2015-Tissue Bank Processing
|
|
|
- Brian Tate
- 6 years ago
- Views:
Transcription
1 2015-Tissue Bank Processing Form Approved OMB No Exp. Date 06/30/2020 Welcome to the 2015 Tissue Processing survey. Please refer to the following instructions as you complete this section of the survey. To facilitate accurate totals, provide counts or mark boxes according to the descriptions given. If the answer is none enter zero. If the information is not obtainable for a specific question enter 999. When you respond to certain questions, subsequent questions may change or disappear based on the services you provide. When you get to the end of a page, click Next to advance to the next page (or click Back to return to the previous page). When you reach the end of the survey click Submit. A survey must be completed in one sitting. Please do NOT include ocular-only and organ-only referrals or donors in this survey. Except where noted, all donations are for transplantation. Do NOT enter percentages unless requested. We are providing a document of definitions for certain terms within the survey. Most definitions are from the AATB Standard A2.000 DEFINITIONS OF TERMS; however, some are new or have been revised. To access pdf versions of the electronic surveys and the NTRUS Definitions of Terms, please go to aatb.org, click on the Standards & Regulatory tab at the top of the page and then click on NTRUS Documents". Paper surveys are for reference only, the final data must be submitted on the electronic survey. If you have any questions regarding the survey, please send an to aatb@aatb.org. Your responses are very important to us, and we appreciate your help! According to the Paperwork Reduction Act of 1995, no persons are required to respond to a collection of information unless it displays a valid OMB control number. The valid OMB control number for this information collection is The time required to complete this information collection is estimated to average 60 minutes per response, including the time to review instructions, search existing data resources, gather the data needed, and complete and review the information collection. If you have comments concerning the accuracy of the time estimate(s) or suggestions for improving this form, please write to: U.S. Department of Health & Human Services, OS/OCIO/PRA, 200 Independence Ave., S.W., Suite 336-E, Washington D.C , Attention: PRA Reports Clearance Officer OMB No
2 To avoid double reporting, include numbers only for your main facility and your satellite facilities (if applicable). The information you are reporting is for the following physical locations(s) by name, city and state. Name City State Location 1 Location 2 Location 3 Location 4 Location 5 Location 6 Location 7 Location 8 Location 9 Location 10 Location 11 Location 12 Location 13 Location 14 Location 15 Location 16 Location 17 Location 18
3 TISSUE FROM DECEASED DONORS Did your facility(ies) process tissue from deceased donors? Yes No Which of the following types of tissue did your tissue bank process? [Check all that apply] musculoskeletal (i.e., bone, cartilage, osteochondral grafts or osteoarticular grafts) soft tissue (i.e., fascia lata, ligaments, tendons, pericardium, nerves, peritoneal membrane or adipose) cardiac tissue vascular tissue skin cellular tissue dura mater other tissue from deceased donors, please specify... other tissue from deceased donors, please specify... other tissue from deceased donors, please specify... How many deceased donors did your tissue bank process? [Count one donor only once] What was the total number of grafts your tissue bank processed from deceased donors? What was the highest number of grafts your tissue bank processed from a deceased donor? How many deceased donors of the following types of tissues did your tissue bank process: [A donor may be counted more than once, depending on tissue types processed.]
4 Musculoskeletal Tissue bone osteochondral grafts fresh/refrigerated (i.e., an allograft consisting of a section, condyle, or plug of bone with an intact articular surface) osteochondral grafts frozen/cryopreserved (i.e., an allograft consisting of a section, condyle, or plug of bone with an intact articular surface) osteoarticular grafts fresh/refrigerated (i.e., a large weight bearing allograft with intact articular surfaces consisting of a joint with associated soft tissue and bone) osteoarticular grafts frozen/cryopreserved (i.e., a large weight bearing allograft with intact articular surfaces consisting of a joint with associated soft tissue and bone) cartilage (e.g., costal, articular) How many deceased donors of the following types of tissues did your tissue bank process: [A donor may be counted more than once, depending on tissue types processed.] Soft Tissue fascia lata ligaments (i.e., patellar) tendons (e.g., Achilles, gracillis, anterior/posterior tibialis, semitendinosus, flexors/extensors, peroneus longus) rotator cuff pericardium nerves peritoneal membrane adipose
5 How many deceased donors of the following types of tissues did your tissue bank process: [A donor may be counted more than once, depending on tissue types processed.] Cardiac Tissue valved conduits non-valved conduits patch graft aortoiliac graft How many deceased donors of the following types of tissues did your tissue bank process: [A donor may be counted more than once, depending on tissue types processed.] Vascular Tissue arteries vein grafts How many deceased donors of the following types of tissues did your tissue bank process: [A donor may be counted more than once, depending on tissue types processed.] Skin split thickness (fresh, cryopreserved) dermal tissue (including acellular/decellularized) lyophilized dehydrated
6 dessicated How many deceased donors of the following types of tissues did your tissue bank process: [A donor may be counted more than once, depending on tissue types processed.] cellular tissue dura mater tissue as a device (i.e., products and combination products requiring PMA or 510k clearance; regulated under the FD&C Act as well as under 21 CFR Part 1271 from Section 361 of the PHSA) tissue as a biological product (i.e., products requiring BLA or IND; regulated under Section 351 of the PHSA and/or the FD&C Act, as well as under 21 CFR Part 1271 from Section 361 of the PHSA) tissue as a drug (i.e., products requiring IND/NDA; regulated under Section 201 of the FD&C Act, as well as under 21 CFR 1271 from Section 361 of the PHSA) Other tissue from deceased donors: Other tissue from deceased donors Number of donors Other tissue 1 Other tissue 2 Other tissue 3 TISSUE FROM LIVING DONORS Did your facility(ies) process tissue from living donors? Yes No
7 Which of the following types of tissue did your tissue bank process? [Check all that apply] How many living donors did your tissue bank process? (Count one donor only once) What was the total number of grafts your tissue bank processed from living donors? What was the highest number of grafts your tissue bank processed from a living donor? living donor tissue (i.e., birth tissue, surgical bone, skin for allogeneic use, autologous parathyroid or autologous bone) other tissue from living donors, please specify... How many living donors of the following types of tissues did your tissue bank process: [A donor may be counted more than once, depending on tissue types processed.] Birth Tissue amniotic membrane (only) chorionic membrane (only) amniotic+chorionic membrane amniotic fluid Wharton s jelly placental/chorionic disc umbilical cord tissue umbilical vein
8 Other birth tissue from living donors: Other birth tissue from living donors Number of donors other tissue 1 other tissue 2 other tissue 3 How many living donors of the following types of tissues did your tissue bank process: [A donor may be counted more than once, depending on tissue types processed.] surgical bone skin for allogeneic use autologous bone autologous parathyroid tissue as a device (i.e., products and combination products requiring PMA or 510k clearance; regulated under the FD&C Act as well as under 21 CFR Part 1271 from Section 361 of the PHSA) tissue as a biological product (i.e., products and combination products requiring PMA or 510k clearance; regulated under the FD&C Act as well as under 21 CFR Part 1271 from Section 361 of the PHSA) tissue as a drug (i.e., products requiring IND/NDA; regulated under Section 201 of the FD&C Act, as well as under 21 CFR 1271 from Section 361 of the PHSA) Other tissue from living donors: Other tissue from living donors Number of donors other tissue 1 other tissue 2
9 other tissue 3 TISSUE FROM DECEASED AND LIVING DONORS How did your tissue bank treat tissues with radiation PRIOR to processing (nonterminal irradiation)? [Check all that apply] we did not treat tissues with radiation prior to processing gamma radiation only, below 1.5 Mrads (15 kgy) gamma radiation only, Mrads (15-25 kgy) gamma radiation only, above 2.5 Mrads (25 kgy) electron beam radiation only Electronic beam radiation only, indicate dose: How did your tissue bank treat tissues with radiation to reduce/eliminate microorganisms as a FINAL treatment (terminal irradiation)? [Check all that apply] we did not treat tissues with radiation as a final treatment gamma radiation only, below 1.5 Mrads (15 kgy) gamma radiation only, Mrads (15-25 kgy) gamma radiation only, above 2.5 Mrads (25 kgy) electron beam radiation only Electronic beam radiation only, indicate dose: TISSUE FROM DECEASED DONORS Did your facility(ies) process tissue from deceased donors? Yes
10 No MUSCULOSKELETAL GRAFTS Indicate how many musculoskeletal GRAFTS were processed using the following methods: electron beam radiation (only) gamma radiation (only) ethylene oxide (only) no terminal sterilization Indicate how many musculoskeletal GRAFTS were processed using the following methods: Types of proprietary/patented processing (only) Allowash ATP BioCleanse Process Clearant Process Tutoplast Process NovaSterilis (supercritical CO2) Other proprietary methods: Other method Number of musculoskeletal grafts processed other method 1 other method 2
11 other method 3 Combinations of Antibiotics and Radiation Musculoskeletal For each combination used specify antibiotic(s), radiation target dose (below 1.5 Mrads, between 1.5 and 2.5 Mrads, and above 2.5 Mrads) and number of grafts processed using these combination methods: Antibiotic(s) (specify) Antibiotic 1 Antibiotic Radiation Target Dose Number of grafts Antibiotic 2 Antibiotic 3 Combinations of Proprietary/Patented Processing then Radiation Musculoskeletal For each combination used please specify proprietary processing method, radiation target dose (below 1.5 Mrads, between 1.5 and 2.5 Mrads, and above 2.5 Mrads) and number of grafts processed using these combination methods: Proprietary/Patented Method (specify) Method Radiation Target Dose Number of grafts Proprietary/patented method 1 Proprietary/patented method 2 Proprietary/patented method 3 Report any other combinations of methods used:
