In Situ Transmission Electron Microscopy Study of Electrochemical Sodiation and Potassiation of Carbon Nanofibers
|
|
|
- Matthew Short
- 6 years ago
- Views:
Transcription
1 pubs.acs.org/nanolett In Situ Transmission Electron Microscopy Study of Electrochemical Sodiation and Potassiation of Carbon Nanofibers Ying Liu, Feifei Fan, Jiangwei Wang, Yang Liu, Hailong Chen, Katherine L. Jungjohann, Yunhua Xu, Yujie Zhu, David Bigio, Ting Zhu,, * and Chunsheng Wang, * Department of Chemical and Biomolecular Engineering, University of Maryland, College Park, Maryland 20742, United States Woodruff School of Mechanical Engineering, Georgia Institute of Technology, Atlanta, Georgia 30332, United States Department of Mechanical Engineering and Materials Science, University of Pittsburgh, Pittsburgh, Pennsylvania 15261, United States Center for Integrated Nanotechnologies, Sandia National Laboratories, Albuquerque, New Mexico 87185, United States Department of Mechanical Engineering, University of Maryland, College Park, Maryland 20742, United States *S Supporting Information ABSTRACT: Carbonaceous materials have great potential for applications as anodes of alkali-metal ion batteries, such as Na-ion batteries and K-ion batteries (NIB and KIBs). We conduct an in situ study of the electrochemically driven sodiation and potassiation of individual carbon nanofibers (CNFs) by transmission electron microscopy (TEM). The CNFs are hollow and consist of a bilayer wall with an outer layer of disorderedcarbon (d-c) enclosing an inner layer of crystalline-carbon (c-c). The d-c exhibits about three times volume expansion of the c-c after full sodiation or potassiation, thus suggesting a much higher storage capacity of Na or K ions in d-c than c-c. For the bilayer CNF-based electrode, a steady sodium capacity of 245 mah/g is measured with a Coulombic efficiency approaching 98% after a few initial cycles. The in situ TEM experiments also reveal the mechanical degradation of CNFs through formation of longitudinal cracks near the c-c/d-c interface during sodiation and potassiation. Geometrical changes of the tube are explained by a chemomechanical model using the anisotropic sodiation/potassiation strains in c-c and d- C. Our results provide mechanistic insights into the electrochemical reaction, microstructure evolution and mechanical degradation of carbon-based anodes during sodiation and potassiation, shedding light onto the development of carbon-based electrodes for NIBs and KIBs. KEYWORDS: Sodium-ion batteries, potassium-ion batteries, carbon nanofibers, sodiation, potassiation, crack The large-scale storage of electrical energy for applications in electrical grids requires battery systems with high energy density and low cost. During the past decade, great effort has been devoted to Li-ion batteries (LIBs). 1,2 However, the limited energy density and high cost still hinder the wide usage of LIBs to store large amounts of electrical energy from intermittent renewable sources such as solar and wind energy. Recently, batteries based on other alkali-metals, such as Na-ion batteries (NIBs) and K-ion batteries (KIBs), 3 5 have attracted great attention, owing to their comparatively high natural abundance and low cost. 3,5 7 Considering the similar chemical properties of Li, Na, and K, the anode and cathode materials used in LIBs are being investigated for their applications in NIBs and KIBs in the past few years. The potential anode materials for NIBs include carbonaceous materials, Sn, Sb, and metal oxides. 3,6 12 Among these candidates, carbonaceous materials 4,13 19 are most promising, given their successful applications in commercial LIBs and particularly low cost, natural abundance, and small volumetric expansion during alloying with alkali-metals, in contrast to other anode materials such as Sn. 10 Graphite has been used as the anode of current commercial LIBs with a theoretical capacity of 372 mah/g. 20 However, only a small amount of Na ions can be stored in the graphite anode of NIBs. Such a limitation has been often attributed to the higher intercalation energy barrier of Na ions. 3,4,21 So far, the highest capacity for carbonaceous anodes reported in NIBs is about 300 mah/g in hard carbon, whose usage is severely limited by its poor cyclability. 19 Recently, the disordered or amorphous carbon materials were found to exhibit much improved cyclability. 4,15,22 For example, the disordered hollow carbon nanowires showed a reversible capacity as high as 251 mah/g with 82.2% capacity retention after almost 400 sodiation-desodiation cycles, 15 which is much better than hard carbon. 16,19 Moreover, carbon is often used to enhance electron Received: March 14, 2014 Revised: May 10, 2014 Published: May 13, American Chemical Society 3445
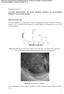
2 Nano s Figure 1. Typical morphology and structure of a pristine hollow CNF with a bilayer wall. (a,b) The wall of CNF consists of a d-c/c-c bilayer. (c) EDP of the bilayer CNF. Two different kinds of spots are shown in the EDP for the {0002} plane of CNF. The two sets of sharp spots (indicated by green arrows) correspond to the diffraction of c-c, while the diffuse spots (indicated by yellow arrows) correspond to the diffraction of d-c. (d) HRTEM image of the CNF with the inset showing the local graphite lattice fringes in the d-c layer. Figure 2. Geometry and structure changes of a bilayer CNF during sodiation under a beam-blank condition. (a,c) Pristine CNF. (b,d) Sodiated CNF. (e) The line scan profiles of C and Na across the CNF after sodiation. The green arrows point to the peak positions of Na and C intensity profiles. (f,g) Electron diffraction patterns (EDPs) of the CNF before and after sodiation. The average spacing of {0002} planes is about 3.38 Å in the pristine CNF. Two sets of patterns form after sodiation; the average spacing of {0002} planes in c-c and d-c increases to 3.67 and 4.16 Å, respectively. 3446
3 Nano s conduction in anodes or cathodes of LIBs and NIBs, as well as to support other active materials in high-capacity nanocomposite anodes for LIBs and NIBs In addition, the application of the carbon-based anodes in KIBs remains largely unexplored, compared with their LIB and NIB counterparts. Therefore, it is essential to understanding the electrochemical reactions and associated structure property relationships of carbon-based anodes during sodiation and potassiation, thereby paving the way for future development of the novel carbon-based or carbonsupported nanocomposite anodes for NIBs and KIBs. In this work, the electrochemical behavior and microstructure evolution of carbon nanofibers (CNFs) during Na + and K + insertions are studied in real time by using a nanoscale battery setup inside a transmission electron microscope (TEM). The CNFs are hollow and consist of a bilayer wall with an outer layer of disordered-carbon (d-c) enclosing an inner layer of crystalline-carbon (c-c). With such bilayer CNFs, the sodiation and potassiation responses of c-c and d-c are directly compared. Longitudinal cracks are frequently observed near the c-c/d-c interface during sodiation and potassiation. The chemomechanical origin of geometrical changes in the sodiated tube is revealed by a computational modeling study. In addition, an in situ TEM study of amorphous Si (a-si) coated CNFs is conducted to further evaluate the mechanical confinement effect on sodiation and potassiation. Figure 1 shows the typical morphology of a pristine hollow CNF, which involves a bilayer structure, that is, a d-c outer layer enclosing a c-c inner layer. The bilayer CNF is a commercial product (PR-19) ordered from the Pyrograf Products, Inc. The diameter of CNFs is typically in the range of nm and the length is about tens of micrometers. The bilayer structure is clearly seen from a zoom-in image in Figure 1b, that is, the c-c inner layer with a dark contrast and the d-c outer layer with a light contrast. Figure 1d shows a high-resolution TEM (HRTEM) image of the bilayer structure. In the d-c layer, the local domains of graphitic lattice fringes (inset in Figure 1d) are clearly visible, implying d-c is not completely disordered. Namely, d-c consists of an assembly of many few-layered graphene patches that are not perfectly aligned in the same orientation. Such a locally ordered structure in d-c is similar to that in hard carbon consisting of stacked graphite layers (termed as graphite nanocrystallites) with microvoids in between. 19,21 The local crystalline order in d-c is also reflected in the electron diffraction pattern (EDP), as shown in Figure 1c. It is interesting to note that two different kinds of spots are shown in the EDP for {0002} planes. The two sets of sharp spots (indicated by green arrows in Figure 1c) arise from the diffraction of c-c, while the diffuse spots from d-c (indicated by the yellow arrow in Figure 1c). Note that the diffuse diffraction spots of d-c are different from the diffraction halo of the pure amorphous phase, which further confirms that the d-c layer is not fully disordered. The unique bilayer structure in CNFs allows us to directly compare the behaviors of c-c and d-c during Na + or K + insertion. The in situ experiments of electrochemically driven sodiation and potassiation of CNFs are conducted by using an all-solid nanobattery setup inside a TEM, and details of the nanobattery setup and operation can be found in previous publications. 