HEALTH PRODUCTS STEWARDSHIP ASSOCIATION COLLECTION LOCATION STANDARDS AGREEMENT ONTARIO
|
|
|
- Brent Noah Strickland
- 5 years ago
- Views:
Transcription
1 1. Introduction HEALTH PRODUCTS STEWARDSHIP ASSOCIATION COLLECTION LOCATION STANDARDS AGREEMENT ONTARIO This Collection Location standards agreement ( Agreement ) applies to the receiving, handling and storage of Pharmaceutical Waste and Sharps Waste generated by the Public in Ontario. This Agreement is entered into between (1) ( Collection Location ) and (2) the Health Products Stewardship Association ( HPSA ) and is effective as of the date this document is signed below ( Effective Date ). 2. Acronyms and Definitions Approved Sharps Container: A hard shell, colour-coded, plastic container that is leak and tamper resistant and is labelled with the universal biohazard symbol. Collection Container: A hard shell plastic container and lid with HPSA markings on the outside used to contain Pharmaceuticals returned by the Public. Collection Location: the premises of each Retail Pharmacy. Customer: A patron of a Retail Pharmacy registered with HPSA who returns Sharps and/or Pharmaceuticals for proper disposal. Designated Person: A Retail Pharmacy owner, licensed pharmacist or pharmacy employee authorized to be responsible for this Agreement. HPSA: Health Products Stewardship Association MRP: Medication Return Program SCP: Sharps Collection Program Medical waste over-packing: Consists of a plastic liner in a medical waste cardboard box into which Sharps containers are placed for storage and proper disposal. Pharmaceutical(s): A drug within the meaning of section 2 of the Food and Drugs Act (Canada) and includes a natural health product within the meaning of the Natural Health Product Regulations made under that Act. Pharmaceutical Waste: Unused and expired medications generated by the Public. Public: Persons who lawfully obtain and possess pharmaceuticals and/or sharps for their own use or for the use of a household member. Retail Pharmacy: Any public-facing community pharmacy which is licensed by the provincial College of Pharmacists, that is accessible to the public with posted business hours and that has signed the certification set out in the Schedule to this Agreement. Sharp(s): A needle, safety engineered needle, lancet, or other instrument designed to puncture the skin for medical purposes and includes anything affixed to the sharp, including a syringe. Sharps Waste: Used Sharps generated by the Public. 3. Terms and Conditions 3.1 General Conditions a) Sharps Waste and Pharmaceutical Waste generated by the Public shall be given to a pharmacist or pharmacy technician who is trained to determine if the waste is acceptable or not. b) Pharmaceutical Waste returned by the Public is acceptable. c) Sharps Waste returned by the Public in Approved Sharps Containers is acceptable. 1
2 d) The Designated Person at the Retail Pharmacy agrees that Sharps and Pharmaceutical waste from the Public will be accepted regardless of whether the member of the Public is a Customer. e) The Retail Pharmacy will offer the HPSA programs free of charge to members of the Public (including the distribution of HPSA Sharps Containers). f) The Retail Pharmacy acting as a Collection Location shall operate in accordance with all applicable municipal, provincial and federal regulations. g) HPSA shall comply with, and shall operate the MRP and SCP in accordance with, all applicable municipal, provincial and federal regulations. 3.2 Public Education a) The Retail Pharmacy may, at the point of display or point of sale of a Pharmaceutical or Sharp product, prominently display education and awareness information about the SCP and the MRP as provided by HPSA. HPSA shall ensure that any such education and awareness information complies with all applicable municipal, provincial and federal regulations and shall not violate any third party interests (including but not limited to their intellectual property rights). b) The Retail Pharmacy may cause its trained pharmacists or pharmacy technicians to educate the Public at their discretion about proper disposal of Sharps and Pharmaceutical Waste. 3.3 Used Sharps Collection by Pharmacy Staff a) The pharmacy shall only accept Sharps that are returned by the Public in an Approved Sharps Container designed to hold needles and other medical devices that contain a Sharp. b) Members of the Public attempting to return Sharps in a non-conforming container shall be provided with an HPSA Approved Sharps Container and instructed to transfer the Sharps from the non-conforming container to the HPSA container and then return it to the pharmacy. c) Pharmacy staff shall inspect each sharps container to ensure the lid is in a closed and locked position and shall deposit all sharps containers in medical waste over-packing provided by HPSA. 3.4 Used Sharps Storage and Disposal a) Once the medical waste box if full, the liner must be securely tied off and the medical waste box closed and secured with the tape provided. b) The weight of each medical waste box should not exceed 23 kg. c) Sharps are to be stored at all times in a location at the Retail Pharmacy where access is controlled and restricted to trained pharmacy employees only. 3.5 Unused and Expired Pharmaceuticals Collection by Pharmacy Staff a) All solid dosage pharmaceuticals should be deposited into the Collection Container and the Retail Pharmacy may cause its pharmacy employees to attempt to recycle as much of the primary and secondary packaging as possible. b) Liquid medications, gels and powders should be deposited directly into the Collection Container in the primary packaging. No free liquids or powder. 3.6 Unused and Expired Pharmaceuticals Storage and Disposal a) The Collection Container lid must be securely closed when the Collection Container is full and ready for disposal. Applying firm pressure to the lid will cause it to close and zip ties are then used to lock it for storage and transportation. b) The weight of each Collection Container of Pharmaceutical Waste shall not exceed 23 kg. c) Narcotics and controlled drugs and substances returned by the Public should be managed according to the requirements of applicable Health Canada regulations and policy. 2
3 d) Collection containers are to be stored at all times in the dispensary where access is controlled and restricted to trained pharmacy employees only. 3.7 Unacceptable Waste The following wastes are not acceptable under the SCP or the MRP: a) Medical waste other than Sharps Waste. b) Pharmaceutical and Sharps Waste generated by the business of the pharmacy (e.g.: short dated, expired, recalled, withdrawn Pharmaceuticals and expired seasonal medications from the dispensary). c) Pharmaceutical and Sharps Waste from hospitals, medical and dental clinics, methadone clinics, long term care facilities and nursing homes. d) Expired pharmaceutical samples from a dentist or doctor s office. e) Veterinary Pharmaceutical Waste from companion animals and agricultural operations. f) Mercury thermometers or any other items containing mercury. g) Aerosol containers (except used inhalers). h) Flu and immunization shots administered on site. 3.8 Training and Record Keeping a) Employees of the Retail Pharmacy that handle Pharmaceuticals and Sharps returned by the Public shall receive annual training on the terms and conditions of this Agreement including the proper collection, handling and storage of Sharps and Pharmaceutical Waste. b) The Retail Pharmacy shall keep one copy of the signed Agreement on file along with any other relevant documentation provided by HPSA and provide HPSA with a signed copy of page 3 of the Agreement. c) The Retail Pharmacy will inform HPSA in writing of any change in its operations that could affect its status as a Collection Location. d) The Collection Location shall keep any shipping document associated with the generation and transportation of Sharps and Pharmaceutical Waste on file for 2 years. e) The Collection Location will attempt to make any reasonable shipping documentation available on file with the Collection Location and not otherwise available to HPSA upon 30 day s advance written request. 