All solid-state Li-ion batteries based on intercalation electrodes and poly (ethylene oxide)-lix electrolytes
|
|
|
- Clifford Gilmore
- 6 years ago
- Views:
Transcription
1 Res.Rep.Fac.Eng.Mie.Univ.,Vol.3,pp (25) 1 Original Paper All solid-state Li-ion batteries based on intercalation electrodes and poly (ethylene oxide)-lix electrolytes Y. Liu a, Y.Ono b, T. Matsumura b, A. Hirano b, T. Ichikawa b,. Imanishi b and Y. Takeda b ( a Satellite Venture Business Laboratory, b Department of Chemistry for Materials) (Received September 2, 25) Abstract All-solid state rechargeable Li-ion batteries based on poly (ethylene oxide) electrolytes are promising power sources for large application such as electric vehicles (EV), which could be likely to require safety and reliability as well as high energy density and long cycle life. However, so far, state-of-the-art electrode materials are limited by the performance factors such as electrochemical instability to lithium insertion/extraction, particularly at the solid/solid interface between the electrodes and the PEO electrolytes. The efforts on conquering above dilemma have been carried out over several decades. Recently, we developed novel or modified insertion type electrodes, which demonstrate good electrochemical behavior with the PEO electrolytes at the elevated temperature. In this paper, preparation and characterization of the electrodes proposed are presented and discussed in detail. Key words: Power sources; Rechargeable lithium ion batteries; All solid-state PEO electrolytes; Insertion electrodes; Energy density; Thermal safety. 1. Introduction Solid-state rechargeable batteries, especially, lithium ion batteries, are principle and promising power sources for a wide variety of electronics. PEO electrolyte formed by dissolving a lithium salt in a host poly (ethylene oxide) (PEO) shows high ionic conductivity (σ >1 4 S cm 1 ) at the elevated temperature. Rechargeable lithium batteries based on the PEO electrolytes have been proposed to overcome low energy density of previously used batteries such as lead acid and nickel metal hydride and to improve safety of conventional lithium-ion batteries with an inflammable liquid electrolyte [1]. The PEO type cells have several benefits such as feasible design and ductile morphology which can absorb volume change for Li insertion/extraction in electrodes. Although the high temperature limitation (>6 ) of the PEO cell is a drawback for applications in the consumer electronic market, it is probably an advantage for numerous other applications such as for electric vehicles (EV). For these large size batteries in EV, safety and reliability are the most important issues. Previously developed lithium polymer batteries contain lithium metal anodes. A lithium battery incident at Los Angeles International Airport on 28 April, 1999 suggests that safety of a lithium battery with a large amount of lithium metal is questionable [2]. In addition, an improvement on the ionic conductivity and Li + transfer number has to be done to achieve a relatively high charge rate. Therefore, so far, research has been extensively concentrated on two major points such as 1) the improvement of ionic conductivity of PEO-LiX complex at the relatively low temperature and 2) searching for novel or modified electrodes which can deliver good interfacial compatibility with the solid PEO electrolytes. By this motivation, in the anode side, researchers have undertaken to replace the lithium metal by a safer one, such as insertion anode, with a high capacity and an excellent reversibility. Lithium-insertion hosts such as carbon, Li-alloy and Li-M-O are promising alternative anodes [3, 4]. Unfortunately, carbon still cannot be adopted successfully with the PEO electrolytes due to the interfacial incompatibility, regardless of its wonderful electrochemical manners in the liquid system. On the other hand, some Li-M-O materials, e.g., spinel Li 4 Ti 5 O 12, demonstrate zero-strain effects for Li-insertion, resulting in long cycling life with the solid PEO electrolytes. However, relatively low capacity and high Li reactive potentials of lithium titanates make them incomparable in the energy density. On the other hand, in the cathode side, the most common cathodes such as LiCoO 2, LiiO 2, LiCo x i 1 x O 2, and LiMn 2 all suffer from side reaction with PEO electrolytes and detrimental phase transitions above 7 that inevitably cause to capacity loss and fade while being oxidized at a high potential. Meanwhile, state-of-the-art electrode materials are limited by the performance factors such as electrochemical instability to
2 2 Y. Liu, Y.Ono, T. Matsumura, A. Hirano, T. Ichikawa,. Imanishi and Y. Takeda lithium insertion/extraction, particularly at the interface between the electrodes and the PEO electrolytes. Therefore, development in the electrode materials in associated with the PEO electrolytes would be urgent. 2. Experimental 2.1 Materials preparations To produce the hexagonal lithium transition-metal nitrides, a given ratio of Li 3 and powders of transition metals was homogeneously mixed in an Ar atmosphere. The mixture was pressed into a tablet with 8 mm in diameter and 5-8 mm in thickness and heated at 7 for 12 h under 2 stream with heating rate of 35 min -1. The reactions were allowed cool down to room temperature normally. For the compounds containing Fe, the heating temperature was increased to 8. The resulting products were ground in a glove box and further treated by high-energy mechanical milling (HEMM) at a rotational speed of 5 rpm for 2 h. The modification for the graphitic carbon was as follows: a given amount of graphitic carbon was dried under vacuum at high temperature, and then was mixed with metallic lithium and selected organic solvent under a certain ratio under inert atmosphere. The mixture was treated by high energy mechanical milling under inert atmosphere. The milled product was further dried under high temperature to entirely remove the residual organic solvent. Ultrafine SnSb alloy powder (particle size <.2 µm) was prepared by chemical precipitation from aqueous solutions containing the reductive agent abh 4 and respective metallic salts in the presence of complexants such as citrates. The Co 3 powder (< 2 µm) was obtained by a thermal decomposition of CoCO 3 at 8 in air. Lii.8 Co.2 O 2 (ca. 1 µm) was prepared from the mixture of Li 2 O 2 (Aldrich) and (i.8 Co.2 )O precursor which was obtained from the decomposition of the hydroxide at 3 for 1 h. The mixture was heated at 7 for 24 h under O 2 gas flow. LiFeP and LiFeP /C (PVC as carbon sources) was prepared as follows: a certain amount of Li 2 CO 3, poly (vinyl chloride) (PVC), H 4 H 2 P and FeC 2 was homogeneously mixed by the aid of THF. The mixture was heated firstly at 35 for 3-6 h and smashed at room temperature. The smashed products were further heated at 7-9 for 16 h under Ar. The final samples were ground and sieved. Powder X-ray diffraction (XRD) patterns were obtained using automated powder diffractometer with Cu K α radiation (Rotaflex RU-2B, Rigaku-denki Corporation). The morphological performance of the materials was characterized with scanning electron microscopy (SEM) and Electron Probe Micro-Analysis (EPMA). 2.2 PEO Electrolytes and cathode film PEO electrolytes (Li/O ratio: 1/18) were prepared under a casting technique. All the procedure was protected by Ar atmosphere in the glove box. A given weight of PEO (MW = ) and Li(CF 3 SO 2 ) 2 was dissolved completely in anhydrous acetonitrile (A). BaTiO 3 (ca..1 µm) was dispersed homogeneously in the solution as filler. After strong stirring overnight the viscous solution was cast into a Teflon dish. After A was slowly and completely evaporated under a 2 flow, the obtained film was further dried at 9 under vacuum at least 8 h. The conductivities of the composite polymer electrolyte were observed as high as 1.7x1-3 S cm -1 at 8 and.82x1-3 S cm -1 at 65 and the decomposition potential was estimated to be more than 4.2 V (vs. Li/Li + ). The cathode electrode consisting of 52 wt.% active hosts (LiFeP or Lii.8 Co.2 O 2 ), 1 wt.% acetylene black(ab) and 38 wt.% PEO-Li(CF 3 SO 2 ) 2 (Li/O ratio: 1/18) were prepared by the casting method similar to PEO film and the thickness was about 2 µm. 2.3 Anode preparations For all the electrodes, a given weight of the electrode components was homogeneously mixed in an agate mortar in a glove box and further pressed onto a 3-mesh stainless steel grid, which served as a current collector. The geometric area of the electrodes was.55 cm 2, and the typical thickness was 1~16 µm. 2.4 Electrochemical and thermal stability (DSC) measurements To evaluate the electrochemical properties of the electrodes, a half-cell containing Li metal as the counter and LiPF 6 / EC+DMC (Ethylene carbonate plus diethyl carbonate as 1:1 in volume) electrolytes (in case of the cells with the liquid electrolytes) was used. Basically all the three layers, including test electrode, separator and Li metal, were stacked in a 225 coin type cell in a glove box. For the cells based on the PEO electrolyte, the separator was replaced by the PEO film, which also served as electrolyte. The full solid-state cell was constituted by replacing lithium metal with the cathode films. A small constant pressure was kept inside cells by means of the i foam as filler. Before the electrochemical test, the PEO based cells were preheated for 2 hrs at a temperature of 75. Unless stated elsewhere, the cells were carried out at a constant current density of ma cm -2. The rest time between charge and discharge was 1 min.
