FOR USE WITH SOFIA ONLY
|
|
|
- Annabel Anderson
- 6 years ago
- Views:
Transcription
1 FOR USE WITH SOFIA ONLY INTENDED USE The employs immunofluorescence technology to detect Group A Streptococcal antigens from throat swabs of symptomatic patients. All negative test results should be confirmed by bacterial culture because negative results do not preclude Group A Strep infection and should not be used as the sole basis for treatment. The test is intended for professional and laboratory use as an aid in the diagnosis of Group A Streptococcal infection. SUMMARY AND EXPLANATION Group A Streptococcus is one of the most common causes of acute upper respiratory tract infection. Early diagnosis and treatment of Group A Streptococcal pharyngitis has been shown to reduce the severity of symptoms and serious complications such as rheumatic fever and glomerulonephritis. 1, 2 Conventional procedures for identification of Group A Streptococcus from throat swabs involve the culture, isolation, and subsequent identification of viable pathogen at 24 to 48 hours or longer for results. 3, 4 PRINCIPLE OF THE TEST The employs immunofluorescence technology that is used with the Sofia analyzer (Sofia) to detect Group A Streptococcal antigen. The involves the extraction of the antigenic components of the Group A Streptococcus (GAS) bacteria. The patient s Swab specimen is placed in the Reagent Tube containing the Reagent Solution, during which time the bacterial antigens are extracted, making them more accessible to the specific antibodies. An aliquot of the extracted specimen is dispensed into the Cassette sample well. From the sample well, the specimen migrates through a test strip containing various unique chemical environments. If Group A Streptococcal antigens are present, they will be bound by antibodies coupled to fluorescent microparticles that migrate through the test strip. The fluorescent microparticles containing bound antigen will be captured by antibodies at a defined location on the test strip where they are detected by Sofia. If antigens are not present, the fluorescent microparticles will not be trapped by the capture antibodies nor detected by Sofia. Note: Depending upon the user s choice, the Cassette, now containing the specimen, is either placed directly inside Sofia for automatically timed development (WALK AWAY Mode) or placed on the counter or bench top for a manually timed development and then placed into Sofia (READ NOW Mode). Sofia scans, measures, and interprets the immunofluorescent signal, using on-board method-specific algorithms. Sofia will then report the test results to the user (Positive, Negative, or Invalid) on its display screen, and it can print out the results via an integrated printer or transmit the results via an LIS connection. Page 1 of 15
2 REAGENTS AND MATERIALS SUPPLIED 25-Test Kit: Individually Packaged Cassettes (25): Polyclonal rabbit anti-group A Streptococcus antibodies Reagent Tubes (25) Reagent Solution Bottles (25): 4M Sodium Nitrite and 0.2M Acetic Acid inside glass ampoule Fixed Volume Pipettes (25) Sterile Rayon Throat Swabs (25) Positive Control Swab (1): Swab is coated with heat-inactivated, non-infectious Group A Streptococcus Negative Control Swab (1): Swab is coated with heat-inactivated, non-infectious Group C Streptococcus Package Insert (1) Quick Reference Instructions (1) QC Card (located on kit box) Printer Paper (1) MATERIALS NOT SUPPLIED IN KIT Timer or watch for use in READ NOW Mode Sofia analyzer Calibration Cassette (supplied with Sofia Installation Pack) WARNINGS AND PRECAUTIONS n For in vitro diagnostic use. Do not use the kit contents beyond the expiration date printed on the outside of the box. Use appropriate precautions in the collection, handling, storage, and disposal of patient samples and used kit contents. 5 Use of Nitrile or Latex (or equivalent) gloves is recommended when handling patient samples. 5 Dispose of containers and used contents in accordance with Federal, State and Local requirements. Do not reuse any used Cassettes, Reagent Tubes, Fixed Volume Pipettes, solutions, or Control Swabs. The user should never open the Foil Pouch of the test Cassette exposing it to the ambient environment until the Cassette is ready for immediate use. Discard and do not use any damaged Cassette or material. The Reagent Solution contains an acidic solution. If the solution contacts the skin or eye, flush with copious amounts of water. Testing should be performed in an area with adequate ventilation. For more information, consult the Safety Data Sheet available on quidel.com. The Reagent Solution Bottle contains glass, break cautiously. If the Reagent Solution Bottle is missing the glass ampoule or the solution is green prior to the breaking of the ampoule, discard and use another Reagent Solution Bottle. To obtain accurate results, the Package Insert instructions must be followed. The Calibration Cassette must be kept in the provided storage pouch between uses. Inadequate or inappropriate specimen collection, storage, and transport may yield false test results. Specimen collection and handling procedures require specific training and guidance. If transport media will be used, use only the Transport Media and configuration recommended in this Package Insert. Use rayon-tipped swabs to collect throat specimens. The performance claims in the Performance Characteristics section were obtained with the Swabs provided in the kit. Use of the provided Swabs is recommended. Do not use calcium alginate, cotton-tipped or wooden shaft swabs. Page 2 of 15
3 Do not write on the barcode of the Cassette. This is used by Sofia to identify the type of test being run and to identify the individual Cassette so as to prevent a second read of the Cassette by the same Sofia. Once a Cassette has been successfully scanned by Sofia, do not attempt to scan the Cassette again in the same Sofia. The barcode on the Cassette contains a unique identifier that will prevent Sofia from performing a second read on a previously scanned Cassette. As the detection reagent is a fluorescent compound, no visible results will form on the test strip. Sofia must be used for result interpretation. KIT STORAGE AND STABILITY Store the kit at room temperature, 59 F to 86 F (15 C to 30 C), out of direct sunlight. Kit contents are stable until the expiration date printed on the outer box. Do not freeze. QUALITY CONTROL There are three types of Quality Control for Sofia and Strep A FIA: Sofia Calibration Check Procedure, built-in procedural control features, and External Controls. Sofia Calibration Check Procedure Note: This is a Calibration Check procedure. The Calibration Check Procedure should be performed every 30 days. Sofia is set to remind the user to complete the Calibration Check Procedure. The Calibration Check is a required function that checks the Sofia optics and calculation systems using a specific Calibration Cassette. This Calibration Cassette is shipped with the Sofia Installation Pack. Refer to the Sofia User Manual for details regarding the Calibration Check Procedure. Important: Ensure that the Calibration Cassette is stored in the provided storage pouch between uses to protect it from exposure to light. 1. To check the calibration of Sofia, select Calibration from the Main Menu. 2. Following the prompts, insert the Calibration Cassette into Sofia and gently close the drawer. Sofia performs the Calibration Check automatically with no user input required. Sofia indicates when the Calibration Check is completed. Select OK to return to the Main Menu. NOTE: If the Calibration Check does not pass, notify the on-site Supervisor or contact Quidel Technical Support for assistance from 7:00 a.m. to 5:00 p.m. Pacific Time at (in the U.S.); (outside the U.S.); Fax: ; custserv@quidel.com (Customer Service); technicalsupport@quidel.com (Technical Support) or contact your local distributor. Page 3 of 15
4 Built-in Procedural Controls The contains two built-in procedural control features. The manufacturer s recommendation for daily control is to document these built-in procedural controls for the first sample tested each day. A control of the extraction procedure is provided by a color change from clear to green as the Reagent Solution is mixed. The color change is an indication of Reagent Solution integrity and is also an indication that the extraction procedure was performed correctly. Each time a test is run in Sofia, a procedural control is interpreted by Sofia and the result is displayed on the Sofia screen. This information is automatically logged in Sofia with each test result. A valid result obtained with the procedural control demonstrates that the extracted specimen flowed correctly and the functional integrity of the Cassette was maintained. This procedural control is interpreted by Sofia after the Cassette has developed for 5 minutes. If the specimen has not flowed correctly, Sofia will indicate that the result is invalid. Should this occur, review the procedure and repeat the test with a new patient sample and a new test Cassette. External Quality Control For example: This result shows that an invalid result had occurred. External Controls may also be used to demonstrate that the reagents and assay procedure perform properly. Quidel recommends that Positive and Negative Controls be run: once for each untrained operator; once for each new shipment of kits-provided that each different lot received in the shipment is tested; and as deemed additionally necessary by your internal quality control procedures, and in accordance with Local, State and Federal regulations or accreditation requirements. The user must first select Run QC on the Main Menu of Sofia and then, when prompted, scan the QC Card (located on the kit box). This card provides information specific to the kit lot, including lot number and expiration date. Sofia will prompt the user to select the desired mode (WALK AWAY or READ NOW) and then to run the External Control Swabs. External Positive and Negative Control Swabs are supplied in the kit and should be tested using the Test Procedure provided in this Package Insert or in the Quick Reference Instructions. Additional External Control Swabs may be obtained separately by contacting Quidel s Customer Support Services at (in the U.S.) or (outside the U.S.). When the QC test is complete, each result will be displayed as Passed or Failed for the Positive Control and the Negative Control. Do not perform patient tests or report patient test results if the QC test does not produce the expected results. Repeat the test or contact Quidel Technical Support before testing patient specimens if a Failed result is obtained with the External Controls. Page 4 of 15
