PY2N20 Material Properties and Phase Diagrams
|
|
|
- Abraham Hensley
- 6 years ago
- Views:
Transcription
1 PY2N20 Mteril Properties nd Phse Digrms ecture 5 P. tmenov, PhD chool of Physics, TCD PY2N20-5
2 Phse Digrms - Introduction How much cn be done with pure elementl compounds? How mny combintions of elements could be imgined? 2 100??? How mny of these combintions will hve the structure (crystllogrphic, nnoscle, microscle, etc. ) of the end members? How is mixing them going to ffect the resulting mechnicl, electronic nd other physicl nd chemicl properties? Cn the properties of the end members be improved on?
3 Phse Digrms Why? When we combine two or more constituents (elements)... wht equilibrium stte do we get? In prticulr, if we specify... c, p, T, but lso H, E (ll re intensive thermodynmic prmeters) - composition (e.g., wt% Cu - wt% Ni), nd - temperture (T ) then... How mny phses do we get? Wht is the composition of ech phse? How much of ech phse do we get? Phse A Phse B Nickel tom Copper tom Does this phse segregtion relly occur for Cu 1-x Ni x?
4 Pure Wter Pure ugr Temperture ( C) Phse Equilibri: olubility imit Introduction olutions solid solutions, single phse Mixtures more thn one phse olubility imit: Mx concentrtion for which only single phse solution occurs. Question: Wht is the solubility limit t 20 C? Answer: 65 wt% sugr. If Co < 65 wt% sugr: syrup If Co > 65 wt% sugr: syrup + sugr ucrose/wter Phse Digrm olubility imit (liquid solution i.e., syrup) (liquid) + (solid sugr) Co =Composition (wt% sugr)
5 Components nd Phses Components: The elements or compounds which re present in the mixture (e.g., Al nd Cu) Phses: The physiclly nd chemiclly distinct mteril regions tht result (e.g., nd b). Aluminum- Copper Alloy b (lighter phse) (drker phse)
6 Temperture ( C) Effect of T & Composition (C o ) Chnging T cn chnge # of phses: pth A to B. Chnging C o cn chnge # of phses: pth B to D. wtersugr system ( liquid solution i.e., syrup) B (100 C,70) 1 phse (liquid) + (solid sugr) A (20 C,70) 2 phses D (100 C,90) 2 phses C o =Composition (wt% sugr)
7 Phse Equilibri imple solution system (e.g., Ni-Cu solution) Crystl tructure Electroneg. r (nm) Ni FCC Cu FCC Both hve the sme crystl structure (FCC) nd hve similr electronegtivities nd tomic rdii (W. Hume Rothery rules) suggesting high mutul solubility. Ni nd Cu re totlly miscible in ll proportions. Hence, the nswer to the erlier question is No
8 Phse Digrms Indicte phses s function of T, C o, nd P. For this course: -binry systems: just 2 components. -independent vribles: T nd C o (P = 1 tm is lmost lwys used). T( C) Phse Digrm for Cu-Ni system (liquid) (FCC solid solution) phses: (liquid) (FCC solid solution) 3 phse fields: + wt% Ni
9 Phse Digrms: Number nd types of phses Rule 1: If we know T nd C o, then we know: - the number nd types of phses present. Exmples: A(1100 C, 60): 1 phse: B (1250 C, 35): 2 phses: + T( C) (liquid) B (1250 C,35) (FCC solid solution) Cu-Ni phse digrm 1100 A(1100 C,60) wt% Ni
10 Composition of phses Rule 2: If we know T nd C o, then we know: - the composition of ech phse. Exmples: C o = 35 wt% Ni At T A = 1320 C: Only iquid () C = C o ( = 35 wt% Ni) At T D = 1190 C: Only olid ( ) C = C o ( = 35 wt% Ni ) At T B = 1250 C: T( C) T A 1300 T B 1200 T D 20 Both nd C = C liquidus ( = 32 wt% Ni here) C = C solidus ( = 43 wt% Ni here) (liquid) Cu-Ni system A B D C o C tie line (solid) C wt% Ni
11 Weight frctions of phses Rule 3: If we know T nd C o, then we know: - the mount of ech phse (given in wt%), vi the so-clled: centre of grvity principle or the lever rule Exmples: C o = 35 wt% Ni At T A : Only iquid () W = 100 wt%, W = 0 At T D : Only olid ( ) At T B : W W W = 0, W = 100 wt% Both nd R + R R = 27 wt% 73wt % T( C) T A 1300 T B 1200 T D 20 (liquid) Cu-Ni system A B R D C o C tie line (solid) C wt% Ni
12 The ever Rule Tie line connects the phses in equilibrium with ech other - essentilly n isotherm T( C) 1300 T B (liquid) R B wt% Ni tie line (solid) 30 C C o C How much of ech phse? Think of it s lever M M R M M R W M M M R C C C C 0 W R R C C 0 C C
13 Cooling in the Cu-Ni Binry ystem Phse digrm: Cu-Ni system. ystem is: - binry i.e., 2 components: Cu nd Ni. - isomorphous i.e., complete solubility of one component in nother; phse field extends from 0 to 100 wt% Ni. Consider C o = 35 wt%ni. T( C) 1300 : 35 wt% Ni : 46 wt% Ni (liquid) (solid) A B C D E 35 C o : 35wt%Ni wt% Ni Cu-Ni system : 32 wt% Ni : 43 wt% Ni : 24 wt% Ni : 36 wt% Ni
14 Cored vs Equilibrium Phses C chnges s we solidify. Cu-Ni cse: Fst rte of cooling: Cored structure First to solidify hs C = 46 wt% Ni. st to solidify hs C = 35 wt% Ni. low rte of cooling: Equilibrium structure First to solidify: 46 wt% Ni st to solidify: < 35 wt% Ni Uniform C : 35 wt% Ni
15 Tensile trength (MP) Elongtion (%E) Mechnicl Properties: Cu-Ni ystem Effect of solid solution strengthening on: - Tensile strength (T) - Ductility (%E,%AR) T for pure Cu Cu Ni T for pure Ni Composition, wt% Ni %E for pure Cu Cu Ni %E for pure Ni Composition, wt% Ni - Mximum s function of Co - Minimum s function of Co
16 Binry Eutectic ystems ευτηκτικός - from Greek esiest to melt
17 Binry-Eutectic ystems Cu-Ag system 3 single phse regions (,, b ) imited solubility: ) : mostly Cu b : mostly Ag T E : No liquid below T E C E : Composition with min. melting T E Eutectic trnsition T( C) TE (C E ) (C E ) + b(c be ) + (liquid) b Cu-Ag system + b b 779 C C E C o, wt% Ag
18 Pb-n Eutectic ystem (1) For 40 wt% n-60 wt% Pb lloy t 150 C, find... - the phses present: - compositions of phses: T( C) W = C O = 40 wt% n C = 11 wt% n C b = 99 wt% n - the reltive mount of ech phse: = W b = = R R R+ = = C b - C O C b - C = C O - C C b - C = = 67 wt% = 33 wt% (liquid) 183 C + b R + b Pb-n system C C o C b Adpted from Fig. 9.8, Cllister 7e. C, wt% n b
19 Pb-n Eutectic ystem (2) For 40 wt% n-60 wt% Pb lloy t 220 C, find... - the phses present: + Pb-n - compositions of phses: T( C) C O = 40 wt% n C = 17 wt% n C = 46 wt% n - the reltive mount of ech phse: W = C - C O C - C = = 6 29 = 21 wt% W = C O - C = 23 C - C = 79 wt% R (liquid) 183 C + b system + b C C o C C, wt% n b
20 Microstructures in Eutectic ystems: I C o < 2 wt% n Result: - t extreme ends - polycrystl of grins i.e., only one solid phse. T( C) T E : C o wt% n : C o wt% n + (Pb-n ystem) b 0 C o (room T solubility limit) 30 C o, wt% n
21 Microstructures in Eutectic ystems: II 2 wt% n < C o < 18.3 wt% n Result: Initilly liquid + then lone finlly two phses polycrystl fine b-phse inclusions T( C) T E + + b : C o wt% n : C o wt% n b Pb-n system C o (sol. limit t T room ) 18.3 (sol. limit t T E ) 30 C o, wt% n
22 Microstructures in Eutectic ystems: III C o = C E Result: Eutectic microstructure (lmellr structure) - lternting lyers (lmelle) of nd b crystls. 300 Pb-n system 200 T E T( C) C : C o wt% n b b Microgrph of Pb-n eutectic microstructure 100 b b: 97.8 wt% n : 18.3 wt%n 160 m Adpted from Fig. 9.14, Cllister 7e C E C, wt% n
23 mellr Eutectic tructure Other possible eutectic structures re: rod-like, globulr nd ciculr.