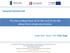
3 All solid-state Li-ion batteries based on intercalation electrodes and poly (ethylene oxide)-lix electrolytes 3 3. Results and discussion 3.1 Anodes a) Cycle b) Cycle 1 1) Li 2.6 Co.4 2) Li 2.6 Co.2 Cu.2 3) Li 2.6 Co.2 i.15 Fe.5 4) Li 2.6 Co.2 Cu.15 Fe Fig.1. Charge and discharge curves of the electrodes based on lithium transition-metal nitrides at the first and tenth cycle, voltage cutoff: -1.4 V, vs. Li/Li +. Electrode composition: 2 wt.% AB, 7 wt.% lithium metal nitrides and 1 wt.% PVDF. Dis. capacity / mah g Li 2.6 Co.4 Li 2.6 Co.2 Cu.2 Li 2.6 Co.2 i.15 Fe.5 Li 2.6 Co.2 Cu.15 Fe Cycle number Fig.2. Cycling performance of the electrodes based on lithium transition-metal nitrides. Electrode compositions are the same as in Fig Electrochemical behavior and thermal stability of the composite electrodes. Lithium metal (transition) nitrides with hexagonal symmetry, P6/mmm, are composed of M (M=Co, Cu, i) substituting lithium between the Li layers of Li 3 [5,6]. Fig.1 shows the electrochemical behavior of the lithium transition-metal nitrides prepared from solid-state reaction and high-energy mechanical milling (HEMM). Due to the vacant sites introduced by the doped transition metals, the fully lithiated nitrides can still allow a small amount of lithium intercalation in the first cycle. The compounds gradually undergo an irreversible transformation from a crystalline phase to an amorphous one in the first Li-extraction stage, as shown in Fig.1a. Compared with Li 2.6 Co.4, the slight increase of the co-doped nitrides in the charge potentials indicates that part Co substituted by Cu, i and Fe may be obstructive to the lithium extraction [7,8]. In the subsequent cycle shown in Fig.1b, the charge potentials become slope and a large amount of lithium can be reversibly re-intercalated and extracted, resulting in high capacities of 7-95 mah g -1 which are about 2-3 times over that of the commercial graphite. The discharge potential curves, on the other hand, demonstrate a hystersis of ca..5 V compared with the charge ones, indicating the different electrochemical kinetics for Li intercalation and extraction. A comparison with Li 2.6 Co.4 in the cycling performance reveals that the co-doped compounds have a remarkably enhanced cyclability shown in Fig.2. The significant improvement in the cycling performance for the co-doped nitrides can be attributed to the improved structural stability in association with the Li extraction degree and the enhanced interfacial compatibility [7,8]. Lithium metal nitrides are very attractive in the packing density and volumetric capacity due to the density similar to the current graphite. A major hindrance to the nitrides as promising anode alternatives for graphite is that they possess Li-rich structure and therefore cannot directly combine with the typically high-potential cathodes such as LiCoO 2 and LiMn 2 to constitute Li-ion cells. This deterrent can be overcome by introducing an appropriate amount of Co 3 into the electrodes containing above lithium metal nitrides. Fig.3 shows charge and discharge curves of the a) Co 3, b) Li 2.6 Co.2 Cu.2 and c) Co 3 -Li 2.6 Co.2 Cu.2 based electrodes vs. lithium under a potential of -1.4 V with liquid electrolytes at room temperature. The Co 3 electrode has a large insertion capacity of 115 mah g -1 along with a potential plateau of about 5 V in the first cycle. However, its capacity retention in the subsequent cycle shown in Fig.3a is below to 18 mah g -1 under the potential range of
4 4 Y. Liu, Y.Ono, T. Matsumura, A. Hirano, T. Ichikawa,. Imanishi and Y. Takeda -1.4V. This is attributed that this material has to be electrochemically activated under a very wide potential as -3 V [9]. The electrochemical behavior of the Li 2.6 Co.2 Cu.2, which has a high extraction capacity as 8 mah g -1 with a potential plateau of V but an insertion capacity low as 23 mahg -1, is obviously in contrast to that of the Co 3, as shown in Fig.3b. In the case of Li 2.6 Co.2 Cu.2 plus Co 3, a very high first charge recovery of ca. 1% shown in Fig3c is remarkable while the cycling is started from an insertion process. Moreover, the composite electrode does not show an obvious potential plateau in the charge stage that reflects the coexistence of two phases in the active hosts similar to that of the Li 2.6 Co.2 Cu.2. Thus, it comes to conclusion that Li 2.6 Co.2 Cu.2 in the composite electrode might be discharged from a delithiated or an amorphous state. The Li-intercalation potential of Co 3 (ca.1.1v) is slightly higher than the Li-extraction potential of lithium metal nitride (ca.v); therefore Co 3 can thermodynamically extract lithium from the nitrides. XRD measurements confirm this point because lithium transfer into Co 3 would be evidenced by a change in the peak intensities for both the hosts. We proposed the reaction mechanism of the composite electrode as follows: Li 2.6 Co.2 Cu.2 + xco 3 Li 2.6-8x Co.2 Cu.2 + 4xLi 2 O + 3xCo (1) a. Co 3 Li extraction b. Li 2.6 Co.2 Cu.2 Li extraction Li insertion c. Li 2.6 Co.2 Cu.2 -Co 3 Li insertion Li extraction Li insertion Cycle 1 Cycle 2 I / ma c. Co 3 cycle 2 cycle b. Li 2.6 Co.2 Cu.2 Cycle 1 Cycle c. Li 2.6 Co.2 Cu.2 -Co 3 Cycle 2 Cycle E / V (Left) Fig.3. Charge and discharge curves of different cells based on a) Co 3, b) Li 2.6 Co.2 Cu.2 and c) Co 3 -Li 2.6 Co.2 Cu.2, voltage cutoff: -1.4 V, vs. Li/Li +. Electrode compositions: a) 8 wt.% Co 3, 1 wt.% AB and 1 wt.% PVDF; b) 7 wt.% Li 2.6 Co.2 Cu.2, 2 wt.% AB and 1 wt.% PVDF and c) 5 wt.% Li 2.6 Co.2 Cu.2, 3 wt % Co 3, 1 wt.% AB and 1 wt.% PVDF. (Right) Fig.4. Cyclic voltammogram of the electrode based on a) Co 3, b) Li 2.6 Co.2 Cu.2 and c) Co 3 -Li 2.6 Co.2 Cu.2 at the scan rate of.5 mv S -1, voltage cutoff: V, vs. Li/Li +. Electrode composites are the same as in Fig.3. Li 2.6-8x Co.2 Cu.2 + zli + + ze - = Li 2.6-8x+z Co.2 Cu.2 (2). Reaction (1) is a chemical reaction and reaction (2) is an electrochemical one. If Li 2.6-8x Co.2 Cu.2 is the composition of the fully charged state, then reaction (2) becomes reversible during charge and discharge even at the first cycle. From the experimental results shown in Fig. 3, we estimated x and z as.16 and 1.7, respectively. These values correspond to 6/4 weight ratio of Li 2.6-8x Co.2 Cu.2 /Co 3 and the reversible capacity of 477 mah g -1. The capacity was calculated by including Li 2.6-x Co.2 Cu.2 and Co 3 as active hosts. We observed a first charge efficiency of 1% and a reversible capacity of ca. 52 mah g -1 for the composite electrode containing of 6 wt. % Li 2.6-x Co.2 Cu.2 plus 37.5 wt % Co 3, as shown in Fig. 3c. The high first cycle efficiency proposed can greatly extend the capacity utilization for the cathode. It is very remarkable because the low charge efficiency of the anodes in the first cycle might consume extra capacity from cathodes and thereby
5 All solid-state Li-ion batteries based on intercalation electrodes and poly (ethylene oxide)-lix electrolytes 5 inevitably reduce the total energy density of the cells. Cyclic voltammogram was further conducted on the electrodes based on different active hosts with a scan rate of.5 mv s -1 in the potential scale of -1.4 V vs. Li/Li +, as shown in Fig.5. There is one cathodic peak at about.95 V in the first Li-insertion process for Co 3 (Fig.4a. However, this peak does not repeat in the subsequent cycle, indicating that Co 3 turns into inert to lithium. In the case of Li 2.6 Co.2 Cu.2, an obvious anodic peak shown in Fig.4b appears at 1.2 V in the first cycle that corresponds to the two-phase transformation. In the subsequent cycle, one cathodic peak at.18 V and two anodic peaks at.78, 1.12 V become visible and remain to be stable. However, the large hystersis in the cathodic and anodic processes seems a drawback and has to be further conquered. In comparison with Li 2.6 Co.2 Cu.2 and Co 3, obviously, the behavior of the composite electrode shown in Fig.4c is highly consistent with that of Li 2.6 Co.2 Cu.2, suggesting that the active host in the composite is mainly associated with lithium metal nitrides. Furthermore, a weak cathodic peak at.95 V corresponding to the Co 3 indicates that the thermodynamically spontaneous reaction may not entirely drive lithium into the Co 3 before the electrochemical cycling. The composite electrodes demonstrated high cycling stability, due to that they could be benefited from the low volume effects of the lithium metal nitrides upon Li insertion and extraction. As measurement, the charge yield of the composite electrode during cycling is always at 1%, indicating that Co 3 remains inactive and stable in the electrode while lithium in the nitrides can be electrochemically intercalated and extracted with high reversibility under a suitable potential window. The composite electrode consisting of Li 2.6-x Co.2 Cu.2 -Co 3 was found to show good electrochemical behavior with the solid PEO electrolytes. A high charge efficiency of 99.6 % and a large capacity of 52 mah g -1 shown in Fig.5 become feasible, indicating that lithium can be electrochemically.5. c ) η 1 = 13 % Cycle 1 Cycle 2 Cycle 3.5. b ) η 1 = 99.6% Cycle 1 Cycle 2 Cycle 3 (Left) Fig.5. Charge and discharge profiles of the SnSb-Li 2.6 Co.4 electrode with PEO at 65 o C. (Right) Fig.6. Charge and discharge profiles of the Co 3 -Li 2.6 Co.2 Cu.2 electrode with PEO at 65 o C a. SnSb-Li 2.6 Co.4 b. Co 3 -Li 2.6 Co.2 Cu Cycle number Filled: Li insertion Open: Li extraction Fig.7. Cycling performance of the composite anodes with PEO electrolytes. intercalated into and extracted from the nitrides with high reversibility while operating with the PEO electrolytes. On the other hand, the composite electrode based on SnSb and Li 2.6 Co.4 also demonstrates a high first charge efficiency of 13 %, which is attributed to the fact that lithium metal nitrides can make a capacity compensation for the ultrafine alloy hosts in the first cycle [1]. As can be seen, the charge-discharge behavior of the SnSb-Li 2.6 Co.4 composite electrode reflects the mixing electrochemical characteristics of these two types of active hosts, as shown in Fig.6. The capacity changes, as a function of cycling, for different anodes with the solid PEO electrolytes are shown in Fig. 7. The SnSb-Li 2.6 Co.4 anode has a large capacity of over 6 mah g -1 in the cycling beginning. However, a gradual deterioration in the cycling performance is always observed. A replacement of Li 2.6 Co.4 with the co-doped nitrides proposed does not bring any progress, indicating that the volume effect from the Li-alloying reaction possesses a morphological instability at the interface while operating with the solid-state PEO electrolytes.
6 6 Y. Liu, Y.Ono, T. Matsumura, A. Hirano, T. Ichikawa,. Imanishi and Y. Takeda Reaction heat / J g -1 Heat Flow / mw mg A:charged to.6 V B:charged to.3 V C:charged to.1 V D:charged to.1 V E: Li/polymer A:charged to.6 V B:charged to.3 V C:charged to.1 V D:charged to.1 V E: Li/polymer b. Co 3 -Li 2.6 Co.2 Cu Temperature / o C a. SnSb-Li 2.6 Co.4 Fig.8. DSC curves of the composite anodes of a) SnSb-Li 2.6 Co.4 and b) Li 2.6 Co.2 Cu.2 -Co 3 with various Li intercalation states (Heating rate: 5 o C/min) a b Metallic lithium Co 3 -Li 2.6 Co.2 Cu.2 SnSb-Li 2.6 Co.4.6 Charged state vs. Li/Li + / V The practical reaction heat for Li/polymer The theoretical reaction heat for Li/polymer Specific reaction heat / J mah -1 By using the Li 2.6-x Co.2 Cu.2 -Co 3 anode, a significantly improved cycling performance was obtained with a high capacity of 5 mah g -1. The charge-discharge efficiency is almost 1% and the capacity fade during cycling is estimated to be only about.37%/cycle. The capacity retention can be further improved by decreasing moisture in the PEO electrolytes since hexagonal lithium metal nitrides suffer from a high sensitivity with H 2 O. The safety of small size lithium-ion cells under normal use is well established. In contrast, the safety of the large size lithium-ion batteries is still questionable, especially in cases of abusive use. Safety of lithium ion batteries is mainly related to the thermal reactivity of the components. Fig.8 shows differential scanning calorimetric curves (DSC) for the composite anodes of Li 2.6 Co.4 -SnSb (a) and Li 2.6 Co.2 Cu.2 -Co 3 (b) with the PEO electrolytes, respectively, as a function of the charged state along with that for Li metal with the PEO electrolytes (1:1 weight ratio) as a reference. For the Li 2.6 Co.4 -SnSb anode, the reaction heat strongly depends on the charged state. The reaction heat is very low for the composite anode charged up to.3 V and appreciable for that charged up to.1 V, as shown in Fig.8a. An endothermic peak in the anode charged to.1 V is observed at 18 o C in the DSC curve, which corresponds to the fusion of lithium metal. That is, the high level insertion of lithium into the Li 2.6 Co.4 -SnSb composite exhibits a lithium metal deposition. On the other hand, the reaction heat of the Li 2.6 Co.2 Cu.2 -Co 3 anode shows exothermic peaks at 25~32 o C, which do not depend on the charged state, as shown in Fi.8b. Fig.9 shows the reaction heat (a) and specific reaction heat (b) of the different anodes at various charged states. The reaction heat of the anode should be compared in terms of energy per discharged capacity, i.e., J mah -1 (a specific reaction heat). Specific reaction heats for the Li 2.6 Co.2 -SnSb anode charged up to.1v vs. Li/Li + and.1 V vs. Li/Li + are.82 J mah -1 and 2.75 J mah -1, respectively. Those for the Li 2.6 Co.2 Cu.2 -Co 3 anode are estimated to be 1.1 J mah -1 compared to that of 1.24 J mah -1 in lithium metal and PEO electrolyte (1:1 weight ratio). In practice, in the case of a lithium metal anode, an excess amount of lithium metal, at least four times compared to the cathode capacity, Fig.9. Reaction heat vs. charged state by a) J g -1 and should be used because of the dendrite formation on b) J mah -1 for different anodes at 65 the anode. Therefore, the reaction heat could be estimated to reach 4.96 J mah -1 or higher. Fig.1 further shows the typical charge and discharge profiles of the full cells based on the Lii.8 Co.2 O 2 cathode and the different anodes of lithium metal, SnSb-Li 2.6 Co.4 and Li 2.6 Co.2 Cu.2 -Co 3, respectively, while operating with the PEO electrolytes at 65. The Lii.8 Co.2 O 2 /Li cell demonstrates a high working potential of V with a capacity of 12 mah g -1, as shown in Fig.1a. Replacing the lithium metal with the insertion anodes could result into a loss in the working potential but an improvement in the safety. In general, for full utilization of lithium storage capacity of the composite anodes, the weight of cathodes has to be much over that of anodes at about 4~5 times. As can be seen from Fig.1b, the high similarity in the potential-capacity trends for the Lii.8 Co.2 O 2 /Li 2.6 Co.2 Cu.2 -Co 3 cell indicates that the amorphous lithium metal nitrides remain high
7 All solid-state Li-ion batteries based on intercalation electrodes and poly (ethylene oxide)-lix electrolytes a b c Voltage cutoff: V Voltage cutoff: V Voltage cutoff: V Fig.1. Charge and discharge profiles of the full cells based on Lii.8 Co.2 O 2 cathode and anode of a) Li, b) Co 3 -Li 2.6 Co.2 Cu.2 and c) SnSb-Li 2.6 Co.4. Electrode compositions of b) and c) are the same as in Fig.9. stability from cycle to cycle. On the other hand, the voltage plateau of the Lii.8 Co.2 O 2 /SnSb-Li 2.6 Co.4 cell slightly increases from the first to the following cycles, which probably is attributed to the two-phase transformation of Li 2.6 Co.4 between the crystal and the amorphous, as shown in Fig.1c. The high initial coulombic efficiency of over 95 % and the large reversible anode capacity above 45 mah g -1 are very remarkable for both the composite anodes, which might be highly favorable for realizing a rather high energy density and can be considered as an ideal power source for HEV & EV. However, bearing in mind that the morphological instability from the Li-alloy host is still questionable, continuous research would be pursued to develop the lithium metal nitrides-co 3 based composite electrodes proposed with low volume effects Modified graphitic carbon as insertion anodes. From the application point of view, the first approach for lithium metal-free anode is to use graphite, as is accomplished in lithium-ion batteries with organic solvent electrolytes. The charge and discharge profile of mesocarbon microbead (MCMB, morphology as shown in Fig.11), a typical graphitic carbon used in the current lithium-ion cells, is shown in Fig.12. The very low charge efficiency of 29.7 % in the first cycle indicates that side reactions occur both in the reduction and oxidation processes which lead to some uncertainty in the determination of the maximum reversible capacity of the graphitic carbon. Consequently, its reversible capacity is.5 a) η 1 = 29.7% Cycle 1 Cycle 2 Cycle 1 Cycle 2 Cycle (Left) Fig.11 SEM images of the MCMB particles. (Right) Fig.12. Charge and discharge profiles of the MCMB electrode with PEO electrolytes at 7 below to 15 mah g -1 and the charge and discharge efficiency in the first several cycles is extremely low. Poor solid/solid interface of the graphite with the PEO electrolytes indicates that structural and interfacial performance has to be improved. To make the graphitic carbon operate well with PEO electrolytes, we design an ionic conducting LiC x layers by directly reacting the opened C-atom with lithium under special conditions (such as high pressure and low temperature) on the surface of the graphitic carbon that can prevent the side reaction Fig.13. Structural images of the modified graphitic carbon.
