3/18/2018. Prof. Steven S. Saliterman Department of Biomedical Engineering, University of Minnesota
|
|
|
- Clara Blair
- 6 years ago
- Views:
Transcription
1 Department of Biomedical Engineering, University of Minnesota ISO Risk Management as Part of Control Human Factors and Usability Engineering Definitions How People Interact with Technology Inherent Safety by Protective Measures Information for Safety Report for Pre-Market Submission IEC Medical Electrical Equipment General requirements for basic safety and essential performance. Risk Management - The systematic application of management policies, procedures and practices to the tasks of analyzing, evaluating and controlling risk. * ISO 14971:2007 Medical Devices - Application of Risk Management to Medical Devices. ISO/TR in ISO TC210 (2013) Expert guidance on application of the standard. EN ISO 14971:2012 applies only to manufacturers with devices intended for the European market. *ISO 14971:2007 Medical Devices 1
2 Product & Process Monitoring Management Leadership Involvement Risk Management Process & Control Risk Management System Support ISO 14971:2007 Adopted from Nolan, N. Boston Scientific, Parenteral Drug Association, Quality Risk Management The Medical Device Experience, D&D Planning Risk Analysis User Needs Input Review DHF Change Verification Process Output Validation Medical Device Risk Management Adopted from Nolan, N. Boston Scientific, Parenteral Drug Association, Quality Risk Management The Medical Device Experience, Requires procedures and practices for analyzing, evaluating, controlling, and monitoring product risks. Management tool: Includes management s role in making product risk-based decisions and reviewing system effectiveness. Connections to, Complaint, CAPA and QS Management reviews. Nolan, N. Boston Scientific, Parenteral Drug Association, Quality Risk Management The Medical Device Experience,
3 Risk Management Plan Risk Management File Risk Analysis Evaluation of Risk Acceptability (Risk/Benefit) Risk Management Report Production and Post Production Information Nolan, N. Boston Scientific, Parenteral Drug Association, Quality Risk Management The Medical Device Experience, Scope of risk management activities, including the intended use of the device and product lifecycle Assignment of responsibilities and authorities Review requirements for risk management Risk acceptability criteria Risk Verification Production activity data collection and review Post Production activity data collection and review. Risk = Severity x Probability Nolan, N. Boston Scientific, Parenteral Drug Association, Quality Risk Management The Medical Device Experience, Abnormal use (unintended use no recourse). Critical task (harm if task not or incorrectly performed). Formative evaluation (assessing user interface & interactions throughout device development). Hazard (potential source of harm). Hazardous situation (hazard plus sequence of events). 3
4 Task (what the user does). Use error (user action or inaction different than the manufacturer expected that could or did cause harm). Use safety (no use-related risk). User (person using the device). User interface (all user device interactions). Human Factors Considerations Outcome User Device Use Adopted from Applying Human Factors and Usability Engineering to Medical Devices. U.S. Department of Health and Human Services Food and Drug Physical hazards (sharp corners or edges). Mechanical hazards (kinetic or potential energy from a moving object). Thermal hazards (high-temperature components). Electrical hazards (electrical current, EMI). 4
5 Chemical hazards (toxic chemicals). Radiation hazards (ionizing and nonionizing). Biological hazards (allergens, bioincompatible agents and infectious agents). Use specific connectors that cannot be connected to the wrong component. Remove features that can be mistakenly selected or eliminate an interaction when it could lead to use error. Improve the detectability or readability of controls, labels, and displays. Automate device functions that are prone to use error when users perform the task manually. Incorporate safety mechanisms such as physical safety guards, shielded elements, or software or hardware interlocks. Include warning screens to advise the user of essential conditions that should exist prior to proceeding with device use, such as specific data entry. Use alerts for hazardous conditions, such as a low battery alert when an unexpected loss of the device s operation could cause harm or death. Use device technologies that require less maintenance or are maintenance free. 5
6 Provide written information, such as warning or caution statements in the user manual that highlight and clearly discuss the use-related hazard. Train users to avoid the use error. 1) Conclusion. 2) Descriptions of intended device users, uses, use environments, and training. 3) Description of device user interface. 4) Summary of known use problems. 5) Analysis of hazards and risks associated with use of the device. 6) Summary of preliminary analyses and evaluations. 7) Description and categorization of critical tasks. 8) Details of human factors validation testing. Standard for electromedical equipment safety. International Electrotechnical Commission (IEC) 3 rd Edition. Image courtesy of MassMEDIC 6
7 Part 1: General requirements for basic safety and essential performance. Part 2: Electromagnetic compatibly. Part 3: Radiation protection in diagnostic X- ray equipment. Part 6: Usability Part 8: Tests and guidance for alarm systems. A standard covering electrical equipment used in medical practice. Covers essential performance & basic safety both fundamental in addressing hazards. Addresses accuracy of power or therapeutic substance delivery and display of physiological data that will effect patient management. Includes: Classification Requirements Test specifications Risk management. If you were the manufacturer of this medical device, what basic safety and essential performance concerns would you have? Image courtesy of Smiths Medical 7
8 Regulating flow of fluids into a patient under pressure generated by a pump. Type 1 - Continuous only. Type 2 - Non-continuous only. Type 3 - Discrete delivery of a bolus. Type 4 - Profile pump General requirements for testing of ME EQUIPMENT Classification of ME EQUIPMENT and ME SYSTEMS ME EQUIPMENT identification, marking and documents Protection against electrical HAZARDS from ME EQUIPMENT Protection against MECHANICAL HAZARDS of ME EQUIPMENT and ME SYSTEMS Protection against unwanted and excessive radiation HAZARDS Protection against excessive temperatures and other HAZARDS Accuracy of controls and instruments and protection against hazardous outputs 8
9 Emissions testing measures Electromagnetic (EM) interference radiated or conducted out of the device. Emissions from the device can cause malfunctions in nearby equipment. Image courtesy of Com-Power Image courtesy of Metaldetectors Susceptibility testing measures the device s immunity to external EM interference conducted or radiated into the device. An example of external interference is Electrostatic Discharge (ESD). Image courtesy of Teseq ISO 14971and a Risk Management Plan. Application of HFE/UE initially reduces the need for design modifications and costly updates after market introduction and offers competitive advantages. A HFE/UE report included in a premarket submission should provide information pertaining to device use safety and effectiveness in summary form. IEC Medical Electrical Equipment basic safety and essential performance. 9