12 SOFT TISSUE GRAFTS Indicate how many soft tissue GRAFTS (i.e., fascia lata, ligaments, tendons, pericardium, nerves, peritoneal membrane or adipose) were processed using the following methods: electron beam radiation (only) gamma radiation (only) ethylene oxide (only) no terminal sterilization Indicate how many soft tissue GRAFTS were processed using the following methods: Types of proprietary/patented processing (only) Allowash ATP BioCleanse Process Clearant Process Tutoplast Process NovaSterilis (supercritical CO2) Other proprietary methods: Other method Number of soft tissue grafts processed other method 1 other method 2 other method 3
13 Combinations of Antibiotics and Radiation Soft Tissue Grafts For each combination used specify antibiotic(s), radiation target dose (below 1.5 Mrads, between 1.5 and 2.5 Mrads, and above 2.5 Mrads) and number of grafts processed using these combination methods: Antibiotic(s) (specify) Antibiotic 1 Antibiotic Radiation Target Dose Number of grafts Antibiotic 2 Antibiotic 3 Combinations of Proprietary/Patented Processing then Radiation Soft Tissue Grafts For each combination used please specify proprietary processing method, radiation target dose (below 1.5 Mrads, between 1.5 and 2.5 Mrads, and above 2.5 Mrads) and number of grafts processed using these combination methods: Proprietary/Patented Method (specify) Method Radiation Target Dose Number of grafts Proprietary/patented method 1 Proprietary/patented method 2 Proprietary/patented method 3 Report any other combinations of methods used:
14 CARDIAC TISSUE GRAFTS Indicate how many cardiac tissue GRAFTS were preserved using the following methods: refrigerated only (i.e., provided for use as fresh) controlled-rate electronic programmable freezing Other methods-cardiac: Other method Number of cardiac tissue grafts preserved other method 1 other method 2 other method 3 Indicate how many cardiac tissue GRAFTS were processed into finished tissue for each of the following types: acellular/decellularized not acellular/decellularized Other type-cardiac: Other type Number of cardiac tissue grafts processed into finished tissue other type 1 other type 2 other type 3
15 VASCULAR GRAFTS Indicate how many vascular tissue GRAFTS were preserved using the following methods: refrigerated only (i.e., provided for use as fresh) controlled-rate electronic programmable freezing Other methods-vascular: Other method Number of vascular tissue grafts preserved other method 1 other method 2 other method 3 Indicate how many vascular tissue GRAFTS were processed into finished tissue for each of the following types: acellular/decellularized not acellular/decellularized Other type-vascular: Other type Number of vascular tissue grafts processed into finished tissue other type 1 other type 2 other type 3
16 Indicate how many skin GRAFTS were processed using the following methods: electron beam radiation (only) gamma radiation (only) ethylene oxide (only) no terminal sterilization Indicate how many skin GRAFTS were processed using the following methods: [Types of proprietary/patented processing (only)] Allowash ATP BioCleanse Process Clearant Process Tutoplast Process NovaSterilis (supercritical CO2) Other proprietary methods-skin: Other method Number of skin grafts processed other method 1 other method 2 other method 3
17 Combinations of Antibiotics and Radiation Skin For each combination used please specify antibiotic(s), radiation target dose (below 1.5 Mrads, between 1.5 and 2.5 Mrads, and above 2.5 Mrads) and number of grafts processed using these combination methods: Antibiotic(s) (specify) Antibiotic 1 Antibiotic Radiation Target Dose Number of grafts Antibiotic 2 Antibiotic 3 Combinations of Proprietary/Patented Processing then Radiation Skin For each combination used please specify proprietary processing method, radiation target dose (below 1.5 Mrads, between 1.5 and 2.5 Mrads, and above 2.5 Mrads) and number of grafts processed using these combination methods: Proprietary/Patented Method (specify) Method Radiation Target Dose Number of grafts Proprietary/patented method 1 Proprietary/patented method 2 Proprietary/patented method 3 Report any other combinations of methods used: Did your facility(ies) process tissue from living donors? Yes No
18 BIRTH TISSUE GRAFTS Indicate how many birth tissue GRAFTS were processed using the following methods: electron beam radiation (only) gamma radiation (only) ethylene oxide (only) no terminal sterilization filtration (only) ultraviolet light (only) Indicate how many birth tissue GRAFTS were processed using the following methods: [Types of proprietary/patented processing (only)] Allowash ATP BioCleanse Process Clearant Process Tutoplast Process NovaSterilis (supercritical CO2) Purion Process Cryotek Process
19 Other proprietary methods-birth tissue: Other method Number of birth tissue grafts processed other method 1 other method 2 other method 3 Combinations of Antibiotics and Radiation Birth Tissue Grafts For each combination used specify antibiotic(s), radiation target dose (below 1.5 Mrads, between 1.5 and 2.5 Mrads, and above 2.5 Mrads) and number of grafts processed using these combination methods: Antibiotic(s) (specify) Antibiotic 1 Antibiotic Radiation Target Dose Number of grafts Antibiotic 2 Antibiotic 3 Combinations of Proprietary/Patented Processing then Radiation Birth tissue grafts For each combination used please specify proprietary processing method, radiation target dose (below 1.5 Mrads, between 1.5 and 2.5 Mrads, and above 2.5 Mrads) and number of grafts processed using these combination methods: Proprietary/Patented Method (specify) Method Radiation Target Dose Number of grafts Proprietary/patented method 1 Proprietary/patented method 2
20 Proprietary/patented method 3 Report any other combinations of methods used: DEMINERALIZED BONE For what applications did your tissue bank process demineralized bone? [Check all that apply] we did not process demineralized bone orthopedic surgery dental/periodontal procedures neurosurgery other applications, please specify... SKIN Did your tissue bank process skin for use as fresh grafts (not cryopreserved)? no, we did not process skin no, we processed skin, but not for use as fresh grafts yes, we processed skin for use as fresh grafts Indicate how much skin (in square feet) was preserved by each of the following methods: refrigerated only controlled-rate electronic programmable freezing heat sink freezing method lyophilized dessicated
21 dehydrated Other methods-skin by square foot: Other method Skin in square feet other method 1 other method 2 other method 3 Indicate how much skin (in square feet) was processed into finished tissue for each of the following types: acellular/decellularized not acellular/decellularized BIRTH TISSUE Did your facility(ies) process birth tissue from living donors? Yes No Indicate how many units of birth tissue were preserved using the following methods; this refers to the preservation method used only for finished tissue: refrigerated only (i.e., provided for use as fresh) simple freezing controlled-rate electronic programmable freezing lyophilized dehydrated
22 dessicated Other methods-birth tissue: Other method Units of birth tissue preserved other method 1 other method 2 other method 3 Indicate how many units of birth tissue were processed into finished tissue for each of the following types: acellular/decellularized not acellular/decellularized For what applications did your tissue bank process birth tissue? [check all that apply] ophthalmic leg/foot ulcers orthopedic dental/periodontal neurosurgical and spine burns general surgical other general uses, please specify... RECOVERY ENTITIES Indicate how many donors of SKIN were recovered by the following: AATB-accredited OPOs/tissue banks
23 non-aatb accredited OPOs/tissue banks health care facilities (e.g., hospital or surgical center) Indicate how many donors of MUSCULOSKELETAL TISSUE were recovered by the following: AATB-accredited OPOs/tissue banks non-aatb accredited OPOs/tissue banks health care facilities (e.g., hospital or surgical center) Indicate how many donors of SOFT TISSUE were recovered by the following: AATB-accredited OPOs/tissue banks non-aatb accredited OPOs/tissue banks health care facilities (e.g., hospital or surgical center) Indicate how many donors of CARDIAC TISSUE were recovered by the following: AATB-accredited OPOs/tissue banks non-aatb accredited OPOs/tissue banks health care facilities (e.g., hospital or surgical center) Indicate how many donors of VASCULAR TISSUE were recovered by the following: AATB-accredited OPOs/tissue banks non-aatb accredited OPOs/tissue banks
24 health care facilities (e.g., hospital or surgical center) Indicate how many donors of BIRTH TISSUE were provided by the following: hospital delivery/birth centers freestanding birth centers (not at a hospital) AATB-accredited OPOs/tissue banks non-aatb accredited OPOs/tissue banks Other provider-birth tissue: Other provider Number of donors other provider 1 other provider 2 other provider 3 Indicate how many donors of birth tissue delivered by: cesarean section vaginally FURTHER MANUFACTURING Did your tissue bank send any human tissue to another tissue bank for further manufacture? Yes No
25 What tissue was sent for further manufacture? [Check all that apply] demineralized bone matrix cancellous bone bone shafts other tissue, please specify... other tissue, please specify... PROCESSING AND DISTRIBUTION - OTHER COUNTRIES Did your tissue bank process any human tissue from non-u.s. sources? Yes No List the non - U.S. countries: Did your tissue bank import human tissue from other countries for processing and distribution in the U.S.? Yes No List the countries from which donors were imported, the number of donors processed, the general types of tissue grafts, and the quantities distributed: Did your tissue bank process tissue from other countries only for distribution by countries other than the U.S. (i.e., processing contract only)? Yes No
26 Indicate the tissue received and the country of origin (tissue from deceased donors): musculoskeletal (i.e., bone, cartilage, osteochondral grafts or osteoarticular grafts) Country of origin soft tissue (i.e., fascia lata, ligaments, tendons, pericardium, nerves, peritoneal membrane or adipose): dura mater cardiac tissue vascular tissue skin Country of origin Country of origin Country of origin Country of origin Country of origin Indicate the tissue received and the country of origin (tissue from living donors): surgical bone Country of origin skin for allogeneic use autologous bone birth tissue Country of origin Country of origin Country of origin
2015-Tissue Storage. Welcome to the 2015 Tissue Storage survey. Please refer to the following instructions as you complete this section of the survey.
