AggiE-Challenge: Final Report
|
|
|
- Anna Holt
- 6 years ago
- Views:
Transcription
1 Faculty Advisors: P. P. Mukherjee, H. Liang Graduate Student Mentors: P. Barai, C. Chen, S. Cho, C. Lopez AggiE-Challenge: Final Report Analysis of Thermo-Mechano-Electrochemical Behavior in Lithium-Ion Batteries Dion Hubble, Benjamin Powell, Deepak Bhatia, Raul Calzada, Jordan Ellington, Wyatt Smitherman, Rhianne Compas, Nathan Kudlaty, Hung Nguyen Fall 13
2 Table of Contents BACKGROUND: INTRODUCTION TO LITHIUM-ION BATTERIES... 2 THERMAL EFFECTS... 3 ELECTROLYTE EFFECTS MECHANICAL EFFECTS METHOD METHOD CONCLUSIONS THERMAL ELECTROLYTE MECHANICAL ACKNOWLEDGEMENTS REFERENCES... 30
3 Background: Introduction to Lithium-Ion Batteries Batteries provide a way to chemically store energy, so that it can be utilized as electrical energy. A battery is essentially an electrochemical transducer: it converts chemical energy to electrical energy, and vice versa (for rechargeable batteries). It consists of two electrodes (an anode and a cathode), and a separator to prevent internal short circuiting. In the case of lithium-ion batteries, the separator allows for the diffusion of lithium ions across the cell through electrolyte, while electrons are forced to travel externally from one electrode to the other. The movement of electrons and lithium ions from one side to the other is the driving mechanism: without a closed circuit, the battery will not discharge lithium ions freely. While the composition of the electrodes determines best-case voltage and capacity characteristics, actual observed voltage and capacity depends on a number of limiting factors. Ambient temperature and electrolyte concentration are naturally coupled and, unless optimized, can form positive feedback loops which decrease battery performance. At high temperatures, this results in thermal runaway, whereas at low temperatures, this results in capacity loss. Additionally, battery degradation naturally occurs during discharge (and recharge) of lithium-ion batteries. All of these effects are accelerated by discharge at high rates. We hope to model and simulate all of these phenomena relating to low-temperature and high-rate discharge of lithium-ion batteries, in order to better understand their origin, and eventually decide how to best optimize a battery for their mitigation. Figure 1. Illustration of operation of a typical Li-ion battery.
4 Thermal Effects Almost all electric vehicles (EV) and hybrid electric vehicles (HEV) produced today incorporate the use of lithium ion batteries in order to store energy in an efficient and practical manner. Because of this, li-ion battery technology must adapt to accommodate a wide range of climates where these vehicles will operate. It has been determined that temperatures at or below 0 C significantly decrease the capacity and operating voltage of lithium ion batteries. High temperature thermal management is currently a key aspect of lithium ion battery research due to dangerous thermal runaway situations, which leaves remedies to poor low temperature performance relatively unexplored. The focus of this research is to understand the mechanisms that create poor low temperature performance and explore possible solutions. It has been observed that capacity can drop by as much as 30-40% or more when operating at 0 C at various C-rates [1]. Attempts to utilize nano-fiber electrode materials to mitigate poor transport effects at low temperatures also yields up to a 40% loss in capacity at 0 C (see Table 1). This kind of performance decrease precludes the use of lithium ion batteries for transportation, defense, and other commercial applications. Table 1. Specific capacity for 70 nm electrode at the indicated temperatures and discharge rates [1]. A study by Zhang, et. al. [2] performed electrochemical impedance spectroscopy (EIS) to study lithium ion battery cycling performance. At subzero temperatures, cell resistance is dominated almost entirely by charge transfer impedance. This concludes that the slow kinetics of the lithium ion cell reactions is the largest contributing factor to poor low temperature operation (see Figure 2).
5 Figure 2. Temperature dependence of the bulk resistance (R b ), solid-state interface resistance (R sei ), and charge transfer resistance (R ct ) as well as the R ct percentange at 3.87 V. [2] The group has chosen to explore the effect of changing electrode and electrolyte properties at various temperatures to observe the effect of battery performance. A one dimensional lithium ion battery modeling software called AutoLion was utilized for all simulations in this study. AutoLion utilizes what is known as a thermally-coupled battery model, which accounts for heat generated during battery discharge and couples the resulting temperature increase to relevant temperature-dependent parameters. This allows it to more accurately reproduce experimental results. The version of the software used here treats the battery as one-dimensional, which is a generally accurate assumption given that most cell sandwiches used in lithium-ion batteries have thicknesses much smaller (~3 orders of magnitude) than their lengths and widths. The first study completed using this software was determining the effect of temperature on the capacity of a standard lithium ion battery (Figure 3).
6 Figure 3. The effect of ambient temperature on the discharge voltage and capacity of a LiCoO2-Graphite cell. From Figure 3, it is seen that temperatures as low as -30 C can cause capacity losses of approximately 70%. These results validate the previous conclusion that low temperatures severely reduce battery performance. In order to determine the cause of such poor low temperature performance, various parameters were manipulated independently. It was first hypothesized that the freezing point of the electrode and electrolyte material were directly related to low temperature performance. Upon study of common cathode materials, it was observed that the freezing point of the individual material didn t have a statistically significant effect on the low temperature behavior of the cell. Varying cathode material as the parameter confounded too many experimental variables to exhibit any significant results. For this reason, we chose to narrow our search to a single cathode material and vary individual cell geometries. Lithium Nickel Cobalt Manganese Oxide (NCM) was chosen due to its stability and high specific power which makes it a common choice for electric vehicles. One parameter we chose to examine is the effect of cathode porosity on battery performance. The porosity was determined to have a significant effect on the self-heating on the battery, which will affect the battery s ability to quickly reach an operating temperature where high capacity and power can be achieved. Figure 4, Figure 5, and Figure 6 show the effect that cathode porosity has on the cell temperature and used capacity during discharge.
7 Temperature ( C) k = 0.50 k = 0.40 k = 0.30 k = 0.20 k = Capacity (mah) Figure 4. Effects of porosity on temperature distribution and capacity for constant Ambient Temperature, 298 K Temperature ( C) k = 0.50 k = 0.40 k = 0.30 k = 0.20 k = Capacity (mah) Figure 5. Effects of porosity on temperature distribution and capacity for constant Ambient Temperature, 273 K.
8 Temperature ( C) k = 0.50 k = 0.40 k = 0.30 k = 0.20 k = Capacity (mah) Figure 6. Effects of porosity on temperature distribution and capacity for constant Ambient Temperature, 323 K. Convective heat transfer experienced in Figures 4-6 was 20 W/m 2 K, to capture typical cell heat transfer in an electric vehicle. Based on the results, decreasing the cathode porosity increased the operating temperature of the cell. These hotter cells displayed maximum capacity at porosities of However, the highest rate of temperature increase was experienced in cells with porosities lower than High heating rates in these low porosity cells (k=0.15) can be utilized to increase performance of nearby cells with the optimal porosity. This free heat generation cell array may increase the low temperature performance of an entire battery pack by utilizing the self-heating aspects of a battery in an efficient manner. Figure 7 shows the temperature difference at any point in time during a discharge cycle for a high porosity cell adjacent to a cell with a porosity of k=0.15. The temperature difference corresponds to the heat flux between the cells.
9 25 20 k = 0.50 k = 0.40 k = 0.30 T ( C) k = time (s) Figure 7. Temperature difference between two adjacent cells at an ambient temperature of 273K with a convective heat transfer coefficient of 20 W/m 2 K. There is a large change in temperature difference between the cells of porosity k=0.2 and k=0.3, even though Figures 4-6 suggest that these porosities allow for the same discharge capacity. Therefore, improved battery performance would result in selecting a battery with a porosity of k=0.3 if considering the use of a free heat generation cell array. Active cooling mechanisms in battery cell arrays could potentially diminish the effects of cell to cell heating. Therefore, it is important to study the effect of convective heat transfer on these phenomena. Figure 8, Figure 9, and Figure 10 show the effect of convective heat transfer coefficient on battery performance with different porosities.
10 Temperature ( C) k = 0.50 k = 0.40 k = 0.30 k = 0.20 k = Capacity (mah) Figure 8. Effects of porosity on temperature distribution and capacity subject to convective heat transfer, 0[W/m 2 -K], adiabatic case Temperature ( C) k = 0.50 k = 0.40 k = 0.30 k = 0.20 k = Capacity (mah) Figure 9. Effects of porosity on temperature distribution and capacity subject to convective heat transfer, 50[W/m 2 -K].
11 Temperature ( C) k = 0.50 k = 0.40 k = 0.30 k = 0.20 k = Capacity (mah) Figure 10. Effects of porosity on temperature distribution and capacity subject to convective heat transfer, 100[W/m 2 -K]. As previously estimated, the adiabatic case (h=0) maximizes cell discharge capacity. This is the best case scenario to maximize heat flux in our free heat generation cell array. Figure 11 shows the temperature difference between a cell with a cathode porosity of k=0.15 compared to cells of various porosities in an adiabatic, room temperature environment. Temperature differences observed are increased with porosity.
12 T ( C) k = 0.50 k = 0.40 k = 0.30 k = time (s) Figure 11 Temperature difference between two adjacent cells at an ambient temperature of 298K with a convective heat transfer coefficient of 0 W/m 2 K. Similar to Figure 7, trade-offs between cell capacity and temperature difference yield that a porosity of k=0.3 would most likely yield the greatest battery performance. Comparing Figure 7 and Figure 11 with Figures 4-6 the ideal porosity value would be 0.30 in order to keep capacity at an optimal level. Selection of an ideal porosity is not recommended without further developments in the free heat generation cell array model. An optimal porosity value may be lower than 0.20 due to increased temperatures (Figures 4-6), thus an adequate thermal model should be developed in order to estimate the heat flux between two adjacent cells. This heat flux should then be used to determine the cell temperature distribution to compare between different porosities. It is likely that a high contact resistance between adjacent cells. This modeling should include varying convective heat transfer coefficient.
