December 2009, March 2010
|
|
|
- Jacob Owen
- 6 years ago
- Views:
Transcription
1 December 2009, March
2 January
3 December
4 December
5 March
6 February
7
8 February 2015 Sysmex Corporation National Institute of Advanced Industrial Science and Technology (AIST) Technology Development Utilizing Sugar Chain Functions The World s First Reagent to Determine the Progression of Hepatic Fibrosis by Measuring Changes in the Sugar Chain Measurement of a Hepatic Fibrosis Glycosylation Marker in Blood Within 17 Minutes Development of Fast and High-Precision Measurement Technology Which Received Approval for Health Insurance Coverage in January 2015 The most common cause of chronic hepatitis in Japan is infection with the hepatitis B virus and hepatitis C virus. The number of infected people is said to be approximately three million nationwide, of which nearly two-thirds are asymptomatic carriers. Chronic hepatitis that develops from infection with the hepatitis virus causes chronic cell destruction in the liver, and can advance to hepatic cirrhosis in 20 to 25 years due to fibrosis of the liver (hepatic fibrosis), and can eventually lead to liver cancer or serious disease. The progression of chronic hepatitis and the likely therapeutic efficacy of treatment can be determined by checking the level of hepatic fibrosis. Today, the mainstay test for hepatic fibrosis is a biopsy in which the state of hepatic fibrosis is ascertained by collecting liver tissue with an inserted needle. However, the test imposes a heavy burden on patients, such as the necessity of hospitalization. Therefore, there is a public need for the development of a simple and high-precision liver fibrosis test such as blood testing. A technology that meets this need is Sysmex Corporation s HISCL M2BPGi reagent which uses a hepatic fibrosis glycosylation marker, a biomarker using sugar chains. NEDO has implemented the world s most advanced research and development projects related to sugar chains since the Technology for the Production and Utilization of Glycoconjugates project started in The National Institute of Advanced Industrial Science and Technology (AIST), which has led these projects, found a new glycosylation marker involved in the progression of hepatic fibrosis. In order to utilize this achievement, AIST started to work with Sysmex Corporation, a manufacturer of clinical examination devices and test reagents, for the practical application of the hepatic fibrosis glycosylation marker, and then developed the HISCL M2BPGi reagent. This reagent received manufacturing and marketing approval in December 2013, was released in March 2014, and received approval for health insurance coverage in January It is expected to be widely used in the future. Automatic immunoassay system used in testing HISCL-5000 (Photo courtesy of Sysmex Corporation) Reagents placed in HISCL-5000 Twenty-four R1 to R3 reagent sample sets can be tested at the same time.
9
10 Topics CASE 04 Medical Biotechnology CASE Success Story Medical Biotechnology Enormous effort and costs are required for the development of new medicine There are long and difficult processes involved in the development of new medicine. During the basic research stage, various new substances that are potential candidates for new medicine are sought. The properties and structures of such substances are investigated, the effectiveness and safety of an enormous number of new substances are verified using laboratory animals, and then the candidates are narrowed down. Separating candidates by quantifying their efficacy, or screening, is a process that requires a lot of time and money. Traditionally, most laboratory animals have been mice, but because there are numerous substances that are potential candidates, investigating the efficacy of all candidates Development of a Procedure That Dramatically Streamlines Essential Screening in Medicine Development Mie University / HASHIMOTO ELECTRONIC INDUSTRY CO., LTD. Innovation Commercialization Venture Support Project, etc. under various conditions using mice is time-consuming and expensive. On the other hand, another type of experimental animal is the zebrafish, which is known to have a genetic structure similar to humans, as is the case with mice. Zebrafish have recently become more commonly used in order to make experimentation convenient. Another advantage is that zebrafish are cheaper to raise than mice. However, there has been one problem in using zebrafish for experimentation as it was not possible to easily create a human disease model with them. A human disease model refers to a group of laboratory animals which can be affected by a disease that also affects humans. When investigating the efficacy of medicine that treats or alleviates the symptoms of a human disease, the animals in the experiment must be able to be affected with the same human disease The ZF Plate, which simplified the observation of zebrafish 2. Zebrafish in a conventional plate (left) and Zebrafish arranged in the ZF Plate (right). (Photo provided by HASHIMOTO ELECTRONIC INDUSTRY CO., LTD.) 3. Aquariums used in the laboratory of Professor Toshio Tanaka at Mie University. Zebrafish with various human diseases are raised in numerous tanks. 4. A semiautomatic workstation for observation of zebrafish developed by HASHIMOTO ELECTRONIC INDUSTRY CO., LTD. 5. An obese zebrafish developed by Professor Toshio Tanaka. (Photo provided by Professor Toshio Tanaka of Mie University) 6. MieKomachi, a zebrafish with a transparent body that is used for observation. (Photo provided by Professor Toshio Tanaka of Mie University) 7 7. Toshio Tanaka, professor at Mie University Graduate School of Medicine. Professor Toshio Tanaka of the Mie University Graduate School of Medicine speculated that if it is possible to use a large amount of zebrafish in drug development screening, then drug development should be able to be performed more conveniently and broadly. At the time, he was participating in NEDO s Grant for Practical Application of University R&D Results program, and he started working to develop of a zebrafish human disease model from NEDO had actually been focusing on the possibility of drug development screening employing zebrafish long before the start of the project. Investigating drug efficacy with large volume observations of zebrafish human disease models Around that time, a technology called genome editing was being established by American researchers. Using new technology that pinpoints and deletes DNA in a genome, it became possible to develop a model organism more simply than in the past. Using this idea, Professor Tanaka succeeded in creating various NEDO s Role zebrafish human disease models. However, he was not satisfied with the results that he obtained. He believed that in order to realize the type of large-scale drug development screening he had envisioned, it would be necessary to have research tools that can evaluate the results of a large number of potential candidates for new medicine by observing a large number of zebrafish. This process would also need to be automated in the future. Therefore, as a first step, Professor Tanaka developed the Zebrafish (ZF) Plate which is capable of observing many zebrafish at the same time. He also selected HASHIMOTO ELECTRONIC INDUSTRY CO., LTD. from Matsusaka City in Mie Prefecture to be a collaborator in his development work. He selected the company because he had heard about their technology to manipulate fine particles and their advanced knowledge of zebrafish. Next, NEDO adopted the development of the ZF Plate under its Innovation Commercialization Venture Support Project which was continued from Through repeated trial and error by developers at Hashimoto, a general purpose ZF Plate was completed. This then led to the development of a procedure for a commonly used centrifugal separator which could be used without expensive accessories. This made it possible to easily perform experiments anywhere using a large number of zebrafish. For The Future Using the new technology to develop functional foods and personalized cancer treatment For research and development venture companies, development for practical application is an extremely risky phase before reaching commercialization. Therefore, support for superior advanced technology seeds that make use of promising original technology is provided to projects with a specific plan to achieve practical application within three to five years. When adopting a project, NEDO considers the novelty of the technology, the level of goal setting, the superiority of patents and know-how, as well as the possibility for practical application. Goals, problems, and steps needed to solve problems are also clarified, and the suitability of research planning, including cost-effectiveness, is evaluated. Furthermore, in addition to the potential for a new market creation effect, the understanding of market needs, and the A well incorporated into a microplate. A circular well is incorporated into a square-shaped space. (Provided by HASHIMOTO ELECTRONIC INDUSTRY CO., LTD.) 9. Mr. Masatoshi Hashimoto, president and representative director of HASHIMOTO ELECTRONIC INDUSTRY CO., LTD. Drug development research employing zebrafish has only just begun, but there are great expectations for the technology to become a driving force in the development of new medicine and an essential research method. Furthermore, there is a wide variety of applications for drug development screening technology in which zebrafish are used. For example, testing active ingredients contained in food products and the effectiveness of anticancer drugs have been investigated. So, use of the technology has also started in the development of functional foods and for personalized cancer treatment. priority of developed products and services, the reliability of the system for commercialization and business planning are also reviewed as part of NEDO s evaluation process. Evaluations are carried out from various points of view, such as the contribution to revitalization of regional economies, in order to strengthen the foundation of creative innovation. 15