5 SPECIMEN COLLECTION AND HANDLING SPECIMEN COLLECTION Use the Rayon-tipped Swabs provided in the kit to collect throat specimens. The performance claims listed in the Performance Characteristics section were obtained with the Swabs provided in the kit. Do not use calcium alginate, cottontipped or wooden shaft swabs. Collect throat specimens by standard clinical methods. Depress the tongue with a tongue blade or spoon. Rub the Swab on the back of the throat, both of the tonsils, the uvula and the posterior pharynx. Be careful not to touch the tongue, sides or top of the mouth with the Swab. Consult standard reference procedures such as the collection method described by Facklam. 6 SPECIMEN TRANSPORT AND STORAGE It is recommended that Swab specimens be processed as soon as possible after collection. Swabs can be held in any clean, dry plastic tube or sleeve up to 24 hours at room temperature (15 C to 30 C), or refrigerated (2 C to 8 C) up to 48 hours. The following transport media and storage conditions have been tested and are also acceptable (Table 1): Transport Media Table 1 Recommended Transport Media Recommended Storage Condition 2 C to 8 C Ambient Temperature BD BBL CultureSwab with Liquid Stuarts Media (#220109)* 48 hours 24 hours Remel BactiSwab with Liquid Amies Media (#R723095)* 48 hours 24 hours *These transport media systems preserve the sample on the Swab tip via contact with a media-moistened sponge. If a culture is desired, lightly streak the Swab on a 5% sheep blood agar plate before using the Swab in the Sofia Strep A FIA. Do not perform the Strep A FIA before streaking the Swab, as the Reagent Solution will destroy the bacteria on the Swab, thereby rendering the organism incapable of successful culturing. Alternatively, two throat Swab specimens can be obtained; in this case one can be used separately for culture and the other for the. TEST PROCEDURE Important: All specimens must be at room temperature (15 C to 30 C) before beginning the test. Gloves should be worn when handling human samples. Do not use the Reagent Solution if it is green prior to breaking the ampoule. Do not open the foil pouch of the test Cassette until it is ready for immediate use. Expiration date: Check expiration on outer box before using. Do not use any test Cassette past the expiration date on the label. 1. Verify that Sofia is set to the desired Mode: WALK AWAY or READ NOW. See the Using Sofia section for more information. 2. Squeeze ONCE to break the glass ampoule inside the Reagent Solution Bottle prior to running the assay. Page 5 of 15
6 3. Vigorously shake the Bottle 5 times to mix the Solutions. Solution should turn green after the ampoule is broken. 4. Remove the cap. Holding Bottle vertically, fill the Reagent Tube to the line [approximately 6 drops]. Fill to Line 5. Immediately add the patient Swab sample to the Reagent Tube. Vigorously mix the solutions by plunging the Swab 5 times in an up and down motion in the Tube. NOTE: Best results are obtained when the specimen is vigorously extracted in the solution. Vigorously plunge up and down 5x 6. Leave the Swab in the Reagent Tube for 1 minute. 1 Leave for 1 minute. 7. Vigorously mix the solution again by plunging the Swab 5 times in an up and down motion in the Tube. Vigorously plunge up and down 5x Page 6 of 15
7 8. Express as much liquid as possible from the Swab by squeezing the sides of the Tube as the Swab is withdrawn. Discard the Swab in accordance with your biohazard waste disposal protocol. 9. Fill the provided Yellow 100 µl Fixed Volume Pipette with the sample: To fill the Fixed Volume Pipette with the sample: a) FIRMLY squeeze the top bulb. b) Still squeezing, place the Pipette tip into the sample. c) With the Pipette tip still in the sample, release pressure on the bulb to fill the Pipette. Pipette Squeeze here Overflow Sample 10. Firmly squeeze the top bulb to empty the contents of the Fixed Volume Pipette into the Cassette sample well. Extra liquid in the overflow bulb is OK. Squeeze here NOTE: The Fixed Volume Pipette is designed to collect and dispense the correct amount of patient sample. Discard the Pipette in your biohazard waste. 11. Proceed to the next section, Using Sofia, to complete the test. STREP A Sample Well USING SOFIA WALK AWAY/READ NOW Modes Refer to the Sofia User Manual for operating instructions. Sofia may be set to two different modes (WALK AWAY and READ NOW). The procedures for each mode are described below. WALK AWAY Mode In WALK AWAY Mode, the user immediately inserts the Cassette into Sofia. The user then returns after 5 minutes to get the test result. In this mode, Sofia will automatically time the test development before scanning and displaying the test result. READ NOW Mode Allow the test to develop for the full 5 minutes BEFORE placing it into Sofia. The user must first place the Cassette onto the counter or bench top for 5 minutes (outside of Sofia) and manually time this development step. Then, the user inserts the Cassette into Sofia. In READ NOW Mode, Sofia will scan and display the test result in less than 1 minute. Note: Results will remain stable for an additional 10 minutes after the recommended development time of 5 minutes. Page 7 of 15
8 Tips for Batch Testing In order to make batch testing easier, the user can prepare one or more Reagent Solution Bottles in advance of testing samples. The user can break the ampoule inside each Reagent Solution Bottle, shake to mix the solutions, and then store the capped Bottles on the bench top at room temperature for up to 12 hours without loss of activity before using with Swab sample(s). Critically important, the user should never open the foil pouch thus exposing the test Cassette to the ambient environment, until it is ready for immediate use. Run Test 1. Input the User ID using the handheld barcode scanner or manually enter the data using the key pad. NOTE: If you mistakenly scan the wrong barcode, use the Arrow Buttons on the Sofia key pad to re-highlight the field. Then simply rescan using the correct barcode, and the previous one will be overwritten with the correct barcode. 2. Input Patient ID and/or Order # using the handheld barcode scanner or manually enter the data using the key pad. 3. Press Start Test and the Sofia drawer will automatically open. 4. Verify that the correct development mode, WALK AWAY or READ NOW, has been selected. Insert the prepared patient test Cassette into the drawer of Sofia and close the drawer. Page 8 of 15
9 5. Sofia will start automatically and display the progress as shown in example below. In the WALK AWAY Mode, the test results will be displayed on the screen in approximately 5 minutes. In the READ NOW Mode, the test results will be displayed on the screen in less than 1 minute. See Interpretation of Results section. For example: This display shows that the test in WALK AWAY mode has 4 minutes, 13 seconds remaining. Sofia will read and display the results in about 5 minutes. INTERPRETATION OF RESULTS When the test is complete, the results will be displayed on the Sofia screen. The results can be automatically printed on the integrated printer, if this option is selected. Test Lines, which are fluorescent, will never be visible to the naked eye. The Sofia screen will display results for the procedural control as being valid or invalid, and will provide a positive or negative result for Strep A. If the procedural control is invalid, retest with a new patient sample and a new test Cassette. Positive Result: Negative Result: For example: This display shows a valid positive result for Strep A. NOTE: A positive result does not rule out co-infections with other pathogens. For example: This display shows a valid negative result for Strep A. NOTE: A negative result does not rule out possible other infections. Invalid Result: For example: This result shows that an invalid result was obtained. Invalid Result: If the test is invalid, a new test should be performed with a new patient sample and a new test Cassette. Page 9 of 15
