Graduate Theses and Dissertations
|
|
|
- Neil Carson
- 5 years ago
- Views:
Transcription
1 University of South Florida Scholar Commons Graduate Theses and Dissertations Graduate School 2009 Using Keeling Plots to trace d ¹³C and d ¹³O through processes of heterotrophic respiration, diffusion and soil water equilibration in artificial C3- and C4-grassland soils Jennifer Chelladurai University of South Florida Follow this and additional works at: Part of the American Studies Commons Scholar Commons Citation Chelladurai, Jennifer, "Using Keeling Plots to trace d ¹³C and d ¹³O through processes of heterotrophic respiration, diffusion and soil water equilibration in artificial C3- and C4-grassland soils" (2009). Graduate Theses and Dissertations. This Thesis is brought to you for free and open access by the Graduate School at Scholar Commons. It has been accepted for inclusion in Graduate Theses and Dissertations by an authorized administrator of Scholar Commons. For more information, please contact scholarcommons@usf.edu.
2 Using Keeling Plots to Trace δ 13 C and δ 18 O of CO 2 Through Processes of Heterotrophic Respiration, Diffusion and Soil Water Equilibration in Artificial C3- and C4-Grassland Soils by Jennifer Chelladurai A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science Department of Geology College of Arts and Sciences University of South Florida Major Professor: Jonathan Wynn, Ph.D. Mark Rains, Ph.D. Mark Stewart, Ph.D. Date of Approval: April 8, 2009 Keywords: stable isotopes, carbon, oxygen, carbonates, fractionation Copyright 2009, Jennifer Chelladurai
3 Dedication I dedicate this thesis to my daughter, Anoushka Chelladurai, whose future I hope will be brighter on account of my successful completion of this Master of Science degree.
4 Acknowledgements I would first and foremost like to acknowledge my husband, for his patience and his hard work that allowed me the time to complete this degree; my mother-in-law for assisting me with childcare and household chores while I was busy, and my daughter for tolerating my frequent absence from her life during my work on this degree. I would also like to acknowledge my parents for listening to my frustrations, and my aunt for inspiring me to complete a Masters degree. I would like to acknowledge my advisor, Jonathan G. Wynn, Ph.D. for answering my questions and for providing help and encouragement when needed throughout my completion of this project and degree. I would also like to acknowledge my committee members, who provided valuable feedback on my thesis and for providing valuable instruction in the writing of theses and papers.
5 Table of Contents List of Tables List of Figures Abstract iii iv vi Introduction 1 Soil Processes and the Global Carbon Cycle 2 Pedogenic Carbonates 3 Controls on Soil Respiration 5 Stable Isotopes as a Tracer of the Carbon Cycle 7 Keeling Plots 11 Cerling Model of Soil CO 2 Diffusion-Production 12 Expectations and Hypotheses 17 Materials and Methods 18 Apparatus 19 Soil Profiles 24 Water Addition 26 Gas Sampling 28 IRMS Analysis 29 Plots 30 Results CO 2 Concentration with Depth 31 δ 13 C Keeling Plots 36 δ 18 O Keeling Plots 46 δ 13 C and δ 18 O with Depth 57 δ 13 C versus δ 18 O 61 Discussion Concentration versus Depth 64 δ 13 C Keeling Plots 68 δ 18 O Keeling Plots 70 Cross plots of δ 13 C versus δ 18 O 72 Possible Issues 73 i
6 Conclusions 75 Future Thoughts 76 References 78 Bibliography 82 Appendices 84 Appendix A: Normalized Values from Utilized Samples of Soil CO 2 85 Appendix B: Data from Early Sampling Dates 89 Appendix C: Effluent ph 93 Appendix D: Watering and Gas Sampling Schedule 95 Appendix E: Additional Keeling Plot Line Equations and R 2 Values 96 Appendix F: Column T3 Problems 101 ii
7 List of Tables Table 1. Apparatus Measurements 24 Table 2. Soil Profile Parameters 26 Table 3. δ 13 C Keeling Plot Line Equations and R 2 Values 39 Table 4. δ 13 C Keeling Plot y-intercepts for Varying Soil Moisture 46 Table 5. δ 18 O Keeling Plot Line Equations and R 2 Values 50 Table 6. δ 18 O Keeling Plot y-intercepts for Varying Soil Moisture 57 iii
8 List of Figures Figure 1. Soil Carbonate Fractionation Diagram 10 Figure 2. Cerling Model CO 2 versus Depth 14 Figure 3. Cerling Model δ 13 C versus Depth 15 Figure 4. Cerling Model Keeling Plot 16 Figure 5. Apparatus 21 Figure 6. Gas Collection Tube 22 Figure 7. Empty Soil Column 23 Figure 8. Soil Profile 25 Figure 9. CO 2 Concentration versus Depth 32 Figure 10. CO 2 Concentration versus Depth in Wet Soil 33 Figure 11. CO 2 Concentration versus Depth in Moist Soil 34 Figure 12. CO 2 Concentration versus Depth in Dry Soil 35 Figure 13. C3 δ 13 C Keeling Plots 37 Figure 14. C4 δ 13 C Keeling Plots 38 Figure 15. C3 δ 13 C Keeling Plots for Wet Soil Conditions 40 Figure 16. C3 δ 13 C Keeling Plots for Moist Soil Conditions 41 Figure 17. C3 δ 13 C Keeling Plots for Dry Soil Conditions 42 Figure 18. C4 δ 13 C Keeling Plots for Wet Soil Conditions 43 Figure 19. C4 δ 13 C Keeling Plots for Moist Soil Conditions 44 iv
9 Figure 20. C4 δ 13 C Keeling Plots for Dry Soil Conditions 45 Figure 21. C3 δ 18 O Keeling Plots 48 Figure 22. C4 δ 18 O Keeling Plots 49 Figure 23. C3 δ 18 O Keeling Plots for Wet Soil Conditions 51 Figure 24. C3 δ 18 O Keeling Plots for Moist Soil Conditions 52 Figure 25. C3 δ 18 O Keeling Plots for Dry Soil Conditions 53 Figure 26. C4 δ 18 O Keeling Plots for Wet Soil Conditions 54 Figure 27. C4 δ 18 O Keeling Plots for Moist Soil Conditions 55 Figure 28. C4 δ 18 O Keeling Plots for Dry Soil Conditions 56 Figure 29. δ 13 C versus Depth 59 Figure 30. δ 18 O versus Depth 60 Figure 31. C3 δ 13 C versus δ 18 O 62 Figure 32. C4 δ 13 C versus δ 18 O 63 Figure 33. δ 18 O versus Depth from Sternberge, et al. (1998) 71 v
10 Using Keeling Plots to Trace δ 13 C and δ 18 O of CO 2 Through Processes of Heterotrophic Respiration, Diffusion and Soil Water Equilibration in Artificial C3- and C4-Grassland Soils Jennifer Chelladurai ABSTRACT Global carbon cycle dynamics and fluxes of CO 2 between biosphere and atmosphere have been progressed through the use of Keeling Plots. Processes that control and effect the isotopic composition of soil-respired CO 2, soil CO 2, and equilibrated soil carbonate are specifically addressed in this study through the use of Keeling Plots. Replicate grassland soil profiles containing either C3 or C4 homogenized organic matter were constructed and maintained under controlled settings to encourage the production of soil-respired CO 2 and the precipitation of pedogenic carbonate. Soil CO 2 was sampled over five months and analyzed with IRMS. Keeling Plots illustrated source CO 2 affected by mixing with atmospheric CO 2 near the surface and equilibration with 13 C-depleted CO 2 at depth in the zone of likely carbonate precipitation. The δ 13 C Keeling Plot intercepts for the surface horizons (~ for C3 profiles and ~ for C4 profiles) follow the vi
11 diffusion-production model when corrected with a constant 4.4 diffusional fractionation, but the Keeling Plot intercepts for developing Bk horizons were curved towards depleted values (~ for C3 profiles and ~ for C4 profiles). This change in isotopic composition with depth deviates from the usual interpretations of Keeling Plots (steady-state, source to background diffusional mixing). δ 18 O Keeling Plot intercepts indicated evaporative enrichment in the surface horizons of C3 and C4 replicate soil profiles. This study uses Keeling Plots as a measure of mixing to assess the efficacy of steady-state diffusionproduction models of soil CO 2 equilibration with soil carbonate. vii
12 Introduction This study specifically addresses the need for increased knowledge of the processes that control the isotopic composition of soil-respired CO 2 and its effect on the δ 13 C and δ 18 O values of soil CO 2 and equilibrated soil carbonate. Much work has focused on the isotopic composition of total soil-respired CO 2 although much of what has been done was carried out in field soils, in which the ability to separate causative factors may be limited. There has also been some work using the isotopic composition of soil-respired CO 2 in pedogenic carbonates to determine paleoenvironmental conditions. In neither of these situations have laboratory-built soils been utilized to measure the isotopic composition of soilrespired CO 2 and to precipitate pedogenic carbonates to test the effect of their precipitation on the isotopic values of carbon and oxygen in the soil CO 2. It is important to fully understand the factors which influence soil CO 2 isotopic composition and its role in the global carbon cycle; and the sources and sinks of atmospheric CO 2 are often determined by stable isotope studies. Knowledge gained on the isotopic processes during soil respiration will ultimately be of use in biogeochemistry, ecosystem studies, and paleoenvironmental reconstruction. The purpose of this thesis is to identify the factors that may influence the carbon and oxygen isotopic composition of soil respired CO 2, and to validate the use of Keeling Plots as a measure of mixing soil respired CO 2 with atmospheric 1
13 CO 2, through the use of artificial soil profiles. This study will also compare the results from collected data to those predicted by the Cerling (1984) CO 2 diffusion production model. This introductory section will cover soil processes and their role in the Global Carbon Cycle, the formation and utilization of pedogenic carbonates in terms of soil respiration, the controls on soil respiration, how stable isotopes can be used to trace the Carbon Cycle, Keeling plots and their use in soil studies, and the use of the Cerling model of CO 2 diffusion-production. Soil Processes and the Global Carbon Cycle Carbon isotope ratios were used to determine that fossil fuel burning increased atmospheric CO 2 by Keeling et al, (1979). Now that this process is known it is important to study the potential carbon sinks. The realization that there was a large terrestrial carbon sink was brought about by studies in the early 1990 s (Tans et al., 1990; Sternberge 1998). A significant component of the carbon cycle is the terrestrial biosphere-atmosphere flux through soil systems, with PgC/year soil respiration globally; soil respiration is the second largest flux of carbon between land and atmosphere (Reichstein et al, 2003). Among the annual total ecosystem respiration, the soil respiration in forests makes up % (Davidson et al., 2006). Soil systems globally contain approximately 1200 PgC in the first meter, in which the carbon is contained in the 2
14 form of soil organic matter (SOM) (Amundson, 2004). Soils also generally contain between 380 and 60,000 ppm of CO 2, depending on various soil properties and the amount of respiration (Amundson, 2004). The primary fluxes involved in the cycling of carbon in soils involve exchange with the atmosphere and with organic matter in the surface litter. This study will isolate the flux of atmospheric exchange. The global flux of carbon into soils from surface litter is estimated at a rate of 4 PgC/year, the flux going from the soils to the atmosphere due to heterotrophic respiration is estimated at a rate of 3.5 PgC/year; and there is an additional flux of carbon going from soils to the atmosphere due to anthropogenic land use, it is estimated at 0.4 PgC/year. This leaves 0.1 PgC/year accumulating in soils in the form of pedogenic carbonates (Amundson, 2004). This study is set up with the intention of growing pedogenic carbonates with the ability to measure the isotopic values and concentrations of the involved soil CO 2. Pedogenic Carbonates Pedogenic carbonates have a number of applications in paleoenvironmental studies, in addition to their relevance to soil CO 2. Pedogenic carbonates typically form in arid to semi-humid environments where the soil ph is 7 or above (Cerling, 1984), and can be found in various forms. They begin as small crystals, and then form small nodules; they can eventually form massive indurated horizons, if conditions permit (Buck, 2005). It is important to 3
15 remember that significant horizons of pedogenic carbonates generally take greater than 100 years to accumulate, and that the carbonate precipitation may be seasonal (Blisnuk, 2005). Because pedogenic mineral accumulations occur over longer time spans they represent a time-averaged isotopic composition (Blisniuk, 2005; Deutz, 2002), but that still may only represent a fraction of the time that it took for the host soil to develop (Deutz, 2002). The isotopic compositions of pedogenic carbonates (CaCO 3 ) in paleosols have been used in interpretations of paleoclimate and paleoelevation. Carbonate minerals can yield isotopic values for δ 13 C and δ 18 O, which can be used to interpret the relative proportions of C3 and C4 flora, and to determine the isotopic composition of meteoric water. This has been applied to study paleoclimate using carbonates from paleosols. The controls on oxygen isotopes in soil systems are less well understood than carbon, but it is known that oxygen isotope compositions of CO 2, DIC, and pedogenic carbonate are related to meteoric water (Dworkin, 2005). The δ 18 O of pedogenic carbonates will be affected by the δ 18 O of rainwater; which itself varies with mean annual air temperature, such that as the temperature increases, so do the δ 18 O values (Cerling, 1984; Schmid, 2006). Given that it does take a significant amount of time to accumulate significantly observable pedogenic carbonates nodules in soils, it is not expected that the soils in this study will produce pedogenic carbonate nodules within the time of this study, although it is expected that fine carbonate crystals will form, 4
