CLIA Complexity: MODERATE
|
|
|
- Augustine Matthews
- 6 years ago
- Views:
Transcription
1 CLIA Complexity: MODERATE INTENDED USE The is intended for the rapid detection of Group A Streptococcal antigen directly from throat swabs and beta-hemolytic colonies recovered from culture. This test is intended for use as an aid in the diagnosis of Group A Streptococcal infection. SUMMARY AND EXPLANATION Group A Streptococcus is the most significant cause of pharyngitis. Early diagnosis and treatment of Group A Streptococcal pharyngitis has been shown to reduce the severity of symptoms and serious complications such as rheumatic fever and glomerulonephritis. Conventional culture methods require hours for results. 1 PRINCIPLES OF THE PROCEDURE is a lateral-flow immunoassay, containing a highly specific and sensitive antibody to Group A Strep antigen. To perform the test, a Throat Swab specimen is collected. Antigen is extracted from the Swab specimen with Reagents A and B. The extracted sample is added to the test Cassette. If the sample contains Strep A antigen, a pink vertical line ( ) forms in the Read Result Window. This pink vertical line, together with the pre-printed blue horizontal line ( ), forms a plus sign (+) to indicate a positive result. If Strep A is not present in the sample, the Read Result Window shows only the pre-printed blue horizontal line, forming a minus sign ( ) to indicate a negative result. As the sample continues to move through the test, the Control Window containing Strep A antigen becomes pink. Pink color in the Control Window indicates that the detection antibody is functionally active and is also evidence that the detection part of the test is functioning properly. The appearance of blue color in the Test Complete Window indicates the completion of the test. This occurs approximately 5 minutes after the addition of the extracted sample to the test Cassette. REAGENTS AND MATERIALS SUPPLIED Individually Packaged test Cassettes (25): Rabbit anti-strep A antibody and heat-inactivated Strep A antigen. Extraction Reagent A (1): 4 M Sodium Nitrite Extraction Reagent B (1): 0.2 M Acetic Acid Sterile Throat Swabs (25) Tubes and Tips (25) Positive Control (1): Heat-inactivated Group A Streptococcus, 0.02% sodium azide. Negative Control (1): Heat-inactivated Group C Streptococcus, 0.02% sodium azide. Package Insert (1) Procedure Card (1) Page 1 of 6
2 STORAGE AND STABILITY Store kit at room temperature, 59 F to 86 F (15 C to 30 C). Kit contents are stable until the expiration date printed on the outer box. Do not freeze. WARNINGS AND PRECAUTIONS For in vitro diagnostic use. Do not use kit contents after the expiration date printed on the outside of the kit. Use appropriate precautions in the collection, storage, handling and disposal of patient samples and used kit contents. Use of Nitrile or Latex gloves is recommended when handling patient samples. 2 Dispose of containers and unused contents in accordance with Federal, State, and Local requirements. DO NOT interchange caps among reagents. The test Cassette must remain sealed in the foil pouch until just prior to use. Reagent A contains 27.6% sodium nitrite and may be harmful if ingested, or absorbed. Reagent B contains an acidic solution. If the solution contacts the skin or eye, flush with large volumes of water. Do not use Reagent B if solution is green prior to mixing with Reagent A in the Tube. If this occurs, contact Quidel Technical Support. Testing should be performed in an area with adequate ventilation. For more information, consult the Safety Data Sheet available on quidel.com. To obtain accurate results, you must follow the Package Insert instructions. SPECIMEN COLLECTION AND STORAGE Collect throat swab specimens by standard clinical methods. When swabbing the throat, be careful not to touch the tongue, sides or top of the mouth with the Swab. Rub the Swab on the back of the throat, on the tonsils and in any other area where there is redness, inflammation or pus. Bloody specimens can create an interfering background and can cause an invalid result. Consult reference procedures such as the collection method described by Facklam. 3 Use rayon-tip or dacron-tip swabs with plastic shafts; individually packaged sterile rayon-tipped Swabs are provided in the kit. Do not use calcium alginate, cotton-tip or wooden shafted swabs. Swab specimens should be processed as soon as possible after collection. However, Swabs can be stored in a clean, dry, sealable plastic tube or in 1 ml or less liquid media, such as modified Stuart s Transport Media, for up to 8 hours at room temperature (15 C to 30 C) or 72 hours refrigerated (2 C to 8 C). Do not use charcoal agar or semi-solid transport media. If culture results are also desired, lightly streak the Swab on a 5% sheep blood agar plate before using the QuickVue+ Strep A Test. The culture plate must be streaked prior to running the QuickVue+ test, as the reagents will kill the bacteria on the Swab. Throat swab specimens can also be obtained by dual Swabs or by two Swabs taken in sequence for the culture procedure. CULTURE CONFIRMATION The can be used to confirm the identification of Group A Streptococcus on SBA culture plates. Lightly touch a colony using a sterile Swab. Do not sweep the plate. Follow the instructions in the TEST PROCEDURE section to test the sample. TEST PROCEDURE Important: Gloves should be worn when handling human samples. BEFORE TESTING: Remove the test Cassette from the foil pouch and place it on a level surface. Put a clean Tube in the Tube Well of the test Cassette. Squeeze 4 DROPS of Reagent A and 4 DROPS of Reagent B into the Tube. The solution should turn green once Reagent B is added. Note: when adding drops, hold bottle vertically so that a complete drop forms. Page 2 of 6
3 Tube Well Test Complete Window Control Window Read Result Window Sample Well Immediately place the Throat Swab into the Tube. Mix solution thoroughly by swirling the Swab 5 times (or vortex briefly). Wait 1 minute. Remove the Tube from the Tube Well. Express all liquid from the Swab head by rolling the Swab against the inside of the Tube and squeezing firmly as it is withdrawn from the Tube. Discard the Swab. Put a clean Tip on the Tube. PERFORM TEST Add 2 DROPS from the Tube to the round Sample Well in the test Cassette. For a valid result the test must be read in 10 minutes or less after adding the sample and there must be ANY shade of blue in the Test Complete Window. If there is no shade of blue in the Test Complete Window at 10 minutes, the result is invalid. INTERPRETATION OF RESULTS Positive Results may be read as early as 5 minutes after or as late as 10 minutes after adding the sample. Negative Results at 5 minutes must be confirmed negative at 10 minutes. Positive Result: The sample contains Group A Streptococcus antigen when you see: A pink and blue plus sign (+) in the large square Read Result Window along with a pink color in the small square Control Window AND Any shade of blue color in the Test Complete Window Note: the combination of any shade of a pink vertical line in the Read Result Window and any blue shade in the Complete Result Window should be interpreted as a positive result. Negative Result: The sample does not contain Group A Streptococcus antigen when you see at 10 minutes after adding the sample: A blue minus sign ( ) in the large square Read Result Window along with a pink color in the small square Control Window AND Any shade of blue color in the Test Complete Window Note: A negative QuickVue+ result indicates a presumptive negative test result for the presence of Group A Streptococcal antigen. Page 3 of 6
4 Invalid Result: The result is invalid if: At 10 minutes, no shade of blue appears in the Test Complete Window OR No pink color appears in the Control Window by 10 minutes OR Background color in the Read Result Window interferes with test interpretation at 10 minutes Note: In the case of an invalid result, a new patient sample should be tested using a new, or contact Quidel Technical Support. QC TESTING PROCEDURE Follow the instructions in the TEST PROCEDURE to dispense the Extraction Reagents into the Tube. Vigorously mix the Control Bottles. Add 1 drop of the Negative or Positive Control into the Tube. Place a clean Swab into the Tube and follow the instructions for testing the patient Swab. QUALITY CONTROL Built-in Control Features The provides three levels of internal procedural controls with each test run. For daily quality control, Quidel recommends documenting that these internal controls were checked for the first sample tested each day. Built-in Extraction Reagent Control: The color of the Extraction Reagent changes from clear to green as the reagents are mixed together. The color change is an internal extraction reagent control and is an indication that the reagents were mixed and functioning properly. Built-in Positive Antigen Control: Pink color in the Control Window serves as a built-in positive antigen control. The appearance of this control indicates that the detection antibody is functionally active and is also evidence that the detection part of the test is functioning