FDA Medical Device HFE Guidance
 W H I T E P A P E R www.makrocare.com U S FDA has established a new Draft Guidance; Applying Human Factors and Usability Engineering to Medical Devices to Optimize Safety and Effectiveness in Design. Manufacturers
W H I T E P A P E R www.makrocare.com U S FDA has established a new Draft Guidance; Applying Human Factors and Usability Engineering to Medical Devices to Optimize Safety and Effectiveness in Design. Manufacturers
ISO INTERNATIONAL STANDARD. Implants for surgery Active implantable medical devices Part 4: Implantable infusion pumps
 INTERNATIONAL STANDARD ISO 14708-4 First edition 2008-11-15 Implants for surgery Active implantable medical devices Part 4: Implantable infusion pumps Implants chirurgicaux Dispositifs médicaux implantables
INTERNATIONAL STANDARD ISO 14708-4 First edition 2008-11-15 Implants for surgery Active implantable medical devices Part 4: Implantable infusion pumps Implants chirurgicaux Dispositifs médicaux implantables
PMA 31-G. English. Printed: Doc-Nr: PUB / / 000 / 00
 PMA 31-G English 1 Information about the documentation 1.1 About this documentation Read this documentation before initial operation or use. This is a prerequisite for safe, trouble-free handling and
PMA 31-G English 1 Information about the documentation 1.1 About this documentation Read this documentation before initial operation or use. This is a prerequisite for safe, trouble-free handling and
(C) QAdvis. IEC and IEC how to make them work (and why so much attention on SW) QAdvis RMD, Prague, November 8th 2016
 IEC 62304 and IEC 82304-1 - how to make them work (and why so much attention on SW) QAdvis RMD, Prague, November 8th 2016 QMS in-the cloud Turn key QMS Digital signatures Efficient and lean Validated and
IEC 62304 and IEC 82304-1 - how to make them work (and why so much attention on SW) QAdvis RMD, Prague, November 8th 2016 QMS in-the cloud Turn key QMS Digital signatures Efficient and lean Validated and
Risk Based Approach To Complaint Handling
 Risk Based Approach To Complaint Handling Khaudeja Bano, M.D. Senior Medical Director, Medical Device Safety Head, AbbVie Inc. Life Sciences Product Complaints Congress Europe Dublin, Ireland December
Risk Based Approach To Complaint Handling Khaudeja Bano, M.D. Senior Medical Director, Medical Device Safety Head, AbbVie Inc. Life Sciences Product Complaints Congress Europe Dublin, Ireland December
TERMS AND DEFINITIONS
 TERMS AND DEFINITIONS Advisory notice A notice issued by the organization, subsequent to delivery of the medical device, to provide supplementary information and/or to advise what action should be taken
TERMS AND DEFINITIONS Advisory notice A notice issued by the organization, subsequent to delivery of the medical device, to provide supplementary information and/or to advise what action should be taken
IEC KHBO, Hobufonds SAFESYS ing. Alexander Dekeyser ing. Kurt Lintermans
 IEC 61508 KHBO, Hobufonds SAFESYS ing. Alexander Dekeyser ing. Kurt Lintermans page 2 PART 1 : GENERAL REQUIREMENTS 1 Scope The first objective of this standard is to facilitate the development of application
IEC 61508 KHBO, Hobufonds SAFESYS ing. Alexander Dekeyser ing. Kurt Lintermans page 2 PART 1 : GENERAL REQUIREMENTS 1 Scope The first objective of this standard is to facilitate the development of application
ISO : Rustam Rakhimov (DMS Lab)
 ISO 26262 : 2011 Rustam Rakhimov (DMS Lab) Introduction Adaptation of IEC 61508 to road vehicles Influenced by ISO 16949 Quality Management System The first comprehensive standard that addresses safety
ISO 26262 : 2011 Rustam Rakhimov (DMS Lab) Introduction Adaptation of IEC 61508 to road vehicles Influenced by ISO 16949 Quality Management System The first comprehensive standard that addresses safety
Regulations governing the application of medical accelerators
 Regulations governing the application of medical accelerators in 50 minutes. marko.mehle@cosylab.com 2 1.The wonderland of STANDARDS AND REGULATIONS 3 Laws and standards Medical devices (and systems) are
Regulations governing the application of medical accelerators in 50 minutes. marko.mehle@cosylab.com 2 1.The wonderland of STANDARDS AND REGULATIONS 3 Laws and standards Medical devices (and systems) are
International Standards and EU regulation of medical device software an update
 International Standards and EU regulation of medical device software an update Sherman Eagles Partner, SoftwareCPR seagles@softwarecpr.com 612 865 0107 1 Who am I? 18 years at Medtronic, retired 2008 Last
International Standards and EU regulation of medical device software an update Sherman Eagles Partner, SoftwareCPR seagles@softwarecpr.com 612 865 0107 1 Who am I? 18 years at Medtronic, retired 2008 Last
The Risk Management + Design Controls Connection: What Device Makers Need to Know
 !!! The Risk Management + Design Controls Connection: What Device Makers Need to Know Jon Speer Founder & VP of QA/RA greenlight.guru Table of Contents 1 Intended Use & User Needs 6 Verification, Validation,
!!! The Risk Management + Design Controls Connection: What Device Makers Need to Know Jon Speer Founder & VP of QA/RA greenlight.guru Table of Contents 1 Intended Use & User Needs 6 Verification, Validation,
MEDICAL DEVICES REGULATORY SYSTEM
 GUIDANCE DOCUMENT MEDICAL DEVICES REGULATORY SYSTEM Table of Contents PART 1: PRE-MARKET ASSESSMENT 9 SECTION 1: INTRODUCTION 9 1.1 Principles and Main Features of A Regulatory Framework of Medical Device
GUIDANCE DOCUMENT MEDICAL DEVICES REGULATORY SYSTEM Table of Contents PART 1: PRE-MARKET ASSESSMENT 9 SECTION 1: INTRODUCTION 9 1.1 Principles and Main Features of A Regulatory Framework of Medical Device
RE: Docket No. FDA 2017 N 0041: Agency Information Collection Activities; Proposed Collection; Comment Request; Safety Assurance Case
 701 Pennsylvania Avenue, NW Suite 800 Washington, D.C. 20004 2654 Tel: 202 783 8700 Fax: 202 783 8750 www.advamed.org Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers
701 Pennsylvania Avenue, NW Suite 800 Washington, D.C. 20004 2654 Tel: 202 783 8700 Fax: 202 783 8750 www.advamed.org Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers
Safety cannot rely on testing
 Standards 1 Computer-based systems (generically referred to as programmable electronic systems) are being used in all application sectors to perform non-safety functions and, increasingly, to perform safety
Standards 1 Computer-based systems (generically referred to as programmable electronic systems) are being used in all application sectors to perform non-safety functions and, increasingly, to perform safety
Industry Experience: Early Collaboration with FDA on Combination Products. Kristi Kistner, Amgen Inc. CMC Strategy Form January 26, 2015
 Industry Experience: Early Collaboration with FDA on Combination Products Kristi Kistner, Amgen Inc. CMC Strategy Form January 26, 2015 Collaboration is working together to achieve shared goals Given the
Industry Experience: Early Collaboration with FDA on Combination Products Kristi Kistner, Amgen Inc. CMC Strategy Form January 26, 2015 Collaboration is working together to achieve shared goals Given the
ISO INTERNATIONAL STANDARD. Implants for surgery Active implantable medical devices Part 2: Cardiac pacemakers