8 8 Y. Liu, Y.Ono, T. Matsumura, A. Hirano, T. Ichikawa,. Imanishi and Y. Takeda Intensity / a.u θ/θ 2:1(C:Li,mol ratio) 8:1 1:1 2:1 24:1 natural Fig.14 XRD of the modified MCMB under different Li/C ratio. in the interface between the electrode and the PEO electrolyte, as shown in Fig.13. Due to high Li-reactivity of its surface, MCMB is chosen and treated by high energy ballmilling with lithium metal by the aid of dodecane ({CH 3 (CH 2 ) 1 CH 3 }) under Ar. The high energy balmilling can make the surface of MCMB become disordered and therefore surface C-atom become open and further can react with Li. Fig.14 shows that the typical crystalline structure of the treated MCMB remains to be unchanged which can permit lithium reversible insertion and extraction. XPS results as shown in Table 1 confirmed the formed LiC x layer on the graphite surface. TEM observation shown in Fig.15 further reveals that the MCMB loss crystal on its surface upon treatment, indicating that the formed LiC x is prevailed by amorphous or micro-crystalline phase. Cyclic voltammogram measurement as shown in Fig.16 indicates that the side Fig.15. TEM images on the surface of the MCMB at left) without modification and right) after modification. irreversible reaction in the first cycle is highly suppressed after the treatment, which is favorable for the electrochemical kinetics during lithium insertion into and extraction from the layered carbon. Finally, Fig.17 shows that the modified MCMB with PEO electrolytes presents an attractive behavior similar to that based on the common liquid electrolytes. Li intercalation into the graphitic hosts possesses several stages below to 2 mv. However, in the real battery testing, only one potential plateau between.2- V can be distinguished, which is correspond to the LiC x compound reaction in an equilibrium phase. The capacity as a function upon the cycles also demonstrates high stability, as shown in Fig.18. The reversible capacity is measured to be about 29 mah g -1, which is very comparable and close to the theory data (LiC 6 ). Furthermore, we compared the thermal stability of the MCMB with metallic lithium while operating with the PEO electrolytes via DSC measurement, as shown in Fig.19. MCMB shows very low reaction heat under high Li utilization with the PEO electrolytes in comparison with lithium metal anode. Such a thermal behavior is highly opposite to the one based on liquid electrolytes, in which the heat generation of graphite is very significant, especially, that of the reaction heat of PVDF and LiC x [11]. This result indicates that graphitic carbon might be a suitable anode candidate in large size rechargeable lithium-ion batteries based on PEO electrolytes for EV in terms of structural stability and thermal reliance stability. Table.1. XPS results of the MCMB with and without treatment. Li 1s C 1s F 1s O 1s Without Binding energy 285 ev, 533. ev, treatment Chemical composition 97.81% 2.19% With Binding energy 56.1 ev, 291 ev, ev treatment Chemical composition 2.23 % 37.5 % 6.2 %
9 All solid-state Li-ion batteries based on intercalation electrodes and poly (ethylene oxide)-lix electrolytes 9 Fig.16. Cyclic voltammogram of the electrode based on a) no modified MCMB, b) Li 2.6 Co.2 Cu.2 and c) Co 3 -Li 2.6 Co.2 Cu.2 at the scan rate of.5 mv S -1, voltage cutoff: V, vs. Li/Li Cathode The selection for proper cathodes is according to the following requirements, such as inexpensive, environmentally benign, wide operating temperature, good capacity/cycling performance, extremely safe. Transition metal oxides, such as LiCoO 2, layered LiiO 2 and LiMn 2 have found application as positive electrode materials for high power applications. These materials provide high potentials (about 4 V vs. Li) and good reversible capacities (about 12 ma h g -1 ). The use of LiMn 2 solves problems related to cost and Co and i toxicity, but the disproportionation of Mn 3+ remains a severe problem. Pervious research reveals that most of the current cathodes suffers from side reaction with PEO electrolytes and detrimental phase transitions above 7 that inevitably cause to capacity loss and fade while being oxidized at a high potential. In this work, in comparison with several cathode materials, such as Li x CoO 2 (.5<x<1,.5-electron reaction, Cap=137 mah g -1 ), Li x Mn 2 (<x<1, liquid electrolyte liquid electrolyte Capacity / mahg Capacity / mahg -1 Fig.17. Comparison in the charge and discharge profiles of the MCMB based on liquid electrolytes and the modified MCMB based on PEO electrolytes. Capacity / mahg After modification based on PEO liquid electrolytes Without modification based on PEO Cycling number Heat Flow / mw mg Li metal with PEO: 46 J g -1, 1.24 J mah -1 graphite with PEO: 16 J g -1, <.8 J mah Temperature / o C graphite with liquid electrolytes 15 J g -1, 4.3 J mah -1 (Left) Fig.18. Cycling performance of different MCMB electrodes with liquid and PEO electrolytes. (Right) Fig.19. DSC curves of different electrode with PEO and liquid electrolytes. o
10 1 Y. Liu, Y.Ono, T. Matsumura, A. Hirano, T. Ichikawa,. Imanishi and Y. Takeda Weight / mg C-LiFeP LiFeP Temperature / Ž TG DTA Heat Flow / ƒêv intensity / a.u Amorphous 12, Carbon , , C-LiFePO LiFeP θ / deg. Fig.21. XRD of the resulting LiFeP and LiFeP /C powders. Fig.2. TG/DTA curves of the LiFeP with and without PVC during heating to 7..5-electron reaction, Cap=148 mah g -1 ) and Li x FeP (<x<1, 1-electron reaction, Cap=17 mah g -1 ), olivine type LiFeP is very attractive due to its capability to operate within a very flat voltage plateau (around 3.5 V), high thermal stability, high working temperature performance similar to PEO electrolytes, low lattice volume changes, low cost and benign environmental properties [12]. However, a significant drawback of the LiFeP is its poor rate capability caused by poor electron conductivity. In this work, we use poly (vinyl chloride) as carbon sources to produce the LiFeP /C composite by means of two pyrolysis reactions. The electronic conductivity of the LiFeP can be substantially enhanced by dispersing fine particle size of the active hosts in the carbon matrix. With this modification, the LiFeP is capable of providing high capacity and high charge rates. TG/DTA analysis conducted on the product upon the heating process shown in Fig.2 reveals that the LiFeP possesses several decomposition reaction related to the starting materials. The PVC does not cause to a reaction with the LiFeP. The carbon contents of the LiFeP /C was confirmed to be about wt% with the TG analysis. XRD technique confirms the crystalline structure of the composite proposed, as shown in Fig.21. As well, pyrolyzed carbon that is prevailed with disordered structure can be found in the product. Carbon coating process does not bring a obvious change in the lattice parameters of the LiFeP, as shown in table.2, Fig.22. EPMA images of elemental distribution of the resulting materials. Fig.23. SEM images of the resulting LiFeP /C powders. indicating the thermal pyrolysis mainly causes to a modification in the morphology. The homogeneous distribution of the active hosts within the carbonaceous matrix is further evidenced by the elemental distribution
11 All solid-state Li-ion batteries based on intercalation electrodes and poly (ethylene oxide)-lix electrolytes 11 mapping from Electron Probe Micro-Analysis (EPMA) shown in Fig.22. SEM observation shown in Fig.23 further reveals that the material possesses high porosity which is favorable for increasing the reactive area and therefore the electrochemical kinetics. It suggests that the introduction of highly dispersed PVC has an effect on preventing the single LiFeP growth during heating process. Meanwhile, the possible aggregation of the active hosts during high temperature could be highly barred, resulting in fine grains of the active hosts which favorable for the electrochemical kinetics. Finally, Fig.24 and 25 show that the LiFeP /C electrode presents a stable working potential at about 3.5 V vs.li/li + and a capacity of 12 mah g -1, as well as excellent cycling stability, probably due to the low lattice volume changes with Li insertion/extraction and good interfacial compatibility with the solid PEO electrolytes. The primary results suggest that the modified olivine type LiFeP might be a good cathode candidate for the PEO electrolytes. Table.2. Lattice parameters of the resulting compounds. a-axis b-axis c-axis Cell volume Crystal structure LiFeP Orthorhombic 2C-LiFeP Orthorhombic cycle Capacity / mahg charge capacity discharge capacity Capacity / mahg Cycling number (Left)Fig.24. Typical charge and discharge profiles of LiFeP /C with PEO electrolytes at 65 o C ( V). (Right) Fig.25. Cycling performance of the LiFeP /C with PEO electrolytes at 65 o C. 4. Conclusions 1) Composite anodes based on hexagonal lithium metal nitrides have been developed to show larger capacity and higher first cycle efficiency over the current graphitic anodes. However, the performance of the composite anodes containing Li-alloy hosts is still in doubt due to that the Li-alloy has a morphological instability along with an increased heating generation dependent on Li-insertion. In contrast, the composite anodes containing lithium transition-metal nitrides and Co 3 demonstrate high first charge efficiency of 1 % and large capacity of 5 mah g -1, as well as high cycling stability and low reaction heating under high Li-utilization with the PEO electrolytes, indicating that they possess potential applications in large size rechargeable lithium-ion batteries for EV in terms of high first-cycle charge efficiency, large capacity and high thermal reliance; 2) Graphitic carbon that is currently used in commercial Li-ion batteries can be successfully operated with the PEO electrolytes by designing an amorphous LiC x layer on its surface which can prevent the side reaction. The material treating process is effective and comparable in production. The modified graphitic carbon demonstrates good electrochemical behavior similar to that in liquid electrolytes. Furthermore, high thermal stability of the graphitic carbon with PEO electrolytes under deep Li utilization suggests that the graphitic carbon might be suitable anode candidates in large size rechargeable lithium-ion batteries for EV based on PEO electrolytes in terms of structural stability and thermal reliance; 3) LiFeP /C composite has been prepared by two pyrolysis processes using poly (vinyl chloride) as carbon sources. The material was confirmed to be of high porosity and homogeneous distribution of active hosts within the carbonaceous matrix. Primarily electrochemical results indicate that the olivine type LiFeP /C is a promising cathode candidate in terms of stable working potential, acceptable capacity, high charge/discharge efficiency and excellent cycle life with the PEO electrolytes at the elevated temperature.