10,29 32 To confirm the negligible effects of electron beam on electrochemical reactions, both beam-on and beamblank experiments (as indicated in figure captions) are performed, showing qualitatively similar behaviors. Figure 2 presents the microstructures of a hollow bilayer CNF before and after the beam-blank sodiation. For the pristine hollow CNF, its inner and outer diameter are respectively 130 nm and 256 nm, and wall thickness is about 63 nm (Figure 2c). After full sodiation, the inner diameter decreases to 116 nm and the outer diameter increases to 263 nm. As a result, the total wall thickness increases to 73.5 nm, owing to sodiation-induced volume expansion. In addition, sodiation causes an increase of the thickness of the c-c layer from 25.5 to 29 nm, which corresponds to an average radial and volume strain of 13.7 and 6%, respectively. Similarly, the thickness of the d-c layer increases from 37.5 to 44.5 nm, which corresponds to an average radial and volume strain of 18.7 and 18.7%, respectively. Similar geometric changes are also observed in potassiated CNFs. Hence, the sodiated/potassiated d-c exhibits about three times volume expansion of c-c, thus suggesting a much higher storage capacity of Na ions in d-c than c-c. Incidentally, a thin layer of Na 2 O(2 3 nm thick) is observed to quickly form on the CNF surface (Figure 2d) while the bulk sodiation is still ongoing, thus suggesting fast surface diffusion of Na on CNFs. 30,31 Figure 2e presents the line scan profiles across the CNF after sodiation, and the green arrows indicate the peak positions of Na and C intensity profiles. Na and C exhibit different spatial distributions, that is, the Na intensity reaches a peak in the middle of the CNF wall and decreases gradually away from the peak, while the C intensity continually increases from the outer to the inner tube wall. Such difference indicates that the c-c and d-c have different capacities of storing Na ions, as further confirmed by EDPs (Figure 2f,g). The EDP of the pristine CNF shows a typical diffraction pattern of the bilayer CNF with an average {0002} plane spacing of 3.38 Å (Figure 2f), which is slightly larger than the theoretical spacing of {0002} plane of 3.35 Å. Such a difference is presumably induced by the partially disordered structure of d-c. After full sodiation, the diffraction pattern of {0002} planes splits into two sets with different lattice spacings (Figure 2g). For c-c, the average spacing of {0002} planes increases to 3.67 Å. This corresponds to an expansion of 8.6% between neighboring graphitic layers, which is slightly larger than that of lithiation ( 7%) 31 and can be correlated to the larger size of Na ions. 3 In contrast, the average spacing of {0002} planes of d-c increases up to about 4.16 Å. This corresponds to an expansion of 23% along [0002] (i.e., c axis) direction, which is about three times that in the sodiated c-c. The larger the increase of the d-spacing along c axis, the more Na ions the carbon system likely stored. However, the quantitative capacity of sodiation in d-c cannot be determined directly by our in situ TEM experiment due to the difficulty of measuring the extremely small electric current. In addition, much less changes are observed in the lattice spacing of {101 0} planes (Figure 2f,g), which indicates that Na ions are most likely inserted in between rather than within the graphitic layers. The EDP after sodiation is consistent with the line scan profiles in Figure 2e, suggesting that more Na ions can be stored in d-c. Compared to c-c, the larger capacity of Na ions storage in d-c might originate from more defects existing in d-c, while the atomic-scale mechanism of Na ion accommodation warrants further study in the future. Potassiation of a bilayer CNF exhibits similar responses, as shown in Supporting Information Figure S1. The average spacing of {0002} planes of d-c increases to 4.18 Å after potassiation, corresponding to an expansion of 24% along the c-axis. Both sodiation and potassiation of the bilayer CNF indicate that d-c has a better capability of storing Na or K ions than c-c, which is desired for the anode of NIBs and KIBs. Galvanostatic discharge charge cycling is performed to directly measure the capacity of the bilayer CNF electrode 3447
4 Nano s Figure 3. Charge discharge curves and cycling stability measured for bilayer CNF electrodes in a coin cell for Na-ion battery and K-ion battery. The electrodes are tested in the voltage range of V at a current density of 50 ma/g, with the electrolyte of 1 M NaPF 6 or 1 M KPF 6 dissolved in the mixture of EC + DMC (1:1 by volume). The specific capacity is calculated based on the mass of d-c, which is about 75% of the total mass of CNFs. (a) Galvanostatic charge discharge profiles in the second, fifth, and twentieth cycle in NIBs. (b) Cycling performance and Coulombic efficiency of bilayer CNF electrodes in NIBs. (c) Galvanostatic charge discharge profiles in the second, third, and fourth cycle in KIBs. (d) Cycling performance and Coulombic efficiency of bilayer CNF electrodes in KIBs. during sodiation and desodiation at a constant current rate of 50 ma/g in a coin cell (Figure 3); experimental details are provided in the Supporting Information. The capacity of c-c during Na insertion/extraction was reported to be extremely small. 21 For bilayer CNFs, our in situ TEM electrochemical studies indicated that Na ions are mainly stored in d-c rather than c-c, based on the different volume changes upon sodiation. Hence, the specific capacity is calculated based on the weight of d-c only, which accounts for about 75% of the total mass of CNFs, as estimated from the average thicknesses of d-c and c-c layers for more than 30 samples. For sodiation of the carbon-based anodes, previous studies indicate that there are two dominant mechanisms of Na insertion: (1) insertion of Na between the graphene layers corresponding to the sloping voltage profile; (2) insertion of Na into the micropores corresponding to a plateau at a low potential. 4 In this work, because the micropores in bilayer CNFs are limited, the discharge charge curve in Figure 3a exhibits a sloping profile in the voltage range of 0 to 1.2 V, which likely corresponds to the insertion of Na ions between the graphene layers in d-c. 4 This is consistent with our TEM results in Figure 2f,g, where Na ions insert in between the graphene layers of stacked graphite patches in d-c. 15 Figure 3b shows the capacity stability and Coulombic efficiency during Na insertion and extraction. The initial discharge and charge capacities are 728 and 263 mah/g, respectively, giving a Coulombic efficiency of 36%. The large irreversible capacity in the first cycle is likely due to electrolyte decomposition to form the solid electrolyte interphase (SEI) at the electrode surface, as well as irreversible insertion of Na into graphitic layers. The capacity decay occurs primarily in the first few cycles, which is possibly attributed to the SEI stabilization, volume adjustment during Na ions insertion/ extraction and partially irreversible Na ions insertion. 4,15 After initial adjustment cycles, a reversible capacity of 245 mah/g is maintained with a Coulombic efficiency approaching 98%, which is in good agreement with other reported results on sodiated disordered carbon. 15,33 Since the inactive c-c can function as a built-in current collector, this bilayer CNF electrode shows excellent cycling stability with 99% capacity retention after 280 cycles, indicating a great potential of d-c in the application for NIBs. In addition, the bilayer CNF electrode also exhibits a 3448
5 Nano s Figure 4. Sodiation-induced crack nucleation and propagation in a hollow bilayer CNF. The experiment was conducted under a beam-on condition with very weak electron-beam exposure. (a) Pristine CNF. (b g) The dynamic process of the sodiation-induced crack nucleation and propagation. Upon sodiation, a crack is observed to nucleate from the contact point between the CNF and Na2O/Na electrolyte, and it extends from one end to the other end along the longitudinal direction. The red arrows indicate the crack and its propagation front. (h,i) EDPs of the CNF before and after the sodiation. (j,k) Zoom-in images showing the cracks close to the c-c/d-c interface. of the sodiation-induced cracks in a bilayer CNF. The sample is tested under beam-on with a very weak electron beam. Upon sodiation, a crack nucleates from the contact point between the CNF and Na2O/Na electrolyte, as indicated by red arrows in Figure 4b. As sodiation proceeds, the crack propagates from one end to the other end of the CNF along its axial direction (Figure 4c,d). Interestingly, the crack initially nucleates on the upper wall of the CNF and then extends to its lower wall, which is likely caused by screw rotation of the moving crack front around the axial direction (Figure 4e g). Such kind of sodiation-induced cracking is frequently observed (5 out of 6 samples) with either beam-on or beam-blank (Figure 2 and Supporting Information Figures S1 3), thus suggesting the insensitivity of cracking to electron beam. Similar to Figure 2f,g, a large increase in the spacing of {0002} planes is observed after sodiation (Figure 4h,i). Figure 4j,k shows the zoom-in images of longitudinal similar sloping profile in the discharge/charge curve in K-ion batteries at the same current rate of 50 ma/g in the voltage range of V, as shown in Figure 3c. Similar to NIBs, the sloping discharge/charge curve likely corresponds to the insertion of K ions between graphene layers in d-c, which is supported by the TEM results in Supporting Information Figure S1c,d. However, Figure 3d shows that the cycle stability of the bilayer CNF electrode for KIBs is not as good as for NIBs, possibly due to the relatively slow kinetics of large K ions. Nonetheless, both our electrochemical testing and in situ TEM results suggest that d-c is a viable material for KIBs. Mechanical degradation in electrodes is usually an important cause of the irreversible capacity loss in rechargeable batteries.34 Our in situ TEM study reveals the mechanical degradation through formation of interface cracks in bilayer CNFs during sodiation and potassiation. Figure 4 shows an in situ TEM result 3449
