A dissertation presented to. the faculty of. the College of Arts and Sciences of Ohio University. In partial fulfillment
|
|
|
- Sibyl Fleming
- 6 years ago
- Views:
Transcription
1 Thermoregulation of Shigella Dysenteriae Factors by RNA Thermometers A dissertation presented to the faculty of the College of Arts and Sciences of Ohio University In partial fulfillment of the requirements for the degree Doctor of Philosophy Andrew B. Kouse August Andrew B. Kouse. All Rights Reserved.
2 2 This dissertation titled Thermoregulation of Shigella Dysenteriae Factors by RNA Thermometers by ANDREW B. KOUSE has been approved for the Department of Biological Sciences and the College of Arts and Sciences by Erin R. Murphy Assistant Professor of Bacteriology Robert Frank Dean, College of Arts and Sciences
3 3 ABSTRACT KOUSE, ANDREW B., Ph.D., August 2014, Molecular and Cellular Biology Thermoregulation of Shigella Dysenteriae Factors by RNA Thermometers Director of Dissertation: Erin R. Murphy Shigella dysenteriae is a gram-negative enteroinvasive pathogen and a causative agent of shigellosis, a severe form of dysentery which afflicts over 90 million people annually. The ability of S. dysenteriae to survival and disease is dependent upon its ability to sense and adapt to the varied environments encountered throughout its infectious cycle. Among the environmental cues sensed by Shigella are temperature and iron, two conditions that vary between the host and non-host environment and that has been shown to influence the expression of genes present in these pathogens. The effect of these regulatory cues on the expression of genes contained within the shu locus, which encodes the heme uptake machinery of Shigella dysenteriae, was investigated in this work. The shu locus was found to encode at least two messages whose transcription was regulated by the ferric uptake regulator (Fur) in an iron-dependent manner. Quantitative PCR (Q-PCR) analyses showed that the levels of the shu transcripts were down-regulated in response to high iron conditions or in the presence of Fur indicating Fur-dependent regulation. In addition, the ability of the shu promoters and 5 untranslated regions to regulate their targets in response to temperature was investigated using a reporter based system. Western blot, Q-PCR and in silico analyses indicated that production of ShuA, the outer-membrane heme receptor encoded within the shu locus, is post-transcriptionally regulated in response to temperature by an RNA thermometer. An RNA thermometer is a
4 4 cis-acting RNA element located within the 5 untranslated region (utr) of the target gene that, under non-permissive temperatures, forms an inhibitory hairpin that occludes the Shine-Delgarno sequence and inhibits ribosomal binding. Further bioinformatics analysis identified an additional 40 RNA thermometers predicted to regulate Shigella dysenteriae genes. Western blot and Q-PCR analyses of several other reporter constructs containing the putative RNA thermometers fused to the reporter gene showed post-transcriptional thermoregulation of the reporter gene, which is indicative of regulation by an RNA thermometer. These data were the first to experimentally demonstrate RNA thermometer regulated gene expression in and Shigella species and suggest that this regulatory method is wide-spread within these important pathogens.
5 5 DEDICATION To my father, Galen Lee Kouse You are missed
6 6 ACKNOWLEDGMENTS I would like to acknowledge the help of several individuals and institutions for the aid throughout my graduate career. First, my advisor, Erin R. Murphy for all of the aid and instruction with planning, projects and planning throughout my graduate career. My lab mates William Broach and Michelle Pate for assistance with many of my projects. Our collaborators, Dr. Franz Narberhaus and Ronan Carrol who helped with the bioinformatics approaches and RNAseq analysis respectively. Finally, I would like to thank Ohio University and the American Heart Association for funding my projects and travel.
7 7 TABLE OF CONTENTS Page Abstract... 3 Dedication... 5 Acknowledgments... 6 List of Tables List of Figures Chapter 1: Introduction Bacterial gene regulation Significance RNA-mediated regulation Shigella Shigella species and evolutionary relationships Shigella mediated diseases Shigella morbidity, mortality and prevalence Treatment of Shigella mediated diseases Infection strategy Shigella and the immune response Master regulators of Shigella virulence Shigella host and non-host environments Conclusion Iron uptake and regulation Iron background Ferrous and ferric iron uptake systems in Shigella The Shigella heme uptake system Temperature Temperature background Thermally regulated Shigella factors Temperature-dependent regulation of Shigella factors Chapter 2: Regulation of the S. dysenteriae shu locus Background and significance Methods Bacterial strains, plasmids, and culture conditions Generation of the Gfp translation reporter plasmids Western blot analysis RNA extraction for Q-PCR and RT-PCR analysis RNA extraction for RNAseq analysis Quantitative real-time polymerase chain reaction Reverse transcriptase PCR analysis of the shua and shut 5 utr Reverse transcriptase PCR analysis of the intergenic shu regions Statistical analysis Results Organization of genes within the shu locus... 74
8 2.3.2 Iron-dependent transcriptional regulation of the shu locus Post-transcriptional thermoregulation of the shu locus Discussion shu locus future directions Chapter 3: Post-transcriptional thermoregulation of shua expression Background and significance Methods Bacterial strains, plasmids and culture conditions Generation of the truncated wild-type and mutant translational reporter plasmids pwt-shua, ps-shua and pd-shua Creation of β-galactosidase reporters pwt-chua, ps-chua and pdchua β-galactosidase assays Western blot analysis RNA extraction Quantitative real-time polymerase chain reaction Alignment and in silico modeling Statistical analysis Results in silico analysis predicts the presence of a 4U RNA thermometer within the shua 5 utr The nucleotide sequence composing the putative shua 4U RNA thermometer is sufficient to confer post-transcriptional thermoregulation Site-directed mutagenesis of the putative 4U element alters thermoregulation of the gfp reporter gene The 5 utr of chua, the E. coli orthologue of shua, contains a 4U RNA thermometer sufficient to confer post-transcriptional thermoregulation Discussion Future directions Chapter 4: Identification of additional RNA thermometers governing regulation of Shigella dysenteriae genes Background Methods Bacterial strains, plasmids, and culture conditions Identification of additional 4U RNA thermometers Isolation and cloning of putative RNA thermometers Western blot analysis RNA isolation Quantitative real-time PCR analysis Alignment and in silico modeling Statistical analysis Results
9 4.3.1 Identification of additional 4U RNA thermometers in S. dysenteriae Background and structure of the predicted RNA thermometers regulating tort, entc, htra and ompa regulation Experimental evaluation of the tort, entc, htra and ompa 4U RNA thermometers Expression of chromosomally encoded S. dysenteriae ompa is subject to post-transcriptionally temperature-dependent regulation Mutational analyses of the ompa 4U RNA thermometer Discussion Future Works Chapter 5: Discussion References
10 10 LIST OF TABLES Page Table 1: Distribution of the Shigella iron uptake systems...43 Table 2: Chapter 2 bacterial strains, primers and plasmids...66 Table 3: Chapter 3 bacterial strains, primers and plasmids Table 4: Chapter 4 bacterial strains, primers and plasmids Table 5: in silico predicted S. dysenteriae 4U RNA thermometers...142
11 11 LIST OF FIGURES Page Figure 1: Trans-encoded srna regulatory mechanisms...18 Caption: Diagram depicting srna regulatory mechanisms. Figure 2: cis-encoded srna regulatory mechanisms...20 Caption: Figure showing cis-encoded RNA regulatory mechanisms. Figure 3: Transcriptional and translational regulation by a riboswitch...22 Caption: General mechanisms for translation and transcription by riboswitches. Figure 4: Regulation of translational efficiency by an RNA thermometer...24 Caption: Diagram showing thermoregulation by an RNA thermometer. Figure 5: Virulence regulation by VirF, VirB and MxiE...25 Caption: Regulation of Shigella virulence pathways by global regulators Figure 6: Shigella evolutionary relationships...32 Caption: Evoluationary relationships between Shigella and E. coli Figure 7: Shigella infection strategy...34 Caption: Shigella infection strategy of human colonic epithelial cells. Figure 8: Schematic of the Type Three Secretion System...38 Caption: Diagram depicting the major components of the Shigella type three secretion system. Figure 9: The Haber-Weiss reaction...42 Caption: Chemical equation showing the formation of reactive oxygen species through the Haber-Weiss reaction. Figure 10: The Shu system...49 Caption: Mechanism of heme uptake by Shigella dysenteriae using the Shu system. Figure 11: Predicted shu locus structure...50 Caption: Structure of the shu locus located on the Shigella dysenteriae chromosome. Figure 12: RNAseq data predicts the presence of two transcripts expressed from the shu locus...77 Caption: Image of transcripts mapped to the shu locus using RNAseq analysis. Figure 13: RT-PCR analysis suggests the presence of two polycistronic transcripts encoded by the shu locus...79
12 12 Caption: An RT-PCR analysis investigating the structure of the shu locus. Figure 14: The shua and shut transcripts contain an extended 5 untranslated region..81 Caption: An RT-PCR experiment maps the length of the shua and shut 5 untranslated region. Figure 15: ShuA production is regulated by iron in a Fur-dependent manner...84 Caption: Western blot analysis showing iron-dependent regulation of ShuA by Fur. Figure 16: The pa-gfp and pt-gfp reporters demonstrate iron-dependent regulation mediated by Fur...85 Caption: Western blot analsyis of Gfp using reporter plasmid constructs containing the promoter and 5 utr of shua and shut translationally fused to gfp. Figure 17: The shu locus is transcriptional regulated by iron via a Fur-dependent mechanism...87 Caption: Q-PCR analysis of several shu transcripts indicating Fur-dependent regulation Figure 18: Fur-dependent regulation of shua and shut is localized to the promoter and 5 utr...88 Caption: The shua and shut reporter constructs show that the Fur-dependent regulation is dependent on the relative promoter and 5 utr regions of each gene. Figure 19: ShuA protein production is influenced by environmental temperature with increased levels detected at 37 C...89 Caption: A Western blot analysis shows that ShuA protein production is thermoregulated. Figure 20: shua and shut promoter and 5 utr are sufficient to confer thermoregulation on Gfp production...89 Caption: The shua and shut reporter constructs confer temperature-dependent regulation on Gfp production. Figure 21: Transcription of shua, shus and shut from the S. dysenteriae chromosome is not significantly affected by temperature...91 Caption: Q-PCR analysis of the shua, shus and shut transcripts indicates that each gene is not transcriptional regulated in response to temperature. Figure 22: Temperature does not affect transcription from the shua reporter but does have a significant effect on the shut reporter...92 Caption: Analsysis of the gfp transcripts form the shua and shut reporter indicates that temperature has an effect on gfp levels transcribed from the shut reporter. Figure 23: in-silico folding analysis of the full-length shua 5 utr Caption: Computer based analysis of the shua 5 utr secondary structure.
13 13 Figure 24: In-silico folding analysis predicts a 4U thermometer occluding the Shine- Dalgarno sequence within the shua mrna Caption: Computer analsysis of the secondary structure surrounding the shua ribosomal binding site indicates the presence of an RNA thermometer. Figure 25: Sequences composing the shua 4U thermometer are sufficient to confer posttranscriptional thermoregulation onto gfp expression Caption: Western blot and Q-PCR analysis shows that nucleotides which compose the predicted RNA thermometer are sufficient to confer post-transcirpitional thermoregulation onto a gfp transcript on a plasmid reporter. Figure 26: Mutational analysis demonstrates that the shua 5 utr contains a functional 4U RNA thermometer Caption: Mutations of the shua reporter construct shows regulation consistent with that from an RNA thermometer. Figure 27: Nucleic acid sequences within the shua 5 utr are conserved among pathogenic E. coli species Caption: An alignment shows that the shua 5 utr and its E. coli orthologue, chua,are significantly conserved across species. Figure 28: A functional 4U RNA thermometer within the 5 utr of E. coli chua confers thermoregulation Caption: A beta-galactosidase assay containing the chua 5 utr fused to a lacz gene shows temperature-dependent regulation of beta-galactosidase activity. Figure 29: Dual regulation controlling the expression of S. dysenteriae shua and chua of pathogenic E. coli Caption: Working model of shua and chua regulation by Fur and an RNA thermometer. Figure 30: Alignment of the putative 4U RNA thermometers Caption: An alignment of the Shigella and E. coli tort, entc, htra and ompa 5 utr shows they are well conserved between these species. Figure 31: In silico predictions of the putative RNA thermometers regulating TorT, EntC, HtrA and OmpA production Caption: Computer based analysis of the secondary structure of the tort, entc, htra and ompa 5 utrs. Figure 32: The predicted RNA thermometer contained within the htra and ompa 5 utr are sufficient to confer post-transcriptional thermoregulation...151
14 Caption: The sequences isolated form the htra and ompa 5 utrs, but not the entc or tort 5 utrs, are sufficient to confer post-transcriptional thermoregulation on a gfp reporter gene. Figure 33: S. dysenteriae ompa is post-transcriptionally thermoregulated Caption: Western blot and Q-PCR analysis shows that chromsomally encoded S. dysenteriae ompa is post-transcriptionally thermoregualted. Figure 34: Mutational analysis of the ompa 5 utr indicates the presence of a 4U RNA thermometer Mutations of the ompa reporter construct shows regulation consistent with that from an RNA thermometer. Figure 35: Model of ompa post-transcriptional regulation Caption: Working model of ompa regulation by an RNA thermometer, RNaseE and MicA. Figure 36: in silico structural analysis of the confirmed 4U RNA thermometers Capation: Computer based analsysis of the secondary structure of all confirmed 4U RNA thermometers. 14
15 15 CHAPTER 1: INTRODUCTION 1.1 Bacterial gene regulation Significance Throughout their life-cycles, bacteria must successfully exploit the nutrients found in their local environment, multiply, and compete with surrounding cells to be competitive and survive in any environment encountered. Bacteria meet these challenges by sensing specific cues within their local environment and producing the required gene products necessary to address their immediate needs(43, 44, 96, 98, 103). Controlled production of bacterial factors requires efficient and precise gene regulation in response to environmental stimuli which is necessary to meet challenges presented to each bacterium. Precise and specific bacterial gene regulation in response to environmental signals is required for a bacterium to adapt to its environment. Generally, precise gene expression allows bacteria to efficiently use the available energy and resources while simultaneously reducing production of energetically expensive and unnecessary factors. Specifically, bacteria modulate their gene expression to meet certain needs including, utilization of available nutrients within their environment, survival of environmental stresses and, in the case of pathogens, establishment of a productive infection(11, 42, 100, 124). Proteins are the most well studied and recognized bacterial gene regulators and are responsible for controlling expression from many of the canonical regulatory circuits(21, 23, 36, 44, 171). The importance of regulatory proteins is seen in the number of systems in which they regulate as well as the conserved nature of many protein
16 16 regulators across bacteria at the genus and family levels(21, 23, 36, 44, 171). While proteins remain the largest class of gene regulators, new research has shown that RNA is also capable of regulating bacterial gene expression(91, 92, 95, 109, 140, 162) RNA-mediated regulation Recent research into RNA-mediated gene expression has shown that RNA regulators play a significant role in controlling bacterial gene expression(142). RNAmediated regulation of bacterial gene expression is governed by a wide variety of molecules collectively termed riboregulators and are defined as any RNA element that influences the expression of a target gene or the activity of a target protein(91, 92, 95, 109, 140, 162). The importance of bacterial riboregulators is highlighted by the number of systems they have been shown to regulate including carbon metabolism, virulence, nutrient acquisition, and stress response (49, 57, 112, 129, 130). Bacterial riboregulators are divided into two classes depending upon where they are encoded relative to their target(s). The most well defined class of riboregulators are the trans-encoded riboregulators, also termed small RNA (srna), which are generally less than 500 nucleotides in length and are encoded distal, or trans, to their target (92). The molecular mechanism by which trans-encoded riboregulators modulate the regulation of their target(s) varies but can be divided into one of several categories. The first category is a small RNA that binds and destabilizes the target message by recruiting RNases to the double stranded region where the srna and mrna interact (Figure 1A) (142). Another method by which srnas regulate their targets is by destabilizing a
17 17 portion of a polycistronic message where a segment of the message is protected from exoribonuclease cleavage due to the formation of a secondary structure which inhibits the ability of the exoribonuclease to progress futher on the transcript (Figure 1B) (142). A third mechanism of srna regulation is translational activation where a srna binds to a region located within the 5 utr of the target message that occludes the ribosomal binding site (Figure 1C) (48). This binding frees the ribosomal binding site allowing for ribosomal binding and translation to occur. Another major class of srna regulators bind to a protein and inhibt its binding to the native target thus modulating protein s (130). A srna exerts its effects on a target mrna by complimentarily base pairing between a small number of nucleotides encoded either consecutively or non-consecutively to the regulated message (98, 162). The flexibility provided by the nature of srna binding to its target allows for a single srna to interact with multiple targets.
18 18 Figure 1: Trans-encoded srna regulatory mechanisms srnas modulate regulation of several genes by destabilization of its entire mrna target (A), differentially regulate transcript stability (B), modulate translational efficiency (C) and alter protein activity (D). Due to the relatively short and non-consecutive nature of srna interaction with its target, however, the RNA chaperone Hfq is often required for a productive interaction between the target mrna and a given srna (3, 30, 48). The exact role of Hfq in facilitating srna-mediated regulation of target gene expression is an area of much dispute. One hypothesis is that by binding both the srna and its mrna target, Hfq increases the local concentration of each RNA species, thus increasing the probability of
19 19 their otherwise unlikely interaction(88). A second hypothesis is that one or both of the RNAs are initially in a conformation that is unfavorable to binding with the other, and that Hfq functions to alter the conformation of one or both of molecules such that binding between the two becomes more favorable(25). Regardless of the mechanism, Hfq allows for a functional interaction between two RNA molecules resulting in a srna-mediated alteration of gene expression from the target mrna molecule. In contrast to the trans-encoded riboregulators, a cis-encoded riboregulator is either encoded anti-sense to its regulatory targets or within the regulated transcript itself(50, 109, 140). Riboregulators encoded anti-sense to their targets are termed antisense srnas (asrna) which resemble trans-encoded srnas in several ways, including some regulatory mechanisms and diversity of function(50, 54). However, one thing that makes asrnas unique is their base-pairing properties. Because they are encoded antisense to their regulated target, asrnas share perfect and extensive complementarity to their regulated transcript and are predicted to not require Hfq to facilitate binding and regulation(50). While anti-sense srnas have been shown to regulate transcript stability and translational efficiency by some of the same mechanisms as cis-encoded srnas, they have also been shown to regulate gene expression by a unique mechanism. Specifically, an anti-sense srna can bind directly to the 5 utr of its target nascent transcript and induce the formation of a terminator sequence that prematurely attenuates transcription(54) (Figure 2A). Additionally, the arrangement of the trans-acting srnas in relation to its target can facilitate regulation by transcriptional interference due to
20 convergent transcription of overlapping sequences of the target mrna and srna(152) (Figure 2B). 20 Figure 2: cis-encoded srna regulatory mechanisms Cis-encoded srnas regulate expression of their target genes by transcriptional attenuation (A) and promoter interference (B) Riboswitches and RNA thermometers represent a subclass of cis-encoded riboregulators and are encoded within the gene they regulate(95, 109). Riboswitches are found within the 5 utr of the regulated transcripts and form two mutually exclusive structures in response to binding of a ligand such as ions, t-rnas or small metabolites (Figure 3) (140). A riboswitch undergoes a conformational change, in response to ligand binding, that alters transcriptional and translational efficiency of the regulated gene (95). Riboswitches work at the transcriptional level by promoting the formation of a terminator
21 21 or anti-terminator structure upon ligand binding which either inhibits or permits transcription of its target respectively(95, 140). Additionally, riboswitches can modulate translational efficiency through permitting or inhibiting access of the ribosome to the ribosomal binding site in response to ligand binding(95, 140).
22 22 Figure 3: Transcriptional and translational regulation by a riboswitch Upon binding of its cognate ligand, the riboswitch structure is altered and regulates translation (A) or transcription (B) of its target gene. Figure legend is listed in (C) Like riboswitches, RNA thermometers are located within the 5 utr of the gene that they regulate; but, they differ from riboswitches in that they regulate gene expression
23 23 in response to temperature(109, 159) (Figure 4). RNA thermometers are further differentiated from riboswitches in that they do not require ligand binding to the regulatory unit in order to modulate target gene expression(109). RNA thermometers control expression of their target gene by assuming two mutually exclusive temperaturedependent conformations that either inhibit or permit ribosomal binding to the Shine- Dalgarno sequence(82, 109). Under temperatures that are not permissive to translation, the closed state of the RNA thermometer predominates resulting in the formation of an inhibitory hairpin that occludes the ribosomal binding site, and thus preventing translation(82, 109). When temperature permits translation from an RNA thermometer, the inhibitory structure enters the open state, liberating the ribosomal binding site and allowing ribosomal binding(82, 109). Together, these regulatory mechanisms function to efficiently control target gene expression.
24 24 Figure 4: Regulation of translational efficiency by an RNA thermometer RNA thermometers regulate target gene expression by forming an inhibitory hairpin at temperatures that are not permissive to translation and block ribosomal binding. When the organism enters an environment with a permissive temperature, the inhibitory structure is destabilized and the ribosomal binding site (RBS) is no longer sequestered, allowing for ribosomal binding and translation. 1.2 Shigella Shigella species and evolutionary relationships Shigella species are Gram-negative, non-spore forming bacilli and are the causative agent of shigellosis, an extreme form of dysentery in humans. Shigella species are members of the Enterobacteriaceae family and are highly related to both Escherichia and Salmonella species, sharing a common phylogenetic clade with each (Figure 5) (118). While Shigella, Escherichia and Salmonella species share a high degree of
25 25 similarity in many housekeeping and virulence genes, Shigella and Escherichia are the most closely related, sharing a genome similarity of approximately 90%(173). Figure 5: Shigella evolutionary relationships Shigella species are a member of the Enterobacteriaceae family and are closely related to both Escherichia, and Salmonella species with which they share a common clade. A phylogentic tree shows the relationships between each Shigella species and species of the genus Escherichia and Salmonella. The genus Shigella is divided into four species, S. boydii, S. sonnei, S. flexneri and S. dysenteriae based on variations within the O-antigen of their respective lipopolysaccharides (72, 96). While highly related, Shigella species display genetic variations that result largely from deletions, insertions, transversions and translocations of DNA sequences(170). Such species-specific variation occurs predominately in genes encoding toxins, nutrient acquisition genes and pathogenicity islands. Alterations in virulence-associated genes account for variability in pathogenicity and epidemiology
26 26 among Shigella species(170). Conserved among all species of Shigella, and what separates them from their closest relatives, is the presence of a 220 kb virulence plasmid(26, 154). The Shigella virulence plasmid contains five pathogenicity islands that encodes virulence-associated genes including those required for adhesion, invasion, and macrophage evasion, as well as for intracellular and intercellular spread of the pathogen; all processes that are essential for virulence of these important pathogens(154) Shigella mediated diseases Shigellosis, the most common disease caused by Shigella species, is an extreme form of dysentery that is initially characterized by watery diarrhea and later progresses to include both blood and mucus in the stool(20, 110). The watery diarrhea initially presented at the onset of shigellosis is caused by two enterotoxins which increase fluid secretion into the intestinal lumen while the invading pathogen, as well as the resulting immune response, causes damage to the intestinal epithelium causing the typical bloody stool associated with shigellosis(20, 110). While shigellosis is the primary disease caused by Shigella species, these pathogens are associated with two additional diseases, Reiter s syndrome and hemolytic uremic syndrome (HUS)(4, 31, 163). Reiter s syndrome is an autoimmune disease which causes inflammation of the knee and other large joints, eyes, urethra and cervix(4, 31). These symptoms are due to the production of an antibody which targets an unknown Shigella antigen and cross-reacts with the human HLA-B27 surface antigen, a part of the major histocompatibility complex encoded by an allele found in a small portion of the human population(4, 31). Both shigellosis and Reiter s
27 27 syndrome can be caused by all Shigella species; however, only Shigella dysenteriae can cause HUS as it is the only species that produces the Shiga toxin responsible for this disease(163). Hemolytic uremic syndrome results in damage to the kidney and microangiopathic hemolysis, a form of hemolytic anemia; combined, they cause lysis of red blood cells and blood in the urine(163). These two symptoms are initiated by the Shiga toxin binding to the glomerulus of the kidney and inducing apoptosis of those cells while simultaneously triggering an immune response responsible for the formation of capillary microthrombi that cause mechanical shearing of passing red blood cells(163). Together, these Shigella associated diseases cause significant morbidity and mortality world-wide Shigella morbidity, mortality and prevalence An accurate estimate of the annual Shigella infection rate is difficult to determine in part because this infection is difficult to distinguish from other gastrointestinal infections based on symptoms alone. The World Health Organization estimates that there are between million Shigella infections annually(20, 83). In addition, nearly 1 million infected individuals do not survive with approximately 60% of these fatalities occurring in children under the age of five(20, 83). Together, these data suggest that Shigella is a serious burden to human health. Organisms of the genus Shigella represent a significant threat to global health; however, the Shigella species attributed to an infection as well as the morbidity and mortality rates associated with a Shigella infection vary by geographical region(83). Over
28 28 99% of all shigellosis cases are reported in developing countries, while the remaining fraction of a percentage is found in developed nations. Recent data indicates that between 60-80% of all shigellosis cases within the developing world are attributed to infection with S. flexneri(83). However, this trend does not follow throughout the developing world as only 50% of all shigellosis cases reported in Sub-Saharan Africa and Southeast Asia are linked to S. flexneri while approximately 20-30% of Shigella isolates are identified as S. dysenteriae (83). While S. flexneri and S. dysenteriae are the most prevalent species responsible for shigellosis in the developing world, S. sonnei is the leading cause of shigellosis in developed countries with approximately 80% of confirmed cases being attributed to this organism(83). The fourth species, S. boydii has rarely been attributed to shigellosis cases and is largely restricted to the Indian subcontinent; the reasons for its reduced association with reported shigellosis cases are unknown(83). The large difference between reported shigellosis cases in the developing and developed world is typically attributed to the mode of Shigella transmission and the sanitary conditions found in these areas of the world. Shigella is transmitted from host-tohost through the fecal-oral route which occurs when infectious agents are shed in fecal matter and are orally ingested by an uninfected host(38, 135). Organisms spread by this mechanism are easily transmitted among hosts in developing nations where access to clean drinking water and proper sanitation are often limited(135). Shigella is especially prone to transmission in these areas because of its exceptionally low infectious dose of organisms; in contrast, most enteropathogens spread by fecal-oral route, like E. coli and Salmonella, have an infectious dose between 10^5-10^8 organisms(61, 89).
29 Treatment of Shigella mediated diseases During the course of a shigellosis infection, treatment options are limited with oral hydration and nutrient replacement being the standard(20, 29, 72). In the case of a known exposure (such as that in a laboratory or clinical setting), a course of antibiotics is recommended to prevent the onset of shigellosis; however, once symptoms are presented the use of antibiotics is contraindicated because it has been linked to increased toxin production which exacerbates existing symptoms and increase the likelihood of developing HUS(20). While antibiotic treatment before the onset of shigellosis still remains a viable option, the increase of multi-drug resistant Shigella strains makes the use of antibiotics to prevent the onset of disease much less effective(20). In addition to the lack of a recommended antibiotic treatment, there is no approved vaccine available to prevent shigellosis infection(29, 72). Because of the lack of an effective antibiotic regiment to treat shigellosis or a vaccine to prevent the disease, research must be performed to identify targets for new and novel treatments. Understanding how Shigella survives within the human host and the molecular mechanisms underlying the production of factors required for survival and virulence may reveal additional therapeutic targets Infection Strategy Shigella species are capable of surviving in the non-host environment for several days to weeks, depending on the environment; however, long-term survival of the population requires the pathogen to infect the human host, its natural reservoir, where it will replicate and cause disease. Infection occurs through the fecal-oral route which is
30 30 initiated following ingestion of Shigella by the next healthy host(110, 138). Following ingestion, Shigella travels to the colon, the site of infection, where it will invade the colonic epithelial cells and replicate(13, 58, 72, 138). The resulting dysentery associated with a shigellosis infection ensures large amounts of the pathogen are shed in the fecal matter allowing the pathogen to start a new round of infection following ingestion by a healthy host(110, 138). The method by which Shigella infects its host has been an area of intense investigation and has shown that the concerted efforts of a large number of molecular mechanisms are required to produce an infection. The first challenge Shigella must overcome on its route to the colon is the acidic environment of the stomach(72, 138). To survive in this highly acidic environment, Shigella contains a well-studied glutamate decarboxylase and at least one glutamate independent system that is not well characterized (90, 99). These systems help to facilitate a stable intracellular ph to relieve acid stress. The combined actions of these systems allows Shigella to resist the acidic conditions long enough to exit the stomach. Next, Shigella travels the length of the intestine to the colon where it invades the epithelial cells and establish a productive infection(72, 96, 138). Within the colon are several areas of aggregated lymphoid tissue called Peyer s patches, which function to screen the intestinal lumen for potential pathogens and initiate an appropriate immune response(93, 138). Within the Peyer s patches are microfold cells (M cells), specialized immune cells that contain a concave pit-like structure on the basolateral membrane and constantly sample the contents of the lumen by endocytosis (Figure 6) (93, 138). Once the intestinal lumen is sampled, the M cells transcytose the sampled contents to the
31 31 basolateral side of the membrane and present it to macrophages waiting in the pit-like structure(93, 138). When an invading pathogen is presented to the macrophage, it is endocytosed, degraded and the resulting antigens are presented to T-cells, activating them(93, 138). Activated T-cells then travel to the mesenteric lymph nodes where the signal is amplified and additional immune cells are recruited to the site of infection(93, 138).
32 Figure 6: Shigella infection strategy To invade the colonic epithelium, Shigella in the intestinal lumen is taken up by Microfold (M) cells and transported across the colonic epithelium (1). Following transcytosis across the intestinal epithelial layer, Shigella is presented to macrophages on the basolateral side of the membrane (2) where Shigella evades macrophage-mediated killing by disrupting the lysosome and inducing macrophage apoptosis using effector molecules secreted from the Type Three Secretion System (T3SS) (3). Now free on the basolateral side of the membrane, Shigella uses the T3SS to secrete effector molecules into the host cell which stimulates actin rearrangement and induces Shigella uptake (4). Once inside the host epithelial cells, Shigella reproduce intracellularly and spread to neighboring cells by actin based motility (5). Following induction of the host immune response, PMN cells are recruited to the site of infection where they phagocytose and lyse Shigella, clearing it from the system (6). 32
33 33 Unlike most other bacteria, Shigella takes advantage of transcytosis by the M cell to gain access to the basolateral side of the membrane where it infects the colonic epithelium(72, 93, 96). Specifically, following endocytosis of Shigella by a macrophage, the bacterium lyses and escapes the macrophage using several conserved virulence factors(72, 96, 138, 149). To escape the macrophage lysosome, Shigella utilizes the type three secretion system (T3SS), which it uses to secrete effector proteins into the macrophage(72, 96, 138) (Figure 7). These secreted effectors pass into the T3SS basal body, which acts to prohibit the secretion of non-effector proteins and also provides the energy for secretion by ATP hydrolysis, and through the needle complex to their targets(72, 96, 138). The secreted effectors function to destabilize the macrophage lysosome and induce macrophage apoptosis allowing Shigella to escape into the basolateral side of the colonic epithelium (72, 96, 138).
34 34 Figure 7: Schematic of the Type Three Secretion System The Shigella Type Three Secretion System is composed of two main components, the base, or basal body, and the needle complex. The basal body screens proteins for a secretion signal and permits entry of these proteins into the needle complex. In addition, the basal body also contains an ATPase domain which provides the energy required for protein secretion. The needle complex prevents premature secretion of the effector proteins until contact with the host epithelial cell or macrophage lysosome is made; following contact, the needle complex delivers the effector proteins to their intended target. Following apoptosis of the macrophage, Shigella uses its T3SS to secrete effector proteins into the colonic epithelial cells and promote its own uptake by inducing localized actin rearrangement resulting in Shigella uptake (Figure 7) (72, 96, 138). The secreted effector proteins work to initially dissociate the actin cytoskeleton from the plasma membrane and recruit additional actin nucleators; together, these actions remodel the actin cytoskeleton and initiate Shigella uptake into a phagosome(72, 96, 138). By using
35 35 the T3SS to secrete virulence factors directly into the host cell, Shigella prohibits the dissemination of immunogenic particles directly into the extra-cellular area thus preventing an immediate immune response(72, 96, 138). Following uptake into eukaryotic epithelial cells, Shigella escapes the phagosome by utilizing the same factors used to disrupt the macrophage lysosome and escapes into the cytoplasm of the colonic epithelial cell(72, 96, 138). Following invasion, Shigella must spread to sites within the host-cell and to neighboring cells to acquire nutrients necessary for survival and proliferation; however, all species of Shigella are non-motile and do not contain a flagella nor cilia to propel the bacterium forward. Instead of using a flagella or cilia for motility, which are energetically expensive, Shigella recruits two host actin nucleators to the old pole of the bacterial membrane(72, 96, 138). These membrane-associated nucelators continuously add actin monomers onto a growing actin chain near the bacterial membrane which results in propelling the bacterium forward in a directional manner (72, 96, 138). Once Shigella comes into contact with the host-cell membrane bordering an adjacent epithelial cell, it induces its uptake into neighboring cells using the T3SS and associated effectors(72, 96, 138). While replicating and spreading within the colonic epithelial cells effectively shields Shigella from extracellular immune cells, the host-cell still mounts an immune response.
