IPS TCON Minute in red Notes from the previous calls in green
|
|
|
- Dustin McCarthy
- 5 years ago
- Views:
Transcription
1 IPS TCON Minute in red Notes from the previous calls in green
2 Attendees 2 Rob Hausam Giorgio Cangioli François Macary Sabutsch, Stefan Gary Dickinson Catherine Chronaki Kai U. Heitmann (excuse) Didi Davis Laura Heermann Langford Philip Scott David DeAbreu Christof Gessner John Moehrke Fernando Portilla Stephen Chu
3 Agenda Make the IPS real Complete and Publish the STU IPS CDA IG Move the IPS IGs to Normative Develop the IPS FHIR IG
4 Agenda 1. IPS PSS review and approval process 2. CDA IG 1. CDA ballot status and ballot reconciliation 2. CDA IG To do list 3. FHIR IG 1. NIB 2. Medical devices 3. AllergyIntolerance 4. Medication related resources 4. Plan for the next weeks 5. AOB < 30 Mins 1 hour
5 IPS PSS REVIEW AND APPROVAL PROCESS 5
6 IPS PSS (for FHIR IG) Approved by SDWG Shared with the International Council Shared with the other co-sponsoring WGs Approved by SD Approved by FMG ( ). Comments add Pharmacy to the list of interested work groups Pharma WG will evaluate in the feb 12 th call ensure that all profiles are reviewed by the work groups that own the resources that are being profiled. Approved by TSC ( ) Pharma WG? (check minutes) 6
7 BALLOT STATUS AND RECONCILIATION
8 IPS STU 1 st ballot Comments: 443 NEG 33 A-S 143 empty 6 A-C 85 A-T 115 A-Q 39 A-A 22
9 IPS STU 1 st ballot Agreed changes % 20% 40% 60% 80% 100%
10 IPS STU ballot#2 results Aff. Neg. Abst. NV Totals 54 (37 for Approval) % of Votes 32.93% 4.27% 50.61% 12.20% Quorum 87.80% Comments: 49 NEG 10 A-C 23 A-Q 0 A-S 4 A-T 11 A-A 1
11 IPS STU ballot#2 results Send to SDWG for approval Single block? Rob will ask for SDWG for the vote next week 11
12 IPS STU ballot#2 results
13 IPS STU ballot#2 results Agreed changes 13
14 CDA IG «TO DO» LIST 14
15 Assigned Define value sets for Absent or Unknown information and then go to harmonization [consider also devices] Rob will take care of this issue Presented at the Round table, harmonization proposal to be prepared. LOINC Code for NEW-LOINC- VERIFICATION_STATUS Rob will issue the request to LOINC. Still in the to do list
16 Assigned Harmonization with Pharma templates (Kai // Giorgio). No updates Observation Results Radiology: Load the value set provided by Francois as example KAI. No updates
17 Not assigned Bind the ISO 3166 country codes to the country part of Addresses (2) Define an IPS Address (datatype) template fragment (3) Define a value set for countries based on ISO 3166 (how?) Add a textual constraint with the ISO (4) Include this datatype element whenever used Add a surgical pathology example from APSR 2.0. François 17
18 Not assigned Prepare the XML schema (xsd) with the IPS extensions Prepare the Sample(s) to be included in the published document Add links to the published value sets Clean-up of the links to the IPS wiki The underlying publication mechanism is a mediawiki where icons always takes you to the file page with the icon. We will try to erase the link in the PDF rendition so that it goes nowhere. At this point in time the creation of a a link to an artefact in the document itself is investigated 18
19 FHIR IPS IG 19
20 FHIR IPS IG FHIR IG based on STU3 Ballot in May Harmonization March To be confirmed at Facilitator s Roundtable Thurs. evening Will want to take our new codes / value sets there for approval for FHIR and CDA Initial proposals due Feb. 28 Final proposals due March 16 FHIR substantive freeze March 25 FHIR final content freeze April 1 20
21 FHIR IPS IG (cont.) Tools Profile creation Forge Sharing artifacts IG Publisher GitHub ( ) Profile under development Simplifier and FHIR registry registry.fhir.org Others? 21
22 Work organization Conventions to be used Identify the components Identify the resources to be profiled Assign components to a responsible person/team Use the CDA IPS as conceptual reference (CEN IPS EN?) Use the calls to review the work done; discuss open issues 22
23 FHIR IPS IG - Naming and identification convention canonical project URL (for profiles) URL (implementation guide) Resource profiles Name: [Name of Resource]-[qualifier-]uv-ips[-version] URL: (lower case] e.g. AllergyIntolerance-uv-ips; Observation-Laboratory-uv-ips E.g. (to be considered the results of the discussion about versioning) Extensions? Value Set 23
24 Agreements Explicit clinical assertion in case of the following section. Not to use emptyreason for No info about allergies/intolerances ; No allergies/intolerances No info about drugs ; No current drug treatment No info about problems ; No current problem No info about procedures ; No history of procedure No info about medical devices, implants ; No current implant No info about immunizations ; No history of immunization Use emptyreason with the preferred value set for the others. Results Past illnesses? Social History 24
25 TO BE DISCUSSED 25
26 Plan 26 7-Feb First analysis of the resources to be used Assign the canonical URLs to all the value set (to be added to the Art Decor value sets) Progress update 14-Feb Composition draft with allergy and MDs Medical devices / AllergyIntolerance Medication related resources Assign the canonical URLs to all the value set (to be added to the Art Decor value sets) 21-Feb NIB approved
27 1. NIB 1. Informational or STU? STU 2. Rob will prepare a draft 2. Medical devices 3. Medication related resources 4. AllergyIntolerance 5. Reassignment of tasks?
28 Composition Medications section entry entry MedicationStatement MedicationStatement Medication Patient Medical Devices section entry DeviceUseStatement Device
29 Issue #1: Methods for representing known absent and unknown in the FHIR IG Proposal to use 2 methods Specific method for the 3 mandatory sections Allergies, Meds, Problems = the one defined in the CDA IG of IPS. Generic method for the non-mandatory sections (Results, Devices, ), relying on Composition.section.emptyReason
30
31 Medical Devices section emptyreason: nilknown Known absent Patient Composition Medical Devices section Unknown Patient emptyreason: unavailable Rationale: Medical devices section is not mandatory. The assertions No device and devices unknown do not need to track by whom (source) and when (assertion date) these statements were made. Simply, when building the patient summary, no devices are included, under the responsibility of the patient summary authoring (person system).