properly. Built-in Negative Background Control: The background area in the Read Result Window should be white to light pink within 10 minutes and not interfere with the reading of the result. A lack of interfering background serves as a built-in negative background control, indicating that there are no immunological interfering substances in the sample. External Quality Control Testing External controls are provided and may also be used to ensure that the reagents are performing properly and that you are able to correctly perform the test procedure. You may also use controls derived from ATCC strain Some commercial controls may contain interfering additives and are not recommended for use in the QuickVue+ test. Positive and Negative Controls can be run with each shipment of a new kit lot number, and as otherwise required by your laboratory's standard quality control procedures. LIMITATIONS OF THE PROCEDURE The contents of this kit are for use in the qualitative detection of Group A Streptococcal antigen from Throat Swabs and culture colonies only. Respiratory infections, including pharyngitis, can be caused by Streptococcus from serogroups other than Group A as well as other pathogens. The QuickVue+ test will not differentiate asymptomatic carriers of Group A Streptococcus from those exhibiting Group A Streptococcal infection. In rare cases, test specimens heavily colonized with Staphylococcus aureus can yield false positive results. Test results must always be evaluated with other data available to the physician. A negative test result might occur if the level of extracted antigen in a sample is below the detection level of the test. Additional follow-up testing using the culture method is recommended if the QuickVue+ test result is negative. 4 Page 4 of 6
5 EXPECTED VALUES Group A Streptococci cause about 19% of all upper respiratory tract infections. Streptococcal pharyngitis is seasonal in nature with the highest prevalence found during the winter and early spring. The highest incidence of this disease is found in crowded populations such as military bases and in school-aged children, and is evenly distributed between males and females. 5 PERFORMANCE CHARACTERISTICS Clinical Sensitivity and Specificity A multi-center evaluation of the was conducted to determine the clinical performance of the test relative to standard sheep blood agar (SBA) culture techniques. Throat swab specimens were collected from patients presenting with pharyngitis. Prior to performance of the QuickVue+ test, each swab specimen was inoculated onto a SBA culture plate containing a bacitracin disk and incubated at 37 C anaerobically for hours for evaluation. All cultures were confirmed for the presence of Group A Streptococcus using commercial latex agglutination assays. Of the seven hundred nineteen (719) specimens that were found negative by SBA culture, seven hundred seven (707) were also negative by the QuickVue+ test. Similarly, of the one hundred fourteen (114) specimens that were confirmed SBA culture positive, one hundred eight (108) were also positive by the QuickVue+ test. The QuickVue+ test correctly identified 100% (5/5) of the 1+ cultures; 95% (20/21) of the 2+ cultures; 100% (31/31) of the 3+ cultures; and 98% (52/53) of the 4+ cultures. The 4 culture positive specimens with less than 10 colonies (rares) were not positive by the QuickVue+ test. Based on this data, specificity was 98% and sensitivity was 95% for the QuickVue+ test. 95% confidence intervals were calculated to be 97%-99% and 91%-99% for specificity and sensitivity, respectively. Overall agreement between SBA culture and QuickVue+ Strep A was 98% (815/833). In addition, the was used to confirm the identification of Group A Streptococcus on SBA culture plates. As a culture confirmation, the QuickVue+ test was 100% sensitive and 100% specific. Physicians' Office Laboratory (POL) Studies An additional evaluation of the QuickVue+ test was conducted at three physicians offices using a panel of coded samples. Testing was performed by physician s office personnel with diverse educational backgrounds and work experience. The panel contained negative, low positive and moderate positive samples. Each sample level was tested in sets of five at each site over a period of 3 days. The results obtained at each site ranged from 97%-100% agreement with the expected results. No significant differences were observed within run, between runs or between sites. Cross-Reactivity Cross-reactivity studies with 53 microorganism strains other than Group A Streptococcus have been performed at levels exceeding 10 7 and produced negative results in the QuickVue+ test. ASSISTANCE If you have any questions regarding the use of this product, please call Quidel s Technical Support Number (in the U.S.) or , Monday through Friday, from 7:00 a.m. to 5:00 p.m., Pacific Time. If outside the U.S., contact your local distributor or technicalsupport@quidel.com. REFERENCES 1. Ruoff K. Streptococcus. In: Manual of Clinical Microbiology, Murray P.R., ed. 6th Edition, 1995, 23: Biosafety in Microbiological and Biomedical Laboratories, 4th Edition. U.S. Department of Health and Human Services, CDC, NIH, Washington, D.C. (1999). 3. Facklam R.R. and Washington II, J.A. Streptococcus and Related Catalase-Negative Gram-Positive Cocci. In: Manual of Clinical Microbiology, Balows A., ed. 5th Edition, 1991, 29: American Academy of Pediatrics. Group A Streptococcal Infections. In: 1994 Redbook: Report of the Committee on Infectious Diseases. Peter G. ed. 23rd Edition, Elk Grove, IL, 1994, p Wannamaker L.W. New England Journal of Medicine, 1970, 282: Page 5 of 6
6 20122 QuickVue+ Strep A 25 Test Kit 20122IN QuickVue+ Strep A 25 Test Kit MDSS GmbH Schiffgraben Hannover, Germany Quidel Corporation McKellar Court San Diego, CA USA quidel.com EN00 (01/15) Catalogue number CE mark of conformity Authorized Representative in the European Community Batch code Use by Manufacturer Temperature limitation Intended use Consult instructions for use For In Vitro diagnostic use Contains sufficient for 25 determinations Contents/Contains Positive Control Negative Control Page 6 of 6
PROCEDURE MANUAL. Prepared By Date Adopted Supersedes Procedure # Review Date Revision Date Signature
 Procedure: CLIA Complexity: Moderate Procedure #: Prepared By Date Adopted Supersedes Procedure # Review Date Revision Date Signature Distributed to # of Copies Distributed to # of Copies This Procedural
Procedure: CLIA Complexity: Moderate Procedure #: Prepared By Date Adopted Supersedes Procedure # Review Date Revision Date Signature Distributed to # of Copies Distributed to # of Copies This Procedural
PROCEDURE MANUAL. Prepared By Date Adopted Supersedes Procedure # Review Date Revision Date Signature
 Procedure: CLIA Complexity: Waived Procedure #: Prepared By Date Adopted Supersedes Procedure # Review Date Revision Date Signature Distributed to # of Copies Distributed to # of Copies This Procedural
Procedure: CLIA Complexity: Waived Procedure #: Prepared By Date Adopted Supersedes Procedure # Review Date Revision Date Signature Distributed to # of Copies Distributed to # of Copies This Procedural
SUMMARY AND EXPLANATION
 This Procedural Bulletin is intended to provide a ready outline reference for performance of the assay. These abbreviated directions for use are not intended to replace the complete package insert. It
This Procedural Bulletin is intended to provide a ready outline reference for performance of the assay. These abbreviated directions for use are not intended to replace the complete package insert. It
Department Of Pathology Point of Care Testing POC Quick Vue In-Line Strep A Version#4
 Department Of Pathology Point of Care Testing POC.014.04 Quick Vue In-Line Strep A Version#4 Printed copies are for reference only. Please refer to the electronic copy for the latest version. A. Purpose:
Department Of Pathology Point of Care Testing POC.014.04 Quick Vue In-Line Strep A Version#4 Printed copies are for reference only. Please refer to the electronic copy for the latest version. A. Purpose:
SAMPLE PROCEDURE 928-8, 07/11
 SAMPLE PROCEDURE This Sample Procedure is not intended as a substitute for your facility s Procedure Manual or reagent labeling, but rather as a model for your use in customizing for your laboratory s
SAMPLE PROCEDURE This Sample Procedure is not intended as a substitute for your facility s Procedure Manual or reagent labeling, but rather as a model for your use in customizing for your laboratory s
REAGENTS AND MATERIALS SUPPLIED Control 2: 25-OH vitamin D in human serum with <0.1% Streptomycin Sulfate (2)
 For use with the Sofia Quantitative Vitamin D FIA test system only For in vitro diagnostic use INTENDED USE The Sofia Quantitative Vitamin D Control Set is intended for use as assayed quality control materials
For use with the Sofia Quantitative Vitamin D FIA test system only For in vitro diagnostic use INTENDED USE The Sofia Quantitative Vitamin D Control Set is intended for use as assayed quality control materials
CLIA Complexity: Waived for Finger-stick Whole Blood. Instructional video available online at quidel.com/sofia2lyme.