6 Nano s cracks, which are located at different places, but all close to the interface between the c-c and d-c layers. Supporting Information Figure S1 shows an in situ TEM result of potassiation of a bilayer CNF, where cracks near the interface are also observed. In contrast, no cracking is observed during the sodiation of monolithic d-c CNFs, being either solid or hollow, as shown in Supporting Information Figure S4. Such different cracking behaviors suggest that the unique bilayer structure, along with the hollow geometry, plays an important role in crack formation during sodiation and potassiation of CNFs. Such interface cracking might be responsible for capacity fading in the initial charge/discharge cycles in Figure To understand the geometrical changes in CNFs, it is essential to elucidate the mechanism of strain generation and accommodation during sodiation (and potassiation). Particularly, one needs to explain the origin of the decreasing inner radius of the tube, as seen from Figure 2c,d. This result is somewhat counterintuitive, because one would expect that the sodiationinduced volume increase tends to cause an outward expansion of the tube that would result in an increase of the inner radius. To explain this unexpected result, we develop a continuum chemomechanical model to simulate the concurrent processes of Na diffusion and mechanical deformation. In the model, the strain at any material point ε ij is assumed to consist of two parts, ε ij = ε c ij + ε m ij, where ε c ij is the chemical strain induced by Na insertion under a stress-free condition and ε m ij is the elastic strain due to chemically induced mechanical deformation. The components of chemical strain ε c ij depend on the underlying atomic processes of Na insertion in c-c and d-c. A physical assignment of the appropriate values for ε c ij is critical to explain the sodiation-induced geometrical changes. We note that c-c consists of parallel graphene layers, and interlayer expansion is the primary deformation mode upon Na insertion. Hence, we assume the chemical strain ε c ij is highly anisotropic with only one nonzero component perpendicular to graphene layers, that is, in the radial direction of a CNF. Similarly, d-c consists of patches of few-layered graphene that are approximately aligned in the radial direction of a CNF. 35 As a result, the chemical strain ε c ij in d-c is similarly assumed to be highly anisotropic with only one nonzero component in the radial direction. In addition, because the longitudinal elongation of the CNFs is negligibly small in TEM experiments, the plane strain condition is assumed in the axial direction. Finite element simulations based on the above chemomechanical model predict the geometrical changes in sodiated bilayer CNFs (Figure 5a,b) that agree with experimental measurements. From the experimental data of volumetric chemical strains (being 6.0 and 18.7% in c-c and d-c layers, respectively) and considering the anisotropic nature of chemical strains as discussed above, we take ε c,c-c r = 0.06, ε c,c-c θ = ε c,c-c z =0 for c-c, and ε c,d-c r = 0.187, ε c,d-c θ = ε c,d-c z = 0 for d-c. In our simplified elastic model, the radial displacement depends on Poisson s ratio (ν = 0.2), but is independent of Young s modulus. 36 The solid line in Figure 5b shows the predicted radial displacement u r as a function radial distance r. It is seen that u r is negative in the c-c layer and so is u r at the c-c/d-c interface. In the c-c layer, u r becomes positive with increasing r and reaches the maximum at the outer surface of the tube. These modeling results are consistent with the experimental measurements (circles in Figure 5b), and their numerical differences arise possibly due to a neglect of plastic strains in our model. It should be noted that previous studies have emphasized the critical role of chemical strain anisotropy in stress generation This work 3450 Figure 5. Chemomechanical modeling of sodiation-induced geometrical changes in a hollow bilayer CNF. (a) Schematic of cross section of the tube before sodiation, where r in and r out are respectively the inner and outer radius of the tube, and r int is the radius of the c-c/d-c interface. (b) Predicted radial displacement u r versus radial distance r (solid line), compared with experimental data (circles). also supports the notation of anisotropic chemical strain, which results from the layered graphene lattice. At the moment of this study, the stress in the system cannot be quantitatively predicted, because the in situ TEM setup is incapable of measuring the mechanical properties of nanosized electrodes. To further understand the coupling between mechanical and electrochemical responses in CNFs, the sodiation behavior of an a-si coated CNF (a-si/cnf) is also investigated, as shown in Figure 6 and Supporting Information Figure S5. The a-si layer is coated on the outer surface of the CNF with a thickness of 19 nm (Figure 6a,d). Because of geometrical confinement of the stiff a-si coating, the sodiated bilayer tends to expand inward to the hollow space of the CNF, resulting in a large decrease of the inner tube diameter (Figure 6b). Similar to the case without coating, sodiation also leads to crack formation along the longitudinal direction of the a-si/cnf (Figure 6c), and cracking occurs near the c-c/d-c interface (Supporting Information Figure S5). Note that the thickness of the a-si coating remains unchanged during sodiation, indicating negligible Na insertion in a-si. This is further confirmed by EDPs of the a-si/cnf before and after sodiation (Figure 6f,g). The sodiated CNF shows a typical split EDP, while no change is observed in the diffraction rings of a-si before and after sodiation. These in situ TEM results suggest that nanoscale a-si may not be a viable anode material for NIBs. A previous theoretical study indicates that Si may serve as an anode material for NIBs, 8 despite the lack of direct experimental support to date. 15 Our in situ TEM study indicates that a-si is electrochemically unfavorable for NIBs. Moreover, the surface coating of d-c or amorphous C has been widely used to enhance the electronic conductivity or serve as a structural support for nanocomposite electrodes of NIBs The present sodiation study of a-si/cnf also suggests that even when d-c is covered with an inactive surface layer, it can still be effectively sodiated or potassiated, likely due to the fast diffusion of Na and K ions at the interface. In conclusion, the electrochemical reactions and associated structure changes of hollow bilayer CNFs during sodiation and potassiation have been studied by in situ TEM. It is shown that d- C exhibits about three times the volume expansion of c-c, thus indicating a larger Na or K ion storage capability in d-c than c-c. Longitudinal cracks are frequently observed near the c-c/d-c interface after sodiation and potassiation. An unexpected shrinkage of the inner tube is explained in terms of anisotropic sodiation strains in c-c and d-c. Moreover, the sodiation study of
7 Nano s Figure 6. Structure changes of an amorphous silicon-coated CNF (a-si/cnf) before and after sodiation. (a,d) A pristine CNF with a-si coating ( 19 nm in thickness). The diameter of the inner wall is about 80 nm. (b) Upon sodiation, the diameter of the inner wall gradually reduces due to the geometric confinement of the stiff a-si coating, and it decrease to 62 nm at the moment of crack formation. (c,e) The crack finally forms along the longitudinal direction of the a-si/cnf system. The thickness of the a-si coating does not change after sodiation, indicating no reaction occurs. (f,g) EDPs of the pristine and sodiated a-si/cnf. The sodiated CNF exhibits a typical split EDP, while no change is observed in the diffraction rings of a-si before and after sodiation, confirming no reaction occurs in a-si. a-si/cnfs demonstrates that the nanoscale a-si is not a viable anode material for NIBs. Our work suggests that disorderedcarbon holds great promise in applications for NIBs and KIBs. The in situ TEM results provide critical insight into the electrochemical reaction and degradation mechanisms of the carbon-based anodes for NIBs and KIBs. AUTHOR INFORMATION Corresponding Authors * ting.zhu@me.gatech.edu. * cswang@umd.edu. Author Contributions Y.L., F.F. and J.W. contributed equally to this work. Notes The authors declare no competing financial interest. ASSOCIATED CONTENT S Supporting Information * ACKNOWLEDGMENTS We acknowledge the support of the Maryland Nano Center and its NispLab. T.Z. acknowledges the support of the NSF Grant CMMI In addition, this work was performed, in part, at the Center for Integrated Nanotechnologies, a U.S. Department The supporting figures showing the sodiation- and potassiationinduced cracks in CNFs under beam blank, as well as sodiationinduced cracks in a-si/cnf. This material is available free of charge via the Internet at
8 Nano s of Energy, Office of Basic Energy Sciences user facility. Sandia National Laboratories is a multiprogram laboratory managed and operated by Sandia Corporation, a wholly owned subsidiary of Lockheed Martin Corporation for the U.S. Department of Energy s National Nuclear Security Administration under Contract DE-AC04-94AL REFERENCES (1) Chan, C. K.; Peng, H.; Liu, G.; McIlwrath, K.; Zhang, X. F.; Huggins, R. A.; Cui, Y. Nat. Nanotechnol. 2008, 3, (2) Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Energy Environ. Sci. 2011, 4, (3) Slater, M. D.; Kim, D.; Lee, E.; Johnson, C. S. Adv. Funct. Mater. 2013, 23, (4) Tang, K.; Fu, L.; White, R. J.; Yu, L.; Titirici, M.-M.; Antonietti, M.; Maier, J. Adv. Energy Mater. 2012, 2, (5) Wessells, C. D.; Peddada, S. V.; Huggins, R. A.; Cui, Y. Nano Lett. 2011, 11, (6) Lee, K. T.; Hong, S. Y.; Kim, Y.; won Park, Y.; Choi, A.; Choi, N.-S. Energy Environ. Sci. 2013, 6, (7) Palomares, V.; Casas-Cabanas, M.; Castillo-Martinez, E.; Han, M. H.; Rojo, T. Energy Environ. Sci. 2013, 6, (8) Chevrier, V. L.; Ceder, G. J. Electrochem. Soc. 2011, 158, A1011 A1014. (9) Ellis, L. D.; Hatchard, T. D.; Obrovac, M. N. J. Electrochem. Soc. 2012, 159, A1801 A1805. (10) Wang, J. W.; Liu, X. H.; Mao, S. X.; Huang, J. Y. Nano Lett. 2012, 12, (11) Xiong, H.; Slater, M. D.; Balasubramanian, M.; Johnson, C. S.; Rajh, T. J. Phys. Chem. Lett. 2011, 2, (12) Cao, Y.; Xiao, L.; Wang, W.; Choi, D.; Nie, Z.; Yu, J.; Saraf, L. V.; Yang, Z.; Liu, J. Adv. Mater. 2011, 23, (13) Alcańtara, R.; Jimeńez-Mateos, J. M.; Lavela, P.; Tirado, J. L. Electrochem. Commun. 2001, 3, (14) Alcańtara, R.; Lavela, P.; Ortiz, G. F.; Tirado, J. L. Electrochem. Solid-State Lett. 2005, 8, A222 A225. (15) Cao, Y.; Xiao, L.; Sushko, M. L.; Wang, W.; Schwenzer, B.; Xiao, J.; Nie, Z.; Saraf, L. V.; Yang, Z.; Liu, J. Nano Lett. 2012, 12, (16) Komaba, S.; Murata, W.; Ishikawa, T.; Yabuuchi, N.; Ozeki, T.; Nakayama, T.; Ogata, A.; Gotoh, K.; Fujiwara, K. Adv. Funct. Mater. 2011, 21, (17) Wenzel, S.; Hara, T.; Janek, J.; Adelhelm, P. Energy Environ. Sci. 2011, 4, (18) Zhao, J.; Zhao, L.; Chihara, K.; Okada, S.; Yamaki, J.