt mines, monitoring is only necessary during the operational phase of the storage cell. After the closure of the salt mine, no after-care measures are necessary, because the salt rock is considered to provide total containment and the waste will only come into contact with the biosphere in the event of an accident or an event in geological time (e.g. earth movement) (Nr. 3.2, Appendix A, WAC). By means of failure scenarios the possible consequences of accidents or geological events have to be included in the site-specific risk assessment. In the site-specific risk assessment, the probability of such accidents or events has to be assessed. During the operational phase, a continuous mercury vapour monitoring system has to be established which should have a minimum sensitivity ensuring the recommended indicative limit value of 0.02mg mercury/m³ (8 hour TWA) [SCOEL 2007] is not exceeded. The vapour detection equipment should be installed at head level and near to the ground as mercury vapour is heavier than air and thus the concentration of mercury is higher at ground level. The monitoring system should be equipped with a visual as well as an acoustic alert system in case the limit value is exceeded. The proper functioning of the monitoring system has to be checked at least once every 12 months.
224 Reference number /2009/530302/ETU/G Regular inspection Apart from the continuous monitoring system, also regular visual inspections of the containers should be carried out by an authorised inspector. These regular visual inspections should also include a control ensuring proper installation of the necessary minimum requirements as stated in the permit for the storage of liquid mercury. The inspection interval should not be more than 12 month. After the detection of a leak, all relevant emergency measures as laid down in internal instructions manuals have to start immediately. The operator of the facility has to take any measures to prevent that workers are exposed to mercury emissions and to avoid mercury or mercury vapour entering the environment. Within one month after the detection of the leak and the subsequent remedial actions an inspection should take place to ensure that the origin of the leak has been eliminated and a proper operation of the storage facility is ensured. A documentation of any leak and the subsequent activities is required. Emergency plans The WAC decision foresees an assessment of the operational phase to identify possible risks for the storage facility as well as for the workers (WAC, Appendix A, and 1.2.9). Potential incidents have to be described, evaluated and appropriate contingency measures have to be implemented. Where there is storage of metallic mercury, emergency plans addressing the specific risks of metallic mercury have to be established and adequate personal protection equipment has to be available. In addition, workers have to be adequately informed and trained in case of any incidents. Environmental minimum requirements No exceeding of current environmental limit values Protection of workers during operational phase (monitoring and regular inspection) Additional facility-related requirements or acceptance criteria Installation of a permanent mercury vapour monitoring systems - with a sensitivity of at least 0.02 mg mercury/m³ - visual and acoustic alert system - annual maintenance and control of the system - sensors have to be installed at ground level and head level Minimum requirements fulfilled Regular visual inspection of the container and the storage site by a certified person - max. interval: 12 months, or - 1 month after detection of a leak Availability of emergency plans and adequate protective equipment suitable for
225 Reference number /2009/530302/ETU/G Environmental minimum requirements Additional facility-related requirements or acceptance criteria metallic mercury Minimum requirements fulfilled Information and training of workers on how to deal with liquid mercury Economic minimum requirements Disposal in salt mines, compared to other storage options, entails moderate costs. The permanent storage of liquid mercury in appropriate containment would result in costs between /t for the storage (see section ) and between 600 1,100 /t mercury (see section 6.4.1) for the container. The economic minimum requirements are fulfilled without additional requirements Feasibility of implementation In the European Community, 5 underground disposal sites in salt rock have been identified which are permitted to accept hazardous waste. The remaining capacity of each underground disposal site would be sufficient for the storage of the expected volume of liquid mercury (700 m³ net, without packaging). According to information from the operators of the mines, storage would probably only be possible in two of these mines (one site is currently not in use, the other sites envisage problems in obtaining a permit). Experience related to the storage of hazardous waste in salt rock is available in Germany in particular. For more than 20 years, Germany has disposed of hazardous waste in salt mines. Up to now, none of the existing facilities has a permit for the storage of pure metallic mercury, since it was excluded from the storage due to its liquid status. However, German salt mines for example have a permit to accept waste containing mercury, such as fluorescent tubes and other mercurycontaining waste (waste code *). Quite extensive information is available on the properties of possible host rocks, information related to the specific behaviour of liquid mercury in underground conditions is still very limited. Extensive experiences, models and simulations are available for the storage of radioactive waste. The models related to the post-closure safety of geological disposal sites are well developed and might also be applicable to liquid mercury. According to experts, the adaption of the radioactive waste models to liquid mercury is expected to take around 3-5 years (see section ) under the precondition that sufficient reliable data on the behaviour of metallic mercury is available. German authorities generally consider the storage in salt mines as safe, but very little is currently
226 Reference number /2009/530302/ETU/G known on the long-term behaviour of metallic mercury under storage conditions in salt rock (e.g. behaviour in case of increased pressure, possible interactions with the host rock). Therefore, according to information from German authorities, a project is planned to test the behaviour of metallic (and probably also of solidified) mercury in salt rock. The outcome of this study is an essential input to the site specific risk assessment for salt mines required for the application for a permit to store liquid mercury. Only based on this information a safe encapsulation of the metallic mercury can be ensured for a timeframe of 1 million years. The intended start of this project is in 2010 (source: questionnaire survey German EPA, personal information by Ms. Hempen). Following the information received from the German EPA, the permanent storage of metallic mercury in a German salt mine before the results of the study are available would not be authorised. Relevant legal requirements like the adaption of the long-term safety assessment and the formal permit to be allowed to store liquid mercury will need additional time. Therefore, it is not expected that this procedure will be finalized before The time for the preparation of disposal cells in the salt mines is expected to be relatively short, since for example, ventilation and monitoring systems have already been installed in mines formerly used for the exploitation of salt. The costs for the implementation of the option depend on the additional requirements which have to be implemented, but they are expected to be comparatively low. Minimum requirements Additional requirements Feasibility fulfilled Capacity Experience Only underground sites which already have experience with the storage of hazardous waste Approval of authorities is given Knowledge of the behaviour of metallic mercury at underground storage conditions Implementation time? Implementation costs?
227 Reference number /2009/530302/ETU/G Summary: option 1l Minimum requirements Additional facility related requirements or acceptance criteria required Minimum requirements fulfilled Technical minimum requirements YES Environmental minimum requirements YES Economic minimum requirements YES Feasibility of implementation YES? The option 1 l permanent storage of liquid mercury in salt mines is promising because there are suitable sites that fulfil the technical, environmental and economic minimum requirements under the precondition that the additional facility related requirements and acceptance criteria are fulfilled. With regard to the feasibility of implementation by 2011 there are doubts due to the fact that reliable information on the long-term behaviour of liquid mercury in salt mines is still lacking. Therefore, problems are expected for availability of this option in good time (expressed by? ).
228 Reference number /2009/530302/ETU/G Option 2l: temporary storage of liquid mercury in salt mines In chapter 6.2.2, an overview is provided on basic characteristics of salt rock formations and its qualification as a permanent disposal facility for hazardous waste. In the following, an evaluation of the option is carried out in view of the minimum requirements described in section 8.1. As already stated above, metallic mercury is only allowed to be stored in facilities which fulfil the requirements of the landfill directive and the WAC decision Technical minimum requirements With regard to the protection of groundwater against mercury, prevention of vapour emissions of mercury and impermeability to gas and liquids of the surroundings, the same requirements apply as for permanent storage of liquid mercury. In contrast to permanent storage, the temporary storage option has to fulfil the technical minimum requirements only over a certain time period, thus a long-term safety verification of the effectiveness of the geological barrier for 1 million years is not necessary for temporary storage. However, during the defined storage time, the relevant technical minimum requirements have to be fulfilled. As a consequence, the effectiveness of the container to prevent mercury emissions will mainly determine the feasibility of this option. The salt rock surrounding is only an additional safety factor in case of spills or any unforeseeable events which would result that the stored mercury would be released from the container. In this case the gas and liquid impermeable salt rock would act as additional geological barrier. Because even in the worst case that not all spilled mercury could be recovered the salt rock system still would act as geological barrier in the long term and prevent the liquid mercury entering the biosphere. The minimum criteria set for the permanent storage related to the depth and minimum thickness of the geological barrier are not relevant for the temporary storage as the container provides the main safety for the storage. The container has to fulfil the minimum requirements as set out in chapter Its main function is to ensure a safe handling and storage. In case of a temporary storage the liquid mercury has to be stored in a way that a subsequent processing of the liquid mercury is not hindered or made impossible. This can be achieved by appropriate containment, which does not, or only to a very limited extent, react with the mercury. Storage of liquid mercury has to be in a separate cell to avoid any reaction of the storage containers with other chemicals. The cells have to be separated by salt barriers or other adequate artificial barriers. In addition, the reversibility of the storage of liquid mercury has to be fulfilled, which means that the
229 Reference number /2009/530302/ETU/G cavities where the liquid mercury is stored have to be stable enough for a defined storage time. Although salt rock has a high creeping potential, the convergence of drifts lasts several hundred years until the drift is closed. The stability and the secure access to the cavities should be guaranteed for at least 100 years. Adequate measures have to be established to avoid spills or leaks allowing liquid mercury to enter other parts of the salt mine. It is recommended that containers are stored in collecting basins which are able to capture the whole amount of the stored liquid mercury. The collecting basins can be constructed as pits in the salt rock or in other construction forms. Adequate linings and slopes should be installed which allow an easy collection of the mercury. Technical minimum requirements Protection of groundwater against mercury Prevention of vapour emissions of mercury Impermeability to gas and liquids of the surroundings Retrievability of waste Additional facility related requirements or acceptance criteria - Cavity stability and secure access to the storage area >100 years - No storage together with other waste - Minimum distance to access shafts and other waste storage areas: 100 m - Storage of the liquid mercury containers in collecting basins able to catch the whole amount of stored mercury Minimum requirements fulfilled Environmental minimum requirements During the whole temporary storage time adequate monitoring, control measures and inspections schemes have to be defined to avoid mercury emissions from the stored mercury due to untight storage containers or improper handling. Proper ventilation is required and in case of any incidents adequate protection equipment and emergency plans have to be available. In additions workers have to be adequately informed and trained in case of any incidents. The same monitoring, inspection and emergency requirements apply as for the permanent storage of metallic mercury in salt mines (see chapter 8.4.2).