32 Composition Medications section entry MedicationStatement medicationcodeableconcept = no medication in use Patient Rationale: Medication section is mandatory. No drug used and drug use unknown are statements about patient drugs, coming from the patient health record, which need to be captured by the patient summary, with a who (source) and a when (assertion date), potentially distinct from the authoring of the summary. Composition Medications section entry MedicationStatement medicationcodeableconcept = medications use unknown Patient
33 For discussion. Cases Assertion unknown case No information at all No medications use Cases Assertion unknown case No information at all No medications use Unknown CDA No medications in use FHIR medicationcodeableconcept = medications use unknown emptyreason: unavailable? medicationcodeableconcept = no medication in use Use the same mechanism of the CDA explicit content and have an entry; even when there is no explicit assertions
34 François proposal Have all the sections specified in the IG present and expressing that there is no content Christof Prefer to omit not required sections Giorgio No in favor of having optional section No strong opinion for the required if known (0..1 R)
35 Issue #2: # scopes for devices between IPS and Trillium II > Choice of the value set for type of medical device CDA IG of IPS: IPS Medical Devices : only implants << Implant, device (physical object).... Trillium II currently uses the regular much broader << Device (physical object)..... which is Update the CDA IPS Medical Devices? Yes Giorgio change the value set definition Note only Kai can make this change; ask Kai
36 We may have different approaches for different sections We can omit some sections when there is no content. Excepting allergies/intolerances 1..1 drugs 1..1 problems 1..1 procedures 0..1 R medical devices 0..1 R Immunizations 0..1 R Non-required section simply omitted
37 allergies/intolerances 1..1 drugs 1..1 problems 1..1 Cannot be omitted procedures 0..1 R medical devices 0..1 R Immunizations 0..1 R Use the same approach of the CDA also for FHIR
38 FUTURE PLAN 38
39
40 Plan 21-Feb (François absent) Problem List & History of Past Illnesses? Allergy Procedure Observation Imaging? Header? 28-Feb Medications Other Observations Pregnancy Implementation Guide 40
41 Plan 7-Mar Additional call to be agreed Friday 9 March ( CET ) 14-Mar (Giorgio Absent)tory Plan of Care Functional Status Advance Directive Value Sets Examples Mar 41
42 Composition General overview Do we need custodian? (Kai suggested to define a minimal guidance for handling the custodians in case of CDA and FHIR) Kai suggests also to keep a semantic alignment between CDA and FHIR Slices for entries Results single or specialized sections?.. (check on C-CDA on FHIR) How to deal with textual translations? To be investigated How to convey translated designations? To be investigated 42
43 General Plan Overview NIB FHIR core and base IG Substantive change freeze: FHIR Content Freeze (core and IGs) Paris meeting 7-Feb 14-Feb 21-Feb 28-Feb 7-Mar 14-Mar 21-Mar 28-Mar 4-Apr 25-feb 25-mar 01-apr 1 Implementation Guide 2 Document 3 Header (to be expanded ) 4 Allergy/Intolerance 5 Medication Summary 6 Devices 7 Immunizations 8 Problem List 9 History past illnesses 10 Pregnancy 11 Procedure 12 Results 13 Social History 14 Plan of Care 15 Functional Status 16 Advance Directives 17 Value sets 18 Publication 43
44 FHIR IPS IG - Components CDA IPS FHIR IPS Notes / Resp. Implementation Guide ImplementationGuide Rob Document Header (to be expanded ) Composition Bundle Patient Provider Organization < > Giorgio Allergy/Intolerance AllergyIntolerance Rob Low priority Emergency contacts Kai to consider what should be the content Medication Summary MedicationStatement Medication François 44
45 FHIR IPS IG - Components CDA IPS FHIR IPS Owner? Devices DeviceUseStatement Device François Immunizations Immunization Giorgio Problem List Condition Rob History past illnesses Condition Rob Pregnancy Condition Rob Procedure Procedure Giorgio 45
46 FHIR IPS IG - Components CDA IPS FHIR IPS Owner? Results Observation Lab / AP => François Imaging => Rob (Giorgio) Social History Plan of Care Functional Status Advance Directive Examples Provenance Data Kai Sylvia (Rob) Giorgio Giorgio 46
47 Paris meeting Agenda Ballot comment resolution and preparing for publishing CDA IG Working on FHIR IG and preparing for ballot in May Trillium II deliverables first available in January What will we re-use? Trillium is re-using IPS value sets 47
48 Paris meeting (cont.) March (dates are confirmed) This is best week prior to ballot content deadline How many days 4 Final content deadline for May ballot March 25 th Phast will be able to host Up to 12 participants 48
49 ACTION LIST
HL7 Standards uptake in Europe and beyond
 HL7 Standards uptake in Europe and beyond estandards Roadmap for the collaborative development of standards for large-scale ehealth Deployment: the case of the International Patient Summary Standard Giorgio
HL7 Standards uptake in Europe and beyond estandards Roadmap for the collaborative development of standards for large-scale ehealth Deployment: the case of the International Patient Summary Standard Giorgio
Project Scope Statement 2015 Version Release 1
 Project Scope Statement 2015 Version Release 1 HL7 Project Management Office Project Services Work Group Point of Contact Name and Email David Hamill (pmo@hl7.org) Co Chairs of Project Services Work Group:
Project Scope Statement 2015 Version Release 1 HL7 Project Management Office Project Services Work Group Point of Contact Name and Email David Hamill (pmo@hl7.org) Co Chairs of Project Services Work Group:
Project Scope Statement for: EHR- S FM + PHR- S FM + RM- ES FP on FHIR
 for: EHR- S FM + PHR- S FM + RM- ES FP on FHIR HL7 Electronic Health Record Work Group EHR = Electronic Health Record EHR- S FM = EHR System Functional Model Release 2 PHR = Personal Health Record PHR-
for: EHR- S FM + PHR- S FM + RM- ES FP on FHIR HL7 Electronic Health Record Work Group EHR = Electronic Health Record EHR- S FM = EHR System Functional Model Release 2 PHR = Personal Health Record PHR-
HL7 C-CDA Consolidated Clinical Document Architecture
 HL7 C-CDA Consolidated Clinical Document Architecture Calvin Beebe Mayo Clinic HL7 C-CDA Resources o Introduction o HL7 CDA Product Management o C-CDA implementation-athon(s) o Access to Value Set(s) for
HL7 C-CDA Consolidated Clinical Document Architecture Calvin Beebe Mayo Clinic HL7 C-CDA Resources o Introduction o HL7 CDA Product Management o C-CDA implementation-athon(s) o Access to Value Set(s) for
Co-Chair Info Meeting
 Co-Chair Info Meeting HL7 Workgroup Meeting May, 2018 Karen Van Hentenryck Health Level Seven, Inc. Reg. U.S. Pat & TM Off Congratulations on your election! Thank you for your willingness to serve 2 Why
Co-Chair Info Meeting HL7 Workgroup Meeting May, 2018 Karen Van Hentenryck Health Level Seven, Inc. Reg. U.S. Pat & TM Off Congratulations on your election! Thank you for your willingness to serve 2 Why
The HL7 Clinical Genomics Work Group
 HL7 Clinical Genomics Domain Information Model The HL7 Clinical Genomics Work Group Prepared by Amnon Shabo (Shvo), PhD HL7 Clinical Genomics WG Co-chair and Modeling Facilitator Pedigree (family health
HL7 Clinical Genomics Domain Information Model The HL7 Clinical Genomics Work Group Prepared by Amnon Shabo (Shvo), PhD HL7 Clinical Genomics WG Co-chair and Modeling Facilitator Pedigree (family health
HL7 EHR Immunization Functional Profile Project. Gary L. Dickinson Mark Janczewski, MD 8 August 2016
 HL7 EHR Immunization Functional Profile Project Gary L. Dickinson Mark Janczewski, MD 8 August 2016 Immunization Functional Profile Project Introduction This is an open project All are welcome! International
HL7 EHR Immunization Functional Profile Project Gary L. Dickinson Mark Janczewski, MD 8 August 2016 Immunization Functional Profile Project Introduction This is an open project All are welcome! International
Dr Philip Scott Centre for Healthcare Modelling & Informatics, University of Portsmouth Chair, HL7 UK
 Dr Philip Scott Centre for Healthcare Modelling & Informatics, University of Portsmouth Chair, HL7 UK What is the new landscape? NHS changes in England Open Standards EU-US collaboration HL7 International
