Infectious Waste Disposal Procedure 25 PA Code 284 Ursinus College Collegeville, PA 19426
|
|
|
- Gerard Melton
- 6 years ago
- Views:
Transcription
1 The purpose of the Infectious Waste Disposal procedure is to establish procedures for the safe handling and proper disposal of infectious waste generated at in accordance with regulation 25 PA Code Chapter 284. As a Generator of Infectious Waste, personnel handling such waste must follow the code requirements for storage and labeling of containers, manifests, and recordkeeping. A quick reference checklist follows the procedure in Appendix A. The Environmental Health & Safety/ Risk Manager manages the infectious waste program. I. Definition of Infectious Waste According to regulation 25 PA Code 271.1, Infectious Waste is defined as a municipal and residual waste which is generated in the diagnosis, treatment, immunization or autopsy of human beings or animals, in research pertaining thereto, in the preparation of human or animal remains for interment or cremation, or in the production or testing of biologicals, and which falls under one or more of the following categories: A. Cultures and stocks. Cultures and stocks of infectious agents and associated biologicals, including the following: cultures from medical and pathological laboratories; cultures and stocks of infectious agents from research and industrial laboratories; wastes from the production of biologicals; discarded live and attenuated vaccines except for residue in emptied containers; and culture dishes, assemblies and devices used to conduct diagnostic tests or to transfer, inoculate and mix cultures. B. Pathological wastes. Human pathological wastes, including tissues, organs and body parts and body fluids that are removed during surgery, autopsy, other medical procedures or laboratory procedures. The term does not include hair, nails or extracted teeth. C. Human blood and body fluid waste and items contaminated with human blood and body fluid waste. D. Animal wastes. Contaminated animal carcasses, body parts, blood, blood products, secretions, excretions and bedding of animals that were known to have been exposed to zoonotic infectious agents or nonzoonotic human pathogens during research (including research in veterinary schools and hospitals), production of biologicals or testing of pharmaceuticals. E. Isolation wastes. Biological wastes and waste contaminated with blood, excretion, exudates or secretions from: 1. Humans who are isolated to protect others from highly virulent diseases. 2. Isolated animals known or suspected to be infected with highly virulent diseases.
2 F. Used sharps. Sharps that have been in contact with infectious agents or that have been used in animal or human patient care or treatment, at medical, research or industrial laboratories, including hypodermic needles, syringes (with or without the attached needle), Pasteur pipettes, scalpel blades, blood vials, needles with attached tubing, culture dishes, suture needles, slides, cover slips and other broken or unbroken glass or plasticware. II. Storage Requirements Infectious waste must be stored in a manner that: A. Maintains the integrity of the container, prevents the leakage or release of waste from the containers and provides protection from water, rain and wind. B. Prevents the spread of infectious agents. C. Affords protection from animals and does not provide a breeding place or a food source for rodents. D. Maintains the waste in a nonputrescent state, using refrigeration (<=7 o C) or freezing (<=-18 o C) when necessary. E. Prevents odors from emanating from the container. F. Prevents unauthorized access to the waste. III. Containers A. In general, containers must be 1. Leak proof 2. Impervious to moisture. B. Sharps containers must be 1. Rigid. 2. Tightly lidded. 3. Puncture resistant. C. Re-use 1. Nonrigid containers may not be re-used. 2. Corrugated fiberboard containers may be re-used if the surface of the container has been protected from direct contact with the waste. 3. Rigid, fiberboard containers may be re-used if the container has been properly disinfected according to DEP guidelines or the surface of the container was protected from direct contact with the waste.
3 D. Bags IV. Labeling If bags are used as the only storage container, 1. Use double or multiple bags. 2. Securely tie the bag. 3. Ensure the bags are constructed of material that meets ASTM standard D and D by should be stamped on the bag or the box of the packaged bags. 4. Ensure the bags have sufficient seam strength equal in resistance to tearing and equally as impermeable as the other parts of the bag. 5. The bags must be fluorescent orange, orange-red, or red in color and labeled per regulations. A. The outermost container for each infectious waste package for off-site transportation must be immediately labeled: 1. Label must be securely attached and legible. 2. Indelible ink used to complete the information on the label. 3. If handwritten, 3 x 5 in size. B. Label information must contain the following: a. Name, address, and telephone number of generator. b. Date the waste was generated. c. Name of transporter. C. The outermost container or bag for each infectious waste package for either onsite or offsite movement must contain the following information: a. The words infectious waste. b. Universal biohazard symbol c. Date waste was generated V. Duration of storage A. Infectious waste, excluding sharps, may be stored at room temperature or in a refrigerator until the storage container is full, but no longer than 30 days from the date waste was first placed in the container. B. A filled infectious waste container may be stored in a freezer for up to 90 days after the date waste was first placed in the container. C. If waste becomes putrescent during the storage period, it must be removed off-site within 24 hours for processing or disposal.
4 D. Used sharp containers may be used until full and no longer than 1 year providing the container meets the general storage requirements; however, the best practice is to remove sharps containers when the containers are 2/3-3/4 full. VI. Waste Removal During the semester, infectious waste should be picked up once a month. The Environmental Health & Safety/Risk Manager communicates the monthly scheduled pickup dates during the academic year; other times, pickups are on a will call basis. Contact the Environmental Health & Safety Risk Manager at VII. Recordkeeping Manifests: The signer of the manifest must ensure all information is correct before signing. The signer will be the one contacted by the DEP in the event of an issue with the waste. A. The following information must be on the manifest: 1. Name, mailing address and telephone of the generator 2. Total number of pages. 3. Each transporter s company name, identification number, PA infectious and chemotherapeutic waste transporter license number and telephone number. 4. Number of containers, types of containers, and the total quantity of the waste by weight or volume. 5. Infectious waste code number for each waste indicated on manifest. 6. United States Department of Transportation proper shipping name, hazard class and identification number for each waste, if applicable. 7. Special instructions and information necessary for proper handling of the waste during transportation, shipping, storage, or disposal, if any. 8. The printed or typed name of the handwritten signature of the generator s authorized representative, and the date of shipment. 9. The printed or typed name and handwritten signature of the initial transporter s authorized representative, and the date of receipt. 10. The designated facility s name, site address, Pennsylvania State permit or identification number and phone number. 11. An authorized representative of the generator shall ensure that the manifest has been completed and shall read the certification statement on the manifest prior to signing the manifest. 12. The generator shall ensure before the waste is transported offsite that the required information on all parts of the manifest are capable of being read.
5 B. Copy 4 of the manifest is retained by the Generator upon shipment of the infectious waste. Please mail this copy to the Purchasing Agent in Facilities Services. C. Copy 1 is mailed to the Generator by the owner/operator of the processing or disposal facility. Currently, the College s vendor, Stericycle, provides this copy via the company s website, The Environmental Health & Safety/Risk Manager ensures this copy is available within the required 20 day period. D. Copy 1 must be received by the Generator within 20 days of the date the generator s waste was accepted by the initial transporter. If the manifest is not received, a. Generator must contact the transporter or owner or operator of the designated facility or both to determine the status of the infectious waste. b. Notify the regional PADEP office by telephone within 1 business day of the status of the shipment. c. If the manifest is not received within 35 days of the date, the generator must notify by telephone the regional PADEP office and submit an exception report to the PADEP s central office that includes 1. a legible copy of the unsigned manifest 2. cover letter explaining the efforts undertaken to located the waste shipment and the results E. All manifests must be maintained for 5 years from the date of shipment.