3.9 Other a) The Collection Location may terminate this Agreement (in whole or for specified Retail Pharmacies) at any time by issuing HPSA with no less than 14 (fourteen) days notice to that effect. b) HPSA shall indemnify the Collection Location and the Retail Pharmacies against all and any loss they may suffer as a result of their participation in the MPR/SCP, this Agreement and/or HPSA s breach, non-compliance or default of its obligations under this Agreement. c) HPSA may terminate this Agreement (in whole or for specified Retail Pharmacies) at any time by issuing the Collection Location with no less than 14 (fourteen) days notice to that effect. [REMAINDER OF PAGE INTENTIONALLY LEFT BLANK] 3
4 IN WITNESS WHEREOF the parties have executed this Agreement as of the date first written above. Health Products Stewardship Association Per: Name: Title: I have the authority to bind the Corporation (Collection Location) Per: Name: Title: I have the authority to bind the Corporation 4
5 SCHEDULE 1 HPSA COLLECTION LOCATION STANDARDS AGREEMENT AND REGISTRATION I UNDERSTAND THE ABOVE TERMS AND CONDITIONS AND AGREE TO FOLLOW THEM IN AN EFFORT TO PROTECT RETAIL PHARMACY STAFF AND THE PUBLIC. I CERTIFY THAT TO MY KNOWLEDGE THE MATERIAL COLLECTED AT THE RETAIL PHARMACY IS COMPRISED OF ONLY POST-CONSUMER PUBLIC WASTE AS DEFINED IN SECTION 2 OF THIS AGREEMENT AND DOES NOT INCLUDE ANY HAZARDOUS WASTE OR OTHER WASTE MATERIAL. Name of Designated Person responsible for this Public waste program Signature Pharmacy Name: Pharmacy Banner/Chain (e.g. Shoppers, Rexall, Independent): College of Pharmacists PHARMACY Permit/Accreditation Number: Phone Number: Fax Number: Street Address: Unit/PO Box/RR#: City: Province: Postal Code: Address: PLEASE SIGN AND RETURN THIS PAGE BY FAX AT , OR A COPY TO info@healthsteward.ca. YOU CAN ALSO QUICKLY COMPLETE THIS FORM AND AGREEMENT ONLINE BY VISITING THE ORIGINAL COPY OF THE ENTIRE AGREEMENT MUST BE KEPT ON FILE AT THE PHARMACY. 5
HEALTH PRODUCTS STEWARDSHIP ASSOCIATION COLLECTION LOCATION STANDARDS AGREEMENT
 1. Introduction HEALTH PRODUCTS STEWARDSHIP ASSOCIATION COLLECTION LOCATION STANDARDS AGREEMENT This Collection Location standards agreement ( Agreement ) applies to the receiving, handling and storage
1. Introduction HEALTH PRODUCTS STEWARDSHIP ASSOCIATION COLLECTION LOCATION STANDARDS AGREEMENT This Collection Location standards agreement ( Agreement ) applies to the receiving, handling and storage
Community Pharmacy Opening/Renovation Audit
 Community Pharmacy Opening/Renovation Audit Date of Audit: Pharmacy Information Pharmacy Name Address City Postal Code Pharmacy Hours Telephone Mon Tues Wed Thurs Fri Sat Sun Fax e-mail Website Pharmacist(s)
Community Pharmacy Opening/Renovation Audit Date of Audit: Pharmacy Information Pharmacy Name Address City Postal Code Pharmacy Hours Telephone Mon Tues Wed Thurs Fri Sat Sun Fax e-mail Website Pharmacist(s)
PROGRAM PLAN FOR THE ONTARIO SHARPS COLLECTION PROGRAM MAY 2013
 PROGRAM PLAN FOR THE ONTARIO SHARPS COLLECTION PROGRAM MAY 2013 EXECUTIVE SUMMARY Ontario Regulation 298/12 "Collection of Pharmaceuticals and Sharps - Responsibility of Producers" came into force on October
PROGRAM PLAN FOR THE ONTARIO SHARPS COLLECTION PROGRAM MAY 2013 EXECUTIVE SUMMARY Ontario Regulation 298/12 "Collection of Pharmaceuticals and Sharps - Responsibility of Producers" came into force on October
STANDARD OPERATING PROCEDURES DIVISION OF COMPARATIVE MEDICINE UNIVERSITY OF SOUTH FLORIDA
 STANDARD OPERATING PROCEDURES DIVISION OF COMPARATIVE MEDICINE UNIVERSITY OF SOUTH FLORIDA SOP#: 014.5 Date Issued: 11/00 Date Revised: 8/15 Page 1 of 5 TITLE: SCOPE: RESPONSIBILITY: PURPOSE: Research
STANDARD OPERATING PROCEDURES DIVISION OF COMPARATIVE MEDICINE UNIVERSITY OF SOUTH FLORIDA SOP#: 014.5 Date Issued: 11/00 Date Revised: 8/15 Page 1 of 5 TITLE: SCOPE: RESPONSIBILITY: PURPOSE: Research
Coast Waste Management Association Compliance and Safety in Handling Hazardous, Regulated and Controlled Waste
 Coast Waste Management Association Compliance and Safety in Handling Hazardous, Regulated and Controlled Waste DATE: October 20 th, 2017 William Yeo Director of Sales, Environmental Solutions, Stericycle
Coast Waste Management Association Compliance and Safety in Handling Hazardous, Regulated and Controlled Waste DATE: October 20 th, 2017 William Yeo Director of Sales, Environmental Solutions, Stericycle
Extended Producer Responsibility for Waste Pharmaceuticals and Sharps in Canada
 Extended Producer Responsibility for Waste Pharmaceuticals and Sharps in Canada 2016 Used Oil and Household Hazardous Waste Training & Conference November 3 2016 Extended Producer Responsibility and product
Extended Producer Responsibility for Waste Pharmaceuticals and Sharps in Canada 2016 Used Oil and Household Hazardous Waste Training & Conference November 3 2016 Extended Producer Responsibility and product
Subj: IMPLEMENTATION AND MANAGEMENT OF PRESCRIPTION DRUG TAKE BACK PROGRAM
 DEPARTMENT OF THE NAVY BUREAU OF MEDICINE AND SURGERY 7700 ARLINGTON BOULEVARD FALLS CHURCH, VA 22042 IN REPLY REFER TO BUMEDINST 6570.4 BUMED-M3/B2 BUMED INSTRUCTION 6570.4 From: Chief, Bureau of Medicine
DEPARTMENT OF THE NAVY BUREAU OF MEDICINE AND SURGERY 7700 ARLINGTON BOULEVARD FALLS CHURCH, VA 22042 IN REPLY REFER TO BUMEDINST 6570.4 BUMED-M3/B2 BUMED INSTRUCTION 6570.4 From: Chief, Bureau of Medicine
TJEMS Drug Box Program Best Practices Reviewed: Updated: November 2015
 TJEMS Drug Box Program Best Practices Reviewed: Updated: November 2015 The TJEMS Drug Box Program Best Practices relate to the use of the TJEMS Drug Box. These best practices sever to provide guidance
TJEMS Drug Box Program Best Practices Reviewed: Updated: November 2015 The TJEMS Drug Box Program Best Practices relate to the use of the TJEMS Drug Box. These best practices sever to provide guidance
10.0 DISPOSAL OF FARM WASTE
 DISPOSAL OF FARM WASTE 10.1 Disposal of Dead Animals 10.1.1 Managing dead animal disposal 10.2 Disposal of Veterinary Waste 10.3 Pesticides 10.2.1 Sharps 10.2.2 Medicine disposal 10.3.1 Pesticide disposal
DISPOSAL OF FARM WASTE 10.1 Disposal of Dead Animals 10.1.1 Managing dead animal disposal 10.2 Disposal of Veterinary Waste 10.3 Pesticides 10.2.1 Sharps 10.2.2 Medicine disposal 10.3.1 Pesticide disposal
Please Sign and Date Above and Initial Each of the Following Pages and Fax Back to or scan & to
 New Client Application and Duplication & Fulfillment Agreement Basic Information (Please print clearly) Company Name: Name of Primary Contact: Phone: Email: Physical Address: City: State: Zip: Billing
New Client Application and Duplication & Fulfillment Agreement Basic Information (Please print clearly) Company Name: Name of Primary Contact: Phone: Email: Physical Address: City: State: Zip: Billing
NORTH CAROLINA VETERINARY MEDICAL BOARD PRACTICE INSPECTION 1611 Jones Franklin Road, Suite 106, Raleigh NC (919) FAX (919)