230 Reference number /2009/530302/ETU/G Environmental minimum requirements No exceeding of current environmental limit values Protection of workers during operational phase Additional facility related requirements or acceptance criteria Installation of permanent mercury vapour monitoring systems - with a sensitivity of at least 0.02mg mercury/m³ - visual and acoustic alert system - annual maintenance and control of the system - sensors have to be installed at ground level and head level Min. requirements fulfilled Regular visual inspection of the container and the storage site by a certified person - max. interval: 12 months, or - 1 month after detection of a leak Availability of emergency plans and adequate protective equipment suitable for metallic mercury Information and training of workers in how to deal with liquid mercury Economic minimum requirements Disposal in salt mines, compared to other storage options, entails moderate costs. Temporary storage of liquid mercury in appropriate containment would result in the same costs as for permanent storage, between 260 and 900/t for the storage and between 600 and 1,100/t for the container. Additional costs will result for the retrieval of the waste after the temporary storage time. The economic minimum requirements are fulfilled without any additional requirements Feasibility of implementation In the European Community, 5 underground disposal sites in salt rock have been identified which are permitted to accept hazardous waste. The remaining capacity of each underground disposal site would be sufficient for the storage of the expected volume of liquid mercury. According to information from the operators of the mines the storage would probably only be possible in two of these mines (one site is currently not in use, the other sites envisage problems in obtaining a permit).
231 Reference number /2009/530302/ETU/G Experience related to the storage of hazardous waste is available in Germany in particular. For more than 20 years, Germany has disposed of hazardous waste in salt mines. But no experience is available for the temporary storage of liquid mercury. The preparation time for the cells and the implementation of the waste acceptance procedure are expected to be relatively short as for example ventilation and monitoring systems are already installed in mines formerly used for the exploitation of salt (see chapter ). The adaptation of these systems to the required standards should be possible within a short time. In Germany, the possibility of long-term storage of liquid mercury in salt mines is already foreseen in national laws (see section 5.3). According to German law, long-term storage in salt mines (landfill class IV) is possible under the precondition that the landfill is adapted for the purpose of disposing of metallic mercury and this aspect is taken into particular consideration in the site-specific safety assessment. In addition, an application for a permit for the temporary storage of liquid mercury is required, including the additional requirements for a safe storage of it. An expertise has to be provided by the owner of the mine to ensure the proper implementation of the minimum requirements. Although such a permit is currently not yet available, there are no doubts that it will be provided within the required time frame. The application time is expected to be not more than 1 year. The costs for the implementation of the option depend on additional requirements which have to be implemented, but they are expected to be comparatively few. Minimum requirements Additional requirements Feasibility fulfilled Capacity Experience Implementation time Approval of authorities is given Implementation costs Only underground sites which already have experience with hazardous waste Expertise on proper implementation of minimum requirements?
232 Reference number /2009/530302/ETU/G Summary: Option 2l Minimum requirements Additional facility-related requirements or acceptance criteria required Minimum requirements fulfilled Technical minimum requirements YES Environmental minimum requirements YES Economic minimum requirements YES Feasibility of implementation YES? Option 2l temporary storage of liquid mercury in salt mines would be a possibility if authorities give their approval for storage of liquid and the minimum requirements relating to the facility and the acceptance criteria are fulfilled. 8.6 Option 3l: permanent storage of liquid mercury in deep underground hard rock formations In chapter 6.2.3, an overview is provided on basic characteristics of hard rock formations and their qualification in accommodating permanent disposal facilities for hazardous waste. In the following, an evaluation of the options in view of the criteria described in chapter 8.1) is carried out. As already stated above, metallic mercury is only allowed to be stored in facilities which fulfil the requirements of the landfill directive and the WAC decision Technical minimum requirements According to Regulation (EC) N 1102/2008 Article 3(1)(a) the permanent storage of liquid mercury in deep underground hard rock formations has to provide an equal level of safety and confinement to those of salt mines. In the case of hard-rock storage, total containment is not possible. Therefore the underground storage needs to be constructed in a way that natural attenuation of the surrounding strata mediates the effect of pollutants to the extent that they have no irreversible negative effects on the environment. As a consequence, the capacity of the artificial barriers (near environment) to attenuate and degrade pollutants will determine the acceptability of a release from such a facility. Hard rock formations are less impermeable against gas and liquids than salt rock, particularly due to possible fractures in the rock body. For the same reason, the hydraulic conductivity is also higher
233 Reference number /2009/530302/ETU/G when compared to salt rock (see section 6.2.3) Within the long term safety assessment for deep underground storage sites in hard rock, it has to be demonstrated that any discharges of hazardous substances from the storage will not reach the biosphere including the upper parts of the groundwater system accessible to the biosphere in amounts or concentrations that will cause adverse effects. Therefore, the water flow paths to and in the biosphere have to be evaluated, including the impact of variability on the geohydraulic system. A Swedish study [Höglund 2009, Höglund 2009A] estimated for a specific site the maximum release of mercury per year in the case of storage of mercury sulphide. The study concluded that g mercury/year would be released as a maximum to the biosphere. Taking into account that the solubility in water of liquid mercury (5.6*10-2 mg/l at 25 C see Annex 4) is by several orders of magnitude higher than the solubility of mercury sulphide (9*10-24 mg/l, see Annex 4), significantly higher release values could be expected. In the case of permanent storage, the containment of the liquid waste mercury and the lining of cavities should not be taken into account for the long-term safety assessment (WAC Decision, Appendix A, Nr ). As a consequence in the long term the geological system (hard rock) has the major barrier function. Although adequate rock structures might be available, the investigations to exclude the presence of possible fractures is very complex and their presence cannot be completely excluded. Therefore mercury might enter the biosphere via fracture systems. Typically, multi-barrier systems are applied in hard rock formations. In the case of liquid mercury, the long-term safety of these barriers cannot be guaranteed and thus mercury entering the biosphere cant not be completed prevented in particular in case of liquid mercury with its higher solubility compared to stabilised waste. Therefore, the technical minimum requirement "equal level of safety and confinement to those of salt mines" is not fulfilled by this option. Technical minimum requirements Additional facility-related requirements or acceptance criteria Geological barrier enables the protection of groundwater against mercury Geological barrier enables the prevention of vapour emissions of mercury Geological barrier ensures impermeability to gas and liquids of the surroundings Firmly encapsulating the waste at the end of the mines' deformation process Equal level of safety and confinement to those of salt mines Minimum requirements fulfilled No No No No No
234 Reference number /2009/530302/ETU/G Summary: option 3l Minimum requirements Additional facility-related requirements or acceptance criteria required Minimum requirements fulfilled Technical minimum requirements no no Environmental minimum requirements / / Economic minimum requirements / / Feasibility of implementation / / For option 3l permanent storage of liquid mercury in deep underground hard rock formations, no sites could be identified in the scope of this study that would fulfil the technical minimum requirements for the storage of liquid mercury. Also involved stakeholders could not suggest any suitable site. 8.7 Option 4l: temporary storage of liquid mercury in deep underground hard rock formations In chapter 6.2.3, an overview is provided on basic characteristics of hard rock formations and their qualification as permanent disposal facilities for hazardous waste. In the following, an evaluation of the options pertaining to the above-described criteria is carried out. As already stated above, metallic mercury is only allowed to be stored in facilities which fulfil the requirements of the landfill directive and the WAC decision Technical minimum requirements With regard to the protection of groundwater against mercury, prevention of vapour emissions of mercury and impermeability to gas and liquids of the surroundings, the same requirements apply as for the permanent storage of liquid mercury in underground hard rock formations. When looking at the assessment of option 3l, it is obvious that hard rock formations do not fulfil the minimum requirements for a permanent safe storage of liquid mercury. In contrast to permanent storage, the temporary storage option has to fulfil the technical minimum requirements only over a certain time period, thus a long-term safety verification of the effectiveness of the geological barrier is not necessary for temporary storage. However, during the defined storage time, the relevant technical minimum requirements have to be fulfilled. As a consequence, the
235 Reference number /2009/530302/ETU/G effectiveness of the artificial barriers (near environment, container) in particular, to attenuate and degrade pollutants will determine the feasibility of this option. According to the information available, it would be possible to build storage cavities fulfilling these requirements, although practical experiences in underground storage sites are not available (see section ). The container has to fulfil the minimum requirements as set out in chapter In the case of a temporary storage, the liquid mercury has to be stored in such a way that a subsequent processing of the liquid mercury is not hindered or made impossible. This can be achieved by appropriate containment, which does not, or only to a very limited extent, react with the mercury. The storage of liquid mercury has to take place in a separate cell to avoid any reaction of the storage containers with other chemicals. The cells have to be separated by adequate artificial barriers. The reversibility of the storage of liquid mercury has to be fulfilled, which means that the cavities where the liquid mercury is stored have to be stable enough for a defined storage time. The stability of cavities is, in particular, given for crystalline hard rock formations. In argillaceous rock, the cavity stability is not given and thus it would have to be stabilised by engineered barriers. The stability of cavities should be guaranteed for at least 100 years. Adequate measures have to be established to avoid spills or leaks allowing liquid mercury to enter the rock. It is recommended to store the containers in collecting basins, which are able to capture the total amount of the stored liquid mercury. Adequate linings (Hg resistant sealing or material able to attenuate mercury like bentonite, see chapter and slopes should be installed, which facilitate an easy collection of the mercury. The most critical point is seen in possible spills or vapour emissions that might result in an intrusion of mercury into the host rock. Mercury, once entering the host rock, might enter the biosphere due to possible fractures in the rock body. Technical minimum requirements Protection of groundwater against mercury Prevention of vapour emissions of mercury Impermeability to gas and liquids of the surroundings Reversibility of storage Additional facility-related requirements or acceptance criteria - Cavity stability and secure access to the storage area for >100 years - No storage together with other waste - Minimum distance to access shafts and other waste storage areas - Storage of the liquid mercury containers in collecting basins able to Minimum requirements fulfilled???
