Trends in Sterile Manufacturing Technologies
|
|
|
- Jasmin Bell
- 6 years ago
- Views:
Transcription
1 Trends in Sterile Manufacturing Technologies ISPE Thailand Annual Meeting Charlotte Enghave Fruergaard
2 Where we come from 1930s Danish Novo and Nordisk Gentofte (later Novo Nordisk) employed the first engineers Pharmaplan was founded as part of the medical care group by Fresenius, Germany After functioning as inhouse consultants at Novo Nordisk for years, NNE (Novo Nordisk Engineering A/S) demerged as an independent company Acquisition of Pharmaplan, a company similar to NNE in DNA. NNE Pharmaplan was founded. With 80 years of experience we are passionate about our services to the pharma and biotech industries. Recent awards 2004 IChemE: Haden Freeman Award for Engineering Excellence 2005 ISPE Facility of the Year Award winner Novo Nordisk s NovoSeven (FVII) facility 2008 ISPE Company of the Year winner 2009 ISPE Facility of the Year Award winner Facility Integration hameln pharmaceuticals, Germany 2009 ISPE Facility of the Year Award winner Operational Excellence R&D division of US biotech company, Switzerland 2009 Emerson: PlantWeb Excellence for DeltaV application for Pronova BioPharma project (KalOmega)
3 Who we are We are the leading consulting and engineering company in the complex field of pharma and biotech. We count close to 1,700 professionals with project experience and knowledge related to pharma and biotech. More than 200 have hands-on development or production experience. We executed 2,929 projects in Our project execution and our staff embody 10,000 years of experience within pharma and biotech
4 Optimal production processes DEVELOP ESTABLISH IMPROVE Process & Product development Project development Investment project CD / BD / DD / CON / C&Q Optimisation, training, revamps, GAP analysis, operational support Company revenue 2012 USD 294M EUR 224M
5 Agenda Market changes forcing technology changes Aseptic/Sterile processes Technology Trends
6 What is OSD and Biotech? Small Molecules vs. Large Molecules Small Molecules OSD Chemical Active Pharmaceutical Ingredient (API) Tablets Oral Solid Dosage Form (OSD) Since 1899 Chemical Synthesis Drying or Granulating Formulation Tableting Packaging Large Molecules Biotech Biologics Active Pharmaceutical Ingredient (API) Injectables Parenteral Dosage Form (aka Sterile Products) Since 1982 Inteferon molecule vs Aspirin Fermentation or Cell Culture Formulation Aseptic Filling Packaging
7 Large Molecules grows faster Small Molecule drugs is the big market Shifting roles: The shift from small to large molecules became visible in 2003 The Economist 2005
8 Market changes forcing technology changes Products New products are more and more classified as high potent and require both a very high level of aseptic processing and operator / environmental protection Batch sizes Small batch sizes of very high value. Based on better diagnostic methods, personalized treatment (1 vial = 1 batch) will increase Processes New products (mainly Biopharmaceuticals) are usually produced by aseptic processes Primary containers Pre-filled syringes and new developed devices are growing fast Automation Elimination of the human factor to avoid direct human impact for all critical process steps (class A operations) in a reproducible, validatable and documentable way
9 Agenda Market changes forcing technology changes Aseptic/Sterile processes Technology Trends
10 From API to Finished Product excl. QA/QC Manufacturing of Sterile Products Assembly & Packaging API Components etc. Wash & Sterilisation (incl. Comp. Prep.) Compounding (Preparation) Filling Barrier Isolator Lyophilizing Assembly Labelling Packaging Finished Products Logistics & Controls in Manufacturing Logistics Material Handling Inspection Controls Filled units
11 Sterile Products Definition Sterile products are products free from living micro organisms Ph. Eur. section Methods of Preparation of Sterile Products It is expected that the principles of good manufacturing practice will have been observed in the design of the process including, in particular, the use of qualified personnel with appropriate training adequate premises suitable production equipment, designed for easy cleaning and sterilisation adequate precautions to minimise the bio burden prior to sterilisation validated procedures for all critical production steps environmental monitoring and in-process testing procedures Sterilisation shall be done in the final container if at all possible Sterility Assurance Level (SAL) of minimum 1:1,000,000
12 Sterile or Aseptic manufacturing? Can the solution tolerate heat treatment? Yes Sterile manufacture Sterilization takes place in the end of the process (= terminal sterilisation) No Aseptic manufacture The solution is sterile filtered and thereafter only comes in contact with sterilised utensils and tanks in a classified environment (grade A/ISO 5 with a grade B/ISO 7 background) Filter pore size is 0.22 µm = mm
13 Why do we have GMP? Manufacturing of pharmaceutical products is all about Risk for the patient This is why we document, train and qualify Traceability is being able to trace BACK especially when something goes wrong (batch numbers and documentation)
14 Washing Process Purpose of washing Secure no leftover from previous product, i.e. no cross contamination Reduction of endotoxin and particles Items to be washed in a utensil washer Utensils Machine parts Hoses Typical process Rinse Wash with or without detergent Several rinse phases. Conductivity measurement Drying
15 Sterilization Process Purpose of sterilization: Secure a product free of living microorganisms with a sterility assurance level of 10-6 or better Terminal sterilization Product produced at least in grade D and sterilized in final container Aseptic preparation Each primary packaging component sterilized individually Solutions sterile filtered All handling and filling of aseptically prepared products must be done in grade A/ISO 5 with grade B/ISO 7 background Items to be sterilized All items going to grade B/ISO 7 including sanitizers and gowning
16 Methods of Sterilization Saturated steam 121 C, minimum 15 minutes, pressure + (Autoclave/SIP) 0,1 bar Hot water 121 C, minimum F 0 15 minutes Dry heat sterilization 160 C, minimum F H 38 minutes Dry heat depyrogenation 250 C, minimum F H 15 minutes, lead to a 3 log reduction of endotoxin VHP sterilization Demonstrate a SAL of 10-6 Filtration Pore size < 0,22 µm Radiation /e-beam A minimum dose of 25 kgy
17 Liquid Compounding Preparation Liquid compounding of Sterile Products is mixing of Water for Injection (WFI) Active Pharmaceutical Ingredient (API) Other raw materials, e.g. Stabilisers (physical/chemical) Preservatives (if multiple use product) Isotonic substances
18 Filling Process To take the sterile product from Compounding and fill it into a primary packing, which must protect the product and keep it sterile until use Multiple Batch Filling: Filling more than one batch between full cleaning/decontamination of the filling line During filling protection against contamination from the following must be avoided: The primary packing itself Surroundings & People
19 Surroundings & People People are the most contaminating source for the product
20 Why is this necessary?
21 Surroundings & People People are the most contaminating source for the product This must be reduced by protecting the filling process: Conventional Clean Room (CCR) Restricted Access Barrier System (RABS) Isolator
22 Material Input/Output Glass Filled and inspected Glass Pistons Combi seals Washing Filling Sterilization Product from Compounding
23 What are Barrier Systems? A physical barrier which separates the operator from the process. As important as the barrier itself are the linked features and processes such as: Properly designed equipment (ergonomics) and HVAC system Material transfer procedures Working procedures and training of the operators Procedures in terms of intervention and accidents That s why it is called a system
24 Definitions CCR Conventional Clean Rooms (CCR) ISO 5 (class A) surrounded by ISO 7 (class B) room 3-6" From HEPAS Pressure difference (15 Pa) between the clean room classes Filling Critical operations Class 10,000 Class with 100 open sensitive products are carried out Mechanism (ISO 7) (ISO 5) under Unidirectional Airflow (class A protection) Nozzle Manipulations (i.e. trouble shooting, change of format parts) are done directly by opening Vial of the machine cladding Operator gets directly Conveyor in contact with critical surfaces (class A area) Gowning of the operator according to class B requirements Material transition Plastic to class B (autoclave, pass box, dry heat oven) curtains Regular wipe sanitization Heavy routine viable monitoring Periodical room sanitization HEPA Filters Machine parts pre-sterilized or disinfected in situ
25 Definitions RABS Restricted Access Passive Barrier System (RABS) HEPA Filters Surrounding clean room class B for the filling operation HVAC HEPA Filters Pressure difference (15 Pa) between the clean room classes All manipulations during Filling production are done via gloves of the Class 100 Mechanism RABS (ISO 5) Class Filling Class ,000 Mechanism Ergonomic Nozzle (ISO 5) designed system for the process (ISO 7) inside (Mock-up studies) Nozzle Vial Transfer of format parts via Rapid Transfer Port Vial (RTP) Conveyor Material transfer via Rapid Transfer Port (RTP) Conveyor or material locks Gowning of the operator according class B requirements Doors with Conventional cleaning and disinfection gloves Same viable monitoring as CCR Locked doors (barrier) during operation The system isolates the operator from the critical areas to increase Sterility Assurance Level (SAL) 3-6" From HEPAS Class 10,000 (ISO 7) Plastic curtains Active
26 Definitions Isolators Isolators ISO 5 (class A) inside isolator Surrounding clean room ISO 8 (class D or C) for the filling operation Positive pressure difference towards the filling room 100,000 (ISO 5) Mechanism Ergonomic designed (ISO 8) system for the process inside (Mock-up studies) Complete closed system Vial with Vaporized Hydrogen Peroxide (VHP) decontamination of all surfaces Conveyor Complete independent HVAC-unit Air All manipulations during production are done via gloves Gowning of the operator according class C or D requirements Material transfer via Rapid Transfer Port (RTP) or material locks Area to be monitored is very limited Very high SAL Doors with gloves Class HEPA Filters Class 100 Nozzle Return Filling
27 The Technologies Barrier Systems Restricted Access Clean Room RABS Barrier Systems Isolator Conventional Clean Room Open RABS (active or passive) Closed RABS Isolator Environment: B/A Complexity: Low Comfort: Low, due to clean room garment Aseptic quality: Low SAL~3 (*) Campaigning unusual (*) Sterility Assurance Level Environment: B No overpressure to surroundings Complexity: High, due to transfer techniques and restricted access by gloves Comfort: Even lower, due to clean room garment and restricted access Aseptic quality: Slightly improved SAL~4 Several days campaign unusual Environment: D Overpressure Complexity: Highest, due to transfer techniques and biodecontamination Comfort: Medium, no clean room garment, but some restrictions Aseptic quality: Highest SAL~6 log Week(s) campaign possible
28 The Technologies Barrier Systems Restricted Access Clean Room RABS Barrier Systems Isolator Conventional Clean Room Open RABS (active or passive) Closed RABS Isolator Currently most installed aseptic production lines are based on this Technology. For new projects not anymore state-of-the-art. (*) Sterility Assurance Level Rather new technology, with large increase in terms of installations within the past years. Since more than ten years developing quite fast, first in Europe, then also USA and Japan. First choice technology for handling of high potent APIs.