10 LIMITATIONS n The contents of this kit are to be used for the qualitative detection of Group A Streptococcal antigens from throat Swab specimens. n The test detects both viable and nonviable Group A Streptococcus bacteria and may yield a positive result in the absence of living organisms. n Respiratory infections, including pharyngitis, can be caused by Streptococcus from serogroups other than Group A, as well as other pathogens. n The will not differentiate asymptomatic carriers of Group A Streptococcus from those exhibiting Streptococcal infection. 7 n A negative test result may occur if the level of antigen in a sample is below the detection limit of the test or if the sample was collected, transported, or stored improperly. n Failure to follow the Test Procedure may adversely affect test performance and/or invalidate the test result. n Patients with symptoms and an antigen negative test should have a follow-up culture. 1 n Test results must be evaluated in conjunction with other clinical data available to the physician. n Negative test results do not rule out possible other infections. n Positive test results do not rule out co-infections with other pathogens. EXPECTED VALUES Group A Streptococcus bacteria are responsible for about 19% of all upper respiratory tract infections. 8 Infection is most prevalent in winter and early spring, with most cases arising in patients living in highly populated areas. Consistent with these figures, in the multi-center clinical study conducted by Quidel during 2011 and 2012, 17.4% (128/736) of the patients presenting with pharyngitis were found to be culture positive for Strep A. Nearly half of these subjects, 46%, were male. The subjects ages ranged from 3-72 years and 88% (647/736) were children (3-17 years of age). PERFORMANCE CHARACTERISTICS Performance vs. Cell Culture The performance of the was compared to standard bacterial culture and identification in a multi-center clinical field study. This study was conducted by health care personnel during 2011 and 2012 at eight (8) distinct sites in various geographical regions within the United States and two (2) sites in Australia. In this multi-center, point-of-care (POC) field trial, two (2) throat Swabs were collected from 736 patients with symptoms suggestive of bacterial pharyngitis. One (1) throat Swab was transported on cold ice packs to a central Reference Laboratory, streaked on a sheep blood agar plate (SBA) and cultured for up to 48 hours. Immediately after streaking, this same Swab was tested in the rapid. The performance of the was determined by comparison of the rapid test result to the corresponding culture result. The results from these analyses are presented in Tables 2, 3a, and 3b. Table 2 Results: Combined Culture Sens. = 90.6% (116/128) Pos Neg Total: (95% CI: 84.3%-94.6%) Sofia Pos Spec. = 96.1% (584/608) Sofia Neg (95% CI: 94.2%-97.3%) Total: PPV = 82.9% (116/140) NPV = 98.0% (584/596) Prev. = 17.4% (128/736) Page 10 of 15
11 Table 3a Results: READ NOW Mode Culture Sens. = 89.3% (100/112) Pos Neg Total: (95% CI: 82.2%-93.8%) Sofia Pos Spec. = 96.0% (549/572) Sofia Neg (95% CI: 94.0%-97.3%) Total: PPV = 81.3% (100/123) NPV = 97.9% (549/561) Prev. = 16.4% (112/684) Table 3b Results: WALK AWAY Mode Culture Sens. = 100% (16/16) Pos Neg Total: (95% CI: 80.6%-100%) Sofia Pos Spec. = 97.2% (35/36) Sofia Neg (95% CI: 85.8%-99.5%) Total: PPV = 94.1% (16/17) NPV = 100% (35/35) Prev. = 30.8% (16/52) Reproducibility Studies The reproducibility of the was evaluated at three (3) different laboratories. Two (2) different operators at each site tested a series of coded, contrived samples, prepared in negative clinical matrix, ranging from low negative to moderate positive Group A Streptococcus. The inter-laboratory agreement (Table 4) for negative samples was 96.7%-100% and 96.7%-100% for positive samples. The intra-laboratory agreement (Table 5) for all samples ranged from 97.5%-99.2%. Site Site Table 4 Reproducibility Study Inter-laboratory Agreement Low Negative (no bacteria) (0 cfu/test) High Negative (C5) (1.5x10 3 cfu/test) Low Positive (C95) (3.0x10 3 cfu/test) Table 5 Reproducibility Study Intra-laboratory Agreement Mod. Positive (C3X) (2.8x10 4 cfu/test) 1 30/30 30/30 28/30 30/ /30 29/30 30/30 30/ /30 28/30 29/30 30/30 Total 90/90 87/90 87/90 90/90 % Overall Agreement (95% CI) Low Negative (no bacteria) (0 cfu/test) 100% (90/90) (95.9%-100%) High Negative (C5) (1.5x10 3 cfu/test) 96.7% (87/90) (90.7%-98.9%) Low Positive (C95) (3.0x10 3 cfu/test) 96.7% (87/90) (90.7%-98.9%) Mod. Positive (C3X) (2.8x10 4 cfu/test) 1 30/30 30/30 28/30 30/ /30 29/30 30/30 30/ /30 28/30 29/30 30/30 100% (90/90) (95.9%-100%) % Overall Agreement (95% CI) 98.3% (118/120) (94.1%-99.5%) 99.2% (119/120) (95.4%-99.9%) 97.5% (117/120) (92.9%-99.1%) Page 11 of 15
12 Limit of Detection The limit of detection (LOD) for the was determined using three (3) strains of Group A Streptococcus pyogenes. The LOD ranged from 9x10 3-2x10 4 colony forming units (cfu)/test (Table 6). Table 6 Limits of Detection for Three Streptococcus pyogenes Strains Strain Bruno [CIP ] CDC-SS-1402 CDC-SS-1460 cfu/test = colony forming units/test Minimum Detectable Level* 1.86x10 4 cfu/test 9.24x10 3 cfu/test 2.34x10 4 cfu/test *The levels of bacteria were determined by limiting dilution, bacterial culture, and colony counting to give cfu/test. Analytical Reactivity Analytical reactivity for the was determined using 15 strains of Streptococcus pyogenes. Each strain listed below in Table 7 produced positive results in the assay. Table 7 Analytical Reactivity Streptococcus pyogenes Strain Strain #1 (ATCC 19615) Strain #2 (ATCC ) Strain #3 (ATCC ) Strain #4 (Field Clinical Isolate) Strain #5 (Field Clinical Isolate) Strain #6 (Field Clinical Isolate) Strain #7 (Field Clinical Isolate) Strain #8 (Field Clinical Isolate) Strain #9 (Field Clinical Isolate) Strain #10 (ATCC ) Strain #11 (ATCC BAA 1315) Strain #12 (ATCC ) Strain #13 (ATCC 12203) Strain #14 (ATCC ) Strain #15 (Field Clinical Isolate) cfu/test = colony forming units/test Test Quantity* 6.5x10 4 cfu/test 7.4x10 4 cfu/test 8.3x10 4 cfu/test 3.1x10 4 cfu/test 7.6x10 4 cfu/test 7.1x10 5 cfu/test 6.3x10 4 cfu/test 6.3x10 4 cfu/test 5.3x10 4 cfu/test 6.5x10 4 cfu/test 7.2x10 4 cfu/test 5.4x10 4 cfu/test 6.9x10 4 cfu/test 5.3x10 4 cfu/test 7.0x10 4 cfu/test *The levels of bacteria were determined by limiting dilution, bacterial culture, and colony counting to give cfu/test. Page 12 of 15
13 Analytical Specificity Cross Reactivity The cross reactivity of the was evaluated with a total of 61 non-group A Streptococcus bacterial and fungal microorganisms, and 26 viral isolates. None of the organisms or viruses listed below in Table 8 showed any sign of cross reactivity in the assay. When the same organisms in Table 8 were pre-mixed with Group A Strep and tested in the Sofia Strep A FIA, all results were positive also indicating that the potential cross-reactants did not interfere with the detection of Strep A. Table 8 Analytical Specificity and Cross Reactivity Organism/Virus Test Quantity* Organism/Virus Test Quantity* Arcanobacterium haemolyticum 3x10 5 cfu/test Streptococcus sp. Group C strain #4 3x10 6 cfu/test Bacteroides fragilis 3x10 7 cfu/test Streptococcus sp. Group C strain #5 3x10 5 cfu/test Bordetella pertussis 3x10 7 cfu/test Streptococcus sp. Group D strain #1 3x10 6 cfu/test Candida albicans 3x10 4 cfu/test Streptococcus sp. Group D strain #2 3x10 6 cfu/test Corynebacterium diphtheria 3x10 5 cfu/test Streptococcus sp. Group D strain #3 3x10 6 cfu/test Corynebacterium pseudodiphtheriticum 3x10 6 cfu/test Streptococcus sp. Group D strain #4 3x10 6 cfu/test Enterococcus faecalis 3x10 6 cfu/test Streptococcus sp. Group D strain #5 3x10 6 cfu/test Enterococcus faecium 3x10 6 cfu/test Streptococcus sp. Group F strain #1 3x10 5 cfu/test Escherichia coli 1.5x10 7 cfu/test Streptococcus sp. Group F strain #2 3x10 6 cfu/test Fusobacterium necrophorum 3x10 6 cfu/test Streptococcus sp. Group F strain #3 3x10 6 cfu/test Haemophilus influenzae 3x10 7 cfu/test Streptococcus sp. Group F strain #4 3x10 5 cfu/test Haemophilus parahaemolyticus 3x10 6 cfu/test Streptococcus sp. Group F strain #5 3x10 5 cfu/test Klebsiella pneumoniae 3x10 7 cfu/test Streptococcus sp. Group G strain #1 3x10 7 cfu/test Moraxella catarrhalis 3x10 6 cfu/test Streptococcus sp. Group G strain #2 3x10 6 cfu/test Neisseria lactamica 3x10 6 cfu/test Streptococcus sp. Group G strain #3 3x10 6 cfu/test Neisseria gonorrhoeae 3x10 6 cfu/test Streptococcus sp. Group G strain #4 3x10 6 cfu/test Neisseria meningitidis 3x10 6 cfu/test Yersinia enterocolitica 3x10 6 cfu/test Neisseria sicca 3x10 7 cfu/test Adenovirus Type 1 3x10 11 TCID50/test Neisseria subflava 3x10 7 cfu/test Adenovirus Type 3 3x10 5 TCID50/test Proteus vulgaris 3x10 7 cfu/test Adenovirus Type 4 1.5x10 2 TCID50/test Pseudomonas aeruginosa 3x10 6 cfu/test Adenovirus Type 5 3x10 5 TCID50/test Serratia marcescens 3x10 7 cfu/test Adenovirus Type 11 3x10 4 TCID50/test Staphylococcus aureus 3x10 6 cfu/test Coronavirus 229E 3x10 4 TCID50/test Staphylococcus epidermidis 3x10 6 cfu/test Coronavirus OC43 3x10 4 TCID50/test Staphylococcus haemolyticus 3x10 5 cfu/test Coxsackievirus B5 (Faulkner) 3x10 6 TCID50/test Staphylococcus intermedius 3x10 5 cfu/test Cytomegalovirus 3x10 3 TCID50/test Staphylococcus saprophyticus 3x10 6 cfu/test Echovirus Type 3 1.5x10 4 TCID50/test Streptococcus anginosus 3x10 6 cfu/test Epstein Barr virus 3x10 7 TCID50/test Streptococcus gordonii 3x10 4 cfu/test Herpes Simplex virus 1 3x10 4 TCID50/test Streptococcus mitis 3x10 4 cfu/test Herpes Simplex virus 2 3x10 4 TCID50/test Streptococcus mutans 3x10 6 cfu/test Influenza A H1N1 3x10 4 TCID50/test Streptococcus oralis 3x10 6 cfu/test Influenza A H3N2 3x10 4 TCID50/test Streptococcus parasanguis 3x10 6 cfu/test Influenza B Hong Kong 3x10 4 TCID50/test Streptococcus pneumoniae 3x10 6 cfu/test Influenza B Panama 1.5x10 4 TCID50/test Streptococcus salivarius 3x10 5 cfu/test Influenza C Taylor 1.5x10 4 TCID50/test Streptococcus sanguinis 3x10 6 cfu/test Measles (Edmonston) 3x10 4 TCID50/test Streptococcus sp. Group B strain #1 3x10 6 cfu/test Mumps (Enders) 3x10 3 TCID50/test Streptococcus sp. Group B strain #2 3x10 6 cfu/test Parainfluenza virus 1 3x10 4 TCID50/test Streptococcus sp. Group B strain #3 3x10 6 cfu/test Parainfluenza virus TCID50/test Streptococcus sp. Group B strain #4 3x10 6 cfu/test Parainfluenza virus 3 3x10 6 TCID50/test Streptococcus sp. Group B strain #5 3x10 6 cfu/test Parainfluenza virus 4A 3x10 4 TCID50/test Streptococcus sp. Group C strain #1 3x10 6 cfu/test Rhinovirus Type 15 3x10 4 TCID50/test Streptococcus sp. Group C strain #2 3x10 6 cfu/test Rhinovirus Type 1B 3x10 3 TCID50/test Streptococcus sp. Group C strain #3 3x10 6 cfu/test cfu/test = colony forming units/test TCID50/test = 50% tissue culture infectious dose *The levels of bacteria were determined by limiting dilution, bacterial culture, and colony counting to give cfu/test. Virus concentrations were determined by standard virology methods, Reed-Muench. Page 13 of 15