13 Electrolyte Effects While the active materials themselves provide a basic framework for energy storage in a lithiumion battery (LIB), charge and discharge would not be possible without a medium for lithium transfer between electrodes in other words, an electrolyte. While the properties of the active materials determine basic parameters such as maximum voltage and maximum capacity of the battery, the properties of the electrolyte have a strong influence on the actual, measured battery performance. Deficiencies in electrolyte properties may lead to loss of capacity, lower observed cell voltage, and poor cycling performance. Conversely, if electrolyte properties are improved, whether through introduction of additives or by changing to a new electrolyte system, overall battery performance will correspondingly improve. Electrolytes used in LIBs consist of a lithium salt, traditionally LiPF 6, dissolved in a mixture of polar, aprotic solvents. Aqueous solvents cannot be used with this salt due to the PF 6 - ion s tendency to react in protic media to form HF. Typical solvents are carbonates, either linear (such as DMC, dimethylcarbonate) or cyclic (such as EC, ethylene carbonate, and PC, propylene carbonate). Cyclic carbonates tend to dissolve lithium more readily, but they also have high viscosities, necessitating the addition of linear carbonates to ensure thorough wetting of all active material. [3] During discharge of a typical LIB, lithium moves out of the porous anode (i.e. graphite) and into the electrolyte, while on the opposite end of the cell, existing lithium ions are pulled from the electrolyte and into the porous cathode (i.e. NCM). This gives rise to two separate driving forces for lithium transfer across the cell: concentration gradient of lithium and electrostatic force. The ions respond to these driving forces through two, electrolyte-dependent parameters: diffusivity and ionic conductivity. Both parameters are strongly dependent on temperature, as well as local concentration. Diffusivity describes the response (flux) of lithium due to a concentration gradient. All chemical species, including ions, tend to move randomly due to thermal energy. If there are more of a particular species in one place relative to another, then more of that species will on average move towards the deficient area than away from it. As each ion moves, it bumps into other particles which change its course and, on average, slow the group s movement. Diffusivity is a measure of how quickly the species, in this case lithium ions, can move to fill a deficient area; therefore, it will depend on both the speed of the ion (temperature) and each ion s immediate surroundings (concentration). Since lithium ions can collide with both solvent molecules, themselves, and counter-ions, diffusivity can be changed for a system by altering either the solvent or the salt composition, as well as overall lithium concentration. In general, lower concentrations lead to higher diffusivities. Conductivity describes the response (current) of lithium due to an electrostatic field. As the positively-charged lithium ions move in response to the potential difference between cathode and anode, they bump into, and lose energy to, their surroundings. Because of this, the ions arrive with less energy than they started with; this energy loss is measured as an Ohmic potential drop. Conductivity is a measure of how much (or rather, how little) energy is lost in transit. If too few ions are present (low concentration), conductivity drops due to a lack of charge carriers: a smaller number of ions must move more quickly to carry the same current, and will lose more energy to collisions. If too many ions are present (high concentration), conductivity decreases because repulsion between ions becomes significant, and the potential experienced by each ion correspondingly decreases. This leads to a sweet spot for
14 conductivity, typically seen just above 1 mol/l for the typical solvent-salt system. In reality, conductivity tends to influence discharge characteristics much more than diffusivity. Charge conservation dictates that all current flowing out of the anode (electrons into a circuit) must be matched by current flowing out of the cathode (lithium ions into the electrolyte). Since it is precisely this movement of ions that produces a concentration gradient in the cell, discharging at higher currents will lead to higher concentration polarization, and therefore a drop in concentration-dependent ionic conductivity. Likewise, operating at lower temperatures will also decrease ionic conductivity through its temperature-dependence. Herein lies the primary difficulty of operating LIBs at high discharge rates and/or low temperatures: electrolyte conductivity is reduced by concentration polarization, causing lower observed voltages, and therefore less capacity available above the cutoff voltage of the system. To illustrate these effects, simulations were performed using AutoLion 1-D. A prismatic cell was set up, with a total capacity of 8.05 Ah. This cell measured 72mm 65.2mm 12mm. Graphite was used as the anode, NCM the cathode, and each had a porosity of Electrode length was 80 µm each. A Celgard porous separator, with a porosity of 0.4 and length of 20 µm, was used. Nominal electrolyte concentration was set at 1.2 mol/l. Bruggeman exponents, which account for tortuosity in porous media, were set at 1.5 for all regions. For some simulations, self-heating was taken into consideration. For these cases, heat transfer coefficient at the surface of the cell was taken to be 20 W/m 2 K, and cell specific heat was assumed to be 1000 J/kg K. All of these parameters are the default settings for an AutoLion Prismatic RED cell. This simulation setup is hereafter referred to as the default cell. A series of simulations were performed in order to demonstrate the overall detrimental effects of high current and/or low temperature. The first study scanned a range of discharge currents, with ambient temperature set to 25 and self-heating allowed. Current was expressed in C-rate; 1C equals the amount of current needed to discharge the entire battery in one hour, 2C is twice that amount, etc. The results are shown below in Figure 12. Expectedly, operating voltage decreases at higher discharge rates, leading to lower capacity at the cutoff voltage of 2.5 V. Interestingly, the 4C discharge run showed a slightly higher total capacity than the 2C discharge; this is because self-heating increased temperature so much that the improvement in transport properties outweighed the unfavorable concentration gradient.
15 Figure 12. Simulation results for default cell at different C-rates and 25 C ambient temperature Next, a similar scan was run over a range of temperatures, from 40 C to -20 C, again with selfheating. A similar drop in operating voltage was observed at lower temperatures, due to the same increase in concentration gradient. Figure 13 below shows the results. Notice the loss of almost 25% capacity at - 20 C, as compared to room temperature. Also notice the voltage recovery effect at low temperature, where operating voltage rapidly drops and then stabilizes back to a higher value. This effect is often seen in experimental data [4] and is due to the rapid self-heating, which improves electrolyte properties enough to recover performance. This effect will be demonstrated later in this report.
16 Figure 13. Simulation results for default cell at different ambient temperatures and 1C discharge rate Then, to demonstrate both low temperature and high-rate discharge effects together, another C- rate scan was run, this time at -10 C, with self-heating. The results are shown in Figure 14 below. Discharge at 4C produces a huge self-heating effect; by the endpoint of discharge, cell temperature has risen by almost 65 C. Correspondingly, the voltage recovery effect is dramatic, leading to a much higher endpoint capacity for the 4C discharge than for either 1C or 2C.
17 Figure 14. Simulation results for default cell at different C-rates and -10 C ambient temperature AutoLion automatically saves electrolyte data, such as concentration and potential, for each separate discharge. To demonstrate the direct role that concentration plays in the above results, two isothermal simulations were performed: one at 25 C and 1C discharge, and one at -10 C and 1C discharge. Electrolyte data was then plotted as a function of position in the cell sandwich for two separate times in each case: 50 seconds into discharge, and at the endpoint when cell voltage reaches the cutoff of 2.5V. Then, the -10 C run was repeated with self-heating, to show the dramatic difference in performance. The isothermal, room temperature run displayed a total capacity of 7246 mah at cutoff voltage. Electrolyte data at 50 seconds is displayed in Figure 15a below. Concentration has not changed much from its initial value of 1.2 mol/l, and ionic conductivity is relatively constant at 1.34 S/m. Electrolyte potential, which lithium ions experience at each individual point, is also relatively constant, indicating almost non-existent polarization due to concentration effects.
18 Figure 15a. Electrolyte concentration, ionic conductivity, and potential for default cell after 50 seconds of 25 C, isothermal, 1C discharge At the endpoint of this discharge, as shown in Figure 15b, concentration has polarized noticeably, but still not much at a maximum deviation of a little over 0.4 mol/l from nominal. Conductivity has dipped slightly as a result, but is still relatively constant at around 1.25 S/m in the anode and 1.35 S/m in the cathode. Electrolyte potential has dropped in value due to the drop in active material potential and the need to maintain a constant driving force for ion transport, but still displays relatively low slope from one end to the other, again indicating very little polarization, even by the endpoint of discharge. Figure 15b. Electrolyte concentration, ionic conductivity, and potential for default cell at endpoint (3329 seconds) of 25 C, isothermal, 1C discharge The isothermal run at -10 C, however, performs much poorer, reaching cutoff voltage after only 2806 mah (35% of rated capacity). Even at only 50 seconds in, as seen in Figure 16a below,
19 concentration has already shifted noticeably from its nominal value, reaching a little over 1.6 mol/l in the anode and a little over 0.7 mol/l in the cathode. Conductivity does not vary too widely yet, though the temperature difference has had an immediate negative effect, dropping average conductivity to 0.56 S/m. As a result, electrolyte potential is already slightly polarized, varying by about 0.2 V across the cell. Figure 16a. Electrolyte concentration, ionic conductivity, and potential for default cell at 50 seconds of -10 C, isothermal, 1C discharge By the end of discharge, the cell has polarized considerably, as Figure 16b shows. Concentration varies from 3.5 mol/l, nearly triple its nominal value, at the far end of the anode to only mol/l at the far end of the cathode. Conductivity drops considerably as a result, reaching a minimum in the anode of only S/m. This lack of conductivity prevents electrolyte potential from adjusting normally, and as a result there is a 1.2 V electrolyte polarization between anode and cathode. This additional polarization manifests as a drop in observed voltage, and results in cutoff voltage being reached at only 35% discharge. This illustrates the positive feedback loop between conductivity and concentration that results in loss of performance at low temperature: initially low conductivity causes large concentration gradients, which results in even lower conductivity, etc.
20 Figure 16b. Electrolyte concentration, ionic conductivity, and potential for default cell at endpoint (1289 seconds) of - 10 C, isothermal, 1C discharge When self-heating is allowed to occur, however, performance improves significantly. This is a direct result of electrolyte effects. To illustrate the voltage recovery effect from temperature increase during discharge, the -10 C run was repeated with self-heating allowed. The new endpoint results can be seen in Figure 16c. This time, the cell reaches 6099 mah of discharge before cutoff voltage is reached, and cell temperature increases from -10 C to 4.4 C. Concentration still varies more than it did at room temperature endpoint, but only reaches 2.23 mol/l in the anode and 0.51 mol/l in the cathode. Conductivity now only varies from 0.38 S/m to 0.90 S/m; in fact, it some regions is has actually increased from its starting value due to self-heating! As a result, electrolyte polarization at the endpoint is only a little over 0.3 V, and the cell has had the chance to discharge much more before the combination of solid phase voltage change and electrolyte polarization drops overall voltage to the cutoff.
