Duodenoscope and Endoscope Reprocessing: Are We Doing Enough to Protect Patients?
|
|
|
- Tracey Harrington
- 6 years ago
- Views:
Transcription
1 Duodenoscope and Endoscope Reprocessing: Are We Doing Enough to Protect Patients? William A. Rutala, Ph.D., M.P.H. Director, Statewide Program for Infection Control and Epidemiology, Research Professor of Medicine, University of North Carolina (UNC) Former Director, Hospital Epidemiology, Occupational Health and Safety Program, UNC Health Care, Chapel Hill
2 DISCLOSURES Consultation (2017) PDI ASP Honoraria (2017) PDI Kennall Grants to UNC or UNC Hospitals (2017) CDC, CMS
3 Endoscope Reprocessing: Are We Doing Enough to Protect Patients? NO
4 Our Responsibility to the Future Prevent All Infectious Disease Transmission by Medical Devices in 5 years
5 Duodenoscopes and Endoscope Reprocessing : A Need to Shift from Disinfection to Sterilization Sources of healthcare-associated pathogens Evaluate the cause of endoscope-related outbreaks Review the outbreaks associated with ERCP and endoscopic procedures Discuss the alternatives that exist today that might improve the safety margin associated with duodenoscope/endoscope reprocessing Describe how to prevent future outbreaks associated with duodenoscopes and other GI endoscopes
6 Sources of Healthcare-Associated Pathogens Weinstein RA. Am J Med 1991:91 (suppl 3B):179S Endogenous flora (SSI, UTI, CLABSI): 40-60% Exogenous: 20-40% (e.g., cross-infection via contaminated hands [staff, visitors]) Other (environment): 20% Medical devices Contact with environmental surfaces (direct and indirect contact)
7 How Can We Prevent All Infections Associated with Medical Devices in 5 Years?
8 Medical/Surgical Devices WA Rutala, DJ Weber, and HICPAC, EH Spaulding believed that how an object will be disinfected depended on the object s intended use (developed 1968). CRITICAL-medical/surgical devices which enter normally sterile tissue or the vascular system or through which blood flows should be sterile. SEMICRITICAL-medical devices that touch mucous membranes or skin that is not intact require a disinfection process (high-level disinfection [HLD]) that kills all microorganisms but high numbers of bacterial spores. NONCRITICAL-medical devices that touch only intact skin require low-level disinfection.
9 Critical Medical/Surgical Devices Rutala et al. ICHE 2014;35:883; Rutala et al. ICHE 2014;35:1068; Rutala et al. AJIC 2016;44:e47 Critical Transmission: direct contact Control measure: sterilization Surgical instruments Enormous margin of safety, rare outbreaks (2 in 60 years) ~85% of surgical instruments <100 microbes Washer/disinfector removes or inactivates million Sterilization kills 1 trillion spores
10 Sterilization Enormous Margin of Safety! 100 quadrillion (10 17 ) margin of safety Sterilization kills 1 trillion spores, washer/disinfector removes or inactivates million; ~100 microbes on surgical instruments
11 Noncritical Medical Devices Rutala et al. AJIC 2016;44:e1; Rutala, Weber. Env Issues NI, Farber 1987 Contact: intact skin (noncritical medical devices, surfaces) Transmission: secondary transmission by contaminating hands/gloves via contact with the environment and transfer to patient Control measures: hand hygiene and low-level disinfection Noncritical devices (stethoscopes, blood pressure cuffs, wound vacuum), rare outbreaks
12 Semicritical Medical Devices Rutala et al. AJIC 2016;44:e47 Semicritical Transmission: direct contact Control measure: high-level disinfection Endoscopes top ECRI list of 10 technology hazards, >130 outbreaks (GI, bronchoscopes) 0 margin of safety Microbial load, Complexity Biofilm Other semicritical devices, rare outbreaks ENT scopes, endocavitary probes (prostate, vaginal, TEE), laryngoscopes, cystoscopes Reduced microbial load, less complex
13 High-Level Disinfection No Margin of Safety 0 margin of safety Microbial contamination : compliant with reprocessing guidelines 10,000 microbes after reprocessing: maximum contamination, minimal cleaning (10 2 )/HLD (10 4 )
14 What are the risks associated with endoscopes?
15 Transmission of Infection by Endoscopy Kovaleva et al. Clin Microbiol Rev : Scope Outbreaks Micro (primary) Pts Contaminated Upper GI 19 Pa, H. pylori, Salmonella Sigmoid/Colon oscopy Pts Infected Cause (primary) Cleaning/Disinfection (C/D) 5 Salmonella, HCV 14 6 Cleaning/Disinfection ERCP 23 P. aeruginosa (Pa) Bronchoscopy 51 Pa, Mtb, Mycobacteria Totals C/D, water bottle, AER C/D, AER, water Based on outbreak data, if eliminated deficiencies associated with cleaning, disinfection, AER, contaminated water and drying would eliminate about 85% of the outbreaks.
16 Preventable Tragedies: Superbugs and How Ineffective Monitoring of Medical Device Safety Fail Patients Minority Staff Report, January 13, 2016, Patty Murray, Ranking Member In January 2015, after several outbreaks of serious infections, Senator Murray initiated an investigation to determine the extent of duodenoscope-linked infections Between 2012 and spring 2015, closed-channel duodenoscopes were linked to at least 25 different incidents of antibiotic-resistant infections that sickened at least 250 patients worldwide None of the manufacturers of the closed-channel duodenoscopes had sufficient data to show that duodenoscopes could be cleaned reliably between uses
17 RECENT ENDOSCOPY-RELATED OUTBREAKS OF MRDO WITHOUT REPROCESSING BREACHES Rutala WA et al. Manuscript in preparation MDRO Scope No. Recovered From Scope Molecular Link Reference P. aeruginosa (VIM-2) Duodenoscope 22 Yes, under forceps elevator Yes Verfaillie CJ, 2015 E. coli (AmpC) Duodenoscope 35 Yes (2 scopes) Yes Wendorf, 2015 K. pneumoniae (OXA) Duodenoscope 12 No Yes Kola A, 2015 E. coli (NDM-CRE) Duodenoscope 39 Yes Yes Epstein L, 2015 K. pneumoniae Duodenoscope 15 No Yes Kim S, 2016 K. pneumoniae Duodenoscope 34 Yes Yes Marsh J, 2015 E. coli Duodenoscope 3 No Unknown Smith Z, 2015 K. pneumoniae Duodenoscope 13 Yes Yes Carbonne A, 2010
18 Carbapenemase-Resistant Enterobacteriaceae (CRE) and Multidrug Resistant Organisms (MDRO) Klebsiella, Enterobacter and E. coli are examples of Enteriobacteriaceae, a normal part of enteric microbes, that have become resistant to carbapenem Healthy people usually do not generally get CRE infections Infections with CRE and MDROs are very difficult to treat and can be deadly Likely that MDR pathogens are acting as a marker or indicator organism for ineffective reprocessing of duodenoscopes
19 Endemic Transmission of Infections Associated with GI Endoscopes May Go Unrecognized CRE and ESBLs Inadequate surveillance of outpatient procedures for healthcare-associated infections Long lag time between colonization and infection Low frequency of infection Pathogens usual enteric flora Risk of some procedures might be lower than others (colonoscopy versus ERCP where normally sterile areas are contaminated in the latter)
20 Reprocesssing Failures Have Led to Patient Notifications and Bloodborne Pathogens Testing Rutala WA, Weber DJ. Infect Control Hosp Epidemiol 2007;28:
21 Because more outbreaks associated with endoscopes than any other reusable medical device, endoscopes top ECRI s list of 10 health technology hazards If we eliminate the risk of disease transmission associated with endoscopes, will eliminate risk associated with all medical and surgical devices
22 GI Endoscopes: Shift from Disinfection to Sterilization Rutala, Weber. JAMA :
23 FDA has mandated a shift from HLD to sterilization in 1992 with dental handpieces
24 HIV Transmission in Dental Settings First case of dentist-to patient transmission; removed molars in 1987, AIDS in 1990, died in 1991 Even though no documented cases of disease transmission, FDA recommends that reusable dental handpieces and related instruments be heat sterilized between each patient use. September 1992.