 NORTH CAROLINA VETERINARY MEDICAL BOARD PRACTICE INSPECTION 1611 Jones Franklin Road, Suite 106, Raleigh NC 27606 (919) 854-5601 FAX (919) 854-5606 FOR OFFICE STAFF ONLY Photo(s): $125.00 Inspection Fee
NORTH CAROLINA VETERINARY MEDICAL BOARD PRACTICE INSPECTION 1611 Jones Franklin Road, Suite 106, Raleigh NC 27606 (919) 854-5601 FAX (919) 854-5606 FOR OFFICE STAFF ONLY Photo(s): $125.00 Inspection Fee
I. Purpose. II. Definitions. Last Approval Date
 Investigational Drugs and Biologics Page 1 of 13 I. Purpose The purpose of this policy is to establish procedures for the proper control, storage, use and handling of investigational drugs and biologics
Investigational Drugs and Biologics Page 1 of 13 I. Purpose The purpose of this policy is to establish procedures for the proper control, storage, use and handling of investigational drugs and biologics
UN 3291 Clinical and Related Wastes Services Specification
 SSS013 UN 3291 Clinical and Related Wastes Services Specification 1. Scope This specification describes the acceptable waste materials and packaging requirements for Daniels Health services for a subset
SSS013 UN 3291 Clinical and Related Wastes Services Specification 1. Scope This specification describes the acceptable waste materials and packaging requirements for Daniels Health services for a subset
Biomedical Waste - Legal Issues. John Tidball
 Biomedical Waste - Legal Issues John Tidball jtidball@millerthomson.com AGENDA Overview of Ontario regulatory regime. On-site treatment of biomedical waste. Biomedical waste generator obligations. Collection
Biomedical Waste - Legal Issues John Tidball jtidball@millerthomson.com AGENDA Overview of Ontario regulatory regime. On-site treatment of biomedical waste. Biomedical waste generator obligations. Collection
PQS performance specification
 PQS performance specification WHO/PQS/E10/SB01.1 Original: English Distribution: General TITLE: Safety box for the disposal of used sharps Specification reference: E10/SB01.1 Product verification protocol:
PQS performance specification WHO/PQS/E10/SB01.1 Original: English Distribution: General TITLE: Safety box for the disposal of used sharps Specification reference: E10/SB01.1 Product verification protocol:
Explanatory Note for Pharmacists, on the Supply of Emergency Prescription-Only Medicines to a Listed Organisation
 Explanatory Note for Pharmacists, on the Supply of Emergency Prescription-Only Medicines to a Listed Organisation Pharmaceutical Society of Ireland Version 1 April 2017 Contents 1. Background 2 2. About
Explanatory Note for Pharmacists, on the Supply of Emergency Prescription-Only Medicines to a Listed Organisation Pharmaceutical Society of Ireland Version 1 April 2017 Contents 1. Background 2 2. About
DRUGS RULES, 1976 DRUGS (IMPORT & EXPORT) RULES, 1976
 DRUGS RULES, 1976 DRUGS (IMPORT & EXPORT) RULES, 1976 DRUGS (IMPORT & EXPORT) RULES, 1976 S R O. 890 (I)/76. In exercise of the powers conferred by Section 43 of the Drugs Act, 1976 (XXX! of 1976), the
DRUGS RULES, 1976 DRUGS (IMPORT & EXPORT) RULES, 1976 DRUGS (IMPORT & EXPORT) RULES, 1976 S R O. 890 (I)/76. In exercise of the powers conferred by Section 43 of the Drugs Act, 1976 (XXX! of 1976), the
GUIDELINES FOR THE USE OF CONTROLLED SUBSTANCES IN RESEARCH MICHIGAN STATE UNIVERSITY ENVIRONMENTAL HEALTH AND SAFETY
 GUIDELINES FOR THE USE OF CONTROLLED SUBSTANCES IN RESEARCH MICHIGAN STATE UNIVERSITY ENVIRONMENTAL HEALTH AND SAFETY REVISION 4.2018 INTRODUCTION In conducting research with controlled substances, University
GUIDELINES FOR THE USE OF CONTROLLED SUBSTANCES IN RESEARCH MICHIGAN STATE UNIVERSITY ENVIRONMENTAL HEALTH AND SAFETY REVISION 4.2018 INTRODUCTION In conducting research with controlled substances, University
Legal Aspects and Process of Biomedical Waste Management Practices in India. *Prakash V **Dr. N. Palaniraj
 Legal Aspects and Process of Biomedical Waste Management Practices in India *Prakash V **Dr. N. Palaniraj *Research Scholar ** Associate Professor, PG & Research Department of Economics, Pachaiyappa s
Legal Aspects and Process of Biomedical Waste Management Practices in India *Prakash V **Dr. N. Palaniraj *Research Scholar ** Associate Professor, PG & Research Department of Economics, Pachaiyappa s
QUALITY AGREEMENT. The following Agreement has been concluded between
 QUALITY AGREEMENT The following Agreement has been concluded between LLC LABORATORIES The contract laboratory and service provider 1625 Trinity Dr., Unit 11 Mississauga, Ontario Canada L5T 1W9 Hereinafter
QUALITY AGREEMENT The following Agreement has been concluded between LLC LABORATORIES The contract laboratory and service provider 1625 Trinity Dr., Unit 11 Mississauga, Ontario Canada L5T 1W9 Hereinafter
*NOTIFY THE DEPARTMENT IN WRITING OF ANY UPDATES
 APPLICATION FOR A PERMIT UNDER CHAPTER 499, FLORIDA STATUTES Florida Department of Business and Professional Regulation Drugs, Devices, and Cosmetics Program 1940 North Monroe Street, Tallahassee FL 323990783
APPLICATION FOR A PERMIT UNDER CHAPTER 499, FLORIDA STATUTES Florida Department of Business and Professional Regulation Drugs, Devices, and Cosmetics Program 1940 North Monroe Street, Tallahassee FL 323990783
- Procedures to Become a Voluntary Steward of the Multi-Material Stewardship Manitoba Program; and - Voluntary Steward Agreement
 - Procedures to Become a Voluntary Steward of the Multi-Material Stewardship Manitoba Program; and - Voluntary Steward Agreement For detailed program information: www.stewardshipmanitoba.org Tel. 1-888-980-9549
- Procedures to Become a Voluntary Steward of the Multi-Material Stewardship Manitoba Program; and - Voluntary Steward Agreement For detailed program information: www.stewardshipmanitoba.org Tel. 1-888-980-9549
BSWH Technician Continuing Education 2016 Part 2: Pharmacy Law - Controlled Substance Security & Drug Supply Chain Security Act (DSCSA) Contact
 BSWH Technician Continuing Education 2016 Part 2: Pharmacy Law - Controlled Substance Security & Drug Supply Chain Security Act (DSCSA) Contact Hours: 1.5 Objectives List some strategies regarding safe
BSWH Technician Continuing Education 2016 Part 2: Pharmacy Law - Controlled Substance Security & Drug Supply Chain Security Act (DSCSA) Contact Hours: 1.5 Objectives List some strategies regarding safe
A report to recommend the award of RFP P for the Loading, Transportation and Recycling/Disposal for Municipal Hazardous or Special Wastes.
 TO: FROM: Members of the Committee of the Whole W. H. Jackson, Director of Utility Services MEETING DATE: September 29, 2008 SUBJECT: and Recycling/Disposal of Municipal Hazardous or Special Wastes PURPOSE
TO: FROM: Members of the Committee of the Whole W. H. Jackson, Director of Utility Services MEETING DATE: September 29, 2008 SUBJECT: and Recycling/Disposal of Municipal Hazardous or Special Wastes PURPOSE
PHS Policy, U.S. Government Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research, and Training:
 Effective: 10/18/2018 Institutional Animal Care and Use Committee Guiding Principles and Procedures Revised: 1/17/2019 Title: Pharmaceutical Management Page 1 of 7 Guiding Principle The use of animals
Effective: 10/18/2018 Institutional Animal Care and Use Committee Guiding Principles and Procedures Revised: 1/17/2019 Title: Pharmaceutical Management Page 1 of 7 Guiding Principle The use of animals
PHARMACY, MEDICINES & POISONS BOARD
 PHARMACY, MEDICINES & POISONS BOARD PHARMACY GUIDELINES FOR INVESTIGATIONAL DRUGS A. Purpose These guidelines are principally derived and adapted from guidelines from various sources within the SADC region.