-i.; Matsumoto, S.; Kuze, S.; Nakane, K. J. Power Sources 2013, 244, (19) Stevens, D.; Dahn, J. J. Electrochem. Soc. 2000, 147, (20) Scrosati, B. Electrochim. Acta 2000, 45, (21) Stevens, D.; Dahn, J. J. Electrochem. Soc. 2001, 148, A803 A811. (22) Shao, Y.; Xiao, J.; Wang, W.; Engelhard, M.; Chen, X.; Nie, Z.; Gu, M.; Saraf, L. V.; Exarhos, G.; Zhang, J.-G.; Liu, J. Nano Lett. 2013, 13, (23) Kim, Y.; Park, Y.; Choi, A.; Choi, N.-S.; Kim, J.; Lee, J.; Ryu, J. H.; Oh, S. M.; Lee, K. T. Adv. Mater. 2013, 25, (24) Qian, J.; Chen, Y.; Wu, L.; Cao, Y.; Ai, X.; Yang, H. Chem. Commun. 2012, 48, (25) Xiao, L.; Cao, Y.; Xiao, J.; Wang, W.; Kovarik, L.; Nie, Z.; Liu, J. Chem. Commun. 2012, 48, (26) Zhu, Y.; Han, X.; Xu, Y.; Liu, Y.; Zheng, S.; Xu, K.; Hu, L.; Wang, C. ACS Nano 2013, 7, (27) Zhu, Y.; Wang, J. W.; Liu, Y.; Liu, X.; Kushima, A.; Liu, Y.; Xu, Y.; Mao, S. X.; Li, J.; Wang, C.; Huang, J. Y. Adv. Mater. 2013, 25, (28) Wang, J. W.; He, Y.; Fan, F.; Liu, X. H.; Xia, S.; Liu, Y.; Harris, C. T.; Li, H.; Huang, J. Y.; Mao, S. X. Nano Lett. 2013, 13, (29) Wang, J. W.; Liu, X. H.; Zhao, K.; Palmer, A.; Patten, E.; Burton, D.; Mao, S. X.; Suo, Z.; Huang, J. Y. ACS Nano 2012, 6, (30) Liu, Y.; Zheng, H.; Liu, X. H.; Huang, S.; Zhu, T.; Wang, J.; Kushima, A.; Hudak, N. S.; Huang, X.; Zhang, S.; Mao, S. X.; Qian, X.; Li, J.; Huang, J. Y. ACS Nano 2011, 5, (31) Liu, X. H.; Wang, J. W.; Liu, Y.; Zheng, H.; Kushima, A.; Huang, S.; Zhu, T.; Mao, S. X.; Li, J.; Zhang, S.; Lu, W.; Tour, J. M.; Huang, J. Y. Carbon 2012, 50, (32) Liu, X. H.; Wang, J. W.; Huang, S.; Fan, F.; Huang, X.; Liu, Y.; Krylyuk, S.; Yoo, J.; Dayeh, S. A.; Davydov, A. V. Nat. Nanotechnol. 2012, 7, (33) Zhou, X.; Guo, Y.-G. ChemElectroChem 2014, 1, (34) Liu, X. H.; Liu, Y.; Kushima, A.; Zhang, S. L.; Zhu, T.; Li, J.; Huang, J. Y. Adv. Energy Mater. 2012, 2, (35) Thomas, P.; Billaud, D. Electrochim. Acta 2001, 46, (36) Hsueh, C. H.; Evans, A. G. J. Appl. Phys. 1983, 54, (37) Huang, S.; Fan, F.; Li, J.; Zhang, S. L.; Zhu, T. Acta Mater. 2013, 61, (38) Liang, W.; Yang, H.; Fan, F.; Liu, Y.; Liu, X. H.; Huang, J. Y.; Zhu, T.; Zhang, S. ACS Nano 2013, 7,
Carbon-Silicon Core-Shell Nanowires as High Capacity Electrode for Lithium Ion Batteries
 Carbon-Silicon Core-Shell Nanowires as High Capacity Electrode for Lithium Ion Batteries NANO LETTERS XXXX Vol. xx, No. x - Li-Feng Cui, Yuan Yang, Ching-Mei Hsu, and Yi Cui* Department of Materials Science
Carbon-Silicon Core-Shell Nanowires as High Capacity Electrode for Lithium Ion Batteries NANO LETTERS XXXX Vol. xx, No. x - Li-Feng Cui, Yuan Yang, Ching-Mei Hsu, and Yi Cui* Department of Materials Science
ALD TiO 2 coated Silicon Nanowires for Lithium Ion Battery Anodes with enhanced Cycling Stability and Coulombic Efficiency
 ALD TiO 2 coated Silicon Nanowires for Lithium Ion Battery Anodes with enhanced Cycling Stability and Coulombic Efficiency Elmira Memarzadeh Lotfabad a, Peter Kalisvaart a,*, Kai Cui b, Alireza Kohandehghan
ALD TiO 2 coated Silicon Nanowires for Lithium Ion Battery Anodes with enhanced Cycling Stability and Coulombic Efficiency Elmira Memarzadeh Lotfabad a, Peter Kalisvaart a,*, Kai Cui b, Alireza Kohandehghan
6th International Conference on Advanced Design and Manufacturing Engineering (ICADME 2016)
 6th International Conference on Advanced Design and Manufacturing Engineering (ICADME 2016) Porous Co3O4 irregular Micro-cubes with lithium storage performances Ting Wanga, Hao Zhengb, Jinsong Chengc,
6th International Conference on Advanced Design and Manufacturing Engineering (ICADME 2016) Porous Co3O4 irregular Micro-cubes with lithium storage performances Ting Wanga, Hao Zhengb, Jinsong Chengc,
SUPPLEMENTARY INFORMATION
 In the format provided by the authors and unedited. DOI: 10.1038/NNANO.2017.27 Revealing the reaction mechanisms of Li-O 2 batteries using environmental transmission electron microscopy Langli Luo, Bin
In the format provided by the authors and unedited. DOI: 10.1038/NNANO.2017.27 Revealing the reaction mechanisms of Li-O 2 batteries using environmental transmission electron microscopy Langli Luo, Bin
Supporting Information P2-Type Na x Cu 0.15 Ni 0.20 Mn 0.65 O 2 Cathodes with High Voltage for High-Power and Long-Life Sodium-Ion Batteries
 Supporting Information P2-Type Na x Cu 0.15 Ni 0.20 Mn 0.65 O 2 Cathodes with High Voltage for High-Power and Long-Life Sodium-Ion Batteries Wenpei Kang,, Denis Y. W. Yu, *, Pui-Kit Lee, Zhenyu Zhang,
Supporting Information P2-Type Na x Cu 0.15 Ni 0.20 Mn 0.65 O 2 Cathodes with High Voltage for High-Power and Long-Life Sodium-Ion Batteries Wenpei Kang,, Denis Y. W. Yu, *, Pui-Kit Lee, Zhenyu Zhang,
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION DOI: 10.1038/NNANO.2012.35 Stable Cycling of Double-Walled Silicon Nanotube Battery Anodes through Solid-Electrolyte Interphase Control Hui Wu, 1* Gerentt Chan, 2* Jang Wook Choi,
SUPPLEMENTARY INFORMATION DOI: 10.1038/NNANO.2012.35 Stable Cycling of Double-Walled Silicon Nanotube Battery Anodes through Solid-Electrolyte Interphase Control Hui Wu, 1* Gerentt Chan, 2* Jang Wook Choi,
Electronic Supplementary Information (ESI) Self-assembly of Polyoxometalate / Reduced Graphene Oxide
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Electronic Supplementary Information (ESI) Self-assembly of Polyoxometalate
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Electronic Supplementary Information (ESI) Self-assembly of Polyoxometalate
Nanocomposites as next-generation anode materials for lithium ion batteries
 Nanocomposites as next-generation anode materials for lithium ion batteries Lorenzo Mangolini Mechanical Engineering Department Materials Science and Engineering Program UC Riverside International Battery
Nanocomposites as next-generation anode materials for lithium ion batteries Lorenzo Mangolini Mechanical Engineering Department Materials Science and Engineering Program UC Riverside International Battery
Orientation-Dependent Interfacial Mobility Governs the Anisotropic Swelling in Lithiated Silicon Nanowires
 pubs.acs.org/nanolett Orientation-Dependent Interfacial Mobility Governs the Anisotropic Swelling in Lithiated Silicon Nanowires Hui Yang, Shan Huang, Xu Huang, Feifei Fan, Wentao Liang,, Xiao Hua Liu,
pubs.acs.org/nanolett Orientation-Dependent Interfacial Mobility Governs the Anisotropic Swelling in Lithiated Silicon Nanowires Hui Yang, Shan Huang, Xu Huang, Feifei Fan, Wentao Liang,, Xiao Hua Liu,
Electronic Supplementary Material
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Material 2D holey cobalt sulfide nanosheets derived
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Material 2D holey cobalt sulfide nanosheets derived
Micron-Sized Nanoporous Antimony with Tunable Porosity for. High Performance Potassium-Ion Batteries
 1 Micron-Sized Nanoporous Antimony with Tunable Porosity for High Performance Potassium-Ion Batteries Yongling An, Yuan Tian, Lijie Ci, Shenglin Xiong, Jinkui Feng,*, Yitai Qian SDU & Rice Joint Center
1 Micron-Sized Nanoporous Antimony with Tunable Porosity for High Performance Potassium-Ion Batteries Yongling An, Yuan Tian, Lijie Ci, Shenglin Xiong, Jinkui Feng,*, Yitai Qian SDU & Rice Joint Center
Supplementary Figure 1. Crystal structures of conventional layered and Li-rich layered manganese oxides. a, The crystal structure of rhombohedral
 Supplementary Figure 1. Crystal structures of conventional layered and Li-rich layered manganese oxides. a, The crystal structure of rhombohedral LiMO 2 (M = Ni, Co, Mn) with the space group R3m. b, The
Supplementary Figure 1. Crystal structures of conventional layered and Li-rich layered manganese oxides. a, The crystal structure of rhombohedral LiMO 2 (M = Ni, Co, Mn) with the space group R3m. b, The
image of ZMO precursor/rgo. Scale bar, 50 nm. (b) XRD patterns of reduced graphene
 Supplementary Figures Supplementary Figure 1 Characterization of ZMO precursor/rgo. (a) STEM image of ZMO precursor/rgo. Scale bar, 50 nm. (b) XRD patterns of reduced graphene oxide (rgo) and ZMO precursor/rgo.
Supplementary Figures Supplementary Figure 1 Characterization of ZMO precursor/rgo. (a) STEM image of ZMO precursor/rgo. Scale bar, 50 nm. (b) XRD patterns of reduced graphene oxide (rgo) and ZMO precursor/rgo.
Doped Si nanoparticles with conformal. and high-rate lithium-ion battery anodes
 Nanotechnology Nanotechnology 26 (2015) 365401 (6pp) doi:10.1088/0957-4484/26/36/365401 Doped Si nanoparticles with conformal carbon coating and cyclizedpolyacrylonitrile network as high-capacity and high-rate
Nanotechnology Nanotechnology 26 (2015) 365401 (6pp) doi:10.1088/0957-4484/26/36/365401 Doped Si nanoparticles with conformal carbon coating and cyclizedpolyacrylonitrile network as high-capacity and high-rate
On the Role of Mechanics in the Design and Performance of Electrode Materials for Energy Storage
 On the Role of Mechanics in the Design and Performance of Electrode Materials for Energy Storage Pradeep R. Guduru School of Engineering, Brown University V. Sethuraman, M. Chon, S. Nadimpalli Collaborators:
On the Role of Mechanics in the Design and Performance of Electrode Materials for Energy Storage Pradeep R. Guduru School of Engineering, Brown University V. Sethuraman, M. Chon, S. Nadimpalli Collaborators:
Sn Wears Super Skin: A New Design For Long Cycling Batteries
 Supporting Information Wears Super Skin: A New Design For Long Cycling Batteries Shuai Kang, Xi Chen, Junjie Niu* Department of Materials Science and Engineering, CEAS, University of Wisconsin-Milwaukee
Supporting Information Wears Super Skin: A New Design For Long Cycling Batteries Shuai Kang, Xi Chen, Junjie Niu* Department of Materials Science and Engineering, CEAS, University of Wisconsin-Milwaukee
The development of advanced lithiumion
 Tough Germanium Nanoparticles under Electrochemical Cycling Wentao Liang, Hui Yang, Feifei Fan, Yang Liu, Xiao Hua Liu, Jian Yu Huang, Ting Zhu,, * and Sulin Zhang, * Department of Engineering Science
Tough Germanium Nanoparticles under Electrochemical Cycling Wentao Liang, Hui Yang, Feifei Fan, Yang Liu, Xiao Hua Liu, Jian Yu Huang, Ting Zhu,, * and Sulin Zhang, * Department of Engineering Science
Supporting Information. Amorphous Red Phosphorus Embedded in Highly Ordered. Mesoporous Carbon with Superior Lithium and Sodium Storage.
