Structure, morphology and corrosion resistance of Ni Mo+PTh composite coatings
|
|
|
- Kerry Sharp
- 5 years ago
- Views:
Transcription
1 Bull. Mater. Sci., Vol. 38, No. 3, June 2015, pp c Indian Academy of Sciences. Structure, morphology and corrosion resistance of Ni Mo+PTh composite coatings J NIEDBAŁA Institute of Non-Ferrous Metals, ul. Sowińskiego 5, Poland MS received 25 July 2014; revised 20 October 2014 Abstract. Ni Mo+PTh composite coatings were prepared from nickel molybdenum galvanic bath with the addition of thiophene (Th) and HClO 4 as result of two processes: induced Ni Mo alloy deposition and PTh polymerization. A scanning electron microscope was used for surface morphology characterization of the coatings. The Scanning Electrochemical Workstation M370 was used to the surface map of the tested composite coatings. The chemical composition of the coatings was determined by the energy-dispersive spectroscopy (EDS) method. It was stated that the surface of the coatings are characterized by the presence of Ni Mo particles and polythiophene agglomerates. Electrochemical corrosion investigations of coatings were carried out in the 5 M KOH solution, using voltammetry and electrochemical impedance spectroscopy (EIS) methods. On the basis of these research works it was found that the composite Ni Mo+PTh coatings are more corrosion resistant in alkaline solution than Ni Mo. The reasons for this are the presence of the polymer on the surface of the coatings and a decrease of corrosion active surface area of the coatings. Keywords. Ni Mo+PTh composite coatings; electropolymerization; electrodeposition; corrosion resistance. 1. Introduction The polymer built-in into the metallic matrix changed the surface morphology of the layer obtained in the electrolytic process. 1 9 The developed surface of the electrolytic layer may be smoothened after polymer building (most often in pores). The polymer particles located on the surface can change surface density of the electrons in the metallic matrix. 2,5 9 The composite coatings containing a metallic matrix and polymers may be produced as a result of (a) cathodic deposition metallic matrix and polymer obtained in cathodic polymerization of monomer, (b) the alternatively deposition metallic matrix on the cathode and electropolymerization and deposition polymer in the anodic process and (c) building polymer particles during matrix deposition. 1,3 11 They are used for different purposes. 10,12 15 It was found that the addition of polymers may improve some properties of the obtained coatings, e.g., the nickel coatings exhibited higher microhardness compared with those prepared using pure nickel. 1 Electrochemical properties of nickel molybdenum coatings are well known. Their wide application is a result of specific properties, exhibit good corrosion resistance in aggressive solutions and also high catalytic activity for many electrochemical processes. 2,16 19 Author for correspondence (jolantan@imn.gliwice.pl) Therefore in this study nickel molybdenum alloys are used as matrix in composite coatings, containing additional polymer component as polythiophene. For determination of the influence the polythiophene in the composite coatings on the corrosion resistance, these coatings were compared with nickel molybdenum coatings. The purpose of this work was to evaluate the suitability of Ni Mo+PTh composite coatings as electrode materials in corrosion resistance investigations in an alkaline solution, with respect to their surface morphology and chemical and phase composition. 2. Experimental Electrolytical coatings with polythiophene (PTh) were prepared from a mixture of two solutions: (I) containing (mol dm 3 ): 7,8 Na 2 MoO ; NiSO ; Na 3 C 6 H 5 O ; ph and (II) containing (mol dm 3 ):HClO and thiophene (Th) C 4 H 4 S (99.9% Aldrich) 0.1. Reagents of analytical purity and twice distilled water were used for the solution. All components of (I) bath were solubilized separately in small volume of distilled water. Ni Mo+PTh coatings were electrodeposited from a mixture containing solutions (I) and (II) in proportion 3:1. Thiophene was freshly distilled and next solubilized in HClO 4. The coatings on a steel substrate (St3S steel, 4 cm 2 plate), preliminarily polished and then etched chemically in HCl solution (v/v 1:1) for 5 min and electrochemically in a 0.8 mol dm 3 solution of sodium gluconate C 6 H 11 NaO 7. The other side of a plate was covered with non-conducting, chemically resistant adhesive. During deposition process the 695
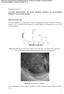
2 696 J Niedbała bath was stirred mechanically (250 rev min 1 ). Temperature of the bath was 298 K and ph was 6 7. The Ni Mo+PTh coatings were deposited under galvanostatic conditions at the current densities of j = 100 (t = 30 min) and 200 ma cm 2 (t = 15 min). Comparative tests were carried out for Ni Mo alloy containing comparable amount of Mo (21.7%). The surface morphology of the coatings was examined using JSM-648 electron microscope, and surface images were recorded at the accelerating voltage of 15 kv. The Scanning Electrochemical Workstation M370 was used to the surface map of the tested composite coatings. The scanning Kelvin probe (SKP) is a non-contact, non-destructive instrument designed to measure the surface work function difference between conducting, coated or semi-conducting materials and a metallic probe. The technique operates using a vibrating capacitance probe, and through a swept backing potential, the work function difference is measured between the scanning probe reference tip and sample surface. The work function can be directly correlated to the surface condition. Using the formula: d 0 = (E k E)εSA 0 ω cos(ωt) IA 0 sin(ωt) 2 /I the distance between the microelectrode and the scanned surface can be determined. The contents of Ni, Mo and C in the coatings were determined by the energydispersive spectroscopy (EDS) method. Electrochemical corrosion resistance investigations were conducted on the coatings in a three-electrode cell, using potentiodynamic and electrochemical impedance spectroscopy (EIS) methods. These measurements were carried out in a 5 M KOH solution, using an Autolab R /PGSTST20 (Eco Chemie B.V., the Netherlands) electrochemical system. Geometric surface area of all electrodes was 1 cm 2.The auxiliary electrode was a platinum mesh and the reference electrode was of the type Hg/HgO/6 M KOH. Open circuit potentials of the coatings were determined using a range of ±0.150 V from the determined value and the recorded potentiodynamic curve with rate v = V min 1. Values of the corrosion potentials and the corrosion currents were determined by the Stern method. The electrochemical impedance measurements were performed at the corrosion potential. In these measurements the amplitude of the ac signal was V. A frequency range from 10 khz to 0.1 Hz was covered with 10 points per decade. All electrochemical investigations were made at 293 K. 3. Results and discussion The Ni Mo+PTh coatings show good adhesion to the substrate and no internal stresses causing their defoliation are observed. The Ni Mo+PTh coatings are characterized by dark-grey, graphite colour. The surface is mat like and porous. It was found that the structure of the Ni Mo coatings is relatively regular with the island character 5 7 (figure 1a). In Ni Mo+PTh coatings the polymer particles are uniformly embedded into the Ni Mo matrix and built into the composite matrix 7,8 (figure 1b). It may be assumed that the nickel molybdenum inductive electrodepositing process and the electropolymerization/electrodeposition process of polymer proceeds parallel and independently. Polymer embedded into the Ni Mo matrix changes the structure. The results show that the deposition process of Ni Mo is different near the deposited polymer. With the grain size of the alloy can be assumed that the process of formation of Ni Mo occurs more easily in the vicinity of the polymer. In these areas, near the polythiophene, the Ni Mo grains are greater (figure 1b). On the surface maps Ni Mo+PTh and Ni Mo coatings was clearly visible differences between character the surface coating with built-in polymer and surface alloy coatings (figure 2). Alloy coatings are less developed and have greater regularity in comparison with the composite coatings with PTh (figure 2a). The thickness of Ni Mo coatings ranges between 30 and 60 µm. Surface of the composite coatings with polymer has a much more developed and porous character. However, comparing the obtained maps with surface morphology, should be noted, that on the maps was confirmed lack of cracks and faults on the surface (figure 2b). The thickness of Ni Mo+PTh coatings varies between 70 and 120 µm. Figure 1. Surface morphology of (a)ni Mo 5 7 and (b) Ni Mo+PTh. 7,8
3 Surface morphology characterization of Ni Mo+PTh coatings 697 Figure 2. coatings. Surface map of the Scanning Kelvin Probe signal recorded for (a)ni Mo and (b)ni Mo+PTh composite Table 1. Chemical composition and corrosion parameters of the Ni Mo and Ni Mo+PTh coatings. Corrosion rate Type of coatings Ni (%) Mo (%) C (%) S (%) PTh ca. (%) j cor (µa cm 2 ) E cor /V R p (k cm 2 ) (mm per year) Ni Mo 78.3 ± ± Ni Mo+PTh ± ± ± ± Ni Mo+PTh ± ± ± ± The chemical analysis of the Ni Mo+PTh coatings was determined by the EDS method (table 1). They showed that for Ni Mo+PTh100 the content of the carbon was 2.8% and for the Ni Mo+PTh200 was almost 2.1 higher (4.9%). It was found that analogical dependence was for sulphur content in the coatings Ni Mo+PTh %S, Ni Mo+PTh %S (table 1). On the basis of the C and S content in the coatings were made calculations of the polythiophene content in Ni Mo+PTh coatings. They showed that it ranged about 4.9% for Ni Mo+PTh100 and 8.6% for Ni Mo+PTh200 the nickel content in the coatings decrease, whereas molybdenum content insignificant increase with the increase in the deposition current density j (table 1). Values of the corrosion parameters were determined from measured dependencies of the j = f(e) behaviour. It was found that for the composite coatings with polythiophene the value of the corrosion current is lower and the value of the corrosion potential is more positive compared with the corresponding values of the Ni Mo coatings (figure 3 and table 1). This suggests greater resistance of the Ni Mo+PTh coatings in alkaline solutions. The results of corrosion tests show that the lowest corrosion rate (0.022 mm per year) exhibit Ni Mo+8.6%PTh coatings (table 1). This value is lower than corrosion rate in case of composite coating with a lower content of polythiophene and Ni Mo alloy. The corrosion current density for this coating is equal µa cm 2 (figure 3, curve 1 and table 1), so this layer could be recommended as protection cover. Corrosion potential of Ni Mo+PTh coatings, with increase contents of polythiophene changes from to V (figure 3). Value of Figure 3. Dependences of log j = f (E) for the (1) Ni Mo+8.6% PTh, (2) Mo+4.9%PTh and (3) Ni 21.7%Mo. potential Ni Mo coatings containing comparable content of Mo is equal V (figure 3). Increase of polythiophene contents in coatings, causes decrease of corrosion current (table 1). Result of this change of corrosion current is increase of polarization resistance from 18.8 to 19.7 k cm 2 for Ni Mo+8.6%PTh. The polarization resistance for Ni Mo coatings was less from investigation coatings (8.6 k cm 2 ). Although the value of potential corrosion was more positive for coatings with less content of polythiophene, the others corrosion parameters (corrosion current and polarization resistance) show the better corrosion resistance for Ni Mo+8.6%PTh coatings (table 1). The results of the EIS investigations were analysed in the form Nyquist (Z = f(z )) (figure 4a c) and Bode (log Z =f (log ω)) and φ = f (log ω) diagrams (figure 5a and b), where Z =((Z ) 2 + (Z ) 2 ) 1/2 and φ = arctangent
4 698 J Niedbała Figure 4. Dependences of Z = f (Z )forthe(a) Ni 21.7%Mo, (b)ni Mo+4.9%PTh and (c) Ni Mo+8.6%PTh coatings. Figure 5. Dependences of log Z = f (log w)for(a)ni 21.7%Mo ( ), Ni Mo+4.9%PTh ( ), Ni Mo+8.6%PTh ( ) andf = f (log w) (b)forni 21.7%Mo ( ), Ni Mo+4.9%PTh ( ), Ni Mo+8.6%PTh ( ).