24 Microstructures in Eutectic ystems: IV 18.3 wt% n < C o < 61.9 wt% n Result: crystls nd n eutectic microstructure Just bove T E : T( C) 300 Pb-n system 200 T E R R + b : C o wt% n b C o, wt% n b primry eutectic eutectic b C = 18.3 wt% n C = 61.9 wt% n W = = 50 wt% R + W = (1- W ) = 50 wt% Just below T E : C = 18.3 wt% n C b = 97.8 wt% n W = = 73 wt% R + W b = 27 wt%
Chapter 9: Phase Diagrams
 Chpter 9: Phse Digrms ISSUES TO ADDRESS... When we combine two elements... wht is the resulting equilibrium stte? In prticulr, if we specify... -- the composition (e.g., wt% Cu - wt% Ni), nd -- the temperture
Chpter 9: Phse Digrms ISSUES TO ADDRESS... When we combine two elements... wht is the resulting equilibrium stte? In prticulr, if we specify... -- the composition (e.g., wt% Cu - wt% Ni), nd -- the temperture
Phase Equilibria: Solubility Limit PHASE DIAGRAMS 10 0 Solubility 8 0 Limit ENT 145 Materials Engineering (liquid) atu (liquid solution 4 0
 Temperture (ºC) ter ugr Temperture (ºC) Phse Equilibri: olubility imit PHE DIGM ENT 145 Mterils Engineering Chpter 9 - olution solid, liquid, or gs solutions, single phse Mixture more thn one phse olubility
Temperture (ºC) ter ugr Temperture (ºC) Phse Equilibri: olubility imit PHE DIGM ENT 145 Mterils Engineering Chpter 9 - olution solid, liquid, or gs solutions, single phse Mixture more thn one phse olubility
Chapter 9: Phase Diagrams
 hpter 9: Phse Digrms ISSUES TO ADDRESS... oncepts of Phse, omponent, Equilibrium Phse digrm In prticulr, if we specify... -- composition (e.g., wt% u - wt% Ni), nd -- temperture (T) then... How mny phses
hpter 9: Phse Digrms ISSUES TO ADDRESS... oncepts of Phse, omponent, Equilibrium Phse digrm In prticulr, if we specify... -- composition (e.g., wt% u - wt% Ni), nd -- temperture (T) then... How mny phses
PY2N20 Material Properties and Phase Diagrams
 PY2N20 Mteril Properties nd Phse Digrms ecture 6 P. Stmenov, PhD School of Physics, TCD PY2N20-6 Microstructures in Eutectic Systems: I C o < 2 wt% Sn Result: - t extreme ends - polycrystl of grins i.e.,
PY2N20 Mteril Properties nd Phse Digrms ecture 6 P. Stmenov, PhD School of Physics, TCD PY2N20-6 Microstructures in Eutectic Systems: I C o < 2 wt% Sn Result: - t extreme ends - polycrystl of grins i.e.,
Course Objectives. MSE 260 Phase Transformations. Prerequisites. Course Outcomes 1/16/2017. Forms of Assessment. Course Outline. Dr.
 ME 26 Phse Trnsformtions Kwme Nkrumh University of cience & Technology, Kumsi, Ghn Dr. Anthony Andrews Deprtment of Mterils Engineering Fculty of Mechnicl nd Chemicl Engineering College of Engineering
ME 26 Phse Trnsformtions Kwme Nkrumh University of cience & Technology, Kumsi, Ghn Dr. Anthony Andrews Deprtment of Mterils Engineering Fculty of Mechnicl nd Chemicl Engineering College of Engineering
Material Properties and Phase Diagrams
 PY2M20 Material Properties and Phase Diagrams ecture 5 P. Stamenov, PhD School of Physics, TCD PY2M20-5 Phase Diagrams - Introduction How much can be done with pure elemental compounds? How many combinations
PY2M20 Material Properties and Phase Diagrams ecture 5 P. Stamenov, PhD School of Physics, TCD PY2M20-5 Phase Diagrams - Introduction How much can be done with pure elemental compounds? How many combinations
Chapter 9: PHASE DIAGRAM
 Chpter 9: PHASE DIAGRAM ISSUES TO ADDRESS... When we combine two elements... wht is the resulting equilibrium stte? In prticulr, if we specify... -- the composition (e.g., wt% Cu - wt% Ni), nd -- the temperture
Chpter 9: PHASE DIAGRAM ISSUES TO ADDRESS... When we combine two elements... wht is the resulting equilibrium stte? In prticulr, if we specify... -- the composition (e.g., wt% Cu - wt% Ni), nd -- the temperture
Chapter 11: Phase Diagrams. Phase Equilibria: Solubility Limit
 Temperature ( C) Water ugar 217/1/4 Chapter 11: Phase Diagrams IUE TO ADDRE... When we combine two elements... what is the resulting equilibrium state? In particular, if we specify... -- the composition
Temperature ( C) Water ugar 217/1/4 Chapter 11: Phase Diagrams IUE TO ADDRE... When we combine two elements... what is the resulting equilibrium state? In particular, if we specify... -- the composition
The Solubility Limit
 Phase Diagrams When we combine two elements... what equilibrium state do we get? In particular, if we specify... --a composition (e.g., wt%cu - wt%ni), and --a temperature (T) then... How many phases do
Phase Diagrams When we combine two elements... what equilibrium state do we get? In particular, if we specify... --a composition (e.g., wt%cu - wt%ni), and --a temperature (T) then... How many phases do
Chapter 9: Phase Diagrams
 Chapter 9: Phase Diagrams When we combine two elements... what equilibrium state do we get? In particular, if we specify... --a composition (e.g., wt% Cu - wt% Ni), and --a temperature (T ) then... How
Chapter 9: Phase Diagrams When we combine two elements... what equilibrium state do we get? In particular, if we specify... --a composition (e.g., wt% Cu - wt% Ni), and --a temperature (T ) then... How
The Stabilities of phase
 The Stabilities of phase What is a phase? A phase is a form of matter that is uniform throughout in chemical composition and physical state Example Fig 1. White phosphorus Fig 2.Black phosphorus Fig 3.
The Stabilities of phase What is a phase? A phase is a form of matter that is uniform throughout in chemical composition and physical state Example Fig 1. White phosphorus Fig 2.Black phosphorus Fig 3.