14 Interfering Substances Several over-the-counter (OTC) products, whole blood, and blood agar were evaluated and did not interfere with the Sofia Strep A FIA at the levels tested (Table 9). Table 9 Non-interfering Substances Substance Concentration Crest Pro-Health Night Mint (Cetylpyridnium chloride) Listerine Antiseptic (Eucalyptol, Menthol, Methyl salicylate, and Thymol) Listerine Cool Mint (Eucalyptol, Menthol, Methyl salicylate, and Thymol) Cepacol Dual Relief Spray (Benzocaine and Menthol) Chloraseptic Max: Sore Throat Relief (Phenol and Glycerin) Children s Dimetapp DM Cold & Cough Elixir (Brompheniramine maleate, Dextromethorphan HBr, and Phenylephrine HCl) Children s Wal-Tap Elixir Cold & Allergy (Brompheniramine maleate and Phenylephrine HCl) Children s Wal-Tap DM Elixir Cold & Cough (Brompheniramine maleate, Dextromethorphan HBr, and Phenylephrine HCl) Rite Aid Tussin CF (Dextromethorphan HBr, Guaifenesin, and Phenylephrine HCl) Robitussin Cough & Cold-CF Max (Dextromethorphan HBr, Guaifenesin, and Phenylephrine HCl) Robitussin Nighttime Cough, Cold, & Flu (Acetaminophen, Diphenhydramine HCl, and Phenylephrine HCl) Cepacol Sore Throat: Cherry Flavor (Benzocaine and Menthol) Halls Cherry Mentholyptus (Menthol) Halls Mentholyptus (Menthol) Ricola Mountain Herb Throat Drops-Sugar Free (Menthol) Sucrets Complete-Vapor Cherry (Dyclonine Hydrochloride and Menthol) Sucrets Complete-Cool Citrus (Dyclonine Hydrochloride and Menthol) Chlorasceptic Throat Drops-Cherry (Phenol and Glycerin) BreathSavers 3 Hour Mint-Spearmint (Cetylpyridnium chloride) Tic Tac Freshmints (Eucalyptol, Menthol, Methylsalicylate, and Thymol) Whole Blood 5% v/v Sheep Blood Agar (5% Sheep Blood) 2.16 mg/ml Horse Blood Agar (5% Horse Blood) 1.67 mg/ml ASSISTANCE If you have any questions regarding the use of this product or if you want to report a test system problem, please call Quidel s Technical Support Number (in the U.S.) or , Monday through Friday, from 7:00 a.m. to 5:00 p.m., Pacific Time. If outside the U.S., contact your local distributor or technicalsupport@quidel.com. REFERENCES 1. American Academy of Pediatrics. [Group Streptococcal Infection]. In: Pickering L.K., Baker C.J., Kimberlin D.W., Long S.S., eds. Red Book: 2009 Report of the Committee on Infectious Diseases. 28th ed. Elk Grove Village, IL: America Academy of Pediatrics, 2009, pp Youmans G.P., Paterson P.Y., and Sommer H.M., Upper Respiratory Tract Infection: General Considerations, in the Biological and Clinical Basis of Infectious Disease, W.B. Saunders Co., Philadelphia, 1980, pp Versalovic J., Carroll K.C., Jorgensen J.H., Funke G., Landry M.L., Warnock D.W., eds. Manual of Clinical Microbiology. 10 th ed. ASM Press, Washington, DC. Chapter 20, Streptococcus, 2011, pp Youmans G.P., Paterson P.Y., and Sommers H.M. The Biological and Clinical Basis of Infectious Disease, 1975, pp Biosafety in Microbiological and Biomedical Laboratories, 5th Edition. U.S. Department of Health and Human Services, CDC, NIH, Washington, DC (2007). 6. Facklam R.R. and Washington J.A. Manual of Clinical Microbiology 5 th Edition, 1991, p Rammelkamp C.H., Jr. Principles of Internal Medicine, 8th Edition, 1977, pp Lauer B.A., Reller L.D., and Mirrett S., Journal of Clinical Microbiology, 17: , Page 14 of 15
15 Tests (multi-language) Tests MDSS GmbH Schiffgraben Hannover, Germany Quidel Corporation McKellar Court San Diego, CA USA quidel.com EN01 (03/15) Catalogue number CE mark of conformity Authorized Representative in the European Community Batch code Use by Manufacturer Temperature limitation Intended use Consult instructions for use For In Vitro diagnostic use Contains sufficient for 25 determinations Contents/Contains Positive control Negative control Page 15 of 15
CLIA Complexity: MODERATE
 CLIA Complexity: MODERATE INTENDED USE The is intended for the rapid detection of Group A Streptococcal antigen directly from throat swabs and beta-hemolytic colonies recovered from culture. This test
CLIA Complexity: MODERATE INTENDED USE The is intended for the rapid detection of Group A Streptococcal antigen directly from throat swabs and beta-hemolytic colonies recovered from culture. This test
QUICK REFERENCE INSTRUCTIONS For use with Sofia and Sofia 2. Rx only
 QUICK REFERENCE INSTRUCTIONS For use with Sofia and Sofia 2. Rx only Test Procedure Study the Package Insert and User Manual thoroughly before using Quick Reference Instructions. This is not a complete
QUICK REFERENCE INSTRUCTIONS For use with Sofia and Sofia 2. Rx only Test Procedure Study the Package Insert and User Manual thoroughly before using Quick Reference Instructions. This is not a complete
PROCEDURE MANUAL. Prepared By Date Adopted Supersedes Procedure # Review Date Revision Date Signature
 Procedure: CLIA Complexity: Moderate Procedure #: Prepared By Date Adopted Supersedes Procedure # Review Date Revision Date Signature Distributed to # of Copies Distributed to # of Copies This Procedural
Procedure: CLIA Complexity: Moderate Procedure #: Prepared By Date Adopted Supersedes Procedure # Review Date Revision Date Signature Distributed to # of Copies Distributed to # of Copies This Procedural
PROCEDURE MANUAL. Prepared By Date Adopted Supersedes Procedure # Review Date Revision Date Signature
 Procedure: CLIA Complexity: Waived Procedure #: Prepared By Date Adopted Supersedes Procedure # Review Date Revision Date Signature Distributed to # of Copies Distributed to # of Copies This Procedural
Procedure: CLIA Complexity: Waived Procedure #: Prepared By Date Adopted Supersedes Procedure # Review Date Revision Date Signature Distributed to # of Copies Distributed to # of Copies This Procedural
SUMMARY AND EXPLANATION
 This Procedural Bulletin is intended to provide a ready outline reference for performance of the assay. These abbreviated directions for use are not intended to replace the complete package insert. It
This Procedural Bulletin is intended to provide a ready outline reference for performance of the assay. These abbreviated directions for use are not intended to replace the complete package insert. It
SAMPLE PROCEDURE 928-8, 07/11
 SAMPLE PROCEDURE This Sample Procedure is not intended as a substitute for your facility s Procedure Manual or reagent labeling, but rather as a model for your use in customizing for your laboratory s
SAMPLE PROCEDURE This Sample Procedure is not intended as a substitute for your facility s Procedure Manual or reagent labeling, but rather as a model for your use in customizing for your laboratory s
QUICK REFERENCE INSTRUCTIONS For use with Sofia and Sofia 2.
 QUICK REFERENCE INSTRUCTIONS For use with Sofia and Sofia 2. Study the Package Insert and User Manual thoroughly before using Quick Reference Instructions. This is not a complete Package Insert. CLIA Complexity:
QUICK REFERENCE INSTRUCTIONS For use with Sofia and Sofia 2. Study the Package Insert and User Manual thoroughly before using Quick Reference Instructions. This is not a complete Package Insert. CLIA Complexity:
REAGENTS AND MATERIALS SUPPLIED Control 2: 25-OH vitamin D in human serum with <0.1% Streptomycin Sulfate (2)
 For use with the Sofia Quantitative Vitamin D FIA test system only For in vitro diagnostic use INTENDED USE The Sofia Quantitative Vitamin D Control Set is intended for use as assayed quality control materials
For use with the Sofia Quantitative Vitamin D FIA test system only For in vitro diagnostic use INTENDED USE The Sofia Quantitative Vitamin D Control Set is intended for use as assayed quality control materials
Department Of Pathology Point of Care Testing POC Quick Vue In-Line Strep A Version#4
 Department Of Pathology Point of Care Testing POC.014.04 Quick Vue In-Line Strep A Version#4 Printed copies are for reference only. Please refer to the electronic copy for the latest version. A. Purpose:
Department Of Pathology Point of Care Testing POC.014.04 Quick Vue In-Line Strep A Version#4 Printed copies are for reference only. Please refer to the electronic copy for the latest version. A. Purpose:
CLIA Complexity: Waived for Finger-stick Whole Blood. Instructional video available online at quidel.com/sofia2lyme.
 Study the Package Insert and User Manual thoroughly before using Quick Reference Instructions. This is not a complete Package Insert. CLIA Complexity: Waived for Finger-stick Whole Blood Instructional
Study the Package Insert and User Manual thoroughly before using Quick Reference Instructions. This is not a complete Package Insert. CLIA Complexity: Waived for Finger-stick Whole Blood Instructional
Strep A Test. Rev ,02/17
 Strep A Test Rev. 3812-6,02/17 Strep A Test CLIA Complexity: Waived 23-200-276 INTENDED USE The Sure-Vue Signature Strep A Test is a color immunochromatographic assay intended for the qualitative detection
Strep A Test Rev. 3812-6,02/17 Strep A Test CLIA Complexity: Waived 23-200-276 INTENDED USE The Sure-Vue Signature Strep A Test is a color immunochromatographic assay intended for the qualitative detection
INVALID. Rev /15 POSITIVE NEGATIVE. Ultra Strep A Test. 2 min 5 min. 10x. 3 drops 3 drops
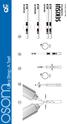 1 3 drops 3 drops Ultra Strep A Test 2 3 4 5 10x 2 min 5 min POSITIVE NEGATIVE INVALID Rev. 3101-2 06/15 Ultra Strep A Test CLIA Complexity: Waived INTENDED USE The OSOM Ultra Strep A Test is a color immunochromatographic
1 3 drops 3 drops Ultra Strep A Test 2 3 4 5 10x 2 min 5 min POSITIVE NEGATIVE INVALID Rev. 3101-2 06/15 Ultra Strep A Test CLIA Complexity: Waived INTENDED USE The OSOM Ultra Strep A Test is a color immunochromatographic
Prepared By Date Adopted Supersedes Procedure # Sofia RSV FIA
 This Procedural Bulletin is intended to provide a ready outline reference for performance of the assay. These abbreviated directions for use are not intended to replace the complete package insert. It
This Procedural Bulletin is intended to provide a ready outline reference for performance of the assay. These abbreviated directions for use are not intended to replace the complete package insert. It
For in vitro diagnostic use.
 FOR USE WITH SOFIA AND SOFIA 2 CLIA Complexity: Waived for children less than 7 years of age. CLIA Complexity: Moderate for pediatric patients 7 to less than 19 years of age. For in vitro diagnostic use.
FOR USE WITH SOFIA AND SOFIA 2 CLIA Complexity: Waived for children less than 7 years of age. CLIA Complexity: Moderate for pediatric patients 7 to less than 19 years of age. For in vitro diagnostic use.