PHARMACY, MEDICINES & POISONS BOARD PHARMACY GUIDELINES FOR INVESTIGATIONAL DRUGS A. Purpose These guidelines are principally derived and adapted from guidelines from various sources within the SADC region.
LABORATORY WASTE DISPOSAL GUIDE
 LABORATORY WASTE DISPOSAL GUIDE Purpose This guide will help lab members properly identify and manage hazardous waste in their laboratory. Identifying the types of waste generated is crucial to handling
LABORATORY WASTE DISPOSAL GUIDE Purpose This guide will help lab members properly identify and manage hazardous waste in their laboratory. Identifying the types of waste generated is crucial to handling
RETURNED MEDICATIONS AND RECLAMATION PROCESSING
 Page 1 of 14 SUBJECT: PURPOSE: POLICY: Returned medications and reclamation processing To define the process for handling products returned to the Pharmacy to ensure that medications are properly destroyed
Page 1 of 14 SUBJECT: PURPOSE: POLICY: Returned medications and reclamation processing To define the process for handling products returned to the Pharmacy to ensure that medications are properly destroyed
Standard Operating Procedures
 Standard Operating Procedures 5.14.1 Investigational Product Handling and Storage History Version Date Author Reason 1.1 23 rd August B Fazekas New procedure 2007 1.2 16 th October 2007 B Fazekas Update
Standard Operating Procedures 5.14.1 Investigational Product Handling and Storage History Version Date Author Reason 1.1 23 rd August B Fazekas New procedure 2007 1.2 16 th October 2007 B Fazekas Update
GUIDELINES FOR REGISTRATION OF IMPORTERS AND FOR THE IMPORTATION OF MEDICINES AND RELATED PRODUCTS
 MEDICINES CONTROL AGENCY 54 Kairaba Avenue, Pipeline, The Gambia. Tel.no. +220 4380632 GUIDELINES FOR REGISTRATION OF IMPORTERS AND FOR THE IMPORTATION OF MEDICINES AND RELATED PRODUCTS Document no: MCA/IMPG/2017/1
MEDICINES CONTROL AGENCY 54 Kairaba Avenue, Pipeline, The Gambia. Tel.no. +220 4380632 GUIDELINES FOR REGISTRATION OF IMPORTERS AND FOR THE IMPORTATION OF MEDICINES AND RELATED PRODUCTS Document no: MCA/IMPG/2017/1
Facility Decommissioning Standard. Safety Resources
 Facility Decommissioning Standard 2017 Safety Resources i Contents 1 Purpose... 1 2 Applicable To... 1 3 Definitions... 1 4 Scope... 2 5 Responsibilities... 3 5.1 College/Division/Department/Unit Heads...
Facility Decommissioning Standard 2017 Safety Resources i Contents 1 Purpose... 1 2 Applicable To... 1 3 Definitions... 1 4 Scope... 2 5 Responsibilities... 3 5.1 College/Division/Department/Unit Heads...
Medical Waste Information Bulletin and Disposal Guide
 Public Health Department Cathy A. Raevsky, Director Andy Miller, M.D., Health Officer Environmental Health 202 Mira Loma Drive T: 530.538.7281 Oroville, California 95965 F: 530.538.5339 buttecounty.net/publichealth
Public Health Department Cathy A. Raevsky, Director Andy Miller, M.D., Health Officer Environmental Health 202 Mira Loma Drive T: 530.538.7281 Oroville, California 95965 F: 530.538.5339 buttecounty.net/publichealth
In-State Licensee Newsletter January 2007
 LOUISIANA BOARD OF WHOLESALE DRUG DISTRIBUTORS 12046 Justice Avenue, Suite C Baton Rouge, LA 70816 Phone: 225-295-8567 Fax: 225-295-8568 Website: www.lsbwdd.org email: lsbwdd@bellsouth.net In-State Licensee
LOUISIANA BOARD OF WHOLESALE DRUG DISTRIBUTORS 12046 Justice Avenue, Suite C Baton Rouge, LA 70816 Phone: 225-295-8567 Fax: 225-295-8568 Website: www.lsbwdd.org email: lsbwdd@bellsouth.net In-State Licensee
MEDICAL NITROUS OXIDE AND ENTONOX COMPLIANCE
 Thursday 15 th April 2010 Dear Agent, MEDICAL NITROUS OXIDE AND ENTONOX COMPLIANCE Medical Nitrous Oxide and Entonox are Schedule 4 - Restricted Substances in accordance with the Federal Medicines Act
Thursday 15 th April 2010 Dear Agent, MEDICAL NITROUS OXIDE AND ENTONOX COMPLIANCE Medical Nitrous Oxide and Entonox are Schedule 4 - Restricted Substances in accordance with the Federal Medicines Act
MANUFACTURE OF INVESTIGATIONAL MEDICINAL PRODUCTS
 ANNEX 13 MANUFACTURE OF INVESTIGATIONAL MEDICINAL PRODUCTS Introduction Medicinal products intended for research and development trials are not at present subject either to marketing or manufacturing Community
ANNEX 13 MANUFACTURE OF INVESTIGATIONAL MEDICINAL PRODUCTS Introduction Medicinal products intended for research and development trials are not at present subject either to marketing or manufacturing Community
Clinical Waste. Policy. Responsibilities
 Clinical Waste Policy Clinical waste is defined as hazardous or offensive waste arising from: - any dental, medical, nursing or veterinary practice, or any other practice or establishment providing medical
Clinical Waste Policy Clinical waste is defined as hazardous or offensive waste arising from: - any dental, medical, nursing or veterinary practice, or any other practice or establishment providing medical
For detailed program information: Steward Services:
 2015: - Procedures to Become a Voluntary Steward with Stewardship Ontario Blue Box Program for Packaging and Printed Paper; and - Voluntary Steward Agreement For detailed program information: www.stewardshipontario.ca
2015: - Procedures to Become a Voluntary Steward with Stewardship Ontario Blue Box Program for Packaging and Printed Paper; and - Voluntary Steward Agreement For detailed program information: www.stewardshipontario.ca
THE SAFE DISPOSAL OF CLINICAL/DOMESTIC WASTE
 Section V THE SAFE DISPOSAL OF CLINICAL/DOMESTIC WASTE The Trust is currently reviewing the requirements of the recent guidelines Health Technical Memorandum Safe Management of Healthcare Waste (HTML 07-01).
Section V THE SAFE DISPOSAL OF CLINICAL/DOMESTIC WASTE The Trust is currently reviewing the requirements of the recent guidelines Health Technical Memorandum Safe Management of Healthcare Waste (HTML 07-01).