Chapter 9: Phase Diagrams
 Chapter 9: Phase Diagrams IUE TO ADDE... Common types of phase diagrams Isomorphous Eutectic Others Phase diagram and microstructure evolution Chapter 9-1 What is a phase? What is a component? Class Exercise
Chapter 9: Phase Diagrams IUE TO ADDE... Common types of phase diagrams Isomorphous Eutectic Others Phase diagram and microstructure evolution Chapter 9-1 What is a phase? What is a component? Class Exercise
Chapter 11: Phase Diagrams
 Chapter 11: Phase Diagrams ISSUES TO ADDRESS... When we combine two elements... what is the resulting equilibrium state? In particular, if we specify... -- the composition (e.g., wt% Cu - wt% Ni), and
Chapter 11: Phase Diagrams ISSUES TO ADDRESS... When we combine two elements... what is the resulting equilibrium state? In particular, if we specify... -- the composition (e.g., wt% Cu - wt% Ni), and
Phase Diagrams. Phases
 Phase Diagrams Reading: Callister Ch. 10 What is a phase? What is the equilibrium i state t when different elements are mixed? What phase diagrams tell us. How phases evolve with temperature and composition
Phase Diagrams Reading: Callister Ch. 10 What is a phase? What is the equilibrium i state t when different elements are mixed? What phase diagrams tell us. How phases evolve with temperature and composition
Lecture 7: Solid State Reactions Phase Diagrams and Mixing
 Lecture 7: Solid State Reactions Phase Diagrams and Mixing Prof Ken Durose, Univ of Liverpool Text book for this lecture: Callister Materials Science and Engineering Learning objectives 1.Solid state reactions
Lecture 7: Solid State Reactions Phase Diagrams and Mixing Prof Ken Durose, Univ of Liverpool Text book for this lecture: Callister Materials Science and Engineering Learning objectives 1.Solid state reactions
CHAPTER 9: PHASE DIAGRAMS
 CHAPTER 9: PHASE DIAGRAMS ISSUES TO ADDRESS... When we combine two elements... what equilibrium state do we get? In particular, if we specify... --a composition (e.g., wt%cu - wt%ni), and --a temperature
CHAPTER 9: PHASE DIAGRAMS ISSUES TO ADDRESS... When we combine two elements... what equilibrium state do we get? In particular, if we specify... --a composition (e.g., wt%cu - wt%ni), and --a temperature
Phase Diagrams, Solid Solutions, Phase Strengthening, Phase Transformations
 Phase Diagrams, Solid Solutions, Phase Strengthening, Phase Transformations Components and Phases Components: The elements or compounds that are mixed initially (Al and Cu). Phases: A phase is a homogenous,
Phase Diagrams, Solid Solutions, Phase Strengthening, Phase Transformations Components and Phases Components: The elements or compounds that are mixed initially (Al and Cu). Phases: A phase is a homogenous,
Phase Diagrams & Phase Tranformation
 ep-16 Phase Diagrams & Phase Tranformation Microstructure - Phases Ferrite Cementite EM micrograph x magnification Plain C steel containing.44 wt. % C Basic Definitions Alloy: A metallic substance that
ep-16 Phase Diagrams & Phase Tranformation Microstructure - Phases Ferrite Cementite EM micrograph x magnification Plain C steel containing.44 wt. % C Basic Definitions Alloy: A metallic substance that
ENGR 151: Materials of Engineering LECTURE #14: PHASE DIAGRAMS
 ENGR 151: Materials of Engineering LECTURE #14: PHASE DIAGRAMS ANNOUNCEMENTS Midterm #2 Monday, May 1. Review on Wednesday, April 26. Chapters 4, 6, 7, 8 TERMINOLOGY Phase: Homogeneous portion of a system
ENGR 151: Materials of Engineering LECTURE #14: PHASE DIAGRAMS ANNOUNCEMENTS Midterm #2 Monday, May 1. Review on Wednesday, April 26. Chapters 4, 6, 7, 8 TERMINOLOGY Phase: Homogeneous portion of a system
Chapter 10. Phase Diagrams
 Chapter 10 Phase Diagrams Chapter 10 Terminology and Unary System Phase Diagrams Issues to Address... When we combine two elements... what equilibrium state do we get? In particular, if we specify... --a
Chapter 10 Phase Diagrams Chapter 10 Terminology and Unary System Phase Diagrams Issues to Address... When we combine two elements... what equilibrium state do we get? In particular, if we specify... --a
Components The elements or compounds which are mixed initially. (e.g. Al & Cu)
 Why Study Phase Diagrams? The purpose of this lecture is to develop an understanding of the phase transformations which occur under conditions of slow cooling; Under such conditions equilibrium is approached,
Why Study Phase Diagrams? The purpose of this lecture is to develop an understanding of the phase transformations which occur under conditions of slow cooling; Under such conditions equilibrium is approached,
ENGR 151: Materials of Engineering LECTURE #15: PHASE DIAGRAMS
 ENGR 151: Materials of Engineering LECTURE #15: PHASE DIAGRAMS TENSILE TESTING VIDEO https://www.youtube.com/watch?v=-qukvzo2jse PROPERTIES OF ISOMORPHOUS ALLOYS Solid solution strengthening For Ni-Cu
ENGR 151: Materials of Engineering LECTURE #15: PHASE DIAGRAMS TENSILE TESTING VIDEO https://www.youtube.com/watch?v=-qukvzo2jse PROPERTIES OF ISOMORPHOUS ALLOYS Solid solution strengthening For Ni-Cu
Phase Diagrams of Pure Substances Predicts the stable phase as a function of P total and T. Example: water can exist in solid, liquid and vapor
 PHASE DIAGRAMS Phase a chemically and structurally homogenous region of a material. Region of uniform physical and chemical characteristics. Phase boundaries separate two distinct phases. A single phase
PHASE DIAGRAMS Phase a chemically and structurally homogenous region of a material. Region of uniform physical and chemical characteristics. Phase boundaries separate two distinct phases. A single phase
The internal structure of a material plays an important part on its mechanical properties.!
 Phase Diagrams The internal structure of a material plays an important part on its mechanical properties.! There is a strong correlation between micro structure and mechanical properties. Definitions Component!
Phase Diagrams The internal structure of a material plays an important part on its mechanical properties.! There is a strong correlation between micro structure and mechanical properties. Definitions Component!
CHAPTER 9: PHASE DIAGRAMS
 CHAPTER 9: PHASE DIAGRAMS ISSUES TO ADDRESS... When we combine two elements... what equilibrium state do we get? In particular, if we specify... --a composition (e.g., wt%cu - wt%ni), and --a temperature
CHAPTER 9: PHASE DIAGRAMS ISSUES TO ADDRESS... When we combine two elements... what equilibrium state do we get? In particular, if we specify... --a composition (e.g., wt%cu - wt%ni), and --a temperature
Effect of Tantalum Additions to a Cobalt-Chromium-Nickel
 Effect of Tntlum Additions to Coblt-Chromium-Nickel Bse Alloy A. P. ROWE, W. C. BIGELOW, nd K. ASGAR University of Michign, School of Dentistry, Ann Arbor, Michign 48104, USA An investigtion by electron
Effect of Tntlum Additions to Coblt-Chromium-Nickel Bse Alloy A. P. ROWE, W. C. BIGELOW, nd K. ASGAR University of Michign, School of Dentistry, Ann Arbor, Michign 48104, USA An investigtion by electron
Phase diagrams. R.D.Makwana,IT,NU. R.D.Makwana,IT,NU
 Phase diagrams Phase?? Phase is region throughout which all properties of material are essentially uniform Uniform region of a system which has uniform physical and chemical characteristics Phase diagram?