36 Shigella and the immune response The intracellular environment provides a niche where Shigella survives and proliferates without being directly exposed to macrophages or other immune cells. However, as a response to invading pathogens the host-cell attempts to induce apoptosis, to prevent intracellular replication of the invading microbe. Additionally, Shigella factors reduce proinflammatory cytokine production inhibiting the ability to recruit immune cells to the site of infection. Shigella must suppress these two processes to maintain its replicative niche and spread throughout the site of infection (96, 138). To inhibit these two processes, additional effector proteins are secreted into the host-cell cytoplasm through the T3SS to prevent apoptosis by inactivating the canonical apoptotic pathways and preventing production of proinflammatory cytokines (96, 138). While Shigella evades immune cells by replicating and spreading within colonic epithelial cells and decrease the host immune response these actions are not enough to completely subvert the host s immune response. The inflammatory response at the site of infection recruits immune cells to the area to clear the pathogen from the system. Among the cells attracted to the site of infection are Polymorphonuclear neutrophils (PMN) cells that clear Shigella form the site of infection(96, 138). In addition to the desired effect of killing Shigella, the resulting inflammatory response also damages host tissue by interrupting tight junctions between the colonic epithelial cells(138). The interruption of these tight junctions adds to the efflux of fluids into the intestinal lumen and allows additional pathogens in the lumen access to the basolateral membrane(138). The inflammatory response also damages the epithelial cells which lead to the formation of
37 37 colonic ulcers that are typical associated with shigellosis(138). Together, this damage to the epithelial cell layer causes the typical profuse bloody diarrhea that is typical of shigellosis Master regulators of Shigella virulence Due to the lack of a good animal model, the actions of the master virulence regulators have been studied extensively in vitro under conditions that mimic the host environment. In these environments, Shigella species are observed to precisely regulate the production of energetically expensive virulence factors in response to conditions which mimic the host environment (56, 72, 106, 147). Specifically, Shigella sense osmotic pressure, ph, temperature and iron to modulate production of the virulence gene cascade(56, 72, 106, 147). These environmental cues control production of the master virulence regulator VirF and its two down-stream co-regulators VirB and MxiE which in turn modulates expression of genes encoded on the Shigella virulence plasmid necessary for invasion of and spread within the host epithelial cell (Figure 8) (71, 138). The VirF gene is an AraC-like transcriptional activator which binds to and induces transcription of two genes: icsa, which recruits host actin nucleators to the old pole of the bacterium facilitating intra- and inter-cellular spread, and VirB (19, 71, 138, 152). VirB, like VirF, is a transcriptional activator and is responsible for the regulation of many Shigella virulence genes by binding to the promoter region and recruiting the transcriptional machinery(18, 64, 148, 152). Specifically, VirB activates transcription of components of the T3SS, icsp, which encodes a protease that modulate the activity of icsa, and mxie
38 38 another regulator of Shigella virulence genes(1, 71, 138). MxiE, a third transcriptional regulator is responsible for activating expression of genes which encode effector proteins secreted from the T3SS (75, 144). Together, the VirF, VirB and MxiE proteins precisely regulate production of the Shigella T3SS, its secreted effectors and actin-based motility (75, 144). A disruption in any of these three processes results in an attenuation of Shigella virulence and inhibits the ability of these pathogens to survive and cause disease. Figure 8: Virulence regulation by VirF, VirB and MxiE The VirF, VirB and MxiE regulatory proteins activate transcription of genes involved in motility (icsa and icsp), as well as the type three secretion system and its effector proteins. Transcription of these system are activated in response to host-associated environmental conditions including ph, temperature, iron and osmotic pressure Shigella host and non-host environments The host range of all Shigella species is limited to humans and higher primates (chimpanzees, monkeys and, gorillas) (65, 72, 110, 138). While there is no permanent natural reservoir outside of the human host, Shigella species survive for several hours to days in salt water, fresh water (such as swimming pools, ponds and lakes) and on contaminated food (69, 70). The length of time in which Shigella survives these conditions is dependent on the conditions of each particular environment. The ability of
39 Shigella species to adapt to and survive outside of the host increases its potential to come into contact with another host and cause disease Conclusion Shigella species are a cause of significant morbidity and mortality world-wide. Through the concerted efforts of many virulence factors, Shigella species invade the colonic epithelium and cause shigellosis, Reiter s syndrome and hemolytic uremic syndrome(4, 4, 31, 72, 138, 149). The production of Shigella virulence factors are tightly regulated in response to environmental cues that mimic the host, including osmolarity, ph, temperature and iron(149). While all of these environmental signals significantly contribute to the virulence gene regulation within Shigella, these studies will examine the impact of both iron availability and temperature on the production of Shigella virulence genes. 1.3 Iron uptake and regulation Iron background Bacteria must acquire the essential nutrient iron in each of the environments encountered in order to survive. Within a bacterial cell, iron plays a significant role in cellular metabolism and is an ideal candidate for use in redox reactions within biological systems due to its relative abundances and the low redox potential between its most common redox states(161). Due to these factors, elemental iron is readily incorporated into metalloproteins and used in redox reactions in several systems, most notably the
40 40 electron transport chain where its low redox potential allows iron to efficiently shuttle electrons(97, 114). In addition to its use in redox reactions and the electron transport chain, iron can be incorporated into iron-sulfur clusters which are widely used to modify protein function in response to oxygen availability by altering the protein conformation in response to the redox state of the iron within the bound iron sulfur cluster(9, 45, 46, 51, 132). To incorporate iron into these required systems, bacteria must first acquire this essential nutrient. In order to satisfy their requirement for iron, bacteria must acquire iron in each of the environments they encounter. Depending on the environment in which the bacteria are located, elemental iron is found in either the ferric (+3) or ferrous (+2) states(161). Ferric iron is typically found under conditions that are similar to the non-host environments which include aerobic conditions and a neutral ph. In the ferric form, iron is bound within large aggregates, which make it difficult for bacteria to access the element and use it as a nutrient(161). To access and use ferric iron, bacteria produce siderophores, molecules with a high affinity for iron, which strip iron away from the aggregate and return the nutrient to the bacterial cell (35, 62, 87, 104, , 136, 137, 161). When iron is in conditions similar to a human host, (an anaerobic environment with an acidic ph) ferric iron is reduced to ferrous iron. In the ferrous form, iron is soluble and does not require a siderophore for acquisition. Ferrous iron is thought to passively diffuse through the outer membrane and be actively transported through the inner membrane using a membrane bound ferrous iron transporter(122, 133, 167). In addition to elemental iron, pathogens also come into contact with iron sequestered within iron binding proteins
41 41 during the course of an infection(27, 84, 117). Iron binding proteins serve dual roles in the host, their native function for which they are produced as well as contribute to the host s immune defense(116). By sequestering iron within the human body into iron binding proteins, the host denies access of this nutrient to pathogenic organisms in an attempt to slow their growth and prevent infection(116). To satisfy the nutritional requirement for iron within the host, many pathogens have acquired systems specialized in the uptake and acquisition of iron from iron binding proteins found at the site of infection(27, 84, 117). Using one or a combination of these systems allows bacteria to acquire iron within the host and non-host environment. Regardless of the iron source, each organism must efficiently regulate its iron uptake systems to satisfy its nutritional requirements and to avoid iron induced toxicity(7, 10, 84, 122). Production of bacterial iron uptake systems is an energetically expensive process; therefore, each iron uptake systems must be produced only when the cognate iron source is available and intracellular iron levels are insufficient(100, 101, 167). Upon the satisfaction of its iron requirements, bacteria must decrease production of their iron uptake systems to inhibit production of non-essential energetically expensive proteins. Additionally, iron uptake systems must be precisely regulated in response to cellular iron levels due to the damaging effects of unbound iron in the cytoplasm. Iron induced toxicity is caused by the Haber-Weiss reaction where ferric iron catalyzes the conversion of elemental oxygen and hydrogen peroxide to a hydroxide and a hydroxyl radical(10). This two-step process requires the conversion of ferric iron to its ferrous form and back again allowing the ferric iron to continuously catalyze this reaction (Figure 9)(10). The
42 42 hydroxyl radical, a reactive oxygen species, generated through the Haber-Weiss reaction damages cellular components including, but not limited to, the cell membrane, proteins, DNA and other cellular components(10). The production of iron uptake systems must be precisely regulated to satisfy iron requirements while minimizing iron induced toxicity. Figure 9: The Haber-Weiss reaction The Haber-Weiss reaction is catalyzed by free iron and produces reactive oxygen species which are harmful to the cell. In this two step reaction, ferric iron first reacts with superoxide to produce elemental oxygen and ferrous iron. In the second step, the ferrous iron reacts with hydrogen peroxide to form ferric iron, hydroxide and a hydroxyl radical. The ferric iron is then free to reinitiate this reaction and the hydroxyl radical can damage macromolecules including DNA, RNA, proteins and lipids Ferrous and Ferric Iron Uptake Systems in Shigella Shigella can satisfy its iron requirements in the host and non-host environments through the production and activity of both ferrous and ferric iron uptake systems. The absolute requirement for iron in Shigella is reflected in the fact that the Shigella genus has a combined six confirmed uptake systems for ferrous and ferric iron; these systems
43 43 include the Feo and Sit systems to utilize ferrous iron and the Ent/Fep, Iuc, Fhu and Iro systems for the acquisition and utilization of ferric iron(62, 87, , 133, 136, 137). These systems are spread throughout the Shigella genus and a single Shigella species does not contain each of these iron uptake systems; although each species does possess a ferrous and ferric iron transport system to acquire iron in the host and non-host environments respectively (Table 1)(35). Table 1 Distribution of the Shigella iron uptake systems Shigella Shigella Iron Transport Systems species Ferrous uptake Ferric uptake Heme uptake Sit Feo Ent/Fep Iro Fhu Iuc Shu S. flexneri S. sonnei S. boydii S. dysenteriae Note: Each iron acquisition system possessed by a Shigella species is denoted by a (+) while a lack of that uptake system within a particular species is denoted by a (-).
44 44 Ferrous iron is thought to passively diffuse through the Shigella outer membrane where it is then actively transported into the cytoplasm through the actions of the Feo or Sit system(35, 122, 133, 134, 167). Genes encoding each of these iron uptake systems are located on the Shigella chromosome and are contained within their own respective operon(35, 122, 133, 134, 167). Both the Sit and Feo systems encode an inner membrane bound ferrous iron transporter which imports iron from the periplasm into the cytoplasm(35, 122, 133, 134, 167). Studies have shown that the Feo system exclusively import iron; however, the Sit system binds and imports both ferrous iron and magnesium with a preference for the latter(35, 133). This preference for magnesium by the Feo transport system could explain why some Shigella species possess two, seemingly redundant, ferrous iron transport systems. Using these two systems, Shigella can satisfy its requirement for iron under conditions which mimic the host environment. Shigella species acquire iron within the non-host environment, where iron is largely in its ferric form, using the Ent/Fep, Iro, Fhu and Iuc transport systems(35, 62, 87, 104, , 136, 137, 161). A total of three siderophores, enterobactin, salmochelin and aerobactin, are produced by Shigella species and are used to acquire ferric iron form large aggregates (35, 62, 87, 104, , 136, 137, 161). Enterobactin and aerobactin are produced solely by genes encoded within the ent and iuc loci respectively; however, salmochelin is generated by the glycosylation of enterobactin by proteins encoded within the iro locus(35, 47, 62, 87, 104, , 136, 137, 161). This modification is thought to allow salmochelin to evade the host siderophore sequestration protein siderocalin and thus increase the probability that salmochelin will deliver iron to the bacteria(35, 104).
45 45 The fourth Shigella ferric iron uptake system, Fhu, does not encode its own siderophore but instead scavenges siderophores produced by other bacteria within the extracellular environment (35, 47). Following iron binding, the resulting siderophore:iron complexes are imported into the cytoplasm by an outer membrane receptor encoded by each uptake system specific for its cognate siderophore(35, 47, 62, 87, 104, , 136, 137, 161). Energy for this process is supplied in a TonB-dependent manner where TonB, and its accessory proteins ExbB and ExbD, harness the proton motive force of the inner membrane and apply it to the outer membrane receptor(35, 122). Once inside the periplasm, the siderophore is bound by a periplasmic binding protein and delivered to an inner membrane bound ABC permease. Each permease consists of a transmembrane channel, which allows for passage of the siderophore into the cytoplasm, and an ATPase, which provides the energy required for transport(35, 47, 120, 122, 161). The Fep, Iro and Fhu systems each possess a periplasmic binding protein and ABC transporter; however, the iuc locus does not encode either of these factors instead relying upon the Fep system for transportation of aerobactin from the periplasm to the cytoplasm(35, 47, 120, 122, 161). Following importation of the siderophore into the cytoplasm, iron is liberated by one of two mechanisms depending on the properties of the siderophore. Iron is freed from both ferrichrome and aerobactin, imported by the Fep and Iuc systems respectively, by reduction of the bound iron to its ferrous state thus reducing the affinity of the siderophore to iron and allowing for its release(35, 47, 120, 122, 161). Iron bound by enterobactin and salmochelin can only be released by degrading these siderophores,
46 46 which is accomplished by proteins encoded by the fep and iuc operons respectively(35, 47, 120, 122, 161). The ferric and ferrous iron uptake systems must be efficiently regulated to produce each system in response to intracellular iron levels to satisfy the nutritional requirement for iron and avoid iron-induced toxicity. The Shigella iron uptake systems are all regulated in response to intracellular availability of free iron, iron that is not sequestered within a molecule; this regulation is mediated by the iron-dependent transcriptional repressor Fur(35, 44, 62, 101). Upon binding of ferrous iron (a requirement for Fur action), which predominates over ferric iron in the reducing environment within the bacterial cell, Fur binds to a consensus sequence within the promoter region of Fur regulated genes and inhibits transcription of the regulated gene by blocking recruitment of the transcriptional machinery(44). Using this mechanism, Fur regulates production of the iron uptake systems as well as virulence factors and other iron dependent proteins(44). As a byproduct of its requirement for ferrous iron to act as a transcriptional repressor, and its high concentration within the cytoplasm, which is estimated at 10,000 copies per cell, Fur is also thought to be a major iron reservoir within the cell because it sequesters a large amount of iron due to its high copy number and affinity for iron.(44). The sequestration of free iron by Fur reduces toxicity by the Haber- Weiss reaction without the production of additional and energetically expensive iron storage proteins. Fur and its homologues are found in many different families of bacteria indicating the significance of iron regulation in bacteria and the importance of Fur and its homologous in iron regulation(44).
47 47 Shigella must not only regulate the production of their uptake systems in response to intracellular iron concentrations but also in response to environmental conditions in which the cognate iron source is located. Currently, no system has been identified that allows Shigella to directly sense the oxidation state of iron; however Shigella contains the Arc and Fnr systems which detect the environmental oxygen levels which is indicative of the redox state of iron(23, 35). Arc is a two-component system which contains a sensor kinase, ArcB, and a negative transcriptional regulator, ArcA (16, 23, 94). Under anoxic conditions, ArcB phosphorylates and activates ArcA allowing for gene regulation in response to oxygen concentration (16, 23, 94). ArcA has been shown to repress activity of the ferric iron uptake systems Ent/Fep and Iuc to maximally produce these systems under conditions that Shigella would encounter ferric iron(23, 35). However, it is unknown if ArcA modulates the production of any of the other iron uptake systems. Fnr is a cytoplasmic protein and global transcriptional activator, inducing expression of genes involved in aerobic metabolism, anaerobic catabolism and nutrient acquisition(35, 77). Transcriptional activity of Fnr is modulated in response to the redox state of a bound iron sulfur cluster that is oxidized under aerobic conditions. Oxidation of Fnr activates the protein, which permits its binding to a consensus sequence within the promoter region of regulated genes and recruits the transcriptional machinery(77). The Fnr protein has been shown to positively regulate the Feo system under the conditions where Shigella is likely to come into contact with ferrous iron; however, the regulation of the Sit or other iron uptake system by Fnr is unknown(35). Together Fur, ArcA and Fnr regulate the ferric and
48 48 ferrous iron uptake machinery in response to oxygen and iron availability and allows for them to utilize their cognate iron sources. The combined action of the ferrous and ferric iron uptake systems allows Shigella to satisfy its nutritional requirements for iron when the nutrient is abundantly available. The efficient regulation of these iron uptake systems in response to iron availability and oxygen levels allows Shigella to maximally produce the proper iron uptake systems based on the nutritional requirements and the state of iron that is available in each environment The Shigella heme uptake system In the non-host environment and the host gastrointestinal tract, iron is relatively abundant and can be utilized using any of the ferrous or ferric iron uptake systems; however, once Shigella is transcytosed to the basolateral side of the intestinal lumen by M cells it enters an environment where free-iron concentrations are exceedingly low(84, 101). To partially satisfy its nutritional requirements for iron, Shigella dysenteriae and select Shigella boydii strains have acquired the ability to utilize heme and hemoglobin, which represent 90% of all iron within the human host, as a source of iron using the Shigella heme uptake (Shu) system (101, 102, 117, 161). In the early stages of infection, Shigella will invade the host epithelial cells and come into contact with unbound heme and hemoproteins which it utilizes as an iron source(117, 161). As the disease progresses, Shigella will come into contact with extracellular heme due to tissue damage and red blood cell lysis by Shigella factors and the resulting immune response (13, 72, 110).
49 49 The shu locus is located on the Shigella chromosome and is predicted by in silico analysis to encode two monocistronic (shua and shus ) and two polycistronic (shutwxy and shuuv) transcripts driven by four independent promoters (Figure 10) (166). ShuA, the outer-membrane heme receptor, first comes into contact with the heme moiety and transports it into the periplasm in a TonB dependent manner (Figure 11) (27, 102). Once inside the periplasm, the heme moiety is bound by ShuT, a periplasmic heme binding protein, and delivered to the ABC permease composed of ShuU, which forms the transmembrane channel, and ShuV, the ATPase which provides energy for heme import into the cytoplasm(28, 39). Inside the cytoplasm, heme is bound by ShuS to prevent ironinduced toxicity by the Haber-Weiss reaction; however, the fate of the heme molecule beyond this point is unknown(164, 168). In addition to these factors, two additional proteins of unknown function are encoded by the shuw and shuy genes. Together, these genes are necessary for heme uptake in Shigella species(27, 28, 39, 102, 164, 168). Figure 10: Predicted shu locus structure The S. dysenteriae Shu system is encoded on the chromosome within a single 8948 nucleotide locus. In silico analysis predicts the eight shu genes are transcribed as two monocistronic (shua and shus) and two polycistronic (shutwxy and shuuv) messages driven by four independent promoters represented by the four arrows.
50 Figure 11: The Shu system Within the host-environment Shigella species utilize the Shigella heme uptake (Shu) system to acquire heme for use as an iron source. The Shu system contains five genes of known function including ShuA/T/U/V/S. ShuA resides on the outer membrane and binds to/imports heme into the periplasm in a TonB dependent manner. Once inside the periplasm, ShuT binds heme and shuttles it to the ABC transporter comprised of a ShuU homodimer, which creates a transmembrane channel, and ShuV, an ATPase. Following entry into the cytoplasm, heme is bound by ShuS to enhance heme utilization or prevent formation of harmful oxygen species by Haber-Weiss reaction. The fate of heme after this step is unknown. 50
51 51 While not tested directly due to the lack of an efficient animal model, several lines of evidence suggest that the Shu system contributes to Shigella virulence. First, ortholgoues of the shu locus are found within pathogenic strains of E. coli but are not present within non-pathogenic strains of these organisms indicating its role in virulence(115, 151, 166). Second, the presence of the E. coli shu orthologue is positively correlated with virulence in these species(66, 115, 151, 166). Third in vitro studies have shown that the shu locus is sufficient to satisfy the requirement for iron in Shigella when heme is the only iron source available(101, 102). These studies indicate that the shu locus both contributes to virulence and that the efficient import of heme by this system is sufficient to satisfy the nutritional requirements for Shigella While regulation of the other Shigella iron uptake systems has been extensively studied, relatively little is known about the regulation of the shu system. Previous reporter studies conducted in E. coli has demonstrated that shua expression is dependent on iron; with little reporter production in the presence of high iron concentrations and increased production under iron stressed conditions(102). Additionally, shua has been shown to be regulated by both Fnr and ArcA in response to oxygen availability(23). These studies represent the accumulation of all knowledge regarding regulation of genes within the shu locus. To more fully understand the regulation of this important system, this study aims to identify the environmental factors and regulatory mechanisms governing production of the Shigella dysenteriae Shu system.
52 Temperature Temperature background Temperature affects a wide range of bacterial processes which are typically due to changes in the conformation of macromolecules including proteins, DNA and RNA in a temperature-dependent manner(36, 59, 75, 82, 84, 124, 126, 172). The alteration of macromolecule conformations can lead to changes in activity, stability and expression of and from these molecules(11, 36, 59, 75, 82, 84, 124, 126, 152, 172). A temperaturedependent alteration of any of these factors can be beneficial to a microbe, such as the production of virulence factors necessary for infection in response to host body temperature(42, 126, 152, 172). Conversely, temperatures that are either too high or low for the bacteria, typically 42 C and 15 C respectively, can be damaging to the bacteria and initiate the heat-shock and cold-shock response (11, 59, 124). To produce necessary factors and survive in a range of temperatures, bacteria must maintain and produce systems that will allow them to cope with each environment encountered. Environmental temperature can act as a regulatory cue, indicating the environment in which the bacteria are located and facilitating production of factors required for each environmental condition. Human pathogens sense the stably maintained, 37 C host body temperature as a sign of host entry. Upon entering the host environment, bacterial pathogens induce production of certain virulence factors in a temperature-dependent manner to establish an infection(81, 113, 152). Outside of the host-environment, bacteria must cope with temperatures that induce cellular stress. When temperatures reach heat shock levels, typically 42 C for most bacteria, heat-shock factors
53 53 are produced to resist the stresses a high temperature places on the bacterium(12, 52, 59, 59, 105). The heat-shock response is designed to decrease production of non-essential proteins while increasing the production of factors which contribute to the degradation or refolding of denatured proteins(12, 52, 59, 59, 105). On the other side of the spectrum, temperatures reach cold-shock levels, usually below 15 C for most bacteria, the bacteria will initiate the cold-shock response. In contrast to heat-shock conditions, low temperatures do not significantly affect protein conformation but globally impact RNA structure(40, 55, 68, 124). At or below 15 C, RNA takes on heavily structured conformations that are inherently unstable under normal physiological conditions(40, 55, 68, 124). These structured conformations significantly alter transcription, translation, RNA stability and ribosome biogenesis(40, 55, 68, 124). To cope with most of these stresses, the cell produces RNA chaperones to alleviate the RNA structure brought on by a decrease in temperatures. To survive and adapt to temperatures in each environment, bacteria must precisely regulate production of factors required at each temperature encountered. Efficient regulation of these factors is accomplished by regulatory mechanisms at every level of gene expression. Temperature modulates gene expression at the transcriptional level through the production of transcription factors which bind to curved DNA in a sequenceindependent manner to modulate recruitment of the transcriptional machinery(36, 152). Additionally, translational efficiency is also affected by temperature due to temperaturedependent effects on RNA structure(55, 84, 109, 124, 159). Cis-encoded regulatory mechanisms collectively termed RNA thermometers permit or inhibit ribosomal access to
54 54 an RNA transcript dependent on its temperature-dependent conformation(55, 84, 109, 159). Finally, temperature can modulate protein activity based on thermally regulated changes in the protein conformation(59, 75, 126). These conformational changes can either inhibit or permit protein activity based on protein structure(59, 75, 126). The thermal regulation of bacterial genes is facilitated by the use of a wide range of mechanisms and directly affects most of the systems required for survival including: heat-shock, cold-shock and virulence systems(12, 68, 81, 152) Thermally regulated Shigella factors Temperature is used as an environmental cue to initiate production of factors required for Shigella virulence(41, 147, 152). Temperature is a major cue for regulation of virulence genes due to the stable, and often elevated, nature of temperature within a human host as compared to that within the non-host environment. At 37 C, the production of the main virulence regulator, VirF and its co-regulator VirB, are significantly increased when compared to lower temperatures (see section 1.2.6)(41, 147, 152). These genes act as transcriptional activators which bind to sequences near the promoters of many Shigella virulence factors and induce their transcription(74, 148). By sensing the environmental temperature, among other factors, and increasing production of virulence factors, Shigella efficiently expresses its virulence genes within the host environment where they will provide the most benefit to this pathogen(41, 147, 152). Shigella species are not constrained to a single geographical region and therefore encounter a wide range of temperatures in the non-host environment. To survive
55 55 conditions outside of the human host, Shigella must produce both heat and cold-shock factors. Unfortunately, very little research has been performed on the heat-shock response in Shigella; however, these systems have been intensively researched in closely related E. coli strains. These studies in E. coli presented here provide insight into how Shigella could resist heat-shock and cold-shock conditions due to the high degree of conservation between heat-shock and cold-shock genes in these two organisms. At temperatures at or above that which induces the heat-shock response in Shigella, 42 C, proteins lose their native tertiary and quaternary structures and become denatured(12, 32, 52, 59). In the denatured state, hydrophobic residues, that are typically located within the proteins interior, are exposed interact forming large aggregates which impede normal cellular processes(12, 32, 52, 59, 67). The heat-shock response allows Shigella to survive above 42 C by producing factors to handle the increased load of denatured proteins. In E. coli, the heat-shock factors DnaK and DnaJ bind to misfolded proteins and prevent their aggregation by recognizing the exposed hydrophobic residues on denatured proteins(12, 52, 67). Inhibition of protein aggregation by DnaK relieves stresses placed on the cell due to misfolded proteins; however, the unfolded proteins must be cleared from the system. Another way that E. coli has been shown to clear misfolded proteins from the system is by refolding them into their native conformations; a function performed by GroES and GroEL(12, 52, 67). In addition to refolding proteins into their native conformation using the GroEL/GroES complex, unfolded proteins can also be cleared from the cell by degradation. E. coli HtrA is a well-studied heat-shock protein and performs two functions dependent on its temperature-dependent conformation(59,
56 56 78). At temperatures below 42 C HtrA acts as a molecular chaperone to prevent the aggregation of unfolded proteins; however, when the heat-shock response is activated at or above 42 C, HtrA undergoes a conformation change to activate is protease function(59, 78). Shigella species are commonly found in warmer climates where they rely upon the heat-shock response to survive the extreme temperatures in the non-host environment; however, Shigella species are also found in much cooler climates where the cold-shock response ensures their survival under these environmental conditions(40, 68). Like the heat-shock response, little research has been conducted on the Shigella cold-shock response; however, this process has also been extensively studied in E. coli which allows us to draw parallels between these two organisms based on the high degree of similarity shared by their cold-shock genes, especially cspd and cspa. An extreme decrease in the environmental temperature, to 15 C and below, places constant stress upon the bacterium and initiates the cold-shock response(40, 68). Upon induction of the cold-shock response, Shigella growth and proliferation are briefly inhibited to acclimate to the change in temperature before these processes are continued; albeit to a much slower degree(40, 68). A decrease in the bacterial growth rate is mediated by the protein CspD which bind s to single-stranded DNA, presumably at the replication forks, and prevents their progression(40, 53, 86, 169). During the acclimation process, production of cold-shock factors is increased to alleviate cellular stress primarily due to the highly structured nature of RNA at low temperatures(68, 73, 123). CspA, whose main function is to act as a molecular chaperone, stimulates proper transcription, translation and mrna decay by
57 57 binding to double stranded RNA and resolving secondary structures in mrna sequences(68, 73, 123). In addition to its role as a molecular chaperone, CspA has also been shown to act as a transcriptional activator of cold-shock proteins by binding to an 11 base-pair region, termed the cold-box, upstream of the target promoter and recruit the transcriptional machinery(123) Temperature-dependent regulation of Shigella factors To survive and produce factors in response to temperature, bacteria must sense and precisely regulate their gene expression in a temperature-dependent manner. Given the need and number of thermally regulated Shigella factors, little is known about how temperature directly regulates Shigella gene expression. Studies have shown that temperature affects the global DNA topology; where, at higher temperatures DNA is in a more relaxed state while at lower temperatures DNA is more highly structured(17, 36, 126). The nucleoid-like protein H-NS, a global transcriptional repressor, binds to promoter regions within the Shigella genome based on the local DNA topology in a sequence independent manner(17, 148, 152). H-NS preferentially binds to curved DNA, which predominates at lower temperatures, and efficiently inhibits recruitment of the transcriptional machinery(17, 148, 152). Thus at lower temperatures, H-NS prevents transcription of thermally regulated genes while at higher temperatures transcriptional inhibition is derepressed allowing for temperature-dependent gene expression(17, 148, 152).
58 58 Transcriptional regulation by H-NS represents the only known mechanism by which temperature directly influences regulation of Shigella genes. Other regulatory mechanisms which are directly affected by temperature, production of alternative sigma factors, alteration of protein activity, RNA thermometers, etc., exist and have been thoroughly researched in other organisms; however, these studies have not been conducted in Shigella(12, 59, 82, 109). This represents a significant gap in overall knowledge about Shigella regulation and physiology. The aims of these studies were to examine the thermoregulation of Shigella virulence factors to more thoroughly understand the effects of temperature on gene expression in Shigella.
59 59 CHAPTER 2: REGULATION OF THE S. DYSENTERIAE SHU LOCUS 2.1 Background and Significance Shigella species must acquire the essential element iron in order to survive within each host and non-host environment encountered during the course of a natural infection. Iron is relatively abundant in the non-host environment and can be easily acquired; however, within the host iron is sequestered within iron binding proteins. These iron binding proteins include heme, hemoglobin, lactoferrin and transferrin; in addition to performing their natural function, these factors also allow the host to sequester iron and deny access of this essential nutrient to invading pathogens (27, 116, 117, 161). The most abundant host-associated iron binding proteins is heme, which represents the largest pool of iron in the human host, approximately 95% of total iron(117). In addition to the large amount of iron contained within heme molecules, Shigella species regularly come into close proximity of heme during an infection within a human host, a proximity that is outwardly manifested by the presentation of bloody diarrhea(13, 72, 110). Specifically, heme is released as a result of both the severe damage to the intestinal epithelium, caused by the invasion and spread of the pathogen within this tissue, as well as damage to endothelial tissue, mediated by activity of the Shiga toxin(13, 72, 110). The abundance, combined with the bacterial mediated exposure makes heme a potentially rich iron source for Shigella during the course of an active infection within the human host. The ability of Shigella to utilize heme as a source of nutrient iron is dependent upon the activity of the Shigella heme uptake (Shu) system(101, 102).
60 60 The Shu system is encoded by eight genes within a single locus the chromosome of all sequenced strains of S. dysenteriae and select strains of S. boydii (Figure 10)(166). The acquisition of heme in Shigella species by the Shu system is a step-wise process that requires uptake into the periplasm, transport across the inner-membrane, storage and utilization of heme bound iron; processes all mediated by specific components of the Shu system(27, 39, 101, 102, 164, 168). The active and selected transport of heme into the periplasm requires the 70kDa outer membrane heme binding protein encoded by the shua gene(27, 101, 102). The action of the ShuA protein is dependent on the TonB system which harnesses the electron motive force of the inner membrane to provide the required energy for heme uptake(27). Upon heme binding to the extracellular portion of ShuA, a conformational change within the protein permits a functional interaction with TonB(27). The energy provided by the TonB system induces a second conformational change in the transmembrane domain of ShuA that initiates the selected transport of heme across the outer membrane of the bacterium(27). Transportation of heme into the cytoplasm by the Shu system requires the ShuT, ShuU and ShuV proteins which are the periplasmic heme binding protein, a transmembrane heme permease and an ATPase respectively(28, 39, 166). ShuT binding of periplasmic heme is thought to serve two functions: 1) to sequester and minimize heme induced toxicity in the periplasm and 2) to deliver periplasmic heme to the inner membrane heme permease composed of two ShuU/V heterodimers(28, 39, 166). Binding of the ShuT:heme complex to the ShuU/V permease induces a conformational change in the inner membrane permease that permits ATP hydrolysis which drives the active
61 61 transport of the heme moiety across the inner membrane(28). Following ATP hydrolysis by the ShuU/V complex, ShuT is released into the periplasm to undergo another round of heme binding(28). Once inside the cytoplasm, heme must either be stored, to prevent heme induced toxicity, or degraded, to release the iron contained within(10, 164, 168). Previous work has shown that the shus gene encodes a cytoplasmic heme binding protein; however, the exact purpose of the ShuS protein is a matter of some debate(164, 168). It has been proposed that ShuS either shuttles heme from the ShuU/V complex to a heme storage protein, that ShuS itself stores the heme, or that the protein functions in some combination of these transport and storage functions(164, 168). While the exact function is unknown, multiple studies have shown that the ShuS protein is not a heme oxygenase and cannot degrade heme; a function attributed to its orthologue in the E. coli Chu system(143, 145, 164, 168). It is of note that no heme oxygenase has been identified in Shigella and therefore the machinery for degrading heme and releasing iron from the heme moiety remains unknown. While studies have identified the mechanisms and function of many of the Shu proteins; the function(s) of ShuX, ShuW and ShuY has not yet been determined. Although no function has been attributed to ShuX, it shares between 94-98% amino acid similarity to ChuX, its orthologueue in pathogenic E. coli species, whose function has been defined(144). In E. coli, ChuX forms a functional dimer which binds and sequester heme in a 1:1 ratio; presumably to prevent heme induced toxicity(144). These results indicate that ShuX, like ShuS, may function as a cytoplasmic heme binding protein. If
62 62 ShuX acts as a heme binding protein, as suggested by studies of ChuX in E. coli, then S. dysenteriae will contain two functionally redundant heme storage proteins, indicating the possibility that ShuX and/or ShuS may serve a different, yet unidentified, function(144). While functional studies of ChuX may provide hints as to the function of ShuX, the ShuW and ShuY ortholgoues in E. coli have not been extensively studied leaving little clues as to the function of these proteins in either species. Interestingly, the shuw gene is predicted to contain a premature translational stop codon within its open reading frame suggesting that it is a pseudogene(166). The shuw gene however, is largely conserved across S. dysenteriae, S. boydii and pathogenic E. coli species (including the premature stop codon) carrying the shu/chu locus, an observation that suggests ShuW may serve a function(166). No research has been conducted on ShuY, and it does not have significant homology to any previously identified heme acquisition proteins. A BLAST search against the ShuY amino acid sequence indicates that ShuY does have significant homology to the NmrA family of oxygen-dependent regulators (data not shown). These limited in silico analyses suggests that ShuY may serve to regulate shu transcription in response to oxygen availability; a mechanism that is not unprecedented(23). Together, the shu genes are necessary to confer heme utilization onto Shigella and E. coli strains(101, 102). The mechanism of heme uptake by the Shu system has been thoroughly examined; however, relatively little is known of the regulatory mechanisms governing expression of the shu genes. A common mechanism that regulates production of iron uptake systems in Shigella, and other gram-negative bacteria, is the iron-dependent
63 63 repression of gene expression mediated by the iron-dependent ferric uptake regulator, Fur where transcription is inhibited at high cytoplasmic iron concentrations(44). In silico analyses of the shu locus suggests the presence of well conserved Fur consensus sequences associated with each predicted shu promoter; however, the effect of Fur on expression of the shu locus is limited to plasmid-based promoter-reporter studies of shua carried out in E. coli(102, 166). These preliminary studies indicate that Fur regulates the expression of shua; however, whether this regulation is direct or indirect remains unknown. A second mechanism that regulates production of many iron uptake systems in Shigella is the direct transcriptional activation or repression by Fnr and ArcA in response to oxygen concentration(23, 77). Recent in vitro evidence has shown that both shua and shut are regulated, either directly or indirectly, in response to oxygen availability by ArcA. These two findings represent the accumulation of all knowledge regarding regulation of the shu locus; more research is required to fully understand the regulation of the Shigella heme uptake system. Heme acquisition by the Shu system allows Shigella to satisfy its requirements for nutrient iron during infection within the human host and heme acquisition systems are thought to increase both fitness and virulence within this environment(27, 63, 107, 107, 119, 151). However, due to the lack of a sufficient animal or cell model to examine the influence of the Shu system on Shigella virulence, this hypothesis cannot be tested directly. While the effect of the Shu system on Shigella virulence cannot be directly observed, there are three lines of evidence that suggest the Shu system influences virulence.