 INTERNATIONAL STANDARD Provläsningsexemplar / Preview ISO 14708-2 First edition 2005-10-01 Implants for surgery Active implantable medical devices Part 2: Cardiac pacemakers Implants chirurgicaux Dispositifs
INTERNATIONAL STANDARD Provläsningsexemplar / Preview ISO 14708-2 First edition 2005-10-01 Implants for surgery Active implantable medical devices Part 2: Cardiac pacemakers Implants chirurgicaux Dispositifs
Deciding When to Submit a 510(k) for a Change to an Existing Device Guidance for Industry and Food and Drug Administration Staff
 Deciding When to Submit a 510(k) for a Change to an Existing Device Guidance for Industry and Food and Drug Administration Staff Document issued on October 25, 2017. The draft of this document was issued
Deciding When to Submit a 510(k) for a Change to an Existing Device Guidance for Industry and Food and Drug Administration Staff Document issued on October 25, 2017. The draft of this document was issued
Water Level Meter User Manual
 Man 054 Water Level Meter User Manual Soil Instruments Limited has an ongoing policy of design review and reserves the right to amend these specifications without notice. Man054 - Water Level Meter - MN1114
Man 054 Water Level Meter User Manual Soil Instruments Limited has an ongoing policy of design review and reserves the right to amend these specifications without notice. Man054 - Water Level Meter - MN1114
Medical Device Software under IEC George Romanski
 Medical Device Software under IEC 62304 George Romanski IEC 62304 Medical Device Software Software Lifecycle Processes Quality Management System* RISK MANAGEMENT Software Safety Classification Development
Medical Device Software under IEC 62304 George Romanski IEC 62304 Medical Device Software Software Lifecycle Processes Quality Management System* RISK MANAGEMENT Software Safety Classification Development
Application of Systems Engineering to Regulatory Compliance Activities for Medical Devices
 Application of Systems Engineering to Regulatory Compliance Activities for Medical Devices Presented by Apoorv Maheshwari INCOSE Healthcare MBSE Challenge Team: Modeling for a Healthy Future Apoorv Maheshwari
Application of Systems Engineering to Regulatory Compliance Activities for Medical Devices Presented by Apoorv Maheshwari INCOSE Healthcare MBSE Challenge Team: Modeling for a Healthy Future Apoorv Maheshwari
Implants for surgery Active implantable medical devices. Part 3: Implantable neurostimulators
 Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 14708-3 Second edition 2017-04 Implants for surgery Active implantable medical devices Part 3: Implantable neurostimulators Implants chirurgicaux
Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 14708-3 Second edition 2017-04 Implants for surgery Active implantable medical devices Part 3: Implantable neurostimulators Implants chirurgicaux
V&V Best Practices. CASSS, CMC Strategy Forum Steven W. Badelt, PhD Managing Partner Suttons Creek, Inc. SUTTONSCREEK.COM
 V&V Best Practices CASSS, CMC Strategy Forum Steven W. Badelt, PhD Managing Partner Suttons Creek, Inc. Solving the problem of Complexity. 21CFR820.30 & FDA Guidance INCOSE System Engineering Handbook
V&V Best Practices CASSS, CMC Strategy Forum Steven W. Badelt, PhD Managing Partner Suttons Creek, Inc. Solving the problem of Complexity. 21CFR820.30 & FDA Guidance INCOSE System Engineering Handbook
HP Standard Supplier Requirements for Safe and Legal Products
 HP Standard 014-2 Supplier Requirements for Safe and Legal Products Document Identifier Revision and Date Last Revalidation Date Abstract Applicability Status HX-00014-02 G, This standard describes HP
HP Standard 014-2 Supplier Requirements for Safe and Legal Products Document Identifier Revision and Date Last Revalidation Date Abstract Applicability Status HX-00014-02 G, This standard describes HP
The Integration and Application of ISO 9001 and the NIAHO Requirements In Medical Equipment Management
 BUSINESS ASSURANCE The Integration and Application of ISO 9001 and the NIAHO Requirements In Medical Equipment Management Randall Snelling 20 October 2017 1 DNV GL 2017 20 October 2017 SAFER, SMARTER,
BUSINESS ASSURANCE The Integration and Application of ISO 9001 and the NIAHO Requirements In Medical Equipment Management Randall Snelling 20 October 2017 1 DNV GL 2017 20 October 2017 SAFER, SMARTER,
SafeDesign: Machine Safety Validation
 SafeDesign: Machine Safety Validation Host: Steve Ludwig Rockwell Automation Safety Business Programs Manager Copyright 2010 Rockwell Automation, Inc. All rights reserved. 1 Today s Agenda 1. Review of
SafeDesign: Machine Safety Validation Host: Steve Ludwig Rockwell Automation Safety Business Programs Manager Copyright 2010 Rockwell Automation, Inc. All rights reserved. 1 Today s Agenda 1. Review of
Implications of the new MDR from a Product Testing and Certification Perspective
 Implications of the new MDR from a Product Testing and Certification Perspective Helping You to Access Global Markets FAST and PREDICTABLY www.test-medical-devices.com 1 Hans Gerd Evering MDR - Implications
Implications of the new MDR from a Product Testing and Certification Perspective Helping You to Access Global Markets FAST and PREDICTABLY www.test-medical-devices.com 1 Hans Gerd Evering MDR - Implications
ISO 22000:2005 SYSTEMKARAN ADVISER & INFORMATION CENTER SYSTEM KARAN ADVISER & INFORMATION CENTER FOOD SAFETY MANAGEMENT SYSTEM ISO 22000:2005
 SYSTEM KARAN ADVISER & INFORMATION CENTER FOOD SAFETY MANAGEMENT SYSTEM ISO 22000:2005 WWW.SYSTEMKARAN.ORG 1 www.systemkaran.org Foreword... 6 Introduction... 7 Food safety management systems Requirements
SYSTEM KARAN ADVISER & INFORMATION CENTER FOOD SAFETY MANAGEMENT SYSTEM ISO 22000:2005 WWW.SYSTEMKARAN.ORG 1 www.systemkaran.org Foreword... 6 Introduction... 7 Food safety management systems Requirements
NEMA Standards Publication NEMA/MITA 1. Good Refurbishment Practices for Medical Imaging Equipment
 TECHNICAL GUIDE: NEMA Standards Publication NEMA/MITA 1 Good Refurbishment Practices for Medical Imaging Equipment Prepared by DITTA the global voice of diagnostic imaging, radiation therapy, healthcare
TECHNICAL GUIDE: NEMA Standards Publication NEMA/MITA 1 Good Refurbishment Practices for Medical Imaging Equipment Prepared by DITTA the global voice of diagnostic imaging, radiation therapy, healthcare
MEASURE FOR MEASURE: QUALITY METRICS
 MEASURE FOR MEASURE: QUALITY METRICS PDA Midwest Chapter Dinner Meeting, Northbrook, IL-9 November 2017 Felicia Ford-Rice, Director, Strategic Compliance 2017 PAREXEL INTERNATIONAL CORP. AGENDA Robust
MEASURE FOR MEASURE: QUALITY METRICS PDA Midwest Chapter Dinner Meeting, Northbrook, IL-9 November 2017 Felicia Ford-Rice, Director, Strategic Compliance 2017 PAREXEL INTERNATIONAL CORP. AGENDA Robust
FCX Department of Occupational Health and Safety Policy
 FCX Department of Occupational Health and Safety Policy Lockout/Tagout/Tryout (LOTOTO) (Control of Hazardous Energy Sources) Approval Date: 08/29/2014 Original Date: 03/09/2009 Policy # FCX-04 Revision
FCX Department of Occupational Health and Safety Policy Lockout/Tagout/Tryout (LOTOTO) (Control of Hazardous Energy Sources) Approval Date: 08/29/2014 Original Date: 03/09/2009 Policy # FCX-04 Revision
DRAFT MEDICAL DEVICE GUIDANCE DOCUMENT
 November 2015 DRAFT DRAFT MEDICAL DEVICE GUIDANCE DOCUMENT REQUIREMENTS FOR LABELLING OF MEDICAL DEVICES Medical Device Authority MINISTRY OF HEALTH MALAYSIA Contents Page Preface... iii 1 Introduction.