25
26 Reason for Endoscope-Related Outbreaks Rutala WA, Weber DJ. Infect Control Hosp Epidemiol 2015;36: Margin of safety with endoscope reprocessing minimal or non-existent Microbial load GI endoscopes contain Cleaning results in 2-6 log 10 reduction High-level disinfection results in 4-6 log 10 reduction Results in a total 6-12 log 10 reduction of microbes Level of contamination after processing: 4log 10 (maximum contamination, minimal cleaning/hld) Complexity of endoscope and endoscope reprocessing Biofilms-unclear if contribute to failure of endoscope reprocessing
27 ENDOSCOPE REPROCESSING: CHALLENGES Complex [elevator channel] bacteria/endoscope Surgical instruments-<10 2 bacteria
28 Mowat AM, Agace WW. Nat Rev Immunology 2014;14:
29 ENDOSCOPE REPROCESSING: CHALLENGES NDM-Producing E. coli Associated ERCP MMWR 2014;62:1051; Epstein et al. JAMA 2014;312: NDM-producing E.coli recovered from elevator channel (elevator channel orients catheters, guide wires and accessories into the endoscope visual field; crevices difficult to access with cleaning brush and may impede effective reprocessing)
30 Bacterial Bioburden Associated with Endoscopes Cleaning Results in 2-6 log 10 Reduction Gastroscope, log 10 CFU Colonoscope, log 10 CFU After procedure Gastro Nursing 1998;22: Am J Inf Cont 1999;27: ~10,000,000,000 or Gastro Endosc 1997;48:137 After cleaning ~100,000 or 10 5
31 Reason for Endoscope-Related Outbreaks Rutala WA, Weber DJ. Infect Control Hosp Epidemiol 2015;36: Margin of safety with endoscope reprocessing minimal or non-existent Microbial load GI endoscopes contain Cleaning results in 2-6 log 10 reduction High-level disinfection results in 4-6 log 10 reduction Results in a total 6-12 log 10 reduction of microbes Level of contamination after processing: 4 log 10 (maximum contamination, minimal cleaning/hld) Complexity of endoscope and endoscope reprocessing Biofilms-unclear if contribute to failure of endoscope reprocessing
32 Reason for Endoscope-Related Outbreaks Rutala WA, Weber DJ. Infect Control Hosp Epidemiol 2015;36: Margin of safety with endoscope reprocessing minimal or non-existent Microbial load GI endoscopes contain Cleaning results in 2-6 log 10 reduction High-level disinfection results in 4-6 log 10 reduction Results in a total 6-12 log 10 reduction of microbes Level of contamination after processing: 4log 10 (maximum contamination, minimal cleaning/hld) Complexity of endoscope and endoscope reprocessing Biofilms-unclear if contribute to failure of endoscope reprocessing
33 What does this off-road driver/vehicle have in common with GI endoscope? 10 Billion particles, complexity
34 FEATURES OF ENDOSCOPES THAT PREDISPOSE TO DISINFECTION FAILURES Rutala WA, Weber DJ. Infect Control Hosp Epidemiol 2015;36: Heat labile Long, narrow lumens (3.5ft, 1-3mm) Right angle bends Rough or pitted surfaces Springs and valves Damaged channels may impede microbial exposure to HLD Heavily contaminated with pathogens, Cleaning (2-6 log 10 reduction) and HLD (4-6 log 10 reduction) essential for patient safe instrument
35 CDC Guideline for Disinfection and Sterilization Rutala, Weber, HICPAC. November
36 MULTISOCIETY GUIDELINE ON REPROCESSING GI ENDOSCOPES, 2017 Petersen et al. Gastro Endoscopy. In press
37 ENDOSCOPE REPROCESSING CDC 2008: Multi-Society Guideline on Endoscope Reprocessing, 2017 PRECLEAN-point-of-use (bedside) remove debris by wiping exterior and aspiration of detergent through air/water and biopsy channels; leak test CLEAN-mechanically cleaned with water and enzymatic cleaner HLD/STERILIZE-immerse scope and perfuse HLD/sterilant through all channels for exposure time (>2% glut at 20m at 20 o C). If AER used, review model-specific reprocessing protocols from both the endoscope and AER manufacturer RINSE-scope and channels rinsed with sterile water, filtered water, or tap water. Flush channels with alcohol and dry DRY-use forced air to dry insertion tube and channels STORE-hang in vertical position to facilitate drying; stored in a manner to protect from contamination
38 Endoscope Reprocessing Methods Ofstead, Wetzler, Snyder, Horton, Gastro Nursing 2010; 33:204
39 Endoscope Reprocessing Methods Ofstead, Wetzler, Snyder, Horton, Gastro Nursing 2010; 33:204 Performed all 12 steps with only 1.4% of endoscopes using manual versus 75.4% of those processed using AER
40 Automated Endoscope Reprocessors AERs automate and standardize endoscope reprocessing steps
41 High-Level Disinfection No Margin of Safety 0 margin of safety Microbial contamination : compliant with reprocessing guidelines 10,000 microbes after reprocessing: maximum contamination, minimal cleaning (10 2 )/HLD (10 4 )
42 Microbial Surveillance of GI Endoscopes Saliou et al. Endoscopy Characteristics of Sample Action Level (TCU>100/scope) or EIP Gastroscope 26.6% Colonoscope 33.7% Duodenoscope 34.7% Echo-endoscope 31.9% AER 27.2% Manual 39.3% Age of endoscope <2 years 18.9% Age of endoscope >2 years 38.8%
43 Visual Inspections of Colonoscopes and Gastroscopes Ofstead et al. Am J Infect Control :e26-e33 All endoscopes (n=20) had visible irregularities (e.g., scratches) Researchers observed fluid (95%), discoloration, and debris in channels
44 Reason for Endoscope-Related Outbreaks Rutala WA, Weber DJ. Infect Control Hosp Epidemiol 2015;36: Margin of safety with endoscope reprocessing minimal or non-existent Microbial load GI endoscopes contain Cleaning results in 2-6 log 10 reduction High-level disinfection results in 4-6 log 10 reduction Results in a total 6-12 log 10 reduction of microbes Level of contamination after processing: 4log 10 (maximum contamination, minimal cleaning/hld) Complexity of endoscope and endoscope reprocessing Biofilms-unclear if contribute to failure of endoscope reprocessing
45 BIOFILMS (Multi-layered bacteria plus exopolysaccharides that cement cell to surface; develop in wet environments; if reprocessing performed promptly after use and endoscope dry the opportunity for biofilm formation is minimal; Pajkos et al. J Hosp Infect 2004;58:224)
46 Reason for Endoscope-Related Outbreaks Rutala WA, Weber DJ. Infect Control Hosp Epidemiol 2015;36: Margin of safety with endoscope reprocessing minimal or non-existent Microbial load GI endoscopes contain Cleaning results in 2-6 log 10 reduction High-level disinfection results in 4-6 log 10 reduction Results in a total 6-12 log 10 reduction of microbes Level of contamination after processing: 4log 10 (maximum contamination, minimal cleaning/hld) Complexity of endoscope and endoscope reprocessing Biofilms-unclear if contribute to failure of endoscope reprocessing
47 What Should We Do Now? Interim Response to ERCP Outbreaks
48 How Can We Prevent ERCP-Related Infections? Rutala WA, Weber DJ. Infect Control Hosp Epidemiol 2015;36: No single, simple and proven technology or prevention strategy that hospitals can use to guarantee patient safety Of course, must continue to emphasize the enforcement of evidenced-based practices, including equipment maintenance and routine audits with at least yearly competency testing of reprocessing staff Must do more or additional outbreaks will continue
49 Education/Training/Competency/Compliance Judie Bringhurst
50 Methods to Prevent GI-Endoscope Related Outbreaks For nearly 40 years have had the opportunity to be part of the infection prevention team and conduct research on disinfection/sterilization at UNC Hospitals and UNC School of Medicine During that time every 2-3 years there have been newsworthy endoscopy-related outbreaks which resulted in meeting with various professional organization, industry and/or government to discuss the outbreak(s) Each time we would focus on strict adherence to cleaning and endoscope reprocessing guidelines and/or a design tweak but the outbreaks continue
51 Endoscope Reproccesing: A Need to Shift from Disinfection to Sterilization
52 High-Level Disinfection No Margin of Safety 0 margin of safety Microbial contamination : compliant with reprocessing guidelines 10,000 microbes after reprocessing: maximum contamination, minimal cleaning (10 2 )/HLD (10 4 )
53 Endoscope Reproccesing: A Need to Shift from Disinfection to Sterilization When Spaulding scheme designed 50 years ago, semicritical items rarely, if ever, penetrated sterile tissue and we did not appreciate the infection risk associated with endoscope reprocessing. Early endoscopes used primarily for diagnostic purposes. New enhancements to include visualization when combined with radiography results in highquality visualization of sterile body sites for treatment (e.g., endoscopic ultrasound with fine needle aspiration for embolization or thermal or alcohol injection ablation of tumors) or nonradiographic peroral endoscopic myotomy. Even when duodenoscope are used for stones or tumors and the area is no longer sterile, the infection risk is unacceptable as demonstrate by >125 published outbreaks and a significant portion of scopes are contaminated after processing. In some cases, endoscopies now replace invasive surgery. Unfortunately, these highly complex devices (e.g., long, narrow lumens, right angle bends, rough or pitted surfaces, springs and valves) exceed the ability of high-level disinfection to eliminate all pathogens (including multidrug-resistant pathogens such as CRE which will have higher mortality).