64 64 1) The entire shu locus is conserved between Shigella and pathogenic E. coli strains but is absent in non-pathogenic strains of E. coli(115, 151, 166). The presence of the Chu system, the E. coli Shu orthologueue, is positively correlated with virulence of these organisms(66, 115, 151, 166). 2) The pathophysiology of shigellosis, specifically the resulting physical damage and bleeding from the colonic epithelium, creates an environment in which S. dysenteriae will encounter heme(72, 110). For this reason, heme is readily available to the pathogen during infection. 3) In vitro studies show the Shu system is sufficient for Shigella to satisfy its iron requirement when heme is the sole iron source available, indicating its role as an efficient iron acquisition system(101, 102). While this does not mean all of the other iron uptake systems are dispensable in vivo, it does indicate the efficiency of this system and its ability to allow Shigella to fully satisfy its iron requirement. Together, these observations suggest that the Shu system gives Shigella species a competitive advantage, against those that lack this system, during an infection by facilitating the utilization of the most abundant source of essential iron within the host. To more fully understand the physiology and virulence of this clinically important pathogen, a full understanding of the regulation of its virulence and nutrient acquisition systems are required. The aim of the studies detailed in this chapter is to identify and characterize the regulatory mechanisms controlling the expression of each gene within the Shigella dysenteriae shu locus.
65 Methods Bacterial strains, plasmids, and culture conditions All bacterial strains and plasmids used in this study are shown in Table: 2. E. coli was routinely cultured in Luria-Bertani (LB) broth (1% tryptone 0.5% yeast extract and 1% NaCl) or on LB agar plates at 37 C. S. dysenteriae was routinely cultured in LB broth or on tryptic soy broth agar plates (Becton Dickenson and Company, Sparks, MD) containing 0.01% (wt/vol) Congo red dye (ISC BioExpress, Kaysville, UT) at the indicated temperature. Iron-stressed growth conditions were achieved by the addition of 200 µg/ml of deferrated ethylenediamine-n,n -bis 2-hydroxyphenylacetic acid (EDDHA) to LB. EDDHA was deferrated as detailed previously(128). Chloramphenicol and Ampicillin were used at a final concentration of 30 µg/ml and 150 µg/ml respectively to select for the presence of reporter or control plasmids. All oligonucleotide primer sequences used in this study are available upon request
66 66 Table 2 Chapter 2 bacterial strains and plasmids Designation Description Reference/ Source Strains S. dysenteriae ND100 Wild-type S. dysenteriae. O-4576S1 containing a spontaneous Str r mutantation (106) shua fur E. coli DH5α Top10 Plasmids O-4576S1 with a chromsomal deletion of shua ND100 with a chromosomal deletion of the fur gene MG1665 derivative containing a enda, hsdr17 and a reca mutation for efficient transformation. Contains Δ(lacZ)M15 enabling blue-white screening. DH5α derivative which also contains an ara14 deletion preventing arabinose catabolism and DE3 which encodes T7 polymerase (102) (106) Invitrogen Invitrogen
67 67 Table 2 (continued) pxg-10 pxg-0 pa-gfp pt-gfp ps-gfp pu-gfp Low-copy reporter containing the constitutive promoter PLtet0-1 driving expression of a gfp reporter; Chl r Derivative of pxg-10 lacking the PLtet0-1 promoter. pxg-10 without the PLtet0-1 promoter with the native shua 5 utr and promoter element translationally fused to gfp; Chl r pxg-10 lacking its constitutive promoter with the S. dysenteriae shut 5 utr and promoter element translationally fused to the gfp reporter; Chl r Derivative of the pxg-10 reporter plasmid with the predicted S. dysenteriae shus 5 utr and promoter translationally fused to and driving expression of the gfp reporter; Chl r The pxg-10 plasmid lacking the constitutive promoter with the putative S. dysenteriae shuu and 5 utr sequence translationally fused to the gfp reporter; Chl r (153) (153) This study This study This study This study
68 Generation of the Gfp translation reporter plasmids Following isolation from E. coli strain Top10 using a Plasmid Midi Kit (Qiagen, Valencia, CA) according to manufacturer protocols, plasmid pxg-10 was digested with AatII and NheI restriction endonucleases (New England BioLabs, Ipswich, MA) to remove the PLtetO-1 promoter and lacz fragment originally encoded on the pxg-10 plasmid (153). A DNA fragment containing the shua, shus, shut and shuu promoter and 5 utr were amplified from the chromosome of wild-type S. dysenteriae by polymerase chain reaction using primers which contain AatII and NheI endonuclease recognition sites respectively. The amplified product was purified using a QIAQuick gel extraction kit (Qiagen) and then digested with AatII and NheI endonucleases and cloned into the digested pxg-10 plasmid backbone to create pa-gfp, ps-gfp, pt-gfp and pu-gfp. The nucleic acid sequence of each reporter was verified by nucleic acid sequencing of both DNA strands Western blot analysis All Western blot analyses were performed using whole cell extracts. Bacterial cultures were grown to stationary phase under the indicated growth conditions and the optical density at 600 nm measured using a ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE). A total of bacterial cells from each culture were pelleted and resuspended in 200 µl of Laemmli protein dye (Bio-Rad) containing 5% 2- mercaptoethanol and were boiled for 10 minutes. Samples were stored frozen at 20 C until use.
69 69 ShuA Western blot analysis was carried out as follows: 15 µl of each whole cell protein preparation was separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) using a 7.5% polyacrylamide gel and was then transferred to a PVDF membrane that had been pre-soaked in methanol for 10 minutes and rinsed three times with water. Following protein transfer, the membrane was blocked by incubation overnight at 4 C in a solution of phosphate buffered saline with 0.1% Tween20 (PBST) and 5% milk. After blocking, the membrane was washed 4 times for 5 minutes each time using PBST and then incubated for 1 hour at 4 C with polyclonal α- ShuA IgG (Custom antibody raised in a rabbit - Fisher Scientific, Waltham, MA) diluted 1:1000 in a solution of PBST with 5% milk. Following a series of washes as described above, the membrane was incubated for 1 hour at 4 C with goat anti-rabbit HRP conjugated IgG (Bio-Rad) diluted 1:10,000 in a solution of PBST with 5% milk. Finally, the membrane was rinsed as above, incubated in Immun-Star regents (Bio-Rad) according to manufacturer protocols and imaged using x-ray film. Gfp Western blot analysis was carried out as detailed for that of ShuA with the following modifications. Following transfer of whole-cell protein samples to the PVDF, as detailed above, the membrane was blocked by incubation overnight at 4 C in a solution of PBST and 10% milk. Next, the membrane was incubated for one hour at 4 C with anti-gfp monoclonal IgG stabilized antibody preparation (Roche, Indianapolis, IN) diluted 1:1000 in a solution of PBST and 5% milk. The membrane was then washed 3 times for 5 minutes, incubated in a solution of PBST and 10% milk for 10 minutes at 4 C and then incubated for 1 hour at 4 C with goat anti-mouse HRP conjugated IgG (Bio-
70 70 Rad) diluted 1:20,000 in PBST with 5% milk. Finally, the membrane was washed and the blot was imaged using Immun-Star WesternC reagents (Bio-Rad) and a ChemiDoc XRS+ Imaging System (Bio-Rad). Contrast was altered uniformly over the entire image using Image Lab software (Bio-Rad) to reduce background noise and did not alter relative band intensity or the banding pattern observed RNA extraction for Q-PCR and RT-PCR analysis After culturing the samples to the growth phase and under the conditions required for each experiment, total RNA was extracted using a Trizol based protocol. First, cells were pelleted and resuspended in μl DEPC treated ddh 2 O, 40 μl 10% SDS, and 2.67 μl 3M sodium acetate (ph 5.2) and vortexed for 15 seconds. Next, cells were incubated to 90 C for 7 minutes and 1 ml of Trizol (Life Technologies, Carlsbad, California) was added. The solution was then added to a phase-lock tube and incubated at room temperature for 5 minutes. Next, 250µL of chloroform was added to the solution, shaken by hand for 1 minute and allowed to incubate at room temperature at 2 minutes. The solution was next centrifuged at 14,000 RPM for 2 minutes and the top aqueous phase containing the purified RNA was removed. To precipitate the RNA, 1mL of absolute ethanol was added to the isolated RNA and was incubated at -80 C overnight. The RNA solution was then centrifuged at max speed for 15 min at 4 C. The ethanol was decanted and ice cold 75% ethanol was added to the RNA pellet; the solution was centrifuged again at max speed for 15 minutes at 4 C. The ethanol was again decanted and the RNA pellet dried in a Vacufuge Concentrator 5301 (Eppendorf, Hauppauge,
71 71 NY). Next, RNA was purified using the TurboDNase kit (Life Technologies) according to manufacturer protocols. The resulting RNA was screened for DNA contamination using a DNA-dependent DNA polymerase RNA extraction for RNAseq analysis WT S. dysenteriae was grown to mid-log phase at 37 C under iron rich and iron poor conditions and RNA was extracted using an RNEasy Mini-kit (Qiagen) according to manufacturer protocols and the samples were treated with TurboDNase according to manufacturer protocols. DNA contamination of the RNA samples was examined by PCR analysis using primers known to specifically amplify a portion of the shua open reading frame. The RNA was then subjected to quality control analysis by the Ohio University Genomics Facility using the 3130xl Genetic Analyzer (Applied Biosystems, Carlsbad, California). Next, an RNA spike, ERCC spike-in mix (Ambion) diluted 1:100 in DEPC water, was added to the RNA samples. The samples were then depleted of ribosomal RNA by sequential treatments with the Ribo-zero rrna removal kit (Epicentre, Madison, WI) and the MICROBExpress rrna removal kit (Ambion) to ensure total removal of rrna. Following ribosomal removal, the samples were again analyzed using the 3130xl Genetic Analyzer and subjected to RNA sequencing performed by the Ohio University Genomics Facility using the Ion Torrent (Life Technologies). Data generated from this experiment was analyzed by Ronan Carrol (University of Southern Florida).
72 Quantitative real-time polymerase chain reaction cdna was generated from 150 ng of total RNA using the iscript cdna Synthesis Kit (Bio-Rad) according to manufacturer protocols. Each cdna sample was diluted 1:10 in water and 5 µl used as template in a 20 µl amplification reaction. Primer concentration and reaction conditions were optimized for each primer set. All quantitative Real-time PCR reactions were carried out using iq SYBR Green Supermix (Bio-Rad). A six point standard curve was generated for each target during each experimental run to ensure that an acceptable efficiency was achieved in the analysis. All expression values were calculated using the ΔΔCt method, normalized to the level of rrsa measured in each sample and expressed relative to the value obtained in the indicated control sample. All reactions were performed in a Bio-Rad CFX96 Real-Time PCR System. All primer sequences were designed using Beacon Designer 7.5 and are available upon request Reverse transcriptase PCR analysis of the shua and shut 5 utr A two-step reverse transcriptase PCR was conducted in a PTC-200 DNA Engine Cycler (Bio-Rad) using SuperScript III Reverse Transcriptase (Invitrogen, Grand Island, New York) according to manufacturer protocols followed by an amplification reaction. Using 150 ng of RNA isolated from wild-type S. dysenteriae cultured to stationary phase under iron-stressed conditions as template, shua specific cdna was generated in the reverse transcription step using primer A R1 that binds within the shua open reading frame. In the amplification step, specific products were amplified in a 50 µl reaction containing 1 µl template (cdna), 5 µl of 2.5 mm dntp, 5 µl of 10 Standard Taq
73 73 Reaction Buffer, 1 µl of Taq DNA Polymerase, 36 µl of RNase free water, 1 µl of primer A R1 and 1 µl of either primer A F1, primer A F2 or primer A F3 at a final concentration of 0.6 µm of each primer. A No-RT control in which the isolated RNA was used as template in the amplification step was performed with each primer pair to screen for DNA contamination in the RNA sample. A positive amplification control using primer pair shua R1 and shua F1 was conducted using S. dysenteriae genomic DNA as template. Amplification products were resolved on a 2% agarose gel with 0.001% ethidium bromide and visualized with a ChemiDoc XRS+ Imaging System (Bio- Rad). The same protocol was used for the analysis of the shut 5 utr, generating shut specific cdna using the T R1 primer which was subsequently paired with the forward primers T F1, T F2 and T F3 during the amplification reaction. All other conditions were the same as used for the identification of the shua 5 utr Reverse transcriptase PCR analysis of the intergenic shu regions RNA was extracted from iron stressed WT S. dysenteriae cultures and used in a cdna reaction using the iscript cdna Synthesis Kit according to manufacturer protocols (Bio-Rad). The cdna was then used in an amplification reaction using the indicated primers in a standard 50 µl PCR reaction containing 1 µl template (cdna), 5 µl of 2.5 mm dntp, 5 µl of 10 Standard Taq Reaction Buffer, 1 µl of Taq DNA Polymerase, 36 µl of RNase free water and 1 µl of each indicated primer. Two controls using DNA and the RNA used in the reverse transcriptase reaction for template were also amplified using the indicated primers. The products were ran on a 2% agarose gel
74 74 containing ethidium bromide and visualized with a ChemiDoc XRS+ Imaging System (Bio-Rad) Statistical analysis Two tailed, two sample Student s t tests, assuming equal variance, were used throughout our studies to determine significance (P 0.05). 2.3 Results Organization of genes within the shu locus Many of the mechanisms governing function of various proteins within the Shu system have been experimentally determined; however, the arrangement and regulation of the genes within the shu locus had not been extensively studied. The shu locus was first identified by the ability of a 27kb region of the wild-type S. dysenteriae genome to confer heme utilization upon an E. coli strain deficient in its uptake(101). In silico and in vitro analysis of the region demonstrated that the S. dysenteriae Shu system is encoded within a single 9kb locus on the chromosome of this pathogen (Figure 10)(101). Further in silico analysis of the shu locus identified four independent putative promoters driving expression of eight genes arranged into two monocistronic (shua and shus) and two polycistronic (shutwxy and shuuv) transcripts(168). While in silico predictions provide an initial idea, experimental determination of the transcriptional landscape within the shu locus is a necessary first step towards understanding the regulatory mechanisms governing Shu protein production.
75 75 To begin characterizing the genetic organization of the shu locus as well as the relative expression level and regulation of each shu gene, an RNAseq analysis was performed. In addition to identifying whether each predicted shu transcript is expressed, RNAseq data will allow for approximate predictions of transcriptional start and stop sites. These data were generated by extracting RNA from WT S. dysenteriae grown to mid-log phase at 37 C under iron stressed conditions. RNA was extracted from these cultures and ribosomal RNA was depleted to enrich for mrna. The resulting mrna was used for RNAseq analysis. Results provided sufficient coverage of the shu locus, except for shuv, and confirmed expression of the shu transcripts (Figure 12). Analysis of the shua transcript agrees well with the in silico prediction suggesting that shua has an unusually long 5 untranslated region, 292 nucleotides according to the RNAseq data compared to 287 with in silico predictions(166). The RNAseq data also supports the position of the shua promoter predicted by in silico analysis(166). Of note is the expression of a polycistronic transcript containing the shua and shus messages that were initially predicted to be encoded separately and driven by independent an independent promoter(166). Transcription of the shutwxy message was identified by RNAseq; however, the reads mapped to this region do not extend to include the predicted shut 5 utr (166). Of note is that RNAseq does not have full coverage of the shuw gene; however, this is most likely due to the decrease in transcripts mapped to that area and not the presence of individual transcripts within the shuw open reading frame due to the low number of reads mapped to this region and the lack of an in silico predicted promoter. In addition, the RNAseq data suggests the shutwxy and shuuv polycistronic transcripts,
76 76 that were predicted to be encoded separately by independent promoters, are transcribed as a single message due to the mapping of reads between the shuy and shuu genes. However, from these data are insufficient to conclude if this transcript extends to shuv due to the low number of reads mapped to this region. Interestingly, an undefined transcript is expressed between the shua and shut transcripts. A BLAST search of this unidentified transcript does not predict any known function but the fact that it overlaps with the shut 5 utr suggests a regulatory function. Analysis of the shu transcripts provided good insight into the transcriptional landscape of the shu locus.
77 77 Figure 12: RNAseq data predicts the presence of two transcripts expressed from the shu locus RNA was extracted from WT Shigella dysenteriae grown at 37 C to mid-log phase under iron stressed conditions. Ribosomal RNA was removed to enrich for mrna that was used in RNAseq analysis. ORF represents the annotated open reading frames from the Shigella dysenteriae SD197 genome deposited onto the Genbank database. A blue arrow predicts a premature translational stop codon within the open reading frame while a yellow arrow does not contain a premature translational stop codon. Individual reads shows each RNA fragment mapped to the shu locus. A green line is an RNA fragment that is encoded on the + strand of the genomic DNA while a red line indicates a fragment transcribed on the strand of the genomic DNA. Coverage denotes any area of the shu locus that has at least one transcript associated with it. Any non-continuous segments of the black line indicate no transcript is associated with that region. A (*) indicates a previously unidentified transcript within the shu locus. These data were generated in biological triplicate. To verify the transcriptional landscape of the shu locus as defined by RNAseq analysis, the transcriptional landscape of the shu locus was directly examined by Northern blot analysis. Briefly, total RNA was isolated from S. dysenteriae and subjected to Northern blot analysis with 32 P labeled probes specific to shua, shus, shut and shuu, the first gene in each predicted transcript. The data generated from these experiments was inconclusive due to apparent cross-reactivity between each shu probe and the 23S and 16S rrna genes and additional probes specific to different regions of the target shu transcripts also showed cross-reactivity to the rrna molecules (data not shown). Ribosomal depletion of the RNA samples also provided poor results as the concentration
78 78 and quality of the resulting RNA were not sufficient to reliably detect the target transcripts with a high degree of certainty (data not shown). In the absence of data from Northern blot analysis, transcript expression from and organization of the shu locus, as indicated by RNAseq data, reverse-transcriptase PCR (RT-PCR) analysis was performed to investigate the shu locus organization by a different method (Figure 13). Briefly, primers were designed to amplify the intergenic regions between each shu gene. The presence of an amplified product would indicate the target genes are encoded on a single transcript, while the absence of a product would suggest the target genes are encoded on separate mrna molecules. Prior to analysis of the cdna, each primer pair was used to amplify DNA in order to validate their functionality. Analysis of the RT-PCR reactions for each primer pair agreed well with the RNAseq results indicating not only that shua and shus are encoded on a single polycistronic message (shuas), but that the same is true for shut, shuw, shux, shuy, shuu and shuv (shutwxyuv) (Figure 13A and 13B) Together, data from both RNAseq and RT-PCR analyses reveal a transcriptional landscape of the shu locus that differs significantly from previously published data(165).
79 Figure 13: RT-PCR analysis suggests the presence of two polycistronic transcripts encoded by the shu locus (A) Schematic of the shu locus with predicted promoters represented by the arrows. Each bracket indicates the PCR products amplified for analysis by the RT-PCR experiment. (B) WT S. dysenteriae was cultured at 37 C to mid-log phase under iron stressed conditions. RNA was extracted from these cells and was used in a Reverse-Transcriptase PCR reaction. Briefly, cdna was synthesized using random oligos in a reversetranscriptase reaction. The cdna generated in the previous step was used as a template to amplify the intergenic region between each shu gene using primers that were specific to the shu open reading frames. In addition to cdna, S. dysenteriae chromosomal DNA (DNA) and the RNA used to generate the cdna (-) were used as template to check the efficacy of each primer pair and to screen for DNA contamination of the RNA samples respectively. Each PCR reaction was ran on a 2% agarose gel to check for amplification products. These experiments were conducted in biological triplicate. 79
80 80 The approximate location of the shut and shua transcriptional start sites was indicated by RNAseq analysis; to more precisely locate the shua and shut promoters and transcriptional start site of these genes, RT-PCR was conducted. The RT-PCR experiment was performed using one reverse primer located within the predicted open reading frame that, in each reaction, was paired with forward primers at sites within and outside of the predicted 5 utr of each gene (Figure 14A and 14C). An amplification product would indicate that the transcript extends at least to the upstream primer binding site while the lack of a product suggests that the 5 utr does not extend to or past the corresponding primer binding site. Analysis of the 5 utr of both shua and shut by RT- PCR indicates that the shua 5 utr is at least 287 but no longer than 328 nucleotides in length, while the shut 5 utr is predicted to be between approximately 90 and 120 nucleotides long (Figure 14B and 14D). The RT-PCR data generated for shua is in good agreement with the RNAseq data (Figure 12); however, the shut data disagrees with the RNAseq data. This discrepancy may be due to processing of the native shut transcript which would be difficult to identify via RNAseq analysis but would be more readily visible using RT-PCR analysis. These analyses were not conducted with shus or shuu, the other two genes predicted to be at the 5 end of independent transcripts, because these genes were shown to be encoded on a single transcript with shua and shut respectively (Figure 12 and 13) (166). Using this technique, the location of an independent transcript containing shus or shuu would be impossible to detect. The combined data from the RNAseq and RT-PCR data not only predicts the approximate transcriptional start sites for
81 shua and shut; but, it can also be used to estimate the promoter region due to the close nature of the promoters to the transcriptional start sites in prokaryotes. 81 Figure 14: The shua and shut transcripts contain an extended 5 untranslated region A and C) Schematic (not drawn to scale) of the S. dysenteriae shua and shut promoters and predicted 5 utr. Arrows represent primer-binding sites used in the cdna synthesis and amplification steps of the reverse transcription polymerase chain reaction (RT-PCR) analyses. The predicted promoter region is boxed and the translational start site (T.S.S.) is indicated. B an D) The predicted shua and shut 5 utr was experimentally identified by a series of RT-PCR analyses using RNA isolated from wild-type S. dysenteriae grown under iron limiting conditions. For each experimental reaction, primer A R1 and T R1 was used to generate the shua and shut specific cdna product respectively. In each amplification step, the reverse primer was paired with the indicated forward primer. A control reaction using RNA as template was conducted with each primer set to ensure that the isolated RNA sample did not contain DNA contamination; a ( ) indicates RNA was used as template in the amplification reaction while a (+) indicates that cdna was used as template in the amplification reaction. DNA was used as template in the amplification step to verify that the indicated primer pair was capable of facilitating target amplification. Following the approximation of putative promoter elements driving expression of shua and shut by RNAseq and RT-PCR analysis, these regions were next identified and
82 82 cloned into translational reporter plasmids to confirm the presence of an active promoter within each. The location of each shu promoter was predicted by in silico analysis using the Bprom software package (data not shown). The indicated promoter and associated 5 utr of the shua and shut genes were cloned and translationally fused to a gfp reporter gene on the plasmid vector pxg-10 creating pa-gfp and pt-gfp respectively. Additionally, the lack of individual transcripts carrying the shus and shuuv message, as determined by the RNAseq and RT-PCR data, does not eliminate the possibility that an independent promoter exists to drive expression of these genes. To investigate this possibility, two additional reporters were created for shuu and shus, pu-gfp and ps-gfp respectively. Since no experimental data exists to approximate the transcriptional start sites of shuu and shus, only in silico analysis was used to predict their respective promoters. These reporters were transformed into wild-type Shigella dysenteriae and the transcriptional and translational activity analyzed by quantitative real-time PCR (Q-PCR) and Western blot analysis. Both transcriptional and translational activity was noted for the pa-gfp and pt-gfp plasmid reporters; however, no activity was observed from the pu-gfp or ps-gfp reporters under conditions tested (data not shown). It is possible that the shus and shuu reporters were non-functional due to an inaccurate estimation of the promoter location. Additional constructs were made that contained a larger area upstream of the predicted translational start sites of each gene; however, none of these reporters produced a detectable product (data not shown). Isolating the shua and shut promoters and 5 untranslated regions from the open reading frame will indicate their ability to regulate expression of their respective genes.
83 Iron-dependent transcriptional regulation of the shu locus The concentration of free iron varies greatly between the iron rich non-host and iron-poor host environments, this variability serves as a cue to influence gene expression(44, 117, 161). Such regulation ensures maximal production of virulence and iron acquisition factors in an environment that the organism will receive the most benefit from each factor produced. The iron-dependent regulation observed in Shigella and many other pathogenic bacteria is facilitated largely by Fur which binds ferrous iron and transcriptionally represses its target genes(44). In addition to regulating Shigella virulence and iron acquisition genes, Fur has been experimentally shown to modulate the iron-dependent regulation of shu homologues in important pathogens including Vibrio cholerae, Pseudomonas aeruginosa, and Bordetella pertussis(44). The fact that Fur has been shown to modulate expression of shu homologues combined with limited in vitro and in silico data on the S. dysenteriae shu locus suggests that shu gene expression may be regulated at the transcriptional level either directly or indirectly, by Fur(101, 166). The effect of any regulatory mechanism on expression of its target gene product should be directly observed at the protein level; thus, Western blot analysis was first performed to determine the effect of iron availability and Fur on ShuA production was analyzed. Western blot analysis was conducted with whole-cell lysates from WT S. dysenteriae or a fur derivative cultured to stationary phase at 37 C in LB, an iron rich media, or in LB treated with the iron chelator EDDHA, to iron stress the cultures, which would stimulate expression of Fur regulated genes. The effect of iron and Fur on production of the ShuA protein was directly analyzed by Western blot using a polyclonal
84 84 α-shua antibody (Figure 15). These results show WT S. dysenteriae minimally produces ShuA protein in an iron rich environment; however, under iron-poor conditions a moderate production of ShuA is observed. When compared to the fur strain grown under iron-rich conditions, ShuA is maximally produced indicating direct or indirect regulation by Fur. Specificity of the α-shua antibody was determined by the lack of a band in a shua knockout. Figure 15: ShuA production is regulated by iron in a Fur-dependent manner WT S. dysenteriae and a shua and fur deletion strains were grown to stationary phase at 37 C in iron rich or iron poor media. Following growth to the indicated growth stage whole cell lysates were created and used in Western blot analysis. ShuA protein was visualized using a polyclonal αshua antibody. These data are representative of a biological triplicate. To observe the effect of iron and Fur on shu promoter activity, whole cell lysates of WT and a fur knockout carrying the reporter plasmids were cultured to stationary phase at 37 C in an iron rich (LB) or iron poor (LB with EDDHA) media. Whole cell lysates were subjected to Western blot analysis with a monoclonal α-gfp antibody. Expression of gfp from pa-gfp reporter plasmid mimics those seen when ShuA is probed directly indicating the pa-gfp translational reporter plasmid accurately represents native levels of the ShuA protein (Figure 16A). Production of Gfp from the shut reporter also mirrors that of ShuA with the least amount of expression from WT S. dysenteriae and
85 85 maximum expression from the fur strain (Figure 16B). The shus and shuu reporter constructs were also examined by Western blot using the α-gfp. In contrast to the shua and shut reporters, no Gfp production was observed from the ps-gfp or pu-gfp under the conditions tested (data not shown). These data indicate that production of the ShuA and ShuT proteins are regulated by iron either directly or indirectly by the transcriptional repressor Fur. A) B) Figure 16: The pa-gfp and pt-gfp reporters demonstrate iron-dependent regulation mediated by Fur Both WT S. dysenteriae and a strain containing a chromosomal fur deletion were cultured to stationary phase in a high iron (LB) or low media. Each strain was harboring the reporter constructs (A) pa-gfp, (B) pt-gfp or the vector control pxg-0. Whole cell lysates were obtained from each culture and were subjected to Western blot analysis using a monoclonal αgfp antibody. These experiments were conducted in biological triplicate and the images are representative of the observed regulation. Fur-dependent repression of its target genes occurs at the transcriptional level by inhibiting the recruitment of the transcriptional machinery; to determine the effects of Fur and iron on the relative abundance of shu transcripts Q-PCR was used(44). Analysis of the shu transcripts was performed using the same strains, reporter constructs and growth conditions as the Western blot analysis to accurately gauge the effects of these conditions on expression of the shu genes. While direct analysis of the Shu proteins was limited to ShuA due to the inhibitive price and time constraints of generating a custom antibody,
86 86 transcript analysis by Q-PCR does not have these restrictions allowing for direct analysis of all shu genes of interest. Investigation of the native shua and shus transcripts by Q- PCR revealed the same expression pattern for each gene with WT S. dysenteriae expressing low and moderate amount of transcripts under high iron and iron limited conditions respectively and the fur strain expressing the highest level of transcripts (Figure 17). Expression of shut was slightly different than that of shua and shus with iron-stressed WT S. dysenteriae and the fur strain expressing the same amount of transcript (Figure 17). When compared to expression of the chromosomally encoded genes, the overall regulatory pattern for pa-gfp and pt-gfp was conserved further validating the translational reporters as valid tools for examining regulation of the shu genes (Figure 18). No gfp transcript was observed from the ps-gfp and pu-gfp reporters or the native shuu promoter. Together these experiments indicate that the shu genes are regulated at the transcriptional level either directly or indirectly by the iron-dependent transcriptional repressor Fur.
87 Figure 17: The shu locus is transcriptional regulated by iron via a Fur-dependent mechanism Quantitative-PCR (Q-PCR) analysis was conducted on RNA extracted from WT S. dysenteriae and a fur deletion mutant were grown at 37 C under iron rich and iron poor conditions. The levels of shua, shus, shut and shuu mrna were expressed relative to the first shua iron rich sample and were normalized to the abundance of rrsa in each sample. A (*) indicates a significant difference between the plus iron and the other two conditions for each gene. Significance levels were set to p 0.05 and error bars represent one standard deviation. These data are representative of biological triplicates and each error bar represents one standard deviation. 87
88 88 Figure 18: Fur-dependent regulation of shua and shut is localized to the promoter and 5 utr Relative amounts of gfp transcript were analyzed by Q-PCR analysis. WT and fur deletion strains of S. dysenteriae harboring either the pa-gfp or pt-gfp reporter plasmid were cultured under iron rich or iron poor conditions at 37 C. The levels of gfp from the reporter plasmid were normalized to the amount of the house-keeping gene rrsa in each sample and set relative to the first plus iron sample for each plasmid. A (*) indicates a significant difference, assuming a significance level of 95% (p 0.05), between the plus iron condition and the minus iron/delta fur conditions. Each error bar represents on standard deviation and these data are representatives of biological triplicates Post-transcriptional thermoregulation of the shu locus To determine the effects of temperature on Shu protein levels, whole cell extracts of wild-type S. dysenteriae both lacking and harboring the pa-gfp, pt-gfp, ps-gfp, or pugfp reporter constructs were extracted and subjected to Western blot analysis. ShuA protein was directly assayed with the same αshua polyclonal antibody used in the iron studies and was found to be significantly upregulated following growth of WT S. dysenteriae at host body temperature, 37 C, as compared to room temperature, 25 C (Figure 19). Additionally, WT S. dysenteriae harboring the shua and shut reporter
89 89 plasmids showed increased levels of Gfp protein at 37 C vs. 25 C (Figure 20). The shus and shuu promoter constructs did not produce Gfp under either condition tested which coincides well with our previous reporter studies indicating that the promoters are either not active under conditions tested or are completely inactive (data not shown). These data indicate that ShuA and ShuT protein levels are modulated in response to temperature. Figure 19: ShuA protein production is influenced by environmental temperature with increased levels detected at 37 C Wild-type S. dysenteriae and S. dysenteriae ΔshuA knockout strain were grown to stationary phase at the temperatures indicated under iron limiting conditions. Western blot analyses were conducted using whole-cell lysates generated from an equivalent number of bacteria grown under each condition and a polyclonal anti-shua antibody. A) B) Figure 20: shua and shut promoter and 5 utr are sufficient to confer thermoregulation on Gfp production A Western blot analysis was performed with monoclonal anti-gfp antibodies and wholecell extracts generated from an equivalent number of wild-type S. dysenteriae carrying either (A) pa-gfp, (B) pt-gfp or the empty vector pxg-0. All strains were cultured under iron-limited conditions to stationary phase at the temperatures indicated. All data are representative of three biological replicates and error bars represent one standard deviation.