Dr Philip Scott Centre for Healthcare Modelling & Informatics, University of Portsmouth Chair, HL7 UK What is the new landscape? NHS changes in England Open Standards EU-US collaboration HL7 International
More Than You Think. HL7 is people, HL7 is ideas, HL7 is collaboration
 More Than You Think HL7 is people, HL7 is ideas, HL7 is collaboration Getting the Most Out of Your Data Using HL7 Clinical Decision Support Standards 2 Speakers: HL7 CDS WG Co-Chairs Robert A Jenders,
More Than You Think HL7 is people, HL7 is ideas, HL7 is collaboration Getting the Most Out of Your Data Using HL7 Clinical Decision Support Standards 2 Speakers: HL7 CDS WG Co-Chairs Robert A Jenders,
HIT Policy Update. A Complimentary Webinar From healthsystemcio.com Sponsored by OnBase by Hyland
 HIT Policy Update A Complimentary Webinar From healthsystemcio.com Sponsored by OnBase by Hyland Your Line Will Be Silent Until Our Event Begins at 12:00 ET Thank You! Housekeeping Moderator Anthony Guerra,
HIT Policy Update A Complimentary Webinar From healthsystemcio.com Sponsored by OnBase by Hyland Your Line Will Be Silent Until Our Event Begins at 12:00 ET Thank You! Housekeeping Moderator Anthony Guerra,
Agenda. Background SNOMED International Collaborations developments Information models Extensions
 Agenda Background SNOMED International Collaborations 2018 developments Information models Extensions SNOMED International International not-for-profit association, based in the UK Owns and maintains SNOMED
Agenda Background SNOMED International Collaborations 2018 developments Information models Extensions SNOMED International International not-for-profit association, based in the UK Owns and maintains SNOMED
Integrating the Healthcare Enterprise International Cardiology Domain Update. Webinar Series 2017
 Integrating the Healthcare Enterprise International Cardiology Domain Update Webinar Series 2017 Presenting Members Jerry Serwer MD, University of Michigan [PC co-chair] David Slotwiner MD, Weill Cornell
Integrating the Healthcare Enterprise International Cardiology Domain Update Webinar Series 2017 Presenting Members Jerry Serwer MD, University of Michigan [PC co-chair] David Slotwiner MD, Weill Cornell
Pre- Decisional Working- Document; Not for Official Use EHR-S FM Release-3 Summer Prototype Plan 7/13. R2 Ballot Reconciled
 Pre- Decisional Working- Document; Not for Official Use EHR-S FM Release-3 Summer Prototype Plan 1 2 EHR Interoperability WG To Participate: Call 1-770-657-9270 PC510269# 2 PM every Tuesday Gary Dickinson,
Pre- Decisional Working- Document; Not for Official Use EHR-S FM Release-3 Summer Prototype Plan 1 2 EHR Interoperability WG To Participate: Call 1-770-657-9270 PC510269# 2 PM every Tuesday Gary Dickinson,
HL7 Clinical Genomics and Structured Documents Work Groups
 HL7 Clinical Genomics and Structured Documents Work Groups CDA Implementation Guide: Genetic Testing Report (GTR) Published as Universal DTSU in February 2013: http://www.hl7.org/documentcenter/public/standards/dstu/cdar2_ig_gentestrpt_dstu_r1_2013feb.zip
HL7 Clinical Genomics and Structured Documents Work Groups CDA Implementation Guide: Genetic Testing Report (GTR) Published as Universal DTSU in February 2013: http://www.hl7.org/documentcenter/public/standards/dstu/cdar2_ig_gentestrpt_dstu_r1_2013feb.zip
IHIC 2011 Orlando, FL
 CDA Implementation Guide for Genetic Testing Report (GTR): Towards a Clinical Genomic Statement IHIC 2011 Orlando, FL Amnon Shabo (Shvo), PhD shabo@il.ibm.com HL7 Clinical Genomics WG Co-chair and Modeling
CDA Implementation Guide for Genetic Testing Report (GTR): Towards a Clinical Genomic Statement IHIC 2011 Orlando, FL Amnon Shabo (Shvo), PhD shabo@il.ibm.com HL7 Clinical Genomics WG Co-chair and Modeling
Data Exchange for Quality Measures (DEQM) Project Call. Thursday, December 20, 2018
 Data Exchange for Quality Measures (DEQM) Project Call Thursday, December 20, 2018 Viet Nguyen, MD Bryn Rhodes Linda Michaelsen Erica Haas, DVM Agenda Brief review of DEQM IG Review progress of ballot
Data Exchange for Quality Measures (DEQM) Project Call Thursday, December 20, 2018 Viet Nguyen, MD Bryn Rhodes Linda Michaelsen Erica Haas, DVM Agenda Brief review of DEQM IG Review progress of ballot
MEANINGFUL USE AND PQRS
 MEANINGFUL USE AND PQRS Understanding the Measures Presented By: Morgan Kuper, Customer Service Supervisor 2016 Meaningful Use Overview Objectives and Measures Exclusions Changes to Specific Objectives
MEANINGFUL USE AND PQRS Understanding the Measures Presented By: Morgan Kuper, Customer Service Supervisor 2016 Meaningful Use Overview Objectives and Measures Exclusions Changes to Specific Objectives
STI ecr Learning Community: Webinar 2. April 27, 2017 Noon 1 p.m. EDT
 STI ecr Learning Community: Webinar 2 April 27, 2017 Noon 1 p.m. EDT STI ecr Learning Community Webinar 2 Please use the link below to register for the webinar: https://attendee.gotowebinar.com/register/1084681796879085313
STI ecr Learning Community: Webinar 2 April 27, 2017 Noon 1 p.m. EDT STI ecr Learning Community Webinar 2 Please use the link below to register for the webinar: https://attendee.gotowebinar.com/register/1084681796879085313
CDISC Controlled Terminology across the Clinical Trial Continuum. Bay Area Implementation Network 6 March 2008
 CDISC Controlled Terminology across the Clinical Trial Continuum Bay Area Implementation Network 6 March 2008 Bron Kisler Co-Founder / Director of Terminology CDISC Terminology Initiative Overview / Background
CDISC Controlled Terminology across the Clinical Trial Continuum Bay Area Implementation Network 6 March 2008 Bron Kisler Co-Founder / Director of Terminology CDISC Terminology Initiative Overview / Background
J P Systems, Inc
 J P Systems, Inc. 1 703 815-0900 info@jpsys.com Requirements Analysis IT Architecture Data Architecture Business Architecture Clinical Terminologies Standards Development Healthcare IT Strategy Interoperability
J P Systems, Inc. 1 703 815-0900 info@jpsys.com Requirements Analysis IT Architecture Data Architecture Business Architecture Clinical Terminologies Standards Development Healthcare IT Strategy Interoperability
Philips HealthSuite Digital Platform and Interoperability
 Philips HealthSuite Digital Platform and Interoperability Developing innovative solutions across the continuum of care Improving patient outcomes, provide better value, and expand access to care. HealthSuite
Philips HealthSuite Digital Platform and Interoperability Developing innovative solutions across the continuum of care Improving patient outcomes, provide better value, and expand access to care. HealthSuite
The Future of Laboratory Implementation Guides
 The Future of Laboratory Implementation Guides Hans Buitendijk,M.Sc, FHL7, Senior Strategist Cerner HL7 Board Member, Chair Policy Advisory Committee, Co-Chair HL7 Orders/Observations Work Group, Co-Chair
The Future of Laboratory Implementation Guides Hans Buitendijk,M.Sc, FHL7, Senior Strategist Cerner HL7 Board Member, Chair Policy Advisory Committee, Co-Chair HL7 Orders/Observations Work Group, Co-Chair
Health Level Seven Updates Durwin Day, Health Care Service Corporation
 Health Level Seven Updates 2017 Durwin Day, Health Care Service Corporation 1 Today s agenda HL7 s Current Activities Events, Publications, NCVHS Recommendations, Attachment Work Group LOINC Demo FHIR
Health Level Seven Updates 2017 Durwin Day, Health Care Service Corporation 1 Today s agenda HL7 s Current Activities Events, Publications, NCVHS Recommendations, Attachment Work Group LOINC Demo FHIR
The Future of Health Data Interoperability is on FHIR: the Argonaut Project
 The Future of Health Data Interoperability is on FHIR: the Argonaut Project Charles Jaffe, MD, PhD CEO, Health Level 7 British Computer Society 10 November 15 The principles underlying FHIR development
The Future of Health Data Interoperability is on FHIR: the Argonaut Project Charles Jaffe, MD, PhD CEO, Health Level 7 British Computer Society 10 November 15 The principles underlying FHIR development
CDISC UK Network face to face Reading
 CDISC UK Network face to face Reading Paul Houston, CDISC Head of European Operations CDISC Update and European Activity 2 CDISC Values and Principals Core value Foster CDISC community is altruistic
CDISC UK Network face to face Reading Paul Houston, CDISC Head of European Operations CDISC Update and European Activity 2 CDISC Values and Principals Core value Foster CDISC community is altruistic