 2015-Tissue Storage Form Approved OMB No. 0990-0457 Exp. Date 06/30/2020 Welcome to the 2015 Tissue Storage survey. Please refer to the following instructions as you complete this section of the survey.
2015-Tissue Storage Form Approved OMB No. 0990-0457 Exp. Date 06/30/2020 Welcome to the 2015 Tissue Storage survey. Please refer to the following instructions as you complete this section of the survey.
2012-Tissue Distribution
 2012-Tissue Distribution Form Approved OMB. 0990-0457 Exp. Date 06/30/2020 Welcome to the 2012 Tissue Distribution survey. Please refer to the following instructions as you complete this section of the
2012-Tissue Distribution Form Approved OMB. 0990-0457 Exp. Date 06/30/2020 Welcome to the 2012 Tissue Distribution survey. Please refer to the following instructions as you complete this section of the
FDA Regulatory, Compliance and Policy Developments: 361 HCT/Ps
 FDA Regulatory, Compliance and Policy Developments: 361 HCT/Ps September 27, 2018 Presentation by: Elaine H. Tseng Partner FDA and Life Sciences Group King & Spalding Discussion with: Thomas Poché Vice
FDA Regulatory, Compliance and Policy Developments: 361 HCT/Ps September 27, 2018 Presentation by: Elaine H. Tseng Partner FDA and Life Sciences Group King & Spalding Discussion with: Thomas Poché Vice
Importing/Exporting Conventional HCT/Ps
 Importing/Exporting Conventional HCT/Ps ISCT/FDA Liaison Meeting January 4, 2007 Scott A. Brubaker, CTBS American Association of Tissue Banks Conventional Tissue Banks! FDA term from the preamble to the
Importing/Exporting Conventional HCT/Ps ISCT/FDA Liaison Meeting January 4, 2007 Scott A. Brubaker, CTBS American Association of Tissue Banks Conventional Tissue Banks! FDA term from the preamble to the
Regulatory Considerations for Human Cell, Tissues, and Cellular and Tissue- Based Products: Minimal Manipulation and Homologous Use
 Regulatory Considerations for Human Cell, Tissues, and Cellular and Tissue- Based Products: Minimal Manipulation and Homologous Use Guidance for Industry and Food and Drug Administration Staff For questions
Regulatory Considerations for Human Cell, Tissues, and Cellular and Tissue- Based Products: Minimal Manipulation and Homologous Use Guidance for Industry and Food and Drug Administration Staff For questions
June 10, In Re: Draft Request for Proposal for Biological Implants, Solicitation Number: VA11916R0139
 June 10, 2016 Henry T. Wells U.S. Department of Veterans Affairs Strategic Acquisition Center 10300 Spotsylvania Ave. Fredericksburg VA 22408 In Re: Draft Request for Proposal for Biological Implants,
June 10, 2016 Henry T. Wells U.S. Department of Veterans Affairs Strategic Acquisition Center 10300 Spotsylvania Ave. Fredericksburg VA 22408 In Re: Draft Request for Proposal for Biological Implants,
HCT/P Regulation vs 361 Products
 HCT/P Regulation - 351 vs 361 Products Presented by: Paul Gadiock February 15, 2017 Arent Fox LLP Washington, DC New York, NY Los Angeles, CA San Francisco, CA 1 Presentation Overview Introduction Public
HCT/P Regulation - 351 vs 361 Products Presented by: Paul Gadiock February 15, 2017 Arent Fox LLP Washington, DC New York, NY Los Angeles, CA San Francisco, CA 1 Presentation Overview Introduction Public
Matrix HD. wound covering. Sterile, room temperature human dermis graft
 Matrix HD wound covering Sterile, room temperature human dermis graft Matrix HD wound covering Sterile, room temperature human dermis graft Matrix HD is an acellular human dermis allograft sterilized using
Matrix HD wound covering Sterile, room temperature human dermis graft Matrix HD wound covering Sterile, room temperature human dermis graft Matrix HD is an acellular human dermis allograft sterilized using
Tissue Engineering For Regenerating Life. Cryopreserved Products. Tissue Corporation
 Tissue Engineering For Regenerating Life Cryopreserved Products Tissue Corporation Tissue Corporation TRC (Tissue regeneration corporation) is a multi-facility institute specializing in the preparation
Tissue Engineering For Regenerating Life Cryopreserved Products Tissue Corporation Tissue Corporation TRC (Tissue regeneration corporation) is a multi-facility institute specializing in the preparation
HANS BIOMED USA, Inc. 140 sylvan Ave Suite #4 Englewood Cliffs, NJ TEL FAX:
 HANS BIOMED USA, Inc. 140 sylvan Ave Suite #4 Englewood Cliffs, NJ 07632 TEL 201 2242333 FA: 201 221 2330 Aug 2, 2016 To Whom it May Concern, Hans Biomed manufactures ExponentT M DBM and PureBone Demineralized
HANS BIOMED USA, Inc. 140 sylvan Ave Suite #4 Englewood Cliffs, NJ 07632 TEL 201 2242333 FA: 201 221 2330 Aug 2, 2016 To Whom it May Concern, Hans Biomed manufactures ExponentT M DBM and PureBone Demineralized
February 17, DSM Biomedical Ms. Brianna Jordan Regulatory Specialist 735 Pennsylvania Drive Exton, Pennsylvania 19341
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control Center WO66-G609 Silver Spring, MD 20993-0002 February 17, 2015 DSM
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control Center WO66-G609 Silver Spring, MD 20993-0002 February 17, 2015 DSM
Human Tissue Importation Practices in Canada
 Human Tissue Importation Practices in Canada October 2006 Human Tissue Importation Practices in Canada Prepared for the Canadian Council for Donation and Transplantation Deloitte Inc. October 2006 1 Table
Human Tissue Importation Practices in Canada October 2006 Human Tissue Importation Practices in Canada Prepared for the Canadian Council for Donation and Transplantation Deloitte Inc. October 2006 1 Table
3/20/2017. R 3 Repair Paradigm REGENERATIVE MEDICINE GLOSSARY OF STEM CELL PLATFORMS. Limitations of Embryonic Stem Cells
 R 3 Repair Paradigm CENTER FOR REGENERATIVE MEDICINE Regenerative Medicine Regulatory And Practice Management Focus Replacement ACL/UCL Reconstruction Regeneration Next generation therapy Repair Rejuvenation
R 3 Repair Paradigm CENTER FOR REGENERATIVE MEDICINE Regenerative Medicine Regulatory And Practice Management Focus Replacement ACL/UCL Reconstruction Regeneration Next generation therapy Repair Rejuvenation
Safa Karandish Office of Tissues and Advanced Therapies Division of Human Tissues. Pharma Conference February
 Safa Karandish Office of Tissues and Advanced Therapies Division of Human Tissues Pharma Conference February 2017 1 Background HCT/P regulatory framework Definitions & exceptions Establishment registration-
Safa Karandish Office of Tissues and Advanced Therapies Division of Human Tissues Pharma Conference February 2017 1 Background HCT/P regulatory framework Definitions & exceptions Establishment registration-
UDI Guidance Document for Medical Devices Containing HCT/P
 LEARNING UDI COMMUNITY UDI Guidance Document for Medical Devices Containing HCT/P WWW.AHRMM.ORG / LUC INTRODUCTION: Human cells, tissues, or cellular or tissue-based products (HCT/Ps) are a precious resource
LEARNING UDI COMMUNITY UDI Guidance Document for Medical Devices Containing HCT/P WWW.AHRMM.ORG / LUC INTRODUCTION: Human cells, tissues, or cellular or tissue-based products (HCT/Ps) are a precious resource
Tissue cleaning and sterilization provide an additional level of safety
 Managing biologics Understanding tissue processing Third in a series on managing bone allografts. In the October issue, articles included were Allografts: Overview of the process; and Donor screening:
Managing biologics Understanding tissue processing Third in a series on managing bone allografts. In the October issue, articles included were Allografts: Overview of the process; and Donor screening:
Tissue banking CHAPTER 40
 CHAPTER 40 Tissue banking Ralph M. Powers 1 & Jeanne V. Linden 2 1 Institute of Regenerative Medicine, LifeNet Health, Virginia Beach, VA, USA 2 Blood and Tissue Resources, Wadsworth Center, New York State
CHAPTER 40 Tissue banking Ralph M. Powers 1 & Jeanne V. Linden 2 1 Institute of Regenerative Medicine, LifeNet Health, Virginia Beach, VA, USA 2 Blood and Tissue Resources, Wadsworth Center, New York State
Human Tissue Intended for Transplantation Skin, corneas, sclera, bone, heart valves, blood vessels, pericardium, tendons, cartilage, fascia
 TITLE / DESCRIPTION: DEPARTMENT: Human Tissue Intended for Transplantation Skin, corneas, sclera, bone, heart valves, blood vessels, pericardium, tendons, cartilage, fascia Surgical Services PERSONNEL:
TITLE / DESCRIPTION: DEPARTMENT: Human Tissue Intended for Transplantation Skin, corneas, sclera, bone, heart valves, blood vessels, pericardium, tendons, cartilage, fascia Surgical Services PERSONNEL:
We invite you to learn more with the enclosed information or through our websites and
 Bioventus LLC 4721 Emperor Blvd., Suite 100 Durham, NC 27703 USA P 800.637.4391 F 888.279.0152 www.bioventussurgical.com Dear Materials Manager, Bioventus LLC, a global leader in orthobiologics, is driven
Bioventus LLC 4721 Emperor Blvd., Suite 100 Durham, NC 27703 USA P 800.637.4391 F 888.279.0152 www.bioventussurgical.com Dear Materials Manager, Bioventus LLC, a global leader in orthobiologics, is driven
Osteobiologic B O N E G R A F T I N G S O L U T I O N S
 Osteobiologic BONE GRAFTING SOLUTIONS The AlloSource Advantage Delivering Quality and Safety * Allografts labeled Sterile R are aseptically processed and packaged and then subjected to gamma irradiation.
Osteobiologic BONE GRAFTING SOLUTIONS The AlloSource Advantage Delivering Quality and Safety * Allografts labeled Sterile R are aseptically processed and packaged and then subjected to gamma irradiation.
We invite you to learn more with the enclosed information or through our websites and
 Bioventus LLC 4721 Emperor Blvd., Suite 100 Durham, NC 27703 USA P 800.637.4391 F 888.279.0152 www.bioventussurgical.com Dear Materials Manager, Bioventus LLC, a global leader in orthobiologics, is driven
Bioventus LLC 4721 Emperor Blvd., Suite 100 Durham, NC 27703 USA P 800.637.4391 F 888.279.0152 www.bioventussurgical.com Dear Materials Manager, Bioventus LLC, a global leader in orthobiologics, is driven
Internationally Standardized Nomenclature for Skin Products DRAFT
 Internationally Standardized Nomenclature for Skin Products DRAFT SkinNomenclature 1 Introduction SkinNomenclature There is growing recognition of the need for a globally consistent nomenclature and a
Internationally Standardized Nomenclature for Skin Products DRAFT SkinNomenclature 1 Introduction SkinNomenclature There is growing recognition of the need for a globally consistent nomenclature and a
Subj: ESTABLISHMENT OF A HUMAN CELL, TISSUE, AND CELLULAR AND TISSUE-BASED PRODUCTS MANAGEMENT PROGRAM
 DEPARTMENT OF THE NAVY BUREAU OF MEDICINE AND SURGERY 7700 ARLINGTON BOULEVARD FALLS CHURCH, VA 22042 IN REPLY REFER TO ` BUMEDINST 6300.21 BUMED-M3 BUMED INSTRUCTION 6300.21 From: Chief, Bureau of Medicine
DEPARTMENT OF THE NAVY BUREAU OF MEDICINE AND SURGERY 7700 ARLINGTON BOULEVARD FALLS CHURCH, VA 22042 IN REPLY REFER TO ` BUMEDINST 6300.21 BUMED-M3 BUMED INSTRUCTION 6300.21 From: Chief, Bureau of Medicine
UNIT CELL PROCESSES UNDERLYING TISSUE ENGINEERING AND REGENERATIVE MEDICINE
 Massachusetts Institute of Technology Harvard Medical School Brigham and Women s Hospital VA Boston Healthcare System 2.79J/3.96J/20.441/HST522J UNIT CELL PROCESSES UNDERLYING TISSUE ENGINEERING AND REGENERATIVE
Massachusetts Institute of Technology Harvard Medical School Brigham and Women s Hospital VA Boston Healthcare System 2.79J/3.96J/20.441/HST522J UNIT CELL PROCESSES UNDERLYING TISSUE ENGINEERING AND REGENERATIVE
Certifications: Table of Contents. Document # Title Page(s)
 Certifications: Table of Contents Document # Title Page(s) 1 ISO 17025: Certificate of Accreditation 2 2 ISO 17025: Scope of Accreditation 3-7 3 FDA: Medical Device Establishment 8 Registration 4 FDA:
Certifications: Table of Contents Document # Title Page(s) 1 ISO 17025: Certificate of Accreditation 2 2 ISO 17025: Scope of Accreditation 3-7 3 FDA: Medical Device Establishment 8 Registration 4 FDA:
South Carolina Donor Registry Q & A
 South Carolina Donor Registry Q & A How do I become a registered organ and tissue donor? There are several ways to register your legal consent to be a donor: 1.) At any SCDMV office or on the SCDMV web
South Carolina Donor Registry Q & A How do I become a registered organ and tissue donor? There are several ways to register your legal consent to be a donor: 1.) At any SCDMV office or on the SCDMV web
FOR IMMEDIATE RELEASE
 FOR IMMEDIATE RELEASE Media Contact: Melanie Battista, Public Relations (TSX-V: LBL) (OTCBB: BLVKF) 16701 N 90th Street, Suite#101 Scottsdale, AZ 85260 480-563-0800 Office News@LatticeBiologics.com www.latticebiologics.com
FOR IMMEDIATE RELEASE Media Contact: Melanie Battista, Public Relations (TSX-V: LBL) (OTCBB: BLVKF) 16701 N 90th Street, Suite#101 Scottsdale, AZ 85260 480-563-0800 Office News@LatticeBiologics.com www.latticebiologics.com
LEADING EVIDENCE BASED PRACTICE GUIDELINES FOR:
 Bioburden Reduction and Control In Tissue Banking LEADING EVIDENCE BASED PRACTICE GUIDELINES FOR: Tissue Recovery Microbial Sampling Processing of Musculoskeletal Tissue Processing of Cardiac Tissue Processing
Bioburden Reduction and Control In Tissue Banking LEADING EVIDENCE BASED PRACTICE GUIDELINES FOR: Tissue Recovery Microbial Sampling Processing of Musculoskeletal Tissue Processing of Cardiac Tissue Processing
An Advancing Allografts Brief October Sterilization of Allografts. Advancing Allografts
 An Advancing Allografts Brief October 24 Sterilization of Allografts Advancing Allografts Ensuring Safety in Tissue Transplantation: The Sterilization of Allografts By Lloyd Wolfinbarger, Jr., PhD Tissue
An Advancing Allografts Brief October 24 Sterilization of Allografts Advancing Allografts Ensuring Safety in Tissue Transplantation: The Sterilization of Allografts By Lloyd Wolfinbarger, Jr., PhD Tissue
A review of Dr. Dinakar Golla s clinical research with AdMatrix surgical grafts for soft tissue repair
 TEAMeffort: Using aggressive surgical techniques in combination with AdMatrix (Lattice Biologics acellular dermal scaffold product) to heal difficult and persistent wounds A review of Dr. Dinakar Golla
TEAMeffort: Using aggressive surgical techniques in combination with AdMatrix (Lattice Biologics acellular dermal scaffold product) to heal difficult and persistent wounds A review of Dr. Dinakar Golla
Certifications: Table of Contents. Document # Title Page(s)
 Certifications: Table of Contents Document # Title Page(s) 1 ISO 17025: Certificate of Accreditation 2 2 ISO 17025: Scope of Accreditation 3-6 3 FDA: Medical Device Establishment 7 Registration 4 FDA:
Certifications: Table of Contents Document # Title Page(s) 1 ISO 17025: Certificate of Accreditation 2 2 ISO 17025: Scope of Accreditation 3-6 3 FDA: Medical Device Establishment 7 Registration 4 FDA:
Inspection of Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps)
 COMPLIANCE PROGRAM GUIDANCE MANUAL Inspection of Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps) 7341.002 Implementation Date: Completion Date: when posted on-going Program/Assignment
COMPLIANCE PROGRAM GUIDANCE MANUAL Inspection of Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps) 7341.002 Implementation Date: Completion Date: when posted on-going Program/Assignment
FDA Approach to the Regulation of Hematopoietic Stem/Progenitor Cells (HPC)
 FDA Approach to the Regulation of Hematopoietic Stem/Progenitor Cells (HPC) Ellen Lazarus, M.D. Medical Officer Division of Human Tissues Office of Cellular, Tissue, and Gene Therapies FDA proposed approach
FDA Approach to the Regulation of Hematopoietic Stem/Progenitor Cells (HPC) Ellen Lazarus, M.D. Medical Officer Division of Human Tissues Office of Cellular, Tissue, and Gene Therapies FDA proposed approach
Regulatory Issues in Human Subjects Research
 Regulatory Issues in Human Subjects Research Ian McNiece, PhD University of Miami Human Subjects Research Require IRB approval Studies of new drugs or applications of drugs require an FDA approved IND
Regulatory Issues in Human Subjects Research Ian McNiece, PhD University of Miami Human Subjects Research Require IRB approval Studies of new drugs or applications of drugs require an FDA approved IND
Development of Regenerative Medicine Products: FDA Perspectives
 資料 3-3 Development of Regenerative Medicine Products: FDA Perspectives Steven R. Bauer, Ph.D. Chief, Cellular and Tissue Therapies Branch Office of Cellular, Tissue and Gene Therapies Center for Biologics
資料 3-3 Development of Regenerative Medicine Products: FDA Perspectives Steven R. Bauer, Ph.D. Chief, Cellular and Tissue Therapies Branch Office of Cellular, Tissue and Gene Therapies Center for Biologics
Quality Manual. Quality Manual. Vera Bioscience / Anu Life Sciences. April 2018
 Quality Manual Vera Bioscience / Anu Life Sciences April 2018 Page 1 of 15 TABLE OF CONTENTS Quality Manual Page 1. Company Overview 3 2. References 3 3. Exemptions, Alternatives and Variances 3 4. General
Quality Manual Vera Bioscience / Anu Life Sciences April 2018 Page 1 of 15 TABLE OF CONTENTS Quality Manual Page 1. Company Overview 3 2. References 3 3. Exemptions, Alternatives and Variances 3 4. General
Processed from Donated Human Tissue by LifeCell Corporation for KCI USA, Inc.
 for wounds instructions for use Processed from Donated Human Tissue by LifeCell Corporation for KCI USA, Inc. marketed by KCI USA, Inc. San Antonio, TX 78219 USA 800.275.4524 www.kci1.com www.graftjacketbykci.com
for wounds instructions for use Processed from Donated Human Tissue by LifeCell Corporation for KCI USA, Inc. marketed by KCI USA, Inc. San Antonio, TX 78219 USA 800.275.4524 www.kci1.com www.graftjacketbykci.com
Tissue Products Catalogue
 Tissue Products Catalogue Tissue Processing: ecoo Technology Our Tissues business segment is focused on processing tissue components such as bones, soft tissues and skin of both human and animal origin.
Tissue Products Catalogue Tissue Processing: ecoo Technology Our Tissues business segment is focused on processing tissue components such as bones, soft tissues and skin of both human and animal origin.
FDA Regulation of Stem Cell Therapies. Regulation of adipose derived stromal preps
 2018 FDA Regulation of Stem Cell Therapies Regulation of adipose derived stromal preps EMERGING NEW FIELD BUT STILL A TODDLER Regenerative medicine technologies for orthopedic medicine application has
2018 FDA Regulation of Stem Cell Therapies Regulation of adipose derived stromal preps EMERGING NEW FIELD BUT STILL A TODDLER Regenerative medicine technologies for orthopedic medicine application has
FDA s Same Surgical Procedure Draft Guidance Could Stifle Use of Autologous Stem Cell Therapies
 FDA s Same Surgical Procedure Draft Guidance Could Stifle Use of Autologous Stem Cell Therapies Executive Summary, June 2015 Life Sciences Practice Group AUTHOR Areta L. Kupchyk Nixon Peabody LLP Washington,
FDA s Same Surgical Procedure Draft Guidance Could Stifle Use of Autologous Stem Cell Therapies Executive Summary, June 2015 Life Sciences Practice Group AUTHOR Areta L. Kupchyk Nixon Peabody LLP Washington,
CBER Regulation of Devices for Cell Therapy
 CBER Regulation of Devices for Cell Therapy Richard D. McFarland, Ph.D., M.D. Associate Director for Policy Office of Cellular, Tissue and Gene Therapies Center for Biologics Evaluation and Research Food
CBER Regulation of Devices for Cell Therapy Richard D. McFarland, Ph.D., M.D. Associate Director for Policy Office of Cellular, Tissue and Gene Therapies Center for Biologics Evaluation and Research Food
Expert in biological products of human origin BLOOD PRODUCTS STABLE PRODUCTS STEM CELLS HUMAN TISSUES MOTHER S MILK
 BLOOD PRODUCTS STABLE PRODUCTS STEM CELLS HUMAN TISSUES MOTHER S MILK Rosalie makes her first apheresis plasma donation. Expert in biological products of human origin 2016 2017 ACTIVITY REPORT MISSION
BLOOD PRODUCTS STABLE PRODUCTS STEM CELLS HUMAN TISSUES MOTHER S MILK Rosalie makes her first apheresis plasma donation. Expert in biological products of human origin 2016 2017 ACTIVITY REPORT MISSION
ALLODERM SELECT ALLODERM SELECT RESTORE
 ALLODERM SELECT ALLODERM SELECT RESTORE Regenerative Tissue Matrix Instructions for Use Processed from donated human tissue by: LifeCell Corporation One Millennium Way Branchburg, NJ 08876-3876 DESCRIPTION
ALLODERM SELECT ALLODERM SELECT RESTORE Regenerative Tissue Matrix Instructions for Use Processed from donated human tissue by: LifeCell Corporation One Millennium Way Branchburg, NJ 08876-3876 DESCRIPTION
TISSUE ENGINEERING AND REGENERATION: TECHNOLOGIES AND GLOBAL MARKETS
 TISSUE ENGINEERING AND REGENERATION: TECHNOLOGIES AND GLOBAL MARKETS HLC101B August 2014 Yojana Jeevane Project Analyst ISBN: 1-56965-894-3 BCC Research 49 Walnut Park, Building 2 Wellesley, MA 02481 USA
TISSUE ENGINEERING AND REGENERATION: TECHNOLOGIES AND GLOBAL MARKETS HLC101B August 2014 Yojana Jeevane Project Analyst ISBN: 1-56965-894-3 BCC Research 49 Walnut Park, Building 2 Wellesley, MA 02481 USA
The Biomechanics of ProLayer Acellular Dermal Matrix: suture retention strength
 The Biomechanics of ProLayer Acellular Dermal Matrix: suture retention strength Reginald Stilwell, B.S., C.T.B.S., Ryan Delaney, M.S. ProLayer, Centennial, CO Basic science volume 2 ProLayer Acellular
The Biomechanics of ProLayer Acellular Dermal Matrix: suture retention strength Reginald Stilwell, B.S., C.T.B.S., Ryan Delaney, M.S. ProLayer, Centennial, CO Basic science volume 2 ProLayer Acellular
Strategies for Ensuring Integrity for Medical Tissues and Devices
 Strategies for Ensuring Integrity for Medical Tissues and Devices RFID technologies offer near-real time safe and secure item-level visibility By Joe Pleshek Business Development Director Terso Solutions,