5 Surface morphology characterization of Ni Mo+PTh coatings 699 Table 2. Corrosion parameters of Ni Mo and Ni Mo+PTh coatings, calculated from EIS method. Type of coatings C dl (µfcm 2 ) R f R ct ( cm 2 ) Ni 21.7%Mo Ni 20.2%Mo+4.9%PTh Ni 23.5%Mo+8.6%PTh (Z /Z ). These investigations confirmed the diversified characteristics of the impedance components (real Z and imaginary Z ) in the alkaline solution. The Nyquist diagram for the Ni 21.7%Mo, Ni 20.2%Mo+4.9%PTh and Ni 23.5%Mo+8.6%PTh composite coatings is characterized by a straight line in the whole range of frequencies (suggesting a diffusion mechanism for the corrosion process) (figure 4a c). It was found that the coatings with 8.6% PTh (figure 4c) are characterized by higher value of the impedance module and phase angle compared with those for the Ni Mo+4.9%PTh and Ni 21.7%Mo coatings (figure 4a and b). Moreover, the dependence φ = f (log ω) forthe coatings with 8.6% PTh (figure 5b) shows a wider range of independence of the phase angle value from the logarithm of angular frequency compared with the Ni Mo+4.9%PTh and Ni Mo coatings (figure 5a). This wider range of independence of φ = f (log ω), implies that the coating is more corrosion resistant (figure 5). For the examined coatings impedance Nyquist diagram (figure 4a c), a semicircle at the high frequencies and linear dependence of impedance components (real Z and imaginary Z ) at the low frequencies are characterized. Nyquist diagram shows activation control at the high frequencies point, however at the low frequencies point at diffusion control of corrosion process. It was found that for this dependence of impedance components (Z and Z ) at particular frequencies, value of charge-transfer resistance is not possible to determine, as described in other papers. The Bode dependences also confirm better corrosion resistance for examined coatings with and without polythiophene (figure 5a and b). Proof of this is the value of doublelayer capacitance C dl (table 2). Proportion of the value of capacitance C dl to capacitance of ideal smooth surface of electrolytic nickel (30 µf cm 2 ) gives a value of factor of electrochemical active surface R f in the corrosion process. Little values of R f, point at blocking of corrosion processes, great value of R f, point at activation of corrosion processes on greater surface. The results of the analysis of R f factors show that for the Ni Mo+8.6% PTh coating blocking of corrosion processes is compared to other investigation coatings. The proof of this is greater value of charge-transfer resistance R ct for this coating (table 2). On the basis of these electrochemical investigations it was found that the coatings with 8.6% polythiophene are more corrosion resistant in alkaline solution than the 4.9% PTh and Ni 21.7% Mo coatings. Probably it is a result of the corrosion reaction, which at-first proceeded in polymer grains presented in Ni Mo+PTh composite coatings, because in polythiophene proceed oxidizing-reduction processes. It limited corrosion in the composite Ni Mo matrix. 4. Conclusion It was found that Ni Mo+PTh coatings are more resistant to corrosion in alkaline solutions than the Ni 21.7% Mo coating. The coatings with 8.6% polythiophene show a more positive value of the corrosion potential, a lower value of the corrosion current and also higher value of the polarization resistance than Ni Mo coating. This is caused by the presence of PTh, which decreased active surface corrosion area. In polythiophene proceed oxidizing-reduction processes and these limited corrosion reaction in alloy matrix. Moreover, parameters determined by the EIS method provide a means for estimating the corrosion resistance of coatings, and confirm the results obtained from potentiodynamic measurements. References 1. Hamid Z A and Ghayad J M 2002 Mater. Lett Karolus M, Niedbała J, Rówiński E, Łągiewka E and Budniok A 2006 J. Achiev. Mater. Manufact. Eng Niedbała J, Budniok A and Łągiewka E 2006 Mater. Sci. Forum Niedbała J, Budniok A and Łągiewka E 2007 Inż. Mater. (Mater. Eng.) Niedbała J, Budniok A and Łągiewka E 2008 Thin Solid Films Niedbała J 2008 Using carbon nanomaterials in clean-energy hydrogen systems, Series: NATO science for peace and security series, Subseries: NATO Science for Peace and Security Series C: Environmental Security, XXXIV, Hardcover, Springer ISBN: Niedbała J 2009 Mater. Chem. Phys Niedbała J 2011 Bull. Mater. Sci Rówiński E, Karolus M and Łągiewka E 2007 Solid State Phenom Lindfors T, Bobacka J, Lewen Stam A and Ivaska A 1998 Electrochim. Acta Karolus M, Rówiński E and Łągiewka E 2006 J. Achiev. Mater. Manuf. Eng Fenelon A M and Breslin C B 2002 Electrochim. Acta Kuwabata S, Masui S, Tomiyori H and Yoneyama H 2000 Electrochim. Acta Levi M D, Gofer Y and Aurbach D 2002 Polym. Adv. Technol Rajagopalan R and Iroh J O 2002 Surf. Eng Birry L and Lasia A 2004 J. Appl. Electrochem Jakšic J M, Vojnovic M V and Krstajic N V 2000 Electrochim. Acta Niedbała J, Popczyk M, Budniok A and Łągiewka E 2004 KSCS 2004 mechanisms of corrosion and corrosion prevention (Helsinki University of Technology) p Niedbała J 2006 Mater. Sci. Forum
ELECTROCHEMICAL SYNTHESIS OF POLYPYRROLE (PPy) and PPy METAL COMPOSITES ON COPPER and INVESTIGATION OF THEIR ANTICORROSIVE PROPERTIES
 ELECTROCHEMICAL SYNTHESIS OF POLYPYRROLE (PPy) and PPy METAL COMPOSITES ON COPPER and INVESTIGATION OF THEIR ANTICORROSIVE PROPERTIES Sibel Zor, Hatice Özkazanç Kocaeli University, Department of Chemistry,
ELECTROCHEMICAL SYNTHESIS OF POLYPYRROLE (PPy) and PPy METAL COMPOSITES ON COPPER and INVESTIGATION OF THEIR ANTICORROSIVE PROPERTIES Sibel Zor, Hatice Özkazanç Kocaeli University, Department of Chemistry,
Corrosion Rate Measurement on C-Steel
 Measurements of corrosion rate on Carbon-steel using Electrochemical (potentiodynamic Polarization, EIS etc.) technique. Corrosion Rate Measurement on C-Steel Abdullah Al Ashraf 1. Introduction: The degradation
Measurements of corrosion rate on Carbon-steel using Electrochemical (potentiodynamic Polarization, EIS etc.) technique. Corrosion Rate Measurement on C-Steel Abdullah Al Ashraf 1. Introduction: The degradation
Electrolytic deposition of Zn-Mn-Mo alloys from a citrate bath
 Indian Journal of Chemical Technology Vol. 17, September 2010, pp. 381-385 Electrolytic deposition of Zn-Mn-Mo alloys from a citrate bath Renu Rastogi* & Archana Pandey Department of Chemistry, Brahmanand
Indian Journal of Chemical Technology Vol. 17, September 2010, pp. 381-385 Electrolytic deposition of Zn-Mn-Mo alloys from a citrate bath Renu Rastogi* & Archana Pandey Department of Chemistry, Brahmanand
Effect of Anodizing Potential on the Surface Morphology and Corrosion Property of AZ31 Magnesium Alloy
 Materials Transactions, Vol. 51, No. 6 (21) pp. 119 to 1113 #21 The Japan Institute of Metals Effect of Anodizing Potential on the Surface Morphology and Corrosion Property of AZ31 Magnesium Alloy S. A.