SUMMARY AND EXPLANATION RSV is a causative agent of highly contagious, acute, viral infection of the respiratory tract in pediatric populations.
 CLIA Complexity: Moderate For in vitro diagnostic use. INTENDED USE The QuickVue RSV 10 test is an immunoassay that allows for the rapid, qualitative detection of respiratory syncytial virus (RSV) antigen
CLIA Complexity: Moderate For in vitro diagnostic use. INTENDED USE The QuickVue RSV 10 test is an immunoassay that allows for the rapid, qualitative detection of respiratory syncytial virus (RSV) antigen
SAMPLE PROCEDURE , 11/07
 SAMPLE PROCEDURE This Sample Procedure is not intended as a substitute for your facility s Procedure Manual or reagent labeling, but rather as a model for your use in customizing for your laboratory s
SAMPLE PROCEDURE This Sample Procedure is not intended as a substitute for your facility s Procedure Manual or reagent labeling, but rather as a model for your use in customizing for your laboratory s
Rapid-VIDITEST RSV. One step RSV Blister test for the detection of Respiratory Syncytial Virus from nasal specimens. Instruction manual
 Rapid-VIDITEST RSV One step RSV Blister test for the detection of Respiratory Syncytial Virus from nasal specimens. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, Vestec, 252 42 Jesenice,
Rapid-VIDITEST RSV One step RSV Blister test for the detection of Respiratory Syncytial Virus from nasal specimens. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, Vestec, 252 42 Jesenice,
CLIA Complexity: WAIVED
 CLIA Complexity: WAIVED INTENDED USE The QuickVue RSV Test is a dipstick immunoassay which allows for the rapid, qualitative detection of respiratory syncytial virus (RSV) antigen (viral fusion protein)
CLIA Complexity: WAIVED INTENDED USE The QuickVue RSV Test is a dipstick immunoassay which allows for the rapid, qualitative detection of respiratory syncytial virus (RSV) antigen (viral fusion protein)
IMMUVIEW RSV ANTIGEN TEST ENGLISH
 IMMUVIEW RSV ANTIGEN TEST ENGLISH Lateral flow test for qualitative detection of respiratory syncytial virus (RSV) in nasal wash, nasopharyngeal swap and throat swap specimens. 1 IMMUVIEW RSV ANTIGEN
IMMUVIEW RSV ANTIGEN TEST ENGLISH Lateral flow test for qualitative detection of respiratory syncytial virus (RSV) in nasal wash, nasopharyngeal swap and throat swap specimens. 1 IMMUVIEW RSV ANTIGEN
Quick Reference Instructions
 PROFILE -V Quick Reference Instructions Reader System Before performing the test, refer to the PROFILE -V MEDTOXScan Drugs of Abuse Test System Insert and MEDTOXScan User Manual for complete operating
PROFILE -V Quick Reference Instructions Reader System Before performing the test, refer to the PROFILE -V MEDTOXScan Drugs of Abuse Test System Insert and MEDTOXScan User Manual for complete operating
Rapid-VIDITEST. RSV Card. One step RSV Card test for the detection of Respiratory Syncytial Virus from nasal specimens. Instruction manual
 Rapid-VIDITEST RSV Card One step RSV Card test for the detection of Respiratory Syncytial Virus from nasal specimens. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, Vestec, 252 42
Rapid-VIDITEST RSV Card One step RSV Card test for the detection of Respiratory Syncytial Virus from nasal specimens. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, Vestec, 252 42
PROCEDURE MANUAL. Prepared By Date Adopted Supersedes Procedure # Review Date Revision Date Signature
 Procedure #: Procedure: CLIA Complexity: Moderate Prepared By Date Adopted Supersedes Procedure # Review Date Revision Date Signature Distributed to # of Copies Distributed to # of Copies This Procedural
Procedure #: Procedure: CLIA Complexity: Moderate Prepared By Date Adopted Supersedes Procedure # Review Date Revision Date Signature Distributed to # of Copies Distributed to # of Copies This Procedural
Guidelines for Laboratory Verification of Performance of the FilmArray Blood Culture Identification (BCID) Panel
 Guidelines for Laboratory Verification of Performance of the FilmArray Blood Culture Identification (BCID) Panel Purpose The Clinical Laboratory Improvement Amendments (CLIA), passed in 1988, establishes
Guidelines for Laboratory Verification of Performance of the FilmArray Blood Culture Identification (BCID) Panel Purpose The Clinical Laboratory Improvement Amendments (CLIA), passed in 1988, establishes
Protocols for Laboratory Verification of Performance of the FilmArray Blood Culture Identification (BCID) Panel
 Protocols for Laboratory Verification of Performance of the FilmArray Blood Culture Identification (BCID) Panel Purpose The Clinical Laboratory Improvement Amendments (CLIA), passed in 1988, establishes
Protocols for Laboratory Verification of Performance of the FilmArray Blood Culture Identification (BCID) Panel Purpose The Clinical Laboratory Improvement Amendments (CLIA), passed in 1988, establishes
Protocols for Laboratory Verification of Performance of the BioFire FilmArray Blood Culture Identification (BCID) Panel
 Protocols for Laboratory Verification of Performance of the BioFire FilmArray Blood Culture Identification (BCID) Panel A Laboratory Protocol for Use with Live s Purpose The Clinical Laboratory Improvement
Protocols for Laboratory Verification of Performance of the BioFire FilmArray Blood Culture Identification (BCID) Panel A Laboratory Protocol for Use with Live s Purpose The Clinical Laboratory Improvement
FOR USE WITH SOLANA INSTRUMENT ONLY
 For the qualitative detection of Group A β-hemolytic Streptococcus (Streptococcus pyogenes) nucleic acids isolated from throat swab specimens obtained from patients with signs and symptoms of pharyngitis,
For the qualitative detection of Group A β-hemolytic Streptococcus (Streptococcus pyogenes) nucleic acids isolated from throat swab specimens obtained from patients with signs and symptoms of pharyngitis,
Sample for Use as a Guide Revised IDEXX ENTEROLERT TEST METHOD FOR THE DETECTION OF ENTEROCOCCI IN WATER
 IDEXX ENTEROLERT TEST METHOD FOR THE DETECTION OF ENTEROCOCCI IN WATER 1 IDEXX ENTEROLERT TEST METHOD FOR THE DETECTION OF ENTEROCOCCI IN WATER 1. Scope and Application 1.1. This method is intended for
IDEXX ENTEROLERT TEST METHOD FOR THE DETECTION OF ENTEROCOCCI IN WATER 1 IDEXX ENTEROLERT TEST METHOD FOR THE DETECTION OF ENTEROCOCCI IN WATER 1. Scope and Application 1.1. This method is intended for
IVD: Approval no AMX (by MHLW in Japan) Ver 1.0 of Nov 2010 Please read this package insert carefully before use.
 IVD: Approval no. 22000AMX01629000 (by MHLW in Japan) Ver 1.0 of Nov 2010 Please read this package insert carefully before use. A Regent for Detecting Adenovirus Antigen General precautions 1. Collecting
IVD: Approval no. 22000AMX01629000 (by MHLW in Japan) Ver 1.0 of Nov 2010 Please read this package insert carefully before use. A Regent for Detecting Adenovirus Antigen General precautions 1. Collecting
Strep A INTENDED USE INTRODUCTION PRINCIPLE COMPONENTS WARNINGS AND PRECAUTIONS FOR SAMPLE WARNINGS AND PRECAUTIONS STORAGE AND STABILITY
 INTENDED USE Strep A ichroma Strep A is a fluorescence Immunoassay (FIA) for the qualitative determination of Streptococcus A in human throat specimen. It is useful as an aid in management and monitoring
INTENDED USE Strep A ichroma Strep A is a fluorescence Immunoassay (FIA) for the qualitative determination of Streptococcus A in human throat specimen. It is useful as an aid in management and monitoring
BD GeneOhm MRSA ACP Lysis Kit
 REF 441638 100 Tests DOPS09-09-V1E1 USA Date: 2010-01 TABLE OF CONTENTS INTENDED USE...3 SUMMARY AND EXPLANATION OF THE PROCEDURE...3 REAGENTS...3 PRECAUTIONS...3 MATERIALS PROVIDED...4 STORAGE, HANDLING
REF 441638 100 Tests DOPS09-09-V1E1 USA Date: 2010-01 TABLE OF CONTENTS INTENDED USE...3 SUMMARY AND EXPLANATION OF THE PROCEDURE...3 REAGENTS...3 PRECAUTIONS...3 MATERIALS PROVIDED...4 STORAGE, HANDLING
CLIA Complexity: Moderate
 This Procedural Bulletin is intended to provide a ready outline reference for performance of the assay. These abbreviated directions for use are not intended to replace the complete Package Insert. It
This Procedural Bulletin is intended to provide a ready outline reference for performance of the assay. These abbreviated directions for use are not intended to replace the complete Package Insert. It
New York State Department of Health - Wadsworth Center Laboratory of Environmental Biology NYS ELAP Laboratory ID 10765
 New York State Department of Health - Wadsworth Center Laboratory of Environmental Biology NYS ELAP Laboratory ID 10765 Division of Environmental Health Sciences Albany, New York NYS DOH LEB-612 Identification
New York State Department of Health - Wadsworth Center Laboratory of Environmental Biology NYS ELAP Laboratory ID 10765 Division of Environmental Health Sciences Albany, New York NYS DOH LEB-612 Identification
E.coli O157:H7 Rapid Test (Card)
 E.coli O157:H7 Rapid Test (Card) Cat. No.:DTS518 Pkg.Size: Intended use The E.coli O157:H7 Rapid Test is a one step coloured chromatographic immunoassay for the qualitative detection of Escherichia coli
E.coli O157:H7 Rapid Test (Card) Cat. No.:DTS518 Pkg.Size: Intended use The E.coli O157:H7 Rapid Test is a one step coloured chromatographic immunoassay for the qualitative detection of Escherichia coli
E. coli O26 Latex Test Kit
 Importance of STEC Determination Shiga toxin-producing Escherichia coli strains (non-o157 STEC) have become an increasing public health concern. Some of the non-o157 STEC possess the same range of virulence
Importance of STEC Determination Shiga toxin-producing Escherichia coli strains (non-o157 STEC) have become an increasing public health concern. Some of the non-o157 STEC possess the same range of virulence
New York State Department of Health - Wadsworth Center Laboratory of Environmental Biology NYS ELAP Laboratory ID 10765
 New York State Department of Health - Wadsworth Center Laboratory of Environmental Biology NYS ELAP Laboratory ID 10765 Division of Environmental Health Sciences Albany, New York NYS DOH LEB-608 Identification
New York State Department of Health - Wadsworth Center Laboratory of Environmental Biology NYS ELAP Laboratory ID 10765 Division of Environmental Health Sciences Albany, New York NYS DOH LEB-608 Identification
Influenza A IgG ELISA
 Influenza A IgG ELISA For the quantitative determination of IgG-class antibodies against Influenza A virus in human serum or plasma (citrate). For Research use Only. Not For Use In Diagnostic Procedures.