 Study the Package Insert and User Manual thoroughly before using Quick Reference Instructions. This is not a complete Package Insert. CLIA Complexity: Waived for Finger-stick Whole Blood Instructional
Study the Package Insert and User Manual thoroughly before using Quick Reference Instructions. This is not a complete Package Insert. CLIA Complexity: Waived for Finger-stick Whole Blood Instructional
Strep A Test. Rev ,02/17
 Strep A Test Rev. 3812-6,02/17 Strep A Test CLIA Complexity: Waived 23-200-276 INTENDED USE The Sure-Vue Signature Strep A Test is a color immunochromatographic assay intended for the qualitative detection
Strep A Test Rev. 3812-6,02/17 Strep A Test CLIA Complexity: Waived 23-200-276 INTENDED USE The Sure-Vue Signature Strep A Test is a color immunochromatographic assay intended for the qualitative detection
INVALID. Rev /15 POSITIVE NEGATIVE. Ultra Strep A Test. 2 min 5 min. 10x. 3 drops 3 drops
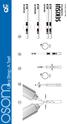 1 3 drops 3 drops Ultra Strep A Test 2 3 4 5 10x 2 min 5 min POSITIVE NEGATIVE INVALID Rev. 3101-2 06/15 Ultra Strep A Test CLIA Complexity: Waived INTENDED USE The OSOM Ultra Strep A Test is a color immunochromatographic
1 3 drops 3 drops Ultra Strep A Test 2 3 4 5 10x 2 min 5 min POSITIVE NEGATIVE INVALID Rev. 3101-2 06/15 Ultra Strep A Test CLIA Complexity: Waived INTENDED USE The OSOM Ultra Strep A Test is a color immunochromatographic
QUICK REFERENCE INSTRUCTIONS For use with Sofia and Sofia 2. Rx only
 QUICK REFERENCE INSTRUCTIONS For use with Sofia and Sofia 2. Rx only Test Procedure Study the Package Insert and User Manual thoroughly before using Quick Reference Instructions. This is not a complete
QUICK REFERENCE INSTRUCTIONS For use with Sofia and Sofia 2. Rx only Test Procedure Study the Package Insert and User Manual thoroughly before using Quick Reference Instructions. This is not a complete
SAMPLE PROCEDURE , 11/07
 SAMPLE PROCEDURE This Sample Procedure is not intended as a substitute for your facility s Procedure Manual or reagent labeling, but rather as a model for your use in customizing for your laboratory s
SAMPLE PROCEDURE This Sample Procedure is not intended as a substitute for your facility s Procedure Manual or reagent labeling, but rather as a model for your use in customizing for your laboratory s
Rapid-VIDITEST RSV. One step RSV Blister test for the detection of Respiratory Syncytial Virus from nasal specimens. Instruction manual
 Rapid-VIDITEST RSV One step RSV Blister test for the detection of Respiratory Syncytial Virus from nasal specimens. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, Vestec, 252 42 Jesenice,
Rapid-VIDITEST RSV One step RSV Blister test for the detection of Respiratory Syncytial Virus from nasal specimens. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, Vestec, 252 42 Jesenice,
QUICK REFERENCE INSTRUCTIONS For use with Sofia and Sofia 2.
 QUICK REFERENCE INSTRUCTIONS For use with Sofia and Sofia 2. Study the Package Insert and User Manual thoroughly before using Quick Reference Instructions. This is not a complete Package Insert. CLIA Complexity:
QUICK REFERENCE INSTRUCTIONS For use with Sofia and Sofia 2. Study the Package Insert and User Manual thoroughly before using Quick Reference Instructions. This is not a complete Package Insert. CLIA Complexity:
QuickVue In-Line Strep A Test Safety Data Sheet Revision Date: April 27, Section 1 Product and Company Identification
 Manufacturer Information Quidel Corporation Phone: 1.800.874.1517 Web: quidel.com 10165 McKellar Court Fax: 1.858.453.4338 E-mail: qehs@quidel.com San Diego, CA 92121 Emergency # (24-Hour): 1.866.519.4752
Manufacturer Information Quidel Corporation Phone: 1.800.874.1517 Web: quidel.com 10165 McKellar Court Fax: 1.858.453.4338 E-mail: qehs@quidel.com San Diego, CA 92121 Emergency # (24-Hour): 1.866.519.4752
E. coli O26 Latex Test Kit
 Importance of STEC Determination Shiga toxin-producing Escherichia coli strains (non-o157 STEC) have become an increasing public health concern. Some of the non-o157 STEC possess the same range of virulence
Importance of STEC Determination Shiga toxin-producing Escherichia coli strains (non-o157 STEC) have become an increasing public health concern. Some of the non-o157 STEC possess the same range of virulence
BD GeneOhm MRSA ACP Lysis Kit
 REF 441638 100 Tests DOPS09-09-V1E1 USA Date: 2010-01 TABLE OF CONTENTS INTENDED USE...3 SUMMARY AND EXPLANATION OF THE PROCEDURE...3 REAGENTS...3 PRECAUTIONS...3 MATERIALS PROVIDED...4 STORAGE, HANDLING
REF 441638 100 Tests DOPS09-09-V1E1 USA Date: 2010-01 TABLE OF CONTENTS INTENDED USE...3 SUMMARY AND EXPLANATION OF THE PROCEDURE...3 REAGENTS...3 PRECAUTIONS...3 MATERIALS PROVIDED...4 STORAGE, HANDLING
Rapid-VIDITEST. RSV Card. One step RSV Card test for the detection of Respiratory Syncytial Virus from nasal specimens. Instruction manual
 Rapid-VIDITEST RSV Card One step RSV Card test for the detection of Respiratory Syncytial Virus from nasal specimens. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, Vestec, 252 42
Rapid-VIDITEST RSV Card One step RSV Card test for the detection of Respiratory Syncytial Virus from nasal specimens. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, Vestec, 252 42
HBeAg and HBeAg Ab ELISA Kit
 HBeAg and HBeAg Ab ELISA Kit Catalog Number KA0290 96 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 Principle of the Assay... 3 General
HBeAg and HBeAg Ab ELISA Kit Catalog Number KA0290 96 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 Principle of the Assay... 3 General
Luteinizing Hormone ELISA kit
 55R-IB19104 Luteinizing Hormone ELISA kit Enzyme Immunoassay for the determination of LH in human serum 1. Principle of the test: The LH ELISA kit is a solid phase direct sandwich method. The samples and
55R-IB19104 Luteinizing Hormone ELISA kit Enzyme Immunoassay for the determination of LH in human serum 1. Principle of the test: The LH ELISA kit is a solid phase direct sandwich method. The samples and
Hepatitis C virus Ab ELISA Kit
 Hepatitis C virus Ab ELISA Kit Catalog Number KA0291 96 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Principle of the Assay... 3 General Information...
