Performance of MEA and amine-blends in the CSIRO PCC Pilot Plant at Loy Yang Power in Australia
|
|
|
- Maria Bishop
- 5 years ago
- Views:
Transcription
1 NOTICE: this is the author s version of a work that was accepted for publication in Fuel. Changes resulting from the publishing process, such as peer review, editing, corrections, structural formatting, and other quality control mechanisms may not be reflected in this document. Changes may have been made to this work since it was submitted for publication. A definitive version was subsequently published in Fuel, vol. 101 (Nov. 2012). Performance of MEA and amine-blends in the CSIRO PCC Pilot Plant at Loy Yang Power in Australia Yuli Artanto a,1, James Jansen a, Pauline Pearson a, Thong Do c, Aaron Cottrell b, Erik Meuleman a and Paul Feron b a CSIRO Energy Technology, Clayton, VIC, Australia, b CSIRO Energy Technology, Newcastle, NSW, Australia c CSIRO Energy Technology, North Ryde, NSW, Australia ABSTRACT Chemical reactive liquid absorption in post-combustion carbon capture (PCC) has sparked an interest of many researchers for improvement in creating high CO 2 absorption capacity and minimizing the reboiler heat duty for regeneration. This paper discusses results from performance trials on different solvents: mono-ethanolamine (MEAbaseline), a mixture of MEA with 2-amino-2-methyl-1-propanol/AMP (Blended Amine 1) and Blended Amine 2 (a propietary solvent developed by Research Institute of Innovative Technology for the Earth (RITE), Japan). The trials were carried out in CSIRO s PCC pilot plant at Loy Yang Power. The pilot plant receives flue gas from this 2,2 GW brown coal-fired power station in the Latrobe Valley, Victoria. The study has shown the benefits of using blended solvents for CO 2 capture in terms of CO 2 recovery and reboiler duty for solvent regeneration. The correlation of CO 2 recovery and process parameters, i.e., liquid and gas (L/G) ratio and lean loading, has been established for the range of solvents tested. The results show that, in general, an increase of L/G ratio increases the CO 2 recovery. It is also noted that a solvent at higher lean loading requires a greater solvent (recycle) flow rate, or vice versa, in order to capture a given amount of CO 2. The effect of reducing the stripper s bottom temperature from 115 to 112 C reduced the CO 2 recovery of both MEA and Blended Amine 1 as their solvent lean loadings are raised. The same temperature change, however, did not significantly decrease CO 2 recovery of Blended Amine 2, as it also shown by insignificant changes in solvent lean loadings. The results also indicate that the reboiler heat duty is dependent upon L/G ratio, solvent lean loading and blending solvent type. For the MEA-115 C, a minimum reboiler heat-duty as a function of L/G ratio is clearly observed and the Blended Amine C has also shown a similar pattern. In constrast, Blended Amine C showed excessive energy duty, which indicates unfavourable condition for this solvent. This is not the case for Blended Amine 2. It is observed that the reboiler heat duty decreases as stripper bottom temperature decreases, which in turn raises the lean loading for both MEA and Blended Amine 1. On the other hand, the lean loading of Blended Amine 2 in the temperature range examined did not affect the reboiler heat duty changes. The investigation also found that the magnitude of reboiler duty is decreasing following the order MEA > Blended Amine 1 > Blended Amine 2. In order to obtain CO 2 recovery of 85%, the Blended Amine C (L/G = 3.60) has similar reboiler duty relative to that of MEA-115 C with L/G ratio of For the Blended Amine 1 (L/G = 3.04), however, the reboiler heat duty increased by 51% at a similar temperature. The temperature reduction to 112 C decreased the reboiler heat duty of both Blended Amines 1 and 2 by 11% (L/G = 4.01) and 14% (L/G = 3.60) respectively. Further raising the CO 2 recovery to 90-95%, in comparison to that of MEA at 115 C with L/G ratio of 4.20, the reboiler heat duty of Blended Amine 1 has risen by 49% (L/G = 3.95) and of Blended Amine 2 dropped by 5% (L/G = 3.52) under a similar temperature. At 112 C, the reboiler heat duty of Blended Amine 1 decreased by 18% (L/G = 5.02). Blended Amine 2 may give a similar reboiler heat duty if run at a larger L/G ratio. Overall, the result of the MEA baseline and blending solvents has shown different behaviours of CO 2 stripping. The distribution of the components of reboiler heat duty may explain this difference. For MEA, the condenser heat, which equals the heat needed for water evaporation, is a major contributor to the 1 Corresponding author: yuli.artanto@csiro.au
2 reboiler duty. For both Blended Amine solvents, the magnitude of sensible heat and the heat of CO 2 desorption are more pronounced than the condenser heat. At the minimum reboiler duty reached by MEA and Blended Amine 1, the condenser heat share is lower than the other reboiler duty components. Therefore, further works involve optimisation of the PCC process with blended Amines and other blends. Keywords: PCC pilot plant, CO 2 recovery, MEA, AMP, Blended Amines 1 and 2, temperature, reboiler heat duty and L/G ratio 1. Introduction The brown coal-fired power generation is the source of about half of Victoria s current greenhouse gas emissions [1]. It is therefore clear that CO 2 -emission reduction strategies aimed at the existing power stations are urgently needed to provide a path towards environmental sustainability of the Victorian brown coal industry. The use of carbon capture and sequestration (CCS) is essential for this path and Victoria is well placed due to the vicinity of vast storage capacity in the Gippsland basin, which equals to more than 100 years of CO 2 produced in Victoria at current rates [2]. Post-combustion capture (PCC) is an important first part of the CCS chain. The implementation of PCC in the Victorian case requires specific focus towards its technological development in regards of three following issues: 1. Brown coal is not sold into a world market due to its high moisture content in contrast to black coal, oil or natural gas. Therefore, it is expected that brown coal prices will remain at low price levels thus continuing to provide the basis for low cost electricity for Victoria. The capture of CO 2 will result in a large increase in the cost of electricity generation, which needs to be addressed; 2. Brown coal flue gases are available at high temperature, have high water content and contain alkaline ash. This provides a challenging environment for chemical absorption processes; 3. The combined process of coal mining, power generation and PCC should use less water than current power generation as ground water level retreats to an unsustainable situation. A wide range of CO 2 separation techniques such as absorption into a liquid, adsorption onto a solid and membrane permeation processes [3] are in principle available to capture CO 2 from flue gases. Due to a high volume flow rate of gas stream from a coal-fired power plant and low CO 2 partial pressure in the gas stream [4], absorption into a liquid is currently the most suitable option for capturing CO 2 [3, 5, 6]. The use of mono-ethanolamine (MEA) as a post-combustion capture (PCC) based solvent has found practical application in CO 2 separation from flue gases [3]. This is due to its low cost, its ability to capture CO 2 from low pressure flue gas and its fast reaction kinetics with CO 2. The PCC unit can also be retrofitted to an existing and/or easily integrated to a new power station [7-9]. A large amount of energy is required for solvent regeneration resulting in a large drop in power station efficiency [6, 8, 9]. Degradation and solvent losses are also identified as an important environmental and cost factor, particularly when it comes to commercial scale. Furthermore, in a financial cost analysis of an MEA-based PCC plant, Veawab et al. [10] concluded that absorption and desorption (solvent regeneration) sections are the major contributors to capital cost investment. They also specified that up to 70% of the total operating costs will be needed to provide the heat duty for the solvent regeneration (desorption section) [10]. The solvent scrubbing technique is considered to be the most advanced post-combustion capture technology [11]. A strategy of optimizing absorber conditions, in order to maintain a higher masstransfer rate by controlling minimum reboiler heat duty required, is a way to introduce a trade-off approach in order to make the PCC plant feasible. In this paper, discussion on initial focus to look at MEA based results as a baseline and an attempt to reduce regeneration energy (reboiler heat duty) 1
3 without impeding the CO 2 capture performance are presented. This may be achieved by utilizing MEA-based blends and/or a new mixture solvent as an alternative solvent. CSIRO has devised a transportable pilot plant based on MEA. For the Latrobe Valley Post-combustion Capture project CSIRO operates the pilot plant, based on amine technology, which is connected to flue gases from Victorian brown coal-fired power station at Loy Yang Power (2.2 GW). The objective of the PCC pilot plant trial program is to set a baseline based on 30 wt-% MEA and benchmark other solvents against this baseline. Up to date, our work has examined two different solvents, i.e., Blended Amine 1, which is a mixture of MEA and 2-amino-2-methyl-1-propanol (AMP) and Blended Amine 2, which is a proprietary solvent. The result for these two solvents are compared to the MEA baseline results in terms of CO 2 recovery and heat duty required. 2. Experimental 2.1. Chemicals and feed gas composition Concentrated MEA and NaOH 32 wt-% are obtained from Water Treatment Services (Aus) Pty Ltd. 20 wt-% MEA and 10 wt-% AMP are blended for making Blended Amine 1. The concentrated AMP is also supplied by the Water Treatment Services (Aus) Pty Ltd. Blended Amine 2 is supplied by RITE, Japan. These solvents (except NaOH 32 wt-%) were diluted with mains water prior to use. Table 1 shows important flue gas constituents and its concentration in the flue gas from Loy Yang Power used during the operation. Furthermore, the flue gas contains significant amounts of SO 2 and NO x. The SO 2 is washed off in the pre-treatment column with 32 wt-% NaOH Description and operation of the PCC pilot plant at Loy Yang Power The transportable PCC pilot plant at Loy Yang Power was designed to capture CO 2 from real flue gases using 30 wt-% MEA. Due to availability of the MEA-based result, it was decided to use the results for this solvent as the baseline case. The PCC pilot plant is able to receive 150 kg/h real flue gas from unit 2. The pilot plant consists of one flue gas pre-treatment unit, two absorber columns to capture the CO 2, a stripper column for stripping off the CO 2 from the solvent and a 120 kw electricboiler unit to generate steam for heating the media in the stripper s reboiler which uses a plate reboiler. The pilot plant has been operated with two absorbers in series (cf. Fig. 1). Two short absorbers were designed to provide a compact plant that is easily transportable. A 200DN stainless steel (211 mm ID) column is used for each absorber. The column height of each absorber column is 940 cm. Each column consists of two packed beds of pall rings, the height of a single bed being 135 cm. The height of the stripper column is 690 cm and it is made from 150DN stainless steel (161 mm ID). The stripper column contains a packed bed of pall rings with a height of 390 cm. During the operation, the level of liquid in each column is kept constant to ensure a constant liquid flow-rate. Water and solvent vapour released through the top of the stripper column are recovered in a 60 kw condenser. The vapour and condensed-liquid are separated in a flash drum situated near the solventfeed tank. The liquid phase is returned to the solvent feed tank after prior mixing with hot lean solvent from the stripper s bottom product. The vapour phase, containing about 98 vol-% CO 2, is delivered back to the flue gas duct. The mass flow of concentrated-co 2 gas is measured with a coriolis meter. 2