Influenza A IgG ELISA For the quantitative determination of IgG-class antibodies against Influenza A virus in human serum or plasma (citrate). For Research use Only. Not For Use In Diagnostic Procedures.
New York State Department of Health - Wadsworth Center Laboratory of Environmental Biology NYS ELAP Laboratory ID 10765
 New York State Department of Health - Wadsworth Center Laboratory of Environmental Biology NYS ELAP Laboratory ID 10765 Division of Environmental Health Sciences Albany, New York NYS DOH LEB-610 Identification
New York State Department of Health - Wadsworth Center Laboratory of Environmental Biology NYS ELAP Laboratory ID 10765 Division of Environmental Health Sciences Albany, New York NYS DOH LEB-610 Identification
Human CoxV-A16 ELISA Kit
 Human CoxV-A16 ELISA Kit For the quantitative in vitro determination of Human Coxsackie virus A16 concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR LABORATORY RESEARCH
Human CoxV-A16 ELISA Kit For the quantitative in vitro determination of Human Coxsackie virus A16 concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR LABORATORY RESEARCH
Clostridium difficile GDH ELISA
 Clostridium difficile GDH ELISA For the qualitative determination of C. difficile glutamate dehydrogenase in human stool. For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number: Size:
Clostridium difficile GDH ELISA For the qualitative determination of C. difficile glutamate dehydrogenase in human stool. For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number: Size:
Accutest ValuPak Immunological Fecal Occult Blood Test
 INTENDED USE The Accutest ValuPak Immunological Fecal Occult Blood (IFOB) Test is a rapid chromatographic immunoassay for the qualitative detection of human occult blood in human fecal specimens. The device
INTENDED USE The Accutest ValuPak Immunological Fecal Occult Blood (IFOB) Test is a rapid chromatographic immunoassay for the qualitative detection of human occult blood in human fecal specimens. The device
FOR IN VITRO DIAGNOSTIC USE
 Ampoules of frozen cells for use in the preparation of Cultured Cell Flasks and Multi-well Plates FOR IN VITRO DIAGNOSTIC USE INTENDED USE Diagnostic Hybrids MRC-5 FreshFrozenCells (human embryonic lung
Ampoules of frozen cells for use in the preparation of Cultured Cell Flasks and Multi-well Plates FOR IN VITRO DIAGNOSTIC USE INTENDED USE Diagnostic Hybrids MRC-5 FreshFrozenCells (human embryonic lung
New York State Department of Health - Wadsworth Center Laboratory of Environmental Biology NYS ELAP Laboratory ID 10765
 New York State Department of Health - Wadsworth Center Laboratory of Environmental Biology NYS ELAP Laboratory ID 10765 Division of Environmental Health Sciences Albany, New York NYS DOH LEB-607 Identification
New York State Department of Health - Wadsworth Center Laboratory of Environmental Biology NYS ELAP Laboratory ID 10765 Division of Environmental Health Sciences Albany, New York NYS DOH LEB-607 Identification
Herpes Simplex 1 IgM (HSV1 IgM)
 DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
Procine CRP ELISA Kit
 Procine CRP ELISA Kit For the quantitative in vitro determination of Procine C-Reactive Protein concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR LABORATORY RESEARCH
Procine CRP ELISA Kit For the quantitative in vitro determination of Procine C-Reactive Protein concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR LABORATORY RESEARCH
REAGENTS AND MATERIALS SUPPLIED
 CLIA Complexity: Waived INTENDED USE The Clearview RSV test is intended for the rapid, qualitative detection of respiratory syncytial virus fusion protein directly from nasopharyngeal swab, and nasal aspirate
CLIA Complexity: Waived INTENDED USE The Clearview RSV test is intended for the rapid, qualitative detection of respiratory syncytial virus fusion protein directly from nasopharyngeal swab, and nasal aspirate
Guinea pig PGI2 ELISA Kit
 Guinea pig PGI2 ELISA Kit For the quantitative in vitro determination of Guinea pig PGI2 concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR LABORATORY RESEARCH USE ONLY.
Guinea pig PGI2 ELISA Kit For the quantitative in vitro determination of Guinea pig PGI2 concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR LABORATORY RESEARCH USE ONLY.
Canine PHA ELISA Kit
 Canine PHA ELISA Kit For the quantitative in vitro determination of Canine Phytohaemagglutinin concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR LABORATORY RESEARCH USE
Canine PHA ELISA Kit For the quantitative in vitro determination of Canine Phytohaemagglutinin concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR LABORATORY RESEARCH USE
Human LENK ELISA Kit
 Human LENK ELISA Kit For the quantitative in vitro determination of Human Leu-enkephalin concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR LABORATORY RESEARCH USE ONLY.
Human LENK ELISA Kit For the quantitative in vitro determination of Human Leu-enkephalin concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR LABORATORY RESEARCH USE ONLY.
Mouse RF IgM ELISA Kit
 Mouse RF IgM ELISA Kit For the quantitative in vitro determination of Mouse rheumatoid factor IgM concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR LABORATORY RESEARCH
Mouse RF IgM ELISA Kit For the quantitative in vitro determination of Mouse rheumatoid factor IgM concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR LABORATORY RESEARCH
Chicken VEGF ELISA Kit
 Chicken VEGF ELISA Kit For the quantitative in vitro determination of Chicken Vascular Endothelial cell Growth Factor concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR
Chicken VEGF ELISA Kit For the quantitative in vitro determination of Chicken Vascular Endothelial cell Growth Factor concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR
Bovine IgG-Ab ELISA Kit
 Bovine IgG-Ab ELISA Kit For the quantitative in vitro determination of Bovine Immunoglobulin G Antibody concentrations in serum - plasma - tissue homogenates - other biological fluids FOR LABORATORY RESEARCH
Bovine IgG-Ab ELISA Kit For the quantitative in vitro determination of Bovine Immunoglobulin G Antibody concentrations in serum - plasma - tissue homogenates - other biological fluids FOR LABORATORY RESEARCH
Guinea pig TIMP-1 ELISA Kit
 Guinea pig TIMP-1 ELISA Kit For the quantitative in vitro determination of Guinea pig tissue inhibitors of metalloproteinase 1 concentrations in serum - plasma - celiac fluid - tissue homogenate - body
Guinea pig TIMP-1 ELISA Kit For the quantitative in vitro determination of Guinea pig tissue inhibitors of metalloproteinase 1 concentrations in serum - plasma - celiac fluid - tissue homogenate - body
Rat GLO ELISA Kit. For the quantitative in vitro determination of Rat Glyoxalase concentrations in
 Rat GLO ELISA Kit For the quantitative in vitro determination of Rat Glyoxalase concentrations in serum - plasma - tissue homogenates - other biological fluids FOR LABORATORY RESEARCH USE ONLY. NOT FOR
Rat GLO ELISA Kit For the quantitative in vitro determination of Rat Glyoxalase concentrations in serum - plasma - tissue homogenates - other biological fluids FOR LABORATORY RESEARCH USE ONLY. NOT FOR
HBeAg and HBeAg Ab ELISA Kit
 HBeAg and HBeAg Ab ELISA Kit Catalog Number KA0290 96 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 Principle of the Assay... 3 General
HBeAg and HBeAg Ab ELISA Kit Catalog Number KA0290 96 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 Principle of the Assay... 3 General
Guinea pig Hyp ELISA Kit
 Guinea pig Hyp ELISA Kit For the quantitative in vitro determination of Guinea pig hydroxyproline concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR LABORATORY RESEARCH
Guinea pig Hyp ELISA Kit For the quantitative in vitro determination of Guinea pig hydroxyproline concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR LABORATORY RESEARCH
Strep Assay. Frequently Asked Questions
 Strep Assay Frequently Asked Questions General What is the sensitivity/specificity of the Lyra Direct Strep Assay? Performance characteristics of the Lyra Direct Strep Assay using the Applied Biosystems
Strep Assay Frequently Asked Questions General What is the sensitivity/specificity of the Lyra Direct Strep Assay? Performance characteristics of the Lyra Direct Strep Assay using the Applied Biosystems
Human CTRP5 ELISA Kit
 Human CTRP5 ELISA Kit For the quantitative in vitro determination of Human C1q And Tumor Necrosis Factor Related Protein 5 concentrations in serum - plasma - tissue homogenates - other biological fluids
Human CTRP5 ELISA Kit For the quantitative in vitro determination of Human C1q And Tumor Necrosis Factor Related Protein 5 concentrations in serum - plasma - tissue homogenates - other biological fluids
Luteinizing Hormone ELISA kit
 55R-IB19104 Luteinizing Hormone ELISA kit Enzyme Immunoassay for the determination of LH in human serum 1. Principle of the test: The LH ELISA kit is a solid phase direct sandwich method. The samples and
55R-IB19104 Luteinizing Hormone ELISA kit Enzyme Immunoassay for the determination of LH in human serum 1. Principle of the test: The LH ELISA kit is a solid phase direct sandwich method. The samples and
Monkey hs-tnt ELISA Kit
 Monkey hs-tnt ELISA Kit For the quantitative in vitro determination of Monkey Hypersensitive troponin T concentrations in serum - plasma - tissue homogenates - other biological fluids FOR LABORATORY RESEARCH
Monkey hs-tnt ELISA Kit For the quantitative in vitro determination of Monkey Hypersensitive troponin T concentrations in serum - plasma - tissue homogenates - other biological fluids FOR LABORATORY RESEARCH
Simplexa EBV Quantitation Standards
 Simplexa EBV Quantitation Standards REF MOL2410 Rev. C Simplexa EBV Quantitation Standards are intended to establish a standard curve with the Simplexa EBV assay on the 3M Integrated Cycler. For in vitro
Simplexa EBV Quantitation Standards REF MOL2410 Rev. C Simplexa EBV Quantitation Standards are intended to establish a standard curve with the Simplexa EBV assay on the 3M Integrated Cycler. For in vitro
Guinea pig 25-OH-VD ELISA Kit
 Guinea pig 25-OH-VD ELISA Kit For the quantitative in vitro determination of Guinea pig 25-Dihydroxy vitamin D concentrations in serum - plasma - tissue homogenates - other biological fluids FOR LABORATORY
Guinea pig 25-OH-VD ELISA Kit For the quantitative in vitro determination of Guinea pig 25-Dihydroxy vitamin D concentrations in serum - plasma - tissue homogenates - other biological fluids FOR LABORATORY
Contamination Prevention and Decontamination
 Contamination Prevention and Decontamination Introduction FilmArray is an automated in vitro diagnostic (IVD) system that utilizes nested multiplex PCR (nmpcr) and high-resolution melting analysis to detect
Contamination Prevention and Decontamination Introduction FilmArray is an automated in vitro diagnostic (IVD) system that utilizes nested multiplex PCR (nmpcr) and high-resolution melting analysis to detect
AmpliSens N.meningitidis / H.influenzae / S.pneumoniae-FRT
 For Professional Use Only AmpliSens N.meningitidis / H.influenzae / S.pneumoniae-FRT PCR kit Instruction Manual AmpliSens Ecoli s.r.o., Studenohorska 12 841 03 Bratislava 47 Slovak Republic Tel.: +421
For Professional Use Only AmpliSens N.meningitidis / H.influenzae / S.pneumoniae-FRT PCR kit Instruction Manual AmpliSens Ecoli s.r.o., Studenohorska 12 841 03 Bratislava 47 Slovak Republic Tel.: +421
PROCEDURE SUMMARY NOTE: Read full instructions for use prior to performing testing.