Strategies for Ensuring Integrity for Medical Tissues and Devices RFID technologies offer near-real time safe and secure item-level visibility By Joe Pleshek Business Development Director Terso Solutions,
Updated main section titles to more closely reflect the names of the families of products supported by ISBT 128. Added New Class: POSTERIOR PART
 ISBT 128 Standard Terminology for Blood, Cellular Therapy, and Version Control Sheet 2014 Version 6.0 November 21, 2014 1 Throughout document 2 2.3.2.2.1 3 3.2.1.1 4 4.1.2 5 10.2 6 10.2.1.1 The ISBT 128
ISBT 128 Standard Terminology for Blood, Cellular Therapy, and Version Control Sheet 2014 Version 6.0 November 21, 2014 1 Throughout document 2 2.3.2.2.1 3 3.2.1.1 4 4.1.2 5 10.2 6 10.2.1.1 The ISBT 128
Stem Cell Therapy for Wound Healing in Diabetic Limb
 Stem Cell Therapy for Wound Healing in Diabetic Limb Tanom Bunaprasert M.D. EC-ATMPs ( EC- Advanced Therapy Medicinal Products) Chulalongkorn Hospital Authority of Laws Public Health Safety act (
Stem Cell Therapy for Wound Healing in Diabetic Limb Tanom Bunaprasert M.D. EC-ATMPs ( EC- Advanced Therapy Medicinal Products) Chulalongkorn Hospital Authority of Laws Public Health Safety act (
Stem Cells, Regenerative Medicine and cgmp (GTP)
 Stem Cells, Regenerative Medicine and cgmp (GTP) Encompass Stem cell based therapies activities Collection source Purification Isolation from other cell types if needed Manipulation Minimal vs Moderate
Stem Cells, Regenerative Medicine and cgmp (GTP) Encompass Stem cell based therapies activities Collection source Purification Isolation from other cell types if needed Manipulation Minimal vs Moderate
Advancing the Science of Gamma Irradiation Continuous improvement in radiation sterilization
 Advancing the Science of Gamma Irradiation Continuous improvement in radiation sterilization Fatima Hasanain, Polymer Materials Specialist www.nordion.com Why are we here today? 2 Overview Radiation sterilization
Advancing the Science of Gamma Irradiation Continuous improvement in radiation sterilization Fatima Hasanain, Polymer Materials Specialist www.nordion.com Why are we here today? 2 Overview Radiation sterilization
TissueMend Soft Tissue Repair Matrix For Biologically Inspired Augmentation of Tendon Healing. Technical Q&A
 TissueMend Soft Tissue Repair Matrix For Biologically Inspired Augmentation of Tendon Healing Technical Q&A TissueMend Soft Tissue Repair Matrix For Biologically Inspired Augmentation of Tendon Healing
TissueMend Soft Tissue Repair Matrix For Biologically Inspired Augmentation of Tendon Healing Technical Q&A TissueMend Soft Tissue Repair Matrix For Biologically Inspired Augmentation of Tendon Healing
Repliform. Tissue Regeneration Matrix. Instructions for Use. Distributed by:
 Repliform Tissue Regeneration Matrix Instructions for Use Distributed by: Boston Scientific Corporation 300 Boston Scientific Way Marlborough, MA 01752 Processed from Donated Human Tissue by: LifeCell
Repliform Tissue Regeneration Matrix Instructions for Use Distributed by: Boston Scientific Corporation 300 Boston Scientific Way Marlborough, MA 01752 Processed from Donated Human Tissue by: LifeCell
Chapter 1 THE CONTRIBUTION OF KENNETH W. SELL TO THE PROGRESS OF TISSUE BANKING. 1.1 Kenneth W. Sell - Reflections on a Successful Life
 Chapter 1 THE CONTRIBUTION OF KENNETH W. SELL TO THE PROGRESS OF TISSUE BANKING 1.1 Kenneth W. Sell - Reflections on a Successful Life L. Kiesow (Legacy Research, Portland, USA) 1.2 Brief History of the
Chapter 1 THE CONTRIBUTION OF KENNETH W. SELL TO THE PROGRESS OF TISSUE BANKING 1.1 Kenneth W. Sell - Reflections on a Successful Life L. Kiesow (Legacy Research, Portland, USA) 1.2 Brief History of the
November 9, AtriCure, Inc. Jonathan McElwee Regulatory Affairs Manager 7555 Innovation Way Mason, Ohio 45040
 November 9, 2018 AtriCure, Inc. Jonathan McElwee Regulatory Affairs Manager 7555 Innovation Way Mason, Ohio 45040 Re: K182565 Trade/Device Name: AtriCure Regulation Number: 21 CFR 882.4250 Regulation Name:
November 9, 2018 AtriCure, Inc. Jonathan McElwee Regulatory Affairs Manager 7555 Innovation Way Mason, Ohio 45040 Re: K182565 Trade/Device Name: AtriCure Regulation Number: 21 CFR 882.4250 Regulation Name:
TRELLIS COLLAGEN RIBBON
 TRELLIS COLLAGEN RIBBON 147321-1 English (en) The following languages are included in this packet: M Wright Medical Technology, Inc. 5677 Airline Rd. Arlington, TN 38002 USA www.wmt.com August 2012 Printed
TRELLIS COLLAGEN RIBBON 147321-1 English (en) The following languages are included in this packet: M Wright Medical Technology, Inc. 5677 Airline Rd. Arlington, TN 38002 USA www.wmt.com August 2012 Printed
BIOFIBER BIOFIBER CM AMBIENT TEMPERATUR. Absorbable Scaffold. Collagen Coated. Absorbable Scaffold
 BIOFIBER Absorbable Scaffold BIOFIBER CM Collagen Coated Absorbable Scaffold A B S O R B A B L E AMBIENT TEMPERATUR B I O L O G I C S C AAND F F OCRYOPRESERVE L D I N G S OSTORAGE L U T I O N S OPTION
BIOFIBER Absorbable Scaffold BIOFIBER CM Collagen Coated Absorbable Scaffold A B S O R B A B L E AMBIENT TEMPERATUR B I O L O G I C S C AAND F F OCRYOPRESERVE L D I N G S OSTORAGE L U T I O N S OPTION
COMMISSION OF THE EUROPEAN COMMUNITIES. Proposal for a DIRECTIVE OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL
 COMMISSION OF THE EUROPEAN COMMUNITIES Brussels, 19.6.2002 COM(2002) 319 final 2002/0128(COD) Proposal for a DIRECTIVE OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL on setting standards of quality and
COMMISSION OF THE EUROPEAN COMMUNITIES Brussels, 19.6.2002 COM(2002) 319 final 2002/0128(COD) Proposal for a DIRECTIVE OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL on setting standards of quality and
07/30/2013. Record of Revisions IRB Page 1 of 17
 Current Protocol Version 7.1 (Continuing Review) Approved July 30, 2013 Version 7.1 (Amendment) September 6, 2012 Version 7.0 (Continuing Review) July 30, 2012 Version 6.0 (Continuing Review) July 30,
Current Protocol Version 7.1 (Continuing Review) Approved July 30, 2013 Version 7.1 (Amendment) September 6, 2012 Version 7.0 (Continuing Review) July 30, 2012 Version 6.0 (Continuing Review) July 30,
Specifically designed for abdominal wall repair STRUCTURAL
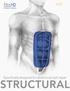 Specifically designed for abdominal wall repair STRUCTURAL The Better Approach To a Better Allograft Better Standards: Board of Directors comprised primarily of surgeons 3-years run by surgeons, for surgeons
Specifically designed for abdominal wall repair STRUCTURAL The Better Approach To a Better Allograft Better Standards: Board of Directors comprised primarily of surgeons 3-years run by surgeons, for surgeons
IS HE SPEAKING ENGLISH? cgmp: : Current Good Manufacturing Practice PHS Act: : Public Health Service Act CFR: : Code of Federal Regulations CP: : Comp
 PREPARING FOR AN FDA INSPECTION August 11, 2011 Kip J. Hanks, Investigator Biologics National Expert FDA Division of Domestic Field Investigations 1 IS HE SPEAKING ENGLISH? cgmp: : Current Good Manufacturing
PREPARING FOR AN FDA INSPECTION August 11, 2011 Kip J. Hanks, Investigator Biologics National Expert FDA Division of Domestic Field Investigations 1 IS HE SPEAKING ENGLISH? cgmp: : Current Good Manufacturing
Meeting with the U.S. FDA Center for Biologics Evaluation and Research Thursday, March 8, 2018, Silver Spring, MD, U.S.A.