16 bridging gaps between soil particles. Even this small amount of carbonate precipitation is expected to affect the isotopic values of the soil CO 2. Controls on Soil Respiration Soil respiration is the primary source of soil CO 2. In natural soils, both root, or autotrophic respiration and heterotrophic respiration (respiration from soil microbes, and other heterotrophs) are the important components of total soil respiration. Soil respiration rate varies considerably depending on a number of outside factors. Seasonal changes, the local climate, and the substrate can all affect soil respiration. Specifically, the factors that influence soil respiration include CO 2 concentration of the surrounding atmosphere, and temperature, soil water availability, and plant growth rates (Davidson et al., 2006). For example, Wan, S. et al. (2007) completed tests of soil respiration in old agricultural fields under atmospheric and enriched CO 2 and found elevated soil respiration in the presence of elevated atmospheric CO 2. This was believed to be fueled by increased plant growth. The study also found that there was no effect on soil respiration in the presence of elevated temperature. However; that is not always the case, and this is a subject of much debate in the global change community. A study by Yutse et al. (2007) found that elevated temperature increased soil respiration in ponderosa pine forests. So the different ecosystems responded differently to the same stimulus in this case. 5
17 Soil respiration can be affected by changing moisture content and decomposition rates as well (Kirschbaum, 2004). The study by Wan, et al (2007) also tested changing moisture levels in the old fields and found no correlation between moisture levels and soil respiration. Once again, the Yutse et al. (2007) study showed differently. The ponderosa pine forests demonstrated no effect of moisture on soil respiration; however, they also analyzed an oak savanna ecosystem, and did find an increase in soil respiration with an increase in moisture, although the oak understory and the open savanna portions responded slightly differently. Adding to this, a study by Pendall et al. (2004) found a decrease in soil respiration in the presence of the combination of increased moisture and increased atmospheric CO 2 concentrations. Because soil respiration involves both plant roots and microbial communities, it varies by ecosystem. By design of this study, many of these environmental controls on respiration rate and processes can be eliminated: root respiration, temperature, substrate and moisture content. By keeping these factors constant in laboratory conditions, it will simplify some of these contradictory issues and allow specific details of heterotrophic soil respiration to be analyzed. Heterotrophic respiration is generally a significant component of total soil respiration and has been reported from numerous field sites to comprise approximately 70 % of the total soil respiration (Buchmann, 2000). 6
18 Stable Isotopes as a Tracer of the Carbon Cycle As carbon moves between different Earth system reservoirs it undergoes isotopic fractionation. Thus, the ratio of 13 C to 12 C will be different in the different reservoirs. As photosynthesis moves carbon from the atmosphere into organic matter there is a very large fractionation, the degree of which will differ according to the photosynthetic pathway. The most common photosynthetic pathways are C3 and C4. CAM (crassulacean acid metabolism) photosynthesis is used only by arid-adapted succulents and is not considered here because a comparatively small percentage of plants use this pathway (only about 10%), and because these plants utilize both C3 and C4 pathways, switching as needed (Clark and Fritz, 1997). C4 plants can account for % of total terrestrial photosynthesis (Ehleringer, 2002), and this fraction varies systemically with environmental conditions, and temporally on several time scales. This difference in the fractionation factor between photosynthetic pathways gives the SOM different starting isotopic values. The isotopic values are written in delta notation: C C - C C 13 sample standard δ C = (1) C Cstandard 7
19 Soil with pure C3 biomass will have a δ 13 C value around -25, whereas pure C4-derived SOM has δ 13 C values of ~ -12. If the organic matter is of mixed composition, containing both C3- and C4-derived organic matter, the δ 13 C value varies proportionally, between -25 and -12. There is some variability in carbon isotope discrimination of C3 and C4 plants that corresponds to physiological and environmental differences (Ehleringer, 2002); but this is not significant enough to disrupt the non-overlapping trend between C3 and C4 plants. These differences in δ 13 C values between photosynthetic pathways are reflected in the isotopic composition of CO 2 respired by heterotrophic consumers of biomass. Both carbon and oxygen isotopic values will be measured on the respired soil CO 2 in this study, each of which responds to different processes. The carbon isotopic signature derives primarily from the decomposing organic matter, i.e. the source of respired C, while the oxygen isotopic signature derives from equilibration of CO 2 with the local meteoric water. When rainwater infiltrates the soil it becomes soil water, and as such may undergo some fractionation, particularly as surface soil water evaporates and leaves behind 18 O- enriched water in the soil. The soil water will also equilibrate with the soil CO 2, imparting a δ 18 O signature to the soil CO 2. CO 2 that is dissolved in the soil water is the source of C for the precipitation of pedogenic carbonates. Some soil CO 2 that remains in a gaseous form diffuses through the soil. During this diffusion process the lighter isotopes 8
20 move quicker, causing an enrichment of heavier isotopes in the soil (Tans, 1998). Diffusional fractionation in soils causes a 4.4 enrichment of δ 13 C values Cerling, 1991). Equations 2 and 3 illustrate the process by which soil CO 2 is dissolved and is incorporated into pedogenic carbonates (from Schlesinger, 1997). 2CO 2 + 2H 2 O 2H + + 2HCO 3 - (2) Ca HCO - 3 CaCO 3 + H 2 O + CO 2 (3) It can be seen from these reactions that there are a number of influential factors that may affect isotopic values of oxygen and carbon in soil systems. In this study soils constructed to replicate semi-arid grassland soils are used, and they were built with the intention of developing a Bk horizon (a subsurface illuvial horizon with an accumulation of carbonates). Soils in which carbon is accumulating in a developing Bk horizon may deplete soil CO 2 of 13 C during this process, because of the fractionation factor between CO 2 and carbonate. The fractionation factor (ε) is the change in δ 13 C value that occurs with each step in the processes leading to the fractionation. As pedogenic carbonates are precipitated in soils from dissolved inorganic carbon (DIC), the ultimate source of their carbon is the soil CO 2, which is then dissolved in the soil water prior to utilization in the carbonate mineral (Figure 1). This occurs by the soil CO 2 first dissolving from a gas in the soil to aqueous CO 2, which entails a 1.1 fractionation factor. Aqueous CO 2 is in equilibrium with bicarbonate (HCO - 3 ) with a 9.0 fractionation factor between these two phases. Equilibration with CO 2-3 has a -0.4 fractionation factor. The final step of precipitating CO 2-3 into 9
21 CaCO 3 comes with a 0.9 fractionation factor. Overall the equilibrium fractionation factor between soil CO 2 gas and CaCO 3 is 10.6, and varies slightly with temperature effects (Clark and Fritz, 1997, p. 120). Figure 1. Soil Carbonate Fractionation Diagram. This diagram from Clark and Fritz, 1997 (p. 120), illustrates the process of carbon from soil-respired CO 2 becoming incorporated in pedogenic carbonate (CaCO 3 ). The δ 13 C of the carbon in each intermediate step is shown, as is the fractionation factor (ε). 10
22 Keeling Plots Keeling Plots are essentially a two-component mixing model that allows the isotopic composition of source CO 2 to be distinguished from soil CO 2 that has undergone mixing with background atmospheric CO 2. The use of Keeling plots began in the late 1950 s and early 1960 s by C. D. Keeling to interpret carbon isotope ratios of ambient CO 2 and to identify the sources that contribute to atmospheric CO 2 concentrations on a regional basis. (Yakir, 2000). The work by Keeling hypothesized that carbon isotope ratios and CO 2 concentration vary proportionally, and with a plot of δ 13 C versus 1/[CO 2 ] it was possible to see a process of the mixing of atmospheric CO 2 and CO 2 from soil and plant respiration (Keeling, 1958 and 1961). The y-intercept of a Keeling Plot represents the δ 13 C of the source CO 2. It was only in the late 1980 s and 1990 s, that the use of Keeling plots for ecosystem respiration became widely known and was used to analyze the isotopic composition of atmospheric CO 2 to determine carbon sources and sinks (Sternberge et al., 1998). Work that has been done using Keeling plots for soil respiration has generally been done in field conditions, not in artificial soils in a laboratory. An example of this can be seen in Mortazavi et al., 2004; in which they utilized soil probes and sampling chambers to collect soil CO 2 samples from 11
23 natural soils. These were used to determine the δ 13 C and δ 18 O of soil efflux via the intercepts of the Keeling plots. There have been other works pertaining to soils, such as work by Liu et al., 2006, who studied the δ 13 C and Δ 14 C in natural soils- and used the Keeling plot to determine at what depths in the soil atmospheric CO 2 had an effect and what depths it was no longer present. Cerling Model of Soil CO 2 diffusion-production Cerling s diffusion-production model is utilized in studies of pedogenic carbonates and their ability to infer past C3/C4 flora compositions. The model utilizes a number of assumed input parameters (including atmospheric CO 2 concentration, diffusion coefficient for CO 2 in the air, free-air porosity, and a tortuosity factor). The diffusion-production equation is solved for steady state conditions, which allows the model calculation of CO 2 concentration profiles in soils (equation 4). The model goes further to predict the δ 13 C CO2 throughout the soil profile, but it assumes the same soil diffusion coefficient throughout the profile. The model solves the diffusion-production equation separately for isotopically substituted molecules of CO 2, and then predicts the ratio of 13 C/ 12 C in soil CO 2 which is converted to δ in equation 5. C 2 * z = φ Lz C * Ds * 2 (4) s *
24 [ ] [ ] ˆ * 1 ˆ * 1 2 * * ˆ * ˆ * 2 * * = a s s s a s s s PDB s C D D z Lz D C D D z Lz D R δ δ φ δ δ φ δ φ β φ β (5) C s * is the concentration of soil CO 2, δ s * is the permil value for soil air, R PDB is the ratio of 13 C/ 12 C in PDB standard, D s * is the diffusion coefficient for CO 2 in soil, φ* is the production rate of CO 2, L is the depth at the base of the soil profile, z is the depth within the soil profile, is the permil value for respired CO 2, is the permil value for atmosphere, D s β is the diffusion coefficient of 13 CO 2 in soil, and C 0 * is the atmospheric concentration of CO 2. δˆφ δˆa Results of this model from Cerling are shown in Figures 2 4, with some standard assumptions. The diffusion-production model demonstrates that atmospheric CO 2 concentration is important at shallow depth (the first 10 cm in the soil from the surface) and when soil respiration value is low because it mixes significantly with soil-respired CO 2 and imparts an isotopic signature on the soil CO 2 indicative of this mixing ratio (Cerling, 1984). Predicted CO 2 concentration with depth is shown in Figure 2; predicted δ 13 C with depth is illustrated in Figure 3, and a Keeling Plot made using these predicted values, is shown in Figure 4. 13
25 Cerling Model CO 2 vs. Depth [CO2], ppm V Depth, cm Figure 2. CO 2 versus depth using the Cerling model for CO 2 diffusion production. Model parameters of the artificial soils in this study and the atmospheric CO 2 value for the lab atmosphere at the time of the study were input into Cerling s model (using the methods of Amundson, 2004). 14
26 Cerling Model δ 13 C vs. Depth δ 13 C CO2, Depth, cm Figure 3. δ 13 C versus depth using the Cerling model for CO 2 diffusion production. Model parameters of the artificial soils in this study and the atmospheric CO 2 value for the lab atmosphere at the time of the study were input into Cerling s model (using the methods of Amundson, 2004). 15
27 Cerling Model Keeling Plot 1/[CO2] Figure 4. Keeling Plot constructed with the values predicted by the Cerling CO2 diffusion production model. The y-intercept represents the source δ 13 C, which is in this model. δ 13 C 16