54 Current Enhanced Methods for Reprocessing Duodenoscopes Rutala WA, Weber DJ. Infect Control Hosp Epidemiol 2015;36: Hospitals performing ERCPs should do one of the following (priority ranked); doing nothing is not an option: Ethylene oxide sterilization after high level disinfection with periodic microbiologic surveillance Double high-level disinfection with periodic microbiologic surveillance High-level disinfection with scope quarantine until negative culture Liquid chemical sterilant processing system using peracetic acid (rinsed with extensively treated potable water) with periodic microbiologic surveillance High-level disinfection with periodic microbiologic surveillance
55 Comparison of High-Level Disinfection and Sterilization Procedures Synder et al. Gastroenterology 2017 Found no significant differences between groups Enhanced disinfection methods did not provide additional protection against contamination However Sterilizer used not FDA cleared with SAL10-6 for duodenoscopes AER was not indicated for reprocessing duodenoscopes Storage in non-ventilated cabinet per AORN, AAMI/ANSI ST91; SGNA
56 Summary of Advantages and Disadvantages of HLD and Sterilization Enhancements for Reprocessing Duodenoscopes Rutala WA, Weber DJ. Infect Control Hosp Epidemiol 2015;36: Method Advantages Disadvantages HLD with ETO, Microbiologic surveillance Major endoscope manufacturer offers ETO as sterilization option Ideally, should be used after standard high-level disinfection Some data demonstrate reduced infection risk with HLD followed by ETO Single-dose cartridge and negativepressure chamber minimizes the potential for gas leak and ETO exposure Simple to operate and monitor Compatible with most medical materials Requires aeration time to remove ETO residue Only 20% of US hospitals have ETO on-site Lengthy cycle/aeration time No microbicidal efficacy data proving SAL 10-6 achieved Studies question microbicidal activity in presence of organic matter/salt ETO is toxic, a carcinogen, flammable May damage endoscope
57 Long-Term Response To ERCP/Endoscope Outbreaks
58 GI Endoscopes: Shift from Disinfection to Sterilization Rutala, Weber. JAMA :
59 What Is the Public Health Benefit? No Endoscopy-Related Infections Rutala, Weber. Am J Infect Control. 2016;44:e1-e6; Rutala, Weber ICHE. 2015;36:643. Margin of Safety-currently nonexistent; sterilization will provide a safety margin (~6 log 10 ). To prevent infections, all duodenoscopes should be devoid of microbial contamination. HLD (6 log 10 reduction) vs Sterilization (12 log 10 reduction=sal 10-6 )
60 FDA Panel, May 2015, Recommended Sterilization of Duodenoscopes (requires FDA-cleared sterilization technology that achieves a SAL 10-6, technology not yet available)
61 Endoscope Reprocessing: A Need to Shift from Disinfection to Sterilization Rutala, Weber. Am J Infect Control. 2016;44:e1-e6; Rutala, Weber ICHE. 2015;36:643. Where are we today-significant risk of being exposed to healthcare pathogens and an infection risk; peer-reviewed literature demonstrates risk and offers recommendations Where do we want to be in the future- eliminate all infections associated with medical /surgical devices How do we get there-modify Spaulding Roadmap for implementation Challenges associated with changeover to sterilization Timelines
62 Disinfection and Sterilization WA Rutala, DJ Weber, and HICPAC, EH Spaulding believed that how an object will be disinfected depended on the object s intended use (developed 1968). CRITICAL - objects which enter normally sterile tissue or the vascular system or through which blood flows should be sterile. SEMICRITICAL - objects that touch mucous membranes or skin that is not intact require a disinfection process (highlevel disinfection [HLD]) that kills all microorganisms but high numbers of bacterial spores. NONCRITICAL -objects that touch only intact skin require lowlevel disinfection (or non-germicidal detergent).
63 Disinfection and Sterilization Rutala, Weber. Am J Infect Control. 2016;44:e1-e6; Rutala, Weber ICHE. 2015;36:643. EH Spaulding believed that how an object will be disinfected depended on the object s intended use (modified). CRITICAL - objects which directly or secondarily (i.e., via a mucous membrane such as duodenoscope, cystoscope, bronchoscope) enter normally sterile tissue or the vascular system or through which blood flows should be sterile. SEMICRITICAL - objects that touch mucous membranes or skin that is not intact require a disinfection process (highlevel disinfection [HLD]) that kills all microorganisms but high numbers of bacterial spores. NONCRITICAL -objects that touch only intact skin require lowlevel disinfection (or non-germicidal detergent).
64 Endoscope Reproccesing: A Need to Shift from Disinfection to Sterilization Rutala, Weber Manuscript in preparation. CRITICAL - objects which directly or secondarily (i.e., via a mucous membrane such as duodenoscope, cystoscope, bronchoscope) enter normally sterile tissue or the vascular system or through which blood flows should be sterile. Duodenoscopes Bronchoscopes Cystoscopes Other GI scopes such as colonoscopies and gastroscopes Many patients need a biopsy, which by definition enters sterile tissue Many patients will have disruptive or non-intact mucous membranes (e.g., gastric ulcers, other erosions)
65 Endoscope Reproccesing: A Need to Shift from Disinfection to Sterilization
66 High-Level Disinfection of Semicritical Objects Rutala, Weber, HICPAC. Exposure Time > 8m-45m (US), 20 o C Germicide Concentration Glutaraldehyde > 2.0% Ortho-phthalaldehyde 0.55% Hydrogen peroxide* 7.5% Hydrogen peroxide and peracetic acid* 1.0%/0.08% Hydrogen peroxide and peracetic acid* 7.5%/0.23% Hypochlorite (free chlorine)* ppm Accelerated hydrogen peroxide 2.0% Peracetic acid 0.2% Glut and isopropanol 3.4%/26% Glut and phenol/phenate** 1.21%/1.93% *May cause cosmetic and functional damage; **efficacy not verified
67 Potential Future Methods to Prevent Endoscope-Related Outbreaks Rutala, Weber. Am J Infect Control. 2016;44:e1-e6; Rutala, Weber ICHE. 2015;36:643. Optimize current low temperature sterilization methods or new LTST proving SAL 10-6 achieved (2 LTS technologies, FDA-cleared) Disposable sterile GI endoscopes (2 manufacturer s) Steam sterilization for GI endoscopes Use of non-endoscope methods to diagnosis or treat disease (e.g., capsule endoscopy, stool or blood tests to detect GI cancer, stool DNA test) Improved GI endoscope design (to reduce or eliminate reprocessing challenges-based on 50y of experience unlikely to resolve problem; closed channel duodenoscopes increased risk)
68 Endoscope Reproccesing: A Need to Shift from Disinfection to Sterilization Rutala, Weber. Am J Infect Control. 2016;44:e1-e6; Rutala, Weber ICHE. 2015;36:643. Where are we today-significant risk of being exposed to healthcare pathogens and an infection risk; peer-reviewed literature demonstrates risk and offers recommendations Where do we want to be in the future-eliminate all infections associated with medical /surgical devices How do we get there-modify Spaulding Roadmap for implementation-peer-reviewed literature recommending transition; FDA Panel; evolution of LTST (FDA-cleared) and single-use endoscopes ($ and reprocessing cost $ ) Timelines-urgent but not more than 5 years (new technology acceptable in terms of sterilization performance, scope performance [disposable], cost, throughput, materials compatibility) Challenges associated with changeover to sterilization
69 Endoscope Reproccesing: A Need to Shift from Disinfection to Sterilization Rutala, Weber. Am J Infect Control. 2016;44:e1-e6; Rutala, Weber ICHE. 2015;36:643. Challenges-urgent but not more than 5 years AAMI should modify the Spaulding classification scheme for critical items from direct contact with sterile tissue to direct or secondary contact with sterile tissue AAMI should incorporate this new modification into the HLD and sterilization guidelines now AAMI should incorporate verbiage that this transition should happen as soon as new sterilization technology (or single use endoscopes) acceptable in terms of sterilization performance, scope performance (disposable), cost, throughput, materials compatibility TJC should ensure implementation of this AAMI recommendation as soon as new sterilization technology are available and acceptable (based on literature and hospital usage)
70 Endoscope Reproccesing: A Need to Shift from Disinfection to Sterilization Rutala, Weber. Am J Infect Control. 2016;44:e1-e6; Rutala, Weber ICHE. 2015;36:643. Challenges-urgent but not more than 5 years Endoscope manufacturer s must make their endoscopes compatible with LTST (e.g., adhesives, lubricants). FDA must ensure endoscope manufacturer s facilitate compatibility with LTST. FDA must clear in a timely manner LTST or single-use, endoscopes when data demonstrate they achieve an SAL 10-6 To protect the public health and prevent endoscope-related infections/outbreaks, FDA should mandate a shift from HLD to sterilization as they did in in 1992 with dental handpieces Manufacturers that submit critical devices to FDA for clearance that secondarily enter normally sterile tissue need to offer a FDA-cleared sterilization method.
71 Endoscope Reproccesing: A Need to Shift from Disinfection to Sterilization Rutala, Weber. Am J Infect Control. 2016;44:e1-e6; Rutala, Weber ICHE. 2015;36:643. Where are we today-significant risk of being exposed to healthcare pathogens and an infection risk; peer-reviewed literature demonstrates risk and offers recommendations Where do we want to be in the future-eliminate all infections associated with medical /surgical devices How do we get there-modify Spaulding Roadmap for implementation-peer-reviewed literature recommending transition; FDA Panel; evolution of LTST (FDA-cleared) and single-use endoscopes ($ and reprocessing cost $ ) Timelines-urgent but not more than 5 years (new technology acceptable in terms of sterilization performance, scope performance [disposable], cost, throughput, materials compatibility) Challenges associated with changeover to sterilization
72 Duodenoscopes and Endoscope Reprocessing : A Need to Shift from Disinfection to Sterilization Sources of healthcare-associated pathogens Evaluate the cause of endoscope-related outbreaks Review the outbreaks associated with ERCP and endoscopic procedures Discuss the alternatives that exist today that might improve the safety margin associated with duodenoscope/endoscope reprocessing Describe how to prevent future outbreaks associated with duodenoscopes and other GI endoscopes
73 Duodenoscope and Endoscope Reprocessing? A Need to Shift from Disinfection to Sterilization Summary Endoscopes represent a significant nosocomial hazard for healthcare-associated infetcions. Narrow or nonexistent margin of safety associated with high-level disinfection of semicritical items due to microbial load, complexity and biofilms. To protect the public health and prevent endoscopy-related (e.g., ERCP) outbreaks, there is an urgent need to shift from HLD to sterilization. AAMI should modify the Spaulding classification to require sterilization of endoscopes that directly or secondarily enter normally sterile tissue. Industry must develop sterilization technology (or single use) and make endoscopes compatible FDA must support this change through mandates and regulatory guidance TJC must enforce this transition when technology is acceptable Professional organizations (APIC, SHEA, ASGE, SGNA, AORN, IAHCSMM, others) must facilitate this change (e.g., guidelines, research, presentations at meetings) Only after transition from HLD to sterilization for endoscopes that contact sterile tissue will we prevent all healthcare-associated infections associated with these medical devices.
