Efficient Ethanol Production from Glucose, Lactose, and Xylose by Recombinant Escherichia colit
|
|
|
- Bethanie Phillips
- 5 years ago
- Views:
Transcription
1 APPLIED AND ENVIRONMENTAL MICROBIOLOGY. Aug p /89/ $02.00/0 Copyright 1989, American Society for Microbiology Vol. 55 No. 8 Efficient Ethanol Production from Glucose, Lactose, and Xylose by Recombinant Escherichia colit FLAVIO ALTERTHUM' AND L. 0. INGRAM2* Department of Microbiology, Inistiti,to de Ciencias Biomnedic as, Univer.sidade de Sao Paiulo, Sao Pal/o, Brazi/ 05508, and Department of Microbiology anid Cell Science, McCarty Hall, Univer-sity of Florida, Gainesville, Florida Received 3 March 1989/Accepted 12 May 1989 Lactose and all of the major sugars (glucose, xylose, arabinose, galactose, and mannose) present in cellulose and hemicellulose were converted to ethanol by recombinant Escherichia coli containing plasmid-borne genes encoding the enzymes for the ethanol pathway from Zymomonas mobilis. Environmental tolerances, plasmid stability, expression of Z. mobilis pyruvate decarboxylase, substrate range, and ethanol production (from glucose, lactose, and xylose) were compared among eight American Type Culture Collection strains. E. coli ATCC 9637(pLOI297), ATCC 11303(pLOI297), and ATCC 15224(pLOI297) were selected for further development on the basis of environmental hardiness and ethanol production. Volumetric ethanol productivities per hour in batch culture were 1.4 g/liter for glucose (12%), 1.3 g/liter for lactose (12%), and 0.64 g/liter for xylose (8%). Ethanol productivities per hour ranged from 2.1 g/g of cell dry weight with 12% glucose to 1.3 g/g of cell dry weight with 8% xylose. The ethanol yield per gram of xylose was higher for recombinant E. coli than commonly reported for Saccharomyces cerevisiae with glucose. Glucose (12%), lactose (12%), and xylose (8%) were converted to (by volume) 7.2% ethanol, 6.5% ethanol, and 5.2% ethanol, respectively. Most fuel ethanol is currently produced from hexose sugars in corn starch or cane syrup by using either Sa(cc(harovmyces cerei'isiae or Zyinomtonas iiobilis (17, 24). However, these are relatively expensive sourses of biomass sugars and have competing value as foods. Starches and sugars represent only a fraction of the total carbohydrates in plants. The dominant forms of plant carbohydrate in stems, leaves, hulls, husks, cobs, etc., are the structural wall polymers, cellulose and hemicellulose (11). Hydrolysis of these polymers releases a mixture of neutral sugars which includes glucose, xylose, mannose, galactose, and arabinose. No organism has been found in nature which can rapidly and efficiently metabolize these different sugars into ethanol or any other single product of value. With the cloning of the genes encoding the enzymes for the ethanol pathway from Z. itnobilis (2-4, 18, 23), it became possible to genetically engineer ethanol production in bacteria which are able to utilize a variety of sugars (8, 9, 30, 31). In most cases, both alcohol dehydrogenase and pyruvate decarboxylase activities were needed for efficient sugar conversion to ethanol (2, 8). Since Z. inobilis pyruvate decarboxylase has a much lower K,,, for pyruvate than competing acidogenic pathways in fermentation, expression of the Z. mobilis enzymes effectively diverted carbon flow in recombinant E. coli (8, 9), Klebsiella planticola (30), and Erw- inia ch-ysanlthemni (31) to ethanol. E. coli has been shown to metabolize all major sugars which are constituents of plant biomass (12), and our studies have focused on this organism (8, 9). Initial studies used strain S17-1 (26) and related strains of E. c oli which contain integrated mobilization elements for plasmid conjugation, recombinants which are unsuitable for commercial application. In this study, we have compared eight strains of E. c oli from the American Type Culture Collection (ATCC) for environmental hardiness and suitability for the further development of commercial ethanol production. * Corresponding author. Florida Agricultural Experiment Station publication MATERIALS AND METHODS Strains and media. E. coli strains were obtained from the ATCC (Table 1). These were grown in a shaking water bath at 30 C in Luria broth (14) containing tryptone (10 g/liter), yeast extract (5 g/liter), sodium chloride (5 g/liter), and a fermentable sugar. Glucose and lactose were added at concentrations of 100 g/liter and xylose was added at a concentration of 80 g/liter unless indicated otherwise. Sugars were autoclaved separately (121 C, 15 min) at double strength in distilled water. Xylose (Sigma Chemical Co., St. Louis, Mo.) solutions were acidic and were neutralized with sodium hydroxide prior to autoclaving; failure to neutralize resulted in extensive browning and decomposition. Similar fermentation results were obtained with sugars which were autoclaved or filter sterilized. Survival in broth and on plates of recombinant strains containing genes encoding the enzymes for the ethanol pathway required the presence of a fermentable sugar. Where indicated, tetracycline was added at a final concentration of 10 mg/liter. Environmental hardiness. Strains were tested for their resistance to sodium chloride, sugars, low ph, and ethanol. Concentrations of sodium chloride and sugars in Table 1 include those in the original medium. The original ph of the medium was 6.8; this was adjusted to lower values with HCI where indicated. Acidified media were sterilized by filtration. Ethanol was added to autoclaved medium after cooling. Sugars were autoclaved separately. Overnight cultures grown in each respective sugar in the absence of test agent were diluted 60-fold into culture tubes (13 by 75 mm) containing 3 ml of test media. Growth was measured as optical density at 550 nm (OD,,() after 48 h. An OD of 1.0 is equivalent to 0.25 mg of cell protein per ml and 0.33 mg of cell dry weight. In tests of environmental hardiness, a final OD below 0.2 reflected less than two doublings and was considered negligible. Sugar utilization. Sugar utilization was tested in two ways. Strains which developed red colonies on MacConkey agar supplemented with 2% carbohydrate were scored positive for sugar utilization (25). Cells were also tested with EC 1943
2 1944 ALTERTHUM AND INGRAM TABLE 1. Growth of E. coli in Luria broth containing 100 g of glucose per liter under chemical and physical stresses Stress Growth" of E. co/i ATCC str-ain: t) NaCI (g/liter) ± ± Ethanol (% by volume) 3.8 +i t) Acidity (ph) ± ± Temperature ( C) ± 'Growth was scored as 0 (lcss thain two dolublings in ODs,,(). + (two to four doublings), or + + (over four doublings). plates (Biolog, Inc., Hayward, Calif.) according to the directions of the manufacturer. The Biolog plates were rapid and convenient, detecting NADH production (conversion of a tetrazolium salt to the insoluble formazan) as a measure of substrate utilization. Both methods were in complete agreement for the 13 sugars examined. Genetic methods and plasmid constructions. Two new plasmids which contained resistance genes for ampicillin and tetracycline as selectable markers were constructed by standard methods (16). The ethanol production operon (PET operon) containing a cryptic Z. inobilis promoter, pyruvate decarboxylase, alcohol hydrogenase, and transcriptional terminator was removed as a 5.2-kilobase EcoRI fragment from ploi (8) and inserted into the EcoRl site of pbr322 to produce ploi The plasmid ploi297 was constructed by inserting the 2.6-kilobase EcoRI fragment from pcos 2EMBL (22) containing the tetracycline resistance gene into the Sall site of ploi295 (9). Cohesive ends were removed by treatment with the Klenow fragment of E. (oli DNA polymerase (16) before ligation. Plasmids were introduced into the different strains of E. (/li by transformation using the calcium chloride procedure of Mandel and Higa (15). Selections were made on solid medium containing 2% glucose and tetracycline. Plasmid stability is expressed as the percentage of cells retaining antibiotic markers after 25 generations of growth in the absence of antibiotic selection. Pyruvate decarboxylase. Pyru vate decarboxylase activity was measured as previously described (3, 18), except that cells were harvested at an OD of 4.0, approximately half of maximal growth. Fermentation experiments. Luria broth was modified for fermentation experiments by the inclusion of potassium phosphate buffer (ph 7.0) at a final concentration of 0.2 M. Phosphate buffer, complex medium components, and sugars were autoclaved separately and mixed after cooling. Tetracycline was included at a concentration of 10 mg/liter. Inocula were grown from freshly isolated colonies for 24 h, washed in the fermentation medium to be tested, and added to an initial OD,,, of approximately 1.0. Fermentations were carried out at 30 or 37 C in 100-ml volumetric flasks containing 80 ml of broth and fitted with rubber septa and 25-gauge needles to allow gas to escape. Fermentation flasks were immersed in a temperature-controlled water bath and stirred by a magnetic stirrer at 100 rpm. Ethanol concentration was measured by gas chromatography as previously described (5) and is expressed as percentage by volume. The conversion efficiency was calculated on the basis of sugar added, assuming that 100% efficiency results in the production of ml of ethanol (10.2 g) per 20 g of glucose or xylose and 13.5 ml of ethanol (10.8 g) per 