6 Appendix A Infectious Waste Checklist Requirement Storage: - Containers maintained, not leaking, puncture proof. - Waste maintained in nonputrescent state - no odors. - Sharps stored in appropriate containers. - Double bags employed when bags are the only storage container. Bags meet strength and certification requirements. - Infectious waste stored for no longer than 30 days at room temperature. - Infectious waste stored for no longer than 90 days in the freezer. - Infectious waste sharps stored for no longer than 1 year. Labeling: - Outermost container of infectious waste for offsite transport labeled immediately with proper label name, address and telephone of generator, and date waste generated in indelible ink - Outermost container of infectious waste properly labeled with biohazard symbol and the words infectious waste. - Stationary containers lined with appropriately colored bag. Containers: - Nonrigid containers not re-used. - Corrugated fiberboard containers only re-used if container protected from direct contact of the waste. Manifests: - Name, address and telephone number of college printed on manifest. - Total number of pages used to complete manifest. - Transporter(s) company name, identification number, PA infectious and chemotherapeutic waste transporter license number and telephone number on manifest. - Number and type of containers and the total quantity of waste by weight of volume listed. - Infectious waste code for each waste. - Proper DOT shipping name, hazard class and identification number if applicable. - Special instructions for handling, storage and disposal if applicable. - Printed/typed name and handwritten signature of generator s authorized signature. - Printed/typed name and handwritten signature of the initial transporter s authorized representative and date of receipt. Completed
Regulated Medical Waste Management and Proper Waste Segregation. State-Specific Information Washington
 Regulated Medical Waste Management and Proper Waste Segregation State-Specific Information Washington Biomedical Waste Regulation In the State of Washington biomedical waste, where regulated, is regulated
Regulated Medical Waste Management and Proper Waste Segregation State-Specific Information Washington Biomedical Waste Regulation In the State of Washington biomedical waste, where regulated, is regulated
BIOMEDICAL WASTE MANAGEMENT POLICY
 BIOMEDICAL WASTE MANAGEMENT POLICY Managing biomedical waste in a safe and environmentally responsible manner is a key focus of the Environmental Compliance and Sustainability office. 1.0 PURPOSE In an
BIOMEDICAL WASTE MANAGEMENT POLICY Managing biomedical waste in a safe and environmentally responsible manner is a key focus of the Environmental Compliance and Sustainability office. 1.0 PURPOSE In an
Regulated Medical Waste Management and Proper Waste Segregation. State-Specific Information Pennsylvania
 Regulated Medical Waste Management and Proper Waste Segregation State-Specific Information Pennsylvania Identifying & Segregating Regulated Medical Waste RMW in Pennsylvania Six Categories of Regulated
Regulated Medical Waste Management and Proper Waste Segregation State-Specific Information Pennsylvania Identifying & Segregating Regulated Medical Waste RMW in Pennsylvania Six Categories of Regulated
Northeastern University Procedure for Disposal of Medical or Biological Waste
 Biohazardous Waste Disposal Fact Sheet Northeastern University Procedure for Disposal of Medical or Biological Waste Fact Sheet #14 August 2016 Revision: Seventh Definition: The State of Massachusetts
Biohazardous Waste Disposal Fact Sheet Northeastern University Procedure for Disposal of Medical or Biological Waste Fact Sheet #14 August 2016 Revision: Seventh Definition: The State of Massachusetts
DOT Hazardous Materials and Regulated Medical Waste Prepared By: George Weishoff
 DOT Hazardous Materials and Regulated Medical Waste Prepared By: George Weishoff Medical Waste Removal & Disposal Introduction Training will include the following: Code of Federal Regulations: Title 49
DOT Hazardous Materials and Regulated Medical Waste Prepared By: George Weishoff Medical Waste Removal & Disposal Introduction Training will include the following: Code of Federal Regulations: Title 49
INFECTIOUS/BIOLOGICAL WASTE MANAGEMENT PROTOCOL
 INFECTIOUS/BIOLOGICAL WASTE MANAGEMENT PROTOCOL UNIVERSITY RISK MANAGEMENT Occupational Safety and Health Programs 19 Hagood Avenue, Suite 908 Charleston, SC 29425 843-792-3604 Revised: November 2016 TABLE
INFECTIOUS/BIOLOGICAL WASTE MANAGEMENT PROTOCOL UNIVERSITY RISK MANAGEMENT Occupational Safety and Health Programs 19 Hagood Avenue, Suite 908 Charleston, SC 29425 843-792-3604 Revised: November 2016 TABLE
Policy & Procedure Manual
 Environmental Health & Safety Policy & Procedure Manual Title: 1. Purpose: To establish policies, work practices, and systematic procedures for the handling, packaging, collection, treatment, and disposal
Environmental Health & Safety Policy & Procedure Manual Title: 1. Purpose: To establish policies, work practices, and systematic procedures for the handling, packaging, collection, treatment, and disposal
Biological and Regulated Medical Waste Plan
 Biological and Regulated Medical Waste Plan TUFTS ENVIRONMENTAL HEALTH AND SAFETY OCTOBER 2016 Table of Contents I. Purpose... 3 II. Responsibilities... 3 III. Sink and sewer discharge of specific medical
Biological and Regulated Medical Waste Plan TUFTS ENVIRONMENTAL HEALTH AND SAFETY OCTOBER 2016 Table of Contents I. Purpose... 3 II. Responsibilities... 3 III. Sink and sewer discharge of specific medical
UNIVERSITY OF RICHMOND REGULATED MEDICAL WASTE MANAGEMENT GUIDELINES
 UNIVERSITY OF RICHMOND REGULATED MEDICAL WASTE MANAGEMENT GUIDELINES October 2016 Table of Contents Section Page I. Introduction.... 1 II. Characteristics of Regulated Medical Waste 1-2 III. Exclusions...2-3
UNIVERSITY OF RICHMOND REGULATED MEDICAL WASTE MANAGEMENT GUIDELINES October 2016 Table of Contents Section Page I. Introduction.... 1 II. Characteristics of Regulated Medical Waste 1-2 III. Exclusions...2-3
WHO NEEDS TO KNOW THIS PROGRAM All NYU employees that generate, handle or transport RMW should be familiar with this written program.
 Title: NYU Regulated Medical Waste Safety Written Program Effective Date: October 2002 Revision Date: April 14, 2017 Issuing Authority: Responsible Officer: VP, Facilities and Construction Management Director
Title: NYU Regulated Medical Waste Safety Written Program Effective Date: October 2002 Revision Date: April 14, 2017 Issuing Authority: Responsible Officer: VP, Facilities and Construction Management Director
Regulated Medical Waste (RMW) Rules, Registration & Reports
 Regulated Medical Waste (RMW) Rules, Registration & Reports 1 Prepared by: Bret Reburn Envi. Specialist 3 Hazardous Waste/UST Compliance & Enforcement Central Regional Office - Trenton, NJ (609) 292-3949
Regulated Medical Waste (RMW) Rules, Registration & Reports 1 Prepared by: Bret Reburn Envi. Specialist 3 Hazardous Waste/UST Compliance & Enforcement Central Regional Office - Trenton, NJ (609) 292-3949
Regulated Medical Waste Management and Proper Waste Segregation. Regulated Medical Waste Management and Proper Waste Segregation.