29 Conflict of GMP vs. operator protection
30 Pro and Cons CCR RABS Isolator Comments Validation, start-up risk Low Low High Complex isolator validation Necessity of experts Low Low High External consultants? Skill of personnel Medium Medium High Aseptic behavior needed in all 3 Investment, equipment Low Medium High Investment, building High High Low FMC high cost countries High High Low FMC elsewhere No real difference Suitability for campaigning Low Medium High Higher productivity with campaigning Sterility Assurance Level (SAL) Low Medium High Regulatory scrutiny High Low Low Risk SAL Media Fill High Medium Low Microbial Sampling Normal Normal Less Suitability for processing potent drugs Not given Limited Very good Maintenance complexity Low Medium High Access for service Easy Restricted Restricted Better for isolator due to garment Realization time Low Medium High
31 Considerations Product Multi product production One product No of individual preparations per Year Containment Preservatives? Explosion proof Batch sizes Multiple batch filling Protein H 2 O 2 sensitivity Price of product
32 Considerations Process Complexity of process Ampoules Vials Syringes Cartridge Powder? Freeze Drying Material Transfer Transfer door/lock α/β-ports Gloves Sterile mounting Integrity testing Replacement period Leak testing H 2 O 2 -decontamination
33 Considerations Working Environment Product and Operator Safety (positive or negative Δ pressure) Working procedures and training of users Procedures in terms of intervention and accidents Surrounding room environment (operator comfort) Energy saving
34 Main Challenges Vaporised Hydrogen Peroxide sensors (VHP-sensors) Inaccurate Biological Indicators BI s are biological D-value determination Needed Log-value VHP as a sterilising agent Surface decontamination Parts with in-direct product contact Harmful to proteins Gloves Integrity testing
35 Technology Evaluation Technology ready! But still some inexpedient issues ~ VHP sensors, biological indicators, gloves. Challenges for experts, not obstacles. Suppliers ready! All major suppliers are of high standard. Filling line, isolator/rabs and facility designed together. Knowledge ready! It seems as the use of properly chosen consultants/experts can minimize both the change over time and the time for and risk of validation. Isolators call for experts Goal/level setting, process development, decision of acceptance criteria, validation and interpretation of results. RABS may be a solution
36 Regulatory requirements The regulatory authorities are demanding more and more barrier systems to eliminate direct operator impact to critical processes: There is no doubt that the operator is biggest risk of a potential particulate and microbiological contamination for the production of pharmaceuticals US-FDA Rick Friedman comments in March 2013: Conventional cleanrooms are on the borderline of compliance ISPE Aseptic Conference Baltimore, March 2013 Rick Friedman, Associate Director, OMPQ, FDA
37 Regulatory Guidelines The regulatory authorities are demanding more and more barrier systems to eliminate direct operator impact to critical processes: EU GMP Guideline (New Annex 1): Manufacture of Medicinal Products, Section Isolator Technology 21. The utilization of isolator technology to minimize human interventions in processing areas may result in a significant decrease in the risk of microbiological contamination of aseptically manufactured products from the environment 122. Restricted access barriers and isolators may be beneficial in assuring the required conditions and minimising direct human interventions into the capping operation.
38 Regulatory Guidelines FDA Guidance Sterile Drug Products Produced by Aseptic Processing, Published version September 2004: ASEPTIC PROCESSING ISOLATORS (Appendix 1) Aseptic processing using isolation systems minimizes the extent of personnel involvement and separates the external cleanroom environment from the aseptic processing line. A well-designed positive pressure isolator, supported by adequate procedures for its maintenance, monitoring, and control, offers tangible advantages* over classical aseptic processing, including fewer opportunities for microbial contamination during processing. However, users should not adopt a false sense of security with these systems. Manufacturers should also be aware of the need to establish new procedures addressing issues unique to isolators.
39 Regulatory Why
40 Regulatory EU Statement What is the general position on the use of isolators and restricted access barriers vs. old conventional thinking? The transfer of materials into the aseptic processing zone and the role of people in the process are key concerns. Robust material transfer strategies together with automation and enhanced product protection (from people) are therefore key to minimising risk. Use of isolators for aseptic processing is therefore to be supported but ultimately it is for industry to select and justify the technologies it used. ISPE Barrier Isolation Technology Conference Berlin, September 2007 Presentation by Ian Thrussell, MHRA
41 Regulatory FDA Statement The FDA would not tell a manufacturer that they must use a specific single technology to assure adherence to aseptic processing requirements. The FDA does indicate its general preference for isolators and provides corresponding regulatory incentives for them. A sound RABS concept also can provide added protection versus traditional processing approaches. Article in PHARMACEUTICAL ENGINEERING MARCH/APRIL 2007 Article made from panel discussion by Rick Friedman and Bob Sausville during ISPE Barrier Isolation Technology Conference Washington, June 2006
42 Why consider Isolator Technology Authorities Less scrutiny Industry State of Art Economical Cheaper per unit produced (with comparable SAL) Manning Less microbiological sampling/testing Risk No scrap of product due to sterility issues or failure in Media fills Environmental Eliminating high class cleanroom environment, less space Cost Reduction of running costs Operator Protection with potent products
43 Why consider Isolator Technology Increasing amount of high potent APIs The protection of the operator as well as the environment for the production of pharmaceutical products becomes more and more important because of the following reasons: The ratio of new APIs which are classified to be high potent is rising continuously: 1990: approx. 5% 2002: approx. 30% 2015:? Existing APIs which were originally classified as not high potent are re-classified to be high potent The importance of operator and environment protection is constantly growing in our society with the result of stricter laws and regulations
44 Summary Isolator Isolator benefits: Higher product quality (e.g. SAL 6 log), reduced risk Better protection of personnel (containment) More comfort for personnel Lower facility cost and running costs (Class C or D) Isolator appropriate for: High output machines Long filling campaigns Expensive products Aseptic and potent drug
45 Conclusion / Recommendation Barrier systems are important to improve the product quality, and if required to provide an operator and environmental protection For new facilities the use of barrier technology is almost mandatory Barrier systems which are using gloves for manipulations have to be designed very well in order to give the operator good ergonomics for all required manipulations In order to avoid gloves and manual handling operations the trend go to a fully automized process An evaluation based on the products and processes about the kind of barrier systems should be done in an early project phase, because this has a huge impact in the overall facility design. The technology is a mature, ready to use, but experts are necessary to secure the right solution and to educate.