12 12 Y. Liu, Y.Ono, T. Matsumura, A. Hirano, T. Ichikawa,. Imanishi and Y. Takeda References [1] Y.Aihara, M.Kodama, K.akahara, H.Okise, K. Murata, J. Power Sources 65, 143 (1997). [2] M.D. Farrington. J. Power Sources 96, 26 (21). [3] D. Fauteux and R. Koksbang, J. Appl. Electrochem. 23, 1 (1993) [4] J. O. Besenhard, J. Yang and M. Winter, J. Power Sources 68, 87 (1997) [5] M. ishijima, T. Kagohashi,. Imanishi, Y. Takeda, O. Yamamoto and S. Kondo, Solid State Ionics, 83, 17 (1996). [6] M. ishijima, T. Kagohashi,. Imanishi, Y. Takeda, O. Yamamoto, J. Power Sources, 68, 51 (1996). [7] Y. Liu, K. Horikawa, M. Fujiyosi,. Imanishi, A. Hirano and Y. Takeda, J. Electrochem. Soc., 151, A145 (24). [8] Y. Liu, T. Matsumura,. Imanishi, T. Ichikawa, A. Hirano, Y. Takeda, Electrochemistry Communications, 6, 632 (24). [9] P. Poizot, S. Laruelle, S. Grugeon, L. Dupont, J-M. Tarascon, ature, 47, 496 (2). [1] J. Yang, Y. Takeda,. Imanishi and O. Yamamoto, J. Electrochem. Soc., 147, 1671 (2) [11] Ph. Biensan, B. Simon, J.P. Peres, A.de. Guiber, M. Broussely, J.M. Bodet and F. Perton, J. Power Sources, 81-82, 96 (1999). [12] A.K. Padhi, K.S. anjundaswamy, J.B. Goodenough, J. Electrochem. Soc. 144 (1997) 1188
Novel negative electrode materials with high capacity density for further rechargeable lithium ion batteries
 Res. Rep. Fac. Eng. Mie Univ., Vol. 29, pp. 65-72 (24) Original Paper ovel negative electrode materials with high capacity density for further rechargeable lithium ion batteries Y. Liu a,. Matsumura a,
Res. Rep. Fac. Eng. Mie Univ., Vol. 29, pp. 65-72 (24) Original Paper ovel negative electrode materials with high capacity density for further rechargeable lithium ion batteries Y. Liu a,. Matsumura a,
Single-crystalline LiFePO 4 Nanosheets for High-rate Li-ion Batteries
 /8 SUPPORTING INFORMATION Single-crystalline LiFePO 4 Nanosheets for High-rate Li-ion Batteries Yu Zhao, Lele Peng, Borui Liu, Guihua Yu* Materials Science and Engineering Program and Department of Mechanical
/8 SUPPORTING INFORMATION Single-crystalline LiFePO 4 Nanosheets for High-rate Li-ion Batteries Yu Zhao, Lele Peng, Borui Liu, Guihua Yu* Materials Science and Engineering Program and Department of Mechanical
Supplementary information. performance Li-ion battery
 Supplementary information The investigation of Ni(OH) 2 /Ni as anode for high performance Li-ion battery Shibing Ni a, Xiaohu Lv a, Tao Li a, Xuelin Yang a,and Lulu Zhang a College of Mechanical and Material
Supplementary information The investigation of Ni(OH) 2 /Ni as anode for high performance Li-ion battery Shibing Ni a, Xiaohu Lv a, Tao Li a, Xuelin Yang a,and Lulu Zhang a College of Mechanical and Material
Methods for Successful Cycling of Alloy
 Methods for Successful Cycling of Alloy Negative Electrodes in Li-ion ion Cells Mark Obrovac, Leif Christensen, Larry Krause, Dinh Ba Le, Jagat Singh, Kevin Eberman, Lowell Jensen, Li Liu, Jehwon Choi,
Methods for Successful Cycling of Alloy Negative Electrodes in Li-ion ion Cells Mark Obrovac, Leif Christensen, Larry Krause, Dinh Ba Le, Jagat Singh, Kevin Eberman, Lowell Jensen, Li Liu, Jehwon Choi,
Electrochemical performance of lithium-rich layered oxides for
 IBA 2013 Electrochemical performance of lithium-rich layered oxides for electric vehicle applications Jay Hyok Song, Andrei Kapylou, Chang Wook Kim, Yong Chan You, and Sun Ho Kang* SAMSUNG SDI Contents
IBA 2013 Electrochemical performance of lithium-rich layered oxides for electric vehicle applications Jay Hyok Song, Andrei Kapylou, Chang Wook Kim, Yong Chan You, and Sun Ho Kang* SAMSUNG SDI Contents
Electronic Supplementary Material (ESI) for Chemical Communications This journal is The Royal Society of Chemistry 2013
 Sodium-ion battery based on ion exchange membranes as electrolyte and separator Chengying Cao, Weiwei Liu, Lei Tan, Xiaozhen Liao and Lei Li* School of Chemical and Chemistry Engineering, Shanghai Jiaotong
Sodium-ion battery based on ion exchange membranes as electrolyte and separator Chengying Cao, Weiwei Liu, Lei Tan, Xiaozhen Liao and Lei Li* School of Chemical and Chemistry Engineering, Shanghai Jiaotong
Lithium Potassium Manganese Mixed Metal Oxide Material for Rechargeable Electrochemical Cells
 Lithium Potassium Manganese Mixed Metal Oxide Material for Rechargeable Electrochemical Cells Terrill B. Atwater 1,2 and Alvin J. Salkind 2,3 1 US Army RDECOM, CERDEC, Ft. Monmouth NJ 2 Rutgers University,
Lithium Potassium Manganese Mixed Metal Oxide Material for Rechargeable Electrochemical Cells Terrill B. Atwater 1,2 and Alvin J. Salkind 2,3 1 US Army RDECOM, CERDEC, Ft. Monmouth NJ 2 Rutgers University,
PVP-Functionalized Nanometer Scale Metal Oxide Coatings for. Cathode Materials: Successful Application to LiMn 2 O 4 Spinel.
 PVP-Functionalized Nanometer Scale Metal Oxide Coatings for Cathode Materials: Successful Application to LiMn 2 O 4 Spinel Nanoparticles Hyesun Lim, Jaephil Cho* Department of Applied Chemistry Hanyang
PVP-Functionalized Nanometer Scale Metal Oxide Coatings for Cathode Materials: Successful Application to LiMn 2 O 4 Spinel Nanoparticles Hyesun Lim, Jaephil Cho* Department of Applied Chemistry Hanyang
Amorphous Metallic Glass as New High Power and Energy Density Anodes For Lithium Ion Rechargeable Batteries
 Amorphous Metallic Glass as New High Power and Energy Density Anodes For Lithium Ion Rechargeable Batteries Meng Ying 1, Li Yi 1,2, E. M. Arroyo 3 and G. Ceder 1,3 1. Singapore-MIT Alliance, Advanced Materials
Amorphous Metallic Glass as New High Power and Energy Density Anodes For Lithium Ion Rechargeable Batteries Meng Ying 1, Li Yi 1,2, E. M. Arroyo 3 and G. Ceder 1,3 1. Singapore-MIT Alliance, Advanced Materials
Tailor Made Carbon and Graphite Based Anode Materials for Lithium Ion Batteries. Heribert Walter, Battery+Storage 2013
 Tailor Made Carbon and Graphite Based Anode Materials for Lithium Ion Batteries Heribert Walter, Battery+Storage 2013 Agenda SGL Group at a Glance Anode Materials Overview Material Synthesis and Modification
Tailor Made Carbon and Graphite Based Anode Materials for Lithium Ion Batteries Heribert Walter, Battery+Storage 2013 Agenda SGL Group at a Glance Anode Materials Overview Material Synthesis and Modification
Factors Governing Life of High-Energy Lithium-Ion Cells
 Factors Governing Life of High-Energy Lithium-Ion Cells D.P. Abraham IBA 2013 March 11, 2013 Barcelona, Spain Research sponsors are both Government and Private Sector 2 Diagnostics Overview Use of characterization
Factors Governing Life of High-Energy Lithium-Ion Cells D.P. Abraham IBA 2013 March 11, 2013 Barcelona, Spain Research sponsors are both Government and Private Sector 2 Diagnostics Overview Use of characterization
Electronic supplementary information. Efficient energy storage capabilities promoted by hierarchically MnCo 2 O 4
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Electronic supplementary information Efficient energy storage capabilities promoted by hierarchically
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Electronic supplementary information Efficient energy storage capabilities promoted by hierarchically
All-solid-state Li battery using a light-weight solid electrolyte
 All-solid-state Li battery using a light-weight solid electrolyte Hitoshi Takamura Department of Materials Science, Graduate School of Engineering, Tohoku University Europe-Japan Symposium, Electrical
All-solid-state Li battery using a light-weight solid electrolyte Hitoshi Takamura Department of Materials Science, Graduate School of Engineering, Tohoku University Europe-Japan Symposium, Electrical
Cycle life performance of lithium-ion pouch cells
 Journal of Power Sources 158 (2006) 679 688 Cycle life performance of lithium-ion pouch cells Karthikeyan Kumaresan, Qingzhi Guo, Premanand Ramadass, Ralph E. White Department of Chemical Engineering,
Journal of Power Sources 158 (2006) 679 688 Cycle life performance of lithium-ion pouch cells Karthikeyan Kumaresan, Qingzhi Guo, Premanand Ramadass, Ralph E. White Department of Chemical Engineering,
Soft silicon anodes for lithium ion batteries
 Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2014 Supplementary Information Soft silicon anodes for lithium ion batteries Qizhen
Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2014 Supplementary Information Soft silicon anodes for lithium ion batteries Qizhen
In Situ IonicÕElectric Conductivity Measurement of La 0.55 Li 0.35 TiO 3 Ceramic at Different Li Insertion Levels
 A1196 Journal of The Electrochemical Society, 151 8 A1196-A1201 2004 0013-4651/2004/151 8 /A1196/6/$7.00 The Electrochemical Society, Inc. In Situ IonicÕElectric Conductivity Measurement of La 0.55 Li
A1196 Journal of The Electrochemical Society, 151 8 A1196-A1201 2004 0013-4651/2004/151 8 /A1196/6/$7.00 The Electrochemical Society, Inc. In Situ IonicÕElectric Conductivity Measurement of La 0.55 Li
Novel concept of rechargeable battery using iron oxide nanorods. anode and nickel hydroxide cathode in aqueous electrolyte
 Supplementary Information for: Novel concept of rechargeable battery using iron oxide nanorods anode and nickel hydroxide cathode in aqueous electrolyte Zhaolin Liu *, Siok Wei Tay and Xu Li Institute
Supplementary Information for: Novel concept of rechargeable battery using iron oxide nanorods anode and nickel hydroxide cathode in aqueous electrolyte Zhaolin Liu *, Siok Wei Tay and Xu Li Institute
Facile, mild and fast thermal-decomposition reduction of graphene oxide in air and its application in high-performance lithium batteries
 Facile, mild and fast thermal-decomposition reduction of graphene oxide in air and its application in high-performance lithium batteries Zhong-li Wang, Dan Xu, Yun Huang, Zhong Wu, Li-min Wang and Xin-bo
Facile, mild and fast thermal-decomposition reduction of graphene oxide in air and its application in high-performance lithium batteries Zhong-li Wang, Dan Xu, Yun Huang, Zhong Wu, Li-min Wang and Xin-bo
Furnace Temperature and Atmosphere Influences on Producing Lithium Iron Phosphate (LiFePO 4 ) Powders for Lithium Ion Batteries
 Furnace Temperature and Atmosphere Influences on Producing Lithium Iron Phosphate (LiFePO 4 ) Powders for Lithium Ion Batteries Abstract: New technologies for creating efficient low cost lithium ion batteries
Furnace Temperature and Atmosphere Influences on Producing Lithium Iron Phosphate (LiFePO 4 ) Powders for Lithium Ion Batteries Abstract: New technologies for creating efficient low cost lithium ion batteries
Solef. Solef PVDF Aqueous Dispersions. for Lithium Batteries
 Solef Solef PVDF Aqueous Dispersions for Lithium Batteries Innovative Polymerization Technology Solef PVDF is a partially fluorinated, semi-crystalline polymer with excellent thermo-mechanical and chemical
Solef Solef PVDF Aqueous Dispersions for Lithium Batteries Innovative Polymerization Technology Solef PVDF is a partially fluorinated, semi-crystalline polymer with excellent thermo-mechanical and chemical
A SOLVENT-FREE COMPOSITE SOLID ELECTROLYTES OF Li 2 CO 3 Al 2 O 3 SYSTEM PREPARED VIA WATER BASED SOL GEL METHOD
 18 TH INTERNATIONAL CONFERENCE ON COMPOSITE MATERIALS A SOLVENT-FREE COMPOSITE SOLID ELECTROLYTES OF Li 2 CO 3 Al 2 O 3 SYSTEM PREPARED VIA WATER BASED SOL GEL METHOD M. Sulaiman 1, *, A.A. Rahman 1, N.S.
