Struvite Recovery from Swine Waste Biogas Digester Effluent through a Stainless Steel Device under Constant ph Conditions 1
|
|
|
- Claribel Stevenson
- 5 years ago
- Views:
Transcription
1 BIOMEDICAL AND ENVIRONMENTAL SCIENCES 22, (2009) Struvite Recovery from Swine Waste Biogas Digester Effluent through a Stainless Steel Device under Constant ph Conditions 1 P. W. ANTON PERERA, WEI-XIANG WU, YING-XU CHEN, AND ZHI-YING HAN 2 College of Environmental and Resource Sciences, Zhejiang University, Hangzhou , Zhejiang, China Objective To investigate the struvite precipitation under constant and non-constant ph conditions and to test a stainless steel device under different operating regimes to maximize the recovery of struvite. Methods The molar ratio of NH + 4 : Mg 2+ : PO 4 was adjusted to 1: 1.2: 1.2 and ph was elevated to 9.0. The absorbance measurement was used to trace the process of struvite crystallization. Wastewater and precipitate analysis was done by standard analytical methods. Results The ph constant experiment reported a significantly higher struvite precipitation (24.6±0.86 g) than the non-constant ph experiment (19.8±1.86 g). The SAR ranged from 5.6 to 8.2 g m -2 h -1 to g m -2 h -1 in ph constant and non-constant experiments, respectively. The highest struvite deposit on the device was found in regime 3 followed by in regimes 2 and 4. The highest PO 4 (97.2%) and NH + 4 (71%) removal was reported in the R1 regime. None of the influent Cu 2+ or Zn 2+ was precipitated on the device. Conclusion A higher struvite yield is evident in ph constant experiments. Moreover, the stainless steel device facilitates the isolation of heavy metal free pure (around 96%) struvite from swine waste biogas digester effluent contaminated with cu 2+ and Zn 2+ and the highest yield is attainable with the device operating at 50 rpm with agitation by a magnetic stirrer. Key words: Struvite recovery; Accumulation device; Swine waste; Constant ph; Struvite precipitation INTRODUCTION The generation of wastewater on a large scale is an inevitable consequence of modern societies. Wastewater is often harmful to humans and environment and it is important to treat it prior to its release. Obligatory anaerobic treatment of domestic and agro-industrial wastewater releases large amounts of phosphorus and nitrogen into wastewater, which is finally disposed into streams, lakes, seas, and land surfaces. These nutrients are directly responsible for the eutrophication, dissolved oxygen depletion and create imbalanced ecosystems in water bodies worldwide [1-3]. Further problems occur owing to the fact that certain forms of nitrogen, such as ammonia, nitrite and nitrate, are toxic to aquatic life or may lead to diseases in those who drink water contaminated with these compounds [4]. Consequently, more stringent standards for nutrient removal, particularly for the phosphorus removal, have been introduced globally [2]. In order to meet the set standards, a variety of wastewater treatment technologies have been developed. In all cases, phosphorus is removed by converting it into a solid fraction. This fraction can be an insoluble salt precipitate, a microbial biomass in activated sludge or a plant biomass in constructed wetlands. As a significant form of nitrogen, ammonia is treated mainly by the process of biological nitrification and denitrification [5-6]. However, none of the above treatments recycles phosphorus or nitrogen as a truly sustainable product. As phosphorus and nitrogen are essential elements of all living organisms, the consumption of these elements is ever increasing [7-8]. The demand for phosphorus alone is projected to increase by 1.5% each year, suggesting that the available phosphorus resources can be exhausted within another years [9]. The recovery of these elements, therefore, is an important priority, which is expected to contribute to sustainable development through saving essential raw materials. The recovery of phosphorus and nitrogen from the wastewater in the form of 1 This research was supported by the National Key Basic Research Project of China (No. 2002CB410807) and the key Project of Science and Technology of Zhejiang Province (No ). 2 Correspondence should be addressed to Zhi-Xiang HAN, Institute of Environmental Science and Technology, College of Environmental and Resource Sciences, Zhejiang University, 268 Kaixuan Road, Hangzhou , Zhejiang, China. Tel: Fax: hanzhiying@zju.edu.cn Biographical note of the first author: P. W. Anton PERERA, born in 1964, Ph. D Scholar, Zhejiang University, majoring in environmental engineering. pwaperera@yahoo.com /2009 CN /Q Copyright 2009 by China CDC 201
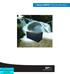
2 202 PERERA ET AL. crystalline struvite (MgNH 4 PO 4 6H 2 O) is found to be a sustainable option because the struvite is considered a possible fertilizer [10-11]. Under high ph conditions, dissolved NH + 4, Mg 2+, and PO 4 ions combine and spontaneously precipitate to form scales that deposit on surfaces during anaerobic digestion and post digestion processes, which becomes a nuisance in the wastewater treatment industry [12-13]. The crystals attach to sludge particles in suspension and to surfaces of equipments, tanks and pipe walls in contact with the digested sludge, causing serious operational problems [14-15]. Several studies have been conducted to identify the fouling propensity of struvite on some materials that can be used in wastewater treatment facilities to minimize the scale deposition. Cast iron [16], metal parts [17], and stainless steel [18] have been found to be more susceptible to struvite scaling than other materials, such as PVC, plastic, Teflon, and acrylic. These findings can be effectively used to design technologies for better struvite recovery, when they are made to occur under controlled conditions. Since the studies on the use of fouling propensity of different materials to increase the struvite recovery are scarce, the authors have undertaken this study. Moreover, the stainless steel is found to be a more favorable material for scaling, and accordingly a device is designed with this material to maximize the recovery. In order to prevent the operational difficulties associated with heavy devices, the device has been designed with stainless steel wire mesh. A previous study of the authors reported that the ph decline occurred in the struvite reaction solution hinders further precipitation, although sufficient amounts of NH + 4, Mg 2+, and PO 4 ions are available [19]. Therefore, a comparative study under constant and non-constant ph conditions was designed by keeping the other conditions identical. The objective of the present study was to evaluate the effect of constant ph on the struvite yield and to assess the performance of the new stainless steel device under different operating regimes in isolating struvite from swine waste biogas digester effluent. Reactor Design MATERIALS AND METHODS A laboratory scale reactor (15 cm diameter 24 cm height) with the working volume of 3 L was used for the struvite precipitation and recovery experiments. The struvite-accumulating device (Fig. 1) comprised of 8 faces of steel mesh was fixed into the reactor. The mesh was woven with a 0.4 mm steel wire forming 1 mm holes. The total surface area of the accumulating faces was 576 cm 2 (8 6 cm 12 cm). This device was placed 4 cm above the bottom of the reactor. FIG. 1. Struvite accumulating device. Quality of Wastewater and Operating Conditions The swine waste biogas digester effluent (SWBDE) was obtained from the Xiasha Swine Farm in Hangzhou of Zhejiang province. The supernatant collected from the effluent discharge pond was stored at 4 for 24 h in a refrigerator to allow the solids to settle and was then separated and stored at 4 until further use. The water quality parameters of the effluent used for the experiment are summarized in Table 1. Two sets of experiments were undertaken to assess the potential of struvite accumulation on the newly designed device and the effect of maintaining a constant ph during the precipitation process. In one set of experiments, the initial ph was elevated to 9.0 and maintained at the same ph value throughout the 15 h experiment period. In the other set, the initial ph was similarly elevated to 9.0 and thereafter no effort was made to maintain a constant ph. The elevation and/or maintenance of ph were done with 1 mol/l NaOH solution. In the other set, the struvite recovery with four different reactor-operating regimes was tested. In the first regime (R 1 ), 3 L of SWBDE was filled into the reactor, and the molar ratio and ph were quickly adjusted and agitated with a magnetic stirrer at 500 rpm (R 1 = agitation + no device). It was operated without the struvite accumulating device (SAD) to assess whether the device influences the precipitation
3 STRUCTIVE RECOVERY BY A STAINLESS STEEL DEVICE 203 TABLE 1 Selected Water Quality Parameters of the Wastewater Used in the Experiments Parameter Concentration/Value ph 7.55 NH 4 + -N (mg L -1 ) 296 NO 3 - -N 1.2 NO 2 - -N (mg L -1 ) 0.28 PO 4 -P (mg L -1 ) 64.2 Mg 2+ (mg L -1 ) 94 Ca 2+ (mg L -1 ) 65 COD Cr (mg L -1 ) 980 SS (g L -1 ) 1.4 Cu 2+ (mg L -1 ) 0.2 Zn 2+ (mg L -1 ) 0.4 of struvite. In the second regime (R 2 ), the device was permanently fixed in the reactor and the magnetic stirrer was also in operation (R 2 = agitation + fixed device). In the third regime (R 3 ), the device coupled with an overhead agitator, which rotates at 50 rpm, and the magnetic stirrer was also in operation in the same reactor (R 3 = agitation + rotating device). The final regime (R 4 ) comprised of a rotating device not agitated by the magnetic stirrer (R 4 = no agitation + rotating device). Except for these operating conditions, the same molar ratio and ph value were used in these experiments. The NH + 4 : Mg 2+ : PO 4 molar ratio of 1:1.2:1.2 which was found in preliminary tests as the best ratio for optimum struvite recovery was used for all the experiments. Regent grade magnesium chloride hexahydrate (MgCl 2 6H 2 O) and potassium di-hydrogen phosphate (KH 2 PO 4 ) were used to bring the NH + 4 : Mg 2+ : PO 4 molar ratio to 1: 1.2: 1.2 in SWBDE at the beginning of each run. Analytical Procedure The crystallization process was monitored by spectrometric absorbance measurements recorded at 385 nm. During the first 20 min of reaction, absorbance was recorded at every 2 min followed by records at every 10 min up to 1 hour and then once in every hour until the end of the 15 h experiment. In the experiment with non-constant ph, the variation of ph was recorded in every 5 min during the initial 30 min of the experiment and thereafter in every 10 min up to 180 min using a Sartorius PB-10 ph meter (Goettingen, Germany). The initial and final NH 4 +, Mg 2+, and PO 4 concentrations of the solution were measured with the standard analytical methods (APHA 1998) [20]. The precipitate deposited on the device was carefully removed with brushing after drying the device at 40 for 48 h. The precipitate settled at the bottom was first air-dried for 2 days and then oven dried at 40 for 48 h. The dried precipitate samples from the device and bottom sediments were dissolved in 0.1 mol/l HCl and analyzed for Mg 2+, NH + 4, and PO 4 content as described elsewhere [21]. Magnesium, copper and zinc concentrations were determined with a thermo elemental solar M6 MK11 atomic absorption spectrophotometer (Thermo Electron Corporation, USA). Orthophosphate (PO 4 ) was measured with the ascorbic acid colorimetric method and ammonia nitrogen was measured with a spectrometer (Unico UV-4802, China). All the analyses were performed in triplicate and the average figures were calculated. Statistical analysis of experimental data was performed using the analytical software SPSS 11.0 (SPSS Inc. USA). RESULTS Evolution of ph during Precipitation Process Previous work revealed that the ph of struvite precipitation solution declines over time and struvite precipitation comes to an equilibrium state even though considerable amounts of Mg 2+, NH 4 +, and PO 4 ions are still available from the solution [19]. In the present study, therefore, struvite recovery was tested at both constant and non-constant ph conditions to evaluate any possible effect of a constant ph on the recovery of struvite. In the struvite recovery experiments with non-constant ph, the evolution of ph over time showed a unique pattern (Fig. 2). All the tested operating regimes showed that the initial ph was sharply declined in the first 15 min, moderately reduced in next min and then maintained stable. Out of the four operating regimes tested, 3 regimes (R 1, R 2, and R 3 ) using the magnetic stirrer, reached almost a similar ph value after 90 minutes of operation. When the reactor was operated no agitated by the magnetic stirrer (R 4 ), the ph was slightly lower than that agitated by the magnetic stirrer. Effect of ph on Struvite Yield A different result was observed when the total precipitation in the ph constant and non-constant experiments was compared. The average amount of total precipitate found in the ph constant experiment
4 204 PERERA ET AL. addition, the total amount precipitated with each operating regime in constant ph experiment was always higher than that precipitated with the operating regimes in the non-constant ph experiment (Figs. 3a and 3b). As indicated in Fig. 2, the final ph of the non-constant ph experiment in R1, R2, and R3 was 7.88 and 7.94 in R4 3 hours after experiment. The availability of the effective free ions became less under these low ph values [22] and consequently, a significantly lower struvite yield was found in the ph non-constant experiments (Fig. 3). Changes in Absorbance Measurements during the Precipitation Experiments FIG. 2. Evolution of ph during struvite precipitation under different reactor operating regimes. FIG. 3. Effect of non-constant (a) and constant (b) ph on the accumulation of struvite under different reactor configurations. after 15 h of reaction was (24.6±0.86 g) significantly higher (P<0.05) than that observed in the non-constant ph experiment (19.8±1.86 g). In Absorbance measurements are used to monitor the nucleation and growth process in precipitation experiments [22]. The absorbance measurement corresponds to the quantity of energy absorbed or transmitted by the matter and, therefore, the evolution of absorbance of a solution at a specific wavelength indicates the advancement of the crystallization process and hence the particle growth. In the present experiments, absorbance measurements were used to investigate the variations in struvite nucleation and growth under constant and non-constant ph conditions with different reactor operating regimes. Just after the initial ph adjustment of both ph constant and non-constant conditions, the absorbance quickly reached the peak in the first 20 min and then gradually maintained a steady state (Figs. 4a and 4b). It was noticeable that the peak absorbance values observed in all the operating regimes in constant ph experiment were comparatively higher than those in the non-constant (variable) ph experiment (Table 2), suggesting that the constant ph condition created more struvite nuclei than the non-constant ph condition when all the other conditions were maintained constant. The highest peak absorbance of 0.43 was achieved by the 14th min and reached nearly stable absorbance of 0.37 after 6 h of reaction in the constant ph experiment with R 1 configuration (Fig. 4b, Table 2). Even though no significant difference was observed in the peak absorbance values, the time taken to reach the peak value in R 4 (18 min) was slightly longer than that of the operating regimes. Similarly, in the non-constant ph experiment, the highest (0.32) and lowest (0.26) peak absorbance values were observed with R 1 and R 4 regimes, respectively and it took 4 min more in R 4 to reach the peak absorbance than in the other regimes (Table 2). It was clear that the absorbance of steady state in R 1 and R 4 was higher than that in R 2 and R 3 in both constant and non-constant ph experiments, indicating the impact of operating regimes on the final absorbance (Figs. 4a and 4b).