46 Agenda Market changes forcing technology changes Aseptic/Sterile processes Technology Trends
47 Industry Trends Overall Avoiding of aseptic handling Trend to avoid any manual aseptic handling of pre-sterilized components. If it cannot be avoided the use of a barrier system is almost mandatory Automation Trend to automize GMP critical processes in order to eliminate the human factor at all Energy efficiency Trend to save energy because it becomes more and more a significant cost factor PAT (Process Analytical Technology) Biopharmaceutical products are becoming more and more expensive, therefore PAT becomes more and more importance to decrease / avoid product loses
48 Technology Trends Equipment cleaning Automation / Validation Clear industry trend to avoid any manual handling steps. Very difficult to achieve reproducible cleaning result by performing cleaning processes of critical equipment parts manually Guiding rail Fixing elements Plungers Stopper chute Stopper hopper Fixing elements Vacuum star wheel Vacuum star wheel Picture courtesy Belimed Sauter AG
49 Technology Trends Stopper treatment Automation Clear industry trend to avoid any manual handling steps, especially after sterilization Aseptic transfer (Use of Barrier Technology) Charging of stoppers into the stopper hopper of a filling machine equipped with a RABS or an isolator is more time consuming compared to a filling line in conventional design. As higher the filling machine speed, and / or as larger the rubber stopper size is, as more relevant this issue gets. Process control A closed automated process combining all or partly the following process steps in one unit provides better process control: Washing (with or without detergents) Siliconization Sterilisation Drying
50 Technology Trends Stopper treatment Lifecycle process: * Cleanroom A/B or Isolator Receiving: Unclassified area Treatment & Transfer: Cleanroom D or C Connection: Rapid Transfer Port ** * Picture courtesy ATEC Steritec GmbH ** Picture courtesy GETINGE-LA CALHENE
51 Technology Trends Stopper treatment Aseptic stopper transfer:
52 Technology Trends Sterilisation technology VHP Passbox: Picture courtesy Metall + Plastic GmbH
53 Technology Trends Sterilisation technology E-Beam: E-Beam is the only continuous sterilization method to supply high speed pre-filled syringe filling machines Lifetime of the emitters are not satisfying for some suppliers, nevertheless it will become the standard sterilization method for tub sterilization A DIN/ISO standard for tubs of pre-filled syringes is currently under examination
54 Technology Trends Sterilisation technology E-Beam: Picture courtesy Metall + Plastic GmbH
55 Technology Trends Disposable Systems Coupling for bag or vessel connection 2. Peristaltic pump when delivery container is not pressurized 3. Bioburden sample bag 4. Sterilizing grade product filter 5. Vent bag 6. Intermediate reservoir bag 7. Disposable manifold 8. Peristaltic dosing pumps 9. Beta-Bag 10. Isolator/RABS wall towards filling 11. Filling needles
56 Technology Trends Single Use Technology Now available from all well known filling machine suppliers Costs are between Euro per set * * Picture courtesy Robert Bosch GmbH
57 Technology Trends Disposable Systems Design Trends: Less important Very important Saving of utilities (CIP/SIP) Saving of investment costs Avoiding of cleaning validation Faster filling machine set up between two batches/products (less filling machine downtime, especially in combination with an isolator) Less product loss at batch end
58 Technology Trends Filling equipment Filling system Peristaltic pump systems are often the preferred system for Biopharmaceuticals and / or Disposable systems Robot / Handling systems Individual positive transport, avoidance of glass to glass contact, minimizing rejects and glass breakage rate and very flexible (fast) format change Performance High speed filling equipment for pre-filled syringes up to units/min. Low speed filling for very small batches with fast format/product change Process Analytical Technology (PAT) 100% check of filling volume Camera inspection for stopper and cap placement
59 Technology Trends Filling equipment Filling machine with handling systems and check weighing:
60 Technology Trends Barrier Technology History (1947):
61 Industry Trends Isolators Barrier Isolator Filling Line Deliveries by Year Year Asia Europe N.America Total ISPE Barrier Isolation Technology Conference Brussels, September 2010 Lysfjord/Porter Final Survey Results 2010
62 Process technology RABS Pictures
63 Technology Trends Freeze Dryer Loading & Unloading Technology Automation Clear industry trend to avoid any manual handling steps, especially during the freeze dryer loading Use of Barrier Technology Loading: Mainly to fulfil GMP purposes Unloading: Mainly to fulfil operator safety requirements Pass-through freeze dryer configuration Increases the overall performance especially when freeze drying cycle is short and number of freeze dryer connected to a filling line is 2 or more Vertical execution Technical area as well as condenser below chamber to create a maintenance access through unclassified areas
64 Technology Trends Freeze Dryer Loading & Unloading Technology Mobile automated cart for freeze dryer loading:
65 Summary Future developments (subjective) Filling Equipment The technical development will go in the direction of handling / robot systems which do not require a direct human intervention...the emergence of the robotics industry, which is developing in much the same way that the computer business did 30 years ago. Think of the manufacturing robots currently used on automobile assembly lines as the equivalent of yesterday's mainframes. Bill Gates; A Robot in Every Home; Sci Am; 2006 RABS RABS technology is on the long-term not a succeeding technology Conventional aseptic filling should become passé soon. Rick Friedman, Director, Div. of Mfg and Quality, FDA-CDER The regulatory requirements for RABS systems will become more strict Isolator Technology of the future Gloves as a weak point of the isolator will more and more disappear The VHP cycle times will become significantly shorter Disposable technology Will increase significantly in the near future
66 ISPE Member Benefits Guidelines Baseline Guide: Sterile Product Manufacturing Facilities (Second Edition) COP s Sterile Products Processing COP Containment COP Biotech COP Webpage
67 Contact Info Charlotte Enghave Fruergaard, PhD Director Chairman ISPE Board of Directors Nybrovej 80, 2820 Gentofte, Denmark Mobile:
68 Abbreviations CCR Conventional Clean Room RABS Restricted Access Barrier System UDF Unidirectional Airflow SAL Sterility Assurance Level VHP Vaporized Hydrogen Peroxide RTP Rapid Transfer Port BI Biological Indicators
Current Trends in Sterile Manufacturing. by Sterling Kline, RA Director of Project Development Integrated Project Services (IPS)
 Current Trends in Sterile Manufacturing by Sterling Kline, RA Director of Project Development Integrated Project Services (IPS) Executive Summary Drug developers go to great lengths to assure that their
Current Trends in Sterile Manufacturing by Sterling Kline, RA Director of Project Development Integrated Project Services (IPS) Executive Summary Drug developers go to great lengths to assure that their
Contract Manufacturing Services for Pre-filled Syringes
 Contract Manufacturing Services for Pre-filled Syringes From Standard Solutions to Complex Products Stefan Czvitkovich, PhD Director Product Partnering Sterile Pharmaceuticals Central Europe CPhI woldwide
Contract Manufacturing Services for Pre-filled Syringes From Standard Solutions to Complex Products Stefan Czvitkovich, PhD Director Product Partnering Sterile Pharmaceuticals Central Europe CPhI woldwide
MONZA ST5 Project. Alberto Penati Patheon Monza EU Engineering Director. The world leader in serving science
 MONZA ST5 Project Alberto Penati Patheon Monza EU Engineering Director The world leader in serving science SUMMARY 1. Monza Site Overview 2. Design Data 3. Philosophy and approach on installation 4. Challenges
MONZA ST5 Project Alberto Penati Patheon Monza EU Engineering Director The world leader in serving science SUMMARY 1. Monza Site Overview 2. Design Data 3. Philosophy and approach on installation 4. Challenges
Why change is inevitable in aseptic manufacturing?
 Why change is inevitable in aseptic manufacturing? Chrissie Fuchs, Marketing & Communication at Fedegari Group Sergio Mauri, Manager, Integrated Projects at Fedegari Group Key words: Aseptic manufacturing
Why change is inevitable in aseptic manufacturing? Chrissie Fuchs, Marketing & Communication at Fedegari Group Sergio Mauri, Manager, Integrated Projects at Fedegari Group Key words: Aseptic manufacturing
Isolators v. RABS: Facility Design Considerations for a Fill-Finish Finish Suite
 APV 2008 Basle Conference Basle, Switzerland: May 28 & 29 2008 Isolators v. RABS: Facility Design Considerations for a Fill-Finish Finish Suite John R. Chester Principal Engineer Global Pharmaceutical
APV 2008 Basle Conference Basle, Switzerland: May 28 & 29 2008 Isolators v. RABS: Facility Design Considerations for a Fill-Finish Finish Suite John R. Chester Principal Engineer Global Pharmaceutical