Materials Transactions, Vol. 51, No. 6 (21) pp. 119 to 1113 #21 The Japan Institute of Metals Effect of Anodizing Potential on the Surface Morphology and Corrosion Property of AZ31 Magnesium Alloy S. A.
J. Mater. Sci. Technol., 2010, 26(11),
 J. Mater. Sci. Technol., 2010, 26(11), 1016-1020. Effects of Current Density on the Microstructure and the Corrosion Resistance of Alumina Coatings Embedded with SiC Nano-particles Produced by Micro-arc
J. Mater. Sci. Technol., 2010, 26(11), 1016-1020. Effects of Current Density on the Microstructure and the Corrosion Resistance of Alumina Coatings Embedded with SiC Nano-particles Produced by Micro-arc
INFLUENCE OF FRICTION STIR WELDING ON CORROSION PROPERTIES OF AW-7020M ALLOY IN SEA WATER
 DOI: 10.1515/adms-2015-0002 K. Dudzik 1, W. Jurczak 2 1 Gdynia Maritime University, Faculty of Marine Engineering, Marine Maintenance Department, Gdynia, Poland 2 Polish Naval Academy, Mechanical Electrical
DOI: 10.1515/adms-2015-0002 K. Dudzik 1, W. Jurczak 2 1 Gdynia Maritime University, Faculty of Marine Engineering, Marine Maintenance Department, Gdynia, Poland 2 Polish Naval Academy, Mechanical Electrical
ELECTRIDEPOSITION AND WEAR BEHAVIOR OF NANO-STRUCTURED Cr-WC COMPOSITE COATINGS FROM A TRIVALENT CHROMIUM BATH
 2nd International Conference on Ultrafine Grained & Nanostructured Materials (UFGNSM) International Journal of Modern Physics: Conference Series Vol. 5 (2012) 737 743 World Scientific Publishing Company
2nd International Conference on Ultrafine Grained & Nanostructured Materials (UFGNSM) International Journal of Modern Physics: Conference Series Vol. 5 (2012) 737 743 World Scientific Publishing Company
Effect of Precorrosion and Temperature on the Formation Rate of Iron Carbonate Film
 7th Pipeline Technology Conference 2012 Effect of Precorrosion and Temperature on the Formation Rate of Iron Carbonate Film Winia Farida a,b, Tor Hemmingsen b, Tonje Berntsen b,c, Patrick Rabindran a a
7th Pipeline Technology Conference 2012 Effect of Precorrosion and Temperature on the Formation Rate of Iron Carbonate Film Winia Farida a,b, Tor Hemmingsen b, Tonje Berntsen b,c, Patrick Rabindran a a
Corrosion and inhibition of Cu-Zn alloys in NaCl solution by using permanganate and phosphate anions
 Int. J. Electrochem. Sci., 2 (2007) 563-571 www.electrochemsci.org Corrosion and inhibition of Cu-Zn alloys in NaCl solution by using permanganate and phosphate anions S. A. M. Refaey 1, *, A. M. Abd El
Int. J. Electrochem. Sci., 2 (2007) 563-571 www.electrochemsci.org Corrosion and inhibition of Cu-Zn alloys in NaCl solution by using permanganate and phosphate anions S. A. M. Refaey 1, *, A. M. Abd El
COMPARATIVE STUDY ON THE CORROSION RESISTANCE OF Zn- 0.2 Pb AND Zn-0.5 Sb GALVANIZED COATINGS. A. Bakhtiari 1, H.R. Asgari 2.
 Association of Metallurgical Engineers of Serbia AMES Scientific paper UDC: 620.193/.197 ; 621.793/.795 ; 667.6 COMPARATIVE STUDY ON THE CORROSION RESISTANCE OF Zn- 0.2 Pb AND Zn-0.5 Sb GALVANIZED COATINGS
Association of Metallurgical Engineers of Serbia AMES Scientific paper UDC: 620.193/.197 ; 621.793/.795 ; 667.6 COMPARATIVE STUDY ON THE CORROSION RESISTANCE OF Zn- 0.2 Pb AND Zn-0.5 Sb GALVANIZED COATINGS
The electrodeposition of Zn-Mo and Zn-Sn-Mo alloys from citrate electrolytes
 Honorata Kazimierczak The electrodeposition of Zn-Mo and Zn-Sn-Mo alloys from citrate electrolytes Supervisor: Assoc. Prof. Piotr Ozga The electrodeposition of Zn-Mo and Zn-Sn-Mo alloys from citrate electrolytes
Honorata Kazimierczak The electrodeposition of Zn-Mo and Zn-Sn-Mo alloys from citrate electrolytes Supervisor: Assoc. Prof. Piotr Ozga The electrodeposition of Zn-Mo and Zn-Sn-Mo alloys from citrate electrolytes
Performance Evaluation of Zinc Deposited Mild Steel in Chloride Medium.
 Int. J. Electrochem. Sci., 6 (2011) 3254-3263 International Journal of ELECTROCHEMICAL SCIENCE www.electrochemsci.org Performance Evaluation of Zinc Deposited Mild Steel in Chloride Medium. Popoola A.P.I
Int. J. Electrochem. Sci., 6 (2011) 3254-3263 International Journal of ELECTROCHEMICAL SCIENCE www.electrochemsci.org Performance Evaluation of Zinc Deposited Mild Steel in Chloride Medium. Popoola A.P.I
Electro catalytic amorphous nickel alloy
 Indian Journal of Chemical Technology Vol. 14, March 2007, pp. 161-165 Electro catalytic amorphous nickel alloy V S Muralidharan Central Electrochemical Research Institute, Karaikudi 630 006, India Received
Indian Journal of Chemical Technology Vol. 14, March 2007, pp. 161-165 Electro catalytic amorphous nickel alloy V S Muralidharan Central Electrochemical Research Institute, Karaikudi 630 006, India Received
NASF SURFACE TECHNOLOGY WHITE PAPERS 80 (7), 1-8 (April 2016) 10th Quarterly Report April - June 2015 AESF Research Project #R-117
 10th Quarterly Report April - June 2015 AESF Research Project #R-117 Electrodeposition of Ni-Fe-Mo-W Alloys by Prof. E.J. Podlaha-Murphy, * and A. Kola Northeastern University Boston, Massachusetts, USA
10th Quarterly Report April - June 2015 AESF Research Project #R-117 Electrodeposition of Ni-Fe-Mo-W Alloys by Prof. E.J. Podlaha-Murphy, * and A. Kola Northeastern University Boston, Massachusetts, USA
Analytical investigation of passive films formed on (UNS N-08028) in Simulated Phosphoric acid at different Temperature
 J. Mater. Environ. Sci. 6 (7) (2015) 1802-1806 Ben Salah et al. Electrochemical and Analytical investigation of passive films formed on (UNS N-08028) in Simulated Phosphoric acid at different Temperature
J. Mater. Environ. Sci. 6 (7) (2015) 1802-1806 Ben Salah et al. Electrochemical and Analytical investigation of passive films formed on (UNS N-08028) in Simulated Phosphoric acid at different Temperature
CORROSION PROPERTIES OF CERMET COATINGS SPRAYED BY HIGH-VELOCITY OXYGEN-FUEL. Dragos UŢU, Iosif HULKA, Viorel-Aurel ŞERBAN, Hannelore FILIPESCU
 Abstract CORROSION PROPERTIES OF CERMET COATINGS SPRAYED BY HIGH-VELOCITY OXYGEN-FUEL Dragos UŢU, Iosif HULKA, Viorel-Aurel ŞERBAN, Hannelore FILIPESCU Politehnica University of Timisoara, Romania, dragosutu@yahoo.com,
Abstract CORROSION PROPERTIES OF CERMET COATINGS SPRAYED BY HIGH-VELOCITY OXYGEN-FUEL Dragos UŢU, Iosif HULKA, Viorel-Aurel ŞERBAN, Hannelore FILIPESCU Politehnica University of Timisoara, Romania, dragosutu@yahoo.com,
Electrochemical study on magnesium anodes in NaCl and CaSO 4 Mg(OH) 2 aqueous solutions
 Electrochimica Acta xxx (2005) xxx xxx Electrochemical study on magnesium anodes in NaCl and CaSO 4 Mg(OH) 2 aqueous solutions Fidel Guadarrama-Muñoz a, Juan Mendoza-Flores a, Ruben Duran-Romero a, J.
Electrochimica Acta xxx (2005) xxx xxx Electrochemical study on magnesium anodes in NaCl and CaSO 4 Mg(OH) 2 aqueous solutions Fidel Guadarrama-Muñoz a, Juan Mendoza-Flores a, Ruben Duran-Romero a, J.
Available online at ScienceDirect. Procedia Engineering 184 (2017 )
 Available online at www.sciencedirect.com ScienceDirect Procedia Engineering 184 (2017 ) 418 422 Advances in Material & Processing Technologies Conference Development of Al-Zn-Cu Alloy for Low Voltage
Available online at www.sciencedirect.com ScienceDirect Procedia Engineering 184 (2017 ) 418 422 Advances in Material & Processing Technologies Conference Development of Al-Zn-Cu Alloy for Low Voltage
Corrosion Behaviour of 18%Ni M250 Grade Maraging Steel under Welded Condition in Hydrochloric Acid Medium
 Portugaliae Electrochimica Acta 2013, 31(1), 21-32 DOI: 10.4152/pea.201301021 PORTUGALIAE ELECTROCHIMICA ACTA ISSN 1647-1571 Corrosion Behaviour of 18%Ni M250 Grade Maraging Steel under Welded Condition
Portugaliae Electrochimica Acta 2013, 31(1), 21-32 DOI: 10.4152/pea.201301021 PORTUGALIAE ELECTROCHIMICA ACTA ISSN 1647-1571 Corrosion Behaviour of 18%Ni M250 Grade Maraging Steel under Welded Condition
Electromagnetic properties of polypyrrole thin film on copper substrate
 Available online at www.scholarsresearchlibrary.com Scholars Research Library Archives of Physics Research, 21, 1 (4): 25-21 (http://scholarsresearchlibrary.com/archive.html) ISSN 976-97 CODEN (USA): APRRC7
Available online at www.scholarsresearchlibrary.com Scholars Research Library Archives of Physics Research, 21, 1 (4): 25-21 (http://scholarsresearchlibrary.com/archive.html) ISSN 976-97 CODEN (USA): APRRC7
Title. Author(s)Tang, Jinwei; Azumi, Kazuhisa. CitationElectrochimica Acta, 56(3): Issue Date Doc URL. Type.