Central Bio-Medical Waste Treatment Facility
 Central Bio-Medical Waste Treatment Facility Central Biomedical Waste Treatment Facility, Swami Ram Cancer Institute and Research Centre Campus, Government Medical College, Rampur Road, Haldwani is the
Central Bio-Medical Waste Treatment Facility Central Biomedical Waste Treatment Facility, Swami Ram Cancer Institute and Research Centre Campus, Government Medical College, Rampur Road, Haldwani is the
Annual Renewal for a Certificate of Authorization for a Health Profession Corporation
 Annual Renewal for a Certificate of Authorization for a Health Profession Corporation College of Chiropodists of Ontario Ontario Regulation 39/02 made under the Regulated Health Professions Act, 1991 states
Annual Renewal for a Certificate of Authorization for a Health Profession Corporation College of Chiropodists of Ontario Ontario Regulation 39/02 made under the Regulated Health Professions Act, 1991 states
Regulated Medical Waste Generator Inspections An Inspector s View Point
 Regulated Medical Waste Generator Inspections An Inspector s View Point Amy Scaffidi, Investigator I Bur. of Hazardous Waste & UST Compliance & Enforcement NJ Dept. of Environmental Protection Phone (609)
Regulated Medical Waste Generator Inspections An Inspector s View Point Amy Scaffidi, Investigator I Bur. of Hazardous Waste & UST Compliance & Enforcement NJ Dept. of Environmental Protection Phone (609)
VILLAGE OF KRONENWETTER FIRE DEPARTMENT
 VILLAGE OF KRONENWETTER FIRE DEPARTMENT 1582 Kronenwetter Drive Kronenwetter, WI 54455 www.kronenwetter.org Dear Fire Department Applicant: On behalf of the Vil1age of Kronenwetter Fire Department, I would
VILLAGE OF KRONENWETTER FIRE DEPARTMENT 1582 Kronenwetter Drive Kronenwetter, WI 54455 www.kronenwetter.org Dear Fire Department Applicant: On behalf of the Vil1age of Kronenwetter Fire Department, I would
East Building, PHH-30 U.S. Department New Jersey Avenue S.E. of Transportation Washington, D.C DOT-SP 16279
 East Building, PHH-30 U.S. Department 1200 New Jersey Avenue S.E. of Transportation Washington, D.C. 20590 Pipeline and Hazardous Materials Safety Administration DOT-SP 16279 1. GRANTEE: (See individual
East Building, PHH-30 U.S. Department 1200 New Jersey Avenue S.E. of Transportation Washington, D.C. 20590 Pipeline and Hazardous Materials Safety Administration DOT-SP 16279 1. GRANTEE: (See individual
Code of Practice for Holder of Certificate of Registration as an Importer and Exporter of Pharmaceutical Products
 Code of Practice for Holder of Certificate of Registration as an Importer and Exporter of Pharmaceutical Products March 2014 Table of Content INTRODUCTION 3 Section 1: GENERAL RESPONSIBILITIES OF HOLDER
Code of Practice for Holder of Certificate of Registration as an Importer and Exporter of Pharmaceutical Products March 2014 Table of Content INTRODUCTION 3 Section 1: GENERAL RESPONSIBILITIES OF HOLDER
SOUTH AFRICAN NATIONAL STANDARD
 ICS 11.020;13.030.30 ISBN 0-626-14901-0 SOUTH AFRICAN NATIONAL STANDARD Management of healthcare waste Published by Standards South Africa 1 dr lategan road groenkloof! private bag x191 pretoria 0001 tel:
ICS 11.020;13.030.30 ISBN 0-626-14901-0 SOUTH AFRICAN NATIONAL STANDARD Management of healthcare waste Published by Standards South Africa 1 dr lategan road groenkloof! private bag x191 pretoria 0001 tel:
Vendor Registration Application Form
 Vendor Registration Application Form Hospitals must complete the Vendor Registration Application Form in full together with all the required supporting documentation. SECTION 1 VENDOR NUMBER HOSPITAL OR
Vendor Registration Application Form Hospitals must complete the Vendor Registration Application Form in full together with all the required supporting documentation. SECTION 1 VENDOR NUMBER HOSPITAL OR
Cytotoxic Waste Sorting, Handling and Disposal
 Approved by: Cytotoxic Waste Sorting, Handling and Disposal Corporate Director, Environmental Supports Environmental Services Operating Standards Manual Number: 3.1.3.14 Date Approved Next Review December
Approved by: Cytotoxic Waste Sorting, Handling and Disposal Corporate Director, Environmental Supports Environmental Services Operating Standards Manual Number: 3.1.3.14 Date Approved Next Review December
WHO NEEDS TO KNOW THIS PROGRAM All NYU employees that generate, handle or transport RMW should be familiar with this written program.
 Title: NYU Regulated Medical Waste Safety Written Program Effective Date: October 2002 Revision Date: April 14, 2017 Issuing Authority: Responsible Officer: VP, Facilities and Construction Management Director
Title: NYU Regulated Medical Waste Safety Written Program Effective Date: October 2002 Revision Date: April 14, 2017 Issuing Authority: Responsible Officer: VP, Facilities and Construction Management Director
Have you ever been convicted of a: Felony? Misdemeanor? Traffic infraction (moving violation)? if yes, please explain
 EMPLOYMENT APPLICATION An Equal Opportunity Employer Date Position Applied for Full Time Part-Time Seasonal Temporary Date you can begin work This application must be filled out, completely in order to
EMPLOYMENT APPLICATION An Equal Opportunity Employer Date Position Applied for Full Time Part-Time Seasonal Temporary Date you can begin work This application must be filled out, completely in order to
The Right Prescription for Working with Investigational Drug Service at BMC
 The Right Prescription for Working with Investigational Drug Service at BMC Andrew Schoch Pharmacy Intern Northeastern University Class of 2010 Hyeseon Hong, Pharm.D. IDS Pharmacy Manager What is Investigational
The Right Prescription for Working with Investigational Drug Service at BMC Andrew Schoch Pharmacy Intern Northeastern University Class of 2010 Hyeseon Hong, Pharm.D. IDS Pharmacy Manager What is Investigational
APPLICATION FOR MARKETING AUTHORIZATION OF A PHARMACEUTICAL PRODUCT FOR HUMAN USE IN UGANDA
 National Drug Authority Plot No. 46-48 Lumumba Avenue, P.O. Box 23096, Kampala, Uganda. email: ndaug@nda.or.ug; website: www.nda.or.ug Doc. No.: DAR/FOM/170 Revision No.: 1 Effective Date: 21 June 2016
National Drug Authority Plot No. 46-48 Lumumba Avenue, P.O. Box 23096, Kampala, Uganda. email: ndaug@nda.or.ug; website: www.nda.or.ug Doc. No.: DAR/FOM/170 Revision No.: 1 Effective Date: 21 June 2016
Management of Hazardous Wastes
 23 Management of Hazardous Wastes Dr. Mohammed Abu Kaff Environment & Public Health Expert Dubai Municipality Dubai. UAE Hazardous wastes have been defined by the United States Environmental Protection
23 Management of Hazardous Wastes Dr. Mohammed Abu Kaff Environment & Public Health Expert Dubai Municipality Dubai. UAE Hazardous wastes have been defined by the United States Environmental Protection
Guide to Wholesaling and Brokering of Medicinal Products for Human Use in Ireland
 Guide to Wholesaling and Brokering of Medicinal Products for Human Use in Ireland IA-G0008-5 17 JUNE 2017 This guide does not purport to be an interpretation of law and/or regulations and is for guidance
Guide to Wholesaling and Brokering of Medicinal Products for Human Use in Ireland IA-G0008-5 17 JUNE 2017 This guide does not purport to be an interpretation of law and/or regulations and is for guidance
STUDY REQUEST FORM AND TRAQ DSS FORM
 Research Road Map April 2017 In This Issue Pharmacy Services for Research Study Request Form and TRAQ DSS FORM Management of Investigational Drugs Pharmacy Standard Operating Procedures IDS Team Qualifications
Research Road Map April 2017 In This Issue Pharmacy Services for Research Study Request Form and TRAQ DSS FORM Management of Investigational Drugs Pharmacy Standard Operating Procedures IDS Team Qualifications
Healthcare Waste Regulatory Challenges. Selin Hoboy November 17, 2011
 Healthcare Waste Regulatory Challenges Selin Hoboy November 17, 2011 Overview What are healthcare wastes? What regulations and agencies affect healthcare waste? How to cope with it all! BMPs What are upcoming
Healthcare Waste Regulatory Challenges Selin Hoboy November 17, 2011 Overview What are healthcare wastes? What regulations and agencies affect healthcare waste? How to cope with it all! BMPs What are upcoming
MEMORANDUM OF UNDERSTANDING
 MEMORUM OF UNDERSTING This Memorandum of Understanding effective the 19 th day of December, 2000, in the Province of Nova Scotia A. PURPOSE BETWEEN The Nova Scotia Department of Environment and Labour
MEMORUM OF UNDERSTING This Memorandum of Understanding effective the 19 th day of December, 2000, in the Province of Nova Scotia A. PURPOSE BETWEEN The Nova Scotia Department of Environment and Labour
The P2D2 Network & IL-IN Sea Grant Passing the Torch Educating the Next Generation for Pharmaceutical Environmental Stewardship
 The P2D2 Network & IL-IN Sea Grant Passing the Torch Educating the Next Generation for Pharmaceutical Environmental Stewardship Susan E. Boehme Illinois-Indiana Sea Grant College Program & Paul Ritter
The P2D2 Network & IL-IN Sea Grant Passing the Torch Educating the Next Generation for Pharmaceutical Environmental Stewardship Susan E. Boehme Illinois-Indiana Sea Grant College Program & Paul Ritter
Waste Management System.