Gloveless Robotic Technologies in Aseptic. Personalized Cytotoxic. Association. Drugs. Sergio Mauri FEDEGARI GROUP
 Gloveless Robotic Technologies in Aseptic PDA: Manufacturing A Global for Personalized Cytotoxic Association Sergio Mauri FEDEGARI GROUP Drugs Presentation outline Pharma outlook trends and the aseptic
Gloveless Robotic Technologies in Aseptic PDA: Manufacturing A Global for Personalized Cytotoxic Association Sergio Mauri FEDEGARI GROUP Drugs Presentation outline Pharma outlook trends and the aseptic
Overview and Introduction Annex 1 Revision
 Overview and Introduction Annex 1 Revision Paul Moody, Inspector Pharmig Current Hot Topics in Pharmaceutical Microbiology, Dublin May 30 th, 2018 Scope History Process of Revision Rationale for Revision
Overview and Introduction Annex 1 Revision Paul Moody, Inspector Pharmig Current Hot Topics in Pharmaceutical Microbiology, Dublin May 30 th, 2018 Scope History Process of Revision Rationale for Revision
Actavis Italy. Nerviano Plant
 Actavis Italy Nerviano Plant Starting out in 1901 in Jerusalem The company known today as Teva was established as a small wholesale drug business by Chaim Salomon, Moshe Levin and Yitschak Elstein. (They
Actavis Italy Nerviano Plant Starting out in 1901 in Jerusalem The company known today as Teva was established as a small wholesale drug business by Chaim Salomon, Moshe Levin and Yitschak Elstein. (They
HVAC and Risk Management RACI Meeting 7 th December Agenda. Design. Project Management. Context. Qualification. Construction
 HVAC and Risk Management RACI Meeting 7 th December 2011 Dr Stephen Firmer Asia Pacific Consultants Pty Ltd Agenda Context Project Management Design Construction Qualification Routine Control /Potential
HVAC and Risk Management RACI Meeting 7 th December 2011 Dr Stephen Firmer Asia Pacific Consultants Pty Ltd Agenda Context Project Management Design Construction Qualification Routine Control /Potential
PDA: A Global. Association. Contamination Control: Particles, Bio-contamination, Aseptic manufacturing. PDA Parenteral 2014 Munich Conference
 PDA Parenteral 2014 Munich Conference PDA: A Global Contamination Control: Particles, Bio-contamination, Bioburden Association and Endotoxins in Aseptic manufacturing. James Drinkwater F Ziel Head of Aseptic
PDA Parenteral 2014 Munich Conference PDA: A Global Contamination Control: Particles, Bio-contamination, Bioburden Association and Endotoxins in Aseptic manufacturing. James Drinkwater F Ziel Head of Aseptic
Review Validation of aseptic processes for pharmaceuticals
 OPEM www.opem.org Oriental Pharmacy and Experimental Medicine 2010 10(4), 231-238 DOI 10.3742/OPEM.2010.10.4.231 Review Validation of aseptic processes for pharmaceuticals Lincy Joseph*, Mathew George
OPEM www.opem.org Oriental Pharmacy and Experimental Medicine 2010 10(4), 231-238 DOI 10.3742/OPEM.2010.10.4.231 Review Validation of aseptic processes for pharmaceuticals Lincy Joseph*, Mathew George
GETINGE ISOFLEX MULTI-PURPOSE ISOLATOR
 GETINGE ISOFLEX MULTI-PURPOSE ISOLATOR 2 GETINGE ISOFLEX THE ANSWER TO YOUR DAILY NEEDS FOR STERILE HANDLING In any sterile operation, maintaining the sterility of goods -- whether you have purchased them
GETINGE ISOFLEX MULTI-PURPOSE ISOLATOR 2 GETINGE ISOFLEX THE ANSWER TO YOUR DAILY NEEDS FOR STERILE HANDLING In any sterile operation, maintaining the sterility of goods -- whether you have purchased them
CONTAINED ASEPTIC TRANSFER VALVES
 1 CONTAINED ASEPTIC TRANSFER VALVES Perform aseptic transfers that maintain critical area integrity. Reduce risk of cross contamination with closed transfers that limit manual intervention. Meet GMP and
1 CONTAINED ASEPTIC TRANSFER VALVES Perform aseptic transfers that maintain critical area integrity. Reduce risk of cross contamination with closed transfers that limit manual intervention. Meet GMP and
CONTAINED ASEPTIC TRANSFER VALVES
 CONTAINED ASEPTIC TRANSFER VALVES Applications Contained filling and dispensing for all production processes. aseptic transfer valves can be integrated into the production of both powder and liquid formulations
CONTAINED ASEPTIC TRANSFER VALVES Applications Contained filling and dispensing for all production processes. aseptic transfer valves can be integrated into the production of both powder and liquid formulations
FDA s Guidance for Industry
 Sterile Drug Products Produced by Aseptic Processing - CGMPs Midwest FDC Conference November 4 2005 Susan Bruederle, Investigator FDA / ORA / Central Region Chicago District / Hinsdale IL FDA s Guidance
Sterile Drug Products Produced by Aseptic Processing - CGMPs Midwest FDC Conference November 4 2005 Susan Bruederle, Investigator FDA / ORA / Central Region Chicago District / Hinsdale IL FDA s Guidance
Microbiological Consideration for Non-Sterile Pharmaceutical
 May 1-3, 2012 Javits Center New York, NY Microbiological Consideration for Non-Sterile Pharmaceutical Dr. Leonard W. Mestrandrea Principal Consultant MESTRANDREA CONSULTING LLC Title Date Microbial Control
May 1-3, 2012 Javits Center New York, NY Microbiological Consideration for Non-Sterile Pharmaceutical Dr. Leonard W. Mestrandrea Principal Consultant MESTRANDREA CONSULTING LLC Title Date Microbial Control
For sterile products manufacturing, Lyoseal is an innovative technology. presented by Mr. Tony Bouzerar from BioCorp offers us detailed
 For sterile products manufacturing, Lyoseal is an innovative technology to improve quality problems with final drug product due to crimping issues and inadequate stopper seating. It eliminates aluminum
For sterile products manufacturing, Lyoseal is an innovative technology to improve quality problems with final drug product due to crimping issues and inadequate stopper seating. It eliminates aluminum
Annex A2. Guidance on Process Validation Scheme for Aseptically Processed Products
 Annex A2 Guidance on Process Validation Scheme for Aseptically Processed Products 1 Table of content 1 PURPOSE... 3 2 SCOPE... 3 3 GENERAL INFORMATION... 3 4 INFORMATION NEEDED FOR ASEPTIC PROCESSES VALIDATION...
Annex A2 Guidance on Process Validation Scheme for Aseptically Processed Products 1 Table of content 1 PURPOSE... 3 2 SCOPE... 3 3 GENERAL INFORMATION... 3 4 INFORMATION NEEDED FOR ASEPTIC PROCESSES VALIDATION...
Complaints Investigations Root Cause Analysis
 Complaints Investigations Root Cause Analysis Dr. Ademola Daramola International Relations Specialist Drugs US FDA India Office New Delhi February 22nd 2018 Information presented in this presentation does
Complaints Investigations Root Cause Analysis Dr. Ademola Daramola International Relations Specialist Drugs US FDA India Office New Delhi February 22nd 2018 Information presented in this presentation does
PDA: A Global. Association Dr. Friedrich Haefele Boehringer Ingelheim Pharma GmbH & Co. KG. Maturing Aseptic Technologies
 Maturing Aseptic Technologies PDA: A Global create more flexible Facilities Association Dr. Friedrich Haefele Boehringer Ingelheim Pharma GmbH & Co. KG Content Changing Environment for Parenteral Manufacturing
Maturing Aseptic Technologies PDA: A Global create more flexible Facilities Association Dr. Friedrich Haefele Boehringer Ingelheim Pharma GmbH & Co. KG Content Changing Environment for Parenteral Manufacturing
Aseptic Process Validation
 Aseptic Process Validation IMB GMP Information Seminar 27 th September 2012 Gerard Sheridan, Inspector Date Insert on Master Slide Slide 1 Overview Guidance Best Practices Common Deficiencies Slide 2 Aseptic
Aseptic Process Validation IMB GMP Information Seminar 27 th September 2012 Gerard Sheridan, Inspector Date Insert on Master Slide Slide 1 Overview Guidance Best Practices Common Deficiencies Slide 2 Aseptic
Sterilizing and Bioburden Filter Risk Assessment in Vaccine Processes as part of QRM
 Sterilizing and Bioburden Filter Risk Assessment in Vaccine Processes as part of QRM DCVMN Workshop, Hyderabad, 4-8 April 2016 G. Somasundaram Associate Director - Technology Management Overview Key Regulatory
Sterilizing and Bioburden Filter Risk Assessment in Vaccine Processes as part of QRM DCVMN Workshop, Hyderabad, 4-8 April 2016 G. Somasundaram Associate Director - Technology Management Overview Key Regulatory
Overview of Inspection Issues with Legacy Products. Barbara Breithaupt Consumer Safety Officer
 Overview of Inspection Issues with Legacy Products Barbara Breithaupt Consumer Safety Officer 1 Overview of the presentation The role of the Quality Unit will be discussed in the context of the inspection
Overview of Inspection Issues with Legacy Products Barbara Breithaupt Consumer Safety Officer 1 Overview of the presentation The role of the Quality Unit will be discussed in the context of the inspection
NO 2 Gas Sterilization and Decontamination: Keeping Pace, from Start to Finish
 NO 2 Gas Sterilization and Decontamination: Keeping Pace, from Start to Finish Evan Goulet, Ph.D., VP of Microbiology Applications Laboratory PDA Midwest: Sterility Assurance and Risk Mitigation October
NO 2 Gas Sterilization and Decontamination: Keeping Pace, from Start to Finish Evan Goulet, Ph.D., VP of Microbiology Applications Laboratory PDA Midwest: Sterility Assurance and Risk Mitigation October
On behalf of the PHSS Pharmaceutical and Healthcare Sciences Society (UK).