 Title Optimization of pulsed electrodeposition of aluminum Author(s)Tang, Jinwei; Azumi, Kazuhisa CitationElectrochimica Acta, 56(3): 1130-1137 Issue Date 2011-01-01 Doc URL http://hdl.handle.net/2115/45134
Title Optimization of pulsed electrodeposition of aluminum Author(s)Tang, Jinwei; Azumi, Kazuhisa CitationElectrochimica Acta, 56(3): 1130-1137 Issue Date 2011-01-01 Doc URL http://hdl.handle.net/2115/45134
Impedance Monitoring of the Anodic Dissolution of PANi during the V 2 O 5 Deposition Step for the PANi/V 2 O 5 Composite Film Preparation
 179 10.1149/1.3490697 The Electrochemical Society Impedance Monitoring of the Anodic Dissolution of PANi during the V 2 O 5 Deposition Step for the PANi/V 2 O 5 Composite Film Preparation K.-I. Park a,
179 10.1149/1.3490697 The Electrochemical Society Impedance Monitoring of the Anodic Dissolution of PANi during the V 2 O 5 Deposition Step for the PANi/V 2 O 5 Composite Film Preparation K.-I. Park a,
EFFECTS OF CURRENT DENSITY ON SIZE AND SURFACE MORPHOLOGY OF HIGH SPEED DIRECT NANO-CRYSTALLINE NICKEL PLATING ON TITANIUM SURFACE
 EFFECTS OF CURRENT DENSITY ON SIZE AND SURFACE MORPHOLOGY OF HIGH SPEED DIRECT NANO-CRYSTALLINE NICKEL PLATING ON TITANIUM SURFACE Noor Zaimah 1, Azieyanti Nurain 1 and Sakhawat Hussain 2 1 Department
EFFECTS OF CURRENT DENSITY ON SIZE AND SURFACE MORPHOLOGY OF HIGH SPEED DIRECT NANO-CRYSTALLINE NICKEL PLATING ON TITANIUM SURFACE Noor Zaimah 1, Azieyanti Nurain 1 and Sakhawat Hussain 2 1 Department
Effects of Bath Temperature on Electrodeposited Permanent Magnetic Co-Pt-W(P) Films
 2214 Bull. Korean Chem. Soc. 2007, Vol. 28, No. 12 Hongliang Ge et al. Effects of Bath Temperature on Electrodeposited Permanent Magnetic Co-Pt-W(P) Films Hongliang Ge, * Qiong Wu, Guoying Wei, Xinyan
2214 Bull. Korean Chem. Soc. 2007, Vol. 28, No. 12 Hongliang Ge et al. Effects of Bath Temperature on Electrodeposited Permanent Magnetic Co-Pt-W(P) Films Hongliang Ge, * Qiong Wu, Guoying Wei, Xinyan
Sealing Mechanism of Anodic Porous Oxide Films Formed on Aluminum in Lithium Hydroxide Solution
 Proceedings of the 12th International Conference on Aluminium Alloys, September 5-9, 2010, Yokohama, Japan 2010 The Japan Institute of Light Metals pp. 1463-1468 1463 Sealing Mechanism of Anodic Porous
Proceedings of the 12th International Conference on Aluminium Alloys, September 5-9, 2010, Yokohama, Japan 2010 The Japan Institute of Light Metals pp. 1463-1468 1463 Sealing Mechanism of Anodic Porous
Carla Sofia Jorge dos Reis
 OPTIMISATION OF AN ANTICORROSION COATING PROCESS USING THE CATHODIC ELECTRODEPOSITION METHOD IN THE AUTOMOTIVE INDUSTRY Carla Sofia Jorge dos Reis INSTITUTO SUPERIOR TÉCNICO UNIVERSIDADE TÉCNICA DE LISBOA
OPTIMISATION OF AN ANTICORROSION COATING PROCESS USING THE CATHODIC ELECTRODEPOSITION METHOD IN THE AUTOMOTIVE INDUSTRY Carla Sofia Jorge dos Reis INSTITUTO SUPERIOR TÉCNICO UNIVERSIDADE TÉCNICA DE LISBOA
Protective Properties of High Temperature Oxide Films on Ni-based Superalloys in 3.5% NaCl Solution
 J. Mater. Sci. Technol., Vol.23 No.4, 2007 541 Protective Properties of High Temperature Oxide Films on Ni-based Superalloys in 3.5% NaCl Solution Huiping BAI and Fuhui WANG State Key Laboratory for Corrosion
J. Mater. Sci. Technol., Vol.23 No.4, 2007 541 Protective Properties of High Temperature Oxide Films on Ni-based Superalloys in 3.5% NaCl Solution Huiping BAI and Fuhui WANG State Key Laboratory for Corrosion
Galvanic corrosion evaluation of 6061 aluminum coupled to CVD coated stainless steel Elizabeth Sikora and Barbara Shaw 6/9/2016
 SHAW AND ASSOCIATES CONSULTING Galvanic corrosion evaluation of 6061 aluminum coupled to CVD coated stainless steel Elizabeth Sikora and Barbara Shaw 6/9/2016 Evaluation of galvanic corrosion of aluminum
SHAW AND ASSOCIATES CONSULTING Galvanic corrosion evaluation of 6061 aluminum coupled to CVD coated stainless steel Elizabeth Sikora and Barbara Shaw 6/9/2016 Evaluation of galvanic corrosion of aluminum
Progress in Organic Coatings
 Progress in Organic Coatings 65 (2009) 229 236 Contents lists available at ScienceDirect Progress in Organic Coatings journal homepage: www.elsevier.com/locate/porgcoat Formation and characteristics of
Progress in Organic Coatings 65 (2009) 229 236 Contents lists available at ScienceDirect Progress in Organic Coatings journal homepage: www.elsevier.com/locate/porgcoat Formation and characteristics of
Microstructure and mechanical properties of nanocrystalline Ni-Mo protective coatings
 IOP Conference Series: Materials Science and Engineering Microstructure and mechanical properties of nanocrystalline Ni-Mo protective coatings To cite this article: A Bigos et al 2012 IOP Conf. Ser.: Mater.
IOP Conference Series: Materials Science and Engineering Microstructure and mechanical properties of nanocrystalline Ni-Mo protective coatings To cite this article: A Bigos et al 2012 IOP Conf. Ser.: Mater.