28 Expectations and Hypotheses Because soil CO 2 equilibrates with soil solution to form pedogenic carbonates and is the source of ecosystem respiration, it is important to fully understand the effect of mixing on the isotopic values of soil CO 2, and how it is influenced by carbonate precipitation and background atmospheric CO 2. This study uses laboratory analysis of artificial soils to test the isotopic values in soils with either C3 or C4 organic matter, at various depths to determine the influence on the isotopic values of soil CO 2 by background atmospheric mixing and carbonate precipitation. It is expected that there will be 4.4 fractionation factor for the CO 2 diffusion through air, from a source of soil-respired CO 2. The soil CO 2 concentration is expected to increase with depth, and the carbon isotopic signatures are expected to reflect the values of the starting soil organic matter mixed with atmospheric CO 2, whereas the oxygen isotopic signatures are expected to reflect equilibration with the water used to irrigate the columns. The diffusivity of soil CO 2 will be dependent on the connectivity of pore space in the soil, and is thus affected by soil type and by the presence of water. If the pore space in a soil is saturated, the diffusion of gas through the soil may be halted (Weerst, 2001). This study will also test the effect of soil moisture on the diffusivity of CO 2 under variable degrees of saturation. 17
29 The Keeling Plots are expected to represent the process of mixing between a homogenous source of respired CO 2 and atmospheric CO 2. If this is the only process involved, the Keeling plots should be linear for both δ 13 C and δ 18 O; any deviation from a linear plot will illuminate processes that fractionate 13 C/ 12 C or 18 O/ 16 O during the production, diffusion, and equilibration. Materials and Methods Replicate soil columns were built with two types of artificial SOM profiles composed of C3 and C4 biomass in sterile mineral soil. By using artificial soils in laboratory conditions, the effects of root respiration have been removed, leaving microbial decomposition of organic matter and evaporation as the primary variables that control the isotopic composition of soil respiration and any precipitation of pedogenic carbonate. Three replicates were built of two types of soil profiles each containing a homogenized grass litter (from C3 or C4 grasses) in a matrix of clean organic-free sand. Profiles were built with fresh grass clippings as the starting organic matter to simulate decomposition and CO 2 concentration that would be typical of a natural grassland soil. In a typical grassland soil with a mean respiration flux rate (F resp ) = 442 g C m -2 yr -1, the CO 2 concentration increases from atmospheric CO 2 concentration at the surface (currently 384 ppm) to about 7,000 ppm at 100 cm depth (Amundson, 2005). 18
30 Periodically, an isotopically-homogenous source of water with 200 mg/l Ca 2+ was added to each profile in order to stimulate both decomposition of the organic matter and precipitation of pedogenic carbonate as the water was evaporated and calcite saturation was reached. The concentration and isotopic composition of CO 2 within the soil profiles were then monitored over ~6 months. Apparatus The soil profiles were contained in six columns that were built using 2.5- inch clear PVC pipe, each pipe measuring 101 cm in length. The columns were measured and marked for the horizons as follows: From the top, cm was to be left empty for watering space; cm was marked for the A1 horizon; cm for the A2 horizon (A horizons are surface horizons that contain humified organic matter and minerals); for the E horizon (E horizons are eluvial horizons that have been leached of organics and minerals such as clay, iron, and aluminum); cm for the Bk horizon (Bk horizons are subsurface soil horizons which accumulate leached materials from the surface horizons and feature the precipitation of carbonates within them); with the bottom remaining space for the C horizon (unweathered material beneath the soil- in this case it is used to assist in draining the columns). Figure 5 illustrates the soil column apparatus, with horizons and the horizon boundary depth in cm. Table 1 contains the measurements for soil horizons in the columns. 19
31 After marking the horizons, holes were drilled (very slowly with a 13/16 Speedbor woodboring bit) in the columns to allow for the installation of the gas sampling tubes. A1, A2, and E horizons each had one gas sampling tube installed in the center of the horizon, and the Bk horizon had two gas sampling tubes (Figure 5). 20
32 Figure 5. Apparatus. Diagram of soil column apparatus with measurements. The horizons are labeled. Columns are made from clear PVC with PVC and CPVC fittings. Gas collection tubes are made with perforated stainless steel tubes. 21
33 No gas sampling tubes were installed in the C horizon. The gas sampling tubes were installed to provide a volume within the soil horizon for collection of CO 2 that is larger than the volume of gas removed during sampling. This is to avoid creating a pressure gradient that may pull CO 2 through the soil during sampling, which could possibly cause a slight isotopic change as the 12 C will diffuse through the soil slightly faster than the 13 C. A Dremel tool with drum sanding bit was used to smooth the edges of the holes that were cut. Figure 6. Gas Collection Tube. Thirty of these tubes were constructed from stainless steel mesh and perforated stainless steel sheet metal. Their purpose was to allow CO2 to collect in the space in a greater volume than would be pulled from the collection tube during sampling. The gas sampling tubes were made using perforated stainless steel sheet (5/16 inch diameter holes, centers 3/8 inches apart; staggered array of holes) and using a hammer to form it around an oak dowel (1/2 inch diameter). Then a fine stainless steel mesh (sst 316, 70 x inches) was cut into slightly smaller rectangles, formed into tubes around an ink pen, and inserted into the perforated 22
34 tubes (this is to keep sand out of the tubes, but to allow gas to exchange between the sampling tubes and pore spaces in the soil). All of the tubes were washed with Nalgene detergent to remove grease and then they were fitted into the columns with CPVC fittings to hold them in place. After removing the black rubber rings from the FIP adapters, septa were added (headspace septa, Teflon/rubber; C , to all of the adapters, which were then screwed on the CPVC 1/2 MIP adapters. The CPVC fittings have rubber septa added to seal the columns from atmospheric gas and to allow the gas sampling needle to remove gas samples from the tubes. 2.5 inch slip cap PVC endcaps were attached to the bottom of each column. In the bottom of each endcap a 3/16 inch hole was drilled. Each of these holes then had a 1.5 inch length of 3/16 thinwall rigid airline tubing added for drainage of excess pore water. A length of flexible airline tubing was then added to each to allow drainage to specific containers. The completed columns are held upright on a monkey lattice and rest on cut pieces of 3 inch PVC pipes. An empty completed column is shown in Figure 7. Figure 7. Completed soil column. Perforated stainless tubes used to collect and sample soil CO 2, via syringe and rubber septa, are visible through the clear PVC. 23
35 Horizon Top Depth (cm) Bottom Depth (cm) Thickness (cm) Depth of Sampling Tubes (cm) A A E Bk (Bk1) 82.5 (Bk2) Table 1. Apparatus Measurements. This table contains the measurements for soil horizons and gas sampling points within the soil columns. Soil Profiles Soil horizons were built within the columns to simulate a simplified grassland soil profile in which the dominant processes were aerobic decomposition of the organic matter, evapotranspiration of the pore water, and precipitation of soil carbonate. The C horizons were filled to the base of the Bk horizons with quartz sand (Quartz play sand from Home Depot) and crushed scoria to improve drainage from the base of the profile. The Bk horizons were then added, each one has 750g Ca-montmorilinite (smectite) clay and 1187g quartz sand. The Bk horizon contained 38.7% smectitic clay to retain Ca 2+ - concentrated water during evaporation from the surface. Next the E horizons were added, each one has pure quartz sand, enough was added to fill that segment; its mass was not measured, but a smaller portion of the sand was measured to obtain the bulk density. This was done by measuring 550 cm 3 of the quartz and obtaining the mass for that volume (the mass was 906.6g). Each A2 horizon was 24
36 filled with a mixture of grass and quartz sand, resulting in 5.0% organic matter. Each A1 horizon was filled with a mixture of grass and quartz sand resulting in 6.2% organic matter. The organic matter in three of the columns consisted of a homogenous C4 grass biomass, collected from grass clippings from the greens of a golf course in Tampa, Florida (Temple Terrace Golf & Country Club); and three of the columns used a relatively homogenous C3 grass biomass, collected from a golf course in St. Andrews, Scotland (the Old Course). Grass was ground in a coffee grinder prior to mixing with the sand to obtain a relatively homogenous consistency and particle size, equivalent for both profiles. The grass biomass had starting isotopic values of ± 0.31 for the δ 13 C of the C3 grass, and ± 0.19 for the δ 13 C of the C4 grass. Predicted δ 13 C CO2 (VPDB) of soil CO 2 respired from these substrates are: (C3) and Two of the filled columns are shown in Figure 8. The bulk density of each horizon is displayed in Table 2. Figure 8. Soil Profile. Two of the completed columns with the soil profiles. The centralized light-colored horizon is the E horizon. The developing Bk horizon is below the E horizon, and the A horizons are above the E horizon. 25
37 Horizon Volume (cm 3 ) Mass (g) Bulk Density (g/cm 3 ) Sand (%) Organic Matter (%) A A E N/A Bk Clay (%) Table 2. Soil Profile Parameters. Bulk density and other parameters of the soil horizons. Column volumes and the mass of added materials for each horizon are given in the table. Water Addition The water used for watering the columns was prepared with the intention of inducing carbonate precipitation within the Bk horizon and with providing a distinct isotopic signature from the water that could be visible in any resulting carbonates. Two clean jugs, one 20 L and one 50 L were filled with 12 MΩ deionized water, and placed open under a fume hood to allow some evaporation (to obtain slightly 18 O-enriched water). After about three months of evaporation time the water was all (about 60 L) placed in a large Rubbermaid bin and 22.2 g of calcium hydroxide was added in order to charge the water with Ca 2+. Because the resulting ph was higher than typical for grasslands, HCl was added to bring down the ph to 6.48, to approximate the ph of slightly buffered rainwater. This water 26
38 was placed back in the jugs and sealed to prevent further evaporation. The starting isotopic value for the water was δ 18 O = ± Predicted δ 18 O CO2 (VSMOW) of CO 2 in oxygen isotopic equilibrium with this water at 25 C is Soil columns were watered regularly with either 175 ml or 650 ml volumes of water; the light watering was intended to wet the profile, and the large watering to flush (see Appendix D for the watering chart). To assist in drainage of the excess pore water, column drainage tubes were attached to a peristaltic pump the day after watering for about 1 hour. Airline tube check valves from Tetra are used to prevent atmospheric gas from entering the columns via the drainage tubes during this pumping. 40W heating lamps were placed on a 12-hr timer above each profile to encourage evaporation of pore water from the surface of the profile. After approximately six months of use it was determined that the peristaltic pump drainage system was not sufficient to drain the columns. To remedy this situation 1½ inch diameter holes were drilled with a hole saw and 1 inch CPVC slip threaded bushings were added. These were sealed with 1 inch PVC threaded caps. Thereafter, subsequent to each watering a ceramic pore water sampler (SG25 porous borosilicate from UMS, Munich, Germany) was inserted with a tube extending from it to a sealed vacuum flask. The flask was kept under vacuum to pull water from the soil columns for approximately 1 hour the day after watering. 27
39 Gas Sampling Gas samples were collected for isotope ratio mass spectrometer (IRMS) analysis by removing a small volume of gas from the gas sampling tube with a gas-tight syringe (Hamilton, or Bee-Stinger). The gas-tight syringe was inserted through the rubber septa into the perforated steel gas collection tubes within the columns. Gas was always sampled from the top to the bottom of each column for consistency in the following pattern: A1 first, A2 second, E third, Bk1 fourth and Bk2 last. The columns were always sampled in the following order: StA1 first, StA2 second, StA3 third, T1 fourth, T2 fifth, and finally T3 (StA referring to C3 biomass columns and T referring to C4 biomass columns). From each column, 7.5 ml of gas was taken from the A1 horizons, and all other horizons had 2 ml of gas taken. The gas samples from the soil columns were then injected from the Gas-tight syringe into Helium-flushed 10 ml Exetainers (Labco, Hertfordshire, UK) in preparation for IRMS analysis. Three samples of ambient lab atmosphere were collected during every sampling session. These were collected by leaving three Exetainers open for several minutes and then sealing them. Samples were analyzed within two hours to avoid leakage from the Exetainer septa. Gas samples for the three IRMS runs used in this study were taken at different time intervals after the columns had been watered. One sampling date was 2 days 28