74 THANK YOU!
Is contamination of bronchoscopes really a problem?
 Is contamination of bronchoscopes really a problem? 1 Is contamination of bronchoscopes really a problem? High Level Disinfection does not methodically clean bronchoscopes What we know 210 More than 210
Is contamination of bronchoscopes really a problem? 1 Is contamination of bronchoscopes really a problem? High Level Disinfection does not methodically clean bronchoscopes What we know 210 More than 210
Controversies in Reprocessing Flexible Endoscopes: High Level Disinfection or Terminal Sterilization????
 Controversies in Reprocessing Flexible Endoscopes: High Level Disinfection or Terminal Sterilization???? HONG KONG HOSPITAL AUTHORITY - ADVANCED COURSE FOR INFECTION CONTROL NURSES NOVEMBER 2017 1 Learning
Controversies in Reprocessing Flexible Endoscopes: High Level Disinfection or Terminal Sterilization???? HONG KONG HOSPITAL AUTHORITY - ADVANCED COURSE FOR INFECTION CONTROL NURSES NOVEMBER 2017 1 Learning
Principles of Disinfection and Sterilization in the outpatient setting
 Module F Principles of Disinfection and Sterilization in the outpatient setting Objectives State the principles of disinfection and sterilization List the current methods for disinfection and sterilization
Module F Principles of Disinfection and Sterilization in the outpatient setting Objectives State the principles of disinfection and sterilization List the current methods for disinfection and sterilization
Prevention of Transmission of the Superbug Carbapenem-Resistant Enterobacteriaceae (CRE) during Gastrointestinal Endoscopy
 Prevention of Transmission of the Superbug Carbapenem-Resistant Enterobacteriaceae (CRE) during Gastrointestinal Endoscopy Sponsored by: Presentation by: Lawrence F. Muscarella, Ph.D. President LFM Healthcare
Prevention of Transmission of the Superbug Carbapenem-Resistant Enterobacteriaceae (CRE) during Gastrointestinal Endoscopy Sponsored by: Presentation by: Lawrence F. Muscarella, Ph.D. President LFM Healthcare
Standards of Infection Prevention in Reprocessing Flexible Gastrointestinal Endoscopes Webinar FAQ
 Standards of Infection Prevention in Reprocessing Flexible Gastrointestinal Endoscopes Webinar FAQ Disclaimer: The Society of Gastroenterology Nurses and Associates, Inc. assume no responsibility for the
Standards of Infection Prevention in Reprocessing Flexible Gastrointestinal Endoscopes Webinar FAQ Disclaimer: The Society of Gastroenterology Nurses and Associates, Inc. assume no responsibility for the
Guidelines for the use of disinfectants in hospital settings. Eleni Tomprou Infection Control Nurse, MSc General Hospital of Athens Polykliniki
 Guidelines for the use of disinfectants in hospital settings Eleni Tomprou Infection Control Nurse, MSc General Hospital of Athens Polykliniki Introduction USA 46.5 million surgical procedures Even more
Guidelines for the use of disinfectants in hospital settings Eleni Tomprou Infection Control Nurse, MSc General Hospital of Athens Polykliniki Introduction USA 46.5 million surgical procedures Even more
Disinfection and Sterilization. Efficacy of Disinfection/Sterilization Influencing Factors
 Recommended Disinfection & How To Assess Disease Transmission When There Is A Failure to Follow Recommended Disinfection and Overview of disinfection and sterilization principles Failure Scenarios Recommended
Recommended Disinfection & How To Assess Disease Transmission When There Is A Failure to Follow Recommended Disinfection and Overview of disinfection and sterilization principles Failure Scenarios Recommended
What s New in Disinfection and Sterilization of Patient Care Equipment A Webber Training Teleclass With Dr. William A. Rutala May 22, 2003 Slide 1
 Slide 1 What s New in Disinfection and Sterilization of Patient-Care Equipment William A. Rutala, Ph.D., M.P.H. University of North Carolina (UNC) Health Care System and UNC at Chapel Hill A Webber Training
Slide 1 What s New in Disinfection and Sterilization of Patient-Care Equipment William A. Rutala, Ph.D., M.P.H. University of North Carolina (UNC) Health Care System and UNC at Chapel Hill A Webber Training
Guidelines for Selection and Use of Disinfectants
 Guidelines for Selection and Use of Disinfectants Ref: (a) APIC Guidelines for Infection Control Practice, American Journal of Infection Control; April 1990, Vol 18, 99-113. To assist health care professionals
Guidelines for Selection and Use of Disinfectants Ref: (a) APIC Guidelines for Infection Control Practice, American Journal of Infection Control; April 1990, Vol 18, 99-113. To assist health care professionals
Improper Scope Reprocessing is Dangerous and has caused Patient Infections. 1-6
 ! Prevention Improper Scope Reprocessing is Dangerous and has caused Patient Infections. 1-6 Meeting the standards of ASGE, SGNA and AAMI, CONMED has a portfolio of products designed to help ensure your
! Prevention Improper Scope Reprocessing is Dangerous and has caused Patient Infections. 1-6 Meeting the standards of ASGE, SGNA and AAMI, CONMED has a portfolio of products designed to help ensure your
QUALITY ASSURANCE IN ENDOSCOPY
 QUALITY ASSURANCE IN ENDOSCOPY Frank Edward Myers III, MA, CIC Kim Delahanty, BSN, MBA/HCM,CIC WHY DO QC ON SCOPES?? The scopes are clean no problem OBJECTIVES Describe the 2 advantages of a testing based
QUALITY ASSURANCE IN ENDOSCOPY Frank Edward Myers III, MA, CIC Kim Delahanty, BSN, MBA/HCM,CIC WHY DO QC ON SCOPES?? The scopes are clean no problem OBJECTIVES Describe the 2 advantages of a testing based
St Boniface Research Centre Winnipeg, Manitoba Canada
 Medical Device Reprocessing: Can we Ban the Biofilm?! St Boniface Research Centre Winnipeg, Manitoba Canada Nancy Olson & Michelle Alfa Dr. Michelle J. Alfa, Ph.D., FCCM Professor, University of Manitoba,
Medical Device Reprocessing: Can we Ban the Biofilm?! St Boniface Research Centre Winnipeg, Manitoba Canada Nancy Olson & Michelle Alfa Dr. Michelle J. Alfa, Ph.D., FCCM Professor, University of Manitoba,
Flexible Endoscopes: The A,B,C s of Monitoring Manual Cleaning Efficacy!
 Flexible Endoscopes: The A,B,C s of Monitoring Manual Cleaning Efficacy! Dr. Michelle J. Alfa, Ph.D., FCCM Medical Director, Clinical Microbiology, Diagnostic Services of Manitoba, Winnipeg, Canada Disclaimers:
Flexible Endoscopes: The A,B,C s of Monitoring Manual Cleaning Efficacy! Dr. Michelle J. Alfa, Ph.D., FCCM Medical Director, Clinical Microbiology, Diagnostic Services of Manitoba, Winnipeg, Canada Disclaimers:
Inactivation of Emerging Pathogens and Continuous Room Decontamination Technologies
 Inactivation of Emerging Pathogens and Continuous Room Decontamination Technologies William A. Rutala, Ph.D., M.P.H. Director, Statewide Program for Infection Control and Epidemiology, Research Professor
Inactivation of Emerging Pathogens and Continuous Room Decontamination Technologies William A. Rutala, Ph.D., M.P.H. Director, Statewide Program for Infection Control and Epidemiology, Research Professor
Hopefully you will learn.. Objectives. Contact Transmission. Pathway of Disease Transmission. Contact Precautions Direct Indirect
 Kathy Zegarski BS, CIC Kettering Medical Center Infection Prevention and Control March 6, 2010 1 2 Objectives Discuss issues and standards associated with infection prevention in the endoscopy setting
Kathy Zegarski BS, CIC Kettering Medical Center Infection Prevention and Control March 6, 2010 1 2 Objectives Discuss issues and standards associated with infection prevention in the endoscopy setting
Case 6. Fight Against Nosocomial Infections
 Case 6 Fight Against Nosocomial Infections Decontamination and microbiological surveillance of endoscopes Prof. dr. Isabel Leroux-Roels Laboratory of Medical Microbiology & Infection Control Team UZ Gent
Case 6 Fight Against Nosocomial Infections Decontamination and microbiological surveillance of endoscopes Prof. dr. Isabel Leroux-Roels Laboratory of Medical Microbiology & Infection Control Team UZ Gent
Administrative Policies and Procedures. Cross Reference: Date Issued: 2/14 Date Reviewed: 9/14 Date: Revised: 12/14 Attachment: None Page of 1 of 6
 Administrative Policies and Procedures Originating Venue: Infection Control Policy No.: IC 2306 Title: Cystoscope Reprocessing Policy & Procedure Cross Reference: Date Issued: 2/14 Date Reviewed: 9/14
Administrative Policies and Procedures Originating Venue: Infection Control Policy No.: IC 2306 Title: Cystoscope Reprocessing Policy & Procedure Cross Reference: Date Issued: 2/14 Date Reviewed: 9/14
Slide 1. Slide 2. Slide 3
 Slide 1 1 Contact Hour CNE Presentation MK-234 Rev. a Slide 2 Successful completion: Participants must attend the entire program, including any resulting Q & A, and submit required documentation. Conflict
Slide 1 1 Contact Hour CNE Presentation MK-234 Rev. a Slide 2 Successful completion: Participants must attend the entire program, including any resulting Q & A, and submit required documentation. Conflict
Contaminated or clean? Your eye can t tell. The 3M Clean-Trace ATP Monitoring System can.