Electrochemical and Semiconducting Behaviour of a Brass Alloy in Borax Solutions
 Electrochemical and Semiconducting Behaviour of a Brass Alloy in Borax Solutions A. Fattah-alhosseini, M. Torkaman, E. Karami Faculty of Engineering, Bu-Ali Sina University, Hamedan 65178-38695, Iran Received
Electrochemical and Semiconducting Behaviour of a Brass Alloy in Borax Solutions A. Fattah-alhosseini, M. Torkaman, E. Karami Faculty of Engineering, Bu-Ali Sina University, Hamedan 65178-38695, Iran Received
Corrosion of Nickel-Chromium Alloy in the Molten Mixture LiF-NaF-KF
 Corrosion of Nickel-Chromium Alloy in the Molten Mixture LiF-NaF-KF Vladimír Danielik*, Pavel Fellner, Marta Ambrová, Oldřich Matal a Institute of Inorganic Chemistry, Technology and Materials, Faculty
Corrosion of Nickel-Chromium Alloy in the Molten Mixture LiF-NaF-KF Vladimír Danielik*, Pavel Fellner, Marta Ambrová, Oldřich Matal a Institute of Inorganic Chemistry, Technology and Materials, Faculty
Threshold Chloride Concentration of Stainless Steels in Simulated Concrete Pore Solution
 5th International Conference on Durability of Concrete Structures Jun 30 Jul 1, 2016 Shenzhen University, Shenzhen, Guangdong Province, P.R.China Threshold Chloride Concentration of Stainless Steels in
5th International Conference on Durability of Concrete Structures Jun 30 Jul 1, 2016 Shenzhen University, Shenzhen, Guangdong Province, P.R.China Threshold Chloride Concentration of Stainless Steels in
Electrodeposition and compositional behaviour of Zn-Ni alloy
 Indian Journal of Chemical Technology Vol. 14, May 2007, pp. 246-252 Electrodeposition and compositional behaviour of Zn-Ni alloy V Thangaraj & A Chitharanjan Hegde* Department of Chemistry, National Institute
Indian Journal of Chemical Technology Vol. 14, May 2007, pp. 246-252 Electrodeposition and compositional behaviour of Zn-Ni alloy V Thangaraj & A Chitharanjan Hegde* Department of Chemistry, National Institute
Studies on Alloys and Composites that Undergo Anomalous Codeposition
 Studies on Alloys and Composites that Undergo Anomalous Codeposition Electrochemical of of South Columbia, SC908 09, 1998 Electrochemical Systems Studied FeNi alloys and FeNiSiO composites Electrodeposition
Studies on Alloys and Composites that Undergo Anomalous Codeposition Electrochemical of of South Columbia, SC908 09, 1998 Electrochemical Systems Studied FeNi alloys and FeNiSiO composites Electrodeposition
Three-dimensional NiFe Layered Double Hydroxide Film for Highefficiency
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Three-dimensional NiFe Layered Double Hydroxide Film for Highefficiency Oxygen Evolution Reaction
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Three-dimensional NiFe Layered Double Hydroxide Film for Highefficiency Oxygen Evolution Reaction
Corrosion inhibition of aluminum and aluminum silicon alloys in sodium hydroxide solutions by methyl cellulose
 Corrosion inhibition of aluminum and aluminum silicon alloys in sodium hydroxide solutions by methyl cellulose Salah Eid*, M. Abdallah, E. M. Kamar, A. Y. El-Etre Chemistry department, Faculty of science,
Corrosion inhibition of aluminum and aluminum silicon alloys in sodium hydroxide solutions by methyl cellulose Salah Eid*, M. Abdallah, E. M. Kamar, A. Y. El-Etre Chemistry department, Faculty of science,
ELECTROCHEMICAL AND SPONTANEOUS PASSIVATION OF THE CoCr ALLOY AS CORROSION PROTECTION
 Applied Engineering Letters Vol. 2, No. 1, 43-47 (2017) e-issn: 2466-4847 ELECTROCHEMICAL AND SPONTANEOUS PASSIVATION OF THE CoCr ALLOY AS CORROSION PROTECTION Łukasz Reimann 1 1 Silesian University of
Applied Engineering Letters Vol. 2, No. 1, 43-47 (2017) e-issn: 2466-4847 ELECTROCHEMICAL AND SPONTANEOUS PASSIVATION OF THE CoCr ALLOY AS CORROSION PROTECTION Łukasz Reimann 1 1 Silesian University of
How initial nucleation influences discharge capacities of Li-O 2 cells
 How initial nucleation influences discharge capacities of Li-O 2 cells Ali Rinaldi 1, Olivia Wijaya 1, Denis Yu 2, Harry.E. Hoster 1 1TUM CREATE Centre for Electromobility #10-02 CREATE Tower, Singapore
How initial nucleation influences discharge capacities of Li-O 2 cells Ali Rinaldi 1, Olivia Wijaya 1, Denis Yu 2, Harry.E. Hoster 1 1TUM CREATE Centre for Electromobility #10-02 CREATE Tower, Singapore
ELECTROCHEMICAL IMPEDANCE SPECTROSCOPY ON THERMAL AGEING EVALUATION OF EPOXY COATING CONTAINING ZINC RICH PRIMER
 ELECTROCHEMICAL IMPEDANCE SPECTROSCOPY ON THERMAL AGEING EVALUATION OF EPOXY COATING CONTAINING ZINC RICH PRIMER Zalilah Sharer 1, John Sykes 2 1 UTM-MPRC, Institute for Oil and Gas, Universiti Teknologi
ELECTROCHEMICAL IMPEDANCE SPECTROSCOPY ON THERMAL AGEING EVALUATION OF EPOXY COATING CONTAINING ZINC RICH PRIMER Zalilah Sharer 1, John Sykes 2 1 UTM-MPRC, Institute for Oil and Gas, Universiti Teknologi
CORROSION of Metals CORROSION CORROSION. Outline ISSUES TO ADDRESS... Why does corrosion occur? What metals are most likely to corrode?
 Outline Corrosion - Introduction Corrosion of Metals - e.g. Rusting of iron in water Electrochemical Cell Electrode Potential in Electrochemical Cell Standard Electromotive Force Example Relative Corrosion
Outline Corrosion - Introduction Corrosion of Metals - e.g. Rusting of iron in water Electrochemical Cell Electrode Potential in Electrochemical Cell Standard Electromotive Force Example Relative Corrosion
Preparation of Trivalent Chromium Coating on 6063 Aluminum Alloy Jian-Zhen HUANG 1,a,* and You-Xiong LUO 1,b
 2017 Joint International Conference on Materials Science and Engineering Application (ICMSEA 2017) and International Conference on Mechanics, Civil Engineering and Building Materials (MCEBM 2017) ISBN:
2017 Joint International Conference on Materials Science and Engineering Application (ICMSEA 2017) and International Conference on Mechanics, Civil Engineering and Building Materials (MCEBM 2017) ISBN:
EFFECT OF VOLUME FRACTION OF (Cr,Fe) 7 C 3 CARBIDES ON CORROSION RESISTANCE OF THE Fe-Cr-C HARDFACING ALLOYS AT
 Association of Metallurgical Engineers of Serbia AMES Scientific paper UD: 621.791.5 EFFET OF VOLUME FRATION OF (r,fe) 7 3 ARBIDES ON ORROSION RESISTANE OF THE Fe-r- HARDFAING ALLOYS AT r = 6 Hamed Sabet
Association of Metallurgical Engineers of Serbia AMES Scientific paper UD: 621.791.5 EFFET OF VOLUME FRATION OF (r,fe) 7 3 ARBIDES ON ORROSION RESISTANE OF THE Fe-r- HARDFAING ALLOYS AT r = 6 Hamed Sabet
Mechanical and magnetic properties of nanostructured CoNiP films
 PRAMANA c Indian Academy of Sciences Vol. 67, No. 2 journal of August 2006 physics pp. 341 349 Mechanical and magnetic properties of nanostructured CoNiP films R N EMERSON 1, C JOSEPH KENNADY 2, and S
PRAMANA c Indian Academy of Sciences Vol. 67, No. 2 journal of August 2006 physics pp. 341 349 Mechanical and magnetic properties of nanostructured CoNiP films R N EMERSON 1, C JOSEPH KENNADY 2, and S
Chapter 3. Electrocatalytic Oxidation of Glucose on Copper Oxide Modified Copper Electrode
 Chapter 3 Electrocatalytic Oxidation of Glucose on Copper Oxide Modified Copper Electrode 3. Electrocatalytic Oxidation of Glucose on Copper Oxide Modified Copper Electrode In order to combat the drawbacks
Chapter 3 Electrocatalytic Oxidation of Glucose on Copper Oxide Modified Copper Electrode 3. Electrocatalytic Oxidation of Glucose on Copper Oxide Modified Copper Electrode In order to combat the drawbacks
Characterization of Coatings on Grey Cast Iron Fabricated by Hot-dipping in Pure Al, AlSi11 and AlTi5 Alloys
 A R C H I V E S o f F O U N D R Y E N G I N E E R I N G Published quarterly as the organ of the Foundry Commission of the Polish Academy of Sciences ISSN (1897-3310) Volume 14 Issue 1/2014 85 90 20/1 Characterization
A R C H I V E S o f F O U N D R Y E N G I N E E R I N G Published quarterly as the organ of the Foundry Commission of the Polish Academy of Sciences ISSN (1897-3310) Volume 14 Issue 1/2014 85 90 20/1 Characterization
MXene-Bonded Activated Carbon as a Flexible. Electrode for High-Performance Supercapacitors
 Supporting information MXene-Bonded Activated Carbon as a Flexible Electrode for High-Performance Supercapacitors Lanyong Yu, Longfeng Hu, Babak Anasori, Yi-Tao Liu, Qizhen Zhu, Peng Zhang, Yury Gogotsi,
Supporting information MXene-Bonded Activated Carbon as a Flexible Electrode for High-Performance Supercapacitors Lanyong Yu, Longfeng Hu, Babak Anasori, Yi-Tao Liu, Qizhen Zhu, Peng Zhang, Yury Gogotsi,
A Novel Electrodeposition Process for Plating Zn-Ni-Cd Alloys
 Journal of The Electrochemical Society, 150 2 C81-C88 2003 0013-4651/2003/150 2 /C81/8/$7.00 The Electrochemical Society, Inc. A Novel Electrodeposition Process for Plating Zn-Ni-Cd Alloys Hansung Kim,
Journal of The Electrochemical Society, 150 2 C81-C88 2003 0013-4651/2003/150 2 /C81/8/$7.00 The Electrochemical Society, Inc. A Novel Electrodeposition Process for Plating Zn-Ni-Cd Alloys Hansung Kim,
Supplementary Information.