11 Topics CASE 05 Medical Biotechnology CASE 05 Success Story Development of Japan s First PET Device Dedicated for Breast Cancer and Able to Perform High-Precision Examinations Without Inflicting Pain Medical Biotechnology SHIMADZU CORPORATION R&D Project on Molecular Imaging Equipment for Treatment of Malignant Tumors A four-layer DOI detector enables high-precision and high-sensitivity examinations. It incorporates technology that is the core of the Elmammo breast cancer- dedicated PET device. (Photo provided by SHIMADZU CORPORATION) 2. During an examination, the patient lies face-down and positions a single breast in the opening at the top in order to have images taken. (Photo provided by SHIMADZU CORPORA- TION) 3. The female-friendly, fresh and clean examination area. (Photo provided by SHIMADZU CORPORA- TION) 8 8. Cancer incident data based on estimates of regional cancer registration nationwide ( ). Created based on Cancer Registration and Statistics from the National Cancer Center s Cancer Information Service. Enabling high-precision examinations without inflicting pain At first glance, Elmammo appears to have the shape of a bed. On the top, there is a single opening with a diameter of approximately 18.5 cm where the examination takes place. A female patient receiving the examination lies face-down. The examination is performed with the patient positioning one breast in the opening at a time and then having a PET image of each breast taken. Currently, with mammography used for breast cancer examinations, a patient s breasts are pressed tightly together before images are taken, resulting in a heavy burden for the patient as she may experience pain and other discomfort. In comparison, using Elmammo does not require a patient to press her breasts together, so she will not experience any pain. Also, the device is capable of taking 3D tomographic PET images of the breasts when they are not pressed together. Furthermore, the device is extremely effective, offering about twice the resolution and ten times the sensitivity of current whole-body positron emission tomography / computed tomography (PET/CT) devices. The biggest contributing factor to realization of such high resolution and sensitivity is that the detectors are arranged in a ring structure. On a side note, Elmammo was originally developed in cooperation with the National Institute of Radiological Sciences for brain examinations. A NEDO project led to it also being used for breast cancer examinations. Among cancer incidence rates for Japanese women, breast cancer is the highest (see accompanying graph), so early detection can mean the difference between life and death. Shimadzu Corporation determined that its device could be used for breast cancer examinations, and therefore applied to participate in NEDO s project. For The Future Listening to user opinions and taking the technology overseas During the early stage of development of the device, it was difficult to simultaneously achieve high resolution and high sensitivity. However, after improvements in technology, hardware, and software, it became possible to achieve both high resolution and high sensitivity. Furthermore, through medical-engineering cooperation with Kyoto University Hospital, the opinions of doctors using the device and patients undergoing examinations were reflected in the development process, making the device more userfriendly. There are future plans to develop domestic and overseas markets for early-stage detection and treatment of breast cancer, which is increasing every year The completed Elmammo together with its three developers. From the right, Keishi Kitamura, who was in charge of the prototype development and is one of the inventors of the four-layer DOI detector (Senior R&D Manager, Radiation Imaging Device Unit, Technology Research Laboratory); Kazumi Tanaka, who was in charge of circuit design and hardware (R&D Manager, Research and Development Department, Medical Systems Division); and Tetsuro Mizuta, who was in charge of software, such as image processing software (Assistant Manager, Research and Development Department, Medical Systems Division). Detecting cancer in its early stage is the difference between life and death It is extremely important to be able to detect and treat cancer in its early stage. This is because the survival rate for the majority of cancers begins to rapidly decline as the disease progresses. Therefore, detection of cancer in its early stage is necessary. At the same time, there is a need to take measures to reduce medical costs associated with the diagnosis and treatment of the disease. Much attention has been given to imaging technology that is capable of detecting functional changes in living cells at the molecular level, so there are great expectations regarding positron emission tomography (PET) devices in fields related to early-stage diagnosis of cancer. 4. When comparing the four-layer DOI detector with conventional detectors, Elmammo has made it possible to distinguish the depth of interactions of gamma rays. (Image provided by SHIMADZU CORPORATION) 5. Traditionally, the shape at the edges of the field of view has been either unclear or distorted. The four-layer DOI detector can clearly and completely capture the shape from the center to the edges of the field of view. (Image provided by SHIMADZU CORPORATION) However, there is still room for growth in the PET device market of the health care industry. Because of this potential, Shimadzu Corporation developed a PET device dedicated for breast cancer called Elmammo in NEDO s R&D of Molecule Imaging Equipment for Malignant Tumor Therapy Support project carried out from FY2006 to FY Phantom image taken by a whole body PET device with a spatial resolution of 3.5 mm. (Image provided by SHIMADZU CORPORATION) 7. Phantom image taken by the Elmammo breast cancer-dedicated PET device with a spatial resolution of less than 1.5 mm. From this, it is possible to distinguish smaller diameters than what is possible with the whole body PET image on the left (from the top left, the diameters are 4.8 mm, 4.0 mm, 3.2 mm, 2.4 mm, 1.6 mm, and mm). (Image provided by SHIMADZU CORPORATION) NEDO s Role In this project, NEDO promoted medical-engineering cooperation by functioning as an intermediary between industry and academia. From medical and industrial perspectives, NEDO promoted the development of molecular imaging technology capable of performing high-precision, early-stage diagnosis of malignant tumors. In particular, a molecular imaging research center was established at Kyoto University Hospital. Information sharing was encouraged between Kyoto University, which was the joint research platform for the project, and participating companies by performing comprehensive progress management and periodically hosting research promotion planning meetings, resulting in the construction of a system that supported the project leaders. The advanced equipment conformed to clinical needs, and was improved based on appropriate evaluation and clinical research (clinical data). The development and evaluation of molecular probes for such advanced equipment were also promoted. In addition, the project was successfully carried out due to management that responded to a changing situation. When an midterm evaluation was conducted in FY2008, the challenges of research and development and final targets were revised based on the results of the evaluation. A supplementary budget was then provided for molecular probe pharmaceutical formulation technology for molecular imaging in order to accelerate development
12 Medical Biotechnology Culture bag attached to cell culture apparatus Adding Momentum to Clinical Applied Research on ES and ips Cells Through Enhanced Qualitative and Quantitative Stabilization Kyoto University Nissan Chemical Industries, Ltd. Nipro Corporation Development to Accelerate the Practical Application of Human Stem Cells / Development of Basic Evaluation Technologies for the Practical Application of Human Stem Cells Development of Innovative Culture Media for Human Pluripotent Stem Cells (ES and ips Cells) and an Automated Cell Culture System To promote regenerative medicine, research is under way using two types of human pluripotent stem cells: embryonic stem (ES) cells and induced pluripotent stem (ips) cells. Clinical application of the research requires development of mass culture and quality control technologies for ES and ips cells. These technologies were successfully established during the course of this project, in which government, industry, and academia collaborated to commercialize world-class products. Working to establish a new method for managing the culture of ES and ips cells and an automated cell culture system ES and ips cells play important roles in regenerative medicine. In a typical laboratory setting, culture dishes and other devices are usually employed to grow these cells, but even with a large dish, only several million to ten million cells can be grown at a time. In regenerative medicine, however, about one billion stem cells per patient are needed. It is also vital that cultured stem cells be free of contaminants such as impurities, toxic substances, and pathogens. In a NEDO project, a collaborative team from industry and academia led by Kyoto University s Institute for Integrated Cell-Material Sciences (icems) worked to address these challenges. It was able to achieve technological breakthroughs that enabled the automated mass culture of stem cells. Realizing 3D stem cell culture using food additives and achieving mass culture using applied culture bag systems Nissan Chemical Industries, Ltd., a team member from industry, developed a three-dimensional (3D) culture system required for large-scale stem cell production. This system promotes growth of stem cells while suspended in a liquid medium, allowing the cells to be cultured with minimum damage. This enables both mass culture and quality control of cells. The food additive gellan gum is used for the stem cell suspension culture. The development of a system to uniformly mix gellan gum with the liquid media led to the commercial application of a 3D culture system. Nipro Corporation, another team member from industry, worked to develop an automated cell culture system. By establishing a closed culture system using culture bags, the company significantly reduced the risk of contamination, including that caused by mixing with other cell lines, relative to the risk of an open system using culture dishes. The company also developed a method to detach and dissociate cell colonies using a small amount of reagent (enzyme) to transfer them between culture bags. This made culture work much less labor-intensive and enabled efficient and large-scale stem cell production. Clinical application of the developed technologies is expected to bring regenerative stem cell-based medicine to many people. Left: Stem cells in 3D culture Top right: FCeM Preparation Kit for Stem Cells developed jointly by Kyoto University and Nissan Chemical Industries, Ltd. Bottom right: An experimental model of an automated stem cell culture system was jointly developed by Kyoto University and Nipro Corporation. The latest model, CELLAFORTE, was launched in April NEDO PROJECT SUCCESS STORIES 2017 SUCCESS STORIES 11