 Regulated Medical Waste Management and Proper Waste Segregation New Jersey New Arkansas Jersey Regulated Medical Waste Management and Proper Waste Segregation The regulations in this presentation are specific
Regulated Medical Waste Management and Proper Waste Segregation New Jersey New Arkansas Jersey Regulated Medical Waste Management and Proper Waste Segregation The regulations in this presentation are specific
Appendix J. University of Alabama at Birmingham Medical Waste Management Plan
 University of Alabama at Birmingham Medical Waste Management Plan I. Definitions per the Alabama Department of Environmental Management Land Division 13-Solid Waste Program, Chapter 335-13-1, Medical Waste
University of Alabama at Birmingham Medical Waste Management Plan I. Definitions per the Alabama Department of Environmental Management Land Division 13-Solid Waste Program, Chapter 335-13-1, Medical Waste
CALIFORNIA CODES HEALTH AND SAFETY CODE SECTION
 CALIFORNIA CODES HEALTH AND SAFETY CODE SECTION 117625-117780 117625. Unless the context requires otherwise, the definitions in this article govern the construction of this part. 117630. "Biohazard bag"
CALIFORNIA CODES HEALTH AND SAFETY CODE SECTION 117625-117780 117625. Unless the context requires otherwise, the definitions in this article govern the construction of this part. 117630. "Biohazard bag"
MEDICAL WASTE MANAGEMENT ACT ATTENTION ALL GENERATORS OF MEDICAL WASTE
 El Dorado County Environmental Management Division Placerville Office: (530) 621-5300 South Lake Tahoe Office: (530) 573-3450 MEDICAL WASTE MANAGEMENT ACT ATTENTION ALL GENERATORS OF MEDICAL WASTE The
El Dorado County Environmental Management Division Placerville Office: (530) 621-5300 South Lake Tahoe Office: (530) 573-3450 MEDICAL WASTE MANAGEMENT ACT ATTENTION ALL GENERATORS OF MEDICAL WASTE The
Employee Environmental, Health & Safety Manual. INFECTIOUS WASTE MANAGEMENT PROGRAM Latest Revision: 05/27/08
 Note: Print document as needed for MATC Departmental purposes; it is user responsibility to ensure use of latest revision by CONTENT checking online at: http://matcmadison.edu/ad/facilities/ehs/ under
Note: Print document as needed for MATC Departmental purposes; it is user responsibility to ensure use of latest revision by CONTENT checking online at: http://matcmadison.edu/ad/facilities/ehs/ under
PROGRAM FOR THE MANAGEMENT AND DISPOSAL OF RADIOACTIVE WASTE
 RADIATION SAFETY DEPARTMENT West Virginia University Health Sciences Center WVU Hospitals Jefferson Memorial Hospital G-139 Health Sciences Center PO Box 9006 Morgantown, WV 26506-9006 Phone: 304-293-3413
RADIATION SAFETY DEPARTMENT West Virginia University Health Sciences Center WVU Hospitals Jefferson Memorial Hospital G-139 Health Sciences Center PO Box 9006 Morgantown, WV 26506-9006 Phone: 304-293-3413
University Health Services Health and Safety
 ADVISORY NO. 10.2: MANAGEMENT OF BIOLOGICAL AND INFECTIOUS WASTES The following recommendations are based on the Ohio Revised Code (ORC), Ohio Administrative Code (OAC) and EPA guidelines regarding biological
ADVISORY NO. 10.2: MANAGEMENT OF BIOLOGICAL AND INFECTIOUS WASTES The following recommendations are based on the Ohio Revised Code (ORC), Ohio Administrative Code (OAC) and EPA guidelines regarding biological
nc.us/swhome/
 Appendix 2 Waste Management Rules in North Carolina http://wastenot.enr.state.nc.us/swhome/12rul.htm#http://wastenot.enr.state. nc.us/swhome/ Home About DWM Contact Us Site Map Search Laws, Rules & Regulations
Appendix 2 Waste Management Rules in North Carolina http://wastenot.enr.state.nc.us/swhome/12rul.htm#http://wastenot.enr.state. nc.us/swhome/ Home About DWM Contact Us Site Map Search Laws, Rules & Regulations
Regulated Medical Waste Management and Proper Waste Segregation. State-Specific Information New Jersey
 Regulated Medical Waste Management and Proper Waste Segregation State-Specific Information New Jersey Identifying & Segregating Regulated Medical Waste RMW in New Jersey Six Categories of Regulated Medical
Regulated Medical Waste Management and Proper Waste Segregation State-Specific Information New Jersey Identifying & Segregating Regulated Medical Waste RMW in New Jersey Six Categories of Regulated Medical
Biomedical Waste Management Guide
 Biomedical Waste Management Guide Yale University Office of Environmental Health & Safety 135 College Street, Suite 100, New Haven, CT 06510 Updated April 2017 Telephone: 203-785-3550 / Fax: 203-785-7588
Biomedical Waste Management Guide Yale University Office of Environmental Health & Safety 135 College Street, Suite 100, New Haven, CT 06510 Updated April 2017 Telephone: 203-785-3550 / Fax: 203-785-7588
UNIVERSITY OF TOLEDO
 UNIVERSITY OF TOLEDO SUBJECT: INFECTIOUS WASTE DISPOSAL Procedure No: HM-08-019 PROCEDURE STATEMENT Departments or laboratories generating infectious waste shall develop procedures for identification,
UNIVERSITY OF TOLEDO SUBJECT: INFECTIOUS WASTE DISPOSAL Procedure No: HM-08-019 PROCEDURE STATEMENT Departments or laboratories generating infectious waste shall develop procedures for identification,
Environmental Management Chapter ALABAMA DEPARTMENT OF ENVIRONMENTAL MANAGEMENT LAND DIVISION MEDICAL WASTE PROGRAM ADMINISTRATIVE CODE
 ALABAMA DEPARTMENT OF ENVIRONMENTAL MANAGEMENT LAND DIVISION MEDICAL WASTE PROGRAM ADMINISTRATIVE CODE CHAPTER 335-17-3 COLLECTION OF MEDICAL WASTE TABLE OF CONTENTS 335-17-3-.01 Collections Of Untreated
ALABAMA DEPARTMENT OF ENVIRONMENTAL MANAGEMENT LAND DIVISION MEDICAL WASTE PROGRAM ADMINISTRATIVE CODE CHAPTER 335-17-3 COLLECTION OF MEDICAL WASTE TABLE OF CONTENTS 335-17-3-.01 Collections Of Untreated
Regulated Medical Waste Generator Inspections An Inspector s View Point
 Regulated Medical Waste Generator Inspections An Inspector s View Point Amy Scaffidi, Investigator I Bur. of Hazardous Waste & UST Compliance & Enforcement NJ Dept. of Environmental Protection Phone (609)
Regulated Medical Waste Generator Inspections An Inspector s View Point Amy Scaffidi, Investigator I Bur. of Hazardous Waste & UST Compliance & Enforcement NJ Dept. of Environmental Protection Phone (609)
Biosafety Level 1 ( BSL1 ) and Biosafety Level 2 ( BSL2 ) Biological Waste Management Guideline
 Implementation Page #: Page 1 of 14 Last Reviewed/Update November 2015 Biosafety Level 1 ( BSL1 ) and Biosafety Level 2 ( BSL2 ) Biological Waste Guideline 1.0 Purpose and Scope The purpose of this Guideline
Implementation Page #: Page 1 of 14 Last Reviewed/Update November 2015 Biosafety Level 1 ( BSL1 ) and Biosafety Level 2 ( BSL2 ) Biological Waste Guideline 1.0 Purpose and Scope The purpose of this Guideline
Regulated Medical Waste Management and Proper Waste Segregation
 Regulated Medical Waste Management and Proper Waste Segregation Connecticut Connecticut Regulated Medical Waste Management and Proper Waste Segregation The regulations in this presentation are specific
Regulated Medical Waste Management and Proper Waste Segregation Connecticut Connecticut Regulated Medical Waste Management and Proper Waste Segregation The regulations in this presentation are specific
Regulated Medical Waste Management and Proper Waste Segregation. State-Specific Information California
 Regulated Medical Waste Management and Proper Waste Segregation State-Specific Information California Learning Topics Objective: Understand how to properly segregate waste streams and properly handle the
Regulated Medical Waste Management and Proper Waste Segregation State-Specific Information California Learning Topics Objective: Understand how to properly segregate waste streams and properly handle the
Biological and Regulated Medical Waste Disposal Policy
 Biological and Regulated Medical Waste Disposal Policy I. Biological Waste II. Regulated Medical Waste Prepared by: Rutgers Environmental Health and Safety 27 Road 1 Building 4086, Livingston Campus Piscataway,