4 Fig. 1 gives a simplified flow diagram of the pilot plant. A portion of flue gases is taken-off by a blower before reaching in chimney. The flue gas is cooled and then flows into the knock-out drum where condensates and particulates are separated. The pilot plant consists of a flue gas pre-treatment unit, which use a caustic solution (32% NaOH), before the flue gas enters the absorber column 2. The average SO 2 concentration in the flue gas from the power station is around 200 ppmv and up to 98% is removed in the pre-treatment column. In series operation mode, the pre-treated flue gas from the pre-treatment column initially flows to the absorber column 2, where it is counter-currently contacted with a CO 2 -rich solvent from the bottom of absorber 1 at around C under atmospheric pressure. Then, the gas of low CO 2 content goes out from the top of absorber 2 to the bottom section of absorber 1 where it also counter-currently contacts with fresh CO 2 -lean solvent pumped from the solvent feed-tank. Absorption and chemical reactions between CO 2, a weak acid, and aqueous CO 2 -contained solvent, a weak base, occurs in these two absorber columns. The gas of low CO 2 content, so-called gas out or lean gas, is emitted from the top of absorber column 1 and is subsequently returned to the power plant s stack. The cold CO 2 -rich solvent stream emerging from the bottom of the absorber column 2 is pre-heated to C (the exact temperature depending on the T approach to be used) in a cross-solvent heat exchanger, utilizing the heat from the hot lean solvent (typically at C) flowing out of the bottom stripper column. This pre-heated solvent will be referred as hot rich solvent. Temperature of the hot lean solvent drops after releasing the heat into the cold rich solvent and is then further reduced with cooling water until it reaches the desired temperature before the lean solvent flows into the solvent-feed tank. From the solvent-feed tank, the solvent is then pumped into the absorber column 1. In the stripper column, water vapour and CO 2 separation from the solvent (through endothermic reaction) have occurred due to heat supplied from the reboiler (typically C). The reboiler heat is obtained from latent heat of the steam (at a steam pressure range of kpa). Typical stripper bottom pressures at given stripper bottom temperatures are presented in Table 2 below. Gas sampling points (indicated by G1 to G5) and liquid sampling points (indicated by L1 to L4) are installed at several points in the pilot plant as shown in Fig. 1 in order to determine species composition in gas streams and CO 2 loading in liquid streams respectively. Flue gas flow-rate at the blower inlet, solvent flow-rate and ph of the rich and lean solvent are monitored Experimental Program Three experimental campaigns with different solvents were carried out at the pilot plant. The aim of the first campaign was to evaluate the performance of 30 wt-% MEA, as a baseline result for our pilot plant. In this baseline operation, it should be noted that adequate CO 2 balance throughout the operation was achieved with typical fluctuations of ± 10% around the zero-point [12]. In the second campaign MEA was blended with AMP that has been reported [13, 14] to be useful in enhancing the loading capacity and lowering the binding energy, thereby decreasing the energy duty. This blended solvent was named Blended Amine 1. In the third campaign a novel proprietary amine blend was trialed; so-called Blended Amine 2, which has been developed by RITE (Japan). The two Blended Amines were examined in the pilot plant aiming at similar percentages of CO 2 recovery as compared to the baseline case with 30 wt-% MEA. Table 3 illustrates a range of processing conditions applied in the pilot plant in order to trial different solvents for CO 2 recovery. The performance of solvent is evaluated based on its ability in capturing CO 2 and the lowest reboiler heat duty necessary for solvent regeneration. In this study, a temperature 3
5 of 115 C was selected as maximum stripper bottom temperature in order to minimize thermal degradation and corrosion which will become excessively high at higher temperature. From Table 4, it can also be seen that there is an upper limit to the pressure in the stripper column because the temperature difference between top packing and bottom cannot be too small. Therefore, using the current design could not do better than that. To increase the pressure further, the packing height in the stripper column would have to be increased in order to improve CO 2 stripping and hence reduce the reboiler heat duty. In our pilot plant, even though stripper bottom temperature reduction is small, however, it can give upper packing temperature significantly different depending on the amount of water vapour flows. This, therefore, gives different amount of reboiler heat duty. In this paper, it also shows that operating the stripper column bottom at a temperature below 112 C gave a little of benefit as the CO 2 recovery will be smaller due to the increased lean loading even though it can give lower reboiler heat duty Gas and liquid analysis Gas analysis is conducted on-line by using a GASMET CEMS. The instrument incorporates a Fourier Transform Infrared spectrometer, a temperature controlled sample cell, and signal processing electronics. The gas analyser is designed for continuous emission monitoring (CEM). Gas components detected are H 2 O, CO 2, CO, N 2 O, NO, NO 2, SO 2, NH 3, HCl, CH 4, C 2 H 4, C 2 H 6, C 3 H 8, C 6 H 14, Formaldehyde, Acetaldehyde, Ethanol, Ethanolamine, HF and O 2. A liquid sample is usually collected within the time period when the absorption process is considered to have reached equilibrium, before the trial is stopped. Liquid samples, which represent lean (L1 and L4) and rich loadings (L2 and L3), are collected into heat and shock resistant glass bottles. The liquid sample is then delivered overnight to CSIRO Clayton laboratories for further analysis to determine CO 2 loading, total/free amine and CO 2 concentrations. An agilent 6850 series gas chromatograph with an automatic liquid sampler (8 samples) is used to determine CO 2 concentration and total/free amine by a volumetric titration method. The instrument is also equipped with an FID detector. The analysis conditions are 1 µl injection, 200 ml/min flowrate, 100:1 split; oven 110 C for 2 min, ramp at 20 o C/min to 250 C then hold for 5 min. The procedure is described below: CO 2 determination: A sample of absorber solution (~2.5 g) is weighed accurately into a vial. The ph of a methanol solution (~50 ml) is adjusted to ph 11 using NaOH (0.5 M). The MEA sample is added to the methanol solution and the mixture is titrated back to ph 11 with NaOH (0.5 M). The second volume of NaOH is used to calculate the amount of CO 2. Amine determination: Free MEA: A sample of MEA solution (~0.25 g) is weighed accurately and diluted with water to ~50 ml. The diluted sample is titrated against HCl (0.1 M) until the equivalence point is reached at ~ ph 4-5. Total MEA: A sample of MEA solution (~0.25 g) is weighed accurately and diluted with water to ~50 ml. A known excess of HCl (20 ml, 0.1 M) is added to the mixture. The mixture is titrated against NaOH 4
6 (0.5 M) until the equivalence point is reached. The volume of NaOH used gives the amount of HCl in excess, hence the amount of HCl that reacted with the MEA can be calculated to determine the total MEA in solution. The errors in the CO 2 loadings, CO 2 and amine concentrations were based on duplicate or triplicate determinations CO 2 recovery and the reboiler duty for solvent regeneration CO 2 recovery is determined based on analysing the gas at the inlet to absorber column 2 (G2), outlet of absorber column 1 (G4) and CO 2 product at the top of the stripper column (G5). When there were large moment to moment fluctuations in the CO 2 product at the top of stripper column, the average CO 2 recovery calculated based on gas analysis (G2 and G4) and liquid analysis was used. Reboiler heat duty for the regeneration of CO 2 -loaded solvent was determined by making an energy balance around the reboiler and/or the stripper column. Two approaches can be used to determine reboiler heat duty in these pilot plant tests. Firstly, actual reboiler heat duty was determined by a manual measurement of steam condensed flow and the steam pressure supplied to the reboiler. This actual reboiler heat duty does incorporate heat loss to the ambient. The actual reboiler heat duty is expressed per amount of CO 2 absorbed. Secondly, the energy supplied by the reboiler can be devided into three contributors [8, 12, 15-17]: the heat required for evaporating the water, the sensible heat to heat up the solvent to reboiler temperature (Q sh ) and the heat of CO 2 desorption (Q des ). The heat required to evaporate the water is actually equivalent to the latent heat of water condensation which can be measured directly around the condenser (Q cond ). The equation can be represented as follows; Q reboiler = = = Q condenser Q cond m H w + Q + Q solvent heat desorption + Q + Q sh des + m c (T w s p - T bottom top ) - m H CO CO 2 2 where Q reboiler is the reboiler heat duty; m w is the amount of water flowing into the condenser; H w is the latent heat of water condensation; ms is the solvent flow rate; c p is the heat capacity of the solvent; T is the temperature of hot lean solvent going out from the bottom of stripper column; bottom T top is the temperature of hot rich solvent entering the top of stripper column; mco is the amount of CO 2 2 produced from the stripper column and H CO is the enthalpy of CO 2 2 desorption. Due to large variations in the ambient temperature and to the fact that the pipelines were not insulated, it is not easy to obtain representative values of actual reboiler heat duty. Therefore, in this paper we express the reboiler heat duty using the second method excluding the heat loss to the ambient Error determination The absolute error or proportional error of each instrumentation/equipment is listed in Table 5. The systematic error for specific gas components is listed in Table 6 below. Errors for CO 2 - lean loading and CO 2 -rich loading were obtained from triplicate measurements. The error for CO 2 - lean loading and CO 2 -rich loading is 0.01 and 0.02 mol CO 2 /mol Amine, respectively. 5
7 The error for CO 2 recovery is varied between 1% to 2%. The error of reboiler heat duty is varied between 0.3 to 0.4 MJ/kg CO 2. The errors for Q cond, Q sh and Q des are 0.1, 0.2 and 0.3 MJ/kg CO Results and discussion 3.1. MEA baseline L/G ratio and lean loading affect CO 2 recovery The effect of varying the L/G ratio on CO 2 recovery at different stripper bottom temperatures, i.e. 112 and 115 C, is illustrated in Fig. 2. The graph implies that for both temperatures, an increase in L/G results in an increase of available solvent to capture CO 2 from the same amount of flue gas. Fig. 2 also shows the effect of the stripper bottom temperature over a range of L/G ratios on CO 2 recovery. Increasing the stripper bottom temperature from 112 C to 115 C elevates the CO 2 recovery over the range of L/G ratios tested. This indicates increasing the bottom stripper temperature led to increasing CO 2 strip-off from the stripper column and hence, lowering of the solvent loading. This suggests that raising the temperatures increases the driving force, which promotes CO 2 transfer from flue gas to the solvent. This finding is supported by Fig. 3. The figure indicates a combination effect between L/G ratio and solvent-lean loading. The figure shows that to obtain higher CO 2 recovery, the plant can be operated in three modes; (i) at a constant L/G ratio, circulate lower solvent-lean loading, (ii) at a constat solvent-lean loading rate, increase L/G ratio and (iii) circulate high solvent-lean loading at L/G ratio as high as possible. It should be noted, however, that to operate the plant at higher L/G ratio will elevate the energy requirement for the pumps and increase plant dimensions. In order to make the solvent leaner, there will have to be an increase in energy required for solvent regeneration in the stripper column (reboiler heat duty). Therefore, there should be a trade-off in order to decide optimum plant operation MEA baseline L/G ratio and lean loading affect reboiler heat duty The dependency of CO 2 recovery upon L/G ratio and lean loading should also translate into a relationship between reboiler heat duty