236 Reference number /2009/530302/ETU/G Technical minimum requirements Additional facility-related requirements or acceptance criteria capture the stored mercury Minimum requirements fulfilled - Proof that in cases of spills and leaks no mercury enters the host rock Environmental minimum requirements During the entire temporary storage time, adequate monitoring, control measures and inspection schemes have to be defined to avoid mercury emissions from the stored mercury due to leaking storage containers or improper handling. Proper ventilation is required and in the case of any incidents, adequate protection equipment and emergency plans have to be available. In addition, workers have to be adequately informed and trained for such incidents. With regard to compliance with environmental limit values, it has to proven by adequate model calculations that possible mercury emissions will not exceed existing environmental limit values. The same monitoring, inspection and emergency requirements apply as for the permanent storage of metallic mercury in salt mines. Environmental minimum requirements No exceeding of existing environmental limit values Protection of workers during operational phase (monitoring and regular inspection) Additional facility-related requirements or acceptance criteria Model calculation to prove the compliance with environmental limit values Installation of a permanent mercury vapour monitoring systems - with a sensitivity of at least 0.02mg mercury/m³ - visual and acoustic alert system - annual maintenance and control of the system - sensors have to be installed at ground level and at head level Minimum requirements fulfilled Regular visual inspection of the containers and the storage site by a certified person - max. interval: 12 months, or - 1 month after detection of a leak Availability of emergency plans and adequate protective equipment suitable for
237 Reference number /2009/530302/ETU/G Environmental minimum requirements Additional facility-related requirements or acceptance criteria metallic mercury Minimum requirements fulfilled Information and training of workers on how to deal with liquid mercury Economic minimum requirements Storage costs for the temporary storage of liquid mercury in hard rock formations are seen as relatively low. However, the costs for the preparation of the cells and the artificial barriers seem to be significantly higher compared to salt rock facilities. Costs are expected in a dimension that this solution - considering also the less sustainable environmental performance of this option - will have difficulties to fulfil economic minimum requirements. However, it cannot be excluded that an economically viable solution can be established in Europe. Cost estimates relating to the preparation of a storage cell are available in section Though there might be feasible hard rock formations for a temporary storage of liquid mercury, the assumed high investment costs to prepare an appropriate cell have to be taken into consideration. Looking at the cost information received from the Swedish Ministry of Environment (see chapter ) the preparation costs and operation costs might be very high for a limited period of time and a limited volume of liquid mercury (700 m³) Feasibility of implementation Euromines 86 indicated that the underground disposal site in Odda, Norway might be an option for the temporary storage of liquid mercury. No information on the precise depth of this disposal site could be identified but one report [Kystverket 2008] indicated that the disposal sites might not fulfil the criteria of several hundreds of meters of depth as stated in the WAC Decision for deep underground hard rock formation. Currently this underground disposal site has only a permit to store e.g. mercury sulphide (see chapter ). Other relevant storage sites could not be identified in the scope of this study. Potential storage sites have to be prepared for the storage of liquid mercury and a permit for a temporary storage has to be issued. It is highly questionable whether within the given timeframe adequate storage facilities will be identified, also preparing adequately the corresponding waste cells will be rather unlikely within the given time frame. 86 Personal Information of Euromines (European Association of Mining Industries)
238 Reference number /2009/530302/ETU/G Summary: option 4l Minimum requirements Additional facility-related requirements or acceptance criteria required Minimum requirements fulfilled Technical minimum requirements YES? Environmental minimum requirements YES Economic minimum requirements? Feasibility of implementation /? Given the experience already gained in storing other hazardous wastes in hard-rock formations, the temporary storage of liquid mercury in hard rock formations would be a possibility in case adequate capacities are available and permits for the storage of liquid mercury are available at the latest until Based on the assessment of the current situation in the EU, however, this solution although feasible seems to be very unlikely to be implemented within the given time frame. 8.8 Option 5l: temporary storage of liquid mercury in aboveground facilities In chapter 6.3, an overview is provided on the current state of the art of above-ground storage of metallic mercury. In the following, an evaluation of the options concerning the criteria described in section 8.1 is presented Technical minimum requirements The Hg-Regulation sets out that temporary storage of liquid mercury is possible at above-ground facilities that are dedicated and equipped for the storage of it. In addition, the Hg-Regulation lays down that all provisions of the landfill directive as well as of the WAC decision (except WAC Nr. 2.4) apply to these facilities. As a consequence, storage sites for the temporary storage of liquid mercury need a valid landfill permit in case the storage takes place for more than 1 year prior to disposal and for more than 3 years prior to recovery or treatment (confirmation of a subsequent disposal or recovery/treatment is required). The provisions set out in the landfill directive in Annex I (General requirements for all classes of
239 Reference number /2009/530302/ETU/G landfills), thus apply for storage facilities for metallic mercury. In addition, the Hg-Regulation sets out that the liquid mercury should be protected against meteoric water. According to Regulation (EC) N 1102/2008 the Seveso Directive (Directive 96/82/EC, see chapter 5.2.2) shall apply for the temporary above ground storage of liquid mercury. The Seveso directive aims at the prevention of major accidents which involve dangerous substances, and the limitation of their consequences for man and the environment, with a view to ensuring high levels of protection (Article 1, Directive 96/82/EC). The Seveso Directive requires that the possible risks of the storage of liquid mercury have to be identified and evaluated in a safety report by taking into consideration the specific properties of liquid mercury. In particular, the risks of accidental release have to be taken into consideration and adequate measures have to be implemented to reduce on the one hand, the risk of accidental releases, and on the other hand to minimize subsequent potential negative effects to the environment. The assessment under the Seveso directive also includes possible scenarios in cases of natural disasters such as floods but also man-made threats such as terrorist attacks. Adequate management plans have to be established to fulfil these requirements. Currently, the storage of liquid mercury takes place in warehouses. In order to protect the stored liquid mercury against meteoric water and to guarantee impermeability towards the soil, the best option for the above-ground storage seems to be construction of a building with engineered barriers to protect the environment against mercury emissions. The protection of the soil can be achieved by sealed floors, with a mercury-resistant sealer, which can prevent the intrusion of mercury into the soil, for example in cases of accidental spills. In addition, the containers have to be stored in areas where in case of an accidental release of the mercury the total amount of the stored mercury can be collected and retrieved. This can either be achieved by storing the containers with the liquid mercury in an appropriate collecting basin or by implementing appropriate other measures, for example by ramped containment dikes that are incorporated into the floor sealant and connected to appropriate collecting basins. Above-ground storage is only seen as temporary storage, therefore the liquid mercury has to be stored in such a way so that a subsequent processing of it is not hindered or made impossible. This can be achieved by appropriate containment that fulfils the minimum requirements as set out in chapter 8.3.2). The storage of liquid mercury has to take place in a separate area to avoid any reaction of the storage containers with other chemicals. The areas have to be separated by adequate barriers, for example concrete walls. In addition adequate fire protection and ventilation systems should by installed. To avoid any unauthorised removal of the stored mercury the storage area should be secured.
240 Reference number /2009/530302/ETU/G Technical minimum requirements Reversibility of storage Protection of mercury against meteoric water Impermeability towards soil Prevention of vapour emissions of mercury Additional facility related requirements or acceptance criteria - Storage in constructed building with engineered barriers to protect the environment against mercury emissions - Storage of the liquid mercury containers in collecting basins able to catch the whole amount of the stored mercury - Hg-resistant sealants for the floor and installation of a slope towards a collection sump Minimum requirements fulfilled - Fire protection system - Ventilation system - No storage together with other waste - Area should be secured to prevent unauthorised removal of the mercury Environmental minimum requirements During the entire temporary storage time, adequate monitoring, control measures and inspection schemes have to be defined to avoid mercury emissions from the stored mercury due to leaking storage containers or improper handling. Proper ventilation is required and in cases of any incidents adequate protection equipment and emergency plans have to be available. In addition, workers have to be adequately informed and trained in such cases. The same monitoring, inspection and emergency requirements apply as for the permanent storage of metallic mercury in salt mines. To avoid any negative impacts of the surrounding area due to mercury emission, in addition to the continuous on site measurements, immission measurements should take place before the temporary storage starts and after 1 year. Based on this information it can be decided if additional measures to protect the environment might be required.
241 Reference number /2009/530302/ETU/G Minimum requirements No exceeding of existing environmental limit values Hg-limit values for air (WHO) Protection of workers during operational phase (monitoring and regular inspection) Additional facility related requirements or acceptance criteria Installation of a regular immission monitoring system of the surrounding of the storage facility Installation of permanent mercury vapour monitoring systems - with a sensitivity of at least 0.02mg mercury/m³ - visual and acoustic alert system - annual maintenance and control of the system - sensors have to be installed at ground level and at head level Min. requirements fulfilled Regular visual inspection of the container and the storage site by a certified person - min. interval: 12 months, or - 1 month after detection of a leak Availability of emergency plans and adequate protective equipment suitable for metallic mercury Information and training of workers on how to deal with liquid mercury Economic minimum requirements The costs related to this option highly depend on the availability of existing facilities and the possibility to adapt these facilities to secure above-ground landfills for the storage of liquid mercury. If existing warehouse facilities can be used which have already implemented parts of the required standards, then the costs seem to be acceptable. If construction of new buildings is necessary, then the costs will be significantly higher Feasibility of implementation Currently, in the European Community no landfill site has been identified which fulfils the requirements for the storage of liquid mercury waste. The most appropriate facility for a central storage is the warehouse of Almadén, which is currently used for the storage of the product liquid mercury. The currently installed capacity for the storage of liquid mercury is below 8,000t.
242 Reference number /2009/530302/ETU/G The owner of the Almadén warehouse has long-term experience with the handling of liquid mercury and has already installed monitoring systems and safety measures to prevent mercury releases from the facility. But the facility does not fulfil the requirements of a landfill and also does not have a permit for the storage of waste. Thus, a permit has to be requested which might be very time consuming. In Germany, the possibility of long-term storage of liquid mercury in above ground landfills dedicated to the storage of hazardous waste is already foreseen in national law (see section 5.3). According to German law, long-term storage in above-ground disposal sites for hazardous waste (landfill class III) is possible under the precondition that the landfill has to be explicitly appointed for the storage of mercury and needs to be operationally and technically equipped for this purpose. Apart from Almadén, other companies also have experience with the storage of liquid mercury as a product. In particular, recycling companies extracting mercury from waste as well as operators of chlor-alkali plants have experience related to the handling and storage of liquid mercury but typically only with smaller amounts. In principal, other companies could also apply for a permit for the temporary storage of liquid mercury. The potential storage sites have, on the one hand, to fulfil the requirements laid down in the landfill directive and the WAC decision (permit as landfill), implement the requirements of the Seveso Directive and they also have to provide adequate storage conditions for liquid mercury. No information is available on existing storage capacities. In particular already permitted landfills could be potential storage sites as they already fulfil the requirements of the landfill directive and WAC Decision. The application process for an amendment/extension of an existing permit is seen by far less time consuming and cost intensive as the application for an entire permit as landfill. The implementation costs and time widely depend on the possibility to use already existing facilities. It is expected that adequate facilities will be available until Minimum requirements Additional requirements Feasibility fulfilled Capacity? Experience Experience with the handling of liquid mercury Approval of authorities is given? Implementation time? Implementation costs?
243 Reference number /2009/530302/ETU/G Summary: option 5l Minimum requirements Additional facility-related requirements or acceptance criteria required Minimum requirements fulfilled Technical minimum requirements YES Environmental minimum requirements YES Economic minimum requirements YES Feasibility of implementation?? Option 5l temporary storage of liquid mercury in above ground facilities would be possible if adequate capacities are available and permits for the storage of liquid mercury are available at the latest by 2011.
244 Reference number /2009/530302/ETU/G Option 6: Pre-treatment In Chapter 7, an overview is provided on different stabilization and solidification techniques for liquid mercury currently available or under development. Based on the information, a screening of the pre-treatment technologies was carried out to identify those which fulfil defined minimum requirements concerning technical, environmental and economic aspects. Afterwards, the feasibility of implementation of the options is checked against criteria like the capacity and time to implement the option. Based on the outcome of the screening analysis one of the following conclusion is relevant: i An appropriate technology for pre-treating elemental mercury is available and already realised to handle the expected quantity of elemental mercury. ii An appropriate technology for pre-treating elemental mercury is available but not realised in a scale to handle the expected quantity of elemental mercury. It is expected that it could be realised by March iii An appropriate technology for pre-treating elemental mercury is available but not realised to handle the expected quantity of elemental mercury. It is not expected that it will be realised by March iv An appropriate technology for pre-treating elemental mercury is not available and is not expected to be in place in a reasonable time to handle the expected quantity of elemental mercury. The appropriate statement will provide an indication of which way Option 6 Pre-treatment should be further considered.