21 Figure 16c Electrolyte concentration, ionic conductivity, and potential for default cell at endpoint (2802 seconds) of -10 C, with self-heating, 1C discharge
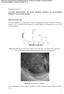
22 Mechanical Effects In a lithium-ion battery, the movement of electrons and lithium is the source of providing electrical power. The process of lithium ions entering and leaving the electrodes is known as lithiation and delithiation. [5] These high concentration gradients can cause significant mechanical stress in the electrodes and can potentially create cracks in the active material. Cracks in active material can cause the growth of Solid Electrolyte Interface (SEI) in those crevices. SEI forms spontaneously at the electrodeelectrolyte interface and places a very critical role in the performance and safety of a Li-ion battery. This formation is both useful and harmful to battery system. During initial cycles, SEI serves as a medium for exchange of li-ions but has a negative effects once the SEI layer begins to become thick. In this research, our group investigated the formation of cracks and developed code to identify any possible cracks that may lead to pieces of the active material to break off from the main electrode. Since these pieces will be covered with SEI, they will also have some trapped Li-ions in them. These ions cannot be released back into the electrolyte and will have negative effects on battery functionality. Method 1 In this research we were interested in quantitatively describing how this loss of active material and the lithium ions trapped in the surrounding SEI affect the capacity and charge rates of these types of batteries. In order to identify any possible particle isolations, our team investigated the following- Step 1 1. Identify broken bonds in the active material through previously developed MATLAB code. 2. Identify cracks that start from the exterior boundary of the active material and traverse towards the interior. 3. Identify cracks that may intersect, resulting in the enclosed area to break off and lead to particle isolation. Several matrices with information on the node locations, broken bond location and the identifiers for the broken bond is stored and processed. These nodes are then plotted for creating a visual aid for the user in the function known as Disp_Orientation. Step 2 The next crucial step involves identifying boundaries of the active material. This is an automated process and will work for any set of data given that the provided data follows the same output format as the one used for testing. This is performed in function idboundary.
23 Figure 17. MATLAB output showing the detected external boundary and all the broken bonds (black lines) connecting different nodes Step 3 Once the external boundaries have been isolated, every nodes neighbours and bonds are automatically mapped in the two functions called- neighbour_elements and neighbor_nodes. Step 4 Figure 18. Output of the data given which would be recreated in Figure 3
24 The final step is to automatically recreate the traces for the given data to show proof of concept. This is automatically performed in the function called Algorithm2. This consists of a series of statements that try to take into account various possible traces that may exist in the active material. Figure 19. Final output of the code to show automatic routing Method 2 A second approach was also utilized to solve the problem of mapping the fractures and cracks in a lithium active material. This approach started out the same as the earlier-described, and operated off the same initial data sets: Coordinates of the nodes used in the FEA The match-up between the node #s and element #s The broken element #s The center and radius of each active material in the FEA However, this approach utilized a radial walk algorithm to find the location and length of each crack or fracture in the active material. The approach of this code is as follows: Step 1 The location of each element midpoint was determined through the use of the External code function. External: A MatLab function which takes in two of the initial data sets (the node coordinates and the match-up between node #s and element #s) and returns a matrix of element locations Because it is the broken elements which create cracks and fragment, this method thus focused on the element locations (rather than the nodes) as the primary means of obtaining its results. Figure 20 demonstrates the graph of nodes in a single material, while Figure 21 shows the graph of the corresponding elements.
25 Figure 20. Nodes in Active Material Figure 21. Elements in Active Material
26 Step 2 The nodes and elements were separated into their materials through the use of the Materialistic code function. Materialistic: A Matlab function which takes in the matrix of elements put out from Elementary, as well as the initial data set of the centers/radii of active materials, and splits up the former into a number of matrices. Each corresponds to its own material. In this way, each material could be analyzed separately in the remaining stages of the process, without different materials polluting the results of each. Figure 22 below demonstrates the output of this function, once plotted on a scatter graph. Figure 22. Material Plot
27 Step 3 Because it is the exterior fractures and cracks (those which extend to the outside of an active material) whose lengths are desired, it was necessary to determine the identity of the exterior elements. This was done through the use of the External code function, which assigned a value of 1 to exterior elements on a certain material and a value of 0 to all others. External: A MatLab function which takes a single material s matrix of element locations (from the outputs of Materialistic) and, element-by-element, determines if each is an exterior element based on the number of elements surrounding it. It outputs this information as 1 or 0. Step 4 The final stage of this process involved the code function Fragmented, which had the task of actually determining two things. 1. Whether or not a broken exterior element actually formed a fragment 2. The length of the crack (or fragment) that was formed In order to perform these two vital tasks, the code had to assume that each and every broken exterior element formed EITHER a crack OR a fragment. Fragmented: A MatLab function that determines crack and fragment locations, as well as the length of each. It does this by starting at a broken exterior elements and walking along any adjacent broken elements. This code served as the most important stage of the whole process. It worked by starting at a single exterior broken element, then searching in a radial pattern for adjacent broken elements. If one is found, it jumps to this element and then repeats the search. This process of searching and jumping (known as walking ) repeats itself continuously, incrementing each time to keep track of the length, until the code jumps to another exterior broken element. When it does, the code realizes that a fragment has been created and it ends itself. A plot of this is shown below in Figure 23, where the broken exterior elements are shown in red and the broken interior elements are shown in blue.
28 Figure 23. Broken Elements in an Active Material By looking at Figure 23 it can be seen that several cracks are apparent in the active material. However, the purpose of this program is to provide this information without any sort of visual information. Therefore, the accompanying information must be produced by the code, the matrix of which is shown below in Table 2.
29 Table 2. Columns 6 and 7 of Final Matrix Each row corresponds to a single broken exterior element, from the material displayed in Figure 5 (moving from bottom-left to top-right). The earlier columns in the final output matrix contain information such as X-position, Y-position, Element No., and other such identifiers. However, these final two columns are the most important. The first tells whether or not the exterior element forms a fragment (1 if true, 0 if false), and the second tells the length of the crack or fragment formed. By looking at Table 1, it can be noticed that no fragments are formed in the displayed material, but that the longest crack formed is somewhere near the bottom of the matrix and has a length of 2.75 units. Both pieces of information can be verified by examining Figure 5, indicating that this code does in fact produce the required deliverables.
30 Conclusions Thermal Extreme low temperatures below 0 C in the harsh winter months cause a significant drop in operating voltage and capacity, resulting in a loss of power and range. Self-heating during cell discharge can increase cell temperature and improve charge and ion transport capabilities, but can create dangerous thermal runaway situations. Cathode porosity was explored as a parameter that affects cell heating. It was found that lower porosity increases the heating rate of the battery during discharge. A porosity of 0.2 to 0.3 has the highest capacity. However, higher porosity cells are more stable in avoiding the possibility of thermal runaway. It is hypothesized that the high heating rate of a low porosity cell in a battery array can offer free heat generation to nearby high porosity cells, resulting in a more efficient battery pack for low temperature operation. The heat flux and convective heat transfer effects need to be modeled in order to confirm the most effective cell porosity and arrangement. Electrolyte Obviously, electrolyte effects play a huge role in observed discharge characteristics. In fact, much of the difference in battery performance between high and low temperatures, and between high and low discharge rates, can be attributed to ion conductivity in the electrolyte and its change with temperature/concentration. Therefore, if one wishes to improve low temperature or high discharge battery performance, it is necessary to optimize the electrolyte conductivity. Several articles in scientific literature have begun to explore this topic [3]. Additionally, effects other than ion transport influence low temperature performance; Zhang et al. have reported that cells with LiBF 4 discharge more fully at low temperature than those with LiPF 6, due to decreased charge-transfer resistance (resistance to intercalation) at the electrodes [6]. These interfacial effects dominate at extremely low discharge rates, although bulk electrolyte properties still have a greater influence at 1C and above [4]. Mechanical Cracks can be induced by many factors, both external and internal to the battery. In this group we investigated the occurrence of diffusion-induced cracks in the active material (ref. Pallabs Paper) and based on that phenomenon, our group developed code to automatically detect any form of particle isolation. There is loss of active lithium ions when isolated in broken chunks of active material, which further reduces the capacity of the battery. Our codes takes in the necessary data and automatically detects areas where two cracks converge and lead to particle isolation. This information would be useful in the future for observing the effects of SEI formation on the area exposed due to cracks, as well as predicting capacity loss over multiple cycles. Two very different approaches for tracing were used and they both show promising results. More test runs are needed in order to prove the robustness of the program.
31 Acknowledgements Thanks to Texas A&M and the Dwight Look College of Engineering for providing the funding necessary to make this AggiE-Challenge project possible. Special thanks to the following people for all of their help: Dr. Mukherjee and Dr. Liang (Faculty Mentors) Pallab Barai, Seongkoo Cho, Chien-Fan Chen, and Carlos Lopez (Graduate Student Mentors) References [1] C. R. Sides, C. R. Martin, Nanostructured electrodes and the Low-Temperature Performance of Li-ion Batteries, Adv. Mater. (2005), 17, 125. [2] S.S. Zhang, K. Xu, T.R. Jow, Electrochemical impedance study on the low temperature of Li-ion batteries, Electrochimica Acta, (2004), 49, [3] Alexandra Lex-Balducci, Wesley Henderson, and Stefano Passerini. "Electrolytes for Lithium-Ion Batteries," in Lithium-Ion Batteries: Advanced Materials and Technologies. CRC Press, [4] Y. Ji, Y. Zhang, C.Y. Wang, Li-Ion Cell Operation at Low Temperatures, J. Electrochem. Soc. (2013), 160, A636. [5] P. Barai and P.P. Mukherjee, Stochastic Analysis of Diffusion Induced Damage in Lithium-Ion Battery Electrodes, J Electrochem. Soc. (2013) 160, A955. [6] S.S. Zhang, K. Xu, T.R. Jow, A new approach toward improved low temperature performance of Liion battery, Electrochem. Comm. (2002), 4, 928.