on the one hand and the L/G ratio and the lean loading on the other. Fig. 4 shows the influence of L/G ratio on the reboiler heat duty of solvent regeneration. The reboiler heat duty of MEA baseline examined at a stripper bottom temperature of 115 C shows a parabolic trend as a function of L/G ratio. This finding was also reported by Mangalapally et al. [16] and Cifre et al. [8], where they presented reboiler heat duty as a function of solvent mass flow rate. Fig. 5 shows the effect of L/G ratio associated with the lean loading on the reboiler heat duty. At stripper bottom temperature of 115 C, the lean loading slightly changes across the L/G ratio ( ) tested from 0.17 to Similar changes in the lean loading (0.21 to 0.27) at different L/G ratio ( ) also noticed at 112 C. It is observed that at similar L/G ratio (3.22 and 3.19 at 115 C and 112 C respectively), the reboiler heat duty did not significantly change within the limits of error as their lean loading also did not change within the limits of error. A similar situation is also noticed for another similar L/G ratio (4.00 and 3.97 at 115 C and 112 C respectively). It shows, however, that the reboiler heat duty significantly increased even though at similar L/G ratio (2.32 and 2.34 at 115 C and 112 C respectively) and similar lean loading (0.19 and 0.21 at 115 C and 112 C respectively) within the limits of error. It is also noticed that the reboiler heat duty of L/G ratio at 4.2 increased as it has a low lean loading of The increase in the reboiler heat duty of both at lower L/G ratios (2.30 and 2.32) and higher L/G ratios (4.2) for 115 C can be explained in considering the three components of reboiler heat duty separately (see sub section 2.5 and Fig. 6A). The increase of reboiler heat duty at L/G ratio of 2.30 and 2.32 may be attributed to the extra heat removed by the cooling water in the condenser (Q cond ) as more water is 6
8 evaporated for stripping-off the CO 2, which in turn makes the solvent leaner (see Fig. 5). In contrast to what is observed at 115 C, Q cond for 112 C is very low compared to Q sh and Q des (see Fig. 6 Fig. 6B), indicating less water was evaporated than that at 115 C. The values of Q sh and Q des do not change within the limits of error from 112 C and 115 C. The major increase of Q cond for the 115 C experiment is associated with the reduction of lean loading to below 0.20 (top packing temperature around 110 C, see Fig. 7). The Q cond, however, does not change significantly at lean loading between 0.20 and The higher Q cond for 115 C (top packing temperature of 105 C) compared to that for 112 C (top packing temperature of 100 C), at a similar lean loading of 0.21, may be attributed to the increase in top packing temperature (cf. Fig. 7) as a consequence of the increase in the steam flow to the top of the stripper column due to the stripper bottom temperature increase. The raise in reboiler heat duty for the L/G above 3.8 is due to an increase in the solvent flow, hence increasing the sensible heat (Q sh ). For L/G at 4.2, not only Q sh, but also Q cond contributes to the increase in reboiler heat duty. The guide-line in Fig. 4 shows that the reboiler heat duty is a minimum at L/G around 3.2 for 115 C stripper bottom temperature. As seen in Fig. 6 Fig. 6, the Q cond contribution at this L/G value is small compared to the Q sh associated with heat up of the solvent and the heat of desorption (Q des ). The amount of Q des varied little with L/G, because the Q des for CO 2 -loadings below 0.5 is relatively constant even with temperature changes [16]. Fig. 4 also indicates that, for the stripper bottom temperature of 112 C, reboiler heat duty is not significantly affected by L/G ratio (2.34 to 3.97) and lean loading (0.21 to 0.27) (cf. Fig. 5). Fig. 7 also indicates that the changes of lean loading (0.21 to 0.27) did not give significant enhancement in Q cond within the limits of error, which in turn reflects a small amount of steam leaving the stripper column A comparison between MEA alone and Blended Amine solvent CO 2 -lean loading and CO 2 - rich loading Table 7 shows lean and rich CO 2 -loadings for MEA alone, Blended Amines 1 and 2 at different stripper bottom temperatures. In general, CO 2 absorption into solvent occurred mainly in absorber column 1 rather than in column 2. This is because the CO 2 -solvent loading into column 1 is lower than that going into column 2 and hence, the driving force of CO 2 -mass transfer in column 1 is higher than that in column 2. For all solvents tested in the pilot plant, the CO 2 -lean loading also reduces as the stripper bottom temperature increases. It can be seen from the Table that at 115 C, MEA has higher lean loading amounts than those of blended amines. The difference of lean loading between MEA and Blended Amine 1, however, was not significantly large at 112 C. Blended Amine 2 has lean loading much lower than those of MEA and Blended Amine 1 at both stripper bottom temperatures. At 112 C, the lean loading values for Blended Amine 1 are also comparable to those for MEA at 115 C. For Blended Amine 2, however, the lean loading did not change as the stripper bottom temperature was reduced from 115 C to 112 C, but, the lean loading did increase as the temperature dropped further to 107 C. There are two factors that can explain the difference among the solvents. The first factor is heat of absorption. It can be assumed that the amount of heat required for reaction with CO 2 is similar to the heat required for breaking the CO 2 -Amine bonds. Among the three alkanolamines tested in the pilot plant, MEA has the highest heat of reaction with CO 2. It requires an energy of about 1920 kj/kg CO 2 [3]. It is noted that Blended Amine 2 properties data cannot be shown as it is a proprietary solvent and readers are referred to papers published by RITE. Our estimation reveals that Blended Amine 1 needs 7
9 kj/kg CO 2, indicating the difference in heat of reaction between MEA and Blended Amine 1 is not large, but this suggests that in the blended amines amine-co 2 bonds break more easily than in the MEA at a similar amount of heat supplied. The second factor is the heat required to generate steam. The steam is needed to create a driving force for CO 2 stripping in the stripper column. Using a model developed in our group [18], we can also estimate an equilibrium CO 2 partial pressure of MEA, AMP and Blended Amine 1 as described in Fig. 8. The Figure indicates that MEA requires a lower operating CO 2 partial pressure than Blended Amine 1 and AMP, hence MEA needs the largest heat consumption to create steam. At a given CO2 loading the order of equilibrium CO 2 partial pressures is Blended Amine 2 > Blended Amine 1 > MEA. In term of rich solvent and cyclic capacity (the difference between rich solvent from column 2 minus lean solvent), Blended Amine 1 has higher capacity than those of MEA and Blended Amine A comparison between MEA alone and Blended Amine solvent L/G ratio and lean loading affect CO 2 recovery and reboiler heat duty It would be desirable to seek a remedy for the MEA solvent s drawback in terms of energy used for regeneration. An examination of a mixture solvent, which was intended to show benefits in terms of adequate CO 2 absorption capacity and lower reboiler heat duty, might be one of the counter-measures to this shortcoming. Pilot plant results from previous workers [5, 19] have shown that a blended solvent has a lower heat of desorption than a single solvent, which can contribute to reduce the reboiler heat duty. In this study, the behavior of Blended Amines 1 and 2 was examined as a function of L/G ratio and of stripper bottom temperature. The effect of changing the stripper bottom temperature on solvent lean loading was also examined. The performance of blended solvents is expressed by the CO 2 recovery and the reboiler duty. They are compared with the MEA baseline. The amine concentration of the blended solvent was maintained constant throughout the campaign. Fig. 9A and 9B show the variation of CO 2 recovery with L/G ratio at different stripper bottom temperatures for both Blended Amine solvents. Fig. 9A indicates that, for both Blended Amines tested under the highest stripper s bottom temperature (115 C) the magnitudes of CO 2 recovery are comparable to that of the MEA baseline at 115 C. Fig. 9B shows that for Blended Amine solvent 1 operating at L/G below 4.0, a stripper bottom temperature reduction from 115 C to 112 C and 109 C significantly decreased the CO 2 recovery compared to those of MEA and Blended Amine 2 at 115 C. A comparable CO 2 recovery to that of MEA at 115 C, however, can be obtained when Blended Amine 1 is circulated at higher L/G ratio. It is noted that for Blended Amine 2, the CO 2 recovery is not greatly lowered when the stripper bottom temperature lowered to112 C.. The effect of lowering the stripper bottom temperature on the CO 2 recovery reductions of Blended Amine 1 may be associated with increasing the solvent lean loading. Fig. 10 shows that a reduction of stripper bottom temperature raises the lean loading. For all temperatures examined, at a relatively constant lean loading, increasing the L/G ratio improves the CO 2 recovery. The figure also shows that operating the stripper bottom temperature at 115 C for Blended Amine 1 resulted in an increase of reboiler heat duty, which in turn produced much leaner solvent compared to that of MEA at 115 C (cf. Table 7). This suggests that 115 C is an unfavourable operating condition for Blended Amine 1. The graph also indicates that operating the stripper bottom temperature at 112 C for this solvent gave a comparable lean loading to that of MEA at 115 C (cf. Table 7) and also significantly lowered the reboiler heat duty compared to that of operating at 115 C. 8
10 For 115 C, as L/G ratio increased, the reboiler heat duty decreased significantly. The reboiler heat duty might be expected increased if the L/G ratio increases. Nevertheless, the magnitude of reboiler heat duty shows large difference across the L/G ratio compared to that of MEA at 115 C. The reboiler heat duty as a function of L/G ratio for Blended Amine 1 at 112 C did not change significantly within the limits of error. The reboiler heat duty for Blended Amine 1 at 112 C is comparable to that for MEA at the same temperature. Further reduction of temperature to 109 C did not lower the reboiler heat duty significantly even though the CO 2 recovery was significantly reduced. It is also observed that the effect of L/G changes is not sensitive to the changes of reboiler heat duty at 109 C, as all L/G values generated similar lean loadings within the limit of error. Fig. 11 also shows that the reduction of stripper bottom temperature for Blended Amine 2 led to a decrease in the reboiler heat duty regardless of L/G ratio value even though the change in temperatures had little effect on the lean loading within the limits of error (except for lean loading at 112 C and L/G ratio 3.56, (cf. Table 7). Both temperatures show similar trends in the increase of reboiler heat duty as a function of increasing the L/G ratio. Further trials could be conducted at lower L/G ratio in order to investigate whether the reboiler heat duty at low L/G ratio increases as in the case of MEA at 115 C and Blended Amine 1 at 112 C. Operating at the L/G ratio lower than 2.00, however, will not benefit CO 2 recovery so that such trials would not have any practical value. It can also be seen in Fig. 12 Fig. 12 that to achieve 85% CO 2 recovery, MEA trialled at 115 C with L/G of 4.12 requires the reboiler heat duty of 5.1 MJ/kg CO 2. Both MEA at 112 C and Blended Amine 1 at 109 C, however, require L/G ratios higher than 6.0 (cf. Fig. 9B) in order to attain similar CO 2 recovery, which may not favourable from a practical point of view. If Blended Amine 1 is operated at 115 C, the reboiler heat duty, unfortunately, rises to 10.5 MJ/kg CO 2, which is also not feasible. However, the reboiler heat duty of Blended Amine 2 at the same temperature with L/G ratio of 3.60 has a similar value to that of MEA. It is also notable that the reduction of temperature to 112 C decreases the reboiler heat duty of both Blended Amines 1 (L/G = 4.01) and 2 (L/G = 3.61) by 11% and 14%, respectively. In order to obtain 90%-95% CO 2 recovery, the reboiler heat duty of MEA at 115 C with L/G = 4.20 increases by 17% compared to that of required to get 85% CO 2 recovery. This increase is mainly due to the increase of condenser duty (cf. Fig. 6A Fig. 6) as indicated earlier. In comparison with MEA at 115 C, the reboiler heat duty of Blended Amines 1 (at L/G = 3.95) is higher by 2.0 MJ/kg CO 2 (increase of 49%) and the reboiler heat duty of Blended Amine 2 (at L/G = 3.52) is a little lower by 0.6 MJ/kg CO 2 (reduction of 5%). If Blended Amine 1 is operated at 112 C with L/G ratio of 5.02, it has a reboiler heat duty 18% lower than that of MEA at 115 C. Blended Amine 2 at 112 C may possibly give a similar reboiler heat duty to that of Blended Amine 1 at 112 C if the L/G ratio is raised, to a higher value than that of MEA at 115 C (L/G of 4.12) with the CO 2 recovery of 85%. 4. The behaviour of component reboiler heat duty distributions for MEA, Blended Amines 1 and 2 It is found (section 3) that the reboiler heat duty trend demonstrates different patterns with regard to changes in L/G ratios and lean loadings for the three solvents. The three energy contributions to the reboiler heat duty (section 2.5) can be used to elucidate the differences in behaviour of reboiler heat duty. 9