245 Reference number /2009/530302/ETU/G Technical, environmental and economic minimum requirements The following figure illustrates the screening process for the selection of suitable pre-treatment options. Figure 8-1: Decision scheme for the selection of suitable pre-treatment processes Technical minimum requirements Technical minimum requirements shall ensure that a technically feasible solution is achievable. If there are only theoretical considerations, a solution cannot be considered as appropriate as experiences on the applicability in the large scale are lacking. A technical minimum requirement requesting a full large-scale application would be desirable as it would clear any uncertainties about usability in the future. However, this requirement would have the disadvantage that technologies, which are currently not yet available in a large scale application but could be realised within the needed time frame would not be considered. The question for the establishment of a technical minimum criteria was therefore, which status of process realisation is required to prove that the process is more developed than just laboratory scale but to leave enough space for promising processes that still need up-scaling. According to various experts judgements, the project team decided to use the following criterion: Available process capable of stabilizing >1kg of elemental mercury can be treated in one batch With this criterion, the possible upgrading potential has to be evaluated additionally for each technology.
Official Journal of the European Union. (Legislative acts) REGULATIONS
 24.5.2017 L 137/1 I (Legislative acts) REGULATIONS REGULATION (EU) 2017/852 OF THE EUROPEAN PARLIAMT AND OF THE COUNCIL of 17 May 2017 on mercury, and repealing Regulation (EC) No 1102/2008 (Text with
24.5.2017 L 137/1 I (Legislative acts) REGULATIONS REGULATION (EU) 2017/852 OF THE EUROPEAN PARLIAMT AND OF THE COUNCIL of 17 May 2017 on mercury, and repealing Regulation (EC) No 1102/2008 (Text with
L 328/28 Official Journal of the European Union DIRECTIVES
 L 328/28 Official Journal of the European Union 6.12.2008 DIRECTIVES DIRECTIVE 2008/99/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 19 November 2008 on the protection of the environment through
L 328/28 Official Journal of the European Union 6.12.2008 DIRECTIVES DIRECTIVE 2008/99/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 19 November 2008 on the protection of the environment through
The European position By Ella Stengler
 Waste Management World November/December 2005 The European position By Ella Stengler November 2, 2005 Where is waste-to-energy, and where is it going? A WTE plant in Mallorca, Spain. European plants operate
Waste Management World November/December 2005 The European position By Ella Stengler November 2, 2005 Where is waste-to-energy, and where is it going? A WTE plant in Mallorca, Spain. European plants operate
SUMMARY. 1 Large household appliances. 2 Small household appliances. 3 Informatics and Telecommunication (IT) equipment. 4 Consuming products
 SUMMARY This technical report presents analytical data and information related to: The characteristics of the Waste Electrical and Electronic Equipment WEEE (composition, hazardous substances/materials,
SUMMARY This technical report presents analytical data and information related to: The characteristics of the Waste Electrical and Electronic Equipment WEEE (composition, hazardous substances/materials,
Identification and documentation of Industrial Emissions Success Stories. - Specific Terms of Reference -
 Identification and documentation of Industrial Emissions Success Stories 1. BACKGROUND - Specific Terms of Reference - Industrial activities play an important role in the economic well-being of Europe,
Identification and documentation of Industrial Emissions Success Stories 1. BACKGROUND - Specific Terms of Reference - Industrial activities play an important role in the economic well-being of Europe,
12. Waste and material flows
 1 Environmental signals 22 12. Waste and material flows policy issue indicator assessment decoupling resource use from economic activity decoupling waste generation from economic activity reducing generation
1 Environmental signals 22 12. Waste and material flows policy issue indicator assessment decoupling resource use from economic activity decoupling waste generation from economic activity reducing generation
Guideline on the implementation of. European Regulation (EC) 1272/2008
 Avenue Jules Bordet, 142 B-1140 Brussels, Belgium Tel: +32 2 761 1653 Fax: +32 2 761 1699 Email: eurobat@kelleneurope.com Guideline on the implementation of European Regulation (EC) 1272/2008 on the classification,
Avenue Jules Bordet, 142 B-1140 Brussels, Belgium Tel: +32 2 761 1653 Fax: +32 2 761 1699 Email: eurobat@kelleneurope.com Guideline on the implementation of European Regulation (EC) 1272/2008 on the classification,
(Consolidated Version) 1 of COUNCIL DIRECTIVE ON WASTE
 (Consolidated Version) 1 of COUNCIL DIRECTIVE ON WASTE THE COUNCIL OF THE EUROPEAN COMMUNITIES,... HAS ADOPTED THIS DIRECTIVE: For the purposes of this Directive: Article 1 (a) "waste" shall mean any substance
(Consolidated Version) 1 of COUNCIL DIRECTIVE ON WASTE THE COUNCIL OF THE EUROPEAN COMMUNITIES,... HAS ADOPTED THIS DIRECTIVE: For the purposes of this Directive: Article 1 (a) "waste" shall mean any substance
The Role Of Sustainable Landfill In Future Waste Management Systems
 The Role Of Sustainable Landfill In Future Waste Management Systems HEIJO SCHARFF, NV Afvalzorg Holding Contact name: Heijo Scharff Organisation: NV Afvalzorg Holding Postal address: PO Box 2, 1566 ZG
The Role Of Sustainable Landfill In Future Waste Management Systems HEIJO SCHARFF, NV Afvalzorg Holding Contact name: Heijo Scharff Organisation: NV Afvalzorg Holding Postal address: PO Box 2, 1566 ZG
Instruments of environmental policy
 Instruments of environmental policy Instruments of environmental policies are related to methods, environmental legislation and administrative procedures developed with a view to reduce negative impacts
Instruments of environmental policy Instruments of environmental policies are related to methods, environmental legislation and administrative procedures developed with a view to reduce negative impacts
IAEA SAFETY STANDARDS for protecting people and the environment. Predisposal Management of Radioactive Waste from Nuclear Fuel Cycle Facilities
 DS447 Date: 20 February 2015 IAEA SAFETY STANDARDS for protecting people and the environment STATUS: SPESS STEP 12 For submission to CSS Predisposal Management of Radioactive Waste from Nuclear Fuel Cycle
DS447 Date: 20 February 2015 IAEA SAFETY STANDARDS for protecting people and the environment STATUS: SPESS STEP 12 For submission to CSS Predisposal Management of Radioactive Waste from Nuclear Fuel Cycle
Baltic Marine Environment Protection Commission
 Baltic Marine Environment Protection Commission HELCOM Recommendation 38/1 Adopted 1 March 2017 having regard to Article 20, Paragraph 1 b) of the Helsinki Convention SEWAGE SLUDGE HANDLING THE COMMISSION,
Baltic Marine Environment Protection Commission HELCOM Recommendation 38/1 Adopted 1 March 2017 having regard to Article 20, Paragraph 1 b) of the Helsinki Convention SEWAGE SLUDGE HANDLING THE COMMISSION,
Guidance on use of Disposal and Recovery Codes (Waste Management Act, 1996 as amended)
 Guidance on use of Disposal and Recovery Codes (Waste Management Act, 1996 as amended) This information is provided as a source of reference for operators completing waste surveys for the EPA. Under each
Guidance on use of Disposal and Recovery Codes (Waste Management Act, 1996 as amended) This information is provided as a source of reference for operators completing waste surveys for the EPA. Under each
Waste-to-Energy in Europe + implementation of the Waste Framework Directive
 Confederation of European Waste-to-Energy Plants Waste-to-Energy in Europe + implementation of the Waste Framework Directive IFAT ENTSORGA 16 th September 2010 Munich Dr. Ella Stengler CEWEP Managing Director
Confederation of European Waste-to-Energy Plants Waste-to-Energy in Europe + implementation of the Waste Framework Directive IFAT ENTSORGA 16 th September 2010 Munich Dr. Ella Stengler CEWEP Managing Director
HBCD Factsheet. Brominated Flame retardant October Hexabromocyclododecane
 HBCD Factsheet Brominated Flame retardant October 2012 Hexabromocyclododecane HBCD Factsheet Hexabromocyclododecane > Introduction Hexabromocyclododecane (HBCD) 1 is a brominated flame retardant used for
HBCD Factsheet Brominated Flame retardant October 2012 Hexabromocyclododecane HBCD Factsheet Hexabromocyclododecane > Introduction Hexabromocyclododecane (HBCD) 1 is a brominated flame retardant used for
Mercury Contaminated land management state of the art. NICOLE Mercury Working Group Paper
 Mercury Contaminated land management state of the art NICOLE Mercury Working Group Paper Context Mercury is a natural element formed primarily through hydrothermal processes. Its occurrence is rare, but
Mercury Contaminated land management state of the art NICOLE Mercury Working Group Paper Context Mercury is a natural element formed primarily through hydrothermal processes. Its occurrence is rare, but
REGULATION (EC) No 2037/2000 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 29 June 2000 on substances that deplete the ozone layer
 2000R2037 EN 24.12.2004 005.001 1 This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents B REGULATION (EC) No 2037/2000 OF THE EUROPEAN
2000R2037 EN 24.12.2004 005.001 1 This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents B REGULATION (EC) No 2037/2000 OF THE EUROPEAN
Discussion of the Environmental Impacts of the Solwara 1 Copper Concentration and Smelting Processes