40 post-watering, one sampling run was 25 days post-watering, and one sampling run was 64 days post-watering. This provided the opportunity to measure the soil respiration in the columns when the soil was wet, moist, and dry. IRMS Analysis IRMS runs were set up as follows: 30 vials were flushed with Helium and were used for the samples from the columns; 6 vials flushed with CO 2 /He were run as standards with the samples. 12 vials were flushed with Helium and had varying increments (4 with 7.5 ml, 4 with 5 ml, and 4 with 2 ml) of a custom CO 2 /He gas mixture (2987 ppm CO 2 in balance of He). The latter samples were added in order to correct sample analysis (which vary significantly in amount of CO 2 ) for linearity effects of the mass spectrometer. Samples of lab atmosphere were added at the end of a run. [CO 2 ], δ 13 C CO2 and δ 18 O CO2 were measured on a Finnigan Delta V IRMS and corrected with respect to a CO 2 /He standard which was calibrated to the VPDB scale via the international NBS-18 and NBS-19 reference materials (limestones). Normalization was as follows: CO 2 samples were measured with respect to replicates of a CO 2 /He mixture, which was calibrated to the VPDB scale via NBS-18 and NBS-19. Concentration of CO 2 was measured for each gas sample by comparison of an equivalent sample of the CO 2 /He gas mixture (2967 ppm CO 2 in balance He). For comparability of water 29
41 and CO 2 data δ 18 O (VPDB) values were converted to δ 18 O (VSMOW) via the equation of Coplen, et al. (1983): δ 18 O VSMOW = δ 18 O PDB (6) A predicted δ 18 O value for CO 2 in equilibrium with the soil water was determined by the equilibrium fractionation factor in O Neil & Adami (1969). Modeled δ 13 C CO2 values follow the diffusion-production model of Cerling (1984). Plots Keeling Plots and all other plots were created in Microsoft Excel XP Pro. The Keeling plots were created by plotting the corrected IRMS results versus the reciprocal of the CO 2 concentration, and then applying Excel s linear regression function to create a trend line which represents the Keeling line. This was done for each horizon, C3 columns together and C4 columns together- resulting in 4 Keeling Plots (two for δ 13 C and two for δ 18 O). Additional plots were made for each sampling day, to illustrate any differences that different soil moisture conditions may have on CO 2 concentration or on the isotopic values of the soil CO 2. 30
42 Results CO 2 Concentration with Depth The CO 2 concentration was measured for each horizon, and the average for each horizon plotted versus the column depth (Figure 9). Soil CO 2 concentration was greater than atmospheric CO 2 concentration (380 ppm) in all horizons during all sampling intervals. Average CO 2 concentration increased from near atmospheric values up to nearly ppm between 70 and 80 cm depth (note this is only an average for replicate profiles and multiple sampling intervals- actual values taken at time of sampling were higher/lower depending on the soil moisture). Figures contain additional CO 2 versus depth plots separated according to one of three moisture regimes, which the sampling occurred in wet, moist, or dry soil. Average CO 2 concentration increased from the A1 horizon to the A2 horizon, and remained fairly steady through the E horizon for the C4 columns, and continued to increase in the C3 columns. C3 columns had a continued increase in CO 2 concentration through the Bk1 sampling point, and then decreased at the Bk2 sampling point. C4 columns decreased at the Bk1 sampling point. The C4 columns only had one sample from the Bk2 sampling points, which showed a significantly greater CO 2 concentration. 31
43 CO 2 Concentration vs. Depth [CO 2 ], ( ppm) A A2 E Depth (cm) Catm = 380 ppm Bk1 Bk1 Bk2 Figure 9. CO2 Concentration versus Depth. Average of [CO2] depth profiles. Sampling points were in the center of each horizon for A1, A2, and E horizons. The Bk horizon contained two sampling points (note that the lower Bk horizon sample was often problematic with extremely high [CO2], likely due to low porosity. The gray triangle is the single sample point that was obtained for the Bk2 horizon in the C4 columns. 32
44 CO 2 Concentration vs. Depth Wet Soil [CO2] (ppm) Depth (cm) A1 A2 E Bk1 C3 C4 Figure 10. Concentration versus Depth in Wet Soil. [CO 2 ] depth profiles for a single sampling time, 2 days after the columns were irrigated with 650 ml of water per column. Sampling points were in the center of each horizon for A1, A2, and E horizons. The Bk horizon contained two sampling points (note that the lower Bk horizon sample was often problematic with extremely high [CO 2 ], likely due to low porosity). 33
45 CO 2 Concentration vs. Depth Moist Soil [CO2] (ppm) Depth (cm) A1 A2 E Bk1 C3 C4 Figure 11. Concentration versus Depth in Moist Soil. [CO 2 ] depth profiles for a single sampling time, 25 days after the columns were irrigated with 650 ml of water per column. Sampling points were in the center of each horizon for A1, A2, and E horizons. The Bk horizon contained two sampling points (note that the lower Bk horizon sample was often problematic with extremely high [CO 2 ], likely due to low porosity). 34
46 Depth (cm) CO 2 Concentration vs. Depth Dry Soil [CO2] (ppm) A1 Bk1 Bk2 A2 E Bk1 C3 C4 Figure 12. Concentration versus Depth in Dry Soil. [CO 2 ] depth profiles for a single sampling time, 64 days after the columns were irrigated with 650 ml of water per column. Sampling points were in the center of each horizon for A1, A2, and E horizons. The Bk horizon contained two sampling points (note that the lower Bk horizon sample was often problematic with extremely high [CO 2 ], likely due to low porosity). 35
47 δ 13 C Keeling Plots Keeling Plots separated for each horizon type but averaged for all sampling periods are shown in Figure 13 and 14, which highlights changes in slope and y-intercept between horizons. Figure 13 shows the δ 13 C Keeling Plots for the C3 columns and Figure 14 shows the Keeling Plots for the C4 columns. In both cases it is clear that the δ 13 C values determined by the y-intercepts on the Keeling Plots (δ 13 C (KP) ) decrease with depth. In all of the δ 13 C Keeling Plots the A and E horizons fit a line well, but the B horizons do not. The y-intercepts become more 13 C-depleted in the B horizons. Keeling Plots shown here consist of all of the data combined from the three gas-sampling days, while Figures contain Keeling Plots for each sampling period. These are organized into one of the three moisture regimes during which the sampling occurred: wet, moist, and dry. Variation in the different moisture regimes produced negligible difference in the δ 13 C (KP), for both the C3 and C4 soil profiles. The Equations and R 2 values for the average Keeling Plot lines are listed in Table 3. The y-intercepts for individual Keeling Plots (in Figures 15 20) that were plotted according to the approximate soil moisture regime are in Table 4. It is important to notice that there is not significant change in y-intercept between the different moisture regimes. 36
48 C3 δ 13 C Keeling Plots /[CO 2 ] Predicted δ 13 C of soil CO 2 is o / oo δ 13 C ( o /oo) Catm = 380 ppm A1 Horizons A2 Horizons E Horizons Bk 1 Horizons Bk 2 Horizons Lab Atmosphere Figure 13. C3 δ 13 C Keeling Plots. Keeling Plots of δ 13 C by horizon for C3 columns (plots summarize 5 months of sampling; variation between sampling intervals is evident). The values for all of the replicate columns are plotted as points and the Keeling Plot lines were plotted using linear regression for all sampling intervals. Notice that the Keeling plot intercepts become more negative for deeper soil horizons (Bk horizon). Predicted δ 13 C of soil CO2 from this C3 biomass is The starting δ 13 C of the C3 biomass was ±
49 C4 δ 13 C Keeling Plots 1/[CO 2 ] oo Predicted δ 13 C of soil CO is -9.4 o / δ 13 C ( o /oo) Catm = 380 ppm A1 Horizons A2 Horizons E Horizons Bk 1 Horizons Lab Atmosphere Figure 14. C4 δ 13 C Keeling Plots. Keeling Plots of δ 13 C by horizon for C4 columns (plots summarize 5 months of sampling; variation between sampling intervals is evident). The values for all of the replicate columns are plotted as points and the Keeling Plot lines were plotted using linear regression for all sampling intervals. Notice that the Keeling plot intercepts become more negative for the Bk horizon. The predicted δ 13 C of soil CO2 from the C4 biomass is The starting δ 13 C of the C4 biomass was ±
Carbon Isotope Systematics in Soil
 Carbon Isotope Systematics in Soil Soil Pathway Summary Organic matter finds it s way into soils and decomposes SOM (Soil Organic Matter) is further decomposed by microbes which emit CO 2 as a bi-product
Carbon Isotope Systematics in Soil Soil Pathway Summary Organic matter finds it s way into soils and decomposes SOM (Soil Organic Matter) is further decomposed by microbes which emit CO 2 as a bi-product
Reactions & Processes Influenced by Soil Wetness. Topics for Consideration. Reactions and Processes Influenced by Soil Wetness
 Reactions & Processes Influenced by Soil Wetness Topics for Consideration A. Organic matter (OM) accumulation B. Some reduction effects induced by soil saturation C. Redoximorphic features D. Calcium carbonate
Reactions & Processes Influenced by Soil Wetness Topics for Consideration A. Organic matter (OM) accumulation B. Some reduction effects induced by soil saturation C. Redoximorphic features D. Calcium carbonate
SUPPLEMENTARY INFORMATION
 Supplementary Figure 1 Simplified schematic representation of carbon cycles of widely different time-scales through northern peatlands and potential impacts of climate warming. Carbon cycles through ecosystems
Supplementary Figure 1 Simplified schematic representation of carbon cycles of widely different time-scales through northern peatlands and potential impacts of climate warming. Carbon cycles through ecosystems
TRANSCRIPT. Thanks Ashlee, my name is Rod, and we re going to open up with a poll question. [Pause for poll]
![TRANSCRIPT. Thanks Ashlee, my name is Rod, and we re going to open up with a poll question. [Pause for poll] TRANSCRIPT. Thanks Ashlee, my name is Rod, and we re going to open up with a poll question. [Pause for poll]](/thumbs/81/84610995.jpg) TRANSCRIPT SLIDE 1 [00:00]: Speaker: Thanks Ashlee, my name is Rod, and we re going to open up with a poll question. SLIDE 2 [00:07]: The question should have popped up on your screen. How large is the
TRANSCRIPT SLIDE 1 [00:00]: Speaker: Thanks Ashlee, my name is Rod, and we re going to open up with a poll question. SLIDE 2 [00:07]: The question should have popped up on your screen. How large is the
Energy & Carbon Sequestration
 Energy & Carbon Sequestration Applications Carbon sequestration To significantly increase the level of carbon sequestration. Can we engineer the system such that microbes will nucleate CaCO 3 into a granular
Energy & Carbon Sequestration Applications Carbon sequestration To significantly increase the level of carbon sequestration. Can we engineer the system such that microbes will nucleate CaCO 3 into a granular
General Groundwater Concepts
 General Groundwater Concepts Hydrologic Cycle All water on the surface of the earth and underground are part of the hydrologic cycle (Figure 1), driven by natural processes that constantly transform water
General Groundwater Concepts Hydrologic Cycle All water on the surface of the earth and underground are part of the hydrologic cycle (Figure 1), driven by natural processes that constantly transform water
Sequestration of Atmospheric Carbon Dioxide as Inorganic Carbon under Semi-Arid Forests
 Sequestration of Atmospheric Carbon Dioxide as Inorganic Carbon under Semi-Arid Forests Murray Moinester, Joel Kronfeld, Israel Carmi Tel Aviv University World Conference on Climate Change, October 24-26,
Sequestration of Atmospheric Carbon Dioxide as Inorganic Carbon under Semi-Arid Forests Murray Moinester, Joel Kronfeld, Israel Carmi Tel Aviv University World Conference on Climate Change, October 24-26,
Ocean Production and CO 2 uptake
 Ocean Production and CO 2 uptake Fig. 6.6 Recall: Current ocean is gaining Carbon.. OCEAN Reservoir size: 38000 Flux in: 90 Flux out: 88+0.2=88.2 90-88.2 = 1.8 Pg/yr OCEAN is gaining 1.8 Pg/yr Sum of the
Ocean Production and CO 2 uptake Fig. 6.6 Recall: Current ocean is gaining Carbon.. OCEAN Reservoir size: 38000 Flux in: 90 Flux out: 88+0.2=88.2 90-88.2 = 1.8 Pg/yr OCEAN is gaining 1.8 Pg/yr Sum of the
ENVIRONMENTAL GEOLOGY - GEOL 406/506
 ENVIRONMENTAL GEOLOGY - GEOL 406/506 Glossary of useful Terms: 1. Abiotic: not living. 2. A b s o r p t i o n: the penetration of atoms, ions, or molecules into the bulk mass of substrate. 3. Acclimation:
ENVIRONMENTAL GEOLOGY - GEOL 406/506 Glossary of useful Terms: 1. Abiotic: not living. 2. A b s o r p t i o n: the penetration of atoms, ions, or molecules into the bulk mass of substrate. 3. Acclimation:
Soil Particle Density Protocol
 Soil Particle Density Protocol Purpose To measure the soil particle density of each horizon in a soil profile Overview Students weigh a sample of dry, sieved soil from a horizon, mix it with distilled
Soil Particle Density Protocol Purpose To measure the soil particle density of each horizon in a soil profile Overview Students weigh a sample of dry, sieved soil from a horizon, mix it with distilled
Water Resources Engineering. Prof. R. Srivastava. Department of Water Resources Engineering. Indian Institute of Technology, Kanpur.