 Ref. Ares(2018)4414549-28/08/2018 EUROPEAN COMMISSION DIRECTORATE-GENERAL FOR HEALTH AND FOOD SAFETY Directorate B - Health systems, medical products and innovation B4 Medical products: quality, safety
Ref. Ares(2018)4414549-28/08/2018 EUROPEAN COMMISSION DIRECTORATE-GENERAL FOR HEALTH AND FOOD SAFETY Directorate B - Health systems, medical products and innovation B4 Medical products: quality, safety
Instructions for Use READY TO USE
 READY TO USE Instructions for Use LifeCell Corporation One Millennium Way Branchburg, NJ 08876-3876 1.908.947.1215 1.800.367.5737 Fax: 1.908.947.1089 E-mail: customerservicegroup@lifecell.com www.lifecell.com
READY TO USE Instructions for Use LifeCell Corporation One Millennium Way Branchburg, NJ 08876-3876 1.908.947.1215 1.800.367.5737 Fax: 1.908.947.1089 E-mail: customerservicegroup@lifecell.com www.lifecell.com
6th Annual Somatic Cell Therapy Symposium Tissue & IND Products
 6th Annual Somatic Cell Therapy Symposium Tissue & IND Products September 27, 2006 D. Michael Strong PhD, BCLD(ABB) Puget Sound Blood Center Northwest Tissue Center 10 Donor Centers Bellevue Central Seattle
6th Annual Somatic Cell Therapy Symposium Tissue & IND Products September 27, 2006 D. Michael Strong PhD, BCLD(ABB) Puget Sound Blood Center Northwest Tissue Center 10 Donor Centers Bellevue Central Seattle
THERMOGRAVIMETRY OF IRRADIATED HUMAN COSTAL CARTILAGE
 2007 International Nuclear Atlantic Conference - INAC 2007 Santos, SP, Brazil, September 30 to October 5, 2007 ASSOCIAÇÃO BRASILEIRA DE ENERGIA NUCLEAR - ABEN ISBN: 978-85-99141-02-1 THERMOGRAVIMETRY OF
2007 International Nuclear Atlantic Conference - INAC 2007 Santos, SP, Brazil, September 30 to October 5, 2007 ASSOCIAÇÃO BRASILEIRA DE ENERGIA NUCLEAR - ABEN ISBN: 978-85-99141-02-1 THERMOGRAVIMETRY OF
Reporting Deviations of Biological Products and HCT/Ps. Ellen Areman Senior Consultant Biologics Consulting Group, Inc.
 Reporting Deviations of Biological Products and HCT/Ps Ellen Areman Senior Consultant Biologics Consulting Group, Inc. Relevant Legislation Public Health Service (PHS) Act Regulates biological products
Reporting Deviations of Biological Products and HCT/Ps Ellen Areman Senior Consultant Biologics Consulting Group, Inc. Relevant Legislation Public Health Service (PHS) Act Regulates biological products
March 22, Atricure, Inc Mark Job Responsible Third Party Official Regulatory Technology Services, LLC th Street, NW Buffalo, MN 55313
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control Center - WO66-G609 Silver Spring, MD 20993-0002 March 22, 2016 Atricure,
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control Center - WO66-G609 Silver Spring, MD 20993-0002 March 22, 2016 Atricure,
Investor Presentation GUY COOK CEO
 Investor Presentation GUY COOK CEO FORWARD LOOKING STATEMENTS This presentation contains certain disclosures that may be deemed forward-looking statements within the meaning of the Private Securities Litigation
Investor Presentation GUY COOK CEO FORWARD LOOKING STATEMENTS This presentation contains certain disclosures that may be deemed forward-looking statements within the meaning of the Private Securities Litigation
APPLICATION FOR SITE INSPECTION Eye Bank Association of America
 APPLICATION FOR SITE INSPECTION Eye Bank Association of America Complete this application to begin the process for inspection and accreditation by the Eye Bank Association of America (EBAA). See the EBAA
APPLICATION FOR SITE INSPECTION Eye Bank Association of America Complete this application to begin the process for inspection and accreditation by the Eye Bank Association of America (EBAA). See the EBAA
How You Can Use Stem Cells
 How You Can Use Stem Cells Dr. Ralph T Bright Macquarie Cosmetic Medicine 21B Bathurst Street Liverpool NSW 2170 www.cosmeticmedicine.com.au (02) 9824 3044 Stem Cell Transfer Many of the people in this
How You Can Use Stem Cells Dr. Ralph T Bright Macquarie Cosmetic Medicine 21B Bathurst Street Liverpool NSW 2170 www.cosmeticmedicine.com.au (02) 9824 3044 Stem Cell Transfer Many of the people in this
Guidance for Industry
 Guidance for Industry Cooperative Manufacturing Arrangements for Licensed Biologics Additional copies of this guidance are available from the Office of Communication, Training and Manufacturers Assistance
Guidance for Industry Cooperative Manufacturing Arrangements for Licensed Biologics Additional copies of this guidance are available from the Office of Communication, Training and Manufacturers Assistance
Anika Therapeutics, Inc.
 Anika Therapeutics, Inc. Jefferies Global Healthcare Conference June 4, 2015 Safe Harbor Statement The statements made in this presentation that are not statements of historical fact are forward looking
Anika Therapeutics, Inc. Jefferies Global Healthcare Conference June 4, 2015 Safe Harbor Statement The statements made in this presentation that are not statements of historical fact are forward looking
ATTACHMENTS. Attachment 1 "Your Rights under the FMLA of 1993" Notice to be posted of employee rights under FMLA.
 File: GCBE-R ATTACHMENTS Attachment 1 "Your Rights under the FMLA of 1993" Notice to be posted of employee rights under FMLA. Attachment 2 "Certification of Health Care Provider" If requested, employee
File: GCBE-R ATTACHMENTS Attachment 1 "Your Rights under the FMLA of 1993" Notice to be posted of employee rights under FMLA. Attachment 2 "Certification of Health Care Provider" If requested, employee
Platelet Concentrate in Total Knees BIOLOGICS. This brochure is for International use only. It is not for distribution in the United States.
 Platelet Concentrate in Total Knees BIOLOGICS This brochure is for International use only. It is not for distribution in the United States. Platelet Concentrate in Total Knees Total Knee Arthroplasty Total
Platelet Concentrate in Total Knees BIOLOGICS This brochure is for International use only. It is not for distribution in the United States. Platelet Concentrate in Total Knees Total Knee Arthroplasty Total
HCT/P Deviations & Reporting. 13 th ANNUAL FDA & The Changing Paradigm for HCT/P Regulation February 13-15, 2017
 HCT/P Deviations & Reporting CASE STUDIES FROM TISSUE AND EYE BANKS AND CELLULAR THERAPY. 13 th ANNUAL FDA & The Changing Paradigm for HCT/P Regulation February 13-15, 2017 Tissue Bank Perspective Ashley
HCT/P Deviations & Reporting CASE STUDIES FROM TISSUE AND EYE BANKS AND CELLULAR THERAPY. 13 th ANNUAL FDA & The Changing Paradigm for HCT/P Regulation February 13-15, 2017 Tissue Bank Perspective Ashley
 Personal Check Money Order Credit Card Visa MC Discover Amex Orthopedic Analysis LLC Laboratory: 2201 W Campbell Park Dr, Suite 215, Chicago IL, 60612 Tel: 312-733-7121 www.orthopedicanalysis.com LYMPHOCYTE
Personal Check Money Order Credit Card Visa MC Discover Amex Orthopedic Analysis LLC Laboratory: 2201 W Campbell Park Dr, Suite 215, Chicago IL, 60612 Tel: 312-733-7121 www.orthopedicanalysis.com LYMPHOCYTE
Sharon Tindle, MS, CQA (ASQ) QA Manager, BMT Tissue Services Mount Sinai Hospital, New York, NY. June 7, 2016
 Sharon Tindle, MS, CQA (ASQ) QA Manager, BMT Tissue Services Mount Sinai Hospital, New York, NY June 7, 2016 1 Brief description of the Mount Sinai Cellular Therapy Laboratory Overview of BM transplant
Sharon Tindle, MS, CQA (ASQ) QA Manager, BMT Tissue Services Mount Sinai Hospital, New York, NY June 7, 2016 1 Brief description of the Mount Sinai Cellular Therapy Laboratory Overview of BM transplant
The Control of Microbial Growth
 The Control of Microbial Growth Sepsis refers to microbial contamination. Asepsis is the absence of significant contamination. Aseptic surgery techniques prevent microbial contamination of wounds. Terminology
The Control of Microbial Growth Sepsis refers to microbial contamination. Asepsis is the absence of significant contamination. Aseptic surgery techniques prevent microbial contamination of wounds. Terminology
The Control of Microbial Growth
 The Control of Microbial Growth Sepsis refers to microbial contamination. Asepsis is the absence of significant contamination. Aseptic surgery techniques prevent microbial contamination of wounds. Terminology
The Control of Microbial Growth Sepsis refers to microbial contamination. Asepsis is the absence of significant contamination. Aseptic surgery techniques prevent microbial contamination of wounds. Terminology
INTERNATIONAL STANDARD
 INTERNATIONAL STANDARD ISO 14630 Fourth edition 2012-12-01 Non-active surgical implants General requirements Implants chirurgicaux non actifs Exigences générales Reference number ISO 14630:2012(E) ISO
INTERNATIONAL STANDARD ISO 14630 Fourth edition 2012-12-01 Non-active surgical implants General requirements Implants chirurgicaux non actifs Exigences générales Reference number ISO 14630:2012(E) ISO
AUTOCLAVE: steam pressure sterilizer