The Interoperability Journey*
 The Interoperability Journey* NETTAB Workshop on Clinical Bioinformatics 14 October 2011, Pavia Amnon Shabo (Shvo), PhD Co-Chair, Medical Informatics Community, IBM Research IBM Haifa Research Lab (HRL)
The Interoperability Journey* NETTAB Workshop on Clinical Bioinformatics 14 October 2011, Pavia Amnon Shabo (Shvo), PhD Co-Chair, Medical Informatics Community, IBM Research IBM Haifa Research Lab (HRL)
Da Vinci. Program Overview. August 15, 2018
 Da Vinci Program Overview August 15, 2018 Interim Antitrust Policy ANSI Antitrust Policy ANSI neither develops standards nor conducts certification programs but instead accredits standards developers and
Da Vinci Program Overview August 15, 2018 Interim Antitrust Policy ANSI Antitrust Policy ANSI neither develops standards nor conducts certification programs but instead accredits standards developers and
Technical Steering Committee
 Technical Steering Committee The HL7 Technical Steering Committee s mission is to provide the technical direction to the HL7 organization to achieve the vision of creating the best and most widely used
Technical Steering Committee The HL7 Technical Steering Committee s mission is to provide the technical direction to the HL7 organization to achieve the vision of creating the best and most widely used
1. Proposed Work Item: HIT Standards for Information Governance White Paper. 2. The Problem
 http://wiki.ihe.net/index.php?title=brief_proposal_template This template is for one or two page IHE workitem proposals for initial review. To create a new proposal, first log in to the Wiki (or create
http://wiki.ihe.net/index.php?title=brief_proposal_template This template is for one or two page IHE workitem proposals for initial review. To create a new proposal, first log in to the Wiki (or create
Integrating RAPID Common Data Elements and Modeling with the Goals of HSPC. MDEpiNet RAPID Sept 14, 2016 Stanley M. Huff, MD
 Integrating RAPID Common Data Elements and Modeling with the Goals of HSPC MDEpiNet RAPID Sept 14, 2016 Stanley M. Huff, MD Why? To help people live the healthiest lives possible. 4 Healthcare Services
Integrating RAPID Common Data Elements and Modeling with the Goals of HSPC MDEpiNet RAPID Sept 14, 2016 Stanley M. Huff, MD Why? To help people live the healthiest lives possible. 4 Healthcare Services
A FHIR Enabled Ecosystem for Health Information Sharing
 A FHIR Enabled Ecosystem for Health Information Sharing Session 269, March 8, 2018 David Hay, Product Strategist, Orion Health FHIR Management Group Co-Chair 1 Conflict of Interest David Hay, MD Currently
A FHIR Enabled Ecosystem for Health Information Sharing Session 269, March 8, 2018 David Hay, Product Strategist, Orion Health FHIR Management Group Co-Chair 1 Conflict of Interest David Hay, MD Currently
Effective participation of Societal Stakeholders in Standardization
 CLC(DG)1318 March 2017 Effective participation of Societal Stakeholders in Standardization Societal Stakeholders Organizations right to submit an Opinion Introduction Standards have a broad impact on society,
CLC(DG)1318 March 2017 Effective participation of Societal Stakeholders in Standardization Societal Stakeholders Organizations right to submit an Opinion Introduction Standards have a broad impact on society,
SHARED HEALTH RECORD(SHR)
 Health Information Data and Messaging Specification HEALTH INFORMATION STANDARDS COMMITTEE FOR ALBERTA SHARED HEALTH RECORD(SHR) MESSAGE AND DATA STANDARD SUMMARY Status: Accepted in Draft Version: 1.10
Health Information Data and Messaging Specification HEALTH INFORMATION STANDARDS COMMITTEE FOR ALBERTA SHARED HEALTH RECORD(SHR) MESSAGE AND DATA STANDARD SUMMARY Status: Accepted in Draft Version: 1.10
Leo Slegers (ING), Guy Rackham(BIAN), Hans Tesselaar (BIAN) BIAN Introduction Webinar, July 20, Datum, Referent
 Standardization driving Flexibility and Agility: BIAN s Service Landscape 1.5 Series of Webinars offered by Banking Industry Architecture Network (BIAN) Leo Slegers (ING), Guy Rackham(BIAN), Hans Tesselaar
Standardization driving Flexibility and Agility: BIAN s Service Landscape 1.5 Series of Webinars offered by Banking Industry Architecture Network (BIAN) Leo Slegers (ING), Guy Rackham(BIAN), Hans Tesselaar
GNSO Policy Development Process
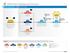 1 Issue Identification 2 Issue Scoping Page 1 of 12 AC Requests an Issue PDP WG Charter Preliminary Issue PUBLIC COMMENT Public Comment Community encouraged to discuss substantive issues before submission
1 Issue Identification 2 Issue Scoping Page 1 of 12 AC Requests an Issue PDP WG Charter Preliminary Issue PUBLIC COMMENT Public Comment Community encouraged to discuss substantive issues before submission
Western Canada Chronic Disease Management Infostructure Initiative
 Western Health Information Collaborative (WHIC) Western Canada Chronic Disease Management Infostructure Initiative CDM HL7 Message Specifications Implementation Guide FINAL - 07/11/2005 1:10 PM Prepared
Western Health Information Collaborative (WHIC) Western Canada Chronic Disease Management Infostructure Initiative CDM HL7 Message Specifications Implementation Guide FINAL - 07/11/2005 1:10 PM Prepared
Clinical Information Interoperability Council (CIIC) Providing a Shared Repository of Detailed Clinical Models for all of Health and Healthcare
 Clinical Information Interoperability Council (CIIC) Providing a Shared Repository of Detailed Clinical Models for all of Health and Healthcare NIH HCS Collaboratory and PCORnet December 1, 2017 Stanley
Clinical Information Interoperability Council (CIIC) Providing a Shared Repository of Detailed Clinical Models for all of Health and Healthcare NIH HCS Collaboratory and PCORnet December 1, 2017 Stanley
In This Issue September 9, 2010
 In This Issue September 9, 2010 Message from the TSC Chair: Success and HL7 HL7 Tooling Report Promoting Attendance at the Sydney WGM: One Group s Strategy Updates from the TSC since the Last TSC Newsletter
In This Issue September 9, 2010 Message from the TSC Chair: Success and HL7 HL7 Tooling Report Promoting Attendance at the Sydney WGM: One Group s Strategy Updates from the TSC since the Last TSC Newsletter
FHIR, Interoperability, and the World of Enablement
 FHIR, Interoperability, and the World of Enablement W. Ed Hammond. Ph.D., FACMI, FAIMBE, FIMIA, FHL7 Director, Duke Center for Health Informatics. DTMI Director, Applied Informatics Research, DHTS Professor,
FHIR, Interoperability, and the World of Enablement W. Ed Hammond. Ph.D., FACMI, FAIMBE, FIMIA, FHL7 Director, Duke Center for Health Informatics. DTMI Director, Applied Informatics Research, DHTS Professor,
Slide 1. Slide 2. Slide 3. Objectives. Who Needs Interoperability? Component 9 Networking and Health Information Exchange
 Slide 1 Component 9 Networking and Health Information Exchange Unit 8 Enterprise Architecture Models This material was developed by Duke University, funded by the Department of Health and Human Services,
Slide 1 Component 9 Networking and Health Information Exchange Unit 8 Enterprise Architecture Models This material was developed by Duke University, funded by the Department of Health and Human Services,
PROPOSED DOCUMENT. Global Harmonization Task Force. Title: Reportable Events During Pre-Market Clinical Investigations
 SG2&5(PD1)N5R10 PROPOSED DOCUMENT Global Harmonization Task Force Title: Reportable Events During Pre-Market Clinical Investigations Authoring Group: Study Groups 2 and 5 Date: 7 June 2011 CONTENTS Reportable
SG2&5(PD1)N5R10 PROPOSED DOCUMENT Global Harmonization Task Force Title: Reportable Events During Pre-Market Clinical Investigations Authoring Group: Study Groups 2 and 5 Date: 7 June 2011 CONTENTS Reportable
ISO/TS TECHNICAL SPECIFICATION. Financial services UNIversal Financial Industry message scheme Part 3: ISO modelling guidelines
 TECHNICAL SPECIFICATION Provläsningsexemplar / Preview ISO/TS 20022-3 First edition 2004-12-15 Financial services UNIversal Financial Industry message scheme Part 3: ISO 20022 modelling guidelines Services
TECHNICAL SPECIFICATION Provläsningsexemplar / Preview ISO/TS 20022-3 First edition 2004-12-15 Financial services UNIversal Financial Industry message scheme Part 3: ISO 20022 modelling guidelines Services