Factors Influencing the Thermal Stability of Lithium Ion Batteries - From Active Materials to State-of-Charge and Degradation
 Factors Influencing the Thermal Stability of Lithium Ion Batteries - From Active Materials to State-of-Charge and Degradation JRC Exploratory Research Workshop Safer Li-Ion Batteries by Preventing Thermal
Factors Influencing the Thermal Stability of Lithium Ion Batteries - From Active Materials to State-of-Charge and Degradation JRC Exploratory Research Workshop Safer Li-Ion Batteries by Preventing Thermal
State of Lithium Ion Battery Research
 State of Lithium Ion Battery Research Professor Vanessa Wood Department of Information Technology and Electrical Engineering ETH Zürich 2/5/2018 1 Lithium ion batteries can be used for many applications
State of Lithium Ion Battery Research Professor Vanessa Wood Department of Information Technology and Electrical Engineering ETH Zürich 2/5/2018 1 Lithium ion batteries can be used for many applications
Supplementary Figure 1. Crystal structures of conventional layered and Li-rich layered manganese oxides. a, The crystal structure of rhombohedral
 Supplementary Figure 1. Crystal structures of conventional layered and Li-rich layered manganese oxides. a, The crystal structure of rhombohedral LiMO 2 (M = Ni, Co, Mn) with the space group R3m. b, The
Supplementary Figure 1. Crystal structures of conventional layered and Li-rich layered manganese oxides. a, The crystal structure of rhombohedral LiMO 2 (M = Ni, Co, Mn) with the space group R3m. b, The
Electrochemical impedance study of initial lithium ion intercalation into graphite powders
 Electrochimica Acta 46 (2001) 1793 1813 www.elsevier.nl/locate/electacta Electrochemical impedance study of initial lithium ion intercalation into graphite powders Chunsheng Wang a, *, A. John Appleby
Electrochimica Acta 46 (2001) 1793 1813 www.elsevier.nl/locate/electacta Electrochemical impedance study of initial lithium ion intercalation into graphite powders Chunsheng Wang a, *, A. John Appleby
Overview of research
 MICDE-TARDEC Faculty Workshop Overview of research Wei Lu Mechanical Engineering University of Michigan, Ann Arbor weilu@umich.edu September 15, 2017 1 Overall of Research Areas Joining of dissimilar materials,
MICDE-TARDEC Faculty Workshop Overview of research Wei Lu Mechanical Engineering University of Michigan, Ann Arbor weilu@umich.edu September 15, 2017 1 Overall of Research Areas Joining of dissimilar materials,
Chapter 7. Evaluation of Electrode Performance by. Electrochemical Impedance
 Chapter 7 Evaluation of Electrode Performance by Electrochemical Impedance Spectroscopy (EIS) 7.1 Introduction A significant fraction of internal resistance of a cell comes from the interfacial polarization
Chapter 7 Evaluation of Electrode Performance by Electrochemical Impedance Spectroscopy (EIS) 7.1 Introduction A significant fraction of internal resistance of a cell comes from the interfacial polarization
THERMOELECTRIC EFFECTS OF SIZE OF MICROCHANNELS ON AN INTERNALLY COOLED LI-ION BATTERY CELL
 Proceedings of the ASME 2016 International Mechanical Engineering Congress and Exposition IMECE2016 November 11-17, 2016, Phoenix, Arizona, USA IMECE2016-65729 THERMOELECTRIC EFFECTS OF SIZE OF MICROCHANNELS
Proceedings of the ASME 2016 International Mechanical Engineering Congress and Exposition IMECE2016 November 11-17, 2016, Phoenix, Arizona, USA IMECE2016-65729 THERMOELECTRIC EFFECTS OF SIZE OF MICROCHANNELS
Application Note. 4D Study of Silicon Anode Volumetric Changes in a Coin Cell Battery using X-ray Microscopy
 4D Study of Silicon Anode Volumetric Changes in a Coin Cell Battery using X-ray Microscopy 4D Study of Silicon Anode Volumetric Changes in a Coin Cell Battery using X-ray Microscopy Authors: Dr. Claus
4D Study of Silicon Anode Volumetric Changes in a Coin Cell Battery using X-ray Microscopy 4D Study of Silicon Anode Volumetric Changes in a Coin Cell Battery using X-ray Microscopy Authors: Dr. Claus
Supplementary Figure 1:
 b a c Supplementary Figure 1: Calibration of the Cs + sputtering rate on composite LiNi 0.7 Mn 0.15 Co 0.15 O 2 electrodes (500 ev ion energy, ~40 na measured sample current): (a) Optical profilometry
b a c Supplementary Figure 1: Calibration of the Cs + sputtering rate on composite LiNi 0.7 Mn 0.15 Co 0.15 O 2 electrodes (500 ev ion energy, ~40 na measured sample current): (a) Optical profilometry
Cycle life performance of lithium-ion pouch cells
 Journal of Power Sources 158 (2006) 679 688 Cycle life performance of lithium-ion pouch cells Karthikeyan Kumaresan, Qingzhi Guo, Premanand Ramadass, Ralph E. White Department of Chemical Engineering,
Journal of Power Sources 158 (2006) 679 688 Cycle life performance of lithium-ion pouch cells Karthikeyan Kumaresan, Qingzhi Guo, Premanand Ramadass, Ralph E. White Department of Chemical Engineering,
Sensitivity Analysis for a Plug-Flow Anaerobic Digester
 Sensitivity Analysis for a Plug-Flow Anaerobic Digester A Master of Engineering Project Presented to the Faculty of the Graduate School of Cornell University in Partial Fulfillment of the Requirements
Sensitivity Analysis for a Plug-Flow Anaerobic Digester A Master of Engineering Project Presented to the Faculty of the Graduate School of Cornell University in Partial Fulfillment of the Requirements
Safe, Inexpensive, Long Life, High Power and Efficiency Batteries For Grid Scale Energy Storage Applications
 Safe, Inexpensive, Long Life, High Power and Efficiency Batteries For Grid Scale Energy Storage Applications Investigators Yi Cui, Associate Professor; Robert Huggins, Professor; Mauro Pasta, Postdoctoral
Safe, Inexpensive, Long Life, High Power and Efficiency Batteries For Grid Scale Energy Storage Applications Investigators Yi Cui, Associate Professor; Robert Huggins, Professor; Mauro Pasta, Postdoctoral
Investigations on polarization losses in planar Solid Oxide Fuel Cells
 Investigations on polarization losses in planar Solid Oxide Fuel Cells S.Senthil Kumar, Akshay Iyer, B. ShriPrakash, S.T. Aruna CSIR National Aerospace Laboratories Bangalore-560017 Presentation at COMSOL
Investigations on polarization losses in planar Solid Oxide Fuel Cells S.Senthil Kumar, Akshay Iyer, B. ShriPrakash, S.T. Aruna CSIR National Aerospace Laboratories Bangalore-560017 Presentation at COMSOL
Novel Materials for Lithium-Ion Batteries
 Novel Materials for Lithium-Ion Batteries John Bradley May 18th 2012 Project Supervisors: Prof. West & Chaou Tan Abstract The effect of carbon coating on two novel battery cathode materials LiMnP 2 O 7
Novel Materials for Lithium-Ion Batteries John Bradley May 18th 2012 Project Supervisors: Prof. West & Chaou Tan Abstract The effect of carbon coating on two novel battery cathode materials LiMnP 2 O 7
The below identified patent application is available for licensing. Requests for information should be addressed to:
 DEPARTMENT OF THE NAVY OFFICE OF COUNSEL NAVAL UNDERSEA WARFARE CENTER DIVISION 1176 HOWELL STREET NEWPORT Rl 02841-1708 IN REPLY REFER TO Attorney Docket No. 300139 15 December 2017 The below identified
DEPARTMENT OF THE NAVY OFFICE OF COUNSEL NAVAL UNDERSEA WARFARE CENTER DIVISION 1176 HOWELL STREET NEWPORT Rl 02841-1708 IN REPLY REFER TO Attorney Docket No. 300139 15 December 2017 The below identified
Battery thermal models for hybrid vehicle simulations
 Journal of Power Sources 110 (2002) 377 382 Battery thermal models for hybrid vehicle simulations Ahmad A. Pesaran National Renewable Energy Laboratory, 1617 Cole Blvd, Golden, CO 80401, USA Abstract This
Journal of Power Sources 110 (2002) 377 382 Battery thermal models for hybrid vehicle simulations Ahmad A. Pesaran National Renewable Energy Laboratory, 1617 Cole Blvd, Golden, CO 80401, USA Abstract This
Hierarchical 3D ZnCo 2 O 4 Nanowire Arrays/Carbon Cloth Anodes for A Novel Class of High-Performance Flexible Lithium-ion Batteries
 Supporting Information Hierarchical 3D ZnCo 2 O 4 Nanowire Arrays/Carbon Cloth Anodes for A Novel Class of High-Performance Flexible Lithium-ion Batteries Bin Liu, Jun Zhang, Xianfu Wang, Gui Chen, Di
Supporting Information Hierarchical 3D ZnCo 2 O 4 Nanowire Arrays/Carbon Cloth Anodes for A Novel Class of High-Performance Flexible Lithium-ion Batteries Bin Liu, Jun Zhang, Xianfu Wang, Gui Chen, Di
Thermal Management of Lithium-ion Batteries
 Thermal Management of Lithium-ion Batteries APEC 2018 Greg Albright 1 What Are We Talking About? Maximize Vehicle Range (battery kwh; regen; charge time) Maximize Performance (power) Minimize Cost ($/mile)
Thermal Management of Lithium-ion Batteries APEC 2018 Greg Albright 1 What Are We Talking About? Maximize Vehicle Range (battery kwh; regen; charge time) Maximize Performance (power) Minimize Cost ($/mile)
Factors Governing Life of High-Energy Lithium-Ion Cells
 Factors Governing Life of High-Energy Lithium-Ion Cells D.P. Abraham IBA 2013 March 11, 2013 Barcelona, Spain Research sponsors are both Government and Private Sector 2 Diagnostics Overview Use of characterization
Factors Governing Life of High-Energy Lithium-Ion Cells D.P. Abraham IBA 2013 March 11, 2013 Barcelona, Spain Research sponsors are both Government and Private Sector 2 Diagnostics Overview Use of characterization
Electroactive Polymer for Controlling Overcharge in Lithium-Ion Batteries
 PSI-SR-1261 Electroactive Polymer for Controlling Overcharge in Lithium-Ion Batteries A. Newman R. Pawle K. White J. Lennhoff A. Newman, R. Pawle, K. White, J. Lennhoff, "Electroactive Polymer for Controlling
PSI-SR-1261 Electroactive Polymer for Controlling Overcharge in Lithium-Ion Batteries A. Newman R. Pawle K. White J. Lennhoff A. Newman, R. Pawle, K. White, J. Lennhoff, "Electroactive Polymer for Controlling
Novel Mn 1.5 Co 1.5 O 4 spinel cathodes for intermediate temperature solid oxide fuel cells
 Novel Mn 1.5 Co 1.5 O 4 spinel cathodes for intermediate temperature solid oxide fuel cells Huanying Liu, a, b Xuefeng Zhu, a * Mojie Cheng, c You Cong, a Weishen Yang a * a State Key Laboratory of Catalysis,