18 TH INTERNATIONAL CONFERENCE ON COMPOSITE MATERIALS A SOLVENT-FREE COMPOSITE SOLID ELECTROLYTES OF Li 2 CO 3 Al 2 O 3 SYSTEM PREPARED VIA WATER BASED SOL GEL METHOD M. Sulaiman 1, *, A.A. Rahman 1, N.S.
Ionic Conductivity and Solid Electrolytes II: Materials and Applications
 Ionic Conductivity and Solid Electrolytes II: Materials and Applications Chemistry 754 Solid State Chemistry Lecture #27 June 4, 2003 References A. Manthiram & J. Kim Low Temperature Synthesis of Insertion
Ionic Conductivity and Solid Electrolytes II: Materials and Applications Chemistry 754 Solid State Chemistry Lecture #27 June 4, 2003 References A. Manthiram & J. Kim Low Temperature Synthesis of Insertion
Terephthalonitrile-derived nitrogen-rich networks for high
 Electronic Supplementary Information Terephthalonitrile-derived nitrogen-rich networks for high performance supercapacitors Long Hao, a Bin Luo, a Xianglong Li, a Meihua Jin, a Yan Fang, a Zhihong Tang,
Electronic Supplementary Information Terephthalonitrile-derived nitrogen-rich networks for high performance supercapacitors Long Hao, a Bin Luo, a Xianglong Li, a Meihua Jin, a Yan Fang, a Zhihong Tang,
Electroactive Polymer for Controlling Overcharge in Lithium-Ion Batteries
 PSI-SR-1261 Electroactive Polymer for Controlling Overcharge in Lithium-Ion Batteries A. Newman R. Pawle K. White J. Lennhoff A. Newman, R. Pawle, K. White, J. Lennhoff, "Electroactive Polymer for Controlling
PSI-SR-1261 Electroactive Polymer for Controlling Overcharge in Lithium-Ion Batteries A. Newman R. Pawle K. White J. Lennhoff A. Newman, R. Pawle, K. White, J. Lennhoff, "Electroactive Polymer for Controlling
Supplementary Figure 1. Photographs of the Suaeda glauca (S. glauca) Bunge at different stages of metal ion absorption. (a) Photographs of S.
 1 2 3 4 5 6 7 Supplementary Figure 1. Photographs of the Suaeda glauca (S. glauca) Bunge at different stages of metal ion absorption. (a) Photographs of S. glauca after absorption of tin salt. (b) Photographs
1 2 3 4 5 6 7 Supplementary Figure 1. Photographs of the Suaeda glauca (S. glauca) Bunge at different stages of metal ion absorption. (a) Photographs of S. glauca after absorption of tin salt. (b) Photographs
Supporting Information
 Supporting Information Low-Temperature Molten-Salt Production of Silicon Nanowires by the Electrochemical Reduction of CaSiO 3 Yifan Dong, Tyler Slade, Matthew J. Stolt, Linsen Li, Steven N. Girard, Liqiang
Supporting Information Low-Temperature Molten-Salt Production of Silicon Nanowires by the Electrochemical Reduction of CaSiO 3 Yifan Dong, Tyler Slade, Matthew J. Stolt, Linsen Li, Steven N. Girard, Liqiang
Safe, Inexpensive, Long Life, High Power and Efficiency Batteries For Grid Scale Energy Storage Applications
 Safe, Inexpensive, Long Life, High Power and Efficiency Batteries For Grid Scale Energy Storage Applications Investigators Yi Cui, Associate Professor; Robert Huggins, Professor; Mauro Pasta, Postdoctoral
Safe, Inexpensive, Long Life, High Power and Efficiency Batteries For Grid Scale Energy Storage Applications Investigators Yi Cui, Associate Professor; Robert Huggins, Professor; Mauro Pasta, Postdoctoral
The below identified patent application is available for licensing. Requests for information should be addressed to:
 DEPARTMENT OF THE NAVY OFFICE OF COUNSEL NAVAL UNDERSEA WARFARE CENTER DIVISION 1176 HOWELL STREET NEWPORT Rl 02841-1708 IN REPLY REFER TO Attorney Docket No. 300139 15 December 2017 The below identified
DEPARTMENT OF THE NAVY OFFICE OF COUNSEL NAVAL UNDERSEA WARFARE CENTER DIVISION 1176 HOWELL STREET NEWPORT Rl 02841-1708 IN REPLY REFER TO Attorney Docket No. 300139 15 December 2017 The below identified
A Novel Lithium-Doping Approach for an Advanced Lithium Ion Capacitor
 A Novel Lithium-Doping Approach for an Advanced Lithium Ion Capacitor Min-Sik Park, Young-Geun Lim, Jin-Hwa Kim, Young-Jun Kim, Jaephil Cho, and Jeom-Soo Kim * Although electrochemical double-layer capacitors
A Novel Lithium-Doping Approach for an Advanced Lithium Ion Capacitor Min-Sik Park, Young-Geun Lim, Jin-Hwa Kim, Young-Jun Kim, Jaephil Cho, and Jeom-Soo Kim * Although electrochemical double-layer capacitors
Summer School June 2-4 th 2015
 MAT4BAT Advanced materials for batteries Summer School June 2-4 th 2015 «Electrode formulation and processing» Dane Sotta (CEA-Liten, France) Mat4Bat Summer School Dane Sotta (CEA) June 3 rd 2015 1 Outline
MAT4BAT Advanced materials for batteries Summer School June 2-4 th 2015 «Electrode formulation and processing» Dane Sotta (CEA-Liten, France) Mat4Bat Summer School Dane Sotta (CEA) June 3 rd 2015 1 Outline
Morphology controlled synthesis of monodispersed manganese. sulfide nanocrystals and their primary application for supercapacitor
 Electronic Supplementary Material (ESI) for Chemical Communications. This journal is The Royal Society of Chemistry 2015 Morphology controlled synthesis of monodispersed manganese sulfide nanocrystals
Electronic Supplementary Material (ESI) for Chemical Communications. This journal is The Royal Society of Chemistry 2015 Morphology controlled synthesis of monodispersed manganese sulfide nanocrystals
Supplemental Information. A Low-Cost and High-Energy Hybrid. Iron-Aluminum Liquid Battery Achieved. by Deep Eutectic Solvents
 JOUL, Volume 1 Supplemental Information A Low-Cost and High-Energy Hybrid Iron-Aluminum Liquid Battery Achieved by Deep Eutectic Solvents Leyuan Zhang, Changkun Zhang, Yu Ding, Katrina Ramirez-Meyers,
JOUL, Volume 1 Supplemental Information A Low-Cost and High-Energy Hybrid Iron-Aluminum Liquid Battery Achieved by Deep Eutectic Solvents Leyuan Zhang, Changkun Zhang, Yu Ding, Katrina Ramirez-Meyers,
Supporting Information
 Supporting Information A Lithium-air fuel cell using copper to catalyze oxygen-reduction based on copper-corrosion mechanism Yonggang Wang Haoshen Zhou* Energy Technology Research Institute, National Institute
Supporting Information A Lithium-air fuel cell using copper to catalyze oxygen-reduction based on copper-corrosion mechanism Yonggang Wang Haoshen Zhou* Energy Technology Research Institute, National Institute
Supporting Information. Oxidation State of Cross-over Manganese Species on the Graphite Electrode of Lithium-ion Cells
 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is The Royal Society of Chemistry 2014 Supporting Information Oxidation State of Cross-over Manganese Species
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is The Royal Society of Chemistry 2014 Supporting Information Oxidation State of Cross-over Manganese Species
MA-SHS of ZrC and ZrB2 in Air from The Zr/B/C Powder. the original is available online at Instructions for use
 Title MA-SHS of ZrC and ZrB2 in Air from The Zr/B/C Powder Author(s)Tsuchida, Takeshi; Yamamoto, Satoshi CitationEURO CERAMICS VIII, PTS 1-3: 85-88 Issue Date 2004 Doc URL http://hdl.handle.net/2115/15876
Title MA-SHS of ZrC and ZrB2 in Air from The Zr/B/C Powder Author(s)Tsuchida, Takeshi; Yamamoto, Satoshi CitationEURO CERAMICS VIII, PTS 1-3: 85-88 Issue Date 2004 Doc URL http://hdl.handle.net/2115/15876
IBM Almaden June 27, Seongmin Ha, Dongho Koo, Kyu Tae Lee * Chemical and Biological Engineering Seoul National University
 IBM Almaden June 27, 2017 Seongmin Ha, Dongho Koo, Kyu Tae Lee * Chemical and Biological Engineering Seoul National University (ktlee@snu.ac.kr) 1) Introduction 2) Failure mechanism of a redox mediator
IBM Almaden June 27, 2017 Seongmin Ha, Dongho Koo, Kyu Tae Lee * Chemical and Biological Engineering Seoul National University (ktlee@snu.ac.kr) 1) Introduction 2) Failure mechanism of a redox mediator
Chemistry 145 Exam number 4 name 11/19/98 # Faraday s constant is 96,500 c/mole of electrons.
 Chemistry 145 Exam number 4 name 11/19/98 # Faraday s constant is 96,500 c/mole of electrons. A.(16) An electrochemical cell is prepared with a strip of manganese metal dipping in to a 1.0 M MnSO 4 solution
Chemistry 145 Exam number 4 name 11/19/98 # Faraday s constant is 96,500 c/mole of electrons. A.(16) An electrochemical cell is prepared with a strip of manganese metal dipping in to a 1.0 M MnSO 4 solution
Effect of Concentrated Electrolyte on High Voltage Aqueous Sodium-ion Battery
 Effect of Concentrated Electrolyte on High Voltage Aqueous Sodium-ion Battery Kosuke Nakamoto, Ayuko Kitajou*, Masato Ito* and Shigeto Okada* (IGSES, Kyushu University, *IMCE, Kyushu University) Oct 6.
Effect of Concentrated Electrolyte on High Voltage Aqueous Sodium-ion Battery Kosuke Nakamoto, Ayuko Kitajou*, Masato Ito* and Shigeto Okada* (IGSES, Kyushu University, *IMCE, Kyushu University) Oct 6.