90 90 Thermoregulation in S. dysenteriae is largely attributed to the direct or indirect actions of H-NS a transcriptional repressor(17, 42). Examination of the relative abundance of shu transcripts in response to temperature by Q-PCR will indicate whether HNS is directly responsible for the temperature-dependent increase of Shu protein levels observed because direct regulation by HNS would show the same pattern of transcriptional regulation of the message compared to the translational regulation of the protein. Direct examination of shua, shut and shus transcripts by Q-PCR showed no significant difference in mrna abundance between transcript levels produced by WT S. dysenteriae cultured at 25 C vs. 37 C (Figure 21). When compared to the native shu genes, gfp transcription from the pa-gfp and pt-gfp plasmids closely mirrors that of shua and shut with equal or significantly more mrna expressed at from WT S. dysenteriae cultured at 25 C as compared to 37 C (Figure 22). Consistent with our previous findings no expression was detected for the chromosomally located shuu or the shus or shuu translational reporter (data not shown). Together, these data indicate that the shu locus is not transcriptionally regulated by H-NS but is post-transcriptionally thermoregulated by an unknown mechanism.
91 91 BLD Figure 21: Transcription of shua, shus and shut from the S. dysenteriae chromosome is not significantly affected by temperature Relative amount of the shua, shus, shut and shuu genes from the S. dysenteriae chromosome was measured by Q-PCR analysis. WT S. dysenteriae the specified reporter plasmid was cultured at 25 C or 37 C to stationary phase.shu transcript levels were expressed relative to the first sample cultured at 25 C for each strain and expression of each sample was normalized to the housekeeping gene rrsa. Error bars represent one standard deviation. These data were collected in triplicate and the data presented are representative of the observed regulation. BLD represents samples that were below the level of detection for this experiment.
92 92 Figure 22: Temperature does not affect transcription from the shua reporter but does have a significant effect on the shut reporter The relative amount of gfp expressed from WT S. dysenteriae carrying the pa-gfp and pt-gfp plasmids was examined by Q-PCR analysis. S. dysenteriae was cultured to stationary phase at either 25 C or 37 C. The total amount of gfp transcript expressed from these strains was expressed relative to the first 25 C sample for each strain and normalized to the house-keeping gene rrsa. A (*) indicates a significant difference, assuming a significance level of 95% (p 0.05), in gfp transcript measured from each strain cultured at 25 C and 37 C. Each error bar represents one standard deviation and these data are representative of a biological triplicate. 2.4 Discussion These represent the first studies to have experimentally validated the organization and transcriptional landscape of the shu locus which encodes the sole heme uptake system in Shigella species and differ significantly from the predicted locus structure(166). In addition this work confirms that the shu locus is regulated by iron, either directly or indirectly by the iron-dependent transcriptional repressor Fur. Finally, these studies are the first to validate the post-transcriptional thermoregulation of a
93 93 bacterial heme uptake system and the first to observe temperature regulation of a Shigella iron uptake system. The transcriptional landscape of the shu locus observed in these studies differs significantly from previously published in silico studies(166). Of note is the presence of a transcript encoding the shua and shus genes on a single message which differs significantly from what was previously reported (Figure 12 and 13). Native shus expression from the S. dysenteriae chromosome is consistently lower than that of shua under all conditions tested, a hallmark of regulation of genes located downstream on an operon. Another interesting finding is that no expression was observed from the predicted shuuv promoter which drives expression of the inner-membrane heme permease and ATPase respectively (data not shown) (28). Curiously, previous in vitro studies have found that the shuuv transcript encodes fully functional proteins; however, in those studies each of the genes was transcribed from non-native, constitutive promoters. These findings indicate that shuu and shuv are encoded on the same transcript as the previously identified shutwxy message (Figures12 and 13). These data were generated using RT- PCR and RNAseq analysis. Together, these data identify what looks like two transcripts produced from the shu locus. While these findings suggest two transcripts were made, the use of random oligos to generate cdna in the RT-PCR reaction could produce an event in vitro where cdna generated from two overlapping but independent transcripts driven by different promoters on the same genomic DNA strand could be amplified into a single product. While this may be a possibility, the evident lack of promoter elements internal to
94 94 the two predicted shu transcripts, based on in silico analysis and cloning experiments, makes this possibility unlikely. The identified shu promoters were cloned and used in regulatory studies that have indicated that the promoter and 5 untranslated region of the shua and shut genes are sufficient to confer transcriptional regulation in response to iron availability and posttranscriptional regulation in response to environmental temperature on a reporter (Figures 16, 18, 20 and 22). Precise regulation of the Shu system likely increases Shigella fitness by maximally expressing these genes under conditions that mimic the human host, the environment in which Shigella encounters heme(161). Specifically, in the non-host environment, the organism will encounter high iron concentrations as well as a variable temperature which will inhibit shu gene expression by Fur and an unknown mechanism respectively. In contrast, the host environment, in which Shigella invades and causes disease, is devoid of free-iron and maintains a steady temperature of 37 C which derepresses transcriptional repression by Fur and increases translation by a temperaturedependent mechanism respectively. These environmental conditions allow for Shigella to conserve energy by repressing production of the Shu system in an environment lacking heme while maximally producing the Shu system under iron starved conditions where it is likely to encounter heme. The iron-dependent regulation of the shu locus is dependent upon Fur in either a direct or indirect manner; however, the thermoregulation observed is mediated by an unknown mechanism. Previous studies have indicated that thermoregulation in Shigella species is facilitated by the temperature-dependent transcriptional regulator H-NS;
95 95 however, the data do not support regulation by this mechanism(17, 148). The shu transcript expressed by S. dysenteriae at 37 C compared to 25 C is not significantly different and is not indicative of regulation by H-NS based on the pattern of transcriptional and translational regulation (Figures 21 and 22). These results indicate that the Shu protein levels are regulated at the post-transcriptional level by a temperaturedependent mechanism. Regulation by this mechanism is indicative of regulation by an RNA thermometer; a cis-encoded riboregulator that modulates translation by forming an inhibitory hairpin that occludes the Shine-Dalgarno sequence at non-permissive temperatures preventing ribosomal binding and translation (Figure 4). RNA thermometers have neither been described in Shigella species nor in the regulation of a bacterial iron uptake system making the putative regulation of the shu genes by an RNA thermometer a unique result. These studies have greatly enhanced our understanding of the Shigella heme uptake system including its organization, transcriptional landscape and regulation. While these finding are significant, additional work is needed to fully describe the molecular details of Fur-dependent iron regulation and post-transcriptional thermoregulation of the shu genes. Completion of these studies would increase our knowledge of bacterial heme uptake systems and potentially expand our understanding of regulatory mechanisms in Shigella.
96 Shu locus future directions These studies indicate that the shu genes are regulated in response to iron in a Fur-dependent manner (Figures 15, 16, 17 and 18). However, these data are insufficient to confirm if this regulation is direct or indirect (Figures 17 and 18). The effect of Fur on shu regulation will be examined by an electromobility shift assay (EMSA) to detect binding of increasing concentrations of Fur to the shu promoters; this assay will determine if the effect of Fur on shu expression is direct or indirect. RNAseq analysis, RT-PCR experiments and in silico predictions have given close approximations as to the transcriptional start and stop locations as well as the predicted promoter sequences; however, the exact ends of each transcript and the promoter sequences have not been identified (Figures 12 and 14). Examination of the transcript ends will be analyzed by rapid amplification of cdna ends (RACE) on the 5 and 3 termini of each identified transcript. The promoters driving transcription of each gene, while predicted and localized have not been fully defined. To determine the nucleotides that are necessary and sufficient to drive transcription of these genes, mutational analyses must be conducted. While the effects of a shua, shus and shut deletion on the effect of Shigella fitness and heme uptake have been studied, deletion experiments of the other shu genes has not been evaluated(28, 39, 101, 102, 164, 168). These studies would provide clues as to the function and requirements of the shuw and shuy genes which currently do not have any function attributed to them. In addition, deletion of shux would provide clues as to the function of its gene product and its relation to it E. coli orthologueue chux. Together,
97 these experiments would greatly increase our understanding of heme uptake in Shigella species. 97
98 98 CHAPTER 3: POST-TRANSCRIPTIONAL THERMOREGULATON OF SHUA EXPRESSION 3.1 Background and Significance During infection within and transit between hosts, Shigella species, like most pathogenic bacteria, must acquire iron to survive. Binding of iron within molecules such as heme, hemoglobin, myoglobin, transferrin and ferritin, effectively reduce the concentration of bioavailable iron within the human host to levels that are too low to support the growth of most bacterial pathogens(108, 139). In response to the incredibly low levels of bio-available iron within the human host, bacteria have evolved to produce specific specialized high affinity uptake systems to mediate the acquisition of nutrient iron from heme and other host-associated iron sources(139, 161). Shigella species contain several conserved iron uptake systems; however, unique to S. dysenteriae is the Shu system, a system dedicated to the utilization of heme and heme containing proteins as sources of nutrient iron(87, 101, 102, 104, 120, 121, 133, 136, 137). The Shu system is encoded within a single chromosomal locus predicted to contain two polycistronic transcripts, shuas and shutwxyuv(166). Transcription of the shu locus is facilitated by two confirmed iron-regulated promoters driving the shutwxy and shuas transcription (Figures 12, 14, 17, 18, 19 and 20). Proteins encoded by genes within the shu locus function in concert to facilitate the uptake and utilization of hemebound iron. The shua gene encodes an outer-membrane heme receptor that binds and imports heme into the periplasm in a TonB dependent manner(27, 101). Once within the periplasm, heme is bound by ShuT and is transported across the inner-membrane to the
99 99 cytoplasmic heme binding protein, ShuS, via the ABC transporter composed of ShuU and ShuV in a coupled action(28, 39, 164, 168). Within the cytoplasm, it is proposed that ShuS either transports heme to a heme-degradation protein to utilize the molecule as a source of nutrient iron, or that the heme is stored by ShuS until needed; however, the fate of imported heme is unknown(164, 168). The functions of the remaining genes within the shu locus, shuwxy have not yet been deduced. The entire shu locus is well conserved amongst strains of S. dysenteriae as well as in several species of pathogen E. coli where the orthologous Chu systems, functions to mediate the utilization of iron from heme(166). While iron is essential for the survival of S. dysenteriae, and heme represents a potentially rich source of iron for the bacterium, both iron and heme are toxic at high concentrations(10). The potential toxicity of iron and heme forces the bacterium to maintain a precise balance between the nutritional requirements for iron and the toxic effects of over-accumulation; as iron disequilibrium can lead to death of the bacterium(10). Furthermore, it is energetically advantageous for the pathogen to produce a heme acquisition system only when it is within the host, as this is the sole environment in which the organism will encounter heme. For these reasons, the production of bacterial heme acquisition and utilization systems is often regulated in response to multiple hostassociated environmental conditions including iron limitation, the presence of heme and/or host body temperature(15, 24, 60, 79, 107, 113, 131, 150). Previous studies have demonstrated that shu genes are maximally expressed under conditions that mimic the human host by iron via the iron-dependent transcriptional repressor Fur and temperature by a suspected RNA thermometer (Figures 15-22). The
100 100 zipper-like RNA thermometers form inhibitory structures that function to occlude the ribosome binding site until the bacterium invades into a warm-blooded host or sudden heat shock causes melting of the inhibitory structure and permits translation initiation(82, 109, 159). Experiments conducted in Chapter 2 have shown that ShuA production is posttranscriptionally thermoregulated (Figures 19-22), which is the regulatory pattern exhibited by genes regulated by an RNA thermometer where RNA base pairing is known to regulate the expression of virulence and heat-shock genes in response to environmental temperature(82, 109, 159). This chapter aims at identifying the regulatory mechanisms driving expression of post-transcriptional thermoregulation of ShuA protein production. 3.2 Methods Bacterial strains, plasmids and culture conditions The bacterial strains and plasmids used in this study are detailed in Table 3. Shigella was cultured in tryptic soy agar plates (Becton Dickenson and Company, Sparks, MD) supplemented with Congo red dye (ISC BioExpress, Kaysville, UT) (0.01% wt.vol) and E. coli was cultured in a rich media (Luria-Bertani) (containing 1% tryptone 0.5% yeast extract and 1% NaCl) using the conditions listed in each experiment. The samples were iron stressed by the addition of the iron chelator deferrated ethylenediamine-n,n - bis 2-hydroxyphenylacetic acid (EDDHA) at a final concentration of 200 µg/ml(128). To select for the presence of plasmids during growth, Ampicillin and Chloramphenicol were added at a final concentration of 150 µg/ml and 30 µg/ml respectively.
101 101 Table 3 Chapter 3 bacterial strains and plasmids Designation Description Reference/ Source Strains S. dysenteriae ND100 Wild-type S. dysenteriae. Spontaneous Str r mutant of clinical strain O-4576S1 (106) shua shua deletion in O-4576S1 (102) fur fur deletion in ND100 (106) E. coli DH5α Top10 Plasmids pxg-10 Daughter strain of MG1665 derivative containing an enda, hsdr17 and a reca mutation for efficient transformation. Contains Δ(lacZ)M15 for blue-white screening DH5α derivative containing the noted DH5 α mutations and an ara14 deletion preventing arabinose catabolism and DE3 which encodes T7 polymerase. Low-copy plasmid with a PLtetO-1 promoter driving expression of a gfp reporter gene; Chl r Invitrogen Invitrogen (153)
102 102 Table 3 (continued) pxg-0 Modification of the pxg-10 plasmid lacking the (153) pwt-shua ps-shua pd-shua pbad2-bgab pagsa pgyra constitutive promoter pxg-10 containing the shua FourU element from S. dysenteriae translationally fused to the gfp reporter; Chl r pwt-shua containing a stabilizing mutation within the FourU element; Chl r pwt-shua containing a destabilizing mutation within the FourU element; Chl r Optimized version of pbad-bgab containing the pbad promoter driving expression of a bgab reporter gene with a nonfunctional ATG; Amp r pbad2-bgab containing the agsa 5 -UTR from Salmonella translationally fused to the bgab reporter; Amp r pbad2-bgab containing the gyra 5 -UTR from E. coli fused to the bgab reporter; Amp r This study This study This study (80) (80) This study pwt-chua pbad2-bgab containing chua 5 -UTR from E. This study coli UTI89 (UPEC) translationally fused to the bgab reporter; Amp r
103 103 Table 3 (continued) ps-chua pwt-chua containing a stabilizing mutation This study pd-chua within the FourU element; Amp r pwt-chua containing a destabilizing mutation within the FourU element; Amp r This study Generation of the truncated wild-type and mutant translational reporter plasmids pwt-shua, ps-shua and pd-shua After extraction of pxg-10 from E. coli strain Top10 with a Plasmid Midi Kit (Qiagen, Valencia, CA) according to manufacturer protocols, the plasmid was digested with NsiI and NheI to maintain the PLtetO-1 constitutive promoter and remove the lacz fragment(153). DNA fragments encoding a portion of the WT shua 5 utr and a mutated version predicted to contain an RNA thermometer was created by the annealing of complementary DNA oligonucleotides generating an insert with nucleic acid sequences at the 5 and 3 ends resembling sites digested with NsiI and NheI endonucleases, respectively. Complementary oligonucleotides were boiled for 10 minutes in 1 STE buffer and allowed to cool to room temperatures. After annealing, the oligonucleotides were ligated into the pxg-10 backbone creating the translational fusion between the inserted sequence and the gfp reporter yielding plasmids pwt-shua, psshua and pd-shua. Each plasmid generated was verified by nucleic acid sequencing of both strands to confirm insertion of the correct DNA fragments.
104 Creation of β-galactosidase reporters pwt-chua, ps-chua and pd-chua 293 nucleotides 5 of the chua translational start site, containing the chua 5 utr and the first two codons of the gene were amplified from uropathogenic E. coli UT189 (UPED NC_ ) via PCR using primers which introduce NheI and EcoRI restriction sites into the amplified product. Plasmid pbad2-bgab and the amplified chua 5 utr were digested using NheI and EcoRI (Thermo Scientific, Fermentas, St. Leon-Rot, Germany) and ligated together forming the translational fusion and generating plasmid pwt-chua. Mutations were introduced into the chua sequence carried on plasmid pwt-chua using the QuikChange mutagenesis kit (Stratagene, La Jolla, USA) according to manufacturer protocols. Specifically, within pwt-chua the thymine at position 19, relative to the bgab translational start site, was mutated to a cytosine to create ps-chua, and an adenine to create pd-chua. Plasmids were sequence verified by Eurofins (Eurofins, Martinsried, Germany). These constructs were made by our collaborator Dr. Franz Narberhaus (Ruhr-Universität Bochum) β-galactosidase assays E. coli DH5α cells carrying the bgab reporter plasmids pwt-chua, ps-chua and pd-chua were grown overnight in 5 ml Luria-Bertani (LB) medium supplemented with ampicillin (150 µg/ml) at 25 C. 1 ml of the stationary phase culture was used to inoculate a 25 ml LB culture which was grown to an OD 600 of 0.5 prior to the addition of 0.01% arabinose (w/v) to induce transcription from each reporter plasmid. Next, 10 ml of each culture was shifted to pre-warmed flasks at 37 C and 400 µl of sample taken
105 105 after 30 minutes of incubation. β-galactosidase assays were performed on each collected sample as described by Rinnenthal et al. (127). Standard deviations were calculated from three independent experiments. These experiments were conducted by our collaborator Dr. Franz Narberhaus (Ruhr-Universität Bochum) Western blot analysis Whole cell lysates were used for Western blot analyses. Samples were generated by growing cultures to stationary phase under the conditions listed for each experiment. A total of 5x10^8 cells were pelleted by centrifugation and the pellet was resuspeneded in 200 µl of Laemmli protein dye (Bio-Rad) containing 5% 2-mercaptoethanol. The samples were then boiled for 10 minutes in a water bath and stored at -20 C. Western blot analysis of Gfp was conducted by adding 15µL of sample to a prepared SDSpolyacrylamide gel containing 7.5% bis-acrylamide and ran for 1 hour at 100V. Next, the gel was transferred to a PVDF membrane and blocked in 1x phosphate buffered saline containing 0.1% Tween20 (PBST) with 10% dry milk (wt/vol) overnight at 4 C. The membrane was then probed with a stabilized IgG αgfp antibody (Roche, Indianapolis, IN) diluted 1:1000in PBST with 5% dry milk for 1 hour at 4 C. The membrane was then washed 3x5 minutes in PBST and blocked in PBST with 10% dry milk for 15 minutes. Next, the membrane was probed with an anti-mouse HRP conjugated IgG (Bio-Rad) diluted 1:20,000 in PBST with 5% milk for 1 hour at 4 C. The membrane was then washed 3x15 minutes with PBST at 4 C and incubated in Immun-Star Western C regents (Bio-Rad). The membrane was imaged using ImageLab software (Bio-Rad) and a
106 106 ChemiDoc XRS+ Imaging System (Bio-Rad). ImageLab software was also used to adjust contrast across the entire gel image to reduce background noise; the relative intensity of the bands was not affected in this manner RNA extraction Following growth of the bacterial culture to the indicated growth phase under the specified growth condition and at the indicated temperature, total RNA was extracted using an RNEasy Mini-kit (Qiagen) according to manufacturer protocols. The purified RNA was then treated with 16 units of amplification grade Dnase I (New England BioLabs) for 1 hour at 37 C. One ml of 100% EtOH, 40 µl of 3 M sodium acetate (ph 5.2) and 100 µl of 1 mm ethylenediaminetetraacetic acid (EDTA) were added to each sample prior to incubation overnight at 80 C. Following precipitation of the RNA by centrifugation at 12,000 g for 15 minutes at 4 C, each sample was washed with cold 75% EtOH, the RNA was pelleted as above and dried. The RNA pellet was resuspended in diethyl polycarbonate (DEPC) treated water and the nucleic acid concentration measured using an ND-1000 spectrophotometer (NanoDrop Technologies). Finally, to ensure the removal of contaminating DNA, each RNA sample was treated using the TURBO DNA-free kit (Ambion, Austin, TX) according to the manufacturer protocols. The lack of DNA in each RNA sample was confirmed by PCR using the purified RNA as template and oligonucleotide primers known to amplify a portion of the shua open reading frame.
107 Quantitative real-time polymerase chain reaction A total of 150 ng of total RNA was reverse-transcribed using the iscript cdna Synthesis Kit (Bio-Rad) while following the protocols obtained with the kit. The cdna samples were diluted 1:10 and a total of 5µL was used per 20µL reaction using iq SYBR Green Supermix (Bio-Rad) and the target specific primers. A standard curve was produced from each reaction to ensure an acceptable efficiency during the reaction. The expression values were calculated using the ΔΔCt method by setting the values relative to a control sample and normalizing towards rrsa, a house keeping gene whose expression is unaffected by temperature, iron and growth phase. The Bio-Rad CFX96 Real-Time PCR System was used to perform each reaction Alignment and in silico modeling Nucleic acid sequences for alignment of the promoters and 5 untranslated regions of shua and chua were acquired from GenBank. Alignments were conducting using Clone Manager 9 software and were recreated in Adobe Illustrator CS4. Predicted structure of shua 5 utr, partial and complete, were obtained using Mfold software ( Statistical analysis Two tailed, two sample Student s t tests, assuming equal variance, were used throughout our studies to determine significance (P 0.05).
108 Results In silico Analysis Predicts the Presence of a 4U RNA Thermometer within the shua 5 utr Given that nucleic acid sequences composing the shua promoter and 5 utr are sufficient to confer post-transcriptional thermoregulation, (Figures 20 and 22) the predicted secondary structure of this region was evaluated by in silico modeling to identify putative regulatory elements. Mfold analysis was used to predict the secondary structure of the entire shua 5 untranslated region ( the most energetically favorable of which is shown in (Figure 23). Ten out of twelve structures generated by Mfold included a short hairpin that is formed by canonical and non-canonical basepairing between four consecutive uracil residues and the predicted Shine-Dalgarno sequence (Figure 24). These characteristics are found in 4U RNA thermometers, a relatively new class of cis-acting riboregulators shown to mediate post-transcriptional thermoregulation of target gene expression by occlusion of the ribosomal binding site at non-permissive temperatures(159).
109 Figure 23: in silico folding analysis of the full-length shua 5 utr The 328 nucleotides upstream of the S. dysenteriae shua transcriptional start site, representing the full-length shua 5 utr, was submitted to the software package Mfold for modeling of the RNA secondary structure. The 4U RNA thermometer is boxed and the ATG start codon are the three nucleotides at the most 3 end of this folding. This folding represents the most thermodynamically stable structure produced. 109
110 110 Figure 24: In-silico folding analysis predicts a 4U thermometer occluding the Shine- Dalgarno sequence within the shua mrna Nucleic acid sequence and secondary structure of nucleotides 29 through +3 of shua as predicted by Mfold analysis ( Boxes indicate the location of the four consecutive uracil residues and putative Shine-Dalgarno sequence within the 4U RNA thermometer, as well as the translational start site (T.S.S.) of shua. This secondary structure is the only predicted structure produced by in silico analysis The Nucleotide Sequence Composing the Putative shua 4U RNA Thermometer is Sufficient to Confer Post-transcriptional Thermoregulation To determine if the nucleotide sequence comprising the predicted RNA thermometer is sufficient to confer post-transcriptional thermoregulation onto reporter gene expression, a 32 nucleotide long insert containing the putative 4U RNA thermometer (Figure 24) was cloned between the constitutive PLtetO-1 promoter and the gfp reporter gene on plasmid pxg-10. Such cloning generates a translational fusion between the first codon of shua and gfp, the transcription of which is driven by the plasmid promoter and protein production is potentially influenced by the
111 111 putative shua 4U thermometer cloned into pxg-10. The newly created plasmid, designated pwt-shua, was introduced into Escherichia coli, and thermoregulation of gfp expression was investigated by Western blot and Q-PCR analyses following growth of the strain to stationary phase at 25 C or 37 C. Western blot analyses demonstrate that Gfp protein levels are increased following growth of E. coli carrying pwt-shua to stationary phase at 37 C as compared to those measured following growth of the strain at 25 C (Figure 25A) while Q-PCR analyses indicate that temperature does not influence the relative abundance of gfp mrna (Figure 25B). Together, these data clearly demonstrate that the predicted 4U RNA thermometer contained within the shua 5 utr is sufficient to confer post-transcriptional thermoregulation onto the production of the Gfp reporter protein.
112 112 A) B) Figure 25: Sequences composing the shua 4U thermometer are sufficient to confer post-transcriptional thermoregulation onto gfp expression A) E. coli strain DH5α containing pwt-shua or pxg-0, as a vector control, was cultured to stationary phase at the temperatures indicated. A Western blot was performed using whole-cell lysates generated from an equal number of cells and anti-gfp monoclonal antibodies. B) Quantitative Real-time PCR was conducted using RNA isolated from E. coli DH5α carrying pwt-shua following growth of the strain to stationary phase at 25 C or 37 C. gfp mrna levels were normalized to rrsa measured in each sample and expressed relative to the amount of gfp transcript measured in the first 25 C sample. All data are representative of three biological replicates and error bars represent one standard deviation. Assuming a confidence interval of 95% (p 0.05), no significant difference exists between the relative levels of gfp transcript measured from pwt-shua following growth of the strain at 25 C or 37 C Site-directed Mutagenesis of the Putative 4U Element Alters Thermoregulation of the gfp Reporter Gene To further investigate the existence of a 4U RNA thermometer within the shua 5 utr, single nucleotide mutations were introduced into the regulatory element cloned within the pwt-shua reporter plasmid. The first mutant construct, ps-shua, contains a
113 113 thymine to cytosine mutation to generate a cytosine-guanine base pair within the predicted RNA thermometer (Figure 26A). This mutation was created to stabilize the closed conformation of the 4U RNA thermometer, a mutation that is predicted to decrease reporter protein production from ps-shua compared to pwt-shua when measured at the permissive temperature of 37 C. E. coli was transformed with psshua and Gfp production compared to that from pwt-shua by Western blot analysis following the growth of each strain at 37 C. Gfp production from ps-shua following growth of the reporter strain at the permissive temperature of 37 C is inhibited as compared to that from the strain carrying pwt-shua (Figure 26C). Q-PCR analysis demonstrates that gfp transcript levels produced from ps-shua and pwt-shua are equivalent under the conditions tested, indicating that the observed difference in Gfp levels does not result from altered transcription or transcript stability of the mutated reporter gene (Figure 26E). Together these data demonstrate that, as expected, mutations that are predicted to stabilize the inhibitory structure within the putative 4U RNA thermometer result in decreased expression of the regulated gene at the permissive temperature of 37 C.
114 114
115 Figure 26: Mutational analysis demonstrates that the shua 5 utr contains a functional 4U RNA thermometer The shua region cloned into pwt-shua was mutagenized to further validate the existence of a predicted 4U element within the shua 5 utr. A) The uracil residue located 19 nucleotides upstream of the gfp translational start site was mutated to a cytosine. This mutation, indicated by the box, is predicted to stabilize the inhibitory structure within the putative 4U RNA thermometer. This mutated sequence was cloned into the gfp translational reporter pxg-10 to generate the stabilized mutant construct designated ps-shua. B) The cytosine residue 17 nucleotides upstream of thegfp translational start site was mutated to an adenine. This mutation, indicated by the box, is predicted to destabilize the inhibitory structure within the putative 4U RNA thermometer. This mutated sequence was cloned into the gfp translational reporter pxg- 10 to generate the destabilized mutant construct designated pd-shua. Western blot analyses were conducted using monoclonal anti-gfp antibodies and whole-cell extracts generated from an equal number of E. coli carrying pwt-shua or ps-shua cultured to stationary phase in LB at the permissive temperature of 37 C (C), and E. coli carrying pwt-shua or pd-shua cultured to stationary phase in LB at the non-permissive temperature of 25 C (D). Quantitative real-time PCR analysis was performed using RNA isolated from E. coli DH5α cells carrying pwt- shua, ps-shua and pd-shua after culturing the strains to stationary phase using the temperatures indicated. gfp transcript levels were normalized to the amount of rrsa in each sample and set relative to the amount of gfp in the first pwt-shua sample. All data are representative of three biological replicates and error bars represent one standard deviation. Assuming a confidence interval of 95% (p 0.05), no significant difference exists between the relative levels of gfp transcript measured from pwt-shua and ps-shua (E) or pd-shua (F) at the temperatures tested. 115 The second mutant construct, pd-shua, contains a cytosine to adenine mutation which is predicted to abolish a canonical cytosine-guanine base-pair and destabilize the inhibitory structure of the 4U RNA thermometer thus allowing for increased Gfp production at the non-permissive temperature of 25 C (Figure 26B). E. coli was transformed with pd-shua and Gfp production compared to that from pwt-shua by Western blot analysis following the growth of each strain at 25 C. Gfp production from pd-shua following growth of the reporter strain at the non-permissive temperature of 25 C is increased as compared to that from the strain carrying pwt-shua (Figure 26D).
116 116 Q-PCR analysis demonstrates that gfp transcript levels expressed from pd-shua and pwt-shua are equivalent under the conditions tested, indicating that the observed difference in Gfp levels does not result from altered transcription or transcript stability of the mutated reporter gene (Figure 26F). Together these data demonstrate that, as expected, mutations that are predicted to destabilize the inhibitory structure within the putative 4U RNA thermometer result in increased expression of the regulated gene at the non-permissive temperature of 25 C. Collectively, these mutagenesis studies demonstrate that thermoregulation conferred by the putative shua 4U RNA thermometer is mediated by differential stability of the identified inhibitory structure. These data strongly support the conclusion that the shua 5 utr harbors a functional 4U RNA thermometer The 5 utr of chua, the E. coli Orthologue of shua, contains a 4U RNA Thermometer Sufficient to Confer Post-transcriptional Thermoregulation An alignment among the 5 utr of shua and its orthologue chua from several strains of pathogenic E. coli shows moderate sequence variation; however, the 4U RNA thermometer and surrounding regions are completely conserved (Figure 27). To determine if the E. coli chua 5 utr confers thermoregulation, this region was amplified from uropathogenic E. coliut189 (UPEC NC_ ) and cloned into plasmid pbad2-bgab, a well-established translational reporter system(80). Specifically, a region containing 293 nucleotides of the chua5 utr and the first two codons of the open reading frame was cloned in frame with the bgab reporter, encoding a heat-stable β-
117 117 galactosidase, to generate the translational reporter plasmid pwt-chua. Expression of bgab from pwt-chua is under control of the pbad arabinose inducible promoter and is potentially influenced by the putative chua 4U RNA thermometer contained within the cloned E. coli sequence (Figure 28A).