 31 st March 2015 Submission of comments on ' Concept paper on the revision of annex 1 of the guidelines on good manufacturing practice manufacture of sterile medicinal products EMA/INS/GMP/735037/2014
31 st March 2015 Submission of comments on ' Concept paper on the revision of annex 1 of the guidelines on good manufacturing practice manufacture of sterile medicinal products EMA/INS/GMP/735037/2014
Webinar. The New EU-GMP Annex 1 draft Dupont. GOP-Innovations your Partner for Practical Training and e-learning. 8 June 2018
 Webinar The New EU-GMP Annex 1 draft Dupont 8 June 2018 GOP-Innovations your Partner for Practical Training and e-learning Milenko Pavičić Pharmaceutical microbiologist, consultant, trainer, coach 10 years
Webinar The New EU-GMP Annex 1 draft Dupont 8 June 2018 GOP-Innovations your Partner for Practical Training and e-learning Milenko Pavičić Pharmaceutical microbiologist, consultant, trainer, coach 10 years
Custom processing services
 Custom processing services Cleaning excellence for your critical environment The only thing in your container is what you add Save wasted resources dedicate your time to the manufacturing process, not
Custom processing services Cleaning excellence for your critical environment The only thing in your container is what you add Save wasted resources dedicate your time to the manufacturing process, not
Process Engineering Applications
 u ' SterUe Pharmaceutical Products Process Engineering Applications Drug Manufacturing Technology Series Kenneth E. Avis, Editor Interpharm Press, Inc. Buffalo Grove, IL CONTENTS Foreword xi 1. Introduction
u ' SterUe Pharmaceutical Products Process Engineering Applications Drug Manufacturing Technology Series Kenneth E. Avis, Editor Interpharm Press, Inc. Buffalo Grove, IL CONTENTS Foreword xi 1. Introduction
ANNEX 1: COMMENT SUBMISSION DOCUMENT
 Submission Comments on: GMP Annex 1: Proposal for amendments to the environmental classification table for particles and associated text, amendment to section 42 concerning acceptance criteria for media
Submission Comments on: GMP Annex 1: Proposal for amendments to the environmental classification table for particles and associated text, amendment to section 42 concerning acceptance criteria for media
Advancements on implementation of single use technology in vaccine manufacturing
 Advancements on implementation of single use technology in vaccine manufacturing October 25 th, 2013 DCVMN Meeting Rio de Janeiro, Brazil Outline Overview of single use products Applications of single
Advancements on implementation of single use technology in vaccine manufacturing October 25 th, 2013 DCVMN Meeting Rio de Janeiro, Brazil Outline Overview of single use products Applications of single
BFS IOA BFS Training 2018 Kunming Qualification and Validation of a BFS-Installation including CIP/SIP
 Qualification and Validation of a BFS-Installation including CIP/SIP Presented by Christoph Bohn and Stefan Kiesel Director Rommelag Pharma Service Senior Manager Rommelag Pharma Service Coyright BFS IOA
Qualification and Validation of a BFS-Installation including CIP/SIP Presented by Christoph Bohn and Stefan Kiesel Director Rommelag Pharma Service Senior Manager Rommelag Pharma Service Coyright BFS IOA
Modular Turnkey Concept for Pharmaceutical Sterile Formulation Facilities
 Presented at the World Batch Forum European Conference Mechelen, Belgium 11-13 October 2004 900 Fox Valley Drive, Suite 204 Longwood, FL 32779-2552 +1.407.774.5764 Fax: +1.407.774.6751 E-mail: info@wbf.org
Presented at the World Batch Forum European Conference Mechelen, Belgium 11-13 October 2004 900 Fox Valley Drive, Suite 204 Longwood, FL 32779-2552 +1.407.774.5764 Fax: +1.407.774.6751 E-mail: info@wbf.org
Production and Test Facilities Via Romagnoli, Fiorenzuola d Arda (PC) - Italy - Tel Fax Containment Systems
 Containment Systems our approach FPS containment approach FPS is specialized in containment systems and fine size reduction equipment for many industrial applications. Our approach to a containment system
Containment Systems our approach FPS containment approach FPS is specialized in containment systems and fine size reduction equipment for many industrial applications. Our approach to a containment system
ABOUT HAMELN PHARMA About us
 Welcome ABOUT HAMELN PHARMA About us With over 60 years of experience hameln pharma is the specialist for contract manufacturing of sterile solutions and suspensions filled in ampoules and vials. 2 ABOUT
Welcome ABOUT HAMELN PHARMA About us With over 60 years of experience hameln pharma is the specialist for contract manufacturing of sterile solutions and suspensions filled in ampoules and vials. 2 ABOUT
Hot Topics in Drug Product Process Validation: A Reviewer s Perspective
 Hot Topics in Drug Product Process Validation: A Reviewer s Perspective Colleen Thomas, Ph.D. Quality Assessment Lead (Acting) FDA/CDER/OPQ/OPF Division of Microbiology Assessment CASSS CMC Strategy Forum
Hot Topics in Drug Product Process Validation: A Reviewer s Perspective Colleen Thomas, Ph.D. Quality Assessment Lead (Acting) FDA/CDER/OPQ/OPF Division of Microbiology Assessment CASSS CMC Strategy Forum
The DPTE System. The original Rapid Transfer Port
 The DPTE System The original Rapid Transfer Port The benchmark for sterile transfer The first choice for risk-free production Manufacturing technologies are pushing productivity further with ever higher
The DPTE System The original Rapid Transfer Port The benchmark for sterile transfer The first choice for risk-free production Manufacturing technologies are pushing productivity further with ever higher
INSPECTOR S OBSERVATIONS AND WARNING LETTER
 Microrite, Inc. San Jose, CA, USA, http://www.microrite.com AIR VELOCITY MEASUREMENTS AND CORRELATION TO SMOKE STUDIES FOR ASEPTIC OPERATIONS KEYWORDS Air Velocity, Air Flows, Smoke Study, Air Pattern
Microrite, Inc. San Jose, CA, USA, http://www.microrite.com AIR VELOCITY MEASUREMENTS AND CORRELATION TO SMOKE STUDIES FOR ASEPTIC OPERATIONS KEYWORDS Air Velocity, Air Flows, Smoke Study, Air Pattern
HISTORY AND MILESTONES
 HISTORY AND MILESTONES HISTORY AND MILESTONES DOC S.r.l., Documentation Organization & Consultancy, was established in 1997 by MASCO group C.E.O. Eng. Alberto Borella and Eng. Paolo Curtò who became Managing
HISTORY AND MILESTONES HISTORY AND MILESTONES DOC S.r.l., Documentation Organization & Consultancy, was established in 1997 by MASCO group C.E.O. Eng. Alberto Borella and Eng. Paolo Curtò who became Managing
Whitepaper. High temperature HEPA filtration. Dr.-Ing. Marc Schmidt, Dr.-Ing. Lothar Gail, Hugo Hemel MSc. Preview
 High temperature HEPA filtration Air filtration challenges and answers for dry heat sterilization tunnels Whitepaper Preview Dr.-Ing. Marc Schmidt, Dr.-Ing. Lothar Gail, Hugo Hemel MSc. Air filtration
High temperature HEPA filtration Air filtration challenges and answers for dry heat sterilization tunnels Whitepaper Preview Dr.-Ing. Marc Schmidt, Dr.-Ing. Lothar Gail, Hugo Hemel MSc. Air filtration
INTEGRATED VPHP DECONTAMINATION SYSTEMS THE EMERGING UTILITY
 INTEGRATED VPHP DECONTAMINATION SYSTEMS THE EMERGING UTILITY John Klostermyer, Ph.D. ISPE Product Show Track 3 Session 3 September 26, 2018 AGENDA 1. BACKGROUND INFORMATION 2. BENEFITS 3. PROCESS 4. BEST
INTEGRATED VPHP DECONTAMINATION SYSTEMS THE EMERGING UTILITY John Klostermyer, Ph.D. ISPE Product Show Track 3 Session 3 September 26, 2018 AGENDA 1. BACKGROUND INFORMATION 2. BENEFITS 3. PROCESS 4. BEST
SCHOTT Pharmaceutical Systems. adaptiq - ready-to-use vials for aseptic processing
 SCHOTT Pharmaceutical Systems adaptiq - ready-to-use vials for aseptic processing Todays market trends require flexible filling concepts and pre sterilized (RTU) containers Targeted medicine e.g. Oncology
SCHOTT Pharmaceutical Systems adaptiq - ready-to-use vials for aseptic processing Todays market trends require flexible filling concepts and pre sterilized (RTU) containers Targeted medicine e.g. Oncology
STERSTAR 2 STATE OF THE ART ON-LINE E-BEAM CONTINUOUS TUB STERILIZATION TUNNEL GETINGE STERSTAR 2 1
 STERSTAR 2 STATE OF THE ART ON-LINE E-BEAM CONTINUOUS TUB STERILIZATION TUNNEL GETINGE STERSTAR 2 1 2 GETINGE STERSTAR 2 GETINGE STERSTAR 2 3 GETINGE S E-BEAM SYSTEMS According to the IAEA (1), over the
STERSTAR 2 STATE OF THE ART ON-LINE E-BEAM CONTINUOUS TUB STERILIZATION TUNNEL GETINGE STERSTAR 2 1 2 GETINGE STERSTAR 2 GETINGE STERSTAR 2 3 GETINGE S E-BEAM SYSTEMS According to the IAEA (1), over the
Streamlined Blow-Fill-Seal Insertion Technology Increases Flexibility and Safety in Aseptic Packaging of Pharmaceutical Liquids
 Streamlined Blow-Fill-Seal Insertion Technology Increases Flexibility and Safety in Aseptic Packaging of Pharmaceutical Liquids Isolators adapted specifically for blow-fill-seal insertion applications
Streamlined Blow-Fill-Seal Insertion Technology Increases Flexibility and Safety in Aseptic Packaging of Pharmaceutical Liquids Isolators adapted specifically for blow-fill-seal insertion applications
Annex 1 to the Good manufacturing practices guide Manufacture of sterile drugs GUI-0119
 Annex 1 to the Good manufacturing practices guide Manufacture of sterile drugs GUI-0119 February 28, 2018 Manufacture of sterile drugs (GUI-0119) Author: Health Canada Date issued: February 28, 2018 Implementation
Annex 1 to the Good manufacturing practices guide Manufacture of sterile drugs GUI-0119 February 28, 2018 Manufacture of sterile drugs (GUI-0119) Author: Health Canada Date issued: February 28, 2018 Implementation
Advanced Aseptic Processing including networking reception at Favrholm Campus
 ISPE Nordic invites you to a conference on Advanced Aseptic Processing including networking reception at Favrholm Campus 7 October 2015 Favrholm Campus Roskildevej 58, 3400 Hillerød, Denmark The Advanced
ISPE Nordic invites you to a conference on Advanced Aseptic Processing including networking reception at Favrholm Campus 7 October 2015 Favrholm Campus Roskildevej 58, 3400 Hillerød, Denmark The Advanced
Dr. Sheelpriya walde Professor Gurunanak college of pharmacy, Nagpur
 Dr. Sheelpriya walde Professor Gurunanak college of pharmacy, Nagpur GMP ( Adopted in 1975 ) In India Good manufacturing practices are the practices required in order to conform to guidelines recommended
Dr. Sheelpriya walde Professor Gurunanak college of pharmacy, Nagpur GMP ( Adopted in 1975 ) In India Good manufacturing practices are the practices required in order to conform to guidelines recommended
How to Handle Excursions in Environmental Monitoring (EM) and Personnel Monitoring (PM) in an Aseptic Processing Plant
 How to Handle Excursions in Environmental Monitoring (EM) and Personnel Monitoring (PM) in an Aseptic Processing Plant Randy Hutt, Ph.D. Associate Director Sterility Assurance and Microbiology Luitpold
How to Handle Excursions in Environmental Monitoring (EM) and Personnel Monitoring (PM) in an Aseptic Processing Plant Randy Hutt, Ph.D. Associate Director Sterility Assurance and Microbiology Luitpold
Vetter Commercial Manufacturing
 A unique blend of capabilities for aseptic fill and finish Vetter Commercial Manufacturing Delivering results across the production chain Answers that work 1 Manufacturing quality is just the beginning.