Realizing the Future that Regenerative Medicine Will Open
 470 Hitachi Review Vol. 65 (2016), No. 9 Featured Articles Realizing the Future that Regenerative Medicine Will Open Shizu Takeda, Ph.D. OVERVIEW: Regenerative medicine is an innovative approach to medicine
470 Hitachi Review Vol. 65 (2016), No. 9 Featured Articles Realizing the Future that Regenerative Medicine Will Open Shizu Takeda, Ph.D. OVERVIEW: Regenerative medicine is an innovative approach to medicine
VISION & STRATEGY FIC LIC. Interview with the President
 VISION & STRATEGY Interview with the President A conversation with Isao Teshirogi, President and CEO about the Shionogi Growth Strategy 2020 (SGS2020) VISION & STRATEGY SGS2020 Growth Strategies Under
VISION & STRATEGY Interview with the President A conversation with Isao Teshirogi, President and CEO about the Shionogi Growth Strategy 2020 (SGS2020) VISION & STRATEGY SGS2020 Growth Strategies Under
Technical Guidance on Development of In Vitro Companion Diagnostics and Corresponding Therapeutic Products
 Administrative Notice December 26, 2013 To: Division of Pharmaceutical Affairs, Prefectural Health Department (Bureau) From: Evaluation and Licensing Division, Pharmaceutical and Food Safety Bureau Ministry
Administrative Notice December 26, 2013 To: Division of Pharmaceutical Affairs, Prefectural Health Department (Bureau) From: Evaluation and Licensing Division, Pharmaceutical and Food Safety Bureau Ministry
Current Topics of Pharmaceutical Regulatory Affairs in Japan
 Pharmaceuticals and Medical Devices Agency Current Topics of Pharmaceutical Regulatory Affairs in Japan Takeyuki SATO Associate Director (International and Product Evaluation Affairs), Centre for Product
Pharmaceuticals and Medical Devices Agency Current Topics of Pharmaceutical Regulatory Affairs in Japan Takeyuki SATO Associate Director (International and Product Evaluation Affairs), Centre for Product
To meet the challenge of providing safe, cost-effective medicines for the world s largest
 An Executive Summary Biopharma in China: New Initiatives, New Opportunities To meet the challenge of providing safe, cost-effective medicines for the world s largest population, the government of China
An Executive Summary Biopharma in China: New Initiatives, New Opportunities To meet the challenge of providing safe, cost-effective medicines for the world s largest population, the government of China
5-Year Strategy for the Creation of Innovative Pharmaceuticals and Medical Devices
 5-Year Strategy for the Creation of Innovative Pharmaceuticals and Medical Devices April 26, 2007 Ministry of Education, Culture, Sports, Science and Technology (MEXT) Ministry of Health, Labour and Welfare
5-Year Strategy for the Creation of Innovative Pharmaceuticals and Medical Devices April 26, 2007 Ministry of Education, Culture, Sports, Science and Technology (MEXT) Ministry of Health, Labour and Welfare
of Innovative Pharmaceuticals* and Takao Inoue 4
 26G-ISMS44 Regulatory Science for R&D Promotion Kazuhiko Mori 1, Takao Yamori 2, Toru Kawanishi 3, 1 Ministry of Health, Labor and Welfare (MHLW), 2 Pharmaceuticals and Medical Devices Agency (PMDA), 3
26G-ISMS44 Regulatory Science for R&D Promotion Kazuhiko Mori 1, Takao Yamori 2, Toru Kawanishi 3, 1 Ministry of Health, Labor and Welfare (MHLW), 2 Pharmaceuticals and Medical Devices Agency (PMDA), 3
PMDA Update: Its current situation and future direction
 PMDA Update: Its current situation and future direction Tatsuya Kondo, M.D. Ph.D. Chief Executive Pharmaceuticals and Medical Devices Agency Contents 1. Organization 2. Recent Approaches for Innovative
PMDA Update: Its current situation and future direction Tatsuya Kondo, M.D. Ph.D. Chief Executive Pharmaceuticals and Medical Devices Agency Contents 1. Organization 2. Recent Approaches for Innovative
Konica Minolta and INCJ Agree to Acquire Ambry Genetics in a Deal Valued at US$1 billion
 News Release Konica Minolta and INCJ Agree to Acquire Ambry Genetics in a Deal Valued at US$1 billion Advances Konica Minolta strategy to establish a leadership position in precision medicine Contributes
News Release Konica Minolta and INCJ Agree to Acquire Ambry Genetics in a Deal Valued at US$1 billion Advances Konica Minolta strategy to establish a leadership position in precision medicine Contributes
Maximizing opportunities towards achieving clinical success D R U G D I S C O V E R Y. Report Price Publication date
 F o r a c l e a r e r m a r k e t p e r s p e c t i v e Early Stage Drug Safety Strategies & Risk Management Maximizing opportunities towards achieving clinical success D R U G D I S C O V E R Y Report
F o r a c l e a r e r m a r k e t p e r s p e c t i v e Early Stage Drug Safety Strategies & Risk Management Maximizing opportunities towards achieving clinical success D R U G D I S C O V E R Y Report
Cell Processing Workstation. In Situ Laser-Mediated Cell Purification and Processing
 LEAP Cell Processing Workstation Cell Processing Workstation In Situ Laser-Mediated Cell Purification and Processing Expanding the Capabilities of Cell Processing Consistent, High-Yield, High-Purity Processing
LEAP Cell Processing Workstation Cell Processing Workstation In Situ Laser-Mediated Cell Purification and Processing Expanding the Capabilities of Cell Processing Consistent, High-Yield, High-Purity Processing
Evaluation of Operating Results for National Institute of Radiological Sciences Estimated at the End of the Third Medium to Long-Term Objective Period
 Evaluation of Operating Results for National Institute of Radiological Sciences Estimated at the End of the Third Medium to Long-Term Objective Period September 2015 Minister of Education, Culture, Sports,
Evaluation of Operating Results for National Institute of Radiological Sciences Estimated at the End of the Third Medium to Long-Term Objective Period September 2015 Minister of Education, Culture, Sports,
Toward Human-oriented Life
 Toward Human-oriented Life 180 Toward Human-oriented Life Minoru Sakairi, Dr. Sci. Keiji Kobashi, Dr. Eng. Yasutaka Hasegawa Kazutoshi Kan OVERVIEW: So that people can live healthy and happy lives, humanoriented
Toward Human-oriented Life 180 Toward Human-oriented Life Minoru Sakairi, Dr. Sci. Keiji Kobashi, Dr. Eng. Yasutaka Hasegawa Kazutoshi Kan OVERVIEW: So that people can live healthy and happy lives, humanoriented
Successful Academia-Pharma Collaboration Drug Discovery and Clinical Development
 Successful Academia-Pharma Collaboration Drug Discovery and Clinical Development Toshio MIYATA Tohoku University Graduate School of Medicine United Centers for Advanced Research and Translational Medicine
Successful Academia-Pharma Collaboration Drug Discovery and Clinical Development Toshio MIYATA Tohoku University Graduate School of Medicine United Centers for Advanced Research and Translational Medicine
Delivering on the Promise of Precision Medicine
 Delivering on the Promise of Precision Medicine Bay Area Council Economic Institute Spring 2016 The Promise of Precision Medicine The Bay Area is one of the world s biggest hubs for innovation in research
Delivering on the Promise of Precision Medicine Bay Area Council Economic Institute Spring 2016 The Promise of Precision Medicine The Bay Area is one of the world s biggest hubs for innovation in research
FY2017 to FY2019 Medium-Term Management Plan. March 29, 2017 Shimadzu Corporation President and CEO Teruhisa Ueda
 FY2017 to FY2019 Medium-Term Management Plan March 29, 2017 Shimadzu Corporation President and CEO Teruhisa Ueda Contents I. Review of the Previous Plan p.1-4 II. Overview of the New Medium-Term Management
FY2017 to FY2019 Medium-Term Management Plan March 29, 2017 Shimadzu Corporation President and CEO Teruhisa Ueda Contents I. Review of the Previous Plan p.1-4 II. Overview of the New Medium-Term Management
2. Purpose and Mission of the icems
 Research Center Project Host institution name Kyoto University Head of host institution Professor Kazuo Oike, Ph. D., President of Kyoto University Title of center project Center name Institute for Integrated
Research Center Project Host institution name Kyoto University Head of host institution Professor Kazuo Oike, Ph. D., President of Kyoto University Title of center project Center name Institute for Integrated
Trend in diagnostic biochip. Eiichiro Ichiishi, M.D., Ph.D. Department of Internal Medicine International University of Health and Welfare Hospital
 Trend in diagnostic biochip development Eiichiro Ichiishi, M.D., Ph.D. Department of Internal Medicine International University of Health and Welfare Hospital Handling of the early stage is important for
Trend in diagnostic biochip development Eiichiro Ichiishi, M.D., Ph.D. Department of Internal Medicine International University of Health and Welfare Hospital Handling of the early stage is important for
6. Vision 3: Leading the Japanese economy forward as a high value-added industry"
 6. Vision 3: Leading the Japanese economy forward as a high value-added industry" Strategic points for realizing the vision Streamlining and rationalization R&D to create innovative drugs Proactively introducing
6. Vision 3: Leading the Japanese economy forward as a high value-added industry" Strategic points for realizing the vision Streamlining and rationalization R&D to create innovative drugs Proactively introducing
A Shimadzu Brand with a Committed Worldwide Client Base
 Outline of the New Medium-Term Management Plan (2008 2010) A Shimadzu Brand with a Committed Worldwide Client Base Towards a Truly Global Business March 24, 2008 Hattori Shigehiko President and CEO 1 I.