 89 90 Discussion of the Environmental Impacts of the Solwara 1 Copper Concentration and Smelting Processes Tongling Non-Ferrous Metals Group copper smelter Image credit: nerin.com 91 Analysis IV Environmental
89 90 Discussion of the Environmental Impacts of the Solwara 1 Copper Concentration and Smelting Processes Tongling Non-Ferrous Metals Group copper smelter Image credit: nerin.com 91 Analysis IV Environmental
This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents
 2000R2037 EN 07.08.2009 010.001 1 This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents B REGULATION (EC) No 2037/2000 OF THE EUROPEAN
2000R2037 EN 07.08.2009 010.001 1 This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents B REGULATION (EC) No 2037/2000 OF THE EUROPEAN
COUNCIL OF THE EUROPEAN UNION. Brussels, 8 February 2010 (OR. en) 11962/1/09 REV 1. Interinstitutional File: 2007/0286 (COD) ENV 494 CODEC 967
 COUNCIL OF THE EUROPEAN UNION Brussels, 8 February 2010 (OR. en) Interinstitutional File: 2007/0286 (COD) 11962/1/09 REV 1 ENV 494 CODEC 967 LEGISLATIVE ACTS AND OTHER INSTRUMENTS Subject: Position of
COUNCIL OF THE EUROPEAN UNION Brussels, 8 February 2010 (OR. en) Interinstitutional File: 2007/0286 (COD) 11962/1/09 REV 1 ENV 494 CODEC 967 LEGISLATIVE ACTS AND OTHER INSTRUMENTS Subject: Position of
HAZARDOUS WASTE ( MANAGEMENT, HANDLING AND TRANSBOUNDARY MOVEMENT) RULES 2008 AMENDMENTS MADE THERE OF
 HAZARDOUS WASTE ( MANAGEMENT, HANDLING AND TRANSBOUNDARY MOVEMENT) RULES 2008 AMENDMENTS MADE THERE OF DEFINITION OF HAZARDOUS WASTES-ITS APPLICABILITY Waste substance is solid, semi-solid or non -aqueous
HAZARDOUS WASTE ( MANAGEMENT, HANDLING AND TRANSBOUNDARY MOVEMENT) RULES 2008 AMENDMENTS MADE THERE OF DEFINITION OF HAZARDOUS WASTES-ITS APPLICABILITY Waste substance is solid, semi-solid or non -aqueous
25th commission meeting, 16 October 2014 WORKING DOCUMENT. Commission for the Environment, Climate Change and Energy
 25th commission meeting, 16 October 2014 ENVE-V-048 WORKING DOCUMENT Commission for the Environment, Climate Change and Energy Towards a circular economy: review of EU waste legislation Rapporteur: Mariana
25th commission meeting, 16 October 2014 ENVE-V-048 WORKING DOCUMENT Commission for the Environment, Climate Change and Energy Towards a circular economy: review of EU waste legislation Rapporteur: Mariana
Waste statistics and accounts in the EU
 Waste statistics and accounts in the EU Main reporting obligations EU regulation on waste statistics (2150/2002/EC ): Waste Framework Directive and related DG Env. Reporting (Directive 75/442/EEC on waste,
Waste statistics and accounts in the EU Main reporting obligations EU regulation on waste statistics (2150/2002/EC ): Waste Framework Directive and related DG Env. Reporting (Directive 75/442/EEC on waste,
EN Official Journal of the European Union L 158/ 7
 30.4.2004 EN Official Journal of the European Union L 158/ 7 REGULATION (EC) No 850/2004 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 29 April 2004 on persistent organic pollutants and amending Directive
30.4.2004 EN Official Journal of the European Union L 158/ 7 REGULATION (EC) No 850/2004 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 29 April 2004 on persistent organic pollutants and amending Directive
UNEP Global Mercury Partnership. Business Plan (October 2011) Mercury supply and storage partnership area
 UNEP Global Mercury Partnership Business Plan (October 2011) Mercury supply and storage partnership area This document describes the business plan for the Mercury supply and storage partnership area within
UNEP Global Mercury Partnership Business Plan (October 2011) Mercury supply and storage partnership area This document describes the business plan for the Mercury supply and storage partnership area within
Appendix W: Solid and Hazardous Waste
 Solid waste, as defined in 40 Code of Federal Regulations (CFR) 261.2, is any discarded material that is typically found in the solid waste stream, including municipal solid waste, construction and demolition
Solid waste, as defined in 40 Code of Federal Regulations (CFR) 261.2, is any discarded material that is typically found in the solid waste stream, including municipal solid waste, construction and demolition
Safety Data Sheet according to Regulation (EC) No 1907/2006 (REACH) Page 1 of 5
 Page 1 of 5 1 Identification of the Substance/Preparation and of the Company Identification of the substance or preparation: CELTROX PC Use of the substance / preparation: Filtration of liquids Filter
Page 1 of 5 1 Identification of the Substance/Preparation and of the Company Identification of the substance or preparation: CELTROX PC Use of the substance / preparation: Filtration of liquids Filter
New Waste Framework Directive
 Practice note Maintained note (pdf generated on 13 March 2009) New Waste Framework Directive This Practice note explains the principal aims and provisions of the new Waste Framework Directive (2008/98/EC),
Practice note Maintained note (pdf generated on 13 March 2009) New Waste Framework Directive This Practice note explains the principal aims and provisions of the new Waste Framework Directive (2008/98/EC),
International Conventions. Regional Training in Hazardous Waste September 30 October 2, 2014 San José, Costa Rica
 International Conventions Regional Training in Hazardous Waste September 30 October 2, 2014 San José, Costa Rica Conventions on Waste In recent years, trade in chemicals and waste has been growing exponentially.
International Conventions Regional Training in Hazardous Waste September 30 October 2, 2014 San José, Costa Rica Conventions on Waste In recent years, trade in chemicals and waste has been growing exponentially.
REACH REGULATION: the new management of chemicals in Europe and operational tools. R egistration. E valuation. A Ch. uthorisation.
 REACH REGULATION: the new management of chemicals in Europe and operational tools R egistration E valuation A Ch uthorisation emicals Index of topics Set of rules before REACH Regulation REACH REGULATION
REACH REGULATION: the new management of chemicals in Europe and operational tools R egistration E valuation A Ch uthorisation emicals Index of topics Set of rules before REACH Regulation REACH REGULATION
GAS CYLINDERS COMPLIANCE WITH THE REVISED PACKAGING WASTE DIRECTIVE 94/62/EC AS AMENDED
 GAS CYLINDERS COMPLIANCE WITH THE REVISED PACKAGING WASTE DIRECTIVE 94/62/EC AS AMENDED Doc 181/13/E EUROPEAN INDUSTRIAL GASES ASSOCIATION AISBL AVENUE DES ARTS 3-5 B 1210 BRUSSELS Tel: +32 2 217 70 98
GAS CYLINDERS COMPLIANCE WITH THE REVISED PACKAGING WASTE DIRECTIVE 94/62/EC AS AMENDED Doc 181/13/E EUROPEAN INDUSTRIAL GASES ASSOCIATION AISBL AVENUE DES ARTS 3-5 B 1210 BRUSSELS Tel: +32 2 217 70 98
EU ECOLABEL FOR LAUNDRY DETERGENTS AND DETERGENTS FOR DISHWASHERS FOR PROFESSIONAL USE - EEB AND BEUC POSITION
 EU ECOLABEL FOR LAUNDRY DETERGENTS AND DETERGENTS FOR DISHWASHERS FOR PROFESSIONAL USE - EEB AND BEUC POSITION Contact: Lukasz Wozniacki, Ecolabel Coordinator for EEB and BEUC lukasz.wozniacki@eeb.org
EU ECOLABEL FOR LAUNDRY DETERGENTS AND DETERGENTS FOR DISHWASHERS FOR PROFESSIONAL USE - EEB AND BEUC POSITION Contact: Lukasz Wozniacki, Ecolabel Coordinator for EEB and BEUC lukasz.wozniacki@eeb.org
LABORATORY ACTIONS, DECISIONS & SAFETY WITHIN THE EUROPEAN UNION
 LABORATORY ACTIONS, DECISIONS & SAFETY WITHIN THE EUROPEAN UNION Kardzhali,, 7-9 7 9 December 2012 Research Center Environment and Health-Regional Health inspectorate George Papageorgiou Chemical Analyst
LABORATORY ACTIONS, DECISIONS & SAFETY WITHIN THE EUROPEAN UNION Kardzhali,, 7-9 7 9 December 2012 Research Center Environment and Health-Regional Health inspectorate George Papageorgiou Chemical Analyst
Waste prevention in Europe. European Environment Agency
 Waste prevention in Europe Özgür Saki European Environment Agency The European Environment Agency An EU institution situated in Copenhagen since 1994 Provides the information necessary to enable policy
Waste prevention in Europe Özgür Saki European Environment Agency The European Environment Agency An EU institution situated in Copenhagen since 1994 Provides the information necessary to enable policy
UN ECE Heavy Metals Protocol Work completed by the Task Force and Workshop to Promote the Ratification
 UN ECE Heavy Metals Protocol Work completed by the Task Force and Workshop to Promote the Ratification Berlin, 12 th /13 th April 12 Katja Kraus Federal Environmental Agency Germany UNECE WGSR September
UN ECE Heavy Metals Protocol Work completed by the Task Force and Workshop to Promote the Ratification Berlin, 12 th /13 th April 12 Katja Kraus Federal Environmental Agency Germany UNECE WGSR September
Hydro CERs and the EU ETS 2009
 Hydro CERs and the EU ETS 2009 September 2010 1 Picture: La Esperanza Hydroelectric CDM Project Contents About Sandbag Climate Campaign 3 Hydro CERs and EU ETS 4 Member State analysis 5 Sectoral Analysis
Hydro CERs and the EU ETS 2009 September 2010 1 Picture: La Esperanza Hydroelectric CDM Project Contents About Sandbag Climate Campaign 3 Hydro CERs and EU ETS 4 Member State analysis 5 Sectoral Analysis
Waste Incineration under the Industrial Emissions Directive
 Waste Incineration under the Industrial Emissions Directive 5 th CEWEP Congress on Waste-to-Energy Antwerpen, 1 July 2010 Filip François European Commission ENV.C3 (Industrial Emissions) 1 Overview IPPC
Waste Incineration under the Industrial Emissions Directive 5 th CEWEP Congress on Waste-to-Energy Antwerpen, 1 July 2010 Filip François European Commission ENV.C3 (Industrial Emissions) 1 Overview IPPC
FINAL REPORT. An initiative. of the
 An initiative of the PROVISION AND ELABORATION OF INFORMATION FOR THE PREPARATION OF THE "IMPLEMENTATION REPORT OF DIRECTIVE 2006/21/EC ON THE MANAGEMENT OF WASTE FROM EXTRACTIVE INDUSTRIES Service Request
An initiative of the PROVISION AND ELABORATION OF INFORMATION FOR THE PREPARATION OF THE "IMPLEMENTATION REPORT OF DIRECTIVE 2006/21/EC ON THE MANAGEMENT OF WASTE FROM EXTRACTIVE INDUSTRIES Service Request