 A set of reagents used to detect the depletion of Complement protein C3 in various animal species. PROCEDURE SUMMARY NOTE: Read full instructions for use prior to performing testing. MicroVue Pan-Specific
A set of reagents used to detect the depletion of Complement protein C3 in various animal species. PROCEDURE SUMMARY NOTE: Read full instructions for use prior to performing testing. MicroVue Pan-Specific
Human Herpes Simplex Virus Type 1 (HSV-1) IgM ELISA Kit
 Human Herpes Simplex Virus Type 1 Catalog No: IRAPKT1412 (HSV-1) IgM ELISA Kit Lot No: SAMPLE INTENDED USE The HSV-1 IgM ELISA is intended for the detection of IgM antibodies to herpes simplex virus HSV-1.
Human Herpes Simplex Virus Type 1 Catalog No: IRAPKT1412 (HSV-1) IgM ELISA Kit Lot No: SAMPLE INTENDED USE The HSV-1 IgM ELISA is intended for the detection of IgM antibodies to herpes simplex virus HSV-1.
3M Rapid Detection RSV Test
 3M Rapid Detection RSV Test IVD FOR IN VITRO DIAGNOSTIC USE INTENDED USE The 3M Rapid Detection RSV Test is a qualitative, immunochromatographic test for the detection of Respiratory Syncytial Virus (RSV)
3M Rapid Detection RSV Test IVD FOR IN VITRO DIAGNOSTIC USE INTENDED USE The 3M Rapid Detection RSV Test is a qualitative, immunochromatographic test for the detection of Respiratory Syncytial Virus (RSV)
Ecoli 360 HCP ELISA ELISA Kit for the Determination of Ecoli-HCP in process-derived Samples
 Ecoli 360 HCP ELISA ELISA Kit for the Determination of Ecoli-HCP in process-derived Samples FOR IN VITRO USE ONLY 1 INTENDED USE The supplied Ecoli 360 HCP ELISA kit is a sandwich ELISA to be performed
Ecoli 360 HCP ELISA ELISA Kit for the Determination of Ecoli-HCP in process-derived Samples FOR IN VITRO USE ONLY 1 INTENDED USE The supplied Ecoli 360 HCP ELISA kit is a sandwich ELISA to be performed
Department of Laboratories St. Louis, MO ISSUE DATE: June 2007 REVISION DATE: May 2012 REVIEWED DATE: May 2018 PRINCIPLE:
 Page 1 of 7 PROCEDURE: LEAD ESA LEADCARE II / HEALTHY KIDS EXPRESS ISSUE DATE: June 2007 REVISION DATE: May 2012 REVIEWED DATE: May 2018 PRINCIPLE: The LEADCARE II System relies on electrochemistry and
Page 1 of 7 PROCEDURE: LEAD ESA LEADCARE II / HEALTHY KIDS EXPRESS ISSUE DATE: June 2007 REVISION DATE: May 2012 REVIEWED DATE: May 2018 PRINCIPLE: The LEADCARE II System relies on electrochemistry and
New York State Department of Health - Wadsworth Center Laboratory of Environmental Biology NYS ELAP Laboratory ID 10765
 New York State Department of Health - Wadsworth Center Laboratory of Environmental Biology NYS ELAP Laboratory ID 10765 Division of Environmental Health Sciences Albany, New York NYS DOH LEB-606 Identification
New York State Department of Health - Wadsworth Center Laboratory of Environmental Biology NYS ELAP Laboratory ID 10765 Division of Environmental Health Sciences Albany, New York NYS DOH LEB-606 Identification
Herpes Simplex 1 IgM (HSV1 IgM)
 DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
Human IgG Rubella ELISA Kit
 Human IgG Rubella ELISA Kit Catalog No: IRAPKT2020 Lot No: SAMPLE INTENDED USE The Rubella IgG ELISA is intended for use in evaluating a patient s serologic status to the rubella virus infection. It is
Human IgG Rubella ELISA Kit Catalog No: IRAPKT2020 Lot No: SAMPLE INTENDED USE The Rubella IgG ELISA is intended for use in evaluating a patient s serologic status to the rubella virus infection. It is
PictArray ToRCH ELISA Kit
 TORCH Instructions PictArray ToRCH ELISA Kit Instructions for testing human serum samples on PictArray ToRCH Panel consisting of Toxoplasma gondii, Rubella, Cytomegalovirus (CMV), Herpes Simplex Virus
TORCH Instructions PictArray ToRCH ELISA Kit Instructions for testing human serum samples on PictArray ToRCH Panel consisting of Toxoplasma gondii, Rubella, Cytomegalovirus (CMV), Herpes Simplex Virus
Sofia Strep A+ FIA Safety Data Sheet Revision Date: June 22, Section 1 Product and Company Identification
 Manufacturer Information Quidel Corporation Phone: 1.8.874.1517 Web: quidel.com 1165 McKellar Court Fax: 1.858.453.4338 E-mail: qehs@quidel.com San Diego, CA 92121 Emergency # (24-Hour): 1.866.519.4752
Manufacturer Information Quidel Corporation Phone: 1.8.874.1517 Web: quidel.com 1165 McKellar Court Fax: 1.858.453.4338 E-mail: qehs@quidel.com San Diego, CA 92121 Emergency # (24-Hour): 1.866.519.4752
Protocols for Laboratory Verification of Performance of the BioFire FilmArray Blood Culture Identification (BCID) Panel
 Protocols for Laboratory Verification of Performance of the BioFire FilmArray Blood Culture Identification (BCID) Panel Laboratory Protocols for Use with Microbiologics Helix Elite Molecular Standards
Protocols for Laboratory Verification of Performance of the BioFire FilmArray Blood Culture Identification (BCID) Panel Laboratory Protocols for Use with Microbiologics Helix Elite Molecular Standards
This kit is intended for Research Use Only. Not for use in diagnostic procedures.
 This kit is intended for Research Use Only. Not for use in diagnostic procedures. Introduction The DRG Herpes Simplex Virus Type 1+2 IgG Enzyme Immunoassay Kit provides materials for determination of IgG-class
This kit is intended for Research Use Only. Not for use in diagnostic procedures. Introduction The DRG Herpes Simplex Virus Type 1+2 IgG Enzyme Immunoassay Kit provides materials for determination of IgG-class
RapidChek SELECT Salmonella Test Kit
 RapidChek SELECT Salmonella Test Kit Part #: 7000188-7000197 AOAC Approved Protocols: This test kit s performance was reviewed by AOAC Research Institute and was found to perform to the manufacturer s
RapidChek SELECT Salmonella Test Kit Part #: 7000188-7000197 AOAC Approved Protocols: This test kit s performance was reviewed by AOAC Research Institute and was found to perform to the manufacturer s
College of Physicians and Surgeons of Saskatchewan Laboratory Quality Assurance Program. Policy Manual Edition
 College of Physicians and Surgeons of Saskatchewan Laboratory Quality Assurance Program Policy Manual 2016 Edition LABORATORY QUALITY ASSURANCE POLICY MANUAL SUMMARY OF POLICY MANUAL CHANGES The following
College of Physicians and Surgeons of Saskatchewan Laboratory Quality Assurance Program Policy Manual 2016 Edition LABORATORY QUALITY ASSURANCE POLICY MANUAL SUMMARY OF POLICY MANUAL CHANGES The following
FOR IN VITRO DIAGNOSTIC USE
 For the qualitative detection of Group A β-hemolytic Streptococcus (Streptococcus pyogenes) nucleic acids isolated from throat swab specimens obtained from patients with signs and symptoms of pharyngitis,
For the qualitative detection of Group A β-hemolytic Streptococcus (Streptococcus pyogenes) nucleic acids isolated from throat swab specimens obtained from patients with signs and symptoms of pharyngitis,
Laboratory kit configured for testing liquid nasopharyngeal wash, aspirate and swab in transport media samples. Date Adopted.