Background: ClincalTrials.gov and the database for Aggregate Analysis of ClincalTrials.gov (AACT)
 Background: ClincalTrials.gov and the database for Aggregate Analysis of ClincalTrials.gov (AACT) Background! What is ClinicalTrials.gov?! ClinicalTrials.gov history! Key reporting requirements! Rationale
Background: ClincalTrials.gov and the database for Aggregate Analysis of ClincalTrials.gov (AACT) Background! What is ClinicalTrials.gov?! ClinicalTrials.gov history! Key reporting requirements! Rationale
CIMI/IHTSDO DCM tooling ecosystem thoughts
 / DCM tooling ecosystem thoughts Thomas Beale openehr Foundation Stan Huff, MD Intermountain Healthcare Introduction These slides describe a possible semantic health modelling environment for /, featuring:
/ DCM tooling ecosystem thoughts Thomas Beale openehr Foundation Stan Huff, MD Intermountain Healthcare Introduction These slides describe a possible semantic health modelling environment for /, featuring:
Inside IHE: Pathology and Laboratory Medicine Webinar Series 2018
 Inside IHE: Pathology and Laboratory Medicine Webinar Series 2018 Presented by Raj Dash, MD, Duke - Planning Committee Co-Chair Riki Merrick, MPH Vernetzt, LLC - Planning Committee Co-Chair Agenda o Intro
Inside IHE: Pathology and Laboratory Medicine Webinar Series 2018 Presented by Raj Dash, MD, Duke - Planning Committee Co-Chair Riki Merrick, MPH Vernetzt, LLC - Planning Committee Co-Chair Agenda o Intro
Disclaimer This webinar may be recorded. This webinar presents a sampling of best practices and overviews, generalities, and some laws.
 Disclaimer This webinar may be recorded. This webinar presents a sampling of best practices and overviews, generalities, and some laws. This should not be used as legal advice. Itentive recognizes that
Disclaimer This webinar may be recorded. This webinar presents a sampling of best practices and overviews, generalities, and some laws. This should not be used as legal advice. Itentive recognizes that
MEDICATION OR OTHER SUBSTANCE FLOW. AHRQ Common Formats for Event Reporting Hospital Version 2.0a
 MEDICATION OR OTHER SUBSTANCE FLOW AHRQ Common Formats for Event Reporting Hospital Version 2.0a AHRQ Common Formats for Event Reporting Hospital Version 2.0a July 2018 Data elements required to be submitted
MEDICATION OR OTHER SUBSTANCE FLOW AHRQ Common Formats for Event Reporting Hospital Version 2.0a AHRQ Common Formats for Event Reporting Hospital Version 2.0a July 2018 Data elements required to be submitted
Igniting Innovation with Cerner s Open Developer Experience
 Igniting Innovation with Cerner s Open Developer Experience Matt Obenhaus Director and Solution Executive, Cerner Open Developer Experience Cerner s SMART on FHIR Ignite API Platform Cloud Based Scalable
Igniting Innovation with Cerner s Open Developer Experience Matt Obenhaus Director and Solution Executive, Cerner Open Developer Experience Cerner s SMART on FHIR Ignite API Platform Cloud Based Scalable
APHL Current S&I Framework Initiatives and Relevance to Public Health
 APHL Current S&I Framework Initiatives and Relevance to Public Health James B. Daniel, MPH Public Health Coordinator Office of the National Coordinator for Health IT June 4, 2014 Beyond Meaningful Use
APHL Current S&I Framework Initiatives and Relevance to Public Health James B. Daniel, MPH Public Health Coordinator Office of the National Coordinator for Health IT June 4, 2014 Beyond Meaningful Use
 http://www.connectingforhealth.nhs.uk/lra Semantic Interoperability I got your message I can understand it I know what you mean I believe you I know what I will do I got your message I understand it Syntax
http://www.connectingforhealth.nhs.uk/lra Semantic Interoperability I got your message I can understand it I know what you mean I believe you I know what I will do I got your message I understand it Syntax
JIC Standards Set - Patient Summary. Introduction January, 2016
 JIC Standards Set - Patient Summary Introduction January, 2016 Background In April 2015, the JIC, comprising the major international Standard Development Organisations (SDOs) in Health Informatics, declared
JIC Standards Set - Patient Summary Introduction January, 2016 Background In April 2015, the JIC, comprising the major international Standard Development Organisations (SDOs) in Health Informatics, declared
2016 Technical Update. August 2016
 2016 Technical Update August 2016 1 Foundational Standards Key Milestones CTR ODM XML Modernize CT Process CDISC Protocol Standards IntraChange Cross-team focus Survey CTR Content Governance: Consistent
2016 Technical Update August 2016 1 Foundational Standards Key Milestones CTR ODM XML Modernize CT Process CDISC Protocol Standards IntraChange Cross-team focus Survey CTR Content Governance: Consistent
ecr Digital Bridge Requirements Workgroup Meeting Minutes
 ecr Digital Bridge Requirements Workgroup Meeting Minutes Date: September 22, 2016 Conference Call: 1-866-774-4804 Time: 12:00PM 1:00PM ET Participant Code: 988-724-4096 Web Link: Web Conference registration:
ecr Digital Bridge Requirements Workgroup Meeting Minutes Date: September 22, 2016 Conference Call: 1-866-774-4804 Time: 12:00PM 1:00PM ET Participant Code: 988-724-4096 Web Link: Web Conference registration:
Welsh Clinical Leadership Training Fellows Scheme. Host Organisation Project Proposal Submission Template 2019
 Welsh Clinical Leadership Training Fellows Scheme Host Organisation Project Proposal Submission Template 2019 Name of Organisation: Project Title: Name of Medical Director: Section 1: 10% criteria Strategic
Welsh Clinical Leadership Training Fellows Scheme Host Organisation Project Proposal Submission Template 2019 Name of Organisation: Project Title: Name of Medical Director: Section 1: 10% criteria Strategic
Criteria Tested and Passed: f.7 - Xmit to Public Health Agencies health care surveys g.2 - Automated Measure Calculation
 PRODUCT UNDER TEST Organization Name: Allscripts Address of Vendor: 222 Merchandise Mart Plaza, Suite 2024 Chicago IL 60654 Test Product Name: Allscripts TouchWorks EHR Test Product Version-with-Release:
PRODUCT UNDER TEST Organization Name: Allscripts Address of Vendor: 222 Merchandise Mart Plaza, Suite 2024 Chicago IL 60654 Test Product Name: Allscripts TouchWorks EHR Test Product Version-with-Release:
Chair of the TMF Reference Model Steering Committee Co-founder of the TMF Reference Model with Lisa Mulcahy Chief Strategy Officer, Phlexglobal
 Karen Roy Chair of the TMF Reference Model Steering Committee Co-founder of the TMF Reference Model with Lisa Mulcahy Chief Strategy Officer, Phlexglobal Paul Fenton Co-chair of the TMF Reference Model
Karen Roy Chair of the TMF Reference Model Steering Committee Co-founder of the TMF Reference Model with Lisa Mulcahy Chief Strategy Officer, Phlexglobal Paul Fenton Co-chair of the TMF Reference Model
The Payer/Provider Perspective on Interoperability Da Vinci
 The Payer/Provider Perspective on Interoperability Da Vinci Lenel James, Business Lead, HIE & Innovation, Blue Cross Blue Shield Association Kirk Anderson, VP, and CTO, Cambia Health Solutions Mark Gingrich,
The Payer/Provider Perspective on Interoperability Da Vinci Lenel James, Business Lead, HIE & Innovation, Blue Cross Blue Shield Association Kirk Anderson, VP, and CTO, Cambia Health Solutions Mark Gingrich,
ISO/TC212/Working Group 2 Progress Report to JCTLM. Neil Greenberg, PhD, DABCC, Convenor, WG2 04 December 2017 Sèvres, France
 ISO/TC212/Working Group 2 Progress Report to JCTLM Neil Greenberg, PhD, DABCC, Convenor, WG2 04 December 2017 Sèvres, France ISO TC212 - Clinical laboratory testing and in vitro diagnostic test systems
ISO/TC212/Working Group 2 Progress Report to JCTLM Neil Greenberg, PhD, DABCC, Convenor, WG2 04 December 2017 Sèvres, France ISO TC212 - Clinical laboratory testing and in vitro diagnostic test systems
Interoperability Update. Denver Marriott Westminster OCTOBER
 Interoperability Update Denver Marriott Westminster OCTOBER 2 5 2017 Bridging the Gap Gary Steiner Dir. Public Health Solutions May 10 th, 2018 Bridging the Gap Problem Definition Cycle of Service for
Interoperability Update Denver Marriott Westminster OCTOBER 2 5 2017 Bridging the Gap Gary Steiner Dir. Public Health Solutions May 10 th, 2018 Bridging the Gap Problem Definition Cycle of Service for
UReady FAQs. 1. How do I access the UReady Continuity Planning tool?