Biological and Regulated Medical Waste Disposal Policy I. Biological Waste II. Regulated Medical Waste Prepared by: Rutgers Environmental Health and Safety 27 Road 1 Building 4086, Livingston Campus Piscataway,
Health Sciences Campus Biomedical Waste Management Standard Operating Procedure (SOP)
 Health Sciences Campus Biomedical Waste Management Standard Operating Procedure (SOP) NOTE: This SOP for biological waste management does not supersede the requirements for radioactive and/or hazardous
Health Sciences Campus Biomedical Waste Management Standard Operating Procedure (SOP) NOTE: This SOP for biological waste management does not supersede the requirements for radioactive and/or hazardous
ENVIRONMENTAL MANAGEMENT GUIDE FOR SMALL LABORATORIES
 United States Office of the EPA 233-B-00-001 Environmental Protection Administrator May 2000 Agency (2131) ENVIRONMENTAL MANAGEMENT GUIDE FOR SMALL LABORATORIES 3.5 Biologically Active Substances and Wastes
United States Office of the EPA 233-B-00-001 Environmental Protection Administrator May 2000 Agency (2131) ENVIRONMENTAL MANAGEMENT GUIDE FOR SMALL LABORATORIES 3.5 Biologically Active Substances and Wastes
You are required to complete this course with a passing score of 85% or higher if you:
 OHS Biosafety BIO301L Medical Waste Management for Labs Training You are required to complete this course with a passing score of 85% or higher if you: Offer medical waste for transport from a UAB campus
OHS Biosafety BIO301L Medical Waste Management for Labs Training You are required to complete this course with a passing score of 85% or higher if you: Offer medical waste for transport from a UAB campus
Biological Materials and Biohazardous/Medical Waste Disposal Program
 Biological Materials and Biohazardous/Medical Waste Disposal Program Document Number: EHS.BIOW.08.02 Effective Date: 7/18/08 Revision Date: 7/15/10 1.0 Purpose and Applicability 1.1. Biological waste is
Biological Materials and Biohazardous/Medical Waste Disposal Program Document Number: EHS.BIOW.08.02 Effective Date: 7/18/08 Revision Date: 7/15/10 1.0 Purpose and Applicability 1.1. Biological waste is
DEPARTMENT OF BIOLOGICAL SCIENCES MEDICAL/BIOHAZARDOUS WASTE DISPOSAL AUTOCLAVE TRAINING
 DEPARTMENT OF BIOLOGICAL SCIENCES MEDICAL/BIOHAZARDOUS WASTE DISPOSAL AUTOCLAVE TRAINING INTRODUCTION In accordance with the California Medical Waste Management Act, Health and Safety Code Stanford University
DEPARTMENT OF BIOLOGICAL SCIENCES MEDICAL/BIOHAZARDOUS WASTE DISPOSAL AUTOCLAVE TRAINING INTRODUCTION In accordance with the California Medical Waste Management Act, Health and Safety Code Stanford University
Regulated Medical Waste Management and Proper Waste Segregation. State-Specific Information Florida
 Regulated Medical Waste Management and Proper Waste Segregation State-Specific Information Florida Biomedical Waste in Florida Regulated by Florida DOH Florida Administrative Code (FAC) 64E-16 Contains
Regulated Medical Waste Management and Proper Waste Segregation State-Specific Information Florida Biomedical Waste in Florida Regulated by Florida DOH Florida Administrative Code (FAC) 64E-16 Contains
Contents. Introduction
 Introduction Contents Medi-Waste is a specialist company that evolved in 1987 providing the collection, transport and disposal of biomedical waste, (including off spec and out of date pharmaceuticals,
Introduction Contents Medi-Waste is a specialist company that evolved in 1987 providing the collection, transport and disposal of biomedical waste, (including off spec and out of date pharmaceuticals,
University Health Services Health and Safety REGULATIONS FOR AUTOCLAVE USE, MAINTENANCE AND RECORDKEEPING
 ADVISORY NO. 10.5: REGULATIONS FOR AUTOCLAVE USE, MAINTENANCE AND RECORDKEEPING INTRODUCTION Using an autoclave to sterilize infectious waste is one method of treatment that is approved by the Ohio EPA.
ADVISORY NO. 10.5: REGULATIONS FOR AUTOCLAVE USE, MAINTENANCE AND RECORDKEEPING INTRODUCTION Using an autoclave to sterilize infectious waste is one method of treatment that is approved by the Ohio EPA.
In-Country shipment : How to safely ship human blood samples from suspected Ebola cases within a country by road, rail and sea
 In-Country shipment : How to safely ship human blood samples from suspected Ebola cases within a country by road, rail and sea Step 1: Before handling the sample, prepare all shipping equipment (1) Manage
In-Country shipment : How to safely ship human blood samples from suspected Ebola cases within a country by road, rail and sea Step 1: Before handling the sample, prepare all shipping equipment (1) Manage
How to safely ship human blood samples from Lassa cases within a country by road, rail and sea
 How to safely ship human blood samples from Lassa cases within a country by road, rail and sea Interim Guidance February 2018 Step 1: Before handling the sample, prepare all shipping equipment Step 1a:
How to safely ship human blood samples from Lassa cases within a country by road, rail and sea Interim Guidance February 2018 Step 1: Before handling the sample, prepare all shipping equipment Step 1a:
How to safely ship human blood samples from suspected Ebola cases within a country by road, rail and sea
 INTERIM GUIDELINE How to safely ship human blood samples from suspected Ebola cases within a country by road, rail and sea 2014 Step 1: Before handling the sample, prepare all shipping equipment Step 1a:
INTERIM GUIDELINE How to safely ship human blood samples from suspected Ebola cases within a country by road, rail and sea 2014 Step 1: Before handling the sample, prepare all shipping equipment Step 1a:
Laboratory Close-Out Guidelines
 Office of Laboratory Safety 2300 I Street, NW Ross Hall, Suite B- 05 Washington, DC 20037 t. 202-994- 8258 I labsafety@gwu.edu Guidelines The Principal Investigator (PI) is responsible for leaving laboratories
Office of Laboratory Safety 2300 I Street, NW Ross Hall, Suite B- 05 Washington, DC 20037 t. 202-994- 8258 I labsafety@gwu.edu Guidelines The Principal Investigator (PI) is responsible for leaving laboratories
Solid Waste Programs. Arizona Department of Environmental Quality Waste Programs Division
 Solid Waste Programs Arizona Department of Environmental Quality Waste Programs Division Tracy Neal Manager, Hazardous & Solid Waste Section March 22, 2018 ADEQ s Solid Waste Programs Solid Waste Facility
Solid Waste Programs Arizona Department of Environmental Quality Waste Programs Division Tracy Neal Manager, Hazardous & Solid Waste Section March 22, 2018 ADEQ s Solid Waste Programs Solid Waste Facility
Legal Aspects and Process of Biomedical Waste Management Practices in India. *Prakash V **Dr. N. Palaniraj
 Legal Aspects and Process of Biomedical Waste Management Practices in India *Prakash V **Dr. N. Palaniraj *Research Scholar ** Associate Professor, PG & Research Department of Economics, Pachaiyappa s
Legal Aspects and Process of Biomedical Waste Management Practices in India *Prakash V **Dr. N. Palaniraj *Research Scholar ** Associate Professor, PG & Research Department of Economics, Pachaiyappa s
LABORATORY WASTE MANAGEMENT PROGRAMMES
 LABORATORY WASTE MANAGEMENT PROGRAMMES Sylvia Wanjiru Kamau INTERNATIONAL LIVESTOCK RESEARCH INSTITUTE LABORATORY MANAGEMENT AND EQUIPMENT OPERATIONS WORKSHOP. 17 TH -21 ST JUNE 2012 TRAINING OBJECTIVES
LABORATORY WASTE MANAGEMENT PROGRAMMES Sylvia Wanjiru Kamau INTERNATIONAL LIVESTOCK RESEARCH INSTITUTE LABORATORY MANAGEMENT AND EQUIPMENT OPERATIONS WORKSHOP. 17 TH -21 ST JUNE 2012 TRAINING OBJECTIVES
Regulated Medical Waste Management and Proper Waste Segregation. Regulated Medical Waste Management and Proper Waste Segregation.