 Waste Management System. Battery recycling Corporate Procedure: CP-WMS 006 Authorised by: Charlotte Winnert Issue number: Three Date of issue: 6 th August 2010 Purpose To define the University of Sheffield
Waste Management System. Battery recycling Corporate Procedure: CP-WMS 006 Authorised by: Charlotte Winnert Issue number: Three Date of issue: 6 th August 2010 Purpose To define the University of Sheffield
Naugatuck Young Men s Christian Association, Inc. Volunteer Application
 Naugatuck Young Men s Christian Association, Inc. Volunteer Application (PLEASE PRINT AND ANSWER ALL QUESTIONS COMPLETELY.) Name: Date of Birth: Last First MI Phone: Home: Work: Cell: Address:_ Street
Naugatuck Young Men s Christian Association, Inc. Volunteer Application (PLEASE PRINT AND ANSWER ALL QUESTIONS COMPLETELY.) Name: Date of Birth: Last First MI Phone: Home: Work: Cell: Address:_ Street
COLLEGE OF VETERINARIANS OF ONTARIO BY-LAWS
 COLLEGE OF VETERINARIANS OF ONTARIO BY-LAWS Only sections concerning Professional Corporations in effect until March 1, 2019. Rev. 3/2016 COLLEGE OF VETERINARIANS OF ONTARIO BY-LAW NO. 1 PREFACE The College
COLLEGE OF VETERINARIANS OF ONTARIO BY-LAWS Only sections concerning Professional Corporations in effect until March 1, 2019. Rev. 3/2016 COLLEGE OF VETERINARIANS OF ONTARIO BY-LAW NO. 1 PREFACE The College
Policy and Procedure Manual
 Policy and Procedure Manual Finance and Accounting Procurement Services F-PS-01.00 SUBJECT/TITLE: PURPOSE: DEFINITION: STATEMENT OF VENDOR OWNED INVENTORY (CONSIGNMENT) POLICY This policy will cover all
Policy and Procedure Manual Finance and Accounting Procurement Services F-PS-01.00 SUBJECT/TITLE: PURPOSE: DEFINITION: STATEMENT OF VENDOR OWNED INVENTORY (CONSIGNMENT) POLICY This policy will cover all
MEDICAL WASTE MANAGEMENT STUDY NOTES
 MEDICAL WASTE MANAGEMENT STUDY NOTES UNIT 1 INTRODUCTION Hospital and other health care establishment has a duty of care for the environment and for public health and have particular responsibility in
MEDICAL WASTE MANAGEMENT STUDY NOTES UNIT 1 INTRODUCTION Hospital and other health care establishment has a duty of care for the environment and for public health and have particular responsibility in
NOTE: Do NOT overfill sharps containers; there should be NO more than 50ml of residual liquid in the mailback kit when returned
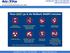 These DO go in the Mailback SHARPS Containers Sharps Waste Such As: Needles & syringes Razor blades Orthodon?c wires Scalpel blades & lancets Glass pipebes, slides & tubes Broken, contaminated glass Staples
These DO go in the Mailback SHARPS Containers Sharps Waste Such As: Needles & syringes Razor blades Orthodon?c wires Scalpel blades & lancets Glass pipebes, slides & tubes Broken, contaminated glass Staples
Ontario Rechargeable Battery Recycling Corporation of Canada 16 Northumberland Toronto, Ontario M6H 1P7
 Ministry of the Environment Ministère de l Environnement AMENDED PROVISIONAL CERTIFICATE OF APPROVAL WASTE MANAGEMENT SYSTEM NUMBER A841566 Ontario Rechargeable Battery Recycling Corporation of Canada
Ministry of the Environment Ministère de l Environnement AMENDED PROVISIONAL CERTIFICATE OF APPROVAL WASTE MANAGEMENT SYSTEM NUMBER A841566 Ontario Rechargeable Battery Recycling Corporation of Canada
Green Label Product Steel Furniture (TGL-21-R2-14)
 Green Label Product Steel Furniture (TGL-21-R2-14) Revision Approved On 18 November 2014 Thailand Environment Institute (TEI) 16/151 Muang Thong Thani, Bond Street, Bangpood, Pakkred, Nonthaburi 11120
Green Label Product Steel Furniture (TGL-21-R2-14) Revision Approved On 18 November 2014 Thailand Environment Institute (TEI) 16/151 Muang Thong Thani, Bond Street, Bangpood, Pakkred, Nonthaburi 11120
Emergency Medical Services Division Policies Procedures Protocols
 Emergency Medical Services Division Policies Procedures Protocols Mobile Intensive Care Unit (MICU) Policies and Procedures (9002.00) I. GENERAL PROVISIONS: A. All Kern County Paramedic Providers shall
Emergency Medical Services Division Policies Procedures Protocols Mobile Intensive Care Unit (MICU) Policies and Procedures (9002.00) I. GENERAL PROVISIONS: A. All Kern County Paramedic Providers shall
TOWN OF GROTON 173 Main Street Groton, Massachusetts
 TOWN OF GROTON 173 Main Street Groton, Massachusetts 01450 978-448-1145 Application for Employment Please read before filling out this application Thank you for your interest in employment with the Town
TOWN OF GROTON 173 Main Street Groton, Massachusetts 01450 978-448-1145 Application for Employment Please read before filling out this application Thank you for your interest in employment with the Town
Vaccine Storage and Handling Protocol, 2014
 Vaccine Storage and Handling Protocol, 2014 Preamble The Ontario Public Health Standards (OPHS) are published by the Minister of Health and Long- Term Care under the authority of the Health Protection
Vaccine Storage and Handling Protocol, 2014 Preamble The Ontario Public Health Standards (OPHS) are published by the Minister of Health and Long- Term Care under the authority of the Health Protection
Application for Full & Part Time At-Will Employment
 Full Time Part Time NAME POSITION APPLIED FOR Application for Full & Part Time At-Will Employment APPLICATION FOR AT-WILL EMPLOYMENT WITH THE CITY OF STURGIS The City of Sturgis is an equal opportunity
Full Time Part Time NAME POSITION APPLIED FOR Application for Full & Part Time At-Will Employment APPLICATION FOR AT-WILL EMPLOYMENT WITH THE CITY OF STURGIS The City of Sturgis is an equal opportunity
University Health Services Health and Safety
 ADVISORY NO. 10.2: MANAGEMENT OF BIOLOGICAL AND INFECTIOUS WASTES The following recommendations are based on the Ohio Revised Code (ORC), Ohio Administrative Code (OAC) and EPA guidelines regarding biological
ADVISORY NO. 10.2: MANAGEMENT OF BIOLOGICAL AND INFECTIOUS WASTES The following recommendations are based on the Ohio Revised Code (ORC), Ohio Administrative Code (OAC) and EPA guidelines regarding biological
BIOMEDICAL WASTE MANAGEMENT POLICY
 BIOMEDICAL WASTE MANAGEMENT POLICY Managing biomedical waste in a safe and environmentally responsible manner is a key focus of the Environmental Compliance and Sustainability office. 1.0 PURPOSE In an
BIOMEDICAL WASTE MANAGEMENT POLICY Managing biomedical waste in a safe and environmentally responsible manner is a key focus of the Environmental Compliance and Sustainability office. 1.0 PURPOSE In an
Michigan Pharmacists Association Annual Convention & Exposition. Michigan Regulations: Collection and Disposal of Rx-Waste
 Michigan Pharmacists Association Annual Convention & Exposition Michigan Regulations: Collection and Disposal of Rx-Waste Michigan Pharmacists Association Annual Convention & Exposition Christine Grossman