5 STRUCTIVE RECOVERY BY A STAINLESS STEEL DEVICE 205 FIG. 4. Variation of absorbance at non-constant (a) and constant (b) ph at different reactor operating regimes. R 1 agitation (with magnetic stirrer) with no device ( ), R 2 agitation with fixed device ( ), R 3 agitation with device rotating at 50 rpm ( ), R 4 No agitation with device rotating at 50 rpm ( ). Parameter TABLE 2 Absorbance and Related Data Observed with Different Operating Regimes under Constant and Non-constant ph Conditions Constant ph Operating Condition Non-constant ph R 1 R 2 R 3 R 4 R 1 R 2 R 3 R 4 Peak Absorbance Time at Peak Absorbance Steady State Absorbance Time to Reach Steady-state (h) Note. R 1: Agitation by magnetic stirrer at 500 rpm without accumulating device; R 2: Agitation by magnetic stirrer at 500 rpm with accumulating device fixed to the reactor; R 3: Agitation by magnetic stirrer at 500 rpm with accumulating device rotating at 50 rpm; R 4: No agitation by magnetic stirrer with accumulating device rotating at 50 rpm. Removal of Struvite Components in Constant ph Condition Since a better performance of precipitate recovery was observed in the constant ph experiment, the removal of NH + 4, Mg 2+, and PO 4 ions under each operating condition was evaluated. The highest PO 4 removal (97.2%) was observed in R 1 and R 2 operating regimes while the highest Mg 2+ removal (97.3%) was achieved in R 1 and R 3 regimes. The highest NH + 4 removal (71.0%) was observed in R 1 regime. The lowest removal of NH + 4, Mg 2+, and PO 4 was reported in R 4 regime, accounting for 69.6%, 95.6%, and 94.7%, respectively (Fig. 5). However, the differences observed in the removal efficiency were not significant. The Rate of Struvite Accumulation on the Device Struvite is well known for plugging pumps and fouling screens and other equipments [14] in wastewater treatment facilities and similarly, we did find that struvite FIG. 5. Percentage removal of NH + 4, Mg 2+, and PO 4 from the precipitation solution after 15 h of reaction with different operating regimes.
6 206 PERERA ET AL. was deposited on the device. As rate of struvite deposition on a surface is a useful criterion for optimizing the recovery in the precipitation reactor, the rate of accumulation was compared in the operating regimes tested. The highest accumulation rate of 8.2 g m -2 h -1, 7.5 g m -2 h -1, and 6.5 g m -2 h -1 was achieved with R 3,, R 2, and R 4, respectively, in the constant ph experiment (Fig. 6). A significantly different accumulation rate was found between R 3 and R 4 only. The same order of accumulation rates accounted for 4.8, 4.5 g h -1 m -2, and 3.6 g h -1 m -2, respectively, and a significant difference in the accumulation rate was observed in the non-constant ph experiment. In addition to the introduction of a device to accumulate struvite, different operating regimes were also studied, in order to identify the most suitable procedure to operate a struvite recovery reactor. It was evident that the presence of the device in the reactor provided a surface for the attachment of crystals formed in the solution as a white color material was deposited on the device. There was 6.4±0.84 g of deposits on the surface of the device in the constant ph experiment while 3.7±0.69 g was found on the device in the non-constant ph experiment. In the reactor with R 4 where the device rotated at 50 rpm but not agitated by a magnetic stirrer, the lowest amount of deposits on the device and the lowest total amount of precipitates were reported after 15 h of reaction in both experiments. DISCUSSION FIG. 6. Rate of struvite accumulation on the device under constant and non-constant ph with different operating regimes. Effects of the Device and Operating Regimes on Struvite Precipitation In order to find out any possible influence of the accumulating device on the overall recovery of struvite, reactor-operating regimes undertaken in this experiment (R 2, R 3, and R 4 ) were compared to those carried out under similar conditions except that the reactor was without device (R 1 ). The average amount of total precipitates (on the device + at the bottom) observed in the non-constant ph experiment with (SAD) was 19.8±1.57 g. An almost similar amount (19.8±3.04 g) was found in the absence of the device, but with the same conditions. Similarly, 24.6±0.89 g and 24.8±0.91 g of total struvite deposits were recorded with and without the device in the constant ph experiment. The statistical analysis suggested that the accumulating device had no significant effect on the total struvite precipitation in both constant and non-constant ph experiments. When the ph and concentration of struvite components are correctly adjusted, the ph decline in the reaction solution can be used to trace the struvite precipitation [8,19]. As the struvite nucleation is a rapid process under super saturation conditions, a greater amount of H + ions is released to the solution and hence a rapid decline of ph can be observed within a short period of time [23]. However, the availability of effective free ions for struvite precipitation becomes limited under these final low ph values (7.88 and 7.94) [24] and consequently a significantly lower struvite yield has been reported in ph non-constant experiments (Fig. 3). In contrast, the availability of struvite component ions is increased at ph 9.0 and above [25] and as a result, the struvite yields reporting a constant ph 9.0 with all the tested operating regimes are higher than those in non-constant ph experiments, suggesting that maintaining a constant ph 9.0 during the precipitation process can enhance the struvite yield. In the present study, the final ph reached a more or less similar value in R 1, R 2, and R 3 regimes and was also slightly lower than that in R 4, suggesting that the difference in ph values between R 1, R 2, R 3, and R 4 is attributed to the variation among the operating conditions. The common operating condition found in R 1, R 2, and R 3 regimes is the agitation with a magnetic stirrer at 500 rpm that gives more opportunities for the struvite components to mix well and react with each other to form more struvites. Stratful et al. [26] reported that mixing is an important for homogeneity of the mixture that is essential for the effectiveness of struvite precipitation reaction. An overhead agitator working at 500 rpm [27] gives the best results and the mixing effect provided by the agitator is comparable to that by a magnetic
7 STRUCTIVE RECOVERY BY A STAINLESS STEEL DEVICE 207 stirrer running at 500 rpm in our experiment. The device rotated at 50 rpm in R 4 could not make sufficient mixing, thus affecting the nucleation and reflecting a relatively lower ph reduction in comparison with that in R 1, R 2, and R 3 regimes (Fig. 2). This phenomenon similarly affected the absorbance measurements (Figs. 4a and 4b), total amount of precipitates and the amount precipitated on the device (Figs. 3a and 3b), as well as the struvite accumulation rate (SAR) on the device (Fig. 6). As suggested by Barett and Parsons [22], absorbance measurements can be used to define the results of our experiment. The lower peak absorbance observed with each tested operating regime in the non-constant ph experiment than in the constant ph experiment was due to the lower nucleation of struvite under uncontrolled ph conditions. The absorbance value of R 1 regime in both experiments remained high as the struvite crystals formed in the reactor were suspended in the solution in the absence of a device to attach to. The lowest absorbance reported with R 3 regime in both experiments (Figs. 4a and 4b) can be explained by the deposition of more struvite nuclei on the device aided by the dual type of mixing: agitation by the magnetic stirrer and rotating device, which was further supported by the fact that the highest amount of struvite in both experiments was deposited on the device in regime R 3 (Figs. 3a and 3b). In the present study, the purity of struvite deposited on the device was nearly 96%, which is in agreement with the reported findings [21]. Although the amount of Cu 2+ and Zn 2+ was low in the wastewater used in the present study (Table 1), nearly 70% and 48% of these elements were settled with struvite at bottom of the reactor (Table 3). However, indiscriminate use of Cu 2+ and Zn 2+ as a growth promoter in swine and other livestock industries is a great concern today. Therefore, struvite recovered from livestock and other wastewaters with higher Cu 2+ and Zn 2+ levels, has the risk of contamination with these two elements. Since the precipitates found on SAD contain no Cu 2+ and Zn 2+ in detectable quantities (Table 3), it is a useful tool to recover uncontaminated phosphorus and nitrogen from the wastewater contaminated with these two heavy metals, and moreover, a cost effective alternative source of phosphate than rock phosphate minerals commonly contaminated with heavy metals such as cadmium and uranium with impurities like magnesium [28]. The reactor with a stainless steel wire mesh device has proved to be a feasible way of recovering fairly pure struvite from swine wastewater biogas digester effluents. Furthermore, it is worth to maintain constant ph as it permits to precipitate more struvite than the nonconstant ph. The SAR observed on the device in the constant ph experiment ranged g m -2 h -1, which weas higher than g m -2 h -1 observed with stainless steel coupons [18]. On the contrary, Suzuki et