20 g of lactose. RESULTS APPL. ENVIRON. MICROBIOL. Environmental hardiness and sugar utilization. Before the introduction of plasmids for ethanol production, the growth of eight different strains of E. (oli was compared for environmental hardiness. Table 1 summarizes the results obtained with medium containing glucose. Similar though not identical results were obtained with media containing lactose and xylose (data not shown). Strains ATCC 8677, ATCC 8739, and ATCC were particularly sensitive to inhibition by sodium chloride. Strains ATCC 8677 and ATCC were inhibited by low concentrations of ethanol. Strains ATCC 9637 and ATCC were the most resistant to low ph, although all strains except ATCC grew for at least two to four doublings at ph 4.0. All strains grew at 45 C. with limited growth at higher temperatures; none could be subcultured above 45 C. All strains grew in media containing 200% glucose, 20%, lactose, or 12% xylose. All strains tested utilized glucose, fructose, galactose, mannose, arabinose, lactose, glucuronic acid, and galacturonic acid. Strain ATCC did not utilize xylose. Maltose and maltotriose were not used by ATCC and ATCC All strains exhibited a weakly positive reaction with cellobiose. Only strain ATCC 9637 utilized sucrose. These results indicated that on the basis of environmental hardiness, ATCC 8677, ATCC 11775, and ATCC were less suitable than the other four strains for further development. Strain ATCC also lacked the ability to metabolize xylose, one of the most abundant sugars in biomass. Growth, plasmid stability, and expression of pyruvate decarboxylase. Recombinant strains harboring plasmids with the genes for ethanol production grew as unusually large colonies which became yellow after 24 to 48 h on solid medium containing a fermentable sugar. In liquid medium, the final cell densities of these recombinants were two to three times higher than that of the control lacking plasmid (Table 2). No transformants were obtained after several attempts from ATCC 14948(pLOI297) or from ATCC 11775(pLOI308-11). Strain ATCC did not utilize xylose, and recombinants of this strain did not grow to higher densities than the control with xylose as the fermentable sugar, although increased growth was observed with lactose and glucose. Plasmid stability was examined after growth in medium containing glucose for 25 generations (Table 3). Both plasmids contained the same replicons and were maintained well in all strains except ATCC 8677 and ATCC The expression of Z. Imobilis pyruvate decarboxylase activity was examined after growth in the presence of
3 VOL. 55, 1989 ETHANOL PRODUCTION BY E. COLI 1945 Sugar Plasmid TABLE 2. Growth of E. coli strains harboring plo1297 and plo Final OD,5, of E. co/i ATCC str-ain: Glucose None plo " plo Lactose None plo plo ( 10.0 Xylose None plo plo No data available. tetracycline (Table 4). With ploi297, Z. inobilis genes are expressed under the control of the E. c(li kac promoter; ploi utilizes the cryptic Z. inobilis promoter for expression of the PET operon. Strains ATCC (ploi297), ATCC 11775(pLOI297), and ATCC (ploi297) contained the highest levels of activity. Comparison of ethanol production during batch fermentation. All genetically engineered strains of E. c(li produced significant amounts of ethanol from sugars (Table 5). Preliminary experiments with strain ATCC 15224(pLOI297) indicated that higher levels of ethanol were produced in medium containing 0.2 M potassium phosphate buffer (ph 7.0). With 15% glucose, higher ethanol levels were produced at 30 C than at 37 C after 48 h. The fermentation of lactose and xylose was examined only at the lower temperature, 30 C. In general, higher levels of ethanol were produced by strains harboring ploi297 than by those with ploi Strains ATCC 11303(pLOI297), ATCC 11775(pLOI297), and ATCC 15224(pLOI297) produced the highest levels of ethanol after 48 h from 15% glucose, 5.8 to 6.9% by volume. Most strains were less tolerant of xylose in initial experiments, and comparisons of fermentation were carried out with 8% xylose. Strains ATCC 9637(pLOI297), ATCC (ploi297), and ATCC 15224(pLOI297) produced the highest levels of ethanol (4.8 to 5.2%) from 8% xylose after 72 h. All strains grew well in 15% lactose. Strains ATCC 11303(pLOI297) and ATCC 15224(pLOI297) produced the highest levels of ethanol from lactose after 48 h (6.1 and 5.6%, respectively). On the basis of these comparative studies, strains ATCC 11303(pLOI297) and ATCC 15224(pLOI297) appeared to be TABLE 3. Stability of ploi297 and ploi after 25 generations of growth with glucose in the absence of antibiotic selection ATCC strain plo1297 % of cells retaining plasmid plo () " No data available. the best constructs for ethanol production. The time courses of growth and ethanol production were examined with both strains in 12% glucose, 12% lactose, and 8% xylose (Fig. 1). Cell mass increased approximately 10-fold, reaching a final density of 3.6 g of dry weight per liter. With xylose, cell mass increased at half the rate observed with glucose or lactose. Ethanol production and growth were approximately linear for the three sugars until the concentration of ethanol reached 5%. To compute the conversion efficiency of sugar to ethanol, final ethanol concentrations after 120 h were averaged from three sets of experiments (Table 6). The final concentration of ethanol in cultures grown with 12% glucose was 7.2% (by volume), representing 94% of theoretical yield from glucose. With 12% lactose, the final ethanol concentration was 6.5%, 80% of the theoretical yield from lactose. With 8% xylose, we consistently obtained yields of 100% and higher. These high yields during slower growth with xylose may reflect the conversion of pyruvate from the catabolism of complex nutrients into ethanol, in addition to conversion of pyruvate from glucose. The rate of ethanol production was computed from the graphs in Fig. 1 and are summarized in Table 6. Volumetric productivity of ethanol ranged from 1.4 g/liter per h for glucose to 0.64 g/liter per h for xylose. Specific productivity of ethanol was highest during the initial growth period for each of the three sugars. The highest productivity was obtained with glucose, 2.1 g of ethanol per g of cell dry weight per h. The highest yield of eth-anot per g of sugar was obtained with xylose, exceeding the maximal theoretical yield for sugar alone. TABLE 4. Expression of Z. mnobilis pyruvate decarboxylase in E. coli strains harboring plo1297 and plo during growth with glucose ATCC strain plo1297 Pyruvate decarboxylase activity" plo h International units per milligram of cell protein. " No data available.
4 1946 ALTERTHUM AND INGRAM APPL. ENVIRON. MICROBIOL. TABLE 5. Ethanol production in batch fermentations from glucose (48 h), xylose (72 h), and lactose (48 h) by E. coli strains harboring plo1297 and plo Carbohydrate a % Ethanol (vol/vol) produced by E. coli ATCC strain: (%) Plasmid C Glucose (15)" ploi plo Glucose (15)" ploi ploi Lactose (15)" ploi plo Xylose (8)"' plo plo 'Incubation with plasmids was at 37'C. "Incubation with plasmids was at 30'C. -, No data available. Experiments were conducted with ATCC 11303(pLOI297) to examine ethanol production from arabinose, galactose, and mannose. Ethanol concentrations of 3 to 4% were obtained after 48 h at 30 C but were not investigated further. These sugars are metabolized by pathways similar to those for glucose and xylose and would be expected to produce analogous yields (12). DISCUSSION Brau and Sahm (2) first demonstrated that ethanol production could be increased in recombinant E. coli by the overexpression of Z. mobilis pyruvate decarboxylase, although very low ethanol concentrations were produced. Subsequent studies by Tolan and Finn extended this work by using two other enteric bacteria (E. chrysanthemi [301 and K. planticola [31]) and achieved much higher levels of ethanol from hexoses, pentoses, and sugar mixtures. Alcohol dehydrogenase is also essential for this conversion, and ethanol production in these previous recombinant systems was dependent on native activities in the host organisms. Mutant E. coli which overproduced native alcohol dehydrogenase produced much higher levels of ethanol with Z. mobilis pyruvate decarboxylase than E. coli recombinants with the native activity did (9). The dependence upon host alcohol dehydrogenase activity was eliminated by combining Z. mobilis genes encoding alcohol dehydrogenase II and pyruvate decarboxylase to form a portable, plasmid-borne operon for ethanol production, the PET operon (8, 9). Considering the vast amount of information available concerning the physiology, genetics, and metabolism of E. coli, this organism is an obvious choice for further exploration of ethanol production. E. coli has an extremely wide substrate range which includes hexoses, pentoses, dissacharides, hexuronic acids, sugar alcohols, purines, pyrimidines, and amino acids, among others. Many potentially useful genes have been cloned from other organisms and expressed in E. coli, the most widely used host for molecular genetics. A variety of factors need to be considered in selecting E. coli strains suitable for ethanol production, including substrate range and environmental hardiness (sugar tolerance, salt tolerance, ethanol tolerance, tolerance to low ph, and thermal tolerance). Strain ATCC 9637 (Waksman strain W) appeared superior in terms of environmental hardiness, C) LJD Jo 9-- LLJ C) >1 -- Time (hours) 6 11, :::a-~-~ Al 9 3 1~~~~~~~~~~~~~~~ 3I3 2a~~~~~~~~~~~~~ Time (hours) CD 12 3 a) cn 9 O 6* a-n a_n 3,3 'UL Time (hours) FIG. 1. Growth and ethanol production from 12% glucose (A), 12% lactose (B), and 8% xylose (C). Symbols:, ethanol; - - -, cell mass; 0, strain ATCC 11303(pLO1297); A, strain ATCC 15224(pLO1297) C-,) tn cn s cn Ln =3 C-, CD 'n W _^ Ln nt C> =:
5 VOL. 55, 1989 TABLE 6. Average kinetic parameters for ethanol production by ATCC 11303(pLO1297) and ATCC 15224(pLOI297) Productivity E Sugar (%) Yield" Efficicncy Ethinol' Volumetric' Specific" (Ce) Glucose (12) Lactose (12) Xylose (8) Grams of ethanol per gram of sugar. "Calculated as: (ethanol produced/theoretical maximum from sugair substrate) x 100. Final ethanol concentration in grams per liter. ' Grams of ethanol per liter pei- hour. Grams of ethanol per g of cell dry weight per hour. although ethanol production from glucose was lower than with other strains. ATCC (Luria strain B) and ATCC (Kepes strain ML308) containing ploi297 produced the highest levels of ethanol and exhibited acceptable levels of environmental hardiness. Plasmids were quite stable in these two constructs, and they were selected as the best candidates for further development of ethanol production. Both constructs expressed the high levels of Z. iniobilis pyruvate decarboxylase which are required for efficient ethanol production (9). All major sugar components of plant biomass were converted to ethanol by recombinant E. coli containing the ethanol pathway from Z. inobilis. The conversion efficiency of glucose and xylose into ethanol exceeded that for S. erev'isiae (13) and pentose-fermenting yeasts systems ( , 28). Xylose was converted to ethanol by recombinant E. coli with a higher efficiency than glucose by S. (-ei-(eil,sia(e (13). The unusually high ethanol yields with xylose (over 100%7 of theoretical values) may include ethanol derived from the catabolism of complex nutrients. Many amino acids and complex-medium components are catabolized to glycolytic intermediates which are converted to pyruvate. This pyruvate could then be converted to ethanol. The ethanol tolerance of E. c oli (6, 7) is lower than that of S. (erevisiae (6, 19) or Z. mobilis (20, 21). However, it is unlikely that this will be a limitation for ethanol production from biomass. The hydrolysis of biomass by a combination of chemical and enzymatic methods typically yields sugar concentrations well below 12% (1). Recombinant E. (olibased ethanol production would result in a small increase in the costs of product recovery. This would be offset by increased ethanol yield and decreased costs of biomass feedstocks. Although further investigations are needed to optimize ethanol production by recombinant E. coli, the conversion rates observed in batch culture for glucose and lactose fermentation compared favorably with those observed for yeasts (13, 29). The conversion rate of xylose to ethanol equaled or exceeded previous rates by yeasts even in expensive systems using added xylose isomerase ( ). The ability of recombinant E. (coli to convert the diversity of sugars present in biomass to ethanol offers potential for the commercial expansion of fuel ethanol production by using alternative feedstocks which do not have competing value as food. Such expansion could involve a blend of current feedstocks with cellulosic biomass from undervalued agricultural products. ETHANOL PRODUCTION BY E. COLI 1947 ACKNOWLEDGMENTS This work was supported in part by the Florida Agriculturtal Experiment Station and by grants from the Department of Agriculture. Alcohol Fuels Program ( ) and from the Department of Energy. Office of Basic Energy Research (FG ER3574). We ar-e graiteful to the Conselho Nacional de Desenvolvimento Cientifico e Teconologico. Brazil (Processo /87) for providing support for Flavio Alterthum. LITERATURE CITED 1. Beck, M. J Faictor-s affecting efficiency of biomass fermentation to ethanol. Biotechnol. Bioeng. Symp. 17: Brau, B., and H. Sahm Cloning and expression of the structural gene for pyruvate decarboxylase of Zyoinoonas iniobilis in Escherichia (oli. Arch. Microbiol. 144: Conway, T., Y. A. Osman, J. I. Konnan, E. M. Hoffmann, and L. 0. Ingram Promoter and nucleotide sequences of the Zv10nomonas mzob/ilius pyruvate decarboxylase. J. Bacteriol. 169: Conway, T., G. W. Sewell, Y. A. Osman, and L. 0. Ingram Cloning and sequencing of the alcohol dehydrogenase 11 gene from Zvinomona)ns miobdilis. J. Bacteriol. 169: Dombek, K. M., and L. 0. Ingram Determination of intracellular concentrations of ethanol in Saccharon'vccs (cereviisima during fermentation. Appl. Environ. Microbiol. 51:197-2)0(. 6. Ingram, L Microbial tolerance to alcohols: role of the cell membrane. Trends Biotechnol. 4: Ingram, L Effects of ethanol on E.sclherichia coli. p In N. van Uden (ed.). Alcohol toxicity in yeasts and bacteria. CRC Press. Inc.. Boca Raton. Fla. 8. Ingram, L. O., and T. Conway Expression of different levels of the ethanologenic enzymes from ZYnlononas,tnohili.s in recombinant strains of Lscherichia (o/i. AppI. Environ. Microbiol. 54: Ingram, L. O., T. Conway, D. P. Clark, G. W. Sewell, and J. F. Preston Genetic engineering of ethanol production in Escher/chia co/i. Appl. Environ. Microbiol. 53: Jeffries, T. W., and H. K. Sreenath Fermentation of hemicellulose sugars and sugar mixtures by Cantcdidai slilioitw(e. Biotechnol. Bioeng. 31: Krull, L. H., and G. 1. Inglet Analysis of neutral carbohydrates in agricultural residues by gas-liquid chromatography. J. Agric. Food Chem. 28: Lin, E. C. C Dissimilatory pathways for sugars, polyols, and carboxylates. p In F. C. Neidhardt, J. L. Ingraham. K. B. Low. B. Magasanik. and M. Schaechter (ed.). E.scherichia coli and Salmontella typlhimumrium, vol. 1. American Society for Microbiology. Washington. D.C. 13. Lovitt, R. W., B. H. Kim, G.-J. Shen, and J. G. Zeikus Solvent production by microorganisms. Crit. Rev. Biotechnol. 7: Luria, S. E., and M. Delbruck Mutations of bacteria from virus sensitivity to virus resistance. Genetics 28: Mandel, M., and A. Higa Calcium dependent bacteriophage DNA infection. J. Mol. Biol. 53: Maniatis, T., E. F. Fritsch, and J. Sambrook Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory. Cold Spring Harbor-. N.Y. 17. Murtagh, J. E Fuel ethanol production-the U.S. experience. Process Biochem. 21: Neale, A. D., R. K. Scopes, R. E. H. Wettenhall, and N. J. Hoogenraad Nucleotide sequence of the pyruvate decairboxylase gene from Zxvnolnonas It10bili.s. Nucleic Acids Res. 15: Ohta, K., and S. Havashida Role of Tween 80 and monolein in a lipid-sterol-protein complex which enhances ethanol tolerance of sake yeasts. Appl. Environ. Microbiol. 46: Ohta, K., K. Supanwong, and S. Hayashida Environmentatl effects on ethanol tolerance of Zymnoinona.s minobilis'. J. Ferm. Technol. 59: Osman, Y. A., and L. 0. Ingram Zv,ynolnona.s iiobilis mutants with an increatsed rate of alcohol pr-oduction. Appl. Environ. Microbiol. 53: Poustka, A., H. R. Rackwitz, A.-M. Firschauf, and H. Lehrach.
6 1948 ALTERTHUM AND INGRAM Selective isolation of cosmid clones by homologous recombination in Escherichia coli. Proc. Natl. Acad. Sci. USA 81: Reynen, M., and H. Sahm Comparison of the structural genes for pyruvate decarboxylase in different Zymomonas mobilis strains. J. Bacteriol. 170: Rosillo-Calle, F., and D. 0. Hall Brazilian alcohol: food versus fuel? Biomass 12: Silhavy, T. J., and J. R. Beckwith Uses of lac fusions for the study of biological problems. Microbiol. Rev. 49: Simon, R., U. Priefer, and A. Puhler A broad host range mobilization system for in i'iio genetic engineering: transposon mutagenesis in gram negative bacteria. Biotechnology 1: Skoog, K., and B. Hahn-Hagerdal Xylose fermentation. AppI. ENVIRON. MICROBIOL. Enzyme Microbiol. Technol. 10: Slininger, P. J., R. J. Bothast, M. R. Okos, and M. R. Ladisch Comparative evaluation of ethanol production by xylosefermenting yeasts presented high xylose concentrations. Biotechnol. Lett. 7: Terrell, S. L., A. Bernard, and R. B. Bailey Ethanol from whey: continuous fermentation with a catabolite repressionresistant Saccharotnvces cerev'isiae mutant. Appl. Environ. Microbiol. 48: Tolan, J. S., and R. K. Finn Fermentation of D-xylose and L-arabinose to ethanol by Erwinia Chrysanthemni. Appl. Environ. Microbiol. 53: Tolan, J. S., and R. K. Finn Fermentation of D-xylose to ethanol by genetically modified Klebsiella planticola. Appl. Environ. Microbiol. 53:
Genetic Engineering of Ethanol Production in Escherichia colit
 APPLIED AND ENVIRONMENTAL MICROBIOLOGY, OCt. 1987, p. 2420-2425 0099-2240/87/102420-06$02.00/0 Copyright 1987, American Society for Microbiology Vol. 53, No. 10 Genetic Engineering of Ethanol Production
APPLIED AND ENVIRONMENTAL MICROBIOLOGY, OCt. 1987, p. 2420-2425 0099-2240/87/102420-06$02.00/0 Copyright 1987, American Society for Microbiology Vol. 53, No. 10 Genetic Engineering of Ethanol Production
Applications of Molecular Biotechnology
 Applications of Molecular Biotechnology Ethanol Production from Cellulosic Biomass David R. Shonnard CM4710 Biochemical Processes November 28, 2003 Environmental Issues in Ethanol Production and Use A.J.
Applications of Molecular Biotechnology Ethanol Production from Cellulosic Biomass David R. Shonnard CM4710 Biochemical Processes November 28, 2003 Environmental Issues in Ethanol Production and Use A.J.