Outline of the New Medium-Term Management Plan (2008 2010) A Shimadzu Brand with a Committed Worldwide Client Base Towards a Truly Global Business March 24, 2008 Hattori Shigehiko President and CEO 1 I.
PHARMACEUTICAL INDUSTRIES IN TOYAMA
 PHARMACEUTICAL INDUSTRIES IN TOYAMA Profile(outline of Toyama Prefecture) Toyama prefecture is located near the center of Japan and is approximately the same distance from the three largest cities in Japan-Tokyo,Nagoya,and
PHARMACEUTICAL INDUSTRIES IN TOYAMA Profile(outline of Toyama Prefecture) Toyama prefecture is located near the center of Japan and is approximately the same distance from the three largest cities in Japan-Tokyo,Nagoya,and
Internationally Renowned Expert in Artificial Liver Device Development Joins HepaLife
 February 5, 2007 Internationally Renowned Expert in Artificial Liver Device Development Joins HepaLife Liver Function of HepaLife's Patented PICM-19 Cell Line Demonstrated to Have Potential Application
February 5, 2007 Internationally Renowned Expert in Artificial Liver Device Development Joins HepaLife Liver Function of HepaLife's Patented PICM-19 Cell Line Demonstrated to Have Potential Application
Growth Strategy for Medical Systems Business
 Growth Strategy for Medical Systems Business Kunimasa Ito, Managing Executive Officer and Medical Systems Division General Manager Market Size (Billion Dollar) Current Status of Business Overview of Diagnostic
Growth Strategy for Medical Systems Business Kunimasa Ito, Managing Executive Officer and Medical Systems Division General Manager Market Size (Billion Dollar) Current Status of Business Overview of Diagnostic
New test for transplant rejection on the horizon
 December 6, 2004 New test for transplant rejection on the horizon Vancouver Researchers at St. Paul s Hospital and Vancouver General Hospital are developing a revolutionary new test to diagnose and facilitate
December 6, 2004 New test for transplant rejection on the horizon Vancouver Researchers at St. Paul s Hospital and Vancouver General Hospital are developing a revolutionary new test to diagnose and facilitate
social, and population-based research conducted and supported by the National Institutes of Health (NIH). The
 Statement by the Ad Hoc Group for Medical Research on FY 2015 Appropriations for the National Institutes of Health Submitted for the record on March 28, 2014, to the Subcommittee on Labor, Health and Human
Statement by the Ad Hoc Group for Medical Research on FY 2015 Appropriations for the National Institutes of Health Submitted for the record on March 28, 2014, to the Subcommittee on Labor, Health and Human
NHS ENGLAND BOARD PAPER
 NHS ENGLAND BOARD PAPER Paper: PB.30.03.2017/06 Title: Creating a genomic medicine service to lay the foundations to deliver personalised interventions and treatments Lead Director: Professor Sir Bruce
NHS ENGLAND BOARD PAPER Paper: PB.30.03.2017/06 Title: Creating a genomic medicine service to lay the foundations to deliver personalised interventions and treatments Lead Director: Professor Sir Bruce
ADVANCE MEDICINE. AstraZeneca Japan President Stefan Woxström shares his healthcare vision. By C Bryan Jones
 ADVANCE MEDICINE AstraZeneca Japan President Stefan Woxström shares his healthcare vision By C Bryan Jones Biopharmaceutical company AstraZeneca says science is at the heart of everything they do. With
ADVANCE MEDICINE AstraZeneca Japan President Stefan Woxström shares his healthcare vision By C Bryan Jones Biopharmaceutical company AstraZeneca says science is at the heart of everything they do. With
Sustaining long-term growth by focusing on our customers
 Strategy in action Sustaining long-term growth by focusing on our customers Our capabilities and customer focused strategy are opening growth opportunities in addressable markets of approximately $8 billion.
Strategy in action Sustaining long-term growth by focusing on our customers Our capabilities and customer focused strategy are opening growth opportunities in addressable markets of approximately $8 billion.
The Life Innovation Policy of Japan and Activities of MEXT
 The Life Innovation Policy of Japan and Activities of MEXT November 2011 Hiroyuki KAMAI Deputy Director, Life Sciences Division Research Promotion Bureau Ministry of Education, Culture, Sports, Science
The Life Innovation Policy of Japan and Activities of MEXT November 2011 Hiroyuki KAMAI Deputy Director, Life Sciences Division Research Promotion Bureau Ministry of Education, Culture, Sports, Science
Future Science Group VISION IN STM PUBLISHING [ BARBARA VALCELLI / ACCUCOMS
 Future Science Group VISION IN STM PUBLISHING [ BARBARA VALCELLI / ACCUCOMS BARBARA@ACCUCOMS.COM Publisher of journals, ebooks and ecommunities Introducing Future Science Group About Future Science Group
Future Science Group VISION IN STM PUBLISHING [ BARBARA VALCELLI / ACCUCOMS BARBARA@ACCUCOMS.COM Publisher of journals, ebooks and ecommunities Introducing Future Science Group About Future Science Group
NETWORKING AND CUREACCELERATOR LIVE!
 RARE DRUG DEVELOPMENT SYMPOSIUM A PARTNERSHIP BETWEEN PENN MEDICINE ORPHAN DISEASE CENTER AND GLOBAL GENES PHILADELPHIA, PENNSYLVANIA NETWORKING AND CUREACCELERATOR LIVE! CureAccelerator Live! Rare Disease
RARE DRUG DEVELOPMENT SYMPOSIUM A PARTNERSHIP BETWEEN PENN MEDICINE ORPHAN DISEASE CENTER AND GLOBAL GENES PHILADELPHIA, PENNSYLVANIA NETWORKING AND CUREACCELERATOR LIVE! CureAccelerator Live! Rare Disease
Regulation of Cell and Gene Therapy Products in Canada
 Regulation of Cell and Gene Therapy Products in Canada Canadian Blood and Bone Marrow Transplant Group June 8, 2018 Nadine Kolas, PhD Senior Policy Analyst Blood, Cells, Tissues and Organs Biologics and
Regulation of Cell and Gene Therapy Products in Canada Canadian Blood and Bone Marrow Transplant Group June 8, 2018 Nadine Kolas, PhD Senior Policy Analyst Blood, Cells, Tissues and Organs Biologics and
National Health Research Institutes
 National Health Research Institutes October 2008 History The founding of the National Health Research Institutes (NHRI) was first proposed in 1988 by members of Academia Sinica. In 1994 a preparatory committee
National Health Research Institutes October 2008 History The founding of the National Health Research Institutes (NHRI) was first proposed in 1988 by members of Academia Sinica. In 1994 a preparatory committee
Overview. FTD Genetics where is it leading us? What comes first or? Family History. AFTD Education Conference and Annual Meeting, White Plains, 2014
 Genetics where is it leading us? Jill Goldman, MS, MPhil, CGC Taub Institute Columbia University Medical Center Overview Why are there so many research studies on the genetics of? How much of is genetic?
Genetics where is it leading us? Jill Goldman, MS, MPhil, CGC Taub Institute Columbia University Medical Center Overview Why are there so many research studies on the genetics of? How much of is genetic?
Research Coverage Report by Shared Research Inc.