 Contaminated or clean? Your eye can t tell. The 3M Clean-Trace ATP Monitoring System can. TM TM Know with confidence that it's clean. We all know the dangers that inadequately cleaned surfaces, endoscopes
Contaminated or clean? Your eye can t tell. The 3M Clean-Trace ATP Monitoring System can. TM TM Know with confidence that it's clean. We all know the dangers that inadequately cleaned surfaces, endoscopes
Monitoring Flexible Endoscope Channels to Assure Cleaning
 Monitoring Flexible Endoscope Channels to Assure Cleaning Dr. Michelle J. Alfa, Ph.D., FCCM Medical Director, Clinical Microbiology, Diagnostic Services of Manitoba, Winnipeg, Canada Disclaimers: Dr. M.
Monitoring Flexible Endoscope Channels to Assure Cleaning Dr. Michelle J. Alfa, Ph.D., FCCM Medical Director, Clinical Microbiology, Diagnostic Services of Manitoba, Winnipeg, Canada Disclaimers: Dr. M.
A GLIMPSE AT THE TRUE COST OF REPROCESSING ENDOSCOPES:
 by Cori L. Ofstead, MSPH; Mariah R. Quick, MPH; John E. Eiland, MS, RN; Steven J. Adams, RN, CRCST A GLIMPSE AT THE TRUE COST OF REPROCESSING ENDOSCOPES: RESULTS OF A PILOT PROJECT INTRODUCTION AND METHODS
by Cori L. Ofstead, MSPH; Mariah R. Quick, MPH; John E. Eiland, MS, RN; Steven J. Adams, RN, CRCST A GLIMPSE AT THE TRUE COST OF REPROCESSING ENDOSCOPES: RESULTS OF A PILOT PROJECT INTRODUCTION AND METHODS
Christina Bradley Endoscope Decontamination A Webber Training Teleclass. Christina Bradley
 ENDOSCOPE DECONTAMINATION Christina Bradley Hospital Infection Research Laboratory City Hospital NHS Trust Dudley Road, Birmingham B18 7QH Hosted by Paul Webber paul@webbertraining.com www.webbertraining.com
ENDOSCOPE DECONTAMINATION Christina Bradley Hospital Infection Research Laboratory City Hospital NHS Trust Dudley Road, Birmingham B18 7QH Hosted by Paul Webber paul@webbertraining.com www.webbertraining.com
Best Practices for Reprocessing Endoscopes
 Best Practices for Reprocessing Endoscopes Reprocessing of Endoscopes Reprocessing Room Standards The resources referenced include: Canadian Standards Association Provincial Infectious Disease Advisory
Best Practices for Reprocessing Endoscopes Reprocessing of Endoscopes Reprocessing Room Standards The resources referenced include: Canadian Standards Association Provincial Infectious Disease Advisory
#003. Liquid Chemical Sterilization Story. The. A Continuing Education Study Guide presented by
 #003 S t u d y G u i d e The Liquid Chemical Sterilization Story A Continuing Education Study Guide presented by Notes The Liquid Chemical Sterilization Story Study Guide #003 Nurses: To receive 1.2
#003 S t u d y G u i d e The Liquid Chemical Sterilization Story A Continuing Education Study Guide presented by Notes The Liquid Chemical Sterilization Story Study Guide #003 Nurses: To receive 1.2
Verification of Environmental Cleaning & the Impact on Patient Safety
 SM 3M Health Care Academy Verification of Environmental Cleaning & the Impact on Patient Safety July 20, 2016 Steve Zeplin, RN, MSN, CSPDT House Keeping Continuing Education Each 1 hour web meeting qualifies
SM 3M Health Care Academy Verification of Environmental Cleaning & the Impact on Patient Safety July 20, 2016 Steve Zeplin, RN, MSN, CSPDT House Keeping Continuing Education Each 1 hour web meeting qualifies
Responses to Questions for SYSTEM 1E Process Monitoring and Validation Webinar
 Responses to Questions for SYSTEM 1E Process Monitoring and Validation Webinar MONITORING THE SYSTEM 1E PROCESSOR Q: I want to confirm that there is no need for a Biological Indicator (BI) and that use
Responses to Questions for SYSTEM 1E Process Monitoring and Validation Webinar MONITORING THE SYSTEM 1E PROCESSOR Q: I want to confirm that there is no need for a Biological Indicator (BI) and that use
Comparison of ANSI/AAMI ST-91 Standards, SGNA Standards and AORN Guidelines on Processing Endoscopes March 9, 2017
 New guidelines for processing endoscopes, published May 2015 Precleaning AORN 2017 Endoscopy Guideline N/A Review N/A 1. Precleaning; 2. Leak testing; 3. Manual cleaning; 4. Rinse after cleaning; 5. Visual
New guidelines for processing endoscopes, published May 2015 Precleaning AORN 2017 Endoscopy Guideline N/A Review N/A 1. Precleaning; 2. Leak testing; 3. Manual cleaning; 4. Rinse after cleaning; 5. Visual
3/26/2015. This quote sums up the activity going on right now with flexible scopes
 This quote sums up the activity going on right now with flexible scopes Flexible Endoscope Cleaning If you want to truly understand something try to change it Kurt Lewis Objectives To understand and make
This quote sums up the activity going on right now with flexible scopes Flexible Endoscope Cleaning If you want to truly understand something try to change it Kurt Lewis Objectives To understand and make
ENDOSCOPY PRACTICE Manual cleaning and Disinfection of flexible endoscopes
 1.0 Purpose Every patient undergoing endoscopy should be examined with clean, disinfected equipment. All endoscopic equipment will be cleaned and disinfected / sterilized according to current recommended
1.0 Purpose Every patient undergoing endoscopy should be examined with clean, disinfected equipment. All endoscopic equipment will be cleaned and disinfected / sterilized according to current recommended
Disinfection and Sterilization
 1 Disinfection and Sterilization William A. Rutala, Ph.D., M.P.H. University of North Carolina (UNC) Health Care System and UNC at Chapel Hill, NC HICPAC Guideline Disinfection and Sterilization Provide
1 Disinfection and Sterilization William A. Rutala, Ph.D., M.P.H. University of North Carolina (UNC) Health Care System and UNC at Chapel Hill, NC HICPAC Guideline Disinfection and Sterilization Provide
Validation Strategy for an Automated Endoscope Reprocessor
 Validation Strategy for an Automated Endoscope Reprocessor November 16, 2010 Bradley Catalone AER Validation Strategy System Approach page 1 AER Validation Strategy AER Scope-specific connectors Requires
Validation Strategy for an Automated Endoscope Reprocessor November 16, 2010 Bradley Catalone AER Validation Strategy System Approach page 1 AER Validation Strategy AER Scope-specific connectors Requires
Disinfection and Sterilization in Health Care Facilities: What Clinicians Need to Know
 HEALTHCARE EPIDEMIOLOGY Robert A. Weinstein, Section Editor INVITED ARTICLE Disinfection and Sterilization in Health Care Facilities: What Clinicians Need to Know William A. Rutala and David J. Weber Hospital
HEALTHCARE EPIDEMIOLOGY Robert A. Weinstein, Section Editor INVITED ARTICLE Disinfection and Sterilization in Health Care Facilities: What Clinicians Need to Know William A. Rutala and David J. Weber Hospital
Draft Guidance for Industry and FDA Staff Processing/Reprocessing Medical Devices in Health Care Settings: Validation Methods and Labeling
 Draft Guidance for Industry and FDA Staff Processing/Reprocessing Medical Devices in Health Care Settings: Validation Methods and Labeling DRAFT GUIDANCE This guidance document is being distributed for
Draft Guidance for Industry and FDA Staff Processing/Reprocessing Medical Devices in Health Care Settings: Validation Methods and Labeling DRAFT GUIDANCE This guidance document is being distributed for
State of Kuwait Ministry of Health Infection Control Directorate. Guidelines for Prevention of Endoscopy-Associated Nosocomial Infection
 State of Kuwait Ministry of Health Infection Control Directorate Guidelines for Prevention of Endoscopy-Associated Nosocomial Infection November 2000 Introduction: There is an increase in number, complexity
State of Kuwait Ministry of Health Infection Control Directorate Guidelines for Prevention of Endoscopy-Associated Nosocomial Infection November 2000 Introduction: There is an increase in number, complexity
Sterilization and Disinfection: Getting It Right. Instructions
 Sterilization and Disinfection: Getting It Right Instructions 1. Print the document. Read the activity and record your answers to the post test questions on the answer sheet. 2. A score of 80 is required
Sterilization and Disinfection: Getting It Right Instructions 1. Print the document. Read the activity and record your answers to the post test questions on the answer sheet. 2. A score of 80 is required
Residual bioburden in reprocessed side-view endoscopes used for endoscopic retrograde cholangiopancreatography (ERCP)
 E12 Residual bioburden in reprocessed side-view endoscopes used for endoscopic retrograde cholangiopancreatography (ERCP) Authors D. L. N. L. Ubhayawardana 1, J. Kottahachchi 1, M. M. Weerasekera 1, I.
E12 Residual bioburden in reprocessed side-view endoscopes used for endoscopic retrograde cholangiopancreatography (ERCP) Authors D. L. N. L. Ubhayawardana 1, J. Kottahachchi 1, M. M. Weerasekera 1, I.