 Emergency Management UReady FAQs 1. How do I access the UReady Continuity Planning tool? Go to www.miami.edu/uready. Click the green Access UReady button. Enter your Cane ID and Password and click login
Emergency Management UReady FAQs 1. How do I access the UReady Continuity Planning tool? Go to www.miami.edu/uready. Click the green Access UReady button. Enter your Cane ID and Password and click login
HL7 Health Care Devices and IEEE PoCD Work Group Meeting Minutes
 2017-01 HL7 Health Care Devices and IEEE 11073 PoCD Work Group Meeting Minutes 2017-01 HL7 Health Care Devices and IEEE 11073 PoCD Work Group Meeting Minutes... 1 Monday Q2... 2 Monday Q3... 3 Monday Q4...
2017-01 HL7 Health Care Devices and IEEE 11073 PoCD Work Group Meeting Minutes 2017-01 HL7 Health Care Devices and IEEE 11073 PoCD Work Group Meeting Minutes... 1 Monday Q2... 2 Monday Q3... 3 Monday Q4...
Name of employee: Revised: Title: Department name: (e.g. Patient Solutions Thrombosis) Org. Unit ID (optional): (e.g ) Location: Job type:
 1. JOB DESCRIPTION Please fill in this form and save it as described in SOP_000151. Background information Name of employee: (John Doe) LEO-id: (AAAUS) Revised: (date and version) Title: (E.g. Specialist,
1. JOB DESCRIPTION Please fill in this form and save it as described in SOP_000151. Background information Name of employee: (John Doe) LEO-id: (AAAUS) Revised: (date and version) Title: (E.g. Specialist,
SUBMISSION BY NORWAY ON APA AGENDA ITEM 7
 SUBMISSION BY NORWAY ON APA AGENDA ITEM 7 The Government of Norway is pleased to submit its views on Modalities and procedures for the effective operation of the committee to facilitate implementation
SUBMISSION BY NORWAY ON APA AGENDA ITEM 7 The Government of Norway is pleased to submit its views on Modalities and procedures for the effective operation of the committee to facilitate implementation
CLINICAL DATA MANAGEMENT
 CLINICAL DATA MANAGEMENT Clinical Data Management is involved in all aspects of processing the clinical data, working with a range of computer applications, database systems to support collection, cleaning
CLINICAL DATA MANAGEMENT Clinical Data Management is involved in all aspects of processing the clinical data, working with a range of computer applications, database systems to support collection, cleaning
NK9c, QUEST Executive Steering Committee Presentation pdf. Jim Murry Chief Information Officer
 1 EXECUTIVE STEERING COMMITTEE UPDATE OCTOBER 2011 Jim Murry Chief Information Officer 1 Agenda Members: Cygan, Ralph MD; Heydt, John MD; Kris Young; Winner, Cynthia RN ; Belmont, Terry ; Issai, Alice
1 EXECUTIVE STEERING COMMITTEE UPDATE OCTOBER 2011 Jim Murry Chief Information Officer 1 Agenda Members: Cygan, Ralph MD; Heydt, John MD; Kris Young; Winner, Cynthia RN ; Belmont, Terry ; Issai, Alice
SDMX as ISO Stuart Feder, Arofan Gregory, Chris Nelson Sep 2013 SDMX Global Conference 2013 OECD, Paris
 SDMX as ISO 17369 Stuart Feder, Arofan Gregory, Chris Nelson 11-13 Sep 2013 SDMX Global Conference 2013 1 Overview What it means How it happened Impact on SDMX 2 What it means Within the international
SDMX as ISO 17369 Stuart Feder, Arofan Gregory, Chris Nelson 11-13 Sep 2013 SDMX Global Conference 2013 1 Overview What it means How it happened Impact on SDMX 2 What it means Within the international
Manufacturers Disclosure Statement for Medical Device Security
 HIMSS Manufacturers Disclosure Statement for Medical Device Security December 8, 2004 Stephen L. Grimes, FACCE Chair, Medical Device Security Workgroup Healthcare Information and Management Systems Society
HIMSS Manufacturers Disclosure Statement for Medical Device Security December 8, 2004 Stephen L. Grimes, FACCE Chair, Medical Device Security Workgroup Healthcare Information and Management Systems Society
Changes in EU Clinical Data Requirements and Expectations
 Changes in EU Clinical Data Requirements and Expectations Waltham, MA (USA) 19 June 2018 Maria E. Donawa, M.D. President, Donawa Lifescience Consulting Srl Rome, Italy Introduction Meeting European clinical
Changes in EU Clinical Data Requirements and Expectations Waltham, MA (USA) 19 June 2018 Maria E. Donawa, M.D. President, Donawa Lifescience Consulting Srl Rome, Italy Introduction Meeting European clinical
APPENDIX E: Supporting Document (1)
 APPENDIX E: Supporting Document (1) MD050 Functional Design Disbursement Voucher Version: Prepared by: 1 of 10 Document History Revision Date Editor(s) Additions / Modifications Document Approval Version
APPENDIX E: Supporting Document (1) MD050 Functional Design Disbursement Voucher Version: Prepared by: 1 of 10 Document History Revision Date Editor(s) Additions / Modifications Document Approval Version
National Projects Requiring HL7 Standards Il Kon Kim, PhD Kyungpook National Univ., IHIS
 National Projects Requiring HL7 Standards 2009. 5. 8. Il Kon Kim, PhD Kyungpook National Univ., IHIS Contents 1. Health Information Exchange (HIE) 2. Clinical Information in BioBank 3. Personal Health
National Projects Requiring HL7 Standards 2009. 5. 8. Il Kon Kim, PhD Kyungpook National Univ., IHIS Contents 1. Health Information Exchange (HIE) 2. Clinical Information in BioBank 3. Personal Health
MEDICAL DEVICE CLINICAL INVESTIGATIONS AND ISO 14155
 MEDICAL DEVICE CLINICAL INVESTIGATIONS AND ISO 14155 EXECUTIVE SUMMARY Medical device regulations around the world generally require manufacturers of most types of medical devices to supply data as part
MEDICAL DEVICE CLINICAL INVESTIGATIONS AND ISO 14155 EXECUTIVE SUMMARY Medical device regulations around the world generally require manufacturers of most types of medical devices to supply data as part
Introduction to HL7 FHIR
 Introduction to HL7 FHIR HIMSS 2017 Grahame Grieve FHIR Product Director FHIR FHIR : Fast Healthcare Interoperability Resources (Pronounced Fire ) The hottest thing interoperability this year Insert other
Introduction to HL7 FHIR HIMSS 2017 Grahame Grieve FHIR Product Director FHIR FHIR : Fast Healthcare Interoperability Resources (Pronounced Fire ) The hottest thing interoperability this year Insert other
PRODUCT UNDER TEST TEST EVENT RESULT. Quality Manual ISO Test Lab Test Report