 Supporting Information Amorphous Red Phosphorus Embedded in Highly Ordered Mesoporous Carbon with Superior Lithium and Sodium Storage Capacity Weihan Li, Zhenzhong Yang, Minsi Li, Yu Jiang, Xiang Wei,
Supporting Information Amorphous Red Phosphorus Embedded in Highly Ordered Mesoporous Carbon with Superior Lithium and Sodium Storage Capacity Weihan Li, Zhenzhong Yang, Minsi Li, Yu Jiang, Xiang Wei,
Supporting Information. Carbon Welding by Ultrafast Joule Heating
 Supporting Information Carbon Welding by Ultrafast Joule Heating Yonggang Yao, 1,(a) Kun Fu, 1,(a) Shuze Zhu, 2 Jiaqi Dai, 1 Yanbin Wang, 1 Glenn Pastel, 1 Yanan Chen, 1 Tian Li, 1 Chengwei Wang, 1 Teng
Supporting Information Carbon Welding by Ultrafast Joule Heating Yonggang Yao, 1,(a) Kun Fu, 1,(a) Shuze Zhu, 2 Jiaqi Dai, 1 Yanbin Wang, 1 Glenn Pastel, 1 Yanan Chen, 1 Tian Li, 1 Chengwei Wang, 1 Teng
Nickel Nanocone-Array Supported Silicon Anode for High- Performance Lithium-Ion Batteries
 Nickel Nanocone-Array Supported Silicon Anode for High- Performance Lithium-Ion Batteries By Shichao Zhang, * Zhijia Du, Ruoxu Lin, Tao Jiang, Guanrao Liu, Xiaomeng Wu, and Dangsheng Weng The development
Nickel Nanocone-Array Supported Silicon Anode for High- Performance Lithium-Ion Batteries By Shichao Zhang, * Zhijia Du, Ruoxu Lin, Tao Jiang, Guanrao Liu, Xiaomeng Wu, and Dangsheng Weng The development
High Performance Lithium Battery Anodes Using Silicon Nanowires
 Supporting Online Materials For High Performance Lithium Battery Anodes Using Silicon Nanowires Candace K. Chan, Hailin Peng, Gao Liu, Kevin McIlwrath, Xiao Feng Zhang, Robert A. Huggins and Yi Cui * *To
Supporting Online Materials For High Performance Lithium Battery Anodes Using Silicon Nanowires Candace K. Chan, Hailin Peng, Gao Liu, Kevin McIlwrath, Xiao Feng Zhang, Robert A. Huggins and Yi Cui * *To
Supporting Information. Exploring Stability of Nonaqueous Electrolytes for Potassium-Ion Batteries
 Supporting Information Exploring Stability of Nonaqueous Electrolytes for Potassium-Ion Batteries Yu Lei,, Lei Qin,, Ruliang Liu,, Kah Chun Lau, Yiying Wu, Dengyun Zhai, *, Baohua Li, and Feiyu Kang *,
Supporting Information Exploring Stability of Nonaqueous Electrolytes for Potassium-Ion Batteries Yu Lei,, Lei Qin,, Ruliang Liu,, Kah Chun Lau, Yiying Wu, Dengyun Zhai, *, Baohua Li, and Feiyu Kang *,
Optimized by Disorder Engineering on Iron doped Ni 3 S 2. nanosheets for Oxygen Evolution Reaction
 Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2018 Supplementary Data Optimized by Disorder Engineering on Iron doped Ni 3 S 2 nanosheets for Oxygen
Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2018 Supplementary Data Optimized by Disorder Engineering on Iron doped Ni 3 S 2 nanosheets for Oxygen
Electronic Supplementary Information (ESI)
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information (ESI) Rationally Designed Hierarchical Porous CNFs/Co
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information (ESI) Rationally Designed Hierarchical Porous CNFs/Co
INITIAL STUDY OF THE MICROSTRUCTURE OF CARBON FIBRES ACTING AS NEGATIVE ELECTRODES IN STRUCTURAL BATTERY COMPOSITES
 Munich, Germany, 26-30 th June 2016 1 INITIAL STUDY OF THE MICROSTRUCTURE OF CARBON FIBRES ACTING AS NEGATIVE ELECTRODES IN STRUCTURAL BATTERY COMPOSITES Fang Liu 1, Masoud Rashidi 2 and Leif E. Asp 3
Munich, Germany, 26-30 th June 2016 1 INITIAL STUDY OF THE MICROSTRUCTURE OF CARBON FIBRES ACTING AS NEGATIVE ELECTRODES IN STRUCTURAL BATTERY COMPOSITES Fang Liu 1, Masoud Rashidi 2 and Leif E. Asp 3
Rapid degradation of methylene blue in a novel
 Title: Rapid degradation of methylene blue in a novel heterogeneous Fe 3 O 4 @rgo@tio 2 -catalyzed photo-fenton system Author names Xiaoling Yang, Wei Chen, Jianfei Huang, Ying Zhou, Yihua Zhu,* and Chunzhong
Title: Rapid degradation of methylene blue in a novel heterogeneous Fe 3 O 4 @rgo@tio 2 -catalyzed photo-fenton system Author names Xiaoling Yang, Wei Chen, Jianfei Huang, Ying Zhou, Yihua Zhu,* and Chunzhong
Elucidating the Phase Transformation of Li4Ti5O12 Lithiation at the Nanoscale
 Elucidating the Phase Transformation of Li4Ti5O12 Lithiation at the Nanoscale Michael G. Verde 1*, Loïc Baggetto 2, Nina Balke 3, Gabriel M. Veith 2, Joon Kyo Seo 4, Ziying Wang 1, Ying Shirley Meng 1*
Elucidating the Phase Transformation of Li4Ti5O12 Lithiation at the Nanoscale Michael G. Verde 1*, Loïc Baggetto 2, Nina Balke 3, Gabriel M. Veith 2, Joon Kyo Seo 4, Ziying Wang 1, Ying Shirley Meng 1*
In situ generation of Li 2 FeSiO 4 coating on MWNT as a high rate cathode material for lithium ion batteries
 Supporting Information: In situ generation of Li 2 FeSiO 4 coating on MWNT as a high rate cathode material for lithium ion batteries Yi Zhao, Jiaxin Li, Ning Wang, Chuxin Wu, Yunhai Ding, Lunhui Guan*
Supporting Information: In situ generation of Li 2 FeSiO 4 coating on MWNT as a high rate cathode material for lithium ion batteries Yi Zhao, Jiaxin Li, Ning Wang, Chuxin Wu, Yunhai Ding, Lunhui Guan*
SUPPORTING INFORMATION. A Rechargeable Aluminum-Ion Battery Based on MoS 2. Microsphere Cathode
 SUPPORTING INFORMATION A Rechargeable Aluminum-Ion Battery Based on MoS 2 Microsphere Cathode Zhanyu Li a, Bangbang Niu a, Jian Liu a, Jianling Li a* Feiyu Kang b a School of Metallurgical and Ecological
SUPPORTING INFORMATION A Rechargeable Aluminum-Ion Battery Based on MoS 2 Microsphere Cathode Zhanyu Li a, Bangbang Niu a, Jian Liu a, Jianling Li a* Feiyu Kang b a School of Metallurgical and Ecological
PHYSICAL REVIEW LETTERS 107,
 Real-time Measurement of Stress and Damage Evolution During Initial Lithiation of Crystalline Silicon M. J. Chon, 1 V.A. Sethuraman, 1 A. McCormick, 1 V. Srinivasan, 2 P. R. Guduru 1,* 1 School of Engineering,
Real-time Measurement of Stress and Damage Evolution During Initial Lithiation of Crystalline Silicon M. J. Chon, 1 V.A. Sethuraman, 1 A. McCormick, 1 V. Srinivasan, 2 P. R. Guduru 1,* 1 School of Engineering,
for Lithium Ion Batteries facile synthesis using L-Arginine as the hydrolysis-controlling agent for the formation of
 1 2 3 4 5 6 7 8 9 10 11 12 High Crystallized Fe 2 O 3 /Graphene Composites as High-performance Anode Materials for Lithium Ion Batteries By Hongwei Zhang, Liang Zhou and Chengzhou Yu* Abstract Fe 2 O 3
1 2 3 4 5 6 7 8 9 10 11 12 High Crystallized Fe 2 O 3 /Graphene Composites as High-performance Anode Materials for Lithium Ion Batteries By Hongwei Zhang, Liang Zhou and Chengzhou Yu* Abstract Fe 2 O 3
Nanocrystalline LiFePO4 as cathode material for lithium battery applications S.C SIAH
 Nanocrystalline LiFePO as cathode material for lithium battery applications Abstract S.C SIAH Engineering Science Programme, National University of Singapore Kent Ridge, Singapore 119260 LiFePO was prepared
Nanocrystalline LiFePO as cathode material for lithium battery applications Abstract S.C SIAH Engineering Science Programme, National University of Singapore Kent Ridge, Singapore 119260 LiFePO was prepared
Microscopic Structural Analysis of Advanced Anode Material for Lithium Battery
 JFE TECHNICAL REPORT No. 22 (Mar. 2017) Microscopic Structural Analysis of Advanced Anode Material for Lithium Battery SIMAUCHI Yutaka *1 OHMORI Shigekazu *2 IKEMOTO Sachi *3 Abstract: analyzed the microstructure
JFE TECHNICAL REPORT No. 22 (Mar. 2017) Microscopic Structural Analysis of Advanced Anode Material for Lithium Battery SIMAUCHI Yutaka *1 OHMORI Shigekazu *2 IKEMOTO Sachi *3 Abstract: analyzed the microstructure
High-Performance Sb/Sb 2 O 3 Anode Materials Using a Polypyrrole Nanowire Network for Na-Ion Batteries
 Anodes High-Performance Sb/Sb 2 O 3 Anode Materials Using a Polypyrrole Nanowire Network for Na-Ion Batteries Do-Hwan Nam, * Kyung-Sik Hong, Sung-Jin Lim, Min-Joong Kim, and Hyuk-Sang Kwon * T hree-dimensional
Anodes High-Performance Sb/Sb 2 O 3 Anode Materials Using a Polypyrrole Nanowire Network for Na-Ion Batteries Do-Hwan Nam, * Kyung-Sik Hong, Sung-Jin Lim, Min-Joong Kim, and Hyuk-Sang Kwon * T hree-dimensional
Supporting Information
 Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2017 Supporting Information Electrocatalysis of Polysulfide Conversion by Sulfur-deficient
Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2017 Supporting Information Electrocatalysis of Polysulfide Conversion by Sulfur-deficient
Electrochemical Properties of Electrodeposited Sn Anodes for Na-Ion Batteries
 pubs.acs.org/jpcc Electrochemical Properties of Electrodeposited Sn Anodes for Na-Ion Batteries Do-Hwan Nam, Kyung-Sik Hong, Sung-Jin Lim, Tae-Hee Kim, and Hyuk-Sang Kwon* Department of Materials Science
pubs.acs.org/jpcc Electrochemical Properties of Electrodeposited Sn Anodes for Na-Ion Batteries Do-Hwan Nam, Kyung-Sik Hong, Sung-Jin Lim, Tae-Hee Kim, and Hyuk-Sang Kwon* Department of Materials Science
Deformation Twinning in Bulk Aluminum with Coarse Grains
 Proceedings of the 12th International Conference on Aluminium Proceedings Alloys, of the September 12th International 5-9, 2010, Yokohama, Conference Japan on 2010 Aluminum The Japan Alloys, Institute
Proceedings of the 12th International Conference on Aluminium Proceedings Alloys, of the September 12th International 5-9, 2010, Yokohama, Conference Japan on 2010 Aluminum The Japan Alloys, Institute
Supplementary Materials for
 advances.sciencemag.org/cgi/content/full/3/6/e1603213/dc1 Supplementary Materials for Compressed glassy carbon: An ultrastrong and elastic interpenetrating graphene network Meng Hu, Julong He, Zhisheng
advances.sciencemag.org/cgi/content/full/3/6/e1603213/dc1 Supplementary Materials for Compressed glassy carbon: An ultrastrong and elastic interpenetrating graphene network Meng Hu, Julong He, Zhisheng
School of Materials Science and Engineering, South China University of Technology,
 Supporting information Zn/MnO 2 Battery Chemistry With H + and Zn 2+ Co-Insertion Wei Sun, Fei Wang, Singyuk Hou, Chongyin Yang, Xiulin Fan, Zhaohui Ma, Tao Gao, Fudong Han, Renzong Hu, Min Zhu *, Chunsheng
Supporting information Zn/MnO 2 Battery Chemistry With H + and Zn 2+ Co-Insertion Wei Sun, Fei Wang, Singyuk Hou, Chongyin Yang, Xiulin Fan, Zhaohui Ma, Tao Gao, Fudong Han, Renzong Hu, Min Zhu *, Chunsheng
High-Rate Alkali-Ion Batteries: from Rational. Synthesis to In-situ Probing
 Supporting Information for Two-Dimensional Holey Co 3 O 4 Nanosheets for High-Rate Alkali-Ion Batteries: from Rational Synthesis to In-situ Probing Dahong Chen,,,# Lele Peng,,# Yifei Yuan,,,# Yue Zhu,
Supporting Information for Two-Dimensional Holey Co 3 O 4 Nanosheets for High-Rate Alkali-Ion Batteries: from Rational Synthesis to In-situ Probing Dahong Chen,,,# Lele Peng,,# Yifei Yuan,,,# Yue Zhu,
FABRICATION AND ELECTROCHEMICAL CHARECTARIZATION OF THE CR2032 COIN CELLS USING THE DEVELOPED PURE AND CARBON COATED
 FABRICATION AND ELECTROCHEMICAL CHARECTARIZATION OF THE CR2032 COIN CELLS USING THE DEVELOPED PURE AND CARBON COATED LiMPO 4 (M= Mn, Co & Ni) NANOPARTICLES CHAPTER VI 181 CHAPTER - VI FABRICATION AND ELECTROCHEMICAL
FABRICATION AND ELECTROCHEMICAL CHARECTARIZATION OF THE CR2032 COIN CELLS USING THE DEVELOPED PURE AND CARBON COATED LiMPO 4 (M= Mn, Co & Ni) NANOPARTICLES CHAPTER VI 181 CHAPTER - VI FABRICATION AND ELECTROCHEMICAL
The Effects of LaF 3 Coating on the Electrochemical Property of Li[Ni 0.3 Co 0.4 Mn 0.3 ]O 2 Cathode Material
![The Effects of LaF 3 Coating on the Electrochemical Property of Li[Ni 0.3 Co 0.4 Mn 0.3 ]O 2 Cathode Material The Effects of LaF 3 Coating on the Electrochemical Property of Li[Ni 0.3 Co 0.4 Mn 0.3 ]O 2 Cathode Material](/thumbs/82/85321953.jpg) 2584 Bull. Korean Chem. Soc. 2009, Vol. 30, No. 11 Su Hyun Yun et al. The Effects of LaF 3 Coating on the Electrochemical Property of Li[ Co 0.4 Cathode Material Su Hyun Yun, Seuk Buom Kim, and Yong Joon
2584 Bull. Korean Chem. Soc. 2009, Vol. 30, No. 11 Su Hyun Yun et al. The Effects of LaF 3 Coating on the Electrochemical Property of Li[ Co 0.4 Cathode Material Su Hyun Yun, Seuk Buom Kim, and Yong Joon
SI GUIDE. File Name: Supplementary Information Description: Supplementary Figures, Supplementary Notes and Supplementary References.