A unique blend of capabilities for aseptic fill and finish Vetter Commercial Manufacturing Delivering results across the production chain Answers that work 1 Manufacturing quality is just the beginning.
Introduction and Background
 Introduction and Background Validation of Biotech Facilities Pacific Biotech Alliance Robert J. Mackey Pacific Biotech Alliance is an established consulting firm in the Pacific Northwest that specializes
Introduction and Background Validation of Biotech Facilities Pacific Biotech Alliance Robert J. Mackey Pacific Biotech Alliance is an established consulting firm in the Pacific Northwest that specializes
USP Chapter 823 USP 32 (old) vs. USP 35 (new)
 USP Chapter 823 USP 32 (old) vs. USP 35 (new) Sally W. Schwarz, MS, BCNP Research Associate Professor of Radiology Washington University School of Medicine St. Louis, MO Why USP Chapter ? FDA has
USP Chapter 823 USP 32 (old) vs. USP 35 (new) Sally W. Schwarz, MS, BCNP Research Associate Professor of Radiology Washington University School of Medicine St. Louis, MO Why USP Chapter ? FDA has
Compounding Aseptic Isolators (CAI) James T Wagner
 Compounding Aseptic Isolators (CAI) James T Wagner jimwagner@cenvironment.com Disclaimer "Although I am a member of the USP Sterile Compounding Expert Committee, I am speaking today in my individual capacity
Compounding Aseptic Isolators (CAI) James T Wagner jimwagner@cenvironment.com Disclaimer "Although I am a member of the USP Sterile Compounding Expert Committee, I am speaking today in my individual capacity
SITE MASTER FILE For MHRA
 ABC CO., LTD. For MHRA Prepared by Date Approved by Date Verified by Date Document No.: SMF, Version No: 01, Effective Date: 09/05/06 Document No.: SMF Version No.: 01 Effective Date: 09/05/06 Page 2 of
ABC CO., LTD. For MHRA Prepared by Date Approved by Date Verified by Date Document No.: SMF, Version No: 01, Effective Date: 09/05/06 Document No.: SMF Version No.: 01 Effective Date: 09/05/06 Page 2 of
While the global pharmaceutical industry tends to be conservative
 A new line at Handai Biken exemplifies the creative use of isolator technology found throughout Japan s pharmaceutical industry. By Jim Akers, Akers Kennedy and Associates, Kazuhito Tanimoto, Shibuya Kogyo
A new line at Handai Biken exemplifies the creative use of isolator technology found throughout Japan s pharmaceutical industry. By Jim Akers, Akers Kennedy and Associates, Kazuhito Tanimoto, Shibuya Kogyo
Microbial contamination is a
 B I O P R O C E S S TECHNICAL Barrier Vial Technology A Global Approach to the Aseptic Filling Process Diego López-Alvarez, Sergi Roura, and J.A. Garcia Microbial contamination is a concern and a constant
B I O P R O C E S S TECHNICAL Barrier Vial Technology A Global Approach to the Aseptic Filling Process Diego López-Alvarez, Sergi Roura, and J.A. Garcia Microbial contamination is a concern and a constant
Environmental Monitoring of Aseptic Processing Areas - 1
 Environmental Monitoring of Aseptic Processing Areas - 1 A war against an invisible enemy DCVMN - Víctor Maqueda - May 30, 31, June 1 2016 1 Training Course Agenda Overview of Environmental Monitoring
Environmental Monitoring of Aseptic Processing Areas - 1 A war against an invisible enemy DCVMN - Víctor Maqueda - May 30, 31, June 1 2016 1 Training Course Agenda Overview of Environmental Monitoring
Guiding Principles for the implementation of fluid management technologies for modern single use aseptic processing
 Guiding Principles for the implementation of fluid management technologies for modern single use aseptic processing Jean-Marc Cappia, Vice President Marketing Fluid Management Technologies Agenda 1. Aseptic
Guiding Principles for the implementation of fluid management technologies for modern single use aseptic processing Jean-Marc Cappia, Vice President Marketing Fluid Management Technologies Agenda 1. Aseptic
Critical Environment Products and Services
 Critical Environment Products and Services For over two decades, EP Scientific products and services have defined clean for environmental sampling containers and services. Our proprietary cleaning methods
Critical Environment Products and Services For over two decades, EP Scientific products and services have defined clean for environmental sampling containers and services. Our proprietary cleaning methods
EU GMP ANNEX 1 CHANGES CLARIFICATION AND IMPACT
 1 EU GMP ANNEX 1 CHANGES CLARIFICATION AND IMPACT UNDERSTAND THE PROGRESSION OF REGULATORY THINKING AND HOW TO SUCCESSFULLY IMPLEMENT THE PROPOSED CHANGES BALTIMORE, MD MAY 6TH AND 7TH, 2019 UNIVERSITY
1 EU GMP ANNEX 1 CHANGES CLARIFICATION AND IMPACT UNDERSTAND THE PROGRESSION OF REGULATORY THINKING AND HOW TO SUCCESSFULLY IMPLEMENT THE PROPOSED CHANGES BALTIMORE, MD MAY 6TH AND 7TH, 2019 UNIVERSITY
Isolator Technology TRUKING TECHNOLOGY LIMITED
 Isolator TRUKING TECHNOLOGY LIMITED Add No.1 xinkang Road,Yutan Town,Ningxiang,Changsha,China P.C 410600 Tel 86 731-87938222 87938255 Fax 86 731-879338201 Web www.truking.cn Any technical renewal will
Isolator TRUKING TECHNOLOGY LIMITED Add No.1 xinkang Road,Yutan Town,Ningxiang,Changsha,China P.C 410600 Tel 86 731-87938222 87938255 Fax 86 731-879338201 Web www.truking.cn Any technical renewal will
AseptiSafe CONTAINED ASEPTIC TRANSFER VALVES. Handling sensitive ingredients and components in aseptic processing
 AseptiSafe CONTAINED ASEPTIC TRANSFER VALVES Handling sensitive ingredients and components in aseptic processing Simple in process sterilization High containment performance for hazardous products www.thechargepoint.com
AseptiSafe CONTAINED ASEPTIC TRANSFER VALVES Handling sensitive ingredients and components in aseptic processing Simple in process sterilization High containment performance for hazardous products www.thechargepoint.com
FDA's Perspectives on Cross- Contamination in CMO Facilities, Considering High Risk Products
 FDA's Perspectives on Cross- Contamination in CMO Facilities, Considering High Risk Products Bo Chi, Ph.D. Biotech Manufacturing Assessment Branch DGMPA/OMPQ/OC/CDER 1 Outline Regulatory framework Regulatory
FDA's Perspectives on Cross- Contamination in CMO Facilities, Considering High Risk Products Bo Chi, Ph.D. Biotech Manufacturing Assessment Branch DGMPA/OMPQ/OC/CDER 1 Outline Regulatory framework Regulatory
Environmental Control Requirements. Gordon Farquharson July 2017
 Environmental Control Requirements Gordon Farquharson July 2017 Learning Objectives Learn how contamination control standards and guidance sets requirements for Critical Parameters ISO-14644-1 Classification
Environmental Control Requirements Gordon Farquharson July 2017 Learning Objectives Learn how contamination control standards and guidance sets requirements for Critical Parameters ISO-14644-1 Classification
EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL. This document is for consultation until 11 December 2015
 Ref. Ares(2015)3808922-15/09/2015 EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL Medicinal Products Quality, Safety and Efficacy Brussels, [date] This document is for consultation until 11
Ref. Ares(2015)3808922-15/09/2015 EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL Medicinal Products Quality, Safety and Efficacy Brussels, [date] This document is for consultation until 11
Containment Barrier Systems
 Containment Barrier Systems As an international leader, focused on Quality, Telstar partners with clients to provide high value on a global basis through innovation, advanced technology, absolute reliability
Containment Barrier Systems As an international leader, focused on Quality, Telstar partners with clients to provide high value on a global basis through innovation, advanced technology, absolute reliability
PROCESS VALIDATION ROCHAPON WACHAROTAYANKUN, PH.D.