Phase diagrams Phase?? Phase is region throughout which all properties of material are essentially uniform Uniform region of a system which has uniform physical and chemical characteristics Phase diagram?
Metallic Materials-Phase Diagrams
 Engineering Alloys Metals and alloys have many useful engineering properties and so have wide spread application in engineering designs. Iron and its alloys (principally steel) account for about 90 percent
Engineering Alloys Metals and alloys have many useful engineering properties and so have wide spread application in engineering designs. Iron and its alloys (principally steel) account for about 90 percent
MME292 Metallic Materials Sessional
 Department of Materials and Metallurgical Engineering angladesh University of Engineering and Technology, Dhaka MME292 Metallic Materials Sessional July 2016 Term Experiment 2 Construction and Interpretation
Department of Materials and Metallurgical Engineering angladesh University of Engineering and Technology, Dhaka MME292 Metallic Materials Sessional July 2016 Term Experiment 2 Construction and Interpretation
PHASE EQUILIBRIUM P + F = C + 2
 PHASE EQUILIBRIUM Component: is either pure metal and/or compound of which an alloy is composed. They refer to the independent chemical species that comprise the system. Solid Solution: It consists of
PHASE EQUILIBRIUM Component: is either pure metal and/or compound of which an alloy is composed. They refer to the independent chemical species that comprise the system. Solid Solution: It consists of
Modeling Diffusion: Flux
 Modeling Diffusion: Flux Flux (#/area/time): J = 1 A dm dt Directional Quantity y Jy kg atoms m 2 or s m 2 s Jx Jz x z Flux can be measured for: --vacancies and interstitials --host (A) atoms --impurity
Modeling Diffusion: Flux Flux (#/area/time): J = 1 A dm dt Directional Quantity y Jy kg atoms m 2 or s m 2 s Jx Jz x z Flux can be measured for: --vacancies and interstitials --host (A) atoms --impurity
MAE 212: Spring 2001 Lecture 14 PHASE DIAGRAMS AND EQUILIBRIUM MICROSTRUCTURES N. Zabaras
 ME 212: Spring 2001 Lecture 14 PHSE DIGRMS ND EQUILIRIUM MICROSTRUCTURES N. Zabaras For more details on the topic read Chapter 9 of the Materials Science for Engineers by J. Shackelford, pp. 304 353. lso
ME 212: Spring 2001 Lecture 14 PHSE DIGRMS ND EQUILIRIUM MICROSTRUCTURES N. Zabaras For more details on the topic read Chapter 9 of the Materials Science for Engineers by J. Shackelford, pp. 304 353. lso
CHAPTER 10 PHASE DIAGRAMS PROBLEM SOLUTIONS
 CHAPTER 10 PHASE DIAGRAMS PROBLEM SOLUTIONS Solubility Limit 10.1 Consider the sugar water phase diagram of Figure 10.1. (a) How much sugar will dissolve in 1000 g of water at 80 C (176 F)? (b) If the
CHAPTER 10 PHASE DIAGRAMS PROBLEM SOLUTIONS Solubility Limit 10.1 Consider the sugar water phase diagram of Figure 10.1. (a) How much sugar will dissolve in 1000 g of water at 80 C (176 F)? (b) If the
Two Components System
 Two Components System Three independent variables: T, P, compositions In general, constant pressure (fixed parameter). P+F=C+1 Simple Eutectic System The binary eutectic phase diagram explains the chemical
Two Components System Three independent variables: T, P, compositions In general, constant pressure (fixed parameter). P+F=C+1 Simple Eutectic System The binary eutectic phase diagram explains the chemical
Chapter 9 Phase Diagrams. Dr. Feras Fraige
 Chapter 9 Phase Diagrams Dr. Feras Fraige Chapter Outline Definitions and basic concepts Phases and microstructure Binary isomorphous systems (complete solid solubility) Binary eutectic systems (limited
Chapter 9 Phase Diagrams Dr. Feras Fraige Chapter Outline Definitions and basic concepts Phases and microstructure Binary isomorphous systems (complete solid solubility) Binary eutectic systems (limited
THERMODYNAMICS OF As, Sb AND Bi DISTRIBUTION DURING REVERB FURNACE SMELTING
 Journl of Mining nd Metllurgy, 38 (1 2) B (2002) 93-102 THERMODYNAMICS OF As, Sb AND Bi DISTRIBUTION DURING REVERB FURNACE SMELTING N.Mitevsk* nd @.D.@ivkovi}** *RTB BOR, Copper Institute, 19210 Bor, Yugoslvi
Journl of Mining nd Metllurgy, 38 (1 2) B (2002) 93-102 THERMODYNAMICS OF As, Sb AND Bi DISTRIBUTION DURING REVERB FURNACE SMELTING N.Mitevsk* nd @.D.@ivkovi}** *RTB BOR, Copper Institute, 19210 Bor, Yugoslvi
The Science and Engineering of Materials, 4 th ed Donald R. Askeland Pradeep P. Phulé. Chapter 8 Solid Solutions and Phase Equilibrium
 The Science and Engineering of Materials, 4 th ed Donald R. Askeland Pradeep P. Phulé Chapter 8 Solid Solutions and Phase Equilibrium Objectives of Chapter 8 The goal of this chapter is to describe the
The Science and Engineering of Materials, 4 th ed Donald R. Askeland Pradeep P. Phulé Chapter 8 Solid Solutions and Phase Equilibrium Objectives of Chapter 8 The goal of this chapter is to describe the
the Phase Diagrams Today s Topics
 MME 291: Lecture 04 Interpretation of the Phase Diagrams Prof. A.K.M.B. Rashid Department of MME BUET, Dhaka Today s Topics Interpretation of phase diagrams Development of microstructures during equilibrium
MME 291: Lecture 04 Interpretation of the Phase Diagrams Prof. A.K.M.B. Rashid Department of MME BUET, Dhaka Today s Topics Interpretation of phase diagrams Development of microstructures during equilibrium
Cu-Ag phase diagram Brazil s map
 Phase Diagrams [9] Cu-Ag phase diagram Brazil s map 1> Some important definitions Component - chemically recognizable species (Fe and C in carbon steel, H2O and NaCl in salted water). A binary alloy contains
Phase Diagrams [9] Cu-Ag phase diagram Brazil s map 1> Some important definitions Component - chemically recognizable species (Fe and C in carbon steel, H2O and NaCl in salted water). A binary alloy contains
PHASE DIAGRAMS. IE-114 Materials Science and General Chemistry Lecture-10
 PHASE DIAGRAMS IE-114 Materials Science and General Chemistry Lecture-10 Importance of Phase Diagrams There is a strong correlation between microstructure and mechanical properties. Phase diagrams provides