Hepatitis C virus Ab ELISA Kit Catalog Number KA0291 96 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Principle of the Assay... 3 General Information...
Strep Assay. Frequently Asked Questions
 Strep Assay Frequently Asked Questions General What is the sensitivity/specificity of the Lyra Direct Strep Assay? Performance characteristics of the Lyra Direct Strep Assay using the Applied Biosystems
Strep Assay Frequently Asked Questions General What is the sensitivity/specificity of the Lyra Direct Strep Assay? Performance characteristics of the Lyra Direct Strep Assay using the Applied Biosystems
Human IL-6 ELISA Set
 Human IL-6 ELISA Set Catalog No. CDK082B Quantity: 10 x 96 tests PRODUCT SPECIFICATIONS : Specificity: Recognizes both natural and recombinant human IL-6 Range: 6.25 pg / ml - 200 pg / ml Sensitivity:
Human IL-6 ELISA Set Catalog No. CDK082B Quantity: 10 x 96 tests PRODUCT SPECIFICATIONS : Specificity: Recognizes both natural and recombinant human IL-6 Range: 6.25 pg / ml - 200 pg / ml Sensitivity:
1. APPROVED MALARIA RDTS
 1. APPROVED MALARIA RDTS There are many different Rapid Diagnostic Tests available in the market. To assist partners of the Three Diseases Fund in the selection of the appropriate RDT, the Fund Manager
1. APPROVED MALARIA RDTS There are many different Rapid Diagnostic Tests available in the market. To assist partners of the Three Diseases Fund in the selection of the appropriate RDT, the Fund Manager
Accutest ValuPak Immunological Fecal Occult Blood Test
 INTENDED USE The Accutest ValuPak Immunological Fecal Occult Blood (IFOB) Test is a rapid chromatographic immunoassay for the qualitative detection of human occult blood in human fecal specimens. The device
INTENDED USE The Accutest ValuPak Immunological Fecal Occult Blood (IFOB) Test is a rapid chromatographic immunoassay for the qualitative detection of human occult blood in human fecal specimens. The device
Sofia Strep A+ FIA Safety Data Sheet Revision Date: June 22, Section 1 Product and Company Identification
 Manufacturer Information Quidel Corporation Phone: 1.8.874.1517 Web: quidel.com 1165 McKellar Court Fax: 1.858.453.4338 E-mail: qehs@quidel.com San Diego, CA 92121 Emergency # (24-Hour): 1.866.519.4752
Manufacturer Information Quidel Corporation Phone: 1.8.874.1517 Web: quidel.com 1165 McKellar Court Fax: 1.858.453.4338 E-mail: qehs@quidel.com San Diego, CA 92121 Emergency # (24-Hour): 1.866.519.4752
Transport Medium for Storage and Transportation of Respiratory Swabs
 For Professional Use Only Transport Medium for Storage and Transportation of Respiratory Swabs Reagent for sampling, transportation, and storage of upper respiratory tract swabs Instruction Manual AmpliSens
For Professional Use Only Transport Medium for Storage and Transportation of Respiratory Swabs Reagent for sampling, transportation, and storage of upper respiratory tract swabs Instruction Manual AmpliSens
PROCEDURE SUMMARY NOTE: Read full instructions for use prior to performing testing.
 A set of reagents used to detect the depletion of Complement protein C3 in various animal species. PROCEDURE SUMMARY NOTE: Read full instructions for use prior to performing testing. MicroVue Pan-Specific
A set of reagents used to detect the depletion of Complement protein C3 in various animal species. PROCEDURE SUMMARY NOTE: Read full instructions for use prior to performing testing. MicroVue Pan-Specific
Human IL-1 alpha ELISA Kit
 Human IL-1 alpha ELISA Kit Catalog No: CDK025B Quantity: 2 x 96 tests SPECIFICITY : RANGE : SENSITIVITY : INCUBATION : SAMPLE TYPES : Recognizes both natural and recombinant human IL-1 alpha 31.2 pg /
Human IL-1 alpha ELISA Kit Catalog No: CDK025B Quantity: 2 x 96 tests SPECIFICITY : RANGE : SENSITIVITY : INCUBATION : SAMPLE TYPES : Recognizes both natural and recombinant human IL-1 alpha 31.2 pg /
FOR IN VITRO DIAGNOSTIC USE
 Ampoules of frozen cells for use in the preparation of Cultured Cell Flasks and Multi-well Plates FOR IN VITRO DIAGNOSTIC USE INTENDED USE Diagnostic Hybrids MRC-5 FreshFrozenCells (human embryonic lung
Ampoules of frozen cells for use in the preparation of Cultured Cell Flasks and Multi-well Plates FOR IN VITRO DIAGNOSTIC USE INTENDED USE Diagnostic Hybrids MRC-5 FreshFrozenCells (human embryonic lung
NATIONAL FOOD SAFETY FOUNDATION
 NATIONAL FOOD SAFETY FOUNDATION RAPID TESTING KITS DETECTION E.COLI 0157 IN FOOD MATICES AND LIQUIDS USING E.COLI RAPID TESTING KIT INTRODUCTION E. coli serotype O157 is a gram-negative rod-shaped bacterium.
NATIONAL FOOD SAFETY FOUNDATION RAPID TESTING KITS DETECTION E.COLI 0157 IN FOOD MATICES AND LIQUIDS USING E.COLI RAPID TESTING KIT INTRODUCTION E. coli serotype O157 is a gram-negative rod-shaped bacterium.