Infection Prevention in Endoscopy, Keeping Patients and Practioners Safe
 Infection Prevention in Endoscopy, Keeping Patients and Practioners Safe August 11, 2015 Cheri Ackert-Burr, RN, MSN, CNOR Clinical Education Manager, Medivators Disclosures 1.Successful completion: Participants
Infection Prevention in Endoscopy, Keeping Patients and Practioners Safe August 11, 2015 Cheri Ackert-Burr, RN, MSN, CNOR Clinical Education Manager, Medivators Disclosures 1.Successful completion: Participants
Competency Guide: Care and Handling of Rigid Endoscopes
 Competency Guide: Care and Handling of Rigid Endoscopes This document details the basic steps to correctly process and maintain a rigid endoscope. This document can be used to perform a competency review
Competency Guide: Care and Handling of Rigid Endoscopes This document details the basic steps to correctly process and maintain a rigid endoscope. This document can be used to perform a competency review
Microbiologic Sampling of the Environment
 William A. Rutala, Ph.D., M.P.H. Director, Statewide Program for Infection Control and Epidemiology and Research Professor of Medicine, University of North Carolina at Chapel Hill, NC, USA Former Director,
William A. Rutala, Ph.D., M.P.H. Director, Statewide Program for Infection Control and Epidemiology and Research Professor of Medicine, University of North Carolina at Chapel Hill, NC, USA Former Director,
8/27/2015. Learning Objectives. Purpose of Sterilization. To destroy all living pathogenic microorganisms
 Learning Objectives Compare and contrast the available methods of sterilization for the healthcare setting Describe the appropriate use and monitoring for sterilization methods Purpose of Sterilization
Learning Objectives Compare and contrast the available methods of sterilization for the healthcare setting Describe the appropriate use and monitoring for sterilization methods Purpose of Sterilization
HemoCheck TM Sample Policy. SUBJECT: Detection of blood residue on various surfaces
 HemoCheck TM Sample Policy SUBJECT: Detection of blood residue on various surfaces DEPARTMENT: Central Service APPROVED BY: EFFECTIVE: REVISED: 07/2016 PURPOSE: To test for detection of blood residue on
HemoCheck TM Sample Policy SUBJECT: Detection of blood residue on various surfaces DEPARTMENT: Central Service APPROVED BY: EFFECTIVE: REVISED: 07/2016 PURPOSE: To test for detection of blood residue on
Products made in Switzerland
 Products made in Switzerland HYGIENIC AND MEDICALLY CLEAR DIFFERENT PRODUCTS BUT THE SAME QUALITY LABEL HYGIENIC AND MEDICALLY CLEAR 2 MDD TECHNOLOGIES FOR: 1. Cleaning 2. Disinfection 3. High Level Disinfection
Products made in Switzerland HYGIENIC AND MEDICALLY CLEAR DIFFERENT PRODUCTS BUT THE SAME QUALITY LABEL HYGIENIC AND MEDICALLY CLEAR 2 MDD TECHNOLOGIES FOR: 1. Cleaning 2. Disinfection 3. High Level Disinfection
Best Practices for High Level Disinfection Part I
 Best Practices for High Level Disinfection Part I Nancy Chobin, RN, AAS, ACSP, CSPM, CFER 2017 Sterile Processing University, LLC. **This in-service has been Approved by the CBSPD for 1.5 CEUs. The Bible
Best Practices for High Level Disinfection Part I Nancy Chobin, RN, AAS, ACSP, CSPM, CFER 2017 Sterile Processing University, LLC. **This in-service has been Approved by the CBSPD for 1.5 CEUs. The Bible
National Endoscopy Programme. Decontamination Standards for Flexible Endoscopes
 National Endoscopy Programme Decontamination Standards for Flexible Endoscopes Updated March 2008 Introduction Providing an effective endoscope decontamination service within a safe environment is an essential
National Endoscopy Programme Decontamination Standards for Flexible Endoscopes Updated March 2008 Introduction Providing an effective endoscope decontamination service within a safe environment is an essential
Infection Control Manual. Table of Contents
 This policy has been adopted by UNC Health Care for its use in infection control. It is provided to you as information only. Infection Control Manual Policy Name Cleaning, Disinfection, and Sterilization
This policy has been adopted by UNC Health Care for its use in infection control. It is provided to you as information only. Infection Control Manual Policy Name Cleaning, Disinfection, and Sterilization
Microfibre cleaning: is it all its been cracked up to be?
 Microfibre cleaning: is it all its been cracked up to be? Disclaimers: Sponsored to give invited presenta/ons at various Na/onal and Interna/onal conferences by; STERIS, 3M, J&J, Healthmark, APIC, CACMID,
Microfibre cleaning: is it all its been cracked up to be? Disclaimers: Sponsored to give invited presenta/ons at various Na/onal and Interna/onal conferences by; STERIS, 3M, J&J, Healthmark, APIC, CACMID,
Disinfection and Sterilization:
 1 Disinfection and Sterilization: Challenges in the XXI Century William A. Rutala, Ph.D., M.P.H. University of North Carolina (UNC) Health Care and UNC at Chapel Hill, NC Disclosure: Advanced Sterilization
1 Disinfection and Sterilization: Challenges in the XXI Century William A. Rutala, Ph.D., M.P.H. University of North Carolina (UNC) Health Care and UNC at Chapel Hill, NC Disclosure: Advanced Sterilization
Sterilization & Chemical Resistance of Healthcare Polymers
 Sterilization & Chemical Resistance of Healthcare Polymers Key Terms & Definitions Sterilization - A process that eliminates or kills all forms of life, including transmissible agents, on a medical device;
Sterilization & Chemical Resistance of Healthcare Polymers Key Terms & Definitions Sterilization - A process that eliminates or kills all forms of life, including transmissible agents, on a medical device;
Clostridium difficile Update Dr. Michelle Alfa, Winnipeg Sponsored by ARJO
 Clostridium difficile: Update Dr. Michelle Alfa Ph.D, FCCM Diagnostic Services of Manitoba St. Boniface General Hospital Site Hosted by Paul Webber paul@webbertraining.com Sponsored by www.arjo.com www.webbertraining.com
Clostridium difficile: Update Dr. Michelle Alfa Ph.D, FCCM Diagnostic Services of Manitoba St. Boniface General Hospital Site Hosted by Paul Webber paul@webbertraining.com Sponsored by www.arjo.com www.webbertraining.com
Environmental Contamination
 John M. Boyce, MD Chief, Infectious Diseases Section Hospital of Saint Raphael and Clinical Professor of Medicine Yale University School of Medicine New Haven, CT Disclosures: Consultant to Soap & Detergent
John M. Boyce, MD Chief, Infectious Diseases Section Hospital of Saint Raphael and Clinical Professor of Medicine Yale University School of Medicine New Haven, CT Disclosures: Consultant to Soap & Detergent
One such problem is blood residual inside the channels of a robotic arm.
 SUBJECT: Robotic Arm Testing for residue blood DEPARTMENT: Central Service APPROVED BY: EFFECTIVE: REVISED: 07/2016 PURPOSE: To test for detection of blood residue inside and outside of a robotic arm and
SUBJECT: Robotic Arm Testing for residue blood DEPARTMENT: Central Service APPROVED BY: EFFECTIVE: REVISED: 07/2016 PURPOSE: To test for detection of blood residue inside and outside of a robotic arm and
Chemical Sterilants and High-Level Disinfectants: Managing Practical, Safe and Effective Use
 Chemical Sterilants and High-Level Disinfectants: Managing Practical, Safe and Effective Use by Donald P. Satchell, Ph.D. Objectives After completion of this self-study activity, the learner will be able
Chemical Sterilants and High-Level Disinfectants: Managing Practical, Safe and Effective Use by Donald P. Satchell, Ph.D. Objectives After completion of this self-study activity, the learner will be able
Parameters exerting Influence on Cleaning of flexible Endoscops
 Parameters exerting Influence on Cleaning of flexible Endoscops Heike Martiny Technische Hygiene Campus Benjamin Franklin Charité Universitätsmedizin Berlin ÖGSV Jahrestagung und wfhss-kongress 03. - 05.
Parameters exerting Influence on Cleaning of flexible Endoscops Heike Martiny Technische Hygiene Campus Benjamin Franklin Charité Universitätsmedizin Berlin ÖGSV Jahrestagung und wfhss-kongress 03. - 05.