CM4125 Bioprocess Engineering Lab: Week 4: Introduction to Metabolic Engineering and Metabolic Flux Analysis
 CM4125 Bioprocess Engineering Lab: Week 4: Introduction to Metabolic Engineering and Metabolic Flux Analysis Instructors: : David R. Shonnard 1, Susan T. Bagley 2 Laboratory Teaching Assistant: : Abraham
CM4125 Bioprocess Engineering Lab: Week 4: Introduction to Metabolic Engineering and Metabolic Flux Analysis Instructors: : David R. Shonnard 1, Susan T. Bagley 2 Laboratory Teaching Assistant: : Abraham
Genetic Engineering for Biofuels Production
 Genetic Engineering for Biofuels Production WSE 573 Spring 2013 Greeley Beck INTRODUCTION Alternative transportation fuels are needed in the United States because of oil supply insecurity, oil price increases,
Genetic Engineering for Biofuels Production WSE 573 Spring 2013 Greeley Beck INTRODUCTION Alternative transportation fuels are needed in the United States because of oil supply insecurity, oil price increases,
Transformation of Escherichia coli With a Chimeric Plasmid
 Transformation of Escherichia coli With a Chimeric Plasmid Now that we have generated recombinant molecules, we must next amplify them by inserting them into an acceptable host so that they may be analyzed
Transformation of Escherichia coli With a Chimeric Plasmid Now that we have generated recombinant molecules, we must next amplify them by inserting them into an acceptable host so that they may be analyzed
MOLECULAR GENETICS: TRANSFORMATION AND CLONING adapted by Dr. D. L. Vogelien
 Introduction MOLECULAR GENETICS: TRANSFORMATION AND CLONING adapted by Dr. D. L. Vogelien The field of molecular genetics has resulted in a number of practical applications that have been of tremendous
Introduction MOLECULAR GENETICS: TRANSFORMATION AND CLONING adapted by Dr. D. L. Vogelien The field of molecular genetics has resulted in a number of practical applications that have been of tremendous
Lab 5/5a Transformation of E. coli with a Recombinant Plasmid
 Lab 5/5a Transformation of E. coli with a Recombinant Plasmid Lab 2 Pre Lab Readiness Familiarity and Proper use of micropipettes Remember the 1 st and 2 nd stops Aseptic Technique Antibiotic Resistance
Lab 5/5a Transformation of E. coli with a Recombinant Plasmid Lab 2 Pre Lab Readiness Familiarity and Proper use of micropipettes Remember the 1 st and 2 nd stops Aseptic Technique Antibiotic Resistance
A Discovery Laboratory Investigating Bacterial Gene Regulation
 Chapter 8 A Discovery Laboratory Investigating Bacterial Gene Regulation Robert Moss Wofford College 429 N. Church Street Spartanburg, SC 29307 mosssre@wofford.edu Bob Moss is an Associate Professor of
Chapter 8 A Discovery Laboratory Investigating Bacterial Gene Regulation Robert Moss Wofford College 429 N. Church Street Spartanburg, SC 29307 mosssre@wofford.edu Bob Moss is an Associate Professor of
MOK. Media Optimization Kit
 MOK Media Optimization Kit The Media Optimization Kit determines the best medium formulation for maximizing accumulation of recombinant proteins expressed in E. coli, utilizing a series of Athena s superior
MOK Media Optimization Kit The Media Optimization Kit determines the best medium formulation for maximizing accumulation of recombinant proteins expressed in E. coli, utilizing a series of Athena s superior
Confirming the Phenotypes of E. coli Strains
 Confirming the Phenotypes of E. coli Strains INTRODUCTION Before undertaking any experiments, we need to confirm that the phenotypes of the E. coli strains we intend to use in the planned experiments correspond
Confirming the Phenotypes of E. coli Strains INTRODUCTION Before undertaking any experiments, we need to confirm that the phenotypes of the E. coli strains we intend to use in the planned experiments correspond
Figure 1. Map of cloning vector pgem T-Easy (bacterial plasmid DNA)
 Texas A&M University-Corpus Christi CHEM4402 Biochemistry II Laboratory Laboratory 6: Ligation & Bacterial Transformation (Bring your text and laptop to class if you wish to work on your assignment during
Texas A&M University-Corpus Christi CHEM4402 Biochemistry II Laboratory Laboratory 6: Ligation & Bacterial Transformation (Bring your text and laptop to class if you wish to work on your assignment during
Introduction to BIOFUELS. David M. Mousdale. CRC Press. Taylor & Francis Group Boca Raton London New York
 Introduction to BIOFUELS David M. Mousdale CRC Press Taylor & Francis Group Boca Raton London New York CRC Press is an imprint of the Taylor & Francis Croup, an informa business Contents Preface Acknowledgments
Introduction to BIOFUELS David M. Mousdale CRC Press Taylor & Francis Group Boca Raton London New York CRC Press is an imprint of the Taylor & Francis Croup, an informa business Contents Preface Acknowledgments
PRODUCT INFORMATION. Composition of SOC medium supplied :
 Product Name : Competent Cell BL21(DE3)pLysS Code No. : DS260 Size : 100 μl 10 Competency : > 5 10 7 cfu/μg (puc19) Supplied product : SOC medium, 1 ml 10 This product is for research use only Description
Product Name : Competent Cell BL21(DE3)pLysS Code No. : DS260 Size : 100 μl 10 Competency : > 5 10 7 cfu/μg (puc19) Supplied product : SOC medium, 1 ml 10 This product is for research use only Description
HiPer Transformation Teaching Kit
 HiPer Transformation Teaching Kit Product Code: HTBM017 Number of experiments that can be performed: 10 Duration of Experiment: 4 days Day 1- Preparation of media and revival of E. coli Host Day 2- Inoculation
HiPer Transformation Teaching Kit Product Code: HTBM017 Number of experiments that can be performed: 10 Duration of Experiment: 4 days Day 1- Preparation of media and revival of E. coli Host Day 2- Inoculation
á62ñ MICROBIOLOGICAL EXAMINATION OF NONSTERILE PRODUCTS: TESTS FOR SPECIFIED MICROORGANISMS
 USP 40 Microbiological Tests / á62ñ Microbiological Examination 1 á62ñ MICROBIOLOGICAL EXAMINATION OF NONSTERILE PRODUCTS: TESTS FOR SPECIFIED MICROORGANISMS INTRODUCTION The tests described hereafter
USP 40 Microbiological Tests / á62ñ Microbiological Examination 1 á62ñ MICROBIOLOGICAL EXAMINATION OF NONSTERILE PRODUCTS: TESTS FOR SPECIFIED MICROORGANISMS INTRODUCTION The tests described hereafter
Q2 (1 point). How many carbon atoms does a glucose molecule contain?
 Q1 (1 point). Name three amino acids that are typically found at the surface of integrated membrane proteins.. Q2 (1 point). How many carbon atoms does a glucose molecule contain? Q3 (1 point). What do
Q1 (1 point). Name three amino acids that are typically found at the surface of integrated membrane proteins.. Q2 (1 point). How many carbon atoms does a glucose molecule contain? Q3 (1 point). What do
IMPORTANCE OF CELLULASES IN BIOETHANOL FROM BIOMASS: A REVIEW
 IMPORTANCE OF CELLULASES IN BIOETHANOL FROM BIOMASS: A REVIEW VERMA NITIN,KUMAR VIVEK,BANSAL M.C. DEPARTMENT OF PAPER TECHNOLOGY INDIAN INSTITUTE OF TECHNOLOGY,ROORKEE INTRODUCTION The continous depletion
IMPORTANCE OF CELLULASES IN BIOETHANOL FROM BIOMASS: A REVIEW VERMA NITIN,KUMAR VIVEK,BANSAL M.C. DEPARTMENT OF PAPER TECHNOLOGY INDIAN INSTITUTE OF TECHNOLOGY,ROORKEE INTRODUCTION The continous depletion
Physical State in Which Naphthalene and Bibenzyl are Utilized by Bacteria
 APPLIED MicRosoLowy, June 1972, p. 1077-1081 Copyright i 1972 American Society for Microbiology Vol. 23, No. 6 Printed in U.S.A. Physical State in Which Naphthalene and Bibenzyl are Utilized by Bacteria
APPLIED MicRosoLowy, June 1972, p. 1077-1081 Copyright i 1972 American Society for Microbiology Vol. 23, No. 6 Printed in U.S.A. Physical State in Which Naphthalene and Bibenzyl are Utilized by Bacteria
Biology 322 Fall 2010 Transfer of genetic information in the bacterium Escherichia coli: Part I
 Biology 322 Fall 2010 Transfer of genetic information in the bacterium Escherichia coli: Part I REQUIRED Reading Assignments: Superbugs on the Hoof http://fire.biol.wwu.edu/trent/trent/superbugs.pdf Triple
Biology 322 Fall 2010 Transfer of genetic information in the bacterium Escherichia coli: Part I REQUIRED Reading Assignments: Superbugs on the Hoof http://fire.biol.wwu.edu/trent/trent/superbugs.pdf Triple
Genetic Improvement of Escherichia coli for Ethanol Production: Chromosomal Integration of Zymomonas mobilis Genes Encoding
 APPLIED AND ENVIRONMENTAL MICROBIOLOGY, Apr. 1991, p. 893-900 Vol. 57, No. 4 0099-2240/91/040893-08$02.00/0 Copyright C 1991, American Society for Microbiology Genetic Improvement of Escherichia coli for
APPLIED AND ENVIRONMENTAL MICROBIOLOGY, Apr. 1991, p. 893-900 Vol. 57, No. 4 0099-2240/91/040893-08$02.00/0 Copyright C 1991, American Society for Microbiology Genetic Improvement of Escherichia coli for
Cell Growth and DNA Extraction- Technion igem HS
 Growing Cells and DNA Extraction Goals 1. Become familiar with the process of growing bacteria 2. Get to know the DNA extraction process 3. Perform miniprep in the lab Keywords 1. Growth stages 6. Techniques
Growing Cells and DNA Extraction Goals 1. Become familiar with the process of growing bacteria 2. Get to know the DNA extraction process 3. Perform miniprep in the lab Keywords 1. Growth stages 6. Techniques
BL21(DE3) expression competent cell pack DS265. Component Code No. Contents Competent cell BL21(DE3) DS250 5 tubes (100 μl/tube)
 Product Name : Code No. : Kit Component : BL21(DE3) expression competent cell pack DS265 Component Code No. Contents Competent cell BL21(DE3) DS250 5 tubes (100 μl/tube) transfromation efficiency: 5 10
Product Name : Code No. : Kit Component : BL21(DE3) expression competent cell pack DS265 Component Code No. Contents Competent cell BL21(DE3) DS250 5 tubes (100 μl/tube) transfromation efficiency: 5 10
Optimization of Agitation Conditions for Maximum Ethanol Production by Coculture
 Kasetsart J. (Nat. Sci.) : - 9 () Optimization of Agitation Conditions for Maximum Ethanol Production by Coculture Arisra Rodmui, Jirasak Kongkiattikajorn* and Yuwapin Dandusitapun ABSTRACT The coculture
Kasetsart J. (Nat. Sci.) : - 9 () Optimization of Agitation Conditions for Maximum Ethanol Production by Coculture Arisra Rodmui, Jirasak Kongkiattikajorn* and Yuwapin Dandusitapun ABSTRACT The coculture
GROWTH OF BACTERIA ON THE SURFACE ANION-EXCHANGE RESIN I. EXPERIMENT WITH BATCH CULTURE
 J. Gen. Appl. Microbiol., 18, 271-283 (1972) GROWTH OF BACTERIA ON THE SURFACE ANION-EXCHANGE RESIN OF I. EXPERIMENT WITH BATCH CULTURE REIKO HATTORI, TSUTOMU HATTORI, AND CHOSEKI FURUSAKA Institute for
J. Gen. Appl. Microbiol., 18, 271-283 (1972) GROWTH OF BACTERIA ON THE SURFACE ANION-EXCHANGE RESIN OF I. EXPERIMENT WITH BATCH CULTURE REIKO HATTORI, TSUTOMU HATTORI, AND CHOSEKI FURUSAKA Institute for
Table of Contents 1. CHAPTER I INTRODUCTION 1 [A] GLUCOSE ISOMERASE 1
![Table of Contents 1. CHAPTER I INTRODUCTION 1 [A] GLUCOSE ISOMERASE 1 Table of Contents 1. CHAPTER I INTRODUCTION 1 [A] GLUCOSE ISOMERASE 1](/thumbs/87/95094148.jpg) Table of Contents 1. CHAPTER I INTRODUCTION 1 [A] GLUCOSE ISOMERASE 1 1.1 Enzymatic versus chemical isomerization 4 1.2 Glucose Isomerase producing microorganisms 5 1.3 Structure of Glucose Isomerase 8
Table of Contents 1. CHAPTER I INTRODUCTION 1 [A] GLUCOSE ISOMERASE 1 1.1 Enzymatic versus chemical isomerization 4 1.2 Glucose Isomerase producing microorganisms 5 1.3 Structure of Glucose Isomerase 8
Fermentation of Hemicellulosic Sugars and Sugar Mixtures by Candida shehatae
 Fermentation of Hemicellulosic Sugars and Sugar Mixtures by Candida shehatae Thomas W. Jeffries* United States Department of Agriculture, Forest Products Laboratory, Madison, Wisconsin 53705-2398 Hassan
Fermentation of Hemicellulosic Sugars and Sugar Mixtures by Candida shehatae Thomas W. Jeffries* United States Department of Agriculture, Forest Products Laboratory, Madison, Wisconsin 53705-2398 Hassan
HI-Control BL21(DE3) & HI-Control 10G Chemically Competent Cells
 HI-Control BL21(DE3) & HI-Control 10G Chemically Competent Cells FOR RESEARCH USE ONLY. NOT FOR HUMAN OR DIAGNOSTIC USE MA156 Rev.31OCT2016 Table of Contents Components & Storage Conditions... 3 HI-Control
HI-Control BL21(DE3) & HI-Control 10G Chemically Competent Cells FOR RESEARCH USE ONLY. NOT FOR HUMAN OR DIAGNOSTIC USE MA156 Rev.31OCT2016 Table of Contents Components & Storage Conditions... 3 HI-Control
Section B: The Genetics of Bacteria
 CHAPTER 18 MICROBIAL MODELS: THE GENETICS OF VIRUSES AND BACTERIA Section B: The Genetics of Bacteria 1. The short generation span of bacteria helps them adapt to changing environments 2. Genetic recombination
CHAPTER 18 MICROBIAL MODELS: THE GENETICS OF VIRUSES AND BACTERIA Section B: The Genetics of Bacteria 1. The short generation span of bacteria helps them adapt to changing environments 2. Genetic recombination
Biotechnology : Unlocking the Mysterious of Life Seungwook Kim Chem. & Bio. Eng.