Novel Mn 1.5 Co 1.5 O 4 spinel cathodes for intermediate temperature solid oxide fuel cells Huanying Liu, a, b Xuefeng Zhu, a * Mojie Cheng, c You Cong, a Weishen Yang a * a State Key Laboratory of Catalysis,
Supplemental Information for:
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 215 Supplemental Information for: A Novel Lithium-sulfur Battery Cathode from Butadiene Rubber-caged
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 215 Supplemental Information for: A Novel Lithium-sulfur Battery Cathode from Butadiene Rubber-caged
Corrosion. Lab. of Energy Conversion & Storage Materials. Produced by K. B. Kim
 Corrosion 대기환경에의한금속소재 (organic film coated steel) 의퇴화현상평가연구 Lab. of Energy Conversion & Storage Materials Produced by K. B. Kim Introduction AC Impedance Spectroscopy Application of AC Impedance to Corrosion
Corrosion 대기환경에의한금속소재 (organic film coated steel) 의퇴화현상평가연구 Lab. of Energy Conversion & Storage Materials Produced by K. B. Kim Introduction AC Impedance Spectroscopy Application of AC Impedance to Corrosion
Supplementary Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Supplementary Information Converting detrimental HF in electrolyte into
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Supplementary Information Converting detrimental HF in electrolyte into
6th International Conference on Advanced Design and Manufacturing Engineering (ICADME 2016)
 6th International Conference on Advanced Design and Manufacturing Engineering (ICADME 2016) Porous Co3O4 irregular Micro-cubes with lithium storage performances Ting Wanga, Hao Zhengb, Jinsong Chengc,
6th International Conference on Advanced Design and Manufacturing Engineering (ICADME 2016) Porous Co3O4 irregular Micro-cubes with lithium storage performances Ting Wanga, Hao Zhengb, Jinsong Chengc,
In Situ Formation of Stable Interfacial Coating for High Performance Lithium Metal Anodes
 In Situ Formation of Stable Interfacial Coating for High Performance Lithium Metal Anodes Haiping Wu 1, Yue Cao 1, Linxiao Geng 2 & Chao Wang 1* 1 Department of Chemistry, University of California Riverside,
In Situ Formation of Stable Interfacial Coating for High Performance Lithium Metal Anodes Haiping Wu 1, Yue Cao 1, Linxiao Geng 2 & Chao Wang 1* 1 Department of Chemistry, University of California Riverside,
Continuous Monitoring of Oxygen Chemical Potential at the Surface of Growing Oxide Scales during High Temperature Oxidation of Metals
 Materials Transactions, Vol. 49, No. 3 (2008) pp. 629 to 636 #2008 The Japan Institute of Metals EXPRESS REGULAR ARTICLE Continuous Monitoring of Oxygen Chemical Potential at the Surface of Growing Oxide
Materials Transactions, Vol. 49, No. 3 (2008) pp. 629 to 636 #2008 The Japan Institute of Metals EXPRESS REGULAR ARTICLE Continuous Monitoring of Oxygen Chemical Potential at the Surface of Growing Oxide
Kuang-Che Hsiao. Supervisor: Prof. Tony West
 New Potential Cathode Materials for Lithium-ion ion Battery - Synthesis and characterization of Li 1+x FePO 4-x N x cathode - Kuang-Che Hsiao Supervisor: Prof. Tony West 08/06/2010 E-mail: dtp09kh@sheffield.ac.uk
New Potential Cathode Materials for Lithium-ion ion Battery - Synthesis and characterization of Li 1+x FePO 4-x N x cathode - Kuang-Che Hsiao Supervisor: Prof. Tony West 08/06/2010 E-mail: dtp09kh@sheffield.ac.uk
Advanced Lithium-ion Battery Manufacturing R&D
 EVS28 KINTEX, Korea, May 3-6, 2015 Advanced Lithium-ion Battery Manufacturing R&D James F. Miller Argonne National Laboratory, Argonne, Illinois, USA 60439 Introduction I. The cost of lithium-ion batteries
EVS28 KINTEX, Korea, May 3-6, 2015 Advanced Lithium-ion Battery Manufacturing R&D James F. Miller Argonne National Laboratory, Argonne, Illinois, USA 60439 Introduction I. The cost of lithium-ion batteries
ENVIRONMENT-FRIENDLY HYDROGEN GAS AS FUEL IN FUEL CELL AND ITS CHALLENGES
 ENVIRONMENT-FRIENDLY HYDROGEN GAS AS FUEL IN FUEL CELL AND ITS CHALLENGES Hydrogen is the simplest and lightest element. Storage is one of the greatest problems for hydrogen. It leaks very easily from
ENVIRONMENT-FRIENDLY HYDROGEN GAS AS FUEL IN FUEL CELL AND ITS CHALLENGES Hydrogen is the simplest and lightest element. Storage is one of the greatest problems for hydrogen. It leaks very easily from
Rechargeable Sodium-ion Batteries with a Cl-doped
 Supplementary Information: Room-Temperature All-solidstate Rechargeable Sodium-ion Batteries with a Cl-doped Na 3 PS 4 Superionic Conductor Iek-Heng Chu a, Christopher Kompella a, Han Nguyen a, Zhuoying
Supplementary Information: Room-Temperature All-solidstate Rechargeable Sodium-ion Batteries with a Cl-doped Na 3 PS 4 Superionic Conductor Iek-Heng Chu a, Christopher Kompella a, Han Nguyen a, Zhuoying
Simulation of Atmospheric Air Micro Plasma Jet for Biomedical Applications
 Simulation of Atmospheric Air Micro Plasma Jet for Biomedical Applications Luke T. Gritter 1, Jeffrey S. Crompton *1, and Kyle C. Koppenhoefer 1 1 AltaSim Technologies, LLC 130 E. Wilson Bridge Rd, Suite
Simulation of Atmospheric Air Micro Plasma Jet for Biomedical Applications Luke T. Gritter 1, Jeffrey S. Crompton *1, and Kyle C. Koppenhoefer 1 1 AltaSim Technologies, LLC 130 E. Wilson Bridge Rd, Suite
FABRICATION AND ELECTROCHEMICAL CHARECTARIZATION OF THE CR2032 COIN CELLS USING THE DEVELOPED PURE AND CARBON COATED
 FABRICATION AND ELECTROCHEMICAL CHARECTARIZATION OF THE CR2032 COIN CELLS USING THE DEVELOPED PURE AND CARBON COATED LiMPO 4 (M= Mn, Co & Ni) NANOPARTICLES CHAPTER VI 181 CHAPTER - VI FABRICATION AND ELECTROCHEMICAL
FABRICATION AND ELECTROCHEMICAL CHARECTARIZATION OF THE CR2032 COIN CELLS USING THE DEVELOPED PURE AND CARBON COATED LiMPO 4 (M= Mn, Co & Ni) NANOPARTICLES CHAPTER VI 181 CHAPTER - VI FABRICATION AND ELECTROCHEMICAL
Supporting Information for
 Supporting Information for Self-stabilized solid electrolyte interface on host-free Li metal anode towards high areal capacity and rate utilization Zhenglin Hu 1,3, Shu Zhang 1, Shanmu Dong*,1, Quan Li
Supporting Information for Self-stabilized solid electrolyte interface on host-free Li metal anode towards high areal capacity and rate utilization Zhenglin Hu 1,3, Shu Zhang 1, Shanmu Dong*,1, Quan Li
From Surface To Cell: Understanding the Lithium Ion Battery. The world leader in serving science
 From Surface To Cell: Understanding the Lithium Ion Battery 1 The world leader in serving science Content Discharge Detail the Li-ion Battery industry drivers & trends Our position in industry and our
From Surface To Cell: Understanding the Lithium Ion Battery 1 The world leader in serving science Content Discharge Detail the Li-ion Battery industry drivers & trends Our position in industry and our
CORROSION of Metals CORROSION CORROSION. Outline ISSUES TO ADDRESS... Why does corrosion occur? What metals are most likely to corrode?
 Outline Corrosion - Introduction Corrosion of Metals - e.g. Rusting of iron in water Electrochemical Cell Electrode Potential in Electrochemical Cell Standard Electromotive Force Example Relative Corrosion
Outline Corrosion - Introduction Corrosion of Metals - e.g. Rusting of iron in water Electrochemical Cell Electrode Potential in Electrochemical Cell Standard Electromotive Force Example Relative Corrosion
All-solid-state Li battery using a light-weight solid electrolyte
 All-solid-state Li battery using a light-weight solid electrolyte Hitoshi Takamura Department of Materials Science, Graduate School of Engineering, Tohoku University Europe-Japan Symposium, Electrical
All-solid-state Li battery using a light-weight solid electrolyte Hitoshi Takamura Department of Materials Science, Graduate School of Engineering, Tohoku University Europe-Japan Symposium, Electrical
Electronic supplementary information. Efficient energy storage capabilities promoted by hierarchically MnCo 2 O 4
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Electronic supplementary information Efficient energy storage capabilities promoted by hierarchically
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Electronic supplementary information Efficient energy storage capabilities promoted by hierarchically
Maximizing Hydrogen Production of A Solid Oxide Electrolyser Cell
 212 International Conference on Clean and Green Energy IPCBEE vol.27 (212) (212) IACSIT Press, Singapore Maximizing Hydrogen Production of A Solid xide Electrolyser Cell Qiong Cai 1+, Claire S. Adjiman
212 International Conference on Clean and Green Energy IPCBEE vol.27 (212) (212) IACSIT Press, Singapore Maximizing Hydrogen Production of A Solid xide Electrolyser Cell Qiong Cai 1+, Claire S. Adjiman
The Preparation of C/Ni Composite Nanofibers with Pores by Coaxial Electrospinning
 2016 International Conference on Intelligent Manufacturing and Materials (ICIMM 2016) ISBN: 978-1-60595-363-2 The Preparation of C/Ni Composite Nanofibers with Pores by Coaxial Electrospinning Yiqiang
2016 International Conference on Intelligent Manufacturing and Materials (ICIMM 2016) ISBN: 978-1-60595-363-2 The Preparation of C/Ni Composite Nanofibers with Pores by Coaxial Electrospinning Yiqiang
FAILURE MECHANISMS OF NANO SILICON ANODES: AN ELECTRODE POROSITY EVOLUTION MODEL
 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2014 Supplementary data for : FAILURE MECHANISMS OF NANO SILICON ANODES: AN ELECTRODE
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2014 Supplementary data for : FAILURE MECHANISMS OF NANO SILICON ANODES: AN ELECTRODE
Experimental Study on Calendaristic Degradation and Self-Discharge of 3.4 Ah Lithium-Sulfur Pouch Cells
 Aalborg Universitet Experimental Study on Calendaristic Degradation and Self-Discharge of 3.4 Ah Lithium-Sulfur Pouch Cells Knap, Vaclav; Stroe, Daniel-Ioan Published in: ECS Transactions DOI (link to
Aalborg Universitet Experimental Study on Calendaristic Degradation and Self-Discharge of 3.4 Ah Lithium-Sulfur Pouch Cells Knap, Vaclav; Stroe, Daniel-Ioan Published in: ECS Transactions DOI (link to
Fundamental Study on Li Metal Dissolution and Deposition on Cu Foil in Nonaqueous Electrolytes with 3DOM Separator
 217 BLI X, Symposium on Energy Storage, June 27-29, 217, at IBM- Research Almaden in San Jose, CA, USA Fundamental Study on Li Metal Dissolution and Deposition on Cu Foil in Nonaqueous Electrolytes with