al. [21] reported that the accumulation speed was 16 g m -2 h -1 on stainless steel rods submerged in a struvite precipitation experiment in which the accumulation rate is higher than that in the present study. However, this difference in accumulation rate could be attributed to the variation of surface roughness occurred in these two precipitation experiments. In our experimental set-up, the reaction time was only 15 h and struvite was accumulated on a stainless-steel wire-mesh surface whereas struvite continued to accumulate on a stainless-steel rod surface for few days as reported above [21]. Once the surface is covered with struvite, it provides a rough surface for further accumulation of struvite in an accelerated rate [18, 21] during the rest of the precipitation process. Therefore, the accumulation rate achieved in our experiment was comparable to the time of the struvite precipitation process. Although a higher struvite accumulation speed has been reported, the weight of the device made of stainless steel bars or coupons would create operational problems. Therefore, the stainless wire mesh device introduced in this study would be more feasible as it reduces the total weight which minimizes the handling and operational difficulties while allowing recovery of a sizable amount of struvite. The other single long-term study using swine wastewater with a struvite accumulation device [29] reported the accumulation rate ranges g m -2 h -1 within the initial 7-month operating period. However, a comparatively higher rate of 12.9 g m -2 h -1 that could be attributed to the increased surface roughness due to continuous struvite accumulation has been observed beyond 7 months of operation. Therefore, the authors predict that a better SAR would be achieved with the long-term operation of the new device tested in this study. Although different wastewaters such as centrate liquor [18], swine wastewater [21,29] and swine waste biogas digester effluent (present study) are used in struvite precipitation experiments, PO 4 and Mg 2+ concentrations vary in a narrow range of mg L -1 and mg L It was reported the NH 4 concentration is 296 mg L -1 in SWBDE and 532 mg L -1 in swine wastewater [29]. However, the PO 4 removal efficiency was 96.7% in the current study and SAD, constant ph and molar ratio was much higher than the reported value (67.3%) [29]. The reactor-operating regime seems to contribute to the struvite accumulation rate on the device. The differences in the amount of precipitates found in each operating regime could be attributed to the different mixing and nucleation conditions. Ohlinger
8 208 PERERA ET AL. TABLE 3 Amounts of Struvite Constituents, Cu 2+ and Zn 2+ Removed from the Solution, Settled at the Bottom and Deposited on the Device (in mmol) with Different Operating Regimes after 15 h of Reaction Fraction Removed from Solution Deposited on Device Settled at Bottom Constituent and Ratio PO 4 Operating Regime R 1 R 2 R 3 R Mg NH Cu Zn NH 4 + : Mg 2+ : PO 4 PO 4 1:1.64:1.64 1:1.64:1.65 1:1.65:1.64 1:1.65: Mg NH Cu Zn NH 4 + : Mg 2+ : PO 4 PO 4-1:1.04:1.02 1:0.98:0.98 1:1.08: Mg NH Cu Zn NH + 4 : Mg 2+ : PO 4 1:1.64:1.64 1:2.47:2.50 1:2.98:2.95 1:2.19:2.21 Note. R 1: Agitation by magnetic stirrer at 500 rpm without accumulating device; R 2: Agitation by magnetic stirrer at 500 rpm with accumulating device fixed to the reactor; R 3 :Agitation by magnetic stirrer at 500 rpm with accumulating device rotating at 50 rpm; R 4 :No agitation by magnetic stirrer with accumulating device rotating at 50 rpm. et al. [30] suggested that the mixing energy disrupts the concentration gradients in boundary layers surrounding the growing crystals and increases crystal formation and growth. Therefore, the lowest yield in R 4 could be a result of the slower rotation of the device, which provides the only way for mixing the struvite components NH 4 +, Mg 2+, and PO 4 in the reaction solution and the mixing energy is insufficient to enhance the formation and growth of struvite crystals. As a result, the struvite nucleation and the total amount of precipitates were decreased. However, the total amount of precipitates in each regime was not proportional to the struvite accumulation rate on the device in the respective regime, suggesting that the conditions created in each operating regime have a different trapping effect on the suspended struvite crystals. This phenomenon is well supported by the observations made in the absorbance experiment. After the initial nucleation, there was no accumulating device in R 1 for the formed crystals to adhere to and consequently, the suspending struvite particles contributed to a higher steady state absorbance. In contrast, the device in R 2 and R 3 provided accumulating surfaces for struvite deposit and as a result the absorbance gradually declined. However, in R 3 regime, both magnetic stirrer and device rotated, depositing more struvite than in R 2, and the latter was only magnetically agitated. In regime R 4, the device rotated only at 50 rpm, which might not have a opportunity for the formed struvite particles to deposit on the device and consequently demonstrated a moderate absorbance. Based on the above observations, it can be concluded that operating regime R 3 is more suitable to isolate the highest amount of almost pure struvite from the reactor. Although the amount of separable struvite was slightly lower in R 2 than in R 3, an overall economic analysis should be done to select the most appropriate operational regime in pilot or commercial scale operation. An increment of the rotation speed or a modification of the reactor in order to enhance mixing is an cost effective option to improve the struvite yield and the nutrient removal efficiency in operating regime 4 (R4). However, these aspects should be investigated before arriving at any conclusions. Furthermore, it is hypothesized that struvite particles have a special affinity to the surface of the device, as the purity of deposits is over 96% despite the fact that there is an equal chance for organic
9 STRUCTIVE RECOVERY BY A STAINLESS STEEL DEVICE 209 matter and other impurities to deposit on the device. CONCLUSION The maintenance of constant ph during the struvite precipitation process enhances the availability of struvite constituents and enables the recovery of a maximum amount of struvite under a given molar ratio. The operation regime, which governs the mixing properties of the reactor, affects the amount of struvite deposits on the device. The highest amount of precipitates on the device could be obtained in a reactor by rotating the device at 50 rpm together with magnetic stirrer working at 500 rpm. The use of an accumulating device has proved to be a promising way to recover Cu 2+ and Zn 2+ free pure struvite although the influent wastewater possesses these heavy metals. An increment of the rotation speed or a modification of the reactor is a cost effective option for further investigation to improve the struvite recovery. A pilot scale and long-term study accompanying economic analysis would be appropriate to determine the maximum SAR and an exact operating regime that ensures the optimum and cost effective recovery of struvite. REFERENCES 1. Lau P S, Tam N F Y, Wong Y S (1997). Wastewater nutrients (N and P) removal by carrageenan and alginate immobilized Chlorella vulgaris Environ Technol 18, Ivanov V, Stabnikov V, Zhuang, et al. (2005). Phosphate removal from the retutned liquor of municipal wastewater treatment plant using iron-reducing bacteria. J Appl Microbiol 98, Jaffer Y, Clark T A, Pearce P, et al. (2002). Potential phosphorus recovery by struvite formation. Water Res 36, Lee S I, Weon S Y, Lee C W, et al. (2003). Removal of nitrogen and phosphate from wastewater by addition of bittern. Chemosphere 51, Tunay O, Kabdasli I, Orhon D, et al. (1997). Ammonia removal by magnesium ammonium phosphate precipitation in industrial wastewaters. Water Sci Technol 36(2-3), Turker M, Celen I (2007). Removal of ammonia as struvite from anaerobic digester effluents and recycling of magnesium and phosphate. Bioresource Technol 98(8), Shu L, Schneider P, Jegatheesan et al. (2006). An economic evaluation of phosphorus recovery as struvite from digester supernatant. Bioresource Technol 97(17), Kofina A N, Koutsoukos P G (2005). Spontaneous Precipitation of Struvite from Synthetic Wastewater Solutions. Cryst Growth Des 5(2), Steen I (1998). Phosphorus availability in the 21st century: management of a non-renewable resource. Phosphorus and Potassium 217, Johnston A E, Rechards I R (2003). Effectiveness of different precipitated phosphates as phosphorus sources for plants. Soil Use Manage 19, Bashan L E D, Bashan Y (2004). Recent advances in removing phosphorus from wastewater and its future use as fertilizer ( ). Water Res 38, Corre K S L, Jones E V, Hobbs P, et al. (2005). Impact of calcium on struvite crystal size, shape and purity. J Cryst Growth 283, Yoshino M, Yao M, Tsuno H, et al. (2003). Removal and recovery of phosphate and ammonium as struvite from supernatant in anaerobic digestion Water Sci Technol 48(1), Ohlinger K N, Young T M, Schroeder E D (1998). Predicting struvite formation in digestion. Wat Res 32(12), Suzuki K, Tanaka Y, Osada T, et al. (2002). Removal of phosphate, magnesium and calcium from swine wastewater through crystallization enhanced by aeration. Water Res 36, Borgerding J (1972). Phosphate deposits in digestion systems. J Water Pollut Control Fed 44, Mohajith K, Bhattarai E, Taiganides P et al. (1989). Struvite deposits in pipes and aerators. Biol wastes 30, Doyle J D, Oldring K, Churchley, et al. (2002). Struvite formation and the fouling propensity of different matrials. Water Res 36, Anton Perera P W, Zhiying H, Yingxu C, et al. (2007). Recovery of nitrogen and phosphorus from swine waste biogas digester effluent. Biomed Environ Sci 20(5), APHA (1998). Standard method for the examination of water and wastewater. 