Circular Economy and Energy Union
 Circular Economy and Energy Union Dr. Ella Stengler CEWEP Managing Director 16 June 2016, Rotterdam 8 th CEWEP Waste-to-Energy Congress 2016 1 Members CEWEP Confederation of European Waste-to-Energy Plants
Circular Economy and Energy Union Dr. Ella Stengler CEWEP Managing Director 16 June 2016, Rotterdam 8 th CEWEP Waste-to-Energy Congress 2016 1 Members CEWEP Confederation of European Waste-to-Energy Plants
TRADE & ENVIRONMENT ISSUES - SECONDARY OR RECYCLED MATERIALS - UPCOMMING BASEL CONVENTION COP-12
 TRADE & ENVIRONMENT ISSUES - SECONDARY OR RECYCLED MATERIALS - UPCOMMING BASEL CONVENTION COP-12 by Ross Bartley BIR Trade & Environment Director JOINT STUDY GROUPS SEMINAR TRADE POLICY ISSUES IN MINING
TRADE & ENVIRONMENT ISSUES - SECONDARY OR RECYCLED MATERIALS - UPCOMMING BASEL CONVENTION COP-12 by Ross Bartley BIR Trade & Environment Director JOINT STUDY GROUPS SEMINAR TRADE POLICY ISSUES IN MINING
COMMISSION DELEGATED DIRECTIVE../ /EU. of XXX
 EUROPEAN COMMISSION Brussels, XXX [ ](2014) XXX draft COMMISSION DELEGATED DIRECTIVE../ /EU of XXX amending, for the purposes of adapting to technical progress, Annex III to Directive 2011/65/EU of the
EUROPEAN COMMISSION Brussels, XXX [ ](2014) XXX draft COMMISSION DELEGATED DIRECTIVE../ /EU of XXX amending, for the purposes of adapting to technical progress, Annex III to Directive 2011/65/EU of the
Continuous Monitoring of Pollution in the Nation s Precipitation
 Continuous Monitoring of Pollution in the Nation s Precipitation Martin Risch and David A. Gay U.S. Geological Survey, Indiana Water Science Center, Indianapolis, IN, mrrisch@usgs.gov NADP Program Office,
Continuous Monitoring of Pollution in the Nation s Precipitation Martin Risch and David A. Gay U.S. Geological Survey, Indiana Water Science Center, Indianapolis, IN, mrrisch@usgs.gov NADP Program Office,
Perspectives and Challenges of the Woodworking Industries in Europe Joint CEI-Bois / EFBWW / EPF Project
 Project carried out with the financial support of the European Commission CEI-Bois We are a responsible Industry Doc P 3 Perspectives and Challenges of the Woodworking Industries in Europe Joint CEI-Bois
Project carried out with the financial support of the European Commission CEI-Bois We are a responsible Industry Doc P 3 Perspectives and Challenges of the Woodworking Industries in Europe Joint CEI-Bois
Perchlorate BMP Regulations R TITLE 22 EMERGENCY REGULATIONS Perchlorate Best Management Practices DEPARTMENT REFERENCE NUMBER: R
 TITLE 22 EMERGENCY REGULATIONS Perchlorate Best Management Practices DEPARTMENT REFERENCE NUMBER: FINDING OF EMERGENCY These emergency regulations are mandated by section 25210.6 of California Health and
TITLE 22 EMERGENCY REGULATIONS Perchlorate Best Management Practices DEPARTMENT REFERENCE NUMBER: FINDING OF EMERGENCY These emergency regulations are mandated by section 25210.6 of California Health and
Statement of the Bundesgütegemeinschaft Kompost e.v. (BGK) on the Green Paper for the Management of Biowaste in the EU
 Köln, den 27.02.2009 Statement of the Bundesgütegemeinschaft Kompost e.v. (BGK) on the Green Paper for the Management of Biowaste in the EU The German Compost Quality Assurance Organisation (BGK) represents
Köln, den 27.02.2009 Statement of the Bundesgütegemeinschaft Kompost e.v. (BGK) on the Green Paper for the Management of Biowaste in the EU The German Compost Quality Assurance Organisation (BGK) represents
POSITION June Circular Economy Proposal for a Directive amending Directive 2008/98/EC on Waste. Parliamentary Draft Report of Simona Bonafè, MEP
 POSITION June 2016 Circular Economy Proposal for a Directive amending Directive 2008/98/EC on Waste Parliamentary Draft Report of Simona Bonafè, MEP The European Aggregates Industry has embraced the imperative
POSITION June 2016 Circular Economy Proposal for a Directive amending Directive 2008/98/EC on Waste Parliamentary Draft Report of Simona Bonafè, MEP The European Aggregates Industry has embraced the imperative
PROPOSED FRAMEWORK FOR A FINANCIAL MECHANISM FOR THE FUTURE MERCURY CONVENTION AND DRAFT OPERATIONAL PROGRAM FOR MERCURY
 GEF Council Meeting November 13 15, 2012 Washington, D.C. GEF/C.43/04 October 15, 2012 Agenda Item 7 PROPOSED FRAMEWORK FOR A FINANCIAL MECHANISM FOR THE FUTURE MERCURY CONVENTION AND DRAFT OPERATIONAL
GEF Council Meeting November 13 15, 2012 Washington, D.C. GEF/C.43/04 October 15, 2012 Agenda Item 7 PROPOSED FRAMEWORK FOR A FINANCIAL MECHANISM FOR THE FUTURE MERCURY CONVENTION AND DRAFT OPERATIONAL
Proposal for a. REGULATION (EU) No / OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL
 EUROPEAN COMMISSION Brussels, 4.11.2010 COM(2010) 597 final 2010/0298 (COD) Proposal for a REGULATION (EU) No / OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL amending Regulation (EC) No 648/2004 as regards
EUROPEAN COMMISSION Brussels, 4.11.2010 COM(2010) 597 final 2010/0298 (COD) Proposal for a REGULATION (EU) No / OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL amending Regulation (EC) No 648/2004 as regards
Machinery for Pesticide Application: Impoving spraying equipment to achieve sustainable use of pesticide
 Workshop Committee on the Internal Market and Consumer Protection Machinery for Pesticide Application: Impoving spraying equipment to achieve sustainable use of pesticide Thursday, 11 December 2008 from
Workshop Committee on the Internal Market and Consumer Protection Machinery for Pesticide Application: Impoving spraying equipment to achieve sustainable use of pesticide Thursday, 11 December 2008 from
COMMISSION REGULATION (EU)
 L 320/8 Official Journal of the European Union 17.11.2012 COMMISSION REGULATION (EU) No 1078/2012 of 16 November 2012 on a common safety method for monitoring to be applied by railway undertakings, infrastructure
L 320/8 Official Journal of the European Union 17.11.2012 COMMISSION REGULATION (EU) No 1078/2012 of 16 November 2012 on a common safety method for monitoring to be applied by railway undertakings, infrastructure
National Standard for Environmental Risk Management of Industrial Chemicals
 National Standard for Environmental Risk Management of Industrial Chemicals Information Paper on the pathway for design and implementation of the National Standard December 2015 CONTENTS Purpose... 3 Overview
National Standard for Environmental Risk Management of Industrial Chemicals Information Paper on the pathway for design and implementation of the National Standard December 2015 CONTENTS Purpose... 3 Overview
THE EUROPEAN PARLIAMENT: ELECTORAL PROCEDURES
 THE EUROPEAN PARLIAMENT: ELECTORAL PROCEDURES The procedures for electing the European Parliament are governed both by European legislation defining rules common to all Member States and by specific national
THE EUROPEAN PARLIAMENT: ELECTORAL PROCEDURES The procedures for electing the European Parliament are governed both by European legislation defining rules common to all Member States and by specific national
CONCEPT PAPER. ASIA-EUROPE ENVIRONMENT FORUM 4 TH ROUNDTABLE Combine or Combust! Co-operating on Chemicals and Hazardous Substances Management
 ASIA-EUROPE ENVIRONMENT FORUM 4 TH ROUNDTABLE Combine or Combust! Co-operating on Chemicals and Hazardous Substances Management 30 November-1 December Brussels, Belgium CONCEPT PAPER SUMMARY A roundtable
ASIA-EUROPE ENVIRONMENT FORUM 4 TH ROUNDTABLE Combine or Combust! Co-operating on Chemicals and Hazardous Substances Management 30 November-1 December Brussels, Belgium CONCEPT PAPER SUMMARY A roundtable
This document is a preview generated by EVS
 EESTI STANDARD EVS-EN 15343:2007 Plastics - Recycled Plastics - Plastics recycling traceability and assessment of conformity and recycled content Plastics - Recycled Plastics - Plastics recycling traceability
EESTI STANDARD EVS-EN 15343:2007 Plastics - Recycled Plastics - Plastics recycling traceability and assessment of conformity and recycled content Plastics - Recycled Plastics - Plastics recycling traceability
This document is a preview generated by EVS
 TECHNICAL SPECIFICATION SPÉCIFICATION TECHNIQUE TECHNISCHE SPEZIFIKATION CEN/TS 15863 July 2012 ICS 13.030.10 English Version Characterisation of waste - Leaching behaviour test for basic characterisation
TECHNICAL SPECIFICATION SPÉCIFICATION TECHNIQUE TECHNISCHE SPEZIFIKATION CEN/TS 15863 July 2012 ICS 13.030.10 English Version Characterisation of waste - Leaching behaviour test for basic characterisation
Copan Italia SPA STERILE PLASTIC APPLICATOR RAYON TIPPED INDIVIDUALLY WRAPPED
 Page n. 1 / 6 Information Sheet SECTION 1. Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Code: Product name 167KS01.US 1.2. Relevant identified uses of
Page n. 1 / 6 Information Sheet SECTION 1. Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Code: Product name 167KS01.US 1.2. Relevant identified uses of
The Industrial Emissions Directive (IED)
 The Industrial Emissions Directive () 2010/75/EU Filip François European Commission, DG Environment Industrial Emissions Unit 02.02.2012 The legal framework concerning industrial emissions in the European
The Industrial Emissions Directive () 2010/75/EU Filip François European Commission, DG Environment Industrial Emissions Unit 02.02.2012 The legal framework concerning industrial emissions in the European
Toyama Framework on Material Cycles
 Toyama Framework on Material Cycles We, the G7 Ministers and high representatives, and European Commissioner responsible for the environment, based on our discussion on resource efficiency and the 3Rs
Toyama Framework on Material Cycles We, the G7 Ministers and high representatives, and European Commissioner responsible for the environment, based on our discussion on resource efficiency and the 3Rs
Material Safety Data Sheet MSDS