11 Fig. 13 shows the three different components of heat duty for MEA, Blended Amines 1 and 2 as a function of L/G ratio at different stripper bottom temperatures and CO 2 recovery. The Q con for Blended Amine 1 at 115 C is higher than any other component energy. Q con for Blended Amine 1 at 115 C possibly slightly increases at L/G ratios higher than This trend is also noticed for the reboiler heat duty for MEA at 115 C and Blended Amine 1 at 112 C, suggesting that the heat of water vaporization for CO 2 stripping, which is equivalent to Q con, is a major factor in increasing the reboiler heat duty. Fig. 13 also shows that the Q con for Blended Amine 1 at all L/G ratios are higher than others when they are trialled at 115 C. MEA at 115 C and Blended Amine 1 at 112 C also reached minimum reboiler heat duty at L/G ratios around Thus the Q con for MEA at 115 C and Blended Amine 1 at 112 C has a similar pattern to their total reboiler heat duty. The Blended Amine 1 at 112 C has slightly lower Q con than MEA at 115 C, suggesting that relatively less heat of water vaporization is required for Blended Amine 1 to obtain similar lean loading to that of MEA at 115 C (cf. Table 7). This is also indicated in Fig. 14. The chart reveals that MEA at 115 C generated more steam compared to Blended Amine 1 at 112 C in order to achieve similar CO 2 lean loading (0.18~0.19). The greater amount of steam generated results in the raising of Q con. As the consequence of increasing the steam flows to the top of the stripper column, the temperature at the top packing in the stripper column also rises. Thus, at the same CO 2 -lean loading (0.18~0.19), MEA at 115 C gave higher Q con than Blended Amine 1 at 112 C as can be noted from Fig. 15. Both Q con for MEA at 112 C and Blended Amine 1 at 109 C are relatively constant at different L/G ratios. The magnitude of the Q con for both of them are also lower compared to others. Nevertheless, for Blended Amine 1 across the operating L/G ratio range up to 4.10, the proportions of condenser heat in the reboiler duty is generally less than those of the individual sensible heat and desorption heat (cf. Fig. 12). Yet, for the highest L/G ratio, the three component energies are similar. For Blended Amine 2 at 112 C and 115 C, in general they have similar pattern of Q con to their reboiler heat duty. The Q con for both temperatures are initially constant and then steadily increase at a higher L/G ratio. It is noticed that Q con at 112 C is lower than that of at 115 C, as expected. The Q con of Blended Amine 2 at 115 is comparable to the value of Q con from MEA at 115 C (at lower L/G ratio) and Blended Amine 1 at 112 C at lower and higher L/G ratios. The magnitude of Q con for Blended Amine 2 at 112 C is also similar within the limits of error compared to that of Blended Amine 1 at 112 C. For Blended Amine 2 at 112 C and 115 C, it is noticed that the sensible heat and the heat of desorption are nearly constant from the lowest L/G ratio up to an L/G ratio of 2.6. In general, the heat of water vaporization is much lower than the other components of reboiler heat duty. The Q con does steadily increase as the L/G ratio increases. For Blended Amine 2, the sensible heat also rises after L/G ratio 0.26, which is attributed to the increasing of solvent flow rate. The heat of desorption is apparently levells-off regardless of L/G ratio values. Blended Amines 1 and 2 are similar in that the magnitude of desorption heat is slightly higher than that of sensible heat. The Figure also shows that for Blended Amine 2, the three energy components make similar contributions to the reboiler heat duty. The sensible heat (Q sh ) for all solvents at different conditions is similar within the limits of error, except for Blended Amine 1 at 115 C, where the Q sh steadily decreases as L/G ratio increases and then starts to level-off above a L/G ratio of 3.0 (within the limits of error). This may be attributed to the temperature difference between the cold-rich solvent entering the stripper column and the hot-lean solvent leaving the stripper bottom not being constant. 10
12 Fig. 13 also shows that MEA at both temperatures has the highest heat of CO 2 desorption (Q des ) compared to the other solvents, suggesting that the Q des has contributed to increase reboiler heat duty for the MEA at both 112 C and 115 C. In this study, the magnitude of desorption heat decreases in the following order: MEA > Blended Amine 1 > Blended Amine 2. For Blended Amine 2, the Q des has more contribution than other component energy to reduce the reboiler heat duty. 5. Conclusion Performance tests of mono-ethanolamine (MEA) and Blended Amine solvents were carried out in CSIRO s transportable PCC pilot plant. The plant is fed with real flue gas from the brown coal-fired power station of Loy Yang Power in the Latrobe Valley, Victoria, Australia. Several campaigns have been devised to determine the solvent performance against the CO 2 absorption capacity (CO 2 recovery) and the requirement of reboiler duty in stripper column. The effect of increasing the stripper bottom temperature is to decrease the lean loading of the solvent. A reduction of lean solvent loading to below 0.20 for all solvents leads to an increase in the reboiler heat duty. This suggests that increasing the lean loading reduced the reboiler heat duty. A value of lean solvent loading above 0.20 for all solvents has less effect on the change of reboiler heat duty. In general, the magnitude of CO 2 recovery for all sets of solvents tested in the pilot plant is dependent on the L/G ratio and solvent lean loading. It is shown that a solvent with a higher lean loading requires a greater solvent (recycle) flow rate, or vice versa, in order to capture a given amount of CO 2. For Blended Amine 1, the operating temperature of stripper bottom at 112 C at L/G ratio between 4.0 to 5.0 is favourable because it can reach high CO 2 recovery at the low reboiler heat duty compared to MEA at 115 C. Even this reboiler heat duty is not higher than that when operating at 109 C. Because Blended Amine 2 has similar recovery for operating at 112 Cand 115 C, a lower temperature will result in reduction of the reboiler heat duty. Blended Amine 1 has higher CO 2 capacity and richer solvent loading than those of MEA and Blended Amine 2. The reboiler duty is also significantly dependent upon the L/G ratio and solvent lean loading. For all solvents at higher temperature, the contribution of Q cond is large compared to that of Q sh and Q des. For MEA and Blended Amine 1, the relationship between Q cond and L/G ratio shows a parabolic trend resembling to that of their total reboiler heat duty. MEA needs the highest reboiler duty followed by Blended Amine 1 and then Blended Amine 2. To achieve a similar CO 2 recovery of 84-85%, the Blended Amines 1 and 2 require 11% and 14% less reboiler duty than that of MEA, respectively ACKNOWLEDGEMENTS The authors gratefully acknowledge the PCC team from RITE and Chiyoda Corporation for their invaluable co-operation during campaign 4 Blended Amine 2, which has been developed by RITE within the COCS project supported by Ministry of Economy, Trade and Industry in Japan. Also thanks to Loy Yang Power for hosting the CSIRO s PCC pilot plant on their site and for having fruitful discussions. CSIRO s contribution in the LVPCC project was performed within CSIRO's Advanced Coal Technology and it is supported by the Victorian State Government through the Energy Technology Innovation Subsidy (ETIS) and Loy Yang Power Management Pty Ltd. 11
13 REFERENCES [1] Energy for Victoria: A Statement by the Minister for Energy and Resources final report submitted to Department of Natural Resources and Environment. 2002; quoted in Energy for Victoria: A Statement by the Minister for Energy and Resources [2] Bradshae J. GEODISC Project 1: Regional Analysis Final Report. APCRC Report, final report submitted to CO2CRC - CRC for Greenhouse gas Technologies. 2003; quoted in GEODISC Project 1: Regional Analysis Final Report. APCRC Report, [3] Kohl AL, Nielsen RB. Gas Purification, 5th. Houston, Texas. Gulf Publishing Company; 1997 [4] Aroonwilas A, Veawab A. Energy Procedia 2009; 1: [5] Idem R, Wilson M, Tontiwachwuthikul P, Chakma A, Veawab A, Aroonwilas A, Gelowitz D. Ind. Eng. Chem. Res. 2006; 45: [6] Chakma A, Mehrotra AK, Nielsen B. Heat Recovery Systems and CHP 1995; 15: 231. [7] Cottrell AJ, McGregor JM, Jansen J, Artanto Y, Dave N, Morgan S, Pearson P, Attalla MI, Wardhaugh L, Yu H, Allport A, Feron PHM. Energy Procedia 2009; 1: [8] Cifre PG, Brechtel K, Unterberger S, Scheffknecht G. "Experimental studies on CO 2 desorption from amine solutions" 2009, Clean Coal Technologies 2009 (CCT2009), Dresden, Germany. [9] Feron PHM. Energy Procedia 2009; 1: [10] Veawab A, Tontiwachwuthikul P, Aroonwilas A, Chakma A, Gale J, Kaya Y. Performance and Cost Analysis for CO 2 Capture from Flue Gas Streams: Absorption and Regeneration Aspects. Greenhouse Gas Control Technologies - 6th International Conference. Oxford: Pergamon; 2003, p.127. [11] Davison J. Energy 2007; 32: [12] Meuleman EEB, Artanto Y, Jansen J, Osborn M, Pearson P, Cottrell AJ, Feron PHM. In: Proceedings of the 35th International Technical Conference on Clean Coal and Fuel Systems. Sheraton Sand Key Clearwater, Florida, USA: June , [13] Xiao J, Li C-W, Li M-H. Chemical Engineering Science 2000; 55: 161. [14] Dey A, Aroonwilas A. Energy Procedia 2009; 1: 211. [15] Goto K, Okabe H, Shimizu S, Onoda M, Fujioka Y. Energy Procedia 2009; 1: [16] Mangalapally HP, Notz R, Hoch S, Asprion N, Sieder G, Garcia H, Hasse H. Energy Procedia 2009; 1: 963. [17] Kvamsdal HM, Jakobsen JP, Hoff KA. Chemical Engineering and Processing: Process Intensification 2009; 48: 135. [18] Puxty G, Rowland R. Environmental Science & Technology 2011; 45: [19] Sakwattanapong R, Aroonwilas A, Veawab A. Ind. Eng. Chem. Res. 2005; 44: [20] Dugas RE. Pilot Plant Study of Carbon Dioxide Capture by Aqueous Monoethanolamine, M.S.E, The University of Texas at Austin,
14 Fig. 1. Typical Flow Diagram of PCC Pilot Plant at Loy Yang Power 13
15 100% 90% 115 o C CO 2 recovery 80% 70% 60% 112 o C 50% 40% L/G (L/Nm 3 ) Fig. 2. Correlation between L/G and CO 2 recovery for two different stripper s bottom temperatures 100% 90% 115 o C CO 2 recovery 80% 70% 60% 112 o C 50% 40% Lean loading (mol CO 2 /mol MEA) L/G = L/G = L/G = Fig. 3. Correlation between lean loading MEA solvent at similar L/G on CO 2 recovery 14
16 6.5 Heat duty (MJ/kg CO 2 ) o C 112 o C L/G (L/Nm 3 ) Fig. 4. Correlation between L/G and reboiler heat duty for two different stripper bottom temperatures Heat duty (MJ/kg CO 2 ) L/G (L/Nm 3 ) Lean loading (mol CO 2 /mol Amine) Duty C Duty C Lean loading C Lean loading C Fig. 5. The effect of L/G ratio and solvent-lean loading on reboiler heat duty 15
17 Component of heat duty (MJ/kg CO 2 ) Component of heat duty (MJ/kg CO 2 ) 6.0 A B L/G (L/Nm 3 ) Total heat duty Q condenser Q solvent heat Q desorption Fig. 6. Reboiler duty distribution as a function of L/G ratio for MEA baseline (A: 115 C, B: 112 C) 16