PHASE DIAGRAMS IE-114 Materials Science and General Chemistry Lecture-10 Importance of Phase Diagrams There is a strong correlation between microstructure and mechanical properties. Phase diagrams provides
12/3/ :12 PM. Chapter 9. Phase Diagrams. Dr. Mohammad Abuhaiba, PE
 Chapter 9 Phase Diagrams 1 2 Learning Objectives 1. Isomorphous and eutectic phase diagrams: a. label various phase regions b. Label liquidus, solidus, and solvus lines 2. Given a binary phase diagram
Chapter 9 Phase Diagrams 1 2 Learning Objectives 1. Isomorphous and eutectic phase diagrams: a. label various phase regions b. Label liquidus, solidus, and solvus lines 2. Given a binary phase diagram
1. Supplementary Figures and Legends a b
 doi:10.1038/nture09540 1. Supplementry Figures nd Legends Supplementry Figure 1. Scnning trnsmission electron microgrphs (STEM) of NCC., STEM prepred from fst evportion of dilute NCC suspension shows individul
doi:10.1038/nture09540 1. Supplementry Figures nd Legends Supplementry Figure 1. Scnning trnsmission electron microgrphs (STEM) of NCC., STEM prepred from fst evportion of dilute NCC suspension shows individul
Cu/Ag Eutectic System
 Eutectic Systems The simplest kind of system with two solid phases is called a eutectic system. A eutectic system contains two solid phases at low temperature. These phases may have different crystal structures,
Eutectic Systems The simplest kind of system with two solid phases is called a eutectic system. A eutectic system contains two solid phases at low temperature. These phases may have different crystal structures,
Equilibrium phase diagram of metallic alloy
 Equilibrium phase diagram of metallic alloy Motivation New structure, concentration (mixing level) (at what temperature? for how long? ) Phase Diagrams - Introduction. Many materials systems can exist
Equilibrium phase diagram of metallic alloy Motivation New structure, concentration (mixing level) (at what temperature? for how long? ) Phase Diagrams - Introduction. Many materials systems can exist
Phase diagrams (cont.) and the Fe-C system
 Phase diagrams (cont.) and the Fe-C system Solidification: Pro-eutectic vs Eutectic Pro-eutectic solidification Ideal liquid, uniform distribution Solid Pb(Sn) () nucleates Solubility limit leads to Sn
Phase diagrams (cont.) and the Fe-C system Solidification: Pro-eutectic vs Eutectic Pro-eutectic solidification Ideal liquid, uniform distribution Solid Pb(Sn) () nucleates Solubility limit leads to Sn
5 a l l o y i n g b e h av i o r
 has dissolved in A to form a substitutional solid solution (igure 5.1b). It can be said also that this is a single-phase alloy. The word phase simply implies that the crystal structure and the alloy composition
has dissolved in A to form a substitutional solid solution (igure 5.1b). It can be said also that this is a single-phase alloy. The word phase simply implies that the crystal structure and the alloy composition
Lecture 3: Solutions: Activities and. Phase Diagrams
 Lecture 3: Solutions: Activities and Lecture plan: Phase Diagrams 21-09-2010 Gibbs phase rule vapour composition two-component phase diagrams phase diagrams in material science: microstructures in isomorphous
Lecture 3: Solutions: Activities and Lecture plan: Phase Diagrams 21-09-2010 Gibbs phase rule vapour composition two-component phase diagrams phase diagrams in material science: microstructures in isomorphous
Phase diagrams wt% of carbon in Fe microstructure of a lead tin alloy of eutectic composition
 Phase diagrams 0.44 wt% of carbon in Fe microstructure of a lead tin alloy of eutectic composition Primer Materials For Science Teaching Spring 2018 28.6.2018 What is a phase? A phase may be defined as
Phase diagrams 0.44 wt% of carbon in Fe microstructure of a lead tin alloy of eutectic composition Primer Materials For Science Teaching Spring 2018 28.6.2018 What is a phase? A phase may be defined as
q p P : Si : : : : Si : : : Si : Si : Si : : Extra free e - Dope Here, energy to get 1/26/10 Lecture Topics: Silicon
 EE 143 Microfbriction Technology Lecture 3 Mterils II 1/26/10 Reding portions throughout Jeger Lecture Topics licon licon Dioxide licon Nitride Aluminum & Other Metls In this course, the focus will be
EE 143 Microfbriction Technology Lecture 3 Mterils II 1/26/10 Reding portions throughout Jeger Lecture Topics licon licon Dioxide licon Nitride Aluminum & Other Metls In this course, the focus will be
Solidification & Binary Phase Diagrams II. Solidification & Binary Phase Diagrams II
 Module 19 Solidification & inary Phase Diagrams II ecture 19 Solidification & inary Phase Diagrams II 1 NPTE Phase II : IIT Kharagpur : Prof. R. N. hosh, Dept of Metallurgical and Materials Engineering
Module 19 Solidification & inary Phase Diagrams II ecture 19 Solidification & inary Phase Diagrams II 1 NPTE Phase II : IIT Kharagpur : Prof. R. N. hosh, Dept of Metallurgical and Materials Engineering
but T m (Sn0.62Pb0.38) = 183 C, so this is a common soldering alloy.
 T m (Sn) = 232 C, T m (Pb) = 327 C but T m (Sn0.62Pb0.38) = 183 C, so this is a common soldering alloy. T m (Au) = 1064 C, T m (Si) = 2550 C but T m (Au0.97Si0.03) = 363 C, so thin layer of gold is used
T m (Sn) = 232 C, T m (Pb) = 327 C but T m (Sn0.62Pb0.38) = 183 C, so this is a common soldering alloy. T m (Au) = 1064 C, T m (Si) = 2550 C but T m (Au0.97Si0.03) = 363 C, so thin layer of gold is used
Introduction of Materials Materials Science SScience
 材料科學導論 Introduction of Materials Science 許正興國立聯合大學電機工程學系 1. Introduction of Materials Science and Engineering 2. Atomic Structure and Bonding 3. Crystal Structures and Crystal Geometry 4. Solidification,
材料科學導論 Introduction of Materials Science 許正興國立聯合大學電機工程學系 1. Introduction of Materials Science and Engineering 2. Atomic Structure and Bonding 3. Crystal Structures and Crystal Geometry 4. Solidification,
solvent: component of a solution present in the greatest amount in alloy.