Electronic Supporting Information
 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2017 Electronic Supporting Information Copyright Royal Society of Chemistry, London,
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2017 Electronic Supporting Information Copyright Royal Society of Chemistry, London,
ELECTROCHEMICAL REDUCTION OF TITANIUM DIOXIDE THIN FILM IN LiCl-KCl-CaCl 2 EUTECTIC MELT
 ELECTROCHEMICAL REDUCTION OF TITANIUM DIOXIDE THIN FILM IN LiCl-KCl-CaCl 2 EUTECTIC MELT Yasushi Katayama Department of Applied Chemistry, Faculty of Science and Technology, Keio University 3-14-1, Hiyoshi,
ELECTROCHEMICAL REDUCTION OF TITANIUM DIOXIDE THIN FILM IN LiCl-KCl-CaCl 2 EUTECTIC MELT Yasushi Katayama Department of Applied Chemistry, Faculty of Science and Technology, Keio University 3-14-1, Hiyoshi,
Natural Graphite versus Synthetic, Silicon and Others in Lithium Ion Battery Anodes
 Natural Graphite versus Synthetic, Silicon and Others in Lithium Ion Battery Anodes George C Hawley President George C Hawley & Associates Supermin123@hotmail.ca Biography George C. Hawley & Associates
Natural Graphite versus Synthetic, Silicon and Others in Lithium Ion Battery Anodes George C Hawley President George C Hawley & Associates Supermin123@hotmail.ca Biography George C. Hawley & Associates
for New Energy Materials and Devices; Beijing National Laboratory for Condense Matter Physics,
 Electronic Supplementary Information Highly efficient core shell CuInS 2 /Mn doped CdS quantum dots sensitized solar cells Jianheng Luo, a Huiyun Wei, a Qingli Huang, a Xing Hu, a Haofei Zhao, b Richeng
Electronic Supplementary Information Highly efficient core shell CuInS 2 /Mn doped CdS quantum dots sensitized solar cells Jianheng Luo, a Huiyun Wei, a Qingli Huang, a Xing Hu, a Haofei Zhao, b Richeng
PHYSICAL REVIEW LETTERS 107,
 Real-time Measurement of Stress and Damage Evolution During Initial Lithiation of Crystalline Silicon M. J. Chon, 1 V.A. Sethuraman, 1 A. McCormick, 1 V. Srinivasan, 2 P. R. Guduru 1,* 1 School of Engineering,
Real-time Measurement of Stress and Damage Evolution During Initial Lithiation of Crystalline Silicon M. J. Chon, 1 V.A. Sethuraman, 1 A. McCormick, 1 V. Srinivasan, 2 P. R. Guduru 1,* 1 School of Engineering,
Supporting Information
 Supporting Information Experimental Methods Pt ALD. The precursor used for ALD was trimethyl-methylcyclopentadienyl-platinum(iv) (MeCpPtMe 3 ) (Strem Chemicals, 99%), which has been widely reported for
Supporting Information Experimental Methods Pt ALD. The precursor used for ALD was trimethyl-methylcyclopentadienyl-platinum(iv) (MeCpPtMe 3 ) (Strem Chemicals, 99%), which has been widely reported for
Supporting Information. Low temperature synthesis of silicon carbide nanomaterials using
 Supporting Information Low temperature synthesis of silicon carbide nanomaterials using solid-state method Mita Dasog, Larissa F. Smith, Tapas K. Purkait and Jonathan G. C. Veinot * Department of Chemistry,
Supporting Information Low temperature synthesis of silicon carbide nanomaterials using solid-state method Mita Dasog, Larissa F. Smith, Tapas K. Purkait and Jonathan G. C. Veinot * Department of Chemistry,
Conductivity and Dielectric Studies of PMMA Composites
 Chem Sci Trans., 2013, 2(S1), S129-S134 Chemical Science Transactions DOI:10.7598/cst2013.26 ISSN/E-ISSN: 2278-3458/2278-3318 RESEARCH ARTICLE Conductivity and Dielectric Studies of PMMA Composites S.
Chem Sci Trans., 2013, 2(S1), S129-S134 Chemical Science Transactions DOI:10.7598/cst2013.26 ISSN/E-ISSN: 2278-3458/2278-3318 RESEARCH ARTICLE Conductivity and Dielectric Studies of PMMA Composites S.
Bonding Technique Using Micro-Scaled Silver-Oxide Particles for In-Situ Formation of Silver Nanoparticles
 Materials Transactions, Vol. 49, No. 12 (28) pp. 2875 to 288 #28 The Japan Institute of Metals Bonding Technique Using Micro-Scaled Silver-Oxide Particles for In-Situ Formation of Silver Nanoparticles
Materials Transactions, Vol. 49, No. 12 (28) pp. 2875 to 288 #28 The Japan Institute of Metals Bonding Technique Using Micro-Scaled Silver-Oxide Particles for In-Situ Formation of Silver Nanoparticles
ABSTRACT. Since carbon dioxide from petroleum-derived fuels has become an environmental
 ABSTRACT KIM, SANGWOOK. Stresses at Electrode-Electrolyte Interface in Lithium-ion Batteries via Multiphysics Modeling (Under the direction of Dr. Hsiao-Ying Shadow Huang.) Since carbon dioxide from petroleum-derived
ABSTRACT KIM, SANGWOOK. Stresses at Electrode-Electrolyte Interface in Lithium-ion Batteries via Multiphysics Modeling (Under the direction of Dr. Hsiao-Ying Shadow Huang.) Since carbon dioxide from petroleum-derived
Electrochemical properties of alkal. Citation Electrochimica Acta (2010), 55(3):
 KURENAI : Kyoto University Researc Title Electrochemical properties of alkal bis(trifluoromethylsulfonyl)amides Author(s) Kubota, Keigo; Tamaki, Kenichiro; N Takuya; Hagiwara, Rika Citation Electrochimica
KURENAI : Kyoto University Researc Title Electrochemical properties of alkal bis(trifluoromethylsulfonyl)amides Author(s) Kubota, Keigo; Tamaki, Kenichiro; N Takuya; Hagiwara, Rika Citation Electrochimica
Morphology of Cadmium Sulfide/Poly(ethylene)Oxide Nanocomposites
 ISSN: 2319-7706 Volume 3 Number 9 (2014) pp. 469-473 http://www.ijcmas.com Original Research Article Morphology of Cadmium Sulfide/Poly(ethylene)Oxide Nanocomposites V.S.Sangawar and R.N.Bhagat* Polymer/Nanomaterials
ISSN: 2319-7706 Volume 3 Number 9 (2014) pp. 469-473 http://www.ijcmas.com Original Research Article Morphology of Cadmium Sulfide/Poly(ethylene)Oxide Nanocomposites V.S.Sangawar and R.N.Bhagat* Polymer/Nanomaterials
Fabrication of Ti-Ni-Zr Shape Memory Alloy by P/M Process
 Materials Transactions, Vol. 5, No. 1 (29) pp. 2446 to 245 #29 The Japan Institute of Metals Fabrication of Ti-Ni-Zr Shape Memory Alloy by P/M Process Akira Terayama 1, Koji Nagai 2; * and Hideki Kyogoku
Materials Transactions, Vol. 5, No. 1 (29) pp. 2446 to 245 #29 The Japan Institute of Metals Fabrication of Ti-Ni-Zr Shape Memory Alloy by P/M Process Akira Terayama 1, Koji Nagai 2; * and Hideki Kyogoku
CHAPTER 2: LITERATURE REVIEW
 CHAPTER 2: LITERATURE REVIEW CHAPTER 2 LITERATURE REVIEW 2.1 Introduction Lithium ion batteries are the prominent choice of power sources in the consumer electronic market today because of the high the
CHAPTER 2: LITERATURE REVIEW CHAPTER 2 LITERATURE REVIEW 2.1 Introduction Lithium ion batteries are the prominent choice of power sources in the consumer electronic market today because of the high the
Metallization deposition and etching. Material mainly taken from Campbell, UCCS
 Metallization deposition and etching Material mainly taken from Campbell, UCCS Application Metallization is back-end processing Metals used are aluminum and copper Mainly involves deposition and etching,
Metallization deposition and etching Material mainly taken from Campbell, UCCS Application Metallization is back-end processing Metals used are aluminum and copper Mainly involves deposition and etching,
EMA4303/5305 Electrochemical Engineering Lecture 05 Applications (1)
 EMA4303/5305 Electrochemical Engineering Lecture 05 Applications (1) Prof. Zhe Cheng Mechanical & Materials Engineering Florida International University Corrosion Definition Electrochemical attack of metals
EMA4303/5305 Electrochemical Engineering Lecture 05 Applications (1) Prof. Zhe Cheng Mechanical & Materials Engineering Florida International University Corrosion Definition Electrochemical attack of metals
Investigation of Alkaline Ion Rocking Chair Batteries. Reza Fathi
 University of Milano-Bicocca Department of Material Science Investigation of Alkaline Ion Rocking Chair Batteries Doctoral dissertation in Materials Science (XVII cycle) of Reza Fathi Supervisor : Prof.
University of Milano-Bicocca Department of Material Science Investigation of Alkaline Ion Rocking Chair Batteries Doctoral dissertation in Materials Science (XVII cycle) of Reza Fathi Supervisor : Prof.
M 3 PO 4 2 -Nanoparticle-Coated LiCoO 2 vs LiCo 0.96 M 0.04 O 2 M = Mg and Zn on Electrochemical and Storage Characteristics
 0013-4651/2008/155 3 /A201/5/$23.00 The Electrochemical Society A201 M 3 PO 4 2 -Nanoparticle-Coated LiCoO 2 vs LiCo 0.96 M 0.04 O 2 M = Mg and Zn on Electrochemical and Storage Characteristics Junho Eom
0013-4651/2008/155 3 /A201/5/$23.00 The Electrochemical Society A201 M 3 PO 4 2 -Nanoparticle-Coated LiCoO 2 vs LiCo 0.96 M 0.04 O 2 M = Mg and Zn on Electrochemical and Storage Characteristics Junho Eom
Progress on Sn-based thin-film anode materials for lithium-ion batteries
 Review SPECIAL ISSUE: New Energy Materials November 2012 Vol.57 No.32: 4119 4130 doi: 10.1007/s11434-012-5303-z Progress on Sn-based thin-film anode materials for lithium-ion batteries HU RenZong, LIU
Review SPECIAL ISSUE: New Energy Materials November 2012 Vol.57 No.32: 4119 4130 doi: 10.1007/s11434-012-5303-z Progress on Sn-based thin-film anode materials for lithium-ion batteries HU RenZong, LIU
Among the commercial aluminum cast alloys, Al-Si-Cu-
 February 2010 Effects of Cd and Sn on double-peak age-hardening behaviors of Al-Si-Cu- Mg cast alloys *Li Runxia, Chen Yujin, Yuan Xiaoguang, Qu Yingdong and Li Rongde (Institute of Material Science and
February 2010 Effects of Cd and Sn on double-peak age-hardening behaviors of Al-Si-Cu- Mg cast alloys *Li Runxia, Chen Yujin, Yuan Xiaoguang, Qu Yingdong and Li Rongde (Institute of Material Science and
Candle Soot as Supercapacitor Electrode Material
 Supporting information Candle Soot as Supercapacitor Electrode Material Bowen Zhang, Daoai Wang, Bo Yu, Feng Zhou and Weimin Liu State Key Laboratory of Solid Lubrication, Lanzhou Institute of Chemical
Supporting information Candle Soot as Supercapacitor Electrode Material Bowen Zhang, Daoai Wang, Bo Yu, Feng Zhou and Weimin Liu State Key Laboratory of Solid Lubrication, Lanzhou Institute of Chemical
Supporting Information. Christina W. Li and Matthew W. Kanan* *To whom correspondence should be addressed.
 Supporting Information CO 2 Reduction at Low Overpotential on Cu Electrodes Resulting from the Reduction of Thick Cu 2 O Films Christina W. Li and Matthew W. Kanan* *To whom correspondence should be addressed.
Supporting Information CO 2 Reduction at Low Overpotential on Cu Electrodes Resulting from the Reduction of Thick Cu 2 O Films Christina W. Li and Matthew W. Kanan* *To whom correspondence should be addressed.