 SI GUIDE File Name: Supplementary Information Description: Supplementary Figures, Supplementary Notes and Supplementary References. File Name: Supplementary Movie 1 Description: (the movie from which Figs.
SI GUIDE File Name: Supplementary Information Description: Supplementary Figures, Supplementary Notes and Supplementary References. File Name: Supplementary Movie 1 Description: (the movie from which Figs.
The Preparation of C/Ni Composite Nanofibers with Pores by Coaxial Electrospinning
 2016 International Conference on Intelligent Manufacturing and Materials (ICIMM 2016) ISBN: 978-1-60595-363-2 The Preparation of C/Ni Composite Nanofibers with Pores by Coaxial Electrospinning Yiqiang
2016 International Conference on Intelligent Manufacturing and Materials (ICIMM 2016) ISBN: 978-1-60595-363-2 The Preparation of C/Ni Composite Nanofibers with Pores by Coaxial Electrospinning Yiqiang
factured pillars, even though the strength is significantly higher than in the bulk. These yield stress values, y
 Abstract The size effect in body-centered cubic (bcc) metals was comprehensively investigated through microcompression tests performed on focused ion beam machined tungsten (W), molybdenum (Mo) and niobium
Abstract The size effect in body-centered cubic (bcc) metals was comprehensively investigated through microcompression tests performed on focused ion beam machined tungsten (W), molybdenum (Mo) and niobium
Patterned Si thin film electrodes for enhancing structural stability
 NANO IDEA Open Access Patterned thin film electrodes for enhancing structural stability Gyu-bong Cho, Jung-pil Noh, Ho-jin Sung, Sang-hun Lee, Yeon-min Im, Hyo-jun Ahn and Ki-won Kim * Abstract A patterned
NANO IDEA Open Access Patterned thin film electrodes for enhancing structural stability Gyu-bong Cho, Jung-pil Noh, Ho-jin Sung, Sang-hun Lee, Yeon-min Im, Hyo-jun Ahn and Ki-won Kim * Abstract A patterned
In Situ Formation of Stable Interfacial Coating for High Performance Lithium Metal Anodes
 In Situ Formation of Stable Interfacial Coating for High Performance Lithium Metal Anodes Haiping Wu 1, Yue Cao 1, Linxiao Geng 2 & Chao Wang 1* 1 Department of Chemistry, University of California Riverside,
In Situ Formation of Stable Interfacial Coating for High Performance Lithium Metal Anodes Haiping Wu 1, Yue Cao 1, Linxiao Geng 2 & Chao Wang 1* 1 Department of Chemistry, University of California Riverside,
Urchin-like V 2 O 3 /C Hollow Nanospheres Hybrid for High-Capacity and Long-Cycle-Life Lithium Storage
 Supporting Information Urchin-like V 2 O 3 /C Hollow Nanospheres Hybrid for High-Capacity and Long-Cycle-Life Lithium Storage Peng Yu, Xu Liu, Lei Wang,* Chungui Tian, Haitao Yu, and Honggang Fu* Key Laboratory
Supporting Information Urchin-like V 2 O 3 /C Hollow Nanospheres Hybrid for High-Capacity and Long-Cycle-Life Lithium Storage Peng Yu, Xu Liu, Lei Wang,* Chungui Tian, Haitao Yu, and Honggang Fu* Key Laboratory
Robust Expandable Carbon Nanotube Scaffold for Ultrahigh-Capacity Lithium-Metal Anodes
 Communication Lithium-Metal Anodes Robust Expandable Carbon Nanotube Scaffold for Ultrahigh-Capacity Lithium-Metal Anodes Zhaowei Sun, Song Jin, Hongchang Jin, Zhenzhen Du, Yanwu Zhu, Anyuan Cao, Hengxing
Communication Lithium-Metal Anodes Robust Expandable Carbon Nanotube Scaffold for Ultrahigh-Capacity Lithium-Metal Anodes Zhaowei Sun, Song Jin, Hongchang Jin, Zhenzhen Du, Yanwu Zhu, Anyuan Cao, Hengxing
Lithium ion batteries (LIBs) currently
 Red Phosphorus Single-Walled Carbon Nanotube Composite as a Superior Anode for Sodium Ion Batteries Yujie Zhu, Yang Wen, Xiulin Fan, Tao Gao, Fudong Han, Chao Luo, Sz-Chian Liou, and Chunsheng Wang*, Department
Red Phosphorus Single-Walled Carbon Nanotube Composite as a Superior Anode for Sodium Ion Batteries Yujie Zhu, Yang Wen, Xiulin Fan, Tao Gao, Fudong Han, Chao Luo, Sz-Chian Liou, and Chunsheng Wang*, Department
Bending-Induced Symmetry Breaking of Lithiation in Germanium Nanowires. 7,31,32 as opposed to the isotropic lithiation in c-ge, 12
 pubs.acs.org/nanolett Bending-Induced Symmetry Breaking of Lithiation in Germanium Nanowires Meng Gu,,# Hui Yang,,# Daniel E. Perea, Ji-Guang Zhang, Sulin Zhang,*, and Chong-Min Wang*, Environmental Molecular
pubs.acs.org/nanolett Bending-Induced Symmetry Breaking of Lithiation in Germanium Nanowires Meng Gu,,# Hui Yang,,# Daniel E. Perea, Ji-Guang Zhang, Sulin Zhang,*, and Chong-Min Wang*, Environmental Molecular
Supporting Information. Multistep Lithiation of Tin Sulfide: An Investigation Using in Situ Electron
 Supporting Information Multistep Lithiation of Tin Sulfide: An Investigation Using in Situ Electron Microscopy Sooyeon Hwang, Zhenpeng Yao, Lei Zhang, Maosen Fu,, Kai He, Liqiang Mai, Chris Wolverton,
Supporting Information Multistep Lithiation of Tin Sulfide: An Investigation Using in Situ Electron Microscopy Sooyeon Hwang, Zhenpeng Yao, Lei Zhang, Maosen Fu,, Kai He, Liqiang Mai, Chris Wolverton,
Supplementary Materials for
 advances.sciencemag.org/cgi/content/full/4/8/eaat4712/dc1 Supplementary Materials for In situ manipulation and switching of dislocations in bilayer graphene Peter Schweizer, Christian Dolle, Erdmann Spiecker*
advances.sciencemag.org/cgi/content/full/4/8/eaat4712/dc1 Supplementary Materials for In situ manipulation and switching of dislocations in bilayer graphene Peter Schweizer, Christian Dolle, Erdmann Spiecker*
Department of Energy Engineering, Hanyang University, Seoul 04763, South Korea
 Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2018 Supplementary Information Development of P3-K 0.69 CrO 2 as an ultra-high-performance
Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2018 Supplementary Information Development of P3-K 0.69 CrO 2 as an ultra-high-performance
produced through electrospinning of 4 ml DMF solution of 0.4 g PVP with the addition of 0.36 g
 Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information (ESI) Encapsulating V 2 O 5 into Carbon
Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information (ESI) Encapsulating V 2 O 5 into Carbon
Oxides for High Performance Lithium-Ion Battery Anodes
 Bacteria Absorption-Based Mn 2 P 2 O 7 -Carbon @ Reduced Graphene Oxides for High Performance Lithium-Ion Battery Anodes Yuhua Yang, 1 Bin Wang, 1,2 Jingyi Zhu, 3 Jun Zhou, 1 Zhi Xu, 1,4 Ling Fan, 1 Jian
Bacteria Absorption-Based Mn 2 P 2 O 7 -Carbon @ Reduced Graphene Oxides for High Performance Lithium-Ion Battery Anodes Yuhua Yang, 1 Bin Wang, 1,2 Jingyi Zhu, 3 Jun Zhou, 1 Zhi Xu, 1,4 Ling Fan, 1 Jian
components and inorganic Li salts from PST-90. When contacting with Li metal, PST-
 Supplementary Figure 1. Proposed mechanism for the formation of organic components and inorganic Li salts from PST-90. When contacting with Li metal, PST- 1 90 first generates high order organopolysulfide
Supplementary Figure 1. Proposed mechanism for the formation of organic components and inorganic Li salts from PST-90. When contacting with Li metal, PST- 1 90 first generates high order organopolysulfide
School of Materials Science and Engineering, Shanghai Jiao Tong University, Shanghai , PR China
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry
Investigation of anode materials for lithium-ion batteries
 University of Wollongong Thesis Collections University of Wollongong Thesis Collection University of Wollongong Year 2006 Investigation of anode materials for lithium-ion batteries Ling Yuan University