INSTRUCTIONS FOR USE LYFO DISK KWIK-STIK KWIK-STIK PLUS. n LYFO DISK Microorganisms n KWIK-STIK Microorganisms n KWIK-STIK Plus Microorganisms
 INSTRUCTIONS FOR USE n LYFO DISK Microorganisms n KWIK-STIK Microorganisms n KWIK-STIK Plus Microorganisms LYFO DISK INTENDED USE LYFO DISK, KWIK-STIK and KWIK-STIK Plus Microorganisms are lyophilized,
INSTRUCTIONS FOR USE n LYFO DISK Microorganisms n KWIK-STIK Microorganisms n KWIK-STIK Plus Microorganisms LYFO DISK INTENDED USE LYFO DISK, KWIK-STIK and KWIK-STIK Plus Microorganisms are lyophilized,
BBL QualiSwab Quality Control Culture Devices
 Revisions Rev from Rev to ECO # 0299 0704 2996-04 SO 0191-5 Notes: 1. BD Cat. Number Various 2. Blank (Sheet) Size : Length: 11 Width: 8.5 Number of Pages: 4 Number of Sheets: 1 Page Size: Length 8.5 Width
Revisions Rev from Rev to ECO # 0299 0704 2996-04 SO 0191-5 Notes: 1. BD Cat. Number Various 2. Blank (Sheet) Size : Length: 11 Width: 8.5 Number of Pages: 4 Number of Sheets: 1 Page Size: Length 8.5 Width
Preparing and Gram-staining a bacteriological smear
 College of Life Sciences and Technology Medical Laboratory Science (Applied Learning) AP 84-205-00 (42) Module 4: Practical 2014-16 Preparing and Gram-staining a bacteriological smear Date Time Class Venue
College of Life Sciences and Technology Medical Laboratory Science (Applied Learning) AP 84-205-00 (42) Module 4: Practical 2014-16 Preparing and Gram-staining a bacteriological smear Date Time Class Venue
Helicobacter pylori IgG CLIA Kit
 Helicobacter pylori IgG CLIA Kit Catalog Number KA3644 96 assays Version: 01 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
Helicobacter pylori IgG CLIA Kit Catalog Number KA3644 96 assays Version: 01 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
QUANTA Lite SS-A ELISA For In Vitro Diagnostic Use CLIA Complexity: High
 Format to CLSI Standards GP2-A5 (Formerly NCCLS) Vol. 26 No. 12 Issue Date: 12/05/11 QUANTA Lite SS-A ELISA 708570 For In Vitro Diagnostic Use CLIA Complexity: High Principles of the Procedure Purified
Format to CLSI Standards GP2-A5 (Formerly NCCLS) Vol. 26 No. 12 Issue Date: 12/05/11 QUANTA Lite SS-A ELISA 708570 For In Vitro Diagnostic Use CLIA Complexity: High Principles of the Procedure Purified
Selective and Enhanced Recovery of Group A and B
 JOURNAL OF CLINICAL MICROBIOLOGY, June 1977, p. 650-655 Copyright C) 1977 American Society for Microbiology Vol. 5, No. 6 Printed in U.S.A. Selective and Enhanced Recovery of Group A and B Streptococci
JOURNAL OF CLINICAL MICROBIOLOGY, June 1977, p. 650-655 Copyright C) 1977 American Society for Microbiology Vol. 5, No. 6 Printed in U.S.A. Selective and Enhanced Recovery of Group A and B Streptococci
ACCESS Immunoassay System. HIV combo QC4 & QC5. For monitoring the system performance of the Access HIV combo assay. B71107A - [EN] /01
![ACCESS Immunoassay System. HIV combo QC4 & QC5. For monitoring the system performance of the Access HIV combo assay. B71107A - [EN] /01 ACCESS Immunoassay System. HIV combo QC4 & QC5. For monitoring the system performance of the Access HIV combo assay. B71107A - [EN] /01](/thumbs/79/78957607.jpg) ACCESS Immunoassay System HIV combo QC4 & QC5 B22822 For monitoring the system performance of the Access HIV combo assay. - [EN] - 2015/01 Table of Contents Access HIV combo QC4 & QC5 1 Intended Use...
ACCESS Immunoassay System HIV combo QC4 & QC5 B22822 For monitoring the system performance of the Access HIV combo assay. - [EN] - 2015/01 Table of Contents Access HIV combo QC4 & QC5 1 Intended Use...
PictArray ToRCH ELISA Kit
 TORCH Instructions PictArray ToRCH ELISA Kit Instructions for testing human serum samples on PictArray ToRCH Panel consisting of Toxoplasma gondii, Rubella, Cytomegalovirus (CMV), Herpes Simplex Virus
TORCH Instructions PictArray ToRCH ELISA Kit Instructions for testing human serum samples on PictArray ToRCH Panel consisting of Toxoplasma gondii, Rubella, Cytomegalovirus (CMV), Herpes Simplex Virus
INSTRUCTIONS FOR USE
 INSTRUCTIONS FOR USE INTENDED USE KWIK-STIK, KWIK-STIK Plus and LYFO DISK microorganisms are intended to be used as controls to verify the performance of assays, reagents or media that are intended to
INSTRUCTIONS FOR USE INTENDED USE KWIK-STIK, KWIK-STIK Plus and LYFO DISK microorganisms are intended to be used as controls to verify the performance of assays, reagents or media that are intended to
E. coli O157 Test Strip Kit Catalog #: 1062
 BIOO FOOD AND FEED SAFETY E. coli O157 Test Strip Kit Catalog #: 1062 BIOO Scientific Corp. 2011 TABLE OF CONTENTS GENERAL INFORMATION... 1 Product Description... 1 Procedure Overview... 1 Kit Contents,
BIOO FOOD AND FEED SAFETY E. coli O157 Test Strip Kit Catalog #: 1062 BIOO Scientific Corp. 2011 TABLE OF CONTENTS GENERAL INFORMATION... 1 Product Description... 1 Procedure Overview... 1 Kit Contents,
Human Collagen Type III (COL3) ELISA
 Human Collagen Type III (COL3) ELISA For the quantitative determination of human COL3 in serum, plasma, cell culture fluid and other biological fluids Cat. No. KT-61018 For Research Use Only. Not for use
Human Collagen Type III (COL3) ELISA For the quantitative determination of human COL3 in serum, plasma, cell culture fluid and other biological fluids Cat. No. KT-61018 For Research Use Only. Not for use
Instructions for use
 Instructions for use SARCOPTES-ELISA 2001 DOG Test to detect the presence of antibodies against the cause of mange of dogs, Sarcoptes scabiei var. canis In-vitro Diagnostic for Dog Contents of the Kit:
Instructions for use SARCOPTES-ELISA 2001 DOG Test to detect the presence of antibodies against the cause of mange of dogs, Sarcoptes scabiei var. canis In-vitro Diagnostic for Dog Contents of the Kit:
Escherichia coli O157:H7 (E.coli O157:H7) ELISA
 Product information User s Manual Escherichia coli O157:H7 (E.coli O157:H7) ELISA Enzyme-linked immune-sorbent assay for the determination of E.coli O157:H7 in food or water. Catalog No.: BE69221 96 Storage:
Product information User s Manual Escherichia coli O157:H7 (E.coli O157:H7) ELISA Enzyme-linked immune-sorbent assay for the determination of E.coli O157:H7 in food or water. Catalog No.: BE69221 96 Storage:
COMMITTEE REPORT. for the full 10 days recommended for bona fide streptococcal infections.' Compared with. many other laboratory diagnostic aids, a
 A Method for Culturing Beta Hemolytic Streptococci from the Throat Downloaded from http://ahajournals.org by on August 20, 2018 EXCEPT in the case of scarlet fever, no completely satisfactory clinical
A Method for Culturing Beta Hemolytic Streptococci from the Throat Downloaded from http://ahajournals.org by on August 20, 2018 EXCEPT in the case of scarlet fever, no completely satisfactory clinical
AAG PRINCIPLE SPECIMEN. REF (150 tests) ANNUAL REVIEW Reviewed by: Date. Date INTENDED USE
 IMMAGE Immunochemistry Systems Chemistry Information Sheet Copyright 2010 Beckman Coulter, Inc. Alpha 1 -Acid Glycoprotein REF 447780 (150 tests) For In Vitro Diagnostic Use ANNUAL REVIEW Reviewed by:
IMMAGE Immunochemistry Systems Chemistry Information Sheet Copyright 2010 Beckman Coulter, Inc. Alpha 1 -Acid Glycoprotein REF 447780 (150 tests) For In Vitro Diagnostic Use ANNUAL REVIEW Reviewed by:
Immunochromatographic test for the qualitative detection of total antibodies against Francisella tularensis in human serum/plasma.