November 2015 DRAFT DRAFT MEDICAL DEVICE GUIDANCE DOCUMENT REQUIREMENTS FOR LABELLING OF MEDICAL DEVICES Medical Device Authority MINISTRY OF HEALTH MALAYSIA Contents Page Preface... iii 1 Introduction.
SAFETY RELATED SYSTEMS
 SAFETY RELATED SYSTEMS Golden Hill Centre School Lane Leyland Preston Lancashire PR25 2TU Tel: 01772 622200 Fax: 01772 622455 Email: contactus@jfnl.co.uk Web: www.jfnuclear.co.uk James Fisher Nuclear Limited
SAFETY RELATED SYSTEMS Golden Hill Centre School Lane Leyland Preston Lancashire PR25 2TU Tel: 01772 622200 Fax: 01772 622455 Email: contactus@jfnl.co.uk Web: www.jfnuclear.co.uk James Fisher Nuclear Limited
Understanding IEC 62304
 Understanding IEC 62304 Co-authored by MethodSense, Inc. and Medical Equipment Compliance Associates, LLC www.methodsense.com 919.313.3960 Understanding IEC 62304 By: Rita King, Chief Executive Officer,
Understanding IEC 62304 Co-authored by MethodSense, Inc. and Medical Equipment Compliance Associates, LLC www.methodsense.com 919.313.3960 Understanding IEC 62304 By: Rita King, Chief Executive Officer,
CDRH Regulation Medical Device Software Validation
 CDRH Regulation Medical Device Software Validation Do-Hyun Kim, Ph.D, Ph.D CEO, BT Solutions, Inc. South Korea CEO, Solutions 4BT, LLC, USA The Transformation of Medical Device Industry for Thailand 4.0:
CDRH Regulation Medical Device Software Validation Do-Hyun Kim, Ph.D, Ph.D CEO, BT Solutions, Inc. South Korea CEO, Solutions 4BT, LLC, USA The Transformation of Medical Device Industry for Thailand 4.0:
ENABLING 21 ST CENTURY HEALTHCARE
 ENABLING 21 ST CENTURY HEALTHCARE CONNECTED EFFICIENT INTELLIGENT PERSONALIZED HCL ERS MEDICAL SERVICES 15+ years of experience in medical devices alone. Several complex mission-critical electro-mechanical
ENABLING 21 ST CENTURY HEALTHCARE CONNECTED EFFICIENT INTELLIGENT PERSONALIZED HCL ERS MEDICAL SERVICES 15+ years of experience in medical devices alone. Several complex mission-critical electro-mechanical
Contractor / Vendor Safety Manual
 Contractor / Vendor Safety Manual All contractors and vendors conducting business at the Salk Institute for Biological Studies, the Institute, at 10010 N. Torrey Pines Road, La Jolla, CA 92037 must: 1.
Contractor / Vendor Safety Manual All contractors and vendors conducting business at the Salk Institute for Biological Studies, the Institute, at 10010 N. Torrey Pines Road, La Jolla, CA 92037 must: 1.
Medical Device Regulatory Framework 9 SEPTEMBER 2015 FUNDISA CONFERENCE JANE ROGERS
 Medical Device Regulatory Framework 9 SEPTEMBER 2015 FUNDISA CONFERENCE JANE ROGERS Key Topics Definitions Essential Principles Classification Conformity Assessment Framework License to Manufacture, Import,
Medical Device Regulatory Framework 9 SEPTEMBER 2015 FUNDISA CONFERENCE JANE ROGERS Key Topics Definitions Essential Principles Classification Conformity Assessment Framework License to Manufacture, Import,
Summary of TL 9000 R4.0 Requirements Beyond ISO 9001:2000
 This summary identifies the additional TL 9000 Release 4.0 requirements beyond those stated in ISO 9001:2000. See the TL 9000 R4.0 Handbook for the actual TL 9000 R4.0 requirements. ISO 9001:2000 section
This summary identifies the additional TL 9000 Release 4.0 requirements beyond those stated in ISO 9001:2000. See the TL 9000 R4.0 Handbook for the actual TL 9000 R4.0 requirements. ISO 9001:2000 section
AEROSPACE STANDARD. Quality Systems - Aerospace - Model for Quality Assurance in Design, Development, Production, Installation and Servicing
 AEROSPACE STANDARD AS9100 Technically equivalent to AECMA pren 9100 Issued 1999-11 Revised 2001-08 Superseding AS9100 REV. A Quality Systems - Aerospace - Model for Quality Assurance in Design, Development,
AEROSPACE STANDARD AS9100 Technically equivalent to AECMA pren 9100 Issued 1999-11 Revised 2001-08 Superseding AS9100 REV. A Quality Systems - Aerospace - Model for Quality Assurance in Design, Development,
Safety in. Cement Industry
 Safety in Cement Industry Safety in Cement Industry Explosion Risk Assessments E xplosions constitute a major issue in the cement industry not only due to the equipment required for handling solid fuels
Safety in Cement Industry Safety in Cement Industry Explosion Risk Assessments E xplosions constitute a major issue in the cement industry not only due to the equipment required for handling solid fuels
DEPARTMENT OF THE ARMY US ARMY MEDICAL DEPARTMENT Fort Huachuca, Arizona
 DEPARTMENT OF THE ARMY US ARMY MEDICAL DEPARTMENT Fort Huachuca, Arizona 85613-7079 *MEDDAC Memo 750-3 MEDDAC Memorandum 8 January 2008 No. 750-3 Maintenance of Supplies and Equipment ELECTRO MAGNETIC
DEPARTMENT OF THE ARMY US ARMY MEDICAL DEPARTMENT Fort Huachuca, Arizona 85613-7079 *MEDDAC Memo 750-3 MEDDAC Memorandum 8 January 2008 No. 750-3 Maintenance of Supplies and Equipment ELECTRO MAGNETIC
1.4 Applicable Regulatory Requirement(s) Any law(s) and regulation(s) addressing the conduct of clinical trials of investigational products.