118 Figure 27: Nucleic acid sequences within the shua 5 utr are conserved among pathogenic E. coli species An alignment of the shua and chua promoter sequence, 5 utr and first nine nucleotides of the coding region are pictured. Nucleotides that are conserved throughout the shuaand chua genes are highlighted. The predicted 35, 10, transcriptional start site (+1), 4U thermometer (4U), Shine-Dalgarno (S.D.) sequence and translational start site (T.S.S.) are indicated by boxes. The alignment was performed using Clone Manager 9. An m is an ambiguous nucleotide and denotes either a thymine or cytosine. All sequences were acquired from GenBank and are as follows: shua from Shigella dysenteriae Sd197 NC_ , chua from Enterohemorrhagic E. coli (EHEC) NC_ , chua from Enteropathogenic E. coli (EPEC) NC_ , chua from Uropathogenic E. coli (UPEC) NC_ , chua from Neonatal Meningitis E. coli (NMEC) NC_ , and chua from Extraintestinal Pathogenic E. coli (ExPEC) NC_
119 119 A) B) C) D) E) Figure 28: A functional 4U RNA thermometer within the 5 utr of E. coli chua confers thermoregulation A) Predicted structure of a portion of the chua mrna molecule containing the start codon (AUG) and preceding 29 nucleotides. This region contains the putative chua4u RNA thermometer. B) The uracil residue located 19 nucleotides upstream of thebgab translational start site was mutated to a cytosine. This mutation, indicated by the box, is predicted to stabilize the inhibitory structure within the putative chua 4U RNA thermometer. The mutated sequence was cloned into the pbad2-bgab translational reporter to generate the stabilized mutant construct designated ps-chua. C) The uracil residue located 19 nucleotides upstream of the bgab translational start site was mutated to an adenine. This mutation, indicated by the box, is predicted to destabilize the inhibitory structure within the putative chua 4U RNA thermometer. The mutated
120 sequence was cloned into the pbad2-bgab translational reporter to generate the destabilized mutant construct designated pd-chua. The influence of temperature on the expression of bgab from the positive and negative control plasmids pagsa and pgyra D)and the translational reporters pwt-chua, ps-chua and pd-chua E) was determined by measuring the β-galactosidase activity form E. coli DH5α carrying each. All date are the average of three replicate experiments. Error bars represent one standard deviation and a *indicates a statistically relevant difference between the indicated strains (p 0.05). 120 E coli DH5α was transformed with pwt-chua and the thermoregulation of bgab activity investigated. A temperature shift from 25 C to 37 C resulted in a significant increase in the β-galactosidase activity measured from pwt-chua (Figure 28E). These data demonstrate that like that of S. dysenteriae shua (Figure 25), sequences within the 5 utr of UPEC chua are sufficient to confer thermoregulation onto the expression of a reporter gene on a translational reporter plasmid. Two control plasmids were utilized in this study. The negative control plasmid, pgyra, carries the reporter bgab translationally fused to the 5 utr of the non-thermoregulated E. coli gyra and is a modification of that used previously (Figure 28D)(159). The positive control plasmid, pagsa, carries the reporter bgab translationally fused to a previously characterized 4U RNA thermometer from Salmonella agsa(80). As expected, a temperature-dependent increase in β-galactosidase activity was also seen from the positive control plasmid pagsa, while no thermoregulation was observed from the negative control plasmid pgyra (Figure 28D). To further characterize the UPEC chua 4U RNA thermometer, point mutations were introduced to stabilize or destabilize the predicted inhibitory structure cloned within the pwt-chua reporter plasmid. Non-pathogenic E. coli DH5α was transformed with
121 121 each mutated reporter plasmid and the influence of the mutation of thermoregulation of the reporter bgab gene evaluated by β-galactosidase analyses. The first mutant construct, ps-chua, carries a thymine to cytosine mutation that introduces a C-G pairing within the predicted RNA thermometer (Figure 28B). As predicted, this stabilizing mutation results in significantly less β-galactosidase activity from the ps-chua translational reporter at the permissive temperature of 37 C as compared to that measured from pwt-chua (Figure 28E). The second mutant construct, pd-chua, carries a thymine to adenine mutation that destabilizes the predicted chua 4U RNA thermometer by disrupting a U-A pairing (Figure 28C). This destabilizing mutation results in significantly more β-galactosidase activity from the pd-chua translational reporter at the non-permissive temperature of 25 C as compared to that measured from pwt-chua (Figure 28E). Together, these data indicate that, like that of S. dysenteriae shua, the expression of UPEC chua is regulated in response to environmental temperature by the activity of a 4U RNA thermometer located within the 5 utr of the gene. 3.4 Discussion This study demonstrates that the expression of S. dysenteriae shua and E. coli chua, genes essential for the utilization of heme as a source of nutrient iron, is modulated in response to environmental temperature by a post-transcriptional regulatory mechanism. This work is the first to demonstrate temperature-dependent post-transcriptional regulation of shua and chua expression and the first to implicate temperature as an
122 122 environmental factor controlling the production of an iron uptake system in Shigella. Finally, it is demonstrated that the observed post-transcriptional thermoregulation of both shua and chua expression is mediated by the activity of a conserved 4U RNA thermometer within the 5 utr of each gene. The conservation of the 4U RNA thermometer between related pathogenic bacterial species (Figure 27) suggests that it provides a selective advantage by increasing the fitness of each species. Multiple regulatory mechanisms control the expression of both S. dysenteriae shua and E. coli chua and this hierarchical regulation likely increases the fitness of each pathogen by limiting the production of the heme binding receptor to environmental conditions mimicking those encountered within the heme containing human body (Figure 29). This work along with previous studies has demonstrated that the expression of shua and chua is regulated by both iron availability and environmental temperature (Figures 15-22, 25, 26 and 28). Dual-regulation of shua and chua likely increases the overall fitness of S. dysenteriae and E. coli by promoting expression of each gene within the host, where heme is going to be encountered, while efficiently preventing expression of each gene in the non-host environment where heme will not be encountered. Specifically, the iron-limitation and environmental temperature (37 C) encountered within the human-host relieves both the Fur-mediated inhibition of shua transcription and 4U RNA thermometer mediated inhibition of shua translation, respectively (Figure 29). Consequently, host-associated conditions are optimal for shua and chua expression and subsequent heme acquisition by S. dysenteriae and pathogenic E. coli. Conversely, the non-host environment encountered during
123 123 transmission, marked by an increase in available iron and a decrease in temperature, will result in Fur-dependent inhibition of shua and chua transcription and 4U RNA thermometer dependent inhibition of translation, respectively (Figure 29). As a result of this dual regulation, shua expression will be efficiently inhibited during transmission through non-heme containing environments.
124 Figure 29: Dual regulation controlling the expression of S. dysenteriae shua and chua of pathogenic E. coli The production of S. dysenteriae ShuA and E. coli ChuA is regulated at the levels of transcription and translation by two independent mechanisms. Transcription of the shua and chua message is inhibited under high-iron conditions by the iron-dependent transcriptional repressor Fur in a temperature-independent manner. However, under ironpoor conditions Fur mediated repression of shua/chua transcription is relieved and mrna is synthesized. Translation of the shua/chua transcript is regulated in response to environmental temperature via a 4U RNA thermometer within the 5 untranslated region of each gene. At a temperature of 25 C, translation of shua/chua is blocked by the formation of an inhibitory structure predicted to occlude the ribosomal binding site and prevents ribosomal binding. At a temperature of 37 C the inhibitory structure within the shua and chua 5 utr is destabilized and efficient translation of each gene proceeds. This model shows that production of ShuA and ChuA occurs only under iron-poor conditions and at 37 C, conditions which mimic the host environment where S. dysenteriae and pathogenic E. coli will encounter heme as a rich source of nutrient iron. 124
125 125 A further advantage that is likely conferred by the regulation of shua and chua expression at both the transcriptional and translational level of gene expression is that such regulation likely facilitates rapid adaptation of the pathogen to changing environmental conditions. It is reasonable to assume that upon being shed from an infected human host, S. dysenteriae and pathogenic E. coli experiences an increase in relative iron-availability and a decrease in environmental temperature. Inhibition of both transcription and translation would likely provide an advantage over regulation only at the level of transcription in this scenario, by preventing the translation of existing shua and chua mrna molecules, thus saving the pathogen the energetic cost of producing the heme receptor in an environment in which the protein will provide no advantage to the bacterium. While shua is regulated at both the transcriptional and translational level of expression, additional regulatory mechanisms could influence shua expression. The 4U element is located within the last 30 nucleotides of the fairly well conserved shua 5 utr that measures over 300 nucleotides (Figure 24). In silico folding analyses predicts a highly structured 5 utr (Figure 23) that may play additional roles in shua regulation. The impact of the remaining 5 utr on shua and chua expression remains under investigation. It is plausible that additional elements within the 5 utr of chua and shua are capable of stabilizing the 4U element and/or regulating expression of each gene in a manner independent of the 4U RNA thermometer. Only two other 4U RNA thermometers, agsa from Salmonella and icrf from Yersinia species have been extensively characterized to date(22, 159). Given the
126 126 conservation and distribution of the shu locus and identified 4U RNA thermometer among S. dysenteriae and several strains of pathogenic E. coli (Figure 26), as well as the predicted distribution of 4U RNA thermometers among both Gram-negative and Grampositive bacterial species, the significance of this study reaches beyond S. dysenteriae gene regulation and into the broader fields of enteric pathogenesis and RNAmediated mechanism of bacterial gene regulation(159, 166). Only after multiple 4U RNA thermometers are identified and characterized will a complete understanding of this potentially wide-spread regulatory element be possible. Future studies aimed at characterizing the regulatory function of the shua 4U RNA thermometer in finer molecular detail as well as the identification and characterization of additional 4U RNA thermometers in Shigella and other bacterial species will not only further the understanding of how this newly recognized regulatory element functions but will also reveal the potentially expansive role that 4U RNA thermometers play in bacterial physiology and pathogenesis. 3.5 Future directions While these experiments have expanded our knowledge of gene regulation in Shigella, further evaluation shua RNA thermometer as well as other RNA thermometers in Shigella species is required. These studies showed that expression of a reporter gene fused to the shua RNA thermometer could be modulated by introducing both stabilizing and destabilizing mutations into the inhibitory structure (Figure 26). While these mutations indicate the presence of an RNA thermometer governing the regulation of
127 127 shua, the effect of these mutations on regulation of the chromosomally located gene are required. Introduction of these mutations into the S. dysenteriae chromosome would allow for the direct evaluation of their effects on production of the native protein. In addition, the effect of the shua mutants on Shigella fitness and ability to acquire heme would indicate the importance of the RNA thermometer in precise regulation of shua. This work has indicated that shua expression is post-transcriptionally thermoregulated by an RNA thermometer and represents the first RNA thermometer discovered in Shigella species. RNA thermometers are a recently identified molecular mechanism but their importance is highlighted by the diverse species they are found in and the number of genes they have been shown to regulate(22, 55, 82, 109, 159). Further research into the effects of RNA thermometers on the regulation of the Shigella genes would greatly improve our knowledge of Shigella regulation. Additionally, with only 4 positively identified 4U RNA thermometers, more research into this subclass of RNA thermometers would identify conserved structural and sequence elements required for the functionality of these RNA thermometers.
128 128 CHAPTER 4: IDENTIFCATION OF ADDITIONAL RNA THERMOMETERS GOVERNING REGULATION OF SHIGELLA DYSENTERIAE GENES 4.1 Background Bacteria must adapt to changes in their local environments, including ph, osmolarity, nutrient availability, oxygen availability and temperature, in order to survive and proliferate(8, 11, 23, 73, 90). Changes in environmental conditions can be rapid and extreme which necessitates the ability of bacteria to sense their local environment and modulate gene expression of factors required to cope with these stresses(8, 11, 23, 42, 73, 90, 109). Efficient environment-specific gene regulation greatly benefits the bacterium by promoting the production of specific factors when their function will provide the greatest advantage to the bacterium and by inhibiting the production of energetically costly factors in an environment where their activity provides the bacterium little or no advantage. A classic example of environment-specific regulation is that of bacterial virulence factors which are often produced in response to conditions that mimic the human host; such regulation ensures maximal production of these factors in the precise environment where they will facilitate the survival and/or virulence of the bacterium(42, 74, 96, 149). The controlled and efficient regulation of bacterial gene expression is of great importance to a pathogen so that virulence factors can be produced at the moment they are required to establish an infection(42, 74, 96, 149). The production of virulence factors by pathogenic bacteria is often regulated by environmental temperature; a regulatory cue which usually differs between the variable non-host environment encountered by a bacterium during transmission and the relatively
129 129 stable host environment experience during infection(17, 41, 81, 82, 109). The stable and often elevated temperature of the host environment, which signals to the pathogen that it has entered into the host, initiates thermoregulation of factors required for virulence and survival(17, 41, 81, 82, 109). The most thoroughly studied mechanisms of thermoregulation control target gene expression at the level of transcription or by influencing the activity of target proteins(17, 41, 73, 78, 123, 148). Temperature directly alter transcription of thermally regulated genes by modifying DNA structure through supercoiling and thus altering local DNA topology and curvature(37, 41, 76, 125, 146, 152, 172). Through alterations of DNA topology due to supercoiling, RNA polymerase efficiency is negatively impacted, resulting in partial or complete inhibition of target gene transcription(37, 76, 172). Temperature also directly modifies DNA curvature where at lower temperatures DNA is typically in a more condensed and curved state compared to a more relaxed state at higher temperature(37, 76, 172). Transcription factors, such as H- NS bind to curved DNA near their target s promoter in a sequence independent manner to regulate their expression(37, 42, 146, 152). At the level of target protein activity, small changes in temperature can affect the conformation of a thermo-sensitive domain, which in turn can significantly alter the activity or stability of the target protein(12, 32, 52, 59). Direct regulation of transcription or protein activity by these mechanisms is subsequently able to indirectly thermoregulate additional downstream factors at every level. While thermoregulation mediated at the level of target gene transcription and target protein activity represents the most well studied mechanisms, recent studies have identified cis-encoded RNA elements that can thermally regulate translation of the
130 130 message on which they are encoded(22, 55, 80, 82, 82, 109, 127, 159). These RNA elements, termed RNA thermometers, are encoded within the 5 untranslated region (utr) of their target genes and form an inhibitory hairpin at certain temperatures that occludes the ribosomal binding site and inhibits translation (Figure 4) (22, 55, 80, 82, 109, 127). At temperatures that are permissive to translation, the hydrogen bonds between the two strands of the inhibitory hairpin are broken and the entire structure is destabilized which allows for ribosomal binding and translation to proceed uninhibited(22, 127, 159). What makes RNA thermometers unique among cis-acting RNA regulators is that they do not require any binding factors to facilitate regulation; unlike riboswitches which require protein or metabolite binding to modulate their expression(22, 55, 80, 82, 95, 109, 127, 140). Thus the intrinsic nature of RNA thermometers represents a simple and metabolically inexpensive mechanism for rapid regulation of a target gene expression in response to changes to environmental temperature. Although RNA thermometers are a relatively newly recognized family of riboregulators, three separate classes have been identified and characterized to date. The first class of RNA thermometer identified mediates the repression of heat-shock gene expression and have been collectively termed ROSE elements(33, 82, 85, ). These thermometers are characterized by three conserved traits: 1) repression of translation at lower temperatures by the formation of an inhibitory hairpin that blocks ribosomal binding that is destabilized at higher temperature, 2) the presence of three or four upstream hairpins that function to stabilize the inhibitory hairpin, and 3) a bulged guanine residue within the inhibitory hairpin that facilitates destabilization of the element
131 131 at increased temperatures(33, 82, 156). Cold-shock thermometers are the second class of RNA thermometers identified and are unique because they permit translation and temperatures that are lower than when translation is inhibited(55, 82). Precise regulation by cold-shock RNA thermometers is dependent upon the formation of an anti-inhibitory structure at lower temperatures that prevents the formation of the inhibitory hairpin. (55, 82) The anti-inhibitory structure forms first following transcription due to its position 5 of the inhibitory hairpin and is stable at lower temperature; however, an increase in temperature results in the destabilization of the antiinhibitory structure and the formation of the more thermally stable inhibitory structure(55, 82). Once formed, the inhibitory structure occludes the ribosomal binding site and by doing so prevents translation by inhibiting binding of the ribosome (55, 82). The final RNA thermometer has been termed the 4U RNA thermometer that mimics the ROSE elements in that their permissive temperatures are higher than their non-permissive temperatures(22, 82, 84, 127, 159). However, the 4U RNA thermometers lack the bulged guanine residue that is characteristic of ROSE elements(22, 80, 82, 84, 127, 159). One defining characteristic of 4U thermometers is that they contain four consecutive uracil residues within the inhibitory hairpin that base-pair both canonically and non-canonically with sequences within the ribosomal binding site(22, 80, 82, 84, 127, 159). The 4U RNA thermometer is the newest class of RNA thermometers and has only been identified in four species, Salmonella enterica, Yersinia pseudotuberculosis, Escherichia coli and Shigella dysenteriae(22, 80, 82, 84, 127, 159). The RNA thermometers characterized to date regulate expression of heat-shock, cold-shock, virulence genes and nutrient
132 132 acquisition systems. Together, this class of riboregulators efficiently regulates production of essential factors in a temperature-dependent manner. Recent studies in the medically relevant pathogen Shigella dysenteriae, which is the causative agent of shigellosis, have identified the first RNA thermometer within the Shigella genus(72, 83, 84, 110). The identified thermometer, controlling expression of the outer membrane heme receptor ShuA, was only the second 4U RNA thermometer identified to date and the only RNA thermometer to control production of a nutrient acquisition system(84). The identification of the first RNA thermometer to control nutrient acquisition and the apparent lack of confirmed RNA thermometers in regulating S. dysenteriae gene expression make it an interesting organism to study RNA thermometers. This study aims at further characterizing RNA thermometers and their role in S. dysenteriae gene expression. 4.2 Methods Bacterial Strains, Plasmids and Culture Conditions All strains and plasmids used in this study are detailed in Table 4. The E. coli strains were used solely for maintaining plasmids and were cultured in a rich media (Luria-Bertani) (containing 1% tryptone 0.5% yeast extract and 1% NaCl) and incubated at 37 C with the appropriate antibiotics to maintain the plasmids. The Shigella dysenteriae strains were used to generate all data shown and were cultured using tryptic soy agar plates (Becton Dickenson and Company, Sparks, MD) supplemented with Congo red dye (ISC BioExpress, Kaysville, UT) (0.01% wt.vol). Strains that required
133 133 iron stressed conditions were supplemented with 150µg/mL final concentration of the iron chelator 4,4 -dipyridyl (Sigma-Aldrich, St. Louis, MO). Plasmids were maintained in each strain by the addition of either Ampicillin or Chloramphenicol at a concentration of 150 µg/ml and 30 µg/ml respectively.
134 134 Table 4 Chapter 4 bacterial strains and plasmids Designation Description Reference/ Source Strains S. dysenteriae ND100 E. coli DH5α Top10 Plasmids pxg-10 pxg-0 Wild-type S. dysenteriae. Spontaneous Str r mutant of clinical strain O-4576S1 Daughter strain of MG1665 derivative containing an enda, hsdr17 and a reca mutation for efficient transformation. Contains Δ(lacZ)M15 for blue-white screening DH5α derivative containing the noted DH5 α mutations and an ara14 deletion preventing arabinose catabolism and DE3 which encodes T7 polymerase. Low-copy plasmid containing a PLtetO-1 constitutive promoter driving expression of a gfp Modification of the pxg-10 plasmid lacking the constitutive promoter (106) Invitrogen Invitrogen (153 (153)
135 135 Table 4 (continued) ptort pentc phtra pompa ps-ompa pd-ompa The pxg-10 plasmid containing the putative tort RNA thermometer translationally fused to the gfp reporter A modified version of the pxg-10 reporter plasmid with the predicted entc RNA thermometer translationally fused to the gfp gene. The htra 5 utr translationally fused to the gfp reporter gene on the reporter plasmid pxg-10. The predicted ompa RNA thermometer translationally fused to the gfp gene on the pxg- 10 plasmid A mutated version of pompa containing a stabilized inhibitory structure within the predicted RNA thermometer A modified version of pompa containing mutations designed to destabilize the ompa RNA thermometer inhibitory structure. This study This study This study This study This study This study
136 Identification of additional 4U RNA thermometers A bioinformatics approach was used to identify additional 4U RNA thermometers was performed by Franz Narberhuas ( Ruhr-Universität Bochum). The algorithm was trained to identify four consecutive uracil residues within 50 nucleotides of an annotated transcriptional start site. To detect occlusion of the ribosomal binding site in-silico predictions of the RNA secondary structure was performed for each positive hit generated by the algorithm. If the majority of the RNA secondary structures predicted by the in silico analysis showed a hairpin with four consecutive uracil residues pairing both canonically and non-canonically with the ribosomal binding site it was labeled as a putative 4U RNA thermometer. Additionally, a second screen was performed by manually searching the Shigella dysenteriae chromosome for potential sequences that resembled a 4U RNA thermometer but were missed in the bioinformatics screen. These screens identified four uracil residues, either consecutive or non-consecutive within the 5 utr of their target gene. These genes were then screened by in silico analysis using the Mfold software package ( Any gene found to have four uracil residues base pair both canonically and non-canonically with the ribosomal binding site was identified as putative RNA thermometers Isolation and cloning of putative RNA thermometers The plasmid pxg-10 was used for analysis of the isolated RNA thermometers. Following extraction with a Plasmid Midi Kit (Qiagen, Valencia, CA) according to manufacturer protocols the plasmid was digested using the restriction enzymes NsiI and
137 137 NheI to conserve the constitutive promoter PLtetO-1 and remove the lacz 5 utr. Next, DNA oligomers encoding the RNA thermometer of interest were designed (Integrated DNA Technologies, Coralville, IA) and annealed together by boiling the complementary oligomers in 1xSTE for 10 minutes and allowing to cool to room temperature. The annealed oligos contained sites at the 5 and 3 ends that mimicked those cut with the restriction enzymes NsiI and NheI respectively. After the oligos were annealed together, they were ligated to the pxg-10 backbone cut with NsiI and NheI to generate the translational gfp reporters. Each reporter construct was verified by DNA sequencing to confirm the creation of the desired product Western blot analysis Proteins were obtained for Western blot analysis by generating whole cell lysates. Samples were created by growing each culture to the growth phase and under the conditions listed in each experiment. For these experiments, 5x10^8 cells carrying the plasmid of interest were pelleted by centrifugation and resuspended in 200 µl of Laemmli protein dye (Bio-Rad) containing 5% 2-mercaptoethanol. Following resuspension, the samples were boiled for 10 minutes and allowed to cool to room temperature. The boiled samples were stored at -20 C until ready for use. Analysis of the Gfp reporter protein by Western blot was conducted by loading 15 µl of each sample onto a 7.5% bis-acrylamide SDS-polyacrylamide gel and run for 1 hour at 100V. After the sample had been run on the gel, proteins were transferred to a PVDF membrane and blocked in 1x phosphate buffered saline supplemented with 0.1%
138 138 Tween20 (PBST) containing 10% dry milk (wt/vol) overnight at 4 C. Following incubation, the membrane was probed using a monoclonal αgfp antibody (Roche, Indianapolis, IN) diluted 1:1000 in PBST with 5% dry milk at 4 C shaking for 1 hour. The membrane was then washed 3x5 minutes with PBST and once with PBST containing 10% dry milk (wt/vol) for 15 minutes. The membrane was then probed with an antimouse HRP conjugated IgG (Bio-Rad) diluted 1:20,000 in PBST with 5% milk for 1 hour at 4 C. Following incubation, the membrane was washed with PBST 3x15 minutes and then incubated with Immun-Star Western C regents (Bio-Rad) according to manufacturer protocols. The membrane was then imaged using the ChemiDoc XRS+ Imaging System (Bio-Rad) in conjunction with the ImageLab software (Bio-Rad). The ImageLab software was also used to adjust contrast to minimize background noise; however, the relative intensity of each band was not manipulated in this manner RNA isolation RNA was isolated from cultures grown to the growth phase and under conditions indicated in each experiment. The RNA was extracted using an RNEasy Mini-kit (Qiagen) according to manager protocols. DNA contamination was eliminated by a Dnase digestion using the TurboDNase kit according to manufacturer protocols. RNA samples were screened for DNA contamination by PCR analysis using primers known to amplify a portion of the shua open reading frame.
139 Quantitative real-time PCR analysis For each Q-PCR reaction, a total of 150 ng of total RNA was reverse transcribed into cdna using the iscript cdna Synthesis Kit (Bio-Rad) according to manufacturer protocols. Each cdna sample was diluted 1:10 and a total of 5 µl was used in each 20µL reaction with iq SYBR Green Supermix (Bio-Rad) with the desired primers. Standard curves were generated for each reaction to verify acceptable efficiency during each reaction. Expression values for each sample were generated using the ΔΔCt method by relativizing to the control sample and normalizing to the quantity of the 16S rrna gene rrsa whose expression is independent of growth phase, iron concentration and temperature. These experiments were performed using the Bio-Rad CFX96 Real-Time PCR System Alignment and in silico modeling Genbank was used to retrieve all sequences used in alignments and in silico modeling of the 5 untranslated regions of selected genes. Alignments were generated using Clone Manager 9 software and recreated in Adobe Illustrator CS4. All in silico modeling was performed using the Mfold software package ( Statistical analysis Two tailed, two sample Student s t tests, assuming equal variance, were used throughout our studies to determine significance (P 0.05).
140 Results Identification of additional 4U RNA thermometers in S. dysenteriae The role of RNA thermometers in Shigella thermoregulation is beginning to be appreciated with the discovery of the S. dysenteriae shua RNA thermometer(84). The identification of the shua 4U RNA thermometer is significant because it represents the first RNA thermometer identified in any Shigella species and it is the first identified RNA thermometer demonstrated to control the production of a protein required for nutrient uptake(84). In addition, the shua RNA thermometer represented only the second characterized 4U RNA thermometer, a subclass of this family of regulators(84, 159). Identification and characterization of additional 4U RNA thermometers in S. dysenteriae will serve a dual purpose: 1) to reveal the extent to which RNA thermometers control Shigella dysenteriae gene expression and 2) to further characterize structural and sequence features that influence the activity of bacterial 4U RNA thermometers. The identification of these regulatory elements within the S. dysenteriae genome represents a necessary first step in this process. To identify additional RNA thermometers, a bioinformatics approach developed by our collaborator Dr. Franz Narberhaus (Ruhr-Universität Bochum) was used to analyze nucleic acid sequences on the S. dysenteriae chromosome and its large virulence plasmid. The algorithm was used to identify four consecutive uracil residues located 3 of the ribosomal binding site and within 50 nucleotides of the translational start site. In addition, a manual search of the chromosome was also conducted to find potential RNA thermometers that had four uracil residues within a six nucleotide region within 50
141 141 nucleotides of the translational start site. The putative RNA thermometers were then further evaluated by in silico analysis of the predicted secondary structure within the 5 utr of each identified gene using the Mfold software. Any element predicted to have four consecutive uracil residues base pairing both canonically and non-canonically with the ribosomal binding site was deemed a putative RNA thermometer. A total of 40 putative 4U RNA thermometers were identified using these screens including shua, a finding that lends credence to the validity of the bioinformatics screen. This group of 40 thermometers was subdivided into a group of 10 which were of particular interest due to their identified cellular functions. Seven of the ten thermometers of interest control either virulence or heat-shock genes, two classes that are traditionally associated with regulation by RNA thermometers(82, 85, 127, 156, 159). Additionally, genes involved in nutrient acquisition were also of interest because of the identification of the 4U RNA thermometer regulating expression of the heme uptake gene shua(84). These 10 genes were chosen for future analysis and are listed in Table 5.
142 142 Table 5 In silico predicted S. dysenteriae 4U RNA thermometers Gene Location Function ospd1 Virulence Plasmid Anti-activator of the TTSS spad Virulence Plasmid Secretion of TTSS effector proteins mxig Virulence Plasmid TTSS structural protein mxii Virulence Plasmid TTSS structural protein ipah 7.8 Virulence Plasmid Escape from macrophage fecd Chromosome Ferric di-citrate uptake tort Chromosome Basic Metabolism entc Chromosome Siderophore synthesis htra Chromosome Heat-shock ompa Chromosome Ion uptake and membrane stability Background and Structure of the predicted RNA thermometers regulating tort, entc, htra and ompa regulation The in silico screen provided a list of genes that were predicted to be regulated by 4U RNA thermometers; from that list tort, entc, htra and ompa were selected to begin our analysis. These genes were selected for two reasons. First, RNA thermometers had previously been shown to regulate production of proteins involved in virulence (tort and ompa)(5, 6, 14, 34), heat-shock (htra)(12, 59, 78) and iron acquisition (entc)(136, 137).
143 143 While these genes have not previously been shown to be regulated by an RNA thermometer, we are interested in protein classes that have previously been identified to be regulated by RNA thermometers to increase our chances of identifying additional 4U RNA thermometers. Second, the in silico structure of each predicted RNA thermometer varied significantly among these four genes; by selecting RNA thermometers with significantly different structures, characteristics associated with and required for a functional 4U RNA thermometer can be identified. The tort gene encodes a periplasmic regulator of the Tor system which produces trimethylamine N-oxide; a final electron acceptor that can be used by Shigella in anaerobic environments(14). In addition, mutational analyses has suggested that deletion of tort corresponds with inappropriate formation of the type three secretion system, a system necessary for host cell invasion and establishment of disease(6, 14). Previous data has determined that TorT production is induced under anaerobic environments; however, the exact molecular mechanism(s) modulating TorT production had not been elucidated(14). The entc protein product is responsible for conversion of chorsimate to isochorsimate, an essential step in the production of the siderophore enterobactin(35). Previous research has shown that the production of EntC, like TorT, is induced in response to anaerobic conditions where iron would be in the ferric state(35, 137). Dual regulation of iron acquisition systems in response to oxygen concentrations and temperature is not unprecedented and could help maximally produce EntC in an environment similar to the host G.I. tract(23, 84). Regulation of an iron uptake system by
144 144 an RNA thermometer is also not unprecedented and has previously been shown to regulate shua expression in Shigella dysenteriae (Chapter 3 of this thesis) (84). If an RNA thermometer is found to regulate EntC production it could indicate that these riboregulators play a central role in governing production of Shigella iron uptake systems. HtrA is a periplasmic heat-shock protein and serves two mutually exclusive functions. At temperatures less than 42 C it acts as a chaperone to prevent the aggregation of misfolded proteins(12, 59, 78). At temperatures greater than 42 C a conformational change occurs within HtrA that allows the protein to act as a serine protease to degrade denatured proteins(12, 59, 78). Regulation of additional heat-shock factors by an RNA thermometer has been previously identified and is not unprecedented(22, 159). The ompa gene encodes an outer-membrane protein that is responsible for ion diffusion through the outer-membrane(5, 34, 141). In addition to ion transport, the OmpA protein has been shown to stabilize the outer-membrane(5, 34, 141). Destabilization of the outer-membrane due to perturbations of OmpA production has been associated with a virulence phenotype that, for Shigella, inhibits the intracellular spread of the pathogen to adjacent cells within the colonic epithelium(5). To maintain efficient and precise OmpA production and prevent outer-membrane destabilization, OmpA has been shown to be post-transcriptionally thermoregulated to maintain even level of protein independent of environmental temperature(2). The mechanism governing the post-transcriptional
145 145 temperature-dependent regulation of OmpA production has not been identified but is indicative of regulation by an RNA thermometer. The function and regulation of the TorT, EntC, HtrA and OmpA proteins was largely studied in E. coli strains but an RNA thermometer had not previously been identified to regulate these genes increasing the significance of these studies. To make sure these genes are well conserved, especially in the 5 utr where an RNA thermometer would be harbored, an alignment was performed between the E. coli and S. dysenteriae orthologue to ensure that the previously identified thermoregulation of some genes which may be regulated by an RNA thermometer would be conserved (Figure 30). These alignments showed good conservation between each orthologueue, with no nucleotide differences in the regions thought to harbor a 4U RNA thermometer. These data support that hypothesis that these sequence regions are important for expression and indicate that any confirmed RNA thermometer found in these studies could have implications outside of the Shigella genus.
146 Figure 30: Alignment of the putative 4U RNA thermometers An alignment between the tort, entc htra and ompa 5 untranslated regions from Shigella dysenteriae and an Enterohemorrhagic strain of E. coli was conducted. The four consecutive uracils residues predicted to be contained within a 4U RNA thermometer are labled as (4U) while the Shine-Dalgarno sequence and the translational start site are labeled as (S.D.) and (T.S.S.) respectively. The alignment was performed in Clonemanater9. All sequences were obtained from Genbank and are as follows: S. dysenteriae tort gene ID: , EHEC tort gene ID: , S. dysenteriae entc gene ID: , EHEC entc gene ID: , S. dysenteriae htra gene ID: , EHEC htra gene ID: , S. dysenteriae ompa gene ID: , EHEC ompa gene ID:
147 147 In addition to the function of each of the selected genes, they were also chosen based on the variability of the 4U RNA thermometer structure predicted by in silico analysis using the Mfold software package (Figure 31). These structures vary greatly in the length of the inhibitory structure that occludes the ribosomal binding site, number of hairpins within the 5 utr of each gene which may act to stabilize the RNA thermometer, and the presence or absence of bulges within the inhibitory structure which have been shown to increase the responsive range of other types of RNA thermometers(82, 85, 109, 127, 159). Discovery of additional RNA thermometers would allow for the identification of characteristics that are necessary for, and/or modulate the activity of functional 4U RNA thermometers.
148 148 Figure 31: In silico predictions of the putative RNA thermometers regulating TorT, EntC, HtrA and OmpA production Nucleic acid sequences composing the 5 utr of S. dysenteriae tort, entc, htra and ompa were submitted to the Mfold software package ( for analysis of the RNA secondary structures. The four uracil residues within the 4U RNA thermometer and ribosomal binding site are indicated by a 4U and RBS respectively and are boxed. These foldings represent the most thermodynamically stable structure for each RNA thermometer Experimental evaluation of the tort, entc, htra and ompa 4U RNA thermometers Chapter 3 of this thesis has indicated the presence of a 4U RNA thermometer that regulates the production of the outer-membrane heme receptor ShuA and has provided a pipe-line for the identification and characterization of additional RNA thermometers. This process requires analysis of thermoregulation from the isolated WT and rationally
149 149 mutated RNA thermometer followed by the confirmation of post-transcriptional thermoregulation of the WT transcript and protein off the chromosome(84). To identify the presence of an RNA thermometer controlling expression from the tort, entc, htra and ompa messages we utilized this process to evaluate thermoregulation and characterize the underlying mechanisms. The 4U RNA thermometer form each selected gene was first isolated from the rest of the 5 utr and natural promoter to analyze its regulatory capability independent of other mechanisms encoded within these regions. Each putative thermometer was cloned into the reporter plasmid pxg-10 between the constitutive plasmid promoter PLtetO- 1and the gfp reporter gene. This cloning strategy creates a translational fusion between the predicted RNA thermometer and the gfp reporter gene such that transcription of the Gfp reporter is driven by the constitutive plasmid promoter and protein production may be influenced by the regulatory activity of the predicted RNA thermometer. The resulting reporter constructs, ptort, pentc, phtra and pompa, were transformed into WT S. dysenteriae and grown to mid-log phase at either 25 C or 37 C and gfp expression was analyzed by Western blot and Q-PCR analysis (Figure 32). Following culturing of each strain to 25 C and 37 C Western blot analyses showed no significant difference in Gfp protein levels from strains carrying ptort or pentc at either temperature tested (Figures 32E and 32G); however, strains carrying phtra and pompa showed a significant increase in Gfp protein production when cultured at 37 C compared to 25 C (Figures 32I and 32K). Q-PCR analysis of each strain found no significant difference in gfp mrna levels from any of the promoter constructs following growth at 25 C and 37 C (Figures 32F,
150 150 32H, 32J and 32L) Post-transcriptional thermoregulation could not be detected from the isolated tort and entc sequences suggesting the lack of a functional RNA thermometer and these sequences were not used in further analyses. The lack of thermoregulation from these isolated sequences could be due to the artificial nature of only cloning the inhibitory structure. In addition, the temperature range that these RNA thermometers could be responsive to may have not been tested. Conversely, ompa and htra sequence cloned into the pompa and phtra plasmids respectively confered post-transcriptional thermoregulation on Gfp protein production indicating the presence of an RNA thermometer controlling the observed regulation. However, before further analyses of the htra RNA thermometer could be conducted, data were published from an independent lab confirming its presence(80). Therefore efforts were focused on the confirmation and characterization of the putative ompa 4U RNA thermometer.