INFECTION PREVENTION PROCESSES 1
 INFECTION PREVENTION PROCESSES 1 PROCESSING INSTRUMENTS, GLOVES, AND OTHER ITEMS As shown in Figure 1, decontamination is the first step in processing soiled (contaminated) instruments, gloves, and other
INFECTION PREVENTION PROCESSES 1 PROCESSING INSTRUMENTS, GLOVES, AND OTHER ITEMS As shown in Figure 1, decontamination is the first step in processing soiled (contaminated) instruments, gloves, and other
ENDORA Endoscope Tracking System
 ENDORA Endoscope Tracking System Procedure Manual Clean Reprocess Dry & Store Procedure Storage Manual Cleaning Workflow Software Designed to Prevent Endoscope Reprocessing Lapses Reprocessing THE COMPLETE
ENDORA Endoscope Tracking System Procedure Manual Clean Reprocess Dry & Store Procedure Storage Manual Cleaning Workflow Software Designed to Prevent Endoscope Reprocessing Lapses Reprocessing THE COMPLETE
Role of the Environmental Surfaces in Disease Transmission: No Touch Technologies Reduce HAIs
 Role of the Environmental Surfaces in Disease Transmission: No Touch Technologies Reduce HAIs William A. Rutala, Ph.D., M.P.H. Director, Hospital Epidemiology, Occupational Health and Safety, UNC Health
Role of the Environmental Surfaces in Disease Transmission: No Touch Technologies Reduce HAIs William A. Rutala, Ph.D., M.P.H. Director, Hospital Epidemiology, Occupational Health and Safety, UNC Health
Antimicrobial Surfaces to Prevent Healthcare-Associated Infection
 Antimicrobial Surfaces to Prevent Healthcare-Associated Infection Matthew P. Muller, MD, PhD, FRCPC Medical Director, Infection Prevention and Control, St. Michael s Hospital Chair, Provincial Infectious
Antimicrobial Surfaces to Prevent Healthcare-Associated Infection Matthew P. Muller, MD, PhD, FRCPC Medical Director, Infection Prevention and Control, St. Michael s Hospital Chair, Provincial Infectious
POLICY FOR THE DECONTAMINATION OF FLEXIBLE ENDOSCOPES
 POLICY FOR THE DECONTAMINATION OF FLEXIBLE ENDOSCOPES Version 2.0 August 2012 Name of Policy: Purpose of Policy: Policy for the Decontamination of Flexible Endoscopes To provide guidance on the decontamination
POLICY FOR THE DECONTAMINATION OF FLEXIBLE ENDOSCOPES Version 2.0 August 2012 Name of Policy: Purpose of Policy: Policy for the Decontamination of Flexible Endoscopes To provide guidance on the decontamination
Summary of Proposed Legislative Provisions
 Summary of Proposed Legislative Provisions The evidence obtained by the Committee on Oversight and Government Reform during its investigation of duodenoscope-related patient infections identified significant
Summary of Proposed Legislative Provisions The evidence obtained by the Committee on Oversight and Government Reform during its investigation of duodenoscope-related patient infections identified significant
Safety, Efficacy & Microbiological Consideration
 Safety, Efficacy & Microbiological Consideration Endoscope Cleaning and Disinfection System (With Optional Cleaning Cycle that Eliminates Manual Cleaning) 1 Medivators is a registered trademark of Minntech
Safety, Efficacy & Microbiological Consideration Endoscope Cleaning and Disinfection System (With Optional Cleaning Cycle that Eliminates Manual Cleaning) 1 Medivators is a registered trademark of Minntech
Disinfection and sterilisation
 Disinfection and sterilisation Mongolia 2011 Prof. Dr. Walter Popp Hospital Hygiene, University Clinics Essen, Germany 1 Term Definition Reduction factor of germs Cleaning Remove dirt including 10-100
Disinfection and sterilisation Mongolia 2011 Prof. Dr. Walter Popp Hospital Hygiene, University Clinics Essen, Germany 1 Term Definition Reduction factor of germs Cleaning Remove dirt including 10-100
AUTOMATED ENDOSCOPE REPROCESSOR. OER-Pro Easy. Fast. Reliable.
 AUTOMATED ENDOSCOPE REPROCESSOR OER-Pro Easy. Fast. Reliable. Olympus Endoscope Reprocessor The only reprocessor designed by an endoscope manufacturer. The OER-Pro provides high-level disinfection of Olympus
AUTOMATED ENDOSCOPE REPROCESSOR OER-Pro Easy. Fast. Reliable. Olympus Endoscope Reprocessor The only reprocessor designed by an endoscope manufacturer. The OER-Pro provides high-level disinfection of Olympus
Water for Instrument Processing
 Water for Instrument Processing by Marcia Frieze, Case Medical Water, the universal solvent Water can dissolve more substances than any other liquid. It is essentially nonionic or neutral. While alkaline
Water for Instrument Processing by Marcia Frieze, Case Medical Water, the universal solvent Water can dissolve more substances than any other liquid. It is essentially nonionic or neutral. While alkaline
Guide for Cleaning, Sterilization and Storage of Introducers for Caldera Medical Implants
 Guide for Cleaning, Sterilization and Storage of Introducers for Caldera Medical Implants M Manufactured by: Caldera Medical, Inc. 5171 Clareton Drive Agoura Hills, CA 91301 U.S. Toll Free: 866-4-CALDERA
Guide for Cleaning, Sterilization and Storage of Introducers for Caldera Medical Implants M Manufactured by: Caldera Medical, Inc. 5171 Clareton Drive Agoura Hills, CA 91301 U.S. Toll Free: 866-4-CALDERA
UNIT Two. Lesson 1C Sterilization
 _ Introduction UNIT Two Lesson 1C Sterilization Pathogenic microorganisms are capable of causing disease when they invade human tissue. Every effort must be made to remove microorganisms from equipment
_ Introduction UNIT Two Lesson 1C Sterilization Pathogenic microorganisms are capable of causing disease when they invade human tissue. Every effort must be made to remove microorganisms from equipment
HIGH-LEVEL DISINFECTANT RAPICIDE. Safety, Efficacy, and Microbiological Considerations
 HIGH-LEVEL DISINFECTANT RAPICIDE PA Safety, Efficacy, and Microbiological Considerations 1 50096-959 Rev D 2011, MEDIVATORS, A Minntech Corporation Business Group All rights reserved. This publication
HIGH-LEVEL DISINFECTANT RAPICIDE PA Safety, Efficacy, and Microbiological Considerations 1 50096-959 Rev D 2011, MEDIVATORS, A Minntech Corporation Business Group All rights reserved. This publication
FREQUENTLY ASKED QUESTIONS FOR THE ADVANTAGE
 FREQUENTLY ASKED QUESTIONS FOR THE ADVANTAGE What is an Automated Endoscope Reprocessor? An Automated Endoscopes Reprocessor (AER) performs all the cycle steps (fresh water preflush, rinsing, disinfection,
FREQUENTLY ASKED QUESTIONS FOR THE ADVANTAGE What is an Automated Endoscope Reprocessor? An Automated Endoscopes Reprocessor (AER) performs all the cycle steps (fresh water preflush, rinsing, disinfection,
Endoscope Washer Disinfector Cleaning Process Challenge Devices Research & Validation
 Endoscope Washer Disinfector Cleaning Process Challenge Devices Research & Validation Richard Bancroft, B.Sc (Hons), FRSB Science & Technical Director STERIS Corporation Registered Authorising Engineer
Endoscope Washer Disinfector Cleaning Process Challenge Devices Research & Validation Richard Bancroft, B.Sc (Hons), FRSB Science & Technical Director STERIS Corporation Registered Authorising Engineer
Irrigation accessories
 Irrigation accessories irrigation The risk of contamination Infection control is a top concern in GI units. The American Society of Gastrointestinal Endoscopy (ASGE) states that crosscontamination and
Irrigation accessories irrigation The risk of contamination Infection control is a top concern in GI units. The American Society of Gastrointestinal Endoscopy (ASGE) states that crosscontamination and
Lumen claims of the STERRAD 100NX Sterilizer: Testing performance limits of this technology
 Lumen claims of the STERRAD 100NX Sterilizer: Testing performance limits of this technology Magda Diab-Elschahawi, Alexander Blacky, Walter Koller Clinical Institute of Hospital Hygiene Medical University
Lumen claims of the STERRAD 100NX Sterilizer: Testing performance limits of this technology Magda Diab-Elschahawi, Alexander Blacky, Walter Koller Clinical Institute of Hospital Hygiene Medical University
Reprocessing of Medical Devices: Concerns about Sharps
 Reprocessing of Medical Devices: Concerns about Sharps Medical Waste Institute Conference May 7, 2008 Chicago, Illinois Ed Krisiunas, MT(ASCP), CIC, MPH WNWN International Contact Info Ed Krisiunas, MT(ASCP),
Reprocessing of Medical Devices: Concerns about Sharps Medical Waste Institute Conference May 7, 2008 Chicago, Illinois Ed Krisiunas, MT(ASCP), CIC, MPH WNWN International Contact Info Ed Krisiunas, MT(ASCP),
The Role of Cleaning and Sterilization in Infection Control:
 CE ONLINE The Role of Cleaning and Sterilization in Infection Control: A Focus on Powered Surgical Instruments A Continuing Education Activity Sponsored By Grant Funds Provided By Welcome to The Role of
CE ONLINE The Role of Cleaning and Sterilization in Infection Control: A Focus on Powered Surgical Instruments A Continuing Education Activity Sponsored By Grant Funds Provided By Welcome to The Role of
EVOTECH endoscope cleaner and reprocessor (ECR) simulated-use and clinical-use evaluation of cleaning efficacy
 RESEARCH ARTICLE Open Access Research article EVOTECH endoscope cleaner and reprocessor (ECR) simulated-use and clinical-use evaluation of cleaning efficacy Michelle J Alfa* 1,2,3, Pat DeGagne 2, Nancy
RESEARCH ARTICLE Open Access Research article EVOTECH endoscope cleaner and reprocessor (ECR) simulated-use and clinical-use evaluation of cleaning efficacy Michelle J Alfa* 1,2,3, Pat DeGagne 2, Nancy
مادة االدوية املرحلة الثالثة أ.م.د. حسام الدين سامل
 مادة االدوية املرحلة الثالثة أ.م.د. حسام الدين سامل 2017-2016 ANTISEPTICS AND DISINFECTANTS Dr. Husam Aldeen Salim General information They have specific use and their selectivity is very low. Disinfectants
مادة االدوية املرحلة الثالثة أ.م.د. حسام الدين سامل 2017-2016 ANTISEPTICS AND DISINFECTANTS Dr. Husam Aldeen Salim General information They have specific use and their selectivity is very low. Disinfectants
BIP Central Venous Catheter
 Bactiguard Infection Protection BIP Central Venous Catheter For prevention of healthcare associated infections Bactiguard benefits Reduced healthcare costs Reduced use of antibiotics Save lives Bloodstream
Bactiguard Infection Protection BIP Central Venous Catheter For prevention of healthcare associated infections Bactiguard benefits Reduced healthcare costs Reduced use of antibiotics Save lives Bloodstream
DEC TM Video Duodenoscope ED34-i10T2. Lock in. Elevate your care.