 Phase Equilibrium Diagrams:- Phase equilibrium diagram is a graphic relationship between temperature and weight ratios of elements and alloys contribute to the built of the diagram. Phase diagrams provide
Phase Equilibrium Diagrams:- Phase equilibrium diagram is a graphic relationship between temperature and weight ratios of elements and alloys contribute to the built of the diagram. Phase diagrams provide
Chapter 10: Phase Diagrams
 hapter 10: Phase Diagrams Show figures 10-1 and 10-3, and discuss the difference between a component and a phase. A component is a distinct chemical entity, such as u, Ni, NiO or MgO. A phase is a chemically
hapter 10: Phase Diagrams Show figures 10-1 and 10-3, and discuss the difference between a component and a phase. A component is a distinct chemical entity, such as u, Ni, NiO or MgO. A phase is a chemically
Introduction to the phase diagram Uses and limitations of phase diagrams Classification of phase diagrams Construction of phase diagrams
 Prof. A.K.M.B. Rashid Department of MME BUET, Dhaka Concept of alloying Classification of alloys Introduction to the phase diagram Uses and limitations of phase diagrams Classification of phase diagrams
Prof. A.K.M.B. Rashid Department of MME BUET, Dhaka Concept of alloying Classification of alloys Introduction to the phase diagram Uses and limitations of phase diagrams Classification of phase diagrams
Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Phase Diagram
 SE104 Structural Materials Phase Diagram Dr. Yu Qiao Department of Structural Engineering, UCSD Introduction Phase: A region in a material that differs in structure and function from other regions. Phase
SE104 Structural Materials Phase Diagram Dr. Yu Qiao Department of Structural Engineering, UCSD Introduction Phase: A region in a material that differs in structure and function from other regions. Phase
Alloys & Their Phase Diagrams. مرجع علمى مهندسى مواد
 Alloys & Their Phase Diagrams Objectives of the class Gibbs phase rule Introduction to phase diagram Practice phase diagram Lever rule Important Observation: One question in the midterm Gibbs phase rule
Alloys & Their Phase Diagrams Objectives of the class Gibbs phase rule Introduction to phase diagram Practice phase diagram Lever rule Important Observation: One question in the midterm Gibbs phase rule
Thermo-Calc based multi-component micro-segregation model and solidification paths calculations
 August 2012 Reserch & Development Thermo-Clc bsed multi-component micro-segregtion model nd solidifiction pths clcultions *Zho Gungwei 1, i XinZhong 1, Xu Dming 1, Guo Jingjie 1, Fu Hengzhi 1, Du Yong
August 2012 Reserch & Development Thermo-Clc bsed multi-component micro-segregtion model nd solidifiction pths clcultions *Zho Gungwei 1, i XinZhong 1, Xu Dming 1, Guo Jingjie 1, Fu Hengzhi 1, Du Yong
Material Properties and Phase Diagrams
 PY2M20 Material Properties and Phase Diagrams Lecture 6 P. Stamenov, PhD School of Physics, TCD PY2M20-6 Microstructures in Eutectic ti Systems: I C o < 2 wt% Sn Result: - at extreme ends - polycrystal
PY2M20 Material Properties and Phase Diagrams Lecture 6 P. Stamenov, PhD School of Physics, TCD PY2M20-6 Microstructures in Eutectic ti Systems: I C o < 2 wt% Sn Result: - at extreme ends - polycrystal
2016 Prelim Essay Question 2
 216 Prelim Essy Question 2 In recent yers, the price of nturl fertilisers for orgnic brown rice production hs risen nd helthy living cmpigns re seeing more consumers switching from nonorgnic white rice
216 Prelim Essy Question 2 In recent yers, the price of nturl fertilisers for orgnic brown rice production hs risen nd helthy living cmpigns re seeing more consumers switching from nonorgnic white rice
the Phase Diagrams Today s Topics
 MME 291: Lecture 03 Introduction to the Phase Diagrams Prof. A.K.M.B. Rashid Department of MME BUET, Dhaka Today s Topics Concept of alloying Classification of alloys Introduction to the phase diagram
MME 291: Lecture 03 Introduction to the Phase Diagrams Prof. A.K.M.B. Rashid Department of MME BUET, Dhaka Today s Topics Concept of alloying Classification of alloys Introduction to the phase diagram
There are many types of alloying systems which they are:
 Phase Equilibrium Diagrams:- Phase equilibrium diagram is a graphic relationship between temperature and weight ratios of elements and alloys contribute to the built of the diagram. Where Phase is a uniform
Phase Equilibrium Diagrams:- Phase equilibrium diagram is a graphic relationship between temperature and weight ratios of elements and alloys contribute to the built of the diagram. Where Phase is a uniform
Two Metals Completely Soluble in the Liquid State and Completely Insoluble in the solid state
 Two Metals Completely Soluble in the Liquid State and Completely Insoluble in the solid state Technically, no two metals are completely insoluble in each other. However, in some cases the solubility is
Two Metals Completely Soluble in the Liquid State and Completely Insoluble in the solid state Technically, no two metals are completely insoluble in each other. However, in some cases the solubility is
MSE 513 Homework #1 Due Jan. 21, 2013
 Reading: My class notes, pgs. 22-27. http://www1.asminternational.org/asmenterprise/apd/help/help.aspx Introduction to Phase diagrams, particularly: o Common terms o Binary diagrams o Features of phase
Reading: My class notes, pgs. 22-27. http://www1.asminternational.org/asmenterprise/apd/help/help.aspx Introduction to Phase diagrams, particularly: o Common terms o Binary diagrams o Features of phase
Introduction to Phase Diagrams. Version 2.1. Andrew Green, MATTER Trevor Myers, UMIST/University of Manchester
 Introduction to Phase Diagrams Version 2.1 Andrew Green, MATTER Trevor Myers, UMIST/University of Manchester Assumed Pre-knowledge This module has been designed as a basic introduction to many of the concepts
Introduction to Phase Diagrams Version 2.1 Andrew Green, MATTER Trevor Myers, UMIST/University of Manchester Assumed Pre-knowledge This module has been designed as a basic introduction to many of the concepts
Engineering materials
 1 Engineering materials Lecture 6 Metal and Alloys Phase Diagrams 2 Metals and alloys Some metals may have more than one crystal structure, a phenomenon known as polymorphism ( 同質異性 ), when found in elemental
1 Engineering materials Lecture 6 Metal and Alloys Phase Diagrams 2 Metals and alloys Some metals may have more than one crystal structure, a phenomenon known as polymorphism ( 同質異性 ), when found in elemental
Schematic representation of the development of microstructure. during the equilibrium solidification of a 35 wt% Ni-65 wt% Cu alloy
 Schematic representation of the development of microstructure during the equilibrium solidification of a 35 wt% Ni-65 wt% Cu alloy At 1300 ºC (point a) the alloy is in the liquid condition This continues
Schematic representation of the development of microstructure during the equilibrium solidification of a 35 wt% Ni-65 wt% Cu alloy At 1300 ºC (point a) the alloy is in the liquid condition This continues
The point at which quantity demanded and quantity supplied come together is known as equilibrium. Price of a slice of pizza $2.00. Demand $2.50 $3.
 Blncing the Mrket The point t which quntity demnded nd quntity supplied come together is known s equilibrium. Finding Equilibrium per slice $3.5 $3. $2.5 $2. $1.5 $1. $.5 Equilibrium Point Equilibrium
Blncing the Mrket The point t which quntity demnded nd quntity supplied come together is known s equilibrium. Finding Equilibrium per slice $3.5 $3. $2.5 $2. $1.5 $1. $.5 Equilibrium Point Equilibrium
Chapter 9. Quadratics
 Chpter 9 Qudrtics Artificil Body Prts 9.1 Solving Qudrtic Equtions by Fctoring 9. Completing the Squre 9.3 The Qudrtic Formul 9.4 Eponentil Functions (Growth nd Decy) Chpter Review Chpter Test 147 Section
Chpter 9 Qudrtics Artificil Body Prts 9.1 Solving Qudrtic Equtions by Fctoring 9. Completing the Squre 9.3 The Qudrtic Formul 9.4 Eponentil Functions (Growth nd Decy) Chpter Review Chpter Test 147 Section
Chapter 3: The Structure of Crystalline Solids
 Chpter 3: The Structure of Crystlline Solids ISSUES TO ADDRESS... How do toms ssemble into solid structures? Exmples of dependence of mteril property on its crystl structure Chpter 3-1 Crystlline mterils...
Chpter 3: The Structure of Crystlline Solids ISSUES TO ADDRESS... How do toms ssemble into solid structures? Exmples of dependence of mteril property on its crystl structure Chpter 3-1 Crystlline mterils...