 Regulated Medical Waste Management and Proper Waste Segregation Maine Maine Arkansas Regulated Medical Waste Management and Proper Waste Segregation The regulations in this presentation are specific to
Regulated Medical Waste Management and Proper Waste Segregation Maine Maine Arkansas Regulated Medical Waste Management and Proper Waste Segregation The regulations in this presentation are specific to
Biosafety Checklist. 07-BiosafetyChecklist-LTC-SOP-v2.0-17Feb of 6
 Title: Biosafety Checklist Origination Date: 31 Jan 1997 Total Pages: 5 Effective Date: 17 Feb 2012 SOP Number LTC-SOP-07 v2.0 Supersedes SOP Written By: ACTG/IMPAACT Lab Tech Committee Dated: 01 Jun 2004
Title: Biosafety Checklist Origination Date: 31 Jan 1997 Total Pages: 5 Effective Date: 17 Feb 2012 SOP Number LTC-SOP-07 v2.0 Supersedes SOP Written By: ACTG/IMPAACT Lab Tech Committee Dated: 01 Jun 2004
COMMENT AND RESPONSE DOCUMENT
 COMMENT AND RESPONSE DOCUMENT PROPOSED REGULATED MEDICAL AND CHEMOTHERAPEUTIC WASTE REGULATIONS (#7-480) JULY 1, 2014 Page 1 of 27 List of Commentators on the Proposed Rulemaking 1. Kenneth J. Warren Warren
COMMENT AND RESPONSE DOCUMENT PROPOSED REGULATED MEDICAL AND CHEMOTHERAPEUTIC WASTE REGULATIONS (#7-480) JULY 1, 2014 Page 1 of 27 List of Commentators on the Proposed Rulemaking 1. Kenneth J. Warren Warren
Biomedical Waste Management Plan
 Biomedical Waste Management Plan USF Biomedical Waste Management Plan for: Facility Address: Facility Phone Number: Facility Contact: Division of Environmental Health and Safety 4202 E. Fowler Ave, OPM
Biomedical Waste Management Plan USF Biomedical Waste Management Plan for: Facility Address: Facility Phone Number: Facility Contact: Division of Environmental Health and Safety 4202 E. Fowler Ave, OPM
Texas Regulations on Medical Waste
 TCEQ REGULATORY GUIDANCE Waste Permits Division RG-001 August 2007 Texas Regulations on Medical Waste This is a guide to regulatory requirements for generators of medical waste, transporters of untreated
TCEQ REGULATORY GUIDANCE Waste Permits Division RG-001 August 2007 Texas Regulations on Medical Waste This is a guide to regulatory requirements for generators of medical waste, transporters of untreated
10.0 DISPOSAL OF FARM WASTE
 DISPOSAL OF FARM WASTE 10.1 Disposal of Dead Animals 10.1.1 Managing dead animal disposal 10.2 Disposal of Veterinary Waste 10.3 Pesticides 10.2.1 Sharps 10.2.2 Medicine disposal 10.3.1 Pesticide disposal
DISPOSAL OF FARM WASTE 10.1 Disposal of Dead Animals 10.1.1 Managing dead animal disposal 10.2 Disposal of Veterinary Waste 10.3 Pesticides 10.2.1 Sharps 10.2.2 Medicine disposal 10.3.1 Pesticide disposal
Clinical Waste. Policy. Responsibilities
 Clinical Waste Policy Clinical waste is defined as hazardous or offensive waste arising from: - any dental, medical, nursing or veterinary practice, or any other practice or establishment providing medical
Clinical Waste Policy Clinical waste is defined as hazardous or offensive waste arising from: - any dental, medical, nursing or veterinary practice, or any other practice or establishment providing medical
BOWDOIN COLLEGE BIOMEDICAL WASTE MANAGEMENT PLAN
 BOWDOIN COLLEGE BIOMEDICAL WASTE MANAGEMENT PLAN This document meets the requirements of Maine DEP 38 MRSA Chapter 900 for the development, implementation and maintenance of a written biomedical waste
BOWDOIN COLLEGE BIOMEDICAL WASTE MANAGEMENT PLAN This document meets the requirements of Maine DEP 38 MRSA Chapter 900 for the development, implementation and maintenance of a written biomedical waste
2/07. PROCEDURE for DISPOSAL of RADIOACTIVE MATERIALS
 2/07 CORNELL UNIVERSITY PROCEDURE for DISPOSAL of RADIOACTIVE MATERIALS This procedure has been developed to ensure the safety of those individuals who handle radioactive waste and to comply with University,
2/07 CORNELL UNIVERSITY PROCEDURE for DISPOSAL of RADIOACTIVE MATERIALS This procedure has been developed to ensure the safety of those individuals who handle radioactive waste and to comply with University,
BIO-MEDICAL WASTE MANAGEMENT (AMENDMENT) RULES -2018
 1 BIO-MEDICAL WASTE MANAGEMENT (AMENDMENT) RULES -2018 BIOMEDICAL WASTE RULES APPLY TO:- All who generate, collect, receive, store, transport, treat, dispose, or handle bio medical waste :- Hospitals,
1 BIO-MEDICAL WASTE MANAGEMENT (AMENDMENT) RULES -2018 BIOMEDICAL WASTE RULES APPLY TO:- All who generate, collect, receive, store, transport, treat, dispose, or handle bio medical waste :- Hospitals,
SECTION 11: SPECIAL WASTES MANAGEMENT
 SECTION 11: SPECIAL WASTES MANAGEMENT A. GENERAL PROVISIONS PART 1 - Infectious Waste 1. All generators of infectious waste shall obtain an Infectious Waste Identification Number for each site or location
SECTION 11: SPECIAL WASTES MANAGEMENT A. GENERAL PROVISIONS PART 1 - Infectious Waste 1. All generators of infectious waste shall obtain an Infectious Waste Identification Number for each site or location
GUIDELINES FOR TRANSPORTING ANTHRAX AND ANTHRAX-CONTAMINATED OBJECTS AND MATERIALS
 RESEARCH AND SPECIAL PROGRAMS ADMINISTRATION GUIDELINES FOR TRANSPORTING ANTHRAX AND ANTHRAX-CONTAMINATED OBJECTS AND MATERIALS What regulations apply to the transportation of anthrax and other infectious
RESEARCH AND SPECIAL PROGRAMS ADMINISTRATION GUIDELINES FOR TRANSPORTING ANTHRAX AND ANTHRAX-CONTAMINATED OBJECTS AND MATERIALS What regulations apply to the transportation of anthrax and other infectious
Healthcare Waste Regulatory Challenges. Selin Hoboy November 17, 2011
 Healthcare Waste Regulatory Challenges Selin Hoboy November 17, 2011 Overview What are healthcare wastes? What regulations and agencies affect healthcare waste? How to cope with it all! BMPs What are upcoming
Healthcare Waste Regulatory Challenges Selin Hoboy November 17, 2011 Overview What are healthcare wastes? What regulations and agencies affect healthcare waste? How to cope with it all! BMPs What are upcoming
RMW Segregation Training H2E Fall Extravaganza October 2, 2009
 RMW Segregation Training H2E Fall Extravaganza October 2, 2009 WASTE STREAMS Regulated Medical Waste Recyclable Waste Solid Waste (Trash) Medical Waste Pick-up by Stericycle AUTOCLAVABLE WASTE: Red Bag
RMW Segregation Training H2E Fall Extravaganza October 2, 2009 WASTE STREAMS Regulated Medical Waste Recyclable Waste Solid Waste (Trash) Medical Waste Pick-up by Stericycle AUTOCLAVABLE WASTE: Red Bag
LABORATORY WASTE DISPOSAL GUIDE
 LABORATORY WASTE DISPOSAL GUIDE Purpose This guide will help lab members properly identify and manage hazardous waste in their laboratory. Identifying the types of waste generated is crucial to handling
LABORATORY WASTE DISPOSAL GUIDE Purpose This guide will help lab members properly identify and manage hazardous waste in their laboratory. Identifying the types of waste generated is crucial to handling