217 BLI X, Symposium on Energy Storage, June 27-29, 217, at IBM- Research Almaden in San Jose, CA, USA Fundamental Study on Li Metal Dissolution and Deposition on Cu Foil in Nonaqueous Electrolytes with
Microscopic Structural Analysis of Advanced Anode Material for Lithium Battery
 JFE TECHNICAL REPORT No. 22 (Mar. 2017) Microscopic Structural Analysis of Advanced Anode Material for Lithium Battery SIMAUCHI Yutaka *1 OHMORI Shigekazu *2 IKEMOTO Sachi *3 Abstract: analyzed the microstructure
JFE TECHNICAL REPORT No. 22 (Mar. 2017) Microscopic Structural Analysis of Advanced Anode Material for Lithium Battery SIMAUCHI Yutaka *1 OHMORI Shigekazu *2 IKEMOTO Sachi *3 Abstract: analyzed the microstructure
Vacuum and Atmospheric Coating and Lamination Techniques Applied to Li-S Battery Fabrication
 Vacuum and Atmospheric Coating and Lamination Techniques Applied to Li-S Battery Fabrication AIMCAL Web Coating Conference Paper AB5, 1:00 PM Wednesday, October 26, 2011 The Rechargeable Battery Company
Vacuum and Atmospheric Coating and Lamination Techniques Applied to Li-S Battery Fabrication AIMCAL Web Coating Conference Paper AB5, 1:00 PM Wednesday, October 26, 2011 The Rechargeable Battery Company
Application Note. Application Note. Electrochemical Impedance Spectroscopy - A Battery Monitoring and Fault Diagnostic Tool Introduction
 Application Note Electrochemical Impedance Spectroscopy - A Battery Monitoring and Fault Diagnostic Tool Introduction Application Note Integration of new battery technologies for portable devices and automotive
Application Note Electrochemical Impedance Spectroscopy - A Battery Monitoring and Fault Diagnostic Tool Introduction Application Note Integration of new battery technologies for portable devices and automotive
Nanocrystalline LiFePO4 as cathode material for lithium battery applications S.C SIAH
 Nanocrystalline LiFePO as cathode material for lithium battery applications Abstract S.C SIAH Engineering Science Programme, National University of Singapore Kent Ridge, Singapore 119260 LiFePO was prepared
Nanocrystalline LiFePO as cathode material for lithium battery applications Abstract S.C SIAH Engineering Science Programme, National University of Singapore Kent Ridge, Singapore 119260 LiFePO was prepared
Corrosion Rate Measurement on C-Steel
 Measurements of corrosion rate on Carbon-steel using Electrochemical (potentiodynamic Polarization, EIS etc.) technique. Corrosion Rate Measurement on C-Steel Abdullah Al Ashraf 1. Introduction: The degradation
Measurements of corrosion rate on Carbon-steel using Electrochemical (potentiodynamic Polarization, EIS etc.) technique. Corrosion Rate Measurement on C-Steel Abdullah Al Ashraf 1. Introduction: The degradation
Reliability of Li-ion Batteries
 IEEE Boston Reliability Seminar 9/11/13 Reliability of Li-ion Batteries Martin Z. Bazant Chemical Engineering & Mathematics MIT Matthew Pinson Peng Bai Dan Cogswell Todd Ferguson Alan Millner Prior funding
IEEE Boston Reliability Seminar 9/11/13 Reliability of Li-ion Batteries Martin Z. Bazant Chemical Engineering & Mathematics MIT Matthew Pinson Peng Bai Dan Cogswell Todd Ferguson Alan Millner Prior funding
Investigations on Fatigue of Li-ion batteries
 Investigations on Fatigue of Li-ion batteries HELMUT EHRENBERG INSTITUTE FOR APPLIED MATERIALS ENERGY STORAGE SYSTEMS (IAM-ESS) KIT University of the State of Baden-Wuerttemberg and National Research Center
Investigations on Fatigue of Li-ion batteries HELMUT EHRENBERG INSTITUTE FOR APPLIED MATERIALS ENERGY STORAGE SYSTEMS (IAM-ESS) KIT University of the State of Baden-Wuerttemberg and National Research Center
Thermal Analysis of a Latent Heat Storage based Battery Thermal Cooling Wrap
 Thermal Analysis of a Latent Heat Storage based Battery Thermal Cooling Wrap J. Chiew *1, C.S. Chin 2, J.B. Jia 1, W.D. Toh 1 1. Clean Energy Research Centre, School of Engineering, Temasek Polytechnic,
Thermal Analysis of a Latent Heat Storage based Battery Thermal Cooling Wrap J. Chiew *1, C.S. Chin 2, J.B. Jia 1, W.D. Toh 1 1. Clean Energy Research Centre, School of Engineering, Temasek Polytechnic,
Investigation of anode materials for lithium-ion batteries
 University of Wollongong Thesis Collections University of Wollongong Thesis Collection University of Wollongong Year 2006 Investigation of anode materials for lithium-ion batteries Ling Yuan University
University of Wollongong Thesis Collections University of Wollongong Thesis Collection University of Wollongong Year 2006 Investigation of anode materials for lithium-ion batteries Ling Yuan University
Development and Application of Liquid-cooled Lithium-ion Battery Pack Thermal Model
 Development and Application of Liquid-cooled Lithium-ion Battery Pack Thermal Model Model based approach by using GT-SUITE Yifan (Flora) Zhou DEP Fan He Optimal Xinran Tao Optimal Meng Li DEP Wei Tao FCA
Development and Application of Liquid-cooled Lithium-ion Battery Pack Thermal Model Model based approach by using GT-SUITE Yifan (Flora) Zhou DEP Fan He Optimal Xinran Tao Optimal Meng Li DEP Wei Tao FCA
PVP-Functionalized Nanometer Scale Metal Oxide Coatings for. Cathode Materials: Successful Application to LiMn 2 O 4 Spinel.
 PVP-Functionalized Nanometer Scale Metal Oxide Coatings for Cathode Materials: Successful Application to LiMn 2 O 4 Spinel Nanoparticles Hyesun Lim, Jaephil Cho* Department of Applied Chemistry Hanyang
PVP-Functionalized Nanometer Scale Metal Oxide Coatings for Cathode Materials: Successful Application to LiMn 2 O 4 Spinel Nanoparticles Hyesun Lim, Jaephil Cho* Department of Applied Chemistry Hanyang
Experiences of PLD Technology for LIB Separators. PICODEON Oy. Neal White
 Experiences of PLD Technology for LIB Separators PICODEON Oy Neal White 1 Outline Introduction to Picodeon Ceramic coating rationale Separator overview Why PLD for LIB separators Current status of Picodeon
Experiences of PLD Technology for LIB Separators PICODEON Oy Neal White 1 Outline Introduction to Picodeon Ceramic coating rationale Separator overview Why PLD for LIB separators Current status of Picodeon
Heat Transfer Simulation to Determine the Impact of Al-5Mg Arc Sprayed Coating onto 7075 T6 Al Alloy Fatigue Performance
 11 th International LS-DYNA Users Conference Simulation (5) Heat Transfer Simulation to Determine the Impact of Al-5Mg Arc Sprayed Coating onto 7075 T6 Al Alloy Fatigue Performance G. D Amours, B. Arsenault,
11 th International LS-DYNA Users Conference Simulation (5) Heat Transfer Simulation to Determine the Impact of Al-5Mg Arc Sprayed Coating onto 7075 T6 Al Alloy Fatigue Performance G. D Amours, B. Arsenault,
Oxides for High Performance Lithium-Ion Battery Anodes
 Bacteria Absorption-Based Mn 2 P 2 O 7 -Carbon @ Reduced Graphene Oxides for High Performance Lithium-Ion Battery Anodes Yuhua Yang, 1 Bin Wang, 1,2 Jingyi Zhu, 3 Jun Zhou, 1 Zhi Xu, 1,4 Ling Fan, 1 Jian
Bacteria Absorption-Based Mn 2 P 2 O 7 -Carbon @ Reduced Graphene Oxides for High Performance Lithium-Ion Battery Anodes Yuhua Yang, 1 Bin Wang, 1,2 Jingyi Zhu, 3 Jun Zhou, 1 Zhi Xu, 1,4 Ling Fan, 1 Jian
High Temperature Ta/MnO 2 Capacitors
 High Temperature Ta/MnO 2 Capacitors Y. Freeman, A. Chacko, P. Lessner, S. Hussey, R. Marques, and J. Moncada KEMET Electronics Corporation, 2835 KEMET Way, Simpsonville, SC 29681, yurifreeman@kemet.com
High Temperature Ta/MnO 2 Capacitors Y. Freeman, A. Chacko, P. Lessner, S. Hussey, R. Marques, and J. Moncada KEMET Electronics Corporation, 2835 KEMET Way, Simpsonville, SC 29681, yurifreeman@kemet.com
Effects of Fluoroethylene Carbonate (FEC) on Anode and Cathode Interfaces at Elevated Temperatures
 Journal of The Electrochemical Society, 162 (9) A1683-A1692 (2015) 0013-4651/2015/162(9)/A1683/10/$33.00 The Electrochemical Society Effects of Fluoroethylene Carbonate (FEC) on Anode and Cathode Interfaces
Journal of The Electrochemical Society, 162 (9) A1683-A1692 (2015) 0013-4651/2015/162(9)/A1683/10/$33.00 The Electrochemical Society Effects of Fluoroethylene Carbonate (FEC) on Anode and Cathode Interfaces
The European Commission s science and knowledge service. Joint Research Centre
 The European Commission s science and knowledge service Joint Research Centre EU-Commission JRC Contribution to EVE IWG M. De Gennaro, E. Paffumi 24th Meeting of the GRPE Informal Working Group on Electric
The European Commission s science and knowledge service Joint Research Centre EU-Commission JRC Contribution to EVE IWG M. De Gennaro, E. Paffumi 24th Meeting of the GRPE Informal Working Group on Electric
Defects and Diffusion
 Defects and Diffusion Goals for the Unit Recognize various imperfections in crystals Point imperfections Impurities Line, surface and bulk imperfections Define various diffusion mechanisms Identify factors
Defects and Diffusion Goals for the Unit Recognize various imperfections in crystals Point imperfections Impurities Line, surface and bulk imperfections Define various diffusion mechanisms Identify factors
Impact Assessment in Safety Testing of Lithium-Ion
 Technology Report Impact Assessment in Safety Testing of Lithium-Ion Secondary Battery Hideki Kawai, Arata Okuyama and Yuichi Aoki ESPEC CORP. Abstract It has been reported that the Lithium-Ion secondary
Technology Report Impact Assessment in Safety Testing of Lithium-Ion Secondary Battery Hideki Kawai, Arata Okuyama and Yuichi Aoki ESPEC CORP. Abstract It has been reported that the Lithium-Ion secondary
Capacity fade study of lithium-ion batteries cycled at high discharge rates
 Journal of Power Sources 117 (2003) 160 169 Capacity fade study of lithium-ion batteries cycled at high discharge rates Gang Ning, Bala Haran, Branko N. Popov * Department of Chemical Engineering, University
Journal of Power Sources 117 (2003) 160 169 Capacity fade study of lithium-ion batteries cycled at high discharge rates Gang Ning, Bala Haran, Branko N. Popov * Department of Chemical Engineering, University
Advanced Analytical Chemistry Lecture 13. Chem 4631
 Advanced Analytical Chemistry Lecture 13 Chem 4631 What is a fuel cell? An electro-chemical energy conversion device A factory that takes fuel as input and produces electricity as output. O 2 (g) H 2 (g)