 Supplementary Information. This file contains additional experimental and analytical information. The organic ionic plastic crystals were synthesised according to the literature procedures. 1-4 Chemicals:
Supplementary Information. This file contains additional experimental and analytical information. The organic ionic plastic crystals were synthesised according to the literature procedures. 1-4 Chemicals:
Acid Extract of Polyalthia Longifolia as a Green Corrosion Inhibitor for Mild Steel in H 2 SO 4 Solution
 IOSR Journal of Applied Chemistry (IOSR-JAC) e-issn: 2278-5736.Volume 8, Issue 2 Ver. II. (Feb. 2015), PP 10-16 www.iosrjournals.org Acid Extract of Polyalthia Longifolia as a Green Corrosion Inhibitor
IOSR Journal of Applied Chemistry (IOSR-JAC) e-issn: 2278-5736.Volume 8, Issue 2 Ver. II. (Feb. 2015), PP 10-16 www.iosrjournals.org Acid Extract of Polyalthia Longifolia as a Green Corrosion Inhibitor
Direct Electroless Silver Plating on Copper Metal from Succinimide Complex Bath Using Imidazole as the Reducing Agent
 Technical Paper Direct Electroless Silver Plating on Copper Metal from Succinimide Complex Bath Using Imidazole as Reducing Agent Hidemi NAWAFUNE*, Keiko SHIROGUCHI**, Shozo MIZUMOTO*, Yasuhito KOHASHI***
Technical Paper Direct Electroless Silver Plating on Copper Metal from Succinimide Complex Bath Using Imidazole as Reducing Agent Hidemi NAWAFUNE*, Keiko SHIROGUCHI**, Shozo MIZUMOTO*, Yasuhito KOHASHI***
Corrosion Protect DLC Coating on Steel and Hastelloy
 Materials Transactions, Vol. 49, No. 6 (2008) pp. 1333 to 1337 #2008 The Japan Institute of Metals Corrosion Protect DLC Coating on Steel and Hastelloy Hironobu Miya and Jie Wang Semiconductor Equipment
Materials Transactions, Vol. 49, No. 6 (2008) pp. 1333 to 1337 #2008 The Japan Institute of Metals Corrosion Protect DLC Coating on Steel and Hastelloy Hironobu Miya and Jie Wang Semiconductor Equipment
New inhibitors for copper corrosion*
 Pure Appl. Chem., Vol. 73, No. 12, pp. 1861 1869, 2001. 2001 IUPAC New inhibitors for copper corrosion* Gy. Vastag 1, E. Szöcs 2, A. Shaban 2, and E. Kálmán 2, 1 University of Novi Sad, Faculty of Natural
Pure Appl. Chem., Vol. 73, No. 12, pp. 1861 1869, 2001. 2001 IUPAC New inhibitors for copper corrosion* Gy. Vastag 1, E. Szöcs 2, A. Shaban 2, and E. Kálmán 2, 1 University of Novi Sad, Faculty of Natural
ELECTROCHEMICAL IMPEDANCE SPECTROSCOPY MEASUREMENTS OF BARRIER COATINGS
 ELECTROCHEMICAL IMPEDANCE SPECTROSCOPY MEASUREMENTS OF BARRIER COATINGS Ken Holyoake, ARMATEC Environmental Ltd, and Jiangnan Yuan, Materials Performance Technologies Ltd SUMMARY Polymer based coatings
ELECTROCHEMICAL IMPEDANCE SPECTROSCOPY MEASUREMENTS OF BARRIER COATINGS Ken Holyoake, ARMATEC Environmental Ltd, and Jiangnan Yuan, Materials Performance Technologies Ltd SUMMARY Polymer based coatings
Electronic supplementary information. Efficient energy storage capabilities promoted by hierarchically MnCo 2 O 4
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Electronic supplementary information Efficient energy storage capabilities promoted by hierarchically
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Electronic supplementary information Efficient energy storage capabilities promoted by hierarchically
ELECTROCHEMICAL REDUCTION OF TITANIUM DIOXIDE THIN FILM IN LiCl-KCl-CaCl 2 EUTECTIC MELT
 ELECTROCHEMICAL REDUCTION OF TITANIUM DIOXIDE THIN FILM IN LiCl-KCl-CaCl 2 EUTECTIC MELT Yasushi Katayama Department of Applied Chemistry, Faculty of Science and Technology, Keio University 3-14-1, Hiyoshi,
ELECTROCHEMICAL REDUCTION OF TITANIUM DIOXIDE THIN FILM IN LiCl-KCl-CaCl 2 EUTECTIC MELT Yasushi Katayama Department of Applied Chemistry, Faculty of Science and Technology, Keio University 3-14-1, Hiyoshi,
The Evaluation of Corrosion Behavior of AISI 347 Stainless Steel to ASTM A335 Low Alloy Steel Dissimilar Welds
 , pp.14-18 The Evaluation of Corrosion Behavior of AISI 347 Stainless Steel to ASTM A335 Low Alloy Steel Dissimilar Welds I. Hajiannia 1, M.Shamanian 2* and M. Kasiri 3 1,3 Department of Materials Engineering,
, pp.14-18 The Evaluation of Corrosion Behavior of AISI 347 Stainless Steel to ASTM A335 Low Alloy Steel Dissimilar Welds I. Hajiannia 1, M.Shamanian 2* and M. Kasiri 3 1,3 Department of Materials Engineering,
What happens if we connect Zn and Pt in HCl solution? Corrosion of platinum (Pt) in HCl. 1. If Zn and Pt are not connected
 Corrosion of platinum (Pt) in HCl Now if we place a piece of Pt in HCl, what will happen? Pt does not corrode does not take part in the electrochemical reaction Pt is a noble metal Pt acts as a reference
Corrosion of platinum (Pt) in HCl Now if we place a piece of Pt in HCl, what will happen? Pt does not corrode does not take part in the electrochemical reaction Pt is a noble metal Pt acts as a reference
Corrosion characteristics of mild steel in aqueous solution of formic acid containing some acetic acid
 Indian Journal of Chemical Technology Vol. 15, March 2008, pp. 174-179 Corrosion characteristics of mild steel in aqueous solution of formic acid containing some acetic acid S K Singh, Ashim K Mukherjee*
Indian Journal of Chemical Technology Vol. 15, March 2008, pp. 174-179 Corrosion characteristics of mild steel in aqueous solution of formic acid containing some acetic acid S K Singh, Ashim K Mukherjee*
Novel concept of rechargeable battery using iron oxide nanorods. anode and nickel hydroxide cathode in aqueous electrolyte
 Supplementary Information for: Novel concept of rechargeable battery using iron oxide nanorods anode and nickel hydroxide cathode in aqueous electrolyte Zhaolin Liu *, Siok Wei Tay and Xu Li Institute
Supplementary Information for: Novel concept of rechargeable battery using iron oxide nanorods anode and nickel hydroxide cathode in aqueous electrolyte Zhaolin Liu *, Siok Wei Tay and Xu Li Institute
The influence of Mg 17 Al 12 phase volume fraction on the corrosion behaviour of AZ91 magnesium alloy. Andrzej Kiełbus* and Grzegorz Moskal
 196 Int. J. Microstructure and Materials Properties, Vol. 4, No. 2, 2009 The influence of Mg 17 Al 12 phase volume fraction on the corrosion behaviour of AZ91 magnesium alloy Andrzej Kiełbus* and Grzegorz
196 Int. J. Microstructure and Materials Properties, Vol. 4, No. 2, 2009 The influence of Mg 17 Al 12 phase volume fraction on the corrosion behaviour of AZ91 magnesium alloy Andrzej Kiełbus* and Grzegorz
IN SITU MONITORING OF REINFORCEMENT CORROSION BY MEANS OF ELECTROCHEMICAL METHODS
 ABSTRACT IN SITU MONITORING OF REINFORCEMENT CORROSION BY MEANS OF ELECTROCHEMICAL METHODS Oskar Klinghoffer, M.Sc. FORCE Institute, Park Allé 345, DK-2605 Brøndby, Denmark The well-known and usually adapted
ABSTRACT IN SITU MONITORING OF REINFORCEMENT CORROSION BY MEANS OF ELECTROCHEMICAL METHODS Oskar Klinghoffer, M.Sc. FORCE Institute, Park Allé 345, DK-2605 Brøndby, Denmark The well-known and usually adapted
POLARIZATION MEASUREMENTS UNDER ATMOSPHERIC CONDITIONS USING A KELVIN PROBE AS A REFERENCE ELECTRODE ABSTRACT
 POLARIZATION MEASUREMENTS UNDER ATMOSPHERIC CONDITIONS USING A KELVIN PROBE AS A REFERENCE ELECTRODE A. de ROOIJ European Space Agency, European Space Technology and Research Centre, Product Assurance
POLARIZATION MEASUREMENTS UNDER ATMOSPHERIC CONDITIONS USING A KELVIN PROBE AS A REFERENCE ELECTRODE A. de ROOIJ European Space Agency, European Space Technology and Research Centre, Product Assurance
Supplementary Materials: A Critical Evaluation of the Influence of the Dark Exchange Current on the Performance of Dye Sensitized Solar Cells
 Supplementary Materials: A Critical Evaluation of the Influence of the Dark Exchange Current on the Performance of Dye Sensitized Solar Cells Rodrigo García Rodríguez, Julio Villanueva Cab, Juan A. Anta
Supplementary Materials: A Critical Evaluation of the Influence of the Dark Exchange Current on the Performance of Dye Sensitized Solar Cells Rodrigo García Rodríguez, Julio Villanueva Cab, Juan A. Anta
Impedance Behavior of LSCF/YDC/LSCF Symmetrical Half Cell Prepared by Plasma Spray
 Impedance Behavior of /YDC/ Symmetrical Half Cell Prepared by Plasma Spray Z. Stoynov 1, D. Vladikova 1, G. Raikova 1*, D. Soysal 2, Z. Ilhan 2, S. Ansar 2 1 Institute of Electrochemistry and Energy Systems
Impedance Behavior of /YDC/ Symmetrical Half Cell Prepared by Plasma Spray Z. Stoynov 1, D. Vladikova 1, G. Raikova 1*, D. Soysal 2, Z. Ilhan 2, S. Ansar 2 1 Institute of Electrochemistry and Energy Systems
Corrosion Resistance of the Ni-Mo Alloy Coatings Related to Coating's Electroplating Parameters
 Int. J. Electrochem. Sci., 10 (2015) 4972-4984 International Journal of ELECTROCHEMICAL SCIENCE www.electrochemsci.org Corrosion Resistance of the Ni-Mo Alloy Coatings Related to Coating's Electroplating
Int. J. Electrochem. Sci., 10 (2015) 4972-4984 International Journal of ELECTROCHEMICAL SCIENCE www.electrochemsci.org Corrosion Resistance of the Ni-Mo Alloy Coatings Related to Coating's Electroplating
METAL FINISHING. (As per revised VTU syllabus: )