FAILURE OF PINUS RADIATA VENEER IN TENSION ACROSS THE GRAIN
 120 NOTE FAILURE OF PINUS RADIATA VENEER IN TENSION ACROSS THE GRAIN A. MICHELLE CARRINGTON, ROGER B. KEEY, Deprtment of Chemicl nd Process Engineering, University of Cnterbury, Christchurch, New Zelnd
120 NOTE FAILURE OF PINUS RADIATA VENEER IN TENSION ACROSS THE GRAIN A. MICHELLE CARRINGTON, ROGER B. KEEY, Deprtment of Chemicl nd Process Engineering, University of Cnterbury, Christchurch, New Zelnd
Slide 1. Slide 2. Slide 3. Chapter 10: Solid Solutions and Phase Equilibrium. Learning Objectives. Introduction
 Slide 1 Chapter 10: Solid Solutions and Phase Equilibrium 10-1 Slide 2 Learning Objectives 1. Phases and the phase diagram 2. Solubility and solid solutions 3. Conditions for unlimited solid solubility
Slide 1 Chapter 10: Solid Solutions and Phase Equilibrium 10-1 Slide 2 Learning Objectives 1. Phases and the phase diagram 2. Solubility and solid solutions 3. Conditions for unlimited solid solubility
[ HOCl] Chapter 16. Problem. Equilibria in Solutions of Weak Acids. Equilibria in Solutions of Weak Acids
![[ HOCl] Chapter 16. Problem. Equilibria in Solutions of Weak Acids. Equilibria in Solutions of Weak Acids [ HOCl] Chapter 16. Problem. Equilibria in Solutions of Weak Acids. Equilibria in Solutions of Weak Acids](/thumbs/72/67425582.jpg) Equilibri in Solutions of Wek Acids Chpter 16 Acid-Bse Equilibri Dr. Peter Wrburton peterw@mun.c http://www.chem.mun.c/zcourses/1011.php The dissocition of wek cid is n equilibrium sitution with n equilibrium
Equilibri in Solutions of Wek Acids Chpter 16 Acid-Bse Equilibri Dr. Peter Wrburton peterw@mun.c http://www.chem.mun.c/zcourses/1011.php The dissocition of wek cid is n equilibrium sitution with n equilibrium
Phase Diagrams. Today s Topics
 MME 291: Lecture 05 Eutectic Type Phase Diagrams Prof. A.K.M.B. Rashid Department of MME BUET, Dhaka Today s Topics Binary eutectic systems with partial miscibility Nomenclatures and invariant reaction
MME 291: Lecture 05 Eutectic Type Phase Diagrams Prof. A.K.M.B. Rashid Department of MME BUET, Dhaka Today s Topics Binary eutectic systems with partial miscibility Nomenclatures and invariant reaction
Materials Engineering. Phase transformation Phase diagrams
 Materials Engineering Phase transformation Phase diagrams Phase Transformation Why is it important for us? o Temperature, chemical composition and pressure can change the properties of materials o Understanding
Materials Engineering Phase transformation Phase diagrams Phase Transformation Why is it important for us? o Temperature, chemical composition and pressure can change the properties of materials o Understanding
CHAPTER 9 PHASE DIAGRAMS
 CHAPTER 9 PHASE DIAGRAMS PROBLEM SOLUTIONS 9.14 Determine the relative amounts (in terms of mass fractions) of the phases for the alloys and temperatures given in Problem 9.8. 9.8. This problem asks that
CHAPTER 9 PHASE DIAGRAMS PROBLEM SOLUTIONS 9.14 Determine the relative amounts (in terms of mass fractions) of the phases for the alloys and temperatures given in Problem 9.8. 9.8. This problem asks that
Building initial configuration. Ideal crystals (I)
 Building initil configurtion. Idel crstls (I) An crstlline solid cn be defined in terms of Brvis lttice which specifies the periodic rr in which the repeted units of the crstl re rrnged. Brvis lttice is
Building initil configurtion. Idel crstls (I) An crstlline solid cn be defined in terms of Brvis lttice which specifies the periodic rr in which the repeted units of the crstl re rrnged. Brvis lttice is
Why do cocktail ice served in expensive restaurants are clear whereas the ice formed in your refrigerator is cloudy?
 Phase Diagrams Why do cocktail ice served in expensive restaurants are clear whereas the ice formed in your refrigerator is cloudy? What is a solder alloy? What is the best composition for solder? How
Phase Diagrams Why do cocktail ice served in expensive restaurants are clear whereas the ice formed in your refrigerator is cloudy? What is a solder alloy? What is the best composition for solder? How
The Iron Iron Carbide (Fe Fe 3 C) Phase Diagram
 The Iron Iron Carbide (Fe Fe 3 C) Phase Diagram Steels: alloys of Iron (Fe) and Carbon (C). Fe-C phase diagram is complex. Will only consider the steel part of the diagram, up to around 7% Carbon. University
The Iron Iron Carbide (Fe Fe 3 C) Phase Diagram Steels: alloys of Iron (Fe) and Carbon (C). Fe-C phase diagram is complex. Will only consider the steel part of the diagram, up to around 7% Carbon. University
Three-Phase Wound-Rotor Induction Machine with Rotor Resistance
 Exercise 2 Three-Phse Wound-Rotor Induction Mchine with Rotor Resistnce EXERCISE OBJECTIVE When you hve completed this exercise, you will know the effects of vrying the rotor resistnce of three-phse wound-rotor
Exercise 2 Three-Phse Wound-Rotor Induction Mchine with Rotor Resistnce EXERCISE OBJECTIVE When you hve completed this exercise, you will know the effects of vrying the rotor resistnce of three-phse wound-rotor
Tanya Aycan Baser and Marcello Baricco
 Glss Rev.Adv.Mter.Sci. forming bility 18(2008) of 1-76 50 (M=none, Al, Nb) bulk metllic glsses 71 GLASS FORMING ABILITY OF (M=NONE, Al, Nb) BULK METALLIC GLASSES Tny Aycn Bser nd Mrcello Bricco Diprtimento
Glss Rev.Adv.Mter.Sci. forming bility 18(2008) of 1-76 50 (M=none, Al, Nb) bulk metllic glsses 71 GLASS FORMING ABILITY OF (M=NONE, Al, Nb) BULK METALLIC GLASSES Tny Aycn Bser nd Mrcello Bricco Diprtimento
Population Distribution
 Nme: Period: Why? Popultion Distriution How does popultion distriution ffect the environment? Alsk contins over 127 million cres of untouched forest lnd. It is the lrgest stte in the United Sttes, yet
Nme: Period: Why? Popultion Distriution How does popultion distriution ffect the environment? Alsk contins over 127 million cres of untouched forest lnd. It is the lrgest stte in the United Sttes, yet
Phase diagrams are diagrammatic representations of the phases present in a
 Chapter 4 What is a binary phase diagram? Phase diagrams are diagrammatic representations of the phases present in a system under specified equilibrium conditions, most often composition, temperature and
Chapter 4 What is a binary phase diagram? Phase diagrams are diagrammatic representations of the phases present in a system under specified equilibrium conditions, most often composition, temperature and
J = D C A C B x A x B + D C A C. = x A kg /m 2
 1. (a) Compare interstitial and vacancy atomic mechanisms for diffusion. (b) Cite two reasons why interstitial diffusion is normally more rapid than vacancy diffusion. (a) With vacancy diffusion, atomic
1. (a) Compare interstitial and vacancy atomic mechanisms for diffusion. (b) Cite two reasons why interstitial diffusion is normally more rapid than vacancy diffusion. (a) With vacancy diffusion, atomic
High strength fine grained structural steel, thermo-mechanically rolled, for high temperature application