151 Figure 32: The predicted RNA thermometer contained within the htra and ompa 5 utr are sufficient to confer post-transcriptional thermoregulation The putative tort (A), entc (B), htra (C), and ompa (D) 4U RNA thermometers were translationally fused to the gfp reporter gene on the plasmid pxg-10 yielding ptort, pentc, phtra, and pompa respectively. The four uracils within the RNA thermometer and ribosomal binding sites are boxed and denoted by a 4U and RBS respectively. The plasmids were transformed into WT S. dysenteriae and grown to mid-log phase at the temperatures indicated. Whole cell lysates from S. dysenteriae carrying ptort (E), pentc (G), phtra (I), and pompa (K) were subjected to Western blot analysis using an anti-gfp monoclonal antibody. Relative levels of gfp expressed from WT S. dysenteriae carrying each strain and cultured under the same conditions as were used for the Western blot analysis was analyzed by Q-PCR. The gfp transcripts were normalized to the housekeeping gene rrsa and were set relative to the first 25 C sample. Error bars represent one standard deviation. 151
152 Expression of chromosomally encoded S. dysenteriae ompa is subject to posttranscriptionally temperature-dependent regulation Previous regulatory studies conducted on the chromosomally encoded E. coli ompa showed it was regulated by an unknown post-transcriptional temperaturedependent mechanism(2). These studies showed that ompa transcript levels were significantly decreased when E. coli was cultured at 37 C as compared to those measured following growth of the strain at 25 C; however, the protein levels were not significantly different between these two growth conditions(2). This post-transcriptional thermoregulation was shown to be mediated by an increase in ribosomal binding to the ompa transcript at 37 C resulting in the same amount of protein produced from less message(2). The mechanism responsible for increased ribosomal binding at 37 C, which we believe is due to the actions of the ompa 4U RNA thermometer, was not identified in that work. Before we can accomplish our goal of identifying the impact of the ompa RNA thermometer on OmpA protein production in vivo, and its influence on S. dysenteriae virulence, we must confirm the post-transcriptional thermoregulation of the chromosomally encoded gene in Shigella. To investigate post-transcriptional thermoregulation of ompa encoded on the S. dysenteriae chromsome, Western blot and Q-PCR analyses were performed to evaluate the protein and transcript abundance respectively. All analyses were performed following growth of the WT S. dysenteriae to the mid-log phase at both 25 C and 37 C. The data generated from these experiments showed significantly decreased amounts of ompa transcript in S. dysenteriae cultured at 37 C as compared to that measured following
153 153 growth of the strain at 25 C (Figure 33B); the same pattern as was previously shown in E. coli(2). Next, Western blot analysis using an α-ompa monoclonal antibody was used to examine total amounts of OmpA produced by S. dysenteriae under each condition being investigated. Western blot analysis demonstrated that there is significantly more OmpA protein produced by S. dysenteriae following growth of the strain at 37 C as compared to OmpA levels measured following growth at 25 C; however, this difference is modest with only a 1.4x change in protein levels between the two temperatures (Figure 33A). Between the change in overall ompa transcript levels from 25 C and 37 C (approximately 6x decreased at 37 C) and the modest increase in protein levels (1.4x) the post-transcriptional thermoregulated mechanism governing this regulation increases expression from the ompa transcript by approximately 8x at 37 C. These data indicate that OmpA protein production is subject to post-transcriptional thermoregulation in both S. dysenteriae and E. coli, an observation that is consistent with a model of regulation by a conserved RNA thermometer within the 5 UTR of the gene.
154 154 A) B) Figure 33: S. dysenteriae ompa is post-transcriptionally thermoregulated WT S. dysenteriae was cultured at 25 C and 37 C to mid-log phase. A) Whole cell lysates were generated and subjected to Western blot analysis using a monoclonal αgfp antibody. B) The same culture used to generate whole-cell lysates for Western blot analysis was also used to isolate RNA for Quantitative-PCR analysis. Total ompa transcript levels were expressed relative to the first sample cultured at 25 C and normalized to the levels of ompa in each sample. A (*) indicates a significant difference, assuming a p-value of.05. Error bars represent one standard deviation and these data are representative of experiments conducted in biological triplicate Mutational analyses of the ompa 4U RNA thermometer To further analyze the putative ompa 4U RNA thermometer, mutations were introduced into the predicted thermometer within the ompa reporter plasmid. These mutants were rationally designed to either stabilize or destabilize the closed conformation of the inhibitory structure. The stabilizing and de-stabilizing mutations were predicted to decrease and increase Gfp protein production from the reporter plasmid, respectively (Figure 34B and 34C). The stabilized and destabilized mutant reporters, designated psompa and pd-ompa respectively, were transformed into WT S. dysenteriae and gfp expression from each was compared to that in the strain carrying p-ompa using Western blot and Q-PCR analysis. After culturing of the strains carrying ps-ompa and pompa at
155 155 the permissive temperature of 37 C, Western blot analysis showed Gfp protein production was inhibited in the strain carrying ps-ompa compared to pompa (Figure 34D). Conversely, Western blot showed an increase in Gfp production from the strain carrying pd-ompa compared to pompa following growth at the inhibitory temperature of 25 C (Figure 34F). To further test that the observed differences in Gfp protein levels between strains carrying the mutant and the wild-type reporters were due to alterations of RNA thermometer stability and not to a change in transcript levels, Q-PCR analysis was conducted using the same strains and culture conditions as the Western blot assay. The resulting Q-PCR analysis did not show any significant difference in gfp transcript levels between the ps-ompa and pompa or the pd-ompa and pompa reporter plasmids under the conditions tested (Figures 34E and 34G).
156 156 Figure 34: Mutational analysis of the ompa 5 utr indicates the presence of a 4U RNA thermometer The RNA thermometer harbored on the pompa plamisd (A) was mutated to stabilize (B) and destabilize (C) the inhibitory structure yielding plasmids ps-ompa and pd-ompa respectively. The mutations made to each structure are highlighted in red. The four uracils within the 4U RNA thermometer and the ribosomal binding site are boxed and denoted by 4U and RBS respectively. Each plasmid was transformed into WT S. dysenteriae and Gfp protein production from the pompa plasmid was compared to that from ps-ompa (D) and pd-ompa (F) using Western blot analysis. Wild-type S. dysenteriae carrying each plasmid was cultured at the temperatures indicated to mid-log phase and Gfp production was analyzed using a monoclonal αgfp antibody. The gfp mrna levels transcribed from the pompa plasmid was compared to that from ps-ompa (E) and pd-ompa (G). The level of gfp was expressed relative to the first sample from strains cultured at 25 C carrying pompa. Each sample was normalized to the housekeeping gene rrsa. Each experiment was performed in biological triplicate and error bars represent one standard deviation. Together, these mutagenesis studies show that the introduction of rational mutations into the isolated ompa sequence in the gfp reporter plasmid modulated gfp expression in a manner expected of a gene regulated by an RNA thermometer. These data strongly support the hypothesis that the ompa 5 utr harbors a functional 4U RNA thermometer.
157 Discussion This work is the first to identify the mechanism of post-transcriptional thermoregulation of ompa, a conserved outer-membrane protein that functions as an ion porin and promotes membrane stability(5, 34, 141). The 4U RNA thermometer responsible for ompa regulation was found using a defined experimental process established during the characterization of the regulatory mechanisms controlling the expression of S. dysenteriae shua, an outer-membrane heme receptor (Chapter 3)(84). These studies show that this pipe-line can be utilized to identify additional 4U RNA thermometers, a required first step before full characterization of this subclass of riboregulators can begin. Studies in E. coli have found that OmpA production is heavily regulated in response to both growth phase and temperature(2, 155). First, the small RNA MicA is transcribed in a growth-phase dependent manner after the cells have entered the late logarithmic stage(155). Studies have shown that following binding to the ompa transcript, MicA recruits RNases which functions to degrade each transcript(155). Additionally, the E. coli ompa transcript was shown to be destabilized when the bacterium was cultured at 37 C, compared to when the bacterium was cultured at 27 C(2). The temperature-dependent destabilization of the E.coli ompa transcript was found to be due to cleavage of the transcript by RNaseE at the higher temperature(2). Destabilization of the transcript lead to an overall decrease in ompa transcript levels at 37 C; however, an increase in ribosomal binding efficiency at 37 C, compared to 27 C, resulted in an even amount of OmpA protein production independent of environmental
158 158 temperature(2). Before the studies described within, the molecular mechanism governing an alteration in ribosomal binding efficiency to the ompa transcript at 37 C was not known; however, this expression pattern fits well with the model of regulation by an RNA thermometer(82, 84, 109, 127, 159). Our bioinformatics analysis suggested that an RNA thermometer may be harbored within the ompa 5 utr (Figure 31) and was experimentally tested by isolating (Figure 32) and mutating (Figure 34) the RNA thermometer. Together these experiments show three independent mechanisms, mediated by MicA(155), RnaseE (2) and a 4U RNA thermometer (Figures 32-34), that regulate OmpA in response to growth phase and environmental temperature. OmpA production must be tightly regulated because previous studies in Shigella have shown that an even level of OmpA protein must be maintained in the cell in order to stabilize the bacterial membrane(5). Destabilization of the bacterial membrane due to an increase or decrease in the total amount of OmpA produces a virulence phenotype that prohibits intracellular spreading of Shigella between colonic epithelial cells(5). The combined efforts of MicA, degradation by RNaseE and the 4U RNA thermometer could maintain a constant level of OmpA independent of temperature and growth phase. MicA could maintain WT OmpA levels in the bacterial membrane by decreasing OmpA production at the later growth stages when the rate of bacterial replication has slowed thus maintaining membrane stability. In addition the actions of RnaseE and the 4U RNA thermometer balance the amount of OmpA protein produced, regardless of environmental temperature, because of the global increase in RNA polymerase activity at higher temperatures. If ompa did not harbor an RNA thermometer an increase in ompa
159 159 transcription would correlate with an increase in OmpA protein production at higher temperatures and destabilize the bacterial membrane. Combined these three mechanisms work together to produce even amounts of OmpA protein independent of temperature or growth phase (Figure 35). Figure 35: Model of ompa post-transcriptional regulation OmpA production is regulated by two confirmed post-transcriptional mechanisms, RNaseE cleavage and message destabilization at 37 C and MicA binding and message degradation at stationary phase and 37 C. We propose that ompa is regulated by a third post-transcriptional mechanism, a FourU RNA thermometer that allows for increased translational efficiency at host body temperature, 37 C. This model accounts for regulation of OmpA production under 25 C and 37 C as well as a low optical density ( OD) and high optical density ( OD). All three mechanisms allow for maximal production of OmpA when the cells are still actively dividing and the transcript has been destabilized by RNaseE cleavage.
160 160 The pipe-line first utilized to identify the RNA thermometer regulating expression of S. dysenteriae shua has been used here to identify and characterize the ompa 4U RNA thermometer. This method has also been applied to putative RNA thermometers in three additional genes, tort, entc and htra, in this study. Of these genes, only htra was found to contain an RNA thermometer, which was characterized by an independent research group (Figure 32) (22). These findings increase the validity of the pipe-line used here by identifying an additional 4U RNA thermometer, found in htra, while rejecting two potential RNA thermometers, predicted to be located within the tort and entc genes. This data presented here indicate that his pipe-line can be used to efficiently identify additional RNA thermometers in bacterial genes. Including the ompa 4U RNA thermometer identified in this work, four additional 4U RNA thermometers have been identified to date; these RNA thermometers were found in agsa, which encodes a heat-shock gene in Salmonella, icrf which encodes a heat-shock factor in Yersinia htra and shua(22, 80, 84, 159). Before analysis of characteristics required to for a functional 4U RNA thermometer additional members of this subclass must first be identified and characterized. By utilizing the pipe-line described in these studies, additional 4U RNA thermometers can be efficiently identified to allow for characterization of this important class of riboregulators. 4.5 Future directions In these works a pipe-line for the identification of 4U RNA thermometers has been utilized that allowed for the positive identification of a RNA thermometer
161 161 controlling expression of ompa. However, in silico and manual screens of the S. dysenteriae chromosome and virulence plasmid identified an additional 36 potential 4U RNA thermometers. By utilizing this pipe-line described here, these additional RNA thermometers can be evaluated for their ability to confer post-transcriptional thermoregulation on their target genes. This study verified the presence of an RNA thermometer within the gene encoding the outer-membrane protein A (Figures 32 and 34); however, additional research is needed to identify the impact of the ompa 4U RNA thermometer on Shigella virulence. To this end, chromosomal mutations which match those of the stabilizing and destabilizing thermometer (Figure 34) are currently being introduced into the S. dysenteriae ompa 5 utr. These mutations were shown to modulate production of a reporter from a reporter plasmid, indicating they have an impact on regulation from this thermometer (Figure 34); and by introducing these mutations into the S. dysenteriae chromosome we hope to modify the regulation of ompa in a temperature-dependent manner. If these modifications are able to post-transcriptionally thermoregulate OmpA protein production, indicative of regulation by an RNA thermometer, these effects will be observable by performing Western blot and Q-PCR analyses. If post-transcriptional thermoregulation is observed, these data would further validate the presence of an RNA thermometer controlling expression of ompa. Additionally, the effect of these mutations on Shigella virulence could be observed, as it has previously been identified that misregulation of ompa impedes the ability of this organism to spread intracellularly(5, 34). By modulating the expression of ompa using these mutations we can observe the
162 162 impact this regulatory factors has on Shigella virulence by performing invasion and spread assays using both WT and mutant ompa strains of S. dysenteriae. If there is a defect in the ability of these bacteria to spread intracellularly in a temperature-dependent manner, as has been previously observed, these data would indicate that the ompa 4U RNA thermometer has a direct role in controlling a virulence factor necessary for Shigella virulence(5, 34).
163 163 CHAPTER 5: DISCUSSION These studies characterized molecular mechanisms regulating expression of virulence genes within the human pathogen Shigella dysenteriae. Specifically, the shu locus was found to be regulated in response to iron by the iron-dependent transcriptional repressor Fur (Figures 15-18). Additionally, the outer-membrane heme receptor, ShuA, and the outer-membrane protein, OmpA, were found to be regulated in response to temperature by an RNA thermometer (Figures 25, 26, 32 and 34), a previously uncharacterized regulatory mechanism in Shigella species. The precise regulation of these genes in response to select environmental conditions allows Shigella to produce these factors under situations that are most advantageous to the bacterium and prevents their misregulation which could likely result in a decrease in fitness and possibly death. The studies detailed in Chapter 2 characterized the organization and transcriptional landscape of the Shigella heme uptake (shu) locus, which is responsible for the acquisition of heme within the human host. Previous in silico studies investigating the organization of the shu locus identified four putative promoters driving expression of two monocistronic (shua and shus) and two polycistronic (shutwxy and shuuv) transcripts (Figure 11) (166). However, the experimental analysis detailed in Chapter 2 indicates that only two polycistronic transcripts (shuas and shutwxyuv) are transcribed from two divergent promoters (Figures12 and 13). The organization of the shu genes into two transcripts with one encoding the outer-membrane heme receptor, shua, and a second containing a periplasmic heme binding protein and a heme specific ABC permease, shut and shuuv respectively, is conserved in shu orthologues in pathogenic E. coli strains and
164 164 Pseudomonas species(111, 166). This conserved organization could allow for the organism to regulate these factors in response to different environmental cues or to a different degree. The outer membrane heme receptor, which is responsible for the first step in heme uptake, could be tightly controlled to allow for heme uptake only when heme is required as an iron source and present in the surrounding environment. Conversely, expression from the operon encoding the periplasmic heme binding protein and a heme specific transporter, encoded by the shutwxyuv operon, could be less tightly regulated facilitating basal levels of expression. Regulation in this manner could prime the cell to begin utilizing heme as an iron source as soon as the outer membrane heme receptor is produced. In addition to the characterization of the transcriptional landscape, these studies also identified two environmental cues which regulate expression form the shu locus. These studies found that both transcripts are regulated in response to iron in a Furdependent manner (Figures 15-18). Fur is a global iron-dependent transcriptional repressor and binds the promoter elements of regulated genes, in a sequence specific manner, and inhibit recruitment of the transcriptional machinery(44). While the exact mechanism by which Fur regulates transcription from the shu promoters is unknown, sequence analysis has identified two putative Fur boxes which overlap the shuas and shutwxyuv promoters suggesting Fur binding and direct regulation (data not shown). The effect of temperature on expression from the shu locus was also investigated. The data in these studies indicates that Shu protein production is regulated at the posttranscriptional level in a temperature-dependent manner (Figures 19-22). Previously, only
165 165 H-NS, a nucleoid-like protein which binds to the promoter regions in a temperaturedependent manner and inhibits recruitment of RNA polymerase, was found to thermoregulate gene expression in Shigella(36). However, data presented here shows that the shu locus is not regulated at the transcriptional level highly suggesting that H-NS is not involved in Shu protein production (Figures 21 and 22). While post-transcriptional thermoregulation of Shigella genes had not previously been reported, this manner of regulation is indicative of an RNA thermometer, a cis-encoded regulatory element that controls translational efficiency in response to temperature by modulating access of the ribosome to the Shine-Dalgarno sequence (Figure 4)(33, 82, 109). Together these studies identified two regulatory signals affecting regulation of the two shu transcripts. Regulation of these transcripts in response to iron (Figures 15-18) and temperature (Figures 19-22), as well as oxygen concentrations which has been previously reported(23), allows for precise regulation of this system in response to hostassociated environmental conditions. By regulating production of the Shu system in response to environments that mimic that of the host, Shigella produces this system when it is most beneficial to the organism. The focus of Chapter 3 was the identification and characterization of the mechanism(s) responsible for the post-transcriptional thermoregulation of the Shigella outer membrane heme receptor (ShuA). These data show that ShuA production is thermoregulated by a 4U RNA thermometer, a subclass of these regulators (Figures 25 and 26). Prior to this study, no RNA thermometer had been identified in Shigella species; additionally, no nutrient acquisition system had been reported to be regulated by an RNA
166 166 thermometer increasing the significance of this discovery. However, one caveat of regulation by an RNA thermometer is that they only regulate translation of the gene which they are associated with. This has implications for the regulation of shus which is encoded downstream of shua on the same transcript. While shus transcript levels are not affected by temperature (Figure 21), it was not possible to identify if ShuS protein production is also regulated in response to temperature indicating the presence of an RNA thermometer regulating ShuS as well. Examination of the ShuS protein levels and the area surrounding the shus ribosomal binding site will provide clues as to whether or not a second RNA thermometer could be encoded on this transcript, an unprecedented finding. In addition to being the first RNA thermometer discovered in Shigella and the first to regulate a nutrient acquisition system, the shua 4U RNA thermometer was also the second identified thermometer within this class(84, 160). While this finding is significant, more 4U RNA thermometers must be found to determine what characteristics define a 4U RNA thermometer and if it is distinctive enough from other RNA thermometers to be considered a separate class. In chapter 4, additional 4U RNA thermometers were identified using in silico analysis of the Shigella dysenteriae chromosome and virulence plasmid. This screen identified 40 potential 4U RNA thermometers, four of which were further identified using a pipe-line developed while investigating the shua RNA thermometer (Table 5). By using this pipe-line on four of the putative 4U RNA thermometers, two additional RNA thermometers, controlling expression of ompa and htra, were identified (Figure 32). These findings, as well as the discovery of the shua RNA thermometer using the pipe-
167 167 line, validate the efficiency of this pipe-line as a tool for identification of additional RNA thermometers. Further research into the ompa RNA thermometer validated its presence and explained the previously identified post-transcriptional thermoregulation. Regulation of OmpA production by an RNA thermometer would allow for stable amounts of OmpA protein within the membrane independent of growth phase or environmental temperature (Figure 35) By using the bioinformatics and manual screen we validated the fifth functional 4U RNA thermometer (Figures 32, 33 and 34) (22, 84, 160). This has allowed us to begin an examination of what characteristics are required for a functional 4U RNA thermometer based on sequence and structural analysis (Figure 36). One characteristic that varies between these predicted RNA thermometers is the length of the putative inhibitory structure, which ranges from the ompa RNA thermometer with 8 nucleotides to the ihrf thermometer which contains 21 nucleotides. Traditionally, the length of an RNA thermometer inhibitory structure correlates with the temperature required to induce expression, with shorter inhibitory structures requiring a lower temperature to permit translation than their longer counterparts(82, 109, 127). This trend seems to hold with the 4U RNA thermometers as the shorter thermometers allow for more protein production throughout the temperature ranges tested (shua Figures 19 and 21, and ompa Figure 32) while the longer thermometers tend to allow protein production only after a certain temperature threshold has been reached(22, 84, 160). A second variable trait observed among these RNA thermometers is the number of hairpins upstream of the inhibitory structure. Upstream hairpins function to stabilize the inhibitory structure in ROSE and
168 168 cold-shock thermometers at temperatures that are not permissive to translation which allows for precise expression of the target gene at the appropriate temperature(55, 82). While several of the identified 4U RNA thermometers do have upstream hairpins associated with them (ihrf, ompa, shua agsa), this trait is not necessary as htra does not require upstream hairpins for precise regulation(80). Finally, the hallmark of a 4U RNA thermometer is the presence of four consecutive uracils that pair both canonically and non-canonically with the ribosomal binding site. The tort thermometer was of particular interest because the four uracil residues do base pair appropriately with the ribosomal binding site but are not consecutive (Figure 31 and 32); which would allow for interesting analysis into the function of these uracils in regulation of a 4U RNA thermometer. However, studies have shown that tort is not regulated in response to temperature (Figure 32). The five confirmed 4U RNA thermometers do contain four consecutive uracil residues indicating that these residues may be required for proper function (Figure 36) (22, 80, 84, 160). This could be due to the unique traits of a guanine and uracil base-pair which allow for a more fluid change in secondary structures. While sequence and structural analysis does give clues to the characteristics required for a functional 4U thermometer, the identification of additional thermometers and mutational analyses are required to properly characterize these regulatory devices.
169 169 Figure 36: in silico structural analysis of the confirmed 4U RNA thermometers The inhibitory structure of the five confirmed 4U RNA thermometers, E. coli htra (A), S. dysenteriae ompa (B), S. dysenteriae shua(c), Yersinia pseudotuberculosis ihrf(d), and Salmonella enteric agsa(e), were submitted for in silico analysis using the mfold software package ( The four uracil residues contained within the RNA thermometer and the ribosomal binding site are boxed and labeled 4U and RBS respectively. The studies detailed in this thesis have identified regulatory cues and characterized the underlying molecular mechanism governing regulation of Shigella dysenteriae virulence factors. Specifically, these studies have characterized the transcriptional landscape and organization of the Shigella dysenteriae shu locus, identified the iron-dependent and Fur-mediated regulation of genes within the shu locus, identified and characterized a 4U RNA thermometer governing regulation of shua and ompa and developed a pipe-line for the efficient Identification of additional 4U RNA thermometers. In completing these works, a further understanding of heme uptake in Shigella dysenteriae and pathogenic E. coli was gained; additionally, these works significantly contribute to the knowledge of Shigella riboregulation by cis-acting regulatory mechanisms. Bu understanding the mechanisms of Shigella gene regulation,
170 170 we are better able to understand the physiology of this organism, how it adapts to the environments it encounters and how Shigella species cause disease.
171 171 REFERENCES 1. Adler B., C. Sasakawa, T. Tobe, S. Makino, K. Komatsu, and M. Yoshikawa A dual transcriptional activation system for the 230 kb plasmid genes coding for virulence-associated antigens of Shigella flexneri. Mol. Microbiol. 3: Afonyushkin T., I. Moll, U. Bläsi, and V. R. Kaberdin Temperaturedependent stability and translation of Escherichia coli ompa mrna. Biochem. Biophys. Res. Commun. 311: Aiba H Mechanism of RNA silencing by Hfq-binding small RNAs. Curr. Opin. Microbiol. 10: Ajene A. N., C. L. Fischer Walker, and R. E. Black Enteric pathogens and reactive arthritis: a systematic review of Campylobacter, salmonella and Shigella-associated reactive arthritis. J. Health Popul. Nutr. 31: Ambrosi C., M. Pompili, D. Scribano, C. Zagaglia, S. Ripa, and M. Nicoletti Outer membrane protein A (OmpA): a new player in shigella flexneri protrusion formation and inter-cellular spreading. PloS One 7:e Ando H., H. Abe, N. Sugimoto, and T. Tobe Maturation of functional type III secretion machinery by activation of anaerobic respiration in enterohaemorrhagic Escherichia coli. Microbiol. Read. Engl. 153: Andrews S. C., A. K. Robinson, and F. Rodríguez-Qui\ nones Bacterial iron homeostasis. FEMS Microbiol. Rev. 27: Andrews S. C., A. K. Robinson, and F. Rodríguez-Qui\ nones Bacterial iron homeostasis. FEMS Microbiol. Rev. 27: Antelmann H., and J. D. Helmann Thiol-based redox switches and gene regulation. Antioxid. Redox Signal. 14: Anzaldi L. L., and E. P. Skaar Overcoming the heme paradox: heme toxicity and tolerance in bacterial pathogens. Infect. Immun. 78: Arsène F., T. Tomoyasu, and B. Bukau The heat shock response of Escherichia coli. Int. J. Food Microbiol. 55: Arsène F., T. Tomoyasu, and B. Bukau The heat shock response of Escherichia coli. Int. J. Food Microbiol. 55: Ashida H., M. Ogawa, H. Mimuro, and C. Sasakawa Shigella infection of intestinal epithelium and circumvention of the host innate defense system. Mol. Mech. Bact. Infect. Gut Baraquet C., L. Théraulaz, M. Guiral, D. Lafitte, V. Méjean, and C. Jourlin- Castelli TorT, a member of a new periplasmic binding protein family, triggers induction of the Tor respiratory system upon trimethylamine N-oxide electron-acceptor binding in Escherichia coli. J. Biol. Chem. 281: Battisti J. M., K. N. Sappington, L. S. Smitherman, N. L. Parrow, and M. F. Minnick Environmental signals generate a differential and coordinated expression of the heme receptor gene family of Bartonella quintana. Infect. Immun. 74: Bauer C. E., S. Elsen, and T. H. Bird Mechanisms for redox control of gene expression. Annu. Rev. Microbiol. 53:
172 17. Beloin C., and C. J. Dorman An extended role for the nucleoid structuring protein H-NS in the virulence gene regulatory cascade of Shigella flexneri. Mol. Microbiol. 47: Beloin C., S. McKenna, and C. J. Dorman Molecular dissection of VirB, a key regulator of the virulence cascade of Shigella flexneri. J. Biol. Chem. 277: Bernardini M. L., J. Mounier, H. d Hauteville, M. Coquis-Rondon, and P. J. Sansonetti Identification of icsa, a plasmid locus of Shigella flexneri that governs bacterial intra- and intercellular spread through interaction with F-actin. Proc. Natl. Acad. Sci. U. S. A. 86: Bhattacharya S. K., and D. Sur An evaluation of current shigellosis treatment. Expert Opin. Pharmacother. 4: Biard D. S. F., M. R. James, A. Cordier, and A. Sarasin Regulation of the Escherichia coli lac operon expressed in human cells. Biochim. Biophys. Acta BBA - Gene Struct. Expr. 1130: Böhme K., R. Steinmann, J. Kortmann, S. Seekircher, A. K. Heroven, E. Berger, F. Pisano, T. Thiermann, H. Wolf-Watz, F. Narberhaus, and P. Dersch Concerted actions of a thermo-labile regulator and a unique intergenic RNA thermosensor control Yersinia virulence. PLoS Pathog. 8:e Boulette M. L., and S. M. Payne Anaerobic Regulation of Shigella flexneri Virulence: ArcA Regulates fur and Iron Acquisition Genes -- Boulette and Payne 189 (19): The Journal of Bacteriology. 24. Braun V Iron uptake mechanisms and their regulation in pathogenic bacteria. Int. J. Med. Microbiol. IJMM 291: Brennan R. G., and T. M. Link Hfq structure, function and ligand binding. Curr. Opin. Microbiol. 10: Buchrieser C., P. Glaser, C. Rusniok, H. Nedjari, H. D Hauteville, F. Kunst, P. Sansonetti, and C. Parsot The virulence plasmid pwr100 and the repertoire of proteins secreted by the type III secretion apparatus of Shigella flexneri. Mol. Microbiol. 38: Burkhard K. A., and A. Wilks Characterization of the outer membrane receptor ShuA from the heme uptake system of Shigella dysenteriae. Substrate specificity and identification of the heme protein ligands. J. Biol. Chem. 282: Burkhard K. A., and A. Wilks Functional characterization of the Shigella dysenteriae heme ABC transporter. Biochemistry (Mosc.) 47: Camacho A. I., J. M. Irache, and C. Gamazo Recent progress towards development of a Shigella vaccine. Expert Rev. Vaccines 12: Caron M.-P., D. A. Lafontaine, and E. Massé Small RNA-mediated regulation at the level of transcript stability. RNA Biol. 7: Carter J. D., and A. P. Hudson Reactive arthritis: clinical aspects and medical management. Rheum. Dis. Clin. North Am. 35:
173 32. Chaudhuri T. K., V. K. Verma, and A. Maheshwari GroEL assisted folding of large polypeptide substrates in Escherichia coli: Present scenario and assignments for the future. Prog. Biophys. Mol. Biol. 99: Chowdhury S., C. Ragaz, E. Kreuger, and F. Narberhaus Temperaturecontrolled structural alterations of an RNA thermometer. J. Biol. Chem. 278: Confer A. W., and S. Ayalew The OmpA family of proteins: roles in bacterial pathogenesis and immunity. Vet. Microbiol. 163: Crosa, J.H. Iron Transport In Bacteria. 36. Dorman C. J H-NS: a universal regulator for a dynamic genome. Nat. Rev. Microbiol. 2: Dorman C. J., N. N. Bhriain, and C. F. Higgins DNA supercoiling and environmental regulation of virulence gene expression in Shigella flexneri. Nature 344: DuPont H. L., M. M. Levine, R. B. Hornick, and S. B. Formal Inoculum size in shigellosis and implications for expected mode of transmission. J. Infect. Dis. 159: Eakanunkul S., G. S. Lukat-Rodgers, S. Sumithran, A. Ghosh, K. R. Rodgers, J. H. Dawson, and A. Wilks Characterization of the periplasmic hemebinding protein shut from the heme uptake system of Shigella dysenteriae. Biochemistry (Mosc.) 44: El-Sharoud W. M., and P. L. Graumann Cold shock proteins aid coupling of transcription and translation in bacteria. Sci. Prog. 90: Falconi M., B. Colonna, G. Prosseda, G. Micheli, and C. O. Gualerzi Thermoregulation of Shigella and Escherichia coli EIEC pathogenicity. A temperature-dependent structural transition of DNA modulates accessibility of virf promoter to transcriptional repressor H-NS. EMBO J. 17: Falconi M., G. Prosseda, M. Giangrossi, E. Beghetto, and B. Colonna Involvement of FIS in the H-NS-mediated regulation of virf gene of Shigella and enteroinvasive Escherichia coli. Mol. Microbiol. 42: Faner M. A., and A. L. Feig Identifying and characterizing Hfq-RNA interactions. Methods San Diego Calif 63: Fillat M. F The FUR (ferric uptake regulator) superfamily: diversity and versatility of key transcriptional regulators. Arch. Biochem. Biophys. 546: Fillebeen C., and K. Pantopoulos Redox control of iron regulatory proteins. Redox Rep. Commun. Free Radic. Res. 7: Fleischhacker A. S., and P. J. Kiley Iron-containing transcription factors and their roles as sensors. Curr. Opin. Chem. Biol. 15: Forman S., M. J. Nagiec, J. Abney, R. D. Perry, and J. D. Fetherston Analysis of the aerobactin and ferric hydroxamate uptake systems of Yersinia pestis. Microbiol. Read. Engl. 153: Fröhlich K. S., and J. Vogel Activation of gene expression by small RNA. Curr. Opin. Microbiol. 12:
174 49. Geissmann T., M. Possedko, E. Huntzinger, P. Fechter, C. Ehresmann, and P. Romby Regulatory RNAs as mediators of virulence gene expression in bacteria. Handb. Exp. Pharmacol Georg J., and W. R. Hess cis-antisense RNA, another level of gene regulation in bacteria. Microbiol. Mol. Biol. Rev. MMBR 75: Georgiou G How to flip the (redox) switch. Cell 111: Georgopoulos C Toothpicks, serendipity and the emergence of the Escherichia coli DnaK (Hsp70) and GroEL (Hsp60) chaperone machines. Genetics 174: Giangrossi M., R. M. Exley, F. Le Hegarat, and C. L. Pon Different in vivo localization of the Escherichia coli proteins CspD and CspA. FEMS Microbiol. Lett. 202: Giangrossi M., G. Prosseda, C. N. Tran, A. Brandi, B. Colonna, and M. Falconi A novel antisense RNA regulates at transcriptional level the virulence gene icsa of Shigella flexneri. Nucleic Acids Res. 38: Giuliodori A. M., F. Di Pietro, S. Marzi, B. Masquida, R. Wagner, P. Romby, C. O. Gualerzi, and C. L. Pon The cspa mrna is a thermosensor that modulates translation of the cold-shock protein CspA. Mol. Cell 37: Gore A. L., and S. M. Payne CsrA and Cra influence Shigella flexneri pathogenesis. Infect. Immun. 78: Gottesman S., C. A. McCullen, M. Guillier, C. K. Vanderpool, N. Majdalani, J. Benhammou, K. M. Thompson, P. C. FitzGerald, N. A. Sowa, and D. J. FitzGerald Small RNA regulators and the bacterial response to stress. Cold Spring Harb. Symp. Quant. Biol. 71: Hale T. L., R. E. Morris, and P. F. Bonventre Shigella infection of henle intestinal epithelial cells: role of the host cell. Infect. Immun. 24: Hansen G., and R. Hilgenfeld Architecture and regulation of HtrA-family proteins involved in protein quality control and stress response. Cell. Mol. Life Sci. CMLS 70: Hantke K Iron and metal regulation in bacteria. Curr. Opin. Microbiol. 4: Hara-Kudo Y., and K. Takatori Contamination level and ingestion dose of foodborne pathogens associated with infections. Epidemiol. Infect. 139: Headley V., M. Hong, M. Galko, and S. M. Payne Expression of aerobactin genes by Shigella flexneri during extracellular and intracellular growth. Infect. Immun. 65: Henderson D. P., and S. M. Payne Vibrio cholerae iron transport systems: roles of heme and siderophore iron transport in virulence and identification of a gene associated with multiple iron transport systems. Infect. Immun. 62: Hensley C. T Transcriptional regulation of shigella virulence plasmidencoded genes by VirB and CRP. 174
175 65. Higgins R., R. Sauvageau, and P. Bonin Shigella flexneri Type 2 Infection in Captive Nonhuman Primates. Can. Vet. J. 26: Hoffmann H., M. W. Hornef, S. Schubert, and A. Roggenkamp Distribution of the outer membrane haem receptor protein ChuA in environmental and human isolates of Escherichia coli. Int. J. Med. Microbiol. IJMM 291: Houry W. A Chaperone-assisted protein folding in the cell cytoplasm. Curr. Protein Pept. Sci. 2: Inouye M., and S. Phadtare Cold shock response and adaptation at nearfreezing temperature in microorganisms. Sci. STKE Signal Transduct. Knowl. Environ. 2004:pe Islam M. S., M. K. Hasan, and S. I. Khan Growth and survival of Shigella flexneri in common Bangladeshi foods under various conditions of time and temperature. Appl. Environ. Microbiol. 59: Islam M. S., F. B. Rezwan, and S. I. Khan Survival of Shigella flexneri in artificial aquatic environment: effects of different physicochemical stress factors. J. Diarrhoeal Dis. Res. 14: Jennison A. V., and N. K. Verma Shigella flexneri infection: pathogenesis and vaccine development. FEMS Microbiol. Rev. 28: Jennison A. V., and N. K. Verma Shigella flexneri infection: pathogenesis and vaccine development. FEMS Microbiol. Rev. 28: Jones P. G., and M. Inouye The cold-shock response--a hot topic. Mol. Microbiol. 11: Jost B Site of transcriptional activation of virb on the large plasmid of Shigella flexneri 2a by VirF, a member of the AraC family of transcriptional activators. Microb. Pathog. 14: Kamp H. D., and D. E. Higgins A protein thermometer controls temperature-dependent transcription of flagellar motility genes in Listeria monocytogenes. PLoS Pathog. 7:e Kelly A., C. Conway, T. O Cróinín, S. G. J. Smith, and C. J. Dorman DNA supercoiling and the Lrp protein determine the directionality of fim switch DNA inversion in Escherichia coli K-12. J. Bacteriol. 188: Kiley P. J., and H. Beinert Oxygen sensing by the global regulator, FNR: the role of the iron-sulfur cluster. FEMS Microbiol. Rev. 22: Kim D. Y., and K. K. Kim Structure and function of HtrA family proteins, the key players in protein quality control. J. Biochem. Mol. Biol. 38: Kirby A. E., D. J. Metzger, E. R. Murphy, and T. D. Connell Heme utilization in Bordetella avium is regulated by RhuI, a heme-responsive extracytoplasmic function sigma factor. Infect. Immun. 69: Klinkert B., A. Cimdins, L. C. Gaubig, J. Roßmanith, U. Aschke-Sonnenborn, and F. Narberhaus Thermogenetic tools to monitor temperature-dependent gene expression in bacteria. J. Biotechnol. 160: Konkel M. E., and K. Tilly Temperature-regulated expression of bacterial virulence genes. Microbes Infect. Inst. Pasteur 2:
176 82. Kortmann J., and F. Narberhaus Bacterial RNA thermometers: molecular zippers and switches. Nat. Rev. Microbiol. 10: Kotloff K. L., J. P. Winickoff, B. Ivanoff, J. D. Clemens, D. L. Swerdlow, P. J. Sansonetti, G. K. Adak, and M. M. Levine Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull. World Health Organ. 77: Kouse A. B., F. Righetti, J. Kortmann, F. Narberhaus, and E. R. Murphy RNA-mediated thermoregulation of iron-acquisition genes in Shigella dysenteriae and pathogenic Escherichia coli. PloS One 8:e Krajewski S. S., M. Nagel, and F. Narberhaus Short ROSE-like RNA thermometers control IbpA synthesis in Pseudomonas species. PloS One 8:e Langklotz S., and F. Narberhaus The Escherichia coli replication inhibitor CspD is subject to growth-regulated degradation by the Lon protease. Mol. Microbiol. 80: Lawlor K. M., and S. M. Payne Aerobactin genes in Shigella spp. J. Bacteriol. 160: De Lay N., D. J. Schu, and S. Gottesman Bacterial small RNA-based negative regulation: Hfq and its accomplices. J. Biol. Chem. 288: Leggett H. C., C. K. Cornwallis, and S. A. West Mechanisms of Pathogenesis, Infective Dose and Virulence in Human Parasites. PLoS Pathog. 8:e Lin J., I. S. Lee, J. Frey, J. L. Slonczewski, and J. W. Foster Comparative analysis of extreme acid survival in Salmonella typhimurium, Shigella flexneri, and Escherichia coli. J. Bacteriol. 177: Lioliou E., C. Romilly, P. Romby, and P. Fechter RNA-mediated regulation in bacteria: from natural to artificial systems. New Biotechnol. 27: Liu J. M., and A. Camilli A broadening world of bacterial small RNAs. Curr. Opin. Microbiol. 13: Mabbott N. A., D. S. Donaldson, H. Ohno, I. R. Williams, and A. Mahajan Microfold (M) cells: important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol. 6: Malpica R., G. R. P. Sandoval, C. Rodríguez, B. Franco, and D. Georgellis Signaling by the arc two-component system provides a link between the redox state of the quinone pool and gene expression. Antioxid. Redox Signal. 8: Mandal M., and R. R. Breaker Gene regulation by riboswitches. Nat. Rev. Mol. Cell Biol. 5: Marteyn B., A. Gazi, and P. Sansonetti Shigella: a model of virulence regulation in vivo. Gut Microbes 3: Mason J. R., and R. Cammack The electron-transport proteins of hydroxylating bacterial dioxygenases. Annu. Rev. Microbiol. 46: Masse E Regulatory roles for small RNAs in bacteria. Curr. Opin. Microbiol. 6:
177 99. Masuda N., and G. M. Church Escherichia coli gene expression responsive to levels of the response regulator EvgA. J. Bacteriol. 184: Matsuoka Y., and K. Shimizu Metabolic regulation in Escherichia coli in response to culture environments via global regulators. Biotechnol. J. 6: Mills M., and S. M. Payne Genetics and regulation of heme iron transport in Shigella dysenteriae and detection of an analogous system in Escherichia coli O157:H7. J. Bacteriol. 177: Mills M., and S. M. Payne Identification of shua, the gene encoding the heme receptor of Shigella dysenteriae, and analysis of invasion and intracellular multiplication of a shua mutant. Infect. Immun. 65: Mitobe J., T. Morita-Ishihara, A. Ishihama, and H. Watanabe Involvement of RNA-binding Protein Hfq in the Post-transcriptional Regulation of inve Gene Expression in Shigella sonnei. J. Biol. Chem. 283: Müller S. I., M. Valdebenito, and K. Hantke Salmochelin, the longoverlooked catecholate siderophore of Salmonella. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 22: Muga A., and F. Moro Thermal adaptation of heat shock proteins. Curr. Protein Pept. Sci. 9: Murphy E. R., and S. M. Payne RyhB, an iron-responsive small RNA molecule, regulates Shigella dysenteriae virulence. Infect. Immun. 75: Murphy E. R., R. E. Sacco, A. Dickenson, D. J. Metzger, Y. Hu, P. E. Orndorff, and T. D. Connell BhuR, a virulence-associated outer membrane protein of Bordetella avium, is required for the acquisition of iron from heme and hemoproteins. Infect. Immun. 70: Nairz M., A. Schroll, T. Sonnweber, and G. Weiss The struggle for iron - a metal at the host-pathogen interface. Cell. Microbiol. 12: Narberhaus F., T. Waldminghaus, and S. Chowdhury RNA thermometers. FEMS Microbiol. Rev. 30: Niyogi S. K Shigellosis. J. Microbiol. Seoul Korea 43: Ochsner U. A., Z. Johnson, and M. L. Vasil Genetics and regulation of two distinct haem-uptake systems, phu and has, in Pseudomonas aeruginosa. Microbiol. Read. Engl. 146 ( Pt 1): Oglesby-Sherrouse A. G., and E. R. Murphy Iron-responsive bacterial small RNAs: variations on a theme. Met. Integr. Biometal Sci. 5: Oh M. H., S. M. Lee, D. H. Lee, and S. H. Choi Regulation of the Vibrio vulnificus hupa gene by temperature alteration and cyclic AMP receptor protein and evaluation of its role in virulence. Infect. Immun. 77: Ohnishi T., V. D. Sled, T. Yano, T. Yagi, D. S. Burbaev, and A. D. Vinogradov Structure-function studies of iron-sulfur clusters and semiquinones in the NADH-Q oxidoreductase segment of the respiratory chain. Biochim. Biophys. Acta 1365:
178 115. Okeke I. N., I. C. A. Scaletsky, E. H. Soars, L. R. Macfarlane, and A. G. Torres Molecular epidemiology of the iron utilization genes of enteroaggregative Escherichia coli. J. Clin. Microbiol. 42: Ong S. T., J. Z. S. Ho, B. Ho, and J. L. Ding Iron-withholding strategy in innate immunity. Immunobiology 211: Otto B. R., A. M. Verweij-van Vught, and D. M. MacLaren Transferrins and heme-compounds as iron sources for pathogenic bacteria. Crit. Rev. Microbiol. 18: Paradis S., M. Boissinot, N. Paquette, S. D. Bélanger, E. A. Martel, D. K. Boudreau, F. J. Picard, M. Ouellette, P. H. Roy, and M. G. Bergeron Phylogeny of the Enterobacteriaceae based on genes encoding elongation factor Tu and F-ATPase beta-subunit. Int. J. Syst. Evol. Microbiol. 55: Paulley J. T., E. S. Anderson, and R. M. Roop Brucella abortus requires the heme transporter BhuA for maintenance of chronic infection in BALB/c mice. Infect. Immun. 75: Payne S. M Synthesis and utilization of siderophores by Shigella flexneri. J. Bacteriol. 143: Payne S. M., D. W. Niesel, S. S. Peixotto, and K. M. Lawlor Expression of hydroxamate and phenolate siderophores by Shigella flexneri. J. Bacteriol. 155: Payne S. M., E. E. Wyckoff, E. R. Murphy, A. G. Oglesby, M. L. Boulette, and N. M. L. Davies Iron and Pathogenesis of Shigella: Iron Acquisition in the Intracellular Environment. BioMetals 19: Phadtare S., and K. Severinov Comparative analysis of changes in gene expression due to RNA melting activities of translation initiation factor IF1 and a cold shock protein of the CspA family. Genes Cells Devoted Mol. Cell. Mech. 14: Phadtare S., and K. Severinov RNA remodeling and gene regulation by cold shock proteins. RNA Biol. 7: Prosseda G., M. Falconi, M. Giangrossi, C. O. Gualerzi, G. Micheli, and B. Colonna The virf promoter in Shigella: more than just a curved DNA stretch. Mol. Microbiol. 51: Quade N., C. Mendonca, K. Herbst, A. K. Heroven, C. Ritter, D. W. Heinz, and P. Dersch Structural basis for intrinsic thermosensing by the master virulence regulator RovA of Yersinia. J. Biol. Chem. 287: Rinnenthal J., B. Klinkert, F. Narberhaus, and H. Schwalbe Direct observation of the temperature-induced melting process of the Salmonella fouru RNA thermometer at base-pair resolution. Nucleic Acids Res. 38: Rogers H. J Iron-Binding Catechols and Virulence in Escherichia coli. Infect. Immun. 7: Romby P., and E. Charpentier An overview of RNAs with regulatory functions in gram-positive bacteria. Cell. Mol. Life Sci. CMLS 67:
179 130. Romeo T., C. A. Vakulskas, and P. Babitzke Post-transcriptional regulation on a global scale: form and function of Csr/Rsm systems. Environ. Microbiol. 15: Rossi M. S., J. D. Fetherston, S. Létoffé, E. Carniel, R. D. Perry, and J. M. Ghigo Identification and characterization of the hemophore-dependent heme acquisition system of Yersinia pestis. Infect. Immun. 69: Rouault T. A., and R. D. Klausner Iron-sulfur clusters as biosensors of oxidants and iron. Trends Biochem. Sci. 21: Runyen-Janecky L. J., S. A. Reeves, E. G. Gonzales, and S. M. Payne Contribution of the Shigella flexneri Sit, Iuc, and Feo iron acquisition systems to iron acquisition in vitro and in cultured cells. Infect. Immun. 71: Runyen-Janecky L., E. Dazenski, S. Hawkins, and L. Warner Role and regulation of the Shigella flexneri sit and MntH systems. Infect. Immun. 74: Sansonetti P. J Slaying the Hydra all at once or head by head? Nat. Med. 4: Schmitt M. P., and S. M. Payne Genetic analysis of the enterobactin gene cluster in Shigella flexneri. J. Bacteriol. 173: Schmitt M. P., and S. M. Payne Genetics and regulation of enterobactin genes in Shigella flexneri. J. Bacteriol. 170: Schroeder G. N., and H. Hilbi Molecular Pathogenesis of Shigella spp.: Controlling Host Cell Signaling, Invasion, and Death by Type III Secretion. Clin. Microbiol. Rev. 21: Skaar E. P The battle for iron between bacterial pathogens and their vertebrate hosts. PLoS Pathog. 6:e Smith A. M., R. T. Fuchs, F. J. Grundy, and T. M. Henkin Riboswitch RNAs: regulation of gene expression by direct monitoring of a physiological signal. RNA Biol. 7: Smith S. G. J., V. Mahon, M. A. Lambert, and R. P. Fagan A molecular Swiss army knife: OmpA structure, function and expression. FEMS Microbiol. Lett. 273: Storz G., J. Vogel, and K. M. Wassarman Regulation by small RNAs in bacteria: expanding frontiers. Mol. Cell 43: Suits M. D. L., N. Jaffer, and Z. Jia Structure of the Escherichia coli O157:H7 heme oxygenase ChuS in complex with heme and enzymatic inactivation by mutation of the heme coordinating residue His-193. J. Biol. Chem. 281: Suits M. D. L., J. Lang, G. P. Pal, M. Couture, and Z. Jia Structure and heme binding properties of Escherichia coli O157:H7 ChuX. Protein Sci. Publ. Protein Soc. 18: Suits M. D. L., G. P. Pal, K. Nakatsu, A. Matte, M. Cygler, and Z. Jia Identification of an Escherichia coli O157:H7 heme oxygenase with tandem functional repeats. Proc. Natl. Acad. Sci. U. S. A. 102:
180 146. Tendeng C., and P. N. Bertin H-NS in Gram-negative bacteria: a family of multifaceted proteins. Trends Microbiol. 11: Tobe T., S. Nagai, N. Okada, B. Adler, M. Yoshikawa, and C. Sasakawa Temperature-regulated expression of invasion genes in Shigella flexneri is controlled through the transcriptional activation of the virb gene on the large plasmid. Mol. Microbiol. 5: Tobe T., M. Yoshikawa, T. Mizuno, and C. Sasakawa Transcriptional control of the invasion regulatory gene virb of Shigella flexneri: activation by virf and repression by H-NS. J. Bacteriol. 175: Tobe T [Modulation of virulence expression in Escherichia coli and Shigella spp. by environmental factors]. Nihon Saikingaku Zasshi Jpn. J. Bacteriol. 62: Torres A. G., and S. M. Payne Haem iron-transport system in enterohaemorrhagic Escherichia coli O157:H7. Mol. Microbiol. 23: Torres A. G., P. Redford, R. A. Welch, and S. M. Payne TonBdependent systems of uropathogenic Escherichia coli: aerobactin and heme transport and TonB are required for virulence in the mouse. Infect. Immun. 69: Tran C. N., M. Giangrossi, G. Prosseda, A. Brandi, M. L. Di Martino, B. Colonna, and M. Falconi A multifactor regulatory circuit involving H-NS, VirF and an antisense RNA modulates transcription of the virulence gene icsa of Shigella flexneri. Nucleic Acids Res. 39: Urban J. H., and J. Vogel Translational control and target recognition by Escherichia coli small RNAs in vivo. Nucleic Acids Res. 35: Venkatesan M. M., M. B. Goldberg, D. J. Rose, E. J. Grotbeck, V. Burland, and F. R. Blattner Complete DNA Sequence and Analysis of the Large Virulence Plasmid of Shigella flexneri. Infect. Immun. 69: Viegas S. C., I. J. Silva, M. Saramago, S. Domingues, and C. M. Arraiano Regulation of the small regulatory RNA MicA by ribonuclease III: a targetdependent pathway. Nucleic Acids Res. 39: Waldminghaus T., A. Fippinger, J. Alfsmann, and F. Narberhaus RNA thermometers are common in alpha- and gamma-proteobacteria. Biol. Chem. 386: Waldminghaus T., L. C. Gaubig, B. Klinkert, and F. Narberhaus The Escherichia coli ibpa thermometer is comprised of stable and unstable structural elements. RNA Biol. 6: Waldminghaus T., L. C. Gaubig, and F. Narberhaus Genome-wide bioinformatic prediction and experimental evaluation of potential RNA thermometers. Mol. Genet. Genomics MGG 278: Waldminghaus T., N. Heidrich, S. Brantl, and F. Narberhaus FourU: a novel type of RNA thermometer in Salmonella. Mol. Microbiol. 65: Waldminghaus T., N. Heidrich, S. Brantl, and F. Narberhaus FourU: a novel type of RNA thermometer in Salmonella. Mol. Microbiol. 65:
181 161. Wandersman C., and P. Delepelaire Bacterial iron sources: from siderophores to hemophores. Annu. Rev. Microbiol. 58: Waters L. S., and G. Storz Regulatory RNAs in Bacteria. Cell 136: Webster K., and E. Schnitzler Hemolytic uremic syndrome. Handb. Clin. Neurol. 120: Wilks A The ShuS protein of Shigella dysenteriae is a heme-sequestering protein that also binds DNA. Arch. Biochem. Biophys. 387: Wyckoff E. E., D. Duncan, A. G. Torres, M. Mills, K. Maase, and S. M. Payne Structure of the Shigella dysenteriae haem transport locus and its phylogenetic distribution in enteric bacteria. Mol. Microbiol. 28: Wyckoff E. E., D. Duncan, A. G. Torres, M. Mills, K. Maase, and S. M. Payne Structure of the Shigella dysenteriae haem transport locus and its phylogenetic distribution in enteric bacteria. Mol. Microbiol. 28: Wyckoff E. E., M. L. Boulette, and S. M. Payne Genetics and environmental regulation of Shigella iron transport systems. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 22: Wyckoff E. E., G. F. Lopreato, K. A. Tipton, and S. M. Payne Shigella dysenteriae ShuS promotes utilization of heme as an iron source and protects against heme toxicity. J. Bacteriol. 187: Yamanaka K., W. Zheng, E. Crooke, Y. H. Wang, and M. Inouye CspD, a novel DNA replication inhibitor induced during the stationary phase in Escherichia coli. Mol. Microbiol. 39: Yang F., J. Yang, X. Zhang, L. Chen, Y. Jiang, Y. Yan, X. Tang, J. Wang, Z. Xiong, J. Dong, Y. Xue, Y. Zhu, X. Xu, L. Sun, S. Chen, H. Nie, J. Peng, J. Xu, Y. Wang, Z. Yuan, Y. Wen, Z. Yao, Y. Shen, B. Qiang, Y. Hou, J. Yu, and Q. Jin Genome dynamics and diversity of Shigella species, the etiologic agents of bacillary dysentery. Nucleic Acids Res. 33: Yang J., M. Tauschek, and R. M. Robins-Browne Control of bacterial virulence by AraC-like regulators that respond to chemical signals. Trends Microbiol. 19: Ye F., T. Brauer, E. Niehus, K. Drlica, C. Josenhans, and S. Suerbaum Flagellar and global gene regulation in Helicobacter pylori modulated by changes in DNA supercoiling. Int. J. Med. Microbiol. IJMM 297: Zhang Y., and K. Lin A phylogenomic analysis of Escherichia coli / Shigella group: implications of genomic features associated with pathogenicity and ecological adaptation. BMC Evol. Biol. 12:
182 !!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!! Thesis and Dissertation Services!
Practice Problems 5. Location of LSA-GFP fluorescence
 Life Sciences 1a Practice Problems 5 1. Soluble proteins that are normally secreted from the cell into the extracellular environment must go through a series of steps referred to as the secretory pathway.
Life Sciences 1a Practice Problems 5 1. Soluble proteins that are normally secreted from the cell into the extracellular environment must go through a series of steps referred to as the secretory pathway.
BA, BSc, and MSc Degree Examinations
 Examination Candidate Number: Desk Number: BA, BSc, and MSc Degree Examinations 2017-8 Department : BIOLOGY Title of Exam: Bacterial pathogenesis Time Allowed: 2 hours Marking Scheme: Total marks available
Examination Candidate Number: Desk Number: BA, BSc, and MSc Degree Examinations 2017-8 Department : BIOLOGY Title of Exam: Bacterial pathogenesis Time Allowed: 2 hours Marking Scheme: Total marks available
Analysis of Shigella strains by Pulsed Field Gel Electrophoresis
 Analysis of Shigella strains by Pulsed Field Gel Electrophoresis Kim N. Pham Public Health Internship Program School of Biological Sciences The University of Texas at Austin Mentor: Ana Maria Valle-Rivera,
Analysis of Shigella strains by Pulsed Field Gel Electrophoresis Kim N. Pham Public Health Internship Program School of Biological Sciences The University of Texas at Austin Mentor: Ana Maria Valle-Rivera,
Viruses. Chapter 19. Biology Eighth Edition Neil Campbell and Jane Reece. PowerPoint Lecture Presentations for
 Chapter 19 Viruses PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from Joan Sharp Copyright
Chapter 19 Viruses PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from Joan Sharp Copyright
Lecture Series 10 The Genetics of Viruses and Prokaryotes
 Lecture Series 10 The Genetics of Viruses and Prokaryotes The Genetics of Viruses and Prokaryotes A. Using Prokaryotes and Viruses for Genetic Experiments B. Viruses: Reproduction and Recombination C.
Lecture Series 10 The Genetics of Viruses and Prokaryotes The Genetics of Viruses and Prokaryotes A. Using Prokaryotes and Viruses for Genetic Experiments B. Viruses: Reproduction and Recombination C.
Viral Genomes. Genomes may consist of: 1. Double Stranded DNA 2. Double Stranded RNA 3. Single-stranded RNA 4. Single-stranded DNA
 Chapter 19 Viral Genomes Genomes may consist of: 1. Double Stranded DNA 2. Double Stranded RNA 3. Single-stranded RNA 4. Single-stranded DNA Genome is usually organized as a single linear or circular molecule
Chapter 19 Viral Genomes Genomes may consist of: 1. Double Stranded DNA 2. Double Stranded RNA 3. Single-stranded RNA 4. Single-stranded DNA Genome is usually organized as a single linear or circular molecule
Bi 8 Lecture 10. Ellen Rothenberg 4 February 2016
 Bi 8 Lecture 10 Bacterial regulation, II Ellen Rothenberg 4 February 2016 Not all bacterial promoters use the same σ factors, and this provides added regulation capability Most sigma factors are related
Bi 8 Lecture 10 Bacterial regulation, II Ellen Rothenberg 4 February 2016 Not all bacterial promoters use the same σ factors, and this provides added regulation capability Most sigma factors are related
Biotechnology and DNA Technology
 11/27/2017 PowerPoint Lecture Presentations prepared by Bradley W. Christian, McLennan Community College CHAPTER 9 Biotechnology and DNA Technology Introduction to Biotechnology Learning Objectives Compare
11/27/2017 PowerPoint Lecture Presentations prepared by Bradley W. Christian, McLennan Community College CHAPTER 9 Biotechnology and DNA Technology Introduction to Biotechnology Learning Objectives Compare
Lac Operon contains three structural genes and is controlled by the lac repressor: (1) LacY protein transports lactose into the cell.
 Regulation of gene expression a. Expression of most genes can be turned off and on, usually by controlling the initiation of transcription. b. Lactose degradation in E. coli (Negative Control) Lac Operon
Regulation of gene expression a. Expression of most genes can be turned off and on, usually by controlling the initiation of transcription. b. Lactose degradation in E. coli (Negative Control) Lac Operon
There are 100 possible points on this exam. THIS EXAM IS CLOSED BOOK. 1. (6 points) Distinguish between the innate and adaptive immune responses:
 BENG 100b: Frontiers in Biomedical Engineering Midterm Examination 28 February 2006 There are 100 possible points on this exam. THIS EXAM IS CLOSED BOOK. SHORT ANSWER (Total=70 points) Read the questions
BENG 100b: Frontiers in Biomedical Engineering Midterm Examination 28 February 2006 There are 100 possible points on this exam. THIS EXAM IS CLOSED BOOK. SHORT ANSWER (Total=70 points) Read the questions
Chapter 18. Viral Genetics. AP Biology
 Chapter 18. Viral Genetics AP Biology What is a virus? Is it alive? DNA or RNA enclosed in a protein coat Viruses are not cells Extremely tiny electron microscope size smaller than ribosomes ~20 50 nm
Chapter 18. Viral Genetics AP Biology What is a virus? Is it alive? DNA or RNA enclosed in a protein coat Viruses are not cells Extremely tiny electron microscope size smaller than ribosomes ~20 50 nm
Bacterial Genetics. Prof. Dr. Asem Shehabi Faculty of Medicine University of Jordan
 Bacterial Genetics Prof. Dr. Asem Shehabi Faculty of Medicine University of Jordan Bacterial Genes-1 All patterns of growth, metabolism, essential cellular structures, biological characteristics of bacteria
Bacterial Genetics Prof. Dr. Asem Shehabi Faculty of Medicine University of Jordan Bacterial Genes-1 All patterns of growth, metabolism, essential cellular structures, biological characteristics of bacteria
LESSON ONE: BAD BACTERIA
 LESSON ONE: BAD BACTERIA KEY QUESTION(S): What are virulence factors? How do bacteria use virulence factors to infect an organism and cause disease? How do bacteria gain or lose virulence factors? OVERALL
LESSON ONE: BAD BACTERIA KEY QUESTION(S): What are virulence factors? How do bacteria use virulence factors to infect an organism and cause disease? How do bacteria gain or lose virulence factors? OVERALL
Virus- infectious particle consisting of nucleic acid packaged in a protein coat.
 Chapter 19 Virus- infectious particle consisting of nucleic acid packaged in a protein coat. Most scientists consider viruses non-living because they cannot reproduce or carry out metabolic activities
Chapter 19 Virus- infectious particle consisting of nucleic acid packaged in a protein coat. Most scientists consider viruses non-living because they cannot reproduce or carry out metabolic activities
BACTERIOPHAGES: STRUCTURE AND PROPERTIES OF BACTERIAL VIRUSES
 BACTERIOPHAGES: STRUCTURE AND PROPERTIES OF BACTERIAL VIRUSES Bacteriophage (phage) are obligate intracellular parasites that multiply inside bacteria by making use of some or all of the host biosynthetic
BACTERIOPHAGES: STRUCTURE AND PROPERTIES OF BACTERIAL VIRUSES Bacteriophage (phage) are obligate intracellular parasites that multiply inside bacteria by making use of some or all of the host biosynthetic
Motivation From Protein to Gene
 MOLECULAR BIOLOGY 2003-4 Topic B Recombinant DNA -principles and tools Construct a library - what for, how Major techniques +principles Bioinformatics - in brief Chapter 7 (MCB) 1 Motivation From Protein
MOLECULAR BIOLOGY 2003-4 Topic B Recombinant DNA -principles and tools Construct a library - what for, how Major techniques +principles Bioinformatics - in brief Chapter 7 (MCB) 1 Motivation From Protein
Name AP Biology Mrs. Laux Take home test #11 on Chapters 14, 15, and 17 DUE: MONDAY, DECEMBER 21, 2009
 MULTIPLE CHOICE QUESTIONS 1. Inducible genes are usually actively transcribed when: A. the molecule degraded by the enzyme(s) is present in the cell. B. repressor molecules bind to the promoter. C. lactose
MULTIPLE CHOICE QUESTIONS 1. Inducible genes are usually actively transcribed when: A. the molecule degraded by the enzyme(s) is present in the cell. B. repressor molecules bind to the promoter. C. lactose
7.1 The lac Operon 7-1
 7.1 The lac Operon The lac operon was the first operon discovered It contains 3 genes coding for E. coli proteins that permit the bacteria to use the sugar lactose Galactoside permease (lacy) which transports
7.1 The lac Operon The lac operon was the first operon discovered It contains 3 genes coding for E. coli proteins that permit the bacteria to use the sugar lactose Galactoside permease (lacy) which transports
Chapter 13A: Viral Basics
 Chapter 13A: Viral Basics 1. Viral Structure 2. The Viral Life Cycle 3. Bacteriophages 1. Viral Structure What exactly is a Virus? Viruses are extremely small entities that are obligate intracellular parasites
Chapter 13A: Viral Basics 1. Viral Structure 2. The Viral Life Cycle 3. Bacteriophages 1. Viral Structure What exactly is a Virus? Viruses are extremely small entities that are obligate intracellular parasites
Chapter 15 Gene Regulation in Prokaryotes
 Chapter 15 Gene Regulation in Prokaryotes 17-1 Sections to study 15.1 The elements of prokaryotic gene expression 15.2 Regulation of transcription initiation via DNA-binding proteins 15.3 RNA-mediated
Chapter 15 Gene Regulation in Prokaryotes 17-1 Sections to study 15.1 The elements of prokaryotic gene expression 15.2 Regulation of transcription initiation via DNA-binding proteins 15.3 RNA-mediated
1/24/2012. Cell. Plasma Membrane
 Chapter 3 Outline Plasma Membrane Cytoplasm and Its Organelles Cell and Gene Expression Protein Synthesis and Secretion DNA Synthesis and Cell Division Cell Basic unit of structure and function in body
Chapter 3 Outline Plasma Membrane Cytoplasm and Its Organelles Cell and Gene Expression Protein Synthesis and Secretion DNA Synthesis and Cell Division Cell Basic unit of structure and function in body
Viruses 11/30/2015. Chapter 19. Key Concepts in Chapter 19
 Chapter 19 Viruses Dr. Wendy Sera Houston Community College Biology 1406 Key Concepts in Chapter 19 1. A virus consists of a nucleic acid surrounded by a protein coat. 2. Viruses replicate only in host
Chapter 19 Viruses Dr. Wendy Sera Houston Community College Biology 1406 Key Concepts in Chapter 19 1. A virus consists of a nucleic acid surrounded by a protein coat. 2. Viruses replicate only in host
Pathogenic Microorganism in Food. Marlia Singgih Wibowo School of Pharmacy ITB
 Pathogenic Microorganism in Food Marlia Singgih Wibowo School of Pharmacy ITB Food Microbiology Analysis Microbiological analysis is important to determine the safety and quality of food. For many years,
Pathogenic Microorganism in Food Marlia Singgih Wibowo School of Pharmacy ITB Food Microbiology Analysis Microbiological analysis is important to determine the safety and quality of food. For many years,
Control of Metabolic Processes
 Control of Metabolic Processes Harriet Wilson, Lecture Notes Bio. Sci. 4 - Microbiology Sierra College As described earlier, the metabolic processes occurring within living organisms (glycolysis, respiration,
Control of Metabolic Processes Harriet Wilson, Lecture Notes Bio. Sci. 4 - Microbiology Sierra College As described earlier, the metabolic processes occurring within living organisms (glycolysis, respiration,
Name 10 Molecular Biology of the Gene Test Date Study Guide You must know: The structure of DNA. The major steps to replication.
 Name 10 Molecular Biology of the Gene Test Date Study Guide You must know: The structure of DNA. The major steps to replication. The difference between replication, transcription, and translation. How
Name 10 Molecular Biology of the Gene Test Date Study Guide You must know: The structure of DNA. The major steps to replication. The difference between replication, transcription, and translation. How
Molecular Genetics Student Objectives
 Molecular Genetics Student Objectives Exam 1: Enduring understanding 3.A: Heritable information provides for continuity of life. Essential knowledge 3.A.1: DNA, and in some cases RNA, is the primary source
Molecular Genetics Student Objectives Exam 1: Enduring understanding 3.A: Heritable information provides for continuity of life. Essential knowledge 3.A.1: DNA, and in some cases RNA, is the primary source
Viruses and Bacteria Notes
 Viruses and Bacteria Notes A. Virus Structure: Viruses are in contrast to bacteria. Viruses are (DNA or RNA) enclosed in a coat called a. Also some viruses have a that helps them infect their host. These
Viruses and Bacteria Notes A. Virus Structure: Viruses are in contrast to bacteria. Viruses are (DNA or RNA) enclosed in a coat called a. Also some viruses have a that helps them infect their host. These
CHAPTER 13 LECTURE SLIDES
 CHAPTER 13 LECTURE SLIDES Prepared by Brenda Leady University of Toledo To run the animations you must be in Slideshow View. Use the buttons on the animation to play, pause, and turn audio/text on or off.