 Basic GMP Requirement PROCESS VALIDATION ROCHAPON WACHAROTAYANKUN, PH.D. Topic Process validation What and Why? Principle of process validation Manufacturing process validation Aseptic process validation
Basic GMP Requirement PROCESS VALIDATION ROCHAPON WACHAROTAYANKUN, PH.D. Topic Process validation What and Why? Principle of process validation Manufacturing process validation Aseptic process validation
Handling of high potency drugs: process and containment
 Environmental Exposure and Health 257 Handling of high potency drugs: process and containment G. Mari, A. Moccaldi & G. Ludovisi Istituto Superiore per la Prevenzione e la Sicurezza del Lavoro (Italian
Environmental Exposure and Health 257 Handling of high potency drugs: process and containment G. Mari, A. Moccaldi & G. Ludovisi Istituto Superiore per la Prevenzione e la Sicurezza del Lavoro (Italian
LOADING SYSTEMS LOADING AND UNLOADING SYSTEMS FOR FREEZE DRYERS
 LOADING SYSTEMS LOADING AND UNLOADING SYSTEMS FOR FREEZE DRYERS LOADING SYSTEMS Automatic loading and unloading systems minimize the risk of contamination through human intervention in the loading and
LOADING SYSTEMS LOADING AND UNLOADING SYSTEMS FOR FREEZE DRYERS LOADING SYSTEMS Automatic loading and unloading systems minimize the risk of contamination through human intervention in the loading and
Controlled dosimetry Fast and accurate process Versatile and easy to use Robust and reliable Meets the highest standards
 THE ART OF RADIOPHARMACEUTICALS: DEDICATED PET RADIOTRACER PREPARATION AND INJECTION Controlled dosimetry Fast and accurate process Versatile and easy to use Robust and reliable Meets the highest standards
THE ART OF RADIOPHARMACEUTICALS: DEDICATED PET RADIOTRACER PREPARATION AND INJECTION Controlled dosimetry Fast and accurate process Versatile and easy to use Robust and reliable Meets the highest standards
Pharmaceutical Compounding Sterile and Nonsterile Preparations. Jeanne Sun, PharmD
 Pharmaceutical Compounding Sterile and Nonsterile Preparations Jeanne Sun, PharmD Agenda Legal Framework Overview Pharmaceutical Compounding Nonsterile Preparations Pharmaceutical Compounding
Pharmaceutical Compounding Sterile and Nonsterile Preparations Jeanne Sun, PharmD Agenda Legal Framework Overview Pharmaceutical Compounding Nonsterile Preparations Pharmaceutical Compounding
Aseptic Processing Practices and Process Validation of Aseptic Operators
 Aseptic Processing Practices and Process Validation of Aseptic Operators CBE Pty Ltd This training program is copyright to CBE Pty Ltd and may not be modified, reproduced, sold, loaned, hired or traded
Aseptic Processing Practices and Process Validation of Aseptic Operators CBE Pty Ltd This training program is copyright to CBE Pty Ltd and may not be modified, reproduced, sold, loaned, hired or traded
A Case Study for an Improved Approach to Cleanroom Disinfection, Minimizing the Impact and Reducing Downtime
 A Case Study for an Improved Approach to Cleanroom Disinfection, Minimizing the Impact and Reducing Downtime James Tucker Global Scientific Affairs Manager Ecolab Life Science A Case Study for an Improved
A Case Study for an Improved Approach to Cleanroom Disinfection, Minimizing the Impact and Reducing Downtime James Tucker Global Scientific Affairs Manager Ecolab Life Science A Case Study for an Improved
EUROPEAN COMMISSION ENTERPRISE AND INDUSTRY DIRECTORATE-GENERAL. EudraLex The Rules Governing Medicinal Products in the European Union
 EUROPEAN COMMISSION ENTERPRISE AND INDUSTRY DIRECTORATE-GENERAL Consumer goods Pharmaceuticals Brussels, 25 November 2008 (rev.) EudraLex The Rules Governing Medicinal Products in the European Union Volume
EUROPEAN COMMISSION ENTERPRISE AND INDUSTRY DIRECTORATE-GENERAL Consumer goods Pharmaceuticals Brussels, 25 November 2008 (rev.) EudraLex The Rules Governing Medicinal Products in the European Union Volume
Contents. Contents (13) 1 Production (23)
 1 Production (23) 1.A Sanitation (27) 1.A.1 Organisational prerequisites (27) 1.A.2 Sources of contamination (28) 1.A.3 Responsibilities and implementation (29) 1.B Personnel hygiene (31) 1.B.1 Clothing
1 Production (23) 1.A Sanitation (27) 1.A.1 Organisational prerequisites (27) 1.A.2 Sources of contamination (28) 1.A.3 Responsibilities and implementation (29) 1.B Personnel hygiene (31) 1.B.1 Clothing
PDA: A Global Association
 Achieving Meticulous Aseptic Standards & Control in a Filling Isolator PDA: A Global Association Lessons for Design Aidan Harrington PhD Senior Consultant DPS Engineering, Cork Ireland Preface Agalloco,
Achieving Meticulous Aseptic Standards & Control in a Filling Isolator PDA: A Global Association Lessons for Design Aidan Harrington PhD Senior Consultant DPS Engineering, Cork Ireland Preface Agalloco,
CLEANING VALIDATION CONFERENCE
 CLEANING VALIDATION CONFERENCE 20 th APRIL 2016 OTTILIAVEJ 8, 2500 VALBY, DENMARK (H. LUNDBECK A/S) Prerequisites for Cleaning Validation How to Prepare for Cleaning Validation This event is organized
CLEANING VALIDATION CONFERENCE 20 th APRIL 2016 OTTILIAVEJ 8, 2500 VALBY, DENMARK (H. LUNDBECK A/S) Prerequisites for Cleaning Validation How to Prepare for Cleaning Validation This event is organized
Single-Use Final Fill: Benefits and Considerations
 Single-Use Final Fill: Benefits and Considerations TENDÊNCIAS DE TECNOLOGIAS DE FABRICAÇÃO E ASSÉPTICA DE MEDICAMENTOS Ana Luísa Lampert Cadore Sales Specialist SU & Aseptic Process Solutions ana.cadore@merckgroup.com
Single-Use Final Fill: Benefits and Considerations TENDÊNCIAS DE TECNOLOGIAS DE FABRICAÇÃO E ASSÉPTICA DE MEDICAMENTOS Ana Luísa Lampert Cadore Sales Specialist SU & Aseptic Process Solutions ana.cadore@merckgroup.com
Vetter Development Service
 A unique blend of expertise for clinical manufacturing Vetter Development Service Supporting your compound s success Answers that work 1 Your molecule s future starts here. By the time your new injectable
A unique blend of expertise for clinical manufacturing Vetter Development Service Supporting your compound s success Answers that work 1 Your molecule s future starts here. By the time your new injectable
School of Pharmacy & Pharmaceutical Sciences QP Forum Thursday 16th April McGee Pharma International
 School of Pharmacy & Pharmaceutical Sciences QP Forum Thursday 16th April 1 Parallel Session - Sterile Facilitator Ms Ann McGee 2 Query Topics Contamination Control Strategy Problems virtually managing
School of Pharmacy & Pharmaceutical Sciences QP Forum Thursday 16th April 1 Parallel Session - Sterile Facilitator Ms Ann McGee 2 Query Topics Contamination Control Strategy Problems virtually managing
DPTE -EN 1 DPTE TRANSFER SYSTEM THE GLOBAL REFERENCE
 DPTE -EN 1 DPTE TRANSFER SYSTEM THE GLOBAL REFERENCE 2 DPTE -EN GETINGE-LA CALHÈNE DPTE SYSTEM A CLEVER TRANSFER SYSTEM The DPTE transfer system enables the user to introduce material into, or to extract
DPTE -EN 1 DPTE TRANSFER SYSTEM THE GLOBAL REFERENCE 2 DPTE -EN GETINGE-LA CALHÈNE DPTE SYSTEM A CLEVER TRANSFER SYSTEM The DPTE transfer system enables the user to introduce material into, or to extract
Presentation Title Author Name
 Presentation Title Author Name 1 An Introduction to West Pharmaceutical Services: West Pharmaceutical Services, Inc. is a leading manufacturer of packaging components and delivery systems for injectable
Presentation Title Author Name 1 An Introduction to West Pharmaceutical Services: West Pharmaceutical Services, Inc. is a leading manufacturer of packaging components and delivery systems for injectable
Contamination Risk Assessment in Aseptic, Non-Sterile and Terminally Sterilized Products March 7 th & 8 th Raleigh, NC
 Contamination Risk Assessment in Aseptic, Non-Sterile and Terminally Sterilized Products March 7 th & 8 th Raleigh, NC Contamination risk levels differ in aseptic, and non-sterile and terminally sterilized
Contamination Risk Assessment in Aseptic, Non-Sterile and Terminally Sterilized Products March 7 th & 8 th Raleigh, NC Contamination risk levels differ in aseptic, and non-sterile and terminally sterilized
ISPE Aseptic Conference February 2014 Washington, D.C.