Medical Waste Information Bulletin and Disposal Guide
 Public Health Department Cathy A. Raevsky, Director Andy Miller, M.D., Health Officer Environmental Health 202 Mira Loma Drive T: 530.538.7281 Oroville, California 95965 F: 530.538.5339 buttecounty.net/publichealth
Public Health Department Cathy A. Raevsky, Director Andy Miller, M.D., Health Officer Environmental Health 202 Mira Loma Drive T: 530.538.7281 Oroville, California 95965 F: 530.538.5339 buttecounty.net/publichealth
MEDICAL WASTE MANAGEMENT PLAN
 MEDICAL WASTE MANAGEMENT PLAN California Polytechnic State University San Luis Obispo, CA 93407 Environmental Health & Safety (805) 756-6662 July 2017 Type of Facility California Polytechnic State University
MEDICAL WASTE MANAGEMENT PLAN California Polytechnic State University San Luis Obispo, CA 93407 Environmental Health & Safety (805) 756-6662 July 2017 Type of Facility California Polytechnic State University
AREAS OF RESPONSIBILITY
 Applies To: UNM Hospitals Responsible Department: Infection Prevention & Control Revised: 2/2017 Procedure Patient Age Group: (X ) N/A ( ) All Ages ( ) Newborns ( ) Pediatric ( ) Adult DESCRIPTION/OVERVIEW
Applies To: UNM Hospitals Responsible Department: Infection Prevention & Control Revised: 2/2017 Procedure Patient Age Group: (X ) N/A ( ) All Ages ( ) Newborns ( ) Pediatric ( ) Adult DESCRIPTION/OVERVIEW
CHAPTER 31 BIOMEDICAL WASTE. by George F. Indest III, JD, MPA, LL.M SCOPE
 CHAPTER 31 BIOMEDICAL WASTE by George F. Indest III, JD, MPA, LL.M SCOPE Every physician's office or clinic in the state of Florida that uses or generates any type of sharps (including needles, blades,
CHAPTER 31 BIOMEDICAL WASTE by George F. Indest III, JD, MPA, LL.M SCOPE Every physician's office or clinic in the state of Florida that uses or generates any type of sharps (including needles, blades,
Central Bio-Medical Waste Treatment Facility
 Central Bio-Medical Waste Treatment Facility Central Biomedical Waste Treatment Facility, Swami Ram Cancer Institute and Research Centre Campus, Government Medical College, Rampur Road, Haldwani is the
Central Bio-Medical Waste Treatment Facility Central Biomedical Waste Treatment Facility, Swami Ram Cancer Institute and Research Centre Campus, Government Medical College, Rampur Road, Haldwani is the
Dartmouth College. Institutional Biosafety Committee. Biohazardous Waste Disposal Guide IBC Approved: 10/3/18
 Biohazardous Waste Disposal IBC Approved: 10/3/18 I. DEFINITION OF BIOHAZARDOUS WASTE: Biohazardous waste is any waste generated from working in biological or biomedical laboratories that may contain infectious
Biohazardous Waste Disposal IBC Approved: 10/3/18 I. DEFINITION OF BIOHAZARDOUS WASTE: Biohazardous waste is any waste generated from working in biological or biomedical laboratories that may contain infectious
PI s Name Date Bldg./Rm#
 PI s Name Date Bldg./Rm# Animal Biosafety Level 3 (ABSL-3) Yes No 1. Is access to the animal facility limited or restricted only to those persons authorized for program or support purposes? Yes No 2. Does
PI s Name Date Bldg./Rm# Animal Biosafety Level 3 (ABSL-3) Yes No 1. Is access to the animal facility limited or restricted only to those persons authorized for program or support purposes? Yes No 2. Does
Dartmouth College. Institutional Biosafety Committee. Biohazardous Waste Disposal Guide IBC Approved: 4/5/17
 Institutional Biosafety Committee Biohazardous Waste Disposal IBC Approved: 4/5/17 I. DEFINITION OF BIOHAZARDOUS WASTE: Biohazardous waste is any waste generated from working in biological or biomedical
Institutional Biosafety Committee Biohazardous Waste Disposal IBC Approved: 4/5/17 I. DEFINITION OF BIOHAZARDOUS WASTE: Biohazardous waste is any waste generated from working in biological or biomedical
Pointers on Shipping: Clinical Samples, Biological Substance Category B and Environmental Test Samples
 Pointers on Shipping: Clinical Samples, Biological Substance Category B and Environmental Test Samples At FedEx, we understand the importance of ensuring the safe shipping of clinical samples such as human
Pointers on Shipping: Clinical Samples, Biological Substance Category B and Environmental Test Samples At FedEx, we understand the importance of ensuring the safe shipping of clinical samples such as human
SECTION.1200 MEDICAL WASTE MANAGEMENT 15A NCAC 13B.1201 DEFINITIONS
 SECTION.1200 MEDICAL WASTE MANAGEMENT 15A NCAC 13B.1201 DEFINITIONS (1) "Blood and body fluids" means liquid blood, serum, plasma, other blood products, emulsified human tissue, spinal fluids, and pleural
SECTION.1200 MEDICAL WASTE MANAGEMENT 15A NCAC 13B.1201 DEFINITIONS (1) "Blood and body fluids" means liquid blood, serum, plasma, other blood products, emulsified human tissue, spinal fluids, and pleural
Biomedical Waste Handling and Disposal
 Approved by: Biomedical Waste Handling and Disposal Corporate Director, Environmental Supports Environmental Services Operating Standards Manual Number: Date Approved Next Review May 3, 2018 Purpose Applicability
Approved by: Biomedical Waste Handling and Disposal Corporate Director, Environmental Supports Environmental Services Operating Standards Manual Number: Date Approved Next Review May 3, 2018 Purpose Applicability
WASTE MANAGEMENT GUIDE. Creighton University Environmental Health and Safety
 WASTE MANAGEMENT GUIDE Creighton University Environmental Health and Safety www.creighton.edu/ehs 402 546 6400 Overview With over 150 teaching and research labs, as well as other areas such as fine arts
WASTE MANAGEMENT GUIDE Creighton University Environmental Health and Safety www.creighton.edu/ehs 402 546 6400 Overview With over 150 teaching and research labs, as well as other areas such as fine arts
Animal Facility Biosafety Level 3 Checklist (date: April 16, 1998)
 Date: Location: Responsible: Project Title: Inspector: _ Animal Facility Biosafety Level 3 Checklist (date: April 16, 1998) These questions are based on the Biosafety Level 3 section of Biosafety in Microbiological
Date: Location: Responsible: Project Title: Inspector: _ Animal Facility Biosafety Level 3 Checklist (date: April 16, 1998) These questions are based on the Biosafety Level 3 section of Biosafety in Microbiological
MEDICAL WASTE MANAGEMENT STUDY NOTES
 MEDICAL WASTE MANAGEMENT STUDY NOTES UNIT 1 INTRODUCTION Hospital and other health care establishment has a duty of care for the environment and for public health and have particular responsibility in
MEDICAL WASTE MANAGEMENT STUDY NOTES UNIT 1 INTRODUCTION Hospital and other health care establishment has a duty of care for the environment and for public health and have particular responsibility in