CO 2 CAPTURE PERFORMANCE OF MEA AND BLENDED AMINE SOLVENTS IN CSIRO S PILOT PLANT WITH FLUE GAS FROM A BROWN COAL-FIRED POWER STATION
 CO 2 CAPTURE PERFORMANCE OF MEA AND BLENDED AMINE SOLVENTS IN CSIRO S PILOT PLANT WITH FLUE GAS FROM A BROWN COAL-FIRED POWER STATION Erik Meuleman, Yuli Artanto, James Jansen, Mick Osborn, Pauline Pearson,
CO 2 CAPTURE PERFORMANCE OF MEA AND BLENDED AMINE SOLVENTS IN CSIRO S PILOT PLANT WITH FLUE GAS FROM A BROWN COAL-FIRED POWER STATION Erik Meuleman, Yuli Artanto, James Jansen, Mick Osborn, Pauline Pearson,
A preliminary evaluation of post-combustion CO 2 capture in a CSIRO pilot plant using MEA at Loy Yang Power in Australia
 A preliminary evaluation of post-combustion CO 2 capture in a CSIRO pilot plant using MEA at Loy Yang Power in Australia Fourth International Conference on Clean Coal Technologies (CCT2009) Dresden, Germany,
A preliminary evaluation of post-combustion CO 2 capture in a CSIRO pilot plant using MEA at Loy Yang Power in Australia Fourth International Conference on Clean Coal Technologies (CCT2009) Dresden, Germany,
CSIRO Post-Combustion Capture Pilot Plant Operations in Australia. CO2CRC Research Symposium 1-3 December 2009 Ashleigh Cousins
 CSIRO Post-Combustion Capture Pilot Plant Operations in Australia CO2CRC Research Symposium 1-3 December 2009 Ashleigh Cousins Overview PCC program at CSIRO Overview of Australian pilot plant operations
CSIRO Post-Combustion Capture Pilot Plant Operations in Australia CO2CRC Research Symposium 1-3 December 2009 Ashleigh Cousins Overview PCC program at CSIRO Overview of Australian pilot plant operations
Analysis of combined process flow sheet modifications for energy efficient CO 2 capture from flue gases using chemical absorption
 Available online at www.sciencedirect.com Energy Procedia 4 (2011) 1331 1338 Energy Procedia 00 (2010) 000 000 www.elsevier.com/locate/procedia www.elsevier.com/locate/xxx GHGT-10 Analysis of combined
Available online at www.sciencedirect.com Energy Procedia 4 (2011) 1331 1338 Energy Procedia 00 (2010) 000 000 www.elsevier.com/locate/procedia www.elsevier.com/locate/xxx GHGT-10 Analysis of combined
CSIRO PCC pilot plant research in Australia
 CSIRO PCC pilot plant research in Australia Aaron Cottrell, PCC pilot plant project manager, CSIRO PCC Science & Technology seminar, Tuesday 26 March 2013 Energy Technology Research Partners CSIRO PCC
CSIRO PCC pilot plant research in Australia Aaron Cottrell, PCC pilot plant project manager, CSIRO PCC Science & Technology seminar, Tuesday 26 March 2013 Energy Technology Research Partners CSIRO PCC
Design Parameters Affecting the Commercial Post Combustion CO 2 Capture Plants
 Available online at www.sciencedirect.com Energy Procedia 37 (2013 ) 1517 1522 GHGT-11 Design Parameters Affecting the Commercial Post Combustion CO 2 Capture Plants Ahmed Aboudheir * and Walid Elmoudir
Available online at www.sciencedirect.com Energy Procedia 37 (2013 ) 1517 1522 GHGT-11 Design Parameters Affecting the Commercial Post Combustion CO 2 Capture Plants Ahmed Aboudheir * and Walid Elmoudir
Available online at ScienceDirect. Energy Procedia 63 (2014 ) GHGT-12. District, Beijing, , China
 Available online at www.sciencedirect.com ScienceDirect Energy Procedia 63 (2014 ) 1399 1406 GHGT-12 Amine based post-combustion capture technology advancement for application in Chinese coal fired power
Available online at www.sciencedirect.com ScienceDirect Energy Procedia 63 (2014 ) 1399 1406 GHGT-12 Amine based post-combustion capture technology advancement for application in Chinese coal fired power
Modelling of post combustion capture plant flexibility
 Modelling of post combustion capture plant flexibility Workshop on operating flexibility of power plants with CCS Hanne Kvamsdal London November 11-12, 2009 1 Outline Background and motivation Dynamic
Modelling of post combustion capture plant flexibility Workshop on operating flexibility of power plants with CCS Hanne Kvamsdal London November 11-12, 2009 1 Outline Background and motivation Dynamic
Post-combustion CO 2 Capture An overview of CSIRO activities
 Post-combustion CO 2 Capture An overview of CSIRO activities Paul Feron 15 May 2012 CSIRO ENERGY TECHNOLOGY / ADVANCED COAL TECHNOLOGY Content Presentation Introduction Overview of PCC programme at CSIRO
Post-combustion CO 2 Capture An overview of CSIRO activities Paul Feron 15 May 2012 CSIRO ENERGY TECHNOLOGY / ADVANCED COAL TECHNOLOGY Content Presentation Introduction Overview of PCC programme at CSIRO
Fluor s Econamine FG Plus SM Technology
 Flue Gas Fluor s Econamine FG Plus SM Technology An Enhanced Amine-Based CO 2 Capture Process NETL Carbon Sequestration Conference May 2003 to Atmos Reflux Condenser 1 Amine Filter Package FI W CW Stripper
Flue Gas Fluor s Econamine FG Plus SM Technology An Enhanced Amine-Based CO 2 Capture Process NETL Carbon Sequestration Conference May 2003 to Atmos Reflux Condenser 1 Amine Filter Package FI W CW Stripper
Comparative evaluation of a new liquid absorbent in a
 Comparative evaluation of a new liquid absorbent in a PCC pilot plant in China PCCC3, Regina, September 2015 ENERGY Paul Feron, Will Conway, Graeme Puxty, Leigh Wardhaugh, Phil Green, Dan Maher, Debra
Comparative evaluation of a new liquid absorbent in a PCC pilot plant in China PCCC3, Regina, September 2015 ENERGY Paul Feron, Will Conway, Graeme Puxty, Leigh Wardhaugh, Phil Green, Dan Maher, Debra
Aspen plus simulation of CO 2 removal from coal and gas fired power plants
 Available online at www.sciencedirect.com Energy Procedia 23 (2012 ) 391 399 Trondheim CCS Conference (TCCS-6) Aspen plus simulation of CO 2 removal from coal and gas fired power plants Udara Sampath P.R.Arachchige
Available online at www.sciencedirect.com Energy Procedia 23 (2012 ) 391 399 Trondheim CCS Conference (TCCS-6) Aspen plus simulation of CO 2 removal from coal and gas fired power plants Udara Sampath P.R.Arachchige
Advances in Engineering Research (AER), volume 111 3rd Annual 2017 International Conference on Sustainable Development (ICSD2017)
 Advances in Engineering Research (AER), volume 111 3rd Annual 2017 International Conference on Sustainable Development (ICSD2017) Amine Regeneration Tests on MEA, DEA and MMEA with Respect to Energy Efficiency
Advances in Engineering Research (AER), volume 111 3rd Annual 2017 International Conference on Sustainable Development (ICSD2017) Amine Regeneration Tests on MEA, DEA and MMEA with Respect to Energy Efficiency
Simulation of CO 2 capture from an aluminium production plant
 Environmental Impact II 729 Simulation of CO 2 capture from an aluminium production plant 1 1 1 1 S. Dayarathna, A. Weerasooriya, S. Hussain, M. Zarsav, A. Mathisen 2, H. Sørensen 2 & M. C. Melaaen 1 1
Environmental Impact II 729 Simulation of CO 2 capture from an aluminium production plant 1 1 1 1 S. Dayarathna, A. Weerasooriya, S. Hussain, M. Zarsav, A. Mathisen 2, H. Sørensen 2 & M. C. Melaaen 1 1
Thermodynamic analysis on post combustion CO 2 capture of natural gas fired power plant
 Thermodynamic analysis on post combustion CO 2 capture of natural gas fired power plant Abstract Zeinab Amrollahi, 1 Ivar S. Ertesvåg, Olav Bolland Department of Energy and Process Engineering, Norwegian
Thermodynamic analysis on post combustion CO 2 capture of natural gas fired power plant Abstract Zeinab Amrollahi, 1 Ivar S. Ertesvåg, Olav Bolland Department of Energy and Process Engineering, Norwegian
Sandhya Eswaran, Song Wu, Robert Nicolo Hitachi Power Systems America, Ltd. 645 Martinsville Road, Basking Ridge, NJ 07920
 ABSTRACT COAL-GEN 2010 Advanced Amine-based CO 2 Capture for Coal-fired Power Plants Sandhya Eswaran, Song Wu, Robert Nicolo Hitachi Power Systems America, Ltd. 645 Martinsville Road, Basking Ridge, NJ
ABSTRACT COAL-GEN 2010 Advanced Amine-based CO 2 Capture for Coal-fired Power Plants Sandhya Eswaran, Song Wu, Robert Nicolo Hitachi Power Systems America, Ltd. 645 Martinsville Road, Basking Ridge, NJ
CONTROL STRTEGIES FOR FLEXIBLE OPERATION OF POWER PLANT INTEGRATED WITH CO2 CAPTURE PLANT
 CONTROL STRTEGIES FOR FLEXIBLE OPERATION OF POWER PLANT INTEGRATED WITH CO2 CAPTURE PLANT Yu-Jeng Lin a, Chun-Cheng Chang a, David Shan-Hill Wong a Shi-Shang Jang a * and Jenq-Jang Ou b a National Tsing-Hua
CONTROL STRTEGIES FOR FLEXIBLE OPERATION OF POWER PLANT INTEGRATED WITH CO2 CAPTURE PLANT Yu-Jeng Lin a, Chun-Cheng Chang a, David Shan-Hill Wong a Shi-Shang Jang a * and Jenq-Jang Ou b a National Tsing-Hua
Available online at Energy Procedia 4 (2011) Energy Procedia 00 (2010) GHGT-10
 Available online at www.sciencedirect.com Energy Procedia 4 (2011) 1683 1690 Energy Procedia 00 (2010) 000 000 Energy Procedia www.elsevier.com/locate/procedia www.elsevier.com/locate/xxx GHGT-10 Optimum
Available online at www.sciencedirect.com Energy Procedia 4 (2011) 1683 1690 Energy Procedia 00 (2010) 000 000 Energy Procedia www.elsevier.com/locate/procedia www.elsevier.com/locate/xxx GHGT-10 Optimum
PRELIMINARY ANALYSIS OF PROCESS FLOW SHEET MODIFICATIONS FOR ENERGY EFFICIENT CO 2 CAPTURE FROM FLUE GASES USING CHEMICAL ABSORPTION
 Distillation Absorption 2010 A.B. de Haan, H. Kooijman and A. Górak (Editors) All rights reserved by authors as per DA2010 copyright notice PRELIMINARY ANALYSIS OF PROCESS FLOW SHEET MODIFICATIONS FOR
Distillation Absorption 2010 A.B. de Haan, H. Kooijman and A. Górak (Editors) All rights reserved by authors as per DA2010 copyright notice PRELIMINARY ANALYSIS OF PROCESS FLOW SHEET MODIFICATIONS FOR
Modelling and Simulation of a Coal-fired Supercritical Power Plant Integrated to a CO 2 Capture Plant
 Energy Technology and Innovation Initiative (ETII) FACULTY OF ENGINEERING UNIVERSITY OF LEEDS Modelling and Simulation of a Coal-fired Supercritical Power Plant Integrated to a CO 2 Capture Plant Elvis
Energy Technology and Innovation Initiative (ETII) FACULTY OF ENGINEERING UNIVERSITY OF LEEDS Modelling and Simulation of a Coal-fired Supercritical Power Plant Integrated to a CO 2 Capture Plant Elvis
Post-Combustion Capture (PCC) R&D and Pilot Plant Operation in Australia. IEA GHG 11th Post Combustion CO 2 Capture Network Meeting
 Post-Combustion Capture (PCC) R&D and Pilot Plant Operation in Australia IEA GHG 11th Post Combustion CO 2 Capture Network Meeting Vienna, Austria, 20-21 May 2008 Overview Clean Coal Technologies in Australia
Post-Combustion Capture (PCC) R&D and Pilot Plant Operation in Australia IEA GHG 11th Post Combustion CO 2 Capture Network Meeting Vienna, Austria, 20-21 May 2008 Overview Clean Coal Technologies in Australia
Optimization of an Existing 130 Tonne per Day CO 2 Capture Plant from a Flue Gas Slipstream of a Coal Power Plant
 Available online at www.sciencedirect.com Energy Procedia 37 (2013 ) 1509 1516 GHGT-11 Optimization of an Existing 130 Tonne per Day CO 2 Capture Plant from a Flue Gas Slipstream of a Coal Power Plant
Available online at www.sciencedirect.com Energy Procedia 37 (2013 ) 1509 1516 GHGT-11 Optimization of an Existing 130 Tonne per Day CO 2 Capture Plant from a Flue Gas Slipstream of a Coal Power Plant
Overall Process Analysis and Optimisation for CO2 Capture from Coal Fired Power Plants based on Phase Change Solvents Forming Two Liquid Phases
 Overall Process Analysis and Optimisation for CO2 Capture from Coal Fired Power Plants based on Phase Change Solvents Forming Two Liquid Phases Ulrich Liebenthala,* ahamburg University of Technology, Institute
Overall Process Analysis and Optimisation for CO2 Capture from Coal Fired Power Plants based on Phase Change Solvents Forming Two Liquid Phases Ulrich Liebenthala,* ahamburg University of Technology, Institute
Fluor's Econamine FG Plus SM Technology
 Fluor's Econamine FG Plus SM Technology An Enhanced Amine-Based CO 2 Capture Process Satish Reddy Jeff Scherffius Stefano Freguia Fluor Enterprises, Inc. Aliso Viejo, CA Christopher Roberts Fluor Ltd.,
Fluor's Econamine FG Plus SM Technology An Enhanced Amine-Based CO 2 Capture Process Satish Reddy Jeff Scherffius Stefano Freguia Fluor Enterprises, Inc. Aliso Viejo, CA Christopher Roberts Fluor Ltd.,
Experimental study on CO 2 absorption into aqueous ammonia-based blended absorbents
 Available online at www.sciencedirect.com ScienceDirect Energy Procedia 61 (2014 ) 2284 2288 The 6 th International Conference on Applied Energy ICAE2014 Experimental study on CO 2 absorption into aqueous