 Biotechnology : Unlocking the Mysterious of Life 2004 Seungwook Kim Chem. & Bio. Eng. Biotechnology in movies Biotechnology is An area of applied bioscience and technology which involves the practical
Biotechnology : Unlocking the Mysterious of Life 2004 Seungwook Kim Chem. & Bio. Eng. Biotechnology in movies Biotechnology is An area of applied bioscience and technology which involves the practical
TD-P Revision 3.0 Creation Date: 6/9/2016 Revision Date: 8/16/2018
 Protocol TD-P Revision 3.0 Creation Date: 6/9/2016 Revision Date: 8/16/2018 Electrotransformation of Agrobacterium tumefaciens Modified from Methods in Molecular Biology Vol. 47 Introduction Agrobacterium
Protocol TD-P Revision 3.0 Creation Date: 6/9/2016 Revision Date: 8/16/2018 Electrotransformation of Agrobacterium tumefaciens Modified from Methods in Molecular Biology Vol. 47 Introduction Agrobacterium
Journal of Experimental Microbiology and Immunology (JEMI) Vol. 6:20-25 Copyright December 2004, M&I UBC
 Preparing Plasmid Constructs to Investigate the Characteristics of Thiol Reductase and Flavin Reductase With Regard to Solubilizing Insoluble Proteinase Inhibitor 2 in Bacterial Protein Overexpression
Preparing Plasmid Constructs to Investigate the Characteristics of Thiol Reductase and Flavin Reductase With Regard to Solubilizing Insoluble Proteinase Inhibitor 2 in Bacterial Protein Overexpression
HiPer Plasmid DNA Cloning Teaching Kit
 HiPer Plasmid DNA Cloning Teaching Kit Product Code: HTBM022 Number of experiments that can be performed: 5 Duration of Experiment: 4 days Day 1- Preparation of media and revival of E. coli Host Day2-
HiPer Plasmid DNA Cloning Teaching Kit Product Code: HTBM022 Number of experiments that can be performed: 5 Duration of Experiment: 4 days Day 1- Preparation of media and revival of E. coli Host Day2-
By two mechanisms: Mutation Genetic Recombination
 Genetics (see text pages 257-259, 267-298) Remember what it is we want to address: How is it that prokaryotes gain new genetic ability? The cells are haploid and reproduce by fission...so how does an genetic
Genetics (see text pages 257-259, 267-298) Remember what it is we want to address: How is it that prokaryotes gain new genetic ability? The cells are haploid and reproduce by fission...so how does an genetic
Effect of glucose and ammonium chloride supplementation and phosphate buffer on Escherichia coli DH5α growth in LB Lennox medium
 Effect of glucose and ammonium chloride supplementation and phosphate buffer on Escherichia coli DH5α growth in LB Lennox medium Wenfa Ng Department of Chemical and Biomolecular Engineering, National University
Effect of glucose and ammonium chloride supplementation and phosphate buffer on Escherichia coli DH5α growth in LB Lennox medium Wenfa Ng Department of Chemical and Biomolecular Engineering, National University
Comparison of Laboratory and Industrial Saccharomyces cerevisiae Strains for Their Inhibitor Resistance and Xylose Utilization
 Comparison of Laboratory and Industrial Saccharomyces cerevisiae Strains for Their Inhibitor Resistance and Xylose Utilization Geng Anli*, Wang Zhankun, Lai Kok Soon and Tan Wei Yi Mark, Goh Kiow Leng
Comparison of Laboratory and Industrial Saccharomyces cerevisiae Strains for Their Inhibitor Resistance and Xylose Utilization Geng Anli*, Wang Zhankun, Lai Kok Soon and Tan Wei Yi Mark, Goh Kiow Leng
GROWTH AND MANOMETRIC STUDIES ON CARBOHYDRATE UTILIZATION
 GROWTH AND MANOMETRIC STUDIES ON CARBOHYDRATE UTILIZATION BY SHIGELLA FLEXNERI' ARVID L. ERLANDSON, JR.,2 AND WILLIAM H. MACKEY Naval Medical Research Institute, National Naval Medical Center, Bethesda,
GROWTH AND MANOMETRIC STUDIES ON CARBOHYDRATE UTILIZATION BY SHIGELLA FLEXNERI' ARVID L. ERLANDSON, JR.,2 AND WILLIAM H. MACKEY Naval Medical Research Institute, National Naval Medical Center, Bethesda,
Cat # Box1 Box2. DH5a Competent E. coli cells CCK-20 (20 rxns) 40 µl 40 µl 50 µl x 20 tubes. Choo-Choo Cloning TM Enzyme Mix
 Molecular Cloning Laboratories User Manual Version 3.3 Product name: Choo-Choo Cloning Kits Cat #: CCK-10, CCK-20, CCK-096, CCK-384 Description: Choo-Choo Cloning is a highly efficient directional PCR
Molecular Cloning Laboratories User Manual Version 3.3 Product name: Choo-Choo Cloning Kits Cat #: CCK-10, CCK-20, CCK-096, CCK-384 Description: Choo-Choo Cloning is a highly efficient directional PCR
artes white paper Efficient Production of Secreted and Active Invertase artes white paper Manfred Suckow, Volker Jenzelewski, Michael Piontek
 Efficient Production of Secreted and Active Invertase Manfred Suckow, Volker Jenzelewski, Michael Piontek ARTES Biotechnology 2017 1 In our today s society, sugar plays an eminent roll. In industrialized
Efficient Production of Secreted and Active Invertase Manfred Suckow, Volker Jenzelewski, Michael Piontek ARTES Biotechnology 2017 1 In our today s society, sugar plays an eminent roll. In industrialized
DNA TRANSFORMATION OF BACTERIA RED COLONY REVISED 3/2003
 DNA TRANSFORMATION OF BACTERIA RED COLONY REVISED 3/2003 Prepared by the Office of Biotechnology, Iowa State University TEACHER PREPARATION AND INSTRUCTION GUIDE Preparation for the DNA transformation
DNA TRANSFORMATION OF BACTERIA RED COLONY REVISED 3/2003 Prepared by the Office of Biotechnology, Iowa State University TEACHER PREPARATION AND INSTRUCTION GUIDE Preparation for the DNA transformation
DNA Cloning with Cloning Vectors
 Cloning Vectors A M I R A A. T. A L - H O S A R Y L E C T U R E R O F I N F E C T I O U S D I S E A S E S F A C U L T Y O F V E T. M E D I C I N E A S S I U T U N I V E R S I T Y - E G Y P T DNA Cloning
Cloning Vectors A M I R A A. T. A L - H O S A R Y L E C T U R E R O F I N F E C T I O U S D I S E A S E S F A C U L T Y O F V E T. M E D I C I N E A S S I U T U N I V E R S I T Y - E G Y P T DNA Cloning
Polyethylene glycol exerted toxicity to growth of Bacillus subtilis NRS-762
 Polyethylene glycol exerted toxicity to growth of Bacillus subtilis NRS-762 Wenfa Ng Department of Chemical and Biomolecular Engineering, National University of Singapore Abstract Email: ngwenfa771@hotmail.com
Polyethylene glycol exerted toxicity to growth of Bacillus subtilis NRS-762 Wenfa Ng Department of Chemical and Biomolecular Engineering, National University of Singapore Abstract Email: ngwenfa771@hotmail.com
BIO440 Genetics Laboratory Transformation
 BIO440 Genetics Laboratory Transformation The transfer of genetic information between bacteria has been occurring for billions of years. Humans first noticed this process in the laboratory in the 1920
BIO440 Genetics Laboratory Transformation The transfer of genetic information between bacteria has been occurring for billions of years. Humans first noticed this process in the laboratory in the 1920
MICROBIOLOGICAL EXAMINATION OF NON-STERILE PRODUCTS: TEST FOR SPECIFIED MICRO-ORGANISMS Test for specified micro-organisms
 5-2-3. Most-probable-number method Prepare and dilute the sample using a method that has been shown to be suitable as described in section 4. Incubate all tubes at 30-35 C for 3-5 days. Subculture if necessary,
5-2-3. Most-probable-number method Prepare and dilute the sample using a method that has been shown to be suitable as described in section 4. Incubate all tubes at 30-35 C for 3-5 days. Subculture if necessary,
Butanol: : A Second Generation Biofuel. Hans P. Blaschek University of Illinois March 6, 2007
 Butanol: : A Second Generation Biofuel Hans P. Blaschek University of Illinois March 6, 2007 Outline Introduction History Rationale Microbe Development and Characterization Genetic and Post-genomic Characterization
Butanol: : A Second Generation Biofuel Hans P. Blaschek University of Illinois March 6, 2007 Outline Introduction History Rationale Microbe Development and Characterization Genetic and Post-genomic Characterization
Production of Cellulase on Mixtures of Xylose and Cellulose in a Fed-Batch Process
 Production of Cellulase on Mixtures of Xylose and Cellulose in a Fed-Batch Process Ali Mohagheghi, Karel Grohmann, and Charles E. Wyman Biotechnolog y Research Branch, Solar Fuels Research Division, Solar
Production of Cellulase on Mixtures of Xylose and Cellulose in a Fed-Batch Process Ali Mohagheghi, Karel Grohmann, and Charles E. Wyman Biotechnolog y Research Branch, Solar Fuels Research Division, Solar
Effects of Oxygen Supply and Mixed Sugar Concentration on D-Ribose Production by a Transketolase-Deficient Bacillus subtilis SPK1