 1.1 Adverse Drug Reaction (ADR) In the pre-approval clinical experience with a new medicinal product or its new usages, particularly as the therapeutic dose(s) may not be established: all noxious and unintended
1.1 Adverse Drug Reaction (ADR) In the pre-approval clinical experience with a new medicinal product or its new usages, particularly as the therapeutic dose(s) may not be established: all noxious and unintended
Tank Scale Service Checklist
 Tank Scale Service Checklist Specifying Service for Optimized Weighing Processes Selecting the right weighing equipment is an important first step to ensuring that your weighing processes are able to meet
Tank Scale Service Checklist Specifying Service for Optimized Weighing Processes Selecting the right weighing equipment is an important first step to ensuring that your weighing processes are able to meet
Post market Surveillance ISO EU Medical Device Regulation
 Post market Surveillance ISO13485 2016 EU Medical Device Regulation Patrick Caines, Ph.D. Baxter Healthcare 15 June 2017 Agenda Post market Regulatory Requirements ISO 13485 2016 Summary of key changes
Post market Surveillance ISO13485 2016 EU Medical Device Regulation Patrick Caines, Ph.D. Baxter Healthcare 15 June 2017 Agenda Post market Regulatory Requirements ISO 13485 2016 Summary of key changes
Medical Devices; Cardiovascular Devices; Classification of the Adjunctive Cardiovascular
 This document is scheduled to be published in the Federal Register on 07/28/2017 and available online at https://federalregister.gov/d/2017-15901, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 07/28/2017 and available online at https://federalregister.gov/d/2017-15901, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
Functional safety Safety instrumented systems for the process industry sector
 BRITISH STANDARD BS IEC 61511-1:2003 Functional safety Safety instrumented systems for the process industry sector Part 1: Framework, definitions, system, hardware and software requirements ICS 25.040.01;
BRITISH STANDARD BS IEC 61511-1:2003 Functional safety Safety instrumented systems for the process industry sector Part 1: Framework, definitions, system, hardware and software requirements ICS 25.040.01;
Q10 PHARMACEUTICAL QUALITY SYSTEM
 Q10 PHARMACEUTICAL QUALITY SYSTEM This draft guidance, when finalized, will represent the Food and Drug Administration's (FDA's) current thinking on this topic. It does not create or confer any rights
Q10 PHARMACEUTICAL QUALITY SYSTEM This draft guidance, when finalized, will represent the Food and Drug Administration's (FDA's) current thinking on this topic. It does not create or confer any rights
A roadmap to ISO implementation
 A roadmap to ISO 14971 implementation Derek Flood *,, Fergal Mc Caffery, Valentine Casey, Ruth McKeever and Peter Rust Dundalk Institute of Technology Dundalk, Ireland ABSTRACT Medical device standards
A roadmap to ISO 14971 implementation Derek Flood *,, Fergal Mc Caffery, Valentine Casey, Ruth McKeever and Peter Rust Dundalk Institute of Technology Dundalk, Ireland ABSTRACT Medical device standards
PRODUCTION AND PERFORMANCE MANAGEMENT DATA HISTORIANS FOR INCIDENT AND DEVIATION MANAGEMENT IN THE REGULATED INDUSTRIES
 PRODUCTION AND PERFORMANCE MANAGEMENT DATA HISTORIANS FOR INCIDENT AND DEVIATION MANAGEMENT IN THE REGULATED INDUSTRIES DATA HISTORIANS FOR INCIDENT AND DEVIATION MANAGEMENT DATA HISTORIANS FOR INCIDENT
PRODUCTION AND PERFORMANCE MANAGEMENT DATA HISTORIANS FOR INCIDENT AND DEVIATION MANAGEMENT IN THE REGULATED INDUSTRIES DATA HISTORIANS FOR INCIDENT AND DEVIATION MANAGEMENT DATA HISTORIANS FOR INCIDENT
Driving Sustainable Resource Management through BS EN ISO 14001:2015. Dr David Smith Technical Director for Business Sustainability, AECOM
 Management through BS EN ISO Dr David Smith Technical Director for Business Sustainability, AECOM March 2017 Structure of Presentation Background Business benefits of Sustainable Resource Management Key
Management through BS EN ISO Dr David Smith Technical Director for Business Sustainability, AECOM March 2017 Structure of Presentation Background Business benefits of Sustainable Resource Management Key
Understanding FDA Guidance On Connected Medical Devices
 Portfolio Media. Inc. 111 West 19 th Street, 5th Floor New York, NY 10011 www.law360.com Phone: +1 646 783 7100 Fax: +1 646 783 7161 customerservice@law360.com Understanding FDA Guidance On Connected Medical
Portfolio Media. Inc. 111 West 19 th Street, 5th Floor New York, NY 10011 www.law360.com Phone: +1 646 783 7100 Fax: +1 646 783 7161 customerservice@law360.com Understanding FDA Guidance On Connected Medical
INCREASING UTILITY: DESIGNING MEDICAL DEVICES AND APPLICATIONS FOR REMOTE MONITORING
 INCREASING UTILITY: DESIGNING MEDICAL DEVICES AND APPLICATIONS FOR REMOTE MONITORING 2011 Design of Medical Devices Conference April 12-14, 2011, Minneapolis, Minnesota, USA Lori Lucke, Anne Mickelson,
INCREASING UTILITY: DESIGNING MEDICAL DEVICES AND APPLICATIONS FOR REMOTE MONITORING 2011 Design of Medical Devices Conference April 12-14, 2011, Minneapolis, Minnesota, USA Lori Lucke, Anne Mickelson,
Update on the IVDR. Sue Spencer
 Update on the IVDR Sue Spencer Caution The new regulations are draft the principles have now been agreed but the Annexes are subject to minor changes Further details will be added later pre and post application
Update on the IVDR Sue Spencer Caution The new regulations are draft the principles have now been agreed but the Annexes are subject to minor changes Further details will be added later pre and post application
ABB drives. Technical guide no.10 Functional safety
 ABB drives Technical guide no.10 Functional safety 2 Technical guide no. 10 - Functional safety ABB drives Technical guide no. 10 Functional safety 3AUA0000048753 REV D EFFECTIVE: 14.3.2011 Copyright 2011
ABB drives Technical guide no.10 Functional safety 2 Technical guide no. 10 - Functional safety ABB drives Technical guide no. 10 Functional safety 3AUA0000048753 REV D EFFECTIVE: 14.3.2011 Copyright 2011
GENERAL PRINCIPLES OF SOFTWARE VALIDATION
 GUIDANCE FOR INDUSTRY GENERAL PRINCIPLES OF SOFTWARE VALIDATION DRAFT GUIDANCE Version 1.1 This guidance is being distributed for comment purposes only. Draft released for comment on: June 9, 1997 Comments
GUIDANCE FOR INDUSTRY GENERAL PRINCIPLES OF SOFTWARE VALIDATION DRAFT GUIDANCE Version 1.1 This guidance is being distributed for comment purposes only. Draft released for comment on: June 9, 1997 Comments
Radiation Shielding Glass RD 30 RD 50
 Radiation Shielding Glass RD 30 RD 50 2 SCHOTT is a leading international technology group in the areas of specialty glass and glass-ceramics. With more than 130 years of outstanding development, materials
Radiation Shielding Glass RD 30 RD 50 2 SCHOTT is a leading international technology group in the areas of specialty glass and glass-ceramics. With more than 130 years of outstanding development, materials
Guide for Class I Manufacturers on Compliance with European Communities (Medical Devices) Regulations, 1994
 Guide for Class I Manufacturers on Compliance with European Communities (Medical Devices) Regulations, 1994 SUR-G0006-2 27 AUGUST 2010 This guide does not purport to be an interpretation of law and/or
Guide for Class I Manufacturers on Compliance with European Communities (Medical Devices) Regulations, 1994 SUR-G0006-2 27 AUGUST 2010 This guide does not purport to be an interpretation of law and/or