Available online at www.sciencedirect.com ScienceDirect Energy Procedia 61 (2014 ) 2284 2288 The 6 th International Conference on Applied Energy ICAE2014 Experimental study on CO 2 absorption into aqueous
CO 2 CAPTURE FROM POST-COMBUSTION GAS BY EMPLOYING MEA ABSORPTION PROCESS EXPERIMENTAL INVESTIGATIONS FOR PILOT STUDIES
 U.P.B. Sci. Bull., Series D, Vol. 74, Iss. 1, 2012 ISSN 1454-2358 CO 2 CAPTURE FROM POST-COMBUSTION GAS BY EMPLOYING MEA ABSORPTION PROCESS EXPERIMENTAL INVESTIGATIONS FOR PILOT STUDIES Adrian Alexandru
U.P.B. Sci. Bull., Series D, Vol. 74, Iss. 1, 2012 ISSN 1454-2358 CO 2 CAPTURE FROM POST-COMBUSTION GAS BY EMPLOYING MEA ABSORPTION PROCESS EXPERIMENTAL INVESTIGATIONS FOR PILOT STUDIES Adrian Alexandru
Modeling post-combustion CO 2 capture with amine solvents
 21st European Symposium on Computer Aided Process Engineering ESCAPE 21 E.N. Pistikopoulos, M.C. Georgiadis and A.C. Kokossis (Editors) 2011 Elsevier B.V. All rights reserved. Modeling post-combustion
21st European Symposium on Computer Aided Process Engineering ESCAPE 21 E.N. Pistikopoulos, M.C. Georgiadis and A.C. Kokossis (Editors) 2011 Elsevier B.V. All rights reserved. Modeling post-combustion
Available online at ScienceDirect. Energy Procedia 63 (2014 ) GHGT-12
 Available online at www.sciencedirect.com ScienceDirect Energy Procedia 63 (2014 ) 1029 1039 GHGT-12 Energetic Evaluation of Different Flow Sheet Modifications of Post- Combustion CO 2 Capture Plant at
Available online at www.sciencedirect.com ScienceDirect Energy Procedia 63 (2014 ) 1029 1039 GHGT-12 Energetic Evaluation of Different Flow Sheet Modifications of Post- Combustion CO 2 Capture Plant at
Performance Review of CASTOR Pilot Plant at Esbjerg
 IEA GHG 11 th Capture Network Meeting 20 21 May 2008, Vienna, Austria Performance Review of CASTOR Pilot Plant at Esbjerg Jacob Nygaard Knudsen, Poul-Jacob Vilhelmsen, Jørgen N. Jensen (DONG Energy) Ole
IEA GHG 11 th Capture Network Meeting 20 21 May 2008, Vienna, Austria Performance Review of CASTOR Pilot Plant at Esbjerg Jacob Nygaard Knudsen, Poul-Jacob Vilhelmsen, Jørgen N. Jensen (DONG Energy) Ole
Energy Requirement for Solvent Regeneration in CO 2
 Energy Requirement for Solvent Regeneration in CO 2 Capture Plants Amy Veawab Andy Aroonwilas Faculty of Engineering, University of Regina Regina, Saskatchewan, Canada S4S A2 Presented at the 9 th International
Energy Requirement for Solvent Regeneration in CO 2 Capture Plants Amy Veawab Andy Aroonwilas Faculty of Engineering, University of Regina Regina, Saskatchewan, Canada S4S A2 Presented at the 9 th International
Modelling of CO 2 capture using Aspen Plus for EDF power plant, Krakow, Poland
 Modelling of CO 2 capture using Aspen Plus for EDF power plant, Krakow, Poland Vipul Gupta vipul.gupta@tecnico.ulisboa.pt Instituto Superior Técnico,Lisboa, Portugal October 2016 Abstract This work describes
Modelling of CO 2 capture using Aspen Plus for EDF power plant, Krakow, Poland Vipul Gupta vipul.gupta@tecnico.ulisboa.pt Instituto Superior Técnico,Lisboa, Portugal October 2016 Abstract This work describes
Dynamic Response of Monoethanolamine (MEA) CO2 Capture Units Robert Brasington and Howard Herzog, Massachusetts Institute of Technology
 CMTC CMTC-151075-PP Dynamic Response of Monoethanolamine (MEA) CO2 Capture Units Robert Brasington and Howard Herzog, Massachusetts Institute of Technology Copyright 2012, Carbon Management Technology
CMTC CMTC-151075-PP Dynamic Response of Monoethanolamine (MEA) CO2 Capture Units Robert Brasington and Howard Herzog, Massachusetts Institute of Technology Copyright 2012, Carbon Management Technology
Econamine FG Plus SM Technology for Post- Combustion CO 2 Capture
 Econamine FG Plus SM Technology for Post- Combustion CO 2 Capture Satish Reddy Presented at: 11 th Meeting of the International Post-Combustion CO2 Capture Network May 20 th -21 th, 2008, Vienna, Austria
Econamine FG Plus SM Technology for Post- Combustion CO 2 Capture Satish Reddy Presented at: 11 th Meeting of the International Post-Combustion CO2 Capture Network May 20 th -21 th, 2008, Vienna, Austria
Available online at Energy Procedia 4 (2011) Energy Procedia 00 (2010) GHGT-10
 Available online at www.sciencedirect.com Energy Procedia 4 (2011) 1395 1402 Energy Procedia 00 (2010) 000 000 Energy Procedia www.elsevier.com/locate/procedia www.elsevier.com/locate/xxx GHGT-10 Integration
Available online at www.sciencedirect.com Energy Procedia 4 (2011) 1395 1402 Energy Procedia 00 (2010) 000 000 Energy Procedia www.elsevier.com/locate/procedia www.elsevier.com/locate/xxx GHGT-10 Integration
Experimental results of Split-Flow Modification for Post-Combustion CO 2 Capture Process
 7 th International Exergy, Energy and Environment Symposium 27 th April 2015 Experimental results of Split-Flow Modification for Post-Combustion CO 2 Capture Process Marcin Stec, Institute for Chemical
7 th International Exergy, Energy and Environment Symposium 27 th April 2015 Experimental results of Split-Flow Modification for Post-Combustion CO 2 Capture Process Marcin Stec, Institute for Chemical
PDU 1 -SCALE EXPERIMENTAL RESULTS OF CO 2 REMOVAL
 Chemical and Process Engineering 2015, 36 (1), 39-48 DOI: 10.1515/cpe-2015-0003 PDU 1 -SCALE EXPERIMENTAL RESULTS OF CO 2 REMOVAL WITH AMP/PZ SOLVENT Dariusz Śpiewak *, Aleksander Krótki, Tomasz Spietz,
Chemical and Process Engineering 2015, 36 (1), 39-48 DOI: 10.1515/cpe-2015-0003 PDU 1 -SCALE EXPERIMENTAL RESULTS OF CO 2 REMOVAL WITH AMP/PZ SOLVENT Dariusz Śpiewak *, Aleksander Krótki, Tomasz Spietz,
Impact of novel PCC solvents on existing and new Australian coal-fired power plants 1 st PCC Conference, Abu-Dhabi
 Impact of novel PCC solvents on existing and new Australian coal-fired power plants 1 st PCC Conference, Abu-Dhabi Dr Narendra Dave Principal Research Engineer CSIRO Energy Technology, North Ryde, Australia
Impact of novel PCC solvents on existing and new Australian coal-fired power plants 1 st PCC Conference, Abu-Dhabi Dr Narendra Dave Principal Research Engineer CSIRO Energy Technology, North Ryde, Australia
Recent Developments of Hitachi s Advanced Solvent Technology for Post-combustion CO 2 Capture
 Recent Developments of Hitachi s Advanced Solvent Technology for Post-combustion CO 2 Capture ABSTRACT Sandhya Eswaran, Song Wu Hitachi Power Systems America, Ltd. 645 Martinsville Road, Basking Ridge,
Recent Developments of Hitachi s Advanced Solvent Technology for Post-combustion CO 2 Capture ABSTRACT Sandhya Eswaran, Song Wu Hitachi Power Systems America, Ltd. 645 Martinsville Road, Basking Ridge,
Innovative Stripper Configurations to Reduce the Energy Cost of CO 2 Capture
 Abstract Innovative Stripper Configurations to Reduce the Energy Cost of CO 2 Capture by Gary T. Rochelle (gtr@che.utexas.edu) Department of Chemical Engineering The University of Texas at Austin Austin,
Abstract Innovative Stripper Configurations to Reduce the Energy Cost of CO 2 Capture by Gary T. Rochelle (gtr@che.utexas.edu) Department of Chemical Engineering The University of Texas at Austin Austin,
by: Steven M. Puricelli and Ernesto Vera-Castaneda MECS, Inc USA
 MECS SOLVR REGENERATIVE SULFUR DIOXIDE TECHNOLOGY by: Steven M. Puricelli and Ernesto Vera-Castaneda MECS, Inc USA Prepared for AMERICAN INSTITUTE OF CHEMICAL ENGINEERS 4798 S. Florida Ave. #253 Lakeland,
MECS SOLVR REGENERATIVE SULFUR DIOXIDE TECHNOLOGY by: Steven M. Puricelli and Ernesto Vera-Castaneda MECS, Inc USA Prepared for AMERICAN INSTITUTE OF CHEMICAL ENGINEERS 4798 S. Florida Ave. #253 Lakeland,
JOURNAL OF APPLIED SCIENCE AND AGRICULTURE
 AENSI Journals JOURNAL OF APPLIED SCIENCE AND AGRICULTURE ISSN 1816-9112 Journal home page: www.aensiweb.com/jasa Effect of Operating Pressure on CO 2 Absorption from Natural Gas in Packed Absorption Column
AENSI Journals JOURNAL OF APPLIED SCIENCE AND AGRICULTURE ISSN 1816-9112 Journal home page: www.aensiweb.com/jasa Effect of Operating Pressure on CO 2 Absorption from Natural Gas in Packed Absorption Column
Advanced CO 2 Capture process using MEA scrubbing: Configuration of a Split Flow and Phase Separation Heat Exchanger
 Available online at www.sciencedirect.com Energy Procedia 37 (2013 ) 1778 1784 GHGT-11 Advanced CO 2 Capture process using MEA scrubbing: Configuration of a Split Flow and Phase Separation Heat Exchanger
Available online at www.sciencedirect.com Energy Procedia 37 (2013 ) 1778 1784 GHGT-11 Advanced CO 2 Capture process using MEA scrubbing: Configuration of a Split Flow and Phase Separation Heat Exchanger
ENVIRONOMIC CONSEQUENCES OF CCS TECHNOLOGY INTEGRATION IN THE CEMENT PROCESS CHAIN
 ENVIRONOMIC CONSEQUENCES OF CCS TECHNOLOGY INTEGRATION IN THE CEMENT PROCESS CHAIN Nela SLAVU 1,2, Cristian DINCA 1, Roxana PATRASCU 1, Energy Generation and Use Department, University POLITEHNICA of Bucharest,
ENVIRONOMIC CONSEQUENCES OF CCS TECHNOLOGY INTEGRATION IN THE CEMENT PROCESS CHAIN Nela SLAVU 1,2, Cristian DINCA 1, Roxana PATRASCU 1, Energy Generation and Use Department, University POLITEHNICA of Bucharest,
Retrofitting a CO2 Capture Unit with a Coal Based Power Plant, Process Simulation and Parametric Study
 Journal of Clean Energy Technologies, Vol. 5, No. 3, May 2017 Retrofitting a CO2 Capture Unit with a Coal Based Power Plant, Process Simulation and Parametric Study Sukanta Kumar Dash and Leena H. Wadibhasme
Journal of Clean Energy Technologies, Vol. 5, No. 3, May 2017 Retrofitting a CO2 Capture Unit with a Coal Based Power Plant, Process Simulation and Parametric Study Sukanta Kumar Dash and Leena H. Wadibhasme
SOLUBILITY OF CARBON DIOXIDE IN AMINE BLEND: EFFECT OF CYCLICS AND AROMATICS
 SOLUBILITY OF CARBON DIOXIDE IN AMINE BLEND: EFFECT OF CYCLICS AND AROMATICS Channarong Wongboonma a, Raphael Idem b, Teeradet Supap b, Uthaiporn Suriyapraphadilok a,c, Chintana Saiwan* a a The Petroleum
SOLUBILITY OF CARBON DIOXIDE IN AMINE BLEND: EFFECT OF CYCLICS AND AROMATICS Channarong Wongboonma a, Raphael Idem b, Teeradet Supap b, Uthaiporn Suriyapraphadilok a,c, Chintana Saiwan* a a The Petroleum
White Rose Research Online URL for this paper: Version: Accepted Version
 This is a repository copy of Performance evaluation and optimisation of post combustion CO2 capture processes for natural gas applications at pilot scale via a verified rate-based model. White Rose Research
This is a repository copy of Performance evaluation and optimisation of post combustion CO2 capture processes for natural gas applications at pilot scale via a verified rate-based model. White Rose Research
Available online at ScienceDirect. Energy Procedia 63 (2014 ) GHGT-12. M. Hossein Sahraei, L.A. Ricardez-Sandoval*
 Available online at www.sciencedirect.com ScienceDirect Energy Procedia 63 (2014 ) 1601 1607 GHGT-12 Simultaneous design and control of the MEA absorption process of a CO 2 capture plant M. Hossein Sahraei,
Available online at www.sciencedirect.com ScienceDirect Energy Procedia 63 (2014 ) 1601 1607 GHGT-12 Simultaneous design and control of the MEA absorption process of a CO 2 capture plant M. Hossein Sahraei,
Effects of Piperazine on Removal of Hydrogen Sulfide from Liquefied Petroleum Gas (LPG) using Aqueous Methyl Diethanol Amine (MDEA)
 Effects of Piperazine on Removal of Hydrogen Sulfide from Liquefied Petroleum Gas (LPG) using Aqueous Methyl Diethanol Amine (MDEA) Matib M and Zoubida L * Safety Engineering Laboratory Industrial and
Effects of Piperazine on Removal of Hydrogen Sulfide from Liquefied Petroleum Gas (LPG) using Aqueous Methyl Diethanol Amine (MDEA) Matib M and Zoubida L * Safety Engineering Laboratory Industrial and
Study evaluates two amine options for gas sweetening
 Study evaluates two amine options for gas sweetening 08/07/2006 A study of two methods for removing H 2S and CO 2 from natural gas has concluded that an arrangement of methyl diethanolamine (MDEA) and
Study evaluates two amine options for gas sweetening 08/07/2006 A study of two methods for removing H 2S and CO 2 from natural gas has concluded that an arrangement of methyl diethanolamine (MDEA) and
Description and evaluation of flowsheet modifications and their interaction for an efficient monoethanolamine based post-combustion CO 2 capture
 CHEMICAL ENGINEERING TRANSACTIONS Volume 21, 2010 Editor J. J. Klemeš, H. L. Lam, P. S. Varbanov Copyright 2010, AIDIC Servizi S.r.l., ISBN 978-88-95608-05-1 ISSN 1974-9791 DOI: 10.3303/CET1021030 175