 Tularemia Rapid Test Cat. No.:DTS745 Pkg.Size:10 Tests Intended use Immunochromatographic test for the qualitative detection of total antibodies against Francisella tularensis in human serum/plasma. Kit
Tularemia Rapid Test Cat. No.:DTS745 Pkg.Size:10 Tests Intended use Immunochromatographic test for the qualitative detection of total antibodies against Francisella tularensis in human serum/plasma. Kit
Clostridium difficile GDH ELISA
 Clostridium difficile GDH ELISA For the qualitative determination of C. difficile glutamate dehydrogenase in human stool. For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number: Size:
Clostridium difficile GDH ELISA For the qualitative determination of C. difficile glutamate dehydrogenase in human stool. For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number: Size:
Mouse Peptide YY (PYY) ELISA
 Mouse Peptide YY (PYY) ELISA For the quantitative determination of mouse PYY in serum, plasma, cell culture fluid and other biological fluids Cat. No. KT-58705 For Research Use Only. Not for use in diagnostic
Mouse Peptide YY (PYY) ELISA For the quantitative determination of mouse PYY in serum, plasma, cell culture fluid and other biological fluids Cat. No. KT-58705 For Research Use Only. Not for use in diagnostic
RUO. Rubella IgG EIA Test Kit Catalog Number:
 An enzyme immunoassay (EIA) for the qualitative and quantitative detection of IgG antibodies to Rubella in human serum or plasma. For professional in vitro diagnostic use only. SUMMARY The is an immunoassay
An enzyme immunoassay (EIA) for the qualitative and quantitative detection of IgG antibodies to Rubella in human serum or plasma. For professional in vitro diagnostic use only. SUMMARY The is an immunoassay
Simplexa EBV Quantitation Standards
 Simplexa EBV Quantitation Standards REF MOL2410 Rev. C Simplexa EBV Quantitation Standards are intended to establish a standard curve with the Simplexa EBV assay on the 3M Integrated Cycler. For in vitro
Simplexa EBV Quantitation Standards REF MOL2410 Rev. C Simplexa EBV Quantitation Standards are intended to establish a standard curve with the Simplexa EBV assay on the 3M Integrated Cycler. For in vitro
Human Myostatin, ELISA Kit (MSTN)
 Human Myostatin, ELISA Kit (MSTN) 96 Tests Catalog Number: MBS733837 Store all reagents at 2-8 C Valid Period: six months For samples: Cell culture fluid & body fluid & tissue homogenate Serum or blood
Human Myostatin, ELISA Kit (MSTN) 96 Tests Catalog Number: MBS733837 Store all reagents at 2-8 C Valid Period: six months For samples: Cell culture fluid & body fluid & tissue homogenate Serum or blood
RapidChek SELECT Salmonella Test Kit
 RapidChek SELECT Salmonella Test Kit Part #: 7000188-7000197 AOAC Approved Protocols: This test kit s performance was reviewed by AOAC Research Institute and was found to perform to the manufacturer s
RapidChek SELECT Salmonella Test Kit Part #: 7000188-7000197 AOAC Approved Protocols: This test kit s performance was reviewed by AOAC Research Institute and was found to perform to the manufacturer s
Human IgG Rubella ELISA Kit
 Human IgG Rubella ELISA Kit Catalog No: IRAPKT2020 Lot No: SAMPLE INTENDED USE The Rubella IgG ELISA is intended for use in evaluating a patient s serologic status to the rubella virus infection. It is
Human IgG Rubella ELISA Kit Catalog No: IRAPKT2020 Lot No: SAMPLE INTENDED USE The Rubella IgG ELISA is intended for use in evaluating a patient s serologic status to the rubella virus infection. It is
Sample for Use as a Guide Revised IDEXX ENTEROLERT TEST METHOD FOR THE DETECTION OF ENTEROCOCCI IN WATER
 IDEXX ENTEROLERT TEST METHOD FOR THE DETECTION OF ENTEROCOCCI IN WATER 1 IDEXX ENTEROLERT TEST METHOD FOR THE DETECTION OF ENTEROCOCCI IN WATER 1. Scope and Application 1.1. This method is intended for
IDEXX ENTEROLERT TEST METHOD FOR THE DETECTION OF ENTEROCOCCI IN WATER 1 IDEXX ENTEROLERT TEST METHOD FOR THE DETECTION OF ENTEROCOCCI IN WATER 1. Scope and Application 1.1. This method is intended for
Sample collection & laboratory methods for identification bacteria. Lab. 2 A.T. Samira
 Sample collection & laboratory methods for identification bacteria Lab. 2 A.T. Samira Learning objectives At the end of the presentation, participants should understand the: Procedures, preparation, processing
Sample collection & laboratory methods for identification bacteria Lab. 2 A.T. Samira Learning objectives At the end of the presentation, participants should understand the: Procedures, preparation, processing
DRG Anti-Tissue Transglutaminase
 Please use only the valid version of the package insert provided with the kit. This kit is intended for Research Use Only. Not for use in diagnostic procedures. NAME AND INTENDED USE Anti-Tissue-Transglutaminase
Please use only the valid version of the package insert provided with the kit. This kit is intended for Research Use Only. Not for use in diagnostic procedures. NAME AND INTENDED USE Anti-Tissue-Transglutaminase
AccuProbe STREPTOCOCCUS PNEUMONIAE CULTURE IDENTIFICATION TEST
 AccuProbe STREPTOCOCCUS PNEUMONIAE CULTURE IDENTIFICATION TEST INTENDED USE IDENTIFICATION TEST is a rapid DNA probe test which utilizes the technique of nucleic acid hybridization for the identification
AccuProbe STREPTOCOCCUS PNEUMONIAE CULTURE IDENTIFICATION TEST INTENDED USE IDENTIFICATION TEST is a rapid DNA probe test which utilizes the technique of nucleic acid hybridization for the identification
Human Selenoprotein P1, Plasma (SEPP1) ELISA
 Human Selenoprotein P1, Plasma (SEPP1) ELISA For the quantitative determination of human SEPP1 in serum, plasma, cell culture fluid and other biological fluids Cat. No. KT-50806 For Research Use Only.
Human Selenoprotein P1, Plasma (SEPP1) ELISA For the quantitative determination of human SEPP1 in serum, plasma, cell culture fluid and other biological fluids Cat. No. KT-50806 For Research Use Only.