Michigan Pharmacists Association Annual Convention & Exposition Michigan Regulations: Collection and Disposal of Rx-Waste Michigan Pharmacists Association Annual Convention & Exposition Christine Grossman
KEEPING SHARP Volume 1, Issue 2
 KEEPING SHARP Volume 1, Issue 2 Medical Waste Update HCA Published by Environmental Health Medical Waste, County of Orange Health Care Agency Save & Comply Medical waste treatment and disposal methods
KEEPING SHARP Volume 1, Issue 2 Medical Waste Update HCA Published by Environmental Health Medical Waste, County of Orange Health Care Agency Save & Comply Medical waste treatment and disposal methods
Guidelines on import procedures for pharmaceutical products
 Guidelines on import procedures for pharmaceutical products INTRODUCTION Public health concerns demand that the manufacture of pharmaceutical products and their subsequent handling within the distribution
Guidelines on import procedures for pharmaceutical products INTRODUCTION Public health concerns demand that the manufacture of pharmaceutical products and their subsequent handling within the distribution
Pharmaceutical Waste Compliance Program Clinician Educators and Managers In-Service
 Pharmaceutical Waste Compliance Program Clinician Educators and Managers In-Service Pharmaceutical Waste Services Stericycle Pharmaceutical Waste Compliance Program Here s our Solution 1. Identification
Pharmaceutical Waste Compliance Program Clinician Educators and Managers In-Service Pharmaceutical Waste Services Stericycle Pharmaceutical Waste Compliance Program Here s our Solution 1. Identification
Laboratory Close-Out Guidelines
 Office of Laboratory Safety 2300 I Street, NW Ross Hall, Suite B- 05 Washington, DC 20037 t. 202-994- 8258 I labsafety@gwu.edu Guidelines The Principal Investigator (PI) is responsible for leaving laboratories
Office of Laboratory Safety 2300 I Street, NW Ross Hall, Suite B- 05 Washington, DC 20037 t. 202-994- 8258 I labsafety@gwu.edu Guidelines The Principal Investigator (PI) is responsible for leaving laboratories
DISPOSAL SERVICES FOR PHARMACEUTICAL WASTES AVAILABLE TO KING COUNTY GENERATORS
 DISPOSAL SERVICES FOR PHARMACEUTICAL WASTES AVAILABLE TO KING COUNTY GENERATORS Summary of Research on Disposal of Pharmaceutical Wastes that are also Hazardous or Dangerous Waste INTERAGENCY REGULATORY
DISPOSAL SERVICES FOR PHARMACEUTICAL WASTES AVAILABLE TO KING COUNTY GENERATORS Summary of Research on Disposal of Pharmaceutical Wastes that are also Hazardous or Dangerous Waste INTERAGENCY REGULATORY
PERSONAL DATA Last Name: First: Middle: Today s Date: City: State: ZIP: Primary Telephone: Secondary Telephone: Address:
 Advantage Surveillance, Inc. is an equal opportunity employer, dedicated to a policy of non-discrimination in employment on any basis including age, sex, color, race, creed, national origin, religion,
Advantage Surveillance, Inc. is an equal opportunity employer, dedicated to a policy of non-discrimination in employment on any basis including age, sex, color, race, creed, national origin, religion,
Application for Employment
 Human Resources Department 131 Hospital Drive Salem, KY 42078 Phone: 270-988-7280 Fax: 270-988-2935 www.lhhs.org Application for Employment Livingston Hospital & Healthcare Services, Inc. (the Hospital
Human Resources Department 131 Hospital Drive Salem, KY 42078 Phone: 270-988-7280 Fax: 270-988-2935 www.lhhs.org Application for Employment Livingston Hospital & Healthcare Services, Inc. (the Hospital
Waste Management System.
 Waste Management System. Battery recycling Corporate Procedure: CP-WMS 006 Authorised by: Charlotte Winnert Issue number: Five Date of issue: 13 June 2013 Purpose To define the University of Sheffield
Waste Management System. Battery recycling Corporate Procedure: CP-WMS 006 Authorised by: Charlotte Winnert Issue number: Five Date of issue: 13 June 2013 Purpose To define the University of Sheffield
Infectious Waste Disposal Procedure 25 PA Code 284 Ursinus College Collegeville, PA 19426
 The purpose of the Infectious Waste Disposal procedure is to establish procedures for the safe handling and proper disposal of infectious waste generated at in accordance with regulation 25 PA Code Chapter
The purpose of the Infectious Waste Disposal procedure is to establish procedures for the safe handling and proper disposal of infectious waste generated at in accordance with regulation 25 PA Code Chapter
DISPOSAL OF HAZARDOUS WASTE, CHEMICAL WASTE AND UNUSED CHEMICALS
 NO. 3071 DISPOSAL OF HAZARDOUS WASTE, CHEMICAL WASTE AND UNUSED CHEMICALS 1.0 PURPOSE. Oklahoma City Community College (OCCC) recognizes that employees have a right and need to know the properties and
NO. 3071 DISPOSAL OF HAZARDOUS WASTE, CHEMICAL WASTE AND UNUSED CHEMICALS 1.0 PURPOSE. Oklahoma City Community College (OCCC) recognizes that employees have a right and need to know the properties and
Regulated Medical Waste Management and Proper Waste Segregation. State-Specific Information New Jersey
 Regulated Medical Waste Management and Proper Waste Segregation State-Specific Information New Jersey Identifying & Segregating Regulated Medical Waste RMW in New Jersey Six Categories of Regulated Medical
Regulated Medical Waste Management and Proper Waste Segregation State-Specific Information New Jersey Identifying & Segregating Regulated Medical Waste RMW in New Jersey Six Categories of Regulated Medical
Custom Incentive Application for Business Customers
 2018 Custom Incentive Application for Business Customers A Cash Incentive Energy Efficiency Program brought to you by: Instructions for Use: For complete instructions, please refer to the Terms and Conditions
2018 Custom Incentive Application for Business Customers A Cash Incentive Energy Efficiency Program brought to you by: Instructions for Use: For complete instructions, please refer to the Terms and Conditions
City of Palmer Human Resources Specialist 231 W. Evergreen Avenue Palmer, AK Phone: Fax: Employment Application
 City of Palmer Human Resources Specialist 231 W. Evergreen Avenue Palmer, AK 99645 Phone: 907-745-3271 Fax: 907-745-0930 Employment Application Application Date: Last Name: First: Middle: Other Names Used
City of Palmer Human Resources Specialist 231 W. Evergreen Avenue Palmer, AK 99645 Phone: 907-745-3271 Fax: 907-745-0930 Employment Application Application Date: Last Name: First: Middle: Other Names Used
City of Lompoc Recycled Water Program Recycled Water Truck Program Application and Permit MUST BE COMPLETED/APPROVED BEFORE PUCHASHING RECYCLED WATER
 City of Lompoc Recycled Water Program Recycled Water Truck Program Application and Permit MUST BE COMPLETED/APPROVED BEFORE PUCHASHING RECYCLED WATER USER INFORMATION: Company Name: Recycled Water Site