 3-D MATIX / 7777 esearch eport by Shared esearch Inc. https://sharedresearch.jp This PDF document is an updated note on the company. A comprehensive version of the report on the company, including this
3-D MATIX / 7777 esearch eport by Shared esearch Inc. https://sharedresearch.jp This PDF document is an updated note on the company. A comprehensive version of the report on the company, including this
Chapter 20 Recombinant DNA Technology. Copyright 2009 Pearson Education, Inc.
 Chapter 20 Recombinant DNA Technology Copyright 2009 Pearson Education, Inc. 20.1 Recombinant DNA Technology Began with Two Key Tools: Restriction Enzymes and DNA Cloning Vectors Recombinant DNA refers
Chapter 20 Recombinant DNA Technology Copyright 2009 Pearson Education, Inc. 20.1 Recombinant DNA Technology Began with Two Key Tools: Restriction Enzymes and DNA Cloning Vectors Recombinant DNA refers
Per Morten Sandset Japan-UiO cooperation within health and care research status and opportunities
 Per Morten Sandset p.m.sandset@medisin.uio.no Japan-UiO cooperation within health and care research status and opportunities Japan-UiO cooperation within health and care Brief presentation of the University
Per Morten Sandset p.m.sandset@medisin.uio.no Japan-UiO cooperation within health and care research status and opportunities Japan-UiO cooperation within health and care Brief presentation of the University
Pre-consultation system at the authority for clinical trials and NDA in Japan
 Pharmaceuticals and Medical Devices Agency Pre-consultation system at the authority for clinical trials and NDA in Japan Takeyuki SATO Associate Director, Centre for Product Evaluation Pharmaceuticals
Pharmaceuticals and Medical Devices Agency Pre-consultation system at the authority for clinical trials and NDA in Japan Takeyuki SATO Associate Director, Centre for Product Evaluation Pharmaceuticals
FDA Regulation of Companion Diagnostics
 FDA Regulation of Companion Diagnostics Paul Radensky October 11, 2017 Disclosure + Slideset drawn from Part I of presentation made by Janice Hogan, HoganLovells, October 2016 + Updated where appropriate
FDA Regulation of Companion Diagnostics Paul Radensky October 11, 2017 Disclosure + Slideset drawn from Part I of presentation made by Janice Hogan, HoganLovells, October 2016 + Updated where appropriate
STRAND NGS 3.0 ALLOWS USERS TO ANALYZE DATA AT NEARLY TWICE THE SPEED
 bio chat STRAND NGS 3.0 ALLOWS USERS TO ANALYZE DATA AT NEARLY TWICE THE SPEED The Bangalore based Strand Life Sciences has been recently in news for the back to back launch of two innovative products.
bio chat STRAND NGS 3.0 ALLOWS USERS TO ANALYZE DATA AT NEARLY TWICE THE SPEED The Bangalore based Strand Life Sciences has been recently in news for the back to back launch of two innovative products.
Comparative Oncology Program
 The Problem: Non-Integrated Cancer Drug Development A Solution: Integration of Informative Non-Clinical Models of Cancer With Clinical Drug Development Efforts Companion Animal Malignancies as Comparative
The Problem: Non-Integrated Cancer Drug Development A Solution: Integration of Informative Non-Clinical Models of Cancer With Clinical Drug Development Efforts Companion Animal Malignancies as Comparative
Establishment and verification of mirnas expression molecules for breast cancer and its distant metastasis Yanhong Gao Associate Professor
 Establishment and verification of mirnas expression molecules for breast cancer and its distant metastasis Yanhong Gao Associate Professor Department of Clinical Biochemistry Chinese PLA General Hospital
Establishment and verification of mirnas expression molecules for breast cancer and its distant metastasis Yanhong Gao Associate Professor Department of Clinical Biochemistry Chinese PLA General Hospital
Regulatory Perspectives of Japan
 International Alliance for Biological Standardization (IABS) Challenge Toward Sound Scientific Regulation of Cell Therapy Products at Kyoto International Conference Center, Kyoto, Japan Regulatory Perspectives
International Alliance for Biological Standardization (IABS) Challenge Toward Sound Scientific Regulation of Cell Therapy Products at Kyoto International Conference Center, Kyoto, Japan Regulatory Perspectives
Report of the Advisory Panel for Promotion of Medical Ventures (Summary)
 Report of the Advisory Panel for Promotion of Medical Ventures (Summary) Report of the Advisory Panel for Promotion of Medical Ventures (Summary) Innovation is a key trigger for Japan s economic growth
Report of the Advisory Panel for Promotion of Medical Ventures (Summary) Report of the Advisory Panel for Promotion of Medical Ventures (Summary) Innovation is a key trigger for Japan s economic growth
Section I: Pharmaceuticals and Medical Devices
 SUPPLEMENT on HEALTHCARE INNOVATION Visionary Goals and Recommendations 51th Japan-U.S. Business Conference Japan-U.S. Business Council / U.S.-Japan Business Council November 14, 2014 The R&D-based pharmaceutical
SUPPLEMENT on HEALTHCARE INNOVATION Visionary Goals and Recommendations 51th Japan-U.S. Business Conference Japan-U.S. Business Council / U.S.-Japan Business Council November 14, 2014 The R&D-based pharmaceutical
Principles of translational medicine: imaging, biomarker imaging, theranostics
 Principles of translational medicine: imaging, biomarker imaging, theranostics Compiled by: Endre Mikus PhD, CEO Budapest, 21/9/2015 Imaging and imaging biomarkers An imaging biomarker is an anatomic,
Principles of translational medicine: imaging, biomarker imaging, theranostics Compiled by: Endre Mikus PhD, CEO Budapest, 21/9/2015 Imaging and imaging biomarkers An imaging biomarker is an anatomic,
REFERENCE CODE GDME1034FPR PUBLICATION DATE DECEMBER 2014 NUCLEAR IMAGING - CURRENT AND FUTURE PLAYERS
 REFERENCE CODE GDME1034FPR PUBLICATION DATE DECEMBER 2014 NUCLEAR IMAGING - 1 1... 2 1.1 List of Tables... 6 1.2 List of Figures... 8 2 Introduction... 9 2.1 Catalyst... 9 2.2 Related Reports... 9 3 Competitive
REFERENCE CODE GDME1034FPR PUBLICATION DATE DECEMBER 2014 NUCLEAR IMAGING - 1 1... 2 1.1 List of Tables... 6 1.2 List of Figures... 8 2 Introduction... 9 2.1 Catalyst... 9 2.2 Related Reports... 9 3 Competitive
TRANSLATIONAL RESEARCH THEORY AND PRACTICE. Ian Weeks Professor in Translational Biochemistry School of Medicine Cardiff University
 TRANSLATIONAL RESEARCH THEORY AND PRACTICE Ian Weeks Professor in Translational Biochemistry School of Medicine Cardiff University Translational research: Bench to bedside Not just clinical trials Encompasses
TRANSLATIONAL RESEARCH THEORY AND PRACTICE Ian Weeks Professor in Translational Biochemistry School of Medicine Cardiff University Translational research: Bench to bedside Not just clinical trials Encompasses
Roche, Roche Molecular Diagnostics and more
 , Molecular and more [Monte Wetzel, PhD] Patients have questions. We provide answers. Group Clear focus on Healthcare Innovation with two strong pillars Pharma Pharma Genentech Chugai Molecular Professional
, Molecular and more [Monte Wetzel, PhD] Patients have questions. We provide answers. Group Clear focus on Healthcare Innovation with two strong pillars Pharma Pharma Genentech Chugai Molecular Professional
Working Together for Better Health Partnering with Boehringer Ingelheim. JOURNEE COLLABORATIVE DE LYONBIOPOLE October 10, 2017
 Working Together for Better Health Partnering with Boehringer Ingelheim JOURNEE COLLABORATIVE DE LYONBIOPOLE October 10, 2017 Welcome to Partnering with Boehringer Ingelheim Our guiding principle Value