 P420M HT High strength fine grined structurl steel, thermo-mechniclly rolled, for high temperture ppliction Specifiction DH-E52-D, edition April 2016 1 P420M HT is high strength thermomechniclly rolled
P420M HT High strength fine grined structurl steel, thermo-mechniclly rolled, for high temperture ppliction Specifiction DH-E52-D, edition April 2016 1 P420M HT is high strength thermomechniclly rolled
Structure study on rapidly solidified hydrogen storage alloy Zr(NiM) 2.1
 Journl of Alloys nd Compounds 293 29 (1999) 107 112 L Structure study on rpidly solidified hydrogen storge lloy Zr(NiM) 2.1 G.L. Lu *, K.Y. Shu, L.S. Chen, X.Y. Song, X.G. Yng, Y.Q. Lei, Q.D. Wng, b b
Journl of Alloys nd Compounds 293 29 (1999) 107 112 L Structure study on rpidly solidified hydrogen storge lloy Zr(NiM) 2.1 G.L. Lu *, K.Y. Shu, L.S. Chen, X.Y. Song, X.G. Yng, Y.Q. Lei, Q.D. Wng, b b
TALAT Lecture Phase Diagrams. 14 pages, 13 Figures. Basic Level
 TALAT Lecture 1203 Phase Diagrams 14 pages, 13 Figures Basic Level prepared by M H Jacobs * Interdisciplinary Research Centre in Materials The University of Birmingham, UK (Based on approach adopted by
TALAT Lecture 1203 Phase Diagrams 14 pages, 13 Figures Basic Level prepared by M H Jacobs * Interdisciplinary Research Centre in Materials The University of Birmingham, UK (Based on approach adopted by
Mohammad Anwar Karim Id :
 Department of Mechanical and Industrial Engineering ME 8109 Casting and Solidification of Materials EFFECTS OF RAPID SOLIDIFICATION ON MICROSTRUCTURE AND PROPERTIES OF AL, MG & TI ALLOYS Winter 2012 Presented
Department of Mechanical and Industrial Engineering ME 8109 Casting and Solidification of Materials EFFECTS OF RAPID SOLIDIFICATION ON MICROSTRUCTURE AND PROPERTIES OF AL, MG & TI ALLOYS Winter 2012 Presented
Binary Phase Diagrams - II
 Binary Phase Diagrams - II Note the alternating one phase / two phase pattern at any given temperature Binary Phase Diagrams - Cu-Al Can you spot the eutectoids? The peritectic points? How many eutectic
Binary Phase Diagrams - II Note the alternating one phase / two phase pattern at any given temperature Binary Phase Diagrams - Cu-Al Can you spot the eutectoids? The peritectic points? How many eutectic
(12) 1. Just one True and False question and a couple of multiple choice calculations, circle one answer for each problem, no partial credit.
 (1) 1. Just one True and False question and a couple of multiple choice calculations, circle one answer for each problem, no partial credit. The next page is left blank for your use, but no partial will
(1) 1. Just one True and False question and a couple of multiple choice calculations, circle one answer for each problem, no partial credit. The next page is left blank for your use, but no partial will
Types of Solids. Chapter 12. Solids and Modern Materials
 Chpter 12 Solids nd 1 Types of Solids Four generl types of solids. Metllic solids shre network of highly deloclized electrons. Ionic solids re sets of ctions nd nions mutully ttrcted to one nother. 2 Bonding
Chpter 12 Solids nd 1 Types of Solids Four generl types of solids. Metllic solids shre network of highly deloclized electrons. Ionic solids re sets of ctions nd nions mutully ttrcted to one nother. 2 Bonding
Metals I. Anne Mertens
 "MECA0139-1: Techniques "MECA0462-2 additives : et Materials 3D printing", Selection", ULg, 19/09/2017 25/10/2016 Metals I Anne Mertens Introduction Outline Metallic materials Materials Selection: case
"MECA0139-1: Techniques "MECA0462-2 additives : et Materials 3D printing", Selection", ULg, 19/09/2017 25/10/2016 Metals I Anne Mertens Introduction Outline Metallic materials Materials Selection: case
In their simplest form, steels are alloys of Iron (Fe) and Carbon (C).
 Iron-Carbon Phase Diagram Its defined as:- A map of the temperature at which different phase changes occur on very slow heating and cooling in relation to Carbon content. is Isothermal and continuous cooling
Iron-Carbon Phase Diagram Its defined as:- A map of the temperature at which different phase changes occur on very slow heating and cooling in relation to Carbon content. is Isothermal and continuous cooling
Supplementary Figures
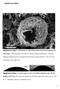 Supplementry Figures Supplementry Figure 1: SEM imge of sphericlly shped closed cell encpsulted y CMG flkes. CMG segregtes to the oil/wter interfce during emulsifiction, stilizing sphericlly shped emulsion
Supplementry Figures Supplementry Figure 1: SEM imge of sphericlly shped closed cell encpsulted y CMG flkes. CMG segregtes to the oil/wter interfce during emulsifiction, stilizing sphericlly shped emulsion
Binary phase diagrams
 inary phase diagrams inary phase diagrams and ibbs free energy curves inary solutions with unlimited solubility Relative proportion of phases (tie lines and the lever principle) Development of microstructure
inary phase diagrams inary phase diagrams and ibbs free energy curves inary solutions with unlimited solubility Relative proportion of phases (tie lines and the lever principle) Development of microstructure
1 Information, Persuasion, and Signalling
 ECON 312: Advertising 1 We will now exmine nother strtegic vrible vilble to firms, tht of dvertising. Industril Orgniztion Advertising 1 Informtion, Persusion, nd Signlling 1.1 Persusion versus Informtion
ECON 312: Advertising 1 We will now exmine nother strtegic vrible vilble to firms, tht of dvertising. Industril Orgniztion Advertising 1 Informtion, Persusion, nd Signlling 1.1 Persusion versus Informtion
CHAPTER9. Phase Diagrams Equilibrium Microstructural Development
 CHAPTER9 Phase Diagrams Equilibrium Microstructural Development The microstructure of a slowly cooled eutectic soft solder ( 38 wt%pb wt % Sn) consists of a lamellar structure of tin-rich solid solution
CHAPTER9 Phase Diagrams Equilibrium Microstructural Development The microstructure of a slowly cooled eutectic soft solder ( 38 wt%pb wt % Sn) consists of a lamellar structure of tin-rich solid solution
MACROSCOPIC INVESTIGATIONS OF THE PRIMARY STRUCTURE OF CONTINUOUS CASTING INGOTS
 Act Metllurgic Slovc, Vol. 20, 2014, No. 2, p. 146-151 146 MACROSCOPIC INVESTIGATIONS OF THE PRIMARY STRUCTURE OF CONTINUOUS CASTING INGOTS Zdzisłw Kudliński 1), Krystin Jniszewski 2)* 1) Silesin University
Act Metllurgic Slovc, Vol. 20, 2014, No. 2, p. 146-151 146 MACROSCOPIC INVESTIGATIONS OF THE PRIMARY STRUCTURE OF CONTINUOUS CASTING INGOTS Zdzisłw Kudliński 1), Krystin Jniszewski 2)* 1) Silesin University
A BEHAVIOR OF ELASTIC AND PLASTIC STRAIN FOR (α+γ) DUAL PHASE STAINLESS STEELS IN ROTATING BENDING TEST
 Copyright JCDS - Interntionl Centre for Diffrction Dt 24, Advnces in X-ry Anlysis, Volume 47. 39 ISSN 197-2 A BEHAVIOR OF ELASTIC AND LASTIC STRAIN FOR (+) DUAL HASE STAINLESS STEELS IN ROTATING BENDING
Copyright JCDS - Interntionl Centre for Diffrction Dt 24, Advnces in X-ry Anlysis, Volume 47. 39 ISSN 197-2 A BEHAVIOR OF ELASTIC AND LASTIC STRAIN FOR (+) DUAL HASE STAINLESS STEELS IN ROTATING BENDING