Advanced Analytical Chemistry Lecture 13 Chem 4631 What is a fuel cell? An electro-chemical energy conversion device A factory that takes fuel as input and produces electricity as output. O 2 (g) H 2 (g)
Batteries for Vehicular Applications
 Batteries for Vehicular Applications Venkat Srinivasan * Staff Scientist Lawrence Berkeley National Laboratory March 2, 2008 *vsrinivasan@lbl.gov Range Specific c Energy (W Wh/kg) 1000 100 10 Relative
Batteries for Vehicular Applications Venkat Srinivasan * Staff Scientist Lawrence Berkeley National Laboratory March 2, 2008 *vsrinivasan@lbl.gov Range Specific c Energy (W Wh/kg) 1000 100 10 Relative
Design and fabrication of all-solid-state rechargeable lithium batteries using ceramic electrolytes
 International Symposium on Electrical Fatigue in Functional Materials September 15, 2014 Sellin, Rügen, Germany Design and fabrication of all-solid-state rechargeable lithium batteries using ceramic electrolytes
International Symposium on Electrical Fatigue in Functional Materials September 15, 2014 Sellin, Rügen, Germany Design and fabrication of all-solid-state rechargeable lithium batteries using ceramic electrolytes
EPSRC Centre for Doctoral Training in Industrially Focused Mathematical Modelling. Solidification of Silicon. Graham Patrick Benham
 EPSRC Centre for Doctoral Training in Industrially Focused Mathematical Modelling Solidification of Silicon Graham Patrick Benham Table of Contents 1. Introduction...2 Background...2 2. Solidification
EPSRC Centre for Doctoral Training in Industrially Focused Mathematical Modelling Solidification of Silicon Graham Patrick Benham Table of Contents 1. Introduction...2 Background...2 2. Solidification
Modeling of Local Cell Degradation in Solid Oxide Fuel Cells: Cumulative Effect of Critical Operating Points
 Modeling of Local Cell Degradation in Solid Oxide Fuel Cells: Cumulative Effect of Critical Operating Points Zacharie Wuillemin, Antonin Faes, Stefan Diethelm, Arata Nakajo, Nordahl Autissier, Jan Van
Modeling of Local Cell Degradation in Solid Oxide Fuel Cells: Cumulative Effect of Critical Operating Points Zacharie Wuillemin, Antonin Faes, Stefan Diethelm, Arata Nakajo, Nordahl Autissier, Jan Van
Improving cyclic performance of Si anode for lithium-ion batteries by forming an intermetallic skin
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 Supporting Information Improving cyclic performance of Si anode for lithium-ion batteries by
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 Supporting Information Improving cyclic performance of Si anode for lithium-ion batteries by
Three-dimensional graphene-based hierarchically porous carbon. composites prepared by a dual-template strategy for capacitive
 Electronic Supplementary Information (ESI) Three-dimensional graphene-based hierarchically porous carbon composites prepared by a dual-template strategy for capacitive deionization Xiaoru Wen, a Dengsong
Electronic Supplementary Information (ESI) Three-dimensional graphene-based hierarchically porous carbon composites prepared by a dual-template strategy for capacitive deionization Xiaoru Wen, a Dengsong
Urchin-like V 2 O 3 /C Hollow Nanospheres Hybrid for High-Capacity and Long-Cycle-Life Lithium Storage
 Supporting Information Urchin-like V 2 O 3 /C Hollow Nanospheres Hybrid for High-Capacity and Long-Cycle-Life Lithium Storage Peng Yu, Xu Liu, Lei Wang,* Chungui Tian, Haitao Yu, and Honggang Fu* Key Laboratory
Supporting Information Urchin-like V 2 O 3 /C Hollow Nanospheres Hybrid for High-Capacity and Long-Cycle-Life Lithium Storage Peng Yu, Xu Liu, Lei Wang,* Chungui Tian, Haitao Yu, and Honggang Fu* Key Laboratory
CHAPTER 4: Oxidation. Chapter 4 1. Oxidation of silicon is an important process in VLSI. The typical roles of SiO 2 are:
 Chapter 4 1 CHAPTER 4: Oxidation Oxidation of silicon is an important process in VLSI. The typical roles of SiO 2 are: 1. mask against implant or diffusion of dopant into silicon 2. surface passivation
Chapter 4 1 CHAPTER 4: Oxidation Oxidation of silicon is an important process in VLSI. The typical roles of SiO 2 are: 1. mask against implant or diffusion of dopant into silicon 2. surface passivation
Development of Phase Change Material/ Cooling Plate Coupled Battery Thermal Management System Using CFD
 Development of Phase Change Material/ Cooling Plate Coupled Battery Thermal Management System Using CFD 1 Mr.D.Omkar, 2 Dr.P.Vijaykumar 1,2 Department of Mechanical Engineering, 1,2 Lakireddy Balireddy
Development of Phase Change Material/ Cooling Plate Coupled Battery Thermal Management System Using CFD 1 Mr.D.Omkar, 2 Dr.P.Vijaykumar 1,2 Department of Mechanical Engineering, 1,2 Lakireddy Balireddy
Finite Element Simulation Research on Medium Plate Multi-Pass Welding Temperature Field
 Send Orders for Reprints to reprints@benthamscience.ae 786 The Open Mechanical Engineering Journal, 2015, 9, 786-790 Open Access Finite Element Simulation Research on Medium Plate Multi-Pass Welding Temperature
Send Orders for Reprints to reprints@benthamscience.ae 786 The Open Mechanical Engineering Journal, 2015, 9, 786-790 Open Access Finite Element Simulation Research on Medium Plate Multi-Pass Welding Temperature
Conductive Filament Formation Failure in a Printed Circuit Board
 Create: 5/17/99 Circuit World, Vol. 25 (3), pp. 6-8, 1999. Conductive Filament Formation Failure in a Printed Circuit Board Abstract Keith Rogers, Craig Hillman, and Michael Pecht CALCE Electronic Products
Create: 5/17/99 Circuit World, Vol. 25 (3), pp. 6-8, 1999. Conductive Filament Formation Failure in a Printed Circuit Board Abstract Keith Rogers, Craig Hillman, and Michael Pecht CALCE Electronic Products
Supporting Information
 Supporting Information Mg 2 B 2 O 5 Nanowires Enabled Multifunctional Solid-State Electrolyte with High Ionic Conductivity, Excellent Mechanical Properties and Flame-retardant Performance Ouwei Sheng,
Supporting Information Mg 2 B 2 O 5 Nanowires Enabled Multifunctional Solid-State Electrolyte with High Ionic Conductivity, Excellent Mechanical Properties and Flame-retardant Performance Ouwei Sheng,
Submitted By Maksim V. Tyufekchiev Somyi Hur. Submitted on April 23, 2013
 PROJECT NUMBER: MQP YW1-YW11 Developing a Low-Cost Methodology for Fabricating All-Solid-State Lithium-Ion Battery A Major Qualifying Project Submitted to the Faculty of WORCESTER POLYTECHNIC INSTITUTE
PROJECT NUMBER: MQP YW1-YW11 Developing a Low-Cost Methodology for Fabricating All-Solid-State Lithium-Ion Battery A Major Qualifying Project Submitted to the Faculty of WORCESTER POLYTECHNIC INSTITUTE
PHOENICS Applications in the Aluminium Smelting Industry Ch. Droste VAW Aluminium-Technologie GmbH Bonn, Germany
 PHOENICS Applications in the Aluminium Smelting Industry Ch. Droste VAW Aluminium-Technologie GmbH 53117 Bonn, Germany The multi-phase fluid system in aluminium reduction cells is exposed to strong electromagnetic
PHOENICS Applications in the Aluminium Smelting Industry Ch. Droste VAW Aluminium-Technologie GmbH 53117 Bonn, Germany The multi-phase fluid system in aluminium reduction cells is exposed to strong electromagnetic
Heat Transfer Analysis for Phase Change Materials (PCMs)
 Abstract Heat Transfer Analysis for Phase Change Materials (PCMs) Y. Tian, C.Y. Zhao* School of Engineering, University of Warwick, CV4 7AL, Coventry, UK *Corresponding author (Tel: +44 4 765339, Email:
Abstract Heat Transfer Analysis for Phase Change Materials (PCMs) Y. Tian, C.Y. Zhao* School of Engineering, University of Warwick, CV4 7AL, Coventry, UK *Corresponding author (Tel: +44 4 765339, Email:
ECEN5017 Guest Lecture
 ECEN5017 Guest Lecture Overview of NREL Battery Lifetime Models & Health Management R&D for Electric Drive Vehicles Kandler Smith kandler.smith@nrel.gov Ahmad A. Pesaran ahmad.pesaran@nrel.gov Center for
ECEN5017 Guest Lecture Overview of NREL Battery Lifetime Models & Health Management R&D for Electric Drive Vehicles Kandler Smith kandler.smith@nrel.gov Ahmad A. Pesaran ahmad.pesaran@nrel.gov Center for
Na-MCl 2 CELL MULTIPHYSICS MODELING: STATUS AND CHALLENGES
 Rémy Christin*, Mikael Cugnet**, Nicola Zanon***, Giorgio Crugnola***, Pascal Mailley**** Na-MCl 2 CELL MULTIPHYSICS MODELING: STATUS AND CHALLENGES *R&D Batteries- Sodium-Nickel and new tech, FIAMM (Aubergenville,
Rémy Christin*, Mikael Cugnet**, Nicola Zanon***, Giorgio Crugnola***, Pascal Mailley**** Na-MCl 2 CELL MULTIPHYSICS MODELING: STATUS AND CHALLENGES *R&D Batteries- Sodium-Nickel and new tech, FIAMM (Aubergenville,
The Pennsylvania State University. The Graduate School. College of Engineering LOW-TEMPERATURE OPERATION OF LI-ION BATTERIES
 The Pennsylvania State University The Graduate School College of Engineering LOW-TEMPERATURE OPERATION OF LI-ION BATTERIES FOR HYBRID AND ELECTRIC VEHICLES A Dissertation in Mechanical Engineering by Yan
The Pennsylvania State University The Graduate School College of Engineering LOW-TEMPERATURE OPERATION OF LI-ION BATTERIES FOR HYBRID AND ELECTRIC VEHICLES A Dissertation in Mechanical Engineering by Yan
TRIBOCORROSION EVALUATION OF PROTECTIVE COATING
 TRIBOCORROSION EVALUATION OF PROTECTIVE COATING Prepared by Duanjie Li, PhD 6 Morgan, Ste156, Irvine CA 92618 P: 949.461.9292 F: 949.461.9232 nanovea.com Today's standard for tomorrow's materials. 2014
TRIBOCORROSION EVALUATION OF PROTECTIVE COATING Prepared by Duanjie Li, PhD 6 Morgan, Ste156, Irvine CA 92618 P: 949.461.9292 F: 949.461.9232 nanovea.com Today's standard for tomorrow's materials. 2014
Supporting Information
 Supporting Information In Situ-formed Li 2 S in Lithiated Graphite Electrodes for Lithium-Sulfur Batteries Yongzhu Fu, Chenxi Zu, Arumugam Manthiram Electrochemical Energy Laboratory & Materials Science
Supporting Information In Situ-formed Li 2 S in Lithiated Graphite Electrodes for Lithium-Sulfur Batteries Yongzhu Fu, Chenxi Zu, Arumugam Manthiram Electrochemical Energy Laboratory & Materials Science
Supplementary Figure 1 The lithium polysulfide distribution on the patterned electrode.