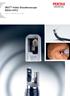 DEC TM Video Duodenoscope ED34-i10T2 Lock in. Elevate your care. Raising the standard of health PENTAX Medical is delivering a unique and innovative advancement in cleaning capabilities for infection prevention
DEC TM Video Duodenoscope ED34-i10T2 Lock in. Elevate your care. Raising the standard of health PENTAX Medical is delivering a unique and innovative advancement in cleaning capabilities for infection prevention
Chapter 7: Control of Microbial Growth
 Chapter 7: Control of Microbial Growth 1. Physical Methods 2. Chemical methods Important Terminology sterilization > commercial sterilization > disinfection = antisepsis > degerming > sanitization Also,
Chapter 7: Control of Microbial Growth 1. Physical Methods 2. Chemical methods Important Terminology sterilization > commercial sterilization > disinfection = antisepsis > degerming > sanitization Also,
Important Terminology
 Chapter 7: Control of Microbial Growth 1. Physical Methods 2. Chemical methods Important Terminology sterilization > commercial sterilization > disinfection = antisepsis > degerming > sanitization Also,
Chapter 7: Control of Microbial Growth 1. Physical Methods 2. Chemical methods Important Terminology sterilization > commercial sterilization > disinfection = antisepsis > degerming > sanitization Also,
8,000 facilities count on it
 Proven Trusted Safe 8,000 facilities count on it 45 countries use it 10 years of experience prove it Proven performance at every level CIDEX OPA Solution is trusted for its performance and professional
Proven Trusted Safe 8,000 facilities count on it 45 countries use it 10 years of experience prove it Proven performance at every level CIDEX OPA Solution is trusted for its performance and professional
CRE is not the first organism we ve had that has become resistant to antibiotics, so why is it so important? CRE resistance is complex because it can
 1 Enterobacteriaceae are a large family of bacteria that are a normal part of a person's digestive system (2). Examples include Escherichia coli and species of the genera Klebsiella, Enterobacter, Serratia,
1 Enterobacteriaceae are a large family of bacteria that are a normal part of a person's digestive system (2). Examples include Escherichia coli and species of the genera Klebsiella, Enterobacter, Serratia,
CSA Z314 Series Standards Understanding the Standards
 CSA Z314 Series Standards Understanding the Standards September 10,2013 Ian Pequegnat Where do standards come from? What influences their development The International Organization for Standardization
CSA Z314 Series Standards Understanding the Standards September 10,2013 Ian Pequegnat Where do standards come from? What influences their development The International Organization for Standardization
Using reusable containers for hospital waste: is there an infection risk?
 Using reusable containers for hospital waste: is there an infection risk? TR Grimmond Terry R Grimmond, FASM, BAgrSc, GrDipAdEd Director, Grimmond and Associates, Microbiology Consultancy, New Zealand
Using reusable containers for hospital waste: is there an infection risk? TR Grimmond Terry R Grimmond, FASM, BAgrSc, GrDipAdEd Director, Grimmond and Associates, Microbiology Consultancy, New Zealand
Bloodborne Pathogens Exposure Control Plan
 Bloodborne Pathogens Exposure Control Plan The Texas Department of Insurance, Division of Workers Compensation (TDI-DWC) Provided by Workplace Safety HS96-089C (10/09) Bloodborne Pathogens Exposure Control
Bloodborne Pathogens Exposure Control Plan The Texas Department of Insurance, Division of Workers Compensation (TDI-DWC) Provided by Workplace Safety HS96-089C (10/09) Bloodborne Pathogens Exposure Control
Important Terminology (pg )
 Number of living microbes 10/18/2016 Chapter 9: Control of Microbial Growth 1. Physical Methods 2. Chemical methods Important Terminology (pg. 263-264) sterilization > commercial sterilization > disinfection
Number of living microbes 10/18/2016 Chapter 9: Control of Microbial Growth 1. Physical Methods 2. Chemical methods Important Terminology (pg. 263-264) sterilization > commercial sterilization > disinfection
High-Level Disinfectant and Sterilant. Rapicide SAFETY, EFFICACY AND MICROBIOLOGICAL CONSIDERATIONS
 High-Level Disinfectant and Sterilant Rapicide SAFETY, EFFICACY AND MICROBIOLOGICAL CONSIDERATIONS Contents Product Description... 3 Intended Use... 3 Safety... 4 Storage, Use and Disposal... 4 Efficacy
High-Level Disinfectant and Sterilant Rapicide SAFETY, EFFICACY AND MICROBIOLOGICAL CONSIDERATIONS Contents Product Description... 3 Intended Use... 3 Safety... 4 Storage, Use and Disposal... 4 Efficacy
Best Practices for High Level Disinfection - Revisited. Objectives. The Bible for Chemicals ST-58 FDA. What is Available? 9/6/2015
 Best Practices for High Level Disinfection - Revisited Nancy Chobin, RN, AAS, ACSP, CSPM President, Sterile Processing University, LLC Copyright 2015 Objectives To define disinfection To review general
Best Practices for High Level Disinfection - Revisited Nancy Chobin, RN, AAS, ACSP, CSPM President, Sterile Processing University, LLC Copyright 2015 Objectives To define disinfection To review general
STERILIZATION VALIDATION IN THIS SECTION REPROCESSING VALIDATIONS FOR REUSABLE MEDICAL DEVICES. Radiation Sterilization Validation
 STERILIZATION VALIDATION All sterilization processes require validation of the efficacy and reproducibility of the process. Depending on the type of sterilization, this may be accomplished by partial,
STERILIZATION VALIDATION All sterilization processes require validation of the efficacy and reproducibility of the process. Depending on the type of sterilization, this may be accomplished by partial,
Reducing SSI s Through Improved Environmental Hygiene- A Threshold ROI Analysis
 Reducing SSI s Through Improved Environmental Hygiene- A Threshold ROI Analysis Executive Summary Faced with the need to reduce healthcare acquired infections (HAIs), providers are using a multi-modal
Reducing SSI s Through Improved Environmental Hygiene- A Threshold ROI Analysis Executive Summary Faced with the need to reduce healthcare acquired infections (HAIs), providers are using a multi-modal
Disinfection of Endoscopes & Accessories. Luis S. Marsano, MD Professor of Medicine University of Louisville Kentucky
 Disinfection of Endoscopes & Accessories Luis S. Marsano, MD Professor of Medicine University of Louisville Kentucky Sources for Transmission of Infections Contaminated Endoscope Bottle of water for Endoscope
Disinfection of Endoscopes & Accessories Luis S. Marsano, MD Professor of Medicine University of Louisville Kentucky Sources for Transmission of Infections Contaminated Endoscope Bottle of water for Endoscope
Safe to Handle? Comparing Manually and Machine-Washed Medical Devices
 Safe to Handle? Comparing Manually and Machine-Washed Medical Devices Kaumudi Kulkarni, Dzelma Kaczorowski, Alex Bonkowski, Stephen Kovach, and Ralph Basile About the Authors Kaumudi Kulkarni, MS, is manager
Safe to Handle? Comparing Manually and Machine-Washed Medical Devices Kaumudi Kulkarni, Dzelma Kaczorowski, Alex Bonkowski, Stephen Kovach, and Ralph Basile About the Authors Kaumudi Kulkarni, MS, is manager
OLYMPUS AUG -1 A9 :02
 OLYMPUS 4718 11 AUG -1 A9 :02 Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm 1061 Rockville, MD 20852 RE: s on Draft Guidance for Industry and FDA Staff " Processing/Reprocessing
OLYMPUS 4718 11 AUG -1 A9 :02 Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm 1061 Rockville, MD 20852 RE: s on Draft Guidance for Industry and FDA Staff " Processing/Reprocessing
Sterilization & Disinfection
 Sterilization & Disinfection Prof. Hanan Habib College of Medicine-KSU Objectives 1- Define the terms sterilization, disinfectant and antiseptic. 2- Classify the different methods of sterilization (physical
Sterilization & Disinfection Prof. Hanan Habib College of Medicine-KSU Objectives 1- Define the terms sterilization, disinfectant and antiseptic. 2- Classify the different methods of sterilization (physical
Reprocessing endoscopes: United States perspective
 Journal of Hospital Infection (2004) 56, S27 S39 www.elsevierhealth.com/journals/jhin Reprocessing endoscopes: United States perspective W. A. Rutala a, *, D. J. Weber b a Hospital Epidemiology, University
Journal of Hospital Infection (2004) 56, S27 S39 www.elsevierhealth.com/journals/jhin Reprocessing endoscopes: United States perspective W. A. Rutala a, *, D. J. Weber b a Hospital Epidemiology, University
Evaluation of a Novel Non-Sterile Glove Dispensing System Uyen Nguyen
 Evaluation of a Novel Non-Sterile Glove Dispensing System Uyen Nguyen Edited by: Michael Diamond Reviewed by: Andrew Duong Disclaimer: The author of this report and declare no conflict of interest with
Evaluation of a Novel Non-Sterile Glove Dispensing System Uyen Nguyen Edited by: Michael Diamond Reviewed by: Andrew Duong Disclaimer: The author of this report and declare no conflict of interest with