 Procedure* BD Veritor System For Rapid Detection of Respiratory Syncytial Virus (RSV) Laboratory kit configured for testing liquid nasopharyngeal wash, aspirate and swab in transport media samples. Prepared
Procedure* BD Veritor System For Rapid Detection of Respiratory Syncytial Virus (RSV) Laboratory kit configured for testing liquid nasopharyngeal wash, aspirate and swab in transport media samples. Prepared
CLIA Complexity: Waived INTENDED USE MATERIALS NOT SUPPLIED SUMMARY AND EXPLANATION WARNINGS AND PRECAUTIONS PRINCIPLE OF THE TEST
 CLIA Complexity: Waived INTENDED USE The RSV test is intended for the rapid, qualitative detection of respiratory syncytial virus fusion protein directly from nasopharyngeal swab, and nasal aspirate specimens
CLIA Complexity: Waived INTENDED USE The RSV test is intended for the rapid, qualitative detection of respiratory syncytial virus fusion protein directly from nasopharyngeal swab, and nasal aspirate specimens
vana, vanb, vanc1, vanc2/3 Open System Reagents for BD MAX
 PI Doc. V1.2 For Laboratory Use Only This product is manufactured and packaged as an Open System Reagent for the BD MAX TM system. It is the responsibility of the end user to determine the analytical performance
PI Doc. V1.2 For Laboratory Use Only This product is manufactured and packaged as an Open System Reagent for the BD MAX TM system. It is the responsibility of the end user to determine the analytical performance
Herpes Simplex Virus Type 1 (HSV-1) IgM ELISA Kit Protocol
 Herpes Simplex Virus Type 1 (HSV-1) IgM ELISA Kit Protocol (Cat. No.:EK-310-93) 330 Beach Road, Burlingame CA Tel: 650-558-8898 Fax: 650-558-1686 E-Mail: info@phoenixpeptide.com www.phoenixpeptide.com
Herpes Simplex Virus Type 1 (HSV-1) IgM ELISA Kit Protocol (Cat. No.:EK-310-93) 330 Beach Road, Burlingame CA Tel: 650-558-8898 Fax: 650-558-1686 E-Mail: info@phoenixpeptide.com www.phoenixpeptide.com
Transport Medium for Storage and Transportation of Respiratory Swabs
 For Professional Use Only Transport Medium for Storage and Transportation of Respiratory Swabs Reagent for sampling, transportation, and storage of upper respiratory tract swabs Instruction Manual AmpliSens
For Professional Use Only Transport Medium for Storage and Transportation of Respiratory Swabs Reagent for sampling, transportation, and storage of upper respiratory tract swabs Instruction Manual AmpliSens
Rapid-VIDITEST E. coli O157:H7
 Rapid-VIDITEST E. coli O157:H7 One Step E.coli O157:H7 Card test. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, 252 50 Vestec, Czech Republic, Tel.: +420 261 090 565, www.vidia.cz
Rapid-VIDITEST E. coli O157:H7 One Step E.coli O157:H7 Card test. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, 252 50 Vestec, Czech Republic, Tel.: +420 261 090 565, www.vidia.cz
Hepatitis C virus Ab ELISA Kit
 Hepatitis C virus Ab ELISA Kit Catalog Number KA0291 96 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Principle of the Assay... 3 General Information...
Hepatitis C virus Ab ELISA Kit Catalog Number KA0291 96 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Principle of the Assay... 3 General Information...
PAB PRINCIPLE. Kit Reorder # ANNUAL REVIEW Reviewed by: Date. Date INTENDED USE
 SYNCHRON CX System(s) Chemistry Information Sheet Copyright 2008 Beckman Coulter, Inc. Prealbumin Kit Reorder # 475106 For In Vitro Diagnostic Use ANNUAL REVIEW Reviewed by: Date Reviewed by: Date PRINCIPLE
SYNCHRON CX System(s) Chemistry Information Sheet Copyright 2008 Beckman Coulter, Inc. Prealbumin Kit Reorder # 475106 For In Vitro Diagnostic Use ANNUAL REVIEW Reviewed by: Date Reviewed by: Date PRINCIPLE
AAG PRINCIPLE SPECIMEN. REF (150 tests) ANNUAL REVIEW Reviewed by: Date. Date INTENDED USE
 IMMAGE Immunochemistry Systems Chemistry Information Sheet Copyright 2010 Beckman Coulter, Inc. Alpha 1 -Acid Glycoprotein REF 447780 (150 tests) For In Vitro Diagnostic Use ANNUAL REVIEW Reviewed by:
IMMAGE Immunochemistry Systems Chemistry Information Sheet Copyright 2010 Beckman Coulter, Inc. Alpha 1 -Acid Glycoprotein REF 447780 (150 tests) For In Vitro Diagnostic Use ANNUAL REVIEW Reviewed by:
FOR IN VITRO DIAGNOSTIC USE
 For the qualitative in vitro diagnostic test for the detection and differentiation of RSV and hmpv viral RNA in nasal and nasopharyngeal swabs from patients with signs and symptoms of respiratory infection.
For the qualitative in vitro diagnostic test for the detection and differentiation of RSV and hmpv viral RNA in nasal and nasopharyngeal swabs from patients with signs and symptoms of respiratory infection.
BBL QualiSwab Quality Control Culture Devices
 Revisions Rev from Rev to ECO # 0299 0704 2996-04 SO 0191-5 Notes: 1. BD Cat. Number Various 2. Blank (Sheet) Size : Length: 11 Width: 8.5 Number of Pages: 4 Number of Sheets: 1 Page Size: Length 8.5 Width
Revisions Rev from Rev to ECO # 0299 0704 2996-04 SO 0191-5 Notes: 1. BD Cat. Number Various 2. Blank (Sheet) Size : Length: 11 Width: 8.5 Number of Pages: 4 Number of Sheets: 1 Page Size: Length 8.5 Width
Product Code: PA-E01 Directions For Use Introduction Intended Use
 TM AllergenControl Peanut Residue Environmental Surface Test Product Code: PA-E01 Directions For Use Introduction The peanut is a ground nut belonging to the legume family. As with other nuts, peanuts
TM AllergenControl Peanut Residue Environmental Surface Test Product Code: PA-E01 Directions For Use Introduction The peanut is a ground nut belonging to the legume family. As with other nuts, peanuts
AmpliSens N.meningitidis / H.influenzae / S.pneumoniae-FRT
 For Professional Use Only AmpliSens N.meningitidis / H.influenzae / S.pneumoniae-FRT PCR kit Instruction Manual AmpliSens Ecoli s.r.o., Studenohorska 12 841 03 Bratislava 47 Slovak Republic Tel.: +421
For Professional Use Only AmpliSens N.meningitidis / H.influenzae / S.pneumoniae-FRT PCR kit Instruction Manual AmpliSens Ecoli s.r.o., Studenohorska 12 841 03 Bratislava 47 Slovak Republic Tel.: +421
Cyfra 21-1 IRMA. Product information Information about other products is available at: Userś Manual DE52100
 Product information Information about other products is available at: www.demeditec.com Userś Manual Cyfra 21-1 IRMA The CYFRA 21.1 IRMA system provides a direct in vitro quantitative determination of
Product information Information about other products is available at: www.demeditec.com Userś Manual Cyfra 21-1 IRMA The CYFRA 21.1 IRMA system provides a direct in vitro quantitative determination of
Sample for use a Guide Revised IDEXX COLILERT -18 TEST METHOD FOR THE SIMULTANEOUS DETECTION OF TOTAL COLIFORMS AND E.
 IDEXX COLILERT -18 TEST METHOD FOR THE SIMULTANEOUS DETECTION OF TOTAL COLIFORMS AND E. COLI IN WATER 1 IDEXX COLILERT -18 TEST METHOD FOR THE SIMULTANEOUS DETECTION OF TOTAL COLIFORMS AND E. COLI IN WATER
IDEXX COLILERT -18 TEST METHOD FOR THE SIMULTANEOUS DETECTION OF TOTAL COLIFORMS AND E. COLI IN WATER 1 IDEXX COLILERT -18 TEST METHOD FOR THE SIMULTANEOUS DETECTION OF TOTAL COLIFORMS AND E. COLI IN WATER
Human IL-6 ELISA Set
 Human IL-6 ELISA Set Catalog No. CDK082B Quantity: 10 x 96 tests PRODUCT SPECIFICATIONS : Specificity: Recognizes both natural and recombinant human IL-6 Range: 6.25 pg / ml - 200 pg / ml Sensitivity:
Human IL-6 ELISA Set Catalog No. CDK082B Quantity: 10 x 96 tests PRODUCT SPECIFICATIONS : Specificity: Recognizes both natural and recombinant human IL-6 Range: 6.25 pg / ml - 200 pg / ml Sensitivity:
1. APPROVED MALARIA RDTS
 1. APPROVED MALARIA RDTS There are many different Rapid Diagnostic Tests available in the market. To assist partners of the Three Diseases Fund in the selection of the appropriate RDT, the Fund Manager
1. APPROVED MALARIA RDTS There are many different Rapid Diagnostic Tests available in the market. To assist partners of the Three Diseases Fund in the selection of the appropriate RDT, the Fund Manager
Actero Salmonella/STEC Enrichment Media Product Information
 Actero Salmonella/STEC Enrichment Media Product Information INTENDED USE: Actero Salmonella/STEC Enrichment Media is a selective medium optimized for an improved enrichment of Salmonella spp. from food
Actero Salmonella/STEC Enrichment Media Product Information INTENDED USE: Actero Salmonella/STEC Enrichment Media is a selective medium optimized for an improved enrichment of Salmonella spp. from food
RealLine HCV Genotype quantitative Str-Format
 Instructions for use REAL TIME PCR DETECTION AND DIFFERENTIATION KIT FOR HEPATITIS C VIRUS GENOTYPES 1, 2 AND 3 RNA WITH QUANTIFICATION Research Use Only (RUO) Str Format VBD0797 48 Tests valid from June
Instructions for use REAL TIME PCR DETECTION AND DIFFERENTIATION KIT FOR HEPATITIS C VIRUS GENOTYPES 1, 2 AND 3 RNA WITH QUANTIFICATION Research Use Only (RUO) Str Format VBD0797 48 Tests valid from June
Helicobacter pylori IgG CLIA Kit
 Helicobacter pylori IgG CLIA Kit Catalog Number KA3644 96 assays Version: 01 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
Helicobacter pylori IgG CLIA Kit Catalog Number KA3644 96 assays Version: 01 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