Working Together for Better Health Partnering with Boehringer Ingelheim JOURNEE COLLABORATIVE DE LYONBIOPOLE October 10, 2017 Welcome to Partnering with Boehringer Ingelheim Our guiding principle Value
Research area in the Strategic Objective Development of optical control technologies and elucidation of biological mechanisms
 Research area in the Strategic Objective Development of optical control technologies and elucidation of biological mechanisms 6.1.5 Development and application of optical technology for spatiotemporal
Research area in the Strategic Objective Development of optical control technologies and elucidation of biological mechanisms 6.1.5 Development and application of optical technology for spatiotemporal
Innovating to Create Value through New Perspectives and Synthesis
 Feature Article Innovating to Create Value through New Perspectives and Synthesis With the corporate mission of Contributing to Society through Healthcare, the Terumo Group has brought valuable innovation
Feature Article Innovating to Create Value through New Perspectives and Synthesis With the corporate mission of Contributing to Society through Healthcare, the Terumo Group has brought valuable innovation
On behalf of the Cystic Fibrosis Foundation (CFF) and the 30,000 people with cystic
 Robert J. Beall, Ph.D. President and Chief Executive Officer Cystic Fibrosis Foundation On behalf of the Cystic Fibrosis Foundation (CFF) and the 30,000 people with cystic fibrosis (CF) in the United States,
Robert J. Beall, Ph.D. President and Chief Executive Officer Cystic Fibrosis Foundation On behalf of the Cystic Fibrosis Foundation (CFF) and the 30,000 people with cystic fibrosis (CF) in the United States,
Konica Minolta to Acquire Invicro (US)
 Konica Minolta to Acquire Invicro (US) Acceleration of expansion of precision medicine business Offering new value for drug discovery and development in immuno-oncology and neurodegenerative disease September
Konica Minolta to Acquire Invicro (US) Acceleration of expansion of precision medicine business Offering new value for drug discovery and development in immuno-oncology and neurodegenerative disease September
Cell and gene therapy: scaling up and moving to mass production
 EDITORIAL Cell and gene therapy: scaling up and moving to mass production Nigel Whittle In order to fulfil the promise of cell therapy, it is important that manufacture of these therapies can be industrialized
EDITORIAL Cell and gene therapy: scaling up and moving to mass production Nigel Whittle In order to fulfil the promise of cell therapy, it is important that manufacture of these therapies can be industrialized
Biotech Patents in Europe
 Biotech Patents in Europe Introduction This circular relates to biotech patent practice in Europe. It is based on our experience of drafting and prosecuting biotech applications. The circular is written
Biotech Patents in Europe Introduction This circular relates to biotech patent practice in Europe. It is based on our experience of drafting and prosecuting biotech applications. The circular is written
MAYO CLINIC CENTER FOR BIOMEDICAL DISCOVERY EXCEPTIONAL RESEARCH LEADS TO EXCEPTIONAL PATIENT CARE
 MAYO CLINIC CENTER FOR BIOMEDICAL DISCOVERY EXCEPTIONAL RESEARCH LEADS TO EXCEPTIONAL PATIENT CARE THE RESEARCH WE DO TODAY WILL DETERMINE THE TYPE OF MEDICAL AND SURGICAL PRACTICE WE CARRY ON AT THE CLINIC
MAYO CLINIC CENTER FOR BIOMEDICAL DISCOVERY EXCEPTIONAL RESEARCH LEADS TO EXCEPTIONAL PATIENT CARE THE RESEARCH WE DO TODAY WILL DETERMINE THE TYPE OF MEDICAL AND SURGICAL PRACTICE WE CARRY ON AT THE CLINIC
Yakult Vision 2020 Phase III Plan ( )
 Yakult Vision 2020 Phase III Plan (2017 2020) May 12, 2017 Yakult Honsha Co., Ltd. Table of Contents Slide No. Outline of Long-term Vision P1-3 (1) Qualitative, quantitative goals P2 (2) Global business
Yakult Vision 2020 Phase III Plan (2017 2020) May 12, 2017 Yakult Honsha Co., Ltd. Table of Contents Slide No. Outline of Long-term Vision P1-3 (1) Qualitative, quantitative goals P2 (2) Global business
Simple, intuitive and accessible MRI solution for preclinical research. M-Series Compact MRI Systems
 Simple, intuitive and accessible MRI solution for preclinical research M-Series Compact MRI Systems Application Oriented Imaging Anatomy and Morphology In vivo soft tissue imaging for morphological characterization.
Simple, intuitive and accessible MRI solution for preclinical research M-Series Compact MRI Systems Application Oriented Imaging Anatomy and Morphology In vivo soft tissue imaging for morphological characterization.
Stem cells and motor neurone disease
 Stem cells and motor neurone disease F Stem cell research has fuelled hope of a treatment for a variety of conditions. This information sheet explains what these cells are and includes details of the current
Stem cells and motor neurone disease F Stem cell research has fuelled hope of a treatment for a variety of conditions. This information sheet explains what these cells are and includes details of the current
Simple, intuitive and accessible MRI solution for preclinical research. M-Series Compact MRI Systems
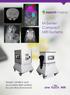 Simple, intuitive and accessible MRI solution for preclinical research M-Series Compact MRI Systems Application Oriented Imaging Molecular Imaging Using Contrast Agents Detection and quantification of
Simple, intuitive and accessible MRI solution for preclinical research M-Series Compact MRI Systems Application Oriented Imaging Molecular Imaging Using Contrast Agents Detection and quantification of
Road map for the PMDA International Vision April 2013 Pharmaceuticals and Medical Devices Agency, Japan 1
 Road map for the PMDA International Vision April 2013 Pharmaceuticals and Medical Devices Agency, Japan 1 1. Introduction The Pharmaceuticals and Medical Devices Agency (PMDA), a Japanese Incorporated
Road map for the PMDA International Vision April 2013 Pharmaceuticals and Medical Devices Agency, Japan 1 1. Introduction The Pharmaceuticals and Medical Devices Agency (PMDA), a Japanese Incorporated
GENOMERA TM MRSA/SA PRODUCTS A NEW ERA IN DIRECT MRSA DNA TESTING. Bringing genetic MRSA results in less than 1 hour!
 GENOMERA TM MRSA/SA PRODUCTS A NEW ERA IN DIRECT MRSA DNA TESTING Bringing genetic MRSA results in less than 1 hour! IMPROVING PATIENT CARE THROUGH EARLIER DETECTION OF MRSA AND SA FAST MRSA AND SA DETECTION
GENOMERA TM MRSA/SA PRODUCTS A NEW ERA IN DIRECT MRSA DNA TESTING Bringing genetic MRSA results in less than 1 hour! IMPROVING PATIENT CARE THROUGH EARLIER DETECTION OF MRSA AND SA FAST MRSA AND SA DETECTION
Future of Stem Cell Engineering. Jaeseung Jeong, Ph.D Department of Bio and Brain Engineering KAIST
 Future of Stem Cell Engineering i Jaeseung Jeong, Ph.D Department of Bio and Brain Engineering KAIST Keywords of Stem Cell Engineering g Embryo and Fetus (Foetus) Adult stem cells and embryonic stem cells
Future of Stem Cell Engineering i Jaeseung Jeong, Ph.D Department of Bio and Brain Engineering KAIST Keywords of Stem Cell Engineering g Embryo and Fetus (Foetus) Adult stem cells and embryonic stem cells
Human ips/es Cell Technology and Its Application to Toxicology Testing
 Human ips/es Cell Technology and Its Application to Toxicology Testing -Especially focusing on in vitro cardiac function toxicity- Atsushi Sanbuissho, PhD Daiichi Sankyo Co., Ltd. Kenji Yasuda, Prof Tokyo