Copyright 2017 Aegis Sciences Corporation All Rights Reserved
 Oral Fluid Collection Healthcare Collection Site Preparation 1. The collection site must have all the supplies needed to complete a specimen collection (e.g. collection kits, ink pens, Custody and Control
Oral Fluid Collection Healthcare Collection Site Preparation 1. The collection site must have all the supplies needed to complete a specimen collection (e.g. collection kits, ink pens, Custody and Control
Herpes Simplex 1 IgM (HSV1 IgM)
 DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
Procine CRP ELISA Kit
 Procine CRP ELISA Kit For the quantitative in vitro determination of Procine C-Reactive Protein concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR LABORATORY RESEARCH
Procine CRP ELISA Kit For the quantitative in vitro determination of Procine C-Reactive Protein concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR LABORATORY RESEARCH
Mouse Coagulation Factor X (F10) ELISA
 Mouse Coagulation Factor X (F10) ELISA For the quantitative determination of mouse F10 in biological samples. Cat. No. KT-52133 For Research Use Only. Not for use in diagnostic procedures. Page 1 of 5
Mouse Coagulation Factor X (F10) ELISA For the quantitative determination of mouse F10 in biological samples. Cat. No. KT-52133 For Research Use Only. Not for use in diagnostic procedures. Page 1 of 5
Guinea pig PGI2 ELISA Kit
 Guinea pig PGI2 ELISA Kit For the quantitative in vitro determination of Guinea pig PGI2 concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR LABORATORY RESEARCH USE ONLY.
Guinea pig PGI2 ELISA Kit For the quantitative in vitro determination of Guinea pig PGI2 concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR LABORATORY RESEARCH USE ONLY.
Canine PHA ELISA Kit
 Canine PHA ELISA Kit For the quantitative in vitro determination of Canine Phytohaemagglutinin concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR LABORATORY RESEARCH USE
Canine PHA ELISA Kit For the quantitative in vitro determination of Canine Phytohaemagglutinin concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR LABORATORY RESEARCH USE
Human LENK ELISA Kit
 Human LENK ELISA Kit For the quantitative in vitro determination of Human Leu-enkephalin concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR LABORATORY RESEARCH USE ONLY.
Human LENK ELISA Kit For the quantitative in vitro determination of Human Leu-enkephalin concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR LABORATORY RESEARCH USE ONLY.
Mouse RF IgM ELISA Kit
 Mouse RF IgM ELISA Kit For the quantitative in vitro determination of Mouse rheumatoid factor IgM concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR LABORATORY RESEARCH
Mouse RF IgM ELISA Kit For the quantitative in vitro determination of Mouse rheumatoid factor IgM concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR LABORATORY RESEARCH
Chicken VEGF ELISA Kit
 Chicken VEGF ELISA Kit For the quantitative in vitro determination of Chicken Vascular Endothelial cell Growth Factor concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR
Chicken VEGF ELISA Kit For the quantitative in vitro determination of Chicken Vascular Endothelial cell Growth Factor concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR
Preparing and Gram-staining a bacteriological smear
 College of Life Sciences and Technology Medical Laboratory Science (Applied Learning) AP 84-205-00 (52) Module 4: Practical 2015-17 Preparing and Gram-staining a bacteriological smear Date Time Class Venue
College of Life Sciences and Technology Medical Laboratory Science (Applied Learning) AP 84-205-00 (52) Module 4: Practical 2015-17 Preparing and Gram-staining a bacteriological smear Date Time Class Venue
Guinea pig TIMP-1 ELISA Kit
 Guinea pig TIMP-1 ELISA Kit For the quantitative in vitro determination of Guinea pig tissue inhibitors of metalloproteinase 1 concentrations in serum - plasma - celiac fluid - tissue homogenate - body
Guinea pig TIMP-1 ELISA Kit For the quantitative in vitro determination of Guinea pig tissue inhibitors of metalloproteinase 1 concentrations in serum - plasma - celiac fluid - tissue homogenate - body
Human CoxV-A16 ELISA Kit
 Human CoxV-A16 ELISA Kit For the quantitative in vitro determination of Human Coxsackie virus A16 concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR LABORATORY RESEARCH
Human CoxV-A16 ELISA Kit For the quantitative in vitro determination of Human Coxsackie virus A16 concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR LABORATORY RESEARCH
Bovine IgG-Ab ELISA Kit
 Bovine IgG-Ab ELISA Kit For the quantitative in vitro determination of Bovine Immunoglobulin G Antibody concentrations in serum - plasma - tissue homogenates - other biological fluids FOR LABORATORY RESEARCH
Bovine IgG-Ab ELISA Kit For the quantitative in vitro determination of Bovine Immunoglobulin G Antibody concentrations in serum - plasma - tissue homogenates - other biological fluids FOR LABORATORY RESEARCH
Status Strep A Strip
 Section 1 Identification Revision Date: March 7, 2017 1.1 Product Name: Status Strep A Strip 1.2 Intended Use: The Status Strep A Direct Group A Streptococcus Antigen Test is a rapid immunochromatographic
Section 1 Identification Revision Date: March 7, 2017 1.1 Product Name: Status Strep A Strip 1.2 Intended Use: The Status Strep A Direct Group A Streptococcus Antigen Test is a rapid immunochromatographic
Guinea pig Hyp ELISA Kit
 Guinea pig Hyp ELISA Kit For the quantitative in vitro determination of Guinea pig hydroxyproline concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR LABORATORY RESEARCH
Guinea pig Hyp ELISA Kit For the quantitative in vitro determination of Guinea pig hydroxyproline concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR LABORATORY RESEARCH
Rat GLO ELISA Kit. For the quantitative in vitro determination of Rat Glyoxalase concentrations in
 Rat GLO ELISA Kit For the quantitative in vitro determination of Rat Glyoxalase concentrations in serum - plasma - tissue homogenates - other biological fluids FOR LABORATORY RESEARCH USE ONLY. NOT FOR
Rat GLO ELISA Kit For the quantitative in vitro determination of Rat Glyoxalase concentrations in serum - plasma - tissue homogenates - other biological fluids FOR LABORATORY RESEARCH USE ONLY. NOT FOR
Human CTRP5 ELISA Kit
 Human CTRP5 ELISA Kit For the quantitative in vitro determination of Human C1q And Tumor Necrosis Factor Related Protein 5 concentrations in serum - plasma - tissue homogenates - other biological fluids
Human CTRP5 ELISA Kit For the quantitative in vitro determination of Human C1q And Tumor Necrosis Factor Related Protein 5 concentrations in serum - plasma - tissue homogenates - other biological fluids
Human Herpes Simplex Virus Type 1 (HSV-1) IgM ELISA Kit
 Human Herpes Simplex Virus Type 1 Catalog No: IRAPKT1412 (HSV-1) IgM ELISA Kit Lot No: SAMPLE INTENDED USE The HSV-1 IgM ELISA is intended for the detection of IgM antibodies to herpes simplex virus HSV-1.
Human Herpes Simplex Virus Type 1 Catalog No: IRAPKT1412 (HSV-1) IgM ELISA Kit Lot No: SAMPLE INTENDED USE The HSV-1 IgM ELISA is intended for the detection of IgM antibodies to herpes simplex virus HSV-1.
Guinea pig Carboxy Terminal. Propeptide of Type 1 Procollagen. (PICP) ELISA kit
 19. If using an automated washer, the operating instructions for washing equipment should be carefully followed. 20. Assay Procedure Preliminary notes: Do not mix reagents from different lots. It is recommended
19. If using an automated washer, the operating instructions for washing equipment should be carefully followed. 20. Assay Procedure Preliminary notes: Do not mix reagents from different lots. It is recommended
Human Angiotensin 2 (Ang2) ELISA
 Human Angiotensin 2 (Ang2) ELISA For the quantitative determination of human Ang2 in serum, plasma, cell culture fluid and other biological fluids Cat. No. KT-52748 For Research Use Only. Not for use in
Human Angiotensin 2 (Ang2) ELISA For the quantitative determination of human Ang2 in serum, plasma, cell culture fluid and other biological fluids Cat. No. KT-52748 For Research Use Only. Not for use in
Serotonin Research ELISA
 Instructions for use Serotonin Research ELISA BA E-5900 Serotonin Research ELISA 1. Intended use and principle of the test Ultra-sensitive Enzyme Immunoassay for the quantitative determination of Serotonin.