FUNDAMENTAL STUDY ON MICROSTRUCTURE OF CeO 2 -DOPED (Na 0.5 Bi 0.5 )TiO 3 CERAMICS
 FUNDAMENTAL STUDY ON MICROSTRUCTURE OF CeO 2 -DOPED (Na 0.5 Bi 0.5 )TiO 3 CERAMICS Rozidawati Awang 1,*, Nurain Ab. Halim 1, Zalita Zainuddin 1, Mohammad Hafizuddin Haji Jumali 1, Muhammad Yahaya 1 and
FUNDAMENTAL STUDY ON MICROSTRUCTURE OF CeO 2 -DOPED (Na 0.5 Bi 0.5 )TiO 3 CERAMICS Rozidawati Awang 1,*, Nurain Ab. Halim 1, Zalita Zainuddin 1, Mohammad Hafizuddin Haji Jumali 1, Muhammad Yahaya 1 and
Materials Chemistry A
 Journal of Materials Chemistry A PAPER Cite this: J. Mater. Chem. A, 2014,2, 14947 High-capacity full lithium-ion cells based on nanoarchitectured ternary manganese nickel cobalt carbonate and its lithiated
Journal of Materials Chemistry A PAPER Cite this: J. Mater. Chem. A, 2014,2, 14947 High-capacity full lithium-ion cells based on nanoarchitectured ternary manganese nickel cobalt carbonate and its lithiated
A R C H I V E S O F M E T A L L U R G Y A N D M A T E R I A L S Volume Issue 2 DOI: /amm
 A R C H I V E S O F M E T A L L U R G Y A N D M A T E R I A L S Volume 60 2015 Issue 2 DOI: 10.1515/amm-2015-0086 S.M. SHIN, G.J. JUNG, WOO-JIN LEE, C.Y. KANG, J.P. WANG, RECOVERY OF ELECTRODIC POWDER
A R C H I V E S O F M E T A L L U R G Y A N D M A T E R I A L S Volume 60 2015 Issue 2 DOI: 10.1515/amm-2015-0086 S.M. SHIN, G.J. JUNG, WOO-JIN LEE, C.Y. KANG, J.P. WANG, RECOVERY OF ELECTRODIC POWDER
Synthesis of Stable Shape Controlled Catalytically Active β-palladium Hydride
 Supporting Information for Synthesis of Stable Shape Controlled Catalytically Active β-palladium Hydride Zipeng Zhao, Xiaoqing Huang, Mufan Li, Gongming Wang, Chain Lee, Enbo Zhu, Xiangfeng Duan, Yu Huang
Supporting Information for Synthesis of Stable Shape Controlled Catalytically Active β-palladium Hydride Zipeng Zhao, Xiaoqing Huang, Mufan Li, Gongming Wang, Chain Lee, Enbo Zhu, Xiangfeng Duan, Yu Huang
CHAPTER-VII SUMMARY AND CONCLUSIONS
 CHAPTER-VII SUMMARY AND CONCLUSIONS Chapter-VII Summary and Conclusions Sr. No. Title Page No. 7.1 Summary 167 7.2 Conclusions.. 171 CHAPTER SEVEN Summary and Conclusions 7.1: Summary The technologies
CHAPTER-VII SUMMARY AND CONCLUSIONS Chapter-VII Summary and Conclusions Sr. No. Title Page No. 7.1 Summary 167 7.2 Conclusions.. 171 CHAPTER SEVEN Summary and Conclusions 7.1: Summary The technologies
Microstructural Evolution of Ti-Mo-Ni-C Powder by Mechanical Alloying
 Materials Transactions, Vol. 50, No. 1 (2009) pp. 117 to 122 #2009 The Japan Institute of Metals Microstructural Evolution of -Mo-Ni-C Powder by Mechanical Alloying Hiroyuki Hosokawa, Kiyotaka Kato, Koji
Materials Transactions, Vol. 50, No. 1 (2009) pp. 117 to 122 #2009 The Japan Institute of Metals Microstructural Evolution of -Mo-Ni-C Powder by Mechanical Alloying Hiroyuki Hosokawa, Kiyotaka Kato, Koji
LITHIUM BATTERIES: RESEARCH, TECHNOLOGY
 ELECTRICAL ENGINEERING DEVELOPMENTS LITHIUM BATTERIES: RESEARCH, TECHNOLOGY AND APPLICATIONS No part of this digital document may be reproduced, stored in a retrieval system or transmitted in any form
ELECTRICAL ENGINEERING DEVELOPMENTS LITHIUM BATTERIES: RESEARCH, TECHNOLOGY AND APPLICATIONS No part of this digital document may be reproduced, stored in a retrieval system or transmitted in any form
PHYSICAL PROPERTIES OF La 0.9 Sr 0.1 Cr 1-X Ni X O 3-δ (X = 0-0.6) SYNTHESIZED VIA CITRATE GEL COMBUSTION
 PHYSICAL PROPERTIES OF La 0.9 Sr 0.1 Cr 1-X Ni X O 3-δ (X = 0-0.6) SYNTHESIZED VIA CITRATE GEL COMBUSTION Anuchit Ruangvittayanon * and Sutin Kuharuangrong Received: Sept 29, 2009; Revised: Nov 17, 2009;
PHYSICAL PROPERTIES OF La 0.9 Sr 0.1 Cr 1-X Ni X O 3-δ (X = 0-0.6) SYNTHESIZED VIA CITRATE GEL COMBUSTION Anuchit Ruangvittayanon * and Sutin Kuharuangrong Received: Sept 29, 2009; Revised: Nov 17, 2009;
Electrochemical and Transport Properties of Ions in Mixtures of. Electroactive Ionic Liquid and Propylene Carbonate with a Lithium
 Supporting Information Electrochemical and Transport Properties of Ions in Mixtures of Electroactive Ionic Liquid and Propylene Carbonate with a Lithium Salt for Lithium-ion Batteries Bruno Gélinas, Myriann
Supporting Information Electrochemical and Transport Properties of Ions in Mixtures of Electroactive Ionic Liquid and Propylene Carbonate with a Lithium Salt for Lithium-ion Batteries Bruno Gélinas, Myriann
Red Phosphorus Nano-Dots on Reduced Graphene Oxide as Flexible High-Performance Anode for Sodium-Ion Batteries
 Red Phosphorus Nano-Dots on Reduced Graphene Oxide as Flexible High-Performance Anode for Sodium-Ion Batteries Yihang Liu 1, Anyi Zhang 2, Chenfei Shen 2, Qingzhou Liu 2, Xuan Cao 2, Yuqiang Ma 2, Liang
Red Phosphorus Nano-Dots on Reduced Graphene Oxide as Flexible High-Performance Anode for Sodium-Ion Batteries Yihang Liu 1, Anyi Zhang 2, Chenfei Shen 2, Qingzhou Liu 2, Xuan Cao 2, Yuqiang Ma 2, Liang
ELECTRICAL PROPERTIES OF FILM AND LITHIUM ION BATTERY COMPOSED OF NATURAL GRAPHITE PARTICLES GROUND UNDER VARIOUS ATMOSPHERES
 ELECTRICAL PROPERTIES OF FILM AND LITIUM ION BATTERY COMPOSED OF NATURAL GRAPITE PARTICLES GROUND UNDER VARIOUS ATMOSPERES Y. Kuga, A. Ueda, Y. Ohira, and K. Ando Muroran Institute of Technology, Department
ELECTRICAL PROPERTIES OF FILM AND LITIUM ION BATTERY COMPOSED OF NATURAL GRAPITE PARTICLES GROUND UNDER VARIOUS ATMOSPERES Y. Kuga, A. Ueda, Y. Ohira, and K. Ando Muroran Institute of Technology, Department
Review Thermal Runaway Reactions mechanisms Issue date : January 2011
 Project HELIOS - High Energy Lithium-Ion Storage Solutions (www.helios-eu.org) Project number: FP7 2333765 (A 3 year project, supported by the European Commission, to study and test the comparative performances
Project HELIOS - High Energy Lithium-Ion Storage Solutions (www.helios-eu.org) Project number: FP7 2333765 (A 3 year project, supported by the European Commission, to study and test the comparative performances
METAL FINISHING. (As per revised VTU syllabus: )
 METAL FINISHING (As per revised VTU syllabus: 2015-16) Definition: It is a process in which a specimen metal (article) is coated with another metal or a polymer in order to modify the surface properties
METAL FINISHING (As per revised VTU syllabus: 2015-16) Definition: It is a process in which a specimen metal (article) is coated with another metal or a polymer in order to modify the surface properties
Supporting Information for Manuscript B516757D
 Supporting Information for Manuscript B516757D 1. UV-Vis absorption spectra Absorbance (a.u.) 0.4 0.2 5F 6F 7F 0.0 300 400 500 Wavelength (nm) Figure S1 UV-Vis spectra of, 5F, 6F and 7F in CHCl 3 solutions
Supporting Information for Manuscript B516757D 1. UV-Vis absorption spectra Absorbance (a.u.) 0.4 0.2 5F 6F 7F 0.0 300 400 500 Wavelength (nm) Figure S1 UV-Vis spectra of, 5F, 6F and 7F in CHCl 3 solutions
APPLICATIONS OF ELECTROCHEMISTRY
 APPLICATIONS OF ELECTROCHEMISTRY SPONTANEOUS REDOX REACTIONS APPLICATIONS OF ELECTROCHEMICAL CELLS BATTERIES A galvanic cell, or series of combined galvanic cells, that can be used as a source of direct
APPLICATIONS OF ELECTROCHEMISTRY SPONTANEOUS REDOX REACTIONS APPLICATIONS OF ELECTROCHEMICAL CELLS BATTERIES A galvanic cell, or series of combined galvanic cells, that can be used as a source of direct
Liquid Solubility of Manganese and Its Influence on Grain Size of Mg-Al Alloys* 1
 Materials Transactions, Vol. 47, No. 8 (2006) pp. 1968 to 1974 #2006 The Japan Institute of Light Metals Liquid Solubility of Manganese and Its Influence on Grain Size of Mg-Al Alloys* 1 Yosuke Tamura,
Materials Transactions, Vol. 47, No. 8 (2006) pp. 1968 to 1974 #2006 The Japan Institute of Light Metals Liquid Solubility of Manganese and Its Influence on Grain Size of Mg-Al Alloys* 1 Yosuke Tamura,
The electrodeposition of Zn-Mo and Zn-Sn-Mo alloys from citrate electrolytes
 Honorata Kazimierczak The electrodeposition of Zn-Mo and Zn-Sn-Mo alloys from citrate electrolytes Supervisor: Assoc. Prof. Piotr Ozga The electrodeposition of Zn-Mo and Zn-Sn-Mo alloys from citrate electrolytes
Honorata Kazimierczak The electrodeposition of Zn-Mo and Zn-Sn-Mo alloys from citrate electrolytes Supervisor: Assoc. Prof. Piotr Ozga The electrodeposition of Zn-Mo and Zn-Sn-Mo alloys from citrate electrolytes
Cu(In,Ga)Se 2 FILM FORMATION FROM SELENIZATION OF MIXED METAL/METAL-SELENIDE PRECURSORS
 Cu(In,Ga)Se 2 FILM FORMATION FROM SELENIZATION OF MIX METAL/METAL-SELENIDE PRECURSORS Rui Kamada, William N. Shafarman, and Robert W. Birkmire Institute of Energy Conversion University of Delaware, Newark,
Cu(In,Ga)Se 2 FILM FORMATION FROM SELENIZATION OF MIX METAL/METAL-SELENIDE PRECURSORS Rui Kamada, William N. Shafarman, and Robert W. Birkmire Institute of Energy Conversion University of Delaware, Newark,
Electrochemical Capacitor of MnFe 2 O 4 with Organic Li-Ion Electrolyte
 1099-0062/2007/10 7 /A171/5/$20.00 The Electrochemical Society Electrochemical Capacitor of MnFe 2 O 4 with Organic Li-Ion Electrolyte Shin-Liang Kuo and Nae-Lih Wu*,z Department of Chemical Engineering,
1099-0062/2007/10 7 /A171/5/$20.00 The Electrochemical Society Electrochemical Capacitor of MnFe 2 O 4 with Organic Li-Ion Electrolyte Shin-Liang Kuo and Nae-Lih Wu*,z Department of Chemical Engineering,
Lower Cost Higher Performance Graphite for LIBs. Prepared by: Dr. Edward R. Buiel President and CEO Coulometrics, LLC. Date: March 23, 2017
 Lower Cost Higher Performance Graphite for LIBs Prepared by: Dr. Edward R. Buiel President and CEO Coulometrics, LLC. Date: March 23, 2017 Outline Company overview Review of natural graphite resources
Lower Cost Higher Performance Graphite for LIBs Prepared by: Dr. Edward R. Buiel President and CEO Coulometrics, LLC. Date: March 23, 2017 Outline Company overview Review of natural graphite resources
Comparison between the article and script of thesis
 Comparison between the article and script of thesis All nanomaterials and a part of results presented and discussed in this article titled Efficient multi-metallic anode catalysts in a PEM water electrolyzer
Comparison between the article and script of thesis All nanomaterials and a part of results presented and discussed in this article titled Efficient multi-metallic anode catalysts in a PEM water electrolyzer
Microstructural and electrochemical properties of rf-sputtered LiMn 2 O 4 thin film cathodes
 Appl Nanosci (2012) 2:401 407 DOI 10.1007/s13204-011-0054-8 REVIEW ARTICLE Microstructural and electrochemical properties of rf-sputtered LiMn 2 O 4 thin film cathodes K. Jayanth Babu P. Jeevan Kumar O.