19th ed, American Public Health Association. Washington, DC. 21. Suzuki K, Tanaka Y, Kuroda K, et al. (2005). Recovery of phosphorous from swine wastewater through crystallization. Bioresource Technol 96, Barret A R, Parsons S A (1998). The influence of magnetic fields on calcium carbonate precipitation. Water Res 32(3), Bouropoulos N C, Koutsoukos P G (2000). Spontaneous precipitation of struvite from aqueous solutions. J Cryst Growth 213 (4), Ali M D I, Schinder P A, Neale Hudson (2005). Thermodynamic and solution chemistry of struvite. J Indian Inst Sci 85, Doyle J D, Parsons S A (2002). Struvite formation, control and recovery. Water Res 36, Stratful I, Scrimshaw M D, Lester J N (2004). Removal of struvite to prevent problems associated with its accumulation in wastewater treatment works. Water Environ Res 76(5), Ali M I, Schneider P A (2006). A fed-batch design approach of struvite system in controlled supersaturation. Chem Eng Sci 61, Driver J (1998). Phosphate recovery for recycling from sewage and animal waste. First International conference on the recovery of phosphate for recycling from sewage and animal wastes. Warwick University, UK, 6-7 March Suzuki K, Tanaka Y, Kuroda K, et al. (2007). Removal and recovery of phosphorous from swine wastewater by crystallization reactor and struvite accumulating device. Bioresource Technol 98, Ohlinger K N, Young T M, Schroeder E D (2000). Post digestion struvite precipitation using a fluidized bed reactor. J Environ Eng 126(4), (Received February 10, 2008 Accepted December 12, 2008)
Recovery of Nitrogen and Phosphorous as Struvite From Swine Waste Biogas Digester Effluent 1
 BIOMEDICAL AND ENVIRONMENTAL SCIENCES 20, 34350 (2007) Recovery of Nitrogen and Phosphorous as Struvite From Swine Waste Biogas Digester Effluent 1 P. W. ANTON PERERA, ZHI-YING HAN, YING-XU CHEN, AND WEI-XIANG
BIOMEDICAL AND ENVIRONMENTAL SCIENCES 20, 34350 (2007) Recovery of Nitrogen and Phosphorous as Struvite From Swine Waste Biogas Digester Effluent 1 P. W. ANTON PERERA, ZHI-YING HAN, YING-XU CHEN, AND WEI-XIANG
REVIEW The Technology of Phosphorous Removal and Recovery from Swine Wastewater by Struvite Crystallization Reaction
 JARQ 40 (4), 341 349 (2006) http://www.jircas.affrc.go.jp REVIEW The Technology of Phosphorous Removal and Recovery from Swine Wastewater by Struvite Crystallization Reaction Kazuyoshi SUZUKI*, Yasuo TANAKA,
JARQ 40 (4), 341 349 (2006) http://www.jircas.affrc.go.jp REVIEW The Technology of Phosphorous Removal and Recovery from Swine Wastewater by Struvite Crystallization Reaction Kazuyoshi SUZUKI*, Yasuo TANAKA,
EFFECT OF INITIAL SOLUTION ph ON SOLUBILITY AND MORPHOLOGY OF STRUVITE CRYSTALS
 EFFECT OF INITIAL SOLUTION ph ON SOLUBILITY AND MORPHOLOGY OF STRUVITE CRYSTALS E. Ariyanto 1, H. M. Ang 1, and T. K. Sen 1 1 Department of Chemical Engineering, Curtin University of Technology GPO BOX
EFFECT OF INITIAL SOLUTION ph ON SOLUBILITY AND MORPHOLOGY OF STRUVITE CRYSTALS E. Ariyanto 1, H. M. Ang 1, and T. K. Sen 1 1 Department of Chemical Engineering, Curtin University of Technology GPO BOX
AMMONIA REMOVAL FROM DIGESTED SLUDGE SUPERNATANT
 AMMONIA REMOVAL FROM DIGESTED SLUDGE SUPERNATANT J. Suschka and S. Popławski University of Bielsko-Biała, Institute of Environmental Protection and Engineering, ul. Willowa 2, 43-309 Bielsko-Biała, Poland
AMMONIA REMOVAL FROM DIGESTED SLUDGE SUPERNATANT J. Suschka and S. Popławski University of Bielsko-Biała, Institute of Environmental Protection and Engineering, ul. Willowa 2, 43-309 Bielsko-Biała, Poland
PHOSPHORUS RECOVERY - LABORATORY SCALE EXPERIMENTS
 PHOSPHORUS RECOVERY - LABORATORY SCALE EXPERIMENTS Jan Suschka and S. Popławski Technical University of Łódź, Filial Bielsko-Biała, Poland, ul. Willowa 2, 43-39 Bielsko-Biała. ABSTARCT Laboratory scale
PHOSPHORUS RECOVERY - LABORATORY SCALE EXPERIMENTS Jan Suschka and S. Popławski Technical University of Łódź, Filial Bielsko-Biała, Poland, ul. Willowa 2, 43-39 Bielsko-Biała. ABSTARCT Laboratory scale
Sustainable Production of Struvite. Using Wastewater for Sustainable Agricultural Activities
 Sustainable Production of Struvite Using Wastewater for Sustainable Agricultural Activities Ceyda Özden, İrem Ülkü, Sibel Uludağ-Demirer Çankaya University, Industrial Engineering, 06530 Ankara, Turkey
Sustainable Production of Struvite Using Wastewater for Sustainable Agricultural Activities Ceyda Özden, İrem Ülkü, Sibel Uludağ-Demirer Çankaya University, Industrial Engineering, 06530 Ankara, Turkey
Impact of calcium on struvite crystal size, shape and purity
 Impact of calcium on struvite crystal size, shape and purity Kristell S. Le Corre 1, Eugenia Valsami-Jones 2, Phil Hobbs 3, Simon A. Parsons 1* 1 School of Water Sciences, Cranfield University, Cranfield
Impact of calcium on struvite crystal size, shape and purity Kristell S. Le Corre 1, Eugenia Valsami-Jones 2, Phil Hobbs 3, Simon A. Parsons 1* 1 School of Water Sciences, Cranfield University, Cranfield
Formation of Struvite Crystals from Domestic Wastewater using Magnesium Carbonate as an Mg Source
 Formation of Struvite Crystals from Domestic Wastewater using Magnesium Carbonate as an Mg Source Modini V 1, Dr. Udayashankara T. H 2 P.G Student, Department of Environmental Engineering, Sri Jayachamarajendra
Formation of Struvite Crystals from Domestic Wastewater using Magnesium Carbonate as an Mg Source Modini V 1, Dr. Udayashankara T. H 2 P.G Student, Department of Environmental Engineering, Sri Jayachamarajendra
Staying Ahead of Struvite Problems in Wastewater Treatment Plants
 Proceedings of the 4 th International Conference on Environmental Pollution and Remediation Prague, Czech Republic, August 11-13, 2014 Paper No. 179 Staying Ahead of Struvite Problems in Wastewater Treatment
Proceedings of the 4 th International Conference on Environmental Pollution and Remediation Prague, Czech Republic, August 11-13, 2014 Paper No. 179 Staying Ahead of Struvite Problems in Wastewater Treatment
WASTEWATER TREATMENT
 WASTEWATER TREATMENT Every community produces both liquid and solid wastes. The liquid portion-wastewater-is essentially the water supply of the community after it has been fouled by a variety of uses.
WASTEWATER TREATMENT Every community produces both liquid and solid wastes. The liquid portion-wastewater-is essentially the water supply of the community after it has been fouled by a variety of uses.
Study on Struvite Precipitation in a Mechanically Stirring Fluidized Bed Reactor
 Study on Struvite Precipitation in a Mechanically Stirring Fluidized Bed Reactor Vincenzo Doino, Kévin Mozet, Hervé Muhr, Edouard Plasari* Ecole Nationale Supérieure des Industries Chimiques de l Institut
Study on Struvite Precipitation in a Mechanically Stirring Fluidized Bed Reactor Vincenzo Doino, Kévin Mozet, Hervé Muhr, Edouard Plasari* Ecole Nationale Supérieure des Industries Chimiques de l Institut
Struvite Scale Potential Determination Using a Computer Model
 Struvite Scale Potential Determination Using a Computer Model K.N. Ohlinger, Ph.D., PE * and R. J. Mahmood, Ph.D., PE * * Office of Water Programs, Department of Civil and Environmental Engineering, California
Struvite Scale Potential Determination Using a Computer Model K.N. Ohlinger, Ph.D., PE * and R. J. Mahmood, Ph.D., PE * * Office of Water Programs, Department of Civil and Environmental Engineering, California
Phosphorus Removal and Recovery from High Phosphorus Wastewater by the HAP Crystallization Process
 ORIENTAL JOURNAL OF CHEMISTRY An International Open Free Access, Peer Reviewed Research Journal www.orientjchem.org ISSN: 0970-020 X CODEN: OJCHEG 2016, Vol. 32, No. (1): Pg. 235-241 Phosphorus Removal
ORIENTAL JOURNAL OF CHEMISTRY An International Open Free Access, Peer Reviewed Research Journal www.orientjchem.org ISSN: 0970-020 X CODEN: OJCHEG 2016, Vol. 32, No. (1): Pg. 235-241 Phosphorus Removal
Efficient and safe production processes in sustainable agriculture and forestry XXXIV CIOSTA CIGR V Conference 2011
 Struvite precipitation from anaerobic co- digestion residues of poultry manure and maize silage Prof.Dr. Göksel N. Demirer Middle East Technical University Department of Environmental Engineering Ankara,
Struvite precipitation from anaerobic co- digestion residues of poultry manure and maize silage Prof.Dr. Göksel N. Demirer Middle East Technical University Department of Environmental Engineering Ankara,
Removal of High C and N Contents in Synthetic Wastewater Using Internal Circulation of Anaerobic and Anoxic/Oxic Activated Sludge Processes
 Removal of High C and N Contents in Synthetic Wastewater Using Internal Circulation of Anaerobic and Anoxic/Oxic Activated Sludge Processes Nittaya Boontian School of Environmental Engineering, Institute
Removal of High C and N Contents in Synthetic Wastewater Using Internal Circulation of Anaerobic and Anoxic/Oxic Activated Sludge Processes Nittaya Boontian School of Environmental Engineering, Institute
Schwing Bioset, Inc. Eric Wanstrom Schwing Bioset, Inc.