University of Wollongong Thesis Collections University of Wollongong Thesis Collection University of Wollongong Year 2006 Investigation of anode materials for lithium-ion batteries Ling Yuan University
Synthesis of Si-Sb-ZnO Composites as High- Performance Anodes for Lithium-ion Batteries
 Li et al. Nanoscale Research Letters (2015) 10:414 DOI 10.1186/s11671-015-1128-4 NANO EXPRESS Synthesis of Si-Sb-ZnO Composites as High- Performance Anodes for Lithium-ion Batteries Yongliang Li, Liang
Li et al. Nanoscale Research Letters (2015) 10:414 DOI 10.1186/s11671-015-1128-4 NANO EXPRESS Synthesis of Si-Sb-ZnO Composites as High- Performance Anodes for Lithium-ion Batteries Yongliang Li, Liang
Lithium Batteries with Nearly Maximum Metal. Storage Supporting Information
 Lithium Batteries with Nearly Maximum Metal Storage Supporting Information Abdul-Rahman O. Raji,, Rodrigo Villegas Salvatierra,, Nam Dong Kim, Xiujun Fan, Yilun Li, Gladys A. L. Silva, Junwei Sha and James
Lithium Batteries with Nearly Maximum Metal Storage Supporting Information Abdul-Rahman O. Raji,, Rodrigo Villegas Salvatierra,, Nam Dong Kim, Xiujun Fan, Yilun Li, Gladys A. L. Silva, Junwei Sha and James
Supporting Information. Kirkendall Diffusion, and their Electrochemical Properties for use in Lithium-ion
 Supporting Information Preparation of Hollow Fe 2 O 3 Nanorods and Nanospheres by Nanoscale Kirkendall Diffusion, and their Electrochemical Properties for use in Lithium-ion Batteries Jung Sang Cho 1,2,
Supporting Information Preparation of Hollow Fe 2 O 3 Nanorods and Nanospheres by Nanoscale Kirkendall Diffusion, and their Electrochemical Properties for use in Lithium-ion Batteries Jung Sang Cho 1,2,
Driven by emerging applications such as electric vehicles, In Situ TEM of Two-Phase Lithiation of Amorphous Silicon Nanospheres
 pubs.acs.org/nanolett In Situ TEM of Two-Phase Lithiation of Amorphous Silicon Nanospheres Matthew T. McDowell, Seok Woo Lee, Justin T. Harris, Brian A. Korgel, Chongmin Wang, William D. Nix, and Yi Cui*,,
pubs.acs.org/nanolett In Situ TEM of Two-Phase Lithiation of Amorphous Silicon Nanospheres Matthew T. McDowell, Seok Woo Lee, Justin T. Harris, Brian A. Korgel, Chongmin Wang, William D. Nix, and Yi Cui*,,
Accumulation (%) Amount (%) Particle Size 0.1
 100 10 Amount (%) 5 50 Accumulation (%) 0 0.1 1 Particle Size (µm) 10 0 Supplementary Figure 1. The particle size distribution of W-15 at% Cr after 20 hours milling. Supplementary Figure 2. a,b, X-ray
100 10 Amount (%) 5 50 Accumulation (%) 0 0.1 1 Particle Size (µm) 10 0 Supplementary Figure 1. The particle size distribution of W-15 at% Cr after 20 hours milling. Supplementary Figure 2. a,b, X-ray
Preparation and characterization of Co BaTiO 3 nano-composite films by the pulsed laser deposition
 Journal of Crystal Growth 289 (26) 48 413 www.elsevier.com/locate/jcrysgro Preparation and characterization of Co BaTiO 3 nano-composite films by the pulsed laser deposition Wu Weidong a,b,, He Yingjie
Journal of Crystal Growth 289 (26) 48 413 www.elsevier.com/locate/jcrysgro Preparation and characterization of Co BaTiO 3 nano-composite films by the pulsed laser deposition Wu Weidong a,b,, He Yingjie
High Rate and Durable, Binder Free Anode Based on Silicon Loaded MoO 3 Nanoplatelets
 Supplementary Information High Rate and Durable, Binder Free Anode Based on Silicon Loaded O 3 Nanoplatelets Alejandro Martinez-Garcia, Arjun Kumar Thapa,Ruvini Dharmadasa,, Tu Q. Nguyen, Jacek Jasinski,
Supplementary Information High Rate and Durable, Binder Free Anode Based on Silicon Loaded O 3 Nanoplatelets Alejandro Martinez-Garcia, Arjun Kumar Thapa,Ruvini Dharmadasa,, Tu Q. Nguyen, Jacek Jasinski,
Energy & Environmental Science
 Energy & Environmental Science PAPER View Article Online View Journal View Issue Cite this: Energy Environ. Sci., 2018, 11, 669 Received 24th January 2018, Accepted 29th January 2018 DOI: 10.1039/c8ee00239h
Energy & Environmental Science PAPER View Article Online View Journal View Issue Cite this: Energy Environ. Sci., 2018, 11, 669 Received 24th January 2018, Accepted 29th January 2018 DOI: 10.1039/c8ee00239h
Safe, Inexpensive, Long Life, High Power and Efficiency Batteries For Grid Scale Energy Storage Applications
 Safe, Inexpensive, Long Life, High Power and Efficiency Batteries For Grid Scale Energy Storage Applications Investigators Yi Cui, Associate Professor; Robert Huggins, Professor; Mauro Pasta, Postdoctoral
Safe, Inexpensive, Long Life, High Power and Efficiency Batteries For Grid Scale Energy Storage Applications Investigators Yi Cui, Associate Professor; Robert Huggins, Professor; Mauro Pasta, Postdoctoral
Supplementary information. performance Li-ion battery
 Supplementary information The investigation of Ni(OH) 2 /Ni as anode for high performance Li-ion battery Shibing Ni a, Xiaohu Lv a, Tao Li a, Xuelin Yang a,and Lulu Zhang a College of Mechanical and Material
Supplementary information The investigation of Ni(OH) 2 /Ni as anode for high performance Li-ion battery Shibing Ni a, Xiaohu Lv a, Tao Li a, Xuelin Yang a,and Lulu Zhang a College of Mechanical and Material
Sb C Nanofibers with Long Cycle Life as an Anode Material for High-performance Sodium-ion Batteries
 Supplementary Material (ESI) for Energy & Environmental Science Sb C Nanofibers with Long Cycle Life as an Anode Material for High-performance Sodium-ion Batteries Lin Wu, a Xiaohong Hu, b* Jiangfeng Qian,
Supplementary Material (ESI) for Energy & Environmental Science Sb C Nanofibers with Long Cycle Life as an Anode Material for High-performance Sodium-ion Batteries Lin Wu, a Xiaohong Hu, b* Jiangfeng Qian,
Supporting Information
 Supporting Information Vapor-Phase Atomic-Controllable Growth of Amorphous Li 2 S for High-Performance Lithium-Sulfur Batteries Xiangbo Meng, David J. Comstock, Timothy T. Fister, and Jeffrey W. Elam *
Supporting Information Vapor-Phase Atomic-Controllable Growth of Amorphous Li 2 S for High-Performance Lithium-Sulfur Batteries Xiangbo Meng, David J. Comstock, Timothy T. Fister, and Jeffrey W. Elam *
Supporting Information
 - 1 - Supporting Information In-situ TEM investigation of congruent phase transition and structural evolution of nanostructured silicon/carbon anode for lithium ion batteries Chong-Min Wang 1*, Xiaolin
- 1 - Supporting Information In-situ TEM investigation of congruent phase transition and structural evolution of nanostructured silicon/carbon anode for lithium ion batteries Chong-Min Wang 1*, Xiaolin
Highly thermally conductive and electrically insulating polymer nanocomposites with boron nitride nanosheet/ionic liquid complexes
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2017 Supplementary Information Highly thermally conductive and electrically insulating polymer nanocomposites
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2017 Supplementary Information Highly thermally conductive and electrically insulating polymer nanocomposites
In-situ Study of Solid Electrolyte Interphase on Silicon Electrodes using PeakForce Tapping Mode AFM in Glove-box
 General Motors R&D BROWN UNIVERSITY In-situ Study of Solid Electrolyte Interphase on Silicon Electrodes using PeakForce Tapping Mode AFM in Glove-box A. Tokranov 1, X. Xiao 2, C. Li 3, S. Minne 3 and B.
General Motors R&D BROWN UNIVERSITY In-situ Study of Solid Electrolyte Interphase on Silicon Electrodes using PeakForce Tapping Mode AFM in Glove-box A. Tokranov 1, X. Xiao 2, C. Li 3, S. Minne 3 and B.