 Water Resources Engineering Prof. R. Srivastava Department of Water Resources Engineering Indian Institute of Technology, Kanpur Lecture # 13 Today we will continue to discuss some of the abstractions
Water Resources Engineering Prof. R. Srivastava Department of Water Resources Engineering Indian Institute of Technology, Kanpur Lecture # 13 Today we will continue to discuss some of the abstractions
Ecosystems. Trophic relationships determine the routes of energy flow and chemical cycling in ecosystems.
 AP BIOLOGY ECOLOGY ACTIVITY #5 Ecosystems NAME DATE HOUR An ecosystem consists of all the organisms living in a community as well as all the abiotic factors with which they interact. The dynamics of an
AP BIOLOGY ECOLOGY ACTIVITY #5 Ecosystems NAME DATE HOUR An ecosystem consists of all the organisms living in a community as well as all the abiotic factors with which they interact. The dynamics of an
Carbon in the Environment
 EES 022: Exploring Earth Name Carbon in the Environment Abstract: Today s Date Lab Due Date You will be collecting data on the carbon stocks within three general land types, a cultivated field, an abandoned
EES 022: Exploring Earth Name Carbon in the Environment Abstract: Today s Date Lab Due Date You will be collecting data on the carbon stocks within three general land types, a cultivated field, an abandoned
This presentation is on the value of reducing emissions and enhancing removals of greenhouse gases related to land use and land cover change in
 This presentation is on the value of reducing emissions and enhancing removals of greenhouse gases related to land use and land cover change in tropical wetland forests. 1 The objective of this presentation
This presentation is on the value of reducing emissions and enhancing removals of greenhouse gases related to land use and land cover change in tropical wetland forests. 1 The objective of this presentation
Tracing carbon isotopes - From the atmosphere to the cave. Summer School on Speleothem Science Jens Fohlmeister
 Tracing carbon isotopes - From the atmosphere to the cave Summer School on Speleothem Science Jens Fohlmeister Heidelberg, 30.07.2013 Villars d 13 C record d 13 C reflects vegetation signal warm, more
Tracing carbon isotopes - From the atmosphere to the cave Summer School on Speleothem Science Jens Fohlmeister Heidelberg, 30.07.2013 Villars d 13 C record d 13 C reflects vegetation signal warm, more
Use of Stable Oxygen Isotopes in Studies of Forest-Atmospheric CO 2 & H 2 O Exchange. Chun-Ta Lai San Diego State University
 Use of Stable Oxygen Isotopes in Studies of Forest-Atmospheric CO 2 & H 2 O Exchange Chun-Ta Lai San Diego State University June 2008 Atmospheric Composition Change Increasing CO 2 Increasing CH 4 Decreasing
Use of Stable Oxygen Isotopes in Studies of Forest-Atmospheric CO 2 & H 2 O Exchange Chun-Ta Lai San Diego State University June 2008 Atmospheric Composition Change Increasing CO 2 Increasing CH 4 Decreasing
Bulk Density Protocol
 Bulk Density Protocol Purpose To measure the soil bulk density of each horizon in your soil profile. Overview Students obtain a soil sample in the field using a container with a measured volume. The soil
Bulk Density Protocol Purpose To measure the soil bulk density of each horizon in your soil profile. Overview Students obtain a soil sample in the field using a container with a measured volume. The soil
SAMPLE CHAPTERS UNESCO EOLSS GROUNDWATER MONITORING. Masanori Ando Musashino University, Japan
 GROUNDWATER MONITORING Masanori Ando Musashino University, Japan Keywords: groundwater, monitoring, sampling, monitoring program, monitoring location, sampling programs, flow measurement, sampling techniques,
GROUNDWATER MONITORING Masanori Ando Musashino University, Japan Keywords: groundwater, monitoring, sampling, monitoring program, monitoring location, sampling programs, flow measurement, sampling techniques,
The Release of Base Metals During Acidic Leaching of Fly Ash
 The Release of Base Metals During Acidic Leaching of Fly Ash George Kazonich and Ann G. Kim U.S. Department of Energy Federal Energy Technology Center P.O. Box 19 Pittsburgh, PA 153 ABSTRACT Since 199,
The Release of Base Metals During Acidic Leaching of Fly Ash George Kazonich and Ann G. Kim U.S. Department of Energy Federal Energy Technology Center P.O. Box 19 Pittsburgh, PA 153 ABSTRACT Since 199,
Installing Equipment for the Hydric Soil Technical Standard
 Installing Equipment for the Hydric Soil Technical Standard Mike Vepraskas, NC State Univ. April 2005 Procedure: 1. Sites: Select at least two spots to monitor. One should be within a hydric soil, or at
Installing Equipment for the Hydric Soil Technical Standard Mike Vepraskas, NC State Univ. April 2005 Procedure: 1. Sites: Select at least two spots to monitor. One should be within a hydric soil, or at
Effects of Long-Term Soil Warming on Aggregate Mass and Physical Protection of Organic Matter
 Effects of Long-Term Soil Warming on Aggregate Mass and Physical Protection of Organic Matter Luis Cartagena, Northwestern University ABSTRACT Global warming may induce accelerated soil organic matter
Effects of Long-Term Soil Warming on Aggregate Mass and Physical Protection of Organic Matter Luis Cartagena, Northwestern University ABSTRACT Global warming may induce accelerated soil organic matter
Principles of Terrestrial Ecosystem Ecology
 E Stuart Chapin III Pamela A. Matson Harold A. Mooney Principles of Terrestrial Ecosystem Ecology Illustrated by Melissa C. Chapin With 199 Illustrations Teehnische Un.fversitSt Darmstadt FACHBEREIGH 10
E Stuart Chapin III Pamela A. Matson Harold A. Mooney Principles of Terrestrial Ecosystem Ecology Illustrated by Melissa C. Chapin With 199 Illustrations Teehnische Un.fversitSt Darmstadt FACHBEREIGH 10
Inputs. Outputs. Component/store. Section of a system where material or energy is held. Something that enters the system (material or energy)
 .. Inputs Something that enters the system (material or energy) Outputs Something that leaves the system (material or energy) Component/store Section of a system where material or energy is held Transfer/flow
.. Inputs Something that enters the system (material or energy) Outputs Something that leaves the system (material or energy) Component/store Section of a system where material or energy is held Transfer/flow
Bulk Density Protocol
 Bulk Density Protocol Purpose To measure the bulk density of each horizon in a soil profile Overview In the field, students collect three soil samples from each horizon in a soil profile using a container
Bulk Density Protocol Purpose To measure the bulk density of each horizon in a soil profile Overview In the field, students collect three soil samples from each horizon in a soil profile using a container
Movement and Storage of Groundwater The Hydrosphere
 Movement and Storage of Groundwater The Hydrosphere The water on and in Earth s crust makes up the hydrosphere. About 97 percent of the hydrosphere is contained in the oceans. The water contained by landmasses
Movement and Storage of Groundwater The Hydrosphere The water on and in Earth s crust makes up the hydrosphere. About 97 percent of the hydrosphere is contained in the oceans. The water contained by landmasses
Soil Respiration and Carbon Sequestration of an Oakgrass Savanna in California: Roles of temperature, soil moisture, rain events and photosynthesis
 001-006 Mission Kearney Foundation of Soil Science: Soil Carbon and California's Terrestrial Ecosystems Final Report: 000, 1/1/003-1/31/00 Soil Respiration and Carbon Sequestration of an Oakgrass Savanna
001-006 Mission Kearney Foundation of Soil Science: Soil Carbon and California's Terrestrial Ecosystems Final Report: 000, 1/1/003-1/31/00 Soil Respiration and Carbon Sequestration of an Oakgrass Savanna
Soil Physical Properties and Wastewater Treatment
 Soil Physical Properties and Wastewater Treatment John R. Buchanan, Ph.D., P. E. Associate Professor Biosystems Engineering and Soil Science Department Soil Physical Properties and Wastewater Treatment
Soil Physical Properties and Wastewater Treatment John R. Buchanan, Ph.D., P. E. Associate Professor Biosystems Engineering and Soil Science Department Soil Physical Properties and Wastewater Treatment
CO 2. and the carbonate system II. Carbon isotopes as a tracer for circulation. The (solid) carbonate connection with. The ocean climate connection
 CO 2 and the carbonate system II Carbon isotopes as a tracer for circulation The (solid) carbonate connection with ocean acidity Climate The ocean climate connection The carbon cycle the carbon cycle involves
CO 2 and the carbonate system II Carbon isotopes as a tracer for circulation The (solid) carbonate connection with ocean acidity Climate The ocean climate connection The carbon cycle the carbon cycle involves
An Introduction into Applied Soil Hydrology
 Klaus Bohne An Introduction into Applied Soil Hydrology Preface Contents 1 Introduction: The soil as a reactor between the earth's atmosphere and the subsurface 1 2 Mechanical composition of mineral soils
Klaus Bohne An Introduction into Applied Soil Hydrology Preface Contents 1 Introduction: The soil as a reactor between the earth's atmosphere and the subsurface 1 2 Mechanical composition of mineral soils
The effect of acetate on the oxygen production of Chlamydomonas reinhardtii
 The effect of acetate on the oxygen production of Chlamydomonas reinhardtii Daniela Castillo, Ivy Chang, Jenny Kim, Monica Ng Abstract Chlamydomonas reinhardtii is a unicellular green alga used for studying
The effect of acetate on the oxygen production of Chlamydomonas reinhardtii Daniela Castillo, Ivy Chang, Jenny Kim, Monica Ng Abstract Chlamydomonas reinhardtii is a unicellular green alga used for studying
Water Related Soil Properties
 Water Related Soil Properties Water is essential for plant growth. Plants extract mineral laden water from the soil. For effective irrigation and drainage it is important that the processes governing soil
Water Related Soil Properties Water is essential for plant growth. Plants extract mineral laden water from the soil. For effective irrigation and drainage it is important that the processes governing soil
Porosity of Compost Water retention capacity of Compost Organic matter content of Compost Buffering capacity of Compost
 Porosity of Compost Water retention capacity of Compost Organic matter content of Compost Buffering capacity of Compost by Page 1/21 Contents What is the effect of compost on soil properties?... 3 Introduction:...