 Schwing Bioset, Inc Eric Wanstrom Schwing Bioset, Inc. Nutrient (struvite) Recovery In partnership with: Nutrient Recovery Integrated Phosphate Management What are the Issues: Waste water inlet www.schwingbioset.com
Schwing Bioset, Inc Eric Wanstrom Schwing Bioset, Inc. Nutrient (struvite) Recovery In partnership with: Nutrient Recovery Integrated Phosphate Management What are the Issues: Waste water inlet www.schwingbioset.com
Domestic Waste Water (Sewage): Collection, Treatment & Disposal
 Domestic Waste Water (Sewage): Collection, Treatment & Disposal Sanitary sewers Storm water sewers Combined sewers Types of sewers: Types of collection system Building sewer/building connections:connected
Domestic Waste Water (Sewage): Collection, Treatment & Disposal Sanitary sewers Storm water sewers Combined sewers Types of sewers: Types of collection system Building sewer/building connections:connected
Struvia Technology for Phosphorus Recovery. o Re-Water Braunschweig November 2015
 Struvia Technology for Phosphorus Recovery o Re-Water Braunschweig 2.-3. November 2015 1 Resourcing the World o Vision of circular economy o Sous-titre o Waste and Waste Water are resources Veolia Water
Struvia Technology for Phosphorus Recovery o Re-Water Braunschweig 2.-3. November 2015 1 Resourcing the World o Vision of circular economy o Sous-titre o Waste and Waste Water are resources Veolia Water
PHOSPHORUS RECOVERY FROM SEWAGE SLUDGE USING THE AQUACRITOX SUPERCRITICAL WATER OXIDATION PROCESS
 14 th European Biosolids and Organic Resources Conference and Exhibition 1 PHOSPHORUS RECOVERY FROM SEWAGE SLUDGE USING THE AQUACRITOX SUPERCRITICAL WATER OXIDATION PROCESS O Callaghan,P. 1 and O Regan,
14 th European Biosolids and Organic Resources Conference and Exhibition 1 PHOSPHORUS RECOVERY FROM SEWAGE SLUDGE USING THE AQUACRITOX SUPERCRITICAL WATER OXIDATION PROCESS O Callaghan,P. 1 and O Regan,
Ostara s Pond Water Treatment Goal: merging cost effective treatment with high water quality through resource recovery
 Ostara s Pond Water Treatment Goal: merging cost effective treatment with high water quality through resource recovery Authors: Ahren Britton, Don Clark and Ram Prasad (Ostara Nutrient Recovery Technologies
Ostara s Pond Water Treatment Goal: merging cost effective treatment with high water quality through resource recovery Authors: Ahren Britton, Don Clark and Ram Prasad (Ostara Nutrient Recovery Technologies
PREDICTION OF STRUVITE FORMATION POTENTIAL IN EBPR DIGESTED SLUDGES
 PREDICTION OF STRUVITE FORMATION POTENTIAL IN EBPR DIGESTED SLUDGES Jacimaria R. Batista and Hyunju (Judy) Jeong University of Nevada Las Vegas 4505 Maryland Parkway, Las Vegas, NV 89154-4015 ABSTRACT
PREDICTION OF STRUVITE FORMATION POTENTIAL IN EBPR DIGESTED SLUDGES Jacimaria R. Batista and Hyunju (Judy) Jeong University of Nevada Las Vegas 4505 Maryland Parkway, Las Vegas, NV 89154-4015 ABSTRACT
Nazim Cicek Page ARDI Final Report. Dr. Nazim Cicek, Biosystems Engineering, University of Manitoba
 Nazim Cicek Page 1 10-10-29 ARDI Final Report Project #: 08-907 PHOSPHORUS RECOVERY THROUGH STRIUVITE PRECIPITATION FROM RAW AND ANAEROBICALLY DIGESTED HOG MANURE Dr. Nazim Cicek, Biosystems Engineering,
Nazim Cicek Page 1 10-10-29 ARDI Final Report Project #: 08-907 PHOSPHORUS RECOVERY THROUGH STRIUVITE PRECIPITATION FROM RAW AND ANAEROBICALLY DIGESTED HOG MANURE Dr. Nazim Cicek, Biosystems Engineering,
Ion exchange for concentration of phosphorus in wastewater and recovery as struvite. Patrick Mullen Dr. Brooke Mayer Marquette University
 Ion exchange for concentration of phosphorus in wastewater and recovery as struvite Patrick Mullen Dr. Brooke Mayer Marquette University About Me 1st year M.S. at Marquette University in Environmental
Ion exchange for concentration of phosphorus in wastewater and recovery as struvite Patrick Mullen Dr. Brooke Mayer Marquette University About Me 1st year M.S. at Marquette University in Environmental
Application of the AGF (Anoxic Gas Flotation) Process
 Application of the AGF (Anoxic Gas Flotation) Process Dennis A. Burke Environmental Energy Company, 6007 Hill Road NE, Olympia, WA 98516 USA (E-mail: dennis@makingenergy.com http//www.makingenergy.com)
Application of the AGF (Anoxic Gas Flotation) Process Dennis A. Burke Environmental Energy Company, 6007 Hill Road NE, Olympia, WA 98516 USA (E-mail: dennis@makingenergy.com http//www.makingenergy.com)
Wastewater Terms for Permit Applications
 Wastewater Terms for Permit Applications Activated Sludge Alkalinity Anaerobic Anoxic Bacteria The term "activated sludge" refers to a brownish flocculent culture of organisms developed in aeration tanks
Wastewater Terms for Permit Applications Activated Sludge Alkalinity Anaerobic Anoxic Bacteria The term "activated sludge" refers to a brownish flocculent culture of organisms developed in aeration tanks
General Information on Nitrogen
 General Information on Nitrogen What is nitrogen? Nitrogen was discovered in 1772 by Daniel Rutherford in Scotland Nitrogen gas makes up nearly 80% of the air we breathe Nitrogen gas is not toxic Nitrogen
General Information on Nitrogen What is nitrogen? Nitrogen was discovered in 1772 by Daniel Rutherford in Scotland Nitrogen gas makes up nearly 80% of the air we breathe Nitrogen gas is not toxic Nitrogen
Research on recovery of phosphorus in high concentrations of phosphorus wastewater by struvite precipitation
 Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2014, 6(6):69-74 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Research on recovery of phosphorus in high concentrations
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2014, 6(6):69-74 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Research on recovery of phosphorus in high concentrations
PAPER No.4 : Environmental Chemistry MODULE No.13 : Advanced Waste Water Treatment
 Subject Chemistry Paper No and Title Module No and Title Module Tag 4, Environmental Chemistry 13, Advanced Waste Water Treatment CHE_P4_M13 Dr. S.K. Garg, Principal, Deen Dayal Upadhyaya College, University
Subject Chemistry Paper No and Title Module No and Title Module Tag 4, Environmental Chemistry 13, Advanced Waste Water Treatment CHE_P4_M13 Dr. S.K. Garg, Principal, Deen Dayal Upadhyaya College, University
EFFECT OF FERROUS SULPHATE ON THE SIMULTANEOUS ORGANIC MATTER AND NUTRIENT REMOVAL PERFORMANCE OF SEQUENCING BATCH REACTOR
 EFFECT OF FERROUS SULPHATE ON THE SIMULTANEOUS ORGANIC MATTER AND NUTRIENT REMOVAL PERFORMANCE OF SEQUENCING BATCH REACTOR Engin Gürtekin Department of Environmental Engineering, Firat University, Elazığ,
EFFECT OF FERROUS SULPHATE ON THE SIMULTANEOUS ORGANIC MATTER AND NUTRIENT REMOVAL PERFORMANCE OF SEQUENCING BATCH REACTOR Engin Gürtekin Department of Environmental Engineering, Firat University, Elazığ,
Study on Effect of Soy sauce wastewater by SBR process Jinlong Zuo1, Xiaoyue Wang1, Xinguo Yang1,Daxiang Chen1,Xuming Wang2*
 6th International Conference on Machinery, Materials, Environment, Biotechnology and Computer (MMEBC 2016) Study on Effect of Soy sauce wastewater by SBR process Jinlong Zuo1, Xiaoyue Wang1, Xinguo Yang1,Daxiang
6th International Conference on Machinery, Materials, Environment, Biotechnology and Computer (MMEBC 2016) Study on Effect of Soy sauce wastewater by SBR process Jinlong Zuo1, Xiaoyue Wang1, Xinguo Yang1,Daxiang
Wastewater Treatment Processes
 Wastewater Treatment Processes (Sep 27 th and 28 th, 2016) by Dr. Arun Kumar (arunku@civil.iitd.ac.in) Objective: To learn about processes used in tertiary treatment Courtesy: Dr. Irene Xagoraraki, MSU,
Wastewater Treatment Processes (Sep 27 th and 28 th, 2016) by Dr. Arun Kumar (arunku@civil.iitd.ac.in) Objective: To learn about processes used in tertiary treatment Courtesy: Dr. Irene Xagoraraki, MSU,
Recovery of Ammonia and Production of High-Grade Phosphates from Side-Stream Digester Effluents Using Gas-Permeable Membranes
 Recovery of Ammonia and Production of High-Grade Phosphates from Side-Stream Digester Effluents Using Gas-Permeable Membranes M.B. Vanotti (&), P.J. Dube, and A.A. Szogi United States Department of Agriculture,
Recovery of Ammonia and Production of High-Grade Phosphates from Side-Stream Digester Effluents Using Gas-Permeable Membranes M.B. Vanotti (&), P.J. Dube, and A.A. Szogi United States Department of Agriculture,
operation of continuous and batch reactors. Contrary to what happens in the batch reactor, the substrate (BOD) of the wastewater in the continuous rea
 The Effect of Ammonia Loading on the Nitrification Kinetic of Aerobic Baffled Continuous Biological Reactor S.R.M. Kutty, M.H. Isa and L.C. Leong Abstract - The purpose of this study is to determine the
The Effect of Ammonia Loading on the Nitrification Kinetic of Aerobic Baffled Continuous Biological Reactor S.R.M. Kutty, M.H. Isa and L.C. Leong Abstract - The purpose of this study is to determine the
CE 370. Wastewater Characteristics. Quality. Wastewater Quality. The degree of treatment depends on: Impurities come from:
 CE 37 Wastewater Characteristics Quality Wastewater Quality The degree of treatment depends on: Influent characteristics Effluent characteristics Impurities come from: Domestic activities Industrial activities
CE 37 Wastewater Characteristics Quality Wastewater Quality The degree of treatment depends on: Influent characteristics Effluent characteristics Impurities come from: Domestic activities Industrial activities
Wastewater Pollutants & Treatment Processes. Dr. Deniz AKGÜL Marmara University Department of Environmental Engineering
 Wastewater Pollutants & Treatment Processes Dr. Deniz AKGÜL Marmara University Department of Environmental Engineering Wastewater combination of the liquid or water carried wastes removed from residences,
Wastewater Pollutants & Treatment Processes Dr. Deniz AKGÜL Marmara University Department of Environmental Engineering Wastewater combination of the liquid or water carried wastes removed from residences,
Best Practice in Sewage and Effluent Treatment Technologies
 Best Practice in Sewage and Effluent Treatment Technologies Contents 1 Wastewater - Introduction 1 1.1 Earth s ecological system 1 1.1.1 Water effect on ecology 2 1.1.2 Wastewater generation 3 1.2 Wastewater
Best Practice in Sewage and Effluent Treatment Technologies Contents 1 Wastewater - Introduction 1 1.1 Earth s ecological system 1 1.1.1 Water effect on ecology 2 1.1.2 Wastewater generation 3 1.2 Wastewater