 METAL FINISHING (As per revised VTU syllabus: 2015-16) Definition: It is a process in which a specimen metal (article) is coated with another metal or a polymer in order to modify the surface properties
METAL FINISHING (As per revised VTU syllabus: 2015-16) Definition: It is a process in which a specimen metal (article) is coated with another metal or a polymer in order to modify the surface properties
Role of Surface Defect Density on the Corrosion Properties of PVD Coatings
 Role of Surface Defect Density on the Corrosion Properties of PVD Coatings Darja Kek Merl Department of Thin Films and Surfaces Jožef Stefan Institute Jamova 39, 1000 Ljubljana, Slovenia Table of contents
Role of Surface Defect Density on the Corrosion Properties of PVD Coatings Darja Kek Merl Department of Thin Films and Surfaces Jožef Stefan Institute Jamova 39, 1000 Ljubljana, Slovenia Table of contents
Electrodeposition of Palladium Coatings from Iminodiacetate Electrolyte
 American Journal of Analytical Chemistry, 2013, 4, 642-646 Published Online November 2013 (http://www.scirp.org/journal/ajac) http://dx.doi.org/10.4236/ajac.2013.411076 Electrodeposition of Palladium Coatings
American Journal of Analytical Chemistry, 2013, 4, 642-646 Published Online November 2013 (http://www.scirp.org/journal/ajac) http://dx.doi.org/10.4236/ajac.2013.411076 Electrodeposition of Palladium Coatings
Surface Analysis of Electrochromic Switchable Mirror Glass Based on Magnesium-Nickel Thin Film in Accelerated Degradation Test
 Materials Transactions, Vol. 52, No. 3 (2011) pp. 464 to 468 #2011 The Japan Institute of Metals Surface Analysis of Electrochromic Switchable Mirror Glass Based on Magnesium-Nickel Thin Film in Accelerated
Materials Transactions, Vol. 52, No. 3 (2011) pp. 464 to 468 #2011 The Japan Institute of Metals Surface Analysis of Electrochromic Switchable Mirror Glass Based on Magnesium-Nickel Thin Film in Accelerated
Impact of Conductive Polymer Cathode Systems with Tantalum. Capacitors. Ta Ta
 Capacitor Seminar: Conductive Cathodes in ntalum and Aluminum SMD Capacitors For the past fifteen years, the tantalum capacitor has had enormous improvements in ESR because of the replacement of MnO 2
Capacitor Seminar: Conductive Cathodes in ntalum and Aluminum SMD Capacitors For the past fifteen years, the tantalum capacitor has had enormous improvements in ESR because of the replacement of MnO 2
Mechanically Assisted Electroless Barrel-plating Ni-P Coatings Deposited on Carbon Steel
 J. Mater. Sci. Technol., 2010, 26(10), 945-950. Mechanically Assisted Electroless Barrel-plating Ni-P Coatings Deposited on Carbon Steel Zhaoxia Ping 1), Guoan Cheng 1) and Yedong He 2) 1) Key Laboratory
J. Mater. Sci. Technol., 2010, 26(10), 945-950. Mechanically Assisted Electroless Barrel-plating Ni-P Coatings Deposited on Carbon Steel Zhaoxia Ping 1), Guoan Cheng 1) and Yedong He 2) 1) Key Laboratory
Mechanism of Building-Up Deposited Layer during Electro-Spark Deposition
 Journal of Surface Engineered Materials and Advanced Technology, 2012, 2, 258-263 http://dx.doi.org/10.4236/jsemat.2012.24039 Published Online October 2012 (http://www.scirp.org/journal/jsemat) Mechanism
Journal of Surface Engineered Materials and Advanced Technology, 2012, 2, 258-263 http://dx.doi.org/10.4236/jsemat.2012.24039 Published Online October 2012 (http://www.scirp.org/journal/jsemat) Mechanism
Corrosion Behaviour of aluminium alloys used in heat-exchangers Joana Catarina Videira Madeira. 1. Introduction to Aluminium Science and Technology
 Corrosion Behaviour of aluminium alloys used in heat-exchangers Joana Catarina Videira Madeira 1. Introduction to Aluminium Science and Technology Although many metals can alloy with aluminium, only few
Corrosion Behaviour of aluminium alloys used in heat-exchangers Joana Catarina Videira Madeira 1. Introduction to Aluminium Science and Technology Although many metals can alloy with aluminium, only few
Electrochemical impedance study of initial lithium ion intercalation into graphite powders
 Electrochimica Acta 46 (2001) 1793 1813 www.elsevier.nl/locate/electacta Electrochemical impedance study of initial lithium ion intercalation into graphite powders Chunsheng Wang a, *, A. John Appleby
Electrochimica Acta 46 (2001) 1793 1813 www.elsevier.nl/locate/electacta Electrochemical impedance study of initial lithium ion intercalation into graphite powders Chunsheng Wang a, *, A. John Appleby
Study on the synergistic effects of additives on the copper corrosion inhibitors
 ISSN(Print) : 2320 1967 ISSN(Online) : 2320 1975 ChemXpress 5(1), 25-31, (2014) Study on the synergistic effects of additives on the copper corrosion inhibitors A.Rahimi*, M.Chaghazardi Electrochemistry
ISSN(Print) : 2320 1967 ISSN(Online) : 2320 1975 ChemXpress 5(1), 25-31, (2014) Study on the synergistic effects of additives on the copper corrosion inhibitors A.Rahimi*, M.Chaghazardi Electrochemistry
1. INTRODUCTION. Further exploitation of the technology led to coatings which offer huge
 1. INTRODUCTION 1.1. GENERAL Surface engineering deals with modification of the surface properties of engineering components as majority of failures originate from the surface. Wear and corrosion are the
1. INTRODUCTION 1.1. GENERAL Surface engineering deals with modification of the surface properties of engineering components as majority of failures originate from the surface. Wear and corrosion are the
components and inorganic Li salts from PST-90. When contacting with Li metal, PST-
 Supplementary Figure 1. Proposed mechanism for the formation of organic components and inorganic Li salts from PST-90. When contacting with Li metal, PST- 1 90 first generates high order organopolysulfide
Supplementary Figure 1. Proposed mechanism for the formation of organic components and inorganic Li salts from PST-90. When contacting with Li metal, PST- 1 90 first generates high order organopolysulfide
MEASURING THE EFFECTIVENESS OF MIGRATING CORROSION INHIBITORS (MCIs) BY ELECTROCHEMICAL TECHNIQUES
 MEASURING THE EFFECTIVENESS OF MIGRATING CORROSION INHIBITORS (MCIs) BY ELECTROCHEMICAL TECHNIQUES By Paul Vrkljan Alla Furman Christophe Chandler Cortec Corporation 4119 White Bear Parkway St. Paul, MN
MEASURING THE EFFECTIVENESS OF MIGRATING CORROSION INHIBITORS (MCIs) BY ELECTROCHEMICAL TECHNIQUES By Paul Vrkljan Alla Furman Christophe Chandler Cortec Corporation 4119 White Bear Parkway St. Paul, MN
Tutorial Corrosion II. Electrochemical characterization with EC-Lab techniques
 Tutorial Corrosion II Electrochemical characterization with EC-Lab techniques 1 OUTLINE 1. Introduction 2. Types of corrosion a) Uniform corrosion b) Localized corrosion 3. Corrosion experiment 4. EC-Lab
Tutorial Corrosion II Electrochemical characterization with EC-Lab techniques 1 OUTLINE 1. Introduction 2. Types of corrosion a) Uniform corrosion b) Localized corrosion 3. Corrosion experiment 4. EC-Lab
Grain Sizes and Surface Roughness in Platinum and Gold Thin Films. L.L. Melo, A. R. Vaz, M.C. Salvadori, M. Cattani
 Journal of Metastable and Nanocrystalline Materials Vols. 20-21 (2004) pp. 623-628 online at http://www.scientific.net 2004 Trans Tech Publications, Switzerland Grain Sizes and Surface Roughness in Platinum
Journal of Metastable and Nanocrystalline Materials Vols. 20-21 (2004) pp. 623-628 online at http://www.scientific.net 2004 Trans Tech Publications, Switzerland Grain Sizes and Surface Roughness in Platinum
EFFECT OF COPPER AND COPPER OXIDE ON CORROSION OF BOILER STEEL
 06 koperoxide www.hbscc.nl - 1 - EFFECT OF COPPER AND COPPER OXIDE ON CORROSION OF BOILER STEEL W.M.M. HUIJBREGTS Mitteilungen der V.G.B., Vol. 51, No.3, pp. 229-235, June 1971 ABSTRACT Electrochemical
06 koperoxide www.hbscc.nl - 1 - EFFECT OF COPPER AND COPPER OXIDE ON CORROSION OF BOILER STEEL W.M.M. HUIJBREGTS Mitteilungen der V.G.B., Vol. 51, No.3, pp. 229-235, June 1971 ABSTRACT Electrochemical
Electrochemical Behavior Analyses of Anodic Oxide Film Obtained on TA2 Pure Titanium in Sulfuric Acid Electrolyte
 157 A publication of CHEMICAL ENGINEERING TRANSACTIONS VOL. 59, 2017 Guest Editors: Zhuo Yang, Junjie Ba, Jing Pan Copyright 2017, AIDIC Servizi S.r.l. ISBN 978-88-95608-49-5; ISSN 2283-9216 The Italian
157 A publication of CHEMICAL ENGINEERING TRANSACTIONS VOL. 59, 2017 Guest Editors: Zhuo Yang, Junjie Ba, Jing Pan Copyright 2017, AIDIC Servizi S.r.l. ISBN 978-88-95608-49-5; ISSN 2283-9216 The Italian
HAND BOOK OF ELECTROPLATING ANODIZING & SURFACE FINISHING TECHNOLOGY
 HAND BOOK OF ELECTROPLATING ANODIZING & SURFACE FINISHING TECHNOLOGY Principles of Electroplating The Electro Chemical Series Electrodeposition Electrochemical Terms Using a Copper Anode Using an Inert
HAND BOOK OF ELECTROPLATING ANODIZING & SURFACE FINISHING TECHNOLOGY Principles of Electroplating The Electro Chemical Series Electrodeposition Electrochemical Terms Using a Copper Anode Using an Inert
Corrosion behavior of composition modulated multilayer Zn Co electrodeposits produced using a single-bath technique
 J Appl Electrochem (2009) 39:339 345 DOI 10.1007/s10800-008-9677-1 ORIGINAL PAPER Corrosion behavior of composition modulated multilayer Zn Co electrodeposits produced using a single-bath technique V.