 Primary zinc/silver oxide button cell (series V...) 1. Identification of the product and of the company undertaking Product details Trade name: Voltage: Electrochemical system: Anode (negative electrode):
Primary zinc/silver oxide button cell (series V...) 1. Identification of the product and of the company undertaking Product details Trade name: Voltage: Electrochemical system: Anode (negative electrode):
5. Dealt with in Brussels by The Transport, Telecommunications and Energy Council Working Party on Land Transport
 Com 63 (2018) final Information Note 1. Proposal Proposal for a Council Decision on the position to be taken on behalf of the European Union at the 55 th Session of the Organisation for International Carriage
Com 63 (2018) final Information Note 1. Proposal Proposal for a Council Decision on the position to be taken on behalf of the European Union at the 55 th Session of the Organisation for International Carriage
EU Energy Winter Package (RED Recast) and Future of Forest Biomass Piotr Borkowski, EUSTAFOR s Executive Director
 EU Energy Winter Package (RED Recast) and Future of Forest Biomass Piotr Borkowski, EUSTAFOR s Executive Director Conference: The Need for Sectoral Education on the European path of B&H 9-10/2/2017, Kupres,
EU Energy Winter Package (RED Recast) and Future of Forest Biomass Piotr Borkowski, EUSTAFOR s Executive Director Conference: The Need for Sectoral Education on the European path of B&H 9-10/2/2017, Kupres,
Seveso Directive 82/501/EEC ("Seveso I")
 Seveso Directive 82/501/EEC ("Seveso I") council directive of 24 June 1982 on the major-accident hazards of certain industrial activities (82/501/EEC) THE COUNCIL OF THE EUROPEAN COMMUNITIES, Having regard
Seveso Directive 82/501/EEC ("Seveso I") council directive of 24 June 1982 on the major-accident hazards of certain industrial activities (82/501/EEC) THE COUNCIL OF THE EUROPEAN COMMUNITIES, Having regard
WASTE STATISTICS IN GERMANY
 STATISTICAL COMMISSION and EUROPEAN ECONOMIC COMMISSION FOR EUROPE COMMISSION OF THE COMMUNITIES CONFERENCE OF EUROPEAN STATISTICIANS EUROSTAT Joint ECE/Eurostat Work Session on Methodological Issues of
STATISTICAL COMMISSION and EUROPEAN ECONOMIC COMMISSION FOR EUROPE COMMISSION OF THE COMMUNITIES CONFERENCE OF EUROPEAN STATISTICIANS EUROSTAT Joint ECE/Eurostat Work Session on Methodological Issues of
OECD RECOMMENDATION ON THE SAFETY OF NANOMATERIALS
 OECD RECOMMENDATION ON THE SAFETY OF NANOMATERIALS Workshop for the Asia-Pacific Region on Nanotechnology and Manufactured Nanomaterials: Safety Issues 10-11 September 2015, Bangkok, Thailand OECD Australia
OECD RECOMMENDATION ON THE SAFETY OF NANOMATERIALS Workshop for the Asia-Pacific Region on Nanotechnology and Manufactured Nanomaterials: Safety Issues 10-11 September 2015, Bangkok, Thailand OECD Australia
Vacancy Notice. 1. The job (7)
 09.11.2015 1(7) Vacancy Notice The European Chemicals Agency (ECHA) is launching this call for expressions of interest in order to establish a reserve list for the following temporary agent profile: Reference
09.11.2015 1(7) Vacancy Notice The European Chemicals Agency (ECHA) is launching this call for expressions of interest in order to establish a reserve list for the following temporary agent profile: Reference
ASSESSING GOOD PRACTICES IN POLICIES AND MEASURES TO MITIGATE CLIMATE CHANGE IN CENTRAL AND EASTERN EUROPE. Elena Petkova
 Workshop on Best Practices in Policies and Measures, 8-10 October 2001, Copenhagen ASSESSING GOOD PRACTICES IN POLICIES AND MEASURES TO MITIGATE CLIMATE CHANGE IN CENTRAL AND EASTERN EUROPE Elena Petkova
Workshop on Best Practices in Policies and Measures, 8-10 October 2001, Copenhagen ASSESSING GOOD PRACTICES IN POLICIES AND MEASURES TO MITIGATE CLIMATE CHANGE IN CENTRAL AND EASTERN EUROPE Elena Petkova
Copan Italia SPA MEDIUM + PERNASAL FLOCKED SWAB
 Page n. 1 / 6 Information Sheet SECTION 1. Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Code: Product name 305C 1.2. Relevant identified uses of the substance
Page n. 1 / 6 Information Sheet SECTION 1. Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Code: Product name 305C 1.2. Relevant identified uses of the substance
Mercury Biogeochemical Cycle
 Mercury Biogeochemical Cycle Hao Zhou 10/09/2017 Today s Discussion Background Compartments Anthropogenic inputs Atmosphere Arctic Soil Freshwater/wetlands Ocean 1 Liquid at room temperature Not biodegradable
Mercury Biogeochemical Cycle Hao Zhou 10/09/2017 Today s Discussion Background Compartments Anthropogenic inputs Atmosphere Arctic Soil Freshwater/wetlands Ocean 1 Liquid at room temperature Not biodegradable
Agenda item 7: Costing of the Regional Plans implementation
 UNITED NATIONS UNEP(DEPI)/MED WG.414/4 UNITED NATIONS ENVIRONMENT PROGRAMME MEDITERRANEAN ACTION PLAN 14 April 2015 Original: English Regional meeting on applying methodology for programmes of measures
UNITED NATIONS UNEP(DEPI)/MED WG.414/4 UNITED NATIONS ENVIRONMENT PROGRAMME MEDITERRANEAN ACTION PLAN 14 April 2015 Original: English Regional meeting on applying methodology for programmes of measures
COMMON IMPLEMENTATION STRATEGY FOR THE WATER FRAMEWORK DIRECTIVE (2000/60/EC)
 COMMON IMPLEMENTATION STRATEGY FOR THE WATER FRAMEWORK DIRECTIVE (2000/60/EC) POLICY SUMMARY to Guidance Document No. 4 Produced by Working Group 2.2 - HMWB EXPLANATORY NOTE This policy summary gives an
COMMON IMPLEMENTATION STRATEGY FOR THE WATER FRAMEWORK DIRECTIVE (2000/60/EC) POLICY SUMMARY to Guidance Document No. 4 Produced by Working Group 2.2 - HMWB EXPLANATORY NOTE This policy summary gives an
LEGAL BASIS COMMON RULES
 THE EUROPEAN PARLIAMENT: ELECTORAL PROCEDURES The procedures for electing the European Parliament are governed both by European legislation defining rules common to all Member States and by specific national
THE EUROPEAN PARLIAMENT: ELECTORAL PROCEDURES The procedures for electing the European Parliament are governed both by European legislation defining rules common to all Member States and by specific national
ISO Environmental management systems Requirements with guidance for use
 INTERNATIONAL STANDARD Environmental management systems Requirements with guidance for use ISO 14001 Third edition 2015-09-15 Systèmes de management environnemental Exigences et lignes directrices pour
INTERNATIONAL STANDARD Environmental management systems Requirements with guidance for use ISO 14001 Third edition 2015-09-15 Systèmes de management environnemental Exigences et lignes directrices pour
Waste Management Planning
 Waste Management Planning Hungary Legal Analysis Justice and Environment 2011 a Dvorakova 13, 602 00, Brno, CZ e info@justiceandenvironment.org 1 t/f 36 1 3228462 / 36 1 4130300 w www.justiceandenvironment.org
Waste Management Planning Hungary Legal Analysis Justice and Environment 2011 a Dvorakova 13, 602 00, Brno, CZ e info@justiceandenvironment.org 1 t/f 36 1 3228462 / 36 1 4130300 w www.justiceandenvironment.org
This document is a preview generated by EVS
 TECHNICAL SPECIFICATION SPÉCIFICATION TECHNIQUE TECHNISCHE SPEZIFIKATION CEN ISO/TS 29001 February 2011 ICS 03.120.10; 75.020 English Version Petroleum, petrochemical and natural gas industries - Sectorspecific
TECHNICAL SPECIFICATION SPÉCIFICATION TECHNIQUE TECHNISCHE SPEZIFIKATION CEN ISO/TS 29001 February 2011 ICS 03.120.10; 75.020 English Version Petroleum, petrochemical and natural gas industries - Sectorspecific
NB: Unofficial translation, legally binding texts are those in Finnish and Swedish Ministry of the Environment, Finland. Climate Change Act 609/2015
 NB: Unofficial translation, legally binding texts are those in Finnish and Swedish Ministry of the Environment, Finland Climate Change Act 609/2015 Section 1 Purpose and goals of the Act (1) The purpose
NB: Unofficial translation, legally binding texts are those in Finnish and Swedish Ministry of the Environment, Finland Climate Change Act 609/2015 Section 1 Purpose and goals of the Act (1) The purpose
6155/18 KS/mb 1 DG E 1A
 Council of the European Union Brussels, 19 February 2018 (OR. en) 6155/18 NOTE From: To: General Secretariat of the Council Permanent Representatives Committee/Council ENV 78 MARE 2 MI 82 AGRI 81 IND 48
Council of the European Union Brussels, 19 February 2018 (OR. en) 6155/18 NOTE From: To: General Secretariat of the Council Permanent Representatives Committee/Council ENV 78 MARE 2 MI 82 AGRI 81 IND 48
Country profile. More from less material resource efficiency in Europe overview of policies, instruments and targets in 32 countries.
 Country profile More from less material resource efficiency in Europe 2015 overview of policies, instruments and targets in 32 countries Lithuania May 2016 This country profile is based on information
Country profile More from less material resource efficiency in Europe 2015 overview of policies, instruments and targets in 32 countries Lithuania May 2016 This country profile is based on information
TO THE COMPLAINTS MECHANISM OF THE EUROPEAN INVESTMENT BANK
 Law at the service of the environment El derecho al servicio del medio ambiente TO THE COMPLAINTS MECHANISM OF THE EUROPEAN INVESTMENT BANK Index 1. The complainant... 1 2. The complaint... 2 3. The facts
Law at the service of the environment El derecho al servicio del medio ambiente TO THE COMPLAINTS MECHANISM OF THE EUROPEAN INVESTMENT BANK Index 1. The complainant... 1 2. The complaint... 2 3. The facts
Revision of Directive 2008/98/EC on waste (Waste Framework Directive) and of Directive 1999/31/EC on landfill of waste. A EURELECTRIC position paper
 Revision of Directive 2008/98/EC on waste (Waste Framework Directive) and of Directive 1999/31/EC on landfill of waste A EURELECTRIC position paper January 2017 EURELECTRIC is the voice of the electricity
Revision of Directive 2008/98/EC on waste (Waste Framework Directive) and of Directive 1999/31/EC on landfill of waste A EURELECTRIC position paper January 2017 EURELECTRIC is the voice of the electricity
3M TM Dyneon TM Fluoropolymers. Up-Cycling. Closing the loop.