Unit Treatment Processes in Water and Wastewater Engineering
 Unit Treatment Processes in Water and Wastewater Engineering T J Casey AQUAVARRA RESEARCH LIMITED 22A Brookfield Avenue Blackrock Co. Dublin. October 2006 Author s Note Water and wastewater treatment technology
Unit Treatment Processes in Water and Wastewater Engineering T J Casey AQUAVARRA RESEARCH LIMITED 22A Brookfield Avenue Blackrock Co. Dublin. October 2006 Author s Note Water and wastewater treatment technology
BASICS OF WASTEWATER TREATMENT
 BASICS OF WASTEWATER TREATMENT Knowing the decisioning criteria relevant to site and drain field suitability, i.e., soil properties, can be enhanced by an understanding of some of the basics of wastewater
BASICS OF WASTEWATER TREATMENT Knowing the decisioning criteria relevant to site and drain field suitability, i.e., soil properties, can be enhanced by an understanding of some of the basics of wastewater
Characterization and Design of Sewage Treatment Plant in Bidar City
 Characterization and Design of Sewage Treatment Plant in Bidar City Harsha Bemalgi Student: Department of Civil Engineering (M.Tech, Environmental Engineering) P.D.A college of Engineering, Gulbarga, India
Characterization and Design of Sewage Treatment Plant in Bidar City Harsha Bemalgi Student: Department of Civil Engineering (M.Tech, Environmental Engineering) P.D.A college of Engineering, Gulbarga, India
STRUVIA. Sustainable recycling of phosphorus from wastewater WATER TECHNOLOGIES
 STRUVIA Sustainable recycling of phosphorus from wastewater WATER TECHNOLOGIES Sustainable recycling of phosphorus: The stakes Phosphorus, a component of DNA, is an essential nutrient for life and to the
STRUVIA Sustainable recycling of phosphorus from wastewater WATER TECHNOLOGIES Sustainable recycling of phosphorus: The stakes Phosphorus, a component of DNA, is an essential nutrient for life and to the
Environ. Eng. Res. 2017; 22(1): pissn eissn X
 Environ. Eng. Res. 2017; 22(1): 12-18 pissn 1226-1025 https://doi.org/10.4491/eer.2016.037 eissn 2005-968X Effects of ph, molar ratios and pre-treatment on phosphorus recovery through struvite crystallization
Environ. Eng. Res. 2017; 22(1): 12-18 pissn 1226-1025 https://doi.org/10.4491/eer.2016.037 eissn 2005-968X Effects of ph, molar ratios and pre-treatment on phosphorus recovery through struvite crystallization
Effect of the start-up length on the biological nutrient removal process
 Water Pollution IX 521 Effect of the start-up length on the biological nutrient removal process F. J. Fernández 1, J. Villaseñor 1 & L. Rodríguez 2 1 Department of Chemical Engineering, ITQUIMA, University
Water Pollution IX 521 Effect of the start-up length on the biological nutrient removal process F. J. Fernández 1, J. Villaseñor 1 & L. Rodríguez 2 1 Department of Chemical Engineering, ITQUIMA, University
Removal of Heavy Metal from Landfill Leachate Using Vertical Flow Construction Wetland
 Removal of Heavy Metal from Landfill Leachate Using Vertical Flow Construction Wetland Mrs. Meenakshi A. Khapre 1 1 (Département of Civil Engineering, JSPM s, Rajarshi Shahu College of Engineering Pune-37,
Removal of Heavy Metal from Landfill Leachate Using Vertical Flow Construction Wetland Mrs. Meenakshi A. Khapre 1 1 (Département of Civil Engineering, JSPM s, Rajarshi Shahu College of Engineering Pune-37,
Struvite recovery options in conventional wastewater treatment plants (WWTPs)
 Burgas Asen Zlatarov University Department of Water Treatment technology Struvite recovery options in conventional wastewater treatment plants (WWTPs) Nenov V., Jemendjiev H., Peeva G., Bonev B., Burgas
Burgas Asen Zlatarov University Department of Water Treatment technology Struvite recovery options in conventional wastewater treatment plants (WWTPs) Nenov V., Jemendjiev H., Peeva G., Bonev B., Burgas
Removal of Nitrogen and Phosphorus from Animal and Municipal Wastewaters as Dittmarite
 Journal of Environmental Science and Engineering B 6 (2017) 295-300 doi:10.17265/2162-5263/2017.06.001 D DAVID PUBLISHING Removal of Nitrogen and Phosphorus from Animal and Municipal Wastewaters as Dittmarite
Journal of Environmental Science and Engineering B 6 (2017) 295-300 doi:10.17265/2162-5263/2017.06.001 D DAVID PUBLISHING Removal of Nitrogen and Phosphorus from Animal and Municipal Wastewaters as Dittmarite
Operating Experience with Ostara Struvite Harvesting Process
 Operating Experience with Ostara Struvite Harvesting Process Authors: Steve Reusser 1, Alan Grooms 1, Aaron Dose 1, Ahren Britton 2, Ram Prasad 2 1. Madison Metropolitan Sewerage District 2. Ostara Nutrient
Operating Experience with Ostara Struvite Harvesting Process Authors: Steve Reusser 1, Alan Grooms 1, Aaron Dose 1, Ahren Britton 2, Ram Prasad 2 1. Madison Metropolitan Sewerage District 2. Ostara Nutrient
Nutrient Cycling in an Aquatic Ecosystem
 Nutrient Cycling in an Aquatic Ecosystem 2.1 Productivity 2.2 Oxygen 2.3 Salinity 2.4 Carbon 2.5 Nitrogen 2.6 Phosphorous 2.7 Iron 2.8 Sulphur 2.9 Silica 2.3 Salinity of Inland Waters The salinity of freshwaters
Nutrient Cycling in an Aquatic Ecosystem 2.1 Productivity 2.2 Oxygen 2.3 Salinity 2.4 Carbon 2.5 Nitrogen 2.6 Phosphorous 2.7 Iron 2.8 Sulphur 2.9 Silica 2.3 Salinity of Inland Waters The salinity of freshwaters
Acidity and Alkalinity:
 Evaluation of Pollution Sources to Lake Glenville Quarterly Report December 2018 Kimberlee K Hall, PhD Environmental Health Program, Western Carolina University Summary Chemical and microbial analysis
Evaluation of Pollution Sources to Lake Glenville Quarterly Report December 2018 Kimberlee K Hall, PhD Environmental Health Program, Western Carolina University Summary Chemical and microbial analysis
American Water College 2010
 Vocabulary Activated Sludge (Part 1) Activated Sludge Sludge particles produced in raw or settled wastewater (primary effluent) by the growth of organisms (including zoogleal bacteria) in aeration tanks
Vocabulary Activated Sludge (Part 1) Activated Sludge Sludge particles produced in raw or settled wastewater (primary effluent) by the growth of organisms (including zoogleal bacteria) in aeration tanks
Treatability of Organic and Radioactive Emerging Contaminants in Stormwater Runoff
 Treatability of Organic and Radioactive Emerging Contaminants in Stormwater Runoff Robert Pitt, Ph.D., P.E., D.WRE, BCEE, University of Alabama Shirley Clark, Ph.D., P.E., D.WRE, Penn State - Harrisburg
Treatability of Organic and Radioactive Emerging Contaminants in Stormwater Runoff Robert Pitt, Ph.D., P.E., D.WRE, BCEE, University of Alabama Shirley Clark, Ph.D., P.E., D.WRE, Penn State - Harrisburg
MULTI PHOSTRIP. Biological phosphorus removal from municipal waste-water in the sidestream
 ! "# %$!# & " ' ( )* ' MULTI PHOSTRIP Biological phosphorus removal from municipal waste-water in the sidestream process Description Application Advantages Operating results References Multi Umwelttechnologie
! "# %$!# & " ' ( )* ' MULTI PHOSTRIP Biological phosphorus removal from municipal waste-water in the sidestream process Description Application Advantages Operating results References Multi Umwelttechnologie
Separation of Ammonia and Phosphate Minerals from Wastewater using Gas-Permeable Membranes. Matias Vanotti
 Separation of Ammonia and Phosphate Minerals from Wastewater using Gas-Permeable Membranes WRRI Water Sustainability Through Nanotechnology Symposium March 15, 2017 Raleigh, NC Matias Vanotti Collaborators:
Separation of Ammonia and Phosphate Minerals from Wastewater using Gas-Permeable Membranes WRRI Water Sustainability Through Nanotechnology Symposium March 15, 2017 Raleigh, NC Matias Vanotti Collaborators:
NEW BIOLOGICAL PHOSPHORUS REMOVAL CONCEPT SUCCESSFULLY APPLIED IN A T-DITCH PROCESS WASTEWATER TREATMENT PLANT
 NEW BIOLOGICAL PHOSPHORUS REMOVAL CONCEPT SUCCESSFULLY APPLIED IN A T-DITCH PROCESS WASTEWATER TREATMENT PLANT ABSTRACT C. Yang*, L. Zhou**, W. Luo***, and L. Johnson**** *Corstar International Corp. 111
NEW BIOLOGICAL PHOSPHORUS REMOVAL CONCEPT SUCCESSFULLY APPLIED IN A T-DITCH PROCESS WASTEWATER TREATMENT PLANT ABSTRACT C. Yang*, L. Zhou**, W. Luo***, and L. Johnson**** *Corstar International Corp. 111
Mid-Halton Wastewater (Sewage) Treatment Plant Expansion And Effluent Sewer Public Information Centre # 1 May 14, 2009
 INFORMATION BRIEF MID-HALTON WASTEWATER (SEWAGE) TREATMENT PLANT EXPANSION AND EFFLUENT SEWER SCHEDULE C CLASS ENVIRONMENTAL ASSESSMENT PUBLIC INFORMATION CENTRE (PIC) # 1 1 Municipal Class Environmental
INFORMATION BRIEF MID-HALTON WASTEWATER (SEWAGE) TREATMENT PLANT EXPANSION AND EFFLUENT SEWER SCHEDULE C CLASS ENVIRONMENTAL ASSESSMENT PUBLIC INFORMATION CENTRE (PIC) # 1 1 Municipal Class Environmental
Maneechotiros Daegi Kim 1, Kyung Jin Min 2,3, Kwanyong Lee 4, Min Sung Yu 2, and Ki Young Park 2
 Environ. Eng. Res. 2016 Research Article http://dx.doi.org/10.4491/eer.2016.037 pissn 1226-1025 eissn 2005-968X In Press, Uncorrected Proof Effects of ph, molar ratios and pre-treatment on phosphorus recovery
Environ. Eng. Res. 2016 Research Article http://dx.doi.org/10.4491/eer.2016.037 pissn 1226-1025 eissn 2005-968X In Press, Uncorrected Proof Effects of ph, molar ratios and pre-treatment on phosphorus recovery
/ Marley MARPAK Modular Biomedia /
 / Marley MARPAK Modular Biomedia / The Marley MARPAK Difference SPX Cooling Technologies is a world leader in the design, manufacturing and construction of cooling products. The design and production of
/ Marley MARPAK Modular Biomedia / The Marley MARPAK Difference SPX Cooling Technologies is a world leader in the design, manufacturing and construction of cooling products. The design and production of
STRUVITE FORMATION FROM WASTEWATER: AFFECTING FACTORS AND NUTRIENT RECOVERY
 9 STRUVITE FORMATION FROM WASTEWATER: AFFECTING FACTORS AND NUTRIENT RECOVERY Nguyen Thi Thuy TRANG, Le Thi Hoang YEN, Le Thi Hong HANH & Bui Xuan THANH Faculty of Environment and Natural Resources, Ho
9 STRUVITE FORMATION FROM WASTEWATER: AFFECTING FACTORS AND NUTRIENT RECOVERY Nguyen Thi Thuy TRANG, Le Thi Hoang YEN, Le Thi Hong HANH & Bui Xuan THANH Faculty of Environment and Natural Resources, Ho
SBR FOR LOW FLOW APPLICATIONS