 J. Microbiol. Biotechnol. (2013), 23(4), 560 564 http://dx.doi.org/10.4014/jmb.1212.12021 First published online January 27, 2013 pissn 1017-7825 eissn 1738-8872 Effects of Oxygen Supply and Mixed Sugar
J. Microbiol. Biotechnol. (2013), 23(4), 560 564 http://dx.doi.org/10.4014/jmb.1212.12021 First published online January 27, 2013 pissn 1017-7825 eissn 1738-8872 Effects of Oxygen Supply and Mixed Sugar
4/3/2013. DNA Synthesis Replication of Bacterial DNA Replication of Bacterial DNA
 4/3/03 3 4 5 6 7 8 9 0 Chapter 8 Microbial Genetics Terminology Genetics: The study of what genes are, how they carry information, how information is expressed, and how genes are replicated Gene: A segment
4/3/03 3 4 5 6 7 8 9 0 Chapter 8 Microbial Genetics Terminology Genetics: The study of what genes are, how they carry information, how information is expressed, and how genes are replicated Gene: A segment
Bacterial genetic exchange : Bacterial Transformation
 Experiment 11 Laboratory to Biology III: Diversity of Microorganisms 1 Experiment 11 Bacterial genetic exchange : Bacterial Transformation Advisor Munti Yuhana myuhana@botinst.unizh.ch Textbook Chapters
Experiment 11 Laboratory to Biology III: Diversity of Microorganisms 1 Experiment 11 Bacterial genetic exchange : Bacterial Transformation Advisor Munti Yuhana myuhana@botinst.unizh.ch Textbook Chapters
The optimization of fermentation conditions particularly physical and chemical
 4.1 Preamble The optimization of fermentation conditions particularly physical and chemical parameters are of primary importance in the development of any fermentation process owing to their impact on
4.1 Preamble The optimization of fermentation conditions particularly physical and chemical parameters are of primary importance in the development of any fermentation process owing to their impact on
SCREENING AND PRESERVATION OF DNA LIBRARIES
 MODULE 4 LECTURE 5 SCREENING AND PRESERVATION OF DNA LIBRARIES 4-5.1. Introduction Library screening is the process of identification of the clones carrying the gene of interest. Screening relies on a
MODULE 4 LECTURE 5 SCREENING AND PRESERVATION OF DNA LIBRARIES 4-5.1. Introduction Library screening is the process of identification of the clones carrying the gene of interest. Screening relies on a
Metabolic Engineering for Fuels and Chemicals
 Metabolic Engineering for Fuels and Chemicals K.T. Shanmugam and Lonnie O. Ingram Dept. of Microbiology and Cell Science University of Florida Gainesville, Florida Florida Center for Renewable Chemicals
Metabolic Engineering for Fuels and Chemicals K.T. Shanmugam and Lonnie O. Ingram Dept. of Microbiology and Cell Science University of Florida Gainesville, Florida Florida Center for Renewable Chemicals
SECOND GENERATION BIOETHANOL FROM Eucalyptus globulus labill AND Nothofagus pumilio USING IONIC LIQUIDS. María Cristina Ravanal E.
 SECOND GENERATION BIOETHANOL FROM Eucalyptus globulus labill AND Nothofagus pumilio USING IONIC LIQUIDS. María Cristina Ravanal E. Centro de Biotecnología y Bioingeniería Universidad de Chile mravanal@ing.uchile.cl
SECOND GENERATION BIOETHANOL FROM Eucalyptus globulus labill AND Nothofagus pumilio USING IONIC LIQUIDS. María Cristina Ravanal E. Centro de Biotecnología y Bioingeniería Universidad de Chile mravanal@ing.uchile.cl
4. Triple-helical DNA structures can result from Hoogsteen (non Watson-Crick) interactions. These interactions are primarily:
 CHEM 4420 Exam I Spring 2012 Page 1 of 5 Name Use complete sentences when requested. There are 100 possible points on this exam. The multiple choice questions are worth 2 points each. All other questions
CHEM 4420 Exam I Spring 2012 Page 1 of 5 Name Use complete sentences when requested. There are 100 possible points on this exam. The multiple choice questions are worth 2 points each. All other questions
Purification and Characterization of a DNA Plasmid Part A CHEM 4581: Biochemistry Laboratory I Version: January 18, 2008
 Purification and Characterization of a DNA Plasmid Part A CHEM 4581: Biochemistry Laboratory I Version: January 18, 2008 INTRODUCTION DNA Plasmids. A plasmid is a small double-stranded, circular DNA molecule
Purification and Characterization of a DNA Plasmid Part A CHEM 4581: Biochemistry Laboratory I Version: January 18, 2008 INTRODUCTION DNA Plasmids. A plasmid is a small double-stranded, circular DNA molecule
Engineering E. coli for xylitol production during growth on xylose
 Engineering E. coli for xylitol production during growth on xylose Olubolaji Akinterinwa & Patrick C. Cirino IBE 2008 Annual Meeting, March 7th 008-17 Advances in Engineering Microbial Metabolism Xylitol
Engineering E. coli for xylitol production during growth on xylose Olubolaji Akinterinwa & Patrick C. Cirino IBE 2008 Annual Meeting, March 7th 008-17 Advances in Engineering Microbial Metabolism Xylitol
7.02 Microbial Genetics in Lab Quiz. Fall, September 27, 2001 ANSWER KEY
 7.02 Microbial Genetics in Lab Quiz Fall, 2001 September 27, 2001 ANSWER KEY This quiz contains 4 questions worth a total of 48 points. Be sure to write your name, Bench letter and Undergraduate TA s (UTA)
7.02 Microbial Genetics in Lab Quiz Fall, 2001 September 27, 2001 ANSWER KEY This quiz contains 4 questions worth a total of 48 points. Be sure to write your name, Bench letter and Undergraduate TA s (UTA)
CopyCutter EPI400 Electrocompetent E. coli CopyCutter EPI400 Chemically Competent E. coli CopyCutter Induction Solution
 CopyCutter EPI400 Electrocompetent E. coli CopyCutter EPI400 Chemically Competent E. coli CopyCutter Induction Solution Cat. Nos. C400EL10, C400CH10, and CIS40025 Available exclusively thru Lucigen. lucigen.com/epibio
CopyCutter EPI400 Electrocompetent E. coli CopyCutter EPI400 Chemically Competent E. coli CopyCutter Induction Solution Cat. Nos. C400EL10, C400CH10, and CIS40025 Available exclusively thru Lucigen. lucigen.com/epibio
Bacterial genetic exchange:transformation
 Experiment 11 Laboratory to Biology III Diversity of Microorganisms / Wintersemester / page 1 Experiment Advisor Reading Objectives Background Literature www. Links Bacterial genetic exchange:transformation
Experiment 11 Laboratory to Biology III Diversity of Microorganisms / Wintersemester / page 1 Experiment Advisor Reading Objectives Background Literature www. Links Bacterial genetic exchange:transformation
Final text for addition to The International Pharmacopoeia
 March 2012 3.3.2 MICROBIOLOGICAL EXAMINATION OF NON-STERILE PRODUCTS: TESTS FOR SPECIFIED MICROORGANISMS Final text for addition to The International Pharmacopoeia This monograph was adopted at the Forty-sixth
March 2012 3.3.2 MICROBIOLOGICAL EXAMINATION OF NON-STERILE PRODUCTS: TESTS FOR SPECIFIED MICROORGANISMS Final text for addition to The International Pharmacopoeia This monograph was adopted at the Forty-sixth
GeNei TM Transformation Teaching Kit Manual
 Teaching Kit Manual Cat No. New Cat No. KT07 107385 KT07A 106220 Revision No.: 00060505 CONTENTS Page No. Objective 3 Principle 3 Kit Description 6 Materials Provided 7 Procedure 9 Observation & Interpretation
Teaching Kit Manual Cat No. New Cat No. KT07 107385 KT07A 106220 Revision No.: 00060505 CONTENTS Page No. Objective 3 Principle 3 Kit Description 6 Materials Provided 7 Procedure 9 Observation & Interpretation
INVESTIGATION ON CONVERSION OF FLOWER WASTES INTO BIOETHANOL AND PERFORMANCE EVALUATION ON SINGLE CYLINDER IC ENGINE
 INVESTIGATION ON CONVERSION OF FLOWER WASTES INTO BIOETHANOL AND PERFORMANCE EVALUATION ON SINGLE CYLINDER IC ENGINE COLLEGE : BAPUJI INSTITUTE OF ENGINEERING AND TECHNOLOGY, DAVANGERE DEPARTMENT : MECHANICAL
INVESTIGATION ON CONVERSION OF FLOWER WASTES INTO BIOETHANOL AND PERFORMANCE EVALUATION ON SINGLE CYLINDER IC ENGINE COLLEGE : BAPUJI INSTITUTE OF ENGINEERING AND TECHNOLOGY, DAVANGERE DEPARTMENT : MECHANICAL
Industrial microbiology
 Industrial microbiology pp. 166-173, 1032-1038, 1039-1045,1046-1050 Ed van Niel Ed.van_Niel@tmb.lth.se We are here Industrial microbiology biotechnology Why the increased interest Microbiological versus
Industrial microbiology pp. 166-173, 1032-1038, 1039-1045,1046-1050 Ed van Niel Ed.van_Niel@tmb.lth.se We are here Industrial microbiology biotechnology Why the increased interest Microbiological versus
QUESTIONSHEET 1. The diagram shows a method of screening fungi for the production of an antibiotic. fungus A fungus B fungus C [2] ...