 PRODUCT UNDER TEST Organization Name: McKesson Corporation Address of Vendor: 5995 Windward Pkwy Alpharetta GA 30005 Test Product Name: Paragon for Hospitals 2015 Certified EHR Test Product Version-with-Release:
PRODUCT UNDER TEST Organization Name: McKesson Corporation Address of Vendor: 5995 Windward Pkwy Alpharetta GA 30005 Test Product Name: Paragon for Hospitals 2015 Certified EHR Test Product Version-with-Release:
MEANINGFUL USE CRITERIA PHYSICIANS
 MEANINGFUL USE CRITERIA PHYSICIANS The first list is of the 25 Stage 1 Meaningful Use criteria for eligible providers (EP) and comes from the proposed rule: "Medicare and Medicaid Programs; Electronic
MEANINGFUL USE CRITERIA PHYSICIANS The first list is of the 25 Stage 1 Meaningful Use criteria for eligible providers (EP) and comes from the proposed rule: "Medicare and Medicaid Programs; Electronic
A Process Model for Project Members Conforming to Enterprise Architecture
 A Process Model for Project Members Conforming to Enterprise Architecture Ralph Foorthuis Sjaak Brinkkemper Frank Hofman Technical Report UU-CS-2008-023 September 2008 Department of Information and Computing
A Process Model for Project Members Conforming to Enterprise Architecture Ralph Foorthuis Sjaak Brinkkemper Frank Hofman Technical Report UU-CS-2008-023 September 2008 Department of Information and Computing
REMIT LNG REPORTING SCHEMA USAGE GUIDELINES
 REMIT LNG REPORTING SCHEMA USAGE GUIDELINES ARIS IMPLEMENTATION AGENCY FOR THE COOPERATION OF ENERGY REGULATORS REVISION 1.0 DRAFT TABLE OF CONTENTS 1 INTRODUCTION... 3 1.1 Purpose... 3 1.1.1 Topics Covered...
REMIT LNG REPORTING SCHEMA USAGE GUIDELINES ARIS IMPLEMENTATION AGENCY FOR THE COOPERATION OF ENERGY REGULATORS REVISION 1.0 DRAFT TABLE OF CONTENTS 1 INTRODUCTION... 3 1.1 Purpose... 3 1.1.1 Topics Covered...
National Health Surveillance Agency. Public Consultation No. 255, dated June 19, 2017 D.O.U of 06/20/2017
 National Health Surveillance Agency www.anvisa.gov.br Public Consultation No. 255, dated June 19, 2017 D.O.U of 06/20/2017 The Board of the National Health Surveillance Agency, in the use of the powers
National Health Surveillance Agency www.anvisa.gov.br Public Consultation No. 255, dated June 19, 2017 D.O.U of 06/20/2017 The Board of the National Health Surveillance Agency, in the use of the powers
Update on the Trillium Bridge Project
 Update on the Trillium Bridge Project Catherine Chronaki euoffice@hl7.org www.trilliumbridge.eu Funded under FP7-610756 ehealth market is demanding! 2 HL7 CDA is a powerful tool for incremental interoperability
Update on the Trillium Bridge Project Catherine Chronaki euoffice@hl7.org www.trilliumbridge.eu Funded under FP7-610756 ehealth market is demanding! 2 HL7 CDA is a powerful tool for incremental interoperability
PDMP & Health IT Integration Initiative
 PDMP & Health IT Integration Initiative Standards and Interoperability Framework Johnathan Coleman, CISSP Initiative Coordinator, PDMP/HITI Office of Standards and Technology (OST)/Office of the Chief
PDMP & Health IT Integration Initiative Standards and Interoperability Framework Johnathan Coleman, CISSP Initiative Coordinator, PDMP/HITI Office of Standards and Technology (OST)/Office of the Chief
HL7 Overview. Byoung-Kee Yi, PhD. Samsung Medical Center Digital Health Dept, SAIHST, SKKU. HL7 Korea ISO/TC215 Health informatics
 HL7 Overview Byoung-Kee Yi, PhD Samsung Medical Center Digital Health Dept, SAIHST, SKKU HL7 Korea ISO/TC215 Health informatics HL7 International Health Level Seven Application layer (layer 7) in OSI model
HL7 Overview Byoung-Kee Yi, PhD Samsung Medical Center Digital Health Dept, SAIHST, SKKU HL7 Korea ISO/TC215 Health informatics HL7 International Health Level Seven Application layer (layer 7) in OSI model
Accelerating Clinical Trials Through Access to Real-World Patient Data
 Accelerating Clinical Trials Through Access to Real-World Patient Data Accelerating Clinical Trials Through Access to Real-World Patient Data Executive Summary: Leveraging Normalized Real-World Patient
Accelerating Clinical Trials Through Access to Real-World Patient Data Accelerating Clinical Trials Through Access to Real-World Patient Data Executive Summary: Leveraging Normalized Real-World Patient
HL7 Plenary EHR In Canada
 HL7 Plenary EHR In Canada September 11, 2006 Dennis Giokas Chief Technology Officer Canada Health Infoway, Inc Agenda Canada Health Infoway What is the EHR and It s Benefits Architecture and Standards
HL7 Plenary EHR In Canada September 11, 2006 Dennis Giokas Chief Technology Officer Canada Health Infoway, Inc Agenda Canada Health Infoway What is the EHR and It s Benefits Architecture and Standards
C Exam Questions Demo IBM. Exam Questions C
 IBM Exam Questions C9560-656 IBM SmartCloud Control Desk V7.5 Service Request Management Implementation Version:Demo 1. What data is stored in the Ticket table? A. Task records B. Solution records C. Ticket
IBM Exam Questions C9560-656 IBM SmartCloud Control Desk V7.5 Service Request Management Implementation Version:Demo 1. What data is stored in the Ticket table? A. Task records B. Solution records C. Ticket
HEALTH INFORMATICS STANDARD: AN EXAMPLE OF APPLICATION. Corso di Informatica Medica
 Università degli Studi di Trieste Corso di Laurea Magistrale in INGEGNERIA CLINICA HEALTH INFORMATICS STANDARD: AN EXAMPLE OF APPLICATION Corso di Informatica Medica Docente Sara Renata Francesca MARCEGLIA
Università degli Studi di Trieste Corso di Laurea Magistrale in INGEGNERIA CLINICA HEALTH INFORMATICS STANDARD: AN EXAMPLE OF APPLICATION Corso di Informatica Medica Docente Sara Renata Francesca MARCEGLIA
HL 7 FHIR and the Future of Interoperability. January 25, pm 3pm ET
 HL 7 FHIR and the Future of Interoperability January 25, 2017 2pm 3pm ET Housekeeping Issues All participants are muted To ask a question or make a comment, please submit via the chat feature and we will
HL 7 FHIR and the Future of Interoperability January 25, 2017 2pm 3pm ET Housekeeping Issues All participants are muted To ask a question or make a comment, please submit via the chat feature and we will
What is a Standards-based Healthcare Services Platform, and Why should it Matter to You?