 Supplementary Figure 1.The lithium polysulfide distribution on the patterned electrode. SEM image of the ITO-carbon electrode after dipping into Li 2 S 8 solution and drying, which shows the random distribution
Supplementary Figure 1.The lithium polysulfide distribution on the patterned electrode. SEM image of the ITO-carbon electrode after dipping into Li 2 S 8 solution and drying, which shows the random distribution
Study of lithium glassy solid electrolyte/electrode interface by impedance analysis
 Bull. Mater. Sci., Vol. 23, No. 3, June 2, pp. 179 183. Indian Academy of Sciences. Study of lithium glassy solid electrolyte/electrode interface by impedance analysis A KARTHIKEYAN, P VINATIER and A LEVASSEUR*
Bull. Mater. Sci., Vol. 23, No. 3, June 2, pp. 179 183. Indian Academy of Sciences. Study of lithium glassy solid electrolyte/electrode interface by impedance analysis A KARTHIKEYAN, P VINATIER and A LEVASSEUR*
A THEORETICAL SIMULATION OF A PEM FUEL CELL WITH 4-SERPENTINE FLOW CHANNEL
 A THEORETICAL SIMULATION OF A PEM FUEL CELL WITH 4-SERPENTINE FLOW CHANNEL B.Sreenivasulu a,*, S.V.Naidu b, V.Dharma Rao c, G.Vasu d a Department of Chemical Engineering,G.V.P College of Engineering, Visakhapatnam
A THEORETICAL SIMULATION OF A PEM FUEL CELL WITH 4-SERPENTINE FLOW CHANNEL B.Sreenivasulu a,*, S.V.Naidu b, V.Dharma Rao c, G.Vasu d a Department of Chemical Engineering,G.V.P College of Engineering, Visakhapatnam
A Study of Two-Phase Flow in a Polymer Electrolyte Fuel Cell Tang.M.Z, K.E.Birgersson
 A Study of Two-Phase Flow in a Polymer Electrolyte Fuel Cell Tang.M.Z, K.E.Birgersson Engineering Science Programme, Faculty of Engineering, National University of Singapore, 10 Kent Ridge Road, Singapore
A Study of Two-Phase Flow in a Polymer Electrolyte Fuel Cell Tang.M.Z, K.E.Birgersson Engineering Science Programme, Faculty of Engineering, National University of Singapore, 10 Kent Ridge Road, Singapore
Effect of Width of Gas/Liquid/Solid Three-Phase Boundary Zone of Discrete Water Film on Atmospheric Corrosion of Metals
 64 The Open Corrosion Journal, 2010, 3, 64-72 Open Access Effect of Width of Gas/Liquid/Solid Three-Phase Boundary Zone of Discrete Water Film on Atmospheric Corrosion of Metals Jia Wang *,1,2 and Jing
64 The Open Corrosion Journal, 2010, 3, 64-72 Open Access Effect of Width of Gas/Liquid/Solid Three-Phase Boundary Zone of Discrete Water Film on Atmospheric Corrosion of Metals Jia Wang *,1,2 and Jing
Tailor Made Carbon and Graphite Based Anode Materials for Lithium Ion Batteries. Heribert Walter, Battery+Storage 2013
 Tailor Made Carbon and Graphite Based Anode Materials for Lithium Ion Batteries Heribert Walter, Battery+Storage 2013 Agenda SGL Group at a Glance Anode Materials Overview Material Synthesis and Modification
Tailor Made Carbon and Graphite Based Anode Materials for Lithium Ion Batteries Heribert Walter, Battery+Storage 2013 Agenda SGL Group at a Glance Anode Materials Overview Material Synthesis and Modification
PHYSICAL REVIEW LETTERS 107,
 Real-time Measurement of Stress and Damage Evolution During Initial Lithiation of Crystalline Silicon M. J. Chon, 1 V.A. Sethuraman, 1 A. McCormick, 1 V. Srinivasan, 2 P. R. Guduru 1,* 1 School of Engineering,
Real-time Measurement of Stress and Damage Evolution During Initial Lithiation of Crystalline Silicon M. J. Chon, 1 V.A. Sethuraman, 1 A. McCormick, 1 V. Srinivasan, 2 P. R. Guduru 1,* 1 School of Engineering,
DYNAMIC SIMULATION OF A PROTON EXCHANGE MEMBRANE FUEL CELL SYSTEM FOR AUTOMOTIVE APPLICATIONS
 DYNAMIC SIMULATION OF A PROTON EXCHANGE MEMBRANE FUEL CELL SYSTEM FOR AUTOMOTIVE APPLICATIONS R. A. Rabbani 1 and M. Rokni 2 1. Technical University of Denmark, Kgs. Lyngby, Denmark; email: raar@mek.dtu.dk
DYNAMIC SIMULATION OF A PROTON EXCHANGE MEMBRANE FUEL CELL SYSTEM FOR AUTOMOTIVE APPLICATIONS R. A. Rabbani 1 and M. Rokni 2 1. Technical University of Denmark, Kgs. Lyngby, Denmark; email: raar@mek.dtu.dk
Transactions on Modelling and Simulation vol 8, 1994 WIT Press, ISSN X
 Invited paper Analysis of stray current corrosion problems using the boundary element method J. Trevelyan* & H.P. Hack& ^ Computational Mechanics, Inc., Billerica, Massachusetts, USA ^ Naval Surface Warfare
Invited paper Analysis of stray current corrosion problems using the boundary element method J. Trevelyan* & H.P. Hack& ^ Computational Mechanics, Inc., Billerica, Massachusetts, USA ^ Naval Surface Warfare
BORAL CONTROL BLADE THERMAL-MECHANICAL ANALYSIS
 BORAL CONTROL BLADE THERMAL-MECHANICAL ANALYSIS Srisharan G.Govindarajan, J.Alex Moreland and Gary L.Solbrekken Department of Mechanical and Aerospace Engineering University of Missouri, Columbia, Missouri,
BORAL CONTROL BLADE THERMAL-MECHANICAL ANALYSIS Srisharan G.Govindarajan, J.Alex Moreland and Gary L.Solbrekken Department of Mechanical and Aerospace Engineering University of Missouri, Columbia, Missouri,
INCLUSION OF POLYVINYLEDENE FLUORIDE POLYMER AS BINDING AGENT FOR GRAPHITE AND LICOO 2 GRANULES
 MATERIALS SCIENCE and TECHNOLOGY Edited by Evvy Kartini et.al. INCLUSION OF POLYVINYLEDENE FLUORIDE POLYMER AS BINDING AGENT FOR GRAPHITE AND LICOO 2 GRANULES T. Nugraha, E. Panjaitan, E. Kartini Center
MATERIALS SCIENCE and TECHNOLOGY Edited by Evvy Kartini et.al. INCLUSION OF POLYVINYLEDENE FLUORIDE POLYMER AS BINDING AGENT FOR GRAPHITE AND LICOO 2 GRANULES T. Nugraha, E. Panjaitan, E. Kartini Center
2. Wet Corrosion: Characteristics, Prevention and Corrosion Rate
 2. Wet Corrosion: Characteristics, Prevention and Corrosion Rate Mighty ships upon the ocean suffer from severe corrosion. Even those that stay at dockside are rapidly becoming oxide Alas, that piling
2. Wet Corrosion: Characteristics, Prevention and Corrosion Rate Mighty ships upon the ocean suffer from severe corrosion. Even those that stay at dockside are rapidly becoming oxide Alas, that piling
On the Role of Mechanics in the Design and Performance of Electrode Materials for Energy Storage
 On the Role of Mechanics in the Design and Performance of Electrode Materials for Energy Storage Pradeep R. Guduru School of Engineering, Brown University V. Sethuraman, M. Chon, S. Nadimpalli Collaborators:
On the Role of Mechanics in the Design and Performance of Electrode Materials for Energy Storage Pradeep R. Guduru School of Engineering, Brown University V. Sethuraman, M. Chon, S. Nadimpalli Collaborators:
Kinetic Characteristics of Different Materials used for Bolting Applications
 Kinetic Characteristics of Different Materials used for Bolting Applications Report Kinetic Characteristics of Different Materials used for Bolting Applications Overview One of the most common problems
Kinetic Characteristics of Different Materials used for Bolting Applications Report Kinetic Characteristics of Different Materials used for Bolting Applications Overview One of the most common problems
Insights into the reversibility of the aluminum graphite battery
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Insights into the reversibility of the aluminum graphite battery Giuseppe
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Insights into the reversibility of the aluminum graphite battery Giuseppe
PASSIVE THERMAL MANAGEMENT OF LITHIUM-ION BATTERIES USING LATENT HEAT STORAGE MATERIALS
 PASSIVE THERMAL MANAGEMENT OF LITHIUM-ION BATTERIES USING LATENT HEAT STORAGE MATERIALS WHITE PAPER Joe Kelly - Materials Scientist, March 2015 Rev: 2 INTRODUCTION As demand steadily grows for more powerful
PASSIVE THERMAL MANAGEMENT OF LITHIUM-ION BATTERIES USING LATENT HEAT STORAGE MATERIALS WHITE PAPER Joe Kelly - Materials Scientist, March 2015 Rev: 2 INTRODUCTION As demand steadily grows for more powerful