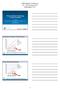 Trends of Barrier Technology in China and Japan Alex Cheng: Shanghai Tofflon Airex Science and Technology Co.,Ltd. Koji Kawaskai: Airex CoLtd. Japan Filling Barrier Isolators Global Deliveries 500 450
Trends of Barrier Technology in China and Japan Alex Cheng: Shanghai Tofflon Airex Science and Technology Co.,Ltd. Koji Kawaskai: Airex CoLtd. Japan Filling Barrier Isolators Global Deliveries 500 450
Design Considerations for TGA Licensed Pharmacies. Presented by Ashley Isbel 11 th August 2015
 Design Considerations for TGA Licensed Pharmacies Presented by Ashley Isbel 11 th August 2015 Focus of the session Primarily of relevance to sterile compounding facilities, particularly those producing
Design Considerations for TGA Licensed Pharmacies Presented by Ashley Isbel 11 th August 2015 Focus of the session Primarily of relevance to sterile compounding facilities, particularly those producing
Services. Premium. See inside to find out more!
 Premium Services Depyrogenation Sterilisation Barcoding & Tare Weighing Particulate Cleaning Surface Treatment Custom Packaging See inside to find out more! Allowing our customers to focus on their core
Premium Services Depyrogenation Sterilisation Barcoding & Tare Weighing Particulate Cleaning Surface Treatment Custom Packaging See inside to find out more! Allowing our customers to focus on their core
Environmental Monitoring of Aseptic Processing Areas - 2
 Environmental Monitoring of Aseptic Processing Areas - 2 A war against an invisible enemy DCVMN - Víctor Maqueda - May 30, 31, June 1 2016 1 Active Air Monitoring (Viables) Air Sieve Samplers Draw air
Environmental Monitoring of Aseptic Processing Areas - 2 A war against an invisible enemy DCVMN - Víctor Maqueda - May 30, 31, June 1 2016 1 Active Air Monitoring (Viables) Air Sieve Samplers Draw air
Allergy Laboratories, Inc. 10/4/13
 Allergy Laboratories, Inc. 10/4/13 SEP 04, 2013 Department of Health and Human Services Public Health Service Food and Drug Administration Office of Regulatory Affairs 12420 Parklawn Drive ELEM-2152 Rockville,
Allergy Laboratories, Inc. 10/4/13 SEP 04, 2013 Department of Health and Human Services Public Health Service Food and Drug Administration Office of Regulatory Affairs 12420 Parklawn Drive ELEM-2152 Rockville,
ANNEX 5 MANUFACTURE OF IMMUNOLOGICAL VETERINARY MEDICINAL PRODUCTS
 ANNEX 5 MANUFACTURE OF IMMUNOLOGICAL VETERINARY MEDICINAL PRODUCTS Principle The manufacture of immunological veterinary medicinal products has special characteristics which should be taken into consideration
ANNEX 5 MANUFACTURE OF IMMUNOLOGICAL VETERINARY MEDICINAL PRODUCTS Principle The manufacture of immunological veterinary medicinal products has special characteristics which should be taken into consideration
Bio-contamination control PHSS Technical Monograph No. 20. Gordon Farquharson, July 2016
 Bio-contamination control PHSS Technical Monograph No. 20 Gordon Farquharson, July 2016 The UK PHSS (Pahramaceuical and Health Care Sciences Socierty prepared Monograph No. 20 in September 2014. This presentation
Bio-contamination control PHSS Technical Monograph No. 20 Gordon Farquharson, July 2016 The UK PHSS (Pahramaceuical and Health Care Sciences Socierty prepared Monograph No. 20 in September 2014. This presentation
How single-use connections advance aseptic processing: Increased process flexibility and reliability, reduced costs
 WHITE PAPER 7004 How single-use connections advance aseptic processing: Increased process flexibility and reliability, reduced costs By John Boehm Business Unit Manager Colder Products Company Today s
WHITE PAPER 7004 How single-use connections advance aseptic processing: Increased process flexibility and reliability, reduced costs By John Boehm Business Unit Manager Colder Products Company Today s
XTREMA HIGH SPEED FILLING & STOPPERING MACHINE
 XTREMA HIGH SPEED FILLING & STOPPERING MACHINE XTREMA HIGH SPEED FILLING & STOPPERING MACHINE FOR TIME HAS COME TO TAKE CONTROL This is the refrain that has driven IMA Life to further innovate. State-of-the-art
XTREMA HIGH SPEED FILLING & STOPPERING MACHINE XTREMA HIGH SPEED FILLING & STOPPERING MACHINE FOR TIME HAS COME TO TAKE CONTROL This is the refrain that has driven IMA Life to further innovate. State-of-the-art
Preparing Your Aseptic Processing Facility for an FDA Inspection. Valerie Welter, Director Quality Bayer HealthCare March 10, 2014
 Preparing Your Aseptic Processing Facility for an FDA Inspection Valerie Welter, Director Quality Bayer HealthCare March 10, 2014 Agenda Regulatory Requirements Establishing your Approach Aseptic Controls
Preparing Your Aseptic Processing Facility for an FDA Inspection Valerie Welter, Director Quality Bayer HealthCare March 10, 2014 Agenda Regulatory Requirements Establishing your Approach Aseptic Controls
Microbiology Testing: USP requirements for Sterile and Nonsterile Preparations Webinar Q&A
 USP Antimicrobial Effectiveness Testing Microbiology Testing: Webinar Q&A 1. If a base ingredient is designed for the purpose of compounding a specific type of preparation does this forgo the necessity
USP Antimicrobial Effectiveness Testing Microbiology Testing: Webinar Q&A 1. If a base ingredient is designed for the purpose of compounding a specific type of preparation does this forgo the necessity
Sterility Assurance Level and Aseptic Manufacturing Process in Pharmaceuticals
 Review Article ISSN 2277-3657 Available online at www.ijpras.com Volume 3, Issue 4 (2014),10-15 International Journal of Pharmaceutical Research & Allied Sciences Sterility Assurance Level and Aseptic
Review Article ISSN 2277-3657 Available online at www.ijpras.com Volume 3, Issue 4 (2014),10-15 International Journal of Pharmaceutical Research & Allied Sciences Sterility Assurance Level and Aseptic
Proposed Revision of USP General Chapter Radiopharmaceuticals for Positron Emission Tomography Compounding <823>
 Proposed Revision of USP General Chapter Radiopharmaceuticals for Positron Emission Tomography Compounding Ravi Ravichandran, Ph.D. Steve Zigler, Ph.D. February 21, 2011 Agenda Rationale for the
Proposed Revision of USP General Chapter Radiopharmaceuticals for Positron Emission Tomography Compounding Ravi Ravichandran, Ph.D. Steve Zigler, Ph.D. February 21, 2011 Agenda Rationale for the
5.1.1 & Changes started in 2008 and were mainly driven under the Chairmanship of Prof Hans van Doorne 11/10/2017
 5.1.1 & 5.1.2 V Iain Fenton-May Chairman Group 1 Methods of Preparation of Sterile Products Changes started in 2008 and were mainly driven under the Chairmanship of Prof Hans van Doorne 1 Methods of Preparation
5.1.1 & 5.1.2 V Iain Fenton-May Chairman Group 1 Methods of Preparation of Sterile Products Changes started in 2008 and were mainly driven under the Chairmanship of Prof Hans van Doorne 1 Methods of Preparation
GUIDE TO INSPECTIONS OF STERILE DRUG SUBSTANCE MANUFACTURERS
 GUIDE TO INSPECTIONS OF STERILE DRUG SUBSTANCE MANUFACTURERS Note: This document is reference material for investigators and other FDA personnel. The document does not bind FDA, and does no confer any
GUIDE TO INSPECTIONS OF STERILE DRUG SUBSTANCE MANUFACTURERS Note: This document is reference material for investigators and other FDA personnel. The document does not bind FDA, and does no confer any
Points to Consider for Aseptic Processing Part 2 May 2016
 Points to Consider for Aseptic Processing Part 2 May 2016 PDA Points to Consider for Aseptic Processing Part 2 Task Force Authors and Contributors Harold Baseman, Valsource, LLC (Co-Chair) Gabriele Gori,
Points to Consider for Aseptic Processing Part 2 May 2016 PDA Points to Consider for Aseptic Processing Part 2 Task Force Authors and Contributors Harold Baseman, Valsource, LLC (Co-Chair) Gabriele Gori,
Guidance for Industry
 Guidance for Industry for the Submission Documentation for Sterilization Process Validation in Applications for Human and Veterinary Drug Products Center for Drug Evaluation and Research (CDER) Center
Guidance for Industry for the Submission Documentation for Sterilization Process Validation in Applications for Human and Veterinary Drug Products Center for Drug Evaluation and Research (CDER) Center
MINIMUM REQUIREMENTS FOR A VENDOR
 MINIMUM REQUIREMENTS FOR A VENDOR When outsourcing the production of sterile products the first step in vendor evaluation is to see if they meet the minimum requirements. We ve developed a group of questions
MINIMUM REQUIREMENTS FOR A VENDOR When outsourcing the production of sterile products the first step in vendor evaluation is to see if they meet the minimum requirements. We ve developed a group of questions
Aseptic Powder Dosing Solutions
 Aseptic Powder Dosing Solutions Delivering Solutions Application Expertise We are thoroughly familiar with your products and applications! Romaco specialises in engineering technologies for solid pharmaceutical
Aseptic Powder Dosing Solutions Delivering Solutions Application Expertise We are thoroughly familiar with your products and applications! Romaco specialises in engineering technologies for solid pharmaceutical