 What is a Standards-based Healthcare Services Platform, and Why should it Matter to You? Session PCM3, February 19, 2017 Stanley M. Huff, MD, CMIO, Intermountain Healthcare 1 Speaker Introduction Stanley
What is a Standards-based Healthcare Services Platform, and Why should it Matter to You? Session PCM3, February 19, 2017 Stanley M. Huff, MD, CMIO, Intermountain Healthcare 1 Speaker Introduction Stanley
Guidance for Industry
 Guidance for Industry SPL Standard for Content of Labeling Technical Qs & As U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) December
Guidance for Industry SPL Standard for Content of Labeling Technical Qs & As U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) December
HL7 FHIR Update. Bob Milius, PhD Principal Research Scientist, CIBMTR IT Co-chair, HL7 Clinical Genomics Work Group
 HL7 FHIR Update Bob Milius, PhD Principal Research Scientist, CIBMTR IT Co-chair, HL7 Clinical Genomics Work Group There are no conflicts of interest to disclose. Health Level 7 International Non-profit,
HL7 FHIR Update Bob Milius, PhD Principal Research Scientist, CIBMTR IT Co-chair, HL7 Clinical Genomics Work Group There are no conflicts of interest to disclose. Health Level 7 International Non-profit,
Update of the Mandate for the Expert Group on International Economic and Social Classifications
 ESA/STAT/AC.234/5 11 March 2011 UNITED NATIONS DEPARTMENT OF ECONOMIC AND SOCIAL AFFAIRS STATISTICS DIVISION Meeting of the Expert Group on International Economic and Social Classifications New York, 18-20
ESA/STAT/AC.234/5 11 March 2011 UNITED NATIONS DEPARTMENT OF ECONOMIC AND SOCIAL AFFAIRS STATISTICS DIVISION Meeting of the Expert Group on International Economic and Social Classifications New York, 18-20
Meeting Minutes. Date: 12/20/2017 Start Time: 3:00pm Stop Time: 4:05pm
 Meeting Name: QIC Location: Board Room Date: 12/20/2017 Start Time: 3:00pm Stop Time: 4:05pm Note Taker: Heather Macdonald Facilitator: Attendees:, Kim Koons, Heather MacDonald, Jil Neuman See attached
Meeting Name: QIC Location: Board Room Date: 12/20/2017 Start Time: 3:00pm Stop Time: 4:05pm Note Taker: Heather Macdonald Facilitator: Attendees:, Kim Koons, Heather MacDonald, Jil Neuman See attached
EXHIBITOR MARKETING TOOLS AND SERVICES
 EXHIBITOR MARKETING TOOLS AND SERVICES Quedah Locket Marketing Manager 212-584-9400 x505 qlocket@rfidjournal.com Alan McIntosh Senior Director of Sales 212-584-9400 x4 amcintosh@rfidjournal.com Key Deadline
EXHIBITOR MARKETING TOOLS AND SERVICES Quedah Locket Marketing Manager 212-584-9400 x505 qlocket@rfidjournal.com Alan McIntosh Senior Director of Sales 212-584-9400 x4 amcintosh@rfidjournal.com Key Deadline
REST-based APIs: Overview and Progress Since #SIIM14
 REST-based APIs: Overview and Progress Since #SIIM14 #DICOMweb, HL7 #FHIR and More Brad Genereaux Agfa HealthCare DICOM WG-27 Industry Co-Chair @integratorbrad Disclosures Employee of Agfa HealthCare DICOM
REST-based APIs: Overview and Progress Since #SIIM14 #DICOMweb, HL7 #FHIR and More Brad Genereaux Agfa HealthCare DICOM WG-27 Industry Co-Chair @integratorbrad Disclosures Employee of Agfa HealthCare DICOM
THE AMRITA EDGE AMRITA THE AMRITA EDGE
 THE EDGE THE EDGE Many hospitals have experienced tremendous challenges and in some cases failures with the implementation of an HIS or EHR system. Often this is the result of an implementation plan that
THE EDGE THE EDGE Many hospitals have experienced tremendous challenges and in some cases failures with the implementation of an HIS or EHR system. Often this is the result of an implementation plan that
Wolf EMR. New Product Features and Roadmap Update. Rowan Helmer, Sr. Product Manager. Western EMR User Conference 2016
 Wolf EMR New Product Features and Roadmap Update Rowan Helmer, Sr. Product Manager Agenda 1. What have we done in 2016? 2. Coming Soon! 3. Roadmap Planning 4. Questions 2 What have we done in 2016? Driving
Wolf EMR New Product Features and Roadmap Update Rowan Helmer, Sr. Product Manager Agenda 1. What have we done in 2016? 2. Coming Soon! 3. Roadmap Planning 4. Questions 2 What have we done in 2016? Driving
Meaningful Use Physician Reporting: Successes and Lessons Learned
 Meaningful Use Physician Reporting: Successes and Lessons Learned NAACCR 2017 Annual Meeting June 2017 Wendy Blumenthal, MPH Cancer Surveillance Branch Division of Cancer Prevention and Control Centers
Meaningful Use Physician Reporting: Successes and Lessons Learned NAACCR 2017 Annual Meeting June 2017 Wendy Blumenthal, MPH Cancer Surveillance Branch Division of Cancer Prevention and Control Centers
HL7 and Service-oriented Architecture (SOA) Ambassador Briefing
 HL7 and Service-oriented Architecture (SOA) Ambassador Briefing February 2010 Topics Understanding Service-oriented Architecture (SOA) The case for Healthcare SOA Standards Introducing HSSP Status of Standards
HL7 and Service-oriented Architecture (SOA) Ambassador Briefing February 2010 Topics Understanding Service-oriented Architecture (SOA) The case for Healthcare SOA Standards Introducing HSSP Status of Standards
Agenda. Project Overview. Workgroups. Outlook. Goals, Motivation, Background Organization, Members, Industry Use
 Agenda Project Overview Goals, Motivation, Background Organization, Members, Industry Use Workgroups Domains and current Scope Interaction with other Activities Outlook LOTAR 2015 All rights reserved 14
Agenda Project Overview Goals, Motivation, Background Organization, Members, Industry Use Workgroups Domains and current Scope Interaction with other Activities Outlook LOTAR 2015 All rights reserved 14
HL7 Working Group Meeting May 2008 Meeting Report
 HL7 Working Group Meeting May 2008 Meeting Report HL7 Working Group Meeting May 08 Report prepared by Heather Grain and Elizabeth Hanley 1. The Working Group Meeting International Participation and Strategy
HL7 Working Group Meeting May 2008 Meeting Report HL7 Working Group Meeting May 08 Report prepared by Heather Grain and Elizabeth Hanley 1. The Working Group Meeting International Participation and Strategy
Dr. Karen DeSalvo Office of the National Coordinator for Health Information Technology U.S. Department of Health and Human Services
 March 20, 2015 Dr. Karen DeSalvo Office of the National Coordinator for Health Information Technology U.S. Department of Health and Human Services RE: A Shared Nationwide Interoperability Roadmap Draft
March 20, 2015 Dr. Karen DeSalvo Office of the National Coordinator for Health Information Technology U.S. Department of Health and Human Services RE: A Shared Nationwide Interoperability Roadmap Draft
Integrating Standards to Achieve Semantic Interoperability. Dr Ken Lunn Director of Data Standards
 Integrating Standards to Achieve Semantic Interoperability Dr Ken Lunn Director of Data Standards www.connectingforhealth.nhs.uk in the National Programme Development Programmes ETP C&B PBR SCR SUS Content
Integrating Standards to Achieve Semantic Interoperability Dr Ken Lunn Director of Data Standards www.connectingforhealth.nhs.uk in the National Programme Development Programmes ETP C&B PBR SCR SUS Content