Human ips/es Cell Technology and Its Application to Toxicology Testing -Especially focusing on in vitro cardiac function toxicity- Atsushi Sanbuissho, PhD Daiichi Sankyo Co., Ltd. Kenji Yasuda, Prof Tokyo
Chairman Cole, Ranking Member DeLauro and distinguished members of the
 Fiscal Year 2019 House Appropriations Committee Subcommittee on Labor, Health and Human Services, Education and Related Agencies Appropriations Testimony Cynthia A. Bens, Senior Vice President, Public
Fiscal Year 2019 House Appropriations Committee Subcommittee on Labor, Health and Human Services, Education and Related Agencies Appropriations Testimony Cynthia A. Bens, Senior Vice President, Public
ORGANIZATION AND ROLE OF A PHASE I ONCOLOGY UNIT. Dr Philippe CASSIER Centre Léon Bérard, Lyon
 ORGANIZATION AND ROLE OF A PHASE I ONCOLOGY UNIT Dr Philippe CASSIER Centre Léon Bérard, Lyon Outline Cancer & Oncology Drug development in oncology Specificity of phase I trials in oncology Study design
ORGANIZATION AND ROLE OF A PHASE I ONCOLOGY UNIT Dr Philippe CASSIER Centre Léon Bérard, Lyon Outline Cancer & Oncology Drug development in oncology Specificity of phase I trials in oncology Study design
Personalised Healthcare Solutions (PHCS) Excellence for your Biomarker-driven Strategies
 Personalised Healthcare Solutions (PHCS) Excellence for your Biomarker-driven Strategies The value of In Vitro Diagnostics The value of diagnostics as an integral part in the healthcare value chain Diagnostics
Personalised Healthcare Solutions (PHCS) Excellence for your Biomarker-driven Strategies The value of In Vitro Diagnostics The value of diagnostics as an integral part in the healthcare value chain Diagnostics
Wider Adoption of Regenerative Medicine Driven by Open Innovation
 Review Vol. 64 (2016), No. 10 693 Featured Articles Wider Adoption of Regenerative Medicine Driven by Open Innovation Kohin Shu, Ph.D., Engineering Masaharu Kiyama Takayuki Nozaki, Ph.D., Medical Science
Review Vol. 64 (2016), No. 10 693 Featured Articles Wider Adoption of Regenerative Medicine Driven by Open Innovation Kohin Shu, Ph.D., Engineering Masaharu Kiyama Takayuki Nozaki, Ph.D., Medical Science
Innovating out of Crisis
 Innovating out of Crisis Shigetaka Komori Chairman and CEO FUJIFILM Holdings Corporation 1 st June, 2015 Business Fields of Fujifilm Group Digital Camera Photo book Optical devise FY2015/3 Digital minilab
Innovating out of Crisis Shigetaka Komori Chairman and CEO FUJIFILM Holdings Corporation 1 st June, 2015 Business Fields of Fujifilm Group Digital Camera Photo book Optical devise FY2015/3 Digital minilab
Conference Exhibitors
 Conference Exhibitors CSM Executive and the 2018 Manitoba Local Organizing Committee would like to thank the following organizations for exhibiting and supporting the Canadian Society of Microbiologists
Conference Exhibitors CSM Executive and the 2018 Manitoba Local Organizing Committee would like to thank the following organizations for exhibiting and supporting the Canadian Society of Microbiologists
About OMICS Group Conferences
 About OMICS Group OMICS Group International is an amalgamation of Open Access publications and worldwide international science conferences and events. Established in the year 2007 with the sole aim of
About OMICS Group OMICS Group International is an amalgamation of Open Access publications and worldwide international science conferences and events. Established in the year 2007 with the sole aim of
REIMAGINING DRUG DEVELOPMENT:
 Biology Reconstructed REIMAGINING DRUG DEVELOPMENT: Accurate Disease Modeling To Drive Successful Therapies Julia Kirshner, CEO julia@zpredicta.com 1 SUCCESS RATES OF DRUG DEVELOPMENT ARE LOW, " PARTICULARLY
Biology Reconstructed REIMAGINING DRUG DEVELOPMENT: Accurate Disease Modeling To Drive Successful Therapies Julia Kirshner, CEO julia@zpredicta.com 1 SUCCESS RATES OF DRUG DEVELOPMENT ARE LOW, " PARTICULARLY
Adis Journals and Newsletters The premier collection of drug-focused medical journals
 adis.com Adis Journals and Newsletters The premier collection of drug-focused medical journals An invaluable resource for all involved in medical research, practice or teaching, drug regulation or reimbursement,
adis.com Adis Journals and Newsletters The premier collection of drug-focused medical journals An invaluable resource for all involved in medical research, practice or teaching, drug regulation or reimbursement,
TRANSFORMING GLOBAL GENETIC DATA INTO MEDICAL DECISIONS
 TRANSFORMING GLOBAL GENETIC DATA INTO MEDICAL DECISIONS THE DOORS ARE OPEN: FEEL FREE TO COME IN CENTOGENE UNLOCKS THE POWER OF GENETIC INSIGHTS TO IMPROVE THE QUALITY OF LIFE OF PATIENTS WITH GENETIC
TRANSFORMING GLOBAL GENETIC DATA INTO MEDICAL DECISIONS THE DOORS ARE OPEN: FEEL FREE TO COME IN CENTOGENE UNLOCKS THE POWER OF GENETIC INSIGHTS TO IMPROVE THE QUALITY OF LIFE OF PATIENTS WITH GENETIC
Federal Funding for Brain Research. Congressional Support Accelerates Discovery
 The American Brain Coalition (ABC) is a non-profit organization that brings together people with disabling brain disorders, the families of those that are affected, and the professionals who research and
The American Brain Coalition (ABC) is a non-profit organization that brings together people with disabling brain disorders, the families of those that are affected, and the professionals who research and
Vision, aims and strategies. Department of Immunology, Genetics and Pathology Uppsala University
 Vision, aims and strategies Department of Immunology, Genetics and Pathology Uppsala University April19,2011 SCOPEOFACTIVITIESATIGP Research at the Department focuses on translational medicine through
Vision, aims and strategies Department of Immunology, Genetics and Pathology Uppsala University April19,2011 SCOPEOFACTIVITIESATIGP Research at the Department focuses on translational medicine through
National Foundation for Women Legislators Annual Meeting September 12, 2015 Tara Ryan Vice President, State Government Advocacy
 From Hope to Cures: The Value of Biopharmaceutical Innovation National Foundation for Women Legislators Annual Meeting September 12, 2015 Tara Ryan Vice President, State Government Advocacy HIV/AIDS: Then
From Hope to Cures: The Value of Biopharmaceutical Innovation National Foundation for Women Legislators Annual Meeting September 12, 2015 Tara Ryan Vice President, State Government Advocacy HIV/AIDS: Then
Information and resources for African Americans living with multiple myeloma and their caregivers
 For African Americans living with Multiple Myeloma Information and resources for African Americans living with multiple myeloma and their caregivers Standing in the Gaap: An initiative created to help
For African Americans living with Multiple Myeloma Information and resources for African Americans living with multiple myeloma and their caregivers Standing in the Gaap: An initiative created to help
Developing Targeted Stem Cell Therapeutics for Cancer. Shawn Hingtgen, Ph.D. Assistant Professor UNC Eshelman School of Pharmacy May 22 nd, 2013
 Developing Targeted Stem Cell Therapeutics for Cancer Shawn Hingtgen, Ph.D. Assistant Professor UNC Eshelman School of Pharmacy May 22 nd, 2013 The Challenge of Drug Delivery for Brain Cancer Stem Cells
Developing Targeted Stem Cell Therapeutics for Cancer Shawn Hingtgen, Ph.D. Assistant Professor UNC Eshelman School of Pharmacy May 22 nd, 2013 The Challenge of Drug Delivery for Brain Cancer Stem Cells
Basic Information. I.1 If your company performs biotechnology research and development, uses a. The Categories of Biotechnology Applications.