 3M TM Dyneon TM Fluoropolymers Up-Cycling. Closing the loop. 02 Introduction Monomer Production Polymer Life cycle Protecting the environment takes more than just good intentions. Chemical recycling End
3M TM Dyneon TM Fluoropolymers Up-Cycling. Closing the loop. 02 Introduction Monomer Production Polymer Life cycle Protecting the environment takes more than just good intentions. Chemical recycling End
The Energy Efficiency Watch Survey
 The Energy Efficiency Watch Survey Christiane Egger OÖ Energiesparverband christiane.egger@esv.or.at, www.esv-en.at www.energy-efficiency-watch.org The Energy Efficiency Watch 2 Project: Reality check
The Energy Efficiency Watch Survey Christiane Egger OÖ Energiesparverband christiane.egger@esv.or.at, www.esv-en.at www.energy-efficiency-watch.org The Energy Efficiency Watch 2 Project: Reality check
Country Questionnaire Prior to the Senior Officials Meeting on the 3R Initiative - INDIA -
 Country Questionnaire Prior to the Senior Officials Meeting on the 3R Initiative - INDIA - [Country Questionnaire Survey] 1. Major developments regarding the strategies, policies and activities on the
Country Questionnaire Prior to the Senior Officials Meeting on the 3R Initiative - INDIA - [Country Questionnaire Survey] 1. Major developments regarding the strategies, policies and activities on the
Experiences in using alternative fuels in Europe and Germany
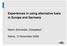 Experiences in using alternative fuels in Europe and Germany Martin Schneider, Düsseldorf Kielce, 13 November 2008 Structure Boundary conditions in waste legislation Use of alternative fuels in the cement
Experiences in using alternative fuels in Europe and Germany Martin Schneider, Düsseldorf Kielce, 13 November 2008 Structure Boundary conditions in waste legislation Use of alternative fuels in the cement
ANNEXES. to the COMMUNICATION FROM THE COMMISSION TO THE EUROPEAN PARLIAMENT AND THE COUNCIL
 EUROPEAN COMMISSION Brussels, 23.7.2014 COM(2014) 520 final ANNEXES 1 to 3 ANNEXES to the COMMUNICATION FROM THE COMMISSION TO THE EUROPEAN PARLIAMENT AND THE COUNCIL Energy Efficiency and its contribution
EUROPEAN COMMISSION Brussels, 23.7.2014 COM(2014) 520 final ANNEXES 1 to 3 ANNEXES to the COMMUNICATION FROM THE COMMISSION TO THE EUROPEAN PARLIAMENT AND THE COUNCIL Energy Efficiency and its contribution
Official Journal of the European Communities COMMISSION RECOMMENDATION. of 12 October (2-butoxyethoxy)ethanol, 2-(2-methoxyethoxy)ethanol,
 L 292/42 13.11.1999 COMMISSION RECOMMDATION of 12 October 1999 on the results of the risk evaluation and on the risk reduction strategies for the substances: 2-(2-butoxyethoxy)ethanol; 2-(2-methoxyethoxy)ethanol;
L 292/42 13.11.1999 COMMISSION RECOMMDATION of 12 October 1999 on the results of the risk evaluation and on the risk reduction strategies for the substances: 2-(2-butoxyethoxy)ethanol; 2-(2-methoxyethoxy)ethanol;
FINAL DRAFT FprEN
 EUROPEAN STANDARD NORME EUROPÉENNE EUROPÄISCHE NORM ICS 49.100 FINAL DRAFT FprEN 12312-8 February 2017 Will supersede EN 12312-8:2005+A1:2009 English Version Aircraft ground support equipment - Specific
EUROPEAN STANDARD NORME EUROPÉENNE EUROPÄISCHE NORM ICS 49.100 FINAL DRAFT FprEN 12312-8 February 2017 Will supersede EN 12312-8:2005+A1:2009 English Version Aircraft ground support equipment - Specific
SAFETY DATA SHEET according to Regulation (EC) No. 1907/2006
 Revision Date 15.03.2013 Version 11.74 SECTION 1. Identification of the substance/mixture and of the company/undertaking 1.1 Product identifier Buffer solution (citric acid/sodium hydroxide/hydrogen chloride),
Revision Date 15.03.2013 Version 11.74 SECTION 1. Identification of the substance/mixture and of the company/undertaking 1.1 Product identifier Buffer solution (citric acid/sodium hydroxide/hydrogen chloride),
Guidance for NORM industrial activities on how to comply with the radioactive substances exemption regime
 Exemption guidance Guidance for NORM industrial activities on how to comply with the radioactive substances exemption regime February 2013 Version 1 Radioactive Substances Act 1993 The Environmental Permitting
Exemption guidance Guidance for NORM industrial activities on how to comply with the radioactive substances exemption regime February 2013 Version 1 Radioactive Substances Act 1993 The Environmental Permitting
Regulations regarding coal ash utilisation in Europe
 Workshop on Environmental and Health Aspects of Coal Ash Utilization International workshop 23 rd 24 th November 2005 Tel-Aviv, Israel Regulations regarding coal ash utilisation in Europe Hans-Joachim
Workshop on Environmental and Health Aspects of Coal Ash Utilization International workshop 23 rd 24 th November 2005 Tel-Aviv, Israel Regulations regarding coal ash utilisation in Europe Hans-Joachim
National TFS Office, Dublin City Council [NTFSO] Guidance for Completing Notification Document (Annex 1A) & Movement Document (Annex 1B)
![National TFS Office, Dublin City Council [NTFSO] Guidance for Completing Notification Document (Annex 1A) & Movement Document (Annex 1B) National TFS Office, Dublin City Council [NTFSO] Guidance for Completing Notification Document (Annex 1A) & Movement Document (Annex 1B)](/thumbs/75/72492936.jpg) National TFS Office, Dublin City Council [NTFSO] Guidance for Completing Notification Document (Annex 1A) & Movement Document (Annex 1B) Introduction A planned shipment subject to the procedure of prior
National TFS Office, Dublin City Council [NTFSO] Guidance for Completing Notification Document (Annex 1A) & Movement Document (Annex 1B) Introduction A planned shipment subject to the procedure of prior
Konferenz Stahl und Recycling Ressourcenpolitik der EU
 Konferenz Stahl und Recycling Ressourcenpolitik der EU Ugo Miretti Unit ENTR F3 Raw Materials, Metals, Minerals and Forest-based industries DG ENTERPRISE AND INDUSTRY Berlin, 12 November 2013 1 1. The
Konferenz Stahl und Recycling Ressourcenpolitik der EU Ugo Miretti Unit ENTR F3 Raw Materials, Metals, Minerals and Forest-based industries DG ENTERPRISE AND INDUSTRY Berlin, 12 November 2013 1 1. The
Petroleum Products in the REACH Regulation Klaas den Haan, Stewardship Conference, SCL 1 June 2015
 ENVIRONMENTAL SCIENCE FOR THE EUROPEAN REFINING INDUSTRY Petroleum Products in the REACH Regulation, Stewardship Conference, SCL 1 June 2015 Disclaimers Considerable efforts have been made to assure the
ENVIRONMENTAL SCIENCE FOR THE EUROPEAN REFINING INDUSTRY Petroleum Products in the REACH Regulation, Stewardship Conference, SCL 1 June 2015 Disclaimers Considerable efforts have been made to assure the
Version 1.0 Revision Date Print Date
 1. Identification of the substance/mixture and of the company/undertaking 1.1 Product identifier Commercial Product Name : Mat.-No./ Genisys-No. : 03707580001 1.2 Relevant identified uses of the substance
1. Identification of the substance/mixture and of the company/undertaking 1.1 Product identifier Commercial Product Name : Mat.-No./ Genisys-No. : 03707580001 1.2 Relevant identified uses of the substance
RECYCLING vs. REUSE ENVIRONMENTAL BENEFITS OF RE-CYCLING VERSUS RE-USE CORRUGATED BOARD PACKAGING AS ILLUSTRATION
 RECYCLING vs. REUSE ENVIRONMENTAL BENEFITS OF RE-CYCLING VERSUS RE-USE CORRUGATED BOARD PACKAGING AS ILLUSTRATION Recycling and reuse of packaging materials should have equal status in European legislation.
RECYCLING vs. REUSE ENVIRONMENTAL BENEFITS OF RE-CYCLING VERSUS RE-USE CORRUGATED BOARD PACKAGING AS ILLUSTRATION Recycling and reuse of packaging materials should have equal status in European legislation.
Health Care Waste Management - To Reduce the Burden of Disease, Health- Care Waste Needs Sound Management, Including Alternatives to Incineration
 Health Care Waste Management - To Reduce the Burden of Disease, Health- Care Waste Needs Sound Management, Including Alternatives to Incineration Fact Sheet No. 281 August 2004 In the last few years there
Health Care Waste Management - To Reduce the Burden of Disease, Health- Care Waste Needs Sound Management, Including Alternatives to Incineration Fact Sheet No. 281 August 2004 In the last few years there
Securing Supply of Resources for European Industries. C. Hebestreit
 Securing Supply of Resources for European Industries C. Hebestreit Minerals: how much do we use? A CAR contains up to 100-150 kg of minerals in rubber (talc, calcium carbonate), plastics (talc, calcium
Securing Supply of Resources for European Industries C. Hebestreit Minerals: how much do we use? A CAR contains up to 100-150 kg of minerals in rubber (talc, calcium carbonate), plastics (talc, calcium
REACH and RoHS updates for EU and Asia Pacific countries Mike McNally Antea Group
 REACH and RoHS updates for EU and Asia Pacific countries Mike McNally Antea Group Agenda Overview REACH RoHS Related regulations Discussion: Key Risks Key Considerations Overview We have witnessed an unprecedented
REACH and RoHS updates for EU and Asia Pacific countries Mike McNally Antea Group Agenda Overview REACH RoHS Related regulations Discussion: Key Risks Key Considerations Overview We have witnessed an unprecedented
FEDERAL REGULATION OF DISPOSAL OF COAL COMBUSTION WASTE FROM COAL FIRED POWER PLANTS
 FEDERAL REGULATION OF DISPOSAL OF COAL COMBUSTION WASTE FROM COAL FIRED POWER PLANTS COST ISSUES AFFECTING STATE ENVIRONMENTAL PROGRAMS BACKGROUND Federal Regulation of Disposal of Coal Ash Coal combustion
FEDERAL REGULATION OF DISPOSAL OF COAL COMBUSTION WASTE FROM COAL FIRED POWER PLANTS COST ISSUES AFFECTING STATE ENVIRONMENTAL PROGRAMS BACKGROUND Federal Regulation of Disposal of Coal Ash Coal combustion
Chapter VII HAZARDOUS WASTE REGULATION. Hazardous wastes are governed by the regulatory program established by the federal
 Chapter VII HAZARDOUS WASTE REGULATION Hazardous wastes are governed by the regulatory program established by the federal Resource Conversation and Recovery Act ( RCRA ), 42 U.S.C. 6901, et seq., and its
Chapter VII HAZARDOUS WASTE REGULATION Hazardous wastes are governed by the regulatory program established by the federal Resource Conversation and Recovery Act ( RCRA ), 42 U.S.C. 6901, et seq., and its
WIND POWER TARGETS FOR EUROPE: 75,000 MW by 2010
 About EWEA EWEA is the voice of the wind industry actively promoting the utilisation of wind power in Europe and worldwide. EWEA members from over 4 countries include 2 companies, organisations, and research
About EWEA EWEA is the voice of the wind industry actively promoting the utilisation of wind power in Europe and worldwide. EWEA members from over 4 countries include 2 companies, organisations, and research