Lithium ion batteries (LIBs) are widely
 Size-Dependent Fracture of Silicon Nanoparticles During Lithiation Xiao Hua Liu, Li Zhong, Shan Huang, Scott X. Mao, Ting Zhu,, * and Jian Yu Huang, * Center for Integrated Nanotechnologies (CINT), Sandia
Size-Dependent Fracture of Silicon Nanoparticles During Lithiation Xiao Hua Liu, Li Zhong, Shan Huang, Scott X. Mao, Ting Zhu,, * and Jian Yu Huang, * Center for Integrated Nanotechnologies (CINT), Sandia
High energy all-solid-state lithium batteries with
 Supporting Information High energy all-solid-state lithium batteries with ultralong cycle life Xiayin Yao, Deng Liu, Chunsheng Wang, Peng Long, Gang Peng, Yong-Sheng Hu, *, Hong Li, Liquan Chen, and Xiaoxiong
Supporting Information High energy all-solid-state lithium batteries with ultralong cycle life Xiayin Yao, Deng Liu, Chunsheng Wang, Peng Long, Gang Peng, Yong-Sheng Hu, *, Hong Li, Liquan Chen, and Xiaoxiong
Elmira Memarzadeh Lotfabad. A thesis submitted in partial fulfillment of the requirements for the degree of. Doctor of Philosophy
 Silicon and Carbon-Based Anode Materials for Lithium and Sodium-Ion Rechargeable Batteries by Elmira Memarzadeh Lotfabad A thesis submitted in partial fulfillment of the requirements for the degree of
Silicon and Carbon-Based Anode Materials for Lithium and Sodium-Ion Rechargeable Batteries by Elmira Memarzadeh Lotfabad A thesis submitted in partial fulfillment of the requirements for the degree of
Amorphous and Nanocrystalline Mg 2 Si Thin Film Electrodes
 Manuscript# IMLB-070, LBNL-52142 Revised to Journal of Power Sources (Dec 13, 2002) Amorphous and Nanocrystalline Mg 2 Si Thin Film Electrodes Seung-Wan Song, a Kathryn A. Striebel, a,z Xiangyun Song a
Manuscript# IMLB-070, LBNL-52142 Revised to Journal of Power Sources (Dec 13, 2002) Amorphous and Nanocrystalline Mg 2 Si Thin Film Electrodes Seung-Wan Song, a Kathryn A. Striebel, a,z Xiangyun Song a
EFFECT OF THE PITCH-BASED CARBON ANODE ON THE IRREVERSIBLE CAPACITY OF LITHIUM-ION SECONDARY BATTERY
 EFFECT OF THE PITCH-BASED CARBON ANODE ON THE IRREVERSIBLE CAPACITY OF LITHIUM-ION SECONDARY BATTERY Weiming Lu and D.D.L. Chung Composite Materials Research Laboratory University at Buffalo The State
EFFECT OF THE PITCH-BASED CARBON ANODE ON THE IRREVERSIBLE CAPACITY OF LITHIUM-ION SECONDARY BATTERY Weiming Lu and D.D.L. Chung Composite Materials Research Laboratory University at Buffalo The State
Supporting Information
 Supporting Information Designing hybrid NiP 2/NiO nanorod arrays for efficient alkaline hydrogen evolution Meng-Ying Wu, Peng-Fei Da, Tong Zhang, Jing Mao,*, Hui Liu,*, and Tao Ling,*, Key Laboratory for
Supporting Information Designing hybrid NiP 2/NiO nanorod arrays for efficient alkaline hydrogen evolution Meng-Ying Wu, Peng-Fei Da, Tong Zhang, Jing Mao,*, Hui Liu,*, and Tao Ling,*, Key Laboratory for
State Key Laboratory of Advanced Electromagnetic Engineering and Technology, School of
 Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2018 Electrocatalysis of polysulfide conversion by conductive RuO 2 Nano Dots for lithium sulfur batteries
Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2018 Electrocatalysis of polysulfide conversion by conductive RuO 2 Nano Dots for lithium sulfur batteries
Application Note. 4D Study of Silicon Anode Volumetric Changes in a Coin Cell Battery using X-ray Microscopy
 4D Study of Silicon Anode Volumetric Changes in a Coin Cell Battery using X-ray Microscopy 4D Study of Silicon Anode Volumetric Changes in a Coin Cell Battery using X-ray Microscopy Authors: Dr. Claus
4D Study of Silicon Anode Volumetric Changes in a Coin Cell Battery using X-ray Microscopy 4D Study of Silicon Anode Volumetric Changes in a Coin Cell Battery using X-ray Microscopy Authors: Dr. Claus
Disket-Nanorings of K 2 Ti 6 O 13 Formed by Self-Spiraling of a Nanobelt
 7547 2008, 112, 7547 7551 Published on Web 04/23/2008 Disket-Nanorings of K 2 Ti 6 O 13 Formed by Self-Spiraling of a Nanobelt Cheng-Yan Xu,,, Yu-Zi Liu,, Liang Zhen, and Zhong Lin Wang*, School of Materials
7547 2008, 112, 7547 7551 Published on Web 04/23/2008 Disket-Nanorings of K 2 Ti 6 O 13 Formed by Self-Spiraling of a Nanobelt Cheng-Yan Xu,,, Yu-Zi Liu,, Liang Zhen, and Zhong Lin Wang*, School of Materials
Dramatically enhanced reversibility of Li 2 O in SnO 2 -based electrodes: the effect of nanostructure on high initial reversible capacity
 Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2015 Electronic supplementary information for Dramatically enhanced reversibility
Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2015 Electronic supplementary information for Dramatically enhanced reversibility
SUPPORTING INFORMATION: Cerium Oxide Nanocrystal Embedded Bimodal. Micro-Mesoporous Nitrogen-Rich Carbon Nanospheres as
 SUPPORTING INFORMATION: Cerium Oxide Nanocrystal Embedded Bimodal Micro-Mesoporous Nitrogen-Rich Carbon Nanospheres as Effective Sulfur Host for Lithium-Sulfur Batteries Lianbo Ma, a Renpeng Chen, a Guoyin
SUPPORTING INFORMATION: Cerium Oxide Nanocrystal Embedded Bimodal Micro-Mesoporous Nitrogen-Rich Carbon Nanospheres as Effective Sulfur Host for Lithium-Sulfur Batteries Lianbo Ma, a Renpeng Chen, a Guoyin
FAILURE MECHANISMS OF NANO SILICON ANODES: AN ELECTRODE POROSITY EVOLUTION MODEL
 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2014 Supplementary data for : FAILURE MECHANISMS OF NANO SILICON ANODES: AN ELECTRODE
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2014 Supplementary data for : FAILURE MECHANISMS OF NANO SILICON ANODES: AN ELECTRODE
Supporting Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Supporting Information Unveiling BiVO 4 Nanorods as a Novel Anode Material
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Supporting Information Unveiling BiVO 4 Nanorods as a Novel Anode Material
Intercalation of Bi nanoparticles into graphite enables ultrafast. and ultra-stable anode material for Sodium-ion
 Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information Intercalation of Bi nanoparticles into
Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information Intercalation of Bi nanoparticles into
Morphology controlled synthesis of monodispersed manganese. sulfide nanocrystals and their primary application for supercapacitor
 Electronic Supplementary Material (ESI) for Chemical Communications. This journal is The Royal Society of Chemistry 2015 Morphology controlled synthesis of monodispersed manganese sulfide nanocrystals
Electronic Supplementary Material (ESI) for Chemical Communications. This journal is The Royal Society of Chemistry 2015 Morphology controlled synthesis of monodispersed manganese sulfide nanocrystals
Graphene/Fe 3 O Quaternary Nanocomposites: Synthesis and Excellent Electromagnetic Absorption Properties
 Graphene/Fe 3 O 4 @Fe/ZnO Quaternary Nanocomposites: Synthesis and Excellent Electromagnetic Absorption Properties Yu Lan Ren, Hong Yu Wu, Ming Ming Lu, Yu Jin Chen, *, Chun Ling Zhu, # Peng Gao *, # Mao
Graphene/Fe 3 O 4 @Fe/ZnO Quaternary Nanocomposites: Synthesis and Excellent Electromagnetic Absorption Properties Yu Lan Ren, Hong Yu Wu, Ming Ming Lu, Yu Jin Chen, *, Chun Ling Zhu, # Peng Gao *, # Mao
Ultra-stretchable, sensitive and durable strain sensors based on. polydopamine encapsulated carbon nanotubes/elastic bands
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry C. This journal is The Royal Society of Chemistry 2018 Ultra-stretchable, sensitive and durable strain sensors based on polydopamine
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry C. This journal is The Royal Society of Chemistry 2018 Ultra-stretchable, sensitive and durable strain sensors based on polydopamine
This journal is The Royal Society of Chemistry S 1
 2013 S 1 Thermochemical analysis on the growth of NiAl 2 O 4 rods Sang Sub Kim, a Yong Jung Kwon, b Gunju Sun, a Hyoun Woo Kim,* b and Ping Wu* c a Department of Materials Science and Engineering, Inha
2013 S 1 Thermochemical analysis on the growth of NiAl 2 O 4 rods Sang Sub Kim, a Yong Jung Kwon, b Gunju Sun, a Hyoun Woo Kim,* b and Ping Wu* c a Department of Materials Science and Engineering, Inha
Uniform Carbon Coating on Silicon Nanoparticles by Dynamic CVD Process for Electrochemical Lithium Storage
 pubs.acs.org/iecr Uniform Carbon Coating on Silicon Nanoparticles by Dynamic CVD Process for Electrochemical Lithium Storage Jinglu Yu, Jun Yang,, * Xuejiao Feng, Hao Jia, Jiulin Wang, and Wei Lu School
pubs.acs.org/iecr Uniform Carbon Coating on Silicon Nanoparticles by Dynamic CVD Process for Electrochemical Lithium Storage Jinglu Yu, Jun Yang,, * Xuejiao Feng, Hao Jia, Jiulin Wang, and Wei Lu School
Title containing nano-crystalline. Author(s) Matsumoto, Naoki; Matsumoto, Ryosuk. Citation Journal of Non-Crystalline Solids (
 Title Estimation of shear-banding containing nano-crystalline resista particl Author(s) Matsumoto, Naoki; Matsumoto, Ryosuk Citation Journal of Non-Crystalline Solids ( Issue Date 2009-01 URL http://hdl.handle.net/2433/88965
Title Estimation of shear-banding containing nano-crystalline resista particl Author(s) Matsumoto, Naoki; Matsumoto, Ryosuk Citation Journal of Non-Crystalline Solids ( Issue Date 2009-01 URL http://hdl.handle.net/2433/88965
Advance on non-equilibrium plasma jets
 Advance on non-equilibrium plasma jets XinPei Lu luxinpei@hotmail.com College of EEE, HUST Outline Overview of the status of Cold Plasma Jets Recent Progress on plasma jet research in our lab A single
Advance on non-equilibrium plasma jets XinPei Lu luxinpei@hotmail.com College of EEE, HUST Outline Overview of the status of Cold Plasma Jets Recent Progress on plasma jet research in our lab A single
Investigation of anode materials for lithium-ion batteries
 University of Wollongong Thesis Collections University of Wollongong Thesis Collection University of Wollongong Year 2006 Investigation of anode materials for lithium-ion batteries Ling Yuan University
University of Wollongong Thesis Collections University of Wollongong Thesis Collection University of Wollongong Year 2006 Investigation of anode materials for lithium-ion batteries Ling Yuan University
Directional Amorphization of Boron Carbide Subjected to Laser Shock Compression
 Supporting Information Directional Amorphization of Boron Carbide Subjected to Laser Shock Compression This PDF file contains: Figures S1 to S4 Supplementary Text : 1. Materials and initial characterization
Supporting Information Directional Amorphization of Boron Carbide Subjected to Laser Shock Compression This PDF file contains: Figures S1 to S4 Supplementary Text : 1. Materials and initial characterization
Effect of heat treatment on electrochemical characteristics of spinel lithium titanium oxide
 Korean J. Chem. Eng., 27(1), 91-95 (2010) DOI: 10.1007/s11814-009-0298-0 RAPID COMMUNICATION Effect of heat treatment on electrochemical characteristics of spinel lithium titanium oxide Sung-Chul Hong*,
Korean J. Chem. Eng., 27(1), 91-95 (2010) DOI: 10.1007/s11814-009-0298-0 RAPID COMMUNICATION Effect of heat treatment on electrochemical characteristics of spinel lithium titanium oxide Sung-Chul Hong*,
Self-Healing Wide and Thin Li Metal Anodes Prepared. Using Calendared Li Metal Powder for Improving Cycle
 Supporting Information Self-Healing Wide and Thin Li Metal Anodes Prepared Using Calendared Li Metal Powder for Improving Cycle Life and Rate Capability Dahee Jin, Jeonghun Oh, Alex Friesen, Kyuman Kim,
Supporting Information Self-Healing Wide and Thin Li Metal Anodes Prepared Using Calendared Li Metal Powder for Improving Cycle Life and Rate Capability Dahee Jin, Jeonghun Oh, Alex Friesen, Kyuman Kim,
Synthesis and Electrochemical Properties of CuFeO 2 as Negative Electrodes for Sodium-Ion Batteries
 American Journal of Physical Chemistry 2015; 4(2): 16-20 Published online April 13, 2015 (http://www.sciencepublishinggroup.com/j/ajpc) doi: 10.11648/j.ajpc.20150402.11 ISSN: 2327-2430 (Print); ISSN: 2327-2449
American Journal of Physical Chemistry 2015; 4(2): 16-20 Published online April 13, 2015 (http://www.sciencepublishinggroup.com/j/ajpc) doi: 10.11648/j.ajpc.20150402.11 ISSN: 2327-2430 (Print); ISSN: 2327-2449