KEEPING SHARP Volume 1, Issue 2
 KEEPING SHARP Volume 1, Issue 2 Medical Waste Update HCA Published by Environmental Health Medical Waste, County of Orange Health Care Agency Save & Comply Medical waste treatment and disposal methods
KEEPING SHARP Volume 1, Issue 2 Medical Waste Update HCA Published by Environmental Health Medical Waste, County of Orange Health Care Agency Save & Comply Medical waste treatment and disposal methods
Medical, Pathological and Low-level Radioactive Waste Management Plan for Incinerator Revised 3-08
 Background Medical, Pathological and Low-level Radioactive Waste Management Plan for Incinerator Revised 3-08 Many WSU laboratories and departments generate medical waste (contaminated solids), pathological
Background Medical, Pathological and Low-level Radioactive Waste Management Plan for Incinerator Revised 3-08 Many WSU laboratories and departments generate medical waste (contaminated solids), pathological
Encl: (1) Requirements for Management of Regulated Medical Waste (2) Extracted Tooth Decision Tree
 DEPARTMENT OF THE NAVY BUREAU OF MEDICINE AND SURGERY 7700 ARLINGTON BOULEVARD FALLS CHURCH, VA 22042 IN REPLY REFER TO BUMEDINST 6280.1C BUMED-M41 BUMED INSTRUCTION 6280. 1C From: Chief, Bureau of Medicine
DEPARTMENT OF THE NAVY BUREAU OF MEDICINE AND SURGERY 7700 ARLINGTON BOULEVARD FALLS CHURCH, VA 22042 IN REPLY REFER TO BUMEDINST 6280.1C BUMED-M41 BUMED INSTRUCTION 6280. 1C From: Chief, Bureau of Medicine
Biosafety Program. I. Policy. Authority. Definitions
 Biosafety Program I. Policy It is the policy of California State University, Fullerton to maintain, insofar as is reasonably possible, an environment that will not adversely affect the health, safety and
Biosafety Program I. Policy It is the policy of California State University, Fullerton to maintain, insofar as is reasonably possible, an environment that will not adversely affect the health, safety and
Animal cell and tissue culture. Lab 1
 Animal cell and tissue culture Lab 1 Tissue culture Laboratory Safety Outline Lab Safety Biohazards Biosafety Levels Biosafety Cabinets Decontamination Biological Waste Introduction A cell culture laboratory
Animal cell and tissue culture Lab 1 Tissue culture Laboratory Safety Outline Lab Safety Biohazards Biosafety Levels Biosafety Cabinets Decontamination Biological Waste Introduction A cell culture laboratory
SOP BIO-006 USE OF AUTOCLAVE FOR STERILIZATION OF MATERIALS AND BIOLOGICAL WASTE
 ENVIRONMENTAL AND EMERGENCY MANAGEMENT Environmental Health and Safety University Crossing Suite 140 Lowell MA 01854 http://www.uml.edu/eem/ SOP BIO-006 USE OF AUTOCLAVE FOR STERILIZATION OF MATERIALS
ENVIRONMENTAL AND EMERGENCY MANAGEMENT Environmental Health and Safety University Crossing Suite 140 Lowell MA 01854 http://www.uml.edu/eem/ SOP BIO-006 USE OF AUTOCLAVE FOR STERILIZATION OF MATERIALS
TITLE 17 REFUSE AND TRASH DISPOSAL 1 CHAPTER 1 REFUSE
 17-1 TITLE 17 REFUSE AND TRASH DISPOSAL 1 CHAPTER 1. REFUSE. CHAPTER 1 REFUSE SECTION 17-101. Refuse defined; definitions. 17-102. Premises to be kept clean. 17-103. Number and size of containers 17-104.
17-1 TITLE 17 REFUSE AND TRASH DISPOSAL 1 CHAPTER 1. REFUSE. CHAPTER 1 REFUSE SECTION 17-101. Refuse defined; definitions. 17-102. Premises to be kept clean. 17-103. Number and size of containers 17-104.
TRANSPORTATION OF INFECTIOUS SUBSTANCES
 TRANSPORTATION OF INFECTIOUS SUBSTANCES 49 CFR Parts 171, 172, 173, 177, and 178 The safe transportation of hazardous materials is a matter of concern to the public, Congress, and Federal, state and local
TRANSPORTATION OF INFECTIOUS SUBSTANCES 49 CFR Parts 171, 172, 173, 177, and 178 The safe transportation of hazardous materials is a matter of concern to the public, Congress, and Federal, state and local
NEW HAMPSHIRE CODE OF ADMINISTRATIVE RULES. (a) The rules in this part shall apply to the management of asbestos waste, both friable and non-friable.
 CHAPTER Env-Sw 900 MANAGEMENT OF CERTAIN WASTES Statutory Authority: RSA 149-M:7 PART Env-Sw 901 ASBESTOS Env-Sw 901.01 Applicability. (a) The rules in this part shall apply to the management of asbestos
CHAPTER Env-Sw 900 MANAGEMENT OF CERTAIN WASTES Statutory Authority: RSA 149-M:7 PART Env-Sw 901 ASBESTOS Env-Sw 901.01 Applicability. (a) The rules in this part shall apply to the management of asbestos
Cytotoxic Waste Sorting, Handling and Disposal
 Approved by: Cytotoxic Waste Sorting, Handling and Disposal Corporate Director, Environmental Supports Environmental Services Operating Standards Manual Number: 3.1.3.14 Date Approved Next Review December
Approved by: Cytotoxic Waste Sorting, Handling and Disposal Corporate Director, Environmental Supports Environmental Services Operating Standards Manual Number: 3.1.3.14 Date Approved Next Review December
State of Rhode Island and Providence Plantations Department of Environmental Management PUBLIC NOTICE
 State of Rhode Island and Providence Plantations Department of Environmental Management PUBLIC NOTICE These Rules and Regulations Governing the Generation, Transportation, Storage, Treatment, Management
State of Rhode Island and Providence Plantations Department of Environmental Management PUBLIC NOTICE These Rules and Regulations Governing the Generation, Transportation, Storage, Treatment, Management
VIII. Biosafety Laboratory Practices and Equipment
 VIII. Biosafety Laboratory Practices and Equipment All laboratory personnel shall engage in good microbiological laboratory practices at all times. The following practices incorporate minimal practices
VIII. Biosafety Laboratory Practices and Equipment All laboratory personnel shall engage in good microbiological laboratory practices at all times. The following practices incorporate minimal practices
Development of a New Classification and Colour Code for Medical Waste Segregation
 Development of a New Classification and Colour Code for Medical Waste Segregation Ramani Bai V. 1*, Lokman Hakim S. 2, Ian Harrison 3, Vanitha G. 4, 1 Department of Civil Engineering, University of Nottingham
Development of a New Classification and Colour Code for Medical Waste Segregation Ramani Bai V. 1*, Lokman Hakim S. 2, Ian Harrison 3, Vanitha G. 4, 1 Department of Civil Engineering, University of Nottingham
Environmental Management System
 Environmental Management System Moreton Bay Research Station (MBRS) Clinical and Related Waste 1. Scope This policy applies to all clinical and related waste (refer to definitions in Section 6) from the
Environmental Management System Moreton Bay Research Station (MBRS) Clinical and Related Waste 1. Scope This policy applies to all clinical and related waste (refer to definitions in Section 6) from the