CHEMICAL ENGINEERING TRANSACTIONS Volume 21, 2010 Editor J. J. Klemeš, H. L. Lam, P. S. Varbanov Copyright 2010, AIDIC Servizi S.r.l., ISBN 978-88-95608-05-1 ISSN 1974-9791 DOI: 10.3303/CET1021030 175
New Model Configuration for Post Combustion Carbon Capture
 New Model Configuration for Post Combustion Carbon Capture Udara S. P. R. Arachchige, Dinesh Kawan, and Morten C. Melaaen Abstract This paper discusses about possible configurations to improve the process
New Model Configuration for Post Combustion Carbon Capture Udara S. P. R. Arachchige, Dinesh Kawan, and Morten C. Melaaen Abstract This paper discusses about possible configurations to improve the process
Amine Plant Energy Requirements & Items impacting the SRU
 Amine Plant Energy Requirements & Items impacting the SRU 10 October 2016 AGRU energy needs Amine energy requirements Regeneration Processing effects Leanness required Determine required leanness Over
Amine Plant Energy Requirements & Items impacting the SRU 10 October 2016 AGRU energy needs Amine energy requirements Regeneration Processing effects Leanness required Determine required leanness Over
Available online at ScienceDirect. Energy Procedia 63 (2014 ) GHGT-12
 Available online at www.sciencedirect.com ScienceDirect Energy Procedia 63 (2014 ) 1678 1685 GHGT-12 Shell Cansolv CO 2 capture technology: Achievement from First Commercial Plant Ajay Singh a *, Karl
Available online at www.sciencedirect.com ScienceDirect Energy Procedia 63 (2014 ) 1678 1685 GHGT-12 Shell Cansolv CO 2 capture technology: Achievement from First Commercial Plant Ajay Singh a *, Karl
Simulation of the Energy Consumption of CO 2 Capture by Aqueous Monoethanolamine in Pilot Plant
 Simulation of the Energy Consumption of CO 2 Capture by Aqueous Monoethanolamine in Pilot Plant Chunli Han, Kirsten Graves, James Neathery & Kunlei Liu (Corresponding author) University of Kentucky Center
Simulation of the Energy Consumption of CO 2 Capture by Aqueous Monoethanolamine in Pilot Plant Chunli Han, Kirsten Graves, James Neathery & Kunlei Liu (Corresponding author) University of Kentucky Center
Removal of CO2 and H2S using Aqueous Alkanolamine Solusions
 Removal of CO2 and H2S using Aqueous Alkanolamine Solusions Zare Aliabad, H., and Mirzaei, S. Abstract This work presents a theoretical investigation of the simultaneous absorption of CO 2 and H 2 S into
Removal of CO2 and H2S using Aqueous Alkanolamine Solusions Zare Aliabad, H., and Mirzaei, S. Abstract This work presents a theoretical investigation of the simultaneous absorption of CO 2 and H 2 S into
Chemical Characterization of MEA Degradation in PCC pilot plants operating in Australia
 Available online at www.sciencedirect.com Energy Procedia 37 (2013 ) 877 882 GHGT-11 Chemical Characterization of MEA Degradation in PCC pilot plants operating in Australia Alicia J. Reynolds a, T. Vincent
Available online at www.sciencedirect.com Energy Procedia 37 (2013 ) 877 882 GHGT-11 Chemical Characterization of MEA Degradation in PCC pilot plants operating in Australia Alicia J. Reynolds a, T. Vincent
Impact of liquid absorption process development on the costs of post-combustion. capture in Australian coal-fired power stations
 Impact of liquid absorption process development on the costs of post-combustion capture in Australian coal-fired power stations N. Dave 1, T. Do, D. Palfreyman 2 and P.H.M. Feron CSIRO Energy Technology,
Impact of liquid absorption process development on the costs of post-combustion capture in Australian coal-fired power stations N. Dave 1, T. Do, D. Palfreyman 2 and P.H.M. Feron CSIRO Energy Technology,
Available online at Energy Procedia 1 (2009) (2008) GHGT-9. Sandra Heimel a *, Cliff Lowe a
 Available online at www.sciencedirect.com Energy Procedia 1 (2009) (2008) 4039 4046 000 000 Energy Procedia www.elsevier.com/locate/procedia www.elsevier.com/locate/xxx GHGT-9 Technology Comparison of
Available online at www.sciencedirect.com Energy Procedia 1 (2009) (2008) 4039 4046 000 000 Energy Procedia www.elsevier.com/locate/procedia www.elsevier.com/locate/xxx GHGT-9 Technology Comparison of
Energy Efficient Solvents for CO 2 Absorption from Flue Gas: Vapor Liquid Equilibrium and Pilot Plant Study
 Available online at www.sciencedirect.com Energy Procedia 37 (2013 ) 2021 2046 GHGT-11 Energy Efficient Solvents for CO 2 Absorption from Flue Gas: Vapor Liquid Equilibrium and Pilot Plant Study Prachi
Available online at www.sciencedirect.com Energy Procedia 37 (2013 ) 2021 2046 GHGT-11 Energy Efficient Solvents for CO 2 Absorption from Flue Gas: Vapor Liquid Equilibrium and Pilot Plant Study Prachi
Reforming Natural Gas for CO 2 pre-combustion capture in Combined Cycle power plant
 Reforming Natural Gas for CO 2 pre-combustion capture in Combined Cycle power plant J.-M. Amann 1, M. Kanniche 2, C. Bouallou 1 1 Centre Énergétique et Procédés (CEP), Ecole Nationale Supérieure des Mines
Reforming Natural Gas for CO 2 pre-combustion capture in Combined Cycle power plant J.-M. Amann 1, M. Kanniche 2, C. Bouallou 1 1 Centre Énergétique et Procédés (CEP), Ecole Nationale Supérieure des Mines
Pilot Test and Simulation of an Advanced Amine Process for CO 2 Capture
 Pilot Test and Simulation of an Advanced Amine Process for CO 2 Capture Xi Chen, Barath Baburao, Frederic Vitse * Alstom Power, 1409 Centerpoint Blvd, Knoxville, TN 37932 Summary An Advanced Amine Process
Pilot Test and Simulation of an Advanced Amine Process for CO 2 Capture Xi Chen, Barath Baburao, Frederic Vitse * Alstom Power, 1409 Centerpoint Blvd, Knoxville, TN 37932 Summary An Advanced Amine Process
ENERGY EFFICIENT SYNTHESIS AND DESIGN FOR CARBON CAPTURE
 Distillation Absorption 2010 A.B. de Haan, H. Kooijman and A. Górak (Editors) All rights reserved by authors as per DA2010 copyright notice ENERGY EFFICIENT SYNTHESIS AND DESIGN FOR CARBON CAPTURE Angelo
Distillation Absorption 2010 A.B. de Haan, H. Kooijman and A. Górak (Editors) All rights reserved by authors as per DA2010 copyright notice ENERGY EFFICIENT SYNTHESIS AND DESIGN FOR CARBON CAPTURE Angelo
Available online at ScienceDirect. Energy Procedia 86 (2016 ) L.V. van der Ham*, P. Khakharia, E.L.V.
 Available online at www.sciencedirect.com ScienceDirect Energy Procedia 86 (2016 ) 106 115 The 8th Trondheim Conference on CO 2 Capture, Transport and Storage Heat-Integrated Liquid Desorption Exchanger
Available online at www.sciencedirect.com ScienceDirect Energy Procedia 86 (2016 ) 106 115 The 8th Trondheim Conference on CO 2 Capture, Transport and Storage Heat-Integrated Liquid Desorption Exchanger
DYNAMIC MODELS AND CONTROL STRATEGIES FOR ABSORPTION- BASED CARBON CAPTURE PROCESSES
 DYNAMIC MODELS AND CONTROL STRATEGIES FOR ABSORPTION- BASED CARBON CAPTURE PROCESSES Ali Abbas Laboratory for Multiscale Systems School of Chemical and Biomolecular Engineering University of Sydney HiPerCap
DYNAMIC MODELS AND CONTROL STRATEGIES FOR ABSORPTION- BASED CARBON CAPTURE PROCESSES Ali Abbas Laboratory for Multiscale Systems School of Chemical and Biomolecular Engineering University of Sydney HiPerCap
Energy Procedia 00 (2008) GHGT-9. Comparison of solvents for post-combustion capture of CO 2 by chemical absorption
 Energy Procedia 00 (2008) 000 000 Energy Procedia www.elsevier.com/locate/xxx GHGT-9 Comparison of solvents for post-combustion capture of CO 2 by chemical absorption Anusha Kothandaraman* a, Lars Nord
Energy Procedia 00 (2008) 000 000 Energy Procedia www.elsevier.com/locate/xxx GHGT-9 Comparison of solvents for post-combustion capture of CO 2 by chemical absorption Anusha Kothandaraman* a, Lars Nord
Addition of Static Mixers Increases Treating Capacity in Central Texas Gas Plant
 Page 1 of 5 Addition of Static Mixers Increases Treating Capacity in Central Texas Gas Plant TRACY G. CARTER, STEVEN D. BEHRENS, Mitchell Gas Services L.P., The Woodlands, Texas JOHN T. (JAY) COLLIE III,
Page 1 of 5 Addition of Static Mixers Increases Treating Capacity in Central Texas Gas Plant TRACY G. CARTER, STEVEN D. BEHRENS, Mitchell Gas Services L.P., The Woodlands, Texas JOHN T. (JAY) COLLIE III,
Optimized CO 2 -flue gas separation model for a coal fired power plant
 INTERNATIONAL JOURNAL OF ENERGY AND ENVIRONMENT Volume 4, Issue 1, 2013 pp.39-48 Journal homepage: www.ijee.ieefoundation.org Optimized CO 2 -flue gas separation model for a coal fired power plant Udara
INTERNATIONAL JOURNAL OF ENERGY AND ENVIRONMENT Volume 4, Issue 1, 2013 pp.39-48 Journal homepage: www.ijee.ieefoundation.org Optimized CO 2 -flue gas separation model for a coal fired power plant Udara
Chemistry of Petrochemical Processes
 Chemistry of Petrochemical Processes ChE 464 Instructor: Dr. Ahmed Arafat, PhD Office: building 45 room 106 E-mail: akhamis@kau.edu.sa www.kau.edu.sa.akhamis files Book Chemistry of Petrochemical Processes
Chemistry of Petrochemical Processes ChE 464 Instructor: Dr. Ahmed Arafat, PhD Office: building 45 room 106 E-mail: akhamis@kau.edu.sa www.kau.edu.sa.akhamis files Book Chemistry of Petrochemical Processes
Progress on CO 2 Capture Pilot Plant at RIST
 IEAGHG/IETS Iron & Steel Industry CCUS & Process Integration Workshop Date: 5th to 7th November 2013 Tokyo Tech Front, Tokyo Institute of Technology, Japan Progress on CO 2 Capture Pilot Plant at RIST
IEAGHG/IETS Iron & Steel Industry CCUS & Process Integration Workshop Date: 5th to 7th November 2013 Tokyo Tech Front, Tokyo Institute of Technology, Japan Progress on CO 2 Capture Pilot Plant at RIST
ADECOS II. Advanced Development of the Coal-Fired Oxyfuel Process with CO 2 Separation
 Fakultät Maschinenwesen Institut für Energietechnik, Professur für Verbrennung, Wärme- & Stoffübertragung ADECOS II Advanced Development of the Coal-Fired Oxyfuel Process with CO 2 S. Grahl, A. Hiller,
Fakultät Maschinenwesen Institut für Energietechnik, Professur für Verbrennung, Wärme- & Stoffübertragung ADECOS II Advanced Development of the Coal-Fired Oxyfuel Process with CO 2 S. Grahl, A. Hiller,
Concentrated Piperazine based Post-Combustion- Capture for Australian coalfired
 CSIRO ENERGY FLAGSHIP Concentrated Piperazine based Post-Combustion- Capture for Australian coalfired power plants Summary Report Aaron Cottrell, Ashleigh Cousins, Sanger Huang, Narendra Dave, Thong Do,
CSIRO ENERGY FLAGSHIP Concentrated Piperazine based Post-Combustion- Capture for Australian coalfired power plants Summary Report Aaron Cottrell, Ashleigh Cousins, Sanger Huang, Narendra Dave, Thong Do,
Development and Cost Estimation of Green Gas Reduction Process for Power Plant
 Development and Cost Estimation of Green Gas Reduction Process for Power Plant Jiyong Kim, Dongwoon Kim and Il Moon Department of Chemical Engineering, Yonsei University, 134 Shinchodong Seodaemoonku,
Development and Cost Estimation of Green Gas Reduction Process for Power Plant Jiyong Kim, Dongwoon Kim and Il Moon Department of Chemical Engineering, Yonsei University, 134 Shinchodong Seodaemoonku,
Optimization of an Existing Coal-fired Power Plant with CO 2 Capture
 Energy and Power Engineering, 2013, 5, 157-161 doi:10.4236/epe.2013.54b030 Published Online July 2013 (http://www.scirp.org/journal/epe) Optimization of an Existing Coal-fired Power Plant with CO 2 Capture
Energy and Power Engineering, 2013, 5, 157-161 doi:10.4236/epe.2013.54b030 Published Online July 2013 (http://www.scirp.org/journal/epe) Optimization of an Existing Coal-fired Power Plant with CO 2 Capture
Optimisation of the Rectisol TM Design with Packing: the Rectisol TM Demonstration Unit
 A publication of CHEMICAL ENGINEERING TRANSACTIONS VOL. 69, 2018 Guest Editors: Elisabetta Brunazzi, Eva Sorensen Copyright 2018, AIDIC Servizi S.r.l. ISBN 978-88-95608-66-2; ISSN 2283-9216 The Italian
A publication of CHEMICAL ENGINEERING TRANSACTIONS VOL. 69, 2018 Guest Editors: Elisabetta Brunazzi, Eva Sorensen Copyright 2018, AIDIC Servizi S.r.l. ISBN 978-88-95608-66-2; ISSN 2283-9216 The Italian
Dynamic Simulation and Control of MEA Absorption Process for CO 2 Capture from Power Plants
 Proceedings of the 6th International Conference on Process Systems Engineering (PSE ASIA) 25-27 June 2013, Kuala Lumpur. Dynamic Simulation and Control of MEA Absorption Process for CO 2 Capture from Power
Proceedings of the 6th International Conference on Process Systems Engineering (PSE ASIA) 25-27 June 2013, Kuala Lumpur. Dynamic Simulation and Control of MEA Absorption Process for CO 2 Capture from Power
A new MAB-series solvent that can break ultimate energy goals of 1.9 GJ/t-CO2 and 190 kwh/t-co2