 SBR FOR LOW FLOW APPLICATIONS The Dutchland Sequencing Batch Reactor uses high quality precast concrete structures to handle all of the work of a continuous flow treatment system. All processes - biological
SBR FOR LOW FLOW APPLICATIONS The Dutchland Sequencing Batch Reactor uses high quality precast concrete structures to handle all of the work of a continuous flow treatment system. All processes - biological
PHOSPHATE REMOVAL AND RECOVERY AS BRUSHITE BY UNSEEDED GRANULATION IN A FLUIDIZED-BED REACTOR
 Proceedings of the 14 th International Conference on Environmental Science and Technology Rhodes, Greece, 3-5 September 215 PHOSPHATE REMOVAL AND RECOVERY AS BRUSHITE BY UNSEEDED GRANULATION IN A FLUIDIZED-BED
Proceedings of the 14 th International Conference on Environmental Science and Technology Rhodes, Greece, 3-5 September 215 PHOSPHATE REMOVAL AND RECOVERY AS BRUSHITE BY UNSEEDED GRANULATION IN A FLUIDIZED-BED
Preparing for Nutrient Removal at Your Treatment Plant
 Summer Seminar Emerging Issues in the Water/Wastewater Industry Preparing for Nutrient Removal at Your Treatment Plant Rajendra P. Bhattarai, P.E., BCEE Austin Water Utility Ana J. Peña-Tijerina, Ph.D.,
Summer Seminar Emerging Issues in the Water/Wastewater Industry Preparing for Nutrient Removal at Your Treatment Plant Rajendra P. Bhattarai, P.E., BCEE Austin Water Utility Ana J. Peña-Tijerina, Ph.D.,
Chapter 4: Advanced Wastewater Treatment for Phosphorous Removal
 ENGI 9605 Advanced Wastewater Treatment Chapter 4: Advanced Wastewater Treatment for Phosphorous Removal Winter 2011 Faculty of Engineering & Applied Science 4.1 Phosphorous in wastewaters 1. Common forms
ENGI 9605 Advanced Wastewater Treatment Chapter 4: Advanced Wastewater Treatment for Phosphorous Removal Winter 2011 Faculty of Engineering & Applied Science 4.1 Phosphorous in wastewaters 1. Common forms
ChemScan PROCESS ANALYZERS
 ChemScan PROCESS ANALYZERS 2002, Applied Spectrometry Associates, Inc. www.chemscan.com ChemScan Application Summary #92 Biological Phosphorous Removal Background Domestic wastewater generally contains
ChemScan PROCESS ANALYZERS 2002, Applied Spectrometry Associates, Inc. www.chemscan.com ChemScan Application Summary #92 Biological Phosphorous Removal Background Domestic wastewater generally contains
CASE STUDY: BREAKTHROUGH CENTRATE TREATMENT AT REDCLIFFE WASTE WATER TREATMENT PLANT
 2007 Virotec International plc All rights reserved. A COMMERCIAL APPLICATION OF VIROSEWAGE TECHNOLOGY CASE STUDY: BREAKTHROUGH CENTRATE TREATMENT AT REDCLIFFE WASTE WATER TREATMENT PLANT We had documented
2007 Virotec International plc All rights reserved. A COMMERCIAL APPLICATION OF VIROSEWAGE TECHNOLOGY CASE STUDY: BREAKTHROUGH CENTRATE TREATMENT AT REDCLIFFE WASTE WATER TREATMENT PLANT We had documented
PO4 Sponge. Phosphorus Removal - Low & High Level Sources
 PO4 Sponge Phosphorus Removal - Low & High Level Sources Phosphorus (P) is a contaminant in streams and lakes that can degrade water bodies, especially when excessive. It contributes to growth of cyanobacteria
PO4 Sponge Phosphorus Removal - Low & High Level Sources Phosphorus (P) is a contaminant in streams and lakes that can degrade water bodies, especially when excessive. It contributes to growth of cyanobacteria
WASTEWATER TREATMENT SYSTEM
 WASTEWATER TREATMENT SYSTEM PrintStudioOne.com Nelson Environmental Inc. The Nelson Environmental OPTAER system is an efficient pond-based wastewater treatment solution utilized in a broad spectrum of
WASTEWATER TREATMENT SYSTEM PrintStudioOne.com Nelson Environmental Inc. The Nelson Environmental OPTAER system is an efficient pond-based wastewater treatment solution utilized in a broad spectrum of
Ch. 5 - Nutrient Cycles and Soils
 Ch. 5 - Nutrient Cycles and Soils What are Nutrient (biogeochemical) Cycles? a process by which nutrients are recycled between living organisms and nonliving environment. The three general types of nutrient
Ch. 5 - Nutrient Cycles and Soils What are Nutrient (biogeochemical) Cycles? a process by which nutrients are recycled between living organisms and nonliving environment. The three general types of nutrient
MARPAK modular biomedia WASTEWATER TREATMENT
 MARPAK modular biomedia WASTEWATER TREATMENT The Marley MARPAK Difference SPX Cooling Technologies is a world leader in the design, manufacturing and construction of evaporative cooling products. The design
MARPAK modular biomedia WASTEWATER TREATMENT The Marley MARPAK Difference SPX Cooling Technologies is a world leader in the design, manufacturing and construction of evaporative cooling products. The design
Wastewater Treatment Processes
 Wastewater Treatment Processes CEL212 Environmental Engineering (2 nd Semester 2010-2011) Dr. Arun Kumar (arunku@civil.iitd.ac.in) Department of Civil Engineering Indian Institute of Technology (Delhi)
Wastewater Treatment Processes CEL212 Environmental Engineering (2 nd Semester 2010-2011) Dr. Arun Kumar (arunku@civil.iitd.ac.in) Department of Civil Engineering Indian Institute of Technology (Delhi)
JEDDAH INDUSTRIAL CITY
 JEDDAH INDUSTRIAL CITY WASTEWATER TREATMENT PLANT A Presentation by : Engr. Mowafaq Al-Sugeir Managing Director ICDOC SAWEA 2007 WORKSHOP, AL-KHOBER 4 December 2007 Built & Being Operated by : on Build-Operate-Transfer
JEDDAH INDUSTRIAL CITY WASTEWATER TREATMENT PLANT A Presentation by : Engr. Mowafaq Al-Sugeir Managing Director ICDOC SAWEA 2007 WORKSHOP, AL-KHOBER 4 December 2007 Built & Being Operated by : on Build-Operate-Transfer
SEASONAL VARIATIONS OF METALS AND OTHER MINERAL CONSTITUENTS OF RIVER YOBE
 Publication of Chemical Society of Nigeria, Kano Chapter SEASONAL VARIATIONS OF METALS AND OTHER MINERAL CONSTITUENTS OF RIVER YOBE 1 Abdulrahman A. Audu and 2 Sagir M. Rabi u* 1 Department of Pure and
Publication of Chemical Society of Nigeria, Kano Chapter SEASONAL VARIATIONS OF METALS AND OTHER MINERAL CONSTITUENTS OF RIVER YOBE 1 Abdulrahman A. Audu and 2 Sagir M. Rabi u* 1 Department of Pure and
ORIGINAL ARTICLE Advanced treatment of swine wastewater by electrodialysis with a tubular ion exchange membrane
 Blackwell Science, LtdOxford, UKASJAnimal Science Journal1344-39412004 Blackwell Publishing Asia Pty LtdOctober 2004755479485Original ArticleElectrodialysis of swine wastewatery. FUKUMOTO and K. HAGA ORIGINAL
Blackwell Science, LtdOxford, UKASJAnimal Science Journal1344-39412004 Blackwell Publishing Asia Pty LtdOctober 2004755479485Original ArticleElectrodialysis of swine wastewatery. FUKUMOTO and K. HAGA ORIGINAL
A STRUVITE CONTROL AND PHOSPHORUS REMOVAL PROCESS FOR CENTRATE: FULL-SCALE TESTING. 850 Pembina Highway Winnipeg, MB
 A STRUVITE CONTROL AND PHOSPHORUS REMOVAL PROCESS FOR CENTRATE: FULL-SCALE TESTING Simon Baker 1, Yoomin Lee 1, Wang Li 1 1 Earth Tech Canada Inc 850 Pembina Highway Winnipeg, MB ABSTRACT The North End
A STRUVITE CONTROL AND PHOSPHORUS REMOVAL PROCESS FOR CENTRATE: FULL-SCALE TESTING Simon Baker 1, Yoomin Lee 1, Wang Li 1 1 Earth Tech Canada Inc 850 Pembina Highway Winnipeg, MB ABSTRACT The North End
Lecture 23. Nitrophosphate Fertilizers Part 1
 Lecture 23 Nitrophosphate Fertilizers Part 1 Introduction Nitrophosphate is the generally accepted term for any fertilizer that is produced by a process involving treatment of phosphate rock with nitric
Lecture 23 Nitrophosphate Fertilizers Part 1 Introduction Nitrophosphate is the generally accepted term for any fertilizer that is produced by a process involving treatment of phosphate rock with nitric
Biological Nitrogen and COD Removal of Nutrient-Rich Wastewater Using Aerobic and Anaerobic Reactors
 J. Water Resource and Protection, 29, 1, 376-38 doi:1.4236/jwarp.29.1545 Published Online November 29 (http://www.scirp.org/journal/jwarp) Biological Nitrogen and COD Removal of Nutrient-Rich Wastewater
J. Water Resource and Protection, 29, 1, 376-38 doi:1.4236/jwarp.29.1545 Published Online November 29 (http://www.scirp.org/journal/jwarp) Biological Nitrogen and COD Removal of Nutrient-Rich Wastewater
Phosphogreen technology. IWA Sweden Seminar Phosphorus recovery from wastewater Malmö, 11 April 2018 Peter Balslev
 Phosphogreen technology IWA Sweden Seminar Phosphorus recovery from wastewater Malmö, 11 April 2018 Peter Balslev Presentation Principles of struvite precipitation Potential in struvite-method PhosphoGreen
Phosphogreen technology IWA Sweden Seminar Phosphorus recovery from wastewater Malmö, 11 April 2018 Peter Balslev Presentation Principles of struvite precipitation Potential in struvite-method PhosphoGreen
EUTROPHICATION. Student Lab Workbook
 EUTROPHICATION Student Lab Workbook THE SCIENTIFIC METHOD 1. Research Background literature research about a topic of interest 2. Identification of a problem Determine a problem (with regards to the topic)
EUTROPHICATION Student Lab Workbook THE SCIENTIFIC METHOD 1. Research Background literature research about a topic of interest 2. Identification of a problem Determine a problem (with regards to the topic)
Water Quality Management for Coastal Aquaculture
 SUB Hamburg A/534446 Water Quality Management for Coastal Aquaculture By Sukumar Bandyopadhyay Former Professor and Emeritus Fellow Indian institute of Technology Kharagpur 2008 DAYA PUBLISHING HOUSE Delhi
SUB Hamburg A/534446 Water Quality Management for Coastal Aquaculture By Sukumar Bandyopadhyay Former Professor and Emeritus Fellow Indian institute of Technology Kharagpur 2008 DAYA PUBLISHING HOUSE Delhi
Presence And Effects Of Aromatic Hydrocarbons On Sewage Treatment Efficiency
 Proceedings of the Annual International Conference on Soils, Sediments, Water and Energy Volume 14 Article 2 January 21 Presence And Effects Of Aromatic Hydrocarbons On Sewage Treatment Efficiency Bozena
Proceedings of the Annual International Conference on Soils, Sediments, Water and Energy Volume 14 Article 2 January 21 Presence And Effects Of Aromatic Hydrocarbons On Sewage Treatment Efficiency Bozena
Removal of Ammonium and Phosphate from Fertilizer Industry Wastewater Using Struvite Precipitation Method
 J. Appl. Environ. Biol. Sci., 7(2)158-162, 217 217, TextRoad Publication ISSN: 29-4274 Journal of Applied Environmental and Biological Sciences www.textroad.com Removal of Ammonium and Phosphate from Fertilizer
J. Appl. Environ. Biol. Sci., 7(2)158-162, 217 217, TextRoad Publication ISSN: 29-4274 Journal of Applied Environmental and Biological Sciences www.textroad.com Removal of Ammonium and Phosphate from Fertilizer