Porosity of Compost Water retention capacity of Compost Organic matter content of Compost Buffering capacity of Compost by Page 1/21 Contents What is the effect of compost on soil properties?... 3 Introduction:...
6 SAMPLING OF WATER QUANTITIES, METHODS, POINTS OF ATTENTION
 6 SAMPLING OF WATER QUANTITIES, METHODS, POINTS OF ATTENTION Water sampling intends to provide information on water quality and quantity, and on hydrodynamic properties of systems under observation. To
6 SAMPLING OF WATER QUANTITIES, METHODS, POINTS OF ATTENTION Water sampling intends to provide information on water quality and quantity, and on hydrodynamic properties of systems under observation. To
groundwater. Because watersheds are complex systems, each tends to respond differently to natural or human activities.
 The private development of Altos del María is located at an altitude between 550 and 1,000 meters above sea level in the environmentally sensitive Cordillera Central of Panama that separates the Pacific
The private development of Altos del María is located at an altitude between 550 and 1,000 meters above sea level in the environmentally sensitive Cordillera Central of Panama that separates the Pacific
Overview. Learning Objectives: This module provides step-by-step instructions in how to do the Bulk Density Protocol.
 Overview This module provides step-by-step instructions in how to do the Bulk Density Protocol. Learning Objectives: After completing this module, you will be able to: Explain why bulk density is worth
Overview This module provides step-by-step instructions in how to do the Bulk Density Protocol. Learning Objectives: After completing this module, you will be able to: Explain why bulk density is worth
Instrumentation for Soil Measurement: Progress and Hurdles
 Instrumentation for Soil Measurement: Progress and Hurdles Colin S. Campbell, Ph.D. 1 Outline Soil moisture sensors Soil water potential Mars update Introduction 2 1 Water Content Measurement ECH 2 O high
Instrumentation for Soil Measurement: Progress and Hurdles Colin S. Campbell, Ph.D. 1 Outline Soil moisture sensors Soil water potential Mars update Introduction 2 1 Water Content Measurement ECH 2 O high
Nutrient Cycling & Soils
 Nutrient Cycling & Soils tutorial by Paul Rich Outline 1. Nutrient Cycles What are nutrient cycles? major cycles 2. Water Cycle 3. Carbon Cycle 4. Nitrogen Cycle 5. Phosphorus Cycle 6. Sulfur Cycle 7.
Nutrient Cycling & Soils tutorial by Paul Rich Outline 1. Nutrient Cycles What are nutrient cycles? major cycles 2. Water Cycle 3. Carbon Cycle 4. Nitrogen Cycle 5. Phosphorus Cycle 6. Sulfur Cycle 7.
Methane baseline concentrations and sources in shallow aquifers from the shale. gas-prone region of the St. Lawrence Lowlands (Quebec, Canada)
 Methane baseline concentrations and sources in shallow aquifers from the shale gas-prone region of the St. Lawrence Lowlands (Quebec, Canada) Anja Moritz 1, Jean-Francois Hélie 2, Daniele L. Pinti 2, Marie
Methane baseline concentrations and sources in shallow aquifers from the shale gas-prone region of the St. Lawrence Lowlands (Quebec, Canada) Anja Moritz 1, Jean-Francois Hélie 2, Daniele L. Pinti 2, Marie
The Carbon cycle. Atmosphere, terrestrial biosphere and ocean are constantly exchanging carbon
 The Carbon cycle Atmosphere, terrestrial biosphere and ocean are constantly exchanging carbon The oceans store much more carbon than the atmosphere and the terrestrial biosphere The oceans essentially
The Carbon cycle Atmosphere, terrestrial biosphere and ocean are constantly exchanging carbon The oceans store much more carbon than the atmosphere and the terrestrial biosphere The oceans essentially
Supplementary Materials
 FIELD METHODS Supplementary Materials Dielectric resistivity-based volumetric soil water content (m 3 H 2 0 / m 3 soil) and temperature sensors (Decagon Instruments, model EC-TM) were installed in the
FIELD METHODS Supplementary Materials Dielectric resistivity-based volumetric soil water content (m 3 H 2 0 / m 3 soil) and temperature sensors (Decagon Instruments, model EC-TM) were installed in the
Hana Santruckova Introduction in the topic of the IP Soil & Water :
 ERASMUS Soil & Water Protokoll Montag 17.09.2012 Hana Santruckova Introduction in the topic of the IP Soil & Water : Soil-Water-Interactions water retention capacity of soil: water doesn't evaporate in
ERASMUS Soil & Water Protokoll Montag 17.09.2012 Hana Santruckova Introduction in the topic of the IP Soil & Water : Soil-Water-Interactions water retention capacity of soil: water doesn't evaporate in
Climate warming accelerates CO 2 -release from subsurface carbon in a sub-arctic peatland
 Climate warming accelerates CO 2 -release from subsurface carbon in a sub-arctic peatland Ellen Dorrepaal Sylvia Toet, Richard van Logtestijn, Elferra Swart Marjan van de Weg, Terry Callaghan, Rien Aerts
Climate warming accelerates CO 2 -release from subsurface carbon in a sub-arctic peatland Ellen Dorrepaal Sylvia Toet, Richard van Logtestijn, Elferra Swart Marjan van de Weg, Terry Callaghan, Rien Aerts
Global Carbon Cycle - II
 Global Carbon Cycle - II OCN 401 - Biogeochemical Systems Reading: Schlesinger, Chapter 11 1. Current status of global CO 2 2. CO 2 speciation in water 3. Atmosphere ocean interactions 4. Ocean acidification
Global Carbon Cycle - II OCN 401 - Biogeochemical Systems Reading: Schlesinger, Chapter 11 1. Current status of global CO 2 2. CO 2 speciation in water 3. Atmosphere ocean interactions 4. Ocean acidification
Draw a ring around the correct answer to complete each sentence.
 1 Fossil fuels contain carbon. The figure below represents a carbon atom. Draw a ring around the correct answer to complete each sentence. (i) The name of the particle with a positive charge is an electron.
1 Fossil fuels contain carbon. The figure below represents a carbon atom. Draw a ring around the correct answer to complete each sentence. (i) The name of the particle with a positive charge is an electron.
River transport and chemistry. Lecture Outline
 OCN 401 Biogeochemical Systems (10.12.17) (Schlesinger & Bernhardt: Chapter 8) River transport and chemistry Lecture Outline 1. Introduction Overview 2. Soil Hydraulics & Stream Hydrology 3. Stream Load
OCN 401 Biogeochemical Systems (10.12.17) (Schlesinger & Bernhardt: Chapter 8) River transport and chemistry Lecture Outline 1. Introduction Overview 2. Soil Hydraulics & Stream Hydrology 3. Stream Load
UAU102F, University of Iceland
 Systems, systems theory, thermodynamics SYSTEMS System A set of components that interact with one another A system must be distinguishable from its environment (i.e. must be able to identify it) Systems
Systems, systems theory, thermodynamics SYSTEMS System A set of components that interact with one another A system must be distinguishable from its environment (i.e. must be able to identify it) Systems
will denote mass of suspension before the filtration in an accumulation tank, m F mass of filtrate, m K mass of produced filtration cake, m S
 6 iltration Alexandra Novotná, Oldřich Holeček English translation by ichal ordač I Basic definitions iltration is a method of separation of solid fluid mixtures. The mixture if pressed through a layer
6 iltration Alexandra Novotná, Oldřich Holeček English translation by ichal ordač I Basic definitions iltration is a method of separation of solid fluid mixtures. The mixture if pressed through a layer
Orono Spectral Solutions, Inc.