CHAPTER 13 LECTURE SLIDES Prepared by Brenda Leady University of Toledo To run the animations you must be in Slideshow View. Use the buttons on the animation to play, pause, and turn audio/text on or off.
Chapter 10 Microbial Genetics: New Genes for Old Germs
 Chapter 10 Microbial Genetics: New Genes for Old Germs Objectives: After reading Chapter Ten, you should understand The structure and complexity of the bacterial chromosome and the significance of plasmids.
Chapter 10 Microbial Genetics: New Genes for Old Germs Objectives: After reading Chapter Ten, you should understand The structure and complexity of the bacterial chromosome and the significance of plasmids.
5. the transformation of the host cell. 2. reject the virus. 4. initiate an attack on the virus.
 Version 001 Bacterial/Viral Genetics mahon (26) 1 This print-out should have 28 questions. Multiple-choice questions may continue on the next column or page find all choices before answering. Holt Bio
Version 001 Bacterial/Viral Genetics mahon (26) 1 This print-out should have 28 questions. Multiple-choice questions may continue on the next column or page find all choices before answering. Holt Bio
Link between iron homeostasis, oxidative mutagenesis and antibiotic resistance evolution
 Thesis of the PhD dissertation Link between iron homeostasis, oxidative mutagenesis and antibiotic resistance evolution Orsolya Katinka Méhi Supervisor: Dr. Csaba Pál, senior research associate PhD School
Thesis of the PhD dissertation Link between iron homeostasis, oxidative mutagenesis and antibiotic resistance evolution Orsolya Katinka Méhi Supervisor: Dr. Csaba Pál, senior research associate PhD School
Bacterial Genetics. Stijn van der Veen
 Bacterial Genetics Stijn van der Veen Differentiating bacterial species Morphology (shape) Composition (cell envelope and other structures) Metabolism & growth characteristics Genetics Differentiating
Bacterial Genetics Stijn van der Veen Differentiating bacterial species Morphology (shape) Composition (cell envelope and other structures) Metabolism & growth characteristics Genetics Differentiating
Unit 1 Human cells. 1. Division and differentiation in human cells
 Unit 1 Human cells 1. Division and differentiation in human cells Stem cells Describe the process of differentiation. Explain how differentiation is brought about with reference to genes. Name the two
Unit 1 Human cells 1. Division and differentiation in human cells Stem cells Describe the process of differentiation. Explain how differentiation is brought about with reference to genes. Name the two
LECTURE PRESENTATIONS
 LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 19 Viruses Lectures by Erin Barley
LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 19 Viruses Lectures by Erin Barley
Genetics Lecture 21 Recombinant DNA
 Genetics Lecture 21 Recombinant DNA Recombinant DNA In 1971, a paper published by Kathleen Danna and Daniel Nathans marked the beginning of the recombinant DNA era. The paper described the isolation of
Genetics Lecture 21 Recombinant DNA Recombinant DNA In 1971, a paper published by Kathleen Danna and Daniel Nathans marked the beginning of the recombinant DNA era. The paper described the isolation of
BACTERIAL GENETICS. How does the DNA in the bacterial cell replicate
 BACTERIAL GENETICS Bacterial genetics is the study of gene structure and function in bacteria. Genetics itself is concerned with determining the number, location, and character of the genes of an organism.
BACTERIAL GENETICS Bacterial genetics is the study of gene structure and function in bacteria. Genetics itself is concerned with determining the number, location, and character of the genes of an organism.
1 Name. 1. (3 pts) What is apoptosis and how does it differ from necrosis? Which is more likely to trigger inflammation?
 1 Name MCB 150 Midterm Eam #1 (100 points total) Please write your full name on each page of the eam!! The eam consists of 17 questions (6 pages). Each has a different point count as indicated. Please
1 Name MCB 150 Midterm Eam #1 (100 points total) Please write your full name on each page of the eam!! The eam consists of 17 questions (6 pages). Each has a different point count as indicated. Please
Genetics Lecture Notes Lectures 17 19
 Genetics Lecture Notes 7.03 2005 Lectures 17 19 Lecture 17 Gene Regulation We are now going to look at ways that genetics can be used to study gene regulation. The issue is how cells adjust the expression
Genetics Lecture Notes 7.03 2005 Lectures 17 19 Lecture 17 Gene Regulation We are now going to look at ways that genetics can be used to study gene regulation. The issue is how cells adjust the expression
CHAPTER 20 DNA TECHNOLOGY AND GENOMICS. Section A: DNA Cloning
 Section A: DNA Cloning 1. DNA technology makes it possible to clone genes for basic research and commercial applications: an overview 2. Restriction enzymes are used to make recombinant DNA 3. Genes can
Section A: DNA Cloning 1. DNA technology makes it possible to clone genes for basic research and commercial applications: an overview 2. Restriction enzymes are used to make recombinant DNA 3. Genes can
Course Descriptions. BIOL: Biology. MICB: Microbiology. [1]
![Course Descriptions. BIOL: Biology. MICB: Microbiology. [1] Course Descriptions. BIOL: Biology. MICB: Microbiology. [1]](/thumbs/72/66855241.jpg) Course Descriptions BIOL: Biology http://www.calendar.ubc.ca/vancouver/courses.cfm?code=biol [1] BIOL 112 (3) Biology of the Cell The principles of cellular and molecular biology using bacterial and eukaryotic
Course Descriptions BIOL: Biology http://www.calendar.ubc.ca/vancouver/courses.cfm?code=biol [1] BIOL 112 (3) Biology of the Cell The principles of cellular and molecular biology using bacterial and eukaryotic
March 15, Genetics_of_Viruses_and_Bacteria_p5.notebook. smallest viruses are smaller than ribosomes. A virulent phage (Lytic)
 Genetics_of_Viruses_and_Bacteria_p5.notebook smallest viruses are smaller than ribosomes Adenovirus Tobacco mosaic virus Bacteriophage Influenza virus envelope is derived from the host cell The capsids
Genetics_of_Viruses_and_Bacteria_p5.notebook smallest viruses are smaller than ribosomes Adenovirus Tobacco mosaic virus Bacteriophage Influenza virus envelope is derived from the host cell The capsids
Session 3 Lecture 1 Dynamics of the GIT Microbiome: Microbial Darwinism
 Session 3 Lecture 1 Dynamics of the GIT Microbiome: Microbial Darwinism 10 14 bacteria representing ~1000 species and many phyla live in a crowded ecological space: The GIT Four major phyla dominate: By
Session 3 Lecture 1 Dynamics of the GIT Microbiome: Microbial Darwinism 10 14 bacteria representing ~1000 species and many phyla live in a crowded ecological space: The GIT Four major phyla dominate: By
Chapter 5. Genetic Models. Organization and Expression of Immunoglobulin Genes 3. The two-gene model: Models to Explain Antibody Diversity
 Chapter 5 Organization and Expression of Immunoglobulin Genes 3 4 5 6 Genetic Models How to account for: ) Vast diversity of antibody specificities ) Presence of Variable regions at the amino end of Heavy
Chapter 5 Organization and Expression of Immunoglobulin Genes 3 4 5 6 Genetic Models How to account for: ) Vast diversity of antibody specificities ) Presence of Variable regions at the amino end of Heavy
Mechanisms of Genetic Variation. Copyright McGraw-Hill Global Education Holdings, LLC. Permission required for reproduction or display.
 16 Mechanisms of Genetic Variation Copyright McGraw-Hill Global Education Holdings, LLC. Permission required for reproduction or display. 1 Mutations: Their Chemical Basis and Effects Stable, heritable
16 Mechanisms of Genetic Variation Copyright McGraw-Hill Global Education Holdings, LLC. Permission required for reproduction or display. 1 Mutations: Their Chemical Basis and Effects Stable, heritable
Thank you and good morning.
 Thank you and good morning. 1 I have no conflicts of interest to disclose. 2 I m going to speak today about basic science research into group B strep virulence. GBS remains an important cause of neonatal
Thank you and good morning. 1 I have no conflicts of interest to disclose. 2 I m going to speak today about basic science research into group B strep virulence. GBS remains an important cause of neonatal
ANTIBODIES. Agents of Immunity
 ANTIBODIES Agents of Immunity - Antibodies are: The Organization What are they? Protective agents of the immune system Neutralize foreign agents called antigens Essential part of the Adaptive Immune System
ANTIBODIES Agents of Immunity - Antibodies are: The Organization What are they? Protective agents of the immune system Neutralize foreign agents called antigens Essential part of the Adaptive Immune System
GENE REGULATION IN PROKARYOTES
 GENE REGULATION IN PROKARYOTES Prepared by Brenda Leady, University of Toledo Copyright (c) The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Gene regulation refers to
GENE REGULATION IN PROKARYOTES Prepared by Brenda Leady, University of Toledo Copyright (c) The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Gene regulation refers to
Viruses. Chapter 19. Biology Eighth Edition Neil Campbell and Jane Reece. PowerPoint Lecture Presentations for
 Chapter 19 Viruses PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from Joan Sharp Copyright
Chapter 19 Viruses PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from Joan Sharp Copyright
1. ADHERE AND DEFEND: Our bacterium has entered the host. Now it needs to adhere and get past the normal microbiota.
 North Seattle College Stage 02 Colonization and Infection This explanatory model will tell the story of how one bacterium adheres to a host and, through binary fission, ends up making two daughter cells.
North Seattle College Stage 02 Colonization and Infection This explanatory model will tell the story of how one bacterium adheres to a host and, through binary fission, ends up making two daughter cells.
Regulation of metabolic pathways
 Regulation of metabolic pathways Bacterial control of gene expression Operon: cluster of related genes with on/off switch Three Parts: 1. Promoter where RNA polymerase attaches 2. Operator on/off, controls
Regulation of metabolic pathways Bacterial control of gene expression Operon: cluster of related genes with on/off switch Three Parts: 1. Promoter where RNA polymerase attaches 2. Operator on/off, controls
The Genetics of Viruses and Bacteria
 The Genetics of Viruses and Bacteria I. A virus is a genome enclosed in a protective coat. A. Viruses are not cells. They are infectious particles consisting of nucleic acid encased in a protein coat and,
The Genetics of Viruses and Bacteria I. A virus is a genome enclosed in a protective coat. A. Viruses are not cells. They are infectious particles consisting of nucleic acid encased in a protein coat and,
Module I: Introduction Lecture 1 4 February, 2010
 Module I: Introduction 20.109 Lecture 1 4 February, 2010 Introduction to: Module Overview Fundamental concepts and techniques in molecular biology A powerful and accessible strategy (SELEX) for identifying
Module I: Introduction 20.109 Lecture 1 4 February, 2010 Introduction to: Module Overview Fundamental concepts and techniques in molecular biology A powerful and accessible strategy (SELEX) for identifying
Enzyme that uses RNA as a template to synthesize a complementary DNA
 Biology 105: Introduction to Genetics PRACTICE FINAL EXAM 2006 Part I: Definitions Homology: Comparison of two or more protein or DNA sequence to ascertain similarities in sequences. If two genes have
Biology 105: Introduction to Genetics PRACTICE FINAL EXAM 2006 Part I: Definitions Homology: Comparison of two or more protein or DNA sequence to ascertain similarities in sequences. If two genes have
Rawan Almujaibel Anas Abu-Humaidan
 8 Rawan Almujaibel...... Anas Abu-Humaidan In the previous lecture the Dr. talked about DNA structure and their 4 types of nitrogen bases. Then he talked about bacterial DNA (chromosomes) and their replication
8 Rawan Almujaibel...... Anas Abu-Humaidan In the previous lecture the Dr. talked about DNA structure and their 4 types of nitrogen bases. Then he talked about bacterial DNA (chromosomes) and their replication
Bioinformatics: Sequence Analysis. COMP 571 Luay Nakhleh, Rice University
 Bioinformatics: Sequence Analysis COMP 571 Luay Nakhleh, Rice University Course Information Instructor: Luay Nakhleh (nakhleh@rice.edu); office hours by appointment (office: DH 3119) TA: Leo Elworth (DH
Bioinformatics: Sequence Analysis COMP 571 Luay Nakhleh, Rice University Course Information Instructor: Luay Nakhleh (nakhleh@rice.edu); office hours by appointment (office: DH 3119) TA: Leo Elworth (DH
Viruses. Chapter 19. Biology Eighth Edition Neil Campbell and Jane Reece. PowerPoint Lecture Presentations for
 Chapter 19 Viruses PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from Joan Sharp Copyright
Chapter 19 Viruses PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from Joan Sharp Copyright
Chapter 27A: Bacteria and Archaea. 1. Extracellular Prokaryotic Structures 2. Intracellular Prokaryotic Structures 3. Genetic Diversity Prokaryotes
 Chapter 27A: Bacteria and Archaea 1. Extracellular Prokaryotic Structures 2. Intracellular Prokaryotic Structures 3. Genetic Diversity Prokaryotes 1. Extracellular Prokaryotic Structures 1 µm 1 µm 3 µm
Chapter 27A: Bacteria and Archaea 1. Extracellular Prokaryotic Structures 2. Intracellular Prokaryotic Structures 3. Genetic Diversity Prokaryotes 1. Extracellular Prokaryotic Structures 1 µm 1 µm 3 µm
1. Extracellular Prokaryotic Structures
 1 µm 1 µm 3 µm 2/11/2015 Chapter 27A: Bacteria and Archaea 1. Extracellular Prokaryotic Structures 2. Intracellular Prokaryotic Structures 3. Genetic Diversity Prokaryotes 1. Extracellular Prokaryotic
1 µm 1 µm 3 µm 2/11/2015 Chapter 27A: Bacteria and Archaea 1. Extracellular Prokaryotic Structures 2. Intracellular Prokaryotic Structures 3. Genetic Diversity Prokaryotes 1. Extracellular Prokaryotic
Answers to Module 1. An obligate aerobe is an organism that has an absolute requirement of oxygen for growth.
 Answers to Module 1 Short Answers 1) What is an obligate aerobe? An obligate aerobe is an organism that has an absolute requirement of oxygen for growth. What about facultative anaerobe? 2) Distinguish
Answers to Module 1 Short Answers 1) What is an obligate aerobe? An obligate aerobe is an organism that has an absolute requirement of oxygen for growth. What about facultative anaerobe? 2) Distinguish
Viruses and Prokaryotes
 Viruses and Prokaryotes Viruses Are they living things? Viruses can reproduce, however, they cannot reproduce without a host cell. They also do not contain cytoplasmic materials and they do not have a
Viruses and Prokaryotes Viruses Are they living things? Viruses can reproduce, however, they cannot reproduce without a host cell. They also do not contain cytoplasmic materials and they do not have a
Unit 2: Metabolism and Survival Sub-Topic (2.7) Genetic Control of Metabolism (2.8) Ethical considerations in the use of microorganisms
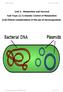 Unit 2: Metabolism and Survival Sub-Topic (2.7) Genetic Control of Metabolism (2.8) Ethical considerations in the use of microorganisms Duncanrig Secondary JHM&MHC 2015 Page 1 of 18 On completion of this
Unit 2: Metabolism and Survival Sub-Topic (2.7) Genetic Control of Metabolism (2.8) Ethical considerations in the use of microorganisms Duncanrig Secondary JHM&MHC 2015 Page 1 of 18 On completion of this
DNA Cloning with Cloning Vectors
 Cloning Vectors A M I R A A. T. A L - H O S A R Y L E C T U R E R O F I N F E C T I O U S D I S E A S E S F A C U L T Y O F V E T. M E D I C I N E A S S I U T U N I V E R S I T Y - E G Y P T DNA Cloning
Cloning Vectors A M I R A A. T. A L - H O S A R Y L E C T U R E R O F I N F E C T I O U S D I S E A S E S F A C U L T Y O F V E T. M E D I C I N E A S S I U T U N I V E R S I T Y - E G Y P T DNA Cloning
Biotechnolog y and DNA Technology
 PowerPoint Lecture Presentations prepared by Bradley W. Christian, McLennan Community College C H A P T E R 9 Biotechnolog y and DNA Technology Introduction to Biotechnology Biotechnology: the use of microorganisms,
PowerPoint Lecture Presentations prepared by Bradley W. Christian, McLennan Community College C H A P T E R 9 Biotechnolog y and DNA Technology Introduction to Biotechnology Biotechnology: the use of microorganisms,
Exam 2 Key - Spring 2008 A#: Please see us if you have any questions!
 Page 1 of 5 Exam 2 Key - Spring 2008 A#: Please see us if you have any questions! 1. A mutation in which parts of two nonhomologous chromosomes change places is called a(n) A. translocation. B. transition.
Page 1 of 5 Exam 2 Key - Spring 2008 A#: Please see us if you have any questions! 1. A mutation in which parts of two nonhomologous chromosomes change places is called a(n) A. translocation. B. transition.
Module I: Introduction
 Module I: Introduction 20.109 Lecture 1 3 February, 2011 Introduction to: Module Overview Fundamental concepts and techniques in molecular biology Appreciating nucleic acids (RNA in particular) as more
Module I: Introduction 20.109 Lecture 1 3 February, 2011 Introduction to: Module Overview Fundamental concepts and techniques in molecular biology Appreciating nucleic acids (RNA in particular) as more
Module 3. Lecture 5. Regulation of Gene Expression in Prokaryotes
 Module 3 Lecture 5 Regulation of Gene Expression in Prokaryotes Recap So far, we have looked at prokaryotic gene regulation using 3 operon models. lac: a catabolic operon which displays induction via negative
Module 3 Lecture 5 Regulation of Gene Expression in Prokaryotes Recap So far, we have looked at prokaryotic gene regulation using 3 operon models. lac: a catabolic operon which displays induction via negative
Section B: The Genetics of Bacteria
 CHAPTER 18 MICROBIAL MODELS: THE GENETICS OF VIRUSES AND BACTERIA Section B: The Genetics of Bacteria 1. The short generation span of bacteria helps them adapt to changing environments 2. Genetic recombination
CHAPTER 18 MICROBIAL MODELS: THE GENETICS OF VIRUSES AND BACTERIA Section B: The Genetics of Bacteria 1. The short generation span of bacteria helps them adapt to changing environments 2. Genetic recombination
Section C: The Control of Gene Expression
 Section C: The Control of Gene Expression 1. Each cell of a multicellular eukaryote expresses only a small fraction of its genes 2. The control of gene expression can occur at any step in the pathway from
Section C: The Control of Gene Expression 1. Each cell of a multicellular eukaryote expresses only a small fraction of its genes 2. The control of gene expression can occur at any step in the pathway from
Therapeutic Proteins BIT 230
 Therapeutic Proteins BIT 230 CLOTTING Haemophilia Benefix Blood Products ANTICOAGULANT THROMBOLYTIC AGENTS tissue plasminogen activator streptokinase Coagulation pathway Factor VIII (Haemophilia A) Factor
Therapeutic Proteins BIT 230 CLOTTING Haemophilia Benefix Blood Products ANTICOAGULANT THROMBOLYTIC AGENTS tissue plasminogen activator streptokinase Coagulation pathway Factor VIII (Haemophilia A) Factor
Antisense RNA Insert Design for Plasmid Construction to Knockdown Target Gene Expression
 Vol. 1:7-15 Antisense RNA Insert Design for Plasmid Construction to Knockdown Target Gene Expression Ji, Tom, Lu, Aneka, Wu, Kaylee Department of Microbiology and Immunology, University of British Columbia
Vol. 1:7-15 Antisense RNA Insert Design for Plasmid Construction to Knockdown Target Gene Expression Ji, Tom, Lu, Aneka, Wu, Kaylee Department of Microbiology and Immunology, University of British Columbia
M I C R O B I O L O G Y WITH DISEASES BY TAXONOMY, THIRD EDITION
 M I C R O B I O L O G Y WITH DISEASES BY TAXONOMY, THIRD EDITION Chapter 7 Microbial Genetics Lecture prepared by Mindy Miller-Kittrell, University of Tennessee, Knoxville The Structure and Replication
M I C R O B I O L O G Y WITH DISEASES BY TAXONOMY, THIRD EDITION Chapter 7 Microbial Genetics Lecture prepared by Mindy Miller-Kittrell, University of Tennessee, Knoxville The Structure and Replication
Viruses CAMPBELL BIOLOGY IN FOCUS SECOND EDITION URRY CAIN WASSERMAN MINORSKY REECE
 CAMPBELL BIOLOGY IN FOCUS URRY CAIN WASSERMAN MINORSKY REECE 17 Viruses Lecture Presentations by Kathleen Fitzpatrick and Nicole Tunbridge, Simon Fraser University SECOND EDITION Overview: A Borrowed Life
CAMPBELL BIOLOGY IN FOCUS URRY CAIN WASSERMAN MINORSKY REECE 17 Viruses Lecture Presentations by Kathleen Fitzpatrick and Nicole Tunbridge, Simon Fraser University SECOND EDITION Overview: A Borrowed Life
Year III Pharm.D Dr. V. Chitra
 Year III Pharm.D Dr. V. Chitra 1 Genome entire genetic material of an individual Transcriptome set of transcribed sequences Proteome set of proteins encoded by the genome 2 Only one strand of DNA serves
Year III Pharm.D Dr. V. Chitra 1 Genome entire genetic material of an individual Transcriptome set of transcribed sequences Proteome set of proteins encoded by the genome 2 Only one strand of DNA serves
Name Per AP: CHAPTER 27: PROKARYOTES (Bacteria) p559,
 AP: CHAPTER 27: PROKARYOTES (Bacteria) p559, 561-564 1. How does the bacterial chromosome compare to a eukaryotic chromosome? 2. What is a plasmid? 3. How fast can bacteria reproduce? 4. What is a bacterial
AP: CHAPTER 27: PROKARYOTES (Bacteria) p559, 561-564 1. How does the bacterial chromosome compare to a eukaryotic chromosome? 2. What is a plasmid? 3. How fast can bacteria reproduce? 4. What is a bacterial
c) Assuming he does not run another endurance race, will the steady-state populations be affected one year later? If so, explain how.
 LS1a Fall 06 Problem Set #8 (100 points total) all questions including the (*extra*) one should be turned in 1. (18 points) Erythrocytes, mature red blood cells, are essential for transporting oxygen to
LS1a Fall 06 Problem Set #8 (100 points total) all questions including the (*extra*) one should be turned in 1. (18 points) Erythrocytes, mature red blood cells, are essential for transporting oxygen to
What is an Aptamer? smallest unit of repeating structure
 What is an Aptamer? apto: mer: to fit smallest unit of repeating structure Aptamers are single stranded folded oligonucleotides that bind to molecular (protein) targets with high affinity and specificity
What is an Aptamer? apto: mer: to fit smallest unit of repeating structure Aptamers are single stranded folded oligonucleotides that bind to molecular (protein) targets with high affinity and specificity
Chapter 2. An Introduction to Genes and Genomes
 PowerPoint Lectures for Introduction to Biotechnology, Second Edition William J.Thieman and Michael A.Palladino Chapter 2 An Introduction to Genes and Genomes Lectures by Lara Dowland Chapter Contents
PowerPoint Lectures for Introduction to Biotechnology, Second Edition William J.Thieman and Michael A.Palladino Chapter 2 An Introduction to Genes and Genomes Lectures by Lara Dowland Chapter Contents
Viruses. Chapter 19. Biology Eighth Edition Neil Campbell and Jane Reece. PowerPoint Lecture Presentations for
 Chapter 19 Viruses PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from Joan Sharp Copyright
Chapter 19 Viruses PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from Joan Sharp Copyright
Basic Concepts and History of Genetic Engineering. Mitesh Shrestha
 Basic Concepts and History of Genetic Engineering Mitesh Shrestha Genetic Engineering AKA gene manipulation, gene cloning, recombinant DNA technology, genetic modification, and the new genetics. A technique
Basic Concepts and History of Genetic Engineering Mitesh Shrestha Genetic Engineering AKA gene manipulation, gene cloning, recombinant DNA technology, genetic modification, and the new genetics. A technique
How to Use This Presentation
 How to Use This Presentation To View the presentation as a slideshow with effects select View on the menu bar and click on Slide Show. To advance through the presentation, click the right-arrow key or
How to Use This Presentation To View the presentation as a slideshow with effects select View on the menu bar and click on Slide Show. To advance through the presentation, click the right-arrow key or
Supervisor:Dr.M.Aslanimehr Presented by :M.Marandi
 Journal Club & MSc Seminar anti adhesin therapy Supervisor:Dr.M.Aslanimehr Presented by :M.Marandi The first stage of microbial infection colonization: the establishment of the pathogen at the appropriate
Journal Club & MSc Seminar anti adhesin therapy Supervisor:Dr.M.Aslanimehr Presented by :M.Marandi The first stage of microbial infection colonization: the establishment of the pathogen at the appropriate
Technical tips Session 4
 Technical tips Session 4 Biotinylation assay: Streptavidin is a small bacterial protein that binds with high affinity to the vitamin biotin. This streptavidin-biotin combination can be used to link molecules
Technical tips Session 4 Biotinylation assay: Streptavidin is a small bacterial protein that binds with high affinity to the vitamin biotin. This streptavidin-biotin combination can be used to link molecules
CRISPR cas : Presented By: Pooya Rashvand Advised By: Dr. M.Aslanimehr
 Journal Club & MSc Seminar CRISPR cas : Presented By: Pooya Rashvand Advised By: Dr. M.Aslanimehr CRISPR - cas : A New tool for Genetic Manipulations from Bacterial Immunity Systems Viral SS DNA RNA Guide
Journal Club & MSc Seminar CRISPR cas : Presented By: Pooya Rashvand Advised By: Dr. M.Aslanimehr CRISPR - cas : A New tool for Genetic Manipulations from Bacterial Immunity Systems Viral SS DNA RNA Guide
B. Incorrect! Ligation is also a necessary step for cloning.
 Genetics - Problem Drill 15: The Techniques in Molecular Genetics No. 1 of 10 1. Which of the following is not part of the normal process of cloning recombinant DNA in bacteria? (A) Restriction endonuclease
Genetics - Problem Drill 15: The Techniques in Molecular Genetics No. 1 of 10 1. Which of the following is not part of the normal process of cloning recombinant DNA in bacteria? (A) Restriction endonuclease
Biological consequences of site specific recombination: integration, excision, deletion
 Biological consequences of site specific recombination: integration, excision, deletion The types of DNA rearrangements promoted by a large number of site specific recombination systems and their physiological
Biological consequences of site specific recombination: integration, excision, deletion The types of DNA rearrangements promoted by a large number of site specific recombination systems and their physiological
OpenStax-CNX module: m Antibodies * OpenStax. Abstract
 OpenStax-CNX module: m44823 1 Antibodies * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section, you will be able to:
OpenStax-CNX module: m44823 1 Antibodies * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section, you will be able to:
Transcriptional regulation of Shigella virulence plasmid-encoded genes by VirB and CRP
 UNLV Theses, Dissertations, Professional Papers, and Capstones 12-2010 Transcriptional regulation of Shigella virulence plasmid-encoded genes by VirB and CRP Christopher Thomas Hensley University of Nevada,
UNLV Theses, Dissertations, Professional Papers, and Capstones 12-2010 Transcriptional regulation of Shigella virulence plasmid-encoded genes by VirB and CRP Christopher Thomas Hensley University of Nevada,
1. A scientist has identified a new membrane receptor. He wishes to determine the hormone-binding
 1. A scientist has identified a new membrane receptor. He wishes to determine the hormone-binding specificity of the receptor. Three different hormones, X, Y, and Z, were mixed with the receptor in separate
1. A scientist has identified a new membrane receptor. He wishes to determine the hormone-binding specificity of the receptor. Three different hormones, X, Y, and Z, were mixed with the receptor in separate
CHAPTER 17 FROM GENE TO PROTEIN. Section C: The Synthesis of Protein
 CHAPTER 17 FROM GENE TO PROTEIN Section C: The Synthesis of Protein 1. Translation is the RNA-directed synthesis of a polypeptide: a closer look 2. Signal peptides target some eukaryotic polypeptides to
CHAPTER 17 FROM GENE TO PROTEIN Section C: The Synthesis of Protein 1. Translation is the RNA-directed synthesis of a polypeptide: a closer look 2. Signal peptides target some eukaryotic polypeptides to
PLNT2530 (2018) Unit 6b Sequence Libraries
 PLNT2530 (2018) Unit 6b Sequence Libraries Molecular Biotechnology (Ch 4) Analysis of Genes and Genomes (Ch 5) Unless otherwise cited or referenced, all content of this presenataion is licensed under the
PLNT2530 (2018) Unit 6b Sequence Libraries Molecular Biotechnology (Ch 4) Analysis of Genes and Genomes (Ch 5) Unless otherwise cited or referenced, all content of this presenataion is licensed under the
Chapter 15 Recombinant DNA and Genetic Engineering. Restriction Enzymes Function as Nature s Pinking Shears
 Chapter 15 Recombinant DNA and Genetic Engineering In this chapter you will learn How restriction enzyme work and why they are essential to DNA technology. About various procedures such as cloning and
Chapter 15 Recombinant DNA and Genetic Engineering In this chapter you will learn How restriction enzyme work and why they are essential to DNA technology. About various procedures such as cloning and
Gene Expression. Chapters 11 & 12: Gene Conrtrol and DNA Technology. Cloning. Honors Biology Fig
 Chapters & : Conrtrol and Technology Honors Biology 0 Cloning Produced by asexual reproduction and so it is genetically identical to the parent st large cloned mammal: Dolly the sheep Animals that are
Chapters & : Conrtrol and Technology Honors Biology 0 Cloning Produced by asexual reproduction and so it is genetically identical to the parent st large cloned mammal: Dolly the sheep Animals that are
Chapter 20 Recombinant DNA Technology. Copyright 2009 Pearson Education, Inc.
 Chapter 20 Recombinant DNA Technology Copyright 2009 Pearson Education, Inc. 20.1 Recombinant DNA Technology Began with Two Key Tools: Restriction Enzymes and DNA Cloning Vectors Recombinant DNA refers
Chapter 20 Recombinant DNA Technology Copyright 2009 Pearson Education, Inc. 20.1 Recombinant DNA Technology Began with Two Key Tools: Restriction Enzymes and DNA Cloning Vectors Recombinant DNA refers
Figure 1. Map of cloning vector pgem T-Easy (bacterial plasmid DNA)
 Texas A&M University-Corpus Christi CHEM4402 Biochemistry II Laboratory Laboratory 6: Ligation & Bacterial Transformation (Bring your text and laptop to class if you wish to work on your assignment during
Texas A&M University-Corpus Christi CHEM4402 Biochemistry II Laboratory Laboratory 6: Ligation & Bacterial Transformation (Bring your text and laptop to class if you wish to work on your assignment during
Applicazioni biotecnologiche
 Applicazioni biotecnologiche Analisi forense Sintesi di proteine ricombinanti Restriction Fragment Length Polymorphism (RFLP) Polymorphism (more fully genetic polymorphism) refers to the simultaneous occurrence
Applicazioni biotecnologiche Analisi forense Sintesi di proteine ricombinanti Restriction Fragment Length Polymorphism (RFLP) Polymorphism (more fully genetic polymorphism) refers to the simultaneous occurrence
1. Two molecules will be attracted to one another by van der Waals interactions as long as...
 1. Two molecules will be attracted to one another by van der Waals interactions as long as... A. they are composed of different types of atoms B. they are composed of the same type of atoms X C. they are
1. Two molecules will be attracted to one another by van der Waals interactions as long as... A. they are composed of different types of atoms B. they are composed of the same type of atoms X C. they are
Bacteria Reproduce Asexually via BINARY FISSION
 An Introduction to Microbial Genetics Today: Intro to Microbial Genetics Lunch pglo! Bacteria Reproduce Asexually via BINARY FISSION But, Bacteria still undergo GENETIC RECOMBINATION (combining DNA from
An Introduction to Microbial Genetics Today: Intro to Microbial Genetics Lunch pglo! Bacteria Reproduce Asexually via BINARY FISSION But, Bacteria still undergo GENETIC RECOMBINATION (combining DNA from
Concept 13.1 Recombinant DNA Can Be Made in the Laboratory
 13 Biotechnology Concept 13.1 Recombinant DNA Can Be Made in the Laboratory It is possible to modify organisms with genes from other, distantly related organisms. Recombinant DNA is a DNA molecule made
13 Biotechnology Concept 13.1 Recombinant DNA Can Be Made in the Laboratory It is possible to modify organisms with genes from other, distantly related organisms. Recombinant DNA is a DNA molecule made
Immunoglobulins. Harper s biochemistry Chapter 49
 Immunoglobulins Harper s biochemistry Chapter 49 Immune system Detects and inactivates foreign molecules, viruses, bacteria and microorganisms Two components with 2 strategies B Lymphocytes (humoral immune
Immunoglobulins Harper s biochemistry Chapter 49 Immune system Detects and inactivates foreign molecules, viruses, bacteria and microorganisms Two components with 2 strategies B Lymphocytes (humoral immune
CHAPTER 2A HOW DO YOU BEGIN TO CLONE A GENE? CHAPTER 2A STUDENT GUIDE 2013 Amgen Foundation. All rights reserved.
 CHAPTER 2A HOW DO YOU BEGIN TO CLONE A GENE? 35 INTRODUCTION In the Program Introduction, you learned that the increase in diabetes in the United States has resulted in a great demand for its treatment,
CHAPTER 2A HOW DO YOU BEGIN TO CLONE A GENE? 35 INTRODUCTION In the Program Introduction, you learned that the increase in diabetes in the United States has resulted in a great demand for its treatment,