ISO INTERNATIONAL STANDARD. Safety of machinery Guards General requirements for the design and construction of fixed and movable guards
 INTERNATIONAL STANDARD ISO 14120 First edition 2002-02-01 Safety of machinery Guards General requirements for the design and construction of fixed and movable guards Sécurité des machines Protecteurs Prescriptions
INTERNATIONAL STANDARD ISO 14120 First edition 2002-02-01 Safety of machinery Guards General requirements for the design and construction of fixed and movable guards Sécurité des machines Protecteurs Prescriptions
CERTIFICATE OF ACCREDITATION
 CERTIFICATE OF ACCREDITATION ANSI-ASQ National Accreditation Board 500 Montgomery Street, Suite 625, Alexandria, VA 22314, 877-344-3044 This is to certify that Element Materials Technology Jupiter, LLC
CERTIFICATE OF ACCREDITATION ANSI-ASQ National Accreditation Board 500 Montgomery Street, Suite 625, Alexandria, VA 22314, 877-344-3044 This is to certify that Element Materials Technology Jupiter, LLC
X30 Xlinks Operator s Manual
 X30 Xlinks Operator s Manual Part Number: AGA5332-EN Rev Number: 1.0 For use with X30 Software Version 3.16 Copyright Topcon Precision Agriculture May 2013 All contents in this manual are copyrighted by
X30 Xlinks Operator s Manual Part Number: AGA5332-EN Rev Number: 1.0 For use with X30 Software Version 3.16 Copyright Topcon Precision Agriculture May 2013 All contents in this manual are copyrighted by
MEDICAL WASTE MANAGEMENT STUDY NOTES
 MEDICAL WASTE MANAGEMENT STUDY NOTES UNIT 1 INTRODUCTION Hospital and other health care establishment has a duty of care for the environment and for public health and have particular responsibility in
MEDICAL WASTE MANAGEMENT STUDY NOTES UNIT 1 INTRODUCTION Hospital and other health care establishment has a duty of care for the environment and for public health and have particular responsibility in
Textvergleich. Verglichene Dokumente MEDIA3917.pdf. ICH_Q10_Step4.pdf
 Textvergleich Verglichene Dokumente MEDIA3917.pdf ICH_Q10_Step4.pdf Übersicht 2270 Wort/Wörter hinzugefügt 1338 Wort/Wörter gelöscht 3558 Wort/Wörter übereinstimmend 157 Block/Blöcke übereinstimmend Blättern
Textvergleich Verglichene Dokumente MEDIA3917.pdf ICH_Q10_Step4.pdf Übersicht 2270 Wort/Wörter hinzugefügt 1338 Wort/Wörter gelöscht 3558 Wort/Wörter übereinstimmend 157 Block/Blöcke übereinstimmend Blättern
EUROPEAN COMMISSION ENTERPRISE AND INDUSTRY DIRECTORATE-GENERAL. EudraLex The Rules Governing Medicinal Products in the European Union
 EUROPEAN COMMISSION ENTERPRISE AND INDUSTRY DIRECTORATE-GENERAL Consumer goods Pharmaceuticals Brussels, 08 April 2008 EudraLex The Rules Governing Medicinal Products in the European Union Volume 4 EU
EUROPEAN COMMISSION ENTERPRISE AND INDUSTRY DIRECTORATE-GENERAL Consumer goods Pharmaceuticals Brussels, 08 April 2008 EudraLex The Rules Governing Medicinal Products in the European Union Volume 4 EU
Safe Supply Chains Help Produce Sustainable Businesses
 Safe Supply Chains Help Produce Sustainable Businesses While international supply chains have created tremendous business opportunities for companies, they have spurred great risks, too. It is no longer
Safe Supply Chains Help Produce Sustainable Businesses While international supply chains have created tremendous business opportunities for companies, they have spurred great risks, too. It is no longer
Part II AUTOMATION AND CONTROL TECHNOLOGIES
 Part II AUTOMATION AND CONTROL TECHNOLOGIES Chapters: 4. Introduction to Automation 5. Industrial Control Systems 6. Hardware Components for Automation and Process Control 7. Numerical Control 8. Industrial
Part II AUTOMATION AND CONTROL TECHNOLOGIES Chapters: 4. Introduction to Automation 5. Industrial Control Systems 6. Hardware Components for Automation and Process Control 7. Numerical Control 8. Industrial
Implementation of Risk Management in the Medical Device Industry
 San Jose State University SJSU ScholarWorks Master's Theses Master's Theses and Graduate Research Fall 2010 Implementation of Risk Management in the Medical Device Industry Rachelo Dumbrique San Jose State
San Jose State University SJSU ScholarWorks Master's Theses Master's Theses and Graduate Research Fall 2010 Implementation of Risk Management in the Medical Device Industry Rachelo Dumbrique San Jose State
On Board Use and Application of Computer based systems
 (Dec 2006 (Corr.1 Oct 2007) (Rev.1 Sept 2010) (Rev.2 June 2016 Complete Revision) On Board Use and Application of Computer based systems 1. Introduction 1.1 Scope These requirements apply to design, construction,
(Dec 2006 (Corr.1 Oct 2007) (Rev.1 Sept 2010) (Rev.2 June 2016 Complete Revision) On Board Use and Application of Computer based systems 1. Introduction 1.1 Scope These requirements apply to design, construction,
Animal Facility Biosafety Level 3 Checklist (date: April 16, 1998)
 Date: Location: Responsible: Project Title: Inspector: _ Animal Facility Biosafety Level 3 Checklist (date: April 16, 1998) These questions are based on the Biosafety Level 3 section of Biosafety in Microbiological
Date: Location: Responsible: Project Title: Inspector: _ Animal Facility Biosafety Level 3 Checklist (date: April 16, 1998) These questions are based on the Biosafety Level 3 section of Biosafety in Microbiological
Post Market Surveillance
 Post Market Surveillance December 6 th, 2017 Michael Maier, Medidee Services Slide 1 Agenda Postmarket surveillance Postmarket clinical follow up (PMCF) Incident reporting What changes with MDR Slide 2
Post Market Surveillance December 6 th, 2017 Michael Maier, Medidee Services Slide 1 Agenda Postmarket surveillance Postmarket clinical follow up (PMCF) Incident reporting What changes with MDR Slide 2
The Impact of Quality Culture on Quality Risk Management. FDA Perspective on Quality Culture; how it Impacts Risk Management
 The Impact of Quality Culture on Quality Risk Management FDA Perspective on Quality Culture; how it Impacts Risk Management Teresa Gorecki Practice Lead Compliance Architects Agenda The WHAT Definitions
The Impact of Quality Culture on Quality Risk Management FDA Perspective on Quality Culture; how it Impacts Risk Management Teresa Gorecki Practice Lead Compliance Architects Agenda The WHAT Definitions
Purchasing Controls. FDA Small Business Regulatory Education for Industry (REdI) Conference Denver, Colorado May 13, 2015
 Purchasing Controls FDA Small Business Regulatory Education for Industry (REdI) Conference Denver, Colorado May 13, 2015 LCDR Samantha Spindel, Ph.D. Premarket Programs Branch Division of Industry and
Purchasing Controls FDA Small Business Regulatory Education for Industry (REdI) Conference Denver, Colorado May 13, 2015 LCDR Samantha Spindel, Ph.D. Premarket Programs Branch Division of Industry and
UVTOP280-FW-SMD. Description. Maximum Rating (T CASE = 25 C) Electro-Optical Characteristics (T CASE = 25 C, I F = 20 ma)
 v2.0 11/2017 UVTOP280-FW-SMD Deep Ultraviolet Light Emission Source 285 nm, 2.0 mw 3535 Ceramic SMD Package Low Thermal Resistance Chemical and Biological Analysis Description UVTOP280-FW-SMD is a deep
v2.0 11/2017 UVTOP280-FW-SMD Deep Ultraviolet Light Emission Source 285 nm, 2.0 mw 3535 Ceramic SMD Package Low Thermal Resistance Chemical and Biological Analysis Description UVTOP280-FW-SMD is a deep
The Full Range for RFID