Instructions for use Serotonin Research ELISA BA E-5900 Serotonin Research ELISA 1. Intended use and principle of the test Ultra-sensitive Enzyme Immunoassay for the quantitative determination of Serotonin.
Mouse Gonadotropin Releasing Hormone (GnRH) ELISA
 KAMIYA BIOMEDICAL COMPANY Mouse Gonadotropin Releasing Hormone (GnRH) ELISA For the quantitative determination of mouse GnRH in serum, plasma, cell culture fluid and other biological fluids Cat. No. KT-58182
KAMIYA BIOMEDICAL COMPANY Mouse Gonadotropin Releasing Hormone (GnRH) ELISA For the quantitative determination of mouse GnRH in serum, plasma, cell culture fluid and other biological fluids Cat. No. KT-58182
Monkey hs-tnt ELISA Kit
 Monkey hs-tnt ELISA Kit For the quantitative in vitro determination of Monkey Hypersensitive troponin T concentrations in serum - plasma - tissue homogenates - other biological fluids FOR LABORATORY RESEARCH
Monkey hs-tnt ELISA Kit For the quantitative in vitro determination of Monkey Hypersensitive troponin T concentrations in serum - plasma - tissue homogenates - other biological fluids FOR LABORATORY RESEARCH
Guinea pig 25-OH-VD ELISA Kit
 Guinea pig 25-OH-VD ELISA Kit For the quantitative in vitro determination of Guinea pig 25-Dihydroxy vitamin D concentrations in serum - plasma - tissue homogenates - other biological fluids FOR LABORATORY
Guinea pig 25-OH-VD ELISA Kit For the quantitative in vitro determination of Guinea pig 25-Dihydroxy vitamin D concentrations in serum - plasma - tissue homogenates - other biological fluids FOR LABORATORY
APB PRINCIPLE SPECIMEN. REF (300 tests) ANNUAL REVIEW Reviewed by: Date. Date INTENDED USE
 IMMAGE Immunochemistry Systems Chemistry Information Sheet Copyright 2010 Beckman Coulter, Inc. Apolipoprotein B REF 447730 (300 tests) For In Vitro Diagnostic Use ANNUAL REVIEW Reviewed by: Date Reviewed
IMMAGE Immunochemistry Systems Chemistry Information Sheet Copyright 2010 Beckman Coulter, Inc. Apolipoprotein B REF 447730 (300 tests) For In Vitro Diagnostic Use ANNUAL REVIEW Reviewed by: Date Reviewed
Salmonella Antigen Detection (In Food)
 DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
RapidChek SELECT Salmonella Enteritidis Test System
 RapidChek SELECT Salmonella Enteritidis Test System Part #: 7000220, 7000221, 7000222, 7000228 AOAC Approved Protocols: This test kit s performance was reviewed by AOAC Research Institute and was found
RapidChek SELECT Salmonella Enteritidis Test System Part #: 7000220, 7000221, 7000222, 7000228 AOAC Approved Protocols: This test kit s performance was reviewed by AOAC Research Institute and was found
Immunoperoxidase Assay for Determination of C3 in Guinea Pig Sera Lot # 2F
 GUINEA PIG C3 ELISA Immunoperoxidase Assay for Determination of C3 in Guinea Pig Sera 40-374-130032 Lot # 2F Directions for Use For Research Use Only For In Vitro Use Only GUINEA PIG COMPLEMENT FACTOR
GUINEA PIG C3 ELISA Immunoperoxidase Assay for Determination of C3 in Guinea Pig Sera 40-374-130032 Lot # 2F Directions for Use For Research Use Only For In Vitro Use Only GUINEA PIG COMPLEMENT FACTOR
Herpes Simplex 1 IgM (HSV1 IgM)
 DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
UBI FMDV NS 3B NEUTRALIZATION BUFFER For UBI FMDV NS 3B NEUTRALIZATION ASSAY (SWINE) or UBI FMDV NS 3B NEUTRALIZATION ASSAY (CATTLE)
 UBI FMDV NS 3B NEUTRALIZATION BUFFER For UBI FMDV NS 3B NEUTRALIZATION ASSAY (SWINE) or UBI FMDV NS 3B NEUTRALIZATION ASSAY (CATTLE) DIRECTION INSERT FOR INVESTIGATIONAL USE ONLY (P/N 100165) CONTENTS
UBI FMDV NS 3B NEUTRALIZATION BUFFER For UBI FMDV NS 3B NEUTRALIZATION ASSAY (SWINE) or UBI FMDV NS 3B NEUTRALIZATION ASSAY (CATTLE) DIRECTION INSERT FOR INVESTIGATIONAL USE ONLY (P/N 100165) CONTENTS
Actero Salmonella Enrichment Media Product Information
 Actero Salmonella Enrichment Media Product Information INTENDED USE: Actero Salmonella Enrichment Media is a selective medium optimized for an improved enrichment of Salmonella spp. from food and environmental
Actero Salmonella Enrichment Media Product Information INTENDED USE: Actero Salmonella Enrichment Media is a selective medium optimized for an improved enrichment of Salmonella spp. from food and environmental
Qualitative determination of Anti-Treponema pallidum antibodies For in vitro use only Store at 2-8 C
 Qualitative determination of Anti-Treponema pallidum antibodies For in vitro use only Store at 2-8 C INTRODUCTION Syphilis is a venereal disease caused by infection with T. pallidum. The microorganism
Qualitative determination of Anti-Treponema pallidum antibodies For in vitro use only Store at 2-8 C INTRODUCTION Syphilis is a venereal disease caused by infection with T. pallidum. The microorganism
QUALITY CONTROL IN. Quality: Quality means meeting the pre-determined requirements of users for a particular substance or service.
 Quality Control in 13 QUALITY CONTROL IN 13.1 INTRODUCTION Quality: Quality means meeting the pre-determined requirements of users for a particular substance or service. ISO: International standard Organization
Quality Control in 13 QUALITY CONTROL IN 13.1 INTRODUCTION Quality: Quality means meeting the pre-determined requirements of users for a particular substance or service. ISO: International standard Organization
Dynamiker Biotechnology (Tianjin) Co., Ltd. Dynamiker Aspergillus Galactomannan Assay. Catalogue No.: DNK User Manual / 96 tests
 Dynamiker Biotechnology (Tianjin) Co., Ltd. DNK-SM-1402-1 A/0 Dynamiker Aspergillus Galactomannan Assay Catalogue No.: DNK-1402-1 User Manual / 96 tests CONTENTS 1. INTENDED USE... 1 2. PRINCIPLE... 1
Dynamiker Biotechnology (Tianjin) Co., Ltd. DNK-SM-1402-1 A/0 Dynamiker Aspergillus Galactomannan Assay Catalogue No.: DNK-1402-1 User Manual / 96 tests CONTENTS 1. INTENDED USE... 1 2. PRINCIPLE... 1