 Page 1 of 14 Orono Spectral Solutions, Inc. STANDARD OPERATING PROCEDURE for ASTM Method DXXXX, Hydrocarbon Contamination in Urea Liquor REVISION # 1 Effective date: April 5, 2012 APPROVED BY Signature
Page 1 of 14 Orono Spectral Solutions, Inc. STANDARD OPERATING PROCEDURE for ASTM Method DXXXX, Hydrocarbon Contamination in Urea Liquor REVISION # 1 Effective date: April 5, 2012 APPROVED BY Signature
River transport and chemistry
 OCN 401 Biogeochemical Systems (10.15.15) (Schlesinger & Bernhardt: Chapter 8) River transport and chemistry Lecture Outline 1. Introduction - Overview 2. Soil Hydraulics & Stream Hydrology 3. Stream Load
OCN 401 Biogeochemical Systems (10.15.15) (Schlesinger & Bernhardt: Chapter 8) River transport and chemistry Lecture Outline 1. Introduction - Overview 2. Soil Hydraulics & Stream Hydrology 3. Stream Load
Mixed-Oak Forest On A Fine-Textured Alfisol
 NRE 430 / EEB 489 D.R. Zak 2003 Lab #3 Mixed-Oak Forest On A Fine-Textured Alfisol Radrick Forest is typical of many of the hardwood forests that occupy dry-mesic to wet-mesic sites in southern Michigan,
NRE 430 / EEB 489 D.R. Zak 2003 Lab #3 Mixed-Oak Forest On A Fine-Textured Alfisol Radrick Forest is typical of many of the hardwood forests that occupy dry-mesic to wet-mesic sites in southern Michigan,
Ecosystems and the Biosphere: Energy Flow Through the Ecosystem and the Recycling of Matter
 Name Ecosystems and the Biosphere: Energy Flow Through the Ecosystem and the Recycling of Matter Overview: An ecosystem is: All of the organisms living on Earth need to carry out life processes such as
Name Ecosystems and the Biosphere: Energy Flow Through the Ecosystem and the Recycling of Matter Overview: An ecosystem is: All of the organisms living on Earth need to carry out life processes such as
Other Septic Tank Effluent Dispersal Options
 Other Septic Tank Effluent Dispersal Options CEE484 Decentralized and Onsite Waste water Management and Reuse April 16, 2007 Department of Civil and Environmental Engineering University of Washington Septic
Other Septic Tank Effluent Dispersal Options CEE484 Decentralized and Onsite Waste water Management and Reuse April 16, 2007 Department of Civil and Environmental Engineering University of Washington Septic
Stable carbon isotopes of DIC and dissolved methane concentrations on the western Iberian Margin
 Stable carbon isotopes of DIC and dissolved methane concentrations on the western Iberian Margin G. Rehder and R.S. Keir GEOMAR D - 24148 Kiel, Germany Introduction The 13 C/ 12 C ratio of total CO 2 (δ
Stable carbon isotopes of DIC and dissolved methane concentrations on the western Iberian Margin G. Rehder and R.S. Keir GEOMAR D - 24148 Kiel, Germany Introduction The 13 C/ 12 C ratio of total CO 2 (δ
Installation of the BAT Mk III Filter Tip
 Installation of the BAT Mk III Filter Tip - Preparation - Installation - Accessories BAT Geosystems AB Box 1060 SE - 186 26 Vallentuna Sweden Phone +46 851170600 Fax +46 851173361 www.bat-gms.com info@bat-gms.com
Installation of the BAT Mk III Filter Tip - Preparation - Installation - Accessories BAT Geosystems AB Box 1060 SE - 186 26 Vallentuna Sweden Phone +46 851170600 Fax +46 851173361 www.bat-gms.com info@bat-gms.com
Pump Tank and Pretreatment Inspection & Troubleshooting. Sara Heger University of Minnesota
 Pump Tank and Pretreatment Inspection & Troubleshooting Sara Heger University of Minnesota sheger@umn.edu Evaluate Presence of Odor Odors are improper venting Check seals Lid Conduit Tank Access a. Access
Pump Tank and Pretreatment Inspection & Troubleshooting Sara Heger University of Minnesota sheger@umn.edu Evaluate Presence of Odor Odors are improper venting Check seals Lid Conduit Tank Access a. Access
Biochemical Oxygen Demand
 Biochemical Oxygen Demand Computer 20 Oxygen available to aquatic organisms is found in the form of dissolved oxygen. Oxygen gas is dissolved in a stream through aeration, diffusion from the atmosphere,
Biochemical Oxygen Demand Computer 20 Oxygen available to aquatic organisms is found in the form of dissolved oxygen. Oxygen gas is dissolved in a stream through aeration, diffusion from the atmosphere,
Diffusion-based Soil Respiration Chamber Measurements
 Vaisala CARBOCAP Carbon Dioxide Probe GMP343: Diffusion-based Soil Respiration Chamber Measurements This application note contains general information about soil respiration and discusses some issues worth
Vaisala CARBOCAP Carbon Dioxide Probe GMP343: Diffusion-based Soil Respiration Chamber Measurements This application note contains general information about soil respiration and discusses some issues worth
an ecosystem is a community of different species interacting with one another and with their nonliving environment of matter and energy
 1 Ecocsystems: Energy Flow and Materials Cycling 2 EVPP 111 Lecture Dr. Largen Spring 2004 Energy Flow and Matter Cycling Energy flow s through ecosystems ecosystems global energy budget physical laws
1 Ecocsystems: Energy Flow and Materials Cycling 2 EVPP 111 Lecture Dr. Largen Spring 2004 Energy Flow and Matter Cycling Energy flow s through ecosystems ecosystems global energy budget physical laws
NUTRIENT CYCLES REVIEW
 52 Name A.P. Environmental Science Date Mr. Romano NUTRIENT CYCLES REVIEW 1. Which of the following chain of events would occur as a result of land clearing/deforestation? (vocabulary check: efflux means
52 Name A.P. Environmental Science Date Mr. Romano NUTRIENT CYCLES REVIEW 1. Which of the following chain of events would occur as a result of land clearing/deforestation? (vocabulary check: efflux means
from volcanoes; carbonate (CaCO 3 + CO 2 + H 2 . The sinks are carbonate rock weathering + SiO2. Ca HCO
 The Carbon Cycle Chemical relations We would like to be able to trace the carbon on Earth and see where it comes and where it goes. The sources are CO 2 from volcanoes; carbonate (CaCO 3 ) formation in
The Carbon Cycle Chemical relations We would like to be able to trace the carbon on Earth and see where it comes and where it goes. The sources are CO 2 from volcanoes; carbonate (CaCO 3 ) formation in
Assessment of Variability in Flux Chamber-based Soil CO 2 Efflux Measurements at Petroleum Remediation Sites
 Assessment of Variability in Flux Chamber-based Soil CO 2 Efflux Measurements at Petroleum Remediation Sites Ellen Porter- CH2M HILL, Calgary Remediation Technologist October 17, 2014 Innovation that Solves
Assessment of Variability in Flux Chamber-based Soil CO 2 Efflux Measurements at Petroleum Remediation Sites Ellen Porter- CH2M HILL, Calgary Remediation Technologist October 17, 2014 Innovation that Solves
Chapter 34 Nature of Ecosystems. Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
 Chapter 34 Nature of Ecosystems 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 34.1 The Biotic Components of Ecosystems Ecosystems Abiotic components include
Chapter 34 Nature of Ecosystems 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 34.1 The Biotic Components of Ecosystems Ecosystems Abiotic components include
Urban Soil Conservation and Management
 Urban Soil Conservation and Management Urban Soil include those located in: Cities in park areas Recreation areas Community gardens Green belts Lawns Septic absorption fields Sediment basins We need a
Urban Soil Conservation and Management Urban Soil include those located in: Cities in park areas Recreation areas Community gardens Green belts Lawns Septic absorption fields Sediment basins We need a
Carbon Dioxide, Alkalinity and ph
 Carbon Dioxide, Alkalinity and ph OCN 623 Chemical Oceanography 31 January 2013 Reading: Libes, Chapter 15, pp. 383 394 (Remainder of chapter will be used with the lecture: Biogenic production, carbonate
Carbon Dioxide, Alkalinity and ph OCN 623 Chemical Oceanography 31 January 2013 Reading: Libes, Chapter 15, pp. 383 394 (Remainder of chapter will be used with the lecture: Biogenic production, carbonate
Tananyag fejlesztés idegen nyelven
 Tananyag fejlesztés idegen nyelven Prevention of the atmosphere KÖRNYEZETGAZDÁLKODÁSI AGRÁRMÉRNÖKI MSC (MSc IN AGRO-ENVIRONMENTAL STUDIES) Calculation of greenhouse effect. The carbon cycle Lecture 11
Tananyag fejlesztés idegen nyelven Prevention of the atmosphere KÖRNYEZETGAZDÁLKODÁSI AGRÁRMÉRNÖKI MSC (MSc IN AGRO-ENVIRONMENTAL STUDIES) Calculation of greenhouse effect. The carbon cycle Lecture 11
Runoff and Its Impact on the Ecosystem
 Runoff and Its Impact on the Ecosystem We are looking at the effects that soil nutrients in runoff, specifically nitrogen, have on bacteria in the soil. Before we started our experiment, we researched
Runoff and Its Impact on the Ecosystem We are looking at the effects that soil nutrients in runoff, specifically nitrogen, have on bacteria in the soil. Before we started our experiment, we researched
Hydrologic Cycle. Rain Shadow:
 Hydrologic Cycle The cyclical movement of water from the ocean to the atmosphere by evaporation, to the surface through precipitation, to streams through runoff and groundwater, and back to the ocean.
Hydrologic Cycle The cyclical movement of water from the ocean to the atmosphere by evaporation, to the surface through precipitation, to streams through runoff and groundwater, and back to the ocean.
Patrick Nimiago Forest Research Institute,Lae
 Patrick Nimiago Forest Research Institute,Lae PRESENTATION CONTENT 1. Why soil survey Is 2. soil What to a survey living and measure or non living thing? 3. Where to sample soil 4. Timing of sample 5.
Patrick Nimiago Forest Research Institute,Lae PRESENTATION CONTENT 1. Why soil survey Is 2. soil What to a survey living and measure or non living thing? 3. Where to sample soil 4. Timing of sample 5.
Lecture 11: Water Flow; Soils and the Hydrologic Cycle
 Lecture 11: Water Flow; Soils and the Hydrologic Cycle Water Flow in Soils Types of Water Flow in Soil Saturated flow: Soil pores completely filled with water; controlled by the hydrostatic potential After
Lecture 11: Water Flow; Soils and the Hydrologic Cycle Water Flow in Soils Types of Water Flow in Soil Saturated flow: Soil pores completely filled with water; controlled by the hydrostatic potential After
Climate and Carbon Cycle Coupling Carbon Cycle - Modern
 Early Cenozoic Carbon Cycle and Climate Coupling: Evidence from δ 13 C & δ 18 O Zachos et al. (2008) Climate and Carbon Cycle Coupling Carbon Cycle - Modern Fluxes and Feedbacks Carbon Isotope Tracers
Early Cenozoic Carbon Cycle and Climate Coupling: Evidence from δ 13 C & δ 18 O Zachos et al. (2008) Climate and Carbon Cycle Coupling Carbon Cycle - Modern Fluxes and Feedbacks Carbon Isotope Tracers
Maximum Surface Storage Provided by Crop Residue
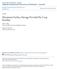 University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Biological Systems Engineering: Papers and Publications Biological Systems Engineering 6-1994 Maximum Surface Storage Provided
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Biological Systems Engineering: Papers and Publications Biological Systems Engineering 6-1994 Maximum Surface Storage Provided
TOOLS AND TIPS FOR MEASURING THE FULL SOIL MOISTURE RELEASE CURVE
 18188-00 6.9.2017 TOOLS AND TIPS FOR MEASURING THE FULL SOIL MOISTURE RELEASE CURVE HOW TO CREATE A FULL MOISTURE RELEASE CURVE USING THE WP4C AND HYPROP Creating a full moisture release curve is challenging.
18188-00 6.9.2017 TOOLS AND TIPS FOR MEASURING THE FULL SOIL MOISTURE RELEASE CURVE HOW TO CREATE A FULL MOISTURE RELEASE CURVE USING THE WP4C AND HYPROP Creating a full moisture release curve is challenging.
BASICS OF WASTEWATER TREATMENT
 BASICS OF WASTEWATER TREATMENT Knowing the decisioning criteria relevant to site and drain field suitability, i.e., soil properties, can be enhanced by an understanding of some of the basics of wastewater
BASICS OF WASTEWATER TREATMENT Knowing the decisioning criteria relevant to site and drain field suitability, i.e., soil properties, can be enhanced by an understanding of some of the basics of wastewater
Ecosystems and Nutrient Cycles Chapters 3
 Ecosystems and Nutrient Cycles Chapters 3 Prokaryotic and Eukaryotic cells Figure 3-2 Prokaryotic cells: Have organelles. Bacteria and Archaea are composed of prokaryotic cells. Eukaryotic cells: cells,
Ecosystems and Nutrient Cycles Chapters 3 Prokaryotic and Eukaryotic cells Figure 3-2 Prokaryotic cells: Have organelles. Bacteria and Archaea are composed of prokaryotic cells. Eukaryotic cells: cells,
COMPARISON OF THE HEAT TRANSFER THROUGH HYDROMX WITH THAT OF WATER
 COMPARISON OF THE HEAT TRANSFER THROUGH HYDROMX WITH THAT OF WATER WITNESS REPORT ON EXPERIMENTAL TESTS Written By Dr Issa Chaer, BEng, PhD, MCIBSE, FInst.R, Reviewed By Mr. Irfan Ahmet Kuran - KODEM Ltd
COMPARISON OF THE HEAT TRANSFER THROUGH HYDROMX WITH THAT OF WATER WITNESS REPORT ON EXPERIMENTAL TESTS Written By Dr Issa Chaer, BEng, PhD, MCIBSE, FInst.R, Reviewed By Mr. Irfan Ahmet Kuran - KODEM Ltd
Rising atmospheric CO 2. The Carbon Cycle
 The Carbon Cycle Rising atmospheric CO 2 I. Introduction: Changes to Global C Cycle (Ch. 15) II. C-cycle overview: pools & fluxes (Ch. 6) III. Controls on GPP (Ch. 5) IV. Controls on NPP (Ch. 6) V. Controls
The Carbon Cycle Rising atmospheric CO 2 I. Introduction: Changes to Global C Cycle (Ch. 15) II. C-cycle overview: pools & fluxes (Ch. 6) III. Controls on GPP (Ch. 5) IV. Controls on NPP (Ch. 6) V. Controls
Erik Lindeberg and Per Bergmo. SINTEF Petroleum Research, NO-7465 Trondheim, Norway
 THE LONG-TERM FATE OF CO 2 INJECTED INTO AN AQUIFER Erik Lindeberg and Per Bergmo SINTEF Petroleum Research, NO-7465 Trondheim, Norway ABSTRACT Assuming that an underground aquifer is capped by a capillary
THE LONG-TERM FATE OF CO 2 INJECTED INTO AN AQUIFER Erik Lindeberg and Per Bergmo SINTEF Petroleum Research, NO-7465 Trondheim, Norway ABSTRACT Assuming that an underground aquifer is capped by a capillary
Figure 1. Location of research sites in the Ameriflux network (from Ameriflux web site,
 CONTEXT - AMERIFLUX NETWORK Figure 1. Location of research sites in the Ameriflux network (from Ameriflux web site, http://public.ornl.gov/ameriflux/). AMERIFLUX OBJECTIVES: Quantify spatial and temporal
CONTEXT - AMERIFLUX NETWORK Figure 1. Location of research sites in the Ameriflux network (from Ameriflux web site, http://public.ornl.gov/ameriflux/). AMERIFLUX OBJECTIVES: Quantify spatial and temporal
E. M. Ryan et al. Correspondence to: Edmund M. Ryan
 Supplement of Geosci. Model Dev., 11, 1909 1928, 2018 https://doi.org/10.5194/gmd-11-1909-2018-supplement Author(s) 2018. This work is distributed under the Creative Commons Attribution 4.0 License. Supplement
Supplement of Geosci. Model Dev., 11, 1909 1928, 2018 https://doi.org/10.5194/gmd-11-1909-2018-supplement Author(s) 2018. This work is distributed under the Creative Commons Attribution 4.0 License. Supplement
VADOSE/W 2D Tutorial
 Elevation 1 Introduction VADOSE/W 2D Tutorial This example illustrates the basic methodology for simulating soil-climate interaction of an engineered soil cover system placed over a waste. The primary
Elevation 1 Introduction VADOSE/W 2D Tutorial This example illustrates the basic methodology for simulating soil-climate interaction of an engineered soil cover system placed over a waste. The primary
Do Now. The ocean contains a large diversity of organisms, but their numbers are starting to decline.