J Appl Electrochem (2009) 39:339 345 DOI 10.1007/s10800-008-9677-1 ORIGINAL PAPER Corrosion behavior of composition modulated multilayer Zn Co electrodeposits produced using a single-bath technique V.
All-solid-state Li battery using a light-weight solid electrolyte
 All-solid-state Li battery using a light-weight solid electrolyte Hitoshi Takamura Department of Materials Science, Graduate School of Engineering, Tohoku University Europe-Japan Symposium, Electrical
All-solid-state Li battery using a light-weight solid electrolyte Hitoshi Takamura Department of Materials Science, Graduate School of Engineering, Tohoku University Europe-Japan Symposium, Electrical
Introduction to Electrochemical Biosensors. Lecture 3
 1 Introduction to Electrochemical Biosensors Lecture 3 2 Summary Introduction to Electrochemical Biosensors Potentiometric sensors Amperometric sensors Oxygen sensing (not a biosensor) Microelectrodes
1 Introduction to Electrochemical Biosensors Lecture 3 2 Summary Introduction to Electrochemical Biosensors Potentiometric sensors Amperometric sensors Oxygen sensing (not a biosensor) Microelectrodes
INFLUENCE OF MURRAYA KOENIGII (CURRY LEAVES) EXTRACT ON THE CORROSION INHIBITION OF CARBON STEEL IN HCl SOLUTION
 http://www.rasayanjournal.com Vol.3, No.1 (2010),74-81 ISSN: 0974-1496 CODEN: RJCABP INFLUENCE OF MURRAYA KOENIGII (CURRY LEAVES) EXTRACT ON THE CORROSION INHIBITION OF CARBON STEEL IN HCl SOLUTION A.
http://www.rasayanjournal.com Vol.3, No.1 (2010),74-81 ISSN: 0974-1496 CODEN: RJCABP INFLUENCE OF MURRAYA KOENIGII (CURRY LEAVES) EXTRACT ON THE CORROSION INHIBITION OF CARBON STEEL IN HCl SOLUTION A.
Arch. Metall. Mater. 63 (2018), 2,
 Arch. Metall. Mater. 63 (2018), 2, 1031-1036 DOI: 10.24425/122438 K. KOŁCZYK* #, W. ZBOROWSKI*, D. KUTYŁA*, A. KWIECIŃSKA*, R. KOWALIK*, P. ŻABIŃSKI* INVESTIGATION OF TWO-STEP METALLIZATION PROCESS OF
Arch. Metall. Mater. 63 (2018), 2, 1031-1036 DOI: 10.24425/122438 K. KOŁCZYK* #, W. ZBOROWSKI*, D. KUTYŁA*, A. KWIECIŃSKA*, R. KOWALIK*, P. ŻABIŃSKI* INVESTIGATION OF TWO-STEP METALLIZATION PROCESS OF
EIS studies of a corrosion inhibitor behavior under multiphase ow conditions
 Corrosion Science 42 (2000) 979±990 EIS studies of a corrosion inhibitor behavior under multiphase ow conditions Y. Chen*, T. Hong, M. Gopal, W.P. Jepson NSF I/UCRC, Corrosion in Multiphase Systems Center,
Corrosion Science 42 (2000) 979±990 EIS studies of a corrosion inhibitor behavior under multiphase ow conditions Y. Chen*, T. Hong, M. Gopal, W.P. Jepson NSF I/UCRC, Corrosion in Multiphase Systems Center,
Laboratory Experiments in Corrosion Engineering II
 Lecture - 40 Laboratory Experiments in Corrosion Engineering II Keywords: Polarization Experiments, Pitting Potentials, Microbial Corrosion. A. Electrochemical tests in a given environment Polarization
Lecture - 40 Laboratory Experiments in Corrosion Engineering II Keywords: Polarization Experiments, Pitting Potentials, Microbial Corrosion. A. Electrochemical tests in a given environment Polarization
FAILURE MECHANISMS OF NANO SILICON ANODES: AN ELECTRODE POROSITY EVOLUTION MODEL
 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2014 Supplementary data for : FAILURE MECHANISMS OF NANO SILICON ANODES: AN ELECTRODE
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2014 Supplementary data for : FAILURE MECHANISMS OF NANO SILICON ANODES: AN ELECTRODE
Electronic Supplementary Material (ESI) for Chemical Communications This journal is The Royal Society of Chemistry 2013
 Sodium-ion battery based on ion exchange membranes as electrolyte and separator Chengying Cao, Weiwei Liu, Lei Tan, Xiaozhen Liao and Lei Li* School of Chemical and Chemistry Engineering, Shanghai Jiaotong
Sodium-ion battery based on ion exchange membranes as electrolyte and separator Chengying Cao, Weiwei Liu, Lei Tan, Xiaozhen Liao and Lei Li* School of Chemical and Chemistry Engineering, Shanghai Jiaotong
International Journal of ChemTech Research CODEN (USA): IJCRGG ISSN: Vol.8, No.8, pp , 2015
 International Journal of ChemTech Research CODEN (USA): IJCRGG ISSN: 0974-4290 Vol.8, No.8, pp 134-142, 2015 Experimental study of inhibition effect, synergistic effect and antagonistic behaviour of L-phenylalanine
International Journal of ChemTech Research CODEN (USA): IJCRGG ISSN: 0974-4290 Vol.8, No.8, pp 134-142, 2015 Experimental study of inhibition effect, synergistic effect and antagonistic behaviour of L-phenylalanine
Effect of heat input on Stellite 6 coatings on a medium carbon steel substrate by laser cladding
 University of Wollongong Research Online Faculty of Engineering and Information Sciences - Papers: Part A Faculty of Engineering and Information Sciences 2015 Effect of heat input on Stellite 6 coatings
University of Wollongong Research Online Faculty of Engineering and Information Sciences - Papers: Part A Faculty of Engineering and Information Sciences 2015 Effect of heat input on Stellite 6 coatings
Effect of Sodium Fluoride on the Stability of Dental Alloys in Artificial Saliva
 Effect of Sodium Fluoride on the Stability of Dental Alloys in Artificial Saliva DANIELA COVACIU ROMONTI 1, INGRID MILOSEV 2, IOANA DEMETRESCU 1 * 1 University Politehnica of Bucharest, Faculty of Applied
Effect of Sodium Fluoride on the Stability of Dental Alloys in Artificial Saliva DANIELA COVACIU ROMONTI 1, INGRID MILOSEV 2, IOANA DEMETRESCU 1 * 1 University Politehnica of Bucharest, Faculty of Applied
The Effect of Reducing Agents on Electroless Copper Plating Process
 Universities Research Journal 2011, Vol. 4, No. 3 The Effect of Reducing Agents on Electroless Copper Plating Process May Zin Oo 1, Sandar Tun 2 and Kyaw Myo Naing 3 Abstract This work is aimed mainly
Universities Research Journal 2011, Vol. 4, No. 3 The Effect of Reducing Agents on Electroless Copper Plating Process May Zin Oo 1, Sandar Tun 2 and Kyaw Myo Naing 3 Abstract This work is aimed mainly
Morphology of Copper Coatings Electroplated in an Ultrasonic Field
 Morphology of Copper Coatings Electroplated in an Ultrasonic Field L. Martins a, J.I. Martins a,b, A.S. Romeira a, M.E. Costa a, J. Costa a, M. Bazzaoui a,b a CISE, Departamento de Engenharia Electrotécnica,
Morphology of Copper Coatings Electroplated in an Ultrasonic Field L. Martins a, J.I. Martins a,b, A.S. Romeira a, M.E. Costa a, J. Costa a, M. Bazzaoui a,b a CISE, Departamento de Engenharia Electrotécnica,
P. Møller Department of Mechanical Engineering, Technical University of Denmark, Lyngby, Denmark
 FIB-SEM investigation of trapped intermetallic particles in anodic oxide films on AA050 aluminium M. Jariyaboon Department of Mechanical Engineering, Technical University of Denmark, Lyngby, Denmark Department
FIB-SEM investigation of trapped intermetallic particles in anodic oxide films on AA050 aluminium M. Jariyaboon Department of Mechanical Engineering, Technical University of Denmark, Lyngby, Denmark Department
The Deposition Characteristics of Accelerated Nonformaldehyde Electroless Copper Plating
 C558 Journal of The Electrochemical Society, 15 8 C558-C562 23 13-4651/23/15 8 /C558/5/$7. The Electrochemical Society, Inc. The Deposition Characteristics of Accelerated Nonformaldehyde Electroless Copper
C558 Journal of The Electrochemical Society, 15 8 C558-C562 23 13-4651/23/15 8 /C558/5/$7. The Electrochemical Society, Inc. The Deposition Characteristics of Accelerated Nonformaldehyde Electroless Copper