BIOMEDICAL WASTE PLAN Health Sciences Campus / Biological Sciences Titusville Campus
 Courtesy of: BIOMEDICAL WASTE PLAN Health Sciences Campus / Biological Sciences Brevard Community College Environmental Health Services 2725 Judge Fran Jamieson Way, Bldg. A Viera, FL 32940 (321) 633-2053
Courtesy of: BIOMEDICAL WASTE PLAN Health Sciences Campus / Biological Sciences Brevard Community College Environmental Health Services 2725 Judge Fran Jamieson Way, Bldg. A Viera, FL 32940 (321) 633-2053
Biosafety Training. WVU Shared Research Facilities 2012
 Biosafety Training WVU Shared Research Facilities 2012 Training Overview DEFINE Biosafety, Biohazard, Biosafety Levels Protection (PPE, biosafety cabinets) CELL CULTURE Hazards (Blood Borne Pathogens)
Biosafety Training WVU Shared Research Facilities 2012 Training Overview DEFINE Biosafety, Biohazard, Biosafety Levels Protection (PPE, biosafety cabinets) CELL CULTURE Hazards (Blood Borne Pathogens)
NOTE: Do NOT overfill sharps containers; there should be NO more than 50ml of residual liquid in the mailback kit when returned
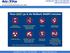 These DO go in the Mailback SHARPS Containers Sharps Waste Such As: Needles & syringes Razor blades Orthodon?c wires Scalpel blades & lancets Glass pipebes, slides & tubes Broken, contaminated glass Staples
These DO go in the Mailback SHARPS Containers Sharps Waste Such As: Needles & syringes Razor blades Orthodon?c wires Scalpel blades & lancets Glass pipebes, slides & tubes Broken, contaminated glass Staples
University of Tennessee Health Science Center. Skills Transfer Center. Exposure Control Plan. Hamilton Eye Institute. 930 Madison Avenue, Third Floor
 University of Tennessee Health Science Center Skills Transfer Center Exposure Control Plan Hamilton Eye Institute 930 Madison Avenue, Third Floor Memphis, Tennessee Skills Transfer Center Hamilton Eye
University of Tennessee Health Science Center Skills Transfer Center Exposure Control Plan Hamilton Eye Institute 930 Madison Avenue, Third Floor Memphis, Tennessee Skills Transfer Center Hamilton Eye
The Mark-Costello Co. Systems and Solutions Since 1956
 The Mark-Costello Co. Systems and Solutions Since 1956 The Mark-Costello Co. was established in the Greater Los Angeles area in 1956 as a Sales Agency/Distributor, with emphasis on designing, supplying
The Mark-Costello Co. Systems and Solutions Since 1956 The Mark-Costello Co. was established in the Greater Los Angeles area in 1956 as a Sales Agency/Distributor, with emphasis on designing, supplying
3R approach towards bio-medical waste management
 3R approach towards bio-medical waste management 7 th IconSWM Conference 15-17 December 2017, Hyderabad, India Anupam Khajuria Researcher United Nations Centre for Regional Development (UNCRD), Japan 1
3R approach towards bio-medical waste management 7 th IconSWM Conference 15-17 December 2017, Hyderabad, India Anupam Khajuria Researcher United Nations Centre for Regional Development (UNCRD), Japan 1
Section : Purpose
 105 CMR 480.000: MINIMUM REQUIREMENTS FOR THE MANAGEMENT OF MEDICAL OR BIOLOGICAL WASTE (STATE SANITARY CODE CHAPTER VIII) Section 480.001: Purpose 480.002: Authority 480.003: Citation 480.004: Scope 480.010:
105 CMR 480.000: MINIMUM REQUIREMENTS FOR THE MANAGEMENT OF MEDICAL OR BIOLOGICAL WASTE (STATE SANITARY CODE CHAPTER VIII) Section 480.001: Purpose 480.002: Authority 480.003: Citation 480.004: Scope 480.010:
EDMONDS COMMUNITY COLLEGE WASHINGTON STATE COMMUNITY COLLEGE DISTRICT 23
 EDMONDS COMMUNITY COLLEGE WASHINGTON STATE COMMUNITY COLLEGE DISTRICT 23 6.5.100 R103 HAZARDOUS WASTE MANAGEMENT PROCEDURE Purpose: To ensure that all hazardous wastes generated are properly identified,
EDMONDS COMMUNITY COLLEGE WASHINGTON STATE COMMUNITY COLLEGE DISTRICT 23 6.5.100 R103 HAZARDOUS WASTE MANAGEMENT PROCEDURE Purpose: To ensure that all hazardous wastes generated are properly identified,
Hazardous Waste Management at Remote (SQG) Sites
 Department: The University of Maine System / Safety and Environmental Management Page 1 of 7 Hazardous Waste Management at Remote (SQG) Sites General In order to maintain a Small Quantity Generator (SQG)
Department: The University of Maine System / Safety and Environmental Management Page 1 of 7 Hazardous Waste Management at Remote (SQG) Sites General In order to maintain a Small Quantity Generator (SQG)
Regulated Waste Management Plan
 Regulated Waste Management Plan November 1, 2016 (First approved: June 1, 2006) Maintained online by the Environmental Health & Safety Committee Regulated Waste Management Plan -1- Section 1: Introduction
Regulated Waste Management Plan November 1, 2016 (First approved: June 1, 2006) Maintained online by the Environmental Health & Safety Committee Regulated Waste Management Plan -1- Section 1: Introduction
Procedure for Health Care Risk Waste Management. Procedure No. 403
 Procedure for Health Care Risk Waste Management Procedure No. 403 Print Name Title Date Prepared by J.G. MacNamara T.S.O. 01/11/04 Reviewed by J. Hoare CATSO 01/11/04 Corporate Authorisation J.G. MacNamara
Procedure for Health Care Risk Waste Management Procedure No. 403 Print Name Title Date Prepared by J.G. MacNamara T.S.O. 01/11/04 Reviewed by J. Hoare CATSO 01/11/04 Corporate Authorisation J.G. MacNamara
Biological Safety Program. For. Otterbein University
 Biological Safety Program For Otterbein University Developed in conformance with the Ohio EPA Infectious Waste Regulation, OAC 3745-27 Table of Contents PURPOSE... 2 GENERAL STATEMENT... 2 POLICY... 3
Biological Safety Program For Otterbein University Developed in conformance with the Ohio EPA Infectious Waste Regulation, OAC 3745-27 Table of Contents PURPOSE... 2 GENERAL STATEMENT... 2 POLICY... 3
Title 40: Protection of Environment PART 60 STANDARDS OF PERFORMANCE FOR NEW STATIONARY SOURCES
 Title 40: Protection of Environment PART 60 STANDARDS OF PERFORMANCE FOR NEW STATIONARY SOURCES [As amended through April 4, 2011] Subpart Ec Standards of Performance for Hospital/Medical/Infectious Waste
Title 40: Protection of Environment PART 60 STANDARDS OF PERFORMANCE FOR NEW STATIONARY SOURCES [As amended through April 4, 2011] Subpart Ec Standards of Performance for Hospital/Medical/Infectious Waste
MODULE 14: Off-site Transport and Storage of Healthcare Waste
 MODULE 14: Off-site Transport and Storage of Healthcare Waste Module Overview Define external transport of healthcare waste Describe requirements for off-site transport of healthcare wastes including training
MODULE 14: Off-site Transport and Storage of Healthcare Waste Module Overview Define external transport of healthcare waste Describe requirements for off-site transport of healthcare wastes including training