 Do Now The ocean contains a large diversity of organisms, but their numbers are starting to decline. What do you think is affecting marine life in the oceans? Why? Provide evidence to support your reasoning!
Do Now The ocean contains a large diversity of organisms, but their numbers are starting to decline. What do you think is affecting marine life in the oceans? Why? Provide evidence to support your reasoning!
The Carbon Cycle: Global to local. Ruth Varner, PhD
 The Carbon Cycle: Global to local Ruth Varner, PhD Atmospheric CO 2 at Mauna Loa Keeling, C.D. and T.P. Whorf. 2004. Atmospheric CO 2 records from sites in the SIO air sampling An Impeccable Record of
The Carbon Cycle: Global to local Ruth Varner, PhD Atmospheric CO 2 at Mauna Loa Keeling, C.D. and T.P. Whorf. 2004. Atmospheric CO 2 records from sites in the SIO air sampling An Impeccable Record of
The Global Carbon Cycle
 The Global Carbon Cycle Tom Bibby September 2003 bibby@imcs.rutgers.edu falko@imcs.rutgers.edu The Carbon Cycle - Look at past climatic change; as controlled by the carbon cycle. - Interpret the influence
The Global Carbon Cycle Tom Bibby September 2003 bibby@imcs.rutgers.edu falko@imcs.rutgers.edu The Carbon Cycle - Look at past climatic change; as controlled by the carbon cycle. - Interpret the influence
Temporal and Spatial Patterns of Water Isotope Ratios (δ 2 H and δ 18 O) in Municipal Tap Water as Measured by TCEA-IRMS
 Naval Research Laboratory Washington, DC 20375-5320 NRL/MR/6110--12-9394 Temporal and Spatial Patterns of Water Isotope Ratios (δ 2 H and δ 18 O) in Municipal Tap Water as Measured by TCEA-IRMS Leila J.
Naval Research Laboratory Washington, DC 20375-5320 NRL/MR/6110--12-9394 Temporal and Spatial Patterns of Water Isotope Ratios (δ 2 H and δ 18 O) in Municipal Tap Water as Measured by TCEA-IRMS Leila J.
Nutrient Cycling in an Aquatic Ecosystem
 Nutrient Cycling in an Aquatic Ecosystem 2.1 Productivity 2.2 Oxygen 2.3 Salinity 2.4 Carbon 2.5 Nitrogen 2.6 Phosphorous 2.7 Iron 2.8 Sulphur 2.9 Silica 2.3 Salinity of Inland Waters The salinity of freshwaters
Nutrient Cycling in an Aquatic Ecosystem 2.1 Productivity 2.2 Oxygen 2.3 Salinity 2.4 Carbon 2.5 Nitrogen 2.6 Phosphorous 2.7 Iron 2.8 Sulphur 2.9 Silica 2.3 Salinity of Inland Waters The salinity of freshwaters
Well Stimulation and Sand Production Management (PGE 489 ) Sandstone Acidizing. By Dr. Mohammed A. Khamis
 Well Stimulation and Sand Production Management (PGE 489 ) Sandstone Acidizing By Dr. Mohammed A. Khamis 23-02-2016 Sandstones Acidizing The goal of sandstone matrix acidizing is to remove siliceous particles
Well Stimulation and Sand Production Management (PGE 489 ) Sandstone Acidizing By Dr. Mohammed A. Khamis 23-02-2016 Sandstones Acidizing The goal of sandstone matrix acidizing is to remove siliceous particles
dissolved fraction particulate fraction Resuspension
 CE4505 SURFACE WATER QUALITY PROJECT 2B. TORCH LAKE RECOVERY MODEL Due 10/29/09 The model that we will use for the next week is shown in Fig. 1 below. This model is a simplification of reality in two major
CE4505 SURFACE WATER QUALITY PROJECT 2B. TORCH LAKE RECOVERY MODEL Due 10/29/09 The model that we will use for the next week is shown in Fig. 1 below. This model is a simplification of reality in two major
Remediation of Brine Spills- What Goes Wrong Kerry Sublette
 Remediation of Brine Spills- What Goes Wrong Kerry Sublette University of Tulsa Spills of produced water or brine on soil result in two types of damage: Excess salinity Creates an osmotic imbalance that
Remediation of Brine Spills- What Goes Wrong Kerry Sublette University of Tulsa Spills of produced water or brine on soil result in two types of damage: Excess salinity Creates an osmotic imbalance that
CEEN Laboratory 1 Mechanical Sieve Analysis Specific Gravity of Soil Solids Gravimetric/Volumetric Relations
 INTRODUCTION CEEN 3160 - Laboratory 1 Mechanical Sieve Analysis Specific Gravity of Soil Solids Gravimetric/Volumetric Relations Grain size analysis is widely used for the classification of soils and for
INTRODUCTION CEEN 3160 - Laboratory 1 Mechanical Sieve Analysis Specific Gravity of Soil Solids Gravimetric/Volumetric Relations Grain size analysis is widely used for the classification of soils and for
Chem 355 Jasperse DISTILLATION
 Chem 355 Jasperse DISTILLATION 1 Background Distillation is a widely used technique for purifying liquids. The basic distillation process involves heating a liquid such that liquid molecules vaporize.
Chem 355 Jasperse DISTILLATION 1 Background Distillation is a widely used technique for purifying liquids. The basic distillation process involves heating a liquid such that liquid molecules vaporize.
Agronomy 406 World Climates
 Agronomy 406 World Climates February 13, 2018 Hydrologic cycle. Team 4 Climate News presentation this Thursday. Review: METED module, Understanding the Hydrologic Cycle Active review session for the midterm
Agronomy 406 World Climates February 13, 2018 Hydrologic cycle. Team 4 Climate News presentation this Thursday. Review: METED module, Understanding the Hydrologic Cycle Active review session for the midterm
Introduction of Dinghushan Forest Ecosystem Research Station
 Introduction of Dinghushan Forest Ecosystem Research Station Dinghushan Station Chinese Academy of Sciences Bayreuth, Germany 2010-04-13 Outline Location of Dinghushan History of Long-term ecological research
Introduction of Dinghushan Forest Ecosystem Research Station Dinghushan Station Chinese Academy of Sciences Bayreuth, Germany 2010-04-13 Outline Location of Dinghushan History of Long-term ecological research
A MODEL FOR SOIL OXYGEN DELIVERY TO WASTEWATER INFILTRATION SURFACES. J. Erickson, E. J. Tyler* ABSTRACT
 #4.44 A MODEL FOR SOIL OXYGEN DELIVERY TO WASTEWATER INFILTRATION SURFACES J. Erickson, E. J. Tyler* ABSTRACT Soil could accept onsite wastewater at rates two to three orders of magnitude higher than the
#4.44 A MODEL FOR SOIL OXYGEN DELIVERY TO WASTEWATER INFILTRATION SURFACES J. Erickson, E. J. Tyler* ABSTRACT Soil could accept onsite wastewater at rates two to three orders of magnitude higher than the
Transport & Transformation of chemicals in an ecosystem, involving numerous interrelated physical, chemical, & biological processes
 OPEN Wetland Ecology Lectures 14-15-16 Wetland Biogeochemistry What is biogeochemical cycling? Transport & Transformation of chemicals in an ecosystem, involving numerous interrelated physical, chemical,
OPEN Wetland Ecology Lectures 14-15-16 Wetland Biogeochemistry What is biogeochemical cycling? Transport & Transformation of chemicals in an ecosystem, involving numerous interrelated physical, chemical,
Orono Spectral Solutions, Inc.
 Page 1 of 10 Orono Spectral Solutions, Inc. STANDARD OPERATING PROCEDURE for ASTM Method DXXXX, Hydrocarbon Contamination in Anhydrous ammonia REVISION # 1 Effective date: April 5, 2012 APPROVED BY Signature
Page 1 of 10 Orono Spectral Solutions, Inc. STANDARD OPERATING PROCEDURE for ASTM Method DXXXX, Hydrocarbon Contamination in Anhydrous ammonia REVISION # 1 Effective date: April 5, 2012 APPROVED BY Signature
Primary Production and Respiration on Land. Production. Respiration. Decomposition
 Primary Production and Respiration on Land Production Respiration Decomposition Photosynthesis: CO 2 + H 2 O + energy = (CH 2 O) + O 2 Respiration: (CH 2 O) + O 2 = CO 2 + H 2 O + energy Carbon balance
Primary Production and Respiration on Land Production Respiration Decomposition Photosynthesis: CO 2 + H 2 O + energy = (CH 2 O) + O 2 Respiration: (CH 2 O) + O 2 = CO 2 + H 2 O + energy Carbon balance
How Ecosystems Work: Energy Flow and Nutrient Cycles
 How Ecosystems Work: Energy Flow and Nutrient Cycles Bubble in your ID and the answer to the 25 questions. You can look up the answers to these question on line. 1. The flow of solar energy through an
How Ecosystems Work: Energy Flow and Nutrient Cycles Bubble in your ID and the answer to the 25 questions. You can look up the answers to these question on line. 1. The flow of solar energy through an
Do not write on the question paper, do not turn in the question paper. Put your answers on a new paper. Unless told otherwise.
 APES Unit 2 Packet Due Sept 10 at the beginning of class Instructions for all homework and labs: Put your name and date on top right corner of all your papers. Put the name of the problem or lab at the
APES Unit 2 Packet Due Sept 10 at the beginning of class Instructions for all homework and labs: Put your name and date on top right corner of all your papers. Put the name of the problem or lab at the
STREAM DISSOLVED OXYGEN IMPROVEMENT FEASIBILITY STUDY
 SALT CREEK / DUPAGE RIVER WORK GROUP STREAM DISSOLVED OXYGEN IMPROVEMENT FEASIBILITY STUDY FOR SALT CREEK AND EAST BRANCH OF THE DUPAGE RIVER DATA REPORT 2007 SOD MEASUREMENT SURVEY SALT CREEK OCTOBER
SALT CREEK / DUPAGE RIVER WORK GROUP STREAM DISSOLVED OXYGEN IMPROVEMENT FEASIBILITY STUDY FOR SALT CREEK AND EAST BRANCH OF THE DUPAGE RIVER DATA REPORT 2007 SOD MEASUREMENT SURVEY SALT CREEK OCTOBER
VADOSE/W 2D Tutorial
 1 Introduction VADOSE/W 2D Tutorial This example illustrates the basic methodology for simulating soil-climate interaction of an engineered soil cover system placed over a waste. The primary objective
1 Introduction VADOSE/W 2D Tutorial This example illustrates the basic methodology for simulating soil-climate interaction of an engineered soil cover system placed over a waste. The primary objective