![QUESTIONSHEET 1. The diagram shows a method of screening fungi for the production of an antibiotic. fungus A fungus B fungus C [2] ... QUESTIONSHEET 1. The diagram shows a method of screening fungi for the production of an antibiotic. fungus A fungus B fungus C [2] ...](/thumbs/77/75444315.jpg) QUESTIONSHEET 1 The diagram shows a method of screening fungi for the production of an antibiotic. test fungus petri dish containing nutrient agar 1 2 3 4 5 6 streaks of different test bacteria The diagrams
QUESTIONSHEET 1 The diagram shows a method of screening fungi for the production of an antibiotic. test fungus petri dish containing nutrient agar 1 2 3 4 5 6 streaks of different test bacteria The diagrams
SECONDARY COLONY FORMATION BY BACILLUS SUBTILIS ON EOSINE
 SECONDARY COLONY FORMATION BY BACILLUS SUBTILIS ON EOSINE METHYLENE BLUE AGAR K. K. SHAH' AND V. N. IYER2 Microbiology Department, S. B. Garda College, Navsari, India Received for publication November
SECONDARY COLONY FORMATION BY BACILLUS SUBTILIS ON EOSINE METHYLENE BLUE AGAR K. K. SHAH' AND V. N. IYER2 Microbiology Department, S. B. Garda College, Navsari, India Received for publication November
SI Results Peculiarities in the consumption of ammonium and glucose by AmtB- strains SI Materials and Methods Strain Construction
 SI Results Peculiarities in the consumption of ammonium and glucose by AmtB - strains. The AmtB - strain failed to consume all the ammonium in the medium under NH 3 -limiting conditions (.5 mm total ammonium
SI Results Peculiarities in the consumption of ammonium and glucose by AmtB - strains. The AmtB - strain failed to consume all the ammonium in the medium under NH 3 -limiting conditions (.5 mm total ammonium
Lecture Series 10 The Genetics of Viruses and Prokaryotes
 Lecture Series 10 The Genetics of Viruses and Prokaryotes The Genetics of Viruses and Prokaryotes A. Using Prokaryotes and Viruses for Genetic Experiments B. Viruses: Reproduction and Recombination C.
Lecture Series 10 The Genetics of Viruses and Prokaryotes The Genetics of Viruses and Prokaryotes A. Using Prokaryotes and Viruses for Genetic Experiments B. Viruses: Reproduction and Recombination C.
Determination of Exclusion Effect in Wild Type and Rop Deficient Mutated pbr322 Co-transformations
 Determination of Exclusion Effect in Wild Type and Rop Deficient Mutated pbr322 Co-transformations Suzana Sabaiduc and Andy Lo Microbiology and Immunology University of British Columbia pbr322 is an expression
Determination of Exclusion Effect in Wild Type and Rop Deficient Mutated pbr322 Co-transformations Suzana Sabaiduc and Andy Lo Microbiology and Immunology University of British Columbia pbr322 is an expression
Isolation and Characterization of Escherichia coli
 Chapter-4 Isolation and Characterization of Escherichia coli 4.1 Sample source and collection of samples: Escherichia coli is known to be a colon bacteria which shows ubiquitous presence in many ecological
Chapter-4 Isolation and Characterization of Escherichia coli 4.1 Sample source and collection of samples: Escherichia coli is known to be a colon bacteria which shows ubiquitous presence in many ecological
Chapter 4. Recombinant DNA Technology
 Chapter 4 Recombinant DNA Technology 5. Plasmid Cloning Vectors Plasmid Plasmids Self replicating Double-stranded Mostly circular DNA ( 500 kb) Linear : Streptomyces, Borrelia burgdorferi Replicon
Chapter 4 Recombinant DNA Technology 5. Plasmid Cloning Vectors Plasmid Plasmids Self replicating Double-stranded Mostly circular DNA ( 500 kb) Linear : Streptomyces, Borrelia burgdorferi Replicon
Growth kinetic and modeling of ethanol production by wilds and mutant Saccharomyces cerevisae MTCC 170
 Available online at www.pelagiaresearchlibrary.com European Journal of Experimental Biology, 2015, 5(4):1-6 ISSN: 2248 9215 CODEN (USA): EJEBAU Growth kinetic and modeling of ethanol production by wilds
Available online at www.pelagiaresearchlibrary.com European Journal of Experimental Biology, 2015, 5(4):1-6 ISSN: 2248 9215 CODEN (USA): EJEBAU Growth kinetic and modeling of ethanol production by wilds
The GeneEditor TM in vitro Mutagenesis System: Site- Directed Mutagenesis Using Altered Beta-Lactamase Specificity
 Promega Notes Magazine Number 62, 1997, p. 02 The GeneEditor TM in vitro Mutagenesis System: Site- Directed Mutagenesis Using Altered Beta-Lactamase Specificity By Christine Andrews and Scott Lesley Promega
Promega Notes Magazine Number 62, 1997, p. 02 The GeneEditor TM in vitro Mutagenesis System: Site- Directed Mutagenesis Using Altered Beta-Lactamase Specificity By Christine Andrews and Scott Lesley Promega
Control of Metabolic Processes
 Control of Metabolic Processes Harriet Wilson, Lecture Notes Bio. Sci. 4 - Microbiology Sierra College As described earlier, the metabolic processes occurring within living organisms (glycolysis, respiration,
Control of Metabolic Processes Harriet Wilson, Lecture Notes Bio. Sci. 4 - Microbiology Sierra College As described earlier, the metabolic processes occurring within living organisms (glycolysis, respiration,
Research Article PRODUCTION OF A THERMOSTABLE EXTRACELLULAR PROTEASE FROM THERMOPHILIC BACILLUS SPECIES
 Research Article PRODUCTION OF A THERMOSTABLE EXTRACELLULAR PROTEASE FROM THERMOPHILIC BACILLUS SPECIES S. Suman *1 and K. Ramesh 1 1 Department of Pharmaceutical Biotechnology, Karnataka College of Pharmacy,
Research Article PRODUCTION OF A THERMOSTABLE EXTRACELLULAR PROTEASE FROM THERMOPHILIC BACILLUS SPECIES S. Suman *1 and K. Ramesh 1 1 Department of Pharmaceutical Biotechnology, Karnataka College of Pharmacy,
Downloaded from
 11.BIOTECHNOLOGY - PRINCIPLES AND PROCESSES Multiple Choice Questions Single Correct Answer Type 1. Rising of dough is due to (a) Multiplication of yeast (b) Production of CO (c) Emulsification (d) Hydrolysis
11.BIOTECHNOLOGY - PRINCIPLES AND PROCESSES Multiple Choice Questions Single Correct Answer Type 1. Rising of dough is due to (a) Multiplication of yeast (b) Production of CO (c) Emulsification (d) Hydrolysis
Overnight Express TM Autoinduction
 About the System Overnight Express Autoinduction System 1 71300-3 71300-4 Overnight Express Autoinduction System 2 71366-3 71366-4 Description The Overnight Express Autoinduction 1 and 2 are designed for
About the System Overnight Express Autoinduction System 1 71300-3 71300-4 Overnight Express Autoinduction System 2 71366-3 71366-4 Description The Overnight Express Autoinduction 1 and 2 are designed for
Genetic Engineering: Transforming Bacteria
 Genetic Engineering: Transforming Bacteria Introduction Activity Introduction In this laboratory experiment, students will transform the phenotype of bacteria through the use of genetic engineering. A
Genetic Engineering: Transforming Bacteria Introduction Activity Introduction In this laboratory experiment, students will transform the phenotype of bacteria through the use of genetic engineering. A
Molecular Genetics Techniques. BIT 220 Chapter 20
 Molecular Genetics Techniques BIT 220 Chapter 20 What is Cloning? Recombinant DNA technologies 1. Producing Recombinant DNA molecule Incorporate gene of interest into plasmid (cloning vector) 2. Recombinant
Molecular Genetics Techniques BIT 220 Chapter 20 What is Cloning? Recombinant DNA technologies 1. Producing Recombinant DNA molecule Incorporate gene of interest into plasmid (cloning vector) 2. Recombinant
Amgen Laboratory Series. Tabs C and E
 Amgen Laboratory Series Tabs C and E Chapter 2A Goals Describe the characteristics of plasmids Explain how plasmids are used in cloning a gene Describe the function of restriction enzymes Explain how to
Amgen Laboratory Series Tabs C and E Chapter 2A Goals Describe the characteristics of plasmids Explain how plasmids are used in cloning a gene Describe the function of restriction enzymes Explain how to
His-Spin Protein Miniprep
 INSTRUCTIONS His-Spin Protein Miniprep Catalog No. P2001 (10 purifications) and P2002 (50 purifications). Highlights Fast 5 minute protocol to purify His-tagged proteins from cell-free extracts Screen
INSTRUCTIONS His-Spin Protein Miniprep Catalog No. P2001 (10 purifications) and P2002 (50 purifications). Highlights Fast 5 minute protocol to purify His-tagged proteins from cell-free extracts Screen
RELATIONSHIPS BETWEEN INITIAL NUTRIENT CONCENTRATION ANDB TOTAL GROWTH1
 RELATIONSHIPS BETWEEN INITIAL NUTRIENT CONCENTRATION ANDB TOTAL GROWTH1 R. E. ECKER2 AND W. R. LOCKHART Department of Bacteriology, Iowa State University, Ames, Iowa Received for publication January 4,
RELATIONSHIPS BETWEEN INITIAL NUTRIENT CONCENTRATION ANDB TOTAL GROWTH1 R. E. ECKER2 AND W. R. LOCKHART Department of Bacteriology, Iowa State University, Ames, Iowa Received for publication January 4,
Optimization of Succinic Acid from Fermentation Process by Using Immobiized Escherichia Coli Cells Via Anaerobic Fermentation
 Optimization of Succinic Acid from Fermentation Process by Using Immobiized Escherichia Coli Cells Via Anaerobic Fermentation Nik Nor Aziati Abdul Aziz Faculty of Chemical Enginnering and Natural Resources,