 Your Global Automation Partner The Full Range for RFID Turck is a global leader in industrial automation technology. Over 4,000 employees in 28 countries strive to deliver the best sensor, connectivity,
Your Global Automation Partner The Full Range for RFID Turck is a global leader in industrial automation technology. Over 4,000 employees in 28 countries strive to deliver the best sensor, connectivity,
Logic Units to ensure safety functions
 Logic Units to ensure safety functions Application of the Machinery Directive 2006/42/EC [1] has been mandatory since 29 December 2009. The directive lists products that are described as "logic units to
Logic Units to ensure safety functions Application of the Machinery Directive 2006/42/EC [1] has been mandatory since 29 December 2009. The directive lists products that are described as "logic units to
CUSTOMER AND SUPPLIER ROLES AND RESPONSIBILITIES FOR 21 CFR 11 COMPLIANCE ASSESSMENT. 21 CFR Part 11 FAQ. (Frequently Asked Questions)
 21 CFR Part 11 FAQ (Frequently Asked Questions) Customer and Supplier Roles and Responsibilities for Assessment of METTLER TOLEDO STARe Software Version 16.00, including: - 21 CFR 11 Compliance software
21 CFR Part 11 FAQ (Frequently Asked Questions) Customer and Supplier Roles and Responsibilities for Assessment of METTLER TOLEDO STARe Software Version 16.00, including: - 21 CFR 11 Compliance software
Building a Safety Case for Automated Mobility: Smart Cities and Autonomous Mobility Getting There Safely
 Building a Safety Case for Automated Mobility: Smart Cities and Autonomous Mobility Getting There Safely Building a Safety Case for Automated Mobility: Smart Cities and Autonomous Mobility Getting There
Building a Safety Case for Automated Mobility: Smart Cities and Autonomous Mobility Getting There Safely Building a Safety Case for Automated Mobility: Smart Cities and Autonomous Mobility Getting There
The In Vitro Diagnostic CRO
 The In Vitro Diagnostic CRO Choose Beaufort Because of Our People, Processes and Proven Experience The value of expertise cannot be overstated, especially when it comes to streamlining complicated in vitro
The In Vitro Diagnostic CRO Choose Beaufort Because of Our People, Processes and Proven Experience The value of expertise cannot be overstated, especially when it comes to streamlining complicated in vitro
The Device Side of Combination Products
 The Device Side of Combination Products Technical and Regulatory Challenges in Life Cycle Management Bob Laughner Associate Director, Combination Products 04 May 2016 What are combination products? Combination
The Device Side of Combination Products Technical and Regulatory Challenges in Life Cycle Management Bob Laughner Associate Director, Combination Products 04 May 2016 What are combination products? Combination
Guidance for Industry - Computerized Systems Used in Clinical Trials
 Page 1 of 14 Regulatory Information Computerized Systems Used in Clinical Trials Guidance for Industry - Computerized Systems Used in Clinical Trials
Page 1 of 14 Regulatory Information Computerized Systems Used in Clinical Trials Guidance for Industry - Computerized Systems Used in Clinical Trials
ISO INTERNATIONAL STANDARD. Road vehicles Test methods for electrical disturbances from electrostatic discharge
 INTERNATIONAL STANDARD ISO 10605 Second edition 2008-07-15 Road vehicles Test methods for electrical disturbances from electrostatic discharge Véhicules routiers Méthodes d'essai des perturbations électriques
INTERNATIONAL STANDARD ISO 10605 Second edition 2008-07-15 Road vehicles Test methods for electrical disturbances from electrostatic discharge Véhicules routiers Méthodes d'essai des perturbations électriques
ISO INTERNATIONAL STANDARD. Needle-free injectors for medical use Requirements and test methods
 INTERNATIONAL STANDARD ISO 21649 First edition 2006-06-01 Needle-free injectors for medical use Requirements and test methods Injecteurs sans aiguille à usage médical Exigences et méthodes d'essai Reference
INTERNATIONAL STANDARD ISO 21649 First edition 2006-06-01 Needle-free injectors for medical use Requirements and test methods Injecteurs sans aiguille à usage médical Exigences et méthodes d'essai Reference
WHO s IMS Organizational Structure Overview
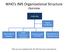 WHO s IMS Organizational Structure Overview Leadership Partner Coordination Information & Planning Health Expertise & Operations Operations Support and Logistics Management & Administration *this structure
WHO s IMS Organizational Structure Overview Leadership Partner Coordination Information & Planning Health Expertise & Operations Operations Support and Logistics Management & Administration *this structure
HotSwap MBP Maintenance Bypass
 ENGLISH HotSwap MBP Maintenance Bypass MBP6K208 MBPK208 Installation and user manual Copyright 202 EATON All rights reserved. Service and suort: Call your local service representative SAFETY INSTRUCTIONS
ENGLISH HotSwap MBP Maintenance Bypass MBP6K208 MBPK208 Installation and user manual Copyright 202 EATON All rights reserved. Service and suort: Call your local service representative SAFETY INSTRUCTIONS
MedDev Rev 4 Medical Devices Regulation. Clinical Evidence Requirements Key Changes and Clarifications. Alan Eller 21 March 2017
 MedDev 2.7.1 Rev 4 Medical Devices Regulation Clinical Evidence Requirements Key Changes and Clarifications Alan Eller 21 March 2017 Copyright 2016 BSI. All rights reserved. 1 Clinical Evidence Requirements
MedDev 2.7.1 Rev 4 Medical Devices Regulation Clinical Evidence Requirements Key Changes and Clarifications Alan Eller 21 March 2017 Copyright 2016 BSI. All rights reserved. 1 Clinical Evidence Requirements
Strategies for Risk Based Validation of Laboratory Systems
 Strategies for Risk Based Validation of Laboratory Systems Video Web Seminar September 23, 2004 Ludwig Huber E-mail: ludwig_huber@agilent.com Today s Agenda Background information: why risk assessment,
Strategies for Risk Based Validation of Laboratory Systems Video Web Seminar September 23, 2004 Ludwig Huber E-mail: ludwig_huber@agilent.com Today s Agenda Background information: why risk assessment,
EMC for Product Committees: A short guide to IEC Guide 107. electromagnetic compatibility. International Electrotechnical Commission
 EMC for Product Committees: A short guide to IEC Guide 107 electromagnetic compatibility International Electrotechnical Commission Short guide to IEC Guide 107 Introduction IEC Guide 107 is required reading
EMC for Product Committees: A short guide to IEC Guide 107 electromagnetic compatibility International Electrotechnical Commission Short guide to IEC Guide 107 Introduction IEC Guide 107 is required reading
Space Environmental Hazards For Space and Launch Systems
 Space Environmental Hazards For Space and Launch Systems Dr. Joseph E. Mazur The Aerospace Corporation Presentation to the 2012 FAA Commercial Space Transportation Conference Physical Sciences Laboratory
Space Environmental Hazards For Space and Launch Systems Dr. Joseph E. Mazur The Aerospace Corporation Presentation to the 2012 FAA Commercial Space Transportation Conference Physical Sciences Laboratory
Institutional Biosafety Committee
 Northern Illinois University Institutional Biosafety Committee Policy Office of Research Compliance, Integrity and Safety 2017 Northern Illinois University Institutional Biosafety Committee Purpose...