AN EXPERIMENTAL AND MATHEMATICAL SIMULATION OF BIOLOGICAL PROCESSES IN A SEWERAGE SYSTEM
 Global NEST Journal, Vol 8, No 1, pp 75-81, 2006 Copyright 2006 Global NEST Printed in Greece. All rights reserved AN EXPERIMENTAL AND MATHEMATICAL SIMULATION OF BIOLOGICAL PROCESSES IN A SEWERAGE SYSTEM
Global NEST Journal, Vol 8, No 1, pp 75-81, 2006 Copyright 2006 Global NEST Printed in Greece. All rights reserved AN EXPERIMENTAL AND MATHEMATICAL SIMULATION OF BIOLOGICAL PROCESSES IN A SEWERAGE SYSTEM
Wastewater chemically enhanced primary treatment combined with adsorption of activated sludge: AS-CEPT in wastewater treatment
 Wastewater chemically enhanced primary treatment combined with adsorption of activated sludge: AS-CEPT in wastewater treatment G. R. Xu, C. L. Qian School of Municipal and Environmental Engineering, Harbin
Wastewater chemically enhanced primary treatment combined with adsorption of activated sludge: AS-CEPT in wastewater treatment G. R. Xu, C. L. Qian School of Municipal and Environmental Engineering, Harbin
Phosphorus Recovery and Nutrient Management with Magnesium Hydroxide: Struvite Recovery
 Phosphorus Recovery and Nutrient Management with Magnesium Hydroxide: Struvite Recovery Alan Bowers Nathaniel Barnes Dept. of Civil & Environmental Engrg. Vanderbilt University Nashville, TN Matthew Madolora
Phosphorus Recovery and Nutrient Management with Magnesium Hydroxide: Struvite Recovery Alan Bowers Nathaniel Barnes Dept. of Civil & Environmental Engrg. Vanderbilt University Nashville, TN Matthew Madolora
Disposal of Sludge with Solid Wastes in Aerobic and Anaerobic Landfill Areas
 CONTACT Günay Kocasoy Turkish National Committee on Solid Wastes Bogazici University 34342, Bebek, Istanbul, TURKEY Tel: +90 212 359 44 76 Fax: +90 212 268 08 98 e-mail: kocasoy@boun.edu.tr Disposal of
CONTACT Günay Kocasoy Turkish National Committee on Solid Wastes Bogazici University 34342, Bebek, Istanbul, TURKEY Tel: +90 212 359 44 76 Fax: +90 212 268 08 98 e-mail: kocasoy@boun.edu.tr Disposal of
Sanitary Sewer Systems. Sewage Collection System. Types of Sewage 10/12/2016. General Overview
 Sanitary Sewer Systems General Overview Sewage Collection System Pipes Pumping stations Maintenance entry points manholes Types of Sewage Sanitary Domestic sewage: human wastes and washwater from public
Sanitary Sewer Systems General Overview Sewage Collection System Pipes Pumping stations Maintenance entry points manholes Types of Sewage Sanitary Domestic sewage: human wastes and washwater from public
Kirill Ukhanov, GE Water & Process Technologies, Russia, describes how advanced membrane technology is helping a Russian refinery to meet stringent
 Kirill Ukhanov, GE Water & Process Technologies, Russia, describes how advanced membrane technology is helping a Russian refinery to meet stringent wastewater requirements. In Russia, there are strict
Kirill Ukhanov, GE Water & Process Technologies, Russia, describes how advanced membrane technology is helping a Russian refinery to meet stringent wastewater requirements. In Russia, there are strict
QUESTIONNAIRE POND TREATMENT PLANT OLOID Agitate, Circulate, Aerate
 Page 1 of 5 In order to quickly clarify whether this energy-saving technology is suitable for your application, please fill out the questionnaire as far as possible and to send us by e-mail. Questionnaire
Page 1 of 5 In order to quickly clarify whether this energy-saving technology is suitable for your application, please fill out the questionnaire as far as possible and to send us by e-mail. Questionnaire
Enhanced Nutrients Removal in Conventional Anaerobic Digestion Processes
 Enhanced Nutrients Removal in Conventional Anaerobic Digestion Processes M. Z. Othman, S. Uludag-Demirer and G.N. Demirer Abstract One of the main challenges for one phase anaerobic digestion processes
Enhanced Nutrients Removal in Conventional Anaerobic Digestion Processes M. Z. Othman, S. Uludag-Demirer and G.N. Demirer Abstract One of the main challenges for one phase anaerobic digestion processes
EFFECT OF WATER DEPTH AND AERATION ON A CONTACT MEDIA CHANNEL PURIFICATION PROCESS FOR WASTEWATER RECLAMATION
 J. Environ. Eng. Manage., 17(5), 339-343 (7) EFFECT OF WATER DEPTH AND AERATION ON A CONTACT MEDIA CHANNEL PURIFICATION PROCESS FOR WASTEWATER RECLAMATION Tzu-Yi Pai, 1, * Chwen-Jeng Tzeng, 2 Chen-Lung
J. Environ. Eng. Manage., 17(5), 339-343 (7) EFFECT OF WATER DEPTH AND AERATION ON A CONTACT MEDIA CHANNEL PURIFICATION PROCESS FOR WASTEWATER RECLAMATION Tzu-Yi Pai, 1, * Chwen-Jeng Tzeng, 2 Chen-Lung
Contents General Information Abbreviations and Acronyms Chapter 1 Wastewater Treatment and the Development of Activated Sludge
 Contents Contents General Information Abbreviations and Acronyms... 6 Chapter 1 Wastewater Treatment and the Development of Activated Sludge... 8 The Importance of Wastewater Treatment... 8 The Scope of
Contents Contents General Information Abbreviations and Acronyms... 6 Chapter 1 Wastewater Treatment and the Development of Activated Sludge... 8 The Importance of Wastewater Treatment... 8 The Scope of
MURDOCH RESEARCH REPOSITORY.
 MURDOCH RESEARCH REPOSITORY http://researchrepository.murdoch.edu.au This is the author's final version of the work, as accepted for publication following peer review but without the publisher's layout
MURDOCH RESEARCH REPOSITORY http://researchrepository.murdoch.edu.au This is the author's final version of the work, as accepted for publication following peer review but without the publisher's layout
AirPrex : Process for Optimization of Biosolids Treatment with the option of Phosphate Recovery
 carbon ǀ nitrogen ǀ phosphorus AirPrex : Process for Optimization of Biosolids Treatment with the option of Phosphate Recovery July 2015 Contents Biological Phosphate-Removal Influence of Phosphate on
carbon ǀ nitrogen ǀ phosphorus AirPrex : Process for Optimization of Biosolids Treatment with the option of Phosphate Recovery July 2015 Contents Biological Phosphate-Removal Influence of Phosphate on
Fig 1. Flex EDR Ammonia Stack
 A municipal wastewater treatment plant contacted Saltworks looking for a reliable, fully controllable ammonia treatment system that could meet any discharge requirements, resist high salinities and rapidly
A municipal wastewater treatment plant contacted Saltworks looking for a reliable, fully controllable ammonia treatment system that could meet any discharge requirements, resist high salinities and rapidly
Appendix C1: Batch Kinetics Tests
 Appendix C1: Batch Kinetics Tests This appendix contains the entire data set for the batch kinetics tests for the potential biofilter components. These tests were performed to provide estimates of optimal
Appendix C1: Batch Kinetics Tests This appendix contains the entire data set for the batch kinetics tests for the potential biofilter components. These tests were performed to provide estimates of optimal
Santa Rosa Creek Water Quality Results 2004
 Santa Rosa Creek Water Quality Results 24 Community Clean Water Institute Site Description: SRC4: Off 3rd Street in downtown Santa Rosa. Behind the Vineyard Hotel just West of Highway 11 along the Prince
Santa Rosa Creek Water Quality Results 24 Community Clean Water Institute Site Description: SRC4: Off 3rd Street in downtown Santa Rosa. Behind the Vineyard Hotel just West of Highway 11 along the Prince
Bio-solar purification : A new process to treat domestic wastewater and to turn water and wastes in a safe reusable form
 Bio-solar purification : A new process to treat domestic wastewater and to turn water and wastes in a safe reusable form www.heliopure.com Camille URBAN R&D Engineer Water, unsuspected real needs 5,000
Bio-solar purification : A new process to treat domestic wastewater and to turn water and wastes in a safe reusable form www.heliopure.com Camille URBAN R&D Engineer Water, unsuspected real needs 5,000
Phosphate Recovery by Crystallization Process Using Magnesium Ammonium Phosphate Crystals as Seed Material
 2014 3rd International Conference on Environment Energy and Biotechnology IPCBEE vol.70 (2014) (2014) IACSIT Press, Singapore DOI: 10.7763/IPCBEE. 2014. V70. 30 Phosphate Recovery by Crystallization Process
2014 3rd International Conference on Environment Energy and Biotechnology IPCBEE vol.70 (2014) (2014) IACSIT Press, Singapore DOI: 10.7763/IPCBEE. 2014. V70. 30 Phosphate Recovery by Crystallization Process
Why Water Quality? FOR IMMEDIATE RELEASE March 26, 2013
 Nutrients Nutrients Microbes Rulers of the World Microbes and Redox Potential Dissolved Oxygen Nitrogen Cycle From Waste to Gas Phosphorus Cycle or Recycle Algae The Miracle of Life Stormwater - Loadings
Nutrients Nutrients Microbes Rulers of the World Microbes and Redox Potential Dissolved Oxygen Nitrogen Cycle From Waste to Gas Phosphorus Cycle or Recycle Algae The Miracle of Life Stormwater - Loadings
Ammonia removal from anaerobically digested dairy manure by struvite precipitation
 Process Biochemistry 40 (2005) 3667 3674 www.elsevier.com/locate/procbio Ammonia removal from anaerobically digested dairy manure by struvite precipitation S. Uludag-Demirer a, *,1, G.N. Demirer b,1, S.
Process Biochemistry 40 (2005) 3667 3674 www.elsevier.com/locate/procbio Ammonia removal from anaerobically digested dairy manure by struvite precipitation S. Uludag-Demirer a, *,1, G.N. Demirer b,1, S.
APPLICATION OF HUMATE SUSTAINABLE ALTERNATIVE FOR REMEDIATION OF WASTEWATER AND GROUNDWATER
 APPLICATION OF HUMATE SUSTAINABLE ALTERNATIVE FOR REMEDIATION OF WASTEWATER AND GROUNDWATER Ewa Lipczynska-Kochany *) and Jan Kochany **) * ) Environmental Consultant, Mississauga, ON Canada **) Conestoga-Rovers
APPLICATION OF HUMATE SUSTAINABLE ALTERNATIVE FOR REMEDIATION OF WASTEWATER AND GROUNDWATER Ewa Lipczynska-Kochany *) and Jan Kochany **) * ) Environmental Consultant, Mississauga, ON Canada **) Conestoga-Rovers
KEYWORDS: Adsorption, Chromium(VI), COD, Tannery Effluent, Banyan Sawdust.
 IJESRT INTERNATIONAL JOURNAL OF ENGINEERING SCIENCES & RESEARCH TECHNOLOGY REMOVAL OF POLLUTION LOAD FROM TANNERY EFFLUENT BY USING BANYAN SAWDUST (FICUS BENGALENSIS) AS AN ADSORBENT Pushpendra Kushwaha1*,
IJESRT INTERNATIONAL JOURNAL OF ENGINEERING SCIENCES & RESEARCH TECHNOLOGY REMOVAL OF POLLUTION LOAD FROM TANNERY EFFLUENT BY USING BANYAN SAWDUST (FICUS BENGALENSIS) AS AN ADSORBENT Pushpendra Kushwaha1*,