Optimization of Succinic Acid from Fermentation Process by Using Immobiized Escherichia Coli Cells Via Anaerobic Fermentation Nik Nor Aziati Abdul Aziz Faculty of Chemical Enginnering and Natural Resources,
Metabolic Engineering of New Routes to Biofuels
 Metabolic Engineering of New Routes to Biofuels Steve Wilkinson Manchester Interdisciplinary Biocentre, Manchester University UK. Overview Metabolism, enzymes, genes Bioethanol production in yeast Metabolic
Metabolic Engineering of New Routes to Biofuels Steve Wilkinson Manchester Interdisciplinary Biocentre, Manchester University UK. Overview Metabolism, enzymes, genes Bioethanol production in yeast Metabolic
Detection of bacterial aggregation by flow cytometry
 Detection of bacterial aggregation by flow cytometry Jack Coleman 1 and Melis McHenry 2 1 Enzo Life Sciences, Farmingdale NY, USA, 2 Beckman Coulter Inc., Miami FL, USA PROTEOSTAT Aggresome Detection Kit
Detection of bacterial aggregation by flow cytometry Jack Coleman 1 and Melis McHenry 2 1 Enzo Life Sciences, Farmingdale NY, USA, 2 Beckman Coulter Inc., Miami FL, USA PROTEOSTAT Aggresome Detection Kit
Genetics. Chapter 9 - Microbial Genetics. Chromosome. Genes. Topics - Genetics - Flow of Genetics - Regulation - Mutation - Recombination
 Chapter 9 - Microbial Genetics Topics - Genetics - Flow of Genetics - Regulation - Mutation - Recombination Genetics Genome (The sum total of genetic material of a cell is referred to as the genome.) Chromosome
Chapter 9 - Microbial Genetics Topics - Genetics - Flow of Genetics - Regulation - Mutation - Recombination Genetics Genome (The sum total of genetic material of a cell is referred to as the genome.) Chromosome
Chapter 9. Biotechnology and DNA Technology
 Chapter 9 Biotechnology and DNA Technology SLOs Compare and contrast biotechnology, recombinant DNA technology, and genetic engineering. Identify the roles of a clone and a vector in making recombined
Chapter 9 Biotechnology and DNA Technology SLOs Compare and contrast biotechnology, recombinant DNA technology, and genetic engineering. Identify the roles of a clone and a vector in making recombined
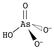 ph and redox potential (pe) are the most important factors controlling arsenic speciation. Phosphate (A) and arsenate (B) speciation are shown as a function of ph for the (V) oxidation states. H 3 PO 4
ph and redox potential (pe) are the most important factors controlling arsenic speciation. Phosphate (A) and arsenate (B) speciation are shown as a function of ph for the (V) oxidation states. H 3 PO 4
Biology 2180 Laboratory #7. Bacterial Growth and Transformation
 Biology 2180 Laboratory #7 Name Bacterial Growth and Transformation Introduction: Most aspects of molecular biology require the use of basic microbiological methods. This section is included for those
Biology 2180 Laboratory #7 Name Bacterial Growth and Transformation Introduction: Most aspects of molecular biology require the use of basic microbiological methods. This section is included for those
March 15, Genetics_of_Viruses_and_Bacteria_p5.notebook. smallest viruses are smaller than ribosomes. A virulent phage (Lytic)
 Genetics_of_Viruses_and_Bacteria_p5.notebook smallest viruses are smaller than ribosomes Adenovirus Tobacco mosaic virus Bacteriophage Influenza virus envelope is derived from the host cell The capsids
Genetics_of_Viruses_and_Bacteria_p5.notebook smallest viruses are smaller than ribosomes Adenovirus Tobacco mosaic virus Bacteriophage Influenza virus envelope is derived from the host cell The capsids
GENETICS OF THE TEMPERATURE-DEPENDENT AND -SENSITIVE REVERTANTS OBTAINED FROM THREONINE-REQUIRING MUTANTS OF BACILLUS SUBTILIS
 J. Gen. Appl. Microbiol., 15, 463-471 (1969) GENETICS OF THE TEMPERATURE-DEPENDENT AND -SENSITIVE REVERTANTS OBTAINED FROM THREONINE-REQUIRING MUTANTS OF BACILLUS SUBTILIS OSAMU KANAMITSU1 AND YONOSUKE
J. Gen. Appl. Microbiol., 15, 463-471 (1969) GENETICS OF THE TEMPERATURE-DEPENDENT AND -SENSITIVE REVERTANTS OBTAINED FROM THREONINE-REQUIRING MUTANTS OF BACILLUS SUBTILIS OSAMU KANAMITSU1 AND YONOSUKE
NCERT. 2. An enzyme catalysing the removal of nucleotides from the ends of DNA is: a. endonuclease b. exonuclease c. DNA ligase d.
 BIOTECHNOLOGY PRINCIPLES AND PROCESSES 75 CHAPTER 11 BIOTECHNOLOGY: PRINCIPLES AND PROCESSES 1. Rising of dough is due to: MULTIPLE-CHOICE QUESTIONS a. Multiplication of yeast b. Production of CO 2 c.
BIOTECHNOLOGY PRINCIPLES AND PROCESSES 75 CHAPTER 11 BIOTECHNOLOGY: PRINCIPLES AND PROCESSES 1. Rising of dough is due to: MULTIPLE-CHOICE QUESTIONS a. Multiplication of yeast b. Production of CO 2 c.
Reading Lecture 3: 24-25, 45, Lecture 4: 66-71, Lecture 3. Vectors. Definition Properties Types. Transformation
 Lecture 3 Reading Lecture 3: 24-25, 45, 55-66 Lecture 4: 66-71, 75-79 Vectors Definition Properties Types Transformation 56 VECTORS- Definition Vectors are carriers of a DNA fragment of interest Insert
Lecture 3 Reading Lecture 3: 24-25, 45, 55-66 Lecture 4: 66-71, 75-79 Vectors Definition Properties Types Transformation 56 VECTORS- Definition Vectors are carriers of a DNA fragment of interest Insert
HOUR EXAM II BIOLOGY 422 FALL, In the spirit of the honor code, I pledge that I have neither given nor received help on this exam.
 Name First Last PID Number - (Please Print) HOUR EXAM II BIOLOGY 422 FALL, 2007 In the spirit of the honor code, I pledge that I have neither given nor received help on this exam. 1 Signature 2 3 4 5 6
Name First Last PID Number - (Please Print) HOUR EXAM II BIOLOGY 422 FALL, 2007 In the spirit of the honor code, I pledge that I have neither given nor received help on this exam. 1 Signature 2 3 4 5 6
Design. Construction. Characterization
 Design Construction Characterization DNA mrna (messenger) A C C transcription translation C A C protein His A T G C T A C G Plasmids replicon copy number incompatibility selection marker origin of replication
Design Construction Characterization DNA mrna (messenger) A C C transcription translation C A C protein His A T G C T A C G Plasmids replicon copy number incompatibility selection marker origin of replication
Volume: 2: Issue-3: July-Sept ISSN EFFECT OF NITROGEN SOURCES ON MICROBIAL PRODUCTION OF XYLITOL. K. Srivani 1 and Y.
 Volume: 2: Issue-3: July-Sept -2011 ISSN 0976-4550 EFFECT OF NITROGEN SOURCES ON MICROBIAL PRODUCTION OF XYLITOL K. Srivani 1 and Y. Pydi Setty 2 1 Department of Chemical Engineering, National Institute
Volume: 2: Issue-3: July-Sept -2011 ISSN 0976-4550 EFFECT OF NITROGEN SOURCES ON MICROBIAL PRODUCTION OF XYLITOL K. Srivani 1 and Y. Pydi Setty 2 1 Department of Chemical Engineering, National Institute
Higher Biology. Unit 2: Homework Booklet Metabolism and Survival
 Higher Biology Unit 2: Homework Booklet Metabolism and Survival 0 1 Sub Topic 2.1: Regulation of Metabolism 1. Membranes can form small compartments within cells. Small compartments have: A high surface
Higher Biology Unit 2: Homework Booklet Metabolism and Survival 0 1 Sub Topic 2.1: Regulation of Metabolism 1. Membranes can form small compartments within cells. Small compartments have: A high surface
Human Viperin Causes Radical SAM Dependent Elongation of E. coli Hinting at its Physiological Role
 Supporting Information Human Viperin Causes Radical SAM Dependent Elongation of E. coli Hinting at its Physiological Role Micah T. Nelp, Anthony P. Young, Branden M. Stepanski, Vahe Bandarian* Department
Supporting Information Human Viperin Causes Radical SAM Dependent Elongation of E. coli Hinting at its Physiological Role Micah T. Nelp, Anthony P. Young, Branden M. Stepanski, Vahe Bandarian* Department
Practical- 6: Fermentative Production of Ethanol
 Practical- 6: Fermentative Production of Ethanol Introduction The overall reaction in fermentation of hexose by Yeast particularly S. cereviceae, can be expressed as under C 6 H 12 O 6 2C 2 H 5 OH + 2CO
Practical- 6: Fermentative Production of Ethanol Introduction The overall reaction in fermentation of hexose by Yeast particularly S. cereviceae, can be expressed as under C 6 H 12 O 6 2C 2 H 5 OH + 2CO
Lac Operon contains three structural genes and is controlled by the lac repressor: (1) LacY protein transports lactose into the cell.
 Regulation of gene expression a. Expression of most genes can be turned off and on, usually by controlling the initiation of transcription. b. Lactose degradation in E. coli (Negative Control) Lac Operon
Regulation of gene expression a. Expression of most genes can be turned off and on, usually by controlling the initiation of transcription. b. Lactose degradation in E. coli (Negative Control) Lac Operon
Executive Summary New Energy Company of Indiana CRADA Completed 1997 Public Release 1999
 Executive Summary New Energy Company of Indiana CRADA Completed 1997 Public Release 1999 CRADA Background The CRADA between the National Renewable Energy Laboratory and the New Energy Company of Indiana
Executive Summary New Energy Company of Indiana CRADA Completed 1997 Public Release 1999 CRADA Background The CRADA between the National Renewable Energy Laboratory and the New Energy Company of Indiana