 A new MAB-series solvent that can break ultimate energy goals of 1.9 GJ/t-CO2 and 190 kwh/t-co2 Kwang Soon Lee, Professor Dept. of Chem. and Biomol. Engng, Sogang Univ., Seoul, Korea The 8 th Korea CCUS
A new MAB-series solvent that can break ultimate energy goals of 1.9 GJ/t-CO2 and 190 kwh/t-co2 Kwang Soon Lee, Professor Dept. of Chem. and Biomol. Engng, Sogang Univ., Seoul, Korea The 8 th Korea CCUS
Start-Up of World s First Commercial Post-Combustion Coal Fired CCS Project: Contribution of Shell Cansolv to SaskPower Boundary Dam ICCS Project
 Available online at www.sciencedirect.com ScienceDirect Energy Procedia 00 (2013) 000 000 www.elsevier.com/locate/procedia GHGT-12 Start-Up of World s First Commercial Post-Combustion Coal Fired CCS Project:
Available online at www.sciencedirect.com ScienceDirect Energy Procedia 00 (2013) 000 000 www.elsevier.com/locate/procedia GHGT-12 Start-Up of World s First Commercial Post-Combustion Coal Fired CCS Project:
Available online at ScienceDirect. Energy Procedia 63 (2014 ) GHGT-12. a Alstom Power, USA
 Available online at www.sciencedirect.com ScienceDirect Energy Procedia 63 (2014 ) 6173 6187 GHGT-12 Advanced Amine Process Technology Operations and Results from Demonstration Facility at EDF Le Havre
Available online at www.sciencedirect.com ScienceDirect Energy Procedia 63 (2014 ) 6173 6187 GHGT-12 Advanced Amine Process Technology Operations and Results from Demonstration Facility at EDF Le Havre
Adsorption based CO 2 capture in the natural gas industry
 Adsorption based CO 2 capture in the natural gas industry Prof Paul A. Webley The University of Melbourne Separation and Sensing Workshop Wednesday 29th November 2017 Outline Motivation and Challenges
Adsorption based CO 2 capture in the natural gas industry Prof Paul A. Webley The University of Melbourne Separation and Sensing Workshop Wednesday 29th November 2017 Outline Motivation and Challenges
THE CANSOLV SO 2 - CO 2 CAPTURE PROCESS
 THE CANSOLV SO 2 - CO 2 CAPTURE PROCESS Carbon Capture Workshop Texas A&M University, Qatar April 2-3, 2012 Niels Fabricius Qatar Shell Copyright of Royal Dutch Shell plc April 2011 Presentation Outline
THE CANSOLV SO 2 - CO 2 CAPTURE PROCESS Carbon Capture Workshop Texas A&M University, Qatar April 2-3, 2012 Niels Fabricius Qatar Shell Copyright of Royal Dutch Shell plc April 2011 Presentation Outline
Example SPC-2: Effect of Increasing Column P on a C3 splitter
 Example SPC-2: Effect of Increasing Column P on a C3 splitter Consider the separation of a mixture of 50 mol/hr of propane C 3 H 8 (1) and 50 mol/hr propene, C 3 H 6 (2) at a pressure of 1.1 bar and a
Example SPC-2: Effect of Increasing Column P on a C3 splitter Consider the separation of a mixture of 50 mol/hr of propane C 3 H 8 (1) and 50 mol/hr propene, C 3 H 6 (2) at a pressure of 1.1 bar and a
Importance of experimental unit for Fluidised Circulating Coal Combustion (FCCC) in the process of capturing CO 2 from combustion gas streams
 4th UNI-SET Energy Clustering Event Universities in the Energy Transition: Focus on Sustainable Transport and Carbon Capture, Storage & Use Importance of experimental unit for Fluidised Circulating Coal
4th UNI-SET Energy Clustering Event Universities in the Energy Transition: Focus on Sustainable Transport and Carbon Capture, Storage & Use Importance of experimental unit for Fluidised Circulating Coal
Dynamic modelling of CO 2 absorption for post combustion capture in coal-fired power plants
 1 2 3 4 Dynamic modelling of CO 2 absorption for post combustion capture in coal-fired power plants A. Lawal a, M. Wang a,*, P. Stephenson b, H. Yeung a a Process Systems Engineering Group, School of Engineering,
1 2 3 4 Dynamic modelling of CO 2 absorption for post combustion capture in coal-fired power plants A. Lawal a, M. Wang a,*, P. Stephenson b, H. Yeung a a Process Systems Engineering Group, School of Engineering,
Removal of Acid Gases from Biomass-to-Liquid Process Syngas Used as Raw Materials for Fischer-Tropsch Technology
 Journal of the Japan Institute of Energy, 93, 17-131(014) 17 Removal of Acid Gases from Biomass-to-Liquid Process Syngas Used as Raw Materials for Fischer-Tropsch Technology Kreangkrai MANEEINTR 1, Thanaphat
Journal of the Japan Institute of Energy, 93, 17-131(014) 17 Removal of Acid Gases from Biomass-to-Liquid Process Syngas Used as Raw Materials for Fischer-Tropsch Technology Kreangkrai MANEEINTR 1, Thanaphat
Field Testing and Independent Review of Post-Combustion CO 2 Capture Technology
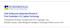 Field Testing and Independent Review of Post-Combustion CO 2 Capture Technology Presented by Phil Boyle, President and COO, Powerspan Corp. McIlvaine Company, Carbon Management Strategies & Technologies
Field Testing and Independent Review of Post-Combustion CO 2 Capture Technology Presented by Phil Boyle, President and COO, Powerspan Corp. McIlvaine Company, Carbon Management Strategies & Technologies
CCS at IFP. from MEA to New Processes for CO 2 Post-Combustion Capture
 Controlled CO 2 Diversified fuels Fuel-efficient vehicles Clean refining Extended reserves CCS at IFP from MEA to New Processes for CO 2 Post-Combustion Capture L. Raynal, E. Lemaire, P. Broutin (IFP)
Controlled CO 2 Diversified fuels Fuel-efficient vehicles Clean refining Extended reserves CCS at IFP from MEA to New Processes for CO 2 Post-Combustion Capture L. Raynal, E. Lemaire, P. Broutin (IFP)
Available online at Energy Procedia 100 (2009) (2008) GHGT-9. Allan Hart and Nimalan Gnanendran*
 Available online at www.sciencedirect.com Energy Procedia 100 (2009) (2008) 697 706 000 000 Energy Procedia www.elsevier.com/locate/procedia www.elsevier.com/locate/xxx GHGT-9 Cryogenic CO 2 Capture in
Available online at www.sciencedirect.com Energy Procedia 100 (2009) (2008) 697 706 000 000 Energy Procedia www.elsevier.com/locate/procedia www.elsevier.com/locate/xxx GHGT-9 Cryogenic CO 2 Capture in
Available online at Energy Procedia 4 (2011) Energy Procedia 00 (2010) GHGT-10
 Available online at www.sciencedirect.com Energy Procedia 4 (2011) 1260 1267 Energy Procedia 00 (2010) 000 000 Energy Procedia www.elsevier.com/locate/procedia www.elsevier.com/locate/xxx GHGT-10 Low-temperature
Available online at www.sciencedirect.com Energy Procedia 4 (2011) 1260 1267 Energy Procedia 00 (2010) 000 000 Energy Procedia www.elsevier.com/locate/procedia www.elsevier.com/locate/xxx GHGT-10 Low-temperature
Experience with CO 2 capture from coal flue gas in pilot-scale: Testing of different amine solvents
 Available online at www.sciencedirect.com Energy Procedia 100 (2009) (2008) 783 790 000 000 www.elsevier.com/locate/procedia www.elsevier.com/locate/xxx GHGT-9 Experience with CO 2 capture from coal flue
Available online at www.sciencedirect.com Energy Procedia 100 (2009) (2008) 783 790 000 000 www.elsevier.com/locate/procedia www.elsevier.com/locate/xxx GHGT-9 Experience with CO 2 capture from coal flue
CRYOGENIC SOLVENT ABATEMENT (VOC s )
 CRYOGENIC SOLVENT ABATEMENT (VOC s ) 1. Introduction The technology for removing volatile organic compounds (V.O.C.s) from gas has been developed to meet the emission limits, decreased during the last
CRYOGENIC SOLVENT ABATEMENT (VOC s ) 1. Introduction The technology for removing volatile organic compounds (V.O.C.s) from gas has been developed to meet the emission limits, decreased during the last
WSA-DC NEXT GENERATION TOPSØE WSA TECHNOLOGY FOR STRONGER SO 2 GASES AND VERY HIGH CONVERSION. Helge Rosenberg Haldor Topsoe
 WSA-DC NEXT GENERATION TOPSØE WSA TECHNOLOGY FOR STRONGER SO 2 GASES AND VERY HIGH CONVERSION Helge Rosenberg Haldor Topsoe Up to now, Topsøe WSA (Wet gas Sulphuric Acid) plants have been in operation
WSA-DC NEXT GENERATION TOPSØE WSA TECHNOLOGY FOR STRONGER SO 2 GASES AND VERY HIGH CONVERSION Helge Rosenberg Haldor Topsoe Up to now, Topsøe WSA (Wet gas Sulphuric Acid) plants have been in operation
Pilot scale demonstration plants of an advanced aqueous amine-based PCC utilizing BASF s OASE blue technology
 Pilot scale demonstration plants of an advanced aqueous amine-based PCC utilizing BASF s OASE blue technology TorstenStoffregen, Linde Engineering Dresden Venice/Italy, 13 th May 2015 10 th CO 2 GEONET
Pilot scale demonstration plants of an advanced aqueous amine-based PCC utilizing BASF s OASE blue technology TorstenStoffregen, Linde Engineering Dresden Venice/Italy, 13 th May 2015 10 th CO 2 GEONET
Kinetic study of a Layout for the Carbon Capture with Aqueous Ammonia without Salt Precipitation
 Downloaded from orbit.dtu.dk on: Jan 12, 2019 Kinetic study of a Layout for the Carbon Capture with Aqueous Ammonia without Salt Precipitation Bonalumi, Davide; Lillia, Stefano; Valenti, Gianluca; Fosbøl,
Downloaded from orbit.dtu.dk on: Jan 12, 2019 Kinetic study of a Layout for the Carbon Capture with Aqueous Ammonia without Salt Precipitation Bonalumi, Davide; Lillia, Stefano; Valenti, Gianluca; Fosbøl,
Available online at ScienceDirect. Energy Procedia 63 (2014 ) GHGT-12
 Available online at www.sciencedirect.com ScienceDirect Energy Procedia 63 (2014 ) 1745 1750 GHGT-12 Characteristics of CO 2 capture system using KIERSOL in the LNG flue gas Yeo Il Yoon a,*, Young Eun
Available online at www.sciencedirect.com ScienceDirect Energy Procedia 63 (2014 ) 1745 1750 GHGT-12 Characteristics of CO 2 capture system using KIERSOL in the LNG flue gas Yeo Il Yoon a,*, Young Eun
CO 2 Capture. John Davison IEA Greenhouse Gas R&D Programme.
 CO 2 Capture John Davison IEA Greenhouse Gas R&D Programme Overview of this Presentation Leading CO 2 capture technologies for power generation Descriptions Main advantages and disadvantages Examples of
CO 2 Capture John Davison IEA Greenhouse Gas R&D Programme Overview of this Presentation Leading CO 2 capture technologies for power generation Descriptions Main advantages and disadvantages Examples of
Energy Procedia 4 (2011) Energy Procedia 00 (2010) GHGT-10
 Energy Procedia 4 (2011) 1729 1736 Energy Procedia 00 (2010) 000 000 Energy Procedia www.elsevier.com/locate/procedia www.elsevier.com/locate/xxx GHGT-10 Study of carbon dioxide capture from industrial
Energy Procedia 4 (2011) 1729 1736 Energy Procedia 00 (2010) 000 000 Energy Procedia www.elsevier.com/locate/procedia www.elsevier.com/locate/xxx GHGT-10 Study of carbon dioxide capture from industrial
F. Vega*, M. Rodríguez-Galán, B. Alonso-Fariñas, B. Navarrete, V. Cortés
 3 rd Oxyfuel Combustion Conference OCC3 Ponferrada (Spain), 9-13 September 2013 F. Vega*, M. Rodríguez-Galán, B. Alonso-Fariñas, B. Navarrete, V. Cortés Assistant Prof. Bernabé Alonso-Fariñas EPE Group,
3 rd Oxyfuel Combustion Conference OCC3 Ponferrada (Spain), 9-13 September 2013 F. Vega*, M. Rodríguez-Galán, B. Alonso-Fariñas, B. Navarrete, V. Cortés Assistant Prof. Bernabé Alonso-Fariñas EPE Group,
PERFORMANCE EVALUATION OF NGCC AND COAL-FIRED STEAM POWER PLANTS WITH INTEGRATED CCS AND ORC SYSTEMS
 Paper ID: 119, Page 1 PERFORMANCE EVALUATION OF NGCC AND COAL-FIRED STEAM POWER PLANTS WITH INTEGRATED CCS AND ORC SYSTEMS Vittorio Tola Department of Mechanical, Chemical and Material Engineering, University
Paper ID: 119, Page 1 PERFORMANCE EVALUATION OF NGCC AND COAL-FIRED STEAM POWER PLANTS WITH INTEGRATED CCS AND ORC SYSTEMS Vittorio Tola Department of Mechanical, Chemical and Material Engineering, University
The Misguided Focus on Low Heat of Absorption Solvents
 Jochen Oexmann Alfons Kather The Misguided Focus on Low Heat of Absorption Solvents Session: Carbon Capture Technologies (I) Institute of Energy Systems Prof. Dr.-Ing. A. Kather 4 th International Conference
Jochen Oexmann Alfons Kather The Misguided Focus on Low Heat of Absorption Solvents Session: Carbon Capture Technologies (I) Institute of Energy Systems Prof. Dr.-Ing. A. Kather 4 th International Conference
FMH606 Master s thesis Ievgeniia Oleksandrivna Vozniuk. Aspen HYSYS process simulation and Aspen ICARUS cost estimation of CO 2 removal plant
 FMH606 Master s thesis 2010 Ievgeniia Oleksandrivna Vozniuk Aspen HYSYS process simulation and Aspen ICARUS cost estimation of CO 2 removal plant Telemark University College Faculty of Technology M.Sc.
FMH606 Master s thesis 2010 Ievgeniia Oleksandrivna Vozniuk Aspen HYSYS process simulation and Aspen ICARUS cost estimation of CO 2 removal plant Telemark University College Faculty of Technology M.Sc.