 APPENDIX 2 SURVEY QUESTIONNAIRE Part I. Basic Information I.1 If your company performs biotechnology research and development, uses a biotechnology process in manufacturing, or produces research tools,
APPENDIX 2 SURVEY QUESTIONNAIRE Part I. Basic Information I.1 If your company performs biotechnology research and development, uses a biotechnology process in manufacturing, or produces research tools,
Multiplex Solution for Better Health
 Multiplex Solution for Better Health About Us About Us Core Technology Technology s Precision Image Code () MicroDisc is manufactured by using a photo lithography fabrication process. This semi-conductor
Multiplex Solution for Better Health About Us About Us Core Technology Technology s Precision Image Code () MicroDisc is manufactured by using a photo lithography fabrication process. This semi-conductor
Personalized Healthcare Diagnostik und Therapie aus einer Hand
 Personalized Healthcare Diagnostik und Therapie aus einer Hand Priv.-Doz. Dr. med. Christian Meisel DVFA Life Science Conference, 17 June 2009 1 The Challenge Personalised healthcare Roche s unique position
Personalized Healthcare Diagnostik und Therapie aus einer Hand Priv.-Doz. Dr. med. Christian Meisel DVFA Life Science Conference, 17 June 2009 1 The Challenge Personalised healthcare Roche s unique position
Annex VIIIB Paragraphs to be included in the Informed Consent Form for the collection and use of biological samples in clinical trials
 DEPARTAMENTO DE MEDICAMENTOS DE USO HUMANO Annex VIIIB Paragraphs to be included in the Informed Consent Form for the collection and use of biological samples in clinical trials Version 20 th December
DEPARTAMENTO DE MEDICAMENTOS DE USO HUMANO Annex VIIIB Paragraphs to be included in the Informed Consent Form for the collection and use of biological samples in clinical trials Version 20 th December
DIAGNOSE AND TREAT WITH ANTIBODIES THAT RECOGNIZE NATIVE HUMAN PROTEIN EPITOPES IN BLOOD AND TISSUE
 DIAGNOSE AND TREAT WITH ANTIBODIES THAT RECOGNIZE NATIVE HUMAN PROTEIN EPITOPES IN BLOOD AND TISSUE EXECUTIVE SUMMARY Oncologists reviewing blood and tissue test results face the same problems: Is the
DIAGNOSE AND TREAT WITH ANTIBODIES THAT RECOGNIZE NATIVE HUMAN PROTEIN EPITOPES IN BLOOD AND TISSUE EXECUTIVE SUMMARY Oncologists reviewing blood and tissue test results face the same problems: Is the
Genetics Faculty of Agriculture and Veterinary Medicine. Instructor: Dr. Jihad Abdallah Topic 16: Biotechnology
 Genetics 10201232 Faculty of Agriculture and Veterinary Medicine Instructor: Dr. Jihad Abdallah Topic 16: Biotechnology 1 Biotechnology is defined as the technology that involves the use of living organisms
Genetics 10201232 Faculty of Agriculture and Veterinary Medicine Instructor: Dr. Jihad Abdallah Topic 16: Biotechnology 1 Biotechnology is defined as the technology that involves the use of living organisms
AMGEN AND CENTOCOR ORTHO BIOTECH PRODUCTS FINALIZE ESA RISK EVALUATION AND MITIGATION STRATEGY (REMS) WITH FDA
 News Release One Amgen Center Drive Thousand Oaks, CA 91320-1799 Telephone (805) 447-1000 Fax (805) 499-3507 www.amgen.com AMGEN AND CENTOCOR ORTHO BIOTECH PRODUCTS FINALIZE ESA RISK EVALUATION AND MITIGATION
News Release One Amgen Center Drive Thousand Oaks, CA 91320-1799 Telephone (805) 447-1000 Fax (805) 499-3507 www.amgen.com AMGEN AND CENTOCOR ORTHO BIOTECH PRODUCTS FINALIZE ESA RISK EVALUATION AND MITIGATION
QPS Neuropharmacology Overview
 HISTOCHEMISTRY HISTOLOGY KO MODELS IN VIVO MODELS BLOOD BRAIN BARRIER IN VITRO MODELS NEUROPHARMACOLOGY OVERVIEW NEUROSCIENCES QPS Neuropharmacology Overview QPS is recognized all over the world as a leading
HISTOCHEMISTRY HISTOLOGY KO MODELS IN VIVO MODELS BLOOD BRAIN BARRIER IN VITRO MODELS NEUROPHARMACOLOGY OVERVIEW NEUROSCIENCES QPS Neuropharmacology Overview QPS is recognized all over the world as a leading
Mirada Case Study. VentureFest, July
 Mirada Case Study VentureFest, July 2015 Synopsis of Mirada Medical Founded in 1999 as a spin-out from the University of Oxford University had 10% equity Headquartered at the OCFI, Oxford Mirada USA based
Mirada Case Study VentureFest, July 2015 Synopsis of Mirada Medical Founded in 1999 as a spin-out from the University of Oxford University had 10% equity Headquartered at the OCFI, Oxford Mirada USA based
Genetic tests are available for hundreds of disorders. DNA testing can pinpoint the exact genetic basis of a disorder.
 Human DNA Analysis Human DNA Analysis There are roughly 6 billion base pairs in your DNA. Biologists search the human genome using sequences of DNA bases. Genetic tests are available for hundreds of disorders.
Human DNA Analysis Human DNA Analysis There are roughly 6 billion base pairs in your DNA. Biologists search the human genome using sequences of DNA bases. Genetic tests are available for hundreds of disorders.
Personalised Medicine Regulatory Issues
 Personalised Medicine Regulatory Issues INFRAFRONTIER / IMPC Stakeholder Meeting Presented by Marisa Papaluca on 14 November 2017 Senior Scientific Advisor, Scientific Committees Regulatory Science Strategy
Personalised Medicine Regulatory Issues INFRAFRONTIER / IMPC Stakeholder Meeting Presented by Marisa Papaluca on 14 November 2017 Senior Scientific Advisor, Scientific Committees Regulatory Science Strategy
Molecular Diagnostics: Market Segmentation and Opportunities
 Molecular Diagnostics: Market Segmentation and Opportunities October 2010 This market report is published by DeciBio, LLC. All rights reserved. Reproduction or redistribution of this report in any form
Molecular Diagnostics: Market Segmentation and Opportunities October 2010 This market report is published by DeciBio, LLC. All rights reserved. Reproduction or redistribution of this report in any form
Undergraduate Research in the Brzustowicz Laboratory Human Genetics Institute Department of Genetics Rutgers University
 Undergraduate Research in the Brzustowicz Laboratory Human Genetics Institute Department of Genetics Rutgers University Genetics plays an important role in the development of many of the major psychiatric
Undergraduate Research in the Brzustowicz Laboratory Human Genetics Institute Department of Genetics Rutgers University Genetics plays an important role in the development of many of the major psychiatric
Japanese Pharmacopoeia s Challenge to the Globalization
 Japanese Pharmacopoeia s Challenge to the Globalization Naoyuki Yabana, Ph.D. Division of Pharmacopoeia and Standards for Drugs, Office of Standards and Guidelines Development, Pharmaceuticals and Agency
Japanese Pharmacopoeia s Challenge to the Globalization Naoyuki Yabana, Ph.D. Division of Pharmacopoeia and Standards for Drugs, Office of Standards and Guidelines Development, Pharmaceuticals and Agency
Gene therapy. Findings by Alert
 Gene therapy Published Mar 21, 2000 Version 1 Findings by Alert Research in gene therapy has increased dramatically during the past 15 years, particularly in the United States. The research has encompassed
Gene therapy Published Mar 21, 2000 Version 1 Findings by Alert Research in gene therapy has increased dramatically during the past 15 years, particularly in the United States. The research has encompassed
NanoPro 1000 System. characterize cell signaling in your smallest samples
 NanoPro 1000 System characterize cell signaling in your smallest samples Technology The NanoPro 1000 system is a capillary-based nano-immunoassay platform. As in Western blot analysis, proteins from complex
NanoPro 1000 System characterize cell signaling in your smallest samples Technology The NanoPro 1000 system is a capillary-based nano-immunoassay platform. As in Western blot analysis, proteins from complex
Healthcare. Healthcare. The Centre for Process Innovation. From innovation to commercialisation
 Healthcare Healthcare The Centre for Process Innovation From innovation to commercialisation The Centre for Process Innovation From innovation to commercialisation The Centre for Process Innovation (CPI)
Healthcare Healthcare The Centre for Process Innovation From innovation to commercialisation The Centre for Process Innovation From innovation to commercialisation The Centre for Process Innovation (CPI)
The flow diagram below shows part of a process to produce a protein, using genetically modified plants.
 1 Some organisms have been genetically modified to produce proteins including hormones and vaccines. The flow diagram below shows part of a process to produce a protein, using genetically modified plants.
1 Some organisms have been genetically modified to produce proteins including hormones and vaccines. The flow diagram below shows part of a process to produce a protein, using genetically modified plants.
PROJECT GROUP FOR AUTOMATION IN MEDICINE AND BIOTECHNOLOGY IN MANNHEIM BIOPROCESS ENGINEERING
 Fraunhofer Institute for Manufacturing Engineering and Automation IPA PROJECT GROUP FOR AUTOMATION IN MEDICINE AND BIOTECHNOLOGY IN MANNHEIM BIOPROCESS ENGINEERING VERGÜTUNG VON ERFINDUNGEN UND TECHNISCHEN
Fraunhofer Institute for Manufacturing Engineering and Automation IPA PROJECT GROUP FOR AUTOMATION IN MEDICINE AND BIOTECHNOLOGY IN MANNHEIM BIOPROCESS ENGINEERING VERGÜTUNG VON ERFINDUNGEN UND TECHNISCHEN
Summary of Medicines Plan
 Summary of Medicines Plan Ministry of Public Health, Welfare and Sport Summary of Medicines Plan 1 Content Introduction 4 1 Accessibility of innovative medicines 5 Go to action 2 Tackle the high price
Summary of Medicines Plan Ministry of Public Health, Welfare and Sport Summary of Medicines Plan 1 Content Introduction 4 1 Accessibility of innovative medicines 5 Go to action 2 Tackle the high price