Northern Illinois University Institutional Biosafety Committee Policy Office of Research Compliance, Integrity and Safety 2017 Northern Illinois University Institutional Biosafety Committee Purpose...
Frequently Asked Questions. Neuraxial Connectors (ISO ) October 19, 2016 (v2)
 Frequently Asked Questions Neuraxial Connectors (ISO 80369-6) October 19, 2016 (v2) 1 Frequently Asked Questions Neuraxial Connectors (ISO 80369-6) 1. What is a small-bore connector? 2. What changes are
Frequently Asked Questions Neuraxial Connectors (ISO 80369-6) October 19, 2016 (v2) 1 Frequently Asked Questions Neuraxial Connectors (ISO 80369-6) 1. What is a small-bore connector? 2. What changes are
ACCIDENT PREVENTION POLICY, SAFETY AND PROTECTION OF HEALTH AND ENVIRONMENT DOCUMENT OF ESSECO SRL
 ACCIDENT PREVENTION POLICY, SAFETY AND PROTECTION OF HEALTH AND ENVIRONMENT DOCUMENT OF ESSECO SRL (In compliance with Art. 2 of Ministry Decree of August 09, 2000) Page 1 of 6 1. INTRODUCTION The ESSECO
ACCIDENT PREVENTION POLICY, SAFETY AND PROTECTION OF HEALTH AND ENVIRONMENT DOCUMENT OF ESSECO SRL (In compliance with Art. 2 of Ministry Decree of August 09, 2000) Page 1 of 6 1. INTRODUCTION The ESSECO
NEMA Standards Publication NEMA/MITA 1. Good Refurbishment Practices for Medical Imaging Equipment
 NEMA Standards Publication NEMA/MITA 1 Good Refurbishment Practices for Medical Imaging Equipment Published by: National Electrical Manufacturers Association 1300 North 17 th Street, Suite 900 Rosslyn,
NEMA Standards Publication NEMA/MITA 1 Good Refurbishment Practices for Medical Imaging Equipment Published by: National Electrical Manufacturers Association 1300 North 17 th Street, Suite 900 Rosslyn,
Brief Summary of Last Lecture. Model checking of timed automata: general approach
 Brief Summary of Last Lecture Formal verification Types: deductive (theorem proving) and algorithmic (model checking) ields proof that a (formal) specification is fulfilled Formalization of specs e.g.
Brief Summary of Last Lecture Formal verification Types: deductive (theorem proving) and algorithmic (model checking) ields proof that a (formal) specification is fulfilled Formalization of specs e.g.
IEC and ISO A cross reference guide
 and A cross reference guide This guide sets out to explain where the details for different safety lifecycle activities can be found in the standards for the Machinery Sector: and. 1 Concept 2 Overall scope
and A cross reference guide This guide sets out to explain where the details for different safety lifecycle activities can be found in the standards for the Machinery Sector: and. 1 Concept 2 Overall scope
A Guide to Ventilation Requirements for Uranium Mines and Mills
 Canadian Nuclear Safety Commission Commission canadienne de sûreté nucléaire REGULATORY GUIDE A Guide to Ventilation Requirements for Uranium Mines and Mills G-221 June 2003 REGULATORY DOCUMENTS The Canadian
Canadian Nuclear Safety Commission Commission canadienne de sûreté nucléaire REGULATORY GUIDE A Guide to Ventilation Requirements for Uranium Mines and Mills G-221 June 2003 REGULATORY DOCUMENTS The Canadian
Implementing Compliant Medical Device Best Practice Business Processes Using Oracle E-Business Suite
 Implementing Compliant Medical Device Best Practice Business Processes Using Oracle E-Business Suite A white paper discussing the compliant use of the Oracle Electronic Record, Electronic Signature (E-Records)
Implementing Compliant Medical Device Best Practice Business Processes Using Oracle E-Business Suite A white paper discussing the compliant use of the Oracle Electronic Record, Electronic Signature (E-Records)
Adam Equipment LBH SERIES. (P.N , Revision B2, January 2010)
 Adam Equipment LBH SERIES (P.N. 700660113, Revision B2, January 2010) Adam Equipment Company 2010 Easy Reference: Model name of the scale: Serial number of the unit: Software revision number (Displayed
Adam Equipment LBH SERIES (P.N. 700660113, Revision B2, January 2010) Adam Equipment Company 2010 Easy Reference: Model name of the scale: Serial number of the unit: Software revision number (Displayed
Copyright. Jeremiah J. Kelly (2015). All rights reserved. Further dissemination without express written consent strictly prohibited.
 Statutory Framework for Devices Medical Devices Investigational Use Application IDE (21 CFR 812) Abbreviated IDE Exempt Pre-Market Approval Applications 510(k) Pre-marketing Notification (21 CFR 807(e))
Statutory Framework for Devices Medical Devices Investigational Use Application IDE (21 CFR 812) Abbreviated IDE Exempt Pre-Market Approval Applications 510(k) Pre-marketing Notification (21 CFR 807(e))
Pre-Clinical Testing for Devices and Diagnostics
 Pre-Clinical Testing for Devices and Diagnostics Larry O. Blankenship Evergreen Research, Inc. April, 2016 1 Many Levels, Many Approaches The requirements differ dramatically by class and category and
Pre-Clinical Testing for Devices and Diagnostics Larry O. Blankenship Evergreen Research, Inc. April, 2016 1 Many Levels, Many Approaches The requirements differ dramatically by class and category and