 Microbiology Chapter 6 Controlling Microbes and Antimicrobial Agents 6:1 Physical Methods for Controlling Microbes DISINFECTION: the process of destroying disease-causing microorganisms STERILIZATION:
Microbiology Chapter 6 Controlling Microbes and Antimicrobial Agents 6:1 Physical Methods for Controlling Microbes DISINFECTION: the process of destroying disease-causing microorganisms STERILIZATION:
BIOTAPE XM TISSUE MATRIX The following languages are included in this packet:
 BIOTAPE XM TISSUE MATRIX 150866-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 -Chinese (sch)
BIOTAPE XM TISSUE MATRIX 150866-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 -Chinese (sch)
Regulation of advanced blood cell therapies
 Regulation of advanced blood cell therapies www.pei.de Clinical trials using cell-based products Substantially manipulated cells and cells for non-homologous use Quality, safety and non-clinical aspects
Regulation of advanced blood cell therapies www.pei.de Clinical trials using cell-based products Substantially manipulated cells and cells for non-homologous use Quality, safety and non-clinical aspects
Freshman/Sophomore Junior Senior
 Freshman/Sophomore Junior Senior Course Recommendations Mathematics (6 courses) MA 1021 MA 1024 (Calculus I) (Calculus IV) MA 1022 MA 2051 (Calculus II) (Differential Equations) MA 1023 MA 2611 (Calculus
Freshman/Sophomore Junior Senior Course Recommendations Mathematics (6 courses) MA 1021 MA 1024 (Calculus I) (Calculus IV) MA 1022 MA 2051 (Calculus II) (Differential Equations) MA 1023 MA 2611 (Calculus
FORWARD LOOKING STATEMENT
 FORWARD LOOKING STATEMENT Statements made in this presentation that look forward in time or that express management's beliefs, expectations, hopes or predictions of the future are forward-looking statements
FORWARD LOOKING STATEMENT Statements made in this presentation that look forward in time or that express management's beliefs, expectations, hopes or predictions of the future are forward-looking statements
Chapter 8 Cell Diversity
 Chapter 8 Cell Diversity Mr. C. Biology 1 Future? Chapter 8 Cell Diversity Cells, Tissues, Organs and Systems Cells have different shapes because they have different jobs to do. A nerve cell is very different
Chapter 8 Cell Diversity Mr. C. Biology 1 Future? Chapter 8 Cell Diversity Cells, Tissues, Organs and Systems Cells have different shapes because they have different jobs to do. A nerve cell is very different
AAMI Standards Monitor Online 16 July 2018
 National Standards New work/call for participation The following documents were recently approved for work by the AAMI Standards Board. If you would like additional information or to join the consensus
National Standards New work/call for participation The following documents were recently approved for work by the AAMI Standards Board. If you would like additional information or to join the consensus
Associate Professor, Division of Nephrology, USP, Sao Paulo-SP, Brazil. Histopathological examinations. III
 11 - ORIGINAL ARTICLE MATERIALS TESTING Comparative study and histomorphometric analysis of bone allografts lyophilized and sterilized by autoclaving, gamma irradiation and ethylene oxide in rats 1 Otavio
11 - ORIGINAL ARTICLE MATERIALS TESTING Comparative study and histomorphometric analysis of bone allografts lyophilized and sterilized by autoclaving, gamma irradiation and ethylene oxide in rats 1 Otavio
PRO-STIM Injectable Inductive Graft Containing DONATED HUMAN TISSUE
 PRO-STIM Injectable Inductive Graft Containing DONATED HUMAN TISSUE 137835-5 The following languages are included in this packet: English (en) For additional information please contact the manufacturer
PRO-STIM Injectable Inductive Graft Containing DONATED HUMAN TISSUE 137835-5 The following languages are included in this packet: English (en) For additional information please contact the manufacturer
PRO-STIM Injectable Inductive Graft Containing DONATED HUMAN TISSUE
 PRO-STIM Injectable Inductive Graft Containing DONATED HUMAN TISSUE 150842-0 The following languages are included in this packet: English (en) For additional information please contact the manufacturer
PRO-STIM Injectable Inductive Graft Containing DONATED HUMAN TISSUE 150842-0 The following languages are included in this packet: English (en) For additional information please contact the manufacturer
February 23, Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm Rockville, MD 20852
 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 In Re: Docket No. FDA 2014 D 1856 0001: Comments to the Draft Guidance Document Titled
Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 In Re: Docket No. FDA 2014 D 1856 0001: Comments to the Draft Guidance Document Titled
4006: Cellular Therapy Infusion
 4006: Cellular Therapy Infusion Registry Use Only Sequence Number: Date Received: Key Fields CIBMTR Center Number: Event date: / / CIBMTR Form 4006 revision 1 (page 1 of 9). Last Updated July, 2016. If
4006: Cellular Therapy Infusion Registry Use Only Sequence Number: Date Received: Key Fields CIBMTR Center Number: Event date: / / CIBMTR Form 4006 revision 1 (page 1 of 9). Last Updated July, 2016. If
What Are Stem Cells? Where Are These Stem Cells?
 While stem cell therapies may seem to have appeared overnight, they have been practiced for some time prior to the recent burst in new applications and stem cell sources. The most recent developments in
While stem cell therapies may seem to have appeared overnight, they have been practiced for some time prior to the recent burst in new applications and stem cell sources. The most recent developments in
Tornier Medical Education
 Tornier Medical Education D E C E M B E R 7, 2 0 0 9 Orthopaedic Uses of Tissue Matrices in Tendon Repair: Why All Tissue Matrices are Not Created Equal John R. Harper, Ph.D, Research Department, LifeCell
Tornier Medical Education D E C E M B E R 7, 2 0 0 9 Orthopaedic Uses of Tissue Matrices in Tendon Repair: Why All Tissue Matrices are Not Created Equal John R. Harper, Ph.D, Research Department, LifeCell
Regulatory Updates on Cellular Therapy Products in Japan
 Regulatory Updates on Cellular Therapy Products in Japan Tetsuya Kusakabe, Ph.D., M.P.H. Review Director Office of Cellular and Tissue-based Products Pharmaceuticals and Medical Devices Agency (PMDA),
Regulatory Updates on Cellular Therapy Products in Japan Tetsuya Kusakabe, Ph.D., M.P.H. Review Director Office of Cellular and Tissue-based Products Pharmaceuticals and Medical Devices Agency (PMDA),
YOUR OWN LIFE
 YOUR OWN LIFE Stromal Tissue (ST), a Regenerative Source There is a worldwide consensus that the isolation and collection of regenerative Mesenchymal Stem Cells (MSC s) from differentiated body tissues
YOUR OWN LIFE Stromal Tissue (ST), a Regenerative Source There is a worldwide consensus that the isolation and collection of regenerative Mesenchymal Stem Cells (MSC s) from differentiated body tissues
Point-of-Care Cell Processing Devices A Manufacturer s Perspective Clinical trials, regulatory compliance, and commercialization.
 Philadelphia, 2013 Point-of-Care Cell Processing Devices A Manufacturer s Perspective Clinical trials, regulatory compliance, and commercialization. Brian Barnes, Ph.D. VP, Clinical and Regulatory Affairs
Philadelphia, 2013 Point-of-Care Cell Processing Devices A Manufacturer s Perspective Clinical trials, regulatory compliance, and commercialization. Brian Barnes, Ph.D. VP, Clinical and Regulatory Affairs
Form 4006 R2.0: Cellular Therapy Infusion
 Key Fields Sequence Number: Date Received: - - CIBMTR Center Number: CIBMTR Research ID: Event date: - - Cellular Therapy Product Identification Questions: 1-27 If more than one type of cell therapy product
Key Fields Sequence Number: Date Received: - - CIBMTR Center Number: CIBMTR Research ID: Event date: - - Cellular Therapy Product Identification Questions: 1-27 If more than one type of cell therapy product
Lesson 7A Specialized Cells, Stem Cells & Cellular Differentiation
 Lesson 7A Specialized Cells, Stem Cells & Cellular Differentiation Learning Goals I can explain the concept of cell differentiation and cell specialization. I can explain how the cell structure relates
Lesson 7A Specialized Cells, Stem Cells & Cellular Differentiation Learning Goals I can explain the concept of cell differentiation and cell specialization. I can explain how the cell structure relates
Key words: radiation sterilization, biomaterials, grafts.
 RADIATION STABILITY OF FIBROUS CONNECTIVE-TISSUE BIOMATERIALS Shangina O.R. Public institution - Russian Center of eye and plastic surgery, Ufa Abstract During the recent years methods of treatment based
RADIATION STABILITY OF FIBROUS CONNECTIVE-TISSUE BIOMATERIALS Shangina O.R. Public institution - Russian Center of eye and plastic surgery, Ufa Abstract During the recent years methods of treatment based