Appl Nanosci (2012) 2:401 407 DOI 10.1007/s13204-011-0054-8 REVIEW ARTICLE Microstructural and electrochemical properties of rf-sputtered LiMn 2 O 4 thin film cathodes K. Jayanth Babu P. Jeevan Kumar O.
What happens if we connect Zn and Pt in HCl solution? Corrosion of platinum (Pt) in HCl. 1. If Zn and Pt are not connected
 Corrosion of platinum (Pt) in HCl Now if we place a piece of Pt in HCl, what will happen? Pt does not corrode does not take part in the electrochemical reaction Pt is a noble metal Pt acts as a reference
Corrosion of platinum (Pt) in HCl Now if we place a piece of Pt in HCl, what will happen? Pt does not corrode does not take part in the electrochemical reaction Pt is a noble metal Pt acts as a reference
Effects of Lead on Tin Whisker Elimination
 Effects of Lead on Tin Whisker Elimination Wan Zhang and Felix Schwager Rohm and Haas Electronic Materials Lucerne, Switzerland inemi Tin Whisker Workshop at ECTC 0 May 30, 2006, in San Diego, CA Efforts
Effects of Lead on Tin Whisker Elimination Wan Zhang and Felix Schwager Rohm and Haas Electronic Materials Lucerne, Switzerland inemi Tin Whisker Workshop at ECTC 0 May 30, 2006, in San Diego, CA Efforts
Electronic and electrochemical properties of Mg 2 Ni alloy doped by Pd atoms *
 Materials Science-Poland, Vol. 25, No. 4, 2007 Electronic and electrochemical properties of Mg 2 Ni alloy doped by Pd atoms * A. SZAJEK 1**, I. OKOŃSKA 2, M. JURCZYK 2 1 Institute of Molecular Physics,
Materials Science-Poland, Vol. 25, No. 4, 2007 Electronic and electrochemical properties of Mg 2 Ni alloy doped by Pd atoms * A. SZAJEK 1**, I. OKOŃSKA 2, M. JURCZYK 2 1 Institute of Molecular Physics,
Structure of Electrodeposited Zn Mn Alloy Coatings
 , pp. 1024 1028 Structure of Electrodeposited Zn Mn Coatings Yasuo TSUCHIYA, Satoshi HASHIMOTO, Yoichi ISHIBASHI, Takayuki URAKAWA, 1) Masaru SAGIYAMA 2) and Yasuo FUKUDA 3) Kokan Keisoku K. K., Minamiwatarida,
, pp. 1024 1028 Structure of Electrodeposited Zn Mn Coatings Yasuo TSUCHIYA, Satoshi HASHIMOTO, Yoichi ISHIBASHI, Takayuki URAKAWA, 1) Masaru SAGIYAMA 2) and Yasuo FUKUDA 3) Kokan Keisoku K. K., Minamiwatarida,
The growth of patterned ceramic thin films from polymer precursor solutions Göbel, Ole
 University of Groningen The growth of patterned ceramic thin films from polymer precursor solutions Göbel, Ole IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you
University of Groningen The growth of patterned ceramic thin films from polymer precursor solutions Göbel, Ole IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you
Characterization of Nano-Scale Fine Precipitates in Al-Mg-Si Alloys for Automotive Applications
 UDC 669. 715 721 782 : 629. 11. 011. 5 Characterization of Nano-Scale Fine Precipitates in Al-Mg-Si Alloys for Automotive Applications Makoto SAGA* 1 Naoki MARUYAMA* 1 Abstract Bake-hadenable Al-Mg-Si
UDC 669. 715 721 782 : 629. 11. 011. 5 Characterization of Nano-Scale Fine Precipitates in Al-Mg-Si Alloys for Automotive Applications Makoto SAGA* 1 Naoki MARUYAMA* 1 Abstract Bake-hadenable Al-Mg-Si
Taigo ONODERA ³, Jun KAWAJI, Akira SATO and Takefumi OKUMURA. 1. Introduction
 Full paper Electrochemical performance of a newly-designed all-solid-state Li ion battery with a LiNi 1/3 Co 1/3 Mn 1/3 O 2 LiVO 3 mono-particle layered cathode and a lamellar LiVO 3 anode Taigo ONODERA
Full paper Electrochemical performance of a newly-designed all-solid-state Li ion battery with a LiNi 1/3 Co 1/3 Mn 1/3 O 2 LiVO 3 mono-particle layered cathode and a lamellar LiVO 3 anode Taigo ONODERA
BATTERY SOLUTIONS WITH KYNAR PVDF LITHIUM-ION FOCUS
 BATTERY SOLUTIONS WITH KYNAR PVDF LITHIUM-ION FOCUS BY 2025, THE WORLD WILL MANUFACTURE 8 BILLION LI-ION CELLS Continued market growth requires rapid advances in higher energy density, higher performance
BATTERY SOLUTIONS WITH KYNAR PVDF LITHIUM-ION FOCUS BY 2025, THE WORLD WILL MANUFACTURE 8 BILLION LI-ION CELLS Continued market growth requires rapid advances in higher energy density, higher performance
Comparison of hydrogen storage properties of pure Mg and milled pure Mg
 Bull. Mater. Sci., Vol. 37, No. 4, June 2014, pp. 831 835. Indian Academy of Sciences. Comparison of hydrogen storage properties of pure Mg and milled pure Mg MYOUNG YOUP SONG a, *, YOUNG JUN KWAK b, SEONG
Bull. Mater. Sci., Vol. 37, No. 4, June 2014, pp. 831 835. Indian Academy of Sciences. Comparison of hydrogen storage properties of pure Mg and milled pure Mg MYOUNG YOUP SONG a, *, YOUNG JUN KWAK b, SEONG
FAYALITE SLAG MODIFIED STAINLESS STEEL AOD SLAG
 FAYALITE SLAG MODIFIED STAINLESS STEEL AOD SLAG Shuigen HUANG, Muxing GUO, Peter Tom JONES, Bart BLANPAIN Department of Metallurgy and Materials Engineering, KU Leuven, 3001 Heverlee, Belgium shuigen.huang@mtm.kuleuven.be,
FAYALITE SLAG MODIFIED STAINLESS STEEL AOD SLAG Shuigen HUANG, Muxing GUO, Peter Tom JONES, Bart BLANPAIN Department of Metallurgy and Materials Engineering, KU Leuven, 3001 Heverlee, Belgium shuigen.huang@mtm.kuleuven.be,
PREPARATION OF ALUMINA MATRIX FOR CERAMIC COMPOSITES BY SOL-GEL METHOD
 PREPARATION OF ALUMINA MATRIX FOR CERAMIC COMPOSITES BY SOL-GEL METHOD Jiayu Xiao, Zhengfang Xie, Zhaohui Chen, Xingye Wang, Wenwei Zheng, and Junzhi Liu Department of material Engineering and Applied
PREPARATION OF ALUMINA MATRIX FOR CERAMIC COMPOSITES BY SOL-GEL METHOD Jiayu Xiao, Zhengfang Xie, Zhaohui Chen, Xingye Wang, Wenwei Zheng, and Junzhi Liu Department of material Engineering and Applied
Storage Characteristics of LiNi 0.8 Co 0.1+x Mn 0.1 x O 2 (x = 0, 0.03, and 0.06) Cathode Materials for Lithium Batteries
 0013-4651/2008/155 3 /A239/7/$23.00 The Electrochemical Society A239 Storage Characteristics of LiNi 0.8 Co 0.1+x Mn 0.1 x O 2 (x = 0, 0.03, and 0.06) Cathode Materials for Lithium Batteries Junho Eom,
0013-4651/2008/155 3 /A239/7/$23.00 The Electrochemical Society A239 Storage Characteristics of LiNi 0.8 Co 0.1+x Mn 0.1 x O 2 (x = 0, 0.03, and 0.06) Cathode Materials for Lithium Batteries Junho Eom,
Influence of TiC on the Viscosity of CaO MgO Al 2 O 3 SiO 2 TiC Suspension System
 , pp. 922 927 Influence of TiC on the Viscosity of CaO MgO Al 2 O 3 SiO 2 TiC Suspension System Guo-Hua ZHANG, 1,2) * Yu-Lan ZHEN 1,2) and Kuo-Chih CHOU 1,2) 1) State Key Laboratory of Advanced Metallurgy,
, pp. 922 927 Influence of TiC on the Viscosity of CaO MgO Al 2 O 3 SiO 2 TiC Suspension System Guo-Hua ZHANG, 1,2) * Yu-Lan ZHEN 1,2) and Kuo-Chih CHOU 1,2) 1) State Key Laboratory of Advanced Metallurgy,
Recycling of spent rechargeable batteries: A review for the lithium-ion batteries
 Recycling of spent rechargeable batteries: A review for the lithium-ion batteries G.G. Papavasileiou, C.S. Psomopoulos *, G.Ch. Ioannidis, S.D. Kaminaris Department of Electrical Engineer, Piraeus University
Recycling of spent rechargeable batteries: A review for the lithium-ion batteries G.G. Papavasileiou, C.S. Psomopoulos *, G.Ch. Ioannidis, S.D. Kaminaris Department of Electrical Engineer, Piraeus University
Stationary Lead-Acid Batteries With Selenium Alloys
 Stationary Lead-Acid Batteries With Selenium Alloys Summary Lead alloys characterized by the addition of selenium exhibit a fine grain structure even at very low antimony contents (less than 2%). This
Stationary Lead-Acid Batteries With Selenium Alloys Summary Lead alloys characterized by the addition of selenium exhibit a fine grain structure even at very low antimony contents (less than 2%). This
Fundamental Chemistry of Sion Power Li/S Battery. Yuriy Mikhaylik Sion Power Corporation, 9040 South Rita Road, Tucson, Arizona, 85747, USA
 Fundamental Chemistry of Sion Power Li/S Battery Yuriy Mikhaylik Sion Power Corporation, 9040 South Rita Road, Tucson, Arizona, 85747, USA Outline Thermodynamics of Li-S Discharge-charge mechanism in the
Fundamental Chemistry of Sion Power Li/S Battery Yuriy Mikhaylik Sion Power Corporation, 9040 South Rita Road, Tucson, Arizona, 85747, USA Outline Thermodynamics of Li-S Discharge-charge mechanism in the
MICROWAVE DIELECTRIC PROPERTIES OF Ba 0.75 Sr 0.25 (Nd x Bi 1-x ) 2 Ti 4 O 12 SOLID SOLUTION
 Original papers MICROWAVE DIELECTRIC PROPERTIES OF Ba 0.75 Sr 0.25 (Nd x Bi 1-x ) 2 Ti 4 O 12 SOLID SOLUTION Long Mingzhu, ZHuang WENDONG*, # Tang Bin, Yu Shengquan, Zhou Xiaohua, Zhang Shuren State Key
Original papers MICROWAVE DIELECTRIC PROPERTIES OF Ba 0.75 Sr 0.25 (Nd x Bi 1-x ) 2 Ti 4 O 12 SOLID SOLUTION Long Mingzhu, ZHuang WENDONG*, # Tang Bin, Yu Shengquan, Zhou Xiaohua, Zhang Shuren State Key
to enable Lithium metal electrodes IBA2013, Barcelona, Spain
 Fluorine free ionic liquid electrolytes to enable Lithium metal electrodes IBA2013, Barcelona, Spain A. S. Best, Martin (Hyun Gook) YOON, G. H. Lane, Y. Shekibi, P. C. Howlett, M. Forsyth & D. R. MacFarlane
Fluorine free ionic liquid electrolytes to enable Lithium metal electrodes IBA2013, Barcelona, Spain A. S. Best, Martin (Hyun Gook) YOON, G. H. Lane, Y. Shekibi, P. C. Howlett, M. Forsyth & D. R. MacFarlane