City of Lompoc Recycled Water Program Recycled Water Truck Program Application and Permit MUST BE COMPLETED/APPROVED BEFORE PUCHASHING RECYCLED WATER USER INFORMATION: Company Name: Recycled Water Site
CITY OF BURLESON. 141 West Renfro Burleson, Texas TEL: (817) FAX: (817) Web Address:
 CITY OF BURLESON 141 West Renfro Burleson, Texas 76028 TEL: (817) 426-9640 FAX: (817) 295-9414 Web www.burlesontx.com AN EQUAL OPPORTUNITY EMPLOYER APPLICATION FOR EMPLOYMENT PLEASE READ FIRST: Thank you
CITY OF BURLESON 141 West Renfro Burleson, Texas 76028 TEL: (817) 426-9640 FAX: (817) 295-9414 Web www.burlesontx.com AN EQUAL OPPORTUNITY EMPLOYER APPLICATION FOR EMPLOYMENT PLEASE READ FIRST: Thank you
Completing. By the end of this chapter, you will be able to: Introduction. Chapter 16
 Chapter 16 Completing Product Processing By the end of this chapter, you will be able to: l Explain the term cold chain l Describe the type of fridge used for correct storage of refrigerated products l
Chapter 16 Completing Product Processing By the end of this chapter, you will be able to: l Explain the term cold chain l Describe the type of fridge used for correct storage of refrigerated products l
The Pharmacy Council under sections 93 and 95 of the Health Professions Regulatory Bodies Act, 2013 (Act 857), grant licences to Pharmacies
 1.0 INTRODUCTION The Pharmacy Council under sections 93 and 95 of the Health Professions Regulatory Bodies Act, 2013 (Act 857), grant licences to Pharmacies The Pharmacy Council issues two types of pharmacy
1.0 INTRODUCTION The Pharmacy Council under sections 93 and 95 of the Health Professions Regulatory Bodies Act, 2013 (Act 857), grant licences to Pharmacies The Pharmacy Council issues two types of pharmacy
(1) Adequately wet asbestos-containing waste material as follows by doing all of the following:
 ACTION: Final DATE: 03/29/2018 9:12 AM 3745-20-05 Standard for asbestos waste handling. [Comment: For dates of non-regulatory government publications, publications of recognized organizations and associations,
ACTION: Final DATE: 03/29/2018 9:12 AM 3745-20-05 Standard for asbestos waste handling. [Comment: For dates of non-regulatory government publications, publications of recognized organizations and associations,
Burnett Dairy Cooperative State Rd. 70 Grantsburg, WI
 Date: Commercial Driver Application An Equal Opportunity Employer Physicals & Drug Screens Required Please fill in ALL Blanks & Provide ALL Information Requested--Print or Type. Name: First Middle Last
Date: Commercial Driver Application An Equal Opportunity Employer Physicals & Drug Screens Required Please fill in ALL Blanks & Provide ALL Information Requested--Print or Type. Name: First Middle Last
Tel: TTY : MASSACHUSETTS BOARD OF REGISTRATION IN PHARMACY
 The Commonwealth of Massachusetts Executive Office of Health and Human Services Department of Public Health Bureau of Health Professions Licensure 239 Causeway Street, Suite 500, Boston, MA 02114 CHARLES
The Commonwealth of Massachusetts Executive Office of Health and Human Services Department of Public Health Bureau of Health Professions Licensure 239 Causeway Street, Suite 500, Boston, MA 02114 CHARLES
Management of Unused Patient Dispensed Medications
 Management of Unused Patient Dispensed Medications TEXAS PRODUCT STEWARDSHIP COUNCIL 1/29/2016 Bloodborne Pathogens Training Jan Harris, MPH Director, Environmental, Health & Safety Sharps Compliance www.sharpsinc.com
Management of Unused Patient Dispensed Medications TEXAS PRODUCT STEWARDSHIP COUNCIL 1/29/2016 Bloodborne Pathogens Training Jan Harris, MPH Director, Environmental, Health & Safety Sharps Compliance www.sharpsinc.com
Ontario s Narcotics Strategy
 Ontario Public Drug Programs Division Ontario s Narcotics Strategy Notice from the Executive Officer: Implementation of the Narcotics Monitoring System The Ministry of Health and Long-Term Care ( ministry
Ontario Public Drug Programs Division Ontario s Narcotics Strategy Notice from the Executive Officer: Implementation of the Narcotics Monitoring System The Ministry of Health and Long-Term Care ( ministry
Application for Employment
 Application for Employment Today s Date: (First MI Last) : City, State Zip: Positions Applied For: SS Number: Cell Phone: Salary Desired: Hours Available to Work List your preference of hours and shifts
Application for Employment Today s Date: (First MI Last) : City, State Zip: Positions Applied For: SS Number: Cell Phone: Salary Desired: Hours Available to Work List your preference of hours and shifts
BRIEFING 1168 Compounding for Phase I Investigational Studies,
 BRIEFING 1168 Compounding for Phase I Investigational Studies, PF 39(5) [Sept. Oct. 2013]. The current proposed chapter in PF 43(3) [May June 2017] is posted online at www.usp.org/usp-nf/notices/compounding-forphase-i-investigational-studies
BRIEFING 1168 Compounding for Phase I Investigational Studies, PF 39(5) [Sept. Oct. 2013]. The current proposed chapter in PF 43(3) [May June 2017] is posted online at www.usp.org/usp-nf/notices/compounding-forphase-i-investigational-studies
LABORATORY WASTE MANAGEMENT PROGRAMMES
 LABORATORY WASTE MANAGEMENT PROGRAMMES Sylvia Wanjiru Kamau INTERNATIONAL LIVESTOCK RESEARCH INSTITUTE LABORATORY MANAGEMENT AND EQUIPMENT OPERATIONS WORKSHOP. 17 TH -21 ST JUNE 2012 TRAINING OBJECTIVES
LABORATORY WASTE MANAGEMENT PROGRAMMES Sylvia Wanjiru Kamau INTERNATIONAL LIVESTOCK RESEARCH INSTITUTE LABORATORY MANAGEMENT AND EQUIPMENT OPERATIONS WORKSHOP. 17 TH -21 ST JUNE 2012 TRAINING OBJECTIVES
MATERIAL SPECIFICATION FOR LATEX MODIFIERS FOR USE IN CONCRETE
 ONTARIO PROVINCIAL STANDARD SPECIFICATION METRIC OPSS 1312 NOVEMBER 2008 MATERIAL SPECIFICATION FOR LATEX MODIFIERS FOR USE IN CONCRETE TABLE OF CONTENTS 1312.01 SCOPE 1312.02 REFERENCES 1312.03 DEFINITIONS
ONTARIO PROVINCIAL STANDARD SPECIFICATION METRIC OPSS 1312 NOVEMBER 2008 MATERIAL SPECIFICATION FOR LATEX MODIFIERS FOR USE IN CONCRETE TABLE OF CONTENTS 1312.01 SCOPE 1312.02 REFERENCES 1312.03 DEFINITIONS
APPLICATION FOR EMPLOYMENT
 Clear Form APPLICATION FOR EMPLOYMENT Thank you for your interest in Howard Payne University. Please answer every question below. Do not leave any item blank. Enter N/A on the line if a question is not
Clear Form APPLICATION FOR EMPLOYMENT Thank you for your interest in Howard Payne University. Please answer every question below. Do not leave any item blank. Enter N/A on the line if a question is not
Environmental Services Conference
 Sharps Compliance Corp. NASDAQ: SMED Credit Suisse Industrial and Environmental Services Conference May 24, 2011 Diana P. Diaz Vice President and Chief Financial Officer 1 Safe Harbor Statement These slides
Sharps Compliance Corp. NASDAQ: SMED Credit Suisse Industrial and Environmental Services Conference May 24, 2011 Diana P. Diaz Vice President and Chief Financial Officer 1 Safe Harbor Statement These slides
